UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the Fiscal year ended March 31, 2007
OR
o Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the transition period from ______ to ______
Commission File Number: 001-32433
PRESTIGE BRANDS HOLDINGS, INC. | ||||||
| (Exact name of Registrant as specified in its charter) | ||||||
Delaware (State or other jurisdiction of incorporation or organization) | 20-1297589 (I.R.S. Employer Identification No.) | |||||
90 North Broadway Irvington, New York 10533 (Address of Principal Executive Offices, including zip code) | ||||||
(914) 524-6810 (Registrant’s telephone number, including area code) | ||||||
| Securities registered pursuant to Section 12(b) of the Act: | ||||||
Title of each class: | Name on each exchange on which registered: | |||||
| Common Stock, par value $.01 per share | New York Stock Exchange | |||||
Securities registered pursuant to Section 12(g) of the Act: None | ||||||
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No þ
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b -2 of the Exchange Act. (Check one)
Large accelerated filer o Accelerated filer þ Non-accelerated filero
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No þ
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold as of the last business day of the Registrant’s most recently completed second fiscal quarter ended September 30, 2006 was $369.7 million.
As of June 1, 2007, the Registrant had 50,005,000 shares of common stock outstanding.
Documents Incorporated by Reference
Portions of the Registrant’s Definitive Proxy Statement for the 2007 Annual Meeting of Stockholders (the “2007 Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent described herein.
Table of Contents
Page | ||
Part I | ||
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 16 |
| Item 1B. | Unresolved Staff Comments | 25 |
| Item 2. | Properties | 25 |
| Item 3. | Legal Proceedings | 26 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 27 |
Part II | ||
| Item 5. | Market for Registrants’ Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 28 |
| Item 6. | Selected Financial Data | 30 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 33 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 51 |
| Item 8. | Financial Statements and Supplementary Data | 51 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 51 |
| Item 9A. | Controls and Procedures | 51 |
| Item 9B. | Other Information | 52 |
Part III | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 53 |
| Item 11. | Executive Compensation | 53 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 53 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 53 |
| Item 14. | Principal Accounting Fees and Services | 53 |
Part IV | ||
| Item 15. | Exhibits and Financial Statement Schedules | 54 |
Trademarks and Trade Names | ||
| Trademarks and trade names used in this Annual Report on Form 10-K are the property of Prestige Brands Holdings, Inc. or its subsidiaries, as the case may be. We have utilized the ® and TM symbols the first time each trademark or trade name appears in this Annual Report on Form 10-K. | ||
Part I.
ITEM 1. BUSINESS
Overview
Unless otherwise indicated by the context, all references in this Annual Report on Form 10-K to “we”, “us”, “our”, “Company” or “Prestige” refer to Prestige Brands Holdings, Inc. and its subsidiaries. Similarly, reference to a year (e.g. “2007”) refers to our fiscal year ended March 31 of that year.
We sell well-recognized, brand name over-the-counter healthcare, household cleaning and personal care products in a global marketplace. We operate in niche segments of these categories where we can use the strength of our brands, our established retail distribution network, a low-cost operating model and our experienced management team as a competitive advantage to grow our presence in these categories and, as a result, grow our sales and profits. Our ultimate success is dependent on our ability to:
| · | Develop effective sales, advertising and marketing programs, |
| · | Grow our existing products lines, |
| · | Acquire new brands, and |
| · | Respond to the technological advances and product introductions of our competitors. |
Our fourteen major brands, set forth in the table below, have strong levels of consumer awareness and retail distribution across all major channels. These brands accounted for approximately 94.1% and 93.3% of our net revenues for 2007 and 2006, respectively.
Major Brands | Market Position(1) | Market Segment | Market Share (1) (%) | ACV(1) (%) | ||||
Over-the-Counter Healthcare: | ||||||||
Chloraseptic® | #1 | Liquid Sore Throat Relief | 44.9 | 95 | ||||
Clear Eyes® | #2 | Redness Relief | 16.0 | 87 | ||||
Compound W® | #2 | Wart Removal | 32.1 | 85 | ||||
Wartner® | #3 | Wart Removal | 12.1 | 67 | ||||
The Doctor’s® NightGuard™ | #1 | Bruxism (Teeth Grinding) | 99.5 | 63 | ||||
The Doctor’s® Brushpicks™ | #2 | Interdental Picks | 27.6 | 47 | ||||
Murine® | #3 | Personal Ear Care | 13.4 | 65 | ||||
Little Remedies®(2) | N/A | Pediatric Healthcare | N/A | 70 | ||||
New-Skin® | #1 | Liquid Bandages | 37.1 | 80 | ||||
Dermoplast® | #3 | Pain Relief Sprays | 31.2 | 62 | ||||
Household Cleaning: | ||||||||
Comet® | #2 | Abrasive Tub and Tile Cleaner | 30.3 | 99 | ||||
Chore Boy® | #1 | Soap Free Metal Scrubbers | 32.8 | 40 | ||||
Spic and Span® | #6 | All Purpose Cleaner | 3.9 | 58 | ||||
Personal Care: | ||||||||
Cutex® | #1 | Nail Polish Remover | 27.4 | 93 | ||||
Denorex® | #4 | Medicated Shampoo | 5.5 | 48 |
| (1) | The data included in this Annual Report on Form 10-K regarding the market share and ranking for our brands, is based on an analysis conducted by the Company, based in part on data generated by the independent market research firm, Information Resources, Inc. (“Information Resources”). Information Resources reports retail sales data in the food, drug and mass merchandise markets. However, Information Resources’ data does not include Wal-Mart point of sale data, as Wal-Mart ceased providing sales data to the industry in 2001. Although Wal-Mart represents a significant portion of the mass merchandise market for us, as well as our competitors, we believe that Wal-Mart’s exclusion from the Information Resources data analyzed by the Company above does not significantly change our market share or ranking relative to our competitors. “Market share” or “market position” is based on sales dollars in the United States, as calculated |
-1-
Our products are sold through multiple channels, including mass merchandisers and drug, grocery, dollar and club stores, which allows us to effectively launch new products across all distribution channels and reduce our exposure to any single distribution channel. We focus our internal resources on our core competencies: (i) marketing, (ii) sales, (iii) customer service and (iv) product development. While we perform the production planning and oversee the quality control aspects of the manufacturing, warehousing and distribution of our products, we outsource the operating elements of these functions to entities which offer expertise in such areas and cost efficiencies due to economies of scale. Our operating model allows us to focus on marketing and product development, which we believe enables us to achieve attractive margins while minimizing capital expenditures and working capital requirements.
We have grown our brand portfolio by acquiring strong and well-recognized brands from larger consumer products and pharmaceutical companies, as well as other brands from smaller private companies. While the brands we have purchased from larger consumer products and pharmaceutical companies have long histories of support and brand development, we believe that at the time we acquired them they were considered “non-core” by their previous owners. Consequently, they did not benefit from the focus of senior level management or strong marketing support. We also believe that the brands we have purchased from smaller private companies have been constrained by the limited resources of their prior owners. After adding a brand to our portfolio, we seek to increase its sales, market share and distribution in both new and existing channels through our established retail distribution network. We pursue this growth through increased advertising and promotion, new marketing strategies, improved packaging and formulations and innovative new products. Our business and business model, however, are faced with various risks that are described in “Risk Factors” in Item 1A of this Annual Report on Form 10-K.
Competitive Strengths
Diversified Portfolio of Well-Recognized and Established Consumer Brands
We own and market well-recognized consumer brands, many of which were established over 60 years ago. Our diverse portfolio of products provides us with multiple sources of growth and minimizes our reliance on any one product or category. We provide significant marketing support to our brands in order to grow our sales and our long-term profitability. The markets in which we sell our products, however, are highly competitive and include numerous national and global manufacturers, distributors, marketers and retailers, many of which have greater resources than we do and may be able to spend more aggressively on advertising and marketing, which may have an adverse effect on our competitive position.
Strong Competitor in Attractive, Niche Categories
We strategically choose to compete in niche product categories that address recurring consumer needs and that we believe are considered “non-core” to larger consumer products and pharmaceutical companies. We believe we are well positioned in these categories due to the long history and consumer awareness of our brands, our strong market positions and our low-cost operating model. However, a significant increase in the number of product introductions by our competitors in these niche markets could have a material adverse effect on our business, financial condition and results from operations.
by Information Resources for the 52 weeks ended March 25, 2007. “ACV” refers to the All Commodity Volume Food Drug Mass Index, as calculated by Information Resources for the 52 weeks ended March 25, 2007. ACV measures the weighted sales volume of stores that sell a particular product out of all the stores that sell products in that market segment generally. For example, if a product is sold by 50% of the stores that sell products in that market segment, but those stores account for 85% of the sales volume in that market segment, that product would have an ACV of 85%. We believe that ACV is a measure of a product’s importance to major retailers. We believe that a high ACV evidences a product’s attractiveness to consumers, as major national and regional retailers will carry products that are attractive to their customers. Lower ACV measures would indicate that a product is not as available to consumers because the major retailers do not carry products for which consumer demand may not be as high. For these reasons, we believe that ACV is an important measure for investors to gauge consumer awareness of the Company’s product offerings.
| (2) | Market share information for market segments in which Little Remedies products compete is not available from Information Resources. |
-2-
Proven Ability to Develop and Introduce New Products
We focus our marketing and product development efforts on identifying underserved consumer needs and then designing products that directly address those needs. Keeping with that philosophy, in late 2007, we introduced Clear Eyes Maximum Redness Relief®, a fast acting formula that lubricates as it relieves redness, and Little Tummys Gripe Water, an herbal supplement with ginger and fennel for safe, gentle relief of infant colic, hiccups and upset stomach. The above were designed to augment our 2006 product introductions that included: Clear Eyes Triple Action Relief, formulated to remove redness, moisturize and relieve irritation; Clear Eyes for Dry Eyes ACR Relief, for long lasting relief from pollen, dust and ragweed; Dermoplast Poison Ivy Treatment, a non-irritating wash that controls the itch and removes oils that cause the rash; as well as Murine Homeopathic Earache Relief, formulated to promote the body’s natural ability to relieve ear pain. Looking forward, in early 2008, we will introduce Comet Spray Gel, a high viscosity mildew stain remover spray, as well as the Murine Earigate® Ear Cleaning System, a natural and hypoallergenic wax removal system with a patented “reverse spray action” that safely rinses away ear wax buildup without harming the user's sensitive eardrums. Although line extensions and new product introductions are important to the overall growth of a brand, our efforts may reduce sales of existing products within that brand. In addition, certain of our product introductions may not be successful, such as Murine Homeopathic Allergy Eye Relief, Murine Homeopathic Tired Eye Relief and Chloraseptic Daily Defense Strips, all of which were introduced in 2006 and discontinued in 2007, as well as Little Teethers® Oral Pain Relief Swabs, which we introduced in February 2005 and discontinued in February 2006.
Efficient Operating Model
To gain operating efficiencies, we directly manage the production planning and quality control aspects of the manufacturing, warehousing and distribution of our products, while we outsource the operating elements of these functions to well-established, lower-cost, third-party providers. This approach allows us to benefit from the core competencies of our third-party providers and maintain a highly variable cost structure, with low overhead, limited working capital requirements and minimal investment in capital expenditures. During 2007, our aggregate gross margin was approximately 52% while our general and administrative expense and our capital expenditures represented less than 9% and 1% of net sales, respectively. This compares slightly less favorably to 2006, when our aggregate gross margin was approximately 53%, and our general and administrative expenses and our capital expenditures represented less than 8% and 1% of net sales, respectively. Our gross margin was impacted by the obsolescence reserves associated with our Chloraseptic inventory and our general and administrative expenses were impacted by the overall growth of the organization. Our operating model, however, requires us to depend on third-party providers for manufacturing and logistics services. The inability or unwillingness of our third-party providers to supply or ship our products may have a material adverse effect on our business, financial condition and results from operations.
Management Team with Proven Ability to Acquire, Integrate and Grow Brands
Our management team has significant experience in consumer product marketing, sales, product development and customer service. We have grown our business through acquisition, integration and expansion of the brands we purchased. Unlike many larger consumer products companies which we believe often entrust their smaller brands to rotating junior employees, we dedicate experienced managers to specific brands. Since the Company has fewer than 100 employees, we seek more experienced personnel to carry the substantial responsibility of brand management. These managers nurture the brands as they grow and evolve.
Growth Strategy
In order to continue to enhance our brands and drive growth we focus our growth strategy on our core competencies: (i) marketing, (ii) sales, (iii) customer service, and (iv) product development efforts. We plan to execute this strategy through:
| · | Investing in Advertising and Promotion. |
We will continue to invest in advertising and promotion to drive the growth of our brands. Our marketing strategy is focused primarily on consumer-oriented programs that include media advertising, targeted coupon programs and in-store advertising. While the absolute level of marketing expenditures differs by brand and category, we typically have increased the amount of investment in our brands after acquiring
-3-
them. For example, after the acquisition of the Dental Concepts line of products in 2006, we expanded consumer promotion programs and increased advertising, which resulted in domestic annual brand sales growth of approximately 26% during 2007. In 2007, we introduced our first dual-action product, Chloraseptic Sore Throat plus Cough Lozenges, as well as 2 sugar free sore throat lozenges. Looking forward, our sore throat relief strip product, originally introduced in 2003, will be reintroduced in June 2007 with a new and improved formulation and new packaging. Given the competition in our industry, there is a risk that our marketing efforts may not result in increased sales and profitability. Additionally, no assurance can be given that we can maintain these increased sales and profitability levels once attained.
| · | Growing our Categories and Market Share with Innovative New Products |
Our strategy is to broaden the categories in which we participate and our share within those categories through ongoing product innovation. As an example, we followed our successful launch in 2005 of an artificial tears product called Clear Eyes for Dry Eyes with another innovative product called Clear Eyes Triple Action Relief, formulated to remove redness, moisturize and relieve irritation in 2006, with yet another product, Clear Eyes Maximum Redness Relief in late 2007. These successful product introductions were the primary drivers of the brand’s continued growth. While there is always a risk that sales of existing products may be reduced by new product introductions, our goal is to grow the overall sales of our brands.
| · | Increasing Distribution Across Multiple Channels |
Our broad distribution base ensures that our products are well positioned across all available channels and that we are able to participate in changing consumer retail trends. In 2005, we expanded our sales in wholesale club stores, introducing customized packaging and sizes of our products designed specifically for this higher growth channel. Comet grew approximately 18% in this channel during 2006. There is a risk however, that we may not be able to maintain or enhance our relationships across distribution channels, which could adversely impact our sales, business, financial condition and results from operations.
| · | Growing Our International Business |
We intend to increase our focus on growing our international business. International sales outside of North America represented approximately 4.6% of revenues in 2007 and approximately 3.4% of our revenues in 2006. We have designed and developed both product and packaging for specific international markets and expect our international revenues to continue to grow as a percentage of total revenues. In addition to Clear Eyes, Murine and Chloraseptic which are currently sold internationally, we license The Procter & Gamble Company to market the Comet brand in Eastern Europe. Since a number of our other brands have previously been sold internationally, we intend to expand the number of brands sold through our existing international distribution network and are actively seeking additional distribution partners for further expansion into other international markets. There is a risk, however, that increasing our focus on international growth may divert attention and resources from implementing our domestic business strategy. There are additional risks associated with the increase of our international business, such as changes in regulatory requirements and currency exchange controls. See “Risk Factors” in Item 1A of this Annual Report on Form 10-K.
| · | Pursuing Strategic Acquisitions |
We have an active corporate development program and intend to continue to investigate strategic add-on acquisitions that enhance our product portfolio. Our management team has a long track record of successfully identifying, acquiring and integrating new brands and we will continue to investigate the acquisition of highly complementary, recognized brands in attractive categories and channels. For example, during 2007 we purchased the Wartner brand of over-the-counter wart treatment products to augment our ownership of Compound W, the number two selling brand in the wart treatment category. Additionally, during 2006, we purchased the Chore Boy brand, which competes in the scrubber and sponge sector of the household cleaning segment, and The Doctor’s brand, which competes in the dental accessories sector of the oral health category, where we previously had a limited presence. While we believe that there will continue to be a strong pipeline of acquisition candidates for us to investigate, strategic fit and relative cost are of the utmost importance in our decision to pursue such opportunities. We believe our business model will allow us to integrate these future acquisitions in an efficient manner, while also providing opportunities to realize significant cost savings. However, there is a risk
-4-
that our operating results could be adversely affected in the event we do not realize all of the anticipated operating synergies and cost savings from any future acquisitions, we do not successfully integrate such acquisitions or we pay too much for these acquisitions. Provisions in our senior credit facility and the indenture governing our senior subordinated notes may limit our ability to engage in strategic acquisitions as well.
Market Position
During 2007, approximately 77% of our net sales were from brands with a number one or number two market position, while during 2006, approximately 74% of our net sales were from brands with a number one or number two market position. Such brands include Chloraseptic, Clear Eyes, Chore Boy, Comet, Compound W, Cutex, Dermoplast (number two in the Pain Relief Spray category in 2006), The Doctor’s and New-Skin.
See the “Business” section on page 1 of this document for information regarding market share and ACV calculations.
Our History and Accomplishments
The Company, through its predecessors-in-interest, was originally formed in 1996, as a joint venture of Medtech Labs and The Shansby Group, to acquire over-the-counter drug brands from American Home Products. Since 2001, our Company’s portfolio of brand name products has expanded from over-the-counter drugs to include household cleaning and personal care products. We have added brands to our portfolio principally by acquiring strong and well-recognized brands from larger consumer products and pharmaceutical companies. In February 2004, GTCR Golder Rauner II, LLC (“GTCR”), a private equity firm, acquired our business from the owners of Medtech Labs and The Shansby Group. In addition, we acquired the Spic & Span business in March 2004.
In April 2004, we acquired Bonita Bay Holdings, Inc., the parent holding company of Prestige Brands International, Inc., which conducted its business under the “Prestige” name. After we completed the Bonita Bay acquisition, we began to conduct our business under the “Prestige” name as well. The Bonita Bay brand portfolio included Chloraseptic, Comet, Clear Eyes and Murine.
In October 2004, we acquired the rights to the Little Remedies brands through our purchase of Vetco, Inc. Vetco is engaged in the development, distribution and marketing of pediatric over-the-counter healthcare products, primarily marketed under the Little Remedies brand name. Vetco’s products include Little Noses® nasal products, Little Tummys® digestive health products, Little Colds® cough/cold remedies and Little Remedies New Parents Survival Kits. The Little Remedies products deliver relief from common childhood ailments without unnecessary additives such as saccharin, alcohol, artificial flavors, coloring dyes or harmful preservatives.
In February 2005, we raised $448.0 million through an initial public offering of 28.0 million shares of common stock. We used the net proceeds of the offering, which were $416.8 million, plus $3.0 million from our revolving credit facility and $8.8 million of cash on hand to (i) repay $100.0 million of our existing senior indebtedness, (ii) to redeem $84.0 million in aggregate principal amount of our existing 9 1/4% senior subordinated notes, (iii) to repurchase an aggregate of 4.7 million shares of our common stock held by the investment funds affiliated with GTCR and TCW/Crescent Mezzanine, LLC (“TWC/Crescent”) for $30.2 million, and (iv) to contribute $199.8 million to our subsidiary, Prestige International Holdings, LLC, which was used to redeem all of its outstanding senior preferred units and class B preferred units.
In October 2005, we acquired the rights to the “Chore Boy” brand of metal cleaning pads, scrubbing sponges, and non-metal soap pads. The brand has over 84 years of history in the scouring pad and cleaning accessories categories.
In November 2005, we acquired Dental Concepts LLC (“Dental Concepts”), a marketer of therapeutic oral care products sold under “The Doctor’s” brand. The business is driven primarily by two niche segments, bruxism
-5-
(nighttime teeth grinding) and interdental cleaning. The Doctor’s NightGuard brand was the first FDA-approved OTC treatment for bruxism and The Doctor’s BrushPicks™ are disposable interdental toothpicks.
In September 2006, we completed the acquisition of Wartner USA B.V. (“Wartner”), the owner of the Wartner brand of over-the-counter wart treatment products. The Company expects that the Wartner brand, which is the number three brand in the United States over-the-counter wart treatment category, will enhance the Company’s leadership position in the category.
During the second half of 2007, we did not consummate any corporate or brand acquisitions. However, in accordance with our strategic plan, we repaid $26.4 million of our senior debt with free cash flow generated from operations. This serves to reduce our interest costs on a going-forward basis, as well as to favorably impact our interest coverage and our debt-to-equity ratios.
Products
We conduct our operations through three principal business segments: (i) over-the-counter healthcare, (ii) household cleaning and (iii) personal care.
Over-the-Counter Healthcare Segment
Our portfolio of over-the-counter healthcare products consists primarily of Clear Eyes, Murine, Chloraseptic, Compound W, Wartner, the Little Remedies line of pediatric healthcare products, The Doctor’s brand of oral care products and first aid products such as New-Skin and Dermoplast. Our other brands in this category include Percogesic®, Momentum®, Freezone®, Mosco®, Outgro®, Sleep-Eze®, Compoz® and Heet®. In 2007, the over-the-counter healthcare segment accounted for 54.8% of our revenues, while in 2006, the over-the-counter healthcare segment accounted for 54.3% of our revenues.
Clear Eyes and Murine
The Clear Eyes and Murine brands were purchased by Bonita Bay Holdings from Abbott Laboratories in December 2002. Since its introduction in 1968, the Clear Eyes brand has been marketed as an effective eye care product that helps take redness away and helps moisturize the eye. Clear Eyes has an ACV of 87%. In February 2007, we introduced Clear Eyes Maximum Redness Relief, while in February 2006, we introduced Clear Eyes Triple Action Relief, and in March 2006 the Clear Eyes for Dry Eyes line was expanded with a new seasonal relief product, Clear Eyes plus ACR Relief. The Murine brand is over 100 years old and its products consist of lubricating, soothing eye drops and ear wax removal aids. The brand was expanded into redness relief in March 2006 with the introduction of Murine for Redness Relief. Clear Eyes and Murine Eye Care are leading brands in the over-the-counter personal eye care category. The 0.5 oz. size of Clear Eyes redness relief eye drops is the number two selling product in the eye redness relief category and Clear Eyes is the number two brand in that category with 16.0 % market share.
Murine Ear Care is the third leading brand in the over-the-counter ear care category with a market share of 13.4%. The ear drop category is composed of products that loosen earwax, treat trapped water (swimmer’s ear) and treat ear aches. In 2008, we look to expand our market share in the ear care category with the introduction of Murine Earigate, Ear Cleaning System, a natural and hypoallergenic wax removal system with a patented “reverse spray action” that safely rinses away ear wax build-up with out harming the user’s sensitive eardrums.
Chloraseptic
Chloraseptic was acquired by Bonita Bay Holdings in March 2000 from Procter & Gamble and was originally developed by a dentist in 1957 to relieve sore throats and mouth pain. Chloraseptic’s 6 oz. cherry liquid sore throat spray is the number one selling product in the sore throat liquids/sprays segment. The Chloraseptic brand has an ACV of 95% and is number one in sore throat liquids/sprays with a 44.9% market share.
Historically, Chloraseptic products were limited to sore throat lozenges and traditional sore throat sprays that were stored and used at home. Since its acquisition, the Chloraseptic product line has been expanded to include portable sprays, gargle, mouth pain sprays and relief strips. In 2007, we introduced our first dual-action product, Chloraseptic Sore Throat plus Cough Lozenges, and our relief strip product, originally introduced in 2003, will be
-6-
reintroduced in June 2007 with a new formulation and new packaging. These product introductions enable us to market Chloraseptic products as a system, encourage consumers to buy multiple types of Chloraseptic products, and increase volume for the entire product line.
Compound W
We acquired Compound W from American Home Products in 1996. The Compound W brand has a long heritage; its wart removal products having been introduced almost 50 years ago. Compound W products are specially designed to provide relief from common and plantar warts and are sold in multiple forms of treatment depending on the consumer’s need, including Fast-Acting Liquid, Fast-Acting Gel, One Step Pads for Kids, One Step Pads for Adults and Freeze Off®. We believe that Compound W is one of the most trusted names in wart removal.
Compound W is the number two wart removal brand in the United States with a 32.1% market share and an ACV of 85%. Since Compound W’s acquisition, we have successfully expanded the wart remover category and enhanced the value associated with the Compound W brand by introducing several new products, such as Compound W Freeze Off, Fast Acting Liquid, One Step Pads for Kids, Waterproof One Step Pads and Invisible Strips Pads. Compound W Freeze Off, a cryogenic wart removal product, has achieved high trade acceptance, as it allows consumers to use a wart freezing treatment similar to that used by doctors.
Wartner
Wartner is the number three brand in the wart removal category with a 12.1% share of the cryogenic segment and an ACV rating of 67%. Launched in 2003, Wartner is recognized by consumers and the trade as the first ever over-the-counter wart freezing (cryogenic therapy) treatment in the U. S and Canada. The brand was acquired from Lil’ Drug Store Products, Inc. in September 2006.
The Doctor’s
The Doctor’s is a line of products designed to help consumers who are highly engaged in oral care wellness to maintain good oral hygiene in between dental office visits. The product line was purchased in November 2005 with the acquisition of Dental Concepts. The business is driven primarily by two niche segments, bruxism (nighttime teeth grinding) and interdental cleaning. The Doctor’s NightGuard brand was the first FDA-approved OTC treatment for bruxism and The Doctor’s BrushPicks are disposable interdental toothpicks. The Doctor’s OraPik is a permanent, interdental pick and mirror. The entire line is supported by national advertising, is distributed in leading food, drug and mass merchandiser retailers and continues to experience sales growth in excess of the dental accessories category.
Little Remedies
Little Remedies markets a full line of pediatric over-the-counter products that contain no alcohol, saccharin, artificial flavors or coloring dyes including: (i) Little Noses, a product line consisting of saline nasal spray/drops, decongestant nose drops, a nasal aspirator for the removal of mucous from nasal passages and moisturizing nasal gel, (ii) Little Colds, a product line consisting of a multi-symptom cold relief formula, sore throat relief Saf-T-Pops®, a cough relief formula, and a combined decongestant plus cough relief formula, and (iii) Little Tummys, a product line consisting of gas relief drops, laxative drops, a nausea relief aid, as well as the recently introduced gripe water, an herbal supplement used to ease discomfort often associated with colic and hiccups.
New-Skin
The brand has a long heritage, with the core product believed by management to be over 100 years old. New-Skin products consist of liquid bandages for small cuts and scrapes that are designed to replace traditional bandages in an effective and easy to use form. Each New-Skin product works by forming a thin, clear, protective covering after it is applied to the skin. New-Skin competes in the liquid bandage segment of the first aid bandage category where it has a 37.1% market share and an 80% ACV.
Dermoplast
We acquired Dermoplast from American Home Products in 1996. Dermoplast is an aerosol spray anesthetic for minor topical pain that was traditionally a “hospital-only” brand dispensed to mothers after giving birth. The primary use in hospitals is for post episiotomy pain, post-partum hemorrhoid pain, and for the relief of female genital itching.
-7-
Since Dermoplast’s acquisition, we have introduced retail versions of the product, a move that has approximately doubled the size of the business. Dermoplast enjoys significant distribution across the drug and mass merchandise channels, with an ACV of 62%. In addition to the traditional hospital uses mentioned above, Dermoplast offers sanitary, convenient first aid relief for pain and itching from minor skin irritations, including sunburn, insect bites, minor cuts, scrapes and burns. Dermoplast is currently offered in two formulas: regular strength and antibacterial strength. In February 2006, we introduced Dermoplast Poison Ivy Treatment as the only poison ivy wash that also contains over-the-counter medicine.
Household Cleaning Segment
Our portfolio of household cleaning brands includes the Comet, Chore Boy and Spic and Span brands. In 2007, the household cleaning segment accounted for 37.4 % of our revenues, while in 2006, the household cleaning segment accounted for 36.3% of our revenues.
Comet
Bonita Bay Holdings acquired Comet from Procter & Gamble in October 2001. Comet was originally introduced in 1956 and is one of the most widely recognized household cleaning brands, with an ACV of 99%. Comet products include different varieties of cleaning powders, sprays and cream, some of which are abrasive and some of which are non-abrasive. Comet competes in the abrasive and non-abrasive tub and tile cleaner sub-category of the household cleaning category that includes abrasive powders and liquids and non-abrasive sprays. The non-abrasive tub and tile cleaner segment is more fragmented and competitive than the abrasive sector and we have been attempting to build momentum in our efforts to increase Comet’s market share in the non-abrasive tub and tile cleaner sector through focused advertising and promotions, including free-standing insert coupons and television advertising.
Since the Comet acquisition, we have expanded the brand’s distribution, increased advertising and promotion and implemented focused marketing initiatives. During 2007, we introduced Comet Spray Gel, a unique mildew stain remover spray product that offers increased cleaning power due to its high viscosity. Previously, we introduced new fragrances, including Comet Lavender Powder Abrasive Cleanser and Comet Orange. We have also extended the brand into underdeveloped demographic targets, and employed new inverted bottle packaging for Comet Soft Cleanser Cream, which improves ease of use. Additionally, multi-packs have been introduced in the warehouse club trade class and an 8oz. Soft Cleanser Cream has been introduced into the dollar store channel extending the brand’s distribution and increasing usage.
Chore Boy
The Chore Boy brand of scrubbing pads and sponges was initially launched in the 1920's. Over the years the line has grown to include metal and non-metal scrubbers that are used for a variety of household cleaning tasks. While many of the brand’s products find use in the kitchen, with cooking clean up in particular, they are also used in clean up jobs in the home work shop, garage, and other areas, including outdoor grill cleaning. The newest additions to the line, launched in 2004, consist of patented mesh materials that clean most surfaces without scratching. Chore Boy products currently are sold in food stores, by mass merchandisers, and in hardware and convenience stores. We acquired the Chore Boy brand in October 2005.
Spic and Span
Spic and Span was introduced in 1925 and is marketed as the complete home cleaner with two product lines consisting of (i) dilutables and (ii) hard surface sprays for counter tops and glass. Each of these products can be used for multi-room and multi-surface cleaning. Since January 2001, the product line has grown from eight to 33 separate items and we have expanded distribution into new channels such as dollar stores.
Personal Care Segment
Our major personal care brands include Denorex dandruff shampoo, Cutex nail products and Prell® shampoo. Other portfolio brands in this segment include EZO® denture cushion, Oxipor VHC® skin-care lotion, Cloverine® skin salve, Zincon® shampoo and Kerodex® barrier cream. While the personal care segment has
-8-
been deemphasized, it accounted for 7.8% of our revenues in 2007 and 9.4% of our revenues in 2006.
Denorex
We acquired Denorex in connection with the Medtech acquisition in February 2004. The Denorex brand was originally launched in 1971 as an effective solution to scalp problems. Denorex competes in the therapeutic segment of the dandruff shampoo category and holds a 5.5% market share. The current lineup of Denorex products includes Daily, for moderate dandruff sufferers and for those with more serious dandruff conditions, Extra Strength, Extra Strength with Conditioner, Therapeutic Strength and Therapeutic Strength with Conditioner.
Cutex
Cutex is an established and trusted brand of nail polish remover. Cutex, with an ACV rating of 93%, has four product lines: (i) Quick and Gentle Liquid Nail Polish Remover, (ii) Cutex Essential Care® Advanced Liquid, also available with a new Pump Action Bottle, (iii) Essential Care Advanced Nail Polish Remover Pads and (iv) Essential Care Twister Nail Polish Remover. Cutex is the number one brand in the nail polish remover category and has a leading 27.4% market share. The main competition comes from a number of private label brands, which collectively have a 54.0% market share.
Prell
Bonita Bay Holdings acquired Prell from Procter & Gamble in November 1999. Prell, which competes in the shampoo category, was launched in 1947 and is a highly recognized shampoo brand. While the shampoo category is fragmented and populated by hundreds of brands, placing a premium on distribution, brand recognition and positioning, we believe Prell has a loyal base of consumers seeking shampoo at the mid-price point segment.
For financial information concerning our business segments, please refer to Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operation and Note 17 to the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Marketing and Sales
Our marketing strategy is based upon the acquisition and the rejuvenation of established consumer brands that possess what we believe to be significant brand value and unrealized potential. Our marketing objective is to increase sales and market share by developing and executing professionally designed, creative and cost-effective advertising and promotional programs. After we acquire a brand, we implement a brand building strategy that uses the brand’s existing consumer awareness to maximize sales of current products and provides for brand growth through product innovation. This brand building process involves the evaluation and enhancement of the existing brand name, the development and introduction of innovative new products and the professional execution of support programs. To ensure consistent growth, all new product concepts are thoroughly researched before launch and supported by an integrated trade, consumer and advertising effort, although advertising is used selectively. Recognizing that financial resources are limited, we allocate our resources to focus on those brands that we believe have the greatest opportunities for growth and financial success. Brand priorities vary from year-to-year and generally revolve around new product introductions.
Customers
Our senior management team and dedicated sales force strive to maintain long-standing relationships with our top 50 domestic customers, which accounted for approximately 77.1% of our combined gross sales for 2007 and 77.9% for 2006. Our sales management team consists of ten people, who focus on our key customer relationships. We also contract with third-party sales management organizations that interface directly with our remaining customers and report directly to members of our sales management team.
We enjoy broad distribution across each of the major retail channels, including mass merchandisers, drug, food, dollar and club stores. The following table sets forth the percentage of gross sales to our top 50 customers across our five major distribution channels during the most recent three-year period:
-9-
Percentage of Gross Sales to Top 50 Customers (1) | ||||||
Channel of Distribution | 2007 | 2006 | 2005 | |||
Mass | 40.1% | 39.1% | 39.1% | |||
Food | 20.4 | 22.4 | 23.0 | |||
Drug | 25.8 | 23.1 | 23.9 | |||
Dollar | 8.1 | 9.6 | 9.4 | |||
Club | 2.6 | 3.3 | 2.8 | |||
Other | 2.9 | 2.5 | 1.8 | |||
(1) Includes estimates for some of our wholesale customers that service more than one distribution channel.
Due to the diversity of our product line, we believe that each of these channels is important to our business and we continue to seek opportunities for growth in each channel.
Our principal customer relationships include Wal-Mart, Walgreens, CVS, Target and Dollar General. For 2007, our top five and ten customers accounted for approximately 43% and 53%, respectively, of our gross sales, while in 2006, our top five and ten customers accounted for approximately 41% and 51%, respectively, of our gross sales. No single customer other than Wal-Mart accounted for more than 10% of our gross sales in either of the two most recent fiscal years and none of our other top five customers accounted for less than 3% of our gross sales in either of the two most recent fiscal years. Our top fifteen customers each purchase products from virtually all of our major product lines.
Our strong customer relationships and product recognition provide us with a number of important benefits including minimizing slotting fees, facilitating new product introductions, ensuring prominent shelf space and shortening payment time after invoicing. We believe that management’s emphasis on strong customer relationships, speed and flexibility, leading sales technology capabilities, including electronic data interchange, e-mail, the Internet, integrated retail coverage, consistent marketing support programs and ongoing product innovation will continue to maximize our competitiveness in the increasingly complex retail environment.
The following table sets forth a list of our primary distribution channels and our principal customers for each channel:
Distribution Channel | Customers | Distribution Channel | Customers | |||
Mass | Kmart | Drug | CVS | |||
| Meijer | Rite Aid | |||||
| Target | Walgreens | |||||
| Wal-Mart | ||||||
Dollar | Dollar General | |||||
Food | Ahold | Family Dollar | ||||
| Kroger | Dollar Tree | |||||
| Publix | ||||||
| Safeway | Club | Costco | ||||
| Supervalu | Sam’s Club | |||||
| BJ’s Wholesale Club | ||||||
Outsourcing and Manufacturing
In order to maximize our competitiveness and efficiently allocate our resources, third-party manufacturers fulfill all of our manufacturing needs. We have found that contract manufacturing maximizes our flexibility and responsiveness to industry and consumer trends while minimizing the need for capital expenditures. We select contract manufacturers based on their core competencies and our perception of the best overall value, including
-10-
factors such as (i) depth of services, (ii) the management team, (iii) manufacturing flexibility, (iv) regulatory compliance and (v) competitive pricing. We also conduct thorough reviews of each potential manufacturer’s facilities, quality standards, capacity and financial stability. We generally only purchase finished products from our manufacturers.
Our primary contract manufacturers provide comprehensive services from product development through the manufacturing of finished goods. They are responsible for such matters as (i) production planning, (ii) product research and development, (iii) procurement, (iv) production, (v) quality testing, and (vi) almost all capital expenditures. In most instances, we provide our contract manufacturers with guidance in the form of (i) product development, (ii) performance criteria, (iii) regulatory guidance, (iv) sourcing of packaging materials and (v) monthly master production schedules. This management approach results in minimal capital expenditures and maximizes our cash flow, which is reinvested to support our marketing initiatives or used for brand acquisitions and/or to repay outstanding indebtedness.
We have relationships with over 40 third-party manufacturers. Of those, our top 10 manufacturers produce items that accounted for 78% of our sales for 2007. We do not have long-term contracts with the manufacturers of products that account for approximately 35% of our sales in 2007. The lack of manufacturing agreements for these products exposes us to the risk that the manufacturer could stop producing our products at any time, for any reason or fail to provide us with the level of products we need to meet our customers’ demands. Should one or more of our manufacturers stop producing product on our behalf, it could have a material adverse effect on our business, financial condition and results from operations.
At March 31, 2007, our largest suppliers of manufactured goods included (i) Vijon Laboratories, (ii) Abbott Laboratories, (iii) Kolmar Canada, (iv) Procter & Gamble, (v) OraSure Technologies and (vi) Humco Holdings. We enter into manufacturing agreements for a majority of our products by sales volume, each of which vary based on the third-party manufacturer and the products being supplied. These agreements explicitly outline the manufacturer’s obligations and product specifications with respect to the brand or brands being produced. The prices for purchase of products under these agreements are subject to change pursuant to the terms of these agreements due to fluctuations in raw material, packaging and labor costs. All of our other products are manufactured on a purchase order basis which are generally based on batch sizes and result in no long-term obligations or commitments.
Warehousing and Distribution
We receive orders from retailers and/or brokers primarily by electronic data interchange, which automatically enters each order into our computer systems and then routes the order to our distribution center. The distribution center will, in turn, send a confirmation that the order was received, fill the order and ship the order to the customer, while sending a shipment confirmation to us. Upon receipt of the confirmation, we send an invoice to the customer.
We manage product distribution in the mainland United States through one facility located in St. Louis, owned and operated by Jacobson / Arthur Wells, Inc. Jacobson / Arthur Wells handles all finished goods storage, as well as the receipt and disposition of customer returns through their Warehousing Specialists subsidiary (“WSI”), and all customer shipments through their Nationwide Logistics subsidiary (“NLI”).
The Storage and Handling Agreement provides that, for the three-year period beginning June 2005, WSI shall provide warehouse services, including without limitation, storage, handling and shipping with respect to our full line of products. The Transportation Management Agreement provides that, for the three-year period beginning August 2005, NLI shall provide (i) complete management services, (ii) claims administration, (iii) proof of delivery, (iv) procurement, (v) report generation, and (vi) automation and tariff compliance services with respect to our full line of products.
If WSI or NLI abruptly stopped providing storage or logistics services to us, our business operations could suffer a temporary disruption while new service providers are engaged. We believe this process could be completed quickly and any temporary disruption resulting therefrom would have an insignificant effect on our operating results and financial condition. However, a serious disruption, such as a flood or fire, to our distribution center
-11-
could damage our inventory and could materially impair our ability to distribute our products to customers in a timely manner or at a reasonable cost. We could incur significantly higher costs and experience longer lead times associated with the distribution of our products to our customers during the time required to reopen or replace our distribution center. As a result, any such serious or prolonged disruption could have a material adverse effect on our business, financial condition and results from operations.
Competition
The business of selling brand name consumer products in the over-the-counter healthcare, household cleaning and personal care categories is highly competitive. These markets include numerous manufacturers, distributors, marketers and retailers that actively compete for consumers’ business both in the United States and abroad. Many of these competitors are larger and have substantially greater resources than we do, and may therefore have the ability to spend more aggressively on advertising and marketing and to respond more effectively to changing business and economic conditions. If this were to occur, our sales, operating results and profitability would be adversely affected.
Our principal competitors vary by industry category. Competitors in the over-the counter healthcare category include Johnson & Johnson, maker of Visine®, which competes with our Clear Eyes and Murine brands; McNeill-PPC, maker of Tylenol® Sore Throat, and Procter & Gamble, maker of Vicks®, each of which compete with our Chloraseptic brand; Schering-Plough, maker of Dr. Scholl’s®, which competes with our Compound W and Wartner brands; Johnson & Johnson, maker of BAND-AID® Brand Liquid Bandage, which competes with our New-Skin brand; GlaxoSmithKline, maker of Debrox®, which competes with our Murine brand; Sunstar America, Inc., maker of GUM® line of oral care products; as well as DenTek® Oral Care, Inc., Power Products, Inc. and Ranir LLC, each of which markets a dental protector for nighttime teeth grinding, and competes with The Doctor’s brand.
Competitors in the household cleaning category include Henkel, maker of Soft Scrub®, and Clorox, maker of Tilex®, each of which competes with our Comet brand, Clorox’s Pine Sol®, which competes with our Spic and Span brand and 3M, maker of Scotch-Brite® and O-Cel-O®, which compete with our Chore Boy brand.
Competitors in the personal care category include Johnson & Johnson, maker of T-Gel® shampoo, and Chattem, maker of Selsun Blue®, which compete with our Denorex brand, as well as Del Laboratories, maker of Sally Hansen®, which competes with our Cutex brand.
We compete on the basis of numerous factors, including brand recognition, product quality, performance, price and product availability at the retail level. Advertising, promotion, merchandising and packaging, the timing of new product introductions and line extensions also have a significant impact on customers’ buying decisions and, as a result, on our sales. The structure and quality of our sales force, as well as sell-through of our products, affects in-store position, wall display space and inventory levels in retail outlets. If we are unable to maintain the inventory levels and in-store positioning of our products in retail stores, our sales and operating results will be adversely affected. Our markets also are highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. An increase in the amount of product introductions by our competitors could have a material adverse effect on our business, financial condition and results from operations.
Regulation
Product Regulation
The formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of our products are subject to extensive regulation by various federal agencies, including the Food and Drug Administration (“FDA”), the Federal Trade Commission (“FTC”), the Consumer Product Safety Commission (“CPSC”), the Environmental Protection Agency (“EPA”), and by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed and sold. Regulatory matters are overseen by a team with appropriate legal and regulatory experience. Our Regulatory and Operations teams work closely with our third-party manufacturers on quality related matters while we monitor their compliance with FDA regulations and perform periodic audits to ensure such compliance. Our management intends to continue this procedure across all of our brands. This continual evaluation process ensures that our manufacturing processes and products are of the
-12-
highest quality and in compliance with all known regulatory requirements. When and if the FDA chooses to audit a particular manufacturing facility, we are notified immediately and updated on the progress of the audit as it proceeds. If we or our manufacturers fail to comply with applicable regulations, we could become subject to significant claims or penalties, which could have a material adverse effect our business, financial condition and results from operations. In addition, the adoption of new regulations or changes in the interpretations of existing regulations may result in significant additional compliance costs or discontinuation of product sales and may also have a material adverse effect on our business, financial condition and results from operations.
All of our over-the-counter drug products are regulated pursuant to the FDA’s monograph system. The monographs, both tentative and final, set out the active ingredients and labeling indications that are permitted for certain broad categories of over-the-counter drug products. When the FDA has finalized a particular monograph, it has concluded that a properly labeled product formulation is generally recognized as safe and effective and not misbranded. A tentative final monograph indicates that the FDA has not made a final determination about products in a category to establish safety and efficacy for a product and its uses. However, unless there is a serious safety or efficacy issue, the FDA will typically exercise enforcement discretion and permit companies to sell products conforming to a tentative final monograph until the final monograph is published. Products that comply with either final or tentative final monograph standards do not require pre-market approval from the FDA.
The Company’s over-the-counter device products are regulated by FDA through a system which usually involves pre-market clearance of new device products. During the review process, the FDA makes an affirmative determination as to the sufficiency of the label directions, cautions and warnings for the devices in question.
In accordance with the Federal Food, Drug and Cosmetic Act (“FDC Act”) and FDA regulations, the manufacturing processes of our third-party drug and device manufacturers must also comply with the FDA’s current Good Manufacturing Processes (“cGMPs”). The FDA inspects our facilities and those of our third-party manufacturers periodically to determine if we and our third-party manufacturers are complying with cGMPs.
Other Regulations
We are also subject to a variety of other regulations in various foreign markets, including regulations pertaining to import/export regulations and antitrust issues. To the extent we decide to commence or expand operations in additional countries, we may be required to obtain an approval, license or certification from the country’s ministry of health or comparable agency. We must also comply with product labeling and packaging regulations that may vary from country-to-country. Government regulations in both our domestic and international markets can delay or prevent the introduction, or require the reformulation or withdrawal, of some of our products. Our failure to comply with these regulations can result in a product being removed from sale in a particular market, either temporarily or permanently. In addition, we are subject to FTC and state regulations, as well as foreign regulations, relating to our product claims and advertising. If we fail to comply with these regulations, we could be subject to enforcement actions and the imposition of penalties which could have a material adverse effect on our business, financial condition and results from operations.
Intellectual Property
We own a number of trademark registrations and applications in the United States, Canada and other foreign countries. The following are some of the most important registered trademarks we own in the United States: Chloraseptic, Chore Boy, Clear Eyes, Cinch, Cloverine, Comet, Compound W, Freeze Off, Compoz, Cutex, The Doctor’s, Denorex, Dermoplast, Essential Care, Freezone, Heet, Kerodex, Little Remedies, Longlast, Momentum, Mosco, Murine, New-Skin, Outgro, Oxipor, Percogesic, Prell, Simple Pad, Simplegel, Sleep-Eze, Spic and Span, Wartner, Vacuum Grip and Zincon. In addition, we have an exclusive royalty bearing license to use the EZO trademark in the United States for the ten year term ending on December 31, 2012, at which time we shall have the right to purchase the trademark for $1,000. While we own the U.S. trademark registration for Kerodex, we have an obligation to pay royalties to Unilever/Scientific with respect to the manufacture and sale of barrier creams sold in the United States under the Kerodex trademark. This royalty obligation, at 1% of Kerodex sales, will continue as long as we make, use or sell products utilizing the Kerodex trademark in the United States.
Our trademarks and trade names are how we convey that the products we sell are “brand name” products. Our ownership of these trademarks and trade names allows us to compete based on the value associated with them.
-13-
Enforcing our proprietary rights in these trademarks and trade names is expensive and if we are not able to effectively enforce our rights, others may be able to dilute our trademarks and trade names and diminish the value associated with our brands, which could have a material adverse effect on our business, financial condition and results from operations.
Other intellectual property rights were acquired from Procter & Gamble and Abbott Laboratories when we acquired the trademarks related to the Comet, Chloraseptic, Clear Eyes, Murine and Prell product lines; however, we did not in all cases obtain title to all of the intellectual property used to manufacture and sell those products. Therefore, we are dependent upon Procter & Gamble, Abbott Laboratories and other third parties for intellectual property used in the manufacture and sale of certain of our products.
We have granted MF Distributions, Inc. an exclusive license (with an option to purchase) to sell Spic and Span and Cinch products in Canada for a royalty. In 2003, we assigned our Italian trademark applications and registrations for Spic and Span and Cinch to Conter, S.p.A., and entered into a concurrent use agreement with Conter with respect to such marks. Conter is also a licensee of the Spic and Span trademark in Benelux, Portugal, Romania and Malta.
We have licensed to Procter & Gamble the right to use the Comet, Spic and Span and Chlorinol® trademarks in the commercial/institutional/industrial segment in the United States and Canada until 2019. We have also licensed to Procter & Gamble the Comet and Chlorinol brands in Russia and specified Eastern European countries until 2015.
Seasonality
The first quarter of our fiscal year typically has the lowest level of revenue due to the seasonal nature of certain of our brands relative to the summer and winter months. In addition, the first quarter is the least profitable quarter due the increased advertising and promotional spending to support those brands with a summer selling season, such as Compound W, Wartner and New-Skin. The Company’s advertising and promotional campaigns in the third quarter influence sales in the fourth quarter winter months. Additionally, the fourth quarter typically has the lowest level of advertising and promotional spending as a percent of revenue.
Employees
We employed 92 individuals as of March 31, 2007. None of our employees are party to collective bargaining agreements. Management believes that its relations with its employees are good.
Backlog Orders
The Company had no backlog orders at March 31, 2006 or 2007.
Available Information
Our Internet address is www.prestigebrandsinc.com. We make available free of charge on or through our Internet website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports, and the Proxy Statement for our annual stockholders’ meetings, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (the “SEC”). The information found on our website shall not be deemed incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act of 1933, as amended (the “Securities Act”), or under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not otherwise be deemed filed under such Acts. Information on our Internet website does not constitute a part of this Annual Report on Form 10-K and is not incorporated herein by reference.
We have adopted a Code of Conduct Policy, Code of Ethics for Senior Financial Employees, Complaint Procedures for Accounting and Auditing Matters, Corporate Governance Guidelines, Audit Committee Pre-Approval Policy, and Charters for our Audit, Compensation, Nominating and Governance, and Strategic Planning Committees. We will provide to any person without charge, upon request, a copy of the foregoing materials. Any
-14-
requests for the foregoing documents from us should be made in writing to:
Prestige Brands Holdings, Inc.
90 North Broadway
Irvington, New York 10533
Attention: Secretary
We intend to disclose future amendments to the provisions of the foregoing documents, policies and guidelines and waivers therefrom, if any, on our Internet website and/or through the filing of a Current Report on Form 8-K with the SEC to the extent required under the Exchange Act.
-15-
ITEM 1A. RISK FACTORS
The high level of competition in our industry, much of which comes from competitors with greater resources, could adversely affect our business, financial condition and results from operations.
The business of selling brand name consumer products in the over-the-counter healthcare, household cleaning and personal care categories is highly competitive. These markets include numerous manufacturers, distributors, marketers and retailers that actively compete for consumers’ business both in the United States and abroad. Many of these competitors are larger and have substantially greater resources than we do, and may therefore have the ability to spend more aggressively on research and development, advertising and marketing, and to respond more effectively to changing business and economic conditions. If this were to occur, it could have a material adverse effect on our business, financial condition and results from operations.
Our principal competitors vary by industry category. Competitors in the over-the counter healthcare category include Johnson & Johnson, maker of Visine®, which competes with our Clear Eyes and Murine brands; McNeill-PPC, maker of Tylenol® Sore Throat and Procter and Gamble, maker of Vicks®, each of which compete with our Chloraseptic brand; Schering-Plough, maker of Dr. Scholl’s®, which competes with our Compound W and Wartner brands; Johnson & Johnson, maker of BAND-AID® Brand Liquid Bandage, which competes with our New-Skin brand; GlaxoSmithKline, maker of Debrox®, which competes with our Murine brand; Sunstar America, Inc., maker of GUM® line of oral care products; as well as DenTek® Oral Care, Inc., Power Products, Inc. and Ranir LLC, each of which markets a dental protector for nighttime teeth grinding, and competes with The Doctor’s brand.
Competitors in the household cleaning category include Henkel, maker of Soft Scrub®, and Clorox, maker of Tilex®, each of which competes with our Comet brand. In addition, Clorox’s Pine Sol® competes with our Spic and Span brand and 3M, maker of Scotch-Brite® and O-Cel-O®, compete with our Chore Boy brand.
Competitors in the personal care category include Johnson & Johnson, maker of T-Gel® shampoo, and Chattem, maker of Selsun Blue® which compete with our Denorex brand, as well as Del Laboratories, maker of Sally Hansen®, which competes with our Cutex brand.
Certain of our product lines that account for a large percentage of our sales have a small market share relative to our competitors. For example, while Clear Eyes has a number two market share position of 16.0% within the allergy/redness eye drop segment, its top competitor, Visine®, has a market share of 41.4%. In contrast, certain of our brands with number two market positions have a similar market share relative to our competitors. For example, Compound W has a number two market position of 32.1% and its top competitor, Dr. Scholl’s Clear Away® and Freeze Away®, have a market position of 43.3%. Also, while Cutex is the number one brand name nail polish remover with a market share of 27.4%, non-branded, private label nail polish removers account, in the aggregate, for 54.0% of the market. Finally, while our New-Skin liquid bandage product has a number one market position of 37.1%, the size of the liquid bandage market is relatively small, particularly when compared to the much larger bandage category. See “Business” section on page 1 of this document for information regarding market share calculations.
We compete for customers’ attention based on a number of factors, including brand recognition, product quality, performance, price and product availability at the retail level. Advertising, promotion, merchandising and packaging, the timing of new product introductions and line extensions also have a significant impact on consumer buying decisions and, as a result, on our sales. The structure and quality of our sales force, as well as sell-through of our products affects in-store position, wall display space and inventory levels in retail stores. If we are unable to maintain the inventory levels and in-store positioning of our products in retail stores, our sales and operating results will be adversely affected. Our markets also are highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. An increase in the amount of product introductions by our competitors could have a material adverse effect on our business, financial condition and results from operations.
In addition, competitors may attempt to gain market share by offering products at prices at or below those typically offered by us. Competitive pricing may require us to reduce prices which may result in lost sales or a
-16-
reduction of our profit margins. Future price adjustments, product changes or new product introductions by our competitors or our inability to react with price adjustments, product changes or new product introductions of our own could result in a loss of market share which could have a material adverse effect on our business, financial condition and results from operations.
We depend on a limited number of customers for a large portion of our gross sales and the loss of one or more of these customers could reduce our gross sales and therefore, could have a material adverse effect on our business, financial condition and results of operations.
For 2007, our top five and ten customers accounted for approximately 43% and 53% of our sales, respectively, while during 2006, our top five and ten customers accounted for approximately 41% and 51% of our sales, respectively. Wal-Mart, which itself accounted for approximately 24.0% and 21.0% in 2007 and 2006, respectively, of our sales, is our only customer that accounted for 10% or more of our sales. We expect that for 2008 and future periods, our top five and ten customers, including Wal-Mart, will, in the aggregate, continue to account for a large portion of our sales. The loss of one or more of our top customers, any significant decrease in sales to these customers, or a significant decrease in our retail display space in any of these customers’ stores, could reduce our sales, and therefore, could have a material adverse effect on our business, financial condition and results from operations.
In addition, our business is based primarily upon individual sales orders. We typically do not enter into long-term contracts with our customers. Accordingly, our customers could cease buying products from us at any time and for any reason. The fact that we do not have long-term contracts with our customers means that we have no recourse in the event a customer no longer wants to purchase products from us. If a significant number of our smaller customers, or any of our significant customers, elect not to purchase products from us, our business, financial condition and results from operations could be adversely affected.
Our risk of doing business internationally increases as we expand our international footprint.
During 2007 and 2006, approximately 4.6% and 3.4%, respectively, of our total revenues were attributable to our international business. We operate in several regions and countries where we have little or no experience, and generally rely on brokers and distributors for the sale of our products. In addition to the risks associated with political instability, changes in the outlook for economic prosperity in these countries could adversely affect the sales of our products in these countries. Other risks of doing business internationally include:
| · | Changes in the legislative or regulatory requirements of the countries or regions where we do business, |
| · | Currency controls which restrict or prohibit the repatriation of earnings to the United States or fluctuations in foreign exchange rates resulting in unfavorable increases in the price of our products or cause increases in the cost of certain products purchased from our foreign third-party manufacturers, |
| · | Regulatory oversight and its impact on our ability to get products registered for sale in certain markets, |
| · | Potential trade restrictions and exchange controls, |
| · | Inability to protect our intellectual property rights in these markets, and |
| · | Increased costs of compliance with general business and tax regulations in these countries or regions. |
We depend on third-party manufacturers to produce the products we sell. If we are unable to maintain these manufacturing relationships or fail to enter into additional relationships, as necessary, we may be unable to meet customer demand and our sales and profitability could suffer as a result.
All of our products are produced by third-party manufacturers. Our ability to retain our current manufacturing relationships and engage in new relationships is critical to our ability to deliver quality products to our customers in a timely manner. Without adequate supplies of quality merchandise, sales would decrease materially and our business would suffer. In the event that our primary third-party manufacturers are unable or unwilling to ship products to us in a timely manner, we would have to rely on secondary manufacturing relationships or identify and qualify new manufacturing relationships. We might not be able to identify or qualify such manufacturers for existing or new products in a timely manner and such manufacturers may not allocate sufficient capacity to us in
-17-
order that we may meet our commitments to customers. In addition, identifying alternative manufacturers without adequate lead times can compromise required product validation and stability protocol, which may involve additional manufacturing expense, delay in production or product disadvantage in the marketplace. The consequences of not securing adequate and timely supplies of merchandise would negatively impact inventory levels, sales and gross margins, and could have a material adverse effect on our business, financial condition and results from operations.
In addition, even if our current manufacturers continue to manufacture our products, they may not maintain adequate quality controls, and therefore, may not be able to continue to produce products that are consistent with our standards or applicable regulatory requirements. If we are forced to rely on products of inferior quality, then our brand recognition and customer satisfaction would likely suffer, leading to a reduction in sales. This sales reduction could have a material adverse effect on our business, financial condition and results from operations. These manufacturers may also increase the cost of the products we purchase which could adversely affect our margins in the event we are unable to pass along these increased costs to our customers. A situation such as this could also have a material adverse effect on our business, financial condition and results from operations.
At March 31, 2007, we had relationships with over 40 third-party manufacturers. Of those, our top 10 manufacturers produced items that accounted for 78% of our sales for 2007. We do not have long-term contracts with the manufacturers of products that accounted for approximately 35% of our sales for 2007. The fact that we do not have long-term contracts with these manufacturers means that they could cease manufacturing these products at any time and for any reason, which could have a material adverse effect on our business, financial condition and results from operations.
Disruption in our main distribution center may prevent us from meeting customer demand and our sales and profitability may suffer as a result.
We manage our product distribution in the continental United States through a primary distribution center in St. Louis, Missouri. A serious disruption, such as a flood or fire, to our primary distribution center could damage our inventory and could materially impair our ability to distribute our products to customers in a timely manner or at a reasonable cost. We could incur significantly higher costs and experience longer lead times during the time required to reopen or replace our primary distribution center. As a result, any such serious disruption could have a material adverse effect on our business, financial condition and results from operations.
Achievement of our strategic objectives requires the acquisition, or potentially the disposition, of certain brands or product lines. Efforts to affect such acquisitions or dispositions may divert our managerial resources away from our business operations.
The majority of our growth has been driven by acquiring other brands and companies. At any given time, we may be engaged in discussions with respect to possible acquisitions that are intended to enhance our product portfolio, enable us to realize cost savings and further diversify our category, customer and channel focus. Our ability to successfully grow through acquisitions depends on our ability to identify, negotiate, complete and integrate suitable acquisition candidates and to obtain any necessary financing. These efforts could divert the attention of our management and key personnel from our business operations. If we complete acquisitions, we may also experience:
| · | Difficulties achieving, or an inability to achieve, our expected returns, |
| · | Difficulties in integrating any acquired companies, personnel and products into our existing business, |
| · | Delays in realizing the benefits of the acquired company or products, |
| · | Higher costs of integration than we anticipated, |
| · | Difficulties in retaining key employees of the acquired business who are necessary to manage the business, |
| · | Difficulties in maintaining uniform standards, controls, procedures and policies throughout our acquired companies, or |
-18-
| · | Adverse customer or shareholder reaction to the acquisition. |
In addition, an acquisition could adversely affect our operating results as a result of higher interest costs from the acquisition related debt and higher amortization expenses related to the acquired intangible assets. The diversion of management’s attention to pursue acquisitions, or our failure to successfully integrate acquired companies into our business, could have a material adverse effect on our business, financial condition and results from operations.
In the event that we decide to sell a brand or product line, we may encounter difficulty finding, or be unable to find, a buyer on acceptable terms in a timely manner. This could cause a delay in our efforts to achieve our strategic objectives.
Regulatory matters governing our industry could have a significant negative effect on our sales and operating costs.
In both our U.S. and foreign markets, we are affected by extensive laws, governmental regulations, administrative determinations, court decisions and similar constraints. Such laws, regulations and other constraints exist at the federal, state or local levels in the United States and at analogous levels of government in foreign jurisdictions.
The formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of our products are subject to extensive regulation by various federal agencies, including (i) the FDA, (ii) the FTC, (iii) the CPSC, (iv) the EPA, and by (v) various agencies of the states, localities and foreign countries in which our products are manufactured, distributed, stored and sold. If we or our third-party manufacturers fail to comply with those regulations, we could become subject to significant penalties or claims, which could materially adversely affect our business, financial condition and results from operations. In addition, the adoption of new regulations or changes in the interpretations of existing regulations may result in significant compliance costs or the cessation of product sales and may adversely affect the marketing of our products, resulting in a significant loss of revenues which could have a material adverse effect on our business, financial condition and results from operations.
In accordance with the FDC Act and FDA regulations, the manufacturing processes of our third-party manufacturers must also comply with the FDA’s cGMPs. The FDA inspects our facilities and those of our third-party manufacturers periodically to determine if we and our third-party manufacturers are complying with cGMPs. A history of past compliance is not a guarantee that future cGMPs will not mandate other compliance steps and associated expense.
If we or our third party-manufacturers fail to comply with federal, state or foreign regulations, we could be required to:
| · | Suspend manufacturing operations, |
| · | Modify product formulations or processes, |
| · | Suspend the sale of products with non-complying specifications, |
| · | Initiate product recalls, or |
| · | Change product labeling, packaging or advertising or take other corrective action. |
Any of the foregoing actions could have a material adverse effect on our business, financial condition and results from operations.
In addition, our failure to comply with FTC or any other federal and state regulations, or with similar regulations in foreign markets, that cover our product claims and advertising, including direct claims and advertising by us, may result in enforcement actions and imposition of penalties or otherwise materially adversely affect the distribution and sale of our products, which could have a material adverse effect on our business, financial condition and results from operations.
-19-
Product liability claims and related negative publicity could adversely affect our sales and operating results.
We may be required to pay for losses or injuries purportedly caused by our products. From time-to-time we have been and may again be subjected to various product liability claims. Claims could be based on allegations that, among other things, our products contain contaminants, include inadequate instructions or warnings regarding their use or inadequate warnings concerning side effects and interactions with other substances. For example, Denorex products contain coal tar which the State of California has determined allegedly causes cancer. Consequently, in order to comply with California law and to mitigate our risks, the Denorex packaging contains a warning to that effect. Any product liability claims may result in negative publicity that may adversely affect our sales and operating results. Also, if one of our products is found to be defective we may be required to recall it. This may result in substantial costs and negative publicity which may adversely affect our sales and operating results. Although we maintain, and require our suppliers and third-party manufacturers to maintain, product liability insurance coverage, potential product liability claims may exceed the amount of insurance coverage or potential product liability claims may be excluded under the terms of the policy, which could have a material adverse effect on our business, financial condition and results from operations. In addition, in the future we may not be able to obtain adequate insurance coverage or we may be required to pay higher premiums and accept higher deductibles in order to secure adequate insurance coverage.
If we are unable to protect our intellectual property rights our ability to compete effectively in the market for our products could be negatively impacted.
The market for our products depends to a significant extent upon the goodwill associated with our trademarks, trade names and patents. Our trademarks and trade names convey that the products we sell are “brand name” products. We believe consumers ascribe value to our brands, some of which are over 100 years old. We own the material trademark, trade names and patents used in connection with the packaging, marketing and sale of our products. This ownership is what prevents our competitors or new entrants to the market from using our valuable brand names and technologies. Therefore, trademark, trade name and patent protection is critical to our business. Although most of our material intellectual property is registered in the United States and in applicable foreign countries, we may not be successful in asserting protection. If we were to lose the exclusive right to use one or more of our intellectual property rights, the loss of such exclusive right could have a material adverse effect on our business, financial condition and results from operations.
Other parties may infringe on our intellectual property rights and may thereby dilute the value of our brands in the marketplace. Brand dilution or the introduction of competitive brands could cause confusion in the marketplace and adversely affect the value that our consumers associate with our brands, and thereby negatively impact our sales. Any such infringement of our intellectual property rights would also likely result in a commitment of our time and resources, financial or otherwise, to protect these rights through litigation or other means. In addition, third parties may assert claims against our intellectual property rights and we may not be able to successfully resolve those claims causing us to lose our ability to use our intellectual property that is the subject of those claims. Such loss could have a material adverse effect on our business, financial condition and results from operations. Furthermore, from time-to-time, we may be involved in litigation in which we are enforcing or defending our intellectual property rights which could require us to incur substantial fees and expenses and have a material adverse effect on our business, financial condition and results from operations.
Virtually all of our assets consist of goodwill and intangibles.
As our financial statements indicate, virtually all of our assets consist of goodwill and intangibles, principally the trademarks, trade names and patents that we have acquired. In the event that the value of those assets became impaired or our business is materially adversely affected in any way, we would not have tangible assets that could be sold to repay our liabilities. As a result, our creditors and investors may not be able to recoup the amount of the indebtedness that they have extended to us or the amount they have invested in us.
-20-
We depend on third parties for intellectual property relating to some of the products we sell, and our inability to maintain or enter into future license agreements may result in our failure to meet customer demand, which would adversely affect our operating results.
We have licenses or manufacturing agreements with third parties that own intellectual property (e.g., formulae, copyrights, trademarks, trade dress, patents and other technology) used in the manufacture and sale of certain of our products. In the event that any such license or manufacturing agreement is terminated as a result of our breach, we may lose the right to use or have reduced rights to use the intellectual property covered by such license or agreement and may have to develop or obtain rights to use other intellectual property. Similarly, our rights could be reduced if the applicable licensor or third-party manufacturer fails to maintain the licensed intellectual property because, in such event, our competitors could obtain the right to use the intellectual property without restriction. If this were to occur, we might not be able to develop or obtain replacement intellectual property in a timely manner. Additionally, any modified products may not be well-received by customers. The consequences of losing the right to use or having reduced rights to such intellectual property could negatively impact our sales due to our failure to meet consumer demand for the affected products or require us to incur costs for development of new or different intellectual property, either of which could have a material adverse effect on our business, financial condition and results from operations. In addition, development of replacement products may be time-consuming and ultimately may not be feasible.
We depend on our key personnel and the loss of the services provided by any of our executive officers or other key employees could harm our business and results of operations.
Our success depends to a significant degree upon the continued contributions of our senior management, many of whom would be difficult to replace. These employees may voluntarily terminate their employment with us at any time. We may not be able to successfully retain existing personnel or identify, hire and integrate new personnel. While we believe we have developed depth and experience among our key personnel, our business may be adversely affected if one or more of these key individuals were to leave. We do not maintain any key-man or similar insurance policies covering any of our senior management or key personnel.
Our substantial indebtedness could adversely affect our financial health and the significant amount of cash we need to service our debt will not be available to reinvest in our business.
We have a significant amount of indebtedness. At March 31, 2007, our total indebtedness, including current maturities, is approximately $463.3 million. Additionally, we have the ability to borrow up to $200.0 million pursuant to our senior credit facility and an additional $60.0 million pursuant to our revolving credit facility.
Our substantial indebtedness could:
| · | Increase our vulnerability to general adverse economic and industry conditions, |
| · | Require us to dedicate a substantial portion of our cash flow from operations to repay our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions and investments and other general corporate purposes, |
| · | Limit our flexibility in planning for, or reacting to, changes in our business and the markets in which we operate, |
| · | Place us at a competitive disadvantage compared to our competitors that have less debt, and |
| · | Limit, among other things, our ability to borrow additional funds on favorable terms or at all. |
The terms of the indenture governing the 9¼% senior subordinated notes and the senior credit facility allow us to issue and incur additional debt upon satisfaction of conditions set forth in the respective agreements. If new debt is added to current debt levels, the related risks described above could increase.
-21-
Our operating flexibility is limited in significant respects by the restrictive covenants in our senior credit facility and the indenture governing our senior subordinated notes.
Our senior credit facility and the indenture governing our senior subordinated notes impose restrictions that could increase our vulnerability to adverse economic and industry conditions by limiting our flexibility in planning for, and reacting to, changes in our business and industry. Specifically, these restrictions limit our ability to, among other things:
| · | Borrow money or issue guarantees, |
| · | Pay dividends, repurchase stock from or make other restricted payments to stockholders, |
| · | Make investments, |
| · | Use assets as security in other transactions, |
| · | Sell assets or merge with or into other companies, |
| · | Enter into transactions with affiliates, |
| · | Sell stock in our subsidiaries, and |
| · | Direct our subsidiaries to pay dividends or make other payments to our company. |
Our ability to engage in these types of transactions is generally limited by the terms of the senior credit facility and the indenture governing the senior subordinated notes, even if we believe that a specific transaction would positively contribute to our future growth, operating results or profitability. However, if we are able to enter into these types of transactions under the terms of the senior credit facility and the indenture, or if we obtain a waiver with respect to any specific transaction, that transaction may cause our indebtedness to increase, may not result in the benefits we anticipate or may cause us to incur greater costs or suffer greater disruptions in our business than we anticipate, and could therefore, have a material adverse effect on our business, financial condition and results from operations.
In addition, the senior credit facility requires us to maintain certain leverage, interest and fixed charge coverage ratios. Although we believe we are on track to meet and/or maintain the financial ratios contained in our credit agreement, our ability to do so may be affected by events outside our control. Covenants in our senior credit facility also require us to use 100% of the proceeds we receive from debt issuances to repay outstanding borrowings under our senior credit facility. Any failure by us to comply with the terms and conditions of the credit agreement and the indenture governing the senior subordinated notes could have a material adverse effect on our business, financial condition and results from operations.
The senior credit facility and the indenture governing the senior subordinated notes contain cross-default provisions that may result in the acceleration of all of our indebtedness.
The senior credit facility and the indenture governing the senior subordinated notes contain provisions that allow the respective creditors to declare all outstanding borrowings under one agreement to be immediately due and payable as a result of a default under the other agreement. Consequently, under the senior credit facility, failure to make a payment required by the indenture governing the senior subordinated notes, among other things, may lead to an event of default under the senior credit facility. Similarly, an event of default or failure to make a required payment at maturity under the senior credit facility, among other things, may lead to an event of default under the indenture governing the senior subordinated notes. If the debt under the senior credit facility and indenture governing the senior subordinated notes were to both be accelerated, the aggregate amount immediately due and payable as of March 31, 2007 would have been approximately $463.3 million. We presently do not have sufficient liquidity to repay these borrowings in the event they were to be accelerated, and we may not have sufficient liquidity in the future to do so. Additionally, we may not be able to borrow money from other lenders to enable us to refinance the indebtedness. At March 31, 2007, the book value of our current assets was $83.8 million. Although the book value of our total assets was $1,063.4 million, approximately $968.1 million was in the form of intangible assets, including goodwill of $310.9 million, a significant portion of which are illiquid and may not be available to satisfy our creditors in the event our debt is accelerated.
-22-
Any failure to comply with the restrictions of the senior credit facility, the indenture governing the senior subordinated notes or any other subsequent financing agreements may result in an event of default. Such default may allow the creditors to accelerate the related debt, as well as any other debt to which the cross-acceleration or cross-default provisions apply. In addition, the lenders may be able to terminate any commitments they had made to supply us with additional funding. As a result, any default by us under our credit agreement, indenture governing the senior subordinated notes or any other financing agreement, could have a material adverse effect on our business, financial condition and results from operations.
Our business is subject to the risk of litigation by employees, consumers, suppliers, stockholders or others through private actions, class actions, administrative proceedings, regulatory actions or other litigation. The outcome of litigation, particularly class action lawsuits and regulatory actions, is difficult to assess or quantify. Plaintiffs in these types of lawsuits may seek recovery of very large or indeterminate amounts, and the magnitude of the potential loss relating to such lawsuits may remain unknown for substantial periods of time. The cost to defend current and future litigation may be significant. There may also be adverse publicity associated with litigation that could decrease customer acceptance of our products, regardless of whether the allegations are valid or whether we are ultimately found liable. As a result, litigation may adversely affect our business, financial condition and results of operations.
The trading price of our common stock may be volatile.
The trading price of our common stock could be subject to significant fluctuations in response to several factors, some of which are beyond our control, including (i) general stock market volatility, (ii) variations in our quarterly operating results, (iii) our leveraged financial position, (iv) potential sales of additional shares of our common stock, (v) general trends in the consumer products industry, (vi) changes by securities analysts in their estimates or investment ratings, (vii) the relative illiquidity of our common stock and (viii) news regarding litigation in which we are or become involved.
Our principal stockholders have the ability to significantly influence our business, which may be disadvantageous to other stockholders and adversely affect the trading price of our common stock.
Entities affiliated with GTCR collectively own approximately 29.9% of our outstanding common stock. As a result, these stockholders, acting together, will have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets and other corporate transactions. Under our amended and restated certificate of incorporation, the GTCR entities and non-employee directors will not have any duty to refrain from engaging directly or indirectly in the same or similar business activities or lines of business that we do. In the event that any GTCR entity or non-employee director, as the case may be, acquires knowledge of a potential transaction or matter which may be a corporate opportunity for itself and us, the GTCR entity or non-employee director, as the case may be, will not have any duty to communicate or offer such corporate opportunity to us and may pursue such corporate opportunity for itself or direct such corporate opportunity to another person. This concentration of stock ownership also may make it difficult for stockholders to replace management. In addition, this significant concentration of stock ownership may adversely affect the trading price for our common stock because investors often perceive disadvantages in owning stock in companies with stockholders who own significant blocks of stock. This concentration of control could be disadvantageous to other stockholders with interests different from those of our officers, directors and principal stockholders and the trading price of shares of our common stock could be adversely affected.
Substantial sales of our common stock by either our controlling stockholder or management or the perception that these sales could occur could cause the price of our common stock to decline.
Sales of substantial amounts of our common stock in the public market by GTCR or management, or the perception that these sales could occur, could adversely affect the price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. Pursuant to an agreement with GTCR, we filed a Registration Statement on Form S-3 (“Form S-3”) with the
-23-
SEC in December 2006. While the Form S-3 has yet to be declared effective by the SEC, once effective, our controlling stockholder will have the ability to sell common stock, up to the maximum number of common shares registered, into the public marketplace. Such a sale could adversely affect the price of our common stock.
We have no current intention of paying dividends to holders of our common stock.
We presently intend to retain our earnings, if any, for use in our operations, to facilitate strategic acquisitions, or to repay our outstanding indebtedness and have no current intention of paying dividends to holders of our common stock. In addition, our debt instruments limit our ability to declare and pay cash dividends on our common stock. As a result, your only opportunity to achieve a return on your investment in our common stock will be if the market price of our common stock appreciates and you sell your shares at a profit.
Our annual and quarterly results from operations may fluctuate significantly and could fall below the expectations of securities analysts and investors due to a number of factors, some of which are beyond our control, resulting in a decline in the price of our securities.
Our annual and quarterly results from operations may fluctuate significantly because of several factors, including:
| · | Increases and decreases in average quarterly revenues and profitability, |
| · | The rate at which we make acquisitions or develop new products and successfully market them, |
| · | Our inability to increase the sales of our existing products and expand their distribution, |
| · | Changes in consumer preferences and competitive conditions, including the effects of competitors’ operational, promotional or expansion activities, |
| · | Seasonality of our products, |
| · | Fluctuations in commodity prices, product costs, utilities and energy costs, prevailing wage rates, insurance costs and other costs, |
| · | Our ability to recruit, train and retain qualified employees, and the costs associated with those activities, |
| · | Changes in advertising and promotional activities and expansion to new markets, |
| · | Negative publicity relating to us and the products we sell, |
| · | Unanticipated increases in infrastructure costs, |
| · | Impairment of goodwill or long-lived assets, |
| · | Changes in interest rates, and |
| · | Changes in accounting, tax, regulatory or other rules applicable to our business. |
Our quarterly operating results and revenues may fluctuate as a result of any of these or other factors. Accordingly, results for any one quarter are not necessarily indicative of results to be expected for any other quarter or for any year, and revenues for any particular future period may decrease. In the future, operating results may fall below the expectations of securities analysts and investors. In that event, the price of our securities could decrease.
We can be affected adversely and unexpectedly by the implementation of new, or changes in the interpretation of existing, accounting principles generally accepted in the United States of America (“GAAP”).
Our financial reporting complies with GAAP, and GAAP is subject to change over time. If new rules or interpretations of existing rules require us to change our financial reporting, our financial condition and results from operations could be adversely affected.
-24-
Identification of material weakness in internal controls over financial reporting may adversely affect our financial results.
We are subject to the ongoing internal control provisions of Section 404 of the Sarbanes-Oxley Act of 2002. Those provisions provide for the identification and reporting of material weaknesses in our system of internal controls over financial reporting. If such a material weakness is identified, it could indicate a lack of controls adequate to generate accurate financial statements. We routinely assess our internal controls over financial reporting, but we cannot assure you that we will be able to timely remediate any material weaknesses that may be identified in future periods, or maintain all of the controls necessary for continued compliance. Likewise, we cannot assure you that we will be able to retain sufficient skilled finance and accounting personnel, especially in light of the increased demand for such personnel among publicly-traded companies.
Provisions in our amended and restated certificate of incorporation and Delaware law may discourage potential acquirers of our company, which could adversely affect the value of our securities.
Our amended and restated certificate of incorporation provides that our board of directors is authorized to issue from time to time, without further stockholder approval, up to 5.0 million shares of preferred stock in one or more series of preferred stock issuances. Our board of directors may establish the number of shares to be included in each series of preferred stock and determine, as applicable, the voting and other powers, designations, preferences, rights, qualifications, limitations and restrictions for such series of preferred stock. The shares of preferred stock could have preferences over our common stock with respect to dividends and liquidation rights. We may issue additional preferred stock in ways which may delay, defer or prevent a change in control of the Company without further action by our stockholders. The shares of preferred stock may be issued with voting rights that may adversely affect the voting power of the holders of our common stock by increasing the number of outstanding shares having voting rights, and by the creation of class or series voting rights.
Our amended and restated certificate of incorporation contains additional provisions that may have the effect of making it more difficult for a third party to acquire or attempt to acquire control of our company. In addition, we are subject to certain provisions of Delaware law that limit, in some cases, our ability to engage in certain business combinations with significant stockholders.
These provisions, either alone, or in combination with each other, give our current directors and executive officers the ability to significantly influence the outcome of a proposed acquisition of the Company. These provisions would apply even if an acquisition or other significant corporate transaction was considered beneficial by some of our stockholders. If a change in control or change in management is delayed or prevented by these provisions, the market price of our securities could decline.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our corporate headquarters are located in Irvington, New York, a suburb of New York City. Primary functions undertaken at the Irvington facility include senior management, marketing, sales, operations, quality control and regulatory affairs, finance and legal. We lease our Irvington facility which expires on April 30, 2009. We also have an administrative center in Jackson, Wyoming. Primary functions undertaken at the Jackson facility include back office functions, such as invoicing, credit and collection, general ledger and customer service. We lease the Jackson facility which expires on December 31, 2007. We conduct business regarding all of our business segments at each of the Irvington, New York and Jackson, Wyoming facilities.
-25-
ITEM 3. LEGAL PROCEEDINGS
The Company and certain of its officers and directors are defendants in a consolidated putative securities class action lawsuit filed in the United States District Court for the Southern District of New York (the “Consolidated Action”). The first of the six consolidated cases was filed on August 3, 2005. Plaintiffs purport to represent a class of stockholders of the Company who purchased shares between February 9, 2005 through November 15, 2005. Plaintiffs also name as defendants the underwriters in the Company’s initial public offering and a private equity fund that was a selling stockholder in the offering. The District Court has appointed a Lead Plaintiff. On December 23, 2005, the Lead Plaintiff filed a Consolidated Class Action Complaint, which asserted claims under Sections 11, 12(a)(2) and 15 of the Securities Act of 1933 and Sections 10(b), 20(a) and 20A of the Securities Exchange Act of 1934. The Lead Plaintiff generally alleged that the Company issued a series of materially false and misleading statements in connection with its initial public offering and thereafter in regard to the following areas: the accounting issues described in the Company’s press release issued on or about November 15, 2005; and the alleged failure to disclose that demand for certain of the Company’s products was declining and that the Company was planning to withdraw several products from the market. Plaintiffs seek an unspecified amount of damages. The Company filed a motion to dismiss the Consolidated Class Action Complaint in February 2006. On July 10, 2006, the Court dismissed all claims against the Company and the individual defendants arising under the Securities Exchange Act of 1934. The parties have commenced the discovery process which is ongoing. On June 1, 2007, a hearing before the Court was held regarding Plaintiffs’ pending motion for class certification in the Consolidated Action on which no decision has been rendered at this time. The Company’s management believes the remaining claims are legally deficient and subject to meritorious defenses. The Company intends to vigorously pursue its defenses; however, the Company cannot reasonably estimate the potential range of loss, if any.
On May 23, 2006, Similasan Corporation filed a lawsuit against the Company in the United States District Court for the District of Colorado in which Similasan alleged false designation of origin, trademark and trade dress infringement, and deceptive trade practices by the Company related to Murine for Allergy Eye Relief, Murine for Tired Eye Relief and Murine for Earache Relief, as applicable. Similasan has requested injunctive relief, an accounting of profits and damages and litigation costs and attorneys’ fees. In response, the Company filed an answer to the complaint with a potentially dispositive motion. In addition to the lawsuit filed by Similasan in the U.S. District Court for the District of Colorado, the Company also received a cease and desist letter from Swiss legal counsel to Similasan and its parent company, Similasan AG, a Swiss company. In the cease and desist letter, Similasan and Similasan AG have alleged a breach of the Secrecy Agreement executed by the Company and demanded that the Company cease and desist from (i) using confidential information covered by the Secrecy Agreement; and (ii) manufacturing, distributing, marketing or selling certain of its homeopathic products. The complaint in the Colorado action has been amended to include allegations relating to the breach of confidentiality and the Company has filed an answer and responsive motions. On February 22, 2007, prior to the Court’s issuance of a decision regarding the pending motions, the Company agreed to a settlement of the litigation with Similasan and Similasan AG and executed a settlement agreement. The terms of the settlement agreement involved a dismissal of the litigation with prejudice and no monetary damages to the Company.
On September 28, 2006, OraSure Technologies, Inc. moved in the Supreme Court of the State of New York for a preliminary injunction prohibiting the Company from selling cryogenic wart removal products under the Wartner brand, which the Company acquired on September 21, 2006. OraSure Technologies is a supplier to the Company for the Company’s Compound W Freeze Off business. The distribution agreement between the parties provides for mediation of contract disputes, followed by arbitration, if necessary. The contract in question is of five years duration ending in December 2007. On October 30, 2006, the Court denied OraSure Technologies’ motion for a preliminary injunction. Subsequently, in a decision and order dated December 20, 2006, the Court denied a motion by OraSure Technologies for a rehearing regarding a preliminary injunction. An appeal was filed by OraSure in the Appellate Division of the Supreme Court of the State of New York on January 29, 2007, and the Company filed a brief with the Court on February 28, 2007. On May 17, 2007, the Appellate Division reversed the decision of the Supreme Court of the State of New York and issued a preliminary injunction prohibiting the marketing and selling of the Wartner brand by the Company until the underlying arbitration with OraSure was concluded. On May 21, 2007, the Company requested that the Appellate Division issue a stay of the preliminary injunction, consider reargument of the Appellate Division’s decision and grant a leave to appeal to the Court of Appeals of the State of New York. In response to the Company’s request for a stay of the preliminary injunction,
-26-
the Appellate Division issued a stay of the preliminary injunction pending the Appellate Division’s consideration of the Company’s motion to reargue and request for leave to appeal to the Court of Appeals. The parties are currently awaiting the decision of the Appellate Division regarding the motions made by the Company. The Company believes the preliminary injunction was granted based upon factual and legal error and is inconsistent with the terms of the distribution agreement with OraSure and applicable law. The Company is also seeking resolution of the matter through arbitration as required pursuant to the distribution agreement. A hearing before the arbitration panel is scheduled to be held in August 2007.
The Company is also involved from time to time in other routine legal matters and other claims incidental to its business. The Company reviews outstanding claims and proceedings internally and with external counsel as necessary to assess probability and amount of potential loss. These assessments are re-evaluated at each reporting period and as new information becomes available to determine whether a reserve should be established or if any existing reserve should be adjusted. The actual cost of resolving a claim or proceeding ultimately may be substantially different than the amount of the recorded reserve. In addition, because it is not permissible under generally accepted accounting principles to establish a litigation reserve until the loss is both probable and estimable, in some cases there may be insufficient time to establish a reserve prior to the actual incurrence of the loss (upon verdict and judgment at trial, for example, or in the case of a quickly negotiated settlement). The Company believes the resolution of routine matters and other incidental claims, taking into account reserves and insurance, will not have a material adverse effect on its business, financial condition or results from operations.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
-27-
ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Prestige Brands Holdings, Inc.’s common stock is listed on The New York Stock Exchange under the symbol “PBH.” Our initial public offering occurred on February 9, 2005, and the first day of trading was February 10, 2005. The high and low prices of the Company’s common stock as reported by The New York Stock Exchange for the Company’s two most recent fiscal years on a quarterly basis were as follows:
Year Ended March 31, 2007 | High | Low | |||||
Quarter Ended: | |||||||
| June 30, 2006 | $ | 12.90 | $ | 8.25 | |||
| September 30, 2006 | 11.55 | 8.50 | |||||
| December 31, 2006 | 13.87 | 10.77 | |||||
| March 31, 2007 | 13.53 | 10.80 | |||||
Year Ended March 31, 2006 | |||||||
Quarter Ended: | |||||||
| June 30, 2005 | $ | 19.67 | $ | 15.80 | |||
| September 30, 2005 | 21.15 | 10.50 | |||||
| December 31, 2005 | 12.50 | 9.39 | |||||
| March 31, 2006 | 13.13 | 10.22 | |||||
Unregistered Sales of Equity Securities and Use of Proceeds
There were no equity securities sold by the Company during the period covered by this Annual Report on Form 10-K that were not registered under the Securities Act of 1933, as amended.
There were no purchases of shares of the Company’s common stock made during the fourth quarter of 2007, by or on behalf of the Company or any “affiliated purchaser,” as defined by Rule 10b-18(a)(3) of the Exchange Act.
Holders
As of June 1, 2007, there were 57 holders of record of our common stock. The number of record holders does not include beneficial owners whose shares are held in the names of banks, brokers, nominees or other fiduciaries.
Dividend Policy
We have not in the past paid, and do not expect for the foreseeable future, to pay dividends on our common stock. Instead, we anticipate that all of our earnings in the foreseeable future will be used in our operations, to facilitate strategic acquisitions, or to pay down our outstanding indebtedness. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon, among other factors, our results from operations, financial condition, capital requirements and contractual restrictions, including restrictions under our senior credit facility and the indenture governing our 91/4% senior subordinated notes, and any other considerations our board of directors deems relevant.
-28-
PERFORMANCE GRAPH
The following graph compares our cumulative total stockholder return since February 9, 2005, the date of our initial public offering, the Peer Group Index, the Russell 2000 Index (in which the Company is included) and the S&P Supercomposite 1500 Household and Personal Products Index. The graph assumes that the value of the investment in the Company’s common stock and each index was $100.00 on February 9, 2005. The graph was also prepared based on the assumption that all dividends paid, if any, were reinvested. The Company has added the Peer Group Index to the performance graph since the Company has recently established a peer group in connection with its review and implementation of executive compensation packages for 2008. Based on the Company's use of the peer group for benchmarking purposes, the Company believes the peer group should be included in the performance graph.
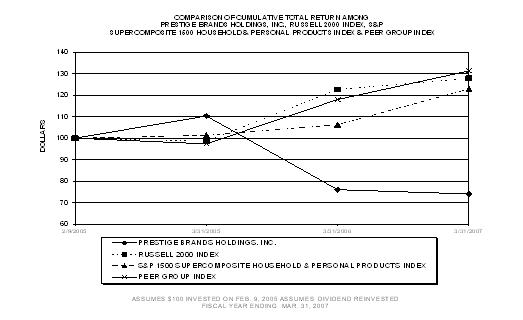
February 9, | March 31 | ||||||||||||
2005 (1) | 2005 | 2006 | 2007 | ||||||||||
| Prestige Brands Holdings | $ | 100.00 | $ | 110.31 | $ | 76.06 | $ | 74.06 | |||||
The Peer Group Index (2) | 100.00 | 97.33 | 117.96 | 131.29 | |||||||||
| The Russell 2000 Index | 100.00 | 98.57 | 122.61 | 127.78 | |||||||||
| The S&P Supercomposite Index | 100.00 | 101.32 | 106.25 | 122.91 | |||||||||
(1) The Company’s initial public offering priced at $16.00 per share on February 9, 2005. Shares of the Company’s common stock closed at $17.75 per share on February 10, 2005, the first day the shares of the Company’s common stock were traded on the NYSE.
(2) The Peer Group Index is a self-constructed peer group consisting of companies in the consumer products industry with comparable revenues and market capitalization, from which the Company has been excluded. Such Peer Group Index was constructed in connection with the Company’s benchmark analysis of executive compensation and is comprised of the following companies: (i) Adams Respiratory Therapeutics Inc, (ii) Alpharma Inc., (iii) Bradley Pharmaceuticals, Inc. (iv) Chattem Inc., (v) Elizabeth Arden, Inc., (vi) Hain Celestial Group, Inc., (vii) Helen of Troy Limited, (viii) Inter Parfums, Inc., (ix) Lifetime Brands, Inc., (x) Maidenform Brands, Inc., (xi) Playtex Products, Inc., and (xii) WD-40 Company.
-29-
ITEM 6. SELECTED FINANCIAL DATA
Prestige Brands Holdings, Inc. and Predecessor
Summary historical financial data for the period from April 1, 2002 to February 5, 2004 is referred to as the “predecessor” information. On February 6, 2004, an indirect subsidiary of Prestige Brands Holdings, Inc. acquired Medtech Holdings, Inc. and The Denorex Company, which at the time were both under common control and management, in a transaction recognized using the purchase method of accounting. The summary financial data after such dates, referred to as “successor” information, includes the financial statement impact of recording fair value adjustments arising from such acquisitions. In addition, the summary financial data includes the effects of The Spic & Span Company, Bonita Bay Holdings, Inc., Vetco Inc. and Dental Concepts LLC and Wartner USA B.V. acquisitions, as well as the acquisition of the Chore Boy trademark, from their respective acquisition dates.
-30-
(In Thousands, except per share data) | Year Ended March 31 | |||||||||
2007 | 2006 | 2005 | ||||||||
Income Statement Data | ||||||||||
| Total revenues | $ | 318,634 | $ | 296,668 | $ | 289,069 | ||||
Cost of sales (1) | 153,147 | 139,430 | 139,009 | |||||||
| Gross profit | 165,487 | 157,238 | 150,060 | |||||||
| Advertising and promotion expenses | 32,005 | 32,082 | 29,697 | |||||||
| Depreciation and amortization | 10,384 | 10,777 | 9,800 | |||||||
| General and administrative | 28,416 | 21,158 | 20,198 | |||||||
Impairment of intangible assets and goodwill | -- | 9,317 | -- | |||||||
| Interest expense, net | 39,506 | 36,346 | 44,726 | |||||||
Other expense (2) | -- | -- | 26,863 | |||||||
| Income before income taxes | 55,176 | 47,558 | 18,776 | |||||||
| Provision for income taxes | 19,098 | 21,281 | 8,556 | |||||||
| Net income | 36,078 | 26,277 | 10,220 | |||||||
Cumulative preferred dividends on Senior Preferred and Class B Preferred units | -- | -- | (25,395 | ) | ||||||
| Net income (loss) available to common stockholders | $ | 36,078 | $ | 26,277 | $ | (15,175 | ) | |||
Net income (loss) per common share: | ||||||||||
| Basic | $ | 0.73 | $ | 0.54 | $ | (0.55 | ) | |||
| Diluted | $ | 0.72 | $ | 0.53 | $ | (0.55 | ) | |||
| Weighted average shares outstanding: | ||||||||||
| Basic | 49,460 | 48,908 | 27,546 | |||||||
| Diluted | 50,020 | 50,008 | 27,546 | |||||||
Year Ended March 31 | ||||||||||
Other Financial Data | 2007 | 2006 | 2005 | |||||||
| Capital expenditures | $ | 540 | $ | 519 | $ | 365 | ||||
| Cash provided by (used in): | ||||||||||
Operating activities | 71,899 | 53,861 | 51,042 | |||||||
| Investing activities | (31,051 | ) | (54,163 | ) | (425,844 | ) | ||||
| Financing activities | (35,290 | ) | 3,168 | 376,743 | ||||||
March 31 | ||||||||||
Balance Sheet Data | 2007 | 2006 | 2005 | |||||||
| Cash and cash equivalents | $ | 13,758 | $ | 8,200 | $ | 5,334 | ||||
| Total assets | 1,063,416 | 1,038,645 | 996,600 | |||||||
Total long-term debt, including current maturities | 463,350 | 498,630 | 495,360 | |||||||
| Stockholders’ equity | 445,334 | 409,407 | 382,047 | |||||||
-31-
(In Thousands, except per share data) | February 6, 2004 to March 31, | April 1, 2003 to February 5, | Year Ended March 31 | |||||||
2004 | 2004 | 2003 | ||||||||
Income Statement Data | (Successor) | (Predecessor) | ||||||||
| Total revenues | $ | 16,876 | $ | 68,402 | $ | 71,734 | ||||
Cost of sales (1) | 9,351 | 26,855 | 27,017 | |||||||
| Gross profit | 7,525 | 41,547 | 44,717 | |||||||
| Advertising and promotion expenses | 1,267 | 10,061 | 11,116 | |||||||
| Depreciation and amortization | 931 | 4,498 | 5,274 | |||||||
| General and administrative | 1,649 | 12,068 | 12,075 | |||||||
| Interest expense, net | 1,725 | 8,157 | 9,747 | |||||||
| Other expense | -- | 1,404 | 685 | |||||||
| Income before income taxes | 1,953 | 5,359 | 5,820 | |||||||
| Provision for income taxes | 724 | 2,214 | 3,287 | |||||||
| Income from continuing operations | 1,229 | 3,145 | 2,533 | |||||||
| Loss from discontinued operations | -- | -- | (5,644 | ) | ||||||
Cumulative effect of change in accounting principle | -- | -- | (11,785 | ) | ||||||
| Net income (loss) | 1,229 | $ | 3,145 | $ | (14,896 | ) | ||||
Cumulative preferred dividends on Senior Preferred and Class B Preferred units | (1,390 | ) | ||||||||
Net loss available to members and common stockholders | $ | (161 | ) | |||||||
Basic and diluted net loss per share | $ | (0.01 | ) | |||||||
Basic and diluted weighted average shares outstanding | 24,472 | |||||||||
February 6, 2004 to March 31, | April 1, 2003 to February 5, | Year Ended March 31 | ||||||||
Other Financial Data: | 2004 | 2004 | 2003 | |||||||
| Capital expenditures | $ | 42 | $ | 66 | $ | 421 | ||||
| Cash provided by (used in): | ||||||||||
| Operating activities | (1,706 | ) | 7,843 | 12,519 | ||||||
| Investing activities | (166,874 | ) | (576 | ) | (2,165 | ) | ||||
| Financing activities | 171,973 | (8,629 | ) | (14,708 | ) | |||||
Balance Sheet Data: | March 31, 2004 | February 5, 2004 | March 31, 2003 | |||||||
| Cash and cash equivalents | $ | 3,393 | $ | 2,868 | $ | 3,530 | ||||
| Total assets | 325,358 | 145,130 | 142,056 | |||||||
Total long-term debt, including current maturities | 148,694 | 71,469 | 81,866 | |||||||
| Members’/Stockholders’ equity | 125,948 | 50,122 | 43,858 | |||||||
(1) | For the period from February 6, 2004 to March 31, 2004 and for 2005, 2006 and 2007, cost of sales includes $1.8 million, $5.3 million, $248,000 and $276,000, respectively, of charges related to the step-up of inventory. |
| (2) | For 2005, other expense includes a loss on debt extinguishment of $26.9 million. |
-32-
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
The following discussion of our financial condition and results of operations should be read together with the “Selected Financial Data” and the consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K. This discussion and analysis may contain forward-looking statements that involve certain risks, assumptions and uncertainties. Future results could differ materially from the discussion that follows for many reasons, including the factors described in Item 1A., “Risk Factors” in this Annual Report on Form 10-K, as well as those described in future reports filed with the SEC.
General
We are engaged in the marketing, sales and distribution of brand name over-the-counter healthcare, household cleaning and personal care products to mass merchandisers, drug stores, supermarkets and club stores primarily in the United States and Canada. We operate in niche segments of these categories where we can use the strength of our brands, our established retail distribution network, a low-cost operating model and our experienced management team as a competitive advantage to grow our presence in these categories and, as a result, grow our sales and profits.
We have grown our brand portfolio by acquiring strong and well-recognized brands from larger consumer products and pharmaceutical companies, as well as other brands from smaller private companies. While the brands we have purchased from larger consumer products and pharmaceutical companies have long histories of support and brand development, we believe that at the time we acquired them they were considered “non-core” by their previous owners and did not benefit from the focus of senior level management or strong marketing support. We believe that the brands we have purchased from smaller private companies have been constrained by the limited resources of their prior owners. After acquiring a brand, we seek to increase its sales, market share and distribution in both existing and new channels. We pursue this growth through increased spending on advertising and promotion, new marketing strategies, improved packaging and formulations and innovative new products.
In February 2005, we raised $448.0 million through an initial public offering (“IPO”) of 28.0 million shares of common stock. The net proceeds of the offering were $416.8 million after deducting $28.0 million of underwriters’ fees and $3.2 million of offering expenses. The net proceeds of $416.8 million plus $3.0 million from our revolving credit facility and $8.8 million of cash on hand went to repay $100.0 million of our existing senior indebtedness (plus a repayment premium of $3.0 million and accrued interest of $0.5 million), to redeem $84.0 million in aggregate principal amount of our existing 91/4% senior subordinated notes (plus a redemption premium of $7.8 million and accrued interest of $3.3 million), to repurchase an aggregate of 4.7 million shares of our common stock held by the GTCR funds and the TCW/Crescent funds for $30.2 million, and to contribute $199.8 million to our subsidiary, Prestige International Holdings, LLC, which was used to redeem all of its outstanding senior preferred units and class B preferred units. We did not receive any of the proceeds from the sale of 4.2 million shares by the selling stockholders as a result of underwriters exercising their over-allotment options.
In October 2005, we completed the acquisition of the “Chore Boy” brand of cleaning pads and sponges. The purchase price of this acquisition was $22.6 million, including direct costs of $400,000. We purchased the Chore Boy brand with funds generated from operations.
In November 2005, we completed the acquisition of Dental Concepts LLC, a marketer of therapeutic oral care products sold under “The Doctor’s” brand. The adjusted purchase price of the ownership interests was approximately $30.2 million, including fees and expenses of the acquisition of $1.3 million. We financed the acquisition price through the utilization of our Revolving Credit Facility in the amount of $30.0 million and cash on hand.
In September 2006, we completed the acquisition of Wartner USA B.V., a privately held Netherlands limited liability company, which owned the intellectual property associated with the “Wartner” brand of over-the-counter wart treatment products. The purchase price of this acquisition was $31.2 million, inclusive of direct
-33-
costs of the acquisition of $216,000. We purchased the Wartner brand with funds generated from operations and the assumption of approximately $5.0 million of contingent payments to the former owner of the Wartner brand.
Impact of Purchase Accounting
The acquisitions of Medtech, Spic and Span, Bonita Bay, Vetco, Dental Concepts and Wartner USA have been accounted for using the purchase method of accounting pursuant to Statement of Financial Accounting Standards No. 141, “Business Combinations” (“Statement No. 141”). As a result, these acquisitions will affect our future results of operations in significant respects. The aggregate acquisition consideration has been allocated to the tangible and intangible assets acquired and liabilities assumed by us based upon their respective fair values as of the acquisition date. A significant portion of the acquisition consideration was allocated to amortizable intangible assets which results in amortization expense in the periods following the acquisitions. In addition, our borrowing to finance these acquisitions has resulted, and will continue to result in significant interest costs until such time that the debt obligations are repaid. For additional information, see “Liquidity and Capital Resources” contained in this Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Critical Accounting Policies and Estimates
The Company’s significant accounting policies are described in the notes to the audited financial statements included elsewhere in this Annual Report on Form 10-K. While all significant accounting policies are important to our consolidated financial statements, certain of these policies may be viewed as being critical. Such policies are those that are both most important to the portrayal of our financial condition and results from operations and require our most difficult, subjective and complex estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses or the related disclosure of contingent assets and liabilities. These estimates are based upon our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates under different conditions. The most critical accounting policies are as follows:
Revenue Recognition
We comply with the provisions of SEC Staff Accounting Bulletin No. 104 “Revenue Recognition,” which states that revenue should be recognized when the following revenue recognition criteria are met: (1) persuasive evidence of an arrangement exists; (2) the product has been shipped and the customer takes ownership and assumes the risk of loss; (3) the selling price is fixed or determinable; and (4) collection of the resulting receivable is reasonably assured. We have determined that the transfer of risk of loss generally occurs when product is received by the customer, and, accordingly recognize revenue at that time. Provision is made for estimated discounts related to customer payment terms and estimated product returns at the time of sale based on our historical experience.
As is customary in the consumer products industry, we participate in the promotional programs of our customers to enhance the sale of our products. The cost of these promotional programs is recorded in accordance with Emerging Issues Task Force 01-09, “Accounting for Consideration Given by a Vendor to a Customer (Including a Reseller of the Vendor’s Products)” as either advertising and promotional expenses or as a reduction of sales. Such costs vary from period-to-period based on the actual number of units sold during a finite period of time. We estimate the cost of such promotional programs at their inception based on historical experience and current market conditions and reduce sales by such estimates. These promotional programs consist of direct to consumer incentives such as coupons and temporary price reductions, as well as incentives to our customers, such as slotting fees and cooperative advertising. We do not provide incentives to customers for the acquisition of product in excess of normal inventory quantities since such incentives increase the potential for future returns, as well as reduce sales in the subsequent fiscal periods.
Estimates of costs of promotional programs are based on (i) historical sales experience, (ii) the current offering, (iii) forecasted data, (iv) current market conditions, and (v) communication with customer purchasing/marketing personnel. At the completion of the promotional program, the estimated amounts are adjusted to actual results. While our promotional expense for the year ended March 31, 2007 was $16.5 million, we participated in 5,900 promotional campaigns, resulting in an average cost of $2,800 per campaign. Of such amount, only 582 payments were in excess of $5,000. We believe that the estimation methodologies employed, combined with the nature of the promotional campaigns, makes the likelihood remote that our obligation would be misstated by a
-34-
material amount. However, for illustrative purposes, had we underestimated the promotional program rate by 10% for the year ended March 31, 2007, our sales and operating income would have been adversely affected by approximately $1.6 million. Net income would have been adversely affected by approximately $1.0 million.
We also periodically run coupon programs in Sunday newspaper inserts or as on-package instant redeemable coupons. We utilize a national clearing house to process coupons redeemed by customers. At the time a coupon is distributed, a provision is made based upon historical redemption rates for that particular product, information provided as a result of the clearing house’s experience with coupons of similar dollar value, the length of time the coupon is valid, and the seasonality of the coupon drop, among other factors. During the year ended March 31, 2007, we had 17 coupon events. The amount recorded against revenues and accrued for these events during the year was $2.7 million, of which $2.3 million was redeemed during the year.
Allowances for Product Returns
Due to the nature of the consumer products industry, we are required to estimate future product returns. Accordingly, we record an estimate of product returns concurrent with the recording of sales. Such estimates are made after analyzing (i) historical return rates, (ii) current economic trends, (iii) changes in customer demand, (iv) product acceptance, (v) seasonality of our product offerings, and (vi) the impact of changes in product formulation, packaging and advertising.
We construct our returns analysis by looking at the previous year’s return history for each brand. Subsequently, each month, we estimate our current return rate based upon an average of the previous six months’ return rate and review that calculated rate for reasonableness giving consideration to the other factors described above. Our historical return rate has been relatively stable; for example, for the years ended March 31, 2007, 2006 and 2005, returns represented 3.7%, 3.5%, and 3.6%, respectively, of gross sales. At March 31, 2007 and 2006, the allowance for sales returns was $1.8 million and $1.9 million, respectively.
While we utilize the methodology described above to estimate product returns, actual results may differ materially from our estimates, causing our future financial results to be adversely affected. Among the factors that could cause a material change in the estimated return rate would be significant unexpected returns with respect to a product or products that comprise a significant portion of our revenues. Based upon the methodology described above and our actual returns’ experience, management believes the likelihood of such an event is remote. As noted, over the last three years, our actual product return rate has stayed within a range of 3.7% to 3.5% of gross sales. An increase of 0.1% in our estimated return rate as a percentage of gross sales would have adversely affected our reported sales and operating income for the year ended March 31, 2007 by approximately $371,000. Net income would have been adversely affected by approximately $228,000.
Allowances for Obsolete and Damaged Inventory
We value our inventory at the lower of cost or market value. Accordingly, we reduce our inventories for the diminution of value resulting from product obsolescence, damage or other issues affecting marketability equal to the difference between the cost of the inventory and its estimated market value. Factors utilized in the determination of estimated market value include (i) current sales data and historical return rates, (ii) estimates of future demand, (iii) competitive pricing pressures, (iv) new product introductions, (v) product expiration dates, and (vi) component and packaging obsolescence.
Many of our products are subject to expiration dating. As a general rule our customers will not accept goods with expiration dating of less than 12 months from the date of delivery. To monitor this risk, management utilizes a detailed compilation of inventory with expiration dating between zero and 15 months and reserves for 100% of the cost of any item with expiration dating of 12 months or less. At March 31, 2007 and 2006, the allowance for obsolete and slow moving inventory represented 5.8% and 2.9%, respectively, of total inventory. The year-over-year increase resulted primarily from obsolescence reserves related to products in the cough and cold category facing expiration dating. Inventory obsolescence costs charged to operations for the years ended March 31, 2007, 2006, and 2005 were 1.0%, 0.2%, and 0.3% of net sales, respectively. A 1.0% increase in our allowance for obsolescence at March 31, 2007 would have adversely affected our reported operating income and net income for the year ended March 31, 2007 by approximately $320,000 and $196,000, respectively.
-35-
Allowance for Doubtful Accounts
In the ordinary course of business, we grant non-interest bearing trade credit to our customers on normal credit terms. We maintain an allowance for doubtful accounts receivable which is based upon our historical collection experience and expected collectibility of the accounts receivable. In an effort to reduce our credit risk, we (i) establish credit limits for all of our customer relationships, (ii) perform ongoing credit evaluations of our customers’ financial condition, (iii) monitor the payment history and aging of our customers’ receivables, and (iv) monitor open orders against an individual customer’s outstanding receivable balance.
We establish specific reserves for those accounts which file for bankruptcy, have no payment activity for 180 days or have reported major negative changes to their financial condition. The allowance for bad debts at March 31, 2007 and 2006 amounted to 0.1% and 0.3%, respectively, of accounts receivable. Bad debt expense (recoveries) for 2007, 2006 and 2005 were $(100,000), $(53,000) and $31,700, respectively, each representing 0.0% of net sales in each of the years.
While management believes that it is diligent in its evaluation of the adequacy of the allowance for doubtful accounts, an unexpected event, such as the bankruptcy filing of a major customer, could have an adverse effect on our future financial results. A 0.1% increase in our bad debt expense as a percentage of sales in 2007 would have resulted in a decrease in reported operating income of approximately $317,000, and a decrease in our reported net income of approximately $195,000.
Valuation of Intangible Assets and Goodwill
Goodwill and intangible assets amounted to $968.1 million and $935.1 million at March 31, 2007 and 2006, respectively. At March 31, 2007, goodwill and intangible assets were apportioned among our three operating segments as follows:
Over-the- Counter Healthcare | Household Cleaning | Personal Care | Consolidated | ||||||||||
| Goodwill | $ | 235,647 | $ | 72,549 | $ | 2,751 | $ | 310,947 | |||||
| Intangible assets | |||||||||||||
| Indefinite lived | 374,070 | 170,893 | -- | 544,963 | |||||||||
| Finite lived | 94,776 | 21 | 17,397 | 112,194 | |||||||||
| 468,846 | 170,914 | 17,397 | 657,157 | ||||||||||
$ | 704,493 | $ | 243,463 | $ | 20,148 | $ | 968,104 | ||||||
Our Clear Eyes, New-Skin, Chloraseptic, Compound W and Wartner brands comprise the majority of the value of the intangible assets within the Over-The-Counter Healthcare segment. Denorex, Cutex and Prell comprised substantially all of the intangible asset value within the Personal Care segment. The Comet, Spic and Span and Chore Boy brands comprise substantially all of the intangible asset value within the Household Cleaning segment.
Goodwill and intangible assets comprise substantially all of our assets. Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed in a purchase business combination. Intangible assets generally represent our trademarks, brand names and patents. When we acquire a brand, we are required to make judgments regarding the value assigned to the associated intangible assets, as well as their respective useful lives. Management considers many factors, both prior to and after, the acquisition of an intangible asset in determining the value, as well as the useful life, assigned to each intangible asset that the Company acquires or continues to own and promote. The most significant factors are:
| · | Brand History |
A brand that has been in existence for a long period of time (e.g., 25, 50 or 100 years) generally warrants a higher valuation and longer life (sometimes indefinite) than a brand that has been in
-36-
existence for a very short period of time. A brand that has been in existence for an extended period of time generally has been the subject of considerable investment by its previous owner(s) to support product innovation and advertising and promotion.
| · | Market Position |
Consumer products that rank number one or two in their respective market generally have greater name recognition and are known as quality product offerings, which warrant a higher valuation and longer life than products that lag in the marketplace.
| · | Recent and Projected Sales Growth |
Recent sales results present a snapshot as to how the brand has performed in the most recent time periods and represent another factor in the determination of brand value. In addition, projected sales growth provides information about the strength and potential longevity of the brand. A brand that has both strong current and projected sales generally warrants a higher valuation and a longer life than a brand that has weak or declining sales. Similarly, consideration is given to the potential investment, in the form of advertising and promotion, which is required to reinvigorate a brand that has fallen from favor.
| · | History of and Potential for Product Extensions |
Consideration also is given to the product innovation that has occurred during the brand’s history and the potential for continued product innovation that will determine the brand’s future. Brands that can be continually enhanced by new product offerings generally warrant a higher valuation and longer life than a brand that has always “followed the leader”.
After consideration of the factors described above, as well as current economic conditions and changing consumer behavior, management prepares a determination of the intangible’s value and useful life based on its analysis of the requirements of Statements No. 141 and No. 142. Under Statement No. 142, goodwill and indefinite-lived intangible assets are no longer amortized, but must be tested for impairment at least annually. Intangible assets with finite lives are amortized over their respective estimated useful lives and must also be tested for impairment.
On an annual basis, or more frequently if conditions indicate that the carrying value of the asset may not be recovered, management performs a review of both the values and useful lives assigned to goodwill and intangible assets and tests for impairment.
Finite-Lived Intangible Assets
As mentioned above, management performs a review annually, or more frequently if necessary, to ascertain the impact of events and circumstances on the estimated useful lives and carrying values of our trademarks and trade names. In connection with this analysis, management:
| · | Reviews period-to-period sales and profitability by brand, |
| · | Analyzes industry trends and projects brand growth rates, |
| · | Prepares annual sales forecasts, |
| · | Evaluates advertising effectiveness, |
| · | Analyzes gross margins, |
| · | Reviews contractual benefits or limitations, |
| · | Monitors competitors’ advertising spend and product innovation, |
| · | Prepares projections to measure brand viability over the estimated useful life of the intangible asset, and |
| · | Considers the regulatory environment, as well as industry litigation. |
Should analysis of any of the aforementioned factors warrant a change in the estimated useful life of the intangible asset, management will reduce the estimated useful life and amortize the carrying value prospectively over the shorter remaining useful life. Management’s projections are utilized to assimilate all of the facts, circumstances and expectations related to the trademark or trade name and estimate the cash flows over its useful life. In the event that the long-term projections indicate that the carrying value is in excess of the undiscounted cash flows expected to result from the use of the intangible assets, management is required to record an
-37-
impairment charge. Once that analysis is completed, a discount rate is applied to the cash flows to estimate fair value. The impairment charge is measured as the excess of the carrying amount of the intangible asset over fair value as calculated using the discounted cash flow analysis. Future events, such as competition, technological advances and reductions in advertising support for our trademarks and trade names could cause subsequent evaluations to utilize different assumptions.
Indefinite-Lived Intangible Assets
In a manner similar to finite-lived intangible assets, on an annual basis, or more frequently if necessary, management analyzes current events and circumstances to determine whether the indefinite life classification for a trademark or trade name continues to be valid. Should circumstance warrant a finite life, the carrying value of the intangible asset would then be amortized prospectively over the estimated remaining useful life.
In connection with this analysis, management also tests the indefinite-lived intangible assets for impairment by comparing the carrying value of the intangible asset to its estimated fair value. Since quoted market prices are seldom available for trademarks and trade names such as ours, we utilize present value techniques to estimate fair value. Accordingly, management’s projections are utilized to assimilate all of the facts, circumstances and expectations related to the trademark or trade name and estimate the cash flows over its useful life. In performing this analysis, management considers the same types of information as listed above in regards to finite-lived intangible assets. Once that analysis is completed, a discount rate is applied to the cash flows to estimate fair value. Future events, such as competition, technological advances and reductions in advertising support for our trademarks and trade names could cause subsequent evaluations to utilize different assumptions.
Goodwill
As part of its annual test for impairment of goodwill, management estimates the discounted cash flows of each reporting unit, which is at the brand level, and one level below the operating segment level, to estimate their respective fair values. In performing this analysis, management considers the same types of information as listed above in regards to finite-lived intangible assets. In the event that the carrying amount of the reporting unit exceeds the fair value, management would then be required to allocate the estimated fair value of the assets and liabilities of the reporting unit as if the unit was acquired in a business combination, thereby revaluing the carrying amount of goodwill. In a manner similar to indefinite-lived assets, future events, such as competition, technological advances and reductions in advertising support for our trademarks and trade names could cause subsequent evaluations to utilize different assumptions.
In estimating the value of trademarks and trade names, as well as goodwill, at March 31, 2007, management applied a discount rate of 9.5%, the Company’s then current weighted-average cost of funds, to the estimated cash flows; however that rate, as well as future cash flows may be influenced by such factors, including (i) changes in interest rates, (ii) rates of inflation, or (iii) sales or contribution margin reductions. In the event that the carrying value exceeded the estimated fair value of either intangible assets or goodwill, we would be required to recognize an impairment charge. Additionally, continued decline of the fair value ascribed to an intangible asset or a reporting unit caused by external factors may require future impairment charges.
During the three month period ended March 31, 2006, we recorded non-cash charges related to the impairment of intangible assets and goodwill of the Personal Care segment of $7.4 million and $1.9 million, respectively, because the carrying amounts of these “branded” assets exceeded their fair market values primarily as a result of declining sales caused by product competition. Should the related fair values of goodwill and intangible assets continue to be adversely affected as a result of declining sales or margins caused by competition, technological advances or reductions in advertising and promotional expenses, the Company may be required to record additional impairment charges. We were not required to record any asset impairment charges during the year ended March 31, 2007.
Stock-Based Compensation
During 2006, we adopted FASB Statement No. 123(R), “Share-Based Payment” (“Statement No. 123(R)”) with the initial grants of restricted stock and options to purchase common stock to employees and directors in accordance with the provisions of the Plan. Statement No. 123(R) requires us to measure the cost of services to be rendered based on the grant-date fair value of the equity award. Compensation expense is to be recognized
-38-
over the period which an employee is required to provide service in exchange for the award, generally referred to as the requisite service period. Information utilized in the determination of fair value includes the following:
| · | Type of instrument (i.e.: restricted shares vs. an option, warrant or performance shares), |
| · | Strike price of the instrument, |
| · | Market price of the Company’s common stock on the date of grant, |
| · | Discount rates, |
| · | Duration of the instrument, and |
| · | Volatility of the Company’s common stock in the public market. |
Additionally, management must estimate the expected attrition rate of the recipients to enable it to estimate the amount of non-cash compensation expense to be recorded in our financial statements. While management uses diligent analysis to estimate the respective variables, a change in assumptions or market conditions, as well as changes in the anticipated attrition rates, could have a significant impact on the future amounts recorded as non-cash compensation expense. The Company recorded non-cash compensation expense of $655,000 and $383,000 during 2007 and 2006, respectively. Assuming no changes in assumptions and no awards authorized by the Compensation Committee of the Board of Directors during 2008, we will record non-cash compensation expense of approximately $800,000. There were no stock-based compensation charges incurred during 2005.
Loss Contingencies
Loss contingencies are recorded as liabilities when it is probable that a liability has been incurred and the amount of such loss is reasonable estimable. Contingent losses are often resolved over longer periods of time and involve many factors including:
| · | Rules and regulations promulgated by regulatory agencies, |
| · | Sufficiency of the evidence in support of our position, |
| · | Anticipated costs to support our position, and |
| · | Likelihood of a positive outcome. |
Recent Accounting Pronouncements
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities - Including an amendment of FASB Statement No. 115” (“Statement No. 159”). Statement No. 159 permits companies to choose to measure certain financial instruments and certain other items at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be recognized in earnings at each subsequent reporting date. Statement No. 159 is effective for interim financial statements issued during the fiscal year beginning after November 15, 2007. The Company is evaluating the impact that the adoption of Statement No. 159 will have on its consolidated financial statements.
In September 2006, the SEC issued Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements” (“SAB No. 108”). SAB No. 108 was issued in an effort to eliminate the diversity in practice surrounding how companies quantify financial statement misstatements. SAB No. 108 requires that errors be quantified using both a balance sheet and income statement approach and evaluated as to materiality giving consideration to all relevant quantitative and qualitative factors. The adoption of SAB No. 108 did not have a material impact on our consolidated financial statements.
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“Statement No. 157”) to address inconsistencies in the definition and determination of fair value pursuant to generally accepted accounting principles (“GAAP”). Statement No. 157 provides a single definition of fair value, establishes a framework for measuring fair value in GAAP and expands disclosures about fair value measurements in an effort to increase comparability related to the recognition of market-based assets and liabilities and their impact on earnings. Statement No. 157 is effective for interim financial statements issued during the fiscal year beginning after November 15, 2007.
-39-
In June 2006, the FASB issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes--an interpretation of FASB Statement 109” (“FIN No. 48”), which clarifies the accounting for uncertainty in income taxes recognized in a company’s financial statements in accordance with FASB Statement 109. FIN No. 48 and prescribes a recognition threshold and measurement attributes for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 is effective for the Company’s interim financial statements issued after April 1, 2007, and is not expected to have a material impact on the Company’s consolidated financial position, results of operations or cash flows.
Management has reviewed and continues to monitor the actions of the various financial and regulatory reporting agencies and is currently not aware of any other pronouncement that could have a material impact on the Company’s consolidated financial position, results of operations or cash flows.
Fiscal 2007 compared to Fiscal 2006
Revenues
2007 Revenues | % | 2006 Revenues | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | $ | 174,704 | 54.8 | $ | 160,942 | 54.3 | $ | 13,762 | 8.6 | ||||||||||
| Household Cleaning | 119,036 | 37.4 | 107,801 | 36.3 | 11,235 | 10.4 | |||||||||||||
| Personal Care | 24,894 | 7.8 | 27,925 | 9.4 | (3,031 | ) | (10.9) | ||||||||||||
$ | 318,634 | 100.0 | $ | 296,668 | 100.0 | $ | 21,966 | 7.4 | |||||||||||
The 7.4% increase in revenues for 2007 versus 2006 was primarily a result of the acquisitions of the Wartner brand, acquired in September of 2006, and the Chore Boy and The Doctor’s brands acquired in October and November 2005, respectively. Excluding the impact of the acquisitions, revenues were up 0.6%. Revenue increases in Over-the-Counter Healthcare and Household Cleaning segments were partially offset by a decrease in the Personal Care segment.
Over-the-Counter Healthcare Segment
Revenues for the Over-the-Counter Healthcare segment increased $13.8 million, or 8.6% for 2007 versus 2006. The increase was primarily attributable to the acquisition of the Wartner and The Doctor’s brands. Excluding the impact of these acquisitions, revenues increased 1.2%. Revenue increases for Clear Eyes, Little Remedies, Murine and Dermoplast were partially offset by revenue declines on Compound W, Chloraseptic and New Skin. The Clear Eyes revenue growth was a result of continued strong consumer consumption trends, the launch of Clear Eyes Triple Action and Maximum Redness Relief, and increased shipments to international customers. The Little Remedies revenue increase was a result of improved consumer consumption. Dermoplast’s revenue increase was due to increased shipments to institutional customers and the launch of Dermoplast Poison Ivy. The Murine revenue increase was primarily the result of improved consumer consumption of the ear product. The revenue decrease for Compound W was primarily a result of weaker consumer consumption primarily in the cryogenic segment of the wart remover category. Chloraseptic’s revenue decrease was primarily due to lower consumer consumption as a result of a weak cough and cold flu season. New Skin’s revenue decrease was the result of softness in the liquid bandage category.
Household Cleaning Segment
Revenues for the Household Cleaning segment increased $11.2 million, or 10.4%, for 2007 versus 2006. Excluding the acquisition of Chore Boy, revenues for this segment increased 2.8% for the period. Comet revenue increased during the period due to strong consumer consumption, expanded distribution and royalty revenues earned from licensing agreements in Eastern Europe and for institutional sales in North America. Revenues for the Spic and Span brand decreased during the period as a result of lower sales to the dollar store channel.
-40-
Personal Care Segment
Revenues of the Personal Care segment declined $3.0 million, or 10.9%, for 2007 versus 2006. The revenue decrease was the result of continued declines in consumer consumption trends for the Cutex, Denorex and Prell brands and was in accordance with management’s expectations.
Gross Profit
2007 Gross Profit | % | 2006 Gross Profit | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | 109,103 | 62.5 | $ | 102,451 | 63.7 | $ | 6,652 | 6.5 | |||||||||||
| Household Cleaning | 46,034 | 38.7 | 42,713 | 39.6 | 3,321 | 7.8 | |||||||||||||
| Personal Care | 10,350 | 41.6 | 12,074 | 43.2 | (1,724 | ) | (14.3) | ||||||||||||
$ | 165,487 | 51.9 | $ | 157,238 | 53.0 | $ | 8,249 | 5.2 | |||||||||||
Gross profit for 2007 increased $8.2 million, or 5.2%, versus 2006. As a percent of total revenue, gross profit decreased from 53.0% for 2006 to 51.9% during 2007. The decrease in gross profit percentage was a result of inventory obsolescence, higher product costs and increased shipments to non-North American distributors which have a lower margin than our domestic markets, partially offset by lower distribution expense. Shipments to markets outside of North America represented 4.6% of total revenues in 2007 versus 3.4% for 2006.
Over-the-Counter Healthcare Segment
Gross profit for 2007 increased $6.6 million, or 6.5%, versus 2006. As a percent of OTC revenue, gross profit decreased from 63.7% for 2006 to 62.5% during 2007. The decrease in gross profit percentage was primarily a result of obsolescence reserves of $2.6 million related to products in the cough and cold category facing expiration dating.
Household Cleaning Segment
Gross profit for 2007 increased $3.3 million, or 7.8%, versus 2006. As a percent of household cleaning revenue, gross profit decreased from 39.6% for 2006 to 38.7% during 2007. The decrease in gross profit percentage is primarily a result of increased product costs partially offset by royalties earned, with no associated costs, from our international and institutional licensing arrangements with Procter & Gamble.
Personal Care Segment
Gross profit for 2007 decreased $1.7 million, or 14.3%, versus 2006. As a percent of personal care revenue, gross profit decreased from 43.2% for 2006 to 41.6% during 2007. The decrease in gross profit percentage is a result of increased promotional pricing allowances and product costs.
Contribution Margin
2007 Contribution Margin | % | 2006 Contribution Margin | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | $ | 84,902 | 48.6 | $ | 80,027 | 49.7 | $ | 4,875 | 6.1 | ||||||||||
| Household Cleaning | 39,355 | 33.1 | 36,218 | 33.6 | 3,137 | 8.7 | |||||||||||||
| Personal Care | 9,225 | 37.1 | 8,911 | 31.9 | 314 | 3.5 | |||||||||||||
$ | 133,482 | 41.9 | $ | 125,156 | 42.2 | $ | 8,326 | 6.7 | |||||||||||
Contribution margin, defined as gross profit less advertising and promotional expenses, increased $8.3 million, or 6.7% for 2007 versus 2006. The contribution margin increase was a result of the increase in sales and gross profit as previously discussed, and a $77,000, or 0.2% reduction in advertising and promotional spending. The reduction in advertising and promotional spending is primarily a result of a $2.0 million reduction in the Personal Care segment mostly offset by an increase of $1.7 million in the Over-the-Counter Healthcare segment and $200,000 in the Household Cleaning segment.
-41-
Over-the-Counter Healthcare Segment
Contribution margin in the Over-the-Counter Healthcare segment increased by $4.9 million, or 6.1%, for 2007 versus 2006. The contribution margin increase was a result of the increase in sales and gross profit as previously discussed, partially offset by a $1.7 million, or an 8.0%, increase in advertising and promotional spending. The increase in advertising and promotional spending was primarily a result of increased media behind The Doctor’s Nightguard, Little Remedies print media and Chloraseptic promotional spending, partially offset by a reduction in Clear Eyes and New Skin media.
Household Cleaning Segment
Contribution margin in the Household Cleaning segment increased by $3.1 million, or 8.7%, for 2007 versus 2006. The contribution margin increase was a result of the sales and gross profit increase previously discussed, slightly offset with a $184,000, or a 2.8% increase in advertising and promotional spending. The increase is a result of a modest reduction of Comet media and promotional spending offset by increased spending resulting from the Chore Boy acquisition.
Personal Care Segment
Contribution margin in the personal care segment was up $314,000, or 3.5% for 2007 versus 2006. The contribution margin increase was primarily the result of a $2.0 million, or 64.4%, reduction in advertising and promotion spending versus 2006, partially offset by the gross profit decline as previously discussed. The reduction in advertising and promotion was due to the Company’s strategic decision to redeploy advertising and promotional funds in support of its growth brands in the other segments.
General and Administrative
General and administrative expenses were $28.4 million for 2007 versus $21.1 million for 2006. The increase was primarily related to additional staff added during the second half of 2006 and employee incentive plan compensation that was not earned in 2006, as well as severance compensation related to the departure of a member of management in 2007, increased stock-based compensation costs in 2007 and increased legal and professional fees in 2007.
Depreciation and Amortization
Depreciation and amortization expense was $10.4 million for 2007 versus $10.8 million for 2006. An increase in amortization related to intangible assets purchased in the Wartner and Dental Concepts acquisitions was partially offset by a reduction of the carrying value of certain trademarks in our Personal Care segment. During the three month period ended March 31, 2006, the Company recognized an asset impairment charge of approximately $7.4 million related to this segment. Depreciation expense decreased by $1.0 million for 2007 versus 2006 due to the absence of depreciation charges for manufacturing equipment that was fully depreciated as of January 31, 2006.
Impairment of Intangible Assets and Goodwill
We performed our impairment analyses of intangible assets and goodwill and determined, in accordance with FASB Statements No. 142 and 144, that no impairment existed in 2007. During the three month period ended March 31, 2006, we recorded non-cash charges related to the impairment of certain intangible assets and goodwill of the Personal Care segment of $7.4 million and $1.9 million, respectively. The impairment charges related to the intangible assets and goodwill were the result of their carrying amounts exceeding their fair market values as a result of declining sales.
Interest Expense
Net interest expense was $39.5 million for 2007 versus $36.3 million for the comparable period of 2006. This represented an increase of $3.2 million, or 8.7%, from 2006. The increase in interest expense was due to the increase in interest rates associated with our variable rate indebtedness. The average cost of funds increased from 6.3% for 2006 to 7.4% for 2007.
Income Taxes
The income tax provision for 2007 was $19.1 million, with an effective rate of 34.6%, compared to $21.3 million, with an effective rate of 44.7% for 2006. During 2006, the Company increased the effective tax rate to 39.1% and adjusted the deferred tax liabilities as a result of the completion of a state nexus study. Fiscal 2007 includes a
-42-
$2.2 million tax benefit resulting from a reduction in the deferred income tax rate to 38.4% from 39.1% as a result of the implementation of initiatives to obtain operational, as well as tax, efficiencies.
Fiscal 2006 compared to Fiscal 2005
Revenues
2006 Revenues | % | 2005 Revenues | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | $ | 160,942 | 54.3 | $ | 159,010 | 55.0 | $ | 1,932 | 1.2 | ||||||||||
| Household Cleaning | 107,801 | 36.3 | 97,897 | 33.9 | 9,904 | 10.1 | |||||||||||||
| Personal Care | 27,925 | 9.4 | 32,162 | 11.1 | (4,237 | ) | (13.2) | ||||||||||||
$ | 296,668 | 100.0 | $ | 289,069 | 100.0 | $ | 7,599 | 2.6 | |||||||||||
Revenues increased by $7.6 million, or 2.6%, to $296.7 million for 2006 from $289.1 million for 2005. The increase was driven by the acquisitions of Vetco, Inc. and Dental Concepts LLC in October 2004 and November 2005, respectively, as well as the Chore Boy brand in October 2005. The Over-the-Counter Healthcare segment had revenues of $160.9 million for 2006, an increase of $1.9 million over revenues of $159.0 million for 2005. The Household Cleaning segment had revenues of $107.8 million for 2006, a $9.9 million increase over revenues of $97.9 million for 2005. The Personal Care segment had revenues of $27.9 million for 2006, a $4.2 million decrease from revenues of $32.2 million for 2005.
Over-the-Counter Healthcare Segment
Revenues of the Over-the-Counter Healthcare segment increased by $1.9 million, or 1.2%, for 2006 as a result of sales related to The Doctor’s brand, which was acquired as part of the Dental Concepts acquisition in November 2005, a full year of revenue from the Little Remedies brand, which was acquired as part of the Vetco acquisition in October 2004, as well as the launch of the Clear Eyes for Dry Eyes product line at the end of 2005. Partially offsetting the increases related to the acquisitions and Clear Eyes were declines in Chloraseptic, Compound W, New-Skin and Murine. The decline in Chloraseptic was generally confined to the quarter ended December 31, 2005, and resulted from a formulation issue related to stability of several batches of the relief strips product line. Declines for Compound W and New-Skin were related to softness in the retail wart remover and liquid bandage categories, respectively. The decline for Murine was a result of decreased consumer consumption and lost distribution.
Household Cleaning Segment
Revenues of the Household Cleaning segment increased $9.9 million, or 10.1%, for 2006 as a result of the Chore Boy acquisition, as well as sales growth in 2006 from both the Comet and Spic and Span brands. The Comet revenue increase was driven by strong retail consumption of powder and sprays and expanded distribution of Comet Cream. The Spic and Span revenue increase was driven by increased distribution within the mass market channel of distribution.
Personal Care Segment
Revenues of the Personal Care segment declined $4.2 million, or 13.2%, for 2006 as a result of the continued decline in consumer consumption of the Denorex brand, attributable to increased competition in the dandruff shampoo category, as well as lower sales of Cutex due to increasing market share of private label brands, as well as a general softness in the nail polish remover category as more women choose to have their nails manicured at salons.
-43-
Gross Profit
2006 Gross Profit | % | 2005 Gross Profit | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | $ | 102,451 | 63.7 | $ | 98,440 | 61.9 | $ | 4,011 | 4.1 | ||||||||||
| Household Cleaning | 42,713 | 39.6 | 35,858 | 36.6 | 6,855 | 19.1 | |||||||||||||
| Personal Care | 12,074 | 43.2 | 15,762 | 49.0 | (3,688 | ) | (23.4) | ||||||||||||
$ | 157,238 | 53.0 | $ | 150,060 | 51.9 | $ | 7,178 | 4.8 | |||||||||||
Gross profit increased by $7.2 million, or 4.8%, to $157.2 million for 2006 from $150.1 million for 2005. As a percentage of revenues, gross profit increased to 53.0% for 2006 from 51.9% in 2005. Excluding the inventory step-up costs of $200,000 in 2006 and $5.3 million in 2005, gross profit would have decreased to 53.1% for 2006 from 53.8% for 2005. The decrease in gross profit resulted primarily from a shift in the sales mix toward the Household Cleaning segment which has a lower gross profit relative to the Over-the-Counter Healthcare and Personal Care segments.
Over-the-Counter Healthcare Segment
Gross profit for the Over-the-Counter Healthcare segment increased by $4.0 million, or 4.1%, for 2006. As a percentage of revenues, gross profit increased to 63.7% for 2006 from 61.9% in 2005. Excluding the inventory step-up costs of $200,000 in 2006 and $2.7 million in 2005, gross profit would have increased to 63.8% for 2006 from 63.6% for 2005. The increase was primarily a result of a favorable product mix, partially offset by higher transportation, packaging and commodity costs.
Household Cleaning Segment
Gross profit for the Household Cleaning segment increased by $6.9 million, or 19.1%, for 2006. As a percentage of revenues, gross profit increased to 39.6% for 2006 from 36.6% in 2005. Excluding charges related to the inventory step-up of $2.4 million for 2005, gross profit as a percentage of revenues would have been 39.6% for 2006 compared to 39.1% for 2005. An increase in transportation costs in 2006 was offset by a favorable product mix.
Personal Care Segment
Gross profit for the Personal Care segment declined by $3.7 million, or 23.4%, for 2006. As a percentage of gross revenues, gross profit decreased to 43.2% for 2006 from 49.0% in 2005. The decrease in gross profit was due to the sales decline, as well as higher product and transportation costs. The increased product costs were the result of higher packaging and raw material costs and the introduction of a “value” size for Denorex in the third quarter of 2005.
Contribution Margin
2006 Contribution Margin | % | 2005 Contribution Margin | % | Increase (Decrease) | % | ||||||||||||||
| OTC Healthcare | $ | 80,027 | 49.7 | $ | 79,897 | 50.2 | $ | 130 | 0.2 | ||||||||||
| Household Cleaning | 36,218 | 33.6 | 30,202 | 30.9 | 6,016 | 19.9 | |||||||||||||
| Personal Care | 8,911 | 31.9 | 10,264 | 31.9 | (1,353 | ) | (13.2) | ||||||||||||
$ | 125,156 | 42.2 | $ | 120,363 | 41.6 | $ | 4,793 | 4.0 | |||||||||||
Contribution margin increased by $4.8 million, or 4.0%, to $125.2 million for 2006 from $120.4 million for 2005. The increase in contribution margin was due to the increased gross profit discussed above and a reduction of advertising and promotion spending in the Personal Care segment, partially offset by increased advertising and promotion spending on the core brands in the Over-the-Counter Healthcare segment and increases associated with the acquisitions of the Little Remedies, Chore Boy and The Doctor’s brands.
-44-
Over-the-Counter Healthcare Segment
Contribution margin for the Over-the-Counter Healthcare segment increased by $130,000, or 0.2%, for 2006 as a result of the increased gross profit discussed above, offset by increased spending behind Chloraseptic, Clear Eyes, Little Remedies and Compound W, as well as by spending behind The Doctor’s brand.
Household Cleaning Segment
Contribution margin for the Household Cleaning segment increased by $6.0 million, or 19.9%, for 2006 as a result of the increased gross profit discussed above, partially offset by an increase in advertising and promotion expenses. The increase in advertising and promotion expenses was a result of increased Comet media spending and advertising and promotion expenditures in support of the Chore Boy brand.
Personal Care Segment
Contribution margin for the Personal Care segment decreased by $1.4 million, or 13.2%, for 2006 as a result of the decrease in gross margin discussed above, partially offset by a reduction of $2.3 million of advertising and promotion expenses. The reduction in advertising and promotion expenses resulted primarily from the reduction of media support behind the Denorex brand.
General and Administrative Expenses
General and administrative expenses increased by $960,000, or 4.8%, to $21.2 million for 2006 from $20.2 million for 2005. The increase was due to accounting and legal costs associated with the Company’s restatement of financial results, initial year testing in connection with compliance with the provisions of Section 404 of the Sarbanes-Oxley Act, increased staffing and stock-based compensation expense resulting from the application of FASB Statement No. 123(R). These increases were partially offset by a significant reduction in employee incentive compensation as a result of the Company’s financial performance in 2006 not meeting internal objectives.
Depreciation and Amortization Expense
Depreciation and amortization expense increased by $1.0 million, or 10.2%, to $10.8 million for 2006 from $9.8 million for 2005. The increase was primarily due to amortization of intangible assets acquired with the Vetco and Dental Concepts acquisitions.
Impairment of Intangible Assets and Goodwill
During the fourth quarter of 2006, we recorded non-cash charges related to the impairment of certain intangible assets and goodwill of the Personal Care segment of $7.4 million and $1.9 million, respectively. We performed our impairment analyses of intangible assets and goodwill and determined, in accordance with FASB Statements No. 142 and 144, that the carrying amounts of these “branded” assets exceeded their fair market values as a result of declining sales.
Net Interest Expense
Net interest expense was $36.3 million in 2006 compared to $44.7 million in 2005. The decrease in interest expense of $8.4 million, or 18.7%, was due to the reduction of indebtedness outstanding, partially offset by higher interest rates on the remaining indebtedness. In February 2005, the Company significantly reduced debt with the proceeds from its IPO.
Loss on Extinguishment of Debt
For 2006 the loss on extinguishment of debt was $0, compared to $26.9 million for 2005. The $26.9 million loss on extinguishment of debt consisted of $19.3 million of charges related to the $184.0 million of debt retired in connection with our IPO and $7.6 million related to the write-off of deferred financing costs associated with the borrowings retired in connection with the Medtech acquisition.
Income Taxes
The provision for income taxes in 2006 was $21.3 million, with an effective rate of 44.7%, compared to a provision of $8.6 million for 2005, with an effective rate of 45.6%. The provision for income taxes in 2006 includes a $2.0 million charge, recorded during the quarter ended March 31, 2006, resulting from an increase in the state tax rate associated with the Company’s deferred tax liability. The increase resulted from the completion of a state tax nexus study during the quarter. The provision for income taxes in 2005 includes a $1.2 million
-45-
charge, recorded during the quarter ended March 31, 2005, resulting from an increase in the Company’s graduated federal income tax rate from 34% to 35% related to its deferred income tax liability.
Liquidity and Capital Resources
Liquidity
We have financed and expect to continue to finance our operations with a combination of internally generated funds and borrowings. In February 2005, we completed an initial public offering that provided the Company with net proceeds of $416.8 million which were used to repay the $100.0 million outstanding under the Tranche C Facility of our Senior Credit Facility, to redeem $84.0 million in aggregate principal amount of our existing 9.25% Senior Notes, to repurchase common stock held by the GTCR funds and the TCW/Crescent funds, and to redeem all of the outstanding senior preferred units and class B preferred units held by previous investors in Prestige International Holdings, LLC, the predecessor-in-interest to Prestige Brands Holdings, Inc. Effective upon the completion of the IPO, we entered into an amendment to the credit agreement that, among other things, allows us to increase the indebtedness we can incur under our Tranche B Term Loan Facility to $200.0 million and allows for an increase in our Revolving Credit Facility up to $60.0 million. Our principal uses of cash are for operating expenses, debt service, acquisitions, working capital and capital expenditures.
Year Ended March 31 | ||||||||||
(In thousands) | 2007 | 2006 | 2005 | |||||||
| Net cash provided by (used in): | ||||||||||
| Operating activities | $ | 71,899 | $ | 53,861 | $ | 51,042 | ||||
| Investing activities | (31,051 | ) | (54,163 | ) | (425,844 | ) | ||||
| Financing activities | (35,290 | ) | 3,168 | 376,743 | ||||||
Fiscal 2007 compared to fiscal 2006
Operating Activities
Net cash provided by operating activities was $71.9 million for 2007 compared to $53.9 million 2006. The $18.0 million increase in net cash provided by operating activities was primarily the result of the following:
| · | An increase of net income of $9.8 million from $26.3 million for 2006 to $36.1 million for 2007, |
| · | An improvement of $22.3 million in the components of operating assets and liabilities as a result of net operating assets and liabilities decreasing by $11.8 million in 2007 compared to an increase of $10.5 million in 2006, offset by |
| · | A decrease in non-cash expenses of $14.1 million from $38.1 million for 2006 to $24.0 million for 2007. |
Investing Activities
Net cash used for investing activities was $31.1 million for 2007 compared to $54.2 million for 2006. The net cash used for investing activities for 2007 was primarily the result of the acquisition of Wartner USA B.V., while during 2006, cash was used primarily for the acquisitions of the Chore Boy brand of cleaning pads and sponges and The Doctor’s brand of therapeutic oral care products.
Financing Activities
Net cash used for financing activities was $35.3 million for 2007 compared to net cash provided by financing activities of $3.2 million for 2006. During 2007, the Company repaid $31.6 million of indebtedness in excess of normal maturities with cash generated from operations. This reduced our outstanding indebtedness to $463.3 million from $498.6 million at March 31, 2006. In November 2005, the Company incurred $30.0 million of indebtedness to fund the acquisition of Dental Concepts LLC. Of such amount, $23.0 million was repaid during 2006.
The Company’s cash flow from operations exceeded net income due to the substantial non-cash charges related to depreciation and amortization of intangibles, increases in deferred income tax liabilities resulting from differences in the amortization of intangible assets and goodwill for income tax and financial reporting purposes, the
-46-
amortization of certain deferred financing costs and stock-based compensation.
Fiscal 2006 compared to fiscal 2005
Operating Activities
Net cash provided by operating activities was $53.9 million for 2006 compared to $51.0 million for 2005. The $2.9 million increase was primarily due to:
| · | An increase in net income of $16.1 million from $10.2 million for 2005 to $26.3 million for 2006, |
| · | A deterioration of $3.4 million in the components of operating assets and liabilities as a result of net operating assets and liabilities increasing by $10.5 million in 2006 compared to an increase of $7.1 million in 2005, and |
| · | A decrease in non-cash expenses of $9.8 million from $47.9 million for 2005 to $38.1 million for 2006. |
Investing Activities
Net cash used in investing activities was $54.2 million for 2006 compared to net cash used of $425.8 million for 2005. The net cash used in investing activities for 2006 was primarily for the acquisitions of the Chore Boy brand and Dental Concepts in October and November 2005, respectively. The net cash used in investing activities for 2005 was primarily for the acquisitions of Bonita Bay and Vetco in April and October 2004, respectively.
Financing Activities
Net cash provided by financing activities was $3.2 million for 2006 compared to $376.7 million for 2005. Net cash provided by financing activities for 2006 was due primarily to $30.0 million of borrowings on the Company’s revolving credit facility to finance the purchase of Dental Concepts, offset by repayments of $23.0 million, and $3.7 million of mandatory principal payments on the term loan. Net cash provided by financing activities for 2005 was primarily a function of the following events: (i) to finance the acquisitions of Bonita Bay and Vetco, the Company borrowed $698.5 million and issued preferred units and common units of $58.7 million, (ii) the repayment of the debt incurred in February 2004 at the time of the Medtech/Denorex acquisition and the repayment of the revolving credit facility and scheduled payments on current debt which all totaled $345.5 million, (iii) the February 2005 IPO raised $416.8 million, and (iv) the repayment of $184.0 million of debt, the repurchase $199.8 million of senior preferred units and class B preferred units, and the repurchase of 4.4 million shares of common stock for $30.2 million, all from the net proceeds of the IPO.
Capital Resources
On February 15, 2005, our initial public offering of common stock resulted in net proceeds of $416.8 million. The proceeds were used to repay the $100.0 million outstanding under the Tranche C facility of our senior credit facility and to redeem $84.0 million in aggregate principal amount of our existing 91/4% senior subordinated notes which were issued in connection with our acquisitions of Bonita Bay and Vetco in 2004.
As of March 31, 2007, we had an aggregate of $463.4 million of outstanding indebtedness, which consisted of the following:
| · | $337.4 million of borrowings under the Tranche B Term Loan Facility, and |
| · | $126.0 million of 9.25% Senior Subordinated Notes due 2012. |
We had $60.0 million of borrowing capacity available under the Revolving Credit Facility at such time, as well as $200.0 million available under the Tranche B Term Loan Facility.
All loans under the Senior Credit Facility bear interest at floating rates, based on either the prime rate, or at our option, the LIBOR rate, plus an applicable margin. As of March 31, 2007, an aggregate of $337.4 million was outstanding under the Senior Credit Facility at a weighted average interest rate of 7.63%.
-47-
In June 2004, we purchased a 5% interest rate cap agreement with a notional amount of $20.0 million which expired in June 2006. In March 2005, we purchased interest rate cap agreements that became effective August 30, 2005, with a total notional amount of $180.0 million and LIBOR cap rates ranging from 3.25% to 3.75%. On May 31, 2006, an interest rate cap agreement with a notional amount of $50.0 million and a 3.25% cap rate expired. Additionally, an interest rate cap agreement with a notional amount of $80.0 million and a 3.50% cap rate expired on May 30, 2007. The remaining agreement, with a notional amount of $50.0 million and a cap rate of 3.75%, terminates on May 30, 2008. The fair value of the interest rate cap agreements was $1.2 million at March 31, 2007.
The Tranche B Term Loan Facility matures in October 2011. We must make quarterly principal payments on the Tranche B Term Loan Facility equal to $887,500, representing 0.25% of the initial principal amount of the term loan. The Revolving Credit Facility matures and the commitments relating to the Revolving Credit Facility terminate in April 2009.
Effective as of December 19, 2006: (i) a Second Supplemental Indenture (“Second Supplemental Indenture”) and (ii) a Guaranty Supplement (“Indenture Guaranty Supplement”) were entered into with the trustee for the holders of the Senior Subordinated Notes. The Second Supplemental Indenture supplements and amends the indenture, dated as of April 6, 2004, as supplemented on October 6, 2004 (“Indenture”). Pursuant to the terms of the Second Supplemental Indenture and the Indenture Guaranty Supplement, the Company agreed to guaranty all of the obligations of Prestige Brands, Inc., an indirect wholly-owned subsidiary of the Company (“PBI”), set forth in the Indenture governing the PBI’s Senior Subordinated Notes. The Second Supplemental Indenture also amended the covenant requiring Prestige Brands International, LLC (“Prestige Brands International”), an indirect wholly-owned subsidiary of the Company, to file periodic reports with the SEC pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended (“Exchange Act”). So long as the Company or any other guarantor is required to file periodic reports under Section 13 or 15(d) of the Exchange Act that are substantially the same as the periodic reports that Prestige Brands International would otherwise be required to file with the SEC pursuant to the Indenture, Prestige Brands International is not required to file such reports.
Also effective as of December 19, 2006, a Joinder Agreement (“Joinder Agreement”) and a Guaranty Supplement (“Credit Agreement Guaranty Supplement”) were entered into with the administrative agent for the lenders under the Senior Credit Facility. Pursuant to the terms of the Joinder Agreement and the Credit Agreement Guaranty Supplement, the Company agreed to become a party to the Pledge and Security Agreement (“Security Agreement”) and the Guaranty (“Credit Agreement Guaranty”), each dated as of April 6, 2004, by PBI and certain of its affiliates in favor of the lenders. The Security Agreement and the Credit Agreement Guaranty secure the performance by PBI of its obligations under the Credit Agreement, dated as of April 6, 2004, as amended (“Credit Agreement”), by granting security interests to PBI's lenders in collateral owned by the Company and certain of its subsidiaries and providing guaranties of such obligations by certain of PBI’s affiliates.
The Revolving Credit Facility and the Tranche B Term Loan Facility contain various financial covenants, including provisions that require us to maintain certain leverage ratios, interest coverage ratios and fixed charge coverage ratios. The Revolving Credit Facility and the Tranche B Term Loan Facility, as well as the Senior Subordinated Notes, contain provisions that accelerate our indebtedness on certain changes in control and restrict us from undertaking specified corporate actions, including, asset dispositions, acquisitions, payment of dividends and other specified payments, repurchasing the Company’s equity securities in the public markets, incurrence of indebtedness, creation of liens, making loans and investments and transactions with affiliates. Specifically, we must:
| · | Have a leverage ratio of less than 5.0 to 1.0 for the quarter ended March 31, 2007, decreasing over time to 3.75 to 1.0 for the quarter ending September 30, 2010, and remaining level thereafter, |
| · | Have an interest coverage ratio of greater than 2.75 to 1.0 for the quarter ended March 31, 2007, increasing over time to 3.25 to 1.0 for the quarter ending March 31, 2010, and |
| · | Have a fixed charge coverage ratio of greater than 1.5 to 1.0 for the quarter ended March 31, 2007, and for each quarter thereafter until the quarter ending March 31, 2011. |
-48-
At March 31, 2007, we were in compliance with the applicable financial and restrictive covenants under the Senior Credit Facility and the Indenture governing the Senior Subordinated Notes.
Our principal sources of funds are anticipated to be cash flows from operating activities and available borrowings under the Revolving Credit Facility and Tranche B Term Loan Facility. We believe that these funds will provide us with sufficient liquidity and capital resources for us to meet our current and future financial obligations, as well as to provide funds for working capital, capital expenditures and other needs for at least the next 12 months. As part of our growth strategy, we regularly review acquisition opportunities and other potential strategic transactions, which may require additional debt or equity financing. If additional financing is required, there are no assurances that it will be available, or if available, that it can be obtained on terms favorable to us or on a basis that is not dilutive to our stockholders.
Commitments
As of March 31, 2007, we had ongoing commitments under various contractual and commercial obligations as follows:
Payments Due by Period | ||||||||||||||||
(In Millions) | Less than | 1 to 3 | 4 to 5 | After 5 | ||||||||||||
Contractual Obligations | Total | 1 Year | Years | Years | Years | |||||||||||
| Long-term debt | $ | 463.4 | $ | 3.6 | $ | 7.1 | $ | 326.7 | $ | 126.0 | ||||||
| Interest on long-term debt (1) | 160.0 | 37.5 | 74.0 | 48.5 | -- | |||||||||||
| Operating leases | 1.5 | 0.7 | 0.8 | -- | -- | |||||||||||
Total contractual cash obligations | $ | 624.9 | $ | 41.8 | $ | 81.9 | $ | 375.2 | $ | 126.0 | ||||||
| (1) | Represents the estimated interest obligations on the outstanding balances of the Revolving Credit Facility, Tranche B Term Loan Facility and Senior Subordinated Notes, together, assuming scheduled principal payments (based on the terms of the loan agreements) were made and assuming a weighted average interest rate of 8.07%. Estimated interest obligations would be different under different assumptions regarding interest rates or timing of principal payments. If interest rates on borrowings with variable rates increased by 1%, interest expense would increase approximately $3.4 million, in the first year. However, given the protection afforded by the interest rate cap agreements, the impact of a one percentage point increase would be limited to $2.7 million. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or financing activities with special-purpose entities.
Inflation
Inflationary factors such as increases in the costs of raw materials, packaging materials, purchased product and overhead may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial condition or results from operations for the periods referred to above, a high rate of inflation in the future could have a material adverse effect on our business, financial condition or results from operations. The recent increase in crude oil prices has had an adverse impact on transportation costs, as well as, certain petroleum based raw materials and packaging material. Although the Company takes efforts to minimize the impact of inflationary factors, including raising prices to our customers, a high rate of pricing volatility associated with crude oil supplies may continue to have an adverse effect on our operating results.
-49-
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLR Act”), including, without limitation, information within Management’s Discussion and Analysis of Financial Condition and Results of Operations. The following cautionary statements are being made pursuant to the provisions of the PSLR Act and with the intention of obtaining the benefits of the “safe harbor” provisions of the PSLR Act. Although we believe that our expectations are based on reasonable assumptions, actual results may differ materially from those in the forward-looking statements.
Forward-looking statements speak only as of the date of this Annual Report on Form 10-K. Except as required under federal securities laws and the rules and regulations of the SEC, we do not have any intention to update any forward-looking statements to reflect events or circumstances arising after the date of this Annual Report on Form 10-K, whether as a result of new information, future events or otherwise. As a result of these risks and uncertainties, readers are cautioned not to place undue reliance on forward-looking statements included in this Annual Report on Form 10-K or that may be made elsewhere from time to time by, or on behalf of, us. All forward-looking statements attributable to us are expressly qualified by these cautionary statements.
These forward-looking statements generally can be identified by the use of words or phrases such as “believe,” “anticipate,” “expect,” “estimate,” “project,” “will be,” “will continue,” “will likely result,” or other similar words and phrases. Forward-looking statements and our plans and expectations are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, and our business in general is subject to such risks. For more information, see “Risk Factors” contained in Item 1A of this Annual Report on Form 10-K. In addition, our expectations or beliefs concerning future events involve risks and uncertainties, including, without limitation:
| · | General economic conditions affecting our products and their respective markets, |
| · | The high level of competition in our industry and markets, |
| · | Our dependence on a limited number of customers for a large portion of our sales, |
| · | Disruptions in our distribution center, |
| · | Acquisitions or other strategic transactions diverting managerial resources, or incurrence of additional liabilities or integration problems associated with such transactions, |
| · | Changing consumer trends or pricing pressures which may cause us to lower our prices, |
| · | Increases in supplier prices, |
| · | Increases in transportation and fuel charges, |
| · | Changes in our senior management team, |
| · | Our ability to protect our intellectual property rights, |
| · | Our dependency on the reputation of our brand names, |
| · | Shortages of supply of sourced goods or interruptions in the manufacturing of our products, |
| · | Our level of debt, and ability to service our debt, |
| · | Any adverse judgments rendered in any pending litigation or arbitration, |
| · | Our ability to obtain additional financing, and |
| · | The restrictions imposed by our senior credit facility and the indenture on our operations. |
-50-
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to changes in interest rates because our senior credit facility is variable rate debt. Interest rate changes, therefore, generally do not affect the market value of such debt, but do impact the amount of our interest payments and, therefore, our future earnings and cash flows, assuming other factors are held constant. At March 31, 2007, we had variable rate debt of approximately $337.4 million related to our Tranche B term loan.
In an effort to protect the Company from the adverse impact that rising interest rates would have on our variable rate debt, we have entered into various interest rate cap agreements to hedge this exposure. In June 2004, we purchased a 5% interest rate cap agreement with a notional amount of $20.0 million which terminated in June 2006. In March 2005, we purchased interest rate cap agreements that became effective August 30, 2005, with a total notional amount of $180.0 million and LIBOR cap rates ranging from 3.25% to 3.75%. On May 31, 2006, an interest rate cap agreement with a notional amount of $50.0 million and a 3.25% cap rate expired. Additionally, an interest rate cap agreement with a notional amount of $80.0 million and a 3.50% cap rate expired on May 30, 2007. The remaining agreement, with a notional amount of $50.0 million and a cap rate of 3.75%, terminates on May 30, 2008.
Holding other variables constant, including levels of indebtedness, a one percentage point increase in interest rates on our variable rate debt would have an adverse impact on pre-tax earnings and cash flows for fiscal 2008 of approximately $3.4 million. However, given the protection afforded by the interest rate cap agreements, the impact of a one percentage point increase would be limited to $2.7 million. The fair value of the interest rate cap agreements was $1.2 million at March 31, 2007.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required by this Item are described in Part IV, Item 15 of this Annual Report on Form 10-K and are presented beginning on page F-1.
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
Disclosure Controls and Procedures
The Company’s management, with the participation of its Chief Executive Officer and the Chief Financial Officer, evaluated the effectiveness of the Company’s disclosure controls and procedures, as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934 (“Exchange Act”) as of March 31, 2007. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that, as of March 31, 2007, the Company’s disclosure controls and procedures were effective to ensure that information required to be disclosed by the Company in the reports the Company files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
-51-
Management’s Annual Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act). Internal control over financial reporting is a process designed by, or under the supervision of the Chief Executive Officer and Chief Financial Officer and effected by the Board of Directors, Management and other personnel, to provide reasonable assurance regarding reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable, not absolute, assurance that the control objectives will be met. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate over time.
Management, with the participation of the Chief Executive Officer and Chief Financial Officer, has assessed the effectiveness of the Company’s internal control over financial reporting as of March 31, 2007. In making its assessment, management has used the criteria established by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control - Integrated Framework (the “COSO Criteria”).
Based on our assessment utilizing the COSO Criteria, management has concluded that the Company’s internal control over financial reporting was effective as of March 31, 2007.
Management’s assessment of the effectiveness of the Company’s internal control over financial reporting, as of March 31, 2007, has been audited by PricewaterhouseCoopers LLP (“PWC”), the Company’s independent registered public accounting firm, who also audited the Company’s consolidated financial statements, as stated in their report included in Part IV, Item 15, of this Annual Report on Form 10-K. PWC has issued an attestation report on management’s assessment of the Company’s internal controls over financial reporting, a copy of which is included in PWC’s report contained in Part IV, Item 15, of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There have been no changes during the quarter ended March 31, 2007 in the Company’s internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
-52-
Part III
Information required to be disclosed by this Item will be contained in the Company’s 2007 Proxy Statement, which is incorporated herein by reference.
Information required to be disclosed by this Item will be contained in the Company’s 2007 Proxy Statement, which is incorporated herein by reference.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information required to be disclosed by this Item will be contained in the Company’s 2007 Proxy Statement, which is incorporated herein by reference.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, DIRECTOR INDEPENDENCE |
Information required to be disclosed by this Item will be contained in the Company’s 2007 Proxy Statement, which is incorporated herein by reference.
Information required to be disclosed by this Item will be contained in the Company’s 2007 Proxy Statement, which is incorporated herein by reference.
-53-
Part IV
(a) (1) Financial Statements
The financial statements and financial statement schedules listed below are set forth at pages F-1 through F-37 of this Annual Report on Form 10-K, which are incorporated herein to this Item as if copied verbatim.
Prestige Brands Holdings, Inc. |
Report of Independent Registered Public Accounting Firm, PricewaterhouseCoopers LLP |
Consolidated Statements of Operations for each of the three years in the period ended March 31, 2007 |
| Consolidated Balance Sheets at March 31, 2007 and 2006 |
Consolidated Statements of Members’ and Stockholders’ Equity and Comprehensive Income for each of the three years in the period ended March 31, 2007 |
Consolidated Statements of Cash Flows for each of the three years in the period ended March 31, 2007 |
| Notes to Consolidated Financial Statements |
| Schedule II—Valuation and Qualifying Accounts |
(a) (2) Financial Statement Schedules
Schedule II - Valuation and Qualifying Accounts listed in (a) (1) above is incorporated herein by reference as if copied verbatim. Schedules other than those listed in the preceding sentence have been omitted as they are either not required, not applicable, or the information has otherwise been shown in the consolidated financial statements or notes thereto.
(b) Exhibits
See Exhibit Index immediately following the F pages to this Annual Report on Form 10-K.
-54-
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PRESTIGE BRANDS HOLDINGS, INC.
By: /s/ PETER J. ANDERSON
Name: Peter J. Anderson
Title: Chief Financial Officer
Date: June 14, 2007
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ MARK PETTIE | Chairman of the Board and Chief Executive Officer | June 14, 2007 | ||
| Mark Pettie | (Principal Executive Officer) | |||
/s/ PETER J. ANDERSON | Chief Financial Officer | June 14, 2007 | ||
| Peter J. Anderson | (Principal Financial Officer and | |||
| Principal Accounting Officer) | ||||
/s/ L. DICK BUELL | Director | June 14, 2007 | ||
| L. Dick Buell | ||||
/s/ JOHN E. BYOM | Director | June 14, 2007 | ||
| John E. Byom | ||||
/s/ GARY E. COSTLEY | Director | June 14, 2007 | ||
| Gary E. Costley | ||||
/s/ DAVID A. DONNINI | Director | June 14, 2007 | ||
| David A. Donnini | ||||
/s/ RONALD B. GORDON | Director | June 14, 2007 | ||
| Ronald B. Gordon | ||||
/s/ VINCENT J. HEMMER | Director | June 14, 2007 | ||
| Vincent J. Hemmer | ||||
/s/ PATRICK M. LONERGAN | Director | June 14, 2007 | ||
| Patrick M. Lonergan | ||||
/s/ PETER C. MANN | Director | June 14, 2007 | ||
| Peter C. Mann | ||||
/s/ RAYMOND P. SILCOCK | Director | June 14, 2007 | ||
| Raymond P. Silcock |
-55-
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Prestige Brands Holdings, Inc.
Audited Financial Statements
March 31, 2007
| Report of Independent Registered Public Accounting Firm, PricewaterhouseCoopers LLP | F-1 | |
Consolidated Statements of Operations for each of the three years in the period ended March 31, 2007 | F-3 | |
| Consolidated Balance Sheets at March 31, 2007 and 2006 | F-4 | |
Consolidated Statements of Members’ and Stockholders’ Equity and Comprehensive Income for each of the three years in the period ended March 31, 2007 | F-5 | |
Consolidated Statements of Cash Flows for each of the three year in the period ended March 31, 2007 | F-9 | |
| Notes to Consolidated Financial Statements | F-11 | |
| Schedule II—Valuation and Qualifying Accounts | F-37 | |
-56-
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Prestige Brands Holdings, Inc.
We have completed integrated audits of Prestige Brands Holdings, Inc.’s 2007 and 2006 consolidated financial statements and of its internal control over financial reporting as of March 31, 2007 and an audit of its 2005 consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our opinions, based on our audits, are presented below.
Consolidated financial statements and financial statement schedule
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of members’ and stockholders’ equity and comprehensive income and of cash flows present fairly, in all material respects, the financial position of Prestige Brands Holdings, Inc. and its subsidiaries at March 31, 2007 and 2006, and the results of their operations and their cash flows for each of the three years in the period ended March 31, 2007 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit of financial statements includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
Internal control over financial reporting
Also, in our opinion, management’s assessment, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 9A, that the Company maintained effective internal control over financial reporting as of March 31, 2007 based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations (“COSO”) of the Treadway Commission, is fairly stated, in all material respects, based on those criteria. Furthermore, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2007, based on criteria established in Internal Control - Integrated Framework issued by COSO. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express opinions on management’s assessment and on the effectiveness of the Company’s internal control over financial reporting based on our audit. We conducted our audit of internal control over financial reporting in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. An audit of internal control over financial reporting includes obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we consider necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinions.
F - 1
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Salt Lake City, Utah
June 14, 2007
F - 2
Prestige Brands Holdings, Inc.
Consolidated Statements of Operations
Year Ended March 31 | ||||||||||
(In thousands, except per share data) | 2007 | 2006 | 2005 | |||||||
Revenues | ||||||||||
| Net sales | $ | 316,847 | $ | 296,239 | $ | 288,918 | ||||
| Other revenues | 1,787 | 429 | 151 | |||||||
| Total revenues | 318,634 | 296,668 | 289,069 | |||||||
Cost of Sales | ||||||||||
| Cost of sales | 153,147 | 139,430 | 139,009 | |||||||
| Gross profit | 165,487 | 157,238 | 150,060 | |||||||
Operating Expenses | ||||||||||
| Advertising and promotion | 32,005 | 32,082 | 29,697 | |||||||
| General and administrative | 28,416 | 21,158 | 20,198 | |||||||
| Depreciation | 744 | 1,736 | 1,899 | |||||||
| Amortization of intangible assets | 9,640 | 9,041 | 7,901 | |||||||
| Impairment of goodwill | -- | 1,892 | -- | |||||||
| Impairment of intangible asset | -- | 7,425 | -- | |||||||
| Total operating expenses | 70,805 | 73,334 | 59,695 | |||||||
| Operating income | 94,682 | 83,904 | 90,365 | |||||||
Other income (expense) | ||||||||||
| Interest income | 972 | 568 | 371 | |||||||
| Interest expense | (40,478 | ) | (36,914 | ) | (45,097 | ) | ||||
| Loss on disposal of equipment | -- | -- | (9 | ) | ||||||
| Loss on extinguishment of debt | -- | -- | (26,854 | ) | ||||||
| Total other income (expense) | (39,506 | ) | (36,346 | ) | (71,589 | ) | ||||
| Income before income taxes | 55,176 | 47,558 | 18,776 | |||||||
| Provision for income taxes | (19,098 | ) | (21,281 | ) | (8,556 | ) | ||||
| Net income | 36,078 | 26,277 | 10,220 | |||||||
Cumulative preferred dividends on Senior Preferred and Class B Preferred Units | -- | -- | (25,395 | ) | ||||||
Net income (loss) available to members and common stockholders | $ | 36,078 | $ | 26,277 | $ | (15,175 | ) | |||
| Basic earnings (loss) per share | $ | 0.73 | $ | 0.54 | $ | (0.55 | ) | |||
| Diluted earnings (loss) per share | $ | 0.72 | $ | 0.53 | $ | (0.55 | ) | |||
Weighted average shares outstanding: Basic | 49,460 | 48,908 | 27,546 | |||||||
| Diluted | 50,020 | 50,008 | 27,546 | |||||||
See accompanying notes.
F - 3
Prestige Brands Holdings, Inc.
Consolidated Balance Sheets
(In thousands)
Assets | March 31, 2007 | March 31, 2006 | |||||
| Current assets | |||||||
| Cash and cash equivalents | $ | 13,758 | $ | 8,200 | |||
| Accounts receivable | 35,167 | 40,042 | |||||
| Inventories | 30,173 | 33,841 | |||||
| Deferred income tax assets | 2,735 | 3,227 | |||||
| Prepaid expenses and other current assets | 1,935 | 701 | |||||
| Total current assets | 83,768 | 86,011 | |||||
| Property and equipment | 1,449 | 1,653 | |||||
| Goodwill | 310,947 | 297,935 | |||||
| Intangible assets | 657,157 | 637,197 | |||||
| Other long-term assets | 10,095 | 15,849 | |||||
| Total Assets | $ | 1,063,416 | $ | 1,038,645 | |||
Liabilities and Stockholders’ Equity | |||||||
| Current liabilities | |||||||
| Accounts payable | $ | 19,303 | $ | 18,065 | |||
| Accrued interest payable | 7,552 | 7,563 | |||||
| Income taxes payable | -- | 1,795 | |||||
| Other accrued liabilities | 10,505 | 4,582 | |||||
| Current portion of long-term debt | 3,550 | 3,730 | |||||
| Total current liabilities | 40,910 | 35,735 | |||||
| Long-term debt | 459,800 | 494,900 | |||||
| Other long-term liabilities | 2,801 | -- | |||||
| Deferred income tax liabilities | 114,571 | 98,603 | |||||
| Total Liabilities | 618,082 | 629,238 | |||||
Commitments and Contingencies - Note 15 | |||||||
Stockholders’ Equity | |||||||
| Preferred stock - $0.01 par value | |||||||
Authorized - 5,000 shares | |||||||
Issued and outstanding - None | -- | -- | |||||
| Common stock - $0.01 par value | |||||||
Authorized - 250,000 shares | |||||||
Issued - 50,060 shares and 50,056 shares at March 31, 2007 and 2006, respectively | 501 | 501 | |||||
| Additional paid-in capital | 379,225 | 378,570 | |||||
Treasury stock, at cost - 55 shares and 18 shares at March 31, 2007 and 2006, respectively | (40 | ) | (30 | ) | |||
| Accumulated other comprehensive income | 313 | 1,109 | |||||
| Retained earnings | 65,335 | 29,257 | |||||
| Total stockholders’ equity | 445,334 | 409,407 | |||||
| Total Liabilities and Stockholders’ Equity | $ | 1,063,416 | $ | 1,038,645 | |||
See accompanying notes.
F - 4
Prestige Brands Holdings, Inc.
Consolidated Statement of Changes in Members’
and Stockholders’ Equity and Comprehensive Income
Senior Preferred Units | Class B Preferred Units | Common Units | Common Stock | ||||||||||||||||||||||
(In thousands) | Units | Amount | Units | Amount | Units | Amount | Shares | Amount | |||||||||||||||||
| Balances at March 31, 2004 | 23 | $ | 17,768 | 107 | $ | 96,807 | 57,902 | $ | 5,273 | -- | $ | -- | |||||||||||||
Issuance of Preferred and Common Units for cash | -- | -- | 58 | 58,385 | 1,839 | 148 | -- | -- | |||||||||||||||||
Issuance of Preferred and Common Units in conjunction with the Bonita Bay Acquisition | -- | -- | -- | 91 | 19 | 1 | -- | -- | |||||||||||||||||
Repurchase/cancellation of Preferred and Common Units in conjunction with the Bonita Bay Acquisition | -- | -- | (2 | ) | -- | (1,987 | ) | (46 | ) | -- | -- | ||||||||||||||
Issuance of restricted Common Units to management for cash | -- | -- | -- | -- | 337 | 235 | -- | -- | |||||||||||||||||
Exchange of Common Units for Common Stock | -- | -- | -- | -- | (58,110 | ) | (5,611 | ) | 26,666 | 267 | |||||||||||||||
Issuance of Common Stock in Initial Public Offering, net | -- | -- | -- | -- | -- | -- | 28,000 | 280 | |||||||||||||||||
| Redemption of Preferred Units | (23 | ) | (17,768 | ) | (163 | ) | (155,283 | ) | -- | -- | -- | -- | |||||||||||||
| Retirement of Common Stock | -- | -- | -- | -- | -- | -- | (4,666 | ) | (47 | ) | |||||||||||||||
| Purchase of Treasury Stock | -- | -- | -- | -- | -- | -- | -- | -- | |||||||||||||||||
| Components of comprehensive income | |||||||||||||||||||||||||
| Net income | -- | -- | -- | -- | -- | -- | -- | -- | |||||||||||||||||
Unrealized gain on interest rate caps, net of income tax expense of $200 | -- | -- | -- | -- | -- | -- | -- | -- | |||||||||||||||||
| Total comprehensive income | -- | -- | -- | -- | -- | -- | -- | -- | |||||||||||||||||
| Balances at March 31, 2005 | -- | $ | - | -- | $ | -- | -- | $ | -- | 50,000 | $ | 500 | |||||||||||||
See accompanying notes.
F - 5
Prestige Brands Holdings, Inc. Consolidated Statement of Changes in Members’ and Stockholders’ Equity and Comprehensive Income (Continued) | |||||||||||||||||||
Additional Paid-in | Treasury Stock | Accumulated Other Comprehensive Income | Retained Earnings (Accumulated | ||||||||||||||||
Capital | Shares | Amount | (Loss) | Deficit) | Total | ||||||||||||||
(In thousands) | |||||||||||||||||||
| Balances at March 31, 2004 | $ | 4,871 | -- | $ | -- | $ | -- | $ | 1,229 | $ | 125,948 | ||||||||
Issuance of Preferred and Common Units for cash | -- | -- | -- | -- | -- | 58,533 | |||||||||||||
Issuance of Preferred and Common Units in conjunction with the Bonita Bay Acquisition | -- | -- | -- | -- | -- | 92 | |||||||||||||
Repurchase/cancellation of Preferred and Common Units in conjunction with the Bonita Bay Acquisition | -- | -- | -- | -- | -- | (46 | ) | ||||||||||||
Issuance of restricted Common Units to management for cash | -- | -- | -- | -- | -- | 235 | |||||||||||||
Exchange of Common Units for Common Stock | 5,344 | -- | -- | -- | -- | -- | |||||||||||||
Issuance of Common Stock in Initial Public Offering, net | 416,552 | -- | -- | -- | -- | 416,832 | |||||||||||||
| Redemption of Preferred Units | (18,315 | ) | -- | -- | -- | (8,469 | ) | (199,835 | ) | ||||||||||
| Retirement of Common Stock | (30,201 | ) | -- | -- | -- | -- | (30,248 | ) | |||||||||||
| Purchase of Treasury Stock | -- | 2 | (4 | ) | -- | -- | (4 | ) | |||||||||||
| Components of comprehensive income | |||||||||||||||||||
| Net income | -- | -- | -- | -- | 10,220 | 10,220 | |||||||||||||
Unrealized gain on interest rate caps, net of income tax expense of $200 | -- | -- | -- | 320 | -- | 320 | |||||||||||||
| Total comprehensive income | -- | -- | -- | -- | -- | 10,540 | |||||||||||||
| Balances at March 31, 2005 | $ | 378,251 | 2 | $ | (4 | ) | $ | 320 | $ | 2,980 | $ | 382,047 | |||||||
F - 6
Prestige Brands Holdings, Inc.
Consolidated Statement of Changes in Members’
and Stockholders’ Equity and Comprehensive Income
(Continued)
Common Stock Par Shares Value | Additional Paid-in Capital | Treasury Stock Shares Amount | Accumulated Other Comprehensive Income | Retained Earnings | Totals | ||||||||||||||||||||
(In thousands) | |||||||||||||||||||||||||
| Balances at March 31, 2005 | 50,000 | $ | 500 | $ | 378,251 | 2 | $ | (4 | ) | $ | 320 | $ | 2,980 | $ | 382,047 | ||||||||||
| Additional costs associated with initial public offering | -- | -- | (63 | ) | -- | -- | -- | -- | (63 | ) | |||||||||||||||
| Stock-based compensation | 56 | 1 | 382 | -- | -- | -- | -- | 383 | |||||||||||||||||
| Purchase of common stock for treasury | -- | -- | -- | 16 | (26 | ) | -- | -- | (26 | ) | |||||||||||||||
| Components of comprehensive income | |||||||||||||||||||||||||
| Net income | -- | -- | -- | -- | -- | -- | 26,277 | 26,277 | |||||||||||||||||
| Amortization of interest rate caps reclassified into earnings, net of income tax expense of $192 | -- | -- | -- | -- | -- | 298 | -- | 298 | |||||||||||||||||
| Unrealized gain on interest rate caps, net of income tax expense of $208 | -- | -- | -- | -- | -- | 491 | -- | 491 | |||||||||||||||||
| Total comprehensive income | -- | -- | -- | -- | -- | -- | -- | 27,066 | |||||||||||||||||
| Balances at March 31, 2006 | 50,056 | $ | 501 | $ | 378,570 | 18 | $ | (30 | ) | $ | 1,109 | $ | 29,257 | $ | 409,407 | ||||||||||
See accompanying notes.
F - 7
Prestige Brands Holdings, Inc.
Consolidated Statement of Changes in Members’
and Stockholders’ Equity and Comprehensive Income
(Continued)
Common Stock Par Shares Value | Additional Paid-in Capital | Treasury Stock Shares Amount | Accumulated Other Comprehensive Income | Retained Earnings | Totals | ||||||||||||||||||||
(In thousands) | |||||||||||||||||||||||||
| Balances at March 31, 2006 | 50,056 | $ | 501 | $ | 378,570 | 18 | $ | (30 | ) | $ | 1,109 | $ | 29,257 | $ | 409,407 | ||||||||||
| Stock-based compensation | 4 | 655 | 655 | ||||||||||||||||||||||
| Purchase of common stock for treasury | 37 | (10 | ) | (10 | ) | ||||||||||||||||||||
| Components of comprehensive income | |||||||||||||||||||||||||
| Net income | 36,078 | 36,078 | |||||||||||||||||||||||
Amortization of interest rate caps reclassified into earnings, net of income tax expense of $429 | 678 | 678 | |||||||||||||||||||||||
Unrealized loss on interest rate caps, net of income tax benefit of $931 | (1,474 | ) | (1,474 | ) | |||||||||||||||||||||
| Total comprehensive income | 35,282 | ||||||||||||||||||||||||
| Balances at March 31, 2007 | 50,060 | $ | 501 | $ | 379,225 | 55 | $ | (40 | ) | $ | 313 | $ | 65,335 | $ | 445,334 | ||||||||||
See accompanying notes.
F - 8
Prestige Brands Holdings, Inc.
Consolidated Statements of Cash Flows
Year Ended March 31 | ||||||||||
(In thousands) | 2007 | 2006 | 2005 | |||||||
Operating Activities | ||||||||||
| Net income | $ | 36,078 | $ | 26,277 | $ | 10,220 | ||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||
| Depreciation and amortization | 10,384 | 10,777 | 9,800 | |||||||
| Amortization of financing costs | 3,257 | 2,649 | 2,943 | |||||||
| Impairment of goodwill and intangible assets | -- | 9,317 | -- | |||||||
| Deferred income taxes | 9,662 | 14,976 | 8,344 | |||||||
| Stock-based compensation | 655 | 383 | -- | |||||||
| Loss on extinguishment of debt | -- | -- | 26,854 | |||||||
| Other | -- | -- | 9 | |||||||
Changes in operating assets and liabilities, net of effects of purchases of businesses | ||||||||||
| Accounts receivable | 4,875 | (1,350 | ) | (7,227 | ) | |||||
| Inventories | 4,292 | (7,156 | ) | 2,922 | ||||||
| Prepaid expenses and other assets | (1,235 | ) | 2,623 | (1,490 | ) | |||||
| Accounts payable | (186 | ) | (6,037 | ) | 5,059 | |||||
| Income taxes payable | (1,795 | ) | 1,795 | -- | ||||||
| Other accrued liabilities | 5,912 | (393 | ) | (6,392 | ) | |||||
| Net cash provided by operating activities | 71,899 | 53,861 | 51,042 | |||||||
Investing Activities | ||||||||||
| Purchases of equipment | (540 | ) | (519 | ) | (365 | ) | ||||
| Purchases of intangible assets | -- | (22,655 | ) | -- | ||||||
| Change in other assets due to purchase price adjustments | 750 | -- | -- | |||||||
| Purchases of businesses, net | (31,261 | ) | (30,989 | ) | (425,479 | ) | ||||
| Net cash used for investing activities | (31,051 | ) | (54,163 | ) | (425,844 | ) | ||||
Financing Activities | ||||||||||
| Proceeds from the issuance of notes | -- | 30,000 | 698,512 | |||||||
| Payment of deferred financing costs | -- | (13 | ) | (24,539 | ) | |||||
| Repayment of notes | (35,280 | ) | (26,730 | ) | (529,538 | ) | ||||
| Prepayment penalty | -- | -- | (10,875 | ) | ||||||
| Payments on interest rate caps | -- | -- | (2,283 | ) | ||||||
| Proceeds from the issuance of equity, net | -- | (63 | ) | 475,554 | ||||||
| Redemption of equity interests | (10 | ) | (26 | ) | (230,088 | ) | ||||
| Net cash provided by (used for) financing activities | (35,290 | ) | 3,168 | 376,743 | ||||||
| Increase in cash | 5,558 | 2,866 | 1,941 | |||||||
| Cash - beginning of period | 8,200 | 5,334 | 3,393 | |||||||
| Cash - end of period | $ | 13,758 | $ | 8,200 | $ | 5,334 | ||||
See accompanying notes.
F - 9
Prestige Brands Holdings, Inc.
Consolidated Statements of Cash Flows
(Continued)
Year Ended March 31 | ||||||||||
2007 | 2006 | 2005 | ||||||||
Supplemental Cash Flow Information | ||||||||||
Purchases of Businesses | ||||||||||
| Fair value of assets acquired, net of cash acquired | $ | 42,115 | $ | 34,706 | $ | 655,542 | ||||
| Fair value of liabilities assumed | (10,854 | ) | (3,717 | ) | (229,971 | ) | ||||
| Purchase price funded with non-cash contributions | -- | -- | (92 | ) | ||||||
| Cash paid to purchase businesses | $ | 31,261 | $ | 30,989 | $ | 425,479 | ||||
| Interest paid | $ | 37,234 | $ | 33,760 | $ | 42,155 | ||||
| Income taxes paid | $ | 11,751 | $ | 2,852 | $ | 2,689 | ||||
See accompanying notes.
F - 10
Prestige Brands Holdings, Inc.
Notes to Consolidated Financial Statements
1. | Business and Basis of Presentation |
Nature of Business
Prestige Brands Holdings, Inc. (referred to herein as the “Company” which reference shall, unless the context requires otherwise, be deemed to refer to Prestige Brands Holdings, Inc. and all of its direct or indirect wholly-owned subsidiaries on a consolidated basis) is engaged in the marketing, sales and distribution of over-the-counter healthcare, personal care and household cleaning brands to mass merchandisers, drug stores, supermarkets and club stores primarily in the United States and Canada. Prestige Brands Holdings, Inc. is a holding company with no assets or operations and is also the parent guarantor of the senior revolving credit facility, senior secured term loan facility and the senior subordinated notes more fully described in Note 9 to the consolidated financial statements.
On April 6, 2004, Prestige International Holdings, LLC, an entity controlled by affiliates of GTCR Golder Rauner II, LLC (“Prestige LLC”), through an indirect wholly-owned subsidiary, acquired all of the outstanding capital stock of Bonita Bay Holdings, Inc. (“Bonita Bay”). On October 6, 2004, Prestige LLC, through an indirect wholly-owned subsidiary, acquired all of the outstanding capital stock of Vetco, Inc. (“Vetco”). On February 9, 2005, the Company became the direct parent company of Prestige LLC, under the terms of an exchange agreement among the Company, Prestige LLC and each holder of common units of Prestige LLC, whereby the holders of common units of Prestige LLC exchanged all of their common units for an aggregate of 26.7 million shares of common stock of the Company. On February 9, 2005, the Company completed an initial public offering. On November 8, 2005, the Company, through a wholly-owned subsidiary, acquired the ownership interests of Dental Concepts LLC (“Dental Concepts”). In addition, on September 21, 2006, the Company, through a wholly-owned subsidiary, acquired the ownership interests of Wartner USA B.V. (“Wartner”).
Basis of Presentation
The Bonita Bay, Vetco, Dental Concepts and Wartner acquisitions were accounted for as purchase transactions. The results of operations and cash flows of Bonita Bay, Vetco, Dental Concepts and Wartner have been reflected in the Company’s consolidated statements of operations and cash flows beginning from their respective acquisition dates. The formation of the Company and exchange of common units for common shares was accounted for as a reorganization of entities under common control. As a result, there was no adjustment to the carrying value of the assets and liabilities. All significant intercompany transactions and balances have been eliminated.
The Company’s fiscal year ends on March 31st of each year. References in these financial statements or notes to a year (e.g., “2007”) means the Company’s fiscal year ended on March 31st of that year.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Although these estimates are based on the Company’s knowledge of current events and actions that the Company may undertake in the future, actual results could differ from those estimates. As discussed below, the Company’s most significant estimates include those made in connection with the valuation of intangible assets, sales returns and allowances, trade promotional allowances and inventory obsolescence.
Cash and Cash Equivalents
The Company considers all short-term deposits and investments with original maturities of three months or less to be cash equivalents. Substantially all of the Company’s cash is held by one bank located in Wyoming. The Company does not believe that, as a result of this concentration, it is subject to any unusual financial risk beyond the normal risk associated with commercial banking relationships.
F - 11
Accounts Receivable
The Company extends non-interest bearing trade credit to its customers in the ordinary course of business. The Company maintains an allowance for doubtful accounts receivable based upon historical collection experience and expected collectibility of the accounts receivable. In an effort to reduce credit risk, the Company (i) has established credit limits for all of its customer relationships, (ii) performs ongoing credit evaluations of customers’ financial condition, (iii) monitors the payment history and aging of customers’ receivables, and (iv) monitors open orders against an individual customer’s outstanding receivable balance.
Inventories
Inventories are stated at the lower of cost or fair value, where cost is determined by using the first-in, first-out method. The Company provides an allowance for slow moving and obsolete inventory, whereby it reduces inventories for the diminution of value, resulting from product obsolescence, damage or other issues affecting marketability, equal to the difference between the cost of the inventory and its estimated market value. Factors utilized in the determination of estimated market value include (i) current sales data and historical return rates, (ii) estimates of future demand, (iii) competitive pricing pressures, (iv) new product introductions, (v) product expiration dates, and (vi) component and packaging obsolescence.
Property and Equipment
Property and equipment are stated at cost and are depreciated using the straight-line method based on the following estimated useful lives:
| Years | |
| Machinery | 5 |
| Computer equipment | 3 |
| Furniture and fixtures | 7 |
| Leasehold improvements | 5 |
Expenditures for maintenance and repairs are charged to expense as incurred. When an asset is sold or otherwise disposed of, the cost and associated accumulated depreciation are removed from the accounts and the resulting gain or loss is recognized in the consolidated statement of operations.
Property and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. An impairment loss is recognized if the carrying amount of the asset exceeds its fair value.
Goodwill
The excess of the purchase price over the fair market value of assets acquired and liabilities assumed in purchase business combinations is classified as goodwill. In accordance with Financial Accounting Standards Board (“FASB”) Statement of Financial Accounting Standards (“Statement”) No. 142, “Goodwill and Other Intangible Assets,” the Company does not amortize goodwill, but performs impairment tests of the carrying value at least annually. The Company tests goodwill for impairment at the “brand” level which is one level below the operating segment level.
Intangible Assets
Intangible assets, which are composed primarily of trademarks, are stated at cost less accumulated amortization. For intangible assets with finite lives, amortization is computed on the straight-line method over estimated useful lives ranging from five to 30 years.
Indefinite lived intangible assets are tested for impairment at least annually, while intangible assets with finite lives are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. An impairment loss is recognized if the carrying amount of the asset exceeds its fair value.
Deferred Financing Costs
The Company has incurred debt issuance costs in connection with its long-term debt. These costs are capitalized as deferred financing costs and amortized using the straight-line method, which approximates the effective interest method, over the term of the related debt.
F - 12
Revenue Recognition
Revenues are recognized in accordance with Securities and Exchange Commission ("SEC") Staff Accounting Bulletin 104, “Revenue Recognition,” when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the product has been shipped and the customer takes ownership and assumes risk of loss; (3) the selling price is fixed or determinable; and (4) collection of the resulting receivable is reasonably assured. The Company has determined that the transfer of risk of loss generally occurs when product is received by the customer and, accordingly, recognizes revenue at that time. Provision is made for estimated discounts related to customer payment terms and estimated product returns at the time of sale based on the Company’s historical experience.
As is customary in the consumer products industry, the Company participates in the promotional programs of its customers to enhance the sale of its products. The cost of these promotional programs varies based on the actual number of units sold during a finite period of time. The Company estimates the cost of such promotional programs at their inception based on historical experience and current market conditions and reduces sales by such estimates. These promotional programs consist of direct to consumer incentives such as coupons and temporary price reductions, as well as incentives to the Company’s customers, such as slotting fees and cooperative advertising. Estimates of the costs of these promotional programs are based on (i) historical sales experience, (ii) the current offering, (iii) forecasted data, (iv) current market conditions, and (v) communication with customer purchasing/marketing personnel. At the completion of the promotional program, the estimated amounts are adjusted to actual results.
Due to the nature of the consumer products industry, the Company is required to estimate future product returns. Accordingly, the Company records an estimate of product returns concurrent with recording sales which is made after analyzing (i) historical return rates, (ii) current economic trends, (iii) changes in customer demand, (iv) product acceptance, (v) seasonality of the Company’s product offerings, and (vi) the impact of changes in product formulation, packaging and advertising.
Costs of Sales
Costs of sales include product costs, warehousing costs, inbound and outbound shipping costs, and handling and storage costs. Shipping, warehousing and handling costs were $24.3 million, $24.5 million and $22.7 million for 2007, 2006 and 2005, respectively.
Advertising and Promotion Costs
Advertising and promotion costs are expensed as incurred. Slotting fees associated with products are recognized as a reduction of sales. Under slotting arrangements, the retailers allow the Company’s products to be placed on the stores’ shelves in exchange for such fees. Direct reimbursements of advertising costs are reflected as a reduction of advertising costs in the period earned.
Stock-based Compensation
During 2006, the Company adopted FASB, Statement No. 123(R), “Share-Based Payment” (“Statement No. 123(R)”) with the grants of restricted stock and options to purchase common stock to employees and directors in accordance with the provisions of the Company’s 2005 Long-Term Equity Incentive Plan (“the Plan”). Statement No. 123(R) requires the Company to measure the cost of services to be rendered based on the grant-date fair value of the equity award. Compensation expense is to be recognized over the period an employee is required to provide service in exchange for the award, generally referred to as the requisite service period. The Company recorded stock-based compensation charges of $655,000 and $383,000 during 2007 and 2006, respectively.
Income Taxes
Income taxes are recorded in accordance with the provisions of FASB Statement No. 109, “Accounting for Income Taxes” (“Statement No. 109”). Pursuant to Statement No. 109, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is established when necessary to reduce deferred tax assets to the amounts expected to be realized.
F - 13
Derivative Instruments
FASB Statement No. 133, “Accounting for Derivative Instruments and Hedging Activities” (“Statement No. 133”), requires companies to recognize derivative instruments as either assets or liabilities in the balance sheet at fair value. The accounting for changes in the fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and further, on the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, a company must designate the hedging instrument, based upon the exposure being hedged, as a fair value hedge, a cash flow hedge or a hedge of a net investment in a foreign operation.
The Company has designated its derivative financial instruments as cash flow hedges because they hedge exposure to variability in expected future cash flows that are attributable to interest rate risk. For these hedges, the effective portion of the gain or loss on the derivative instrument is reported as a component of other comprehensive income (loss) and reclassified into earnings in the same line item associated with the forecasted transaction in the same period or periods during which the hedged transaction affects earnings. Any ineffective portion of the gain or loss on the derivative instruments is recorded in results of operations immediately.
Earnings Per Share
Basic earnings per share is calculated based on income available to common stockholders and the weighted-average number of shares outstanding during the reporting period. Diluted earnings per share is calculated based on income available to common stockholders and the weighted-average number of common and potential common shares outstanding during the reporting period. Potential common shares, composed of the incremental common shares issuable upon the exercise of stock options, stock appreciation rights and unvested restricted shares, are included in the earnings per share calculation to the extent that they are dilutive.
Fair Value of Financial Instruments
The carrying value of cash, accounts receivable and accounts payable at both March 31, 2007 and 2006 approximates fair value due to the short-term nature of these instruments. The carrying value of long-term debt at both March 31, 2007 and 2006 approximates fair value based on interest rates for instruments with similar terms and maturities.
Recently Issued Accounting Standards
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities - Including an amendment of FASB Statement No. 115” (“Statement No. 159”). Statement No. 159 permits companies to choose to measure certain financial instruments and certain other items at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be recognized in earnings at each subsequent reporting date. Statement No. 159 is effective for the Company’s interim financial statements issued after April 1, 2008. The Company is evaluating the impact that the adoption of Statement No. 159 will have on its consolidated financial statements.
In September 2006, the SEC issued Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements” (“SAB No. 108”). SAB No. 108 was issued in an effort to eliminate the diversity in practice surrounding how companies quantify financial statement misstatements. SAB No. 108 requires that errors be quantified using both a balance sheet and income statement approach and evaluated as to materiality giving consideration to all relevant quantitative and qualitative factors. The adoption of SAB No. 108 did not have a material impact on the Company’s consolidated financial statements.
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“Statement No. 157”) to address inconsistencies in the definition and determination of fair value pursuant to generally accepted accounting principles (“GAAP”). Statement No. 157 provides a single definition of fair value, establishes a framework for measuring fair value in GAAP and expands disclosures about fair value measurements in an effort to increase comparability related to the recognition of market-based assets and liabilities and their impact on earnings. Statement No. 157 is effective for the Company’s interim financial statements issued after April 1, 2008. The Company is evaluating the impact that the adoption of Statement No. 157 will have on its consolidated financial statements.
F - 14
In June 2006, the FASB issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes--an interpretation of FASB Statement 109” (“FIN No. 48”), which clarifies the accounting for uncertainty in income taxes recognized in a company’s financial statements in accordance with FASB Statement 109. FIN No. 48 prescribes a recognition threshold and measurement attributes for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 is effective for the Company’s interim financial statements issued after April 1, 2007, and is not expected to have a material impact on the Company’s consolidated financial position, results of operations or cash flows.
Management has reviewed and continues to monitor the actions of the various financial and regulatory reporting agencies and is currently not aware of any other pronouncement that could have a material impact on the Company’s consolidated financial position, results of operations or cash flows.
2. | Acquisition of Businesses |
Acquisition of Bonita Bay
On April 6, 2004, the Company acquired all of the outstanding capital stock of Bonita Bay for a purchase price of approximately $561.3 million (including working capital adjustments totaling $1.1 million). The Bonita Bay acquisition, including fees and expenses related to the financing of $22.7 million, and funds used to repay $154.4 million of outstanding debt obligations, was financed through the following sources:
(In thousands) Revolving Credit Facility | $ | 3,512 | ||
| Tranche B Term Loan Facility | 355,000 | |||
| Tranche C Term Loan Facility | 100,000 | |||
| 9.25% Senior Subordinated Notes | 210,000 | |||
| Issuance of Preferred and Common units | 58,579 | |||
| Total sources of funds | $ | 727,091 |
The total purchase price of the Bonita Bay acquisition (which included cash of $379.2 million paid to the selling stockholders, Prestige LLC Class B Preferred Units valued at an aggregate of $91,000 and Prestige LLC Common Units valued at an aggregate of $1,000, assumed debt and accrued interest which was retired of $176.9 million and acquisition costs of $3.6 million) was allocated to the acquired assets and liabilities as set forth in the following table:
(In thousands) Cash | $ | 4,304 | ||
| Accounts receivable | 13,186 | |||
| Inventories | 16,185 | |||
| Prepaid expenses and other current assets | 1,391 | |||
| Property and equipment | 2,982 | |||
| Goodwill | 217,234 | |||
| Intangible assets | 352,460 | |||
| Accounts payable and accrued liabilities | (21,189 | ) | ||
| Long-term debt | (172,898 | ) | ||
| Deferred income taxes | (34,429 | ) | ||
$ | 379,226 |
As a result of the Bonita Bay acquisition, the Company recorded indefinite lived trademarks of $340.7 million and $11.8 million of trademarks with an estimated weighted average useful life of seven years. Additionally, the Company recorded goodwill of $217.2 million that is not deductible for income tax purposes.
F - 15
Acquisition of Vetco, Inc.
On October 6, 2004, the Company acquired all the outstanding stock of Vetco, Inc. for a purchase price of approximately $50.6 million. To finance the acquisition, the Company used cash on hand of approximately $20.6 million and borrowed an additional $12.0 million on its Revolving Credit Facility and $18.0 million on its Tranche B Term Loan Facility. The results from operations of Vetco, Inc. have been included within the Company’s consolidated financial statements as a component of the over-the-counter healthcare segment commencing October 6, 2004.
The total purchase price of the Vetco acquisition was allocated to the acquired assets and liabilities as set forth in the following table:
(In thousands) Accounts receivable | $ | 2,136 | ||
| Inventories | 910 | |||
| Prepaid expenses and other current assets | 37 | |||
| Property and equipment | 5 | |||
| Goodwill | 21,858 | |||
| Intangible assets | 27,158 | |||
| Accounts payable and accrued liabilities | (1,455 | ) | ||
$ | 50,649 |
As a result of the Vetco acquisition, the Company recorded $27.0 million of trademarks with an estimated useful life of 20 years and $158,000 related to a 5-year non-compete agreement with the former owner of Vetco. Additionally, the Company recorded goodwill of $21.9 million that will be fully deductible for income tax purposes.
Acquisition of Dental Concepts, LLC
On November 8, 2005, the Company acquired all of the ownership interests of Dental Concepts, a marketer of therapeutic oral care products sold under “The Doctor’s” brand. The Doctor’s product line has been fully integrated in the Company’s operations and has benefited from its business model of outsourced manufacturing. Additionally, the Company has increased product sales as a result of a targeted marketing and advertising program. The results from operations of Dental Concepts have been included within the Company’s consolidated financial statements as a component of the over-the-counter healthcare segment commencing November 8, 2005.
The purchase price of the ownership interests was approximately $30.2 million (net of cash acquired of $280,000), including fees and expenses of the acquisition of $1.3 million. The Company financed the acquisition price through the utilization of its senior revolving credit facility in the amount of $30.0 million and cash on hand.
The following table summarizes the estimated fair values of the assets acquired and the liabilities assumed at the date of acquisition, which includes a purchase price adjustment of $750,000 that was recorded in 2007.
(In thousands) | ||||
| Accounts receivable | $ | 2,774 | ||
| Inventories | 1,707 | |||
| Prepaid expenses and other current assets | 172 | |||
| Property and equipment | 546 | |||
| Goodwill | 6,362 | |||
| Intangible assets | 22,395 | |||
| Accounts payable and accrued liabilities | (3,717 | ) | ||
$ | 30,239 | |||
As a result of the Dental Concepts acquisition, the Company recorded a trademark valued at $22.4 million with an estimated useful life of 20 years. Goodwill resulting from this transaction was $6.4 million and it is estimated that such amount will be fully deductible for income tax purposes.
F - 16
Acquisition of Wartner USA BV
On September 21, 2006, the Company completed the acquisition of the ownership interests of Wartner USA B.V., the owner of the Wartner brand of over-the-counter wart treatment products. The Company expects that the Wartner brand, which is the #3 brand in the United States over-the-counter wart treatment category, will enhance the Company’s leadership in the category. Additionally, the Company believes that the brand will benefit from a targeted advertising and marketing program, as well as the Company’s business model of outsourcing manufacturing and the elimination of redundant operations. The results from operations of the Wartner brand have been included within the Company’s consolidated financial statements as a component of the over-the-counter healthcare segment commencing September 21, 2006.
The purchase price of the ownership interests was approximately $31.2 million, including fees and expenses of the acquisition of $216,000 and the assumption of approximately $5.0 million of contingent payments, with an estimated fair value of $3.8 million, owed to the former owner of Wartner through 2011. The Company funded the cash acquisition price from operating cash flows.
The following table summarizes the estimated fair values of the assets acquired and the liabilities assumed at the date of acquisition; however, the final purchase price will not be determined until all preliminary valuations have been finalized. Consequently, the allocation of the purchase price is subject to refinement.
(In thousands) | ||||
| Inventory | $ | 769 | ||
| Intangible assets | 29,600 | |||
| Goodwill | 11,746 | |||
| Accrued liabilities | (3,854 | ) | ||
| Deferred tax liabilities | (7,000 | ) | ||
$ | 31,261 | |||
The amount allocated to intangible assets of $29.6 million includes $17.8 million related to the Wartner brand trademark which the Company estimates to have a useful life of 20 years, as well as $11.8 million related to a patent estimated to have a useful life of 14 years. Goodwill resulting from this transaction was $11.7 million, inclusive of a deferred income tax liability recorded for the difference between the assigned values of assets acquired and liabilities assumed, and their respective taxes bases. As discussed above, this recorded amount is subject to change as additional information becomes available; however, it is estimated that of such amount, approximately $4.7 million will be deductible for income tax purposes.
The following table sets forth the unaudited results of the Company’s operations on a pro forma basis as if the acquisition of Wartner had been completed on April 1, 2005, and the acquisition of Dental Concepts, which was acquired in November 2005, had been completed on April 1, 2004. It also includes the pro forma results from operations of Vetco, Inc., which was acquired in October 2004, as if the acquisition of Vetco, Inc. had been completed on April 1, 2004. The pro forma financial information is not necessarily indicative of the operating results that the combined entities would have achieved, nor is it necessarily indicative of the operating results that may be expected in the future.
F - 17
Year Ended March 31 | ||||||||||
(In thousands, except per share data) | 2007 | 2006 | 2005 | |||||||
(Unaudited Pro forma) | ||||||||||
| Revenues | $ | 326,103 | $ | 315,276 | $ | 308,062 | ||||
| Income before provision for income taxes | $ | 55,340 | $ | 47,368 | $ | 20,730 | ||||
| Net income | $ | 36,178 | $ | 26,161 | $ | 11,418 | ||||
Cumulative preferred dividends on Senior Preferred and Class B Preferred Units | -- | -- | (25,395 | ) | ||||||
Net income (loss) available to members and common stockholders | $ | 36,178 | $ | 26,161 | $ | (13,977 | ) | |||
| Basic earnings per share | $ | 0.73 | $ | 0.53 | $ | (0.51 | ) | |||
| Diluted earnings per share | $ | 0.72 | $ | 0.52 | $ | (0.51 | ) | |||
Weighted average shares outstanding: Basic | 49,460 | 48,908 | 27,546 | |||||||
| Diluted | 50,020 | 50,008 | 27,546 | |||||||
Accounts Receivable |
Accounts receivable consist of the following (in thousands):
March 31 | |||||||
2007 | 2006 | ||||||
| Accounts receivable | $ | 35,274 | $ | 40,140 | |||
| Other receivables | 1,681 | 1,870 | |||||
| 36,955 | 42,010 | ||||||
Less allowances for discounts, returns and uncollectible accounts | (1,788 | ) | (1,968 | ) | |||
$ | 35,167 | $ | 40,042 | ||||
Inventories |
Inventories consist of the following (in thousands):
March 31 | |||||||
2007 | 2006 | ||||||
| �� | |||||||
| Packaging and raw materials | $ | 2,842 | $ | 3,278 | |||
| Finished goods | 27,331 | 30,563 | |||||
$ | 30,173 | $ | 33,841 | ||||
Inventories are shown net of allowances for obsolete and slow moving inventory of $1.8 million and $1.0 million at March 31, 2007 and 2006, respectively.
F - 18
Property and equipment consist of the following (in thousands):
March 31 | |||||||
2007 | 2006 | ||||||
| Machinery | $ | 1,480 | $ | 3,722 | |||
| Computer equipment | 566 | 987 | |||||
| Furniture and fixtures | 247 | 303 | |||||
| Leasehold improvements | 372 | 340 | |||||
| 2,665 | 5,352 | ||||||
| Accumulated depreciation | (1,216 | ) | (3,699 | ) | |||
$ | 1,449 | $ | 1,653 | ||||
6. Goodwill
A reconciliation of the activity affecting goodwill by operating segment is as follows (in thousands):
Over-the-Counter | Household | Personal | |||||||||||
Healthcare | Cleaning | Care | Consolidated | ||||||||||
| Balance - March 31, 2005 | $ | 217,539 | 72,549 | $ | 4,643 | $ | 294,731 | ||||||
| Additions | 5,096 | -- | -- | 5,096 | |||||||||
| Impairments | -- | -- | (1,892 | ) | (1,892 | ) | |||||||
| Balance - March 31, 2006 | 222,635 | 72,549 | 2,751 | 297,935 | |||||||||
| Additions | 13,012 | -- | -- | 13,012 | |||||||||
| Balance - March 31, 2007 | $ | 235,647 | $ | 72,549 | $ | 2,751 | $ | 310,947 | |||||
During the year ended March 31, 2006 and in conjunction with the annual test for goodwill impairment, the Company recorded a $1.9 million charge to adjust the carrying amount of goodwill related to one of the reporting units in the personal care segment to its fair value as determined by use of discounted cash flow methodologies.
7. Intangible Assets
On October 28, 2005, the Company acquired the “Chore Boy” brand of cleaning pads and sponges for $22.7 million, including direct costs of $405,000. The acquired trademark was assigned an indefinite life.
During 2006, management determined that declining sales in the Company’s personal care segment might be indicative of an impairment of the Company’s intangible assets. Accordingly, in connection with its annual impairment tests of goodwill and indefinite-lived intangibles in accordance with Statement No. 142, management also performed an impairment analysis for all of the Company’s finite-lived intangible assets in accordance with Statement No. 144. As a result of this analysis, the Company recorded a $7.4 million charge to adjust the carrying amount of certain trademarks related to the personal care segment to their fair values as determined by use of discounted cash flow methodologies. As discussed in Note 6, the Company also recorded a related impairment charge to goodwill.
F - 19
A reconciliation of the activity affecting intangible assets is as follows (in thousands):
Year Ended March 31, 2007 | |||||||||||||
Indefinite Lived | Finite Lived | Non Compete | |||||||||||
Trademarks | Trademarks | Agreement | Totals | ||||||||||
Carrying Amounts | |||||||||||||
| Balance - March 31, 2006 | $ | 544,963 | $ | 109,870 | $ | 196 | $ | 655,029 | |||||
| Additions | -- | 29,600 | -- | 29,600 | |||||||||
| Balance - March 31, 2007 | $ | 544,963 | $ | 139,470 | $ | 196 | $ | 684,629 | |||||
Accumulated Amortization | |||||||||||||
| Balance - March 31, 2006 | $ | -- | $ | 17,779 | $ | 53 | $ | 17,832 | |||||
| Additions | -- | 9,596 | 44 | 9,640 | |||||||||
| Balance - March 31, 2007 | $ | -- | $ | 27,375 | $ | 97 | $ | 27,472 | |||||
Year Ended March 31, 2006 | |||||||||||||
Indefinite Lived | Finite Lived | Non Compete | |||||||||||
Trademarks | Trademarks | Agreement | Totals | ||||||||||
Carrying Amounts | |||||||||||||
| Balance - March 31, 2005 | $ | 522,346 | $ | 94,900 | $ | 158 | $ | 617,404 | |||||
| Additions | 22,617 | 22,395 | 38 | 45,050 | |||||||||
| Impairments | -- | (7,425 | ) | -- | (7,425 | ) | |||||||
| Balance - March 31, 2006 | $ | 544,963 | $ | 109,870 | $ | 196 | $ | 655,029 | |||||
Accumulated Amortization | |||||||||||||
| Balance - March 31, 2005 | $ | -- | $ | 8,775 | $ | 16 | $ | 8,791 | |||||
| Additions | -- | 9,004 | 37 | 9,041 | |||||||||
| Balance - March 31, 2006 | $ | -- | $ | 17,779 | $ | 53 | $ | 17,832 | |||||
At March 31, 2007, intangible assets are expected to be amortized over a period of five to 30 years as follows (in thousands):
Year Ending March 31 | ||||
| 2008 | $ | 10,507 | ||
| 2009 | 10,502 | |||
| 2010 | 9,086 | |||
| 2011 | 9,071 | |||
| 2012 | 9,071 | |||
| Thereafter | 63,957 | |||
$ | 112,194 | |||
F - 20
8. Other Accrued Liabilities
Other accrued liabilities consist of the following (in thousands):
March 31 | ||||||||||
2007 | 2006 | |||||||||
| Accrued marketing costs | $ | 5,687 | $ | 2,513 | ||||||
| Accrued payroll | 3,721 | 813 | ||||||||
| Accrued commissions | 335 | 248 | ||||||||
| Other | 762 | 1,008 | ||||||||
$ | 10,505 | $ | 4,582 | |||||||
F - 21
9. Long-Term Debt
Long-term debt consists of the following (in thousands):
March 31 | |||||||
2007 | 2006 | ||||||
| Senior revolving credit facility (“Revolving Credit Facility”), which expires on April 6, 2009 and is available for maximum borrowings of up to $60.0 million. The Revolving Credit Facility bears interest at the Company’s option at either the prime rate plus a variable margin or LIBOR plus a variable margin. The variable margins range from 0.75% to 2.50% and at March 31, 2007, the interest rate on the Revolving Credit Facility was 9.5% per annum. The Company is also required to pay a variable commitment fee on the unused portion of the Revolving Credit Facility. At March 31, 2007, the commitment fee was 0.50% of the unused line. The Revolving Credit Facility is collateralized by substantially all of the Company’s assets. | $ | -- | $ | 7,000 | |||
| Senior secured term loan facility (“Tranche B Term Loan Facility”) that bears interest at the Company’s option at either the prime rate plus a margin of 1.25% or LIBOR plus a margin of 2.25%. At March 31, 2007, the applicable interest rate on the Tranche B Term Loan Facility was 7.63%. Principal payments of $887,500 plus accrued interest are payable quarterly. In February 2005, the Tranche B Term Loan Facility was amended to increase the additional amount available thereunder by $50.0 million to $200.0 million, all of which is available at March 31, 2007. Current amounts outstanding under the Tranche B Term Loan Facility mature on April 6, 2011, while amounts borrowed pursuant to the amendment will mature on October 6, 2011. The Tranche B Term Loan Facility is collateralized by substantially all of the Company’s assets. | 337,350 | 365,630 | |||||
Senior Subordinated Notes that bear interest at 9.25% which is payable on April 15th and October 15th of each year. The Senior Subordinated Notes mature on April 15, 2012; however, the Company may redeem some or all of the Senior Subordinated Notes on or prior to April 15, 2008 at a redemption price equal to 100%, plus a make-whole premium, and after April 15, 2008 at redemption prices set forth in the indenture governing the Senior Subordinated Notes. The Senior Subordinated Notes are unconditionally guaranteed by Prestige Brands Holdings, Inc., and its domestic wholly-owned subsidiaries other than Prestige Brands, Inc., the issuer. Each of these guarantees is joint and several. There are no significant restrictions on the ability of any of the guarantors to obtain funds from their subsidiaries. | 126,000 | 126,000 | |||||
| 463,350 | 498,630 | ||||||
| Current portion of long-term debt | (3,550 | ) | (3,730 | ) | |||
$ | 459,800 | $ | 494,900 | ||||
Effective as of December 19, 2006: (i) a Second Supplemental Indenture (“Second Supplemental Indenture”), and (ii) a Guaranty Supplement (“Indenture Guaranty Supplement”) were entered into with the trustee for the holders of the Senior Subordinated Notes. The Second Supplemental Indenture supplements and amends the indenture,
F - 22
dated as of April 6, 2004, as supplemented on October 6, 2004 (“Indenture”). Pursuant to the terms of the Second Supplemental Indenture and the Indenture Guaranty Supplement, the Company agreed to guaranty all of the obligations of Prestige Brands, Inc., an indirect wholly-owned subsidiary of the Company (“PBI”), set forth in the Indenture governing PBI’s Senior Subordinated Notes. The Second Supplemental Indenture also amended the covenant requiring Prestige Brands International, LLC (“Prestige Brands International”), an indirect wholly-owned subsidiary of the Company, to file periodic reports with the SEC pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended (“Exchange Act”). So long as the Company or any other guarantor is required to file periodic reports under Section 13 or 15(d) of the Exchange Act that are substantially the same as the periodic reports that Prestige Brands International would otherwise be required to file with the SEC pursuant to the Indenture, Prestige Brands International is not required to file such reports.
Also effective as of December 19, 2006, a Joinder Agreement (“Joinder Agreement”) and a Guaranty Supplement (“Credit Agreement Guaranty Supplement”) were entered into with the administrative agent for the lenders under the Senior Credit Facility. Pursuant to the terms of the Joinder Agreement and the Credit Agreement Guaranty Supplement, the Company agreed to become a party to the Pledge and Security Agreement (“Security Agreement”) and the Guaranty (“Credit Agreement Guaranty”), each dated as of April 6, 2004, by PBI and certain of its affiliates in favor of the lenders. The Security Agreement and the Credit Agreement Guaranty secure the performance by PBI of its obligations under the Credit Agreement, dated as of April 6, 2004, as amended (“Credit Agreement”), by granting security interests to PBI's lenders in collateral owned by the Company and certain of its subsidiaries and providing guaranties of such obligations by certain of PBI’s affiliates.
The Revolving Credit Facility and the Tranche B Term Loan Facility (together the “Senior Credit Facility”) contain various financial covenants, including provisions that require the Company to maintain certain leverage ratios, interest coverage ratios and fixed charge coverage ratios. The Senior Credit Facility and the Senior Subordinated Notes also contain provisions that restrict the Company from undertaking specified corporate actions, such as asset dispositions, acquisitions, dividend payments, repurchase of common shares outstanding, changes of control, incurrence of indebtedness, creation of liens, making of loans and transactions with affiliates. Additionally, the Senior Credit Facility and the Senior Subordinated Notes contain cross-default provisions whereby a default pursuant to the terms and conditions of either indebtedness will cause a default on the remaining indebtedness. At March 31, 2007, the Company was in compliance with its applicable financial and other covenants under the Senior Credit Facility and the Indenture.
Future principal payments required in accordance with the terms of the Senior Credit Facility and the Senior Subordinated Notes are as follows (in thousands):
Year Ending March 31 | ||||
| 2008 | $ | 3,550 | ||
| 2009 | 3,550 | |||
| 2010 | 3,550 | |||
| 2011 | 3,550 | |||
| 2012 | 323,150 | |||
| Thereafter | 126,000 | |||
$ | 463,350 | |||
In an effort to mitigate the impact of changing interest rates, the Company entered into interest rate cap agreements with various financial institutions. In June 2004, the Company purchased a 5% interest rate cap with a notional amount of $20.0 million which expired in June 2006. In March 2005, the Company purchased interest rate cap agreements with a total notional amount of $180.0 million and cap rates ranging from 3.25% to 3.75%. On May 31, 2006, an interest rate cap agreement with a notional amount of $50.0 million and a 3.25% cap rate expired. Additionally, an interest rate cap agreement with a notional amount of $80.0 million and a 3.50% cap rate expired on May 30, 2007. The remaining agreement, with a notional amount of $50.0 million and a cap rate of 3.75%, terminates on May 30, 2008. The Company is accounting for the interest rate cap agreements as cash flow hedges. The fair value of the interest rate cap agreements, which is included in other long-term assets, was $1.2 million and $3.3 million at March 31, 2007 and 2006, respectively.
F - 23
10. Stockholders’ Equity
The Company is authorized to issue 250.0 million shares of common stock, $0.01 par value per share, and 5.0 million shares of preferred stock, $0.01 par value per share. The Board of Directors may direct the issuance of the undesignated preferred stock in one or more series and determine preferences, privileges and restrictions thereof.
Each share of common stock has the right to one vote on all matters submitted to a vote of stockholders. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared or paid on the Company’s common stock through March 31, 2007.
Prior to the Company’s initial public offering in February 2005, Prestige International Holdings, LLC (“Prestige LLC”), then the top tier entity, had four classes of units: Senior Preferred Units, Class A Preferred Units, Class B Preferred Units and Common Units. A “unit” is an equity interest of a unitholder in the profits, losses and distributions of the Company.
On February 6, 2004, certain senior executive officers purchased an aggregate of 5.3 million common units of Prestige LLC at $.10 per unit. These units were purchased on the same day and at the same price that GTCR and TCW/Crescent Partners, the Company’s unrelated equity investors (the “Sponsors”), purchased 50.0 million common units. On March 17, 2004, other executive officers purchased an aggregate of 405,000 common units at a price of $.10 per unit and on April 6, 2004, two additional employees purchased an aggregate of 50,000 common units at a price of $.10 per unit. Each of the above-referenced purchase transactions by management were conducted at fair market value based upon the price paid by the Sponsors and the fact that such purchases were made at the same price and at the same time or shortly thereafter. Certain of these shares are subject to vesting requirements over a period of 5 years. No compensation cost was recorded in connection with the issuance of these units as these units were purchased by management at fair value. As of March 31, 2007, there were approximately 295,000 shares of unvested restricted stock related to these purchases.
On April 6, 2004, the Company acquired all of the outstanding capital stock of Bonita Bay for a purchase price of approximately $564.9 million, inclusive of acquisition costs of $3.6 million. The total purchase price consideration for the Bonita Bay included cash of $379.2 million paid to the selling stockholders, the issuance of Prestige LLC Class B Preferred Units valued at an aggregate of $91,000 and Prestige LLC Common Units valued at an aggregate of $1,000, as well as the assumption of debt and accrued interest of $176.9 million.
On November 1, 2004, certain non-executive employees purchased an aggregate of 337,000 common units of Prestige LLC, for $0.70 per unit, which was equal to fair market value, and which vest over a period of 5 years. This determination was based on a contemporaneous valuation that utilized traditional methodologies, including market multiples, comparable transactions and discounted cash flow. Prestige LLC relied on this fair market value analysis in setting the $0.70 per unit price for the purchases. Prestige LLC awarded a total cash bonus of $235,000 to allow employees to purchase such units. In connection therewith, Prestige LLC recorded a bonus expense of $235,000. In this regard, all employee purchases were conducted at fair market value based upon the contemporaneous valuation. As of March 31, 2007, there were approximately 37,000 shares of unvested restricted stock related to these employee purchases.
On February 9, 2005, the Company became the direct parent company of Prestige LLC, under the terms of an exchange agreement among the Company, Prestige LLC and each holder of common units of Prestige LLC. Pursuant to the exchange agreement, the holders of common units of Prestige LLC exchanged all their common units for an aggregate of 26.7 million shares of common stock of the Company.
On February 9, 2005, the Company completed its initial public offering, pursuant to which it sold 28.0 million shares of its common stock and selling stockholders sold 4.2 million shares of common stock at a price of $16.00 per share. The offering resulted in proceeds to the Company of approximately $416.8 million, net of $3.1 million of issuance costs. In connection with the offering, the Company retired 4.7 million shares of its common stock
F - 24
for an aggregate cost of $30.2 million. Upon completion of the initial public offering, there were 50.0 million shares of the Company’s common stock issued and outstanding.
On February 15, 2005, a portion of the net proceeds from the initial public offering were used to redeem all of Prestige LLC’s outstanding Senior Preferred Units and Class B Preferred Units for $199.8 million, which included cumulative and liquidating dividends of $26.8 million. The cumulative dividends were based on an 8% per year rate of return.
On July 29, 2005, each of the Company’s four independent members of the Board of Directors received an award of 6,222 shares of common stock in accordance with Company’s directors’ compensation arrangements. The common stock had a fair value of $11.25 per share, the closing price of the Company’s common stock on July 28, 2005. Of such amount, 1,778 shares represent a one-time grant of unrestricted shares, while the remaining 4,444 shares represent restricted shares that vest over a two year period.
On August 4, 2005, the Company named a new President and Chief Operating Officer. In connection therewith, the Board of Directors granted this individual 30,888 shares of restricted common stock with a fair market value of $12.95 per share, the closing price of the common stock on August 4, 2005, and options to purchase an additional 61,776 shares of common stock at an exercise price of $12.95 per share. During 2007, compensation costs of $142,000 were reversed upon the departure of this member of management.
On October 1, 2005, the Company’s Board of Directors authorized the grant of 156,000 shares of restricted common stock with a fair market value of $12.32 per share, the closing price of the Company’s common stock on September 30, 2005, to employees. The issuance of such shares is contingent upon the Company’s attainment of certain revenue and earnings per share targets. Additionally, in the event that an employee terminates his or her employment with the Company prior to October 1, 2008, the vesting date, the shares will be forfeited.
On August 15, 2006, each of two new independent members to the Company’s Board of Directors received an award of 2,119 unrestricted shares of common stock in accordance with Company’s directors’ compensation arrangements. The common stock had a fair value of $9.44 per share, the closing price of the Company’s common stock on August 14, 2006.
During 2007 and 2006, the Company repurchased 6,000 and 16,000 shares, respectively, of restricted common stock from former employees pursuant to the provisions of the various employee stock purchase agreements at an average purchase price of $1.70 per share. Additionally, during 2007, the Company recovered 30,888 shares of restricted stock upon the departure of a former member of management. All of such shares have been recorded as treasury stock.
F - 25
11. Earnings Per Share
The following table sets forth the computation of basic and diluted earnings per share (in thousands):
Year Ended March 31 | ||||||||||
2007 | 2006 | 2005 | ||||||||
Numerator | ||||||||||
| Net income | $ | 36,078 | $ | 26,277 | $ | 10,220 | ||||
Cumulative preferred dividends on Senior Preferred and Class B Preferred Units | -- | -- | (25,395 | ) | ||||||
Net income (loss) available to members and common stockholders | $ | 36,078 | $ | 26,277 | $ | (15,175 | ) | |||
Denominator | ||||||||||
| Denominator for basic earnings per share | 49,460 | 48,908 | 27,546 | |||||||
Dilutive effect of unvested restricted common stock and stock appreciation rights issued to employees and directors | 560 | 1,100 | -- | |||||||
| Denominator for diluted earnings per share | 50,020 | 50,008 | 27,546 | |||||||
Earnings per Common Share: | ||||||||||
| Basic | $ | 0.73 | $ | 0.54 | $ | (0.55 | ) | |||
| Diluted | $ | 0.72 | $ | 0.53 | $ | (0.55 | ) | |||
Outstanding employee stock options to purchase an aggregate of 61,800 shares of common stock at March 31, 2006 were not included in the computation of diluted earnings per share because their exercise price was greater than the average market price of the common stock, and therefore, their inclusion would be antidilutive. There were no such stock options outstanding at March 31, 2007. At March 31, 2007, 373,000 restricted shares, issued to management and employees are unvested; however, such shares are included in the calculation of diluted earnings per share. Additionally, at March 31, 2007, 254,000 shares of restricted stock granted to management and employees, as well as 16,000 stock appreciation rights have been excluded from the calculation of both basic and diluted earnings per share since vesting of such shares is subject to contingencies. At March 31, 2006, 734,000 restricted shares issued to management and employees were unvested; however, such shares were included in the calculation of diluted earnings per share. Additionally, at March 31, 2006, 180,000 shares of restricted stock granted to management and employees have been excluded from the calculation of both basic and diluted earnings per share since vesting of such shares is subject to contingencies.
12. Related Party Transactions
Effective February 6, 2004, the Company entered into an agreement with an affiliate of GTCR Golder Rauner II, LLC (“GTCR”), a private equity firm and an investor in the Company, whereby the GTCR affiliate was to provide management and advisory services to the Company for an aggregate annual compensation of $4.0 million. The agreement was terminated in February 2005. The total fee paid to the GTCR affiliate during 2005 was $3.4 million.
13. | Share-Based Compensation |
In connection with the Company’s initial public offering, the Board of Directors adopted the 2005 Long-Term Equity Incentive Plan (“Plan”) which provides for the grant, to a maximum of 5.0 million shares, of stock options,
F - 26
restricted stock units, deferred stock units and other equity-based awards. Directors, officers and other employees of the Company and its subsidiaries, as well as others performing services for the Company, are eligible for grants under the Plan. The Company believes that such awards better align the interests of its employees with those of its stockholders.
During 2006, the Company adopted Statement No. 123(R) with the initial grants of restricted stock and options to purchase common stock to employees and directors in accordance with the provisions of the Plan. Compensation costs charged against income, and the related tax benefits recognized were $655,000 and $253,000, respectively, for 2007 and $383,000 and $150,000, respectively, for 2006.
Restricted Shares
Restricted shares granted under the plan generally vest in 3 to 5 years, contingent on attainment of Company performance goals, including both revenue and earnings per share growth targets. Certain restricted share awards provide for accelerated vesting if there is a change of control. The fair value of nonvested restricted shares is determined as the closing price of the Company’s common stock on the day preceding the grant date. The weighted-average grant-date fair values during 2007 and 2006 were $9.83 and $12.29, respectively.
A summary of the Company’s restricted shares granted under the Plan is presented below:
Nonvested Shares | Shares (000) | Weighted- Average Grant-Date Fair Value | |||||
| Granted | 211.6 | $ | 12.29 | ||||
| Vested | (7.1) | 11.25 | |||||
| Forfeited | (6.5) | 12.32 | |||||
| Nonvested at March 31, 2006 | 198.0 | 12.32 | |||||
| Granted | 156.5 | 9.83 | |||||
| Vested | (13.1) | 10.67 | |||||
| Forfeited | (47.0) | 12.47 | |||||
| Nonvested at March 31, 2007 | 294.4 | $ | 11.05 | ||||
Options
The Plan provides that the exercise price of the option granted shall be no less than the fair market value of the Company’s common stock on the date the option is granted. Options granted have a term of no greater than 10 years from the date of grant and vest in accordance with a schedule determined at the time the option is granted, generally 3 to 5 years. Certain option awards provide for accelerated vesting if there is a change in control.
The fair value of each option award is estimated on the date of grant using the Black-Scholes Option Pricing Model (“Black-Scholes Model”) that uses the assumptions noted in the following table. Expected volatilities are based on the historical volatility of the Company’s common stock and other factors, including the historical volatilities of comparable companies. The Company uses appropriate historical data, as well as current data, to estimate option exercise and employee termination behaviors. Employees that are expected to exhibit similar exercise or termination behaviors are grouped together for the purposes of valuation. The expected terms of the options granted are derived from management’s estimates and information derived from the public filings of companies similar to the Company and represent the period of time that options granted are expected to be outstanding. The risk-free rate represents the yield on U.S. Treasury bonds with a maturity equal to the expected term of the granted option. The weighted-average grant-date fair value of the options granted during the year
F - 27
ended March 31, 2006 was $5.02. There were no options granted during the year ended March 31, 2007.
Year Ended March 31 | |||||||
2007 | 2006 | ||||||
| Expected volatility | -- | 31.0% | |||||
| Expected dividends | -- | -- | |||||
| Expected term in years | -- | 6.0 | |||||
| Risk-free rate | -- | 4.2% | |||||
A summary of option activity under the Plan is as follows:
Options | Shares (000) | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value (000) | |||||||||
| Granted | 61.8 | $ | 12.95 | 5.0 | $ | -- | |||||||
| Exercised | -- | -- | -- | -- | |||||||||
| Forfeited or expired | -- | -- | -- | -- | |||||||||
| Outstanding at March 31, 2006 | 61.8 | 12.95 | 4.3 | -- | |||||||||
| Granted | -- | -- | -- | -- | |||||||||
| Exercised | -- | -- | -- | -- | |||||||||
| Forfeited or expired | (61.8) | 12.95 | 4.3 | -- | |||||||||
| Outstanding at March 31, 2007 | -- | $ | -- | -- | $ | -- | |||||||
| Exercisable at March 31, 2007 | -- | $ | -- | -- | $ | -- | |||||||
Since the exercise price of the option exceeded the Company’s closing stock price of $12.17 at March 31, 2006, the aggregate intrinsic value of outstanding options was $0 at March 31, 2006.
Stock Appreciation Rights (“SARS”)
During 2007, the Board of Directors granted SARS to a group of selected executives. The terms of the SARS provide that on the vesting date, the executive will receive the excess of the market price of the stock award over the market price of the stock award on the date of issuance. The Board of Directors, in its sole discretion, may settle the Company’s obligation to the executive in shares of the Company’s common stock, cash, other securities of the Company or any combination thereof.
The Plan provides that the issuance price of a SAR shall be no less than the market price of the Company’s common stock on the date the SAR is granted. SARS may be granted with a term of no greater than 10 years from the date of grant and will vest in accordance with a schedule determined at the time the SAR is granted, generally 3 to 5 years. The weighted-average grant date fair value of the SARS granted during 2007 was $3.68. The fair value of each SAR award was estimated on the date of grant using the Black-Scholes Model using the assumptions noted in the following table.
Year Ended March 31, 2007 | ||
| Expected volatility | 50.0% | |
| Expected dividends | -- | |
| Expected term in years | 2.75 | |
| Risk-free rate | 5.0% |
F - 28
A summary of SARS activity under the Plan is as follows:
SARS | Shares (000) | Grant Date Stock Price | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value (000) | |||||||||
| Granted | 16.1 | $ | 9.97 | 2.0 | $ | -- | |||||||
| Forfeited or expired | -- | -- | -- | -- | |||||||||
| Outstanding at March 31, 2007 | 16.1 | $ | 9.97 | 2.0 | $ | 30,300 | |||||||
| Exercisable at March 31, 2007 | -- | $ | -- | -- | $ | -- | |||||||
Since the closing market price of the Company’s common stock on March 31, 2007 of $11.85 exceeded the market price of the Company’s stock on the grant date, the aggregate intrinsic value of outstanding SARS was $30,300 at March 31, 2007.
At March 31, 2007 and 2006, there were $1.4 million and $1.2 million, respectively, of unrecognized compensation costs related to nonvested share-based compensation arrangements under the Plan based on management’s estimate of the shares that will ultimately vest. The Company expects to recognize such costs over the next 2.5 years. However, the restricted shares vest upon the attainment of Company performance goals and if such goals are not met, no compensation costs would ultimately be recognized and any previously recognized compensation cost would be reversed. The total fair value of shares vested during 2007 and 2006 was $104,000 and $80,000, respectively. There were no options exercised during 2007 or 2006; hence there were no tax benefits realized during these periods. At March 31, 2007, there were 4.7 million shares available for issuance under the Plan.
14. Income Taxes
The provision (benefit) for income taxes consists of the following (in thousands):
Year Ended March 31 | ||||||||||
2007 | 2006 | 2005 | ||||||||
| Current | ||||||||||
| Federal | $ | 7,547 | $ | 5,043 | $ | (544 | ) | |||
| State | 1,739 | 1,056 | 654 | |||||||
| Foreign | 150 | 206 | 102 | |||||||
| Deferred | ||||||||||
| Federal | 10,391 | 10,621 | 7,495 | |||||||
| State | (729 | ) | 4,355 | 849 | ||||||
$ | 19,098 | $ | 21,281 | $ | 8,556 | |||||
F - 29
The principal components of the Company’s deferred tax balances are as follows (in thousands):
March 31 | |||||||
2007 | 2006 | ||||||
Deferred Tax Assets | |||||||
| Allowance for doubtful accounts and sales returns | $ | 982 | $ | 1,975 | |||
| Inventory capitalization | 420 | 524 | |||||
| Inventory reserves | 731 | 420 | |||||
| Net operating loss carryforwards | 1,052 | 2,402 | |||||
| Property and equipment | 95 | 325 | |||||
| State income taxes | 4,545 | 5,319 | |||||
| Accrued liabilities | 286 | 233 | |||||
| Other | 347 | 168 | |||||
Deferred Tax Liabilities | |||||||
| Intangible assets | (120,096 | ) | (106,342 | ) | |||
| Interest rate caps | (198 | ) | (400 | ) | |||
$ | (111,836 | ) | $ | (95,376 | ) | ||
At March 31, 2007, Medtech Products, Inc., a wholly-owned subsidiary of the Company, had a net operating loss carryforward of approximately $2.6 million which may be used to offset future taxable income of the consolidated group and which begins to expire in 2020. The net operating loss carryforward is subject to an annual limitation as to usage under Internal Revenue Code Section 382 of approximately $240,000.
A reconciliation of the effective tax rate compared to the statutory U.S. Federal tax rate is as follows (in thousands):
Year Ended March 31 | |||||||||||||||||||
2007 | 2006 | 2005 | |||||||||||||||||
% | % | % | |||||||||||||||||
Income tax provision at statutory rate | $ | 19,312 | 35.0 | $ | 16,645 | 35.0 | $ | 6,384 | 34.0 | ||||||||||
| Foreign tax provision | (69 | ) | (0.1 | ) | 59 | 0.1 | 102 | 0.5 | |||||||||||
State income taxes, net of federal income tax benefit | 2,029 | 3.7 | 2,096 | 4.4 | 901 | 4.8 | |||||||||||||
Increase in net deferred tax liability resulting from an increase in federal tax rate to 35% | -- | -- | -- | -- | 1,147 | 6.2 | |||||||||||||
Increase (decrease) in net deferred tax liability resulting from an increase (decrease) in the effective state tax rate | (2,200 | ) | (4.0 | ) | 2,019 | 4.2 | -- | -- | |||||||||||
| Goodwill | -- | -- | 461 | 1.0 | -- | -- | |||||||||||||
| Other | 26 | 0.0 | 1 | 0.0 | 22 | 0.1 | |||||||||||||
| Provision for income taxes | $ | 19,098 | 34.6 | $ | 21,281 | 44.7 | $ | 8,556 | 45.6 | ||||||||||
The Company and certain of its officers and directors are defendants in a consolidated putative securities class action lawsuit filed in the United States District Court for the Southern District of New York (the “Consolidated Action”). The first of the six consolidated cases was filed on August 3, 2005. Plaintiffs purport to represent a class of stockholders of the Company who purchased shares between February 9, 2005 through November 15,
F - 30
2005. Plaintiffs also name as defendants the underwriters in the Company’s initial public offering and a private equity fund that was a selling stockholder in the offering. The District Court has appointed a Lead Plaintiff. On December 23, 2005, the Lead Plaintiff filed a Consolidated Class Action Complaint, which asserted claims under Sections 11, 12(a)(2) and 15 of the Securities Act of 1933 and Sections 10(b), 20(a) and 20A of the Securities Exchange Act of 1934. The Lead Plaintiff generally alleged that the Company issued a series of materially false and misleading statements in connection with its initial public offering and thereafter in regard to the following areas: the accounting issues described in the Company’s press release issued on or about November 15, 2005; and the alleged failure to disclose that demand for certain of the Company’s products was declining and that the Company was planning to withdraw several products from the market. Plaintiffs seek an unspecified amount of damages. The Company filed a motion to dismiss the Consolidated Class Action Complaint in February 2006. On July 10, 2006, the Court dismissed all claims against the Company and the individual defendants arising under the Securities Exchange Act of 1934. The parties have commenced the discovery process which is ongoing. On June 1, 2007, a hearing before the Court was held regarding Plaintiffs’ pending motion for class certification in the Consolidated Action on which no decision has been rendered at this time. The Company’s management believes the remaining claims are legally deficient and subject to meritorious defenses. The Company intends to vigorously pursue its defenses; however, the Company cannot reasonably estimate the potential range of loss, if any.
On May 23, 2006, Similasan Corporation filed a lawsuit against the Company in the United States District Court for the District of Colorado in which Similasan alleged false designation of origin, trademark and trade dress infringement, and deceptive trade practices by the Company related to Murine for Allergy Eye Relief, Murine for Tired Eye Relief and Murine for Earache Relief, as applicable. Similasan has requested injunctive relief, an accounting of profits and damages and litigation costs and attorneys’ fees. In response, the Company filed an answer to the complaint with a potentially dispositive motion. In addition to the lawsuit filed by Similasan in the U.S. District Court for the District of Colorado, the Company also received a cease and desist letter from Swiss legal counsel to Similasan and its parent company, Similasan AG, a Swiss company. In the cease and desist letter, Similasan and Similasan AG have alleged a breach of the Secrecy Agreement executed by the Company and demanded that the Company cease and desist from (i) using confidential information covered by the Secrecy Agreement; and (ii) manufacturing, distributing, marketing or selling certain of its homeopathic products. The complaint in the Colorado action has been amended to include allegations relating to the breach of confidentiality and the Company has filed an answer and responsive motions. On February 22, 2007, prior to the Court’s issuance of a decision regarding the pending motions, the Company agreed to a settlement of the litigation with Similasan and Similasan AG and executed a settlement agreement. The terms of the settlement agreement involved a dismissal of the litigation with prejudice and no monetary damages to the Company.
On September 28, 2006, OraSure Technologies, Inc. moved in the Supreme Court of the State of New York for a preliminary injunction prohibiting the Company from selling cryogenic wart removal products under the Wartner brand, which the Company acquired on September 21, 2006. OraSure Technologies is a supplier to the Company for the Company’s Compound W Freeze Off business. The distribution agreement between the parties provides for mediation of contract disputes, followed by arbitration, if necessary. The contract in question is of five years duration ending in December 2007. On October 30, 2006, the Court denied OraSure Technologies’ motion for a preliminary injunction. Subsequently, in a decision and order dated December 20, 2006, the Court denied a motion by OraSure Technologies for a rehearing regarding a preliminary injunction. An appeal was filed by OraSure in the Appellate Division of the Supreme Court of the State of New York on January 29, 2007, and the Company filed a brief with the Court on February 28, 2007. On May 17, 2007, the Appellate Division reversed the decision of the Supreme Court of the State of New York and issued a preliminary injunction prohibiting the marketing and selling of the Wartner brand by the Company until the underlying arbitration with OraSure was concluded. On May 21, 2007, the Company requested that the Appellate Division issue a stay of the preliminary injunction, consider reargument of the Appellate Division’s decision and grant a leave to appeal to the Court of Appeals of the State of New York. In response to the Company’s request for a stay of the preliminary injunction, the Appellate Division issued a stay of the preliminary injunction pending the Appellate Division’s consideration of the Company’s motion to reargue and request for leave to appeal to the Court of Appeals. The parties are currently awaiting the decision of the Appellate Division regarding the motions made by the Company. The Company believes the preliminary injunction was granted based upon factual and legal error and is inconsistent with the terms of the distribution agreement with OraSure and applicable law. The Company is also seeking resolution of the matter through arbitration as required pursuant to the distribution agreement. A hearing before
F - 31
the arbitration panel is scheduled to be held in August 2007.
The Company is also involved from time to time in other routine legal matters and other claims incidental to its business. The Company reviews outstanding claims and proceedings internally and with external counsel as necessary to assess probability and amount of potential loss. These assessments are re-evaluated at each reporting period and as new information becomes available to determine whether a reserve should be established or if any existing reserve should be adjusted. The actual cost of resolving a claim or proceeding ultimately may be substantially different than the amount of the recorded reserve. In addition, because it is not permissible under generally accepted accounting principles to establish a litigation reserve until the loss is both probable and estimable, in some cases there may be insufficient time to establish a reserve prior to the actual incurrence of the loss (upon verdict and judgment at trial, for example, or in the case of a quickly negotiated settlement). The Company believes the resolution of routine matters and other incidental claims, taking into account reserves and insurance, will not have a material adverse effect on its business, financial condition or results from operations.
Lease Commitments
The Company has operating leases for office facilities and equipment in New York, New Jersey and Wyoming, which expire at various dates through 2011.
The following summarizes future minimum lease payments for the Company’s operating leases (in thousands):
Facilities | Equipment | Total | ||||||||
Year Ending March 31, | ||||||||||
| 2008 | $ | 612 | $ | 120 | $ | 732 | ||||
| 2009 | 501 | 113 | 614 | |||||||
| 2010 | 75 | 85 | 160 | |||||||
| 2011 | -- | 25 | 25 | |||||||
$ | 1,188 | $ | 343 | $ | 1,531 | |||||
Rent expense for 2007, 2006 and 2005 was $565,000, $584,000 and $512,000 respectively.
The Company’s sales are concentrated in the areas of over-the-counter healthcare, personal care and household cleaning products. The Company sells its products to mass merchandisers, food and drug accounts, and dollar and club stores. During 2007, 2006 and 2005, approximately 57%, 61%, and 64%, respectively, of the Company’s total sales were derived from its four major brands. During 2007, 2006 and 2005, approximately 24%, 21%, and 24%, respectively, of the Company’s net sales were made to one customer. At March 31, 2007, approximately 22% of accounts receivable were owed by the same customer.
The Company manages product distribution in the continental United States through a main distribution center in St. Louis, Missouri. A serious disruption, such as a flood or fire, to the main distribution center could damage the Company’s inventories and could materially impair the Company’s ability to distribute its products to customers in a timely manner or at a reasonable cost. The Company could incur significantly higher costs and experience longer lead times associated with the distribution of its products to its customers during the time that it takes the Company to reopen or replace its distribution center. As a result, any such disruption could have a material adverse affect on the Company’s sales and profitability.
The Company has relationships with over 40 third-party manufacturers. Of those, the top 10 manufacturers produced items that accounted for approximately 78% of the Company’s gross sales for 2007. The Company does not have long-term contracts with 3 of these manufacturers and certain manufacturers of various smaller brands, which collectively, represented approximately 35% of the Company’s gross sales for 2007. The lack of manufacturing agreements for these products exposes the Company to the risk that a manufacturer could stop producing the Company’s products at any time, for any reason or fail to provide the Company with the level of products the Company needs to meet its customers’ demands. Without adequate supplies of merchandise to sell
F - 32
to the Company’s customers, sales would decrease materially and the Company’s business would suffer.
17. Business Segments
Segment information has been prepared in accordance with FASB Statement No. 131, “Disclosures about Segments of an Enterprise and Related Information.” The Company’s operating and reportable segments consist of (i) Over-the-Counter Healthcare, (ii) Household Cleaning and (iii) Personal Care.
There were no inter-segment sales or transfers during any of the periods presented. The Company evaluates the performance of its operating segments and allocates resources to them based primarily on contribution margin. The table below summarizes information about the Company’s operating and reportable segments (in thousands).
Year Ended March 31, 2007 | |||||||||||||
Over-the-Counter | Household | Personal | |||||||||||
Healthcare | Cleaning | Care | Consolidated | ||||||||||
| Net sales | $ | 174,704 | $ | 117,249 | $ | 24,894 | $ | 316,847 | |||||
| Other revenues | -- | 1,787 | -- | 1,787 | |||||||||
| Total revenues | 174,704 | 119,036 | 24,894 | 318,634 | |||||||||
| Cost of sales | 65,601 | 73,002 | 14,544 | 153,147 | |||||||||
| Gross profit | 109,103 | 46,034 | 10,350 | 165,487 | |||||||||
| Advertising and promotion | 24,201 | 6,679 | 1,125 | 32,005 | |||||||||
| Contribution margin | $ | 84,902 | $ | 39,355 | $ | 9,225 | 133,482 | ||||||
| Other operating expenses | 38,800 | ||||||||||||
| Operating income | 94,682 | ||||||||||||
| Other (income) expense | 39,506 | ||||||||||||
| Provision for income taxes | 19,098 | ||||||||||||
| Net income | $ | 36,078 | |||||||||||
F - 33
Year Ended March 31, 2006 | |||||||||||||
Over-the-Counter | Household | Personal | |||||||||||
Healthcare | Cleaning | Care | Consolidated | ||||||||||
| Net sales | $ | 160,942 | $ | 107,372 | $ | 27,925 | $ | 296,239 | |||||
| Other revenues | -- | 429 | -- | 429 | |||||||||
| Total revenues | 160,942 | 107,801 | 27,925 | 296,668 | |||||||||
| Cost of sales | 58,491 | 65,088 | 15,851 | 139,430 | |||||||||
| Gross profit | 102,451 | 42,713 | 12,074 | 157,238 | |||||||||
| Advertising and promotion | 22,424 | 6,495 | 3,163 | 32,082 | |||||||||
| Contribution margin | $ | 80,027 | $ | 36,218 | $ | 8,911 | 125,156 | ||||||
| Other operating expenses | 41,252 | ||||||||||||
| Operating income | 83,904 | ||||||||||||
| Other (income) expense | 36,346 | ||||||||||||
| Provision for income taxes | 21,281 | ||||||||||||
| Net income | $ | 26,277 | |||||||||||
Year Ended March 31, 2005 | |||||||||||||
Over-the-Counter | Household | Personal | |||||||||||
Healthcare | Cleaning | Care | Consolidated | ||||||||||
| Net sales | $ | 159,010 | $ | 97,746 | $ | 32,162 | $ | 288,918 | |||||
| Other revenues | -- | 151 | -- | 151 | |||||||||
| Total revenues | 159,010 | 97,897 | 32,162 | 289,069 | |||||||||
| Cost of sales | 60,570 | 62,039 | 16,400 | 139,009 | |||||||||
| Gross profit | 98,440 | 35,858 | 15,762 | 150,060 | |||||||||
| Advertising and promotion | 18,543 | 5,656 | 5,498 | 29,697 | |||||||||
| Contribution margin | $ | 79,897 | $ | 30,202 | $ | 10,264 | 120,363 | ||||||
| Other operating expenses | 29,998 | ||||||||||||
| Operating income | 90,365 | ||||||||||||
| Other (income) expense | 71,589 | ||||||||||||
| Provision for income taxes | 8,556 | ||||||||||||
| Net income | $ | 10,220 | |||||||||||
During 2007, approximately 95% of the Company’s sales were made to customers in the United States and Canada, while during each of 2006 and 2005, approximately 97% of the Company’s sales were made to customers in the United States and Canada. Other than the United States, no individual geographical area accounted for more than 10% of net sales in any of the periods presented. At March 31, 2007, substantially all of the Company’s long-term assets were located in the United States of America and have been allocated to the
F - 34
operating segments as follows:
Over-the-Counter | Household | Personal | |||||||||||
Healthcare | Cleaning | Care | Consolidated | ||||||||||
| Goodwill | $ | 235,647 | $ | 72,549 | $ | 2,751 | $ | 310,947 | |||||
| Intangible assets | |||||||||||||
| Indefinite lived | 374,070 | 170,893 | -- | 544,963 | |||||||||
| Finite lived | 94,776 | 21 | 17,397 | 112,194 | |||||||||
| 468,846 | 170,914 | 17,397 | 657,157 | ||||||||||
$ | 704,493 | $ | 243,463 | $ | 20,148 | $ | 968,104 | ||||||
18. Unaudited Quarterly Financial Information
Unaudited quarterly financial information for 2007 and 2006 is as follows:
Year Ended March 31, 2007
Quarterly Period Ended | |||||||||||||
(In thousands, except for per share data) | June 30, 2006 | September 30, 2006 | December 31, 2006 | March 31, 2007 | |||||||||
| Total revenues | $ | 75,923 | $ | 84,551 | $ | 80,124 | $ | 78,036 | |||||
| Cost of sales | 36,325 | 41,259 | 36,766 | 38,797 | |||||||||
| Gross profit | 39,598 | 43,292 | 43,358 | 39,239 | |||||||||
| Operating expenses | |||||||||||||
| Advertising and promotion | 7,402 | 9,455 | 8,952 | 6,196 | |||||||||
| Depreciation and amortization | 2,413 | 2,412 | 2,804 | 2,755 | |||||||||
| General and administrative | 6,434 | 7,259 | 7,068 | 7,655 | |||||||||
| 16,249 | 19,126 | 18,824 | 16,606 | ||||||||||
| Operating income | 23,349 | 24,166 | 24,534 | 22,633 | |||||||||
| Net interest expense | (9,792 | ) | (9,743 | ) | (10,156 | ) | (9,815 | ) | |||||
| Income before income taxes | 13,557 | 14,423 | 14,378 | 12,818 | |||||||||
| Provision for income taxes | (5,301 | ) | (5,639 | ) | (3,735 | ) | (4,423 | ) | |||||
| Net income | $ | 8,256 | $ | 8,784 | $ | 10,643 | $ | 8,395 | |||||
| Net income per share: | |||||||||||||
| Basic | $ | 0.17 | $ | 0.18 | $ | 0.21 | $ | 0.17 | |||||
| Diluted | $ | 0.17 | $ | 0.18 | $ | 0.21 | $ | 0.17 | |||||
| Weighted average shares outstanding: | |||||||||||||
| Basic | 49,372 | 49,451 | 49,535 | 49,607 | |||||||||
| Diluted | 50,005 | 49,994 | 50,024 | 50,027 | |||||||||
F - 35
Year Ended March 31, 2006
Quarterly Period Ended | |||||||||||||
(In thousands, except for per share data) | June 30, 2005 | September 30, 2005 | December 31, 2005 | March 31, 2006 | |||||||||
| Total revenues | $ | 63,453 | $ | 73,345 | $ | 79,856 | $ | 80,014 | |||||
| Cost of sales | 28,949 | 35,549 | 38,726 | 36,206 | |||||||||
| Gross profit | 34,504 | 37,796 | 41,130 | 43,808 | |||||||||
| Operating expenses | |||||||||||||
| Advertising and promotion | 8,705 | 10,217 | 7,385 | 5,775 | |||||||||
| Depreciation and amortization | 2,631 | 2,635 | 2,834 | 2,694 | |||||||||
| General and administrative | 4,911 | 4,117 | 6,159 | 5,954 | |||||||||
Other expenses (1) | -- | -- | -- | 9,317 | |||||||||
| 16,247 | 16,969 | 16,378 | 23,740 | ||||||||||
| Operating income | 18,257 | 20,827 | 24,752 | 20,068 | |||||||||
| Net interest expense | (8,510 | ) | (8,671 | ) | (9,526 | ) | (9,639 | ) | |||||
| Income before income taxes | 9,747 | 12,156 | 15,226 | 10,429 | |||||||||
| Provision for income taxes | (3,818 | ) | (4,782 | ) | (5,881 | ) | (6,800 | ) | |||||
| Net income | $ | 5,929 | $ | 7,374 | $ | 9,345 | $ | 3,629 | |||||
| Net income per share: | |||||||||||||
| Basic | $ | 0.12 | $ | 0.15 | $ | 0.19 | $ | 0.07 | |||||
| Diluted | $ | 0.12 | $ | 0.15 | $ | 0.19 | $ | 0.07 | |||||
| Weighted average shares outstanding: | |||||||||||||
| Basic | 48,722 | 48,791 | 48,929 | 49,077 | |||||||||
| Diluted | 49,998 | 49,949 | 50,010 | 50,008 | |||||||||
(1) | Consists of a $7.4 million charge for the impairment of intangible assets and a $1.9 million charge for the impairment of goodwill. |
F - 36
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
(In Thousands) | Balance at Beginning of Year | Amounts Charged to Expense | Deductions | Other | Balance at End of Year | ||||||||||||||
Year Ended March 31, 2007 | |||||||||||||||||||
Reserves for sales returns and allowance | $ | 1,868 | $ | 12,611 | $ | (12,726 | ) | $ | -- | $ | 1,753 | ||||||||
Reserves for trade promotions | 1,671 | 2,974 | (2,484 | ) | -- | 2,161 | |||||||||||||
Reserves for consumer coupon redemptions | 283 | 2,674 | (2,556 | ) | -- | 401 | |||||||||||||
Allowance for doubtful accounts | 100 | 100 | (165 | ) | -- | 35 | |||||||||||||
Allowance for inventory obsolescence | 1,019 | 3,096 | (2,397 | ) | 136 | (1 | ) | 1,854 | |||||||||||
Year Ended March 31, 2006 | |||||||||||||||||||
Reserves for sales returns and allowance | $ | 1,652 | $ | 13,040 | $ | (13,056 | ) | $ | 232 | (2 | ) | $ | 1,868 | ||||||
Reserves for trade promotions | 1,493 | 2,522 | (2,481 | ) | 137 | (2 | ) | 1,671 | |||||||||||
Reserves for consumer coupon redemptions | 290 | 2,680 | (2,687 | ) | -- | 283 | |||||||||||||
Allowance for doubtful accounts | 250 | 1 | (92 | ) | (59 | ) | (2 | ) | 100 | ||||||||||
Allowance for inventory obsolescence | 1,450 | 76 | (526 | ) | 19 | (2 | ) | 1,019 | |||||||||||
| Pecos returns reserve | 242 | -- | (242 | ) | -- | -- | |||||||||||||
Year Ended March 31, 2005 | |||||||||||||||||||
Reserves for sales returns and allowance | $ | 687 | $ | 10,245 | $ | (9,280 | ) | $ | -- | $ | 1,652 | ||||||||
Reserves for trade promotions | 1,163 | 10,120 | (11,660 | ) | 1,870 | (3 | ) | 1,493 | |||||||||||
Reserves for consumer coupon redemptions | 266 | 2,265 | (2,891 | ) | 650 | (3 | ) | 290 | |||||||||||
Allowance for doubtful accounts | 60 | 32 | (33 | ) | 191 | (3 | ) | 250 | |||||||||||
Allowance for inventory obsolescence | 124 | 769 | (266 | ) | 823 | (3 | ) | 1,450 | |||||||||||
| Pecos returns reserve | 1,186 | -- | (944 | ) | -- | 242 | |||||||||||||
(1) As a result of the acquisition of Dental Concepts LLC, the Company recorded an allowance for inventory obsolescence in purchase accounting.
(2) As a result of the acquisition of Dental Concepts LLC, the Company recorded allowance for sales returns, promotional allowances and bad debts in purchase accounting.
(3) As a result of the acquisition of Bonita Bay Holdings, Inc. and Vetco, Inc., the Company recorded allowances for doubtful accounts and inventory obsolescence in purchase accounting.
F - 37
EXHIBIT INDEX
EXHIBIT NO. DESCRIPTION
| 2.1 | Asset Sale and Purchase Agreement, dated July 22, 2005, by and among Reckitt Benckiser Inc., Reckitt Benckiser (Canada) Inc., Prestige Brands Holdings, Inc. and The Spic and Span Company (filed as Exhibit 2.1 to Prestige Brands Holdings, Inc.’s Form 8-K filed on July 28, 2005).+ | |
| 2.2 | Unit Purchase Agreement, dated as of November 9, 2005, by and between Prestige Brands Holdings, Inc., and each of Dental Concepts LLC, Richard Gaccione, Combined Consultants DBPT Gordon Wade, Douglas A.P. Hamilton, Islandia L.P., George O’Neill, Abby O’Neill, Michael Porter, Marc Cole and Michael Lesser (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 14, 2006).+ | |
| 2.3 | Stock Sale and Purchase Agreement, dated as of September 21, 2006, by Lil’ Drug Store Products, Inc., Wartner USA B.V., Lil’ Drug Store Products, Inc.’s shareholders set forth on the signature thereto, and Medtech Products Inc. (filed as Exhibit 2.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on November 9, 2006).+ | |
| 3.1 | Amended and Restated Certificate of Incorporation of Prestige Brands Holdings, Inc. (filed as Exhibit 3.1 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on February 8, 2005).+ | |
| 3.2 | Amended and Restated Bylaws of Prestige Brands Holdings, Inc., as amended (filed as Exhibit 3.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on August 9, 2006).+ | |
| 4.1 | Form of stock certificate for common stock (filed as Exhibit 4.1 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+ | |
| 4.2 | Indenture, dated April 6, 2004, among Prestige Brands, Inc., each Guarantor thereto and U.S. Bank National Association, as Trustee (filed as Exhibit 4.1 to Prestige Brands, Inc.’s Form S-4 filed on July 6, 2004).+ | |
| 4.3 | Form of 9¼% Senior Subordinated Note due 2012 (contained in Exhibit 4.2 to this Annual Report on Form 10-K).+ | |
| 4.4 | Supplemental Indenture, dated as of October 6, 2004, among Vetco, Inc., Prestige Brands, Inc. and U.S. Bank, National Association (filed as Exhibit 4.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+ | |
| 4.5 | Second Supplemental Indenture, dated as of December 19, 2006, by and among Prestige Brands, Inc., U.S. Bank, National Association, Prestige Brands Holdings, Inc., Dental Concepts LLC and Prestige International Holdings, LLC (filed as Exhibit 4.2 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+ | |
| 10.1 | Credit Agreement, dated April 6, 2004, among Prestige Brands, Inc., Prestige Brands International, LLC, the Lenders thereto, the Issuers thereto, Citicorp North America, Inc., as Administrative Agent and as Tranche C Agent, Bank of America, N.A., as Syndication Agent, and Merrill Lynch Capital, a division of Merrill Lynch Business Financial Services Inc., as Documentation Agent (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.2 | Form of Amendment No. 1 to the Credit Agreement, dated as of April 6, 2004, among Prestige Brands, Inc., Prestige Brands International, LLC, the Lenders thereto, the Issuers thereto, Citicorp North America, Inc., as administrative agent, Bank of America, N.A., as syndication agent, and Merrill Lynch Capital, a division of Merrill Lynch Business Financial Services, Inc., as documentation agent (filed as Exhibit 10.1.1 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on February 8, 2005).+ |
| 10.3 | Pledge and Security Agreement, dated April 6, 2004, by Prestige Brands, Inc. and each of the Grantors party thereto, in favor of Citicorp North America, Inc. as Administrative Agent and Tranche C Agent (filed as Exhibit 10.2 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.4 | Joinder Agreement, dated as of December 19, 2006, by Prestige Brands Holdings, Inc., Prestige International Holdings, LLC and Dental Concepts LLC in favor of Citicorp North America, Inc., as Administrative Agent, to the Pledge and Security Agreement, dated as of April 6, 2004, by Prestige Brands, Inc. and its subsidiaries and affiliates listed on the signature pages thereof in favor of Citicorp North America, Inc., as Administrative Agent (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+ | |
| 10.5 | Guaranty, dated as of April 6, 2004, by Prestige Brands International, LLC and each of the other entities listed on the signature pages thereof in favor of Citicorp North America, Inc., as Administrative Agent (filed as Exhibit 10.2 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+ | |
| 10.6 | Guaranty Supplement, dated as of December 19, 2006, by Prestige Brands Holdings, Inc., Prestige International Holdings, LLC and Dental Concepts LLC in favor of Citicorp North America, Inc., as Administrative Agent, to the Guaranty, dated as of April 6, 2004, among Prestige Brands International, LLC and certain subsidiaries and affiliates of Prestige Brands, Inc. listed on the signature pages thereof in favor of Citicorp North America, Inc., as Administrative Agent (filed as Exhibit 10.3 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+ | |
| 10.7 | Securityholders Agreement, dated February 6, 2004, among Medtech/Denorex, LLC, GTCR Fund VIII, L.P., GTCR Fund VIII/B, L.P., GTCR Co-Invest II, L.P., GTCR Capital Partners, L.P., the TCW/Crescent Purchasers and the TCW/Crescent Lenders thereto, each Executive thereto and each of the Other Securityholders thereto (filed as Exhibit 10.11 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.8 | First Amendment and Acknowledgement to Securityholders Agreement, dated April 6, 2004, to the Securityholders Agreement, dated February 6, 2004, among Medtech/Denorex, LLC, GTCR Fund VIII, L.P., GTCR Fund VIII/B, L.P., GTCR Co-Invest II, L.P., GTCR Capital Partners, L.P., the TCW/Crescent Purchasers and the TCW/Crescent Lenders thereto, each Executive thereto and each of the Other Securityholders thereto (filed as Exhibit 10.12 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.9 | Registration Rights Agreement, dated February 6, 2004, among Medtech/Denorex, LLC, GTCR Fund VIII, L.P., GTCR Fund VIII/B, L.P., GTCR Co-Invest II, L.P., GTCR Capital Partners, L.P., the TCW/Crescent Purchasers and the TCW/Crescent Lenders thereto, each Executive thereto and each of the Other Securityholders thereto (filed as Exhibit 10.13 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.10 | First Amendment and Acknowledgement to Registration Rights Agreement, dated April 6, 2004, to the Registration Rights Agreement, dated February 6, 2004, among Medtech/Denorex, LLC, GTCR Fund VIII, L.P., GTCR Fund VIII/B, L.P., GTCR Co-Invest II, L.P., GTCR Capital Partners, L.P., the TCW/Crescent Purchasers and the TCW/Crescent Lenders thereto, each Executive thereto and each of the Other Securityholders thereto (filed as Exhibit 10.14 to Prestige Brands Holdings, Inc.’s Form S-1 filed on July 28, 2004).+ | |
| 10.11 | Omnibus Consent and Amendment to Securityholders Agreement, Registration Rights Agreement, Senior Management Agreements and Unit Purchase Agreement, dated as of July 6, 2004 (filed as Exhibit 10.29.1 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on November 12, 2004).+ | |
| 10.12 | Form of Exchange Agreement by and among Prestige Brands Holdings, Inc., Prestige International Holdings, LLC and the common unit holders listed on the signature pages thereto (filed as Exhibit 10.39 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+ | |
| 10.13 | Employment Agreement, dated as of January 19, 2007, by and between Prestige Brands Holdings, Inc. and Mark Pettie (filed as Exhibit 10.5 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+@ | |
| 10.14 | Senior Management Agreement, dated as of March 21, 2006, between Prestige Brands Holdings, Inc., Prestige Brands, Inc. and Peter C. Mann (filed as Exhibit 99.1 to Prestige Brands Holdings, Inc.’s Form 8-K filed on March 23, 2006).+@ | |
| 10.15 | Form of Amended and Restated Senior Management Agreement, dated as of January 28, 2005, by and among Prestige International Holdings, LLC, Prestige Brands Holdings, Inc., Prestige Brands, Inc., and Peter J. Anderson (filed as Exhibit 10.29.7 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+@ |
| 10.16 | Executive Employment Agreement, dated as of January 17, 2006, between Prestige Brands Holdings, Inc. and Charles N. Jolly (filed as Exhibit 10.35 to Prestige Brands Holdings, Inc.’s Form 10-K filed on June 14, 2006).+@ | |
| 10.17 | Letter Agreement between Prestige Brands Holdings, Inc. and James E. Kelly*@ | |
| 10.18 | Executive Employment Agreement, dated as of August 21, 2006, between Prestige Brands Holdings, Inc. and Jean A. Boyko (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on November 9, 2006).+@ | |
| 10.19 | Form of Amended and Restated Senior Management Agreement, dated as of January 28, 2005, by and among Prestige International Holdings, LLC, Prestige Brands Holdings, Inc., Prestige Brands, Inc., and Gerard F. Butler (filed as Exhibit 10.29.8 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+@ | |
| 10.20 | Letter Agreement, dated December 22, 2006, among Prestige Brands Holdings, Inc., Prestige Brands, Inc. and Gerard F. Butler (filed as Exhibit 10.4 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on February 9, 2007).+# | |
| 10.21 | Form of Amended and Restated Senior Management Agreement, dated as of January 28, 2005, by and among Prestige International Holdings, LLC, Prestige Brands Holdings, Inc., Prestige Brands, Inc., and Michael A. Fink (filed as Exhibit 10.29.9 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+@ | |
| 10.22 | Letter Agreement, dated April 13, 2007, by and among Prestige Brands Holdings, Inc., Prestige Brands, Inc. and Michael A. Fink.*# | |
| 10.23 | Form of Amended and Restated Senior Management Agreement, dated as of January 28, 2005, by and among Prestige International Holdings, LLC, Prestige Brands Holdings, Inc., Prestige Brands, Inc., and Charles Shrank (filed as Exhibit 10.29.10 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+@ | |
| 10.24 | Form of Amended and Restated Senior Management Agreement, dated as of January 28, 2005, by and among Prestige International Holdings, LLC, Prestige Brands Holdings, Inc., Prestige Brands, Inc., and Eric M. Millar (filed as Exhibit 10.29.11 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+@ | |
| 10.25 | Prestige Brands Holdings, Inc. 2005 Long-Term Equity Incentive Plan (filed as Exhibit 10.38 to Prestige Brands Holdings, Inc.’s Form S-1/A filed on January 26, 2005).+# | |
| 10.26 | Form of Restricted Stock Grant Agreement (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on August 9, 2005).+# | |
| 10.27 | Form of Performance Share Grant Agreement (filed as Exhibit 10.3 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on November 9, 2006).+# | |
| 10.28 | Form of Nonqualified Stock Option Agreement *# | |
| 10.29 | Contract Manufacturing Agreement, dated February 1, 2001, among The Procter & Gamble Manufacturing Company, P&G International Operations SA, Prestige Brands International, Inc. and Prestige Brands International (Canada) Corp. (filed as Exhibit 10.31 to Prestige Brands, Inc.’s Form S-4/A filed on August 4, 2004).+** | |
| 10.30 | Patent and Technology License Agreement, dated October 2, 2001, between The Procter & Gamble Company and Prestige Brands International, Inc. (filed as Exhibit 10.29 to Prestige Brands, Inc.’s Form S-4/A filed on August 19, 2004).+** | |
| 10.31 | Amendment No. 4 and Restatement of Contract Manufacturing Agreement, dated May 1, 2002, by and between The Procter & Gamble Company and Prestige Brands International, Inc. (filed as Exhibit 10.33 to Prestige Brands, Inc.’s Form S-4/A filed on August 4, 2004).+** | |
| 10.32 | Manufacturing Agreement, dated December 30, 2002, by and between Prestige Brands International, Inc. and Abbott Laboratories (filed as Exhibit 10.32 to Prestige Brands, Inc.’s Form S-4/A filed on August 4, 2004).+** | |
| 10.33 | Distribution Agreement, dated April 24, 2003, by and between Medtech Holdings, Inc. and OraSure Technologies, Inc. (filed as Exhibit 10.27 to Prestige Brands, Inc.’s Form S-4/A filed on August 4, 2004).+** | |
| 10.34 | Amendment No. 1 to Distribution Agreement, dated as of February 10, 2006, between OraSure Technologies, Inc., Medtech Holdings, Inc. and Medtech Products Inc. (filed as Exhibit 10.2 to Prestige Brands Holdings, Inc.’s Form 8-K filed on September 29, 2006).+ | |
| 10.35 | Amendment No. 1 dated April 30, 2003 to the Patent and Technology License Agreement, dated October 2, 2001, between The Procter & Gamble Company and Prestige Brands International, Inc. (filed as Exhibit 10.30 to Prestige Brands, Inc.’s Form S-4/A filed on August 19, 2004).+ | |
| 10.36 | Storage and Handling Agreement dated April 13, 2005 by and between Warehousing Specialists, Inc. and Prestige Brands, Inc. (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 8-K filed on April 15, 2005).+ | |
| 10.37 | Transportation Management Agreement dated April 13, 2005 by and between Prestige Brands, Inc. and Nationwide Logistics, Inc. (filed as Exhibit 10.2 to Prestige Brands Holdings, Inc.’s Form 8-K filed on April 15, 2005).+ | |
| 10.38 | Trademark License and Option to Purchase Agreement, dated September 8, 2005, by and among The Procter & Gamble Company and Prestige Brands Holdings, Inc. (filed as Exhibit 10.1 to Prestige Brands Holdings, Inc.’s Form 8-K filed on September 12, 2005).+ | |
| 10.39 | Exclusive Supply Agreement, dated as of September 18, 2006, among Medtech Products Inc., Pharmacare Limited, Prestige Brands Holdings, Inc. and Aspen Pharmacare Holdings Limited (filed as Exhibit 10.2 to Prestige Brands Holdings, Inc.’s Form 10-Q filed on November 9, 2006).+ | |
| 21.1 | Subsidiaries of the Registrant.* | |
| 23.1 | Consent of PricewaterhouseCoopers LLP.* | |
| 31.1 | Certification of Principal Executive Officer of Prestige Brands Holdings, Inc. pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
| 31.2 | Certification of Principal Financial Officer of Prestige Brands Holdings, Inc. pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
| 32.1 | Certification of Principal Executive Officer of Prestige Brands Holdings, Inc. pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934 and Section 1350 of Chapter 63 of Title 18 of the United States Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* | |
| 32.2 | Certification of Principal Financial Officer of Prestige Brands Holdings, Inc. pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934 and Section 1350 of Chapter 63 of Title 18 of the United States Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| * | Filed herewith. |
| ** | Certain confidential portions have been omitted pursuant to a confidential treatment request separately filed with the Securities and Exchange Commission. |
| + | Incorporated herein by reference. |
| @ | Represents a management contract. |
| # | Represents a compensatory plan. |