UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) |
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2013
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) |
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 000-51820
Alexza Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| | |
| Delaware | | 77-0567768 |
(State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification Number) |
2091 Stierlin Court
Mountain View, California 94043
(Address of Principal Executive Offices including Zip Code)
Registrant’s telephone number, including area code:
(650) 944-7000
Securities registered pursuant to Section 12 (b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| Common Stock, par value $0.0001 per share | | Nasdaq Global Market |
Securities registered pursuant to Section 12 (g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (of for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of Form 10-K or any amendments to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer ¨ | | Accelerated filer ¨ | | Non-accelerated filer ¨ | | Smaller reporting company þ |
| | (Do not check if a smaller reporting company) |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value of the voting and non-voting stock held by non-affiliates of the Registrant was $73,873,744 based on the closing sale price of the Registrant’s common stock on The NASDAQ Global Market on June 30, 2013. Shares of the Registrant’s common stock beneficially owned by each executive officer and director of the Registrant and by each person known by the Registrant to beneficially own 10% or more of its outstanding common stock have been excluded, in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes. The number of outstanding shares of the Registrant’s common stock as of March 14, 2014 was 17,283,053.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for the 2014 Annual Meeting of Stockholders to be filed within 120 days after the end of the Registrant’s fiscal year ended December 31, 2013 are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent stated therein.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2013
TABLE OF CONTENTS
2
The names “Alexza Pharmaceuticals,” “Alexza,” “Staccato” and “ADASUVE” are trademarks of Alexza Pharmaceuticals, Inc. We have registered these trademarks with the U.S. Patent and Trademark Office and other ex-U.S. trademark offices. All other trademarks, trade names and service marks appearing in this Annual Report on Form 10-K are the property of their respective owners.
PART I.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements under “Business,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Annual Report constitute forward-looking statements. In some cases, you can identify forward-looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Examples of these statements include, but are not limited to, statements regarding: the ability of us and our collaborators to effectively and profitably commercialize ADASUVE, estimated product revenues and royalties associated with the sales of ADASUVE, the timing of the commercial launch of ADASUVE in various countries, the adequacy of our capital to support our operations, our ability to raise additional funds and the potential terms of such potential financings, our collaborators’ and our ability to implement and assess the ADASUVE REMS program, the timing and outcome of the ADASUVE post-marketing studies, the prospects of our receiving approval to market ADASUVE in Latin America, Russia, the other Commonwealth of Independent States countries and other countries, the implications of interim or final results of our other clinical trials, the progress and timing of our research programs, including clinical testing, the extent to which our issued and pending patents may protect our products and technology, the potential of our product candidates to lead to the development of safe or effective therapies, our ability to enter into collaborations, our future operating expenses, our future losses, our future expenditures and the sufficiency of our cash resources. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. While we believe that we have a reasonable basis for each forward-looking statement contained in this Annual Report, we caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain.
In addition, you should refer to the “Risk Factors” section of this Annual Report for a discussion of other important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosures we make on related subjects in our Quarterly Reports on Form 10-Q and in our Current Reports on Form 8-K.
BACKGROUND
We are a pharmaceutical company focused on the research, development and commercialization of novel proprietary products for the acute treatment of central nervous system conditions. The Staccato® system, our proprietary technology, is the foundation for our first approved product, ADASUVE® (Staccato loxapine), and all of our product candidates. TheStaccatosystem vaporizes excipient-free drugs to form a condensation aerosol that, when inhaled, allows for rapid systemic drug delivery. Because of the particle size of the aerosol, the drug is quickly absorbed through the deep lung into the bloodstream, providing speed of therapeutic onset that is comparable to intravenous, or IV, administration but with greater ease, patient comfort and convenience.
3
ADASUVE has been developed for the treatment of agitation associated with schizophrenia or bipolar disorder and has been approved for marketing in the United States by the U.S. Food and Drug Administration, or FDA, and in the European Union, or EU, by the European Commission, or EC. In the United States and the EU, ADASUVE is approved for similar indications. It is approved with a different number of dose strengths and has different risk mitigation and management plans in the United States and the EU. ADASUVE is our only approved product.
We have one product candidate in active development, AZ-002(Staccato alprazolam), which is being developed for the treatment of acute repetitive seizures, sometimes called cluster seizures or ARS. We expect to initiate a Phase 2a proof-of-concept study in patients with ARS in the second quarter of 2014.
We have retained all rights to theStaccato system and to our product candidates other than ADASUVE and AZ-104 (Staccato loxapine, low-dose). For the U.S. market, we have licensed the exclusive rights to commercialize ADASUVE to Teva Pharmaceuticals USA, Inc., or Teva. For Europe, Latin America, Russia and the other Commonwealth of Independent States countries, or the Ferrer Territories, we have licensed the exclusive rights to commercialize ADASUVE to Grupo Ferrer Internacional S.A., or Ferrer. We intend to develop certain product candidates internally and to identify external resources or collaborators to develop and commercialize other product candidates.
We believe that, based on our cash, cash equivalents and marketable securities balance at December 31, 2013, remaining proceeds available under the Convertible Promissory Note and Agreement to Lend, dated as of May 7, 2013, between us and Teva, or the Teva Note, estimated product revenues and milestone payments associated with the sale of ADASUVE, the proceeds from our March 2014 royalty securitization financing, and our current expected cash usage, we have sufficient capital resources to meet our anticipated cash needs for at least the next twelve months. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate, or to alter our operations.
AGITATION — ADASUVE
Background
Episodes of agitation afflict many people suffering from major psychiatric disorders, including schizophrenia and bipolar disorder. In the United States, approximately 2.4 million adults have schizophrenia and approximately 5.7 million adults have bipolar disorder. Of these patients, approximately 900,000 adult patients with schizophrenia and 5 million adult patients with bipolar disorder are currently receiving pharmaceutical treatment and are the target patient population for ADASUVE. Agitation in these patients generally escalates over time, with patients initially feeling uncomfortable, tense and restless, and as the agitation intensifies, their behavior appears more noticeable to others. From the healthcare professional’s perspective, agitation, if not treated quickly and effectively, may escalate unpredictably and poses a serious safety risk to staff and the patients themselves. More than 90% of patients with schizophrenia and bipolar disorder will experience agitation in their lifetimes. Based on our market research with caregivers of patients with schizophrenia or bipolar disorder, we believe that patients with schizophrenia or bipolar disorder experience an average of 11 to 12 agitation episodes each year.
While patients seek treatment at different points along the agitation continuum, once they present at a medical setting they generally need treatment urgently. Our primary market research indicates that approximately 50% of treated acute agitation episodes are treated in a hospital setting. In the hospital setting, patients are routinely treated in medical emergency departments, psychiatric emergency services and inpatient psychiatric units, which are the settings where ADASUVE may be used if such facilities are enrolled in the ADASUVE REMS program.
Based on our market research, we estimate that between 10% and 30% of the treated patients receive an intramuscular injection to treat their agitation. Although there are no approved oral medications to treat agitation, current treatment guidelines recommend the use of oral medications over IM injections. Oral medications are non-coercive to the extent that patients cooperate to take the medication. Our market research data also indicate that: (i) over 95% of psychiatrists surveyed believe that there is a “need” and approximately 40-45% believe that there is a “significant need” for a novel therapy to treat agitation episodes in the medical setting that can be
4
administered non-invasively with a fast onset, (ii) approximately 75% of psychiatrists surveyed would be “likely” or “very likely” to use a fast-acting, non-invasive product if available in a hospital setting and (iii) as many as 73% of patients surveyed “could use” and “would use” a fast-acting product.
Commercialization Strategy Overview
Our global commercialization strategy for ADASUVE is to use strategic collaborations to commercialize ADASUVE, while maintaining control and primary responsibility for commercial manufacturing. We currently have two commercialization collaborations, one with Teva and one with Ferrer. We are continuing to seek additional commercialization collaborations to commercialize ADASUVE in territories outside the United States and the Ferrer Territories.
In December 2012, the FDA approved our New Drug Application, or NDA, forStaccato loxapine, as ADASUVE (loxapine) Inhalation Powder 10 mg for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. We transferred the ADASUVE NDA and related regulatory documents to Teva in June 2013, together with responsibility for post-approval commitments and conditions related to the U.S. ADASUVE approval. In March 2014, Teva announced the U.S. launch of ADASUVE.
In February 2013, the EC granted marketing authorization for ADASUVE in the EU. ADASUVE, 4.5 mg and 9.1 mg inhalation powder loxapine, pre-dispensed, is authorized in the EU for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. The ADASUVE marketing authorization requires that patients receive regular treatment immediately after control of acute agitation symptoms, and that ADASUVE is administered only in a hospital setting under the supervision of a healthcare professional. The ADASUVE marketing authorization also states that a short-acting beta-agonist bronchodilator treatment should be available for treatment of possible severe respiratory side-effects, such as bronchospasm. The EC granted the marketing authorization for ADASUVE on the basis of the positive opinion issued by the European Medicines Agency, or EMA, in December 2012 and is valid in all 28 EU Member States, plus Iceland, Liechtenstein and Norway.
We have primary responsibility for certain post-approval commitments related to the EU ADASUVE approval. Two of the commitments related to the EU approval have been completed.
We are, and expect to continue to be, responsible for the commercial manufacturing of ADASUVE in our facility in Mountain View, California for commercialization in the United States, the Ferrer Territories and in any potential future territories. This facility has been inspected by the U.S. and EU authorities and operates under applicable U.S. and EU regulations.
We began manufacturing commercial quantities of ADASUVE in the second quarter of 2013 and will continue to manufacture commercial product for Ferrer and Teva. Shipments for 2013 consisted of the following:
| | | | | | | | | | | | | | | | |
| | | Q2 2013 | | | Q3 2013 | | | Q4 2013 | | | Total | |
EU Units | | | 13,370 | | | | 14,405 | | | | 11,863 | | | | 39,638 | |
U.S. Units | | | — | | | | — | | | | 9,307 | | | | 9,307 | |
| | | | | | | | | | | | | | | | |
Total Units | | | 13,370 | | | | 14,405 | | | | 21,170 | | | | 48,945 | |
| | | | | | | | | | | | | | | | |
Because our current license and supply agreements provide our collaborators with an acceptance period during which they may reject any product that does not conform to agreed-upon specifications, revenue for units shipped may be recognized in a period subsequent to the period in which the units are shipped.
Commercialization Strategy and Post-Approval Commitments — United States
In May 2013, we entered into a License and Supply Agreement with Teva, or the Teva Agreement, which provides Teva with an exclusive license to develop and commercialize ADASUVE in the United States. Under the terms of the Teva Agreement, Teva is responsible for all U.S. development, regulatory and commercialization activities for ADASUVE, including the U.S. post-approval clinical studies. Teva also has rights to conduct additional clinical trials of ADASUVE for potential new indications.
5
We are responsible for manufacturing and supplying ADASUVE to Teva for clinical trials and commercial sales. Teva has the exclusive rights to commercialize ADASUVE in the United States and the co-exclusive rights, with us and our affiliates, to manufacture the product.
As a condition of the approval of ADASUVE in the United States, a Risk Evaluation and Mitigation Strategy, or REMS, program including “elements to assure safe use,” is required to be implemented and periodically assessed. Among other requirements in the REMS, ADASUVE is only available in healthcare facilities and hospitals enrolled in the ADASUVE REMS program. As an additional condition of U.S. approval, ADASUVE is subject to a post-marketing commitment and a number of post-marketing studies, including a 10,000 patient Phase 4 observational clinical study designed to gather patient safety data based on the “real-world” use of ADASUVE in the hospital setting and a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients. The data derived from any post-marketing study or trial could result in additional restrictions on the commercialization of ADASUVE through changes to the approved ADASUVE label, changes to the approved REMS, the imposition of additional post-marketing studies or trials, or even the withdrawal of the approval of ADASUVE from the market.
ADASUVE is being marketed by the Teva Select Brands team, or TSB, of Teva’s U.S. Specialty Pharmaceuticals business, which is a subsidiary of Teva Pharmaceutical Industries, Ltd. In March 2014, TSB announced the U.S. launch of ADASUVE, with dedicated sales and medical science liaison teams focusing on hospitals in the United States that treat a high volume of patients with agitation. Teva has set the per-dose price of ADASUVE and has received a C-Code from the Centers for Medicare and Medicaid Services, or CMS, to aid in reimbursement for use of ADASUVE in the hospital through the Outpatient Prospective Payment System, or OPPS. Teva has also launched the website for the ADASUVE REMS program (www.adasuverems.com) to provide information about the program and to allow healthcare facilities to complete the enrollment in the REMS program in order to purchase ADASUVE from specialty wholesalers. In March 2014, we completed a royalty securitization financing, in which we transferred our rights to U.S. royalty and milestone payments under the Teva Agreement to a wholly-owned subsidiary. The subsidiary then sold $45.0 million in notes backed by the royalty and milestone payments in a private placement to institutional accredited investors. The notes have no other recourse to us, and we did not guarantee the notes.
Commercialization Strategy and Post-Approval Commitments — European Union and additional Ferrer Territory Countries
In October 2011, we entered into a commercial collaboration with Ferrer pursuant to a Collaboration, License and Supply Agreement, or the Ferrer Agreement, to commercialize ADASUVE in the Ferrer Territories. We supply ADASUVE to Ferrer for all of its commercial sales and receive a specified per-unit transfer price. We were responsible for gaining initial EU marketing authorization for ADASUVE and are responsible for specific EU post-marketing commitments required by the marketing authorization for ADASUVE. Ferrer is responsible for satisfying all other regulatory, pricing and reimbursement requirements to market and sell ADASUVE in the EU Member States and the non-EU countries of the Ferrer Territories.
The Ferrer Territories include the EU Member States and countries outside of the EU. In the EU, Ferrer has initiated a launch plan based on estimated market opportunity, timing of pricing and projected reimbursement, and coordination with distribution collaborators. ADASUVE is now available in Germany, Austria, Spain and Romania. Ferrer anticipates additional EU Member State launches in 2014 and 2015. In the non-EU Ferrer Territories, Ferrer is interacting with various regulatory agencies and working on the required independent regulatory submissions.
In countries where Ferrer does not have a direct commercial presence, it is seeking to establish distribution agreements with companies that it believes have sales capabilities in the hospital and/or psychiatry markets. As of March 2014, Ferrer had established ADASUVE distribution agreements with AOP Orphan Pharmaceuticals AG, Galenica SA and Medivir AB to serve such countries.
As a part of its education, market conditioning and commercialization strategy, Ferrer is building awareness about agitation and ADASUVE at national and regional medical conferences, hosting medical symposia and regional educational events, and exhibiting at medical meetings with a branded ADASUVE booth in applicable
6
territories. Also, in each country in the Ferrer Territory, we expect Ferrer to use a combination of medical science liaisons and sales representatives, as appropriate, to target hospitals and medical settings that Ferrer believes treat a high volume of patients with agitation, and to call on physicians and clinicians to educate them about agitation and promote ADASUVE.
As a condition of the ADASUVE marketing authorization in the EU, we are responsible for the conduct and funding of post-authorization studies, including (i) a benzodiazepine interaction study, which has been completed, (ii) a controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, with two doses of ADASUVE, which has been completed, (iii) a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients, (iv) an observational clinical trial, and (v) a drug utilization clinical trial. Results of the benzodiazepine interaction study and the thorough QTc study have been submitted to the EMA.
The benzodiazepine interaction study was a drug-drug interaction study with ADASUVE and lorazepam. The objective of this study was to compare the safety and pharmacodynamic profiles of concurrent administration of single doses of ADASUVE and intramuscular lorazepam compared to that of each drug administered alone. The study enrolled 22 subjects.
The primary endpoints of the study were the maximum effect and area under the concentration-time curve from baseline to two hours post-treatment value, in respirations per minute and pulse oximetry among the treatment groups. The three treatment groups were (a) ADASUVE and lorazepam, (b) ADASUVE alone and (c) lorazepam alone. There were no statistically significant differences observed among the three treatment groups on the two primary endpoints.
Secondary endpoints of the study included sitting blood pressure, heart rate, sedation, and psychomotor measures of attention, information processing speed, reaction time, and coordination. With the exception of sedation and psychomotor measures, there were no statistically significant differences observed on these secondary measures.
The controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, was a QT/QTc study of two doses. A pre-authorization clinical study indicated that clinically relevant QT prolongation does not appear to be associated with a single dose of ADASUVE. The potential risk of QTc prolongation following repeat dosing was unknown. The objective of this study was to assess the potential effects on the QT interval of two consecutive doses of ADASUVE administered two hours apart, in relation to placebo and an active control in healthy volunteers. The study enrolled 60 subjects and 48 subjects completed the study.
ADASUVE given as two 10 mg doses, two hours apart, did not cause significant electrocardiogram changes. Results for formulas used to analyze the QT interval were confirmatory, as were findings for absolute values and change from baseline values. Heart rate and other standard measures were also unaffected. Assay sensitivity was confirmed with significant effects observed for moxyfloxicin, the active control in the study. We believe that ADASUVE is not associated with clinically relevant QT prolongation under the dosing conditions studied.
Commercialization Strategy — Other Countries
We continue to seek additional strategic collaborators to commercialize ADASUVE in countries outside of the United States and the Ferrer Territories.
Competition
ADASUVE competes with various injectable formulations of other antipsychotic and benzodiazepine drugs and oral, orally disintegrating tablet and liquid formulations of other antipsychotic drugs and benzodiazepine drugs. Only the injectable antipsychotics are approved for the treatment of agitation.
STACCATO SYSTEM
Background
Acute and intermittent medical conditions are characterized by a rapid onset of symptoms that are temporary and severe, and that occur at irregular intervals, unlike the symptoms of chronic medical conditions
7
that continue at a relatively constant level over time. Approved drugs for the treatment of many acute and intermittent conditions, such as antipsychotics to treat agitation, triptans to treat migraine headaches and benzodiazepines to treat anxiety or epilepsy, are typically delivered either in tablets, by injections or by other formulations. Traditional oral inhalation technologies are also being developed to treat these conditions. These delivery methods have the following advantages and disadvantages:
| | • | | Oral Tablets. Oral tablets or capsules are convenient and cost effective, but they generally do not provide rapid onset of action. Oral tablets may require at least one to four hours to achieve peak plasma levels. Also, some drugs, if administered as a tablet or capsule, do not achieve adequate or consistent bioavailability due to the degradation of the drug by the stomach or liver or inability to be absorbed into the bloodstream. Orally-disintegrating technology is often incorporated into oral tablets to enhance the dissolution characteristics of a formulation, and most orally disintegrating tablets are bioequivalent to conventional oral dosage forms of the original drug. |
| | • | | Injections. Intravenous, or IV, or intramuscular, or IM, injections provide a more rapid onset of action than oral tablets and can sometimes be used to titrate potent drugs with very rapid changes in effect. Titration refers to the ability to administer an initial dose of medication to determine if that dose is effective and, if not, to administer additional doses until the medication has had an adequate effect. However, with a few exceptions, injections generally are administered by trained medical personnel in a medical care setting. Other forms of injections result in an onset of action that is generally substantially slower than IV injection, although often faster than oral administration. All forms of injections are invasive, can be painful to some patients and are often expensive. In addition, many drugs are not water soluble and can be difficult to formulate in an injectable form. |
| | • | | Traditional Oral Inhalation. Traditional dry powder and aerosolized inhalation delivery systems have been designed and used primarily for local delivery of drugs to the airways, not to the deep lung for rapid systemic drug delivery. Certain recent variants of these systems, however, can provide systemic delivery of drugs, either for the purpose of rapid onset of action or to enable noninvasive delivery of drugs that are not orally bioavailable. Nevertheless, many of these systems have difficulty in generating appropriate drug particle sizes or consistent emitted doses for deep lung delivery. To achieve appropriate drug particle sizes and consistent emitted doses, most traditional inhalation systems require the use of excipients and additives such as detergents, stabilizers and solvents, which may cause toxicity or allergic reactions. Many traditional inhalation devices require patient coordination to deliver the correct drug dose, leading to potentially wide variations in the drug delivered to a patient. |
As a result of these limitations, we believe there is a significant unmet medical and patient need for products for the treatment of acute and intermittent conditions that can be delivered in precise amounts, provide rapid therapeutic onset, and are noninvasive and easy to use.
Our Solution:Staccato System
OurStaccatosystem rapidly vaporizes an excipient-free drug compound to form a proprietary condensation aerosol that is inhaled and rapidly achieves systemic blood circulation via deep lung absorption. TheStaccatosystem consistently creates aerosol particles averaging one to three and one-half microns in size, which is the most appropriate size for deep lung inhalation and absorption into the bloodstream for systemic effect.
We believe ourStaccatosystem matches delivery characteristics and product attributes to clinical and patient needs for acute and intermittent conditions, with the following advantages:
| | • | | Rapid Onset. The aerosol produced with theStaccato system is designed to be rapidly absorbed through the deep lung with a speed of therapeutic onset comparable to an IV injection, generally achieving peak plasma levels of drug in two to five minutes. |
| | • | | Ease of Use. TheStaccatosystem is breath actuated, and a patient simply inhales to administer the drug dose. Unlike injections, theStaccatosystem is noninvasive and may not require caregiver assistance. The aerosol produced with theStaccatosystem is relatively insensitive to patient inhalation rates. Unlike many other inhalation technologies, the patient does not need to learn a special breathing pattern. In addition, theStaccato device is small and easily portable. |
8
| | • | | Consistent Particle Size and Dose. TheStaccato system uses rapid heating of the drug film to create consistent and appropriate particle sizes for deep lung inhalation and absorption into the bloodstream. TheStaccato system also produces a consistent high emitted dose, regardless of the patient’s breathing pattern. |
| | • | | Potential for Broad Applicability. TheStaccatosystem can deliver both water-soluble and water-insoluble drugs and eliminates the need for excipients and additives such as detergents, stabilizers and solvents, avoiding the side effects that may be associated with the excipients or additives. |
| | • | | Design Flexibility. TheStaccatosystem can incorporate multiple features, including, for example, a lockout system to potentially enhance safety, the convenience of patient titration, and a variety of dose administration regimens. |
Drug Candidates Based on the Staccato System
We combine small molecule drugs with ourStaccatosystem to create proprietary products and product candidates. We believe that the drugs we are currently using or studying are no longer eligible for patent protection as chemical entities or have their patent protection expiring in the next several years. These drugs have been widely used, and we believe their biological activity and safety are well understood and characterized. We have received composition of matter patent protection on theStaccatoaerosolized forms of these drugs. We also intend to collaborate with pharmaceutical companies to develop new chemical entities, including compounds that might otherwise not be suitable for development because of limitations of traditional delivery methods.
Since our inception, we have screened more than 400 drug compounds, identifying approximately 200 drug compounds that demonstrate initial vaporization feasibility for delivery with our technology. We believe that a number of these drug compounds, when delivered by theStaccato system, would have a desirable therapeutic profile for the treatment of various acute and intermittent conditions. We are initially focusing on developing proprietary products by combining ourStaccato system with small molecule drugs that have been in use for many years and are well-characterized to create Staccato-based aerosolized forms of these drugs.
Since 2004, we have filed six Investigational New Drug Applications, or INDs, and have received a U.S. NDA approval and EU marketing authorization for ADASUVE, based on theStaccato technology. In addition to ADASUVE, our only product approved for marketing, and AZ-002, our only product candidate in active development, we have in the past allocated resources to the development of the following product candidates, none of which are currently in active development: (i) AZ-007 (Staccato zaleplon) for the treatment of insomnia in patients who have difficulties falling asleep, including patients who awake in the middle of the night and have difficulty falling back asleep, (ii) AZ-104 (Staccato loxapine, low-dose) for the treatment of patients suffering from acute migraine headaches, (iii) AZ-003 (Staccato fentanyl) for the treatment of patients with acute pain, including patients with breakthrough cancer pain and postoperative patients with acute pain episodes and (iv) Staccato nicotine which is designed to help smokers quit by addressing both the chemical and behavioral components of nicotine addiction by delivering nicotine replacement via inhalation. During 2014, we are assessing ourStaccato-based pipeline and other possible product candidates, with the goal of identifying an additional product candidate for active development in 2014.
Staccato System
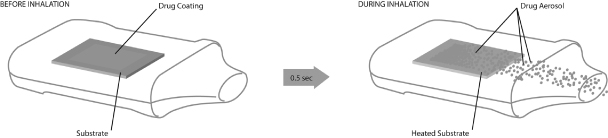
We invented theStaccato system. Our product candidates employing theStaccatosystem consist of three core components: (1) a heat source that includes an inert metal substrate; (2) a thin film of an excipient-free drug compound, also known as an active pharmaceutical ingredient, or API, coated on the substrate; and (3) an airway through which the patient inhales. The left panel of the illustration below depicts these core components prior to patient inhalation.
9
The right panel of the illustration below depicts theStaccatosystem during patient inhalation: (1) the heated substrate has reached peak temperature in less than one half second after the start of patient inhalation; (2) the thin drug film has been vaporized; and (3) the drug vapor has subsequently cooled and condensed into excipient-free drug aerosol particles that are being drawn into the patient’s lungs. The entireStaccatosystem actuation occurs in less than one second.
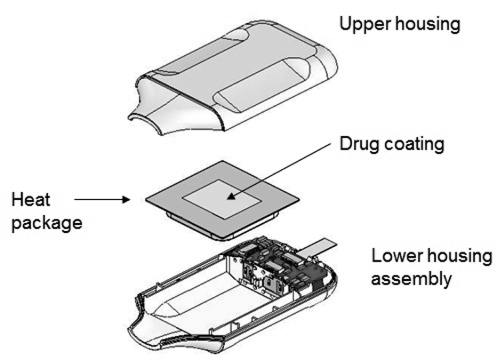
ADASUVE, AZ-002 and AZ-104 use the same disposable, single-dose delivery device. The single dose delivery device consists of a metal substrate that is chemically heated through a battery-initiated reaction of energetic materials. The device is portable and easy to carry. A diagram of the single dose delivery device is shown below:
We control final assembly of theStaccato technology-based products in an effort to maintain quality, to reduce the risk for supply interruptions and to allow for potentially more cost-effective manufacturing. We continue to undertake engineering and development efforts to improve the commercial manufacturability of our single dose device.
AZ-003, AZ-007 andStaccatonicotine use our multiple dose delivery technology, with a device consisting of a reusable controller and a disposable dose cartridge. We have designed the multiple dose delivery platform to meet the specific needs of each product candidate. The AZ-003 dose cartridge currently contains 25 separate metal substrates, each coated with the API, which rapidly heat upon application of electric current from the controller. In the current design for AZ-003, 25 micrograms of drug compound are coated on each metal substrate. The device is portable and easy to carry, with dimensions of approximately five inches in length, two and one-half inches in width and one inch in thickness. The controller weighs approximately four ounces, and the
10
dose cartridge weighs approximately one ounce. AZ-007 and Staccato nicotine dose cartridge and reusable controller designs are still in development, but contemplate a similar concept of a disposable dose cartridge and a reusable controller.
ACTIVE DEVELOPMENT PROGRAM
Acute Repetitive Seizures Program — AZ-002 (Staccato alprazolam)
We are developing AZ-002 (Staccatoalprazolam) for the treatment of acute repetitive seizures, or ARS, which are clusters of epileptic seizures that occur over a short period of time. The API of AZ-002 is alprazolam, a generic benzodiazepine drug. Alprazolam is currently approved in oral formulations in the United States for use in the management of anxiety disorder, for the short term relief of symptoms of anxiety, for anxiety associated with depression, and for the treatment of panic disorder with or without agoraphobia, or an abnormal fear of being in public places.
Market Opportunity
Epilepsy, a disorder of recurrent seizures, affects approximately 2.5 million Americans, which means it is the third most common neurological disorder in the United States. ARS refers to seizures that are serial, clustered, or crescendo, and distinct from the patient’s usual seizure pattern despite treatment from anti-epileptic drugs. ARS occurs in a small subset of patients with epilepsy who regularly experience breakthrough seizures in flurries or clusters, despite treatment with a regimen of anti-epileptic drugs. The socioeconomic effects of seizure clustering include missed school and work, as well as greater use of health care resources.
Among the implications of ARS are concerns for patient safety. Prolonged or recurrent seizure activity persisting for 30 minutes or more may result in serious injury, health impacts and death that correlate directly with seizure duration. ARS, if left untreated, has been reported to evolve into a state of persistent seizure, or status epilepticus, which has a 3% mortality rate in children and 26% in adults.
Our market research among neurologists and caregivers of ARS patients indicates that patients may experience clusters of seizures that vary from weekly to several months between clusters of seizures. Seizures can be preceded by a sense of perceptual disturbance and can last from one to two seconds to a couple of minutes. Patients typically experience three or four seizures per cluster, and the interval between seizures can vary from half an hour to two hours. In the intervals between seizures, the majority of patients are able to follow simple instructions and participate in a conversation.
Benzodiazepines are considered to be medications of first choice for the treatment of acute seizures. Clinical advantages of benzodiazepines include relatively rapid onset of action, high efficacy and minimal toxicity. The rapidity by which a medication can be delivered to the systemic circulation and then to the brain plays a significant role in reducing the time needed to treat seizures and reducing the likelihood of damage to the central nervous system. Improvements in quality of life reported anecdotally by families of individuals treated with benzodiazepines for epileptic seizures include reduced emergency room visits and hospitalizations, reduced disruption of daily activities, reduced time lost from work or school, and increased sense of “control.”
Current standard of care for ARS is the rectal gel formulation of diazepam, which must be administered by a caregiver or healthcare professional. In our market research, patients surveyed have commented that they find that the rectal gel takes longer to work than they would like and that the route of administration is sub-optimal and cannot be used in public. Intravenous benzodiazepines are rapidly acting, but must be administered by a healthcare professional in a medical facility.
The ability to treat quickly is clinically imperative to prevent an epileptic event from evolving into status epilepticus or causing other serious complications. A product that can be administered easily in the home setting to effectively treat ARS may result in avoiding a trip to the hospital for treatment or diminishing the need to use the rectal formulation.
If approved, AZ-002 could replace use of IV or rectal benzodiazepines currently used in treating patients who experience ARS. The potential benefits of AZ-002 as compared to IV or rectal delivery may include a faster delivery to the blood stream, compared to rectal delivery, and greater ease of use. AZ-002 could be administered
11
after a first seizure in a cluster with the aim of preventing further seizures. The caregiver could provide dosing assistance between cluster seizures. We believe that alprazolam’s ability to inhibit anxiety, or anxiolytic action, may provide additional therapeutic benefits to ARS patients.
We own full development and commercial rights to AZ-002, and we believe AZ-002 could qualify for orphan drug status. Orphan drug designation is available for drugs intended to treat rare diseases or conditions (i.e., generally those that affect fewer than 200,000 individuals in the United States at the time designation is sought). Drugs that receive orphan designation may be eligible for certain research tax credits and exemptions from certain fees and, upon approval, may receive 7 years of non-patent market exclusivity in the United States.
Development Status
Clinical Trials
We intend to initiate a Phase 2a proof-of-concept study with AZ-002 in the second quarter of 2014. The Phase 2a clinical trial will be an in-clinic, randomized, double-blind, proof-of-concept evaluation of patients suffering from repetitive seizures. The primary aim of the clinical trial will be to assess the safety and efficacy of a single dose of AZ-002 at different dose strengths.
We have completed three clinical trials with AZ-002. In 2005, we completed a Phase 1 clinical trial of AZ-002 in healthy volunteers. The purpose of this trial was to assess the safety, tolerability and pharmacokinetic properties of AZ-002. Using a dose escalation design, five doses (0.125 mg to 2.0 mg) of AZ-002 or placebo were studied in a total of 50 subjects. Results from the trial showed that AZ-002 was generally well tolerated at all doses. There were no serious adverse events observed across all dose groups, and all of the side effects were rated as mild or moderate in severity. Reported side effects included dizziness, sleepiness, fatigue and unpleasant taste. Across all doses, the pharmacokinetic analyses revealed that dose proportional plasma concentration of alprazolam and peak plasma levels were generally reached within the first few minutes after dosing.
In 2008, we released the preliminary results from our Phase 2a clinical trial with AZ-002 in patients with panic disorder. The study did not meet its two primary endpoints, which were the effect of AZ-002 on the incidence of a doxapram-induced panic attack and the effect of AZ-002 on the duration of a doxapram-induced panic attack, both as compared with placebo. There were no serious adverse events in the clinical trial, and AZ-002 was well tolerated in the study patient population. As a result of these studies, we made the decision to stop development of AZ-002 for the possible treatment of panic attacks in patients with panic disorder.
In 2009, we completed an abuse liability study with AZ-002. The objective of this clinical study was to compare the potential abuse liability of AZ-002, as compared to that of oral immediate-release alprazolam andStaccato placebo. This study was a Phase 1, single-center, randomized, double-blind, placebo-controlled, crossover abuse liability study of three inhaled doses of AZ-002, 0.5 mg, 1 mg, and 2 mg, three oral doses of immediate-release alprazolam (1 mg, 2 mg, and 4 mg), andStaccato placebo. The subjects participated in the study as outpatients. After completion of the screening/training session, two qualifying sessions evaluated effects of oral alprazolam 2 mg and oral placebo, and subjects who demonstrated a preference for alprazolam were identified. This phase of the study was double-blind. Only those subjects whose abuse liability measures indicated greater preference for oral alprazolam versus oral placebo were continued in the study. The subsequent phase of the study followed a crossover schedule planned according to a seven-treatment, seven-period Latin Square design. This phase of the study employed a double-blind, double-dummy design and included seven experimental sessions during which the effects of three doses of oral immediate-release alprazolam, three doses of AZ-002, andStaccatoplacebo were evaluated. A total of 14 subjects completed the study. The primary outcome measure was the categorical response to the question: “Rate the degree to which you would like to take the drug again.” Secondary outcome measures included questions from other questionnaires and rating scales.
Study validity (active controls statistically different from placebo) was confirmed for the primary outcome endpoint and was supported by the secondary outcome endpoint results.
Results of this study demonstrated that the abuse potential of 2 mg AZ-002 is generally similar to that of 4 mg oral alprazolam, but greater than that of 1 mg and 2 mg oral alprazolam; the abuse potential of 1 mg AZ-002 is generally similar to that of 1 mg and 2 mg oral alprazolam, but less than that of 4 mg oral alprazolam; the
12
abuse potential of 0.5 mg AZ-002 is generally similar to that of 1 mg oral alprazolam, but less than that of 2 mg and 4 mg oral alprazolam. Overall, there was no apparent increase in abuse potential for AZ-002 compared with oral alprazolam, based on the results of the primary outcome endpoint. However, the FDA or other regulatory agencies may disagree with the results of this study, find the results inconclusive, or may require additional studies to confirm the lack of abuse potential of AZ-002.
Preclinical Studies
Alprazolam has been approved for marketing in oral tablet form. There are publicly available safety pharmacology, systemic toxicology, carcinogenicity and reproductive toxicology data that we believe we will be able to use for our regulatory filings. Therefore, our preclinical development plan was primarily focused on assessing the local tolerability of AZ-002. To date, our two preclinical inhalation toxicology studies with AZ-002 have indicated that it is generally well tolerated.
OUR PRODUCT CANDIDATE DEVELOPMENT AND COMMERCIAL STRATEGY
Key elements of our strategy include:
| | • | | Focus on Acute and Intermittent Conditions. We focus our development efforts on product candidates based on ourStaccatosystem that are intended to address important unmet medical and patient needs in the treatment of acute and intermittent conditions in which we believe theStaccato technology has the potential to change clinical practice or provide value to patients and healthcare professionals, for example, by offering rapid onset, ease of use, noninvasive administration and, in some cases, patient titration of dosage. |
| | • | | Establish Strategic Collaborations/Relationships. When appropriate, we intend to strategically collaborate with pharmaceutical and other companies for development funding or commercialization efforts. Collaborations, such as those with Ferrer and Teva, provide resources to address markets that may require greater sales or marketing resources than we are able to provide, or specific expertise to maximize the value of some product candidates. We may also choose to commercialize a product on our own with or without the services of a contract sales organization. We may also enter into strategic collaborations with other pharmaceutical companies to combine ourStaccatosystem with their proprietary compounds. |
| | • | | Retain and Control Product Manufacturing. We own all manufacturing rights to our product candidates. We complete the final manufacture and assembly of our product candidates and any future products internally, potentially enabling greater intellectual property protection and economic return from our future products. We believe controlling the final manufacture and assembly has the potential to maintain the quality ofStaccato technology-based products, reduce the risk of supply interruptions and allow for more cost-effective manufacturing through global supply of the finished product. |
Licensing Collaborations — Product Candidates
Cypress Agreement
In August 2010 we entered into a license and development agreement, or the Cypress Agreement, with Cypress Bioscience, Inc., or Cypress, forStaccato nicotine. According to the terms of the Cypress Agreement, Cypress paid us a non-refundable upfront payment of $5.0 million to acquire the worldwide license for theStaccatonicotine technology.
On December 31, 2013, the Cypress Agreement automatically terminated by the terms of the agreement, and all rights to the Staccato nicotine technology reverted back to us.
13
Research and Development
Research and development expenditures made to advance our product candidates and develop our manufacturing capabilities and for general research efforts during the last three years ended December 31, 2013, were as follows (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Product candidate expenses | | $ | 12,432 | | | $ | 18,216 | | | $ | 25,686 | |
General research | | | 6,650 | | | | 3,633 | | | | 2,576 | |
| | | | | | | | | | | | |
Total research and development | | $ | 19,082 | | | $ | 21,849 | | | $ | 28,262 | |
| | | | | | | | | | | | |
MANUFACTURING
We manufacture ADASUVE with components supplied by qualified vendors. We own all manufacturing rights to our product candidates and, subject to Teva’s co-exclusive right with us and our affiliates, to ADASUVE. The drug product manufacturing portion of the process is completed at our facility in Mountain View, California. We believe that manufacturing ADASUVE and any future products will potentially enable greater intellectual property protection, economies of scale and decrease the risk of supply interruptions. We believe our manufacturing facility will have sufficient capacity to manufacture commercial scale batches of ADASUVE and to manufacture materials for toxicology studies and clinical trials for any future studies of our product candidates for at least the next two years.
For our single dose delivery platform utilized by ADASUVE, after inspection and release of incoming components, we assemble the components of the product and spray-coat the exterior of the heat package with a thin film of API (loxapine, in the case of ADASUVE). We then assemble the plastic airway housings around the coated heat package and insert the completed product in a pharmaceutical-grade foil pouch.
We believe we have developed quality assurance, quality control systems and regulatory compliance appropriate to the design, manufacture, packaging, labeling and storage of ADASUVE and our product candidates in compliance with applicable regulations and the appropriate level of current Good Manufacturing Practice, or cGMP. These systems include extensive requirements with respect to design, quality management, quality planning and organization, product design, manufacturing facilities, equipment, purchase and handling of components, production and process controls, packaging and labeling controls, device evaluation, distribution and record keeping.
In 2007, we completed the construction of a cGMP compliant manufacturing facility located in Mountain View, California. In November 2007, we received a pharmaceutical manufacturing license from the California State Food and Drug Branch for this facility. The license was renewed in January 2013 and is valid until January 31, 2015.
In August 2012, the Spanish authorities, on behalf of the EMA, determined that our facility complied with the principles and guidelines of Good Manufacturing Practice set forth in Directive 2003/94/EC and issued an EU Certificate of Good Manufacturing Practices Compliance of a Manufacturer for our facility. This initial certificate is valid for three years, through May 15, 2015. In November 2012, the FDA completed a Pre-Approval Inspection of our facility, as part of the review process for the ADASUVE NDA. All issues related to this and other previous inspections were satisfactorily resolved, and in December 2012, we received FDA approval to market ADASUVE in the United States.
We outsource the production of the components of our single dose delivery platform, including the printed circuit boards, the molded plastic airways and the heat packages. We currently use single source suppliers for these components, as well as for the API used in ADASUVE and AZ-002. We do not carry a significant inventory of these components, and establishing additional or replacement suppliers for any of these components may not be accomplished quickly, or at all, and could cause significant additional expense. Any supply interruption from our vendors would limit our ability to manufacture ADASUVE, including for our post-approval
14
clinical trials, and/or could delay clinical trials for, and regulatory approval of our product candidates that are in development. In October 2013, Autoliv ASP, Inc., or Autoliv, notified us that they were terminating, effective October 2016, the agreement pursuant to which Autoliv supplies heat packages to us. We are seeking new manufacturers for the heat packages.
The controller for our multiple dose delivery design includes the battery power source for heating the individual metal substrates, a microprocessor that directs the electric current to the appropriate metal substrate at the appropriate time, and an icon-based liquid crystal display that shows pertinent information to the user, such as the number of doses remaining in the dose cartridge and the controller status. We may need to develop modified versions of our devices for future product candidates.
Autoliv ASP, Inc.
In November 2007, we entered into a manufacturing and supply agreement, or the Manufacture Agreement, with Autoliv, relating to the commercial supply of chemical heat packages for our single doseStaccatodevice. Autoliv developed these chemical heat packages for us pursuant to a development agreement executed in October 2005.
Currently, Autoliv manufactures, assembles and tests the chemical heat packages solely for us in conformance with our specifications. We pay Autoliv a specified purchase price, which varies based on annual quantities ordered by us, per chemical heat package delivered. The Manufacture Agreement provides that during the term of the Manufacture Agreement, Autoliv will be our exclusive supplier of chemical heat packages. In addition, the Manufacture Agreement grants Autoliv the right to negotiate for the right to supply commercially any second generation chemical heat package, or a second generation product, and provides that we will pay Autoliv certain royalty payments if we manufacture second generation products ourselves or if we obtain second generation products from a third party manufacturer. Upon the expiration or termination of the Manufacture Agreement we will also be required, on an ongoing basis, to pay Autoliv certain royalty payments related to the manufacture of the chemical heat packages by us or third party manufacturers.
In June 2010 and February 2011, we entered into agreements to amend the terms of the Manufacture Agreement, or the Amendments. Under the terms of the first of the Amendments, we paid Autoliv $4.0 million and issued Autoliv a $4.0 million unsecured promissory note in return for a production line for the commercial manufacture of chemical heat packages. Each production line is comprised of two identical and self-sustaining “cells”, and the first such cell was completed, installed and qualified in connection with such Amendment. Under the terms of the second of the Amendments, the original $4.0 million note was cancelled and a new unsecured promissory note with a reduced principal amount of $2.8 million, or the Second Note, was issued and production on the second cell ceased. In the event that we request completion of the second cell of the first production line for the commercial manufacture of chemical heat packages, Autoliv will complete, install and fully qualify such second cell for a cost to us of $1.2 million and Autoliv will transfer ownership of such cell to us upon the payment in full of such $1.2 million and the Second Note.
The provisions of the Amendments supersede (a) our obligation set forth in the Manufacture Agreement to reimburse Autoliv for certain expenses related to the equipment and tooling used in production and testing of the chemical heat packages in an amount of up to $12.0 million upon the earliest of December 31, 2011, 60 days after the termination of the Manufacture Agreement or 60 days after approval by the FDA of an NDA filed by us, and (b) the obligation of Autoliv to transfer possession of such equipment and tooling.
At our request, Autoliv will manufacture up to two additional production lines for the commercial manufacture of chemical heat packages at a cost not to exceed $2.4 million for each additional line.
In October 2013, Autoliv notified us that they were terminating, effective October 2016, the Manufacture Agreement. Upon termination of the Manufacture Agreement, we will retain full ownership of the production equipment for commercial manufacture of chemical heat packages developed for us by Autoliv, and Autoliv’s obligations under the Manufacture Agreement will terminate in full. Prior to October 2016 we and Autoliv will remain fully obligated to perform pursuant to the terms of the Manufacture Agreement. We are analyzing manufacturing options for the chemical heat packages subsequent to October 2016.
15
GOVERNMENT REGULATION
FDA Product Approval Process
The testing, manufacturing, labeling, advertising, promotion, distribution, export and marketing of our product candidates are subject to extensive regulation by governmental authorities in the United States and other countries. Our product candidates include drug compounds incorporated into our delivery device and are considered “combination products” in the United States. We have agreed with the FDA that our product candidates will be reviewed by the FDA’s Center for Drug Evaluation and Research. The FDA regulates pharmaceutical products in the United States under the Federal Food, Drug and Cosmetic Act, or FDCA. The steps required before a drug may be approved for marketing in the United States generally include:
| | • | | preclinical laboratory studies and animal tests; |
| | • | | the submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials commence; |
| | • | | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product; |
| | • | | the submission to the FDA of an NDA; |
| | • | | satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made to assess compliance with cGMP. In addition, the FDA may inspect clinical trial sites that generated the data in support of the NDA; and |
| | • | | FDA review and approval of the NDA. |
The testing and approval process requires substantial time, effort and financial resources, and the receipt and timing of any approval is uncertain. Preclinical studies include laboratory evaluations of the product candidate, as well as animal studies to assess the potential safety and efficacy of the product candidate. The results of the preclinical studies, together with manufacturing information and analytical data, are submitted to the FDA as part of the IND, which must become effective before clinical trials may commence. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the trials as outlined in the IND prior to that time. In that case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed.
Clinical trials typically begin with the administration of the product candidates to healthy volunteers or patients under the supervision of a qualified principal investigator. Before a clinical trial may begin, an independent institutional review board, or IRB, at or servicing each institution must review and approve each clinical trial. The IRB will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution.
Clinical trials typically are conducted in three sequential phases prior to approval, but the phases may overlap. A fourth, or post-approval, phase may include additional clinical studies. These phases generally include the following:
| | • | | Phase 1. Phase 1 clinical trials involve the initial introduction of the drug into human subjects, frequently healthy volunteers. These studies are designed to determine the metabolism and pharmacologic actions of the drug in humans, the adverse effects associated with increasing doses and, if possible, to gain early evidence of effectiveness. In Phase 1 clinical trials, the drug is usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution, metabolism, excretion and pharmacodynamics. |
| | • | | Phase 2. Phase 2 clinical trials usually involve studies in a limited patient population to (1) evaluate the efficacy of the drug for specific, targeted indications; (2) determine dosage tolerance and optimal dosage; and (3) identify possible adverse effects and safety risks. Although there are no statutory or regulatory definitions for Phase 2a and Phase 2b, Phase 2a is commonly used to describe a Phase 2 clinical trial designed to evaluate efficacy, adverse effects and safety risks and Phase 2b is commonly used to describe a subsequent Phase 2 clinical trial that also evaluates dosage tolerance and optimal dosage. |
16
| | • | | Phase 3. If a compound is found to be potentially effective and to have an acceptable safety profile in Phase 2 clinical trials, the clinical trial program will be expanded to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population at geographically dispersed clinical trial sites. Phase 3 clinical trials usually include several hundred to several thousand patients. |
| | • | | Phase 4. Phase 4 clinical trials are studies required of, or agreed to by, a sponsor that are conducted after the FDA has approved a product for marketing. These studies are used to gain additional information from the treatment of patients in the intended therapeutic indication and to verify a clinical benefit in the case of drugs approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement. These clinical trials are often referred to as Phase 3/4 post-approval clinical trials. Failure to promptly conduct Phase 4 clinical trials could result in withdrawal of approval for products approved under accelerated approval regulations. |
In the case of products for the treatment of severe or life threatening diseases, the initial clinical trials are sometimes conducted in patients rather than in healthy volunteers. Since these patients are already afflicted with the target disease, it is possible that such clinical trials may provide evidence of efficacy traditionally obtained in Phase 2 clinical trials. These trials are referred to frequently as Phase 1/2 clinical trials. The FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
The results of preclinical studies and clinical trials, together with detailed information on the manufacture and composition of the product, are submitted to the FDA in the form of an NDA requesting approval to market the product. Generally, regulatory approval of a new drug by the FDA may follow one of three routes. The most traditional of these routes is the submission of a full NDA under Section 505(b)(1) of the FDCA. A second route, which is possible where an applicant chooses to rely in part on the FDA’s conclusion about the safety and effectiveness of previously approved drugs, is to submit a more limited NDA described in Section 505(b)(2) of the FDCA. The final route is the submission of an Abbreviated New Drug Application for products that are shown to be therapeutically equivalent to previously approved drug products as permitted under Section 505(j) of the FDCA. We do not expect any of our product candidates to be submitted under Section 505(j). Both Section 505(b)(1) and Section 505(b)(2) applications are required by the FDA to contain full reports of investigations of safety and effectiveness. However, in contrast to a traditional NDA submitted pursuant to Section 505(b)(1) in which the applicant submits all of the data demonstrating safety and effectiveness, an application submitted pursuant to Section 505(b)(2) can rely upon findings by the FDA that the reference drug is safe and effective. As a consequence, the preclinical and clinical development programs leading to the submission of an NDA under Section 505(b)(2) may be less expensive to carry out and may be concluded in a shorter period of time than programs required for a Section 505(b)(1) application. In its review of any NDA submissions, however, the FDA has broad discretion to require an applicant to generate additional data related to safety and efficacy, and it is impossible to predict the number or nature of the studies that may be required before the FDA will grant approval. Notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), certain brand-name pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA changes its interpretation of Section 505(b)(2), this could delay or even prevent the FDA from approving any Section 505(b)(2) NDA that we submit.
To the extent that a Section 505(b)(2) applicant is relying on the FDA’s findings for an already-approved reference product, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the FDA’s publication,Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. A certification that the new product will not infringe the reference product’s Orange Book-listed patents or that such patents are invalid is called a paragraph IV certification, and could be challenged in court by the patent owner or holder of the application of the reference product. This could delay the approval of any Section 505(b)(2) application we submit. In addition, any period of non-patent exclusivity applicable to the reference product might delay approval of any Section 505(b)(2) application we submit. Any Section 505(b)(1) or Section 505(b)(2) application we submit for a drug product containing a previously approved API might be eligible for three years of marketing exclusivity, provided new clinical
17
investigations that were conducted or sponsored by us are deemed to be essential to the FDA’s approval of the application. Five years of data exclusivity are granted if the FDA approves an NDA for a new chemical entity. In addition, we are required to list in the Orange Book the patents that claim the approved drug product or drug substance, or that cover an approved method-of-use of the drug product. In order for a generic or 505(b)(2) applicant to rely on the FDA’s approval of any NDA we submit, the generic or 505(b)(2) applicant must certify to any Orange Book listed patents and might be subject to any non-patent exclusivity covering our approved drug product.
For the ADASUVE NDA, we followed, and in future submissions for ADASUVE and our other product candidates we plan to follow the development pathway permitted under the FDCA that we believe will maximize the commercial opportunities for these product candidates. We are currently pursuing the Section 505(b)(2) application route for our product candidates. As such, we have engaged and intend to continue to engage in discussions with the FDA to determine which, if any, portions of our development program can be modified, based on previous FDA findings of a drug’s safety and effectiveness.
Before approving an NDA, the FDA inspects the facilities at which the product is manufactured, whether ours or our third party manufacturers’, and will not approve the product unless the manufacturing facility complies with cGMP or, where applicable, the Quality System Regulation, or QSR. The FDA reviews all NDA’s submitted before it accepts them for filing and may request additional information rather than accept an NDA for filing. Once the NDA submission has been accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA has 10 months in which to complete its initial review of a standard NDA and respond to the applicant, and six months for a priority NDA. Those time frames run from the date that FDA accepts an NDA for filing if the NDA is for a New Molecular Entity, or NME, and from the date of NDA submission for a non-NME NDA. The FDA does not always meet the PDUFA goal dates for standard and priority NDAs. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may delay approval of an NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product. Also, if regulatory approval of a product is granted, such approval may include limitations on the indicated uses for which such product may be marketed. Once approved, the FDA may withdraw the product approval if compliance with regulatory requirements and conditions of approvals are not maintained or if safety problems occur after the product reaches the marketplace. In addition, the FDA may require testing, including Phase 4 studies or clinical trials, and surveillance programs to monitor the approved products, and may limit further marketing of the product based on the results of these post-marketing programs.
Post-Marketing Regulations
ADASUVE and any other product candidates that receive NDA approval will be limited to those diseases and conditions for which the product is effective, as demonstrated through clinical trials and as specified in the approved labeling. Even if that regulatory approval is obtained, a marketed product, its manufacturer and its manufacturing facilities, and those who market and promote the product, are subject to continual review and periodic inspections by the FDA and, in our case, the State of California. Discovery of previously unknown problems with a medicine, device, manufacturer or facility may result in restrictions on the marketing or manufacturing of an approved product, including costly recalls or withdrawal of the product from the market. The FDA has broad post-market regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, withdraw approvals, and through the Department of Justice, to initiate court actions seeking to seize products, enjoin violations and impose criminal penalties.
Other Governmental Regulations
In addition to regulation by the FDA and certain state regulatory agencies, the United States Drug Enforcement Administration, or DEA, imposes various registration, recordkeeping and reporting requirements, procurement and manufacturing quotas, labeling and packaging requirements, security controls and a restriction on prescription refills on certain pharmaceutical products under the Controlled Substances Act, or CSA. The DEA regulates drug substances as Schedule I, II, III, IV or V substances, with FDA approved drugs in Schedules II through V. A principal factor in determining the particular requirements, if any, applicable to a substance
18
under the CSA is its actual or potential abuse profile. Substances controlled in Schedule I and II have the highest potential for abuse and Schedule V substances the lowest potential for abuse relative to the substances in other schedules. Alprazolam and zaleplon, the APIs in AZ-002 and AZ-007, respectively, are regulated as Schedule IV substances and fentanyl, the API in AZ-003, is regulated as a Schedule II substance. Each of these product candidates is subject to DEA regulations relating to registration, security, recordkeeping, and distribution procedures, and, if approved, prescription restrictions and the DEA regulations may impact the availability of the scheduled substance for clinical trials and commercial distribution. As a Schedule II substance, fentanyl is subject to additional controls, including quotas on the amount of product that can be manufactured and additional limitations on prescription refills. We have received necessary registrations from the DEA for the manufacture of AZ-002, AZ-003 and AZ-007. The DEA periodically inspects facilities for compliance with its rules and regulations. Failure to comply with current and future CSA requirements and regulations of the DEA could lead to a variety of sanctions, including revocation, or denial of renewal, of DEA registrations, injunctions, or civil or criminal penalties, and could harm our business and financial condition.
The single dose design of ourStaccatosystem uses what we refer to as “energetic materials” to generate the rapid heating necessary for vaporizing the drug while avoiding degradation. Manufacture of products containing these types of materials is controlled by the Bureau of Alcohol, Tobacco, Firearms and Explosives, or ATF, under 18 United States Code Chapter 40 and implementing regulations. Technically, the energetic materials used in ourStaccatosystem are classified as “low explosives,” and we have been granted a license/permit by the ATF for their manufacture.
Additionally, due to inclusion of the energetic materials in ourStaccatosystem, shipments of the single dose design of ourStaccatosystem have been evaluated to determine if they are regulated by the Department of Transportation, or DOT, under Section 173.56, Title 49 of the United States Code of Federal Regulations. The single dose version of ourStaccato device has been granted “Not Regulated as an Explosive” status by the DOT.
We have received funding for one or more research projects from a funding agency of the United States government, and inventions conceived or first actually reduced to practice in performance of the research project, or subject inventions, are subject to the rights and limitations of certain federal statutes and various implementing regulations known generally and collectively as the “Bayh-Dole Requirements.” As a funding recipient, we are subject to certain reporting requirements for subject inventions, and certain limitations are placed on assignment of the invention rights. In addition, the federal government retains a non-exclusive, irrevocable, paid-up license to practice any subject invention and, in exceptional cases, the federal government may seek to take title to the invention.
We will be subject to a variety of foreign regulations governing clinical trials and the marketing of any future products. The conduct of clinical trials in the EU is governed by Directive 2001/20/EC which imposes obligations and procedures that are similar to those provided in applicable U.S. laws. European Union Good Clinical Practice rules and EU Good Laboratory Practice obligations must also be respected during conduct of the trials. The conduct of clinical trials must be approved by the competent regulatory authorities and the competent Ethics Committees in the EU Member States in which clinical trials take place.
In the EU, in order to obtain marketing authorization for a medicinal product, applicants are required to submit applications for marketing authorization based on the ICH Common Technical Document to the relevant competent authorities, and must demonstrate the quality, safety and efficacy of the medicinal product. As part of the applications applicants must include a demonstration that studies have been conducted with the medicinal product in the pediatric population as provided by a Pediatric Investigation Plan approved by the Pediatric Committee of the EMA. Alternatively, confirmation that the applicant has been granted a waiver or deferral for the conduct of these studies must be provided.
Similarly to the United States, marketing authorization holders and manufacturers of medicinal products in the EU are subject to comprehensive regulatory oversight by the EMA and the competent authorities of the EU Member States. This oversight is conducted both before and after grant of manufacturing and marketing authorizations. It includes control of compliance with EU cGMP rules.
Failure to comply with EU and EU Member State laws governing the conduct of clinical trials, grant of marketing authorization for medicinal products and the marketing of such products, both before and after grant of
19
marketing authorization, could result in administrative, civil or criminal penalties. These penalties could include refusal to authorize the conduct of clinical trials, refusal to grant marketing authorization, product withdrawals and recalls, product seizures, suspension or withdrawal of the marketing authorization, fines and criminal penalties.
Outside the United States and the EU, our ability to market a product depends upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. In any country, however, we will only be permitted to commercialize our products if the appropriate regulatory authority is satisfied that we have presented adequate evidence of safety, quality and efficacy. Whether or not FDA approval or EC authorization has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing of the product in those countries. The time needed to secure approval may be longer or shorter than that required for FDA approval. The regulatory approval and oversight process in other countries includes all of the risks associated with the FDA process described above.
Federal Anti-Kickback, False Claims Act & Federal Physician Payment Sunshine Act
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of U.S. state and federal laws are relevant to certain marketing practices in the pharmaceutical industry. These laws include the Federal Anti-Kickback Statute, false claims statutes, and the Federal Physician Payment Sunshine Act. We are subject to these laws and they may affect our business. The Federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good or service for which payment may be made under federal health care programs such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the Federal Anti-Kickback Statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. The Federal Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 and subsequent legislation, or collectively, the Healthcare Reform Act or PPACA, among other things, amends the intent requirement of the Federal Anti-Kickback Statute. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions; however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny.
The Federal False Claims Act prohibits, among other things, any person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment, or knowingly making, or causing to be made, a false record or statement material to a false or fraudulent claim. Several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, also may implicate the Federal False Claims Act. Federal False Claims Act violations may result in imprisonment, criminal fines, civil monetary damages and penalties and exclusion from participation in federal healthcare programs. The majority of U.S. states also have statutes or regulations similar to the Federal Anti-Kickback Statute and False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs. A number of states have Anti-Kickback Statutes that apply regardless of the payor.
In addition, the Federal Physician Payment Sunshine Act requires extensive tracking of physician and teaching hospital payments, maintenance of a payments database, and public reporting of the payment data. The system for reporting is called Open Payments and applies to pharmaceutical companies. This is the first year for
20
reporting under the Open Payments system. CMS recently announced that, starting February 28, 2014, Open Payments registration and data submission for the Federal Physician Payment Sunshine Act will open with a two-phased approach for reporting 2013 payments. Phase 1 (February 18, 2014 — March 31, 2014) includes user registration in CMS’ Enterprise Portal and submission of corporate profile information and aggregate 2013 payment data. Phase 2 (begins in May and extends for at least 30 days) includes industry registration in the Open Payments system, submission of detailed 2013 payment data, and legal attestation to the accuracy of the data. Failure to comply with the reporting obligations may result in civil monetary penalties.
Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also prohibit certain marketing related activities including the provision of gifts, meals, or other items to certain health care providers. In addition, some states require pharmaceutical companies to implement compliance programs or marketing codes.
In the EU, the advertising and promotion of our products is also subject to EU and EU Member States’ laws concerning promotion of medicinal products, interactions with physicians, misleading and comparative advertising and unfair commercial practices, as well as other EU Member State legislation that may apply to the advertising and promotion of medicinal products. These laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics (SmPC) as approved by the competent authorities. The SmPC is the document that provides information to physicians concerning the safe and effective use of the medicinal product. It forms an intrinsic and integral part of the marketing authorization granted for the medicinal product. Promotion of a medicinal product that does not comply with the SmPC is considered to constitute off-label promotion. The off-label promotion of medicinal products is prohibited in the EU. The applicable laws at EU level and in the individual EU Member States also prohibit the direct-to-consumer advertising of prescription-only medicinal products. Violations of the rules governing the promotion of medicinal products in the EU could be penalized by administrative measures, fines and imprisonment. These laws may further limit or restrict communications concerning the advertising and promotion of our products to the general public and may also impose limitations on our promotional activities with healthcare professionals.
Failure to comply with the EU Member State laws implementing the Community Code on medicinal products, EU rules governing the promotion of medicinal products, interactions with physicians, misleading and comparative advertising and unfair commercial practices, with the EU Member State laws that apply to the promotion of medicinal products, statutory health insurance, bribery and anti-corruption or with other applicable regulatory requirements can result in enforcement action by the EU Member State authorities, which may include any of the following: fines, imprisonment, orders forfeiting products or prohibiting or suspending their supply to the market, or requiring the manufacturer to issue public warnings, or to conduct a product recall.
Interactions between pharmaceutical companies and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct in the individual EU Member States. The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medicinal products is prohibited in the EU. The provision of benefits or advantages to physicians is also governed by the national anti-bribery laws of the EU Member States. One example is the UK Bribery Act 2010. This Act applies to any company incorporated in or “carrying on business” in the UK, irrespective of where in the world the alleged bribery activity occurs. This Act could have implications for our interactions with physicians in and outside the UK. Violation of these laws could result in substantial fines and imprisonment.
Payments made to physicians in certain EU Member States must be publically disclosed. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer, his/her competent professional organization, and/or the competent authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
21
United States Healthcare Reform
In March 2010, the Healthcare Reform Act, or PPACA, was adopted in the United States. This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Healthcare Reform Act contains a number of provisions that are expected to impact our business and operations, in some cases in ways we cannot currently predict. Changes that may affect our business include those governing enrollment in federal and private healthcare programs, new Medicare reimbursement methods and rates, increased rebates and taxes on pharmaceutical products, and revised fraud and abuse and enforcement requirements. These changes will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
Additional provisions of the Healthcare Reform Act, some of which became effective in 2011, may negatively affect our future revenues. For example, the Healthcare Reform Act makes changes to the Medicaid Drug Rebate Program, including increasing the minimum rebate from 15.1% to 23.1% of the average manufacturer price, or AMP, for most innovator products and from 11% to 13% for non-innovator products changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs, and capped the total rebate amount for innovator drugs at 100% of the average manufacturer price. We expect that the increased minimum rebate of 23.1% will apply to ADASUVE following its commercialization in the United States.
The Healthcare Reform Act also expanded the Public Health Service’s 340B drug pricing discount program, in which we expect to participate. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. The Healthcare Reform Act expanded the 340B program to include additional types of covered entities: certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals, each as defined by the Healthcare Reform Act. The Healthcare Reform Act exempts orphan drugs — those designated under section 526 of the FDCA — from the ceiling price requirements for these newly-eligible entities. We believe our product candidate in active development, AZ-002 (Staccato alprazolam), could qualify for orphan drug status. However, we have not applied for, andAZ-002 may not be granted, orphan drug status. The Health Resources and Services Administration, or HRSA, which administers the 340B program, issued a final regulation to implement the orphan drug exception in July 2013. The final regulation interprets the orphan drug exception narrowly. It exempts orphan drugs from the ceiling price requirements for the newly-eligible entities only when the orphan drug is used for its orphan indication. The newly-eligible entities are entitled to purchase orphan drugs at the ceiling price when the orphan drug is not used for its orphan indication. The final regulation, which became effective October 1, 2013, is subject to a pending lawsuit that seeks to block its implementation. The narrow scope of the orphan drug exception in HRSA’s final regulation will increase the complexity of compliance, will make compliance more time-consuming, and could negatively impact our results of operations if we successfully commercialize AZ-002 (Staccato alprazolam) and AZ-002 qualifies for orphan drug status.
The Healthcare Reform Act also obligates the Secretary of the HHS to create regulations and processes to improve the integrity of the 340B program and to update the agreement that manufacturers must sign to participate in the 340B program to obligate a manufacturer to offer the 340B price to covered entities if the manufacturer makes the drug available to any other purchaser at any price and to report to the government the ceiling prices for its drugs. HRSA is expected to issue a comprehensive proposed regulation in 2014 that will address many aspects of the 340B program. When that regulation is finalized, it could affect our obligations under the 340B program in ways we cannot anticipate. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
Many of the Healthcare Reform Act’s most significant reforms take effect in 2014 and thereafter, and the details will be shaped significantly as the various provisions become active, especially given the political nature of the law. In 2012, the Supreme Court of the United States heard challenges to the constitutionality of the individual mandate and the viability of certain provisions of the Healthcare Reform Act. The Supreme Court’s decision upheld most of the Healthcare Reform Act and determined that requiring individuals to maintain
22
“minimum essential” health insurance coverage or pay a penalty to the Internal Revenue Service was within Congress’s constitutional taxing authority. However, the Supreme Court struck down a provision in the Healthcare Reform Act that penalized states that choose not to expand their Medicaid programs through an increase in the Medicaid eligibility income limit from a state’s current eligibility levels to 133% of the federal poverty limit. As a result of the Supreme Court’s ruling, it is unclear whether states will expand their Medicaid programs by raising the income limit to 133% of the federal poverty level and whether there will be more uninsured patients in 2014 than anticipated when Congress passed the Healthcare Reform Act. For each state that does not choose to expand its Medicaid program, there will be fewer insured patients overall. The reduction in the number of insured patients could impact our sales, business and financial condition.
PHARMACEUTICAL PRICING AND REIMBURSEMENT
In both domestic and foreign markets, our ability to commercialize successfully and/or attract strategic collaborators for ADASUVE and our other product candidates depends in significant part on the availability of adequate coverage and reimbursement from third-party payors, including, in the United States, governmental payors such as the Medicare and Medicaid programs, managed care organizations, and private health insurers.
In the United States, the Medicare program is administered by the Centers for Medicare & Medicaid Services, or CMS. Coverage and reimbursement for products and services under Medicare are determined in accordance with the Social Security Act and pursuant to regulations promulgated by CMS, as well as the agency’s subregulatory coverage and reimbursement determinations. Medicare Part B provides limited coverage of outpatient drugs and biologicals that are furnished “incident to” a physician’s services. Generally, “incident to” drugs and biologicals are covered only if they satisfy certain criteria, including that they are of the type that is not usually self-administered by the patient and they are reasonable and necessary for a medically accepted diagnosis or treatment. CMS and Medicare contractors may limit coverage of ADASUVE for beneficiaries in accordance with the approved product labeling. In the past, state Medicaid programs were required to cover drugs and biologicals of manufacturers that entered into a Medicaid Drug Rebate Agreement, although such drugs and biologicals were still subject to prior authorization or other utilization controls. On July 15, 2013, CMS issued its Final Rule addressing prescription drug coverage for the Medicaid expansion population. The Final Rule dramatically restricts mandatory coverage of prescription drugs for the expansion Medicaid enrollees by essentially eliminating the requirement that states must cover each covered outpatient drug subject to a national rebate agreement. Although drugs provided to the new Medicaid expansion population still are subject to manufacturer rebates, state Medicaid programs are no longer required to cover all drugs subject to the national rebate agreement. Instead, states are required to cover a minimum of drugs consistent with the applicable “benchmark” plan in the state coverage for the Medicaid expansion population. Private payors have their own processes for determining whether or not a drug or biological will be covered, often based on the available medical literature.
Medicare Part B pays providers that administer covered Part B drugs and biologicals under a payment methodology using average sales price, or ASP, information. Manufacturers, including us when we or any collaborator commercialize one of our products, are required to provide ASP information to CMS on a quarterly basis. This information is used to compute Medicare payment rates, updated quarterly based on this ASP information. Until enough ASP data are available to calculate a payment rate, reimbursement is wholesale acquisition cost plus six percent. There also is a mechanism for comparison of the ASP for a product to the widely available market price and the Medicaid Average Manufacturer Price for the product, which could cause further decreases in Medicare payment rates.
We anticipate that ADASUVE will be used only in hospital inpatient and hospital outpatient settings that are enrolled in the ADASUVE REMS and that have immediate access on-site to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation). The Medicare statute establishes the payment rate for new drugs and biologicals administered in hospital outpatient departments that are granted “pass-through status” at the rate applicable in physicians’ offices (i.e., ASP plus six percent) for two to three years after FDA approval. ADASUVE has pass-through status under OPPS starting January 1, 2014. CMS establishes the payment rates for drugs and biologicals that do not havepass-through status by regulation. For 2014, these drugs are reimbursed at ASP plus six percent if they have an
23
average cost per day exceeding $90; drugs with an average cost per day of less than $90 are not separately reimbursed in the hospital outpatient department setting. Drugs furnished in the hospital inpatient setting generally are not separately reimbursed but are paid for as part of the payment for the inpatient stay. CMS granted a C-code for ADASUVE, which will provide for reimbursement for the product used in emergency departments under a hospital’s OPPS.
The statutory methodology under which CMS establishes reimbursement rates is subject to change, particularly because of budgetary pressures facing the Medicare program and the federal government. As of April 1, 2013, Medicare payments for all items and services, including drugs and biologicals, were reduced by up to 2% under the sequestration required by the Budget Control Act of 2011, Pub. L. No. 112-25 as amended by the American Taxpayer Relief Act of 2012, Pub. L. 112-240, unless Congress acts to prevent the cuts. Congress also could enact further cuts, and, in fact, under the Bipartisan Budget Act of 2013, the cuts originally scheduled through 2021 were extended through 2023. The Medicare Modernization Act of 2003 made changes in reimbursement methodology that reduced the Medicare reimbursement rates for many drugs. In the past, Congress has considered additional reductions in Medicare reimbursement for drugs as part of legislation to reduce the budget deficit. Similar legislation could be enacted in the future. The Medicare regulations and interpretive determinations that determine how drugs and services are covered and reimbursed also are subject to change.
Third-party payors other than Medicare have a variety of methodologies for paying for drugs and biologicals. Payors also are increasingly considering new metrics as the basis for reimbursement rates, ASP, AMP and Actual Acquisition Cost. The existing data for reimbursement based on these metrics is relatively limited, although certain states have begun to survey acquisition cost data for the purpose of setting Medicaid reimbursement rates. CMS has made draft National Average Drug Acquisition Cost, or NADAC, and draft National Average Retail Price, or NARP, data publicly available on at least a monthly basis. In July 2013, CMS suspended the publication of draft NARP data, pending funding decisions. In November 2013, CMS moved to publishing final rather than draft NADAC data and has since made updated NADAC data publicly available on a weekly basis. Therefore, it may be difficult to project the impact of these evolving reimbursement mechanics on the willingness of payors to cover ADASUVE.
Third-party payors are increasingly challenging the prices charged for medical products and services and examining their cost effectiveness, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost effectiveness of any future product candidates. Even with studies, our product candidates may be considered less safe, less effective or less cost effective than existing products, and third-party payors therefore may not provide coverage and reimbursement for our product candidates, in whole or in part. Teva is responsible for conducting any cost-effectiveness studies for ADASUVE in the United States.
Political, economic and regulatory influences are subjecting the healthcare industry in the United States to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals and enactments to change the healthcare system in ways that could impact our ability to sell our products profitably. We anticipate that the U.S. Congress, state legislatures and the private sector will continue to consider and may adopt healthcare policies intended to curb rising healthcare costs. These cost containment measures include:
| | • | | controls on government funded reimbursement for medical products and services; |
| | • | | new or increased requirements to pay prescription drug rebates to government health care programs; |
| | • | | controls on healthcare providers; |
| | • | | challenges to the pricing of medical products and services or limits or prohibitions on reimbursement for specific products and therapies through other means; |
| | • | | requirements that health care providers try less expensive products or generics before a more expensive branded product; |
| | • | | changes in or reform of drug importation laws; |
24
| | • | | expansion of use of managed care systems in which healthcare providers contract to provide comprehensive healthcare for a fixed cost per person; and |
| | • | | public funding for cost effectiveness research, which may be used by government and private third party payors to make coverage and payment decisions. |
If we commercialize future product candidates ourselves, we expect to participate in the Medicaid Drug Rebate program, established by the Omnibus Budget Reconciliation Act of 1990 and amended by the Veterans Health Care Act of 1992 as well as subsequent legislation. We would also expect to participate in and have certain price reporting obligations to several state Medicaid supplemental rebate programs and other governmental pricing programs. Once we begin to participate in the Medicaid Drug Rebate program, we will be required to pay a rebate to each state Medicaid program for our covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having federal funds being made available to the states for our drugs under Medicaid and Medicare Part B. Those rebates will be based on pricing data that we will report on a monthly and quarterly basis to CMS, the federal agency that administers the Medicaid Drug Rebate program. These data will include the AMP and, in the case of innovator products, such asStaccato-technology based products, the best price, or BP, for each drug. We expect that a significant portion of Teva’s revenue from sales of ADASUVE will be obtained through government payors, including Medicaid, and any failure to qualify for reimbursement for ADASUVE under those programs would have a material adverse effect on future revenues from sales of ADASUVE.
Federal law also requires that a company that participates in the Medicaid rebate program report ASP information to CMS for certain categories of drugs that are paid under Part B of the Medicare program. Manufacturers calculate ASP based on a statutorily defined formula and interpretations of the statute by CMS as to what should or should not be considered in computing ASP. An ASP for each National Drug Code for a product that is subject to the ASP reporting requirement must be submitted to CMS no later than 30 days after the end of each calendar quarter. CMS uses these submissions to determine payment rates for drugs under Medicare Part B. Changes affecting the calculation of ASP could affect the ASP calculations for our products and the resulting Medicare payment rate, and could negatively impact our results of operations, once we begin to participate in the Medicare program.
Once we or any collaborator begin to participate in government pricing programs, we or any collaborator will be liable for errors associated with our submission of pricing data. If we or any collaborator are found to have knowingly submitted false AMP, ASP, or BP information to the government, we or any collaborator may be liable for civil monetary penalties in the amount of $100,000 per item of false information. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, the statute provides for civil monetary penalties of up to $10,000 for each misrepresentation for each day in which the misrepresentation was applied. Failure to submit monthly/quarterly AMP, ASP, and BP data on a timely basis could result in a civil monetary penalty of $10,000 per day for each day the information is late beyond the due date. In the event that CMS were to terminate our rebate agreement after we or any collaborator begin to participate in the Medicaid program, no federal payments would be available under Medicaid or Medicare Part B for our covered outpatient drugs.
Federal law requires that any company that participates in the Medicaid Drug Rebate Program also participate in the Public Health Service’s 340B drug pricing discount program in order for federal funds to be available for the manufacturer’s drugs under Medicaid and Medicare Part B. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients. The 340B ceiling price is calculated using a statutory formula, which is based on the AMP and rebate amount for the covered outpatient drug as calculated under the Medicaid rebate program. Changes to the definition of AMP and the Medicaid rebate amount under PPACA and CMS’s issuance of final regulations implementing those changes also could affect our 340B ceiling price calculations and negatively impact our results of operations once we begin to participate in the 340B program.
25
As described above, the Healthcare Reform Act expanded the 340B program to include additional types of covered entities but exempts “orphan drugs” — those designated under section 526 of the FDCA — from the ceiling price requirements for these newly-eligible entities. We believe our product candidate in active development, AZ-002 (Staccato alprazolam), could qualify for orphan drug status. HRSA issued a final regulation to implement the orphan drug exception in July 2013. The final regulation interprets the orphan drug exception narrowly. It exempts orphan drugs from the ceiling price requirements for the newly-eligible entities only when the orphan drug is used for its orphan indication. The newly-eligible entities are entitled to purchase orphan drugs at the ceiling price when the orphan drug is not used for its orphan indication. The final regulation, which became effective October 1, 2013, is subject to a pending lawsuit that seeks to block its implementation. The narrow scope of the orphan drug exception in HRSA’s final regulation will increase the complexity of compliance, will make compliance more time-consuming, and could negatively impact our results of operations if we successfully commercialize AZ-002 (Staccato alprazolam) and AZ-002 qualifies for orphan drug status.
In order to be eligible to have our products paid for with federal funds under the Medicaid and Medicare Part B programs and purchased by certain federal agencies and grantees, we anticipate that we will participate in the Department of Veterans Affairs, or VA, Federal Supply Schedule, or FSS, pricing program, established by Section 603 of the Veterans Health Care Act of 1992. Under this program, we will be obligated to make our product available for procurement on an FSS contract and charge a price to four federal agencies — VA, Department of Defense, Public Health Service, and Coast Guard — that is no higher than the statutory Federal Ceiling Price, or FCP. The FCP is based on the non-federal average manufacturer price, which we will be required to calculate and report to the VA on a quarterly and annual basis.
We expect to experience pricing pressures in the United States in connection with the sale of ADASUVE due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals. We are unable to predict what additional legislation, regulations or policies, if any, relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation, regulations or policies would have on our business. Any cost containment measures, including those listed above, or other healthcare system reforms that are adopted could have a material adverse effect on our ability to operate profitably.
In the EU the sole legal instrument at the EU level governing the pricing and reimbursement of medicinal products is Council Directive 89/105/EEC, or the Price Transparency Directive. The aim of this Directive is to ensure that pricing and reimbursement mechanisms established in the EU Member States are transparent and objective, do not hinder the free movement and trade of medicinal products in the EU and do not hinder, prevent or distort competition on the market. The Price Transparency Directive does not provide any guidance concerning the specific criteria on the basis of which pricing and reimbursement decisions are to be made in individual EU Member States. Neither does it have any direct consequence for pricing nor reimbursement levels in individual EU Member States. The EU Member States are free to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices and/or reimbursement levels of medicinal products for human use. An EU Member State may approve a specific price or level of reimbursement for the medicinal product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the medicinal product on the market, including volume-based arrangements and reference pricing mechanisms.
Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States. These EU Member States include the United Kingdom, France, Germany and Sweden. The HTA process in the EU Member States is governed by the national laws of these countries. HTA is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. HTA generally focuses on the clinical efficacy and effectiveness, safety, cost, and cost-effectiveness of individual medicinal products as well as their potential implications for the healthcare system. Those elements of medicinal products are compared with other treatment options available on the market.
26
The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product vary among EU Member States.
In 2011, Directive 2011/24/EU was adopted at EU level. This Directive concerns the application of patients’ rights in cross-border healthcare. The Directive is intended to establish rules for facilitating access to safe and high-quality cross-border healthcare in the EU. It also provides for the establishment of a voluntary network of national authorities or bodies responsible for HTA in the individual EU Member States. The purpose of the network is to facilitate and support the exchange of scientific information concerning HTAs. This could lead to harmonization among EU Member States of the criteria taken into account in the conduct of HTA and their impact on pricing and reimbursement decisions.
Pricing for ADASUVE has already been granted in the individual countries in the EU in which it has been launched, namely Germany, Austria, Spain and Romania.
PATENTS AND PROPRIETARY RIGHTS
We actively seek to patent the technologies, inventions and improvements we consider important to the development of our business. In addition, we rely on trade secrets and contractual arrangements to protect our proprietary information. Some areas for which we seek patent protection include:
| | • | | theStaccatosystem and its components; |
| | • | | methods of using theStaccatosystem; |
| | • | | the aerosolized form of drug compounds produced by theStaccatosystem; and |
| | • | | methods of making and using the drug containing aerosols, including methods of administering the aerosols to a patient. |
As of March 18, 2014, we held 195 issued and allowed U.S. and international patents. Most of our patents are directed to compositions for delivery of an aerosol comprising drugs other than our primary product candidates described below, and cover the process for producing those aerosols using theStaccatosystem. As of February 4, 2014, we held 17 additional pending patent applications in the United States. As of February 4, 2014, we also held 8 pending corresponding foreign patent applications that will permit us to pursue additional patents outside of the United States. The claims in these various patents and patent applications are directed to various aspects of our drug delivery devices and their components, methods of using our devices, drug containing aerosol compositions and methods of making and using such compositions.
ADASUVE (Staccato loxapine)
One of our issued U.S. patents covers compositions for delivery of a condensation aerosol comprising loxapine and covers the process for producing such condensation aerosol using theStaccatosystem technology. This patent expires in October 2021. An application for patent term extension has been applied for, which could extend the expiration for the patent to 2026. Counterparts to this patent are in force in a number of foreign jurisdictions, including Europe. We also have four other U.S. patents directed to condensation aerosol compositions for delivery of loxapine, kits containing devices for forming such compositions and methods of administering such compositions. In total, we reference 12 patents for ADASUVE in the Orange Book. The latest expiration date for the Orange Book listed patents is 2026.
AZ-002 (Staccato alprazolam)
One of our issued U.S. patents covers compositions for delivery of a condensation aerosol comprising alprazolam and covers the process for producing such condensation aerosol using theStaccatosystem technology. This patent will not expire until 2022. Counterparts to this patent are in force in a number of foreign jurisdictions, including Europe. We also have three other U.S. patents directed to condensation aerosol compositions for delivery of alprazolam, kits containing devices for forming such compositions, and methods of administering such compositions.
27
AZ-007 (Staccato zaleplon)
One of our issued U.S. patents covers compositions for delivery of a condensation aerosol comprising zaleplon and covers the process for producing such condensation aerosol using theStaccatosystem technology. This patent will not expire until 2022. Counterparts to this patent are in force in a number of foreign jurisdictions, including Europe. We also have three other U.S. patents directed to condensation aerosol compositions for delivery of zaleplon, kits containing devices for forming such compositions, and methods of administering such compositions.
AZ-003 (Staccato fentanyl)
One of our issued U.S. patents covers compositions for delivery of a condensation aerosol comprising fentanyl and covers the process for producing such condensation aerosol using theStaccatosystem technology. This patent will not expire until 2022. Counterparts to this patent are in force in a number of foreign jurisdictions, including Europe. We also have three other U.S. patents directed to condensation aerosol compositions for delivery of fentanyl, kits containing devices for forming such compositions, and methods of administering such compositions.
Staccato nicotine
Two of our U.S. issued patents cover the apparatus and methods for producing condensation aerosol. One of these patents will not expire until 2026. Two of our U.S. patent applications cover compositions for delivery of a condensation aerosol comprising nicotine and nicotine formulations. One of our U.S. patent applications covers a method of treating nicotine craving by administering a condensation aerosol comprising nicotine.
COMPETITION
The pharmaceutical and biotechnology industries are intensely competitive. Many pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations are actively engaged in research and development of products targeting the same markets as ADASUVE and our other product candidates. Many of these organizations have substantially greater financial, research, drug development, manufacturing and marketing resources than we have. Large pharmaceutical companies in particular have extensive experience in clinical testing, obtaining regulatory approvals for drugs, and commercial capabilities. Our ability to compete successfully will depend largely on our ability to:
| | • | | develop products that are superior to other products in the market; |
| | • | | attract and retain qualified scientific, product development, manufacturing, quality and commercial personnel; |
| | • | | obtain patent and/or other proprietary protection covering our future products and technologies; |
| | • | | obtain required regulatory approvals; |
| | • | | successfully collaborate with pharmaceutical and biotechnology companies in the development and commercialization of new products; and |
| | • | | successfully commercialize or enter into agreements that result in third parties successfully commercializing our approved products. |
We expect ADASUVE and any future products we develop to compete on the basis of, among other things, product efficacy, speed of onset and safety, time to market, price, extent of adverse side effects experienced and convenience of treatment procedures. One or more of our competitors may develop products based upon the principles underlying our proprietary technologies earlier than we do, obtain approvals for such products from the FDA, the EC or third country authorities more rapidly than we do or develop alternative products or therapies that are safer, more effective and/or more cost effective than ADASUVE or any future products developed by us. In addition, our ability to compete may be affected if insurers and other third-party payors encourage the use of generic or other products through other routes of administration. ADASUVE competes with various injectable
28
formulations of other antipsychotic and benzodiazepine drugs and oral, orally disintegrating tablet and liquid formulations of other antipsychotic drugs and benzodiazepine drugs. Only the injectable antipsychotics are approved for the treatment of agitation.
Any future products developed by us would compete with a number of alternative drugs and therapies, including the following:
| | • | | AZ-002 would compete with the oral tablet form of alprazolam and with other benzodiazepines in various formulations, including IV and rectal gel; |
| | • | | AZ-007 would compete with non-benzodiazepine GABA-A receptor agonists; |
| | • | | AZ-104 would compete with available anti-migraine treatments; |
| | • | | AZ-003 would compete with injectable and other forms of fentanyl and various generic oxycodone, hydrocodone and morphine products; and |
| | • | | Staccato Nicotine would compete with other treatments for smoking cessation. |
Many of these existing drugs have substantial current sales and long histories of effective and safe use. As patent protection expires for these drugs, we will also compete with their generic versions. In addition to currently marketed drugs and their generic versions, we believe there are a number of drug candidates in clinical trials that, if approved in the future, would compete with ADASUVE or any future product candidates we may develop. In addition, after patent protection and non-patent exclusivity expire for our products, we may face direct generic competition, or competition from sponsors relying to some extent on our approval by filing NDAs under section 505(b)(2) of the FDCA.
CORPORATE INFORMATION
We were incorporated in the state of Delaware on December 19, 2000 as FaxMed, Inc. In June 2001, we changed our name to Alexza Corporation and in December 2001 we became Alexza Molecular Delivery Corporation. In July 2005, we changed our name to Alexza Pharmaceuticals, Inc.
Employees
As of February 13, 2014, we had 90 full time employees, 13 of whom held Ph.D. or M.D. degrees, 15 of whom were engaged in full time research and development activities and 51 of whom were engaged in commercial manufacturing, supply chain and quality functions and we had no part-time employees. None of our employees are represented by a labor union, and we consider our employee relations to be good.
Available Information
Our website address is www.alexza.com; however, information found on, or that can be accessed through, our website is not incorporated by reference into this Annual Report. We file electronically with the Securities and Exchange Commission, or SEC, our Annual Report, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available free of charge on or through our website copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov. You may also read and copy any of our materials filed with the SEC at the SEC’s Public References Room at 100 F Street, NW, Washington, DC 20549. Information regarding the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330.
29
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this Annual Report, before deciding whether to invest in shares of our common stock. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations. The occurrence of any of the following risks could harm our business, financial condition or results of operations. In such case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
Our near-term prospects are dependent on ADASUVE. If we or our collaborators are unable to successfully commercialize ADASUVE for the acute treatment of agitation in adults with schizophrenia or bipolar disease, our ability to generate significant revenue or achieve profitability will be adversely affected.
ADASUVE is our only product approved for marketing in the United States or the EU, and our ability to generate revenue in the near term is entirely dependent upon sales of ADASUVE. We do not have any product approved for marketing outside of the United States or the EU. We or our collaborators may not be able to successfully commercialize ADASUVE for a number of reasons, including:
| | • | | we or our collaborators may not be able to establish or demonstrate in the medical community the safety and efficacy of ADASUVE and any potential advantages over existing therapeutics and products currently in clinical development; |
| | • | | doctors may be hesitant to prescribe ADASUVE until results from our post-approval studies are available or other long term data regarding efficacy and safety become available; |
| | • | | results from our post-approval studies may fail to verify the clinical benefit of ADASUVE for the treatment of agitation in patients with bipolar disorder and schizophrenia or may reveal unforeseen safety issues; |
| | • | | our limited experience in marketing, selling and distributing ADASUVE; |
| | • | | reimbursement and coverage policies of government and private payers such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators; |
| | • | | the relative price of ADASUVE as compared to alternative treatment options; |
| | • | | the reliability of our estimates, including the frequency of agitation in many patients with bipolar disorder and schizophrenia; |
| | • | | we or our collaborators may not have adequate financial or other resources to successfully commercialize ADASUVE; |
| | • | | we may be unable to secure an adequate replacement manufacturer for sufficient quantities of the chemical heat packages in ADASUVE by October 2016 or the costs for such manufacturer to produce the chemical heat packages may be higher than the cost from Autoliv; and |
| | • | | we may not be able to manufacture ADASUVE in commercial quantities or at acceptable costs. |
If we are unable to successfully commercialize ADASUVE for the treatment of agitation in adults with schizophrenia or bipolar disorder, our ability to generate revenue from royalties and product sales and achieve profitability will be adversely affected and our stock price would likely decline.
We have a history of net losses. We expect to continue to incur substantial and increasing net losses for the foreseeable future, and we may never achieve or maintain profitability.
We are not profitable and have incurred significant net losses in each year since our inception, including net losses of $39.7 million, $28.0 million, and $40.5 million for the years ended December 31, 2013, 2012, and 2011,
30
respectively. As of December 31, 2013, we had an accumulated deficit of $374.2 million and a stockholders’ deficit of $24.0 million. We expect to continue to incur substantial net losses and negative operating cash flow through at least 2016. These losses and negative operating cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital.
Because of the numerous risks and uncertainties associated with the commercialization of ADASUVE by Teva and Ferrer, our ability to manufacture commercial quantities of ADASUVE and conduct pharmaceutical product development, we are unable to predict accurately the timing or amount of future revenues or expenses, or when, or if, we will be able to achieve or maintain profitability. To date we have not generated any significant product or royalty revenue. We have financed our operations primarily through the sale of equity securities, equipment financing, debt financing, collaboration and licensing agreements, and government grants. The size of our future net losses will depend, in part, on the rate of growth or contraction of our expenses and the level and rate of growth, if any, of our revenues. Revenues from strategic collaborations are uncertain because we may not enter into any additional strategic collaborations. If we or our collaborators are unable to successfully commercialize ADASUVE or one or more of our product candidates or if sales revenue from ADASUVE or any product candidate that receives marketing approval is insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability.
Our operating results are unpredictable and may fluctuate. If our operating results are below the expectations of securities analysts or investors, the trading price of our stock could decline.
Our operating results are difficult to predict and will likely fluctuate from quarter to quarter and year to year. Due to the recent approval of ADASUVE for the treatment of agitation associated with schizophrenia and bipolar disorder in adults in the United States and the EU and the lack of historical sales data, ADASUVE sales will be difficult to predict from period to period. We believe that our quarterly and annual results of operations may be negatively affected by a variety of factors, including:
| | • | | a failure to achieve a sufficient level of demand by patients and healthcare providers for ADASUVE; |
| | • | | the timing and level of investment in our or our collaborators’ sales and marketing efforts to support ADASUVE sales; |
| | • | | the timing and level of investment in our or our collaborators’ research and development activities involving ADASUVE; and |
| | • | | expenditures we may incur to acquire or develop additional products. |
In addition, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award, and recognize the cost as an expense over the employee’s requisite service period. As the variables that we use as a basis for valuing these awards change over time, including our underlying stock price, the magnitude of the expense that we must recognize may vary significantly. Any such variance from one period to the next could cause a significant fluctuation in our operating results.
For these reasons, it is difficult for us to accurately forecast future profits or losses. As a result, it is possible that in some quarters our operating results could be below the expectations of securities analysts or investors, which could cause the trading price of our common stock to decline, perhaps substantially.
We will need substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
We will need to raise additional capital to fund our operations, to develop our product candidates and to develop and expand our commercial manufacturing capabilities. Our future capital requirements will be substantial and will depend on many factors including:
| | • | | Teva’s success in commercializing ADASUVE in the United States; |
| | • | | Teva’s executing the ADASUVE REMS program to the satisfaction of the FDA; |
| | • | | Ferrer’s success in commercializing ADASUVE in the Ferrer Territories; |
31
| | • | | the terms and success of any future licensing arrangement that we may enter into for the commercial rights for ADASUVE outside the United States and the Ferrer Territories; |
| | • | | the development costs for our other product candidates; |
| | • | | the cost and timing of complying with our post-approval commitments; |
| | • | | the cost and timing of complying with the process for renewal of marketing authorization in the EU; |
| | • | | the scope, rate of progress, results and costs of our preclinical studies, clinical trials and other research and development activities; |
| | • | | the scope, rate of progress, results and costs of our commercial manufacturing development and commercial manufacturing activities; |
| | • | | payments received under our collaborations with Ferrer and Teva and any future strategic collaborations; |
| | • | | the continuation of the Ferrer and Teva collaborations under their agreed terms; |
| | • | | the filing, prosecution and enforcement of patent claims; and |
| | • | | the costs associated with commercializing our other product candidates, if they receive regulatory approval. |
We believe that, based on our cash, cash equivalents and marketable securities balance at December 31, 2013, remaining proceeds available under the Teva Note, estimated product revenues and milestone payments associated with the sale of ADASUVE, the proceeds from our March 2014 royalty securitization financing and our current expected cash usage, we have sufficient capital resources to meet our anticipated cash needs for at least the next twelve months. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate, or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could exhaust our available financial resources sooner than we currently expect. The key assumptions underlying these estimates include:
| | • | | continuation of our Teva and Ferrer collaborations; |
| | • | | no unexpected costs related to the development of our commercial manufacturing capability; and |
| | • | | no unbudgeted growth in the number of our employees during this period. |
We may never be able to generate a sufficient amount of product or royalty revenue to cover our expenses. We did not generate any product revenues until the third quarter of 2013 and have not generated any royalty revenues through 2013. In addition, any royalty and milestone payments payable under the Teva Agreement will first be applied to repaying principal and interest on the $45.0 million of non-recourse notes that our wholly-owned subsidiary issued in our March 2014 royalty securitization financing before we receive as income any royalty or milestone payments under the Teva Agreement. Until we generate sufficient revenues to cover expenses, we expect to finance our future cash needs through public or private equity offerings, debt financings, strategic collaborations or licensing arrangements. Any financing transaction may contain unfavorable terms. For example, the terms of certain warrants we have issued in previous financings could require us to pay warrant holders a significant portion of the proceeds in a change of control transaction, potentially materially reducing the proceeds available to holders of our common stock. If we raise additional funds by issuing equity securities our stockholders’ equity will be diluted and debt financing, if available, may involve restrictive covenants. If we raise additional funds through strategic collaborations, we may be required to relinquish rights to ADASUVE, our product candidates or our technologies, or to grant licenses on terms that are not favorable to us. Complying with the terms of the foregoing rights and restrictions may make it more difficult to complete certain types of transactions and result in delays to our fundraising efforts.
We do not have sales and marketing capabilities, and consequently must rely on commercial collaborations to sell our products, and we and our collaborators may be unable to generate significant product revenue.
In December 2012, the FDA granted marketing approval for ADASUVE in the United States for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. The approval of ADASUVE
32
is our first regulatory approval. We do not have a sales and marketing organization and as a company, we do not have significant experience in the sales and distribution of pharmaceutical products.
We have exclusively licensed the ADASUVE U.S. commercialization rights to Teva, which we believe will have a significant impact on the ultimate success of ADASUVE in the United States. Teva announced the U.S. launch of ADASUVE in March 2014. If Teva is unable to commercialize ADASUVE successfully in the United States or Teva is unable to fulfill the post-marketing obligations that were required by the FDA as part of the ADASUVE NDA approval, our revenue will suffer and our stock price may decline. Teva’s commercialization efforts could also have an effect on investors’ perception of potential sales of ADASUVE outside the United States, which could also cause a decline in our stock price and may make it more difficult for us to enter into additional strategic collaborations.
There are risks involved with utilizing Teva to sell our product. By licensing the U.S. commercialization rights to Teva, we may generate fewer revenues from the royalties and milestone payments under the Teva Agreement than if we commercialized ADASUVE on our own. Additionally, Teva may not fulfill its obligations or carry out selling and marketing activities as diligently as we would like. We could also become involved in disputes with Teva, which could lead to delays in or termination of commercialization programs and time-consuming and expensive litigation or arbitration. If Teva terminates or breaches its agreement, or otherwise fails to complete its obligations in a timely manner, the chances of successfully selling or marketing ADASUVE would be materially and adversely affected. After May 7, 2014, Teva has the right to terminate the Teva Agreement with or without cause, upon 120 days notice. If Teva exercises this right, our business and operations and stock price may be negatively affected.
In February 2013, the EC granted a marketing authorization for ADASUVE, as ADASUVE (Staccato loxapine) 4.5 mg or 9.1 mg, inhalation powder, pre-dispensed. In October 2011, we entered into a commercial collaboration with Ferrer pursuant to the Ferrer Agreement, to commercialize ADASUVE in the Ferrer Territories. In July 2013, Ferrer launched ADASUVE in the EU, with the first commercial sale in Germany, and currently sells ADASUVE in Germany, Austria, Spain and Romania. We expect Ferrer to launch ADASUVE in additional EU Member States in 2014 and 2015. If Ferrer is unable to commercialize ADASUVE successfully in the various Ferrer Territories or we are unable to fulfill the post-marketing authorization commitments that were required as part of the marketing authorization granted for ADASUVE in the EU, our revenue will suffer and our stock price may decline.
We also intend to seek international distribution collaborators in addition to Ferrer for ADASUVE and our product candidates. If we are unable to enter into an international distribution collaboration, we will be unable to generate revenues from countries outside the United States and the Ferrer Territories.
If we enter into additional strategic collaborations, we may be required to relinquish important rights to and control over the development of ADASUVE or our product candidates or otherwise be subject to terms unfavorable to us.
Our relationships with Teva and Ferrer are, and any other strategic collaborations with pharmaceutical or biotechnology companies we may establish will be, subject to a number of risks including:
| | • | | business combinations or significant changes in a strategic collaborator’s business strategy may adversely affect a strategic collaborator’s willingness or ability to complete its obligations under any arrangement; |
| | • | | we may not be able to control the amount and timing of resources that our strategic collaborators devote to the development or commercialization of ADASUVE or our product candidates; |
| | • | | strategic collaborators may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new version of a product candidate for clinical testing; |
| | • | | strategic collaborators may not pursue further development and commercialization of products resulting from the strategic collaboration arrangement or may elect to discontinue research and development programs; |
33
| | • | | strategic collaborators may not commit adequate resources to the marketing and distribution of any future products, limiting our potential revenues from these products; |
| | • | | disputes may arise between us and our strategic collaborators that result in the delay or termination of the research, development or commercialization of ADASUVE or our product candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources; |
| | • | | strategic collaborators may experience financial difficulties; |
| | • | | strategic collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| | • | | strategic collaborators could independently move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors; and |
| | • | | strategic collaborators, including Teva or Ferrer, could terminate the arrangement or allow it to expire, which would delay and may increase the cost of developing our product candidates or commercializing ADASUVE. |
The REMS program for ADASUVE imposes, and any REMS on any other approved products may impose, regulatory burdens on the distribution and sales of ADASUVE or any other approved products and also on healthcare providers that may make the products commercially unattractive or impractical.
As a condition of FDA approval, Teva is required to have a REMS program for ADASUVE, and we may be required to implement a REMS program for any other product candidates we may develop. A REMS may include various elements, such as distribution of a medication guide or a patient package insert; implementation of a communication plan to educate healthcare providers of the drug’s risks; imposition of limitations on who may prescribe or dispense the drug, including training and certification requirements; or other measures that the FDA deems necessary to assure the safe use of the drug. The FDA has a wide degree of discretion in deciding which elements are necessary for the safe use of a product, and it may impose elements that significantly burden our ability to commercialize the product, or that burden healthcare providers to the extent that use of the product is severely curtailed.
For ADASUVE, the REMS program contains measures to ensure that the product is administered only in healthcare facilities enrolled in the ADASUVE REMS program that have immediate on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation). The REMS program may not allow commercialization and use of ADASUVE in a commercially feasible manner. In the future, the FDA could impose additional REMS elements, such as if the REMS proves inadequate in managing the risk of bronchospasm associated with ADASUVE or if new safety risks emerge, and such additional elements could substantially burden or even eliminate our ability to commercialize ADASUVE in a feasible manner.
If we or our collaborators are unable to successfully complete the ADASUVE post-marketing studies required by the FDA and EC, or if data generated from the post-marketing studies indicate safety concerns, our sales could be diminished and our ability to generate a profit could be negatively affected.
As a condition of U.S. ADASUVE approval, there are several required post-marketing studies, including a 10,000 patient observational clinical trial that is designed to gather patient safety data based on the “real-world” use of ADASUVE in the hospital setting and a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients. The data derived from any post-approval study or trial could result in additional restrictions on the commercialization of ADASUVE through changes to the approved ADASUVE label, additional goals or elements in the REMS program, the imposition of additional post-approval studies or trials, or even the withdrawal of the approval of ADASUVE. Our business, operations and stock price may be negatively affected if any of these or similar events occur.
As a condition of the ADASUVE marketing authorization in the EU, we are responsible for the conduct and funding of post-authorization studies, including (i) a benzodiazepine interaction study, which has been
34
completed, (ii) a controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, with two doses of ADASUVE, which has been completed, (iii) a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients, (iv) an observational clinical trial, and (v) a drug utilization clinical trial. Results of the benzodiazepine interaction study and the thorough QTc study have been submitted to the EMA.
If we or our collaborators are unable to fulfill the FDA or EC post-approval obligations, or do not fulfill these obligations within an appropriate time, or if the EMA determines from the results of our completed benzodiazepine interaction study or our completed thorough QTc study that ADASUVE poses or may pose actual or possible safety risks to some patients, the NDA could be suspended, withdrawn, or limited, and the current marketing authorization in the EU could be varied, suspended or withdrawn and our business, operations and stock price may be negatively affected.
If we do not produce our commercial devices cost effectively, we will never be profitable.
ADASUVE and ourStaccatosystem-based product candidates contain electronic and other components in addition to the API. The cost to produce ADASUVE and our product candidates, and any additional approved products, will likely be higher per dose than the cost to produce intravenous or oral tablet products. This higher cost of goods may prevent us or our collaborators from ever selling any products at a profit. The development and production of our technology entail a number of technical challenges, including achieving adequate dependability in our production, that may be expensive or time consuming to solve. Any delay in or failure to develop and manufacture any future products in a cost effective way could prevent us from generating any meaningful revenues and prevent us from becoming profitable.
In October 2011, we committed to sell ADASUVE to Ferrer for a fixed transfer price, which is below our current production costs, and in May 2013, we committed to supply ADASUVE to Teva at a price based on costs of commercial production, which transfer price will convert to a fixed price upon achievement of costs equal to a specified per-unit price. Our future manufacturing costs per unit will be dependent on future demand of ADASUVE. If we and our collaborators do not generate sufficient demand, our manufacturing costs will exceed the fixed transfer price and will result in losses. In October 2013, Autoliv notified us that they were terminating, effective October 2016, the Manufacture Agreement. Prior to October 2016, Autoliv and we remain fully obligated to perform pursuant to the terms of both the Manufacture Agreement and the Second Note. However, Autoliv may not continue to perform its obligations in the same manner during the termination period. If we are unable to secure an adequate replacement manufacturer for sufficient quantities of the chemical heat packages in ADASUVE by October 2016 or if the costs for such manufacturer to produce the chemical heat packages are higher than the cost from Autoliv, it will significantly impact our ability to produce our commercial devices or to produce them at quantities or at a cost to allow us to become profitable.
If our products do not receive adequate coverage and reimbursement from third-party payors, our sales could be diminished and our ability to sell our products profitably could be negatively affected.
Sales of ADASUVE will be dependent on the availability and extent of coverage and reimbursement from third-party payers, including government healthcare programs and private insurance plans. We will rely in large part on the reimbursement and coverage by federal and state sponsored government programs, such as Medicare and Medicaid in the United States and equivalent programs in other countries. Medicare, the dominant federal health insurance program for the elderly in the United States, may limit coverage of ADASUVE for beneficiaries in accordance with the boxed warning against use of the drug in elderly patients with dementia-related psychosis. Governments and private payors may regulate prices, reimbursement levels and/or access to ADASUVE and any other products we may market to control costs or to affect levels of use of our products.
Third-party payors are increasingly challenging the prices charged for medical products and services and examining their cost effectiveness, in addition to their safety and efficacy. We or our collaborators may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost effectiveness of ADASUVE and any future products. Even with studies, ADASUVE and our product candidates may be considered less safe, less effective or less cost effective than existing products, and some third-party payors therefore may not provide
35
coverage and reimbursement for our product candidates, in whole or in part. We cannot predict actions third party payors may take, or whether they will limit the coverage and level of reimbursement for our products or refuse to provide any coverage at all.
Although Teva has been granted a reimbursement code for ADASUVE by the Centers for Medicare & Medicaid Services, or CMS, we cannot predict the availability or level of coverage and reimbursement for ADASUVE or our product candidates. A reduction in coverage and/or reimbursement for our products could have a material adverse effect on our product sales and results of operations.
The availability and amount of reimbursement for ADASUVE and our product candidates and the manner in which government and private payors may reimburse for our potential products is uncertain.
Many of the patients in the United States who seek treatment with ADASUVE or any other of our products that are approved for marketing will be eligible for Medicare benefits. Other patients may be covered by private health plans. The Medicare program is administered by CMS, and coverage and reimbursement for products and services under Medicare are determined pursuant to statute, regulations promulgated by CMS, and CMS’s subregulatory coverage and reimbursement determinations. CMS’s regulations and interpretive determinations are subject to change, as are the procedures and criteria by which CMS makes coverage and reimbursement determinations and the reimbursement amounts established by statute, particularly because of budgetary pressures facing the Medicare program. For example, we anticipate that ADASUVE will be used only in the hospital inpatient and hospital outpatient settings. In the hospital inpatient setting, Medicare does not provide separate reimbursement for drugs but pays for them as part of the payment for the hospital stay. In the hospital outpatient setting, the statute establishes the payment rate for new drugs and biologicals administered incident to a physician’s service that are granted “pass-through status” at the rate applicable in physicians’ offices (i.e., ASP plus six percent) for two to three years after FDA approval. For drugs and biologicals that do not have pass-through status, CMS establishes the payment rates by regulation. For 2014, these drugs are reimbursed at ASP plus six percent if they have an average cost per day exceeding $90; drugs with an average cost per day of less than $90 are not separately reimbursed. In future years, CMS could change both the payment rate and the average cost threshold, and these changes could adversely affect payment for ADASUVE. In addition, the President has proposed and Congress has considered amending the statute to reduce Medicare’s payment rates for drugs and biologicals, and if such legislation is enacted, it could adversely affect payment for ADASUVE. Moreover, ADASUVE is different from many drugs covered by Medicare Part B because it is administered by a healthcare professional through a disposable inhaler.
Effective April 1, 2013, Medicare payments for all items and services, including drugs and biologicals, were reduced by up to 2% under the sequestration (i.e., automatic spending reductions) required by the Budget Control Act of 2011, Pub. L. No. 112-25, or BCA, as amended by the American Taxpayer Relief Act of 2012, Pub. L. 112-240, or ATRA. The BCA requires sequestration for most federal programs, excluding Medicaid, Social Security, and certain other programs, because Congress failed to enact legislation by January 15, 2012, to reduce federal deficits by $1.2 trillion over ten years. The BCA caps the cuts to Medicare payments or items and services at 2%, and requires the cuts to be implemented on the first day of the first month following the issuance of a sequestration order. The ATRA delayed implementation of sequestration from January 2, 2013, to March 1, 2013, and as a result, the Medicare cuts took effect April 1, 2013, and will remain in effect unless Congress enacts legislation to cancel the cuts. The cuts were originally scheduled to occur through 2021, but under the Bipartisan Budget Act of 2013, these cuts were extended through 2023. These cuts could adversely impact payment for ADASUVE and related procedures.
In March 2014, Teva announced the U.S. launch of ADASUVE. We expect ADASUVE to experience pricing pressures in the United States due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals. We cannot be sure that reimbursement amounts, or the lack of reimbursement, will not reduce the demand for, or the price of, ADASUVE or any future products. If reimbursement is not available or is available only to limited levels, we or any collaborator may not be able to effectively commercialize ADASUVE or any future products, In addition, if we or any collaborator fail to successfully secure and maintain reimbursement coverage for ADASUVE or any future products or are significantly delayed in doing so, we or any collaborator will have difficulty achieving market acceptance of our products and our business will be harmed.
36
Payors also are increasingly considering new metrics as the basis for reimbursement rates, such as ASP, AMP and Actual Acquisition Cost. The existing data for reimbursement based on these metrics is relatively limited, although certain states have begun to survey acquisition cost data for the purpose of setting Medicaid reimbursement rates. CMS has made draft National Average Drug Acquisition Cost, or NADAC, and draft National Average Retail Price, or NARP, data publicly available on at least a monthly basis. In July 2013, CMS suspended the publication of draft NARP data, pending funding decisions. In November 2013, CMS moved to publishing final rather than draft NADAC data and has since made updated NADAC data publicly available on a weekly basis. Therefore, it may be difficult to project the impact of these evolving reimbursement mechanics on the willingness of payors to cover ADASUVE or any future products. As discussed below, once we or any collaborator begin to participate in government pricing programs, recent legislative changes to the 340B drug pricing program, and the Medicaid Drug Rebate program also could impact our revenues. We anticipate that a significant portion of our revenue from sales of ADASUVE will be obtained through government payors, including Medicaid, and any failure to qualify for reimbursement for ADASUVE under those programs would have a material negative effect on revenues from sales of ADASUVE.
The EU Member States are free to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices and/or reimbursement levels of medicinal products for human use. An EU Member State may approve a specific price or level of reimbursement for the medicinal product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the medicinal product on the market, including volume-based arrangements and reference pricing mechanisms. We anticipate that pricing and reimbursement decisions concerning ADASUVE in the EU will have a significant impact on the sales of the product in the EU. Failure to obtain pricing and reimbursement for ADASUVE at an appropriate level in any of the EU Member States would, in part due to EU parallel trade rules, have a material adverse effect on revenues from sales of ADASUVE.
Healthcare law and policy changes, including those based on recently enacted legislation, may impact our business in ways that we cannot currently predict and these changes could have a material adverse effect on our business and financial condition.
Healthcare costs have risen significantly over the past decade. In March 2010, the Healthcare Reform Act, or PPACA, was adopted in the United States. The Healthcare Reform Act substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Healthcare Reform Act contains a number of provisions that are expected to impact our business and operations, in some cases in ways we cannot currently predict. Changes that may affect our business include those governing enrollment in federal and private healthcare programs, new Medicare reimbursement methods and rates, increased rebates and taxes on pharmaceutical products, expansion of the 340B program, and revised fraud and abuse and enforcement requirements. These changes will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
We anticipate that with Teva’s commercialization of ADASUVE in the United States or with other potential product candidates, some of our revenue and the revenue from our collaborators may be derived from U.S. government healthcare programs, including Medicare. We expect that the Healthcare Reform Act and other healthcare reform measures that may be adopted in the future could have an adverse effect on our industry generally and the ability to successfully commercialize ADASUVE or our product candidates or could limit or eliminate our spending on development projects.
The Healthcare Reform Act made significant changes to the Medicaid Drug Rebate program, in which we expect to participate. Effective March 23, 2010, rebate liability expanded from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well. With regard to the amount of the rebates owed, the Healthcare Reform Act increased the minimum Medicaid rebate from 15.1% to 23.1% of the average manufacturer price for most innovator products and from 11% to 13% for non-innovator products; changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs; and capped the total rebate amount for innovator drugs at 100% of the average manufacturer price. We expect that the increased minimum rebate of 23.1% will apply to ADASUVE. In addition, the Healthcare Reform
37
Act and subsequent legislation changed the definition of average manufacturer price. In 2012, the Centers for Medicare and Medicare Services, or CMS, the federal agency that administers the Medicare and Medicaid Drug Rebate program, issued proposed regulations to implement the changes to the Medicaid Drug Rebate program under the Healthcare Reform Act but has not yet issued final regulations. CMS is currently expected to release the final regulations in 2014. Finally, the Healthcare Reform Act requires pharmaceutical manufacturers of branded prescription drugs to pay a branded prescription drug fee to the federal government beginning in 2011. Each individual pharmaceutical manufacturer pays a prorated share of the branded prescription drug fee of $3.0 billion in 2014 (and set to increase in ensuing years), based on the dollar value of its branded prescription drug sales to certain federal programs identified in the law. Additional provisions of the Healthcare Reform Act, some of which became effective in 2011, may negatively affect our future revenues.
The Healthcare Reform Act also expanded the Public Health Service’s 340B drug pricing discount program, which we expect to participate in. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. The Healthcare Reform Act expanded the 340B program to include additional types of covered entities: certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals, each as defined by the Healthcare Reform Act. The Healthcare Reform Act exempts “orphan drugs” — those designated under section 526 of the FDCA — from the ceiling price requirements for these newly-eligible entities. We believe our product candidate in active development, AZ-002 (Staccato alprazolam), could qualify for orphan drug status. The Health Resources and Services Administration, or HRSA, which administers the 340B program, issued a final regulation to implement the orphan drug exception in July 2013. The final regulation interprets the orphan drug exception narrowly. It exempts orphan drugs from the ceiling price requirements for the newly-eligible entities only when the orphan drug is used for its orphan indication. The newly-eligible entities are entitled to purchase orphan drugs at the ceiling price when the orphan drug is not used for its orphan indication. The final regulation, which became effective October 1, 2013, is subject to a pending lawsuit that seeks to block its implementation. The narrow scope of the orphan drug exception in HRSA’s final regulation will increase the complexity of compliance, will make compliance more time-consuming, and could negatively impact our results of operations if we successfully commercialize AZ-002 (Staccato alprazolam) and AZ-002 qualifies for orphan drug status. We have not applied for, andAZ-002 may not be granted, orphan drug status.
The Healthcare Reform Act also obligates the Secretary of the HHS to create regulations and processes to improve the integrity of the 340B program and to update the agreement that manufacturers must sign to participate in the 340B program to obligate a manufacturer to offer the 340B price to covered entities if the manufacturer makes the drug available to any other purchaser at any price and to report to the government the ceiling prices for its drugs. HRSA is expected to issue a comprehensive proposed regulation in 2014 that will address many aspects of the 340B program. When that regulation is finalized, it could affect our obligations under the 340B program in ways we cannot anticipate. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
Many of the Healthcare Reform Act’s most significant reforms take effect in 2014 and thereafter, and the details will be shaped significantly as the various provisions become active, especially given the political nature of the law. In 2012, the Supreme Court of the United States heard challenges to the constitutionality of the individual mandate and the viability of certain provisions of the Healthcare Reform Act. The Supreme Court’s decision upheld most of the Healthcare Reform Act and determined that requiring individuals to maintain “minimum essential” health insurance coverage or pay a penalty to the Internal Revenue Service was within Congress’s constitutional taxing authority. However, the Supreme Court struck down a provision in the Healthcare Reform Act that penalized states that choose not to expand their Medicaid programs through an increase in the Medicaid eligibility income limit from a state’s current eligibility levels to 133% of the federal poverty limit. As a result of the Supreme Court’s ruling, it is unclear whether states will expand their Medicaid programs by raising the income limit to 133% of the federal poverty level and whether there will be more uninsured patients in 2014 than anticipated when Congress passed the Healthcare Reform Act. For each state that does not choose to expand its Medicaid program, there will be fewer insured patients overall. The reduction in the number of insured patients could impact the sales, business and financial condition.
38
While the constitutionality of key provisions of the Healthcare Reform Act was upheld by the Supreme Court, legislative changes to it remain possible. We expect that the Healthcare Reform Act, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and on our ability to successfully commercialize our product candidates or could limit or eliminate our future spending on development projects.
In addition to the Healthcare Reform Act, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to keep these costs down while expanding individual healthcare benefits. Certain of these changes could impose limitations on the prices we will be able to charge for ADASUVE or any other product candidates that are approved or the amounts of reimbursement available for these products from governmental agencies or third-party payors, or may increase the tax obligations on life sciences companies such as ours. While it is too early to predict specifically what effect the Health Reform Act and its implementation or any future legislation or policies will have on our business, we believe that healthcare reform may have an adverse effect on our business and financial condition.
If we or any collaborator fail to comply with reporting and payment obligations under the Medicaid Drug Rebate program or other governmental pricing programs, including programs developed by countries outside the United States, after we or any collaborator begin to participate in such programs, we or any collaborator could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We expect to participate, or any collaborator to participate, in the Medicaid Drug Rebate program, established by the Omnibus Budget Reconciliation Act of 1990 and amended by the Veterans Health Care Act of 1992 as well as subsequent legislation. We also expect to participate, or any collaborator to participate, in and have certain price reporting obligations to several state Medicaid supplemental rebate programs, and we anticipate that we will have obligations to report average sales price, or ASP, for the Medicare program for future product candidates. Under the Medicaid Drug Rebate program, we will be required to pay a rebate to each state Medicaid program for our covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having federal funds being made available to the states for our drugs under Medicaid and Medicare Part B. Those rebates will be based on pricing data that we will report on a monthly and quarterly basis to CMS, the federal agency that administers the Medicaid Drug Rebate program. These data will include the average manufacturer price, or AMP, and, in the case of innovator products, the best price, or BP, for each drug. The rebate liability resulting from this reporting will negatively impact our financial results.
The PPACA made significant changes to the Medicaid Drug Rebate program. Effective March 23, 2010, rebate liability expanded from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well. With regard to the amount of the rebates owed, the PPACA increased the minimum Medicaid rebate from 15.1% to 23.1% of the average manufacturer price for most innovator products and from 11% to 13% for non-innovator products; changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs; and capped the total rebate amount for innovator drugs at 100% of the average manufacturer price. We expect that the increased minimum rebate of 23.1% will apply to ADASUVE. In addition, the PPACA and subsequent legislation changed the definition of AMP. Finally, the PPACA requires pharmaceutical manufacturers of branded prescription drugs to pay a new branded prescription drug fee to the federal government beginning in 2011. Each individual pharmaceutical manufacturer will pay a prorated share of the branded prescription drug fee of $3.0 billion in 2014 (and set to increase in ensuing years) based on the dollar value of its branded prescription drug sales to certain federal programs identified in the law. Additional provisions of the Healthcare Reform Act, some of which became effective in 2011, may negatively affect our future revenues.
On July 15, 2013, CMS issued its Final Rule addressing prescription drug coverage for the Medicaid expansion population under the PPACA. The Final Rule dramatically restricts mandatory coverage of prescription drugs for the expansion Medicaid enrollees by essentially eliminating the requirement that states must cover each covered outpatient drug subject to a national rebate agreement. Although drugs provided to the
39
new Medicaid expansion population still are subject to manufacturer rebates, state Medicaid programs are no longer required to cover all drugs subject to the national rebate agreement. Instead, states are required to cover a minimum of drugs consistent with the applicable “benchmark” plan in the state coverage for the Medicaid expansion population.
In the future, Congress could enact legislation that further increases Medicaid drug rebates or other costs and charges associated with participating in the Medicaid Drug Rebate program. The issuance of regulations and coverage expansion by various governmental agencies relating to the Medicaid Drug Rebate program will increase our costs and the complexity of compliance, will be time-consuming, and could have a material adverse effect on our results of operations.
Federal law requires that any company that participates in the Medicaid Drug Rebate Program also participate in the Public Health Service’s 340B drug pricing discount program in order for federal funds to be available for the manufacturer’s drugs under Medicaid and Medicare Part B. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients. The 340B ceiling price is calculated using a statutory formula, which is based on the average manufacturer price and rebate amount for the covered outpatient drug as calculated under the Medicaid rebate program. Changes to the definition of average manufacturer price and the Medicaid rebate amount under PPACA and CMS’s issuance of final regulations implementing those changes also could affect our 340B ceiling price calculations and negatively impact our results of operations once we or any collaborator begin to participate in the 340B program.
Compliance with the regulations associated with the 340B program will increase our costs and the complexity of compliance, will be time-consuming, and could have a material adverse effect on our results of operations once we or any collaborator begin to participate in the 340B program.
As described above, the Healthcare Reform Act expanded the 340B program to include additional types of covered entities but exempts “orphan drugs” — those designated under section 526 of the FDCA — from the ceiling price requirements for these newly-eligible entities. We believe our product candidate in active development, AZ-002 (Staccato alprazolam), could qualify for orphan drug status. HRSA issued a final regulation to implement the orphan drug exception in July 2013. The final regulation interprets the orphan drug exception narrowly. It exempts orphan drugs from the ceiling price requirements for the newly-eligible entities only when the orphan drug is used for its orphan indication. The newly-eligible entities are entitled to purchase orphan drugs at the ceiling price when the orphan drug is not used for its orphan indication. The final regulation, which became effective October 1, 2013, is subject to a pending lawsuit that seeks to block its implementation. The narrow scope of the orphan drug exception in HRSA’s final regulation will increase the complexity of compliance, will make compliance more time-consuming, and could negatively impact our results of operations if we successfully commercialize AZ-002 (Staccato alprazolam) and AZ-002 qualifies for orphan drug status.
The Healthcare Reform Act also obligates the Secretary of the HHS to create regulations and processes to improve the integrity of the 340B program and to update the agreement that manufacturers must sign to participate in the 340B program to obligate a manufacturer to offer the 340B price to covered entities if the manufacturer makes the drug available to any other purchaser at any price and to report to the government the ceiling prices for its drugs. HRSA is expected to issue a comprehensive proposed regulation in 2014 that will address many aspects of the 340B program. When that regulation is finalized, it could affect our obligations under the 340B program in ways we cannot anticipate. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
Federal law also requires that a company that participates in the Medicaid Drug Rebate Program report average sales price, or ASP, information to CMS for certain categories of drugs that are paid under Part B of the Medicare program. We anticipate that ADASUVE will fall into that category. Manufacturers calculate ASP based on a statutorily defined formula and interpretations of the statute by CMS as to what should or should not be considered in computing ASP. An ASP for each National Drug Code for a product that is subject to the ASP
40
reporting requirement must be submitted to CMS no later than 30 days after the end of each calendar quarter. CMS uses these submissions to determine payment rates for drugs under Medicare Part B. Once we or any collaborator begin to participate in the Medicare program, changes affecting the calculation of ASP could affect the ASP calculations for our products and the resulting Medicare payment rate, and could negatively impact our results of operations once we begin to participate in the Medicare program.
Pricing and rebate calculations vary among products and programs. The calculations are complex and are often subject to interpretation by governmental or regulatory agencies and the courts. Once we or any collaborator begin to participate in the Medicaid program, the Medicaid rebate amount will be computed each quarter based on our submission to the CMS of our current AMP and BP for the quarter. If we become aware that our reporting for prior quarters was incorrect, or has changed as a result of recalculation of the pricing data, we or any collaborator will be obligated to resubmit the corrected data for a period not to exceed 12 quarters from the quarter in which the data originally were due. Such restatements and recalculations would serve to increase our costs for complying with the laws and regulations governing the Medicaid rebate program. Once we begin to participate in the Medicaid program, any corrections to our rebate calculations could result in an overage or underage in our rebate liability for past quarters, depending on the nature of the correction. Price recalculations also may affect the price that we or any collaborator will be required to charge certain safety-net providers under the Public Health Service 340B drug discount program.
Once we or any collaborator begin to participate in government pricing programs, we or any collaborator will be liable for errors associated with our submission of pricing data. In addition to retroactive rebates and the potential for 340B program refunds, if we or any collaborator are found to have knowingly submitted false average manufacturer price, average sales price, or best price information to the government, we or any collaborator may be liable for civil monetary penalties in the amount of $100,000 per item of false information. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, the statute provides for civil monetary penalties of up to $10,000 for each misrepresentation for each day in which the misrepresentation was applied. Failure to submit monthly/quarterly average manufacturer price, average sales price, and best price data on a timely basis could result in a civil monetary penalty of $10,000 per day for each day the information is late beyond the due date. In the event that CMS were to terminate our rebate agreement after we or any collaborator begin to participate in the Medicaid program, no federal payments would be available under Medicaid or Medicare Part B for our covered outpatient drugs.
In September 2010, CMS and the Office of the Inspector General indicated that they intend to pursue more aggressively companies who fail to report these data to the government in a timely manner. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. We cannot assure you that our or any collaborator’s submissions, once we or any collaborator begin to submit pricing data to CMS, will not be found by CMS to be incomplete or incorrect.
Federal law requires that for a company to be eligible to have its products paid for with federal funds under the Medicaid and Medicare Part B programs, as well as to be purchased by certain federal agencies and grantees, it also must participate in the Department of Veterans Affairs (VA) Federal Supply Schedule, or FSS, pricing program. To participate, we or any collaborator will be required to enter into an FSS contract with the VA, under which we must make our innovator “covered drugs,” such as ADASUVE or other product candidates, available to the “Big Four” federal agencies — the VA, the Department of Defense, or DoD, the Public Health Service, and the Coast Guard — at pricing that is capped pursuant to a statutory federal ceiling price, or FCP, formula set forth in Section 603 of the Veterans Health Care Act of 1992, or VHCA. The FCP is based on a weighted average wholesaler price known as the “non-federal average manufacturer price,” or Non-FAMP, which manufacturers are required to report on a quarterly and annual basis to the VA. If a company misstates Non-FAMPs or FCPs it must restate these figures. Pursuant to the VHCA, knowing provision of false information in connection with a Non-FAMP filing can subject a manufacturer to penalties of $100,000 for each item of false information.
FSS contracts are federal procurement contracts that include standard government terms and conditions, separate pricing for each product, and extensive disclosure and certification requirements. All items on FSS contracts are subject to a standard FSS contract clause that requires FSS contract price reductions under certain
41
circumstances where pricing is reduced to an agreed “tracking customer.” Further, in addition to the “Big Four” agencies, all other federal agencies and some non-federal entities are authorized to access FSS contracts. FSS contractors are permitted to charge FSS purchasers other than the Big Four agencies “negotiated pricing” for covered drugs that is not capped by the FCP; instead, such pricing is negotiated based on a mandatory disclosure of the contractor’s commercial “most favored customer” pricing. We cannot anticipate the pricing structure we will enter into with respect to our products. The FSS contract price may have a material adverse effect on future revenues from sales of ADASUVE.
Once we or any collaborator enter into an FSS contract, if we or any collaborator overcharge the government in connection with the FSS contract, whether due to a misstated FCP or otherwise, we or any collaborator will be required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges could result in allegations under the Federal False Claims Act and other laws and regulations. Unexpected refunds to the government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If we or any collaborators fail to gain market acceptance among physicians, patients, third-party payors and the medical community, we will not become profitable.
TheStaccatosystem is a fundamentally new method of drug delivery. ADASUVE or any future product based on ourStaccatosystem may not gain market acceptance among physicians, patients, third-party payors and the medical community. If these products do not achieve an adequate level of acceptance, we will not meet our revenue guidance nor will we generate sufficient product or royalty revenues to become profitable. The degree of market acceptance of ADASUVE or any of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| | • | | the ability of our collaborators’ sales forces to convince potential purchasers of ADASUVE’s advantages over other treatments; |
| | • | | demonstration of acceptable quality, safety and efficacy in clinical trials and meeting applicable regulatory standards for approval; |
| | • | | the existence, prevalence and severity of any side effects; |
| | • | | potential or perceived advantages or disadvantages compared to alternative treatments; |
| | • | | therapeutic or other improvements of ADASUVE over existing or future drugs used to treat the same or similar conditions; |
| | • | | perceptions about the relationship or similarity between ADASUVE or our product candidates and the parent drug compound upon which ADASUVE or our product candidate is based; |
| | • | | the timing of market entry relative to competitive treatments; |
| | • | | the ability to produce ADASUVE or any future products in commercial quantities at an acceptable cost, or at all; |
| | • | | the ability to offer ADASUVE or any future products for sale at competitive prices; |
| | • | | relative convenience, product dependability and ease of administration; |
| | • | | the restrictions imposed on ADASUVE by the REMS program and labeling requirements; |
| | • | | the strength of marketing and distribution support; |
| | • | | acceptance by patients, the medical community or third-party payors; |
| | • | | the sufficiency of coverage and reimbursement of ADASUVE or our product candidates by governmental and other third-party payors; and |
| | • | | the product labeling, including the package insert, and the marketing restrictions required by the FDA or regulatory authorities in other countries. |
42
We are subject to significant ongoing regulatory obligations and oversight, which may result in significant additional expense and limit our and our collaborators’ ability to commercialize our products.
We and our collaborators are subject to significant ongoing regulatory obligations, such as safety reporting requirements, periodic and annual reporting requirements, and regulatory oversight of the promotion and marketing of our products. In addition, the manufacture, labeling, packaging, distribution, import, export, adverse event reporting, storage, advertising, promotion and recordkeeping for ADASUVE and any of our product candidates that may be approved by the FDA or foreign regulatory authorities will be subject to extensive and ongoing regulatory requirements. Teva has agreed to take responsibility at its expense to complete several post-marketing requirements that were a condition to FDA approval of ADASUVE, including the responsibility for conducting a 10,000 patient observational clinical trial designed to gather patient safety data based on the real-world use of ADASUVE, as well as a clinical program addressing the safety and efficacy of ADASUVE in agitated adolescent patients. As a condition of grant of EU marketing authorization for ADASUVE by the EC, we are responsible for the conduct and funding of post-authorization studies, including (i) a benzodiazepine interaction study, which has been completed, (ii) a controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, with two doses of ADASUVE, which has been completed, (iii) a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients, (iv) an observational clinical trial, and (v) a drug utilization clinical trial.
The FDA and foreign regulatory authorities may also impose significant restrictions on the indicated uses or marketing of our future products, or impose requirements for burdensome post-approval study commitments. For example, ADASUVE’s U.S. labeling contains a “boxed warning” regarding the risks of bronchospasm caused by the product and the increased risk of death for elderly patients with dementia-related psychosis. Boxed warnings are used to highlight warning information that is especially important to the prescriber. Products with boxed warnings are subject to more restrictive advertising and promotion regulations than products without such warnings. The terms of any product approval, including labeling, may be more restrictive than we desire and could affect the commercial potential of the product. If we become aware of previously unknown problems with any of our products in the United States or overseas or at our contract manufacturers’ facilities, a regulatory agency may impose labeling changes or restrictions on our products, our collaborators, our manufacturers or on us. In such an instance, we could experience a significant drop in the sales of the affected products, our product revenues and reputation in the marketplace may suffer, and we could become the target of lawsuits.
The FDA and other governmental authorities, including foreign regulatory authorities, also actively enforce regulations prohibiting off-label promotion, and governments have levied large civil and criminal fines against companies for alleged improper promotion. Governments have also required companies to enter into corporate integrity agreements and/or non-prosecution agreements that impose significant reporting and other burdens on the affected companies.
We and our commercial collaborators are also subject to regulation by regional, national, state and local agencies, including the DEA, the Department of Justice, the Federal Trade Commission, the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies, as well as governmental authorities in those foreign countries in which we may in the future commercialize our products. The FDCA, the Public Health Service Act, the Social Security Act, and other federal and state statutes and regulations govern to varying degrees the research, development, manufacturing and commercial activities relating to prescription pharmaceutical products, including preclinical testing, approval, production, labeling, sale, distribution, import, export, post-market surveillance, advertising, dissemination of information, promotion, marketing, and pricing to government purchasers and government healthcare programs. Any manufacturing, licensing, or commercialization collaborators we have or may in the future have, including Teva and Ferrer, will be subject to many of the same requirements.
The Federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical companies on one hand and prescribers, purchasers and formulary managers on the other.
43
Further, the Healthcare Reform Act, among other things, amends the intent requirement of the Federal Anti-Kickback Statute. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Healthcare Reform Act provides that the government may assert that a claim including items or services resulting from a violation of the Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common manufacturer business arrangements and activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration may be subject to scrutiny if they do not qualify for an exemption or safe harbor. We intend to comply with the exemptions and safe harbors whenever possible, but our practices or those of our commercial collaborators may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability and may be subject to scrutiny.
The Federal False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Many pharmaceutical and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under these laws for a variety of alleged marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug payment rates under government healthcare programs. Companies have been prosecuted for causing false claims to be submitted because of the marketing of their products for unapproved uses. Pharmaceutical and other healthcare companies have also been prosecuted on other legal theories of Medicare and Medicaid fraud.
The majority of U.S. states also have statutes or regulations similar to the Federal Anti-Kickback Statute and Federal False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also prohibit certain marketing related activities including the provision of gifts, meals, or other items to certain health care providers. In addition, California, Connecticut, Nevada and Massachusetts require pharmaceutical companies to implement compliance programs or marketing codes.
Compliance with various federal and state laws is difficult and time consuming, and companies that violate them may face substantial penalties. The potential sanctions include civil monetary penalties, exclusion of a company’s products from reimbursement under government programs, criminal fines and imprisonment. Because of the breadth of these laws and the lack of extensive legal guidance in the form of regulations or court decisions, it is possible that some of our business activities or those of our commercial collaborators could be subject to challenge under one or more of these laws. Such a challenge could have a material adverse effect on our business and financial condition and growth prospects.
We or our commercial collaborators could become subject to government investigations and related subpoenas. Such subpoenas are often associated with previously filed qui tam actions, or lawsuits filed under seal under the Federal False Claims Act. Qui tam actions are brought by private plaintiffs suing on behalf of the federal government for alleged Federal False Claims Act violations. The time and expense associated with responding to such subpoenas, and any related qui tam or other actions, may be extensive, and we cannot predict the results of our review of the responsive documents and underlying facts or the results of such actions. Responding to government investigations, defending any claims raised, and any resulting fines, restitution, damages and penalties, settlement payments or administrative actions, as well as any related actions brought by stockholders or other third parties, could have a material impact on our reputation, business and financial condition and divert the attention of our management from operating our business.
The number and complexity of both federal and state laws continues to increase, and additional governmental resources are being added to enforce these laws and to prosecute companies and individuals who are believed to be violating them. In particular, the Healthcare Reform Act includes a number of provisions aimed at strengthening the government’s ability to pursue anti-kickback and false claims cases against
44
pharmaceutical manufacturers and other healthcare entities, including substantially increased funding for healthcare fraud enforcement activities, enhanced investigative powers, amendments to the False Claims Act that make it easier for the government and whistleblowers to pursue cases for alleged kickback and false claim violations and, beginning in March 2014 for payments made on or after August 1, 2013, public reporting of payments by pharmaceutical manufacturers to physicians and teaching hospitals nationwide. While it is too early to predict what effect these changes will have on our business, we anticipate that government scrutiny of pharmaceutical sales and marketing practices will continue for the foreseeable future and subject us and our commercial collaborators to the risk of government investigations and enforcement actions. Responding to a government investigation or enforcement action would be expensive and time-consuming, and could have a material adverse effect on our business and financial condition and growth prospects.
Similar restrictions are imposed on the promotion and marketing of medicinal products in the EU and other countries. The applicable laws at EU level and in the individual EU Member States require promotional materials and advertising concerning medicinal products to comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities. The SmPC is the document that provides information to physicians concerning the safe and effective use of a medicinal product. Promotion of a medicinal product which does not comply with the SmPC is considered to constitute off-label promotion. The off-label promotion of medicinal products is prohibited in the EU. The applicable laws at both EU level and in the individual EU Member States also prohibit the direct-to-consumer advertising of prescription-only medicinal products. Violations of the rules governing the promotion of medicinal products in the EU could be penalized by administrative measures, fines and imprisonment.
Interactions between pharmaceutical companies and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct in the individual EU Member States. The provision of any inducement to physicians to prescribe, recommend, endorse, order, purchase, supply, use or administer a medicinal product is prohibited. A number of EU Member States have introduced additional rules requiring pharmaceutical companies to publically disclose their interactions with physicians and to obtain approval from employers, professional organizations and/or competent authorities before entering into agreements with physicians. Violations of these rules could lead to the imposition of fines or imprisonment.
Laws, including those governing promotion, marketing and anti-kickback provisions, industry regulations and professional codes of conduct are often strictly enforced. Increasing regulatory scrutiny of the promotional activities of pharmaceutical companies has been observed in a number of EU Member States. The Bribery Act in the United Kingdom entered into force on 1 July 2011. This Act applies to any company incorporated in or “carrying on business” in the UK, irrespective of where in the world the alleged bribery activity occurs. Even though we strive for complete and continuous adherence to all laws and rules during our promotion and marketing activities, this Act could have implications for our interactions or those of Ferrer with physicians both in and outside the UK.
Payments made to physicians in certain EU Member States must be publically disclosed and this obligation will expand to other EU Member States. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer, his/her competent professional organization, and/or the competent authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment. Even in those countries where we are not directly responsible for the promotion and marketing of our products, inappropriate activity by our international distribution collaborators can have implications for us.
If we or any collaborators fail to comply with applicable federal, state, local, or foreign regulatory requirements, we or they could be subject to a range of regulatory actions that could affect our or any collaborators’ ability to commercialize our products and could harm or prevent sales of the affected products, or could substantially increase the costs and expenses of commercializing and marketing our products. Any threatened or actual government enforcement action could also generate adverse publicity and require that we devote substantial resources that could otherwise be used in other aspects of our business.
45
We could be adversely affected by violations of applicable anti-corruption laws such as the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act of 2010.
Anti-corruption laws, such as the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act of 2010, generally prohibit directly or indirectly giving, offering, or promising anything of value to improperly induce the recipient to act, or refrain from acting, in a manner that would confer a commercial advantage. The anti-bribery provisions of the U.S. Foreign Corrupt Practices Act generally prohibit directly or indirectly giving, offering or promising an inducement to a public official (broadly interpreted) to corruptly influence the official’s actions in order to obtain a commercial advantage. The U.K. Bribery Act of 2010 prohibits both domestic and international bribery, as well as bribery in both the private and public sectors. In addition, an organization that “fails to prevent bribery” by anyone associated with the organization may be charged under the U.K. Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. In recent years, the U.S. Government has brought enforcement actions that resulted in significant monetary penalties against several multinational healthcare companies for violations of the U.S. Foreign Corrupt Practices Act stemming from illegal payments made to non-U.S. healthcare professionals. We plan to adopt and implement policies and procedures to ensure that those involved in the marketing, sale, and distribution of our products are both aware of these legal requirements and committed to complying therewith. However, we cannot assure that these policies and procedures will protect us from potentially illegal acts committed by individual employees or agents. If we were found to be liable for anti-corruption law violations, we could be subject to criminal or civil penalties or other consequences that could have a material adverse effect on our business and financial condition.
If we do not establish additional strategic collaborations, we will have to undertake additional development and future commercialization efforts on our own, which would be costly and delay our ability to commercialize any future products.
An element of our business strategy is our intent to selectively collaborate with pharmaceutical, biotechnology and other companies to obtain assistance for the development and commercialization of ADASUVE and our product candidates. In October 2011, we entered into the Ferrer Agreement with Ferrer for the commercialization of ADASUVE in the Ferrer Territories. In May 2013, we entered into a commercial collaboration with Teva, granting Teva an exclusive license to develop and commercialize ADASUVE in the United States. We may never enter into additional strategic collaborations with third parties to develop and commercialize ADASUVE or our product candidates. Other than Teva and Ferrer, we do not currently have any strategic collaborations for ADASUVE or any of our product candidates.
We face significant competition in seeking appropriate strategic collaborators, and these strategic collaborations can be intricate and time consuming to negotiate and document. We may not be able to negotiate additional strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing strategic collaborations. We are currently seeking collaborations to commercialize ADASUVE outside of the United States and the Ferrer Territories. If we are unable to negotiate additional strategic collaborations for ADASUVE outside of the United States and the Ferrer Territories, we may be unable to maximize ADASUVE’s commercial potential.
If we are unable to negotiate additional collaborations for ADASUVE or our product candidates we may be forced to curtail the development of a particular candidate, reduce or delay its development program, or one or more of our other development programs, delay its commercialization, or undertake development or commercialization activities at our own expense. In addition, we will bear all the risk related to the development of a product candidate. If we elect to increase our expenditures to fund development or commercialization activities on our own, we will need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring ADASUVE or our product candidates to market successfully and generate revenue or profit.
46
ADASUVE and any of our product candidates approved for marketing remain subject to ongoing regulatory review in the United States, the EU or in other countries. If we or any collaborators fail to comply with the regulations, we could lose these approvals, and the sale of any future products could be suspended. If approval is denied or limited in a country, or if a country imposes post-marketing requirements, that decision could negatively affect our ability to market our product in such countries.
Even with regulatory approval to market a particular product candidate, the FDA, the EC or another foreign regulatory authority could condition approval on conducting additional costly post-approval studies or trials or could limit the scope of our approved labeling or could impose burdensome post-approval obligations, such as those required in the United States and in the post-marketing obligations required as part of a marketing authorization in the EU. Moreover, the product may later cause adverse effects that limit or prevent its widespread use, force us to withdraw it from the market, cause the FDA, the EC or another foreign regulatory authority to impose additional obligations or restriction on marketing, or impede or delay our ability to obtain regulatory approvals in additional countries. In addition, we will continue to be subject to FDA, EMA, EC and other foreign regulatory authority regulations, as well as periodic inspections to ensure adherence to applicable regulations. After receiving marketing approval, the FDA, the EC and other foreign regulatory authorities could impose extensive regulatory requirements on the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution, and record keeping related to the product. The approval of the ADASUVE NDA requires us to implement, administer and assess at regular intervals a REMS program that, among other things, limits the use of ADASUVE to healthcare facilities enrolled in the ADASUVE REMS program.
As a condition to FDA approval of ADASUVE, Teva also has several post-approval commitments and requirements, including a 10,000 patient observational clinical trial designed to gather patient safety data based on the real-world use of ADASUVE, as well as a clinical program addressing the safety and efficacy of ADASUVE in agitated adolescent patients.
As a condition to marketing authorization for ADASUVE granted by the EC in the EU, we are responsible for the conduct and funding of post-marketing studies, including (i) a benzodiazepine interaction study, which has been completed, (ii) a controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, with two doses of ADASUVE, which has been completed, (iii) a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients, (iv) an observational clinical study, and (v) a drug utilization study. Results of the benzodiazepine interaction study and the thorough QTc study have been submitted to the EMA.
The costs associated with development and approval of study protocols and the completion of studies and clinical trials are significant. There are risks involved with relying on our own capabilities to perform the tasks required by the post-market studies for ADASUVE, as well as with entering into arrangements with third parties to perform these services. If Teva enters into an arrangement with a third party or parties to perform the tasks required for the ADASUVE post-market studies and trials, the expense of such outsourcing could be significant, decreasing the profitability of ADASUVE. Additionally, any third party with whom we may collaborate may not fulfill its obligations or carry out activities sufficiently to satisfy FDA, EC, EMA or other U.S. or foreign regulatory authority standards, which could result in increased expenses needed to remediate any deficiencies or could even result in an FDA, EC, EMA or other U.S. or foreign regulatory authority enforcement action, including the imposition of civil money penalties and the withdrawal of approval of the product in the U.S. Finally, the data derived from any post-market study or trial could result in additional restrictions on the commercialization of ADASUVE through changes to the approved ADASUVE label, a more burdensome REMS program, the imposition of additional post-market studies or trials, or could even lead to the withdrawal of the approval of the product.
If we or any collaborators fail to comply with the regulatory requirements of the FDA, the EMA, the EC or other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any future products, suppliers or manufacturing processes are discovered, we or our collaborators could be subject to administrative or judicially imposed sanctions, including:
| | • | | restrictions on the products, suppliers or manufacturing processes; |
| | • | | warning letters or untitled letters; |
47
| | • | | injunctions, consent decrees, or the imposition of civil or criminal penalties; |
| | • | | product seizures, detentions or import or export bans; |
| | • | | voluntary or mandatory product recalls and publicity requirements; |
| | • | | variation, suspension or withdrawal of regulatory approvals; |
| | • | | required variations of the clinical trial protocol |
| | • | | suspension or termination of any clinical trials of the products; |
| | • | | total or partial suspension of production; |
| | • | | refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications; and |
| | • | | denial of permission to file an application or supplement in a jurisdiction. |
If we or our collaborators were subject to administrative or judicially-imposed sanctions arising out of enforcement of the FDA, EMA, EC or other applicable U.S. and foreign laws, it could impair our ability to manufacture and the ability of our collaborators to successfully market ADASUVE, even if such an enforcement action does not relate specifically to ADASUVE. Such enforcement could have a significant impact on our financial condition and results.
Problems with the third parties that manufacture the API in ADASUVE or our product candidates may delay our clinical trials or subject us to liability.
We do not currently own or operate manufacturing facilities for clinical or commercial production of the API used in ADASUVE or any of our product candidates. We have no experience in API manufacturing, and we lack the resources and the capability to manufacture any of the APIs used in ADASUVE and our product candidates, on either a clinical or commercial scale. As a result, we rely on third parties to supply the API used in ADASUVE and each of our product candidates. We expect to continue to depend on third parties to supply the API for ADASUVE and our current and future product candidates and to supply the API for ADASUVE in commercial quantities.
An API manufacturer must meet high precision and quality standards for that API to meet regulatory specifications and comply with regulatory requirements. A contract manufacturer is subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign authorities to ensure strict compliance with cGMP and other applicable government regulations and corresponding foreign standards. Additionally, a contract manufacturer must pass a pre-approval inspection by the FDA and corresponding foreign authorities to ensure strict compliance with cGMP prior to the FDA’s or corresponding foreign authorities’ approval of any product candidate for marketing. A contract manufacturer’s failure to conform to cGMP could result in a refusal by the FDA or a corresponding foreign authority to approve or a delay in their approval of a product candidate for marketing. We are ultimately responsible for confirming that the APIs used in ADASUVE and our product candidates are manufactured in accordance with applicable regulations.
Our third-party suppliers may not carry out their contractual obligations or meet our deadlines. In addition, the API they supply to us may not meet our specifications and quality policies and procedures or they may not be able to supply the API in commercial quantities. If we need to find alternative suppliers of the API used in ADASUVE or any of our product candidates, we may not be able to contract for such supplies on acceptable terms, if at all. Any such failure to supply or delay caused by such contract manufacturers would have an adverse effect on our ability to continue clinical development of our product candidates or commercialize ADASUVE.
If our third-party drug suppliers fail to achieve and maintain high manufacturing standards in compliance with cGMP regulations, we could be subject to certain product liability claims in the event such failure to comply resulted in defective products that caused injury or harm.
48
Unless our preclinical studies demonstrate an acceptable safety profile of our product candidates, we will not be able to pursue clinical development or commercialize our product candidates.
We must satisfy the FDA and other regulatory authorities abroad, through extensive preclinical studies, that our product candidates have an acceptable safety profile. OurStaccatosystem creates a condensation aerosol from drug compounds, and there currently are no approved products that use a similar method of drug delivery other than ADASUVE. Companies developing other inhalation products have not defined or successfully completed the types of preclinical studies we believe will be required for submission to regulatory authorities as we seek approval to conduct our clinical trials. We may not have conducted or may not conduct in the future the types of preclinical testing ultimately required by regulatory authorities, or future preclinical tests may indicate that our product candidates are not safe for use in humans. Preclinical testing is expensive, can take many years and has an uncertain outcome. In addition, success in initial preclinical testing does not ensure that later preclinical testing will be successful.
We may experience numerous unforeseen events during, or as a result of, the preclinical testing process, which could delay or prevent our ability to develop or commercialize our product candidates, including:
| | • | | our preclinical testing may produce inconclusive or negative safety results, which may require us to conduct additional preclinical testing or to abandon product candidates that we believed to be promising; |
| | • | | our product candidates may have unfavorable pharmacology, toxicology or carcinogenicity; and |
| | • | | our product candidates may cause undesirable side effects. |
Any such events would increase our costs and could delay or prevent our ability to conduct clinical testing and commercialize our product candidates, which could adversely impact our business, financial condition and results of operations.
Failure or delay in commencing or completing clinical trials for our product candidates could harm our business.
Other than for ADASUVE, we have not completed all the clinical trials necessary to support an application with the FDA or other regulatory authorities abroad for approval to market any of our product candidates other than for ADASUVE in the United States and the European Union. Future clinical trials may be delayed or terminated as a result of many factors, including:
| | • | | insufficient financial resources to fund such trials; |
| | • | | delays or failure in reaching agreement on acceptable clinical trial contracts or clinical trial protocols with prospective sites; |
| | • | | regulators, Ethics Committees or IRBs may not authorize us to commence a clinical trial; |
| | • | | regulators, Ethics Committees or IRBs may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or concerns about patient safety; |
| | • | | we may suspend or terminate our clinical trials if we believe that they expose the participating patients to unacceptable health risks; |
| | • | | we may experience slower than expected patient enrollment or lack of a sufficient number of patients who meet the enrollment criteria for our clinical trials; |
| | • | | patients may not complete clinical trials due to safety issues, side effects, dissatisfaction with the product candidate, or other reasons; |
| | • | | we may have difficulty in maintaining contact with patients after treatment, preventing us from collecting the data required by our study protocol; |
| | • | | product candidates may demonstrate a lack of efficacy during clinical trials; |
| | • | | we may experience governmental or regulatory delays, failure to obtain regulatory approval or changes in regulatory requirements, policy and guidelines; and |
| | • | | we may experience delays in our ability to manufacture clinical trial materials in a timely manner as a result of ongoing process and design enhancements to ourStaccatosystem. |
49
Any delay in commencing or completing clinical trials for our product candidates could delay commercialization of our product candidates and harm our business, financial condition and results of operations. It is possible that none of our product candidates will successfully complete clinical trials or receive regulatory approval, which would severely harm our business, financial condition and results of operations.
If our product candidates do not meet safety and efficacy endpoints in clinical trials, they will not receive regulatory approval, and we will be unable to market them.
The clinical development and regulatory approval process is extremely expensive and takes many years. Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. Initial results may not be confirmed upon full analysis of the detailed results of a trial. Product candidates in later stage clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials with acceptable endpoints. Whether an approval will be granted, and the timing of such approval cannot be accurately predicted. If we fail to obtain regulatory approval for ADASUVE in markets outside of the United States and the Ferrer Territories or for our other product candidates in any markets where we seek regulatory approval, we will be unable to market and sell them in those locations and therefore we may never be profitable.
As part of the regulatory process, we must conduct clinical trials for each product candidate to demonstrate safety and efficacy to the satisfaction of the FDA, the EMA, the EC and other regulatory authorities abroad. The number and design of clinical trials that will be required varies depending on the product candidate, the condition being evaluated, the trial results and regulations applicable to any particular product candidate. In June 2008, we announced that our Phase 2a proof-of-concept clinical trial of AZ-002 (Staccatoalprazolam) did not meet either of its two primary endpoints. In September 2009, we announced that our Phase 2b clinical trial of AZ-104 (Staccatoloxapine, low-dose) for the treatment of migraine did not meet its primary endpoint.
If our product candidates fail to show a clinically significant benefit compared to placebo, they will not be approved for marketing.
The design of our clinical trials is based on many assumptions about the expected effect of our product candidates, and if those assumptions prove incorrect, the clinical trials may not produce statistically significant results. For example, in 2008 we released the preliminary results from our Phase 2a clinical trial with AZ-002 in patients with panic disorder, a separate indication from our current AZ-002 indication of ARS. The study did not meet its two primary endpoints, which were the effect of AZ-002 on the incidence of a doxapram-induced panic attack and the effect of AZ-002 on the duration of a doxapram-induced panic attack, both as compared with placebo. OurStaccatosystem is not similar to other approved drug delivery methods, and there is no precedent for the application of detailed regulatory requirements to our product candidates. We cannot assure you that the design of, or data collected from, the clinical trials of our product candidates will be sufficient to support the FDA, the EC and other foreign regulatory approvals.
Regulatory authorities may not approve our product candidates even if they meet safety and efficacy endpoints in clinical trials.
The FDA, the EC and other foreign regulatory agencies can delay, limit or deny marketing approval for many reasons, including:
| | • | | a product candidate may not be considered to have a favorable risk-benefit ratio; |
| | • | | the manufacturing processes or facilities we have selected may not meet the applicable requirements; and |
| | • | | changes in their approval policies or adoption of new regulations may require additional work on our part. |
Part of the regulatory approval process includes compliance inspections of manufacturing facilities to ensure adherence to applicable regulations and guidelines. The regulatory agency may delay, limit or deny marketing approval of our other product candidates as a result of such inspections. Any delay in, or failure to receive or maintain, approval for any of our product candidates could prevent us from ever generating meaningful revenues or achieving profitability.
50
Our product candidates may not be approved even if they achieve their endpoints in clinical trials. Regulatory agencies, including the FDA, the EMA, the EC or their advisors may disagree with our trial design and our interpretations of data from preclinical studies and clinical trials. Regulatory agencies may change requirements for approval even after a clinical trial design has been approved. For example, ADASUVE and our other product candidates combine drug and device components in a manner that the FDA considers to meet the definition of a combination product under FDA regulations. The FDA exercises significant discretion over the regulation of combination products, including the discretion to require separate marketing applications for the drug and device components in a combination product. ADASUVE and our product candidates are being regulated as drug products under the NDA process administered by the FDA. The FDA could in the future require additional regulation of ADASUVE or our product candidates under the medical device provisions of the FDCA. Our systems are designed to comply with the QSR, which sets forth the FDA’s cGMP requirements for medical devices, and other applicable government regulations and corresponding foreign standards. If we fail to comply with these regulations, it could have a material adverse effect on our business and financial condition.
Regulatory agencies also may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies, such as the FDA’s requirement that we perform a Phase 4 observational study and a study in adolescent patients for ADASUVE. Similarly, as a condition to EC approval of ADASUVE, we are responsible for the conduct and funding of post-authorization studies, including (i) a benzodiazepine interaction study, which has been completed, (ii) a controlled study to determine ADASUVE’s effect on cardiac rhythms, or a thorough QTc study, with two doses of ADASUVE, which has been completed, (iii) a clinical program designed to evaluate the safety and efficacy of ADASUVE in agitated adolescent patients, (iv) an observational clinical study, and (v) a drug utilization study. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
We rely on third parties to conduct our preclinical studies and our clinical trials. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for our product candidates, or we may be delayed in doing so.
We rely on third parties, such as contract research organizations, medical institutions, academic institutions, clinical investigators and contract laboratories, to conduct our preclinical studies and clinical trials. We are responsible for confirming that our preclinical studies are conducted in accordance with applicable regulations and that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. The FDA requires us to comply with regulations and standards, commonly referred to as good laboratory practices, or GLP, for conducting and recording the results of our preclinical studies and with good clinical practices, or GCP, for conducting, monitoring, recording and reporting the results of clinical trials, to assure that data and reported results are accurate and that the clinical trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines, fail to comply with the FDA’s GLP or GCP regulations and standards, do not adhere to our clinical trial protocols or otherwise fail to generate reliable clinical data, we may need to enter into new arrangements with alternative third parties and our clinical trials may be extended, delayed or terminated or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the product candidate being tested in such trials.
If we experience problems with the manufacturers of components of ADASUVE or our product candidates, our ability to supply ADASUVE and our other product candidates will be impaired, our sales may be lower than expected and our development programs may be delayed and we may be subject to liability.
We outsource the manufacturing of the components of ourStaccatosystem, including the printed circuit boards, the plastic airways, and the chemical heat packages to be used in our commercial single dose device. We have no experience in the manufacturing of components, other than our chemical heat packages, and we currently lack the resources and the capability to manufacture them, on either a clinical or commercial scale. As a result, we rely on third parties to supply these components. We expect to continue to depend on third parties to supply these components for ADASUVE and our current product candidates and any devices based on theStaccato system we develop in the foreseeable future.
51
The third-party suppliers of the components of ourStaccatosystem must meet high precision and quality standards for our finished devices to comply with regulatory requirements. A contract manufacturer is subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign authorities to ensure that our finished devices remain in strict compliance with the QSR, and other applicable government regulations and corresponding foreign standards. We are ultimately responsible for confirming that the components used in theStaccatosystem are manufactured in accordance with specifications, standards and procedures necessary to ensure that our finished devices comply with the QSR or other applicable regulations.
Our third party suppliers may not comply with their contractual obligations or meet our deadlines, or the components they supply to us may not meet our specifications and quality policies and procedures. If we need to find alternative suppliers of the components used in theStaccato system, we may not be able to contract for such components on acceptable terms, if at all. Any such failure to supply or delay caused by such contract manufacturers would have an adverse effect on our ability to manufacture commercial quantities of ADASUVE and on our ability to continue clinical development of our product candidates or commercialize ADASUVE.
In addition, the heat packages used in the single dose version of ourStaccatosystem are manufactured using certain energetic, or highly combustible, materials that are used to generate the rapid heating necessary for vaporizing the drug compound while avoiding degradation. Manufacture of products containing energetic materials is regulated by the U.S. government. We have entered into a manufacture agreement with Autoliv for the manufacture of the heat packages in the commercial design of our single dose version of ourStaccatosystem. If Autoliv fails to manufacture the heat packages to the necessary specifications, or does not carry out its contractual obligations to supply our heat packages to us, or if the FDA requires different manufacturing or quality standards than those set forth in our manufacture agreement, our clinical trials or commercialization efforts may be delayed, suspended or terminated while we seek additional suitable manufacturers of our heat packages, which may prevent us from commercializing ADASUVE or our product candidates that utilize the single dose version of theStaccatosystem. In October 2013, Autoliv notified us of their intent to terminate, effective October 2016, the Manufacture Agreement. Prior to October 2016, Autoliv and we remain fully obligated to perform pursuant to the terms of both the Manufacture Agreement and the Second Note. However, Autoliv may not continue to perform its obligations in the same manner during the termination period. If we are unable to secure an adequate replacement manufacturer for sufficient quantities of the chemical heat packages in ADASUVE or if the costs for such manufacturer to produce the chemical heat packages are higher than the cost from Autoliv, it will significantly impact our ability to produce our commercial devices or to produce them at quantities or at a cost to allow us to become profitable.
Product candidates that we may develop may require expensive carcinogenicity tests.
We combine small molecule drugs with ourStaccatosystem to create proprietary product candidates. Some of these drugs may not have previously undergone carcinogenicity testing that is now generally required for marketing approval. We may be required to perform carcinogenicity testing with product candidates incorporating drugs that have not undergone carcinogenicity testing or may be required to do additional carcinogenicity testing for drugs that have undergone such testing. Any carcinogenicity testing we are required to complete will increase the costs to develop a particular product candidate and may delay or halt the development of such product candidate.
If some or all of our patents expire, are invalidated or are unenforceable, or if some or all of our patent applications do not yield issued patents or yield patents with narrow claims, competitors may develop competing products using our or similar intellectual property and our business will suffer.
Our success will depend in part on our ability to obtain and maintain patent and trade secret protection for our technologies, ADASUVE and our product candidates both in the United States and other countries. We do not know whether any patents will issue from any of our pending or future patent applications. In addition, a third party may successfully circumvent our patents. Our rights under any issued patents may not provide us with sufficient protection against competitive products or otherwise cover commercially valuable products or processes.
52
The degree of protection for our proprietary technologies, ADASUVE and our product candidates is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| | • | | we might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
| | • | | we might not have been the first to file patent applications for these inventions; |
| | • | | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| | • | | the claims of our issued patents may be narrower than as filed and not sufficiently broad to prevent third parties from circumventing them; |
| | • | | it is possible that none of our pending patent applications will result in issued patents; |
| | • | | we may not develop additional proprietary technologies or drug candidates that are patentable; |
| | • | | our patent applications or patents may be subject to interference, opposition or similar administrative proceedings; |
| | • | | any patents issued to us or our potential strategic collaborators may not provide a basis for commercially viable products or may be challenged by third parties in the course of litigation or administrative proceedings such as reexaminations or interferences; and |
| | • | | the patents of others may have an adverse effect on our ability to do business. |
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The United States Patent and Trademark Office has developed regulations and procedures to govern administration of the Leahy-Smith Act, but many of the substantive changes to patent law associated with the Leahy-Smith Act, particularly the first inventor to file provisions, only became effective 18 months after its enactment. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
Even if valid and enforceable patents cover ADASUVE, our product candidates and our technologies, the patents will provide protection only for a limited amount of time.
The Teva Agreement provides Teva with the first right to enforce certain of the Alexza patents listed in the FDA Orange Book as well as product-specific patents related to ADASUVE that may be identified and prosecuted during the term of the Teva Agreement. While Teva may not settle any such enforcement action without our consent, there can be no assurance that Teva would enforce the ADASUVE patents in the same manner or with the same strategy as we might if we maintained the sole right to control litigation against potential third-party infringers. Our current patents or any future patents that may be issued regarding ADASUVE or our product candidates or methods of using them, can be challenged by our competitors who can argue that our patents are invalid and/or unenforceable. Third parties may challenge our rights to, or the scope or validity of, our patents. Patents also may not protect ADASUVE or our product candidates if competitors devise ways of making these or similar product candidates without legally infringing our patents. The FDCA and the FDA regulations and policies provide incentives to manufacturers to challenge patent validity or create modified, non-infringing versions of a drug or device in order to facilitate the approval of generic substitutes. These same types of incentives encourage manufacturers to submit new drug applications that rely on literature and clinical data not prepared for or by the drug sponsor.
Our potential strategic collaborators’ ability to obtain patents is uncertain because, to date, some legal principles remain unresolved, there has not been a consistent policy regarding the breadth or interpretation of
53
claims allowed in patents in the United States, and the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Furthermore, the policies governing pharmaceutical and medical device patents outside the United States may be even more uncertain. Changes in either patent laws or interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
We also rely on trade secrets to protect our technology, especially where we do not believe that patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. The employees, consultants, contractors, outside scientific collaborators and other advisors of our company and our strategic collaborators may unintentionally or willfully disclose our confidential information to competitors. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming and the outcome is unpredictable. Failure to protect or maintain trade secret protection could adversely affect our competitive business position.
Our research and development collaborators may have rights to publish data and other information in which we have rights. In addition, we sometimes engage individuals or entities to conduct research that may be relevant to our business. The ability of these individuals or entities to publish or otherwise publicly disclose data and other information generated during the course of their research is subject to certain contractual limitations. These contractual provisions may be insufficient or inadequate to protect our trade secrets and may impair our patent rights. If we do not apply for patent protection prior to such publication or if we cannot otherwise maintain the confidentiality of our technology and other confidential information, then our ability to receive patent protection or protect our proprietary information may be jeopardized.
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend time and money and could shut down some of our operations.
Our commercial success depends in part on not infringing patents and proprietary rights of third parties. Others have filed, and in the future are likely to file, patent applications covering products that are similar to ADASUVE or our product candidates, as well as methods of making or using similar or identical products. We are aware of certain pending U.S. patent applications related generally to a systemic respiratory delivery of loxapine. If these patent applications result in issued patents and we wish to use the claimed technology, we would need to obtain a license from the third party. We may not be able to obtain these licenses at a reasonable cost, if at all.
In addition, administrative proceedings, such as interferences and reexaminations before the U.S. Patent and Trademark Office, could limit the scope of our patent rights. We may incur substantial costs and diversion of management and technical personnel as a result of our involvement in such proceedings. In particular, our patents and patent applications may be subject to interferences in which the priority of invention may be awarded to a third party. We do not know whether our patents and patent applications would be entitled to priority over patents or patent applications held by such a third party. Our issued patents may also be subject to reexamination proceedings. We do not know whether our patents would survive reexamination in light of new questions of patentability that may be raised following their issuance.
Third parties may assert that we are employing their proprietary technology or their proprietary products without authorization. In addition, third parties may already have or may obtain patents in the future and claim that use of our technologies or our products infringes these patents. We could incur substantial costs and diversion of management and technical personnel in defending against any of these claims. Furthermore, parties making claims against us may be able to obtain injunctive or other equitable relief, which could effectively block our ability to further develop, commercialize and sell any future products and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and obtain one or more licenses from third parties. We may not be able to obtain these licenses at a reasonable cost, if at all. In that event, we could encounter delays in product introductions while we attempt to develop alternative methods or products. In the event we cannot develop alternative methods or products, we may be effectively blocked from developing, commercializing or selling any future products. Defense of any lawsuit or failure to obtain any of these licenses would be expensive and could prevent us from commercializing any future products.
54
We review from time to time publicly available information concerning the technological development efforts of other companies in our industry. If we determine that these efforts violate our intellectual property or other rights, we intend to take appropriate action, which could include litigation. Any action we take could result in substantial costs and diversion of management and technical personnel in enforcing our patents or other intellectual property rights against others. Furthermore, the outcome of any action we take to protect our rights may not be resolved in our favor.
Competition in the pharmaceutical industry is intense. If our competitors are able to develop and market products that are more effective, safer or less costly than ADASUVE or any future products that we may develop, our commercial opportunity will be reduced or eliminated.
We face competition from established as well as emerging pharmaceutical and biotechnology companies, academic institutions, government agencies and private and public research institutions. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than ADASUVE or any future products that we may develop and commercialize. In addition, significant delays in the development or commercialization of ADASUVE or our product candidates could allow our competitors to bring products to market before us and impair our ability to commercialize ADASUVE or our product candidates.
We anticipate that ADASUVE will compete with various injectable formulations of other antipsychotic and benzodiazepine drugs and oral, orally-disintegrating tablet and liquid formulations of other antipsychotic drugs and benzodiazepine drugs. Only the injectable antipsychotics are approved for the treatment of agitation.
We anticipate that, if approved, AZ-002 would compete with the oral tablet forms of alprazolam and possibly IV, oral and rectal forms of other benzodiazepines.
We anticipate that, if approved, AZ-007 would compete with non-benzodiazepine GABA-A receptor agonists. We are aware of more than 13 approved generic versions of zolpidem, or zaleplon, oral tablets, as well as at least one insomnia product, a version of zolpidem intended to treat middle of the night awakening, that is approved by the FDA.
We anticipate that, if approved, AZ-104 would compete with currently marketed triptan drugs and with other migraine headache treatments.
We anticipate that, if approved, AZ-003 would compete with some of the available forms of fentanyl, including injectable fentanyl, oral transmucosal and nasal fentanyl formulations and ionophoretic transdermal delivery of fentanyl. We are also aware of two fentanyl products approved by regulatory agencies in the United States or abroad. In addition, if approved, AZ-003 would compete with various generic opioid drugs, such as oxycodone, hydrocodone and morphine, or combination products including one or more of such drugs.
We anticipate that, if approved,Staccato Nicotine would compete with other treatments for smoking cessation, some of which are available over the counter.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Established pharmaceutical companies may invest heavily to discover quickly and develop novel compounds or drug delivery technology that could make ADASUVE or our product candidates obsolete. Smaller or early stage companies may also prove to be significant competitors, particularly through strategic collaborations with large and established companies. In addition, these third parties compete with us in recruiting and retaining qualified scientific, sales, marketing, and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA approval or discovering, developing and commercializing products before we do. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition will suffer.
55
If we lose our key personnel or are unable to attract and retain additional personnel, we may be unable to develop or commercialize ADASUVE or our product candidates.
We are highly dependent on our President and Chief Executive Officer, Thomas B. King, the loss of whose services might adversely impact the achievement of our objectives. In addition, recruiting and retaining qualified clinical, scientific and engineering personnel to manage clinical trials of our product candidates and to perform future research and development work will be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. Although we believe we will be successful in attracting and retaining qualified personnel, competition for experienced management and clinical, scientific and engineering personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms. In addition, we do not have employment agreements with any of our employees, and they could leave our employment at will. We have change of control agreements with our executive officers and vice presidents that provide for certain benefits upon termination or a change in role or responsibility in connection with a change of control of our company. We do not maintain life insurance policies on any employees. Failure to attract and retain personnel would prevent us from developing and commercializing ADASUVE and our product candidates.
If plaintiffs bring product liability claims or lawsuits against us or our collaborators, we may incur substantial liabilities and may be required to limit commercialization of ADASUVE or product candidates that we may develop.
As the supplier of ADASUVE to Teva and Ferrer, we are obligated to deliver commercial supply from a qualified manufacturing facility in accordance with certain specifications. In addition, we are obligated to supply ADASUVE free from product defects or manufacturing defects from our manufacture of ADASUVE. The development, manufacture, testing, marketing and sale of combination pharmaceutical and medical device products, like ADASUVE, entail significant risk of product liability claims, lawsuits, safety alerts or recalls. We may be held liable if any product we develop or manufacture causes injury or is found otherwise unsuitable or unsafe during product testing, manufacturing, marketing or sale, including, but not limited to quality issues, component failures, manufacturing flaws, unanticipated or improper uses of ADASUVE or any future products, design defects, inadequate disclosure of product-related risks or product-related information, or for unlawful, unfair or fraudulent competition or business practices relating to such products. Side effects of, or design or manufacturing defects in, the products tested or commercialized by us or any collaborator could result in exacerbation of a clinical trial participant or patient’s condition, serious injury or impairment or even death. This could result in product liability claims, lawsuits, safety alerts and/or recalls for ADASUVE or any future products, including those in clinical testing, to be commercialized, or already commercialized. Product liability claims or lawsuits may be brought by individuals seeking relief for themselves, by persons seeking to represent a class of claimants/plaintiffs, or by a large number of individual claimants in a coordinated or mass litigation. We cannot predict the frequency, outcome, or cost to defend or resolve product liability claims or lawsuits.
While we have not had to defend against any product liability claims or lawsuits to date, we face greater risk of product liability claims or lawsuits as we or any collaborator commercialize ADASUVE or other future products. As ADASUVE or any future product is more widely prescribed, we believe it is likely that product liability claims or lawsuits will eventually be brought against us. Regardless of merit or eventual outcome, such claims or lawsuits may result in decreased demand for any products or product candidates that we may develop, injury to our reputation, withdrawal of clinical trials, issuance of safety alerts, recall of products under investigation or already commercialized, costs and legal fees to defend and resolve litigation, injunctive relief, disgorgement of profits, or substantial monetary awards to clinical trial participants or patients, loss of revenue, and the inability to commercialize any products we develop. Safety alerts or recalls could result in the FDA or similar government agencies in the United States, or abroad, investigating or bringing enforcement actions regarding any products and/or practices, with resulting significant costs and negative publicity, all of which could materially adversely affect us.
Product liability insurance is expensive, can be difficult to obtain and may not be available in the future on acceptable terms, if at all. We currently have product liability insurance that covers commercial product and clinical trials. Partly as a result of product liability lawsuits related to pharmaceutical and medical device
56
products, product liability and other types of insurance have become more difficult and costly for pharmaceutical and medical device companies to obtain. Insurance may be prohibitively expensive, or may not fully cover our potential liabilities. Inability to maintain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims or lawsuits could impede or negatively affect the commercialization of ADASUVE or our product candidates. If we are sued for any injury caused by any product, our liability could exceed our insurance coverage and total assets. In addition, there is no guarantee that insurers will pay for defense and indemnity of claims or lawsuits or that coverage will be adequate or otherwise available.
A successful claim or lawsuit brought against us in excess of available insurance coverage could subject us to significant liabilities and could have a materially adverse effect on our business, financial condition, results of operations and growth prospects. Such claims could also harm our reputation and the reputation of ADASUVE or any future products, adversely affecting our ability to develop and market any products successfully. In addition, defending a product liability claim or lawsuit is expensive and can divert the attention of key employees from operating our business.
Product recalls and safety alerts may be issued at our discretion or at the discretion of our suppliers, collaborators, government agencies, and other entities that have regulatory authority for medical device and pharmaceutical sales. Any recall of ADASUVE could materially adversely affect our business by rendering us unable to sell ADASUVE for some time, causing us to incur significant recall costs and by adversely affecting our reputation. A recall could also result in product liability claims.
Our product candidates AZ-002, AZ-003 and AZ-007 contain drug substances that are regulated by the U.S. Drug Enforcement Administration. Failure to comply with applicable regulations and requirements could harm our business.
The Controlled Substances Act imposes various registration, recordkeeping and reporting requirements, procurement and manufacturing quotas, labeling and packaging requirements, security controls and a restriction on prescription refills on certain pharmaceutical products. The DEA regulates drug substances as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest potential for abuse and Schedule V substances the lowest potential for abuse relative to the other schedules in the CSA. Alprazolam, the API in AZ-002, is regulated as a Schedule IV substance, fentanyl, the API in AZ-003, is regulated as a Schedule II substance, and zaleplon, the API in AZ-007, is regulated as a Schedule IV substance. Each of these product candidates is subject to DEA regulations relating to registration, security, record keeping and reporting, distribution and, if approved, physician prescription procedures, and DEA regulations may impact the availability of the scheduled substance available for clinical trials and commercial distribution. As a Schedule II substance, fentanyl is subject to more stringent controls, including quotas on the amount of product that can be manufactured as well as a prohibition on the refilling of prescriptions without a new prescription from the physician. The DEA periodically inspects facilities for compliance with its rules and regulations. Failure to comply with current and future regulations of the DEA could lead to a variety of sanctions, including revocation, or denial of renewal, of DEA registrations, injunctions, or civil or criminal penalties and could harm our business, financial condition and results of operations.
The single dose version of our Staccato system contains materials that are regulated by the U.S. government, and failure to comply with applicable regulations could harm our business.
The single dose version of ourStaccatosystem uses energetic materials to generate the rapid heating necessary for vaporizing the drug, while avoiding degradation. Manufacture of products containing energetic materials is controlled by the ATF. Technically, the energetic materials used in ourStaccatosystem are classified as “low explosives,” and the ATF has granted us a license/permit for the manufacture of such low explosives. Additionally, due to inclusion of the energetic materials in ourStaccatosystem, the DOT, might regulate shipments of the single dose version of ourStaccatosystem. However, the DOT has granted the single dose version of ourStaccatosystem “Not Regulated as an Explosive” status. Failure to comply with the current and future regulations of the ATF or DOT could subject us to future liabilities and could harm our business, financial condition and results of operations. Furthermore, these regulations could restrict our ability to expand our facilities or construct new facilities or could require us to incur other significant expenses in order to maintain compliance.
57
We use hazardous chemicals and highly combustible materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development processes involve the controlled use of hazardous materials, including chemicals. We also use energetic materials in the manufacture of the chemical heat packages that are used in our single dose devices. Our operations produce hazardous waste. We cannot eliminate the risk of accidental contamination or discharge or injury from these materials. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these materials. We could be subject to civil damages in the event of an improper or unauthorized release of, or exposure of individuals to, hazardous materials. In addition, claimants may sue us for injury or contamination that results from our use of these materials and our liability may exceed our total assets. Compliance with environmental and other laws and regulations may be expensive, and current or future regulations may impair our research, development or production efforts.
Certain of our suppliers are working with these types of hazardous and energetic materials in connection with our component manufacturing agreements. In the event of a lawsuit or investigation, we could be held responsible for any injury caused to persons or property by exposure to, or release of, these hazardous and energetic materials. Further, under certain circumstances, we have agreed to indemnify our suppliers against damages and other liabilities arising out of development activities or products produced in connection with these agreements.
We identified a material weakness in our internal control over financial reporting, and our business and stock price may be adversely affected if we do not adequately address this weakness or if we have other material weaknesses or significant deficiencies in our internal control over financial reporting.
We did not adequately implement certain controls relating to our review and verification of internally prepared reports and analyses utilized in the financial statement closing process. Therefore, we identified a material weakness in our internal control over financial reporting relating to the financial statement closing process as of December 31, 2013. The existence of this or one or more other material weaknesses or significant deficiencies could result in errors in our financial statements, and substantial costs and resources may be required to rectify any internal control deficiencies. If we cannot produce reliable financial reports, investors could lose confidence in our reported financial information, the market price of our stock could decline significantly, we may be unable to obtain additional financing to operate and expand our business, and our business and financial condition could be harmed.
We will need to implement additional systems, procedures and controls in the future as we grow and to satisfy new reporting requirements as a commercial entity.
Numerous laws and regulations affect commercial companies, including, but not limited to, the Federal Anti-Kickback, False Claims Act, the Federal Physician Payment Sunshine Act, the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act of 2010. The rules make it more difficult and costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage as compared to the policies generally available to public companies. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or our board committees or as executive officers.
Compliance with the Federal Anti-Kickback, False Claims Act, the Federal Physician Payment Sunshine Act, the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act of 2010 and other regulations will continue to increase our costs and require additional management resources. As we grow, we will need to continue to implement additional reporting systems, procedures and controls to satisfy new reporting requirements. We currently do not have an internal audit group. In addition, we may need to hire additional legal and accounting staff with appropriate experience and technical knowledge, and we cannot assure you that if additional staffing is necessary that we will be able to do so in a timely fashion.
58
Our business is subject to complex corporate governance, public disclosure and accounting requirements that could adversely affect our business and financial results.
We are subject to changing rules and regulations of federal and state governments, the SEC, and the NASDAQ Global Market. These entities have issued a significant number of new and increasingly complex requirements and regulations over the course of the last several years and continue to develop additional regulations and requirements. On July 21, 2010, the Dodd-Frank Wall Street Reform and Protection Act, or the Dodd-Frank Act, was enacted. The Dodd-Frank Act contains significant corporate governance and executive compensation-related provisions, some of which the SEC, has implemented by adopting additional rules and regulations in areas such as the compensation of executives, referred to as “say-on-pay.” We cannot assure you that we are or will be in compliance with all potentially applicable regulations. If we fail to comply with the Sarbanes Oxley Act of 2002, the Dodd-Frank Act and associated SEC rules, or any other regulations or if our interpretations of these rules and regulations differ from the regulating bodies, we could be subject to a range of consequences, including restrictions on our ability to sell equity securities or otherwise raise capital funds, the de-listing of our common stock from the NASDAQ Global Market, suspension or termination of our clinical trials, restrictions on future products or our manufacturing processes, significant fines, or other sanctions or litigation. Any of such consequences could have a material adverse effect on our business, results of operations and the price of our common stock. Our efforts to comply with these requirements have resulted in, and are likely to continue to result in, an increase in expenses and a diversion of management’s time from other business activities.
Our facility is located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster could damage our facility and equipment, which could cause us to curtail or cease operations.
Our facility, which is the location where the final manufacturing of our product occurs, is located in the San Francisco Bay Area near known earthquake fault zones and, therefore, is vulnerable to damage from earthquakes. We are also vulnerable to damage from other types of disasters, such as power loss, fire, floods and similar events. If any disaster were to occur, our ability to operate our business could be seriously impaired. We currently may not have adequate insurance to cover our losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business, financial condition and results of operations.
Significant disruptions of information technology systems or breaches of data security could adversely affect our business.
We are increasingly dependent on information technology systems and infrastructure, including mobile technologies, to operate our business. In the ordinary course of our business, we collect, store and transmit large amounts of confidential information, including intellectual property, proprietary business information and personal information. It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. We have also outsourced elements of our information technology infrastructure, and as a result we manage a number of third party vendors who may or could have access to our confidential information. The size and complexity of our information technology systems, and those of third-party vendors with whom we contract, make such systems potentially vulnerable to breakdown, malicious intrusion, security breaches and other cyber attacks. In addition, the prevalent use of mobile devices that access confidential information increases the risk of data security breaches, which could lead to the loss of confidential information, trade secrets or other intellectual property. While we have implemented security measures to protect our data security and information technology systems, such measures may not prevent the adverse effect of such events. Significant disruptions of our information technology systems or breaches of data security could adversely affect our business.
Regulations related to conflict minerals could adversely impact our business.
The Dodd-Frank Wall Street Reform and Consumer Protection Act contains provisions to improve transparency and accountability concerning the supply of tin, tantalum, tungsten and gold, known as conflict
59
minerals, originating from the Democratic Republic of Congo, or the DRC, and adjoining countries. As a result, in August 2012 the SEC adopted annual disclosure and reporting requirements for public companies that use conflict minerals mined from the DRC and adjoining countries in their products. We have determined that we use at least one of these conflict minerals in the manufacture of ADASUVE and our other product candidates, although we have not yet determined the source of the conflict minerals that we use. These new disclosure requirements require us to use diligent efforts to determine which conflict minerals we use and the source of those conflict minerals, and disclose the results of our findings beginning in May 2014. There are and will be costs associated with complying with these disclosure requirements, including those costs incurred in conducting diligent efforts to determine which conflict minerals we use and the sources of conflict minerals used in ADASUVE and our other product candidates. Further, the implementation of these rules could adversely affect the sourcing, supply and pricing of materials used in ADASUVE and our other product candidates. As there may be only a limited number of suppliers offering conflict free conflict minerals, and we cannot be sure that we will be able to obtain necessary conflict free conflict minerals in sufficient quantities or at competitive prices. In addition, we may face reputational challenges if we determine that ADASUVE and our other product candidates contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all conflict minerals used in ADASUVE and our other product candidates through the procedures we may implement. If we determine to redesign ADASUVE and our other product candidates to not use conflict minerals, we would incur costs associated with doing so.
Risks Relating to Owning Our Common Stock
Our stock price has been and may continue to be extremely volatile.
Our common stock price has experienced large fluctuations. In addition, the trading prices of life science and biotechnology company stocks in general have experienced extreme price fluctuations in recent years. The valuations of many life science companies without consistent product revenues and earnings are extraordinarily high based on conventional valuation standards, such as price to revenue ratios. These trading prices and valuations may not be sustained. Any negative change in the public’s perception of the prospects of life science or biotechnology companies could depress our stock price regardless of our results of operations. Other broad market and industry factors may decrease the trading price of our common stock, regardless of our performance. Market fluctuations, as well as general political and economic conditions such as terrorism, military conflict, recession or interest rate or currency rate fluctuations, also may decrease the trading price of our common stock. In addition, our stock price could be subject to wide fluctuations in response to various factors, including:
| | • | | the success of the commercial launches of ADASUVE in the United States and the Ferrer Territories; |
| | • | | our and our collaborators’ ability to complete and implement our post-approval commitments for ADASUVE; |
| | • | | the process and outcome of our post-approval commitments for ADASUVE; |
| | • | | our ability to manufacture ADASUVE at a cost effective price; |
| | • | | actual or anticipated regulatory approvals or non-approvals of our product candidates or competing products; |
| | • | | actual or anticipated cash depletion of our financial resources; |
| | • | | actual or anticipated results and timing of our clinical trials; |
| | • | | changes in laws or regulations applicable to ADASUVE or our product candidates; |
| | • | | changes in the expected or actual timing of our development programs such as for AZ-002, including delays or cancellations of clinical trials for our product candidates; |
| | • | | period to period fluctuations in our operating results; |
| | • | | announcements of new technological innovations or new products by us or our competitors; |
| | • | | changes in financial estimates or recommendations by securities analysts; |
60
| | • | | conditions or trends in the life science and biotechnology industries; |
| | • | | changes in the market valuations of other life science or biotechnology companies; |
| | • | | developments in domestic and international governmental policy or regulations; |
| | • | | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures or capital commitments; |
| | • | | additions or departures of key personnel; |
| | • | | difficulty, or increased costs, associated with replacing Autoliv as the supplier of chemical heat packages for ADASUVE and other product candidates; |
| | • | | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| | • | | sales of our common stock (or other securities) by us; and |
| | • | | sales and distributions of our common stock by our stockholders. |
In the past, stockholders have often instituted securities class action litigation after periods of volatility in the market price of a company’s securities. If a stockholder files a securities class action suit against us, we would incur substantial legal fees, and our management’s attention and resources would be diverted from operating our business in order to respond to the litigation.
If we sell shares of our common stock in future financings, existing common stockholders will experience immediate dilution and, as a result, our stock price may go down.
We will need to raise additional capital to fund our operations to develop our product candidates and to develop our manufacturing capabilities. We may obtain such financing through the sale of our equity securities from time to time. As a result, our existing common stockholders will experience immediate dilution upon any such issuance. For example, in February 2012, we issued 4,400,000 shares of our common stock and warrants to purchase up to an additional 4,400,000 shares of our common stock in an underwritten public offering; in March 2012, we issued 241,936 shares of our common stock in a private placement to Ferrer; in July 2012 we issued 80,429 shares of our common stock to Azimuth in consideration for its execution and delivery of the Purchase Agreement; in August and September 2012, we issued an aggregate of 3,489,860 shares of our common stock to Azimuth under the Purchase Agreement; and in May 2013, we issued 1,437,481 shares of our common stock to Azimuth under the Purchase Agreement. If we enter into other financing transactions in which we issue equity securities in the future, our existing common stockholders will experience immediate dilution upon any such issuance.
If we fail to maintain compliance with the listing requirements of The NASDAQ Global Market, we may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is currently listed on The NASDAQ Global Market. To maintain the listing of our common stock on The NASDAQ Global Market, we are required to meet certain listing requirements, including, among others, either: (i) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $5 million and stockholders’ equity of at least $10 million; or (ii) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors, affiliates and 10% or more stockholders) of at least $15 million and a total market value of listed securities of at least $50 million. On January 31, 2012, we received a notice from The NASDAQ Stock Market indicating that our common stock had not met the $1.00 per share minimum closing bid price requirement for 30 consecutive business days and that, if we were unable to demonstrate compliance with this requirement during the applicable grace periods, our common stock would be delisted after that time. We were notified that we had regained compliance with the minimum closing bid requirement on June 27, 2012 after our one-for-ten reverse stock split.
61
This reverse stock split may not prevent our common stock from dropping back down below The NASDAQ Global Market minimum closing bid price requirement in the future. It is also possible that we would otherwise fail to satisfy another NASDAQ Global Market requirement for continued listing of our common stock. As of March 14, 2014, the total market value of our publicly held shares of our common stock (excluding shares held by our executive officers, directors, affiliates and 10% or more stockholders) was $87.0 million, the total market value of our listed securities was $88.3 million and the closing bid price of our common stock was $5.11 per share. As of December 31, 2013, we had a stockholders’ deficit of $24.0 million.
| Item 1B. | Unresolved Staff Comments |
None.
We lease a building with 64,104 square feet of manufacturing, office, and laboratory facilities in Mountain View, California, which we began to occupy in the fourth quarter of 2007. The lease expires on March 31, 2018, and we have two options to extend the lease for five years each.
None.
| Item 4. | Mine Safety Disclosures |
None.
62
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
| Item 5A. | Quarterly Stock Price Information and Registered Stockholders |
Our common stock trades on The NASDAQ Global Market under the symbol “ALXA.” The following table sets forth, for the periods indicated, the high and low sales prices of our common stock.
| | | | | | | | |
2013 | | High | | | Low | |
First Quarter | | $ | 5.15 | | | $ | 4.03 | |
Second Quarter | | | 6.65 | | | | 4.15 | |
Third Quarter | | | 5.52 | | | | 4.16 | |
Fourth Quarter | | | 5.35 | | | | 4.12 | |
| | |
2012 | | High | | | Low | |
First Quarter | | $ | 9.90 | | | $ | 4.61 | |
Second Quarter | | | 7.60 | | | | 2.55 | |
Third Quarter | | | 5.15 | | | | 2.91 | |
Fourth Quarter | | | 6.46 | | | | 3.78 | |
As of December 31, 2013, there were 95 holders of record of our common stock. We have not paid cash dividends on our common stock since our inception, and we do not anticipate paying any in the foreseeable future.
Recent Sales of Unregistered Securities
None
63
Performance Graph
The following graph compares the cumulative 5-year total return provided to stockholders of Alexza Pharmaceuticals, Inc.’s common stock relative to the cumulative total returns of the NASDAQ Composite index and the NASDAQ Biotechnology index. An investment of $100 (with reinvestment of all dividends) is assumed to have been made in our common stock and in each of the indexes on December 31, 2008 and its relative performance is tracked through December 31, 2013.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Alexza Pharmaceuticals, Inc., the NASDAQ Composite Index,
and the NASDAQ Biotechnology Index
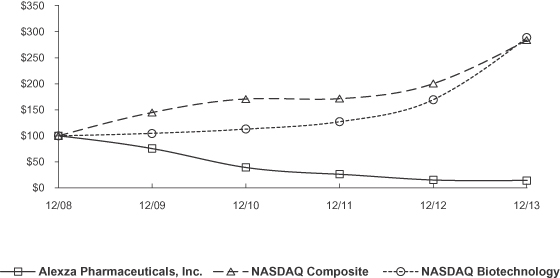
| * | $100 invested on 12/31/08 in stock or index, including reinvestment of dividends. |
Fiscal year ending December 31.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12/08 | | | 12/09 | | | 12/10 | | | 12/11 | | | 12/12 | | | 12/13 | |
Alexza Pharmaceuticals, Inc. | | | 100.00 | | | | 75.71 | | | | 39.43 | | | | 26.18 | | | | 15.62 | | | | 14.92 | |
NASDAQ Composite | | | 100.00 | | | | 144.88 | | | | 170.58 | | | | 171.30 | | | | 199.99 | | | | 283.39 | |
NASDAQ Biotechnology | | | 100.00 | | | | 104.67 | | | | 112.89 | | | | 127.04�� | | | | 169.50 | | | | 288.38 | |
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
| Item 5B. | Use of Proceeds from the Sale of Registered Securities |
None.
None.
64
| Item 6. | Selected Financial Data |
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (In thousands, except per share data) | |
Consolidated Statement of Comprehensive Loss Data: | | | | | | | | | | | | | | | | | | | | |
Revenue | | $ | 47,839 | | | $ | 4,070 | | | $ | 5,660 | | | $ | 42,876 | | | $ | 9,514 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Cost of goods sold | | | 11,209 | | | | — | | | | — | | | | — | | | | — | |
Research and development | | | 19,082 | | | | 21,849 | | | | 28,262 | | | | 33,528 | | | | 39,778 | |
General and administrative | | | 15,778 | | | | 11,093 | | | | 11,766 | | | | 14,000 | | | | 15,406 | |
Restructuring charges | | | — | | | | — | | | | — | | | | — | | | | 2,037 | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 46,069 | | | | 32,942 | | | | 40,028 | | | | 47,528 | | | | 57,221 | |
Gain/(loss) from operations | | | 1,770 | | | | (28,872 | ) | | | (34,368 | ) | | | (4,652 | ) | | | (47,707 | ) |
Change in fair value of contingent consideration liability | | | (39,913 | ) | | | 1,900 | | | | (4,000 | ) | | | 4,838 | | | | (7,983 | ) |
Interest income/(expense) and other income/(expense), net | | | (1,472 | ) | | | (1,006 | ) | | | (2,163 | ) | | | (1,667 | ) | | | (375 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net loss | | | (39,615 | ) | | | (27,978 | ) | | | (40,531 | ) | | | (1,481 | ) | | | (56,065 | ) |
Consideration paid in excess of carrying value of the noncontrolling interest in Symphony Allegro, Inc. | | | — | | | | — | | | | — | | | | — | | | | (61,566 | ) |
Loss attributed to noncontrolling interest in Symphony Allegro, Inc. | | | — | | | | — | | | | — | | | | — | | | | 13,987 | |
| | | | | | | | | | | | | | | | | | | | |
Net loss attributable to Alexza common stockholders | | $ | (39,615 | ) | | $ | (27,978 | ) | | $ | (40,531 | ) | | $ | (1,481 | ) | | $ | (103,644 | ) |
| | | | | | | | | | | | | | | | | | | | |
Basic and diluted net loss per share attributable to Alexza common stockholders | | $ | (2.38 | ) | | $ | (2.24 | ) | | $ | (5.97 | ) | | $ | (0.27 | ) | | $ | (26.84 | ) |
| | | | | | | | | | | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share attributable to Alexza common stockholders | | | 16,669 | | | | 12,472 | | | | 6,787 | | | | 5,542 | | | | 3,861 | |
| | | | | | | | | | | | | | | | | | | | |
Other Comprehensive Loss | | | | | | | | | | | | | | | | | | | | |
Change in unrealized income (loss) on marketable securities | | $ | 1 | | | $ | — | | | $ | (2 | ) | | $ | 3 | | | $ | (29 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | (39,614 | ) | | | (27,978 | ) | | | (40,533 | ) | | | (1,478 | ) | | | (56,094 | ) |
Comprehensive loss attributable to noncontrolling interest in Symphony Allegro, Inc. | | | — | | | | — | | | | — | | | | — | | | | 13,987 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive loss attributable to Alexza common stockholders | | $ | (39,614 | ) | | $ | (27,978 | ) | | $ | (40,533 | ) | | $ | (1,478 | ) | | $ | (42,107 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 | |
| | | (In thousands) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and marketable securities | | $ | 25,884 | | | $ | 17,715 | | | $ | 16,903 | | | $ | 41,449 | | | $ | 19,916 | |
Working capital (deficit) | | | 16,015 | | | | 4,900 | | | | (7,396 | ) | | | 8,031 | | | | (3,830 | ) |
Total assets | | | 47,072 | | | | 40,551 | | | | 48,605 | | | | 68,482 | | | | 46,174 | |
Noncurrent portion of financing obligations | | | 10,744 | | | | — | | | | — | | | | — | | | | — | |
Accumulated deficit | | | (374,228 | ) | | | (334,613 | ) | | | (306,635 | ) | | | (266,104 | ) | | | (264,623 | ) |
Total stockholders’ equity (deficit) | | | (23,975 | ) | | | 2,573 | | | | (9,692 | ) | | | 12,290 | | | | (7,126 | ) |
65
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements that are based upon current expectations. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential” or “continue” or the negative of these terms or other comparable terminology. Forward-looking statements involve risks and uncertainties. Our actual results and the timing of events could differ materially from those discussed in our forward-looking statements as a result of many factors, including those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K.
Overview
We are a pharmaceutical company focused on the research, development and commercialization of novel proprietary products for the acute treatment of central nervous system conditions. The Staccato® system, our proprietary technology, is the foundation for our first approved product ADASUVE®, orStaccato loxapine, and all of our product candidates. TheStaccatosystem vaporizes excipient-free drugs to form a condensation aerosol that, when inhaled, allows for rapid systemic drug delivery. Because of the particle size of the aerosol, the drug is quickly absorbed through the deep lung into the bloodstream, providing speed of therapeutic onset that is comparable to intravenous, or IV, administration but with greater ease, patient comfort and convenience.
ADASUVE has been developed for the treatment of agitation associated with schizophrenia or bipolar disorder and has been approved for marketing in the United States by the U.S. Food and Drug Administration, or FDA, and in the European Union, or EU, by the European Commission, or EC. In the United States and the EU, ADASUVE is approved for similar indications. It is approved with a different number of dose strengths and has different risk mitigation and management plans in the United States and the EU. ADASUVE is our only approved product.
We have one product candidate in active development: AZ-002(Staccato alprazolam), which is being developed for the treatment of acute repetitive seizures, sometimes called cluster seizures or ARS. We expect to initiate a Phase 2a proof-of-concept study in patients with ARS in the second quarter of 2014.
In addition to ADASUVE, our only product approved for marketing, and AZ-002, our only product candidate in active development, we have in the past allocated resources to the development of the following product candidates, none of which are currently in active development: (i) AZ-007 (Staccato zaleplon) for the treatment of insomnia in patients who have difficulties falling asleep, including patients who awake in the middle of the night and have difficulty falling back asleep, (ii) AZ-104 (Staccato loxapine, low-dose) for the treatment of patients suffering from acute migraine headaches, (iii) AZ-003 (Staccato fentanyl) for the treatment of patients with acute pain, including patients with breakthrough cancer pain and postoperative patients with acute pain episodes and (iv) Staccato nicotine which is designed to help smokers quit by addressing both the chemical and behavioral components of nicotine addiction by delivering nicotine replacement via inhalation.
We have retained all rights to theStaccato system and to our product candidates other than ADASUVE and AZ-104. For the U.S. market, we have licensed the exclusive rights to commercialize ADASUVE to Teva Pharmaceuticals USA, Inc., or Teva. For Europe, Latin America, Russia and the other Commonwealth of Independent States countries, or the Ferrer Territories, we have licensed the exclusive rights to commercialize ADASUVE to Grupo Ferrer Internacional S.A., or Ferrer. We intend to develop certain product candidates internally and to identify external resources or collaborators to develop and commercialize other product candidates.
We believe that, based on our cash, cash equivalents and marketable securities balance at December 31, 2013, remaining proceeds available under the Convertible Promissory Note and Agreement to Lend, dated as of May 7, 2013, between us and Teva, or the Teva Note, estimated product revenues and milestone payments associated with the sale of ADASUVE, the proceeds from our March 2014 royalty securitization financing and our current expected cash usage, we have sufficient capital resources to meet our anticipated cash needs for at least the next twelve months. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate, or to alter our operations.
66
AGITATION — ADASUVE
Commercialization Strategy Overview
Our global commercialization strategy for ADASUVE is to secure strategic collaborations to commercialize ADASUVE, while maintaining control and primary responsibility for commercial manufacturing. We currently have two commercialization collaborations. We are continuing to seek additional commercialization collaborations to commercialize ADASUVE in territories outside the United States and the Ferrer Territories.
In December 2012, the FDA approved our New Drug Application, or NDA, forStaccato loxapine, as ADASUVE (loxapine) Inhalation Powder 10 mg for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. We transferred the ADASUVE NDA and related regulatory documents to Teva in June 2013, and Teva has responsibility for post-approval commitments and conditions related to the U.S. ADASUVE approval. In March 2014, Teva announced the U.S. launch of ADASUVE.
In February 2013, the EC granted marketing authorization for ADASUVE in the EU. ADASUVE, 4.5 mg and 9.1 mg inhalation powder loxapine, pre-dispensed, is authorized in the EU for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. The ADASUVE marketing authorization requires that patients receive regular treatment immediately after control of acute agitation symptoms, and that ADASUVE is administered only in a hospital setting under the supervision of a healthcare professional. The ADASUVE marketing authorization also states that a short-acting beta-agonist bronchodilator treatment should be available for treatment of possible severe respiratory side-effects, such as bronchospasm. The EC granted the marketing authorization for ADASUVE on the basis of the positive opinion issued by the European Medicines Agency, or EMA, in December 2012 and is valid in all 28 EU Member States, plus Iceland, Liechtenstein and Norway.
We are, and expect to continue to be, responsible for the commercial manufacturing of ADASUVE in our facility in Mountain View, California for commercialization in the United States, the Ferrer Territories and in any potential future territories. This facility has been inspected by the U.S. and EU authorities and operates under applicable U.S. and EU regulations. Two of these commitments have been completed.
We began manufacturing commercial quantities of ADASUVE in the second quarter of 2013 and will continue to manufacture commercial product for Ferrer and Teva. Shipments for 2013 consisted of the following:
| | | | | | | | | | | | | | | | |
| | | Q2 2013 | | | Q3 2013 | | | Q4 2013 | | | Total | |
EU Units | | | 13,370 | | | | 14,405 | | | | 11,863 | | | | 39,638 | |
U.S. Units | | | — | | | | — | | | | 9,307 | | | | 9,307 | |
| | | | | | | | | | | | | | | | |
Total Units | | | 13,370 | | | | 14,405 | | | | 21,170 | | | | 48,945 | |
| | | | | | | | | | | | | | | | |
Because our current license and supply agreements provide our collaborators with an acceptance period during which they may reject any product that does not conform to agreed-upon specifications, revenue for units shipped may be recognized in a period subsequent to the period in which the units are shipped.
Financing update
In May 2010 we entered into a Loan and Security Agreement, or Loan Agreement, with Hercules Technology Growth Capital, Inc., or Hercules. Under the terms of the Loan Agreement, we borrowed $15.0 million at an interest rate of the higher of (i) 10.75% or (ii) 6.5% plus the prime rate as reported in the Wall Street Journal, with a maximum interest rate of 14%. We made interest only payments through February 2011, following which we repaid the loan in full over 33 equal monthly installments. In conjunction with the Loan Agreement, we issued to Hercules a five-year warrant to purchase 37,639 shares of our common stock at a price of $26.90 per share. The warrant is fully exercisable, expires in May 2015 and remains outstanding.
In August 2010 we entered into a license and development agreement, or the Cypress Agreement, with Cypress Bioscience, Inc., or Cypress, forStaccato nicotine. According to the terms of the Cypress Agreement, Cypress paid us a non-refundable upfront payment of $5.0 million to acquire the worldwide license for the
67
Staccato nicotine technology. On December 31, 2013, the Cypress Agreement automatically terminated by its terms, and all rights to theStaccato nicotine technology reverted back to us.
On July 20, 2012, we entered into a committed equity line of credit with Azimuth Opportunity, L.P., or Azimuth, pursuant to which we were granted the ability to sell up to $20.0 million of our common stock over an approximately 24-month period pursuant to the terms of a Common Stock Purchase Agreement, or the Purchase Agreement, at a discount of 5% to the volume-weighted average price of our common stock over a preceding period of trading days. In addition to the foregoing amounts, in consideration for Azimuth’s execution and delivery of the Purchase Agreement, we issued to Azimuth 80,429 shares of our common stock on July 23, 2012. In August and September 2012, we utilized $13.6 million under the facility by issuing 3,489,860 shares of our common stock to Azimuth at an average price of $3.88 per share, which resulted in $13.4 million in net proceeds. In May 2013, we utilized the remaining $6.4 million available under the facility by issuing 1,437,481 shares of our common stock to Azimuth at an average price of $4.48 per share, which resulted in $6.3 million of net proceeds to us. Shares sold under this facility were sold pursuant to a shelf registration statement declared effective by the Securities and Exchange Commission, or SEC, on July 3, 2012.
In February 2012, we issued an aggregate of 4,400,000 shares of our common stock and warrants to purchase up to an additional 4,400,000 shares of our common stock in an underwritten public offering. Net proceeds from the offering were approximately $20.4 million, after deducting offering expenses. The warrants are exercisable at $5.00 per share and will expire on February 23, 2017. The shares of common stock and warrants were sold pursuant to a shelf registration statement declared effective by the SEC on May 20, 2010. We agreed to customary obligations, including indemnification.
In May 2013, we entered into a License and Supply Agreement with Teva, or the Teva Agreement. Concurrent with entering into the Teva Agreement, we entered into the Teva Note. Under the terms of the Teva Note, we may, upon written notice to Teva, draw upon the Teva Note to fund agreed operating budgets related to ADASUVE. The aggregate draw-downs may total up to $25.0 million and will be due and payable on the fifth anniversary of the signing of the Teva Note. We may prepay, from time to time, up to one-half of the total amounts advanced plus the related interest outstanding at any time prior to the maturity date. At the maturity date, Teva will have the right to convert the then outstanding amounts into shares of Alexza common stock at a conversion price of $4.4833 per share. The Teva Note bears simple interest of 4% per year. The effective interest rate of the Teva Note, which takes into consideration the beneficial conversion feature and the right-to-borrow discounts, is 13.9%. We have two years from the effective date of the Teva Note to receive advances. In 2013, we drew $15.0 million against the Teva Note, in March 2014, we drew an additional $5.0 million against the Teva Note and we anticipate drawing the remaining amount.
In March 2014, we completed a royalty securitization financing, which consisted of a private placement to qualified institutional buyers and accredited institutional investors of $45.0 million of non-recourse notes issued by our wholly-owned subsidiary, or the Notes, and warrants to purchase 345,661 shares of our common stock at a price of $0.01 per share exercisable for five years from the date of issuance. The Notes bear interest at 12.25% per annum payable quarterly beginning June 15, 2014. All royalty and milestone payments under the Teva Agreement, after paying interest, administrative fees, and any applicable taxes, will be applied to principal and interest payments on the Notes until the Notes have been paid in full. From the proceeds of the transaction, we established a $6.9 million interest reserve account to cover potential shortfall in interest payments. Any remaining amounts in the interest reserve account will be released to us commencing June 15, 2015, subject to meeting certain net sales targets for ADASUVE, or, if not previously released, on or after December 15, 2015, irrespective of ADASUVE net sales. All other payments of principal and interest on the Notes will be made from royalty revenues from sales in the U.S. of ADASUVE, as well as potential U.S. commercialization and regulatory milestone payments due to Alexza. The Notes are secured by the right to receive royalty and milestone payments under the Teva Agreement and our equity ownership in the wholly-owned subsidiary. The Notes have no other recourse to us. The Notes may not be redeemed at our option until after March 18, 2016, and may be redeemed after that date subject to the achievement of certain milestones and the payment of a redemption premium for any redemption occurring prior to March 19, 2019. The Notes are not convertible into Alexza equity, nor have we guaranteed them. After fees and expenses, the net proceeds to us were approximately $41 million before the establishment of the interest reserve account.
68
Critical Accounting Estimates and Judgments
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments related to development costs. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making assumptions about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 of the notes to consolidated financial statements, we believe the following accounting policies are critical to the process of making significant estimates and judgments in preparation of our financial statements.
Revenue Recognition
We recognize revenue when (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred or services have been rendered; (iii) the fee is fixed or determinable; and (iv) collectability is reasonably assured. Prior to the second quarter of 2013, our revenue consisted primarily of amounts earned from collaboration agreements and under research grants with the National Institutes of Health. Beginning in the second quarter of 2013, we also have revenue from product sales.
For collaboration agreements, revenues for non-refundable upfront license fee payments, where we continue to have performance obligations, are recognized as performance occurs and obligations are completed. Revenues for non-refundable upfront license fee payments where we do not have significant future performance obligations are recognized when the agreement is signed and the payments are due.
For multiple element arrangements, such as collaboration agreements in which a collaborator may purchase several deliverables, we account for each deliverable as a separate unit of accounting if both of the following criteria are met: (i) the delivered item or items have value to the customer on a standalone basis; and (ii) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We evaluate how the consideration should be allocated among the units of accounting and allocate revenue to each non-contingent element based upon the relative selling price of each element. We determine the relative selling price for each deliverable using (i) vendor-specific objective evidence, or VSOE, of selling price if it exists; (ii) third-party evidence, or TPE, of selling price if it exists; or (iii) our best estimated selling price for that deliverable if neither VSOE nor TPE of selling price exists for that deliverable. We then recognize the revenue allocated to each element when the four basic revenue criteria described above are met for each element.
For milestone payments received in connection with our collaboration agreements, we have elected to adopt the milestone method of accounting under Financial Accounting Standards Board Accounting Standards Codification 605-28,Milestone Method. Under the milestone method, revenues for payments which meet the definition of a milestone will be recognized as the respective milestones are achieved.
We recognize product revenue as follows:
| | • | | Persuasive Evidence of an Arrangement. We currently sell product through license and supply agreements with our collaborators, Ferrer and Teva. Persuasive evidence of an arrangement is generally determined by the receipt of an approved purchase order from the collaborator in connection with the terms of the license and supply agreements. |
| | • | | Delivery. Typically, ownership of the product passes to the collaborator upon shipment. Our current license and supply agreements also provide our collaborators with an acceptance period during which they may reject any product which does not conform to agreed-upon specifications. Because ADASUVE is a new product, a new technology and our first product to be commercialized, and because we do not have a history of producing product to collaborator specifications, we will not consider delivery to have |
69
| | occurred until after the collaborator acceptance period has ended or the collaborator has positively accepted the product. Once we have demonstrated over the course of time an ability to reliably produce the product to collaborator specifications, we will consider delivery to have occurred upon shipment in the absence of any other relevant shipment or acceptance terms. |
| | • | | Sales Price Fixed or Determinable. Sales prices for product shipments are determined by the license and supply agreements and documented in the purchase orders. After the collaborator acceptance period has ended or the collaborator has positively accepted the product, our collaborators do not have any product return or replacement rights, including for expired products. |
| | • | | Collectability. Payment for the product is contractually obligated under the license and supply agreements. We will monitor payment histories for our collaborators and specific issues as they arise to determine whether collection is probable for a specific transaction and defer revenue as necessary. |
Royalty revenue from our collaboration agreements will be recognized as we receive information from our collaborators regarding product sales.
Significant management judgment is used in the determination of revenue to be recognized and the period in which it is recognized.
Inventory
Inventory is stated at standard cost, which approximates actual cost, determined on a first-in first-out basis, not in excess of market value. Inventory includes the direct costs incurred to manufacture products combined with allocated manufacturing overhead, which consists of indirect costs, including labor and facility overhead. We are in the early stages of commercialization and have incurred significantly higher than normal indirect costs in the production of our inventory due to start-up manufacturing costs and low production volumes and expect to continue to incur higher than normal indirect costs until we get closer to our normal manufacturing capacity. The carrying cost of inventory is reduced so as to not be in excess of market value of the inventory as determined by the contractual transfer prices to Ferrer and Teva. The excess over the market value is written off to cost of goods sold. All material purchases and costs associated with the manufacturing process incurred prior to the approval of ADASUVE in December 2012 were expensed as a component of research and development expense.
Preclinical Study and Clinical Trial Accruals
We estimate our preclinical study and clinical trial expenses based on our estimates of the services received pursuant to contracts with multiple research institutions and clinical research organizations that conduct and manage preclinical studies and clinical trials on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Preclinical study and clinical trial expenses include the following:
| | • | | fees paid to contract research organizations in connection with preclinical studies; |
| | • | | fees paid to contract research organizations and other clinical sites in connection with clinical trials; and |
| | • | | fees paid to contract manufacturers in connection with the production of components and drug materials for preclinical studies and clinical trials. |
We record accruals for these preclinical study and clinical trial costs based upon the estimated amount of work completed. All such costs are charged to research and development expenses based on these estimates. Costs related to patient enrollment in clinical trials are accrued as patients are entered in the trial. We monitor patient enrollment levels and related activities to the extent possible through internal reviews, correspondence and discussions with research institutions and clinical research organizations. However, if we have incomplete or inaccurate information, we may underestimate or overestimate activity levels associated with various preclinical studies and clinical trials at a given point in time. In this event, we could record significant research and development expenses in future periods when the actual activity level becomes known. To date, we have not made any material adjustments to our estimates of preclinical study and clinical trial costs. We make good faith estimates which we believe to be accurate, but the actual costs and timing of clinical trials are highly uncertain, subject to risk and may change depending upon a number of factors, including our clinical development plan.
70
Share-Based Compensation
We currently use the Black-Scholes option pricing model to determine the fair value of stock options and purchase rights issued under our 2005 Employee Stock Purchase Plan. The determination of the fair value of share-based payment awards on the date of grant using an option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rates and expected dividends.
The estimated fair value of restricted stock unit awards is calculated based on the market price of our common stock on the date of grant, reduced by the present value of dividends expected to be paid on our common stock prior to vesting of the restricted stock unit. Our current estimate assumes no dividends will be paid prior to the vesting of the restricted stock unit.
We estimate the expected term of options based on the historical term periods of options that have been granted but are no longer outstanding and the estimated terms of outstanding options. We estimate the volatility of our stock based on our actual historical volatility since our initial public offering. We base the risk-free interest rate that we use in the option pricing model on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options. We do not anticipate paying any cash dividends in the foreseeable future and therefore use an expected dividend yield of zero in the option pricing model.
We are required to estimate forfeitures at the time of grant and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. We use historical data to estimate pre-vesting option forfeitures and record share-based compensation expense only for those awards that are expected to vest. All share-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods.
If factors change and we employ different assumptions for estimating share-based compensation expense in future periods or if we decide to use a different valuation model, the expenses in future periods may differ significantly from what we have recorded in the current period and could materially affect our operating loss, net loss and net loss per share.
Contingent Consideration Liability
In August 2009, we completed our purchase of all of the outstanding equity of Symphony Allegro Inc., or Allegro, and in exchange we: (i) issued to the former stockholders of Allegro 1,000,000 shares of our common stock; (ii) issued to the former stockholders of Allegro 5-year warrants to purchase 500,000 shares of our common stock with an exercise price of $22.60 per share; and (iii) will pay to the former stockholders of Allegro certain percentages of cash payments that may be generated from future collaboration transactions pertaining to ADASUVE/AZ-104 (Staccatoloxapine) or AZ-002 (Staccatoalprazolam).
We estimate the fair value of the liability associated with the contingent cash payments to the former stockholders of Allegro, or contingent consideration liability, on a quarterly basis using a probability-weighted discounted cash flow model. We derive multiple cash flow scenarios for each of the product candidates subject to the cash payments and apply a probability to each of the scenarios. These probability and risk adjusted weighted average cash flows are then discounted utilizing our estimated weighted average cost of capital, or WACC. Our WACC considers our cash position, competition, risk of substitute products, and risk associated with the financing of the development projects. We determined the initial discount rate to be 18% and applied this rate to the probability adjusted cash flow scenarios and, in 2013, reduced the discount rate to 16.5% to reflect our reduced WACC.
We record any changes in the fair value of the contingent consideration liability in earnings in the period of the change. Certain events including, but not limited to, clinical trial results, FDA approval or non-approval of our submissions, the timing and terms of any strategic collaboration agreement, the commercial success of ADASUVE, AZ-104 or AZ-002 and the discount rate assumption could have a material impact on the fair value of the contingent consideration liability, and as a result, our results of operations and financial position.
71
Results of Operations
Comparison of Years Ended December 31, 2013, 2012 and 2011
Revenue
Revenue for the years ended December 31, 2013, 2012 and 2011 consisted of the following (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Licensing revenue | | $ | 42,800 | | | $ | — | | | $ | — | |
Amortization of deferred revenue | | | 2,915 | | | | 4,070 | | | | 5,660 | |
Milestone revenue | | | 1,250 | | | | — | | | | — | |
Product revenue | | | 874 | | | | — | | | | — | |
| | | | | | | | | | | | |
Total revenue | | $ | 47,839 | | | $ | 4,070 | | | $ | 5,660 | |
| | | | | | | | | | | | |
Licensing revenue in 2013 consists of the $40.0 million upfront payment and the fair value of the right-to-borrow asset we received from Teva upon signing of the Teva Agreement in May 2013.
Amortization of deferred revenues in 2013 includes the amortization of upfront payments received from our Collaboration, License and Supply Agreement with Ferrer, or the Ferrer Agreement. Amortization of deferred revenue in 2012 and 2011 includes the amortization of upfront payments from both our Ferrer Agreement and the Cypress Agreement, which terminated automatically as of December 31, 2013.
Milestone revenue in 2013 resulted from milestone payment associated with the first commercial sale in Germany under the Ferrer Agreement.
We began shipping commercial units and recognizing revenue on ADASUVE product shipments in the second quarter of 2013.
In 2014, we expect product revenue to increase over 2013 levels due to the continued rollout of ADASUVE in Europe and the commercialization of ADASUVE in the United States and our consequent receipt of royalties. We also expect to recognize milestone payments from Ferrer for the first commercial sale in specified countries in the Ferrer Territories.
Cost of Goods Sold
Costs of goods sold include the direct costs incurred to manufacture products sold combined with allocated manufacturing overhead, which consists of indirect costs, including labor and facility overhead. We are in the early stages of commercialization and have incurred significantly higher than normal indirect costs in the production of our inventory due to manufacturing start-up costs and low production volumes and expect to continue to incur higher than normal indirect costs until we get closer to our normal manufacturing capacity.
The carrying cost of inventory is reduced so as to not be in excess of market value as determined by the contractual transfer prices to Ferrer and Teva. These amounts are expensed to cost of goods sold. All costs associated with the manufacturing process incurred prior to the first commercial product produced in the second quarter of 2013 were expensed as a component of research and development expense.
Cost of goods sold for 2013 consist mainly of start-up and excess manufacturing costs during the period in addition to the cost of the units shipped. We expect our costs of goods sold to decrease as a percentage of product sales in 2014 because we anticipate an increase in the number of units shipped to Ferrer and Teva. However, we will continue to incur high unabsorbed production costs until we achieve higher consistent production levels.
Research and Development Expenses
Research and development expenses are identified as costs directly attributable to the development of our product and product candidates or as general research. Direct costs consist of personnel costs directly associated
72
with a candidate, preclinical study costs, clinical trial costs, related clinical drug and device development and manufacturing costs, contract services and other research expenditures. Overhead, facility costs and other support service expenses are allocated to each product or candidate or to general research, and the allocation is based on employee time spent on each program.
The following table allocates our expenditures between product and product candidate costs or general research, based on our internal records and estimated allocations of employee time and related expenses (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Product candidate expenses | | $ | 12,432 | | | $ | 18,216 | | | $ | 25,686 | |
General research | | | 6,650 | | | | 3,633 | | | | 2,576 | |
| | | | | | | | | | | | |
Total research and development | | $ | 19,082 | | | $ | 21,849 | | | $ | 28,262 | |
| | | | | | | | | | | | |
The decrease in research and development expenses in 2013 as compared to 2012 is primarily due to expenses related to quality and manufacturing being classified as cost of goods sold or inventory in 2013, partially offset by additional expenses associated with our post-approval commitments related to the Marketing Authorization Application, or MAA, approval. The decrease in 2012 as compared to 2011 was a result of the suspension of development of our AZ-007 andStaccato nicotine product candidates, in 2011 and the first quarter of 2012, respectively, as well as our efforts to conserve cash resources, including a 38% reduction in our workforce in February 2012. These reductions were partially offset by approximately $1.1 million and $0.9 million of cash bonuses and share-based compensation expense incurred in 2012 that were not incurred in 2011, as a result of our meeting certain corporate goals in 2012.
In 2014, we expect our research and development expenses to be higher than in 2013 due to anticipated clinical expenses related to development of AZ-002, additional pre-clinical studies and post-approval commitments related to the MAA approval.
General and Administrative Expenses.
General and administrative expenses were $15.8 million, $11.1 million and $11.8 million for the years ended December 31, 2013, 2012 and 2011, respectively. General and administrative expenses consist principally of salaries and related personnel costs, including share-based compensation, in executive, finance, accounting, business development, legal and human resources functions. Other general and administrative expenses include facility and information technology costs not otherwise included in research and development expenses, patent related costs and professional fees for legal, consulting and accounting services.
The increase in 2013 as compared to 2012 was primarily due to pre-commercialization efforts such as market research, including pricing and market segmentation studies, during 2013, and an increase in headcount and external expenses to support the increased operational activity as a result of the approval of the ADASUVE NDA in December 2012. In 2012, we recognized a non-cash contra expense of $1.4 million related to the termination of our lease and associated subleases of one of our Mountain View facilities. The non-cash contra expense was a result of the reversal of deferred rent liability, net of the accelerated depreciation of the fixed assets, related to the facility.
The decrease in 2012 expenses as compared to 2011 was primarily due to our efforts to conserve cash balance as well as a one-time non-cash contra expense. Our cash conservation efforts included the workforce reduction discussed above and the non-cash contra expense of $1.4 million discussed above. These were partially offset by approximately $0.9 million and $1.0 million of cash bonuses and share-based compensation expense in 2012 that were not incurred in 2011, as a result of our meeting certain corporate goals in 2012.
We expect our general and administrative expenses to increase significantly in 2014 as we increase our infrastructure to support the continued commercial rollout of ADASUVE.
73
Change in the Fair Value of Contingent Consideration Liability.
In connection with our acquisition of all of the outstanding equity of Allegro in the third quarter of 2009, we are obligated to pay the former stockholders of Allegro certain percentages of cash receipts that may be generated from future collaboration transactions for ADASUVE, AZ-104 and/or AZ-002. We measure the fair value of this contingent consideration liability on a recurring basis. Any changes in the fair value of this contingent consideration liability are recognized in earnings in the period of the change. Certain events, including, but not limited to, the timing and terms of a collaboration, clinical trial results, regulatory approval or nonapproval of our submissions, and the commercial success of ADASUVE, AZ-104, and/or AZ-002, could have a material impact on the fair value of the contingent consideration liability, and as a result, our results of operations.
In 2013, we updated the contingent liability fair value model primarily to reflect the impact of the terms of the Teva Agreement, specifically the probability that ADASUVE would be commercialized by a third party. Had we commercialized ADASUVE internally, we would not have been required to pay the former stockholders of Allegro percentages of revenues earned. In addition, we reduced the discount rate used to compute the fair value of the contingent consideration liability from 18.0% to 16.5% to reflect the reduction in our estimated weighted average cost of capital. These changes resulted in non-operating, non-cash losses of $39.9 million in 2013.
In 2012, we increased the probability that we would internally commercialize ADASUVE in the United States rather than enter into a commercial collaboration that would result in upfront milestone and royalty payments. This change in assumption was partially offset by the approval of the ADASUVE NDA in December 2012 and the December 2012 positive opinion of the EMA Committee for Medicinal Products for Human recommending that ADASUVE be granted European Union centralized marketing authorization by the EC. Marketing authorization for ADASUVE was granted by the EC on February 20, 2013. These changes resulted in non-operating, non-cash gains of $1.9 million in 2012.
In October 2011, we entered into the Ferrer Agreement. As a result of this agreement, we revised upwards the probabilities, amounts and timing of certain cash flows for ADASUVE. We recognized a non-operating loss of $4.0 million to reflect the increase in the contingent consideration liability during the year ended December 31, 2011.
Interest and Other Income, Net.
Interest and other income, net, primarily represents income earned on our cash, cash equivalents, marketable securities balances, and restricted cash. Interest and other income, net was $26,000, $420,000 and $26,000 for the years ended December 31, 2013, 2012 and 2011, respectively. In 2012, the amount primarily consisted of gains on the retirement of fixed assets as we sold certain equipment no longer being utilized. We expect interest income to remain nominal through 2014 because we expect the low interest rate environment to continue and because we expect to focus on investing with a priority on liquidity and capital preservation.
Interest Expense.
Interest expense, which consists of interest on our financing obligations and in 2013 also included amortization of the right-to-borrow asset received in connection with the Teva Note, was $1.5 million, $1.4 million and $2.2 million in the years ended December 31, 2013, 2012 and 2011, respectively. The increase in 2013 as compared to 2012 was due to the drawdowns against the Teva Note, partially offset by the declining balances from the Hercules note. The decrease in 2012 as compared to 2011 was due to the declining debt balances as a result of our monthly payments. We expect interest expense to increase in 2014 due to the interest related to the $15.0 million of drawdowns on the Teva Note in 2013, the $5.0 million drawdown on the Teva Note in March 2014, future anticipated advances on the Teva Note, the amortization of the right-to-borrow asset and the beneficial conversion feature related to the Teva Note and the March 2014 royalty securitization financing.
Liquidity and Capital Resources
Since inception, we have financed our operations primarily through private placements and public offerings of equity securities, debt financings, revenues primarily from licensing agreements and government grants, and
74
payments from Allegro. We have received additional funding from interest earned on investments, as described below, and funds received upon exercises of stock options and exercises of purchase rights under our 2005 Employee Stock Purchase Plan. As of December 31, 2013, we had $25.9 million in cash, cash equivalents and marketable securities. Our cash and cash equivalents balances are held in money market accounts, investment grade commercial paper and government-backed securities. Cash in excess of immediate requirements is invested with regard to liquidity, capital preservation and yield.
Net cash used in operating activities was $(0.6) million, $(18.2) million and $(34.4) million in 2013, 2012 and 2011, respectively. The net cash used in each of these periods primarily reflects net loss for these periods, offset in part by depreciation, non-cash share-based compensation, the change in fair value of the contingent consideration liability, and non-cash changes in operating assets and liabilities. The other liabilities change in each year was related to the amortization of our deferred rent liability, which is a result of the straight line method of recognizing our facility rent expense and the recognition of tenant improvement reimbursements from the landlord as a deferred rent liability. In 2013, we received a payment of $40.0 million under the Teva Agreements. In 2012, we received a payment of $10.0 million under the Ferrer Agreement resulting in the decrease in accounts receivables. In 2012, the decrease in our deferred revenues was the result of the amortization of upfront payments on the Ferrer Agreement and the Cypress Agreement. In 2011, the Ferrer Agreement resulted in increases to other receivables and deferred revenues.
Net cash provided by (used in) investing activities was $(10.3) million, $2.0 million and $25.1 million in 2013, 2012 and 2011, respectively. Investing activities consist primarily of purchases and maturities of marketable securities and capital purchases. In 2013, we had purchases, net of maturities, of marketable securities of $8.6 million. During 2012 and 2011 we had maturities, net of purchases, of marketable securities of $2.0 million and $25.6 million, respectively. Purchases of property and equipment were $1.8 million, $0.5 million and $0.5 million in 2013, 2012 and 2011, respectively.
Net cash provided by financing activities was $10.5 million, $19.1 million and $10.6 million in 2013, 2012 and 2011, respectively. Financing activities consist primarily of proceeds from financing arrangements, the sale of our common stock and warrants to purchase common stock and financing obligations. In 2013, we received proceeds of $15.0 million from drawdown on the Teva Note. In 2013, 2012 and 2011, we received net proceeds from the issuance of common stock and warrants to purchase common stock of $6.3 million, $35.2 million and $16.1 million, respectively. In 2013, 2012 and 2011, payments on financing obligations were $5.8 million, $6.1 million and $5.6 million, respectively. In 2013 and 2012, we made payments of $10.3 million and $5.0 million, respectively, to the former stockholders of Allegro as a result of the receipt of the $40.0 million upfront fee from Teva and a $1.3 million in a milestone payment from Ferrer in 2013 and the receipt of the $10.0 million upfront fee in accordance with the Ferrer Agreement in 2012. Cash from financing activities in 2013 and 2012 was also impacted by the classifying of $5.1 million of cash as restricted cash in 2012 due to Hercules’ requirement that we establish a restricted access account equal to the principal balance of the Hercules debt and the reversal of that restricted cash in 2013 as Hercules removed the restricted access account requirement.
We believe that with current cash, cash equivalents and marketable securities balances, borrowing available under the Teva Note, estimated product revenues and milestone payments associated with the sale of ADASUVE, the proceeds from our March 2014 royalty securitization financing, and our current expected cash usage, we have sufficient capital resources to meet our anticipated cash needs, at our current cost levels for at least the next twelve months. Changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate or to alter our operations. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect. The key assumptions underlying these estimates include:
| | • | | expenditures related to the ADASUVE post-approval commitments to both the FDA and EC during this period being within budgeted levels; |
| | • | | expenditures related to ADASUVE commercial manufacturing during this period being within budgeted levels; |
| | • | | no unbudgeted growth in the number of our employees during this period; and |
| | • | | no material shortfall in our budgeted revenues. |
75
Our forecast of the period of time that our financial resources will be adequate to support operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed in “Risk Factors.” In light of the numerous risks and uncertainties associated with the commercialization of ADASUVE, the commercial manufacturing of ADASUVE, the development of our product candidates and the extent to which we enter into additional strategic collaborations with third parties to participate in development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials. Our future funding requirements will depend on many factors, including:
| | • | | the cost and timing of the development of our commercialization abilities; |
| | • | | the commercial success of ADASUVE or any other product candidates that are approved for marketing; |
| | • | | the cost, timing and outcomes of regulatory approvals or non-approvals; |
| | • | | the scope, rate of progress, results and costs of our preclinical studies, clinical trials and other research and development activities; |
| | • | | the terms and timing of any additional distribution, strategic collaboration or licensing agreements that we may establish; |
| | • | | the number and characteristics of product candidates that we pursue; |
| | • | | the cost of manufacturing ADASUVE in commercial quantities; |
| | • | | the cost of establishing clinical supplies of our product candidates; |
| | • | | the cost of preparing, filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| | • | | the extent to which we acquire or invest in businesses, products or technologies, although we currently have no commitments or agreements relating to any of these types of transactions. |
We will need to raise additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all. If we are unable to raise additional funds when needed, we may not be able to successfully commercialize ADASUVE, perform our post-approval commitments to the FDA and EC, manufacture commercial quantities of ADASUVE or continue development of our product candidates or we could be required to delay, scale back or eliminate some or all of our development programs, or other operations. We may seek to raise additional funds through public or private financing, strategic collaborations or other arrangements. Any additional equity financing may be dilutive to stockholders and debt financing, if available, may involve restrictive covenants. Applicable listing standards may affect our ability to consummate certain types of offerings of our securities in the future. Our February 2012 underwritten public offering involved the sale of 4,400,000 shares of our common stock and warrants to purchase an additional 4,400,000 shares of our common stock. Our March 2014 royalty securitization financing involved the issuance of warrants to purchase 345,661 shares of our common stock and requires interest payments by our wholly-owned subsidiary without recourse to us beginning in June 2014. If we raise funds through additional collaborative or licensing arrangements, we may be required to relinquish, on terms that are not favorable to us, rights to some of our technologies or product candidates that we would otherwise seek to develop or commercialize ourselves. Our failure to raise capital when needed may harm our business, financial condition, results of operations, and prospects.
Contractual Obligations
Facility Lease
We lease a 64,104 square foot manufacturing, office and laboratory facility in Mountain View, California, which we began to occupy in the fourth quarter of 2007. The lease expires on March 31, 2018, and we have two options to extend the lease for five years each. We believe that the Mountain View facility is sufficient for our office, manufacturing and laboratory needs for at least the next three years.
76
Autoliv
In November 2007, we entered into a manufacturing and supply agreement, or the Manufacture Agreement, with Autoliv relating to the commercial supply of chemical heat packages that can be incorporated into ourStaccato device. Autoliv had developed these chemical heat packages for us pursuant to a development agreement between Autoliv and us executed in October 2005.
In June 2010 and February 2011, we entered into agreements to amend the terms of the Manufacture Agreement, or the Amendments. Under the terms of the first of the Amendments, we paid Autoliv $4.0 million and issued Autoliv a $4.0 million unsecured promissory note in return for a production line for the commercial manufacture of chemical heat packages. A production line is comprised of two identical and self-sustaining “cells,” and the first such cell has been completed, installed and qualified. Under the terms of the second of the Amendments, the original $4.0 million note was cancelled and a new unsecured promissory note was issued with a reduced principal amount of $2.8 million, or the Second Note, and production on the second cell halted. The Second Note is payable in 48 equal monthly installments of approximately $68,000 and the last payment is scheduled for December 2014.
In the event that we request completion of the second cell of the first production line for the commercial manufacture of chemical heat packages, Autoliv will complete, install and fully qualify such second cell for a cost to us of $1.2 million and Autoliv will transfer ownership of such cell to us upon the payment in full of such $1.2 million and the Second Note. At our request, Autoliv will manufacture up to two additional production lines for the commercial manufacture of chemical heat packages at a cost not to exceed $2.4 million for each additional line.
We will pay Autoliv a specified purchase price, which varies based on annual quantities ordered by us, per chemical heat package delivered. The initial term of the Manufacture Agreement expired on December 31, 2012, at which time the Manufacture Agreement renewed for a five-year term, and will continue to automatically renew for successive five-year renewal terms unless we or Autoliv notify the other party no less than 36 months prior to the end of the then-current renewal term that such party wishes to terminate the Manufacture Agreement.
In October 2013, we received from Autoliv notification of their intent to terminate, effective October 2016, the Manufacturing Agreement. At such time, we will retain full ownership of the production lines for commercial manufacture of chemical heat packages developed for us by Autoliv, and Autoliv’s obligations under the Manufacturing Agreement will terminate in full. Prior to October 2016 we and Autoliv will remain fully obligated to perform pursuant to the terms of the Manufacture Agreement.
Our scheduled future minimum contractual payments, net of sublease income, including interest at December 31, 2013, are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | Total | | | Less Than
1 Year | | | 1-3 Years | | | 3-5 Years | | | Thereafter | |
Operating lease obligations | | $ | 14,226 | | | $ | 3,502 | | | $ | 6,485 | | | $ | 4,239 | | | $ | — | |
Term note obligations | | | 18,540 | | | | 815 | | | | — | | | | 17,725 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
| | $ | 32,766 | | | $ | 4,317 | | | $ | 6,485 | | | $ | 21,964 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
As part of our purchase of all of the outstanding equity of Allegro in August 2009, we agreed to pay to the former stockholders of Allegro certain percentages of cash payments that may be generated from future collaboration transactions pertaining to ADASUVE/AZ-104 (Staccatoloxapine) or AZ-002 (Staccatoalprazolam). In 2013, we made payments to the former stockholders of Allegro of $10.0 million as a result of the $40.0 million upfront payment we received from Teva and $0.3 million as a result of a $1.25 million milestone payment received from Ferrer. In January 2012, we made a payment to the former stockholders of Allegro of $5.0 million as a result of the $10.0 million upfront payment we received from Ferrer.
Off-Balance Sheet Arrangements
None.
77
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Our exposure to market risk is confined to our cash, cash equivalents, which have maturities of less than three months, and marketable securities. The primary objective of our investment activities is to preserve our capital to fund operations. We also seek to maximize income from our investments without assuming significant risk. To achieve our objectives, we maintain a portfolio of cash equivalents and marketable securities in a variety of securities of high credit quality. As of December 31, 2013, we had cash, cash equivalents, marketable securities and restricted cash of $25.9 million. The securities in our investment portfolio are not leveraged, are classified as available for sale and are, due to their very short-term nature, subject to minimal interest rate risk. We currently do not hedge interest rate exposure. Because of the short-term maturities of our investments, we do not believe that an increase in market rates would have a material negative impact on the realized value of our investment portfolio. We actively monitor changes in interest rates. We perform quarterly reviews of our investment portfolio and believe we have no exposure related to: mortgage and other asset backed securities, European sovereign debt, or auction rate securities.
78
| Item 8. | Financial Statements and Supplementary Data |
ALEXZA PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
79
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Alexza Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Alexza Pharmaceuticals, Inc. (the “Company”) as of December 31, 2013 and 2012, and the related consolidated statements of loss and comprehensive loss, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2013. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Alexza Pharmaceuticals, Inc. at December 31, 2013 and 2012, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Redwood City, California
March 25, 2014
80
ALEXZA PHARMACEUTICALS, INC
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
| ASSETS | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 17,306 | | | $ | 17,715 | |
Marketable securities | | | 8,578 | | | | — | |
Restricted cash | | | — | | | | 5,051 | |
Receivables | | | 129 | | | | — | |
Inventory | | | 3,447 | | | | — | |
Prepaid expenses and other current assets | | | 1,453 | | | | 852 | |
| | | | | | | | |
Total current assets | | | 30,913 | | | | 23,618 | |
Property and equipment, net | | | 14,991 | | | | 16,531 | |
Other assets | | | 1,168 | | | | 402 | |
| | | | | | | | |
Total assets | | $ | 47,072 | | | $ | 40,551 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 3,789 | | | $ | 2,147 | |
Accrued clinical trial liabilities | | | 178 | | | | 96 | |
Other accrued liabilities | | | 4,736 | | | | 3,599 | |
Current portion of contingent consideration liability | | | 2,500 | | | | 3,500 | |
Current portion of financing obligations | | | 780 | | | | 6,461 | |
Current portion of deferred revenues | | | 2,915 | | | | 2,915 | |
| | | | | | | | |
Total current liabilities | | | 14,898 | | | | 18,718 | |
Deferred rent | | | 6,405 | | | | 8,059 | |
Noncurrent portion of contingent consideration liability | | | 36,700 | | | | 6,100 | |
Noncurrent portion of deferred revenues | | | 2,185 | | | | 5,101 | |
Noncurrent portion of financing obligations | | | 10,744 | | | | — | |
Other liabilities | | | 115 | | | | — | |
Commitments (See Note 6) | | | | | | | | |
Stockholders’ equity (deficit): | | | | | | | | |
Preferred stock, $0.0001 par value, 5,000,000 shares authorized at December 31, 2013 and 2012; no shares issued and outstanding at December 31, 2013 or 2012 | | | — | | | | — | |
Common stock, $0.0001 par value; 200,000,000 shares authorized at December 31, 2013 and 2012, respectively; 17,279,804 and 15,767,525 shares issued and outstanding at December 31, 2013 and 2012, respectively | | | 2 | | | | 2 | |
Additional paid-in capital | | | 350,250 | | | | 337,184 | |
Accumulated other comprehensive income | | | 1 | | | | — | |
Accumulated deficit | | | (374,228 | ) | | | (334,613 | ) |
| | | | | | | | |
Total stockholders’ (deficit) equity | | | (23,975 | ) | | | 2,573 | |
| | | | | | | | |
Total liabilities and stockholders’ equity (deficit) | | $ | 47,072 | | | $ | 40,551 | |
| | | | | | | | |
See accompanying notes.
81
ALEXZA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF LOSS AND COMPREHENSIVE LOSS
(In thousands, except per share amounts)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Revenue | | $ | 47,839 | | | $ | 4,070 | | | $ | 5,660 | |
Operating expenses: | | | | | | | | | | | | |
Cost of goods sold | | | 11,209 | | | | — | | | | — | |
Research and development | | | 19,082 | | | | 21,849 | | | | 28,262 | |
General and administrative | | | 15,778 | | | | 11,093 | | | | 11,766 | |
| | | | | | | | | | | | |
Total operating expenses | | | 46,069 | | | | 32,942 | | | | 40,028 | |
| | | | | | | | | | | | |
Income (loss) from operations | | | 1,770 | | | | (28,872 | ) | | | (34,368 | ) |
(Loss) gain on change in fair value of contingent consideration liability | | | (39,913 | ) | | | 1,900 | | | | (4,000 | ) |
Interest and other income/expense, net | | | 26 | | | | 420 | | | | 26 | |
Interest expense | | | (1,498 | ) | | | (1,426 | ) | | | (2,189 | ) |
| | | | | | | | | | | | |
Net loss | | $ | (39,615 | ) | | $ | (27,978 | ) | | $ | (40,531 | ) |
| | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (2.38 | ) | | $ | (2.24 | ) | | $ | (5.97 | ) |
| | | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share | | | 16,669 | | | | 12,472 | | | | 6,787 | |
| | | | | | | | | | | | |
Other Comprehensive Loss: | | | | | | | | | | | | |
Change in unrealized (loss)/gain on marketable securities | | | 1 | | | | — | | | | (2 | ) |
| | | | | | | | | | | | |
Comprehensive loss | | $ | (39,614 | ) | | $ | (27,978 | ) | | $ | (40,533 | ) |
| | | | | | | | | | | | |
See accompanying notes.
82
ALEXZA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional
Paid in
Capital | | | Accumulated
Other
Comprehensive
(Loss) Income | | | Accumulated
Deficit | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | | Amount | | | | | |
Balance at January 1, 2011 | | | 5,976,591 | | | $ | 1 | | | $ | 278,391 | | | $ | 2 | | | $ | (266,104 | ) | | $ | 12,290 | |
Issuance of common stock and common stock warrants for cash | | | 1,192,703 | | | | — | | | | 15,941 | | | | — | | | | — | | | | 15,941 | |
Issuance of common stock for cash under the Company’s Equity Incentive Plan | | | 98 | | | | — | | | | 2 | | | | — | | | | — | | | | 2 | |
Issuance of common stock for cash under the Company’s Employee Stock Purchase Plan | | | 24,998 | | | | — | | | | 202 | | | | — | | | | — | | | | 202 | |
Issuance of common stock upon vesting of restricted stock units | | | 19,202 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense related to consultant stock options | | | — | | | | — | | | | 17 | | | | — | | | | — | | | | 17 | |
Compensation expense related to fair value of employee share based awards | | | — | | | | — | | | | 2,389 | | | | — | | | | — | | | | 2,389 | |
Comprehensive loss | | | — | | | | — | | | | — | | | | (2 | ) | | | — | | | | (2 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (40,531 | ) | | | (40,531 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 7,213,592 | | | $ | 1 | | | $ | 296,942 | | | $ | — | | | $ | (306,635 | ) | | $ | (9,692 | ) |
Issuance of common stock and common stock warrants for cash | | | 4,400,000 | | | | 1 | | | | 20,230 | | | | — | | | | — | | | | 20,231 | |
Issuance of common stock for cash | | | 3,812,225 | | | | — | | | | 14,929 | | | | — | | | | — | | | | 14,929 | |
Issuance of common stock for cash under the Company’s Employee Stock Purchase Plan | | | 18,409 | | | | — | | | | 82 | | | | — | | | | — | | | | 82 | |
Issuance of common stock upon vesting of restricted stock units | | | 323,299 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense related to fair value of employee share based awards | | | — | | | | — | | | | 5,001 | | | | — | | | | — | | | | 5,001 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (27,978 | ) | | | (27,978 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2012 | | | 15,767,525 | | | $ | 2 | | | $ | 337,184 | | | $ | — | | | $ | (334,613 | ) | | $ | 2,573 | |
Issuance of common stock for cash | | | 1,437,481 | | | | — | | | | 6,347 | | | | — | | | | — | | | | 6,347 | |
Issuance of common stock for cash under the Company’s Equity Incentive Plan | | | 10,317 | | | | — | | | | 36 | | | | — | | | | — | | | | 36 | |
Issuance of common stock for cash under the Company’s Employee Stock Purchase Plan | | | 54,494 | | | | — | | | | 200 | | | | — | | | | — | | | | 200 | |
Issuance of common stock upon vesting of restricted stock units | | | 9,987 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation expense related to fair value of employee share based awards | | | — | | | | — | | | | 3,371 | | | | — | | | | — | | | | 3,371 | |
Beneficial conversion feature of convertible debt | | | — | | | | — | | | | 3,112 | | | | — | | | | — | | | | 3,112 | |
Comprehensive income | | | — | | | | — | | | | — | | | | 1 | | | | — | | | | 1 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (39,615 | ) | | | (39,615 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 | | | 17,279,804 | | | $ | 2 | | | $ | 350,250 | | | $ | 1 | | | $ | (374,228 | ) | | $ | (23,975 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
83
ALEXZA PHARMACEUTICALS, INC
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Cash flows from operating activities: | |
Net loss | | $ | (39,615 | ) | | | (27,978 | ) | | $ | (40,531 | ) |
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
Share-based compensation expense | | | 3,371 | | | | 5,001 | | | | 2,406 | |
Change in fair value of contingent consideration liability | | | 39,913 | | | | (1,900 | ) | | | 4,000 | |
Recognition of right to borrow asset | | | (2,800 | ) | | | — | | | | — | |
Amortization of debt discount, deferred interest and right to borrow asset | | | 1,079 | | | | 447 | | | | 602 | |
Amortization of discount on available-for-sale securities | | | — | | | | 1 | | | | 204 | |
Depreciation | | | 3,323 | | | | 4,336 | | | | 4,432 | |
(Gain)/loss on disposal of property and equipment | | | (15 | ) | | | (415 | ) | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Receivables | | | (129 | ) | | | 10,000 | | | | (10,000 | ) |
Inventory | | | (3,447 | ) | | | — | | | | — | |
Prepaid expenses and other current assets | | | (601 | ) | | | (203 | ) | | | 316 | |
Other assets | | | — | | | | 200 | | | | (147 | ) |
Accounts payable | | | 1,642 | | | | (1,456 | ) | | | 822 | |
Accrued clinical trial expense and other accrued liabilities | | | 1,122 | | | | 559 | | | | (498 | ) |
Deferred revenues | | | (2,916 | ) | | | (2,618 | ) | | | 6,303 | |
Other liabilities | | | (1,539 | ) | | | (4,215 | ) | | | (2,335 | ) |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (612 | ) | | | (18,241 | ) | | | (34,426 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchase of available-for-sale securities | | | (19,227 | ) | | | — | | | | (28,465 | ) |
Maturities of available-for-sale securities | | | 10,650 | | | | 2,000 | | | | 54,036 | |
Purchases of property and equipment | | | (1,784 | ) | | | (452 | ) | | | (496 | ) |
Proceeds from disposal of property and equipment | | | 16 | | | | 425 | | | | — | |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | (10,345 | ) | | | 1,973 | | | | 25,075 | |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from issuance of common stock, common stock warrants and exercise of stock options and stock purchase rights | | | 6,583 | | | | 35,242 | | | | 16,145 | |
Payment of contingent payment to Symphony Allegro Holdings, LLC | | | (10,313 | ) | | | (5,000 | ) | | | — | |
Change in current restricted cash | | | 5,051 | | | | (5,051 | ) | | | — | |
Proceeds from term loans | | | 15,000 | | | | — | | | | — | |
Payments of term loans | | | (5,773 | ) | | | (6,110 | ) | | | (5,563 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 10,548 | | | | 19,081 | | | | 10,582 | |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (409 | ) | | | 2,813 | | | | 1,231 | |
| | | | | | | | | | | | |
Cash and cash equivalents at beginning of period | | | 17,715 | | | | 14,902 | | | | 13,671 | |
Cash and cash equivalents at end of period | | $ | 17,306 | | | $ | 17,715 | | | $ | 14,902 | |
| | | | | | | | | | | | |
Supplemental disclosures of cash flow information | | | | | | | | | | | | |
Cash paid for interest | | $ | 863 | | | $ | 979 | | | $ | 1,631 | |
| | | | | | | | | | | | |
Non cash investing and financing activities: | | | | | | | | | | | | |
Debt discount related to Teva Note | | $ | 4,405 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
Reversal of Note Payable to Autoliv | | $ | — | | | $ | — | | | $ | 1,200 | |
| | | | | | | | | | | | |
See accompanying notes.
84
ALEXZA PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. The Company
Business
We were incorporated in the state of Delaware on December 19, 2000 as FaxMed, Inc., changed our name to Alexza Corporation in June 2001 and in December 2001 became Alexza Molecular Delivery Corporation. In July 2005, we changed our name to Alexza Pharmaceuticals, Inc.
We are a pharmaceutical company focused on the research, development, and commercialization of novel proprietary products for the acute treatment of central nervous system conditions. We operate in one business segment. Our facilities and employees are currently located in the United States. In 2012, we transitioned out of the development stage.
Reverse Stock Split
On June 12, 2012, we effected a 1-for-10 reverse stock split of our outstanding common stock resulting in a reduction of our total common stock issued and outstanding from 119.6 million shares to 12.0 million shares. The reverse stock split affected all of the stockholders of our common stock uniformly, and did not materially affect any stockholder’s percentage of ownership interest. The par value of our common stock remained unchanged at $0.0001 per share and the number of authorized shares of common stock remained the same after the reverse stock split.
As the par value per share of our common stock remained unchanged at $0.0001 per share, a total of $11,000 was reclassified from common stock to additional paid-in capital. In connection with this reverse stock split, the number of shares of common stock reserved for issuance under our equity incentive, stock option and employee stock purchase plans (see Note 10) as well as the shares of common stock underlying outstanding stock options, restricted stock units and warrants were also proportionately reduced while the exercise prices of such stock options and warrants were proportionately increased. All references to shares of common stock and per share data for all periods presented in the accompanying financial statements and notes thereto have been adjusted to reflect the reverse stock split on a retroactive basis.
Authorized Shares
On July 28, 2011, we filed a Certificate of Amendment to our Restated Certificate of Incorporation to increase the total number of authorized shares from 105,000,000 to 205,000,000 and to increase the total number of authorized shares of common stock from 100,000,000 to 200,000,000.
Underwritten Public Offering
On February 23, 2012, we issued an aggregate of 4,400,000 shares of our common stock and warrants to purchase up to an additional 4,400,000 shares of our common stock in an underwritten public offering. Net proceeds from the offering were $20,231,000, after deducting offering expenses. The warrants are exercisable beginning February 24, 2013, at an exercise price of $5.00 per share, and will expire on February 23, 2017. The shares of common stock and warrants were sold pursuant to a shelf registration statement declared effective by the Securities and Exchange Commission on May 20, 2010. We agreed to customary obligations, including indemnification.
Private Placement
On March 5, 2012, we entered into an amendment to the Collaboration, License and Supply Agreement, or the Ferrer Agreement, with Grupo Ferrer Internacional, S.A., or Ferrer (See Note 8). Ferrer and we agreed to eliminate a future potential milestone payment in exchange for Ferrer’s purchase of $3,000,000 of our common stock. On March 15, 2012 Ferrer purchased 241,936 shares of our common stock for $12.40 per share. We classified $1,452,000 of the proceeds as deferred revenue and are recognizing the amount into revenue over the estimated performance period of the Ferrer Agreement (see Note 8).
85
Registered Direct Equity Issuances
On May 6, 2011, we issued an aggregate of 1,192,703 shares of its common stock and warrants to purchase up to an additional 417,445 shares of its common stock in a registered direct offering. Net proceeds from the offering were approximately $15,941,000, after deducting offering expenses. The warrants are exercisable at $17.55 per share and will expire on May 6, 2016. The securities were sold pursuant to a shelf registration statement declared effective by the SEC on May 20, 2010. We agreed to customary obligations regarding registration, including indemnification and maintenance of the registration statement.
Equity Financing Facility
In July 2012, we entered into a committed equity line of credit with Azimuth Opportunity, L.P., or Azimuth, pursuant to which we were granted the ability to sell up to $20,000,000 of our common stock over an approximately 24-month period pursuant to the terms of a Common Stock Purchase Agreement, or the Purchase Agreement. In addition to the foregoing amounts, in consideration for the performance of Azimuth’s obligations under the Purchase Agreement, we issued to Azimuth 80,429 shares of its common stock on July 23, 2012. In August and September 2012, we utilized $13,600,000 under the facility by issuing 3,489,860 shares of our common stock to Azimuth at an average price of $3.88 per share, which resulted in $13,393,000 in net proceeds to us. In May 2013, we utilized the remaining $6,400,000 available under the facility by issuing 1,437,481 shares of our common stock to Azimuth at an average price of $4.48 per share, which resulted in $6,347,000 of net proceeds to us. Shares were issued to Azimuth at a discount of 5% to the volume-weighted average price of our common stock over a preceding period of trading days. Shares sold under this facility were sold pursuant to a shelf registration statement declared effective by the SEC on July 3, 2012.
2. Summary of Significant Accounting Policies
Basis of Presentation
We have prepared the accompanying consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP. Our fiscal year ends on December 31. The accompanying consolidated financial statements include our accounts and those of our wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Three levels of inputs, of which the first two are considered observable and the last unobservable, may be used to measure fair value which are the following:
| | • | | Level 1 — Quoted prices in active markets for identical assets or liabilities. |
| | • | | Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | • | | Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
86
The following table represents the fair value hierarchy for our financial assets (cash equivalents and marketable securities) by major security type and contingent consideration liability measured at fair value on a recurring basis as of December 31, 2013 and 2012 (in thousands):
| | | | | | | | | | | | | | | | |
December 31, 2013 | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets | | | | | | | | | | | | | | | | |
Money market funds | | $ | 11,345 | | | $ | — | | | $ | — | | | $ | 11,345 | |
Corporate debt securities | | | — | | | | 12,003 | | | | — | | | | 12,003 | |
| | | | | | | | | | | | | | | | |
Total assets | | $ | 11,345 | | | $ | 12,003 | | | $ | — | | | $ | 23,348 | |
| | | | | | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | | | | | |
Contingent consideration liability | | $ | — | | | $ | — | | | $ | 39,200 | | | $ | 39,200 | |
| | | | | | | | | | | | | | | | |
Total liabilities | | $ | — | | | $ | — | | | $ | 39,200 | | | $ | 39,200 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
December 31, 2012 | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets | | | | | | | | | | | | | | | | |
Money market funds | | $ | 17,307 | | | $ | — | | | $ | — | | | $ | 17,307 | |
| | | | | | | | | | | | | | | | |
Total assets | | $ | 17,307 | | | $ | — | | | $ | — | | | $ | 17,307 | |
| | | | | | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | | | | | |
Contingent consideration liability | | $ | — | | | $ | — | | | $ | 9,600 | | | $ | 9,600 | |
| | | | | | | | | | | | | | | | |
Total liabilities | | $ | — | | | $ | — | | | $ | 9,600 | | | $ | 9,600 | |
| | | | | | | | | | | | | | | | |
Our available-for-sale debt securities are valued utilizing a multi-dimensional relational model. Inputs, listed in approximate order of priority for use when available, include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data. There were no transfers between Level 1 and Level 2 measurements during the year ended December 31, 2013, and there were no changes in our valuation technique.
Contingent Consideration Liability
In connection with the exercise of our option to purchase all of the outstanding equity of Symphony Allegro, Inc., or Allegro, in 2009, we are obligated to make contingent cash payments to the former Allegro stockholders related to certain payments received by us from future collaboration agreements pertaining to ADASUVE/AZ-104 (Staccatoloxapine) or AZ-002 (Staccato alprazolam). In order to estimate the fair value of the liability associated with the contingent cash payments, we prepared several cash flow scenarios for ADASUVE, AZ-104 and AZ-002, which are subject to the contingent payment obligation. Each potential cash flow scenario consisted of assumptions of the range of estimated milestone and license payments potentially receivable from such collaborations and assumed royalties received from future product sales. Based on these estimates, we computed the estimated payments to be made to the former Allegro stockholders. Payments were assumed to terminate in accordance with current agreement terms or, if no agreements exist, upon the expiration of the related patents.
The projected cash flow assumptions for ADASUVE in the United States are based on the License and Supply Agreement, or the Teva Agreement, between us and Teva Pharmaceuticals USA, Inc., or Teva, (see Note 8) and on internally and externally developed product sales forecasts. The timing and extent of the projected cash flows for ADASUVE for the territories licensed to Ferrer are based on the Ferrer Agreement (see Note 8). The timing and extent of the projected cash flows for the remaining territories for ADASUVE and worldwide territories for AZ-002 and AZ-104 were based on internal estimates for potential milestones and multiple product royalty scenarios and are also consistent in structure to the most recently negotiated collaboration agreements.
We then assigned a probability to each of the cash flow scenarios based on several factors, including: the product candidate’s stage of development, preclinical and clinical results, technological risk related to the
87
successful development of the different drug candidates, estimated market size, market risk and potential collaboration interest to determine a risk adjusted weighted average cash flow based on all of these scenarios. These probability and risk adjusted weighted average cash flows were then discounted utilizing our estimated weighted average cost of capital, or WACC. Our WACC considered our cash position, competition, risk of substitute products, and risk associated with the financing of the development projects. In 2013, we reduced the discount rate from 18.0% to 16.5% to reflect our current estimated WACC. The change in discount rate increased the net loss for 2013 by approximately $3,600,000 or $0.22 per share.
The fair value measurement of the contingent consideration liability is based on significant inputs not observed in the market and thus represents a Level 3 measurement. Level 3 measurements are valued based on unobservable inputs that are supported by little or no market activity and reflect our assumptions in measuring fair value.
We record any changes in the fair value of the contingent consideration liability in earnings in the period of the change. Certain events including, but not limited to, the timing and terms of any collaboration agreement, clinical trial results, approval or non-approval of any future regulatory submissions and the commercial success of ADASUVE, AZ-104 or AZ-002 could have a material impact on the fair value of the contingent consideration liability, and as a result, our results of operations and financial position for the impacted period.
During the year ended December 31, 2011, we modified the assumptions regarding the timing and probability of certain cash flows primarily to reflect the ADASUVE commercial collaboration entered into with Ferrer in October 2011 (see Note 8). The changes in these assumptions and the effect of the passage of one year on the present value computation resulted in a $4,000,000 increase to the contingent consideration liability in the year ended December 31, 2011. The changes in these assumptions resulted in an increase to net loss per share of $0.59 for the year ended December 31, 2011.
During the year ended December 31, 2012, we modified the assumptions and the timing and extent of certain cash flows regarding the increased probability that we will commercialize ADASUVE in the United States internally without a collaborator and the timing of the commercial launch of ADASUVE in the United States. This change in assumptions resulted in a decrease to the contingent consideration liability as the former Allegro stockholders do not receive payments from us for product revenues generated by us. We also modified assumptions to reflect the approval of the ADASUVE NDA in December 2012 and the December 2012 positive opinion by the Committee for Medicinal Products for Human recommending that ADASUVE be granted European Union centralized marketing authorization by the European Commission, resulting in an increase in the contingent consideration liability. The changes in these assumptions resulted in a decrease to net loss per share of $0.15 for the year ended December 31, 2012.
During the year ended December 31, 2013, we modified the assumptions regarding the timing and amount of certain cash flows primarily to reflect (i) the increased probability in the first quarter of 2013 that we would license the U.S. commercialization rights to ADASUVE to a third party, (ii) the impact of the licensing and the terms of the Teva Agreement in the second quarter of 2013, (iii) the change in the projected ADASUVE launch date to the first quarter of 2014 and (iv) the projected timing of certain future revenues and the potential expansion of the ADASUVE label. These changes in assumptions, the change in the WACC in 2013 described above, and the effects of the passage of a year on the present value computation resulted in an increase to the net loss of $39,913,000, or $2.39 per share, for the year ended December 31, 2013.
The following table represents a reconciliation of the change in the fair value measurement of the contingent consideration liability for the years ended December 31, 2013 and 2012 (in thousands).
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Beginning balance | | $ | 9,600 | | | $ | 16,500 | |
Payments made | | | (10,313 | ) | | | (5,000 | ) |
Adjustments to fair value measurement | | | 39,913 | | | | (1,900 | ) |
| | | | | | | | |
Ending balance | | $ | 39,200 | | | $ | 9,600 | |
| | | | | | | | |
88
The $5,000,000 payment in 2012 was the result of our receipt of the $10,000,000 upfront payment from Ferrer (see Note 8). The $10,313,000 of aggregate payments in 2013 were the result of our receipt of the $40,000,000 upfront payment from Teva (see Note 8) and our receipt of the $1,250,000 milestone payment from Ferrer associated with the launch of ADASUVE in Germany.
Financing Obligations
We have estimated the fair value of our financing obligations (see Note 7) using the net present value of the payments discounted at an interest rate that is consistent with its estimated current borrowing rate for similar long-term debt. We believe the estimates used to measure the fair value of the financing obligations constitute Level 3 inputs.
At December 31, 2013 and 2012, the estimated fair value of our financing obligations was $9,342,000 and $6,254,000, respectively and had book values of $11,524,000 and $6,461,000, respectively. Our payment commitments associated with these debt instruments may vary with changes in interest rates and are comprised of interest payments and principal payments. The fair value of our debt will fluctuate with movements of interest rates, increasing in periods of declining rates of interest and declining in periods of increasing rates of interest.
Concentration of Credit Risk
Financial instruments that potentially subject us to credit risk consist of cash, cash equivalents and marketable securities to the extent of the amounts recorded on the balance sheets. Our cash, cash equivalents and marketable securities are placed with high credit-quality U.S. financial institutions and issuers. We believe that its established guidelines for investment of its excess cash maintain liquidity through its policies on diversification and investment maturity.
Cash Equivalents and Marketable Securities
We determine the appropriate classification of our investments at the time of purchase. These securities are recorded as either cash equivalents or marketable securities.
We consider all highly liquid investments with original maturities of three months or less from date of purchase to be cash equivalents. Cash equivalents consist of interest-bearing instruments including obligations of U.S. government agencies, high credit rating corporate borrowers and money market funds, which are carried at market value.
All other investments are classified as available-for-sale marketable securities. We view our available-for-sale investments as available for use in current operations. Accordingly, we have classified all investments as short-term marketable securities, even though the stated maturity date may be one year or more beyond the current balance sheet date. Marketable securities are carried at estimated fair value with unrealized gains or losses included in accumulated other comprehensive income (loss) in stockholders’ (deficit) equity.
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion are included in interest and other income (expense), net. Realized gains and losses, if any, are also included in interest and other income (expense), net. The cost of all securities sold is based on the specific-identification method. Interest and dividends are included in interest income.
We review our investments for other than temporary decreases in market value on a quarterly basis. To date, we have not recorded any charges related to other-than-temporary impairments.
Restricted Cash
In January 2012, we and Hercules Technology Growth Capital, Inc., or Hercules, amended the Loan and Security Agreement between Hercules and us entered into in May 2010, or the Loan Agreement, to require us to maintain an amount equal to the outstanding principal balance of the loan in a restricted account (See Note 7). Upon an event of default, as defined in the Loan Agreement, Hercules had the ability to access the funds. On January 3, 2013, Hercules removed the requirement to maintain the restricted account and the funds were no longer classified as restricted cash.
89
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Property and equipment are depreciated using the straight-line method over the estimated life of the asset, generally three years for computer equipment and five years for manufacturing equipment and laboratory equipment and seven years for furniture. Leasehold improvements are amortized over the estimated useful life or the remaining lease term, whichever is shorter.
Impairment of Long-Lived Assets
We review long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition are less than its carrying amount. The impairment loss, if recognized, would be based on the excess of the carrying value of the impaired asset over its respective fair value. Through December 31, 2013, we have not recorded an impairment of a long-lived asset.
Revenue Recognition
We recognize revenue when (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred or services have been rendered; (iii) the fee is fixed or determinable; and (iv) collectability is reasonably assured. Prior to the second quarter of 2013, our revenue consisted primarily of amounts earned from collaboration agreements and under research grants with the National Institutes of Health. Beginning in the second quarter of 2013, we also have revenue from product sales.
For collaboration agreements, revenues for non-refundable upfront license fee payments, where we continue to have performance obligations, are recognized as performance occurs and obligations are completed. Revenues for non-refundable upfront license fee payments where we do not have significant future performance obligations are recognized when the agreement is signed and the payments are due.
For multiple element arrangements, such as collaboration agreements in which a collaborator may purchase several deliverables, we account for each deliverable as a separate unit of accounting if both of the following criteria are met: (i) the delivered item or items have value to the customer on a standalone basis; and (ii) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We evaluate how the consideration should be allocated among the units of accounting and allocate revenue to each non-contingent element based upon the relative selling price of each element. We determine the relative selling price for each deliverable using (i) vendor-specific objective evidence, or VSOE, of selling price if it exists; (ii) third-party evidence, or TPE, of selling price if it exists; or (iii) our best estimated selling price for that deliverable if neither VSOE nor TPE of selling price exists for that deliverable. We then recognize the revenue allocated to each element when the four basic revenue criteria described above are met for each element.
For milestone payments received in connection with our collaboration agreements, we have elected to adopt the milestone method of accounting under Financial Accounting Standards Board Accounting Standards Codification 605-28, Milestone Method. Under the milestone method, revenues for payments which meet the definition of a milestone will be recognized as the respective milestones are achieved.
We recognize product revenue as follows:
| | • | | Persuasive Evidence of an Arrangement. We currently sell product through license and supply agreements with our collaborators, Ferrer and Teva. Persuasive evidence of an arrangement is generally determined by the receipt of an approved purchase order from the collaborator in connection with the terms of the license and supply agreements. |
| | • | | Delivery. Typically, ownership of the product passes to the collaborator upon shipment. Our current license and supply agreements also provide our collaborators with an acceptance period during which they may reject any product which does not conform to agreed-upon specifications. Because ADASUVE |
90
| | is a new product, a new technology and our first product to be commercialized, and because we do not have a history of producing product to collaborator specifications, we will not consider delivery to have occurred until after the collaborator acceptance period has ended or the collaborator has positively accepted the product. Once we have demonstrated over the course of time an ability to reliably produce the product to collaborator specifications, we will consider delivery to have occurred upon shipment in the absence of any other relevant shipment or acceptance terms. |
| | • | | Sales Price Fixed or Determinable. Sales prices for product shipments are determined by the license and supply agreements and documented in the purchase orders. After the collaborator acceptance period has ended or the collaborator has positively accepted the product, our collaborators do not have any product return or replacement rights, including for expired products. |
| | • | | Collectability. Payment for the product is contractually obligated under the license and supply agreements. We will monitor payment histories for our collaborators and specific issues as they arise to determine whether collection is probable for a specific transaction and defer revenue as necessary. |
Royalty revenue from our collaboration agreements will be recognized as we receive information from our collaborators regarding product sales.
Significant management judgment is used in the determination of revenue to be recognized and the period in which it is recognized.
Inventory
Inventory is stated at standard cost, which approximates actual cost, determined on a first-in first-out basis, not in excess of market value. Inventory includes the direct costs incurred to manufacture products combined with allocated manufacturing overhead, which consists of indirect costs, including labor and facility overhead. We are in the early stages of commercialization and have incurred significantly higher than normal indirect costs in the production of our inventory due to start-up manufacturing costs and low production volumes. The carrying cost of inventory is reduced so as to not be in excess of the market value of the inventory as determined by the contractual transfer prices to Ferrer and Teva. The excess over the market value is expensed to cost of goods sold. All costs associated with the ADASUVE manufacturing process incurred prior to regulatory approval and the beginning of commercial manufacturing were expensed as a component of research and development expense. If information becomes available that suggests that all or certain of the inventory may not be realizable, we may be required to expense a portion, or all, of the capitalized inventory into cost of goods sold. Inventory, which is stated at the lower of cost or estimated market value, consists of the following at December 31, 2013 (in thousands):
| | | | |
Raw materials | | $ | 3,044 | |
Work in process | | | — | |
Finished goods | | | 403 | |
| | | | |
Inventory | | $ | 3,447 | |
| | | | |
Research and Development
Research and development expenses include personnel and facility-related expenses, outside contracted services including clinical trial costs, manufacturing and process development costs, research costs and other consulting services. Research and development costs are expensed as incurred.
Clinical development costs are a significant component of research and development expenses. We have a history of contracting with third parties that perform various clinical trial activities on our behalf in the ongoing development of its product candidates. The financial terms of these contracts are subject to negotiations and may vary from contract to contract and may result in uneven payment flow. We accrue and expense costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites.
91
Income Taxes
We utilize the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities and are measured using enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
Share-Based Compensation
Compensation cost for employee share-based awards is based on the grant-date fair value and is recognized on a ratable basis over the requisite service periods of the awards, which are generally the vesting periods or, for performance-based options, the expected period during which the performance criteria is expected to be met. We issue employee share-based awards in the form of stock options and restricted stock units under our equity incentive plans and stock purchase rights under our employee stock purchase plan.
Stock Options, Stock Purchase Rights and Restricted Stock Units
During the years ended December 31, 2013, 2012 and 2011, the weighted average fair value per share of the employee stock options (excluding options issued in the Exchange Program as defined in Note 10), restricted stock units and stock purchase rights granted were:
| | | | | | | | | | | | |
| | | 2013 | | | 2012 | | | 2011 | |
Stock Options | | $ | 3.46 | | | $ | 2.60 | | | $ | 10.60 | |
Restricted Stock Units | | | 4.67 | | | | 4.96 | | | | 13.30 | |
Stock Purchase Rights | | | 1.76 | | | | 3.86 | | | | 8.20 | |
The estimated grant date fair values of the stock options (excluding options issued in the Exchange Program) and stock purchase rights were calculated using the Black-Scholes valuation model. The Black-Scholes model requires the use of a number of highly subjective and complex assumptions in determining the fair value of stock-based awards as follows:
Weighted-Average Expected Term We determine the expected term of stock options granted through a combination of our own historical exercise experience and expected future exercise activities and post-vesting termination behavior. Under the Employee Stock Purchase Plan, the expected term of employee stock purchase plan shares is equal to the offering period.
Volatility We utilize our historical volatility to determine future volatility for the purpose of determining share-based payments for all options granted.
Risk-Free Interest Rate We utilize U.S. Treasury zero-coupon issues with remaining terms similar to the expected term of the options or purchase rights on the respective grant dates to determine our risk-free interest rate.
Dividend Yield We have never declared or paid any cash dividends and do not plan to pay cash dividends in the foreseeable future, and, therefore, use an expected dividend yield of zero in the valuation model.
Forfeiture Rate We use historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods.
92
During the years ended December 31, 2013, 2012 and 2011, we calculated the estimated grant date fair value of stock options (excluding those issued in the Exchange Program as defined in Note 10) and stock purchase rights using the following assumptions:
| | | | | | |
| | | 2013 | | 2012 | | 2011 |
Stock Option Plans | | | | | | |
Weighted-average expected term | | 5.0 Years | | 5.0 Years | | 5.0 Years |
Expected volatility | | 99% | | 98% | | 90% |
Risk-free interest rate | | 0.86% | | 0.62% | | 1.52% |
Dividend yield | | 0% | | 0% | | 0% |
Employee Stock Purchase Plan | | | | | | |
Weighted-average expected term | | 0.5 Years | | 0.6 Years | | 1.45 Years |
Expected volatility | | 80% | | 98% | | 87% |
Risk-free interest rate | | 0.70% | | 0.14% | | 0.59% |
Dividend yield | | 0% | | 0% | | 0% |
In January 2011, as described in Note 10, we commenced a voluntary stock option Exchange Program. The Exchange Program did not result in incremental expense, as the fair value of the New Options (as defined in Note 10) granted was equal to or less than the fair values of the Original Options (as defined in Note 10) measured immediately prior to the date the New Options were granted and the Original Options were cancelled. The estimated grant date fair value of the New Options was calculated using the Black-Scholes valuation model. At the time of exchange, the exercise price of the Original Options was in excess of the market price, therefore the expected term of the Original Options granted was determined using the Monte Carlo Simulation method. The expected term of the New Options granted was determined using the “shortcut” method, as illustrated in the Securities and Exchange Commission’s Staff Accounting Bulletin No. 107, because the terms of the New Options are unique as compared to the existing awards and we do not have historical experience under the terms of the New Options. Under this approach, the expected term is estimated to be the average of the vesting term and the contractual term of the option. All other assumptions have been calculated using the historical methodologies we applied to all other stock option awards.
The number of shares underlying the options included in the Exchange Program in 2011 and the weighted average assumptions utilized in the Black-Scholes valuation model were as follows:
| | | | |
| | | Original
Options | | New
Options |
Number of shares | | 212,843 | | 80,890 |
Expected term | | 4.7 years | | 3.4 years |
Expected volatility | | 94% | | 98% |
Risk-free interest rate | | 1.96% | | 1.38% |
Dividend yield | | 0% | | 0% |
Restricted Stock Units The estimated fair value of restricted stock unit awards is calculated based on the market price of our common stock on the date of grant, reduced by the present value of dividends expected to be paid on our common stock prior to vesting of the restricted stock unit. Our estimate assumes no dividends will be paid prior to the vesting of the restricted stock unit.
In 2013, certain restricted stock unit awards were issued that vest based on market conditions. The estimated fair value of these awards was calculated using a binomial lattice model with a term of 10 years, an expected volatility of 99.4%, a risk-free interest rate of 1.94% and a dividend yield of 0%.
As of December 31, 2013, there was $3,930,000, $1,856,000 and $70,000 total unrecognized compensation costs related to non-vested stock option awards, restricted stock unit awards and stock purchase rights, respectively, which are expected to be recognized over a weighted average period of 2.1 years, 3.2 years and 0.3 years, respectively.
93
3. Net Loss per Share
Basic and diluted net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period less weighted average shares subject to repurchase, of which there were none in 2013, 2012 or 2011. Outstanding stock options, warrants, and unvested restricted stock units are not included in the diluted net loss per share calculation for the years ended December 31, 2013, 2012 and 2011 as the inclusion of such shares would have had an anti-dilutive effect.
Potentially anti-dilutive securities include the following (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Outstanding stock options | | | 1,657 | | | | 840 | | | | 563 | |
Unvested restricted stock units | | | 315 | | | | 120 | | | | 133 | |
Warrants to purchase common stock | | | 6,462 | | | | 5,582 | | | | 1,895 | |
Convertible debt | | | 651 | | | | — | | | | — | |
4. Cash Equivalents and Marketable Securities
The amortized cost, fair value and unrealized gain/(loss) for our financial assets by major security type as of December 31, 2013 and 2012 are as follows (in thousands):
| | | | | | | | | | | | |
December 31, 2013 | | Amortized
Cost | | | Fair
Value | | | Unrealized
Gain/
(Loss) | |
Money market funds | | $ | 11,345 | | | $ | 11,345 | | | $ | — | |
Corporate debt securities | | | 12,002 | | | | 12,003 | | | | 1 | |
| | | | | | | | | | | | |
Total | | $ | 23,347 | | | $ | 23,348 | | | $ | 1 | |
Less amounts classified as cash equivalents | | $ | (14,770 | ) | | $ | (14,770 | ) | | $ | — | |
| | | | | | | | | | | | |
Total investments | | $ | 8,577 | | | $ | 8,578 | | | $ | 1 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
December 31, 2012 | | Amortized
Cost | | | Fair
Value | | | Unrealized
Gain/
(Loss) | |
Money market funds | | $ | 17,307 | | | $ | 17,307 | | | $ | — | |
| | | | | | | | | | | | |
Total | | $ | 17,307 | | | $ | 17,307 | | | $ | — | |
Less amounts classified as cash equivalents | | $ | (17,307 | ) | | $ | (17,307 | ) | | $ | — | |
| | | | | | | | | | | | |
Total investments | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
94
5. Balance Sheet Components
Property and equipment consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Lab equipment | | $ | 9,035 | | | $ | 8,900 | |
Manufacturing equipment | | | 10,502 | | | | 9,181 | |
Computer equipment and software | | | 4,706 | | | | 4,779 | |
Furniture | | | 816 | | | | 816 | |
Leasehold improvements | | | 18,899 | | | | 18,719 | |
| | | | | | | | |
| | | 43,958 | | | | 42,395 | |
Less: accumulated depreciation | | | (28,967 | ) | | | (25,864 | ) |
| | | | | | | | |
| | $ | 14,991 | | | $ | 16,531 | |
| | | | | | | | |
Other accrued liabilities consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Accrued compensation | | $ | 3,435 | | | $ | 2,166 | |
Accrued professional fees | | | 796 | | | | 658 | |
Other | | | 505 | | | | 775 | |
| | | | | | | | |
| | $ | 4,736 | | | $ | 3,599 | |
| | | | | | | | |
6. Commitments
Operating Leases
We lease one building, at 2091 Stierlin Court, or the 2091 Building, and we formerly leased an additional building at 2023 Stierlin Court, or the 2023 Building, both in Mountain View, California, and both of which we began to occupy in 2007. We recognize rental expense on the facilities on a straight line basis over the initial term of the lease. Differences between the straight line rent expense and actual rent payments are classified as deferred rent liability on the balance sheet. The lease for the 2091 Building expires on March 31, 2018, and we have two options to extend the lease for five years each.
In January 2010, we entered into an agreement to sublease a portion of the 2023 Building from March 1, 2010 through February 28, 2014. In August 2010, we entered into an agreement to sublease approximately 2,500 square feet of the 2091 Building to Cypress Bioscience, Inc., or Cypress, and to provide certain administrative, facility and information technology support for a period of 12 months and on a month-to-month basis thereafter. The Cypress sublease was terminated in October 2012.
On March 30, 2012, we terminated the lease for the 2023 Building, totaling 41,290 square feet, and concurrently cancelled the subleases associated with the 2023 Building. At the time of the termination, we recorded a non-cash contra-expense of $1,421,000 in general and administrative expenses, which is the net effect of reversing $2,073,000 of deferred rent liability associated with the 2023 Building lease and subleases and accelerating $652,000 of depreciation of fixed assets associated with the 2023 Building.
The 2091 Building lease, as amended, included tenant improvement reimbursements from the landlord. We have recorded all tenant improvements as additions to property and equipment and are amortizing the improvements over the shorter of the estimated useful life of the improvement or the remaining life of the lease. The reimbursements received from the landlord are included in deferred rent liability and amortized over the life of the lease as a contra-expense.
95
Future minimum lease payments under non-cancelable operating leases, at December 31, 2013 were as follows (in thousands):
| | | | |
| | | Lease
Payments | |
2014 | | $ | 3,502 | |
2015 | | | 3,197 | |
2016 | | | 3,287 | |
2017 | | | 3,386 | |
2018 | | | 853 | |
| | | | |
Total minimum payments | | $ | 14,225 | |
| | | | |
Rental expense for the year ended December 31, 2013 was $2,286,000. Rental expense for the year ended December 31, 2012, net of sublease income and exclusive of the impact of reversing the deferred rent liability and acceleration of deprecation described above, was $1,972,000. Rental expense, net of sublease income, was $2,000,000 for the year ended December 31, 2011. Rental income from the sublease agreements was $0, $340,000 and $1,584,000, for the years ended December 31, 2013, 2012, and 2011, respectively.
Manufacturing and Supply Agreement
In November 2007, we entered into a Manufacturing and Supply Agreement, or the Manufacture Agreement, with Autoliv ASP, Inc., or Autoliv, relating to the commercial supply of chemical heat packages that can be incorporated into ourStaccato device, or the Chemical Heat Packages. Autoliv had developed these Chemical Heat Packages for us pursuant to a development agreement between Autoliv and us. Under the terms of the Manufacture Agreement, Autoliv agreed to develop a manufacturing line capable of producing 10 million Chemical Heat Packages a year.
In June 2010 and February 2011, we entered into agreements to amend the terms of the Manufacture Agreement, or together the Amendments. Under the terms of the first Amendment, we paid Autoliv $4,000,000 and issued Autoliv a $4,000,000 unsecured promissory note in return for a production line for the commercial manufacture of Chemical Heat Packages. Each production line is comprised of two identical and self-sustaining “cells,” and the first such cell was completed, installed and qualified in connection with such Amendment. Under the terms of the second Amendment, the original $4,000,000 note was cancelled and a new note was issued with a reduced principal amount of $2,800,000, or the New Note, and production on the second cell ceased. The New Note is payable in 48 equal monthly installments of approximately $68,000. In the event that we request completion of the second cell of the first production line for the commercial manufacture of Chemical Heat Packages, Autoliv will complete, install and fully qualify such second cell for a cost to us of $1,200,000 and Autoliv will transfer ownership of such cell to us upon the payment in full of such $1,200,000 and the New Note.
Subject to certain exceptions, Autoliv has agreed to manufacture, assemble and test the Chemical Heat Packages solely for us in conformance with our specifications. We will pay Autoliv a specified purchase price, which varies based on annual quantities we order, per Chemical Heat Package delivered. In October 2013, Autoliv notified us of their intent to terminate, effective October 2016, the Manufacture Agreement. Prior to October 2016, we and Autoliv remain fully obligated to perform pursuant to the terms of both the Manufacture Agreement and the New Note. The Manufacture Agreement provides that during the term of the Manufacture Agreement, Autoliv will be our exclusive supplier of the Chemical Heat Packages. In addition, the Manufacture Agreement grants Autoliv the right to negotiate for the right to supply commercially any second generation Chemical Heat Package, or a Second Generation Product, and provides that we will pay Autoliv certain royalty payments if we manufacture Second Generation Products itself or if we obtain Second Generation Products from a third party manufacturer. Upon the termination of the Manufacture Agreement, we will be required, on an ongoing basis, to pay Autoliv certain royalty payments related to the manufacture of the Chemical Heat Packages by us or third party manufacturers.
96
7. Financing Obligations
Hercules Technology Growth Capital
In May 2010, we entered into a Loan Agreement with Hercules. Under the terms of the Loan Agreement, we borrowed $15,000,000 at an interest rate of the higher of (i) 10.75% or (ii) 6.5% plus the prime rate as reported in the Wall Street Journal, with a maximum interest rate of 14% and issued to Hercules a secured term promissory note evidencing the loan. We made interest-only payments through January 2011 and beginning in February 2011 the loan was repaid in 33 equal monthly installments. The loan was paid in full in October 2013.
In conjunction with the loan, we issued to Hercules a five-year warrant to purchase 37,639 shares of our common stock at a price of $26.90 per share. The warrant was immediately exercisable and expires in May 2015. We estimated the fair value of this warrant as of the issuance date to be $921,000 which was recorded as a debt discount to the loan and consequently a reduction to the carrying value of the loan. The fair value of the warrant was calculated using the Black-Scholes option valuation model, and was based on the contractual term of the warrant of five years, a risk-free interest rate of 2.31%, expected volatility of 84% and a 0% expected dividend yield. We also recorded fees paid to Hercules as a debt discount, which further reduced the carrying value of the loan. The debt discount was amortized to interest expense over the life of the loan.
In January 2012, we and Hercules amended the Loan Agreement to require us to maintain an amount equal to the outstanding principal balance of the loan in a restricted account that we classified as restricted cash. On January 3, 2013, Hercules removed the requirement to maintain the restricted account and the funds were no longer classified as restricted cash.
Autoliv ASP, Inc.
In June 2010, in return for transfer to us of all right, title and interest in a production line for the commercial manufacture of chemical heat packages completed or to be completed by Autoliv on our behalf, we paid Autoliv $4,000,000 in cash and issued Autoliv a $4,000,000 unsecured promissory note. In February 2011, we entered into an agreement to amend the terms of the unsecured promissory note. Under the terms of that amendment, the original $4,000,000 note was cancelled and the New Note was issued with a reduced principal amount of $2,800,000.
The New Note bears interest beginning on January 1, 2011 at 8% per annum and is being paid in 48 consecutive and equal monthly installments of approximately $68,000.
Teva Pharmaceuticals USA, Inc.
In May 2013, concurrent with the Teva Agreement (see Note 8), we entered into a Convertible Promissory Note and Agreement to Lend, dated as of May 7, 2013, between us and Teva, or the Teva Note. Under the terms of the Teva Note, we may, upon written notice to Teva, draw upon the Teva Note to fund agreed operating budgets related to ADASUVE. The aggregate drawdowns may total up to $25,000,000 and will be due and payable, together with all interest, on the fifth anniversary of the signing of the Teva Note. We may prepay, from time to time, up to one-half of the total amounts advanced plus the related interest outstanding at any time prior to the maturity date. At any time prior to five days before the maturity date, Teva will have the right to convert the then outstanding amounts into shares of our common stock at a conversion price of $4.4833 per share. The Teva Note bears simple interest of 4% per year. We have two years from the effective date of the Teva Note to receive advances.
In the third quarter of 2013, we drew down $10,000,000 against the Teva Note and, in the fourth quarter of 2013, we drew down an additional $5,000,000 against the Teva Note. At the time of the drawdowns, the contractual conversion price was less than the market value of our common stock. As a result, we calculated the value of the beneficial conversion feature of the convertible note and recorded an increase to additional paid-in-capital and a discount on the Teva Note of $3,112,000 which will be amortized to interest expense over the life of the borrowing. Additionally, we reclassified the relative portion of the unamortized right-to-borrow (see Note 8) against the Teva Note in the amount of $1,293,000 which will also be amortized to interest expense over the life of the borrowing. The effective interest rate of the first and second drawdowns against the Teva Note, which take into consideration the beneficial conversion features and the right-to-borrow discounts, is 13.9% and 9.9%, respectively.
97
Future scheduled principal payments under the debt obligations as of December 31, 2013 are as follows (in thousands):
| | | | |
| | | Total | |
2014 | | $ | 781 | |
2015 | | | — | |
2016 | | | — | |
2017 | | | — | |
2018 | | | 15,000 | |
| | | | |
Total | | $ | 15,781 | |
| | | | |
8. License Agreements
Cypress Bioscience, Inc.
In August 2010, we entered into a license and development agreement with Cypress Bioscience, Inc., or Cypress, forStaccatonicotine, or the Cypress Agreement. According to the terms of the Cypress Agreement, Cypress paid us a non-refundable upfront payment of $5,000,000 to acquire the worldwide license for theStaccatonicotine technology. In January 2011, Cypress was acquired by Ramius Value and Opportunity Advisors LLC; Royalty Pharma, U.S. Partner, LP; Royalty Pharma U.S. Partners 2008, LP; and RP Investment Corporation, or collectively, Royalty Pharma, at which time Royalty Pharma became Cypress’ successor in interest to the Cypress Agreement. As Royalty Pharma did not sell or license theStaccato nicotine technology by December 31, 2013, the Cypress Agreement automatically terminated and all rights to theStaccato nicotine technology reverted back to us at that date.
For revenue recognition purposes, we viewed the Cypress Agreement as a multiple element arrangement. Multiple element arrangements are analyzed to determine whether the various performance obligations, or elements, can be separated or whether they must be accounted for as a single unit of accounting. We evaluated the Cypress Agreement to determine whether the delivered elements under the arrangement had value on a stand-alone basis and whether objective and reliable evidence of fair value of the undelivered items existed. We accounted for the deliverables as a single unit of accounting. We did not recognize revenues under the Cypress Agreement in 2013 and recognized $1,259,000 of revenue under the Cypress Agreement in 2012. At December 31, 2013, we had no deferred revenues related to the Cypress Agreement.
Grupo Ferrer Internacional, S.A.
On October 5, 2011, we and Ferrer entered into the Ferrer Agreement to commercialize ADASUVE in Europe, Latin America, Russia and the Commonwealth of Independent States countries, or the Ferrer Territories. Under the terms of the Ferrer Agreement, we received an upfront cash payment of $10,000,000, of which $5,000,000 was paid to the former stockholders of Allegro. The Ferrer Agreement provided for up to an additional $51,000,000 in additional milestone payments, contingent on approval of Marketing Authorization Applications, or MAAs, individual country commercial sales initiation and royalty payments based on cumulative net sales targets in the Ferrer Territories. We were responsible for the marketing authorization for ADASUVE. The application for marketing authorization was submitted to the European Medicines Agency, or EMA, for an opinion regarding the potential authorization of ADASUVE and subsequent decision by the European Commission, or EC. We are also responsible for all post-authorization clinical studies required by the EMA and EC. Ferrer will be responsible for satisfaction of all other regulatory and pricing requirements to market and sell ADASUVE in the Ferrer Territories. Ferrer will have the exclusive rights to commercialize the product in the Ferrer Territories. We will supply ADASUVE to Ferrer for all of its commercial sales, and will receive a specified per-unit transfer price paid in Euros. Either party may terminate the Ferrer Agreement for the other party’s uncured material breach or bankruptcy. The Ferrer Agreement continues in effect on a country-by-country basis until the later of the last to expire patent covering ADASUVE in such country or 12 years after first commercial sale. The Ferrer Agreement is subject to earlier termination in the event the parties mutually agree, by a party in the event of an uncured material breach by the other party or upon the bankruptcy or insolvency of either party.
98
In March 2012, we entered into an amendment to the Ferrer Agreement. We and Ferrer agreed to eliminate the potential MAA approval milestone payment in exchange for Ferrer’s purchase of 241,936 shares of our common stock for $12.40 per share for a total of $3,000,000, which reflected a premium on the fair value of our common stock of approximately $1,452,000.
We evaluated whether the delivered elements under the Ferrer Agreement have value on a stand-alone basis and allocated revenue to the identified units of accounting based on relative fair value. We determined that the license and the development and regulatory services are a single unit of accounting as the licenses were determined not to have stand-alone value. We have begun to deliver all elements of the arrangement and are recognizing the $10,000,000 upfront payment as revenue ratably over the estimated performance period of the agreement of four years. The $1,452,000 premium received from the sale of common stock to Ferrer is additional consideration received pursuant to the Ferrer Agreement and does not pertain to a separate deliverable or element of the arrangement, and thus is being deferred and recognized as revenue in a manner consistent with the $10,000,000 upfront payment.
The Ferrer Agreement provides for us to receive up to $48,000,000 of additional payments related to first commercial sales in nine identified countries and to cumulative net sales targets in the Ferrer Territories. The cumulative net sales targets will be recognized as royalty revenue when each target is earned and payable to us. The first commercial sales payments will be recognized utilizing the milestone method of revenue recognition. We believe each of these milestones is substantive as there is uncertainty that the milestones will be met, the milestone can only be achieved as a result of our past performance and the achievement of the milestone will result in additional payment to us. We will recognize milestone revenue upon first commercial sales in each of these identified countries. In 2013, we received and recognized revenue on the first milestone in the amount of $1,250,000, of which $312,500 was paid to the former stockholders of Allegro (see Note 2).
We recognized $2,915,000, $2,811,000 and $625,000 of license revenue related to the Ferrer Agreement in the years ended December 31, 2013, 2012 and 2011, respectively. At December 31, 2013 we had deferred revenue of $5,101,000 related to the Ferrer Agreement.
Teva Pharmaceuticals USA, Inc.
In May 2013, we entered into the Teva Agreement to provide Teva with an exclusive license to develop and commercialize ADASUVE in the United States. Under the terms of the Teva Agreement, Teva will be responsible for all U.S. development, regulatory and commercialization activities for ADASUVE, including the U.S. post-approval clinical studies and any additional clinical trials for new indications. Teva has the full right to sublicense its rights and obligations under the Teva Agreement. We are responsible for manufacturing and supplying ADASUVE to Teva for clinical trials and commercial sales. Teva has the exclusive rights to commercialize ADASUVE in the United States and the co-exclusive rights (with us and our affiliates) to manufacture the product.
We received an upfront cash payment of $40,000,000 from Teva, $10,000,000 of which was paid to the former stockholders of Allegro. We are eligible to receive up to $195,000,000 in additional payments contingent on Teva’s successful completion of the ADASUVE post-approval studies in the United States and Teva achieving specified net sales targets. In addition to these payments, we will supply ADASUVE to Teva for all of its clinical trials and commercial sales, and we will receive a specified per-unit transfer price in an amount of the greater of our costs of commercial production or a specified per-unit price. Teva will make tiered royalty payments based on net commercial sales of ADASUVE in the United States. In March 2014, Teva announced the U.S. launch of ADASUVE.
Unless earlier terminated, the Teva Agreement continues in effect until the later of the last to expire patent covering ADASUVE in the United States or a specified number of years after first commercial sale. The Agreement is subject to earlier termination in the event the parties mutually agree, by a party in the event of an uncured material breach by the other party or upon the bankruptcy or insolvency of either party. Teva may also terminate the Teva Agreement in the event the FDA requires withdrawal or suspension of ADASUVE from the market due to safety reasons or, subject to a specified period of notice, for Teva’s convenience at any time following the first anniversary of the Teva Agreement.
99
We evaluated whether the delivered elements under the Teva Agreement have value on a stand-alone basis and allocated revenue to the identified units of accounting based on relative fair value. We determined that the license fees are a single unit of accounting and valued the license based on its best estimate of selling price, as VSOE and TPE of the selling price could not be determined. The selling price was estimated using discounted projected cash flows related to the licensed territory. We have delivered the license to Teva and recognized the $40,000,000 non-refundable upfront payment as revenue.
As described in Note 7, in connection with the Teva Agreement, we received a right-to-borrow under the Teva Note. We have the ability to draw on the Teva Note in amounts not to exceed $25,000,000, over a two year period. As outlined in Note 7, the Teva Note has a fixed conversion price and interest rate. This right-to-borrow was considered additional consideration provided by Teva to us pursuant to the Teva Agreement. We performed an analysis using a Monte Carlo simulation model with a volatility rate of 70% and an estimated debt yield of 15%. Based on this analysis, we determined that the fair value of this right-to-borrow was $2,800,000. We recorded this amount as revenue and as another asset upon entering into the Teva Note. The asset is being amortized to interest expense over the two year period during which we are able to exercise the right-to-borrow. As we draw on the Teva Note, the relative portion of the unamortized right-to-borrow is accounted for as a discount on the borrowing and will be amortized to interest expense over the life of the borrowing. In 2013, we drew down $15,000,000 against the Teva Note and reclassified $1,293,000 of the unamortized right-to-borrow as a discount on the borrowing to be amortized to interest expense over the life of the borrowing. We recorded $752,000 in interest expense related to the right-to-borrow in 2013.
As noted above, we are eligible to receive up to $195,000,000 of additional payments from Teva related to Teva’s successful completion of the ADASUVE post-approval studies in the United States and Teva achieving specified net sales targets. The payments related to net sales targets will be recognized as royalty revenue when each target is earned and payable to us. The payment related to the completion of the ADASUVE post-approval studies will be recognized upon completion of the studies when the payment is earned and payable to us.
9. Warrants
In May 2010, in conjunction with the Loan Agreement with Hercules, we issued to Hercules a five-year warrant to purchase 37,639 shares of our common stock at a price of $26.90 per share. The warrant expires in May 2015. At December 31, 2013, this warrant remained outstanding and exercisable.
In August 2010, we issued an aggregate of 668,518 shares of our common stock and warrants to purchase up to an additional 334,258 shares of our common stock in a registered direct offering. These securities were sold as units with each unit consisting of (i) one share of common stock and (ii) a warrant to purchase 0.5 of a share of common stock, at a purchase price of $27.00 per unit. The warrants are exercisable at $33.00 per share and expire five years after August 2010. At December 31, 2013, these warrants remained outstanding and exercisable.
In May 2011, we issued an aggregate of 1,192,703 shares of our common stock and warrants to purchase up to an additional 417,445 shares of our common stock in a registered direct offering. The warrants are exercisable at $17.55 per share and will expire on May 6, 2016. At December 31, 2013, these warrants remained outstanding and exercisable.
In February 2012, we issued an aggregate of 4,400,000 shares of our common stock and warrants to purchase up to an additional 4,400,000 shares of our common stock in an underwritten public offering. The warrants are exercisable beginning February 24, 2013, at an exercise price of $5.00 per share, and will expire on February 23, 2017. At December 31, 2013, these warrants remained outstanding and exercisable.
All outstanding warrants include a provision that allows the warrant holder to net share settle the warrant. In no circumstances will we issue shares in excess of the number of shares underlying the warrant. The outstanding warrants are classified as stockholders’ equity and are indexed to our common stock.
10. Equity Incentive Plans
2005 Equity Incentive Plan
In December 2005, our Board of Directors adopted the 2005 Equity Incentive Plan, or the 2005 Plan, and authorized for issuance thereunder 108,879 shares of common stock. The 2005 Plan became effective upon the
100
closing of our initial public offering on March 8, 2006. The 2005 Plan is an amendment and restatement of our previous stock option plans.
Stock options issued under the 2005 Plan generally vest over 4 years, vesting is generally based on service time, and have a maximum contractual term of 10 years. Restricted stock units granted to non-employee directors, which are granted in lieu of paying director fees in cash, generally vest one year after the date of grant. Prior to vesting, restricted stock units do not have dividend equivalent rights, do not have voting rights and the shares underlying the restricted units are not considered issued and outstanding. Shares are issued on the date the restricted stock units vest.
The 2005 Plan provides for annual reserve increases on the first day of each fiscal year commencing on January 1, 2007 and ending on January 1, 2015. The annual reserve increases will be equal to the lesser of (i) 2% of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year, or (ii) 100,000 shares of common stock. Our Board of Directors has the authority to designate a smaller number of shares by which the authorized number of shares of common stock will be increased prior to the last day of any calendar year.
In May 2008, our stockholders approved an amendment to the Plan to increase the number of shares of our stock reserved for issuance under the 2005 Plan by an additional 150,000 shares. In July 2011, following stockholder approval, the 2005 Plan was amended to increase the shares of common stock reserved for issuance pursuant to the 2005 Plan by 750,000 shares of common stock as well as to increase the number of shares that can be issued as incentive stock options pursuant to the 2005 Plan. In May 2013, our stockholders approved an amendment to the 2005 Plan to increase the shares reserved by an additional 2,200,000 shares.
2005 Non-Employee Directors’ Stock Option Plan
In December 2005, our Board of Directors adopted the 2005 Non-Employee Directors’ Stock Option Plan, or the Directors’ Plan, and authorized for issuance thereunder 25,000 shares of common stock. The Directors’ Plan provides for the automatic grant of nonstatutory stock options to purchase shares of common stock to our non-employee directors, which vest over four years and have a term of 10 years. The Directors’ Plan provides for an annual reserve increase to be added on the first day of each fiscal year, commencing on January 1, 2007 and ending on January 1, 2015. The annual reserve increases will be equal to the number of shares subject to options granted during the preceding fiscal year less the number of shares that revert back to the share reserve during the preceding fiscal year. Our Board of Directors has the authority to designate a smaller number of shares by which the authorized number of shares of common stock will be increased prior to the last day of any calendar year. In May 2013, our stockholders approved an amendment to the Directors’ Plan to increase the shares reserved by an additional 200,000 shares.
2011 Employee Stock Option Exchange Program
On January 21, 2011, we commenced a voluntary employee stock option exchange program, or the Exchange Program, to permit our eligible employees to exchange some or all of their eligible outstanding options, or Original Options to purchase our common stock with an exercise price greater than or equal to $23.70 per share, whether vested or unvested, for a lesser number of new stock options, or New Options. In accordance with the terms and conditions of the Exchange Program, on February 22, 2011, or the Grant Date, we accepted outstanding options to purchase an aggregate of 212,843 shares of our common stock, with exercise prices ranging from $23.80 to $117.00, and issued, in exchange, an aggregate of 80,890 New Options with an exercise price of $12.30. The New Options vested 33% on February 22, 2012 with the balance of the shares vesting in a series of twenty-four successive equal monthly installments thereafter, and have a term of five years. The exchange resulted in a decrease in our common stock subject to outstanding stock options by 131,953 shares, which increased the number of shares available to be issued under the 2005 Plan. The Exchange Program did not result in incremental share-based compensation.
101
The following table sets forth the summary of option activity under our share-based compensation plans:
| | | | | | | | |
| | | Outstanding Options | |
| | | Number of
Shares | | | Weighted Average
Exercise Price | |
Balance as of January 1, 2013 | | | 1,038,245 | | | $ | 12.24 | |
Options granted | | | 1,369,030 | | | | 4.49 | |
Options exercised | | | (10,317 | ) | | | 3.48 | |
Options cancelled | | | (275,454 | ) | | | 10.31 | |
| | | | | | | | |
Balance as of December 31, 2013 | | | 2,121,504 | | | $ | 7.53 | |
| | | | | | | | |
At December 31, 2013, options to exercise 607,000 shares of our common stock, at a weighted average exercise price of $15.22 were exercisable.
The intrinsic value of options exercised is calculated based on the difference between the exercise price and the quoted market price of our stock on the exercise date. The total intrinsic value of options exercised during the years ended December 31, 2013, 2012 and 2011 was $13,000, $0, and $0, respectively. None of our options have expired.
Information regarding the stock options outstanding at December 31, 2013 is summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Outstanding | | | Exercisable | |
Exercise Price | | Number of
Shares | | | Remaining
Contractual
Life (In
Years) | | | Aggregate
Intrinsic
Value | | | Number
of Shares | | | Remaining
Contractual
Life (In
Years) | | | Aggregate
Intrinsic
Value | |
$3.32 — $3.50 | | | 384,882 | | | | 8.53 | | | $ | 485,000 | | | | 132,783 | | | | 8.48 | | | $ | 167,000 | |
$4.08 — $4.41 | | | 154,100 | | | | 9.36 | | | | 59,000 | | | | — | | | | — | | | | — | |
$4.42 — $4.42 | | | 788,500 | | | | 9.24 | | | | 244,000 | | | | — | | | | — | | | | — | |
$4.44 — $12.30 | | | 402,350 | | | | 7.95 | | | | 21,000 | | | | 95,744 | | | | 3.31 | | | | 1,000 | |
$12.70 — $117.00 | | | 391,672 | | | | 6.82 | | | | — | | | | 378,728 | | | | 6.80 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2,121,504 | | | | 8.43 | | | $ | 809,000 | | | | 607,255 | | | | 6.62 | | | $ | 168,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The intrinsic value noted in the table above is calculated as the difference between the market value as of December 31, 2013 and the exercise price of the shares. The market value as of December 31, 2013, the last trading date of 2013, was $4.73 as reported by The NASDAQ Stock Market.
Information with respect to unvested share units (restricted stock units) as of December 31, 2013 is as follows:
| | | | | | | | |
| | | Number
of Shares | | | Weighted
Average
Grant Date
Fair Value | |
Outstanding at January 1, 2013 | | | — | | | $ | — | |
Granted | | | 590,495 | | | | 4.67 | |
Released | | | (17,795 | ) | | | 4.20 | |
Canceled | | | (51,650 | ) | | | 4.68 | |
| | | | | | | | |
Outstanding at December 31, 2013 | | | 521,050 | | | $ | 4.68 | |
| | | | | | | | |
The total value of restricted stock units released during the years ended December 31, 2013, 2012 and 2011 was $75,000, $1,443,000 and $272,000, respectively.
102
We authorized shares of common stock for issuance under the 2005 Plan and the Directors’ Plan as follows.
| | | | |
Year | | Number of Shares | |
2011 | | | 857,500 | |
2012 | | | 120,000 | |
2013 | | | 2,319,812 | |
The shares authorized for issuance in 2013 include the annual reserve increase of 119,812 shares and the stockholder approved increase of 2,200,000 shares. As of December 31, 2013, 950,396 and 166,249 shares remained available for issuance under the 2005 Plan and the Directors’ Plan, respectively.
On January 1, 2014 an additional 100,000 and 58,751 shares were authorized for issuance under the evergreen provisions of the 2005 Plan and the Directors’ Plan, respectively.
2005 Employee Stock Purchase Plan
In December 2005, our Board of Directors adopted the 2005 Employee Stock Purchase Plan (“ESPP”) and authorized for issuance thereunder 50,000 shares of common stock. The ESPP allows eligible employee participants to purchase shares of our common stock at a discount through payroll deductions. The ESPP consists of a fixed offering period, generally twenty-four months with four purchase periods within each offering period. Purchases are generally made on the last trading day of each October and April. Employees purchase shares at each purchase date at 85% of the market value of our common stock on their enrollment date or the end of the purchase period, whichever price is lower.
The ESPP provides for annual reserve increases on the first day of each fiscal year commencing on January 1, 2007 and ending on January 1, 2015. The annual reserve increases will be equal to the lesser of (i) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, or (ii) 75,000 shares of common stock. Our Board of Directors has the authority to designate a smaller number of shares by which the authorized number of shares of common stock will be increased prior to the last day of any calendar year. In May 2011, our Compensation Committee terminated the then current offering period under the ESPP and resolved to begin a new offering period in August 2011 and also amended the ESPP to reduce the time period of each offering period from twenty-four to six months.
In July 2011, following stockholder approval, the ESPP was amended to, among other changes, modify the annual automatic increase in shares reserved for the plan to an amount equal to the least of (i) one percent (1%) of the total number of shares of common stock outstanding on December 31st of the preceding calendar year, (ii) 75,000 shares of common stock and (iii) an amount determined by our Board of Directors. The new offering period under the ESPP began on August 15, 2011 and the related purchase occurred on April 30, 2012.
Pursuant to the ESPP, on January 1, 2013, 2012 and 2011 an additional 75,000, 72,136 and 25,000 shares, respectively, were reserved for issuance. We issued 54,494, 18,409 and 24,998 shares at weighted average prices of $3.67, $4.44 and $8.10, during the years ended December 31, 2013, 2012 and 2011, respectively. At December 31, 2013, 74,632 shares were available for issuance under the ESPP.
On January 1, 2014 an additional 75,000 shares were reserved for issuance under the ESPP.
11. 401(k) Plan
We sponsor a 401(k) Plan that stipulates that eligible employees can elect to contribute to the 401(k) Plan, subject to certain limitations. In 2013, we matched employee contributions of a total of $96,000.
12. Income Taxes
There is no provision for income taxes because we have incurred operating losses since inception and applied a full valuation allowance against all deferred tax assets.
103
The reported amount of income tax expense (benefit) attributable to operations for the year differs from the amount that would result from applying domestic federal statutory tax rates to loss before income taxes from operations as summarized below (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2013 | | | 2012 | | | 2011 | |
Federal tax benefit at statutory rate | | $ | (13,469 | ) | | $ | (9,512 | ) | | $ | (13,781 | ) |
State tax benefit net of federal effect | | | (2,311 | ) | | | (1,632 | ) | | | (2,365 | ) |
Research and development credits | | | (532 | ) | | | (254 | ) | | | (1,669 | ) |
Other permanent differences | | | 14 | | | | (30 | ) | | | 11 | |
Share-based compensation | | | 650 | | | | 1,040 | | | | 902 | |
Adjustment to basis in subsidiary | | | 15,899 | | | | (757 | ) | | | 1,593 | |
Change in valuation allowance | | | 251 | | | | 11,016 | | | | 15,233 | |
Other | | | — | | | | 129 | | | | 76 | |
| | | | | | | | | | | | |
Total | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
Deferred income taxes reflect the net tax effects of the temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amount used for income tax purposes. The deferred tax assets were calculated using an effective tax rate of 40%. Significant components of our deferred tax assets are as follows (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2013 | | | 2012 | |
Federal and state net operating loss carryforwards | | $ | 114,643 | | | $ | 113,216 | |
Federal and state research and development credit carryforwards | | | 14,977 | | | | 14,611 | |
Accrued liabilities | | | 8,085 | | | | 6,073 | |
Capitalized research and development costs | | | 13,845 | | | | 18,610 | |
Other | | | 2,648 | | | | 1,960 | |
| | | | | | | | |
Total deferred tax assets | | | 154,198 | | | | 154,470 | |
Valuation allowance | | | (154,198 | ) | | | (154,470 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
Our accounting for deferred taxes involves the evaluation of a number of factors concerning the realizability of our net deferred tax assets. We primarily considered such factors as our history of operating losses, the nature of our deferred tax assets and the timing, likelihood and amount, if any, of future taxable income during the periods in which those temporary differences and carryforwards become deductible. At present, we do not believe that it is more likely than not that the deferred tax assets will be realized; accordingly, a full valuation allowance has been established and no deferred tax asset is shown in the accompanying balance sheets. The valuation allowance increased by approximately $(272,000), $10,552,000 and $14,628,000 during the years ended December 31, 2013, 2012 and 2011, respectively.
As of December 31, 2013 we had federal net operating loss carryforwards of approximately $290,060,000. We also had federal research and development tax credit carryforwards of approximately $10,073,000. The net operating loss and tax credit carryforwards will expire at various dates beginning in 2020, if not utilized.
As of December 31, 2013, we had state net operating loss carryforwards of approximately $284,136,000 which will begin to expire in 2014. We also had state research and development tax credit carryforwards of approximately $4,904,000, which have no expiration.
As of December 31, 2013, approximately $1,393,000 of deferred tax assets is attributable to certain employee stock option deductions and the federal and state net operating loss carryforward has been adjusted
104
accordingly. When realized, the benefit of the tax deduction related to these options will be accounted for as a credit to stockholders’ equity rather than as a reduction of the income tax provision.
A limitation may apply to the use of the net operating loss and credit carryforwards, under provisions of the Internal Revenue Code that are applicable if we experience an “ownership change”. That may occur, for example, as a result of trading in our stock by institutional investors as well issuance of new equity. Should these limitations apply, the carryforwards would be subject to an annual limitation, resulting in a substantial reduction in the gross deferred tax assets before considering the valuation allowance. As of December 31, 2013, we have not performed an analysis to determine if our net operating loss and credit carryforwards would be subject to such limitations.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| | | | |
| |
Balance at January 1, 2011 | | | 2,958 | |
Additions based on tax positions taken during a prior period | | | — | |
Reductions based on tax positions taken during a prior period | | | (277 | ) |
Additions based on tax positions taken during the current period | | | 253 | |
Reductions based on tax positions taken during the current period | | | — | |
Reductions related to settlement of tax matters | | | — | |
Reductions related to a lapse of applicable statute of limitations | | | — | |
| | | | |
| |
Balance at December 31, 2011 | | $ | 2,934 | |
Additions based on tax positions taken during a prior period | | | — | |
Reductions based on tax positions taken during a prior period | | | (16 | ) |
Additions based on tax positions taken during the current period | | | 96 | |
Reductions based on tax positions taken during the current period | | | — | |
Reductions related to settlement of tax matters | | | — | |
Reductions related to a lapse of applicable statute of limitations | | | — | |
| | | | |
| |
Balance at December 31, 2012 | | $ | 3,014 | |
Additions based on tax positions taken during a prior period | | | — | |
Reductions based on tax positions taken during a prior period | | | (231 | ) |
Additions based on tax positions taken during the current period | | | 36 | |
Reductions based on tax positions taken during the current period | | | — | |
Reductions related to settlement of tax matters | | | — | |
Reductions related to a lapse of applicable statute of limitations | | | — | |
| | | | |
| |
Balance at December 31, 2013 | | $ | 2,819 | |
| | | | |
If we eventually are able to recognize these uncertain tax positions, the unrecognized tax benefits would not reduce the effective tax rate if we are applying a full valuation allowance against the deferred tax assets, as is our current policy.
We have not incurred any material tax interest or penalties as of December 31, 2013. We do not anticipate any significant change within 12 months of this reporting date of its uncertain tax positions. We are subject to taxation in the United States and various states jurisdictions. There are no other ongoing examinations by taxing authorities at this time. Our various tax years starting with 2001 to 2013 remain open in various taxing jurisdictions.
105
13. Quarterly Results (Unaudited)
The following table is in thousands, except per share amounts:
| | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
| | | March 31 | | | June 30 | | | September 30 | | | December 31 | |
Fiscal 2013 | | | | | | | | | | | | | | | | |
Revenues | | $ | 729 | | | $ | 43,635 | | | $ | 2,166 | | | $ | 1,309 | |
Income/(Loss) from operations | | | (9,603 | ) | | | 31,069 | | | | (10,339 | ) | | | (9,357 | ) |
Net loss | | | (20,712 | ) | | | (799 | ) | | | (12,412 | ) | | | (5,692 | ) |
Basic and diluted net loss per share | | | (1.31 | ) | | | (0.05 | ) | | | (0.72 | ) | | | (0.33 | ) |
Fiscal 2012 | | | | | | | | | | | | | | | | |
Revenues | | $ | 1,884 | | | $ | 728 | | | $ | 729 | | | $ | 729 | |
Loss from operations | | | (4,393 | ) | | | (7,186 | ) | | | (6,393 | ) | | | (10,900 | ) |
Net loss | | | (3,827 | ) | | | (6,957 | ) | | | (6,921 | ) | | | (10,273 | ) |
Basic and diluted net loss per share | | | (0.42 | ) | | | (0.58 | ) | | | (0.52 | ) | | | (0.65 | ) |
14. Subsequent Events
Teva Draw Down
In March 2014, we drew down an additional $5.0 million against the Teva note.
Royalty Securitization Financing
In March 2014, we completed a royalty securitization financing, which consisted of a private placement to qualified institutional buyers and accredited institutional investors of $45.0 million of non-recourse notes issued by our wholly-owned subsidiary, or the Notes, and warrants to purchase 345,661 shares of our common stock at a price of $0.01 per share exercisable for five years from the date of issuance. The Notes bear interest at 12.25% per annum payable quarterly beginning June 15, 2014. All royalty and milestone payments under the Teva Agreement, after paying interest, administrative fees, and any applicable taxes, will be applied to principal and interest payments on the Notes until the Notes have been paid in full. From the proceeds of the transaction, we established a $6.9 million interest reserve account to cover potential shortfall in interest payments. Any remaining amounts in the interest reserve account will be released to us commencing June 15, 2015, subject to meeting certain net sales targets for ADASUVE, or, if not previously released, on or after December 15, 2015, irrespective of ADASUVE net sales. All other payments of principal and interest on the Notes will be made from royalty revenues from sales in the U.S. of ADASUVE, as well as potential U.S. commercialization and regulatory milestone payments due to Alexza. The Notes are secured by the right to receive royalty and milestone payments under the Teva Agreement and our equity ownership in the wholly-owned subsidiary. The Notes have no other recourse to us. The Notes may not be redeemed at our option until after March 18, 2016, and may be redeemed after that date subject to the achievement of certain milestones and the payment of a redemption premium for any redemption occurring prior to March 19, 2019. The Notes are not convertible into Alexza equity, nor have we guaranteed them. After fees and expenses, the net proceeds to us were approximately $41 million before the establishment of the interest reserve account.
106
| Item 9. Changes | in and Disagreements With Accountants on Accounting and Financial Disclosure |
Not Applicable.
| Item 9A. Controls | and Procedures |
Evaluation of Disclosure Controls and Procedures:
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of December 31, 2013, the end of the period covered by this report, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective at the reasonable assurance level. We have identified a material weakness in our internal control over financial reporting. The principal factor that contributed to this material weakness was the ineffective review and verification of internally prepared reports and analyses utilized in the financial statement closing process.
Management’s Annual Report on Internal Control Over Financial Reporting:
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2013 based on the framework inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 Framework) (the COSO criteria). Based on that evaluation, our management concluded that our internal control over financial reporting was ineffective as of December 31, 2013 due to the identification of a material weakness.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
We identified a material weakness related to the ineffective review and verification of internally prepared reports and analyses utilized in the financial statement closing process. This material weakness was identified during the quarter ended December 31, 2013. This material weakness did not result in the restatement of prior quarterly or annually filed financial statements.
To remediate the material weakness described above, we have begun and will continue to implement remedial measures to improve and develop internal controls, processes and procedures in the financial statement close process.
107
Changes in Internal Control Over Financial Reporting:
Other than the changes discussed above, there has been no change in our internal control over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. Other | Information |
None.
108
PART III
| Item 10. Directors | and Executive Officers of the Registrant |
The information required by this Item concerning our directors is incorporated by reference to the information to be set forth in the sections entitled “Proposal No. 1 — Election of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive Proxy Statement for the 2014 Annual Meeting of Stockholders to be filed within 120 days after the end of our fiscal year ended December 31, 2013, or the Proxy Statement. The information required by this Item concerning our executive officers is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled “Executive Officers.” The information required by this item concerning compliance with Section 16(a) of the Exchange Act, our code of business conduct and ethics, the procedures by which security holders may recommend nominees for our board of directors and certain information related to our Audit and Ethics Committee is incorporated by reference to the information to be set forth in the sections entitled “Information Regarding the Board of Directors and Corporate Governance” and “Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement.
| Item 11. Executive | Compensation |
The information required by this Item 11 is incorporated by reference to the information to be set forth in the sections entitled “Executive Compensation” and “Information Regarding the Board of Directors and Corporate Governance” in the Proxy Statement.
| Item 12. Security | Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this Item 12 with respect to stock ownership of certain beneficial owners and management is incorporated by reference to the information to be set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” in the Proxy Statement.
Securities Authorized For Issuance Under Equity Compensation Plans
We maintain our 2005 Equity Incentive Plan, or the 2005 Plan, 2005 Non-Employee Directors’ Stock Option Plan, or the Directors’ Plan, and 2005 Employee Stock Purchase Plan, or the ESPP, pursuant to which we may grant equity awards to eligible persons.
109
The following table gives information about equity awards under the 2005 Plan, the Directors’ Plan and the ESPP as of December 31, 2013:
| | | | | | | | | | | | |
Plan Category | | (a)
Number of Securities
to be Issued upon
Exercise of
Outstanding Options,
Warrants and Rights | | | (b)
Weighted-Average
Exercise Price of
Outstanding
Options,
Warrants and
Rights | | | (c)
Number of Securities Remaining
Available for Future Issuance
Under Equity Compensation
Plans (Excluding Securities
Reflected in Column (a)) | |
Equity compensation plans approved by security holders | | | 2,642,554 | | | $ | 6.05 | | | | 1,191,277 | (1)(2) |
Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total | | | 2,642,554 | | | $ | 6.05 | | | | 1,191,277 | |
| | | | | | | | | | | | |
| (1) | The 2005 Plan incorporates an evergreen formula pursuant to which on each January 1st, the aggregate number of shares reserved for issuance under the 2005 Plan will increase by a number equal to the least of (i) 100,000 shares, (ii) 2% of the outstanding shares on December 31st of the preceding calendar year, or (iii) an amount determined by our Board. |
The Directors’ Plan incorporates an evergreen formula pursuant to which on each January 1st, the aggregate number of shares reserved for issuance under the Directors’ Plan will increase by the number of shares subject to options granted during the preceding calendar year less the number of shares that revert back to the share reserve during the preceding calendar year.
The ESPP incorporates an evergreen formula pursuant to which on each January 1st, the aggregate number of shares reserved for issuance under the ESPP will increase by a number equal to the least of (i) 75,000 shares, (ii) 1% of the outstanding shares on December 31st of the preceding calendar year, or (iii) an amount determined by our Board.
| (2) | Of these shares, 950,396 shares remained available as of December 31, 2013 under the 2005 Plan, 166,249 shares under the Directors’ Plan and 74,632 under the ESPP. |
| Item 13. Certain | Relationships and Related Transactions and Director Independence |
The information required by this Item 13 is incorporated by reference to the information to be set forth in the sections entitled “Certain Relationships and Related Transactions and Director Independence” and “Information Regarding the Board of Directors and Corporate Governance” in the Proxy Statement.
| Item 14. Principal | Accountant Fees and Services |
The information required by this Item 14 is incorporated by reference to the information to be set forth in the sections entitled “Principal Accountant Fees and Services” and “Pre-Approval Policies and Procedures” in the Proxy Statement.
110
PART IV
| Item 15. Exhibits | and Financial Statement Schedules |
(a) 1. Financial Statements
See Index to Financial Statements under Item 8 on page [71]
(a) 2. Financial Statement Schedules
All schedules are omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Financial Statements or notes thereto.
(a) 3. Exhibits
111
EXHIBIT INDEX
| | |
Exhibit
Number | | Description of Document |
| 3.1 | | Restated Certificate of Incorporation(23) |
| 3.2 | | Certificate of Amendment to Restated Certificate of Incorporation(23) |
| 3.3 | | Certificate of Amendment to Restated Certificate of Incorporation(23) |
| 3.4 | | Amended and Restated Bylaws(1) |
| 3.5 | | Amendment to Amended and Restated Bylaws(5) |
| 4.1 | | Specimen Common Stock Certificate(1) |
| 4.2 | | Second Amended and Restated Investors’ Rights Agreement between Registrant and certain holders of Preferred Stock dated November 5, 2004(1) |
| 4.3 | | Reference is made to exhibits 3.1, 3.2, 3.3, 3.4, and 3.5 |
| 10.1* | | Form of Director/Officer Indemnification Agreement entered into between Registrant and each of its directors and officers(7) |
| 10.2*¿ | | Form of Amended Change of Control Agreement between Registrant and each of its officers |
| 10.3.1* | | 2005 Equity Incentive Plan, as amended(27) |
| 10.3.2* | | Form of Option Grant Notice, Form of Option Agreement and Form of Notice of Exercise to 2005 Equity Incentive Plan(1) |
| 10.3.3* | | Form of Notice of Grant of Stock Options to 2005 Equity Incentive Plan(15) |
| 10.3.4* | | Form of Option Agreement to 2005 Equity Incentive Plan(15) |
| 10.3.5* | | Form of Option Agreement to 2005 Equity Incentive Plan(15) |
| 10.3.6* | | Form of Notice of Grant of Award and Stock Unit Award Agreement to 2005 Equity Incentive Plan(11) |
| 10.4.1 | | 2005 Non-Employee Directors’ Stock Option Plan, as amended(30) |
| 10.4.2 | | Form of Option Grant Notice, Form of Option Agreement and Form of Notice of Exercise to 2005 Non-Employee Directors’ Stock Option Plan(1) |
| 10.5.1* | | 2005 Employee Stock Purchase Plan, as amended(18) |
| 10.5.2* | | Form of Offering Document to 2005 Employee Stock Purchase Plan(1) |
| 10.6 | | Warrant to Purchase shares of Series B Preferred Stock issued to Silicon Valley Bank dated March 20, 2002(1) |
| 10.7 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated January 7, 2003, as amended on March 4, 2003(1) |
| 10.8 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated September 19, 2003(1) |
| 10.9 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated April 7, 2004(1) |
| 10.10.1 | | Lease Agreement between the Brittania, LLC and Registrant dated August 25, 2006(2) |
| 10.10.2 | | First Amendment to Lease between Britannia Hacienda VIII LLC and Registrant dated May 4, 2007(3) |
| 10.10.3† | | Second Amendment to Lease between Britannia Hacienda VIII LLC and Registrant dated August 28, 2007(4) |
| 10.10.4 | | Partial Lease Termination Agreement between the Registrant and Britannia Hacienda VIII LLC dated February 7, 2012(21) |
| 10.11.1† | | Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc., dated November 2, 2007(5) |
112
| | |
| 10.11.2† | | Amendment No. 1 to Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc. dated June 30, 2010(12) |
| 10.11.3† | | Amendment No. 2 to Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc. dated February 15, 2011(20) |
| 10.12 | | Promissory Note issued by Registrant to Autoliv ASP, Inc. dated June 30, 2010(12) |
| 10.13 | | Promissory Note issued by Registrant to Autoliv ASP, Inc. dated February 15, 2011(20) |
| 10.14* | | Offer Letter between Registrant and Michael Simms, dated January 23, 2008(5) |
| 10.15 | | Stock and Warrant Purchase Agreement between Registrant and Biomedical Investment Fund Pte Ltd., dated March 26, 2008(6) |
| 10.16 | | Warrant to Purchase shares of Common Stock issued to Biomedical Investment Fund Pte Ltd. dated March 27, 2008(6) |
| 10.17 | | Amended and Restated Purchase Option Agreement by and among Registrant, Symphony Allegro Holdings LLC and Symphony Allegro, Inc. dated June 15, 2009(8) |
| 10.18 | | Warrant Purchase Agreement between Registrant and Symphony Allegro Holdings LLC dated June 15, 2009(8) |
| 10.19 | | Amended and Restated Registration Rights Agreement between Registrant and Symphony Allegro Holdings LLC dated June 15, 2009(8) |
| 10.20 | | Form of Warrants to Purchase Shares of Common Stock, dated August 26, 2009(9) |
| 10.21 | | Letter Agreement among Registrant, Symphony Allegro Holdings LLC, Symphony Capital Partners, L.P. and Symphony Strategic Partners, LLC, dated August 26, 2009(9) |
| 10.22 | | Securities Purchase Agreement by and among Registrant and the purchasers identified therein, dated September 29, 2009(10) |
| 10.23 | | Form of Warrants to Purchase shares of Common Stock, dated October 5, 2009(10) |
| 10.24.1 | | Loan and Security Agreement between Registrant and Hercules Technology Growth Capital, Inc. dated May 4, 2010(12) |
| 10.24.2 | | Amendment No. 1 to Loan and Security Agreement between Registrant and Hercules Technology Growth Capital, Inc. dated September 20, 2010(13) |
| 10.24.3 | | Amendment No. 2 to Loan and Security Agreement among Registrant, Symphony Allegro, Inc. and Hercules Technology Growth Capital, Inc. dated January 25, 2012(21) |
| 10.25 | | Warrant issued by Registrant to Hercules Technology Growth Capital, Inc. dated May 4, 2010(12) |
| 10.26 | | Form of Warrant to Purchase Shares of Common Stock dated August 10, 2010(14) |
| 10.27 | | Securities Purchase Agreement dated May 3, 2011(17) |
| 10.28 | | Form of Warrant to Purchase Shares of Common Stock dated May 6, 2011(17) |
| 10.29.1† | | Collaboration, License and Supply Agreement between Registrant and Grupo Ferrer Internacional, S.A. dated October 5, 2011(21) |
| 10.29.2 | | Amendment to Collaboration, License and Supply Agreement between Registrant and Grupo Ferrer, Internacional, S.A. dated March 5, 2012(21) |
| 10.30 | | Form of Warrant to Purchase Shares of Common Stock dated February 23, 2012(19) |
| 10.31* | | Form of Retention Bonus letter agreement between Registrant and each of Thomas B. King, James V. Casella, Michael J. Simms and Mark K. Oki dated March 23, 2012(22) |
| 10.32* | | Form of Issuance of an Officer RSU Retention Grant letter agreement between Registrant and each of Thomas B. King, James V. Casella, Michael J. Simms and Mark K. Oki dated March 23, 2012(22) |
| 10.33* | | 2012 Cash Bonus Plan(22) |
| 10.34 | | Common Stock Purchase Agreement between Registrant and Azimuth Opportunity, L.P. dated July 20, 2012(24) |
113
| | |
| 10.35 | | Summary of Compensation Arrangements with Non-Employee Directors(16) |
| 10.36 | | Amendment to License & Development Agreement between Alexza Pharmaceuticals, Inc. and Royalty Pharma dated January 4, 2013(25) |
| 10.37* | | 2013 Cash Bonus Plan(26) |
| 10.38 | | License and Supply Agreement between Registrant and Teva Pharmaceuticals USA, Inc. dated May 7, 2013(29) |
| 10.39 | | Convertible Promissory Note and Agreement to Lend between Registrant and Teva Pharmaceuticals USA, Inc. dated May 7, 2013(28) |
| 14.1 | | Alexza Pharmaceuticals, Inc. Code of Business Conduct for Employees, Executive Officers and Directors(2) |
| 21.1¿ | | Subsidiaries of Registrant |
| 23.1¿ | | Consent of Independent Registered Public Accounting Firm |
| 24.1¿ | | Power of Attorney included on the signature pages hereto |
| 31.1¿ | | Section 302 Certification of principal executive officer. |
| 31.2¿ | | Section 302 Certification of principal financial officer. |
| 32.1‡ | | Section 1350 Certifications of CEO and CFO. |
| 101.INS | | XBRL Instance Document (filed electronically herewith). |
| 101.SCH | | XBRL Taxonomy Extension Schema Document (filed electronically herewith). |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document (filed electronically herewith). |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document (filed electronically herewith). |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document (filed electronically herewith). |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document (filed electronically herewith). |
| * | Management contract or compensation plan or arrangement. |
| † | Confidential treatment has been granted with respect to certain portions of this exhibit. This exhibit omits the information subject to this confidentiality request. Omitted portions have been filed separately with the SEC. |
| (1) | Incorporated by reference to exhibits to our Registration Statement on Form S-1 filed on December 22, 2005, as amended (File No. 333-130644). |
| (2) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 29, 2007. |
| (3) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on August 13, 2007. |
| (4) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 1, 2007. |
| (5) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 17, 2008. |
| (6) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on March 17, 2008. |
| (7) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on July 14, 2008. |
| (8) | Incorporated by reference to our Current Report on Form 8-K/A (File No. 000-51820) as filed with the SEC on June 26, 2009. |
| (9) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 26, 2009. |
114
| (10) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on September 30, 2009. |
| (11) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 10, 2009. |
| (12) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on July 26, 2010. |
| (13) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 9, 2010. |
| (14) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 5, 2010. |
| (15) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on February 22, 2011. |
| (16) | Incorporated by reference to our Annual Report on Form 10-K/A (File No. 000-51820) as filed with the SEC on April 28, 2011. |
| (17) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on May 3, 2011. |
| (18) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 2, 2011. |
| (19) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on February 17, 2012. |
| (20) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 15, 2011. |
| (21) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 12, 2012. |
| (22) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on May 10, 2012. |
| (23) | Incorporated by reference to exhibits to our Registration Statement on Form S-3 filed on June 26, 2012 (File No. 333-182341). |
| (24) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on July 23, 2012. |
| (25) | Incorporated by reference to our Annual Report on Form 10-K filed on March 26, 2013 (File No. 000-51820). |
| (26) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on April 2, 2013. |
| (27) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on May 22, 2013. |
| (28) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on August 14, 2013. |
| (29) | Incorporated by reference to our Quarterly Report on Form 10-Q/A (File No. 000-51820) as filed with the SEC on October 23, 2013. |
| (30) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 6, 2013. |
115
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
|
| ALEXZA PHARMACEUTICALS, INC. |
| |
| By: | | /S/ THOMAS B. KING |
| | Thomas B. King |
| | President and Chief Executive Officer |
Dated: March 25, 2014
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Thomas B. King and Mark K. Oki, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Exchange Act, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated on March , 2014.
| | |
Signature | | Title |
/s/ THOMAS B. KING Thomas B. King | | President, Chief Executive Officer and Director (Principal Executive Officer) |
| |
/s/ MARK K. OKI Mark K. Oki | | Senior Vice President, Finance, Chief Financial Officer and Secretary (Principal Accounting Officer, Principal Financial Officer) |
| |
/s/ J. KEVIN BUCHI J. Kevin Buchi | | Director |
| |
/s/ DEEPIKA R. PAKIANATHAN Deepika R. Pakianathan | | Director |
| |
/s/ J. LEIGHTON READ J. Leighton Read | | Director |
| |
/s/ GORDON RINGOLD Gordon Ringold | | Director |
| |
/s/ ISAAC STEIN Isaac Stein | | Director |
| |
/s/ JOSEPH L. TURNER Joseph L. Turner | | Director |
116
EXHIBIT INDEX
| | |
Exhibit
Number | | Description of Document |
| 3.1 | | Restated Certificate of Incorporation(23) |
| 3.2 | | Certificate of Amendment to Restated Certificate of Incorporation(23) |
| 3.3 | | Certificate of Amendment to Restated Certificate of Incorporation(23) |
| 3.4 | | Amended and Restated Bylaws(1) |
| 3.5 | | Amendment to Amended and Restated Bylaws(5) |
| 4.1 | | Specimen Common Stock Certificate(1) |
| 4.2 | | Second Amended and Restated Investors’ Rights Agreement between Registrant and certain holders of Preferred Stock dated November 5, 2004(1) |
| 4.3 | | Reference is made to exhibits 3.1, 3.2, 3.3, 3.4, and 3.5 |
| 10.1* | | Form of Director/Officer Indemnification Agreement entered into between Registrant and each of its directors and officers(7) |
| 10.2*¿ | | Form of Amended Change of Control Agreement between Registrant and each of its officers |
| 10.3.1* | | 2005 Equity Incentive Plan, as amended(27) |
| 10.3.2* | | Form of Option Grant Notice, Form of Option Agreement and Form of Notice of Exercise to 2005 Equity Incentive Plan(1) |
| 10.3.3* | | Form of Notice of Grant of Stock Options to 2005 Equity Incentive Plan(15) |
| 10.3.4* | | Form of Option Agreement to 2005 Equity Incentive Plan(15) |
| 10.3.5* | | Form of Option Agreement to 2005 Equity Incentive Plan(15) |
| 10.3.6* | | Form of Notice of Grant of Award and Stock Unit Award Agreement to 2005 Equity Incentive Plan(11) |
| 10.4.1 | | 2005 Non-Employee Directors’ Stock Option Plan(30) |
| 10.4.2 | | Form of Option Grant Notice, Form of Option Agreement and Form of Notice of Exercise to 2005 Non-Employee Directors’ Stock Option Plan(1) |
| 10.5.1* | | 2005 Employee Stock Purchase Plan, as amended(18) |
| 10.5.2* | | Form of Offering Document to 2005 Employee Stock Purchase Plan(1) |
| 10.6 | | Warrant to Purchase shares of Series B Preferred Stock issued to Silicon Valley Bank dated March 20, 2002(1) |
| 10.7 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated January 7, 2003, as amended on March 4, 2003(1) |
| 10.8 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated September 19, 2003(1) |
| 10.9 | | Warrant to Purchase shares of Series C Preferred Stock issued to Silicon Valley Bank dated April 7, 2004(1) |
| 10.10.1 | | Lease Agreement between the Brittania, LLC and Registrant dated August 25, 2006(2) |
| 10.10.2 | | First Amendment to Lease between Britannia Hacienda VIII LLC and Registrant dated May 4, 2007(3) |
| 10.10.3† | | Second Amendment to Lease between Britannia Hacienda VIII LLC and Registrant dated August 28, 2007(4) |
| 10.10.4 | | Partial Lease Termination Agreement between the Registrant and Britannia Hacienda VIII LLC dated February 7, 2012(21) |
| 10.11.1† | | Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc., dated November 2, 2007(5) |
117
| | |
| 10.11.2† | | Amendment No. 1 to Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc. dated June 30, 2010(12) |
| 10.11.3† | | Amendment No. 2 to Manufacturing and Supply Agreement between Registrant and Autoliv ASP, Inc. dated February 15, 2011(20) |
| 10.12 | | Promissory Note issued by Registrant to Autoliv ASP, Inc. dated June 30, 2010(12) |
| 10.13 | | Promissory Note issued by Registrant to Autoliv ASP, Inc. dated February 15, 2011(20) |
| 10.14* | | Offer Letter between Registrant and Michael Simms, dated January 23, 2008(5) |
| 10.15 | | Stock and Warrant Purchase Agreement between Registrant and Biomedical Investment Fund Pte Ltd., dated March 26, 2008(6) |
| 10.16 | | Warrant to Purchase shares of Common Stock issued to Biomedical Investment Fund Pte Ltd. dated March 27, 2008(6) |
| 10.17 | | Amended and Restated Purchase Option Agreement by and among Registrant, Symphony Allegro Holdings LLC and Symphony Allegro, Inc. dated June 15, 2009(8) |
| 10.18 | | Warrant Purchase Agreement between Registrant and Symphony Allegro Holdings LLC dated June 15, 2009(8) |
| 10.19 | | Amended and Restated Registration Rights Agreement between Registrant and Symphony Allegro Holdings LLC dated June 15, 2009(8) |
| 10.20 | | Form of Warrants to Purchase Shares of Common Stock, dated August 26, 2009(9) |
| 10.21 | | Letter Agreement among Registrant, Symphony Allegro Holdings LLC, Symphony Capital Partners, L.P. and Symphony Strategic Partners, LLC, dated August 26, 2009(9) |
| 10.22 | | Securities Purchase Agreement by and among Registrant and the purchasers identified therein, dated September 29, 2009(10) |
| 10.23 | | Form of Warrants to Purchase shares of Common Stock, dated October 5, 2009(10) |
| 10.24.1 | | Loan and Security Agreement between Registrant and Hercules Technology Growth Capital, Inc. dated May 4, 2010(12) |
| 10.24.2 | | Amendment No. 1 to Loan and Security Agreement between Registrant and Hercules Technology Growth Capital, Inc. dated September 20, 2010(13) |
| 10.24.3 | | Amendment No. 2 to Loan and Security Agreement among Registrant, Symphony Allegro, Inc. and Hercules Technology Growth Capital, Inc. dated January 25, 2012(21) |
| 10.25 | | Warrant issued by Registrant to Hercules Technology Growth Capital, Inc. dated May 4, 2010(12) |
| 10.26 | | Form of Warrant to Purchase Shares of Common Stock dated August 10, 2010(14) |
| 10.27 | | Securities Purchase Agreement dated May 3, 2011(17) |
| 10.28 | | Form of Warrant to Purchase Shares of Common Stock dated May 6, 2011(17) |
| 10.29.1† | | Collaboration, License and Supply Agreement between Registrant and Grupo Ferrer Internacional, S.A. dated October 5, 2011(21) |
| 10.29.2 | | Amendment to Collaboration, License and Supply Agreement between Registrant and Grupo Ferrer, Internacional, S.A. dated March 5, 2012(21) |
| 10.30 | | Form of Warrant to Purchase Shares of Common Stock dated February 23, 2012(19) |
| 10.31* | | Form of Retention Bonus letter agreement between Registrant and each of Thomas B. King, James V. Casella, Michael J. Simms and Mark K. Oki dated March 23, 2012(22) |
| 10.32* | | Form of Issuance of an Officer RSU Retention Grant letter agreement between Registrant and each of Thomas B. King, James V. Casella, Michael J. Simms and Mark K. Oki dated March 23, 2012(22) |
| 10.33* | | 2012 Cash Bonus Plan(22) |
| 10.34 | | Common Stock Purchase Agreement between Registrant and Azimuth Opportunity, L.P. dated July 20, 2012(24) |
118
| | |
| 10.35 | | Summary of Compensation Arrangements with Non-Employee Directors(16) |
| 10.36 | | Amendment to License & Development Agreement between Alexza Pharmaceuticals, Inc. and Royalty Pharma dated January 4, 2013(25) |
| 10.37* | | 2013 Cash Bonus Plan(26) |
| 10.38 | | License and Supply Agreement between Registrant and Teva Pharmaceuticals USA, Inc. dated May 7, 2013(29) |
| 10.39 | | Convertible Promissory Note and Agreement to Lend between Registrant and Teva Pharmaceuticals USA, Inc. dated May 7, 2013(28) |
| 14.1 | | Alexza Pharmaceuticals, Inc. Code of Business Conduct for Employees, Executive Officers and Directors(2) |
| 21.1¿ | | Subsidiaries of Registrant |
| 23.1¿ | | Consent of Independent Registered Public Accounting Firm |
| 24.1¿ | | Power of Attorney included on the signature pages hereto |
| 31.1¿ | | Section 302 Certification of principal executive officer. |
| 31.2¿ | | Section 302 Certification of principal financial officer. |
| 32.1‡ | | Section 1350 Certifications of CEO and CFO. |
| 101.INS | | XBRL Instance Document (filed electronically herewith). |
| 101.SCH | | XBRL Taxonomy Extension Schema Document (filed electronically herewith). |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document (filed electronically herewith). |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document (filed electronically herewith). |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document (filed electronically herewith). |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document (filed electronically herewith). |
| * | Management contract or compensation plan or arrangement. |
| † | Confidential treatment has been granted with respect to certain portions of this exhibit. This exhibit omits the information subject to this confidentiality request. Omitted portions have been filed separately with the SEC. |
| (1) | Incorporated by reference to exhibits to our Registration Statement on Form S-1 filed on December 22, 2005, as amended (File No. 333-130644). |
| (2) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 29, 2007. |
| (3) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on August 13, 2007. |
| (4) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 1, 2007. |
| (5) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 17, 2008. |
| (6) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on March 17, 2008. |
| (7) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on July 14, 2008. |
| (8) | Incorporated by reference to our Current Report on Form 8-K/A (File No. 000-51820) as filed with the SEC on June 26, 2009. |
| (9) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 26, 2009. |
119
| (10) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on September 30, 2009. |
| (11) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 10, 2009. |
| (12) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on July 26, 2010. |
| (13) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 9, 2010. |
| (14) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 5, 2010. |
| (15) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on February 22, 2011. |
| (16) | Incorporated by reference to our Annual Report on Form 10-K/A (File No. 000-51820) as filed with the SEC on April 28, 2011. |
| (17) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on May 3, 2011. |
| (18) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on August 2, 2011. |
| (19) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on February 17, 2012. |
| (20) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 15, 2011. |
| (21) | Incorporated by reference to our Annual Report on Form 10-K (File No. 000-51820) as filed with the SEC on March 12, 2012. |
| (22) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on May 10, 2012. |
| (23) | Incorporated by reference to exhibits to our Registration Statement on Form S-3 filed on June 26, 2012 (File No. 333-182341). |
| (24) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on July 23, 2012. |
| (25) | Incorporated by reference to our Annual Report on Form 10-K filed on March 26, 2013 (File No. 000-51820). |
| (26) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on April 2, 2013. |
| (27) | Incorporated by reference to our Current Report on Form 8-K (File No. 000-51820) as filed with the SEC on May 22, 2013. |
| (28) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on August 14, 2013. |
| (29) | Incorporated by reference to our Quarterly Report on Form 10-Q/A (File No. 000-51820) as filed with the SEC on October 23, 2013. |
| (30) | Incorporated by reference to our Quarterly Report on Form 10-Q (File No. 000-51820) as filed with the SEC on November 6, 2013. |
120


