Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| FOR THE FISCAL YEAR ENDED DECEMBER 31, 2008 | ||
| OR | ||
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
001-32410
(Commission File Number)
(Commission File Number)
CELANESE CORPORATION
(Exact Name of Registrant as Specified in its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) | 98-0420726 (I.R.S. Employer Identification No.) | |
| 1601 West LBJ Freeway, Dallas, TX (Address of Principal Executive Offices) | 75234-6034 (Zip Code) |
(972) 443-4000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act
| Name of Each Exchange | ||
Title of Each Class | on Which Registered | |
Series A Common Stock, par value $0.0001 per share | New York Stock Exchange | |
4.25% Convertible Perpetual Preferred Stock, par value $0.01 per share (liquidation preference $25.00 per share) | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act
None
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15 (d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein and will not be contained, to the best of the Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer þ | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined inRule 12-b2 of the Act). Yes o No þ
The aggregate market value of the registrant’s common stock held by non-affiliates as of June 30, 2008 (the last business day of the registrants’ most recently completed second fiscal quarter) was $6,927,608,530.
The number of outstanding shares of the registrant’s Series A Common Stock, $0.0001 par value, as of February 6, 2009 was 143,505,708.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of the registrant’s Definitive Proxy Statement relating to the 2009 annual meeting of shareholders, to be filed with the Securities and Exchange Commission, are incorporated by reference into Part III.
CELANESE CORPORATION
Form 10-K
For the Fiscal Year Ended December 31, 2008
TABLE OF CONTENTS
2
Table of Contents
Special Note Regarding Forward-Looking Statements
Certain statements in this Annual Report are forward-looking in nature as defined in the Private Securities Litigation Reform Act of 1995. These statements, and other written and oral forward-looking statements made by the Company from time to time, may relate to, among other things, such matters as planned and expected capacity increases and utilization; anticipated capital spending; environmental matters; legal proceedings; exposure to, and effects of hedging of, raw material and energy costs and foreign currencies; global and regional economic, political, and business conditions; expectations, strategies, and plans for individual assets and products, segments, as well as for the whole Company; cash requirements and uses of available cash; financing plans; pension expenses and funding; anticipated restructuring, divestiture, and consolidation activities; cost reduction and control efforts and targets and integration of acquired businesses. These plans and expectations are based upon certain underlying assumptions, and are in turn based upon internal estimates and analyses of current market conditions and trends, management plans and strategies, economic conditions, and other factors. Actual results could differ materially from expectations expressed in the forward-looking statements if one or more of the underlying assumptions and expectations proves to be inaccurate or is unrealized. Certain important factors that could cause actual results to differ materially from those in the forward-looking statements are included with such forward-looking statements and in “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Forward-Looking Statements May Prove Inaccurate.”
| Item 1. | Business |
Basis of Presentation
In this Annual Report onForm 10-K, the term “Celanese” refers to Celanese Corporation, a Delaware corporation, and not its subsidiaries. The terms the “Company,” “we,” “our” and “us” refer to Celanese and its subsidiaries on a consolidated basis. The term “Celanese US” refers to our subsidiary, Celanese US Holdings LLC, a Delaware limited liability company, formerly known as BCP Crystal US Holdings Corp., a Delaware corporation, and not its subsidiaries. The term “Purchaser” refers to our subsidiary, Celanese Europe Holding GmbH & Co. KG, formerly known as BCP Crystal Acquisition GmbH & Co. KG, a German limited partnership, and not its subsidiaries, except where otherwise indicated.
Overview
Celanese Corporation was formed in 2004 when affiliates of The Blackstone Group purchased 84% of the ordinary shares of Celanese GmbH, formerly known as Celanese AG, a diversified German chemical company. Celanese Corporation was incorporated in 2005 under the laws of the state of Delaware and its shares are traded on the New York Stock Exchange under the symbol “CE”. During the period from2005-2007, Celanese Corporation purchased the remaining 16% interest in Celanese GmbH.
We are a leading global integrated producer of chemicals and advanced materials. We are one of the world’s largest producers of acetyl products, which are intermediate chemicals for nearly all major industries, as well as a leading global producer of high performance engineered polymers that are used in a variety of high-value end-use applications. As an industry leader, we hold geographically balanced global positions and participate in diversified end-use markets. Our operations are primarily located in North America, Europe and Asia. We combine a demonstrated track record of execution, strong performance built on our principles and objectives, and a clear focus on growth and value creation.
Our large and diverse global customer base primarily consists of major companies in a broad array of industries. For the year ended December 31, 2008, approximately 28% of our net sales were to customers located in North America, 43% to customers in Europe and Africa, 26% to customers in Asia and Australia and 3% to customers in South America. We have property, plant and equipment in the United States of $732 million and outside the United States of $1,739 million. For more information regarding our property, plant and equipment, see Note 9 and Note 25 to the consolidated financial statements.
Market Industry
This Annual Report onForm 10-K includes industry data obtained from industry publications and surveys as well as our own internal company surveys. Third-party industry publications, surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable. The statements
3
Table of Contents
regarding Celanese’s market position in this document are based on information derived from, among others, the2008 Stanford Research Institute International Chemical Economics Handbook,CMAI 2008 World Methanol Analysis, Tecnon OrbiChemAcetic Acid and Vinyl Acetate World Surveythird quarter 2008 report and Synthetic Latex Polymer Reports from Kline and Co.
Segment Overview
We operate principally through four business segments: Advanced Engineered Materials, Consumer Specialties, Industrial Specialties and Acetyl Intermediates. For further details on our business segments, see Note 25 to the consolidated financial statements. The table below illustrates each segment’s net sales to external customers for the year ended December 31, 2008, as well as each segment’s major products and end-use markets.
| Advanced | ||||||||
| Engineered Materials | Consumer Specialties | Industrial Specialties | Acetyl Intermediates | |||||
2008 Net Sales(1) | $1,061 million | $1,155 million | $1,406 million | $3,199 million | ||||
Key Products | • Polyacetal products (“POM”) • Ultra-high molecular weight polyethylene (“GUR®”) • Liquid crystal polymers (“LCP”) • Polyphenylene sulfide (“PPS”) • Polybutylene terephthalate (“PBT”) • Polyethylene terephthalate (“PET”) • Long fiber reinforced thermoplastics (“LFRT”) | • Acetate tow • Acetate flake • Sunett® sweetener • Sorbates | • Polyvinyl alcohol (“PVOH”) • Polyvinyl acetate • Conventional emulsions • Vinyl acetate ethylene emulsions • Low-density polyethylene resins (“LDPE”) • Ethylene vinyl acetate (“EVA”) resins and compounds | • Acetic acid • Vinyl acetate monomer (“VAM”) • Acetic anhydride • Acetaldehyde • Ethyl acetate • Butyl acetate • Formaldehyde | ||||
Major End-Use Markets | • Fuel system components • Conveyor belts • Battery separators • Electronics • Seat belt mechanisms • Other automotive • Appliances and electronics • Filtrations • Coatings • Medical Devices • Telecommunications | • Filter products • Beverages • Confections • Baked goods • Pharmaceuticals | • Paints • Coatings • Adhesives • Building products • Glass fibers • Textiles • Paper • Flexible packaging • Lamination products • Medical tubing • Automotive parts | • Paints • Coatings • Adhesives • Lubricants • Detergents • Pharmaceuticals • Films • Textiles • Inks • Plasticizers • Esters • Solvents |
| (1) | Consolidated net sales of $6,823 million for the year ended December 31, 2008 also includes $2 million in net sales from Other Activities, which is attributable to our captive insurance companies. Acetyl Intermediates’ net sales exclude inter-segment sales of $676 million for the year ended December 31, 2008. |
Advanced Engineered Materials
Our Advanced Engineered Materials segment develops, produces and supplies a broad portfolio of high performance technical polymers for application in automotive and electronics products, as well as other consumer and industrial applications. Together with our strategic affiliates, we are a leading participant in the global technical polymers industry. The primary products of Advanced Engineered Materials are POM, PPS, LFRT, PBT, PET, GUR® and LCP. POM, PPS, LFRT, PBT and PET are used in a broad range of products including automotive components, electronics, appliances and industrial applications. GUR® is used in battery separators, conveyor belts, filtration equipment, coatings and medical devices. Primary end markets for LCP are electrical and electronics.
4
Table of Contents
Consumer Specialties
Our Consumer Specialties segment consists of our Acetate Products and Nutrinova businesses. Our Acetate Products business primarily produces and supplies acetate tow, which is used in the production of filter products. We also produce acetate flake which is processed into acetate fiber in the form of a tow band. Our Nutrinova business produces and sells Sunett®, a high intensity sweetener, and food protection ingredients, such as sorbates, for the food, beverage and pharmaceuticals industries.
Industrial Specialties
Our Industrial Specialties segment includes our Emulsions, PVOH and AT Plastics businesses. Our Emulsions business is a global leader which produces a broad product portfolio, specializing in vinyl acetate ethylene emulsions, and is a recognized authority on low VOC (volatile organic compounds), an environmentally-friendly technology. As a global leader, our PVOH business produces a broad portfolio of performance PVOH chemicals engineered to meet specific customer requirements. Our emulsions and PVOH products are used in a wide array of applications including paints and coatings, adhesives, building and construction, glass fiber, textiles and paper. AT Plastics offers a complete line of low-density polyethylene and specialty ethylene vinyl acetate resins and compounds. AT Plastics’ products are used in many applications including flexible packaging films, lamination film products, hot melt adhesives, medical tubing, automotive carpeting and solar cell encapsulation films.
Acetyl Intermediates
Our Acetyl Intermediates segment produces and supplies acetyl products, including acetic acid, VAM, acetic anhydride and acetate esters. These products are generally used as starting materials for colorants, paints, adhesives, coatings, medicines and more. Other chemicals produced in this segment are organic solvents and intermediates for pharmaceutical, agricultural and chemical products.
Competitive Strengths
We benefit from a number of competitive strengths, including the following:
Leading Market Positions
We believe that we are a leading global integrated producer of acetyl, acetate and vinyl emulsion products. Advanced Engineered Materials and our strategic affiliates, Polyplastics Co., Ltd. (“Polyplastics”) and Korea Engineering Plastics Co., Ltd. (“KEPCO”), are leading producers and suppliers of engineered polymers in North America, Europe and the Asia/Pacific region. Our leadership positions are based on our large share of global production capacity, operating efficiencies, proprietary technology and competitive cost structures in our major product lines.
Proprietary Production Technology and Operating Expertise
Our production of acetyl products employs industry-leading proprietary and licensed technologies, including our proprietary AOPlustm technology for the production of acetic acid and VAntagetm and VAntage Plustm vinyl acetate monomer technology. AOPlustm enables increased raw material efficiencies, lower operating costs and the ability to expand plant capacity with minimal investment. VAntagetm and VAntage Plustm enable significant increases in production efficiencies, lower operating costs and increases in capacity at ten to fifteen percent of the cost of building a new plant.
Low Cost Producer
Our competitive cost structures are based on production and purchasing economies of scale, vertical integration, technical expertise and the use of advanced technologies.
Global Reach
We operate thirty-two production facilities throughout the world. We participate in strategic alliances which operate nine additional facilities. Our infrastructure of manufacturing plants, terminals, warehouses and sales offices provides us with a competitive advantage in anticipating and meeting the needs of our global and local customers in well-established and growing markets, while our geographic diversity reduces the potential impact of
5
Table of Contents
volatility in any individual country or region. We have a strong, growing presence in Asia, particularly in China, and we have a defined strategy to continue this growth. For more information regarding our financial information with respect to our geographic areas, see Note 25 to the consolidated financial statements.
Growth
We aggressively align with our customers and their markets to capture growth. We are quickly expanding in Asia, the fastest-growing region in the world, in order to meet increasing demand for our products. As part of our strategy, we also continue to develop new products and industry-leading production technologies that deliver value-added solutions for our customers.
Strategic Investments
Our strategic investments have enabled us to gain access, minimize costs and accelerate growth in new markets, while also generating significant cash flow and earnings. Our equity investments and cost investments represent an important component of our growth strategy. See Note 8 to the consolidated financial statements and the “Investments” subheading of Item 1 for additional information on our equity and cost investments.
Diversified Products and End-Use Markets
We offer our customers a broad range of products in a wide variety of end-use markets. Our diversified product lines include paints and coatings, textiles, automotive applications, consumer and medical applications, performance industrial applications, filter media, paper and packaging, chemical additives, construction, consumer and industrial adhesives, and food and beverage applications. This product and market diversity reduces the potential impact of volatility in any individual market segment.
Business Strategies
Our strategic foundation is based on the following four pillars which are focused on increasing operating cash flows, improving profitability, delivering high return on investments and increasing shareholder value:
Focus
We focus on businesses where we have a sustainable and proven competitive advantage. We continue to optimize our business portfolio in order to achieve market, cost and technology leadership while expanding our product mix into higher value-added products.
Investment
We leverage and build on advantaged positions that optimize our portfolio of products. In order to increase our competitive advantage, we have invested in our core group of businesses through acquisitions; growth in Asia bolstered by our integrated chemical complex in Nanjing, China; and new applications of our advanced engineered polymers products.
Redeployment
We divest non-core assets and revitalize underperforming businesses. We have divested or exited businesses where we no longer maintain a competitive advantage. We also continue to make key strategic decisions to revitalize businesses that have significant potential for improved performance and enhanced efficiency.
Underlying all of these strategies is a culture of execution and productivity. We continually seek ways to reduce costs, increase productivity and improve process technology. Our commitment to operational excellence is an integral part of our strategy to maintain our cost advantage and productivity leadership.
Business Segments
Advanced Engineered Materials
Our Advanced Engineered Materials segment develops, produces and supplies a broad portfolio of high-performance technical polymers.
6
Table of Contents
Advanced Engineered Materials’ technical polymers have chemical and physical properties enabling them, among other things, to withstand extreme temperatures, resist chemical reactions with solvents and withstand fracturing or stretching. These products are used in a wide range of performance-demanding applications in the automotive and electronics sectors as well as in other consumer and industrial goods.
Advanced Engineered Materials works in concert with its customers to enable innovations and develop new or enhanced products. Advanced Engineered Materials focuses its efforts on developing new markets and applications for its product lines, often developing custom formulations to satisfy the technical and processing requirements of a customer’s applications. For example, Advanced Engineered Materials has collaborated with fuel system suppliers to develop an acetal copolymer with the chemical and impact resistance necessary to withstand exposure to hot diesel fuels in the new generation of common rail diesel engines. The product can also be used in automotive fuel sender units where it remains stable at the high operating temperatures present in direct-injection diesel engines and can meet the requirements of the new generation of bio fuels.
Advanced Engineered Materials’ customer base consists primarily of a large number of plastic molders and component suppliers, which typically supply original equipment manufacturers (“OEMs”). Advanced Engineered Materials works with these molders and component suppliers as well as directly with the OEMs to develop and improve specialized applications and systems.
Prices for most of these products, particularly specialized product grades for targeted applications, generally reflect the value added in complex polymer chemistry, precision formulation and compounding, and the extensive application development services provided. These specialized product lines are not typically susceptible to cyclical swings in pricing.
Key Products
POM is sold under the trademark Hostaform® in all regions but North America, where it is sold under the trademark Celcon®. Polyplastics and KEPCO are leading suppliers of POM and other engineering resins in the Asia/Pacific region. POM is used for mechanical parts, including door locks and seat belt mechanisms, in automotive applications and in electrical, consumer and medical applications such as drug delivery systems and gears for large appliances.
The primary raw material for POM is formaldehyde, which is manufactured from methanol. Advanced Engineered Materials currently purchases formaldehyde in the United States from our Acetyl Intermediates segment and, in Europe, manufactures formaldehyde from purchased methanol.
GUR® is an engineered material used in heavy-duty automotive and industrial applications such as car battery separator panels and industrial conveyor belts, as well as in specialty medical and consumer applications, such as sports equipment and prostheses. GUR® micro powder grades are used for high-performance filters, membranes, diagnostic devices, coatings and additives for thermoplastics and elastomers. GUR® fibers are also used in protective ballistic applications.
Celstran® and Compel® are long fiber reinforced thermoplastics, which impart extra strength and stiffness, making them more suitable for larger parts than conventional thermoplastics and are used in automotive, transportation and industrial applications.
Polyesters such as Celanex® PBT, Celanex® PET, Vandar®, a series of PBT-polyester blends and Riteflex®, a thermoplastic polyester elastomer, are used in a wide variety of automotive, electrical and consumer applications, including ignition system parts, radiator grilles, electrical switches, appliance and sensor housings, light emitting diodes (“LEDs”) and technical fibers. Raw materials for polyesters vary. Base monomers, such as dimethyl terephthalate and purified terephthalic acid (“PTA”), are widely available with pricing dependent on broader polyester fiber and packaging resins market conditions. Smaller volume specialty co-monomers for these products are typically supplied by a limited number of companies.
Liquid crystal polymers, such as Vectra®, are used in electrical and electronics applications and for precision parts with thin walls and complex shapes or on high-heat cookware applications.
7
Table of Contents
Fortron®, a PPS product, is used in a wide variety of automotive and other applications, especially those requiring heatand/or chemical resistance, including fuel system parts, radiator pipes and halogen lamp housings, often replacing metal. Other possible application fields include non-woven filtration devices such as coal fired power plants. Fortron® is manufactured by Fortron Industries LLC, Advanced Engineered Materials’ 50% owned strategic venture with Kureha Corporation of Japan.
Facilities
Advanced Engineered Materials has polymerization, compounding and research and technology centers in Germany, Brazil, China and the United States.
Markets
The following table illustrates the destination of the net sales of the Advanced Engineered Materials segment by geographic region for the years ended December 31, 2008, 2007 and 2006.
Net Sales to External Customers by Destination — Advanced Engineered Materials
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| $ | Segment | $ | Segment | $ | Segment | |||||||||||||||||||
| (In millions, except percentages) | ||||||||||||||||||||||||
| North America | 365 | 34 | % | 388 | 38 | % | 311 | 34 | % | |||||||||||||||
| Europe/Africa | 553 | 52 | % | 517 | 50 | % | 500 | 55 | % | |||||||||||||||
| Asia/Australia | 106 | 10 | % | 88 | 8 | % | 55 | 6 | % | |||||||||||||||
| South America | 37 | 4 | % | 37 | 4 | % | 49 | 5 | % | |||||||||||||||
| Total | 1,061 | 1,030 | 915 | |||||||||||||||||||||
Advanced Engineered Materials’ sales in the Asian market are made directly and through its strategic affiliates. Polyplastics, KEPCO and Fortron Industries are accounted for under the equity method and therefore not included in Advanced Engineered Materials’ consolidated net sales. If Advanced Engineered Materials’ portion of the sales made by these strategic affiliates were included in the table above, the percentage of sales sold in Asia/Australia would be substantially higher. A number of Advanced Engineered Materials’ POM customers, particularly in the appliance, electrical components and certain sections of the electronics/telecommunications fields, have moved tooling and molding operations to Asia, particularly southern China. In addition to our Advanced Engineered Materials affiliates, we directly service Asian demand offering our customers global solutions.
Advanced Engineered Materials’ principal customers are consumer product manufacturers and suppliers to the automotive industry. These customers primarily produce engineered products, and Advanced Engineered Materials collaborates with its customers to assist in developing and improving specialized applications and systems. Advanced Engineered Materials has long-standing relationships with most of its major customers, but also uses distributors for its major products, as well as a number of electronic marketplaces to reach a larger customer base. For most of Advanced Engineered Materials’ product lines, contracts with customers typically have a term of one to two years.
Competition
Advanced Engineered Materials’ principal competitors include BASF AG (“BASF”), E. I. DuPont de Nemours and Company (“DuPont”), DSM N.V., Sabic Innovative Plastics and Solvay S.A. Smaller regional competitors include Asahi Kasei Corporation, Mitsubishi Gas Chemicals, Inc., Chevron Phillips Chemical Company, L.P., Braskem S.A., Lanxess AG, Teijin, Sumitomo, Inc. and Toray Industries Inc.
Consumer Specialties
The Consumer Specialties segment consists of our Acetate Products and Nutrinova businesses. Our Acetate Products business primarily produces and supplies acetate tow, which is used in the production of filter products. We
8
Table of Contents
also produce acetate flake which is processed into acetate fiber in the form of a tow band. Our Nutrinova business produces and sells Sunett®, a high intensity sweetener, and food protection ingredients, such as sorbates, for the food, beverage and pharmaceutical industries.
Key Products
Acetate tow is used primarily in cigarette filters. We produce acetate flake by processing wood pulp with acetic anhydride. We purchase wood pulp that is made from reforested trees from major suppliers and produce acetic anhydride internally. The acetate flake is then further processed into acetate fiber in the form of a tow band. According to the 2008 Stanford Research Institute International Chemical Economics Handbook, we are the world’s leading producer of acetate tow, including production of our China ventures.
Sales of acetate tow amounted to approximately 12%, 11% and 9% of our consolidated net sales for the years ended December 31, 2008, 2007 and 2006, respectively.
We have an approximate 30% interest in three manufacturing China ventures, which are accounted for as cost method investments (Note 8) that produce acetate flake and tow. Our partner in each of the ventures is the Chinesestate-owned tobacco entity, China National Tobacco Corporation. In addition, approximately 10% of our 2008 acetate tow sales were sold directly to China, the largest single market for acetate tow in the world.
Acesulfame potassium, a high intensity sweetener marketed under the trademark Sunett®, is used in a variety of beverages, confections and dairy products throughout the world. Sunett® pricing for targeted applications reflects the value added by Nutrinova, through consistent product quality and reliable supply. Nutrinova’s strategy is to be the most reliable and highest quality producer of this product, to develop new product applications and expand into new markets.
Nutrinova’s food ingredients business consists of the production and sale of food protection ingredients, such as sorbic acid and sorbates, and high intensity sweeteners worldwide. Nutrinova’s food protection ingredients are mainly used in foods, beverages and personal care products. The primary raw materials for these products are ketene and crotonaldehyde. Sorbates pricing is extremely sensitive to demand and industry capacity and is not necessarily dependent on the prices of raw materials.
Facilities
Acetate Products has production sites in the United States, Mexico, the United Kingdom and Belgium, and participates in three manufacturing ventures in China.
Nutrinova has production facilities in Germany, as well as sales and distribution facilities in all major world markets.
Markets
The following table illustrates the destination of the net sales of the Consumer Specialties segment by geographic region for the years ended December 31, 2008, 2007 and 2006.
Net Sales to External Customers by Destination — Consumer Specialties
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| $ | Segment | $ | Segment | $ | Segment | |||||||||||||||||||
| (In millions, except percentages) | ||||||||||||||||||||||||
| North America | 194 | 17 | % | 201 | 18 | % | 204 | 23 | % | |||||||||||||||
| Europe/Africa | 497 | 43 | % | 427 | 39 | % | 248 | 28 | % | |||||||||||||||
| Asia/Australia | 413 | 36 | % | 437 | 39 | % | 395 | 45 | % | |||||||||||||||
| South America | 51 | 4 | % | 46 | 4 | % | 29 | 4 | % | |||||||||||||||
| Total | 1,155 | 1,111 | 876 | |||||||||||||||||||||
9
Table of Contents
Sales of acetate tow are principally to the major tobacco companies that account for a majority of worldwide cigarette production. Our contracts with most of our customers are entered into on an annual basis.
Nutrinova primarily markets Sunett® to a limited number of large multinational and regional customers in the beverage and food industry under long-term and annual contracts. Nutrinova markets food protection ingredients primarily through regional distributors to small and medium sized customers and directly through regional sales offices to large multinational customers in the food industry.
Competition
Acetate Products’ principal competitors include Daicel Chemical Industries Ltd. (“Daicel”), Eastman Chemical Corporation (“Eastman”) and Rhodia S.A.
The principal competitors for Nutrinova’s Sunett® sweetener are Holland Sweetener Company, The NutraSweet Company, Ajinomoto Co., Inc., Tate & Lyle PLC and several Chinese manufacturers. In sorbates, Nutrinova competes with Nantong AA, Daicel, Yu Yao/Ningbo, Yancheng AmeriPac and other Chinese manufacturers of sorbates.
Industrial Specialties
Our Industrial Specialties segment includes our Emulsions, PVOH and AT Plastics businesses. Our Emulsions business is a global leader which produces a broad product portfolio, specializing in vinyl acetate ethylene emulsions and is a recognized authority on low VOC, an environmentally-friendly technology. As a global leader, our PVOH business produces and sells a broad portfolio of performance PVOH chemicals engineered to meet specific customer requirements. AT Plastics offers a complete line of low-density polyethylene and specialty, ethylene vinyl acetate resins and compounds.
Key Products
The products in our Emulsions business include conventional vinyl and acrylate based emulsions and high-pressure vinyl acetate ethylene emulsions. Emulsions are made from VAM, acrylate esters and styrene. Our Emulsions business is a leading producer of polyvinyl acetate and vinyl acetate ethylene emulsions in Europe. These products are a key component of water-based architectural coatings, adhesives, non-wovens, textiles, glass fiber and other applications.
Sales from the Emulsions business amounted to approximately 13%, 14% and 14% of our consolidated net sales for the years ended December 31, 2008, 2007 and 2006, respectively.
PVOH is used in adhesives, building products, paper coatings, films and textiles. The primary raw material to produce PVOH is VAM, while acetic acid is produced as a by-product. Products are sold on a global basis and prices vary depending on industry segment and end-use application. According to industry sources on PVOH, we are the largest North American producer of PVOH and the third largest producer in the world.
AT Plastics produces low-density polyethylene and EVA resins and compounds that are used in the manufacture of hot melt adhesives, automotive carpeting, lamination film products, flexible packaging films, medical tubing and solar cell encapsulation films. EVA resins and compounds are produced in high-pressure reactors from ethylene and VAM.
Facilities
Emulsions has production sites in the United States, Canada, China, Spain, Sweden, the Netherlands and Germany. PVOH has production sites in the United States and Spain along with sales and distribution facilities in Europe, Asia and South America. During 2008, we shut down our Slovenia and United Kingdom facilities. AT Plastics has a production facility in Edmonton, Alberta, Canada.
10
Table of Contents
Markets
The following table illustrates the destination of the net sales of the Industrial Specialties segment by geographic region for years ended December 31, 2008, 2007 and 2006.
Net Sales to External Customers by Destination — Industrial Specialties
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| $ | Segment | $ | Segment | $- | Segment | |||||||||||||||||||
| (In millions, except percentages) | ||||||||||||||||||||||||
| North America | 617 | 44 | % | 583 | 43 | % | 605 | 47 | % | |||||||||||||||
| Europe/Africa | 684 | 48 | % | 674 | 50 | % | 601 | 47 | % | |||||||||||||||
| Asia/Australia | 81 | 6 | % | 69 | 5 | % | 58 | 5 | % | |||||||||||||||
| South America | 24 | 2 | % | 20 | 2 | % | 17 | 1 | % | |||||||||||||||
| Total | 1,406 | 1,346 | 1,281 | |||||||||||||||||||||
Industrial Specialties’ products are sold to a diverse group of regional and multinational customers. Customers for emulsions are manufacturers of water-based paints and coatings, adhesives, paper, building and construction products, glass fiber, non-wovens and textiles. The customers of the PVOH business, who purchase mainly under multi-year contracts, are primarily engaged in the production of adhesives, paper, films, building products and textiles. Customers of AT Plastics are primarily engaged in the manufacture of adhesives, automotive components, packaging materials, print media and solar energy products.
Competition
Principal competitors in the Emulsions business include The Dow Chemical Company (“Dow”), Rohm & Haas Company, BASF, Dairen, Wacker and several smaller regional manufacturers.
Principal competitors in the PVOH business include Kuraray Co., Ltd., DuPont, Chang Chun Petrochemical Co., Ltd., The Nippon Synthetic Chemical Industry Co., Ltd. and several Chinese manufacturers.
Principal competitors for the AT Plastics EVA resins and compounds business include DuPont, ExxonMobil Chemical, Arkema and several Asian manufacturers.
Acetyl Intermediates
Our Acetyl Intermediates segment produces and supplies acetyl products, including acetic acid, VAM, acetic anhydride and acetate esters. Other chemicals produced in this segment are organic solvents and intermediates for pharmaceutical, agricultural and chemical products.
Key Products
Acetyls. Acetyls products include acetic acid, VAM, acetic anhydride and acetaldehyde. Acetic acid is primarily used to manufacture VAM, PTA and other acetyl derivatives. VAM is used in a variety of adhesives, paints, films, coatings and textiles. Acetic anhydride is a raw material used in the production of cellulose acetate, detergents and pharmaceuticals. Acetaldehyde is a major feedstock for the production of polyols. Acetaldehyde is also used in other organic compounds such as pyridines, which are used in agricultural products. We manufacture acetic acid, VAM and acetic anhydride for our own use, as well as for sale to third parties.
Acetic acid and VAM, our basic acetyl intermediates products, are impacted by global supply/demand fundamentals and are cyclical in nature. The principal raw materials in these products are ethylene, which we purchase from numerous sources; carbon monoxide, which we purchase under long-term contracts; and methanol, which we purchase under long-term and short-term contracts. With the exception of carbon monoxide, these raw materials are commodity products available from a wide variety of sources.
11
Table of Contents
Our production of acetyl products employs leading proprietary and licensed technologies, including our proprietary AOPlustm technology for the production of acetic acid and VAntagetm and VAntage Plustm vinyl acetate monomer technology.
Solvents and Derivatives. Solvents and derivatives products include a variety of solvents, formaldehyde and other chemicals, which in turn are used in the manufacture of paints, coatings, adhesives and other products.
Many solvents and derivatives products are derived from our production of acetic acid. Primary products are:
| • | Ethyl acetate, an acetate ester that is a solvent used in coatings, inks and adhesives and in the manufacture of photographic films and coated papers; and | |
| • | Butyl acetate, an acetate ester that is a solvent used in inks, pharmaceuticals and perfume. |
Formaldehyde and formaldehyde derivative products are derivatives of methanol and are made up of the following products:
| • | Formaldehyde, paraformaldehyde and formcels are primarily used to produce adhesive resins for plywood, particle board, coatings, POM engineering resins and a compound used in making polyurethane; | |
| • | Amines such as methyl amines, monisopropynol amines and butyl amines are used in agrochemicals, herbicides and the treatment of rubber and water; and | |
| • | Special solvents, such as crotonaldehyde, which are used by the Nutrinova business line for the production of sorbates, as well as raw materials for the fragrance and food ingredients industry. |
Solvents and derivatives are commodity products characterized by cyclicality in pricing. The principal raw materials used in solvents and derivatives products are acetic acid, various alcohols, methanol, ethylene and ammonia. We manufacture many of these raw materials for our own use as well as for sales to third parties, including our competitors in the solvents and derivatives business. We purchase ethylene from a variety of sources. We manufacture acetaldehyde in Europe for our own use, as well as for sale to third parties.
Sales from acetyl products amounted to approximately 35%, 34% and 33% of our consolidated net sales for the years ended December 31, 2008, 2007 and 2006, respectively. Sales from solvents and derivatives products amounted to approximately 12%, 12% and 13% of our consolidated net sales for the years ended December 31, 2008, 2007 and 2006, respectively.
Facilities
Acetyl Intermediates has production sites in the United States, China, Mexico, Singapore, Spain, France and Germany. We also participate in a strategic venture in Saudi Arabia that produces methanol and methyl tertiary-butyl ether (“MTBE”). Over the last few years, we have continued to shift our production capacity to lower cost production facilities while expanding in growth markets, such as China.
12
Table of Contents
Markets
The following table illustrates net sales by destination of the Acetyl Intermediates segment by geographic region for the years ended December 31, 2008, 2007 and 2006.
Net Sales to External Customers by Destination — Acetyl Intermediates
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| $ | Segment | $ | Segment | $ | Segment | |||||||||||||||||||
| (In millions, except percentages) | ||||||||||||||||||||||||
| North America | 743 | 23 | % | 685 | 23 | % | 685 | 26 | % | |||||||||||||||
| Europe/Africa | 1,198 | 37 | % | 1,183 | 40 | % | 1,075 | 40 | % | |||||||||||||||
| Asia/Australia | 1,142 | 36 | % | 968 | 33 | % | 814 | 30 | % | |||||||||||||||
| South America | 116 | 4 | % | 119 | 4 | % | 110 | 4 | % | |||||||||||||||
Total(1) | 3,199 | 2,955 | 2,684 | |||||||||||||||||||||
| (1) | Excludes inter-segment sales of $676 million, $660 million and $667 million for the years ended December 31, 2008, 2007 and 2006, respectively. |
Acetyl Intermediates markets its products both directly to customers and through distributors.
Acetic acid, VAM and acetic anhydride are global businesses which have several large customers. Generally, we supply these global customers under multi-year contracts. The customers of acetic acid, VAM and acetic anhydride produce polymers used in water-based paints, adhesives, paper coatings, polyesters, film modifiers, pharmaceuticals, cellulose acetate and textiles. We have long-standing relationships with most of these customers.
Solvents and derivatives are sold to a diverse group of regional and multinational customers both under multi-year contracts and on the basis of long-standing relationships. The customers of solvents and derivatives are primarily engaged in the production of paints, coatings and adhesives. We manufacture formaldehyde for our own use as well as for sale to a few regional customers that include manufacturers in the wood products and chemical derivatives industries. The sale of formaldehyde is based on both long and short-term agreements. Specialty solvents and amines are sold globally to a wide variety of customers, primarily in the coatings and resins and the specialty products industries. These products serve global markets in the synthetic lubricant, agrochemical, rubber processing and other specialty chemical areas.
Competition
Our principal competitors in the Acetyl Intermediates segment include Atofina S.A., BASF, British Petroleum PLC, Chang Chun Petrochemical Co., Ltd., Daicel, Dow, Eastman, DuPont, LyondellBasell Industries, Nippon Gohsei, Perstorp Inc., Rohm & Haas Company, Jiangsu Sopo Corporation (Group) Ltd., Showa Denko K.K., and Kuraray Co. Ltd.
Other Activities
Other Activities primarily consists of corporate center costs, including financing and administrative activities such as legal, accounting and treasury functions, interest income and expense associated with our financing activities, and our captive insurance companies. Our two wholly-owned captive insurance companies are a key component of our global risk management program, as well as a form of self-insurance for our property, liability and workers compensation risks. The captive insurance companies issue insurance policies to our subsidiaries to provide consistent coverage amid fluctuating costs in the insurance market and to lower long-term insurance costs by avoiding or reducing commercial carrier overhead and regulatory fees. The captive insurance companies retain risk at levels approved by management and obtain reinsurance coverage from third parties to limit the net risk retained. One of the captive insurance companies also insures certain third-party risks.
13
Table of Contents
Investments
We have a significant portfolio of strategic investments, including a number of ventures in Asia, North America and Europe. In aggregate, these strategic investments enjoy significant sales, earnings and cash flow. We have entered into these strategic investments in order to gain access to local markets, minimize costs and accelerate growth in areas we believe have significant future business potential. See Note 8 to the consolidated financial statements for additional information.
The table below represents our significant strategic ventures as of December 31, 2008:
Location | Ownership | Segment | Partner(s) | Year Entered | ||||||||||
Equity Method Investments | ||||||||||||||
| KEPCO | South Korea | 50% | Advanced Engineered Materials | Mitsubishi Gas Chemical Company, Inc./Mitsubishi Corporation | 1999 | |||||||||
| Polyplastics Co., Ltd. | Japan | 45% | Advanced Engineered Materials | Daicel Chemical Industries Ltd. | 1964 | |||||||||
| Fortron Industries LLC | US | 50% | Advanced Engineered Materials | Kureha Corporation | 1992 | |||||||||
Cost Method Investments | ||||||||||||||
| National Methanol Co. | Saudi Arabia | 25% | Acetyl Intermediates | Saudi Basic Industries Corporation (“SABIC”)/ CTE Petrochemicals | 1981 | |||||||||
| Kunming Cellulose Fibers Co. Ltd. | China | 30% | Consumer Specialties | China National Tobacco Corporation | 1993 | |||||||||
| Nantong Cellulose Fibers Co. Ltd. | China | 31% | Consumer Specialties | China National Tobacco Corporation | 1986 | |||||||||
| Zhuhai Cellulose Fibers Co. Ltd. | China | 30% | Consumer Specialties | China National Tobacco Corporation | 1993 | |||||||||
Major Equity Method Investments
Korea Engineering Plastics Co. Ltd. Founded in 1987, KEPCO is the leading producer of polyacetal in South Korea. Mitsubishi Gas Chemical Company, Inc. owns 40% and Mitsubishi Corporation owns 10% of KEPCO. KEPCO operates a POM plant in Ulsan, South Korea and participates with Polyplastics and Mitsubishi Gas Chemical Company, Inc. in a world-scale POM facility in Nantong, China.
Polyplastics Co., Ltd. Polyplastics is a leading supplier of engineered plastics in the Asia-Pacific region. Polyplastics’ principal production facilities are located in Japan, Taiwan, Malaysia and China. We believe Polyplastics is the largest producer and marketer of POM in the Asia-Pacific region.
Fortron Industries LLC. Fortron Industries LLC is a leading global producer of PPS. Production facilities are located in Wilmington, North Carolina. We believe Fortron has the leading technology in linear polymer applications.
Major Cost Method Investments
National Methanol Co. (“Ibn Sina”). With production facilities in Saudi Arabia, National Methanol Co. represents approximately 2% of the world’s methanol production capacity and is the world’s eighth largest producer of MTBE. Methanol and MTBE are key global commodity chemical products. We indirectly own a 25% interest in National Methanol Co. through CTE Petrochemicals, a joint venture with Texas Eastern Arabian Corporation Ltd., with the remainder held by SABIC (50%). SABIC has responsibility for all product marketing.
China Acetate Products ventures. We hold approximately 30% ownership interests (50% board representation) in three separate Acetate Products production entities in China: the Nantong, Kunming and Zhuhai Cellulose Fiber Companies. In each instance, the Chinese state-owned tobacco entity, China National Tobacco Corporation controls the remainder. The China ventures fund operations using operating cash flows.
These cost investments where we own greater than a 20% ownership interest are accounted for under the cost method of accounting because we cannot exercise significant influence over these entities. We determined that we
14
Table of Contents
cannot exercise significant influence over these entities due to local government investment in and influence over these entities, limitations on our involvement in the day-to-day operations and the present inability of the entities to provide timely financial information prepared in accordance with US generally accepted accounting principles.
Other Equity Investments
InfraServs. We hold ownership interests in several InfraServ entities located in Germany. InfraServs own and develop industrial parks and provideon-site general and administrative support to tenants. The table below represents our equity investments in InfraServ ventures as of December 31, 2008:
Company | Ownership % | |||
| InfraServ GmbH & Co. Gendorf KG | 39.0 | % | ||
| InfraServ GmbH & Co. Knapsack KG | 28.2 | % | ||
| InfraServ GmbH & Co. Hoechst KG | 31.2 | % | ||
Raw Materials and Energy
We purchase a variety of raw materials and energy from sources in many countries for use in our production processes. We have a policy of maintaining, when available, multiple sources of supply for materials. However, some of our individual plants may have single sources of supply for some of their raw materials, such as carbon monoxide, steam and acetaldehyde. Although we have been able to obtain sufficient supplies of raw materials, there can be no assurance that unforeseen developments will not affect our raw material supply. Even if we have multiple sources of supply for a raw material, there can be no assurance that these sources can make up for the loss of a major supplier. There cannot be any guarantee that profitability will not be affected should we be required to qualify additional sources of supply to our specifications in the event of the loss of a sole supplier. In addition, the price of raw materials varies, often substantially, from year to year.
A substantial portion of our products and raw materials are commodities whose prices fluctuate as market supply/demand fundamentals change. Our production facilities rely largely on fuel oil, natural gas and electricity for energy. Most of the raw materials for our European operations are centrally purchased by one of our subsidiaries, which also buys raw materials on behalf of third parties. We manage our exposure through forward purchase contracts, long-term supply agreements and multi-year purchasing and sales agreements. During 2008, we did not enter into any commodity financial derivative contracts. See Note 2 and Note 22 to the consolidated financial statements for additional information.
We also currently purchase and lease supplies of various precious metals, such as rhodium, used as catalysts for the manufacture of Acetyl Intermediates products. For precious metals, the leases are distributed between a minimum of three lessors per product and are divided into several contracts.
Research and Development
All of our businesses conduct research and development activities to increase competitiveness. Our businesses are innovation-oriented and conduct research and development activities to develop new, and optimize existing, production technologies, as well as to develop commercially viable new products and applications. We consider the amount spent during each of the last three fiscal years on research and development activities to be adequate to drive our strategic initiatives.
Intellectual Property
We attach great importance to patents, trademarks, copyrights and product designs in order to protect our investment in research and development, manufacturing and marketing. Our policy is to seek the widest possible protection for significant product and process developments in our major markets. Patents may cover products, processes, intermediate products and product uses. We also seek to register trademarks extensively as a means of protecting the brand names of our products, which brand names become more important once the corresponding patents have expired. We protect our trademarks vigorously against infringement and also seek to register design protection where appropriate.
In most industrial countries, patent protection exists for new substances and formulations, as well as for unique applications and production processes. However, we do business in regions of the world where intellectual property
15
Table of Contents
protection may be limited and difficult to enforce. We maintain strict information security policies and procedures wherever we do business. Such information security policies and procedures include data encryption, controls over the disclosure and safekeeping of confidential information, as well as employee awareness training. Moreover, we monitor our competitors and vigorously challenge patent and trademark infringement. For example, Acetyl Intermediates maintains a strict patent enforcement strategy, which has resulted in favorable outcomes in a number of patent infringement matters in Europe, Asia and the United States. We are currently pursuing a number of matters relating to the infringement of our acetic acid patents. Some of our earlier acetic acid patents expired in 2007; other patent applications covering acetic acid are presently pending.
Neither our business as a whole nor any particular segment is materially dependent upon any one particular patent, trademark, copyright or trade secret.
Trademarks
AOPlustm, VAntagetm, VAntage Plustm, BuyTiconaDirecttm, Celanex®, Celcon®, Celstran®, Celvol®, Celvolit®, Compel®, Erkol®, GUR®, Hostaform®, Impet®, Impet-HI®, Mowilith®, Nutrinova®, Riteflex®, Sunett®, Vandar®, Vectra®, Vinamul®, EcoVAEtm, Duroset®,, Acetex® and certain other products and services named in this document are registered trademarks and service marks of Celanese. Fortron® is a registered trademark of Fortron Industries LLC, a venture of Celanese.
Environmental and Other Regulation
Matters pertaining to environmental and other regulations are discussed in Item 1A. Risk Factors, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, and Note 16 and Note 23 to the consolidated financial statements.
Employees
As of December 31, 2008, we had approximately 8,350 employees worldwide from continuing operations, compared to 8,400 as of December 31, 2007. This represents a decrease of less than 1%. The following table sets forth the approximate number of employees on a continuing basis as of December 31, 2008, 2007 and 2006.
| Employees as of December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
North America | 4,100 | 4,350 | 4,700 | |||||||||
| thereof US | 3,050 | 3,200 | 3,300 | |||||||||
| thereof Canada | 250 | 250 | 500 | |||||||||
| thereof Mexico | 800 | 900 | 900 | |||||||||
Europe | 3,500 | 3,500 | 3,900 | |||||||||
| thereof Germany | 1,700 | 1,700 | 2,600 | |||||||||
Asia | 700 | 500 | 250 | |||||||||
Rest of World | 50 | 50 | 50 | |||||||||
Total Employees | 8,350 | 8,400 | 8,900 | |||||||||
Many of our employees are unionized, particularly in Germany, Canada, Mexico, Brazil, Belgium and France. However, in the United States, less than one quarter of our employees are unionized. Moreover, in Germany and France, wages and general working conditions are often the subject of centrally negotiated collective bargaining agreements. Within the limits established by these agreements, our various subsidiaries negotiate directly with the unions and other labor organizations, such as workers’ councils, representing the employees. Collective bargaining agreements between the German chemical employers associations and unions relating to remuneration generally have a term of one year, while in the United States a three year term for collective bargaining agreements is typical. We offer comprehensive benefit plans for employees and their families and believe our relations with employees are satisfactory.
16
Table of Contents
Backlog
We do not consider backlog to be a significant indicator of the level of future sales activity. In general, we do not manufacture our products against a backlog of orders. Production and inventory levels are based on the level of incoming orders as well as projections of future demand. Therefore, we believe that backlog information is not material to understanding our overall business and should not be considered a reliable indicator of our ability to achieve any particular level of revenue or financial performance.
Available Information — Securities and Exchange Commission (“SEC”) Filings and Corporate Governance Materials
We make available free of charge, through our internet website (www.celanese.com), our annual reports onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as soon as reasonably practicable after electronically filing such material with, or furnishing it to, the SEC. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers, including Celanese Corporation, that electronically file with the SEC athttp://www.sec.gov.
We also make available free of charge, through our internet website, our Corporate Governance Guidelines of our Board of Directors and the charters of each of the committees of the Board. Such materials are also available in print upon the written request of any shareholder to Celanese Corporation, 1601 West LBJ Freeway, Dallas, Texas,75234-6034, Attention: Investor Relations.
| Item 1A. | Risk Factors |
Many factors could have an effect on our financial condition, cash flows and results of operations. We are subject to various risks resulting from changing economic, environmental, political, industry, business and financial conditions. The factors described below represent our principal risks.
Risks Related to Our Business
The worldwide economic downturn and difficult conditions in the global capital and credit markets have affected and may continue to adversely affect our business, as well as the industries of many of our customers and suppliers, which are cyclical in nature. We do not expect these conditions to improve in the near future.
Some of the markets in which our end-use customers participate, such as the automotive, electrical, construction and textile industries, are cyclical in nature, thus posing a risk to us which is beyond our control. These markets are highly competitive, to a large extent driven by end-use markets, and may experience overcapacity, all of which may affect demand for and pricing of our products.
Recent declines in consumer and business confidence and spending, together with severe reductions in the availability and cost of credit and volatility in the capital and credit markets, have adversely affected the business and economic environment in which we operate and the profitability of our business. Our business is exposed to risks associated with the creditworthiness of our key suppliers, customers and business partners. In particular, we are exposed to risks associated with the ongoing decline of the automotive, textile and housing markets. These conditions have resulted in financial instability or other adverse effects at many of our suppliers, customers or business partners. The consequences of such adverse effects could include the interruption of production at the facilities of our customers, the reduction, delay or cancellation of customer orders, delays in or the inability of customers to obtain financing to purchase our products, delays or interruptions of the supply of raw materials we purchase and bankruptcy of customers, suppliers or other creditors. Any of these events may adversely affect our cash flow, profitability and financial condition.
Moreover, the current worldwide financial crisis has reduced the availability of liquidity and credit to fund or support the continuation and expansion of business operations worldwide. Many lenders and institutional investors have reduced and, in some cases, ceased to provide funding to borrowers. Continued disruption of the credit markets has affected and could continue to adversely affect our customer’s access to credit which supports the continuation
17
Table of Contents
and expansion of their businesses worldwide and could result in contract cancellations or suspensions, payment delays or defaults by our customers.
We are a company with operations around the world and are exposed to general economic, political and regulatory conditions and risks in the countries in which we have significant operations.
We operate in the global market and have customers in many countries. We have major facilities primarily located in North America, Europe and Asia, and hold interests in ventures that operate in Germany, China, Japan, South Korea and Saudi Arabia. Our principal customers are similarly global in scope, and the prices of our most significant products are typically world market prices. Consequently, our business and financial results are affected, directly and indirectly, by world economic, political and regulatory conditions.
In addition to the worldwide economic downturn, conditions such as the uncertainties associated with war, terrorist activities, epidemics, pandemics or political instability in any of the countries in which we operate could affect us by causing delays or losses in the supply or delivery of raw materials and products, as well as increasing security costs, insurance premiums and other expenses. These conditions could also result in or lengthen economic recession in the United States, Europe, Asia or elsewhere.
Moreover, changes in laws or regulations, such as unexpected changes in regulatory requirements (including import or export licensing requirements), or changes in the reporting requirements of the United States, German or European Union (“EU”) governmental agencies, could increase the cost of doing business in these regions. Any of these conditions may have an effect on our business and financial results as a whole and may result in volatile current and future prices for our securities, including our stock.
In particular, we have invested significant resources in China and other Asian countries. This region’s growth has slowed and we may fail to realize the anticipated benefits associated with our investment there and our financial results may be adversely impacted.
We are subject to risks associated with the increased volatility in the prices and availability of key raw materials and energy.
We purchase significant amounts of natural gas, ethylene and methanol from third parties for use in our production of basic chemicals in the Acetyl Intermediates segment, principally formaldehyde, acetic acid and VAM. We use a portion of our output of these chemicals, in turn, as inputs in the production of further products in all our segments. We also purchase significant amounts of wood pulp for use in our production of cellulose acetate in the Consumer Specialties segment. The price of many of these items is dependent on the available supply of such item and may increase significantly as a result of production disruptions or strikes. For example, the unplanned shutdown of our Clear Lake, Texas facility together with other tight supply conditions caused a shortage of acetic acid and increased the price for such product during 2007.
We are exposed to any volatility in the prices of our raw materials and energy. Although we have agreements providing for the supply of natural gas, ethylene, wood pulp, electricity and fuel oil, the contractual prices for these raw materials and energy vary with market conditions and may be highly volatile. Factors which have caused volatility in our raw material prices in the past and which may do so in the future include:
| • | Shortages of raw materials due to increasing demand, e.g., from growing uses or new uses; | |
| • | Capacity constraints, e.g., due to construction delays, strike action or involuntary shutdowns; | |
| • | The general level of business and economic activity; and | |
| • | The direct or indirect effect of governmental regulation. |
If we are not able to fully offset the effects of higher energy and raw material costs, or if such commodities were unavailable, it could have a significant adverse effect on our financial results.
18
Table of Contents
Failure to develop new products and production technologies or to implement productivity and cost reduction initiatives successfully may harm our competitive position.
Our operating results, especially in our Consumer Specialties and Advanced Engineered Materials segments, depend significantly on the development of commercially viable new products, product grades and applications, as well as production technologies. If we are unsuccessful in developing new products, applications and production processes in the future, our competitive position and operating results may be negatively affected. Likewise, we have undertaken and are continuing to undertake initiatives in all segments to improve productivity and performance and to generate cost savings. These initiatives may not be completed or beneficial or the estimated cost savings from such activities may not be realized.
Recent federal regulations aimed at increasing security at certain chemical production plants and similar legislation that may be proposed in the future could, if passed into law, require us to relocate certain manufacturing activities and require us to alter or discontinue our production of certain chemical products, thereby increasing our operating costs and causing an adverse effect on our results of operations.
Regulations have recently been issued by the Department of Homeland Security (“DHS”) aimed at decreasing the risk, and effects, of potential terrorist attacks on chemical plants located within the United States. Pursuant to these regulations, these goals would be accomplished in part through the requirement that certain high-priority facilities develop a prevention, preparedness, and response plan after conducting a vulnerability assessment. In addition, companies may be required to evaluate the possibility of using less dangerous chemicals and technologies as part of their vulnerability assessments and prevention plans and implementing feasible safer technologies in order to minimize potential damage to their facilities from a terrorist attack. We have registered certain of our sites with DHS in accordance with these regulations, and are conducting vulnerability assessments for our sites and until that is done we cannot state with certainty the costs associated with any security plans that DHS may require. These regulations may be revised further, and additional legislation may be proposed in the future on this topic. It is possible that such future legislation could contain terms that are more restrictive than what has recently been passed and which would be more costly to us. We cannot predict the final form of currently pending legislation, or other related legislation that may be passed and can provide no assurance that such legislation will not have an adverse effect on our results of operations in a future reporting period.
Environmental regulations and other obligations relating to environmental matters could subject us to liability for fines,clean-ups and other damages, require us to incur significant costs to modify our operations and increase our manufacturing and delivery costs.
Costs related to our compliance with environmental laws and regulations, and potential obligations with respect to contaminated sites may have a significant negative impact on our operating results. These include obligations related to sites currently or formerly owned or operated by us, or where waste from our operations was disposed. We also have obligations related to the indemnity agreement contained in the demerger and transfer agreement between Celanese GmbH and Hoechst, also referred to as the demerger agreement, for environmental matters arising out of certain divestitures that took place prior to the demerger.
Our operations are subject to extensive international, national, state, local and other supranational laws and regulations that govern environmental and health and safety matters, including the Comprehensive Environmental Response, Compensation and Liability Act of 1980 (“CERCLA”) and the Resource Conservation and Recovery Act of 1976 (“RCRA”). We incur substantial capital and other costs to comply with these requirements. If we violate them, we can be held liable for substantial fines and other sanctions, including limitations on our operations as a result of changes to or revocations of environmental permits involved. Stricter environmental, safety and health laws, regulations and enforcement policies could result in substantial costs and liabilities to us or limitations on our operations and could subject our handling, manufacture, use, reuse or disposal of substances or pollutants to more rigorous scrutiny than at present. Consequently, compliance with these laws and regulations could result in significant capital expenditures as well as other costs and liabilities, which could cause our business and operating results to be less favorable than expected.
19
Table of Contents
We are also involved in several claims, lawsuits and administrative proceedings relating to environmental matters. An adverse outcome in any of them may negatively affect our earnings and cash flows in a particular reporting period.
Changes in environmental, health and safety regulations in the jurisdictions where we manufacture and sell our products could lead to a decrease in demand for our products.
New or revised governmental regulations and independent studies relating to the effect of our products on health, safety and the environment may affect demand for our products and the cost of producing our products.
Canada recently included vinyl acetate monomer (“VAM”) as one of approximately 200 chemicals being assessed as part of the Canadian Government’s Chemicals Management Plan under the Canadian Environmental Protection Act (“CEPA”). On May 16, 2008, Health Canada published a draft screening risk assessment and draft risk management scope document for VAM that concluded, using a ’precautionary approach’, that VAM be listed as a “toxic substance” under CEPA. On January 27, 2009, after consideration of data submitted by Celanese and other manufacturers and users of VAM and further consideration of a risk assessment prepared by the European Union, Health Canada published its final decision overruling its May 16, 2008 draft assessment of VAM. Health Canada concluded that VAM does not meet the regulatory requirements for being listed as a toxic substance under CEPA.
The Registration, Evaluation, Authorization and Restriction of Chemicals (“REACh”), which established a system to register and evaluate chemicals manufactured in, or imported to, the European Union, became effective on June 1, 2007. VAM is one of the chemicals that the European Chemicals Agency (“ECHA”) will regulate under REACh. ECHA will likely rely on the work of the EU-Working group on classification and labeling of dangerous substances. After extensive study, the EU-Working Group agreed that VAM should be classified in the EU as showing limited evidence of a carcinogenic effect. In addition, a risk assessment was performed on VAM by the European Chemicals Bureau of the European Commission. Risk reduction strategies for human health and the environment were finalized without the imposition of any restrictions or burdens atypical to an industrial chemical. The EU-Working Group conclusion has been reviewed and substantively approved by EU Scientific Committee on Health and Environmental Risks.
We can provide no assurance that the EU classifications on VAM will not be revised in the future, or that other chemicals we produce will not be classified in a manner that would adversely affect demand for such products. Such negative classifications could have an adverse affect on our business and results of operations.
We are a producer of formaldehyde and plastics derived from formaldehyde. Several studies have investigated possible links between formaldehyde exposure and various end points including leukemia. The International Agency for Research on Cancer (“IARC”), a private research agency, has reclassified formaldehyde from Group 2A (probable human carcinogen) to Group 1 (known human carcinogen) based on studies linking formaldehyde exposure to nasopharyngeal cancer, a rare cancer in humans. We expect the results of IARC’s review will be examined and considered by government agencies with responsibility for setting worker and environmental exposure standards and labeling requirements. If such agencies give strong consideration to IARC’s findings in setting such standards and requirements, it could have an adverse effect on our business.
Other pending initiatives will potentially require toxicological testing and risk assessments of a wide variety of chemicals, including chemicals used or produced by us. These initiatives include the Voluntary Children’s Chemical Evaluation Program, High Production Volume Chemical Initiative and Chemical Assessment and Management Program (“ChAMP”) in the United States, as well as various European Commission programs, such as REACh.
The above-mentioned assessments in the United States, Canada and Europe may result in heightened concerns about the chemicals involved and additional requirements being placed on the production, handling, labeling or use of the subject chemicals. Such concerns and additional requirements could increase the cost incurred by our customers to use our chemical products and otherwise limit the use of these products, which could lead to a decrease in demand for these products. Such a decrease in demand would likely have an adverse impact on our business and results of operations.
20
Table of Contents
Our production facilities handle the processing of some volatile and hazardous materials that subject us to operating risks that could have a negative effect on our operating results.
Our operations are subject to operating risks associated with chemical manufacturing, including the related storage and transportation of raw materials, products and waste. These risks include, among other things, pipeline and storage tank leaks and ruptures, explosions and fires and discharges or releases of toxic or hazardous substances.
These operating risks can cause personal injury, property damage and environmental contamination, and may result in the shutdown of affected facilities and the imposition of civil or criminal penalties. The occurrence of any of these events may disrupt production and have a negative effect on the productivity and profitability of a particular manufacturing facility and our operating results and cash flows.
Production at our manufacturing facilities could be disrupted for a variety of reasons, which could prevent us from producing enough of our products to maintain our sales and satisfy our customers’ demands.
A disruption in production at our manufacturing facilities could have a material adverse effect on our business. Disruptions could occur for many reasons, including fire, natural disasters, weather, unplanned maintenance or other manufacturing problems, disease, strikes, transportation interruption, government regulation or terrorism. Alternative facilities with sufficient capacity or capabilities may not be available, may cost substantially more or may take a significant time to start production, each of which could negatively affect our business and financial performance. If one of our key manufacturing facilities is unable to produce our products for an extended period of time, our sales may be reduced by the shortfall caused by the disruption and we may not be able to meet our customers’ needs, which could cause them to seek other suppliers. For example, during 2007, production was disrupted for an extended period of time at our Clear Lake, Texas facility that produces primarily acetic acid and VAM. The disruption was caused by an unplanned outage of our acetic acid unit. Because of this disruption, the volumes of our Acetyl Intermediates segment were lower than we had expected for 2007 as we were unable to fully offset the lost production. Similar outages could occur in the future from unexpected disruptions at any of our other manufacturing facilities of key products. Such outages could have an adverse effect on our results of operations in future reporting periods.
We may experience unexpected difficulties in the relocation of our Ticona plant from Kelsterbach to the Rhine Main area, which may increase our costs, delay the transition or disrupt our ability to supply our customers.
We have agreed with Frankfurt, Germany Airport (“Fraport”) to relocate our Kelsterbach, Germany business, resolving several years of legal disputes related to the planned Frankfurt airport expansion. As a result of the settlement, we will transition Ticona’s operations from Kelsterbach to another location in Germany by mid-2011. In July 2007, we announced that we would relocate the Kelsterbach, Germany business to the Hoechst Industrial Park in the Rhine Main area. Over a five-year period, Fraport agreed to pay Ticona a total of €670 million to offset the costs associated with the transition of the business from its current location and the closure of the Kelsterbach plant. While the settlement and related payment amount are meant to be cost-neutral and represent the actual amount we will require to select a site, build new production facilities, demolish old production facilities and transition business activities according to schedule and without any disruptions to customer supply, we may encounter unexpected costs or other difficulties during the relocation process that bring the total costs of the relocation to an amount greater than the compensation provided by Fraport. The relocation of these facilities represents a major logistical undertaking, and we may have underestimated the amount that will be required to carry out every aspect of the relocation. We may lose the services of valuable experienced employees during the transition if they decide not to work at the new location. The construction of the new facilities may not be complete on time or may face cost overruns. If our costs relating to the relocation exceed the amount of payments from Fraport or if the relocation causes other unexpected difficulties, our expenses may increase or supplies to our customers may be disrupted.
If supply to our customers is disrupted for an extended period, this could negatively impact the reputation of this business and result in the loss of customers. Such effects could have an adverse impact on our results of operations in future periods.
21
Table of Contents
Our significant non-US operations expose us to global exchange rate fluctuations that could adversely impact our profitability.
As we conduct a significant portion of our operations outside the United States, fluctuations in currencies of other countries, especially the Euro, may materially affect our operating results. For example, changes in currency exchange rates may decrease our profits in comparison to the profits of our competitors on the same products sold in the same markets and increase the cost of items required in our operations.
A substantial portion of our net sales is denominated in currencies other than the US dollar. In our consolidated financial statements, we translate our local currency financial results into US dollars based on average exchange rates prevailing during a reporting period or the exchange rate at the end of that period. During times of a strengthening US dollar our reported international sales, earnings, assets and liabilities will be reduced because the local currency will translate into fewer US dollars.
In addition to currency translation risks, we incur a currency transaction risk whenever one of our operating subsidiaries enters into either a purchase or a sales transaction using a currency different from the operating subsidiary’s functional currency. Given the volatility of exchange rates, we may not be able to manage our currency transaction and translation risks effectively, and volatility in currency exchange rates may expose our financial condition or results of operations to a significant additional risk. Since a portion of our indebtedness is and will be denominated in currencies other than US dollars, a weakening of the US dollar could make it more difficult for us to repay our indebtedness.
We use financial instruments to hedge our exposure to foreign currency fluctuations, but we cannot guarantee that our hedging strategies will be effective. Failure to effectively manage these risks could have an adverse impact on our financial position, results of operations and cash flows.
Significant changes in pension fund investment performance or assumptions relating to pension costs may have a material effect on the valuation of pension obligations, the funded status of pension plans and our pension cost.
The cost of our pension plans is incurred over long periods of time and involve many uncertainties during those periods of time. Our funding policy for pension plans is to accumulate plan assets that, over the long run, will approximate the present value of projected benefit obligations. Our pension cost is materially affected by the discount rate used to measure pension obligations, the level of plan assets available to fund those obligations at the measurement date and the expected long-term rate of return on plan assets. Significant changes in investment performance or a change in the portfolio mix of invested assets can result in corresponding increases and decreases in the valuation of plan assets, particularly equity securities, or in a change of the expected rate of return on plan assets. During the previous fiscal year, the value of our plan assets declined significantly due to the decline in the overall equity markets. A change in the discount rate would result in a significant increase or decrease in the valuation of pension obligations, affecting the reported funded status of our pension plans as well as the net periodic pension cost in the following fiscal years. In recent years, an extended duration strategy in the asset portfolio has been implemented to minimize the influence of liability volatility due to interest rate movements. Similarly, changes in the expected return on plan assets can result in significant changes in the net periodic pension cost for subsequent fiscal years. If the value of our pension fund’s portfolio declines or does not perform as expected or if our experience with the fund leads us to change our assumptions regarding the fund, we may be required to contribute additional capital to the fund.
Our future success will depend in part on our ability to protect our intellectual property rights. Our inability to enforce these rights could reduce our ability to maintain our market position and our profit margins.
We attach great importance to patents, trademarks, copyrights and product designs in order to protect our investment in research and development, manufacturing and marketing. Our policy is to seek the widest possible protection for significant product and process developments in our major markets. Patents may cover products, processes, intermediate products and product uses. Protection for individual products extends for varying periods in accordance with the date of patent application filing and the legal life of patents in the various countries. The protection afforded, which may also vary from country to country, depends upon the type of patent and its scope of
22
Table of Contents
coverage. As patents expire, the products and processes described and claimed in those patents become generally available for use by the public. We also seek to register trademarks extensively as a means of protecting the brand names of our products, which brand names become more important once the corresponding patents have expired. Our continued growth strategy may bring us to regions of the world where intellectual property protection may be limited and difficult to enforce. If we are not successful in protecting our trademark or patent rights, our revenues, results of operations and cash flows may be adversely affected.
Provisions in our second amended and restated certificate of incorporation and second amended and restated bylaws, as well as any shareholders’ rights plan, may discourage a takeover attempt.
Provisions contained in our second amended and restated certificate of incorporation and bylaws could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our shareholders. Provisions of our second amended and restated certificate of incorporation and bylaws impose various procedural and other requirements, which could make it more difficult for shareholders to effect certain corporate actions. For example, our second amended and restated certificate of incorporation authorizes our Board of Directors to determine the rights, preferences, privileges and restrictions of unissued series of preferred stock, without any vote or action by our shareholders. Thus, our Board of Directors can authorize and issue shares of preferred stock with voting or conversion rights that could adversely affect the voting or other rights of holders of our Series A common stock. These rights may have the effect of delaying or deterring a change of control of our company. In addition, a change of control of our company may be delayed or deterred as a result of our having three classes of directors (each class elected for a three year term) or as a result of any shareholders’ rights plan that our Board of Directors may adopt. These provisions could limit the price that certain investors might be willing to pay in the future for shares of our Series A common stock.
Risks Related to the Acquisition of Celanese GmbH, formerly Celanese AG
The amounts of the fair cash compensation and of the guaranteed annual payment offered under the domination and profit and loss transfer agreement (“Domination Agreement”) may be increased, which may further reduce the funds the Purchaser can otherwise make available to us.
Several minority shareholders of Celanese GmbH have initiated special award proceedings seeking the court’s review of the amounts of the fair cash compensation and of the guaranteed annual payment offered under the Domination Agreement. On March 14, 2005, the Frankfurt District Court dismissed on grounds of inadmissibility the motions of all minority shareholders regarding the initiation of these special award proceedings. In January 2006, the Frankfurt Higher District Court ruled that the appeals were admissible, and the proceedings will therefore continue. On December 12, 2006, the Frankfurt District Court appointed an expert to help determine the value of Celanese GmbH. As a result of these proceedings, the amounts of the fair cash compensation and of the guaranteed annual payment could be increased by the court, and the Purchaser would be required to make such payments within two months after the publication of the court’s ruling. Any such increase may be substantial. All minority shareholders would be entitled to claim the respective higher amounts. This may reduce the funds the Purchaser can make available to us and, accordingly, diminish our ability to make payments on our indebtedness. See Note 23 to the consolidated financial statements for further information.
The Purchaser may be required to compensate Celanese GmbH for annual losses, which may reduce the funds the Purchaser can otherwise make available to us.
Under the Domination Agreement, the Purchaser is required, among other things, to compensate Celanese GmbH for any annual loss incurred, determined in accordance with German accounting requirements, by Celanese GmbH at the end of the fiscal year in which the loss was incurred. This obligation to compensate Celanese GmbH for annual losses will apply during the entire term of the Domination Agreement. If Celanese GmbH incurs losses during any period of the operative term of the Domination Agreement and if such losses lead to an annual loss of Celanese GmbH at the end of any given fiscal year during the term of the Domination Agreement, the Purchaser will be obligated to make a corresponding cash payment to Celanese GmbH to the extent that the respective annual loss is not fully compensated for by the dissolution of profit reserves accrued at the level of Celanese GmbH during the term of the Domination Agreement. The Purchaser may be able to reduce or avoid cash payments to Celanese GmbH by off-setting against such loss compensation claims by Celanese GmbH any valuable counterclaims against
23
Table of Contents
Celanese GmbH that the Purchaser may have. If the Purchaser is obligated to make cash payments to Celanese GmbH to cover an annual loss, we may not have sufficient funds to make payments on our indebtedness when due and, unless the Purchaser is able to obtain funds from a source other than annual profits of Celanese GmbH, the Purchaser may not be able to satisfy its obligation to fund such shortfall. See Note 23 to the consolidated financial statements.
We and two of our subsidiaries have taken on certain obligations with respect to the Purchaser’s obligation under the Domination Agreement and intercompany indebtedness to Celanese GmbH, which may diminish our ability to make payments on our indebtedness.
Our subsidiaries, Celanese International Holdings Luxembourg S.à r.l. (“CIH”), formerly Celanese Caylux Holdings Luxembourg S.C.A., and Celanese US, have each agreed to provide the Purchaser with financing so that the Purchaser is at all times in a position to completely meet its obligations under, or in connection with, the Domination Agreement. In addition, Celanese has guaranteed (i) that the Purchaser will meet its obligation under the Domination Agreement to compensate Celanese GmbH for any annual loss incurred by Celanese GmbH during the term of the Domination Agreement; and (ii) the repayment of all existing intercompany indebtedness of Celanese’s subsidiaries to Celanese GmbH. Further, under the terms of Celanese’s guarantee, in certain limited circumstances Celanese GmbH may be entitled to require the immediate repayment of some or all of the intercompany indebtedness owed by Celanese’s subsidiaries to Celanese GmbH. If CIHand/or Celanese US are obligated to make payments under their obligations to the Purchaser or Celanese GmbH, as the case may be, or if the intercompany indebtedness owed to Celanese GmbH is accelerated, we may not have sufficient funds for payments on our indebtedness when due.
Risks Related to Our Indebtedness
See also “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Liquidity — Contractual Obligations.”
Our level of indebtedness could diminish our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or the chemicals industry and prevent us from meeting obligations under our indebtedness.
Our total indebtedness is approximately $3.5 billion as of December 31, 2008.
Our debt could have important consequences, including:
| • | increasing vulnerability to general economic and industry conditions including exacerbating any adverse business effects that are determined to be material adverse effects under our senior credit facility; | |
| • | requiring a substantial portion of cash flow from operations to be dedicated to the payment of principal and interest on indebtedness, therefore reducing our ability to use our cash flow to fund operations, capital expenditures and future business opportunities; | |
| • | exposing us to the risk of increased interest rates as certain of our borrowings are at variable rates of interest; | |
| • | limiting our ability to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions and general corporate or other purposes; and | |
| • | limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors who have less debt. |
A breach of a covenant or other provision in any debt instrument governing our current or future indebtedness could result in a default under that instrument and, due to cross-default provisions, could result in a default under our senior credit facility. Upon the occurrence of an event of default under the senior credit facility, the lenders could elect to declare all amounts outstanding to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders could proceed against the collateral, if any, granted to them to secure the indebtedness. If the lenders under the senior credit facility were to accelerate the payment of the indebtedness, there is no guarantee that our assets or cash flow would be sufficient to repay in full our outstanding indebtedness.
24
Table of Contents
Moreover, the terms of our existing debt do not fully prohibit us or our subsidiaries from incurring substantial additional indebtedness in the future. If new debt, including amounts available under our senior credit agreement, is added to our current debt levels, the related risks that we now face could intensify. See also “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Liquidity — Contractual Obligations.”
Our variable rate indebtedness subjects us to interest rate risk, which could cause our debt service obligations to increase significantly and affect our operating results.
Certain of our borrowings are at variable rates of interest and expose us to interest rate risk. If interest rates were to increase, our debt service obligations on our variable rate indebtedness would increase, net of the impacts of hedges in place. In April 2007, we, through certain of our subsidiaries, entered into a senior credit agreement. The new senior credit agreement consists of $2,280 million of US dollar denominated and €400 million of Euro denominated term loans due 2014, a $650 million revolving credit facility terminating in 2013 and a $228 million credit-linked revolving facility terminating in 2014. Borrowings under the new senior credit agreement bear interest at a variable interest rate based on LIBOR (for US dollars) or EURIBOR (for Euros), as applicable, or, for US dollar denominated loans under certain circumstances, a base rate, in each case plus an applicable margin. The applicable margin for the term loans and any loans under the credit-linked revolving facility is 1.75%, subject to potential reductions as defined in the new senior credit agreement. The term loans under the new senior credit agreement are subject to amortization at 1% of the initial principal amount per annum, payable quarterly, commencing in July 2007. The remaining principal amount of the term loans will be due on April 2, 2014.
An increase in interest rates could have an adverse impact on our future results of operations and cash flows. See also “Quantitative and Qualitative Disclosures About Market Risk — Interest Rate Risk Management.”
We may not be able to generate sufficient cash to service our indebtedness, and may be forced to take other actions to satisfy obligations under our indebtedness, which may not be successful.
Our ability to satisfy our cash needs depends on cash on hand, receipt of additional capital, including possible additional borrowings, and receipt of cash from our subsidiaries by way of distributions, advances or cash payments.
Our ability to make scheduled payments on or to refinance our debt obligations depends on the financial condition and operating performance of our subsidiaries, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We may not be able to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital or restructure or refinance our indebtedness. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. In the absence of such operating results and resources, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. The senior credit agreement governing our indebtedness restricts our ability to dispose of assets and use the proceeds from the disposition. We may not be able to consummate those dispositions or to obtain the proceeds which we could realize from them and these proceeds may not be adequate to meet any debt service obligations then due.
Restrictive covenants in our debt instruments may limit our ability to engage in certain transactions and may diminish our ability to make payments on our indebtedness.
The senior credit agreement governing our indebtedness contains various covenants that limit our ability to engage in specified types of transactions. The covenants contained in the senior credit agreement limit our ability to, among other things, incur additional indebtedness, pay dividends on or make other distributions on or repurchase capital stock or make other restricted payments, make investments and sell certain assets. Such restrictions in our debt instruments could result in us having to obtain the consent of our lenders in order to take certain actions. Recent disruptions in credit markets may prevent us from or make it more difficult or more costly for us to obtain such
25
Table of Contents
consents from our lenders. Our ability to expand our business or to address declines in our business may be limited if we are unable to obtain such consents.
In addition, the senior credit agreement requires us to maintain a maximum first lien senior secured leverage ratio no greater than 3.90 to 1.00 if there are outstanding borrowings under the revolving credit facility. Our ability to meet this financial ratio can be affected by events beyond our control, and we may not be able to meet this test at all.
A breach of any of these covenants could result in a default under the senior credit agreement. Upon the occurrence of an event of default under the senior credit agreement, the lenders could elect to declare all amounts outstanding under the senior credit agreement to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders under the senior credit agreement could proceed against the collateral granted to them to secure that indebtedness. Our subsidiaries have pledged a significant portion of our assets as collateral under the senior credit agreement. If the lenders under the senior credit agreement accelerate the repayment of borrowings, we may not have sufficient assets to repay amounts borrowed under the senior credit agreement as well as their other indebtedness, which could have a material adverse effect on the value of our stock.
The terms of our senior credit agreement limit the ability of BCP Crystal and its subsidiaries to pay dividends or otherwise transfer their assets to us.
Our operations are conducted through our subsidiaries and our ability to pay dividends is dependent on the earnings and the distribution of funds from our subsidiaries. However, the terms of our senior credit agreement limit the ability of BCP Crystal and its subsidiaries to pay dividends or otherwise transfer their assets to us. Accordingly, our ability to pay dividends on our stock is similarly limited.
| Item 1B. | Unresolved Staff Comments |
None.
26
Table of Contents
| Item 2. | Properties |
Description of Property
As of December 31, 2008, we had numerous production and manufacturing facilities throughout the world. We also own or lease other properties, including office buildings, warehouses, pipelines, research and development facilities and sales offices. We continuously review and evaluate our facilities as a part of our strategy to optimize our business portfolio. The following table sets forth a list of our principal production and other facilities throughout the world as of December 31, 2008.
Site | Leased/Owned | Products/Functions | ||
Corporate Offices | ||||
| Budapest, Hungary | Leased | Administrative offices | ||
| Dallas, Texas, US | Leased | Corporate headquarters | ||
| Kronberg/Taunus, Germany | Leased | Administrative offices | ||
| Mexico City, Mexico | Leased | Administrative offices | ||
Mexico City, Mexico(1) | Owned | Administrative offices | ||
Advanced Engineered Materials | ||||
| Auburn Hills, Michigan, US | Leased | Automotive Development Center | ||
| Bishop, Texas, US | Owned | POM, GUR®, Compounding | ||
| Florence, Kentucky, US | Owned | Compounding | ||
| Kelsterbach, Germany | Owned | LFRT, POM, Compounding | ||
Oberhausen, Germany(5) | Leased | GUR® | ||
| Fuji City, Japan | Venture owned by Polyplastics Co., Ltd., a cost method investment | POM, PBT, LCP, Compounding | ||
| Kuantan, Malaysia | Venture owned by Polyplastics Co., Ltd., a cost method investment | POM, Compounding | ||
| Shelby, North Carolina, US | Owned | LCP, PBT, PET, Compounding | ||
| Suzano, Brazil | Owned | Compounding | ||
| Ulsan, South Korea | Venture owned by Korea Engineering Plastics Co., Ltd., an equity method investment | POM | ||
| Wilmington, North Carolina, US | Venture owned by Fortron Industries LLC, an equity method investment | PPS | ||
| Winona, Minnesota, US | Owned | LFRT | ||
Nanjing, China(3) | Leased | GUR® | ||
Consumer Specialties | ||||
| Kunming, China | Venture owned by Kunming Cellulose Fibers Co. Ltd., a cost method investment | Acetate tow, Acetate flake | ||
| Lanaken, Belgium | Owned | Acetate tow | ||
| Nantong, China | Venture owned by Nantong Cellulose Fibers Co. Ltd., a cost method investment | Acetate tow, Acetate flake | ||
| Narrows, Virginia, US | Owned | Acetate tow, Acetate flake | ||
| Ocotlán, Jalisco, Mexico | Owned | Acetate tow, Acetate flake | ||
| Spondon, Derby, UK | Owned | Acetate tow, Acetate flake | ||
Frankfurt am Main, Germany(4) | Venture owned by InfraServ GmbH & Co. Hoechst KG, an equity method investment | Sorbates, Sunett® sweetener | ||
| Zhuhai, China | Venture owned by Zhuhai Cellulose Fibers Co. Ltd., a cost method investment | Acetate tow, Acetate flake | ||
Industrial Specialties | ||||
| Boucherville, Quebec, Canada | Owned | Conventional emulsions |
27
Table of Contents
Site | Leased/Owned | Products/Functions | ||
Calvert City, Kentucky, US(5) | Leased | PVOH | ||
| Enoree, South Carolina, US | Owned | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
| Edmonton, Alberta, Canada | Owned | LDPE, EVA | ||
Frankfurt am Main, Germany(4) | Venture owned by InfraServ GmbH & Co. Hoechst KG, an equity method investment | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
| Geleen, Netherlands | Owned | Vinyl acetate ethylene emulsions | ||
Guardo, Spain(6) | Owned | PVOH, Polyvinyl acetate | ||
| Meredosia, Illinois, US | Owned | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
Nanjing, China(3) | Leased | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
Pasadena, Texas, US(5) | Leased | PVOH | ||
Koper, Slovenia(6) | Owned | Conventional emulsions | ||
Tarragona, Spain(2) | Venture owned by Complejo Industrial Taqsa AIE, a cost method investment | Conventional emulsions, Vinyl acetate ethylene emulsions, PVOH | ||
| Perstorp, Sweden | Owned | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
Warrington, UK(6) | Owned | Conventional emulsions, Vinyl acetate ethylene emulsions | ||
Acetyl Intermediates | ||||
| Bay City, Texas, US | Leased | VAM | ||
| Bishop, Texas, US | Owned | Formaldehyde | ||
Cangrejera, Veracruz, Mexico(7) | Owned | Acetic anhydride, Ethyl acetate, VAM | ||
| Clear Lake, Texas, US | Owned | Acetic acid, VAM | ||
Frankfurt am Main, Germany(4) | Venture owned by InfraServ GmbH & Co. Hoechst KG, an equity method investment | Acetaldehyde, VAM, Butyl acetate | ||
Nanjing, China(3) | Leased | Acetic acid, Acetic anhydride | ||
Pampa, Texas, US(6) | Owned | Acetic acid, Acetic anhydride, Ethyl acetate | ||
| Pardies, France | Owned | Acetic acid, VAM | ||
Roussillon, France(5) | Leased | Acetic anhydride | ||
| Jubail, Saudi Arabia | Venture owned by National Methanol Company (Ibn Sina), a cost method investment | Methyl tertiary-butyl ether, Methanol | ||
Jurong Island, Singapore(5) | Leased | Acetic acid, Butyl acetate, Ethyl acetate, VAM | ||
Tarragona, Spain(2) | Venture owned by Complejo Industrial Taqsa AIE, a cost method investment | VAM |
| (1) | Site is no longer operational and is currently held for sale. | |
| (2) | Multiple Celanese business segments conduct operations at the Tarragona facility. Celanese owns its assets at the facility but shares ownership in the land. Celanese’s ownership percentage in the land is 15%. |
28
Table of Contents
| (3) | Multiple Celanese business segments conduct operations at the Nanjing facility. Celanese owns the assets on this site, but utilizes the land through the terms of a long-term land lease. | |
| (4) | Multiple Celanese business segments conduct operations at the Frankfurt facility. | |
| (5) | Celanese owns the assets on this site, but utilizes the land through the terms of a long-term land lease. | |
| (6) | Site is no longer operating as of December 31, 2008. | |
| (7) | The Company has announced its decision to shut down the Cangrejera VAM production unit effective at the end of February 2009. |
We believe that our current facilities are adequate to meet the requirements of our present and foreseeable future operations. We continue to review our capacity requirements as part of our strategy to maximize our global manufacturing efficiency.
See Note 8 to the consolidated financial statements for more information on our cost and equity method investments.
For information on environmental issues associated with our properties, see “Business — Environmental and Other Regulation” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Environmental Matters.” Additional information with respect to our property, plant and equipment, and leases is contained in Note 9 and Note 21 to the consolidated financial statements.
| Item 3. | Legal Proceedings |
We are involved in a number of legal proceedings, lawsuits and claims incidental to the normal conduct of our business, relating to such matters as product liability, antitrust, past waste disposal practices and release of chemicals into the environment. While it is impossible at this time to determine with certainty the ultimate outcome of these proceedings, lawsuits and claims, we believe, based on the advice of legal counsel, that adequate provisions have been made and that the ultimate outcomes will not have a material adverse effect on our financial position, but may have a material adverse effect on our results of operations or cash flows in any given accounting period. See Note 23 to the consolidated financial statements for a discussion of legal proceedings.
| Item 4. | Submission of Matters to a Vote of Security Holders |
No matters were submitted to a vote of security holders during the fourth quarter of 2008.
29
Table of Contents
PART II
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our Series A common stock has traded on the New York Stock Exchange under the symbol “CE” since January 21, 2005. The closing sale price of our Series A common stock, as reported by the New York Stock Exchange, on February 6, 2009 was $11.92. The following table sets forth the high and low intraday sales prices per share of our common stock, as reported by the New York Stock Exchange, for the periods indicated.
| Price Range | ||||||||
| High | Low | |||||||
2008 | ||||||||
| Quarter ended March 31, 2008 | $ | 43.72 | $ | 31.76 | ||||
| Quarter ended June 30, 2008 | $ | 50.99 | $ | 39.50 | ||||
| Quarter ended September 30, 2008 | $ | 47.02 | $ | 24.68 | ||||
| Quarter ended December 31, 2008 | $ | 27.76 | $ | 5.71 | ||||
2007 | ||||||||
| Quarter ended March 31, 2007 | $ | 32.00 | $ | 24.50 | ||||
| Quarter ended June 30, 2007 | $ | 39.43 | $ | 30.59 | ||||
| Quarter ended September 30, 2007 | $ | 42.49 | $ | 30.70 | ||||
| Quarter ended December 31, 2007 | $ | 44.77 | $ | 35.16 | ||||
Holders
No shares of Celanese’s Series B common stock are issued and outstanding. As of February 6, 2009, there were 72 holders of record of our Series A common stock, and one holder of record of our perpetual preferred stock. By including persons holding shares in broker accounts under street names, however, we estimate our shareholder base to be approximately 56,000 as of February 6, 2009.
Dividend Policy
Our Board of Directors adopted a policy of declaring, subject to legally available funds, a quarterly cash dividend on each share of our Series A common stock at an annual rate of $0.16 per share unless our Board of Directors, in its sole discretion, determines otherwise. Pursuant to this policy, we paid quarterly dividends of $0.04 per share on February 1, 2008, May 1, 2008, August 1, 2008 and November 1, 2008 and similar quarterly dividends during each quarter of 2007. The annual cash dividend declared and paid during the years ended December 31, 2008 and 2007 were $24 million and $25 million, respectively. Dividends payable to holders of our Series A common stock cannot be declared or paid nor can any funds be set aside for the payment thereof, unless we have paid or set aside funds for the payment of all accumulated and unpaid dividends with respect to the shares of our preferred stock, as described below. Our Board of Directors may, at any time, modify or revoke our dividend policy on our Series A common stock.
We are required under the terms of the preferred stock to pay scheduled quarterly dividends, subject to legally available funds. For so long as the preferred stock remains outstanding, (1) we will not declare, pay or set apart funds for the payment of any dividend or other distribution with respect to any junior stock or parity stock and (2) neither we, nor any of our subsidiaries, will, subject to certain exceptions, redeem, purchase or otherwise acquire for consideration junior stock or parity stock through a sinking fund or otherwise, in each case unless we have paid or set apart funds for the payment of all accumulated and unpaid dividends with respect to the shares of preferred stock and any parity stock for all preceding dividend periods. Pursuant to this policy, we paid quarterly dividends of $0.265625 per share on our 4.25% convertible perpetual preferred stock on February 1, 2008, May 1, 2008, August 1, 2008 and November 1, 2008 and similar quarterly dividends during each quarter of 2007.
30
Table of Contents
On January 5, 2009, we declared a cash dividend of $0.265625 per share on our 4.25% convertible perpetual preferred stock amounting to $3 million and a cash dividend of $0.04 per share on its Series A common stock amounting to $6 million. Both cash dividends are for the period November 1, 2008 to January 31, 2009 and were paid on February 1, 2009 to holders of record as of January 15, 2009.
Based on the number of outstanding shares as of December 31, 2008, the cash dividends to be paid in 2009 are expected to result in annual dividend payments similar to that paid in 2008.
The amount available to us to pay cash dividends is restricted by our senior credit agreement. Any decision to declare and pay dividends in the future will be made at the discretion of our Board of Directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our Board of Directors may deem relevant.
Celanese Purchases of its Equity Securities
The table below sets forth information regarding repurchases of our Series A common stock during the three months ended December 31, 2008:
| Approximate Dollar | ||||||||||||||||
| Total Number of | Value of Shares | |||||||||||||||
| Total Number | Average | Shares Purchased as | Remaining that may be | |||||||||||||
| of Shares | Price Paid | Part of Publicly | Purchased Under | |||||||||||||
Period | Purchased(1) | per Share | Announced Program | the Program | ||||||||||||
| October 1, 2008 | 44,221 | $ | 26.71 | — | $ | 122,300,000 | ||||||||||
| (1) | Relates to shares employees have elected to have withheld to cover their minimum withholding requirements for personal income taxes related to the vesting of restricted stock units. No shares were purchased during the three months ended December 31, 2008 under our previously announced stock repurchase plan. |
31
Table of Contents
Performance Graph
The following Performance Graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that we specifically incorporate it by reference into such filing.
Comparison of Cumulative Total Return
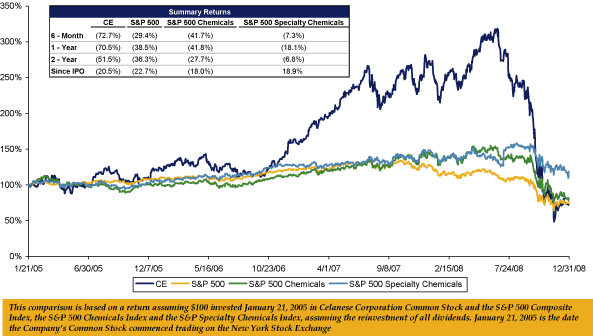
32
Table of Contents
Equity Compensation Plans
Securities Authorized for Issuance Under Equity Compensation Plans
The following information is provided as of December 31, 2008 with respect to equity compensation plans:
| Number of Securities | ||||||||||||
| Remaining Available for | ||||||||||||
| Number of Securities to be | Weighted Average | Future Issuance Under | ||||||||||
| Issued upon Exercise of | Exercise Price of | Equity Compensation | ||||||||||
| Outstanding Options, | Outstanding Options, | Plans (excluding securities | ||||||||||
Plan Category | Warrants and Rights | Warrants and Rights | reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | — | — | — | |||||||||
| Equity compensation plans not approved by security holders: | ||||||||||||
| Stock options | 7,015,759 | $ | 19.35 | 285,517 | ||||||||
| Restricted stock units | 1,603,766 | — | 285,517 | |||||||||
| Total | 8,619,525 | 285,517 | ||||||||||
Recent Sales of Unregistered Securities
In December 2007, we adopted a deferred compensation plan whereby we offered certain of our senior employees and directors the opportunity to defer a portion of their compensation in exchange for a future payment amount equal to their deferments plus or minus certain amounts based upon the market-performance of specified measurement funds selected by the participant. These deferred compensation obligations may be considered securities of Celanese. Participants were required to make deferral elections under the plan prior to January 1 of the year such deferrals will be withheld from their compensation. We relied on the exemption from registration provided by Section 4(2) of the Securities Act in making this offer to a select group of employees, fewer than 35 of which were non-accredited investors under the rules promulgated by the Commission.
| Item 6. | Selected Financial Data |
The balance sheet data shown below as of December 31, 2008 and 2007, and the statements of operations and cash flow data for the years ended December 31, 2008, 2007 and 2006, all of which are set forth below, are derived from the consolidated financial statements included elsewhere in this document and should be read in conjunction with those financial statements and the notes thereto. The balance sheet data as of December 31, 2006, 2005 and 2004 and as of March 31, 2004 and the statements of operations and cash flow data for the year ended December 31, 2005, the nine months ended December 31, 2004 and the three months ended March 31, 2004 shown below were derived from previously issued financial statements, adjusted for applicable discontinued operations.
The balance sheet, statement of operations and cash flow data for periods prior to April 1, 2004 in the tables below represent the results of the Predecessor, which refers to Celanese GmbH, formerly known as Celanese AG, and its consolidated subsidiaries. The balance sheet, statement of operations and cash flow data for periods subsequent to April 1, 2004 in the tables below represent the results of the Successor, which refers to Celanese Corporation and its consolidated subsidiaries. The results of the Successor are not comparable to the results of the Predecessor due to the difference in the basis of presentation of purchase accounting as compared to historical cost. Furthermore, the Successor and the Predecessor have different accounting policies with respect to certain matters.
33
Table of Contents
| Successor | Predecessor | ||||||||||||||||||||||||
| Nine Months | Three Months | ||||||||||||||||||||||||
| Ended | Ended | ||||||||||||||||||||||||
| Year Ended December 31, | December 31, | March 31, | |||||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | 2004 | ||||||||||||||||||||
| (In $ millions, except per share data) | |||||||||||||||||||||||||
Statement of Operations Data: | |||||||||||||||||||||||||
| Net sales | 6,823 | 6,444 | 5,778 | 5,270 | 3,206 | 1,058 | |||||||||||||||||||
| Other (charges) gains, net | (108 | ) | (58 | ) | (10 | ) | (61 | ) | (78 | ) | (28 | ) | |||||||||||||
| Operating profit | 440 | 748 | 620 | 486 | 17 | 39 | |||||||||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 434 | 447 | 526 | 276 | (230 | ) | 62 | ||||||||||||||||||
| Earnings (loss) from continuing operations | 372 | 336 | 319 | 214 | (292 | ) | 48 | ||||||||||||||||||
| Earnings (loss) from discontinued operations | (90 | ) | 90 | 87 | 63 | 39 | 30 | ||||||||||||||||||
| Net earnings (loss) | 282 | 426 | 406 | 277 | (253 | ) | 78 | ||||||||||||||||||
| Earnings (loss) per share from continuing operations — basic | 2.44 | 2.11 | 1.95 | 1.32 | (2.94 | ) | 0.97 | ||||||||||||||||||
| Earnings (loss) per share from continuing operations — diluted | 2.28 | 1.96 | 1.86 | 1.29 | (2.94 | ) | 0.97 | ||||||||||||||||||
Statement of Cash Flows Data: | |||||||||||||||||||||||||
| Net cash provided by (used in): | |||||||||||||||||||||||||
| Operating activities | 586 | 566 | 751 | 701 | (62 | ) | (102 | ) | |||||||||||||||||
| Investing activities | (201 | ) | 143 | (268 | ) | (907 | ) | (1,811 | ) | 91 | |||||||||||||||
| Financing activities | (499 | ) | (714 | ) | (108 | ) | (144 | ) | 2,686 | (43 | ) | ||||||||||||||
Balance Sheet Data (at the end of period): | |||||||||||||||||||||||||
Trade working capital(1) | 685 | 827 | 824 | 758 | 743 | 689 | |||||||||||||||||||
| Total assets | 7,166 | 8,058 | 7,895 | 7,445 | 7,410 | 6,613 | |||||||||||||||||||
| Total debt | 3,533 | 3,556 | 3,498 | 3,437 | 3,387 | 587 | |||||||||||||||||||
| Shareholders’ equity (deficit) | 182 | 1,062 | 787 | 235 | (112 | ) | 2,622 | ||||||||||||||||||
Other Financial Data: | |||||||||||||||||||||||||
| Depreciation and amortization | 350 | 291 | 269 | 267 | 165 | 64 | |||||||||||||||||||
Capital expenditures(3) | 267 | 306 | 244 | 203 | 134 | 29 | |||||||||||||||||||
Cash basis dividends paid per common share(2) | 0.16 | 0.16 | 0.16 | 0.08 | — | — | |||||||||||||||||||
| (1) | Trade working capital is defined as trade accounts receivable from third parties and affiliates net of allowance for doubtful accounts, plus inventories, less trade accounts payable to third parties and affiliates. Trade working capital is calculated in the table below: |
| Successor | Predecessor | |||||||||||||||||||||||||||||
| As of December 31, | March 31, | |||||||||||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | 2004 | |||||||||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||||||||
| Trade receivables, net | 631 | 1,009 | 1,001 | 919 | 866 | 810 | ||||||||||||||||||||||||
| Inventories | 577 | 636 | 653 | 650 | 603 | 491 | ||||||||||||||||||||||||
| Trade payables | (523 | ) | (818 | ) | (830 | ) | (811 | ) | (726 | ) | (612 | ) | ||||||||||||||||||
| Trade working capital | 685 | 827 | 824 | 758 | 743 | 689 | ||||||||||||||||||||||||
| (2) | In the nine months ended December 31, 2004, Celanese GmbH declared and paid a dividend of €0.12 ($0.14) per share for the year ended December 31, 2003. Dividends paid to Celanese and its consolidated subsidiaries eliminate in consolidation. | |
| (3) | Amounts include accrued capital expenditures. Amounts do not include capital expenditures related to capital lease obligations or capital expenditures related to the relocation of our Ticona plant in Kelsterbach. See Note 24 and Note 28 to the consolidated financial statements. |
34
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
In this Annual Report onForm 10-K, the term “Celanese” refers to Celanese Corporation, a Delaware corporation, and not its subsidiaries. The terms the “Company,” “we,” “our” and “us” refer to Celanese and its subsidiaries on a consolidated basis. The term “Celanese US” refers to our subsidiary Celanese US Holdings LLC, a Delaware limited liability company, formally known as BCP Crystal US Holdings Corp., a Delaware corporation, and not its subsidiaries. The term “Purchaser” refers to our subsidiary, Celanese Europe Holding GmbH & Co. KG, formerly known as BCP Crystal Acquisition GmbH & Co. KG, a German limited partnership, and not its subsidiaries, except where otherwise indicated.
You should read the following discussion and analysis of the financial condition and the results of operations together with the consolidated financial statements and the accompanying notes to the consolidated financial statements, which were prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
Investors are cautioned that the forward-looking statements contained in this section involve both risk and uncertainty. Several important factors could cause actual results to differ materially from those anticipated by these statements. Many of these statements are macroeconomic in nature and are, therefore, beyond the control of management. See “Forward-Looking Statements May Prove Inaccurate” below.
Reconciliation of Non-US GAAP Measures: We believe that using non-US GAAP financial measures to supplement US GAAP results is useful to investors because such use provides a more complete understanding of the factors and trends affecting the business other than disclosing US GAAP results alone. In this regard, we disclose net debt, which is a non-US GAAP financial measure. Net debt is defined as total debt less cash and cash equivalents. We use net debt to evaluate the capital structure. Net debt is not a substitute for any US GAAP financial measure. In addition, calculations of net debt contained in this report may not be consistent with that of other companies. The most directly comparable financial measure presented in accordance with US GAAP in our financial statements for net debt is total debt. For a reconciliation of net debt to total debt, see “Financial Highlights” below.
Forward-Looking Statements May Prove Inaccurate
Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) and other parts of this Annual Report contain certain forward-looking statements and information relating to us that are based on the beliefs of our management as well as assumptions made by, and information currently available to, us. When used in this document, words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan” and “project” and similar expressions, as they relate to us are intended to identify forward-looking statements. These statements reflect our current views with respect to future events, are not guarantees of future performance and involve risks and uncertainties that are difficult to predict. Further, certain forward-looking statements are based upon assumptions as to future events that may not prove to be accurate. We assume no obligation to revise or update any forward-looking statements for any reason, except as required by law.
See the Risk Factors section under Part 1, Item 1A for a description of risk factors that could significantly affect our financial results. In addition, the following factors could cause our actual results to differ materially from those results, performance or achievements that may be expressed or implied by such forward-looking statements. These factors include, among other things:
| • | changes in general economic, business, political and regulatory conditions in the countries or regions in which we operate; | |
| • | the length and depth of product and industry business cycles particularly in the automotive, electrical, electronics and construction industries; | |
| • | changes in the price and availability of raw materials, particularly changes in the demand for, supply of, and market prices of ethylene, methanol, natural gas, wood pulp, fuel oil and electricity; | |
| • | the ability to pass increases in raw material prices on to customers or otherwise improve margins through price increases; |
35
Table of Contents
| • | the ability to maintain plant utilization rates and to implement planned capacity additions and expansions; | |
| • | the ability to reduce production costs and improve productivity by implementing technological improvements to existing plants; | |
| • | increased price competition and the introduction of competing products by other companies; | |
| • | changes in the degree of intellectual property and other legal protection afforded to our products; | |
| • | compliance costs and potential disruption or interruption of production due to accidents or other unforeseen events or delays in construction of facilities; | |
| • | potential liability for remedial actions under existing or future environmental regulations; | |
| • | potential liability resulting from pending or future litigation, or from changes in the laws, regulations or policies of governments or other governmental activities in the countries in which we operate; | |
| • | changes in currency exchange rates and interest rates; and | |
| • | various other factors, both referenced and not referenced in this document. |
Many of these factors are macroeconomic in nature and are, therefore, beyond our control. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, our actual results, performance or achievements may vary materially from those described in this Annual Report as anticipated, believed, estimated, expected, intended, planned or projected. We neither intend nor assume any obligation to update these forward-looking statements, which speak only as of their dates.
Overview
During 2008, we made significant strides in strengthening our competitive position. As detailed below, we advanced our expansion efforts in Asia, successfully generated cash and used it to deliver value for our shareholders, settled legacy litigation matters, carried out our sustainability commitments and fulfilled our role as a responsible corporate citizen.
2008 Highlights:
| • | Opened a customer application development center in Shanghai, China, to support growth in the region for Advanced Engineered Materials’ engineering polymers business. | |
| • | Our Board of Directors authorized us to repurchase up to $500 million of our Series A common stock. During the year ended December 31, 2008, we repurchased 9,763,200 shares of our Series A common stock for $378 million pursuant to this authorization. | |
| • | Signed an agreement to establish a 20,000 square-meter integrated technology and marketing facility in Shanghai, China. The facility will combine the headquarters for our Asia businesses, customer application development and research and development center. | |
| • | Successfully started up our newly constructed 20,000 ton ultra-high molecular weight polyethylene (“GUR®”) facility, 100,000 ton acetic anhydride facility and 300,000 ton vinyl acetate monomer (“VAM”) facility, all located at our integrated chemical complex in Nanjing, China. | |
| • | Our Nutrinova business and BRAIN AG, a leading European “white” biotech company, identified all-natural compounds for high intensity sweeteners and sweetness enhancers. | |
| • | Introduced EcoVAEtm, a new vinyl acetate/ethylene emulsion technology designed to facilitate the manufacture of high quality, eco-friendly paints for North America and Asia. | |
| • | Resolved certain legacy litigation matters by entering into a settlement agreement for $107 million related to sales by the polyester staple fibers business, which Hoechst AG sold to KoSa, Inc. in 1998. In addition, we paid €17 million to settle Sorbates antitrust actions with the European Commission. |
36
Table of Contents
| • | Announced plans to build a new Vectra® liquid crystal polymer (“LCP”) production facility co-located at our integrated chemical complex in Nanjing, China. Construction for the LCP production facility and start-up dates will depend on market conditions. | |
| • | Began construction of the world’s largest polyacetal plant in Hoechst Industrial Park. The state-of-the-art facility is expected to be operational in 2011 and will replace Ticona’s existing production operations in Kelsterbach, Germany. | |
| • | Received Green Partner certification from Sony Corporation at our Ticona plant in Shelby, North Carolina. The certification recognizes suppliers’ cooperation of eco-friendly products and their ability to meet established regulations for environment-related substances found in components of products that bear the Sony name. | |
| • | Announced the shutdown of the VAM production unit in Cangrejera, Mexico and the assessment of the potential closure of acetic acid and vinyl acetate monomer (“VAM”) production in Pardies, France and certain other actions. | |
| • | Released our 2008 Sustainability Report, which details our industry-leading commitment to safety, health and the environment across our worldwide operations. The report also highlights best practices driven by our employees around the world. | |
| • | Reached an agreement with the Frankfurt, Germany Airport to receive an advance payment of €322 million associated with the relocation of our Ticona business in Kelsterbach, Germany. This advance payment will be in lieu of payments of €200 million and €140 million originally scheduled to be paid in June 2009 and June 2010, respectively. |
During the first half of 2008, the US and Europe began to experience challenging economic conditions that resulted in significant inflation in raw material and energy pricing. Despite the challenges, we were able to implement higher pricing on continued strong demand and higher volumes associated with our growth strategy in Asia, all of which contributed to growth in net sales during the period.
During the second half of 2008, recessionary trends stemming from the US credit crisis quickly spread to other regions of the world. This triggered an unexpected reduction in consumer and industrial demand that caused our customers to sharply lower their inventories during the last few months of the year. As a result, demand for our products declined dramatically and we aggressively managed our global production capacity to align with the current environment. During this period of economic uncertainty, we remained focused on executing our long-term strategy to increase the value of Celanese.
2009 Outlook
We expect inventory destocking to diminish in 2009, but do not foresee a short-term recovery in the global economic environment. We expect earnings to improve from fourth quarter levels throughout the year as the impact of destocking and the negative effects of lower-of-cost or market and first-in, first-out inventory accounting decrease.
We are taking aggressive actions in response to the expected prolonged weak demand environment. These actions include an assessment of our current manufacturing footprint and reductions of our overall fixed cost structure. Initial fixed cost reductions have been initiated and are estimated to result in between $100 million and $120 million of cost savings annually.
Expansion in China
The Nanjing complex brings world-class scale to one site for the production of acetic acid, VAM, acetic anhydride, emulsions, Celstran® long fiber reinforced thermoplastic (“LFRT”), GUR®, Vectra® LCP and compounding. We believe the Nanjing complex will further enhance our capabilities to better meet the growing needs of our customers in a number of industries across Asia.
The acetic acid facility located in our Nanjing, China complex achieved normal operations in June 2007 and we commenced production of vinyl acetate emulsions at the complex during the fourth quarter of 2007. During the
37
Table of Contents
first quarter of 2008, we commissioned the startup of our LFRT unit in Nanjing. Our newly constructed 20,000 ton GUR® facility, 100,000 ton acetic anhydride facility and 300,000 ton VAM facility started up during the third quarter of 2008. Operations for the compounding plant at the complex are expected to begin during 2009.
In 2008, we announced our plans to build an LCP production facility at our Nanjing complex. Construction for the LCP production facility and start-up dates will depend on market conditions.
Financial Highlights
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
Statement of Operations Data: | ||||||||||||
| Net sales | 6,823 | 6,444 | 5,778 | |||||||||
| Gross profit | 1,256 | 1,445 | 1,309 | |||||||||
| Selling, general and administrative expenses | (540 | ) | (516 | ) | (536 | ) | ||||||
| Other (charges) gains, net | (108 | ) | (58 | ) | (10 | ) | ||||||
| Operating profit | 440 | 748 | 620 | |||||||||
| Equity in net earnings of affiliates | 54 | 82 | 76 | |||||||||
| Interest expense | (261 | ) | (262 | ) | (293 | ) | ||||||
| Refinancing expenses | — | (256 | ) | (1 | ) | |||||||
| Dividend income — cost investments | 167 | 116 | 79 | |||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 434 | 447 | 526 | |||||||||
| Earnings (loss) from continuing operations | 372 | 336 | 319 | |||||||||
| Earnings (loss) from discontinued operations | (90 | ) | 90 | 87 | ||||||||
| Net earnings | 282 | 426 | 406 | |||||||||
Other Data: | ||||||||||||
| Depreciation and amortization | 350 | 291 | 269 | |||||||||
Operating margin(1) | 6.4 | % | 11.6 | % | 10.7 | % | ||||||
| Earnings from continuing operations before tax and minority interests as a percentage of net sales | 6.4 | % | 6.9 | % | 9.1 | % | ||||||
| (1) | Defined as operating profit divided by net sales. |
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
Balance Sheet Data: | ||||||||
| Short-term borrowings and current installments of long-term debt — third party and affiliates | 233 | 272 | ||||||
| Plus: Long-term debt | 3,300 | 3,284 | ||||||
| Total debt | 3,533 | 3,556 | ||||||
| Less: Cash and cash equivalents | 676 | 825 | ||||||
| Net debt | 2,857 | 2,731 | ||||||
Summary of Consolidated Results — Year Ended December 31, 2008 compared with Year Ended December 31, 2007
The challenging economic environment in the United States and Europe during the first half of 2008 resulted in higher raw material and energy costs which enabled price increase initiatives across all segments. During the second half of 2008, the US credit crisis accelerated the economic slowdown and its spread to other regions of the world. Despite the halt in demand, we were able to maintain the majority of our enacted price increases through the
38
Table of Contents
remainder of 2008. As a result, increased prices improved net sales by 8%. Favorable foreign currency impacts also had a positive impact on net sales of 3%.
Net sales declined 5% due to decreased volumes. Lower volumes were primarily a result of decreased demand stemming from the global economic downturn. As demand declined, particularly during the fourth quarter of 2008, our customers began destocking to reduce their inventory levels. In response, we aggressively managed our global production capacity to align with the current environment. We expect destocking trends to continue to a lesser extent into the first quarter of 2009. Decreased volumes in our acetate flake and tow businesses were not significantly impacted by the economic downturn. Rather, decreased flake volumes were the result of our strategic decision to shift our flake production to our China ventures, which we account for as cost investments. The full impact of this shift has been realized during 2008 and thus the resulting trend of diminishing volumes is not expected to continue.
Gross profit declined as higher raw material, energy and freight costs more than offset increases in net sales during the period. The uncertain economic environment resulted in higher natural gas, ethylene, methanol and other commodity prices during the first nine months of the year. Our freight costs also increased, primarily due to increased rates driven by higher energy prices. Late in 2008, raw material and energy prices declined and we expect that decline to positively impact gross profit during the first quarter of 2009.
Other (charges) gains, net increased $50 million during 2008 as compared to 2007:
| Year Ended | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Employee termination benefits | (21 | ) | (32 | ) | ||||
| Plant/office closures | (7 | ) | (11 | ) | ||||
| Deferred compensation triggered by Exit Event | — | (74 | ) | |||||
| Insurance recoveries associated with plumbing cases | — | 4 | ||||||
| Insurance recoveries associated with Clear Lake, Texas | 38 | 40 | ||||||
| Resolution of commercial disputes with a vendor | — | 31 | ||||||
| Asset impairments | (115 | ) | (9 | )(1) | ||||
| Ticona Kelsterbach plant relocation | (12 | ) | (5 | ) | ||||
| Sorbates antitrust actions | 8 | — | ||||||
| Other | 1 | (2 | ) | |||||
| Total Other (charges) gains, net | (108 | ) | (58 | ) | ||||
| (1) | Includes $6 million of goodwill impairment |
Other charges increased in 2008 compared to 2007 and includes a long-lived asset impairment loss of $92 million in connection with the potential closure of our acetic acid and VAM production facility in Pardies, France, our VAM production unit in Cangrejera, Mexico and certain other facilities. Consideration of this potential capacity reduction was necessitated by the significant change in the global economic environment and anticipated lower customer demand. Following the initial assessment of this capacity reduction, we determined we would shut down the Cangrejera VAM production unit effective at the end of February 2009.
In addition, we recognized $23 million of long-lived asset impairment losses and $13 million of employee termination benefits in 2008 related to the shutdown of our Pampa, Texas facility.
Selling, general and administrative expenses increased $24 million during 2008 primarily due to business optimization and finance improvement initiatives.
Operating profit decreased due to lower gross profit and higher other charges and selling, general and administrative costs. The absence of a $34 million gain on the sale of our Edmonton, Alberta, Canada facility during 2007 also contributed to lower operating profit in 2008 as compared to 2007.
Equity in net earnings of affiliates decreased $28 million during 2008, primarily due to reduced earnings from our Advanced Engineered Materials’ affiliates resulting from higher raw material and energy costs and decreased demand.
39
Table of Contents
Our effective income tax rate for 2008 was 15% compared to 25% in 2007. The effective income tax rate decreased in 2008 due to: 1) a decrease in the valuation allowance 2) tax credits generated on foreign jurisdictions and 3) the US tax impact of foreign operations.
The loss from discontinued operations of $90 million during 2008 primarily relates to a legal settlement agreement we entered into during 2008. Under the settlement agreement, we agreed to pay $107 million to resolve certain legacy items. Because the legal proceeding related to sales by the polyester staple fibers business which Hoechst AG sold to KoSa, Inc. in 1998, the impact of the settlement is reflected within discontinued operations in the current period. See the “Polyester Staple Antitrust Litigation” section in Note 23 of the consolidated financial statements.
Summary of Consolidated Results — Year Ended December 31, 2007 compared with Year Ended December 31, 2006
For the year ended December 31, 2007, net sales increased by 12% to $6,444 million compared to the same period in 2006 as increases in pricing and favorable currency impacts more than offset lower overall volumes. Overall pricing contributed to a 6% increase in net sales primarily due to a tight global supply of acetyl, PVOH and emulsion products, and higher acetate tow and flake prices. Favorable currency impacts (particularly related to the Euro) also contributed to a 4% increase in net sales and the acquisition of Acetate Products Limited (“APL”) in 2007 increased net sales by $227 million. Strong volume increases due to increased market penetration from several of Advanced Engineered Material’s key products and the successful startup of our acetic acid unit in Nanjing, China helped to partially offset the decreases in volumes in our Acetyl Intermediates and Industrial Specialties segments resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility.
Gross profit as a percentage of net sales remained relatively flat for the year ended December 31, 2007 (22.4%) compared to the same period in 2006 (22.7%). The slight decrease was primarily due to lower overall volumes and higher energy and raw material costs more than offsetting the higher overall prices and favorable currency impacts (particularly related to the Euro).
Selling, general and administrative expenses decreased by $20 million for the year ended December 31, 2007 compared to the same period in 2006. The decrease was primarily due to the absence of executive severance and legal costs associated with the Squeeze-Out of $23 million and long-term incentive plan expenses of $20 million, both recorded in 2006. Selling, general and administrative expenses also decreased $5 million due to lowerstock-based compensation expenses during the year ended December 31, 2007. The decreases were partially offset by $15 million of additional expenses related to finance improvement initiatives and $6 million related to the revised deferred compensation plan expenses.
Other (charges) gains, net increased $48 million during 2007 as compared to 2006:
| Year Ended | ||||||||
| December 31, | ||||||||
| 2007 | 2006 | |||||||
| (In $ millions) | ||||||||
| Employee termination benefits | (32 | ) | (12 | ) | ||||
| Plant/office closures | (11 | ) | 1 | |||||
| Deferred compensation triggered by Exit Event | (74 | ) | — | |||||
| Insurance recoveries associated with plumbing cases | 4 | 5 | ||||||
| Insurance recoveries associated with Clear Lake, Texas | 40 | — | ||||||
| Resolution of commercial disputes with a vendor | 31 | — | ||||||
| Asset impairments | (9 | )(1) | — | |||||
| Ticona Kelsterbach plant relocation | (5 | ) | — | |||||
| Other | (2 | ) | (4 | ) | ||||
| Total Other (charges) gains, net | (58 | ) | (10 | ) | ||||
| (1) | Includes $6 million of goodwill impairment |
40
Table of Contents
Other charges for employee termination benefits and plant/office closures include charges related to: 1) our plan to simplify and optimize our Emulsions and PVOH businesses to become a leader in technology and innovation and grow in both new and existing markets and 2) charges related to the sale of our Pampa, Texas facility. Additionally, we recorded a $74 million deferred compensation charge in May 2007 as a result of the triggering of an Exit Event, as defined in Note 20 to the consolidated financial statements. Also included in other charges are $40 million in insurance recoveries we received during the fourth quarter of 2007 in partial satisfaction of claims that we made based on losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. In addition, during the fourth quarter of 2007, we received $31 million as a one-time payment in resolution of commercial disputes with a vendor.
The net $128 million improvement in operating profit during the year ended December 31, 2007 as compared to 2006 is a result of higher net sales, lower selling, general and administrative expenses and a $34 million gain recorded on the sale of our Edmonton, Alberta, Canada facility during 2007, offset by higher other charges, as discussed above.
Equity in net earnings of affiliates increased 8% in the year ended December 31, 2007 compared to the same period in 2006 due primarily to improved performance from our InfraServ affiliates.
Interest expense decreased $31 million for the year ended December 31, 2007 compared to the same period in 2006. The decrease was primarily related to lower interest rates on the new senior credit agreement compared to the interest rates on the senior discount notes and senior subordinated notes, which were repaid in April 2007 in conjunction with the debt refinancing (see Note 14 to the consolidated financial statements for more information). These decreases were partially offset by an increase in interest expense due to additional China financing activities in 2007.
Refinancing expenses incurred during 2007 related to our debt refinancing. As a result of the refinancing, we expensed $207 million of premiums paid on early redemption of debt. In addition, we expensed $33 million of unamortized deferred financing costs and premiums related to the former $2,454 million senior credit facility, senior discount notes and senior subordinated notes and $16 million of debt issuance and other refinancing expenses.
Income tax expense decreased by $93 million to $110 million for the year ended December 31, 2007. The effective tax rate for continuing operations for the year ended December 31, 2007 was 25%, which is less than the combined US federal and state statutory rate of 39%. The effective tax rate for 2007 was favorably impacted by (1) an increase in unrepatriated low-taxed earnings including tax holidays and (2) the tax benefit related to German Tax Reform of $39 million recorded during the year ended December 31, 2007. These benefits are partially offset by the accounting treatment of recent tax law changes in Mexico. See Note 19 to the consolidated financial statements for additional information.
Earnings from discontinued operations primarily relate to Acetyl Intermediates’ sale of its oxo products and derivatives businesses in February 2007, the shut down of its Edmonton, Alberta, Canada methanol operations during the second quarter of 2007 and its pentaerythritol (“PE”) operations, which were discontinued during the third quarter of 2006. As a result, revenues and expenses related to these businesses and operations are reflected as a component of discontinued operations.
41
Table of Contents
Selected Data by Business Segment — Year Ended December 31, 2008 Compared with Year Ended December 31, 2007 and Year Ended December 31, 2007 Compared with Year Ended December 31, 2006
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||||||||||
| Change | Change | |||||||||||||||||||||||
| 2008 | 2007 | in $ | 2007 | 2006 | in $ | |||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||
Net sales | ||||||||||||||||||||||||
| Advanced Engineered Materials | 1,061 | 1,030 | 31 | 1,030 | 915 | 115 | ||||||||||||||||||
| Consumer Specialties | 1,155 | 1,111 | 44 | 1,111 | 876 | 235 | ||||||||||||||||||
| Industrial Specialties | 1,406 | 1,346 | 60 | 1,346 | 1,281 | 65 | ||||||||||||||||||
| Acetyl Intermediates | 3,875 | 3,615 | 260 | 3,615 | 3,351 | 264 | ||||||||||||||||||
| Other Activities | 2 | 2 | — | 2 | 22 | (20 | ) | |||||||||||||||||
| Inter-segment Eliminations | (676 | ) | (660 | ) | (16 | ) | (660 | ) | (667 | ) | 7 | |||||||||||||
| Net sales | 6,823 | 6,444 | 379 | 6,444 | 5,778 | 666 | ||||||||||||||||||
Other (charges) gains, net | ||||||||||||||||||||||||
| Advanced Engineered Materials | (29 | ) | (4 | ) | (25 | ) | (4 | ) | 6 | (10 | ) | |||||||||||||
| Consumer Specialties | (2 | ) | (4 | ) | 2 | (4 | ) | — | (4 | ) | ||||||||||||||
| Industrial Specialties | (3 | ) | (23 | ) | 20 | (23 | ) | (11 | ) | (12 | ) | |||||||||||||
| Acetyl Intermediates | (78 | ) | 72 | (150 | ) | 72 | — | 72 | ||||||||||||||||
| Other Activities | 4 | (64 | ) | 68 | (64 | ) | (5 | ) | (59 | ) | ||||||||||||||
| Inter-segment Eliminations | — | (35 | ) | 35 | (35 | ) | — | (35 | ) | |||||||||||||||
| Other (charges) gains, net | (108 | ) | (58 | ) | (50 | ) | (58 | ) | (10 | ) | (48 | ) | ||||||||||||
Operating profit | ||||||||||||||||||||||||
| Advanced Engineered Materials | 32 | 133 | (101 | ) | 133 | 145 | (12 | ) | ||||||||||||||||
| Consumer Specialties | 190 | 199 | (9 | ) | 199 | 165 | 34 | |||||||||||||||||
| Industrial Specialties | 47 | 28 | 19 | 28 | 44 | (16 | ) | |||||||||||||||||
| Acetyl Intermediates | 309 | 616 | (307 | ) | 616 | 456 | 160 | |||||||||||||||||
| Other Activities | (138 | ) | (228 | ) | 90 | (228 | ) | (190 | ) | (38 | ) | |||||||||||||
| Operating profit | 440 | 748 | (308 | ) | 748 | 620 | 128 | |||||||||||||||||
Earnings (loss) from continuing operations before tax and minority interests | ||||||||||||||||||||||||
| Advanced Engineered Materials | 69 | 189 | (120 | ) | 189 | 201 | (12 | ) | ||||||||||||||||
| Consumer Specialties | 237 | 235 | 2 | 235 | 185 | 50 | ||||||||||||||||||
| Industrial Specialties | 47 | 28 | 19 | 28 | 43 | (15 | ) | |||||||||||||||||
| Acetyl Intermediates | 434 | 694 | (260 | ) | 694 | 519 | 175 | |||||||||||||||||
| Other Activities | (353 | ) | (699 | ) | 346 | (699 | ) | (422 | ) | (277 | ) | |||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 434 | 447 | (13 | ) | 447 | 526 | (79 | ) | ||||||||||||||||
Depreciation and amortization | ||||||||||||||||||||||||
| Advanced Engineered Materials | 76 | 69 | 7 | 69 | 65 | 4 | ||||||||||||||||||
| Consumer Specialties | 53 | 51 | 2 | 51 | 39 | 12 | ||||||||||||||||||
| Industrial Specialties | 62 | 59 | 3 | 59 | 59 | — | ||||||||||||||||||
| Acetyl Intermediates | 150 | 106 | 44 | 106 | 101 | 5 | ||||||||||||||||||
| Other Activities | 9 | 6 | 3 | 6 | 5 | 1 | ||||||||||||||||||
| Depreciation and amortization | 350 | 291 | 59 | 291 | 269 | 22 | ||||||||||||||||||
42
Table of Contents
Factors Affecting Year Ended December 31, 2008 Segment Net Sales Compared to Year Ended December 31, 2007
The tables below set forth the percentage increase (decrease) in net sales attributable to each of the factors indicated in each of our business segments.
| Volume | Price | Currency | Other | Total | ||||||||||||||||
| (In percentages) | ||||||||||||||||||||
| Advanced Engineered Materials | (4 | ) | 3 | 4 | — | 3 | ||||||||||||||
| Consumer Specialties | (6 | ) | 7 | 1 | 2 | (b) | 4 | |||||||||||||
| Industrial Specialties | (10 | ) | 11 | 4 | (1 | )(c) | 4 | |||||||||||||
| Acetyl Intermediates | (3 | ) | 7 | 3 | — | 7 | ||||||||||||||
Total Company(a) | (5 | ) | 8 | 3 | — | 6 | ||||||||||||||
Factors Affecting Year Ended December 31, 2007 Segment Net Sales Compared to Year Ended December 31, 2006
| Volume | Price | Currency | Other | Total | ||||||||||||||||
| (In percentages) | ||||||||||||||||||||
| Advanced Engineered Materials | 9 | (1 | ) | 5 | — | 13 | ||||||||||||||
| Consumer Specialties | (4 | ) | 4 | 1 | 26 | (b) | 27 | |||||||||||||
| Industrial Specialties | (1 | ) | 2 | 5 | (1 | )(c) | 5 | |||||||||||||
| Acetyl Intermediates | (5 | ) | 9 | 4 | — | 8 | ||||||||||||||
Total Company(a) | (2 | ) | 6 | 4 | 4 | 12 | ||||||||||||||
| (a) | Includes the effects of the captive insurance companies. | |
| (b) | Includes net sales from the APL acquisition. | |
| (c) | Includes loss of sales related to the AT Plastics’ Films business. |
Summary by Business Segment — Year Ended December 31, 2008 Compared with Year Ended December 31, 2007
Advanced Engineered Materials
| Year Ended December 31, | Change | |||||||||||
| 2008 | 2007 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,061 | 1,030 | 31 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (4 | )% | ||||||||||
Price | 3 | % | ||||||||||
Currency | 4 | % | ||||||||||
Other | 0 | % | ||||||||||
| Operating profit | 32 | 133 | (101 | ) | ||||||||
| Operating margin | 3.0 | % | 12.9 | % | ||||||||
| Other (charges) gains, net | (29 | ) | (4 | ) | (25 | ) | ||||||
| Earnings (loss) from continuing operations before tax and minority interests | 69 | 189 | (120 | ) | ||||||||
| Depreciation and amortization | 76 | 69 | 7 | |||||||||
Our Advanced Engineered Materials segment develops, produces and supplies a broad portfolio of high performance technical polymers for application in automotive and electronics products, as well as other consumer and industrial applications. Together with our strategic affiliates, we are a leading participant in the global technical polymers industry. The primary products of Advanced Engineered Materials are POM, PPS, LFRT, PBT, PET,
43
Table of Contents
GUR® and LCP. POM, PPS, LFRT, PBT and PET are used in a broad range of products including automotive components, electronics, appliances and industrial applications. GUR® is used in battery separators, conveyor belts, filtration equipment, coatings and medical devices. Primary end markets for LCP are electrical and electronics.
Net sales increased 3% during 2008 as compared to 2007 primarily as a result of implemented pricing increases combined with favorable foreign currency impacts. Increases in net sales were partially offset by lower volumes due to significant weakness in the US and European automotive and housing industries. Extended plant shutdowns enacted by major car manufacturers during the fourth quarter of 2008 contributed significantly to the volume decline.
Operating profit declined $101 million primarily due to higher raw material, freight and energy costs. Raw material costs increased on higher prices while freight costs increased as a result of increased freight rates and larger shipments to Asia. Raw material costs declined late in 2008 and we expect to realize a benefit from the lower prices early in 2009 as our higher-cost inventory levels diminish. Higher depreciation and amortization expense and increased other charges also contributed to lower operating profit. Depreciation and amortization expense are higher in 2008 due to thestart-up of the GUR® and LFRT units in Asia. Other charges consist primarily of a $16 million long-lived asset impairment loss related to the potential closure of certain Advanced Engineered Materials’ facilities and $12 million related to the relocation of our Ticona plant in Kelsterbach. See “Ticona Kelsterbach Plant Relocation” below.
Earnings from continuing operations before tax and minority interests decreased due to decreased operating profit and decreased equity in net earnings of affiliates. Equity in net earnings of affiliates decreased $18 million during 2008, primarily due to reduced earnings from our Advanced Engineered Materials’ affiliates resulting from higher raw material and energy costs and decreased demand.
Ticona Kelsterbach Plant Relocation
In 2007, we finalized a settlement agreement with the Frankfurt, Germany Airport (“Fraport”) to relocate our Kelsterbach, Germany, business, resolving several years of legal disputes related to the planned Frankfurt airport expansion. As a result of the settlement, we will transition Ticona’s operations from Kelsterbach to the Hoechst Industrial Park in the Rhine Main area by mid-2011. Over a five-year period, Fraport will pay Ticona a total of €670 million to offset the costs associated with the transition of the business from its current location and the closure of the Kelsterbach plant. In June 2008, we received €200 million ($311 million) from Fraport under this agreement. Amounts received from Fraport are accounted for as deferred proceeds and are included in noncurrent other liabilities in the consolidated balance sheets.
In February 2009, we announced the Fraport supervisory board approved the acceleration of the 2009 and 2010 payments of €200 million and €140 million, respectively, required by the settlement agreement signed in June 2007. On February 5, 2009, we received a discounted amount of approximately €322 million, excluding value-added tax of €59 million.
Below is a summary of the cash flow activity and financial statement impact associated with the Ticona plant relocation:
| Year Ended | Total From | |||||||||||
| December 31, | Inception through | |||||||||||
| 2008 | 2007 | December 31, 2008 | ||||||||||
| (In $ millions) | ||||||||||||
| Proceeds received from Fraport | 311 | — | 337 | |||||||||
| Costs expensed | 12 | 5 | 17 | |||||||||
Costs capitalized(1) | 202 | 40 | 243 | |||||||||
| (1) | Includes an increase in accrued capital expenditures of $17 million and $19 million during the years ended December 31, 2008 and 2007, respectively. |
44
Table of Contents
Consumer Specialties
| Year Ended December 31, | Change | |||||||||||
| 2008 | 2007 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,155 | 1,111 | 44 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (6 | )% | ||||||||||
Price | 7 | % | ||||||||||
Currency | 1 | % | ||||||||||
Other | 2 | % | ||||||||||
| Operating profit | 190 | 199 | (9 | ) | ||||||||
| Operating margin | 16.4 | % | 17.9 | % | ||||||||
| Other (charges) gains, net | (2 | ) | (4 | ) | 2 | |||||||
| Earnings (loss) from continuing operations before tax and minority interests | 237 | 235 | 2 | |||||||||
| Depreciation and amortization | 53 | 51 | 2 | |||||||||
Our Consumer Specialties segment consists of our Acetate Products and Nutrinova businesses. Our Acetate Products business primarily produces and supplies acetate tow, which is used in the production of filter products. We also produce acetate flake which is processed into acetate fiber in the form of a tow band. Our Nutrinova business produces and sells Sunett®, a high intensity sweetener, and food protection ingredients, such as sorbates, for the food, beverage and pharmaceuticals industries.
Net sales increased 4% to $1,155 million during the year ended December 31, 2008 driven primarily by pricing actions in our Acetate Products business and an additional month of sales from our Acetate Products Limited (“APL”) acquisition, which was acquired on January 31, 2007, offset by lower volumes. Lower volumes are a direct result of our strategic decision to shift acetate flake production to our China ventures, which are accounted for as cost method investments. The full impact of this shift has been realized during 2008 and thus the resulting trend of diminishing volumes is not expected to continue. Lower flake volumes were partially offset by a 5% increase in tow volumes as we were able to capture a portion of the growth in global tow demand.
The increase in net sales due to higher sales prices during 2008 was offset most significantly by higher energy costs, and to a lesser extent, higher raw material and freight costs. Operating profit, as compared to 2007, declined primarily due to the absence of a $22 million gain on the sale of our Edmonton, Alberta, Canada facility in 2007. Other charges during 2007 includes $3 million of deferred compensation plan expenses and $5 million of other restructuring charges, offset by insurance recoveries of $5 million for partial satisfaction of the business interruption losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility.
Earnings from continuing operations before tax and minority interests of $237 million increased from 2007 as increased dividends from our China ventures more than offset the decline in operating profit. Increased dividends are the result of increased volumes and higher prices, as well as efficiency improvements.
45
Table of Contents
Industrial Specialties
| Year Ended December 31, | Change | |||||||||||
| 2008 | 2007 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,406 | 1,346 | 60 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (10 | )% | ||||||||||
Price | 11 | % | ||||||||||
Currency | 4 | % | ||||||||||
Other | (1 | )% | ||||||||||
| Operating profit | 47 | 28 | 19 | |||||||||
| Operating margin | 3.3 | % | 2.1 | % | ||||||||
| Other (charges) gains, net | (3 | ) | (23 | ) | 20 | |||||||
| Earnings (loss) from continuing operations before tax and minority interests | 47 | 28 | 19 | |||||||||
| Depreciation and amortization | 62 | 59 | 3 | |||||||||
Our Industrial Specialties segment includes our Emulsions, PVOH and AT Plastics businesses. Our Emulsions business is a global leader which produces a broad product portfolio, specializing in vinyl acetate ethylene emulsions, and is a recognized authority on low VOC (volatile organic compounds), an environmentally-friendly technology. As a global leader, our PVOH business produces a broad portfolio of performance PVOH chemicals engineered to meet specific customer requirements. Our emulsions and PVOH products are used in a wide array of applications including paints and coatings, adhesives, building and construction, glass fiber, textiles and paper. AT Plastics offers a complete line of low-density polyethylene and specialty ethylene vinyl acetate resins and compounds. AT Plastics’ products are used in many applications including flexible packaging films, lamination film products, hot melt adhesives, medical tubing, automotive carpeting and solar cell encapsulation films.
Net sales increased by 4% during 2008 as increased prices and favorable foreign currency impacts more than offset volume reductions. Pricing actions implemented by all business lines late in 2007 and during 2008 contributed to the increase in net sales. Volumes declined primarily on decreased demand across all regions due to the global economic downturn combined with the temporary shutdown of our AT Plastics plant late in 2008. The overall volume decline was partially offset by increased emulsions volumes at our Nanjing, China facility, which began operating late in 2008.
Increased net sales were more than offset by higher raw material and energy costs during 2008. The $19 million increase in operating profit was primarily due to lower other charges and the absence of the $7 million loss on the divestiture of our AT Plastics’ Films business in 2007. During 2007, we initiated a plan to simplify and optimize our Emulsions and PVOH businesses to focus on technology and innovation. Other charges during 2008 includes a charge of $3 million for employee termination benefits and accelerated depreciation related to this plan. Other charges during 2007 includes a charge of $14 million for employee termination benefits, $3 million for an impairment of long-lived assets and $5 million of accelerated depreciation expense for our shuttered United Kingdom plant related to this plan. Other charges in 2007 also include $6 million of goodwill impairment and receipt of $7 million in insurance recoveries in partial satisfaction of the business interruption losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility.
46
Table of Contents
Acetyl Intermediates
| Year Ended December 31, | �� | Change | ||||||||||
| 2008 | 2007 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 3,875 | 3,615 | 260 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (3 | )% | ||||||||||
Price | 7 | % | ||||||||||
Currency | 3 | % | ||||||||||
Other | 0 | % | ||||||||||
| Operating profit | 309 | 616 | (307 | ) | ||||||||
| Operating margin | 8.0 | % | 17.0 | % | ||||||||
| Other (charges) gains, net | (78 | ) | 72 | (150 | ) | |||||||
| Earnings (loss) from continuing operations before tax and minority interests | 434 | 694 | (260 | ) | ||||||||
| Depreciation and amortization | 150 | 106 | 44 | |||||||||
Our Acetyl Intermediates segment produces and supplies acetyl products, including acetic acid, VAM, acetic anhydride and acetate esters. These products are generally used as starting materials for colorants, paints, adhesives, coatings, medicines and more. Other chemicals produced in this segment are organic solvents and intermediates for pharmaceutical, agricultural and chemical products.
Net sales increased by 7% during 2008 primarily due to increased prices and favorable foreign currency impacts, partially offset by lower volumes. Our formula-based pricing arrangements benefited from higher ethylene and methanol costs during the first nine months of 2008. Market tightness in the Americas and favorable foreign currency impacts in Europe also contributed to the increase in net sales. Reduced volumes offset the increase in net sales as the slowdown of the global economy caused customers to slow production and diminish current inventory levels, particularly in Asia during the fourth quarter. We expect the impact of our customers’ destocking initiatives to continue to a lesser extent during 2009. Ethylene and methanol prices decreased during the fourth quarter of 2008 on slowed global demand.
Operating profit declined $307 million primarily as a result of higher ethylene, methanol and energy prices, increased other charges, increased depreciation and amortization and the absence of a $12 million gain on the sale of our Edmonton facility in 2007. Other charges increased during 2008 partially due to $76 million of long-lived asset impairment losses recognized in 2008 related to the potential closure of our acetic acid and VAM production facility in Pardies, France, our VAM production unit in Cangrejera, Mexico (which we subsequently decided to shut down effective at the end of February 2009) and certain other facilities. Other charges in 2008 also includes $23 million of long-lived asset impairment and $13 million of severance and retention charges related to the shutdown of our Pampa, Texas facility. Also contributing to the increase was the absence of a one-time payment of $31 million received in 2007 in resolution of commercial disputes with a vendor and a $25 million decrease in insurance recoveries received in partial satisfaction of the losses resulting from the temporary outage of the acetic acid unit at our Clear Lake, Texas facility. Increased depreciation and amortization expense during 2008 is the result of accelerated depreciation associated with the shutdown of our Pampa, Texas facility and a full year of depreciation for our acetic acid plant in Nanjing, China, which started up in mid-2007.
Earnings from continuing operations before tax and minority interest differs from operating profit primarily as a result of dividend income from our cost investment, National Methanol Co. (“Ibn Sina”). Increased dividend income of $41 million during 2008 had a positive impact on earnings from continuing operations before tax and minority interest. Ibn Sina increased their dividends as a result of higher earnings from expanding margins for methanol and methyl tertiary-butyl ether.
47
Table of Contents
Other Activities
Other Activities primarily consists of corporate center costs, including finance and administrative activities, and our captive insurance companies.
Net sales remained flat in 2008 as compared to 2007. We do not expect third-party revenues from our captive insurance companies to increase significantly in the near future.
The operating loss for Other Activities improved $90 million during 2008 as compared to 2007 due to lower other charges, partially offset by higher selling, general and administrative expenses. Other charges decreased principally due to the release of reserves related to the Sorbates antitrust actions settlement of $8 million and the absence of $59 million of deferred compensation plan costs which were incurred during 2007. Selling, general and administrative expenses increased due to additional spending on business optimization and finance improvement initiatives during 2008.
The loss from continuing operations before tax and minority interests decreased $346 million during 2008. The significant decrease was primarily due to the absence of $256 million of refinancing costs incurred in 2007 and the decrease in the operating loss discussed above.
Summary by Business Segment — Year Ended December 31, 2007 Compared with Year Ended December 31, 2006
Advanced Engineered Materials
| Year Ended December 31, | Change | |||||||||||
| 2007 | 2006 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,030 | 915 | 115 | |||||||||
| Net sales variance: | ||||||||||||
Volume | 9 | % | ||||||||||
Price | (1 | )% | ||||||||||
Currency | 5 | % | ||||||||||
Other | 0 | % | ||||||||||
| Operating profit | 133 | 145 | (12 | ) | ||||||||
| Operating margin | 12.9 | % | 15.8 | % | ||||||||
| Other (charges) gains, net | (4 | ) | 6 | (10 | ) | |||||||
| Earnings (loss) from continuing operations before tax and minority interests | 189 | 201 | (12 | ) | ||||||||
| Depreciation and amortization | 69 | 65 | 4 | |||||||||
Advanced Engineered Materials’ net sales increased 13% for the year ended December 31, 2007 compared to the same period in 2006 primarily due to volume growth in all major business lines and favorable currency impacts partially offset by a slight decline in pricing. Overall volume for the year increased, principally driven by increased market penetration, successful implementation of new projects and a continued strong business environment, particularly in Europe. Advanced Engineered Materials experienced a slight decline in average pricing primarily driven by a larger mix of sales from lower priced products.
Operating profit decreased to $133 million for the year ended December 31, 2007 compared to $145 million for the same period in 2006 as higher volumes and favorable currency impacts were more than offset by higher energy costs, increased other charges and slightly lower average pricing. Other charges increased primarily due to $2 million of deferred compensation plan expenses and $5 million of Kelsterbach plant relocation costs, both incurred in 2007.
Earnings from continuing operations before tax and minority interests decreased 6% to $189 million for the year ended December 31, 2007 compared to the same period in 2006 primarily due to lower operating profits in 2007 driven by increased other charges as discussed above.
48
Table of Contents
Consumer Specialties
| Year Ended December 31, | Change | |||||||||||
| 2007 | 2006 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,111 | 876 | 235 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (4 | )% | ||||||||||
Price | 4 | % | ||||||||||
Currency | 1 | % | ||||||||||
Other | 26 | % | ||||||||||
| Operating profit | 199 | 165 | 34 | |||||||||
| Operating margin | 17.9 | % | 18.8 | % | ||||||||
| Other (charges) gains, net | (4 | ) | — | (4 | ) | |||||||
| Earnings (loss) from continuing operations before tax and minority interests | 235 | 185 | 50 | |||||||||
| Depreciation and amortization | 51 | 39 | 12 | |||||||||
Consumer Specialties’ net sales increased 27% for the year ended December 31, 2007 compared to the same period in 2006 primarily driven by additional net sales from the APL acquisition completed on January 31, 2007. Net sales for APL were $227 million during the year ended December 31, 2007. Higher pricing for acetate tow and flake products, higher Sunett® sweetener volumes and favorable currency impacts for the Nutrinova business also contributed to the overall increase in net sales. Higher Sunett® sweetener volumes reflected the continued growth in the global beverage and confectionary markets. These increases were partially offset by lower acetate flake volumes and Nutrinova’s exit of non-core lower margin trade business during the fourth quarter of 2006. The decrease in acetate flake volumes was due primarily to the shift in production of acetate flake to our China ventures.
Operating profit increased $34 million for the year ended December 31, 2007 compared to the same period in 2006. Higher overall pricing more than offset increases in raw material costs. Higher overall costs were primarily due to price increases in wood pulp, acetyls (used as raw materials in acetate flake production) and acetate flake as well as expenses associated with the continued integration of APL. Other charges during the year ended December 31, 2007 includes $3 million of deferred compensation plan expenses, $5 million of other restructuring charges and insurance recoveries of $5 million for partial satisfaction of the losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. Also included in operating profit for the year ended December 31, 2007 is a $22 million gain related to the sale of our Edmonton, Alberta, Canada facility.
Earnings from continuing operations before tax and minority interests increased $50 million to $235 million for the year ended December 31, 2007 compared to the same period in 2006. The increase was driven principally by the changes in operating profit discussed above and an increase of $16 million in dividends received from our China ventures during the year ended December 31, 2007 compared to the same period in 2006.
Depreciation and amortization increased $12 million to $51 million for the year ended December 31, 2007 compared to the same period in 2006 primarily driven by the acquisition of APL in 2007.
49
Table of Contents
Industrial Specialties
| Year Ended December 31, | Change | |||||||||||
| 2007 | 2006 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 1,346 | 1,281 | 65 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (1 | )% | ||||||||||
Price | 2 | % | ||||||||||
Currency | 5 | % | ||||||||||
Other | (1 | )% | ||||||||||
| Operating profit | 28 | 44 | (16 | ) | ||||||||
| Operating margin | 2.1 | % | 3.4 | % | ||||||||
| Other (charges) gains, net | (23 | ) | (11 | ) | (12 | ) | ||||||
| Earnings (loss) from continuing operations before tax and minority interests | 28 | 43 | (15 | ) | ||||||||
| Depreciation and amortization | 59 | 59 | — | |||||||||
Industrial Specialties’ net sales for the year ended December 31, 2007 increased 5% to $1,346 million compared to the same period in 2006 primarily driven by pricing increases and favorable currency impacts partially offset by a slight decrease in volumes. Higher overall pricing, particularly in our emulsions and PVOH products, was primarily due to market tightness and increasing raw material costs which allowed for upward movement in pricing across all regions. Lower volumes were primarily driven by the tight supply of VAM, a major raw material used in the production of emulsions products. This was a result of the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility and other global planned and unplanned production outages in the chemical industry during the year ended December 31, 2007. The increase in net sales was partially offset by the absence of net sales from the AT Plastics’ Films business, which was divested during the third quarter of 2007.
Operating profit decreased $16 million to $28 million for the year ended December 31, 2007 compared to the same period in 2006. Higher prices and favorable currency impacts were more than offset by the increase in other charges. Other charges for the year ended December 31, 2007 included $14 million of employee termination benefits, $3 million for an impairment of long-lived assets and $5 million of accelerated depreciation expense for our shuttered United Kingdom plant. These increases were a result of our plan to simplify and optimize our Emulsions and PVOH businesses to become a leader in technology and innovation and grow in both new and existing markets. Other charges for the year ended December 31, 2007 also included $6 million of goodwill impairment. The increase in other charges was partially offset by insurance recoveries of $7 million for partial satisfaction of the losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. Additionally, operating profit decreased by $7 million during the year ended December 31, 2007 as a result of the loss on the divestiture of our AT Plastics’ Films business.
Earnings from continuing operations before tax and minority interests decreased $15 million to $28 million for the year ended December 31, 2007 compared to the same period in 2006 principally driven by lower operating profits.
50
Table of Contents
Acetyl Intermediates
| Year Ended | ||||||||||||
| December 31, | Change | |||||||||||
| 2007 | 2006 | in $ | ||||||||||
| (In $ millions, except percentages) | ||||||||||||
| Net sales | 3,615 | 3,351 | 264 | |||||||||
| Net sales variance: | ||||||||||||
Volume | (5 | )% | ||||||||||
Price | 9 | % | ||||||||||
Currency | 4 | % | ||||||||||
Other | 0 | % | ||||||||||
| Operating profit | 616 | 456 | 160 | |||||||||
| Operating margin | 17.0 | % | 13.6 | % | ||||||||
| Other (charges) gains, net | 72 | — | 72 | |||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 694 | 519 | 175 | |||||||||
| Depreciation and amortization | 106 | 101 | 5 | |||||||||
Acetyl Intermediates’ net sales for the year ended December 31, 2007 increased 8% to $3,615 million compared to the same period in 2006 driven by pricing increases and favorable currency impacts partially offset by lower volumes. Tight supply of acetyl products caused by global planned and unplanned production outages in the industry and higher methanol and ethylene prices were the drivers of the price increases. Lower volumes in 2007 were the result of lower product availability due to the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. However, the decrease in volumes from the Clear Lake, Texas facility was partially offset by the successful startup of our acetic acid plant in Nanjing, China and externally procured product.
Operating profit increased to $616 million for the year ended December 31, 2007 compared to $456 million in the same period in 2006. Higher pricing, favorable currency impacts and an improvement in other charges were partially offset by higher raw material costs. Other charges for the year ended December 31, 2007 included $4 million of employee termination benefits and $5 million of expenses related to accelerated depreciation expense, both associated with the shutdown of our Pampa, Texas plant, and $10 million for deferred compensation plan expenses. These charges were more than offset by $31 million related to a one-time payment received in resolution of commercial disputes with a vendor and insurance recoveries of $28 million for partial satisfaction of the losses resulting from the temporary unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. Acetyl Intermediates also received $35 million of insurance recoveries from our captive insurance companies relating to the unplanned outage of the acetic acid unit at our Clear Lake, Texas facility. This amount is included in Acetyl Intermediates’ other charges but is properly eliminated in our consolidated statements of operations. Operating profit also includes a gain on the sale of our Edmonton facility of $12 million.
Earnings from continuing operations before tax and minority interests increased $175 million to $694 million for the year ended December 31, 2007 compared to the same period in 2006 primarily driven by higher operating profit and an increase in dividend income from our cost investments. Dividend income from Ibn Sina cost investment increased $24 million due to increased earnings for the year ended December 31, 2007 compared to the same period in 2006.
Other Activities
Net sales for Other Activities decreased to $2 million from $22 million for the year ended December 31, 2007 compared to the same period in 2006. This decrease was principally driven by the decrease in third-party revenues from our captive insurance companies.
Operating loss increased to $228 million for the year ended December 31, 2007 compared to $190 million in the same period in 2006. This increase was principally driven by an increase in other charges more than offsetting a decrease in selling, general and administrative expenses. Other charges increased primarily due to $59 million of deferred compensation plan costs expensed in 2007. Selling, general and administrative expenses decreased for the
51
Table of Contents
year ended December 31, 2007 primarily due to the absence of executive severance and legal costs associated with the Squeeze-Out of $23 million and long-term incentive plan expenses of $20 million recorded in 2006.
Loss from continuing operations before tax and minority interests increased $277 million to $699 million for the year ended December 31, 2007 compared to the same period in 2006. The increase was primarily driven by the increase in operating loss discussed above and higher refinancing expenses incurred in 2007, partially offset by lower interest expense. During the year ended December 31, 2007, we incurred $256 million of refinancing expenses associated with the April 2, 2007 debt refinancing. Interest expense decreased $31 million during the year ended December 31, 2007 compared to the same period in 2006 primarily related to lower interest rates on the new senior credit agreement compared to the interest rates on the senior discount notes and senior subordinated notes, which were repaid in April 2007 in conjunction with the debt refinancing. In addition, during the year ended December 31, 2007, we incurred $26 million of mark-to-market loss on the cross currency swap and the Euro denominated term loan that had been used as a hedge of our net investment in our European subsidiaries.
Liquidity and Capital Resources
Our primary source of liquidity is cash generated from operations, available cash and cash equivalents and dividends from our portfolio of strategic investments. In addition, we have a $650 million revolving credit facility and a $228 million credit-linked revolving facility to assist, if required, in meeting our working capital needs and other contractual obligations. In excess of 20 lenders participate in our revolving credit facility, each with a commitment of not more than 10% of the $650 million commitment. Further, Lehman Brothers Holdings, Inc., which filed for protection under Chapter 11 of the United States Bankruptcy Code in September 2008, is not a lender under our revolving credit facility and we do not have any other material direct exposure to Lehman Brothers Holdings, Inc.
While our contractual obligations, commitments and debt service requirements over the next several years are significant, we continue to believe we will have available resources to meet our liquidity requirements, including debt service, for the remainder of 2009. If our cash flow from operations is insufficient to fund our debt service and other obligations, we may be required to use other means available to us such as increasing our borrowings, reducing or delaying capital expenditures, seeking additional capital or seeking to restructure or refinance our indebtedness. There can be no assurance, however, that we will continue to generate cash flows at or above current levels or that we will be able to maintain our ability to borrow under our revolving credit facilities.
On a stand-alone basis, Celanese Corporation has no material assets other than the stock of our subsidiaries and no independent external operations of our own. As such, we generally will depend on the cash flow of our subsidiaries to meet our obligations under our preferred stock, our Series A common stock and our senior credit agreement.
Cash Flows
Cash and cash equivalents as of December 31, 2008 were $676 million, which was a decrease of $149 million from December 31, 2007. Cash and cash equivalents as of December 31, 2007 were $825 million, which was an increase of $34 million from December 31, 2006. See below for details on the change in cash and cash equivalents from December 31, 2007 to December 31, 2008 and the change in cash and cash equivalents from December 31, 2006 to December 31, 2007.
Net Cash Provided by Operating Activities
Cash flow provided by operating activities increased $20 million to a cash inflow of $586 million in 2008 from a cash inflow of $566 million for the same period in 2007. Operating cash flows were favorably impacted by positive trade working capital changes ($202 million), lower cash taxes paid ($83 million) and the absence of adjustments to cash for discontinued operations. Adjustments to cash for discontinued operations of $84 million during 2007 related primarily to working capital changes of the oxo products and derivatives businesses and the shut down of our Edmonton, Alberta, Canada methanol facility. Offsetting the increase in cash flows were an increase in net cash interest paid ($78 million), cash spent on legal settlements ($134 million) and decreased operating profit during the period.
Cash flow provided by operating activities decreased to a cash inflow of $566 million in 2007 compared to a cash inflow of $751 million for the same period in 2006. The decrease in operating cash flows was primarily due to
52
Table of Contents
adjustments to cash for discontinued operations and an increase in working capital, partially offset by an increase in earnings from continuing operations. Earnings from continuing operations increased to $336 million during the year ended December 31, 2007 compared with $319 million for the same period in 2006.
Net Cash Provided by/Used in Investing Activities
Net cash from investing activities decreased from a cash inflow of $143 million in 2007 to a cash outflow of $201 million in 2008. Net cash from investing activities decreased primarily due to cash spent in settlement of our cross currency swaps of $93 million (see Note 22 to the consolidated financial statements) and the absence of proceeds from the sale of our oxo products and derivatives businesses during 2007. These amounts were offset by net cash received on the sale of marketable securities ($111 million) and the excess of cash received from Fraport over amounts spent in connection with the Ticona Kelsterbach plant relocation.
Net cash from investing activities improved to a cash inflow of $143 million in 2007 compared to a cash outflow of $268 million in 2006. The increase in cash inflow was primarily due to the proceeds from the sale of our oxo products and derivatives businesses partially offset by the cash outflow for the APL acquisition. Additionally, our cash outflow for capital expenditures during the year ended December 31, 2007 was $44 million higher compared to the same period in 2006. During the year ended December 31, 2006, we increased restricted cash by $42 million related to the anticipated payment to minority shareholders for their remaining Celanese GmbH, formerly Celanese AG, shares. During the year ended December 31, 2007, as a result of the completion of the Squeeze-Out (see Note 4 to the consolidated financial statements) and the payment to minority shareholders for their remaining Celanese GmbH shares, restricted cash decreased $46 million.
Our cash outflow for capital expenditures were $274 million, $288 million and $244 million for the years ended December 31, 2008, 2007 and 2006, respectively, excluding amounts related to the relocation of our Ticona plant in Kelsterbach. Capital expenditures were primarily related to major replacements of equipment, capacity expansions, major investments to reduce future operating costs and environmental, health and safety initiatives. Capital expenditures in 2008, 2007 and 2006 included costs for the expansion of our Nanjing, China site into an integrated chemical complex. Cash outflows for capital expenditures are expected to be approximately $175 million in 2009, excluding amounts related to the relocation of our Ticona plant in Kelsterbach.
On February 5, 2009, we received approximately €322 million of cash from Fraport in connection with the Ticona Kelsterbach plant relocation, excluding value-added tax of €59 million. We anticipate related cash outflows for capital expenditures in 2009 will range from $350 to $370 million.
Net Cash Used in Financing Activities
Net cash from financing activities increased to a cash outflow of $499 million in 2008 compared to a cash outflow of $714 million during 2007. The increase primarily relates to the absence of cash outflows attributable to the debt refinancing in 2007. Also contributing to the increase, cash spent to repurchase shares was $25 million less during 2008 than during 2007. Decreased cash received for stock option exercises of $51 million partially offset the increase.
Net cash from financing activities decreased to a cash outflow of $714 million in 2007 compared to a cash outflow of $108 million in the same period in 2006. The decrease primarily relates to the repurchase of shares of our Series A common stock and the debt refinancing as discussed in Note 17 and Note 14 to the consolidated financial statements, respectively. This decrease was partially offset by $69 million of proceeds received from the exercise of stock options. Primarily as a result of the debt refinancing, we incurred a net cash outflow of $119 million related to repayments of our debt and $240 million for various refinancing expenses during the year ended December 31, 2007. Furthermore, we paid a total of $403 million to repurchase shares of our Series A common stock during the year ended December 31, 2007.
In addition, exchange rate effects on cash and cash equivalents decreased to an unfavorable currency effect of $35 million in 2008 compared to a favorable impact of $39 million in 2007 and a favorable impact of $26 million in 2006.
53
Table of Contents
Debt and Other Obligations
As of December 31, 2008, we had total debt of $3,533 million and cash and cash equivalents of $676 million, resulting in net debt of $2,857 million, a $126 million increase over December 31, 2007. Decreased cash of $149 million and noncash increases in debt of $102 million primarily resulting from new capital lease obligations were partially offset by net cash paydowns on debt of $98 million and favorable foreign currency impacts of $27 million.
Senior Credit Facilities
Our senior credit agreement consists of $2,280 million of US dollar-denominated and €400 million of Euro-denominated term loans due 2014, a $650 million revolving credit facility terminating in 2013 and a $228 million credit-linked revolving facility terminating in 2014. As of December 31, 2008, there were no outstanding borrowings or letters of credit issued under the revolving credit facility; accordingly, $650 million remained available for borrowing. As of December 31, 2008, there were $91 million of letters of credit issued under the credit-linked revolving facility and $137 million remained available for borrowing. Our senior credit agreement requires us to maintain a maximum first lien senior secured leverage ratio not greater than 3.90 to 1.00 if there are outstanding borrowings under the revolving credit facility. The first lien senior secured leverage ratio is calculated as the ratio of consolidated first lien senior secured debt to earnings before interest, taxes, depreciation and amortization, subject to adjustments identified in the credit agreement. See Note 14 to the consolidated financial statements for additional information regarding our senior credit facilities.
Commitments Relating to Share Capital
Our Board of Directors adopted a policy of declaring, subject to legally available funds, a quarterly cash dividend on each share of our Series A common stock at an annual rate of $0.16 per share unless our Board of Directors in its sole discretion determines otherwise. For the years ended December 31, 2008, 2007 and 2006, we paid $24 million, $25 million and $26 million, respectively, in cash dividends on our Series A common stock. On January 5, 2009, we declared a $6 million cash dividend which was paid on February 1, 2009.
Holders of our perpetual preferred stock are entitled to receive, when, as and if declared by our Board of Directors, out of funds legally available, quarterly cash dividends at the rate of 4.25% per annum, or $0.265625 per share of liquidation preference. Dividends on the preferred stock are cumulative from the date of initial issuance. The preferred stock is convertible, at the option of the holder, at any time into approximately 1.26 shares of our Series A common stock, subject to adjustments, per $25.00 liquidation preference of the preferred stock. For the years ended December 31, 2008, 2007 and 2006, we paid $10 million annually of cash dividends on our preferred stock. On January 5, 2009, we declared a $3 million cash dividend on our convertible perpetual preferred stock, which was paid on February 1, 2009.
Based upon the number of outstanding shares as of December 31, 2008, the cash dividends to be paid in 2009 are expected to result in annual dividend payments similar to that paid in 2008.
54
Table of Contents
Contractual Debt and Cash Obligations
The following table sets forth our fixed contractual debt and cash obligations as of December 31, 2008.
| Expiration per Period | ||||||||||||||||||||
| Less Than | After 5 | |||||||||||||||||||
Fixed Contractual Debt and Cash Obligations | Total | 1 Year | Years 2 & 3 | Years 4 & 5 | Years | |||||||||||||||
| (In $ millions) | ||||||||||||||||||||
| Term loans facility | 2,794 | 28 | 57 | 57 | 2,652 | |||||||||||||||
Interest payments on debt(1) | 1,128 | 204 | 369 | 253 | 302 | |||||||||||||||
| Capital lease obligations | 211 | 11 | 41 | 23 | 136 | |||||||||||||||
Other debt(2) | 530 | 194 | 91 | 58 | 187 | |||||||||||||||
| Fixed contractual debt obligations | 4,663 | 437 | 558 | 391 | 3,277 | |||||||||||||||
| Operating leases | 140 | 45 | 48 | 25 | 22 | |||||||||||||||
| Unconditional purchase obligations | 2,291 | 527 | 717 | 399 | 648 | |||||||||||||||
FIN 48 obligations, including interest and penalties(3) | 218 | — | — | — | 218 | |||||||||||||||
| Other contractual obligations | 165 | 47 | 35 | 18 | 65 | |||||||||||||||
| Fixed contractual debt and cash obligations | 7,477 | 1,056 | 1,358 | 833 | 4,230 | |||||||||||||||
| (1) | Future interest expense is calculated using the rate in effect on January 2, 2009. | |
| (2) | Does not include a $2 million reduction due to purchase accounting. | |
| (3) | Due to uncertainties in the timing of the effective settlement of tax positions with the respective taxing authorities, we are unable to determine the timing of payments related to our Financial Accounting Standards Board (“FASB”) Interpretation No. 48, “Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109(“FIN 48”) obligations, including interest and penalties. These amounts are therefore reflected in “After 5 Years”. |
Other Debt. Other debt of $530 million is primarily made up of fixed rate pollution control and industrial revenue bonds, short-term borrowings from affiliated companies and other bank obligations.
Unconditional Purchase Obligations. Unconditional Purchase Obligations include take or pay contracts. We do not expect to incur any material losses under these contractual arrangements. In addition, these contracts may include variable price components.
Other Contractual Obligations. Other Contractual Obligations primarily includes committed capital spending and fines associated with the US antitrust settlement described in Note 23 to the consolidated financial statements.
Contractual Guarantees and Commitments
As of December 31, 2008, we have contractual guarantees and commitments as follows:
| Expiration per Period | ||||||||||||||||||||
| Less Than | After 5 | |||||||||||||||||||
Contractual Guarantees and Commitments | Total | 1 Year | Years 2 & 3 | Years 4 & 5 | Years | |||||||||||||||
| (In $ millions) | ||||||||||||||||||||
| Financial guarantees | 26 | 8 | 16 | 2 | — | |||||||||||||||
| Standby letters of credit | 91 | 91 | — | — | — | |||||||||||||||
| Contractual guarantees and commitments | 117 | 99 | 16 | 2 | — | |||||||||||||||
We are secondarily liable under a lease agreement which we assigned to a third party. The lease expires on April 30, 2012. The lease liability for the period from January 1, 2009 to April 30, 2012 is estimated to be approximately $26 million.
55
Table of Contents
Standby letters of credit of $91 million outstanding as of December 31, 2008 are irrevocable obligations of an issuing bank that ensure payment to third parties in the event that certain subsidiaries fail to perform in accordance with specified contractual obligations. The likelihood is remote that material payments will be required under these agreements.
Other Obligations
Deferred Compensation. In April 2007, certain participants in our 2004 deferred compensation plan elected to participate in a revised program, which includes both cash awards and restricted stock units. Under the revised program, participants relinquished their cash awards of up to $30 million that would have contingently accrued from2007-2009 under the original plan. See additional discussion of the revised program in Note 20 to the consolidated financial statements. Based on current participation in the revised program, the awards aggregate to approximately $27 million plus notional earnings and will be recognized as expense through December 31, 2010. We expensed $8 million and $6 million during the years ended December 31, 2008 and 2007, respectively, related to the revised program.
In December 2008, we entered into time-vesting cash awards of $22 million with Celanese’s executive officers and certain other key employees. Each award of cash vests 30% on October 14, 2009, 30% on October 14, 2010 and 40% on October 14, 2011. In its sole discretion, the compensation committee of the Board of Directors may at any time convert all or a portion of the cash award to an award of time-vesting restricted stock units.
Pension and Other Postretirement Obligations. Our contributions for pension and postretirement benefits are preliminarily estimated to be $40 million and $35 million, respectively, in 2009.
Domination Agreement. The domination and profit and loss transfer agreement (the “Domination Agreement”) was approved at the Celanese GmbH, formerly known as Celanese AG, extraordinary shareholders’ meeting on July 31, 2004. The Domination Agreement between Celanese GmbH and the Purchaser became effective on October 1, 2004 and cannot be terminated by the Purchaser in the ordinary course of business until September 30, 2009. Our subsidiaries, Celanese International Holdings Luxembourg S.à r.l. (“CIH”), formerly Celanese Caylux Holdings Luxembourg S.C.A., and Celanese US, have each agreed to provide the Purchaser with financing to strengthen the Purchaser’s ability to fulfill its obligations under, or in connection with, the Domination Agreement and to ensure that the Purchaser will perform all of its obligations under, or in connection with, the Domination Agreement when such obligations become due, including, without limitation, the obligation to compensate Celanese GmbH for any statutory annual loss incurred by Celanese GmbH during the term of the Domination Agreement. If CIHand/or Celanese US are obligated to make payments under such guarantees or other security to the Purchaser, we may not have sufficient funds for payments on our indebtedness when due. We have not had to compensate Celanese GmbH for an annual loss for any period during which the Domination Agreement has been in effect.
Purchases of Treasury Stock
In February 2008, our Board of Directors authorized the repurchase of up to $400 million of our Series A common stock. This authorization was increased to $500 million in October 2008. The authorization gives management discretion in determining the conditions under which shares may be repurchased. This repurchase program does not have an expiration date. During the year ended December 31, 2008, we repurchased 9,763,200 shares of our Series A common stock at an average purchase price of $38.68 per share for a total of approximately $378 million in connection with this authorization.
These purchases reduced the number of shares outstanding and the repurchased shares may be used by us for compensation programs utilizing our stock and other corporate purposes. We account for treasury stock using the cost method and include treasury stock as a component of Shareholders’ equity.
Plumbing Actions
We are involved in a number of legal proceedings and claims incidental to the normal conduct of our business. As of December 31, 2008 and 2007, there were reserves of $64 million and $65 million, respectively, related to plumbing action litigation. Although it is impossible at this time to determine with certainty the ultimate outcome of these matters, we believe, based on the advice of legal counsel, that adequate provisions have been made and that the
56
Table of Contents
ultimate outcome will not have a material adverse effect on our financial position, but could have a material adverse effect on our results of operations or cash flows in any given accounting period.
Off-Balance Sheet Arrangements
We have not entered into any material off-balance sheet arrangements.
Market Risks
Please see “Quantitative and Qualitative Disclosure about Market Risk” under Item 7A of thisForm 10-K for additional information about our Market Risks.
Critical Accounting Policies and Estimates
Our consolidated financial statements are based on the selection and application of significant accounting policies. The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues, expenses and allocated charges during the reporting period. Actual results could differ from those estimates. However, we are not currently aware of any reasonably likely events or circumstances that would result in materially different results.
We believe the following accounting polices and estimates are critical to understanding the financial reporting risks present in the current economic environment. These matters, and the judgments and uncertainties affecting them, are also essential to understanding our reported and future operating results. See Note 2 to the consolidated financial statements for a more comprehensive discussion of our significant accounting policies.
Recoverability of Long-Lived Assets
We assess the recoverability of long-lived assets, excluding goodwill and indefinite-lived intangible assets, whenever events or circumstances indicate that the carrying value of the long-lived asset may not be recoverable. Examples of a change in events or circumstances include, but are not limited to, a decrease in the market price of a long-lived asset, a history of cash flow losses related to the use of the long-lived asset or a significant adverse change in the extent or manner in which a long-lived asset is being used. To assess the recoverability of long-lived assets, excluding goodwill and indefinite-lived intangible assets, we compare the carrying amount of the long-lived asset or group of long-lived assets to the future net undiscounted cash flows expected to be generated by the long-lived asset or group of long-lived assets. If such long-lived assets are considered impaired, the impairment recognized is measured as the amount by which the carrying amount of the long-lived assets exceeds the fair value of the long-lived assets.
We assess the recoverability of the carrying value of our goodwill and other indefinite-lived intangible assets annually during the third quarter of our fiscal year using June 30 balances or whenever events or changes in circumstances indicate that the carrying amount of the asset may not be fully recoverable. Recoverability of goodwill and other indefinite-lived intangible assets is measured using a discounted cash flow model. We periodically engage a third-party valuation consultant to assist us with this process.
The development of future net undiscounted cash flow projections and the discounted cash flow projections require management projections related to sales and profitability trends, other future results of operations and discount rates. These projections, which require significant judgment, are consistent with projections we use to manage our operations internally. To the extent that changes in the current business environment result in adjusted management projections, impairment losses may occur in future periods.
Income Taxes
We regularly review our deferred tax assets for recoverability and establish a valuation allowance based on historical taxable income, projected future taxable income, applicable tax strategies, and the expected timing of the reversals of existing temporary differences. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Such evaluations require significant management
57
Table of Contents
judgments. Valuation allowances have been established primarily on net operating loss carryforwards and other deferred tax assets in the US, the United Kingdom and Canada.
We record accruals for income taxes and associated interest that may become payable in future years as a result of audits by tax authorities. We recognize tax benefits when it is more likely than not (likelihood of greater than 50%), based on technical merits, that the position will be sustained upon examination. Tax positions that meet the more-likely-than-not threshold are measured using a probability weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement. Whether the more-likely-than-not recognition threshold is met for a tax position is a matter of judgment based on the individual facts and circumstances of that position evaluated in light of all available evidence. The actual outcome of the future tax consequence could differ from our estimate due to changes in future circumstances and may have a material impact on our consolidated results of operations, financial position or cash flows.
Benefit Obligations
We have pension and other postretirement benefit plans covering substantially all employees who meet eligibility requirements. With respect to its US qualified defined benefit pension plan, minimum funding requirements are determined by the Employee Retirement Income Security Act based on years of serviceand/or compensation. Various assumptions are used in the calculation of the actuarial valuation of the employee benefit plans. These assumptions include the weighted average discount rate, compensation levels, expected long-term rates of return on plan assets and trends in health care costs. In addition to the above mentioned assumptions, actuarial consultants use subjective factors such as withdrawal and mortality rates to estimate the projected benefit obligation. The actuarial assumptions used may differ materially from actual results due to changing market and economic conditions, higher or lower withdrawal rates or longer or shorter life spans of participants. These differences may result in a significant impact to the amount of pension expense recorded in future periods.
The amounts recognized in the consolidated financial statements related to pension and other postretirement benefits are determined on an actuarial basis. A significant assumption used in determining our pension expense is the expected long-term rate of return on plan assets. As of December 31, 2008, we assumed an expected long-term rate of return on plan assets of 8.5% for the US qualified defined benefit pension plan, which represents approximately 83% and 78% of our pension plan assets and liabilities, respectively. On average, the actual return on these plan assets over the long-term (15 to 20 years) has exceeded 9.0%.
We estimate a 25 basis point decline in the expected long-term rate of return for the US qualified defined benefit pension plan to increase pension expense by an estimated $5 million in 2008. Another estimate that affects our pension and other postretirement benefit expense is the discount rate used in the annual actuarial valuations of pension and other postretirement benefit plan obligations. At the end of each year, we determine the appropriate discount rate, used to determine the present value of future cash flows currently expected to be required to settle the pension and other postretirement benefit obligations. The discount rate is generally based on the yield on high-quality corporate fixed-income securities. As of December 31, 2008, we increased the discount rate to 6.50% from 6.30% as of December 31, 2007 for the US plans. We estimate that a 50 basis point decline in our discount rate will decrease our annual pension expenses by an estimated $1 million, and increase our benefit obligations by approximately $144 million for our US pension plan. In addition, the same basis point decline in our discount rate will also increase our annual expenses and benefit obligations by less than $1 million and $8 million respectively, for our US postretirement medical plans. We estimate that a 50 basis point decline in the discount rate for the non-US pension and postretirement medical plans will increase pension and other postretirement benefit annual expenses by approximately $1 million and less than $1 million, respectively, and will increase our benefit obligations by approximately $30 million and $2 million, respectively.
Other postretirement benefit plans provide medical and life insurance benefits to retirees who meet minimum age and service requirements. The key determinants of the accumulated postretirement benefit obligation (“APBO”) are the discount rate and the healthcare cost trend rate. The healthcare cost trend rate has a significant effect on the reported amounts of APBO and related expense. For example, increasing or decreasing the healthcare cost trend rate by one percentage point in each year would result in the APBO as of December 31, 2008, and the 2008 postretirement benefit cost to change by approximately $3 million and $(3) million, respectively. See Note 15 to the consolidated financial statements for additional information.
58
Table of Contents
Accounting for Commitments and Contingencies
We are subject to a number of legal proceedings, lawsuits, claims, and investigations, incidental to the normal conduct of our business, relating to and including product liability, patent and intellectual property, commercial, contract, antitrust, past waste disposal practices, release of chemicals into the environment and employment matters, which are handled and defended in the ordinary course of business. We routinely assess the likelihood of any adverse judgments or outcomes to these matters as well as ranges of probable and reasonably estimable losses. Reasonable estimates involve judgments made by us after considering a broad range of information including: notifications, demands, settlements which have been received from a regulatory authority or private party, estimates performed by independent consultants and outside counsel, available facts, identification of other potentially responsible parties and their ability to contribute, as well as prior experience. With respect to environmental liabilities, it is our policy to accrue through fifteen years, unless we have government orders or other agreements that extend beyond fifteen years. A determination of the amount of loss contingency required, if any, is assessed in accordance with FASB Statement of Financial Accounting Standards No. 5,Contingencies and Commitments,and recorded if probable and estimable after careful analysis of each individual matter. The required reserves may change in the future due to new developments in each matter and as additional information becomes available.
Financial Reporting Changes
See Note 3 to the consolidated financial statements for information regarding recent accounting pronouncements.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Market Risks
Our financial market risk consists principally of exposure to currency exchange rates, interest rates and commodity prices. Exchange rate and interest rate risks are managed with a variety of techniques, including use of derivatives. We have in place policies of hedging against changes in currency exchange rates, interest rates and commodity prices as described below. Contracts to hedge exposures are primarily accounted for under Statement of Financial Accounting Standards (“SFAS”) No. 133,Accounting for Derivative Instruments and Hedging Activities(“SFAS No. 133”) amended by SFAS No. 138,Accounting for Certain Derivative Instruments and Certain Hedging Activitiesand SFAS No. 148,Amendment of Statement 133 on Derivative Instruments and Hedging Activities.
Interest Rate Risk Management
We use interest rate swap agreements to manage the interest rate risk of our total debt portfolio and related overall cost of borrowing. To reduce the interest rate risk inherent in our variable rate debt, we utilize interest rate swap agreements to convert a portion of our variable rate debt to a fixed rate obligation. These interest rate swap agreements are designated as cash flow hedges.
In March 2007, in anticipation of the April 2007 debt refinancing, we entered into various US dollar and Euro interest rate swap agreements, which became effective on April 2, 2007, with notional amounts of $1.6 billion and €150 million, respectively. The notional amount of the $1.6 billion US dollar interest rate swaps decreased by $400 million effective January 2, 2008 and decreased by another $200 million effective January 2, 2009. To offset the declines, we entered into US dollar interest rate swaps with a combined notional amount of $400 million which became effective on January 2, 2008 and an additional US dollar interest rate swap with a notional amount of $200 million which will become effective April 2, 2009.
As of December 31, 2008, we had $2.3 billion, €454 million and CNY 1.5 billion of variable rate debt, of which $1.6 billion and €150 million is hedged with interest rate swaps, which leaves $674 million, €304 million and CNY 1.5 billion of variable rate debt subject to interest rate exposure. Accordingly, a 1% increase in interest rates would increase annual interest expense by approximately $13 million.
See Note 22 to the consolidated financial statements for further discussion of our interest rate risk management and the related impact on our financial position and results of operations.
59
Table of Contents
Foreign Exchange Risk Management
The primary business objective of this hedging program is to maintain an approximately balanced position in foreign currencies so that exchange gains and losses resulting from exchange rate changes, net of related tax effects, are minimized. It is our policy to minimize currency exposures and to conduct operations either within functional currencies or using the protection of hedge strategies. Accordingly, we enter into foreign currency forwards and swaps to minimize our exposure to foreign currency fluctuations. From time to time we may also hedge our currency exposure related to forecasted transactions. Forward contracts are not designated as hedges under SFAS No. 133.
The following table indicates the total US dollar equivalents of net foreign exchange exposure related to (short) long foreign exchange forward contracts outstanding by currency. All of the contracts included in the table below will have approximately offsetting effects from actual underlying payables, receivables, intercompany loans or other assets or liabilities subject to foreign exchange remeasurement.
| 2009 Maturity | ||||
| (In $ millions) | ||||
Currency | ||||
| Euro | 145 | |||
| British pound sterling | (115 | ) | ||
| Mexican peso | 80 | |||
| Singapore dollar | 26 | |||
| Canadian dollar | 21 | |||
| Japanese yen | 9 | |||
| Brazilian real | (7 | ) | ||
| Swedish krona | 6 | |||
| Hungarian forint | (5 | ) | ||
| Other | (3 | ) | ||
| Total | 157 | |||
Additionally, a portion of our assets, liabilities, revenues and expenses are denominated in currencies other than the US dollar, principally the Euro. Fluctuations in the value of these currencies against the US dollar, particularly the value of the Euro, can have a direct and material impact on the business and financial results. For example, a decline in the value of the Euro versus the US dollar results in a decline in the US dollar value of our sales and earnings denominated in Euros due to translation effects. Likewise, an increase in the value of the Euro versus the US dollar would result in an opposite effect.
To protect the foreign currency exposure of a net investment in a foreign operation, we entered into cross currency swaps with certain financial institutions in 2004. The cross currency swaps and the Euro-denominated portion of the senior term loan were designated as a hedge of a net investment of a foreign operation. We dedesignated the net investment hedge due to the debt refinancing in April 2007 and redesignated the cross currency swaps and new senior EURO term loan in July 2007. As a result, we recorded $26 million ofmark-to-market losses related to the cross currency swaps and the new senior Euro term loan during this period.
Under the terms of the cross currency swap arrangements, we paid approximately €13 million in interest and received approximately $16 million in interest on June 15 and December 15 of each year. The fair value of the net obligation under the cross currency swaps was included in current Other liabilities in the consolidated balance sheets as of December 31, 2007. Upon maturity of the cross currency swap arrangements in June 2008, we owed €276 million ($426 million) and were owed $333 million. In settlement of the obligation, we paid $93 million (net of interest of $3 million) in June 2008.
During the year ended December 31, 2008, we dedesignated €385 million of the €400 million euro-denominated portion of the term loan, previously designated as a hedge of a net investment of a foreign operation. Prior to this dedesignation, we had been using external derivative contracts to offset foreign currency exposures on
60
Table of Contents
intercompany loans. The foreign currency exposure resulting from dedesignation of €385 million of the hedge of a net investment of a foreign operation is expected to offset the foreign currency exposure on certain intercompany loans, decreasing the need for external derivative contracts and reducing our exposure to external counterparties. The remaining €15 million euro-denominated portion of the term loan continues to be designated as a hedge of a net investment of a foreign operation.
See Note 22 to the consolidated financial statements for further discussion of our foreign exchange risk management and the related impact on our financial position and results of operations.
Commodity Risk Management
We have exposure to the prices of commodities in its procurement of certain raw materials. We manage its exposure primarily through the use of long-term supply agreements and derivative instruments. We regularly assess our practice of purchasing a portion of its commodity requirements forward and utilization of other raw material hedging instruments, in addition to forward purchase contracts, in accordance with changes in market conditions. Forward purchases and swap contracts for raw materials are principally settled through actual delivery of the physical commodity. For qualifying contracts, we have elected to apply the normal purchases and normal sales exception of SFAS No. 133, as amended, as it was probable at the inception and throughout the term of the contract that they would not settle net and would result in physical delivery. As such, realized gains and losses on these contracts are included in the cost of the commodity upon the settlement of the contract.
In addition, we occasionally enter into financial derivatives to hedge a component of a raw material or energy source. Typically, these types of transactions do not qualify for hedge accounting. These instruments are marked to market at each reporting period and gains (losses) are included in Cost of sales in the consolidated statements of operations. We recognized no gain or loss from these types of contracts during the year ended December 31, 2008 and less than $1 million during each of the years ended December 31, 2007 and 2006, respectively. As of December 31, 2008, we did not have any open financial derivative contracts for commodities.
| Item 8. | Financial Statements and Supplementary Data |
Our consolidated financial statements and supplementary data are included in pages F-2 through of this Annual Report onForm 10-K. See accompanying Item 15. Exhibits and Financial Statement Schedules and Index to the consolidated financial statements onpage F-1.
61
Table of Contents
Quarterly Financial Information
CELANESE CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| Three Months Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| 2008 | 2008 | 2008 | 2008 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| (In $ millions, except per share data) | ||||||||||||||||
| Net sales | 1,846 | 1,868 | 1,823 | 1,286 | ||||||||||||
| Other (charges) gains, net | (16 | ) | (7 | ) | (1 | ) | (84 | ) | ||||||||
| Operating profit (loss) | 234 | 207 | 151 | (152 | ) | |||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 218 | 247 | 152 | (183 | ) | |||||||||||
| Earnings (loss) from continuing operations | 145 | 203 | 164 | (140 | ) | |||||||||||
| Earnings (loss) from discontinued operations | — | (69 | ) | (6 | ) | (15 | ) | |||||||||
| Net earnings (loss) | 145 | 134 | 158 | (155 | ) | |||||||||||
| Earnings (loss) per share — basic | 0.93 | 0.87 | 1.05 | (1.09 | ) | |||||||||||
| Earnings (loss) per share — diluted | 0.87 | 0.80 | 0.97 | (1.09 | ) | |||||||||||
| Three Months Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| 2007 | 2007 | 2007 | 2007 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| (In $ millions, except per share data) | ||||||||||||||||
| Net sales | 1,555 | 1,556 | 1,573 | 1,760 | ||||||||||||
| Other (charges) gains, net | (1 | ) | (105 | ) | (12 | ) | 60 | |||||||||
| Operating profit | 206 | 71 | 147 | 324 | ||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 171 | (168 | ) | 131 | 313 | |||||||||||
| Earnings (loss) from continuing operations | 122 | (124 | ) | 130 | 208 | |||||||||||
| Earnings (loss) from discontinued operations | 79 | 7 | (2 | ) | 6 | |||||||||||
| Net earnings (loss) | 201 | (117 | ) | 128 | 214 | |||||||||||
| Earnings (loss) per share — basic | 1.25 | (0.76 | ) | 0.84 | 1.39 | |||||||||||
| Earnings (loss) per share — diluted | 1.15 | (0.76 | ) | 0.76 | 1.27 | |||||||||||
For a discussion of material events affecting performance in each quarter, see Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations. All amounts in the table above have been properly adjusted for the effects of discontinued operations.
62
Table of Contents
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Under the supervision and with the participation of our management, including the Chief Executive Officer and Chief Financial Officer, we have evaluated the effectiveness of our disclosure controls and procedures pursuant to Exchange ActRule 13a-15(b) as of the end of the period covered by this report. Based on that evaluation, as of December 31, 2008, the Chief Executive Officer and Chief Financial Officer have concluded that these disclosure controls and procedures are effective.
Changes in Internal Control Over Financial Reporting
None.
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal controls over financial reporting for the Company. Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our consolidated financial statements; providing reasonable assurance that receipts and expenditures of company assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on our consolidated financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our consolidated financial statements would be prevented or detected.
Management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of December 31, 2008. KPMG LLP has audited this assessment of our internal control over financial reporting; their report is included below.
63
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Celanese Corporation:
We have audited Celanese Corporation and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying report of management on internal control over financial reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Celanese Corporation and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Celanese Corporation and subsidiaries as of December 31, 2008 and 2007, and the related consolidated statements of operations, shareholders’ equity and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2008, and our report dated February 12, 2009 expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Dallas, Texas
February 12, 2009
64
Table of Contents
| Item 9B. | Other Information |
None.
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this Item 10 is incorporated herein by reference from the section captioned “Corporate Governance”, “Our Management Team,” and “Section 16(a) Beneficial Ownership Reporting Compliance” of the Company’s definitive proxy statement for the 2009 annual meeting of stockholders to be filed not later than March 23, 2009 with the Securities and Exchange Commission pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended (the “2009 Proxy Statement”).
| Item 11. | Executive Compensation |
The information required by this Item 11 is incorporated by reference from the section captioned “Executive Compensation” of the 2009 Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this Item 12 is incorporated by reference from the section captioned “Stock Ownership Information” of the 2009 Proxy Statement. The information required by Item 201(d) ofRegulation S-K is submitted in a separate section of thisForm 10-K. See Item 5 —Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities, above.
| Item 13. | Certain Relationships and Related Transactions and Director Independence |
The information required by this Item 13 is incorporated by reference from the section captioned “Certain Relationships and Related Party Transactions” of the 2009 Proxy Statement.
| Item 14. | Principal Accounting Fees and Services |
The information required by this Item 14 is incorporated by reference from the section captioned “Ratification of Independent Auditors — Audit and Related Fees” of the 2009 Proxy Statement.
PART IV
| Item 15. | Exhibits and Financial Statement Schedule |
1. Financial Statements. The reports of our independent registered public accounting firm and our consolidated financial statements are listed below and begin onpage F-1 of this Annual Report onForm 10-K.
| Page Number | ||
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Consolidated Statements of Operations | F-3 | |
| Consolidated Balance Sheets | F-4 | |
| Consolidated Statements of Shareholders’ Equity and Comprehensive Income (Loss) | F-5 | |
| Consolidated Statements of Cash Flows | F-6 | |
| Notes to Consolidated Financial Statements | F-7 |
2. Financial Statement Schedule.
The financial statement schedule required by this item is included as an Exhibit to this Annual Report onForm 10-K.
3. Exhibit List.
65
Table of Contents
See Index to Exhibits following our consolidated financial statements contained in this Annual Report onForm 10-K.
PLEASE NOTE: It is inappropriate for readers to assume the accuracy of, or rely upon any covenants, representations or warranties that may be contained in agreements or other documents filed as Exhibits to, or incorporated by reference in, this Annual Report. Any such covenants, representations or warranties may have been qualified or superseded by disclosures contained in separate schedules or exhibits not filed with or incorporated by reference in this Annual Report, may reflect the parties’ negotiated risk allocation in the particular transaction, may be qualified by materiality standards that differ from those applicable for securities law purposes, and may not be true as of the date of this Annual Report or any other date and may be subject to waivers by any or all of the parties. Where exhibits and schedules to agreements filed or incorporated by reference as Exhibits hereto are not included in these exhibits, such exhibits and schedules to agreements are not included or incorporated by reference herein.
66
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused the report to be signed on its behalf by the undersigned, thereunto duly authorized.
CELANESE CORPORATION
| By: | /s/ David N. Weidman |
Name: David N. Weidman
| Title: | Chairman of the Board of |
Directors and Chief Executive
Officer
Date: February 12, 2009
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Steven M. Sterin, his true and lawful attorney-in-fact with power of substitution and resubstitution to sign in his name, place and stead, in any and all capacities, to do any and all things and execute any and all instruments that such attorney may deem necessary or advisable under the Securities Exchange Act of 1934 and any rules, regulations and requirements of the US Securities and Exchange Commission in connection with the Annual Report onForm 10-K and any and all amendments hereto, as fully for all intents and purposes as he might or could do in person, and hereby ratifies and confirms said attorney-in-fact, acting alone, and his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||||
/s/ David N. Weidman David N. Weidman | Chairman of the Board of Directors, Chief Executive Officer (Principal Executive Officer) | February 12, 2009 | ||||
/s/ Steven M. Sterin Steven M. Sterin | Senior Vice President, Chief Financial Officer (Principal Financial Officer) | February 12, 2009 | ||||
/s/ Miguel A. Desdin Miguel A. Desdin | Vice President, Controller (Principal Accounting Officer) | February 12, 2009 | ||||
/s/ James E. Barlett James E. Barlett | Director | February 12, 2009 | ||||
/s/ David F. Hoffmeister David F. Hoffmeister | Director | February 12, 2009 | ||||
/s/ Martin G. McGuinn Martin G. McGuinn | Director | February 12, 2009 | ||||
67
Table of Contents
Signature | Title | Date | ||||
/s/ Paul H. O’Neill Paul H. O’Neill | Director | February 12, 2009 | ||||
/s/ Mark C. Rohr Mark C. Rohr | Director | February 12, 2009 | ||||
/s/ Daniel S. Sanders Daniel S. Sanders | Director | February 12, 2009 | ||||
/s/ Farah M. Walters Farah M. Walters | Director | February 12, 2009 | ||||
/s/ John K. Wulff John K. Wulff | Director | February 12, 2009 | ||||
68
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| ANNUAL CELANESE CORPORATION CONSOLIDATED FINANCIAL STATEMENTS | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-7 | ||||
| F-12 | ||||
| F-14 | ||||
| F-16 | ||||
| F-18 | ||||
| F-18 | ||||
| F-19 | ||||
| F-21 | ||||
| F-21 | ||||
| F-22 | ||||
| F-23 | ||||
| F-23 | ||||
| F-24 | ||||
| F-26 | ||||
| F-31 | ||||
| F-34 | ||||
| F-35 | ||||
| F-37 | ||||
| F-41 | ||||
| F-45 | ||||
| F-45 | ||||
| F-49 | ||||
| F-56 | ||||
| F-56 | ||||
| F-60 | ||||
| F-61 | ||||
| F-61 | ||||
| F-62 | ||||
| F-62 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Celanese Corporation:
We have audited the accompanying consolidated balance sheets of Celanese Corporation and subsidiaries (the “Company”) as of December 31, 2008 and 2007, and the related consolidated statements of operations, shareholders’ equity and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2008. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Celanese Corporation and subsidiaries as of December 31, 2008 and 2007, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2008, in conformity with US generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 12, 2009 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
As discussed in Note 22 to the consolidated financial statements, the Company adopted Statement of Financial Accounting Standards No. 157,Fair Value Measurements, during the year ended December 31, 2008.
As discussed in Note 19 to the consolidated financial statements, the Company adopted Financial Accounting Standards Board Interpretation No. 48,Accounting for Uncertainty in Income Taxes, during the year ended December 31, 2007.
As discussed in Note 15 to the consolidated financial statements, the Company adopted Statement of Financial Accounting Standards No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans, during the year ended December 31, 2006.
As discussed in Note 20 to the consolidated financial statements, the Company adopted Statement of Financial Accounting Standards No. 123 (revised 2004),Share-Based Payment, during the year ended December 31, 2006.
/s/ KPMG LLP
Dallas, Texas
February 12, 2009
F-2
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions, except for share and per share data) | ||||||||||||
| Net sales | 6,823 | 6,444 | 5,778 | |||||||||
| Cost of sales | (5,567 | ) | (4,999 | ) | (4,469 | ) | ||||||
| Gross profit | 1,256 | 1,445 | 1,309 | |||||||||
| Selling, general and administrative expenses | (540 | ) | (516 | ) | (536 | ) | ||||||
| Amortization of intangible assets (primarily customer relationships) | (76 | ) | (72 | ) | (66 | ) | ||||||
| Research and development expenses | (80 | ) | (73 | ) | (65 | ) | ||||||
| Other (charges) gains, net | (108 | ) | (58 | ) | (10 | ) | ||||||
| Foreign exchange gain (loss), net | (4 | ) | 2 | (3 | ) | |||||||
| Gain (loss) on disposition of businesses and assets, net | (8 | ) | 20 | (9 | ) | |||||||
| Operating profit | 440 | 748 | 620 | |||||||||
| Equity in net earnings of affiliates | 54 | 82 | 76 | |||||||||
| Interest expense | (261 | ) | (262 | ) | (293 | ) | ||||||
| Refinancing expenses | — | (256 | ) | (1 | ) | |||||||
| Interest income | 31 | 44 | 37 | |||||||||
| Dividend income — cost investments | 167 | 116 | 79 | |||||||||
| Other income (expense), net | 3 | (25 | ) | 8 | ||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 434 | 447 | 526 | |||||||||
| Income tax (provision) benefit | (63 | ) | (110 | ) | (203 | ) | ||||||
| Minority interests | 1 | (1 | ) | (4 | ) | |||||||
| Earnings (loss) from continuing operations | 372 | 336 | 319 | |||||||||
| Earnings (loss) from operation of discontinued operations | (120 | ) | 40 | 130 | ||||||||
| Gain (loss) on disposal of discontinued operations | 6 | 52 | 5 | |||||||||
| Income tax (provision) benefit, discontinued operations | 24 | (2 | ) | (48 | ) | |||||||
| Earnings (loss) from discontinued operations | (90 | ) | 90 | 87 | ||||||||
| Net earnings (loss) | 282 | 426 | 406 | |||||||||
| Cumulative preferred stock dividend | (10 | ) | (10 | ) | (10 | ) | ||||||
| Net earnings (loss) available to common shareholders | 272 | 416 | 396 | |||||||||
| Earnings (loss) per common share — basic: | ||||||||||||
| Continuing operations | 2.44 | 2.11 | 1.95 | |||||||||
| Discontinued operations | (0.61 | ) | 0.58 | 0.55 | ||||||||
| Net earnings (loss) — basic | 1.83 | 2.69 | 2.50 | |||||||||
| Earnings (loss) per common share — diluted: | ||||||||||||
| Continuing operations | 2.28 | 1.96 | 1.86 | |||||||||
| Discontinued operations | (0.55 | ) | 0.53 | 0.50 | ||||||||
| Net earnings (loss) — diluted | 1.73 | 2.49 | 2.36 | |||||||||
| Weighted average shares — basic | 148,350,273 | 154,475,020 | 158,597,424 | |||||||||
| Weighted average shares — diluted | 163,471,873 | 171,227,997 | 171,807,599 | |||||||||
See the accompanying notes to the consolidated financial statements.
F-3
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions, except | ||||||||
| share amounts) | ||||||||
ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | 676 | 825 | ||||||
| Trade receivables — third party and affiliates (net of allowance for doubtful accounts — 2008: $25; 2007: $18) | 631 | 1,009 | ||||||
| Non-trade receivables | 328 | 437 | ||||||
| Inventories | 577 | 636 | ||||||
| Deferred income taxes | 24 | 70 | ||||||
| Marketable securities, at fair value | 6 | 46 | ||||||
| Other assets | 42 | 40 | ||||||
| Total current assets | 2,284 | 3,063 | ||||||
| Investments in affiliates | 789 | 814 | ||||||
| Property, plant and equipment (net of accumulated depreciation — 2008: $1,141; 2007: $838) | 2,472 | 2,362 | ||||||
| Deferred income taxes | 27 | 10 | ||||||
| Marketable securities, at fair value | 94 | 209 | ||||||
| Other assets | 357 | 309 | ||||||
| Goodwill | 779 | 866 | ||||||
| Intangible assets, net | 364 | 425 | ||||||
| Total assets | 7,166 | 8,058 | ||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Short-term borrowings and current installments of long-term debt — third party and affiliates | 233 | 272 | ||||||
| Trade payables — third party and affiliates | 523 | 818 | ||||||
| Other liabilities | 574 | 888 | ||||||
| Deferred income taxes | 15 | 30 | ||||||
| Income taxes payable | 24 | 23 | ||||||
| Total current liabilities | 1,369 | 2,031 | ||||||
| Long-term debt | 3,300 | 3,284 | ||||||
| Deferred income taxes | 122 | 265 | ||||||
| Uncertain tax positions | 218 | 220 | ||||||
| Benefit obligations | 1,167 | 696 | ||||||
| Other liabilities | 806 | 495 | ||||||
| Minority interests | 2 | 5 | ||||||
| Commitments and contingencies | ||||||||
| Shareholders’ equity: | ||||||||
| Preferred stock, $0.01 par value, 100,000,000 shares authorized (2008 and 2007: 9,600,000 issued and outstanding) | — | — | ||||||
| Series A common stock, $0.0001 par value, 400,000,000 shares authorized (2008: 164,107,394 issued and 143,505,708 outstanding; 2007: 162,941,287 issued and 152,102,801 outstanding) | — | — | ||||||
| Series B common stock, $0.0001 par value, 100,000,000 shares authorized (2008 and 2007: 0 shares issued and outstanding) | — | — | ||||||
| Treasury stock, at cost — (2008: 20,601,686 shares; 2007: 10,838,486 shares) | (781 | ) | (403 | ) | ||||
| Additional paid-in capital | 495 | 469 | ||||||
| Retained earnings | 1,047 | 799 | ||||||
| Accumulated other comprehensive income (loss), net | (579 | ) | 197 | |||||
| Total shareholders’ equity | 182 | 1,062 | ||||||
| Total liabilities and shareholders’ equity | 7,166 | 8,058 | ||||||
See the accompanying notes to the consolidated financial statements.
F-4
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| Shares | Shares | Shares | ||||||||||||||||||||||
| Outstanding | Amount | Outstanding | Amount | Outstanding | Amount | |||||||||||||||||||
| (In $ millions, except share data) | ||||||||||||||||||||||||
Preferred stock | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 9,600,000 | — | 9,600,000 | — | 9,600,000 | — | ||||||||||||||||||
| Issuance of preferred stock | — | — | — | — | — | — | ||||||||||||||||||
| Balance as of the end of the period | 9,600,000 | — | 9,600,000 | — | 9,600,000 | — | ||||||||||||||||||
Series A common stock | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 152,102,801 | — | 158,668,666 | — | 158,562,161 | — | ||||||||||||||||||
| Issuance of Series A common stock | — | — | 7,400 | — | — | — | ||||||||||||||||||
| Stock option exercises | 1,056,368 | — | 4,265,221 | — | 106,505 | — | ||||||||||||||||||
| Purchases of treasury stock | (9,763,200 | ) | — | (10,838,486 | ) | — | — | — | ||||||||||||||||
| Stock awards | 109,739 | — | — | — | — | — | ||||||||||||||||||
| Balance as of the end of the period | 143,505,708 | — | 152,102,801 | — | 158,668,666 | — | ||||||||||||||||||
Treasury stock | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 10,838,486 | (403 | ) | — | — | — | — | |||||||||||||||||
| Purchases of treasury stock, including related fees | 9,763,200 | (378 | ) | 10,838,486 | (403 | ) | — | — | ||||||||||||||||
| Balance as of the end of the period | 20,601,686 | (781 | ) | 10,838,486 | (403 | ) | — | — | ||||||||||||||||
Additional paid-in capital | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 469 | 362 | 337 | |||||||||||||||||||||
| Indemnification of demerger liability | 2 | �� | 4 | 3 | ||||||||||||||||||||
| Stock-based compensation, net of tax | 15 | 15 | 20 | |||||||||||||||||||||
| Stock option exercises, net of tax | 10 | 88 | 2 | |||||||||||||||||||||
| Restricted stock unit withholding | (1 | ) | — | — | ||||||||||||||||||||
| Balance as of the end of the period | 495 | 469 | 362 | |||||||||||||||||||||
Retained earnings | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 799 | 394 | 24 | |||||||||||||||||||||
| Net earnings (loss) | 282 | 426 | 406 | |||||||||||||||||||||
| Series A common stock dividends | (24 | ) | (25 | ) | (26 | ) | ||||||||||||||||||
| Preferred stock dividends | (10 | ) | (10 | ) | (10 | ) | ||||||||||||||||||
| Adoption of FIN 48 (Note 19) | — | 14 | — | |||||||||||||||||||||
| Balance as of the end of the period | 1,047 | 799 | 394 | |||||||||||||||||||||
Accumulated other comprehensive income (loss), net | ||||||||||||||||||||||||
| Balance as of the beginning of the period | 197 | 31 | (126 | ) | ||||||||||||||||||||
| Unrealized gain (loss) on securities | (23 | ) | 17 | 13 | ||||||||||||||||||||
| Foreign currency translation | (130 | ) | 70 | 5 | ||||||||||||||||||||
| Unrealized gain (loss) on interest rate swaps | (79 | ) | (41 | ) | 2 | |||||||||||||||||||
| Pension and postretirement benefits | (544 | ) | 120 | 269 | ||||||||||||||||||||
| Adjustment to initially apply FASB Statement No. 158, net of tax | — | — | (132 | ) | ||||||||||||||||||||
| Balance as of the end of the period | (579 | ) | 197 | 31 | ||||||||||||||||||||
Total shareholders’ equity | 182 | 1,062 | 787 | |||||||||||||||||||||
Comprehensive income (loss) | ||||||||||||||||||||||||
| Net earnings (loss) | 282 | 426 | 406 | |||||||||||||||||||||
| Other comprehensive income (loss), net of tax: | ||||||||||||||||||||||||
| Unrealized gain (loss) on securities | (23 | ) | 17 | 13 | ||||||||||||||||||||
| Foreign currency translation | (130 | ) | 70 | 5 | ||||||||||||||||||||
| Unrealized gain (loss) on interest rate swaps | (79 | ) | (41 | ) | 2 | |||||||||||||||||||
| Pension and postretirement benefits | (544 | ) | 120 | 269 | ||||||||||||||||||||
| Total comprehensive income (loss), net of tax | (494 | ) | 592 | 695 | ||||||||||||||||||||
See the accompanying notes to the consolidated financial statements.
F-5
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
Operating activities | ||||||||||||
| Net earnings (loss) | 282 | 426 | 406 | |||||||||
| Adjustments to reconcile net earnings (loss) to net cash provided by operating activities: | ||||||||||||
| Other (charges) gains, net of amounts used | 111 | 30 | (34 | ) | ||||||||
| Depreciation, amortization and accretion | 360 | 311 | 323 | |||||||||
| Deferred income taxes, net | (69 | ) | 23 | 125 | ||||||||
| (Gain) loss on disposition of businesses and assets, net | 1 | (74 | ) | (8 | ) | |||||||
| Refinancing expense | — | 256 | — | |||||||||
| Other, net | 35 | (1 | ) | 61 | ||||||||
| Operating cash provided by (used in) discontinued operations | 3 | (84 | ) | 10 | ||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Trade receivables — third party and affiliates, net | 339 | (69 | ) | (9 | ) | |||||||
| Inventories | 21 | (27 | ) | 19 | ||||||||
| Other assets | 53 | 66 | (5 | ) | ||||||||
| Trade payables — third party and affiliates | (265 | ) | (11 | ) | (22 | ) | ||||||
| Other liabilities | (285 | ) | (280 | ) | (115 | ) | ||||||
| Net cash provided by operating activities | 586 | 566 | 751 | |||||||||
Investing activities | ||||||||||||
| Capital expenditures on property, plant and equipment | (274 | ) | (288 | ) | (244 | ) | ||||||
| Acquisitions and related fees, net of cash acquired | — | (269 | ) | — | ||||||||
| Proceeds from sale of businesses and assets, net | 9 | 715 | 23 | |||||||||
| Proceeds from Ticona Kelsterbach plant relocation | 311 | — | 26 | |||||||||
| Capital expenditures related to Ticona Kelsterbach plant relocation | (185 | ) | (21 | ) | — | |||||||
| Proceeds from sale of marketable securities | 202 | 69 | 95 | |||||||||
| Purchases of marketable securities | (91 | ) | (59 | ) | (65 | ) | ||||||
| Changes in restricted cash | — | 46 | (42 | ) | ||||||||
| Settlement of cross currency swap agreements | (93 | ) | — | — | ||||||||
| Investing cash provided by (used in) discontinued operations | — | — | (18 | ) | ||||||||
| Other, net | (80 | ) | (50 | ) | (43 | ) | ||||||
| Net cash provided by (used in) investing activities | (201 | ) | 143 | (268 | ) | |||||||
Financing activities | ||||||||||||
| Short-term borrowings (repayments), net | (64 | ) | 30 | 13 | ||||||||
| Proceeds from long-term debt | 13 | 2,904 | 38 | |||||||||
| Repayments of long-term debt | (47 | ) | (3,053 | ) | (125 | ) | ||||||
| Refinancing costs | — | (240 | ) | — | ||||||||
| Purchases of treasury stock, including related fees | (378 | ) | (403 | ) | — | |||||||
| Stock option exercises | 18 | 69 | 2 | |||||||||
| Series A common stock dividends | (24 | ) | (25 | ) | (26 | ) | ||||||
| Preferred stock dividends | (10 | ) | (10 | ) | (10 | ) | ||||||
| Other, net | (7 | ) | 14 | — | ||||||||
| Net cash used in financing activities | (499 | ) | (714 | ) | (108 | ) | ||||||
| Exchange rate effects on cash and cash equivalents | (35 | ) | 39 | 26 | ||||||||
| Net increase (decrease) in cash and cash equivalents | (149 | ) | 34 | 401 | ||||||||
| Cash and cash equivalents at beginning of period | 825 | 791 | 390 | |||||||||
| Cash and cash equivalents at end of period | 676 | 825 | 791 | |||||||||
See the accompanying notes to the consolidated financial statements.
F-6
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
| 1. | Description of the Company |
Celanese Corporation and its subsidiaries (collectively the “Company”) is a leading global integrated chemical and advanced materials company. The Company’s business involves processing chemical raw materials, such as methanol, carbon monoxide and ethylene, and natural products, including wood pulp, into value-added chemicals, thermoplastic polymers and other chemical-based products.
Definitions
In this Annual Report onForm 10-K, the term “Celanese” refers to Celanese Corporation, a Delaware corporation, and not its subsidiaries. The term “Celanese US” refers to the Company’s subsidiary, Celanese US Holdings LLC, a Delaware limited liability company, formerly known as BCP Crystal US Holdings Corp., a Delaware corporation, and not its subsidiaries. The term “Purchaser” refers to the Company’s subsidiary, Celanese Europe Holding GmbH & Co. KG, formerly known as BCP Crystal Acquisition GmbH & Co. KG, a German limited partnership, and not its subsidiaries, except where otherwise indicated. The term “Original Shareholders” refers, collectively, to Blackstone Capital Partners (Cayman) Ltd. 1, Blackstone Capital Partners (Cayman) Ltd. 2, Blackstone Capital Partners (Cayman) Ltd. 3 and BA Capital Investors Sidecar Fund, L.P. The term “Advisor” refers to Blackstone Management Partners, an affiliate of The Blackstone Group.
Basis of Presentation
The consolidated financial statements contained in this Annual Report were prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) for all periods presented. The consolidated financial statements and other financial information included in this Annual Report, unless otherwise specified, have been presented to separately show the effects of discontinued operations.
| 2. | Summary of Accounting Policies |
• Consolidation principles
The consolidated financial statements have been prepared in accordance with US GAAP for all periods presented and include the accounts of the Company and its majority owned subsidiaries over which the Company exercises control. All significant intercompany accounts and transactions have been eliminated in consolidation.
• Estimates and assumptions
The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues, expenses and allocated charges during the reporting period. Significant estimates pertain to impairments of goodwill, intangible assets and other long-lived assets, purchase price allocations, restructuring costs and other (charges) gains, net, income taxes, pension and other postretirement benefits, asset retirement obligations, environmental liabilities and loss contingencies, among others. Actual results could differ from those estimates.
• Cash and cash equivalents
All highly liquid investments with original maturities of three months or less are considered cash equivalents.
• Inventories
Inventories, including stores and supplies, are stated at the lower of cost or market. Cost for inventories is determined using thefirst-in, first-out (“FIFO”) method. Cost includes raw materials, direct labor and manufacturing overhead. Cost for stores and supplies is primarily determined by the average cost method.
• Investments in marketable securities
The Company classifies its investments in debt and equity securities as “available-for-sale” and reports those investments at their fair market values in the consolidated balance sheets as Marketable Securities, at fair value.
F-7
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Unrealized gains or losses, net of the related tax effect on available-for-sale securities, are excluded from earnings and are reported as a component of Accumulated other comprehensive income (loss), net until realized. The cost of securities sold is determined by using the specific identification method.
A decline in the market value of any available-for-sale security below cost that is deemed to be other-than-temporary results in a reduction in the carrying amount to fair value. The impairment is charged to earnings and a new cost basis for the security is established. To determine whether impairment is other-than-temporary, the Company considers whether it has the ability and intent to hold the investment until a market price recovery and evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. Evidence considered in this assessment includes the reasons for the impairment, the severity and duration of the impairment, changes in value subsequent to year end and forecasted performance of the investee.
• Investments in affiliates
Accounting Principles Board (“APB”) Opinion No. 18,The Equity Method of Accounting for Investments in Common Stock, stipulates that the equity method should be used to account for investments whereby an investor has “the ability to exercise significant influence over operating and financial policies of an investee”, but does not exercise control. APB Opinion No. 18 generally considers an investor to have the ability to exercise significant influence when it owns 20% or more of the voting stock of an investee. Financial Accounting Standards Board (“FASB”) Interpretation No. 35,Criteria for Applying the Equity Method of Accounting for Investments in Common Stock, which was issued to clarify the criteria for applying the equity method of accounting to 50% or less owned companies, lists circumstances under which, despite 20% ownership, an investor may not be able to exercise significant influence. Certain investments where the Company owns greater than a 20% ownership and cannot exercise significant influence or control are accounted for under the cost method (Note 8).
The Company assesses the recoverability of the carrying value of its investments whenever events or changes in circumstances indicate a loss in value that is other than a temporary decline. A loss in value of an equity-method or cost-method investment which is other than a temporary decline will be recognized as the difference between the carrying amount of the investment and its fair value.
The Company’s estimates of fair value are determined based on a discounted cash flow model. The Company periodically engages third-party valuation consultants to assist with this process.
• Property, plant and equipment, net
Land is recorded at historical cost. Buildings, machinery and equipment, including capitalized interest, and property under capital lease agreements, are recorded at cost less accumulated depreciation. Depreciation is calculated on a straight-line basis over the following estimated useful lives of depreciable assets:
| Land Improvements | 20 years | |||
| Buildings and Building Improvements | 30 years | |||
| Machinery and Equipment | 20 years |
Leasehold improvements are amortized over ten years or the remaining life of the respective lease, whichever is shorter.
Accelerated depreciation is recorded when the estimated useful life is shortened. Ordinary repair and maintenance costs, including costs for planned maintenance turnarounds, that do not extend the useful life of the asset are charged to earnings as incurred. Fully depreciated assets are retained in property and depreciation accounts until sold or otherwise disposed. In the case of disposals, assets and related depreciation are removed from the accounts, and the net amounts, less proceeds from disposal, are included in earnings.
The Company also leases property, plant and equipment under operating and capital leases. Rent expense for operating leases, which may have escalating rentals or rent holidays over the term of the lease, is recorded on a
F-8
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
straight-line basis over the lease term. Amortization of capital lease assets is included as a component of depreciation expense.
Assets acquired in business combinations are recorded at their fair values and depreciated over the assets’ remaining useful lives or the Company’s policy lives, whichever is shorter.
The Company assesses the recoverability of the carrying amount of its property, plant and equipment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. An impairment loss would be assessed when estimated undiscounted future cash flows from the operation and disposition of the asset group are less than the carrying amount of the asset group. Asset groups have identifiable cash flows and are largely independent of other asset groups. Measurement of an impairment loss is based on the excess of the carrying amount of the asset group over its fair value. Fair value is measured using discounted cash flows or independent appraisals, as appropriate. Impairment losses are recorded in depreciation expense or Other (charges) gains, net depending on the facts and circumstances.
• Goodwill and other intangible assets
Trademarks and trade names, customer-related intangible assets and other intangibles with finite lives are amortized on a straight-line basis over their estimated useful lives. The weighted average amortization period is 8 years. The excess of the purchase price over fair value of net identifiable assets and liabilities of an acquired business (“goodwill”) and other indefinite-lived intangible assets are not amortized, but rather tested for impairment, at least annually. The Company tests for goodwill and indefinite-lived intangible asset impairment during the third quarter of its fiscal year using June 30 balances.
The Company assesses the recoverability of the carrying value of goodwill at least annually or whenever events or changes in circumstances indicate that the carrying amount of the goodwill of a reporting unit may not be fully recoverable. Recoverability is measured at the reporting unit level based on the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 142,Goodwill and Other Intangible Assets. The Company’s estimates of fair value are determined based on a discounted cash flow model. The Company periodically engages third-party valuation consultants to assist with this process. Impairment losses are recorded in other operating expense or Other (charges) gains, net depending on the facts and circumstances.
The Company assesses recoverability of other indefinite-lived intangible assets at least annually or whenever events or changes in circumstances indicate that the carrying amount of the indefinite-lived intangible asset may not be fully recoverable. Recoverability is measured by a comparison of the carrying value of the indefinite-lived intangible asset over its fair value. Any excess of the carrying value of the indefinite-lived intangible asset over its fair value is recognized as an impairment loss. The Company’s estimates of fair value are determined based on a discounted cash flow model. The Company periodically engages third-party valuation consultants to assist with this process. Impairment losses are recorded in other operating expense or Other (charges) gains, net depending on the facts and circumstances.
The Company assesses the recoverability of finite-lived intangible assets in the same manner as for property, plant and equipment as described above. Impairment losses are recorded in amortization expense or Other (charges) gains, net depending on the facts and circumstances.
• Financial instruments
On January 1, 2008, the Company adopted the provisions of SFAS No. 157,Fair Value Measurements(“SFAS No. 157”) for financial assets and liabilities. On January 1, 2009, the Company applied the provisions of SFAS No. 157 for non-recurring fair value measurements of non-financial assets and liabilities, such as goodwill, indefinite-lived intangible assets, property, plant and equipment and asset retirement obligations. The adoption of SFAS No. 157 for non-recurring fair value measurements of non-financial assets and liabilities did not have a material impact on the Company’s financial position, results of operations or cash flows. SFAS No. 157 defines fair value, and increases disclosures surrounding fair value calculations.
F-9
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company manages its exposures to currency exchange rates, interest rates and commodity prices through a risk management program that includes the use of derivative financial instruments (Note 22). The Company does not use derivative financial instruments for speculative trading purposes. The fair value of all derivative instruments is recorded as assets or liabilities at the balance sheet date. Changes in the fair value of these instruments are reported in income or Accumulated other comprehensive income (loss), net, depending on the use of the derivative and whether it qualifies for hedge accounting treatment under the provisions of SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities, as amended (“SFAS No. 133”).
Gains and losses on derivative instruments qualifying as cash flow hedges are recorded in Accumulated other comprehensive income (loss), net, to the extent the hedges are effective, until the underlying transactions are recognized in income. To the extent effective, gains and losses on derivative and non-derivative instruments used as hedges of the Company’s net investment in foreign operations are recorded in Accumulated other comprehensive income (loss), net as part of the cumulative translation adjustment. The ineffective portions of cash flow hedges and hedges of net investment in foreign operations, if any, are recognized in income immediately. Derivative instruments not designated as hedges are marked to market at the end of each accounting period with the change in fair value recorded in income.
• Concentrations of credit risk
The Company is exposed to credit risk in the event of nonpayment by customers and counterparties. The creditworthiness of customers and counterparties is subject to continuing review, including the use of master netting agreements, where the Company deems appropriate. The Company minimizes concentrations of credit risk through its global orientation in diverse businesses with a large number of diverse customers and suppliers. In addition, credit risks arising from derivative instruments is not significant because the counterparties to these contracts are primarily major international financial institutions and, to a lesser extent, major chemical companies. Where appropriate, the Company has diversified its selection of counterparties. Generally, collateral is not required from customers and counterparties and allowances are provided for specific risks inherent in receivables.
• Deferred financing costs
The Company capitalizes direct costs incurred to obtain debt financings and amortizes these costs using a method that approximates the effective interest rate method over the terms of the related debt. Upon the extinguishment of the related debt, any unamortized capitalized debt financing costs are immediately expensed.
• Environmental liabilities
The Company manufactures and sells a diverse line of chemical products throughout the world. Accordingly, the Company’s operations are subject to various hazards incidental to the production of industrial chemicals including the use, handling, processing, storage and transportation of hazardous materials. The Company recognizes losses and accrues liabilities relating to environmental matters if available information indicates that it is probable that a liability has been incurred and the amount of loss can be reasonably estimated. Depending on the nature of the site, the Company accrues through fifteen years, unless the Company has government orders or other agreements that extend beyond fifteen years. If the event of loss is neither probable nor reasonably estimable, but is reasonably possible, the Company provides appropriate disclosure in the notes to the consolidated financial statements if the contingency is considered material. The Company estimates environmental liabilities on acase-by-case basis using the most current status of available facts, existing technology, presently enacted laws and regulations and prior experience in remediation of contaminated sites. Recoveries of environmental costs from other parties are recorded as assets when their receipt is deemed probable.
An environmental reserve related to cleanup of a contaminated site might include, for example, a provision for one or more of the following types of costs: site investigation and testing costs, cleanup costs, costs related to soil and water contamination resulting from tank ruptures and post-remediation monitoring costs. These reserves do not take into account any claims or recoveries from insurance. There are no pending insurance claims for any
F-10
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
environmental liability that are expected to be material. The measurement of environmental liabilities is based on the Company’s periodic estimate of what it will cost to perform each of the elements of the remediation effort. The Company utilizes third parties to assist in the management and development of cost estimates for its sites. Changes to environmental regulations or other factors affecting environmental liabilities are reflected in the consolidated financial statements in the period in which they occur (Note 16).
• Legal fees
The Company accrues for legal fees related to loss contingency matters when the costs associated with defending these matters can be reasonably estimated and are probable of occurring. All other legal fees are expensed as incurred.
• Revenue recognition
The Company recognizes revenue when title and risk of loss have been transferred to the customer, generally at the time of shipment of products, and provided that four basic criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured. Should changes in conditions cause the Company to determine revenue recognition criteria are not met for certain transactions, revenue recognition would be delayed until such time that the transactions become realizable and fully earned. Payments received in advance of meeting the above revenue recognition criteria are recorded as deferred revenue.
• Research and development
The costs of research and development are charged as an expense in the period in which they are incurred.
• Insurance loss reserves
The Company has two wholly owned insurance companies (the “Captives”) that are used as a form of self insurance for property, liability and workers compensation risks. One of the Captives also insures certain third-party risks. The liabilities recorded by the Captives relate to the estimated risk of loss which is based on management estimates and actuarial valuations, and unearned premiums, which represent the portion of the third-party premiums written applicable to the unexpired terms of the policies in-force. Liabilities are recognized for known claims when sufficient information has been developed to indicate involvement of a specific policy and the Company can reasonably estimate its liability. In addition, liabilities have been established to cover additional exposure on both known and unasserted claims. Estimates of the liabilities are reviewed and updated regularly. It is possible that actual results could differ significantly from the recorded liabilities. Premiums written are recognized as revenue based on the terms of the policies. Capitalization of the Captives is determined by regulatory guidelines.
• Reinsurance receivables
The Captives enter into reinsurance arrangements to reduce their risk of loss. The reinsurance arrangements do not relieve the Captives from their obligations to policyholders. Failure of the reinsurers to honor their obligations could result in losses to the Captives. The Captives evaluate the financial condition of their reinsurers and monitor concentrations of credit risk to minimize their exposure to significant losses from reinsurer insolvencies and to establish allowances for amounts deemed non-collectible.
• Income taxes
The provision for income taxes has been determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes and net operating loss and tax credit carry forwards. The amount of deferred taxes on these temporary differences is determined using the tax rates that are expected to apply to the period when the asset is
F-11
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
realized or the liability is settled, as applicable, based on tax rates and laws in the respective tax jurisdiction enacted as of the balance sheet date.
The Company reviews its deferred tax assets for recoverability and establishes a valuation allowance based on historical taxable income, projected future taxable income, applicable tax strategies, and the expected timing of the reversals of existing temporary differences. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company adopted the provisions of FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes — an interpretation of FASB Statement No. 109(“FIN 48”) on January 1, 2007. The Company considers many factors when evaluating and estimating its tax positions and tax benefits, which may require periodic adjustments and which may not accurately anticipate actual outcomes. Tax positions are recognized only when it is more likely than not (likelihood of greater than 50%), based on technical merits, that the positions will be sustained upon examination. Tax positions that meet the more-likely-than-not threshold are measured using a probability weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement. Whether the more-likely-than-not recognition threshold is met for a tax position is a matter of judgment based on the individual facts and circumstances of that position evaluated in light of all available evidence.
• Minority interests
Minority interests in the equity and results of operations of the entities consolidated by the Company are shown as a separate line item in the consolidated financial statements. The entities included in the consolidated financial statements that have minority interests are as follows:
| Ownership Percentage | ||||||||
| as of December 31, | ||||||||
| 2008 | 2007 | |||||||
| Celanese Polisinteza d.o.o. | 76 | % | 76 | % | ||||
| Synthesegasanlage Ruhr GmbH | 50 | % | 50 | % | ||||
The Company has a 60% voting interest and the right to appoint a majority of the board of management of Synthesegasanlage Ruhr GmbH, which results in the Company controlling this entity and, accordingly, the Company is consolidating this entity in its consolidated financial statements.
• Accounting for purchasing agent agreements
A subsidiary of the Company acts as a purchasing agent on behalf of the Company, as well as third parties. The entity arranges sale and purchase agreements for raw materials on a commission basis. Accordingly, the commissions earned on these third-party sales are classified as a reduction to Selling, general and administrative expenses.
• Functional and reporting currencies
For the Company’s international operations where the functional currency is other than the US dollar, assets and liabilities are translated using period-end exchange rates, while the statement of operations amounts are translated using the average exchange rates for the respective period. Differences arising from the translation of assets and liabilities in comparison with the translation of the previous periods or from initial recognition during the period are included as a separate component of Accumulated other comprehensive income (loss), net.
• Reclassifications
The Company has reclassified certain prior period amounts to conform to the current year presentation.
| 3. | Recent Accounting Pronouncements |
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements — an amendment of ARB No. 51(“SFAS No. 160”) to establish accounting and reporting standards for
F-12
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements and establishes a single method of accounting for changes in a parent’s ownership interest in a subsidiary that does not result in deconsolidation. The Company adopted SFAS No. 160 on January 1, 2009. This standard had no material impact on the Company’s financial position, results of operations or cash flows.
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations (“SFAS No. 141(R)”). SFAS No. 141(R) establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, the goodwill acquired and any non-controlling interest in the acquiree. This statement also establishes disclosure requirements to enable the evaluation of the nature of the financial effect of the business combination. SFAS No. 141(R) is effective for all business combinations for which the acquisition date is on or after the beginning of the first fiscal year after December 15, 2008, with the exception of the accounting for valuation allowances on deferred taxes and acquired tax contingencies. SFAS No. 141(R) amends SFAS No. 109,Accounting for Income Taxes, such that adjustments made to valuation allowances on deferred taxes and acquired tax contingencies associated with acquisitions that closed prior to the effective date of SFAS No. 141(R) would also apply the provisions of SFAS No. 141(R). The Company adopted SFAS No. 141(R) on January 1, 2009. Beginning in 2009, the Company will record adjustments to preacquisition tax contingencies as a component of income tax expense in the consolidated statement of operations.
In March 2008, the FASB issued SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities, an amendment of FASB Statement No. 133 (“SFAS No. 161”). SFAS No. 161 requires enhanced disclosures about a company’s derivative and hedging activities. These enhanced disclosures will discuss: (a) how and why a company uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under FASB Statement No. 133 and its related interpretations and (c) how derivative instruments and related hedged items affect a company’s financial position, results of operations and cash flows. The Company adopted SFAS No. 161 on January 1, 2009. This standard had no impact on the Company’s financial position, results of operations or cash flows.
In April 2008, the FASB issued FASB Staff Position (“FSP”)FAS No. 142-3,Determination of the Useful Life of Intangible Assets(“FSPFAS No. 142-3”). FSPFAS No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142,Goodwill and Other Intangible Assets. The intent of this FSP is to improve the consistency between the useful life of a recognized intangible asset and the period of expected cash flows used to measure the fair value of the asset under SFAS No. 141 (revised 2007),Business Combinations, and other US GAAP. The Company adopted FSPFAS No. 142-3 on January 1, 2009. This standard had no material impact on the Company’s financial position, results of operations or cash flows.
In May 2008, the FASB issued SFAS No. 162,The Hierarchy of Generally Accepted Accounting Principles, (“SFAS No. 162”), which became effective November 15, 2008. SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with US GAAP. This standard had no impact on the Company’s financial position, results of operations or cash flows.
In September 2008, the EITF issued EITFNo. 08-6,Equity Method Investment Accounting Considerations(“EITF 08-6”).EITF 08-6 addresses the effect of SFAS No. 141(R) and SFAS No. 160 on the equity method of accounting. The Company adoptedEITF 08-6 on January 1, 2009. This standard had no impact on the Company’s financial position, results of operations or cash flows.
In October 2008, the FASB issued FSP FASNo. 157-3,Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active(“FSP FASNo. 157-3”), to provide guidance on determining the fair value of
F-13
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
financial instruments in inactive markets. FSP FASNo. 157-3 became effective for the Company upon issuance. This standard had no impact on the Company’s financial position, results of operations or cash flows.
| 4. | Acquisitions, Ventures, Divestitures and Asset Sales |
Acquisitions
On January 31, 2007, the Company completed the acquisition of the cellulose acetate flake, tow and film business of Acetate Products Limited (“APL”), a subsidiary of Corsadi B.V. The purchase price for the transaction was approximately £57 million ($112 million), in addition to direct acquisition costs of approximately £4 million ($7 million). As contemplated prior to the closing of the acquisition, the Company closed the acquired tow production plant at Little Heath, United Kingdom in September 2007. In accordance with the Company’s sponsor services agreement dated January 26, 2005, as amended, the Company paid the Advisor $1 million in connection with the acquisition. The acquired business is included in the Company’s Consumer Specialties segment.
On April 6, 2004, the Company acquired 84% of Celanese GmbH. During 2005, the Company acquired an additional 14% of Celanese GmbH. On May 30, 2006, Celanese GmbH’s shareholders approved a transfer to the Purchaser of all shares owned by minority shareholders against payment of cash compensation in the amount of €66.99 per share (the “Squeeze-Out”). As a result of the effective registration of the Squeeze-Out in the commercial register in Germany in December 2006, the Company acquired the remaining 2% of Celanese GmbH in January 2007. The Company’s current ownership percentage in Celanese GmbH is 100%.
Ventures
In March 2007, the Company entered into a strategic partnership with Accsys Technologies PLC (“Accsys”), and its subsidiary, Titan Wood, to become the exclusive supplier of acetyl products to Titan Wood’s technology licensees for use in wood acetylation. In connection with this partnership, in May 2007, the Company acquired 8,115,883 shares of Accsys’ common stock representing approximately 5.45% of the total voting shares of Accsys for €22 million ($30 million). The investment was treated as an available-for-sale security and was included in Marketable securities on the Company’s consolidated balance sheets. On November 20, 2007, the Company and Accsys announced that they agreed to amend their business arrangements so that each company would have a nonexclusive “at-will” trading and supply relationship to give both companies greater flexibility. As part of this amendment, the Company subsequently sold all of its shares of Accsys stock for approximately €20 million ($30 million), which resulted in a cumulative loss of $3 million.
Divestitures
In July 2008, the Company sold its 55.46% interest in Derivados Macroquimicos S.A. de C.V. (“DEMACSA”) for proceeds of $3 million. DEMACSA produces cellulose ethers at an industrial complex in Zacapu, Michoacan, Mexico and is included in the Company’s Acetyl Intermediates segment. In June 2008, the Company recorded an impairment loss of $1 million to Cost of sales in the consolidated statements of operations. As a result, the proceeds from the sale approximated the carrying value of DEMACSA on the date of the sale. The Company concluded the sale of DEMACSA is not a discontinued operation due to certain forms of continuing involvement between the Company and DEMACSA subsequent to the sale.
In August 2007, the Company sold its Films business of AT Plastics, located in Edmonton and Westlock, Alberta, Canada, to British Polythene Industries PLC (“BPI”) for $12 million. The Films business manufactures products for the agricultural, horticultural and construction industries. The Company recorded a loss on the sale of $7 million during the year ended December 31, 2007. The Company maintained ownership of the Polymers business of AT Plastics, which concentrates on the development and supply of specialty resins and compounds. AT Plastics is included in the Company’s Industrial Specialties segment. The Company concluded that the sale of the Films business of AT Plastics is not a discontinued operation due to the level of continuing cash flows between the Films business and AT Plastics’ Polymers business subsequent to the sale. Under the terms of the purchase
F-14
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
agreement, the Company entered into a two year sales agreement to continue selling product to BPI through August 2009.
In December 2006, the Company sold its preferred interest in Pemeas GmbH, a venture formed in April 2004 to advance the commercialization of the Company’s fuel cell technology to BASF AG. The Company received net proceeds from the sale of €9 million and recognized a gain of €8 million ($11 million). This amount is included in Other income (expense), net in the consolidated statements of operations.
In connection with the Company’s strategy to optimize its portfolio and divest non-core operations, the Company announced on December 13, 2006 its agreement to sell its Acetyl Intermediates segment’s oxo products and derivatives businesses, including European Oxo GmbH (“EOXO”), a 50/50 venture between Celanese GmbH and Degussa AG (“Degussa”), to Advent International, for a purchase price of €480 million ($636 million) subject to final agreement adjustments and the successful exercise of the Company’s option to purchase Degussa’s 50% interest in EOXO. On February 23, 2007, the option was exercised and the Company acquired Degussa’s interest in the venture for a purchase price of €30 million ($39 million), in addition to €22 million ($29 million) paid to extinguish EOXO’s debt upon closing of the transaction. The Company completed the sale of its oxo products and derivatives businesses, including the acquired 50% interest in EOXO, on February 28, 2007. The sale included the oxo and derivatives businesses at the Oberhausen, Germany, and Bay City, Texas facilities as well as portions of its Bishop, Texas facility. Also included were EOXO’s facilities within the Oberhausen and Marl, Germany plants. The former oxo products and derivatives businesses acquired by Advent International was renamed Oxea. Taking into account agreed deductions by the buyer for pension and other employee benefits and various costs for separation activities, the Company received proceeds of approximately €443 million ($585 million) at closing. The transaction resulted in the recognition of a $47 million pre-tax gain, recorded to Gain (loss) on disposal of discontinued operations, which includes certain working capital and other adjustments, in 2007. Due to certain lease-back arrangements between the Company and the buyer and related environmental obligations of the Company, approximately $51 million of the transaction proceeds attributable to the fair value of the underlying land at Bay City ($1 million) and Oberhausen (€36 million) is included in deferred proceeds in noncurrent Other liabilities, and divested land with a book value of $14 million (€10 million at Oberhausen and $1 million at Bay City) remains in Property, plant and equipment, net in the Company’s consolidated balance sheets.
Subsequent to closing, the Company and Oxea have certain site service and product supply arrangements. The site services include, but are not limited to, administrative, utilities, health and safety, waste water treatment and maintenance activities for terms which range up to fifteen years. Product supply agreements contain initial terms of up to fifteen years. The Company has no contractual ability through these agreements or any other arrangements to significantly influence the operating or financial policies of Oxea. The Company concluded, based on the nature and limited projected magnitude of the continuing business relationship between the Company and Oxea, the divestiture of the oxo products and derivatives businesses should be accounted for as a discontinued operation.
Third-party net sales include $5 million and $35 million for the years ended December 31, 2007 and 2006, respectively, that would have been eliminated upon consolidation were the divestiture not accounted for as a discontinued operation. These amounts relate to sales from the continuing operations of the Company to the divested businesses.
In accordance with the Company’s sponsor services agreement dated January 26, 2005, as amended, the Company paid the Advisor $6 million in connection with the sale of the oxo products and derivatives businesses.
During the third quarter of 2006, the Company discontinued its pentaerythritol (“PE”) operations, which were included in the Acetyl Intermediates segment. During the second quarter of 2007, the Company discontinued its Edmonton, Alberta, Canada methanol operations, which were included in the Acetyl Intermediates segment. As a result, the earnings (loss) from operations related to the Edmonton methanol and PE operations are accounted for as discontinued operations.
F-15
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Asset Sales
In May 2008, shareholders of the Company’s Koper, Slovenia legal entity voted to approve the April 2008 decision by the Company to permanently shut down this emulsions production site. The decision to shut down the site resulted in employee severance of less than $1 million, which is included in Other (charges) gains, net, in the consolidated statements of operations during the year ended December 31, 2008. Currently, the facility is idle and the existing fixed assets, including machinery and equipment, buildings and land are being marketed for sale. The Koper, Slovenia legal entity is included in the Company’s Industrial Specialties segment.
In December 2007, the Company sold the assets at its Edmonton, Alberta, Canada facility to a real estate developer for approximately $35 million. As part of the agreement, the Company will retain certain environmental liabilities associated with the site. The Company derecognized $16 million of asset retirement obligations which were transferred to the buyer. As a result of the sale, the Company recorded a gain of $37 million for the year ended December 31, 2007, of which a gain of $34 million was recorded to Gain (loss) on disposition of businesses and assets, net in the consolidated statements of operation.
In July 2007, the Company reached an agreement with Babcock & Brown, a worldwide investment firm which specializes in real estate and utilities development, to sell the Company’s Pampa, Texas, facility. The Company ceased operations at the site in December 2008. Proceeds received upon certain milestone events will be treated as deferred proceeds and included in noncurrent Other liabilities in the Company’s consolidated balance sheets until the transaction is complete (expected to be in 2010), as defined in the sales agreement. These operations are included in the Company’s Acetyl Intermediates segment. During the second half of 2008, the Company determined that two of the milestone events, which are outside of the Company’s control, were unlikely to be achieved. The Company performed a discounted cash flow analysis which resulted in a $23 million long-lived asset impairment loss recorded to Other (charges) gains, net, in the consolidated statements of operations during the year ended December 31, 2008 (Note 18).
| 5. | Securities Available for Sale |
The Company’s captive insurance companies and pension-related trusts hold available-for-sale securities for capitalization and funding requirements, respectively. The Company recorded a net realized gain (loss) of $0 million, $1 million and $(1) million recorded to Other income (expense), net in the consolidated statements of operations for the years ended December 31, 2008, 2007 and 2006, respectively.
F-16
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The amortized cost, gross unrealized gain, gross unrealized loss and fair values for available-for-sale securities by major security type were as follows:
| Gross | Gross | |||||||||||||||
| Amortized | Unrealized | Unrealized | Fair | |||||||||||||
| Cost | Gain | Loss | Value | |||||||||||||
| (In $ millions) | ||||||||||||||||
As of December 31, 2008 | ||||||||||||||||
| Debt securities | ||||||||||||||||
| US government | 35 | 17 | — | 52 | ||||||||||||
| US corporate | 3 | — | — | 3 | ||||||||||||
| Total debt securities | 38 | 17 | — | 55 | ||||||||||||
| Equity securities | 55 | — | (13 | ) | 42 | |||||||||||
| Money market deposits and other securities | 3 | — | — | 3 | ||||||||||||
| Total | 96 | 17 | (13 | ) | 100 | |||||||||||
As of December 31, 2007 | ||||||||||||||||
| Debt securities | ||||||||||||||||
| US government | 67 | 5 | — | 72 | ||||||||||||
| US corporate | 33 | — | (1 | ) | 32 | |||||||||||
| Total debt securities | 100 | 5 | (1 | ) | 104 | |||||||||||
| Equity securities | 78 | 26 | — | 104 | ||||||||||||
| Money market deposits and other securities | 1 | — | — | 1 | ||||||||||||
| Mortgage-backed securities | 46 | — | — | 46 | ||||||||||||
| Total | 225 | 31 | (1 | ) | 255 | |||||||||||
Fixed maturities as of December 31, 2008 by contractual maturity are shown below. Actual maturities could differ from contractual maturities because borrowers may have the right to call or prepay obligations, with or without call or prepayment penalties.
| Amortized | Fair | |||||||
| Cost | Value | |||||||
| (In $ millions) | ||||||||
| Within one year | 6 | 6 | ||||||
| From one to five years | — | — | ||||||
| From six to ten years | — | — | ||||||
| Greater than ten years | 35 | 52 | ||||||
| Total | 41 | 58 | ||||||
Proceeds received from fixed maturities that mature within one year are expected to be reinvested into additional securities upon such maturity.
F-17
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 6. | Receivables, Net |
| As of | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Trade receivables — third party and affiliates | 656 | 1,027 | ||||||
| Allowance for doubtful accounts — third party and affiliates | (25 | ) | (18 | ) | ||||
| Trade receivables — third party and affiliates, net | 631 | 1,009 | ||||||
| Non-trade receivables: | ||||||||
| Reinsurance receivables | 33 | 35 | ||||||
| Income taxes receivable | 88 | 73 | ||||||
| Derivatives | 54 | 29 | ||||||
| Other | 153 | 300 | ||||||
| Receivables, net | 959 | 1,446 | ||||||
As of December 31, 2008 and 2007, the Company had no significant concentrations of credit risk since the Company’s customer base is dispersed across many different industries and geographies.
| 7. | Inventories |
| As of | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Finished goods | 434 | 500 | ||||||
| Work-in-process | 24 | 29 | ||||||
| Raw materials and supplies | 119 | 107 | ||||||
| Inventories | 577 | 636 | ||||||
During December 2008, the Company recorded a $14 million charge to reduce its inventories to the lower-of-cost or market.
F-18
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 8. | Investments |
Equity Method
The Company’s equity investments and ownership interests are as follows:
| Share of Earnings (Loss) | ||||||||||||||||||||||||||||||
| Ownership Percentage | Carrying Value | Year Ended | ||||||||||||||||||||||||||||
| as of December 31, | as of December 31, | December 31, | ||||||||||||||||||||||||||||
| Segment | 2008 | 2007 | 2008 | 2007 | 2008 | 2007 | 2006 | |||||||||||||||||||||||
| (In percentages) | (In $ millions) | |||||||||||||||||||||||||||||
European Oxo GmbH(1) | Acetyl Intermediates | — | — | — | — | — | 2 | 10 | ||||||||||||||||||||||
| Erfei, A.I.E. | Acetyl Intermediates | 45.0 | 45.0 | 1 | 1 | — | (1 | ) | — | |||||||||||||||||||||
| Advanced Engineered | ||||||||||||||||||||||||||||||
| Fortron Industries LLC | Materials | 50.0 | 50.0 | 77 | 73 | 4 | 16 | 14 | ||||||||||||||||||||||
| Korea Engineering | Advanced Engineered | |||||||||||||||||||||||||||||
| Plastics Co., Ltd. | Materials | 50.0 | 50.0 | 145 | 170 | 12 | 14 | 13 | ||||||||||||||||||||||
| Advanced Engineered | ||||||||||||||||||||||||||||||
| Polyplastics Co., Ltd. | Materials | 45.0 | 45.0 | 189 | 170 | 19 | 25 | 26 | ||||||||||||||||||||||
| Advanced Engineered | ||||||||||||||||||||||||||||||
| Una SA | Materials | 50.0 | 50.0 | 2 | — | 2 | — | — | ||||||||||||||||||||||
| InfraServ GmbH & Co. Gendorf KG | Other Activities | 39.0 | 39.0 | 28 | 30 | 4 | 5 | 4 | ||||||||||||||||||||||
| InfraServ GmbH & Co. Hoechst KG | Other Activities | 31.2 | 31.2 | 137 | 154 | 10 | 18 | 14 | ||||||||||||||||||||||
| InfraServ GmbH & Co. Knapsack KG | Other Activities | 28.2 | 28.2 | 22 | 23 | 4 | 4 | 1 | ||||||||||||||||||||||
Sherbrooke Capital Health and Wellness, L.P.(2) | Consumer Specialties | 10.0 | 10.0 | 4 | 4 | (1 | ) | — | 1 | |||||||||||||||||||||
| Total | 605 | 625 | 54 | 83 | 83 | |||||||||||||||||||||||||
| (1) | The Company divested this investment in February 2007 (Note 4). The share of earnings (loss) for this investment is included in Earnings (loss) from operation of discontinued operations on the consolidated statements of operations. | |
| (2) | The Company accounts for its 10% ownership interest in Sherbrooke Capital Health and Wellness, L.P. under the equity method of accounting because the Company is able to exercise significant influence. |
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Affiliates totals: | ||||||||||||
| Net earnings | 121 | 204 | 197 | |||||||||
| Company’s share: | ||||||||||||
Net earnings(1) | 54 | 83 | 83 | |||||||||
| Dividends and other distributions | 64 | 57 | 109 | |||||||||
| (1) | Amount does not include a $1 million and a $3 million liquidating dividend from Clear Lake Methanol Partners for the years ended December 31, 2007 and 2006, respectively. |
F-19
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The total assets and liabilities associated with the Company’s equity method investments are as follows:
| As of | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Total assets | 3,163 | 2,916 | ||||||
| Total liabilities | 1,853 | 1,576 | ||||||
Cost Method
The Company’s investments accounted for under the cost method of accounting are as follows:
| Ownership Percentage | Carrying Value as of | |||||||||||||||||
| as of December 31, | December 31, | |||||||||||||||||
| Segment | 2008 | 2007 | 2008 | 2007 | ||||||||||||||
| (In percentages) | (In $ millions) | |||||||||||||||||
| National Methanol Company (“Ibn Sina”) | Acetyl Intermediates | 25 | 25 | 54 | 54 | |||||||||||||
| Kunming Cellulose Fibers Co. Ltd. | Consumer Specialties | 30 | 30 | 14 | 15 | |||||||||||||
| Nantong Cellulose Fibers Co. Ltd. | Consumer Specialties | 31 | 31 | 77 | 77 | |||||||||||||
| Zhuhai Cellulose Fibers Co. Ltd. | Consumer Specialties | 30 | 30 | 14 | 15 | |||||||||||||
| InfraServ GmbH & Co. Wiesbaden KG | Other Activities | 8 | 8 | 6 | 6 | |||||||||||||
| Other | 19 | 22 | ||||||||||||||||
| Total | 184 | 189 | ||||||||||||||||
Certain cost investments where the Company owns greater than a 20% ownership interest are accounted for under the cost method of accounting because the Company cannot exercise significant influence over these entities. The Company determined that it cannot exercise significant influence over these entities due to local government investment in and influence over these entities, limitations on the Company’s involvement in the day-to-day operations and the present inability of the entities to provide timely financial information prepared in accordance with US GAAP.
During 2007, the Company wrote-off its remaining €1 million ($1 million) cost investment in European Pipeline Development Company B.V. (“EPDC”) and expensed €7 million ($9 million), included in Other income (expense), net, associated with contingent liabilities that became payable due to the Company’s decision to exit the pipeline development project. In June 2008, the outstanding contingent liabilities were resolved and the Company recognized a gain of €2 million ($2 million), included in Other income (expense), net, in the consolidated statements of operations to remove the remaining accrual. The investment in EPDC related to the construction of a pipeline system, solely dedicated to the transportation of propylene, which was to connect Rotterdam via Antwerp, Netherlands, with the Company’s Oberhausen and Marl production facilities in Germany. However, on February 15, 2007, EPDC shareholders voted to cease the pipeline project as originally envisaged and go into liquidation. The Company was a 12.5% shareholder of EPDC.
During 2007, the Company fully impaired its $5 million cost investment in Elemica Corporation (“Elemica”). Elemica is a network for the global chemical industry developed by 22 of the leading chemical companies in the world for the benefit of the entire industry. The Company is a 1.83% shareholder of Elemica through its preferred share holdings. As part of Elemica’s planned capital reorganization in 2007, its board of directors has proposed to convert all outstanding preferred stock into shares of Elemica’s common stock. Based on the Company’s analysis of
F-20
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Elemica’s proposed capital reorganization, past earnings performance and business prospects, the Company concluded that its cost investment in Elemica was impaired. The impairment was included in Other income (expense), net in the consolidated statements of operations.
| 9. | Property, Plant and Equipment, Net |
| As of | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Land | 61 | 69 | ||||||
| Land improvements | 44 | 45 | ||||||
| Buildings | 362 | 353 | ||||||
| Machinery and equipment | 2,702 | 2,404 | ||||||
| Construction in progress | 444 | 329 | ||||||
| Property, plant and equipment, gross | 3,613 | 3,200 | ||||||
| Less: accumulated depreciation | (1,141 | ) | (838 | ) | ||||
| Property, plant and equipment, net | 2,472 | 2,362 | ||||||
Assets under capital leases amounted to $233 million and $112 million, less accumulated amortization of $38 million and $7 million, as of December 31, 2008 and 2007, respectively. Interest costs capitalized were $6 million, $9 million and $6 million during the years ended December 31, 2008, 2007 and 2006, respectively. Depreciation expense was $255 million, $209 million and $191 million during the years ended December 31, 2008, 2007 and 2006, respectively.
At December 31, 2008, the Company had assets held for sale with a net book value of $2 million.
During 2008, certain long-lived assets were impaired (Note 18).
| 10. | Goodwill |
| Advanced | ||||||||||||||||||||
| Engineered | Consumer | Industrial | Acetyl | |||||||||||||||||
| Materials | Specialties | Specialties | Intermediates | Total | ||||||||||||||||
| (In $ millions) | ||||||||||||||||||||
| Goodwill as of December 31, 2006 | 256 | 240 | 52 | 327 | 875 | |||||||||||||||
| Acquisitions | 1 | 19 | — | 3 | 23 | |||||||||||||||
| Adjustments to preacquisition tax uncertainties | (12 | ) | (15 | ) | — | (14 | ) | (41 | ) | |||||||||||
| Sale of businesses | — | — | (1 | ) | (42 | ) | (43 | ) | ||||||||||||
Adoption of FIN 48(1) | 15 | 6 | (1 | ) | (22 | ) | (2 | ) | ||||||||||||
Goodwill impairment(2) | — | — | (6 | ) | — | (6 | ) | |||||||||||||
| Exchange rate changes | 17 | 14 | 3 | 26 | 60 | |||||||||||||||
| Goodwill as of December 31, 2007 | 277 | 264 | 47 | 278 | 866 | |||||||||||||||
| Adjustments to preacquisition tax uncertainties | (9 | ) | 2 | (12 | ) | (30 | ) | (49 | ) | |||||||||||
| Exchange rate changes | (10 | ) | (14 | ) | (1 | ) | (13 | ) | (38 | ) | ||||||||||
| Goodwill as of December 31, 2008 | 258 | 252 | 34 | 235 | 779 | |||||||||||||||
| (1) | See Note 19 for additional discussion of FIN 48. |
F-21
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| (2) | In connection with the Company’s annual goodwill impairment test, the Company recorded an impairment of $6 million in the polyvinyl alcohol (“PVOH”) reporting unit. The PVOH reporting unit is included in the Industrial Specialties segment. |
In connection with the Company’s annual goodwill impairment test, the Company did not record an impairment loss for goodwill during the nine months ended September 30, 2008. In addition, based on the significant adverse change in the US and global business climate, the Company performed a goodwill impairment test during the three months ended December 31, 2008. Based on the results of this goodwill impairment test, the Company did not record an impairment loss for goodwill.
| 11. | Intangible Assets, Net |
| Customer- | Covenants | |||||||||||||||||||||||
| Trademarks | Related | �� | not to | |||||||||||||||||||||
| and | Intangible | Developed | Compete | |||||||||||||||||||||
| Trade names | Licenses | Assets | Technology | and Other | Total | |||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||
Gross Asset Value | ||||||||||||||||||||||||
| As of December 31, 2006 | 79 | — | 523 | 13 | 12 | 627 | ||||||||||||||||||
| Acquisitions | 2 | — | 10 | — | — | 12 | ||||||||||||||||||
| Divestitures | (1 | ) | — | (17 | ) | (1 | ) | — | (19 | ) | ||||||||||||||
| Exchange rate changes | 5 | — | 46 | — | — | 51 | ||||||||||||||||||
| As of December 31, 2007 | 85 | — | 562 | 12 | 12 | 671 | ||||||||||||||||||
Acquisitions(1) | — | 28 | — | — | — | 28 | ||||||||||||||||||
| Divestitures | — | — | — | — | — | — | ||||||||||||||||||
| Exchange rate changes | (3 | ) | 1 | (25 | ) | — | — | (27 | ) | |||||||||||||||
| As of December 31, 2008 | 82 | 29 | 537 | 12 | 12 | 672 | ||||||||||||||||||
Accumulated Amortization | ||||||||||||||||||||||||
| As of December 31, 2006 | (1 | ) | — | (149 | ) | (8 | ) | (6 | ) | (164 | ) | |||||||||||||
| Current period amortization | — | — | (68 | ) | (1 | ) | (3 | ) | (72 | ) | ||||||||||||||
| Divestitures | 1 | — | 5 | — | — | 6 | ||||||||||||||||||
| Exchange rate changes | — | — | (16 | ) | — | — | (16 | ) | ||||||||||||||||
| As of December 31, 2007 | — | — | (228 | ) | (9 | ) | (9 | ) | (246 | ) | ||||||||||||||
| Current period amortization | — | (3 | ) | (71 | ) | (1 | ) | (1 | ) | (76 | ) | |||||||||||||
| Exchange rate changes | — | — | 14 | — | — | 14 | ||||||||||||||||||
| As of December 31, 2008 | — | (3 | ) | (285 | ) | (10 | ) | (10 | ) | (308 | ) | |||||||||||||
| Intangible assets, net as of December 31, 2008 | 82 | 26 | 252 | 2 | 2 | 364 | ||||||||||||||||||
| (1) | Acquisition of a sole and exclusive license to patents and patent applications related to acetic acid. The license will be amortized over 10 years. |
Estimated amortization expense for the succeeding five fiscal years is approximately $73 million in 2009, $64 million in 2010, $59 million in 2011, $45 million in 2012 and $29 million in 2013. The Company’s trademarks and trade names have an indefinite life. Accordingly, no amortization is recorded on these intangible assets. As a result of the Company’s annual impairment test on indefinite-lived intangible assets, the Company did not record an impairment loss for indefinite-lived intangible assets during the year ended December 31, 2008.
F-22
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 12. | Current Other Liabilities |
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Salaries and benefits | 107 | 157 | ||||||
| Environmental (Note 16) | 19 | 19 | ||||||
| Restructuring (Note 18) | 32 | 40 | ||||||
| Insurance | 34 | 41 | ||||||
| Sorbates litigation (Note 23) | 1 | 170 | ||||||
| Asset retirement obligations | 9 | 16 | ||||||
| Derivatives | 65 | 129 | ||||||
| Current portion of benefit obligations | 57 | 56 | ||||||
| Interest | 56 | 60 | ||||||
| Other | 194 | 200 | ||||||
| Current Other liabilities | 574 | 888 | ||||||
| 13. | Noncurrent Other Liabilities |
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Environmental (Note 16) | 79 | 96 | ||||||
| Insurance | 85 | 78 | ||||||
| Deferred revenue | 56 | 71 | ||||||
| Deferred proceeds (Note 4, Note 28) | 370 | 93 | ||||||
| Asset retirement obligations | 40 | 31 | ||||||
| Derivatives | 76 | 37 | ||||||
| Other | 100 | 89 | ||||||
| Noncurrent Other liabilities | 806 | 495 | ||||||
Changes in asset retirement obligations are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Balance at beginning of year | 47 | 59 | 54 | |||||||||
| Additions | 6 | (2) | — | 10 | ||||||||
| Accretion | 3 | 5 | 3 | |||||||||
| Payments | (6 | ) | (6 | ) | (2 | ) | ||||||
| Divestitures | — | (16 | )(1) | — | ||||||||
| Purchase accounting adjustments | — | 3 | — | |||||||||
| Revisions to cash flow estimates | 1 | (2 | ) | (7 | ) | |||||||
| Exchange rate changes | (2 | ) | 4 | 1 | ||||||||
| Balance at end of year | 49 | 47 | 59 | |||||||||
| (1) | Relates to the sale of the Edmonton, Alberta, Canada plant (Note 4). | |
| (2) | Primarily relates to long-lived assets impaired during the year ended December 31, 2008 (Note 18) which management no longer considers to have an indeterminate life. |
F-23
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Included in the asset retirement obligations for each of the years ended December 31, 2008, 2007 and 2006 is $10 million related to a business acquired in 2005. The Company has a corresponding receivable for this amount included in noncurrent Other assets as of December 31, 2008.
The Company has identified but not recognized asset retirement obligations related to certain of its existing operating facilities. Examples of these types of obligations include demolition, decommissioning, disposal and restoration activities. Legal obligations exist in connection with the retirement of these assets upon closure of the facilities or abandonment of the existing operations. However, the Company currently plans on continuing operations at these facilities indefinitely and therefore a reasonable estimate of fair value cannot be determined at this time. In the event the Company considers plans to abandon or cease operations at these sites, an asset retirement obligation will be reassessed at that time. If certain operating facilities were to close, the related asset retirement obligations could significantly affect the Company’s results of operations and cash flows.
| 14. | Debt |
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
Short-term borrowings and current installments of long-term debt — third party and affiliates | ||||||||
| Current installments of long-term debt | 81 | 44 | ||||||
| Short-term borrowings, principally comprised of amounts due to affiliates | 152 | 228 | ||||||
| Short-term borrowings and current installments of long-term debt — third party and affiliates | 233 | 272 | ||||||
Long-term debt | ||||||||
| Senior Credit Facilities: Term Loan facility due 2014 | 2,794 | 2,855 | ||||||
| Term notes 7.125%, due 2009 | 14 | 14 | ||||||
| Pollution control and industrial revenue bonds, interest rates ranging from 5.7% to 6.7%, due at various dates through 2030 | 181 | 181 | ||||||
| Obligations under capital leases and other secured borrowings due at various dates through 2024 | 211 | 110 | ||||||
| Other bank obligations, interest rates ranging from 4.33% to 7.05%, due at various dates through 2014 | 181 | 168 | ||||||
| Subtotal | 3,381 | 3,328 | ||||||
| Less: Current installments of long-term debt | 81 | 44 | ||||||
| Long-term debt | 3,300 | 3,284 | ||||||
Senior Credit Facilities
The Company’s senior credit agreement consists of $2,280 million of US dollar-denominated and €400 million of Euro-denominated term loans due 2014, a $650 million revolving credit facility terminating in 2013 and a $228 million credit-linked revolving facility terminating in 2014. Borrowings under the senior credit agreement bear interest at a variable interest rate based on LIBOR (for US dollars) or EURIBOR (for Euros), as applicable, or, for US dollar-denominated loans under certain circumstances, a base rate, in each case plus an applicable margin. The applicable margin for the term loans and any loans under the credit-linked revolving facility is 1.75%, subject to potential reductions as defined in the senior credit agreement. As of December 31, 2008, the applicable margin was 1.5% and continues to be subject to potential adjustments as defined in the senior credit agreement. The term loans
F-24
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
under the senior credit agreement are subject to amortization at 1% of the initial principal amount per annum, payable quarterly. The remaining principal amount of the term loans is due on April 2, 2014.
As of December 31, 2008, there were no outstanding borrowings or letters of credit issued under the revolving credit facility; accordingly, $650 million remained available for borrowing. As of December 31, 2008, there were $91 million of letters of credit issued under the credit-linked revolving facility and $137 million remained available for borrowing.
The senior credit agreement is guaranteed by Celanese Holdings LLC, a subsidiary of Celanese Corporation, and certain domestic subsidiaries of Celanese US, and is secured by a lien on substantially all assets of Celanese US and such guarantors, subject to certain agreed exceptions, pursuant to the Guarantee and Collateral Agreement, dated as of April 2, 2007, by and among Celanese Holdings LLC, Celanese US, certain subsidiaries of Celanese US and Deutsche Bank AG, New York Branch, as Administrative Agent and as Collateral Agent.
Our senior credit agreement requires us to maintain a maximum first lien senior secured leverage ratio not greater than 3.90 to 1.00 if there are outstanding borrowings under the revolving credit facility. The first lien senior secured leverage ratio is calculated as the ratio of consolidated first lien senior secured debt to earnings before interest, taxes, depreciation and amortization, subject to adjustments identified in the credit agreement. The Company is in compliance with all of the covenants related to its debt agreements as of December 31, 2008.
Debt Refinancing
In April 2007, the Company, through certain of its subsidiaries, entered into a new senior credit agreement. Proceeds from the new senior credit agreement, together with available cash, were used to retire the Company’s $2,454 million amended and restated (January 2005) senior credit facilities, which consisted of $1,626 million in term loans due 2011, a $600 million revolving credit facility terminating in 2009 and a $228 million credit-linked revolving facility terminating in 2009, and to retire all of the Company’s 9.625% senior subordinated notes due 2014 and 10.375% senior subordinated notes due 2014 (the “Senior Subordinated Notes”) and 10% senior discount notes due 2014 and 10.5% senior discount notes due 2014 (the “Senior Discount Notes”) as discussed below.
Substantially all of the Senior Discount Notes and Senior Subordinated Notes were tendered in the first quarter of 2007. The remaining outstanding Senior Discount Notes and Senior Subordinated Notes not tendered in conjunction with the Tender Offers were redeemed by the Company in May 2007 through optional redemption allowed in the indentures.
As a result of the refinancing, the Company incurred premiums paid on early redemption of debt, accelerated amortization and other refinancing expenses. The components of refinancing expenses are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Premium paid on early redemption of debt | — | 207 | — | |||||||||
| Accelerated amortization of premiums and deferred financing costs on early redemption and prepayment of debt | — | 33 | 1 | |||||||||
| Debt issuance costs and other | — | 16 | — | |||||||||
| Refinancing expenses | — | 256 | 1 | |||||||||
In connection with the refinancing, the Company recorded deferred financing costs of $39 million related to the new senior credit agreement, which are included in noncurrent Other assets on the consolidated balance sheets and are being amortized over the term of the new senior credit agreement. The deferred financing costs consist of $23 million of costs incurred to acquire the new senior credit agreement and $16 million of debt issue costs existing prior to the refinancing.
F-25
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For the years ended December 31, 2008, 2007 and 2006, the Company recorded amortization of deferred financing costs, which is classified in Interest expense, of $7 million, $8 million and $13 million, respectively. As of December 31, 2008 and 2007, respectively, the Company had $32 million and $39 million of net deferred financing costs.
Principal payments scheduled to be made on the Company’s debt, including short-term borrowings, are as follows:
| (In $ millions) | ||||
| 2009 | 233 | |||
| 2010 | 100 | |||
| 2011 | 89 | |||
| 2012 | 65 | |||
| 2013 | 73 | |||
| Thereafter | 2,973 | |||
| Total | 3,533 | |||
| 15. | Benefit Obligations |
The Company adopted the provisions of SFAS No. 158,Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans an amendment of FASB Statements No. 87, 88, 106, and 132(R) (“SFAS No. 158”), on December 31, 2006 which caused the Company to recognize the unfunded status of its benefit plans of $113 million in the December 31, 2006 consolidated balance sheet, with a corresponding adjustment to Accumulated other comprehensive income (loss), net. The Company’s adoption of SFAS No. 158 had no impact to the consolidated statements of operations for the year ended December 31, 2006.
Pension obligations. Pension obligations are established for benefits payable in the form of retirement, disability and surviving dependent pensions. The benefits offered vary according to the legal, fiscal and economic conditions of each country. The commitments result from participation in defined contribution and defined benefit plans, primarily in the US. Benefits are dependent on years of service and the employee’s compensation. Supplemental retirement benefits provided to certain employees are nonqualified for US tax purposes. Separate trusts have been established for some nonqualified plans. Pension costs under the Company’s retirement plans are actuarially determined.
The Company sponsors defined benefit pension plans in North America, Europe and Asia. As of December 31, 2008, the Company’s US qualified pension plan represents approximately 83% and 78% of the Company’s pension plan assets and liabilities, respectively. Independent trusts or insurance companies administer the majority of these plans. The US qualified defined benefit plan’s actual return on assets for the year ended December 31, 2008 was a loss of 18% versus an expected long-term rate of asset return assumption of 8.5%. The overall performance of the portfolio suffered due to the significant declines in the global equity markets. The returns on equity investments for this plan were negative while the returns on fixed income investments were positive.
The Company sponsors various defined contribution plans in North America, Europe and Asia covering certain employees. Employees may contribute to these plans and the Company will match these contributions in varying amounts. The Company’s matching contribution to the defined contribution plans are based on specified percentages of employee contributions and aggregated $13 million, $12 million and $11 million for the years ended December 31, 2008, 2007 and 2006, respectively.
The Company participates in multiemployer defined benefit pension plans in Europe covering certain employees. The Company’s contributions to the multiemployer defined benefit pension plans are based on specified percentages of employee contributions and aggregated $7 million, for each of the years ended December 31, 2008, 2007 and 2006.
Other postretirement obligations. Certain retired employees receive postretirement healthcare and life insurance benefits under plans sponsored by the Company, which has the right to modify or terminate these plans at any time. The cost for coverage is shared between the Company and the retiree. The cost of providing retiree
F-26
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
health care and life insurance benefits is actuarially determined and accrued over the service period of the active employee group. The Company’s policy is to fund benefits as claims and premiums are paid. The US plan was closed to new participants effective January 1, 2006.
The following tables set forth the benefit obligations, the fair value of the plan assets and the funded status of the Company’s pension and postretirement benefit plans; and the amounts recognized in the Company’s consolidated financial statements:
| Postretirement Benefits | ||||||||||||||||
| Pension Benefits | as of | |||||||||||||||
| as of December 31, | December 31, | |||||||||||||||
| 2008 | 2007 | 2008 | 2007 | |||||||||||||
| (In $ millions) | ||||||||||||||||
Change in projected benefit obligation | ||||||||||||||||
| Projected benefit obligation at beginning of period | 3,264 | 3,343 | 306 | 343 | ||||||||||||
| Service cost | 31 | 38 | 2 | 2 | ||||||||||||
| Interest cost | 195 | 187 | 17 | 19 | ||||||||||||
| Participant contributions | — | 1 | 22 | 17 | ||||||||||||
| Plan amendments | — | 4 | 2 | — | ||||||||||||
Actuarial gains(1) | (107 | ) | (123 | ) | (14 | ) | (18 | ) | ||||||||
| Special termination benefits | — | — | — | — | ||||||||||||
| Divestitures | — | — | — | — | ||||||||||||
| Settlements | (19 | ) | (40 | ) | — | — | ||||||||||
| Benefits paid | (222 | ) | (215 | ) | (58 | ) | (66 | ) | ||||||||
| Federal subsidy on Medicare Part D | — | — | 6 | 6 | ||||||||||||
| Curtailments | (1 | ) | (1 | ) | (2 | ) | (1 | ) | ||||||||
| Foreign currency exchange rate changes | (68 | ) | 69 | (6 | ) | 4 | ||||||||||
| Other | — | 1 | — | — | ||||||||||||
| Projected benefit obligation at end of period | 3,073 | 3,264 | 275 | 306 | ||||||||||||
Change in plan assets | ||||||||||||||||
| Fair value of plan assets at beginning of period | 2,875 | 2,802 | — | — | ||||||||||||
| Actual return on plan assets | (448 | ) | 209 | — | — | |||||||||||
| Employer contributions | 48 | 48 | 35 | 49 | ||||||||||||
| Participant contributions | — | 1 | 23 | 17 | ||||||||||||
| Settlements | (22 | ) | (28 | ) | — | — | ||||||||||
| Benefits paid | (222 | ) | (215 | ) | (58 | ) | (66 | ) | ||||||||
| Foreign currency exchange rate changes | (61 | ) | 57 | — | — | |||||||||||
| Other | — | 1 | — | — | ||||||||||||
| Fair value of plan assets at end of period | 2,170 | 2,875 | — | — | ||||||||||||
Funded status and net amounts recognized | ||||||||||||||||
| Plan assets less than benefit obligation | (903 | ) | (389 | ) | (275 | ) | (306 | ) | ||||||||
| Unrecognized prior service cost | 1 | 3 | 1 | (1 | ) | |||||||||||
| Unrecognized actuarial (gain) loss | 502 | (53 | ) | (80 | ) | (71 | ) | |||||||||
| Net amount recognized in the consolidated balance sheets | (400 | ) | (439 | ) | (354 | ) | (378 | ) | ||||||||
Amounts recognized in the consolidated balance sheets consist of: | ||||||||||||||||
| Noncurrent Other assets | 8 | 7 | — | — | ||||||||||||
| Current Other liabilities | (22 | ) | (21 | ) | (35 | ) | (34 | ) | ||||||||
| Pension obligations | (889 | ) | (375 | ) | (240 | ) | (272 | ) | ||||||||
| Accrued benefit liability | (903 | ) | (389 | ) | (275 | ) | (306 | ) | ||||||||
| Net actuarial (gain) loss | 502 | (53 | ) | (80 | ) | (71 | ) | |||||||||
| Prior service (benefit) cost | 1 | 3 | 1 | (1 | ) | |||||||||||
Other comprehensive (income) loss(2) | 503 | (50 | ) | (79 | ) | (72 | ) | |||||||||
| Net amount recognized in the consolidated balance sheets | (400 | ) | (439 | ) | (354 | ) | (378 | ) | ||||||||
| (1) | Primarily relates to change in discount rates. |
F-27
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| (2) | Amount shown net of tax in the consolidated statements of shareholders’ equity and comprehensive income (loss). See Note 19 for the related tax associated with the pension and postretirement benefit obligations. |
A summary of pension plans with projected benefit obligations in excess of plan assets is shown below:
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Projected benefit obligation | 2,924 | 3,146 | ||||||
| Fair value of plan assets | 2,014 | 2,750 | ||||||
Included in the above table are pension plans with accumulated benefit obligations in excess of plan assets as detailed below:
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Accumulated benefit obligation | 2,797 | 618 | ||||||
| Fair value of plan assets | 1,985 | 308 | ||||||
The accumulated benefit obligation for all defined benefit pension plans was $2,967 million and $3,147 million as of December 31, 2008 and 2007, respectively.
The following table sets forth the Company’s net periodic pension cost:
| Pension Benefits | Postretirement Benefits | |||||||||||||||||||||||
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, | December 31, | |||||||||||||||||||||||
| 2008 | 2007 | 2006 | 2008 | 2007 | 2006 | |||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||
Components of net periodic benefit cost | ||||||||||||||||||||||||
| Service cost | 31 | 38 | 40 | 1 | 2 | 2 | ||||||||||||||||||
| Interest cost | 195 | 187 | 183 | 17 | 19 | 20 | ||||||||||||||||||
| Expected return on plan assets | (218 | ) | (216 | ) | (207 | ) | — | — | — | |||||||||||||||
| Amortization of prior service cost | — | — | 1 | — | — | — | ||||||||||||||||||
| Recognized actuarial (gain) loss | 1 | 1 | 2 | (4 | ) | (2 | ) | — | ||||||||||||||||
| Curtailment (gain) loss | (2 | ) | (1 | ) | 1 | — | (1 | ) | (1 | ) | ||||||||||||||
| Settlement (gain) loss | 3 | (12 | ) | — | — | — | — | |||||||||||||||||
| Special termination benefits | — | — | 3 | — | — | — | ||||||||||||||||||
| Net periodic benefit cost | 10 | (3 | ) | 23 | 14 | 18 | 21 | |||||||||||||||||
Amortization of the actuarial (gain) loss into net periodic cost in 2009 is expected to be $1 million and $(5) million for pension benefits and postretirement benefits, respectively.
Included in the pension obligations above are accrued liabilities relating to supplemental retirement plans for certain employees amounting to $224 million and $230 million as of December 31, 2008 and 2007, respectively. Pension expense relating to these plans included in net periodic benefit cost totaled $15 million, $14 million and $15 million for the years ended December 31, 2008, 2007 and 2006, respectively. To fund these obligations, nonqualified trusts were established which hold marketable securities valued at $97 million and $121 million as of December 31, 2008 and 2007, respectively. In addition to holding marketable securities, the nonqualified trusts hold investments in insurance contracts of $67 million and $74 million as of December 31, 2008 and 2007, respectively, which are included in noncurrent Other assets in the consolidated balance sheets.
F-28
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Valuation
The Company uses the corridor approach in the valuation of the defined benefit plans and other postretirement benefits. The corridor approach defers all actuarial gains and losses resulting from variances between actual results and economic estimates or actuarial assumptions. For defined benefit pension plans, these unrecognized gains and losses are amortized when the net gains and losses exceed 10% of the greater of the market-related value of plan assets or the projected benefit obligation at the beginning of the year. For other postretirement benefits, amortization occurs when the net gains and losses exceed 10% of the accumulated postretirement benefit obligation at the beginning of the year. The amount in excess of the corridor is amortized over the average remaining service period to retirement date for active plan participants or, for retired participants, the average remaining life expectancy.
The following tables set forth the principal weighted-average assumptions used:
| Pension Benefits | Postretirement Benefits | |||||||||||||||
| as of | as of | |||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2008 | 2007 | 2008 | 2007 | |||||||||||||
| (In percentages) | ||||||||||||||||
Weighted-average assumptions used to determine benefit obligations | ||||||||||||||||
| Discount rate: | ||||||||||||||||
| US plans | 6.50 | 6.30 | 6.40 | 6.00 | ||||||||||||
| International plans | 5.84 | 5.42 | 6.11 | 5.31 | ||||||||||||
| Combined | 6.41 | 6.16 | 6.37 | 5.93 | ||||||||||||
| Rate of compensation increase: | �� | |||||||||||||||
| US plans | 4.00 | 4.00 | N/A | N/A | ||||||||||||
| International plans | 3.24 | 3.15 | N/A | N/A | ||||||||||||
| Combined | 3.90 | 3.66 | N/A | N/A | ||||||||||||
| Pension Benefits | Postretirement Benefits | |||||||||||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||||||||||
| 2008 | 2007 | 2006 | 2008 | 2007 | 2006 | |||||||||||||||||||
| (In percentages) | ||||||||||||||||||||||||
Weighted-average assumptions used to determine net cost | ||||||||||||||||||||||||
| Discount rate: | ||||||||||||||||||||||||
| US plans | 6.30 | 5.88 | 5.63 | 6.00 | 5.88 | 5.63 | ||||||||||||||||||
| International plans | 5.42 | 4.70 | 4.54 | 5.31 | 4.80 | 4.97 | ||||||||||||||||||
| Combined | 6.16 | 5.86 | 5.46 | 5.93 | 5.79 | 5.57 | ||||||||||||||||||
| Expected return on plan assets: | ||||||||||||||||||||||||
| US plans | 8.50 | 8.50 | 8.50 | N/A | N/A | N/A | ||||||||||||||||||
| International plans | 5.68 | 6.59 | 6.30 | N/A | N/A | N/A | ||||||||||||||||||
| Combined | 8.05 | 8.20 | 8.17 | N/A | N/A | N/A | ||||||||||||||||||
| Rate of compensation increase: | ||||||||||||||||||||||||
| US plans | 4.00 | 4.00 | 4.00 | N/A | N/A | N/A | ||||||||||||||||||
| International plans | 3.15 | 3.18 | 3.26 | N/A | N/A | N/A | ||||||||||||||||||
| Combined | 3.66 | 3.73 | 3.81 | N/A | N/A | N/A | ||||||||||||||||||
The expected rate of return assumptions for plan assets are based mainly on historical performance achieved over a long period of time (15 to 20 years) encompassing many business and economic cycles. Adjustments, upward and
F-29
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
downward, may be made to those historical returns to reflect future capital market expectations; these expectations are typically derived from expert advice from the investment community and surveys of peer company assumptions.
The Company determines its assumption for the discount rates to be used for the purposes of computing annual service and interest costs for its US plans based on the yields of high quality corporate/government bonds with a duration appropriate to the duration of the plan obligations.
The Company determines its discount rates in the Euro zone using the iBoxx Euro Corporate AA Bond indices with appropriate adjustments for the duration of the plan obligations. In other international locations, the Company determines its discount rates based on the yields of high quality government bonds with a duration appropriate to the duration of the plan obligations.
On January 1, 2008, the Company’s health care cost trend assumption for US postretirement medical plan’s net periodic benefit cost was 9% for the first two years declining 0.5% per year to an ultimate rate of 5%. On January 1, 2007, the health care cost trend rate was 8.5% per year declining 1% per year to an ultimate rate of 5%.
Assumed health care cost trend rates have a significant effect on the amounts reported for the health care plans. A one-percentage-point increase or decrease in the assumed health care cost trend rate would impact postretirement obligations by $3 million and $(3) million, respectively. The effect of a one percent increase or decrease in the assumed health care cost trend rate would have a less than $1 million impact on service and interest cost.
Plan Assets
The following tables set forth the target asset allocation for the Company’s pension plans and the asset allocation:
| Weighted | ||||||||||||
| Average | Percentage of | |||||||||||
| Target | Plan Assets as of | |||||||||||
| Allocation | December 31, | |||||||||||
Asset Category — US | 2009 | 2008 | 2007 | |||||||||
| Equity securities | 72 | % | 56 | % | 68 | % | ||||||
| Debt securities | 28 | % | 44 | % | 30 | % | ||||||
| Real estate and other | — | — | 2 | % | ||||||||
Total | 100 | % | 100 | % | 100 | % | ||||||
| Weighted | ||||||||||||
| Average | Percentage of | |||||||||||
| Target | Plan Assets as of | |||||||||||
| Allocation | December 31, | |||||||||||
Asset Category — International | 2009 | 2008 | 2007 | |||||||||
| Equity securities | 21 | % | 17 | % | 39 | % | ||||||
| Debt securities | 74 | % | 72 | % | 48 | % | ||||||
| Real estate and other | 5 | % | 11 | % | 13 | % | ||||||
Total | 100 | % | 100 | % | 100 | % | ||||||
The financial objectives of the qualified pension plans are established in conjunction with a comprehensive review of each plan’s liability structure. The Company’s asset allocation policy is based on a detailed asset/liability analysis. In developing investment policy and financial goals, consideration is given to the plan’s demographics, the returns and risks associated with alternative investment strategies, and the current and projected cash, expense and funding ratios of the plan. The investment policy must also comply with local statutory requirements as determined by each country. A formal asset/liability mix study of the plan is undertaken every 3 to 5 years or whenever there has been a material change in plan demographics, benefit structure or funding status and investment market. The Company has adopted a long-term investment horizon such that the risk and duration of investment losses are weighed against the long-term
F-30
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
potential for appreciation of assets. Although there cannot be complete assurance that these objectives will be realized, it is believed that the likelihood for their realization is reasonably high, based upon the asset allocation chosen and the historical and expected performance of the asset classes utilized by the plans. The intent is for investments to be broadly diversified across asset classes, investment styles, investment managers, developed and emerging markets, business sectors and securities in order to moderate portfolio volatility and risk. Investments may be in separate accounts, commingled trusts, mutual funds and other pooled asset portfolios provided they all conform to fiduciary standards.
External investment managers are hired to manage the pension assets. An investment consultant assists with the screening process for each new manager hired. Over the long-term, the investment portfolio is expected to earn returns that exceed a composite of market indices that are weighted to match each plan’s target asset allocation. Long-term is considered 3 to 5 years; however, incidences of underperformance are analyzed. The portfolio return should also (over the long term) meet or exceed the return used for actuarial calculations in order to minimize future pension contributions and escalation in pension expense.
Employer contributions for pension benefits and postretirement benefits are preliminarily estimated to be $40 million and $35 million, respectively, in 2009. The table below reflects pension benefits expected to be paid from the plan or from the Company’s assets. The postretirement benefits represent the Company’s share of the benefit cost.
| Postretirement | ||||||||||||
| Benefit | ||||||||||||
| Pension | Expected | |||||||||||
| Benefit | Federal | |||||||||||
| Payments(1) | Payments | Subsidy | ||||||||||
| (In $ millions) | ||||||||||||
| 2009 | 215 | 35 | 11 | |||||||||
| 2010 | 210 | 28 | 7 | |||||||||
| 2011 | 209 | 24 | 7 | |||||||||
| 2012 | 209 | 21 | 8 | |||||||||
| 2013 | 211 | 19 | 8 | |||||||||
| 2014-2018 | 1,108 | 96 | 14 | |||||||||
| (1) | Payments are expected to be made primarily from plan assets. |
Other Obligations
The following table represents additional benefit liabilities and other similar obligations:
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Long-term disability | 33 | 36 | ||||||
| Other | 5 | 13 | ||||||
| Total | 38 | 49 | ||||||
| 16. | Environmental |
General
The Company is subject to environmental laws and regulations worldwide which impose limitations on the discharge of pollutants into the air and water and establish standards for the treatment, storage and disposal of solid and hazardous wastes. The Company believes that it is in substantial compliance with all applicable environmental laws and regulations. The Company is also subject to retained environmental obligations specified in various
F-31
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
contractual agreements arising from the divestiture of certain businesses by the Company or one of its predecessor companies.
For the years ended December 31, 2008, 2007 and 2006, the Company’s expenditures, including expenditures for legal compliance, internal environmental initiatives and remediation of active, orphan, divested and US Superfund sites (as defined below) were $78 million, $83 million and $71 million, respectively. The Company’s capital project-related environmental expenditures for the years ended December 31, 2008, 2007 and 2006 were $13 million, $14 million and $5 million, respectively. Environmental reserves for remediation matters were $98 million and $115 million as of December 31, 2008 and 2007, respectively, which represents the Company’s best estimate of its liability.
Remediation
Due to its industrial history and through retained contractual and legal obligations, the Company has the obligation to remediate specific areas on its own sites as well as on divested, orphan or US Superfund sites. In addition, as part of the demerger agreement between the Company and Hoechst, a specified portion of the responsibility for environmental liabilities from a number of Hoechst divestitures was transferred to the Company. The Company provides for such obligations when the event of loss is probable and reasonably estimable.
For the years ended December 31, 2008, 2007 and 2006, the total remediation efforts charged to Cost of sales in the consolidated statements of operations were $3 million, $4 million and $6 million, respectively. The Company believes that environmental remediation costs will not have a material adverse effect on the financial position of the Company, but may have a material adverse effect on the results of operations or cash flows in any given accounting period.
The Company did not record any insurance recoveries related to these matters for the reported periods. There are no receivables for insurance recoveries as of December 31, 2008 and 2007, however, as of December 31, 2008, there is an $8 million receivable from a former owner of an acquired business.
German InfraServs
On January 1, 1997, coinciding with a reorganization of the Hoechst businesses in Germany, real estate service companies (“InfraServs”) were created to own directly the land and property and to provide various technical and administrative services at each of the manufacturing locations. The Company has manufacturing operations at the InfraServ location in Frankfurt am Main-Hoechst, Germany and holds interests in the companies which own and operate the former Hoechst sites in Gendorf, Knapsack and Wiesbaden.
InfraServs are liable for any residual contamination and other pollution because they own the real estate on which the individual facilities operate. In addition, Hoechst, and its legal successors, as the responsible party under German public law, is liable to third parties for all environmental damage that occurred while it was still the owner of the plants and real estate. The contribution agreements entered into in 1997 between Hoechst and the respective operating companies, as part of the divestiture of these companies, provide that the operating companies will indemnify Hoechst, and its legal successors, against environmental liabilities resulting from the transferred businesses. Additionally, the InfraServs have agreed to indemnify Hoechst, and its legal successors, against any environmental liability arising out of or in connection with environmental pollution of any site. Likewise, in certain circumstances the Company could be responsible for the elimination of residual contamination on a few sites that were not transferred to InfraServ companies, in which case Hoechst, and its legal successors, must reimburse the Company for two-thirds of any costs so incurred.
The InfraServ partnership agreements provide that, as between the partners, each partner is responsible for any contamination caused predominantly by such partner. Any liability, which cannot be attributed to an InfraServ partner and for which no third party is responsible, is required to be borne by the InfraServ partnership. In view of this potential obligation to eliminate residual contamination, the InfraServs, primarily relating to equity and cost affiliates which are not consolidated by the Company, have reserves of $84 million and $88 million as of December 31, 2008 and 2007, respectively.
F-32
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
If an InfraServ partner defaults on its respective indemnification obligations to eliminate residual contamination, the owners of the remaining participation in the InfraServ companies have agreed to fund such liabilities, subject to a number of limitations. To the extent that any liabilities are not satisfied by either the InfraServs or their owners, these liabilities are to be borne by the Company in accordance with the demerger agreement. However, Hoechst, and its legal successors, will reimburse the Company for two-thirds of any such costs. Likewise, in certain circumstances the Company could be responsible for the elimination of residual contamination on several sites that were not transferred to InfraServ companies, in which case Hoechst, and its legal successors, must also reimburse the Company for two-thirds of any costs so incurred. The German InfraServs are owned partially by the Company, as noted below, and the remaining ownership is held by various other companies. The Company’s ownership interest and environmental liability participation percentages for such liabilities which cannot be attributed to an InfraServ partner were as follows as of December 31, 2008:
Company | Ownership % | Liability % | ||||||
| InfraServ GmbH & Co. Gendorf KG | 39.0 | % | 10.0 | % | ||||
| InfraServ GmbH & Co. Knapsack KG | 28.2 | % | 22.0 | % | ||||
| InfraServ GmbH & Co. Hoechst KG | 31.2 | % | 40.0 | % | ||||
| InfraServ GmbH & Co. Wiesbaden KG | 7.9 | % | 0.0 | % | ||||
| InfraServ Verwaltungs GmbH | 100.0 | % | 0.0 | % | ||||
US Superfund Sites
In the US, the Company may be subject to substantial claims brought by US federal or state regulatory agencies or private individuals pursuant to statutory authority or common law. In particular, the Company has a potential liability under the US Federal Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended, and related state laws (collectively referred to as “Superfund”) for investigation and cleanup costs at approximately 50 sites. At most of these sites, numerous companies, including certain companies comprising the Company, or one of its predecessor companies, have been notified that the Environmental Protection Agency, state governing bodies or private individuals consider such companies to be potentially responsible parties (“PRP”) under Superfund or related laws. The proceedings relating to these sites are in various stages. The cleanup process has not been completed at most sites and the status of the insurance coverage for most of these proceedings is uncertain. Consequently, the Company cannot accurately determine its ultimate liability for investigation or cleanup costs at these sites. As of December 31, 2008 and 2007, the Company had provisions totaling $11 million and $13 million, respectively, for US Superfund sites and utilized $2 million, $1 million and $1 million of these reserves during the years ended December 31, 2008, 2007 and 2006, respectively. Additional provisions and adjustments recorded during the years ended December 31, 2008, 2007 and 2006 approximately offset these expenditures.
As events progress at each site for which it has been named a PRP, the Company accrues, as appropriate, a liability for site cleanup. Such liabilities include all costs that are probable and can be reasonably estimated. In establishing these liabilities, the Company considers its shipment of waste to a site, its percentage of total waste shipped to the site, the types of wastes involved, the conclusions of any studies, the magnitude of any remedial actions that may be necessary and the number and viability of other PRPs. Often the Company will join with other PRPs to sign joint defense agreements that will settle, among PRPs, each party’s percentage allocation of costs at the site. Although the ultimate liability may differ from the estimate, the Company routinely reviews the liabilities and revises the estimate, as appropriate, based on the most current information available.
Hoechst Liabilities
In connection with the Hoechst demerger, the Company agreed to indemnify Hoechst, and its legal successors, for the first €250 million of future remediation liabilities for environmental damages arising from 19 specified divested Hoechst entities. As of December 31, 2008 and 2007, reserves of $27 million and $27 million, respectively, for these matters are included as a component of the total environmental reserves. As of December 31, 2008 and
F-33
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
2007, the Company, has made total cumulative payments of $48 million and $46 million, respectively. If such future liabilities exceed €250 million, Hoechst, and its legal successors, will bear such excess up to an additional €500 million. Thereafter, the Company will bear one-third and Hoechst, and its legal successors, will bear two-thirds of any further environmental remediation liabilities. Where the Company is unable to reasonably determine the probability of loss or estimate such loss under this indemnification, the Company has not recognized any liabilities relative to this indemnification.
| 17. | Shareholders’ Equity |
Preferred Stock
The Company has $240 million aggregate liquidation preference of outstanding 4.25% convertible perpetual preferred stock. Holders of the preferred stock are entitled to receive, when, as and if, declared by the Company’s Board of Directors, out of funds legally available, cash dividends at the rate of 4.25% per annum of liquidation preference, payable quarterly in arrears, commencing on May 1, 2005. Dividends on the preferred stock are cumulative from the date of initial issuance. Accumulated but unpaid dividends accumulate at an annual rate of 4.25%. The preferred stock is convertible, at the option of the holder, at any time into approximately 1.26 shares of Series A common stock, subject to adjustments, per $25.00 liquidation preference of preferred stock and upon conversion will be recorded in the consolidated statements of shareholders’ equity and comprehensive income (loss).
During 2008 and 2007, the Company declared and paid $10 million of cash dividends in each period on its 4.25% convertible perpetual preferred stock.
Dividends
During 2005, the Company’s Board of Directors adopted a policy of declaring, subject to legally available funds, a quarterly cash dividend on each share of the Company’s Series A common stock at an annual rate of $0.16 per share unless the Company’s Board of Directors, in its sole discretion, determines otherwise. Further, such dividends payable to holders of the Company’s Series A common stock cannot be declared or paid nor can any funds be set aside for the payment thereof, unless the Company has paid or set aside funds for the payment of all accumulated and unpaid dividends with respect to the shares of the Company’s preferred stock, as described above. Additionally, the amount available to pay cash dividends is restricted by our senior credit agreement.
During 2008, 2007 and 2006, the Company declared and paid $24 million, $25 million and $26 million, respectively, of cash dividends to holders of its Series A common stock.
Treasury Stock
In conjunction with the April 2007 debt refinancing (Note 14), the Company, through its wholly-owned subsidiary CIH, repurchased 2,021,775 shares of its outstanding Series A common stock in a modified “Dutch Auction” tender offer from public shareholders, which expired on April 3, 2007, at a purchase price of $30.50 per share. The total price paid for these shares was $62 million. The Company also separately purchased, through its wholly-owned subsidiary CIH, 329,011 shares of the Company’s Series A common stock at $30.50 per share from the investment funds associated with The Blackstone Group L.P. The total price paid for these shares was $10 million.
In June 2007, the Company’s Board of Directors authorized the repurchase of up to $330 million of its Series A common stock. During 2007, the Company repurchased 8,487,700 shares of its Series A common stock at an average purchase price of $38.88 per share for a total of $330 million pursuant to this authorization. The Company completed repurchasing shares related to this authorization during July 2007.
In February 2008, the Company’s Board of Directors authorized the repurchase of up to $400 million of the Company’s Series A common stock. This authorization was increased to $500 million in October 2008. The authorization gives management discretion in determining the conditions under which shares may be repurchased.
F-34
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
During the year ended December 31, 2008, the Company repurchased 9,763,200 shares of its Series A common stock at an average purchase price of $38.68 per share for a total of $378 million pursuant to this authorization.
These purchases reduced the number of shares outstanding and the repurchased shares may be used by the Company for compensation programs utilizing the Company’s stock and other corporate purposes. The Company accounts for treasury stock using the cost method and includes treasury stock as a component of Shareholders’ equity.
Accumulated Other Comprehensive Income (Loss), Net
Accumulated other comprehensive income (loss), net, which is displayed in the consolidated statements of shareholders’ equity, represents net earnings (loss) plus the results of certain shareholders’ equity changes not reflected in the consolidated statements of operations. Such items include unrealized gain (loss) on marketable securities, foreign currency translation, certain pension and postretirement benefit obligations and unrealized gain (loss) on interest rate swaps.
The after-tax components of Accumulated other comprehensive income (loss), net are as follows:
| Accumulated | ||||||||||||||||||||
| Unrealized | Unrealized | Other | ||||||||||||||||||
| Gain (Loss) on | Foreign | Pension and | Gain (Loss) | Comprehensive | ||||||||||||||||
| Marketable | Currency | Postretirement | on Interest | Income | ||||||||||||||||
| Securities | Translation | Benefits | Rate Swaps | (Loss), Net | ||||||||||||||||
| (In $ millions) | ||||||||||||||||||||
| Balance as of December 31, 2005 | (4 | ) | 12 | (136 | ) | 2 | (126 | ) | ||||||||||||
| Current-period change | 13 | 5 | 137 | 2 | 157 | |||||||||||||||
| Balance as of December 31, 2006 | 9 | 17 | 1 | 4 | 31 | |||||||||||||||
| Current-period change | 17 | 70 | 120 | (41 | ) | 166 | ||||||||||||||
| Balance as of December 31, 2007 | 26 | 87 | 121 | (37 | ) | 197 | ||||||||||||||
| Current-period change | (23 | )(1) | (130 | ) | (544 | ) | (79 | ) | (776 | ) | ||||||||||
| Balance as of December 31, 2008 | 3 | (43 | ) | (423 | ) | (116 | ) | (579 | ) | |||||||||||
| (1) | Includes a net reclassification adjustment of ($2) million to the consolidated statements of operations. |
| 18. | Other (Charges) Gains, Net |
Other (charges) gains, net includes provisions for restructuring and other expenses and income incurred outside the normal course of operations. Restructuring provisions represent costs related to severance and other benefit programs related to major activities undertaken to fundamentally redesign the business operations, as well as costs incurred in connection with decisions to exit non-strategic businesses. These measures are based on formal management decisions, establishment of agreements with employees’ representatives or individual agreements with affected employees, as well as the public announcement of the restructuring plan. The related reserves reflect certain estimates, including those pertaining to separation costs, settlements of contractual obligations and other closure costs. The Company reassesses the reserve requirements to complete each individual plan under existing restructuring programs at the end of each reporting period. Actual experience may be different from these estimates.
F-35
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The components of Other (charges) gains, net are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Employee termination benefits | (21 | ) | (32 | ) | (12 | ) | ||||||
| Plant/office closures | (7 | ) | (11 | ) | 1 | |||||||
| Deferred compensation triggered by Exit Event (Note 20) | — | (74 | ) | — | ||||||||
| Insurance recoveries associated with plumbing cases | — | 4 | 5 | |||||||||
| Insurance recoveries associated with Clear Lake, Texas (Note 29) | 38 | 40 | — | |||||||||
| Resolution of commercial disputes with a vendor | — | 31 | — | |||||||||
| Asset impairments | (115 | ) | (9 | )(1) | — | |||||||
| Ticona Kelsterbach plant relocation (Note 28) | (12 | ) | (5 | ) | — | |||||||
| Sorbates antitrust actions (Note 23) | 8 | — | — | |||||||||
| Other | 1 | (2 | ) | (4 | ) | |||||||
| Total Other (charges) gains, net | (108 | ) | (58 | ) | (10 | ) | ||||||
| (1) | Includes $6 million of goodwill impairment (Note 10). |
2008
Other (charges) gains, net for asset impairments includes long-lived asset impairment losses of $92 million related to the potential closure of the Company’s acetic acid and vinyl acetate monomer (“VAM”) production facility in Pardies, France, the VAM production unit in Cangrejera, Mexico (which the Company subsequently decided to shut down effective at the end of February 2009) and certain other facilities. Of the $92 million recorded in December 2008, $76 million relates to the Acetyl Intermediates segment and $16 million relates to the Advanced Engineered Materials segment. Consideration of this potential capacity reduction was necessitated by the significant change in the global economic environment and anticipated lower customer demand.
Additionally, the Company recognized $23 million of long-lived asset impairment losses related to the shutdown of the Company’s Pampa, Texas facility (Acetyl Intermediates segment).
Other (charges) gains, net for employee termination benefits includes severance and retention charges of $13 million related to the sale of the Company’s Pampa, Texas facility and $8 million of severance and retention charges related to other business optimization plans undertaken by the Company.
2007
Other (charges) gains, net for employee termination benefits and plant/office closures include charges related to the Company’s plan to simplify and optimize its Emulsions and PVOH businesses (Industrial Specialties segment) to become a leader in technology and innovation and grow in both new and existing markets. Other (charges) gains, net for employee termination benefits and plant/office closures also includes charges related to the sale of the Company’s Pampa, Texas facility. In addition, the Company recorded an impairment of long-lived assets of $3 million during the year ended December 31, 2007.
In December 2007, the Company received a one-time payment in resolution of commercial disputes with a vendor.
2006
Other (charges) gains, net includes charges related to severance associated with the relocation of corporate offices of $4 million, severance payments in connection with the lockout settlement at the Meredosia, Illinois plant of $5 million and severance and relocation expenses related to restructuring at AT Plastics (Industrial Specialties segment) of $4 million.
F-36
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
These charges were offset by $5 million of income related to insurance recoveries associated with plumbing cases.
The changes in the restructuring reserves by business segment are as follows:
| Advanced | ||||||||||||||||||||||||
| Engineered | Consumer | Industrial | Acetyl | |||||||||||||||||||||
| Materials | Specialties | Specialties | Intermediates | Other | Total | |||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||
Employee Termination Benefits | ||||||||||||||||||||||||
| Restructuring reserve as of December 31, 2006 | 4 | 13 | 5 | 8 | — | 30 | ||||||||||||||||||
| Restructuring additions | — | 13 | 9 | 18 | 2 | 42 | ||||||||||||||||||
| Cash payments | (2 | ) | (22 | ) | (3 | ) | (11 | ) | — | (38 | ) | |||||||||||||
| Currency translation adjustment | — | 1 | 1 | 1 | — | 3 | ||||||||||||||||||
| Restructuring reserve as of December 31, 2007 | 2 | 5 | 12 | 16 | 2 | 37 | ||||||||||||||||||
| Restructuring additions | 2 | 2 | 2 | 14 | 3 | 23 | ||||||||||||||||||
| Cash payments | (2 | ) | (6 | ) | (6 | ) | (9 | ) | (4 | ) | (27 | ) | ||||||||||||
| Currency translation adjustment | — | — | (2 | ) | (1 | ) | (1 | ) | (4 | ) | ||||||||||||||
| Restructuring reserve as of December 31, 2008 | 2 | 1 | 6 | 20 | — | 29 | ||||||||||||||||||
Plant/Office Closures | ||||||||||||||||||||||||
| Restructuring reserve as of December 31, 2006 | 1 | — | — | 2 | 2 | 5 | ||||||||||||||||||
| Restructuring additions | — | 1 | 1 | — | — | 2 | ||||||||||||||||||
| Cash payments | — | (2 | ) | — | — | (1 | ) | (3 | ) | |||||||||||||||
| Currency translation adjustment | — | 4 | — | — | — | 4 | ||||||||||||||||||
| Restructuring reserve as of December 31, 2007 | 1 | 3 | 1 | 2 | 1 | 8 | ||||||||||||||||||
| Restructuring additions | — | — | — | — | — | — | ||||||||||||||||||
| Cash payments | — | (1 | ) | (1 | ) | (1 | ) | (1 | ) | (4 | ) | |||||||||||||
| Currency translation adjustment | — | (1 | ) | — | — | — | (1 | ) | ||||||||||||||||
| Restructuring reserve as of December 31, 2008 | 1 | 1 | — | 1 | — | 3 | ||||||||||||||||||
| Total Restructuring reserves as of December 31, 2008 | 3 | 2 | 6 | 21 | — | 32 | ||||||||||||||||||
| 19. | Income Taxes |
Earnings (loss) from continuing operations before tax and minority interests by jurisdiction are as follows:
| Year Ended | ||||||||||||
| December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| US | 135 | (111 | ) | 116 | ||||||||
| Foreign | 299 | 558 | 410 | |||||||||
| Total | 434 | 447 | 526 | |||||||||
F-37
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The income tax provision (benefit) consists of the following:
| Year Ended | ||||||||||||
| December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Current: | ||||||||||||
| US | 62 | (9 | ) | 38 | ||||||||
| Foreign | 92 | 163 | 29 | |||||||||
| Total current | 154 | 154 | 67 | |||||||||
| Deferred: | ||||||||||||
| US | (37 | ) | 17 | 80 | ||||||||
| Foreign | (54 | ) | (61 | ) | 56 | |||||||
| Total deferred | (91 | ) | (44 | ) | 136 | |||||||
| Income tax provision | 63 | 110 | 203 | |||||||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the consolidated deferred tax assets and liabilities were as follows:
| As of | ||||||||
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Pension and postretirement obligations | 304 | 229 | ||||||
| Accrued expenses | 195 | 92 | ||||||
| Inventory | 8 | 2 | ||||||
| Net operating loss and tax credit carryforwards | 279 | 214 | ||||||
| Other | 192 | 33 | ||||||
| Subtotal | 978 | 570 | ||||||
Valuation allowance(1) | (652 | ) | (311 | ) | ||||
| �� | ||||||||
| Deferred tax assets | 326 | 259 | ||||||
| Depreciation and amortization | 322 | 384 | ||||||
| Investments | 41 | 26 | ||||||
| Interest | — | 7 | ||||||
| Other | 49 | 57 | ||||||
| Deferred tax liabilities | 412 | 474 | ||||||
| Net deferred tax assets (liabilities) | (86 | ) | (215 | ) | ||||
| (1) | Includes deferred tax asset valuation allowances primarily for the Company’s deferred tax assets in the US, the United Kingdom and China, as well as other foreign jurisdictions. These valuation allowances relate primarily to net operating loss carryforward benefits and other net deferred tax assets, all of which may not be realizable. |
For the year ended December 31, 2008, the valuation allowance increased by $341 million, consisting of: (1) charges to income tax expense of $11 million for continuing operations and discontinued operations, (2) an increase of $223 million allocated to accumulated other comprehensive income, (3) an increase of $41 million
F-38
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
related to CTA, (4) an increase of $16 million related to additional paid in capital and (5) $50 million of other increases related to preacquisition deferred tax assets, FIN 48 and other adjustments to deferred taxes.
A reconciliation of the significant differences between the US federal statutory tax rate of 35% and the effective income tax rate on income from continuing operations is as follows:
| Year Ended | ||||||||||||
| December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Income tax provision computed at US federal statutory tax rate | 152 | 156 | 184 | |||||||||
| Increase (decrease) in taxes resulting from: | ||||||||||||
| Change in valuation allowance | (5 | ) | 9 | 4 | ||||||||
| Equity income and dividends | (17 | ) | 8 | 5 | ||||||||
| Expenses not resulting in tax benefits | 18 | 19 | 15 | |||||||||
| US tax effect of foreign earnings and dividends | (5 | ) | 27 | 28 | ||||||||
Other foreign tax rate differentials(1) | (84 | ) | (98 | ) | (59 | ) | ||||||
| Legislative changes | 3 | (21 | ) | — | ||||||||
| State income taxes and other | 1 | 10 | 26 | |||||||||
| Income tax provision | 63 | 110 | 203 | |||||||||
| (1) | Includes impact of earnings from China and Singapore subject to tax holidays which expire between 2008 and 2013. |
Federal and state income taxes have not been provided on accumulated but undistributed earnings of $2.5 billion as of December 31, 2008, as such earnings have been permanently reinvested in the business. The determination of the amount of the unrecognized deferred tax liability related to the undistributed earnings is not practicable.
The effective tax rate for continuing operations for the year ended December 31, 2008 was 15% compared to 25% for the year ended December 31, 2007. The effective tax rate for 2008 was favorably impacted by: (1) an increase in unrepatriated low-taxed earnings, including tax holidays, (2) tax credits generated in foreign jurisdictions and (3) the US tax impact of foreign operations.
The Company operates under tax holidays in various countries which are effective through December 2013. In China, one of the Company’s entities has a tax holiday that provides for a zero percent tax rate in 2007 and 2008. For 2009 through 2011, the Company’s tax rate will be 50% of the statutory rate, or 12.5% based on the 2008 statutory rate of 25%. In Singapore, one of the Company’s entities has a tax holiday that provides for a zero percent tax rate through 2010. For 2011 through 2013, the Company’s tax rate will be 50% of the statutory rate, or 9% based on the current statutory rate of 18%. The impact of these tax holidays decreased foreign taxes $22 million for the year ended December 31, 2008.
The Corporate Tax Reform Act of 2008 was signed by the German Federal President in August 2007. The Act reduced the Company’s combined corporate statutory tax rate from 40% to 30% while imposing limitations on the deductibility of certain expenses, including interest expense. The Company recognized a tax benefit of approximately $39 million in 2007 related to the statutory rate reduction on its German net deferred tax liabilities.
Mexico enacted the 2008 Fiscal Reform Bill on October 1, 2007. Effective January 1, 2008, the bill repealed the existing asset-based tax and established a dual income tax system consisting of a new minimum flat tax (the “IETU”) and the existing regular income tax system. The IETU system taxes companies on cash basis net income, consisting only of certain specified items of revenue and expense, at a rate of 16.5%, 17% and 17.5% for 2008, 2009
F-39
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
and 2010 forward, respectively. In general, companies must pay the higher of the income tax or the IETU, although unlike the previous asset tax, the IETU is not creditable against future income tax liabilities. The Company has determined that it will primarily be subject to the IETU in future periods, and as such it has recorded tax expense of approximately $20 million in 2007 for the deferred tax effects of the new IETU system.
As of December 31, 2008, the Company had US federal net operating loss carryforwards of approximately $298 million which will begin to expire in 2021. Of this amount, $53 million relates to the preacquisition period and is subject to limitation. Of the remaining $245 million, $153 million is subject to limitation as a result of the change in stock ownership in May 2006. This limitation is not expected to have a material impact on utilization of the net operating loss carryforwards.
The Company also had foreign net operating loss carryforwards as of December 31, 2008 of approximately $435 million for China, Germany, Mexico and other foreign jurisdictions with various expiration dates. Net operating losses in China have various carryforward periods and begin expiring in 2011. Net operating losses in Germany have no expiration date. Net operating losses in Mexico have a ten year carryforward period and begin to expire in 2009. However, these losses are not available for use under the new IETU tax regulations in Mexico. As the IETU is the primary system upon which the Company will be subject to tax in future periods, no deferred tax asset has been reflected in the consolidated balance sheets as of December 31, 2008 for these income tax loss carryforwards.
The Company adopted the provisions of FIN 48 effective January 1, 2007. FIN 48 clarifies the accounting for income taxes by prescribing a minimum recognition threshold a tax benefit is required to meet before being recognized in the financial statements. FIN 48 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. As a result of the implementation of FIN 48, the Company increased Retained earnings by $14 million and decreased Goodwill by $2 million. In addition, certain tax liabilities for unrecognized tax benefits, as well as related potential penalties and interest, were reclassified from current liabilities to noncurrent liabilities. Liabilities for unrecognized tax benefits as of December 31, 2008 relate to various US and foreign jurisdictions.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
| Balance as of January 1, 2008 | 200 | 193 | ||||||
| Increases in tax positions for the current year | — | 2 | ||||||
| Increases in tax positions for prior years | 7 | 28 | ||||||
| Decreases in tax positions of prior years | (10 | ) | (21 | ) | ||||
| Settlements | (2 | ) | (2 | ) | ||||
| Balance as of December 31, 2008 | 195 | 200 | ||||||
Included in the unrecognized tax benefits as of December 31, 2008 and 2007 are $71 million and $56 million, respectively, of tax benefits that, if recognized, would reduce the Company’s effective tax rate.
The Company recognizes interest and penalties related to unrecognized tax benefits in the provision for income taxes. As of December 31, 2008 and 2007, the Company has recorded a liability of approximately $38 million and $36 million, respectively, for interest and penalties. This amount includes an increase of approximately $2 million and $13 million for the years ended December 31, 2008 and 2007, respectively.
The Company operates in the US (including multiple state jurisdictions), Germany and approximately 40 other foreign jurisdictions including Canada, China, France, Mexico and Singapore. Examinations are ongoing in a number of those jurisdictions including, most significantly, in Germany for the years 2001 to 2004. During the
F-40
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
quarter ended March 31, 2007, the Company received final assessments in Germany for the prior examination period, 1997 to 2000. The effective settlement of those examinations resulted in a reduction to Goodwill of approximately $42 million with a net expected cash outlay of $29 million. The Company’s US federal income tax returns for 2003 and beyond are open tax years not currently under examination. Unrecognized tax benefits are not expected to change significantly over the next 12 months.
The tax provision (benefit) amounts allocated to other comprehensive income (loss), net, were $5 million, $10 million and $11 million for the years ended December 31, 2008, 2007 and 2006 respectively. Of these amounts, $5 million, $10 million and $11 million were attributable to the pension and postretirement benefits component of other comprehensive income (loss), net. The income tax provision (benefit) associated with Accumulated other comprehensive income (loss), net is dependent upon the tax jurisdiction in which the items arise and accordingly could result in an effective tax rate that is different from the overall consolidated effective income tax rate on the consolidated statements of operations.
| 20. | Stock-Based and Other Management Compensation Plans |
In December 2004, the Company approved a stock incentive plan for executive officers, key employees and directors, a deferred compensation plan for executive officers and key employees as well as other management incentive programs.
The stock incentive plan allows for the issuance or delivery of up to 16,250,000 shares of the Company’s Series A common stock through the award of stock options, restricted stock units (“RSUs”) and other stock-based awards as may be approved by the Company’s Compensation Committee of the Board of Directors.
Deferred Compensation
The 2004 deferred compensation plan provides an aggregate maximum amount payable of $196 million. The initial component of the deferred compensation plan vested in 2004 and was paid in the first quarter of 2005. In May 2007, the Original Shareholders sold their remaining equity interest in the Company triggering an Exit Event, as defined by the plan. Cash compensation of $74 million, representing the participants’ 2005 and 2006 contingent benefits, was paid to the participants during the year ended December 31, 2007. Participants continuing in the 2004 deferred compensation plan (see below for discussion regarding certain participant’s decision to participate in a revised program) continue to vest in their 2008 and 2009 time-based and performance-based entitlements as defined in the deferred compensation plan. During the years ended December 31, 2008, 2007 and 2006, the Company recorded compensation expense of $3 million, $84 million and $19 million, respectively, associated with this plan. The remaining amount payable under the plan is $1 million.
On April 2, 2007, certain participants in the Company’s deferred compensation plan elected to participate in a revised program, which includes both cash awards and restricted stock units (see Restricted Stock Units below). Under the revised program, participants relinquished their cash awards of up to $30 million that would have contingently accrued from2007-2009 under the original plan. In lieu of these awards, the revised deferred compensation program provides for a future cash award in an amount equal to 90% of the maximum potential payout under the original plan, plus growth pursuant to one of three participant-selected notional investment vehicles, as defined in the associated agreements. Participants must remain employed through 2010 to vest in the new award. The Company will make award payments under the revised program in the first quarter of 2011, unless participants elect to further defer the payment of their individual awards. Based on current participation in the revised program, the awards aggregate to approximately $27 million plus notional earnings and will be recognized as expense through December 31, 2010. The Company expensed $8 million and $6 million during the years ended December 31, 2008 and 2007, respectively, related to the revised program.
In December 2007, the Company adopted a deferred compensation plan whereby certain of the Company’s senior employees and directors were offered the opportunity to defer a portion of their compensation in exchange for a future payment amount equal to their deferments plus or minus certain amounts based upon the market-
F-41
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
performance of specified measurement funds selected by the participant. Participants are required to make deferral elections under the plan prior to January 1 of the year such deferrals will be withheld from their compensation. The Company expensed $1 million during the year ended December 31, 2008 related to this plan.
In December 2008, the Company granted time-vesting cash awards of $22 million with the Company’s executive officers and certain other key employees. Each award of cash vests 30% on October 14, 2009, 30% on October 14, 2010 and 40% on October 14, 2011. In its sole discretion, the compensation committee of the Board of Directors may at any time convert all or a portion of the cash award to an award of time-vesting restricted stock units.
Long-Term Incentive Plan
Effective January 1, 2004, the Company adopted a long-term incentive plan (the “LTIP Plan”) which covers certain members of management and other key employees of the Company. The LTIP Plan is a three-year cash based plan in which awards are based on annual and three-year cumulative targets (as defined in the LTIP Plan). In February 2007, $26 million was paid to the LTIP plan participants. During the year ended December 31, 2006, the Company recorded expense of $19 million related to the LTIP Plan. There are no additional amounts due under the LTIP Plan.
Stock Options
The Company adopted the provisions of SFAS No. 123(R),Share-Based Payments (“SFAS No. 123(R)”), on January 1, 2006. The Company elected the modified prospective transition method under which compensation costs are recognized for all stock-based payments granted prior to, but not yet vested as of January 1, 2006 and all stock-based payments granted subsequent to January 1, 2006. Compensation costs are based on the grant-date fair value estimated in accordance with SFAS No. 123(R).
The Company has a stock-based compensation plan that makes awards of stock options to certain employees. It is the Company’s current policy to grant options with an exercise price equal to the average of the high and low price of the Company’s Series A common stock on the grant date. The options issued have a ten-year term and vest on a graded basis over periods ranging from one to five years. The estimated value of the Company’s stock-based awards less expected forfeitures is amortized over the awards’ respective vesting period on a straight-line basis.
The fair value of each option granted is estimated on the grant date using the Black-Scholes option pricing method. The weighted average assumptions used in the model are outlined in the following table:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Risk-free interest rate | 3.3 | % | 4.6 | % | ||||
| Estimated life in years | 7.7 | 6.8 | ||||||
| Dividend yield | 0.38 | % | 0.42 | % | ||||
| Volatility | 31.4 | % | 27.5 | % | ||||
The computation of the expected volatility assumption used in the Black-Scholes calculations for new grants is based on the Company’s historical volatilities and volatilities of peer companies. When establishing the expected life assumptions, the Company reviews annual historical employee exercise behavior of option grants with similar vesting periods and the expected life assumptions of peer companies. The Company utilizes the review of peer companies based on its own lack of extensive history.
F-42
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of changes in stock options outstanding is as follows:
| Year Ended December 31, 2008 | ||||||||||||||||
| Weighted- | Weighted- | |||||||||||||||
| Average | Average | |||||||||||||||
| Grant | Remaining | Aggregate | ||||||||||||||
| Number of | Price in | Contractual | Intrinsic | |||||||||||||
| Options | $ | Term | Value | |||||||||||||
| (In millions) | (In years) | (In $ millions) | ||||||||||||||
| Outstanding at beginning of year | 8.1 | 18.72 | 7.5 | 192 | ||||||||||||
| Granted | 0.2 | 42.12 | ||||||||||||||
| Exercised | (1.1 | ) | 17.35 | |||||||||||||
| Forfeited | (0.2 | ) | 22.72 | |||||||||||||
| Outstanding at end of year | 7.0 | 19.35 | 6.6 | — | ||||||||||||
| Options exercisable at end of year | 5.3 | 16.41 | 6.2 | — | ||||||||||||
The weighted-average grant-date fair value of stock options granted during the years ended December 31, 2008 and 2007 was $16.78 and $14.42, respectively, per option. As of December 31, 2008, the Company had approximately $12 million of total unrecognized compensation expense related to stock options, excluding estimated forfeitures, to be recognized over the remaining vesting periods of the options. Cash received from stock option exercises was $18 million during the year ended December 31, 2008. There was no tax benefit realized from stock option exercises during the year ended December 31, 2008.
Restricted Stock Units
Performance-based RSUs. Participants in the revised deferred compensation program also received an award of RSUs. The RSUs, which were granted in April 2007, cliff vest on December 31, 2010 and have a fair value of $23.63 per unit. The number of RSUs that ultimately vest depends on market performance targets measured by comparison of the Company’s stock performance versus a defined peer group. The ultimate award will range from zero to 263,030 RSUs, based on the market performance measurement at the cliff vesting date. The market performance feature is factored into the estimated fair value per unit, and compensation expense for the award is based on the maximum RSUs of 263,030. Dividends on RSUs are earned in accordance with the Company’s common stock dividend policy and are reinvested in additional RSUs.
In addition, under the 2007 Long-term Performance Program, the Company granted RSUs with a fair value of $21.30 per unit to certain employees. The RSUs vest annually in equal tranches beginning September 30, 2008 through September 30, 2011. The RSUs contain the same market performance criteria as those described above, with an ultimate award that will range from zero to 947,361 RSUs. The awards include acatch-up provision that provides for vesting on September 30, 2012 of previously unvested amounts, subject to certain maximums. Dividends on RSUs are earned in accordance with the Company’s common stock dividend policy and are reinvested in additional RSUs.
F-43
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of changes in RSUs outstanding under the revised deferred compensation program and the 2007 Long-term Performance Program during the year ended December 31, 2008 is presented as follows:
| Performance-based RSU’s | ||||||||
| Weighted | ||||||||
| Average | ||||||||
| Number of | Fair Value in | |||||||
| Units | $ | |||||||
| (Units in thousands) | ||||||||
| Nonvested at beginning of year | 1,119 | 21.84 | ||||||
| Granted | — | — | ||||||
| Vested | (143 | ) | 21.30 | |||||
| Forfeited | (85 | ) | 21.30 | |||||
| Nonvested at end of year | 891 | 21.98 | ||||||
In December 2008, the Company granted RSUs to certain employees with a fair value of $12.65 per unit. The RSUs vest on October 14, 2011. The number of RSUs that ultimately vest is dependent upon the achievement of 1) internal profitability targets and 2) market performance targets measured by the comparison of the Company’s stock performance versus a defined peer group. The ultimate award will range from zero to 669,150 RSUs. The market performance feature is factored into the estimated fair value per unit.
The Company also made a grant in December 2008 of 200,000 performance units to be settled in cash to the Company’s Chief Executive Officer. The terms of the performance units are substantially similar to the performance-based RSUs granted in December 2008. The value of the performance units is equivalent to the value of one share of the Company’s Series A common stock and any amounts that may vest under the performance unit award agreement are to be settled in cash, rather than shares of the Company’s Series A common stock. The compensation committee of the Board of Directors may elect to convert all or any portion of the performance units award to an award of an equivalent value of performance-based RSUs.
Fair value for the Company’s performance-based RSUs was estimated at the grant date using a Monte Carlo simulation approach. Monte Carlo simulation was utilized to randomly generate future stock returns for the Company and each company in the designated peer group for each grant based on company-specific dividend yields, volatilities and stock return correlations. These returns were used to calculate future RSU vesting percentages and the simulated values of the vested RSUs were then discounted to present value using a risk-free rate, yielding the expected value of these RSUs.
The range of assumptions used in the Monte Carlo simulation approach is outlined in the following table:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Risk-free interest rate | 1.05 | % | 4.53 — 4.55 | % | ||||
| Dividend yield | 0.00 — 12.71 | % | 0.00 — 2.76 | % | ||||
| Volatility | 20 — 70 | % | 20 — 45 | % | ||||
Time-based RSUs. During 2007 and 2008, the Company granted non-employee Directors time-based RSUs that generally vest one year after grant. During 2008, the Company granted time-based RSUs to certain employees that vest ratably over time intervals ranging from one to four years. The fair value of the time-based RSUs was equal to the average of the high and low price of the Company’s Series A common stock on the grant date.
F-44
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of changes in time-based RSUs outstanding during the year ended December 31, 2008 is as follows:
| Employee Time-based RSUs | Director Time-Based RSUs | |||||||||||||||
| Weighted | Weighted | |||||||||||||||
| Average | Average | |||||||||||||||
| Number of | Fair Value in | Number of | Fair Value in | |||||||||||||
| Units | $ | Units | $ | |||||||||||||
| Shares in 000’s | ||||||||||||||||
| Nonvested at beginning of year | — | — | 23 | 32.61 | ||||||||||||
| Granted | 106 | 39.35 | 15 | 44.02 | ||||||||||||
| Vested | — | — | (23 | ) | 32.61 | |||||||||||
| Forfeited | (1 | ) | 39.53 | — | — | |||||||||||
| Nonvested at end of year | 105 | 39.34 | 15 | 44.02 | ||||||||||||
As of December 31, 2008, there was approximately $19 million of unrecognized compensation cost related to RSUs, excluding estimated forfeitures, which will be amortized on a straight-line basis over the remaining vesting periods.
| 21. | Leases |
Total rent expense charged to operations under all operating leases was $96 million, $122 million and $109 million for the years ended December 31, 2008, 2007 and 2006, respectively. Future minimum lease payments under non-cancelable rental and lease agreements which have initial or remaining terms in excess of one year as of December 31, 2008 are as follows:
| Capital | Operating | |||||||
| (In $ millions) | ||||||||
| 2009 | 38 | 45 | ||||||
| 2010 | 55 | 27 | ||||||
| 2011 | 33 | 21 | ||||||
| 2012 | 33 | 14 | ||||||
| 2013 | 30 | 11 | ||||||
| Later years | 228 | 22 | ||||||
| Sublease income | — | (34 | ) | |||||
| Minimum lease commitments | 417 | 106 | ||||||
| Less amounts representing interest | 206 | |||||||
| Present value of net minimum lease obligations | 211 | |||||||
The Company expects that, in the normal course of business, leases that expire will be renewed or replaced by other leases.
| 22. | Financial Instruments |
Interest Rate Risk Management
To reduce the interest rate risk inherent in the Company’s variable rate debt, the Company utilizes interest rate swap agreements to convert a portion of the variable rate debt to a fixed rate obligation. These interest rate swap agreements are designated as cash flow hedges. If an interest rate swap agreement is terminated prior to its maturity, the amount previously recorded in Accumulated other comprehensive income (loss), net is recognized into earnings over the period that the hedged transaction impacts earnings. If the hedging relationship is discontinued because it is probable that the forecasted transaction will not occur according to the original strategy, any related amounts previously recorded in Accumulated other comprehensive income (loss), net are recognized into earnings immediately.
F-45
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As of December 31, 2006, the Company had an interest rate swap agreement in place with a notional value of $300 million. On March 29, 2007, in connection with the April 2007 debt refinancing, the Company terminated this interest rate swap agreement and recognized a gain of $2 million related to amounts previously recorded in Accumulated other comprehensive income (loss), net.
In March 2007, in anticipation of the April 2007 debt refinancing, the Company entered into various US dollar and Euro interest rate swap agreements, which became effective on April 2, 2007, with notional amounts of $1.6 billion and €150 million, respectively. The notional amount of the $1.6 billion US dollar interest rate swaps decreased by $400 million effective January 2, 2008 and decreased by another $200 million effective January 2, 2009. To offset the declines, the Company entered into US dollar interest rate swaps with a combined notional amount of $400 million which became effective on January 2, 2008 and an additional US dollar interest rate swap with a notional amount of $200 million which will become effective April 2, 2009.
The Company recognized interest (expense) income from hedging activities relating to interest rate swaps of ($18) million, $6 million and $1 million for the years ended December 31, 2008, 2007 and 2006, respectively. The Company recorded a net loss of less than $1 million to Other income (expense), net for the ineffective portion of the interest rate swap agreements for each of the years ended December 31, 2008, 2007 and 2006. The Company recorded an unrealized loss on interest rate swaps of $79 million during the year ended December 31, 2008.
Foreign Exchange Risk Management
Certain entities have receivables and payables denominated in currencies other than their respective functional currencies, which creates foreign exchange risk. The Company enters into foreign currency forwards and swaps to minimize its exposure to foreign currency fluctuations. The currently outstanding foreign currency contracts are hedging booked exposure, however the Company may from time to time hedge its currency exposure related to forecasted transactions. Forward contracts are not designated as hedges under SFAS No. 133.
The following table indicates the total US dollar equivalents of net foreign exchange exposure related to (short) long foreign exchange forward contracts outstanding by currency. All of the contracts included in the table below will have approximately offsetting effects from actual underlying payables, receivables, intercompany loans or other assets or liabilities subject to foreign exchange remeasurement.
| 2009 Maturity | ||||
| (In $ millions) | ||||
Currency | ||||
| Euro | 145 | |||
| British pound sterling | (115 | ) | ||
| Mexican peso | 80 | |||
| Singapore dollar | 26 | |||
| Canadian dollar | 21 | |||
| Japanese yen | 9 | |||
| Brazilian real | (7 | ) | ||
| Swedish krona | 6 | |||
| Hungarian forint | (5 | ) | ||
| Other | (3 | ) | ||
| Total | 157 | |||
To protect the foreign currency exposure of a net investment in a foreign operation, the Company entered into cross currency swaps with certain financial institutions in 2004. The cross currency swaps and the Euro-denominated portion of the senior term loan were designated as a hedge of a net investment of a foreign operation. The Company dedesignated the net investment hedge due to the debt refinancing in April 2007 and redesignated the
F-46
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
cross currency swaps and new senior Euro term loan in July 2007. As a result, the Company recorded $26 million of mark-to-market losses related to the cross currency swaps and the new senior Euro term loan during this period.
Under the terms of the cross currency swap arrangements, the Company paid approximately €13 million in interest and received approximately $16 million in interest on June 15 and December 15 of each year. The fair value of the net obligation under the cross currency swaps was included in current Other liabilities in the consolidated balance sheets as of December 31, 2007. Upon maturity of the cross currency swap agreements in June 2008, the Company owed €276 million ($426 million) and was owed $333 million. In settlement of the obligation, the Company paid $93 million (net of interest of $3 million) in June 2008.
During the year ended December 31, 2008, the Company dedesignated €385 million of the €400 Euro-denominated portion of the term loan, previously designated as a hedge of a net investment of a foreign operation. Prior to this dedesignation, the Company had been using external derivative contracts to offset foreign currency exposures on intercompany loans. The foreign currency exposure resulting from dedesignation of €385 million of the hedge of a net investment of a foreign operation is expected to offset the foreign currency exposure on certain intercompany loans, decreasing the need for external derivative contracts and reducing the Company’s exposure to external counterparties. The remaining €15 million Euro-denominated portion of the term loan continues to be designated as a hedge of a net investment of a foreign operation.
The effective portion of the gain (loss) on the derivative (cross currency swaps) is recorded in Accumulated other comprehensive income (loss), net. For the years ended December 31, 2008, 2007 and 2006, the amount charged to Accumulated other comprehensive income (loss), net was $(19) million, $(19) million and $(23) million, respectively. The gain (loss) related to items excluded from the assessment of hedge effectiveness of the cross currency swaps are recorded to Other income (expense), net in the consolidated statements of operations. For the years ended December 31, 2008, 2007 and 2006, the amount charged to Other income (expense), net in the consolidated statements of operations was $1 million, $(6) million and $5 million, respectively.
Commodity Risk Management
The Company has exposure to the prices of commodities in its procurement of certain raw materials. The Company manages its exposure primarily through the use of long-term supply agreements and derivative instruments. The Company regularly assesses its practice of purchasing a portion of its commodity requirements forward and utilization of other raw material hedging instruments, in addition to forward purchase contracts, in accordance with changes in market conditions. Forward purchases and swap contracts for raw materials are principally settled through actual delivery of the physical commodity. For qualifying contracts, the Company has elected to apply the normal purchases and normal sales exception of SFAS No. 133, as amended, as it was probable at the inception and throughout the term of the contract that they would not settle net and would result in physical delivery. As such, realized gains and losses on these contracts are included in the cost of the commodity upon the settlement of the contract.
In addition, the Company occasionally enters into financial derivatives to hedge a component of a raw material or energy source. Typically, these types of transactions do not qualify for hedge accounting. These instruments are marked to market at each reporting period and gains (losses) are included in Cost of sales in the consolidated statements of operations. The Company recognized no gain or loss from these types of contracts during the year ended December 31, 2008 and less than $1 million during each of the years ended December 31, 2007 and 2006, respectively. As of December 31, 2008, the Company did not have any open financial derivative contracts for commodities.
Fair Value of Financial Instruments
As discussed in Note 2, the Company adopted certain provisions of SFAS No. 157 on January 1, 2008. SFAS No. 157 establishes a three-tiered fair value hierarchy that prioritizes inputs to valuation techniques used in fair value calculations. The three levels of inputs are defined as follows:
Level 1 — unadjusted quoted prices for identical assets or liabilities in active markets accessible by the Company
F-47
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Level 2 — inputs that are observable in the marketplace other than those inputs classified as Level 1
Level 3 — inputs that are unobservable in the marketplace and significant to the valuation
SFAS No. 157 requires the Company to maximize the use of observable inputs and minimize the use of unobservable inputs. If a financial instrument uses inputs that fall in different levels of the hierarchy, the instrument will be categorized based upon the lowest level of input that is significant to the fair value calculation.
The Company’s financial assets and liabilities are measured at fair value on a recurring basis and include securities available for sale and derivative financial instruments. Securities available for sale include US government and corporate bonds, mortgage-backed securities and equity securities. Derivative financial instruments include interest rate swaps and foreign currency forwards and swaps.
Marketable Securities. Where possible, the Company utilizes quoted prices in active markets to measure debt and equity securities; such items are classified as Level 1 in the hierarchy and include equity securities and US government bonds. When quoted market prices for identical assets are unavailable, varying valuation techniques are used. Common inputs in valuing these assets include, among others, benchmark yields, issuer spreads, forward mortgage-backed securities trade prices and recently reported trades. Such assets are classified as Level 2 in the hierarchy and typically include mortgage-backed securities, corporate bonds and other US government securities.
Derivatives. Derivative financial instruments are valued in the market using discounted cash flow techniques. These techniques incorporate Level 1 and Level 2 inputs such as interest rates and foreign currency exchange rates. These market inputs are utilized in the discounted cash flow calculation considering the instrument’s term, notional amount, discount rate and credit risk. Significant inputs to the derivative valuation for interest rate swaps and foreign currency forwards and swaps are observable in the active markets and are classified as Level 2 in the hierarchy.
The following fair value hierarchy table presents information about the Company’s assets and liabilities measured at fair value on a recurring basis:
| Fair Value Measurement as of | ||||||||||||
| December 31, 2008 Using | ||||||||||||
| Quoted Prices in | ||||||||||||
| Active Markets for | Significant Other | As of | ||||||||||
| Identical Assets | Observable Inputs | December 31, | ||||||||||
| (Level 1) | (Level 2) | 2008 | ||||||||||
| (In $ millions) | ||||||||||||
Assets | ||||||||||||
| Marketable securities | 42 | 58 | 100 | |||||||||
| Current derivatives (included in Non-trade receivables) | — | 54 | 54 | |||||||||
| Total assets | 42 | 112 | 154 | |||||||||
Liabilities | ||||||||||||
| Current derivatives (included in current Other liabilities) | — | (65 | ) | (65 | ) | |||||||
| Noncurrent derivatives (included in noncurrent Other liabilities) | — | (76 | ) | (76 | ) | |||||||
| Total liabilities | — | (141 | ) | (141 | ) | |||||||
F-48
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Summarized below are the carrying values and estimated fair values of financial instruments that are not carried at fair value on our consolidated balance sheets:
| As of December 31, | ||||||||||||||||
| 2008 | 2007 | |||||||||||||||
| Carrying | Fair | Carrying | Fair | |||||||||||||
| Amount | Value | Amount | Value | |||||||||||||
| (In $ millions) | ||||||||||||||||
| Cost investments | 184 | — | 189 | — | ||||||||||||
| Insurance contracts in nonqualified pension trusts | 67 | 67 | 74 | 74 | ||||||||||||
| Long-term debt, including current installments of long-term debt | 3,381 | 2,404 | 3,328 | 3,211 | ||||||||||||
In general, the cost investments included in the table above are not publicly traded and their fair values are not readily determinable; however, the Company believes the carrying values approximate or are less than the fair values.
As of December 31, 2008 and 2007, the fair values of cash and cash equivalents, receivables, trade payables, short-term debt and the current installments of long-term debt approximate carrying values due to the short-term nature of these instruments. These items have been excluded from the table. Additionally, certain noncurrent receivables, principally insurance recoverables, are carried at net realizable value.
The fair value of long-term debt and debt-related financial instruments is based upon valuations from third-party banks and market quotations.
23. Commitments and Contingencies
The Company is involved in a number of legal proceedings, lawsuits and claims incidental to the normal conduct of business, relating to such matters as product liability, antitrust, past waste disposal practices and release of chemicals into the environment. While it is impossible at this time to determine with certainty the ultimate outcome of these proceedings, lawsuits and claims, the Company is actively defending those matters where the Company is named as a defendant. Additionally, the Company believes it has determined its best estimate, based on the advice of legal counsel, that adequate reserves have been made and that the ultimate outcomes will not have a material adverse effect on the financial position of the Company; however, the ultimate outcome of any given matter may have a material impact on the results of operations or cash flows of the Company in any given reporting period.
Plumbing Actions
CNA Holdings, Inc. (“CNA Holdings”), a US subsidiary of the Company, which included the US business now conducted by the Ticona business which is included in the Advanced Engineered Materials segment, along with Shell Oil Company (“Shell”), E.I. DuPont de Nemours and Company (“DuPont”) and others, has been a defendant in a series of lawsuits, including a number of class actions, alleging that plastics manufactured by these companies that were utilized in the production of plumbing systems for residential property were defective or caused such plumbing systems to fail. Based on, among other things, the findings of outside experts and the successful use of Ticona’s acetal copolymer in similar applications, CNA Holdings does not believe Ticona’s acetal copolymer was defective or caused the plumbing systems to fail. In many cases CNA Holdings’ potential future exposure may be limited by invocation of the statute of limitations since CNA Holdings ceased selling the resin for use in the plumbing systems in site-built homes during 1986 and in manufactured homes during 1990.
In November 1995, CNA Holdings, DuPont and Shell entered into national class action settlements which called for the replacement of plumbing systems of claimants who have had qualifying leaks, as well as reimbursements for certain leak damage. In connection with such settlement, the three companies had agreed to fund these replacements and reimbursements up to an aggregate amount of $950 million. As of December 31,
F-49
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
2008, the aggregate funding is $1,103 million, due to additional contributions and funding commitments made primarily by other parties.
During the period between 1995 and 2001, CNA Holdings was also named as a defendant in the following putative class actions:
| • | Cox, et al. v. Hoechst Celanese Corporation, et al.,No. 94-0047 (Chancery Ct., Obion County, Tennessee) (class was certified). | |
| • | Couture, et al. v. Shell Oil Company, et al.,No. 200-06-000001-985 (Quebec Superior Court, Canada). | |
| • | Dilday, et al. v. Hoechst Celanese Corporation, et al., No. 15187 (Chancery Ct., Weakley County, Tennessee). | |
| • | Furlan v. Shell Oil Company, et al., No. C967239 (British Columbia Supreme Court, Vancouver Registry, Canada). | |
| • | Gariepy, et al. v. Shell Oil Company, et al., No. 30781/99 (Ontario Court General Division, Canada). | |
| • | Shelter General Insurance Co., et al. v. Shell Oil Company, et al., No. 16809 (Chancery Ct., Weakley County, Tennessee). | |
| • | St. Croix Ltd., et al. v. Shell Oil Company, et al., No. 1997/467 (Territorial Ct., St. Croix Division, the US Virgin Islands). | |
| • | Tranter v. Shell Oil Company, et al., No. 46565/97 (Ontario Court General Division, Canada). |
In addition, between 1994 and 2008 CNA Holdings was named as a defendant in numerous non-class actions filed in Arizona, Florida, Georgia, Louisiana, Mississippi, New Jersey, Tennessee and Texas, the US Virgin Islands and Canada of which ten are currently pending. In all of these actions, the plaintiffs have sought recovery for alleged damages caused by leaking polybutylene plumbing. Damage amounts have generally not been specified but these cases generally do not involve (either individually or in the aggregate) a large number of homes.
As of December 31, 2008, the Company had remaining accruals of $64 million, of which $2 million is included in current Other liabilities in the consolidated balance sheets. As of December 31, 2007, the Company had remaining accruals of $65 million, of which $3 million was included in current Other liabilities in the consolidated balance sheets.
The Company reached settlements with CNA Holdings’ insurers specifying their responsibility for these claims. During the year ended December 31, 2007, the Company received $23 million of insurance proceeds from various CNA Holdings’ insurers as full satisfaction for their responsibility for these claims.
Plumbing Insurance Indemnifications
Celanese GmbH entered into agreements with insurance companies related to product liability settlements associated with Celcon® plumbing claims. These agreements, except those with insolvent insurance companies, require the Company to indemnifyand/or defend these insurance companies in the event that third parties seek additional monies for matters released in these agreements. The indemnifications in these agreements do not provide for time limitations.
In certain of the agreements, Celanese GmbH received a fixed settlement amount. The indemnities under these agreements generally are limited to, but in some cases are greater than, the amount received in settlement from the insurance company. The maximum exposure under these indemnifications is $95 million. Other settlement agreements have no stated limits.
F-50
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
There are other agreements whereby the settling insurer agreed to pay a fixed percentage of claims that relate to that insurer’s policies. The Company has provided indemnifications to the insurers for amounts paid in excess of the settlement percentage. These indemnifications do not provide for monetary or time limitations.
Sorbates Antitrust Actions
In May 2002, the European Commission informed Hoechst AG (“Hoechst”) of its intent to officially investigate the sorbates industry. In early January 2003, the European Commission served Hoechst, Nutrinova, Inc., a US subsidiary of Nutrinova Nutrition Specialties & Food Ingredients GmbH and previously a wholly owned subsidiary of Hoechst (“Nutrinova”), and a number of competitors of Nutrinova with a statement of objections alleging unlawful, anticompetitive behavior affecting the European sorbates market. In October 2003, the European Commission ruled that Hoechst, Chisso Corporation, Daicel Chemical Industries Ltd. (“Daicel”), The Nippon Synthetic Chemical Industry Co. Ltd. and Ueno Fine Chemicals Industry Ltd. operated a cartel in the European sorbates market between 1979 and 1996. The European Commission imposed a total fine of €138 million on such companies, of which €99 million was assessed against Hoechst and its legal successors. The case against Nutrinova was closed. Pursuant to the Demerger Agreement with Hoechst, Celanese GmbH was assigned the obligation related to the sorbates antitrust matter; however, Hoechst, and its legal successors, agreed to indemnify Celanese GmbH for 80% of any costs Celanese GmbH incurred relative to this matter. Accordingly, Celanese GmbH recognized a receivable from Hoechst from this indemnification. In June 2008, the Court of First Instance of the European Communities (Fifth Chamber) reduced the fine against Hoechst to €74.25 million and in July 2008, Hoechst paid the €74.25 million fine. In August 2008, the Company paid Hoechst €17 million, including interest of €2 million, in satisfaction of its 20% obligation with respect to the fine.
Based on the advice of external counsel and a review of the existing facts and circumstances relating to the sorbates antitrust matters, including the settlement of the European Union’s investigation, as well as civil claims filed and settled, the Company released its accruals related to the settled sorbates antitrust matters and the indemnification receivables resulting in a gain of $8 million, net, included in Other (charges) gains, net, in the consolidated statements of operations. As of December 31, 2007, the Company had indemnification receivables of $137 million included in current Other assets and accruals of $170 million included in current Other liabilities in the consolidated balance sheets.
In addition, in 2004 a civil antitrust action styledFreeman Industries LLC v. Eastman Chemical Co., et. al.was filed against Hoechst and Nutrinova, Inc. in the Law Court for Sullivan County in Kingsport, Tennessee. The plaintiff sought monetary damages and other relief for alleged conduct involving the sorbates industry. The trial court dismissed the plaintiff’s claims and upon appeal the Supreme Court of Tennessee affirmed the dismissal of the plaintiff’s claims. In December 2005, the plaintiff lost an attempt to amend its complaint and the entire action was dismissed with prejudice by the trial court. Plaintiff’s counsel has subsequently filed a new complaint with new class representatives in the District Court of the District of Tennessee. The Company’s motion to strike the class allegations was granted in April 2008 and the plaintiff’s appeal of such ruling is currently pending.
Acetic Acid Patent Infringement Matters
On May 9, 1999, Celanese International Corporation filed a private criminal action styledCelanese International Corporation v. China Petrochemical Development Corporationagainst China Petrochemical Development Corporation (“CPDC”) in the Taiwan Kaoshiung District Court alleging that CPDC infringed Celanese International Corporation’s patent covering the manufacture of acetic acid. Celanese International Corporation also filed a supplementary civil brief which, in view of changes in Taiwanese patent laws, was subsequently converted to a civil action alleging damages against CPDC based on a period of infringement of ten years,1991-2000, and based on CPDC’s own data which was reported to the Taiwanese securities and exchange commission. Celanese International Corporation’s patent was held valid by the Taiwanese patent office. On August 31, 2005, the District Court held that CPDC infringed Celanese International Corporation’s acetic acid patent and awarded Celanese International Corporation approximately $28 million (plus interest) for the period of 1995 through 1999. In October 2008, the High Court, on appeal, reversed the District Court’s $28 million award to the Company. The Company is appealing. On January 16, 2006, the District Court
F-51
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
awarded Celanese International Corporation $800,000 (plus interest) for the year 1990. In January 2009, the High Court, on appeal, affirmed the District Court’s award and CPDC appealed on February 5, 2009. On June 29, 2007, the District Court awarded Celanese International Corporation $60 million (plus interest) for the period of 2000 through 2005. CPDC is appealing this award.
Domination Agreement
On October 1, 2004, a Domination Agreement between Celanese GmbH and the Purchaser became operative. When the Domination Agreement became operative, the Purchaser became obligated to offer to acquire all outstanding Celanese GmbH shares from the minority shareholders of Celanese GmbH in return for payment of fair cash compensation. The amount of this fair cash compensation was determined to be €41.92 per share, plus interest, in accordance with applicable German law. Until the Squeeze-Out was registered in the commercial register in Germany on December 22, 2006, any minority shareholder who elected not to sell its shares to the Purchaser was entitled to remain a shareholder of Celanese GmbH and to receive from the Purchaser a gross guaranteed annual payment on its shares of €3.27 per Celanese GmbH share less certain corporate taxes in lieu of any dividend. For the year ended December 31, 2006, a charge of €3 million ($4 million) was recorded to Other income (expense), net for the anticipated guaranteed annual payment.
On June 1, 2006, the guaranteed annual payment for the fiscal year ended September 30, 2005, which amounted to €3 million, was paid. In addition, pursuant to a settlement agreement entered into with plaintiff shareholders in March 2006, the Purchaser paid €1 million on June 30, 2006, the guaranteed annual payment for the fiscal year ended September 30, 2006, to those shareholders who signed a letter waiving any further rights with respect to such guaranteed annual payment that ordinarily would become due and payable after the 2007 annual general meeting. Between June 30, 2006, and January 17, 2007, the Purchaser paid a total amount of less than €1 million to minority shareholders who required early payment of the guaranteed annual payment for the fiscal year ended September 30, 2006, by submitting such waiver letter after June 30, 2006.
On January 17, 2007, the Purchaser made, pursuant to a settlement agreement entered into with plaintiff shareholders in December 2006, the following guaranteed annual payments: (i) a total amount of €1 million was paid to all minority shareholders who had not yet requested early payment of the guaranteed annual payment for the fiscal year ended on September 30, 2006, and (ii) a total amount of €1 million representing the pro rata share of the guaranteed annual payment for the first five months of the fiscal year ending September 30, 2007 was paid to all minority shareholders.
The Domination Agreement cannot be terminated by the Purchaser in the ordinary course of business until September 30, 2009. The Company’s subsidiaries, Celanese International Holdings Luxembourg S.à r.l. (“CIH”), formerly Celanese Caylux Holdings Luxembourg S.C.A., and Celanese US, have each agreed to provide the Purchaser with financing to strengthen the Purchaser’s ability to fulfill its obligations under, or in connection with, the Domination Agreement and to ensure that the Purchaser will perform all of its obligations under, or in connection with, the Domination Agreement when such obligations become due, including, without limitation, the obligation to compensate Celanese GmbH for any statutory annual loss incurred by Celanese GmbH during the term of the Domination Agreement. If CIHand/or Celanese US are obligated to make payments under such guarantees or other security to the Purchaser, the Company may not have sufficient funds for payments on its indebtedness when due. The Company has not had to compensate Celanese GmbH for an annual loss for any period during which the Domination Agreement has been in effect.
The amounts of the fair cash compensation and of the guaranteed annual payment offered under the Domination Agreement are under court review in special award proceedings. As a result of these proceedings, either amount could be increased by the court so that all former Celanese GmbH shareholders, including those who have already tendered their shares into the mandatory offer and have received the fair cash compensation could claim the respective higher amounts. Certain former Celanese GmbH shareholders may initiate such proceedings also with respect to the Squeeze-Out compensation. In this case, former Celanese GmbH shareholders who ceased to be shareholders of Celanese
F-52
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
GmbH due to the Squeeze-Out are entitled, pursuant to a settlement agreement between the Purchaser and certain former Celanese GmbH shareholders, to claim for their shares the higher of the compensation amounts determined by the court in these different proceedings. Payments these shareholders already received as compensation for their shares will be offset so that those shareholders who ceased to be shareholders of Celanese GmbH due to the Squeeze-Out are not entitled to more than the higher of the amount set in the two court proceedings.
Shareholder Litigation
The amounts of the fair cash compensation and of the guaranteed annual payment offered under the Domination Agreement may be increased in special award proceedings initiated by minority shareholders, which may further reduce the funds the Purchaser can otherwise make available to the Company. As of March 30, 2005, several minority shareholders of Celanese GmbH had initiated special award proceedings seeking the court’s review of the amounts of the fair cash compensation and of the guaranteed annual payment offered under the Domination Agreement. As a result of these proceedings, the amount of the fair cash consideration and the guaranteed annual payment offered under the Domination Agreement could be increased by the court so that all minority shareholders, including those who have already tendered their shares into the mandatory offer and have received the fair cash compensation could claim the respective higher amounts. The court dismissed all of these proceedings in March 2005 on the grounds of inadmissibility. Thirty-three plaintiffs appealed the dismissal, and in January 2006, twenty-three of these appeals were granted by the court. They were remanded back to the court of first instance, where the valuation will be further reviewed. On December 12, 2006, the court of first instance appointed an expert to help determine the value of Celanese GmbH. In the first quarter of 2007, certain minority shareholders that received €66.99 per share as fair cash compensation also filed award proceedings challenging the amount they received as fair cash compensation.
As a result of the special proceedings discussed above, amounts paid as fair cash compensation to certain minority shareholders of Celanese GmbH could be increased by the court such that minority shareholders could be awarded amounts in excess of the fair cash compensation they have previously received.
The Company received applications for the commencement of award proceedings filed by 79 shareholders against the Purchaser with the Frankfurt District Court requesting the court to set a higher amount for the Squeeze-Out compensation. The motions are based on various alleged shortcomings and mistakes in the valuation of Celanese GmbH done for purposes of the Squeeze-Out. On May 11, 2007, the court of first instance appointed a common representative for those shareholders that have not filed an application on their own.
Polyester Staple Antitrust Litigation
CNA Holdings, the successor in interest to Hoechst Celanese Corporation (“HCC”), Celanese Americas Corporation and Celanese GmbH (collectively, the “Celanese Entities”) and Hoechst, the former parent of HCC, were named as defendants in two actions (involving 25 individual participants) filed in September 2006 by US purchasers of polyester staple fibers manufactured and sold by HCC. The actions allege that the defendants participated in a conspiracy to fix prices, rig bids and allocate customers of polyester staple sold in the United States. These actions were consolidated in a proceeding by a Multi-District Litigation Panel in the United States District Court for the Western District of North Carolina styledIn re Polyester Staple Antitrust Litigation, MDL 1516. On June 12, 2008 the court dismissed these actions against all Celanese Entities in consideration of a payment by the Company of $107 million. This proceeding related to sales by the polyester staple fibers business which Hoechst AG sold to KoSa, Inc. in 1998. Accordingly, the impact of this settlement is reflected within discontinued operations in the consolidated statements of operations. The Company also previously entered into tolling arrangements with four other alleged US purchasers of polyester staple fibers manufactured and sold by the Celanese Entities. These purchasers were not included in the settlement and one such company filed suit against the Company in December 2008 in the Western District of North Carolina entitledMilliken & Company v. CNA Holdings, Inc., Celanese Americas Corporation and Hoechst AG (No. 8-CV-00578).
F-53
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In 1998, HCC sold its polyester staple business as part of the sale of its Film & Fibers Division to KoSa B.V., f/k/a Arteva B.V. and a subsidiary of Koch Industries, Inc. (“KoSa”). In March 2001 the US Department of Justice (“DOJ”) commenced an investigation of possible price fixing regarding the sales of polyester staple fibers in the US subsequent to the period the Celanese Entities were engaged in the polyester staple fiber business. The Celanese Entities were never named in these DOJ actions. As a result of the DOJ action, during August of 2002, Arteva Specialties, S.a.r.l., a subsidiary of KoSa, (“Arteva Specialties”) pled guilty to criminal violation of the Sherman Act related to anti-competitive conduct occurring after the 1998 sale of the polyester staple fiber business and paid a fine of $29 million. In a complaint pending against the Celanese Entities and Hoechst in the United States District Court for the Southern District of New York, Koch Industries, Inc., KoSa, Arteva Specialties and Arteva Services S.a.r.l. seek damages in excess of $371 million which includes indemnification for all damages related to the defendants’ alleged participation in, and failure to disclose, the alleged conspiracy.
Guarantees
The Company has agreed to guarantee or indemnify third parties for environmental and other liabilities pursuant to a variety of agreements, including asset and business divestiture agreements, leases, settlement agreements and various agreements with affiliated companies. Although many of these obligations contain monetaryand/or time limitations, others do not provide such limitations.
As indemnification obligations often depend on the occurrence of unpredictable future events, the future costs associated with them cannot be determined at this time.
The Company has accrued for all probable and reasonably estimable losses associated with all known matters or claims that have been brought to its attention. These known obligations include the following:
• Demerger Obligations
The Company has obligations to indemnify Hoechst, and its legal successors, for various liabilities under the Demerger Agreement, including for environmental liabilities associated with contamination arising under 19 divestiture agreements entered into by Hoechst prior to the demerger.
The Company’s obligation to indemnify Hoechst, and its legal successors, is subject to the following thresholds:
| • | The Company will indemnify Hoechst, and its legal successors, against those liabilities up to €250 million; | |
| • | Hoechst, and its legal successors, will bear those liabilities exceeding €250 million, however the Company will reimburse Hoechst, and its legal successors, for one-third of those liabilities for amounts that exceed €750 million in the aggregate. |
The aggregate maximum amount of environmental indemnifications under the remaining divestiture agreements that provide for monetary limits is approximately €750 million. Three of the divestiture agreements do not provide for monetary limits.
Based on the estimate of the probability of loss under this indemnification, the Company had reserves of $27 million and $27 million as of December 31, 2008 and 2007, respectively, for this contingency. Where the Company is unable to reasonably determine the probability of loss or estimate such loss under an indemnification, the Company has not recognized any related liabilities.
The Company has also undertaken in the Demerger Agreement to indemnify Hoechst and its legal successors for liabilities that Hoechst is required to discharge, including tax liabilities, which are associated with businesses that were included in the demerger but were not demerged due to legal restrictions on the transfers of such items. These indemnities do not provide for any monetary or time limitations. The Company has not provided for any reserves associated with this indemnification as it is not probable or estimable. The Company has not made any
F-54
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
payments to Hoechst or its legal successors during the years ended December 31, 2008 and 2007, respectively, in connection with this indemnification.
• Divestiture Obligations
The Company and its predecessor companies agreed to indemnify third-party purchasers of former businesses and assets for various pre-closing conditions, as well as for breaches of representations, warranties and covenants. Such liabilities also include environmental liability, product liability, antitrust and other liabilities. These indemnifications and guarantees represent standard contractual terms associated with typical divestiture agreements and, other than environmental liabilities, the Company does not believe that they expose the Company to any significant risk.
The Company has divested numerous businesses, investments and facilities through agreements containing indemnifications or guarantees to the purchasers. Many of the obligations contain monetaryand/or time limitations, ranging from one year to thirty years. The aggregate amount of guarantees provided for under these agreements is approximately $2.3 billion as of December 31, 2008. Other agreements do not provide for any monetary or time limitations.
Based on historical claims experience and its knowledge of the sites and businesses involved, the Company believes that it is adequately reserved for these matters. As of December 31, 2008 and 2007, the Company has reserves in the aggregate of $33 million and $27 million, respectively, for these matters.
• Other Obligations
The Company is secondarily liable under a lease agreement that the Company assigned to a third party. The lease expires on April 30, 2012. The lease liability for the period from January 1, 2009 to April 30, 2012 is estimated to be approximately $26 million.
The Company has agreed to indemnify various insurance carriers for amounts not in excess of the settlements received from claims made against these carriers subsequent to the settlement. The aggregate amount of guarantees under these settlements which is limited in term is approximately $10 million.
Asbestos Claims
As of December 31, 2008, Celanese Ltd. and/or CNA Holdings, Inc., both US subsidiaries of the Company, are defendants in approximately 559 asbestos cases. During the year ended December 31, 2008, 66 new cases were filed against the Company, 137 cases were resolved, and 4 cases were added after further analysis by outside counsel. Because many of these cases involve numerous plaintiffs, the Company is subject to claims significantly in excess of the number of actual cases. The Company has reserves for defense costs related to claims arising from these matters. The Company believes that there is no significant exposure related to these matters.
Purchase Obligations
In the normal course of business, the Company enters into commitments to purchase goods and services over a fixed period of time. The Company maintains a number of “take-or-pay” contracts for purchases of raw materials and utilities. As of December 31, 2008, there were outstanding future commitments of $2,291 million under take-or-pay contracts. The Company does not expect to incur any losses under these contractual arrangements and historically has not incurred any material losses related to these contracts. Additionally, as of December 31, 2008, there were outstanding commitments relating to capital projects of $33 million.
F-55
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 24. | Supplemental Cash Flow Information |
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
| Cash paid for: | ||||||||||||
| Taxes, net of refunds | 98 | 181 | 101 | |||||||||
Interest, net of amounts capitalized(1) | 259 | 414 | 239 | |||||||||
| Noncash investing and financing activities: | ||||||||||||
| Fair value adjustment to securities available for sale, net of tax | (25 | ) | 17 | 13 | ||||||||
| Capital lease obligations | 103 | 80 | 5 | |||||||||
| Accrued capital expenditures | (7 | ) | 18 | — | ||||||||
| Asset retirement obligations | 8 | 4 | 10 | |||||||||
| Accrued Ticona Kelsterbach plant relocation costs | 17 | 19 | — | |||||||||
| (1) | Amount includes premiums paid on early redemption of debt and related issuance costs, net of amounts capitalized, of $217 million for the year ended December 31, 2007. |
| 25. | Business and Geographical Segments |
The Company operates through the following business segments:
Advanced Engineered Materials
The Company’s Advanced Engineered Materials segment develops, produces and supplies a broad portfolio of high performance technical polymers for application in automotive and electronics products as well as other consumer and industrial applications. Together with the Company’s strategic affiliates, we are a leading participant in the global technical polymers industry. The primary products of Advanced Engineered Materials are used in a broad range of products including automotive components, electronics, appliances, industrial applications, battery separators, conveyor belts, filtration equipment, coatings, medical devices, electrical and electronics.
Consumer Specialties
The Company’s Consumer Specialties segment consists of the Acetate Products and Nutrinova businesses. The Acetate Products business primarily produces and supplies acetate tow, which is used in the production of filter products. We also produce acetate flake which is processed into acetate fiber in the form of a tow band. Our Nutrinova business produces and sells Sunett®, a high intensity sweetener, and food protection ingredients, such as sorbates, for the food, beverage and pharmaceuticals industries.
Industrial Specialties
The Company’s Industrial Specialties segment includes our Emulsions, PVOH and AT Plastics businesses. Our Emulsions business is a global leader which produces a broad product portfolio, specializing in vinyl acetate ethylene emulsions, and is a recognized authority on low VOC (volatile organic compounds), an environmentally-friendly technology. As a global leader, our PVOH business produces a broad portfolio of performance PVOH chemicals engineered to meet specific customer requirements. Our emulsions and PVOH products are used in a wide array of applications including paints and coatings, adhesives, building and construction, glass fiber, textiles and paper. AT Plastics offers a complete line of low-density polyethylene and specialty ethylene vinyl acetate resins and compounds. AT Plastics’ products are used in many applications including flexible packaging films, lamination film products, hot melt adhesives, medical tubing, automotive carpeting and solar cell encapsulation films.
F-56
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Acetyl Intermediates
The Company’s Acetyl Intermediates segment produces and supplies acetyl products, including acetic acid, VAM, acetic anhydride and acetate esters. These products are generally used as starting materials for colorants, paints, adhesives, coatings, medicines and more. Other chemicals produced in this segment are organic solvents and intermediates for pharmaceutical, agricultural and chemical products.
Other Activities
Other Activities primarily consists of corporate center costs, including financing and administrative activities such as legal, accounting and treasury functions and interest income or expense associated with financing activities of the Company, and the captive insurance companies.
The segment management reporting and controlling systems are based on the same accounting policies as those described in the summary of significant accounting policies in Note 2. The Company evaluates performance based on operating profit, net earnings (loss), cash flows and other measures of financial performance reported in accordance with US GAAP.
F-57
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Sales and revenues related to transactions between segments are generally recorded at values that approximate third-party selling prices.
| Advanced | ||||||||||||||||||||||||||||
| Engineered | Consumer | Industrial | Acetyl | Other | ||||||||||||||||||||||||
| Materials | Specialties | Specialties | Intermediates | Activities | Eliminations | Consolidated | ||||||||||||||||||||||
| (In $ millions) | ||||||||||||||||||||||||||||
As of and for the year ended December 31, 2008: | ||||||||||||||||||||||||||||
| Net sales | 1,061 | 1,155 | 1,406 | 3,875 | (1) | 2 | (676 | ) | 6,823 | |||||||||||||||||||
| Other (charges) gains, net | (29 | ) | (2 | ) | (3 | ) | (78 | ) | 4 | — | (108 | ) | ||||||||||||||||
| Equity in net earnings of affiliates | 37 | — | — | 3 | 14 | — | 54 | |||||||||||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 69 | 237 | 47 | 434 | (353 | ) | — | 434 | ||||||||||||||||||||
| Depreciation and amortization | 76 | 53 | 62 | 150 | 9 | — | 350 | |||||||||||||||||||||
Capital expenditures(2) | 55 | 49 | 67 | 86 | 10 | — | 267 | |||||||||||||||||||||
| Goodwill and intangible assets | 398 | 309 | 73 | 363 | — | — | 1,143 | |||||||||||||||||||||
| Total assets | 1,867 | 995 | 903 | 2,197 | 1,204 | — | 7,166 | |||||||||||||||||||||
As of and for the year ended December 31, 2007: | ||||||||||||||||||||||||||||
| Net sales | 1,030 | 1,111 | 1,346 | 3,615 | (1) | 2 | (660 | ) | 6,444 | |||||||||||||||||||
| Other (charges) gains, net | (4 | ) | (4 | ) | (23 | ) | 72 | (64 | ) | (35 | )(3) | (58 | ) | |||||||||||||||
| Equity in net earnings of affiliates | 55 | 3 | — | 6 | 18 | — | 82 | |||||||||||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 189 | 235 | 28 | 694 | (699 | ) | — | 447 | ||||||||||||||||||||
| Depreciation and amortization | 69 | 51 | 59 | 106 | 6 | — | 291 | |||||||||||||||||||||
Capital expenditures(2) | 59 | 43 | 63 | 130 | 11 | — | 306 | |||||||||||||||||||||
| Goodwill and intangible assets | 445 | 339 | 97 | 410 | — | — | 1,291 | |||||||||||||||||||||
| Total assets | 1,734 | 1,137 | 963 | 2,542 | 1,682 | — | 8,058 | |||||||||||||||||||||
As of and for the year ended December 31, 2006: | ||||||||||||||||||||||||||||
| Net sales | 915 | 876 | 1,281 | 3,351 | (1) | 22 | (667 | ) | 5,778 | |||||||||||||||||||
| Other (charges) gains, net | 6 | — | (11 | ) | — | (5 | ) | — | (10 | ) | ||||||||||||||||||
| Equity in net earnings of affiliates | 53 | 3 | — | 8 | 12 | — | 76 | |||||||||||||||||||||
| Earnings (loss) from continuing operations before tax and minority interests | 201 | 185 | 43 | 519 | (422 | ) | — | 526 | ||||||||||||||||||||
| Depreciation and amortization | 65 | 39 | 59 | 101 | 5 | — | 269 | |||||||||||||||||||||
| Capital expenditures | 27 | 75 | 30 | 105 | 7 | — | 244 | |||||||||||||||||||||
| (1) | Includes $676 million, $660 million and $667 million of intersegment sales eliminated in consolidation for the years ended December 31, 2008, 2007 and 2006, respectively. | |
| (2) | Excludes expenditures related to the relocation of the Company’s Ticona plant in Kelsterbach and includes a decrease in accrued capital expenditures of $7 million and an increase of $18 million for the years ended December 31, 2008 and 2007, respectively (Note 24). | |
| (3) | Represents insurance recoveries received from the Company’s captive insurance companies related to the Clear Lake, Texas facility (Note 29) that eliminates in consolidation. |
F-58
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Geographical Segments
Revenues and noncurrent assets are presented based on the location of the business. The following table presents financial information based on the geographic location of the Company’s facilities:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
Net sales | ||||||||||||
| United States | 1,719 | 1,754 | 1,803 | |||||||||
Non-United States | 5,104 | 4,690 | 3,975 | |||||||||
| Total | 6,823 | 6,444 | 5,778 | |||||||||
SignificantNon-United States net sales sources include: | ||||||||||||
| Germany | 2,469 | 2,348 | 1,974 | |||||||||
| Singapore | 783 | 762 | 771 | |||||||||
| Belgium | 478 | 295 | 228 | |||||||||
| Mexico | 391 | 349 | 303 | |||||||||
| China | 393 | 182 | 14 | |||||||||
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
Property, plant and equipment, net | ||||||||
| United States | 733 | 788 | ||||||
Non-United States | 1,739 | 1,574 | ||||||
| Total | 2,472 | 2,362 | ||||||
SignificantNon-United States property, plant and equipment, net sources include: | ||||||||
| Germany | 682 | 493 | ||||||
| Singapore | 111 | 91 | ||||||
| Canada | 117 | 126 | ||||||
| Mexico | 105 | 133 | ||||||
| China | 493 | 383 | ||||||
F-59
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 26. | Transactions and Relationships with Affiliates and Related Parties |
The Company is a party to various transactions with affiliated companies. Entities in which the Company has an investment accounted for under the cost or equity method of accounting, are considered affiliates; any transactions or balances with such companies are considered affiliate transactions. The following tables represent the Company’s transactions and balances with affiliates for the periods presented:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| (In $ millions) | ||||||||||||
Statements of Operations | ||||||||||||
Purchases from affiliates(1)(2) | 131 | 126 | 159 | |||||||||
Sales to affiliates(1) | 36 | 126 | 290 | |||||||||
| Interest income from affiliates | 2 | 1 | 1 | |||||||||
| Interest expense to affiliates | 9 | 7 | 5 | |||||||||
| (1) | Purchases and sales from/to affiliates are accounted for at prices which, in the opinion of the Company, approximate those charged to third-party customers for similar goods or services. | |
| (2) | Primarily includes utilities and services purchased from InfraServ Hoechst. |
Refer to Note 8 for additional information related to dividends received from affiliates.
| As of December 31, | ||||||||
| 2008 | 2007 | |||||||
| (In $ millions) | ||||||||
Balance Sheets | ||||||||
| Trade and other receivables from affiliates | 8 | 15 | ||||||
| Current notes receivable (including interest) from affiliates | 9 | 15 | ||||||
| Noncurrent notes receivable (including interest) from affiliates | 9 | 7 | ||||||
| Total receivables from affiliates | 26 | 37 | ||||||
| Accounts payable and other liabilities due affiliates | 18 | 22 | ||||||
| Short-term borrowings from affiliates | 103 | 200 | ||||||
| Total due affiliates | 121 | 222 | ||||||
The Company has agreements with certain affiliates, primarily InfraServ entities, whereby excess affiliate cash is lent to and managed by the Company, at variable interest rates governed by those agreements.
For the year ended 2007, the Company made payments to the Advisor of $7 million in accordance with the sponsor services agreement dated January 26, 2005, as amended. These payments were related primarily to the sale of the oxo products and derivatives businesses and the acquisition of APL (Note 4).
F-60
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| 27. | Earnings (Loss) Per Share |
| Year Ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| Basic | Diluted | Basic | Diluted | Basic | Diluted | |||||||||||||||||||
| (In $ millions, except for share and per share data) | ||||||||||||||||||||||||
| Earnings (loss) from continuing operations | 372 | 372 | 336 | 336 | 319 | 319 | ||||||||||||||||||
| Earnings (loss) from discontinued operations | (90 | ) | (90 | ) | 90 | 90 | 87 | 87 | ||||||||||||||||
| Net earnings (loss) | 282 | 282 | 426 | 426 | 406 | 406 | ||||||||||||||||||
| Less: cumulative preferred stock dividend | (10 | ) | — | (10 | ) | — | (10 | ) | — | |||||||||||||||
| Net earnings (loss) available to common shareholders | 272 | 282 | 416 | 426 | 396 | 406 | ||||||||||||||||||
| Weighted average shares — basic | 148,350,273 | 148,350,273 | 154,475,020 | 154,475,020 | 158,597,424 | 158,597,424 | ||||||||||||||||||
| Dilutive stock options | — | 2,559,268 | — | 4,344,644 | — | 1,205,413 | ||||||||||||||||||
| Dilutive restricted stock | — | 504,439 | — | 362,130 | — | — | ||||||||||||||||||
| Assumed conversion of preferred stock | — | 12,057,893 | — | 12,046,203 | — | 12,004,762 | ||||||||||||||||||
| �� | ||||||||||||||||||||||||
| Weighted average shares — diluted | 148,350,273 | 163,471,873 | 154,475,020 | 171,227,997 | 158,597,424 | 171,807,599 | ||||||||||||||||||
| Per share: | ||||||||||||||||||||||||
| Earnings (loss) from continuing operations | 2.44 | 2.28 | 2.11 | 1.96 | 1.95 | 1.86 | ||||||||||||||||||
| Earnings (loss) from discontinued operations | (0.61 | ) | (0.55 | ) | 0.58 | 0.53 | 0.55 | 0.50 | ||||||||||||||||
| Net earnings (loss) | 1.83 | 1.73 | 2.69 | 2.49 | 2.50 | 2.36 | ||||||||||||||||||
The following securities were not included in the computation of diluted net earnings per share as their effect would have been antidilutive:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Stock options | 2,298,159 | 336,133 | 1,915,289 | |||||||||
| Restricted stock units | 90,625 | — | — | |||||||||
| Total | 2,388,784 | 336,133 | 1,915,289 | |||||||||
| 28. | Ticona Kelsterbach Plant Relocation |
On November 29, 2006, the Company reached a settlement with the Frankfurt, Germany, Airport (“Fraport”) to relocate its Kelsterbach, Germany, business, resolving several years of legal disputes related to the planned Frankfurt airport expansion. The final settlement agreement was approved by the Fraport supervisory board on May 8, 2007 at an extraordinary board meeting. The final settlement agreement was signed on June 12, 2007. In July 2007, the Company announced that it would relocate the Kelsterbach, Germany, business to the Hoechst Industrial Park in the Rhine Main area by mid-2011. Over a five-year period, Fraport will pay Ticona a total of €670 million to
F-61
Table of Contents
CELANESE CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
offset the costs associated with the transition of the business from its current location and the closure of the Kelsterbach plant. The payment amount was increased by €20 million to €670 million in consideration of the Company’s agreement to waive certain obligations of Fraport set forth in the settlement agreement. In June 2008, the Company received €200 million ($311 million) from Fraport under this agreement. Amounts received from Fraport are accounted for as deferred proceeds and are included in noncurrent Other liabilities in the consolidated balance sheets. See Note 30 for a description of proceeds received in advance of the original terms of the agreement.
Below is a summary of the financial statement impact associated with the Ticona Kelsterbach plant relocation:
| Year Ended | Total From | |||||||||||
| December 31, | Inception Through | |||||||||||
| 2008 | 2007 | December 31, 2008 | ||||||||||
| (In $ millions) | ||||||||||||
| Proceeds received from Fraport | 311 | — | 337 | |||||||||
| Costs expensed | 12 | 5 | 17 | |||||||||
Costs capitalized(1) | 202 | 40 | 243 | |||||||||
| (1) | Includes increase in accrued capital expenditures of $17 million and $19 million for the years ended December 31, 2008 and 2007, respectively. |
| 29. | Insurance Recoveries |
In May 2007, the Company announced that it had an unplanned outage at its Clear Lake, Texas acetic acid facility. At that time, the Company originally expected the outage to last until the end of May. Upon restart of the facility, additional operating issues were identified which necessitated an extension of the outage for further, more extensive repairs. In July 2007, the Company announced that the further repairs were unsuccessful on restart of the unit. All repairs were completed in early August 2007 and normal production capacity resumed. During the years ended December 31, 2008 and 2007, the Company recorded $38 million and $40 million, respectively, of insurance recoveries from its reinsurers in partial satisfaction of claims that the Company made based on losses resulting from the outage. These insurances recoveries are included in Other (charges) gains, net in the consolidated statements of operations (Note 18).
In October 2008, the Company declared force majeure on its specialty polymers products produced at its AT Plastics facility in Edmonton, Alberta, Canada as a result of certain events and subsequent cessation of production. The Company intends to replace damaged long-lived assets. Any contingent liabilities associated with the outage may be mitigated by the Company’s insurance policies.
| 30. | Subsequent Events |
On January 5, 2009, the Company declared a cash dividend of $0.265625 per share on its 4.25% convertible perpetual preferred stock amounting to $3 million and a cash dividend of $0.04 per share on its Series A common stock amounting to $6 million. Both cash dividends are for the period November 1, 2008 to January 31, 2009 and were paid on February 1, 2009 to holders of record as of January 15, 2009.
On February 2, 2009, the Company announced the Fraport supervisory board approved the acceleration of the 2009 and 2010 payments of €200 million and €140 million, respectively, required by the settlement agreement signed in June 2007. On February 5, 2009, the Company received a discounted amount of approximately €322 million, excluding value-added tax of €59 million.
On February 12, 2009, the Company announced it will shut down its VAM production unit in Cangrejera, Mexico and cease VAM production at Cangrejera effective the end of February 2009. The Company believes this capacity reduction is necessitated by the significant change in the global economic environment, the cost structure of its VAM unit operations in Cangrejera and anticipated lower demand. The Company recorded an impairment loss as it relates to the Cangrejera VAM production unit of $4 million for the three months ended December 31, 2008.
F-62
Table of Contents
INDEX TO EXHIBITS
Exhibits will be furnished upon request for a nominal fee, limited to reasonable expenses.
| Exhibit | ||
Number | Description | |
| 3.1 | Second Amended and Restated Certificate of Incorporation (Incorporated by reference to Exhibit 3.1 to the Current Report onForm 8-K filed on January 28, 2005). | |
| 3.2 | Third Amended and Restated By-laws, effective as of October 23, 2008 (Incorporated by reference to Exhibit 3.1 to the Current Report onForm 8-K filed on October 29, 2008). | |
| 3.3 | Certificate of Designations of 4.25% Convertible Perpetual Preferred Stock (Incorporated by reference to Exhibit 3.2 to the Current Report onForm 8-K filed on January 28, 2005). | |
| 4.1 | Form of certificate of Series A Common Stock (Incorporated by reference to Exhibit 4.1 to the Registration Statement onForm S-1 (FileNo. 333-120187), filed on January 13, 2005). | |
| 4.2 | Form of certificate of 4.25% Convertible Perpetual Preferred Stock (Incorporated by reference to Exhibit 4.2 to the Registration Statement onForm S-1 (FileNo. 333-120187) filed on January 13, 2005). | |
| 10.1 | Credit Agreement, dated April 2, 2007, among Celanese Holdings LLC, Celanese US Holdings LLC, the subsidiaries of Celanese US Holdings LLC from time to time party thereto as borrowers, the Lenders party thereto, Deutsche Bank AG, New York Branch, as administrative agent and as collateral agent, Merrill Lynch Capital Corporation as syndication agent, ABN AMRO Bank N.V., Bank of America, N.A., Citibank NA, and JP Morgan Chase Bank NA, as co-documentation agents (incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed with the SEC on April 5, 2007). | |
| 10.2 | Guarantee and Collateral Agreement, dated April 2, 2007, by and among Celanese Holdings LLC, Celanese US Holdings LLC, certain subsidiaries of Celanese US Holdings LLC and Deutsche Bank AG, New York Branch (incorporated by reference to Exhibit 10.2 to the Current Report onForm 8-K filed with the SEC on April 5, 2007). | |
| 10.3 | Celanese Corporation 2004 Stock Incentive Plan (Incorporated by reference to Exhibit 10.7 to the Current Report onForm 8-K filed on January 28, 2005). | |
| 10.4 | Celanese Corporation Deferred Compensation Plan (Incorporated by reference to Exhibit 10.21 to the Registration Statement onForm S-1 (FileNo. 333-120187) filed on January 3, 2005). | |
| 10.5 | Amendment to Celanese Corporation Deferred Compensation Plan (incorporated by reference to Exhibit 10.2 to the Current Report onForm 8-K filed with the SEC on April 3, 2007). | |
| 10.6 | Deferred Compensation Plan-Master Plan Document adopted December 7, 2007 (Incorporated by reference to Exhibit 10.6 to the Annual Report onForm 10-K filed on February 29, 2008). | |
| 10.7 | Form of Nonqualified Stock Option Agreement (for employees) (Incorporated by reference to Exhibit 10.5 to the Current Report onForm 8-K filed on January 28, 2005). | |
| 10.8 | Form of Amendment Two to Nonqualified Stock Option Agreement (for executive officers), dated January 20, 2009, (Incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed on January 26, 2009). | |
| 10.9 | Form of Nonqualified Stock Option Agreement (for non-employee directors) (Incorporated by reference to Exhibit 10.6 to the Current Report onForm 8-K filed on January 28, 2005). | |
| 10.10 | Form of Director Performance-Based Restricted Stock Unit Agreement between Celanese Corporation and award recipient (Incorporated by reference to Exhibit 10.1 to the Quarterly Report onForm 10-Q filed on July 27, 2007). | |
| 10.11 | Form of 2007 Deferral Agreement between Celanese Corporation and award recipient, dated as of April 2, 2007 (incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed with the SEC on April 3, 2007). | |
| 10.12 | Form of Performance-Based Restricted Stock Unit Agreement between Celanese Corporation and award recipient, dated as of April 2, 2007 (incorporated by reference to Exhibit 10.3 to the Current Report onForm 8-K filed with the SEC on April 3, 2007). | |
| 10.13 | Form of Performance-Vesting Restricted Stock Unit Award Agreement, together with a schedule identifying substantially identical agreements between the Company and each of its executive officers identified thereon (Incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed on January 26, 2009). |
Table of Contents
| Exhibit | ||
Number | Description | |
| 10.14 | Form of Time-Vesting Cash Award Agreement, together with a schedule identifying substantially identical agreements between the Company and each of its executive officers identified thereon (Incorporated by reference to Exhibit 10.3 to the Current Report onForm 8-K filed on January 26, 2009). | |
| 10.15 | Summary of pension benefits for David N. Weidman (Incorporated by reference to Exhibit 10.34 to the Annual Report ofForm 10-K filed on March 31, 2005). | |
| 10.16 | Separation Agreement, dated as of July 5, 2007, between Celanese Corporation and Lyndon B. Cole (Incorporated by reference to Exhibit 10.2 to the Quarterly Report onForm 10-Q filed on July 27, 2007). | |
| 10.17 | Offer letter agreement, effective April 18, 2005 between Curtis S. Shaw and Celanese Corporation (Incorporated by reference to Exhibit 10.23 to the Quarterly Report onForm 10-Q filed on May 16, 2005. | |
| 10.18 | Offer Letter Agreement, dated June 27, 2007, between Celanese Corporation and Sandra Beach Lin (Incorporated by reference to Exhibit 10.3 to the Quarterly Report onForm 10-Q filed on July 27, 2007). | |
| 10.19 | Offer Letter Agreement, dated May 21, 2008, between the Company and Michael L. Summers (Incorporated by reference to Exhibit 10.2 to the Quarterly Report onForm 10-Q filed on July 23, 2008). | |
| 10.20 | Amended and Restated Employment Agreement, dated as of July 26, 2007 between Celanese Corporation and John J. Gallagher III (Incorporated by reference to Exhibit 10.1 to the Quarterly Report onForm 10-Q filed on October 24, 2007). | |
| 10.21 | Nonqualified Stock Option Agreement, dated as of January 25, 2005, between Celanese Corporation and Blackstone Management Partners IV L.L.C. (Incorporated by reference from Exhibit 10.23 to the Annual Report onForm 10-K filed on March 31, 2005). | |
| 10.22 | Share Purchase and Transfer Agreement and Settlement Agreement, dated August 19, 2005 between Celanese Europe Holding GmbH & Co. KG, as purchaser, and Paulson & Co. Inc., and Arnhold and S. Bleichroeder Advisers, LLC, each on behalf of its own and with respect to shares owned by the investment funds and separate accounts managed by it, as the sellers (Incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed on August 19, 2005). | |
| 10.23 | Translation of Letter of Intent, dated November 29, 2006, among Celanese AG, Ticona GmbH and Fraport AG (Incorporated by reference to Exhibit 99.2 to the Current Report onForm 8-K filed November 29, 2006). | |
| 10.24† | Purchase Agreement dated as of December 12, 2006 by and among Celanese Ltd. and certain of its affiliates named therein and Advent Oxo (Cayman) Limited, Oxo Titan US Corporation, Drachenfelssee 520. V V GMBH and Drachenfelssee 521. V V GMBH (Incorporated by reference to Exhibit 10.27 to the Annual Report ofForm 10-K filed on February 21, 2007). | |
| 10.25 | First Amendment to Purchase Agreement dated February 28, 2007, by and among Advent Oxea Cayman Ltd., Oxea Corporation, Drachenfelssee 520. V V GmbH, Drachenfelssee 521. V V GmbH, Celanese Ltd., Ticona Polymers Inc. and Celanese Chemicals Europe GmbH (Incorporated by reference to Exhibit 10.6 to the Quarterly Report onForm 10-Q filed on May 9, 2007). | |
| 10.26 | Second Amendment to Purchase Agreement effective as of July 1, 2007 by and among Advent Oxea Cayman Ltd., Oxea Corporation, Oxea Holdings GmbH, Oxea Deutschland GmbH, Oxea Bishop, LLC, Oxea Japan KK, Oxea UK Ltd., Celanese Ltd., and Celanese Chemicals Europe GmbH (Incorporated by reference to Exhibit 10.2 to the Quarterly Report onForm 10-Q filed on October 24, 2007). | |
| 10.27 | Compensation Letter Agreement, dated March 27, 2007 by and between Jim Alder and Celanese Corporation (Incorporated by reference to Exhibit 10.31 to the Annual Report onForm 10-K filed on February 29, 2008 | |
| 10.28 | Change in Control Agreement, dated April 1, 2008, between the Company and David N. Weidman, together with a schedule identifying other substantially identical agreements between the Company and each of its name executive officers identified thereon and identifying the material differences between each of those agreements and the filed Changed of Control Agreement (Incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed on April 7, 2008). | |
| 10.29 | Change in Control Agreement, dated April 1, 2008 between the Company and Sandra Beach Lin, together with a schedule identifying other substantially identical agreements between the Company and each of its executive officers identified thereon and identifying the material differences between each of those agreements and the filed Change of Control Agreement (Incorporated by reference to Exhibit 10.2 to the Quarterly Report onForm 10-Q filed on April 23, 2008). |
Table of Contents
| Exhibit | ||
Number | Description | |
| 10.30 | Change in Control Agreement, dated April 1, 2008, between the Company and Curtis S. Shaw (Incorporated by reference to Exhibit 10.3 to the Quarterly Report onForm 10-Q filed on April 23, 2008). | |
| 10.31 | Change in Control Agreement, dated May 1, 2008, between the Company and Christopher W. Jensen (Incorporated by reference to Exhibit 10.1 to the Quarterly Report onForm 10-Q filed on July 23, 2008). | |
| 10.32 | Change in Control Agreement, dated June 5, 2008, between the Company and Michael L. Summers (Incorporated by reference to Exhibit 10.1 to the Quarterly Report onForm 10-Q filed on October 22, 2008). | |
| 10.33 | Agreement and General Release, dated September 25, 2008, between the Company and Curtis S. Shaw (Incorporated by reference to Exhibit 10.2 to the Quarterly Report onForm 10-Q filed on October 22, 2008). | |
| 10.34 | Agreement and General Release, dated March 28, 2008, between the Company and William P. Antonace (Incorporated by reference to Exhibit 10.4 to the Quarterly Report onForm 10-Q filed on October 22, 2008). | |
| 10.35 | Form of Long-Term Incentive Claw-Back Agreement (Incorporated by reference to Exhibit 10.1 to the Current Report onForm 8-K filed on January 26, 2009). | |
| 21.1* | List of subsidiaries of Celanese Corporation | |
| 23.1* | Report on Financial Statement Schedule and Consent of Independent Registered Public Accounting Firm, KPMG LLP | |
| 31.1* | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1* | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2* | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 99.1* | Financial Statement schedule regarding Valuation and Qualifying Accounts |
| * | Filed herewith | |
| † | Portions of this exhibit have been omitted pursuant to a request for confidential treatment filed with the Securities and Exchange Commission underRule 24b-2 of the Securities Exchange Act of 1934, as amended. The omitted portions of this exhibit have been separately filed with the Securities and Exchange Commission. |