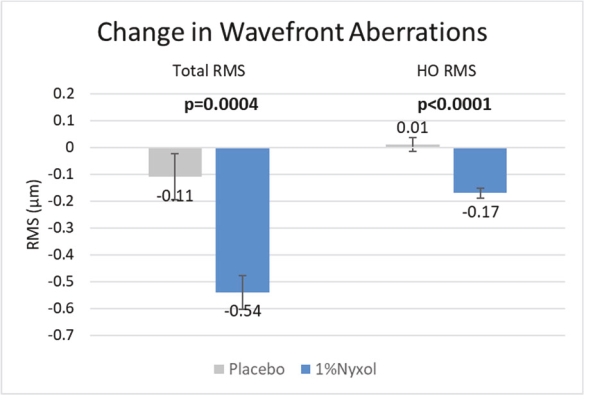
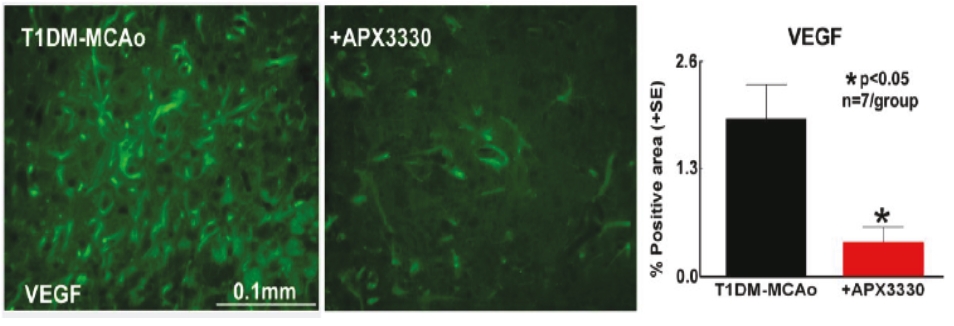
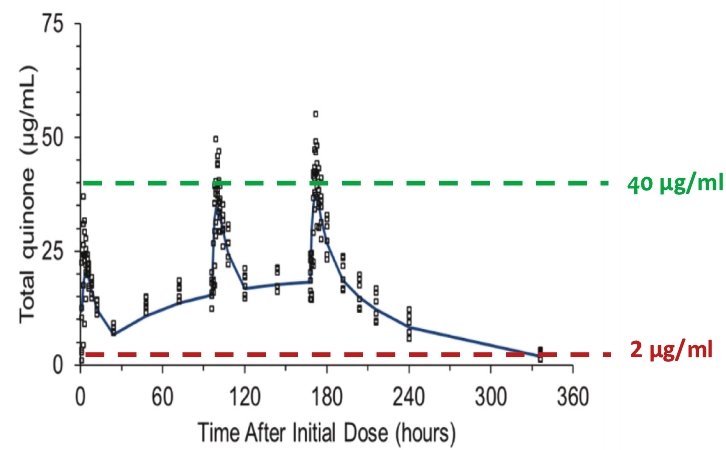
As of June 22, 2020, Ocuphire’s patent estate relating to Nyxol contains nine U.S. patents, five pending U.S. non-provisional patent applications, two pending international patent applications, as well as issued patents in Australia, Europe, Japan, and Mexico and pending patent applications in Australia, Canada, Europe, and Japan.
Ocuphire’s U.S. Patents 9,795,560 and 10,278,918 and counterpart Australian, European, and Japanese patents each contain composition of matter claims to aqueous phentolamine mesylate formulations and are scheduled to expire in year 2034. A counterpart patent application directed to aqueous phentolamine mesylate is pending in Canada, where a patent, if granted, based on this pending patent application would expire in year 2034. In the same patent family, Ocuphire also has 2 pending U.S. patent applications with additional claims to aqueous phentolamine mesylate formulations, whereby such patents, if granted, would expire in year 2034.
Ocuphire’s U.S. Patent Nos. 9,089,560 and 9,789,088 contain claims directed to methods of improving visual performance using, for example, phentolamine mesylate and are scheduled to expire in year 2034. Counterpart patents have issued in Australia and Japan, which are scheduled to expire in year 2034. Counterpart patent applications are pending in Australia, Canada, Europe, and Japan, where the Australian patent application has been allowable, and the European Patent Office has deemed the claims in Ocuphire’s European patent application to be allowable. These patents, if granted, would expire in year 2034.
Ocuphire’s pending international patent application PCT/US2019/056324 is directed to treating glaucoma and other medical disorders using phentolamine. These patents, if granted, would expire in year 2039. Ocuphire’s pending international patent application PCT/US2019/058182 is directed to methods of treating presbyopia, mydriasis, and other medical disorders; such patents, if granted, would expire in year 2039. Currently, two U.S. patent applications are pending based on international patent application PCT/US2019/058182, one with claims to treating presbyopia and the other U.S. application with claims to treating mydriasis.
The remaining five of Ocuphire’s U.S. patents are scheduled to expire in year 2020 and have claims to methods of use or ophthalmic formulations containing an ophthalmic artificial tear solution. Ocuphire’s issued patent in Mexico is scheduled to expire in year 2025 and has claims to ophthalmic formulations.
Ocuphire has registered trademark protection in the United States for the mark NYXOL®.
APX3330
The patent estate that Ocuphire has in-licensed for APX3330 and related compounds contains five U.S. patents, four pending U.S. non-provisional patent applications, and one pending international patent application, as well as issued patents in Europe, Japan, Canada, and Australia, and pending patent applications in Europe, Japan, and Canada. The license is for the use and commercialization of APX3330 and related composition of matter compounds covered by the subject patents and patent applications in the field of human health uses for ophthalmic and diabetes mellitus indications.
In-licensed U.S. patent 9,040,505 has claims to methods of treating diabetic retinopathy and other diseases using, for example, APX3330 and is scheduled to expire in year 2030. Counterpart patents have issued in Europe, Japan, Australia, and Canada, which are scheduled to expire in year 2028, and there is a related pending U.S. patent application that, if issued as a patent, would expire in year 2028. International patent application PCT/US2019/017023 has claims to methods of treating wAMD and other diseases using, for example, APX3330, along with other formulations such as APX2009 and APX2014. These patents, if granted, would expire in year 2039. The U.S. and certain foreign countries permit extension of patent term for up to five years to compensate for patent term lost during the government regulatory review process for a new medicine. If U.S. patent 9,040,505 qualifies for the full five years of patent term extension, the expiration of U.S. patent 9,040,505 would be in year 2035. Whether U.S. patent 9,040,505 qualifies for the full five years of patent term extension depends in part on the date of FDA approval for the new medicine, because a U.S. patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval.
In-licensed patent applications directed to use of a Ref-1 inhibitor, such as APX3330, in combination therapy to treat retinal diseases and in monotherapy or combination therapy to reduce neuronal sensitivity and/or treat other indications are pending in the U.S., Europe, Japan, and Canada. Patents, if granted, would expire in year 2038.
Patents that Ocuphire has in-licensed to derivatives of APX3330 include U.S. patents 9,089,605; 9,193,700; 9,877,936; and 10,154,973 and counterpart patents in Europe, Japan, China, and Canada that are scheduled to