ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with the unaudited condensed consolidated financial statements and notes thereto included elsewhere in this Quarterly Report on Form 10-Q.
Overview
We are a commercial-stage biopharmaceutical company committed to discovering, developing and commercializing small-molecule and protein therapeutics for large-market as well as orphan indications targeting inflammation, complement-mediated diseases, disorders of the central nervous system, and immune-related diseases, including cancers.
Our drug product OMIDRIA® is marketed in the United States for use during cataract surgery or intraocular lens replacement to maintain pupil size by preventing intraoperative miosis (pupil constriction) and to reduce postoperative pain. In our pipeline we have clinical-stage development programs focused on complement-associated thrombotic microangiopathies, complement-mediated glomerulonephropathies, and addictive and compulsive disorders. In addition, we have a diverse group of preclinical programs and two platforms: one capable of unlocking new G protein-coupled receptor (GPCR) drug targets and the other used to generate antibodies. For OMIDRIA and each of our product candidates and our programs, we have retained control of all commercial rights.
Commercial Product - OMIDRIA® (phenylephrine and ketorolac intraocular solution) 1%/0.3%
OMIDRIA is approved by the FDA for use during cataract surgery or intraocular lens replacement to maintain pupil size by preventing intraoperative miosis (pupil constriction) and to reduce postoperative ocular pain. Outside of the U.S., we have received approval from the European Commission (EC) to market OMIDRIA in the European Economic Area (EEA) for use during cataract surgery and other intraocular lens replacement procedures for maintenance of intraoperative mydriasis (pupil dilation), prevention of intraoperative miosis and reduction of acute postoperative ocular pain.
OMIDRIA is a proprietary drug product containing two active pharmaceutical ingredients: ketorolac, an anti-inflammatory agent, and phenylephrine, a mydriatic, or pupil dilating, agent. Cataract and other lens replacement surgery involves replacement of the original lens of the eye with an artificial intraocular lens. These procedures are typically performed to replace a lens opacified by a cataract and/or to correct a refractive error. OMIDRIA is added to standard irrigation solution used during cataract and lens replacement surgery and is delivered intracamerally, or within the anterior chamber of the eye, to the site of the surgical trauma throughout the procedure. Preventing pupil constriction is essential for these procedures and, if miosis occurs, the risk of damaging structures within the eye and other complications increases, as does the operating time required to perform the procedure.
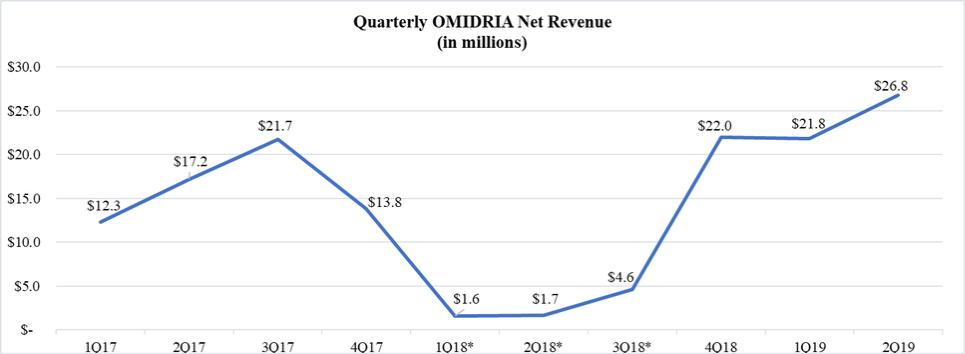
We launched OMIDRIA in the U.S. in the second quarter of 2015 and sell OMIDRIA primarily through wholesalers which, in turn, sell to ambulatory surgery centers (ASCs) and hospitals. The Centers for Medicare & Medicaid Services (CMS), the federal agency responsible for administering the Medicare program, granted transitional pass-through reimbursement status for OMIDRIA in 2014, effective from January 1, 2015 through December 31, 2017. Pass-through status allows for separate payment (i.e., outside the packaged payment rate for the surgical procedure) under Medicare Part B. In March 2018, the Consolidated Appropriations Act of 2018 (the Appropriations Act) was signed into law. The Appropriations Act included a provision by which Congress extended pass-through reimbursement status for a small number of drugs, including OMIDRIA, used during procedures performed on Medicare Part B fee-for-service patients for an additional two years, running from October 1, 2018 until October 1, 2020.
We continue to pursue permanent separate reimbursement for OMIDRIA. In the 2019 final rule for CMS’ outpatient prospective payment system (OPPS), CMS indicated that, in the ASC setting, it will separately pay for certain non-opioid drugs used during surgery that have an FDA-approved indication for postoperative pain relief and are currently packaged with the procedure in calendar year 2019. In its OPPS proposed rule for 2020, CMS indicates an intention to continue its policy of paying separately for non-opioid drugs indicated for postoperative pain relief when