UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2024
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File No. 001-34186
VANDA PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | 03-0491827 |
(State or other jurisdiction of
incorporation or organization) | (I.R.S. Employer
Identification No.) |
2200 Pennsylvania Avenue NW, Suite 300 E
Washington, DC 20037
(Address of principal executive offices)
(202) 734-3400
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Exchange Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value $0.001 per share | VNDA | The Nasdaq Global Market |
| Series A Junior Participating Preferred Stock Purchase Right, par value $0.001 per share | - | The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | | ☐ | | Accelerated filer | | ☒ |
| | | | | | |
| Non-accelerated filer | | ☐ | | Smaller reporting company | | ☐ |
| | | | | | |
| | | | Emerging growth company | | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of
the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C.
7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☒ No ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes ☐ No ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
As of June 30, 2024, the last business day of the registrant’s last completed second quarter, the aggregate market value of the Common Stock held by non-affiliates of the registrant was approximately $319.5 million based on the closing price of the registrant’s Common Stock, as reported by The Nasdaq Global Market, on such date. Shares of Common Stock held by each executive officer and director have been excluded since such persons may be deemed affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares of the registrant’s Common Stock, par value $0.001 per share, outstanding as of February 7, 2025 was 58,316,044.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant’s proxy statement with respect to the registrant’s 2025 Annual Meeting of Stockholders, which is to be filed pursuant to Regulation 14A within 120 days after the end of the registrant’s fiscal year ended December 31, 2024, are incorporated by reference into Part III of this Form 10-K.
Vanda Pharmaceuticals Inc.
Form 10-K
Table of Contents
| | | | | | | | |
| | | Page |
|
| | |
| | |
| Item 1 | | |
| Item 1A | | |
| Item 1B | | |
| Item 1C | | |
| Item 2 | | |
| Item 3 | | |
| Item 4 | | |
|
| Item 5 | | |
| Item 6 | | |
| Item 7 | | |
| Item 7A | | |
| Item 8 | | |
| Item 9 | | |
| Item 9A | | |
| Item 9B | | |
| Item 9C | | |
|
| Item 10 | | |
| Item 11 | | |
| Item 12 | | |
| Item 13 | | |
| Item 14 | | |
|
| Item 15 | | |
| Item 16 | | |
| |
| |
PART I
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K (Annual Report) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (Exchange Act). Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “project,” “target,” “goal,” “likely,” “will,” “would,” and “could,” or the negative of these terms and similar expressions or words, identify forward-looking statements. Forward-looking statements are based upon current expectations and assumptions that involve risks, changes in circumstances and uncertainties. If the risks, changes in circumstances or uncertainties materialize or the assumptions prove incorrect, the results of Vanda Pharmaceuticals Inc. (we, our, the Company or Vanda) may differ materially from those expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The forward-looking statements in this Annual Report may include, but are not limited to, statements about:
•our ability to commercialize Fanapt® (iloperidone) oral tablets for the acute treatment of manic or mixed episodes associated with bipolar I disorder;
•our ability to continue to generate United States (U.S.) sales of Fanapt® oral tablets for the treatment of schizophrenia;
•our ability to obtain approval from the FDA for BysantiTM (milsaperidone) for bipolar I disorder, schizophrenia and major depressive disorder (MDD);
•our ability to continue to commercialize HETLIOZ® (tasimelteon) capsules for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) in the U.S., in light of existing and potential generic competition, and Europe and HETLIOZ® capsules and oral suspension (HETLIOZ LQ®) for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome (SMS) in the U.S.;
•our ability to obtain approval from the U.S. Food and Drug Administration (FDA) for HETLIOZ® beyond the currently approved indications;
•our ability to increase market awareness of Non-24 and SMS and market acceptance of HETLIOZ®;
•our ability to commercialize PONVORY® (ponesimod) tablets for the treatment of adults with relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease in the U.S. and Canada;
•our ability to obtain approval from the FDA for PONVORY® beyond the currently approved indications;
•our ability to obtain approval from the FDA for tradipitant for the treatment of gastroparesis and motion sickness;
•our level of success in commercializing Fanapt® and HETLIOZ® in new markets;
•our ability to overcome the continued reimbursement and patient access challenges we face as a result of third-party payor coverage;
•the impact of public health crises, epidemics, pandemics or similar events on our business and operations, including our revenue, our supply chain, our commercial activities, our ongoing and planned clinical trials and our regulatory activities;
•our dependence on third-party manufacturers to manufacture Fanapt®, HETLIOZ®, HETLIOZ LQ® and PONVORY® in sufficient quantities and quality;
•our ability to prepare, file, prosecute, defend and enforce any patent claims and other intellectual property rights;
•our ability to maintain rights to develop and commercialize our products under our license agreements;
•our ability to obtain and maintain regulatory approval of our products, and the labeling for any approved products;
•our expectations regarding the timing and success of preclinical studies and clinical trials;
•the safety and efficacy of our products;
•regulatory developments in the U.S., Europe and other jurisdictions;
•limitations on our ability to utilize some or all of our prior net operating losses and orphan drug and research and development credits;
•our expectations regarding the size and growth of the current and potential markets for our products and our ability to serve those markets;
•our expectations regarding trends with respect to our revenues, costs, expenses, liabilities and cash, cash equivalents and marketable securities;
•our ability to identify or obtain rights to new products;
•our ability to attract and retain key scientific or management personnel;
•our expectations regarding the cost, time frame, outcome, insurance coverage and effects of any litigation or other dispute;
•our ability to obtain the capital necessary to fund our research and development or commercial activities;
•potential losses incurred from product liability claims made against us; and
•the use of our existing cash, cash equivalents and marketable securities.
All forward-looking statements in this report are expressly qualified in their entirety by the cautionary statements contained throughout this report. We caution you not to rely too heavily on such forward-looking statements. Each forward-looking statement speaks only as of the date of this Annual Report, and we undertake no obligation, and specifically decline any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
We encourage you to read Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, and our consolidated financial statements contained in this Annual Report. We also encourage you to read Summary of Principal Risk Factors below and Part I, Item 1A of this Annual Report, entitled Risk Factors, which contains a more complete discussion of the risks and uncertainties associated with our business. In addition to the risks described in this Annual Report, other unknown or unpredictable factors also could affect our results. Therefore, the information in this report should be read together with other reports and documents that we file with the Securities and Exchange Commission from time to time, including on Form 10-Q and Form 8-K, which may supplement, modify, supersede or update those risk factors. As a result of these factors, we cannot assure you that the forward-looking statements in this report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
SUMMARY OF PRINCIPAL RISK FACTORS
This summary briefly lists the principal risks and uncertainties facing our business, which are only a select portion of those risks. A more complete discussion of those risks and uncertainties is set forth in Part I, Item 1A of this Annual Report, entitled Risk Factors. Additional risks not presently known to us or that we currently deem immaterial may also affect us. If any of these risks occur, our business, financial condition or results of operations could be materially and adversely affected.
Our business is subject to the following principal risks and uncertainties:
Risks Related to our Business and Industry
•We are dependent on the commercial success of Fanapt®, HETLIOZ® and PONVORY®.
•We face generic competition for HETLIOZ®.
•Future performance of Fanapt®, HETLIOZ® and PONVORY® may be impacted by a number of factors including competing products or unanticipated safety issues.
•We are subject to uncertainty relating to pricing and reimbursement policies in the U.S.
•We have encountered third-party payors that refuse to cover or reimburse prescriptions written for HETLIOZ®.
•The FDA may not approve our New Drug Application (NDA) filing for the use of tradipitant for patients with gastroparesis or the FDA may determine that our clinical trial results for tradipitant for the treatment of gastroparesis do not demonstrate adequate safety and substantial evidence of efficacy.
•The FDA may not accept our NDA filing for the use of tradipitant for patients with motion sickness or the FDA may determine that our clinical trial results for tradipitant for the treatment of motion sickness do not demonstrate adequate safety and substantial evidence of efficacy.
•Global economic conditions may have an adverse effect on our business.
•Global health crises and pandemics may adversely impact our business.
•The FDA may not approve our supplemental New Drug Applications (sNDA) for HETLIOZ® for the treatment of jet lag disorder or insomnia.
•We may be unable to enter into third-party collaborations to develop and commercialize our products, or collaborations we enter into with any such third party may not be commercially successful.
•Even after we obtain regulatory approvals of a product, acceptance of the product in the marketplace is uncertain.
•Our ability to successfully launch PONVORY® in the U.S. and Canada is uncertain.
•We rely on, and will continue to rely on, outsourcing arrangements for many of our activities, including preclinical and clinical development and supply of Fanapt®, HETLIOZ®, HETLIOZ LQ®, PONVORY® and our other products.
•We may experience disruptions to our Fanapt®, HETLIOZ®, HETLIOZ LQ® or PONVORY® supply chains.
•We may fail to comply with government regulations regarding the sale and marketing of our products.
•We may fail to comply with regulations and obligations related to the ongoing oversight of our products regarding, among other things, development, manufacturing, labeling, recordkeeping and reporting.
•We may not market or distribute our products in a manner compliant with federal or state healthcare fraud and abuse laws.
•We rely on a limited number of specialty pharmacies for distribution of HETLIOZ® in the U.S., and the loss of one or more of these specialty pharmacies or their failure to distribute HETLIOZ® effectively would materially harm our business.
•Our revenues from Fanapt® and PONVORY® are substantially dependent on sales through a limited number of customers.
•We face substantial competition, which may result in others developing or commercializing products before or more successfully than we do.
•FDA and foreign regulatory approval of our products is uncertain.
•Our products may cause undesirable side effects or have other properties that could delay, prevent or result in the revocation of their regulatory approval or limit their marketability.
•Clinical trials for our products are expensive and their outcomes are uncertain.
•Our ability to use net operating loss carryforwards and tax credit carryforwards to offset future taxable income is dependent on generating future taxable income and may be limited, including as a result of transactions involving our common stock.
•Our contract research organizations (CROs), third-party vendors and investigators may not successfully carry out their duties or we may lose our relationships with CROs, third-party vendors and investigators.
•We rely on a limited number of third-party manufacturers to formulate and manufacture our products and these manufacturers may not be able to satisfy our demand and alternative sources may not be available.
•Materials necessary to manufacture our products may not be available on commercially reasonable terms, or at all.
•We may lose key scientists or management personnel or fail to recruit additional highly skilled personnel.
•We may be subject to product liability lawsuits.
•European Union (E.U.) Member States tend to impose strict price controls, which may delay or prevent the further commercial launch or impede the commercial success of HETLIOZ® in Europe and adversely affect our future results of operations.
•We may not be able to effectively market and sell our future products, if approved, in the U.S.
•Healthcare legislative reform measures or developments arising from changes in political climate may have a material adverse effect on our business and results of operations.
•We are subject to stringent laws, rules, regulations, policies, industry standards and contractual obligations regarding data privacy and security in foreign jurisdictions which are subject to change and reinterpretation.
Risks Related to Intellectual Property and Other Legal Matters
•Our rights to develop and commercialize our products are subject, in part, to the terms and conditions of licenses or sublicenses granted to us by other pharmaceutical companies.
•Our efforts to protect the proprietary nature of the intellectual property related to our products may not be adequate.
•We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful.
•We may not be able to obtain protection under the Hatch-Waxman Act and similar foreign legislation to extend our patents and to obtain market exclusivity for our products.
•Generic company competitors have received FDA approval of generic versions of HETLIOZ® in the U.S.
•We may not be successful in the development of products for our own account.
•Litigation or third-party claims of intellectual property infringement could require us to divert resources and may prevent or delay our drug discovery and development efforts.
Overview
Vanda Pharmaceuticals Inc. (we, our, the Company or Vanda) is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients.
We strive to advance novel approaches to bring important new medicines to market through responsible innovation. We are committed to the use of technologies that support sound science, including genetics and genomics, in drug discovery, clinical trials and the commercial positioning of our products.
Our commercial portfolio is currently comprised of three products: Fanapt® for the acute treatment of manic or mixed episodes associated with bipolar I disorder and the treatment of schizophrenia, HETLIOZ® for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) and for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome (SMS) and PONVORY® for the treatment of relapsing forms of multiple sclerosis (RMS) including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. HETLIOZ® is the first product approved by the United States Food and Drug Administration (FDA) for patients with Non-24 and for patients with SMS. In addition, we have a number of drugs and/or additional indications for current products in development, including:
•Fanapt® (iloperidone) long acting injectable (LAI) formulation for the treatment of schizophrenia;
•BysantiTM (milsaperidone), the active metabolite of Fanapt® (iloperidone), for the acute treatment of manic or mixed episodes associated with bipolar I disorder and for the treatment of schizophrenia and major depressive disorder (MDD);
•HETLIOZ® (tasimelteon) for the treatment of jet lag disorder, insomnia, pediatric insomnia, delayed sleep phase disorder (DSPD) and pediatric Non-24;
•PONVORY® (ponesimod) for the treatment of psoriasis and ulcerative colitis;
•Tradipitant (VLY-686), a small molecule neurokinin-1 (NK-1) receptor antagonist, for the treatment of gastroparesis, motion sickness and atopic dermatitis;
•Imsidolimab, an IL-36R antagonist, for the treatment of generalized pustular psoriasis (GPP);
•VTR-297, a small molecule histone deacetylase (HDAC) inhibitor for the treatment of onychomycosis and hematologic malignancies and with potential use as a treatment for several oncology indications;
•Portfolio of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) activators and inhibitors, including VSJ-110 for the treatment of dry eye and ocular inflammation and VPO-227 for the treatment of secretory diarrhea disorders, including cholera;
•VQW-765, a small molecule nicotinic acetylcholine receptor partial agonist, for the treatment of social/performance anxiety and psychiatric disorders; and
•Antisense oligonucleotide (ASO) molecules, including VCA-894A for the treatment of Charcot-Marie-Tooth Disease, Type 2S (CMT2S), caused by cryptic slice site variants within the IGHMBP2 gene and VGT-1849A for the treatment of polycythemia vera (PV), a form of a rare hematologic malignancy.
We were incorporated in 2002 and commenced operations in 2003. We are headquartered in Washington, D.C.
Our Strategy
Our goal is to further solidify our position as a leading global biopharmaceutical company focused on developing and commercializing innovative therapies addressing high unmet medical needs through the application of our drug development expertise and our pharmacogenetics and pharmacogenomics expertise. The key elements of our strategy to accomplish this goal are to:
•Maximize the commercial success of Fanapt®, HETLIOZ®, and PONVORY®;
•Enter into strategic partnerships to supplement our capabilities and to extend our commercial reach;
•Pursue the clinical development and regulatory approval of our products, including tradipitant;
•Apply our pharmacogenetics and pharmacogenomics expertise to differentiate our products;
•Expand our product portfolio through the identification and acquisition of additional products; and
•Utilize novel and innovative approaches in pursuit of each of these strategies.
Commercialized Products
Our commercial product portfolio consists of:
| | | | | | | | | | | | | | | | | | | | |
| Product | | Indication | | 2024 Net Sales (in millions) | | Geography |
| | Schizophrenia (tablets)
Manic or mixed episodes associated with bipolar I disorder (tablets) | | $94.3 | | United States
Israel (schizophrenia only) |
| | | | | | |
| | Non-24 (capsules)
Nighttime sleep disturbances in SMS (capsules and HETLIOZ LQ® oral suspension) | | $76.7 | | United States
Europe (Non-24 in blind patients only) |
| | | | | | |
| | Relapsing forms of multiple sclerosis (tablets) | | $27.8 | | United States
Canada |
| | | | | | |
Fanapt® for schizophrenia (tablets)
Fanapt® is a product approved for the treatment of schizophrenia. In May 2009, the FDA granted U.S. marketing approval of Fanapt® for the acute treatment of schizophrenia in adults. At that time, we had certain worldwide exclusive rights relating to Fanapt®, which we obtained pursuant to a sublicense agreement entered into with Novartis Pharma AG (Novartis) in June 2004. In October 2009, we amended and restated our sublicense agreement with Novartis pursuant to which Novartis retained exclusive commercialization rights to all formulations of Fanapt® in the U.S. and Canada. In January 2010, Novartis launched Fanapt® in the U.S. On December 31, 2014, Novartis transferred all the U.S. and Canadian commercial rights to the Fanapt® franchise to us as part of a settlement agreement. Additionally, our distribution partners launched Fanapt® in Israel in 2014. In May 2016, the FDA approved a supplemental New Drug Application (sNDA) for Fanapt® for the maintenance treatment of schizophrenia in adults.
Schizophrenia is a chronic, debilitating mental disorder characterized by hallucinations, delusions, racing thoughts and other psychotic symptoms (collectively referred to as “positive symptoms”), as well as moodiness, anhedonia (inability to feel pleasure), loss of interest, eating disturbances and withdrawal (collectively referred to as “negative symptoms”), and attention and memory deficits (collectively referred to as “cognitive symptoms”). Schizophrenia develops in late adolescence or early adulthood in approximately 1% of the world’s population. Most schizophrenia patients today are treated with drugs known as “atypical” antipsychotics, which were first approved in the U.S. in the late 1980s. These antipsychotics have been named “atypical” for their ability to treat a broader range of negative symptoms than the first-generation “typical” antipsychotics, which were introduced in the 1950s and are now generic. Atypical antipsychotics are generally regarded as having improved side effect profiles and efficacy relative to typical antipsychotics. See Competition below for a discussion of commonly prescribed atypical antipsychotics in addition to Fanapt®.
Fanapt® for bipolar I disorder (tablets)
In April 2024, the FDA approved Fanapt® tablets for the acute treatment of manic or mixed episodes associated with bipolar I disorder in adults. We initiated the commercial launch of Fanapt® for the acute treatment of bipolar I disorder in adults in the third quarter of 2024.
Bipolar disorders are brain disorders that cause changes in a person’s mood, energy and ability to function. Bipolar disorder is a category that includes three different conditions - bipolar I, bipolar II and cyclothymic disorder. People with bipolar disorders have extreme and intense emotional states that occur at distinct times, called mood episodes. These mood episodes are categorized as manic, hypomanic or depressive. People with bipolar disorders generally have periods of normal mood as well. See Competition below for a discussion of commonly prescribed atypical antipsychotics in addition to Fanapt®.
HETLIOZ® for Non-24 (capsules)
In January 2014, HETLIOZ® capsules were approved in the U.S. for the treatment of adults with Non-24. Non-24 is a serious, rare and chronic circadian rhythm sleep-wake disorder characterized by the inability to entrain (synchronize) the master body clock with the 24-hour day-night cycle. HETLIOZ® is the first FDA approved treatment for Non-24. HETLIOZ® is a melatonin agonist of the human MT1 and MT2 receptors, with greater specificity for MT2. These receptors are thought to be involved in the control of circadian rhythms. HETLIOZ® is believed to reset the master body clock in the suprachiasmatic nucleus, located in the hypothalamus, resulting in the entrainment and alignment of the body’s melatonin and cortisol rhythms to the 24-hour day-night cycle.
Most people have a master body clock that naturally runs longer than 24 hours and light is the primary environmental cue that resets it to 24 hours each day. Individuals with Non-24 have a master body clock that is not reset, and continually delays, resulting in prolonged periods of misalignment between their circadian rhythms and the 24-hour day-night cycle, including the timing of melatonin and cortisol secretion. As a result of this misalignment, Non-24 is associated with significant disruption of the sleep-wake cycle and impairments in social and occupational functioning, and marked subjective distress. Individuals with Non-24 cycle in and out of phase and suffer from disrupted nighttime sleep patterns and/or excessive daytime sleepiness.
HETLIOZ® was launched commercially in the U.S. in April 2014. In addition, in July 2015, the European Commission (EC) granted centralized marketing authorization with unified labeling for HETLIOZ® for the treatment of Non-24 in totally blind adults and included post-marketing commitments related to a pediatric investigation plan. This authorization was renewed in July 2020 for an unlimited duration and is valid in the 27 countries that are members of the European Union (E.U.), as well as European Economic Area members Iceland, Liechtenstein and Norway. HETLIOZ® was launched commercially in Germany in August 2016.
In January 2010, the FDA granted orphan drug designation status for HETLIOZ® in Non-24 in blind individuals. The FDA grants orphan drug designation to drugs that may provide significant therapeutic advantage over existing treatments and target conditions affecting 200,000 or fewer U.S. patients per year. Orphan drug designation provides potential financial and regulatory incentives, including study design assistance, tax credits, waiver of FDA user fees, and up to seven years of market exclusivity upon marketing approval. In February 2011, the European Medicines Agency (EMA) designated HETLIOZ® as an orphan medicinal product for the same indication.
Non-24 affects a majority of totally blind individuals, or approximately 80,000 people in the U.S. Blind individuals who develop Non-24 lack the light sensitivity necessary to synchronize the master body clock in the brain with the 24-hour day-night cycle. Non-24 also can affect sighted individuals. As with the totally blind, Non-24 in sighted individuals appears to be a comorbidity with certain other conditions. For example, a comorbidity has been established between psychiatric mood disorders and Non-24. Hospitalized individuals with neurological and psychiatric disorders can become insensitive to social cues, which may predispose them to the development of Non-24. This recognition of comorbidity led us to an initiative to engage with the psychiatric community. Patients diagnosed with traumatic brain injury, including concussions, frequently suffer from sleep disorders, some of which may be circadian rhythm sleep-wake disorders, including Non-24.
While there are no EC approved treatments for Non-24 other than HETLIOZ®, and the FDA has approved generics for the treatment of Non-24, there are a number of drugs approved and prescribed for patients with sleep disorders. The most commonly prescribed drugs are hypnotics. See Competition below for a discussion of commonly prescribed drugs for patients with sleep disorders.
HETLIOZ® for SMS (capsules and oral suspension)
In December 2020, HETLIOZ® capsules and oral suspension (HETLIOZ LQ®) were approved in the U.S. for the treatment of nighttime sleep disturbances in SMS in adults 16 years and older and children 3 years to 15 years old, respectively. HETLIOZ® capsules, for adults with SMS, were immediately available after approval and the HETLIOZ LQ® liquid formulation, for children with SMS, became available in the first quarter of 2021. SMS is a developmental disorder that is caused by a small deletion of human chromosome 17p. In more rare cases, SMS is caused by a point mutation in the RAI1 gene, which resides in the deleted region. HETLIOZ® is the first FDA-approved medication for patients with SMS.
In April 2010, the FDA granted orphan drug designation status for HETLIOZ® in the treatment of sleep disorder in SMS. SMS is estimated to affect 1/15,000-25,000 births in the U.S. SMS is not usually inherited but rather is caused by a de-novo deletion. Patients with SMS present with a number of physical, mental and behavioral problems. The most common symptom of SMS is a severe sleep disorder associated with significant disruption in the lives of patients and their families. In September 2023, the EMA designated HETLIOZ® as an orphan medicinal product for the treatment of SMS.
While there are no FDA approved treatments for patients with SMS other than HETLIOZ®, there are a number of drugs approved and prescribed for patients with sleep disorders that may be used to treat patients with SMS. The most commonly prescribed drugs are hypnotics. See Competition below for a discussion of commonly prescribed drugs for patients with sleep disorders.
PONVORY® for relapsing multiple sclerosis (tablets)
PONVORY® is a product approved for the treatment of relapsing forms of MS (RMS), including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease, in adults. In December 2023, we purchased the right to market and sell PONVORY® in the U.S. and Canadian markets from Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company. In March 2021, the FDA granted U.S. marketing approval of PONVORY® for the treatment of RMS in adults. Health Canada approved PONVORY® for the treatment of RMS in April 2021. MS is a chronic autoimmune inflammatory disease of the central nervous system (CNS) in which immune cells attack myelin (the protective casing that insulates nerve cells), damaging or destroying it and causing inflammation. This affects how the CNS processes information and communicates with the rest of the body, causing the neurologic signs and symptoms of MS. Symptoms vary by person, but common symptoms include fatigue, balance and walking problems, numbness or tingling, dizziness and vertigo, vision problems, bladder and bowel problems and weakness.
PONVORY® was launched commercially in the U.S. in April 2021 and in Canada in November 2021 by one of the Johnson & Johnson Companies. There are a number of drugs approved and prescribed to treat patients with MS. See Competition below for a discussion of these commonly prescribed drugs.
Research and Development
We have built a research and development organization that includes extensive expertise in the scientific disciplines of pharmacogenetics and pharmacogenomics. We operate cross-functionally and are led by an experienced research and development management team. We use rigorous project management techniques to assist us in making disciplined strategic research and development program decisions and to help limit the risk profile of our product pipeline. We also access relevant market information and key opinion leaders in creating target product profiles and, when appropriate, as we advance our programs towards commercialization. We engage third parties to conduct portions of our preclinical research. In addition, we utilize multiple clinical sites to conduct our clinical trials; however, we are not substantially dependent upon any one of these sites for our clinical trials nor do any of them conduct a major portion of our clinical trials.
Our product pipeline currently consists of the following products in clinical development or under regulatory review:
Fanapt® for schizophrenia (LAI)
In October 2018, we enrolled our first patient in a pharmacokinetic study of the LAI formulation of Fanapt®. This pharmacokinetic study is ongoing and will serve to inform the dosing for a later clinical study of Fanapt® LAI for the treatment of schizophrenia. We initiated a Phase III program for the LAI formulation of Fanapt® in the fourth quarter of 2024.
BysantiTM (Milsaperidone)
In 2021, we initiated a bioequivalence study of Fanapt® and milsaperidone, the active metabolite of iloperidone. We believe that milsaperidone represents a potential improvement over the clinical profile of Fanapt® and has the potential to create sustained, long-term value in the treatment of psychiatric disorders, including bipolar I disorder and schizophrenia, the indications already approved for Fanapt®, as well as new indications such as MDD. We initiated a Phase III clinical study for milsaperidone for MDD in the fourth quarter of 2024.
HETLIOZ® for jet lag disorder
In March and May 2018, respectively, we announced the results of our JET8 and JET studies for the treatment of jet lag disorder. In the JET8 clinical study, HETLIOZ® demonstrated significant and clinically meaningful benefits in nighttime and daytime symptoms of jet lag disorder, including improvement in sleep time and benefits in measurements of next day alertness. The JET study showed effectiveness in treating travelers who traveled either five or eight time zones from Washington, D.C. to London and San Francisco or Los Angeles to London, respectively. The results support the previously reported pivotal JET5 and JET8 Phase III clinical studies, which demonstrated improvements in patients who experienced circadian advances of five and eight hours, respectively. Additionally, in September 2018, we announced results from a driving study, which demonstrated that tasimelteon did not impair measures of driving performance.
The FDA accepted the filing of our sNDA for HETLIOZ® for the treatment of jet lag disorder in December 2018. The FDA determined the target action date under the Prescription Drug User Fee Act Amendments of 2017 to be August 16, 2019 and, on that date, we received a complete response letter (CRL) from the FDA. The FDA asserted in the CRL that the measures demonstrating improved sleep were of unclear clinical significance. We met with the FDA to discuss the CRL in a Post Action meeting and in 2022 we requested the opportunity for a hearing with the FDA on the approvability of the jet lag disorder sNDA. We filed a lawsuit against the FDA in September 2022 demanding that the FDA immediately publish in the Federal Register a notice of opportunity for a hearing on the jet lag disorder sNDA. The FDA then published the notice in the Federal Register in October 2022. We have asked the U.S. District Court for the District of Columbia (DC District Court) to, among other things, compel the FDA to comply with its obligations and declare that the FDA’s lack of compliance violates the FDCA and the FDA regulations. In January 2024, the DC District Court held an oral argument on dispositive cross-motions, following which the DC District Court granted our motion for summary judgment. The DC District Court ruled that the FDA violated the statute and ordered the FDA to either finally resolve our application or commence a hearing on or before March 5, 2024. In March 2024, we and the FDA filed a consent motion for entry of final judgment in our favor on our Administrative Procedure Act claim for the FDA’s unreasonable delay in resolving the hearing request, following which the FDA refused to hold a hearing or approve our sNDA for HETLIOZ® in the treatment of jet lag disorder. We subsequently filed a petition for review in the U.S. Court of Appeals for the District of Columbia Circuit (DC Circuit). In January 2025, the DC Circuit held an oral argument on the petition. Our petition remains pending.
Jet lag disorder is a common circadian disorder frequently observed in millions of travelers who cross multiple time zones. Jet lag disorder is characterized by nighttime sleep disruption, a decrease in daytime alertness and impairment to social and occupational functioning. Jet lag disorder symptoms are more severe during eastward travel. U.S. Department of Commerce, International Trade Administration reports state that more than 20 million U.S. residents make trips abroad each year to overseas destinations in Europe, the Middle East and Asia.
HETLIOZ® for insomnia
HETLIOZ® is effective in improving sleep onset difficulty in people with primary insomnia with the effect observed as early as the first night of treatment. A Phase III, multi-center, placebo-controlled, 4-week trial evaluated patients with chronic primary insomnia. Two studies of transient insomnia induced by phase advance of the sleep-wake cycle were also conducted with five-hour and eight-hour phase advance, which showed a significant effect the first night in improving sleep parameters. In July 2023, the FDA accepted our sNDA for HETLIOZ® in insomnia for filing and set a target action date of March 4, 2024 under the Prescription Drug User Fee Act (PDUFA) for its decision. On March 4, 2024, we received a CRL from the FDA. In June 2024, we received a Notice of Opportunity for a Hearing, and we accepted the opportunity for a hearing in July 2024. In August 2024, we filed for summary judgment requesting approval or a hearing on approvability of tasimelteon to treat insomnia associated with difficulties with sleep initiation. In October 2024, we received a Proposed Order denying a hearing on approvability for the insomnia sNDA and we submitted a response to the Proposed Order in December 2024.
HETLIOZ® for DSPD
A Phase III clinical study of HETLIOZ® in DSPD is ongoing. DSPD is a circadian rhythm disorder in which a person’s sleep is delayed beyond the socially acceptable or conventional bedtime. This delay in falling asleep causes difficulty in waking up at the desired time and affects social and occupational functioning. DSPD is likely the most prevalent circadian-rhythm sleep disorder, affecting approximately 1% of the population, and there is no FDA approved treatment at this time.
PONVORY®
The mechanism of action of PONVORY® makes it also a potential therapeutic candidate for the treatment of a diverse group of inflammatory/autoimmune disorders including but not limited to ulcerative colitis, psoriasis, Crohn’s disease, atopic dermatitis, eosinophilic esophagitis and alopecia areata. In a randomized placebo controlled clinical study, PONVORY® has been shown to reduce the symptoms and signs of psoriasis. Investigational New Drug (IND) applications for PONVORY® in the treatments of psoriasis and ulcerative colitis were accepted by the FDA in the fourth quarter of 2024.
HETLIOZ® for pediatric Non-24
We plan to develop HETLIOZ® for the treatment of pediatric Non-24. A pharmacokinetic study of the HETLIOZ® pediatric liquid formulation was completed in the first quarter of 2018.
Tradipitant for gastroparesis
In December 2018, we announced results from a Phase II randomized clinical study (2301) of tradipitant as a monotherapy in the treatment of gastroparesis. Several symptom severity scales were used to assess gastroparesis symptoms, including the Gastroparesis Symptom Index (GCSI), Patients Assessment of Upper Gastrointestinal Disorders-Symptoms (PAGI-SYM), and Patient Global Impression of Change (PGI-C) as well as a Clinician Global Impression of Severity (CGI-S). Tradipitant met the primary endpoint of the study of change in nausea score as measured by patient daily diaries and also met the related endpoint of improvement in the number of nausea free days. Tradipitant also showed significant improvement in most of the secondary endpoints studied, including several key scales reflecting overall gastroparesis symptoms, specifically GCSI, PAGI-SYM, CGI-S, and PGI-C.
In February 2022, we announced results from our Phase III clinical study, VP-VLY-686-3301, evaluating the efficacy and safety of tradipitant in treating the symptoms of gastroparesis. The study did not meet its prespecified primary endpoint, which was the difference between drug and placebo on the change of the severity of nausea from baseline at week 12 of treatment. Both treatment arms showed significant improvements from baseline on nausea as well as the other core symptoms of gastroparesis. When restricting the analysis in the group of patients that used no rescue medications at baseline and adjusting for poor compliance, we identified strong evidence of a drug effect across a number of symptoms and across the duration of the study, including a significant and meaningful effect at the prespecified primary endpoint of nausea change at week 12. The FDA may not view this data as constituting substantial evidence of efficacy for tradipitant in any indication for the treatment of gastroparesis or its symptoms, for any length of treatment. The open-label phase of the study remains open.
We believe that tradipitant has a well-established safety profile, as demonstrated by the results of extensive testing in animals and humans. Despite these results, however, the FDA informed us in December 2018 that in order to treat patients beyond 12 weeks, we would have to conduct a nine-month non-rodent chronic toxicity study. This currently limits our ability to collect safety data in humans for more than 12 weeks. The non-rodent study required by the FDA necessitates the sacrifice of dozens of animals and we have disputed the necessity of a nine-month non-rodent chronic toxicity study. In February 2019, we filed a lawsuit in the U.S. District Court for the District of Columbia (DC District Court) challenging the FDA’s position, but we ultimately did not prevail. Despite our disagreement with the FDA, the preclinical package has allowed us to continue to conduct all of the efficacy studies necessary for New Drug Application (NDA) filing. Moreover, in July 2020, the FDA authorized tradipitant through an expanded access program (EAP) for a single patient. An EAP allows a patient to request the use of tradipitant, prior to NDA approval, for up to six months with an option to request renewal. Since then, certain patients who experienced a benefit in tradipitant studies have requested and received expanded access, while others have been denied treatment under the EAP. The EAP is ongoing and a number of patients have initiated treatment. Although this EAP is not intended for data collection, we collect safety data from this cohort of expanded access patients and included this data in the NDA that we submitted for tradipitant for patients with gastroparesis. In December 2023, the FDA accepted our NDA for tradipitant in gastroparesis for filing and set a PDUFA target action date of September 18, 2024. On September 18, 2024, we received a CRL from the FDA. In January 2025, we received a Notice of Opportunity for a Hearing, and we have accepted the opportunity for a hearing. Tradipitant is the first novel drug to be accepted for review by the FDA for gastroparesis in over 30 years and, if approved, will be the first novel drug to be approved by the FDA for the treatment of gastroparesis in over 40 years. The lack of long-term (i.e., more than 12 weeks in humans) safety data would likely impact the FDA’s willingness to approve tradipitant for a chronic indication. However, because long-term safety data is not normally a requirement for short-term indications, and with a preclinical profile that has not precluded clinical development, we believe the package was complete for any NDA filing to treat patients for 12 weeks or less. For example, the FDA has communicated to us that it is considering an indication for the short-term relief of nausea in gastroparesis. While this short-term indication is not preferred, we would consider accepting this limited indication while continuing to pursue a chronic indication. However, the FDA may
not deem the safety information sufficient even for a short-term indication. Moreover, FDA authorization of an EAP is not a guarantee of or a step towards obtaining full FDA approval of an NDA.
Gastroparesis is a serious medical condition characterized by delayed gastric emptying associated with the symptoms of nausea, vomiting, bloating, fullness after meals and abdominal pain, along with significant impairment of social and occupational functioning. A paper by Rey et al published in the January 2012 Journal of Neurogastroenterology and Motility estimated the prevalence of gastroparesis in the U.S. to be approximately six million patients, many of whom remain undiagnosed.
Tradipitant for motion sickness
In July 2019, we reported tradipitant was effective in treating motion sickness in a randomized double blind placebo controlled Phase II clinical study conducted in the Pacific Ocean. The study had two primary endpoints: percentage of participants vomiting, and Motion Sickness Severity Scale (MSSS) Worst score. In the overall population, a significantly higher percentage of participants experienced vomiting in the placebo arm as compared to the tradipitant arm. The MSSS Worst score endpoint also favored tradipitant, but the difference did not reach statistical significance. The protocol for a pivotal Phase III motion sickness study was discussed with the FDA at the end of Phase II meeting, and the FDA agreed with the adequacy of the program design to support an NDA. In May 2023, we announced positive results from the first Phase III clinical study of tradipitant in motion sickness, confirming the previously reported results demonstrating that tradipitant is effective in the prevention of vomiting associated with motion sickness. In May 2024, we announced positive results from the second Phase III study of tradipitant in motion sickness, confirming the previously reported results of two efficacy studies demonstrating that tradipitant is effective in the prevention of vomiting associated with motion sickness. In addition, we submitted an NDA for tradipitant in the prevention of vomiting associated with motion sickness in the fourth quarter of 2024.
Motion sickness is a disorder that arises often as a response to real or perceived movement, as occurring during vehicular travel. Vomiting is the most disturbing symptom of motion sickness, although the disorder is often accompanied by a constellation of symptoms that includes nausea, sweating, pallor, headache and anorexia. Motion sickness is one of the most prevalent episodic disorders in the world, whose prevalence has dramatically increased with world population mobility over the last 100 years. It is reported that approximately 30% of the general population suffers from motion sickness under ordinary travel conditions that include sea, air and land travel.
Tradipitant for atopic dermatitis
We announced results in September 2017 from a randomized Phase II clinical study of tradipitant as a monotherapy in the treatment of patients with atopic dermatitis. Tradipitant was shown to improve the intensity of the worst itch patients experienced, as well as atopic dermatitis disease severity. On the pre-specified primary endpoint of Average Itch Visual Analog Scale (VAS), tradipitant showed improvement over placebo, but this improvement was not significant due to high placebo effect and the lack of sensitivity of this measure.
In June 2018, we initiated EPIONE, a Phase III clinical study of tradipitant for pruritus in atopic dermatitis. In October 2019, we began enrolling patients in EPIONE 2, a second Phase III clinical study of tradipitant in atopic dermatitis. We announced results of EPIONE in February 2020. The EPIONE study did not meet its primary endpoint in reduction of pruritus across the overall study population. However, the antipruritic effect of tradipitant was robust in the mild atopic dermatitis population. The EPIONE study continued to demonstrate that tradipitant is safe and well-tolerated. The EPIONE 2 study was placed on hold in 2020.
Atopic dermatitis is a chronic, relapsing inflammatory skin disorder characterized by the symptom of intense and persistent pruritus or itch. Other clinical features include erythema, excoriation, edema, lichenification, oozing and xerosis. Atopic dermatitis is a common skin disorder affecting millions of people worldwide. Currently, there are very few safe systemic treatments available for atopic dermatitis, representing a significant unmet medical need in this population. A 2015 Decision Resources Group report estimated that 9.8 million individuals were diagnosed with atopic dermatitis in the U.S., of which approximately 6.4 million were drug-treated atopic dermatitis patients.
Imsidolimab for GPP
Imsidolimab has successfully completed two global Phase III clinical studies for the treatment of GPP. In January 2025, we entered into an exclusive global license agreement with AnaptysBio, Inc. (Anaptys) under which we acquired the worldwide rights to develop, manufacture, and commercialize imsidolimab, an IL-36R antagonist.
GPP is a rare, chronic, systemic autoinflammatory disease that is potentially life-threatening, if left untreated. During a GPP flare, individuals experience the sudden eruption of painful pustules. These pustules appear over large areas of the skin, accompanied by redness, severe itchiness, and dry, cracked, or scaly skin. People with GPP may also experience more general symptoms such as fever, headache, extreme tiredness, or a burning sensation on the skin.
VTR-297
In the fourth quarter of 2018, we initiated a clinical study in patients with hematologic malignancies. Enrollment in the Phase I/II clinical study (1101) of VTR-297 in hematologic malignancies is ongoing. VTR-297 is a small molecule HDAC inhibitor with potential use as a treatment for several oncology indications.
In January 2024, the FDA approved our Investigational New Drug (IND) application to evaluate VTR-297 for the treatment of onychomycosis, a fungal infection of the nail. The Phase I clinical study for VTR-297 for the treatment of onychomycosis was initiated in April 2024. The study is fully enrolled.
Portfolio of CFTR activators and inhibitors
A clinical program in VSJ-110 is ongoing. We are evaluating VSJ-110 for the treatment of allergic conjunctivitis. VSJ-110 is a small molecule nanomolar potency CFTR activator. VSJ-110 has shown efficacy in a dry eye model and exhibited anti-inflammatory properties in both in vitro and in vivo assays. An ongoing proof of concept study for VSJ-110 indicates an effect in improving the signs (fluorescein corneal staining) of dry eye disease.
In addition, an early stage CFTR inhibitor program is planned for VPO-227 for the treatment of secretory diarrhea disorders, including cholera. We believe that VPO-227 has the potential to be an orally administered treatment for cholera. In October 2022, VPO-227 was granted orphan drug designation by the FDA for the treatment of cholera. We have also received approval to proceed in a Phase I study of VPO-227 for the treatment of cholera in Bangladesh, a country where the treatment of cholera remains a significant and unmet need.
VQW-765
We are evaluating VQW-765 for the treatment of psychiatric disorders. In December 2022, we announced results from our Phase II clinical study, VP-VQW-765-2201 (Study 2201), of a single-dose treatment to alleviate acute performance anxiety in social situations. In the clinical study, 230 volunteers with prior history of performance anxiety were randomized to receive a single dose of VQW-765 or placebo and were challenged with the standardized Trier Social Stress Test (TSST). The TSST creates an acute stress by requiring participants to make an interview-style presentation in front of a panel who provides no feedback or encouragement. Participants who received VQW-765 showed numerically lower stress levels compared to those who received placebo. A significant relationship was also seen between exposure to VQW-765 (amount of drug measured in blood) and the clinical response.
VQW-765 is a Phase II alpha-7 nicotinic acetylcholine receptor partial agonist that we licensed from Novartis on December 31, 2014 pursuant to a settlement agreement.
Other products
ASO Molecules
In 2022, we announced a research and development collaboration agreement with OliPass Corporation (OliPass) to jointly develop a set of ASO molecules based on OliPass’ proprietary modified peptide nucleic acids. The collaboration focuses on editing and modifying gene expression using ASOs in disease states where the expression of genes is either altered or the sequence of the expressed genes can be altered for therapeutic benefit. OliPass’ unique OliPass Peptide Nucleic Acids technology provides the delivery platform to enable these gene expression modifications.
ASOs may have broad applicability in addressing a number of disorders, from nervous system treatments to systemic treatments. In June 2023, VCA-894A, an ASO molecule, was granted orphan designation by the FDA for the treatment of a patient with CMT2S, caused by cryptic splice site variants within IGHMBP2. In January 2024, we announced that the FDA had approved the Investigational New Drug (IND) application to evaluate VCA-894A for the treatment of a patient with CMT2S. CMT2S is a rare subtype of Charcot-Marie-Tooth disease (CMT), an inherited peripheral neuropathy for which there is no available treatment. The estimated prevalence of CMT is 1 in 2,500 individuals, with varying clinical features dependent on the various genetic variants of CMT. The prevalence of CMT2S is estimated to be less than 1 in 1,000,000 worldwide. In addition, in December 2024, the FDA granted orphan drug designation for VGT-1849A, an ASO-based JAK2 inhibitor for the treatment of polycythemia vera (PV), a form of a rare hematologic malignancy that is estimated to affect 1 in 2,000 Americans.
For more detailed information regarding our clinical trial results and regulatory activities for our products, please refer to our SEC filings and press releases, which can be found on the SEC EDGAR website and on our website www.vandapharma.com. Information contained on those websites is not incorporated by reference into this Annual Report or any other report or document that we file with the SEC.
License Agreements
Our rights to develop and commercialize certain of our products are subject to the terms and conditions of licenses granted to us by other pharmaceutical companies.
Fanapt®
Pursuant to the terms of a settlement agreement with Novartis, Novartis transferred all U.S. and Canadian rights in the Fanapt® franchise to us on December 31, 2014. We paid directly to Sanofi S.A. (Sanofi) a fixed royalty of 3% of net sales through December 2019 related to manufacturing know-how. No further royalties on manufacturing know-how are payable by us. We are also obligated to pay Sanofi a fixed royalty on Fanapt® net sales equal up to 6% on Sanofi know-how not related to manufacturing under certain conditions for a period of up to 10 years in markets where the new chemical entity (NCE) patent has expired or was not issued. We are obligated to pay this 6% royalty on net sales in the U.S. through November 2026. No further royalties on know-how not related to manufacturing will be payable by us for net sales in the U.S. after November 2026. We may lose our rights to develop and commercialize Fanapt® if we fail to comply with certain requirements in the Titan license agreement regarding our financial condition, or if we fail to comply with certain diligence obligations regarding our development or commercialization activities.
HETLIOZ®
In February 2004, we entered into a license agreement with Bristol-Myers Squibb (BMS) under which we received an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize HETLIOZ®. We have paid BMS $37.5 million in upfront fees and milestone obligations. We have no remaining milestone obligations to BMS. Additionally, we are obligated to make royalty payments on HETLIOZ® net sales to BMS. The royalty period in each territory where we commercialize HETLIOZ® is 10 years following the first commercial sale in the territory. In territories outside the U.S., the royalty is 5% on net sales. In the U.S., the royalty on net sales decreased from 10% to 5% in December 2022. This U.S. royalty ended in April 2024. We are also obligated under the license agreement to pay BMS a percentage of any sublicense fees, upfront payments and milestone and other payments (excluding royalties) that we receive from a third party in connection with any sublicensing arrangement, at a rate which is in the mid-twenties. We are obligated to use commercially reasonable efforts to develop and commercialize HETLIOZ®.
Either party may terminate the HETLIOZ® license agreement under certain circumstances, including a material breach of the agreement by the other. In the event we terminate our license, or if BMS terminates our license due to our breach, all rights licensed and developed by us under this agreement will revert or otherwise be licensed back to BMS on an exclusive basis.
Tradipitant (VLY-686)
In April 2012, we entered into a license agreement with Eli Lilly and Company (Lilly) pursuant to which we acquired an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize an NK-1 receptor antagonist, tradipitant, for all human indications. Lilly is eligible to receive future payments based upon achievement of specified development, regulatory approval and commercialization milestones as well as tiered-royalties on net sales at percentage rates up to the low double digits. We have paid Lilly $5.0 million in upfront fees and development milestones, including a $2.0 million development milestone paid in December 2023 for the filing of the first
marketing authorization for tradipitant in the U.S. or the E.U. As of December 31, 2024, remaining milestones include $10.0 million and $5.0 million milestones for the first approval of a marketing authorization for tradipitant in the U.S. and the E.U., respectively, and up to $80.0 million for sales milestones. We are obligated to use commercially reasonable efforts to develop and commercialize tradipitant.
Either party may terminate the agreement under certain circumstances, including a material breach of the agreement by the other. In the event that we terminate the agreement, or if Lilly terminates the agreement due to our breach or for certain other reasons set forth in the agreement, all rights licensed and developed by us under the agreement will revert or otherwise be licensed back to Lilly on an exclusive basis, subject to payment by Lilly to us of a royalty on net sales of products that contain tradipitant.
Imsidolimab
In January 2025, we entered into an exclusive global license agreement with Anaptys under which we acquired the worldwide rights to develop, manufacture, and commercialize imsidolimab, an IL-36R antagonist. The agreement grants us exclusive rights to imsidolimab, which has successfully completed two global Phase III clinical studies for the treatment of GPP. Under the terms of the agreement, we made an upfront payment of $10 million to Anaptys and an additional $5 million for existing drug supply. Anaptys is eligible to receive up to $35 million in future regulatory approval and sales milestones. Additionally, we are obligated to pay Anaptys a 10% royalty on global net sales of imsidolimab. We are obligated to use commercially reasonable efforts to develop and commercialize imsidolimab.
Either party may terminate the agreement under certain circumstances, including a material breach by the other party. In the event that we terminate the agreement, or if Anaptys terminates the agreement due to our breach, all rights licensed and developed by us under the agreement will revert or otherwise be licensed back to Anaptys on an exclusive basis.
Portfolio of CFTR activators and inhibitors
In March 2017, we entered into a license agreement with the University of California San Francisco (UCSF), under which we acquired an exclusive worldwide license to develop and commercialize a portfolio of CFTR activators and inhibitors. Pursuant to the license agreement, we will develop and commercialize the CFTR activators and inhibitors and are responsible for all development costs under the license agreement, including current pre-investigational new drug development work. UCSF is eligible to receive future payments based upon achievement of specified development, regulatory approval and commercialization milestones as well as single-digit tiered-royalties on net sales. We have paid UCSF $1.6 million in upfront fees and development milestones. As of December 31, 2024, remaining milestones include $11.9 million for development milestones and $33.0 million for future regulatory approval and sales milestones. Included in the $11.9 million in development milestones are $1.1 million of milestone obligations due upon the conclusion of clinical studies for each licensed product, not to exceed $3.2 million in total for the CFTR portfolio.
Either party may terminate the agreement under certain circumstances. In the event that we terminate the agreement, or if UCSF terminates the agreement due to our breach or for certain other reasons set forth in the agreement, all rights licensed and developed by us under the agreement will revert or otherwise be licensed back to UCSF. Termination will not relieve us of our obligation to pay royalties or other payments owed, if any, to UCSF under the terms of the agreement.
VQW-765
In connection with the settlement agreement with Novartis relating to Fanapt®, we received an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize VQW-765, a Phase II alpha-7 nicotinic acetylcholine receptor partial agonist. Pursuant to the license agreement, we are obligated to use commercially reasonable efforts to develop and commercialize VQW-765 and are responsible for all development costs. We have no milestone obligations, but Novartis is eligible to receive tiered-royalties on net sales at percentage rates up to the mid-teens.
Either party may terminate the agreement under certain circumstances, including a material breach of the agreement by the other. In the event that we terminate the agreement, or if Novartis terminates the agreement due to our breach or for certain other reasons set forth in the agreement, all rights licensed and developed by us under the agreement will revert or otherwise be licensed back to Novartis on an exclusive basis, subject to payment by Novartis to us of a royalty on net sales of products that contain VQW-765.
Patents and Proprietary Rights; Hatch-Waxman Protection
Our products are protected from unauthorized use by others only to the extent that our products are covered through regulatory protections or by valid and enforceable patents, either licensed to us by others or generated through our activities internally, that give us sufficient proprietary rights. Accordingly, securing patents, regulatory data package protection, and other proprietary rights are an essential element of our business strategies.
PONVORY®, tradipitant and VQW-765 are covered by NCE and other patents and patent applications related to their respective medicinal uses. In addition, NCE patent protection has been sought for VTR-297 and CFTR activators and inhibitors. Patent applications for these active ingredients remain pending. Although the NCE patents protecting Fanapt® and HETLIOZ® have expired, Fanapt® remains protected by additional patents and HETLIOZ® and HETLIOZ LQ® remain protected by additional patents, some of which we have asserted against current generic competitors. For more on the license and sublicense arrangements related to these active ingredients, see License Agreements above. For more on patent litigation, see Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” in Part I, Item 1A of this Annual Report, each of which is incorporated herein by reference. In addition, we have filed for patents based on our own discoveries that seek to provide additional protection for Fanapt® and HETLIOZ® and HETLIOZ LQ®.
A comprehensive list of active patents for our U.S. commercial products is available in the Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) and is also provided in the table below. Members of these patent families are also issued or pending in a number of territories, such as Europe and Japan. The patents in the table below that are marked with “*” are the subject of ongoing patent litigation. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” in Part I, Item 1A of this Annual Report, each of which is incorporated herein by reference, for additional information.
| | | | | | | | | | | | | | |
| Product | | Number | | Type |
Fanapt® | | US 8,586,610* | | Method of treatment |
| | US 8,652,776 | | Method of treatment |
| | US 8,999,638 | | Method of treatment |
| | US 9,072,742 | | Method of treatment |
| | US 9,074,254 | | Method of treatment |
| | US 9,074,255 | | Method of treatment |
| | US 9,074,256 | | Method of treatment |
| | US 9,138,432* | | Method of treatment |
| | US 9,157,121 | | Method of treatment |
HETLIOZ® | | US 9,060,995 | | Method of treatment |
| | US 9,539,234 | | Method of treatment |
| | US 9,549,913 | | Method of treatment |
| | US 9,730,910* | | Method of treatment |
| | US 9,855,241 | | Method of treatment |
| | | US RE46,604* | | Method of treatment |
| | US 10,071,977 | | Drug substance |
| | US 10,149,829* | | Method of treatment |
| | US 10,179,119 | | Method of treatment |
| | US 10,376,487* | | Method of treatment |
| | US 10,449,176 | | Method of treatment |
| | US 10,610,510 | | Method of treatment |
| | US 10,610,511 | | Method of treatment |
| | US 10,829,465 | | Drug substance |
| | | | | | | | | | | | | | |
| Product | | Number | | Type |
| | US 10,945,988 | | Method of treatment |
| | US 10,980,770 | | Method of treatment |
| | US 11,141,400 | | Method of treatment |
| | US 11,266,622 | | Method of treatment |
| | US 11,285,129* | | Method of treatment |
| | US 11,566,011 | | Drug substance |
| | US 11,633,377 | | Method of treatment |
| | US 11,759,446 | | Method of treatment |
| | US 11,760,740 | | Drug substance |
| | US 11,786,502 | | Method of treatment |
| | US 11,826,339 | | Method of treatment |
| | US 11,833,130 | | Method of treatment |
| | US 11,850,229 | | Method of treatment |
| | US 11,918,556* | | Method of treatment |
| | US 11,918,557 | | Method of treatment |
| | US 12,049,457 | | Drug substance |
HETLIOZ LQ® | | US 9,539,234 | | Method of treatment |
| | US 9,730,910* | | Method of treatment |
| | US 10,071,977 | | Drug substance |
| | US 10,149,829* | | Method of treatment |
| | US 10,179,119* | | Method of treatment |
| | US 10,376,487* | | Method of treatment |
| | US 10,610,510* | | Method of treatment |
| | US 10,610,511 | | Method of treatment |
| | US 10,829,465 | | Drug substance |
| | US 10,980,770* | | Method of treatment |
| | US 11,141,400 | | Method of treatment |
| | US 11,202,770 | | Drug formulation |
| | US 11,266,622* | | Method of treatment |
| | US 11,285,129* | | Method of treatment |
| | US 11,566,011 | | Drug substance |
| | US 11,633,377 | | Method of treatment |
| | US 11,759,446* | | Method of treatment |
| | US 11,760,740 | | Drug substance |
| | US 11,786,502 | | Method of treatment |
| | US 11,826,339 | | Method of treatment |
| | US 11,833,130 | | Method of treatment |
| | US 11,850,229* | | Method of treatment |
| | US 11,918,556 | | Method of treatment |
| | US 11,918,557 | | Method of treatment |
| | US 12,049,457 | | Drug substance |
PONVORY® | | US 8,273,779 | | Method of treatment |
| | US 9,062,014 | | Drug substance |
| | US 10,220,023 | | Method of treatment |
| | | | | | | | | | | | | | |
| Product | | Number | | Type |
| | US RE43,728 | | New chemical entity |
| | US 11,951,097 | | Method of treatment |
Fanapt®
The NCE patent for Fanapt®, which expired in 2016 in the U.S. and in 2010 in other countries, was owned by Sanofi. Other patents and patent applications relating to Fanapt® are owned by us.
Fanapt® metabolites, formulations, genetic markers and uses are the subject of numerous patent filings in which protection has been sought in the U.S., Europe, and other markets. In November 2013, a U.S. patent (U.S. 8,586,610) directed to a method of treating patients with Fanapt® based on genotype was issued to us by the U.S. Patent and Trademark Office (USPTO). This patent, which was listed in the Orange Book in January 2015, is set to expire in 2027, and potentially further extends the U.S. marketing exclusivity for Fanapt®. Additional method of treatment patents have been issued in the U.S. and listed in the Orange Book, with the latest expiration date in December 2031.
We have also filed and plan on filing additional patent applications covering the use of iloperidone (Fanapt® active ingredient) LAI formulations. Patents for the microsphere LAI formulation of Fanapt® expired in 2022 in some markets in Europe and expired in 2024 in the U.S. Patents for the aqueous microcrystals LAI formulation of Fanapt® expired in 2023 in the U.S. and in some markets in Europe. We have pending patent applications covering the use of iloperidone and plan on filing additional applications based on discoveries made throughout the development plan of this molecule.
We filed several Hatch-Waxman lawsuits in the Delaware District Court against a number of generic company competitors asserting infringement of two of our Fanapt® patents. The litigation has been resolved with respect to all but one of these competitors. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” in Part I, Item 1A of this Annual Report, each of which is incorporated herein by reference, for additional information.
In Europe, the law provides for 10 years of regulatory exclusivity (with the potential for an additional year if the drug is developed for a significant new indication). No generic versions of Fanapt® would be permitted to be marketed or sold during the applicable regulatory exclusivity period in most European countries. Outside the U.S. and Europe, similar regulatory package protection periods may be available and could protect Fanapt® from generic competition for varying numbers of years depending upon the country.
HETLIOZ® and HETLOZ LQ®
Our rights to the NCE patent covering HETLIOZ® capsules and oral suspension (HETLIOZ LQ®) and related intellectual property have been acquired through a license with BMS. HETLIOZ® and its formulations, genetic markers and uses are the subject of numerous patent filings for which protection has been sought in selected countries worldwide. The NCE patent covering HETLIOZ® expired in December 2022 in the U.S., which is inclusive of a five-year extension granted under the Hatch-Waxman Act in October 2018. Corresponding NCE patent protection has expired in most other markets. The USPTO has also issued 25 method of treatment patents for HETLIOZ® that will expire between 2033 and 2041 and five drug substance patents that will expire in 2035. Additionally, the USPTO has issued a drug formulation patent for HETLIOZ LQ® that will expire in 2040. We also have other pending patent applications covering methods of treatment and compositions of tasimelteon (HETLIOZ® active ingredient) oral suspensions.
We filed several Hatch-Waxman lawsuits in the U.S. District Court for the District of Delaware (Delaware District Court) against Teva Pharmaceuticals USA, Inc. (Teva), Apotex Inc. (Apotex), MSN Pharmaceuticals, Inc. and MSN Laboratories Private Limited (MSN) asserting infringement of patents covering HETLIOZ® 20 mg capsules. In January 2022, we entered into a license agreement with MSN and Impax Laboratories LLC resolving the lawsuits against MSN. The consolidated lawsuits against Teva and Apotex were tried in March 2022. In December 2022, the Delaware District Court ruled that Teva and Apotex did not infringe U.S. Patent No. RE46,604, and that the asserted claims of U.S. Patent Nos. RE46,604; 9,730,910; 10,149,829; and 10,376,487 were invalid. We appealed the decision to the U.S. Court of Appeals for the Federal Circuit (Federal Circuit) and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S. Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied
our petition. We have also filed Hatch-Waxman lawsuits in U.S. District Court for the District of New Jersey against each of Teva and Apotex and in the U.S. District Court for the Southern District of Florida against Apotex, in each case, asserting infringement of U.S. Patent No. 11,285,129, which is a method of administration patent that was not litigated in the prior litigation. The New Jersey cases have been transferred to the Delaware District Court, where they remain pending, and the Florida case was voluntarily dismissed. We have also filed patent infringement lawsuits against each of Teva and Apotex in the Delaware District Court, in each case, asserting infringement of U.S. Patent No. 11,918,556, another method of administration patent that was not litigated in the prior litigation. These lawsuits do not affect the sale of HETLIOZ® in the E.U. and there is no generic litigation pending outside of the U.S. with respect to HETLIOZ®. Furthermore, this litigation does not relate to the HETLIOZ LQ® oral suspension formulation.
In July 2024, we filed a Hatch-Waxman lawsuit against MSN in the Delaware District Court asserting that the U.S. Patent Nos. 10,179,119, 11,266,622, 11,285,129, 11,850,229, 10,610,510, 10,980,770, and 11,759,446 will be infringed by MSN’s generic version of HETLIOZ LQ® for which MSN is seeking FDA approval. Our lawsuit remains pending, and a trial is scheduled to begin on June 15, 2026.
See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” in Part I, Item 1A of this Annual Report, each of which is incorporated herein by reference, for additional information.
In Europe, the law provides for 10 years of data exclusivity (with the potential for an additional year if a medicine is developed for a significant new indication). In addition, Europe provides for 10 years of market exclusivity for orphan indications. As such, in Europe, data or market exclusivity will provide protection for HETLIOZ® for at least 10 years from approval. It is also possible that the protection through a basic patent (i.e., a patent that protects a product as such, a process to obtain a product, or an application of a product) in Europe could be extended for up to five years by the issuance of a supplementary protection certificate (SPC). A completed Pediatric Investigation Plan (PIP) could further extend SPC protection for an additional six months or the market exclusivity in an orphan indication for two additional years. Thus, a PIP could provide a total of 12 years of market exclusivity for an orphan indication. The European Patent Office has granted our patent application directed to the 20 mg/day dose. This patent will expire in 2027 and provides the basis for an SPC. Other pending patent applications in Europe, if granted, may offer additional protection for HETLIOZ®.
Outside the U.S. and Europe, data exclusivity will protect HETLIOZ® from generic competition for varying numbers of years depending on the country.
Additional patent applications directed to specific sleep disorders and to methods of treating patients with HETLIOZ®, if issued, could provide exclusivity for such indications and methods of treatment.
PONVORY®
Janssen obtained patent protection for PONVORY® and its formulations in selected countries worldwide, including the U.S. and Canada. In December 2023, we acquired all rights that Janssen had in U.S. and Canadian patents related to PONVORY®, pending U.S. and Canada patent applications related to PONVORY®, and any further U.S. and Canadian derivative patents and patent applications arising from the foregoing patents and pending patent applications. The NCE protecting PONVORY® in the U.S. will expire in March 2026. The NCE patent covering the active ingredient in PONVORY® (Reissue Patent No. 43,728) expired in November 2024, but an application for patent term extension (PTE) pursuant to the Hatch-Waxman Act was submitted to extend this patent’s term to November 2029. The PTE application was granted on January 31, 2025. The USPTO has granted additional patents, including a further patent directed to a crystalline form of the active ingredient in PONVORY®, which will expire in May 2032 as a result of the awarded patent term adjustment. The USPTO has also issued four method of treatment patents for PONVORY®, of which one expired in November 2024, and the remaining three will expire between December 2025 and October 2042. Also, a number of patent applications covering further methods of treatment remain pending at the USPTO. Because PONVORY® was granted NCE exclusivity, no generic applications can be filed until one year prior to the expiration of that exclusivity, in March 2025.
In Canada, the Patented Medicines (Notice of Compliance) Regulations (PM(NOC) Regulations) create a regime analogous to the Hatch-Waxman Act and link the regulatory approval process for generic and biosimilar drugs to the adjudication of innovator patent rights. To be eligible for protection under the PM(NOC) Regulations, patents must first be listed on the Patent Register in connection with an innovator’s drug submission to Health Canada. A generic or biosimilar
manufacturer must then provide notice to the innovator of its plans to market a drug that it compared to the innovator’s patented drug in the Health Canada approval process. Within 45 days of receiving such a notice of allegation, an innovator drug company may commence patent infringement proceedings against the generic or biosimilar manufacturer. The commencement of an action by the innovator under the PM(NOC) Regulations may stay Health Canada’s regulatory approval of the generic or biosimilar drug for a period of 24 months. It is also possible that protection through a patent (i.e., a patent claiming a medicinal ingredient, or the combination of all medicinal ingredients, or uses thereof) in Canada can be extended for up to two years by the issuance of a Certificate of Supplementary Protection (CSP). Health Canada’s Patent Register lists Canadian Patent Nos. 2740313 and 2968180 for PONVORY®, which are respectively directed to the crystalline form of ponesimod, the active ingredient in PONVORY® and methods of treatment. These listed patents will respectively expire in October 2029 and, as a result of the CSP granted for 2968180, in April 2036. The ponesimod compound patent Canadian Patent No. 2545582 expired in November 2024.
Canada also employs a data exclusivity regime for innovative drugs that provides an eight-year period of data protection from the date of market approval by Health Canada. An additional six months of data exclusivity is provided for drugs studied in clinical trials relating to use in pediatric populations. Drug submissions seeking approval based on a comparison to an innovative drug cannot be filed during the first six years of the data exclusivity period. Generic or biosimilar drug submissions remain on hold until expiry of the innovator’s data protection term, unless the innovative product is a patented drug subject to further protection under the PM(NOC) Regulations. The six-year no-file date for PONVORY® is April 28, 2027, and data protection for ponesimod ends April 28, 2029 according to the Canadian Register of Innovative Drugs, the Products for Human Use - Active Data Protection Period. Canada has no distinct drug submission process for biosimilar or orphan drug products.
Tradipitant
Lilly owns the NCE patent as well as patent applications directed to polymorphic forms of, and methods of making tradipitant. This patent protection was sought in the U.S. and in other countries worldwide. These patents and patent applications have been licensed to us. The NCE patent covering tradipitant expired in April 2023, except in the U.S., where it expired in June 2024, subject to any extension that may be received under the Hatch-Waxman Act. We have filed additional patent applications based on discoveries made during recent studies with tradipitant.
Imsidolimab
Anaptys owns patents and patent applications covering imsidolimab, an IL-36R antagonist, which have been licensed to us under an exclusive global license agreement. These patents and applications include claims directed to antibodies that inhibit the interleukin-36 receptor, methods of use and formulations. Patent protection for imsidolimab has been sought in the U.S. and other countries. In the U.S., the patent protection may be subject to extension under the Biologics Price Competition and Innovation Act (BPCIA), which provides a framework similar to the Hatch-Waxman Act for small molecules, allowing for potential exclusivity extensions for biologics. The patents protecting imsidolimab are expected to provide exclusivity into the late 2030s. We are committed to advancing the development of imsidolimab and may file additional patent applications based on discoveries made during its development. For more on the license and sublicense arrangements related to imsidolimab, see License Agreements above.
VTR-297
VTR-297 is a small molecule HDAC inhibitor with potential use as a treatment for several oncology indications. We have pending patent applications covering the use of VTR-297 and plan on filing additional applications based on discoveries made throughout the development plan of this molecule.
Portfolio of CFTR activators and inhibitors
Our portfolio of CFTR activators and inhibitors may have broad applicability in addressing a number of high unmet medical needs, including chronic dry eye, constipation, polycystic kidney disease, cholestasis and secretory diarrheas. We plan on filing applications based on discoveries made throughout the development plan of these product candidates.
VQW-765
Novartis owns the NCE patent as well as patent applications directed to methods of using VQW-765, VQW-765 formulations, and combinations of VQW-765 with other active pharmaceutical ingredients. In connection with the settlement
agreement with Novartis relating to Fanapt®, we received an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize VQW-765, a Phase II alpha-7 nicotinic acetylcholine receptor partial agonist. The NCE patent expired normally in 2023 in the U.S., Europe and other markets.
Other patents
Aside from the NCE patents and other in-licensed patents discussed above, we have obtained or filed numerous patents and patent applications, most of which have been filed in key markets including the U.S., relating to our products and product candidates. In addition, we have filed numerous other patent applications relating to drugs not presently in clinical studies. The claims in these various patents and patent applications are directed to compositions of matter, including claims covering other products, pharmaceutical compositions and methods of use.
Proprietary know-how
For proprietary know-how that is not appropriate for patent protection, processes for which patents are difficult to enforce and any other elements of our discovery process that involve proprietary know-how and technology that are not covered by patent applications, we generally rely on trade secret protection and confidentiality agreements to protect our interests. We require all of our employees, relevant consultants and advisors to enter into confidentiality agreements. Where it is necessary to share our proprietary information or data with outside parties, our policy is to make available only that information and data required to accomplish the desired purpose and only pursuant to a duty of confidentiality on the part of those parties.
Marketing and Sales
Fanapt® oral tablets were approved in the U.S. for the treatment of schizophrenia in May 2009 and commercially launched in the U.S. in January 2010. Fanapt® tablets for the acute treatment of bipolar I disorder in adults were approved in April 2024 and commercially launched in the U.S. in the third quarter of 2024. We continue to explore the regulatory path and commercial opportunity for Fanapt® oral formulation in other regions.
HETLIOZ® capsules were approved in the U.S. for the treatment of Non-24 in January 2014 and HETLIOZ® capsules and oral suspension were approved for the treatment of nighttime sleep disturbances in SMS in December 2020. We commercially launched HETLIOZ® capsules in the U.S. in April 2014 and the oral suspension (HETLIOZ LQ®) in March 2021. Additionally, HETLIOZ® capsules were approved in the E.U. for the treatment of Non-24 in totally blind adults in July 2015 and, in August 2016, we commercially launched HETLIOZ® in Germany. Given the range of potential indications for HETLIOZ®, we may pursue one or more partnerships for the development and commercialization of HETLIOZ® worldwide.
PONVORY® tablets were approved in the U.S. for the treatment of RMS in adults in March 2021 and commercially launched in the U.S. by Janssen in April 2021. PONVORY® tablets were approved in Canada for the treatment of RMS in adults in April 2021 and commercially launched in Canada by Janssen in November 2021. We acquired the U.S. and Canadian rights to PONVORY® in December 2023 from Janssen and we transitioned U.S. operations of PONVORY® during 2024.
Major Customers
Our revenues are generated from product sales and are concentrated in a limited number of specialty pharmacies, specialty distributors and wholesalers. There were four major customers that each accounted for more than 10% of total revenues for 2024 and, as a group, represented 69% of total revenues for the year ended December 31, 2024.
Competition
The pharmaceutical industry, in particular, is highly competitive and includes a number of established large and mid-sized companies with greater financial, technical and personnel resources than we have and significantly greater commercial infrastructures than we have. Our market segment also includes several smaller emerging companies whose activities are directly focused on our target markets and areas of expertise. Our products, once approved for commercial use, will compete with numerous therapeutic treatments offered by these competitors. While we believe that our products will have certain favorable features, existing and new treatments may also possess advantages. Additionally, the development of other drug technologies and methods of disease prevention are occurring at a rapid pace. These developments may render our products or technologies obsolete or noncompetitive.
We believe the competitors for Fanapt®, HETLIOZ® and PONVORY® are as follows:
•For Fanapt® in the treatment of schizophrenia, the atypical antipsychotics competitors are Risperdal® (risperidone), including the LAI formulation Risperdal Consta®, and Invega® Sustenna® (paliperidone), each by Johnson & Johnson, the LAI formulation Zyprexa® RelprevvTM (olanzapine) by Cheplapharm, Abilify® (aripiprazole) by Otsuka America Pharmaceutical Inc., Abilify Maintena® (the LAI formulation of Abilify®), Abilify Asimtufi® and Rexulti® (brexpiprazole), all by Lundbeck/Otsuka America Pharmaceutical Inc., Geodon® (ziprasidone) by Viatris, Inc., Saphris® (asenapine) and Vraylar® (cariprazine), both by AbbVie Inc., Latuda® (lurasidone) by Sunovion Pharmaceuticals Inc., Aristada® (aripiprazole lauroxil) extended-release injectable suspension and Lybalvi® (olanzapine and samidorphan), both by Alkermes, plc, Caplyta® (lumapteperone) by Intra-Cellular Therapies, Inc., CobenfyTM (xanomeline/trospium chloride) by BMS, Secuado® (asenapine) by Noven Pharmaceuticals and generic clozapine and quetiapine, as well as the typical antipsychotics haloperidol, chlorpromazine, thioridazine, and sulpiride (all of which are generic). For Fanapt® in the treatment of bipolar I disorder, competitors include Lithobid® (lithium) by ANI Pharmaceuticals, Depakote® (divalproex sodium) and Vraylar® (cariprazine), both by AbbVie Inc., Lamictal® (lamotrigine by GlaxoSmithKline, Seroquel® (quetiapine fumarate) by AstraZeneca, Risperdal® (risperidone), including the LAI formulation Risperdal Consta®, by Johnson & Johnson, Abilify® (aripiprazole) by Otsuka America Pharmaceutical Inc., Abilify Maintena® (the LAI formulation of Abilify®) and Abilify Asimtufi®, both by Lundbeck/Otsuka America Pharmaceutical Inc., Latuda® (lurasidone) by Sunovion Pharmaceuticals Inc., Zyprexa® (olanzapine) and Symbyax® (olanzapine/fluoxetine), both by Lilly, Caplyta® (lumapteperone) by Intra-Cellular Therapies, Inc., Lybalvi® (olanzapine and samidorphan) by Alkermes, plc, Geodon® (ziprasidone) by Viatris, Inc. and Equetro® (carbamazepine) by Validus Pharmaceuticals LLC.
•For HETLIOZ® in the treatment of nighttime sleep disturbances in SMS, there are no FDA approved direct competitors. For HETLIOZ® in the treatment of Non-24, Teva and Apotex have launched their respective Abbreviated New Drug Application (ANDA) products at risk, and the FDA has also approved the ANDA for MSN. In addition, sedative-hypnotic treatments for certain sleep-related disorders include, Ambien® (zolpidem), including Ambien CR®, by Cosette Pharmaceuticals, Inc., Lunesta® (eszopiclone) by Woodward Pharma Services LLC, Rozerem® (ramelteon) by Takeda Pharmaceuticals Company Limited, Silenor® (doxepin) by Currax Pharmaceuticals LLC, Belsomra® (suvorexant) by Merck & Co., Inc., Dayvigo® (lemborexant) by Eisai Inc., generic products such as agomelatine, zaleplon, trazodone and doxepin, and over-the-counter remedies such as Benadryl® and Tylenol PM®. The class of melatonin agonist treatments includes Rozerem® (ramelteon) by Takeda Pharmaceuticals Company Limited, and the food supplement melatonin. Shift work and excessive sleepiness disorder treatments include Nuvigil® (armodafinil) and Provigil® (modafinil) both by Apotex.
•For PONVORY® in the treatment of RMS, the competitors include Avonex® (interferon beta-1a), Tysabri® (natalizumab), Tecfidera® (Dimethyl fumarate) and Plegridy® (peginterferon beta-1a), all by Biogen Inc., Vumerity® (diroximel fumerate) by Biogen Inc./Alkermes, plc, Betaseron® (interferon beta-1b) by Bayer Healthcare Pharmaceuticals Inc., Rebif® (interferon beta-1a) and Mavenclad® (cladribine), both by Merck KGaA, Extavia® (interferon beta-1b), Mayzent® (siponimod), Gilenya® (fingolimod) and Kesimpta® (ofatumumab), all by Novartis AG, Lemtrada® (alemtuzumab) and Aubagio® (teriflunomide), both by Sanofi, Ocrevus® (ocrelizumab) by Roche Holding AG/Biogen Inc., Zeposia® (ozanimod) by BMS, Briumvi® (ubiltuximab) by TG Therapeutics, Inc., Tyruko® (natalizumab) by Sandoz Group AG and Copaxone® (glatiramer acetate) by Teva.
Our ability to compete successfully will depend, in part, on our ability to utilize our pharmacogenetics and pharmacogenomics and drug development expertise to identify, develop, secure rights to and obtain regulatory approvals for promising pharmaceutical products before others are able to develop competitive products. Our ability to compete successfully will also depend on our ability to attract and retain skilled and experienced personnel. Additionally, our ability to compete may be affected because insurers and other third-party payors in some cases seek to encourage the use of cheaper, generic products, which could make our products less attractive.
Manufacturing
We currently utilize a virtual supply manufacturing and distribution chain, meaning that we do not have our own facilities to manufacture commercial or clinical trial supplies of drugs, and we do not have our own distribution facilities. Instead, we contract with third parties to source critical raw materials and for the manufacture, warehousing, order management, billing and collection and distribution of our products and product candidates.
We expect to continue to rely solely on third-party manufacturers to manufacture drug substance and final drug products for both clinical development and commercial sale. However, there are numerous factors that could cause interruptions in the supply of our products, including regulatory reviews, changes in our sources for manufacturing, disputes with a
manufacturer, or financial instability of manufacturers, all of which could negatively impact our operations and our financial results.
We have agreements in place with Patheon Pharmaceuticals Inc. and Patheon Inc. (collectively, Patheon), subsidiaries of Thermo Fisher Scientific, for the manufacture of Fanapt® oral tablets and HETLIOZ® capsules.
As part of a settlement agreement in 2014, we assumed Novartis’ manufacturing agreement with Patheon for the manufacture of commercial supplies of Fanapt®. In May 2016, we entered into a new manufacturing agreement with Patheon for the manufacture of commercial supplies of Fanapt® 1, 2, 4, 6, 8, 10 and 12 mg tablets at Patheon’s Mississauga, Ontario, Canada manufacturing site. Under the Fanapt® manufacturing agreement, we are responsible for supplying the active pharmaceutical ingredient (iloperidone) and have agreed to order from Patheon at least 70% of the total expected yearly production of new units of Fanapt® tablets for the U.S. and other specified countries each year for the term of the agreement. Patheon is responsible for manufacturing the Fanapt® 1, 2, 4, 6, 8, 10 and 12 mg tablets, conducting quality control and stability testing, and packaging the Fanapt® tablets. The Fanapt® manufacturing agreement had an initial term of five years and automatically renews after the initial term for successive terms of one year each, unless either party gives notice of its intention to terminate the agreement at least 12 months prior to the end of the then current term. Either party may terminate the Fanapt® manufacturing agreement under certain circumstances upon specified written notice to the other party.
In January 2014, we entered into a manufacturing agreement with Patheon for the manufacture of commercial supplies of HETLIOZ® 20 mg capsules at Patheon’s Cincinnati, Ohio manufacturing site. Under the HETLIOZ® manufacturing agreement, we are responsible for supplying the active pharmaceutical ingredient (tasimelteon) for HETLIOZ® to Patheon and have agreed to order from Patheon at least 80% of the total expected yearly production of new units of HETLIOZ® capsules. Patheon is responsible for manufacturing the HETLIOZ® 20 mg capsules, conducting quality control and stability testing, and packaging the HETLIOZ® capsules. The HETLIOZ® manufacturing agreement had an initial term of five years and automatically renews after the initial term for successive terms of one year each, unless either party gives notice of its intention to terminate the agreement at least 12 months prior to the end of the then current term. Either party may terminate the HETLIOZ® manufacturing agreement under certain circumstances upon specified written notice to the other party.
In December 2020, we entered into a non-exclusive manufacturing agreement for the manufacture of commercial supplies of both 48 mL and 158 mL HETLIOZ LQ® bottles. The HETLIOZ LQ® manufacturing agreement has an initial term of five years and automatically renews after the initial term for successive terms of one year each, unless either party gives notice of its intention to terminate the agreement at least 12 months prior to the end of the then current term.
In December 2024, we entered into a non-exclusive manufacturing agreement of PONVORY®. The PONVORY® manufacturing agreement has an initial term of three years and automatically renews after the initial term until the termination or expiration of any outstanding project agreements.
Government Regulation
Government authorities in the U.S. at the federal, state and local levels and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, recordkeeping, promotion, advertising, distribution, marketing and export and import of pharmaceutical products. A new drug must be approved by the FDA through the NDA process before it may be legally marketed in the U.S. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign laws and regulations require the expenditure of substantial time and financial resources. Moreover, failure to comply with applicable requirements may result in, among other things, warning letters, clinical holds, civil or criminal penalties, recall or seizure of products, injunction, disbarment, partial or total suspension of production or withdrawal of the product from the market. Any judicial, administrative or other governmental enforcement action could have a material adverse effect on our business.
U.S. government regulation
U.S. drug development and regulation
In the U.S., the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act (FDCA) and related regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or post-approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning
letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and/or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on our business.
Once a drug candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND application sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor must also include a protocol detailing, among other things, the objectives of the first phase of clinical trials, the parameters to be used in monitoring the safety of the trial, and the effectiveness criteria to be evaluated should the first phase lend itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns about on-going or proposed clinical trials or non-compliance with specific FDA requirements, and the trials may not begin or continue until the FDA notifies the sponsor that the hold has been lifted.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with FDA good clinical practice (GCP) requirements, which include a requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and/or effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. An Institutional Review Board (IRB) at each institution participating in the clinical trial must review and approve each protocol before a clinical trial may commence at the institution and must also approve the information regarding the trial as well as the consent form that must be provided to each trial subject or his or her legal representative, monitor the study until completed and otherwise comply with all applicable IRB regulations.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined in certain cases:
•Phase I: The compound is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain an early indication of its effectiveness. In most cases, initial Phase I clinical trials are conducted with healthy volunteers. However, where the compound being evaluated is for the treatment of severe or life-threatening diseases, such as cancer, and especially when the product may be too toxic to ethically administer to healthy volunteers, the initial human testing may be conducted on patients with the target disease or condition. Sponsors sometimes subdivide their Phase I clinical trials into Phase Ia and Phase Ib clinical trials. Phase Ib clinical trials are typically aimed at confirming dosage, pharmacokinetics and safety in a larger number of patients. Some Phase Ib studies evaluate biomarkers or surrogate markers that may be associated with efficacy in patients with specific types of diseases or conditions.
•Phase II: This phase involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases or conditions and to confirm dosage tolerance and appropriate dosage.
•Phase III: Phase III clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population, generally at geographically dispersed clinical study sites. These clinical trials, often referred to as “pivotal” clinical trials, are intended to establish the overall risk-benefit ratio of the compound and provide, if appropriate, an adequate basis for product labeling.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including any finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected, serious harm to study subjects. In addition, clinical trials may be overseen by a Data and Safety Monitoring Board (DSMB), an independent group of qualified experts organized by the sponsor. Depending on its charter, the DSMB may determine whether a trial may move forward at designated check points based on access to certain data from the trial.
Post-approval trials may also be conducted after a drug receives initial marketing approval. These trials, often referred to as “Phase IV” trials, are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of such clinical trials as a condition of approval of an NDA.
During the development of a new drug, sponsors are given several opportunities to meet with the FDA. These meetings can provide an opportunity for the sponsor to share information about the progress of the application or clinical trials, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. These meetings may occur prior to the submission of an IND, at the end of Phase I and Phase II clinical trials, or before an NDA is ultimately submitted. Sponsors typically use the meetings at the end of the Phase II trials to discuss Phase II clinical results and present plans for the pivotal Phase III clinical trials that they believe will support approval of the new drug. Meetings at other times may be scheduled upon request.
Concurrent with clinical trials, companies typically complete additional animal or other non-clinical studies, develop additional information about the chemistry and physical characteristics of the drug, and finalize a process for manufacturing the product in commercial quantities in accordance with the FDA’s current Good Manufacturing Practices (cGMP) requirements. The manufacturing process must consistently produce quality batches of the drug and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate the effectiveness of the packaging and that the compound does not undergo unacceptable deterioration over its shelf life.
While the IND is active, progress reports summarizing the results of ongoing clinical trials and nonclinical studies performed since the last progress report must be submitted on at least an annual basis to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans and any clinically important, increased incidence of a serious adverse reaction compared to that listed in the protocol or investigator brochure.
There are also requirements governing the submission of certain clinical trials and completed trial results to public registries. Sponsors of certain clinical trials of FDA-regulated products are required to register and disclose specified clinical trial registration and results information, which is made publicly available at www.clinicaltrials.gov. Failure to properly report clinical trial results can result in civil monetary penalties. Disclosure of clinical trial results can often be delayed until the new product or new indication being studied has been approved.
U.S. review and approval process
The results of product development, preclinical and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA. The submission of an NDA is subject to the payment of substantial user fees; a waiver of which may be obtained under certain limited circumstances.
The FDA reviews NDAs to determine, among other things, whether the product is safe and effective for its intended use and whether it is manufactured in a cGMP-compliant manner, which will assure and preserve the product’s identity, strength, quality and purity. Under Prescription Drug User Fee Act Amendments of 2022, the FDA has a goal of 10 months from the date of “filing” of a standard, completed NDA for a new molecular entity to review and act on the submission. This review typically takes 12 months from the date the NDA is submitted to the FDA because the FDA has 60 days to make a “filing” decision after the application is submitted. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
The FDA may refer an application for a new drug to an advisory committee within the FDA. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether and under what conditions the application should be approved. The FDA is not bound by the recommendations of such an advisory committee, but it considers advisory committee recommendations carefully when making decisions.
Before approving an NDA, the FDA will also inspect the facility where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. Before approving an NDA, the FDA may also inspect one or more clinical trial sites to assure compliance with GCP requirements.
After the FDA evaluates an NDA, it will issue an approval letter or a CRL. A CRL indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A CRL usually describes the specific deficiencies in the NDA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase III trial or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. If a CRL is issued, the sponsor must resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not satisfy the criteria for approval. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications.
The Pediatric Research Equity Act (PREA) requires IND sponsors to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under the PREA, original NDAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or the FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The sponsor or FDA may also request a waiver of the requirement to submit pediatric assessments. A waiver may be granted for one of several reasons, including because the necessary studies are impossible or highly impracticable. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
If a drug receives FDA approval, the approval may be limited to specific diseases and dosages, which could restrict the commercial value of the product. In addition, the FDA may require testing and surveillance programs to monitor the safety of approved products that have been commercialized, and may require a sponsor to conduct post-marketing clinical trials, which are designed to further assess a drug’s safety and effectiveness after NDA approval. The FDA may also place other conditions on approval, including a requirement for a risk evaluation and mitigation strategy (REMS) to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescribing or dispensing of products. Marketing approval may be withdrawn for non-compliance with REMS or other regulatory requirements, or if problems occur following initial marketing.
Post-approval requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the drug reaches the market. Later discovery of previously unknown problems with a drug may result in restrictions on the drug or even complete withdrawal of the drug from the market. After approval, some types of changes to the approved drug, such as adding new indications, certain manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP regulations and other laws and regulations.
Our approved products are, and any additional product manufactured or distributed by us following FDA approval will be, subject to continuing regulation by the FDA, including, among other things, recordkeeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information regarding approved drugs that are placed on the market, and imposes requirements and restrictions on drug manufacturers, such as those related to direct-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities and promotional activities involving the internet. Discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product for a certain indication or withdrawal of the product from the market as well as possible civil or criminal sanctions. Failure to comply with the applicable governmental requirements at any time during the product development process, approval process or after approval, may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical holds on post-marketing clinical trials, enforcement letters, product recalls, product seizures, total or partial suspension of
production or distribution, injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties, any of which could have a material adverse effect on our business.
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, generally a disease or condition that affects fewer than 200,000 individuals in the U.S or, if it affects more than 200,000 individuals in the U.S., there is no reasonable expectation that sales of the drug will be sufficient to offset the cost of developing and making the drug available in the U.S. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and development expenses and a waiver of the NDA application user fee.
Expedited development and review programs
The FDA has a fast track designation program that is intended to expedite or facilitate the process for reviewing new drug products that meet certain criteria. Specifically, new drugs are eligible for fast track designation if they are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. With regard to a fast track product, the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
Any product submitted to the FDA for approval, including a product with a fast track designation, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval.
A product is eligible for priority review if it is intended to treat a serious condition, and if approved, would provide a significant improvement in safety or efficacy compared to currently marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug designated for priority review in an effort to facilitate the review. The FDA endeavors to review applications with priority review designations within six months of the filing date, as compared to 10 months for review of NDAs under its current PDUFA review goals.
In addition, a product may be eligible for accelerated approval. Drugs intended to treat serious or life-threatening diseases or conditions may be eligible for accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. Drugs receiving accelerated approval may be subject to expedited withdrawal procedures if the sponsor fails to conduct the required post-marketing trials or if such trials fail to verify the predicted clinical benefit. In addition, the FDA requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
The Food and Drug Administration Safety and Innovation Act established a category of drugs referred to as “breakthrough therapies” that may be eligible to receive breakthrough therapy designation. A sponsor may seek FDA designation of a compound as a “breakthrough therapy” if the product is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the fast track program features, as well as more intensive FDA interaction and guidance. The breakthrough therapy designation is a distinct status from
both accelerated approval and priority review, which can also be granted to the same drug if relevant criteria are met. If a product is designated as a breakthrough therapy, the FDA will work to expedite the development and review of such drug.
Fast track designation, priority review and breakthrough therapy designation do not change the standards for approval but may expedite the development or approval process. However, even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Marketing exclusivity
The FDA provides periods of regulatory exclusivity, which provide the holder of an approved NDA limited protection from new competition in the marketplace for the innovation represented by its approved drug for a period of three or five years following the FDA’s approval of the NDA. Five years of exclusivity are available to NCEs. An NCE is a drug that contains no active moiety that has been approved by the FDA in any other NDA. An active moiety is the molecule or ion, excluding those appended portions of the molecule that cause the drug to be an ester, salt, including a salt with hydrogen or coordination bonds, or other noncovalent, or not involving the sharing of electron pairs between atoms, derivatives, such as a complex (i.e., formed by the chemical interaction of two compounds), chelate (i.e., a chemical compound), or clathrate (i.e., a polymer framework that traps molecules), of the molecule, responsible for the therapeutic activity of the drug substance. During the exclusivity period, the FDA may not accept for review or approve an ANDA or a 505(b)(2) NDA submitted by another company that contains the previously approved active moiety. An ANDA or 505(b)(2) application, however, may be submitted one year before NCE exclusivity expires if it includes a certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid. If a product is not eligible for the NCE exclusivity, it may be eligible for three years of exclusivity. Three-year exclusivity is available to the holder of an NDA, including a 505(b)(2) NDA, for a particular condition of approval, or change to a marketed product, such as a new formulation, route of administration or indication for a previously approved product, if one or more new clinical trials, other than bioavailability or bioequivalence trials, was essential to the approval of the application and was conducted or sponsored by the applicant. This three-year exclusivity period protects against FDA approval of ANDAs and 505(b)(2) NDAs for the condition of the new drug’s approval. As a general matter, three-year exclusivity granted for a new drug does not prohibit the FDA from approving additional ANDAs or 505(b)(2) NDAs for generic versions of the original, unmodified drug product. Five-year and three-year exclusivity will not delay the submission or approval of an NDA where an applicant obtained a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and efficacy.
Pediatric exclusivity is another type of marketing exclusivity available in the U.S. Pediatric exclusivity provides for an additional six months of marketing exclusivity attached to another period of exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA. The issuance of a written request does not require the sponsor to undertake the described clinical trials. In addition, orphan drug designation, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
Orange Book listing, the Hatch-Waxman Act
In seeking approval for a drug through an NDA, applicants are required to submit to the FDA each patent with at least one claim that covers the applicant’s drug substance (active ingredient), drug product (formulation or composition) or a method of using the drug which is sought or has been granted in the application. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Orange Book, which potential competitors seeking approval of ANDAs must certify against. An ANDA provides for marketing of a drug that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. ANDA applicants are not required to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug, other than the requirement for bioequivalence testing. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved drug in the Orange Book. Specifically, the applicant must certify for each listed patent that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new drug. A certification that the new drug will not infringe the already approved drug’s listed patents or that such patents are invalid is called a Paragraph IV certification. If the applicant does not challenge the listed patents through a Paragraph IV certification, the ANDA application will not be approved until all the listed patents claiming the referenced drug have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
In addition to patent exclusivity, the NDA holder may be entitled to additional exclusivities. The ANDA application also will not be approved until any non-patent exclusivity listed in the Orange Book for the referenced drug has expired. For example, the FDA provides an NCE exclusivity period of five years following approval of a drug containing no previously approved active ingredients. For drugs that have NCE exclusivity, ANDAs for generic versions of those drugs cannot be submitted until four years following the original drug approval. Federal law also provides for a period of three years of exclusivity following approval of a listed drug that contains previously approved active ingredients but is approved in a new dosage form, route of administration or combination, or for a new use, the approval of which was required to be supported by new clinical trials conducted by or for the sponsor, during which the FDA cannot grant effective approval of an ANDA based on that listed drug.
Fraud and abuse laws and other U.S. regulatory matters
Pharmaceutical companies are subject to broadly applicable fraud and abuse and other healthcare laws and regulations, in addition to the FDCA, that may constrain the business or financial arrangements and relationships through which these companies market, sell and distribute the products for which they obtain marketing approval. Some of the laws and regulations that may affect the ability of pharmaceutical companies to operate are described below.
Anti-kickback laws
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease, or order of any health care item or service reimbursable under federal healthcare programs such as Medicare and Medicaid. The term “remuneration” has been broadly interpreted to include anything of value, and the government can establish a violation of the Anti-Kickback Statute without proving that a person or entity had actual knowledge of the law or specific intent to violate it. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, patients, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly. Failure to meet all of the requirements of a particular statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute, but the legality of the arrangement will be evaluated on a case-by-case basis based on the totality of the facts and circumstances. Violations of the Anti-Kickback Statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs, such as Medicare and Medicaid. A number of states also have anti-kickback laws that establish similar prohibitions that may apply to items or services reimbursed by government programs, as well as any third-party payors, including commercial payors, known as “all-payor” laws, and in some cases may apply regardless of whether there is reimbursement by a third-party payor.
Prescription Drug Marketing Act
As part of the sales and marketing process, pharmaceutical companies frequently provide healthcare providers with samples of approved drugs. The Prescription Drug Marketing Act of 1987 (PDMA), as modified by the Prescription Drug Amendments of 1992 and the FDA Modernization Act of 1997, along with regulations promulgated thereunder, imposes, among other things, requirements and limitations upon the distribution of drugs and drug samples, and prohibits states from licensing distributors of prescription drugs unless the state licensing program meets certain federal guidelines that include minimum standards for storage and handling, as well as recordkeeping and other requirements. Violations of the PDMA may result in criminal and civil penalties. In addition, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively known as the Affordable Care Act or ACA), discussed in more detail in Pharmaceutical Coverage, Pricing and Reimbursement and Healthcare Reform below, imposes annual reporting requirements related to sample distribution.
False Claims Act
The federal False Claims Act prohibits, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment of government funds and knowingly making, or causing to be made or used, a false record or
statement to get a false claim paid. Certain marketing practices may implicate the False Claims Act, including promotion of pharmaceutical products for unapproved uses, providing free product to customers with the expectation that customers would bill federal programs for the product, or inflating prices reported to private price publication services used to set drug reimbursement rates under federal healthcare programs. In addition, the ACA amended the Social Security Act to provide that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false claim for purposes of the False Claims Act. Actions under the False Claims Act may be brought by the government or as a qui tam action by private individuals who may receive financial awards if their claims are successful. False Claims Act liability is potentially significant in the healthcare industry because the statute provides for treble damages and monetary penalties of $5,500 to $11,000 per false claim or statement, adjusted for inflation as applicable, with respect to violations occurring after November 2, 2015. Violations of the False Claims Act are also punishable by exclusion from participation in federal healthcare programs, such as Medicare and Medicaid. Pharmaceutical and other life sciences companies often resolve allegations without admissions of liability for significant and sometimes material amounts to avoid the uncertainty of treble damages and per claim penalties that may be awarded in litigation. These companies may be required, however, to enter into corporate integrity agreements with the government, which may impose substantial costs on companies to ensure compliance.
Health Insurance Portability and Accountability Act of 1996
The Health Insurance Portability and Accountability Act of 1996 (HIPAA), includes federal criminal statutory provisions that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH) and their implementing regulations, impose certain requirements and restrictions on certain types of entities relating to the privacy and security of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable not only to covered entities (e.g., certain health care providers and health plans), but also to business associates (i.e., independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity). HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
Physician Payments Sunshine Act
The Physician Payments Sunshine Act (Sunshine Act), as amended by the SUPPORT Act and its implementing regulations, requires certain applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually (with certain exceptions) to the Centers for Medicare & Medicaid Services (CMS) information related to direct or indirect payments or other “transfers of value” made to physicians, certain non-physician practitioners, such as nurse practitioners and physician assistants, and teaching hospitals (“covered recipients”), as well as payments or other transfers of value provided to a third party on behalf of a covered recipient. The Sunshine Act also requires applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians and their immediate family members and related payments or other “transfers of value.” Failure to report relevant data may result in civil fines and/or penalties.
Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act (FCPA), prohibits U.S. corporations and their representatives and intermediaries from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Violation of the FCPA could result in substantial civil and criminal penalties and remedies, including fines, disgorgement, debarment and/or imprisonment.
Analogous state laws
Analogous state fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, can apply to the business practices of pharmaceutical companies, including but not limited to research, distribution, sales or marketing arrangements as well as claims involving healthcare items or services reimbursed by non-governmental third-party payors, and are generally broad and are enforced by many different federal and state agencies as well as through private actions. In addition to requiring reporting transfers of value, some states have imposed price reporting requirements. These state laws apply to items
and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. In addition, a number of states require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products and to report gifts and payments to individual physicians in the states. Other states restrict when pharmaceutical companies may provide meals to prescribers or engage in other marketing-related activities or require pharmaceutical companies to implement compliance programs or marketing codes of conduct, and file periodic reports or disclosures with states. Compliance with these laws requires significant resources and companies that do not comply may face civil penalties or other consequences.
Many state laws govern the privacy and security of personal information in specified circumstances. For example, the California Consumer Privacy Act (CCPA), which became effective on January 1, 2020, as further amended by the California Privacy Rights Act, along with corresponding regulations, established a new legal framework governing covered businesses’ collection and use of personal information of California residents by, among other things, creating an expanded definition of covered personal information, establishing new privacy rights for California residents, imposing an opt-in standard for certain disclosures of personal information about minors, and creating a new and potentially severe statutory damages framework for businesses subject to certain data breaches resulting from the failure to implement and maintain reasonable security procedures and practices. While properly collected clinical trial data and all protected health information governed by HIPAA are exempt from the current version of the CCPA, other personal information may be applicable and possible changes to the CCPA may broaden its scope.
Foreign regulation
Foreign drug development, review and approval processes
Regardless of whether a sponsor obtains FDA approval for a product, it must obtain approval by the comparable regulatory authorities of foreign countries before it can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement also vary greatly from country to country. Although governed by the applicable country, clinical trials conducted outside of the U.S. typically are administered with the three-Phase sequential process that is discussed above under U.S. drug development and regulation. However, the foreign equivalent of an IND is not a prerequisite to performing pilot studies or Phase I clinical trials.
Under E.U. regulatory systems, a sponsor may submit Marketing Authorization Applications either under a centralized or decentralized procedure. The centralized procedure, which is available for drugs produced by biotechnology or which are highly innovative, provides for the grant of a single marketing authorization that is valid for all E.U. Member States. This authorization is referred to as a marketing authorization approval. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining Member States. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval. This procedure is referred to as the mutual recognition procedure. In addition, regulatory approval of prices is required in most countries other than the U.S. Companies face the risk that the resulting prices would be insufficient to generate an acceptable return.
Foreign fraud and abuse laws and other regulatory matters
Outside the U.S., companies are subject to similar regulations, including with respect to transparency, bribery and other laws mentioned above. In some foreign countries, including major markets in the E.U. and Japan, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take nine to 12 months or longer after the receipt of regulatory marketing approval for a product. To obtain reimbursement or pricing approval in some countries, companies may be required to conduct a clinical trial that compares the cost-effectiveness of their product to other available therapies, which can be costly and time-consuming.
The collection and processing of personal data in the E.U. is governed by the General Data Protection Regulation (GDPR), which became applicable in May 2018. The GDPR applies to personal data processing carried out by a controller or processor (i) established in the E.U. or (ii) offering goods or services to E.U. individuals, or monitoring their behavior within the E.U., regardless of the controller’s or processor’s location. The GDPR implements stringent operational requirements for controllers and processors of personal data, including, for example, the implementation of transparent information for data subjects regarding the processing of their personal data, appropriate legal basis for the processing of personal data, which may include the obtaining of valid consent in certain circumstances, expanded individual data subject rights, limitations on retention of personal data, increased requirements pertaining to data security and confidentiality, mandatory data breach notification to the competent supervisory authority and higher standards for controllers and processors to demonstrate their compliance with
the GDPR through appropriate documentation. The GDPR provides that E.U. Member States may supplement the GDPR with their own additional laws and regulations in relation to certain processing of personal data, in particular regarding sensitive personal data (e.g., genetic, biometric or health data), which could result in differences between E.U. Member States, limit a company’s ability to collect, use and share such personal data or cause their costs to increase, and harm their reputation, business and financial condition. Further, the U.K.’s exit from the E.U., often referred to as Brexit, has created uncertainty with regard to applicable data protection regulation in the U.K. While the transition period has now concluded, decisions are still to be made on how to adapt the U.K. data protection provisions further to Brexit. Companies are also subject to evolving and strict rules on the transfer of personal data out of the E.U., in particular to the U.S. Failure to comply with the GDPR may result in fines of up to the higher of €20,000,000 or 4% of total worldwide annual revenue of the preceding financial year, and other administrative penalties.
Pharmaceutical Coverage, Pricing and Reimbursement and Healthcare Reform
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs in the U.S. such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity, and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may also limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor’s decision to provide coverage for a drug product does not imply that the reimbursement rate ultimately paid will be adequate. Third party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. There have been a number of federal and state proposals during the last several years regarding the pricing of pharmaceutical and biopharmaceutical products, limiting coverage and reimbursement for drugs and other medical products, government control and other changes to the healthcare system in the U.S. By way of example, the ACA was passed in 2010 and made significant changes to the coverage and payment for products under government health care programs. Among the provisions of the ACA of importance to pharmaceutical companies are:
•added an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee does not apply to sales of certain products approved exclusively for orphan indications;
•expanded the eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level;
•expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program (MDRP) by increasing the minimum rebate for both branded and generic drugs and extending rebate liability to prescriptions for individuals enrolled in Medicaid managed care plans and otherwise made amendments to the MDRP;
•expanded the types of entities eligible for the 340B drug discount program;
•established the Medicare Part D coverage gap discount program by requiring manufacturers to provide a point‑of‑sale‑discount off the negotiated price of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D;
•established a new Patient‑Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
•added a requirement to annually report product samples that manufacturers and distributors provide to physicians;
•expanded healthcare fraud and abuse laws, including the False Claims Act and the federal Anti-Kickback Statute, and enhanced penalties for noncompliance; and
•established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. In June 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes include the Budget Control Act of 2011, which, among other things, included aggregate reductions of Medicare payments to providers that started in 2013 and will stay in effect through 2032 unless additional congressional action is taken. More recently, in March 2021, President Biden signed into law the American Rescue Plan Act of 2021, which, beginning January 1, 2024, eliminated the statutory Medicaid drug rebate cap, previously set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs. Additionally, in December 2020, CMS issued a final rule that materially modifies current MDRP regulations by, among other things, broadening the definitions of what constitutes a “line extension.” A “line extension” drug may be subject to a higher Medicaid rebate, and broadening this definition is likely to subject a greater number of drugs to the higher rebate. These new definitions became effective on January 1, 2022. In September 2024, CMS issued a final rule that implemented regulations to, among other things, reflect the removal of the rebate cap in accordance with the American Rescue Plan Act of 2021.
Most significantly, in August 2022, President Biden signed the Inflation Reduction Act of 2022 (IRA) into law. This statute marks the most significant action by Congress with respect to the pharmaceutical industry since adoption of the ACA in 2010. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare, with prices that can be negotiated subject to a cap, with the first drug price negotiations effective January 1, 2026; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation beginning October 1, 2022; and replaces the Medicare Part D coverage gap discount program with a new discounting program beginning January 1, 2025. The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On March 15, 2023 and June 30, 2023, HHS issued guidance regarding implementation of the Medicare drug price negotiation program in initial price applicability year 2026. More recently, in October 2024, HHS released guidance related to implementation for initial price applicability year 2027. In August 2024, CMS announced prices for the first ten drugs under the drug price negotiation program, with the new prices going into effect in 2026, and is expected to announce the next fifteen drugs to be selected for the second round of negotiations by February 1, 2025. Several manufacturers and industry groups have challenged the drug price negotiation program for Medicare Parts B and D in federal court. These lawsuits are ongoing, and additional lawsuits may be filed in the future related to provisions of the IRA. It is unknown whether such litigation or other litigation, if brought, will be successful, or whether there will be future changes to the IRA. Moreover, the recent change in presidential administrations in the U.S. may introduce additional unpredictability regarding the future of the IRA. For these and other reasons, it is currently unclear how the IRA will be effectuated, and while the impact of the IRA on the pharmaceutical industry cannot yet be fully determined, it is likely to be significant.
The cost of prescription pharmaceuticals in the U.S. is likely to remain the subject of considerable discussion. There have been several Congressional inquiries and proposed and enacted legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare, and reform government program reimbursement methodologies for drug products. The likelihood of implementation of these and other reform initiatives is uncertain. In the coming years, additional legislative and regulatory changes could be made to governmental health programs that could significantly impact pharmaceutical companies and the success of our product candidates. We expect that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria, new payment methodologies and additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our product candidates.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures. Some measures encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing. The IRA, as well as other federal, state and foreign healthcare reform measures that have been and may be adopted in the future, could have a material adverse effect on our business.
These healthcare reforms, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the price for any approved product and/or the level of reimbursement physicians receive for administering any approved product. Reductions in reimbursement levels may negatively impact the prices we can charge or the frequency with which products are prescribed or administered. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
Similarly, pricing and reimbursement and the containment of healthcare costs has become a priority in a number of foreign jurisdictions. In the E.U., pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies, or so-called health technology assessments, in order to obtain reimbursement or pricing approval. For example, the E.U. provides options for its Member States to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. E.U. Member States may approve a specific price for a drug product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other Member States allow companies to fix their own prices for drug products but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not provide favorable reimbursement and pricing arrangements.
See the risk factor entitled “If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs in the U.S., we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects” in Part I, Item 1A of this Annual Report for additional information regarding our participation in federal healthcare programs and related compliance obligations.
Human Capital
We had 368 full-time employees as of December 31, 2024, compared with 203 employees as of December 31, 2023. None of our employees are represented by a labor union. We have not experienced any work stoppages and consider our employee relations to be good. Our human capital objectives include attracting, training and retaining employees in a manner that supports innovation across our business.
Corporate Information
We were incorporated in Delaware in 2002. Our principal executive offices are located at 2200 Pennsylvania Avenue NW, Suite 300E, Washington, D.C. 20037, and our telephone number is (202) 734-3400. Our website address is www.vandapharma.com, and the information contained in, or that can be accessed through, our website is not incorporated by reference in this Annual Report and should not be considered a part of this Annual Report.
Available Information
We file annual, quarterly, and current reports, proxy statements, and other documents with the Securities and Exchange Commission (SEC) under the Securities Exchange Act of 1934 (Exchange Act). The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers, including us, that file electronically with the SEC.
We also make available free of charge on our Internet website at www.vandapharma.com our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and, if applicable, amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
Our code of business conduct and ethics, other corporate policies and procedures, and the charters of our Audit Committee, Compensation Committee and Nominating/Corporate Governance Committee are available at our corporate website at www.vandapharma.com. To access these documents from the main page of our website, click on “Investors” at the top of the page, then click on “Learn More” under “Corporate Governance” and then click on the desired document. We intend
to satisfy the disclosure requirements under Item 5.05 of Form 8‑K regarding amendments to, or waivers from, provisions of our code of business conduct and ethics by posting such information on the website address and location specified above.
None of the information contained on our website or www.sec.gov is incorporated by reference into this Annual Report or any other report or document filed with the SEC unless expressly stated otherwise therein.
Our business, financial condition and operating results can be affected by a number of factors, whether currently known or unknown, including but not limited to those described below, any one or more of which could, directly or indirectly, cause our actual operating results and financial condition to vary materially from past, or anticipated future, operating results and financial condition. Any of these factors, in whole or in part, could materially and adversely affect our business, financial condition, operating results and the price of our common stock.
The following discussion of risk factors contains forward-looking statements. These risk factors may be important to understanding any statement in this annual report on Form 10-K (Annual Report) or elsewhere. The following information should be read in conjunction with the consolidated financial statements and related notes in Part II, Item 8, Financial Statements and Supplementary Data and Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Because of the following risk factors, as well as other risk factors affecting our financial condition and operating results, past financial performance should not be considered to be a reliable indicator of future performance, and investors should not use historical trends to anticipate results or trends in future periods.
Risks Related to our Business and Industry
We are dependent on the commercial success of Fanapt®, HETLIOZ® and PONVORY®. In the U.S., HETLIOZ® competes with generic versions of HETLIOZ® and we could experience increased generic competition in the near term.
We are substantially dependent upon the commercial success of Fanapt® oral tablets for the acute treatment of mixed or manic episodes associated with bipolar I disorder and the treatment of schizophrenia, HETLIOZ® capsules for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24), HETLIOZ® capsules and oral suspension (HETLIOZ LQ®) for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome (SMS) and PONVORY® oral tablets for the treatment of relapsing forms of multiple sclerosis (RMS) in adults.
In the fourth quarter of 2014, we acquired the U.S. commercial rights to Fanapt®, and began selling, marketing and distributing Fanapt® in the U.S. for the treatment of schizophrenia in adults. On April 2, 2024, the FDA approved Fanapt® tablets for the acute treatment of manic or mixed episodes associated with bipolar I disorder in adults. We initiated the commercial launch of Fanapt® for the acute treatment of bipolar I disorder in adults in the third quarter of 2024.
In January 2014, the U.S. Food and Drug Administration (FDA) approved our New Drug Application (NDA) for HETLIOZ® for the treatment of Non-24 and in April 2014, we commenced the U.S. commercial launch of HETLIOZ®. In July 2015, the European Commission (EC) granted centralized marketing authorization with unified labeling for HETLIOZ® for the treatment of Non-24 in totally blind adults, and in August 2016 we commenced the commercial launch of HETLIOZ® in Germany. This authorization, which was renewed in July 2020 for an unlimited duration, is valid in the 27 countries that are members of the European Union (E.U.), as well as European Economic Area members Iceland, Liechtenstein and Norway. In December 2020, the FDA approved our NDA and supplemental New Drug Application (sNDA) for HETLIOZ® for the treatment of nighttime sleep disturbances in SMS in adults and children, respectively. HETLIOZ® capsules, for adults with SMS, were immediately available after approval and the HETLIOZ LQ® liquid formulation, for children with SMS, became available in March 2021.
In December 2022, the U.S. District Court for the District of Delaware (Delaware District Court) ruled in favor of certain generic drug companies in our patent litigation alleging that the companies’ generic versions of HETLIOZ® capsules, for which they were seeking FDA approval, infringed our patents covering HETLIOZ®. We appealed the decision to the U.S. Court of Appeals for the Federal Circuit (Federal Circuit). In May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition. The FDA has approved Abbreviated New Drug Applications (ANDA) for generic versions of HETLIOZ® for Teva Pharmaceuticals USA, Inc. (Teva), Apotex Inc. (Apotex) and MSN Pharmaceuticals Inc. and
MSN Laboratories Private Limited (MSN). Teva and Apotex have launched their generic versions of HETLIOZ® at risk in the U.S., and MSN has launched its generic version as well. HETLIOZ® could face even more competition from other generic companies in the U.S. in the near term in light of the patent litigation rulings against us. Sales of generic versions of HETLIOZ® have resulted in and could continue to result in a reduction in the demand for HETLIOZ® and/or the price at which we can sell it and/or create volatility in net product sales in future periods, which would have a material and adverse impact on our revenues and results of operations. Our expansion and development of HETLIOZ® outside the U.S. is generally not subject to the adverse patent ruling in the U.S.
PONVORY® tablets were approved in the U.S. for the treatment of RMS in adults in March 2021 and commercially launched in the U.S. by Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company in April 2021. PONVORY® tablets were approved in Canada for the treatment of relapsing forms of multiple sclerosis (RMS) in adults in April 2021 and commercially launched in Canada by Janssen in November 2021. In December 2023, we acquired the U.S. and Canadian rights to PONVORY® from Janssen. Janssen was responsible for the continued marketing and sale of PONVORY® during a transition period. We initiated our commercial launch of PONVORY® in RMS in the third quarter of 2024.
Our ability to generate significant product revenue from sales of Fanapt®, HETLIOZ® and PONVORY® both in the U.S. and abroad, in the near term will depend on, among other things, our ability to:
•defend our patents and intellectual property from generic competition;
•properly price and obtain adequate coverage and reimbursement of these products by governmental authorities, private health insurers, managed care organizations and other third-party payors;
•gain broad acceptance of our products from physicians, health care payors, patients, pharmacists and the medical community;
•minimize the impact of disruptions caused by public health crises;
•maintain commercial manufacturing arrangements with third-party manufacturers;
•produce, through a validated process, sufficiently large quantities of inventory of our products to meet demand;
•continue to maintain and grow a wide variety of internal sales, distribution and marketing capabilities sufficient to sustain sales trajectories of our products;
•maintain compliance with ongoing labeling, packaging, storage, advertising, promotion, recordkeeping, safety and other post-market requirements;
•obtain regulatory approval to expand the labeling of our approved products for additional indications;
•obtain regulatory approval for HETLIOZ® or Fanapt® in additional countries;
•maintain our existing regulatory approval for HETLIOZ® in Europe and PONVORY® in Canada;
•adequately protect against and effectively respond to any claims by holders of patents and other intellectual property rights that our products infringe their rights; and
•adequately protect against and effectively respond to any unanticipated adverse effects or unfavorable publicity that develops in respect to our products, as well as the emergence of new or existing competitive products, which may be proven to be more clinically effective and cost-effective.
We expect to continue to incur significant expenses and to utilize a substantial portion of our cash resources as we continue to commercialize our commercial products, evaluate foreign market opportunities for Fanapt® and HETLIOZ® and continue to grow our operational capabilities, both domestically and abroad. This activity represents a significant investment in the commercial success of Fanapt®, HETLIOZ® and PONVORY®, which is uncertain.
If our continued commercial efforts are not successful with respect to our commercial products in the U.S., Europe, Canada or other jurisdictions in which these products may be approved for sale, our ability to generate increased product sales revenue may be adversely affected.
The cost of growing and maintaining a sales, marketing and distribution organization may exceed its cost effectiveness. If we fail to continue to develop sales, marketing and distribution capabilities, if sales efforts are not effective or if costs of developing sales, marketing and distribution capabilities exceed their cost effectiveness, our business, results of operations and financial condition could be materially adversely affected.
As a result of the decision in favor of generic drug companies in connection with our HETLIOZ® patent litigation, we have faced generic competition in the near term and our revenues and results of operations could be further affected by the launch of additional generic versions of HETLIOZ® in the U.S.
Between April 2018 and March 2021, we filed numerous Hatch-Waxman lawsuits in the Delaware District Court against Teva, Apotex and MSN asserting that our patents would be infringed by their generic versions of HETLIOZ®. In January 2022, we entered into a license agreement with MSN and Impax Laboratories LLC (Impax), resolving the lawsuits against MSN. A trial was held in March 2022 in the Delaware District Court to resolve the consolidated lawsuits against Teva and Apotex.
In December 2022, following conclusion of the trial, the Delaware District Court issued its ruling in favor of Teva and Apotex, finding that their use of a generic HETLIOZ®, for which they were seeking FDA approval, did not infringe one of our HETLIOZ® patents and the asserted claims of certain of our other HETLIOZ® patents were invalid. We appealed the decision to the Federal Circuit and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S. Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition. Teva and Apotex have since launched their generic versions at risk and MSN has launched its generic version as well. The commercial launch of the generic versions, and potential increased competition from additional generic entrants in the near term, have resulted in and could continue to have a material and adverse impact on our revenues and results of operations.
Further, although we are pursuing additional remedies in other courts, including seeking injunctions against Apotex and Teva, we may not be successful in any such efforts, which will be costly and time-consuming to pursue. Specifically, we have filed Hatch-Waxman lawsuits in the U.S. District Court for the District of New Jersey against each of Teva and Apotex, and in the U.S. District Court for the Southern District of Florida against Apotex, asserting infringement of U.S. Patent No. 11,285,129, which is a method of administration patent that was not litigated in the prior litigation. The New Jersey cases have been transferred to the Delaware District Court, where they remain pending, and the Florida case was voluntarily dismissed. We have also filed patent infringement lawsuits against each of Teva and Apotex in the U.S. District Court for the District of Delaware, in each case, asserting infringement of U.S. Patent No. 11,918,556, another method of administration patent that was not litigated in the prior litigation. These lawsuits do not affect the sale of HETLIOZ® in the E.U. and there is no generic litigation pending outside of the U.S. with respect to HETLIOZ®. These efforts will also require considerable attention of management and could, even if ultimately successful, negatively impact our results of operations. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” each of which is incorporated herein by reference, for additional information.
In addition, while we believe that HETLIOZ® is difficult to manufacture and that building capacity to manufacture HETLIOZ® is time-consuming and expensive, which may limit the amount of tasimelteon supply available to generic companies, we do not have direct visibility into the supply levels of any of the generic companies and we rely on our own experience together with information from third parties, which may not be reliable. The generic companies could potentially find or develop sources of qualified tasimelteon supply that are not known to us and that are more efficient or inexpensive than our sources. Furthermore, generic companies could potentially convince our suppliers or third-party manufacturers to prioritize supply to the generic companies ahead of any applicable contractual commitments to supply us. Such circumstances could have a material and adverse impact on our revenues and results of operations directly in the U.S. and potentially outside of the U.S. as well if supply costs and availability are affected.
Future performance of Fanapt®, HETLIOZ® and PONVORY® may be impacted by a number of factors including competing products or unanticipated safety issues. If Fanapt®, HETLIOZ® or PONVORY® is not successful in gaining broad commercial acceptance, our business would be harmed.
Future performance of Fanapt®, HETLIOZ® and PONVORY® sales will be dependent on several factors, including our ability to educate physicians and to increase physician awareness of the benefits of our products relative to competing products. The degree of further market acceptance of any of our products, including with respect to new indications, or market acceptance of approved product candidates among physicians, patients, health care payors and the medical community, will depend on a number of factors, including but not limited to:
•the impact and outcome of our pending patent litigation and appeals efforts;
•the commercialization and pricing of any generic version of HETLIOZ® on the market;
•acceptable evidence of safety and efficacy;
•relative convenience and ease of administration;
•the prevalence and severity of any adverse side effects;
•availability of alternative treatments;
•market awareness of the condition to be treated; and
•pricing and cost effectiveness.
In addition, commercial products are subject to continual review by the FDA, and we cannot assure that newly discovered or reported safety issues will not arise. With the use of any newly marketed drug by a wider patient population, serious adverse events may occur from time to time that initially do not appear to relate to the drug itself. Any safety issues could cause us to suspend or cease marketing of our approved products, cause us to modify how we market our approved products, subject us to substantial liabilities and adversely affect our revenues and financial condition. In the event of a withdrawal of our commercial products from the market, our revenues would decline significantly and our business would be seriously harmed.
With the launch of generic versions of HETLIOZ® and further generic versions possible, it may not be viable for us to invest in market education to grow the U.S. market and our ability to maintain current promotional efforts and attract favorable commercial terms in several aspects of our business will likely be adversely affected as we face increased generic competition.
We are subject to uncertainty relating to pricing and reimbursement policies in the U.S., including recent and future health reform measures, which, if not favorable for our products, could hinder or prevent our products’ commercial success.
Our ability to commercialize our products successfully depends in part on the coverage and reimbursement levels with governmental authorities, private health insurers and other third-party payors. In determining whether to reimburse our products and at what level, third-party payors consider factors that include the efficacy, cost effectiveness and safety of our products, as well as the availability of other treatments including generic prescription drugs and over-the-counter alternatives. We expect to continue to face pressure to make unfavorable pricing modifications, such as discounts or rebates. Negotiating favorable reimbursement can be a time consuming and expensive process, and there is no guarantee that we will be able to reach pricing terms with third-party payors at levels that are profitable to us. Certain third-party payors also have reimbursement or coverage processes that we believe are difficult to navigate and require prior authorization for, or even refuse to provide, reimbursement for our products, and others may do so in the future. Our business may be materially adversely affected if our patients are not able to receive approval for reimbursement of our products from third-party payors on a broad, timely or satisfactory basis; if reimbursement is subject to difficult reimbursement or coverage processes or prior authorization requirements; or if reimbursement is not maintained at satisfactory levels. In addition, our business could be adversely affected if third-party payors limit or reduce the indications for, or conditions under which, or the patient populations for whom, our products may be reimbursed. Moreover, as discussed further below and above in Part I, Item 1 under the heading Pharmaceutical Coverage, Pricing and Reimbursement and Healthcare Reform, changes in insurance coverage or reimbursement levels by third-party payors, or in the type of such coverage held by patients, may materially harm our business and commercialization efforts.
We expect to experience pricing pressures in connection with the sale of our current and future products due to the healthcare reforms discussed below and above in Part I, Item 1 under the heading Pharmaceutical Coverage, Pricing and Reimbursement and Healthcare Reform, as well as the trend toward initiatives aimed at reducing healthcare costs, the increasing influence of managed care, the scrutiny of pharmaceutical pricing, the ongoing debates on reducing government spending and additional legislative proposals. There has been significant scrutiny of pharmaceutical pricing and the resulting costs of pharmaceutical products that could cause significant operational and reimbursement changes for the pharmaceutical industry. There have been a number of federal and state efforts to address drug costs, which generally have focused on increasing transparency around drug costs or limiting drug prices, price increases or other related costs.
Most significantly, in August 2022, President Biden signed the Inflation Reduction Act of 2022 (IRA) into law. This statute marks the most significant action by Congress with respect to the pharmaceutical industry since adoption of the ACA in 2010. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare, with prices that can be negotiated subject to a cap, with the first drug price negotiations effective January 1, 2026; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation beginning October 1, 2022; and replaces the Medicare Part D coverage gap discount program with a new discounting program beginning January 1, 2025. The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On March 15, 2023 and June 30, 2023, HHS issued guidance regarding implementation of the Medicare drug price negotiation program in initial price applicability year 2026. More recently, in October 2024, HHS released guidance related to implementation for initial price applicability year 2027. In August
2024, CMS announced prices for the first ten drugs under the drug price negotiation program, with the new prices going into effect in 2026, and is expected to announce the next fifteen drugs to be selected for the second round of negotiations by February 1, 2025. Several manufacturers and industry groups have challenged the drug price negotiation program for Medicare Parts B and D in federal court. These lawsuits are ongoing, and additional lawsuits may be filed in the future related to provisions of the IRA. It is unknown whether such litigation or other litigation, if brought, will be successful, or whether there will be future changes to the IRA. Moreover, the recent change in presidential administrations in the U.S. may introduce additional unpredictability regarding the future of the IRA. For these and other reasons, it is currently unclear how the IRA will be effectuated, and while the impact of the IRA on the pharmaceutical industry cannot yet be fully determined, it is likely to be significant.
Healthcare reform efforts or any future legislation or regulatory actions aimed at controlling and reducing healthcare costs, including through measures designed to limit reimbursement, restrict access or impose unfavorable pricing modifications on pharmaceutical products, could impact our ability to obtain or maintain reimbursement for our products at satisfactory levels, or at all, which could materially harm our business and financial results.
We have encountered third-party payors that refuse to cover or reimburse prescriptions written for HETLIOZ® and patients who are unable to navigate the coverage or reimbursement processes established by these third-party payors. If this trend continues, the commercial success of HETLIOZ® may be limited, and our business and results of operations may be materially harmed.
We have encountered third-party payors that refuse to cover or reimburse prescriptions written for HETLIOZ®. This rate may increase further as a result of the entry into the market of generic versions of HETLIOZ®. Additionally, we are aware of patients who are experiencing difficulties navigating coverage processes established by third-party payors, making it difficult for them to fill a prescription for HETLIOZ®. The revenue that we receive from HETLIOZ® is significantly less than it would be if third-party payors were to remove or lessen these reimbursement challenges and hurdles and approve a greater percentage of the prescriptions written for HETLIOZ®. Our business may be materially adversely affected if this trend continues and large numbers of patients cannot fill their HETLIOZ® prescriptions due to coverage or reimbursement challenges.
If the FDA does not approve our NDA filing for the use of tradipitant for patients with gastroparesis; or if the FDA determines that our clinical trial results for tradipitant for the treatment of gastroparesis do not demonstrate adequate safety and substantial evidence of efficacy, continued development of tradipitant may be significantly delayed or terminated, our business will be significantly harmed, and the market price of our stock could decline.
In February 2022, we announced results from our Phase III clinical study, VP-VLY-686-3301, evaluating the efficacy and safety of tradipitant in treating the symptoms of gastroparesis. The study did not meet its prespecified primary endpoint, which was the difference between drug and placebo on the change of the severity of nausea from baseline at week 12 of treatment. Both treatment arms showed significant improvements from baseline on nausea as well as the other core symptoms of gastroparesis. When restricting the analysis in the group of patients that used no rescue medications at baseline and adjusting for poor compliance, we identified strong evidence of a drug effect across a number of symptoms and across the duration of the study, including a significant and meaningful effect at the prespecified primary endpoint of nausea change at week 12. The FDA may not view this data as constituting substantial evidence of efficacy for tradipitant in any indication for the treatment of gastroparesis or its symptoms, for any length of treatment. Any adverse developments or results or perceived adverse developments or results with respect to our regulatory submission or the tradipitant clinical program in gastroparesis will significantly harm our business and could cause the market price of our stock to decline. Examples of such potential adverse developments include, but are not limited to:
•the FDA determining that it believes additional clinical studies are required with respect to tradipitant for the treatment of gastroparesis;
•safety, efficacy or other concerns arising from clinical or non-clinical studies in this program; or
•the FDA determining that the tradipitant clinical trial program for gastroparesis does not demonstrate adequate safety and substantial evidence of efficacy.
We believe that tradipitant has a well-established safety profile, as demonstrated by the results of extensive testing in animals and humans. Despite these results, however, the FDA informed us in December 2018 that in order to treat patients beyond 12 weeks, we would have to conduct a nine-month non-rodent chronic toxicity study. This currently limits our ability to collect safety data in humans for more than 12 weeks. The non-rodent study required by the FDA necessitates the sacrifice of dozens of animals and we have disputed the necessity of a nine-month non-rodent chronic toxicity study. In February 2019, we filed a lawsuit in the U.S. District Court for the District of Columbia (DC District Court) challenging the FDA’s position, but
we ultimately did not prevail. Despite our disagreement with the FDA, the preclinical package has allowed us to continue to conduct all of the efficacy studies necessary for NDA filing. Moreover, in July 2020, the FDA authorized tradipitant through an expanded access program (EAP) for a single patient. An EAP allows a patient to request the use of tradipitant, prior to NDA approval, for up to six months with an option to request renewal. Since then, certain patients who experienced a benefit in tradipitant studies have requested and received expanded access, while others have been denied treatment under the EAP. The EAP is ongoing and a number of patients have initiated treatment. Although this EAP is not intended for data collection, we collect safety data from this cohort of expanded access patients and included this data in the NDA that we submitted for tradipitant for patients with gastroparesis. In December 2023, the FDA accepted our NDA for tradipitant in gastroparesis for filing and set a PDUFA target action date of September 18, 2024. On September 18, 2024, we received a CRL from the FDA. In January 2025, we received a Notice of Opportunity for a Hearing, and we have accepted the opportunity for a hearing. Tradipitant is the first novel drug to be accepted for review by the FDA for gastroparesis in over 30 years and, if approved, will be the first novel drug to be approved by the FDA for the treatment of gastroparesis in over 40 years. The lack of long-term (i.e., more than 12 weeks in humans) safety data would likely impact the FDA’s willingness to approve tradipitant for a chronic indication. However, because long-term safety data is not normally a requirement for short-term indications, and with a preclinical profile that has not precluded clinical development, we believe the package was complete for any NDA filing to treat patients for 12 weeks or less. For example, the FDA has communicated to us that it is considering an indication for the short-term relief of nausea in gastroparesis. While this short-term indication is not preferred, we would consider accepting this limited indication while continuing to pursue a chronic indication. However, the FDA may not deem the safety information sufficient even for a short-term indication. Moreover, FDA authorization of an EAP is not a guarantee of or a step towards obtaining full FDA approval of an NDA. We plan to continue to pursue the marketing authorization for tradipitant but our business will be materially adversely impacted if we are not able to agree with the FDA on a regulatory path to approval for tradipitant or the FDA delays or denies approval of our NDA filing.
If the FDA does not accept our NDA filing for the use of tradipitant for patients with motion sickness; or if the FDA determines that our clinical trial results for tradipitant for the treatment of motion sickness do not demonstrate adequate safety and substantial evidence of efficacy, continued development of tradipitant will be significantly delayed or terminated, our business will be significantly harmed, and the market price of our stock could decline.
In May 2023, we announced positive results from the first Phase III study of tradipitant in motion sickness, confirming the previously reported results demonstrating that tradipitant is effective in the prevention of vomiting associated with motion sickness. In May 2024 we announced positive results from the second Phase III study of tradipitant in motion sickness, confirming the previously reported results of two efficacy studies demonstrating tradipitant is effective in prevention of vomiting associated with motion sickness. Any adverse developments or results or perceived adverse developments or results with respect to our regulatory submission or the tradipitant clinical program in motion sickness will significantly harm our business and could cause the market price of our stock to decline. Examples of such potential adverse developments include, but are not limited to:
•the FDA determining that it believes additional clinical studies are required with respect to tradipitant for the treatment of motion sickness;
•safety, efficacy or other concerns arising from clinical or non-clinical studies in this program; or
•the FDA determining that the tradipitant clinical trial program for motion sickness does not demonstrate adequate safety and substantial evidence of efficacy.
See the risk factor entitled “If the FDA does not approve our NDA filing for the use of tradipitant for patients with gastroparesis; or if the FDA determines that our clinical trial results for tradipitant for the treatment of gastroparesis do not demonstrate adequate safety and substantial evidence of efficacy, continued development of tradipitant may be significantly delayed or terminated, our business will be significantly harmed, and the market price of our stock could decline” for additional details regarding tradipitant.
Global economic conditions may have an adverse effect on our business.
Financial instability or a general decline in economic conditions in the U.S. and other countries caused by political instability and conflict and economic challenges caused by general health crises have led to market disruptions, including significant volatility in commodity prices, credit and capital market instability and supply chain interruptions, which have caused record inflation globally and could adversely affect our operations. Increased inflation may result in increases in our operating costs (including our labor costs), reduced liquidity and limits on our ability to access credit or otherwise raise capital on acceptable terms, if at all. Existing free trade laws and regulations, such as the United States-Mexico-Canada Agreement, provide certain beneficial duties and tariffs for qualifying imports and exports, subject to compliance with the applicable
classification and other requirements. Changes in laws or policies governing the terms of trade, and in particular increased trade restrictions, tariffs or taxes on imports from countries where we manufacture products, such as Canada, China and Mexico, could have a material adverse effect on our business and financial results. For example, in February 2025, the U.S. government imposed or threatened to impose new tariffs on imported products from Mexico, Canada and China. The impact of these tariffs is subject to a number of factors, including the effective date and duration of such tariffs, changes in the amount, scope and nature of the tariffs in the future, any retaliatory responses to such actions that the target countries may take and any mitigating actions that may become available. Despite recent trade negotiations between the U.S. and the Mexican, Canadian and Chinese governments, given the uncertainty regarding the scope and duration of any new tariffs, as well as the potential for additional tariffs or trade barriers by the U.S., Mexico, Canada, China or other countries, we can provide no assurance that any strategies we implement to mitigate the impact of such tariffs or other trade actions will be successful. In addition, the U.S. Federal Reserve has raised, and may again raise, interest rates in response to concerns about inflation, which, coupled with reduced government spending and volatility in financial markets, may have the effect of further increasing economic uncertainty and heightening these risks. Economic conditions, and uncertainty as to the general direction of the macroeconomic environment, are beyond our control and may make any necessary debt or equity financing more difficult, costly and dilutive. While we believe we have adequate capital resources to meet current working capital and capital expenditure requirements, an economic downturn or significant increase in our expenses could require additional financing on less than attractive rates or on terms that are excessively dilutive to existing stockholders. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our stock price and could require us to delay or abandon clinical development plans.
As discussed in the risk factor entitled “We are subject to uncertainty relating to pricing and reimbursement policies in the U.S., including recent and future health reform measures, which, if not favorable for our products, could hinder or prevent our products’ commercial success”, sales of our products are dependent, in large part, on reimbursement from government health administration authorities, private health insurers, distribution partners and other organizations. In the event of economic decline, these organizations may be unable to satisfy their reimbursement obligations or may delay payment. In addition, federal and state health authorities may further reduce Medicare and Medicaid reimbursements, and private insurers may further increase their scrutiny of claims. A reduction in the availability or extent of reimbursement could negatively affect our product sales and revenue.
In addition, we rely on third parties for several important aspects of our business. For example, we use third parties for sales, distribution, medical affairs and clinical research, and we rely upon several single source providers of raw materials and contract manufacturers for the manufacture of our products. During challenging and uncertain economic times and in tight credit markets, there may be a disruption or delay in the performance of our third-party contractors, suppliers or partners. If such third parties are unable to satisfy their commitments to us, our business and results of operations would be adversely affected.
Global health crises and pandemics may adversely impact our business.
Global health crises and pandemics could lead to the implementation of various responses, including government-imposed quarantines, travel restrictions and other public health safety measures that may negatively impact productivity and disrupt our business.
The COVID-19 pandemic impacted clinical research globally, including delays in our development programs. While our clinical trials have since resumed patient enrollment, we may experience future disruptions as a result of other health crises that could adversely impact our sales activities, supply chain, our ongoing and planned clinical trials, and other regulatory activities, including:
•curtailment of our sales force or patient access to healthcare providers, which may reduce the number of prescription refills or new patient starts, thereby adversely affecting our revenues;
•interruption of, or delays in receiving, supplies of the active pharmaceutical ingredients that our contract manufacturing organizations use to manufacture our products and any related interruption of, or delays in receiving, supplies of our products from these organizations, due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems;
•delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
•diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials;
•delays or difficulties in enrolling patients in our clinical trials;
•interruption of key clinical trial activities, such as clinical trial site data monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others or interruption of clinical trial subject visits and study procedures (such as procedures that are deemed non-essential), which may impact the integrity of subject data and clinical study endpoints;
•limitations on our employee resources or those of third-party clinical research organizations towards the development of our products, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; and
•interruption or delays in the operations of regulatory agencies, which may impact review and approval timelines.
If the FDA does not approve our sNDAs for HETLIOZ® for the treatment of jet lag disorder or insomnia, continued development of tasimelteon for the treatment of jet lag disorder and insomnia may be significantly delayed or terminated, our business will be significantly harmed, and the market price of our stock could decline.
In December 2018, we announced that the FDA had accepted the HETLIOZ® sNDA for the treatment of jet lag disorder. We received a complete response letter (CRL) in August 2019 in which the FDA asserted that the measures of the study were of unclear clinical significance and declined to approve our sNDA. We met with the FDA to discuss the CRL in a Post Action meeting and in 2022 we requested the opportunity for a hearing with the FDA on the approvability of the jet lag disorder sNDA. We filed a lawsuit against the FDA in September 2022 demanding that the FDA immediately publish in the Federal Register a notice of opportunity for a hearing on the jet lag disorder sNDA. The FDA then published the notice in the Federal Register in October 2022. We have asked the U.S. District Court for the District of Columbia (DC District Court) to, among other things, compel the FDA to comply with its obligations and declare that the FDA’s lack of compliance violates the FDCA and the FDA regulations. In January 2024, the DC District Court held an oral argument on dispositive cross-motions, following which the DC District Court granted our motion for summary judgment. The DC District Court ruled that the FDA violated the statute and ordered the FDA to either finally resolve our application or commence a hearing on or before March 5, 2024. In March 2024, we and the FDA filed a consent motion for entry of final judgment in our favor on our Administrative Procedure Act claim for the FDA’s unreasonable delay in resolving the hearing request, following which the FDA refused to hold a hearing or approve our sNDA for HETLIOZ® in the treatment of jet lag disorder. We subsequently filed a petition for review in the U.S. Court of Appeals for the District of Columbia Circuit (DC Circuit). In January 2025, the DC Circuit held an oral argument on the petition. Our petition remains pending. We continue to pursue FDA approval for HETLIOZ® for the treatment of jet lag disorder.
In July 2023, the FDA accepted our sNDA for HETLIOZ® for insomnia for filing. On March 4, 2024, we received a CRL from the FDA. In June 2024, we received a Notice of Opportunity for a Hearing and accepted an opportunity for a heading in July 2024. In August 2024, we filed for summary judgment requesting approval or a hearing on approvability of tasimelteon to treat insomnia associated with difficulties with sleep initiation. In October 2024, we received a Proposed Order denying a hearing on approvability for the insomnia sNDA, and we submitted a response to the Proposed Order in December 2024. We continue to pursue FDA approval for HETLIOZ® for the treatment of insomnia.
Any additional adverse developments or results or perceived adverse developments or results with respect to our regulatory submissions for jet lag disorder or insomnia will significantly harm our business and could cause the market price of our stock to decline. Examples of such potential adverse developments include, but are not limited to:
•the FDA determining that additional clinical studies are required with respect to the jet lag disorder or insomnia programs;
•safety, efficacy or other concerns arising from clinical or non-clinical studies in the jet lag disorder or insomnia programs, or the manufacturing processes or facilities used for the jet lag disorder or insomnia programs; or
•the FDA determining that the jet lag disorder or insomnia programs raise safety concerns or do not demonstrate substantial evidence of efficacy.
We may enter into third-party collaborations from time to time in order to develop and commercialize our products. If we are unable to identify or enter into an agreement with any material third-party collaborator, if our collaborations with any such third party are not commercially successful or if our agreement with any such third party is terminated or allowed to expire, we could be adversely affected financially or our business reputation could be harmed.
Our business strategy includes entering into collaborations with third parties for the commercialization of our products. While we are not currently party to any material commercial collaborative arrangements, areas in which we may potentially
enter into third-party collaboration arrangements include joint sales and marketing arrangements for sales and marketing in certain E.U. countries and elsewhere outside of the U.S., and future product development arrangements. If we are unable to identify or enter into an agreement with any material third-party collaborator, our business, results of operations or financial condition could be adversely affected. The launch of generic versions of HETLIOZ® and further generic competition may make it more difficult for us to identify or attract third-party collaborators and obtain favorable commercial terms in any such agreement or arrangement. Any arrangements we do enter into may not be scientifically or commercially successful. The termination of any of these arrangements might adversely affect our ability to develop, commercialize and market our products.
The success of our collaboration arrangements will depend heavily on the efforts and activities of our collaborators. Our collaborators may have significant discretion in determining the efforts and resources that they will apply to these collaborations. We expect that the risks we face in connection with these future collaborations will include the following:
•our collaboration agreements are expected to be for fixed terms and subject to termination under various circumstances, including, in many cases, on short notice without cause;
•our collaborators may develop and commercialize, either alone or with others, products and services that are similar to or competitive with our products that are the subject of their collaboration with us; and
•our collaborators may change the focus of their commercialization efforts.
In recent years, there have been a significant number of mergers and consolidations in the pharmaceutical and biotechnology industries, some of which have resulted in the participant companies reevaluating and shifting the focus of their business following the completion of these transactions. The ability of our products to reach their potential could be limited if any of our future collaborators decreases or fails to increase spending relating to such products.
Collaborations with pharmaceutical companies and other third parties often are terminated or allowed to expire by the other party. Any such termination or expiration with respect to our future collaborations could adversely affect us financially as well as harm our business reputation.
Even after we obtain regulatory approvals of a product, acceptance of the product in the marketplace is uncertain and failure to achieve commercial acceptance will prevent or delay our ability to generate significant revenue from such product.
Even after obtaining regulatory approvals for the sale of our products, the commercial success of these products will depend, among other things, on their acceptance by physicians, patients, third-party payors and other members of the medical community as therapeutic and cost-effective alternatives to competing products and treatments. The degree of market acceptance of any product will depend on a number of factors, including the demonstration of its safety and efficacy, its cost-effectiveness, its potential advantages over other therapies, the reimbursement policies of government and third-party payors with respect to such product, our ability to attract and maintain corporate partners, including pharmaceutical companies, to assist in commercializing our products, receipt of regulatory clearance of marketing claims for the uses that we are developing and the effectiveness of our marketing and distribution capabilities. If our approved products fail to gain market acceptance or do not become widely accepted by physicians, patients, third-party payors and other members of the medical community, it is unlikely that we will ever become profitable on a sustained basis or achieve significant revenues. Generic competition may also adversely affect our ability to grow our markets and obtain acceptance of our products in the marketplace.
We completed the acquisition of PONVORY® in December 2023 and initiated our commercial launch of PONVORY® in the third quarter of 2024. Our ability to successfully launch PONVORY® in the U.S. and Canada is uncertain, and we may not realize all of the anticipated benefits of the acquisition, those benefits make take longer to realize than expected or we may encounter significant integration difficulties.
We acquired the U.S. and Canadian rights to PONVORY® in December 2023 and we initiated our commercial launch of PONVORY® in the third quarter of 2024. Our ability to realize the anticipated benefits of the acquisition will depend, to a large extent, on our capacity to capitalize on potential growth opportunities and synergies. The process may be disruptive to our business and the expected benefits may not be achieved within the anticipated time frame, or at all. The failure to meet the challenges involved and to realize the anticipated benefits of the acquisition could adversely affect our business, financial condition and results of operations.
Our ability to realize the anticipated benefits of the transaction will require us to overcome a number of difficulties, including, among others:
•difficulties in achieving anticipated business opportunities and growth prospects from the acquisition;
•challenges related to public and market perception of PONVORY® and/or our acquisition of the product; and
•potential unknown liabilities, adverse consequences, unforeseen increased expenses or other unanticipated problems associated with the acquisition.
Many of these factors are outside of our control, and any one of them could result in increased costs, decreased expected revenues and further diversion of management time and energy, which could materially harm our business, financial condition and results of operations.
In addition, we have no Canadian operations and no history of commercializing products in Canada. As a result, we will have to either build our own Canadian sales force or enter into an agreement with one or more third-party collaborators for the sale and distribution of PONVORY® in Canada. There is no guarantee that we will be successful in building our own Canadian sales force or that we will be able to identify or enter into an agreement with any such third-party collaborator on favorable terms, or at all.
All of these factors could decrease or delay the expected accretive effect of the acquisition and negatively impact our stock price and harm our business. As a result, it cannot be assured that the acquisition of PONVORY® will result in the full realization of the benefits anticipated from the transaction within the anticipated time frames, or at all.
We rely on, and will continue to rely on, outsourcing arrangements for many of our activities, including preclinical and clinical development and supply of Fanapt®, HETLIOZ®, HETLIOZ LQ®, PONVORY® and our other products.
We rely on outsourcing arrangements for a significant portion of our activities, including distribution, preclinical and clinical research and development, data collection and analysis and manufacturing. We have limited control over these third parties and we cannot guarantee that they will perform their obligations in an effective and timely manner.
Disruptions to our Fanapt®, HETLIOZ®, HETLIOZ LQ® or PONVORY® supply chains could materially affect our level of success in commercializing these products, thereby reducing our future earnings and prospects.
A loss or disruption with any one of our manufacturers or suppliers could disrupt the supply of Fanapt®, HETLIOZ®, HETLIOZ LQ® or PONVORY®, possibly for a significant time period, and we may not have sufficient inventories to maintain supply before the manufacturer or supplier could be replaced or the disruption is resolved. In addition, marketed drugs and their contract manufacturing organizations are subject to continual review, including review and approval by regulatory authorities of their manufacturing facilities and the manufacturing processes, which can result in delays in the regulatory approval process and/or commercialization. Introducing a replacement or backup manufacturer or supplier for our products requires a lengthy regulatory and commercial process, including FDA approval of chemistry, manufacturing and controls (CMC) changes, and there can be no guarantee that we could obtain necessary regulatory approvals in a timely fashion, or at all. In addition, it is difficult to identify and select qualified suppliers and manufacturers with the necessary technical capabilities, and establishing new supply and manufacturing sources involves a lengthy and technical engineering process.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs in the U.S., we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
In U.S. markets, our ability to commercialize our products successfully, and to attract commercialization partners for our products, should we choose to do so, depends in significant part on the availability of adequate financial coverage and reimbursement from third-party payors, including, in the U.S., governmental payors such as the Medicare and Medicaid programs, managed care organizations, and private health insurers. We therefore participate in, and have drug price reporting, payment, and other compliance obligations under, these programs.
We participate in the Medicaid Drug Rebate Program (MDRP). Under the MDRP, we are required to pay a rebate to each state Medicaid program for our covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having our drugs eligible for coverage under Medicaid and Medicare Part B. Those rebates are based on pricing data that are reported by us on a monthly and quarterly basis to the Centers for Medicare & Medicaid Services (CMS). If we become aware that our MDRP submissions for a prior period were incorrect or have changed as a result of recalculation of the pricing data, we must resubmit the corrected data for up to three years after those data originally were due. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the MDRP and the 340B program discussed below. Pursuant to the IRA, certain figures we report under the MDRP
will also be used to compute rebates under Medicare Part D triggered by price increases that outpace inflation. If we fail to provide information timely or are found to have knowingly submitted false information to CMS, we may be subject to civil monetary penalties and other sanctions, including termination from the MDRP.
Federal law requires that any company that participates in the MDRP also participate in the Public Health Service Act’s 340B drug pricing discount program (340B program), in order for the manufacturer’s drugs to be eligible for coverage under Medicaid and Medicare Part B. The 340B program is administered by the Health Resources and Services Administration (HRSA) and requires us to agree to charge statutorily defined covered entities no more than the 340B “ceiling price” for our covered drugs when used in an outpatient setting. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as certain small rural hospitals and hospitals that serve a disproportionate share of low-income patients. The ACA expanded the 340B program to include additional entity types, including certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals, but exempts drugs designated under section 526 of the Federal Food, Drug and Cosmetic Act as “orphan drugs” from the ceiling price requirements for these covered entities. The 340B ceiling price is calculated using a statutory formula, which is based on pricing data we report under the MDRP and the rebate amount for the covered outpatient drug as calculated under the MDRP. In general, products subject to Medicaid price reporting and rebate liability are also subject to the 340B ceiling price requirement. We must report 340B ceiling prices to HRSA on a quarterly basis, and HRSA publishes them to 340B covered entities and state Medicaid programs. HRSA has finalized regulations regarding the calculation of the 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities for 340B eligible drugs. HRSA has also finalized an administrative dispute resolution process through which 340B covered entities may pursue claims against participating manufacturers for overcharges. A recent court decision in the District Court of South Carolina, Genesis Health Care, Inc. v. Becerra, found that HRSA’s definition of “patient” as applied to the 340B Program was too restrictive compared to the plain language of the statute, and may result in covered entities expanding the number of individuals considered eligible to receive drugs purchased through the 340B Program, resulting in higher volumes of drugs purchased at the discounted 340B ceiling price. Recently, HRSA has stated that it has received inquiries from manufacturers related to different rebate models, and some manufacturers have commenced litigation to challenge the legality of HRA’s position regarding proposed rebate models. The outcome of such litigation and impact on participating manufacturers is unknown at this time. In addition, legislation may be introduced that, if passed, would further expand the 340B program, such as adding further covered entities or requiring participating manufacturers to agree to provide 340B discounted pricing on drugs when used in an inpatient setting.
In order for products to be eligible for coverage under the Medicaid and Medicare Part B programs and to be purchased by certain federal agencies and grantees, we must also participate in the Department of Veterans Affairs Federal Supply Schedule (FSS), pricing program. As a participant, we must list our covered (innovator and authorized generic) drugs on an FSS contract and charge no more than Federal Ceiling Price (FCP), to the Department of Veterans Affairs, Department of Defense, Public Health Service, and Coast Guard when those agencies purchase from the FSS contract or a depot contract. FCP is calculated based on non-federal average manufacturer price data, which we are required to submit quarterly and annually. In addition, because our products are available in the retail and specialty pharmacy setting, we are required to provide rebates to the Department of Defense for prescriptions dispensed to Tricare beneficiaries from Tricare retail network pharmacies under the Tricare Retail Refund Program. If a manufacturer participating in the FSS program fails to provide timely information or is found to have knowingly submitted false information, or fails to provide information on a timely basis, the manufacturer may be subject to civil monetary penalties.
Individual states continue to consider and have enacted legislation to limit the growth of healthcare costs, including the cost of prescription drugs and combination products. A number of states have either implemented or are considering implementation of drug price transparency legislation that may prevent or limit our ability to take price increases at certain rates or frequencies. Requirements under such laws include advance notice of planned price increases, reporting price increase amounts and factors considered in taking such increases, wholesale acquisition cost information disclosure to prescribers, purchasers, and state agencies, and new product notice and reporting. Such legislation could limit the price or payment for certain drugs, and a number of states are authorized to impose civil monetary penalties or pursue other enforcement mechanisms against manufacturers for the untimely, inaccurate, or incomplete reporting of drug pricing information or for otherwise failing to comply with drug price transparency requirements. If we are found to have violated state law requirements, we may become subject to penalties or other enforcement mechanisms, which could have a material adverse effect on our business.
Pricing and rebate calculations vary among products and programs. The calculations are complex and will often be subject to interpretation by us, governmental or regulatory agencies and the courts. We may be liable for errors associated with our submission of pricing data. If we are found to have knowingly submitted false pricing data to the Medicaid program or the FSS pricing program, or fail to submit pricing data on a timely basis, we may be subject to significant civil monetary penalties.
Such failure could also be grounds for CMS to terminate our Medicaid drug rebate agreement, which is the agreement under which we would participate in the MDRP. In the event that CMS terminates our rebate agreement, our products may no longer be eligible for coverage under Medicaid or Medicare Part B. There can be no assurance that our submissions will not be found to be incomplete or incorrect.
Efforts to ensure that our business arrangements with third parties, and our business generally, will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. In addition, the requirements and penalties described above may affect our ability to profitably sell any product for which we obtain marketing approval.
We are subject to ongoing regulatory obligations and oversight of our products, and any failure by us to maintain compliance with applicable regulations may result in adverse consequences including the suspension of the manufacturing, marketing and sale of our respective products, the incurrence of significant additional expense and other limitations on our ability to commercialize our respective products.
We are subject to ongoing regulatory requirements and review, including periodic audits pertaining to the development, manufacture, labeling, packaging, adverse event reporting, distribution, storage, marketing, promotion, recordkeeping and export of our products. Failure to comply with such regulatory requirements or the later discovery of previously unknown problems with the manufacture, distributions and storage of our products, or our third-party contract manufacturing facilities or processes by which we manufacture our products may result in restrictions on our ability to develop, manufacture, market, distribute or sell our products, including potential withdrawal of our products from the market. Any such restriction could slow or stop production development or result in decreased sales, damage to our reputation or the initiation of lawsuits against us or our third-party contract manufacturers. We may also be subject to additional sanctions, including, but not limited, to the following:
•Warning letters, public warnings and untitled letters;
•Court-ordered seizures or injunctions;
•Civil or criminal penalties, or criminal prosecutions;
•Variation, suspension or withdrawal of regulatory approvals for our products;
•Changes to the package insert of our products, such as additional warnings regarding potential side effects or potential limitations on the current dosage or administration;
•Requirements to communicate with physicians and other customers about concerns related to actual or potential safety, efficacy, or other issues involving our products;
•Implementation of risk mitigation programs and post-approval obligations;
•Restrictions on our continued manufacturing, marketing, distribution or sale of our products;
•Temporary or permanent closing of the facilities of our third-party contract manufacturers;
•Interruption or suspension of clinical trials; and
•Refusal by regulators to consider or approve applications for additional indications.
Any of the above sanctions could have a material adverse impact on our revenues or our reputation, and cause us to incur significant additional expenses.
In addition, if our products face any safety or efficacy issues, including drug interaction problems, under the federal Food, Drug, and Cosmetic Act (FDCA), the FDA has broad authority to force us to take any number of actions, including, but not limited to, the following:
•Requiring us to conduct post-approval clinical studies to assess product efficacy or known risks or new signals of serious risks, or to evaluate unexpected serious risks;
•Mandating changes to a product’s label;
•Requiring us to implement a risk evaluation and mitigation strategy (REMS) where necessary to assure safe use of the drug; or
•Removing an already approved product from the market.
Further, our partners, including our licensors, are subject to similar requirements and obligations as well as the attendant risks and uncertainties. If our partners, including our licensors, suffer material and adverse effects from such risks and uncertainties, our rights and benefits for our licensed products could be negatively impacted, which could have a material adverse effect on our business.
If our products are marketed or distributed in a manner that violates federal or state healthcare fraud and abuse laws, marketing disclosure laws or other federal or state laws and regulations, we may be subject to civil or criminal penalties.
In addition to FDA and related regulatory requirements, our general operations, and the research, development, manufacture, sale and marketing of our products, are subject to extensive additional federal and state healthcare regulation, including the federal Anti-Kickback Statute, the Prescription Drug Marketing Act, the federal False Claims Act (FCA), the federal Health Insurance Portability and Accountability Act of 1996, the federal Physician Payments Sunshine Act and the Foreign Corrupt Practices Act (and their state analogues), as discussed above in Part I, Item 1 under the heading Government Regulation - Fraud and abuse laws and other U.S. regulatory matters. If we or our partners, such as licensors, fail to comply with any federal and state laws or regulations governing our industry, we could be subject to administrative, criminal and civil penalties and a range of regulatory actions that could adversely affect our ability to commercialize our products, harm or prevent sales of our products, or substantially increase the costs and expenses of commercializing and marketing our products, all of which could have a material adverse effect on our business, financial condition and results of operations. In recent years, CMS has been actively proposing and implementing changes to the list of business practices that are protected by safe harbors. There is inherent risk and uncertainty in any changing regulatory environment as companies work to transition business practices to conform with new regulations.
Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws, and private individuals have been active in bringing so-called “whistleblower” lawsuits on behalf of the government (as Relators) under the FCA and similar regulations in other countries. In addition, incentives exist under applicable U.S. law that encourage employees and physicians to report violations of rules governing promotional activities for pharmaceutical products. These incentives have led to, and could continue to lead to, FCA lawsuits, which attempt to recoup moneys paid by government agencies and extract penalties from manufacturers. For example, federal enforcement agencies have recently pursued enforcement actions against pharmaceutical companies’ product and patient assistance programs, including relationships with specialty pharmacies, and support for charitable foundations providing patients with co-pay assistance. In addition, Relators have filed lawsuits involving manufacturer reimbursement support services as well as promotion of pharmaceutical products beyond labeled claims. Some FCA lawsuits have resulted in government enforcement authorities obtaining significant civil and criminal settlements. Such lawsuits, whether with or without merit, are typically time-consuming and costly to defend. Such suits may also result in related shareholder lawsuits, which are also time-consuming and costly to defend. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report, which is incorporated herein by reference, for information regarding ongoing litigation related to similar matters.
Further, the FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. A product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. The FDA also regulates the content of promotional material, including, among other things, the presentation of efficacy information, the types of comparative claims that can be made to distinguish products from those with similar indications, and the balance of risk information provided. For drug products that are approved by the FDA under the FDA’s accelerated approval regulations, unless otherwise informed by the FDA, the sponsor must submit promotional materials at least 30 days prior to the intended time of initial dissemination of the promotional materials, which delays and may negatively impact a company’s ability to implement changes to its marketing materials, thereby negatively impacting revenues. For other products, the FDA does not review promotional materials prior to dissemination but does issue “Untitled Letters” or “Warning Letters” if it objects to content that has been used promotionally. The FDA may also withdraw approval of drug products under certain conditions. In particular, the FDA may withdraw approval of a drug if, among other things, the promotional materials are false or misleading, or other evidence demonstrates that the drug is not shown to be safe or effective under its conditions of use.
In recent years, in addition to federal legislation related to transparency reporting of transfers of value to healthcare providers and healthcare organizations, several states have enacted legislation requiring pharmaceutical companies to file periodic reports. Several states have adopted legislation requiring pharmaceutical companies to establish marketing and
promotional compliance programs or codes of conduct or to file periodic reports with the state or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Several states have also adopted laws that prohibit certain marketing-related activities, including the provision of gifts, meals or other items to certain healthcare providers.
We have developed and implemented a corporate compliance program based on what we believe are current best practices in the pharmaceutical industry; however, relevant compliance laws are broad in scope and there may not be regulations, guidance or court decisions that definitively interpret these laws in the context of particular industry practices. We cannot guarantee that we, our employees, our partners, our consultants or our contractors are or will be in compliance with all federal and state regulations. If we, our partners, or our representatives fail to comply with any of these laws or regulations, a range of fines, penalties or other sanctions and regulatory actions could be imposed on us, including, but not limited to, restrictions on how we market and sell our products, significant fines, exclusions from government healthcare programs, including Medicare and Medicaid, litigation, or other sanctions. Even if it is not determined that we have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could also have a material adverse effect on our business, financial condition and results of operations. Such investigations or suits have resulted in, and may continue to result in, related shareholder lawsuits, which can also have a material adverse effect on our business.
Our partners, including our licensors, are subject to similar requirements and obligations as well as the attendant risks and uncertainties. If our partners, including our licensors, suffer material and adverse effects from such risks and uncertainties, our rights and benefits for our licensed products could be negatively impacted, which could have a material adverse effect on our business.
We rely on a limited number of specialty pharmacies for distribution of HETLIOZ® in the U.S., and the loss of one or more of these specialty pharmacies or their failure to distribute HETLIOZ® effectively would materially harm our business.
HETLIOZ® is available for distribution through a limited number of specialty pharmacies in the U.S. A specialty pharmacy is a pharmacy that specializes in the dispensing of medications for complex or chronic conditions that often require a high level of patient education and ongoing management. The use of specialty pharmacies involves certain risks, including, but not limited to, risks that these specialty pharmacies will:
•not provide us accurate or timely information regarding their inventories, the number of patients who are using HETLIOZ® or complaints about HETLIOZ®;
•reduce their efforts or discontinue to sell or support or otherwise not effectively sell or support HETLIOZ®, particularly in light of the recent entry into the market of generic versions of HETLIOZ®;
•not devote the resources necessary to sell HETLIOZ® in the volumes and within the time frames that we expect;
•be unable to satisfy financial obligations to us or others; or
•cease operations.
In addition, if one or more of our specialty pharmacies do not fulfill their contractual obligations to us, or refuse or fail to adequately serve patients, or their agreements are terminated without adequate notice, shipments of HETLIOZ®, and associated revenues, would be adversely affected. We expect that it would take a significant amount of time if we were required to replace one or more of our specialty pharmacies.
Our revenues from Fanapt® and PONVORY® are substantially dependent on sales through a limited number of customers, and such revenues may fluctuate from quarter to quarter.
We sell Fanapt® primarily through a limited number of pharmaceutical wholesalers in the U.S. We sell PONVORY® through a limited number of specialty pharmacies and specialty distributors in the U.S., which may not be identical to the historic distribution network used by Janssen when selling PONVORY® in the U.S. The use of pharmaceutical wholesalers, specialty pharmacies and specialty distributors involves certain risks, including, but not limited to, risks that these specialty pharmacies, specialty distributors and pharmaceutical wholesalers will:
•not provide us accurate or timely information regarding their inventories, demand from customers buying Fanapt® or PONVORY® or complaints about Fanapt® or PONVORY®;
•reduce their efforts or discontinue to sell or support or otherwise not effectively sell or support Fanapt® or PONVORY®;
•not devote the resources necessary to sell Fanapt® or PONVORY® in the volumes and within the time frames that we expect;
•be unable to satisfy financial obligations to us or others; or
•cease operations.
Additionally, our reliance on a small number of wholesalers, specialty pharmacies and specialty distributors, could cause revenues to fluctuate from quarter to quarter based on the buying patterns of these customers. In addition, if any of these customers fails to pay on a timely basis or at all, our business, financial condition and results of operations could be materially adversely affected.
We face substantial competition, which may result in others developing or commercializing products before or more successfully than we do.
Our future success will depend on our ability to demonstrate and maintain a competitive advantage with respect to our products and our ability to identify and develop additional products. Large, fully integrated pharmaceutical companies, either alone or together with collaborative partners, have substantially greater financial resources and have significantly greater experience than we do in:
•developing products;
•undertaking preclinical testing and clinical trials;
•obtaining FDA and other regulatory approvals of products; and
•manufacturing, marketing and selling products.
These companies may invest heavily and quickly to discover and develop novel products that could make our products obsolete. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA or foreign regulatory approval or commercializing superior products or other competing products before we do. Technological developments or the approval by the FDA or foreign regulators of new therapeutic indications for existing products may make our products obsolete or may make them more difficult to market successfully, either of which could have a material adverse effect on our business, results of operations and financial condition.
Our products, if successfully developed and approved for commercial sale, will compete with a number of drugs and therapies currently manufactured and marketed by other biotechnology companies, including major pharmaceutical companies. Our products may also compete with new products currently under development by others or with products that may cost less than our products. Physicians, patients, third-party payors and the medical community may not accept or utilize any of our products that may be approved. If Fanapt®, HETLIOZ®, PONVORY® and our other products, if and when approved, do not achieve significant market acceptance, our business, results of operations and financial condition would be materially adversely affected. See Part I, Item 1, Competition, for a discussion of the primary competitors for our commercial products.
In addition, we may face competition from newly developed generic products. Under the Hatch-Waxman Act, newly approved drugs and indications may benefit from a statutory period of non-patent marketing exclusivity. The Hatch-Waxman Act seeks to stimulate competition by providing incentives to generic pharmaceutical manufacturers to introduce non-infringing forms of patented pharmaceutical products and to challenge patents on branded pharmaceutical products. If we are unsuccessful at challenging an ANDA filed pursuant to the Hatch-Waxman Act, cheaper generic versions of our products, which may be favored by insurers and third-party payors, may be launched commercially, which would significantly harm our business. In December 2022, the Delaware District Court ruled in favor of Teva and Apotex in our patent litigation relating to their filing of ANDAs for generic versions of HETLIOZ® in the U.S. We appealed the decision to the Federal Circuit, and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition. We have also filed Hatch-Waxman lawsuits in U.S. District Court for the District of New Jersey against each of Teva and Apotex and in the U.S. District Court for the Southern District of Florida against Apotex, in each case, asserting infringement of U.S. Patent No. 11,285,129, which is a method of administration patent that was not litigated in the prior litigation. The New Jersey cases have been transferred to the Delaware District Court, where they remain pending, and the Florida case was voluntarily dismissed. We have also filed patent infringement lawsuits against each of Teva and Apotex in the U.S. District Court for the District of Delaware, in each case, asserting infringement of U.S. Patent No. 11,918,556, another method of administration patent that was not litigated in the prior litigation. The FDA has approved ANDAs for Teva, Apotex and MSN, and Teva and Apotex have
launched their generic versions of HETLIOZ® at risk in the U.S., and MSN has launched its generic version as well. In addition, other potential competitors may be successful in obtaining ANDA approval and launching their own generic versions.
In July 2024, we filed a Hatch-Waxman lawsuit against MSN in the Delaware District Court asserting that U.S. Patent Nos. 10,179,119, 11,266,622, 11,285,129, 11,850,229, 10,610,510, 10,980,770, and 11,759,446 will be infringed by MSN’s generic version of HETLIOZ LQ® for which MSN is seeking FDA approval. A trial is scheduled to begin on June 15, 2026, and a 30-month stay is in place until December 3, 2026. We also have Orphan Drug Exclusivity for HETLIOZ LQ® until December 1, 2027, thus no ANDAs for generic HETLIOZ LQ® can be approved prior to December 1, 2027.
To obtain an ANDA approval for a generic drug, the generic company needs to show, among other things, that its version of the product is bioequivalent to the Reference Listed Drug (RLD). This usually requires the generic company to conduct bioequivalence studies comparing its product to the RLD, and to retain sufficient samples of the RLD used in testing after a study is complete. In recent years, U.S. federal lawmakers and the FDA have considered proposals and enacted legislation to facilitate the generic drug company’s access to samples and foster generic competition. For example, the Creating and Restoring Equal Access to Equivalent Samples Act (CREATES Act) allows a biosimilar or generic product developer to bring a civil action against a brand drug manufacturer for failing to provide samples of the brand product for comparative testing “on commercially reasonable, market-based terms.” The developer could receive injunctive relief and a monetary award “sufficient to deter the license holder from failing to provide other eligible product developers with sufficient quantities of a covered product on commercially reasonable, market-based terms” in certain cases. While the full impact of the CREATES Act is unclear at this time, its provisions do have the potential to facilitate the development and future approval of generic versions of our products, introducing generic competition that could have a material adverse effect on our business, results of operations and financial condition.
Certain states have also taken similar actions. For example, in 2018, Maine passed a new law that requires brand drug manufacturers to make samples of drugs distributed in the state available for sale in Maine at a price no greater than wholesale acquisition cost and without any restriction that would block or delay a biosimilar and generic drug application in a manner inconsistent with federal law. The state may seek injunctive relief and attorney’s fees from a drug manufacturer who fails to comply with this requirement.
FDA and foreign regulatory approval of our products is uncertain.
The research, testing, manufacturing and marketing of products such as those that we have developed or that we are developing are subject to extensive regulation by federal, state and local government authorities, including the FDA, as well as foreign regulatory authorities in jurisdictions in which we seek approval. To obtain regulatory approval of such products, we must demonstrate to the satisfaction of the applicable regulatory agency that, among other things, the product is safe and effective for its intended use. In addition, we must show that the manufacturing facilities used to produce such products are in compliance with current good manufacturing practices (cGMPs).
The process of obtaining FDA and other required regulatory approvals and clearances can take many years and will require us to expend substantial time and capital. Despite the time and expense expended, regulatory approval is never guaranteed. The number of preclinical and clinical trials that will be required for FDA or foreign regulatory approval varies depending on the product, the disease or condition that the product is in development for, and the requirements applicable to that particular product. The FDA or applicable foreign regulatory agency can delay, limit or deny approval of a product for many reasons, including that:
•the FDA or foreign agency may not believe a product is shown to be safe or effective;
•the FDA or foreign agency may interpret data from preclinical and clinical trials in different ways than we do;
•the FDA or foreign agency may not approve our or our partners’ manufacturing processes or facilities;
•the FDA or foreign agency may not approve a product for all the indications we request;
•the FDA or foreign agency may change its approval policies or adopt new regulations;
•the FDA or foreign agency may not meet, or may extend, the PDUFA date or its foreign equivalent with respect to a particular NDA or foreign application; and
•the FDA or foreign agency may not agree with our regulatory approval strategies or components of the regulatory filings, such as clinical trial designs.
For example, if certain of our methods for analyzing trial data are not accepted by the FDA or the applicable foreign agency, we may fail to obtain regulatory approval for our products.
Additionally, the approval procedure varies among countries and jurisdictions and can involve additional trials, and the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or jurisdictions or by the FDA.
Any delay or failure to obtain regulatory approvals for our products will result in increased costs, could diminish competitive advantages that we may attain and would adversely affect the marketing and sale of our products. Other than Fanapt® in the U.S., Mexico and Israel, HETLIOZ® and HETLIOZ LQ® in the U.S. and HETLIOZ® in the European countries covered by the EC’s centralized marketing authorization and PONVORY® in the U.S. and Canada, we have not received, and may never receive, regulatory approval to market any of our products in any jurisdiction.
Even following regulatory approval of our products, the FDA or the applicable foreign agency may impose limitations on the indicated uses for which such products may be marketed, subsequently withdraw approval or take other actions against us or such products that are adverse to our business. The FDA and foreign agencies generally approve drugs for use in specific indications. An approval for a more limited indication reduces the size of the potential market for the product. Product approvals, once granted, may be withdrawn or modified if problems occur after initial marketing.
We and our partners are also subject to numerous federal, state, local and foreign laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the environment and the use and disposal of hazardous substances used in connection with discovery, research and development work. In addition, we cannot predict the extent to which new governmental regulations might significantly impede the discovery, development, production and marketing of our products. We or our partners may be required to incur significant costs to comply with current or future laws or regulations, and we may be adversely affected by the cost of such compliance or the inability to comply with such laws or regulations.
Our products may cause undesirable side effects or have other properties that could delay, prevent or result in the revocation of their regulatory approval or limit their marketability.
Undesirable side effects caused by our products could interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, and in turn prevent us from commercializing or continuing the commercialization of such products and generating revenues from their sale. We will continue to assess the side effect profile of our products in ongoing clinical development programs. However, we cannot predict whether the commercial use of our approved products (or our products in development, if and when they are approved for commercial use) will produce undesirable or unintended side effects that have not been evident in the use of, or in clinical trials conducted for, such products to date. For example, despite the positive results of the completed trials for Fanapt®, HETLIOZ® and PONVORY®, as well as the FDA’s approval of the NDA for HETLIOZ® for the treatment of Non-24 in January 2014, the NDA for Fanapt® for the treatment of schizophrenia in May 2009, the EC’s grant of the centralized marketing authorization for HETLIOZ® for the treatment of Non-24 in totally blind adults in July 2015, the FDA’s approval of the sNDA and NDA for HETLIOZ® capsule and liquid formulation for the treatment of adults and children, respectively, with nighttime sleep disturbances in SMS in December 2020, and the NDA for PONVORY® for the treatment of RMS in March 2021 and Health Canada’s approval of PONVORY® for the treatment of RMS in April 2021, we are uncertain whether any of these products will ultimately prove to be effective and safe in humans long term and in all uses. Frequently, products that have shown promising results in clinical trials have suffered significant setbacks in later clinical trials or even long after they are approved for commercial sale. Additionally, incidents of product misuse may occur. These events, among others, could result in product recalls, product liability actions or withdrawals or additional regulatory controls, any of which could have a material adverse effect on our business, results of operations and financial condition.
In addition, if after receiving marketing approval of a product, we or others identify undesirable side effects caused by such product, we could face one or more of the following:
•regulatory authorities may require us to implement a REMS, such as the addition of labeling statements (e.g., “black box” warning or a contraindication);
•regulatory authorities may withdraw their approval of the product;
•we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product; and
•our or the product’s reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the product, which in turn could delay or prevent us from generating significant revenues from its sale.
Clinical trials for our products are expensive and their outcomes are uncertain. Any failure or delay in completing clinical trials for our products could severely harm our business.
Preclinical studies and clinical trials required to demonstrate the safety and efficacy of our products are time-consuming and expensive and together take several years to complete. Before obtaining regulatory approvals for the commercial sale of any of our products, we must demonstrate through preclinical testing and clinical trials that such product is safe and effective for use in humans. We have incurred, and we will continue to incur, substantial expense for, and devote a significant amount of time to, preclinical testing and clinical trials.
Historically, the results from preclinical testing and early clinical trials often have not predicted results of later clinical trials. A number of new drugs have shown promising results in clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals. Clinical trials conducted by us or by third parties on our behalf may not demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals for our products. Regulatory authorities may not permit us to undertake any additional clinical trials for our products, may force us to stop any ongoing clinical trials and it may be difficult to design efficacy studies for our products in new indications.
Clinical development efforts performed by us may not be successfully completed or completed in a timely manner. Completion of clinical trials may take several years or more. The length of time can vary substantially with the type, complexity, novelty and intended use of the products and the size of the prospective patient population. Our ability to enroll patients in, and the commencement and rate of completion of, clinical trials for our products may be affected by many factors, including:
•the size and nature of the patient population;
•the design of the trial protocol for our clinical trials;
•the eligibility and exclusion criteria for the trial in question;
•the availability of competing therapies and competing clinical trials, and physician and patient perception of our product candidates and our other product candidates being studied in relation to these other potential options;
•the availability of raw materials and the possibility of raw materials expiring prior to their use;
•difficulty in maintaining contact with patients after treatment, resulting in incomplete data;
•poor effectiveness of our products during clinical trials;
•unforeseen safety issues or side effects;
•the number and location of clinical sites in our clinical trials;
•the proximity and availability of clinical trial sites for prospective patients;
•the availability of time and resources at the institutions where clinical trials are and will be conducted;
•the availability of adequate financing to fund ongoing clinical trial expenses;
•the study endpoints that rely on subjective patient reported outcomes;
•the impact of global health crises; and
•governmental or regulatory delays and changes in regulatory requirements and guidelines.
If we fail to complete our clinical trials successfully or have difficulty enrolling a sufficient number of patients for them, we may not receive the regulatory approvals needed to market that product. Any such failure or difficulty could have a material adverse effect on our business.
We may not be able to achieve sustained profitability.
We have been engaged in identifying and developing drug products since March 2003, which has required, and will continue to require, significant research and development expenditures. The continued commercialization of our products will also require substantial additional expenditures.
As of December 31, 2024, we had an accumulated deficit of $174.3 million and we cannot estimate with precision the extent of our future income or loss. We may not succeed in maintaining or gaining additional market acceptance of our products in the U.S. and we may not succeed in commercializing Fanapt® or HETLIOZ® outside of the U.S or PONVORY® in Canada. We may be unable to fully develop, obtain regulatory approval for, commercialize, manufacture, market, sell and derive revenue from our products in the timeframes we project, if at all, and our inability to do so would materially and adversely impact the market price of our common stock and our ability to raise capital and continue operations.
There can be no assurance that we will achieve sustained profitability, which depends on many factors, including but not limited to, our ability to obtain regulatory approval for our products and achieve success in commercializing them in the U.S., Europe, Canada and our other target jurisdictions, as well as other factors described in this Annual Report.
In addition, the amount we spend on developing, obtaining and maintaining regulatory approval for and commercializing our products, among other expenditures described in this Annual Report, will impact our profitability.
Our ability to use net operating loss carryforwards and tax credit carryforwards to offset future taxable income is dependent on generating future taxable income and may be limited, including as a result of transactions involving our common stock.
We have recorded deferred tax assets based on our assessment that it is more likely than not that we will be able to realize the benefits of our net operating losses and other favorable tax attributes. Realization of deferred tax assets involves significant judgments and estimates, which are subject to change, and ultimately depends on generating sufficient taxable income of the appropriate character during the appropriate periods. Changes in circumstances may affect the likelihood of such realization, which in turn may trigger the need for additional valuation allowance against our deferred tax assets and adversely affect our net income and financial condition. In addition, we are potentially subject to ongoing and periodic tax examinations and audits in various jurisdictions, including with respect to the amount of our net operating losses and any limitation thereon. An adjustment to such net operating loss carryforwards or other tax attributes, including an adjustment from a taxing authority, could result in higher tax costs, penalties and interest, thereby adversely impacting our financial condition.
Certain of our tax attributes, including net operating losses (NOLs) and credit carryforwards, would be subject to limitation under Section 382 and 383 should an ownership change as defined under Section 382 of the Internal Revenue Code of 1986, as amended (IRC), occur. The limitation resulting from a change in ownership could affect our ability to utilize NOLs and credit carryforwards (tax attributes) to offset future taxable income. In general, an ownership change occurs if the aggregate stock ownership of certain stockholders increases by more than 50 percentage points over such stockholders’ lowest percentage ownership during the testing period (generally three years). Transactions involving our common stock, even those outside our control, such as purchases or sales by investors, within the testing period could result in an ownership change. A limitation on our ability to utilize some or all of our NOLs or credit carryforwards could have a material adverse effect on our results of operations and cash flows. An ownership change occurred in the year ended December 31, 2014. We believe that the ownership change in 2014 will not impact our ability to utilize NOL and credit carryforwards; however, future ownership changes may cause our existing tax attributes to have additional limitations.
If we fail to adequately fund our research and development activities and commercialization efforts, we may be unable to continue operations or we may be forced to share our rights to commercialize our products with third parties on terms that may not be attractive to us.
Our activities will necessitate significant uses of working capital throughout 2025 and beyond. It is uncertain whether cash provided by our operating activities, together with our existing funds, will be sufficient to meet our long-term operating needs. As of December 31, 2024, our total cash and cash equivalents and marketable securities were $374.6 million. Our long-term capital requirements are expected to depend on many factors, including, among others:
•our level of success in commercializing Fanapt®, HETLIOZ® and PONVORY®, as well as other products that may be approved, globally;
•outcomes of ongoing and potential patent litigation;
•costs of developing and maintaining sales, marketing and distribution channels and our ability to sell our products;
•market acceptance of our products;
•costs of establishing and maintaining manufacturing capabilities for commercial quantities of our products;
•the number of potential formulations and products in development;
•progress with preclinical studies and clinical trials;
•time and costs involved in obtaining regulatory (including FDA) approval;
•costs of preparing, filing, prosecuting, maintaining and enforcing patent, trademark and other intellectual property claims;
•cost of evaluating and acquiring new products from third parties;
•competing technological and market developments;
•costs of recruiting and retaining employees and consultants;
•costs of training physicians; and
•legal, accounting, insurance and other professional and business-related costs.
As a result, we may need to raise additional capital to fund our anticipated operating expenses and execute on our business plans. In our capital-raising efforts, we may seek to sell debt securities or additional equity securities, obtain a bank credit facility, or enter into partnerships or other collaboration agreements. The sale of additional equity or debt securities, if convertible, could result in dilution to our stockholders and may also result in a lower price for our common stock. The incurrence of indebtedness would result in increased fixed obligations and could also result in covenants that could restrict our operations, including potentially limiting our ability to license product rights or enter into product development collaborations. However, we may not be able to raise additional funds on acceptable terms, or at all. If additional financing is not available when required or is not available on acceptable terms, we may be unable to fund our operations and planned growth, develop or enhance our technologies or products, take advantage of business opportunities or respond to competitive market pressures, any of which would materially harm our business, financial condition and results of operations.
If our contract research organizations (CROs), third-party vendors and investigators do not successfully carry out their duties or if we lose our relationships with CROs, third-party vendors and investigators, our drug development efforts could be delayed.
Our arrangements with CROs, third-party vendors and investigators are critical to our success in bringing our products to the market. We are generally dependent on CROs, third-party vendors and investigators for preclinical testing and clinical trials related to our drug discovery and development efforts and we will likely continue to depend on them to assist in our future discovery and development efforts. These parties are not our employees and we cannot control the amount or timing of resources that they devote to our programs. As such, they may not complete activities on schedule or may not conduct our clinical trials in accordance with regulatory requirements or our stated protocols. The parties with which we contract for execution of our clinical trials play a significant role in the conduct of the trials and the subsequent collection and analysis of data. If they fail to devote sufficient time and resources to our drug development programs or if their performance is substandard, it will delay the development, approval and commercialization of our products. Moreover, these parties may also have relationships with other commercial entities, some of which may compete with us. If they assist our competitors, it could harm our competitive position.
Our CROs, third-party vendors and investigators could merge with or be acquired by other companies or experience financial or other setbacks unrelated to our collaboration that could, nevertheless, materially adversely affect our business, results of operations and financial condition.
If we lose our relationship with any one or more of these parties, we could experience a significant delay in both identifying another comparable provider and contracting for its services. We may be unable to retain an alternative provider on reasonable terms, if at all. Even if we locate an alternative provider, it is likely that this provider may need additional time to respond to our needs and may not provide the same type or level of service as the original provider. In addition, any provider that we retain will be subject to current Good Laboratory Practices as set forth in 21 Code of Federal Regulations (C.F.R.) Part 58 and Good Clinical Practices as set forth in 21 C.F.R. Part 50, 54, and 312, and similar international standards and we do not have control over compliance with these regulations by these providers. Consequently, if these practices and standards are not adhered to by these providers, the development and commercialization of our products could be delayed.
We rely on a limited number of third-party manufacturers to formulate and manufacture our products, and our business will be seriously harmed if these manufacturers are not able to satisfy our demand and alternative sources are not available.
We do not have an in-house manufacturing capability and depend completely on a small number of third-party manufacturers and active pharmaceutical ingredient formulators for the manufacture of our products. Therefore, we are dependent on third parties for our formulation development and manufacturing of our products. This may expose us to the risk of not being able to directly oversee the production and quality of the manufacturing process and provide ample commercial supplies to successfully launch and maintain the marketing of our products. Furthermore, these third-party contractors, whether foreign or domestic, may experience regulatory compliance difficulties, mechanical shut downs, employee strikes, or other unforeseeable events that may delay or limit production. Our inability to adequately establish, supervise and conduct (either ourselves or through third parties) all aspects of the formulation and manufacturing processes would have a material adverse effect on our ability to develop and commercialize our products. In addition, if we are not able to continue to operate our business relationships in a manner that is sufficiently profitable for us and our suppliers, certain members of our supply chain could compete with us through supply to competitors, such as generic drug companies, through breach of our agreements or otherwise.
We have agreements in place with Patheon Pharmaceuticals Inc. and Patheon Inc. (collectively, Patheon), subsidiaries of Thermo Fisher Scientific, for the manufacture of Fanapt® and HETLIOZ®. In January 2014, we entered into a manufacturing agreement with Patheon for the manufacture of commercial supplies of HETLIOZ® 20 mg capsules at Patheon’s Cincinnati, Ohio manufacturing site. In May 2016, we entered into a manufacturing agreement with Patheon for the manufacture of commercial supplies of Fanapt® tablets at Patheon’s Mississauga, Ontario, Canada manufacturing site. Additionally, in December 2020, we entered into a non-exclusive third-party manufacturing agreement for the manufacture of commercial supplies of HETLIOZ LQ®. During December 2024, we entered into a non-exclusive third-party manufacturing agreement for the manufacture of commercial supplies of PONVORY®. We do not have exclusive long-term agreements with any other third-party manufacturers of our products. If our current manufacturers, or any other third-party manufacturer, is unable or unwilling to perform its obligations under our manufacturing agreements for any reason, we may not be able to locate alternative acceptable manufacturers or formulators or enter into favorable agreements with them. Any inability to acquire sufficient quantities of our products in a timely manner from these third parties could adversely affect sales of our products, delay clinical trials and prevent us from developing our products in a cost-effective manner or on a timely basis. In addition, manufacturers of our products are subject to cGMP and similar foreign standards and we do not have control over compliance with these regulations by our manufacturers. If one of our contract manufacturers fails to maintain compliance, the production of our products could be interrupted, resulting in delays and additional costs. Moreover, if the facilities of such manufacturers do not pass a pre-approval or post-approval plant inspection, the FDA will not grant approval for our products and may institute restrictions on the marketing or sale of our products. Similarly, if we change contract manufacturers, the FDA must approve these contract manufacturers or any other CMC changes before our products can be manufactured.
Our manufacturing strategy presents the following additional risks:
•because most of our third-party manufacturers and formulators are located outside of the U.S., there may be difficulties in importing our products or their components into the U.S. as a result of, among other things, FDA import inspections, incomplete or inaccurate import documentation or defective packaging; and
•because of the complex nature of our products, our manufacturers may not be able to successfully manufacture our products in a cost-effective and/or timely manner.
Materials necessary to manufacture our products may not be available on commercially reasonable terms, or at all, which may delay the development, regulatory approval and commercialization of our products.
We rely on manufacturers to purchase from third-party suppliers the materials necessary to produce our products for clinical trials and commercialization. Suppliers may not sell these materials to such manufacturers at the times we need them or on commercially reasonable terms. We do not have any control over the process or timing of the acquisition of these materials by these manufacturers. Moreover, we currently do not have any agreements for the commercial production of these materials. If the manufacturers are unable to obtain these materials for our clinical trials, including due to supply chain issues caused by global health crises, product testing, potential regulatory approval of our products and commercial scale manufacturing could be delayed, significantly affecting our ability to further develop and commercialize our products. If we or our manufacturers are unable to purchase these materials for our products, there would be a shortage in supply or the commercial launch of such products would be delayed, which would materially and adversely affect our ability to generate revenues from the sale of such products.
If we cannot identify, or enter into licensing arrangements for, new products, our ability to develop a diverse product portfolio will be limited.
A component of our business strategy is acquiring rights to develop and commercialize products discovered or developed by other pharmaceutical and biotechnology companies for which we may find effective uses and markets through our unique pharmacogenetics and pharmacogenomics expertise. Competition for the acquisition of these products is intense. If we are not able to identify opportunities to acquire rights to commercialize additional products, we may not be able to develop a diverse portfolio of products. Additionally, it may take substantial human and financial resources to secure commercial rights to promising products. Moreover, if other firms develop pharmacogenetics and pharmacogenomics capabilities, we may face increased competition in identifying and acquiring additional products.
If we lose key scientists or management personnel, or if we fail to recruit additional highly skilled personnel, our ability to identify, develop, and commercialize new products will be impaired.
We are highly dependent on principal members of our management team and scientific staff, including our Chief Executive Officer, Mihael H. Polymeropoulos, M.D. These executives each have significant pharmaceutical industry experience. The loss of any such executives, including Dr. Polymeropoulos, or any other principal member of our management team or scientific staff, would impair our ability to identify, develop and market new products. Our management and other employees may voluntarily terminate their employment with us at any time. The loss of the services of these or other key personnel, or the inability to attract and retain additional qualified personnel, could result in delays to development or approval, loss of sales and diversion of management resources. In addition, we depend on our ability to attract and retain other highly skilled personnel, including research scientists. Competition for qualified personnel is intense, and the process of hiring and integrating such qualified personnel is often lengthy. We may be unable to recruit such personnel on a timely basis, if at all, which would negatively impact our development and commercialization programs.
Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or any of our employees. This lack of insurance means that we may not have adequate compensation for the loss of the services of these individuals.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
The risk that we may be sued on product liability claims is inherent in the development and sale of pharmaceutical products. For example, we face a risk of product liability exposure related to the testing of our products in clinical trials and will face even greater risks upon commercialization of our products. We believe that we may be at a greater risk of product liability claims relative to other pharmaceutical companies because certain of our products are intended to treat central nervous system disorders, among others, and it is possible that we may be held liable for the behavior and actions of patients who use our products. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and we may be forced to limit or forego further commercialization of one or more of our products. Although we maintain product liability insurance, our aggregate coverage limit under this insurance is $30.0 million, and while we believe this amount of insurance is sufficient to cover our product liability exposure, these limits may not be high enough to fully cover potential liabilities. As our development activities and commercialization efforts progress and we sell our products, this coverage may be inadequate, we may be unable to obtain adequate coverage at an acceptable cost, we may be unable to get adequate coverage at all or our insurer may disclaim coverage as to a future claim. This could prevent the commercialization or limit the commercial potential of our products. Even if we are able to maintain insurance that we believe is adequate, our results of operations and financial condition may be materially adversely affected by a product liability claim. Uncertainties resulting from the initiation and continuation of products liability litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Product liability litigation and other related proceedings may also require significant management time.
E.U. Member States tend to impose strict price controls, which may delay or prevent the further commercial launch or impede the commercial success of HETLIOZ® in Europe and adversely affect our future results of operations.
In the E.U., prescription drug pricing and reimbursement are subject to governmental control and reimbursement mechanisms used by private and public health insurers in the E.U. vary by Member State. For the public systems, reimbursement is determined by law and/or by guidelines established by the responsible national authority. As elsewhere, inclusion in reimbursement catalogues focuses on the medical usefulness, need, quality and economic benefits to patients and the health care system. Acceptance for reimbursement comes with cost, use and often volume restrictions, which can vary by Member State. Although we have received marketing authorization for HETLIOZ® capsules from the EC, pricing negotiations
with governmental authorities may take a considerable amount of time in those Member States that impose price controls. For example, we launched HETLIOZ® commercially in Germany in August 2016, and concluded our pricing negotiations with German authorities in October 2017. In addition, to obtain reimbursement or pricing approval for HETLIOZ® in some Member States, we may be required to conduct an additional clinical trial that compares the cost-effectiveness of HETLIOZ® to other available therapies.
Some Member States require approval of the sale price of a drug before it can be marketed. In others, the pricing review period begins after marketing or product licensing approval is granted. In some Member States, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we may be subject to lengthy price regulations that delay or prevent the commercial launch of HETLIOZ® in a particular Member State and negatively impact the revenues that are generated from the sale of HETLIOZ® in that country. If reimbursement of HETLIOZ® is unavailable or limited in scope or amount, or if pricing for HETLIOZ® is set at unsatisfactory levels or takes too long to establish, or if there is competition from lower priced cross-border sales, our results of operations will be negatively affected.
We may not be able to effectively market and sell our future products, if approved, in the U.S.
We plan to continue to build our sales and marketing capabilities in the U.S. to commercialize future products, if approved. Our current sales and marketing capabilities in the U.S. may not be adequate to support the commercialization of future products and we would expect to build such capabilities by investing significant amounts of financial and management resources. Furthermore, the cost of establishing and maintaining marketing and sales capabilities may not be justifiable in light of the revenues generated by any future products.
If we are unable to establish and maintain adequate sales and marketing capabilities for future products or are unable to do so in a timely manner, we may not be able to generate product revenues from these products, which may prevent us from reaching or maintaining profitability.
Healthcare legislative reform measures or developments arising from changes in the political climate may have a material adverse effect on our business and results of operations.
In the U.S., there have been and continue to be a number of legislative initiatives to contain healthcare costs. Most significantly, in August 2022, President Biden signed the IRA into law. This statute marks the most significant action by Congress with respect to the pharmaceutical industry since adoption of the ACA in 2010. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare, with prices that can be negotiated subject to a cap, with the first drug price negotiations effective January 1, 2026; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation beginning October 1, 2022; and replaces the Medicare Part D coverage gap discount program with a new discounting program beginning January 1, 2025. The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On March 15, 2023 and June 30, 2023, HHS issued guidance regarding implementation of the Medicare drug price negotiation program in initial price applicability year 2026. More recently, in October 2024, HHS released guidance related to implementation for initial price applicability year 2027. In August 2024, CMS announced prices for the first ten drugs under the drug price negotiation program, with the new prices going into effect in 2026, and is expected to announce the next fifteen drugs to be selected for the second round of negotiations by February 1, 2025. Several manufacturers and industry groups have challenged the drug price negotiation program for Medicare Parts B and D in federal court. These lawsuits are ongoing, and additional lawsuits may be filed in the future related to provisions of the IRA. It is unknown whether such litigation or other litigation, if brought, will be successful, or whether there will be future changes to the IRA. Moreover, the recent change in presidential administrations in the U.S. may introduce additional unpredictability regarding the future of the IRA. For these and other reasons, it is currently unclear how the IRA will be effectuated, and while the impact of the IRA on the pharmaceutical industry cannot yet be fully determined, it is likely to be significant.
Healthcare reforms are discussed above in Part I, Item 1 under the heading Pharmaceutical Coverage, Pricing and Reimbursement and Healthcare Reform and in the risk factor entitled “We are subject to uncertainty relating to pricing and reimbursement policies in the U.S., including recent and future health reform measures, which, if not favorable for our products, could hinder or prevent our products’ commercial success.”
These healthcare reforms, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the price for any approved product and/or the level of reimbursement physicians receive for administering any approved product which could affect our business strategy or commercial prospects. Reductions in
reimbursement levels may negatively impact the prices we can charge or the frequency with which products are prescribed or administered. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
Changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade, manufacturing, development and investment, and any negative sentiments towards the U.S. as a result of such changes, could also adversely affect our business.
We are subject to stringent laws, rules, regulations, policies, industry standards and contractual obligations regarding data privacy and security in foreign jurisdictions and may be subject to additional related laws, rules, regulations, policies, industry standards and contractual obligations in other jurisdictions into which we expand. Many of these provisions are subject to change and reinterpretation depending on the jurisdiction and could result in claims, changes to our business practices, monetary penalties, increased cost of operations or other harm to our business activities.
The regulatory framework for privacy and personal information security issues worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. Various foreign government bodies and agencies have adopted or are considering adopting laws, rules, regulations and standards limiting, or laws, rules, regulations and standards regarding, the collection, distribution, use, disclosure, storage, security and other processing of personal information.
Outside of the U.S., legal requirements relating to the collection, storage, processing and transfer of personal data continue to evolve. For example, the collection and use of health data and other personal data is governed in the E.U. by the General Data Protection Regulation (GDPR), which became applicable in May 2018. The GDPR applies to personal data processing carried out by a controller or processor (i) established in the E.U. or (ii) offering goods or services to E.U. individuals, or monitoring their behavior within the E.U., regardless of the controller’s or processor’s location. The GDPR implements stringent operational requirements for controllers and processors of personal data, including, for example, the implementation of transparent information for data subjects regarding the processing of their personal data, appropriate legal basis for the processing of personal data, which may include the obtaining of valid consent in certain circumstances, expanded individual data subject rights, limitations on retention of personal data, increased requirements pertaining to data security and confidentiality, mandatory data breach notification to the competent supervisory authority and higher standards for controllers and processors to demonstrate their compliance with the GDPR through appropriate documentation. The GDPR provides that E.U. Member States may supplement the GDPR with their own additional laws and regulations in relation to certain processing of personal data, in particular regarding sensitive personal data, (e.g., genetic, biometric or health data), which could result in differences between E.U. Member States, limit our ability to collect, use and share such personal data or cause our costs to increase, and harm our reputation, business and financial condition. Failure to comply with the GDPR may result in fines up to the higher of €20,000,000 or 4% of total worldwide annual revenue of the preceding financial year and other administrative penalties. The GDPR may increase our responsibility and liability in relation to health data and other personal data that we may collect and process, and we may be required to implement additional measures in an effort to comply with the GDPR and with other laws, rules, regulations and standards in the E.U., including those of E.U. Member States, relating to privacy and data protection. This may be onerous and if our efforts to comply with GDPR or other applicable E.U. laws, rules, regulations and standards are not successful, or are perceived to be unsuccessful, it could adversely affect our business.
In July 2020, the European Court of Justice (ECJ) invalidated the E.U.-U.S. Privacy Shield, which had enabled the transfer of personal data from the E.U. to the U.S. for companies that had self-certified to the Privacy Shield. While we did not rely on the Privacy Shield, the ECJ decision also raised questions about the continued validity of the European Commission’s then in effect Standard Contractual Clauses, on which we relied.
The EC has presented a new set of Standard Contractual Clauses, to be included in agreements involving personal data transfers outside the E.U. In addition, in July 2023, the European Commission adopted its adequacy decision for the E.U.-U.S. Data Privacy Framework (DPF). U.S. companies with the DPF certification can lawfully transfer personal data from the E.U. to the U.S. without additional transfer mechanisms. However, the validity of such mechanism is currently challenged by NYOB, an Austrian non-profit organization seeking to enforce digital rights (particularly privacy, and data protection rights) in the E.U. In addition, E.U. regulators have issued additional guidance regarding considerations and requirements that we and other companies must consider and undertake when using the Standard Contractual Clauses. Uncertainty regarding the validity of the existing transfer mechanisms could restrict our activities in those jurisdictions, limit our ability to provide our products and services in those jurisdictions, require us to modify our policies and practices, and cause us to engage in additional contractual negotiations, or increase our costs and obligations and impose limitations upon our ability to efficiently transfer personal data from the E.U. to the U.S. for conducting our business activities.
Risks related to intellectual property and other legal matters
Our rights to develop and commercialize our products are subject, in part, to the terms and conditions of licenses or sublicenses granted to us by other pharmaceutical companies.
Our rights to our product portfolio are based, in part, on patents and other intellectual property licensed from third parties. These third parties may generally terminate the license agreements under certain circumstances, including a material breach of the agreement by the other. In the event we terminate our license, or if the third party terminates our license due to our breach, rights to the intellectual property revert back to the licensor. Any termination or reversion of our rights to develop or commercialize our products would have a material adverse effect on our business.
If our efforts to protect the proprietary nature of the intellectual property related to our products are not adequate, we may not be able to compete effectively in our markets.
Method of treatment patents protect the use of a product for the method specified in the patent claims. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for a use that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our patented methods, physicians may prescribe these products “off-label.” Although off-label prescriptions may infringe or contribute to the infringement of method of treatment patents, such infringement may be difficult to prevent.
Our patents and patent applications may be challenged or fail to result in issued patents and our existing or future patents may be too narrow to prevent third parties from developing or designing around these patents. In addition, we generally rely on trade secret protection and confidentiality agreements to protect certain proprietary know-how that is not patentable, for processes for which patents are difficult to enforce and for any other elements of our drug development processes that involve proprietary know-how, information and technology that is not covered by patent applications. While we require all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information and technology to enter into confidentiality agreements, we cannot be certain that this know-how, information and technology will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Further, the laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the U.S. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the U.S. and abroad. If we are unable to protect or defend the intellectual property related to our technologies, we will not be able to establish or maintain a competitive advantage in our market.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. Our competitors may seek to market generic versions of any approved products by submitting ANDAs to the FDA in which they claim that patents owned or licensed by us are invalid, unenforceable and/or not infringed. In December 2022, the Delaware District Court ruled in favor of Teva and Apotex in our patent litigation relating to their filing of ANDAs for generic versions of HETLIOZ® in the U.S. We appealed the decision to the Federal Circuit, and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report, which is incorporated herein by reference, for additional information.
Alternatively, our competitors may seek approval to market their own products similar to, or otherwise competitive with, our products. In these circumstances, we may need to defend and/or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid and/or unenforceable. Even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. In addition, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful.
Even where laws provide protection or we are able to obtain patents, costly and time-consuming litigation may be necessary to enforce and determine the scope of our proprietary rights, and the outcome of such litigation would be uncertain. Moreover, any actions we may bring to enforce our intellectual property rights against our competitors could provoke them to bring counterclaims against us, and some of our competitors have substantially greater intellectual property portfolios than we have. To counter infringement or unauthorized use of any patents we may obtain, we may be required to file infringement claims, which can be expensive and time-consuming to litigate. In addition, if we or one of our future collaborators were to initiate legal proceedings against a third party to enforce a patent covering one of our products, current product candidates, or one of our future products, the defendant could counterclaim that the patent is invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace and challenges to validity of patents in certain foreign jurisdictions are common as well. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or lack of statutory subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant material information from the U.S. Patent and Trademark Office (USPTO), or made a materially misleading statement, during prosecution. We may assert the patents in Hatch-Waxman litigation against the party filing the ANDA to keep the competing product off of the market until the patents expire but there is a risk that we will not succeed. The party filing the ANDA may also counterclaim in the litigation that our patents are not valid or unenforceable, and the court may find one or more claims of our patents invalid or unenforceable. If this occurs, a competing generic product could be marketed prior to expiration of our patents listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the “Orange Book,” which would harm our business.
We have been and continue to be involved in a number of lawsuits with a variety of generic drug manufacturers who have filed ANDAs relating to certain of our patents. In December 2022, the Delaware District Court ruled in favor of Teva and Apotex in our patent litigation relating to their filing of ANDAs for generic versions of HETLIOZ® in the U.S. We appealed the decision to the Federal Circuit, and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition. We have also filed Hatch-Waxman lawsuits in U.S. District Court for the District of New Jersey against each of Teva and Apotex and in the U.S. District Court for the Southern District of Florida against Apotex, in each case, asserting infringement of U.S. Patent No. 11,285,129, which is a method of administration patent that was not litigated in the prior litigation. The New Jersey cases have been transferred to the Delaware District Court, where they remain pending, and the Florida case was voluntarily dismissed. We have also filed patent infringement lawsuits against each of Teva and Apotex in the U.S. District Court for the District of Delaware, in each case, asserting infringement of U.S. Patent No. 11,918,556 another method of administration patent that was not litigated in the prior litigation. We have also filed a Hatch-Waxman lawsuit against MSN in the Delaware District Court asserting that U.S. Patent Nos. 10,179,119, 11,266,622, 11,285,129, 11,850,229, 10,610,510, 10,980,770, and 11,759,446 will be infringed by MSN’s generic version of HETLIOZ LQ® for which MSN is seeking FDA approval. A trial is scheduled to begin on June 15, 2026, and a 30-month stay is in place until December 3, 2026. We also have Orphan Drug Exclusivity for HETLIOZ LQ® until December 1, 2027, thus no ANDAs for generic HETLIOZ LQ® can be approved prior to December 1, 2027. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report, which is incorporated herein by reference, for additional information.
If we do not obtain protection under the Hatch-Waxman Act and similar foreign legislation to extend our patents and to obtain market exclusivity for our products, our business will be harmed.
The Hatch-Waxman Act provides for an extension of patent term for drugs for a period of up to five years to compensate for time spent in development. The Fanapt® U.S. New Chemical Entity (NCE) patent, the primary patent covering the product as a new composition of matter, received the full five-year patent term extension under the Hatch-Waxman Act and so this patent in the U.S. expired in November 2016. In November 2013, a patent directed to a method of treating patients with Fanapt® based on genotype was issued to us by the USPTO. This patent, which was listed in the Orange Book in January 2015, is set to expire in 2027. Additional U.S. patents directed to methods of treating patients with Fanapt®, which are set to expire between 2025 and 2031, were issued to us in 2015. The HETLIOZ® U.S. NCE patent received the full five-year patent term extension under the Hatch-Waxman Act and so, assuming that we continue to have rights under our license agreement with respect to this product, this patent in the U.S. expired in December 2022. We also own HETLIOZ® U.S. method of treatment patents (directed to the approved method of treatment as described in the HETLIOZ® label approved by the FDA), which expire normally between 2033 and 2041, and drug substance patents that expire in 2035. Additionally, the USPTO has issued a drug formulation patent for HETLIOZ LQ® that will expire in 2040. With respect to PONVORY®, term extension of the NCE patent
pursuant to the Hatch-Waxman Act was granted on January 31, 2025 and received the full five-year patent term extension, extending the term of this patent to November 2029. The USPTO has granted additional patents, including a further patent directed to a crystalline form of the active ingredient in PONVORY®, which will expire in May 2032 in view of awarded patent term adjustment. The USPTO has also issued four method of treatment patents for PONVORY®, one of which expired in November 2024, and the remaining three expiring between December 2025 and October 2042. Also, a number of patent applications covering further methods of treatment remain pending at the USPTO.
In December 2022, the Delaware District Court ruled in favor of Teva and Apotex in our patent litigation relating to their filing of ANDAs for generic versions of HETLIOZ® in the U.S. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” each of which is incorporated herein by reference, for additional information.
The E.U. provides that companies that receive regulatory approval for a new medicinal product will have a 10-year period of regulatory data protection and market protection for that product (with the possibility of a further one-year extension under certain conditions), beginning on the date of such European regulatory approval, regardless of when the European NCE patent covering such product expires. A generic version of the approved drug that refers to the approved drug’s regulatory data may not be marketed or sold in Europe during such market protection period. This legislation is of material importance with respect to Fanapt®, since the European NCE patent for Fanapt® has expired.
Assuming we gain a five-year patent term restoration for tradipitant, and that we continue to have rights under our license agreement with respect to this product, we would have exclusive rights to tradipitant’s U.S. NCE patent until 2029. Assuming we gain a five-year patent term restoration for VQW-765, and that we continue to have rights under our license agreement with respect to this product, we would have exclusive rights to VQW-765’s U.S. NCE patent until 2028.
There is no assurance that we will receive the extensions of our patents or other exclusive rights available under the Hatch-Waxman Act or similar foreign legislation. Such extensions may not be granted because of, for example, the failure to exercise due diligence during the testing phase or regulatory review process, the failure to apply within applicable deadlines, the failure to apply prior to expiration of relevant patents, or any other failure to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we fail to receive such extensions or exclusive rights, our ability to prevent competitors from manufacturing, marketing and selling generic versions of our products will be materially impaired.
Generic company competitors have received FDA approval of generic versions of HETLIOZ® in the U.S., which has harmed our business. We have not prevailed in our litigation against these competitors to date, and if additional efforts to protect our patents are unsuccessful, our business will be further harmed.
The FDCA, as amended by the Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Amendments, permits the FDA to approve ANDAs for generic versions of brand name drugs like HETLIOZ®. We refer to the process of generic drug applications as the “ANDA process.” The ANDA process permits competitor companies to obtain marketing approval for a drug product with the same active ingredient, dosage form, strength, route of administration, and labeling as the approved brand name drug, but without having to conduct and submit clinical studies to establish the safety and efficacy of the proposed generic product. In place of such clinical studies, an ANDA applicant needs to submit data demonstrating that its product is bioequivalent to the brand name product, usually based on pharmacokinetic studies.
As an alternate path to FDA approval for modifications of products previously approved by the FDA, an applicant may submit an NDA, under Section 505(b)(2) of the FDCA (enacted as part of the Hatch-Waxman Amendments). This statutory provision permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference from the owner of the data. The Hatch-Waxman Amendments permit the applicant to rely upon the FDA findings of safety and effectiveness of a drug that has obtained FDA approval based on preclinical or clinical studies conducted by others. In addition to relying on FDA prior findings of safety and effectiveness for a referenced drug product, the FDA may require companies to perform additional preclinical or clinical studies to support approval of the modification to the referenced product.
If an application for a generic version of a branded product or a Section 505(b)(2) application relies on a prior FDA finding of safety and effectiveness of a previously-approved product, including an alternative strength thereof, the applicant is
required to certify to the FDA concerning any patents listed for the referenced product in the Orange Book. Specifically, the applicant must certify in the application that:
I.there is no patent information listed for the reference drug;
II.the listed patent has expired for the reference drug;
III.the listed patent for the reference drug has not expired, but will expire on a particular date and approval is sought after patent expiration; or
IV.the listed patent for the reference drug is invalid, unenforceable, or will not be infringed by the manufacture, use or sale of the product for which the ANDA or 505(b)(2) NDA is submitted.
The Hatch-Waxman Amendments require an applicant for a drug product that relies, in whole or in part, on the FDA’s prior approval of HETLIOZ®, to notify us of its application, a “paragraph IV” notice, if the applicant is seeking to market its product prior to the expiration of the patents that both claim HETLIOZ® and are listed in the Orange Book. A bona fide paragraph IV notice may not be given under the Hatch-Waxman Amendments until after the generic company receives from the FDA an acknowledgement letter stating that its ANDA is sufficiently complete to permit a substantive review.
The paragraph IV notice is required to contain a detailed factual and legal statement explaining the basis for the applicant’s opinion that the proposed product does not infringe our patents, that the relevant patents are invalid, or both. After receipt of a valid notice, the branded product manufacturer has the option of bringing a patent infringement suit in federal district court against any generic company seeking approval for its product within 45 days from the date of receipt of each notice. If such a suit is commenced within this 45-day period, the Hatch-Waxman Amendments provide for a 30-month stay on FDA’s ability to give final approval to the proposed generic product, which period begins on the date the paragraph IV notice is received. Generally, during a period of time in which generic applications may be submitted for a branded product based on a product’s regulatory exclusivity status, if no patents are listed in the Orange Book before the date on which a complete ANDA application for a product (excluding an amendment or supplement to the application) is submitted, an ANDA application could be approved by FDA without regard to a stay. For products entitled to five-year exclusivity status, the Hatch-Waxman Amendments provide that an ANDA application may be submitted after four years following FDA approval of the branded product if it contains a certification of patent invalidity or non-infringement to a patent listed in the Orange Book. In such a case, the 30-month stay runs from the end of the five-year exclusivity period. Statutory stays may be shortened or lengthened if either party fails to cooperate in the litigation and it may be terminated if the court decides the case in less than 30 months. If the litigation is resolved in favor of the ANDA applicant before the expiration of the 30-month period, the stay will be immediately lifted and the FDA’s review of the application may be completed. Such litigation is often time-consuming and costly, and may result in generic competition if such patents are not upheld or if the generic competitor is found not to infringe such patents.
Between April 2018 and March 2021, we filed numerous Hatch-Waxman lawsuits in the Delaware District Court against Teva, Apotex and MSN asserting that U.S. Patent Nos. RE46,604 (‘604 Patent) , 9,060,995, 9,539,234, 9,549,913, 9,730,910 (‘910 Patent), 9,844,241, 10,071,977, 10,149,829 (‘829 Patent), 10,376,487 (‘487 Patent), 10,449,176, 10,610,510, 10,610,511, 10,829,465, and 10,611,744 would be infringed by their generic versions of HETLIOZ®, for which they were seeking FDA approval. In January 2022, we entered into a license agreement with MSN and Impax resolving the lawsuits against MSN. The license agreement grants MSN and Impax a non-exclusive license to manufacture and commercialize MSN’s version of HETLIOZ® in the U.S. effective as of March 13, 2035, unless prior to that date we obtain pediatric exclusivity for HETLIOZ®, in which case the license will be effective as of July 27, 2035. The license agreement also provides that MSN and Impax may launch a generic version of HETLIOZ® earlier under certain limited circumstances. In January 2023, MSN and its commercial partner, Amneal Pharmaceuticals, Inc., informed us of their belief that such circumstances have occurred and have since launched their generic version. We disagreed with this position and sought to defend our legal rights to exclusivity for HETLIOZ®. The consolidated lawsuits against Teva and Apotex went to trial in March 2022.
In December 2022, the Delaware District Court ruled that Teva and Apotex did not infringe the ‘604 Patent, and that the asserted claims of the ‘604 Patent, ‘910 Patent, ‘829 Patent and ‘487 Patent were invalid. We appealed the decision to the Federal Circuit and in May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling. In August 2023, the Federal Circuit denied our request for a rehearing. In January 2024, we filed a petition for a writ of certiorari with the U.S. Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied our petition.
We have also filed Hatch-Waxman lawsuits in U.S. District Court for the District of New Jersey against each of Teva and Apotex and in the U.S. District Court for the Southern District of Florida against Apotex, in each case, asserting infringement of U.S. Patent No. 11,285,129, a method of administration patent that was not litigated in the prior litigation. The
New Jersey cases have been transferred to the Delaware District Court, where they remain pending, and the Florida case was voluntarily dismissed. We have also filed patent infringement lawsuits against each of Teva and Apotex in the U.S. District Court for the District of Delaware, in each case, asserting infringement of U.S. Patent No. 11,918,556, another method of administration patent that was not litigated in the prior litigation. While we are pursuing additional remedies, we cannot be certain of the success, timing or efforts involved in connection with these efforts. If any of the generic manufacturers has adequate supply available and is successful, such generic competition in the short term could have a material and adverse impact on our revenues and our stock price.
We may also face challenges to the validity of our patents through a procedure known as inter partes review. Inter partes review is a trial proceeding conducted through the Patent Trial and Appeal Board, of the USPTO. Such a proceeding could be introduced against us within the statutory one-year window triggered by service of a complaint for infringement related to an ANDA filing or at any time by an entity not served with a complaint. Such proceedings may review the patentability of one or more claims in a patent on specified substantive grounds such as allegations that a claim is obvious on the basis of certain prior art.
We intend to continue to vigorously enforce our intellectual property rights relating to HETLIOZ®, but we cannot predict the outcome of the pending lawsuits, our appeal, or any subsequently filed lawsuits or inter partes review. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report and the risk factor entitled “We are, have been, and may continue to be, involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful, and third parties may challenge the validity or enforceability of our patents and they may be successful,” each of which is incorporated herein by reference, for additional information.
Any significant degree of generic market entry would limit our U.S. sales, which would have a significant adverse impact on our business and results of operations. In addition, even if a competitor’s effort to introduce a generic product is ultimately unsuccessful, the perception that such development is in progress and/or news related to such progress could materially affect the perceived value of our company and our stock price. For example, our stock price suffered a significant decline following our announcement of the Delaware District Court’s ruling in favor of Teva and Apotex.
We may not be successful in the development of products for our own account.
In addition to our business strategy of acquiring rights to develop and commercialize products, we may develop products for our own account by applying our technologies to off-patent drugs as well as developing our own proprietary molecules. Because we will be funding the development of such programs, there is a risk that we may not be able to continue to fund all such programs to completion or to provide the support necessary to perform the clinical trials, obtain regulatory approvals or market any approved products. We expect the development of products for our own account to consume substantial resources. If we are able to develop commercial products on our own, the risks associated with these programs may be greater than those associated with our programs with collaborative partners.
Litigation or third-party claims of intellectual property infringement could require us to divert resources and may prevent or delay our drug discovery and development efforts.
Our commercial success depends, in part, on our not infringing the patents and proprietary rights of third parties. Third parties may assert that we are employing their proprietary technology without authorization. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
Furthermore, parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to develop and commercialize one or more of our products. Defense of these claims, regardless of their merit, would divert substantial financial and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, obtain one or more licenses from third parties or pay royalties. In addition, even in the absence of litigation, we may need to obtain additional licenses from third parties to advance our research or allow commercialization of our products. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to develop and commercialize further one or more of our products.
In addition, in the future we could be required to initiate litigation to enforce our proprietary rights against infringement by third parties. Prosecution of these claims to enforce our rights against others could divert substantial financial and employee resources from our business. If we fail to enforce our proprietary rights against others, our business will be harmed.
As described elsewhere in these risk factors and in Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report, incorporated herein by reference, we have initiated lawsuits to enforce our patent rights against certain generic pharmaceutical companies.
General Risk Factors
Our stock price has been highly volatile and may be volatile in the future, and purchasers of our common stock could incur substantial losses.
The realization of any of the risks described in these risk factors or other unforeseen risks could have a dramatic and adverse effect on the market price of our common stock. Between January 1, 2024 and December 31, 2024, the high and low sale prices of our common stock as reported on The Nasdaq Global Market varied between $3.46 and $6.75. Additionally, market prices for securities of biotechnology and pharmaceutical companies, including ours, have historically been very volatile. The market for these securities has, from time to time, experienced significant price and volume fluctuations for reasons that were unrelated to the operating performance of any one company.
The following factors, in addition to the other risk factors described in this section, may also have a significant impact on the market price of our common stock:
•our level of success in commercializing our products;
•our level of success in executing our commercialization strategies;
•publicity regarding actual or potential litigation involving us and the outcome of any such litigation;
•publicity regarding actual or potential testing or trial results relating to products under development by us or our competitors;
•the outcome of regulatory review relating to products under development by us or our competitors;
•regulatory developments in the U.S. and foreign countries;
•newly enacted healthcare legislation or changes to existing legislation;
•developments concerning any collaboration or other strategic transaction we may undertake;
•announcements of patent issuances or denials, technological innovations or new commercial products by us or our competitors;
•safety issues with our products or those of our competitors;
•announcements of technological innovations or new therapeutic products or methods by us or others;
•actual or anticipated variations in our quarterly operating results;
•changes in estimates of our financial results or recommendations by securities analysts or failure to meet such financial expectations;
•changes in government regulations or policies;
•changes in patent legislation or patent decisions or adverse changes to patent law;
•additions or departures of key personnel or members of our board of directors;
•the publication of negative research or articles about our company, our business or our products by industry analysts or others;
•market rumors or press reports;
•publicity regarding actual or potential transactions involving us; and
•economic, political and other external factors beyond our control.
We have been, and may in the future be, subject to litigation, which could harm our stock price, business, results of operations and financial condition.
We have been the subject of litigation in the past and may be subject to litigation in the future. In the past, following periods of volatility in the market price of their stock, many companies, including us, have been the subjects of securities class action litigation. Any such litigation can result in substantial costs and diversion of management’s attention and resources and
could harm our stock price, business, results of operations and financial condition. For example, our stock price suffered a significant decline following our announcement of the Delaware District Court’s ruling in favor of Teva and Apotex. As a result of these factors, holders of our common stock might be unable to sell their shares at or above the price they paid for such shares.
If there are substantial sales of our common stock, our stock price could decline.
A small number of institutional investors and private equity funds hold a significant number of shares of our common stock. Sales by these stockholders of a substantial number of shares, or the expectation of such sales, could cause a significant reduction in the market price of our common stock.
In addition to our outstanding common stock, as of December 31, 2024, there were a total of 7,253,587 shares of our common stock that we have registered and are obligated to issue upon the exercise of currently outstanding options and settlement of restricted stock unit awards granted under our 2006 and 2016 Equity Incentive Plans. Upon the exercise of these options or settlement of the shares underlying these restricted stock units, as the case may be, in accordance with their respective terms, these shares may be resold freely, subject to restrictions imposed on our affiliates under Rule 144. If significant sales of these shares occur in short periods of time, these sales could reduce the market price of our common stock. Any reduction in the trading price of our common stock could impede our ability to raise capital on attractive terms, if at all.
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. We currently have research coverage by securities and industry analysts. If one or more analysts who cover us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, interest in the purchase of our stock could decrease, which could cause our stock price or trading volume to decline.
Our common stock may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in previous offerings. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in previous offerings, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors.
Our business could be negatively affected as a result of the actions of activist stockholders.
Proxy contests have been waged against many companies in the biopharmaceutical industry, including us, over the last several years. If faced with a proxy contest or other type of shareholder activism, we may not be able to respond successfully to the contest or dispute, which would be disruptive to our business. Even if we are successful, our business could be adversely affected by a proxy contest or shareholder dispute involving us for several reasons, including, among others:
•responding to proxy contests and other actions by activist stockholders can be costly and time-consuming, disrupting operations and diverting the attention of management and employees;
•perceived uncertainties as to future direction may result in the loss of potential acquisitions, collaborations or in-licensing opportunities, and may make it more difficult to attract and retain qualified personnel and business partners; and
•if individuals are elected to our board of directors with a specific agenda, it may adversely affect our ability to effectively and timely implement our strategic plan and create additional value for our stockholders.
These actions could cause our stock price to experience periods of volatility.
Anti-takeover provisions in our charter and bylaws and under Delaware law, and the adoption of a rights plan, could prevent or delay a change in control of our company.
We are a Delaware corporation and the anti-takeover provisions of Section 203 of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change of control would be beneficial to our existing stockholders. In addition, our amended and restated certificate of incorporation and bylaws may discourage, delay or prevent a change in our management or control over us that stockholders may consider favorable. Our amended and restated certificate of incorporation and bylaws:
•authorize the issuance of “blank check” preferred stock that could be issued by our board of directors to thwart a takeover attempt;
•do not provide for cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors;
•establish a classified board of directors, as a result of which the successors to the directors whose terms have expired will be elected to serve from the time of election and qualification until the third annual meeting following their election;
•require that directors only be removed from office for cause;
•provide that vacancies on the board of directors, including newly created directorships, may be filled only by a majority vote of directors then in office;
•limit who may call special meetings of stockholders;
•prohibit stockholder action by written consent, requiring all actions to be taken at a meeting of the stockholders; and
•establish advance notice requirements for nominating candidates for election to the board of directors or for proposing matters that can be acted upon by stockholders at stockholder meetings.
Moreover, in April 2024, our board of directors adopted a rights agreement which provided each stockholder of record as of the close of business on April 29, 2024 a right for each outstanding share of common stock of the Company held by such stockholder (each, a Right), which entitles the registered holder to purchase from the Company one one-thousandth of a share of Series A Junior Participating Preferred Stock, par value $0.001 per share, of the Company at an exercise price of $25.00, subject to adjustment. The complete terms of the Rights are set forth in the Rights Agreement, dated as of April 17, 2024, between the Company and Equiniti Trust Company, LLC, as rights agent (Rights Agent), as amended by that certain Amendment No. 1 to the Rights Agreement, by and between the Company and the Rights Agent, and that certain Amendment No. 2 to the Rights Agreement, by and between the Company and the Rights Agent (as amended, the Rights Agreement). The Rights Agreement has a one-year term, expiring on April 16, 2025, and could have the effect of discouraging, delaying or preventing a change in management or control over us. While there is no plan to do so at this time, our board of directors may choose to adopt a new rights plan in the future.
Changes to tax regulations to which we are subject could adversely affect us.
We are subject to tax laws, treaties and regulations in the countries in which we operate, and these laws and treaties are subject to interpretation. New legislation or regulation that could affect our tax burden could be enacted by any governmental authority. We cannot predict the timing or extent of such tax-related developments which could have a negative impact on our financial results. We have taken, and will continue to take, tax positions based on our interpretation of such tax laws. However, a challenge by a taxing authority, our ability to utilize tax benefits such as carryforwards or tax credits, or a deviation from other tax-related assumptions may cause our actual financial results to deviate from previous estimates.
Future transactions may harm our business or the market price of our stock.
We regularly review potential transactions related to technologies, products or product rights and businesses complementary to our business. These transactions could include:
•mergers;
•acquisitions;
•asset purchases;
•strategic alliances;
•licensing agreements; and
•co-promotion and similar agreements.
We may choose to enter into one or more of these transactions at any time, which may cause substantial fluctuations in the market price of our stock. Moreover, depending upon the nature of any transaction, we may experience a charge to earnings, which could also materially adversely affect our results of operations and could harm the market price of our stock. It is too early to tell whether our December 2023 acquisition of PONVORY® from Janssen will yield the results that we expect. If we experience difficulties integrating PONVORY® into our portfolio of approved products, or we are unable to achieve market acceptance of PONVORY®, our business and results of operations may be materially harmed.
We may undertake strategic acquisitions in the future, and difficulties integrating such acquisitions could damage our ability to achieve or sustain profitability.
Although we have no experience in acquiring businesses, we may acquire businesses or assets that complement or augment our existing business. If we acquire businesses with promising products or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to move one or more products through preclinical and/or clinical development to regulatory approval and commercialization. Integrating any newly acquired businesses or technologies could be expensive and time-consuming, resulting in the diversion of resources from our current business. We may not be able to integrate any acquired business successfully. We cannot assure that, following an acquisition, we will achieve revenues, specific net income or loss levels that justify the acquisition or that the acquisition will result in increased earnings, or reduced losses, for the combined company in any future period. Moreover, we may need to raise additional funds through public or private debt or equity financing to acquire any businesses, which would result in dilution for stockholders or the incurrence of indebtedness and may not be available on terms which would otherwise be acceptable to us. We may not be able to operate acquired businesses profitably or otherwise implement our growth strategy successfully.
Our operating results may fluctuate significantly due to a number of factors which make our future results difficult to predict and could cause our operating results to fall below expectations or our guidance.
Our operating results will continue to be subject to fluctuations and are affected by numerous factors, including:
•product sales;
•cost of product sales;
•the rate at which third-party payors approve coverage for our products;
•marketing and other expenses;
•manufacturing or supply issues;
•the timing and amount of royalties or milestone payments;
•our addition or termination of development programs;
•variations in the level of expenses related to our products or future development programs;
•regulatory developments affecting our products or those of our competitors;
•our execution of collaborative, licensing or other arrangements, and the timing of payments we may make or receive under these arrangements;
•any intellectual property infringement or other lawsuit in which we may become involved; and
•the timing and recognition of stock-based compensation expense.
If our operating results fall below the expectations of investors or securities analysts or below any guidance we may provide, the price of our common stock could decline substantially. Furthermore, any fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially. We believe that comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
We are increasingly dependent on information technology systems, infrastructure and data. Cybersecurity breaches could expose us to liability, damage our reputation, compromise our confidential information or otherwise adversely affect our business.
We are increasingly dependent upon information technology systems, infrastructure and data. Our computer systems have been and will continue to be attacked, including from service interruption or destruction, malicious intrusion, phishing,
ransomware, nation-state attacks and random attack. Security breaches pose a risk that sensitive data, including intellectual property, trade secrets or personal information may be exposed to unauthorized persons or to the public. Cyber-attacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyber-attacks could include the deployment of harmful malware, denial-of service, social engineering and other means to affect service reliability and threaten data confidentiality, integrity and availability. Our cybersecurity controls may not function as intended or our logging may be insufficient to fully investigate an incident. Our key business partners face similar risks, and a security breach of their systems could adversely affect our security posture. While we continue to invest in data protection and information technology, we may not be able to effectively prevent, detect or respond to data breaches, which could adversely affect our business and operations and/or result in the loss of critical or sensitive information, which could result in financial, legal, business or reputational harm. In addition, the technologies we depend on may not operate as intended or generate intended efficiencies, which may similarly impact our business.
Our internal computer systems, or those of our collaborators, CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates.
Despite the implementation of security measures, our internal computer systems and those of our collaborators, CROs, and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Information security risks have significantly increased in recent years, in part, due to the proliferation of new technologies and the increased sophistication and activities of organized crime, hackers, terrorists and other external parties, including foreign state actors. As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any information security breaches.
A system failure, accident or security breach could result in a material disruption of our independent drug development programs. For example, the loss of clinical trial data from ongoing or future clinical trials for any of our product candidates could result in delays in regulatory approval efforts and significantly increase costs to recover or reproduce the data. Our information security systems are also subject to laws and regulations requiring that we take measures to protect the privacy and security of certain information we gather and use in our business. For example, federal and state laws, including, without limitation, state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and storage of personal information. To the extent that any disruption or security breach were to result in a loss of or damage to data or applications, or inappropriate disclosure of confidential or proprietary information or personal health information, we could incur substantial liability, our reputation would be damaged and the further development of our product candidates could be delayed.
Natural disasters, public health crises, political crises, severe weather events, and other catastrophic events or other events outside of our control may damage our facilities or the facilities of third parties on which we depend and could impact our ability to sell products.
An earthquake or other natural disaster or power shortages or outages could disrupt operations or impair critical systems. We, our suppliers, third-party service providers and customers are vulnerable to damage from natural disasters, including fire, floods or monsoons, power loss, communications failures, public health crises, such as pandemics and epidemics, political crises, such as terrorism, war, political instability or other conflict and similar events. If any disaster were to occur, our ability to operate our business could be seriously, or potentially completely, impaired. In addition, the nature of our activities could cause significant delays in our research programs and commercial activities and make it difficult for us to recover from a disaster. The insurance we maintain may not be adequate to cover our losses resulting from disasters or other business interruptions. Accordingly, an earthquake or other disaster could materially and adversely harm our ability to conduct business.
| | | | | |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
Cybersecurity Risk Management and Strategy
We recognize the importance of assessing, identifying, and managing material risks associated with cybersecurity threats, as such term is defined in Item 106(a) of Regulation S-K. These risks include, among other things, operational risks,
intellectual property theft, fraud, extortion, harm to employees or customers, violation of privacy or security laws and other litigation and legal risk and reputational risks. We have implemented several cybersecurity processes, technologies and controls to aid in our efforts to assess, identify and manage such material risks.
Our process for identifying and assessing material risks from cybersecurity threats operates alongside our broader overall risk assessment process, covering all company risks. As part of this process, appropriate personnel will collaborate with subject matter specialists, as necessary, to gather insights for identifying and assessing material cybersecurity threat risks, their severity and potential mitigations.
We also have a cybersecurity specific risk assessment process, which helps identify our cybersecurity threat risks. As part of this process, and our processes to provide for the availability of critical data and systems, maintain regulatory compliance, identify and manage our risks from cybersecurity threats and to protect against, detect and respond to cybersecurity incidents, as such term is defined in Item 106(a) of Regulation S-K, we undertake the below listed activities, among others:
•compare our processes to benchmark standards, such as those set by the National Institute of Standards and Technology (NIST);
•closely monitor emerging data protection laws and implement changes to our processes designed to comply;
•conduct annual customer data handling and use requirements training for employees;
•conduct annual cybersecurity management and incident training for employees involved in our systems and processes that handle sensitive data;
•through policy, practice and contract, as applicable, require employees, as well as third-parties who provide services on our behalf, to treat customer information and data with care;
•run tabletop exercises to simulate a response to a cybersecurity incident and use the findings to improve our processes and technologies;
•conduct regular network and endpoint monitoring, vulnerability assessments, and penetration testing to improve our information systems, as such term is defined in Item 106(a) of Regulation S-K;
•leverage the NIST incident handling framework to help us govern, identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident; and
•carry information security risk insurance that provides protection against the potential losses arising from a cybersecurity incident.
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate brand and reputational damage.
As part of the above processes, we may engage with assessors, consultants, auditors and other third-parties, including by having a third-party review our cybersecurity program to help identify areas for continued focus, improvement and/or compliance.
Our processes also address cybersecurity threat risks associated with our use of third-party service providers, including those in our supply chain, our CROs or those who have access to our customer and employee data or our systems. Third-party risks are included within our broader overall risk assessment process, as well as our cybersecurity-specific risk identification program, both of which are discussed above. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third-parties that have access to our systems, data or facilities that house such systems or data, and continually monitor cybersecurity threat risks identified through such diligence.
We describe whether and how risks from identified cybersecurity threats have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition, under the risk factors entitled “We are increasingly dependent on information technology systems, infrastructure and data. Cybersecurity breaches could expose us to liability, damage our reputation, compromise our confidential information or otherwise adversely affect our business,” and “Our internal computer systems, or those of our collaborators, CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of development programs for our product candidates,” in Part I, Item 1A of this Annual Report on Form 10-K, each of which is incorporated herein by reference.
Cybersecurity Governance
Cybersecurity is an important part of our risk management processes and an area of increasing focus for our Board and management.
Our Audit Committee is responsible for the oversight of risks from cybersecurity threats. At least quarterly, the Audit Committee receives an overview from management of our cybersecurity threat risk management and strategy processes covering topics such as data security posture, results from third-party assessments, progress towards pre-determined risk-mitigation-related goals, our incident response plan and material cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. Members of the Audit Committee are also encouraged to regularly engage in ad hoc conversations with management on cybersecurity-related news events and discuss any updates to our cybersecurity risk management and strategy programs. Material cybersecurity threat risks are also considered during separate Board meeting discussions of important matters like risk management, business continuity planning, brand management, and other relevant matters.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by a team of senior level management, including our President, Chief Executive Officer and Chairman of the Board, Senior Vice President, Chief Financial Officer and Treasurer, Senior Vice President, General Counsel and Secretary, and VP of Information Technology. Such individuals collectively have significant prior work experience in various roles involving managing information security, developing cybersecurity strategy and implementing effective information and cybersecurity programs.
These members of management are informed about and monitor the prevention, mitigation, detection, and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan.
As discussed above, these members of management report to the Audit Committee about cybersecurity threat risks, among other cybersecurity related matters.
Our headquarters office consists of a total of 43,462 square feet of office space located at 2200 Pennsylvania Avenue, N.W. in Washington, D.C. under operating leases and subleases that expire between 2026 and 2028 and certain of these leases are subject to renewal options. In addition, we have 2,880 square feet of office space in London, England under an operating lease that has a lease term ending in 2026, and other short-term leases. We believe that these facilities are suitable and adequate to meet our anticipated near-term needs. We anticipate that following the expiration of the leases, additional or alternative space will be available at commercially reasonable terms.
Information with respect to this item may be found in Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this annual report on Form 10-K, which is incorporated herein by reference.
| | | | | |
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
| | | | | |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is quoted on The Nasdaq Global Market under the symbol “VNDA.” As of February 7, 2025, there were ten holders of record of our common stock. The number of holders of record of our common stock does not reflect the number of beneficial holders whose shares are held by depositors, brokers or other nominees.
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
The following graph shows the cumulative five-year total return on our common stock relative to the cumulative total returns of the Nasdaq Composite Index and the iShares Biotechnology Index. An investment of $100 (with reinvestment of dividends) is assumed to have been made in our common stock and in each of the indexes on December 31, 2019 and its
relative performance is tracked through December 31, 2024. The comparisons in the table are required by the Securities and Exchange Commission (SEC) and are not intended to forecast or be indicative of possible future performance of our common stock. We have never paid cash dividends to our stockholders and do not plan to pay dividends in the foreseeable future. The following graph and related information is being furnished solely to accompany this annual report on Form 10-K pursuant to Item 201(e) of Regulation S-K and shall not be deemed “soliciting materials” or to be “filed” with the SEC (other than as provided in Item 201), nor shall such information be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof, and irrespective of any general incorporation language in any such filing.
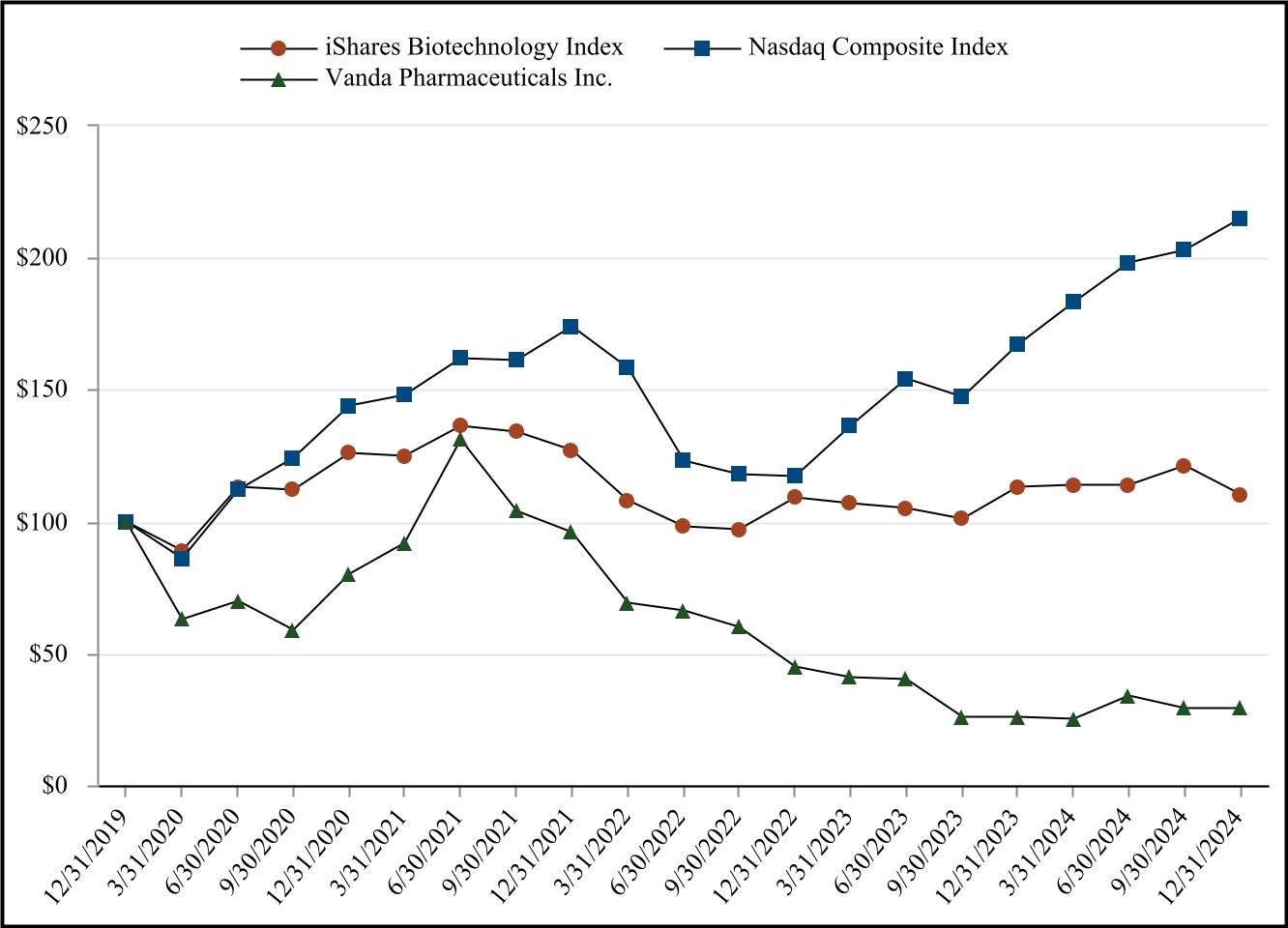
| | | | | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes appearing in this annual report on Form 10-K (Annual Report). This discussion and analysis generally addresses 2024 and 2023 items and year-to-year comparisons between 2024 and 2023. Discussions of 2022 items and year-to-year comparisons between 2023 and 2022 that are not included in this Annual Report can be found in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report include historical information and other information with respect to our plans and strategy for our business and contain forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including but not limited to those set forth under Part I, Item 1A, Risk Factors, and elsewhere in this Annual Report.
Overview
Vanda Pharmaceuticals Inc. (we, our, us or Vanda) is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients.
We strive to advance novel approaches to bring important new medicines to market through responsible innovation. We are committed to the use of technologies that support sound science, including genetics and genomics, in drug discovery, clinical trials and the commercial positioning of our products.
Our commercial portfolio is currently comprised of three products: Fanapt® for the acute treatment of manic or mixed episodes associated with bipolar I disorder and the treatment of schizophrenia, HETLIOZ® for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) and for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome (SMS) and PONVORY® for the treatment of relapsing forms of multiple sclerosis (RMS) including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. HETLIOZ® is the first product approved by the United States Food and Drug Administration (FDA) for patients with Non-24 and for patients with SMS. In addition, we have a number of drugs and/or indications for current products in development, including:
•Fanapt® (iloperidone) long acting injectable (LAI) formulation for the treatment of schizophrenia;
•BysantiTM (milsaperidone), the active metabolite of Fanapt® (iloperidone), for the acute treatment of manic or mixed episodes associated with bipolar I disorder and for the treatment of schizophrenia and major depressive disorder (MDD);
•HETLIOZ® (tasimelteon) for the treatment of jet lag disorder, insomnia, pediatric insomnia, delayed sleep phase disorder (DSPD) and pediatric Non-24;
•PONVORY® (ponesimod) for the treatment of psoriasis and ulcerative colitis;
•Tradipitant (VLY-686), a small molecule neurokinin-1 (NK-1) receptor antagonist, for the treatment of gastroparesis, motion sickness and atopic dermatitis;
•Imsidolimab, an IL-36R antagonist, for the treatment of generalized pustular psoriasis (GPP);
•VTR-297, a small molecule histone deacetylase (HDAC) inhibitor for the treatment of onychomycosis and hematologic malignancies and with potential use as a treatment for several oncology indications;
•Portfolio of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) activators and inhibitors, including VSJ-110 for the treatment of dry eye and ocular inflammation and VPO-227 for the treatment of secretory diarrhea disorders, including cholera;
•VQW-765, a small molecule nicotinic acetylcholine receptor partial agonist, for the treatment of social/performance anxiety and psychiatric disorders; and
•Antisense oligonucleotide (ASO) molecules, including VCA-894A for the treatment of Charcot-Marie-Tooth Disease, Type 2S (CMT2S), caused by cryptic slice site variants within the IGHMBP2 gene and VGT-1849A for the treatment of polycythemia vera (PV), a form of a rare hematologic malignancy.
Operational Highlights
Fanapt®
•Fanapt® was approved in the second quarter of 2024 for the acute treatment of bipolar I disorder. We initiated the commercial launch of Fanapt® in this indication in the third quarter of 2024. In the fourth quarter of 2024, as compared to the fourth quarter of 2023, new patient starts, as reflected by new to brand prescriptions (NBRx), increased by over 160% and Fanapt® net product sales increased by 18%.
•We initiated a Phase III program for the long acting injectable (LAI) formulation of Fanapt® in the fourth quarter of 2024.
•We plan to initiate a study of the Fanapt® LAI as a once-a-month injectable for the treatment of hypertension to address both treatment resistance and treatment compliance.
•We submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in the fourth quarter of 2024 for Fanapt® for bipolar I disorder and schizophrenia.
BysantiTM
•We expect to submit a New Drug Application (NDA) for Bysanti™ to the FDA for the treatments of acute bipolar I disorder and schizophrenia in the first quarter of 2025. Exclusivity, including pending patent applications, could extend into the 2040s.
•We initiated a Phase III clinical study for Bysanti™ as a once-daily adjunctive treatment for MDD in the fourth quarter of 2024. Results are expected in 2026.
HETLIOZ®
•We have initiated clinical programs for HETLIOZ® in pediatric insomnia and DSPD and these programs are ongoing.
•Our MAA for HETLIOZ® and HETLIOZ LQ® for SMS is pending with the EMA.
PONVORY®
•We initiated the commercial launch of PONVORY® for the treatment of relapsing forms of multiple sclerosis in the third quarter of 2024.
•Investigational New Drug (IND) applications for PONVORY® in the treatments of psoriasis and ulcerative colitis were accepted by the FDA in the fourth quarter of 2024.
Tradipitant
•The NDA for tradipitant for the treatment of motion sickness was submitted to the FDA in the fourth quarter of 2024.
•We initiated a clinical trial to study tradipitant in the prevention of vomiting induced by a GLP-1 analog, Wegovy (semaglutide), in the fourth quarter of 2024.
•We have accepted the opportunity for a hearing with the FDA on the approvability of the NDA for tradipitant for the treatment of symptoms of gastroparesis.
Imsidolimab
•In February 2025, we announced that we entered into an exclusive, global license agreement with AnaptysBio, Inc. (Anaptys) for the development and commercialization of imsidolimab (IL-36R antagonist mAb). We expect to initiate and complete the technology transfer activities in 2025 and immediately begin preparing the Biologics License Application (BLA) and MAA for GPP for the US and EU. The imsidolimab BLA for GPP is expected to be submitted to the FDA in 2025.
Early-Stage Program Highlights
•VQW-765, an alpha-7 nicotinic acetylcholine receptor partial agonist, is currently in clinical development for the treatment of acute performance anxiety in social situations. We expect to initiate a Phase III program in 2025.
•The IND application for VCA-894A in the treatment of CMT2S, an inherited peripheral neuropathy for which there is no available treatment, was accepted by the FDA in 2024. Previously in 2023, VCA-894A was granted Orphan Drug Designation for the same indication. The Phase I clinical study for VCA-894A expects to enroll the patient by mid-2025.
•In December 2024, we announced that the FDA has granted Orphan Drug Designation for VGT-1849A, a selective ASO-based JAK2 inhibitor for the treatment of polycythemia vera (PV), a form of a rare hematologic malignancy that is estimated to affect 1 in 2,000 Americans.
Since we began operations, we have devoted substantially all of our resources to the in-licensing, clinical development and commercialization of our products. Our ability to generate meaningful product sales and achieve profitability largely depends on our level of success in commercializing Fanapt® and HETLIOZ® in the U.S. and Europe and PONVORY® in the U.S and Canada, on our ability, alone or with others, to complete the development of our products and to obtain the regulatory approvals for and to manufacture, market and sell our products. The results of our operations will vary significantly from year-to-year and quarter-to-quarter and depend on a number of factors, including risks related to our business, risks related to our industry, and other risks that are detailed in Part I, Item 1A, Risk Factors, of this Annual Report.
Critical Accounting Policies and Estimates
The preparation of our consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements, as well as the reported revenues and expenses during the reported periods. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
A summary of our significant accounting policies appears in the notes to our audited consolidated financial statements for the year ended December 31, 2024 included in this Annual Report. However, we believe that the following accounting policies are important to understanding and evaluating our reported financial results as they involve the most significant judgments and estimates used in the preparation of our consolidated financial statements, and we have accordingly included them in this discussion.
Revenue from net product sales. We account for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance and collectability of consideration is probable. We recognize revenue when control of the product is transferred to the customer in an amount that reflects the consideration we expect to be entitled to in exchange for those product sales, which is typically once the product physically arrives at the customer. Sales tax, value-added taxes and usage-based taxes are excluded from revenues.
Fanapt® is available in the U.S. for distribution through a limited number of wholesalers and is available in retail pharmacies. HETLIOZ® is available in the U.S. for distribution through a limited number of specialty pharmacies and is not available in retail pharmacies. PONVORY® is available in the U.S. for distribution primarily through a limited number of specialty distributors and specialty pharmacies. We invoice and record revenue when our customers, wholesalers, specialty pharmacies and specialty distributors, receive product from the third-party logistics warehouse, which is the point at which control is transferred to the customer. Revenues and accounts receivable are concentrated with these customers. Outside the U.S., we have a distribution agreement for the commercialization of Fanapt® in Israel and sell HETLIOZ® in Germany. Receivables are carried at transaction price, net of allowance for credit losses. Allowance for credit losses is measured using historical loss rates based on the aging of receivables and incorporating current conditions and forward-looking estimates.
The transaction price is determined based upon the consideration to which we will be entitled in exchange for transferring product to the customer. Our product sales are recorded net of applicable product revenue allowances for which reserves are established and include discounts, rebates, chargebacks, service fees, co-pay assistance and product returns that are applicable for various government and commercial payors. Where appropriate, our estimates of variable consideration included in the transaction price consider a range of possible outcomes. Allowances for rebates, chargebacks and co-pay assistance are based upon the insurance benefits of the end customer, which are estimated using historical activity and, where available, actual and pending prescriptions for which we have validated the insurance benefits. Variable consideration may be constrained and is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the respective underlying contracts. If actual results in the future vary from our estimates, we adjust our estimate in the period identified, which would affect net product sales in the period such variances become known.
During the year ended December 31, 2024, we constrained the variable consideration for HETLIOZ® net product sales. The constrained revenue relates to the uncertainties of payor utilization, patient demand and chargeback and rebate amounts, including Medicaid, related to the elevated levels of inventory on hand at the specialty pharmacies.
Reserves for variable consideration are classified as product revenue allowances on the Consolidated Balance Sheets, with the exception of prompt-pay discounts, which are classified as reductions of accounts receivable. The reserve for product returns for which the product may not be returned for a period of greater than one year from the balance sheet date is included as a component of other non-current liabilities in the Consolidated Balance Sheets. Uncertainties related to variable consideration are generally resolved in the quarter subsequent to period end, with the exception of Medicaid rebates, which are dependent upon the timing of when states submit reimbursement claims, Medicare inflationary rebates, which are expected to be billed on an annual basis beginning in 2025, and product returns that are resolved during the product expiry period specified in the customer contract. Due to increased inventory stocking at specialty pharmacy customers of HETLIOZ® in 2024 and 2023, the time it takes to resolve these uncertainties could be longer than we have historically experienced. We currently record sales allowances for the following:
•Prompt-pay: Wholesalers, specialty pharmacies and specialty distributors, our direct customers, are generally offered discounts for prompt payment. We expect that these direct customers will earn prompt payment discounts and, therefore, we deduct the full amount of these discounts from total product sales when revenues are recognized.
•Rebates: Allowances for rebates include mandated discounts under the Medicaid Drug Rebate Program as well as contracted rebate programs with other payors, including the Medicare Part D inflationary rebate effective October 1, 2022. Rebate amounts owed after the final dispensing of the product to a benefit plan participant are based upon contractual agreements or legal requirements with public sector benefit providers, such as Medicaid and Medicare. The allowances for rebates are based on statutory or contracted discount rates and estimated patient utilization.
•Chargebacks: Chargebacks are discounts that occur when contracted indirect customers purchase directly from wholesalers, specialty pharmacies and specialty distributors. Contracted indirect customers, which currently consist primarily of Public Health Service institutions and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The wholesaler, specialty pharmacy or specialty distributor, in turn, charges back the difference between the price initially paid by the wholesaler, specialty pharmacy or specialty distributor and the discounted price paid to the wholesaler, specialty pharmacy or specialty distributor by the contracted customer.
•Medicare Part D rebates: The Medicare Part D prescription drug benefit requires manufacturers to fund approximately 70% of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients for applicable drugs. We account for the Medicare Part D coverage gap using a point of sale model. Estimates for expected Medicare Part D rebates are based, in part, on historical activity and, where available, actual and pending prescriptions when we have validated the insurance benefits. Beginning January 1, 2025, the Medicare Part D coverage gap discount program was replaced with a new discounting program under the Inflation Reduction Act of 2022. The Medicare Part D benefit redesign is expected to result in higher discounts for our Medicare payor segment relative to the current Medicare Part D prescription drug coverage gap discount program. Under the redesigned Medicare Part D program, applicable drugs dispensed to applicable beneficiaries will be subject to manufacturer discounts of 10% during the initial coverage phase and 20% during the catastrophic coverage phase. Manufacturers may be eligible for phase-in of discounts, such as for applicable beneficiaries who are Low Income Subsidy (LIS) eligible under section 1860D-14(a) of the Social Security Act. We are a specified manufacturer whose applicable drugs for applicable beneficiaries who are LIS eligible will be subject to lower applicable discounts during the phase-in period.
•Service fees: We receive sales order management, data and distribution services from certain customers, for which we are assessed fees. These fees are based on contracted terms and are known amounts. We accrue service fees at the time of revenue recognition, resulting in a reduction of product sales and the recognition of an accrued liability, unless it is a payment for a distinct good or service from the customer in which case the fair value of those distinct goods or services are recorded as selling, general and administrative expense.
•Co-pay assistance: Patients who have commercial insurance and meet certain eligibility requirements may receive co-pay assistance. Co-pay assistance utilization is based on information provided by our third-party administrator.
•Product returns: We generally offer direct customers a limited right to return, as contractually defined with our customers. We consider several factors in the estimation process, including expiration dates of product shipped to customers, inventory levels within the distribution channel, product shelf life, historical return activity, including
activity for product sold for which the return period has past, prescription trends and other relevant factors. We do not expect returned products to be resalable. There was no right of return asset as of December 31, 2024 or 2023.
The following table summarizes sales discounts and allowance activity as of and for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| (in thousands) | Rebates &
Chargebacks | | Discounts,
Returns
and Other | | Total |
| Balances at December 31, 2021 | 31,854 | | | 9,601 | | | 41,455 | |
| Provision related to current period sales | 92,109 | | | 30,636 | | | 122,745 | |
| Adjustments for prior period sales | (2,647) | | | 1,396 | | | (1,251) | |
| Credits/payments made | (83,857) | | | (31,609) | | | (115,466) | |
| Balances at December 31, 2022 | 37,459 | | | 10,024 | | | 47,483 | |
| Provision related to current period sales | 85,916 | | | 28,488 | | | 114,404 | |
| Adjustments for prior period sales | (267) | | | 276 | | | 9 | |
| Credits/payments made | (82,957) | | | (28,361) | | | (111,318) | |
| Balances at December 31, 2023 | 40,151 | | | 10,427 | | | 50,578 | |
| Provision related to current period sales | 82,233 | | | 33,449 | | | 115,682 | |
| Adjustments for prior period sales | (3,246) | | | 3 | | | (3,243) | |
| Credits/payments made | (69,199) | | | (31,488) | | | (100,687) | |
| Balances at December 31, 2024 | $ | 49,939 | | | $ | 12,391 | | | $ | 62,330 | |
The provision for rebates and chargebacks of $82.2 million and $85.9 million for the years ended December 31, 2024 and 2023, respectively, and their ending balances at December 31, 2024 and 2023, primarily represent Medicaid rebates. The provision for discounts, returns and other of $33.4 million and $28.5 million for the years ended December 31, 2024 and 2023, primarily represents wholesaler distribution fees applicable to sales of Fanapt® and estimated product returns of Fanapt®, and co-pay assistance costs and prompt pay discounts applicable to the sales of all of our commercial products. The ending balances of discounts, returns and other as of December 31, 2024 and 2023 primarily represent estimated product returns of Fanapt® and wholesaler distribution fees applicable to sales of Fanapt®.
Stock-based compensation. Compensation costs for all stock-based awards to employees and directors are measured based on the grant date fair value of those awards and recognized over the period during which the employee or director is required to perform service in exchange for the award. We use the Black-Scholes-Merton option pricing model to determine the fair value of stock options. The determination of the fair value of stock options on the date of grant using an option pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include the expected stock price volatility over the expected term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rate and expected dividends. Expected volatility rates are based on the historical volatility of our publicly traded common stock and other factors. The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. We have never paid cash dividends to our stockholders and do not plan to pay dividends in the foreseeable future. As stock-based compensation expense recognized in the Consolidated Statements of Operations is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Research and development expenses. Research and development expenses consist primarily of fees for services provided by third parties in connection with the clinical trials, costs of contract manufacturing services for clinical trial use, milestone payments made under licensing agreements prior to regulatory approval, costs of materials used in clinical trials and research and development, costs for regulatory consultants and filings, depreciation of capital resources used to develop products, related facilities costs, and salaries, other employee-related costs and stock-based compensation for research and development personnel. We expense research and development costs as they are incurred for products in the development stage, including manufacturing costs and milestone payments made under license agreements prior to FDA approval. Upon and subsequent to FDA approval, manufacturing and milestone payments made under license agreements are capitalized. Milestone payments are accrued when it is deemed probable that the milestone event will be achieved. Costs related to the acquisition of intellectual property are expensed as incurred if the underlying technology is developed in connection with our research and development efforts and has no alternative future use.
Clinical trials are inherently complex, often involve multiple service providers and can include payments made to investigator physicians at study sites. Because billing for services often lags delivery of service by a substantial amount of time, we are often required to estimate a significant portion of our accrued clinical expenses. Our assessments include, but are not limited to: (i) an evaluation by the project manager of the work that has been completed during the period, (ii) measurement of progress prepared internally and/or provided by the third-party service provider, (iii) analyses of data that justify the progress, and (iv) management’s judgment. In the event that we do not identify certain costs that have begun to be incurred or we under- or over-estimate the level of services performed or the costs of such services, our reported expenses for such period would be too low or too high.
Intangible assets and impairment of long-lived assets. Our intangible assets consist of capitalized license costs for products approved by the FDA or costs to acquire already commercialized products. We amortize our intangible assets on a straight-line basis over the estimated useful economic life of the related product patents. We assess the impairment of intangible assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include significant underperformance relative to expected historical or projected future operating results, a significant adverse change in legal or regulatory factors that could affect the value or patent life, including our ability to defend and enforce patent claims and other intellectual property rights, and significant negative industry or economic trends. When we determine that the carrying value of our intangible assets may not be recoverable based upon the existence of one or more of the indicators of impairment, we measure any impairment based on the amount that carrying value exceeds fair value.
As a result of the unfavorable events and subsequent developments in the 2022 and 2023 related to the HETLIOZ® patent litigation (see Note 18, Legal Matters, to the consolidated financial statements included in Part II, Item 8 of this Annual Report) we performed impairment reviews for our HETLIOZ® asset group in those years and determined, based upon our review of undiscounted cash flows, that the carrying value of our HETLIOZ® asset group, inclusive of the intangible asset, is recoverable. Accordingly, we have not recorded an intangible asset impairment charge in any period. The litigation and subsequent developments do not affect the sale of HETLIOZ® in the E.U. and there is no generic litigation pending outside of the U.S. with respect to HETLIOZ®. Furthermore, the litigation and subsequent events do not relate to the HETLIOZ LQ® oral suspension formulation. Our expected cash flows continue to support our estimated useful economic life of the intangible asset through 2035.
Income taxes. We assess the need for a valuation allowance against our deferred tax assets each quarter through the review of all available positive and negative evidence. Deferred tax assets are reduced by a tax valuation allowance when, in the opinion of management, it is more likely than not that some portion of the deferred tax assets will not be realized. The analysis is highly dependent upon historical and projected pretax income. In 2024, we generated our first pretax loss since 2017, largely due to expenditures on the commercial launches of Fanapt® in bipolar I disorder and PONVORY® in RMS. Projected taxable income includes significant assumptions related to revenue, which could be affected by the success of the commercial launches of Fanapt® in bipolar I disorder and PONVORY® in RMS and HETLIOZ® generic competition, commercial and research and development activities, and our ability to obtain regulatory approval from the FDA for products or new indications in development, among other factors. Tax benefits are recognized from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefit recognized in the financial statements for a particular tax position is based on the largest benefit that is more likely than not to be realized upon settlement. As of December 31, 2024, after considering all available positive and negative evidence, we concluded, consistent with prior periods, that it was more likely than not that substantially all of our deferred tax assets in the U.S. are realizable in future periods. We maintain a valuation allowance against certain state net deferred tax assets. If our results are not in line with our projections or if our projections change, the conclusion about the appropriateness of the valuation allowance could change in a future period. An increase in the valuation allowance would result in a non-cash income tax expense during the period of change. Any such adjustment could have a material impact on our results of operations.
Recent Accounting Pronouncements
See Note 2, Summary of Significant Accounting Policies, to the consolidated financial statements included in Part II, Item 8 of this Annual Report for information on recent accounting pronouncements.
Results of Operations
We anticipate that our results of operations will fluctuate for the foreseeable future due to several factors, including our and our partners’ ability to continue to successfully commercialize our products, including activities related to Fanapt® for the acute treatment of manic or mixed episodes associated with bipolar I disorder in adults which was approved in April 2024 and
acquired rights to PONVORY® in the U.S. and Canada in December 2023, the impact of the Medicare Part D benefit redesign effective January 1, 2025 under the Inflation Reduction Act of 2022, any possible payments made or received pursuant to license agreements, progress of our research and development efforts, the timing and outcome of clinical trials and related possible regulatory approvals and the status of existing and future potential litigation involving our products and intellectual property.
For HETLIOZ®, the FDA has approved ANDAs for Teva and Apotex, both of which have since launched their generic versions of HETLIOZ® at risk in the U.S. In December 2022, the U.S. District Court for the District of Delaware (Delaware District Court) ruled in favor of Teva and Apotex in our patent litigation relating to their filing of ANDAs for generic versions of HETLIOZ® in the U.S. The Federal Circuit affirmed this ruling, and the U.S. Supreme Court denied our petition for a writ of certiorari in April 2024. The FDA has also approved the ANDA for MSN Pharmaceuticals, Inc. and MSN Laboratories Private Limited (MSN). The license agreement that we entered into when we settled our patent litigation with MSN (MSN/Impax License Agreement) grants MSN and Impax Laboratories LLC (Impax) a non-exclusive license to manufacture and commercialize MSN’s generic version of HETLIOZ® in the U.S. effective as of March 13, 2035, unless prior to that date we obtain pediatric exclusivity for HETLIOZ®, in which case the license will be effective as of July 27, 2035, or earlier under certain limited circumstances. In January 2023, MSN and its commercial partner, Amneal Pharmaceuticals, Inc., informed us of their belief that such circumstances had occurred and have since launched their generic version. In April 2024, we filed litigation against MSN, Impax, and Amneal alleging fraudulent inducement of the license agreement. See Note 18, Legal Matters, to the consolidated financial statements in Part II, Item 8 of this Annual Report. HETLIOZ® could face even more competition from other generic companies in the U.S. in the near term in light of the patent litigation rulings against us. In addition, sales of generic versions of HETLIOZ® have resulted in and could continue to result in a reduction in the demand for HETLIOZ® and/or the price at which we can sell it and/or create volatility in net product sales in future periods, which could have a material impact on our revenues and results of operations.
On September 18, 2024, the FDA declined to approve our NDA of tradipitant for the treatment of symptoms in gastroparesis, providing us with a Complete Response Letter (CRL). We will continue to pursue the marketing authorization for tradipitant and will continue to support the expanded access program that is currently serving several dozen patients with gastroparesis.
Year ended December 31, 2024 compared to year ended December 31, 2023
Revenues. Total revenues increased by $6.1 million, or 3%, to $198.8 million for the year ended December 31, 2024 compared to $192.6 million for the year ended December 31, 2023. The impact of the Medicare Part D benefit redesign under the Inflation Reduction Act of 2022, which begins January 1, 2025, is uncertain and will affect all of our products. Revenues may decline in future periods, potentially significantly, as a result of the Medicare Part D program benefit redesign. Revenue from net product sales were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | Net
Change | | Percent |
Fanapt® net product sales | $ | 94,297 | | | $ | 90,873 | | | $ | 3,424 | | | 4 | % |
HETLIOZ® net product sales | 76,675 | | | 100,167 | | | (23,492) | | | (23) | % |
PONVORY® net product sales | 27,800 | | | 1,600 | | | 26,200 | | | 1638 | % |
| Total net product sales | $ | 198,772 | | | $ | 192,640 | | | $ | 6,132 | | | 3 | % |
Fanapt® net product sales increased by $3.4 million, or 4%, to $94.3 million for the year ended December 31, 2024 compared to $90.9 million for the year ended December 31, 2023. The increase to net product sales was attributable to an increase in price net of deductions and volume. We initiated the commercial launch of Fanapt® for bipolar I disorder in adults in the third quarter of 2024.
HETLIOZ® net product sales decreased by $23.5 million, or 23%, to $76.7 million for the year ended December 31, 2024 compared to $100.2 million for the year ended December 31, 2023. The decrease to net product sales was attributable to a decrease in volume, partially offset by an increase in price, net of deductions, including the impact of changes in constrained revenue. Our HETLIOZ® net product sales as reported for the three months ended March 31, 2023 reflected higher unit sales as compared to recent prior periods. The higher unit sales during the three months ended March 31, 2023 resulted in a significant increase of inventory stocking at specialty pharmacy customers at March 31, 2023. During the remainder of 2023, although there was continued destocking at specialty pharmacy customers, inventory levels remained elevated relative to inventory levels prior to the entrance of generic competition and remained elevated throughout 2024. Going forward, HETLIOZ® net product sales may reflect lower unit sales as a result of reduction of the elevated inventory levels at specialty pharmacy customers or
may be variable depending upon when specialty pharmacy customers need to purchase again. Further, HETLIOZ® net product sales will likely decline in future periods, potentially significantly, related to continued generic competition in the U.S. We constrained HETLIOZ® net product sales for the years ended December 31, 2024 and 2023 to an amount not probable of significant revenue reversal. The amount of revenue recognized during the year ended December 31, 2024 related to change in estimates on revenue constrained during 2023 was $1.3 million. HETLIOZ® net product sales could experience variability in future periods as the remaining uncertainties associated with variable consideration related to inventory stocking by specialty pharmacy customers are resolved.
In December 2023, we completed the acquisition of the U.S. and Canadian rights to PONVORY® from Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company. PONVORY® net product sales were $27.8 million for the year ended December 31, 2024 and $1.6 million for the year ended December 31, 2023, which reflects sales during the post-acquisition period. We initiated the commercial launch of PONVORY® in RMS in the third quarter of 2024. During the fourth quarter of 2024, we recognized approximately $3.0 million of variable consideration that may be subject to dispute but that we believe is not probable of significant revenue reversal.
Cost of goods sold. Cost of goods sold decreased by $3.5 million, or 24%, to $11.3 million for the year ended December 31, 2024 compared to $14.8 million for the year ended December 31, 2023. Cost of goods sold includes third-party manufacturing costs of product sold, third-party royalty costs and distribution and other costs. Third-party royalty costs were 6% of Fanapt® net product sales and 5% of HETLIOZ® net product sales in Germany. Third-party royalty costs on HETLIOZ® net product sales in the U.S. decreased from 10% to 5% in December 2022 and ended in April 2024. There were no-third party royalty costs on net sales of PONVORY®. We evaluate the risk of excess inventory and product expiry by evaluating current and future product demand relative to product shelf life and build demand forecasts by considering factors such as, but not limited to, overall market potential, market share, market acceptance, patient usage, and generic competition. Our inventory balance consisted of $2.0 million of Fanapt® product, $7.3 million of HETLIOZ® product and $0.2 million of PONVORY® product as of December 31, 2024. Our inventory balance consisted of $3.0 million of Fanapt® product and $7.2 million of HETLIOZ® product as of December 31, 2023.
Research and development expenses. Research and development expenses decreased by $2.4 million, or 3%, to $74.4 million for the year ended December 31, 2024 compared to $76.8 million for the year ended December 31, 2023. The decrease was primarily due to a decrease associated with our Fanapt®, tradipitant and other development programs, which include expenses incurred on product discovery such as ASO, partially offset by an increase in expenses associated with our PONVORY® and CFTR development programs.
The following table summarizes the costs of our product development initiatives for the years ended December 31, 2024 and 2023.
| | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
| Direct project costs (1) | | | |
Fanapt® | $ | 9,401 | | | $ | 11,306 | |
| Milsaperidone | 6,872 | | | 6,186 | |
HETLIOZ® | 10,528 | | | 8,978 | |
PONVORY® | 5,300 | | | 839 | |
| Tradipitant | 23,608 | | | 32,781 | |
| CFTR | 6,344 | | | 1,490 | |
| VTR-297 | 2,642 | | | 1,595 | |
| VQW-765 | 743 | | | 988 | |
| Other | 1,272 | | | 4,844 | |
| Total direct project costs | 66,710 | | | 69,007 | |
| Indirect project costs (1) | | | |
| Stock-based compensation | 2,960 | | | 3,323 | |
| Other indirect overhead | 4,761 | | | 4,493 | |
| Total indirect project costs | 7,721 | | | 7,816 | |
| Total research and development expense | $ | 74,431 | | | $ | 76,823 | |
(1)We record direct costs, including personnel costs and related benefits, on a project-by-project basis. Many of our research and development costs are not attributable to any individual project because we share resources across several
development projects. We record indirect costs that support a number of our research and development activities in the aggregate, including stock-based compensation.
We expect to incur significant research and development expenses as we continue to develop our products. In addition, we expect to incur licensing costs in the future that could be substantial, as we continue our efforts to expand our product pipeline.
Selling, general and administrative expenses. Selling, general and administrative expenses increased by $33.5 million, or 30%, to $146.4 million for the year ended December 31, 2024 compared to $112.9 million for the year ended December 31, 2023. The increase in selling, general and administrative expenses was primarily the result of an increase in spending on commercial activities related to our commercial launches of Fanapt® in bipolar disorder and PONVORY® in RMS, as well as legal and other corporate activities. During 2024, we commenced a host of commercial activities as part of our commercial launches of Fanapt® in bipolar disorder and PONVORY® in RMS, including an expansion of our sales force and the development of prescriber awareness and comprehensive marketing programs. Selling, general and administrative expenses may increase in future periods as a result of these ongoing commercial launches, which were initiated in the third quarter of 2024.
Intangible asset amortization. Intangible asset amortization was $7.3 million for the year ended December 31, 2024 compared to $2.1 million for the year ended December 31, 2023. Amortization expense increased in 2024 due to amortization on the intangible asset from the rights to PONVORY® in the U.S. and Canada which were acquired in December 2023.
Other income, net. Other income, net was $17.7 million for the year ended December 31, 2024 compared to $20.3 million for the year ended December 31, 2023. Other income primarily consists of investment income on our marketable securities.
Provision for income taxes. We recorded an income tax benefit of $4.0 million and provision for income taxes of $3.8 million for the years ended December 31, 2024 and 2023, respectively. Our income tax expense or benefit is determined by applying the statutory tax rates in jurisdictions where we operate to each period’s income before income taxes. Adjustments are made for permanent differences in taxability or deductibility of pretax items as well as for other items, such as tax credits that are generated from our research and development activities. See Note 15, Income Taxes, to the consolidated financial statements in Part II, Item 8 of this Annual Report for additional information.
Liquidity and Capital Resources
As of December 31, 2024, our total cash and cash equivalents and marketable securities were $374.6 million compared to $388.3 million at December 31, 2023. Our cash and cash equivalents are deposits in operating accounts and highly liquid investments with an original maturity of 90 days or less at date of purchase and consist of investments in money market funds with commercial banks and financial institutions, and commercial paper of high-quality corporate issuers. Our marketable securities consist of investments in government-sponsored and corporate enterprises and commercial paper.
Our liquidity resources as of December 31, 2024 and 2023 are summarized as follows:
| | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 |
| Cash and cash equivalents | $ | 102,316 | | | $ | 135,821 | |
| Marketable securities: | | | |
| U.S. Treasury and government agencies | 227,830 | | | 185,115 | |
| Corporate debt | 44,497 | | | 67,328 | |
| | | |
| Total marketable securities | 272,327 | | | 252,443 | |
| Total cash, cash equivalents and marketable securities | $ | 374,643 | | | $ | 388,264 | |
As of December 31, 2024, we maintained all of our cash, cash equivalents and marketable securities in two financial institutions. Deposits held with these institutions may exceed the amount of insurance provided on such deposits, but we do not anticipate any losses with respect to such deposits.
In the normal course of our business, we regularly enter into agreements with third-party vendors under fee service arrangements which generally may be terminated on 90 days’ notice without incurring additional charges, other than charges for work completed or materials procured but not paid for through the effective date of termination and other costs incurred by our contractors in closing out work in progress as of the effective date of termination. Our non-cancellable purchase commitments for agreements longer than one year primarily relate to commitments for data services. Various other long-term agreements entered into for services with other third-party vendors, such as inventory purchase commitments, are cancellable in nature or contain variable commitment terms within the agreement that are within our control.
We also have long-term contractual obligations related to our leases and license agreements. See Note 8, Leases, and Note 11, Commitments and Contingencies, respectively, to the consolidated financial statements in Part II, Item 8 of this Annual Report for more information about these commitments.
We do not have any off-balance sheet arrangements.
Based on our current operating plans, which include costs and expenses in connection with our U.S. commercial activities, continued clinical development of tradipitant and our other products, pursuit of regulatory approval of tradipitant, pursuit of further regulatory approvals for our currently approved products, and payments due upon achievement of milestones under our license agreements, we believe that our cash, cash equivalents and marketable securities and cash received from product sales will be sufficient for at least the next 12 months. Our future cash requirements and the adequacy of our available funds will depend on many factors, primarily including our ability to generate revenue, the scope and costs of our commercial, manufacturing and process development activities, the magnitude of our discovery, preclinical and clinical development programs, and potential costs to acquire or license the rights to additional products.
We may need or desire to obtain additional capital to finance our operations through debt, equity or alternative financing arrangements. We may also seek capital through collaborations or partnerships with other companies. The issuance of debt could require us to grant liens on certain of our assets that may limit our flexibility and debt securities may be convertible into common stock. If we raise additional capital by issuing equity securities, the terms and prices for these financings may be much more favorable to the new investors than the terms obtained by our existing stockholders. These financings may also significantly dilute the ownership of our existing stockholders. If we are unable to obtain additional financing, we may be required to reduce the scope of our future activities, which could harm our business, financial condition and operating results. There can be no assurance that any additional financing required in the future will be available on acceptable terms, if at all.
Cash Flow
The following table summarizes our net cash flows from operating, investing and financing activities for the years ended December 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | Net Change | | |
| Net cash provided by (used in): | | | | | | | |
| Operating activities: | | | | | | | |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | (21,409) | | | |
| Non-cash charges | 9,701 | | | 11,039 | | | (1,338) | | | |
| Net change in operating assets and liabilities | (6,558) | | | (747) | | | (5,811) | | | |
| Operating activities | (15,757) | | | 12,801 | | | (28,558) | | | |
| Investing activities: | | | | | | | |
| | | | | | | |
| Asset acquisition | (4,229) | | | (100,665) | | | 96,436 | | | |
| Purchases of property and equipment | (490) | | | (383) | | | (107) | | | |
| Net purchases, sales and maturities of marketable securities | (12,711) | | | 88,992 | | | (101,703) | | | |
| Investing activities | (17,430) | | | (12,056) | | | (5,374) | | | |
| Financing activities: | | | | | | | |
| | | | | | | |
| Principal payments on finance leases | (155) | | | — | | | (155) | | | |
| | | | | | | |
| Financing activities | (155) | | | — | | | (155) | | | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (163) | | | 47 | | | (210) | | | |
| Net change in cash, cash equivalents and restricted cash | $ | (33,505) | | | $ | 792 | | | $ | (34,297) | | | |
Operating Activities. Cash flows used in operating activities during the year ended December 31, 2024 were $15.8 million, a decrease of $28.6 million compared to cash provided by operating activities of $12.8 million during the year ended December 31, 2023. The decrease reflects a decrease of $21.4 million in net income, a decrease of $1.3 million in non-cash charges primarily due to an increase in deferred income taxes, partially offset by higher intangible asset amortization due to the PONVORY® acquisition, and a decrease of $5.8 million from the net change in operating assets and liabilities. The decrease from net change in operating assets and liabilities includes an increase in accounts receivable, product revenue allowances, and prepaid expenses due to timing.
Investing Activities. Cash flows used in investing activities during the year ended December 31, 2024 were $17.4 million, a decrease in cash of $5.4 million compared to $12.1 million during the year ended December 31, 2023.
Financing Activities. Financing activities include principal payments for our finance lease liabilities and exercise of stock options. Cash flows provided by financing activities during the year ended December 31, 2024 were $0.2 million. There were no principal payments on finance leases during the year ended December 31, 2023 or exercises of stock options during the years ended December 31, 2024 and 2023.
| | | | | |
| ITEM 7A. | QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK |
Our exposure to market risk is currently confined to our cash and cash equivalents, marketable securities and restricted cash. We currently do not hedge interest rate exposure. We have not used derivative financial instruments for speculation or trading purposes.
We deposit our cash with financial institutions that we consider to be of high credit quality and purchase marketable securities that are generally investment grade, liquid, short-term fixed income securities and money-market instruments denominated in U.S. dollars. Our marketable securities consist of commercial paper, corporate notes and U.S. government agency notes and have maturities of less than two years. We do not believe that an increase in market rates would have any significant impact on the realized value of our cash equivalents and marketable securities.
We are also exposed to risks related to changes in foreign currency exchange rates relating to our foreign operations. The functional currency of our international subsidiaries is the local currency. We are exposed to foreign currency risk to the extent that we enter into transactions denominated in currencies other than our subsidiaries’ respective functional currencies. We are also exposed to unfavorable fluctuations of the U.S. dollar, which is our reporting currency, against the currencies of our operating subsidiaries when their respective financial statements are translated into U.S. dollars for inclusion in our consolidated financial statements. We do not currently hedge our foreign currency exchange rate risk. Foreign currency has not had, nor do we believe that a decrease or increase in any foreign currency exchange rates would have, a material impact on our results of operations.
| | | | | |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The consolidated financial statements and related financial statement schedules required to be filed are listed in the Index to Consolidated Financial Statements and are incorporated in Part IV, Item 15 of this annual report on Form 10-K.
| | | | | |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| | | | | |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including the Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (Exchange Act)) as of December 31, 2024. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective as of December 31, 2024, the end of the period covered by this annual report on Form 10-K (Annual Report), to ensure that the information required to be disclosed by us in the reports that we file or submit under the
Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosures.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as defined in the Exchange Act Rule 13a-15(f). Management conducted an assessment of our internal control over financial reporting based on the original framework established in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control — Integrated Framework. Based on the assessment, management concluded that, as of December 31, 2024, our internal control over financial reporting was effective. The effectiveness of our internal control over financial reporting as of December 31, 2024 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report included in this Annual Report.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the fourth quarter of 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| | | | | |
| ITEM 9B. | OTHER INFORMATION |
During the fiscal quarter ended December 31, 2024, none of our directors or officers informed us of the adoption, modification or termination of a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as those terms are defined in Regulation S-K, Item 408. Furthermore, during the fiscal quarter ended December 31, 2024, we did not adopt or terminate a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as those terms are defined in Regulation S-K, Item 408.
| | | | | |
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
PART III
| | | | | |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information required under this item will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference.
| | | | | |
| ITEM 11. | EXECUTIVE COMPENSATION |
Information required under this item will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference, except that information required by Item 407(e)(5) of Regulation S-K will be deemed furnished in this Form 10-K and will not be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference into such filing.
| | | | | |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information required under this item will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference.
Securities Authorized for Issuance under Equity Incentive Plans
Information regarding securities authorized for issuance under equity incentive plans will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference pursuant to General Instruction G(3) to Form 10-K.
| | | | | |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information required under this item will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference.
| | | | | |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Information required under this item will be contained in our Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2024 and is incorporated herein by reference.
PART IV
| | | | | |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The consolidated financial statements filed as part of this annual report on Form 10-K are listed in the Index to Consolidated Financial Statements. Certain schedules are omitted because they are not applicable, or not required, or because the required information is included in the consolidated financial statements or notes thereto. The Exhibits are listed in the Exhibit Index.
| | | | | |
| ITEM 16. | Form 10-K Summary |
None.
Signatures
Pursuant to the requirements of Section 13 and 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this annual report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | Vanda Pharmaceuticals Inc. |
| | | | |
| February 14, 2025 | | By: | | /s/ Mihael H. Polymeropoulos, M.D. |
| | | | Mihael H. Polymeropoulos, M.D. |
| | | | President, Chief Executive Officer and Chairman of the Board of Directors |
Pursuant to the requirements of the Securities Exchange Act of 1934, this annual report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | | | |
| /s/ Mihael H. Polymeropoulos, M.D. | | President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) | | February 14, 2025 |
| Mihael H. Polymeropoulos, M.D. | | |
| | | | |
| /s/ Kevin Moran | | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | | February 14, 2025 |
| Kevin Moran | | |
| | | | |
| /s/ Richard W. Dugan | | Lead Independent Director | | February 14, 2025 |
| Richard W. Dugan | | |
| | | | |
| /s/ Anne Sempowski Ward | | Director | | February 14, 2025 |
| Anne Sempowski Ward | | |
| | | | |
| /s/ Phaedra Chrousos | | Director | | February 14, 2025 |
| Phaedra Chrousos | | |
| | | | |
| /s/ Stephen Ray Mitchell | | Director | | February 14, 2025 |
| Stephen Ray Mitchell | | |
| | | | |
| /s/ Tage Honoré | | Director | | February 14, 2025 |
| Tage Honoré | | |
| | | | |
Vanda Pharmaceuticals Inc.
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Vanda Pharmaceuticals Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Vanda Pharmaceuticals Inc. and its subsidiaries (the “Company”) as of December 31, 2024 and 2023, and the related consolidated statements of operations, of comprehensive income (loss), of changes in stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2024, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Allowances for the Medicaid Drug Rebate Program
As described in Note 2 to the consolidated financial statements, the allowances for rebates include mandated discounts under the Medicaid Drug Rebate Program as well as contracted rebate programs with other payors. Rebate amounts owed after the final dispensing of the product to a benefit plan participant are based upon contractual agreements or legal requirements with public sector benefit providers, such as Medicaid and Medicare. Allowances for rebates are based upon the insurance benefits of the end customer, which are estimated using historical activity. The allowance for rebates is based on statutory or contracted discount rates and estimated patient utilization. The Company has recorded product revenue allowances of $60.9 million as of December 31, 2024, of which a significant portion relates to allowances for Medicaid drug rebates.
The principal considerations for our determination that performing procedures relating to the allowances for the Medicaid Drug Rebate Program is a critical audit matter are (i) the significant judgment by management due to the significant measurement uncertainty when developing the estimate of the allowances and (ii) a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating assumptions related to historical activity, statutory or contracted discount rates, and estimated patient utilization.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the allowances for the Medicaid Drug Rebate Program. These procedures also included, among others, (i) developing an independent estimate of the allowances for Medicaid drug rebates by utilizing third-party information related to patient utilization and historical activity; (ii) comparing the independent estimate to management’s estimate to evaluate the reasonableness of management’s estimate; and (iii) testing, on a sample basis, rebate claims processed by the Company, including evaluating those claims for consistency with the statutory or contractual terms of the Medicaid Drug Rebate Program.
Income Taxes - Realizability of U.S. Federal Deferred Tax Assets
As described in Note 15 to the consolidated financial statements, the Company has approximately $81.4 million of net deferred tax assets as of December 31, 2024, of which a significant portion relates to the U.S. federal jurisdiction. The Company assesses the need for a valuation allowance against its deferred tax assets each quarter through the review of all available positive and negative evidence. Deferred tax assets are reduced by a tax valuation allowance when, in the opinion of management, it is more likely than not that some portion of the deferred tax assets will not be realized. The analysis is highly dependent upon historical and projected pretax income. As of December 31, 2024, after considering all available positive and negative evidence, including but not limited to cumulative income in recent periods, historical, current and future projected results and significant risks and uncertainties related to forecasts, the Company concluded, consistent with prior periods, that it is more likely than not that substantially all of its deferred tax assets in the U.S. are realizable in future periods.
The principal considerations for our determination that performing procedures relating to the realizability of U.S. federal deferred tax assets is a critical audit matter are (i) the significant judgment by management in determining whether the U.S. federal deferred tax assets are more likely than not to be realized in the future and (ii) a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating audit evidence relating to management’s assessment of the realizability of U.S. federal deferred tax assets.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s assessment of the realizability of U.S. federal deferred tax assets, including controls over projected results, including projected pretax income. These procedures also included, among others, (i) evaluating the positive and negative
evidence available to support management’s assessment of the realizability of U.S. federal deferred tax assets; (ii) testing the completeness and accuracy of underlying data used in management’s assessment; and (iii) evaluating the reasonableness of management’s projected results, including projected pretax income. Evaluating the reasonableness of management’s projected results of the Company involved considering (i) the current and past performance of the Company; (ii) the consistency with external market and industry data; and (iii) the consistency with evidence obtained in other areas of the audit.
/s/ PricewaterhouseCoopers LLP
Washington, District of Columbia
February 14, 2025
We have served as the Company’s auditor since 2003.
VANDA PHARMACEUTICALS INC.
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | | | |
| (in thousands, except for share and per share amounts) | December 31, 2024 | | December 31, 2023 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 102,316 | | | $ | 135,821 | |
| Marketable securities | 272,327 | | | 252,443 | |
| Accounts receivable, net | 47,101 | | | 34,155 | |
| Inventory | 1,726 | | | 1,357 | |
| Prepaid expenses and other current assets | 15,420 | | | 9,170 | |
| Total current assets | 438,890 | | | 432,946 | |
| Property and equipment, net | 2,132 | | | 2,037 | |
| Operating lease right-of-use assets | 5,602 | | | 7,103 | |
| Finance lease right-of-use assets | 4,943 | | | — | |
| Intangible assets, net | 114,096 | | | 121,369 | |
| Deferred tax assets | 81,440 | | | 75,000 | |
| Non-current inventory and other | 9,101 | | | 9,985 | |
| Total assets | $ | 656,204 | | | $ | 648,440 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| Current liabilities: | | | |
| Accounts payable and accrued liabilities | $ | 39,086 | | | $ | 38,460 | |
| Product revenue allowances | 60,895 | | | 49,237 | |
| | | |
| Total current liabilities | 99,981 | | | 87,697 | |
| Operating lease non-current liabilities | 4,944 | | | 7,006 | |
| Finance lease non-current liabilities | 3,146 | | | — | |
| Other non-current liabilities | 9,587 | | | 8,827 | |
| Total liabilities | 117,658 | | | 103,530 | |
| Commitments and contingencies (Notes 11 and 18) | | | |
| Stockholders’ equity: | | | |
| Preferred stock, $0.001 par value; 20,000,000 shares authorized, and no shares issued or outstanding at December 31, 2024 and 2023, respectively | — | | | — | |
| Common stock, $0.001 par value; 150,000,000 shares authorized; 58,310,644 and 57,534,499 shares issued and outstanding at December 31, 2024 and 2023, respectively | 58 | | | 58 | |
| Additional paid-in capital | 712,706 | | | 700,274 | |
| Accumulated other comprehensive income (loss) | 74 | | | (30) | |
| Accumulated deficit | (174,292) | | | (155,392) | |
| Total stockholders’ equity | 538,546 | | | 544,910 | |
| Total liabilities and stockholders’ equity | $ | 656,204 | | | $ | 648,440 | |
The accompanying notes are an integral part of these consolidated financial statements.
VANDA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands, except for share and per share amounts) | 2024 | | 2023 | | 2022 |
| Revenues: | | | | | |
| Net product sales | $ | 198,772 | | | $ | 192,640 | | | $ | 254,382 | |
| Total revenues | 198,772 | | | 192,640 | | | 254,382 | |
| Operating expenses: | | | | | |
| Cost of goods sold excluding amortization | 11,314 | | | 14,796 | | | 24,282 | |
| Research and development | 74,431 | | | 76,823 | | | 85,770 | |
| Selling, general and administrative | 146,414 | | | 112,883 | | | 136,485 | |
| Intangible asset amortization | 7,273 | | | 2,090 | | | 1,516 | |
| Total operating expenses | 239,432 | | | 206,592 | | | 248,053 | |
| Income (loss) from operations | (40,660) | | | (13,952) | | | 6,329 | |
| Other income, net | 17,739 | | | 20,291 | | | 4,971 | |
| Income (loss) before income taxes | (22,921) | | | 6,339 | | | 11,300 | |
| Provision (benefit) for income taxes | (4,021) | | | 3,830 | | | 5,025 | |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | 6,275 | |
| Net income (loss) per share: | | | | | |
| Basic | $ | (0.33) | | | $ | 0.04 | | | $ | 0.11 | |
| Diluted | $ | (0.33) | | | $ | 0.04 | | | $ | 0.11 | |
| Weighted average shares outstanding: | | | | | |
| Basic | 58,149,087 | | | 57,380,975 | | | 56,461,877 | |
| Diluted | 58,149,087 | | | 57,557,911 | | | 56,983,171 | |
The accompanying notes are an integral part of these consolidated financial statements.
VANDA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | 6,275 | |
| Other comprehensive income (loss): | | | | | |
| Net foreign currency translation gain (loss) | (34) | | | 28 | | | (39) | |
| Change in net unrealized gain (loss) on marketable securities | 178 | | | 1,461 | | | (1,271) | |
| Tax benefit (provision) on other comprehensive income (loss) | (40) | | | (326) | | | 292 | |
| Other comprehensive income (loss), net of tax | 104 | | | 1,163 | | | (1,018) | |
| Comprehensive income (loss) | $ | (18,796) | | | $ | 3,672 | | | $ | 5,257 | |
The accompanying notes are an integral part of these consolidated financial statements.
VANDA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-in Capital | | Accumulated Other
Comprehensive
Income (Loss) | | Accumulated
Deficit | | Total |
| (in thousands, except for share amounts) | Shares | | Par Value | | | | |
| Balances at December 31, 2021 | 55,900,855 | | | $ | 56 | | | $ | 669,223 | | | $ | (175) | | | $ | (164,176) | | | $ | 504,928 | |
| | | | | | | | | | | |
| Issuance of common stock from the exercise of stock options and settlement of restricted stock units | 882,909 | | | 1 | | | 733 | | | — | | | — | | | 734 | |
| Stock-based compensation expense | — | | | — | | | 16,279 | | | — | | | — | | | 16,279 | |
| Net income | — | | | — | | | — | | | — | | | 6,275 | | | 6,275 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (1,018) | | | — | | | (1,018) | |
| Balances at December 31, 2022 | 56,783,764 | | | 57 | | | 686,235 | | | (1,193) | | | (157,901) | | | 527,198 | |
| Issuance of common stock from the exercise of stock options and settlement of restricted stock units | 750,735 | | | 1 | | | (1) | | | — | | | — | | | — | |
| Stock-based compensation expense | — | | | — | | | 14,040 | | | — | | | — | | | 14,040 | |
| Net income | — | | | — | | | — | | | — | | | 2,509 | | | 2,509 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 1,163 | | | — | | | 1,163 | |
| Balances at December 31, 2023 | 57,534,499 | | | 58 | | | 700,274 | | | (30) | | | (155,392) | | | 544,910 | |
| | | | | | | | | | | |
| Issuance of common stock from the exercise of stock options and settlement of restricted stock units | 776,145 | | | — | | | — | | | — | | | — | | | — | |
| Stock-based compensation expense | — | | | — | | | 12,432 | | | — | | | — | | | 12,432 | |
| Net loss | — | | | — | | | — | | | — | | | (18,900) | | | (18,900) | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 104 | | | — | | | 104 | |
| Balances at December 31, 2024 | 58,310,644 | | | $ | 58 | | | $ | 712,706 | | | $ | 74 | | | $ | (174,292) | | | $ | 538,546 | |
The accompanying notes are an integral part of these consolidated financial statements.
VANDA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Cash flows from operating activities | | | | | |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | 6,275 | |
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | |
| Depreciation of property and equipment | 859 | | | 920 | | | 1,217 | |
| Stock-based compensation | 12,432 | | | 14,040 | | | 16,279 | |
| Amortization of premiums and accretion of discounts on marketable securities | (6,994) | | | (8,799) | | | (2,963) | |
| Loss (gain) on sales of marketable securities | — | | | 655 | | | — | |
| Intangible asset amortization | 7,273 | | | 2,090 | | | 1,516 | |
| Deferred income taxes | (6,482) | | | (1,286) | | | 1,130 | |
| Other non-cash adjustments, net | 2,613 | | | 3,419 | | | 2,456 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | (13,012) | | | (707) | | | (1,089) | |
| Prepaid expenses and other assets | (6,816) | | | 8,523 | | | (6,136) | |
| Inventory | (29) | | | (771) | | | (4,479) | |
| Accounts payable and other liabilities | 1,708 | | | (10,984) | | | 11,793 | |
| Product revenue allowances | 11,591 | | | 3,192 | | | 5,985 | |
| Net cash provided by (used in) operating activities | (15,757) | | | 12,801 | | | 31,984 | |
| Cash flows from investing activities | | | | | |
| | | | | |
| Asset acquisition | (4,229) | | | (100,665) | | | — | |
| Purchases of property and equipment | (490) | | | (383) | | | (679) | |
| Purchases of marketable securities | (364,773) | | | (512,606) | | | (349,258) | |
| Sales and maturities of marketable securities | 352,062 | | | 601,598 | | | 399,862 | |
| Net cash provided by (used in) investing activities | (17,430) | | | (12,056) | | | 49,925 | |
| Cash flows from financing activities | | | | | |
| Principal payments on finance leases | (155) | | | — | | | — | |
| | | | | |
| Proceeds from exercise of stock options | — | | | — | | | 734 | |
| Net cash provided by (used in) financing activities | (155) | | | — | | | 734 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (163) | | | 47 | | | 265 | |
| Net change in cash, cash equivalents and restricted cash | (33,505) | | | 792 | | | 82,908 | |
| Cash, cash equivalents and restricted cash | | | | | |
| Beginning of year | 136,290 | | | 135,498 | | | 52,590 | |
| End of year | $ | 102,785 | | | $ | 136,290 | | | $ | 135,498 | |
The accompanying notes are an integral part of these consolidated financial statements.
VANDA PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Organization and Presentation
Business Organization
Vanda Pharmaceuticals Inc. (the Company or Vanda) is a global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. The Company commenced its operations in 2003.
The Company’s commercial portfolio is currently comprised of three products: Fanapt® for the acute treatment of manic or mixed episodes associated with bipolar I disorder and the treatment of schizophrenia, HETLIOZ® for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) and for the treatment of nighttime sleep disturbances in Smith-Magenis syndrome (SMS) and PONVORY® for the treatment of relapsing forms of multiple sclerosis (RMS) including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. HETLIOZ® is the first product approved by the United States Food and Drug Administration (FDA) for patients with Non-24 and for patients with SMS. In addition, the Company has a number of drugs and/or additional indications for current products in development, including:
•Fanapt® (iloperidone) long acting injectable (LAI) formulation for the treatment of schizophrenia;
•BysantiTM (milsaperidone), the active metabolite of Fanapt® (iloperidone), for the acute treatment of manic or mixed episodes associated with bipolar I disorder and for the treatment of schizophrenia and major depressive disorder (MDD);
•HETLIOZ® (tasimelteon) for the treatment of jet lag disorder, insomnia, pediatric insomnia, delayed sleep phase disorder (DSPD) and pediatric Non-24;
•PONVORY® (ponesimod) for the treatment of psoriasis and ulcerative colitis;
•Tradipitant (VLY-686), a small molecule neurokinin-1 (NK-1) receptor antagonist, for the treatment of gastroparesis, motion sickness and atopic dermatitis;
•Imsidolimab, an IL-36R antagonist, for the treatment of generalized pustular psoriasis (GPP);
•VTR-297, a small molecule histone deacetylase (HDAC) inhibitor for the treatment of onychomycosis and hematologic malignancies and with potential use as a treatment for several oncology indications;
•Portfolio of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) activators and inhibitors, including VSJ-110 for the treatment of dry eye and ocular inflammation and VPO-227 for the treatment of secretory diarrhea disorders, including cholera;
•VQW-765, a small molecule nicotinic acetylcholine receptor partial agonist, for the treatment of social/performance anxiety and psychiatric disorders; and
•Antisense oligonucleotide (ASO) molecules, including VCA-894A for the treatment of Charcot-Marie-Tooth Disease, Type 2S (CMT2S), caused by cryptic slice site variants within the IGHMBP2 gene and VGT-1849A for the treatment of polycythemia vera (PV), a form of a rare hematologic malignancy.
Basis of Presentation
The accompanying consolidated financial statements include the accounts of Vanda Pharmaceuticals Inc. and its wholly owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP). All intercompany accounts and transactions have been eliminated in consolidation.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenue and expenses during the reported periods. Management continually re-
evaluates its estimates, judgments and assumptions, and management’s evaluation could change. Actual results could differ from those estimates under different assumptions or conditions.
Cash, Cash Equivalents and Restricted Cash
For purposes of the Consolidated Balance Sheets and Consolidated Statements of Cash Flows, cash equivalents represent highly-liquid investments with a maturity date of three months or less at the date of purchase. Cash and cash equivalents include investments in money market funds with commercial banks and financial institutions, and commercial paper of high-quality corporate issuers. Restricted cash relates primarily to amounts held as collateral for letters of credit for office space leases at the Company’s Washington, D.C. headquarters.
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the Consolidated Balance Sheets to the total end of period cash, cash equivalents and restricted cash reported within the Consolidated Statements of Cash Flows for the years ended December 31, 2024 and 2023:
| | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 |
| Cash and cash equivalents | $ | 102,316 | | | $ | 135,821 | |
| | | |
| Restricted cash included in non-current inventory and other | 469 | | | 469 | |
| Total cash, cash equivalents and restricted cash | $ | 102,785 | | | $ | 136,290 | |
Marketable Securities
The Company classifies all of its marketable securities as available-for-sale securities. The Company’s investment policy requires the selection of high-quality issuers. Available-for-sale securities are carried at fair market value, with unrealized gains and losses reported as a component of stockholders’ equity in accumulated other comprehensive income (loss). At each balance sheet date, the Company assesses available-for-sale securities in an unrealized loss position to determine whether it intends to sell or if it is more likely than not that the Company will be required to sell the security before recovery of its amortized cost basis. If either of the criteria regarding intent or requirement to sell is met, the security’s amortized cost basis is written down to fair value. The Company also reviews its available-for-sale securities in an unrealized loss position to determine whether the unrealized loss is the result of a change in creditworthiness or other factors. If declines in the value of available for-sale securities are determined to be credit-related, a loss is recorded in earnings in the current period. Interest and dividend income is recorded when earned and included in other income. Premiums and discounts on marketable securities are amortized and accreted, respectively, to earliest call date and maturity, respectively, and included in other income. The Company uses the specific identification method in computing realized gains and losses on the sale of investments, which would be included in the Consolidated Statements of Operations when generated. All available-for-sale marketable securities are available for use in current operations and are classified as current.
Inventory
Inventory, which is recorded at the lower of cost or net realizable value, includes the cost of third-party manufacturing and other direct and indirect costs and is valued using the first-in, first-out method. The Company evaluates the risk of excess inventory and product expiry by evaluating current and future product demand relative to product shelf life, taking into account all possible alternative uses for the inventory available in the ordinary course of business. The Company builds demand forecasts by considering factors such as, but not limited to, overall market potential, market share, market acceptance, patient usage and generic competition. The Company capitalizes inventory costs associated with its products upon regulatory approval when, based on management’s judgment, future commercialization is considered probable and the future economic benefit is expected to be realized; otherwise, such costs are expensed as research and development. Inventory levels are evaluated for the amount of inventory that would be sold within one year. At certain times, the level of inventory can exceed the forecasted level of cost of goods sold for the next 12 months. The Company classifies the estimate of such inventory as non-current.
Asset Acquisitions
The Company evaluates acquisitions of assets and other similar transactions to assess whether or not the transaction should be accounted for as a business combination or asset acquisition by first applying a screen to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets. If the screen is met, the transaction is accounted for as an asset acquisition. If determined to be an asset acquisition, the Company accounts for the transaction under Accounting Standards Codification (ASC) 805-50, which requires the recognition of assets acquired and liabilities assumed on a relative fair value basis based on the acquisition cost, which includes transaction costs in addition to consideration given. Any excess consideration transferred over the fair value of the net assets acquired is allocated to the identifiable assets based on relative fair values.
See Note 3, PONVORY® Acquisition, for further discussion of the Company’s acquisition of the United States (U.S.) and Canadian rights to PONVORY® from Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company, which the Company accounted for as an asset acquisition under ASC 805-50.
Intangible Assets
Costs incurred for products not yet approved by the FDA and for which no alternative future use exists are recorded as research and development expense. Obligations for milestone payments to other pharmaceutical companies that may result in a capitalized intangible asset are recognized when it is deemed probable that the milestone event will occur. In the event a product has been approved by the FDA or an alternative future use exists for a product, patent and license costs are capitalized and amortized on a straight-line basis over the estimated useful economic life of the related product patents. For intangible assets related to HETLIOZ®, the estimated useful life is through 2035, which is the estimated economic useful life of the related product patents.
Useful lives for acquired intangible assets accounted for under ASC 805 are generally estimated based on the market participant methodology. For intangible assets related to PONVORY®, the estimated useful life is through 2042, which is the estimated economic useful life of the related acquired product patents. Intangible assets related to Fanapt® have been fully amortized on a straight-line basis to 2016. The Fanapt® transaction represented reacquired rights, and therefore did not reflect the impact of additional Fanapt® patents solely owned by the Company with varying expiration dates, the latest of which is December 2031.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation of most property and equipment is provided on a straight-line basis over the estimated useful lives of the assets. Leasehold improvements are amortized using a straight-line basis over the lesser of the estimated useful lives of the assets or the terms of the related leases. The costs of additions and improvements are capitalized, and repairs and maintenance costs are charged to operations in the period incurred. Upon retirement or disposition of property and equipment, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is reflected in the Consolidated Statement of Operations for that period.
Leases
The Company determines if an arrangement contains a lease at inception. Right-of-use (ROU) assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from that lease. For leases with a term greater than 12 months, ROU assets and liabilities are recognized at the lease commencement date based on the estimated present value of lease payments over the lease term. The lease term includes the option to extend the lease when it is reasonably certain the Company will exercise that option. When available, the Company uses the rate implicit in the lease to discount lease payments to present value. When the implicit rate is not available, the Company uses its incremental borrowing rate based on information available at the lease commencement date, including publicly available data for instruments with similar characteristics, to determine the present value of lease payments. The Company does not combine lease and non-lease elements for office or vehicle leases.
Impairment of Long-Lived Assets
The Company evaluates if events and circumstances have occurred that indicate the remaining estimated useful life of its long-lived assets may warrant revision or that the remaining balance of these assets may not be recoverable. In evaluating for recoverability, the Company estimates the future undiscounted cash flows expected to result from the use of the assets and their eventual disposition. In the event that the balance of any asset exceeds the future undiscounted or discounted cash flow estimate, impairment is recognized based on the excess of the carrying amounts of the asset above its estimated fair value. No impairment was recognized for the years ended December 31, 2024, 2023 and 2022.
Accounts Payable and Accrued Liabilities
The Company’s management is required to estimate accrued liabilities as part of the process of preparing financial statements. The estimation of accrued liabilities involves identifying services that have been performed on the Company’s behalf, and then estimating the level of service performed and the associated cost incurred for such services as of each balance sheet date in the financial statements. Accrued liabilities include research and development expenses, such as accrued costs under contracts with clinical monitors, data management organizations and investigators in conjunction with clinical trials, fees to contract manufacturers in conjunction with the production of clinical materials, consulting and professional fees, such as lawyers and fees for marketing and other commercialization activities, accrued compensation and employee benefits, such as accrued bonus, royalties payable under licensing agreements, and other accrued fees. Pursuant to management’s assessment of the services that have been performed on clinical trials and other contracts, the Company recognizes these expenses as the services are provided.
Revenue from Net Product Sales
The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance and collectability of consideration is probable. The Company recognizes revenue when control of the product is transferred to the customer in an amount that reflects the consideration the Company expects to be entitled to in exchange for those product sales, which is typically once the product physically arrives at the customer. Sales taxes, value-added taxes, and usage-based taxes are excluded from revenues.
The Company’s net product sales consist of sales of Fanapt®, HETLIOZ® and PONVORY®. Net sales by product for the years ended December 31, 2024, 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
(in thousands) | 2024 | | 2023 | | 2022 |
Fanapt® net product sales | $ | 94,297 | | | $ | 90,873 | | | $ | 94,727 | |
HETLIOZ® net product sales | 76,675 | | | 100,167 | | | 159,655 | |
PONVORY® net product sales | 27,800 | | | 1,600 | | | — | |
| Total net product sales | $ | 198,772 | | | $ | 192,640 | | | $ | 254,382 | |
The Company’s HETLIOZ® net product sales as reported for the three months ended March 31, 2023 reflected higher unit sales as compared to recent prior periods. The higher unit sales during the three months ended March 31, 2023 resulted in a significant increase of inventory stocking at specialty pharmacy customers at March 31, 2023. During the remainder of 2023, although there was continued destocking at specialty pharmacy customers, inventory levels remained elevated relative to inventory levels prior to the entrance of generic competition and remained elevated throughout 2024. Going forward, HETLIOZ® net product sales may reflect lower unit sales as a result of reduction of the elevated inventory levels at specialty pharmacy customers or may be variable depending upon when specialty pharmacy customers need to purchase again. Further,
HETLIOZ® net product sales will likely decline in future periods, potentially significantly, related to continued generic competition in the U.S. The Company constrained HETLIOZ® net product sales for the years ended December 31, 2024 and 2023 to an amount not probable of significant revenue reversal. The amount of revenue recognized during the year ended December 31, 2024 related to changes in estimates on revenue constrained during 2023 was $1.3 million. HETLIOZ® net product sales could experience variability in future periods as the remaining uncertainties associated with variable consideration related to inventory stocking by specialty pharmacy customers are resolved. The Company's PONVORY® net product sales for the year ended December 31, 2024 include approximately $3.0 million of variable consideration that may be subject to dispute but that the Company believes is not probable of significant revenue reversal.
Fanapt® is available in the U.S. for distribution through a limited number of wholesalers and is available in retail pharmacies. HETLIOZ® is available in the U.S. for distribution through a limited number of specialty pharmacies and is not available in retail pharmacies. PONVORY® is available in the U.S. for distribution primarily through a limited number of specialty distributors and specialty pharmacies. The Company invoices and records revenue when its customers, wholesalers, specialty pharmacies and specialty distributors, receive product from the third-party logistics warehouse, which is the point at which control is transferred to the customer. Revenues and accounts receivable are concentrated with these customers. Outside the U.S., the Company has a distribution agreement for the commercialization of Fanapt® in Israel and sells HETLIOZ® in Germany. Receivables are carried at transaction price, net of allowance for credit losses. Allowance for credit losses is measured using historical loss rates based on the aging of receivables and incorporating current conditions and forward-looking estimates.
The following table presents each major customer that represented more than 10% of total revenues for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| Percent of Net Product Sales | 2024 | | 2023 | | 2022 |
| Customer A | 21 | % | | 15 | % | | * |
| Customer B | 17 | % | | 14 | % | | 11 | % |
| Customer C | 16 | % | | 15 | % | | 11 | % |
| Customer D | 15 | % | | 16 | % | | 13 | % |
| Customer E | * | | 20 | % | | 36 | % |
| Customer F | * | | * | | 16 | % |
| Total net product sales from major customers | 69 | % | | 80 | % | | 87 | % |
*Represents less than 10% of respective balance.
The following table presents each major customer that represented more than 10% of accounts receivable, net, as of December 31, 2024 and 2023:
| | | | | | | | | | | |
| | December 31, |
| Percent of Accounts Receivable, Net | 2024 | | 2023 |
| Customer A | 21 | % | | 34 | % |
| Customer B | 27 | % | | 16 | % |
| Customer C | 18 | % | | 21 | % |
| Customer D | * | | 19 | % |
| Customer E | 18 | % | | * |
| Customer F | * | | * |
| Total accounts receivable, net from major customers | 84 | % | | 90 | % |
*Represents less than 10% of respective balance.
The transaction price is determined based upon the consideration to which the Company will be entitled in exchange for transferring product to the customer. The Company’s product sales are recorded net of applicable product revenue allowances for which reserves are established and include discounts, rebates, chargebacks, service fees, co-pay assistance and product returns that are applicable for various government and commercial payors. Where appropriate, the Company’s estimates of variable consideration included in the transaction price consider a range of possible outcomes. Allowances for rebates, chargebacks and co-pay assistance are based upon the insurance benefits of the end customer, which are estimated using historical activity and, where available, actual and pending prescriptions for which the Company has validated the insurance benefits. Variable consideration may be constrained and is included in the transaction price if, in the Company’s judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Overall, these reserves reflect the Company’s best estimates of the amount of consideration to which it is entitled based on the terms of the respective underlying contracts. If actual results in the future vary from the Company’s estimates, it adjusts its estimates in the period identified, which would affect net product sales in the period such variances become known. During the year ended December 31, 2024, the Company constrained the variable consideration for HETLIOZ® net product sales. The constrained revenue relates to the uncertainties of payor utilization, patient demand and chargeback and rebate amounts, including Medicaid, related to the elevated levels of inventory on hand at the specialty pharmacies.
Reserves for variable consideration are classified as product revenue allowances on the Consolidated Balance Sheets, with the exception of prompt-pay discounts which are classified as reductions of accounts receivable. The reserve for product returns for which the product may not be returned for a period of greater than one year from the balance sheet date is included as a component of other non-current liabilities in the Consolidated Balance Sheets. Uncertainties related to variable consideration are generally resolved in the quarter subsequent to period end, with the exception of Medicaid rebates, which are dependent upon the timing of when states submit reimbursement claims, Medicare inflationary rebates, which are expected to be billed on an annual basis beginning in 2025, and product returns that are resolved during the product expiry period specified in the customer contract. Due to increased inventory stocking at specialty pharmacy customers of HETLIOZ® in 2024 and 2023, the time it takes to resolve these uncertainties could be longer than the Company has historically experienced. The Company currently records sales allowances for the following:
•Prompt-pay: Wholesalers, specialty pharmacies and specialty distributors, the Company’s direct customers, are generally offered discounts for prompt payment. The Company expects that these direct customers will earn prompt payment discounts and, therefore, deducts the full amount of these discounts from total product sales when revenues are recognized.
•Rebates: Allowances for rebates include mandated discounts under the Medicaid Drug Rebate Program as well as contracted rebate programs with other payors, including the Medicare Part D inflationary rebate effective October 1, 2022. Rebate amounts owed after the final dispensing of the product to a benefit plan participant are based upon contractual agreements or legal requirements with public sector benefit providers, such as Medicaid and Medicare. The allowances for rebates are based on statutory or contracted discount rates and estimated patient utilization.
•Chargebacks: Chargebacks are discounts that occur when contracted indirect customers purchase directly from wholesalers, specialty pharmacies and specialty distributors. Contracted indirect customers, which currently consist primarily of Public Health Service institutions and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The wholesaler, specialty pharmacy or specialty distributor, in turn, charges back the difference between the price initially paid by the wholesaler, specialty pharmacy or specialty distributor and the discounted price paid to the wholesaler, specialty pharmacy or specialty distributor by the contracted customer.
•Medicare Part D Rebates: The Medicare Part D prescription drug benefit requires manufacturers to fund approximately 70% of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients for applicable drugs. The Company accounts for the Medicare Part D coverage gap using a point of sale model. Estimates for expected Medicare Part D rebates are based, in part, on historical activity and, where available, actual and pending prescriptions when the Company has validated the insurance benefits. Beginning January 1, 2025, the Medicare Part D coverage gap discount program was replaced with a new discounting program under the Inflation Reduction Act of 2022. The Medicare Part D benefit redesign is expected to result in higher discounts for our Medicare payor segment relative to the current Medicare Part D prescription drug coverage gap discount program. Under the redesigned Medicare Part D program, applicable drugs dispensed to applicable beneficiaries will be subject to manufacturer discounts of 10% during the initial coverage phase and 20% during the catastrophic coverage phase. Manufacturers may be eligible for phase-in of discounts, such as for applicable beneficiaries who are Low Income Subsidy (LIS) eligible under section 1860D-14(a) of the Social
Security Act. The Company is a specified manufacturer whose applicable drugs for applicable beneficiaries who are LIS eligible will be subject to lower applicable discounts during the phase-in period.
•Service Fees: The Company receives sales order management, data and distribution services from certain customers, for which it is assessed fees. These fees are based on contracted terms and are known amounts. The Company accrues service fees at the time of revenue recognition, resulting in a reduction of product sales and the recognition of an accrued liability, unless it is a payment for a distinct good or service from the customer in which case the fair value of those distinct goods or services are recorded as selling, general and administrative expense.
•Co-pay Assistance: Patients who have commercial insurance and meet certain eligibility requirements may receive co-pay assistance. Co-pay assistance utilization is based on information provided by the Company’s third-party administrator.
•Product Returns: The Company generally offers direct customers a limited right to return, as contractually defined with its customers. The Company considers several factors in the estimation process, including expiration dates of product shipped to customers, inventory levels within the distribution channel, product shelf life, historical return activity, including activity for product sold for which the return period has past, prescription trends and other relevant factors. The Company does not expect returned products to be resalable. There was no right of return asset as of December 31, 2024 or 2023. The following table summarizes activity for product returns as of and for the years ended December 31, 2024, 2023 and 2022, all of which relates to sales of Fanapt®:
| | | | | |
| (in thousands) | Reserve for Product Returns |
| Balances at December 31, 2021 | $ | 4,551 | |
| Additions | 4,332 | |
| Credits/payments | (3,739) | |
| Balances at December 31, 2022 | 5,144 | |
| Additions | 3,001 | |
| Credits/payments | (2,934) | |
| Balances at December 31, 2023 | 5,211 | |
| Additions | 2,780 | |
| Credits/payments | (3,060) | |
| Balances at December 31, 2024 | $ | 4,931 | |
Cost of Goods Sold
Cost of goods sold includes royalties payable, the cost of inventory sold, costs to write down inventory to net realizable value, manufacturing and supply chain costs and product shipping and handling costs related to sales of HETLIOZ® and Fanapt® to the Company’s distribution partners.
Research and Development Expenses
Research and development expenses consist primarily of fees for services provided by third parties in connection with the clinical trials, costs of contract manufacturing services for clinical trial use, milestone payments made under licensing agreements prior to regulatory approval, costs of materials used in clinical trials and research and development, costs for regulatory consultants and filings, depreciation of capital resources used to develop products, related facilities costs, and salaries, other employee-related costs and stock-based compensation for research and development personnel. The Company expenses research and development costs as they are incurred for products in the development stage, including manufacturing costs and milestone payments made under license agreements prior to FDA approval. Upon and subsequent to FDA approval, manufacturing and milestone payments made under license agreements are capitalized. Milestone payments are accrued when it is deemed probable that the milestone event will be achieved. Costs related to the acquisition of intellectual property are expensed as incurred if the underlying technology is developed in connection with the Company’s research and development efforts and has no alternative future use.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist of salaries, other employee-related costs and stock-based compensation, and facilities and third-party expenses. Selling, general and administrative expenses are associated with the
activities of the corporate, finance, accounting, information technology, business development, commercial support, trade and distribution, sales, marketing, legal, medical affairs and human resource functions. Additionally, selling, general and administrative expenses include an estimate for the annual Affordable Care Act fee.
Stock-Based Compensation
Compensation costs for all stock-based awards to employees and directors are measured based on the grant date fair value of those awards and recognized over the period during which the employee or director is required to perform service in exchange for the award. The Company recognizes the expense over the award’s vesting period. The fair value of stock options granted and restricted stock units (RSUs) awarded are amortized using the straight-line method. As stock-based compensation expense recognized in the Consolidated Statements of Operations is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Advertising Expense
The Company expenses the costs of advertising, including branded promotional expenses, as incurred. Branded advertising expenses, recorded in selling, general and administrative expenses, were $1.9 million, $0.5 million and $2.6 million for the years ended December 31, 2024, 2023 and 2022, respectively.
Foreign Currency
The reporting currency of the Company is the U.S. dollar. The functional currency of the Company’s international subsidiaries is the local currency. Assets and liabilities denominated in foreign currencies, including intercompany balances for which settlement is anticipated in the foreseeable future, are translated at exchange rates in effect at the balance sheet date. Foreign currency equity balances are translated at historical rates. Revenues and expenses denominated in foreign currencies are translated at average exchange rates for the respective periods. Foreign currency translation adjustments are recorded in accumulated other comprehensive income (loss).
Transactions denominated in currencies other than functional currency are recorded based on exchange rates at the time such transactions arise. Changes in exchange rates with respect to amounts recorded in the Consolidated Balance Sheets related to these items will result in unrealized foreign currency transaction gains and losses based upon period-end exchange rates. The Company also records realized foreign currency transaction gains and losses upon settlement of the transactions. Foreign currency transaction gains and losses are included in other income and were not material for the years ended December 31, 2024, 2023 and 2022, respectively.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Under the asset and liability method, current income tax expense or benefit is the amount of income taxes expected to be payable or refundable for the current year. A deferred income tax asset or liability is recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and tax credits and loss carryforwards. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion of the deferred tax assets will not be realized. Tax rate changes are reflected in income during the period such changes are enacted. Changes in ownership may limit the amount of net operating loss (NOL) carryforwards and tax credits that can be utilized in the future to offset taxable income. Tax benefits are recognized from an uncertain tax position only if it is more likely than not that the position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefit recognized in the financial statements for a particular tax position is based on the largest benefit that is more likely than not to be realized upon settlement. Interest and penalties related to income taxes are recognized as a component of income tax expense in the Consolidated Statements of Operations, and cumulative accrued interest and penalties are recognized within the related liability line items in the Consolidated Balance Sheets.
Certain Risks and Uncertainties
The Company’s products under development require approval from the FDA or other international regulatory agencies prior to commercial sales. There can be no assurance the products will receive the necessary clearance. If the Company is denied clearance or clearance is delayed, it may have a material adverse impact on the Company.
The Company’s products are concentrated in rapidly changing, highly competitive markets, which are characterized by rapid technological advances, increasing generic competition, changes in customer requirements and evolving regulatory requirements and industry standards. Any failure by the Company to anticipate or to respond adequately to technological developments in its industry, challenges from new generic market entrants, changes in customer or regulatory requirements or changes in industry standards, or any significant delays in the development or introduction of products or services could have a material adverse effect on the Company’s business, operating results and future cash flows.
The Company depends on single source suppliers for critical raw materials for manufacturing, as well as other components required for the administration of its products. The loss of these suppliers could delay the clinical trials or prevent or delay commercialization of the products.
Concentrations of Credit Risk
Financial instruments, which potentially subject the Company to significant concentrations of credit risk, consist primarily of cash, cash equivalents and marketable securities. The Company places its cash, cash equivalents and marketable securities with highly rated financial institutions. At December 31, 2024, the Company maintained all of its cash, cash equivalents and marketable securities in two financial institutions. Deposits held with these institutions may exceed the amount of insurance provided on such deposits. Generally, these deposits may be redeemed upon demand, and the Company believes there is minimal risk of losses on such balances.
Recent Accounting Pronouncements
In November 2023, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which is intended to provide enhanced segment disclosures. The standard will require disclosures about significant segment expenses and other segment items and identifying the Chief Operating Decision Maker and how they use the reported segment profitability measures to assess segment performance and allocate resources. These enhanced disclosures are required for all entities on an interim and annual basis, even if they have only a single reportable segment. The standard is effective for years beginning after December 15, 2023, and interim periods within annual periods beginning after December 15, 2024 and early adoption is permitted. The adoption of this standard for the year ended December 31, 2024 did not have a material impact on the Company’s consolidated financial results, but resulted in enhanced disclosures as included in Note 17, Segments.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which is intended to provide enhancements to annual income tax disclosures. The standard will require more detailed information in the rate reconciliation table and for income taxes paid, among other enhancements. The standard is effective for years beginning after December 15, 2024 and early adoption is permitted. The Company is evaluating this standard to determine if adoption will have a material impact on the Company’s consolidated financial statements.
In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Topic 220-40), which addresses the disaggregation of income statement expenses. This standard is effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027. The Company is evaluating this standard to determine if adoption will have a material impact on the Company’s consolidated financial statements.
3. PONVORY® Acquisition
On December 7, 2023, the Company entered into an Asset Purchase Agreement (the Purchase Agreement) to acquire the U.S. and Canadian rights to PONVORY® from Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company, and the closing of the transaction took place simultaneously with signing. PONVORY® is a once-daily oral selective sphingosine-1-phosphate receptor 1 modulator, indicated to treat adults with relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. The total consideration for the acquisition was $104.9 million consisting of cash paid to Janssen and acquisition-related transaction costs. The Purchase Agreement includes customary representations, warranties and covenants, as well as standard mutual indemnities covering losses arising from any material breach of the Purchase Agreement or inaccuracy of representations and warranties. Janssen has agreed to indemnify the Company against losses arising from its activities prior to the closing, and the Company has agreed to indemnify Janssen against losses arising from the Company’s activities pertaining to PONVORY® after the closing. Simultaneously and in connection with the Purchase Agreement, the parties also entered into certain supporting agreements, including a customary transition agreement, pursuant to which, during a transition period, Janssen will continue PONVORY® operations. The Company announced in May 2024 that ownership of the U.S. New Drug Application (NDA) and Investigational New Drug applications for PONVORY® had been transferred to Vanda from a Johnson & Johnson Company, fully allowing the Company to commercialize PONVORY® in the U.S.
The acquisition of PONVORY® has been accounted for as an asset acquisition in accordance with ASC 805-50 because substantially all of the fair value of the assets acquired is concentrated in a single asset, the PONVORY® product rights. The PONVORY® products rights consist of certain patents and trademarks, regulatory approvals, marketing assets, and other records, and are considered a single asset as they are inextricably linked. The total consideration of $104.9 million was fully allocated to the acquired intangible asset for the U.S. and Canadian rights to PONVORY®. The straight-line method is used to amortize the intangible asset, as disclosed in Note 9, Intangible Assets.
4. Marketable Securities
The following is a summary of the Company’s available-for-sale marketable securities as of December 31, 2024, which all have contractual maturities of less than two years:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Market
Value |
| U.S. Treasury and government agencies | $ | 227,720 | | | $ | 371 | | | $ | (261) | | | $ | 227,830 | |
| Corporate debt | 44,506 | | | 9 | | | (18) | | | 44,497 | |
| | | | | | | |
| Total marketable securities | $ | 272,226 | | | $ | 380 | | | $ | (279) | | | $ | 272,327 | |
The following is a summary of the Company’s available-for-sale marketable securities as of December 31, 2023, which all have contractual maturities of less than two years:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Market
Value |
| U.S. Treasury and government agencies | $ | 185,168 | | | $ | 227 | | | $ | (280) | | | $ | 185,115 | |
| Corporate debt | 67,352 | | | 2 | | | (26) | | | 67,328 | |
| | | | | | | |
| Total marketable securities | $ | 252,520 | | | $ | 229 | | | $ | (306) | | | $ | 252,443 | |
5. Fair Value Measurements
Authoritative guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. These tiers include:
•Level 1 — defined as observable inputs such as quoted prices in active markets
•Level 2 — defined as inputs other than quoted prices in active markets that are either directly or indirectly observable
•Level 3 — defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions
The Company’s assets classified in Level 1 and Level 2 as of December 31, 2024 and 2023 consist of cash equivalents and available-for-sale marketable securities. The valuation of Level 1 instruments is determined using a market approach and is
based upon unadjusted quoted prices for identical assets in active markets. The valuation of Level 2 instruments is also determined using a market approach based upon quoted prices for similar assets in active markets, or other inputs that are observable for substantially the full term of the financial instrument. Level 2 securities include certificates of deposit, commercial paper and corporate notes that use as their basis readily observable market parameters.
The Company held certain assets that are required to be measured at fair value on a recurring basis as of December 31, 2024, as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | Fair Value Measurement as of December 31, 2024 Using |
| (in thousands) | Total Fair Value | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) |
| U.S. Treasury and government agencies | $ | 227,830 | | | $ | 227,830 | | | $ | — | | | $ | — | |
| Corporate debt | 44,497 | | | — | | | 44,497 | | | — | |
| | | | | | | |
| Total assets measured at fair value | $ | 272,327 | | | $ | 227,830 | | | $ | 44,497 | | | $ | — | |
The Company held certain assets that are required to be measured at fair value on a recurring basis as of December 31, 2023, as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | Fair Value Measurement as of December 31, 2023 Using |
| (in thousands) | Total Fair Value | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) |
| U.S. Treasury and government agencies | $ | 209,103 | | | $ | 209,103 | | | $ | — | | | $ | — | |
| Corporate debt | 107,108 | | | — | | | 107,108 | | | — | |
| | | | | | | |
| Total assets measured at fair value | $ | 316,211 | | | $ | 209,103 | | | $ | 107,108 | | | $ | — | |
Total assets measured at fair value as of December 31, 2024 include no cash equivalents. Total assets measured at fair value as of December 31, 2023 include $63.8 million cash equivalents.
The Company also has financial assets and liabilities, not required to be measured at fair value on a recurring basis, which primarily consist of cash, accounts receivable, restricted cash, accounts payable and accrued liabilities and product revenue allowances, the carrying values of which materially approximate their fair values.
6. Inventory
Inventory consisted of the following as of December 31, 2024 and 2023:
| | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 |
| Current assets | | | |
| Work-in-process | $ | — | | | $ | 27 | |
| Finished goods | 1,726 | | | 1,330 | |
| Total inventory, current | $ | 1,726 | | | $ | 1,357 | |
| Non-Current assets | | | |
| Raw materials | $ | 934 | | | $ | 934 | |
| Work-in-process | 6,236 | | | 7,177 | |
| Finished goods | 617 | | | 737 | |
| Total inventory, non-current | 7,787 | | | 8,848 | |
| Total inventory | $ | 9,513 | | | $ | 10,205 | |
The Company’s inventory balance consisted of $2.0 million of Fanapt® product, $7.3 million of HETLIOZ® product and $0.2 million of PONVORY® product as of December 31, 2024. The Company’s inventory balance consisted of $3.0 million of Fanapt® product and $7.2 million of HETLIOZ® product as of December 31, 2023.
7. Property and Equipment
The following is a summary of the Company’s property and equipment, at cost, as of December 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | |
| (in thousands) | Estimated
Useful Life
(Years) | | December 31, 2024 | | December 31, 2023 |
| Computer and other equipment | 3 | | $ | 6,734 | | | $ | 6,284 | |
| Furniture and fixtures | 5 - 7 | | 1,497 | | | 1,496 | |
| Leasehold improvements | 5 - 11 | | 5,468 | | | 5,438 | |
| Total property and equipment, gross | | | 13,699 | | | 13,218 | |
| Accumulated depreciation and amortization | | (11,567) | | | (11,181) | |
| Total property and equipment, net | | | $ | 2,132 | | | $ | 2,037 | |
Depreciation expense was $0.9 million, $0.9 million and $1.2 million for the years ended December 31, 2024, 2023 and 2022, respectively.
8. Leases
The Company’s long-term leases primarily include operating leases and subleases for office space in Washington, D.C. and London, England and vehicle finance leases for its fleet program. The Company recognized ROU assets and lease liabilities related to fixed payments for these long-term leases in its Consolidated Balance Sheets as of December 31, 2024 and 2023. The Company also has short-term leases, including office space in Berlin, Germany.
In June 2011, the Company entered into an operating lease agreement under which it leases 33,534 square feet of office space for its headquarters at 2200 Pennsylvania Avenue, N.W. in Washington, D.C. Subject to the prior rights of other tenants, the Company has the right to renew the lease for five years following its expiration in July 2028. As of December 31, 2024, the renewal period has not been included in the lease term. The Company has the right to sublease or assign all or a portion of the premises, subject to standard conditions. The lease may be terminated early by the Company or the landlord under certain circumstances.
In June 2016, the Company entered into a sublease agreement under which it subleases an additional 9,928 square feet of office space for its headquarters at 2200 Pennsylvania Avenue, N.W. in Washington, D.C. The sublease term began in January 2017 and ends in July 2026 but may be terminated earlier by either party under certain circumstances. The Company has the right to sublease or assign all or a portion of the premises, subject to standard conditions.
In May 2016, the Company entered into an operating lease agreement under which it leases 2,880 square feet of office space in London, England. In November 2022, the Company extended the non-cancellable portion of the lease term from 2023 to 2026.
In August 2024, the Company entered into a master lease agreement for vehicles to be utilized by the Company’s sales force. The individual car leases commence upon delivery of the vehicles, which began in the fourth quarter of 2024, and were determined to be finance leases upon lease commencement. The contractual period of each lease is three years.
The following is a summary of the Company’s ROU assets and lease liabilities as of December 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | Classification on the Balance Sheet | | December 31, 2024 | | December 31, 2023 |
| Assets | | | | | | |
| Operating lease assets | | Operating lease right-of-use assets | | $ | 5,602 | | | $ | 7,103 | |
| Finance lease assets | | Finance lease right-of-use assets | | 4,943 | | | — | |
| Total lease assets | | | | $ | 10,545 | | | $ | 7,103 | |
| | | | | | | |
| Liabilities | | | | | | |
| Operating lease current liabilities | | Accounts payable and accrued liabilities | | $ | 2,456 | | | $ | 2,398 | |
| Finance lease current liabilities | | Accounts payable and accrued liabilities | | 1,814 | | | — | |
| Operating lease non-current liabilities | | Operating lease non-current liabilities | | 4,944 | | | 7,006 | |
| Finance lease non-current liabilities | | Finance lease non-current liabilities | | 3,146 | | | — | |
| Total lease liabilities | | | | $ | 12,360 | | | $ | 9,404 | |
| | | | | | | |
| Weighted average remaining lease term, operating leases | | | | 3.3 | | 4.3 |
| Weighted average discount rate, operating leases | | | | 8.2 | % | | 8.2 | % |
| Weighted average remaining lease term, finance leases | | | | 2.9 | | — | |
| Weighted average discount rate, finance leases | | | | 6.4 | % | | — | % |
The components of lease expense for the years ended December 31, 2024, 2023 and 2022 was as follows:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Operating lease cost: | | | | | |
| Fixed lease cost | $ | 2,223 | | | $ | 2,216 | | | $ | 2,226 | |
| Short-term lease cost | 426 | | | 396 | | | 389 | |
| Finance lease cost: | | | | | |
| Amortization | 171 | | | — | | | — | |
| Interest | 32 | | | — | | | — | |
| Total lease costs | $ | 2,852 | | | $ | 2,612 | | | $ | 2,615 | |
Supplemental cash flow information related to leases for the years ended December 31, 2024, 2023 and 2022 was as follows: | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Cash paid for amounts included in measurement of lease liabilities: | | | | | |
| Operating cash flows from operating leases | $ | 2,723 | | | $ | 2,659 | | | $ | 2,581 | |
| Operating cash flows from finance leases | 32 | | | — | | | — | |
| Financing cash flows from finance leases | 155 | | | — | | | — | |
| Right-of-use assets obtained in exchange for lease obligations: | | | | | |
| | | | | |
| Finance leases | 5,114 | | | — | | | — | |
The table below reconciles the Company’s future cash obligations to lease liabilities recorded on the balance sheet as of December 31, 2024:
| | | | | | | | | | | | | | | | |
| | |
| (in thousands) | | Operating Leases | | Finance Leases | | |
| 2025 | | $ | 2,792 | | | $ | 1,877 | | | |
| 2026 | | 2,409 | | | 1,877 | | | |
| 2027 | | 2,159 | | | 1,689 | | | |
| 2028 | | 1,099 | | | — | | | |
| 2029 | | — | | | — | | | |
| Thereafter | | — | | | — | | | |
| Total minimum lease payments | | 8,459 | | | 5,443 | | | |
| Less: amount of lease payments representing interest | | (1,059) | | | (483) | | | |
| Present value of future minimum lease payments | | 7,400 | | | 4,960 | | | |
| Less: current obligations under leases | | (2,456) | | | (1,814) | | | |
| Lease non-current liabilities | | $ | 4,944 | | | $ | 3,146 | | | |
9. Intangible Assets
HETLIOZ®. In January 2014, the Company announced that the FDA had approved the NDA for HETLIOZ®. As a result of this approval, the Company met a milestone under its license agreement with Bristol-Myers Squibb (BMS) that required the Company to make a license payment of $8.0 million to BMS. In April 2018, the Company met its final milestone under its license agreement with BMS when cumulative worldwide sales of HETLIOZ® reached $250.0 million. As a result of the achievement of this milestone, the Company made a payment to BMS of $25.0 million in 2018. These milestone payments were determined to be additional consideration for the acquisition of HETLIOZ® and capitalized as an intangible asset and are being amortized on a straight-line basis over the estimated economic useful life of the related product patents.
PONVORY®. On December 7, 2023, the Company acquired the U.S. and Canadian rights to PONVORY® from Janssen. The total purchase price was allocated to the acquired intangible asset for the U.S. and Canadian rights to PONVORY®. See Note 3, PONVORY® Acquisition, for additional details. The PONVORY® intangible asset is being amortized on a straight-line basis over the estimated economic useful life of the related product rights. During the first quarter of 2024, the estimated useful life for the PONVORY® intangible asset was changed from 2035 to 2042 based on a change in the estimated economic useful life of the related product rights.
The following is a summary of the Company’s amortizing intangible assets as of December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2024 |
| (in thousands) | Estimated
Useful Life | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net
Carrying
Amount |
HETLIOZ® | 2035 | | $ | 33,000 | | | $ | 17,400 | | | $ | 15,600 | |
PONVORY® | 2042 | | 104,894 | | | 6,398 | | | 98,496 | |
| Total amortizing intangible assets | | | $ | 137,894 | | | $ | 23,798 | | | $ | 114,096 | |
The following is a summary of the Company’s amortizing intangible assets as of December 31, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2023 |
| (in thousands) | Estimated
Useful Life | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net
Carrying
Amount |
HETLIOZ® | 2035 | | $ | 33,000 | | | $ | 15,937 | | | $ | 17,063 | |
PONVORY® | 2035 | | 104,894 | | | 588 | | | 104,306 | |
| Total amortizing intangible assets | | | $ | 137,894 | | | $ | 16,525 | | | $ | 121,369 | |
As of December 31, 2024 and 2023, the Company also had $27.9 million of fully amortized intangible assets related to Fanapt®. Intangible assets are amortized over their estimated useful economic life using the straight-line method. Amortization expense for the years ended December 31, 2024, 2023 and 2022 was as follows:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
HETLIOZ® | $ | 1,463 | | | $ | 1,502 | | | $ | 1,516 | |
PONVORY® | 5,810 | | | 588 | | | — | |
| Total amortization expense | $ | 7,273 | | | $ | 2,090 | | | $ | 1,516 | |
The following is a summary of the future intangible asset amortization schedule as of December 31, 2024:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Total | | 2025 | | 2026 | | 2027 | | 2028 | | 2029 | | Thereafter |
HETLIOZ® | $ | 15,600 | | | $ | 1,463 | | | $ | 1,463 | | | $ | 1,463 | | | $ | 1,463 | | | $ | 1,463 | | | $ | 8,285 | |
PONVORY® | 98,496 | | | 5,544 | | | 5,544 | | | 5,544 | | | 5,544 | | | 5,544 | | | 70,776 | |
| Total amortization expense | $ | 114,096 | | | $ | 7,007 | | | $ | 7,007 | | | $ | 7,007 | | | $ | 7,007 | | | $ | 7,007 | | | $ | 79,061 | |
10. Accounts Payable and Accrued Liabilities
The following is a summary of the Company’s accounts payable and accrued liabilities as of December 31, 2024 and 2023:
| | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 |
| Research and development expenses | $ | 11,962 | | | $ | 15,691 | |
| Consulting and other professional fees | 9,226 | | | 4,404 | |
| Compensation and employee benefits | 8,465 | | | 6,413 | |
| Operating lease liabilities | 2,456 | | | 2,398 | |
| Finance lease liabilities | 1,814 | | | — | |
| Royalties payable | 1,665 | | | 2,409 | |
| | | |
| Accounts payable and other accrued liabilities | 3,498 | | | 7,145 | |
| Total accounts payable and accrued liabilities | $ | 39,086 | | | $ | 38,460 | |
11. Commitments and Contingencies
Guarantees and Indemnifications
The Company has entered into a number of standard intellectual property indemnification agreements in the ordinary course of its business. Pursuant to these agreements, the Company indemnifies, holds harmless, and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, generally the Company’s business partners or customers, in connection with any U.S. patent or any copyright or other intellectual property infringement claim by any third party with respect to the Company’s products. The term of these indemnification agreements is generally perpetual from the date of execution of the agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited. Since inception, the Company has not incurred costs to defend lawsuits or settle claims related to these indemnification agreements. The Company also indemnifies its officers and directors for certain events or occurrences, subject to certain conditions.
License Agreements
The Company’s rights to develop and commercialize its products are subject to the terms and conditions of licenses granted to the Company by other pharmaceutical companies.
Fanapt®. Pursuant to the terms of a settlement agreement with Novartis Pharma AG (Novartis), Novartis transferred all U.S. and Canadian rights in the Fanapt® franchise to the Company on December 31, 2014. The Company paid directly to Sanofi S.A. (Sanofi) a fixed royalty of 3% of net sales through December 2019 related to manufacturing know-how. The Company is also obligated to pay Sanofi a fixed royalty on Fanapt® net sales equal to 6% on Sanofi know-how not related to manufacturing under certain conditions for a period of up to 10 years in markets where the new chemical entity (NCE) patent has expired or was not issued. The Company is obligated to pay this 6% royalty on net sales in the U.S. through November 2026.
HETLIOZ®. In February 2004, the Company entered into a license agreement with BMS under which it received an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize HETLIOZ®. As of December 31, 2024, the Company has paid BMS $37.5 million in upfront fees and milestone obligations, including $33.0 million of regulatory approval and commercial milestones capitalized as intangible assets (see Note 9, Intangible Assets). The Company has no remaining milestone obligations to BMS. Additionally, the Company is obligated to make royalty payments on HETLIOZ® net sales to BMS. The royalty period in each territory where the Company commercializes HETLIOZ® is 10 years following the first commercial sale in the territory. In territories outside the U.S., the royalty is 5% on net sales. In the U.S., the royalty on net sales decreased from 10% to 5% in December 2022. This U.S. royalty ended in April 2024. The Company is also obligated under the license agreement to pay BMS a percentage of any sublicense fees, upfront payments and milestone and other payments (excluding royalties) that it receives from a third party in connection with any sublicensing arrangement, at a rate which is in the mid-twenties. The Company is obligated to use its commercially reasonable efforts to develop and commercialize HETLIOZ®.
Tradipitant. In April 2012, the Company entered into a license agreement with Eli Lilly and Company (Lilly) pursuant to which the Company acquired an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize an NK-1 receptor antagonist, tradipitant, for all human indications. Lilly is eligible to receive future payments based upon achievement of specified development, regulatory approval and commercialization milestones as well as tiered-royalties on net sales at percentage rates up to the low double digits. As of December 31, 2024, the Company has paid Lilly $5.0 million in upfront fees and development milestones, including a $2.0 million milestone paid to Lilly during the year ended December 31, 2023 for the filing of the first application for marketing authorization for tradipitant in either the U.S. or European Union (E.U.). As of December 31, 2024, remaining milestone obligations include $10.0 million and $5.0 million milestones for the first approval of an application for marketing authorization for tradipitant in the U.S. and E.U., respectively, and up to $80.0 million for sales milestones. The Company is obligated to use its commercially reasonable efforts to develop and commercialize tradipitant.
Imsidolimab. In January 2025, the Company entered into an exclusive global license agreement with Anaptys under which it acquired the worldwide rights to develop, manufacture, and commercialize imsidolimab, an IL-36R antagonist. Under the terms of the agreement, the Company made an upfront payment of $10 million to Anaptys and an additional $5 million for existing drug supply. Anaptys is eligible to receive up to $35 million in future regulatory approval and sales milestones. Additionally, the Company is obligated to pay Anaptys a 10% royalty on global net sales of imsidolimab. The Company is obligated to use its commercially reasonable efforts to develop and commercialize imsidolimab.
Portfolio of CFTR activators and inhibitors. In March 2017, the Company entered into a license agreement with the University of California San Francisco (UCSF), under which the Company acquired an exclusive worldwide license to develop and commercialize a portfolio of CFTR activators and inhibitors. Pursuant to the license agreement, the Company will develop and commercialize the CFTR activators and inhibitors and is responsible for all development costs, including current pre-investigational new drug development work. UCSF is eligible to receive future payments based upon achievement of specified development and commercialization milestones as well as single-digit royalties on net sales. As of December 31, 2024, the Company has paid UCSF $1.6 million in upfront fees and development milestones. As of December 31, 2024, remaining milestone obligations include $11.9 million for development milestones and $33.0 million for future regulatory approval and sales milestones. Included in the $11.9 million of development milestones are $1.1 million of milestone obligations due upon the conclusion of clinical studies for each licensed product, not to exceed $3.2 million in total for the CFTR portfolio.
VQW-765. In connection with a settlement agreement with Novartis relating to Fanapt®, the Company received an exclusive worldwide license under certain patents and patent applications, and other licenses to intellectual property, to develop and commercialize VQW-765, a Phase II alpha-7 nicotinic acetylcholine receptor partial agonist. Pursuant to the license
agreement, the Company is obligated to use its commercially reasonable efforts to develop and commercialize VQW-765 and is responsible for all development costs. The Company has no milestone obligations, but Novartis is eligible to receive tiered-royalties on net sales at percentage rates up to the mid-teens.
Other Agreements
Olipass. In September 2022, the Company entered into an agreement with OliPass Corporation (OliPass) to jointly develop a set of ASO molecules based on OliPass’ proprietary modified peptide nucleic acids. As consideration for entering into the arrangement, the Company paid OliPass an upfront fee of $3.0 million, which was recorded as research and development expense in 2022. The Company is funding the research and development activities and has the option to license jointly developed intellectual property upon successful development.
Shareholder Rights Plan. On April 17, 2024, the Company’s board of directors authorized and declared a dividend distribution of one right (each, a Right) for each outstanding share of common stock of the Company to stockholders of record as of the close of business on April 29, 2024 (the Record Date). Each Right entitles the registered holder to purchase from the Company, subject to certain conditions which have not yet occurred, one one-thousandth of a share of Series A Junior Participating Preferred Stock, par value $0.001 per share (the Preferred Stock), of the Company at an exercise price of $25.00 (the Exercise Price), subject to adjustment. The complete terms of the Rights are set forth in a Rights Agreement, dated as of April 17, 2024, between the Company and Equiniti Trust Company, LLC, as rights agent (the Rights Agent), as amended by that certain Amendment No. 1 to the Rights Agreement, and that certain Amendment No. 2 to the Rights Agreement, each, by and between the Company and the Rights Agent (as amended, the Rights Agreement).
In general terms, subject to certain enumerated exceptions, the Rights Agreement works by imposing a significant penalty upon any person or group that acquires beneficial ownership of 10% or more of the shares of common stock without the prior approval of the board of directors. In general, any person will be deemed to beneficially own any securities (a) as to which such person has any agreement, arrangement or understanding with another person for the purpose of acquiring, holding, voting or disposing of any shares of Common Stock or (b) that are the subject of a derivative transaction or constitute a derivative security. As a result, the overall effect of the Rights Agreement and the issuance of the Rights may be to render more difficult or discourage a merger, tender or exchange offer or other business combination involving the Company that is not approved by the board of directors. However, neither the Rights Agreement nor the Rights should interfere with any merger, tender or exchange offer or other business combination approved by the board of directors.
Purchase Commitments
In the normal course of its business, the Company regularly enters into agreements with third-party vendors under fee service arrangements, which generally may be terminated on 90 days’ notice without incurring additional charges, other than charges for work completed or materials procured but not paid for through the effective date of termination and other costs incurred by the Company’s contractors in closing out work in progress as of the effective date of termination. The Company’s non-cancellable purchase commitments for agreements longer than one year primarily relate to commitments for data services. Various other long-term agreements entered into for services with other third-party vendors, such as inventory purchase commitments, are cancellable in nature or contain variable commitment terms within the agreement that are within our control.
12. Accumulated Other Comprehensive Income (Loss)
The accumulated balances related to each component of other comprehensive income (loss), net of taxes, were as follows for the years ended December 31, 2024 and 2023:
| | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 |
| Foreign currency translation | $ | (13) | | | $ | 21 | |
| Unrealized gain (loss) on marketable securities | 87 | | | (51) | |
| Accumulated other comprehensive income (loss) | $ | 74 | | | $ | (30) | |
13. Stock-Based Compensation
As of December 31, 2024, there were 7,253,587 shares subject to outstanding options and RSUs under the 2006 Equity Incentive Plan (2006 Plan) and the Amended and Restated 2016 Equity Incentive Plan (2016 Plan, and together with the 2006 Plan, Plans). The 2006 Plan expired by its terms in April 2016, and the Company adopted the 2016 Plan. Outstanding options under the 2006 Plan remain in effect and the terms of the 2006 Plan continue to apply, but no additional awards can be granted
under the 2006 Plan. In June 2016, the Company’s stockholders approved the 2016 Plan. The 2016 Plan has been amended a number of times since to increase the number of shares reserved for issuance, among other administrative changes. Each of the amendments to the 2016 Plan was approved by the Company’s stockholders. There is a total of 15,690,000 shares of common stock authorized for issuance under the 2016 Plan, 4,725,935 shares of which remained available for future grant as of December 31, 2024.
Stock Options
The Company has granted option awards under the Plans with service conditions (service option awards) that are subject to terms and conditions established by the compensation committee of the board of directors. Service option awards have 10-year contractual terms. Service option awards granted to employees and new directors upon their election vest and become exercisable over four years, with the first 25% of the shares subject to service option awards vesting on the first anniversary of the grant date and the remaining 75% of the shares subject to the service option awards in 36 equal monthly installments thereafter. Subsequent annual service option awards granted to directors vest and become exercisable in full on the first anniversary of the grant date. Service option awards granted to executive officers and certain other employees provide for partial acceleration of vesting if the executive officer or employee is subject to an involuntary termination, and full acceleration of vesting if the executive officer or employee is subject to an involuntary termination within 24 months after a change in control of the Company. Service option awards granted to directors provide for accelerated vesting if there is a change in control of the Company or if the director’s service terminates as a result of the director’s death or total and permanent disability.
As of December 31, 2024, $2.9 million of unrecognized compensation costs related to unvested service option awards are expected to be recognized over a weighted average period of 0.8 years. No option awards are classified as a liability as of December 31, 2024.
A summary of option activity under the Plans for the year ended December 31, 2024 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
(in thousands, except for share and per share amounts) | Number of
Shares | | Weighted Average
Exercise Price at Grant Date | | Weighted Average
Remaining Term
(Years) | | Aggregate
Intrinsic
Value |
| Outstanding at December 31, 2023 | 4,792,506 | | | $ | 12.95 | | | 6.00 | | $ | — | |
| Granted | 190,514 | | | 5.41 | | | | | |
| | | | | | | |
| Expired | (379,439) | | | 12.02 | | | | | |
| | | | | | | |
| Outstanding at December 31, 2024 | 4,603,581 | | | 12.71 | | | 5.60 | | — | |
| Exercisable at December 31, 2024 | 3,778,833 | | | 13.73 | | | 5.05 | | — | |
| Vested and expected to vest at December 31, 2024 | 4,560,920 | | | 12.76 | | | 5.58 | | — | |
The weighted average grant-date fair value of options granted was $2.95, $3.53 and $5.18 per share for the years ended December 31, 2024, 2023 and 2022, respectively. There were no options exercised for the year ended December 31, 2024 and 2023. The total intrinsic value of options exercised was $1.6 million for the year ended December 31, 2022. There were no proceeds from the exercise of stock options for the year ended December 31, 2024 and 2023. Proceeds from the exercise of stock options amounted to $0.7 million for the year ended December 31, 2022.
Restricted Stock Units
An RSU is a stock award that entitles the holder to receive shares of the Company’s common stock as the award vests. The fair value of each RSU is based on the closing price of the Company’s stock on the date of grant. The Company has granted RSUs under the Plans with service conditions (service RSUs) that are subject to terms and conditions established by the compensation committee of the board of directors. Service RSUs granted to employees and new directors upon their election vest in four equal annual installments. Subsequent annual service RSUs granted to directors vest on the first anniversary of the date of grant. Service RSUs granted to executive officers and certain other employees provide for accelerated vesting if the executive officer or employee is subject to an involuntary termination within 24 months after a change in control of the Company. Service RSUs granted to directors provide for accelerated vesting if there is a change in control of the Company.
As of December 31, 2024, $11.4 million of unrecognized compensation costs related to unvested service RSUs are expected to be recognized over a weighted average period of 1.6 years. No RSUs are classified as a liability as of December 31, 2024.
A summary of RSU activity for the Plans for the year ended December 31, 2024 is as follows:
| | | | | | | | | | | |
| Number of
Shares | | Weighted
Average
Grant Date Fair Value |
| Unvested at December 31, 2023 | 1,905,310 | | | $ | 10.87 | |
| Granted | 1,652,903 | | | 4.48 | |
| Forfeited | (132,062) | | | 7.91 | |
| Vested | (776,145) | | | 11.54 | |
| Unvested at December 31, 2024 | 2,650,006 | | | 6.82 | |
The weighted average grant-date fair value of RSUs granted was $4.48, $6.96 and $11.24 per share for the years ended December 31, 2024, 2023 and 2022, respectively. The total fair value of the RSUs that vested during the years ended December 31, 2024, 2023 and 2022 was $9.0 million, $11.3 million, and $11.6 million, respectively.
Stock-Based Compensation Expense
Stock-based compensation expense recognized for the years ended December 31, 2024, 2023 and 2022 was comprised of the following:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Research and development | $ | 2,960 | | | $ | 3,323 | | | $ | 3,964 | |
| Selling, general and administrative | 9,472 | | | 10,717 | | | 12,315 | |
| Total stock-based compensation expense | $ | 12,432 | | | $ | 14,040 | | | $ | 16,279 | |
The fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton option pricing model that uses the assumptions noted in the following table. Expected volatility rates are based on the historical volatility of the Company’s publicly traded common stock and other factors. The expected terms are determined based on a combination of historical exercise data and hypothetical exercise data for unexercised stock options. The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. The Company has never paid cash dividends to its stockholders and does not plan to pay dividends in the foreseeable future. Assumptions used in the Black-Scholes-Merton option pricing model for employee and director stock options granted during the years ended December 31, 2024, 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Expected dividend yield | — | % | | — | % | | — | % |
| Weighted average expected volatility | 50 | % | | 47 | % | | 46 | % |
| Weighted average expected term (years) | 6.27 | | 6.16 | | 6.05 |
| Weighted average risk-free rate | 4.52 | % | | 3.89 | % | | 2.03 | % |
14. Employee Benefit Plan
The Company has a defined contribution plan under IRC Section 401(k). This plan covers substantially all employees who meet minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pre-tax basis. Currently, the Company matches fifty percent up to the first six percent of employee contributions. All matching contributions have been paid by the Company. The Company match vests over a 4-year period and amounted to $1.0 million, $1.0 million and $1.1 million for the years ended December 31, 2024, 2023 and 2022, respectively.
15. Income Taxes
The following is a summary of the domestic and foreign components of income (loss) before income taxes for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Domestic | $ | (23,042) | | | $ | 6,184 | | | $ | 11,216 | |
| Foreign | 121 | | | 155 | | | 84 | |
| Total income (loss) before income taxes | $ | (22,921) | | | $ | 6,339 | | | $ | 11,300 | |
The following is a summary of the provision (benefit) for income taxes for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Current | | | | | |
| Federal | $ | 1,654 | | | $ | 2,577 | | | $ | — | |
| State | 749 | | | 2,447 | | | 3,710 | |
| Foreign | 58 | | | 91 | | | 185 | |
| Deferred | | | | | |
| Federal | (4,760) | | | (2,240) | | | 1,238 | |
| State | (1,719) | | | 970 | | | (111) | |
| Foreign | (3) | | | (15) | | | 3 | |
| Provision (benefit) for income taxes | $ | (4,021) | | | $ | 3,830 | | | $ | 5,025 | |
The Company assesses the need for a valuation allowance against its deferred tax asset each quarter through the review of all available positive and negative evidence. Deferred tax assets are reduced by a tax valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The analysis is highly dependent upon historical and projected pretax income. As of December 31, 2024, after considering all available positive and negative evidence, including but not limited to cumulative income in recent periods, historical, current and future projected results and significant risks and uncertainties related to forecasts, the Company concluded, consistent with prior periods, that it is more likely than not that substantially all of its deferred tax assets in the U.S. are realizable in future periods. The Company maintains a valuation allowance against certain state net deferred tax assets.
The Company generated a pretax loss for the year ended December 31, 2024. If the Company continues to generate pretax losses and/or if the Company’s projections indicate pretax losses in future periods, the conclusion about the appropriateness of the valuation allowance could change in a future period. An increase in the valuation allowance would result in a non-cash income tax expense during the period of change. The analysis to determine that a valuation allowance is necessary is highly dependent upon historical and future projected earnings, among other factors. Any such adjustment could have a material impact on the Company’s results of operations.
The following is a reconciliation between the federal statutory tax rate and the Company’s effective tax rate for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2024 | | 2023 | | 2022 |
| Federal tax at statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State taxes | 4.3 | % | | 5.0 | % | | 4.8 | % |
| Change in valuation allowance | (1.5) | % | | 22.6 | % | | 1.0 | % |
| Research and development credit | 12.8 | % | | (69.3) | % | | (25.9) | % |
| Orphan drug credit | 1.0 | % | | (4.5) | % | | (0.9) | % |
| Section 162(m) limitation | (4.5) | % | | 17.2 | % | | 9.4 | % |
| Other tax rate changes | (0.5) | % | | (23.4) | % | | 1.7 | % |
| Other changes in state deferred taxes | 1.0 | % | | 12.7 | % | | — | % |
| Uncertain tax positions | (5.9) | % | | 41.0 | % | | 24.5 | % |
| Stock-based compensation | (7.1) | % | | 30.2 | % | | 5.8 | % |
| Settlements | (0.1) | % | | 2.7 | % | | 2.6 | % |
| Non-deductible items | (2.6) | % | | 5.5 | % | | 0.3 | % |
| Other items | (0.3) | % | | (0.3) | % | | 0.2 | % |
| Effective tax rate | 17.6 | % | | 60.4 | % | | 44.5 | % |
The following is a summary of the components of the Company’s net deferred tax assets and the related tax valuation allowance as of December 31, 2024 and 2023.
| | | | | | | | | | | |
| (in thousands) | December 31, 2024 | | December 31, 2023 |
| Deferred tax assets | | | |
| Net operating loss carryforwards | $ | 5,962 | | | $ | 5,897 | |
| Stock-based compensation | 3,606 | | | 4,187 | |
| Accrued expenses | 2,113 | | | 1,453 | |
| Allowance for returns and credit losses | 1,716 | | | 1,748 | |
| Research and development and orphan drug credit carryforwards | 36,214 | | | 38,559 | |
| Capitalized research and development expenses | 38,393 | | | 29,036 | |
| Other | 4,537 | | | 5,037 | |
| Total deferred tax assets | 92,541 | | | 85,917 | |
| Deferred tax liabilities | | | |
| Intangible assets | (961) | | | (812) | |
| Other | (1,253) | | | (1,558) | |
| Total deferred tax liabilities | (2,214) | | | (2,370) | |
| Deferred tax assets, net | 90,327 | | | 83,547 | |
| Less: Valuation allowance | 8,887 | | | 8,547 | |
| Net deferred tax assets | $ | 81,440 | | | $ | 75,000 | |
The following is a summary of changes in the Company’s tax valuation allowance for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | Balance at Beginning of Year | | Additions | | Reductions | | Balance at End of Year |
| Year Ended | | | | | | | |
| December 31, 2024 | $ | 8,547 | | | $ | 340 | | | $ | — | | | $ | 8,887 | |
| December 31, 2023 | 7,136 | | | 1,411 | | | — | | | 8,547 | |
| December 31, 2022 | 7,025 | | | 111 | | | — | | | 7,136 | |
The Company has NOL and other tax credit carryforwards in several jurisdictions. As of December 31, 2024 and 2023, the Company had utilized all of its federal NOL carryforwards. As of December 31, 2024 the Company has deferred tax
assets of $18.3 million and $17.9 million related to U.S. federal and state research and development credits and orphan drug credits, respectively. Both of these tax attributes will begin to expire in 2031. In addition, the Company has $6.0 million of deferred tax assets relating to other U.S. NOL carryforwards, which primarily relate to the District of Columbia. NOLs for the District of Columbia will begin to expire in 2032 and state NOLs will begin to expire in 2034.
Cash paid for income taxes was $2.4 million and $3.6 million for the years ended December 31, 2024 and 2023. Cash paid for income taxes for the year ended December 31, 2022 was not material. Cash taxes beginning in 2023 include federal tax payments as a result of utilizing the Company’s remaining federal net operating loss carryover. For federal tax purposes, utilization of the Company’s tax credits is limited to 75 percent of federal income tax each year.
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Unrecognized tax benefits at the beginning of the year | $ | 18,312 | | | $ | 15,485 | | | $ | 12,935 | |
| Increases (decreases) related to prior year tax positions | (159) | | | 919 | | | (75) | |
| Increases related to current year tax positions | 742 | | | 2,003 | | | 2,895 | |
| Settlements | — | | | — | | | (270) | |
| Statute lapses | $ | (99) | | | $ | (95) | | | $ | — | |
| Unrecognized tax benefits at the end of the year | $ | 18,796 | | | $ | 18,312 | | | $ | 15,485 | |
The amount of uncertain tax benefits that, if recognized, would impact the effective tax rate is $18.8 million. Unrecognized tax benefits are not expected to change materially over the next 12 months. Generally, the tax years 2021 through 2023 remain open to examination by the major taxing jurisdiction to which the Company is subject. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service, or state or foreign tax authorities, to the extent utilized in a future period.
Certain tax attributes of the Company, including NOLs and credit carryforwards, would be subject to limitation under Section 382 and 383 should an ownership change as defined under Section 382 of the Internal Revenue Code of 1986, as amended (IRC) occur. The limitation resulting from a change in ownership could affect the Company’s ability to utilize its NOLs and credit carryforwards (tax attributes) to offset future taxable income. An ownership change occurred in the year ended December 31, 2014. The Company believes that the ownership change in 2014 will not impact its ability to utilize NOL and credit carryforwards; however, future ownership changes may cause the Company’s existing tax attributes to have additional limitations.
16. Earnings per Share
Basic earnings per share (EPS) is calculated by dividing the net income (loss) by the weighted average number of shares of common stock outstanding. Diluted EPS is computed by dividing the net income (loss) by the weighted average number of shares of common stock outstanding, plus potential outstanding common stock for the period. Potential outstanding common stock includes stock options and shares underlying RSUs, but only to the extent that their inclusion is dilutive, as calculated using the treasury stock method.
The following table presents the calculation of basic and diluted net income (loss) per share of common stock for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands, except for share and per share amounts) | 2024 | | 2023 | | 2022 |
| Numerator: | | | | | |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | 6,275 | |
| Denominator: | | | | | |
| Weighted average shares outstanding, basic | 58,149,087 | | | 57,380,975 | | | 56,461,877 | |
| Effect of dilutive securities | — | | | 176,936 | | | 521,294 | |
| Weighted average shares outstanding, diluted | 58,149,087 | | | 57,557,911 | | | 56,983,171 | |
| Net income (loss) per share, basic and diluted: | | | | | |
| Basic | $ | (0.33) | | | $ | 0.04 | | | $ | 0.11 | |
| Diluted | $ | (0.33) | | | $ | 0.04 | | | $ | 0.11 | |
| Antidilutive securities excluded from calculations of diluted net income (loss) per share | 6,493,140 | | | 6,464,057 | | | 4,786,891 | |
The Company incurred a net loss for the year ended December 31, 2024 causing inclusion of any potentially dilutive securities to have an anti-dilutive effect, resulting in dilutive loss per share and basic loss per share attributable to common stockholders being equivalent.17. Segments
The Company operates in one reportable segment, which includes all activities related to the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. The determination of a single reportable segment is consistent with the consolidated financial information regularly provided to the Company’s chief operating decision maker (CODM), which is its chief executive officer, who reviews and evaluates consolidated net income (loss) for purposes of assessing performance, making operating decisions, allocating resources and planning and forecasting for future periods. The measure of segment assets is reported on the balance sheet as total assets.
The following table presents the segment revenue and significant expense categories included within the product segment’s measure of profit or loss for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in thousands) | 2024 | | 2023 | | 2022 |
| Revenue | $ | 198,772 | | | $ | 192,640 | | | $ | 254,382 | |
| Less: | | | | | |
| Cost of goods sold, excluding amortization | 11,314 | | | 14,796 | | | 24,282 | |
| Research and development | 74,431 | | | 76,823 | | | 85,770 | |
| Selling, general and administrative | 146,414 | | | 112,883 | | | 136,485 | |
| Intangible asset amortization | 7,273 | | | 2,090 | | | 1,516 | |
| Other income | (17,739) | | | (20,291) | | | (4,971) | |
| Provision (benefit) for income taxes | (4,021) | | | 3,830 | | | 5,025 | |
| Net income (loss) | $ | (18,900) | | | $ | 2,509 | | | $ | 6,275 | |
Foreign sales were not material for each of the years ended December 31, 2024, 2023 and 2022.
18. Legal Matters
HETLIOZ®. Between April 2018 and March 2021, the Company filed numerous Hatch-Waxman lawsuits in the U.S. District Court for the District of Delaware (Delaware District Court) against Teva Pharmaceuticals USA, Inc. (Teva), MSN Pharmaceuticals Inc. and MSN Laboratories Private Limited (MSN) and Apotex Inc. and Apotex Corp. (Apotex, and collectively with Teva and MSN, the HETLIOZ® Defendants) asserting that U.S. Patent Nos. RE46,604 (‘604 Patent), 9,060,995, 9,539,234, 9,549,913, 9,730,910 (‘910 Patent), 9,844,241, 10,071,977, 10,149,829 (‘829 Patent), 10,376,487 (‘487 Patent), 10,449,176, 10,610,510, 10,610,511, 10,829,465, and 10,611,744 will be infringed by the HETLIOZ® Defendants’ generic versions of HETLIOZ® for which they were seeking FDA approval. As initially disclosed in the Company’s Current
Report on Form 8-K filed with the Securities and Exchange Commission on January 14, 2022, in January 2022, the Company entered into a license agreement with MSN and Impax Laboratories LLC (Impax) resolving the lawsuits against MSN (the MSN/Impax License Agreement). The MSN/Impax License Agreement grants MSN and Impax a non-exclusive license to manufacture and commercialize MSN’s generic version of HETLIOZ® in the U.S. effective as of March 13, 2035, unless prior to that date the Company obtains pediatric exclusivity for HETLIOZ®, in which case the license will be effective as of July 27, 2035. The MSN/Impax License Agreement also provides that MSN and Impax may launch a generic version of HETLIOZ® earlier under certain limited circumstances. In January 2023, MSN and its commercial partner, Amneal Pharmaceuticals, Inc. (Amneal), informed the Company of their belief that such circumstances have occurred and have since launched their generic version. The Company disagreed with this position and sought to defend its legal rights to exclusivity for HETLIOZ®. The consolidated lawsuits against the remaining HETLIOZ® Defendants were tried in March 2022.
In December 2022, the Delaware District Court ruled that Teva and Apotex did not infringe the ‘604 Patent, and that the asserted claims of the ‘604, ‘910, ‘829 and ‘487 Patents were invalid. In December 2022, the Company appealed the Delaware District Court’s decision to the U.S. Court of Appeals for the Federal Circuit (Federal Circuit) and an oral argument for the appeal was held in March 2023. In May 2023, a three-judge panel of the Federal Circuit affirmed the Delaware District Court’s ruling, and in June 2023, the Company requested a rehearing or rehearing en banc from the Federal Circuit. In August 2023, the Federal Circuit denied the Company’s petition for a rehearing. In January 2024, the Company filed a petition for a writ of certiorari with the U.S. Supreme Court to review the Federal Circuit’s decision. In April 2024, the U.S. Supreme Court denied the Company’s petition for a writ of certiorari.
In December 2022, the Company filed patent infringement lawsuits, including Hatch-Waxman Act claims, against each of Teva and Apotex in the U.S. District Court for the District of New Jersey (NJ District Court) asserting that U.S. Patent No. 11,285,129, a method of administration patent that was not litigated in the Delaware District Court cases (‘129 Patent), will be infringed by Teva’s and Apotex’ generic versions of HETLIOZ®, each of which was approved by the FDA. The Company asked the NJ District Court to, among other things, order that the effective date of the FDA’s approval of Teva’s and Apotex’ generic versions of HETLIOZ® be a date that is no earlier than the expiration of the ‘129 Patent, or such later date that the NJ District Court may determine, and enjoin each of Teva and Apotex from the commercial manufacture, use, import, offer for sale and/or sale of their generic versions of HETLIOZ® until the expiration of the ‘129 Patent, or such later date that the NJ District Court may determine. In February 2023, the case was transferred to the Delaware District Court. In April 2023, Teva and Apotex moved for judgment on the pleadings. In June 2024, the Delaware District Court denied those motions, allowing the Company’s lawsuit to proceed. The Company’s lawsuit remains pending.
In January 2023, the Company filed a lawsuit in the NJ District Court against Teva challenging Teva’s advertising and marketing practices related to its at risk launch of its generic version of HETLIOZ® for the single indication of Non-24. The Company believes that Teva’s advertising and marketing practices related to its generic version of HETLIOZ® promote its product for uses beyond the limited labeling that Teva sought, and the FDA approved. The Company seeks to, among other things, enjoin Teva from engaging in false and misleading advertising and recover monetary damages. In December 2023, the case was transferred to the Delaware District Court following Teva’s motion to transfer and dismiss the case earlier that year. In February 2025, the Delaware District Court denied Teva’s motion to dismiss, allowing the Company’s lawsuit to proceed. The Company’s lawsuit remains pending.
In January 2023, the Company filed a lawsuit in the U.S. District Court for the District of Columbia (DC District Court) against the FDA challenging the FDA’s approval of Teva’s Abbreviated New Drug Application (ANDA) for its generic version of HETLIOZ® capsules under the Administrative Procedure Act (APA), the Food, Drug, and Cosmetic Act (FDCA) and FDA regulations. Under the FDCA, every ANDA must contain information to show that the labeling proposed for the generic drug is the same as the labeling approved for the listed drug. The labeling and packaging for HETLIOZ® includes Braille, but Teva’s generic version does not. On this basis, the Company believes that Teva’s approved labeling does not comply with applicable requirements. The Company has asked the DC District Court to, among other things, vacate the FDA’s approval of Teva’s ANDA, declare that the approval of the ANDA was unlawful, arbitrary, and capricious and compel the FDA to order Teva to recall its generic HETLIOZ® product. In February 2023, Teva intervened in the lawsuit as a defendant. In September 2023, the Company amended its lawsuit to request that the DC District Court set aside the FDA’s July 2023 denial of the Company’s citizen petition, originally filed with the FDA in January 2023. In April 2024, the Company filed a motion for summary judgment. In February 2025, the DC District Court denied the Company’s motion and granted the FDA and Teva’s cross motions for summary judgment. The Company is evaluating options with respect to this litigation.
In September 2023, the Company filed a lawsuit in the DC District Court against the FDA challenging the FDA’s approval of MSN’s ANDA for its generic version of HETLIOZ® capsules under the APA, the FDCA, FDA regulations and the Appointments Clause of the U.S. Constitution. The Company believes that MSN’s underlying approval data, particularly its bioequivalence studies, are faulty. On this basis, the Company asked the DC District Court to, among other things, vacate the
FDA’s approval of MSN’s ANDA, declare that the approval of the ANDA was unlawful, arbitrary, and capricious, is unconstitutional under the Appointments Clause, and compel the FDA to order MSN to recall its generic HETLIOZ® product. In December 2023, the Company filed a motion for summary judgment. In January 2024, the FDA opposed the Company’s motion and moved to waive the administrative record, following which the court held an oral argument on the cross-motions. The DC District Court issued an order compelling the FDA to serve the administrative record and set deadlines for further proceedings. In April 2024, the Company filed a motion for summary judgment. In July 2024, the DC District Court held an oral argument on the motion to dismiss that the FDA filed in January 2024, which the Company opposed in February 2024. In September 2024, the DC District Court granted in part the FDA’s motion to dismiss as to the Company’s APA claims and denied the FDA’s motion to dismiss as to the Company’s claims under the Appointments Clause. The Company’s lawsuit remains pending.
In April 2024, the Company filed a lawsuit in the Delaware District Court against MSN, Amneal, and Impax alleging claims for false advertising in violation of the Lanham Act and unfair competition under several state laws as well as claims for breach of express representation and fraudulent inducement of a license agreement. In July 2024, the defendants filed a motion to dismiss and in September 2024, the Company opposed the motion. The Company’s lawsuit remains pending.
In December 2024, the Company filed a lawsuit in the Delaware District Court against each of Teva and Apotex in the Delaware District Court, in each case, asserting U.S. Patent No. 11,918,556 (‘556 Patent), a method of administration patent that was not litigated in the prior Delaware District Court cases, will be infringed by Teva’s and Apotex’s generic versions of HETLIOZ®, each of which was approved by the FDA. The Company has asked the Delaware District Court to, among other things, order that the effective date of the FDA’s approval of Teva’s and Apotex’ generic versions of HETLIOZ® be a date that is no earlier than the expiration of the ‘556 Patent, or such later date that the Delaware District Court may determine, and enjoin each of Teva and Apotex from the commercial manufacture, use, import, offer for sale and/or sale of their generic versions of HETLIOZ® until the expiration of the ‘556 Patent, or such later date that the Delaware District Court may determine. The Company’s lawsuit remains pending.
HETLIOZ LQ®. In July 2024, the Company filed a Hatch-Waxman lawsuit against MSN in the Delaware District Court asserting that U.S. Patent Nos. 10,179,119, 11,266,622, 11,285,129, 11,850,229, 10,610,510, 10,980,770, and 11,759,446 (together, the Asserted Patents) will be infringed by MSN’s generic version of HETLIOZ LQ® for which MSN is seeking FDA approval. The Company has asked the Delaware District Court to, among other things, enter judgment that MSN has infringed at least one claim of each of the Asserted Patents by submitting or causing to be submitted its ANDA to obtain FDA approval for the commercial manufacture, use, import, offer for sale and/or sale in the U.S. of its generic version of HETLIOZ LQ® before the expiration of each of the Asserted Patents, enter judgment that the use of MSN’s generic version of HETLIOZ LQ® in the U.S. before the expiration of each of the Asserted Patents will directly infringe at least one claim of each of the Asserted Patents, order that the effective date of any approval by the FDA of MSN’s generic version of HETLIOZ LQ® be a date that is no earlier than the expiration of the last expiring Asserted Patent(s), or such later date as the Court may determine, enjoin MSN from the commercial manufacture, use, import, offer for sale and/or sale of its generic version of HETLIOZ LQ® until the expiration of each of the Asserted Patents or such later date as the Court may determine, and award monetary damages, to the extent applicable. The Company’s lawsuit remains pending, and a trial is scheduled to begin on June 15, 2026.
Other Matters. From April 2022 to July 2024, the Company filed eighteen lawsuits in the DC District Court against the FDA to compel the FDA to produce records under the Freedom of Information Act (FOIA) regarding, among other matters: the FDA’s denial of the Company’s supplemental New Drug Application (sNDA) for HETLIOZ® in the treatment of jet lag disorder; cases in which the FDA waived its putative requirement of a 9-month non-rodent toxicity study before drugs can be tested on human patients for extended durations; communications external to and within the FDA relating to tradipitant, HETLIOZ® and Fanapt®; a warning letter that the FDA sent to the Company concerning its webpages for HETLIOZ® and Fanapt®; the FDA’s removal of a clinical trials design presentation from its website; discipline reviews relating to the FDA’s evaluations of the Company’s sNDA for HETLIOZ® and a third-party sNDA for jet lag; internal standard operating procedures or guidance relating to the FDA’s processing of incoming FOIA requests; and bioequivalence and other study reports submitted relating to the FDA’s consideration of tasimelteon ANDAs. Eight of these lawsuits have since been resolved in the Company’s favor and the other ten remain outstanding. The FDA has failed to respond and provide the requested documents within the statutory timeframe with respect to each of these ten outstanding cases. The Company has asked the DC District Court to, among other things, compel the FDA to comply with its obligations and declare that its lack of compliance violates FOIA.
In April 2022, the Company filed a lawsuit in the U.S. District Court for the District of Maryland (MD District Court) against the Centers for Medicare & Medicaid Services (CMS) and the Administrator of CMS challenging CMS’ rule broadly interpreting the defined terms “line extension” and “new formulation” under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (ACA), which went into effect in January 2022 (the Rule). The Company believes that the Rule is unlawful and contrary to the intent of Congress when it passed the ACA. Under
the Rule, certain of the Company’s products would be treated as line extensions and new formulations subject to enhanced rebates, despite the statutory text and CMS’ own long-standing practice, under which such products would not constitute line extensions or new formulations. In March 2023, the MD District Court ruled that CMS’ interpretation of the terms was reasonable and consistent with Congress’ intent. In April 2023, the Company appealed the ruling to the U.S. Court of Appeals for the Fourth Circuit (Fourth Circuit). In January 2024, the Fourth Circuit held an oral argument. In April 2024, the Fourth Circuit ruled against the Company. In September 2024, the Company filed a petition for a writ of certiorari with the U.S. Supreme Court to review the Fourth Circuit’s decision. In January 2025, the U.S. Supreme Court denied the Company’s petition for a writ of certiorari.
In May 2022, the Company filed a lawsuit in the DC District Court against the FDA challenging the FDA’s denial of Fast Track designation for tradipitant. In October 2021, the Company submitted to the FDA a request for Fast Track designation for tradipitant under the Food and Drug Administration Modernization Act of 1997 (FDAMA). The FDAMA provides for expedited development and review of drugs that receive Fast Track designation from the FDA. Under the FDAMA, the FDA must designate a drug as a Fast Track product if it both (1) is intended to treat a serious or life-threatening disease or condition and (2) demonstrates the potential to address unmet medical needs for such disease or condition. Although Fast Track designation is non-discretionary when the criteria are satisfied, the FDA denied the Company’s request for Fast Track designation. The Company does not believe that the FDA based its decision on the relevant criteria. Therefore, among other reasons, the Company maintains that the FDA’s denial is unlawful. The Company has asked the DC District Court to, among other things, set aside and vacate the FDA’s denial. An oral argument was held in January 2023. In August 2023, the DC District Court ruled against the Company. In September 2023, the Company appealed the ruling to the U.S. Court of Appeals for the District of Columbia Circuit and in September 2024, the Court of Appeals held an oral argument. In December 2024, the Court of Appeals affirmed the DC District Court’s ruling. The Company is currently evaluating options with respect to this litigation.
In September 2022, the Company filed a lawsuit in the DC District Court against the FDA to compel the FDA to comply with two separate non-discretionary obligations under the FDCA and its implementing regulations: an obligation to publish a notice of an opportunity for a hearing on the Company’s sNDA for HETLIOZ® in the treatment of jet lag disorder in the Federal Register within 180 days of the filing of the sNDA, and a separate obligation to publish the same notice within 60 days of the request for a hearing. The FDA published the notice of an opportunity for a hearing on October 11, 2022. The Company has asked the DC District Court to, among other things, compel the FDA to comply with its obligations and declare that its lack of compliance violates the FDCA and the FDA regulations. In January 2024, the DC District Court held an oral argument on dispositive cross-motions, following which the DC District Court granted in part the Company’s motion for summary judgment. The DC District Court ruled that the FDA violated the statute and ordered the FDA to either finally resolve the Company’s application or commence a hearing on or before March 5, 2024. In March 2024, the Company and the FDA filed a consent motion for entry of final judgment in the Company’s favor on its Administrative Procedure Act claim for the FDA’s unreasonable delay in resolving the hearing request, following which the FDA refused to hold a hearing or approve the Company’s sNDA for HETLIOZ® in the treatment of jet lag disorder. The Company subsequently filed a petition for review in the U.S. Court of Appeals for the District of Columbia Circuit (DC Circuit). Under the FDCA, the FDA has an obligation to either approve an sNDA or to hold a hearing on the application’s approvability. The Company’s petition asks the DC Circuit to set aside the FDA’s order refusing to hold a hearing and refusing approval. In January 2025, the DC Circuit held an oral argument. The Company’s petition remains pending.
In May 2023, the Company filed a lawsuit in the U.S. Court of Federal Claims (Federal Claims Court) against the federal government for the uncompensated taking and misuse of the Company’s trade secrets and confidential information. The Company believes that the FDA violated the Fifth Amendment’s due process clause by improperly providing confidential details from the Company’s drug master files for HETLIOZ® and Fanapt® to generic drug manufacturers during the FDA’s review of the manufacturers’ ANDAs. The Company has asked the Federal Claims Court to, among other things, declare that the FDA’s disclosure of the Company’s confidential commercial information constitutes a taking for purposes of the Fifth Amendment and award just compensation. The federal government filed a motion to dismiss the complaint, which the Company opposed. In January 2024, the Federal Claims Court held an oral argument on the motion to dismiss, following which the Federal Claims Court issued a decision denying in part the government’s motion, allowing the Company’s takings claim to proceed. In April 2024, the government filed a judgment on the pleadings, to which the Company opposed in July 2024 and the government replied in August 2024. In December 2024, the Federal Claims Court held an oral argument on the motion for judgment on the pleadings and in January 2025, the Federal Claims Court ruled against the Company. In February 2025, the Company appealed the ruling.
In February 2024, the Company filed a lawsuit in the DC District Court against the FDA to compel the FDA to comply with its statutory obligations under the FDCA and its implementing regulations, and to challenge the FDA’s complete response letter and 60-day filing regulations, which the Company believes do not absolve the FDA of its statutory responsibilities. Under
the FDCA, the FDA has an obligation to either approve the Company’s sNDA for HETLIOZ® in the treatment of insomnia characterized by difficulties with sleep initiation within 180 days of the filing of the sNDA or give the Company a notice of an opportunity for a hearing. The Company submitted the sNDA on May 4, 2023. The Company has asked the DC District Court to, among other things, compel the FDA to comply with its obligations, declare that its lack of compliance violates the FDCA and the FDA regulations and declare the FDA’s complete response letter and 60-day filing regulations unlawful. In June 2024, the Company filed a motion for summary judgment and the FDA published a notice of opportunity for a hearing. In July 2024, the FDA opposed the Company’s motion for summary judgment. In September 2024, the DC District Court held an oral argument on the dispositive cross motions. The Company's lawsuit remains pending.
In March 2024, the Company filed a petition for review in the U.S. Court of Appeals for the District of Columbia Circuit (DC Circuit) seeking review of the FDA’s final order refusing to hold a hearing or to approve the Company’s sNDA for HETLIOZ® in the treatment of jet lag disorder. Under the FDCA, the FDA has an obligation to either approve an sNDA or to hold a hearing on the application’s approvability. The Company’s petition asks the DC Circuit to set aside the FDA’s order refusing to hold a hearing and refusing approval. In January 2025, the DC Circuit held an oral argument. The Company’s petition remains pending.
On April 22, 2024, a purported stockholder of the Company filed a lawsuit in the Court of Chancery of the State of Delaware (Delaware Chancery Court) against the members of the Company’s board of directors and the Rights Agent, along with the Company as nominal defendant (collectively, the Defendants), captioned Steamfitters Local 449 Pension Fund v. Mihael H. Polymeropoulos, et al., CA No. 2024-0416-KSJM. The lawsuit contended, among other things, that the members of the Company’s board of directors breached their fiduciary duties in adopting the Rights Agreement. The lawsuit sought relief declaring, in part, that provisions of the Rights Agreement be deemed unenforceable and sought to enjoin the use of such provisions as well as damages, costs, and other remedies, and also sought to enjoin for 30 days the Company’s 2024 Annual Meeting of Stockholders (the Annual Meeting) that was held on May 17, 2024. At a hearing on May 7, 2024, the Delaware Chancery Court denied the plaintiff’s request to enjoin the Annual Meeting. A three-day trial in the case was scheduled to begin on November 4, 2024. On August 7, 2024, the Company amended the Rights Agreement to, among other things, clarify certain of the provisions that were the subject of the lawsuit. On August 12, 2024, the parties filed with the Delaware Chancery Court a stipulation and order dismissing the lawsuit with prejudice, pursuant to which, the plaintiff agreed that the amendment to the Rights Agreement mooted the plaintiff’s claims. The plaintiff filed a petition in the Delaware Chancery Court for an award of attorney’s fees and reimbursement of expenses, which the Company is opposing. In February 2025, the Delaware Chancery Court held a hearing, and a decision remains pending.
In August 2024, the Company filed a lawsuit against the FDA in the DC District Court challenging FDA decisionmakers’ authority under the Appointments Clause of the U.S. Constitution to render a decision on the Company’s new drug application for tradipitant to treat symptoms of gastroparesis. In September 2024, the Company filed a motion for a preliminary injunction to enjoin the FDA from subjecting the Company’s NDA for tradipitant for the treatment of symptoms of gastroparesis to a final decision prior to the PDUFA target date of September 18, 2024. In September 2024, the DC District Court denied the motion. The Company filed a motion for summary judgment in December 2024, and in January 2025, the FDA opposed the motion and cross-moved to dismiss and for summary judgment. The Company’s lawsuit remains pending.
In January 2025, the Company and a co-plaintiff filed a lawsuit against the FDA in the Middle District of Florida District Court (Florida District Court) challenging the FDA’s partial clinical hold preventing the Company from studying the effects of tradipitant for the prevention of motion sickness for more than 90 total doses per patient over a period of two years. The FDA based its partial clinical hold on the Company’s refusal to perform a repeat-dose toxicity study in a non-rodent species of at least a 6-month duration. However, under the FDA Modernization Act 2.0, nonclinical studies may include non-animal alternatives. The Company believes that the FDA’s basis for the partial clinical hold is contrary to law and in excess of the FDA’s statutory authority. The Company and co-plaintiff have asked the Florida District Court to, among other things, set aside the FDA’s partial clinical hold and declare the FDA’s partial clinical hold as unlawful. The Company’s lawsuit remains pending.
VANDA PHARMACEUTICALS INC.
EXHIBIT INDEX
| | | | | | | | |
Exhibit
Number | | Description |
| | |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 3.3 | | |
| | |
| 4.1 | | |
| | |
| 4.2 | | |
| | |
| 4.3 | | |
| | |
| 4.4 | | |
| | |
| 4.5 | | |
| | |
| 10.1 | | |
| | |
| 10.2† | | |
| | |
| 10.3† | | |
| | |
| 10.4† | | |
| | |
| 10.5† | | |
| | |
| 10.6† | | |
| | |
| 10.7 | | |
| | |
| 10.8 | | |
| | |
| | | | | | | | |
Exhibit
Number | | Description |
| 10.9 | | |
| | |
| 10.10 | | |
| | |
| 10.11 | | |
| | |
10.12‡ | | |
| | |
| 10.13 | | |
| | |
| 10.14 | | |
| | |
| 10.15 | | |
| | |
| 10.16 | | |
| | |
| 10.17 | | |
| | |
| 10.18 | | |
| | |
| 10.19 | | |
| | |
| 10.20 | | |
| | |
| 10.21 | | |
| | |
| 10.22 | | |
| | |
| 10.23† | | |
| | |
| 10.24† | | |
| | |
10.25‡ | | |
| | |
| | | | | | | | |
Exhibit
Number | | Description |
| 10.26 | | |
| | |
| 10.27 | | |
| | |
| 10.28 | | |
| | |
| 10.29 | | |
| | |
| 10.30 | | |
| | |
| 10.31 | | |
| | |
| 10.32† | | |
| | |
| 10.33† | | |
| | |
| 10.34† | | |
| | |
| 10.35† | | |
| | |
| 10.36 | | |
| | |
| 10.37 | | |
| | |
| 10.38 | | |
| | |
| 10.39† | | |
| | |
| 10.40† | | |
| | |
| 10.41† | | |
| | |
| 10.42† | | |
| | |
| | | | | | | | |
Exhibit
Number | | Description |
| 10.43† | | |
| | |
| 10.44† | | |
| | |
| 10.45† | | |
| | |
| 10.46† | | |
| | |
| 10.47‡ | | |
| | |
| 19.1* | | |
| | |
| 21.1 | | |
| | |
| 23.1* | | |
| | |
| 31.1* | | |
| | |
| 31.2* | | |
| | |
| 32.1** | | |
| | |
| 97 | | |
| | |
| 101* | | The following financial information from this annual report on Form 10-K for the fiscal year ended December 31, 2024, formatted in Inline Extensible Business Reporting Language (iXBRL) and furnished electronically herewith: (i) Consolidated Balance Sheets as of December 31, 2024 and 2023; (ii) Consolidated Statements of Operations for the years ended December 31, 2024, 2023 and 2022; (iii) Consolidated Statements of Comprehensive Income (Loss) for the years ended December 31, 2024, 2023 and 2022; (iv) Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2024, 2023 and 2022; (v) Consolidated Statements of Cash Flows for the years ended December 31, 2024, 2023 and 2022; and (vi) Notes to Consolidated Financial Statements. |
| | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
| | |
| † | | Indicates management contract or compensatory plan or arrangement. |
| | |
| ‡ | | Portions of this exhibit have been omitted under rules of the Securities and Exchange Commission permitting the confidential treatment of select information. |
| | |
| * | | Filed herewith. |
| | |
| ** | | Furnished herewith. |





