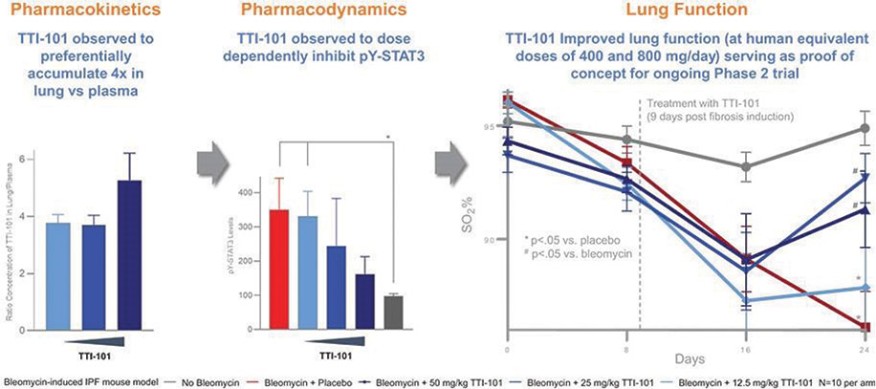
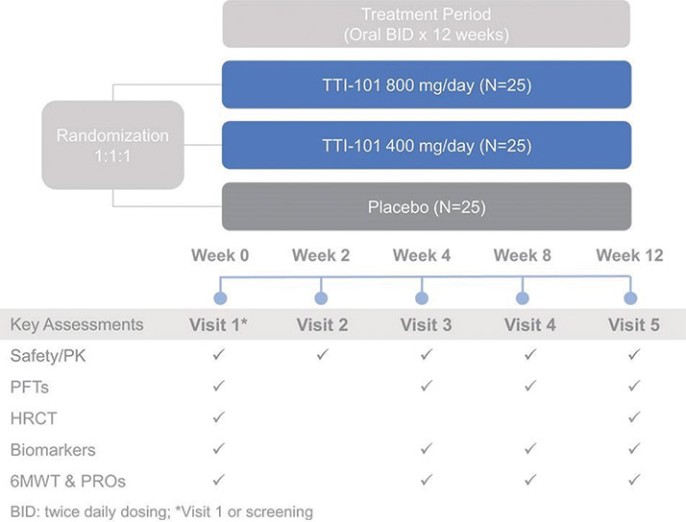
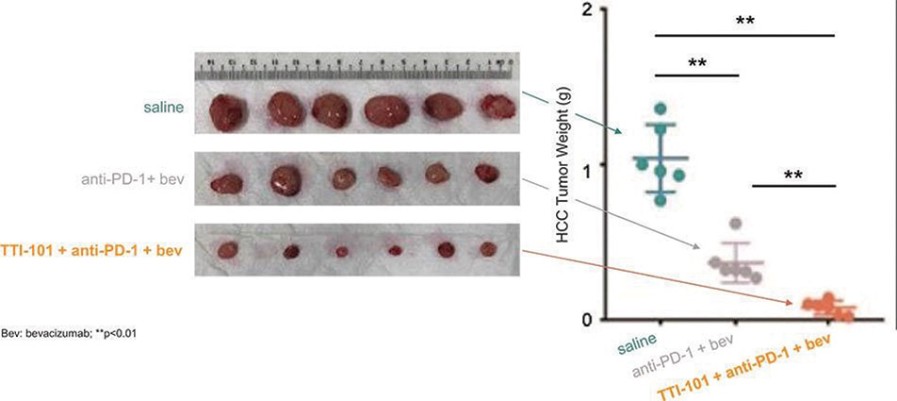
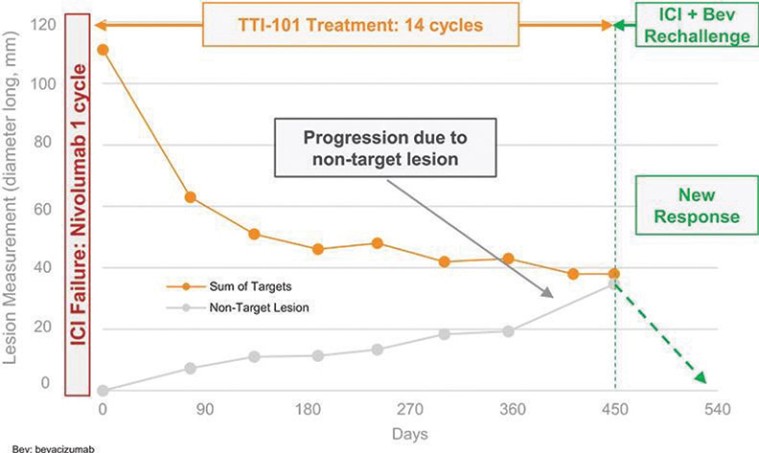
Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, Turkey, the United Kingdom and Hong Kong, all expiring on July 18, 2034.
The fourth patent family in-licensed from BCM relates to methods of using TTI-101 to reduce the risk or severity of or prevent allergic reaction. This family includes one pending U.S. patent application and 14 issued foreign patents in Australia, Canada, Belgium, France, Germany, Ireland, Italy, Netherlands, Norway, Spain, Sweden, Switzerland, Turkey, United Kingdom and Hong Kong, all expiring on July 18, 2034.
Tvardi co-owns one patent family with BCM. This family is directed to methods of using TTI-101 to treat insulin resistance. This patent family includes one pending U.S. patent application and four pending foreign patent application in Canada, China, Europe and Japan. If issued, the patent applications in this patent family are expected to expire on December 3, 2040.
In addition to the above, TTI-101 is protected by seven patent families owned by Tvardi.
The first patent family Tvardi owns relates to self-emulsifying drug dispersion formulation of TTI-101 and includes three issued U.S. patents and one issued foreign patent in Eurasia, all expiring on January 22, 2041, and three pending foreign patent applications in China, Europe and Hong Kong.
The second patent family Tvardi owns relates to spray-dried dispersion tablets of TTI-101 and includes one pending U.S. patent application and 15 pending patent applications in Argentina, Pakistan, Taiwan, Australia, Brazil, Canada, Eurasia, Europe, India, Israel, Japan, Korea, Mexico, New Zealand and Singapore. If issued, patents in this family are expected to expire on March 1, 2043.
The third patent family Tvardi owns relates to highly pure compositions of TTI-101 and includes one pending U.S. patent application, three pending foreign patent applications in Argentina, Pakistan and Taiwan, and one pending PCT application. If issued, patents in this family are expected to expire on July 18, 2043.
The fourth patent family Tvardi owns relates to methods of treating cancer using a combination of TTI-101 and an immune checkpoint inhibitor such as anti-PD-1 antibody and anti-PD-L1 antibody and includes one pending U.S. patent application and 8 pending foreign patent applications in Australia, Canada, Eurasia, Europe, Japan, Mexico, New Zealand and Singapore. If issued, patents in this family are expected to expire on March 3, 2043.
The fifth patent family Tvardi owns relates to methods of treating non-viral liver cancer with TTI-101 and includes one pending PCT application. If issued, patents in this family are expected to expire on December 11, 2043.
The sixth patent family Tvardi owns relates to methods of treating cancer with certain doses of TTI-101 and includes one pending PCT application. If issued, patents in this family are expected to expire on September 5, 2044.
The seventh patent family Tvardi owns relates to methods of treating cancer with TTI-101 in certain patient populations and includes one pending U.S. provisional patent application. If issued, patents in this family are expected to expire on February 29, 2045.
TTI-109 is protected by three patent families owned by Tvardi.
The first patent family claims the TTI-109 compound and includes one issued U.S. patent expiring on June 9, 2043, one pending U.S. patent application, three pending foreign patent applications in Argentina, Pakistan and Taiwan and one pending PCT application. If issued, patents in this family are expected to expire on June 9, 2043.
The second patent family relates to methods of treating cancer with TTI-109 in certain patient populations and includes one pending U.S. provisional patent application. If issued, patents in this family are expected to expire on February 29, 2045.
The third patent family relates to solid forms of TTI-109 and includes one pending U.S. provisional patent application. If issued, patents in this family are expected to expire on December 2, 2044.
Tvardi cannot predict whether the patent applications it pursues or may license in the future will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide any protection from competitors. Even if its pending patent applications are granted as issued patents, those patents, as well as any patents Tvardi may license in the future from third parties now