UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the fiscal year ended December 31, 2013.
| |
o | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. |
For the transition period from to .
Commission File Number
001-35342
NEWLINK GENETICS CORPORATION
(Exact name of Registrant as specified in Its Charter)
|
| | |
| Delaware | | 42-1491350 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
2503 South Loop Drive
Ames, Iowa 50010
(515) 296-5555
(Address, including zip code, and telephone number, including area code, of principal executive offices)
|
| | |
| Securities registered pursuant to Section 12(b) of the Act: | Common Stock, par value $0.01 | |
| Name of each exchange on which registered: | The Nasdaq Stock Market LLC | |
| Securities registered pursuant to Section 12(g) of the Act: | None | |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark whether the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
| | |
Large accelerated filer o | | Accelerated filer x |
| | | |
Non-accelerated filer o | | Smaller reporting company o |
| (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the common stock held by non-affiliates of the registrant based on the closing sale price of the registrant’s common stock on June 30, 2013, as reported by the NASDAQ Stock Market, was $342,253,457. Shares of the registrant’s common stock beneficially owned by each executive officer and director of the registrant and by each person known by the registrant to beneficially own 10% or more of its outstanding common stock have been excluded, in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 7, 2014, there were 27,861,429 shares of the registrant’s Common Stock, par value $0.01 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference to our definitive Proxy Statement for our 2014 Annual Meeting of Stockholders (the “2014 Proxy Statement”).
NewLink Genetics Corporation
Table of Contents
|
| | | |
| | | | Page |
| Part I | |
| | Item 1. | | |
| | Item 1A. | | |
| | Item 1B. | | |
| | Item 2. | | |
| | Item 3. | | |
| | Item 4. | | |
| Part II | |
| | Item 5. | | |
| | Item 6. | | |
| | Item 7. | | |
| | Item 7A. | | |
| | Item 8. | | |
| | Item 9. | | |
| | Item 9A. | | |
| | Item 9B. | | |
| Part III | |
| | Item 10. | | |
| | Item 11. | | |
| | Item 12. | | |
| | Item 13. | | |
| | Item 14. | | |
| Part IV | |
| | Item 15. | | |
| Signatures | |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, and such statements are subject to the “safe harbor” created by those sections. Forward-looking statements involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Annual Report on Form 10-K, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this Annual Report on Form 10-K include, among other things, statements regarding the following: plans to develop and commercialize our product candidates; ongoing and planned preclinical studies and clinical trials, including the timing for completion of enrollment of one of our Phase 3 clinical trials for HyperAcute Pancreas and outcomes of both of our Phase 3 clinical trial for HyperAcute Pancreas; the timing for completion of enrollment and outcomes of our other ongoing clinical studies; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; the clinical utility of any potential products; our plans to leverage our existing technologies to discover and develop additional product candidates; our ability to quickly and efficiently identify and develop product candidates; our commercialization, marketing and manufacturing capabilities and strategy; our intellectual property position; the potential benefits of strategic collaboration agreements and our ability to enter into strategic arrangements; our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; and other risks and uncertainties, including those listed under the caption “Risk Factors.”
The forward-looking statements in this Annual Report on Form 10-K represent our views as of the date of this Annual Report on Form 10-K. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These statements involve known and unknown risks and uncertainties that may cause our, or our industry's results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Factors that may cause or contribute to such differences include, among other things, those discussed under the captions “Business,” “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements not specifically described above also may be found in these and other sections of this report.
We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so, even if new information becomes available, except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Annual Report on Form 10-K.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. You are also advised to consult any further disclosures we make on related subjects in our Quarterly Reports on Form 10-Q, and our Current Reports on Form 8-K.
PART I
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing novel immunotherapeutic products to improve treatment options for patients with cancer. Our portfolio includes both biologic and small-molecule immunotherapy product candidates. Our biologic product candidates are based on our proprietary HyperAcute® immunotherapy technology, which is designed to stimulate the human immune system. Our small-molecule product candidates are focused on breaking the immune system's tolerance to cancer by inhibiting the indoleamine-(2,3)-dioxygenase, or IDO, pathway. We believe that our immunotherapeutic technologies have the potential to lead to multiple product candidates, targeting a wide range of oncology indications that can be used either alone or in combination with other therapies.
Our lead product candidate, HyperAcute Pancreas immunotherapy (algenpantucel-L) or HyperAcute Pancreas, is being studied in two Phase 3 clinical trials; one in surgically-resected pancreatic cancer patients that is being performed under a Special Protocol Assessment, or SPA, with the United States Food and Drug Administration, or FDA, and one in patients with locally advanced pancreatic cancer. We initiated these trials based on encouraging Phase 2 data that suggests improvement in both disease-free and overall survival. We have also received Fast Track and Orphan Drug designations from the FDA for this product candidate for the adjuvant treatment of surgically-resected pancreatic cancer and Orphan Medicinal Product designation for this product candidate from the European Commission. Our additional HyperAcute product candidates in clinical development include HyperAcute Lung immunotherapy (tergenpumatucel-L), or HyperAcute Lung, HyperAcute Melanoma immunotherapy (dorgenmeltucel-L), or HyperAcute Melanoma, HyperAcute Prostate immunotherapy or HyperAcute Prostate and HyperAcute Renal immunotherapy, or HyperAcute Renal. To date, our HyperAcute platform product candidates have been administered to more than 500 patients with cancer, either as a monotherapy or in combination with other therapies.
Our HyperAcute immunotherapy platform consists of novel biologic products designed to stimulate the patient's immune system to recognize and attack cancer cells. HyperAcute product candidates are composed of human, tumor-specific cancer cell lines. These cells have been modified to express alpha-Gal, a carbohydrate to which humans have preexisting immunity. These alpha-Gal-modified cancer cells stimulate an immune response that “educates” the immune system to attack a patient’s own cancer cells. Unlike some immunotherapies, HyperAcute immunotherapies do not require the harvesting of an individual’s tissue or cancer cells in order to produce the vaccine and are manufactured using established tumor-specific human cell lines. In addition, HyperAcute immunotherapies use intact whole cells rather than cell fragments or purified proteins, which we believe results in the stimulation of an enhanced, multi-faceted immune response against the patient’s cancer.
Our indoleamine 2,3-dioxygenase, or IDO, pathway inhibitor platform is focused on developing small molecule drugs that disrupt mechanisms by which tumors evade the patient’s immune system. IDO pathway inhibitors are a class of immune checkpoint inhibitors akin to antibodies targeting cytotoxic T-lymphocyte antigen 4, or CTLA-4 and programmed cell death protein, or PD-1, which represent a potential breakthrough in cancer therapy. Our IDO pathway inhibitors are orally administered small molecules and can potentially be used alone or in combination with other cancer therapies including other checkpoint inhibitors such as CTLA-4 and PD-1, cancer vaccines such as our HyperAcute vaccines and chemotherapy regimens, for potential increased activity. We are actively developing two IDO pathway inhibitors with broad potential across multiple tumor types. The most advanced is indoximod, currently in multiple phase 2 clinical trials in breast cancer and prostate cancer. Our second IDO pathway inhibitor, NLG919, is currently in a Phase 1 clinical trial in patients with solid tumors.
The IDO pathway regulates immune response by suppressing T cell function and enabling local tumor immune escape. Recent studies indicate that the IDO pathway is active in many cancers, both within tumor cells as a direct defense against T cell attack, and also within antigen-presenting cells, or APCs, in tumor-draining lymph nodes resulting in peripheral tolerance to tumor-associated antigens, or TAAs. Cancers may use the IDO pathway to facilitate survival, growth, invasion, and metastasis of malignant cells expressing TAAs that might otherwise be recognized and attacked by the immune system.
In addition to oncology products, we have received grants to develop and commercialize vaccines to control infectious disease. This effort leverages HyperAcute immunotherapy technology, which is applicable to enhancing vaccines for influenza and other pathogens.
Founded in 1999 and headquartered in Ames, Iowa, we have a clinical, research and development staff that is developing our pipeline of product candidates for potential commercialization. In addition to our internal efforts, we have established clinical, research and development collaborations with other companies and organizations. We intend to build our own commercial
organization through which we intend to market our products in the United States. Outside the United States, we intend to seek commercialization and distribution collaborators for our product candidates. At our facilities in the United States, we manufacture the clinical trial supplies for our current product candidates.
Our HyperAcute Cancer Immunotherapy Technology
Our HyperAcute immunotherapy platform consists of novel biologic products designed to stimulate the patient's immune system to recognize and attack cancer cells. HyperAcute product candidates are composed of human, tumor-specific cancer cell lines. These cells have been modified to express alpha-Gal, a carbohydrate to which humans have preexisting immunity. These alpha-Gal-modified cancer cells stimulate an immune response that “educates” the immune system to attack a patient's own cancer cells.
We believe that, compared to some prior immunotherapy approaches, our proprietary HyperAcute immunotherapy technology offers several advantages including:
| |
| • | a robust inherent immune response that harnesses the human body's naturally protective and rapid immune reaction to the alpha-Gal carbohydrate to fight cancer; |
| |
| • | a complex targeted approach that is multi-faceted and involves combined antibody-mediated and multi-cellular responses; and |
| |
| • | an allogeneic, or non-patient specific, approach, in which we manufacture products from genetically modified, allogeneic cells from previously established cell lines, which permits an easier scale-up of the manufacturing process compared to an autologous, or patient specific, approach involving a patient's own cells. |
We believe our HyperAcute immunotherapies operate by exploiting a natural barrier present in humans that protects against infections. This barrier is related to the enzyme alpha-GT. The presence of this enzyme results in the expression of a carbohydrate called alpha-Gal on the surface of affected cells. Introducing alpha-Gal expressing cells to the immune system activates an immune response from preexisting antibodies against alpha-Gal.
Unlike some immunotherapies, HyperAcute immunotherapies do not require the harvesting of an individual's tissue or cancer cells in order to produce the vaccine. HyperAcute immunotherapies are manufactured using established tumor-specific human cell lines. In addition, HyperAcute immunotherapies use intact whole cells rather than cell fragments or purified proteins, which we believe results in the stimulation of an enhanced multi-faceted immune response against the patient's cancer.

HyperAcute immunotherapy product candidates are composed of irradiated, live, allogeneic human cancer cells modified to express the gene that makes alpha-Gal. Exposure to alpha-Gal stimulates the human immune system to attack and destroy the immunotherapy cells on which alpha-Gal is present by activating complement, an important component of the immune system that is capable of cell destruction. After destruction, we believe the resulting cellular fragments bound by anti-alpha-Gal antibodies are processed by the immune system to elicit an enhanced multi-faceted immune response to TAAs common to both the immunotherapy and the patient's tumor cells.
In the case of a HyperAcute cancer immunotherapy, this process results in immune cells that are educated to attack a patient's own cancer cells by virtue of the antigens that the immunotherapy and these tumor cells share and by a more generalized activation of the immune system. Our scientists have shown in mouse models of cancer that the immune system responds after a HyperAcute injection by attacking all similar cancer cells, including those that have no alpha-Gal carbohydrate.
HyperAcute immunotherapies are designed to break tolerance and enable longer duration of anti-tumor effect. We believe that our HyperAcute immunotherapy technology induces a unique combination of advantageous immunologic effects.
HyperAcute Pipeline
We have multiple HyperAcute immunotherapy product candidates currently under development specific to multiple types of cancer.
| |
| • | Algenpantucel-L is currently being studied in two Phase 3 trials: |
| |
| ▪ | IMPRESS (Immunotherapy for Pancreatic Resectable Cancer Survival Study) |
| |
| ▪ | PILLAR (Pancreatic Immunotherapy with algenpantucel-L for Locally Advanced non-Resectable) |
| |
| • | Tergenpumatucel-L is being investigated in progressive or relapsed non-small cell lung cancer |
| |
| • | Dorgenmeltucel-L is being investigated in advanced melanoma |
| |
| • | HyperAcute Renal is currently in a Phase 1 trial in renal cancer |
We have also created HyperAcute immunotherapies for prostate and breast cancers.
Our IDO Pathway Inhibitor Product Candidate
The IDO pathway inhibitor platform is focused on developing small molecule drugs that disrupt mechanisms by which tumors evade the patient’s immune system. IDO pathway inhibitors are a class of immune checkpoint inhibitors akin to antibodies targeting CTLA-4 and PD-1, which represent a potential breakthrough in cancer therapy. Blocking IDO has been shown preclinically to reduce immunosuppression and enhance activation offering the potential for enhanced anti-tumor response without additional toxicities. IDO has demonstrated preclinical synergy in combination with other immunotherapies including other checkpoint inhibitors and cancer vaccines as well as chemotherapy or radiation.

The IDO pathway regulates immune response by suppressing T cell function and enabling local tumor immune escape. Recent studies indicate that the IDO pathway is active in many cancers, both within tumor cells as a direct defense against T cell attack, and also within antigen-presenting cells in tumor-draining lymph nodes resulting in peripheral tolerance to TAAs. Cancers may use the IDO pathway to facilitate survival, growth, invasion, and metastasis of malignant cells expressing TAAs that might otherwise be recognized and attacked by the immune system.
Our IDO pathway inhibitors are orally administered small molecules and can potentially be used alone or in combination with other cancer therapies including other checkpoint inhibitors such as CTLA-4 and PD-1, cancer vaccines such as our HyperAcute vaccines and chemotherapy regimens for potential increased activity.
We are actively developing two IDO pathway inhibitors with broad potential across multiple tumor types. The most advanced is indoximod, which is being studied in combination with docetaxel in metastatic breast cancer and as follow-on therapy after sipuleucel-T treatment in metastatic, castrate-resistant prostate cancer. The second IDO pathway inhibitor, NLG919, has shown promising preclinical results and is currently in a Phase 1 clinical trial in patients with solid tumors.
Our IDO pathway inhibitors, indoximod and NLG919, are being investigated as part of various combination regimens including with other checkpoint inhibitors, cancer vaccines and chemotherapy in metastatic breast cancer, metastatic prostate cancer, and other cancers.
Our Strategy
Our strategy is to discover, develop and commercialize immunotherapeutic products for the treatment of cancer where the needs of patients are unmet by current therapies. The critical components of our business strategy include:
| |
| • | Complete the Phase 3 clinical trial of algenpantucel-L, our lead HyperAcute pancreas immunotherapy product candidate, and gain regulatory approval. |
| |
| • | Develop sales and marketing infrastructure to commercialize algenpantucel-L product candidate in the United States and establish commercial collaborations in other regions. |
| |
| • | Expand the target market for our algenpantucel-L in locally advanced patients. |
| |
| • | Advance our HyperAcute Lung and HyperAcute Melanoma product candidates through additional clinical trials. |
| |
| • | Expand our HyperAcute manufacturing capabilities. |
| |
| • | Investigate our HyperAcute technology in additional oncology indications. |
| |
| • | Further develop for commercialization Indoximod (D-1MT) and NLG919, two small-molecule product candidates focused on modulating the IDO pathway by different mechanisms of action. |
| |
| • | Strengthen our pipeline of immune stimulatory drug candidates. |
Cancer Market Overview
Cancer is the second-leading cause of death in the United States; the American Cancer Society estimated that 580,350 deaths would occur in 2013. Despite a number of advancements in the diagnosis and treatment of cancer over the past decade, overall five-year survival rates from all cancer types is 68% for the period spanning 2002-2008 according to the American Cancer Society.
Cancer is characterized by abnormal cells that grow and proliferate, forming masses called tumors. Under certain circumstances, these proliferating cells can metastasize, or spread, throughout the body and produce deposits of tumor cells called metastases. As the tumors grow, they may cause tissue and organ failure and, ultimately, death. To be effective, cancer therapies must eliminate or control the growth of the cancer.
The specialized cells of the immune system recognize specific chemical structures called antigens. Generally, foreign antigens trigger an immune response that results in the removal of disease-causing agents from the body. Cancer cells, however, frequently display antigens that are also found on normal cells. The immune system may not be able to distinguish between tumors and normal cells and, thus, may be unable to mount a strong anti-cancer response. Tumors also have various defense mechanisms that may prevent the immune system from fully activating.
Current therapies, such as surgery, radiation, hormone treatments and chemotherapy, do not address this evasive characteristic of cancer and may not have the desired therapeutic effect. Active immunotherapies stimulate the immune system, the body's natural mechanism for fighting disease, and may overcome some of the limitations of current standard-of-care cancer therapies.
Limitations of Current Cancer Therapies
We believe current cancer treatment alternatives suffer from a number of limitations that impair their effectiveness including:
| |
| • | Toxicity. Chemotherapeutic agents are highly toxic to the human body and often cause a variety of side effects, which may include nausea and vomiting, bleeding, anemia and mucositis. Targeted therapeutics may have fewer systemic toxicities, but still tend to have off-target effects such as gastrointestinal inflammation, severe skin reactions and breathing difficulties. These effects limit a patient's ability to tolerate treatment thereby depriving the patient of the potential benefit of additional treatments or treatment combinations that might otherwise destroy or prevent the growth of cancer cells. Once educated as to the limited efficacy, limited increased survival and potentially significant toxicity of existing treatment alternatives, patients diagnosed with terminal cancer often choose to limit or forego therapy in order to avoid further compromising their quality of life. Patients with advanced stage cancer often cannot tolerate cancer therapy, and certain therapies have been shown to hasten death in some cases as the patient's health deteriorates. |
| |
| • | Development of resistance. While many current therapeutic approaches may be effective against a particular target, the overall impact of these therapies on treating cancer is limited because the abundance and diversity of tumor cells are believed to enable cancers to adapt and become resistant to these treatments over time resulting in reduced longer-term efficacy. |
| |
| • | Short-term approach. Incremental survival benefit is the primary objective of many currently marketed and development-stage cancer therapeutics. In general, many drugs show modest impact on overall survival or only affect progression-free survival. Other than surgical tumor removal, curative intent is often not a focus or realistic potential outcome of many current cancer therapies. |
| |
| • | Immune system suppression. Cancer is difficult to treat in part because cancer cells use sophisticated strategies to evade the immune system. Current approaches to cancer treatment generally involve introduction of an agent, such as a chemical, an antibody or radiation. These agents cause cell apoptosis (programmed cell death) or inhibit the proliferation of all cells, including immune cells, thereby indirectly suppressing the immune system. A weakened immune system not only further inhibits the body's natural ability to fight cancer, but also causes patients to become more susceptible to infections and other diseases. |
Potential Advantages of HyperAcute Immunotherapy Over Current Cancer Therapies
We believe our HyperAcute immunotherapy has the following potential advantages over existing therapies, which may enable us to develop commercial products that extend both survival and quality of life for cancer patients:
| |
| • | Robust, innate immune response. Our HyperAcute immunotherapy technology is designed to fight cancer by activating the human body's naturally protective and rapid immune response to the alpha-Gal carbohydrate. |
| |
| • | Complex, multi-targeted approach. We believe our HyperAcute immunotherapy technology attacks cancer through several mechanisms. Initially, by introducing allogeneic, whole cancer cells incorporating alpha-Gal to the body, our HyperAcute immunotherapy is designed to teach the immune system to attack specific cancer cells, such as pancreas, lung or melanoma cancer cells, with both antibody mediated and cellular immune responses. Secondly, by using multiple whole cancer cell lines, our HyperAcute immunotherapy targets multiple tumor proteins simultaneously, which we believe increases the probability of stimulating an effective immune response to the heterogeneous cells that are present in cancer. |
| |
| • | Favorable safety profile. We have not observed significant additional systemic toxicities when HyperAcute immunotherapy has been added to standard chemotherapy or radiation regimens. Our HyperAcute immunotherapy technology is designed to stimulate a strong or robust immune response to specific cancer cells while limiting the risks of off-target effects. |
| |
| • | Broad applicability. We believe that the novel mechanism of action, good tolerability and favorable safety profile will enable our HyperAcute product candidates to have potential benefits across multiple disease stages and tumor types and in combination with other therapies. |
| |
| • | Potential application as single agent adjuvant therapy. We believe many patients who are too ill to tolerate the toxicities and off target effects of other cancer treatments may be able to benefit from our HyperAcute product candidates. We also believe that the safety profile of our HyperAcute immunotherapies may make them suitable for use in patients with low risk of recurrence or metastasis who choose not to receive chemotherapy due to its toxicity relative to the potential therapeutic benefits. |
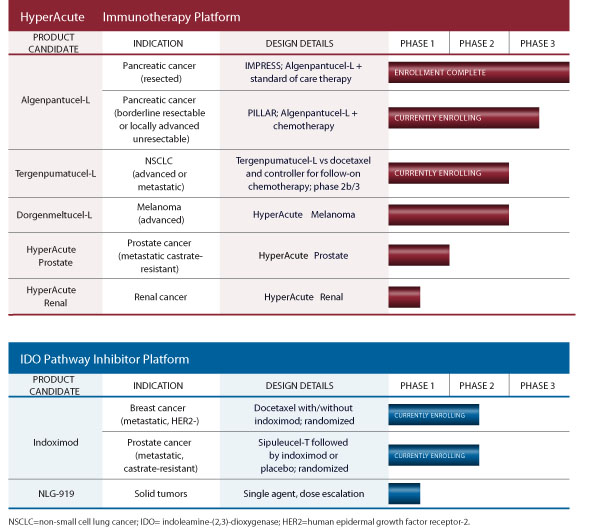
Our Product Pipeline
The chart below summarizes our current product candidates and their stages of development. Our product candidate pipeline includes biologic and small molecule immunotherapy product candidates intended to treat a wide range of oncology indications.
Our Algenpantucel-L (HyperAcute Pancreas) Cancer Immunotherapy Product Candidate
Our lead HyperAcute product candidate, algenpantucel-L, is an investigational immunotherapy for pancreatic cancer. The product consists of two pancreatic cancer cell lines that have been genetically modified to express alpha-Gal carbohydrates on cell surface molecules.1 Upon injection into the patient, the alpha-Gal stimulates an immune response against pancreatic cancer-specific antigens in the tumor cell lines. The patient’s immune system then targets the patient's own pancreatic cancer cells, destroying them. In the adjuvant setting, the immune response targets and eradicates residual tumor cells in conjunction with chemotherapy or chemotherapy and chemoradiation.
Algenpantucel-L is currently being studied in combination with standard of care in two phase 3 trials:
| |
| • | IMPRESS (Immunotherapy for Pancreatic Resectable Cancer Survival Study) |
| |
| • | PILLAR (Pancreatic Immunotherapy with Algenpantucel-L for Locally Advanced Non-Resectable) |
Our Phase 3 IMPRESS study in surgically-resected pancreatic cancer patients is being performed under an SPA with the FDA. Algenpantucel-L has also received Fast Track and Orphan Drug designations from the FDA for the adjuvant treatment of surgically-resected pancreatic cancer.
In May 2010, we initiated our Phase 3 IMPRESS clinical trial for algenpantucel-L. This trial is an open-label, randomized, controlled, multi-center Phase 3 clinical trial, evaluating Stage I and Stage II surgically-resected pancreatic cancer patients, according to the American Joint Committee on Cancer classification system, or AJCC system, who have no detectable disease by a CT scan. The primary endpoint of the clinical trial is overall survival, with secondary endpoints of disease-free survival, safety, toxicity and immunological responses. The SPA we have entered into with the FDA contemplated that we would enroll up to 722 patients, which, under the statistical plan contained in the SPA would enable us to demonstrate statistically significant improvement in median overall survival at the end of the trial. We completed enrollment in September 2013 with 722 patients and project the first and second interim analyses of data from this study will occur in early 2014 and late 2014, respectively.
We initiated the IMPRESS trial based on encouraging interim data from our Phase 2 clinical trial that was fully enrolled in March 2010. As of June 2012, all patients in this Phase 2 study had reached at least 24 months of follow-up with a median follow-up period of approximately 33 months. The study met its primary objective with an established median disease--free survival of 14.1 months. The secondary endpoint of overall survival showed one-year overall survival to be 86 percent. As of June 2012, interim efficacy data for the 26 patients receiving high dose therapy demonstrated median disease-free survival of 15.3 months and a one-year overall survival rate of 96 percent. To date, algenpantucel-L has demonstrated good tolerability and a favorable safety profile in the Phase 2 study. The most common treatment-related adverse reactions (reported by at least 5 percent of patients) for the product candidate included injection site reactions (40%), induration (19%), injection site pain (10%), pyrexia (9%), erythema (7%), fatigue (19%), nausea (6%), lymphopenia (6%) and pruritus (5%). All of these events were grade three or less. The common terminology criteria, or CTC, of the National Cancer Institute, or NCI, categorizes adverse events into five grades, where grade one is mild, grade two is moderate, grade three is severe, grade four is life-threatening and grade five is death.
In 2013, we launched a Phase 3 study for algenpantucel-L in patients whose pancreatic cancer is locally advanced (PILLAR). This trial is expected to enroll 280 patients with borderline or locally advanced unresectable disease with the primary endpoint for the study being overall survival after treatment with a regimen of chemotherapy (either FOLFIRINOX or gemcitabine/nab-paclitaxel) with or without algenpantucel-L.
Market Opportunity
Pancreatic cancer is the fourth leading cause of cancer death in the United States and is one of the most difficult cancers to treat. Over 46,420 Americans are expected to be diagnosed with pancreatic cancer in 2014, and the incidence rates have been increasing by 1.5% per year since 2004. Approximately 20% of those patients will be eligible for surgical resection. Adjuvant therapy with chemotherapy or chemoradiation is standard of care postresection. However, the 5-year survival rate for these patients is less than 15%.
Current treatment recommendations are primarily palliative in nature, usually including combined modality treatments with gemcitabine, infusional 5-FU chemotherapy and external-beam radiation therapy (50 Gy). These treatments are rarely effective in shrinking a tumor to the point where it can be successfully resected. For patients with locally advanced, unresectable/borderline resectable pancreatic cancer, options include chemotherapy with or without chemoradiation. There is a trend towards using combination regimens that have shown benefits for conversion to resectability and increased survival. However, many of these regimens are associated with considerable toxicity. There remains a critical need for novel therapies that can improve outcomes for patients with pancreatic cancer.
Clinical Trials
Phase 3 Clinical Trial (IMPRESS)
In May 2010, we initiated our IMPRESS Phase 3 clinical trial for algenpantucel-L. This trial is an open-label, randomized, controlled, multi-center Phase 3 clinical trial, evaluating 722 patients with Stage I and Stage II (AJCC) cancer, who have no detectable disease by a CT scan. The primary endpoint of the clinical trial is overall survival, with secondary endpoints of disease-free survival, safety, toxicity and immunological responses. In September 2013, we completed the enrollment of 722 patients in this clinical trial. Based on our discussions with the FDA, we believe this number of patients will enable us to demonstrate statistically significant improvement in median overall survival at the end of the trial.
Current adjuvant standard-of-care regimens for post-resection pancreatic cancer patients include gemcitabine alone or a combination of gemcitabine plus 5-FU based chemoradiotherapy. In our Phase 3 clinical trial, 50% of the patients receive standard adjuvant therapy with algenpantucel-L and 50% receive standard adjuvant therapy without algenpantucel-L. Data from our Phase 2 clinical trial demonstrated a statistically significant improvement in disease-free survival at one year for the high dose (300 million cells) arm of the study. Therefore, we selected the 300 million cell dose as the treatment dose for our Phase 3 clinical trial. In addition, we reasoned empirically that considering the observed dose response, higher doses of treatment might provide further benefit. We therefore modified the treatment schedule for all patients receiving algenpantucel-L to increase the number of immunotherapy treatments from 12 to up to 18 treatments given every two weeks over a period of approximately six months followed by six monthly injections. Patients in the study are being monitored with periodic imaging to check for recurrences for at least five years after surgery or until death occurs.
The clinical trial includes interim evaluations for both overall survival and disease-free survival when approximately one-half of the expected number of deaths have occurred and, if needed, again when approximately three-quarters of the expected number of deaths have occurred. Our SPA specifies that if results show a highly statistically significant effect on survival we may stop the trial and apply for marketing approval. Our statistical modeling indicates that a 45% or 30% improvement in overall survival, relative to controls through the period when one-half or three-quarters, respectively, of the expected number of deaths have occurred, would be highly statistically significant. Overall survival refers to the duration of life after surgery. Disease-free survival refers to the period of time after surgical resection when no evidence of disease is detected.
When initially diagnosed, patients eligible for our Phase 3 clinical trials have localized tumors that can potentially be completely removed based upon strict imaging criteria. In addition, the patients are generally strong enough to survive a major surgical procedure that involves an inherent significant risk of death. Patients are not eligible to participate in this trial until pathology and post-operative imaging studies indicate that they are without clinical evidence of residual tumor as observed by a CT scan. As a result, patients admitted to the trials have minimal residual tumor burden and possess generally intact immune systems, characteristics that we believe improve the likelihood of meaningful response.
Phase 3 Clinical Trial (PILLAR)
The recent emergence of combination therapy with FOLFIRINOX (leucovorin, 5-FU, oxaliplatin and irinotecan) has been promising in the metastatic disease and is being tested extensively in the locoregional disease setting. We believe that the addition of algenpantucel-L immunotherapy to FOLFIRINOX combination chemotherapy has the potential to improve survival of patients in this population. To assess this potential treatment combination we recently initiated the PILLAR Phase 3 clinical study that will assess overall survival after treatment with a regimen of FOLFIRINOX with or without algenpantucel-L immunotherapy in subjects who have borderline resectable or locally advanced unresectable pancreatic cancer. In addition to overall survival, secondary endpoints include assessment of progression free survival after treatment with a regimen of FOLFIRINOX with or without algenpantucel-L immunotherapy correlative scientific studies of subject samples to determine the mechanism of any observed anti-tumor effect. In these studies human humoral and cellular immune responses to algenpantucel-L will be evaluated.
Phase 2 Clinical Trial
We have completed an open-label, two-armed Phase 2 clinical trial, referred to as NLG-0205, in which algenpantucel-L was given in doses of either 100 million cells or 300 million cells approximately twice monthly for six months in combination with the standard-of-care treatment regimen, which consisted of gemcitabine chemotherapy plus 5-FU based chemoradiotherapy. We enrolled patients for this clinical trial at 16 different sites in the United States. Patients in this clinical trial had been diagnosed with Stage I and Stage II pancreatic adenocarcinoma, according to the AJCC system, and subsequently underwent surgical resection to remove all visible tumors with curative intent. There were no other exclusion criteria relative to pre-operative disease status. The primary endpoint of this clinical trial was to evaluate disease-free survival with secondary endpoints of overall survival and toxicity. We also wanted to determine if a superior dosing regimen could be identified.
We enrolled 44 patients in the 100 million cell dose cohort, or low dose group, and 26 patients in the 300 million cell dose cohort, or high dose group. The baseline patient characteristics of both cohorts were similar in terms of age, gender and disease state. Patient enrollment was complete in March 2010.
As of June 2012, all patients had reached at least 24 months of follow-up with a median follow-up period of approximately 33 months. To date, algenpantucel-L` has demonstrated good tolerability and a favorable safety profile. The most common non-serious adverse events observed were fatigue, local injection site skin reactions and injection site pain. The nature and frequency of the adverse events observed in this clinical trial are consistent with the adverse events observed in all clinical trials for other HyperAcute immunotherapies. The study met its primary objective with an established median disease-free survival (DFS) of 14.1 months. The analyses of the secondary endpoint of overall survival for the entire study showed one-year overall survival of 86 percent and a Kaplan-Meier estimate predicts a median overall survival at 24.1 months. Subgroup analysis showed that patients receiving 300 million cells/dose had a 12-month DFS of 81 percent while those receiving 100 million cells/dose had a 12-month DFS of 51 percent (p=0.02, Fisher's exact), and a one-year overall survival rate of 96 percent and 79 percent for the respective cohorts.
These results compare favorably to the outcomes of prior clinical trials in surgically-resected pancreatic cancer patients. Of these clinical trials, we believe the study known as RTOG 97-04, a 538-patient (451 evaluable patients) clinical trial conducted by the Radiation Therapy Oncology Group, is the most comparable with respect to baseline patient characteristics and treatment regimen even though our trial population had a higher frequency of lymphatic node invasion (68% vs. 81% in our trial). One treatment arm in RTOG 97-04 received gemcitabine chemotherapy plus 5-FU based chemoradiotherapy, which is the current standard-of-care treatment regimen, and we believe this treatment arm provides the best comparison to our NLG-0205 study. In RTOG 97-04, the 221 patients in the standard-of-care treatment arm had one-year disease-free survival of less than 50 percent and a one-year overall survival rate of 69 percent based on Kaplan-Meier analysis.
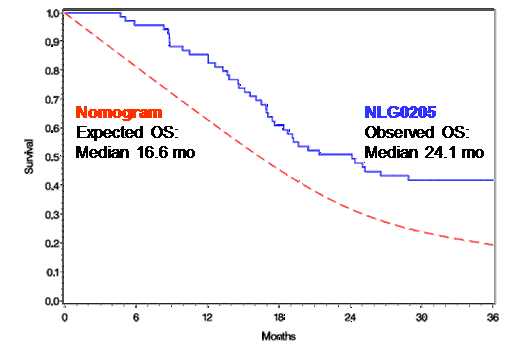
Kaplan-Meier Analysis of Overall Survival in our Phase 2 Study
Kaplan-Meier analysis is a statistical method of predicting survival rates using study survival data. Nomogram analysis is a method for determining expected survival rates using patient baseline characteristics and other prognostic indicators. As shown in the graph below, the Kaplan-Meier-calculated overall survival in our Phase 2 clinical trial compares favorably to projected survival calculated by nomogram analysis.
Kaplan-Meier Plot of Overall Survival for
NLG-0205 Versus Expected Distribution
The dotted line represents projected survival of patients enrolled in NLG-0205 based on the prognostic Brennan et al. Nomogram (Brennan et al., Ann Surg, 2004, 240:293), also known as the Memorial Sloan Kettering Cancer Center nomogram. The solid blue line represents the Kaplan-Meier estimated survival curve for patients enrolled in NLG-0205 as of June 2012. At 12 months after surgery overall survival for the combined patient population in NLG-0205 is 86%. The observed median overall
survival is 24.1 months for the combined patient population in NLG-0205 versus 16.6 months predicted by the Brennan et.al. Nomogram.
Data from the NLG-0205 study has been stratified into high dose and low dose groups on the basis of statistically significant differential responses to algenpantucel-L immunotherapy. We believe 300 million cells is the largest practically attainable treatment dose based on clinician observation; however, we have tested a 100 million cell dose as a means to reduce the number of injections needed during therapy. The patterns of response in patients treated with these two doses have become distinct during the study.
Patients in the high dose group of NLG-0205 demonstrated an improved disease-free survival compared to patients in the low dose group or the current standard-of-care RTOG 97-04 chemoradiotherapy protocol alone. The data from NLG-0205 demonstrate a statistically significant difference between high and low dose groups in terms of disease-free survival (p=0.02).
In addition, an increased overall survival at one year for 300 million cell dose patients compared to low dose patients was observed (96% vs. 79%, p=0.053). These data demonstrate that patients in the high dose group have both a higher disease-free survival and a trend towards higher overall survival at one year compared to patients in the low dose group. Notably, patients treated at both dose levels in NLG-0205 compare favorably to the 63% one-year overall survival calculated by the Memorial Sloan Kettering Cancer Center nomogram analysis of the NLG-0205 patient population. Furthermore, both dose levels in NLG-0205 compare favorably to the 69% one-year overall survival observed for the 221 patients who received gemcitabine plus 5-FU based chemoradiotherapy in the RTOG 97-04 study.
Survival Outcome of NLG0205 vs predicted and RTOG 97-04
|
| | | | |
| | | Disease-Free Survival at 1 year | | Overall Survival at 1 year |
| Predicted Brennan et al., 2005 nomogram | | Not Applicable | | 63% |
| RTOG 97-04 (221 patients) | | <50%* | | 69% |
| NLG-0205-100 million cell dose group | | 51% | | 79% |
| NLG-0205-300 million cell dose group | | 81% | | 96% |
________________________________
| |
| * | Disease-free survival at 1 year was not reported. However, from the median disease-free survival of 11.4 months, we have inferred that disease-free survival at 1 year is less than 50%. |
Analysis of Historical Controls
Baseline patient characteristics are key factors to consider in reviewing clinical trials. Not all patients have an identical disease state and, in the context of surgically-resected pancreatic cancer patients, certain patient characteristics have been shown to have a significant impact on a patient's prognosis of disease progression and survival. Prognostic indicators for Stage I/II pancreatic cancer have been analyzed during the development of the AJCC system. The principal prognostic indicators have been validated and demonstrate that baseline data on tumors, nodal involvement and metastasis inform meaningful predictions of likely outcomes for patients. These characteristics include:
Nodal status: refers to the presence of cancer in the nearby lymph nodes. When cancer enters the lymph nodes, there is an increased risk that the cancer will spread, or metastasize, to other regions of the body via the lymphatic system. As such, nodal status is an indicator of disease progression and thereby a prognostic indicator of survival. A study completed by Hsu et al. and published in the Annals of Surgical Oncology in 2010 reported that resected pancreatic cancer patients who received adjuvant chemoradiotherapy with positive lymph nodes prior to resection had a median overall survival 8.5 months less than that of patients with negative nodes. Further, a study conducted by Lim et al. published in Annals of Surgery in 2003 demonstrated that patients with greater than four positive lymph nodes had median overall survival 9.4 months less than that of patients with no positive lymph nodes.
Degree of local invasion: refers to the extension of tumors into peripancreatic tissues including neural, vascular, or lymphatic structures or surrounding organs. Larger, higher-staged tumors are associated with a higher degree of local invasion, advanced disease and a poorer prognosis. As it relates to pancreatic cancer, patients with smaller, less invasive tumors have a greater median overall survival as reported by Gebhardt et al. in Langenbeck's Archives of Surgery in 2000. In the Gebhardt study, patients with pancreatic cancer that had invaded the lymph vessels, blood vessels and perineural tissues had a median overall survival of 16.8 months, 7.2 months and 4.8 months less, respectively, than patients with cancer that had not invaded these tissues.
Tumor stage: refers to the size and peripancreatic extension of pancreatic cancer. T1 is defined as less than two centimeters in diameter and limited to the pancreas; T2 is defined as greater than two centimeters in diameter and limited to the
pancreas; T3 is defined as a tumor that has extended beyond the pancreas; and T4 tumors are defined as unresectable. The T3 tumor stage is associated with poorer prognosis and increased risk of death compared to T1-T2 tumors in resected pancreatic cancer patients who receive adjuvant chemoradiotherapy as reported by Hsu et al., where T3 patients had a median overall survival that was 8.3 months less than T1-T2 patients.
Tumor grade: refers to abnormalities of cancer cells relative to healthy cells. Tumor cells considered undifferentiated, or having a higher tumor grade, have little to no resemblance to the cells from which they originated (in this case pancreatic cells). Tumors classified as G1 or G2 are considered low grade tumors with well and moderately differentiated cells, respectively. Tumors classified as G3 or G4 are considered high grade tumors with poorly or undifferentiated cells, respectively. Many factors are considered in determining tumor grade, including the structure and growth pattern of the cells. Tumor grade is determined by a pathologist via biopsy of the tumor. Higher degrees of cancer cell abnormality are associated with a poorer disease prognosis; in fact, high tumor grade is an independent predictor of survival. The study conducted by Lim et al. referred to above showed that patients with poorly differentiated (G3), or higher grade, tumors of the pancreas had median overall survival of 22.8 months less than patients with well differentiated (G1), or lower grade, tumors.
Ca 19-9 markers: refers to the post-operative concentration of the tumor marker carbohydrate antigen 19-9. The concentration of Ca 19-9 markers is associated with significant risk of early, distant metastasis. A study conducted by Kinsella et al. published in American Journal of Clinical Oncology in 2008 reported that pancreatic cancer patients with high post-operative Ca 19-9 levels, defined as greater than 70 units per milliliter, had a median overall survival 16.8 month less than patients with Ca 19-9 marker levels lower than 70 units per milliliter.
Our Phase 2 clinical trial did not compare the outcomes of patients who received algenpantucel-L plus the standard-of-care treatment regimen to the standard-of-care alone. Therefore, we believe it is important to evaluate the patient characteristics and clinical results of NLG-0205 relative to those of prior clinical trials in surgically-resected pancreatic cancer patients.
|
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Study | | Nodal Status (%N+) | | Local Invasion | | Tumor Stage (T3/T4) | | High Tumor Grade | | Ca 19-9 (≥ 180 U/mL) | | Disease-Free Survival Median (Months) | | Overall Survival Median (Months) | | Overall Survival at 1 Year |
| RTOG 97-04 2008(1) | | 68 |
| % | | Not reported | | 81 |
| % | | 32%* | | 14%(2) | | 11/4/2003 | | 20.5(3) |
| | 69 |
| % |
| Treatment Arm: | | | | | | | | | | | | | | | | |
| Gemcitabine + 5FU + | | | | | | | | | | | | | | | | |
| Radiation (221 patients) | | | | | | | | | | | | | | | | |
| NLG-0205 | | 81 |
| % | | 90%* | | 83 |
| % | | 36%* | | 17%* | | 14.1 |
| | | 24.1 |
| | 86 |
| % |
| Gemcitabine + 5-FU + | | | | | | | | | | | | | | | | |
| Radiation + Algenpantucel-L | | | | | | | | | | | | | | | | |
| (69 patients) | | | | | | | | | | | | | | | | |
________________________________
| |
| (1) | Regine et al., JAMA 2008; 299(9): 1019-1026. |
| |
| (2) | Includes only the 124 patients who tested positive for the Lewis antigen (patients who test negative for the antigen do not express Ca 19-9). |
| |
| (3) | Regine et al. study in JAMA only reports overall survival and disease-free survival for patients with pancreatic head tumors. The median overall survival of patients in the standard-of-care treatment arm of RTOG 97-04 is 18.8 months. |
* Calculation excludes unknowns.
U.S.-based comparator studies
In terms of historical comparisons between NLG-0205 and other resectable pancreatic cancer trials with curative intent, we believe RTOG 97-04 represents the most appropriate comparator study. This clinical trial enrolled 538 patients at 164 U.S. and Canadian institutions from July 1998 to July 2002 with follow-up through August 2006. The objective of RTOG 97-04 was to determine if the addition of gemcitabine to adjuvant 5-FU chemoradiation would improve survival for patients with resected pancreatic adenocarcinoma. In their primary analysis of a 451 patient sub-population, 221 of which received gemcitabine, the RTOG 97-04 investigators determined that the addition of gemcitabine to adjuvant 5-FU-based chemoradiation was associated with a survival benefit for patients with resected pancreatic cancer, although this benefit was not statistically significant. Based on the subpopulation analysis of this study, we believe that this study demonstrated limited benefit. The results of RTOG 97-04 were presented at the 2006 American Society of Clinical Oncologists, or ASCO, annual meeting and published in JAMA in March 2008.
|
| | | | | | | | | | | | | | | | | | | | | |
| Study | | | Nodal Status (%N+) | | Local Invasion | | Tumor Stage (T3/T4) | | High Tumor Grade | | Ca 19-9 (≥ 180 U/mL) | | Disease-Free Survival Median (Months) | | Overall Survival Median (Months) | | Overall Survival at 1 Year |
| RTOG 97-04 2008(1) | | 68 | % | | Not reported | | 81 | % | | 32%* | | 14%(2) | | 11.4(3) |
| | 20.5(3) | | 69 | % |
| Treatment Arm: | | | | | | | | | | | | | | | | |
| Gemcitabine + 5FU + | | | | | | | | | | | | | | | | |
| Radiation (221 patients) | | | | | | | | | | | | | | | | |
| NLG-0205 | | 81 | % | | 90%* | | 83 | % | | 36%* | | 17%* | | 14.3 |
| | -(4) | | 86 | % |
| Gemcitabine + 5-FU + | | | | | | | | | | | | | | | | |
| Radiation + Algenpantucel-L | | | | | | | | | | | | | | | | |
| (70 patients) | | | | | | | | | | | | | | | | |
________________________________
| |
| (1) | Regine et al., JAMA 2008; 299(9): 1019-1026. |
| |
| (2) | Includes only the 124 patients who tested positive for the Lewis antigen (patients who test negative for the antigen do not express Ca 19-9). |
| |
| (3) | Regine et al. study in JAMA only reports overall survival and disease-free survival for patients with pancreatic head tumors. The median overall survival of patients in the standard-of-care treatment arm of RTOG 97-04 is 18.8 months. |
| |
| (4) | Not calculable as of September 1, 2011. |
| |
| * | Calculation excludes unknowns. |
Because comparisons between specific studies can have limited validity, researchers have developed statistical tools to evaluate the likely impact of therapies on overall survival. One such tool is the Brennan et. al. Nomogram developed at Memorial Sloan Kettering Cancer Center for surgically resected Stage I/II patients based on the interaction of baseline patient characteristics and other prognostic indicators.
Among the U.S.-based comparator studies, RTOG 97-04 baseline patient characteristics are the most similar to NLG-0205 baseline patient characteristics; both studies enrolled patients primarily at major medical centers in the United States, and NLG-0205 incorporates the addition of algenpantucel-L to a chemoradiotherapy protocol highly similar to that used in RTOG 97-04. As calculated by applying Brennan et. al. Nomogram analysis to the patient characteristics and other prognostic indicators of the respective studies, the projected overall survival of patients in the RTOG 97-04 study is higher than the projected overall survival of patients in the NLG-0205 study.
European studies
Several European studies have evaluated post-surgical adjuvant therapy, but important differences prevent a meaningful comparison between these studies and NLG-0205. Differences in surgical approaches between the United States and Europe may lead to differences in patient selection between trials. For example, according to Picozzi, in Business Briefing: US Gastroenterology Review in 2005, less than 3% of Stage I/II pancreatic cancer patients receive surgery in the United Kingdom. Gebhardt, in Langenbeck's Archives of Surgery (2000) 385:14-20, notes that the surgery frequency is approximately 20% in the United States. Furthermore, the major European studies have not followed harmonized or standardized study regimens, so generating meaningful conclusions about specific therapeutic regimens is difficult.
Our Tergenpumatucel-L (HyperAcute Lung) Cancer Immunotherapy Product Candidate
Tergenpumatucel-L is an investigational HyperAcute immunotherapy for non-small cell lung cancer, or NSCLC. The product consists of three NSCLC cell lines that have been genetically modified to express alpha-Gal carbohydrates on cell surface molecules. Upon injection into the patient, the alpha-Gal stimulates a rapid and powerful immune response against NSCLC-specific antigens in the tumor cell lines. The patient’s immune system then targets the patient's own NSCLC cancer cells, destroying them. In a phase 2b/3 study in progressive or relapsed NSCLC, two different dosing schedules of tergenpumatucel-L are currently being compared to docetaxel with a primary endpoint of overall survival. This study is also evaluating the potential for chemosensitization to salvage regimens post tergenpumatucel-L exposure.
Market Opportunity
According to the American Cancer Society, NSCLC is the leading cause of cancer death among both men and women in the United States, with almost 140,000 Americans expected to have died from this devastating disease in 2013. Of more than 180,000 newly diagnosed patients in 2013, approximately 40% presented with metastatic disease, for which there are significant limitations in treatment options. Best supportive care is the only option for many of these patients with NSCLC, and survival outcomes remain poor. The 5-year survival rates for advanced NSCLC are only 5% for Stage IIIB disease and 1% for Stage IV disease, which underscores an urgent need for innovative therapies.
Phase 1/2 Clinical Trial
Tergenpumatucel-L was studied in a Phase 1/2, single-arm, open-label clinical trial that fully enrolled with 54 patients at the NCI. This clinical trial was for patients with refractory, recurrent or metastatic NSCLC. Its primary endpoint was to assess tumor response rate after administration of tergenpumatucel-L, and the secondary endpoint was to assess overall survival. For the Phase 1 portion of this clinical trial, a positive response included stable disease for 16 weeks in patients who had enrolled after having previously shown progressive disease. A total of 17 patients in the Phase 1 portion and 37 patients in the Phase 2 portion were injected with tergenpumatucel-L. Of the 37 patients in the Phase 2 portion, only 28 were evaluated for clinical response. In the Phase 1 portion, four cohorts of patients each received injections of 3 million, 10 million, 30 million, or 100 million cells every four weeks for four doses, and one cohort of three patients received an initial dose of 500 million cells, followed by injections of 300 million cells every two weeks for up to seven doses. In the Phase 2 portion, the 28 patients evaluated received injections of 300 million cells every two weeks for up to eight doses.
The Phase 1 portion of our Phase 1/2 clinical trial for our tergenpumatucel-L immunotherapy demonstrated a favorable safety profile, with no dose limiting toxicities at any of the five escalating dose levels. There have been no reported CTC grade four adverse events attributed to tergenpumatucel-L. The most common treatment-related adverse reactions (reported by at least 5% of patients) for tergenpumatucel-L, were injection site reaction (88%), induration (51%), fatigue (25%), urticaria (10%), anemia (5%), pruritus (7%), lymphopenia (7%), elevated serum amylase (5%), edema (5%), skin pain (5%) and dyspnea (5%). The clinical trial involved a dose escalation from approximately three million up to 300 million cells in repeat dosing. Only a single dose escalation has been required by the FDA in all subsequent clinical trials of our other HyperAcute candidates conducted to date.
Results of our Phase 1/2 clinical trial for tergenpumatucel-L, based on the analysis of 45 patients, were encouraging. As of January 2012, the results for the 28 patients evaluated in the Phase 2 clinical trial group showed a median progression-free survival of 3.4 months, median overall survival of 11.3 months, and a one-year survival rate of 46%. Median overall survival data from the Phase 2 clinical trial group was better than the Phase 1 clinical trial group (11.3 versus 7.6 months), a comparison that would be consistent with study drug dose dependency.
Immunological studies showed that tergenpumatucel-L induced the increase of IFN-gamma secreted post immunization by peripheral blood lymphocytes in 11 of 18 tested patients. Patients that responded with increased IFN-gamma secretion post immunization had significantly increased overall survival when compared to non-responding patients (21.9 vs 7.2 months p=0.044).
Prior Phase 3 studies suggest that in the refractory, recurrent or metastatic NSCLC setting (second line therapy), the median overall survival of patients receiving best supportive care was 4.6 months and the median overall survival of patients receiving pemetrexed or docetaxel (Taxotere) therapy was approximately eight months. The following table shows comparative results for second-line treatment in advanced NSCLC with pemetrexed, docetaxel and tergenpumatucel-L.
Treatment Options and Clinical Outcomes in 2nd Line Advanced Stage NSCLC
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Overall Survival (Months) | | 12 Month Survival | | Serious Adverse Events (CTC Grade 3 or 4) Attributed to Therapy | |
| Therapy | | | | Nausea | | Fatigue | | Anemia | | Neutropenia |
| Best supportive care(1) | | 4.6 |
| | | 11 |
| % | | — |
| | | — |
| | | — |
| | | — |
| |
| Docetaxel(1) | | 7.5 |
| | | 37 |
| % | | 1.8 |
| % | | 5.4 |
| % | | 4.3 |
| % | | 40.2 |
| % |
| Pemetrexed(2) | | 8.3 |
| | | 30 |
| % | | 2.6 |
| % | | 5.3 |
| % | | 4.2 |
| % | | 5.3 |
| % |
| HyperAcute Lung(3) | | 11.3 |
| | | 46 |
| % | | — | % | | — | % | | — | % | | — | % |
________________________________
| |
| (1) | Prospective Randomized Trial of Docetaxel versus Best Supportive Care in Patients with Non-Small-Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy. Shepherd et al., Journal of Clinical Oncology, Volume 18, No. 10 (May), 2000: pp 2095-2103 |
| |
| (2) | Randomized Phase III Trial of Pemetrexed Versus Docetaxel in Patients with Non-Small-Cell Lung Cancer Previously Treated with Chemotherapy. Hanna et al., Journal of Clinical Oncology 2004 May 1; 22(9):1589-97 |
| |
| (3) | Data from NLG-0101 clinical trial Patients 18-45 |
Given the favorable safety profile of tergenpumatucel-L, and the 11.3 month median overall survival observed in the Phase 2 study, tergenpumatucel-L compares favorably to current standard-of-care cytotoxic chemotherapy.
In 2013, enrollment started on our phase 2B/3 clinical trial in non-small cell lung cancer. When available, the results of the Phase 2B portion of the study will assist in the design of the Phase 3 portion of the study.
Our Dorgenmeltucel-L (HyperAcute Melanoma) Cancer Immunotherapy Product Candidate
Our dorgenmeltucel-L (HyperAcute Melanoma) product candidate was studied in an investigator-initiated Phase 2 clinical trial in 25 patients with advanced melanoma. In this trial, dorgenmeltucel-L was administered in combination with an eight-week course of PEG-Intron, a man-made immune modulator that has been tested for the treatment of melanoma. Dorgenmeltucel-L consists of a group of three allogeneic melanoma tumor cell lines that were modified to express the gene that makes alpha-GT. These three cell lines each possess collections of known melanoma antigens so that the immune response they stimulate will provide broad coverage. Each of the modified cell lines is grown separately in large cultures, harvested, irradiated and packaged. Approximately 50 million cells of each dorgenmeltucel-L cell line are given by intradermal injection with each treatment.
Market Opportunity
According to the American Cancer Society, the incidence of melanoma in the United States has been increasing for the last 30 years. Most cases of melanoma (84%) are diagnosed at an early stage, when curative treatment with surgical excision is possible. However, melanoma can recur after surgery, and about 13% of patients present with regionally advanced or metastatic disease. For patients with stage IIIC disease, 5-year survival has been reported to be 27%. Only 16% of patients with metastatic disease will survive for 5 years or more.
Phase 2 Clinical Trial
We provided dorgenmeltucel-L product in support of a Phase 2 investigator-initiated clinical trial studying HyperAcute Melanoma in combination with an eight week course of PEG-Intron for patients with advanced melanoma. The trial completed enrolling 25 patients in September 2010. The treatment consisted of 12 weekly injections of dorgenmeltucel-L with PEG-Intron co-administered in weeks five through 12. This was the first time that one of our HyperAcute immunotherapies was combined with another approved immunotherapy, in this case PEG-Intron. The primary objective of this clinical trial was to conduct correlative scientific studies of patient tumor and peripheral blood samples to determine the mechanism of any observed anti-tumor effect involving the innate and cell-mediated host immune response to HyperAcute immunotherapy alone and combined with PEG-Intron. Although the number of patients in this clinical trial is modest, the results to date are encouraging.
As of June 2012, vitiligo had been observed in four out of 25 (16%) patients. Vitiligo is an autoimmune condition in which the patient's immune system attacks melanoctyes, the cells responsible for skin pigmentation and potential melanoma cancer cells. Two prior clinical trials of immunotherapies conducted by others suggest that the development of vitiligo was correlated with a favorable response to therapy in melanoma patients. All patients evaluated developed autoimmune antibodies. Other than vitiligo, no other clinically apparent autoimmune disorder has been reported in any patient to date. These observations suggest an immunological response to the dorgenmeltucel-L. Dorgenmeltucel-L has demonstrated good tolerability and a favorable safety profile, with no systemic, drug-related serious adverse events characterized by the investigators as possibly or probably attributable to our product candidate. The most common treatment-related adverse events reported included injection site reactions (92%), induration (76%), pruritis (16%), fatigue (52%), fever (32%), diarrhea (52%), nausea (44%), vomiting (8%), rigors or chills (8%), head pain (52%), muscle pain (52%) and anorexia (28%). By Response Evaluation Criteria in Solid Tumors, or RECIST criteria, of 16 stage IV patients, there were two complete responders (CR), two with stable disease (SD) and two with no evidence of disease (NED) after resection. For the one stage II and eight stage III patients, three of nine (3/9) remain NED, with one patient with slowly progressive disease remaining alive at 30 months. The median overall survival is 29 months, with 50% of the patients surviving for two years. In conclusion, combination immunotherapy with dorgenmeltucel-L plus PEG-Intron shows signs of clinical efficacy with tumor regression and concomitant immune activation.
We currently are developing new clinical trial designs exploring new dosing regimens to evaluate the efficacy of dorgenmeltucel-L either as a stand-alone or combination therapy in these new settings, in part because we believe, based on the statistically significant improvements in outcomes resulting from higher doses in our Phase 2 clinical trials with algenpantucel-L (HyperAcute Pancreas), that higher doses may lead to better outcomes for patients. Specifically, in the Phase2 clinical trial of our dorgenmeltucel-L product candidate, we employed a weekly dose of 150 million cells for 12 weeks during the course of the treatment. After enrollment was completed in the Phase 2 clinical trial of algenpantucel-L, data from our Phase 2 clinical trial of algenpantucel-L (HyperAcute Pancreas) showed a statistically significant improvement in disease-free survival when comparing patients receiving doses of 100 million cells to those receiving doses of 300 million cells. Our Phase 3 HyperAcute Pancreas clinical trial protocol was amended to administer 18 injections of 300 million cells to patients over a 12 month period. Similarly, we intend to employ a 24 month treatment schedule employing the 300 million cell dosing level in our next Phase 2B dorgenmeltucel-L clinical trial, which we plan to initiate in late 2013. This will represent a three-fold increase in total dose compared to the initial Phase 2 dorgenmeltucel-L clinical trial.
Our Other HyperAcute Cancer Immunotherapy Product Candidates and Indications
We believe we have developed a process to efficiently discover and develop new tumor-specific HyperAcute immunotherapies for other solid tumor types. We initiated clinical development for our HyperAcute Prostate, HyperAcute Breast and HyperAcute Renal product candidates and are developing our HyperAcute immunotherapy technology for other indications.
Our HyperAcute Prostate Cancer Immunotherapy Product Candidate
Prostate cancer is one of the most common forms of cancer affecting men. The American Cancer Society estimates that there will be an estimated 233,000 new cases of prostate cancer in the United States in 2014. Increased screening over the past few decades has enabled physicians to detect prostate cancer in its early, more treatable stages. Nonetheless, while overall five-year survival rates for cases of prostate cancer approach 100%, the outlook for advanced and metastasized (distant) cases is poor with five-year survival rate of about 30%, according to the American Cancer Society.
HyperAcute Prostate is an investigational HyperAcute immunotherapy for prostate cancer. The product consists of prostate cancer cell lines that have been genetically modified to express alpha-Gal carbohydrates on cell surface molecules.1 Upon injection into the patient, the alpha-Gal stimulates an immune response against prostate cancer-specific antigens in the tumor cell lines. The patient's immune system then targets the patient's own prostate cancer cells, destroying them. We have completed an open-label, single-center Phase 1 clinical trial for our HyperAcute Prostate product candidate. This clinical trial enrolled eight patients with hormone refractory prostate cancer that had recurred or no longer responded to standard treatment. Study participants received 12 bi-weekly intradermal injections of HyperAcute Prostate, which consists of two separate allogeneic prostate cancer cell lines that were selected based on antigen profiles and modified to express the gene that makes alpha-GT. The primary endpoint for this clinical trial was safety and efficacy of administration. We successfully completed this clinical trial in August 2008. We observed no dose-limiting toxicities and only one serious adverse event (CTC grade three anemia) was reported by the investigator as possibly attributable to HyperAcute Prostate. Median survival was 25.1 months with one treated patient surviving more than 70 months with stable Prostate Specific Antigen, or PSA, and unchanged bone metastasis since 2007. Three of 8 patients showed PSA stabilization for more than 100 days. Although we currently do not have an active Investigational New Drug application, or IND for this indication due to resource constraints, we believe HyperAcute Prostate could provide a valuable treatment alternative for many prostate cancer patients.
Our HyperAcute Breast Cancer Immunotherapy Product Candidate
According to the American Cancer Society, carcinoma of the breast is the second leading cause of cancer death in women in the United States with approximately 232,670 new cases and 40,040 deaths expected to occur in 2014. Increased access to improved screening methods has had a major impact on reducing deaths from this disease; however, despite these interventions, patients continue to present with nodal or metastatic lesions that carry poor prognoses.
We initiated an open-label, single-center Phase 1 clinical trial for our HyperAcute Breast product candidate. Three patients were enrolled in this clinical trial. Due to resource constraints, the clinical trial was suspended.
HyperAcute Breast consists of two allogeneic breast cancer cell lines genetically modified to express the gene that makes alpha-GT. The cell lines selected for inclusion in this drug represent both estrogen receptor positive and estrogen receptor negative examples of disease. The cell lines of the drug are manufactured with growth nutrient in media by concentrating, irradiating and compounding in a cryopreservative solution. Although we currently do not have an active IND for this indication, we believe HyperAcute Breast could provide a valuable treatment alternative for many breast cancer patients.
Our HyperAcute Renal Cell Cancer Immunotherapy Product Candidate
The American Cancer Society estimated that approximately 63,920 new cases of kidney cancer would be diagnosed the United States in 2014 with renal cell carcinoma accounting for approximately 90% of all diagnoses. The American Cancer Society predicted there were approximately 13,860 deaths from kidney and renal pelvis cancer in the United States in 2013. Depending on stage at diagnosis, a wide variety of treatments may be used including surgery, radiation, targeted molecules, immunotherapy and chemotherapy, either individually or in combination. Surgery is most often used in Stages I, II, and III, with partial nephrectomy often the treatment of choice in tumors up to 7 centimeters.
Unless part of an investigational study, adjuvant therapy is not frequently used as the currently available treatment options have not demonstrated improved survival. We are interested in expanding treatment options in renal cancer, initially targeting patients with minimal disease after surgery. We initiated a phase I clinical study for this product candidate in December 2013.
Our IDO Pathway Inhibitor Technology
The IDO pathway inhibitor platform is focused on developing small molecule drugs that disrupt mechanisms by which tumors evade the patient's immune system. IDO pathway inhibitors are a class of immune checkpoint inhibitors akin to the antibodies targeting CTLA-4 and PD-1, which represent potential breakthroughs in cancer therapy. Our IDO pathway inhibitors are orally administered small molecules and can potentially be used alone or in combination with other cancer therapies.
The IDO pathway regulates immune response by suppressing T cell function and enabling local tumor immune escape.7,8 Recent studies indicate that the IDO pathway is active in many cancers, both within tumor cells as a direct defense against T cell attack, and also within antigen-presenting cells in tumor-draining lymph nodes resulting in peripheral tolerance to TAAs. Cancers may use the IDO pathway to facilitate survival, growth, invasion, and metastasis of malignant cells expressing TAAs that might otherwise be recognized and attacked by the immune system.
We are actively developing two IDO pathway inhibitors with broad potential across multiple tumor types. The most advanced is indoximod, which is being studied in combination with docetaxel in metastatic breast cancer and as follow-on therapy after sipuleucel-T treatment in metastatic, castrate-resistant prostate cancer. The second IDO pathway inhibitor, NLG919, has shown promising preclinical results and is currently in a Phase 1 clinical trial in patients with solid tumors.
We believe that immune system failure is a fundamental reason for the inability of the human body to successfully fight cancer cells. Research into the inability of the immune system to respond to cancerous tumors indicates that tumors can induce the human immune system to tolerate the existence of the tumor. This immune tolerance and suppression represents a major barrier to successful treatment of cancer and is a significant target for new therapeutics.
Scientific understanding of the process leading to immune tolerance is in its early stages. We believe IDO is part of a system that may be used by some tumors as a mechanism to evade the immune system. IDO is an enzyme that regulates immune response by suppressing effector T-cell function by breaking down the essential amino acid tryptophan. Expression of IDO, either directly by tumors or by dendritic cells in tumor-draining lymph nodes, has been shown in animal studies to induce immune tolerance to tumors, and inhibition of IDO has been shown in these studies to prevent this induction of tolerance. IDO is rarely expressed by the majority of normal tissues, but it is overexpressed in many types of human tumors.
Cytotoxic chemotherapy places substantial stress on established, tumor-induced tolerance. Several factors can potentially contribute to this result: (1) dying tumor cells release waves of TAAs for processing and presentation, (2) many chemotherapeutic regimens induce a period of transient lymphopenia and homeostatic recovery during which T-cells may become more susceptible to breaking tolerance, and (3) certain regimens can transiently deplete or inactivate tumor-protective T-regulatory cells. Despite producing these challenges to tolerance, most chemotherapeutic agents do not appear to trigger a protective immune response against established tumors. This shortcoming of traditional chemotherapy has been attributed, in part, to the ability of tumors to rapidly reestablish tolerance following each cycle of chemotherapy. We believe a potential mechanism underlying the failed opportunity is IDO expression by APCs in tumor-draining lymph nodes, which are thereby converted to an immunosuppressive and tolerance-inducing milieu. Preclinical data have demonstrated that IDO pathway inhibitors have anti-tumor effects in combination with a number of radiotherapy, chemotherapeutic drugs or other immunotherapy drug candidates and may work better together than either type of treatment alone.
The ability to acutely eliminate the protective IDO mechanism by administering IDO pathway inhibitor drugs, such as indoximod, may provide a therapeutic window in which to break tolerance in tumors and reverse the inhibition of immune cells. Additionally, we believe that once immune cells are restored to normal function, they can assist in the rejection of tumors.
We believe our IDO pathway inhibitor technology has the following potential advantages in combating cancers:
| |
| • | Potential to break immune tolerance. The immune tolerance to cancerous cells represents a key barrier to the treatment of cancer. To date, few available therapies have addressed the immune escape mechanisms of cancer. We believe inhibition of the IDO pathway has the potential to break a key immune escape mechanism of cancer cells and significantly enhance patient outcomes. |
| |
| • | Tolerability. In early-stage clinical development, we have observed an encouraging safety profile. We believe inhibition of the IDO pathway will selectively enhance the immune response against cancer cells given the limited expression of IDO in normal cells. |
| |
| • | Oral bioavailability. Unlike many cancer therapies which require intravenous administration, our IDO pathway inhibitors are orally bioavailable, a significant advantage in ease of administration for patients and physicians. |
| |
| • | Synergy with existing cancer therapies. Inhibiting the IDO pathway in conjunction with immunotherapy or chemotherapy has the potential to enhance the therapeutic effect of these agents. Used in combination with |
chemotherapy IDO inhibitors have the potential to delay or disrupt the reacquisition of immune tolerance to tumor antigens during the period following chemotherapy. In addition, preclinical studies have demonstrated the synergistic potential of IDO pathway inhibitors in combination with other cancer immunotherapies, including other checkpoint pathway inhibitors as well as cancer vaccines . The safety profile in humans is conducive to exploring combination therapy and the available animal data does not indicate significant additive or synergistic toxicities with many common oncology therapies.
Our IDO Pathway Inhibitor Product Candidates
We are actively developing two IDO pathway inhibitors with broad potential across multiple tumor types. The most advanced is indoximod, which is being studied in combination with docetaxel in metastatic breast cancer and as follow-on therapy after sipuleucel-T treatment in metastatic, castrate-resistant prostate cancer. The second IDO pathway inhibitor, NLG919, has shown promising preclinical results and is currently in a Phase 1 clinical trial in patients with solid tumors. Our IDO pathway inhibitor drug candidates operate through different mechanisms of action and thus offer the potential to achieve stronger blockade of such pathway when administered in a combination therapy.
Our first and most advanced IDO pathway inhibitor is indoximod, a small-molecule, orally bioavailable product candidate. Preclinical experiments have demonstrated a strong, synergistic anti-tumor effect without increased toxicity when indoximod was administered in combination with a number of currently available chemotherapeutic agents. Indoximod is currently being evaluated for the treatment of a broad range of solid tumors in chemotherapeutic and immunotherapeutic combinations in two Phase 1B/2 clinical trials. The second IDO pathway inhibitor is NLG919, an orally bioavailable, small-molecule drug candidate. Preclinical experiments have demonstrated potent inhibition of the IDO pathway, increased antitumor and immunostimulatory effect when co-administered with vaccination and chemotherapy regimens, as well as when administered in combination with indoximod.
Clinical Trials
Phase 1B/2 Clinical Trials
We currently have two Phase 1B/2 clinical trials enrolling patients to evaluate indoximod in combination with other approved therapies. The first clinical trial has primary endpoints that assess safety and efficacy of indoximod in combination with an Ad-p53 autologous dendritic cell vaccine for solid malignancies with p53 mutations, such as lung, breast and colon cancers. Enrollment for the Phase 1B dose escalation portion of this clinical trial is completed. The Phase 2 clinical trial portion of the indoximod/Ad-p53 study will expand primarily to enroll patients with metastatic breast cancer. The second clinical trial has primary endpoints that assess safety and efficacy of indoximod in combination with Taxotere for patients with advanced stage solid tumors for which Taxotere is the standard-of-care, such as metastatic breast, prostate, ovarian and lung cancers. Enrollment is completed for the Phase 1B dose escalation portion of this clinical trial. The Phase 2 clinical trial portion of the indoximod/Taxotere study will expand primarily to enroll patients with metastatic breast cancer. We believe indoximod has the potential to have a synergistic therapeutic effect in combination with Ad-p53 or Taxotere without adding systemic safety complications. The clinical trials are being co-sponsored by the NCI's Division of Cancer Treatment and Diagnosis under a Cooperative Research and Development Agreement and are taking place at the Moffitt Cancer and Research Institute in Tampa, Florida.
According to the American Cancer Society, carcinoma of the breast is the second leading cause of cancer death in women in the United States with approximately 232,670 new cases and 40,000 deaths predicted to occur in 2014.
The disease comes in different forms depending on whether the tumor is driven by signaling through the estrogen receptor (approximately 70% of patients), the HER2/neu receptor (approximately 15-20% of patients), or neither. In the early stages, breast cancer may have no symptoms and can be detected only through mammography screening. During the later phases, symptoms may include tenderness, swelling, lumps, and skin irritation. Treatment of breast cancer typically includes surgery to remove tumors and lymph nodes. Usually a combination of radiation, chemotherapy or hormonal therapy is used post-surgery. Metastatic breast cancer can be treated with a variety of monotherapy or combination drug regimens.
According to the American Cancer Society the 5-year relative survival for women diagnosed with localized breast cancer (cancer that has not spread to lymph nodes or other locations outside the breast) is 99%; if the cancer has spread to nearby lymph nodes (regional stage) or distant lymph nodes or organs (distant stage), the survival rate falls to 84% or 23%, respectively.
Phase 1 Clinical Trials
We have completed two Phase 1 clinical trials of indoximod as a single agent. These Phase 1 clinical trials were open to all tumor types and enrolled patients with a wide variety of cancers. The principal goal of these trials was to demonstrate that patients can tolerate the drug and that increasing quantities of the drug can be administered without inducing toxicity that would prevent the attainment of efficacy. We have observed autoimmune hypophysitis in a small subset of patients previously sensitized to immunotherapy (ipilimumab) and in one immunotherapy-naive patient receiving high dose indoximod. Autoimmune hypophysitis is a disease that most commonly occurs with chronic inflammation of the pituitary gland and may be characterized by diminished production of one or more hormones by the pituitary gland. Autoimmune hypophysitis can be successfully managed by hormone replacement therapy during acute or chronic phases.
We have had few serious adverse events with indoximod in the Phase 1 studies, limited primarily to the hypophysitis, and are proceeding with Phase 1B/2 studies. We have observed one reported CTC grade four (cerebrovascular ischemia) and two reported CTC grade three (lymphopenia) serious adverse events characterized by the investigators possibly, probably or definitely attributable to indoximod in the clinical trial combining Indoximod with Ad-p53. There have been no reported serious adverse events characterized by the investigators as attributable to Indoximod in the clinical trial combining Indoximod with docetaxel.
We have started a Phase 1 clinical trial for NLG919 as a single agent. The trial is a dose-escalation study to evaluate safety, pharmacokinetics, pharmacodynamics and initial evidence of activity of NLG919 as determined by overall response rates in up to 36 cancer patients with recurrent advanced solid tumors. In prelinical studies, NLG919 has demonstrated encouraging activity in solid tumor models and shown that IDO pathway inhibition is critical to reversal of the local immune suppression which impairs immunological detection and destruction of tumors. NLG919 inhibits the IDO pathway by a complementary, yet different mechanism of action than indoximod.
Infectious Disease (BioProtection Systems Corporation)
Historically, in addition to our oncology programs, we have carried out an infectious disease program through our subsidiary, BioProtection Systems Corporation, or BPS. BPS was founded by us in 2005 to research, develop and commercialize vaccines to control the spread of emerging lethal viruses and infectious diseases, improve the efficacy of existing vaccines and provide rapid-response prophylactic and therapeutic treatment for pathogens likely to enter the human population through either pandemics or even acts of bioterrorism. In 2010, we owned a majority of BPS' common stock on an as-converted basis. On January 7, 2011, we acquired the remaining minority interest in BPS and BPS became a wholly-owned subsidiary of us.
BPS is based upon three core technologies, each of which can be leveraged into the infectious disease or biodefense fields. The first is our HyperAcute immunotherapy technology, which is currently focused on enhancing vaccines for influenza. The second technology, based on a yellow fever virus, is in licensed from the University of California at San Francisco. The third technology is replication competent recombinant Vesicular Stomatitus Virus, or rVSV, an advanced vaccine technology developed for the Marburg and Ebola viruses and licensed from the Public Health Agency of Canada.
BPS Grants and Contracts with the United Stated Government
Grants and contracts between BPS and the United States Government accounted for substantially all of our revenue in each of the last three fiscal years. We had no material revenue from external customers outside the United States for any of the last three fiscal years.
BPS is continuing work on a research and development contract with the Department of Defense, or DOD, for the study of alpha-Gal adjuvant technology for the biodefense field with an initial focus on improving existing vaccines for influenza. The contract provides that BPS may receive reimbursements of up to approximately $7.1 million, of which BPS had submitted reimbursement requests for approximately $4.7 million as of December 31, 2013. On September 17, 2013, BPS entered into an amendment to the contract extending the contract period to September 24, 2014.
Manufacturing
We manufacture our HyperAcute immunotherapies at our facilities in Ames, Iowa. We believe this facility is adequate to supply all of the Phase 3 clinical trial drug requirements for at least two HyperAcute product candidates as well as initial commercial quantities of HyperAcute Pancreas for the U.S. market. We have made manufacturing process improvements that we believe will significantly increase our production capacity
We currently contract with Regis Technologies, and with University of Iowa Pharmaceutical Services for the manufacture of our indoximod drug product. We believe that many suppliers would be available for the production of this product, if required. We currently have no plans to build our own manufacturing capacity to support this product.
We currently contract the pre-clinical synthesis and drug product manufacturing of NLG919, our new IDO pathway inhibitor product candidate. We believe that many suppliers would be available for the production of this product, if required. We currently have no plans to build our own manufacturing capacity to support this product.
Sales and Marketing
We currently own exclusive worldwide commercial rights to our HyperAcute Immunotherapy and IDO pathway inhibitor product candidates. If we obtain approval for any of these product candidates, we intend to build a commercial infrastructure targeting oncologists and cancer centers in the United States. In addition, we may pursue collaborations or co-promotion arrangements with pharmaceutical and biotechnology companies to complement these efforts or for particular indications.
We expect that our commercial infrastructure would be a fully integrated and highly experienced team, consisting of sales, marketing, medical affairs, market access and other positions necessary for a successful product launch in the U.S. market. Our lead product candidate, algenpantucel-L is anticipated to be approved for patients with resected pancreatic cancer. We anticipate that a moderately sized, focused and highly experienced sales team will be sufficient to reach key institutions and cancer centers treating pancreatic cancer patients. Additional support for our commercialization efforts may be provided by both medical affairs and market access professionals, who will provide the needed education, reimbursement support and other key functions needed to support an oncology product launch in the United States. We anticipate the need to hire personnel in advance of the approval of any of our product candidates.
Outside the United States, we may enter into out-licensing agreements with other pharmaceutical or biotechnology firms to develop and commercialize our product candidates in foreign markets.
Competition
The biopharmaceutical industry is highly competitive. Given the significant unmet patient need for new therapies, oncology is an area of focus for many public and private biopharmaceutical companies, public and private universities and research organizations actively engaged in the discovery and research and development of products for cancer. As a result, there are and will likely continue to be extensive research and substantial financial resources invested in the discovery and development of new oncology products. In addition, there are a number of multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same cancer indications as our product candidates.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drugs, obtaining FDA and other regulatory approvals, and the commercialization of those products. Accordingly, our competitors may be more successful in obtaining approval for drugs and achieving widespread market acceptance. Our competitors' drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available.
Immunotherapy Products for Cancer
The cancer immunotherapy landscape is broad but still in the early stages of development as a class of therapeutics with only one FDA-approved active cellular immunotherapy product, Dendreon Corporation's Provenge for the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer. We estimate that there are over 100 cancer immunotherapy products in clinical development by approximately 70 public and private biotechnology and pharmaceutical companies. Altogether, trials of these product candidates target at least 23 different cancer types. Of this universe,
several large public biopharmaceutical companies are developing or have commercialized cancer immunotherapy products, including Dendreon Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Merck & Co., Inc., Merck KGaA and Sanofi-Aventis. The cancer immunotherapy product landscape includes numerous immunotherapeutic approaches including but not limited to anti-idiotype, whole cell, DNA, peptide/antigen, viral, tumor lysate, shed antigens, and dendritic cell. To the extent applicable, cancer immunotherapies are also distinguished by whether or not they are derived from autologous or allogeneic sources. Different approaches to cancer immunotherapy design have the potential to confer corresponding advantages and disadvantages based on their respective immunostimulatory mechanisms, formulation characteristics, manufacturing requirements, and logistical demands.
Algenpantucel-L (HyperAcute Pancreas)
There are no anti-cancer drugs currently approved by the FDA for patients with resected pancreatic cancer. However, there are several companies actively marketing anti-cancer drugs indicated for patients with advanced pancreatic cancer including Celgene Corporation with Abraxane (nab-paclitaxel) and Genentech/Astellas with Tarceva (erlotonib). Additionally, there are numerous generic drugs approved for advanced pancreatic cancer including gemcitabine, fluorouracil and mytomycin C. A number of companies have active clinical trials ongoing in pancreatic cancer including AB Science SA, Amgen Inc., Astellas Pharma, BioSante Pharmaceuticals, Inc., Celgene Corporation, Immunomedics, Inc., Lorus Therapeutics Inc., Sanofi-Aventis, and Threshold Pharmaceuticals, Inc. among other companies.
Tergenpumatucel-L (HyperAcute Lung)
There are numerous companies actively marketing FDA approved drugs for patients with NSCLC. These include Celgene wtih Abraxane (nab-paclitaxel), Genentech/Roche with Avastin (bevacizumab), Eli Lilly with Alimta (premetrexed), Astellas/Genentech with Tarceva (erlotinib) and Pfizer with Zalkori (crizotinib). Additionally, there are a number of generic drugs approved by the FDA for use in NSCLC, including cisplatin, docetaxel, paclitaxel, gemcitabine, vinorelbine and methotrexate. There are a large number of companies with active clinical trials ongoing in lung cancer including Abbott Laboratories, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim GmbH, BioNumerik Pharmaceuticals, Inc., Celgene, GlaxoSmithKline, NovaRx Corporation, Onyx Pharmaceuticals, Inc., Pfizer Inc., and Regeneron Pharmaceuticals, Inc. among other companies.
Dorgenmeltucel-L (HyperAcute Melanoma)
Excision is the preferred treatment for early stage, localized melanoma, and there are several marketed therapeutics indicated for advanced melanoma including Merck's Intron A, Novartis AG / Prometheus Laboratories Inc.'s Proleukin as well as cisplatin and dacarbazine, which are available through several generic pharmaceuticals firms. Bristol-Myers Squibb's immunotherapy ipilimumab was recently approved by the FDA as was Roche/Daiichi Sankyo's drug, vemurafenid. In addition, there are a number of companies with active clinical trials ongoing in advanced melanoma including Amgen, Astellas Pharma, Eli Lilly, Onyx, Roche, Synta Pharmaceuticals Corp., and Vical Inc., among other companies.
Intellectual Property
We believe that patent protection and trade secret protection are important to our business and that our future success will depend, in part, on our ability to maintain our technology licenses, maintain trade secret protection, obtain and maintain patents and operate without infringing the proprietary rights of others both in the United States and abroad. We believe that obtaining identical patents and protection periods for a given technology throughout all markets of the world will be difficult because of differences in patent laws. In addition, the protection provided by non-U.S. patents, if any, may be weaker than that provided by U.S. patents. We have established and continue to build proprietary positions for our HyperAcute technology and our IDO pathway inhibitor technology in the United States and abroad. As of December 31, 2013, our patent portfolio included ten patent families relating to our HyperAcute technology and eighteen patent families relating to our IDO pathway inhibitor technology.
There are two principal families of patents and patent applications relating to our HyperAcute product candidates and HyperAcute technology. The first patent family is exclusively licensed from Central Iowa Health System and includes three pending patent applications and 23 registered U.S. and foreign patents related to the HyperAcute Technology. This patent family is expected to provide basic composition of matter patent protection and methods of use of such compositions extending until 2024 and has already resulted in granted patents in U.S. (US 8,5551,474 and US 8,535,658), Europe (EP 1549353 B1), Mexico (278681), Japan (4966496) and Canada (2501744), all covering pharmaceutical compositions for inhibiting pre-established tumor growth comprising attenuated allogeneic tumor cells modified with alpha-Gal. Our patent is issued from this family in the U.S. and contains claims to methods of making master cell banks of HyperAcute allogeneic cells (US 7,763,461). Similar composition claims as well as methods of use for treating pre-established tumors are currently being further pursued in the U.S. and China.
The second principal family of patents is exclusively licensed from Drexel University and includes two U.S. patents (US 6,361,775 and US 5,879,675) relating to the use of alpha-Gal in viral and cancer vaccines. These patents expire in 2014 and 2016, respectively in the United States. Related patents in this family have also been granted in Canada and Europe and expire in 2015. We exclusively license from Central Iowa Health System or own several other patents relating to alpha-Gal technology, which we believe provide additional barriers to entry in the space occupied by our HyperAcute Technology. Additional coverage includes issued patents relating to gene therapy technology and the use of xenogeneic cells having alpha-Gal expiring in 2016 (US 7,005,126); and two applications issued in the United States (US Patents No. 7,998,486 and 8,357,777) and four pending applications in both the United States and Europe covering isolated tumor antigens comprising alpha-Gal residues. The issued United States patent expire in 2027 and 2028, while the pending applications are projected to expire in 2027. Additional patent applications have been filed covering the use of carbohydrate-modified glycoproteins (US 61/800,623 and US 13/463,420), chemosensitization by Hyper Actute vaccines (US 61/831,048). and correlates of efficacy to tumor vaccine (US61/823,873).
Our IDO pathway inhibitor technology patent portfolio contains several key U.S. patent families exclusively licensed from Georgia Health Sciences University, formerly known as the Medical College of Georgia Research Institute. The first patent family contains five issued U.S. patents expiring in 2018, 2019 and 2021. This family contains patents having claims to pharmaceutical compositions of 1-methyl-tryptophan (US 8,198,265), methods of increasing T cell activation (US 6,451,840) and methods of augmenting rejection of tumor cells (US 6,482,416) by administering an IDO inhibitor. The second patent family contains three issued U.S. patents directed to pharmaceutical compositions of indoximod (US 8,232,313, expires in 2024) and to methods of using indoximod to treat cancer (US 7,598,287 and US 8,580,844 expires in 2027and 2016, respectively). Related applications directed to the use of indoximod to activate T cells are granted in Australia and are pending in Canada.
We believe additional barriers to entry in the IDO space are provided through exclusive licenses with Lankenau Institute for Medical Research (LIMR) and various NewLink-owned inventions, in which we are pursuing patent protection for specific combination therapies targeting the IDO pathway, as well as protection for novel families of inhibitor compounds and second generation products. Four patent families that cover different classes of IDO inhibitor compounds and methods of treatment of cancer have been licensed from LIMR and are represented by several international pending cases and the issued patents US 7,714,139, US 8,476,454, CA 2520586, US 7,705,022, US 8,008,281, JP 4921965, CN ZL200480014321.1, CA 2520172, US 8,383,613, US 8,389,568. An additional patent family co-owned by us and LIMR covers compositions and methods of use of another family of IDO inhibitor compounds (PCT/US2009/041609). Additionally, three patent families covering compositions of matter and methods of use of different classes of IDO inhibitor compounds are fully owned by us (PCT/US2008/085167, PCT/US2010/054289 and PCT/US2012/033245. Among these patent families, PCT/US2012/033245 covers the IDO inhibitor compound NLG919, currently in clinical development, and its national counterparts are set to provide protection at least until 2032.
In order to protect the confidentiality of our technology, including trade secrets and know-how and other proprietary technical and business information, we require all of our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the use or disclosure of confidential information. The agreements also oblige our employees, consultants, advisors and collaborators to assign or license to us ideas, developments, discoveries and inventions made by such persons in connection with their work with us. We cannot be sure that these agreements will maintain confidentiality, will prevent disclosure, or will protect our proprietary information or intellectual property, or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The pharmaceutical industry is highly competitive and patents have been applied for by, and issued to, other parties relating to products or new technologies that may be competitive with those being developed by us. Therefore, any of our product candidates may give rise to claims that it infringes the patents or proprietary rights of other parties now or in the future. Furthermore, to the extent that we, our consultants, or manufacturing and research collaborators, use intellectual property owned by others in work performed for us, disputes may also arise as to the rights to such intellectual property or in related or resulting know-how and inventions. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties. A license required under any such patents or proprietary rights may not be available to us, or may not be available on acceptable terms. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that we are prevented from the development, manufacture or sale of products requiring such licenses. In addition, we could incur substantial costs in defending ourselves in legal proceedings instituted before patent and trademark offices in the United States, the European Union, or other ex-U.S. territories, or in a suit brought against us by a private party based on such patents or proprietary rights, or in a suit by us asserting our patent or proprietary rights against another party, even if the outcome is not adverse to us.
Licensing Agreements
Following are licensing agreements covering technologies and intellectual property rights useful to our HyperAcute product candidates and technologies:
Central Iowa Health System License Agreement
We are a party to a license agreement, or the CIHS Agreement, dated August 2, 2001, as amended on December 30, 2013 with the Central Iowa Health System, or CIHS. The CIHS Agreement grants to us an exclusive, worldwide license to make, have made, use, import, sell and offer for sale products that are covered by certain CIHS patent rights, proprietary information or know-how relating to our HyperAcute immunotherapy technology. The license is subject to CIHS's retained right to use, and to permit other academic and research institutions to use, the CIHS patent rights and information for non-commercial bona fide research purposes. The license is also subject to certain rights of and obligations to the United States government under applicable law, to the extent that such intellectual property was created using funding provided by a United States federal agency. We may grant sublicenses under the license, so long as the sublicense is subordinate to, and complies with, the CIHS Agreement.
In partial consideration of the license under the CIHS Agreement, we entered into a stock purchase agreement with CIHS, under which we issued to CIHS shares of our common stock and granted CIHS certain rights related to ownership of such shares. In addition, we must reimburse CIHS for out-of-pocket costs incurred for patent prosecution and maintenance. If we or our sublicensees commercialize a licensed product, we also have the obligation to pay CIHS royalties as a low single-digit percentage of net sales of the licensed product, subject to annual minimum royalties and a reduction for any royalty payments we must make to third parties. If we grant a sublicense under the licenses granted by CIHS, we must pay to CIHS, in addition to the royalties described above, a percentage of certain consideration paid by the sublicensee to us. We have no obligation to pay fees to CIHS as a result of payments between us and our affiliates.
Under the CIHS Agreement, we must use commercially reasonable efforts to develop and commercialize licensed products, to obtain necessary regulatory approvals and to launch and market such products in specified markets. As part of such efforts, we must deliver to CIHS certain information including an annual progress report detailing our progress towards commercial use of licensed products. At specific dates after the effective date we must satisfy certain obligations to conduct specified development on the licensed product, expend specified amounts on development of the licensed technology, or raise specific minimum amounts of equity capital. We are obligated to use commercially reasonable efforts to negotiate appropriate sponsored research programs with researchers at CIHS. If CIHS concludes that we have not met any of these obligations, and we fail to cure such failure, CIHS may either terminate the agreement or convert the license to a non-exclusive license. In addition, if CIHS determines that we have failed to use commercially reasonable efforts to, or to grant sublicenses to, develop or commercialize a licensed product in a particular field within the licensed field of use and we fail to cure such failure, CIHS may terminate, or convert the license to a non-exclusive license with respect to such particular field.
Unless terminated earlier, the CIHS Agreement will remain in effect until the expiration of all of our royalty obligations under the agreement. Our royalty obligations expire on a country-by-country and a licensed product-by-licensed product basis upon the later of (i) the expiration of the last to expire valid claim within the licensed patents covering a licensed product in a country or (ii) 12 years following the first commercial sale of a licensed product in a country. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect that the last patent will expire under this agreement in 2023, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. We may terminate the agreement, or specific patents covered by the agreement, after written notice to CIHS or for CIHS' uncured material breach of the agreement. CIHS has the right to terminate for our uncured material breach of the agreement after written notice. Upon termination of the agreement we may sell our existing inventory of licensed products for a period of three months after such termination. We have the right to assign the CIHS Agreement to any affiliate or in connection with the transfer of all or substantially all of our assets relating to the agreement, but any other assignment requires CIHS' written consent, which consent shall not be unreasonably withheld.
On December 30, 2013, we entered into an amendment to the CIHS Agreement, or the CIHS Amendment. Under the terms of the CIHS Amendment, certain provisions of the CIHS Agreement were amended, in part to clarify that we are not obligated to pay fees to CIHS as a result of payments between us and our affiliates with respect to the intellectual property rights licensed by CIHS to us pursuant to the CIHS Agreement. The CIHS Amendment includes, without limitation, clarifications to (i) the definitions of “Third Party(ies)” and “Net Sales”, (ii) provisions with respect to our obligations to take actions to enable CIHS to meet its obligations under certain federal statutes and (iii) provisions with respect to the sublicensing fee payable by us to CIHS. In addition, the CIHS Amendment updates the list of patents that are licensed to us under the CIHS Agreement. We entered into the CIHS Amendment in connection with our sublicensing of certain intellectual property to our wholly-owned foreign subsidiary.
Drexel University License Agreement
We are a party to a license agreement, or the Drexel Agreement, dated October 13, 2004 with Drexel University, or Drexel. The Drexel Agreement grants us, and our affiliates, an exclusive, worldwide license, under specified Drexel patent rights relating to compositions and methods for vaccines based on alpha-Gal epitopes, to make, have made, use, import, sell and offer for sale vaccine products that are covered by such patent rights, or that use related Drexel technical information, for use in the diagnosis and treatment of cancer, viral and other infectious disease. The license is subject to Drexel's retained right to use, and to permit other non-profit organizations to use, those patent rights and technical information for educational and non-commercial research purposes. The license is also subject to certain rights of and obligations to the United States government under applicable law, to the extent that such intellectual property was created using funding provided by a United States federal agency. We may grant sublicenses under the license, pursuant to a sublicense agreement in form acceptable to Drexel and subject to certain additional conditions and obligations.
In consideration of our license under the Drexel Agreement, we have paid and are obligated to continue to pay specified license fees, potential milestone payments in an aggregate amount up to approximately $1 million for each licensed product, annual license maintenance fees, reimbursement of patent prosecution costs, and royalty payments as a low single-digit percentage of “net sales” of any licensed product that is commercialized, subject to minimum royalty payments. Royalty rates vary depending on the type of licensed product, the territory where it is sold and whether the licensed product is combined with other technologies. In addition, if we grant a sublicense under the license granted by Drexel, we must pay Drexel a percentage of the consideration paid by the sublicensee to us.
In accordance with a development plan included in the Drexel Agreement, we are obligated to use commercially reasonable efforts to develop and market products covered by the license as soon as practicable. In addition, we must either market licensed products within five years of the date of the agreement, or demonstrate that we have made and continue to make bona fide, good faith, ongoing efforts to develop and market licensed products.
Unless terminated earlier, the Drexel Agreement shall remain in effect until the expiration or abandonment of all the licensed Drexel patents. Pending the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2015, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. We may terminate the Drexel Agreement on written notice to Drexel. Drexel has the right to terminate for the uncured breach of our obligations under the agreement or for certain other reasons. If the Drexel Agreement terminates we may, in certain circumstances, sell any remaining inventory of licensed products for a period of six months after termination. We may not assign the Drexel Agreement except with Drexel's written consent, not to be unreasonably withheld or delayed.
Following are licensing agreements covering technologies and intellectual property rights useful to our IDO pathway inhibitor technology and product candidate:
LIMR Exclusive License Agreement (IDO-1)
We are a party to a license agreement dated July 7, 2005, as amended May 22, 2006 and September 11, 2007, or the IDO-1 Agreement, with Lankenau Institute for Medical Research, or LIMR. The IDO-1 Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to inhibitors of indoleamine 2,3-dioxygenase, or IDO-1, and related LIMR technology, to make, have made, use, and sell products that are covered by such patent rights for use in the field of animal and human therapeutics and diagnostics. Such license is subject to LIMR's retained right to use such LIMR patent rights and technology for its non-commercial educational and research purposes. In addition, the license is subject to certain rights of and obligations to the U.S. government under applicable law, to the extent that such intellectual property was created using funding provided by a U.S. federal agency. We may grant sublicenses under the LIMR Licenses, provided that each sublicense materially conforms to the IDO-1 Agreement and is expressly subject to its terms.
In consideration of such license grant, we are obligated to pay to LIMR specified license fees, annual license maintenance fees, reimbursement of past patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $1.36 million for each licensed product, and royalties as a low single-digit percentage of net sales of the licensed products if a licensed product is commercialized. In addition, if we grant a sublicense under the IDO-1 Agreement, we must to pay to LIMR a percentage of the consideration received by us from the sublicensee.
Under the IDO-1 Agreement, we are obligated to use commercially reasonable efforts to develop and market the licensed products, and to achieve certain milestones by agreed-upon deadlines. If we breach our obligations and fail to cure such breach, LIMR may reduce our license to a non-exclusive license or revoke the license in its entirety.
Unless terminated earlier, the IDO-1 Agreement shall remain in effect until the expiration of the last licensed LIMR patents. Pending the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2024, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. LIMR may terminate the agreement for our failure to make payments due, bankruptcy or similar proceedings. Upon termination of the agreement, we may sell our current inventory of licensed products and those licensed products in the process of manufacture, subject to the terms of the agreement. We have the right to assign the IDO-1 Agreement in connection with an acquisition, merger, consolidation, operation of law or the transfer of all or substantially all of our assets or equity relating to the agreement, but any other assignment requires the express prior written consent of LIMR, not to be unreasonably withheld.
Georgia Health Sciences University License Agreement
We are a party to a License Agreement dated September 13, 2005, or the GHS Agreement, with Georgia Health Sciences University, or GHS, which was formerly known as the Medical College of Georgia Research Institute.The GHS Agreement was amended on April 27, 2006 and February 13, 2007. The GHS Agreement grants us, including our affiliates, an exclusive, worldwide license, under specified GHS patent rights and related technology to make, use, import, sell and offer for sale products that are covered by licensed patent rights or incorporates or uses licensed technology in all medical applications.
Such license is subject to GHS's retained right to use, and to permit its academic research collaborators to use, such GHS patent rights and technology for research and educational purposes. In addition, the license is subject to certain rights of and obligations to the U.S. government under applicable law, to the extent that such intellectual property was created using funding provided by a U.S. federal agency. We may grant sublicenses under such license, subject to the prior approval of GHSURI, not to be unreasonably withheld or delayed.
In consideration of such license grant, we are obligated to pay to GHS specified license fees (including issuing shares of our common stock), annual license maintenance fees, reimbursement of patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $2.8 million per licensed product, and royalties as a single-digit percentage of net sales of the licensed products, subject to minimum royalty payments and royalty rates depending on the type of license product. In addition, if we grant a sublicense under the license granted by GHS, we must pay to GHS a percentage of the consideration we receive from the sublicensee.
Under the agreement, we are obligated to make certain investments toward the further development of licensed products within specified time periods. If we fail to make the required investment, GHS may convert our license in the oncology field to a non-exclusive license. In addition, if we fail to develop the licensed products in a non-cancer field, specifically infectious disease or diagnostics, GHS may convert our license in such field to a non-exclusive license.
Unless terminated earlier, the GHS Agreement will remain in effect until the expiration of the last licensed GHS patents. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2027, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. GHS may terminate this agreement for our uncured material breach, bankruptcy or similar proceedings. For a period of one year following the termination of the agreement, we may sell our licensed products that are fully manufactured and part of our normal inventory at the date of termination. We have the right to assign the GHS Agreement to our affiliates or in connection with the transfer of all or substantially all of our assets relating to the agreement, but any other assignment requires the prior written consent of GHS.
LIMR Exclusive License Agreement (IDO-2)
We are a party to a license agreement, or the LIMR IDO-2 Agreement, executed December 21, 2007 with LIMR. The LIMR IDO-2 Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to inhibitors of the target Indoleamine 2,3 Dioxygenase-2, or IDO-2, and under related LIMR know-how or technology, to make, have made, use, import, sell and offer for sale products and services that are covered by such patent rights, for all uses. Such license is subject to LIMR's retained non-exclusive right to use such LIMR patent rights and technical information for internal non-commercial, educational and research purposes only. In addition, the license is subject to certain rights of and obligations to the U.S. government under applicable law, to the extent that such intellectual property was created using funding provided by a U.S. federal agency. We may grant sublicenses under the LIMR IDO-2 license, provided that each sublicense complies with the terms of the LIMR IDO-2 Agreement.
In consideration of such license grant, we have paid to LIMR an upfront license fee and annual license maintenance fees, and are obligated to pay LIMR annual license maintenance fees, potential milestone payments in an aggregate amount up to approximately $1.52 million per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement, and, if a licensed product is commercialized, royalties as a low single-digit percentage of “net sales” of the licensed product,
subject to reduction for our royalty payments to third parties. In addition, if we grant a sublicense under the licenses granted by LIMR, we must pay to LIMR a percentage of the consideration paid by the sublicensee to us. The payment provisions of the LIMR IDO-2 Agreement provide that, in the event a product for which we have payment obligations under the LIMR IDO-2 Agreement is also covered by payment obligations under the LIMR IDO-1 Agreement, we will not be obligated to pay both such obligations but rather will pay to LIMR the higher of the amounts owed under the two agreements.
Under the LIMR IDO-2 Agreement, we have agreed to use our commercially reasonable efforts to develop and exploit products covered by the license. We have the obligation, at our expense and in our reasonable discretion, to conduct the prosecution and maintenance of the LIMR patent rights licensed to us under the agreement. In addition, LIMR granted us the exclusive option to obtain exclusive, worldwide licenses on commercially reasonable terms to future inventions and discoveries of LIMR related to IDO-2 or inhibitors of IDO-2.
Concurrently with, and as an obligation under, the LIMR IDO-2 Agreement, we entered into a cooperative research and development agreement with LIMR, or the CRADA Agreement. Under the CRADA agreement, we agree to provide funding to LIMR in support of IDO research for one year and renewable at our option.
Unless terminated earlier, the LIMR IDO-2 Agreement shall continue until the expiration of the last valid LIMR patent licensed under the agreement. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2027, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. We may terminate the Agreement on written notice to LIMR. LIMR has the right to terminate for our uncured breach, bankruptcy or similar proceedings. Upon termination of the agreement, we may sell our current inventory of licensed products and those licensed products in the process of manufacture, subject to the terms of the agreement. We may assign the LIMR Agreement in connection with the transfer of all or substantially all of our assets or equity, or by reason of acquisition, merger, consolidation or operation of law, but any other assignment requires LIMR's written consent, which shall not be unreasonably withheld.
LIMR Exclusive License Agreement (IDO)
We are a party to a license agreement, or the LIMR IDO Agreement, dated April 23, 2009 with LIMR. The LIMR IDO Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to IDO inhibitors, and under related LIMR know-how or technology, to make, have made, use, import, sell and offer for sale products and services that are covered by such patent rights, for all uses. Such license is subject to LIMR's retained non-exclusive right to use such LIMR patent rights and technical information for internal non-commercial, educational and research purposes only. In addition, the license is subject to certain rights of and obligations to the U.S. government under applicable law, to the extent that such intellectual property was created using funding provided by a U.S. federal agency. We may grant sublicenses under the LIMR IDO license, provided that each sublicense complies with the terms of the LIMR IDO Agreement.
In consideration of such license grant, we are obligated to pay LIMR potential milestone payments in an aggregate amount up to approximately $610,000 per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement or LIMR IDO-2 Agreement, and royalties as a low single-digit percentage of “net sales” of the licensed product, subject to reduction for our royalty payments to third parties and to LIMR under the IDO-1 Agreement or LIMR IDO-2 Agreement. In addition, if we grant a sublicense under the licenses granted by LIMR, we must pay to LIMR a percentage of the consideration paid by the sublicensee to us.
Under the LIMR IDO Agreement, we have agreed to use our commercially reasonable efforts to develop and exploit products covered by the license. We have the right and responsibility, at our expense and in our reasonable discretion, to conduct the prosecution and maintenance of the LIMR patent rights licensed to us under the agreement.
Unless terminated earlier, the LIMR IDO Agreement shall continue until the expiration of the last valid LIMR patent licensed under the agreement. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2029, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. We may terminate the Agreement on written notice to LIMR. LIMR has the right to terminate for our uncured breach, bankruptcy or similar proceedings. Upon termination of the agreement, we may sell our current inventory of licensed products and those licensed products in the process of manufacture, subject to the terms of the agreement. We may assign the LIMR IDO Agreement in connection with the transfer of all or substantially all of our assets or equity, or by reason of acquisition, merger, consolidation or operation of law, but any other assignment requires LIMR's written consent, which shall not be unreasonably withheld.
Bresagen Patent License Agreement
We are a party to a license agreement, or the Bresagen Agreement, dated March 1, 2006 with Bresagen Xenograft Marketing Ltd, or Bresagen. The Bresagen Agreement grants us a non-exclusive, non-sublicensable license to specified Bresagen patent rights for use in testing microbial and cancer vaccines in the U.S. In consideration of such license grant, we are obligated to pay Bresagen an up-front license fee and an annual license fee.
Unless terminated earlier, the Bresagen Agreement shall continue for an initial period of eight years, which may be extended an additional five years upon agreement of the parties. We may terminate the Agreement upon agreement in writing with Bresagen. Bresagen has the right to terminate for our uncured breach, insolvency, change of control without consent or similar proceedings. Upon termination of the agreement, all of our rights under the license are terminated. We may assign the Bresagen Agreement in connection with the transfer of all or substantially all of our assets by reason of acquisition, merger, purchase or otherwise with notice to Bresagen, but any other assignment requires Bresagen's written consent.
Following are licensing agreements to which BPS is a party covering technologies and intellectual property rights applicable to BPS's development of vaccines for the biodefense field:
The Ohio State University License Agreement
We are a party to a license agreement dated June 28, 2013 with The Ohio State University, or OSU. This agreement grants us exclusive rights in the United States and its territories, in all fields of use, to patents related to pharmaceutical compositions and vaccines comprising antigens associated with L-Rhamnose and Forssman epitopes, including patent application US 13/463,420, which is co-owned by us and OSU.
In consideration of the license grant, we are obligated to pay OSU specified license fees and are obligated to pay to OSU specified annual license maintenance fees, patent prosecution and maintenance costs, potential milestone payments in an aggregate amount up to approximately $2.75 million for the first licensed product, and royalties as a low single-digit percentage of net sales of the licensed products if a licensed product is commercialized, with minimum royalties for the first three years in which a licensed product generates revenue. In addition, if we grant a sublicense under this agreement, we must also pay to OSU a percentage of certain consideration received by us from the sublicensee. Under this agreement, we are obligated to use commercially reasonable efforts to develop and market the licensed products and to use commercially reasonable efforts to achieve certain milestones by agreed-upon deadlines.
Unless terminated earlier, the agreement will continue until the expiration of the last licensed patent rights. We may terminate the agreement at any time for any reason with 90 days prior written notice. OSU may immediately terminate the agreement if we materially breach our obligations to OSU and do not cure the breach within the specified period, or if we or our affiliates or sublicensees participates in a challenge to the validity, enforceability or scope of the licensed patents. The agreement will also terminate if we agree with OSU that the agreement should be terminated or if we cease business operations or become involved in any bankruptcy, insolvency or liquidation-related activities. If the agreement is terminated, we will lose our license from OSU to the licensed patents but we will retain our rights as owners of all licensed patents that are co-owned by us and OSU.
Regents of the University of California License Agreement
BPS is a party to a license agreement dated July 29, 2008, or the California License, with the Regents of the University of California, or California. The California License grants BPS an exclusive, worldwide license, under specified California patent rights relating to technology based on yellow fever virus, to make, use, import, sell and offer for sale products that are covered by licensed patent rights in the field of human healthcare. The license is subject to California's retained right to use the California patent rights and technology for research purposes. The license is also subject to certain rights of and obligations to the United States government under applicable law. BPS may grant sublicenses under the California license, provided that each sublicense is consistent with the terms and conditions of the California License.
In consideration of the license grant, BPS must pay to California a specified license issue fee, annual license maintenance fees, patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $285,000 per licensed product, and royalties as a low single-digit percentage of net sales of the licensed product, which royalty rate varies depending on the territory. In addition, if BPS grants a sublicense under the licenses granted by California, BPS may be required to pay to California a percentage of certain consideration BPS receives from the sublicensee. BPS is obligated to use commercially reasonable efforts to develop and market the licensed products, and to achieve certain milestones by agreed-upon deadlines. If BPS breaches its obligations and fails to cure the breach, California may terminate the California License or reduce BPS's rights under the license.
Unless terminated earlier, the California License will remain in effect until the expiration or abandonment of the last of the California patent rights. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we expect the last patent will expire under this agreement in 2024, excluding any patent term extensions or additional patents issued that are included under the license. This agreement will terminate automatically upon the filing, by or against BPS, for relief under the United States Bankruptcy Code or upon the filing of a legal action, by or on behalf of BPS, claiming that any portion of the California License is invalid or unenforceable. California may terminate this agreement for BPS's uncured material breach. BPS may terminate this agreement upon written notice to California. Upon termination of the agreement, BPS may sell any previously made licensed product for a period of 120 days after termination. BPS has the right to assign the California License to its affiliates or in connection with a merger, acquisition, or the transfer of all or substantially all of its assets relating to the agreement, but any other assignment requires the prior written consent of California.
Her Majesty the Queen in Right of Canada License Agreement
BPS is a party to a license agreement dated May 4, 2010, or the Canada License, with the Her Majesty the Queen in Right of Canada, or Canada. The Canada License grants BPS a worldwide, personal, non-transferable, sole, revocable, royalty-bearing license for commercialization of specified Canada patent rights relating to technology based on rVSV. The license is subject to Canada's retained right to use the Canada patent rights and technology to improve the patent rights, carryout educational purposes, and development of the patent rights where BPS cannot obtain regulatory approval or meet demand. BPS may grant sublicenses under the Canada license, provided that each sublicense is consistent with the terms and conditions of the Canada License and contain certain mandatory sublicensing provisions.
In consideration of the license grant, BPS must pay to Canada specified patent and signing fees, annual license maintenance fees, patent prosecution costs, potential milestone payments in an aggregate amount up to approximately C$205,000 per licensed product, and royalties as a low single-digit percentage of the sales price of the licensed products sold by BPS, which royalty rate varies depending on the type of licensed product. In addition, if BPS grants a sublicense under the licenses granted by Canada, BPS is required to pay to Canada a percentage of certain consideration BPS receives from the sublicensee. BPS is obligated to use commercially reasonable efforts to develop and market the licensed products. If BPS breaches its obligations and fails to cure the breach, Canada may terminate the Canada License.
Unless terminated earlier, the Canada License will remain in effect until the expiration of the last of the Canada patent rights. Pending the status of certain patent applications and the payment of appropriate maintenance, renewal, annuity or other governmental fees, we currently expect the last patent will expire under this agreement in 2023, excluding any patent term adjustments or patent term extensions or additional patents issued that are included under the license. Canada may terminate this agreement for BPS's failure to use commercially reasonable efforts to commercialize, failure to pay, breach of confidentiality, cessation of business, criminal conviction or other breach of its obligations under the agreement. BPS may not assign the Canada License to a third party without the prior written consent of Canada, not to be unreasonably withheld. This agreement will terminate automatically if BPS assigns the Canada License without prior written consent or if BPS files for bankruptcy or similar proceedings.
Government Regulation
We operate in a highly regulated industry that is subject to significant federal, state, local and foreign regulation. Our present and future business has been, and will continue to be, subject to a variety of laws including, the Federal Food, Drug, and Cosmetic Act, or FDC Act, and the Public Health Service Act, among others.
The FDC Act and other federal and state statutes and regulations govern the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of our products. As a result of these laws and regulations, product development and product approval processes are very expensive and time consuming.
FDA Approval Process
In the United States, pharmaceutical products, including biologics, are subject to extensive regulation by the FDA. The FDC Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, or biologic license applications, or BLAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of a notice of claimed investigational exemption or an investigational new drug application, or IND, which must
become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug or biologic for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation as well as animal trials to assess the characteristics and potential pharmacology and toxicity of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not objected to the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations, good clinical practices, or GCP, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The clinical trial protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
Clinical trials to support NDAs or BLAs, which are applications for marketing approval, are typically conducted in three sequential Phases, but the Phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population, to determine the effectiveness of the drug for a particular indication or indications, dosage tolerance and optimum dosage, and identify common adverse effects and safety risks.
If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. Before proceeding with a Phase 3 clinical trial, sponsors may seek a written agreement from the FDA regarding the design, size, and conduct of a clinical trial. This is known as a Special Protocol Assessment, or SPA. SPAs help establish up front agreement with the FDA about the adequacy of the design of a clinical trial to support a regulatory approval, but the agreement is not binding if new circumstances arise. In addition, even if an SPA remains in place and the trial meets its endpoints with statistical significance, the FDA could determine that the overall balance of risks and benefits for the product candidate is not adequate to support approval, or only justifies approval for a narrow set of clinical uses or approval with restricted distribution or other burdensome post-approval requirements or limitations.
In the case of product candidates for severe or life-threatening diseases such as cancer, the initial human testing is often conducted in patients rather than in healthy volunteers. Since these patients already have the target disease, these studies may provide initial evidence of efficacy traditionally obtained in Phase 2 clinical trials and thus these trials are frequently referred to as Phase 1B clinical trials. Additionally, when product candidates can do damage to normal cells, it is not ethical to administer such drugs to healthy patients in a Phase 1 clinical trial. After completion of the required clinical testing, an NDA or, in the case of a biologic, a BLA, is prepared and submitted to the FDA. FDA approval of the marketing application is required before marketing of the product may begin in the U.S. The marketing application must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product's pharmacology, chemistry, manufacture, and controls.
The FDA has 60 days from its receipt of an NDA or BLA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of marketing applications. Most such applications for non-priority drug products are reviewed within ten months of their acceptance for filing. The review process may be extended by the FDA for three additional months to consider new information submitted
during the review or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving a marketing application, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practices, or cGMPs, is satisfactory and the marketing application (the NDA or, in the case of biologics, the BLA) contains data that provide substantial evidence that the drug is safe and effective in the indication studied. Manufacturers of biologics also must comply with FDA's general biological product standards.
After the FDA evaluates the marketing application and the manufacturing facilities, it issues an approval letter, or a complete response letter. A complete response letter outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed in a resubmission of the marketing application, FDA will re-initiate review. If it is satisfied that the deficiencies have been addressed, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. It is not unusual for the FDA to issue a complete response letter because it believes that the drug is not safe enough or effective enough or because it does not believe that the data submitted are reliable or conclusive.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of approval of the marketing application, the FDA may require substantial post-approval testing and surveillance to monitor the drug's safety or efficacy and may impose other conditions, including labeling restrictions which can materially affect the potential market and profitability of the drug. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Fast Track Designation
A Fast Track product is a new drug or biologic intended for the treatment of a serious or life-threatening condition that demonstrates the potential to address unmet medical needs for this condition. Under the FDA's Fast Track program, the sponsor of a new drug or biologic may request that the FDA designate the drug or biologic as a Fast Track product at any time during the development of the product, prior to a new drug application submission. Fast Track designation enables a company to file their application for approval on a rolling basis and potentially qualify for priority review.
The FDA may condition approval of an application for a Fast Track product on a commitment to do post-approval studies to validate the surrogate endpoint or confirm the effect on the clinical endpoint and require prior review of all promotional materials. In addition, the FDA may withdraw approval of a Fast Track product in an expedited manner on a number of grounds, including the sponsor's failure to conduct any required post-approval study in a timely manner. On October 1, 2010 the FDA approved our application for Fast Track product designation for HyperAcute Pancreas.
Orphan Drug Designation
We were granted Orphan Drug designation for HyperAcute Pancreas on October 21, 2010 by the FDA. The FDA grants Orphan Drug designation to drugs intended to treat a rare disease or condition, which for this program is defined as having a prevalence of less than 200,000 individuals in the United States.
Orphan drug designation does not shorten the regulatory review and approval process for an orphan drug, nor does it give that drug any advantage in the regulatory review and approval process. However, if an orphan drug later receives the first approval for the indication for which it has designation, the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years in the United States. Orphan Drug exclusive marketing rights may be lost if the FDA determines that our request for designation was materially defective or if we are unable to assure sufficient quantity of our drug.
Additional benefits of Orphan Drug designation include clinical tax research incentives and exemption from application filing fees. Although obtaining approval to market a product with orphan drug exclusivity may be advantageous, we cannot be certain:
| |
| • | that we will be the first to obtain approval for any other drugs or indications for which we obtain Orphan Drug designation; |
| |
| • | that Orphan Drug designation will result in any commercial advantage or reduce competition; or |
| |
| • | that the limited exceptions to this exclusivity will not be invoked by the FDA. |
The Hatch-Waxman Act
In seeking approval for marketing of a drug or biologic through an NDA or BLA, respectively, applicants are required to list with the FDA each patent with claims that cover the applicant's product or FDA approved method of using this product. Upon approval of a product, each of the patents listed in the application for the drug is then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application, or ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. ANDA applicants are not required to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug product, other than the requirement for bioequivalence testing. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA's Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. A certification that the new product will not infringe the already approved product's listed patents or that such patents are invalid is called a Paragraph IV certification. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA applicant and patent holders once the ANDA has been accepted for filing by the FDA. The NDA applicant and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification notification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. Federal law provides a period of five years following approval of a drug containing no previously approved active moiety, during which ANDAs for generic versions of those drugs cannot be submitted unless the submission contains a Paragraph IV challenge to a listed patent, in which case the submission may be made four years following the original product approval. Federal law provides for a period of three years of exclusivity following approval of a listed drug that contains previously approved active ingredients but is approved in a new dosage form, route of administration or combination, or for a new use, the approval of which was required to be supported by new clinical trials conducted by or for the sponsor, during which the FDA cannot grant effective approval of an ANDA based on that listed drug.
Other Regulatory Requirements
Once an NDA or BLA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement, or in the case of biologics, a new BLA or BLA supplement, before the change can be implemented. An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA and BLA supplements as it does in reviewing NDAs and BLAs. We cannot be certain that the FDA or any other regulatory agency will grant approval for our product candidates for any other indications or any other product candidate for any indication on a timely basis, if at all.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA or BLA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies, and surveillance
to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to access compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Priority Review
Under FDA policies, a drug or biologic candidate is eligible for priority review, or review within a six-month time frame from the acceptance for filing of an NDA or BLA, if the drug candidate provides a significant improvement compared to marketed drugs in the treatment, diagnosis or prevention of a disease. A Fast Track designated drug or biologic candidate ordinarily meets the FDA's criteria for priority review.
U.S. Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Federal and State Healthcare Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to increase the transparency of and restrict certain marketing practices in the pharmaceutical and medical device industries in recent years. These laws include the Physician Payment Sunshine Act, anti-kickback statutes and false claims statutes.
Federal and state healthcare laws, including fraud and abuse and health information privacy and security laws, are also applicable to our business. We could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected. The laws that may affect our ability to operate include: the federal Anti-Kickback Statute, which prohibits soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; and the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters and was amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. There are also state law equivalents of each of the above federal laws, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Regulation in the European Union
Drugs are also subject to extensive regulation outside of the United States. In the E.U., for example, there is a centralized approval procedure that authorizes marketing of a product in all countries of the E.U. (which includes most major countries in Europe). If this procedure is not used, approval in one country of the E.U. can be used to obtain approval in another country of the E.U. under two simplified application processes, the mutual recognition procedure or the decentralized procedure, both of which rely on the principle of mutual recognition. After receiving regulatory approval through any of the European registration procedures, pricing and reimbursement approvals are also required in most countries.
Similar to the United States, a system for Orphan Drug designation exists in the E.U. Orphan designation does not shorten the regulatory review and approval process for an orphan drug, nor does it give that drug any advantage in the regulatory review and approval process. However, if an orphan drug later receives approval for the indication for which it has designation, the relevant regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for ten years in the E.U.
On November 4-5, 2012, the Committee for Orphan Medical Products, or COMP, recommended to the European Commission that HyperAcute Pancreas (algenpantucel-L) be designated as an orphan medicinal product, and the recommendation was formally accepted by the European Commission.
Price Controls
In many of the markets where we may do business in the future, the prices of pharmaceutical products are subject to direct price controls (by law) and to reimbursement programs with varying price control mechanisms. In the United States, the Medicare program is administered by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services. Coverage and reimbursement for products and services under Medicare are determined pursuant to regulations promulgated by CMS and pursuant to CMS's subregulatory coverage and reimbursement determinations. It is difficult to predict how CMS may apply those regulations and subregulatory determinations to newly approved products, especially novel products, and those regulations and interpretive determinations are subject to change. Moreover, the methodology under which CMS makes coverage and reimbursement determinations is subject to change, particularly because of budgetary pressures facing the Medicare program. Medicare regulations and interpretive determinations also may determine who may be reimbursed for certain services.
In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from member state to member state. Some jurisdictions operate positive or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products, as exemplified by the role of the National Institute for Health and Clinical Excellence in the United Kingdom, which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert commercial pressure on pricing within a country.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances and biological materials. We may incur significant costs to comply with such laws and regulations now or in the future.
Legal Proceedings
We are not currently a party to any legal proceedings that are expected to have a material impact on our operations.
Employees
As of December 31, 2013 we had 104 employees. None of our employees are subject to a collective bargaining agreement or represented by a labor or trade union, and we believe that our relations with our employees are good.
Facilities
Our executive offices and manufacturing facilities are located in the Iowa State University Research Park in Ames, Iowa. In June 2010, we completed the expansion of a 22,500 square foot facility, which includes executive offices as well as approximately 14,000 feet dedicated to manufacturing, testing and product storage. The manufacturing portion of the facility became operational on October 17, 2010. The lease expires January 31, 2015, and we have the option to extend the lease for three additional five-year periods upon the same terms as the base lease. In addition, we continue to occupy a small pilot manufacturing and office facility in the same research park under the terms of a lease that expires October 31, 2014.
On November 14, 2011, we entered into a Memorandum of Agreement, or the Memorandum, with Iowa State University Research Park Corporation, or ISURP. The Memorandum is an addendum to the lease, or the Lease, dated September 30, 2009 between us and ISURP covering our facilities in Ames, Iowa. The Memorandum added approximately 26,000 square feet of additional space to the Lease. During 2012 operating rents to ISURP increased by $211,000. During 2012 ISURP provided a building allowance of $622,000 and assisted in securing an additional $456,000 in debt financing for us.
On May 30, 2013, we entered into a Standard Design-Build Agreement, or the Story Agreement, with Story Construction Co. to provide remodeling services with respect to approximately 11,700 square feet of existing manufacturing and quality control
space at our headquarters in Ames, Iowa. Our obligations under the Story Agreement constitute a purchase obligation of approximately $1.0 million. The full amount is due upon substantial completion of the work.
On February 24, 2014, we signed a lease for a commercial facility in Austin, Texas. We anticipate conducting activities at this location associated with expansion of our commercialization efforts as well as supporting the continued growth of our clinical operations activities.
Corporate Information
We were incorporated in the state of Delaware on June 4, 1999 under the name “NewLink Genetics Corporation.”
Available Information
Our website address is www.linkp.com; however, information found on, or that can be accessed through, our website is not incorporated by reference into this Annual Report on Form 10-K. We make available free of charge on or through our website copies of our Annual Report, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
Investing in our common stock involves a high degree of risk. In evaluating our business, investors should carefully consider the following risk factors. These risk factors contain, in addition to historical information, forward-looking statements that involve substantial risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward-looking statements, and the differences could be material. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below. The order in which the following risks are presented is not intended to reflect the magnitude of the risks described. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Business Risks
Risks Relating to Clinical Development and Commercialization of Our Product Candidates
Our near term prospects are highly dependent on HyperAcute Pancreas. If we fail to complete, or fail to demonstrate safety and efficacy in, clinical trials, fail to obtain regulatory approval or fail to successfully commercialize HyperAcute Pancreas, our business would be harmed and the value of our common stock would likely decline.
We must be evaluated in light of the uncertainties and complexities affecting a development stage biopharmaceutical company. We have not completed clinical development for any of our products. Our most advanced product candidate is HyperAcute Pancreas. The United State Food and Drug Administration, or FDA, must approve HyperAcute Pancreas before it can be marketed or sold. Our ability to obtain FDA approval of HyperAcute Pancreas depends on, among other things, completion of our Phase 3 clinical trial, whether our Phase 3 clinical trial of HyperAcute Pancreas demonstrates statistically significant achievement of the clinical trial endpoints with no significant safety issues and whether the FDA agrees that the data from our Phase 3 clinical trial of HyperAcute Pancreas is sufficient to support approval. The final results of our Phase 3 clinical trials of HyperAcute Pancreas may not meet the FDA’s requirements to approve the product for marketing, and the FDA may otherwise determine that our manufacturing processes, facilities or raw materials are insufficient to warrant approval. We may need to conduct more clinical trials than we currently anticipate. Furthermore, even if we do receive FDA approval, we may not be successful in commercializing HyperAcute Pancreas. If any of these events occur, our business could be materially harmed and the value of our common stock would likely decline.
If our product candidates do not meet safety and efficacy endpoints in clinical trials, they will not receive regulatory approval, and we will be unable to market them. We have not completed testing any of our product candidates in controlled clinical trials.
The clinical development and regulatory approval process is expensive and time-consuming. The timing of any future product approval cannot be accurately predicted. If we fail to obtain regulatory approval for our current or future product candidates, we will be unable to market and sell them and therefore we may never be profitable.
As part of the regulatory process, we must conduct clinical trials for each product candidate to demonstrate safety and efficacy to the satisfaction of the FDA and other regulatory authorities abroad. The number and design of clinical trials that will be required varies depending on the product candidate, the condition being evaluated, the trial results and regulations applicable to any particular product candidate.
Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. Initial results may not be confirmed upon full analysis of the detailed results of a trial. Product candidates in later stage clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials with acceptable endpoints.
In particular, there have been no control groups in our clinical trials completed to date. While comparisons to results from other reported clinical trials can assist in predicting the potential efficacy of our HyperAcute Pancreas product candidate, there are many factors that affect the outcome for patients in clinical trials, some of which are not apparent in published reports, and results from two different trials cannot always be reliably compared. As a result, we are studying HyperAcute Pancreas in combination with the current standard-of-care in direct comparison to the current standard-of-care alone in the same trial and will need to show a statistically significant benefit when added to the current standard-of-care in order for HyperAcute Pancreas to be approved as a marketable drug. Patients in our Phase 3 study who do not receive HyperAcute Pancreas may not have results similar to patients studied in the other studies we have used for comparison to our Phase 2 studies. If the patients in our Phase 3 study
who receive standard-of-care without HyperAcute Pancreas have results which are better than the results predicted by the other large studies, we may not demonstrate a sufficient benefit from HyperAcute Pancreas to allow the FDA to approve it for marketing.
Our HyperAcute product candidates are based on a novel technology, which may raise development issues we may not be able to resolve, regulatory issues that could delay or prevent approval or personnel issues that may keep us from being able to develop our product candidates.
Our HyperAcute product candidates are based on our novel HyperAcute immunotherapy technology. In the course of developing this technology and these product candidates, we have encountered difficulties in the development process. There can be no assurance that additional development problems, which we may not be able to resolve or which may cause significant delays in development, will not arise in the future.
Regulatory approval of novel product candidates such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to our and regulatory agencies’ lack of experience with them. This may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, including post-approval studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions. For example, the two cell lines that comprise HyperAcute Pancreas are novel and complex therapeutics that we have endeavored to better characterize so that their identity, strength, quality, purity and potency may be compared among batches created from different manufacturing methods. We currently lack the manufacturing capacity necessary for larger-scale production. If we make any changes to our current manufacturing methods or cannot design assays that satisfy the FDA’s expectations regarding the equivalency of such therapeutics in the laboratory, the FDA may require us to undertake additional clinical trials.
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
Our Special Protocol Assessment, or SPA, with the FDA relating to our HyperAcute Pancreas Phase 3 clinical trial does not guarantee any particular outcome from regulatory review of the trial or the product candidate, including any regulatory approval.
The protocol for our HyperAcute Pancreas Phase 3 clinical trial was reviewed by the FDA under its SPA process, which allows for FDA evaluation of a clinical trial protocol intended to form the primary basis of an efficacy claim in support of an NDA or BLA, and provides an agreement that the study design, including trial size, clinical endpoints and/or data analyses are acceptable to the FDA. However, the SPA agreement is not a guarantee of approval. The FDA retains the right to require additional Phase 3 testing and we cannot be certain that the design of, or data collected from, the HyperAcute Pancreas Phase 3 clinical trial will be adequate to demonstrate the safety and efficacy of HyperAcute Pancreas for the treatment of patients with pancreatic cancer, or otherwise be sufficient to support FDA or any foreign regulatory approval. In addition, the survival rates, duration of response and safety profile required to support FDA approval are not specified in the HyperAcute Pancreas Phase 3 clinical trial protocol and will be subject to FDA review. Although the SPA agreement calls for review of interim data at certain times prior to completion, there is no assurance that any such review, even if such interim data are positive, will result in early approval. Further, the SPA agreement is not binding on the FDA if public health concerns unrecognized at the time the SPA agreement was entered into become evident, other new scientific concerns regarding product safety or efficacy arise, or if we fail to comply with the agreed upon trial protocols. In addition, the SPA agreement may be changed by us or the FDA on written agreement of both parties, and the FDA retains significant latitude and discretion in interpreting the terms of the SPA agreement and the data and results from the HyperAcute Pancreas Phase 3 clinical trial. As a result, we do not know how the FDA will interpret the parties’ respective commitments under the SPA agreement, how it will interpret the data and results from the HyperAcute Pancreas Phase 3 clinical trial, or whether HyperAcute Pancreas will receive any regulatory approvals as a result of the SPA agreement or the HyperAcute Pancreas Phase 3 clinical trial. Therefore, significant uncertainty remains regarding the clinical development and regulatory approval process for HyperAcute Pancreas for the treatment of patients with pancreatic cancer.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we must focus on research programs and product candidates for the specific indications that we believe are the most scientifically and commercially promising. As a result, we have in the past determined to let certain of our development projects remain idle including by allowing the applicable Investigational New Drug application, or IND, to lapse into inactive status, and we may in the future decide to forego or delay pursuit of opportunities with
other product candidates or other indications that later prove to have greater scientific or commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable scientific or commercial products or profitable market opportunities. In addition, we may spend valuable time and managerial and financial resources on research programs and product candidates for specific indications that ultimately do not yield any scientifically or commercially viable products. Furthermore, our resource allocation decisions, and our decisions about whether and how to develop or commercialize any particular product candidate may be based on evaluations of the scientific and commercial potential or target market for the particular product candidate that later prove to be materially inaccurate. If we enter into collaborations, licensing or other royalty arrangements to develop or commercialize any particular product candidate, we may relinquish valuable rights to that product candidate in situations where it would have been more advantageous for us to retain sole rights to development and commercialization.
We may face delays in completing our clinical trials, or we may not be able to complete them at all.
We have not completed all the clinical trials necessary to support an application with the FDA for approval to market any of our product candidates. Our current and future clinical trials may be delayed or terminated as a result of many factors, including:
| |
| • | we may experience delays or failure in reaching agreement on acceptable clinical trial contracts or clinical trial protocols with prospective sites; |
| |
| • | regulators or institutional review boards may not authorize us to commence a clinical trial; |
| |
| • | regulators or institutional review boards may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or concerns about patient safety; |
| |
| • | we may suspend or terminate our clinical trials if we believe that they expose the participating patients to unacceptable health risks; |
| |
| • | our clinical trials may have slower than expected patient enrollment or lack of a sufficient number of patients that meet the enrollment criteria for our clinical trials; |
| |
| • | patients may not complete clinical trials due to safety issues, side effects, dissatisfaction with the product candidate, or other reasons; |
| |
| • | we may experience difficulty in maintaining contact with patients after treatment, preventing us from collecting the data required by our study protocol; |
| |
| • | product candidates may demonstrate a lack of efficacy during clinical trials; |
| |
| • | we may experience governmental or regulatory delays, failure to obtain regulatory approval or changes in regulatory requirements, policy and guidelines; |
| |
| • | enrollment in and conduct of our clinical trials may be adversely affected by competition with ongoing clinical trials and scheduling conflicts with participating clinicians; and |
| |
| • | we may experience delays in achieving study endpoints and completing data analysis for a trial. |
In addition, we rely on academic institutions, physician practices and clinical research organizations to conduct, supervise or monitor some or all aspects of clinical trials involving our product candidates. We have less control over the timing and other aspects of these clinical trials than if we conducted the monitoring and supervision entirely on our own. Third parties may not perform their responsibilities for our clinical trials on our anticipated schedule or consistent with a clinical trial protocol or applicable regulations. We also may rely on clinical research organizations to perform our data management and analysis. They may not provide these services as required or in a timely or compliant manner.
Moreover, our development costs will increase if we are required to complete additional or larger clinical trials for the HyperAcute product candidates or the IDO pathway inhibitor product candidates prior to FDA approval. If the delays or costs are significant, our financial results and ability to commercialize the HyperAcute product candidates, indoximod, NLG919 or other future product candidates will be adversely affected.
If we encounter difficulties enrolling patients in our clinical trials, our clinical trials could be delayed or otherwise adversely affected.
Clinical trials for our product candidates require us to identify and enroll a large number of patients with the disease under investigation. We may not be able to enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a study, to complete our clinical trials in a timely manner. Patient enrollment is affected by factors including:
| |
| • | severity of the disease under investigation; |
| |
| • | design of the trial protocol; |
| |
| • | the size of the patient population; |
| |
| • | eligibility criteria for the study in question; |
| |
| • | perceived risks and benefits of the product candidate under study; |
| |
| • | availability of competing therapies and clinical trials; |
| |
| • | efforts to facilitate timely enrollment in clinical trials; |
| |
| • | patient referral practices of physicians; |
| |
| • | the ability to monitor patients adequately during and after treatment; and |
| |
| • | proximity and availability of clinical trial sites for prospective patients. |
In particular, the inclusion of critically ill patients in our clinical trials may result in deaths or other adverse medical events for reasons that may not be related to the product candidate we are testing or, in those trials where our product candidate is being tested in combination with one or more other therapies, for reasons that may be attributable to such other therapies, but which can nevertheless negatively affect clinical trial results. In addition, we have experienced difficulties enrolling patients in certain of our smaller clinical trials due to lack of referrals and may experience similar difficulties in the future.
If we have difficulty enrolling a sufficient number or diversity of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have an adverse effect on our business.
Regulatory authorities may not approve our product candidates even if they meet safety and efficacy endpoints in clinical trials.
We have discussions with and obtain guidance from regulatory authorities regarding certain aspects of our clinical development activities. These discussions are not binding commitments on the part of regulatory authorities. Under certain circumstances, regulatory authorities may revise or retract previous guidance during the course of our clinical activities or after the completion of our clinical trials. A regulatory authority may also disqualify a clinical trial in whole or in part from consideration in support of approval of a potential product for commercial sale or otherwise deny approval of that product. Prior to regulatory approval, a regulatory authority may elect to obtain advice from outside experts regarding scientific issues and/or marketing applications under a regulatory authority review. In the United States, these outside experts are convened through the FDA’s Advisory Committee process, which would report to the FDA and make recommendations that may differ from the views of the FDA. Should an Advisory Committee be convened, it would be expected to lengthen the time for obtaining regulatory approval, if such approval is obtained at all.
The FDA and other foreign regulatory agencies can delay, limit or deny marketing approval for many reasons, including:
| |
| • | a product candidate may not be considered safe or effective; |
| |
| • | our manufacturing processes or facilities may not meet the applicable requirements; and |
| |
| • | changes in their approval policies or adoption of new regulations may require additional work on our part. |
Any delay in, or failure to receive or maintain, approval for any of our product candidates could prevent us from ever generating meaningful revenues or achieving profitability.
Our product candidates may not be approved even if they achieve their endpoints in clinical trials. Regulatory agencies, including the FDA, or their advisors may disagree with our trial design and our interpretations of data from preclinical studies and clinical trials. Regulatory agencies may change requirements for approval even after a clinical trial design has been approved. Regulatory agencies also may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the trials are not well designed.
Clinical trials must be conducted in accordance with the FDA’s current Good Clinical Practices, or cGCP, or other applicable foreign government guidelines and are subject to oversight by the FDA, other foreign governmental agencies and Institutional Review Boards at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with product candidates produced under current Good Manufacturing Practices, or cGMP, and may require large numbers of test subjects. Clinical trials may be suspended by the FDA, other foreign governmental agencies, or us for various reasons, including:
| |
| • | deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with regulatory requirements or clinical protocols; |
| |
| • | deficiencies in the clinical trial operations or trial sites; |
| |
| • | the product candidate may have unforeseen adverse side effects; |
| |
| • | the time required to determine whether the product candidate is effective may be longer than expected; |
| |
| • | fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial treatments; |
| |
| • | the product candidate may not appear to be more effective than current therapies; |
| |
| • | the quality or stability of the product candidate may fall below acceptable standards; or |
| |
| • | insufficient quantities of the product candidate to complete the trials. |
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to Institutional Review Boards for reexamination, which may impact the costs, timing or successful completion of a clinical trial. Due to these and other factors, our HyperAcute product candidates, indoximod, NLG919 and other product candidates could take a significantly longer time to gain regulatory approval for any additional indications than we expect or we may never gain approval for additional indications, which could reduce our revenue by delaying or terminating the commercialization of our HyperAcute product candidates, indoximod, NLG919 and other product candidates for additional indications.
Some of our product candidates have been or in the future may be studied in clinical trials co-sponsored by the National Cancer Institute, or NCI, or in investigator-initiated clinical trials, which means we have little control over the conduct of such trials.
Our indoximod product candidate has been studied in two Phase 1B/2 clinical trials co-sponsored by the National Cancer Institute. We are currently supplying our indoximod product candidate in support of a Phase 2 investigator-initiated clinical trial, and we provided clinical supply of our HyperAcute Melanoma product candidate in support of a Phase 2 investigator-initiated clinical trial. We may continue to supply and otherwise support similar trials in the future. However, because we are not the sponsors of these trials, we do not control the protocols, administration or conduct of these trials, including follow-up with patients and ongoing collection of data after treatment, and, as a result, are subject to risks associated with the way these types of trials are conducted, in particular should any problems arise. These risks include difficulties or delays in communicating with investigators or administrators, procedural delays and other timing issues and difficulties or differences in interpreting data.
If we cannot demonstrate the safety of our product candidates in preclinical and/or other non-clinical studies, we will not be able to initiate or continue clinical trials or obtain approval for our product candidates.
In order to move a product candidate not yet being tested in humans into a clinical trial, we must first demonstrate in preclinical testing that the product candidate is safe. Furthermore, in order to obtain approval, we must also demonstrate safety in various preclinical and non-clinical tests. We may not have conducted or may not conduct in the future the types of preclinical and other non-clinical testing ultimately required by regulatory authorities, or future preclinical tests may indicate that our product candidates are not safe for use in humans. Preclinical testing is expensive, can take many years and have an uncertain outcome. In addition, success in initial preclinical testing does not ensure that later preclinical testing will be successful. We may experience numerous unforeseen events during, or as a result of, the preclinical testing process, which could delay or prevent our ability to develop or commercialize our product candidates, including:
| |
| • | our preclinical testing may produce inconclusive or negative safety results, which may require us to conduct additional preclinical testing or to abandon product candidates that we believed to be promising; |
| |
| • | our product candidates may have unfavorable pharmacology, toxicology or carcinogenicity; |
| |
| • | our product candidates may cause undesirable side effects; and |
| |
| • | the FDA or other regulatory authorities may determine that additional safety testing is required. |
Any such events would increase our costs and could delay or prevent our ability to commercialize our product candidates, which could adversely impact our business, financial condition and results of operations.
Even if approved, the HyperAcute product candidates, indoximod, NLG919 or any other product we may commercialize and market may be later withdrawn from the market or subject to promotional limitations.
We may not be able to obtain the labeling claims necessary or desirable for the promotion of our products. We may also be required to undertake post-marketing clinical trials. If the results of such post-marketing studies are not satisfactory, the FDA or a comparable agency in a foreign country may withdraw marketing authorization or may condition continued marketing on commitments from us that may be expensive and/or time consuming to fulfill. In addition, if we or others identify adverse side effects after any of our products are on the market, or if manufacturing problems occur, regulatory approval may be withdrawn and reformulation of our products, additional clinical trials, changes in labeling of our products and additional marketing applications may be required. Any reformulation or labeling changes may limit the marketability of our products.
We will need to develop or acquire additional capabilities in order to commercialize any product candidates that obtain FDA approval, and we may encounter unexpected costs or difficulties in doing so.
We will need to acquire additional capabilities and effectively manage our operations and facilities to successfully pursue and complete future research, development and commercialization efforts. Currently, we have no experience in preparing applications for marketing approval, commercial-scale manufacturing, managing of large-scale information technology systems or managing a large-scale distribution system. We will need to add personnel and expand our capabilities, which may strain our existing managerial, operational, regulatory compliance, financial and other resources.
To do this effectively, we must:
| |
| • | train, manage and motivate a growing employee base; |
| |
| • | accurately forecast demand for our products; and |
| |
| • | expand existing operational, financial and management information systems. |
We plan to increase our manufacturing capacity, which may include negotiating and entering into arrangements for third-party contract manufacturing for some or all of our commercial manufacturing requirements, and seek FDA approval for our production process simultaneously with seeking approval for the marketing and sale of our HyperAcute Pancreas product candidate. Should we not receive timely approval of our production process, our ability to produce the immunotherapy products following regulatory approval for sale could be delayed, which would further delay the period of time when we would be able to generate revenues from the sale of such products, if we are even able to generate revenues at all.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate significant product revenue.
We do not have a sales organization and have no experience in the sales and distribution of pharmaceutical products. There are risks involved with establishing our own sales capabilities and increasing our marketing capabilities, as well as entering into arrangements with third parties to perform these services. Developing an internal sales force is expensive and time consuming and could delay any product launch. On the other hand, if we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us could potentially be lower than if we market and sell any products that we develop ourselves.
We may establish our own specialty sales force and/or engage other biopharmaceutical or other healthcare companies with established sales, marketing and distribution capabilities to sell, market and distribute any future products. We may not be able to establish a specialty sales force or establish sales, marketing or distribution relationships on acceptable terms. Factors that may inhibit our efforts to commercialize any future products without strategic collaborators or licensees include:
| |
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| |
| • | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any future products; |
| |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Because the establishment of sales, marketing and distribution capabilities depends on the progress towards commercialization of our product candidates, and because of the numerous risks and uncertainties involved with establishing those capabilities, we are unable to predict when, if ever, we will establish our own sales, marketing and distribution capabilities. If we are not able to collaborate with third parties and are unsuccessful in recruiting sales, marketing and distribution personnel or in building the necessary infrastructure, we will have difficulty commercializing our product candidates, which would adversely affect our business and financial condition.
Failure to attract and retain key personnel could impede our ability to develop our products and to obtain new collaborations or other sources of funding.
Because of the specialized scientific nature of our business, our success is highly dependent upon our ability to attract and retain qualified scientific and technical personnel, consultants and advisors. We are highly dependent on the principal members of our scientific and management staff, particularly Dr. Charles J. Link, Jr. and Dr. Nicholas N. Vahanian. The loss of their services might significantly delay or prevent the achievement of our research, development, and business objectives. We do not maintain key-man life insurance with respect to any of our employees, nor do we intend to secure such insurance.
We will need to recruit a significant number of additional personnel in order to achieve our operating goals. In order to pursue product development and marketing and sales activities, if any, we will need to hire additional qualified scientific personnel to perform research and development, as well as personnel with expertise in clinical testing, government regulation, manufacturing, marketing and sales. We also rely on consultants and advisors to assist in formulating our research and development strategy and adhering to complex regulatory requirements. We face competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities and other research institutions. There can be no assurance that we will be able to attract and retain such individuals on acceptable terms, if at all. If the personnel that have contingently agreed to join us do not join us it will be difficult or impossible for us to execute our business plan in a timely manner. Additionally, our principal facilities are located in Iowa, which may make attracting and retaining qualified scientific and technical personnel from outside of Iowa difficult. The failure to attract and retain qualified personnel, consultants and advisors could have a material adverse effect on our business, financial condition and results of operations.
Risks Relating to Manufacturing Activities
We have never manufactured our product candidates at commercial scale, and there can be no assurance that such products can be manufactured in compliance with regulations at a cost or in quantities necessary to make them commercially viable.
We have no experience in commercial-scale manufacturing, the management of large-scale information technology systems or the management of a large-scale distribution system. We may develop our manufacturing capacity by expanding our current facilities, by entering into third-party contract manufacturing arrangements, or by some combination of the foregoing. Expanding our current facilities would require substantial additional funds and we would need to hire and train significant numbers of qualified employees to staff these facilities. We may not be able to develop commercial-scale manufacturing facilities that are sufficient to produce materials for additional later-stage clinical trials or commercial use. Contracting for third-party commercial manufacturing would require expertise and qualified personnel to manage such relationships and may increase our expenses related to, and decrease our direct control over, procuring a sufficient supply of our product candidates for commercial sale.
If we are unable to manufacture or contract for a sufficient supply of our product candidates on acceptable terms, or if we encounter delays or difficulties in the scale-up of our manufacturing processes or our relationships with other manufacturers, our preclinical and human clinical testing schedule would be delayed. This in turn would delay the submission of product candidates for regulatory approval and thereby delay the market introduction and subsequent sales of any products that receive regulatory approval, which would have a material adverse effect on our business, financial condition and results of operations. Furthermore, we or our contract manufacturers must supply all necessary documentation in support of each Biologics License Application, or BLA, and each NDA, on a timely basis and must adhere to Good Laboratory Practice, or GLP and cGMP regulations enforced by the FDA through its facilities inspection program. If these facilities cannot pass a pre-approval plant inspection, the FDA approval of the products will not be granted.
We and our contract manufacturers are subject to significant regulation with respect to manufacturing of our products.
All entities involved in the preparation of a therapeutic drug for clinical trials or commercial sale, including our existing contract manufacturer for indoximod and the components used in the HyperAcute product candidates, our contract manufacturer for NLG919 and any contract manufacturer that we may use in the future for manufacturing related to clinical trials or commercial sale are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Our facilities and quality systems and the facilities and quality systems of some or all of our third party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of the HyperAcute product candidates, indoximod, NLG919 or any of our other potential products. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of the HyperAcute product candidates, indoximod, NLG919 or any of our other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business. In addition, to the extent that we rely on foreign contract manufacturers, as we do for NLG919, we are subject to additional risks including the need to comply with export and import regulations.
We currently rely on relationships with third-party contract manufacturers, which limits our ability to control the availability of, and manufacturing costs for, our product candidates in the near-term.
We intend to rely upon contract manufacturers for indoximod, for NLG919 and for components of or finished HyperAcute product candidates, for commercial sale if any are approved for sale. Problems with any of our facilities or processes, or our contract manufacturers’ facilities or processes, could prevent or delay the production of adequate supplies of antigen, components of or finished HyperAcute product candidates, indoximod or NLG919. This could delay or reduce commercial sales and materially harm our business. We do not currently have experience with the manufacture of products at commercial scale, or the management of relationships related to commercial-scale contract manufacturing, and we may incur substantial costs to develop the capability to manufacture products at commercial scale or to negotiate and enter into relationships with third-party contract manufacturers. Any prolonged delay or interruption in the operations of our facilities or our current or future contract manufacturers’ facilities could result in cancellation of shipments, loss of components in the process of being manufactured or a shortfall in availability of a product. A number of factors could cause interruptions, including the inability of a supplier to provide raw materials, equipment malfunctions or failures, damage to a facility due to natural disasters, changes in regulatory requirements or standards that require modifications to our manufacturing processes, action by the regulatory authorities or by us that results in the halting or slowdown of production of components or finished product due to regulatory issues, a contract manufacturer going out of business or failing to produce product as contractually required or other similar factors. Because manufacturing processes are highly complex and are subject to a lengthy regulatory approval process, alternative qualified production capacity and sufficiently trained or qualified personnel may not be available on a timely or cost-effective basis or at all. Difficulties or delays in our contract manufacturers’ production of drug substances could delay our clinical trials, increase our costs, damage our reputation and cause us to lose revenue and market share if we are unable to timely meet market demand for any products that are approved for sale.
Further, if our current or future contract manufacturers are not in compliance with regulatory requirements at any stage, including post-marketing approval, we may be fined, forced to remove a product from the market and/or experience other adverse consequences, including delays, which could materially harm our business.
We replicate all biological cells for our products internally and utilize a single manufacturing site to manufacture our clinical product candidates. Any disruption in the operations of our manufacturing facility would have a significant negative impact on our ability to manufacture products for clinical testing and would result in increased costs and losses.
We have thus far elected to replicate all biological cells for our products internally using a complex process. The disruption of our operations could result in manufacturing delays due to the inability to purchase the cell lines from outside sources. We have only one manufacturing facility in which we can manufacture clinical products. In the event of a physical catastrophe at our manufacturing or laboratory facilities, we could experience costly delays in reestablishing manufacturing capacity, due to a lack of redundancy in manufacturing capability.
Our current manufacturing facility contains highly specialized equipment and utilizes complicated production processes developed over a number of years, which would be difficult, time-consuming and costly to duplicate or may be impossible to duplicate. Any prolonged disruption in the operations of our manufacturing facility would have a significant negative impact on our ability to manufacture products for clinical testing on our own and would cause us to seek additional third-party manufacturing contracts, thereby increasing our development costs. We may suffer losses as a result of business interruptions that exceed the coverage available under our insurance policies or any losses may be excluded under our insurance policies. Certain events, such as natural disasters, fire, political disturbances, sabotage or business accidents, which could impact our current or future facilities, could have a significant negative impact on our operations by disrupting our product development efforts until such time as we are able to repair our facility or put in place third-party contract manufacturers to assume this manufacturing role.
We have experienced bacterial and mycoplasm contaminations in lots produced at our facilities, and we destroyed the contaminated lots and certain overlapping lots. We may experience additional contaminated lots at our facilities, and we will destroy any contaminated lots that we detect, which could result in significant delay or additional expense in our operations.
Our facilities are located in areas where floods and tornados are known to occur, and the occurrence of a flood, tornado or other catastrophic disaster could damage our facilities and equipment, which could cause us to curtail or cease operations.
Our principal facilities and manufacturing operations are located in Ames, Iowa, which is susceptible to floods and tornados, and these facilities are therefore vulnerable to damage or disruption from floods and tornados. We are also vulnerable to damage from other types of disasters, such as power loss, fire and similar events. If any disaster were to occur, our ability to operate our business could be seriously impaired. We currently carry business personal property insurance in the amount of $9.5 million in the aggregate, but this policy does not cover disasters such as floods and earthquakes. We may not have adequate
insurance to cover our losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business and financial condition.
Significant disruptions of information technology systems or breaches of data security could adversely affect our business.
We are increasingly dependent on information technology systems and infrastructure, including mobile technologies, to operate our business. In the ordinary course of our business, we collect, store and transmit large amounts of confidential information, including intellectual property, proprietary business information and personal information. It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. We have also outsourced elements of our information technology infrastructure, and as a result we manage a number of third party vendors who may or could have access to our confidential information. The size and complexity of our information technology systems, and those of third-party vendors with whom we contract, make such systems potentially vulnerable to breakdown, malicious intrusion, security breaches and other cyber attacks. In addition, the prevalent use of mobile devices that access confidential information increases the risk of data security breaches, which could lead to the loss of confidential information, trade secrets or other intellectual property. While we have implemented security measures to protect our data security and information technology systems, such measures may not prevent the adverse effect of such events. Significant disruptions of our information technology systems or breaches of data security could adversely affect our business.
Risks Relating to Regulation of Our Industry
The industry within which we operate and our business are subject to extensive regulation, which is costly and time consuming and which may subject us to unanticipated delays.
The research, design, testing, manufacturing, labeling, marketing, distribution and advertising of biologic and pharmaceutical products such as our product candidates are subject to extensive regulation by governmental regulatory authorities in the United States and other countries. The drug development and approval process is generally lengthy, expensive and subject to unanticipated delays. Data obtained from preclinical and clinical testing are subject to varying interpretations that could delay, limit or prevent regulatory approval. In addition, delays or rejections may be encountered based upon changes in regulatory policy for product approval during the period of development and regulatory review of each submitted application for approval. To obtain approval for a product candidate, we must demonstrate to the satisfaction of the regulatory authorities that the product candidate is safe, pure, potent and effective, which typically takes several years or more depending upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. There can be no assurance that we will not encounter problems in clinical trials that would cause us or the regulatory authorities to delay or suspend clinical trials. Any such delay or suspension could have a material adverse effect on our business, financial condition and results of operations.
There can be no assurance that clinical studies for any of our product candidates currently under development will be completed successfully or within any specified time period, if at all. Further, there can also be no assurance that such testing will show any product to be safe, pure, potent or effective. There can be no assurance that we will not encounter problems in clinical trials that will cause us to delay or suspend clinical trials.
Regardless of how much time and resources we devote to development of a product candidate, there can be no assurance that regulatory approval will be obtained for that product candidate. To date, the FDA has approved only one active cellular cancer immunotherapy product, even though several have been, and currently are in, clinical development. Further, even if such regulatory approval is obtained, we, our products and any contract manufacturers or commercial collaborators of ours will be subject to continual regulatory review in both the United States and other countries. Later discovery of previously unknown problems with regard to a product, distributor or manufacturer may result in restrictions, including withdrawal of the product from the market and/or disqualification or decertification of the distributor or manufacturer.
We cannot predict when, if ever, we might submit for regulatory review our product candidates currently under development. Once we submit our potential products for review, there can be no assurance that regulatory approvals for any pharmaceutical products developed by us will be granted on a timely basis, if at all.
The FDA and comparable agencies in foreign countries impose substantial requirements on the introduction of new biologic and pharmaceutical products through lengthy and detailed preclinical and clinical testing procedures, sampling activities and other costly and time-consuming compliance procedures. Clinical trials are vigorously regulated and must meet requirements for FDA review and oversight and requirements under GCP guidelines. A new drug may not be marketed in the United States until the FDA has approved it. There can be no assurance that we will not encounter delays or rejections or that the FDA will not make policy changes during the period of product development and FDA regulatory review of each submitted BLA and NDA. A delay
in obtaining or failure to obtain such approvals would have a material adverse effect on our business, financial condition and results of operations. Even if regulatory approval were obtained, it would be limited as to the indicated uses for which the product may be promoted or marketed. A marketed product, its manufacturer and the facilities in which it is manufactured are subject to continual review and periodic inspections. If marketing approval is granted, we would be required to comply with FDA requirements for manufacturing, labeling, advertising, record keeping and reporting of adverse experiences and other information. In addition, we would be required to comply with federal and state anti-kickback and other health care fraud and abuse laws that pertain to the marketing of pharmaceuticals. Failure to comply with regulatory requirements and other factors could subject us to regulatory or judicial enforcement actions, including product recalls or seizures, injunctions, withdrawal of the product from the market, civil penalties, criminal prosecution, refusals to approve new products and withdrawals of existing approvals, as well as enhanced product liability exposure, any of which could have a material adverse effect on our business, financial condition and results of operations. Sales of our products outside the United States will be subject to foreign regulatory requirements governing clinical trials, marketing approval, manufacturing and pricing. Non-compliance with these requirements could result in enforcement actions or penalties or could delay introduction of our products in certain countries.
The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement outside the United States vary greatly from country to country. The time required to obtain approvals outside the United States may differ from that required to obtain FDA approval. We may not obtain foreign regulatory approvals on a timely basis, or at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA and foreign regulatory authorities could require additional testing. Failure to comply with these regulatory requirements or obtain required approvals could impair our ability to develop foreign markets for our products and may have a material adverse effect on our results of operations and financial condition.
We are also subject to laws generally applicable to businesses, including but not limited to, federal, state and local regulations relating to wage and hour matters, employee classification, mandatory healthcare benefits, unlawful workplace discrimination and whistle-blowing. Any actual or alleged failure to comply with any regulation applicable to our business or any whistle-blowing claim, even if without merit, could result in costly litigation, regulatory action or otherwise harm our business, results of operations, financial condition, cash flow and future prospects.
The availability and amount of reimbursement for our product candidates, if approved, and the manner in which government and private payors may reimburse for our potential product, are uncertain.
In both United States and foreign markets, sales of our proposed products will depend in part on the availability of reimbursement from third-party payors such as government health administration authorities, private health insurers and other organizations. Our future levels of revenues and profitability may be affected by the continuing efforts of governmental and third party payors to contain or reduce the costs of health care. We cannot predict the effect that private sector or governmental health care reforms may have on our business, and there can be no assurance that any such reforms will not have a material adverse effect on our business, financial condition and results of operations.
In addition, in both the United States and elsewhere, sales of prescription drugs are dependent in part on the availability of reimbursement to the consumer from third-party payors, such as government and private insurance plans. Third-party payors are increasingly challenging the price and cost-effectiveness of medical products and services. Significant uncertainty exists as to the reimbursement status of newly approved health care products. There can be no assurance that our proposed products will be considered cost-effective or that adequate third-party reimbursement will be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Legislation and regulations affecting the pricing of pharmaceuticals may change before any of our proposed products are approved for marketing. Adoption of such legislation could further limit reimbursement for medical products and services. As a result, we may elect not to market future products in certain markets.
Moreover, while we are in clinical trials, we will not be reimbursed for any of our materials used during the clinical trials.
The biopharmaceutical industry is subject to significant regulation and oversight in the United States, in addition to approval of products for sale and marketing.
In addition to FDA restrictions on marketing of biopharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the biopharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for the purchase,
lease, or order of any health care item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Recently, several pharmaceutical and other health care companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws, which could have a material adverse effect on our business, financial condition and results of operations.
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. We expect to face pricing pressure globally from managed care organizations, institutions and government agencies and programs, which could negatively affect the sales and profit margins for our HyperAcute product candidates, indoximod, NLG919 or any other of our product candidates that may be approved for marketing.
In particular, there have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs. Most recently, in March 2010 the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the PPACA, was enacted, which includes measures to significantly change the way health care is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
| |
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| |
| • | requirements to report certain financial arrangements with physicians and others, including reporting any “transfer of value” made or distributed to prescribers and other healthcare providers and reporting any investment interests held by physicians and their immediate family members; |
| |
| • | a licensure framework for follow-on biologic products, also known as biosimilars; |
| |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| |
| • | creation of the Independent Payment Advisory Board which will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs and those recommendations could have the effect of law even if Congress does not act on the recommendations; and |
| |
| • | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
Many of the details regarding the implementation of the PPACA are yet to be determined, and at this time, it remains unclear the full effect that the PPACA would have on our business. The regulations that are ultimately promulgated and their implementation are likely to have considerable impact on the way we conduct our business and may require us to change current strategies.
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and designed to encourage importation from other countries and bulk purchasing. Legally-mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and
individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
In addition, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While we cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to generate revenues. In addition, legislation has been introduced in Congress that, if enacted, would permit more widespread importation or re-importation of pharmaceutical products from foreign countries into the United States, including from countries where the products are sold at lower prices than in the United States. Such legislation, or similar regulatory changes, could put competitive pressure on our ability to profitably price our products, which, in turn, could adversely affect our business, results of operations, financial condition and prospects. Alternatively, in response to legislation such as this, we might elect not to seek approval for or market our products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue we generate from our product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, changing policies and agency funding, staffing and leadership. We cannot be sure whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. For example, average review times at the FDA for marketing approval applications have fluctuated over the last ten years, and we cannot predict the review time for any of our submissions with any regulatory authorities. In addition, review times can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
We use hazardous materials in our business and must comply with environmental laws and regulations, which can be expensive.
Our research and development involves the controlled use of hazardous materials, chemicals, various active microorganisms and volatile organic compounds, and we may incur significant costs as a result of the need to comply with numerous laws and regulations. We are subject to laws and regulations enforced by the FDA, the Drug Enforcement Agency, the Environmental Protection Act, the Toxic Substances Control Act, the Food, Drug and Cosmetic Act, the Resource, Conservation and Recovery Act, and other current and potential federal, state, local and foreign laws and regulations governing the use, manufacture, storage, handling and disposal of our products, materials used to develop and manufacture our product candidates, and resulting waste products. Although we believe that our safety procedures for handling and disposing of such materials, and for killing any unused microorganisms before disposing of them, comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources.
Financial Risks
We have a history of net losses. We expect to continue to incur increasing net losses for the foreseeable future, and we may never achieve or maintain profitability.
We are not profitable and have incurred significant net losses in each year since our inception, including net losses of $31.2 million, $23.3 million and $18.1 million for the years ended December 31, 2013, 2012 and 2011, respectively. As of December 31, 2013, we had an accumulated deficit of $136.0 million. Our losses have resulted principally from costs incurred in our research and development activities. We anticipate that our operating losses will substantially increase over the next several years as we expand our discovery, research and development activities, including the Phase 2 and Phase 3 clinical development of the HyperAcute product candidates and Phase 2 clinical development of indoximod.
Because of the numerous risks and uncertainties associated with biopharmaceutical product development and commercialization, we are unable to accurately predict the timing or amount of future expenses or when, or if, we will be able to achieve or maintain profitability. Currently, we have no products approved for commercial sale, and to date we have not generated any product revenue. We have financed our operations primarily through the sale of equity securities, government grants, economic development loans and capital lease and equipment financing. The size of our future net losses will depend, in part, on the rate of growth or contraction of our expenses and the level and rate of growth, if any, of our revenues. Our ability to achieve profitability is dependent on our ability, alone or with others, to complete the development of our products successfully, obtain the required regulatory approvals, manufacture and market our proposed products successfully or have such products manufactured and
marketed by others and gain market acceptance for such products. There can be no assurance as to whether or when we will achieve profitability.
We will require substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
Development of our HyperAcute product candidates, indoximod, NLG919 and any other product candidates will require substantial additional funds to conduct research, development and clinical trials necessary to bring such product candidates to market and to establish manufacturing, marketing and distribution capabilities, internally or through collaborations with third parties. Our future capital requirements will depend on many factors, including, among others:
| |
| • | the scope, rate of progress, results and costs of our preclinical studies, clinical trials and other research and development activities; |
| |
| • | the scope, rate of progress and costs of our manufacturing development and commercial manufacturing activities; |
| |
| • | the cost, timing and outcomes of regulatory proceedings (including FDA review of any BLA or NDA we file); |
| |
| • | payments required with respect to development milestones we achieve under our in-licensing agreements; |
| |
| • | the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; |
| |
| • | the costs associated with commercializing our product candidates, if they receive regulatory approval; |
| |
| • | the cost and timing of developing our ability to establish sales and marketing capabilities; |
| |
| • | competing technological efforts and market developments; |
| |
| • | changes in our existing research relationships; |
| |
| • | our ability to establish collaborative arrangements to the extent necessary; |
| |
| • | revenues received from any existing or future products; and |
| |
| • | payments received under any future strategic collaborations. |
We anticipate that we will continue to generate significant losses for the next several years as we incur expenses to complete our clinical trial programs for our product candidates, build commercial capabilities, develop our pipeline and expand our corporate infrastructure. We believe that our existing cash and cash equivalents and certificates of deposit, including the proceeds from our follow-on public offering that closed on February 4, 2013, and the proceeds received to date from our at-the-market offering under our Sales Agreement, dated as of September 5, 2013, with Cantor Fitzgerald & Co., or the ATM Offering, will allow us to fund our operating plan through the end of 2014, although not through commercialization and launch of revenue producing products. However, our operating plan may change as a result of factors currently unknown to us.
There can be no assurance that our revenue and expense forecasts will prove to be accurate, and any change in the foregoing assumptions could require us to obtain additional financing earlier than anticipated. There is a risk of delay or failure at any stage of developing a product candidate, and the time required and costs involved in successfully accomplishing our objectives cannot be accurately predicted. Actual drug research and development costs could substantially exceed budgeted amounts, which could force us to delay, reduce the scope of or eliminate one or more of our research or development programs.
We are party to license agreements with various parties pursuant to which we have obtained licenses to certain patents, patent applications and other intellectual property related to our product candidates and product development efforts. Pursuant to most of these license agreements, we are obligated to make aggregate payments ranging from approximately $200,000 to $2.8 million per license (and in some cases, for each product candidate in such license) upon achievement of development and regulatory approval milestones specified in the applicable license. The timing of our achievement of these events and corresponding milestone payments to our licensors are subject to factors relating to the clinical and regulatory development and commercialization of our product candidates, many of which are beyond our control. We may become obligated to make a milestone payment when we do not have the cash on hand to make such payment, which could require us to delay our clinical trials, curtail our operations, scale back our commercialization or marketing efforts or seek funds to meet these obligations on terms unfavorable to us.
We may never be able to generate a sufficient amount of product revenue to cover our expenses. Until we do, we expect to seek additional funding through public or private equity or debt financings, collaborative relationships, capital lease transactions or other available financing transactions. However, there can be no assurance that additional financing will be available on acceptable terms, if at all, and such financings could be dilutive to existing stockholders. Moreover, in the event that additional funds are obtained through arrangements with collaborators such arrangements may require us to relinquish rights to certain of our technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves.
If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs. Our failure to obtain adequate financing when needed and on acceptable terms would have a material adverse effect on our business, financial condition and results of operations.
We have a forgivable loan that may have to be repaid if we do not achieve job creation goals.
In March 2010, we entered into a $400,000 forgivable loan agreement with the City of Ames, Iowa and the Ames Chamber of Commerce that requires us to create or retain at least 70 full-time jobs located in Ames, Iowa as of March 10, 2012 and to create or retain at least 150 full-time positions located in Ames, Iowa as of March 10, 2015. If, as of March 10, 2015, we have fulfilled the terms of the loan agreement, the loan will be forgiven. If on March 10, 2012 and March 10, 2015, we have failed to create or retain at least 70 full-time jobs and 150 full-time jobs in Ames, Iowa, respectively, we will be required to repay approximately $3,100 per job not created or retained following the respective date. As of December 31, 2013, we had created or retained an aggregate of 102 full-time jobs in Ames, Iowa, and prior to March 10, 2012, we had created or retained at least 70 full-time jobs in Ames, Iowa.
We have not yet met all the job creation requirements of the City of Ames loan. If we cannot or do not comply with these and all other requirements under this loan, we may be obligated to pay principal and interest on this loan immediately.
Even though we have received governmental support in the past, we may not continue to receive support at the same level or at all.
We have received significant financial assistance from state and local governments, primarily in the form of forgivable loans. There can be no assurance that we will continue to receive the same level of assistance from these or other government agencies, if at all.
Through our subsidiary, BioProtection Systems Corporation, or BPS, we also have an ongoing contract with the United States Department of Defense and pending applications with the National Institutes of Health. The termination of a United States government grant, contract or relationship as a result of our failure to satisfy any of our obligations under the grants or contracts would have a negative impact on our operations and harm our reputation and ability to procure government contracts. Additionally, there can be no assurance that we will secure comparable contracts with, or grants from, the United States government in the future.
Changes in our effective income tax rate could adversely affect our results of operations in the future.
We may become subject to income taxes in the United States or foreign jurisdictions, and our effective income tax rate, as well as our relative domestic and international tax liabilities, will depend in part on the allocation of any future income among different jurisdictions. In addition, various factors may have favorable or unfavorable effects on our effective income tax rate in individual jurisdictions or in the aggregate. These factors include whether tax authorities agree with our interpretations of existing tax laws, any required accounting for stock options and other share-based compensation, changes in tax laws and rates, our future levels of research and development spending, changes in accounting standards, changes in the mix of any future earnings in the various tax jurisdictions in which we may operate, the outcome of any examinations by the U.S. Internal Revenue Service or other tax authorities, the accuracy of our estimates for unrecognized tax benefits and realization of deferred tax assets and changes in overall levels of pre-tax earnings. The effect on our income tax liabilities resulting from the above-mentioned factors or other factors could have a material adverse effect on our results of operations.
Risks Relating to Competitive Factors
We compete in an industry characterized by extensive research and development efforts and rapid technological progress. New discoveries or commercial developments by our competitors could render our potential products obsolete or non-competitive.
New developments occur and are expected to continue to occur at a rapid pace, and there can be no assurance that discoveries or commercial developments by our competitors will not render some or all of our potential products obsolete or non-competitive, which would have a material adverse effect on our business, financial condition and results of operations.
We expect to compete with fully integrated and well-established pharmaceutical and biotechnology companies in the near and long term. Most of these companies have substantially greater financial, research and development, manufacturing and marketing experience and resources than we do and represent substantial long-term competition for us. Such companies may succeed in discovering and developing pharmaceutical products more rapidly than we do or pharmaceutical products that are safer, more effective or less costly than any that we may develop. Such companies also may be more successful than we are in production and marketing. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biotechnology companies. Academic institutions, governmental agencies and other public and private research organizations also conduct clinical trials, seek patent protection and establish collaborative arrangements for the development of oncology products.
We will face competition based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capabilities, reimbursement coverage, price and patent position. There can be no assurance that our competitors will not develop safer and more effective products, commercialize products earlier than we do, or obtain patent protection or intellectual property rights that limit our ability to commercialize our products.
There can be no assurance that our issued patents or pending patent applications, if issued, will not be challenged, invalidated or circumvented or that the rights granted thereunder will provide us with proprietary protection or a competitive advantage.
Our competitors may develop and market products that are less expensive, more effective, safer or reach the market sooner than our product candidates, which may diminish or eliminate the commercial success of any products we may commercialize.
The biopharmaceutical industry is highly competitive. There are many public and private biopharmaceutical companies, public and private universities and research organizations actively engaged in the discovery and research and development of products for cancer. Given the significant unmet patient need for new therapies, oncology is an area of focus for large and small companies as well as research institutions. As a result, there are and will likely continue to be extensive research and substantial financial resources invested in the discovery and development of new oncology products. In addition, there are a number of multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same cancer indications as our product candidates, and several large public biopharmaceutical companies have approved or are developing cancer immunotherapy products, including Dendreon Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline plc, Merck & Co., Merck KGaA, Celgene Corporation and Sanofi-Aventis.
There are several marketed products indicated for pancreatic cancer, including Eli Lilly and Company’s Gemzar®, Astellas Pharma’s Tarceva®, Teva Pharmaceutical Industries Limited’s streptozocin, and fluorouracil, or 5-FU, and mitomycin which are marketed by several generic pharmaceutical firms. There are numerous marketed therapeutics indicated for NSCLC, including Roche AG’s Avastin®, Eli Lilly’s Alimta® and Gemzar, Astellas Pharma’s Tarceva, AstraZeneca’s Iressa®, and Sanofi-Aventis’ Taxotere and Eloxatin, as well as generically available platinum-based chemotherapeutics (cisplatin and carboplatin) and mitotic inhibitors (paclitaxel and venorelbine). There are also several marketed therapeutics indicated for advanced melanoma, including Merck’s Intron A and Novartis/Prometheus Laboratories’ Proleukin®, as well as cisplatin and dacarbazine, which are available generically. Bristol-Myers Squibb’s immunotherapy ipilimumab was recently approved by the FDA as was Roche/Daiichi Sankyo’s drug, vemurafenid.
In addition, there are a number of companies with active clinical trials ongoing in pancreatic cancer including AB Science SA, Amgen Inc., Astellas Pharma, BioSante Pharmaceuticals, Inc., Celgene Corporation, Immunomedics, Inc., Lorus Therapeutics Inc., Sanofi-Aventis and Threshold Pharmaceuticals, Inc., a number of companies with active clinical trials ongoing in NSCLC, including Abbott Laboratories, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, BioNumerik Pharmaceuticals, Inc., Celgene, GlaxoSmithKline, NovaRx Corporation, Onyx Pharmaceuticals, Inc., Pfizer Inc. and Regeneron Pharmaceuticals, Inc., and a number of companies with active clinical trials ongoing in advanced melanoma, including Amgen, Astellas Pharma, Eli Lilly, Onyx, Roche, Synta Pharmaceuticals Corp., and Vical Inc. among other companies.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drugs, obtaining FDA and other regulatory approvals, and the commercialization of those products. Accordingly, our competitors may be more successful in obtaining approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the significant expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available.
There are many different approaches to using immunotherapies to treat cancer, including anti-idiotype, whole cell, DNA, peptide/antigen, viral, tumor lysate, shed antigens, and dendritic cell. Cancer immunotherapies are also distinguished by whether or not they are derived from autologous or allogeneic sources. Each of the various approaches to cancer immunotherapy have potential advantages and disadvantages based on factors such as their immunostimulatory mechanisms, formulation characteristics, manufacturing requirements, and treatment regimens.
We also compete with other clinical-stage companies and institutions for clinical trial participants, which could reduce our ability to recruit participants for our clinical trials. Delay in recruiting clinical trial participants could adversely affect our ability to bring a product to market prior to our competitors. Further, research and discoveries by others may result in breakthroughs
that render our HyperAcute product candidates, indoximod, NLG919 or our other potential products obsolete even before they begin to generate any revenue.
In addition, our competitors may obtain patent protection or FDA approval and commercialize products more rapidly than we do, which may impact future sales of any of our product candidates that receive marketing approval. If the FDA approves the commercial sale of any of our product candidates, we will also be competing with respect to marketing capabilities and manufacturing efficiency, areas in which we have limited or no experience. We expect that competition among products approved for sale will be based, among other things, on product efficacy, price, safety, reliability, availability, patent protection, and sales, marketing and distribution capabilities. Our profitability and financial position will suffer if our products receive regulatory approval, but cannot compete effectively in the marketplace.
If any of our product candidates are approved and commercialized, we may face competition from generic products if the product candidate is a small molecule drug, or biosimilars if the product candidate is a biologic. The route to market for generic versions of small molecule drugs was established with the passage of the Hatch-Waxman Amendments in 1984 and for biosimilars with the passage of the PPACA in March 2010. The PPACA establishes a pathway for the FDA approval of follow-on biologics and provides 12 years of marketing exclusivity for reference products and an additional six months of exclusivity if pediatric studies are conducted. In Europe, the European Medicines Agency has issued guidelines for approving products through an abbreviated pathway, and biosimilars have been approved in Europe. If a biosimilar version of one of our potential products were approved in the United States or Europe, it could have a negative effect on sales and gross profits of the potential product and our financial condition.
Our biodefense product candidates face significant competition for United States government funding for both development and procurement of medical countermeasures for biological, chemical and nuclear threats, diagnostic testing systems and other emergency preparedness countermeasures. Competitors include (but are not limited to) Crucell Holland BV, Janssen Pharmaceuticals, Integrated Biotherapeutics, Okarios, GlaxoSmithKline, Profectus BioSciences, SIGA Technologies, Sarepta Therapeutics, Acambis, Bavarian Nordic, Mapp BioPharmaceutical, and Novartis. Academic institutions, government agencies, private research organizations and public research organizations are also conducting research and filing patents toward commercialization of products. In addition, we may not be able to compete effectively if our product candidates do not satisfy government procurement requirements with respect to biodefense products.
Our future products, if any, may not be accepted in the marketplace; therefore, we may not be able to generate significant revenue, if any.
Even if the HyperAcute product candidates, indoximod, NLG919 or any of our other potential products are approved for sale, physicians and the medical community may not ultimately use them or may use them only in applications more restricted than we expect. Our products, if successfully developed, will compete with a number of traditional products and immunotherapies manufactured and marketed by major pharmaceutical and other biotechnology companies. Our products will also compete with new products currently under development by such companies and others. Physicians will prescribe a product only if they determine, based on experience, clinical data, side effect profiles and other factors, that it is beneficial as compared to other products currently in use. Many other factors influence the adoption of new products, including marketing and distribution restrictions, course of treatment, adverse publicity, product pricing, the views of thought leaders in the medical community and reimbursement by government and private third party payors.
Risks Relating to Our Arrangements with Third Parties
We rely on third parties to conduct our preclinical studies and our clinical trials. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for our product candidates, or we may be delayed in doing so.
We do not have the ability to conduct preclinical studies or clinical trials independently for our product candidates. We must rely on third parties, such as contract research organizations, medical institutions, academic institutions, clinical investigators and contract laboratories, to conduct our preclinical studies and clinical trials. We are responsible for confirming that our preclinical studies are conducted in accordance with applicable regulations and that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. The FDA requires us to comply with GLP for conducting and recording the results of our preclinical studies and cGCP for conducting, monitoring, recording and reporting the results of clinical trials, to assure that data and reported results are accurate and that the clinical trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines, fail to comply with cGCP, do not adhere to our clinical trial protocols or otherwise fail to generate reliable clinical data, we may need to enter into new arrangements with alternative third parties and our clinical
trials may be more costly than expected or budgeted, extended, delayed or terminated or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the product candidate being tested in such trials.
Further, if our contract manufacturers are not in compliance with regulatory requirements at any stage, including post-marketing approval, we may be fined, forced to remove a product from the market and/or experience other adverse consequences, including delays, which could materially harm our business.
If we fail to enter into any needed collaboration agreements for our product candidates, we may be unable to commercialize them effectively or at all.
To successfully commercialize the HyperAcute product candidates, indoximod, NLG919 or any other potential product, we will need substantial financial resources as well as expertise and physical resources and systems. We may elect to develop some or all of these physical resources and systems and expertise ourselves or we may seek to collaborate with another company that can provide some or all of such physical resources and systems as well as financial resources and expertise. Such collaborations are complex and any potential discussions may not result in a definitive agreement for many reasons. For example, whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of our clinical trials, the potential market for the HyperAcute product candidates and indoximod, the costs and complexities of manufacturing and delivering the HyperAcute product candidates, indoximod, NLG919 or any other potential product to patients, the potential of competing products, the existence of uncertainty with respect to ownership or the coverage of our technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. If we were to determine that a collaboration for the HyperAcute product candidates, indoximod, NLG919 or any other potential product is necessary and were unable to enter into such a collaboration on acceptable terms, we might elect to delay or scale back the commercialization of the HyperAcute product candidates, indoximod, NLG919 or any other potential product in order to preserve our financial resources or to allow us adequate time to develop the required physical resources and systems and expertise ourselves.
If we enter into a collaboration agreement we consider acceptable, the collaboration may not proceed as quickly, smoothly or successfully as we plan. The risks in a collaboration agreement include the following:
| |
| • | the collaborator may not apply the expected financial resources, efforts or required expertise in developing the physical resources and systems necessary to successfully commercialize the HyperAcute product candidates, indoximod, NLG919, or any other potential product; |
| |
| • | the collaborator may not invest in the development of a sales and marketing force and the related infrastructure at levels that ensure that sales of the HyperAcute product candidates, indoximod, NLG919, or any other potential product reach their full potential; |
| |
| • | disputes may arise between us and a collaborator that delay the commercialization or adversely affect its sales or profitability of the HyperAcute product candidates, indoximod, NLG919 or any other potential product; or |
| |
| • | the collaborator may independently develop, or develop with third parties, products that could compete with the HyperAcute product candidates, indoximod, NLG919 or any other potential product. |
If we enter into one or more collaborations for our HyperAcute product candidates, indoximod, NLG919 or any of our other product candidates, we will be dependent on our collaborators’ performance of their responsibilities and their cooperation with us. Our collaborators may not perform their obligations under our agreements with them or otherwise cooperate with us. We cannot control whether our collaborators will devote the necessary resources to the activities contemplated by our collaborative agreements, nor can we control the timing of their performance. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us. Disputes may arise between us and our collaborators that delay the development and commercialization of our product candidates that are difficult and costly to resolve, or may not be resolved. In addition, a collaborator for the HyperAcute product candidates, indoximod, NLG919, or any other potential product may have the right to terminate the collaboration at its discretion. Any termination may require us to seek a new collaborator, which we may not be able to do on a timely basis, if at all, or require us to delay or scale back the commercialization efforts. The occurrence of any of these events could adversely affect the commercialization of the HyperAcute product candidates, indoximod, NLG919, or any other potential product and materially harm our business and stock price by delaying the sale of any product that may be approved by the FDA, by slowing the growth of such sales, by reducing the profitability of the product and/or by adversely affecting the reputation of the product.
We rely on a single manufacturer for a key component used in the manufacture of our HyperAcute immunotherapy product candidates, which could impair our ability to manufacture and supply our products.
The manufacturing process for our HyperAcute immunotherapy product candidates has one component that we obtain from a single manufacturer. If our current supplier is unable to continue supplying the component for our clinical trials, or to supply the component at quantities insufficient for commercial sale, we may need to utilize an alternative manufacturer. If we utilize an alternative manufacturer, we may be required to demonstrate comparability of the drug product before releasing the product for clinical use. The loss of our current supplier could result in manufacturing delays for the component substitution, and we may need to accept changes in terms or price from our existing supplier in order to avoid such delays.
We may explore strategic collaborations that may never materialize or may fail.
We may, in the future, periodically explore a variety of possible strategic collaborations in an effort to gain access to additional product candidates or resources. At the current time, we cannot predict what form such a strategic collaboration might take. We are likely to face significant competition in seeking appropriate strategic collaborators, and these strategic collaborations can be complicated and time consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing strategic collaborations.
If we enter into one or more strategic collaborations, we may be required to relinquish important rights to and control over the development of our product candidates or otherwise be subject to unfavorable terms.
Any future strategic collaborations we enter into could subject us to a number of risks, including:
| |
| • | we may be required to undertake the expenditure of substantial operational, financial and management resources; |
| |
| • | we may be required to issue equity securities that would dilute our existing stockholders’ percentage ownership; |
| |
| • | we may be required to assume substantial actual or contingent liabilities; |
| |
| • | we may not be able to control the amount and timing of resources that our strategic collaborators devote to the development or commercialization of our product candidates; |
| |
| • | strategic collaborators may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new version of a product candidate for clinical testing; |
| |
| • | strategic collaborators may not pursue further development and commercialization of products resulting from the strategic collaboration arrangement or may elect to discontinue research and development programs; |
| |
| • | strategic collaborators may not commit adequate resources to the marketing and distribution of our product candidates, limiting our potential revenues from these products; |
| |
| • | disputes may arise between us and our strategic collaborators that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources; |
| |
| • | strategic collaborators may experience financial difficulties; |
| |
| • | strategic collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| |
| • | business combinations or significant changes in a strategic collaborator’s business strategy may also adversely affect a strategic collaborator’s willingness or ability to complete its obligations under any arrangement; |
| |
| • | strategic collaborators could decide to move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors; and |
| |
| • | strategic collaborators could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing our product candidates. |
Risks Relating to Protecting Our Intellectual Property
If we are unable to protect our proprietary rights or to defend against infringement claims, we may not be able to compete effectively or operate profitably.
Our success will depend, in part, on our ability to obtain patents, operate without infringing the proprietary rights of others and maintain trade secrets, both in the United States and other countries. Patent matters in the biotechnology and pharmaceutical industries can be highly uncertain and involve complex legal and factual questions. Accordingly, the validity, breadth, and enforceability of our patents and the existence of potentially blocking patent rights of others cannot be predicted, either in the United States or in other countries.
There can be no assurance that we will discover or develop patentable products or processes or that patents will issue from any of the currently pending patent applications or that claims granted on issued patents will be sufficient to protect our technology or adequately cover the actual products we may actually sell. Potential competitors or other researchers in the field may have filed patent applications, been issued patents, published articles or otherwise created prior art that could restrict or block our efforts to obtain additional patents. There also can be no assurance that our issued patents or pending patent applications, if issued, will not be challenged, invalidated, rendered unenforceable or circumvented or that the rights granted hereunder will provide us with proprietary protection or competitive advantages. Our patent rights also depend on our compliance with technology and patent licenses upon which our patent rights are based and upon the validity of assignments of patent rights from consultants and other inventors that were, or are, not employed by us.
In addition, competitors may manufacture and sell our potential products in those foreign countries where we have not filed for patent protection or where patent protection may be unavailable, not obtainable or ultimately not enforceable. In addition, even where patent protection is obtained, third party competitors may challenge our patent claims in the various patent offices, for example via opposition in the European Patent Office or reexamination or interference proceedings in the United States Patent and Trademark Office, or USPTO. The ability of such competitors to sell such products in the United States or in foreign countries where we have obtained patents is usually governed by the patent laws of the countries in which the product is sold.
We will incur significant ongoing expenses in maintaining our patent portfolio. Should we lack the funds to maintain our patent portfolio or to enforce our rights against infringers, we could be adversely impacted. Even if claims of infringement are without merit, any such action could divert the time and attention of management and impair our ability to access additional capital and/or cost us significant funds to defend.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The United States Patent and Trademark Office has developed regulations and procedures to govern administration of the Leahy-Smith Act, but many of the substantive changes to patent law associated with the Leahy-Smith Act, particularly the first inventor to file provisions, only became effective 18 months after its enactment. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
We may be subject to litigation with respect to the ownership and use of intellectual property that will be costly to defend or pursue and uncertain in its outcome.
Our success also will depend, in part, on our refraining from infringing patents or otherwise violating intellectual property owned or controlled by others. Pharmaceutical companies, biotechnology companies, universities, research institutions, and others may have filed patent applications or have received, or may obtain, issued patents in the United States or elsewhere relating to aspects of our technology. It is uncertain whether the issuance of any third-party patents will require us to alter our products or processes, obtain licenses, or cease certain activities. Some third-party applications or patents may conflict with our issued patents or pending applications. Any such conflict could result in a significant reduction of the scope or value of our issued or licensed patents.
In addition, if patents issued to other companies contain blocking, dominating or conflicting claims and such claims are ultimately determined to be valid, we may be required to obtain licenses to these patents or to develop or obtain alternative non-infringing technology and cease practicing those activities, including potentially manufacturing or selling any products deemed to infringe those patents. If any licenses are required, there can be no assurance that we will be able to obtain any such licenses on commercially favorable terms, if at all, and if these licenses are not obtained, we might be prevented from pursuing the development and commercialization of certain of our potential products. Our failure to obtain a license to any technology that we may require to commercialize our products on favorable terms may have a material adverse impact on our business, financial condition and results of operations.
Litigation, which could result in substantial costs to us (even if determined in our favor), may also be necessary to enforce any patents issued or licensed to us or to determine the scope and validity of the proprietary rights of others. There can be no assurance that our issued or licensed patents would be held valid by a court of competent jurisdiction or that any third party would be found to infringe our patents.
In addition, if our competitors file patent applications in the United States that claim technology also claimed by us, we may have to participate in interference proceedings to determine priority of invention. These proceedings, if initiated by the USPTO, could result in substantial cost to us, even if the eventual outcome is favorable to us. Such proceedings can be lengthy, are costly to defend and involve complex questions of law and fact the outcomes of which are difficult to predict. An adverse outcome with respect to a third party claim or in an interference proceeding could subject us to significant liabilities, require us to license disputed rights from third parties, or require us to cease using such technology, any of which could have a material adverse effect on our business, financial condition and results of operations.
We also rely on trade secrets to protect technology, especially where patent protection is not believed to be appropriate or obtainable or where patents have not issued. We attempt to protect our proprietary technology and processes, in part, with confidentiality agreements and assignment of invention agreements with our employees and confidentiality agreements with our consultants and certain contractors. There can be no assurance that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets will not otherwise become known or be independently discovered by competitors. We may fail in certain circumstances to obtain the necessary confidentiality agreements, or their scope or term may not be sufficiently broad to protect our interests.
If our trade secrets or other intellectual property become known to our competitors, it could result in a material adverse effect on our business, financial condition and results of operations. To the extent that we or our consultants or research collaborators use intellectual property owned by others in work for us, disputes may also arise as to the rights to related or resulting know-how and inventions.
Risks Relating to Our Exposure to Litigation
We are exposed to potential product liability or similar claims, and insurance against these claims may not be available to us at a reasonable rate in the future.
Our business exposes us to potential liability risks that are inherent in the testing, manufacturing and marketing of human therapeutic products. Clinical trials involve the testing of product candidates on human subjects or volunteers under a research plan, and carry a risk of liability for personal injury or death to patients due to unforeseen adverse side effects, improper administration of the product candidate, or other factors. Many of these patients are already seriously ill and are therefore particularly vulnerable to further illness or death.
We currently carry clinical trial liability insurance in the amount of $5 million in the aggregate, but there can be no assurance that we will be able to maintain such insurance or that the amount of such insurance will be adequate to cover claims. We could be materially and adversely affected if we were required to pay damages or incur defense costs in connection with a claim outside the scope of indemnity or insurance coverage, if the indemnity is not performed or enforced in accordance with its terms, or if our liability exceeds the amount of applicable insurance. In addition, there can be no assurance that insurance will continue to be available on terms acceptable to us, if at all, or that if obtained, the insurance coverage will be sufficient to cover any potential claims or liabilities. Similar risks would exist upon the commercialization or marketing of any products by us or our collaborators.
Regardless of their merit or eventual outcome, product liability claims may result in:
| |
| • | decreased demand for our product; |
| |
| • | injury to our reputation and significant negative media attention; |
| |
| • | withdrawal of clinical trial volunteers; |
| |
| • | distraction of management; and |
| |
| • | substantial monetary awards to plaintiffs. |
We may become involved in securities class action litigation that could divert management’s attention and adversely affect our business and could subject us to significant liabilities.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of biopharmaceutical companies. These broad market fluctuations as well a broad range of other factors, including the realization of any of the risks described in this “Risk Factor,” section of this Annual Report on Form 10-K, may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and pharmaceutical companies generally experience significant stock price volatility. We may become involved in
this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business. Any adverse determination in any such litigation or any amounts paid to settle any such actual or threatened litigation could require that we make significant payments.
Risks Related to Ownership of Our Common Stock
The market price of our common stock may be highly volatile, and could decline significantly.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including those described elsewhere in this “Risk Factors” section of this this Annual Report on Form 10-K and the following:
| |
| • | new products, product candidates or new uses for existing products introduced or announced by our strategic collaborators, or our competitors, and the timing of these introductions or announcements; |
| |
| • | actual or anticipated results from and any delays in our clinical trials, including our Phase 3 clinical trial of our HyperAcute Pancreas product candidate, as well as results of regulatory reviews relating to the approval of our product candidates; |
| |
| • | variations in the level of expenses related to any of our product candidates or clinical development programs, including relating to the timing of invoices from, and other billing practices of, our clinical research organizations and clinical trial sites; |
| |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
| |
| • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| |
| • | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures and capital commitments; |
| |
| • | additions or departures of key scientific or management personnel; |
| |
| • | conditions or trends in the biotechnology and biopharmaceutical industries; |
| |
| • | actual or anticipated changes in earnings estimates, development timelines or recommendations by securities analysts; actual and anticipated fluctuations in our quarterly operating results; |
| |
| • | the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| |
| • | deviations from securities analysts’ estimates or the impact of other analyst ratings downgrades by any securities analysts who follow our common stock; |
| |
| • | other events or factors, including those resulting from war, incidents of terrorism, natural disasters or responses to these events; |
| |
| • | changes in accounting principles; |
| |
| • | discussion of us or our stock price by the financial and scientific press and in online investor communities; |
| |
| • | general economic and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies; and |
| |
| • | sales of common stock by us or our stockholders in the future, as well as the overall trading volume of our common stock. |
In addition, the stock market in general and the market for biotechnology and biopharmaceutical companies in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
Our principal stockholders and management own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval.
As of December 31, 2013, our executive officers, directors and principal stockholders, together with their respective affiliates, owned approximately 42.2% of our common stock, including shares subject to outstanding options and warrants that are exercisable within 60 days after December 31, 2013. These stockholders will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of our Board of Directors, future issuances of our common stock or other securities, declarations of dividends on our common stock and approval of other significant corporate transactions. This concentration of ownership could have the effect of delaying or preventing a change in
our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the fair market value of our common stock. In addition, sales of shares beneficially owned by executive officers and directors and their affiliates could be viewed negatively by third parties and have a negative impact on our stock price. Moreover, we cannot assure you as to how these shares may be distributed and subsequently voted.
A significant portion of our total outstanding shares of common stock is restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur in the future. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. Certain holders of outstanding shares of our common stock that have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to meet compliance obligations.
As a public company, we incur significant legal, accounting and other expenses to comply with reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC and The NASDAQ Stock Market. Meeting the requirements of these rules and regulations entails significant legal and financial compliance costs, makes some activities more difficult, time-consuming or costly and may also place undue strain on our personnel, systems and resources. Our management and other personnel devote a substantial amount of time to these compliance requirements. In addition, these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on our Board of Directors, our board committees or as executive officers.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our ability to produce accurate financial statements and on our stock price.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we are required to publish a report by our management on our internal control over financial reporting. To achieve compliance with Section 404, we have engaged in a process to document and evaluate our internal control over financial reporting, which has been both costly and challenging. To maintain compliance on an ongoing basis, we will need to dedicate internal resources, engage outside consultants and adopt a detailed work plan. Despite our effort, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We do not expect to pay any cash dividends for the foreseeable future. Investors may never obtain a return on their investment.
You should not rely on an investment in our common stock to provide dividend income. We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. In addition, our ability to pay cash dividends is currently prohibited by the terms of one of our debt financing arrangements, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our common stock.
Provisions in our certificate of incorporation, our by-laws or Delaware law might discourage, delay or prevent a change in control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our certificate of incorporation, our by-laws or Delaware law may have the effect of deterring unsolicited takeovers or delaying or preventing a change in control of our company or changes in our management, including transactions in which our stockholders might otherwise receive a premium for their shares over then current market prices. In addition, these provisions may limit the ability of stockholders to approve transactions that they may deem to be in their best interest. These provisions include:
| |
| • | the division of our Board of Directors into three classes with staggered, three-year terms; |
| |
| • | advance notice requirements for stockholder proposals and nominations; |
| |
| • | the inability of stockholders to act by written consent or to call special meetings; |
| |
| • | limitation on the ability of stockholders to remove directors or amend our by-laws; and |
| |
| • | the ability of our Board of Directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could include the right to approve an acquisition or other change in our control or could be used to institute a rights plan, also known as a poison pill, that would work to dilute the stock ownership of a potential hostile acquirer, likely preventing acquisitions that have not been approved by our Board of Directors. |
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
Our stockholders may be diluted, and the prices of our securities may decrease, by the exercise of outstanding stock options and warrants or by future issuances of securities by us.
We may issue additional common stock, preferred stock, restricted stock units, or securities convertible into or exchangeable for our common stock. Furthermore, substantially all shares of common stock for which our outstanding stock options or warrants are exercisable are, once they have been purchased, eligible for immediate sale in the public market. The issuance of additional common stock, preferred stock, restricted stock units, or securities convertible into or exchangeable for our common stock or the exercise of stock options or warrants would dilute existing investors and could adversely affect the price of our securities. In addition, such securities may have rights senior to the rights of securities held by existing investors.
Our ability to use our net operating loss carryforwards and certain other tax attributes is limited by Sections 382 and 383 of the Internal Revenue Code.
Sections 382 and 383 of the Internal Revenue Code limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset any future taxable income or tax if the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. State net operating loss carryforwards (and certain other tax attributes) may be similarly limited. A Section 382 ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
Based on Section 382 ownership change analyses, we believe that, from its inception through December 31, 2011, we experienced Section 382 ownership changes in September 2001 and March 2003 and our subsidiary experienced Section 382 ownership changes in January 2006 and January 2011. These ownership changes limit our ability to utilize federal net operating loss carryforwards (and certain other tax attributes) that accrued prior to the respective ownership changes of us and our subsidiary.
Additional analysis will be required to determine whether changes in our ownership since December 31, 2011, changes in our ownership that resulted from our follow-on public offering and/or changes in our ownership that resulted from our ongoing ATM Offering have caused or will cause another ownership change to occur, and the conclusions will depend on information that currently may not be available to us. Any such change could result in significant limitations on all of our net operating loss carryforwards and other tax attributes.
Additional ownership changes may occur in the future as a result of events over which we will have little or no control, including purchases and sales of our equity by our 5% stockholders, the emergence of new 5% stockholders, additional equity offerings or redemptions of our stock or certain changes in the ownership of any of our 5% stockholders.
Accounting pronouncements may impact our reported results of operations and financial position.
United States generally accepted accounting principles, or GAAP, and related implementation guidelines and interpretations can be highly complex and involve subjective judgments. Changes in these rules or their interpretation, the adoption of new pronouncements or the application of existing pronouncements to changes in our business could significantly alter our reported financial statements and results of operations.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If no securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our stock, publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
We implemented a new accounting software, or ERP, system in the third quarter of 2013, and will continue to develop its application to our business processes as we prepare to commercialize, and we may experience unforeseen difficulties or delays related to implementation of the new system.
We implemented a new enterprise resource planning, or ERP, system in the third quarter of 2013, which we have used for financial reporting purposes and certain manufacturing purposes beginning in the fourth quarter of 2013. We expect to use the ERP system for other manufacturing and finance functions in 2014. If we encounter unforeseen difficulties in the use of the ERP system, we may experience delays in financial reporting, weaknesses in internal controls or unanticipated expenses.
| |
| Item 1B. | UNRESOLVED STAFF COMMENTS |
None.
We conduct our primary operations at leased facilities described below.
|
| | | | | | |
| Location | | Operations Conducted | | Approximate Square Feet | | Lease Expiration Date |
| Ames, Iowa | | Executive offices, research and development, and manufacturing | | 53,000 | | 2018 |
We believe that our administrative office space is adequate to meet our needs for the foreseeable future. We also believe that our research and development facilities and our manufacturing facility, together with third party manufacturing facilities, will be adequate for our on-going activities.
On February 24, 2014, we signed a lease for a commercial facility in Austin, Texas. We anticipate conducting activities at this location associated with expansion of our commercialization efforts as well as supporting the continued growth of our clinical operations activities.
None.
| |
| Item 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
| |
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES |
Our common stock is quoted on The Nasdaq Stock Market, LLC under the symbol “NLNK.” The following table sets forth the range of high and low sales prices for our common stock on The Nasdaq Stock Market, LLC for the periods indicated since November 11, 2011.
|
| | | | |
| | | High | | Low |
| Fiscal 2013 | | | | |
| First Quarter | | $12.70 | | $11.40 |
| Second Quarter | | 22.56 | | 11.27 |
| Third Quarter | | 20.16 | | 15.78 |
| Fourth Quarter | | 23.50 | | 16.04 |
| | | | | |
| Fiscal 2012 | | | | |
| First Quarter | | $10.85 | | $6.33 |
| Second Quarter | | 18.00 | | 8.75 |
| Third Quarter | | 16.55 | | 11.00 |
| Fourth Quarter | | 16.50 | | 10.60 |
| | | | | |
| Fiscal 2011 | | | | |
| Fourth Quarter | | | | |
| (November 11, 2011 to December 31, 2011) | | $7.81 | | $6.25 |
| | | | | |
As of March 7, 2014, we had 146 stockholders of record of our common stock.
Dividend Policy
We have never paid cash dividends. We do not expect to declare or pay any cash dividends on our common stock in the near future. We intend to retain all earnings, if any, to invest in our operations. The payment of future dividends is within the discretion of our board of directors and will depend upon our future earnings, if any, our capital requirements, financial condition and other relevant factors.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plans is incorporated by reference to Item 12 of Part III of the Annual Report on Form 10-K.
Our Stock Performance
The following graph compares cumulative total return of our Common Stock with the cumulative total return of (i) the NASDAQ Stock Market-United States, and (ii) the NASDAQ Biotechnology Index. The graph assumes (a) $100 was invested on November 11, 2011 in our Common Stock, the stocks comprising the NASDAQ Stock Market-United States and the stocks comprising the NASDAQ Biotechnology Index, and (b) the reinvestment of dividends. The comparisons shown in the graph are based on historical data and the stock price performance shown in the graph is not necessarily indicative of, or intended to forecast, future performance of our stock.
* $100 invested on November 11, 2011 in stock or index, including reinvestment of dividends.
|
| | | | | | | | |
| Cumulative Total Return | | |
| | | 11/11/2011 | | 12/31/2011 | | 12/31/2012 | | 12/31/2013 |
| NewLink Genetics Corporation | | $100 | | $99 | | $177 | | $311 |
| NASDAQ Composite | | $100 | | $97 | | $113 | | $156 |
| NASDAQ Biotechnology | | $100 | | $110 | | $145 | | $240 |
|
| | | | | | | | | | | | | | | | |
| Date* | | Transaction Type | | Closing Price** | | Beginning No. Of Shares*** | | Dividend per Share | | Dividend Paid | | Shares Reinvested | | Ending Shares | | Cum. Total Return |
| 11-Nov-11 | | Begin | | $7.08 | | 14.124 | | | | | | | | 14.124 | | $100.0 |
| 31-Dec-11 | | Year End | | $7.04 | | 14.124 | | | | | | | | 14.124 | | $99.4 |
| 31-Dec-12 | | Year End | | $12.50 | | 14.124 | | | | | | | | 14.124 | | $176.6 |
| 31-Dec-13 | | Year End | | $22.01 | | 14.124 | | | | | | | | 14.124 | | $310.9 |
* Specified ending dates are ex-dividends dates.
** All Closing Prices and Dividends are adjusted for stock splits and stock dividends.
*** ‘Begin Shares’ based on $100 investment.
Recent Sales of Unregistered Securities
None
Repurchases of Equity Securities
There were no repurchases of equity securities in the year ending December 31, 2013.
Use of Proceeds
Not applicable.
| |
| Item 6. | SELECTED FINANCIAL DATA |
You should read the following selected consolidated financial data together with our financial statements and the related notes appearing at the end of this Annual Report on Form 10-K and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this annual report.
We derived the annual consolidated financial data from our audited financial statements, the last three years of which are included elsewhere in this Annual Report on Form 10-K. We derived the summary statement of operations data for the years ended December 31, 2009 and 2010 and the balance sheet data as of December 31, 2009, 2010 and 2011 from our audited financial statements not included in this annual report.
Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
|
| | | | | | | | | | | | | | | | | | | | | |
| | | | |
| | | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | (in thousands, except per share data) | |
| Statement of operations data: | | | | | | | | | | | |
| Grant revenue | | $ | 1,093 |
| | $ | 1,687 |
| | $ | 1,872 |
| | $ | 2,079 |
| | $ | 934 |
| |
| Operating expenses: | | | | | | | | | | | |
| Research and development | | 22,713 |
| | 17,838 |
| | 14,255 |
| | 12,666 |
| | 7,578 |
| |
| General and administrative | | 9,521 |
| | 7,108 |
| | 5,679 |
| | 6,074 |
| | 3,705 |
| |
| Total operating expenses | | 32,234 |
| | 24,946 |
| | 19,934 |
| | 18,740 |
| | 11,283 |
| |
| Loss from operations | | (31,141 | ) | | (23,259 | ) | | (18,062 | ) | | (16,661 | ) | | (10,349 | ) | |
| Other income and expense: | | | | | | | | | | | |
| Miscellaneous income | | 112 |
| | (38 | ) | | 5 |
| | 71 |
| | 19 |
| |
| Interest income | | 12 |
| | 14 |
| | 11 |
| | 75 |
| | 132 |
| |
| Interest expense | | (33 | ) | | (38 | ) | | (42 | ) | | (47 | ) | | (9 | ) | |
| Other income (expense), net | | 91 |
| | (62 | ) | | (26 | ) | | 99 |
| | 142 |
| |
| Net loss before taxes | | (31,050 | ) | | (23,321 | ) | | (18,088 | ) | | (16,562 | ) | | (10,207 | ) | |
| Income tax expense | | (130 | ) | | — |
| | — |
| | — |
| | — |
| |
| Net loss | | (31,180 | ) | | (23,321 | ) | | (18,088 | ) | | (16,562 | ) | | (10,207 | ) | |
| Less net loss attributable to noncontrolling interest | | — |
| | — |
| | 1 |
| | 349 |
| | 233 |
| |
| Net loss attributable to NewLink | | $ | (31,180 | ) | | $ | (23,321 | ) | | $ | (18,087 | ) | | $ | (16,213 | ) | | $ | (9,974 | ) | |
| Net loss per share—basic and diluted | | $ | (1.23 | ) | | $ | (1.12 | ) | | $ | (2.98 | ) | | $ | (4.84 | ) | | $ | (3.16 | ) | |
| Weighted average shares outstanding—basic and diluted | | 25,275 |
| | 20,779 |
| | 6,065 |
| | 3,352 |
| | 3,160 |
| |
|
| | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | (in thousands) | |
| Balance sheet data: (1) | | | | | | | | | | | |
| Cash, cash equivalents, and certificates of deposit | | $ | 61,540 |
| | $ | 21,744 |
| | $ | 41,980 |
| | $ | 12,841 |
| | $ | 17,209 |
| |
| Working capital | | 60,094 |
| | 20,470 |
| | 32,124 |
| | 11,377 |
| | 15,657 |
| |
| Total assets | | 70,557 |
| | 29,429 |
| | 48,379 |
| | 20,078 |
| | 22,667 |
| |
| Royalty obligations, notes payable and obligations under capital leases | | 7,222 |
| | 7,382 |
| | 7,156 |
| | 7,294 |
| | 6,113 |
| |
| Convertible preferred stock | | — |
| | — |
| | — |
| | 62,775 |
| | 55,164 |
| |
| Deficit accumulated during the development stage | | (135,977 | ) | | (104,797 | ) | | (81,476 | ) | | (63,389 | ) | | (47,176 | ) | |
| Total equity (deficit) | | $ | 58,327 |
| | $ | 17,927 |
| | $ | 36,773 |
| | $ | (52,019 | ) | | $ | (40,786 | ) | |
_________________________________
| |
| (1) | From January 1, 2014 through February 13, 2014, we sold 1,017,217 shares of common stock raising a total of $27.6 million in net proceeds. The data presented above does not include these proceeds. |
| |
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis should be read in conjunction with the consolidated financial statements and notes thereto included in Part II, Item 8 of this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K, our actual results may differ materially from those anticipated in these forward-looking statements. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Annual Report on Form 10-K. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K completely.
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing novel immunotherapeutic products to improve treatment options for patients with cancer. Our portfolio includes biologic and small-molecule immunotherapy product candidates intended to treat a wide range of oncology indications. Our lead product candidate, HyperAcute Pancreas cancer immunotherapy (algenpantucel-L), or HyperAcute Pancreas, is being studied in two Phase 3 clinical trials; one in surgically-resected pancreatic cancer patients that is being performed under a Special Protocol Assessment, or SPA, with the United States Food and Drug Administration, or FDA, and one in patients with locally advanced pancreatic cancer. We initiated these trials based on encouraging Phase 2 data that suggest improvement in both disease-free and overall survival. We have also received Fast Track and Orphan Drug designations from the FDA for this product candidate for the adjuvant treatment of surgically-resected pancreatic cancer and Orphan Medicinal Product designation for this product candidate from the European Commission. The primary endpoint for our IMPRESS (Immunotherapy for Pancreatic Resectable cancer Survival Study) Phase 3 trial with algenpantucel-L for patients with surgically-resected pancreatic cancer is overall survival and, as determined by the SPA, the first interim analysis will be conducted when 222 deaths are reported for the study. This triggering event for the first interim analysis has not yet occurred. Our additional product candidates in clinical development include our HyperAcute Lung cancer immunotherapy (tergenpumatucel-L), or HyperAcute Lung, our HyperAcute Melanoma cancer immunotherapy (dorgenmeltucel-L), or HyperAcute Melanoma, our HyperAcute Prostate cancer immunotherapy, or HyperAcute Prostate, our HyperAcute Renal cancer immunotherapy, or HyperAcute Renal, 1-methyl-D-tryptophan (D-IMT), or indoximod, our lead indoleamine-(2.3)-dioxygenase, or IDO pathway inhibitor product candidate, and NLG919, our second IDO pathway inhibitor, which is currently in a Phase I clinical trial in patients with solid tumors. To date, our HyperAcute product candidates have been dosed in more than 500 cancer patients, either as a monotherapy or in combination with other therapies, and have demonstrated a favorable safety profile.
Our HyperAcute product candidates are based on our proprietary HyperAcute immunotherapy technology, which is designed to stimulate the human immune system. Our HyperAcute product candidates use allogeneic (non-patient specific) cells from previously established human cancer cell lines rather than cells derived from the individual patient. We believe our approach enables a simpler, more consistent and scalable manufacturing process than therapies based on patient specific tissues or cells. Our product candidates are designed to harness multiple components of the innate immune system to combat cancer, either as a monotherapy or in combination with current treatment regimens without incremental toxicity. We are also conducting small-molecule based research and development with an aim to produce new drugs capable of breaking the immune system’s tolerance to cancer through inhibition of the IDO pathway. We are currently studying our lead IDO pathway inhibitor product candidate, indoximod, in multiple Phase 2 studies in breast cancer and prostate cancer. We believe that our immunotherapeutic technologies will enable us to discover, develop and commercialize multiple product candidates that can be used either alone or in combination to enhance or potentially replace current therapies to treat cancer with underserved patient populations for significant market potential.
BioProtection Systems Corporation, or BPS, was founded by us as a subsidiary in 2005 to research, develop and commercialize vaccines to control the spread of emerging lethal viruses and infectious diseases, improve the efficacy of existing vaccines and provide rapid-response prophylactic and therapeutic treatment for pathogens most likely to enter the human population through pandemics or acts of bioterrorism. BPS is based on three core technologies, each of which can be leveraged into the infectious disease or biodefense fields. The first is our HyperAcute immunotherapy technology, which is currently focused on enhancing vaccines for influenza. The second technology is based on a yellow fever virus. The third technology is a replication competent recombinant Vesicular Stomatitus Vaccine, or rVSV, an advanced vaccine technology developed for the Marburg and Ebola viruses.
We are a development stage company and have incurred significant losses since our inception. As of December 31, 2013, we had an accumulated deficit of $136.0 million. We incurred a net loss of $31.2 million, $23.3 million and $18.1 million for the years ended December 31, 2013, 2012, and 2011, respectively. We expect our losses to increase over the next several years as we advance into late-stage clinical trials and pursue regulatory approval of our product candidates. In addition, if one or more of our product candidates are approved for marketing, we will incur significant expenses for the initiation of commercialization activities.
On October 25, 2011, we filed a Certificate of Amendment of the Restated Certificate of Incorporation with the Secretary of State of Delaware effecting a 2.1-for-one reverse split of our Common Stock. All share and per share amounts have been retroactively restated in the accompanying financial statements and notes for all periods presented.
Initial Public Offering and Follow-On Offering
On November 16, 2011, we completed our initial public offering, or IPO, raising a total of $37.6 million in net proceeds after deducting underwriting discounts and commissions of $3.0 million and offering expenses of $2.9 million.
On February 4, 2013, we completed a follow-on public offering, raising a total of $49.0 million after deducting $3.1 million in underwriter discounts and commissions and offering expenses of $300,000.
We entered into a Sales Agreement, with Cantor Fitzgerald & Co., dated as of September 5, 2013, under which we may sell up to $60.0 million in shares of our common stock in one or more placements at prevailing market prices for our common stock, or the ATM Offering. Any such sales would be effected pursuant to our registration statement on Form S-3 (333-185721), declared effective by the Securities and Exchange Commission, or SEC, on January 4, 2013. As of December 31, 2013, we had sold 816,621 shares of common stock raising a total of $17.4 million in net proceeds under the ATM Offering. Subsequent to December 31, 2013 and through February 13, 2014, we sold an additional 1,017,217 shares of common stock under the ATM Offering raising an additional $27.6 million in net proceeds.
Financial Overview
Revenues
From our inception through December 31, 2013, we have not generated any revenue from product sales. We have generated $8.5 million in grant revenue from our inception through December 31, 2013, which is primarily attributable to research and development being performed by our subsidiary, BPS, under contracts and grants with the Department of Defense, or DOD, and the National Institutes of Health, or NIH.
In the future, we may generate revenue from a variety of sources, including product sales if we develop products which are approved for sale, license fees, and milestone, research and development and royalty payments in connection with strategic collaborations or licenses of our intellectual property. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the timing and amount of license fees, research and development reimbursements, milestone and other payments we may receive under potential strategic collaborations, and the amount and timing of payments we may receive upon the sale of any products, if approved, to the extent any are successfully commercialized. We do not expect to generate revenue from product sales for several years, if ever. If we fail to complete the development of our product candidates in a timely manner or to obtain regulatory approval for them, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely affected.
Research and Development Expenses
Research and development expenses consist of expenses incurred in connection with the discovery and development of our product candidates. These expenses consist primarily of:
| |
| • | employee-related expenses, which include salaries, bonuses, benefits and share-based compensation; |
| |
| • | the cost of acquiring and manufacturing clinical trial materials; |
| |
| • | expenses incurred under agreements with contract research organizations, investigative sites and consultants that conduct our clinical trials and a substantial portion of our preclinical studies; |
| |
| • | facilities, depreciation of fixed assets and other allocated expenses, which include direct and allocated expenses for rent and maintenance of research facilities and equipment; |
| |
| • | license fees for and milestone payments related to in-licensed products and technology; and |
| |
| • | costs associated with non-clinical activities and regulatory approvals. |
We expense research and development expenses as incurred.
Product candidates in late stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size, duration and complexity of later stage clinical trials. We plan to increase our research and development expenses for the foreseeable future as we seek to complete development of our most advanced product candidates, and to further advance our earlier-stage research and development projects. For the years ended December 31, 2013, 2012 and 2011 and from our inception through December 31, 2013, we have incurred $22.7 million, $17.8 million, $14.3 million and $100.9 million, respectively, in research and development expenses. The following tables summarize our research and development expenses for the periods indicated:
Research and Development Expenses by Product (In thousands) |
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Cumulative from
June 4, 1999
(inception)
through
December 31, | |
| | 2013 | | 2012 | | 2011 | | 2013 | |
| HyperAcute immunotherapy technology | $ | 16,241 |
| | $ | 11,957 |
| | $ | 10,044 |
| | $ | 71,343 |
| |
| IDO pathway inhibitor technology | 4,772 |
| | 4,153 |
| | 2,663 |
| | 19,849 |
| |
| Other research and development | 1,700 |
| | 1,728 |
| | 1,548 |
| | 9,677 |
| |
| Total research and development expenses | $ | 22,713 |
| | $ | 17,838 |
| | $ | 14,255 |
| | $ | 100,869 |
| |
Research and Development Expenses by Category (In thousands) |
| | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Cumulative
from
June 4, 1999 (inception) through December 31, | |
| | 2013 | | 2012 | | 2011 | | 2013 | |
| Compensation | $ | 9,273 |
| | $ | 7,619 |
| | $ | 6,709 |
| | $ | 47,287 |
| |
| Equipment, supplies and occupancy | 5,846 |
| | 4,932 |
| | 4,321 |
| | $ | 30,378 |
| |
| Outside clinical and other | 7,594 |
| | 5,287 |
| | 3,225 |
| | $ | 23,204 |
| |
| Total research and development expenses | $ | 22,713 |
| | $ | 17,838 |
| | $ | 14,255 |
| | $ | 100,869 |
| |
At this time, we cannot accurately estimate or know the nature, specific timing or costs necessary to complete clinical development activities for our product candidates. We are subject to the numerous risks and uncertainties associated with developing biopharmaceutical products including the uncertain cost and outcome of ongoing and planned clinical trials, the possibility that the FDA or another regulatory authority may require us to conduct clinical or non-clinical testing in addition to trials that we have planned, rapid and significant technological changes, frequent new product and service introductions and enhancements, evolving industry standards in the life sciences industry and our future need for additional capital. In addition, we currently have limited clinical data concerning the safety and efficacy of our product candidates. A change in the outcome of any of these variables with respect to the development of any of our product candidates could result in a significant change in the costs and timing of our research and development expenses.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive, finance, business development, information technology, legal and human resources functions. Other general and administrative expenses include facility costs not otherwise associated with research and development expenses, intellectual property prosecution and defense costs and professional fees for legal, consulting, auditing and tax services.
We anticipate that our general and administrative expenses will continue to increase over the next several years for, among others, the following reasons:
| |
| • | we expect our general and administrative expenses to increase as a result of increased payroll, expanded infrastructure and higher consulting, legal, auditing and tax services and investor relations costs, and director and officer insurance premiums associated with being a public company; |
| |
| • | we expect to incur increased general and administrative expenses to support our research and development activities, which we expect to expand as we continue to advance the clinical development of our product candidates; and |
| |
| • | we expect to incur increased expenses related to the planned sales and marketing of our product candidates, which may include recruiting a specialty sales force, in anticipation of commercial launch before we receive regulatory approval, if any, of a product candidate. |
Interest Income and Interest Expense
Interest income consists of interest earned on our cash and cash equivalents and certificates of deposit. The primary objective of our investment policy is capital preservation. We expect our interest income to increase as we invest the net proceeds from our offerings pending their use in our operations.
Interest expense consists primarily of interest, amortization of debt discount and amortization of deferred financing costs associated with our notes payable and obligations under capital leases.
Tax Loss Carryforwards
The valuation allowance for deferred tax assets as of December 31, 2013 and 2012 was $25.2 million and $23.1 million, respectively. The net change in the total valuation allowance for the years ended December 31, 2013 and 2012 was an increase of $2.0 and $4.3 million, respectively. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected taxable income, and tax planning strategies in making this assessment. Valuation allowances have been established for the entire amount of the net deferred tax assets as of December 31, 2013 and 2012, due to the uncertainty of future recoverability.
As of December 31, 2013 and December 31, 2012, we had federal net operating loss carryforwards of $88.4 million and $94.5 million and federal research credit carryforwards of $4.3 million and $2.9 million, respectively, that expire at various dates from 2020 through 2032. Sections 382 and 383 of the Internal Revenue Code limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset any future taxable income or tax if the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. State net operating loss carryforwards (and certain other tax attributes) may be similarly limited. An ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
Based on a preliminary analysis, we believe that, from its inception through December 31, 2011, we experienced Section 382 ownership changes in September 2001 and March 2003 and our subsidiary experienced Section 382 ownership changes in January 2006 and January 2011. These ownership changes limit our ability to utilize federal net operating loss carryforwards (and certain other tax attributes) that accrued prior to the respective ownership changes of us and our subsidiary.
Additional analysis will be required to determine whether changes in our ownership since December 31, 2011 and/or changes in our ownership that resulted from our follow-on offerings have caused or will cause another ownership change to occur. Any such change could result in significant limitations on all of our net operating loss carryforwards and other tax attributes.
Even if another ownership change has not occurred, additional ownership changes may occur in the future as a result of events over which we will have little or no control, including purchases and sales of our equity by our 5% stockholders, the emergence of new 5% stockholders, additional equity offerings or redemptions of our stock or certain changes in the ownership of any of our 5% stockholders.
Income tax expense was $130,000, $0, $0 and $130,000 for the years ended December 31, 2013, 2012 and 2011 and from inception, respectively. Income tax expense differs from the amount that would be expected after applying the statutory U.S. federal income tax rate primarily due to changes in the valuation allowance for deferred taxes.
Critical Accounting Policies and Significant Judgments and Estimates
We have prepared our financial statements in accordance with United States generally accepted accounting principles, or GAAP. Our preparation of these financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, expenses and related disclosures at the date of the financial statements, as well as revenues and expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions. We have reviewed our critical accounting policies and estimates with the Audit Committee of our Board of Directors.
While our significant accounting policies are described in more detail in note 2 to our consolidated financial statements included later in this annual report, we believe the following accounting policies to be critical in the preparation of our financial statements.
Expenses Accrued Under Contractual Arrangements with Third Parties; Accrued Clinical Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued clinical expenses include:
| |
| • | fees paid to contract research organizations in connection with clinical trials; |
| |
| • | fees paid to investigator sites in connection with clinical trials; |
| |
| • | fees paid to contract manufacturers in connection with the production of clinical trial materials; and |
| |
| • | fees paid to vendors in connection with preclinical development activities. |
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and contract research organizations that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low in any particular period.
Share-Based Compensation
We are required to estimate the grant-date fair value of stock options, stock awards and restricted stock issued to employees and recognize this cost over the period these awards vest. We estimate the fair value of each award granted using the Black-Scholes option pricing model. The Black-Scholes model requires the input of subjective assumptions, including the expected stock price volatility, the calculation of expected term and the fair value of the underlying common stock on the date of grant, among other inputs. Generally, we have issued employee awards that vest over time. For these awards, we record compensation cost on a straight-line basis over the vesting period. We issue awards which typically vest 20% to 25% on the first anniversary date of issuance with the remaining options vesting ratably over the next 36 to 48 months, as determined by the Board of Directors at the
time of grant. We calculate the fair value of the award on the grant date, which is the date the award is authorized by the Board of Directors or Chief Executive Officer and the employee has an understanding of the terms of the award.
We have issued awards to nonemployee consultants and advisers. All grants to nonemployees are valued using the same fair value method that we use for grants to employees. The compensation cost on these awards is recognized through the later of the vesting of the award or completion of services by the nonemployee.
We recorded noncash share-based compensation expense for employee and nonemployee stock options and stock awards of $4.4 million, $3.2 million, and $2.5 million during 2013, 2012 and 2011, respectively. As of December 31, 2013, the total compensation cost related to nonvested option awards not yet recognized was $7.0 million and the weighted average period over which it is expected to be recognized was 2.7 years. We expect to continue to grant stock options, stock awards and restricted stock in the future, which will increase our share-based compensation expense in future periods. If any of the assumptions used in the Black-Scholes model change significantly, share-based compensation expense may differ materially in the future from that recorded in the current period.
The following table summarizes our assumptions used in the Black-Scholes model for option grants during the last three years:
| Black-Scholes Model Assumptions |
| | | | | | |
| | | Years Ended December 31, |
| | |
| | | 2013 | | 2012 | | 2011 |
| Exercise price | | $11.79-$22.61 | | $6.87-$15.16 | | $7.00-$10.02 |
| Risk-free interest rate | | .89%-2.14% | | .98%-2.05% | | 0.1%-3.1% |
| Expected dividend yield | | — | | — | | — |
| Expected volatility | | 61.4%-67.3% | | 63.0%-73.6% | | 62.6%-67.9% |
| Expected term (in years) | | 4.8-7.0 | | 5.8-7.0 | | 5.0-7.5 |
Exercise Price. Prior to the IPO, our stock options were granted with an exercise price at or above the then current fair value of our common stock as determined by the Board of Directors. As an input to making this determination, the Board of Directors obtained a third-party valuation. See “Common Stock Fair Value” below. Subsequent to the IPO, we have used the fair value of our common stock on the date of grant based on the current market price.
Expected Volatility. We estimate future expected volatility for each stock option valuation utilizing volatility rates of similar publicly traded companies considered to be in the same peer group. We use a combination of our own data and peer group data to compute our volatility and will continue to do so until we have sufficient information to rely solely on our own volatility. In prior years we relied only on volatility from peer companies. The volatility is calculated over a period of time commensurate with the expected term for the options granted.
Expected Term (in Years). The expected term of a stock option is the period of time for which the option is expected to be outstanding. We have a large number of options outstanding. We use historical exercise and option expiration data to estimate the expected term assumption for the Black-Scholes grant-date valuation. We believe this historical data is currently the best estimate of the expected term of awards.
Risk-Free Interest Rate. We use the average yield on current U.S. Treasury instruments with terms that approximate the expected term of the stock options being valued.
Expected Dividend Yield. The expected dividend yield for all of our stock option grants is 0%, as we have not declared a cash dividend since inception, and do not expect to do so in the foreseeable future.
Forfeitures. The share-based compensation expense recognized has been reduced for estimated forfeitures. The estimated forfeiture rate is based on historical experience of our option plan, which we expect to continue at the current level, and any adjustments in the forfeiture rate in the future will result in a cumulative adjustment in the period that this estimate is changed. Ultimately, the total compensation expense recognized for any given share-based award over its vesting period will only be for those shares that actually vest.
Common Stock Fair Value. Due to the absence of an active market for our common stock prior to the IPO, the fair value of our common stock for purposes of determining the exercise price for stock option grants prior to the IPO was determined by our Board of Directors, with the assistance of our management, in good faith based on a number of objective and subjective factors including:
| |
| • | the prices of our convertible preferred stock sold to outside investors in arms-length transactions, and the rights, preferences and privileges of our convertible preferred stock as compared to those of our common stock, including the liquidation preference of our convertible preferred stock; |
| |
| • | our results of operations, financial position and the status of our research and development efforts; |
| |
| • | our stage of development and business strategy; |
| |
| • | the lack of liquidity of our private stock as a private company; |
| |
| • | valuations performed by an unrelated valuation specialist prepared in accordance with methodologies outlined in the AICPA Technical Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation”; |
| |
| • | the likelihood of achieving a liquidity event for the shares of our common stock and underlying stock options, such as an initial public offering, given prevailing market conditions; |
| |
| • | the material risks related to our business; and |
| |
| • | the composition of and changes to our management team. |
The fair value of the common stock was determined by the Board of Directors in good faith until November 16, 2011, when our common stock initiated trading on the NASDAQ Stock Market. Following that date, the fair value of the common stock has been the quoted market price as listed on the public exchange.
Fair Value Estimates
After taking into account all of the assumptions and estimates described in our application of the probability-weighted expected return method and the guideline public company method within the market approach, we determined the fair value of our common stock to be approximately $4.24 per share as of December 31, 2009, approximately $4.37 per share as of March 31, 2010, approximately $4.73 per share as of June 30, 2010, approximately $8.44 per share as of September 30, 2010 and approximately $10.02 per share as of December 31, 2010. The following table lists grants of options to purchase shares of common stock with GAAP measurement dates in 2009, 2010 and 2011.
|
| | | | | | | | | | | | | | | | | |
| Options Granted on Shares of Common Stock |
| Approval Date(1) | | GAAP Measurement Date(2) | | Number of shares | | Exercise price per share | | Common Stock values | | Intrinsic value per share |
| July 16, 2008 | | September 2, 2009 | | 173,304 |
| | $ | 2.10 |
| | $ | 2.21 |
| | $ | 0.11 |
|
| August 6, 2008 | | September 2, 2009 | | 233,327 |
| | 2.10 |
| | 2.21 |
| | 0.11 |
|
| May 13, 2009 | | September 2, 2009 | | 1,079,519 |
| | 2.10 |
| | 2.21 |
| | 0.11 |
|
| December 4, 2009 | | March 3, 2010 | | 812,617 |
| | 2.96 |
| | 4.37 |
| | 1.41 |
|
| March 3, 2010 | | June 2, 2010 | | 478,669 |
| | 3.07 |
| | 4.73 |
| | 1.66 |
|
| June 2, 2010 | | October 8, 2010 | | 6,189 |
| | 4.01 |
| | 8.44 |
| | 4.43 |
|
| October 8, 2010(3)(5) | | January 19, 2011 | | 12,615 |
| | 7.16 |
| | 10.02 |
| | 2.86 |
|
| December 9, 2010(4)(5) | | April 14, 2011 | | 76,185 |
| | 10.02 |
| | 10.02 |
| | — |
|
| January 19, 2011(5) | | January 19, 2011 | | 33,804 |
| | 10.02 |
| | 10.02 |
| | — |
|
| April 14, 2011(5) | | April 14, 2011 | | 11,902 |
| | 10.02 |
| | 10.02 |
| | — |
|
| April 14, 2011 | | November 10, 2011 | | 406,632 |
| | 7.00 |
| | 7.00 |
| | — |
|
| July 29, 2011 | | November 10, 2011 | | 15,951 |
| | 7.00 |
| | 7.00 |
| | — |
|
| October 7, 2011 | | November 10, 2011 | | 47,619 |
| | 7.00 |
| | 7.00 |
| | — |
|
| October 19, 2011 | | November 10, 2011 | | 23,809 |
| | $ | 7.00 |
| | $ | 7.00 |
| | $ | — |
|
_________________________________
| |
| (1) | The Approval Date is the date on which the Board of Directors authorized, and we had a legal obligation to issue, an option grant. |
| |
| (2) | The GAAP Measurement Date is the first date at which the number of shares and exercise price per share were known and the awards were communicated to all recipients. The GAAP Measurement Date occurs subsequent to the Approval Date due to the timing of the completion and approval of third-party valuation reports of our common stock. We utilize |
the common stock value from the most recent valuation report that has been completed and approved at the time of the GAAP Measurement Date. Due to the significance of the number of option grants on September 2, 2009, management also obtained a third-party valuation on that date.
| |
| (3) | The options were granted on October 8, 2010 . The GAAP Measurement Date did not occur until January 19, 2011, which was the date the exercise price per share was determined for accounting purposes based on completion and approval of the September 30, 2010 common stock valuation report and the awards were communicated to all recipients. |
| |
| (4) | The options were granted on December 9, 2010. The GAAP Measurement Date did not occur until April 14, 2011, which was the date the exercise price per share based on completion and approval of the December 31, 2010 common stock valuation report and the awards were communicated to all recipients. We do not believe the fair value of our common stock on April 14, 2011 was materially different than the value at December 31, 2010; therefore the December 31, 2010 value was used to measure the stock option award. |
| |
| (5) | We do not believe the fair values of our common stock on January 19, 2011 or April 14, 2011 was materially different than the value at December 31, 2010; therefore the December 31, 2010 value was used to measure the stock option award. |
Based on the December 31, 2013 price of $22.01 per share, the intrinsic value of stock options outstanding at December 31, 2013, was $72.3 million, of which $57.9 million and $14.4 million related to stock options that were vested and unvested, respectively, at that date.
Results of Operations
Comparison of the Years Ended December 31, 2013 and 2012
Revenues. Revenues for the year ended December 31, 2013 were $1.1 million, decreasing from $1.7 million for the same period in 2012. The decrease in revenue of $594,000 was due to a decrease in billings by BPS under various DOD contracts and NIH grants.
Research and Development Expenses. Research and development expenses for the year ended December 31, 2013 were $22.7 million, increasing from $17.8 million for the same period in 2012. The $4.9 million increase was due to a $2.3 million increase in outside clinical and other expenses including contract development costs for NLG919, contract manufacturing costs for indoximod, consulting fees, and direct development expenses for our clinical trial activities, accompanied by a $1.6 million increase in personnel-related expenses due to increased staffing levels and compensation increases and a $914,000 increase in equipment, supplies and occupancy costs to support our expanding clinical trials.
General and Administrative Expenses. General and administrative expenses for the year ended December 31, 2013 were $9.5 million, increasing from $7.1 million for the same period in 2012. The $2.4 million increase was due to a $1.3 million increase in other costs including legal and consulting fees, travel, dues, subscription and licensing fees, accompanied by a $1.1 million increase in personnel-related expenses due to increased staffing levels and compensation increases and a $21,000 increase in equipment, supplies and occupancy costs.
Income tax expense. Income tax expense for the year ended December 31, 2013 was $130,000 increasing from $0 for the same period in 2012. The increase was due to our Federal alternative minimum tax liability incurred in 2013.
Net Loss. Net loss for the year ended December 31, 2013 was $31.2 million, increasing from $23.3 million for the same period in 2012 due to a $4.9 million increase in research and development expense and a $2.4 million increase in general and administrative expense. The weighted average common shares outstanding for 2013 were 25.3 million, resulting in a loss per share of $1.23, as compared to 20.8 million and $1.12 per share for 2012. The increase in the number of weighted average common shares outstanding was attributable to shares issued in our public offering in February 2013 and, to a lesser extent, shares issued in our ATM Offering during the fourth quarter of 2013.
Comparison of the Years Ended December 31, 2012 and 2011
Revenues. Revenues for the twelve months ended December 31, 2012 were $1.7 million, decreasing from $1.9 million for the same period in 2011. The decrease in revenue of $185,000 was due to a decrease in billings and by BPS under various DOD contracts and NIH grants.
Research and Development Expenses. Research and development expenses for the twelve months ended December 31, 2012 were $17.8 million, increasing from $14.3 million for the same period in 2011. The $3.6 million increase was due to a $2.1 million increase in outside clinical and other expenses including contract development costs for NLG919, contract manufacturing
costs for indoximod and direct development expenses for our clinical trial activities, accompanied by a $910,000 increase in personnel-related expenses due to increased staffing levels and compensation increases and a $611,000 increase in equipment, supplies and occupancy costs to support our expanding clinical trials.
General and Administrative Expenses. General and administrative expenses for the twelve months ended December 31, 2012 were $7.1 million, decreasing from $5.7 million for the same period in 2011. The $1.4 million increase was due to a $986,000 increase in other costs including consulting fees, director's and officer's insurance and software subscription fees, accompanied by a $423,000 increase in personnel-related expenses due to increased staffing levels and compensation increases and a $19,000 increase in equipment, supplies and occupancy costs.
Net Loss. Net loss for the year ended December 31, 2012 was $23.3 million, increasing from $18.1 million for the same period in 2011 due to a $3.6 million increase in research and development expense and a $1.4 million increase in general and administrative expense. The weighted average common shares outstanding for 2012 were 20.8 million, resulting in a loss per share of $1.12, as compared to 6.1 million and $2.98 per share for 2011. The increase in the number of weighted average common shares outstanding was primarily attributable to shares issued in our public offering in November 2011, and the related conversion of all of our outstanding preferred stock to common stock.
Liquidity and Capital Resources
Before our IPO, we funded our operations principally through the private placement of equity securities, debt financing and interest income. As of December 31, 2013, we have received proceeds, net of offering costs, of $76.3 million from the issuance of convertible preferred stock, including $7.5 million from the sale of 1.5 million shares of Series D preferred stock in July 2009, $30.0 million from the sale of 6.0 million shares of Series C preferred stock in during the course of 2008 and 2009, and $21.4 million from the sale of 684,624 shares of Series E preferred stock during the course of 2010 and the first half of 2011, of which $8.6 million was issued to acquire the minority interest in BPS.
Since our IPO, we have funded our operations principally through public offerings of common stock. On November 16, 2011, we received proceeds, net of offering costs, of $37.6 million from the issuance of 6,200,000 shares of common stock in our IPO. On February 4, 2013, we received proceeds, net of offering costs, of $49.0 million from the issuance of 4,600,000 shares of common stock in our follow-on offering. As of December 31, 2013, we had sold 816,621 shares of common stock in our ATM Offering, raising a total of $17.4 million in net proceeds. Subsequent to December 31, 2013 and through February 13, 2014, we sold an additional 1,017,217 shares of common stock under the ATM Offering raising an additional $27.6 million in net proceeds.
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
Sources and Uses of Cash (in thousands) |
| | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2013 | | 2012 | | 2011 |
| Net cash used in development activities | | $ | (25,818 | ) | | $ | (20,471 | ) | | $ | (12,949 | ) |
| Net cash used in investing activities | | (141 | ) | | (277 | ) | | (502 | ) |
| Net cash provided by financing activities | | 67,000 |
| | 1,508 |
| | 42,369 |
|
| Net increase (decrease) in cash and cash equivalents | | $ | 41,041 |
| | $ | (19,240 | ) | | $ | 28,918 |
|
For the years ended December 31, 2013, 2012 and 2011, we used cash of $25.8 million, $20.5 million and $12.9 million, respectively, for our development activities. The use of cash in these periods primarily resulted from our net losses adjusted for non-cash items and changes in operating assets and liabilities.
For the years ended December 31, 2013, 2012 and 2011, our investing activities used cash of $141,000, $277,000 and $502,000, respectively. The cash used by investing activities in the year ended December 31, 2013 was due to $1.4 million in purchases of property and equipment offset by the combined net sale of investments for $1.2 million. The cash used by investing activities in the year ended December 31, 2012 was due to $1.3 million in purchases of property and equipment offset by the combined net sale of investments for $1.0 million. The cash used by investing activities in the year ended December 31, 2011 was due to $281,000 in purchases of property and equipment accompanied by the combined net purchase of investments for $221,000.
For the years ended December 31, 2013, 2012 and 2011, our financing activities provided $67.0 million, $1.5 million and $42.4 million, respectively. The cash provided by financing activities in the year ended December 31, 2013 was primarily due to the sale and issuance of common stock for net proceeds of $67.2 million offset by payments on long-term obligations of $215,000. The cash provided by financing activities in the year ended December 31, 2012 was primarily due to the sale and issuance of common stock for net proceeds of $1.3 million accompanied by proceeds on notes payable of $456,000 and offset by payments on long-term obligations of $229,000. The cash provided by financing activities in the year ended December 31, 2011 was primarily due to the sale and issuance of common stock in our IPO for net proceeds of $37.6 million accompanied by the sale and issuance of Series E Preferred stock for net proceeds of $5.0 million offset by payments on long-term financing obligations of $218,000.
Obligations under our Royalty and Loan Agreements
March 2012 Iowa Economic Development Authority Loan
In March 2005, we entered into a $6.0 million forgivable loan agreement with the Iowa Department of Economic Development, or the IDED. Under the agreement, in the absence of default, there were no principal or interest payments due until the completion date for the project. The agreement provided us with financial assistance for research and product development activities at our Iowa State University Research Park facility. Additionally, under the agreement, we were obligated to pay a minimum of 0.25% royalties on all gross revenues of any products that we bring to market with a cumulative maximum royalty amount due of $3.2 million. Substantially all of our assets were pledged to secure this loan. This loan was converted into a royalty obligation under the terms of a settlement agreement entered into on March 26, 2012, or the IEDA Agreement, with the Iowa Economic Development Authority, or the IEDA, as successor in interest to the IDED.
Under the terms of the IEDA Agreement, the forgivable loan agreement between us and IEDA (as successor to IDED) was terminated and we were thereby released from the forgivable loan agreement's job creation, project expenditure, royalty and other requirements in exchange for agreeing to pay a royalty of 0.50% on all gross revenues of any products that we bring to market, with a cumulative maximum royalty obligation due of $6.8 million. Additionally, under the IEDA Agreement, the IEDA released its security interest in our assets. We are obligated to maintain our business substantially in the State of Iowa until the royalty obligation under the IEDA Agreement is satisfied.
September 2007 IDED High Quality Job Creation Program Tax Credit
In September 2007, we entered into a master contract and associated funding agreement, or the HQJC Agreement, with the IDED under its high quality job creation program. Under the HQJC Agreement, as amended, we received a tax credit of $414,000, which was refunded to us between March 2006 and October 2009. We fulfilled all of the job creation and investment requirements of the HQJC Agreement, as amended, on March 18, 2013.
2009 and 2012 Iowa State University Research Park Loans
In 2009, the Company executed a promissory note in favor of Iowa State University Research Park, or ISURP, in an original principal amount of $800,000, which is due in monthly installments through March 2018. The note represents amounts owed by the Company to ISURP for certain improvements that were made to facilities the Company leases from ISURP. The principal and interest owed under the note is amortized over an eight-year period. Interest is payable monthly under this promissory note, initially at a rate of 3.0% per annum and increasing to 5.0% per annum after five years from the date the improvements were completed. ISURP may accelerate all amounts owed under the note upon an event of default, including the Company’s uncured material breach of the terms of the note or the lease or upon early termination of the lease. In the event of a default under the note, amounts owed under the note will bear interest at 8.0% per annum. The balance outstanding under the 2009 note was $449,000 and $546,000 at December 31, 2013 and December 31, 2012, respectively.
In 2012, the Company executed a promissory note in favor of ISURP in an original principal amount of $456,000, which is due in monthly installments through September, 2020. The note represents amounts owed by the Company to ISURP for certain additional improvements that were made to facilities the Company leases from ISURP. The principal and interest owed under the note is amortized over an eight-year period. Interest is payable monthly under this promissory note, initially at a rate of 3.0% per annum and increasing to 5.0% per annum after five years from the date the improvements were completed. ISURP may accelerate all amounts owed under the note upon an event of default, including the Company’s uncured material breach of the terms of the note or the lease or upon early termination of the lease. In the event of a default under the note, amounts owed under the note will bear interest at 8.0% per annum. The balance outstanding under the 2012 note was $392,000 and $443,000 at December 31, 2013 and December 31, 2012, respectively.
March 2010 City of Ames Forgivable Loan
In March 2010, we entered into a $400,000 forgivable loan agreement with the City of Ames, Iowa and the Ames Chamber of Commerce, jointly, as lenders. The project provides us with financial assistance to construct new facilities within the Ames city limits. In the absence of a default, there are no principal or interest payments due until the expected completion date for the project, which is March 10, 2015.
The project calls for our creating or retaining at least 70 full-time jobs located in Ames, Iowa as of March 10, 2012 and the creation or maintenance of at least 150 full-time positions located in Ames, Iowa as of March 10, 2015. The agreement also calls for our entering into a five-year building lease with the option for extension for an additional five years of not less than 20,000 square feet within the corporate limits of the City of Ames by March 10, 2015. If, as of March 10, 2015, we have fulfilled the terms of the loan agreement, the loan will be forgiven. If on March 10, 2015, we have failed to create or retain at least 150 full-time jobs in Ames, Iowa, respectively, we will be required to repay approximately $3,100 per job not created or retained following such date. As of December 31, 2013, we had created or retained an aggregate of 102 full-time jobs in Ames, Iowa. As of December 31, 2013, $300,000 of the total $400,000 forgivable loan was advanced to us with the final $100,000 pending certification to the City of Ames regarding the creation of a threshold level of jobs. In the event of default, including failure to repay any amounts under the loan when due, we will be required to repay the note, including 6.5% interest per annum, beginning at the date of default.
Operating Capital Requirements
We anticipate that we will continue to generate significant operating losses for the next several years as we incur expenses related to the research and development of our HyperAcute immunotherapy and IDO pathway inhibitor product candidates, build commercial capabilities and expand our corporate infrastructure. Including the funds received on February 4, 2013 from our follow-on offering and the funds received from our ATM Offering, we believe that we have sufficient cash and cash equivalents and certificates of deposit to fund our operations through at least the end of 2014.
We may seek to sell additional equity or debt securities or obtain a credit facility if our available cash and cash equivalents are insufficient to satisfy our liquidity requirements or if we develop additional opportunities to do so. The sale of additional equity and debt securities may result in additional dilution to our shareholders. If we raise additional funds through the issuance of debt securities or preferred stock, these securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we were unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could harm our business.
Because of the numerous risks and uncertainties associated with research, development and commercialization of biopharmaceutical products, we are unable to estimate the exact amounts of our working capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| |
| • | the scope, progress, results and costs of clinical trials for our product candidates, and discovery and development activities related to new product candidates; |
| |
| • | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; |
| |
| • | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| |
| • | the cost of manufacturing our product candidates and any products we commercialize; |
| |
| • | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
| |
| • | whether, and to what extent, we are required to repay our outstanding government provided loans; |
| |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
| |
| • | the timing, receipt and amount of sales of, or royalties on, our future products, if any. |
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at December 31, 2013:
Contractual Obligations Due (in thousands) |
| | | | | | | | | | | | | | | | | | | | |
| | | Total | | Less than 1 Year | | 1 to 3 Years | | 3 to 5 Years | | More than 5 Years |
| Short and long-term debt (including interest) (1) | | $ | 7,228 |
| | $ | 177 |
| | $ | 659 |
| | $ | 276 |
| | $ | 6,116 |
|
| Operating lease obligations | | 1,443 |
| | 610 |
| | 635 |
| | 198 |
| | — |
|
| Capital lease obligations | | 86 |
| | 38 |
| | 48 |
| | — |
| | — |
|
| Total contractual cash obligations | | $ | 8,757 |
| | $ | 825 |
| | $ | 1,342 |
| | $ | 474 |
| | $ | 6,116 |
|
(1) Short and long-term debt includes an accrued royalty obligation of $6.0 million for which the timing of payment is uncertain. See section “March 2005 Iowa Department of Economic Development Loan” above.
Under the license agreements described below under the heading “Financial Obligations Related to Licensing and Development—In-Licensing Agreements” we are obligated to make potential milestone payments as listed in the following table. These obligations are contingent upon achieving the applicable milestone event, the timing of which cannot presently be determined.
|
| | |
| Licensor | | Aggregate potential milestone payments |
| Drexel University | | $1 million per licensed product |
| | | |
| Lankenau Institute for Medical Research under the IDO-1 Agreement (1) | | $1.36 million per licensed product |
| | | |
| Lankenau Institute for Medical Research under the LIMR IDO-2 Agreement (1) | | $1.52 million per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement |
| | | |
| Lankenau Institute for Medical Research under the 2009 LIMR Agreement (1) | | $610,000 per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement or LIMR IDO-2 Agreement |
| | | |
| Georgia Health Sciences University | | $2.8 million per licensed product |
| | | |
| Regents of the University of California | | $285,000 per licensed product |
| | | |
| The Ohio State University | | $2.75 million for first licensed product |
| | | |
| Her Majesty the Queen in Right of Canada | | C$205,000 per licensed product |
_________________________________
(1) As defined below under the heading “Financial Obligations Related to Licensing and Development—In-Licensing Agreements”.
We have made payments of approximately $88,000, $255,000, $89,000, and $2.9 million under all of the in-licensing agreements for the years ended December 31, 2013, 2012, 2011, and from inception, respectively, which are listed below under the heading “Financial Obligations Related to Licensing and Development—In-licensing Agreements”.
Financial Obligations Related to Licensing and Development
In-Licensing Agreements
We are subject to a number of licensing agreements with respect to certain of the technologies that underlie our intellectual property. Unless otherwise noted, these agreements typically provide that we have exclusive rights to the use and sublicensing of the technologies in question for the duration of the intellectual property patent protection in question, subject to us meeting our
financial and other contractual obligations under the agreements. Certain of the key licensing agreements with significant financial obligations include the following:
Central Iowa Health Systems. We are a party to a license agreement, or the CIHS Agreement, dated August 2, 2001, as amended December 30, 2013, with the Central Iowa Health System, or CIHS. The CIHS Agreement grants to us an exclusive, worldwide license to make, have made, use, import, sell and offer for sale products that are covered by certain CIHS patent rights, proprietary information or know-how relating to our HyperAcute immunotherapy technology. In partial consideration of the license under the CIHS Agreement, we entered into a stock purchase agreement with CIHS, under which we issued to CIHS shares of our common stock and granted CIHS certain rights related to ownership of such shares.
In addition, we must reimburse CIHS for out-of-pocket costs incurred for patent prosecution and maintenance. If we or our sublicensees commercialize a licensed product, we also have the obligation to pay CIHS royalties as a low single-digit percentage of net sales of the licensed product, subject to annual minimum royalties and a reduction for any royalty payments we must make to third parties. If we grant a sublicense under the licenses granted by CIHS, we must pay to CIHS, in addition to the royalties described above, a percentage of certain consideration paid by the sublicensee to us. Under the CIHS Agreement, we must use commercially reasonable efforts to develop and commercialize licensed products, to obtain necessary regulatory approvals and to launch and market such products in specified markets. We have no obligation to pay fees to CIHS as a result of payments between us and our affiliates.
Drexel University. We are party to a license agreement, or the Drexel Agreement, dated October 13, 2004 with Drexel University, or Drexel. The Drexel Agreement grants us, and our affiliates, an exclusive, worldwide license, under specified Drexel patent rights relating to compositions and methods for vaccines based on alpha-Gal epitopes, to make, have made, use, import, sell and offer for sale vaccine products that are covered by such patent rights, or that use related Drexel technical information, for use in the diagnosis and treatment of cancer, viral and other infectious disease.
In consideration of our license under the Drexel Agreement, we have paid and are obligated to continue to pay specified license fees, potential milestone payments in an aggregate amount up to approximately $1 million for each licensed product, annual license maintenance fees, reimbursement of patent prosecution costs, and royalty payments as a low single-digit percentage of “net sales” of any licensed product that is commercialized, subject to minimum royalty payments. Royalty rates vary depending on the type of licensed product, the territory where it is sold and whether the licensed product is combined with other technologies. In addition, if we grant a sublicense under the license granted by Drexel, we must pay Drexel a percentage of the consideration paid by the sublicensee to us. In accordance with a development plan included in the Drexel Agreement, we are obligated to use commercially reasonable efforts to develop and market products covered by the license as soon as practicable.
Lankenau Institute for Medical Research—IDO-1. We are a party to a license agreement dated July 7, 2005, as amended May 22, 2006 and September 11, 2007, or the IDO-1 Agreement, with Lankenau Institute for Medical Research, or LIMR. The IDO-1 Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to inhibitors of IDO and related LIMR technology, to make, have made, use, and sell products that are covered by such patent rights for use in the field of animal and human therapeutics and diagnostics.
In consideration of the license grant, we are obligated to pay to LIMR specified license fees, annual license maintenance fees, reimbursement of past patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $1.36 million for each licensed product, and royalties as a low single-digit percentage of net sales of the licensed products if a licensed product is commercialized. In addition, if we grant a sublicense under the IDO-1 Agreement, we must to pay to LIMR a percentage of the consideration received by us from the sublicensee. Under the IDO-1 Agreement, we are obligated to use commercially reasonable efforts to develop and market the licensed products, and to achieve certain milestones by agreed-upon deadlines.
LIMR—IDO-2. We are a party to a license agreement, or the LIMR IDO-2 Agreement, executed December 21, 2007 with LIMR. The LIMR IDO-2 Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to inhibitors of the target IDO and under related LIMR know-how or technology, to make, have made, use, import, sell and offer for sale products and services that are covered by such patent rights, for all uses.
In consideration of the license grant, we have paid to LIMR an upfront license fee and annual license maintenance fees, and are obligated to pay LIMR annual license maintenance fees, potential milestone payments in an aggregate amount up to approximately $1.52 million per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement, and, if a licensed product is commercialized, royalties as a low single-digit percentage of “net sales” of the licensed product, subject to reduction for our royalty payments to third parties. In addition, if we grant a sublicense under the licenses granted by
LIMR, we must pay to LIMR a percentage of the consideration paid by the sublicensee to us. Under the LIMR IDO-2 Agreement, we have agreed to use our commercially reasonable efforts to develop and exploit products covered by the license.
2009 LIMR Exclusive License Agreement. We are a party to a license agreement, or the 2009 LIMR Agreement, dated April 23, 2009 with LIMR. The 2009 LIMR Agreement grants us an exclusive, worldwide license, under specified LIMR patent rights relating to IDO inhibitors, and under related LIMR know-how or technology, to make, have made, use, import, sell and offer for sale products and services that are covered by such patent rights, for all uses. In consideration of such license grant, we are obligated to pay LIMR potential milestone payments in an aggregate amount up to approximately $610,000 per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement or LIMR IDO-2 Agreement, and royalties as a low single-digit percentage of “net sales” of the licensed product, subject to reduction for our royalty payments to third parties and to LIMR under the IDO-1 Agreement or LIMR IDO-2 Agreement. In addition, if we grant a sublicense under the licenses granted by LIMR, we must pay to LIMR a percentage of the consideration paid by the sublicensee to us.
Georgia Health Sciences University. We are a party to a License Agreement dated September 13, 2005, or the GHS Agreement, with Georgia Health Sciences University, or GHS, which was formerly known as the Medical College of Georgia Research Institute. The GHS Agreement was amended on April 27, 2006 and February 13, 2007. The GHS Agreement grants us, including our affiliates, an exclusive, worldwide license, under specified GHS patent rights and related technology to make, have made, use, import, sell and offer for sale products that are covered by licensed patent rights or incorporates or uses licensed technology in all medical applications.
In consideration of such license grant, we are obligated to pay to GHS specified license fees (including issuing shares of our common stock), annual license maintenance fees, reimbursement of patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $2.8 million per licensed product, and royalties as a single-digit percentage of net sales of the licensed products, subject to minimum royalty payments and royalty rates depending on the type of license product. In addition, if we grant a sublicense under the license granted by GHS, we must pay to GHS a percentage of the consideration we receive from the sublicensee. Under the agreement, we are obligated to make certain investments toward the further development of licensed products within specified time periods.
Regents of the University of California License Agreement. BPS is a party to a license agreement dated July 29, 2008, or the California License, with the Regents of the University of California, or California. The California License grants BPS an exclusive, worldwide license, under specified California patent rights relating to technology based on yellow fever virus, to make, use, import, sell and offer for sale products that are covered by licensed patent rights in the field of human healthcare.
In consideration of the license grant, BPS must pay to California a specified license issue fee, annual license maintenance fees, patent prosecution costs, potential milestone payments in an aggregate amount up to approximately $285,000 per licensed product, and royalties as a low single-digit percentage of net sales of the licensed product, which royalty rate varies depending on the territory. In addition, if BPS grants a sublicense under the licenses granted by California, BPS may be required to pay to California a percentage of certain consideration BPS receives from the sublicensee. BPS is obligated to use commercially reasonable efforts to develop and market the licensed products, and to achieve certain milestones by agreed-upon deadlines.
The Ohio State University License Agreement. We are a party to a license agreement dated June 28, 2013 with The Ohio State University, or OSU. This agreement grants us exclusive rights in the United States and its territories, in all fields of use, to patents related to pharmaceutical compositions and vaccines comprising antigens associated with L-Rhamnose and Forssman epitopes including patent application US 13/463,420, which is co-owned by us and OSU.
In consideration of the license grant, we were obligated to pay OSU specified license fees and are obligated to pay to OSU specified annual license maintenance fees, patent prosecution costs and maintenance costs, potential milestone payments in an aggregate amount up to approximately $2.75 million for the first licensed product, and royalties as a low single-digit percentage of net sales of the licensed products if a licensed product is commercialized, with minimum royalties for the first three years in which a licensed product generates revenue. In addition, if we grant a sublicense under this agreement, we must also pay to OSU a percentage of certain consideration received by us from the sublicensee. Under this agreement, we are obligated to use commercially reasonable efforts to develop and market the licensed products and to use commercially reasonable effort to achieve certain milestones by agreed-upon deadlines.
Her Majesty the Queen in Right of Canada License Agreement. BPS is a party to a license agreement dated May 4, 2010, or the Canada License, with the Her Majesty the Queen in Right of Canada, or Canada. The Canada License grants BPS a worldwide, personal, non-transferable, sole, revocable, royalty-bearing license for commercialization of specified Canada patent rights relating to technology based on rVSV.
In consideration of the license grant, BPS must pay to Canada a specified patent and signing fees, annual license maintenance fees, patent prosecution costs, potential milestone payments in an aggregate amount up to approximately C$205,000 per licensed product, and royalties as a low single-digit percentage of the sales price of the licensed products sold by BPS, which royalty rate varies depending on the type of licensed product. In addition, if BPS grants a sublicense under the licenses granted by Canada, BPS is required to pay to Canada a percentage of certain consideration BPS receives from the sublicensee. BPS is obligated to use commercially reasonable efforts to develop and market the licensed products.
Bresagen Patent License Agreement. We are a party to a license agreement, or the Bresagen Agreement, dated March 1, 2006 with Bresagen Xenograft Marketing Ltd, or Bresagen. The Bresagen Agreement grants us a non-exclusive, non-sublicensable license to specified Bresagen patent rights for use in testing microbial and cancer vaccines in the U.S. In consideration of such license grant, we are obligated to pay Bresagen an up front license fee and an annual license fee.
Collaborative Agreements with Medical Institutions
We have entered into numerous agreements with various medical institutions for the performance of clinical trials for various products. They typically call for the payment of fees by us for the performance of the clinical trials and the maintenance of confidentiality as to the associated technology.
We entered into a formal Cooperative Research and Development Agreement, or CRADA, with the National Cancer Institute, or NCI, on March 27, 2012 to continue our collaboration on the development of indoximod as an anti-cancer agent. Upon execution of this agreement, we acquired rights to any inventions or raw data generated by the NCI pursuant to the informal joint research under the Letter of Intent dating retroactively to May 23, 2007.
In consideration of the CRADA, we paid an upfront services fee of $60,000 and were obligated to pay an annual fee of $20,000 during the term of the CRADA. We were also obligated to provide funding to support the development of indoximod in an amount up to $250,000 upon FDA approval of a New Drug Application, or NDA, covering indoximod and an additional $250,000 upon completion of product launch of a pharmaceutical product containing indoximod under such approved NDA. On October 31, 2013, we terminated this CRADA in accordance with its terms.
Patents and Trademarks
As noted above, we presently have an extensive portfolio of patents and patent applications (and certain trademark registrations) with the United States Patent and Trademark Office. During the years ended December 31, 2013, 2012 and 2011, we incurred expenses related to the filing, maintenance, and initiation of our patent portfolio of $636,000, $285,000 and $410,000, respectively, for an increase of 123% for the 2013 period as compared to the same period in 2012, and a decrease of 31% for the 2012 period as compared to the same period in 2011. We anticipate these expenses will decrease in 2014 compared to 2013. Increased costs in 2013 compared to 2012 were due to multiple international filings and activity on patent families related to IDO inhibitors.
BioProtection Systems Corporation
We formed BioProtection Systems Corporation, or BPS, as a subsidiary in 2005 to research, develop and commercialize vaccines to control the spread of emerging lethal viruses and infectious diseases, improve the efficacy of existing vaccines and provide rapid response prophylactic and therapeutic treatment for pathogens that might be targeted to the human population through acts of bioterrorism. At December 31, 2010, we owned shares of BPS Series A common stock representing approximately 64% of BPS’s common stock on an as-converted basis, assuming conversion into BPS Series B common stock of all outstanding BPS Series A and BPS Series B preferred stock. On December 1, 2010, we entered into an agreement to acquire all of the noncontrolling interest in BPS, as described in more detail below.
Acquisition of BioProtection Systems Corporation
On January 7, 2011, we acquired all of the minority interest in BPS, by merging a newly-formed subsidiary of ours with BPS, with BPS as the surviving corporation. In connection with this transaction, we issued an aggregate of 276,304 shares of our Series E preferred stock to the former holders of BPS Series B common stock, BPS Series A preferred stock and BPS Series B preferred stock (other than us). As a result of this transaction, BPS became a wholly-owned subsidiary of us and our note was
converted into Series B preferred stock of BPS. All options to purchase shares of BPS stock became options to purchase a total of 50,513 shares of our common stock.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Recent Accounting Pronouncements
None considered applicable or likely to have a material impact.
Basic and Diluted Net Loss Attributable to Common Stockholders per Common Share
We calculated net loss per share in accordance with Accounting Standards Codification (ASC) 260, Earnings per Share. We determined that our preferred stock outstanding prior to the IPO represented participating securities in accordance with ASC 260. We had a net loss for all periods presented; accordingly, the inclusion of common stock options would be anti-dilutive.
Dilutive common stock equivalents would include the dilutive effect of convertible securities, common stock options, warrants for convertible securities and warrants for common stock equivalents. Potentially dilutive common stock equivalents total approximately 4.5 million, 3.8 million, and 3.5 million as of December 31, 2013, 2012 and 2011, respectively. Potentially dilutive common stock equivalents were excluded from the diluted earnings per share denominator for all periods because of their anti-dilutive effect. Therefore, the weighted average shares used to calculate both basic and diluted earnings per share are the same.
| |
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to market risk related to changes in interest rates. As of December 31, 2013 and December 31, 2012, we had cash and cash equivalents and certificates of deposit of $61.5 million and $21.7 million, respectively, consisting of money market funds, treasury bills and bank certificates of deposit. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term marketable securities. Our certificates of deposit are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% change in interest rates would not have a material effect on the fair market value of our portfolio. We expect to have the ability to hold our certificates of deposit until maturity, and therefore we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investments. We do not currently have any auction rate securities.
Our long-term debt and our capital lease obligations bear interest at fixed rates. Any change in interest rates would have an immaterial (or no) impact on our financial statements.
| |
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The report of KPMG LLP, our independent registered public accounting firm, the financial statements of us and our consolidated subsidiaries and the notes thereto are included beginning on page F-1.
| |
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None
| |
| Item 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures.
We carried out an evaluation required by the Securities Exchange Act of 1934, as amended, or the Exchange Act, under the supervision and with the participation of our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13a-15(e) of the Exchange Act, as of December 31, 2013. Based on this evaluation, our chief executive officer and chief financial officer concluded that, as of December 31, 2013, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the SEC and to provide reasonable assurance that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosures.
Management’s Report on Internal Control over Financial Reporting.
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) of the Exchange Act. Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2013 based on criteria established in Internal Control — Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission, or the COSO Framework. As a result of this assessment, management concluded that, as of December 31, 2013, our internal control over financial reporting was effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of the consolidated financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance
with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of our internal control over financial reporting as of December 31, 2013, was audited by our independent registered public accounting firm, KPMG LLP, as stated in its report, which is included in this filing on page F-1.
Changes in Internal Control Over Financial Reporting.
We implemented a new enterprise resource planning, or ERP, system in the third quarter of 2013. As of December 31, 2013, the ERP system was used for certain manufacturing and finance purposes and we expect the ERP system to be used for other manufacturing and finance functions in 2014. The new ERP system did not eliminate any existing controls over financial reporting. In addition, the ERP system can support internal controls over some items that our previous accounting system did not support.
With the exception of the new ERP system, there were no changes in our internal control over financial reporting during the period covered by this Annual Report on Form 10-K that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Controls.
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all internal control issues and instances of fraud, if any, have been detected.
| |
| Item 9B. | OTHER INFORMATION |
None.
PART III
| |
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item concerning our directors and nominees is incorporated by reference to our definitive Proxy Statement for our 2014 Annual Meeting of Stockholders, or the 2014 Proxy Statement.
| |
| Item 11. | EXECUTIVE COMPENSATION |
The information required by this item concerning the compensation of our directors and executive officers is incorporated by reference to the 2014 Proxy Statement.
| |
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item concerning securities authorized under our equity compensation plans and security ownership of certain beneficial owners is incorporated by reference to our 2014 Proxy Statement.
Securities Authorized For Issuance Under Equity Compensation Plans
We maintain our 2009 Equity Incentive Plan, 2010 Non-Employee Directors’ Stock Award Plan and 2010 Employee Stock Purchase Plan, each of which was approved by the Company’s security holders, pursuant to which we may grant equity awards to eligible persons.
The following table gives information about equity awards under the foregoing plans as of December 31, 2013:
|
| | | | | | |
| Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | |
| Equity compensation plans approved by security holders | 4,486,564 |
| $5.89 | 1,234,358 |
| (1)(2) |
| Equity compensation plans not approved by security holders | — |
| $0.00 | — |
| |
| Total | 4,486,564 |
| $5.89 | 1,234,358 |
| |
(1) The 2009 Equity Incentive Plan incorporates an evergreen formula pursuant to which, on each January 1st, the aggregate number of shares reserved for issuance under the plan will increase by a number equal to 4% of the outstanding shares on December 31st of the preceding calendar year, or such lesser amount (or no shares) as determined by our Board.
(2) Of these shares, as of December 31, 2013, 671,348 shares remained available under the 2009 Equity Incentive Plan, 266,202 shares remained available under the 2010 Non-Employee Directors’ Stock Award Plan and 296,808 shares remained available under the 2010 Employee Stock Purchase Plan.
| |
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this item concerning transactions with related persons is incorporated by reference to our 2014 Proxy Statement.
| |
| Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this item concerning fees and services of accountants and auditors is incorporated by reference to our 2014 Proxy Statement.
PART IV
| |
| Item 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
Item 15(a)
(1) Financial Statements
(2) Financial Statement Schedules
Schedules have been omitted because of the absence of conditions under which they are required or because the required information is included in the financial statements or notes thereto beginning on page F-1 of this report.
(3) Exhibits
The following exhibits are filed with this form 10-K or incorporated herein by reference to the document set forth next to the exhibit listed below. Where so indicated, exhibits that were previously filed are incorporated by reference.
|
| | | | | | |
| | | | Incorporated By Reference | |
| Exhibit Number | | Description | Form | Filing Date | Number | Filed Herewith |
| 3.1 | | Amended and Restated Certificate of Incorporation filed on November 16, 2011 | 8-K | 11/18/2011 | 3.1 | |
| 3.2 | | Certificate of Amendment to Restated Certificate of Incorporation filed on May 10, 2013 | 8-K | 5/14/2013 | 3.1 | |
| 3.3 | | Amended and Restated Bylaws | 8-K | 11/18/2011 | 3.2 | |
| 4.1 | | Form of the Registrant's Common Stock Certificate | S-1/A | 10/26/2011 | 4.1 | |
| 4.2 | | Reference is made to Exhibits 3.1, 3.2 and 3.3 | | | | |
| 4.3 | | Amended and Restated Investor Rights Agreement by and between the Company and certain holders of the Company's capital stock dated as of December 1, 2010 | 10-Q | 5/10/2012 | 4.3 | |
| 10.2 | † | Form of Indemnity Agreement by and between the Registrant and its directors and executive officers | S-1/A | 11/8/2011 | 10.11 | |
| 10.3 | † | 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.2 | |
| 10.4.1 | † | Form of Stock Option Agreement under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.3 | |
| 10.4.2 | † | Form of Stock Option Grant Notice under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.4 | |
| 10.4.3 | † | Form of Stock Bonus Agreement under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.5 | |
| 10.5 | † | Amended and Restated 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.6 | |
| 10.5.1 | † | Form of Stock Option Agreement under 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.7 | |
| 10.5.2 | † | Form of Stock Option Grant Notice under 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.8 | |
| 10.6 | † | 2010 Employee Stock Purchase Plan | 8-K | 5/14/2013 | 10.2 | |
| 10.7 | † | 2010 Non-Employee Directors' Stock Award Plan | 8-K | 5/14/2013 | 10.1 | |
|
| | | | | | |
| 10.8 | † | Employment Agreement, dated as of December 6, 2010, by and between the Registrant and Charles J. Link, Jr. | S-1 | 12/21/2010 | 10.12 | |
| 10.9 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Nicholas N. Vahanian | S-1 | 12/21/2010 | 10.13 | |
| 10.10 | † | Employment Agreement, dated as of June 26, 2008, by and between the Registrant and Gordon H. Link, Jr. | S-1 | 12/21/2010 | 10.14 | |
| 10.11 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Gordon H. Link, Jr. | S-1 | 12/21/2010 | 10.15 | |
| 10.12 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Kenneth Lynn | S-1 | 12/21/2010 | 10.16 | |
| 10.13 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and W. Jay Ramsey | S-1 | 12/21/2010 | 10.17 | |
| 10.14 | † | Form of Employee Proprietary Information and Inventions Agreement | S-1 | 12/21/2010 | 10.18 | |
| 10.15 | † | Promissory Note dated May 2, 2008 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.19 | |
| 10.16 | † | Promissory Note dated April 18, 2000 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.20 | |
| 10.17 | † | Promissory Note dated August 20, 2008 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.21 | |
| 10.18 | † | Promissory Note dated July 2008 by and between the Registrant and Gordon Link | S-1/A | 2/28/2011 | 10.22 | |
| 10.19 | † | Amendment Agreement dated July 1, 2010 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.23 | |
| 10.20 | † | Amendment Agreement dated July 1, 2010 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.24 | |
| 10.21 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.25 | |
| 10.22 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.26 | |
| 10.23 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Gordon Link | S-1/A | 2/28/2011 | 10.27 | |
| 10.24 | † | Acknowledgment Agreement dated November 24, 2010 by and between BioProtection Systems Corporation and Charles Link | S-1/A | 2/28/2011 | 10.28 | |
| 10.25 | † | Acknowledgment Agreement dated November 23, 2010 by and between BioProtection Systems Corporation and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.29 | |
| 10.26 | * | License Agreement dated July 7, 2005 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.30 | |
| 10.26.1 | * | First Amendment to License Agreement dated May 22, 2006 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.31 | |
| 10.26.2 | * | Second Amendment to License Agreement September 11, 2007 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.32 | |
| 10.27 | * | Exclusive License Agreement executed December 21, 2007 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.33 | |
| 10.28 | * | Exclusive License Agreement effective April 23, 2009 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.34 | |
| 10.29 | * | License Agreement dated October 13, 2004 by and between the Registrant and Drexel University | S-1/A | 11/8/2011 | 10.36 | |
| 10.30 | * | License Agreement dated August 2, 2001 by and between the Registrant and Central Iowa Health System | S-1/A | 11/8/2011 | 10.37 | |
| 10.31 | * | License Agreement dated September 13, 2005 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.46 | |
| 10.31.1 | * | First Amendment to License Agreement dated April 27, 2006 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.47 | |
| 10.31.2 | * | Second Amendment to License Agreement dated April 27, 2006 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.48 | |
| 10.31.3 | * | Third Amendment to License Agreement dated February 13, 2007 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.49 | |
|
| | | | | | |
| 10.32 | * | Patent License Agreement dated March 1, 2006 by and between the Registrant and Bresagen Xenograft Marketing Ltd. | S-1/A | 11/8/2011 | 10.50 | |
| 10.33 | * | Exclusive License Agreement dated July 29, 2008 by and between the Regents of the University of California and BioProtection Systems Corporation | S-1/A | 11/8/2011 | 10.66 | |
| 10.34 | * | Sole License Agreement executed May 4, 2010 by and between Her Majesty the Queen in Right of Canada and BioProtection Systems Corporation | S-1/A | 11/8/2011 | 10.67 | |
| 10.35 | * | Letter of Intent for Cooperative Research and Development Agreement (CRADA #2166) dated May 7, 2007 by and between the Registrant and National Cancer Institute | S-1/A | 11/8/2011 | 10.38 | |
| 10.35.1 | | Amendment No. 1 to Letter of Intent for CRADA #2166 dated January 17, 2008 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.39 | |
| 10.35.2 | | Amendment No. 2 to Letter of Intent for CRADA #2166 dated July 7, 2008 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.40 | |
| 10.35.3 | | Amendment No. 3 to Letter of Intent for CRADA #2166 dated March 24, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.41 | |
| 10.35.4 | | Amendment No. 4 to Letter of Intent for CRADA #2166 dated October 28, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.42 | |
| 10.35.5 | | Amendment No. 5 to Letter of Intent for CRADA #2166 dated December 16, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.43 | |
| 10.35.6 | | Amendment No. 6 to Letter of Intent for CRADA #2166 dated June 29, 2010 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.44 | |
| 10.35.7 | | Amendment No. 7 to Letter of Intent for CRADA #2166 dated November 26, 2010 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.45 | |
| 10.35.8 | | Amendment No. 8 to Letter of Intent for CRADA #2166 dated June 2, 2011 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.79 | |
| 10.36 | | Lease dated September 1, 2000 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.46 | |
| 10.37 | | Sublease Agreement effective February 1, 2001 by and between the Registrant and Iowa State Innovation System | S-1 | 12/21/2010 | 10.47 | |
| 10.38 | | Memorandum of Agreement dated December 6, 2005 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.48 | |
| 10.39 | | Memorandum of Agreement dated April 13, 2006 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.49 | |
| 10.40 | | Memorandum of Agreement dated February 20, 2008 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.50 | |
| 10.41 | | Memorandum of Agreement dated May 1, 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.51 | |
| 10.42 | | Memorandum of Agreement dated March 24, 2010 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.52 | |
| 10.43 | | Lease dated September 30, 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.53 | |
| 10.44 | | Lease dated August 10, 2005 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.82 | |
| 10.45 | | Memorandum of Agreement dated September 29, 2011 by and between the Registrant and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.84 | |
| 10.46 | | Memorandum of Agreement dated September 29, 2011 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.83 | |
| 10.47 | | Memorandum of Agreement dated November 14, 2011 by and between NewLink Genetics Corporation and Iowa State University Research Park Corporation | 8-K | 11/18/2011 | 10.1 | |
| 10.48 | | Promissory Note executed in 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.54 | |
| 10.49 | | Forgivable Loan Agreement dated March 10, 2010 by and between the Registrant and City of Ames, Iowa | S-1 | 12/21/2010 | 10.55 | |
| 10.50 | | Iowa Values Fund Agreement dated March 18, 2005 by and between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.56 | |
|
| | | | | | |
| 10.51 | | Master Contract dated December 29, 2005 by and between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.58 | |
| 10.52 | | Contract Amendment dated April 21, 2009 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.59 | |
| 10.53 | | Contract Amendment dated August 19, 2010 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.57 | |
| 10.54 | | Contract Amendment dated August 19, 2010 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.60 | |
| 10.55 | | Contract Amendment effective February 17, 2011 between the Registrant and Iowa Department of Economic Development | S-1/A | 9/14/2011 | 10.77 | |
| 10.56 | | Contract Amendment effective February 17, 2011 between the Registrant and Iowa Department of Economic Development | S-1/A | 9/14/2011 | 10.78 | |
| 10.57 | | Contract No. W911NF-08-C-0044 dated May 5, 2008 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.68 | |
| 10.57.1 | | Amendment to Contract No. W911NF-08-C-0044 dated February 12, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.69 | |
| 10.58 | * | Contract No. HDTRA1-09-C-0014 dated September 25, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 11/8/2011 | 10.70 | |
| 10.58.1 | | Amendment of Contract No. HDTRA1-09-C-0014 dated September 20, 2011 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 10/4/2011 | 10.80 | |
| 10.59 | | Contract No. W911NF-09-C-0072 dated July 31, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.71 | |
| 10.59.1 | | Amendment to Contract No. W911NF-09-C-0072 dated April 21, 2010 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.72 | |
| 10.60 | | Grant Number 5U01AI066327-05 issued August 26, 2009 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 2/28/2011 | 10.73 | |
| 10.61 | | Grant Number 1R43AI084350-01A1 issued April 6, 2010 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 2/28/2011 | 10.74 | |
| 10.62 | | Grant Number 5R43AI084350-02 issued March 24, 2011 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 10/4/2011 | 10.81 | |
| 10.63 | | Agreement and Plan of Merger dated December 1, 2010 by and between the Registrant, BPS Merger Sub, Inc., BioProtection Systems Corporation and BPS Stockholder Representative, LLC | S-1/A | 2/28/2011 | 10.75 | |
| 10.64 | | Certificate of Merger of BPS Merger Sub, Inc. into BioProtection Systems Corporation filed on January 7, 2011 | S-1/A | 2/28/2011 | 10.76 | |
| 10.65 | | Standard Design-Build Agreement dated November 14, 2011 by and between NewLink Genetics Corporation and Story Construction Co. | 8-K | 11/18/2011 | 10.2 | |
| 10.66 | | NewLink Genetics Corporation 401(k) Prototype Plan and Trust, effective as of January 1, 2005 | 8-K | 3/12/2012 | 10.2 | |
| 10.67 | | NewLink Genetics Corporation 401(k) Adoption Agreement, effective as of January 1, 2005 | 8-K | 3/12/2012 | 10.3 | |
| 10.68 | | Material Modification to the NewLink Genetics Corporation 401(k) Adoption Agreement, effective as of January 1, 2011 | 8-K | 3/12/2012 | 10.4 | |
| 10.69 | | Settlement Agreement with the Iowa Economic Development Authority, effective as of March 26, 2013 | 8-K | 3/28/2012 | 10.1 | |
| 10.70 | † | 2012 Target Bonus Awards | 8-K | 3/28/2012 | 10.2 | |
| 10.71 | * | Cooperative Research and Development Agreement between the Company and the National Cancer Institute, effective as of March 27, 2012 | 10-Q | 5/10/2012 | 10.6 | |
| 10.72 | | Letter of Resignation of Executive Vice President of Business Development, effective as of June 27, 2012 | 10-Q | 8/14/2012 | 10.2 | |
| 10.73 | † | Offer of Employment for Head of Business Development, effective as of July 26, 2012 | 10-Q | 8/14/2012 | 10.3 | |
| 10.74 | † | 2013 Salaries and Target Bonus Awards | 8-K | 11/7/2012 | 10.1 | |
|
| | | | | | |
| 10.75 | † | 2012 Named Executive Officer Bonus Awards | 8-K | 12/21/2012 | 10.1 | |
| 10.76 | | Underwriting Agreement, effective as of January 30, 2013 | 8-K | 1/31/2013 | 1.1 | |
| 10.77 | | Memorandum of Agreement dated May 7, 2012 by and between the Registrant and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.1 | |
| 10.78 | | Memorandum of Agreement dated May 7, 2012 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.2 | |
| 10.79 | | Memorandum of Agreement dated November 6, 2012 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.3 | |
| 10.80 | † | 2013 Target Bonus Awards | 8-K | 4/5/2013 | 10.1 | |
| 10.81 | | Memorandum of Agreement dated April 15, 2013 by and between the Registrant and Iowa State University Research Park Corporation | 10-Q | 5/8/2013 | 10.1 | |
| 10.82 | | Story Construction Contract | 10-Q | 8/8/2013 | 10.1 | |
| 10.83 | | Memorandum of Agreement; Addendum to the Lease Between ISU Research Park Corporation and NewLink Genetics Corporation Dated March 1, 2010 | 10-Q | 8/8/2013 | 10.2 | |
| 10.84 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Charles J. Link, Jr. | 8-K | 8/14/2013 | 10.1 | |
| 10.85 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Nicholas N. Vahanian | 8-K | 8/14/2013 | 10.2 | |
| 10.86 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Gordon H. Link, Jr. | 8-K | 8/14/2013 | 10.3 | |
| 10.87 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and W. Jay Ramsey | 8-K | 8/14/2013 | 10.4 | |
| 10.88 | | Controlled Equity OfferingSM Sales Agreement, dated September 5, 2013, by and between NewLink Genetics Corporation and Cantor Fitzgerald & Co. | 8-K | 9/5/2013 | 10.1 | |
| 10.89 | | Amendment of Contract No. HDTRA1-09-C-0014, by and between BioProtection Systems Corporation and the United States Department of Defense, dated as of September 18, 2013 | 10-Q | 11/12/2013 | 10.1 | |
| 10.90 | | License Agreement Amendment, by and between NewLink Genetics Corporation and Georgia Health Sciences University Research Institute, dated as of July 13, 2013 | 10-Q | 11/12/2013 | 10.2 | |
| 10.91 | † | 2013 Bonus Awards | 8-K | 1/8/2014 | 10.1 | |
| 10.92 | † | 2014 Salaries, Bonus Targets and Equity Awards | 8-K | 1/8/2014 | 10.2 | |
| 10.93 | | Memorandum of Agreement, dated January 4, 2014, by and between the Registrant and Iowa State University Research Park Corporation | | | | X |
| 10.94 | * | Amendment to License Agreement dated December 30, 2013, by and between Registrant and Central Iowa health System | | | | X |
| 10.95 | † | Employment Agreement, dated as of March 11, 2014, by and between the Registrant and Brian Wiley | | | | X |
| 10.96 | † | Employment Agreement, dated as of March 11, 2014, by and between the Registrant and Carl W. Langren | | | | X |
| 21.1 | | Subsidiary Information | | | | X |
| 23.1 | | Consent of KPMG LLP, independent registered public accounting firm | | | | X |
| 24.1 | | Power of Attorney (included on signature page hereto) | | | | X |
| 31.1 | | Rule 13a-14(a)/15d-14(a) Certification | | | | X |
| 31.2 | | Rule 13a-14(a)/15d-14(a) Certification | | | | X |
| 32.1 | # | Section 1350 Certification | | | | X |
| 101.INS | | XBRL Instance Document (filed electronically herewith) | | | | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document (filed electronically herewith) | | | | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document (filed electronically herewith) | | | | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document (filed electronically herewith) | | | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document (filed electronically herewith) | | | | |
|
| | | | | | |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document (filed electronically herewith) | | | | |
| | | | | | | |
____________________
|
| |
| † | Indicates management contract or compensatory plan. |
| * | Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 19434, as amended. |
| # | The certifications attached as Exhibit 32.1 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of NewLink Genetics Corporation under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned thereunto duly authorized.
|
| | | |
| NEWLINK GENETICS CORPORATION | | |
| | | |
| By: | /s/ Charles J. Link, Jr. | By: | /s/ Gordon H. Link, Jr. |
| Charles J. Link, Jr. | Gordon H. Link, Jr. |
| Chief Executive Officer | Chief Financial Officer and Secretary |
| (Principal Executive Officer) | (Principal Financial Officer) |
| Date: March 12, 2014 | Date: March 12, 2014 |
| | | |
| | | By: | /s/ Carl W. Langren |
| | Carl W. Langren |
| | Vice President Finance |
| | (Principal Accounting Officer) |
| | Date: March 12, 2014 |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints Charles J. Link, Jr. and Gordon H. Link, Jr., and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated:
|
| | | | | | | | |
| | Name | | | | Title | | Date |
| | | | | |
| /s/ Charles J. Link, Jr. | | Chief Executive Officer, Chairman of Board of Directors and Director | | March 12, 2014 |
| Charles J. Link, Jr. | | (Principal Executive Officer) | | |
| /s/ Gordon H. Link, Jr. | | Chief Financial Officer and Secretary | | March 12, 2014 |
| Gordon H. Link, Jr. | | (Principal Financial Officer) | | |
| /s/ Thomas A. Raffin | | Director | | March 12, 2014 |
| Thomas A. Raffin | | | | |
| /s/ Ernest J. Talarico, III | | Director | | March 12, 2014 |
| Ernest J. Talarico, III | | | | |
| /s/ Lota Zoth | | Director | | March 12, 2014 |
| Lota Zoth | | | | |
| /s/ Joseph Saluri | | Director | | March 12, 2014 |
| Joseph Saluri | | | | |
| /s/ Paul R. Edick | | Director | | March 12, 2014 |
| Paul R. Edick | | | | |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
NewLink Genetics Corporation:
We have audited the accompanying consolidated balance sheets of NewLink Genetics Corporation and subsidiaries (a development stage enterprise) (the Company) as of December 31, 2013 and 2012, and the related consolidated statements of operations, stockholders' equity (deficit), and cash flows for each of the years in the three-year period ended December 31, 2013, and for the period from June 4, 1999 (inception) through December 31, 2013. We also have audited the Company's internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control Over Financial Reporting included in Item 9A. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the Company's internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of NewLink Genetics Corporation and subsidiaries (a development stage enterprise) as of December 31, 2013 and 2012, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2013, and for the period from June 4, 1999 (inception) through December 31, 2013, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
/s/ KPMG LLP
Des Moines, Iowa
March 12, 2014
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Balance Sheets (In thousands, except share and per share data) |
| | | | | | | | | |
| | | December 31, 2013 | | December 31, 2012 | |
| Assets | | | | | |
| Current assets: | | | | | |
| Cash and cash equivalents | | $ | 61,291 |
| | $ | 20,250 |
| |
| Certificates of deposit | | 249 |
| | 1,494 |
| |
| Prepaid expenses | | 773 |
| | 907 |
| |
| State research and development credit receivable | | 329 |
| | 542 |
| |
| Other receivables | | 1,328 |
| | 196 |
| |
| Total current assets | | 63,970 |
| | 23,389 |
| |
| Leasehold improvements and equipment: | | | | | |
| Leasehold improvements | | 5,588 |
| | 5,085 |
| |
| Computer equipment | | 1,133 |
| | 636 |
| |
| Lab equipment | | 3,724 |
| | 3,297 |
| |
| Total leasehold improvements and equipment | | 10,445 |
| | 9,018 |
| |
| Less accumulated depreciation and amortization | | (3,858 | ) | | (2,978 | ) | |
| Leasehold improvements and equipment, net | | 6,587 |
| | 6,040 |
| |
| | | | | | |
| Total assets | | $ | 70,557 |
| | $ | 29,429 |
| |
| See accompanying notes to consolidated financial statements. | |
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Balance Sheets (In thousands, except share and per share data) |
| | | | | | | | | |
| | | December 31, 2013 | | December 31, 2012 | |
| Liabilities and Stockholders' Equity | | |
| | |
| |
| Current liabilities: | | |
| | |
| |
| Accounts payable | | $ | 612 |
| | $ | 972 |
| |
| Accrued expenses | | 2,861 |
| | 1,659 |
| |
| Income taxes payable | | 130 |
| | — |
| |
| Current portion of deferred rent | | 84 |
| | 84 |
| |
| Obligations under capital leases | | 35 |
| | 55 |
| |
| Current portion of long term debt | | 154 |
| | 149 |
| |
| Total current liabilities | | 3,876 |
| | 2,919 |
| |
| Long term liabilities: | | |
| | |
| |
| Royalty obligation payable to Iowa Economic Development Authority | | 6,000 |
| | 6,000 |
| |
| Notes payable to Iowa State University Research Park | | 687 |
| | 840 |
| |
| Note payable to City of Ames | | 300 |
| | 300 |
| |
| Obligations under capital leases | | 46 |
| | 38 |
| |
| Deferred rent, excluding current portion | | 1,321 |
| | 1,405 |
| |
| Total long-term liabilities | | 8,354 |
| | 8,583 |
| |
| Total liabilities | | 12,230 |
| | 11,502 |
| |
| Commitments | | | | | |
| Stockholders' Equity | | | | | |
| Blank check preferred stock, $0.01 par value: Authorized shares — 5,000,000 at December 31, 2013 and 2012; issued and outstanding shares — 0 at December 31, 2013 and 2012 | | — |
| | — |
| |
| Common stock, $0.01 par value: Authorized shares — 75,000,000 at December 31, 2013 and 38,833,334 at December 31, 2012; issued and outstanding shares — 26,573,023 at December 31, 2013, and 20,985,192 at December 31, 2012 | | 266 |
| | 210 |
| |
| Additional paid-in capital | | 194,038 |
| | 122,514 |
| |
| Deficit accumulated during the development stage | | (135,977 | ) | | (104,797 | ) | |
| Total stockholders' equity | | 58,327 |
| | 17,927 |
| |
| Total liabilities and stockholders' equity | | $ | 70,557 |
| | $ | 29,429 |
| |
| See accompanying notes to consolidated financial statements. | |
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise)
Consolidated Statements of Operations (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | Cumulative from
June 4, 1999
(inception)
through
December 31, | |
| | | 2013 | | 2012 | | 2011 | | 2013 | |
| Grant revenue | | $ | 1,093 |
| | $ | 1,687 |
| | $ | 1,872 |
| | $ | 8,497 |
| |
| Operating expenses: | | |
| | |
| | |
| | |
| |
| Research and development | | 22,713 |
| | 17,838 |
| | 14,255 |
| | 100,869 |
| |
| General and administrative | | 9,521 |
| | 7,108 |
| | 5,679 |
| | 46,464 |
| |
| Total operating expenses | | 32,234 |
| | 24,946 |
| | 19,934 |
| | 147,333 |
| |
| Loss from operations | | (31,141 | ) | | (23,259 | ) | | (18,062 | ) | | (138,836 | ) | |
| Other income and expense: | | |
| | |
| | |
| | |
| |
| Miscellaneous income | | 112 |
| | (38 | ) | | 5 |
| | 432 |
| |
| Forgiveness of debt | | — |
| | — |
| | — |
| | 449 |
| |
| Interest income | | 12 |
| | 14 |
| | 11 |
| | 1,779 |
| |
| Interest expense | | (33 | ) | | (38 | ) | | (42 | ) | | (214 | ) | |
| Other income (expense), net | | 91 |
| | (62 | ) | | (26 | ) | | 2,446 |
| |
| Net loss before taxes | | (31,050 | ) | | (23,321 | ) | | (18,088 | ) | | (136,390 | ) | |
| Income tax expense | | (130 | ) | | — |
| | — |
| | (130 | ) | |
| Net loss | | (31,180 | ) | | (23,321 | ) | | (18,088 | ) | | (136,520 | ) | |
| Less net loss attributable to noncontrolling interest | | — |
| | — |
| | 1 |
| | 583 |
| |
| Net loss attributable to NewLink | | $ | (31,180 | ) | | $ | (23,321 | ) | | $ | (18,087 | ) | | $ | (135,937 | ) | |
| Net loss per common share, basic and diluted | | $ | (1.23 | ) | | $ | (1.12 | ) | | $ | (2.98 | ) | | | |
| Weighted-average common shares outstanding, basic and diluted | | 25,275,179 |
| | 20,779,450 |
| | 6,064,542 |
| | | |
| See accompanying notes to consolidated financial statements. |
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Stockholders’ Equity (Deficit) (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Common Stock | | | | | | | | |
| | | | Deficit Accumulated During the Development Stage | | Total NewLink Genetics Stockholders' Equity | | | | |
| | Preferred Stock Series A | | Number of Common Shares Outstanding | | Common Stock | | Additional Paid-in Capital | | Notes Receivable For Common Stock | | Treasury Stock | | Non- Controlling Interest | | Total Stockholders' Equity (Deficit) |
| Balance at June 4, 1999 | $ | — |
| | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
|
| Initial stock issuance (November 11, 1999) | — |
| | 1,904,762 |
| | 19 |
| | 21 |
| | (13 | ) | | — |
| | — |
| | 27 |
| | — |
| | 27 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (64 | ) | | (64 | ) | | — |
| | (64 | ) |
| Balance at December 31, 1999 | — |
| | 1,904,762 |
| | 19 |
| | 21 |
| | (13 | ) | | — |
| | (64 | ) | | (37 | ) | | — |
| | (37 | ) |
| Loan payment | — |
| | — |
| | — |
| | — |
| | 13 |
| | — |
| | — |
| | 13 |
| | — |
| | 13 |
|
| Common stock issuance (April 18 and June 13 ,2000) | — |
| | 686,762 |
| | 6 |
| | 145 |
| | (104 | ) | | — |
| | — |
| | 47 |
| | — |
| | 47 |
|
| Issuance of 420,000 shares of Series A preferred stock (net of offering costs) (August 29, 2000) | 989 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 989 |
| | — |
| | 989 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (236 | ) | | (236 | ) | | — |
| | (236 | ) |
| Balance at December 31, 2000 | 989 |
| | 2,591,524 |
| | 25 |
| | 166 |
| | (104 | ) | | — |
| | (300 | ) | | 776 |
| | — |
| | 776 |
|
| Repurchase of common stock and settlement of notes receivable (January 29, 2001) | — |
| | (131,806 | ) | | — |
| | — |
| | 18 |
| | (33 | ) | | — |
| | (15 | ) | | — |
| | (15 | ) |
| Issuance of common stock (August 2, 2001) | — |
| | 95,238 |
| | 1 |
| | 49 |
| | — |
| | — |
| | — |
| | 50 |
| | — |
| | 50 |
|
| Deemed dividend due to sale of Series AA preferred shares (September 26, 2001) | 41 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (41 | ) | | — |
| | — |
| | — |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (1,448 | ) | | (1,448 | ) | | — |
| | (1,448 | ) |
| Balance at December 31, 2001 | 1,030 |
| | 2,554,956 |
| | 26 |
| | 215 |
| | (86 | ) | | (33 | ) | | (1,789 | ) | | (637 | ) | | — |
| | (637 | ) |
| Receipt of payment on note receivable (April 5, September 4 and October 5, 2002) | — |
| | — |
| | — |
| | — |
| | 33 |
| | — |
| | — |
| | 33 |
| | — |
| | 33 |
|
| Issuance of common stock from exercise of stock options (July 26, 2002) | — |
| | 23,810 |
| | 1 |
| | 12 |
| | — |
| | — |
| | — |
| | 13 |
| | — |
| | 13 |
|
| Issuance of dividend paid in common stock (October 18, 2002) | — |
| | 20,998 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of stock options to nonemployees | — |
| | — |
| | — |
| | 14 |
| | — |
| | — |
| | — |
| | 14 |
| | — |
| | 14 |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (6 | ) | | — |
| | — |
| | — |
| | (6 | ) | | — |
| | (6 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (2,253 | ) | | (2,253 | ) | | — |
| | (2,253 | ) |
| Balance at December 31, 2002 | $ | 1,030 |
| | 2,599,764 |
| | $ | 27 |
| | $ | 235 |
| | $ | (53 | ) | | $ | (33 | ) | | $ | (4,042 | ) | | $ | (2,836 | ) | | $ | — |
| | $ | (2,836 | ) |
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Stockholders’ Equity (Deficit) (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Common Stock | | | | | | | | |
| | | | Deficit Accumulated During the Development Stage | | Total NewLink Genetics Stockholders' Equity | | | | |
| | Preferred Stock Series A | | Number of Common Shares Outstanding | | Common Stock | | Additional Paid-in Capital | | Notes Receivable For Common Stock | | Treasury Stock | | Non- Controlling Interest | | Total Stockholders' Equity (Deficit) |
| Balance at December 31, 2002 (brought forward) | $ | 1,030 |
| | 2,599,764 |
| | $ | 27 |
| | $ | 235 |
| | $ | (53 | ) | | $ | (33 | ) | | $ | (4,042 | ) | | $ | (2,836 | ) | | $ | — |
| | $ | (2,836 | ) |
| Issuance of common stock for compensation (March 20, 2003) | — |
| | 9,524 |
| | — |
| | 46 |
| | — |
| | — |
| | — |
| | 46 |
| | — |
| | 46 |
|
| Receipt of payment on note receivable (January 1, April 4, July 9, and September 29, 2003) | — |
| | — |
| | — |
| | — |
| | 7 |
| | — |
| | — |
| | 7 |
| | — |
| | 7 |
|
| Issuance of common stock from exercise of warrants (various dates through March 2003) | — |
| | 235,537 |
| | 2 |
| | 1,110 |
| | — |
| | — |
| | — |
| | 1,112 |
| | — |
| | 1,112 |
|
| Issuance of dividend paid in common stock | — |
| | 20,998 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of stock options | — |
| | — |
| | — |
| | 15 |
| | — |
| | — |
| | — |
| | 15 |
| | — |
| | 15 |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (5 | ) | | — |
| | — |
| | — |
| | (5 | ) | | — |
| | (5 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (2,979 | ) | | (2,979 | ) | | — |
| | (2,979 | ) |
| Balance at December 31, 2003 | 1,030 |
| | 2,865,823 |
| | 29 |
| | 1,401 |
| | (46 | ) | | (33 | ) | | (7,021 | ) | | (4,640 | ) | | — |
| | (4,640 | ) |
| Receipt of payment on note receivable (February 25, 2004 and July 15, 2004) | — |
| | — |
| | — |
| | — |
| | 5 |
| | — |
| | — |
| | 5 |
| | — |
| | 5 |
|
| Issuance of stock options | — |
| | — |
| | — |
| | 57 |
| | — |
| | — |
| | — |
| | 57 |
| | — |
| | 57 |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (5 | ) | | — |
| | — |
| | — |
| | (5 | ) | | — |
| | (5 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (3,669 | ) | | (3,669 | ) | | — |
| | (3,669 | ) |
| Balance at December 31, 2004 | 1,030 |
| | 2,865,823 |
| | 29 |
| | 1,453 |
| | (41 | ) | | (33 | ) | | (10,690 | ) | | (8,252 | ) | | — |
| | (8,252 | ) |
| Receipt of payment on note receivable | — |
| | — |
| | — |
| | — |
| | 1 |
| | — |
| | — |
| | 1 |
| | — |
| | 1 |
|
| Issuance of stock options | — |
| | — |
| | — |
| | 2 |
| | — |
| | — |
| | — |
| | 2 |
| | — |
| | 2 |
|
| Issuance of dividend paid in common stock (September 23, 2005) | — |
| | 41,995 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of common stock for OncoRx acquisition (June 21, 2005) | — |
| | 61,953 |
| | 1 |
| | 353 |
| | — |
| | — |
| | — |
| | 354 |
| | — |
| | 354 |
|
| Issuance of common stock to consultants (April 4 and June 1, 2005) | — |
| | 8,946 |
| | — |
| | 51 |
| | — |
| | — |
| | — |
| | 51 |
| | — |
| | 51 |
|
| Issuance of 593,247 shares of Series BB preferred stock (net of offering costs of $36,114) (January and February 2005) | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (82 | ) | | — |
| | — |
| | — |
| | (82 | ) | | — |
| | (82 | ) |
| Issuance of subsidiary preferred stock | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 2,278 |
| | 2,278 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (4,770 | ) | | (4,770 | ) | | — |
| | (4,770 | ) |
| Balance at December 31, 2005 | $ | 1,030 |
| | 2,978,717 |
| | $ | 30 |
| | $ | 1,777 |
| | (40 | ) | | $ | (33 | ) | | $ | (15,460 | ) | | $ | (12,696 | ) | | $ | 2,278 |
| | $ | (10,418 | ) |
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Stockholders’ Equity (Deficit) (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Common Stock | | | | | | | | |
| | | | Deficit Accumulated During the Development Stage | | Total NewLink Genetics Stockholders' Equity | | | | |
| | Preferred Stock Series A | | Number of Common Shares Outstanding | | Common Stock | | Additional Paid-in Capital | | Notes Receivable For Common Stock | | Treasury Stock | | Non- Controlling Interest | | Total Stockholders' Equity (Deficit) |
| Balance at December 31, 2005 (brought forward) | $ | 1,030 |
| | 2,978,717 |
| | $ | 30 |
| | $ | 1,777 |
| | $ | (40 | ) | | $ | (33 | ) | | $ | (15,460 | ) | | $ | (12,696 | ) | | $ | 2,278 |
| | $ | (10,418 | ) |
| Share-based compensation | — |
| | — |
| | — |
| | 22 |
| | — |
| | — |
| | — |
| | 22 |
| | — |
| | 22 |
|
| Issuance of common stock for OncoRx acquisition (March 22, 2006) | — |
| | 61,953 |
| | 1 |
| | 129 |
| | — |
| | — |
| | — |
| | 130 |
| | — |
| | 130 |
|
| Issuance of dividend paid in common stock (September 25, 2006) | — |
| | 20,996 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of subsidiary preferred and common stock, net of deposits | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 257 |
| | 257 |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (4 | ) | | — |
| | — |
| | — |
| | (4 | ) | | — |
| | (4 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (5,318 | ) | | (5,318 | ) | | — |
| | (5,318 | ) |
| Balance at December 31, 2006 | 1,030 |
| | 3,061,666 |
| | 31 |
| | 1,924 |
| | (40 | ) | | (33 | ) | | (20,778 | ) | | (17,866 | ) | | 2,535 |
| | (15,331 | ) |
| Share-based compensation | — |
| | — |
| | — |
| | 58 |
| | — |
| | — |
| | — |
| | 58 |
| | — |
| | 58 |
|
| Exercise of stock options | — |
| | 15,476 |
| | — |
| | 12 |
| | — |
| | — |
| | — |
| | 12 |
| | — |
| | 12 |
|
| Receipt of payment on note receivable | — |
| | — |
| | — |
| | — |
| | 2 |
| | — |
| | — |
| | 2 |
| | — |
| | 2 |
|
| Issuance of common stock for license milestone (August 2, 2007) | — |
| | 11,905 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of dividend paid in common stock | — |
| | 20,996 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Retire treasury stock | — |
| | — |
| | — |
| | (33 | ) | | — |
| | 33 |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of subsidiary common stock, net of deposits | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 8 |
| | 8 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (7,582 | ) | | (7,582 | ) | | — |
| | (7,582 | ) |
| Balance at December 31, 2007 | 1,030 |
| | 3,110,043 |
| | 31 |
| | 1,961 |
| | (38 | ) | | — |
| | (28,360 | ) | | (25,376 | ) | | 2,543 |
| | (22,833 | ) |
| Share-based compensation | — |
| | — |
| | — |
| | 86 |
| | — |
| | — |
| | — |
| | 86 |
| | — |
| | 86 |
|
| Exercise of stock options | — |
| | 15,298 |
| | — |
| | 19 |
| | — |
| | — |
| | — |
| | 19 |
| | — |
| | 19 |
|
| Issuance of common stock for license milestone (September 8, 2008) | — |
| | 2,381 |
| | — |
| | 5 |
| | — |
| | — |
| | — |
| | 5 |
| | — |
| | 5 |
|
| Issuance of dividend paid in common stock | — |
| | 21,000 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of subsidiary common stock, net of deposits | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (1 | ) | | (1 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (8,842 | ) | | (8,842 | ) | | — |
| | (8,842 | ) |
| Balance at December 31, 2008 | $ | 1,030 |
| | 3,148,722 |
| | $ | 31 |
| | $ | 2,071 |
| | $ | (38 | ) | | $ | — |
| | $ | (37,202 | ) | | $ | (34,108 | ) | | $ | 2,542 |
| | $ | (31,566 | ) |
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Stockholders’ Equity (Deficit) (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Common Stock | | | | | | | | |
| | | | Deficit Accumulated During the Development Stage | | Total NewLink Genetics Shareholders' Equity | | | | |
| | Preferred Stock Series A | | Number of Common Shares Outstanding | | Common Stock | | Additional Paid-in Capital | | Notes Receivable For Common Stock | | Treasury Stock | | Non- Controlling Interest | | Total
Stockholders' Equity
(Deficit) |
| Balance at December 31, 2008 (brought forward) | $ | 1,030 |
| | 3,148,722 |
| | $ | 31 |
| | $ | 2,071 |
| | $ | (38 | ) | | $ | — |
| | $ | (37,202 | ) | | $ | (34,108 | ) | | $ | 2,542 |
| | $ | (31,566 | ) |
| Share-based compensation | — |
| | — |
| | — |
| | 929 |
| | — |
| | — |
| | — |
| | 929 |
| | — |
| | 929 |
|
| Exercise of stock options | — |
| | 7,138 |
| | — |
| | 15 |
| | — |
| | — |
| | — |
| | 15 |
| | — |
| | 15 |
|
| Issuance of dividend paid in common stock | — |
| | 20,998 |
| | 1 |
| | (1 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Issuance of subsidiary common stock net of deposits | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 3 |
| | 3 |
|
| Issuance of 30,000 shares of subsidiary Series B preferred stock (November 16, 2009) | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 40 |
| | 40 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (9,974 | ) | | (9,974 | ) | | (233 | ) | | (10,207 | ) |
| Balance at December 31, 2009 | 1,030 |
| | 3,176,858 |
| | 32 |
| | 3,014 |
| | (38 | ) | | — |
| | (47,176 | ) | | (43,138 | ) | | 2,352 |
| | (40,786 | ) |
| Share-based compensation | — |
| | — |
| | — |
| | 1,525 |
| | — |
| | — |
| | — |
| | 1,525 |
| | — |
| | 1,525 |
|
| Exercise of stock options | — |
| | 141,573 |
| | 1 |
| | 81 |
| | — |
| | — |
| | — |
| | 82 |
| | — |
| | 82 |
|
| Exercise of warrants for common stock | — |
| | 178,571 |
| | 2 |
| | 1,998 |
| | — |
| | — |
| | — |
| | 2,000 |
| | — |
| | 2,000 |
|
| Receipt of payment and forgiveness of note receivable | — |
| | — |
| | — |
| | — |
| | 25 |
| | — |
| | — |
| | 25 |
| | 121 |
| | 146 |
|
| Issuance of common stock for OncoRX acquisition (July 29, 2010) | — |
| | 173,469 |
| | 2 |
| | 817 |
| | — |
| | — |
| | — |
| | 819 |
| | — |
| | 819 |
|
| Issuance of common stock for license termination (September 3, 2010) | — |
| | 23,810 |
| | — |
| | 201 |
| | — |
| | — |
| | — |
| | 201 |
| | — |
| | 201 |
|
| Issuance of dividend paid in common stock | — |
| | 20,996 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (2 | ) | | — |
| | — |
| | — |
| | (2 | ) | | — |
| | (2 | ) |
| Conversion of preferred stock to common stock | — |
| | 14,915 |
| | — |
| | 114 |
| | — |
| | — |
| | — |
| | 114 |
| | — |
| | 114 |
|
| Repurchase and retirement of common stock (December 20, 2010) | — |
| | (102,110 | ) | | (1 | ) | | (374 | ) | | — |
| | — |
| | — |
| | (375 | ) | | (94 | ) | | (469 | ) |
| Issuance of subsidiary common stock net of deposits | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 2 |
| | 2 |
|
| Issuance of 555,930 shares of subsidiary Series B Preferred Stock (September 7, 2010) | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 911 |
| | 911 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (16,213 | ) | | (16,213 | ) | | (349 | ) | | (16,562 | ) |
| Balance at December 31, 2010 | $ | 1,030 |
| | 3,628,082 |
| | $ | 36 |
| | $ | 7,374 |
| | $ | (13 | ) | | $ | — |
| | $ | (63,389 | ) | | $ | (54,962 | ) | | $ | 2,943 |
| | $ | (52,019 | ) |
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Stockholders’ Equity (Deficit) (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Common Stock | | | | | | | | |
| | | | Deficit Accumulated During the Development Stage | | Total NewLink Genetics Shareholders' Equity | | | | |
| | Preferred Stock Series A | | Number of Common Shares Outstanding | | Common Stock | | Additional Paid-in Capital | | Notes Receivable For Common Stock | | Treasury Stock | | Non- Controlling Interest | | Total
Stockholders' Equity
(Deficit) |
| Balance at December 31, 2010 (brought forward) | $ | 1,030 |
| | 3,628,082 |
| | $ | 36 |
| | $ | 7,374 |
| | $ | (13 | ) | | $ | — |
| | $ | (63,389 | ) | | $ | (54,962 | ) |
| $ | 2,943 |
| | $ | (52,019 | ) |
| Share-based compensation | — |
| | 11,905 |
| | — |
| | 2,545 |
| | — |
| | — |
| | — |
| | 2,545 |
| | — |
| | 2,545 |
|
| Exercise of stock options | — |
| | 9,320 |
| | — |
| | 19 |
| | — |
| | — |
| | — |
| | 19 |
| | — |
| | 19 |
|
| Receipt of payment on note receivable | — |
| | — |
| | — |
| | — |
| | 13 |
| | — |
| | — |
| | 13 |
| | — |
| | 13 |
|
| Issuance of dividend paid in common stock | — |
| | 22,580 |
| | 1 |
| | (1 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Accretion of redemption feature of preferred stock | — |
| | — |
| | — |
| | (8 | ) | | — |
| | — |
| | — |
| | (8 | ) | | — |
| | (8 | ) |
| Conversion of preferred stock to common stock (November 16, 2011) | (1,030 | ) | | 10,719,353 |
| | 107 |
| | 76,313 |
| | — |
| | — |
| | — |
| | 75,390 |
| | — |
| | 75,390 |
|
| Issuance of 6,200,000 shares of common stock (net of offering costs of $5,845) (November 16, 2011) | — |
| | 6,200,000 |
| | 62 |
| | 37,493 |
| | — |
| | — |
| | — |
| | 37,555 |
| | — |
| | 37,555 |
|
| Acquisition of noncontrolling interest | — |
| | — |
| | — |
| | (5,692 | ) | | — |
| | — |
| | — |
| | (5,692 | ) | | (2,942 | ) | | (8,634 | ) |
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (18,087 | ) | | (18,087 | ) | | (1 | ) | | (18,088 | ) |
| Balance at December 31, 2011 | — |
| | 20,591,240 |
| | 206 |
| | 118,043 |
| | — |
| | — |
| | (81,476 | ) | | 36,773 |
| | — |
| | 36,773 |
|
| Share-based compensation | — |
| | 13,000 |
| | — |
| | 3,195 |
| | — |
| | — |
| | — |
| | 3,195 |
| | — |
| | 3,195 |
|
| Exercise of stock options | — |
| | 341,429 |
| | 4 |
| | 883 |
| | — |
| | — |
| | — |
| | 887 |
| | — |
| | 887 |
|
| Sale of shares under stock purchase plan | — |
| | 39,523 |
| | — |
| | 239 |
| | — |
| | — |
| | — |
| | 239 |
| | — |
| | 239 |
|
| Purchase of fractional shares | — |
| | — |
| | — |
| | (4 | ) | | — |
| | — |
| | — |
| | (4 | ) | | — |
| | (4 | ) |
| Reduction of IPO offering costs | — |
| | — |
| | — |
| | 158 |
| | — |
| | — |
| | — |
| | 158 |
| | — |
| | 158 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (23,321 | ) | | (23,321 | ) | | — |
| | (23,321 | ) |
| Balance at December 31, 2012 | — |
| | 20,985,192 |
| | 210 |
| | 122,514 |
| | — |
| | — |
| | (104,797 | ) | | 17,927 |
| | — |
| | 17,927 |
|
| Share-based compensation | — |
| | — |
| | — |
| | 4,365 |
| | — |
| | — |
| | — |
| | 4,365 |
| | — |
| | 4,365 |
|
| Exercise of stock options | — |
| | 107,541 |
| | 1 |
| | 441 |
| | — |
| | — |
| | — |
| | 442 |
| | — |
| | 442 |
|
| Sale of shares under stock purchase plan | — |
| | 63,669 |
| | 1 |
| | 415 |
| | — |
| | — |
| | — |
| | 416 |
| | — |
| | 416 |
|
| Issuance of 4,600,000 shares of common stock (net of offering costs of $3,524,405) (February 4, 2013) | — |
| | 4,600,000 |
| | 46 |
| | 48,870 |
| | — |
| | — |
| | — |
| | 48,916 |
| | — |
| | 48,916 |
|
| Issuance of shares under at the market offering (net of offering costs of $402,542) | — |
| | 816,621 |
| | 8 |
| | 17,433 |
| | — |
| | — |
| | — |
| | 17,441 |
| | — |
| | 17,441 |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (31,180 | ) | | (31,180 | ) | | — |
| | (31,180 | ) |
| Balance at December 31, 2013 | $ | — |
| | 26,573,023 |
| | $ | 266 |
| | $ | 194,038 |
| | $ | — |
| | $ | — |
| | $ | (135,977 | ) | | $ | 58,327 |
| | $ | — |
| | $ | 58,327 |
|
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation and Subsidiaries (A Development Stage Enterprise) Consolidated Statements of Cash Flows (In thousands, except share and per share data) |
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | Cumulative from
June 4, 1999
(inception)
through
December 31, | |
| | | 2013 | | 2012 | | 2011 | | 2013 | |
| Cash Flows From Development Activities | | | | | | | | | |
| Net loss | | $ | (31,180 | ) | | $ | (23,321 | ) | | $ | (18,088 | ) | | $ | (136,520 | ) | |
| Adjustments to reconcile net loss to net cash used in development activities: | | | | | | | | | |
| Share-based compensation | | 4,365 |
| | 3,195 |
| | 2,545 |
| | 12,909 |
| |
| Depreciation and amortization | | 891 |
| | 788 |
| | 687 |
| | 4,640 |
| |
| Loss on sale of fixed assets | | 2 |
| | 38 |
| | — |
| | 40 |
| |
| In-process research and development expenses | | — |
| | — |
| | — |
| | 1,629 |
| |
| Forgiveness of debt | | — |
| | — |
| | — |
| | (449 | ) | |
| Forgiveness of notes receivable from related parties | | — |
| | — |
| | — |
| | 350 |
| |
| Changes in operating assets and liabilities: | | |
| | |
| | | | |
| |
| Prepaid expenses | | 134 |
| | (498 | ) | | 550 |
| | (773 | ) | |
| State research and development credit receivable | | 213 |
| | (340 | ) | | 28 |
| | (329 | ) | |
| Other receivables | | (1,132 | ) | | — |
| | 416 |
| | (1,328 | ) | |
| Accounts payable | | (359 | ) | | (700 | ) | | 637 |
| | (619 | ) | |
| Income taxes payable | | 130 |
| | — |
| | — |
| | 130 |
| |
| Accrued expenses and deferred rent | | 1,118 |
| | 367 |
| | 276 |
| | 4,266 |
| |
| Net cash used in development activities | | (25,818 | ) | | (20,471 | ) | | (12,949 | ) | | (116,054 | ) | |
| Cash Flows From Investing Activities | | | | | | | | | |
| Purchase of certificates of deposit | | (249 | ) | | (1,992 | ) | | (2,490 | ) | | (13,531 | ) | |
| Sale of certificates of deposit | | 1,494 |
| | 2,988 |
| | 2,269 |
| | 13,282 |
| |
| Notes receivable from related parties | | — |
| | — |
| | — |
| | (350 | ) | |
| Purchase of equipment | | (1,386 | ) | | (1,323 | ) | | (281 | ) | | (9,478 | ) | |
| Proceeds on sale of fixed assets | | — |
| | 50 |
| | — |
| | 50 |
| |
| Cash paid for OncoRx | | — |
| | — |
| | — |
| | (120 | ) | |
| Net cash used in investing activities | | (141 | ) | | (277 | ) | | (502 | ) | | (10,147 | ) | |
| Cash Flows From Financing Activities | | | | | | | | | |
| Cash received from noncontrolling interest investment | | — |
| | — |
| | — |
| | 3,479 |
| |
| Issuance of common stock, net of offering costs | | 67,215 |
| | 1,285 |
| | 37,574 |
| | 109,660 |
| |
| Repurchase of common stock | | — |
| | (4 | ) | | 13 |
| | (505 | ) | |
| Proceeds from preferred stock | | — |
| | — |
| | 5,000 |
| | 67,743 |
| |
| Proceeds from notes payable | | — |
| | 456 |
| | — |
| | 8,215 |
| |
| Principal payments on debt | | (149 | ) | | (107 | ) | | (92 | ) | | (625 | ) | |
| Payments under capital lease obligations | | (66 | ) | | (122 | ) | | (126 | ) | | (475 | ) | |
| Net cash provided by financing activities | | 67,000 |
| | 1,508 |
| | 42,369 |
| | 187,492 |
| |
| Net increase (decrease) in cash and cash equivalents | | 41,041 |
| | (19,240 | ) | | 28,918 |
| | 61,291 |
| |
| Cash and cash equivalents at beginning of period | | 20,250 |
| | 39,490 |
| | 10,572 |
| | — |
| |
| Cash and cash equivalents at end of period | | $ | 61,291 |
| | $ | 20,250 |
| | $ | 39,490 |
| | $ | 61,291 |
| |
| Supplemental disclosure of cash flows information: | | | | | | | | | |
| Cash paid for interest | | $ | 34 |
| | $ | 38 |
| | $ | 42 |
| | $ | 176 |
| |
| Noncash financing and investing activities: | | | | | | | | | |
| Accretion on redeemable preferred stock | | — |
| | — |
| | 8 |
| | 113 |
| |
| Purchased leasehold improvements and equipment in accounts payable | | — |
| | 53 |
| | 481 |
| | — |
| |
| Common stock issued to shareholders of OncoRx as part of acquisition | | — |
| | — |
| | — |
| | 1,654 |
| |
| Issuance of common stock dividend to Series AA preferred shareholders | | — |
| | — |
| | 1 |
| | 6 |
| |
| Assets acquired under capital lease | | 54 |
| | — |
| | 80 |
| | 596 |
| |
| Reduction of IPO offering costs | | — |
| | 158 |
| | — |
| | 158 |
| |
See accompanying notes to consolidated financial statements.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
1. Description of Business and Development Stage Activities
On June 4, 1999, NewLink Genetics Corporation (NewLink) was incorporated as a Delaware corporation. NewLink was formed for the purpose of developing treatments for cancer and other diseases. NewLink initiated operations in April of 2000, which primarily consist of research and development.
In 2005, NewLink created a wholly owned subsidiary, BioProtection Systems Corporation (BPS). NewLink contributed certain licensing agreements and other intangible assets for BPS to create vaccines against potential biological terror threats. On January 7, 2011, NewLink acquired all of the minority interest in BPS, by merging a newly-formed subsidiary of NewLink with BPS, with BPS as the surviving corporation resulting in NewLink owning all the outstanding capital stock of BPS. See note 13.
In 2013, NewLink created a wholly owned subsidiary, NewLink International (NI). NewLink plans to conduct all or a portion of its operations outside of the United States through NI.
NewLink and its subsidiaries (the Company) are development stage enterprises and are devoting substantially all of their efforts toward research and development. The Company has never earned revenue from sales of its drugs under development. The Company incurred a net loss of $31.2 million for the year ended December 31, 2013, and from June 4, 1999 (inception) through December 31, 2013 has generated a cumulative deficit of $136.0 million. The Company has managed its liquidity needs during its development stage to date through a series of capital market transactions. On November 16, 2011, the Company completed its initial public offering (IPO) of common stock pursuant to a Registration Statement on Form S-1 that was declared effective on November 10, 2011. The Company sold 6,200,000 shares of common stock at a price of $7.00 per share raising a total of $37.6 million in net proceeds after underwriting discounts and commissions of $3.0 million and offering expenses of $2.9 million. Costs directly associated with the IPO were capitalized and recorded as deferred IPO costs prior to the closing of the IPO. These costs have been recorded as a reduction of the proceeds received in arriving at the amount to be recorded in additional paid-in capital. Upon the closing of the IPO, 14,270,113 shares of the Company’s convertible preferred stock automatically converted into 10,719,353 shares of common stock, which also reflected conversion price adjustments to our preferred stock. During the years ended December 31, 2013 and 2012, the Company received equity financing of $67.2 million and $1.3 million, respectively, through common stock offerings. On February 4, 2013, the Company completed a follow-on offering of its common stock. The Company sold 4,600,000 shares of common stock at a price of $11.40 per share raising a total of $49.0 million in net proceeds.
NewLink entered into a sales agreement with Cantor Fitzgerald & Co., dated as of September 5, 2013, under which NewLink may sell up to $60.0 million in shares of its common stock in one or more placements at prevailing market prices for its common stock (the ATM Offering). Any such sales would be effected pursuant to its registration statement on Form S-3 (333-185721), declared effective by the SEC on January 4, 2013. As of December 31, 2013, the Company had sold 816,621 shares of common stock under the ATM Offering, raising a total of $17.4 million in net proceeds. Subsequent to December 31, 2013 and through February 13, 2014, the Company sold an additional 1,017,217 shares of common stock under the ATM Offering, raising an additional $27.6 million in net proceeds. See note 16.
The accompanying financial statements as of and for the year ended December 31, 2013 have been prepared assuming the Company will continue as a going concern. As noted above, the Company successfully raised net proceeds of $37.6 million from its IPO, completed a follow-on offering of its common stock raising net proceeds of $49.0 million, raised an additional $17.4 million in net proceeds from the ATM Offering prior to December 31, 2013 and raised an additional $27.6 million in net proceeds from the ATM Offering subsequent to December 31, 2013 and through February 13, 2014. See note 16. The Company's cash and cash equivalents after these offerings are expected to be adequate to satisfy the Company's liquidity requirements through December 31, 2014, although not through commercialization and launch of revenue producing products. If available liquidity becomes insufficient to meet the Company’s operating obligations as they come due, the Company's plans include pursuing alternative funding arrangements and/or reducing expenditures as necessary to meet the Company’s cash requirements. However, there is no assurance that, if required, the Company will be able to raise additional capital or reduce discretionary spending to provide the required liquidity. Failure by the Company to successfully execute its plans or otherwise address its liquidity needs may have a material adverse effect on its business and financial position, and may materially affect the Company’s ability to continue as a going concern.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
2. Significant Accounting Policies
(a) Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. generally accepted accounting principles (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
(b) Principles of Consolidation
The consolidated financial statements include the financial statements of NewLink and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
(c) Cash and Cash Equivalents
For the purposes of the consolidated statements of cash flows, the Company considers all highly liquid debt instruments with an original maturity of three months or less to be cash equivalents. Cash equivalents of $61.3 million and $20.3 million at December 31, 2013 and 2012, respectively, consist of money market accounts and treasury bills.
(d) Certificates of Deposit
Certificates of deposit have original maturities of greater than three months. Certificates of deposit are classified as held-to-maturity with due dates through 2014 and are presented at amortized cost, which approximates fair value.
(e) Leasehold Improvements and Equipment, and Deferred Rent
Leasehold improvements and equipment are capitalized as we believe they have alternative future uses and are stated at cost. Equipment under capital leases is stated at the present value of minimum lease payments. Depreciation on all leasehold improvements and equipment is calculated on the straight-line method over the shorter of the lease term or estimated useful life of the asset. Computer equipment has useful lives of three to five years and lab equipment has useful lives of three to seven years.
Deferred rent reflects improvement allowances from the Company's lessors deferred to be recognized as part of lease expense over the remaining term of the lease, which is recognized on a straight-line basis. During 2012, the Company added leasehold improvements to a new facility under an operating lease. As part of the lease, the lessor approved a tenant improvement allowance of $622,000 for improvements made to the facility. Total deferred rents were $1.4 million and $1.5 million as of December 31, 2013, and 2012, respectively.
(f) Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset group to future net undiscounted cash flows expected to be generated by the asset group, primarily relating to proceeds for selling the assets. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
(g) Revenue Recognition
The Company receives payments from government entities under its grants and contracts with the National Institute of Health and the Department of Defense. These agreements provide the Company cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues are recognized in the period during which the related costs are incurred, provided that the conditions under which the cost reimbursement was provided have been met and the Company has only perfunctory obligations outstanding. During the years ended December 31, 2013, 2012, and 2011 and from inception through December 31, 2013, the Company has earned $1.1 million, $1.7 million, $1.9 million and $8.5 million in grant revenue, respectively.
(h) Expenses Accrued Under Contractual Arrangements with Third Parties; Accrued Clinical Expenses
The Company estimates its accrued expenses through a process of reviewing open contracts and purchase orders, communicating with personnel to identify services that have been performed and estimating the level of service performed and
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
the associated cost incurred for the service that may not be invoiced from the provider. The estimates of accrued expenses as of each balance sheet date are based on facts and circumstances known at that time. Such estimates are periodically confirmed with the service providers to verify accuracy.
The Company bases its expenses related to clinical trials on estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and contract research organizations that conduct and manage clinical trials on behalf of the Company. Invoicing from third-party contractors for services performed can lag several months. The Company accrues the costs of services rendered in connection with third-party contractor activities based on its estimate of management fees, site management and monitoring costs and data management costs as contracted. Differences between actual clinical trial costs and estimated clinical trial costs have not been material and are adjusted for in the period in which they become known.
(i) Research and Development
Research and development costs are expensed as incurred. Certain research and development expenses are refundable from the state of Iowa without regard to income. State research and development credits of $329,000, $283,000, $160,000 and $2.4 million at December 31, 2013, 2012 and 2011, and from inception through December 31, 2013, respectively, are reflected as a reduction of research and development expenses on the accompanying consolidated statements of operations.
(j) Patents
The Company generally applies for patent protection on processes and products. Patent application costs are expensed as incurred as a component of general and administrative expense, as recoverability of such expenditures is uncertain.
(k) Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operating results in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. As of December 31, 2013 and 2012, the Company has not recognized any uncertain tax positions.
(l) Share-Based Compensation
The Company is required to estimate the grant-date fair value of share-based compensation options issued to employees and recognize this cost over the period these awards vest. For purposes of estimating the grant-date fair value, the date of grant is the effective date on which the Board of Directors or the Chief Executive Officer, as applicable, grants the award, and the employee has a mutual understanding of the terms of the award. The service inception date is typically the date of grant. The Company estimates the fair value of each option granted using the Black-Scholes option pricing model. Generally, the Company has issued employee awards with a graded vesting schedule that vest over time. For these awards, the Company records compensation cost on a straight-line basis over the vesting period for the entire award.
The Company has issued awards to nonemployee consultants and advisors. All grants to nonemployees are valued using the same fair value method that the Company uses for grants to employees. The cost recognized on these awards is determined on the later of the vesting of the award or completion of services by the nonemployee.
Following is a description of the inputs for the Black-Scholes model:
Exercise Price
The Company’s stock options granted prior to the IPO were granted with an exercise price as determined by the NewLink Board of Directors in good faith with the assistance of a third-party appraisal report and an evaluation of milestones achieved. Subsequent to the IPO, the Company uses the quoted market price as listed on the public exchange on the date of grant. If Incentive Stock Options (ISO) are granted to a 10% stockholder in the Company, the exercise price shall not be less than 110% of the common stock’s fair market value on the date of grant.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
Expected Term (in Years)
The expected term of a stock option is the period of time for which the option is expected to be outstanding. The Company uses historical exercise and option expiration data to estimate the expected term for the Black-Scholes grant-date valuation.
Risk-Free Interest Rate
The Company uses the average yield on current U.S. Treasury instruments with terms that approximate the expected term of the stock options being valued.
Expected Dividend Yield
The expected dividend yield for all of the Company’s stock option grants is 0%, as the Company has not declared a cash dividend since inception and has no plans to declare a dividend.
Expected Volatility
The Company estimates future expected volatility for each stock option valuation utilizing volatility rates of similar publicly traded companies considered to be in the same peer group. The Company plans to continue using peer group data until it has sufficient information to rely solely on its own volatility. The volatility is calculated over a period of time commensurate with the expected term for the options granted.
Forfeitures
The share-based compensation expense has been reduced for estimated forfeitures. The estimated forfeiture rate is based on historical experience of the Company’s option plan, which the Company expects to continue at the current level, and any adjustments in the forfeiture rate in the future will result in a cumulative adjustment in the period that this estimate is changed. Ultimately, the total compensation expense recognized for any given stock-based award over its vesting period will only be for those shares that actually vest.
(m) Segments
The Company operates in one segment. NewLink and its subsidiaries BPS and NI conduct research and development activities based from facilities located in Ames, Iowa. The Ames location also includes corporate headquarters for NewLink and BPS. The companies conduct preclinical and clinical research in the biopharmaceutical industry. Management uses cash flow as the primary measure to manage its business and does not segment its business for internal reporting or decision-making.
(n) Financial Instruments and Concentrations of Credit Risk
The fair values of cash and cash equivalents, certificates of deposit, receivables and accounts payable, which are recorded at cost, approximate fair value based on the short-term nature of these financial instruments. The fair value of notes payable and capital lease obligations was $1.2 million and $1.4 million as of December 31, 2013 and 2012, respectively, and was determined using Level 3 inputs. The Company is unable to estimate the fair value of the royalty obligation based on future product sales as the timing of payments, if any, is uncertain.
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents, and certificates of deposit. Cash and cash equivalents are held by financial institutions and are federally insured up to certain limits. At times, the Company’s cash and cash equivalents balance exceeds the federally insured limits. To limit the credit risk, the Company invests its excess cash primarily in high quality securities such as money market funds.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
3. Leases
(a) Capital Leases
The following is an analysis of the leased property under capital leases by major class (in thousands):
|
| | | | | | | | | |
| | | Asset balances at December 31, | |
| Class of property | | 2013 | | 2012 | |
| Lab equipment | | $ | 489 |
| | $ | 489 |
| |
| Leasehold improvements | | 27 |
| | 27 |
| |
| Computer equipment | | 54 |
| | — |
| |
| Total property under capital leases | | 570 |
| | 516 |
| |
| Less accumulated depreciation and amortization | | 293 |
| | 211 |
| |
| Capital leased assets, net | | $ | 277 |
| | $ | 305 |
| |
The depreciation and amortization reflected above has been recorded in both research and development and general administrative expense in the consolidated statements of operations.
The following is a schedule by years of the future minimum lease payments under capital leases together with the present value of the net minimum lease payments as of December 31, 2013 (in thousands):
|
| | | |
| Year ending December 31: | |
| 2014 | $ | 38 |
|
| 2015 | 36 |
|
| 2016 | 12 |
|
| Total minimum lease payments | 86 |
|
| Less amount representing interest | 4 |
|
| Present value of net minimum lease payments | $ | 82 |
|
(b) Operating Leases
The Company has certain facilities operating leases that are being leased on a month-to-month basis. The Company entered into a lease for additional space in October 2009 expiring in 2015. Under the terms of the lease, the Company has the option to extend the lease for three additional five-year periods upon the same terms as the base lease. Additionally, in November of 2011, the Company entered into an amendment to the October 2009 lease adding additional space and extending the term on the additional space through 2017. Additionally, in April of 2013, the Company entered into an amendment to the October 2009 lease extending the term on a portion of the additional space through 2018. Lease expense is recognized on a straight-line basis. Rental expense for operating leases during the years ended December 31, 2013, 2012 and 2011, and from inception through December 31, 2013, was $766,000, $707,000, $601,000 and $4.5 million respectively.
Future minimum lease payments under the noncancelable operating leases (with initial or remaining lease terms in excess of one year) as of December 31, 2013 are as follows (in thousands):
|
| | | |
| Year ending December 31: | |
| 2014 | $ | 610 |
|
| 2015 | 329 |
|
| 2016 | 306 |
|
| 2017 | 164 |
|
| 2018 | 34 |
|
| Total | $ | 1,443 |
|
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
4. Long-Term Debt and Conversion to Royalty Obligation
March 2005 Iowa Department of Economic Development Loan
In March 2005, the Company entered into a $6.0 million forgivable loan agreement with the Iowa Department of Economic Development (the IDED). Under the agreement, in the absence of default, there were no principal or interest payments due until the completion date for the project. The balance outstanding under the loan agreement was $6.0 million as of December 31, 2011. This loan was converted into a royalty obligation under the terms of a settlement agreement entered into on March 26, 2012, (the IEDA Agreement) with the Iowa Economic Development Authority (the IEDA), as successor in interest to the IDED.
March 2012 Iowa Economic Development Authority Royalty Obligation
Under the terms of the IEDA Agreement the Company agreed to pay a 0.5% royalty on future product sales up to a cap of $6.8 million in exchange for IEDA’s release of the Company's job creation and project expenditure obligations and their release of the security interest in substantially all of the Company's assets. As no payments are expected in the next 12 months, the entire accrued royalty obligation of $6.0 million is considered long-term as of December 31, 2013.
2009 and 2012 Iowa State University Research Park Loans
In 2009, the Company executed a promissory note in favor of Iowa State University Research Park (ISURP) in an original principal amount of $800,000, which is due in monthly installments through March, 2018. The note represents amounts owed by the Company to ISURP for certain improvements that were made to facilities the Company leases from ISURP. The principal and interest owed under the note is amortized over an eight-year period. Interest is payable monthly under this promissory note, initially at a rate of 3.0% per annum and increasing to 5.0% per annum after five years from the date the improvements were completed. ISURP may accelerate all amounts owed under the note upon an event of default, including the Company’s uncured material breach of the terms of the note or the lease or upon early termination of the lease. In the event of a default under the note, amounts owed under the note will bear interest at 8.0% per annum. The balance outstanding under the 2009 note was $449,000 and $546,000 at December 31, 2013 and December 31, 2012, respectively.
In 2012, the Company executed a promissory note in favor of ISURP in an original principal amount of $456,000, which is due in monthly installments through September 2020. The note represents amounts owed by the Company to ISURP for certain additional improvements that were made to facilities the Company leases from ISURP. The principal and interest owed under the note is amortized over an eight-year period. Interest is payable monthly under this promissory note, initially at a rate of 3.0% per annum and increasing to 5.0% per annum after five years from the date the improvements were completed. ISURP may accelerate all amounts owed under the note upon an event of default, including the Company’s uncured material breach of the terms of the note or the lease or upon early termination of the lease. In the event of a default under the note, amounts owed under the note will bear interest at 8.0% per annum. The balance outstanding under the 2012 note was $392,000 and $443,000 at December 31, 2013 and December 31, 2012, respectively.
March 2010 City of Ames Forgivable Loan
In March 2010, the Company entered into a $400,000 forgivable loan agreement with the City of Ames, Iowa and the Ames Chamber of Commerce, jointly, as lenders. The project provides the Company with financial assistance to construct new facilities within the Ames city limits. In the absence of a default, there are no principal or interest payments due until the expected completion date for the project, which is March 10, 2015.
The project calls for the Company to create or retain at least 70 full-time jobs located in Ames, Iowa as of March 10, 2012 and to create or maintain at least 150 full-time positions located in Ames, Iowa as of March 10, 2015. The agreement also calls for the Company to enter into a five-year building lease with the option for extension for an additional five years of not less than 20,000 square feet within the corporate limits of the City of Ames by March 10, 2015. If, as of March 10, 2015, the Company has fulfilled the terms of the loan agreement, the loan will be forgiven. If on March 10, 2015, the Company has failed to create or retain at least 150 full-time jobs in Ames, Iowa, the Company will be required to repay approximately $3,100 per job not created or retained following such date. As of December 31, 2013, $300,000 of the total $400,000 forgivable loan was advanced to the Company with the final $100,000 pending certification to the City of Ames regarding the creation of a threshold level of jobs. In the event of default, including failure to repay any amounts under the loan when due, the Company will be required to repay the note, including 6.5% interest per annum, beginning at the date of default.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
5. Common Stock
The holders of common stock are entitled to one vote per share on all matters to be voted upon by the NewLink stockholders. Subject to preferences applicable to outstanding preferred stock, the holders of common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by the NewLink Board of Directors.
In the event of liquidation, dissolution, or winding up of NewLink, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities subject to prior distribution rights of the preferred stock.
6. Preferred Stock
As of December 31, 2013 and 2012, the Company had no outstanding preferred stock. The NewLink Board of Directors has the authority to issue up to 5,000,000 shares of preferred stock in one or more series and to fix the voting power and such designations, preferences, and rights of such series, subject to approval of outstanding preferred series shareholders.
7. Common Stock Equity Incentive Plans
In April 2000, the stockholders approved NewLink’s 2000 Equity Incentive Plan (the 2000 Plan), and in July 2009, the stockholders approved NewLink’s 2009 Equity Incentive Plan (the 2009 Plan). Following the approval of the 2009 Plan, all options outstanding under the 2000 Plan are effectively included under the 2009 Plan. Under the provisions of the 2009 Plan, NewLink may grant the following types of common stock awards:
| |
| • | Nonstatutory Stock Options |
| |
| • | Stock Appreciation Rights |
Awards under the 2009 Plan, as amended, may be made to officers, employees, members of the NewLink Board of Directors, advisors, and consultants to NewLink. As of December 31, 2013 there were 5,733,514 shares of common stock authorized for the 2009 Plan and 671,348 shares remained available for issuance. As of December 31, 2012 there were 4,895,139 shares of common stock authorized for the 2009 plan and 614,665 shares remained available for issuance.
On January 7, 2011, stockholders authorized an increase of 714,286 shares of common stock available for issuance under the 2009 Plan. On January 1, 2012 an additional 823,649 shares of common stock were added to the shares reserved for future issuance under the 2009 Plan. On January 1, 2013, an additional 838,375 shares of common stock were added to the shares reserved for future issuance under the 2009 Plan. Subsequent to year end, on January 1, 2014 an additional 1,066,340 shares of commons stock were added to the shares reserved for future issuance under the 2009 Plan. The shares added to the reserve on January 1, 2012, January 1, 2013, and January 1, 2014 were added pursuant to an “evergreen provision”, in accordance with which, on January 1 of each year, from 2012 to (and including) 2019, a number of shares of Common Stock in an amount equal to 4% of the total number of shares of Common Stock outstanding on December 31 of the preceding calendar year, or such lesser amount of shares (or no shares) approved by the NewLink Board of Directors, was added or will be added to the shares reserved under the 2009 Plan.
Under the terms of the Company's 2010 Non-Employee Directors’ Stock Option Plan (the Directors’ Plan), which became effective on November 10, 2011, 238,095 shares of common stock were reserved for future issuance. On May 9, 2013, an additional 161,905 shares of common stock were added to the reserve. As of December 31, 2013, 266,202 shares remained available for issuance under the Directors' Plan.
Under the terms of the Company's 2010 Employee Stock Purchase Plan, (the 2010 Purchase Plan), which became effective on November 10, 2011, 214,285 shares of common stock were reserved for future issuance. On May 9, 2013, an additional 185,715 shares of common stock were added to the reserve. As of December 31, 2013, 296,808 shares remained available for issuance under the plan. During the years ending December 31, 2013 and 2012, 63,669 and 39,523 shares of common stock, respectively, were purchased under the terms of the 2010 Purchase Plan.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
Stock Options
Share-based employee compensation expense for the years ended December 31, 2013, 2012 and 2011, and from inception through December 31, 2013 was $4.4 million, $3.2 million, $2.5 million and $12.9 million, respectively, and is allocated between research and development and general and administrative expenses within the consolidated statements of operations, giving rise to a related tax benefit of $0 for all periods. As of December 31, 2013, the total compensation cost related to nonvested option awards not yet recognized was $7.0 million and the weighted average period over which it is expected to be recognized was 2.7 years.
The NewLink Board of Directors determines the vesting period for each stock option award. Generally, stock options awarded to date under the 2009 Plan vest 20% or 25% on the first anniversary date of issuance with the remaining options vesting ratably over the next 36 to 48 months, though some options have effective vesting periods that begin prior to the date of grant. In such cases, compensation expense was recognized for the vested portion of the award upon grant. The stock options may include provisions for early exercise of options. If any shares acquired are unvested, they are subject to repurchase at NewLink’s discretion until they become vested.
The following table summarizes the stock option activity for the year ended December 31, 2013:
|
| | | | | | | | | |
| | | Number of options | | Weighted average exercise price | | Weighted average remaining contractual term (years) |
| Outstanding at beginning of period | | 3,752,413 |
| | $ | 4.34 |
| | |
| Options granted | | 860,250 |
| | 12.54 |
| | |
| Options exercised | | (107,541 | ) | | 4.11 |
| | |
| Options forfeited | | (18,558 | ) | | 10.25 |
| | |
| Options expired | | — |
| | — |
| | |
| Outstanding at end of period | | 4,486,564 |
| | $ | 5.89 |
| | 6.8 |
| Options exercisable at end of period | | 3,193,753 |
| | $ | 3.87 |
| | 6.1 |
Based on the December 31, 2013 price of $22.01 per share, the intrinsic value of stock options outstanding at December 31, 2013, was $72.3 million, of which $57.9 million and $14.4 million related to stock options that were vested and unvested, respectively, at that date.
The following table summarizes options that were granted during the years ended December 31, 2013, 2012 and 2011, and the range of assumptions used to estimate the fair value of those stock options using a Black-Scholes valuation model:
|
| | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2013 | | 2012 | | 2011 |
| Number of options granted | | 860,250 |
| | 672,079 |
| | 664,323 |
|
| Risk-free interest rate | | .89%-2.14% |
| | .98%-2.05% |
| | 0.1%-3.1% |
|
| Expected dividend yield | | — |
| | — |
| | — |
|
| Expected volatility | | 61.4%-67.3% |
| | 63.05%-73.6% |
| | 62.6%-67.9% |
|
| Expected term (in years) | | 4.8-7.0 |
| | 5.8-7.0 |
| | 5.0-7.5 |
|
| Weighted average grant-date fair value per share | | $7.50 | | $ | 5.02 |
| | $ | 7.12 |
|
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
The following table summarizes the intrinsic value of options exercised and the fair value of awards vested during the years ended December 31, 2013, 2012 and 2011:
|
| | | | | | | | |
| | | Years Ended December 31, |
| | | 2013 | | 2012 | | 2011 |
| Intrinsic value of options exercised | | $1.6 million | | $3.6 million | | $ | 194,000 |
|
| Fair value of awards vested | | $3.0 million | | $2.5 million | | $7.8 million |
|
NewLink does not have a formal policy regarding the source of shares issued upon exercise of stock options. NewLink expects shares issued upon future stock option exercises to be issued from treasury shares or new shares.
8. Income Taxes
The tax effects of temporary differences that give rise to significant portions of deferred tax assets and the deferred tax liability at December 31, 2013 and 2012 are presented below (in thousands):
|
| | | | | | | | |
| | | Year Ended December 31, |
| | | 2013 | | 2012 |
| Deferred tax assets: | | | | |
| Net operating loss carryforwards | | $ | 18,556 |
| | $ | 19,853 |
|
| Federal research credits | | 4,322 |
| | 2,912 |
|
| Share-based compensation | | 1,748 |
| | — |
|
| Deferred rent | | 295 |
| | 313 |
|
| Minimum tax credits | | 120 |
| | — |
|
| Accrued compensation | | 107 |
| | 76 |
|
| Leasehold improvements and equipment | | 20 |
| | — |
|
| Gross deferred tax assets | | 25,168 |
| | 23,154 |
|
| Less valuation allowance | | (25,168 | ) | | (23,137 | ) |
| Net deferred tax assets | | — |
| | 17 |
|
| Deferred tax liability: | | | | |
| Leasehold improvements and equipment | | — |
| | (17 | ) |
| Total net deferred tax assets | | $ | — |
| | $ | — |
|
The valuation allowance for deferred tax assets as of December 31, 2013 and 2012 was $25.2 million and $23.1 million, respectively. The net change in the total valuation allowance for the years ended December 31, 2013 and 2012 was an increase of $2.0 million and $4.3 million, respectively. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected taxable income, and tax planning strategies in making this assessment. Valuation allowances have been established for the entire amount of the net deferred tax assets as of December 31, 2013 and 2012, due to the uncertainty of future recoverability.
Federal operating loss carryforwards as of December 31, 2013 of approximately $88.4 million and federal research credit carryforwards of approximately $4.3 million expire at various dates from 2020 through 2032. Sections 382 and 383 of the Internal Revenue Code limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset any future taxable income or tax if the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. State net operating loss carryforwards (and certain other tax attributes) may be similarly
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
limited. An ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
From its inception through December 31, 2011, NewLink experienced Section 382 ownership changes in September 2001 and March 2003 and BPS experienced Section 382 ownership changes in January 2006 and January 2011 and the reported deferred tax assets reported reflect these expected limitations. These ownership changes limit NewLink's ability to utilize federal net operating loss carryforwards (and certain other tax attributes) that accrued prior to the respective ownership changes of NewLink and our subsidiary. Additional ownership changes may occur in the future as a result of events over which the Company will have little or no control, including purchases and sales of the Company’s equity by our 5% stockholders, the emergence of new 5% stockholders, additional equity offerings or redemptions of the Company’s stock or certain changes in the ownership of any of the Company’s 5% stockholders.
Income tax expense was $130,000, $0, $0, and $130,000 for the years ended December 31, 2013, 2012 and 2011, and for the period from inception through December 31, 2013. Income tax expense differs from the amount that would be expected after applying the statutory U.S. federal income tax rate primarily due to changes in the valuation allowance for deferred taxes.
9. Net Loss per Common Share
Basic net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding for the period, without consideration of common stock equivalents. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of common share equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, preferred stock, stock options and warrants are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following table presents the computation of basic and diluted net loss per common share (in thousands, except share and per share data):
|
| | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2013 | | 2012 | | 2011 | |
| Historical net loss per share | | | | | | | |
| Numerator | | | | | | | |
| Net loss attributable to common stockholders | | $ | (31,180 | ) | | $ | (23,321 | ) | | $ | (18,087 | ) | |
| Denominator | | | | | | | |
| Weighted-average common shares outstanding (basic and diluted) | | 25,275,179 |
| | 20,779,450 |
| | 6,064,542 |
| |
| Basic and diluted net loss per share | | $ | (1.23 | ) | | $ | (1.12 | ) | | (2.98 | ) | |
Potentially dilutive securities not included in the calculation of diluted net loss per common share because to do so would be anti-dilutive are common stock options of 4,486,564, 3,752,413, and 3,515,051 as of December 31, 2013, 2012 and 2011, respectively.
10. Licensing Agreements
The Company is a party to a number of licensing agreements with respect to certain of the technologies that underlie its intellectual property. These agreements typically provide that the Company has exclusive rights to the use and sublicensing of the technologies in question for the duration of the intellectual property patent protection in question, subject to the Company meeting its financial and other contractual obligations under the agreements. The Company recognizes expense under its licensing agreements in the period the obligation is incurred. These agreements typically provide for a license fee based on a percent of sales and annual minimum royalties. For additional information regarding how the Company records payments under these agreements, see note 2(i) above. The Company has made payments of approximately $88,000, $255,000, $89,000, and $2.9 million under all of the in-licensing agreements for the years ended December 31, 2013, 2012, 2011, and from inception, respectively, which is recorded as a component of general and administrative expenses.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
Under certain license agreements the Company is obligated to make potential milestone payments as listed in the following table. In addition to the milestone payments, each license is paid as a low single digit percentage of net sales of the licensed product, subject to annual minimum royalties. These obligations are contingent upon achieving the applicable milestone event, the timing of which cannot presently be determined. The milestone payments and royalty payments are in place through at least the expiration of certain of the Company's patents, which is currently 2029 and beyond.
|
| | |
| Licensor | | Aggregate potential milestone payments |
| Drexel University | | $1 million per licensed product |
| | | |
| Lankenau Institute for Medical Research under the IDO-1 Agreement | | $1.36 million per licensed product |
| | | |
| Lankenau Institute for Medical Research under the LIMR IDO-2 Agreement | | $1.52 million per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement |
| | | |
| Lankenau Institute for Medical Research under the 2009 LIMR Agreement | | $610,000 per licensed product, subject to reductions if milestones also qualify under the IDO-1 Agreement or LIMR IDO-2 Agreement |
| | | |
| Georgia Health Sciences University | | $2.8 million per licensed product |
| | | |
| Regents of the University of California | | $285,000 per licensed product |
| | | |
| Her Majesty the Queen in Right of Canada | | C$205,000 per licensed product |
| | | |
| The Ohio State University | | $2.75 million for first licensed product |
11. Employee Benefit Plans
The Company sponsors a 401(k) plan, which includes a defined contribution feature. The Company's defined contribution was $198,000, $136,000, $157,000 and $1.0 million for the years ended December 31, 2013, 2012 and 2011 and from inception through December 31, 2013, respectively. The Company made discretionary contributions to the plan of $144,000, $152,000, $136,000 and $985,000 for the years ended December 31, 2013, 2012 and 2011 and from inception through December 31, 2013, respectively.
The Company has approved employment agreements for certain executives, dated October 29, 2010, as amended August 13, 2013, that provide for the payment of 6 to 24 months of base salary, bonus, and group health insurance premiums plus accrued obligations upon termination of the executive in certain circumstances. The agreements include provisions to accelerate the vesting of stock options subject to certain events including those related to a change in control. The Company entered into the August 2013 amendments to the foregoing agreements, upon recommendation by the Compensation Committee of the Board of Directors, to align the terms of certain termination benefits with termination terms for executive officers at similarly situated companies to the Company.
12. Related-Party Transactions
On January 7, 2011, we acquired all of the minority interest in BPS, by merging a newly-formed subsidiary of ours with BPS, with BPS as the surviving corporation. In connection with this transaction, we issued an aggregate of 276,304 shares of our Series E preferred stock to the former holders of BPS Series B common stock, BPS Series A preferred stock and BPS Series B preferred stock (other than the Company). As a result of this transaction, BPS became a wholly-owned subsidiary of the Company and our note was converted into Series B preferred stock of BPS. All options to purchase shares of BPS stock became options to purchase a total of 50,513 shares of our common stock.
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
In 2013, NewLink created a wholly owned subsidiary, NewLink International (NI). NewLink plans to conduct all or a portion of its operations outside of the United States through NI.
Certain purchasing activities are outsourced to a company owned by an immediate family member of the Company’s VP of Finance. Total purchases through this related party were $59,000, $35,000 and $22,000 and $386,000 for the years ended December 31, 2013, 2012 and 2011 and from inception through December 31, 2013. The Company paid fees to this related party for consulting services of approximately $0, $0, $0 and $7,000 for the years ended December 31, 2013, 2012 and 2011 and from inception through December 31, 2013, respectively. Amounts payable were $0 and $0 as of December 31, 2013 and 2012, respectively.
13. Acquisition of BioProtection Systems Corporation
On January 7, 2011, NewLink acquired all of the minority interest in BPS, by merging a newly-formed subsidiary of NewLink’s with BPS, with BPS as the surviving corporation. In connection with this transaction, NewLink issued an aggregate of 276,304 shares of NewLink’s Series E preferred stock with a value of $8.6 million to the former holders of BPS Series B common stock, Series A preferred stock and Series B preferred stock (other than NewLink). As a result of this transaction, BPS became a wholly-owned subsidiary of NewLink. All options to purchase shares of BPS common stock became options to purchase NewLink’s common stock. The net assets of BPS had a book value of $2.9 million. The remaining amount paid of $5.7 million was recorded as a reduction of additional paid-in capital. No gain or loss was recorded as a result of this transaction.
14. Reverse Stock Split
On October 19, 2011, the Company’s board of directors approved a 2.1-for-one reverse split of the Company’s common stock to be effected prior to the effective date of the Company’s IPO. In connection with the reverse split, the Company filed a Certificate of Amendment of the Restated Certificate of Incorporation with the Secretary of State of Delaware on October 25, 2011 making the reverse split effective. All share and per share amounts have been retroactively restated in the accompanying financial statements and notes for all periods presented.
15. Quarterly Financial Information (Unaudited)
|
| | | | | | | | | | | | | | | | | |
| | | First | | Second | | Third | | Fourth | |
| | | (In thousands, except per share data) | |
| | | | | | | | | | |
| Year Ended December 31, 2013 | | | | | | | | | |
| Grant revenue | | $ | 302 |
| | $ | 232 |
| | $ | 265 |
| | $ | 294 |
| |
| Loss from operations | | (8,042 | ) | | (7,069 | ) | | (8,117 | ) | | (7,913 | ) | |
| Net loss | | (7,934 | ) | | (7,077 | ) | | (8,123 | ) | | (8,046 | ) | |
| Net loss per share | | $ | (0.33 | ) | | $ | (0.28 | ) | | $ | (0.32 | ) | | $ | (0.31 | ) | |
| | | | | | | | | | |
| Year Ended December 31, 2012 | | | | | | | | | |
| Grant revenue | | $ | 471 |
| | $ | 590 |
| | $ | 327 |
| | $ | 299 |
| |
| Loss from operations | | (4,817 | ) | | (6,301 | ) | | (5,848 | ) | | (6,293 | ) | |
| Net loss | | (4,842 | ) | | (6,309 | ) | | (5,851 | ) | | (6,319 | ) | |
| Net loss per share | | $ | (0.23 | ) | | $ | (0.31 | ) | | $ | (0.28 | ) | | $ | (0.30 | ) | |
| | | | | | | | | | |
NewLink Genetics Corporation
and Subsidiaries
(A Development Stage Enterprise)
Notes to Consolidated Financial Statements
16. Subsequent Events
NewLink entered into a sales agreement with Cantor Fitzgerald & Co., dated as of September 5, 2013, under which NewLink may sell up to $60.0 million in shares of its common stock in one or more placements at prevailing market prices for its common stock. Any such sales would be effected pursuant to its registration statement on Form S-3 (333-185721), declared effective by the SEC on January 4, 2013. Subsequent to December 31, 2013 and through February 13, 2014, the Company sold an additional 1,017,217 shares of common stock under the ATM Offering raising an additional $27.6 million in net proceeds.
On February 24, 2014, NewLink signed a lease for a commercial facility in Austin, Texas. The Company anticipates conducting activities at this location associated with expansion of the Company's commercialization efforts as well as supporting the continued growth of its clinical operations activities.
On January 2, 2014, NewLink approved grants of restricted stock unit awards to certain of the named executive officers for extraordinary performance in 2013.
Index to Exhibits
The following exhibits are filed with this form 10-K or incorporated herein by reference to the document set forth next to the exhibit listed below. Where so indicated, exhibits that were previously filed are incorporated by reference.
|
| | | | | | |
| | | | Incorporated By Reference | |
| Exhibit Number | | Description | Form | Filing Date | Number | Filed Herewith |
| 3.1 | | Amended and Restated Certificate of Incorporation filed on November 16, 2011 | 8-K | 11/18/2011 | 3.1 | |
| 3.2 | | Certificate of Amendment to Restated Certificate of Incorporation filed on May 10, 2013 | 8-K | 5/14/2013 | 3.1 | |
| 3.3 | | Amended and Restated Bylaws | 8-K | 11/18/2011 | 3.2 | |
| 4.1 | | Form of the Registrant's Common Stock Certificate | S-1/A | 10/26/2011 | 4.1 | |
| 4.2 | | Reference is made to Exhibits 3.1, 3.2 and 3.3 | | | | |
| 4.3 | | Amended and Restated Investor Rights Agreement by and between the Company and certain holders of the Company's capital stock dated as of December 1, 2010 | 10-Q | 5/10/2012 | 4.3 | |
| 10.2 | † | Form of Indemnity Agreement by and between the Registrant and its directors and executive officers | S-1/A | 11/8/2011 | 10.11 | |
| 10.3 | † | 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.2 | |
| 10.4.1 | † | Form of Stock Option Agreement under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.3 | |
| 10.4.2 | † | Form of Stock Option Grant Notice under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.4 | |
| 10.4.3 | † | Form of Stock Bonus Agreement under 2000 Equity Incentive Plan | S-1 | 12/21/2010 | 10.5 | |
| 10.5 | † | Amended and Restated 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.6 | |
| 10.5.1 | † | Form of Stock Option Agreement under 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.7 | |
| 10.5.2 | † | Form of Stock Option Grant Notice under 2009 Equity Incentive Plan | S-1 | 12/21/2010 | 10.8 | |
| 10.6 | † | 2010 Employee Stock Purchase Plan | 8-K | 5/14/2013 | 10.2 | |
| 10.7 | † | 2010 Non-Employee Directors' Stock Award Plan | 8-K | 5/14/2013 | 10.1 | |
| 10.8 | † | Employment Agreement, dated as of December 6, 2010, by and between the Registrant and Charles J. Link, Jr. | S-1 | 12/21/2010 | 10.12 | |
| 10.9 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Nicholas N. Vahanian | S-1 | 12/21/2010 | 10.13 | |
| 10.10 | † | Employment Agreement, dated as of June 26, 2008, by and between the Registrant and Gordon H. Link, Jr. | S-1 | 12/21/2010 | 10.14 | |
| 10.11 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Gordon H. Link, Jr. | S-1 | 12/21/2010 | 10.15 | |
| 10.12 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and Kenneth Lynn | S-1 | 12/21/2010 | 10.16 | |
| 10.13 | † | Employment Agreement, dated as of November 22, 2010, by and between the Registrant and W. Jay Ramsey | S-1 | 12/21/2010 | 10.17 | |
| 10.14 | † | Form of Employee Proprietary Information and Inventions Agreement | S-1 | 12/21/2010 | 10.18 | |
| 10.15 | † | Promissory Note dated May 2, 2008 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.19 | |
| 10.16 | † | Promissory Note dated April 18, 2000 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.20 | |
| 10.17 | † | Promissory Note dated August 20, 2008 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.21 | |
| 10.18 | † | Promissory Note dated July 2008 by and between the Registrant and Gordon Link | S-1/A | 2/28/2011 | 10.22 | |
| 10.19 | † | Amendment Agreement dated July 1, 2010 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.23 | |
| 10.20 | † | Amendment Agreement dated July 1, 2010 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.24 | |
| 10.21 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Charles Link | S-1/A | 2/28/2011 | 10.25 | |
|
| | | | | | |
| 10.22 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.26 | |
| 10.23 | † | Acknowledgment Agreement dated November 24, 2010 by and between the Registrant and Gordon Link | S-1/A | 2/28/2011 | 10.27 | |
| 10.24 | † | Acknowledgment Agreement dated November 24, 2010 by and between BioProtection Systems Corporation and Charles Link | S-1/A | 2/28/2011 | 10.28 | |
| 10.25 | † | Acknowledgment Agreement dated November 23, 2010 by and between BioProtection Systems Corporation and Nicholas Vahanian | S-1/A | 2/28/2011 | 10.29 | |
| 10.26 | * | License Agreement dated July 7, 2005 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.30 | |
| 10.26.1 | * | First Amendment to License Agreement dated May 22, 2006 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.31 | |
| 10.26.2 | * | Second Amendment to License Agreement September 11, 2007 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.32 | |
| 10.27 | * | Exclusive License Agreement executed December 21, 2007 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.33 | |
| 10.28 | * | Exclusive License Agreement effective April 23, 2009 by and between the Registrant and Lankenau Institute for Medical Research | S-1/A | 11/8/2011 | 10.34 | |
| 10.29 | * | License Agreement dated October 13, 2004 by and between the Registrant and Drexel University | S-1/A | 11/8/2011 | 10.36 | |
| 10.30 | * | License Agreement dated August 2, 2001 by and between the Registrant and Central Iowa Health System | S-1/A | 11/8/2011 | 10.37 | |
| 10.31 | * | License Agreement dated September 13, 2005 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.46 | |
| 10.31.1 | * | First Amendment to License Agreement dated April 27, 2006 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.47 | |
| 10.31.2 | * | Second Amendment to License Agreement dated April 27, 2006 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.48 | |
| 10.31.3 | * | Third Amendment to License Agreement dated February 13, 2007 by and between the Registrant and Medical College of Georgia Research Institute, Inc. | S-1/A | 11/8/2011 | 10.49 | |
| 10.32 | * | Patent License Agreement dated March 1, 2006 by and between the Registrant and Bresagen Xenograft Marketing Ltd. | S-1/A | 11/8/2011 | 10.50 | |
| 10.33 | * | Exclusive License Agreement dated July 29, 2008 by and between the Regents of the University of California and BioProtection Systems Corporation | S-1/A | 11/8/2011 | 10.66 | |
| 10.34 | * | Sole License Agreement executed May 4, 2010 by and between Her Majesty the Queen in Right of Canada and BioProtection Systems Corporation | S-1/A | 11/8/2011 | 10.67 | |
| 10.35 | * | Letter of Intent for Cooperative Research and Development Agreement (CRADA #2166) dated May 7, 2007 by and between the Registrant and National Cancer Institute | S-1/A | 11/8/2011 | 10.38 | |
| 10.35.1 | | Amendment No. 1 to Letter of Intent for CRADA #2166 dated January 17, 2008 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.39 | |
| 10.35.2 | | Amendment No. 2 to Letter of Intent for CRADA #2166 dated July 7, 2008 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.40 | |
| 10.35.3 | | Amendment No. 3 to Letter of Intent for CRADA #2166 dated March 24, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.41 | |
| 10.35.4 | | Amendment No. 4 to Letter of Intent for CRADA #2166 dated October 28, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.42 | |
| 10.35.5 | | Amendment No. 5 to Letter of Intent for CRADA #2166 dated December 16, 2009 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.43 | |
| 10.35.6 | | Amendment No. 6 to Letter of Intent for CRADA #2166 dated June 29, 2010 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.44 | |
| 10.35.7 | | Amendment No. 7 to Letter of Intent for CRADA #2166 dated November 26, 2010 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.45 | |
|
| | | | | | |
| 10.35.8 | | Amendment No. 8 to Letter of Intent for CRADA #2166 dated June 2, 2011 by and between the Registrant and National Cancer Institute | S-1/A | 10/4/2011 | 10.79 | |
| 10.36 | | Lease dated September 1, 2000 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.46 | |
| 10.37 | | Sublease Agreement effective February 1, 2001 by and between the Registrant and Iowa State Innovation System | S-1 | 12/21/2010 | 10.47 | |
| 10.38 | | Memorandum of Agreement dated December 6, 2005 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.48 | |
| 10.39 | | Memorandum of Agreement dated April 13, 2006 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.49 | |
| 10.40 | | Memorandum of Agreement dated February 20, 2008 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.50 | |
| 10.41 | | Memorandum of Agreement dated May 1, 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.51 | |
| 10.42 | | Memorandum of Agreement dated March 24, 2010 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.52 | |
| 10.43 | | Lease dated September 30, 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.53 | |
| 10.44 | | Lease dated August 10, 2005 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.82 | |
| 10.45 | | Memorandum of Agreement dated September 29, 2011 by and between the Registrant and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.84 | |
| 10.46 | | Memorandum of Agreement dated September 29, 2011 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | S-1/A | 10/26/2011 | 10.83 | |
| 10.47 | | Memorandum of Agreement dated November 14, 2011 by and between NewLink Genetics Corporation and Iowa State University Research Park Corporation | 8-K | 11/18/2011 | 10.1 | |
| 10.48 | | Promissory Note executed in 2009 by and between the Registrant and Iowa State University Research Park Corporation | S-1 | 12/21/2010 | 10.54 | |
| 10.49 | | Forgivable Loan Agreement dated March 10, 2010 by and between the Registrant and City of Ames, Iowa | S-1 | 12/21/2010 | 10.55 | |
| 10.50 | | Iowa Values Fund Agreement dated March 18, 2005 by and between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.56 | |
| 10.51 | | Master Contract dated December 29, 2005 by and between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.58 | |
| 10.52 | | Contract Amendment dated April 21, 2009 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.59 | |
| 10.53 | | Contract Amendment dated August 19, 2010 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.57 | |
| 10.54 | | Contract Amendment dated August 19, 2010 between the Registrant and Iowa Department of Economic Development | S-1 | 12/21/2010 | 10.60 | |
| 10.55 | | Contract Amendment effective February 17, 2011 between the Registrant and Iowa Department of Economic Development | S-1/A | 9/14/2011 | 10.77 | |
| 10.56 | | Contract Amendment effective February 17, 2011 between the Registrant and Iowa Department of Economic Development | S-1/A | 9/14/2011 | 10.78 | |
| 10.57 | | Contract No. W911NF-08-C-0044 dated May 5, 2008 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.68 | |
| 10.57.1 | | Amendment to Contract No. W911NF-08-C-0044 dated February 12, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.69 | |
| 10.58 | * | Contract No. HDTRA1-09-C-0014 dated September 25, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 11/8/2011 | 10.70 | |
| 10.58.1 | | Amendment of Contract No. HDTRA1-09-C-0014 dated September 20, 2011 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 10/4/2011 | 10.80 | |
| 10.59 | | Contract No. W911NF-09-C-0072 dated July 31, 2009 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.71 | |
|
| | | | | | |
| 10.59.1 | | Amendment to Contract No. W911NF-09-C-0072 dated April 21, 2010 by and between BioProtection Systems Corporation and the United States Department of Defense | S-1/A | 2/28/2011 | 10.72 | |
| 10.60 | | Grant Number 5U01AI066327-05 issued August 26, 2009 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 2/28/2011 | 10.73 | |
| 10.61 | | Grant Number 1R43AI084350-01A1 issued April 6, 2010 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 2/28/2011 | 10.74 | |
| 10.62 | | Grant Number 5R43AI084350-02 issued March 24, 2011 by and between BioProtection Systems Corporation and the National Institutes of Health | S-1/A | 10/4/2011 | 10.81 | |
| 10.63 | | Agreement and Plan of Merger dated December 1, 2010 by and between the Registrant, BPS Merger Sub, Inc., BioProtection Systems Corporation and BPS Stockholder Representative, LLC | S-1/A | 2/28/2011 | 10.75 | |
| 10.64 | | Certificate of Merger of BPS Merger Sub, Inc. into BioProtection Systems Corporation filed on January 7, 2011 | S-1/A | 2/28/2011 | 10.76 | |
| 10.65 | | Standard Design-Build Agreement dated November 14, 2011 by and between NewLink Genetics Corporation and Story Construction Co. | 8-K | 11/18/2011 | 10.2 | |
| 10.66 | | NewLink Genetics Corporation 401(k) Prototype Plan and Trust, effective as of January 1, 2005 | 8-K | 3/12/2012 | 10.2 | |
| 10.67 | | NewLink Genetics Corporation 401(k) Adoption Agreement, effective as of January 1, 2005 | 8-K | 3/12/2012 | 10.3 | |
| 10.68 | | Material Modification to the NewLink Genetics Corporation 401(k) Adoption Agreement, effective as of January 1, 2011 | 8-K | 3/12/2012 | 10.4 | |
| 10.69 | | Settlement Agreement with the Iowa Economic Development Authority, effective as of March 26, 2013 | 8-K | 3/28/2012 | 10.1 | |
| 10.70 | † | 2012 Target Bonus Awards | 8-K | 3/28/2012 | 10.2 | |
| 10.71 | * | Cooperative Research and Development Agreement between the Company and the National Cancer Institute, effective as of March 27, 2012 | 10-Q | 5/10/2012 | 10.6 | |
| 10.72 | | Letter of Resignation of Executive Vice President of Business Development, effective as of June 27, 2012 | 10-Q | 8/14/2012 | 10.2 | |
| 10.73 | † | Offer of Employment for Head of Business Development, effective as of July 26, 2012 | 10-Q | 8/14/2012 | 10.3 | |
| 10.74 | † | 2013 Salaries and Target Bonus Awards | 8-K | 11/7/2012 | 10.1 | |
| 10.75 | † | 2012 Named Executive Officer Bonus Awards | 8-K | 12/21/2012 | 10.1 | |
| 10.76 | | Underwriting Agreement, effective as of January 30, 2013 | 8-K | 1/31/2013 | 1.1 | |
| 10.77 | | Memorandum of Agreement dated May 7, 2012 by and between the Registrant and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.1 | |
| 10.78 | | Memorandum of Agreement dated May 7, 2012 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.2 | |
| 10.79 | | Memorandum of Agreement dated November 6, 2012 by and between BioProtection Systems Corporation and Iowa State University Research Park Corporation | 10-K | 3/15/2013 | 10.3 | |
| 10.80 | † | 2013 Target Bonus Awards | 8-K | 4/5/2013 | 10.1 | |
| 10.81 | | Memorandum of Agreement dated April 15, 2013 by and between the Registrant and Iowa State University Research Park Corporation | 10-Q | 5/8/2013 | 10.1 | |
| 10.82 | | Story Construction Contract | 10-Q | 8/8/2013 | 10.1 | |
| 10.83 | | Memorandum of Agreement; Addendum to the Lease Between ISU Research Park Corporation and NewLink Genetics Corporation Dated March 1, 2010 | 10-Q | 8/8/2013 | 10.2 | |
| 10.84 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Charles J. Link, Jr. | 8-K | 8/14/2013 | 10.1 | |
| 10.85 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Nicholas N. Vahanian | 8-K | 8/14/2013 | 10.2 | |
| 10.86 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and Gordon H. Link, Jr. | 8-K | 8/14/2013 | 10.3 | |
| 10.87 | † | First Amendment to Employment Agreement, dated as of August 13, 2013, by and between Registrant and W. Jay Ramsey | 8-K | 8/14/2013 | 10.4 | |
|
| | | | | | |
| 10.88 | | Controlled Equity OfferingSM Sales Agreement, dated September 5, 2013, by and between NewLink Genetics Corporation and Cantor Fitzgerald & Co. | 8-K | 9/5/2013 | 10.1 | |
| 10.89 | | Amendment of Contract No. HDTRA1-09-C-0014, by and between BioProtection Systems Corporation and the United States Department of Defense, dated as of September 18, 2013 | 10-Q | 11/12/2013 | 10.1 | |
| 10.90 | | License Agreement Amendment, by and between NewLink Genetics Corporation and Georgia Health Sciences University Research Institute, dated as of July 13, 2013 | 10-Q | 11/12/2013 | 10.2 | |
| 10.91 | † | 2013 Bonus Awards | 8-K | 1/8/2014 | 10.1 | |
| 10.92 | † | 2014 Salaries, Bonus Targets and Equity Awards | 8-K | 1/8/2014 | 10.2 | |
| 10.93 | | Memorandum of Agreement, dated January 4, 2014, by and between the Registrant and Iowa State University Research Park Corporation | | | | X |
| 10.94 | * | Amendment to License Agreement dated December 30, 2013, by and between Registrant and Central Iowa health System | | | | X |
| 10.95 | † | Employment Agreement, dated as of March 11,2014, by and between Registrant and Brian Wiley | | | | X |
| 10.96 | † | Employment Agreement, dated as of March 11,2014, by and between Registrant and Carl Langren | | | | X |
| 21.1 | | Subsidiary Information | | | | X |
| 23.1 | | Consent of KPMG LLP, independent registered public accounting firm | | | | X |
| 24.1 | | Power of Attorney (included on signature page hereto) | | | | X |
| 31.1 | | Rule 13a-14(a)/15d-14(a) Certification | | | | X |
| 31.2 | | Rule 13a-14(a)/15d-14(a) Certification | | | | X |
| 32.1 | # | Section 1350 Certification | | | | X |
| 101.INS | | XBRL Instance Document (filed electronically herewith) | | | | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document (filed electronically herewith) | | | | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document (filed electronically herewith) | | | | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document (filed electronically herewith) | | | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document (filed electronically herewith) | | | | |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document (filed electronically herewith) | | | | |
| | | | | | | |
____________________
|
| |
| † | Indicates management contract or compensatory plan. |
| * | Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 19434, as amended. |
| # | The certifications attached as Exhibit 32.1 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of NewLink Genetics Corporation under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing. |