UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
☑ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-35050
ENDOCYTE, INC.
(Exact name of Registrant as specified in its charter)
Delaware |
| 35-1969-140 |
(State or other jurisdiction of |
| (I.R.S. Employer |
3000 Kent Avenue, Suite A1-100
West Lafayette, IN 47906
(Address of Registrant’s principal executive offices)
Registrant’s telephone number, including area code: (765) 463-7175
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Name of Each Exchange on Which Registered |
Common Stock, $.001 par value |
| NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☑
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ☐ | Accelerated filer ☑ | Non-accelerated filer ☐ | Smaller reporting company ☐ Emerging Growth Company ☐ |
|
| (Do not check if a smaller reporting company) |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☑ No
The aggregate market value of the registrant’s common stock, $0.001 par value per share, held by non-affiliates of the registrant, based upon the closing price of the Common Stock on the Nasdaq Global Market on June 30, 2017, was approximately $57.2 million (excludes shares of the registrant’s common stock held as of such date by officers, directors and stockholders that the registrant has concluded are or were affiliates of the registrant, exclusion of such shares should not be construed to indicate that the holder of any such shares possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.)
Number of shares of the registrant’s Common Stock, $0.001 par value, outstanding on February 23, 2018: 48,349,930
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement to be delivered to stockholders in connection with the 2018 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
ENDOCYTE, INC.
ANNUAL REPORT ON FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2017
This annual report contains certain statements that are forward-looking statements within the meaning of federal securities laws. When used in this report, the words “may,” “will,” “should,” “could,” “would,” “anticipate,” “estimate,” “expect,” “plan,” “believe,” “predict,” “potential,” “project,” “target,” “forecast,” “intend” and similar expressions are intended to identify forward-looking statements. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those projected. These risks and uncertainties include the important risks and uncertainties that may affect our future operations that we describe in Part I, Item 1A — Risk Factors of this report, including, but not limited to, statements regarding the progress and timing of clinical trials, the safety and efficacy of our product candidates, the goals of our development activities, estimates of the potential markets for our product candidates, estimates of the capacity of manufacturing and other facilities to support our product candidates, projected cash needs and our expected future revenues, operations, expenditures, and cash position. Readers of this report are cautioned not to place undue reliance on these forward-looking statements. While we believe the assumptions on which the forward-looking statements are based are reasonable, there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this report.
i
Overview
We are a biopharmaceutical company and leader in developing targeted therapies for the treatment of cancer. We use drug conjugation technology to create novel therapeutics and companion imaging agents for personalized targeted therapies. Our agents actively target receptors that are over-expressed on diseased cells relative to healthy cells, such as prostate specific membrane antigen, or PSMA, in prostate cancer. This targeted approach is designed to safely enable the delivery of highly potent drug payloads. The companion imaging agents are designed to identify patients whose disease over-expresses the target of the therapy and who are therefore more likely to benefit from treatment.
On September 29, 2017, we entered into a Development and License Agreement, or the License Agreement, with ABX advanced biochemical compounds – Biomedizinische Forschungsreagenzien GmbH, or ABX, pursuant to which we acquired exclusive worldwide rights to develop and commercialize PSMA‑617 agents, including the drug candidate known as 177Lu-PSMA‑617, a radioligand therapeutic, or RLT. Under the terms of the License Agreement, we will be responsible for, and bear the future costs of, worldwide development and commercialization of PSMA‑617, which ABX will supply. As consideration for the exclusive license, on September 29, 2017, we made an upfront cash payment of approximately $11.9 million to ABX, consisting of $12.0 million less an immaterial expense reimbursement amount, and issued to ABX 2,000,000 shares of our common stock and warrants to purchase, in the aggregate, 4,000,000 shares of our common stock. The License Agreement obligates us to pay ABX regulatory milestone payments of up to $25.0 million, sales milestone payments of up to $135.0 million, and tiered royalties based on percentages of net sales beginning in the mid-teens and not to exceed the mid-twenties.
177Lu-PSMA‑617 in Prostate Cancer. Following a successful End of Phase 2 meeting with the U.S. Food and Drug Administration, or FDA, we finalized the phase 3 VISION trial design and registration plan for 177Lu-PSMA-617. The trial will include two interim assessments of efficacy, which could potentially lead to an early approval for 177Lu-PSMA-617. We intend to initiate, in the second quarter of 2018, the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 in up to 750 patients with progressive PSMA-positive metastatic castration-resistant prostate cancer, or mCRPC, who have received at least one novel androgen axis drug, or NAAD, and at least one taxane regimen. For development in the U.S., we acquired an Investigational New Drug, or IND, application from the prior sponsor, RadioMedix, in December 2017. 177Lu-PSMA‑617 utilizes a high affinity targeting ligand to direct potent radiotherapy to prostate cancer cells. The specific targeting of this therapy comes from the “ligand” portion of the RLT, which is a small molecule designed to bind to PSMA, a protein highly expressed on the cell surface of most prostate cancer cells but absent on most normal cells. The PSMA targeting ligand in 177Lu-PSMA‑617 is chemically attached to a therapeutic radioactive atom called Lutetium‑177 (177Lu), which releases an energetic beta particle designed to precisely deliver cell-killing radiation to the site of disease. Unlike traditional external beam radiotherapy, 177Lu-PSMA‑617, which is administered as a systemic injection, has been designed to directly target multiple sites of PSMA-positive prostate cancer throughout the body, including the bone and soft tissue, while bypassing the PSMA-negative cells. Prior to treatment with 177Lu-PSMA‑617, the patient’s expression of PSMA can be determined using imaging technology, allowing for personalization of treatment so that the optimum course of therapy might be selected. As highlighted in roughly 20 peer reviewed publications of studies in the post-chemotherapy compassionate use setting, 177Lu-PSMA‑617 demonstrated a prostate-specific antigen, or PSA, response (defined as greater than 50% decline from baseline) in 40% to 60% of patients, and a Response Evaluation Criteria in Solid Tumors, or RECIST, response rate in soft tissue disease of between 40% and 50%.
At the European Society for Medical Oncology Congress in September 2017, Dr. Michael Hofman of the Peter MacCallum Cancer Center in Melbourne, Australia presented the results of an open-label, single-arm, non-randomized pilot trial of 177Lu-PSMA‑617 in 30 mCRPC patients. Primary endpoints included safety and efficacy as defined by PSA response, quality of life, and imaging response. The results showed a 57% PSA response rate (>50% reduction) and 71% interim RECIST response rate in soft tissue lesions in patients who had previously failed such conventional therapies as docetaxel, cabazitaxel, enzalutamide and abiraterone. Median overall survival was 12.7 months. The drug was well-tolerated, with a grade 3 or higher hematoxicity attributable to 177Lu-PSMA‑617 occurring in five (17%) patients and no renal toxicity. Significantly improved quality of life scores and reduction in pain scores were recorded in 37% and 43%
1
of patients, respectively. This trial has subsequently been expanded to 50 patients from the original 30, with updated results expected to be presented at the annual American Society of Clinical Oncology, or ASCO, meeting in June 2018.
CAR T-Cell Therapy. We are also developing a unique therapeutic approach that involves the re-targeting of potent immune cells, called chimeric antigen receptor T-cells, or CAR T-cells, to fight cancer. CAR T-cell therapies may be characterized as either allogeneic CAR T-cells, which are those that are engineered using T-cells from a single donor that are utilized in multiple patients, or autologous CAR T-cells, which are those that are engineered using a patient’s own T-cells. Our program utilizes an autologous approach. Traditional CAR T-cell therapies rely on the activity and specificity of T-cells that have been engineered to recognize a single naturally expressed target that, ideally, is only present on cancer cells, with no cross-reactivity to or targeting of healthy tissues. Our alternative strategy relies on an adaptor-controlled CAR T-cell that expresses a high affinity for a molecule called fluorescein isothiocyanate, or FITC, which is not naturally present in the human body. The activity and specificity of these adaptor-controlled CAR T-cells is dependent upon the administration of our proprietary CAR T adaptor molecules, or CAMs, that, once administered, bridge the CAR T-cell to the targeted cancer cell. The CAM attracts the CAR T-cell to the site of disease, causing the anti-cancer immune response of a traditional CAR T-cell therapy. However, unlike existing CAR T-cell technologies, our unique CAM-dependent technology makes possible the engineering of a single universal CAR T-cell that can be used to treat a wide range of cancer types. This is accomplished through the use of multiple CAMs, each of which is designed to bind the FITC molecule to a specific cancer type. In addition to enabling the treatment of multiple cancer types with an adaptor-controlled CAR T-cell, this controlling adaptor molecule technology is also designed to facilitate novel control strategies intended to increase the safety of CAR T-cell therapy.
In March 2017, we announced our collaboration with Seattle Children’s Research Institute, or SCRI, and Dr. Michael Jensen for the development of our technology in the CAR T-cell immunotherapy setting. The aim of the research collaboration is to join our CAM technology with the CAR T-cell immunotherapy research efforts at the Ben Towne Center for Childhood Cancer Research at SCRI, to move these potentially enabling technologies more quickly to patients in the clinic. In October 2017, we announced that we are focusing our adaptor-controlled CAR T-cell program on a targeted effort to generate proof-of-concept data. Our CAM is targeted to the folate receptor which is overexpressed in osteosarcoma, among other solid tumors. Osteosarcoma is a type of bone cancer with a high unmet need for new treatment alternatives, usually occurring in children and young adults. In collaboration with Dr. Jensen, our current strategy is to pursue an IND application in the United States to enroll osteosarcoma patients in a phase 1 trial. Pre-clinical evaluations have been completed, and clinical evaluation is expected to begin in the fourth quarter of 2018.
We currently have no commercial products and we have not received regulatory approval for, nor have we generated commercial revenue from, any of our product candidates.
Our Technology Platforms
We use drug conjugation technology to create novel therapeutics and companion imaging agents for personalized targeted therapies. Our targeting technology now includes advancements in both RLTs as well as adaptor-controlled CAR T-cell therapy.
Radioligand therapy. We have expanded our technology platform to RLTs. In this modality, a high affinity cancer targeting ligand is attached to a therapeutic radioactive atom, such as lutetium-177 or actinium-225. Lutetium-177 is a beta particle, which is much smaller than an alpha particle but can travel further to deliver the radioactive doses to more cells than an alpha particle. Actinium-225 is an alpha particle, which is much larger and delivers higher doses than beta particles but has a much smaller range for targeting cells. See “Pipeline” for information about our lutetium-177 RLT and actinium-225 RLT clinical trials. Once injected into a patient, our RLTs target tumors while largely bypassing healthy cells. Once inside the tumor, the radioactive atoms release energetic particles to precisely deliver a lethal dose of radiation to the cancer cells with minimal side effects to surrounding healthy tissue. PSMA-617, which targets PSMA receptors on mCRPC, is our most advanced RLT.
2
Rendering of PSMA-617 RLT:
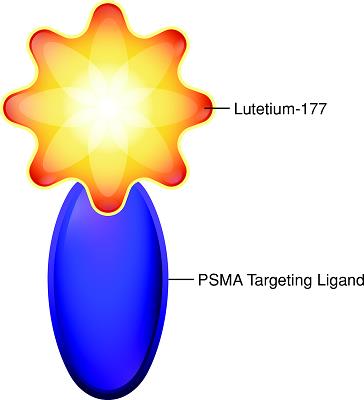
We are developing 177Lu-PSMA-617, an RLT, to direct potent radiotherapy to prostate cancer cells. The specific targeting of this therapy comes from the “ligand” portion of the RLT, which is a small molecule designed to bind to PSMA, a protein highly expressed on the cell surface of most prostate cancer cells but absent on most normal cells. The PSMA targeting ligand in 177Lu-PSMA-617, is chemically attached to the therapeutic radioactive atom lutetium-177 which releases an energetic beta particle to precisely deliver cell killing radiation to the site of disease. Unlike traditional external beam radiotherapy, 177Lu-PSMA-617 is administered as a systemic injection where it can directly target multiple sites of PSMA-positive prostate cancer throughout the body, including the bone and soft tissue, while bypassing the PSMA-negative cells. The expression of PSMA prior to treatment with 177Lu-PSMA-617 can be determined using whole body PSMA-directed imaging, allowing for personalization of treatment so that the best course of therapy might be selected. Based upon the EC1169 phase 1 trial, which completed enrollment in October 2017 and which selected patients for PSMA positivity in mCRPC patients, along with Dr. Michael Hofman’s patient selection for PSMA positivity in the 177Lu-PSMA‑617 Australian trial, it is believed that approximately 80-85% of mCRPC patients have PSMA-positive lesions and no PSMA-negative progressing lesions.
CAR T-cell therapy using our CAM technology. We have leveraged our targeting ligand conjugate technology platform to develop a novel therapeutic approach to CAR T-cell therapy by using specially engineered autologous CAR T-cells that bind a molecule called FITC. Since FITC is not naturally present in the human body, these CAR T-cells will only recognize and kill cancer cells following the administration of our proprietary CAMs. Each CAM is constructed with one FITC molecule together with a distinctive tumor-targeting molecule, or ligand, that can specifically bind to cancer cells. By administering our CAMs, a patient’s tumor is rapidly “painted” with the FITC molecule which then attracts, and bridges, the CAR T-cell to the cancer cell to potentially cause a powerful, localized, and controllable anti-cancer immune response. Unlike existing CAR T-cell technologies, which target a single protein on the cell surface (often CD19 on certain lymphomas and leukemias), our unique CAM-dependent approach makes possible the engineering of a single universal CAR T-cell that can be directed to target multiple proteins and
3
consequently treat various cancer types. This is accomplished by developing multiple CAMs, each one containing a distinctive cancer targeting ligand, but the same FITC molecule. By design, the controlled adaptor approach allows for novel control strategies to increase the safety of the potent immune response of CAR T-cell therapy. Therefore, our CAR T-cell therapy program offers three potentially distinct advantages over existing approved CAR T-cell therapies:
1. | Control: Provides the health-care provider a degree of control over the body’s immune response to the therapy, both with respect to safety/tolerability (control of cytokine release syndrome or neurotoxicity) and efficacy (dose titration, dose skip, dose maintain, rescue agent); |
2. | Universal: Provides the potential for a single CAR T-cell to be targeted to multiple proteins on the cell surface, addressing heterogeneity of disease inherent in many malignancies; and |
3. | Safety: Provides for T-cell response activation to sufficiently drive efficacy, but not to the point of causing T-Cell exhaustion. |
Rendering of FITC CAR T-cell binding to cancer cells using our CAM technology:
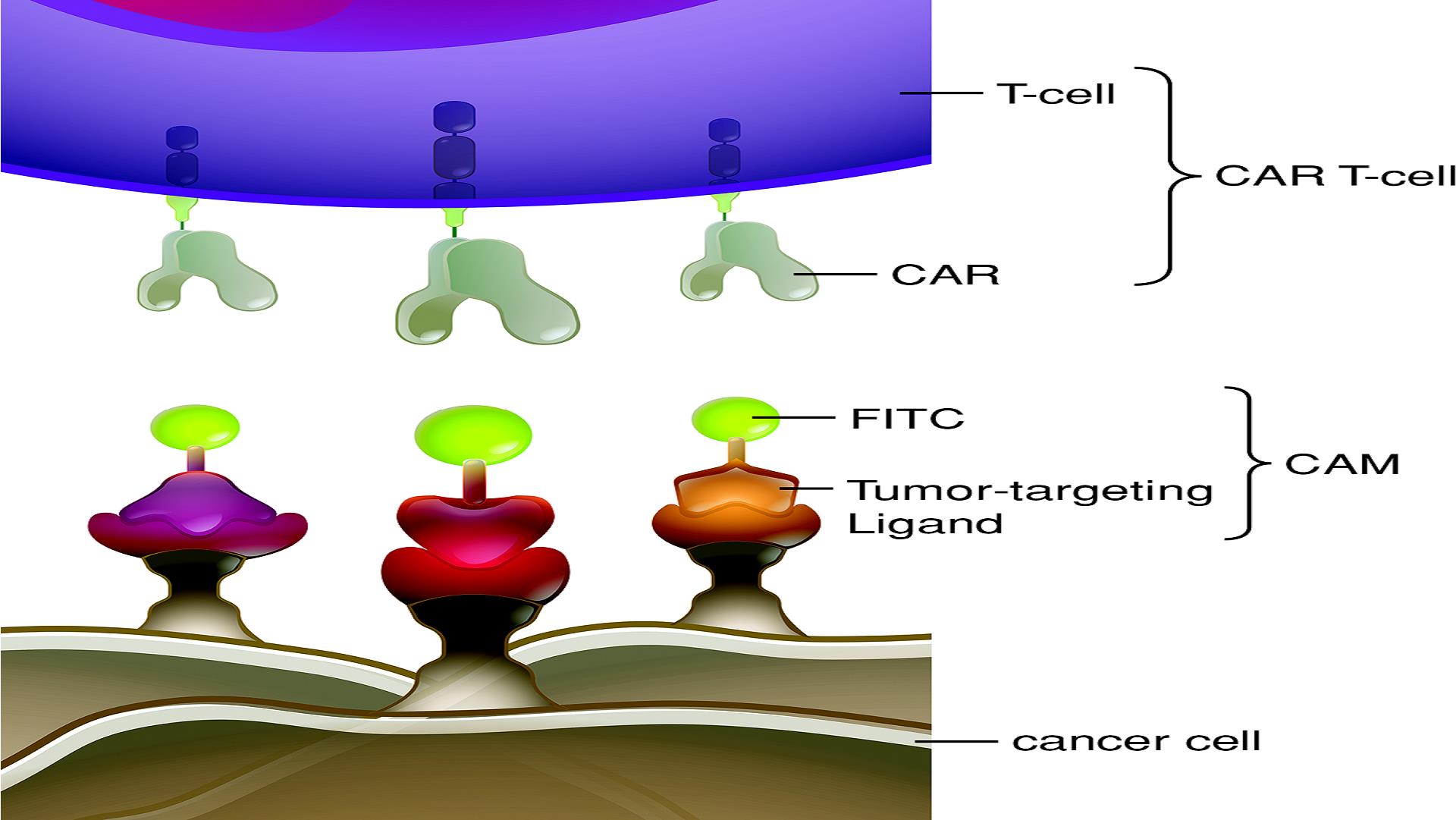
We own or have rights to issued patents or patent applications worldwide covering our core technology, therapeutic product candidates and companion imaging agents. We recently in-licensed a patent portfolio directed to PSMA-617 from ABX. Patent applications directed to PSMA-617 are pending in the United States and numerous other jurisdictions.
4
Our Strategy
Since our inception, we have been focused on the development of our targeting ligand conjugate technology candidates. In June 2017, we discontinued clinical development of EC1456, our second-generation folate-targeted product candidate, and narrowed the focus of our phase 1 clinical development of EC1169, a PSMA-targeted product candidate, to include only the cohort of taxane-exposed mCRPC patients, which completed enrollment in October 2017. In the fourth quarter of 2017, we determined not to invest further resources in the development of EC1169 beyond the completion of the phase 1 taxane-exposed cohort. In addition, in June 2017, we reduced our workforce by approximately 40% to align resources to focus on our highest value opportunities while maintaining key capabilities.
On September 29, 2017, we entered into the License Agreement with ABX, and on October 2, 2017, we announced our plan to primarily focus our resources on the development of 177Lu-PSMA‑617 and on a targeted effort to generate proof-of-concept data for our adaptor-controlled CAR T-cell program, and to explore out-licensing opportunities for all other development programs, such as EC2629, our dual-targeted folate-pro pyrrolobenzodiazepine, or pro-PBD, DNA crosslinker drug.
The principal components of our business strategy are to:
· | Develop 177Lu-PSMA-617 for mCRPC. Following a successful End of Phase 2 meeting with the FDA, we finalized the phase 3 VISION trial design for 177Lu-PSMA-617. The trial will include two interim assessments of efficacy, which could potentially lead to an early approval for 177Lu-PSMA-617. We intend to initiate the VISION trial in the second quarter of 2018 and will enroll up to 750 patients. For development in the U.S., we acquired an IND application from the prior sponsor, RadioMedix, in December 2017. Unlike traditional external beam radiotherapy, 177Lu-PSMA‑617, which is administered as a systemic injection, has been designed to directly target multiple sites of PSMA-positive prostate cancer throughout the body, including both the bone and soft tissue, while bypassing the PSMA-negative cells. Targeting both metastatic bone and soft tissue disease is potentially an important differentiating feature compared to the existing radiotherapy alternative in mCRPC which targets only bone disease. Prior to treatment with 177Lu-PSMA‑617, the patient’s expression of PSMA can be determined using imaging technology, allowing for personalization of treatment so that the optimum course of therapy might be selected. Based upon our EC1169 phase 1 trial, which selected patients for PSMA positivity, along with Dr. Michael Hofman’s patient selection for PSMA positivity in the 177Lu-PSMA‑617 Australian trial, we believe that approximately 80-85% of mCRPC patients have PSMA-positive lesions and no PSMA-negative progressing lesions. |
· | Develop proof-of-concept data for our CAR T-cell therapy through collaboration with Seattle Children’s Research Institute. The aim of our research collaboration is to join our adaptor technology with the CAR T-cell immunotherapy research efforts at the Ben Towne Center for Childhood Cancer Research at SCRI, to move these potentially enabling technologies more quickly to patients in the clinic. This controlled adaptor molecule technology involves the re-targeting of potent immune cells called CAR T-cells to fight cancer and is also designed to facilitate novel control strategies intended to increase the safety of CAR T-cell therapy. In October 2017, we announced that we are focusing the scope of our CAR T-cell program on a targeted effort to generate proof-of-concept data. In collaboration with Dr. Jensen, our current strategy is to pursue an IND application in the United States. Pre-clinical evaluations have been completed, and clinical evaluation is expected to begin in the fourth quarter of 2018. |
· | Seek opportunities to out-license other development programs. We continue to seek opportunities for potential out-licensing of our other development programs, including our legacy targeting ligand conjugate technology programs. |
· | Develop companion imaging agents for each of our therapies. We believe that there is a significant opportunity to create targeted therapies where individual patients are selected based on the use of non-invasive diagnostic tools. Our companion imaging agents may lower the risk of development of our product candidates by allowing us to select for our clinical trials only those patients whose disease over-expresses the receptor targeted by our product candidates. This benefit may, following any regulatory approval, extend to clinical |
5
practice by giving physicians the information they need to prescribe our product candidates to patients who are most likely to respond to our therapy. |
· | Build commercial capabilities and partner to maximize the value of our product candidates. We have retained or acquired all worldwide commercial rights to our product candidates. If we obtain the required regulatory approvals to commercialize our oncology product candidates in the United States and Europe, we intend to do so through our own focused sales force that we would build in connection with such commercialization efforts, or by co-promoting these product candidates in collaboration with one or more larger pharmaceutical companies that have established capabilities in commercializing cancer therapies. Outside of the United States and Europe, we will consider partnering with established international pharmaceutical companies to maximize the value of our pipeline. |
Precision Medicine
We believe the future of medicine includes not only safer and more effective drugs, but also the ability to identify the appropriate therapy for a particular patient. We are committed to this approach, which is commonly referred to as personalized medicine or precision medicine. Our technology allows us to create companion imaging agents intended for use with our therapeutics.
Receptor Targets. We are developing novel therapeutics and companion imaging agents to identify cancer disease receptor targets, including PSMA receptor targets. These receptors are over-expressed on diseased cells. Our targeted therapeutics and companion imaging agents bind to these receptors and are internalized through a process known as endocytosis. This approach seeks to deliver potent therapy to diseased cells, while minimizing the impact on healthy cells.
Rendering of PSMA-617 RLT binding to PSMA receptors:
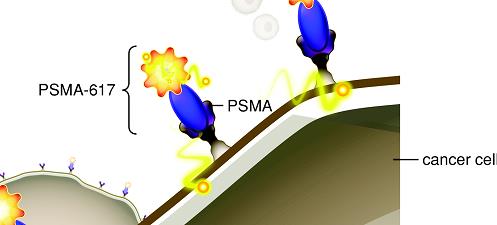
Companion Imaging Agents. Companion imaging agents are available or can be created for our therapeutics, which are designed to target the same diseased cells as the therapeutic and is easily seen with widely available nuclear imaging equipment. The companion imaging agent allows for real-time, full-body assessment of the receptor target without requiring an invasive tissue biopsy. Using full-body imaging, the receptor expression can be measured in every tumor
6
and monitored throughout treatment. Potential key advantages of companion imaging agents over tissue-based samples include:
· | minimally invasive (not requiring biopsy); |
· | real-time assessment of tumor receptor expression (as opposed to analysis based on archived tissue); |
· | greater sensitivity as the companion imaging agent binds to all forms of the receptor (tissue sample analysis may understate receptor expression by not recognizing all forms of receptor); |
· | greater specificity (tissue sample analysis may overstate receptor expression); and |
· | full-body evaluation (as opposed to samples of tumor which may or may not be indicative of all areas of disease). |
Pipeline
We own or have licensed worldwide commercial rights to a radioligand therapy platform and an adaptor-controlled CAR T-cell therapy platform with the potential for treating cancer and selecting patients who would likely benefit from the treatment. Our current focus is on the development of 177Lu-PSMA‑617 and on a targeted effort to generate proof-of-concept data for our CAR T-cell program. We are exploring out-licensing opportunities for other development programs, such as EC2629. A summary of our pipeline, with targeted timelines shown in the dotted lines, is as follows:
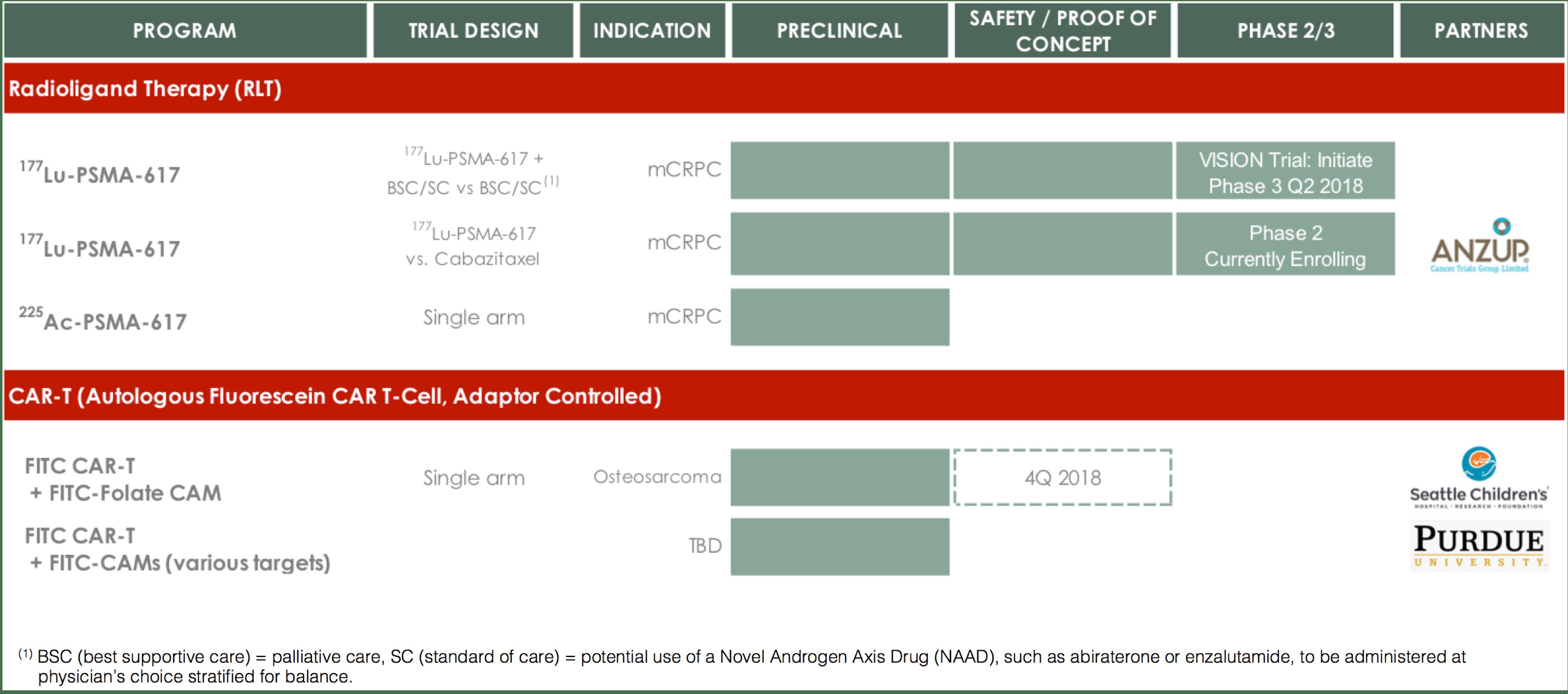
Radioligand therapy for the targeted treatment of prostate cancer
Following a successful End of Phase 2 meeting with the FDA, we finalized the phase 3 VISION trial design for 177Lu-PSMA-617. The trial will include two interim assessments of efficacy, which could potentially lead to an early approval for 177Lu-PSMA-617. We intend to initiate the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617, in the second quarter of 2018. VISION will enroll up to 750 patients with PSMA-positive scans, randomized in a 2:1 ratio to receive either 177Lu-PSMA-617 and best supportive care alone or in combination with a NAAD (physician’s choice) versus best supportive care alone or in combination with a NAAD (physician’s choice). Best supportive care alternatives are palliative in nature. Patients treated with 177Lu-PSMA-617 will receive 7.4 GBq intravenously every six weeks for a maximum of six cycles. After four cycles, patients will be assessed for evidence of response, residual disease and tolerance to 177Lu-PSMA-617. If the patient meets the criteria, the investigator may administer two additional cycles. Patients will be monitored throughout the 6-10 month treatment period for survival, disease progression and adverse events. The trial will be stratified by the physician’s choice of using
7
a NAAD or not, so the use of NAAD’s will be balanced between trial arms. The primary endpoint of the study will be overall survival, or OS. Secondary endpoints include radiographic progression free survival, overall response rate determined by RECIST criteria, and time to first symptomatic skeletal event. Interim efficacy analyses of OS will be conducted at 50% and 70% of the 489 targeted events. There has been extensive clinical experience with 177Lu-PSMA-617 in over 400 patients, the studies of which have shown consistent results in pre-treated patient populations that have been diagnosed with PSMA-positive disease. Enrollment of the trial is expected to begin in the second quarter of 2018 and is expected to be completed in 18-24 months.
On February 26, 2018, we also announced an agreement with ITM Isotopen Technologien München AG, or ITM, which will provide clinical supply of no-carrier-added lutetium for the manufacturing of 177Lu-PSMA-617 for the VISION trial.
In addition, we entered into a three-party agreement in October 2017, with the University of Sydney, or the University, and ANZUP, a cooperative cancer trials group operating in Australia and New Zealand pursuing research in genito-urinary malignancies, in which ANZUP will sponsor and undertake jointly with the University a randomized phase 2 multi-center TheraP trial of 177Lu-PSMA-617 versus cabazitaxel in 200 mCRPC patients. Under the three-party agreement, we will provide the PSMA-617 precursor molecule and financial support for the trial. We will have access to data generated from the trial, which is a potentially important supportive trial for future regulatory submissions. The primary financial obligations of the trial, along with labeling PSMA-617 with Lutetium-177, will be the responsibility of the University and ANZUP. The TheraP trial commenced enrollment in the first quarter of 2018.
Adaptor-Controlled CAR T-cell Immunotherapy in Cancer
Our first CAR T-cell therapy clinical trial will be led by Dr. Michael Jensen at SCRI and will assess folate-receptor (FR)-positive osteosarcoma patients. We will use a high affinity CAR T-cell therapy design plus our folate FITC CAM, EC17. Patients will be selected based on the presence of folate receptor positive disease. Following administration of the FITC-targeted CAR T-cells, the protocol provides for intra-patient dose escalation of our tumor-targeting CAMs. Patients will be monitored following each CAM dose to assess immune response, providing for rapid feedback on activity and safety of the therapy. This innovative design is intended to gradually build the immune response, thereby allowing for the potential to maximize antitumor activity with the intent to avoid severe cytokine release syndrome as well as CAR T-cell exhaustion. Pre-clinical evaluations have been completed, and clinical evaluation is expected to begin in the fourth quarter of 2018.
Competition
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching and marketing products designed to address various types of cancer and other indications we treat or may treat in the future. We are currently developing cancer therapeutics that will compete with other drugs and therapies that currently exist or are being developed. Also, certain of our product candidates may be clinically developed not as an initial first line therapy but as a therapy for patients whose tumors have developed resistance to first line chemotherapy, which limits its potential addressable market. Products we may develop in the future are also likely to face competition from other drugs and therapies.
Many of our competitors have significantly greater financial, manufacturing, marketing and drug development resources than we do. Large biopharmaceutical companies, in particular, have extensive experience in clinical testing and in obtaining regulatory approvals for drugs. Additional mergers and acquisitions in the biopharmaceutical industry may result in even more resources being concentrated by our competition. Competition may increase further as a result of advances in the commercial applicability of technologies currently being developed and a greater availability of capital investment in those fields. These companies may also have significantly greater research and marketing capabilities than we do. Some of the companies developing products which may compete with our product candidates include: Adaptimmune Therapeutics PLC; Affimed N.V.; AstraZeneca PLC; Atara Biotherapeutics, Inc.; Atridia Pty LTD; Autolus Limited; Bayer AG; Bellicum Pharmaceuticals, Inc.; BioNTech AG; Bluebird Bio Inc.; Cancer Targeted Technology LLC; Celgene Corporation; Cellectis S.A.; Celyad S.A.; Editas Medicine, Inc.; ESSA Pharma Inc.; Gilead Sciences, Inc.; GlaxoSmithKline plc; Immatics Biotechnologies GmbH; Immunocore Limited; Innocrin
8
Pharmaceuticals Inc.; Intellia Therapeutics, Inc.; Intrexon Corporation; Janssen Biotech, Inc.; Johnson & Johnson; Juno Therapeutics, Inc.; MedImmune, Inc.; Merck & Co., Inc.; MorphoSys AG; Novartis AG; Progenics Pharmaceuticals, Inc.; Pfizer, Inc., Roche Holding AG; Suzhou Kintor Pharmaceuticals, Inc.; Takara Bio, Inc.; TRACON Pharmaceuticals, Inc.; Unum Therapeutics, Inc.; Zenith Pharmaceuticals Ltd; and Zymeworks Inc. In addition, many universities and private and public research institutes are active in cancer research, the results of which may result in direct competition with our product candidates. For example, the German Center of Cancer Research and University Medical Center Heidelberg, the owners of the patent rights to PSMA‑617 (which have been exclusively licensed to ABX and, in turn, exclusively sublicensed to us under the License Agreement), may continue to engage in research relating to RLTs or other cancer therapies, which could result in competition for 177Lu-PSMA‑617 or other product candidates that we advance from PSMA‑617.
In certain instances, the drugs which will compete with our product candidates are widely available or established, existing standards of care. To compete effectively with these drugs, our product candidates will need to demonstrate advantages that lead to improved clinical safety or efficacy compared to these competitive products. We cannot assure you that we will be able to achieve competitive advantages versus alternative drugs or therapies. If our competitors’ market products are more effective, safer or less expensive than our product candidates or reach the market sooner than our product candidates, we may not achieve commercial success.
We believe that our ability to successfully compete will depend on, among other things:
· | our ability to design and successfully execute appropriate clinical trials; |
· | our ability to recruit and enroll patients for our clinical trials; |
· | the results of our clinical trials and the efficacy and safety of our product candidates; |
· | the speed at which we develop our product candidates; |
· | achieving and maintaining compliance with regulatory requirements applicable to our business; |
· | the timing and scope of regulatory approvals, including labeling; |
· | adequate levels of reimbursement under private and governmental health insurance plans, including Medicare; |
· | our ability to protect intellectual property rights related to our product candidates; |
· | our ability to commercialize and market any of our product candidates that may receive regulatory approval; |
· | our ability to have any partners manufacture and sell commercial quantities of any approved product candidates to the market; |
· | acceptance of our product candidates by physicians, other healthcare providers and patients; |
· | our ability to maintain access to capital to fully fund our clinical programs; and |
· | the cost of treatment in relation to alternative therapies. |
In addition, the biopharmaceutical industry is characterized by rapid technological change. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive
9
from our research approach and proprietary technologies. Also, because our research approach integrates many technologies, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively.
Manufacturing
To date, our product candidates have been manufactured in small quantities for pre-clinical studies and clinical trials by third-party manufacturers. If the FDA, or other regulatory agencies approve any of our product candidates for commercial sale, we expect that we would continue to rely, at least initially, on third-party manufacturers to produce commercial quantities of any approved product candidates. These manufacturers may not be able to successfully increase the manufacturing capacity for any approved product candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the regulatory agencies must review and approve. Additionally, any third-party manufacturer we retain to manufacture our product candidates on a commercial scale must pass a pre-approval inspection for conformance to the cGMP before we can obtain approval of our product candidates. If we are unable to successfully increase the manufacturing capacity for a product candidate in conformance with current Good Manufacturing Practice, or cGMP, the regulatory approval or commercial launch of such products may be delayed or there may be a shortage in supply.
Our product candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could seriously harm our business. Contract manufacturers may encounter difficulties involving production yields, quality control and assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and non-U.S. authorities to ensure strict compliance with cGMP and other applicable government regulations and corresponding foreign standards; however, we do not have control over third-party manufacturers’ compliance with these regulations and standards.
Sales and Marketing
Our operations to date have been limited to organizing and staffing our company, acquiring, developing and securing our technology, undertaking pre-clinical testing and clinical studies of our product candidates and engaging in research and development under collaboration agreements. We have not yet demonstrated an ability to obtain full regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, it is difficult to predict our future success and the viability of any commercial programs that we may take forward.
Research and Development; Raw Materials
For information regarding the amount spent by us on company-sponsored research and development activities during each of the last three fiscal years, see Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations herein.
We procure raw material components for our product candidates from a core group of principal suppliers. In order to meet our clinical trial needs, we are required to enter into agreements with third party manufacturing facilities for certain production needs and for fill and finish capabilities. On February 26, 2018, we announced an agreement with ITM, which will provide clinical supply of no-carrier-added lutetium for the manufacturing of 177Lu-PSMA-617 for the VISION trial.
Employees
In June 2017, we reduced our workforce by 40% to align resources to focus on our highest value opportunities while maintaining key capabilities. As of December 31, 2017, we had a total of 44 full-time employees, 33 of whom were engaged in research and development activities. None of our employees are represented by a labor union or subject to a
10
collective bargaining agreement. We have not experienced a work stoppage and consider our relations with our employees to be good.
Government Regulation
Government authorities in the United States (including federal, state and local authorities) and in other countries extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, distribution, post-approval monitoring and reporting, advertising and promotion, and export and import of pharmaceutical products, such as those we are developing. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
United States Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and related regulations. Drugs are also subject to other federal, state and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition of a clinical hold on trials by the FDA or an Institutional Review Board, or IRB, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The Investigational New Drug Process
An IND is a request for authorization from the FDA to administer an investigational drug to humans. Such authorization must be secured before commencing clinical trials of any new drug candidate in humans in the U.S.
The central focus of the initial IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of animal studies or other human studies, as appropriate, as well as manufacturing information, analytical data and any available clinical data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials as outlined in the IND. In such a case, the IND may be placed on clinical hold until any outstanding concerns or questions are resolved.
Clinical trials involve the administration of the investigational drug to patients under the supervision of qualified investigators in accordance with Good Clinical Practices, or GCP. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical site’s IRB before the trials may be initiated. All participants in clinical trials must provide their informed consent in writing prior to their enrollment in the trial.
The clinical investigation of an investigational drug is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of a clinical investigation are as follows:
· | Phase 1. Phase 1 involves the initial introduction of an investigational new drug into humans. Phase 1 clinical trials are typically closely monitored and may be conducted in patients with the target disease or condition or healthy volunteers. These studies are designed to evaluate the safety, tolerability, metabolism and pharmacologic actions of the investigational drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on efficacy. During phase 1 clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacologic effects may be obtained to permit the design of |
11
scientifically valid phase 2 clinical trials. The total number of participants included in phase 1 clinical trials varies, but generally ranges from 20 to 80. |
· | Phase 2. Phase 2 includes the clinical trials conducted to preliminarily or further evaluate the efficacy of the investigational drug for a particular indication in patients with the disease or condition under study, and to further define safety, tolerability, and optimal dosage. Phase 2 clinical trials can be uncontrolled or controlled trials, and are closely monitored and conducted in a limited patient population, usually involving no more than several hundred participants. |
· | Phase 3. Phase 3 clinical trials are typically well-controlled clinical trials conducted in an expanded patient population generally at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting efficacy of the drug has been obtained, and are intended to further evaluate dosage, safety, and effectiveness to establish the overall benefit-risk relationship of the investigational drug, and to provide an adequate basis for product approval by the FDA and other regulatory agencies. Phase 3 clinical trials usually involve several hundred to several thousand participants. |
The decision to terminate development of an investigational drug may be made by either a health authority body such as the FDA or by us for various reasons. Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a Data Safety Monitoring Board, or DSMB. This group provides a recommendation of whether or not a trial may move forward at pre-specified check points based on access that only the DSMB maintains to available data from the trial. Suspension or termination of development during any phase of clinical trials can occur if it is determined that the participants or patients are being exposed to an unacceptable health risk. We may decide to suspend or terminate development based on evolving business objectives and/or the competitive climate.
In addition, there are requirements and industry guidelines that require the posting of ongoing clinical trials on public registries and the disclosure of designated clinical trial results and related payments to healthcare professionals.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational drug information is submitted to the FDA in the form of a New Drug Application, or NDA, except under limited circumstances, requesting approval to market the product for one or more indications.
The NDA Approval Process
In order to obtain approval to market a drug in the United States, an NDA must be submitted to the FDA that provides data establishing the safety and effectiveness of the investigational drug for the proposed indication to the FDA’s satisfaction. The application includes all relevant data available from pertinent pre-clinical and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators.
The FDA will initially review the NDA for completeness before it accepts the NDA for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
12
Based on phase 3 clinical trial results submitted in an NDA, upon the request of an applicant a priority review designation may be granted to a product by the FDA, which sets the target date for FDA action on the application at six months. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a safe and effective therapy where no satisfactory alternative therapy exists, or a significant improvement compared to marketed products is possible. If criteria are not met for priority review, the standard FDA review period is ten months. The timing of the priority or standard review period begins after the initial 60 day decision regarding acceptability of the submission for filing and substantive review. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval. Other designations, such as breakthrough or fast track designation, are also potentially available and can help expedite development and approval of investigational new drugs.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to develop our product candidates or companion imaging agents and secure necessary governmental approvals, which could delay or preclude us from marketing our products. Even if the FDA approves a product candidate or companion imaging agent, it may limit the approved indications for use or place other conditions on approval that could restrict commercial application, such as a requirement that we implement special risk management measures through a Risk Evaluation and Mitigation Strategy. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Regulation
After regulatory approval, if any, of a drug product is obtained, we may be required to comply with a number of post-approval requirements. For example, as a condition of approval of an NDA, the FDA may require post-marketing testing, including phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug. In addition, as a holder of an approved NDA, we would be required to report, among other things, certain adverse reactions and production problems to the FDA, to provide updated safety and effectiveness information, and to comply with requirements concerning advertising and promotional labeling for any of our products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to assure and preserve the drug product’s identity, strength, quality and purity. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural, substantive and record-keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
13
Europe/Rest of World Government Regulation
In addition to regulations in the United States, we are subject, either directly or through our distribution partners, to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and other applicable regulatory requirements.
To obtain regulatory approval of an investigational drug under European Union regulatory systems, we must submit a marketing authorization application to the European Medicines Agency, or EMA. This application is similar to the NDA in the United States, with the exception of, among other things, country-specific document requirements. Medicines can be authorized in the European Union by using either the centralized authorization procedure or national authorization procedures, and our product candidates and companion imaging agents fall under the centralized authorization procedure.
The European Commission may grant conditional marketing authorization for a product that addresses an unmet medical need while additional development work is being completed. Conditional marketing authorization is subject to annual review, and such authorization may be limited or even withdrawn if subsequent development work is not satisfactory or fails to confirm the clinical benefit of the product.
The European Commission implemented the centralized procedure for the approval of human medicines to facilitate marketing authorizations that are valid throughout the European Union. This procedure results in a single marketing authorization issued by the European Commission that is valid across the European Union, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human medicines that: are derived from biotechnology processes, such as genetic engineering; contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions; and are officially designated orphan medicines.
Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the EMA’s Committee for Medicinal Products for Human Use, or CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials must be conducted in accordance with GCP and the other applicable regulatory requirements.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
14
Compliance
During all phases of development (pre- and post-marketing), failure to comply with the applicable regulatory requirements may result in administrative or judicial sanctions. These sanctions could include the following actions by the FDA or other regulatory authorities: imposition of a clinical hold on trials; refusal to approve pending applications; withdrawal of an approval; warning letters; product recalls; product seizures; total or partial suspension of production or distribution; product detention or refusal to permit the import or export of products; injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our product candidates. Future FDA and state inspections or inspections by other regulatory authorities may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s or other regulatory authorities’ policies may change, which could delay or prevent regulatory approval of our products under development.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the third-party coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government health programs, such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Consolidation and integration among healthcare providers and health plans has increased their purchasing power and impacted reimbursement. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain FDA approvals. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In 2003, the United States government enacted legislation providing a partial prescription drug benefit for Medicare recipients, which became effective at the beginning of 2006. Government payment for some of the costs of prescription drugs may increase demand for any products for which we receive marketing approval. However, to obtain payments under this program, we would be required to sell products to Medicare recipients through prescription drug plans operating pursuant to this legislation. These plans will likely negotiate discounted prices for our products. Federal, state and local governments in the United States continue to consider legislation to limit the growth of healthcare costs, including the cost of prescription drugs. The Patient Protection and Affordable Care Act, or PPACA, and its amendment, the Health Care and Education Reconciliation Act, were enacted in 2010. Since then, other legislative changes have been proposed and adopted, including the Budget Control Act of 2011, which requires reductions to Medicare payments to providers of 2% per fiscal year from April 2013 through 2024 unless additional congressional action is taken. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers. We expect additional state and federal healthcare reform
15
measures to be adopted in the future, which could limit payments or otherwise negatively impact reimbursement for pharmaceuticals such as the drug candidates that we are developing.
We also face uncertainties that might result from modification or repeal of any of the provisions of the PPACA, including as a result of current and future executive orders and legislative actions. The repeal of PPACA’s individual mandate may reduce the number of insured patients in the U.S. and have an adverse impact on revenue received by healthcare providers. Further, the impact of de-regulation efforts on healthcare providers is uncertain. Shortly after taking office, President Trump began a series of initiatives to reduce government regulation. Executive Order 13771 requires federal agencies to identify two existing regulations to be repealed whenever a new regulation is proposed (referred to as the “2-for-1” Executive Order). The 2-for-1 Executive Order requires each federal agency to appoint a Regulatory Reform Officer and Regulatory Reform Task Forces to ensure that agencies effectively carry out these regulatory reform initiatives. At this point, it is unclear what the impact on regulation of reimbursement the 2-for-1 Executive Order or its results will have. We cannot predict what other healthcare programs and regulations will ultimately be implemented at the federal or state level or the effect any future legislation or regulation in the United States may have on our business.
Different pricing and reimbursement schemes exist in other countries. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member countries allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, the Veterans Health Care Act of 1992 and the PPACA, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Under the Veterans Health Care Act, or VHCA, drug companies are required to offer certain drugs at a reduced price to a number of federal agencies including the U.S. Department of Veterans Affairs and the U.S. Department of Defense, the Public Health Service and certain private Public Health Service designated entities in order to participate in other federal funding programs, including Medicare and Medicaid. Legislative changes purport to require that discounted prices be offered for certain U.S. Department of Defense purchases for its TRICARE program via a rebate system. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations.
16
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities or register their sales representatives, as well as prohibiting pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical companies for use in sales and marketing, and prohibiting certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
Patents and Proprietary Rights
Because of the length of time and expense associated with bringing new products to market, biopharmaceutical companies have traditionally placed considerable importance on obtaining and maintaining patent protection for significant new technologies, products and processes.
Our success depends in part on our ability to protect the proprietary nature of our radioligand therapy and adaptor-controlled CAR T-cell platform technologies, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. As a matter of policy, we seek to protect our proprietary position by, among other methods, filing and prosecuting United States and foreign patent applications related to our proprietary technology and our in-licensed assets, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
We have filed patent applications directed to our two main areas of focus: anti-tumor therapeutics, including applications covering radiotherapy, and diagnostics; and adaptor-controlled CAR T-cell therapeutics and diagnostics, in many major developed markets around the world.
We have recently in-licensed an asset known as PSMA-617 from ABX. The patent portfolio directed to PSMA-617 was filed in 2014 and thus, absent patent term adjustments or extensions, is set to expire in 2034. The patent applications directed to PSMA-617 are owned by the German Center of Cancer Research and University Medical Center Heidelberg, which have licensed those assets to ABX, which in turn has sublicensed those assets to us.
The License Agreement with ABX can be terminated by either party if the other party becomes insolvent or commits a material breach of its obligations and, within 60 days following notice of such breach, fails to cure the breach (or, for breaches that cannot be cured within 60 days, fails to commence substantial remedial actions). Any material breach that is specific to a particular country triggers termination rights only as to that country, provided that any material breach relating to any country designated as a “major market” is treated as a breach as to the entire world. We have the right to terminate the License Agreement in our discretion following any event that is reasonably likely to have a material adverse effect on the commercial potential in the U.S. of any product covered by the license. Absent such an event, however, our discretionary right to terminate the ABX license is conditioned upon our having met certain investment and diligence obligations and, under some circumstances, would result in acceleration of any unpaid development milestone payments. See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Contractual Obligations and Commitments for a description of our payment obligations under the ABX license agreement.
Based on research we fund at Purdue University, we have entered into three exclusive, worldwide in-licenses for a number of patents and patent applications owned by the Purdue Research Foundation related to select folate-targeted technology, select technology related to PSMA, and select technology related to other ligands. The folate-technology license was originally entered into on July 17, 1998 with an effective date as of December 21, 1995, and was restated on October 21, 1998. The PSMA license was entered into on March 1, 2010. The license to other technologies was entered
17
into in 2014 with an effective date of July 1, 2013. Under these three licenses, over 80 patents and published applications are licensed to us.
We may terminate the Purdue Research Foundation licenses without cause with 60 days notice for the folate and PSMA licenses and three months notice for the license directed to other ligands. Purdue Research Foundation may terminate any of the licenses for material default by us which is not cured within 90 days notice, or upon 60 days notice, for the folate and PSMA licenses, in the event we fail to meet public demand for approved products covered by the licensed patents after a six month cure period following commercial introduction. The Purdue Research Foundation may terminate the license to other ligands if we fail to make a payment within 60 days of notice under the specific terms of the license. The folate and PSMA licenses also contain provisions allowing Purdue Research Foundation to terminate upon our bankruptcy. We have annual minimum royalty obligations to Purdue Research Foundation, as well as royalty obligations based on sales of products that are designed, developed or tested using the licensed technology, and milestone obligations based upon the achievement of specific scientific, clinical and regulatory milestones. See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Contractual Obligations and Commitments for a description of our payment obligations under the license agreements with the Purdue Research Foundation.
We collaborate with Purdue University on CAR T-cell therapy research and are thus co-owners on some patent applications with the Purdue Research Foundation.
Most of our portfolio consists of intellectual property we exclusively license from ABX, Purdue Research Foundation or which we own ourselves. Generally, the intellectual property licensed from Purdue Research Foundation is early stage and relates to methods that were invented in the laboratory of Professor Philip Low. Internally, we typically develop these methods further and refine them to determine the commercial applicability. Additionally, these early-stage patents often provide us protection from competitors while we evaluate commercial possibilities of a specific program. For example, some of the patents we licensed from Purdue Research Foundation cover drug conjugates of PSMA.
Due to the use of federal funds in the development of some folate-related technology, the U.S. government has the irrevocable, royalty-free, paid-up right to practice and have practiced certain patents throughout the world, should it choose to exercise such rights.
Our development strategy also employs lifecycle management positions. For example, we explore the potential for filing patent applications claiming methods of treatment combining our molecules with other treatments. Such strategies may provide additional patent protection even after the expiration of composition of matter patent protection for our intellectual property. As a result, our general guiding strategy is to obtain patent coverage for innovations that show a clinical benefit to patients and an economic opportunity for us.
In addition, in October 2007, we entered into an exclusive worldwide license with R&D Biopharmaceuticals to research, develop, and commercialize products containing conjugates of folate receptor targeting compounds and tubulysin compounds. In February 2011, this licensing agreement was assigned by R&D Biopharmaceuticals to Trientlgasse.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office, or USPTO in granting a patent, or may be shortened if a patent is terminally disclaimed over another patent.
The patent term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent.
18
The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other non-U.S. jurisdictions to extend the term of a patent that covers an approved drug. In the future, if our pharmaceutical products receive FDA approval, we expect to apply for patent term extensions on patents covering those products.
While we pursue patent protection and enforcement of our product candidates and aspects of our technologies when appropriate, we also rely on trade secrets, know-how and continuing technological advancement to develop and maintain our competitive position. To protect this competitive position, we regularly enter into confidentiality and proprietary information agreements with third parties, including employees, independent contractors, suppliers and collaborators. Our employment policy requires each new employee to enter into an agreement containing provisions generally prohibiting the disclosure of confidential information to anyone outside of us and providing that any invention conceived by an employee within the scope of his or her employment duties is our exclusive property. We have a similar policy with respect to independent contractors, generally requiring independent contractors to enter into an agreement containing provisions generally prohibiting the disclosure of confidential information to anyone outside of us and providing that any invention conceived by an independent contractor within the scope of his or her services is our exclusive property, with the exception of contracts with universities and colleges that may be unable to make such assignments. Furthermore, our know-how that is accessed by third parties through collaborations and research and development contracts and through our relationships with scientific consultants is generally protected through confidentiality agreements with the appropriate parties. We cannot, however, assure you that these protective arrangements will be honored by third parties, including employees, independent contractors, suppliers and collaborators, or that these arrangements will effectively protect our rights relating to unpatented proprietary information, trade secrets and know-how. In addition, we cannot assure you that other parties will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our proprietary information and technologies.
There can be no assurance that any of our patent applications or any that we in-license will result in granted patents. Additionally, there can be no assurance that our patents or any that we in-license (collectively “Patents”) will provide significant protection, competitive advantage or commercial benefit. The validity and enforceability of patents issued to biopharmaceutical companies has proven highly uncertain. For example, legal considerations surrounding the validity of patents in the fields of biopharmaceuticals are in transition, and we cannot assure you that the historical legal standards surrounding questions of validity will continue to be applied or that current defenses relating to issued Patents in these fields will be sufficient in the future. In addition, we cannot assure you as to the degree and range of protections any of our Patents, if issued, may afford us or whether Patents will be issued. For example, Patents which may issue to us may be subjected to further governmental review that may ultimately result in the reduction of their scope of protection, and pending Patent applications of ours or those that we in-license may have their requested breadth of protection significantly limited before being issued, if issued at all. Further, since publication of discoveries in scientific or patent literature often lags behind actual discoveries, we cannot assure you that we were the first creator of inventions covered by pending Patent applications of ours or those that we in-license, or that we, or the applicants for the assets we in-license, were the first to file patent applications for these inventions.
Many biopharmaceutical companies and university and research institutions have filed patent applications or have received patents in our areas of product development. Many of these entities’ applications, patents and other intellectual property rights could prevent us from obtaining Patents or could call into question the validity of any of our Patents, if issued, or could otherwise adversely affect our ability to develop, manufacture or commercialize our product candidates. In addition, certain parts of our technology originated from third-party sources. These third-party sources include academic, government and other research laboratories, as well as the public domain. If the use of technology incorporated into or used to produce our product candidates is challenged, or if a conflicting patent issued to others is upheld in the courts or if a conflicting patent application filed by others is issued as a patent and is upheld, we may be unable to market one or more of our product candidates, or we may be required to obtain a license to market those product candidates. To contend with these possibilities, we may have to enter into license agreements in the future with third parties for technologies that may be useful or necessary for the manufacture or commercialization of some of our product candidates. In addition, we are routinely in discussions with academic and commercial entities that hold patents on technology or processes that we may find necessary in order to engage in some of our activities. However, we cannot
19
assure you that these licenses, or any others that we may be required to obtain to market our product candidates, will be available on commercially reasonable terms, if at all, or that we will be able to develop alternative technologies if we cannot obtain required licenses.
To protect our rights to any of our issued Patents and proprietary information, we may need to litigate against infringing third parties, or avail ourselves of the courts or participate in hearings to determine the scope and validity of those Patents or other proprietary rights. These types of proceedings are often costly and could be very time-consuming to us, and we cannot assure you that the deciding authorities will rule in our favor. An unfavorable decision could allow third parties to use our technology without being required to pay us licensing fees or may compel us to license needed technologies to avoid infringing third-party patent and proprietary rights, or even could result in the invalidation or a limitation in the scope of our Patents or forfeiture of the rights associated with our Patents or pending Patent applications of ours or those that we in-license. Although we believe that we would have valid defenses to allegations that our current product candidates, production methods and other activities infringe the valid and enforceable intellectual property rights of any third parties, we cannot be certain that a third party will not challenge our position in the future. Even if some of these activities were found to infringe a third party’s patent rights, we may be found to be exempt from infringement under 35 U.S.C. §271(e) to the extent that these are found to be pre-commercialization activities related to our seeking regulatory approval for a product candidate. However, the scope of protection under 35 U.S.C. §271(e) is uncertain and we cannot assure you that any defense under 35 U.S.C. §271(e) would be successful. Further, the defense under 35 U.S.C. §271(e) is only available for pre-commercialization activities, and could not be used as a defense for sale and marketing of any of our product candidates. There has been, and we believe that there will continue to be, significant litigation in the biopharmaceutical and pharmaceutical industries regarding patent and other intellectual property rights.
Corporate Information
We were incorporated in the State of Indiana in 1995 and we were reincorporated in the State of Delaware in 2001. Our principal executive offices are located at 3000 Kent Avenue, Suite A1-100, West Lafayette, Indiana 47906, and our telephone number is (765) 463-7175.
Available Information
Our Internet address is www.endocyte.com. We routinely post important information for investors on our website in the “Investors & News” section. We use this website as a means of disclosing material information in compliance with our disclosure obligations under Regulation FD. Accordingly, investors should monitor the “Investors & News” section of our website, in addition to following our press releases, Securities and Exchange Commission, or SEC, filings, public conference calls, presentations and webcasts. Investors can easily find or navigate to pertinent information about us, free of charge, on our website, including:
· | our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after we electronically file such material with or furnish it to the SEC; |
· | announcements of investor conferences and events at which our executives talk about our products and competitive strategies. Archives of some of these events are also available; |
· | press releases on quarterly earnings, product announcements, legal developments and other material news that we may post from time to time; |
· | corporate governance information, including our Corporate Governance Principles, Code of Ethics and Business Conduct, information concerning our Board of Directors and its committees, including the charters of the Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee, and other governance-related policies; |
· | stockholder services information, including ways to contact our transfer agent; and |
20
· | opportunities to sign up for email alerts and RSS feeds to have information provided in real time. |
The information available on our website is not incorporated by reference in, or a part of, this or any other report we file with or furnish to the SEC.
The name “Endocyte” and our logo are our trademarks. All other trademarks and trade names appearing in this annual report are the property of their respective owners.
Executive Officers of the Registrant
The following table sets forth certain information concerning our executive officers as of March 1, 2018:
Name |
| Age |
| Position |
Michael A. Sherman |
| 51 |
| President and Chief Executive Officer |
Michael T. Andriole |
| 45 |
| Chief Financial Officer |
Philip S. Low, Ph.D. |
| 70 |
| Chief Science Officer |
Alison A. Armour, M.D. |
| 54 |
| Chief Medical Officer |
Beth A. Taylor |
| 52 |
| Vice President of Finance and Chief Accounting Officer |
Christopher P. Leamon, Ph.D. |
| 51 |
| Vice President of Research |
Katherine K. Parker |
| 53 |
| Vice President of Human Resources |
Erik C. Chelius |
| 55 |
| Vice President of CMC |
Michael A. Sherman has served as our President and Chief Executive Officer since June 2016. He served as our Chief Financial Officer from October 2006 to February 2017 and as our Chief Operating Officer from June 2014 to June 2016. From December 1994 to October 2006, Mr. Sherman served in various executive roles, but most recently as Vice President of Finance and Strategic Planning from May 2004 to October 2006, of Guidant Corporation, a cardiovascular device manufacturer acquired by Boston Scientific Corporation, a medical device company, in April 2006. Mr. Sherman serves as Vice Chairman on the Board of Trustees for the Indianapolis Children’s Museum. He also served on the Board of Directors at Mead Johnson Nutrition from February 2015 until June 2017. Mr. Sherman holds a B.A. in economics from DePauw University and an M.B.A. from the Amos Tuck School, Dartmouth College.
Michael T. Andriole has served as our Chief Financial Officer since February 2017. From June 2001 to February 2017, Mr. Andriole served in various executive roles at Eli Lilly and Company, a large pharmaceutical company that develops, manufactures and markets pharmaceutical products on a global basis, and most recently as Vice President, Corporate Business Development from December 2014 to February 2017. Previous roles included Director, European Development and Strategic Planning and Director, Corporate Finance and Investment Banking. Mr. Andriole holds a B.S. in Finance from Xavier University’s Williams College of Business and an M.B.A. from Kelley School of Business, Indiana University.
Philip S. Low, Ph.D. is one of our founders and has served as our Chief Science Officer since April 1998 and as a member of our Board of Directors since December 1995. Dr. Low has served on the faculty at Purdue University since August 1976, where he is currently the Director of the Purdue Institute for Drug Discovery and the Ralph C. Corley Distinguished Professor of Chemistry. Dr. Low holds a B.S. in chemistry from Brigham Young University and a Ph.D. in biochemistry from the University of California, San Diego.
Alison A. Armour, M.D. has served as our Chief Medical Officer since August 2015. Prior to joining us, Dr. Armour served as Vice President of Oncology and led the Tykerb, a breast cancer drug, program at GlaxoSmithKline PLC and Novartis AG from August 2011 to July 2015. From August 2004 to August 2011 she was Global Medical Science Director at AstraZeneca PLC. Before joining the pharmaceutical industry, Dr. Armour established herself as a thought leader in medical and radiation oncology. Dr. Armour holds her B.Sc. in Biochemistry, M.B., Ch.B., MSc., and Doctorate of Medicine from the University of Glasgow, MRCP from the Member of the Royal College of Physicians Glasgow, and her FRCR from the Royal College of Radiologists London, UK.
21
Beth A. Taylor, C.P.A. has served as our Vice President of Finance and Chief Accounting Officer since August 2016. She previously served as our Corporate Controller from January 2011 to August 2016. She served as Corporate Controller of Author Solutions, Inc. from December 2009 to December 2010, as Vice President of Accounting, Financial Reporting and Control at Harlan Laboratories, Inc. from July 2007 to May 2009 and in various roles at Republic Airways Holdings, Inc. from May 1999 through June 2007, including Vice President and Corporate Controller from August 2004 through June 2007. Ms. Taylor holds a B.S. in Accounting from Kelley School of Business, Indiana University.
Christopher P. Leamon, Ph.D. has served as our Vice President of Research since April 2000. From February 1999 to April 2000, Dr. Leamon served as our Director of Biology and Biochemistry. Prior to joining us, Dr. Leamon was employed in the pharmaceutical industry where he conducted discovery research in the field of peptide, oligonucleotide, liposome and DNA drug delivery for GlaxoWellcome, a healthcare company, and Isis Pharmaceuticals, a biomedical pharmaceutical company. Dr. Leamon holds a B.S. in chemistry from Baldwin Wallace College and a Ph.D. in biochemistry from Purdue University.
Katherine K. Parker has served as our Vice President of Human Resources since January 2015. From December 2013 to January 2015, she served as our Senior Director of Human Resources. Prior to December 2013, Ms. Parker served as Principal for Auxilius International, LLC from August 2010 to December 2013. She also has served as President/Owner of Auxilius Heavy Industries, LLC, a wind turbine maintenance, cleaning and repair company, from August 2013 to the present. Prior to her entrepreneurial endeavors, Ms. Parker worked at Hyundai Motor America for 21 years, most recently as Vice President of Human Resources and Administrative Services from April 2005 to April 2009. Ms. Parker holds a B.A. in history from the University of California, Los Angeles and an M.B.A. from California State University, Dominguez Hills.
Erik C. Chelius has served as our Vice President of CMC since January 2018. He previously served as our Senior Director of CMC from September 2014 until January 2018 and as a Senior Research Scientist for us from 2011 to 2014. Prior to joining us, Dr. Chelius worked in the Development and Manufacturing departments of Eli Lilly & Co. from 1989 through 2010. He also has contract manufacturing experience gained while employed at Evonik from 2010 through 2011. Dr. Chelius holds a B.S. in Chemistry from Marquette University as well as M.S. and Ph.D. degrees in Chemistry from Northwestern University.
22
Risk factors which could cause actual results to differ from our expectations and which could negatively impact our financial condition and results of operations are discussed below and elsewhere in this report. Additional risks and uncertainties not presently known to us or that are currently not believed to be significant to our business may also affect our actual results and could harm our business, financial condition, results of operations, cash flows or stock price. If any of these risks or uncertainties actually occurs, our business, financial condition, results of operations, cash flows or stock price could be materially and adversely affected.
Risks Related to Our Business and Industry
We have incurred significant losses in each year since our inception, other than in 2014, and we anticipate that we will continue to incur significant losses for the foreseeable future. We may never again achieve or sustain profitability.
We are a clinical-stage biopharmaceutical company with a limited operating history. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We have not generated any revenue from product sales to date. For the year ended December 31, 2017, we had a net loss of $55.1 million and a retained deficit of $309.0 million. Other than in 2014, we have incurred significant losses in each year since our inception in December 1995. We expect to continue to incur significant operating expenses for the next several years as we pursue the advancement of our product candidates through the research, development, regulatory and commercialization processes. As such, we are subject to all of the risks incident to the creation of new biopharmaceutical products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. If our product candidates fail in clinical trials, or do not gain regulatory approval, or fail to achieve market acceptance, we may never again be profitable. Even if we achieve profitability again in the future, we may not be able to sustain profitability in subsequent periods.
We currently have no approved products, which makes it difficult to assess our future viability.
To date, we have not derived any revenue from the sales of our products. Our operations to date have been limited to acquiring, developing and securing our technology, undertaking pre-clinical studies and clinical trials of our product candidates and engaging in research and development under collaboration agreements. We have not yet demonstrated an ability to obtain regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, it is difficult to predict our future success and the viability of any commercial programs that we may choose to take forward. While we have in the past derived revenues from payments under collaboration agreements, all of such agreements have been terminated, and we have no current sources of revenue except for the recognition of remaining deferred revenue related to the upfront payment under the agreement with Nihon Medi-Physic Co., LTD., or NMP.
Our restructuring activities and refocused development program efforts may not be successful, and our restructuring activities and changes in our development program efforts may cause uncertainty regarding the future of our business and may adversely impact employee hiring and retention, our stock price and our results of operations and financial condition.
In June 2017, we discontinued clinical development of EC1456, our second-generation folate-targeted small molecule drug conjugate, or SMDC, and narrowed the focus of our phase 1 clinical development of EC1169, a PSMA-targeted SMDC, to include only the cohort of taxane-exposed mCRPC patients, which completed enrollment in October 2017 and for which we do not intend to invest further resources beyond the completion of the phase 1 taxane-exposed cohort. In addition, in June 2017, we reduced our workforce by approximately 40% to align resources to focus on our highest value opportunities while maintaining key capabilities. On October 2, 2017, we announced our plan to focus our resources on the development of 177Lu-PSMA-617 and on a targeted effort to generate proof-of-concept data for our CAR T-cell therapy program, and to explore out-licensing opportunities for all other development programs.
23
Our ability to achieve the anticipated benefits, including the anticipated levels of cost savings and efficiency, of our restructuring activities within expected timeframes is subject to many estimates, assumptions and uncertainties. Additional restructuring or reorganization activities may also be required in the future, which could further increase the risks associated with these activities. There is no assurance that we will successfully implement, or fully realize the anticipated impact of, our restructuring or execute successfully on our refocused development program, in the timeframes we desire or at all. If we fail to realize the anticipated benefits from these measures, or if we incur charges or costs in amounts that are greater than anticipated, our financial condition and operating results may be adversely affected.
In addition, the changes in focus of our development program may not be successful and we may have to terminate other clinical and pre-clinical efforts. Further, although the workforce reduction is intended to align resources to focus on highest value opportunities while maintaining key capabilities, those opportunities may not prove to be of high value and those capabilities may not be sufficient.
The changes to our development program and the workforce reduction measures, as well as the potential for additional changes or activities in the future, may introduce uncertainty regarding our prospects and may result in disruption of our business. As a result of these actions, we incurred significant expenses and charges, including site close-out expenses, employee termination benefits and fixed asset impairment charges, and we may incur additional expenses and charges related to these actions. In addition, these changes and measures could distract our employees, decrease employee morale and make it more difficult to retain and hire new talent, and harm our reputation. These changes and activities caused our stock price to decline, and may cause it to further decline in the future. As a result of these or other similar risks, our business, results of operations and financial condition may be adversely affected.
We are highly dependent on the success of PSMA-617 product candidates, and we cannot give any assurance that we will successfully complete its clinical development, or that it will receive regulatory approval or be successfully commercialized.
In September 2017, we entered into a License Agreement with ABX, pursuant to which we acquired exclusive worldwide rights to develop and commercialize PSMA-617, including the drug candidate known as 177Lu-PSMA-617, an RLT. We intend to initiate, in the second quarter of 2018, the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 and will enroll 750 patients with progressive PSMA-positive mCRPC. The VISION trial may not be successful, and 177Lu-PSMA-617 may never receive regulatory approval or be successfully commercialized. We may fail to obtain necessary marketing approvals for 177Lu-PSMA-617 from the FDA or other regulatory authorities if our clinical development programs for 177Lu-PSMA-617 fail to demonstrate that it is safe and effective to the satisfaction of such authorities, or if we have inadequate financial or other resources to advance 177Lu-PSMA-617 through the necessary development activities. Even if 177Lu-PSMA-617 receives regulatory approval, we may not be successful in marketing it for a number of reasons, including the introduction by our competitors of more clinically-effective or cost-effective alternatives or failure in our sales and marketing efforts. Any failure to obtain approval of 177Lu-PSMA-617 and successfully commercialize it would have a material and adverse impact on our business.
We cannot give any assurance that we will successfully complete the clinical development of any of our other product candidates, or that they will receive regulatory approval or be successfully commercialized.
We have terminated or significantly limited the development programs for product candidates other than PSMA-617 and CAR T-cell therapy product candidates. With respect to any other product candidates that we may pursue, they may never receive regulatory approval or be successfully commercialized. We may fail to obtain necessary marketing approvals from the FDA or other regulatory authorities if our clinical development programs fail to demonstrate that they are safe and effective to the satisfaction of such authorities, or if we have inadequate financial or other resources to advance our product candidates through the necessary development activities. Even if any of our product candidates receive regulatory approval, we may not be successful in marketing them for a number of reasons, including the introduction by our competitors of more clinically-effective or cost-effective alternatives or failure in our sales and marketing efforts.
24
The results of clinical trials may not be predictive of future results, and those trials may not satisfy the requirements of the FDA or other regulatory authorities.
The clinical trials of our product candidates are, and the manufacturing and marketing of any approved products will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States, Europe and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through pre-clinical testing and clinical trials that the product candidate is safe and effective for use in each indication for which we intend to market such product candidate. This process takes many years and requires the expenditure of substantial financial and human resources and may include post-marketing trials and surveillance. To date, we have not completed any randomized phase 3 clinical trials.
Positive results from pre-clinical studies and early clinical trials, such as those of 177Lu-PSMA-617, should not be relied upon as evidence that later-stage or large-scale clinical trials will succeed. Like our past history with respect to certain product candidates, a number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, even after promising results in earlier trials. We will be required to demonstrate with substantial evidence through adequate and well-controlled clinical trials that our product candidates are safe and effective for use in the target population before the regulatory authorities will approve our product candidates for commercial sale, and we cannot assure you that we will be able to do so.
In addition to the phase 3 clinical trial of 177Lu-PSMA-617 that we intend to initiate in the second quarter of 2018, certain third party investigators, including Dr. Michael Hofman of the Peter MacCallum Cancer Center in Melbourne, Australia, are conducting clinical trials of 177Lu-PSMA-617 and other product candidates containing PSMA-617. In addition, the German institutions that own the patent rights to PSMA‑617 have retained the right, under their license to ABX (under which we are the exclusive sublicensee), to conduct clinical trials of compounds containing PSMA-617 at their premises in Heidelberg, Germany, subject to our approval of the trial protocol. Current or possible future clinical trials of compounds containing PSMA-617 that are conducted by third party investigators outside of our control (in whole or in part) may generate clinical data that hinders our ability to obtain regulatory approvals for the development and/or commercialization of 177Lu-PSMA-617.
Further, our product candidates may not be approved even if they achieve the primary endpoints in phase 3 clinical trials or registration trials. The FDA or other regulatory authorities may disagree with our trial design or the interpretation of data from pre-clinical studies and clinical trials. In addition, the FDA and other regulatory authorities may change requirements for the approval of our product candidates even after reviewing and providing non-binding comments on a protocol for a pivotal phase 3 clinical trial that has the potential to result in approval. Regulatory authorities may also approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
There is a high risk that our development and clinical activities will not result in commercial products, and we may be required to invest significant additional resources in our current development and clinical programs, to the exclusion of others, before it is known whether one or more of our product candidates will receive regulatory approval or be commercially introduced.
Our product candidates are in various stages of development and are prone to the risks of failure inherent in biopharmaceutical development. Development of our product candidates could be discontinued due to insufficient efficacy or unacceptable toxicity. In many cases, even if we ultimately obtain regulatory approval to market a product candidate, we will need to complete significant additional clinical trials before we can demonstrate that the product candidate is safe and effective to the satisfaction of the regulatory authorities involved. Clinical trials are expensive and uncertain processes that take years to complete. Failure can occur at any stage of the process. Further, even if a product candidate receives the required regulatory approvals, we cannot assure you that it will be successful commercially. In addition, while we invest in the technology and indications that we believe are most promising, financial and resource
25
constraints may require us to forego or delay opportunities that may ultimately have greater commercial potential than those programs we are currently actively developing.
We may not achieve research, development and commercialization goals in the time frames that we publicly estimate, which could have an adverse impact on our business and could cause our stock price to decline.
We set goals, and make public statements regarding our expectations, regarding the timing of certain accomplishments, developments and milestones under our research and development programs. The actual timing of these events can vary significantly due to a number of factors, including, without limitation, the amount of time, effort and resources committed to our programs by us and any collaborators and the uncertainties inherent in the regulatory approval process. As a result, there can be no assurance that we or any collaborators will make regulatory submissions or receive regulatory approvals as planned or that we or any collaborators will be able to adhere to our current schedule for the achievement of key milestones under any of our programs. If we or any collaborators fail to achieve one or more of the milestones described above as planned, our business could be materially adversely affected and the price of our common stock could decline.
The coverage and reimbursement status of newly approved biopharmaceuticals is uncertain, and failure to obtain adequate coverage and adequate reimbursement of our product candidates could limit our ability to generate revenue.
There is significant uncertainty related to the third-party coverage and reimbursement of newly approved drugs. The commercial success of our product candidates, if approved, in both domestic and international markets will depend in part on the availability of coverage and adequate reimbursement from third-party payors, including government payors, such as the Medicare and Medicaid programs, and managed care organizations. Government and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and, as a result, they may not cover or provide adequate payment for our product candidates. These payors may conclude that our product candidates are less safe, less effective or less cost-effective than existing or later introduced products, and third-party payors may not approve our product candidates for coverage and reimbursement or may cease providing coverage and reimbursement for these product candidates. Consolidation and integration among healthcare providers and health plans has increased their purchasing power and impacted reimbursement, and could continue to do so in the future. Because each country has one or more payment systems, obtaining reimbursement in the United States and internationally may take significant time and cause us to spend significant resources. The failure to obtain coverage and adequate reimbursement for our product candidates or healthcare cost containment initiatives that limit or deny reimbursement for our product candidates may significantly reduce any future product revenue.
In the United States and in other countries, there have been and we expect there will continue to be a number of legislative and regulatory proposals to change the healthcare system in ways that could significantly affect our business. International, federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. The U.S. government and other governments have shown significant interest in pursuing healthcare reform. Such government-adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third-party payors. In addition, in some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. The continuing efforts of U.S. and other governments, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce healthcare costs may adversely affect our ability to set satisfactory prices for our products, to generate revenues, and to achieve and maintain profitability.
In some countries, particularly in the European Union, prescription drug pricing is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of our product candidates to other available therapies. If reimbursement of our product candidates is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability of our products in such country.
26
Our development activities could be delayed or stopped for a number of reasons, many of which are outside our control, which could materially harm our financial results and the commercial prospects for our product candidates.
Each of our clinical trials requires the investment of substantial expense and time, and the timing of the commencement, continuation and completion of these clinical trials may be subject to significant delays relating to various causes. We do not know whether our current clinical trials will be completed on schedule, or at all, and we cannot guarantee that our future planned clinical trials will begin on time, or at all. Clinical trials must be conducted in accordance with FDA or applicable foreign government guidelines and are subject to oversight by the FDA, foreign governmental agencies and independent institutional review boards, or IRBs, at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with supplies of our product candidates produced under current Good Manufacturing Practice, or cGMP, and other requirements in foreign countries, and may require large numbers of test patients. Our current and planned clinical trials could be substantially delayed or prevented by several factors, including:
· | limited number of, and competition for, suitable sites to conduct our clinical trials; |
· | government or regulatory delays and changes in regulatory requirements, policy and guidelines; |
· | delay or failure to obtain sufficient supplies of the product candidate for, or other drugs used in, our clinical trials as a result of our suppliers’ non-compliance with cGMP, or for other reasons; |
· | delay or failure to reach agreement on acceptable clinical trial agreement terms with prospective sites or investigators; and |
· | delay or failure to obtain IRB approval to conduct a clinical trial at a prospective site. |
The completion of our clinical trials could also be substantially delayed or prevented by several factors, including:
· | slower than expected rates of patient recruitment and enrollment; |
· | unforeseen safety issues; |
· | lack of efficacy evidenced during clinical trials, which risk may be heightened given the advanced state of disease and lack of response to prior therapies of patients in certain clinical trials; |
· | termination of our clinical trials by an IRB at one or more clinical trial sites; |
· | inability or unwillingness of patients or medical investigators to follow our clinical trial protocols; and |
· | inability to monitor patients adequately during or after treatment or high patient dropout rates. |
Our clinical trials may be suspended or terminated at any time by the FDA, other regulatory authorities or us. For example, in June 2017 we ended clinical development of EC1456 and stopped enrollment in our EC1456 phase 1b trial, as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. In December 2017, we stopped enrollment in our EC1456 ovarian cancer surgical trial. We cannot assure you that we will not terminate our current and future development programs.
Failure or significant delay in completing clinical trials for our product candidates could materially harm our financial results and the commercial prospects for our product candidates.
27
Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval or commercialization.
Common side effects of our product candidates include abdominal pain, vomiting, constipation, nausea, fatigue, loss of appetite, hematologic events and peripheral sensory neuropathy. Because our product candidates have been tested in relatively small patient populations and for limited durations to date, additional side effects may be observed as their development progresses.
177Lu-PSMA-617 is designed to target PSMA, a protein highly expressed on the surface of most prostate cancer cells but absent on most normal cells. However, the fact that PSMA is expressed on some normal cells may result in off-target toxicity due to the delivery of 177Lu, the cell-killing radioactive atom in 177Lu-PSMA-617, to those normal cells.
Undesirable side effects caused by any of our product candidates could cause us or regulatory authorities to interrupt, delay or discontinue clinical trials and could result in the denial, cancellation or withdrawal of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications. This, in turn, could prevent us from commercializing our product candidates and generating revenues from their sale. In addition, if one of our products receives marketing approval and we or others later identify undesirable side effects caused by that product:
· | regulatory authorities may withdraw their approval of the product; |
· | we may be required to recall the product, change the way the product is administered, conduct additional clinical trials or change the labeling of the product; |
· | the product may be rendered less competitive and sales may decrease; or |
· | our reputation may suffer generally both among clinicians and patients. |
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the product, which in turn could delay or prevent us from generating revenues from the sale of the product.
We may not obtain government regulatory approval to market our product candidates.
We may seek approval to market certain of our product candidates in both the United States and in non-U.S. jurisdictions. Prior to commercialization, each product candidate will be subject to an extensive and lengthy governmental regulatory approval process in the United States and in other countries. In order to market our products in the European Union and many other non-U.S. jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. We may not receive the approvals necessary to commercialize our product candidates in any market and we may withdraw applications for approval before acted upon by the regulatory authority.
We may not be able to obtain regulatory approval for any product candidates, or even if approval is obtained, the labeling for such products may place restrictions on their use that could materially negatively impact the marketability and profitability of the product subject to such restrictions. Satisfaction of these regulatory requirements, which includes satisfying regulatory authorities that the product is both safe and effective for its intended uses, typically takes several years or more depending upon the type, complexity, novelty and safety profile of the product and requires the expenditure of substantial resources. Uncertainty with respect to meeting the regulatory requirements governing our product candidates may result in excessive costs or extensive delays in the regulatory approval process, adding to the already lengthy review process.
Also, the approval procedure varies among countries and can involve additional testing and data review. The time and safety and efficacy data required to obtain foreign regulatory approval may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure
28
approval by regulatory agencies in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in other jurisdictions, including approval by the FDA. The failure to obtain regulatory approval in any jurisdiction could materially harm our business.
We may require substantial additional funding which may not be available to us on acceptable terms, or at all.
As we work to advance product candidates through pre-clinical and clinical development, our future funding requirements will depend on many factors, including but not limited to:
· | our need to expand our research and development activities; |
· | the rate of progress and cost of our clinical trials and the need to conduct clinical trials beyond those planned; |
· | the costs associated with establishing a sales force and commercialization capabilities; |
· | the costs of acquiring, licensing or investing in businesses, product candidates and technologies; |
· | the costs and timing of seeking and obtaining approval from regulatory authorities; |
· | our ability to maintain, defend and expand the scope of our intellectual property portfolio; |
· | our need and ability to hire additional management and scientific and medical personnel; |
· | the effect of competing technological and market developments; and |
· | the economic and other terms and timing of collaboration, licensing or other arrangements into which we have entered or may enter into in the future. |
Until we can generate a sufficient amount of revenue to finance our cash requirements, which we may never do, and if we would require additional funding, we expect to finance future cash needs primarily through public or private equity or debt financings or other sources. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs, or enter into collaboration or other arrangements with other companies to provide such funding for one or more of such clinical trials or programs in exchange for our affording such partner commercialization or other rights to the product candidates that are the subject of such clinical trials or programs.
Furthermore, we may incur unexpected expenses, experience timing delays or encounter other circumstances that result in us needing additional funds sooner than planned. Also, we may seek additional capital due to favorable market conditions or other strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights.
We may seek the additional capital necessary to fund our operations through public or private equity or debt financings or other sources, such as strategic partnerships or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of the current stockholders will be diluted and the terms may include liquidation or other preferences that adversely affect their rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures, or declaring
29
dividends, or which impose financial covenants on us that limit our operating flexibility to achieve our business objectives. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. In addition, we cannot assure you that additional funds will be available to us on favorable terms or at all.
If our competitors develop and market products that are more effective, safer or less expensive than our product candidates, our commercial opportunities will be negatively impacted.
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching and marketing products designed to address various types of cancer and other indications we treat or may treat in the future. We are currently developing cancer therapeutics that will compete with other drugs and therapies that currently exist or are being developed. Also, certain of our product candidates may be clinically developed not as an initial first line therapy but as a therapy for patients whose tumors have developed resistance to first line chemotherapy, which limits its potential addressable market. Products we may develop in the future are also likely to face competition from other drugs and therapies.
Many of our competitors have significantly greater financial, manufacturing, marketing and drug development resources than we do. Large biopharmaceutical companies, in particular, have extensive experience in clinical testing and in obtaining regulatory approvals for drugs. Additional mergers and acquisitions in the biopharmaceutical industry may result in even more resources being concentrated by our competition. Competition may increase further as a result of advances in the commercial applicability of technologies currently being developed and a greater availability of capital investment in those fields. These companies may also have significantly greater research and marketing capabilities than we do. Some of the companies developing products which may compete with our product candidates include Adaptimmune Therapeutics PLC; Affimed N.V.; AstraZeneca PLC; Atara Biotherapeutics, Inc.; Atridia Pty LTD; Autolus Limited; Bayer AG; Bellicum Pharmaceuticals, Inc.; BioNTech AG; Bluebird Bio Inc.; Cancer Targeted Technology LLC; Celgene Corporation; Cellectis S.A.; Celyad S.A.; Editas Medicine, Inc.; ESSA Pharma Inc.; Gilead Sciences, Inc.; GlaxoSmithKline plc; Immatics Biotechnologies GmbH; Immunocore Limited; Innocrin Pharmaceuticals Inc.; Intellia Therapeutics, Inc.; Intrexon Corporation; Janssen Biotech, Inc.; Johnson & Johnson; Juno Therapeutics, Inc.; MedImmune, Inc.; Merck & Co., Inc.; MorphoSys AG; Novartis AG; Progenics Pharmaceuticals, Inc.; Pfizer, Inc., Roche Holding AG; Suzhou Kintor Pharmaceuticals, Inc.; Takara Bio, Inc.; TRACON Pharmaceuticals, Inc.; Unum Therapeutics, Inc.; Zenith Pharmaceuticals Ltd; and Zymeworks Inc. In addition, many universities and private and public research institutes are active in cancer research, the results of which may result in direct competition with our product candidates. For example, the German Center of Cancer Research and University Medical Center Heidelberg, the owners of the patent rights to PSMA‑617 (which have been exclusively licensed to ABX and, in turn, exclusively sublicensed to us under the License Agreement), may continue to engage in research relating to RLTs or other cancer therapies, which could result in competition for 177 Lu-PSMA‑617 or other product candidates that we advance from PSMA‑617.
In certain instances, the drugs which will compete with our product candidates are widely available or established, existing standards of care. To compete effectively with these drugs, our product candidates will need to demonstrate advantages that lead to improved clinical safety or efficacy compared to these competitive products. We cannot assure you that we will be able to achieve competitive advantages versus alternative drugs or therapies. If our competitors’ market products are more effective, safer or less expensive than our product candidates or reach the market sooner than our product candidates, we may not achieve commercial success.
We believe that our ability to successfully compete will depend on, among other things:
· | our ability to design and successfully execute appropriate clinical trials; |
· | our ability to recruit and enroll patients for our clinical trials; |
· | the results of our clinical trials and the efficacy and safety of our product candidates; |
30
· | the speed at which we develop our product candidates; |
· | achieving and maintaining compliance with regulatory requirements applicable to our business; |
· | the timing and scope of regulatory approvals, including labeling; |
· | adequate levels of reimbursement under private and governmental health insurance plans, including Medicare; |
· | our ability to protect intellectual property rights related to our product candidates; |
· | our ability to commercialize and market any of our product candidates that may receive regulatory approval; |
· | our ability to have any partners manufacture and sell commercial quantities of any approved product candidates to the market; |
· | acceptance of our product candidates by physicians, other healthcare providers and patients; and |
· | the cost of treatment in relation to alternative therapies. |
In addition, the biopharmaceutical industry is characterized by rapid technological change. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. Also, because our research approach integrates many technologies, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively.
If we fail to attract and retain key management and scientific personnel, we may be unable to successfully develop or commercialize our product candidates.
Our success as a specialized scientific business depends on our continued ability to attract, retain and motivate highly qualified management and scientific and clinical personnel. The loss of the services of any of our senior management could delay or prevent the commercialization of our product candidates.
We may not be able to attract or retain qualified management and scientific personnel in the future due to the intense competition for a limited number of qualified personnel among biopharmaceutical, biotechnology, pharmaceutical and other businesses. In addition, the impact on employee morale experienced in connection with our workforce reduction in June 2017, in which we reduced our workforce by approximately 40%, could make it more difficult for us to retain current employees or to attract new employees when needed. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede the achievement of our research and development objectives, our ability to raise additional capital and our ability to implement our business strategy.
If we evolve from a company primarily involved in clinical development to a company also involved in commercialization, we may encounter difficulties in managing our growth and expanding our operations successfully.
If we are able to advance our product candidates through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. If our operations expand, we expect that we may need to manage additional relationships with such third parties, as well as additional collaborators and suppliers.
31
Maintaining these relationships and managing our future growth will impose significant added responsibilities on members of our management and other personnel. We must be able to: manage our development efforts effectively; manage our clinical trials effectively; hire, train and integrate additional management, development, administrative and sales and marketing personnel; improve our managerial, development, operational and finance systems; and expand our facilities, all of which may impose a strain on our administrative and operational infrastructure. We may also begin to expand our capabilities or enter into contractual relationships during the later stage clinical trial or regulatory approval process, and then have to reduce our capabilities or terminate those relationships if the trials or approval processes are terminated.
Even if we are able to obtain regulatory approval of our products, we may be unable to successfully market and sell them unless we establish sales, marketing and distribution capabilities.
We currently have no sales or distribution capabilities and only limited marketing capabilities. If any of our product candidates receive regulatory approval, we intend to build a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize our product candidates, which will be expensive and time consuming. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. We may choose to collaborate with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. To the extent that we enter into co-promotion or other licensing arrangements, our product revenue is likely to be lower than if we directly marketed or sold our products and will depend in whole or in part upon the efforts of such third parties, which may not be successful and are generally not within our control. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
We rely on third parties to conduct clinical trials for our product candidates and plan to rely on third parties to conduct future clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, it may cause delays in commencing and completing clinical trials of our product candidates or we may be unable to obtain regulatory approval for or commercialize our product candidates.
Clinical trials must meet applicable FDA and foreign regulatory requirements. We do not have the ability to independently conduct phase 2 or phase 3 clinical trials for any of our product candidates. We rely on third parties, such as medical institutions, clinical investigators and contract laboratories, to conduct all of our clinical trials of our product candidates; however, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with its investigational plan and protocol. Moreover, the FDA and other regulatory authorities require us to comply with regulations and standards, commonly referred to as Good Clinical Practices, for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical trials. Our reliance on third parties does not relieve us of these responsibilities and requirements.
We or the third parties we rely on may encounter problems in clinical trials that may cause us or the FDA or foreign regulatory agencies to delay, suspend or terminate our clinical trials at any phase. These problems could include the possibility that we may not be able to manufacture sufficient quantities of materials for use in our clinical trials, conduct clinical trials at our preferred sites, enroll a sufficient number of patients for our clinical trials at one or more sites, or begin or successfully complete clinical trials in a timely fashion, if at all. Furthermore, we, the FDA or foreign regulatory agencies may suspend clinical trials of our product candidates at any time if we or they believe the subjects participating in the trials are being exposed to unacceptable health risks, whether as a result of adverse events occurring in our trials or otherwise, or if we or they find deficiencies in the clinical trial process or conduct of the investigation.
The FDA and foreign regulatory agencies could also require additional clinical trials before or after granting marketing approval for any products, which would result in increased costs and significant delays in the development and commercialization of such products and could result in the withdrawal of such products from the market after obtaining marketing approval. Our failure to adequately demonstrate the safety and efficacy of a product candidate in
32
clinical development could delay or prevent obtaining marketing approval of the product candidate and, after obtaining marketing approval, data from post-approval trials could result in the product being withdrawn from the market, either of which would have a material adverse effect on our business.
We rely on third parties to manufacture and supply our product candidates.
We do not currently own or operate manufacturing facilities for the clinical or commercial production of our product candidates. We lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. Although we entered into agreements with ABX and ITM for the supply to us of PSMA-617 and no-carrier-added lutetium, respectively, we do not have any long-term supply arrangements with any third-party manufacturers and we obtain our raw materials on a purchase order-basis. We expect to continue to depend on third-party contract manufacturers for the manufacture of our product candidates for the foreseeable future. If for some reason our contract manufacturers cannot perform as agreed, we may be unable to replace them in a timely manner and the production of our product candidates would be interrupted, resulting in delays in clinical trials and additional costs. We have no experience with managing the manufacturing of commercial quantities of any of our product candidates and scaling-up production to commercial quantities could take us significant time and result in significant costs. Switching manufacturers may be difficult because the number of potential manufacturers is limited and the FDA and other regulatory authorities must approve any replacement manufacturer prior to manufacturing our product candidates. Such approval would require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our product candidates after receipt of regulatory approval to manufacture any of our product candidates.
To date, our product candidates have been manufactured in small quantities for pre-clinical studies and clinical trials by third-party manufacturers. If the FDA or other regulatory agencies approve any of our product candidates for commercial sale, we expect that we would continue to rely, at least initially, on third-party manufacturers to produce commercial quantities of any approved product candidates. These manufacturers may not be able to successfully increase the manufacturing capacity for any approved product candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation trials, which the regulatory agencies must review and approve. Additionally, any third-party manufacturer we retain to manufacture our product candidates on a commercial scale must pass a pre-approval inspection for conformance to the cGMP before we can obtain approval of our product candidates. If we are unable to successfully increase the manufacturing capacity for a product candidate in conformance with cGMP, the regulatory approval or commercial launch of such products may be delayed or there may be a shortage in supply.
Our product candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could seriously harm our business. Contract manufacturers may encounter difficulties involving production yields, quality control and assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and non-U.S. authorities to ensure strict compliance with cGMP and other applicable government regulations and corresponding foreign standards; however, we do not have control over third-party manufacturers’ compliance with these regulations and standards.
We are subject to risks associated with the availability of key raw materials, such as the radioisotopes used in the manufacture of our products.
The manufacture of our RLTs and companion imaging agents requires the use of raw materials which are subject, at times, to global supply constraints that have the potential to delay our work on the products incorporating those raw materials. For example, any limitation on our ability to source adequate supply of lutetium-177 for 177Lu-PSMA-617 could prevent us from gathering sufficient data in clinical trials, or to the extent that we obtain regulatory approval for marketing for this product candidate, a limited supply may prevent us from meeting commercial demands. Supply constraints for lutetium-177 could also materially increase the manufacturing costs of 177Lu-PSMA-617, which would increase the cost of our clinical trials and reduce the commercial potential of the product candidate.
33
In addition, we plan to use etarfolatide that employs Tc-99m in our development of imaging capabilities and there have been historical periods in which global supply was not sufficient to satisfy worldwide demand for use in various nuclear medicine diagnostics utilized by healthcare providers. If we are not able to obtain sufficient quantities of Tc-99m for use in etarfolatide, we may not be able to gather sufficient data on etarfolatide to use in future clinical trials or to possibly seek the approval of etarfolatide. In addition, to the extent the approval of our product candidates depends on the screening and monitoring of the patient population with a companion imaging agent such as etarfolatide in our clinical trials, we would experience a corresponding delay in approval and commercialization of these product candidates if we are not able to obtain sufficient Tc-99m.
If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, we could be forced to pay substantial damage awards.
The use of any of our product candidates in clinical trials, and the sale of any approved products, may expose us to product liability claims. We currently maintain product liability insurance coverage in an amount which we believe is adequate for our clinical trials currently in progress and those recently completed. We monitor the amount of coverage we maintain as the size and design of our clinical trials evolve and intend to adjust the amount of coverage we maintain accordingly. However, we cannot assure you that such insurance coverage will fully protect us against some or all of the claims to which we might become subject. We might not be able to maintain adequate insurance coverage at a reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a claim is brought against us, we might be required to pay legal and other expenses to defend the claim, as well as uncovered damages awards resulting from a claim brought successfully against us. Furthermore, whether or not we are ultimately successful in defending any such claims, we might be required to direct financial and managerial resources to such defense and adverse publicity could result, all of which could harm our business.
Each of our product candidates will remain subject to ongoing regulatory review even if it receives marketing approval. If we or any collaborators and contractors fail to comply with continuing regulations, we or they may be subject to enforcement action that could adversely affect us.
If any of our product candidates become approved products, they will continue to be subject to pervasive regulation by the FDA and other regulatory authorities. We and any collaborators and contractors will continue to be subject to regulatory requirements governing among other things the manufacture, packaging, sale, promotion, adverse event reporting, storage and recordkeeping of our approved products. Although we have not received any notice that we are the subject of any regulatory enforcement action, it is possible that we may be in the future and that could have a material adverse effect on our business. We may be slow to adapt, or may never adapt, to changes in existing regulatory requirements or adoption of new regulatory requirements. If we or any collaborators or contractors fail to comply with the requirements of the FDA and other applicable governmental or regulatory authorities or previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we or the collaborator or contractor could be subject to administrative or judicially imposed sanctions, including: fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
We deal with hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our activities involve the controlled storage, use, and disposal of hazardous materials, including corrosive, explosive and flammable chemicals, biologic waste and various radioactive compounds. We are subject to federal, state, and local laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. Although we believe that our safety procedures for the handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials.
In the event of an accident, state or federal authorities may curtail our use of these materials, and we could be liable for any civil damages, which may exceed our financial resources and may seriously harm our business. We currently maintain insurance covering hazardous waste clean-up costs in an amount we believe to be sufficient for typical risks
34
regarding our handling of these materials, however, this amount of coverage may not be sufficient to cover extraordinary or unanticipated events. Additionally, an accident could damage, or force us to temporarily shut down, our operations.
Risks Related to Intellectual Property
We may be at risk for cyber attacks or other security breaches that could compromise our intellectual property, trade secrets or other sensitive business information and expose us to liability, which would cause our business and reputation to suffer.
Cyber attacks or security breaches could compromise confidential, business critical information, cause a disruption in our operations, harm our reputation or increase our stock trading risk. We have attractive information assets, including intellectual property, trade secrets and other sensitive, business critical information, including personally identifiable information of our employees. Our employees, some of whom have access to such information, frequently receive “phishing” emails intended to trick recipients into surrendering their user names and passwords. Phishing is a fraud method in which the perpetrator sends out legitimate-looking emails in an attempt to gather personal, business, financial or other information from recipients. To date, we have found no evidence of unauthorized access to our employees’ accounts, but cannot preclude the possibility that sensitive information has been accessed, publicly disclosed, lost or stolen.
We have cybersecurity technologies, processes and practices that are designed to protect networks, computers, programs and data from attack, damage or unauthorized access, but we cannot assure you that they will be effective or will work as designed. Our cybersecurity is continuously reviewed, maintained and upgraded in response to possible security breach incidents. Notwithstanding these measures, a cyber attack could compromise our networks and data centers and/or result in access, disclosure, or other loss of information, which could result in legal claims or proceedings, investigations, potential liabilities under laws that protect the privacy of personal information, delays and impediments to our discovery and development efforts, damage to our reputation and a negative impact on our financial results.
Our proprietary rights may not adequately protect our technologies and product candidates.
Our commercial success will depend in part on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, product candidates, and any future products in the United States and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. The laws of some foreign countries do not protect our proprietary rights to the same extent or in the same manner as U.S. laws, and we may encounter significant problems in protecting and defending our proprietary rights in these countries. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, product candidates and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
We apply for patents covering both our technologies and product candidates, as we deem appropriate. However, we may fail to apply for patents on important technologies or product candidates in a timely fashion, or at all. The existing patent rights that we own or license, and any future patents rights, may not be sufficiently broad to prevent others from practicing our technologies or from developing competing products and technologies. We cannot be certain that patent applications will be approved or that any patents issued will adequately protect our or our licensors’ intellectual property. With respect to PSMA‑617 and CAR T-cell therapies, for example, other than in the PSMA-617 family for Australia, New Zealand, and Singapore, patents have yet to issue and the patents may not issue at all, or if they do issue, they may be challenged. In addition, we often do not ultimately control the patent prosecution of subject matter that we license from others, including those licensed from Purdue Research Foundation, a non-profit organization which manages the intellectual property of Purdue University. In addition, we have licensed intellectual property from other third parties, such as ABX, where we were not involved in preparing, drafting or filing the patent applications. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we would over our own and would need to involve such third parties in legal proceedings to enforce these intellectual property rights. Moreover, the patent positions of biopharmaceutical companies are highly uncertain and involve complex legal and
35
factual questions for which important legal principles are often evolving and remain unresolved. As a result, the validity and enforceability of patents cannot be predicted with certainty. In addition, we do not know whether:
· | we or our licensors were the first to make the inventions covered by each of our issued patents and pending patent applications; |
· | we or our licensors were the first to file patent applications for these inventions; |
· | any of our product candidates will be Orange Book eligible; |
· | others will independently develop similar or alternative technologies or duplicate any of our technologies; |
· | any of our or our licensors’ pending patent applications will result in issued patents; |
· | any of our or our licensors’ patents will be valid or enforceable; |
· | any patents issued to us or our licensors and collaboration partners will provide us with any competitive advantages, or will be challenged by third parties; |
· | we will develop additional proprietary technologies that are patentable; |
· | the U.S. government will exercise any of its statutory rights to our intellectual property that was developed with government funding; or |
· | our business may infringe the patents or other proprietary rights of others. |
The actual protection afforded by a patent varies based on products or processes, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country, the validity and enforceability of the patent and the financial ability of us or a third party to enforce the patent and other intellectual property. Our ability to maintain and solidify our proprietary position for our products will depend on our success in obtaining effective claims and enforcing those claims once granted. Our issued patents and those that may issue in the future, or those licensed to us, may be challenged, narrowed, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages against competitors with similar products. Due to the extensive amount of time required for the development, testing and regulatory review of a potential product, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
We also rely upon unpatented proprietary know-how and continuing technological innovation and other trade secrets in connection with the development of our technologies and product candidates. While it is our policy to enter into agreements imposing confidentiality obligations upon our employees and third parties, including our collaboration partners, to protect our intellectual property, these confidentiality obligations may be breached, may not provide meaningful protection for our trade secrets or proprietary know-how, or adequate remedies may not be available in the event of an unauthorized access, use or disclosure of our trade secrets and know-how. In addition, others could obtain knowledge of our trade secrets through independent development or other access by legal means. Further, non-U.S. courts are sometimes less willing than U.S. courts to protect trade secrets.
The failure of patent rights or confidentiality agreements to protect our processes, technology, trade secrets or proprietary know-how or the failure of adequate legal remedies for related actions could have a material adverse effect on our business, results of operations, financial condition and liquidity.
36
The intellectual property protection for our product candidates is dependent on third parties.
While we do have the right and responsibility under the License Agreement to control the prosecution and maintenance of the patent rights covering PSMA‑617, we are subject to certain consent and cooperation obligations to ABX and/or the owners of the patent rights. With respect to patent applications relating to our product candidates that incorporate patents licensed from Purdue Research Foundation, the right and obligation to prosecute and maintain the patents and patent applications covered by these license agreements are generally retained by Purdue Research Foundation. Generally, we do not have the right to prosecute and maintain such patents in our territories, unless Purdue Research Foundation elects not to file, prosecute or maintain any or all of such patents, however, our most recent master license agreement for future potential technology provides us lead prosecution responsibility. We would need to determine, with our other potential partners, who would be responsible for the prosecution of patents relating to any joint inventions. If any of our licensing partners who maintain such rights fail to appropriately prosecute and maintain patent protection for any of our product candidates, our ability to develop and commercialize those product candidates may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
If we breach any of the agreements under which we license commercialization rights to our product candidates or technology from third parties, we could lose license rights that are important to our business.
We license the use, development and commercialization rights for some of our product candidates, and we expect to enter into similar licenses in the future. For example, we licensed exclusive worldwide rights from ABX and Purdue Research Foundation, pursuant to license agreements, which enables us to use PSMA-617 and adaptor-controlled CAR T-cell therapies, respectively, in the treatment of cancer. Under these licenses, we are subject to development and commercialization obligations, diligence obligations, sublicense revenue sharing requirements, royalty payments, and other obligations. If we fail to comply with any of these obligations or otherwise breach a license agreement or any other current or future licenses, our licensing partners may have the right to terminate the license in whole or in part or to terminate the exclusive nature of the license. In addition, if ABX fails to comply with its obligations under its license agreement with the owners of the patent rights covering PSMA-617, our rights under the License Agreement with ABX could be materially impaired. The loss of any current or future licenses or the exclusivity rights provided therein would materially harm our financial condition and operating results.
The patent protection for our product candidates may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
The patents for our product candidates have varying expiration dates and, if these patents expire, we may be subject to increased competition and we may not be able to recover our development costs or market any of our approved products profitably. In some of the larger potential market territories, such as the United States and Europe, patent term extension or restoration may be available to compensate for time taken during aspects of the product’s development and regulatory review. However, we cannot be certain that such an extension will be granted, or if granted, what the applicable time period or the scope of patent protection afforded during any extension period will be. In addition, even though some regulatory authorities may provide some other exclusivity for a product under their own laws and regulations, we may not be able to qualify the product or obtain the exclusive time period. If we are unable to obtain patent term extension/restoration or some other exclusivity, we could be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. Furthermore, we may not have sufficient time to recover our development costs prior to the expiration of patents.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where there is no patent protection for our product candidates to develop their own products and further, may export otherwise infringing products to territories where there is patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have rights to any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
37
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult to stop the infringement of the patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business.
If we are sued for infringing intellectual property rights of third parties, litigation will be costly and time-consuming and could prevent us from developing or commercializing our product candidates.
Our commercial success depends, in part, on our not infringing the patents and proprietary rights of other parties and not breaching any collaboration or other agreements we have entered into with regard to our technologies and product candidates. Numerous third-party U.S. and non-U.S. issued patents and pending applications exist in the areas of targeted therapy and targeted diagnostics, including RLTs, CAR T-cell therapies, cytotoxic agents and other active compounds and formulations comprising such compounds.
Because patent applications can take several years to issue, if they are issued at all, there may currently be pending applications, unknown to us, that may result in issued patents that cover our technologies or product candidates. It is uncertain whether the issuance of any third-party patent would require us to alter our products or processes, obtain licenses or cease activities related to the development or commercialization of our product candidates. If we wish to use the technology or compound claimed in issued and unexpired patents owned by others, we may need to obtain a license from the owner, enter into litigation to challenge the validity of the patents or incur the risk of litigation in the event that the owner asserts that any of our product candidates infringe its patents. The failure to obtain a license to technology or the failure to challenge an issued patent that we may require to discover, develop or commercialize our products may have a material adverse impact on us.
There is a substantial amount of litigation involving intellectual property in the biopharmaceutical industry generally. If a third party asserts that our products or technologies infringe its patents or other proprietary rights, we could face a number of risks that could seriously harm our results of operations, financial condition and competitive position, including:
· | infringement and other intellectual property claims, which would be costly and time-consuming to defend, whether or not the claims have merit, and which could delay the regulatory approval process and divert management’s attention from our business; |
· | substantial damages for past infringement, which we may have to pay if a court determines that our product candidates or technologies infringe a third party’s patent or other proprietary rights; |
· | a court prohibiting us from selling or licensing our technologies or our product candidates unless the third party licenses its patents or other proprietary rights to us on commercially reasonable terms, which it is not required to do; |
· | if a license is available from a third party, we may have to pay substantial royalties or lump sum payments or grant cross-licenses to our patents or other proprietary rights to obtain that license; and |
· | redesigning our products so they do not infringe, which may not be possible or may require substantial monetary expenditure and time. |
Although we are not currently a party to any legal proceedings relating to our intellectual property, in the future, third parties may file claims asserting that our technologies or products infringe on their intellectual property. We cannot predict whether third parties will assert these claims against us or against the current or future licensors of technology licensed to us, or whether those claims will harm our business. If we are forced to defend against these claims, whether they are with or without any merit, whether they are resolved in favor of or against us or our licensors, we may face costly litigation and diversion of management’s attention and resources. As a result of these disputes, we may have to
38
develop costly non-infringing technology, or enter into licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, if at all, which could seriously harm our business or financial condition.
One or more third-party patents or patent applications may conflict with patent applications to which we have rights. Any such conflict may substantially reduce the coverage of any rights that may issue from the patent applications to which we have rights. If third parties file patent applications in technologies that also claim technology to which we have rights, we may have to participate in interference proceedings with the USPTO, or non-U.S. patent regulatory authorities, as applicable, to determine priority of invention.
We may become involved in lawsuits to enforce patents or other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe the patents or other intellectual property rights related to our product candidates. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. To the extent such claims relate to patent rights held by our licensors, they would have to file such an infringement lawsuit since we do not have the independent right to enforce those third parties’ intellectual property. In addition, in an infringement proceeding, a court may decide that a patent is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that the patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of the patents at risk of being invalidated or interpreted narrowly and could put patent applications at risk of not issuing.
Interference proceedings brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents and patent applications or those of our current or future licensors or collaborators. An unfavorable outcome could require us to cease using the technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if a prevailing party does not offer us a license on terms that are acceptable to us. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction of our management and other employees. We may not be able to prevent, alone or with our licensors or collaborators, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential and proprietary information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Risks Related to Ownership of Our Common Stock
The price of our common stock has been volatile and our shares may suffer a decline in value.
Since becoming a public company in February 2011, we have experienced volatility in the trading price of our common stock. Factors that could cause volatility in the market price of our common stock include, but are not limited to, the risk factors identified above as well as:
· | results from, supplemental analyses of and any delays in, our current or planned clinical trials; |
· | announcements of approval or non-approval by any regulatory authorities of any of our product candidates, or delays in any regulatory authority review processes; |
· | other regulatory actions affecting us or our industry; |
· | litigation or public concern about the safety of our product candidates; |
· | failure or discontinuation of any of our research or clinical trial programs; |
39
· | withdrawal of regulatory approval applications; |
· | delays in the commercialization of our product candidates; |
· | our ability to effectively partner with collaborators to develop or sell our products; |
· | market conditions in the pharmaceutical, biopharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
· | actual and anticipated fluctuations in our quarterly operating results; |
· | developments or disputes concerning our intellectual property or other proprietary rights; |
· | introduction of technological innovations or new products by us or our competitors; |
· | issues in supply or manufacturing of our product candidates; |
· | market acceptance of our product candidates; |
· | deviations in our operating results from the estimates of securities analysts; |
· | coverage and reimbursement policies of governments and other third-party payors; |
· | sales of our common stock by our officers, directors or significant stockholders; |
· | price and volume fluctuations in the overall stock market from time to time; |
· | general economic conditions and trends; |
· | major catastrophic events; |
· | our ability to expand our operations, domestically and internationally, and the amount and timing of expenditures related to this expansion; and |
· | additions or departures of key personnel. |
In addition, the stock markets in general, and the markets for biopharmaceutical, pharmaceutical and biotechnology stocks in particular, have experienced extreme volatility that has been often unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action and other litigation against the issuer.
Sales of substantial amounts of our shares could adversely affect the market price of our common stock.
Sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur, could cause the market price of our common stock to decline. These sales could also make it more difficult for us to raise capital by selling equity or equity-related securities in the future at a time and price that we deem appropriate.
As of December 31, 2017, there were 48,203,529 shares of our common stock outstanding. All of the outstanding shares are freely transferable without restriction under the Securities Act of 1933, as amended, or the Securities Act, unless held by our “affiliates” as that term is used in Rule 144 promulgated under the Securities Act or unless issued in
40
an unregistered offering. Such shares may be sold in the public market pursuant to Rule 144, another exemption from registration or an effective registration statement under the Securities Act.
We do not intend to pay dividends on our common stock, and, consequently, your ability to achieve a return on your investment will depend on appreciation, if any, in the price of our common stock.
We have never declared or paid any cash dividend on our common stock and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business. In addition, any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Provisions in our certificate of incorporation and bylaws and under Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Our certificate of incorporation and bylaws contain provisions that could depress the trading price of our common stock by acting to discourage, delay or prevent a change of control of our company or changes in our management that our stockholders may deem advantageous. These provisions include:
· | establishing a classified board so that not all members of our Board of Directors are elected at one time; |
· | authorizing “blank check” preferred stock that our Board of Directors could issue to increase the number of outstanding shares to discourage a takeover attempt; |
· | eliminating the ability of stockholders to call a special stockholder meeting; |
· | eliminating the ability of stockholders to act by written consent; |
· | being subject to provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers; |
· | providing that our Board of Directors is expressly authorized to make, alter or repeal our bylaws; and |
· | establishing advance notice requirements for nominations for elections to our Board of Directors or for proposing other matters that can be acted upon by stockholders at stockholder meetings. |
If we fail to maintain proper internal controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
We are subject to the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, which requires management to assess and report annually on the effectiveness of internal control over financial reporting and identify any material weaknesses in internal control over financial reporting, and our independent registered public accounting firm to issue an attestation report as to the effectiveness of internal control over financial reporting.
If we identify one or more material weaknesses in our internal control over financial reporting, or if we are unable to conclude that we have effective internal control over financial reporting or if our independent auditors are unwilling or unable to provide us with an attestation report on the effectiveness of internal control over financial reporting, investors may lose confidence in our operating results, our stock price could decline and we may be subject to litigation or regulatory enforcement actions.
Our ability to use net operating losses to offset future taxable income is subject to certain limitations.
41
Under Section 382 of the U.S. Internal Revenue Code, or Code, a corporation that experiences a more-than 50 percent ownership change over a three-year testing period is subject to limitations on its ability to utilize its pre-change net operating losses to offset future taxable income. We experienced such an ownership change in August 2011. As a result, the future use of our net operating losses, after giving effect to net unrealized built-in gains, is currently limited to approximately $218.7 million for 2017. Any available but unused amounts will become available for use in all successive years, subject to certain limitations. Utilization of these net operating loss carryforwards would require us to generate future taxable income prior to their expiration. Furthermore, the utilization of the net operating loss carryforwards could be limited beyond our generation of taxable income if a change in the underlying ownership of our common stock has occurred, resulting in a limitation on the amounts that could be utilized in any given period under Section 382 of the Code. If not used, the net operating loss carryforwards will begin expiring in the year 2021. At December 31, 2017, we recorded a full valuation allowance against our deferred tax assets of approximately $95.9 million, as we believe it is more likely than not that the deferred tax assets will not be fully realized.
Item 1B.Unresolved Staff Comments
None.
Our offices are located in two primary leased facilities, a 21,927 square foot facility in West Lafayette, Indiana in the Purdue University Research Park and a 7,600 square foot corporate office space in Indianapolis, Indiana. The West Lafayette facility includes both administrative and research laboratory space. The lease for this facility expires June 30, 2018, and we do not foresee risk in obtaining an extension to this lease agreement on similar terms or having a disruption to our operations. The Indianapolis offices are used exclusively for corporate and administrative functions. The lease for this facility was renewed in early 2018 and expires in February 2019. We believe the existing facilities are sufficient to meet our current and near-term needs.
We are not currently a party to any material legal proceedings.
Item 4.Mine Safety Disclosures
Not applicable.
42
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information and Stockholders
Shares of our common stock are traded on The Nasdaq Global Market (symbol ECYT). The following table sets forth the high and low sale prices per share for our common stock on The Nasdaq Global Market for the periods indicated:
|
|
|
|
|
|
|
|
|
| High |
| Low |
| ||
2016 |
|
|
|
|
|
|
|
First Quarter |
| $ | 3.99 |
| $ | 2.65 |
|
Second Quarter |
| $ | 4.33 |
| $ | 3.06 |
|
Third Quarter |
| $ | 3.38 |
| $ | 2.90 |
|
Fourth Quarter |
| $ | 3.49 |
| $ | 2.52 |
|
2017 |
|
|
|
|
|
|
|
First Quarter |
| $ | 2.74 |
| $ | 1.98 |
|
Second Quarter |
| $ | 2.98 |
| $ | 1.43 |
|
Third Quarter |
| $ | 1.55 |
| $ | 1.17 |
|
Fourth Quarter |
| $ | 6.55 |
| $ | 1.63 |
|
As of February 23, 2018, there were 93 stockholders of record of our common stock. The number of record holders is based upon the actual number of holders registered on the books of the company at such date and does not include holders of shares in “street name” or persons, partnerships, associations, corporations or other entities identified in security position listings maintained by depositories.
We have never declared or paid any dividends on our capital stock. We currently intend to retain all future earnings for the operation and expansion of our business and, therefore, we do not anticipate declaring or paying cash dividends for the foreseeable future. The payment of dividends, if any, will be at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements, any limitations on payment of dividends in our future debt agreements, and other factors that our Board of Directors may deem relevant.
Unregistered Sales of Securities
We did not have any unregistered sales of securities in the fourth quarter of 2017.
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities in the fourth quarter of 2017.
Comparative Stock Performance Graph
The information included under the heading “Comparative Stock Performance Graph” in this Item 5 of Part II of this Annual Report on Form 10-K shall not be deemed to be “soliciting material” or subject to Regulation 14A or 14C, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act.
43
Set forth below is a graph comparing the total cumulative returns of ECYT, the Nasdaq Composite Index and the Nasdaq Biotechnology Index. The graph assumes $100 was invested on December 31, 2012 in our common stock and each of the indices and that all dividends, if any, are reinvested.
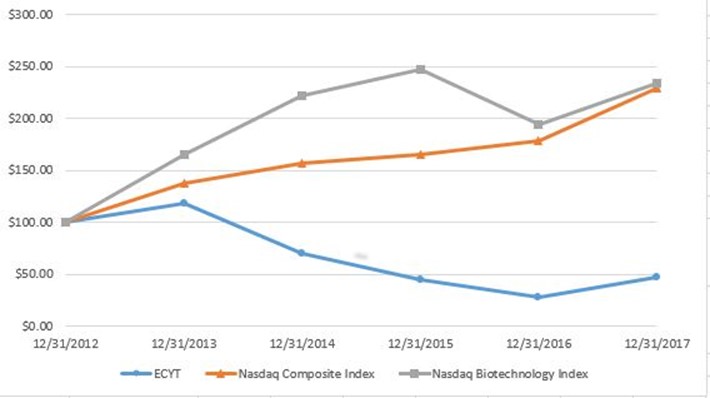
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 12/31/12 |
| 12/31/13 |
| 12/31/14 |
| 12/31/15 |
| 12/31/16 |
| 12/31/17 |
| ||||||
Endocyte, Inc. |
| $ | 100.00 |
| $ | 118.93 |
| $ | 70.04 |
| $ | 44.65 |
| $ | 28.40 |
| $ | 47.66 |
|
NASDAQ Composite Index |
| $ | 100.00 |
| $ | 138.32 |
| $ | 156.85 |
| $ | 165.84 |
| $ | 178.28 |
| $ | 228.63 |
|
NASDAQ Biotechnology Index |
| $ | 100.00 |
| $ | 165.61 |
| $ | 222.08 |
| $ | 247.44 |
| $ | 193.79 |
| $ | 234.60 |
|
44
Item 6.Selected Financial Data
We have derived the selected statement of operations data for the years ended December 31, 2015, 2016 and 2017 and selected balance sheet data as of December 31, 2016 and 2017 from our audited financial statements and related notes included in this annual report. We have derived the selected statement of operations data for the years ended December 31, 2013 and 2014 and the selected balance sheet data as of December 31, 2013, 2014 and 2015 from our audited financial statements not included in this annual report. Our historical results are not necessarily indicative of the results to be expected for any future period. The following selected financial data should be read in conjunction with Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and our financial statements and related notes included elsewhere in this annual report.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| |||||||||||||
|
| 2013 |
| 2014 |
| 2015 |
| 2016 |
| 2017 |
| |||||
|
|
| (In thousands, except share and per share amounts) |
| ||||||||||||
Statement of operations data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
| $ | 64,871 |
| $ | 70,353 |
| $ | 70 |
| $ | 70 |
| $ | 70 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 57,899 |
|
| 41,650 |
|
| 26,309 |
|
| 27,492 |
|
| 25,867 |
|
General and administrative |
|
| 25,314 |
|
| 23,677 |
|
| 15,734 |
|
| 17,298 |
|
| 13,770 |
|
Acquired in-process research and development |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 16,539 |
|
Total operating expenses |
|
| 83,213 |
|
| 65,327 |
|
| 42,043 |
|
| 44,790 |
|
| 56,176 |
|
Income (loss) from operations |
|
| (18,342) |
|
| 5,026 |
|
| (41,973) |
|
| (44,720) |
|
| (56,106) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
| 464 |
|
| 578 |
|
| 652 |
|
| 861 |
|
| 1,029 |
|
Other income (expense), net |
|
| (154) |
|
| (145) |
|
| 51 |
|
| (29) |
|
| 13 |
|
Total other income, net |
|
| 310 |
|
| 433 |
|
| 703 |
|
| 832 |
|
| 1,042 |
|
Income (loss) before income taxes |
|
| (18,032) |
|
| 5,459 |
|
| (41,270) |
|
| (43,888) |
|
| (55,064) |
|
Income tax (benefit) expense (1) |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
Net income (loss) |
| $ | (18,032) |
| $ | 5,459 |
| $ | (41,270) |
| $ | (43,888) |
| $ | (55,064) |
|
Basic net income (loss) per share |
| $ | (0.50) |
| $ | 0.14 |
| $ | (0.98) |
| $ | (1.04) |
| $ | (1.25) |
|
Diluted net income (loss) per share |
| $ | (0.50) |
| $ | 0.13 |
| $ | (0.98) |
| $ | (1.04) |
| $ | (1.25) |
|
Shares used in computation of: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share (2) |
|
| 36,036,996 |
|
| 40,242,352 |
|
| 41,939,504 |
|
| 42,210,643 |
|
| 43,900,257 |
|
Diluted net income (loss) per share (2) |
|
| 36,036,996 |
|
| 41,758,101 |
|
| 41,939,504 |
|
| 42,210,643 |
|
| 43,900,257 |
|
(1) | For 2014 we reported net income; however, we had no income tax expense that year due to net operating losses in prior years. We had no income tax expense in 2013, 2015, 2016 or 2017 because we incurred net losses in each of those years. |
(2) | For 2013, 2015, 2016 and 2017, diluted weighted-average common shares outstanding were identical to basic weighted-average common shares outstanding and diluted loss per share was identical to basic loss per share because common share equivalents were excluded from the calculations of diluted weighted-average common shares outstanding for those years, as their effect was anti-dilutive. For 2014, 1,515,749 shares were added to basic weighted-average common shares outstanding to calculate diluted weighted-average shares outstanding because of their dilutive effect. |
45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| As of December 31, |
| |||||||||||||
|
| 2013 |
| 2014 |
| 2015 |
| 2016 |
| 2017 |
| |||||
|
| (In thousands) |
| |||||||||||||
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and investments |
| $ | 148,853 |
| $ | 206,831 |
| $ | 173,600 |
| $ | 138,207 |
| $ | 97,471 |
|
Working capital |
|
| 59,668 |
|
| 120,153 |
|
| 168,687 |
|
| 134,694 |
|
| 94,027 |
|
Total assets |
|
| 162,858 |
|
| 212,801 |
|
| 178,386 |
|
| 143,494 |
|
| 100,762 |
|
Total debt |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
Total stockholders’ equity |
|
| 88,229 |
|
| 205,004 |
|
| 171,346 |
|
| 137,147 |
|
| 95,484 |
|
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes included in this annual report. Certain amounts presented in this discussion and analysis are presented in thousands and may be rounded for purposes of calculating to the summed rounded amount.
Overview
We are a biopharmaceutical company and leader in developing targeted therapies for the treatment of cancer. We use drug conjugation technology to create novel therapeutics and companion imaging agents for personalized targeted therapies. Our agents actively target receptors that are over-expressed on diseased cells relative to healthy cells, such as prostate specific membrane antigen, or PSMA, in prostate cancer. This targeted approach is designed to safely enable the delivery of highly potent drug payloads. The companion imaging agents are designed to identify patients whose disease over-expresses the target of the therapy and who are therefore more likely to benefit from treatment.
In June 2017, we ended clinical development of EC1456 and stopped enrollment in our EC1456 phase 1b trial as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. In December 2017, we stopped enrollment in our EC1456 ovarian cancer surgical trial. In addition, in June 2017, we narrowed the focus of our phase 1 EC1169 development program (for which enrollment was completed in October 2017, but for which we currently do not intend to invest further resources beyond the completion of the phase 1 taxane-exposed cohort), refocused our efforts on pre-clinical programs, and reduced our workforce by approximately 40% to align resources to focus on our highest value opportunities while maintaining key capabilities. We recorded $2.3 million of restructuring expenses for the year ended December 31, 2017, as follows:
· | included in research and development expenses were expenses for employee termination benefits of $0.9 million, $0.9 million for the remaining EC1456 phase 1b trial expenses, including site close-out expenses, $0.3 million related to other restructuring expenses, and $0.1 million related to fixed asset impairment charges; and |
· | included in general and administrative expenses were expenses for employee termination benefits of $0.1 million. |
As of December 31, 2017, we had paid all severance and other costs related to the restructuring activities, and had a clinical trial accrual balance related to the EC1456 phase 1b trial termination of $0.1 million, which is expected to be fully paid by the end of the first quarter of 2018.
On September 29, 2017, we entered into a Development and License Agreement, or the License Agreement, with ABX advanced biochemical compounds – Biomedizinische Forschungsreagenzien GmbH, or ABX, pursuant to which we acquired exclusive worldwide rights to develop and commercialize PSMA-617 agents, including the product candidate known as 177Lu-PSMA-617, a radioligand therapeutic, or RLT. Under the terms of the License Agreement,
46
we will be responsible for, and bear the future costs of, worldwide development and commercialization of PSMA-617. As consideration for the exclusive license, we made an upfront cash payment on September 29, 2017 of approximately $11.9 million to ABX, consisting of $12.0 million less an immaterial expense reimbursement amount, and issued to ABX 2,000,000 shares of our common stock and warrants to purchase, in the aggregate, 4,000,000 shares of our common stock. The License Agreement also obligates us to pay ABX regulatory milestone payments of up to $25.0 million, sales milestone payments of up to $135.0 million, and tiered royalties, beginning in the mid-teens and not to exceed the mid-twenties, based on percentages of net sales. We recorded $16.5 million of acquired in-process research and development, or IPR&D, expenses related to the License Agreement for the year ended December 31, 2017 consisting of the following:
· | $12.0 million related to the upfront payment to ABX; |
· | $3.8 million related to the fair value of common stock and warrant shares issued; and |
· | $0.7 million of acquisition costs consisting primarily of legal and professional fees. |
In October 2017, we announced our plan to focus our resources on the development of 177Lu-PSMA-617 and on a targeted effort to generate proof-of-concept data for our chimeric antigen receptor T-cell, or CAR T-cell, therapy program, and to explore out-licensing opportunities for all other development programs, including EC2629, our dual-targeted folate-pro pyrrolobenzodiazepine, or pro-PBD, DNA crosslinker drug.
We intend to initiate, in the second quarter of 2018, the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 and will enroll up to 750 patients with progressive PSMA-positive metastatic castration-resistant prostate cancer, or mCRPC. 177Lu-PSMA-617 utilizes a high affinity targeting ligand to direct potent radiotherapy to prostate cancer cells. The specific targeting of this therapy comes from the “ligand” portion of the RLT, which is a small molecule designed to bind to PSMA, a protein highly expressed on the cell surface of most prostate cancer cells but absent on most normal cells. The PSMA targeting ligand in 177Lu-PSMA-617 is chemically attached to a therapeutic radioactive atom called Lutetium-177 (177Lu), which releases an energetic beta particle designed to precisely deliver cell-killing radiation to the site of disease. Unlike traditional external beam radiotherapy, 177Lu-PSMA-617, which is administered as a systemic injection, has been designed to directly target multiple sites of PSMA-positive prostate cancer throughout the body, including the bone and soft tissue, while bypassing the PSMA-negative cells. Prior to treatment with 177Lu-PSMA-617, the patient’s expression of PSMA can be determined using imaging technology, allowing for personalization of treatment so that the optimum course of therapy might be selected. As highlighted in roughly 20 peer reviewed publications of trials in the post-chemotherapy compassionate use setting, 177Lu-PSMA-617 demonstrated a prostate-specific antigen, or PSA, response (defined as greater than 50% decline from baseline) in 40% to 60% of patients, and a Response Evaluation Criteria in Solid Tumors, or RECIST, response rate in soft tissue disease of between 40% and 50%.
In October 2017, we entered into an agreement with RadioMedix, Inc., a biotechnology company focused on innovative targeted radiopharmaceuticals for diagnosis, monitoring and therapy of cancer, which enabled the transfer of a U.S. Investigational New Drug application of 177Lu-PSMA-617 from the prior sponsor, RadioMedix, to us. This transfer helped accelerate our successful End of Phase 2 trial meeting with the U.S Food and Drug Administration, or FDA, in early 2018 to confirm our phase 3 trial design and registration plan for 177Lu-PSMA-617.
On February 26, 2018, we also announced an agreement with ITM Isotopen Technologien München AG, which will provide clinical supply of no-carrier-added lutetium for the manufacturing of 177Lu-PSMA-617 for the VISION trial.
At the European Society for Medical Oncology Congress in September 2017, Dr. Michael Hofman of the Peter MacCallum Cancer Center in Melbourne, Australia presented the results of an open-label, single-arm, non-randomized pilot trial of 177Lu-PSMA-617 in 30 mCRPC patients. Primary endpoints included safety and efficacy as defined by PSA response, quality of life, and imaging response. The results showed a 57% PSA response rate (>50% reduction) and 71% interim RECIST response rate in soft tissue lesions in patients who had previously failed such conventional therapies as docetaxel, cabazitaxel, enzalutamide and abiraterone. Median overall survival was 12.7 months. The drug was well-tolerated, with a grade 3 or higher hematoxicity attributable to 177Lu-PSMA-617 occurring in five (17%) patients and no renal toxicity. Significantly improved quality of life scores and reduction in pain scores were recorded in 37% and 43% of patients, respectively. This trial has subsequently been expanded to 50 patients from the original 30, with updated results expected to be presented at the annual American Society of Clinical Oncology, or ASCO, meeting in June 2018.
47
In addition, we entered into a three-party agreement in October 2017, with the University of Sydney, or the University, and ANZUP, a cooperative cancer trials group operating in Australia and New Zealand pursuing research in genito-urinary malignancies, in which ANZUP sponsors jointly with the University a randomized phase 2 multi-center TheraP trial of 177Lu-PSMA-617 versus cabazitaxel in 200 mCRPC patients. The TheraP trial commenced enrollment in the first quarter of 2018. Under the three-party agreement, we provide the PSMA-617 precursor molecule and financial support for the trial. We will have access to data generated from the trial, which is a potentially important supportive trial for future regulatory submissions. The primary financial obligations of the trial, along with labeling PSMA-617 with Lutetium-177, will be the responsibility of the University and ANZUP.
We are also developing a unique therapeutic approach that involves the re-targeting of potent immune cells, called CAR T-cells, to fight cancer. CAR T-cell therapies may be characterized as either allogeneic CAR T-cells, which are those that are engineered using T-cells from a single donor that are utilized in multiple patients, or autologous CAR T-cells, which are those that are engineered using a patient’s own T-cells. Our program utilizes an autologous approach. Traditional CAR T-cell therapies rely on the activity and specificity of T-cells that have been engineered to recognize a single naturally expressed target that, ideally, is only present on cancer cells, with no cross-reactivity to or targeting of healthy tissues. Our alternative strategy relies on a single universal CAR T-cell that expresses a high affinity for a molecule called fluorescein isothiocyanate, or FITC, which is not naturally present in the human body. The activity and specificity of these universal CAR T-cells is dependent upon the administration of our proprietary CAR T adaptor molecules, or CAMs, that “paint” a patient’s cancer cells with FITC by conjugating it to a tumor-targeting ligand. The FITC molecule then attracts the universal CAR T-cell to the site of disease, causing the anti-cancer immune response of a traditional CAR T-cell therapy. However, unlike existing CAR T-cell technologies, our unique CAM-dependent technology makes possible the engineering of a single universal CAR T-cell that can be used to treat a wide range of cancer types. This is accomplished through the use of multiple CAMs, each of which is designed to bind the FITC molecule to a specific cancer type. In addition to enabling the treatment of multiple cancer types with a single universal CAR T-cell, this adaptor technology is also designed to facilitate novel control strategies intended to increase the safety of CAR T-cell therapy. In March 2017, we announced our collaboration with Seattle Children’s Research Institute, or SCRI, and Dr. Michael Jensen for the development of our technology in the CAR T-cell immunotherapy setting. The aim of the research collaboration is to join our adapter technology with the CAR T-cell immunotherapy research efforts at the Ben Towne Center for Childhood Cancer Research at SCRI, to move these potentially enabling technologies more quickly to patients in the clinic. In October 2017, we announced that we are limiting the scope our CAR T-cell therapy program to a targeted effort to generate proof-of-concept data. Pre-clinical evaluations have been completed, and clinical evaluation is expected to begin in the fourth quarter of 2018.
For the year ended December 31, 2017, we had a net loss of $55.1 million and a retained deficit of $309.0 million. We expect to continue to incur significant operating expenses for the next several years as we pursue the advancement of our product candidates through the research, development, regulatory and, potentially, the commercialization processes. During the years ended December 31, 2017 and 2016, our revenue related to the amortization of the $1.0 million non-refundable upfront payment from Nihon Medi-Physic Co., LTD., or NMP, as well as annual minimum royalty payments received in 2016 and 2017. See Note 12 Collaboration and Other Arrangements to Consolidated Financial Statements contained in Part II, Item 8 herein, for further information regarding remaining contractual obligations.
Our operating costs were higher for the year ended December 31, 2017 compared to the year ended December 31, 2016, primarily due to an increase in acquired IPR&D expenses related to the License Agreement for PSMA-617, and, to a lesser extent, an increase in trial expenses related to EC1169, an increase in expenses related to the termination of the EC1465 phase 1b trial, including remaining trial and site close-out expenses, an increase in expenses related to pre-clinical work and general research, including the development of EC2629, an increase in legal and professional fees related to the License Agreement, an increase in expenses related to consulting and other fees related to the development of PSMA-617, and an increase in expenses related to our CAR T-cell therapy program. These increases were partially offset by a decrease in compensation expense due to the resignation of our former Chief Executive Officer in June 2016, lower compensation expense in the year ended December 31, 2017 due to employee terminations, including terminations as a result of the workforce reduction, a decrease in manufacturing expense for EC1169 and EC1456, and a decrease in trial expenses for EC1456 unrelated to the trial termination.
48
As of December 31, 2017, our cash, cash equivalents and investments were $97.5 million. We believe that our current cash balance will be sufficient to fund our activities into the second half of 2019 through many important milestones, which include our focus on clinical development of 177Lu-PSMA-617 and our effort to generate proof-of-concept data on our CAR T-cell therapy program.
Revenue
To date, we have generated no revenue from sales of our product candidates. All of our revenue has been derived from license fees, research and development expense reimbursement, milestone payments and government grants.
In the future, we may generate revenue from a combination of direct sales of our product candidates, license fees, research and development expense reimbursement, milestone payments and royalties in connection with strategic collaborations. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the timing and amount of license fees, achievement of performance-based milestones and other payments received under such collaborations, and the amount and timing of payments that we receive upon the sale of our product candidates to the extent any are successfully commercialized. If we or any strategic partners fail to complete the development of our product candidates in a timely manner or at all, or fail to obtain regulatory approval for them, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely affected.
Research and Development Expenses
Research and development expenses consist of expenses incurred in connection with the ongoing development of our novel therapeutics and companion imaging agents for personalized targeted therapies, including:
· | employee-related expenses, which include salaries, benefits and stock-based compensation expense; |
· | facility costs related to research activities; |
· | expenses incurred under agreements with contract research organizations, investigative sites and consultants that conduct our clinical trials and a portion of our pre-clinical studies; |
· | the cost of acquiring and manufacturing clinical trial materials; |
· | license fees for and milestone payments related to in-licensed products and technology; |
· | costs associated with non-clinical activities and regulatory approvals; and |
· | research supplies. |
We expense research and development costs as incurred. License fees and milestone payments related to in-licensed products and technology and research supplies are expensed if it is determined that they have no alternative future use.
Our internal resources, employees and infrastructure are not directly tied to any individual research project and are typically deployed across multiple projects. The following table sets forth costs incurred on a program-specific basis for our novel therapeutics and companion imaging agents, excluding personnel-related costs. Discovery research includes such costs for projects where no lead candidate has yet been identified. All employee-related expenses for those
49
employees working in research and development functions are included in research and development employee compensation.
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| |||||||
|
| 2015 |
| 2016 |
| 2017 |
| |||
|
| (in thousands) |
| |||||||
Vintafolide (EC145) |
| $ | 1,488 |
| $ | (26) |
| $ | — |
|
Etarfolatide (EC20) |
|
| 259 |
|
| 285 |
|
| 187 |
|
Folate-Tubulysin (EC1456) (1) |
|
| 3,488 |
|
| 3,452 |
|
| 2,567 |
|
PSMA Imaging (EC0652) and Therapeutic (EC1169) (2) |
|
| 1,231 |
|
| 3,952 |
|
| 4,524 |
|
Folate-Aminopterin (EC2319) (2) |
|
| 173 |
|
| 324 |
|
| 955 |
|
Folate-pro pyrrolobenzodiazepine, or proPBD, DNA Crosslinker (EC2629) (2) |
|
| — |
|
| 635 |
|
| 1,020 |
|
PSMA-617 RLT (3) |
|
| — |
|
| — |
|
| 485 |
|
CAR T-cell Therapy |
|
| — |
|
| — |
|
| 230 |
|
Discovery research |
|
| 4,882 |
|
| 4,402 |
|
| 4,345 |
|
Research and development employee compensation (1) |
|
| 14,788 |
|
| 14,468 |
|
| 11,554 |
|
Total research and development expenses |
| $ | 26,309 |
| $ | 27,492 |
| $ | 25,867 |
|
(1) The development of EC1456 ceased in June 2017 as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. The June 2017 restructuring activities also included a 40% reduction in headcount.
(2) The development of these programs has ceased, as we announced, in October 2017, our plan to focus our resources on the development of 177Lu-PSMA-617 and on a targeted effort to generate proof-of-concept data for our CAR T-cell therapy program, and to explore out-licensing opportunities for all other development programs, including EC2629.
(3) Excludes acquired IPR&D expenses.
The following table identifies the current status of our major research and development projects and our currently expected near-term milestone timing:
Project |
| Status |
| Expected Near-Term Milestones |
PSMA-targeted radioligand therapeutic (PSMA-617) |
| End of Phase 2 |
| Initiation of phase 3 trial expected in second quarter of 2018 |
Adaptor-controlled chimeric antigen receptor T-cell (CAR T-cell) therapy |
| Pre-Clinical |
| Initiation of phase 1 trial in collaboration with SCRI expected in fourth quarter of 2018 |
General and Administrative Expenses
General and administrative expenses consist principally of salaries, benefits and stock-based compensation for personnel in executive, finance, business development, legal and human resources functions. Other general and administrative expenses include facility costs related to administrative functions, patent filing and prosecution costs, and professional service fees. We incurred compensation expenses of $2.8 million for noncash stock compensation and $0.8 million for a cash payment related to the resignation of our former Chief Executive Officer in June 2016.
Restructuring Costs included in Research and Development and General and Administrative Expenses
We terminated the PROCEED trial in May 2014 after the interim futility analysis indicated that vintafolide did not demonstrate efficacy on the pre-specified outcome of progression-free survival for the treatment of PROC. As a result, we ceased our pre-launch commercial activities and implemented staff reductions in Europe and in the U.S. All restructuring expenses related to the PROCEED trial termination and related staff reductions were paid by December 31, 2016.
50
In June 2017, we ended clinical development of EC1456 and stopped enrollment in our EC1456 phase 1b trial as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. In December 2017, we stopped enrollment in our EC1456 ovarian cancer surgical trial. In addition, in June 2017, we narrowed the focus of our phase 1 EC1169 development program (for which enrollment was complete in October 2017, but for which we currently do not intend to invest further resources beyond the completion of the phase 1 taxane-exposed cohort), refocused our efforts on pre-clinical programs, and reduced our workforce by approximately 40% to align resources to focus on our highest value opportunities while maintaining key capabilities. All employee and contract termination expenses were recorded and paid in the year ended December 31, 2017.
See Note 15 - Restructuring Costs of the Notes to Consolidated Financial Statements contained in Part II, Item 8 herein, for further information regarding restructuring costs.
Other Income (Expense), Net
Other income and expense consists primarily of gains or losses on disposal of equipment, charitable contributions, gains or losses on foreign currency exchange, and state franchise tax fees.
Interest Income, Net
Interest income consists of interest earned on our cash, cash equivalents and investments. The primary objective of our investment policy is capital preservation. Interest expense consists primarily of interest related to capital lease obligations.
Critical Accounting Policies and Significant Judgments and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts, identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We estimate our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Expense accruals related to clinical trial activity typically comprise the majority of these accruals. Examples of estimated expenses related to clinical trial activity include:
· | fees paid to contract research organizations in connection with clinical trials; and |
· | fees paid to investigative sites in connection with clinical trials. |
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and contract research organizations that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under certain contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services are performed and the level of effort to be expended in each period. If the actual timing
51
of the performance of services or the level of the effort varies from our estimate, we adjust the accrual accordingly. Although our estimates in the past have not been materially different from amounts actually incurred, and we do not expect our estimates to be materially different from amounts actually incurred in the future, if our estimate of the status and timing of services performed differs from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. In the event that a clinical trial is terminated early, we record, in the period of termination, an accrual for the estimated remaining costs to complete the trial. Based on our level of clinical trial expenses for the twelve months ended December 31, 2017, a five percent change in our estimate could result in an adjustment to our accrued clinical trial expense in future periods of approximately $34,000.
Our significant accounting policies are described in more detail in Note 2 of the Notes to Consolidated Financial Statements contained in Part II, Item 8 herein. See also Note 10 of the Notes to Consolidated Financial Statements contained in Part II, Item 8 herein for further information regarding our stock-based compensation.
Results of Operations
Comparison of Years Ended December 31, 2016 and 2017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| $ Increase/ |
| % Increase/ |
| ||||||
|
| 2016 |
| 2017 |
| (Decrease) |
| (Decrease) |
| ||||
|
| (In thousands) |
| ||||||||||
Statement of operations data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
| $ | 70 |
| $ | 70 |
| $ | — |
|
| — |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 27,492 |
|
| 25,867 |
|
| (1,625) |
|
| (6) | % |
General and administrative |
|
| 17,298 |
|
| 13,770 |
|
| (3,528) |
|
| (20) | % |
Acquired in-process research and development |
|
| — |
|
| 16,539 |
|
| 16,539 |
|
| 100 | % |
Total operating expenses |
|
| 44,790 |
|
| 56,176 |
|
| 11,386 |
|
| 25 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
| (44,720) |
|
| (56,106) |
|
| (11,386) |
|
| (25) | % |
Interest income, net |
|
| 861 |
|
| 1,029 |
|
| 168 |
|
| 20 | % |
Other income (expense), net |
|
| (29) |
|
| 13 |
|
| 42 |
|
| 145 | % |
Net loss |
| $ | (43,888) |
| $ | (55,064) |
| $ | (11,176) |
|
| (25) | % |
Revenue
Our revenue of $70,000 in the years ended December 31, 2016 and 2017 related to the amortization of the $1.0 million non-refundable upfront payment from NMP as well as annual minimum royalty payments received in 2016 and 2017.
Research and Development
The decrease in research and development expenses for the year ended December 31, 2017 compared to the year ended December 31, 2016 was primarily attributable to a decrease of $3.1 million in compensation expense as a result of employee terminations since December 31, 2016, including those resulting from the June 2017 restructuring activities, a decrease of $2.1 million in manufacturing expenses related to EC1169 and EC1456, and a $0.6 million decrease in EC1456 trial expenses unrelated to the trial termination. These decreases were partially offset by an increase of $1.9 million in EC1169 trial expenses, an increase of $0.9 million in expenses related to the EC1456 phase 1b trial termination, including trial and site close out expenses, an increase of $0.7 million in expenses related to pre-clinical work and general research, including costs related to EC2629, an increase of $0.5 million of expenses related to consulting and other fees related to the development of PSMA-617, and an increase of $0.2 million related to the CAR T-cell therapy program.
52
Included in research and development expenses were stock-based compensation charges of $4.2 million and $2.3 million for the years ended December 31, 2016 and 2017, respectively.
Research and development expenses included $1.1 million and $0.8 million for the years ended December 31, 2016 and 2017, respectively, for company-funded research at Purdue University, the primary employer of our Chief Science Officer.
General and Administrative
The decrease in general and administrative expense in the year ended December 31, 2017 compared to the year ended December 31, 2016 was primarily attributable to a $3.6 million decrease in compensation expense related to the resignation of our former Chief Executive Officer in June of 2016, and a decrease of $0.4 million in other compensation and benefit expense as a result of employee terminations since December 31, 2016, including those resulting from the June 2017 restructuring activities. These decreases were partially offset by a net increase of $0.5 million in other administrative fees, including legal and professional fees, primarily related to the License Agreement.
Included in general and administrative expenses were stock-based compensation charges of $5.0 million and $1.8 million for the years ended December 31, 2016 and 2017, respectively.
Acquired In-Process Research and Development
The increase in acquired IPR&D expenses in the year ended December 31, 2017 compared to the year ended December 31, 2016 related to the expenses incurred as a result of the License Agreement. We recorded $16.5 million of acquired IPR&D expenses in the year ended December 31, 2017, which included $12.0 million for an upfront payment to ABX, $3.8 million related to the fair value of common stock and warrant shares issued, and $0.7 million related to acquisition costs consisting primarily of legal and professional fees.
Interest Income, Net
The increase in interest income, net in 2017 compared to 2016 resulted from an increase of $501,000 due to an increase in the interest rate yield during the year ended December 31, 2017 as compared to the year ended December 31, 2016, partially offset by a decrease of $333,000 due to a decrease in the average short-term investment balances in the year ended December 31, 2017 as compared to the year ended December 31, 2016. There were no long-term investment balances at December 31, 2016 or 2017.
Other Income (Expense), Net
The change in other income (expense), net in the year ended December 31, 2017 as compared to the year ended December 31, 2016 resulted primarily from a decrease in charitable contributions in the year ended December 31, 2017 as compared to the year ended December 31, 2016.
53
Comparison of Years Ended December 31, 2015 and 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| $ Increase/ |
| % Increase/ | ||||||
|
| 2015 |
| 2016 |
| (Decrease) |
| (Decrease) | ||||
|
| (In thousands) |
| |||||||||
Statement of operations data: |
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
| $ | 70 |
| $ | 70 |
| $ | — |
| — |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 26,309 |
|
| 27,492 |
|
| 1,183 |
| 4 | % |
General and administrative |
|
| 15,734 |
|
| 17,298 |
|
| 1,564 |
| 10 | % |
Total operating expenses |
|
| 42,043 |
|
| 44,790 |
|
| 2,747 |
| 7 | % |
Loss from operations |
|
| (41,973) |
|
| (44,720) |
|
| (2,747) |
| (7) | % |
Interest income, net |
|
| 652 |
|
| 861 |
|
| 209 |
| 32 | % |
Other income (expense), net |
|
| 51 |
|
| (29) |
|
| (80) |
| (157) | % |
Net loss |
| $ | (41,270) |
| $ | (43,888) |
| $ | (2,618) |
| (6) | % |
Revenue
Our revenue of $70,000 in the years ended December 31, 2015 and 2016 related to the amortization of the $1.0 million non-refundable upfront payment from NMP as well as annual minimum royalty payments received in 2015 and 2016.
Research and Development
The increase in research and development expenses for the year ended December 31, 2016 compared to the year ended December 31, 2015 was primarily attributable to an increase of $2.7 million related to the phase 1 trial and the drug manufacturing expense for EC1169, and an increase of $0.3 million related to general research and other pre-clinical work, partially offset by a decrease of $1.5 million related to the TARGET trial which was completed in 2015, and a decrease of $0.3 million in employee compensation.
Included in research and development expenses were stock-based compensation charges of $4.4 million and $4.2 million for the years ended December 31, 2015 and 2016, respectively.
Research and development expenses included expense of $1.0 million and $1.1 million for the years ended December 31, 2015 and 2016, respectively, for company-funded research at Purdue University, the primary employer of our Chief Science Officer.
General and Administrative
The increase in general and administrative expense in the year ended December 31, 2016 compared to the year ended December 31, 2015 was primarily attributable to a $3.6 million increase in compensation expense related to the resignation of our former Chief Executive Officer in June of 2016, partially offset primarily by a decrease in legal and professional fees of $1.1 million and a decrease of $0.5 million of other employee compensation expense.
Included in general and administrative expenses were stock-based compensation charges of $2.5 million and $5.0 million for the years ended December 31, 2015 and 2016, respectively.
Interest Income, Net
The increase in interest income, net in 2016 compared to 2015 resulted from an increase of $344,000 in the interest rate yield during the year ended December 31, 2016 as compared to the year ended December 31, 2015, partially offset by a decrease of $135,000 in the average short-term and long-term investment balances in the year ended December 31, 2016 as compared to the year ended December 31, 2015.
54
Other Income (Expense), Net
The increase in other expense, net in the year ended December 31, 2016 as compared to the year ended December 31, 2015 resulted primarily from a favorable vendor lawsuit settlement in the year ended December 31, 2015, that did not recur in the year ended December 31, 2016, which was partially offset by a loss on foreign currency exchange in the year ended December 31, 2015 as well as a decrease in charitable contributions in the year ended December 31, 2016 as compared to the year ended December 31, 2015.
Liquidity and Capital Resources
We have funded our operations principally through sales of equity and debt securities, revenue from strategic collaborations, grants and loans. As of December 31, 2017, we had cash, cash equivalents and investments of $97.5 million. The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| |||||||
|
| 2015 |
| 2016 |
| 2017 |
| |||
|
|
| (in thousands) |
| ||||||
Net cash used in operating activities |
| $ | (32,218) |
| $ | (34,651) |
| $ | (33,659) |
|
Net cash provided by investing activities |
|
| 1,490 |
|
| 50,119 |
|
| 15,368 |
|
Net cash provided by financing activities |
|
| 627 |
|
| 329 |
|
| 5,622 |
|
Effect of exchange rate |
|
| (1) |
|
| — |
|
| — |
|
Net increase (decrease) in cash and cash equivalents |
| $ | (30,102) |
| $ | 15,797 |
| $ | (12,669) |
|
Operating Activities
The cash used in operating activities in the year ended December 31, 2015 primarily resulted from our net loss adjusted for non-cash items, primarily consisting of stock-based compensation, depreciation, accretion of bond premiums, and changes in operating assets and liabilities, primarily due to a decrease in the receivable from Merck relating to the former collaboration agreement and a decrease in clinical trial accruals due to closing out the PROCEED and TARGET trials. The cash used in operating activities for the year ended December 31, 2016 primarily resulted from our net loss adjusted for non-cash items, primarily consisting of stock-based compensation, depreciation, accretion of bond premiums, and changes in operating assets and liabilities. The cash used in operating activities for the year ended December 31, 2017 primarily resulted from our net loss adjusted for acquired IPR&D expense and non-cash items, primarily consisting of stock-based compensation, depreciation, accretion of bond discounts, loss on disposal of fixed assets, and changes in operating assets and liabilities.
Investing Activities
The cash provided by investing activities for the year ended December 31, 2015 was due to the net result of the sale, maturities and purchases of investments, which were partially offset by capital expenditures for equipment of $0.3 million. The cash provided by investing activities for the year ended December 31, 2016 was due to the net result of the sales, maturities and purchases of investments, which was partially offset by capital expenditures for equipment of $0.7 million. The cash provided by investing activities for the year ended December 31, 2017 was due to the net result of the sales, maturities and purchases of investments, which was partially offset by purchases of $12.8 million for acquired IPR&D and capital expenditures for equipment of $0.1 million. As our investments matured in the years ended December 31, 2016 and 2017, a portion of these funds were used to fund operations and excess funds were maintained in shorter-term investment accounts due to the competitive nature of the interest rates.
Financing Activities
The cash provided by financing activities in each of 2015 and 2016 primarily resulted from net proceeds from the exercise of stock options and purchases of stock under our employee stock purchase program, or the ESPP. The cash provided by financing activities in 2017 primarily resulted from net proceeds of $4.6 million from the exercise of a
55
warrant issued in connection with the License Agreement and net proceeds from the exercise of stock options and purchase of stock under the ESPP.
Operating Capital Requirements
We expect to continue to incur significant operating losses for the next several years as we pursue the advancement of our product candidates through the research, development, regulatory and, potentially, the commercialization processes.
As of December 31, 2017, our cash, cash equivalents and investments were $97.5 million. We believe that our current cash balance will be sufficient to fund our activities into the second half of 2019 through many important milestones, which include our focus on clinical development of 177Lu-PSMA-617 and our effort to generate proof-of-concept data on our CAR T-cell therapy program, but because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amounts of our working capital requirements. Our future funding requirements will depend on many factors, including but not limited to:
· | the number and characteristics of the product candidates we pursue; |
· | the scope, progress, results and costs of researching and developing our product candidates and conducting pre-clinical and clinical trials; |
· | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; |
· | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
· | the cost of manufacturing any product candidates we successfully commercialize; |
· | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; |
· | the timing, payments, receipts and amount of sales of, or royalties on, our product candidates, if any; and |
· | the costs we may incur and revenues we may generate in connection with in-licensing and out-licensing activities. |
We may seek to sell additional equity or debt securities or obtain new loans or credit facilities. We have an effective shelf Registration Statement on Form S-3 that, in addition to the registration of 6,000,000 shares of our common stock that may be resold by certain stockholders from time to time, also registers up to $150 million of equity and debt securities that may be offered and sold by us from time to time. The sale of additional equity securities by us may result in additional dilution to our stockholders. If we raise additional funds through the issuance of debt securities or convertible preferred stock, these securities may have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we were unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could materially harm our business.
56
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at December 31, 2017:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Less than |
| 1 to 3 |
| 3 to 5 |
| More than |
| ||||
|
| Total |
| 1 Year |
| Years |
| Years |
| 5 Years |
| |||||
|
| (In thousands) |
| |||||||||||||
Operating lease obligations |
| $ | 305 |
| $ | 305 |
| $ | — |
| $ | — |
| $ | — |
|
Total contractual cash obligations |
| $ | 305 |
| $ | 305 |
| $ | — |
| $ | — |
| $ | — |
|
In addition to the obligations shown in the above table, we have certain obligations under licensing agreements with third parties. In October 2007, we entered into an exclusive worldwide license with R&D Biopharmaceuticals to research, develop, and commercialize products containing conjugates of folate receptor targeting compounds and tubulysin compounds. In February 2011, this licensing agreement was assigned by R&D Biopharmaceuticals to Trientlgasse. Under this license agreement, we may be required to make $5.9 million in additional contingent payments upon the achievement of specific scientific, clinical and regulatory milestones, in addition to royalties upon commercial sales. We paid an upfront fee of $300,000 as research and development and pay $25,000 in annual maintenance fees unless we pay a milestone in a given year. We did not make any milestone payments in the years ended December 31, 2016 or 2017.
Pursuant to our exclusive license agreement with Purdue Research Foundation relating to folate, we are obligated to pay an annual minimum royalty of $12,500 until commercial sales commence, following which time the payment of low single digit royalty rates will commence. We do not anticipate incurring liabilities for vintafolide royalty payments. Pursuant to our exclusive license agreement with Purdue Research Foundation relating to PSMA, we are obligated to pay minimum annual royalty payments of $15,000 until commercial sales commence, following which time the payment of low single digit royalty rates will commence, along with a minimum annual royalty payment of $100,000. In addition, a milestone payment of $500,000 is payable upon approval of a new drug application in the U.S. and low single digit sales-based royalty rates are also payable when commercial sales commence. During 2016, we paid a penalty because we did not meet the last milestone related to the PSMA license agreement. No penalties were paid in the year ended December 31, 2017. There are no material obligations greater than five years.
In 2014, we entered into a Master License Agreement with Purdue Research Foundation. Under this license, we have the right to exclusively license patents and technology discovered under company funded research at Purdue University. If we decide to move forward with development of one or more of these technologies, we will be obligated to make an acceptance payment, and we will also be obligated to make contingent payments upon the achievement of specific scientific, clinical and regulatory milestones. Certain scientific, clinical and regulatory milestones, in addition to internal resource targets, must be met by us to avoid fees as consideration for waivers of potentially missed milestones. We are obligated to pay annual minimum payments until commercial sales commence, following which time the payment of low single digit royalty rates will commence, along with an annual royalty payment.
In September 2017, we entered into the License Agreement with ABX that grants us exclusive worldwide rights to develop and commercialize PSMA-617 agents. Under the terms of the License Agreement, we will be responsible for, and bear the future costs of, worldwide development and commercialization of PSMA-617. The License Agreement obligates us to pay ABX regulatory milestone payments of up to $25.0 million, sales milestone payments of up to $135.0 million, and tiered royalties, beginning in the mid-teens and not to exceed the mid-twenties, based on percentages of net sales.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under rules promulgated by the Securities and Exchange Commission.
57
Tax Loss Carryforwards
As of December 31, 2017, we had net operating loss carryforwards of approximately $247.5 million and $315.8 million for federal and state income taxes, respectively, that may be used to offset future taxable income. We also have research and development and orphan drug tax credit carryforwards of approximately $12.0 million and $7.5 million, respectively, to offset future federal income taxes and approximately $4.5 million in research and development tax credit carryforwards to offset future state income taxes. We experienced an ownership change in August 2011. As a result, the future use of our net operating loss carryforwards, after giving effect to net unrealized built-in gains, will be limited to approximately $218.7 million for 2017. Any available but unused amounts will become available for use in all successive years, subject to certain limitations. Utilization of these net operating loss carryforwards would require us to generate future taxable income prior to their expiration. Furthermore, the utilization of the net operating loss carryforwards could be limited beyond our generation of taxable income if a change in the underlying ownership of our common stock has occurred, resulting in a limitation on the amounts that could be utilized in any given period under Section 382 of the Code. At December 31, 2017, we recorded a full valuation allowance against our net deferred tax assets of approximately $95.9 million, as we believe it is more likely than not that the net deferred tax assets will not be fully realized.
On December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act, or the 2017 Tax Act, which, among other provisions, reduces the corporate income tax rate from 35% to 21% effective January 1, 2018. During the fourth quarter of 2017, we revalued our net deferred tax assets using the newly enacted rate resulting in a reduction of our net deferred tax assets of $33.1 million. We also reduced our valuation allowance by $33.1 million, resulting in no income tax expense being recognized as a result of the revaluation.
Our estimate of the impact of the 2017 Tax Act is based upon our analysis and interpretations of currently available information. Uncertainties remain regarding the impact of the 2017 Tax Act due to future regulatory and rulemaking processes, prospects of additional corrective or supplemental legislation, and potential trade or other litigation. These uncertainties, along with our completion of the calculations and potential changes in our initial assumptions as new information becomes available, could cause the actual charge to ultimately differ from the provisional amount recorded in 2017 related to the enactment of the 2017 Tax Act.
The impact of the 2017 Tax Act does not have an effect on our liquidity, financial condition or results of operations, as we are in a net loss position, are currently not paying federal income taxes and our deferred tax assets have a full valuation allowance against them.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2016 and December 31, 2017 we had cash, cash equivalents and investments of $138.2 million and $97.5 million, respectively. The investments consisted primarily of U.S. Treasuries, corporate debt securities and cash equivalents. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term marketable securities. Our short-term investments are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10 percent change in interest rates would not have a material effect on the fair market value of our portfolio. We have the ability to hold our short-term investments until maturity, and therefore we do not expect that our results of operations or cash flows would be adversely affected by any change in market interest rates on our investments. We carry our investments based on publicly available information. We do not currently have any investment securities for which a market is not readily available or active.
We do not believe that any credit risk is likely to have a material impact on the value of our assets and liabilities.
58
Item 8.Financial Statements and Supplementary Data
ENDOCYTE, INC.
INDEX TO FINANCIAL STATEMENTS
|
| Page |
| 60 | |
| 61 | |
Consolidated Balance Sheets as of December 31, 2016 and 2017 |
| 63 |
| 64 | |
| 65 | |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2015, 2016 and 2017 |
| 66 |
| 67 |
59
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Endocyte, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Endocyte, Inc. (the Company) as of December 31, 2017 and 2016, the related consolidated statements of operations and comprehensive income (loss), stockholders' equity (deficit), and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 27, 2018 expressed an unqualified opinion thereon.
Adoption of ASU No. 2016-09
As discussed in Note 3 to the consolidated financial statements, the Company changed its method of accounting for estimated forfeitures and the income tax effects of share-based payment awards in 2017 due to the adoption of ASU No. 2016-09, Improvements to Employee Share-Based Payment Accounting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.

We have served as the Company’s auditor since 2001.
Indianapolis, Indiana
February 27, 2018
60
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Endocyte, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Endocyte, Inc.’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Endocyte, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2017 and 2016, the related consolidated statements of operations and comprehensive income (loss), stockholders' equity (deficit), and cash flows for each of the three years in the period ended December 31, 2017, and the related notes and our report dated February 27, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
61
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.

Indianapolis, Indiana
February 27, 2018
62
ENDOCYTE, INC.
|
|
|
|
|
|
|
|
|
| December 31, |
| ||||
|
| 2016 |
| 2017 |
| ||
Assets |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents |
| $ | 31,228,192 |
| $ | 18,559,130 |
|
Short-term investments |
|
| 106,979,224 |
|
| 78,912,297 |
|
Receivables |
|
| 55,074 |
|
| 273,044 |
|
Prepaid expenses |
|
| 1,737,308 |
|
| 751,255 |
|
Other assets |
|
| 255,912 |
|
| 77,077 |
|
Total current assets |
|
| 140,255,710 |
|
| 98,572,803 |
|
Property and equipment, net |
|
| 3,205,077 |
|
| 2,182,399 |
|
Other noncurrent assets |
|
| 33,567 |
|
| 7,067 |
|
Total assets |
| $ | 143,494,354 |
| $ | 100,762,269 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
Accounts payable |
| $ | 1,381,545 |
| $ | 376,394 |
|
Accrued wages and benefits |
|
| 2,705,475 |
|
| 2,533,133 |
|
Accrued clinical trial expenses |
|
| 861,293 |
|
| 689,985 |
|
Accrued expenses and other liabilities |
|
| 613,861 |
|
| 946,668 |
|
Total current liabilities |
|
| 5,562,174 |
|
| 4,546,180 |
|
Other liabilities, net of current portion |
|
| 2,873 |
|
| — |
|
Deferred revenue, net of current portion |
|
| 781,944 |
|
| 731,944 |
|
Total liabilities |
|
| 6,346,991 |
|
| 5,278,124 |
|
Stockholders’ equity: |
|
|
|
|
|
|
|
Common stock: $0.001 par value, 100,000,000 shares authorized; 42,377,522 and 48,203,529 shares issued and outstanding at December 31, 2016 and December 31, 2017 |
|
| 42,378 |
|
| 48,204 |
|
Additional paid-in capital |
|
| 390,768,742 |
|
| 404,454,909 |
|
Accumulated other comprehensive loss |
|
| (41,196) |
|
| (64,433) |
|
Retained deficit |
|
| (253,622,561) |
|
| (308,954,535) |
|
Total stockholders’ equity |
|
| 137,147,363 |
|
| 95,484,145 |
|
Total liabilities and stockholders’ equity |
| $ | 143,494,354 |
| $ | 100,762,269 |
|
See accompanying notes.
63
ENDOCYTE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| |||||||
|
| 2015 |
| 2016 |
| 2017 |
| |||
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
| $ | 70,000 |
| $ | 70,000 |
| $ | 70,000 |
|
Total revenue |
|
| 70,000 |
|
| 70,000 |
|
| 70,000 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 26,309,475 |
|
| 27,492,185 |
|
| 25,866,845 |
|
General and administrative |
|
| 15,733,462 |
|
| 17,298,139 |
|
| 13,769,853 |
|
Acquired in-process research and development |
|
| — |
|
| — |
|
| 16,539,347 |
|
Total operating expenses |
|
| 42,042,937 |
|
| 44,790,324 |
|
| 56,176,045 |
|
Loss from operations |
|
| (41,972,937) |
|
| (44,720,324) |
|
| (56,106,045) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
| 651,543 |
|
| 861,212 |
|
| 1,029,497 |
|
Other income (expense), net |
|
| 51,645 |
|
| (28,461) |
|
| 12,398 |
|
Net loss |
|
| (41,269,749) |
|
| (43,887,573) |
|
| (55,064,150) |
|
Net loss per share – basic and diluted |
| $ | (0.98) |
| $ | (1.04) |
| $ | (1.25) |
|
Items included in other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
Unrealized gain on foreign currency translation |
|
| 50,592 |
|
| — |
|
| — |
|
Unrealized gain (loss) and amounts reclassified to net loss on available-for-sale securities |
|
| 13,937 |
|
| 38,203 |
|
| (23,237) |
|
Other comprehensive income (loss) |
|
| 64,529 |
|
| 38,203 |
|
| (23,237) |
|
Comprehensive loss |
| $ | (41,205,220) |
| $ | (43,849,370) |
| $ | (55,087,387) |
|
Weighted-average number of common shares used in net loss per share calculation – basic and diluted |
|
| 41,939,504 |
|
| 42,210,643 |
|
| 43,900,257 |
|
See accompanying notes.
64
ENDOCYTE, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accumulated |
|
|
|
|
|
|
| ||
|
|
|
|
|
|
| Additional |
| Other |
|
|
|
|
|
| |||
|
| Common Stock |
| Paid-In |
| Comprehensive |
| Retained |
|
|
|
| ||||||
|
| Shares |
| Amount |
| Capital |
| Income (Loss) |
| Deficit |
| Total |
| |||||
Balances December 31, 2014 |
| 41,784,692 |
| $ | 41,785 |
| $ | 373,571,500 |
| $ | (143,928) |
| $ | (168,465,239) |
| $ | 205,004,118 |
|
Exercise of stock options |
| 146,046 |
|
| 146 |
|
| 379,144 |
|
| — |
|
| — |
|
| 379,290 |
|
Stock-based compensation |
| 29,190 |
|
| 29 |
|
| 6,862,917 |
|
| — |
|
| — |
|
| 6,862,946 |
|
Employee stock purchase plan |
| 74,805 |
|
| 75 |
|
| 304,928 |
|
| — |
|
| — |
|
| 305,003 |
|
Net loss |
| — |
|
| — |
|
| — |
|
| — |
|
| (41,269,749) |
|
| (41,269,749) |
|
Net realized loss on foreign exchange translation |
| — |
|
| — |
|
| — |
|
| 50,592 |
|
| — |
|
| 50,592 |
|
Unrealized gain on securities |
| — |
|
| — |
|
| — |
|
| 13,937 |
|
| — |
|
| 13,937 |
|
Balances December 31, 2015 |
| 42,034,733 |
| $ | 42,035 |
| $ | 381,118,489 |
| $ | (79,399) |
| $ | (209,734,988) |
| $ | 171,346,137 |
|
Exercise of stock options |
| 129,638 |
|
| 130 |
|
| 260,952 |
|
| — |
|
| — |
|
| 261,082 |
|
Stock-based compensation |
| 126,580 |
|
| 127 |
|
| 9,157,528 |
|
| — |
|
| — |
|
| 9,157,655 |
|
Employee stock purchase plan |
| 86,571 |
|
| 86 |
|
| 231,773 |
|
| — |
|
| — |
|
| 231,859 |
|
Net loss |
| — |
|
| — |
|
| — |
|
| — |
|
| (43,887,573) |
|
| (43,887,573) |
|
Unrealized gain on securities |
| — |
|
| — |
|
| — |
|
| 38,203 |
|
| — |
|
| 38,203 |
|
Balances December 31, 2016 |
| 42,377,522 |
| $ | 42,378 |
| $ | 390,768,742 |
| $ | (41,196) |
| $ | (253,622,561) |
| $ | 137,147,363 |
|
Reclassification of impact of ASU 2016-09 (See Note 3) |
| — |
|
| — |
|
| 267,824 |
|
| — |
|
| (267,824) |
|
| — |
|
Balances at January 1, 2017 |
| 42,377,522 |
| $ | 42,378 |
| $ | 391,036,566 |
| $ | (41,196) |
| $ | (253,890,385) |
| $ | 137,147,363 |
|
Exercise of stock options |
| 378,030 |
|
| 378 |
|
| 1,087,513 |
|
| — |
|
| — |
|
| 1,087,891 |
|
Issuance of common stock in connection with development and license agreement |
| 2,000,000 |
|
| 2,000 |
|
| 2,818,000 |
|
| — |
|
| — |
|
| 2,820,000 |
|
Issuance of common stock in connection with exercise of warrant to purchase common stock |
| 3,278,000 |
|
| 3,278 |
|
| 5,364,871 |
|
| — |
|
| — |
|
| 5,368,149 |
|
Stock-based compensation |
| 114,365 |
|
| 114 |
|
| 4,075,903 |
|
| — |
|
| — |
|
| 4,076,017 |
|
Employee stock purchase plan |
| 55,612 |
|
| 56 |
|
| 72,056 |
|
| — |
|
| — |
|
| 72,112 |
|
Net loss |
| — |
|
| — |
|
| — |
|
| — |
|
| (55,064,150) |
|
| (55,064,150) |
|
Unrealized loss on securities |
| — |
|
| — |
|
| — |
|
| (23,237) |
|
| — |
|
| (23,237) |
|
Balances December 31, 2017 |
| 48,203,529 |
| $ | 48,204 |
| $ | 404,454,909 |
| $ | (64,433) |
| $ | (308,954,535) |
| $ | 95,484,145 |
|
See accompanying notes.
65
ENDOCYTE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| |||||||
|
| 2015 |
| 2016 |
| 2017 |
| |||
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
|
|
|
|
|
|
|
|
|
Net loss |
| $ | (41,269,749) |
| $ | (43,887,573) |
| $ | (55,064,150) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
| 886,710 |
|
| 919,809 |
|
| 922,670 |
|
Stock-based compensation |
|
| 6,920,220 |
|
| 9,322,127 |
|
| 4,170,645 |
|
Acquired in-process research and development |
|
| — |
|
| — |
|
| 16,539,347 |
|
Loss on disposal of property and equipment |
|
| 10,639 |
|
| — |
|
| 106,217 |
|
Accretion of bond premium (discount) |
|
| 1,310,238 |
|
| 395,116 |
|
| (161,399) |
|
Net realized foreign currency translation loss |
|
| 51,944 |
|
| — |
|
| — |
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
Receivables |
|
| 856,372 |
|
| 191,555 |
|
| 1,433 |
|
Prepaid expenses and other assets |
|
| (96,582) |
|
| (517,318) |
|
| 915,499 |
|
Accounts payable |
|
| (60,713) |
|
| (252,605) |
|
| (1,025,199) |
|
Accrued wages, benefits and other liabilities |
|
| (777,204) |
|
| (771,866) |
|
| (13,717) |
|
Deferred revenue |
|
| (50,000) |
|
| (50,000) |
|
| (50,000) |
|
Net cash used in operating activities |
|
| (32,218,125) |
|
| (34,650,755) |
|
| (33,658,654) |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
| (341,616) |
|
| (713,839) |
|
| (82,455) |
|
Proceeds from disposal of property and equipment |
|
| — |
|
| — |
|
| 15,727 |
|
Purchases of investments |
|
| (86,281,946) |
|
| (139,932,358) |
|
| (106,724,911) |
|
Purchase of acquired in-process research and development |
|
| — |
|
| — |
|
| (12,770,565) |
|
Proceeds from sale and maturities of investments |
|
| 88,114,199 |
|
| 190,765,053 |
|
| 134,930,000 |
|
Net cash provided by investing activities |
|
| 1,490,637 |
|
| 50,118,856 |
|
| 15,367,796 |
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
Stock repurchase |
|
| (57,274) |
|
| (164,472) |
|
| (94,628) |
|
Proceeds from exercise of warrant to purchase common stock |
|
| — |
|
| — |
|
| 4,556,421 |
|
Proceeds from the exercise of stock options |
|
| 379,290 |
|
| 261,082 |
|
| 1,087,891 |
|
Proceeds from stock purchases under employee stock purchase plan |
|
| 305,003 |
|
| 231,859 |
|
| 72,112 |
|
Net cash provided by financing activities |
|
| 627,019 |
|
| 328,469 |
|
| 5,621,796 |
|
Effect of exchange rate |
|
| (1,352) |
|
| — |
|
| — |
|
Net increase (decrease) in cash and cash equivalents |
|
| (30,101,821) |
|
| 15,796,570 |
|
| (12,669,062) |
|
Cash and cash equivalents at beginning of period |
|
| 45,533,443 |
|
| 15,431,622 |
|
| 31,228,192 |
|
Cash and cash equivalents at end of period |
| $ | 15,431,622 |
| $ | 31,228,192 |
| $ | 18,559,130 |
|
See accompanying notes.
66
1. Nature of Business and Organization
Endocyte, Inc. (the “Company”) is a biopharmaceutical company and leader in developing targeted therapies for the treatment of cancer. The Company uses drug conjugation technology to create novel therapeutics and companion imaging agents for personalized targeted therapies. The agents actively target receptors that are over-expressed on diseased cells relative to healthy cells, such as prostate specific membrane antigen (“PSMA”) in prostate cancer. This targeted approach is designed to safely enable the delivery of highly potent drug payloads. The companion imaging agents are designed to identify patients whose disease over-expresses the target of the therapy and who are therefore more likely to benefit from treatment.
In June 2017, the Company discontinued clinical development of EC1456 and stopped enrollment in its EC1456 phase 1b trial as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. In December 2017, the Company stopped enrollment in its EC1456 ovarian cancer surgical trial. In addition, in June 2017, the Company narrowed the focus of its phase 1 clinical development of EC1169 to include only the cohort of taxane-exposed metastatic castration-resistant prostate cancer (“mCRPC”) patients, which completed enrollment in October 2017. The Company does not intend to invest further resources in the development of EC1169 beyond the completion of the phase 1 taxane-exposed cohort. In addition, in June 2017, the Company reduced its workforce by approximately 40% to align resources to focus on the Company’s highest value opportunities while maintaining key capabilities.
In September 2017, the Company entered into a Development and License Agreement (the “License Agreement”) with ABX advanced biochemical compounds – Biomedizinische Forschungsreagenzien GmbH (“ABX”), pursuant to which the Company acquired exclusive worldwide rights to develop and commercialize PSMA-617 agents, including the product candidate known as 177Lu-PSMA-617, a radioligand therapeutic (“RLT”). Following a successful End of Phase 2 meeting with the U.S. Food and Drug Administration (“FDA”), the Company finalized the phase 3 VISION trial design and registration plan for 177Lu-PSMA-617. The trial will include two interim assessments of efficacy, which could potentially lead to an early approval for 177Lu-PSMA-617. The Company intends to initiate, in the second quarter of 2018, the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 in up to 750 patients with progressive PSMA-positive mCRPC who have received at least one novel androgen axis drug (NAAD) and at least one taxane regimen. On October 2, 2017, the Company announced its plan to primarily focus its resources on the development of 177Lu-PSMA‑617 and on a targeted effort to generate proof-of-concept data for the adaptor-controlled CAR T-cell program, and to explore out-licensing opportunities for all other development programs, such as EC2629.
The Company had two wholly-owned subsidiaries, Endocyte Europe B.V. and Endocyte Europe GmbH, which were formed to assist with the administration of applications with the European Commission (“EC”) and commercial pre-launch activities in Europe. The applications were withdrawn in May 2014 and the commercial pre-launch activities in Europe ceased. The Company dissolved Endocyte Europe GmbH in the fourth quarter of 2015 and dissolved Endocyte Europe B.V. in the first quarter of 2016.
2. Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements include the accounts of Endocyte, Inc. and its subsidiaries and all intercompany amounts have been eliminated as of December 31, 2015. The accompanying financial statements for the years ended December 31, 2016 and 2017 include only the accounts of Endocyte, Inc. as the Company dissolved Endocyte Europe GmbH and Endocyte Europe B.V. in the fourth quarter of 2015 and the first quarter of 2016, respectively. There were no intercompany transactions in the years ended December 31, 2016 or 2017 and no intercompany balances as of December 31, 2016 or 2017. The consolidated financial statements are prepared in conformity with U.S. generally accepted accounting principles (“GAAP”) for annual financial information to Form 10-
67
K. Subsequent events have been evaluated through the date of issuance, which is the same as the date this Form 10-K is filed with the Securities and Exchange Commission (the “SEC”).
Use of Estimates
The preparation of financial statements in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual amounts may differ from those estimates.
Cash and Cash Equivalents
The Company considers cash and all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. Cash equivalents consist primarily of money market instruments, U.S. government treasury obligations, corporate debt securities and repurchase agreements that are maintained by an investment manager.
Investments
Investments consist primarily of investments in U.S. Treasuries and corporate debt securities, which could also include commercial paper, that are maintained by an investment manager. Management determines the appropriate classification of marketable securities at the time of purchase and reevaluates such classification as of each balance sheet date. Available-for-sale securities are carried at fair value, with the unrealized gains and losses reported in other comprehensive income. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in other income. The Company considers and accounts for other-than-temporary impairments according to the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 320, Investments — Debt and Equity Securities (“ASC 320”). The cost of securities sold is based on the specific-identification method. Discounts and premiums on debt securities are amortized to interest income and expensed over the term of the security.
Property and Equipment
Property and equipment are stated at cost and are being depreciated using the straight-line method over estimated useful lives, which range from three to seven years.
Licenses and Patents
Licenses and patent costs are expensed as incurred as the Company does not believe there is an alternate future use for the costs. Licenses are classified as research and development expenses and patents are classified as general and administrative expenses in the consolidated statements of operations.
Long-Lived Assets
The Company reviews long-lived assets, including property and equipment, for impairment when events or changes in business conditions indicate that their full carrying value may not be fully recoverable.
Leases
The Company evaluates all leases to determine whether they should be accounted for as operating or capital leases.
68
Revenue Recognition
The Company recognizes revenues from license and collaboration agreements when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the fee is fixed or determinable, and there is reasonable assurance that the related amounts are collectible in accordance with ASC Topic 605, Revenue Recognition (“ASC 605”). The Company’s license and collaboration agreements may contain multiple elements, including grants of licenses to intellectual property rights, agreement to provide research and development services and other deliverables. The deliverables under such arrangements are evaluated under ASC Subtopic 605-25, Multiple-Element Arrangements (“ASC 605-25”). Under ASC 605-25, each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration that is fixed or determinable, excluding contingent milestone payments and royalties, is then allocated to each separate unit of accounting based on the relative selling price of each deliverable. In general, the consideration allocated to each unit of accounting is recognized as the related goods or services are delivered, limited to the consideration that is not contingent upon future deliverables.
Upfront payments for licensing the Company’s intellectual property are evaluated to determine if the licensee can obtain stand-alone value from the license separate from the value of the research and development services and other deliverables in the arrangement to be provided by the Company. If at the inception of an arrangement the Company determines that the license does not have stand-alone value separate from the research and development services or other deliverables, the license, services and other deliverables are combined as one unit of account and upfront payments are recorded as deferred revenue on the balance sheet and are recognized in a manner consistent with the final deliverable. Subsequent to the inception of an arrangement, the Company evaluates the remaining deliverables for separation as items in the arrangement are delivered. When stand-alone value is identified, the related consideration is recorded as revenue in the period in which the license or other intellectual property rights are delivered.
In those circumstances where research and development services or other deliverables are combined with the license, and multiple services are being performed such that a common output measure to determine a pattern of performance cannot be discerned, the Company recognizes amounts received on a straight line basis over the performance period. Such amounts are recorded as collaboration revenue. Any subsequent reimbursement payments, which are contingent upon the Company’s future research and development expenditures, will be recorded as collaboration revenue and will be recognized on a straight-line basis over the performance period using the cumulative catch up method. The costs associated with these activities are reflected as a component of research and development expense in the statements of operations in the period incurred. In the event of an early termination of a collaboration agreement, any deferred revenue is recognized in the period in which all obligations of the Company under the agreement have been fulfilled.
Milestone payments under collaborative arrangements are triggered either by the results of the Company’s research and development efforts, achievement of regulatory goals or by specified sales results by a third-party collaborator. Milestones related to the Company’s development-based activities may include initiation of various phases of clinical trials and applications and acceptance for product approvals by regulatory agencies. Due to the uncertainty involved in meeting these development-based milestones, the determination is made at the inception of the collaboration agreement whether the development-based milestones are considered to be substantive (i.e. not just achieved through passage of time). In addition, the amounts of the payments assigned thereto are considered to be commensurate with the enhancement of the value of the delivered intellectual property as a result of the Company’s performance. Because the Company’s involvement is necessary to the achievement of development-based milestones, the Company would account for development-based milestones as revenue upon achievement of the substantive milestone events. Milestones related to sales-based activities may be triggered upon events such as first commercial sale of a product or when sales first achieve a defined level. Since these sales-based milestones would be achieved after the completion of the Company’s development activities, the Company would account for the sales-based milestones in the same manner as royalties, with revenue recognized upon achievement of the milestone. Royalties based on reported sales of licensed products will be recognized based on contract terms when reported sales are reliably measurable and collectability is reasonably assured.
69
To date, none of the Company’s products have been approved and therefore the Company has not earned any royalty revenue from product sales. In territories where the Company and a collaborator may share profit, the revenue would be recorded in the period earned.
The Company often is required to make estimates regarding drug development and commercialization timelines for compounds being developed pursuant to a collaboration agreement. Because the drug development process is lengthy and the Company’s collaboration agreements typically cover activities over several years, this approach often results in the deferral of significant amounts of revenue into future periods. In addition, because of the many risks and uncertainties associated with the development of drug candidates, the Company’s estimates regarding the period of performance may change in the future. Any change in the Company’s estimates or a termination of the arrangement could result in substantial changes to the period over which the revenues are recognized.
Research and Development Expenses
Research and development expenses represent costs associated with the ongoing development of novel therapeutics and companion imaging agents for personalized targeted therapies and include salaries and employee benefits, supplies, facility costs related to research activities, and expenses for clinical trials. The Company records accruals for clinical trial expenses based on the estimated amount of work completed. The Company monitors patient enrollment levels and related activities to the extent possible through internal reviews, correspondence, and discussions with research organizations. In the event that a clinical trial is terminated early, the Company records, in the period of termination, an accrual for the estimated remaining costs to complete and close out the trial pursuant to ASC Topic 420, Exit or Disposal Cost Obligations, as a terminated trial does not provide any future economic benefit to the Company. See Note 15 – Restructuring Costs of the Notes to Consolidated Financial Statements contained herein for costs incurred during the years ended December 31, 2015, 2016, and 2017 related to the Company’s restructuring activities.
Upfront payments made in connection with business collaborations and research and development arrangements are evaluated under ASC Subtopic 730-20, Research and Development Arrangements. Amounts related to future research and development are capitalized as prepaid research and development and are expensed over the service period based upon the level of services provided. As of December 31, 2017, the Company had approximately $0.1 million of capitalized research and development costs included in prepaid expenses.
Acquired In-Process Research and Development Expense
The Company has acquired and may continue to acquire the rights to develop and commercialize new drug candidates. In accordance with ASC Subtopic 730-25, Accounting for Research and Development Costs, the upfront payments to acquire a new drug compound, as well as future milestone payments when paid or payable, are immediately expensed as acquired in-process research and development (“IPR&D”) in transactions other than a business combination provided that the drug has not achieved regulatory approval for marketing and, absent obtaining such approval, has no alternative future use. Upon obtaining regulatory approval for marketing, any related milestone payments may be capitalized and amortized over the life of the asset.
Stock-Based Compensation
The Company accounts for its stock-based compensation pursuant to ASC Topic 718, Compensation — Stock Compensation (“ASC 718”), which requires the recognition of the fair value of stock-based compensation in net income. Stock-based compensation consists of stock options, which are granted at exercise prices at or above the fair market value of the Company’s common stock on the dates of grant, service-based restricted stock units (“RSUs”), performance-based RSUs (“PRSUs”), and shares available for purchase under the Company’s 2010 Employee Stock Purchase Plan (“ESPP”). For PRSUs issued by the Company, stock-based compensation expense would be recognized if and when the Company determines that it is probable that the performance conditions will be achieved. For RSUs and stock options issued by the Company, stock-based compensation expense is recognized ratably over the service period. The Company
70
recognizes compensation cost based on the grant-date fair value estimated in accordance with the provisions of ASC 718.
Net Loss Per Share
The Company calculates basic net loss per share based on the weighted-average number of outstanding common shares. The Company calculates diluted net loss per share based on the weighted-average number of outstanding common shares plus the effect of dilutive securities.
Income Taxes
The Company accounts for income taxes under the liability method in accordance with the provision of ASC Topic 740, Income Taxes (“ASC 740”). ASC 740 requires recognition of deferred taxes to provide for temporary differences between financial reporting and tax basis of assets and liabilities. Deferred taxes are measured using enacted tax rates expected to be in effect in a year in which the basis difference is expected to reverse. The Company continues to record a valuation allowance for the full amount of deferred tax assets, which would otherwise be recorded for tax benefits relating to operating loss and tax credit carryforwards, as realization of such deferred tax assets cannot be determined to be more likely than not.
The Company accounts for uncertain income tax positions recognized in the financial statements in accordance with Accounting Standards Update (“ASU”) No. 2009-06. This guidance prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return and provides guidance on derecognition of tax benefits, classification on the balance sheet, interest and penalties, accounting in interim periods, disclosure, and transition.
Segment Information
Operating segments are defined as components of an enterprise engaging in business activities for which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company had performed clinical trials globally and established a subsidiary in The Netherlands to assist in the administration of filing applications in Europe and a subsidiary in Switzerland for commercial pre-launch activities in Europe. The applications filed in Europe were withdrawn in May 2014 and the pre-launch activities in Europe ceased. The Company dissolved Endocyte Europe GmbH in the fourth quarter of 2015 and dissolved Endocyte Europe B.V. in the first quarter of 2016. All long-lived assets are held in the U.S. The Company views its operations and manages its business in one operating segment.
3. New Accounting Pronouncements
Recently Issued Accounting Standards
In January 2017, the FASB issued ASU 2017-01, Clarifying the Definition of a Business, an update to ASC Topic 805, Business Combinations. This guidance is intended to clarify the definition of a business as it relates to the evaluation of whether a set of transferred assets and activities are accounted for as a business combination or as an asset acquisition. This update was effective for the Company in the year ended December 31, 2017, as the Company elected early adoption. As a result, the Company considered ASU 2017-01 when evaluating the License Agreement. In the year ended December 31, 2017, the Company determined that the set of transferred assets and activities included in the License Agreement did not meet the definition of a business and accounted for the License Agreement as an asset acquisition. The adoption of this guidance did not have a material impact on the Company’s financial statements.
In March 2016, the FASB issued ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, an update to ASC Topic 718, Stock Compensation. This guidance involves improving several aspects of the accounting for share-based payment transactions, including classification of awards as either equity or liabilities, classification on the
71
statement of cash flows, the method of accounting for forfeitures and requiring entities to recognize all income tax effects of awards in the income statement when the awards vest or are settled. This update was effective for the Company for interim and annual reporting periods beginning January 1, 2017. In the year ended December 31, 2017, the Company adopted this guidance using the modified retrospective method. As a result, the Company has elected to account for forfeitures as they occur and no longer estimates the number of awards expected to be forfeited. The cumulative effect related to the change in accounting for forfeitures was a $0.3 million increase to the opening balance of retained deficit at January 1, 2017. Additionally, as a result of the adoption, the Company recognized the excess tax benefits of awards that have vested or settled that had previously not been recognized as the related tax deduction had increased the Company’s net operating loss carryforward. The Company determined, consistent with its accounting for existing net operating losses, that a full valuation allowance was required for the excess tax benefits. As such, the Company recognized an increase in its net operating loss carryforward deferred tax asset of $0.8 million and the valuation allowance against the net operating loss carryforward was also increased by $0.8 million, which resulted in no impact to the financial statements. The adoption of this guidance did not have a material impact on the Company’s financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, an update to ASC Topic 842, Leases. This guidance requires lessees to recognize leases as assets and liabilities on their balance sheets but recognize expenses on their income statements in a manner similar to the current accounting guidance. For lessors, the guidance also modifies the classification criteria and the accounting for sales-type and direct finance leases. This update is effective for the Company for interim and annual reporting periods beginning January 1, 2019 unless it elects early adoption. The Company is currently evaluating the impact, if any, the adoption of this guidance will have on its consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606), to clarify the principles used to recognize revenue for all entities. Under ASU 2014-09, an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In order to do so, an entity would follow the five-step process for in-scope transactions: 1) identify the contract with a customer, 2) identify the separate performance obligations in the contract, 3) determine the transaction price, 4) allocate the transaction price to the separate performance obligations in the contract, and 5) recognize revenue when (or as) the entity satisfies a performance obligation. In August 2015, the FASB issued ASU 2015-14, which defers the effective date of ASU 2014-09 by one year. Therefore, ASU 2014-09 will become effective for the Company for interim and annual reporting periods beginning after December 15, 2017. Early adoption is permitted, but not any earlier than the original effective date of December 15, 2016. An entity can apply the new revenue standard retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. In April 2016, the FASB issued ASU 2016-10, an update to Topic 606, which clarifies how entities should identify performance obligations and evaluate licensing. In May 2016, the FASB issued ASU 2016-12, an update to Topic 606, which clarifies guidance on transition, collectability, noncash consideration and the presentation of sales and other similar taxes. In December 2016, the FASB issued ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers, which affects narrow aspects of the guidance issued in ASU 2014-09. The Company currently has a limited number of contracts with customers and only one revenue stream, which relates to collaboration and licensing arrangements, and which represents all of the revenue earned in the year ended December 31, 2017. The Company will adopt the new standard effective January 1, 2018. The Company has identified all of its contracts and has completed a preliminary review of the estimated impact of the adoption of this ASU, which is not expected to have a material impact on the Company’s consolidated financial statements.
4. Net Loss per Share
Basic net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration for common stock equivalents. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-
72
average number of common share equivalents outstanding for the period determined using the treasury-stock method and the if-converted method. For purposes of this calculation, stock options, warrants, PRSUs, RSUs and shares to be purchased under the ESPP are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
Common stock equivalents
As of December 31, 2015, 2016, and 2017 the following number of potential common stock equivalents were outstanding:
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| ||||
|
| 2015 |
| 2016 |
| 2017 |
|
Outstanding common stock options |
| 5,686,815 |
| 6,447,594 |
| 5,878,660 |
|
Outstanding warrants |
| 34,647 |
| 34,647 |
| 722,000 |
|
Outstanding PRSUs |
| 213,758 |
| — |
| — |
|
Outstanding RSUs |
| 351,414 |
| 394,132 |
| 1,383,770 |
|
Shares to be purchased under the ESPP |
| 4,236 |
| 4,394 |
| 2,634 |
|
Total |
| 6,290,870 |
| 6,880,767 |
| 7,987,064 |
|
These common stock equivalents were excluded from the determination of diluted net loss per share due to their anti-dilutive effect on earnings. The increase in warrants was due to warrants issued in connection with the License Agreement. For additional information on the outstanding warrants, see Note 8 – Warrants of the Notes to Consolidated Financial Statements contained herein.
5. Other Comprehensive Income (Loss)
The accumulated balances related to each component of other comprehensive income (loss) were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign |
|
|
|
| Accumulated |
| ||
|
| Currency |
| Unrealized Net |
| Other |
| |||
|
| Translation |
| Gains (Losses) |
| Comprehensive |
| |||
|
| Gains (Losses) |
| on Securities |
| Gains (Losses) |
| |||
Balance at December 31, 2014 |
| $ | (50,592) |
| $ | (93,336) |
| $ | (143,928) |
|
Unrealized gain (loss) |
|
| (1,352) |
|
| 18,841 |
|
| 17,489 |
|
Net amount reclassified to net loss |
|
| 51,944 |
|
| (4,904) |
|
| 47,040 |
|
Other comprehensive income |
|
| 50,592 |
|
| 13,937 |
|
| 64,529 |
|
Balance at December 31, 2015 |
| $ | — |
| $ | (79,399) |
| $ | (79,399) |
|
Unrealized gain |
|
| — |
|
| 36,908 |
|
| 36,908 |
|
Net amount reclassified to net loss |
|
| — |
|
| 1,295 |
|
| 1,295 |
|
Other comprehensive income |
|
| — |
|
| 38,203 |
|
| 38,203 |
|
Balance at December 31, 2016 |
| $ | — |
| $ | (41,196) |
| $ | (41,196) |
|
Unrealized loss |
|
| — |
|
| (23,237) |
|
| (23,237) |
|
Other comprehensive loss |
|
| — |
|
| (23,237) |
|
| (23,237) |
|
Balance at December 31, 2017 |
| $ | — |
| $ | (64,433) |
| $ | (64,433) |
|
The assets and liabilities of foreign operations were translated into U.S. dollars using the current exchange rate. For those operations, changes in exchange rates generally did not affect cash flows, which resulted in translation adjustments made in stockholders’ equity rather than to net loss. The accumulated balance of translation adjustments for Endocyte Europe B.V. were reclassified out of accumulated other comprehensive income (loss) and recognized as foreign currency translation losses reported in other income (expense) as the Company determined that the liquidation of Endocyte Europe
73
B.V. was substantially complete as of December 31, 2015. There were no transactions from foreign operations in the years ended December 31, 2016 or 2017.
Reclassifications Out of Accumulated Other Comprehensive Income (Loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amount Reclassified from Accumulated |
|
|
| |||||||
|
| Other Comprehensive Income (Loss) |
| Affected Line Item in the |
| |||||||
|
| Year Ended |
| Year Ended |
| Year Ended |
| Consolidated |
| |||
Details about Accumulated Other |
| December 31, |
| December 31, |
| December 31, |
| Statements of Operations and |
| |||
Comprehensive Income (Loss) Components |
| 2015 |
| 2016 |
| 2017 |
| Comprehensive Income (Loss) |
| |||
Unrealized Net Gains (Losses) on Securities |
| $ | (4,904) |
| $ | 1,295 |
| $ | — |
| Other income (expense) |
|
Foreign Currency Translation Losses |
|
| 51,944 |
|
| — |
|
| — |
| Other income (expense) |
|
Total Reclassifications for the Period |
| $ | 47,040 |
| $ | 1,295 |
| $ | — |
|
|
|
6. Investments
The Company applies the fair value measurement and disclosure provisions of ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”). ASC 820, which defines fair value, establishes a framework for measuring fair value in GAAP, and expands disclosures about fair value measurements. Investments consist primarily of investments with original maturities greater than three months, but no longer than 24 months when purchased.
ASC 820 establishes a three-level valuation hierarchy for fair value measurements. These valuation techniques are based upon the transparency of inputs (observable and unobservable) to the valuation of an asset or liability as of the measurement date. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect the Company’s market assumptions. These two types of inputs create the following fair value hierarchy:
Level 1 — Valuation is based on quoted prices for identical assets or liabilities in active markets.
Level 2 — Valuation is based on quoted prices for similar assets or liabilities in active markets, or other inputs that are observable for the asset or liability, either directly or indirectly, for the full term of the financial instrument.
Level 3 — Valuation is based upon other unobservable inputs that are significant to the fair value measurement.
The fair value of the Company’s fixed income securities is based on a market approach using quoted market values.
The following table summarizes the fair value of cash and cash equivalents and investments as of December 31, 2016:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair Value |
| |
Description |
| Cost |
| Level 1 |
| Level 2 |
| (Carrying Value) |
| ||||
Cash |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
| $ | 6,249,703 |
| $ | 6,249,703 |
| $ | — |
| $ | 6,249,703 |
|
Cash equivalents (original maturity of 3 months or less) |
|
|
|
|
|
|
|
|
|
|
|
|
|
FDIC insured deposits and money market funds |
|
| 24,978,489 |
|
| 24,978,489 |
|
| — |
|
| 24,978,489 |
|
Cash and cash equivalents |
| $ | 31,228,192 |
| $ | 31,228,192 |
| $ | — |
| $ | 31,228,192 |
|
Short-term investments (due within 1 year) |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government treasury obligations |
| $ | 86,078,622 |
| $ | 86,053,755 |
| $ | — |
| $ | 86,053,755 |
|
Corporate obligations |
|
| 20,941,799 |
|
| — |
|
| 20,925,469 |
|
| 20,925,469 |
|
Total short-term investments |
| $ | 107,020,420 |
| $ | 86,053,755 |
| $ | 20,925,469 |
| $ | 106,979,224 |
|
74
The following table summarizes the fair value of cash and cash equivalents and investments as of December 31, 2017:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair Value |
| |
Description |
| Cost |
| Level 1 |
| Level 2 |
| (Carrying Value) |
| ||||
Cash |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
| $ | 2,544,972 |
| $ | 2,544,972 |
| $ | — |
| $ | 2,544,972 |
|
Cash equivalents (original maturity of 3 months or less) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Repurchase agreements |
|
| 10,000,000 |
|
| — |
|
| 10,000,000 |
|
| 10,000,000 |
|
Money market funds |
|
| 6,014,158 |
|
| 6,014,158 |
|
| — |
|
| 6,014,158 |
|
Cash and cash equivalents |
| $ | 18,559,130 |
| $ | 8,559,130 |
| $ | 10,000,000 |
| $ | 18,559,130 |
|
Short-term investments (due within 1 year) |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government treasury obligations |
| $ | 63,034,548 |
| $ | 62,970,115 |
| $ | — |
| $ | 62,970,115 |
|
Corporate obligations |
|
| 15,942,182 |
|
| — |
|
| 15,942,182 |
|
| 15,942,182 |
|
Total short-term investments |
| $ | 78,976,730 |
| $ | 62,970,115 |
| $ | 15,942,182 |
| $ | 78,912,297 |
|
All securities held at December 31, 2016 and 2017, were classified as available-for-sale as defined by ASC 320.
Total unrealized gross gains were $8,257 for the year ended December 31, 2016. There were no unrealized gross gains for the year ended December 31, 2017. Total unrealized gross losses were $49,453 and $64,433 for the years ended December 31, 2016 and 2017, respectively. The Company does not consider any of the unrealized losses to be other-than-temporary impairments because the Company has the intent and ability to hold investments until they recover in value. There were no total realized gross gains for the year ended December 31, 2016 or 2017. Total realized gross losses were $53 for the year ended December 31, 2016. There were no total realized gross losses for the year ended December 31, 2017.
7. Property and Equipment
Property and equipment consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
| Estimated |
| December 31, |
| ||||
|
| Useful Lives |
| 2016 |
| 2017 |
| ||
Laboratory equipment |
| 7 |
| $ | 6,666,506 |
| $ | 5,626,280 |
|
Office equipment and software |
| 3 – 7 |
|
| 1,347,768 |
|
| 1,245,232 |
|
Leasehold improvements |
| 7 |
|
| 424,176 |
|
| 424,176 |
|
Assets not in service |
|
|
|
| 19,000 |
|
| 66,809 |
|
|
|
|
|
| 8,457,450 |
|
| 7,362,497 |
|
Less accumulated depreciation |
|
|
|
| (5,252,373) |
|
| (5,180,098) |
|
|
|
|
| $ | 3,205,077 |
| $ | 2,182,399 |
|
Assets not in service represent new laboratory equipment, computer equipment and software that were not installed and ready to use at December 31, 2016 and excess equipment held for sale at December 31, 2017. The total amount of depreciation expense for the years ended December 31, 2015, 2016 and 2017 were $886,710, $919,809 and $922,670 respectively.
8. Warrants
In connection with the License Agreement, the Company issued to ABX on September 29, 2017, two warrants to purchase up to 4,000,000 shares of the Company’s common stock, at a per share exercise price of $1.39, which is equal to the average closing price of the Company’s common stock during the 30 calendar days prior to September 29,
75
2017. The Company accounted for the warrants at fair value in stockholders’ equity. The warrants contain a conversion feature in the case of certain mergers or consolidations by the Company. Immediately upon issuance, ABX assigned the warrants to an affiliate and certain related parties, which exercised a warrant for 3,278,000 shares on September 29, 2017, resulting in proceeds to the Company in the amount of approximately $4.6 million. The remaining warrant, covering an aggregate of 722,000 shares, remained outstanding as of December 31, 2017, is exercisable until September 29, 2027, and is subject to restrictions on transfer. This warrant was valued using the Black-Scholes model utilizing a ten-year term, the Company’s historic volatility of 91.1%, and an interest rate of 2.28% which is the risk free interest rate of a treasury bond with the same term as the warrants, which are level 2 fair value measurements. There were no other outstanding warrants as of December 31, 2017.
9. Leases
Future minimum lease payments for noncancellable operating leases as of December 31, 2017, are as follows:
|
|
|
|
|
2018 |
| $ | 304,523 |
|
2019 |
|
| — |
|
2020 |
|
| — |
|
2021 |
|
| — |
|
2022 |
|
| — |
|
Thereafter |
|
| — |
|
Total minimum lease payments |
| $ | 304,523 |
|
Rent expense for operating leases was $736,249, $743,323 and $749,406 for the years ended December 31, 2015, 2016 and 2017, respectively.
10. Stockholders’ Equity
Issuances Related to the License Agreement
In connection with the License Agreement, the Company issued to ABX on September 29, 2017, 2,000,000 unregistered shares of the Company’s common stock and two warrants to purchase up to 4,000,000 shares of the Company’s common stock, one of which warrants to purchase 3,278,000 shares was exercised on that same day. Pursuant to a Registration Rights Agreement entered into with ABX, the Company registered for resale these 6,000,000 shares of common stock with the SEC on a Form S-3 Registration Statement that was declared effective on October 24, 2017.
Stock-Based Compensation Plans
The Company has had stock-based compensation plans since 1997. The awards made under the plans adopted in 1997 and 2007 consisted of stock options. The 2010 Equity Incentive Plan (the “2010 Plan”), which is the only plan under which awards may currently be made, authorizes awards in the form of stock options, stock appreciation rights, restricted stock, RSUs, PRSUs, and performance units and performance shares. Awards under the 2010 Plan may be made to employees, directors and certain consultants as determined by the compensation committee of the board of directors. There were 11,003,563 and 11,850,563 shares of common stock authorized and reserved under these plans at December 31, 2016 and December 31, 2017, respectively.
Stock Options
Under the various plans, employees have been granted incentive stock options, while directors and consultants have been granted non-qualified options. The plans allow the holder of an option to purchase common stock at the exercise price, which was at or above the fair value of the Company’s common stock on the date of grant.
76
Generally, options granted under the 1997 and 2007 plans in connection with an employee’s commencement of employment vested over a four-year period with one-half of the shares subject to the grant vesting after two years of employment and the remaining options vesting monthly over the remainder of the four-year period. Options granted under the 1997 and 2007 plans for performance or promotions vested monthly over a four-year period. Generally, options granted under the 2010 Plan vest annually over a three-year or four-year period. Unexercised stock options terminate on the tenth anniversary date after the date of grant. The Company recognizes stock-based compensation expense over the requisite service period of the individual grantees, which generally equals the vesting period. The Company utilizes a Black-Scholes option-pricing model to estimate the value of stock options. The Black-Scholes model allows the use of a range of assumptions related to volatility, risk-free interest rate, employee exercise behavior and dividend yield. Expected volatilities used in the model beginning in 2015 are based on historical volatility of the Company’s stock prices.
The Company is using the “simplified” method for “plain vanilla” options to estimate the expected term of the stock option grants. Under this approach, the weighted-average expected life is presumed to be the average of the vesting term and the contractual term of the option. The risk-free interest rate assumption is derived from the weighted-average yield of a U.S. Treasury security with the same term as the expected life of the options, and the dividend yield assumption is based on historical experience and the Company’s estimate of future dividend yields.
The weighted-average value of the individual options granted during 2015, 2016 and 2017 were determined using the following assumptions:
|
|
|
|
|
|
|
|
|
| Year Ended December 31, |
| ||||
|
| 2015 |
| 2016 |
| 2017 |
|
Expected volatility |
| 106.38 | % | 98.83 | % | 92.98 | % |
Risk-free interest rate |
| 1.55 | % | 1.47 | % | 2.15 | % |
Weighted-average expected life (in years) |
| 6.4 |
| 6.6 |
| 6.9 |
|
Dividend yield |
| 0.00 | % | 0.00 | % | 0.00 | % |
The resulting value of options granted was $2,838,053 and $1,600,031 for the years ended December 31, 2016 and 2017, respectively, which will be amortized into income over the remaining requisite service period. The Company executed a Separation Agreement and Release of Claims with its former Chief Executive Officer, P. Ron Ellis, in connection with his resignation from the Company in June 2016. Under this agreement and Mr. Ellis’ original Severance Agreement, the Company incurred additional stock-based compensation expense of $2,800,000 related to the modification of Mr. Ellis’ options and RSUs. The vesting of each stock option and RSU, other than fully vested awards, was modified and the exercise period of each stock option was extended. In determining the additional expense related to the modification of Mr. Ellis’ awards, the Company revalued the modified options in accordance with ASC 718 using the Black-Scholes model. In addition, the Company adopted ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, for interim and annual reporting periods beginning January 1, 2017. As a result, the Company elected to account for forfeitures as they occur and no longer estimates the number of awards expected to be forfeited. The Company recognized total stock-based compensation cost in the amount of $6,920,220, $9,322,127 and $4,170,645 for the years ended December 31, 2015, 2016 and 2017, respectively.
77
The Company’s stock option activity and related information are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted-Average |
|
|
|
|
|
|
|
|
|
|
| Remaining |
|
|
|
|
|
|
|
| Weighted-Average |
| Contractual Term |
| Aggregate |
| ||
|
| Options |
| Exercise Price |
| (In Years) |
| Intrinsic Value |
| ||
Outstanding at January 1, 2015 |
| 5,096,674 |
| $ | 7.29 |
|
|
|
|
|
|
Granted during year |
| 1,126,672 |
|
| 5.23 |
|
|
|
|
|
|
Exercised during year |
| (146,046) |
|
| 2.60 |
|
|
|
|
|
|
Expired during year |
| (156,160) |
|
| 11.03 |
|
|
|
|
|
|
Forfeited during year |
| (234,325) |
|
| 8.05 |
|
|
|
|
|
|
Outstanding at December 31, 2015 |
| 5,686,815 |
| $ | 6.87 |
| 6.52 |
| $ | 1,432,306 |
|
Exercisable at December 31, 2015 |
| 3,391,069 |
|
| 6.46 |
| 5.37 |
|
| 1,333,875 |
|
Outstanding at January 1, 2016 |
| 5,686,815 |
|
| 6.87 |
|
|
|
|
|
|
Granted during year |
| 1,100,997 |
|
| 3.21 |
|
|
|
|
|
|
Exercised during year |
| (129,638) |
|
| 2.01 |
|
|
|
|
|
|
Expired during year |
| (28,369) |
|
| 7.65 |
|
|
|
|
|
|
Forfeited during year |
| (182,211) |
|
| 4.45 |
|
|
|
|
|
|
Outstanding at December 31, 2016 |
| 6,447,594 |
| $ | 6.41 |
| 6.17 |
| $ | 33,283 |
|
Exercisable at December 31, 2016 |
| 4,666,628 |
|
| 6.76 |
| 5.34 |
|
| 33,283 |
|
Outstanding at January 1, 2017 |
| 6,447,594 |
|
| 6.41 |
|
|
|
|
|
|
Granted during year |
| 883,392 |
|
| 2.29 |
|
|
|
|
|
|
Exercised during year |
| (378,030) |
|
| 2.88 |
|
|
|
|
|
|
Expired during year |
| (716,436) |
|
| 8.02 |
|
|
|
|
|
|
Forfeited during year |
| (357,860) |
|
| 4.53 |
|
|
|
|
|
|
Outstanding at December 31, 2017 |
| 5,878,660 |
| $ | 5.93 |
| 5.83 |
| $ | 3,357,028 |
|
Exercisable at December 31, 2017 |
| 4,370,748 |
|
| 6.79 |
| 4.92 |
|
| 1,319,246 |
|
The following is a rollforward of the Company’s nonvested stock options from January 1, 2015 to December 31, 2017.
|
|
|
|
|
|
|
|
|
|
| Weighted-Average |
| |
|
| Options |
| Grant Date Value |
| |
Nonvested stock options at January 1, 2015 |
| 2,401,396 |
| $ | 7.07 |
|
Granted during year |
| 1,126,672 |
|
| 4.34 |
|
Vested during year |
| (997,997) |
|
| 6.48 |
|
Forfeited during year |
| (234,325) |
|
| 6.37 |
|
Nonvested at December 31, 2015 |
| 2,295,746 |
| $ | 6.05 |
|
Nonvested stock options at January 1, 2016 |
| 2,295,746 |
| $ | 6.05 |
|
Granted during year |
| 1,100,997 |
|
| 2.58 |
|
Vested during year |
| (1,433,566) |
|
| 5.74 |
|
Forfeited during year |
| (182,211) |
|
| 3.57 |
|
Nonvested at December 31, 2016 |
| 1,780,966 |
| $ | 4.41 |
|
Nonvested stock options at January 1, 2017 |
| 1,780,966 |
| $ | 4.41 |
|
Granted during year |
| 883,392 |
|
| 1.81 |
|
Vested during year |
| (798,586) |
|
| 5.03 |
|
Forfeited during year |
| (357,860) |
|
| 3.63 |
|
Nonvested at December 31, 2017 |
| 1,507,912 |
| $ | 2.75 |
|
The total grant date value of stock options vested during 2015, 2016 and 2017 was $6,467,294, $8,233,220 and $4,015,344 respectively. As of December 31, 2016 and December 31, 2017, the total remaining unrecognized
78
compensation cost related to stock options granted was $4.2 million and $2.1 million respectively, which is expected to be recognized over a weighted average period of approximately 1.4 and 1.6 years, respectively. The intrinsic value of options exercised was $126,651 and $635,925 for the years ended December 31, 2016 and December 31, 2017, respectively.
Restricted Stock Units
In May 2011, the Company adopted and granted awards under a performance-based RSU program (the “2011 PRSU Program”) under the 2010 Plan. All PRSU awards expired in the second quarter of 2016 when the performance deadline of May 26, 2016 passed.
RSUs are service-based awards that will vest and be paid in the form of one share of the Company’s common stock for each RSU, generally in two, three or four equal annual installments beginning on the first anniversary of the date of grant of an RSU. As of December 31, 2017, the Company had 1,383,770 RSU awards outstanding. As of December 31, 2016 and 2017, the total remaining unrecognized compensation cost related to RSUs was $1.1 million and $5.7 million, respectively, each of which is expected to be recognized over a weighted average period of approximately 1.4 years.
The following table sets forth the number of RSUs that were granted, vested and forfeited in the periods indicated:
|
|
|
|
|
|
|
|
|
|
| Weighted-Average |
| |
|
| Restricted |
| Grant Date |
| |
|
| Stock Units |
| Fair Value |
| |
Outstanding at January 1, 2015 |
| 161,439 |
| $ | 11.11 |
|
Granted during year |
| 260,976 |
|
| 5.17 |
|
Vested during year |
| (40,333) |
|
| 11.11 |
|
Forfeited during year |
| (30,668) |
|
| 8.07 |
|
Outstanding at December 31, 2015 |
| 351,414 |
| $ | 7.03 |
|
Outstanding at January 1, 2016 |
| 351,414 |
|
| 7.03 |
|
Granted during year |
| 254,538 |
|
| 3.21 |
|
Vested during year |
| (177,078) |
|
| 6.71 |
|
Forfeited during year |
| (34,742) |
|
| 4.13 |
|
Outstanding at December 31, 2016 |
| 394,132 |
|
| 4.96 |
|
Outstanding at January 1, 2017 |
| 394,132 |
| $ | 4.96 |
|
Granted during year |
| 1,300,068 |
|
| 4.90 |
|
Vested during year |
| (158,112) |
|
| 5.35 |
|
Forfeited during year |
| (152,318) |
|
| 3.15 |
|
Outstanding at December 31, 2017 |
| 1,383,770 |
| $ | 5.05 |
|
Employee Stock Purchase Plan
Effective January 1, 2014, the Company implemented the ESPP. At January 1, 2017, 825,154 common shares were available for issuance under the ESPP. Shares may be issued under the ESPP twice a year. In the year ended December 31, 2017, plan participants purchased 55,612 shares of common stock under the ESPP at an average purchase price of $1.30 per share. At December 31, 2017, 769,542 shares were available for issuance under the ESPP.
79
11. Income Taxes
A reconciliation of the expected income tax benefit (expense) computed using the federal statutory income tax rate to the Company’s effective income tax rate is as follows for the years ended December 31, 2015, 2016, and 2017:
|
|
|
|
|
|
|
|
|
| December 31, |
| ||||
|
| 2015 |
| 2016 |
| 2017 |
|
Income tax computed at federal statutory tax rate |
| 34.0 | % | 34.0 | % | 34.0 | % |
State taxes, net of federal benefit |
| 3.2 | % | 3.3 | % | 3.6 | % |
International operations |
| (4.8) | % | (0.7) | % | — |
|
Research and development credits |
| 3.8 | % | 4.0 | % | 2.9 | % |
Orphan drug credits |
| 0.9 | % | 0.6 | % | 0.3 | % |
Equity compensation |
| (2.0) | % | (3.7) | % | (1.1) | % |
Tax reform |
| — |
| — |
| (60.3) | % |
Change in valuation allowance |
| (35.1) | % | (37.5) | % | 20.6 | % |
Total |
| — |
| — |
| — |
|
At December 31, 2017, the Company had net operating loss carryforwards totaling approximately $247,493,000 and $315,755,000 for federal and state income taxes, respectively, that may be used to offset future taxable income. If not used, the net operating loss carryforwards and research and development credits will begin expiring in the year 2021. The Company has determined that it experienced a change in ownership as defined under Section 382 of the U.S. Internal Revenue Code (the “Code”), as a result of the public offering in August 2011. As a result, the future use of its net operating losses, after giving effect to net unrealized built-in gains, will be limited to approximately $218,700,000 for 2017. Any available but unused amounts will become available for use in all successive years, subject to certain limitations. Utilization of these net operating loss carryforwards would require the Company to generate future taxable income prior to their expiration. Furthermore, the utilization of the net operating loss carryforwards could be limited beyond the Company's generation of taxable income if a change in the underlying ownership of the Company's common stock has occurred, resulting in a limitation on the amounts that could be utilized in any given period under Section 382 of the Code.
80
Net deferred tax assets and liabilities are comprised of the following:
|
|
|
|
|
|
|
|
|
| December 31, |
| ||||
|
| 2016 |
| 2017 |
| ||
Deferred tax assets |
|
|
|
|
|
|
|
Net operating loss carryforwards |
| $ | 78,021,000 |
| $ | 62,921,000 |
|
Research and development credit carryforwards |
|
| 14,143,000 |
|
| 16,456,000 |
|
Orphan drug credit |
|
| 7,235,000 |
|
| 7,518,000 |
|
Stock options |
|
| 7,105,000 |
|
| 4,766,000 |
|
Intangibles |
|
| — |
|
| 4,045,000 |
|
Other |
|
| 562,000 |
|
| 334,000 |
|
Deferred tax assets |
|
| 107,066,000 |
|
| 96,040,000 |
|
Deferred tax liabilities |
|
|
|
|
|
|
|
Property and equipment |
|
| (337,000) |
|
| (166,000) |
|
Deferred tax liabilities |
| $ | (337,000) |
| $ | (166,000) |
|
Net deferred tax asset before valuation allowance |
| $ | 106,729,000 |
| $ | 95,874,000 |
|
Less valuation allowance |
|
| (106,729,000) |
|
| (95,874,000) |
|
Net deferred tax assets |
| $ | — |
| $ | — |
|
For the year ended December 31, 2017, net operating loss carryforwards in the table above have been reduced by $2.3 million of uncertain tax liabilities. All deferred tax asset and liability balances have been revalued due to the 2017 Tax Act (as defined and discussed further below). A reconciliation of the beginning and ending amount of the unrecognized tax positions is as follows:
|
|
|
|
|
|
| Unrecognized Tax Positions | |
Balance at January 1, 2016 | $ | — |
Additions based on tax positions related to the current year |
| 3,405,000 |
Additions for tax positions of prior years |
| — |
Reductions for tax positions of prior years |
| — |
Settlements |
| — |
Balance at January 1, 2017 |
| 3,405,000 |
Additions based on tax positions related to the current year |
| — |
Additions for tax positions of prior years |
| — |
Reductions for tax positions of prior years |
| — |
Impact of 2017 Tax Act |
| (1,131,000) |
Settlements |
| — |
Balance at December 31, 2017 | $ | 2,274,000 |
At December 31, 2017 there were no uncertain tax liabilities that if recognized would affect the annual effective tax rate, due to the Company’s full valuation allowance. The Company does not recognize interest accrued related to unrecognized tax liabilities.
On December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act (the “2017 Tax Act”) which, among other provisions, reduces the corporate income tax rate from 35% to 21% effective January 1, 2018. During the fourth quarter of 2017, the Company revalued its net deferred tax assets using the newly enacted rate, resulting in a reduction of its net deferred tax assets of $33.1 million. The Company also reduced its valuation allowance by $33.1 million, resulting in no income tax expense being recognized as a result of the revaluation.
The Company’s estimate of the impact of the 2017 Tax Act is based upon its analysis and interpretations of currently available information. Uncertainties remain regarding the impact of the 2017 Tax Act due to future regulatory
81
and rulemaking processes, prospects of additional corrective or supplemental legislation, and potential trade or other litigation. These uncertainties, along with the Company’s completion of the calculations and potential changes in its initial assumptions as new information becomes available, could cause the actual charge to ultimately differ from the provisional amount recorded in 2017 related to the enactment of the 2017 Tax Act.
12. Collaboration and Other Arrangements
ABX Development and License Agreement
In September 2017, the Company entered into the License Agreement with ABX that grants the Company exclusive worldwide rights to develop and commercialize PSMA-617 agents. Under the terms of the License Agreement, the Company will be responsible for, and bear the future costs of, worldwide development and commercialization of PSMA-617. As consideration for the exclusive license, the Company made an upfront cash payment on September 29, 2017 of approximately $11.9 million to ABX, consisting of $12.0 million less an immaterial expense reimbursement amount, and issued to ABX 2,000,000 shares of the Company’s common stock (see Note 10 – Stockholders’ Equity of the Notes to Consolidated Financial Statements for additional information regarding this issuance) and two warrants to purchase, in the aggregate, 4,000,000 shares of the Company’s common stock (see Note 8 – Warrants of the Notes to Consolidated Financial Statements for additional information regarding the warrants). The License Agreement also obligates the Company to pay ABX regulatory milestone payments of up to $25.0 million, sales milestone payments of up to $135.0 million, and tiered royalties, beginning in the mid-teens and not to exceed the mid-twenties, based on percentages of net sales.
The Company has accounted for the License Agreement as an asset acquisition and as a result, the upfront payment was expensed in the year ended December 31, 2017 as acquired IPR&D expense as the acquired asset did not have alternative future use. Any future regulatory milestone payments when paid or payable will be expensed as acquired IPR&D, provided that the drug has not achieved regulatory approval for marketing and, absent obtaining such approval, has no alternative future use. If the Company obtains regulatory approval for marketing for 177Lu-PSMA-617 or any other PSMA-617 agents, any related milestone payments may be capitalized as an intangible asset and amortized over the life of the asset. The Company recorded $16.5 million of acquired IPR&D expenses related to the License Agreement in the year ended December 31, 2017 consisting of the following:
· | $12.0 million related to the upfront payment to ABX; |
· | $3.8 million related to the fair value of common stock and warrant shares issued; and |
· | $0.7 million of acquisition costs consisting primarily of legal and professional fees. |
The Company intends to initiate, in the second quarter of 2018, the VISION trial, an international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 and will enroll 750 patients with progressive PSMA-positive mCRPC. In October 2017, the Company entered into an agreement with RadioMedix, Inc., a biotechnology company focused on innovative targeted radiopharmaceuticals for diagnosis, monitoring and therapy of cancer, which enabled the transfer of a U.S. Investigational New Drug application of 177Lu-PSMA-617 from the prior sponsor, RadioMedix, to the Company. This transfer helped accelerate the Company’s successful End of Phase 2 trial meeting with the FDA in early 2018 to confirm the phase 3 trial design and registration plan for 177Lu-PSMA-617.
In addition, under a three-party agreement, entered into in October 2017, among the Company, the University of Sydney (the “University”) and ANZUP, a cooperative cancer trials group operating in Australia and New Zealand pursuing research in genito-urinary malignancies, ANZUP sponsors jointly with the University a randomized phase 2 multi-center TheraP trial of 177Lu-PSMA-617 versus cabazitaxel in 200 mCRPC patients. The TheraP trial commenced enrollment in the first quarter of 2018. Under the three-party agreement, the Company provides the PSMA-617 precursor molecule and financial support for the trial. The Company will have access to data generated from the trial, which is a potentially important supportive trial for future regulatory submissions. The primary financial obligations of the trial, along with labeling PSMA-617 with Lutetium-177, will be the responsibility of the University and ANZUP.
82
NMP License and Commercialization Agreement
In August 2013, the Company entered into a license and commercialization agreement with Nihon Medi-Physic Co., LTD. (“NMP”) that grants NMP the right to develop and commercialize etarfolatide in Japan for use in connection with any folate receptor-targeted small molecule drug conjugate (“SMDC”) in Japan. The Company received a $1.0 million non-refundable upfront payment, is eligible for up to $4.5 million based on the successful achievement of regulatory goals for etarfolatide in five different cancer indications and is eligible to receive double-digit percentage royalties on sales of etarfolatide in Japan.
For revenue recognition purposes, the Company viewed the agreement with NMP as a multiple element arrangement. Multiple element arrangements are analyzed to determine whether the various performance obligations, or elements, can be separated or whether they must be accounted for as a single unit of accounting. The Company has identified the deliverables related to the collaboration with NMP, which include the license granted to NMP, as well as the obligation to provide pre-clinical and clinical supply of etarfolatide, to provide rights to NMP if a product is developed that replaces etarfolatide, the obligation for the Company to provide clinical data to NMP during the contract period and the coordination of development and commercialization efforts between the Company or any of its potential sub-licensees for any folate receptor-targeted SMDC and NMP for etarfolatide in Japan. The Company’s deliverables will be accounted for as a single unit of account, therefore the non-refundable upfront payment is being recognized on a straight-line basis over the performance period. This determination was made because the successful development of etarfolatide in Japan requires the ongoing participation by the Company, including the development of the related folate receptor-targeted SMDC therapeutic drug. The performance period over which the revenue will be recognized continues from the date of execution of the agreement through the end of 2033, the estimated termination date of the contract which is when the Company’s performance obligations will be completed. Any significant changes in the timing of the performance period could result in a change in the revenue recognition period. The Company had deferred revenue related to the agreement of approximately $0.8 million at December 31, 2017. Subsequent to the inception of the NMP arrangement, the Company evaluates the remaining deliverables for separation as items in the arrangement are delivered.
The arrangement with NMP includes milestone payments of up to approximately $4.5 million and the milestones are based on the commencement of clinical trials in Japan for specific and non-specific indications and filing for approval in Japan for specific and non-specific indications. The Company evaluated each of these milestone payments and believes that all of the milestones are substantive as there is substantial performance risk that must occur in order for them to be met because the Company must complete additional clinical trials which show a positive outcome or receive approval from a regulatory authority and would be commensurate with the enhancement of value of the underlying intellectual property. To date, the products have not been approved in Japan and no revenue has been recognized related to the regulatory milestones or royalties as continued development of any folate receptor-targeted SMDC is still an opportunity that the Company could pursue in the future.
NMP has the right to terminate the collaboration agreement on 90 days notice prior to the first commercial sale in Japan and six months notice after the first commercial sale in Japan. NMP also has the right to terminate the agreement on six months notice if the Company fails to launch any folate receptor-targeted SMDC therapeutic drug after receiving regulatory approval in Japan. NMP and the Company each have the right to terminate the agreement due to the material breach or insolvency of the other party. Upon termination of the agreement depending on the circumstances, the parties have varying rights and obligations with respect to licensing and related regulatory materials and data.
Other Agreements
In October 2007, the Company entered into an exclusive worldwide license with R&D Biopharmaceuticals to research, develop, and commercialize products containing conjugates of folate receptor targeting compounds and tubulysin compounds. In February 2011, this licensing agreement was assigned by R&D Biopharmaceuticals to
83
Trientlgasse. The Company paid an upfront fee of $300,000 as research and development and pays $25,000 in annual maintenance fees unless a milestone is paid in a given year. The Company could pay $5,900,000 in additional contingent payments upon the achievement of specific scientific, clinical, and regulatory milestones, in addition to royalties upon commercial sales. All payments have been expensed as research and development as incurred, as there is no alternate future use for this technology.
In December 1995, as amended in October 1998, the Company entered into an exclusive license agreement with Purdue Research Foundation, which licenses the right under certain patents to the Company. The Company is obligated to pay an annual minimum royalty of $12,500 until commercial sales commence, following which time the payment of low single digit royalty rates will commence. All payments have been expensed as incurred. The Company does not anticipate incurring any liabilities for vintafolide royalty payments.
In December 2009, the Company entered into a financial term sheet to be incorporated into a written license agreement with Purdue Research Foundation for a patent related to prostate cancer. The agreement was signed and became effective on March 1, 2010. Pursuant to the exclusive license agreement, the Company is subject to minimum annual royalty payments of $15,000, payable until first commercial sale, following which time the minimum annual royalty payments increase to $100,000 and low single digit sales-based royalty rates are also payable when commercial sales commence. In addition, a milestone payment of $500,000 is payable upon approval of a new drug application in the U.S. In 2014, the Company paid a penalty as a milestone was not met. In 2015, the Company accrued a penalty which was paid in the first quarter of 2016 as an additional milestone was not met. There were no additional penalties or milestone payments accrued or paid in the years ended December 31, 2016 or 2017.
In 2014, the Company entered into a Master License Agreement with Purdue Research Foundation. Under this license, the Company has the right to exclusively license patents and technology discovered under company funded research at Purdue University. If the Company decides to move forward with development of one or more of these technologies, the Company will be obligated to make an acceptance payment and the Company will also be obligated to make contingent payments upon the achievement of specific scientific, clinical and regulatory milestones. Certain scientific, clinical and regulatory milestones, in addition to internal resource targets must be met by the Company to avoid fees as consideration for waivers of potentially missed milestones. The Company is obligated to pay annual minimum payments until commercial sales commence, following which time the payment of low single digit royalty rates will commence, along with an annual royalty payment.
13. Related-Party Transactions
The Company funds research at Purdue University, the employer of one of its founders and current Chief Science Officer. Amounts included in research and development expenses were $1,000,000, $1,069,000, and $781,000 for the years ended December 31, 2015, 2016 and 2017, respectively.
The Company leases a facility from Purdue Research Foundation, a nonprofit organization affiliated with Purdue University, the employer of the Company’s Chief Science Officer. The prior lease agreements have generally been renewed annually, and the current lease agreement expires June 30, 2018 with an option to extend the term. The amounts paid to Purdue Research Foundation related to the lease of this facility were $588,000, $597,000 and $609,000 for the years ended December 31, 2015, 2016 and 2017, respectively.
At December 31, 2017, the Company had a balance of $29,600 in accounts payable and other liabilities due to Purdue University and Purdue Research Foundation related to the license agreements, the lease agreement and other operating expenses.
In September 2011, the Company entered into exclusive worldwide licenses with On Target Laboratories, L.L.C. (“On Target”) to develop and commercialize products relating to the compound comprising the Company’s Folate and DUPA ligands and other certain licensed patents. On Target’s Chief Executive Officer is the brother of the Company’s
84
Chief Science Officer. On Target is solely responsible for conducting research and development, seeking regulatory approval and commercialization of products. The Company received nonrefundable upfront license fees totaling $191,000 from On Target in December 2011. The Company has determined that the deliverables under this agreement do not meet the criteria required for separate accounting units for the purposes of revenue recognition, and as a result, the Company recognized revenue from non-refundable, upfront fees when the performance condition of delivering the licenses and certain consultation services had been achieved and there was reasonable assurance of collectability. If On Target fails to meet minimum spend requirements on research and development, the Company has the right to terminate the agreement. The Company will be entitled to receive minimum royalty payments annually based on net sales, but there is no guarantee that a commercial product will be developed and approved for commercial sales. The Company will also be entitled to reimbursement of expenses relating to patent expenses and payments to the inventors and Purdue University if certain milestones are met. During 2011, the Company received a $50,000 reimbursement from On Target for payments made to inventors for issued patents. The Company received $20,000 in annual maintenance license fees during each of 2015, 2016 and 2017, and received $21,044, $14,397 and $6,349 for reimbursable research and development expenses for 2015, 2016 and 2017, respectively.
14. Retirement Plans
The Company maintains a 401(k) retirement savings plan to provide retirement benefits for substantially all of its employees. Participants in the plan may elect to contribute a portion of their annual compensation to the plan, limited to the maximum allowed by the Code. Prior to January 1, 2014 the Company did not match 401(k) contributions. Effective January 1, 2014, the Company implemented a matching contribution for the 401(k) contributions. For each of the years ended December 31, 2015, 2016, and 2017, the Company had approximately $0.3 million of expense related to the 401(k) employer match.
15. Restructuring Costs
In June 2017, the Company refocused its clinical development efforts and aligned its resources to focus on the Company’s highest value opportunities while maintaining key capabilities. The Company’s restructuring activities included a reduction of its workforce by approximately 40%, as well as stopping enrollment in its EC1456 phase 1b trial as the assessment of trial data did not yield the level of clinical activity necessary to support continued advancement of EC1456. In December 2017, the Company stopped enrollment in its EC1456 ovarian cancer surgical trial. Pursuant to ASC Topic 420, Exit or Disposal Cost Obligations, the Company recorded $2.3 million of restructuring expenses in the year ended December 31, 2017 as follows:
· | included in research and development expenses were expenses for employee termination benefits of $0.9 million, $0.9 million for the remaining EC1456 phase 1b trial expenses, including site close-out expenses, $0.3 million related to other restructuring expenses, and $0.1 million related to fixed asset impairment charges; and |
· | included in general and administrative expenses were expenses for employee termination benefits of $0.1 million. |
As of December 31, 2017, the Company had paid all severance and other costs related to the restructuring activities, and had a clinical trial accrual balance related to the EC1456 phase 1b trial termination of $0.1 million, which is expected to be fully paid by the end of the first quarter of 2018.
85
The following table summarizes the restructuring accrual related to the June 2017 restructuring activities for the year ended December 31, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EC1456 |
|
|
|
|
| |||
|
| Employee |
| Phase 1b Trial |
| Other |
|
|
| |||
|
| Termination |
| Termination |
| Restructuring |
|
|
| |||
|
| Accrual |
| Accrual |
| Costs Accrual |
| Total | ||||
Balance, January 1, 2017 |
| $ | — |
| $ | — |
| $ | — |
| $ | — |
Charges for the year ended December 31, 2017 |
|
| 1,029,400 |
|
| 947,100 |
|
| 126,500 |
|
| 2,103,000 |
Revised estimates in the year ended December 31, 2017 |
|
| — |
|
| (30,000) |
|
| — |
|
| (30,000) |
Amounts paid in the year ended December 31, 2017 |
|
| (1,029,400) |
|
| (810,200) |
|
| (126,500) |
|
| (1,966,100) |
Balance, December 31, 2017 |
| $ | — |
| $ | 106,900 |
| $ | — |
| $ | 106,900 |
The Company terminated the PROCEED trial in May 2014 after the interim futility analysis indicated that vintafolide did not demonstrate efficacy on the pre-specified outcome of progression-free survival for the treatment of platinum-resistant ovarian cancer. As a result, the Company ceased its pre-launch commercial activities in Europe and implemented staff reductions in Europe and in the U.S. At December 31, 2016 and 2017, the Company had no remaining accrual balance related to the PROCEED trial termination.
The following table summarizes the restructuring accruals related to the PROCEED trial termination for the years ended December 31, 2015 and 2016:
|
|
|
|
|
|
| PROCEED |
| |
|
| Trial |
| |
|
| Termination |
| |
|
| Accrual |
| |
Balance, January 1, 2015 |
| $ | 1,300,000 |
|
Revised estimates in the year ended December 31, 2015 |
|
| 134,000 |
|
Amounts paid in the year ended December 31, 2015 |
|
| (1,387,400) |
|
Balance, December 31, 2015 |
| $ | 46,600 |
|
Balance, January 1, 2016 |
| $ | 46,600 |
|
Revised estimates in the year ended December 31, 2016 |
|
| (15,800) |
|
Amounts paid in the year ended December 31, 2016 |
|
| (30,800) |
|
Balance, December 31, 2016 |
| $ | — |
|
86
16. Selected Quarterly Financial Data (unaudited)
The following tables summarize the unaudited statements of operations for each quarter of 2017 and 2016 (in thousands except share and per share amounts):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Quarters Ended |
| ||||||||||
|
| March 31, |
| June 30, |
| September 30, |
| December 31, |
| ||||
2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
| $ | 12 |
| $ | 13 |
| $ | 33 |
| $ | 12 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 7,994 |
|
| 8,655 |
|
| 4,090 |
|
| 5,128 |
|
General and administrative |
|
| 3,745 |
|
| 3,306 |
|
| 3,011 |
|
| 3,708 |
|
Acquired in-process research and development |
|
| — |
|
| — |
|
| 16,493 |
|
| 46 |
|
Loss from operations |
|
| (11,727) |
|
| (11,948) |
|
| (23,561) |
|
| (8,870) |
|
Interest income, net |
|
| 235 |
|
| 234 |
|
| 265 |
|
| 295 |
|
Other income (expense), net |
|
| 3 |
|
| (30) |
|
| 29 |
|
| 11 |
|
Net loss |
| $ | (11,489) |
| $ | (11,744) |
| $ | (23,267) |
| $ | (8,564) |
|
Net loss per share-basic and diluted (1) (2) |
| $ | (0.27) |
| $ | (0.28) |
| $ | (0.55) |
| $ | (0.18) |
|
Weighted average common shares outstanding – basic and diluted (2) |
|
| 42,434,709 |
|
| 42,503,584 |
|
| 42,636,567 |
|
| 47,979,127 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Quarters Ended |
| ||||||||||
|
| March 31, |
| June 30, |
| September 30, |
| December 31, |
| ||||
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
| $ | 12 |
| $ | 13 |
| $ | 33 |
| $ | 12 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 6,531 |
|
| 6,788 |
|
| 5,985 |
|
| 8,188 |
|
General and administrative |
|
| 3,820 |
|
| 7,394 |
|
| 2,988 |
|
| 3,096 |
|
Loss from operations |
|
| (10,339) |
|
| (14,169) |
|
| (8,940) |
|
| (11,272) |
|
Interest income, net |
|
| 189 |
|
| 208 |
|
| 232 |
|
| 232 |
|
Other expense, net |
|
| (3) |
|
| (1) |
|
| — |
|
| (25) |
|
Net loss |
| $ | (10,153) |
| $ | (13,962) |
| $ | (8,708) |
| $ | (11,065) |
|
Net loss per share-basic and diluted (1) (2) |
| $ | (0.24) |
| $ | (0.33) |
| $ | (0.21) |
| $ | (0.26) |
|
Weighted average common shares outstanding – basic and diluted (2) |
|
| 42,109,828 |
|
| 42,178,537 |
|
| 42,263,311 |
|
| 42,289,453 |
|
(1) | Per common share amounts for the quarters and full years have been calculated separately. Accordingly, the sum of quarterly amounts may not equal the annual amount due to differences in the weighted average common shares outstanding during each period, principally due to the effect of share issuances by the Company during the year. |
(2) | Diluted weighted average common shares outstanding are identical to basic weighted average common shares outstanding and diluted net loss per share is identical to basic net loss per share for all quarters of 2016 and 2017 because common share equivalents are excluded from the calculations of diluted weighted average common shares outstanding for those quarters, as their effect is anti-dilutive. |
87
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A.Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
As of December 31, 2017, management, with the participation of our Chief Executive Officer and Chief Financial Officer, performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended. Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosures. Because of inherent limitations, any controls and procedures, no matter how well designed and operated, can provide only reasonable, and not absolute, assurance of achieving the desired control objective. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2017, the design and operation of our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended December 31, 2017, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) of the Exchange Act. Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on criteria established in the Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework). Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment. Based on this evaluation, our management concluded that, as of December 31, 2017, our internal control over financial reporting was effective based on such criteria.
Attestation on Internal Control over Financial Reporting
Ernst & Young LLP, our independent registered public accounting firm, has audited our consolidated financial statements for the year ended December 31, 2017 and has issued an audit report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2017, which is included in Item 8 of this Annual Report on Form 10-K.
88
89
Item 10.Directors, Executive Officers and Corporate Governance
Directors
The information required by this item is set forth in our definitive Proxy Statement to be used in connection with the 2018 Annual Meeting of Stockholders (the “2018 Proxy Statement”) and incorporated herein by reference.
Executive Officers
Information regarding our executive officers is set forth in Item 1 of Part I of this annual report under the caption “Executive Officers of the Registrant.”
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our directors and officers and other employees, including our principal executive officer, principal financial officer and principal accounting officer. This code is publicly available through the Investors & News section of our website at www.endocyte.com. To the extent permissible under applicable law, the rules of the SEC or Nasdaq listing standards, we intend to post on our website any amendment to the code of business conduct and ethics, or any grant of a waiver from a provision of the code of business conduct and ethics, that requires disclosure under applicable law, the rules of the SEC or Nasdaq listing standards.
Audit Committee
The information required by this item relating to our audit committee is set forth in the 2018 Proxy Statement and incorporated herein by reference.
Section 16(a) Beneficial Ownership Reporting Compliance
The information required by this item relating to our compliance with Section 16(a) of the Exchange Act is set forth in the 2018 Proxy Statement and incorporated herein by reference.
Corporate Governance
The information required by this item relating to the procedures by which stockholders may recommend nominees to the board of directors is set forth in the 2018 Proxy Statement and incorporated herein by reference.
Item 11.Executive Compensation
The information required by this item is set forth in the 2018 Proxy Statement and incorporated herein by reference.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item with respect to security ownership of certain beneficial owners and management is set forth in the 2018 Proxy Statement and incorporated herein by reference.
90
Equity Compensation Plan Information
The following table provides information regarding our equity compensation plans as of December 31, 2017:
|
|
|
|
|
|
|
|
|
|
| Equity Compensation Plan Information |
| |||||
|
|
|
|
|
|
| Number of Securities |
|
|
|
|
|
|
|
| Remaining Available for |
|
|
| Number of Securities |
|
|
|
| Future Issuance Under |
|
|
| to be Issued Upon |
| Weighted Average |
| Equity Compensation |
| |
|
| Exercise of |
| Exercise Price of |
| Plans (Excluding |
| |
|
| Outstanding Options, |
| Outstanding Options, |
| Securities Reflected |
| |
|
| Warrants and Rights |
| Warrants and Rights |
| in Column (a)) |
| |
Plan Category |
| (a) |
| (b) |
| (c) |
| |
Equity compensation plans approved by security holders: (1) |
| 7,262,430 | (2) | $ | 5.93 | (3) | 3,126,259 | (4) |
Equity compensation plans not approved by security holders: |
| — |
|
|
|
| — |
|
Total |
| 7,262,430 |
| $ | 5.93 |
| 3,126,259 |
|
(1) | Consists of the 2007 Stock Plan, the 2010 Equity Incentive Plan, and the 2010 Employee Stock Purchase Plan (“ESPP”). |
(2) | Includes shares which may be issued pursuant to: |
· | 5,878,660 outstanding stock options; and |
· | 1,383,770 outstanding restricted stock units, or RSUs. |
(3) | Represents the weighted average exercise price of outstanding stock options. Does not take into account the outstanding RSUs which, once earned and vested, will be settled in shares of our common stock on a one-for-one basis at no additional cost. |
(4) | Consists of shares available for awards under the 2010 Equity Incentive Plan and shares available for purchase under the ESPP. As of December 31, 2017, there were no shares available for awards under the 1997 Stock Plan or the 2007 Stock Plan. The original number of shares available for awards under the 2010 Equity Incentive Plan was 1,498,929. The 2010 Equity Incentive Plan provides for an annual increase in the number of shares available for issuance under that plan, if approved by the Board of Directors. Between the date of stockholder approval of the 2010 Equity Incentive Plan, January 10, 2011, and December 31, 2017, an aggregate of 8,055,000 shares have become available for issuance under the 2010 Equity Incentive Plan pursuant to the annual increase provision. The 2010 Equity Incentive Plan also provides that shares of common stock subject to outstanding awards under the 1997 Stock Plan or the 2007 Stock Plan that expire, terminate, are forfeited or are repurchased become available for issuance under the 2010 Equity Incentive Plan. Between January 10, 2011 and December 31, 2017, an aggregate of 100,490 shares underlying outstanding awards under the 1997 Stock Plan and the 2007 Stock Plan expired, terminated, were forfeited or were repurchased and therefore became available under the 2010 Equity Incentive Plan. Effective January 1, 2018, the Board of Directors increased the number of shares available for awards under the 2010 Equity Incentive Plan by 1,446,000 shares pursuant to the annual increase provision of the 2010 Equity Incentive Plan. The original number of shares available for purchase under the ESPP was 261,780. The ESPP provides for an annual increase in the number of shares available for purchase under that plan, if approved by the Board of Directors. Between the date of stockholder approval of the ESPP, January 10, 2011, and December 31, 2017, an aggregate of 778,000 shares have become available for purchase under the ESPP pursuant to the annual increase provision. The number of shares related to the ESPP shown in this column consists of 769,542 shares remaining available for issuance under the ESPP as of December 31, 2017. |
Item 13.Certain Relationships and Related Transactions, and Director Independence
The information required by this item is set forth in the 2018 Proxy Statement and incorporated herein by reference.
91
Item 14.Principal Accounting Fees and Services
The information required by this item is set forth in the 2018 Proxy Statement and incorporated herein by reference.
92
Item 15.Exhibits, Financial Statement Schedules
(a) 1. Financial Statements
The following financial statements of Endocyte, Inc. and its subsidiaries are set forth in Part II, Item 8.
|
|
|
|
| Page |
| 60 | |
| 61 | |
Consolidated Balance Sheets as of December 31, 2016 and 2017 |
| 63 |
| 64 | |
| 65 | |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2015, 2016 and 2017 |
| 66 |
| 67 |
2. Financial Statement Schedules
Financial statement schedules are omitted because they are not applicable or the required information is shown in the financial statements or the notes thereto.
3. Exhibits
93
|
|
| |
Exhibit |
| Description+ | |
| 3.1 |
|
| |
| 3.2 |
|
| |
| 4.1 |
|
| |
| 4.2 |
|
| |
| 4.3 |
|
| |
| 4.4 |
|
| |
| 4.5 |
|
| |
| 4.6 |
|
| |
| 10.1 | * |
| |
| 10.2 | * |
| |
| 10.3 | * |
| |
| 10.4 | * |
| |
| 10.5 | * |
| |
| 10.6 | * |
| |
| 10.7 | * |
| |
| 10.8 | * |
| |
| 10.9 |
|
| |
| 10.10 |
|
| |
| 10.11 | ** |
| |
94
|
|
| |
Exhibit |
| Description + | |
| 10.12 | ** |
| |
| 10.13 | *** |
| |
| 10.14 | ** |
| |
| 10.15 |
|
| |
| 10.16 | *** |
| |
| 10.17 | ** |
| |
| 10.18 | ** |
| |
| 10.19 | *** |
| |
| 10.20 |
|
| |
| 10.21 |
|
| |
| 10.22 |
|
| |
| 10.23 | * |
| |
| 10.24 | * |
| |
| 10.25 | * |
| |
| 10.26 | * |
| Schedule of Terms for Change in Control and Severance Agreements with Executive Officers |
| 10.27 |
|
| |
| 23.1 |
|
| Consent of Ernst & Young, LLP, Independent Registered Public Accounting Firm. |
| 31.1 |
|
| |
| 31.2 |
|
| |
| 32.1 |
|
| |
95
|
|
| |
Exhibit |
| Description + | |
| 101 |
|
| The following materials from Registrant’s Annual Report on Form 10-K for the year ended December 31, 2017, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2016 and 2017, (ii) Consolidated Statements of Operations and Comprehensive Income (Loss) for the years ended December 31, 2015, 2016 and 2017, (iii) Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2015, 2016 and 2017, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2016 and 2017 and (v) Notes to Consolidated Financial Statements. |
+ Unless otherwise indicated, exhibits incorporated by reference herein were originally filed under SEC File No. 001-35050.
* Indicates management contracts or compensatory plans or arrangements.
** The Securities and Exchange Commission has granted our request that certain provisions of this exhibit be treated as confidential. Such material has been redacted from the exhibit as filed.
*** Application has been made to the Securities and Exchange Commission to seek confidential treatment of certain provisions of this exhibit. Omitted material for which confidential treatment has been requested has been filed separately with the Securities and Exchange Commission.
None.
96
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ENDOCYTE, INC. | |
|
|
|
| By: | /s/ Michael A. Sherman |
|
| Michael A. Sherman |
|
| President and Chief Executive Officer |
Date: February 27, 2018 |
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
| Title |
| Date |
|
|
|
|
|
/s/ Michael A. Sherman |
| President and Chief Executive Officer |
| February 27, 2018 |
Michael A. Sherman |
| (Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Michael T. Andriole |
| Chief Financial Officer |
| February 27, 2018 |
Michael T. Andriole |
| (Principal Financial Officer) |
|
|
|
|
|
|
|
/s/ Beth A. Taylor |
| Vice President of Finance and Chief Accounting Officer |
| February 27, 2018 |
Beth A. Taylor |
| (Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ John C. Aplin |
| Chairman of the Board of Directors |
| February 27, 2018 |
John C. Aplin |
|
|
|
|
|
|
|
|
|
/s/ Philip S. Low |
| Director and Chief Science Officer |
| February 27, 2018 |
Philip S. Low |
|
|
|
|
|
|
|
|
|
/s/ Keith E. Brauer |
| Director |
| February 27, 2018 |
Keith E. Brauer |
|
|
|
|
|
|
|
|
|
/s/ Ann F. Hanham |
| Director |
| February 27, 2018 |
Ann F. Hanham |
|
|
|
|
|
|
|
|
|
/s/ Marc D. Kozin |
| Director |
| February 27, 2018 |
Marc D. Kozin |
|
|
|
|
|
|
|
|
|
/s/ Peter D. Meldrum |
| Director |
| February 27, 2018 |
Peter D. Meldrum |
|
|
|
|
|
|
|
|
|
/s/ Fred A. Middleton |
| Director |
| February 27, 2018 |
Fred A. Middleton |
|
|
|
|
|
|
|
|
|
/s/ Lesley Russell |
| Director |
| February 27, 2018 |
Lesley Russell |
|
|
|
|
97