UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended December 25, 2010 |
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | for the transition period from to . |
Commission file number: 001-34507
VITAMIN SHOPPE, INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 11-3664322 |
(State or Other Jurisdiction of Incorporation or Organization) | | (IRS Employer Identification No.) |
2101 91st Street
North Bergen, New Jersey 07047
(Addresses of Principal Executive Offices, including Zip Code)
(800) 223-1216
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of Class | | Name of the exchange on which registered |
Common Stock, $0.01 par value per share | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. x Yes ¨ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer ¨ | | Accelerated filer x | | Non-accelerated filer ¨ | | Smaller reporting company ¨ |
(Do not check if smaller reporting company)
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) ¨ Yes x No
As of March 4, 2011, Vitamin Shoppe, Inc. had 28,735,024 shares of common stock outstanding.
TABLE OF CONTENTS
2
Forward-Looking Statements
Statements in this document that are not historical facts are hereby identified as “forward looking statements” for the purposes of the safe harbor provided by Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Section 27A of the Securities Act of 1933, as amended (the “Securities Act”). Vitamin Shoppe, Inc., (“VSI”), Vitamin Shoppe Industries Inc. (“Industries”) and VS Direct Inc. (“Direct,” and, together with VSI and Industries, the “Company,” “we,” “us” or “our”) caution readers that such “forward looking statements”, including without limitation, those relating to the Company’s future business prospects, revenue, new stores, working capital, liquidity, capital expenditures, capital needs, leverage levels, interest costs and income, wherever they occur in this document or in other statements attributable to the Company, are estimates reflecting the judgment of the Company’s senior management and involve a number of risks and uncertainties that could cause the Company’s actual results to differ materially from those suggested by the “forward looking statements.” You can identify these statements by forward-looking words such as “may,” “expect,” “intend,” “anticipate,” “plan,” “believe,” “seek,” “estimate,” “outlook,” “trends,” “future benefits,” “strategies,” “goals” and similar words. Such “forward looking statements” should, therefore, be considered in light of the factors set forth throughout this annual report on Form 10-K, including “Item 7—Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Risk Factors.”
Moreover, the Company, through its senior management, may from time to time make “forward looking statements” about matters described herein or other matters concerning the Company. You should consider our forward-looking statements in light of the risks and uncertainties that could cause the Company’s actual results to differ materially from those which are management’s current expectations or forecasts. These risks and uncertainties include, but are not limited to, industry based factors such as the level of competition in the vitamin, mineral and supplement (“VMS”) industry, continued demand from the primary markets the Company serves, the availability of raw materials, as well as factors more specific to the Company such as restrictions imposed by the Company’s debt including financial covenants and limitations on the Company’s ability to incur additional indebtedness, the Company’s future capital requirements, and risk associated with economic conditions generally. See “Item 1A—Risk Factors” for further discussion.
The Company disclaims any intent or obligation to update “forward looking statements” to reflect changed assumptions, the occurrence of unanticipated events, or changes to future operating results over time.
Electronic Access to Company Reports
Our investor website can be accessed atwww.vitaminshoppe.com under “Investor Relations.” Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished to the Securities and Exchange Commission (the “SEC”) pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge on our investor website under the caption “SEC Filings” promptly after we electronically file such materials with, or furnish such materials to, the SEC. No information contained on any of our websites is intended to be included as part of, or incorporated by reference into, this Annual Report on Form 10-K. Information relating to corporate governance at our Company, including our Corporate Governance Guidelines, our Code of Ethics and Business Practices for all directors, officers, and employees, and information concerning our directors, Committees of the Board, including Committee charters, and transactions in Company securities by directors and executive officers, is available at our investor website under the captions “Corporate Governance” and “SEC Filings.” Paper copies of these filings and corporate governance documents are available to stockholders free of charge by written request to Investor Relations, The Vitamin Shoppe, Inc., 2101 91st Street, North Bergen, New Jersey, 07047. Documents filed with the SEC are also available on the SEC’s website atwww.sec.gov.
3
PART I
Unless the context requires otherwise, references in this annual report on Form 10-K to the “Company,” “we,” “us” and “our” refer to Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and VS Direct Inc. References to “VMS” mean vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products. References to “Fiscal” or “Fiscal Year” mean the fifty-two weeks ended for all years covered under this report.
Overview of our Company
We are a leading specialty retailer and direct marketer of vitamins, minerals, herbs, supplements, sports nutrition and other health and wellness products. For each of the past six years, we have been the fastest growing national VMS specialty retailer while maintaining our position as the second largest in retail sales in our industry. We market over 700 different nationally recognized brands as well as our proprietary Vitamin Shoppe, BodyTech and MD Select brands. We believe we offer the greatest variety of products among VMS retailers with approximately 8,000 stock keeping units (“SKUs”) offered in our typical store and an additional 12,000 SKUs available through our Internet and other direct sales channels. We target the dedicated, well-informed VMS consumer and differentiate ourselves by providing high quality products at competitive prices in an educational and high-touch customer service environment. We believe our extensive product offerings, together with our well-known brand name and emphasis on product education and customer service, help us bond with our target customer and serve as a foundation for strong customer loyalty.
We sell our products through two business segments: retail and direct. In our retail segment, we have leveraged our successful store economic model by opening a total of 184 new stores from the beginning of fiscal year 2007 through fiscal year 2010. As of March 4, 2011, we operated 493 stores in 39 states and the District of Columbia, located in high-traffic regional retail centers. In our direct segment, we sell our products directly to consumers through our websites, primarily www.vitaminshoppe.com, and our catalog. Our websites and our catalog complement our in-store experience by extending our retail product offerings and by enabling us to access customers outside our retail markets and those who prefer to shop online.
On February 16, 2011, we announced executive management promotions that will become effective April 4, 2011. Richard Markee, currently our Chief Executive Officer and Chairman of the Board, will continue to be Executive Chairman for our company as well as maintain his position as Chairman of the Board, while Anthony Truesdale, currently our company’s President and Chief Merchandising Officer, will assume the role of Chief Executive Officer. Michael Archbold, currently our Executive Vice President, Chief Financial Officer and Chief Operating Officer, has been named President of our company and will maintain his position as Chief Operating Officer. In addition, Brenda Galgano has been appointed Chief Financial Officer and will join our company April 4, 2011. Ms. Galgano was most recently Chief Financial Officer and Treasurer at the grocer The Great Atlantic & Pacific Tea Company, Inc. (“A&P”).
On November 2, 2009, we completed an initial public offering (“IPO”). Prior to and in connection with the IPO, VS Parent, Inc. (our former Parent company) merged into VS Holdings, Inc., with VS Holdings, Inc. being renamed as Vitamin Shoppe, Inc. (the “Merger”). All common shares and warrants previously issued by VS Parent, Inc., were converted to common shares of Vitamin Shoppe, Inc., at approximately a 1.8611-for-one split, and all preferred shares issued by VS Parent, Inc. were converted on a one-to-one basis to preferred shares of Vitamin Shoppe, Inc. In addition, as the Merger was between entities under common control all financial statements presented herein were retroactively restated to reflect the Merger as if it occurred prior to December 29, 2007.
Segment Information
We sell our products through two business segments: retail, which is our retail store format, and direct, which consists of our internet and catalog formats.
Retail. We believe we operate a unique retail store format in the VMS industry, which has been successful in diverse geographic and demographic markets, ranging from urban locations in New York City to suburban locations in Plantation, Florida and Manhattan Beach, California, as well as to resort locations in Hawaii. Our stores carry a broad selection of VMS products and are staffed with experienced and knowledgeable associates who are able to educate our customers about product features and assist in product selection.
Direct. We sell our products directly to consumers through our websites, primarily www.vitaminshoppe.com. Our websites and our catalog complement our in-store experience by extending our retail product offerings with an additional 12,000 SKUs that are not available in our stores and enable us to access customers outside our retail markets and those who prefer to shop online. Catalog sales were not material in 2010, and are expected to remain immaterial in the future, as customers migrate to our website and stores. In 2010 we increased the number of active online customers, defined by shopping frequency, by approximately 141,000 to 669,000.
4
Industry
The VMS industry in the U.S. is highly fragmented, and based on the most current information available from the Nutrition Business Journal (“NBJ”) and public filings with the SEC, no single industry participant accounted for more than 5% of total industry sales in 2009. Retailers of VMS products primarily include specialty retailers and mass merchants, such as drugstores and supermarkets. The specialty retailers typically cater to the more sophisticated VMS customer by focusing on selection and customer service, while the mass merchants generally offer a limited assortment comprised of more mainstream products with less customer care. Specialty retailers comprised the largest segment of the market in 2009, with 37% market share, sales of which are forecasted to grow by 5.0% annually through 2017, according to the NBJ.
According to the NBJ, the U.S. nutritional supplements industry was a $26.9 billion retail market in 2009, and is projected to grow at an approximately a 5.4% average annual growth rate through 2017. Positive industry trends include an aging U.S. population, rising healthcare costs and the increased use of preventive measures. In addition, the increased focus on diet and nutrition, along with growing fitness and wellness program participation, serves as a positive trend for the nutritional supplements industry.
Competitive Strengths
We believe we are well positioned to capitalize on the favorable VMS industry dynamics as a result of the following competitive strengths:
Most Extensive Product Selection, Including a Strong Assortment of Proprietary Brands. We believe we have the most complete and authoritative merchandise assortment and market the broadest product selection in the VMS industry, with over 20,000 competitively priced SKUs from a combination of over 700 different nationally recognized brands and our proprietary brands. Our proprietary brand merchandise accounted for approximately 24% of our net sales in fiscal 2010, and provides our customers the opportunity to purchase VMS products at a great value while affording us higher gross margins.
Value-Added Customer Service. We believe we offer the highest degree of customer service in the VMS retail industry, aided by the deep product knowledge of our experienced store associates. We place a strong emphasis on employee training and customer service and view our sales associates as health and wellness information stewards who educate our customers while assisting them with their product selections.
Highly Refined Real Estate Strategy. We apply demanding criteria to our retail site selection. We locate our stores exclusively in attractive stand-alone locations or endcap (corner) positions in retail centers. We believe that the location and visibility of our real estate is our single most effective and efficient customer acquisition strategy.
Attractive, Loyal Customer Base. We have a large and growing base of loyal customers who proactively manage their long-term health and wellness through the use of supplements. Our no-fee Healthy Awards Program promotes brand loyalty among our customers and allows our customers to earn points redeemable for future purchases, approximately 74% of which are redeemed annually.
Multi-Channel Retailer. We are a multi-channel retailer, distributing products through our retail stores, our websites and our catalog, enabling us to access customers outside our retail markets and those who prefer to shop online. This business model affords us multiple touch points with our customers, which allows us to gather data and communicate with them in person, through our call center and via the web.
Experienced Management Team with Proven Track Record. We have assembled a management team across a broad range of disciplines with extensive experience in building leading national specialty retailers.
Business Strategy
We intend to continue to pursue the following key strategies to drive customer traffic and grow our sales:
| | • | | enhance our customers’ shopping experience by continuing to offer a broad selection of VMS products and providing them with a convenient shopping experience, value-added customer service and low prices. We continue to expand categories complementary to our core VMS and Sports Nutrition products such as our sports nutrition accessories. These categories have shown strong growth in Fiscal 2009 and Fiscal 2010, and we intend to continue to foster that growth into Fiscal 2011. We continue to strengthen our assortment by adding brands and line extensions in growing categories such as Vitamin D, Essential Fatty Acids and Superfood products while reducing offerings in shrinking categories. As a result, we have added approximately 1,400 items over the course of a year and removed roughly the same number. We will review these products along with our entire product mix on an ongoing basis to best ensure the relevancy of our product mix; |
| | • | | utilize our extensive customer database to improve customer loyalty, facilitate direct marketing and increase cross-sell opportunities. In addition, over the past 24 months we have significantly increased the number of emails sent to our |
5
customers as well as the number of email addresses in our database, with the majority of information being obtained from our Healthy Awards information queries;
| | • | | continue to invest in associate training and provide employees with opportunities to grow within our Company. In 2010 we held our sixth product education conference, attended by our store and district managers, and a large body of our vendors. It is our current plan to continue this conference as an annual event. In addition, we have increased the number of courses available through our Vitamin Shoppe University, and intend to increase them further in the coming Fiscal year; |
| | • | | increase sales of our direct business segment by enhancing the features and functionality of our websites and providing our customers with a more personalized shopping experience. We are going to continue to enhance our Web platform attributes for customer tracking and marketing ability, which allows us, among other things, to track purchases by customer. In addition, we will continue to increase our paid key word search terms, and will continue to add these search terms to various shopping search engines; and |
| | • | | increase sales and profitability through the maturation of the 184 stores we opened since the beginning of 2007 through Fiscal 2010. In Fiscal 2010, comparable stores outperformed their business plans, resulting in a 7.1% increase in comparable store sales (a store is included in the comparable store sales calculation after 410 days of operations). The increase in our store base has improved shopping convenience to our customers, and we plan to selectively open approximately 40 new stores per year over the next few years. |
Retail Stores
We believe we operate a unique retail store format in the VMS industry by locating our retail stores in high-traffic areas, instead of a mall-based retail format similar to our competitors. Many of our stores are freestanding, which further enhance store visibility. Our retail store format has been successful in diverse geographic and demographic markets, ranging from urban locations in New York City and Chicago, to suburban locations in Plantation, Florida and Manhattan Beach, California, as well as to resort locations in Hawaii. Our stores carry a broad selection of VMS products and are staffed with highly experienced and knowledgeable associates who are able to educate our customers about product features and assist in product selection.
6
Store Counts and Locations
We plan to open approximately 48 new stores in Fiscal 2011. The following table shows the change in our network of stores for the Fiscal Years 2006 through 2010:
| | | | | | | | | | | | | | | | | | | | |
| | | Fiscal Year | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
Stores open at beginning of year | | | 438 | | | | 401 | | | | 341 | | | | 306 | | | | 275 | |
Stores opened | | | 47 | | | | 39 | | | | 62 | | | | 36 | | | | 32 | |
Stores closed | | | (1 | ) | | | (2 | ) | | | (2 | ) | | | (1 | ) | | | (1 | ) |
| | | | | | | | | | | | | | | | | | | | |
Stores open at end of year | | | 484 | | | | 438 | | | | 401 | | | | 341 | | | | 306 | |
| | | | | | | | | | | | | | | | | | | | |
Our stores typically require three to four years to mature, generating lower store level sales and store contribution in the initial years than our mature stores. As a result, new stores generally have a negative impact on our overall operating margin. As our recently opened stores mature, we expect them to contribute meaningfully to our sales and store contribution. The following table reflects our store count by state at December 25, 2010:
| | | | | | | | | | |
State | | Stores Open at
December 25, 2010 | | | State | | Stores Open at
December 25, 2010 | |
Alabama | | | 4 | | | Minnesota | | | 4 | |
Arizona | | | 9 | | | Missouri | | | 2 | |
California | | | 56 | | | Nevada | | | 3 | |
Colorado | | | 8 | | | New Hampshire | | | 3 | |
Connecticut | | | 9 | | | New Jersey | | | 24 | |
Delaware | | | 2 | | | New Mexico | | | 2 | |
District of Columbia | | | 1 | | | New York | | | 59 | |
Florida | | | 61 | | | North Carolina | | | 14 | |
Georgia | | | 12 | | | Ohio | | | 15 | |
Hawaii | | | 5 | | | Oregon | | | 4 | |
Iowa | | | 1 | | | Pennsylvania | | | 15 | |
Idaho | | | 1 | | | Rhode Island | | | 1 | |
Illinois | | | 23 | | | South Carolina | | | 8 | |
Indiana | | | 7 | | | Tennessee | | | 8 | |
Kansas | | | 2 | | | Texas | | | 40 | |
Kentucky | | | 4 | | | Vermont | | | 1 | |
Louisiana | | | 3 | | | Virginia | | | 22 | |
Maryland | | | 15 | | | Washington | | | 7 | |
Massachusetts | | | 12 | | | Wisconsin | | | 6 | |
Michigan | | | 11 | | | | | | | |
| | | | | | | | | | |
| | | | | | Total | | | 484 | |
| | | | | | | | | | |
As of March 4, 2011, we leased the property for all of our 493 stores. Our typical lease terms are ten years, with one or two five-year renewal options. We do not believe that any individual store property is material to our financial condition or results of operations. Of the leases for our stores, 10 expire in Fiscal 2011, 26 expire in Fiscal 2012, 60 expire in Fiscal 2013, 79 expire in Fiscal 2014, 62 expire in Fiscal 2015, and the balance expire in Fiscal 2016 or thereafter.
Our primary warehouse and distribution center and corporate headquarters are consolidated into a leased, 230,000 square-foot state-of-the-art facility. The initial lease term for the facility, which commenced in 2002, is for 15 years, with one five-year renewal option. In addition to our primary facility, we entered into an agreement with a west coast third party logistics facility during Fiscal 2010, which we began fully utilizing during the third fiscal quarter of Fiscal 2010. The agreement is for a period of three years, and supplies certain of our stores in the western United States with our most popular products.
7
We believe that all of our current facilities are in good condition and are suitable and adequate for our current and reasonably anticipated future needs.
8
Other
We organize our products by category enabling easy comparisons between different brands within each product sub-category, including our Vitamin Shoppe and BodyTech proprietary brands. In addition, our stores are staffed with highly experienced and knowledgeable health enthusiasts, many of whom are regular and informed VMS consumers. Our associates are trained to educate our customers about product features and assist our customers in product selection. To further educate our customers, our stores are equipped with Health Notes, as well as a library whereby our customers can freely read health related literature and purchase books for their personal libraries.
Products
We offer a broad selection of vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products with over 20,000 SKUs from over 700 national brands. Our products are sold under our Vitamin Shoppe, BodyTech and MD Select brand names, including Ultimate Man, Ultimate Woman and Whey Tech, and under nationally recognized third party brand names, including Solgar®, Twinlab®, EAS® and Nature’s Way®. This variety is designed to provide our customers with a vast selection of products to fit their specific needs. Sales of our Vitamin Shoppe and BodyTech branded products account for approximately 24% of our net sales in Fiscal 2010.
Key Product Categories
Based on data collected from our merchandise systems, below is a comparison of our net merchandise sales by major product category and the respective percentage of our total net merchandise sales for the periods shown (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | |
Product Category | | Fiscal 2010 | | | Fiscal 2009 | | | Fiscal 2008 | |
| | | Dollars | | | % | | | Dollars | | | % | | | Dollars | | | % | |
Vitamins, Minerals and Herbs | | $ | 302,568 | | | | 40 | % | | $ | 242,210 | | | | 36 | % | | $ | 209,698 | | | | 35 | % |
Specialty Supplements and Sports Nutrition | | | 393,349 | | | | 53 | % | | | 381,434 | | | | 57 | % | | | 336,397 | | | | 56 | % |
Other | | | 51,130 | | | | 7 | % | | | 47,031 | | | | 7 | % | | | 51,190 | | | | 9 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 747,047 | | | | 100 | % | | | 670,674 | | | | 100 | % | | | 597,285 | | | | 100 | % |
Delivery Revenue | | | 4,435 | | | | | | | | 3,821 | | | | | | | | 4,255 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 751,482 | | | | | | | $ | 674,495 | | | | | | | $ | 601,540 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Vitamins and Minerals
Vitamins and minerals are taken to maintain health, proactively to improve health and in support of specific health conditions. These products help prevent nutrient deficiencies that can occur when diet alone does not provide all the necessary vitamins and minerals our bodies need. The vitamin and mineral product category includes multi-vitamins, which many consider to be a foundation of a healthy regimen, lettered vitamins, such as Vitamin A, C, D, E, and B-complex, along with major and trace minerals such as calcium, magnesium, chromium and zinc. With approximately 4,000 SKUs, a wide range of potency levels and multiple delivery systems, our customers have many choices to fit their individual needs. Our vitamin and mineral products are available in tablets, capsules, vegi-capsules, softgels, gelcaps, liquids and powders.
Herbs
Herbs offer a natural remedy and are taken to address specific conditions. Certain herbs can be taken to help support specific body systems, including ginkgo to support brain activity and milk thistle to help maintain proper liver function, as well as other less common herbs such as holy basil for stress relief and blood sugar control, turmeric for inflammation support and black cohosh for menopause support. Herbal products include whole herbs, standardized extracts, herbs designed for single remedies, herb combination formulas and teas. With over 7,000 SKUs, a wide range of potency levels and multiple delivery systems, our customers have many choices to fit their individual needs. Our herb products are available in tablets, capsules, vegi-capsules, soft gels, gelcaps, liquids, tea bags and powders.
9
Specialty Supplements
Specialty supplements help supply higher levels of nutrients than diet alone can provide, help people stay healthy, and support specific conditions and life stages such as childhood, pregnancy, menopause and aging. Categories of specialty supplements include essential fatty acids, probiotics and condition specific formulas. Certain specialty supplements, such as organic greens, psyllium fiber and soy proteins, are taken for added support during various life stages and are intended to supplement vital nutrients absent in an individual’s diet. Flax seed oils and folic acid are specifically useful during pregnancy. Super antioxidants, such as coenzyme Q-10, grapeseed extract and pycnogenol, are taken to address specific conditions. High ORAC (oxygen radical absorptive capacity) fruit concentrates like gogi, mangosteen, pomegranate and blueberry are taken to supplement high levels of natural nutrients not available in modern diets. Other specialty supplement formulas are targeted to support specific organs, biosystems and body functions. For example, we offer Ultimate Memory Aid for brain function, Sleep Naturally for sleeplessness and various enzyme combinations for other support systems. We offer over 5,000 supplement SKUs available in tablets, capsules, vegi-capsules, soft gels, gelcaps, sublingual and liquid forms.
Sports Nutrition
Our sports nutrition consumers are looking for products to help maintain or supplement a healthy lifestyle. These products are used in conjunction with cardiovascular conditioning, weight training and sports activities. Major categories in sports nutrition include protein and weight gain powders, meal replacements, nutrition bars, sport drinks and pre and post-workout supplements to either add energy or enhance recovery after exercise. Our sports nutrition products are offered in many convenient forms, such as powders, tablets, capsules, soft gels and liquids. Our sports nutrition consumers include the sports enthusiast, weekend warrior, endurance athlete, marathoner and serious bodybuilder, as well as those seeking to maintain a healthy fitness level. We offer over 2,000 SKUs in sports nutrition.
Other
Our “Other” category represents all other product classifications we stock that do not fit within the previously described categories. These products include natural beauty and personal care, natural pet food, low carb foods and diet and weight management supplements. Natural beauty and personal care products offer an alternative to traditional products that often contain synthetic and/or other ingredients that our customers find objectionable. We offer over 2,000 SKUs for our other category. Our customers choose these products over more traditional products because they contain organic and natural ingredients, are produced without the use of pesticides or animal testing and are more closely aligned with the health and wellness goals of our customers. Our wide variety of diet and weight management products range from low calorie bars, drinks and meal replacements to energy tablets, capsules and liquids. Our natural pet products include nutritionally balanced foods and snacks along with condition specific supplements such as glucosamine for joint health.
Access to New Products and New Product Development
A key component of customer satisfaction is the introduction of new products. We identify customer trends through interactions with our customers, attending trade shows, contacting vendors and generally being active within the marketplace. We maintain close relationships with our third party branded manufacturers, which allows us to be at the forefront of introducing new third party branded products within the industry. In addition, we maintain a product development group that is staffed with knowledgeable employees who oversee our development of new Vitamin Shoppe branded products. During the past three Fiscal years, we focused on, and will continue to focus on, developing Vitamin Shoppe branded product offerings for beauty care, condition-specific and branded blended specialty supplements (which are designed to assist with certain conditions, for example, sleep difficulties) and functional foods and beverages (offering further benefits beyond nourishment and hydration, such as additional vitamins and minerals). We are also focusing on enhancing our Vitamin Shoppe branded product offerings under our BodyTech label as we see a significant opportunity for expansion in the sports nutrition category. We incurred $2.1 million, $1.5 million and $1.4 million of research and development costs for the Fiscal 2010, Fiscal 2009 and Fiscal 2008, respectively.
Suppliers and Inventory
Optimum Nutrition is the only supplier from whom we purchased at least 5% of our merchandise during Fiscal 2010, and Nature’s Value, Inc. was the only supplier from whom we purchased at least 5% of our merchandise during Fiscal 2009 and 2008. We purchased approximately 6% of our total merchandise from Optimum Nutrition during Fiscal 2010, and 6%, and 7% of our total merchandise from Nature’s Value, Inc. during Fiscal 2009 and 2008, respectively.
We consider numerous factors in supplier selection, including, but not limited to, quality, price, credit terms, and product offerings. As is customary in our industry, we generally do not have long-term contracts with any supplier and most suppliers may discontinue selling to us at any time.
10
We strive to maintain sufficient inventory to enable us to provide a high level of service to our customers. Inventory, accounts receivable and accounts payable levels, payment terms and return policies are in accordance with standard business procedures. We maintain a distribution center which we use in conjunction with a just-in-time inventory ordering system that we use to replenish our stores based upon customer demand of a given product or products. Our working capital requirements for merchandise inventory will continue to increase as we continue to open additional stores. Currently, our practice is to establish an inventory level of approximately $165,000 to $185,000 in cost for each of our stores, a portion of which is vendor-financed, based upon agreed credit terms, with the remainder being purchased in cash. Thirty day payment terms are extended to us by some of our suppliers allowing us to effectively manage our inventory and working capital. We believe that our buying power enables us to receive favorable pricing terms and enhances our ability to obtain high demand merchandise.
Warehouse and Distribution
Our primary distribution facility is a state of the art warehouse facility which provides operating space of approximately 180,000 square feet and gives us great control over supervision costs and distribution center related inventory levels. In addition, through a combination of improved technology, processes, controls and layout, we have greatly improved our pick accuracy rates and net inventory accuracy rates. The primary facility currently operates two shifts, seven days a week, and offers the ability to expand our schedule and capacity to meet future demand. In addition, we warehouse and distribute from a west coast third party logistics provider with operating space of approximately 20,000 square feet, which operates one shift seven days a week, to service certain of our west coast stores. With minor physical changes, and systems enhancements to our primary facility, along with our west coast distribution operations, we believe we have sufficient warehousing and distribution capacity for the next several years.
Regulatory and Quality Control
The Food and Drug Administration (“FDA”) is the regulatory authority charged with overseeing the products marketed by us and the products found in our stores. The Federal Trade Commission (“FTC”) regulates the advertising of the products marketed by us and the products found in our stores.
Our Scientific and Regulatory Affairs (“S&RA”) department reviews all aspects of our Company’s FDA and FTC regulatory processes, ensuring compliance with regulations. We have established processes to review the underlying safety and efficacy of our Vitamin Shoppe and BodyTech branded products. These processes include review of the ingredients’ safety information, product formulation, product form, product labeling, the efficacy and claim support for the product and any marketing materials. All consumer communications that deal with product and health issues must be approved by S&RA prior to being disseminated to the public.
We have standard procedures whereby all potential Vitamin Shoppe contract manufacturers are reviewed and approved before they can supply any of our Vitamin Shoppe or BodyTech branded products. In addition, all potential new products are vetted and approved prior to being accepted into our Vitamin Shoppe or BodyTech branded product line.
Our three primary suppliers for our Vitamin Shoppe and BodyTech branded products are Nature’s Value, Inc., Rasi Laboratories, Inc., and Main Street Ingredients, and Softgel Technology, Inc, which together produce over half of our Vitamin Shoppe and BodyTech branded products. We have long-term relationships with these suppliers of over ten years. There are numerous contract manufacturers in our industry and we do not believe it would be difficult to source our products from other vendors, should all of our three primary suppliers cease providing us with supplies. Our relationships with manufacturers require that all Vitamin Shoppe and BodyTech branded products not be adulterated or misbranded under any provisions of the Federal Food, Drug, and Cosmetic Act (“FDCA”) and the regulations promulgated thereunder. This includes, but is not limited to, compliance with applicable Good Manufacturing Practices (“GMP”). This means that ingredients in our products must be tested for identity, purity, quality, strength, and composition before being incorporated into our Vitamin Shoppe or BodyTech branded products, and that our final Vitamin Shoppe and BodyTech branded products must again be tested for identity, purity, quality, strength, and composition prior to being released. All of these products require a certificate of analysis, which includes certification to 100% of label claim.
We have established a standard quality control operating procedure that calls for on-site audits of our contract manufacturers’ facilities and processes, and have established an internal team that will audit each of these facilities and work with our contract manufacturers to resolve any noncompliance with dietary supplement GMP regulations. We require that our manufacturers have certificates of analysis (such as for microbe testing and label testing).
Additionally, we have established standard quality control operating procedures to review vendors of third party products and require them to carry adequate insurance policies to satisfy our standards. We further review each new product proposed to be carried by us to assure the safety of the ingredients. We reject those products that we believe may be unsafe. Our third party manufacturers and distributors and contract manufacturers deliver finished products to our warehouse and distribution centers, which then supply our retail and direct channels with products.
11
Healthy Awards Program
Our Healthy Awards Program, which we established over 14 years ago, encourages our customers to make repeat purchases and enables us to enhance customer loyalty. The program is open to customers across our two distribution channels and is free of charge to join. Members of the program earn one point for every dollar they spend, starting with the first purchase upon joining the program. If a member accumulates over 100 points between January 1 and December 31 in a calendar year, the member will receive a special credit certificate in January of the following year to use on any single purchase made before March 31 of that year. We signed up approximately 1.2 million new members in our new and existing stores in Fiscal 2010. The number of active members between retail and online shoppers has grown to approximately 4.1 million as of December 25, 2010.
We utilize our Healthy Awards Program database to track customer purchasing patterns across our two business segments, analyze market and industry trends and create targeted merchandising and marketing strategies. In addition, it provides us with customer and demographic data that has been used to assist us in the selection of future store locations.
Marketing
We believe our high quality real estate is one of our primary marketing tools, as we ensure that our stores are located in high-visibility areas. We advertise in national magazines, and do local advertising via direct mail, radio and television for new stores. We also do niche radio advertising and occasional television advertising. We also exhibit at many consumer trade shows across the country. Additionally, we conduct targeted marketing efforts by mailing offers and promotional announcements to members of our Healthy Awards Program.
We promote our Vitamin Shoppe and BodyTech branded products through our retail channel by placing the products in strategic and highly visible locations in our stores. Our retail and promotional activities promote our Vitamin Shoppe and BodyTech branded products as the “best value” brand of our in-store products.
Competition
The U.S. nutritional supplements retail industry is highly competitive and fragmented. According to the NBJ and public filings with the SEC, no single retailer accounted for more than 5% of total industry sales in 2009. Competition is based primarily on quality, product assortment, price, customer service, marketing support and availability of new products. We compete with publicly and privately owned companies with broad geographical market coverage and product categories. We compete with other specialty and mass market retailers including Vitamin World®, GNC®, Whole Foods®, Costco® and Wal-Mart®, Internet and mail order companies including Puritan’s Pride®, Vitacost.com®, Bodybuilding.com®, Doctors Trust®, Swanson® and iHerb® in addition to a variety of independent health and vitamin stores.
Insurance and Risk Management
We purchase insurance to cover standard risks in our industry, including policies to cover general and products liability, workers compensation, travel liability, auto liability and other casualty and property risks. Our insurance rates are based on our safety record as well as trends in the insurance industry.
We face an inherent risk of exposure to product liability claims in the event that, among other things, the use of our products results in injury. With respect to product liability coverage, we carry insurance coverage typical of our industry and product lines. Our coverage involves self-insured retentions with primary and excess liability coverage above the retention amount. We have the ability to refer claims to our contract manufacturers, third party vendors and their respective insurers to pay the costs associated with any claims arising from such contract manufacturers’ or third party vendors’ products. Our insurance covers any claims that are not adequately covered by a contract manufacturer’s or third party vendor’s insurance and provides for excess secondary coverage above the limits provided by our contract manufacturers or third party vendors. We believe we have obtained a prudent amount of insurance for the insurable risks associated with our business. Our experience is that our insurance costs have increased in the past, and may increase in the future.
Trademarks and Other Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized proprietary brand names under which we market our products. We own material trademarks or trade names that we use in conjunction with the sale of our products, including the Vitamin Shoppe, BodyTech, MD Select, and Eco Shoppe brand names. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We protect our intellectual property rights through a variety of methods including trademark and trade secret laws, as well as confidentiality agreements and proprietary information agreements with vendors, employees, consultants and others who have access to our proprietary information. Protection of our intellectual property often affords us the opportunity to enhance our position in the
12
marketplace by precluding our competitors from using or otherwise exploiting our technology and brands. The carrying value of our trademark, which is an indefinite lived intangible asset, was $68.8 million at both December 25, 2010 and December 26, 2009.
Sales from International Sources
For the last three years, less than 0.5% of our sales have been derived from international sources.
Employees
As of December 25, 2010, we had a total of 2,220 full-time and 1,361 part-time employees, of whom 3,083 were employed in our retail channel and 498 were employed in corporate, distribution and direct channel support functions. None of our employees belongs to a union or is a party to any collective bargaining or similar agreement. We consider our relationships with our employees to be good.
Environmental
We are subject to numerous federal, state, local and foreign laws and regulations governing our operations, including the handling, transportation and disposal of our products and our non-hazardous and hazardous substances and wastes, as well as emissions and discharges into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for corrective action, penalties or the imposition of other liabilities. Changes in environmental laws or the interpretation thereof or the development of new facts could also cause us to incur additional capital and operational expenditures to maintain compliance with environmental laws and regulations. We also are subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties. The presence of contamination from such substances or wastes could also adversely affect our ability to utilize our leased properties. Compliance with environmental laws and regulations has not had a material effect upon our earnings or financial position; however, if we violate any environmental obligation, it could have a material adverse effect on our business or financial performance.
Government Regulation
The formulation, manufacturing, processing, labeling, packaging, advertising and distribution of our products are subject to regulation by several federal agencies, including the FDA, the FTC, the Consumer Product Safety Commission, the U.S. Department of Agriculture (“DOA”) and the Environmental Protection Agency (“EPA”). These activities are also regulated by various agencies of the states and localities in which our products are sold. Pursuant to the FDCA, the FDA regulates the processing, formulation, safety, manufacture, packaging, labeling and distribution of dietary supplements (including vitamins, minerals, and herbs) and cosmetics. The FTC has jurisdiction to regulate the advertising of these products.
The FDCA has been amended several times with respect to dietary supplements, in particular by the Dietary Supplement Health and Education Act of 1994 (“DSHEA”). DSHEA established a new framework governing the composition, safety, labeling and marketing of dietary supplements. “Dietary supplements” are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances for human use to supplement the diet, as well as concentrates, metabolites, constituents, extracts or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. New dietary ingredients (i.e., not marketed in the U.S. prior to October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without being “chemically altered.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. There is no certainty that the FDA will accept any particular evidence of safety for any new dietary ingredient. The FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients.
DSHEA permits “statements of nutritional support” to be included in labeling for dietary supplements without premarket FDA approval. Such statements must be submitted to the FDA within 30 days of marketing and must bear a label disclosure that “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.” Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect body structure, function or well-being, but may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat, or prevent a disease. A company that uses a statement of nutritional support in labeling must possess scientific evidence substantiating that the statement is truthful and not misleading. If the FDA were to determine that a particular statement of nutritional support was an unacceptable drug claim or an unauthorized version of a disease claim for a food product, or if the FDA were to determine that a particular claim was not adequately supported by existing scientific data or was false or misleading, we would be prevented from using that claim.
13
In addition, DSHEA provides that so-called “third-party literature,” e.g. a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may be used “in connection with the sale of a dietary supplement to consumers” without the literature being subject to regulation as labeling. Such literature must not be false or misleading; the literature may not “promote” a particular manufacturer or brand of dietary supplement; and a balanced view of the available scientific information on the subject matter must be presented. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any dissemination could subject our product to regulatory action as an illegal drug. The FDA adopted final regulations regarding GMP, in manufacturing, packing, or holding dietary ingredients and dietary supplements, authorized by DSHEA on June 25, 2007. GMP regulations require dietary supplements to be prepared, packaged and held in compliance with strict rules, and require quality control provisions similar to those in the GMP regulations for drugs. We or our third party manufacturers may not be able to comply with these new rules without incurring substantial additional expenses.
The FDA has broad authority to enforce the provisions of the FDCA applicable to foods, dietary supplements, and cosmetics including powers to issue a public warning letter to a company, to publicize information about illegal products, to request a recall of illegal products from the market, and to request the Department of Justice to initiate a seizure action, an injunction action, or a criminal prosecution in the United States courts. The regulation of foods, dietary supplements and cosmetics may increase or become more restrictive in the future.
Legislation has been passed that imposes substantial new regulatory requirements for dietary supplements. One new law requires adverse event reporting, and some post-market surveillance requirements on the OTC (over the counter) and dietary supplement industries. Other legislation expected to be introduced in the current Congress could impose new requirements which could raise our costs and hinder our business.
The FTC exercises jurisdiction over the advertising of foods, dietary supplements and cosmetics. In recent years, the FTC has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. As a result of our efforts to comply with applicable statutes and regulations, we have from time to time reformulated, eliminated or relabeled certain of our products and revised certain provisions of our sales and marketing program. The FTC has broad authority to enforce its laws and regulations applicable to foods, dietary supplements and cosmetics, including the ability to institute enforcement actions which often result in consent decrees, injunctions, and the payment of civil penalties by the companies involved. Failure to comply with the FTC’s laws and regulations could impair our ability to market our products.
You should carefully consider the following factors, in addition to other information in this Annual Report on Form 10-K, in evaluating our Company and our business.
Unfavorable publicity or consumer perception of our products and any similar products distributed by other companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, resulting in decreased sales.
We are highly dependent upon consumer perception regarding the safety and quality of our products, as well as similar products distributed by other companies. Consumer perception of products can be significantly influenced by adverse publicity in the form of published scientific research, national media attention or other publicity, whether or not accurate, that associates consumption of our products or any other similar products with illness or other adverse effects, or questions the benefits of our or similar products or that claims that any such products are ineffective. A product may be received favorably, resulting in high sales associated with that product that may not be sustainable as consumer preferences change. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. Such research or publicity could have a material adverse effect on our ability to generate sales. For example, sales of some of our products, such as those containing Ephedra, were initially strong, but decreased as a result of negative publicity and an ultimate ban by the FDA. As a result of the above factors, our operations may fluctuate significantly from quarter-to-quarter and year to year.
Our failure to appropriately respond to changing consumer preferences and demand for new products and services could significantly harm our customer relationships and sales.
Our business is subject to changing consumer trends and preferences. Our failure to accurately predict or react to these trends could negatively impact consumer opinion of us as a source for the latest products, which in turn could harm our customer relationships and cause us to lose market share. The success of our new product offerings depends upon a number of factors, including our ability to:
| | • | | anticipate customer needs; |
| | • | | innovate and develop new products; |
| | • | | successfully commercialize new products in a timely manner; |
| | • | | price our products competitively with retail and online competitors; |
14
| | • | | deliver our products in sufficient volumes and in a timely manner; and |
| | • | | differentiate our product offerings from those of our competitors. |
If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could be rendered obsolete, which could have a material adverse effect on our sales and operating results.
We may incur material product liability claims, which could increase our costs and adversely affect our reputation, sales and operating income.
As a retailer and direct marketer of products designed for human consumption, we are subject to product liability claims if the use of our products is alleged to have resulted in injury or include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. Most of our products are vitamins, minerals, herbs and other ingredients that are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the United States. Our products could contain contaminated substances, and some of our products contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur. While we attempt to manage these risks by obtaining indemnification agreements and insurance, our insurance policies may not be sufficient or available and/or third parties may not satisfy their commitments to us. A product liability claim against us could result in increased costs and could adversely affect our reputation with our customers, which in turn could adversely affect our financial performance. See “Business—Legal Proceedings.”
We may not be able to obtain insurance coverage in the future at current rates.
Our current insurance program is consistent with both our past level of coverage and our risk management policies. While we believe we will be able to obtain product liability insurance in the future, because of increased selectivity by insurance providers we may only be able to obtain such insurance at increased rates and/or with reduced coverage levels which could reduce our income from operations.
Compliance with new and existing governmental regulations could increase our costs significantly and adversely affect our operating income.
The processing, formulation, manufacturing, packaging, labeling, advertising and distribution of our products are subject to federal laws and regulation by one or more federal agencies, including the FDA, Federal Trade Commission (“FTC”), the Department of Agriculture (“DOA”) and the Environmental Protection Agency (“EPA”). These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products are sold. Regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost sales and increased costs to us. The FDA may not accept the evidence of safety for any new ingredients that we may want to market, may determine that a particular product or product ingredient presents an unacceptable health risk, may determine that a particular statement of nutritional support on our products, or that we want to use on our products, is an unacceptable drug claim or an unauthorized version of a food “health claim,” or the FDA or the FTC may determine that particular claims are not adequately supported by available scientific evidence. Any such regulatory determination would prevent us from marketing particular products or using certain statements on our products which could adversely affect our sales of those products. The FDA also could require us to remove a particular product from the market. For example, in April 2004, the FDA banned the sale of products containing Ephedra. We stopped selling Ephedra-based products in June 2003. Sales of products containing Ephedra amounted to approximately $10.9 million, or 4% of our net sales, in 2002. Any recall or removal of products we sell could result in additional costs to us and the loss of future sales from any products that we are required to remove from the market. Any such product recalls or removals could also lead to liability and substantial costs. Delayed product introduction, product recalls or similar issues as a result of governmental regulation may arise from time to time, which may have a material adverse effect on our sales and operating results.
In addition, from time to time, Congress, the FDA, the FTC or other federal, state, local or foreign legislative and regulatory authorities may impose additional laws or regulations that apply to us, repeal laws or regulations that we consider favorable to us or impose more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations or to predict the effect additional governmental regulation, when and if it occurs, would have on our business in the future. Such developments could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or other new requirements. Any such developments could increase our costs significantly and could have a material adverse effect on our business, financial condition and results of operations. For example, legislation has been passed by Congress to, among other things, impose substantial new regulatory requirements for dietary supplements, including adverse event reporting, and post-market surveillance requirements, which could raise our costs and negatively impact our business. In addition, the FDA has adopted rules on good manufacturing practices (“GMP”) in manufacturing, packaging, or holding dietary ingredients and dietary supplements, which apply to the products we distribute. These regulations will require dietary supplements to be prepared, packaged and held in compliance with stricter rules, and require quality control provisions similar to those in the drug GMP regulations. We or our third-
15
party manufacturers may not be able to comply with the new rules without incurring additional expenses, which could be significant. See “Business—Government Regulation.”
We rely on contract manufacturers to produce all of the Vitamin Shoppe and BodyTech branded products we sell. Disruptions in our contract manufacturers’ systems or losses of manufacturing certifications could adversely affect our sales and customer relationships.
Our contract manufacturers produce 100% of our Vitamin Shoppe and BodyTech branded products. Any significant disruption in those operations for any reason, such as regulatory requirements and loss of certifications, power interruptions, fires, hurricanes, war or threats of terrorism could adversely affect our sales and customer relationships.
Increase in the price and shortage of supply of key raw materials could adversely affect our business.
Our products are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, it could result in a significant increase to us in the prices our contract manufacturers and third party manufacturers charge us for our Vitamin Shoppe and BodyTech branded products and third party products. Raw material prices may increase in the future and we may not be able to pass on such increases to our customers. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our results of operations and financial condition. In addition, if we no longer are able to obtain products from one or more of our suppliers on terms reasonable to us or at all, our revenues could suffer. We purchased over 10% of our Vitamin Shoppe and BodyTech branded products from Nature’s Value, Inc. Events such as the threat of terrorist attacks or war, or the perceived threat thereof, may also have a significant impact on raw material prices and transportation costs for our products. In addition, the interruption in supply of certain key raw materials essential to the manufacturing of our products, may have an adverse impact on our supplier’s ability to provide us with the necessary products needed to maintain our customer relationships and an adequate level of sales.
We rely primarily on a single warehouse and distribution facility to distribute most of the products we sell. Disruptions to our warehouse and distribution facility could adversely affect our business.
Our primary warehouse and distribution operations are currently concentrated in a single location adjacent to our corporate headquarters in New Jersey. Although we have added a west coast distribution logistics solution to our operations during 2010 to service certain of our west coast stores, any significant disruption to our primary distribution center operations for any reason, such as a flood, fire or hurricane, could adversely affect our product distributions and sales until such time as we are able to secure an alternative distribution method.
Our new store base, or any stores opened in the future, may not achieve sales and operating levels consistent with our mature store base on a timely basis or at all. In addition, our growth strategy includes the addition of a significant number of new stores each year. We may not be able to successfully implement this strategy on a timely basis or at all, and our business could be adversely affected if we are unable to successfully negotiate favorable lease terms.
Since the beginning of 2007, we have aggressively pursued new store growth by opening 184 new stores through Fiscal 2010 in existing and new markets. Historically, our new stores have reached sales that are consistent with our mature stores over the course of a three to four year period. New stores opened since the beginning of 2007, or any new stores to be opened in the future, may not achieve sales and operating levels consistent with our mature store base in this time frame or at all. The failure of our new store base to achieve sales and operating levels consistent with our mature store base on a timely basis will have an adverse effect on our financial condition and operating results. As of March 4, 2011, we leased 493 stores along with our corporate headquarters and distribution facility. The store leases are generally for a term of ten years and we have options to extend most leases for a minimum of five years. Our business, financial condition, and operating results could be adversely affected if we are unable to continue to negotiate acceptable lease and renewal terms.
In addition, our growth continues to depend, in part, on our ability to open and operate new stores successfully. The success of this strategy depends upon, among other things, the identification of suitable sites for store locations, the negotiation of acceptable lease terms, the hiring, training and retention of competent sales personnel, and the effective management of inventory to meet the needs of new and existing stores on a timely basis. Our proposed expansion will also place increased demands on our operational, managerial and administrative resources. These increased demands could cause us to operate our business less effectively, which in turn could cause deterioration in the financial performance of our existing stores. Further, our new store openings may result in reduced net sales volumes in the direct channel, as well as in our existing stores in those markets. We expect to fund our expansion through cash flow from operations and, if necessary, by borrowings under our revolving credit facility. If we experience a decline in performance, we may slow or discontinue store openings. If we fail to successfully implement these strategies, our financial condition and operating results may be adversely affected.
We operate in a highly competitive industry. Our failure to compete effectively could adversely affect our sales and growth prospects.
16
The U.S. nutritional supplements retail industry is a large and highly fragmented industry. In 2009, no single industry participant accounted for more than 5% of total industry sales. We compete primarily against other specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations and mail order companies. This market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. As certain products become more mainstream, we experience increased competition for those products. For example, as the trend in favor of low carb products developed, we experienced increased competition for our low carb products from supermarkets, drug stores, mass merchants and other food companies. Increased competition from companies that distribute through retail, internet or wholesale channels could have a material adverse effect on our financial condition and results of operations. Certain of our competitors may have significantly greater financial, technical and marketing resources than we do. In addition, our competitors may be more effective and efficient in introducing new products. We may not be able to compete effectively, and any of the factors listed above may cause price reductions, reduced margins and losses of our market share.
If we fail to protect our brand name, competitors may adopt tradenames that dilute the value of our brand name.
We may be unable or unwilling to strictly enforce our trademark in each jurisdiction in which we do business. In addition, because of the differences in foreign trademark laws concerning proprietary rights, our trademarks may not receive the same degree of protection in foreign countries as they do in the United States. Also, we may not always be able to successfully enforce our trademarks against competitors, or against challenges by others. Our failure to successfully protect our trademarks could diminish the value and efficacy of our past and future marketing efforts, and could cause customer confusion, which could, in turn, adversely affect our sales and profitability. Moreover, we may be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses or prevent us from selling or using some aspect of our products.
Our indebtedness could adversely affect our financial health.
Our indebtedness could have important consequences on our operations. For example, it could:
| | • | | increase our vulnerability to general adverse economic, industry and competitive conditions; |
| | • | | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, new store growth and other capital expenditures, research and development efforts and other general corporate purposes; |
| | • | | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| | • | | place us at a competitive disadvantage compared to our competitors that have less debt; and |
| | • | | limit our ability to borrow additional funds. |
Recent legislation regarding healthcare may adversely impact our results of operations
We currently provide medical and dental insurance benefits to substantially all of our full-time employees. The Patient Protection and Affordable Care Act, signed into law in March 2010, may cause the cost of providing medical insurance to our employees to increase. We may not be able to pass these costs on to our customers, which could have an adverse impact on our results of operations and cash flows.
| Item 1B. | Unresolved Staff Comments |
None.
As of March 4, 2011, there were 493 retail stores open in the United States. See “Item 1—Business—Store Counts and Locations” for additional information on the growth in our network of stores for Fiscal 2006 through 2010 and the location of our stores as of December 25, 2010. As of March 4, 2011, we leased the property for all of our 493 stores. We do not believe that any individual store property is material to our financial condition or results of operation. Of the leases for our stores, 10 expire in Fiscal 2011, 26 expire in Fiscal 2012, 60 expire in Fiscal 2013, 79 expire in Fiscal 2014, 62 expire in Fiscal 2015, and the balance expire in Fiscal 2016 or thereafter. We have options to extend most of these leases for a minimum of five years.
Our corporate offices, along with our primary warehouse and distribution center, are housed in one state-of-the-art facility. The initial lease term for the facility, entered into in Fiscal 2002, was for 15 years, with one five-year renewal option. In addition to our primary distribution facility, we entered into an agreement with a west coast third party logistic solution facility for a period of three years, which we began using in our operations in the third fiscal quarter of 2010.
We believe that all of our current facilities are in good condition and are suitable and adequate for our current and reasonably anticipated future needs.
17
Dwight Thompson v. The Vitamin Shoppe and Consolidated Actions. On February 25, 2005, a former store manager in California, Dwight Thompson, filed suit against the Vitamin Shoppe in the Superior Court of the State of California for the County of Orange (Case No. 05CC00048) on behalf of himself and other “similarly situated” current and former California store managers and assistant store managers, who were employed by the Company on or after April 14, 2006 alleging causes of action for alleged wage and hour violations. Almost one year later, on July 7, 2006, the same group of plaintiffs’ attorneys who were representing Thompson filed another wage and hour lawsuit against The Vitamin Shoppe based on substantively identical allegations in the Orange County Superior Court, entitled Estel v. The Vitamin Shoppe Industries Inc. (Case No. 06CC07852) (the “Estel Action”). In January 2008, the Court consolidated the Thompson and Estel actions. On October 6, 2010, the Company reached agreement with Plaintiffs to settle and resolve all individual and class claims for amounts, in the aggregate, not material to the Company. The Court gave preliminarily approval on December 20, 2010, and final approval of the class settlement is set for March 11, 2011. All settlement amounts were fully accrued in Fiscal 2010 and paid to the disbursing agent during February 2011.
California District Attorney’s Letter. On May 17, 2007, the Company received a letter from the Napa County (California) District Attorney alleging that six of the Company’s private label products contain levels of lead that, pursuant to California’s Proposition 65, Cal. Health & Safety Code section 25249.5 et seq., (“Proposition 65”) require the products to bear a warning when sold in California. The letter claims that 12 other public prosecutors in California, including the California Attorney General, “are involved in a joint investigation of dietary supplements containing lead in amounts that expose users to lead in excess of 0.50 micrograms (ug) per day.” The letter demands that the Company immediately cease all sales of these products in California unless it provides a warning to consumers. It also notes that Proposition 65 provides for civil penalties of up to $2,500 per violation per day. The Company has met with the California Attorney General and certain District Attorneys, and is investigating these allegations and consulting with its third party suppliers of these products. The Company has withdrawn certain named products from the California market and has provided warnings with respect to other products still available in California pending discussions with the public prosecutors. The Napa County District Attorney has expressed concerns on several occasions as to the method of warning employed by the Company and the completeness of its implementation. The Company has revised its warnings and reviewed its procedures for implementing warnings. The Company has responded to numerous requests for information and has met in person with representatives of the Napa County District Attorney and the California Attorney General to attempt to resolve this matter. As of December 25, 2010, the Company does not believe the financial statement impact of this matter will be material.
The People of the State of California v. 21st Century Healthcare, Inc. On October 22, 2008, a private enforcer named Vicky Hamilton sent over 70 manufacturers and retailers of multivitamin products, including the Company, various Sixty-Day Notices of Violation of Proposition 65, Cal. Health & Safety Code section 25249.5 et seq. alleging that certain products contain lead and lead compounds and were sold in California without a Proposition 65 warning threatening litigation pertaining to two of the Company’s multivitamin products. On December 23, 2008, the California Attorney General and nine California District Attorneys filed a complaint on behalf of the People of the State of California against a number of companies who received notices of violation from Ms. Hamilton, including the Company in Alameda County Superior Court. The action alleges violations of both Proposition 65 and the UCL and supplants the litigation Ms. Hamilton sought to bring against the Company on the claims stated in her Notice of Violation. Penalties under Proposition 65 may be assessed at the maximum rate of $2,500 per violation per day. Penalties under the UCL may be assessed at the same rate and are cumulative to those available under Proposition 65. Injunctive relief and attorneys fees are also available. The Company is investigating the claims in the action and has been discussing them with the California Attorney General and District Attorneys. At this time it is premature to determine the extent of any potential loss. Accordingly, as of December 25, 2010, the Company has not accrued any liabilities related to this litigation.
J.C. Romero v. ErgoPharm Inc., Proviant Technologies Inc., VS Holdings Inc, d/b/a Vitamin Shoppe, and General Nutrition Centers Inc. On April 27, 2009, plaintiff, a professional baseball player, filed a complaint against us, among others, in Superior Court of New Jersey (Law Division/Camden County). Plaintiff alleges that he purchased from one of our stores and consumed 6-OXO Extreme, which was manufactured by a third party, and in August 2008, allegedly tested positive for a banned substance. Plaintiff served a 50 game suspension imposed by Major League Baseball. The seven count complaint asserts, among other things, claims for negligence, strict liability, misrepresentation, breach of implied warranty and violations of the New Jersey Consumer Fraud Act, and seeks unspecified monetary damages, including lost income during the suspension. The Company denies any and all liability and intends to vigorously defend these claims. Any liabilities that may arise from this matter are not probable or reasonably estimable at this time. Accordingly, as of December 25, 2010, the Company has not accrued any liabilities related to this litigation.
The Company is party to various lawsuits arising from time to time in the normal course of business, many of which are covered by insurance. Except as described above, as of December 25, 2010, the Company was not party to any material legal proceedings. Although the impact of the final resolution of these matters on the Company’s financial condition, results of operations or cash flows is not known, management does not believe that the resolution of these lawsuits will have a material adverse effect on the financial condition, results of operations or liquidity of the Company.
18
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Since October 28, 2009 our common stock has been traded on the New York Stock Exchange (“NYSE”) under the trading symbol “VSI”. At December 25, 2010, there were 28,627,897 common shares outstanding, and the closing sale price of our common stock was $33.74. Also as of that date, we had approximately 39 common shareholders of record. The table below sets forth the high and low closing sale prices of our common stock for the periods indicated:
| | | | | | | | |
Fiscal period | | High | | | Low | |
2009 Quarter ended: | | | | | | | | |
December | | $ | 22.63 | | | $ | 17.57 | |
| | |
2010 Quarter ended: | | | | | | | | |
March | | $ | 23.65 | | | $ | 19.55 | |
June | | | 27.88 | | | | 21.12 | |
September | | | 27.49 | | | | 23.39 | |
December | | | 34.07 | | | | 27.08 | |
Stock Performance Graph
The line graph below compares the cumulative total stockholder return on the Company’s common stock with the S&P Retail Index (RLX), the Russell 2000 Index (RUT), and the NYSE Composite Index (NYA) for the period covering the Company’s initial public offering on October 28, 2009 through December 31, 2010. The graph assumes an investment of $100 made at the closing of trading on October 28, 2009, in (i) The Company’s common stock, (ii) the stocks comprising the RLX, (iii) the stocks comprising the RUT, and (iv) the stocks comprising the NYA. All values assume reinvestment of the full amount of all dividends, if any, into additional shares of the same class of equity securities at the frequency with which dividends are paid on such securities during the applicable time period.
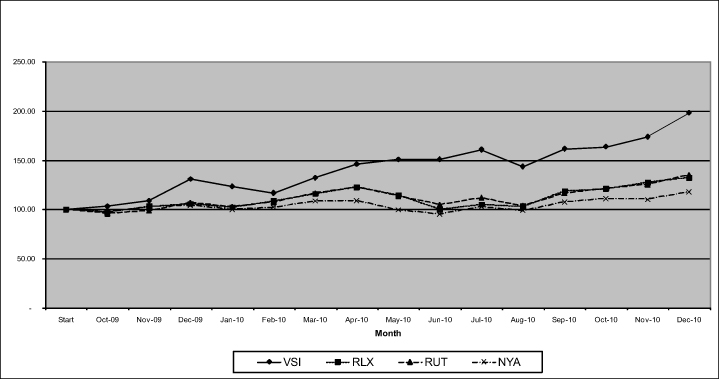
19
| Item 6. | Selected Financial Data |
We have derived the selected financial data presented below from our consolidated financial statements for the Fiscal Years ended December 25, 2010, December 26, 2009, December 27, 2008, December 29, 2007, and December 30, 2006. Financial results for all periods are based on a 52-week period. The selected financial information for the Fiscal Years ended December 25, 2010, December 26, 2009 and December 27, 2008 presented below, should be read in conjunction with such consolidated financial statements and notes included herein and in conjunction with Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations. As a result of the Merger discussed in Item 1- Business, amounts for fiscal years 2006 through 2008 have been retroactively adjusted to reflect the Merger as if it occurred at the beginning of Fiscal 2006 (the year VS Parent was created).
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | | | December 29,
2007 | | | December 30,
2006 | |
| | | (data presented in thousands, except for share and per share data and number of stores) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 751,482 | | | $ | 674,495 | | | $ | 601,540 | | | $ | 537,872 | | | $ | 486,026 | |
Cost of goods sold | | | 501,948 | | | | 457,573 | | | | 405,659 | | | | 360,346 | | | | 326,523 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 249,534 | | | | 216,922 | | | | 195,881 | | | | 177,526 | | | | 159,503 | |
Selling, general and administrative expenses | | | 189,872 | | | | 173,144 | | | | 158,713 | | | | 143,544 | | | | 128,821 | |
Related party expenses | | | — | | | | 2,446 | | | | 1,523 | | | | 1,365 | | | | 1,356 | |
| | | | | | | | | | | | | | | | | | | | |
Income from operations | | | 59,662 | | | | 41,332 | | | | 35,645 | | | | 32,617 | | | | 29,326 | |
Loss on extinguishment of debt and other (1) | | | 1,349 | | | | 2,016 | | | | — | | | | — | | | | (366 | ) |
Interest expense, net | | | 9,517 | | | | 18,636 | | | | 21,137 | | | | 22,045 | | | | 21,788 | |
| | | | | | | | | | | | | | | | | | | | |
Income before provision for income taxes | | | 48,796 | | | | 20,680 | | | | 14,508 | | | | 10,572 | | | | 7,904 | |
Provision for income taxes | | | 19,550 | | | | 8,014 | | | | 6,341 | | | | 3,792 | | | | 3,242 | |
| | | | | | | | | | | | | | | | | | | | |
Net income | | | 29,246 | | | | 12,666 | | | | 8,167 | | | | 6,780 | | | | 4,662 | |
Preferred stock dividends in arrears (2) | | | — | | | | 7,692 | | | | 9,279 | | | | 9,105 | | | | 8,412 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | 29,246 | | | $ | 4,974 | | | $ | (1,112 | ) | | $ | (2,325 | ) | | $ | (3,750 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding (2): | | | | | | | | | | | | | | | | | | | | |
Basic | | | 27,390,419 | | | | 16,238,338 | | | | 14,175,906 | | | | 14,175,906 | | | | 14,175,906 | |
Diluted | | | 28,338,788 | | | | 17,748,371 | | | | 14,175,906 | | | | 14,175,906 | | | | 14,175,906 | |
Net income (loss) per share: | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 1.07 | | | $ | 0.31 | | | $ | (0.08 | ) | | $ | (0.16 | ) | | $ | (0.26 | ) |
Diluted | | $ | 1.03 | | | $ | 0.28 | | | $ | (0.08 | ) | | $ | (0.16 | ) | | $ | (0.26 | ) |
| | | | | |
Other Financial Data: | | | | | | | | | | | | | | | | | | | | |
Depreciation and amortization of fixed and intangible assets | | $ | 21,112 | | | $ | 21,095 | | | $ | 17,483 | | | $ | 14,882 | | | $ | 13,728 | |
| | | | | |
Operating Data: | | | | | | | | | | | | | | | | | | | | |
Number of stores at end of period | | | 484 | | | | 438 | | | | 401 | | | | 341 | | | | 306 | |
Net sales per store (3) | | $ | 1,380 | | | $ | 1,361 | | | $ | 1,303 | | | $ | 1,355 | | | $ | 1,332 | |
Comparable store sales growth (4) | | | 7.1 | % | | | 5.2 | % | | | 6.2 | % | | | 6.2 | % | | | 6.6 | % |
| | | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Working capital | | $ | 75,959 | | | $ | 50,416 | | | $ | 52,285 | | | $ | 51,175 | | | $ | 38,248 | |
Total assets | | | 485,717 | | | | 469,257 | | | | 463,705 | | | | 428,330 | | | | 411,670 | |
Total debt, including capital lease obligations | | | 75,794 | | | | 123,946 | | | | 186,382 | | | | 165,000 | | | | 171,500 | |
Stockholders’ equity | | | 297,696 | | | | 234,351 | | | | 168,483 | | | | 159,789 | | | | 153,516 | |
| (1) | For Fiscal 2010, loss on extinguishment of debt includes the write-off of deferred financing fees and a portion of the unrecognized loss on our terminated interest rate swap of $0.9 million and $0.4 million, respectively, related to the repurchase of a portion of our floating rate notes during Fiscal 2010. For Fiscal 2009, loss on extinguishment of debt includes $0.4 million for the premium on the repurchase of a portion of floating rate notes, along with the write-off of the related portions of deferred financing fees and a portion of the unrecognized loss of our terminated interest rate swap of $0.7 million and $0.6 million, respectively, as well as a $0.3 million write-off of deferred financing fees related to the repayment of our former revolving credit facility which was terminated in September 2009. |
20
| (2) | Preferred dividends in arrears are restated for all periods presented as a result of the Merger as described in Item 1. In addition, all shares presented take into effect the approximately 1.8611-for-one split which resulted from the Merger. |
| (3) | Net sales per store is calculated by dividing retail net sales by the number of stores open at the end of the period. |
| (4) | A store is included in comparable store sales after 410 days of operation. |
21
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following Management’s Discussion and Analysis of our Financial Condition and Results of Operations should be read in conjunction with the consolidated financial statements and notes thereto included as part of this Annual Report on Form 10-K. The discussion in this section contains forward-looking statements that are based upon current expectations. We sometimes identify forward-looking statements with such words as “may,” “expect,” “intend,” “anticipate,” “plan,” “believe,” “seek,” “estimate,” “outlook,” “trends,” “future benefits,” “strategies,” “goals” and similar words concerning future events. The forward-looking statements contained herein, include, without limitation, statements concerning future revenue sources and concentration, gross profit margins, selling and marketing expenses, research and development expenses, general and administrative expenses, capital resources, capital expenditures, new stores, additional financings or borrowings and additional losses and are subject to risks and uncertainties including those discussed below and elsewhere in this Annual Report on Form 10-K that could cause actual results to differ materially from the results contemplated by these forward-looking statements. We also urge you to carefully review the risk factors set forth in “Item 1A – Risk Factors.”
Overview
We are a leading specialty retailer and direct marketer of vitamins, minerals, herbs, supplements, sports nutrition and other health and wellness products. For each of the past three years, we have been the second largest in retail sales and the fastest growing national VMS specialty retailer. We market over 700 different nationally recognized brands as well as our proprietary Vitamin Shoppe, BodyTech and MD Select brands. We believe we offer the greatest variety of products among VMS retailers with approximately 8,000 SKUs offered in our typical store and an additional 12,000 SKUs available through our Internet and catalog direct sales channels. Our broad product offering enables us to provide our target customers with a selection of products not readily available at other specialty VMS retailers or mass merchants, such as supermarkets and drugstore chains. We target the dedicated, well-informed VMS consumer and differentiate ourselves by providing high quality products at competitive prices in an educational and high-touch customer service environment. We believe our extensive product offering, together with our well-known brand name and emphasis on product education and customer service, help us bond with our target customer and serve as a foundation for strong customer loyalty.
On November 2, 2009, we completed an IPO. Prior to and in connection with the IPO, VS Parent, Inc. merged into VS Holdings, Inc., with VS Holdings being renamed as Vitamin Shoppe, Inc. All common shares and warrants previously held by VS Parent, Inc., were converted to common shares of Vitamin Shoppe, Inc., at approximately a 1.8611-for-one split, resulting in 15,231,446 common shares issued and outstanding at October 27, 2009. Also in connection with the IPO, 36,969 preferred shares previously held by VS Parent Inc., along with accumulated dividends in arrears, were converted into 3,764,720 common shares of Vitamin Shoppe, Inc., with the remaining 41,899 preferred shares being redeemed for cash of approximately $72.5 million. In addition, 7,666,667 new shares of common stock were issued in connection with the IPO at a price of $17 per share, resulting in net proceeds from the offering of approximately $121.2 million, net of underwriters’ commissions.
Trends and Other Factors Affecting Our Business
Our performance is affected by trends that affect the VMS industry, including demographic, health and lifestyle preferences. Changes in these trends and other factors, which we may not foresee, may also impact our business. For example, our industry is subject to potential regulatory actions, such as the ban on Ephedra by the Food and Drug Administration, and other legal matters that affect the viability of a given product. Volatile consumer trends, such as those described in the following paragraph, as well as the overall impact on consumer spending, can dramatically affect purchasing patterns. Our business allows us to respond to changing industry trends by introducing new products and adjusting our product mix and sales incentives. We will continue to diversify our product lines to offer items less susceptible to the effects of economic conditions and not as readily substitutable, such as teas, lotions and spring water.
Sales of weight management products are generally more sensitive to consumer trends, resulting in higher volatility than our other products. Our sales of weight management products have been significantly influenced by the rapid increase and subsequent decline of products such as products containing Ephedra, low carb products, and certain thermogenic products. For instance, the demand for low carb products overall have been on a consistent decline since early Fiscal 2006, which we believe was due to a change in demand for low carb products and the wider availability of popular products in the marketplace, and as such we have shifted our focus more to weight management products. During this decline in demand for low carb products we have continued to launch new weight management products. Moreover, as the rate of obesity increases and as the general public becomes increasingly more health conscious, we expect the demand for weight management products, albeit volatile, to continue to be strong in the near term. Accordingly, we will continue to offer the highest quality products available in this segment.
In addition to the weight management product lines, we intend to continue our focus in meeting the demands of an increasingly aging population, the effects of increasing costs of traditional healthcare and a rapidly growing fitness conscious public.
22
We believe that the aging of the U.S. population provides us with an area of opportunity. The U.S. Census Bureau reports that the number of individuals in the 50 and over age group is expected to increase to 115 million people in 2018 from 94 million people in 2008, representing more than twice the overall population growth rate. Moreover, it is estimated that by 2030 the 65 or older group will comprise 20% of the population. The increase in the population of this age group, coupled with the need for increased supplementation as digestive abilities wane, provides us with an enhanced sales opportunity. For example, anti-degenerative supplements, such as chondroitin sulfate, have demonstrated consistent increases in sales growth. We will continue to offer products such as chondroitin sulfate to meet the demands of this market segment.
We believe that as the costs of healthcare continue to increase, lower-cost alternatives to prescription drugs and preventative supplementation will continue to be an option for the American consumer. According to the Center for Medicare and Medicaid Services, medical spending as a percentage of GDP was 17.62% in 2009, and is projected to reach 20% of GDP by 2018. As an increasing number of the population seeks to avoid costly medical issues and focuses on prevention through diet, supplementation and exercise, we expect the demand in this market segment to provide us with continued opportunities. For example, lower-cost alternatives to expensive cholesterol lowering medications such as fish oil (essential fatty acids), are experiencing increasing popularity.
Our historical results have also been significantly influenced by our new store openings. Since the beginning of 2007, we have opened 193 stores and operate 493 stores located in 39 states and the District of Columbia as of March 4, 2011.
Our stores typically require three to four years to mature, generating lower store level sales and store contribution in the initial years than our mature stores. As a result, new stores generally have a negative impact on our overall operating margin and sales per square foot. As our recently opened stores mature, we expect them to contribute meaningfully to our sales and store contribution.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Critical accounting policies are those that are the most important portrayal of our financial condition and results of operations, and require our most difficult, subjective and complex judgments as a result of the need to make estimates about the effect of matters that are inherently uncertain. While our significant accounting policies are described in more detail in the notes to our financial statements, our most critical accounting policies, discussed below, pertain to revenue recognition, inventories, impairment of long-lived assets, goodwill and other intangible assets, deferred sales for our Healthy Awards Program, stock-based compensation, and income taxes. In applying such policies, we must use some amounts that are based upon our informed judgments and best estimates. Estimates, by their nature, are based on judgments and available information. The estimates that we make are based upon historical factors, current circumstances and the experience and judgment of management. We evaluate our assumptions and estimates on an ongoing basis.
Revenue Recognition. We recognize revenue upon sale of our products when merchandise is sold “over-the-counter” in retail stores or upon delivery to a direct customer, net of sales returns. In addition, we classify all amounts billed to customers that represent shipping fees as sales. To arrive at net sales, gross sales are reduced by deferred sales, actual customer returns, and a provision for estimated future customer returns, which is based on management’s review of historical and current customer returns. The net amounts reserved for sales returns were $0.1 million at both December 25, 2010 and December 26, 2009. Sales taxes collected from customers are presented on a net basis and as such are excluded from revenue.
23
Inventories. Inventories are stated at the lower of cost or market value. Cost is determined using the moving weighted average method. As applied to inventories, cost means in principle the sum of the applicable expenditures and charges directly or indirectly incurred in bringing the product to its existing condition and location. Finished goods inventory includes costs on freight on internally transferred merchandise, rent for the distribution center and costs associated with our buying department and distribution facility, including payroll which are capitalized into inventory and then expensed as merchandise is sold. In addition, the cost of inventory is reduced by purchase discounts and allowances received from certain of our vendors. We adjust our inventory to reflect situations in which the cost of inventory is not expected to be recovered. We regularly review our inventory, including when a product is close to expiration and not expected to be sold, when a product has reached its expiration date, or when a product is not expected to be saleable. In determining the reserves for these products we consider factors such as the amount of inventory on hand and its remaining shelf life, and current and expected market conditions, including management forecasts and levels of competition. We have evaluated the current level of inventory considering historical trends and other factors, and based on our evaluation, have recorded adjustments to reflect inventory at net realizable value. These adjustments are estimates, which could vary significantly from actual results if future economic conditions, customer demand or competition differ from expectations. These estimates require us to make assessments about the future demand for our products in order to categorize the status of such inventory items as slow moving, expiring, obsolete or in excess of need. These future estimates are subject to the ongoing accuracy of management’s forecasts of market conditions, industry trends and competition. We are also subject to volatile changes in specific product demand as a result of unfavorable publicity, government regulation and rapid changes in demand for new and improved products or services. Obsolescence reserves were $1.8 million and $1.4 million at December 25, 2010 and December 26, 2009, respectively.
Long-Lived Assets. We evaluate long-lived assets, including fixed assets and intangible assets with finite useful lives, periodically for impairment whenever events or changes in circumstances indicate that the carrying amount of any such asset may not be recoverable. If the sum of our estimated undiscounted future cash flows is less than the asset’s carrying value, we recognize an impairment loss, measured as the amount by which the carrying value exceeds the fair value of the asset. These estimates of cash flow require significant management judgment and certain assumptions about future volume, sales and expense growth rates, devaluation and inflation. As such, these estimates may differ from actual cash flows. During Fiscal 2010 we recognized an impairment charge of approximately $1.3 million (which was charged to selling, general and administrative expenses) on fixed assets related to three underperforming retail locations currently still in use in our retail operations. There were no charges related to the impairment of fixed assets for the remaining periods presented.
Goodwill and Other Intangible Assets. On an annual basis, or whenever impairment indicators exist, we perform an evaluation of goodwill and indefinite lived intangible assets. In the absence of any impairment indicators, goodwill and other indefinite lived intangible assets are tested in the fourth quarter of each fiscal year. With regards to goodwill, our tests are based on our two reporting units, and utilize the discounted cash flow method, based on our current operating projections, as well as the market multiples method. For those intangible assets which have definite lives, we amortize their cost on a straight-line basis over their estimated useful lives, the periods of which vary based on their particular contractual terms. Judgments regarding the existence of impairment indicators are based on market conditions and operational performance of the business. Future events could cause us to conclude that impairment indicators exist, and therefore that goodwill and other intangible assets are impaired.
Our impairment test involves calculating the fair value of both our reporting units (our segments) using the discounted cash flow method along with the market multiples method which is used for additional validation of the value calculated. The use of the discounted cash flow valuation method requires us to make certain assumptions and estimates regarding certain industry trends and future profitability of our reporting units. It is our policy to conduct goodwill impairment testing from information based on our most current business projections, which include projected future revenues and cash flows. The cash flows utilized in the discounted cash flow analysis are based on five-year financial forecasts developed internally by our management. Cash flows for each unit are discounted using an internally derived weighted average cost of capital which reflects the costs of borrowing for the funding of each unit as well as the risk associated with the units themselves and the industry they perform in. We also conduct the test using a 10% decrease in our revenue projections as an additional sensitivity test to ensure the business unit’s fair value is greater than its carrying value even in a less favorable environment. If the carrying amount of a reporting unit exceeds its fair value, we would compare the implied fair value of the reporting unit goodwill with its carrying value. To compute the implied fair value, we would assign the fair value of the reporting unit to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination. The excess of the fair value of a reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill. If the carrying value of the reporting unit goodwill exceeded the implied fair value of the reporting unit goodwill, we would record an impairment loss to write down such goodwill to its implied fair value. The valuation of goodwill and indefinite-lived intangible assets is affected by, among other things, our business plan for the future and estimated results of future operations. Changes in the business plan or operating results that are different than the estimates used to develop the valuation of the assets may impact their valuation.
24
We have tested our goodwill and indefinite-lived intangibles for impairment in the fourth quarter of each fiscal year presented and concluded there was no impairment relative to such assets. Accordingly, there is no impairment expense recorded in any of the periods presented.
Deferred Sales. Our frequent buyer program allows customers to earn points toward free merchandise based on the volume of purchases. Points are earned each year under our frequent buyer program and are redeemable within the first three months of the following year or they expire. We defer sales as points are earned, based on historical redemption data as well as marketing data within the current period, and record a liability for points earned based on the value of points that are expected to be redeemed. Net increases to deferred sales were $1.5 million, $1.3 million and $1.8 million for the years ended December 25, 2010, December 26, 2009 and December 27, 2008, respectively. The balance for the deferred sales liability was $15.9 million and $14.4 million at December 25, 2010 and December 26, 2009, respectively.
Stock-Based Compensation. We account for our stock-based compensation based on fair value recognition requirements, as defined by generally accepted accounting principles. Stock-based compensation cost is measured at the grant date based on the fair value of awards and is recognized as expense over the vesting period, net of anticipated forfeitures. With the exception of restricted shares determining the fair value of stock-based awards at the grant date requires considerable judgment, including estimating expected volatility, expected term and risk-free rate. The expected volatility is derived from the average volatility of similar actively traded companies over our expected holding periods. Generally, the expected holding period of non performance based options is calculated using the simplified method using the vesting term of 4 years and the contractual term of 10 years, resulting in a holding period of 6.25 years. Certain limited grants have contractual terms of 7.5 years, and, as such, have a calculated holding period of 4.81 years. Our performance based grants vest annually over four years depending on a particular year’s attainment of certain internal financial performance metrics. For accounting purposes, performance based grants are measured, and expense is calculated and recorded, subsequent to the determination that the achievement of the pre-established performance targets are probable, over the relevant service period. The target metrics underlying the vesting of performance based options are established each year. The vesting requirements for performance-based options permit a catch-up of vesting should the target not be achieved in a calendar year but achieved in a subsequent calendar year, over the four year vesting period. Accordingly, the holding period for performance based options is calculated using the vesting term of 1 year and the remainder of the contractual term of 10 years, depending on which year of the four year grant is currently vesting; e.g. 25% of the grant vesting in year two of the grant would have a holding period calculated using 1 year and the remaining 9 years of the contractual term. The simplified method was chosen as a means to determine the Company’s holding period as, prior to November 2009, there was no historical option exercise experience due to the Company being privately held. As of December 25, 2010, there is insufficient information for purposes of determining a Company specific holding period due to the Company being a relatively new publicly owned company. The risk-free interest rate is derived from the average yields of zero-coupon U.S. Treasury Strips for the expected holding period of each of the Company’s stock option grants. Compensation expense resulting from the granting of restricted shares is based on the grant date fair value of those common shares and is recognized generally over the four year vesting period. If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past.
Similar to stock options, our Employee Stock Purchase Plan (“ESPP”) is accounted for based on fair value recognition requirements. ESPP participation occurs quarterly (the “Participation Period”) and the expense of which is subject to employee participation in the plan. Under the ESPP, participating employees are allowed to purchase shares at 85% of the lower of the market price of the Company’s common stock at either the first or last trading day of the Participation Period. Compensation expense related to the ESPP is based on the estimated fair value of the discount and purchase price offered on the estimated shares to be purchased under the ESPP. Expense is calculated quarterly, based on the employee contributions made over the three-month Participation Period, using volatility and risk free rates applicable to that three month period.
Amounts charged to expense were $4.1 million, $3.0 million and $2.4 million for stock-based compensation for Fiscal 2010, Fiscal 2009, and Fiscal 2008, respectively. The weighted average fair value for grants for Fiscal 2010, Fiscal 2009, and Fiscal 2008 was $11.91, $7.70, and $7.92, respectively.
Income Taxes. Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. We periodically assess the realizability of deferred tax assets and the adequacy of deferred tax liabilities, based on the results of local, state, federal or foreign statutory tax audits or estimates and judgments used.
Realization of deferred tax assets associated with net operating loss and tax credit carryforwards is dependent upon generating sufficient taxable income prior to their expiration in the applicable tax jurisdiction. We periodically review the recoverability of tax assets recorded on our balance sheet, and provide valuation allowances as we deem necessary, to reduce deferred tax assets to the amount that more likely than not will be realized. Deferred tax assets could be reduced in the future if our estimates of taxable income during the carryforward period are significantly reduced or alternative tax strategies are no longer viable.
25
We account for our tax positions based on the provisions of the accounting literature related to accounting for uncertainty in income tax positions. Such literature provides guidance for the recognition threshold and measurement attribute for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This requires that we recognize the largest amount that would more likely than not be unsustainable upon audit by the relevant taxing authority for the impact of an uncertain income tax position on our income tax return. An uncertain income tax position will be recognized if it has less than a 50% likelihood of being sustained. The tax positions are analyzed periodically (at least quarterly) and adjustments are made as events occur that warrant adjustments for those positions. We record interest expense and penalties payable to relevant tax authorities as income tax expense. See Note 7 to our consolidated financial statements for more information on income taxes.
General Definitions for Operating Results
Net Sales consist of sales, net of sales returns and deferred sales, from comparable stores and non comparable stores, as well as sales made directly to our internet and catalog customers. A store is included in comparable store sales after 410 days of operation.
Cost of goods sold,which excludes depreciation and amortization which is included withinSelling, general and administrative expenses, includes the cost of inventory sold, costs of warehousing and distribution and store occupancy costs. Warehousing and distribution costs include freight on internally transferred merchandise, rent for the distribution center and costs associated with our buying department and distribution facility, including payroll, which are capitalized into inventory and then expensed as merchandise is sold. Store occupancy costs include rent, common area maintenance, real estate taxes and utilities.
Gross profitis net sales minus cost of goods sold.
Selling, general and administrative expensesconsist of depreciation and amortization of fixed and intangible assets, operating payroll and related benefits, advertising and promotion expense, and other selling, general and administrative expenses.
Related party expenses consist of management fees incurred and paid to IPC Manager II, LLC (our former controlling shareholder).
Income from operations consists of gross profit minus selling, general and administrative expenses, and related party expenses.
Loss on extinguishment of debtrepresents expenses incurred in connection with the redemption or repayment of debt.
Interest expense, net includes interest on our second priority senior secured floating rate notes (the “Notes”) along with interest on our swap and the amortization of the unrealized loss portion of that swap, interest on the revolving credit facility, letters of credit fees, interest on our capital leases, as well as amortization of financing costs, offset with interest income earned from highly liquid investments (investments purchased with an original maturity of three months or less).
26
Key Performance Indicators and Statistics
We use a number of key indicators of financial condition and operating results to evaluate the performance of our business, including the following (in thousands):
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Net sales | | $ | 751,482 | | | $ | 674,495 | | | $ | 601,540 | |
Increase in comparable store net sales | | | 7.1 | % | | | 5.2 | % | | | 6.2 | % |
Gross profit as a percent of net sales | | | 33.2 | % | | | 32.2 | % | | | 32.6 | % |
Income from operations | | $ | 59,662 | | | $ | 41,332 | | | $ | 35,645 | |
The following table shows the growth in our network of stores for Fiscal 2010, 2009 and 2008:
| | | | | | | | | | | | |
| | | Fiscal Year | |
| | | 2010 | | | 2009 | | | 2008 | |
Stores open at beginning of year | | | 438 | | | | 401 | | | | 341 | |
Stores opened | | | 47 | | | | 39 | | | | 62 | |
Stores closed | | | (1 | ) | | | (2 | ) | | | (2 | ) |
| | | | | | | | | | | | |
Stores open at end of year | | | 484 | | | | 438 | | | | 401 | |
| | | | | | | | | | | | |
27
Results of Operations
The information presented below is for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008 and was derived from our audited consolidated financial statements, which, in the opinion of management, includes all adjustments necessary for a fair presentation of our financial position and operating results for such periods and as of such dates. The following table summarizes our results of operations for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008 as a percentage of net sales:
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Net sales | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
Cost of goods sold | | | 66.8 | % | | | 67.8 | % | | | 67.4 | % |
| | | | | | | | | | | | |
Gross profit | | | 33.2 | % | | | 32.2 | % | | | 32.6 | % |
Selling, general and administrative expenses | | | 25.3 | % | | | 25.7 | % | | | 26.4 | % |
Related party expenses | | | 0.0 | % | | | 0.4 | % | | | 0.3 | % |
| | | | | | | | | | | | |
Income from operations | | | 7.9 | % | | | 6.1 | % | | | 5.9 | % |
Loss on extinguishment of debt | | | 0.2 | % | | | 0.3 | % | | | 0.0 | % |
Interest expense, net | | | 1.2 | % | | | 2.7 | % | | | 3.4 | % |
| | | | | | | | | | | | |
Income before provision for income taxes | | | 6.5 | % | | | 3.1 | % | | | 2.5 | % |
Provision for income taxes | | | 2.6 | % | | | 1.2 | % | | | 1.1 | % |
| | | | | | | | | | | | |
Net income | | | 3.9 | % | | | 1.9 | % | | | 1.4 | % |
| | | | | | | | | | | | |
The net sales results presented for the years ended December 25, 2010, December 26, 2009, and December 27, 2008, are each based on a 52-week period (“Fiscal 2010,” “Fiscal 2009,” and “Fiscal 2008”).
Comparison of Fiscal 2010 with Fiscal 2009 Net Sales
Net sales increased $77.0 million, or 11.4%, to $751.5 million for Fiscal 2010 compared to $674.5 million for Fiscal 2009. The increase was the result of an increase in our comparable store sales, sales from our new non-comparable stores, as well as an increase in our direct sales.
Retail
Net sales from our retail stores increased $71.8 million, or 12.0%, to $668.0 million for Fiscal 2010 compared to $596.3 million for Fiscal 2009. We operated 484 stores as of December 25, 2010 compared to 438 stores as of December 26, 2009. Our overall store sales increased due to non-comparable store sales of $29.7 million, as well as an increase in comparable store sales growth of $42.1 million, or 7.1% (Comparable store sales include only those stores open more than 410 days). The increase in comparable store sales was primarily due to an increase in customer count. Our overall sales increased primarily in the categories of specialty supplements, which increased $7.3 million; herbs, which increased $9.7 million; vitamins and minerals, which increased $10.1 million; and sports nutrition, which increased $39.6 million.
Within the supplements category, sales in essential fatty acids, or EFAs, and CoQ10/Ubiquinol continued to exhibit strong growth. The growth in net sales in our vitamin and minerals category increased largely due to the introduction of new special formulations for men and women as well as an increase in sales of Vitamin D. Product sales in the sports nutrition category continues to be among our fastest growing categories and has been so for over four consecutive fiscal years. We expect this trend to continue based on the growth of the fitness-conscious market.
28
Direct
Net sales to our direct customers increased $5.2 million, or 6.7%, to $83.5 million for Fiscal 2010 compared to $78.2 million for Fiscal 2009. The increase in direct sales during Fiscal 2010 was comprised of an increase in our internet sales of $7.6 million, offset by a decrease in our catalog sales. The increase in web-based sales was largely due to a greater influx of customers gained as a result of an increase in promotional activity through certain of our online store-fronts. We have reduced our catalog circulation and catalog customer prospecting as we believe catalog purchasing in general is declining in popularity as a purchasing medium, especially in the wake of the growth of online shopping. In addition, as we continue to open more stores in new markets, some catalog customers choose to shop at our retail locations.
Cost of Goods Sold
Cost of goods sold, which includes product, warehouse and distribution and occupancy costs, increased $44.4 million, or 9.7%, to $501.9 million for Fiscal 2010 compared to $457.6 million for Fiscal 2009. The dollar increase was primarily due to an increase in sales, as well as an increase in occupancy costs for the year ended December 25, 2010, as compared to the year ended December 26, 2009. Cost of goods sold as a percentage of net sales decreased to 66.8% for the year ended December 25, 2010, compared to 67.8% for the year ended December 26, 2009. The decrease of cost of goods sold as a percentage of net sales was due to decreases in product costs of approximately 0.7% as a percentage of net sales, and a decrease in occupancy costs of 0.3% as a percentage of net sales. The decrease in product costs as a percentage of net sales was due primarily to a lower level of promotional activity during the year ended December 25, 2010, as compared to the year ended December 26, 2009. The decrease in occupancy costs as a percentage of sales during Fiscal 2010 reflects the maturation of our newer stores as the increase in comparable store sales more than offsets the increase in our store occupancy costs.
Gross Profit
As a result of the foregoing, gross profit increased $32.6 million, or 15.0%, to $249.5 million for Fiscal 2010 compared to $216.9 million for Fiscal 2009.
Selling, General and Administrative Expenses
Selling, general and administrative expenses, including operating payroll and related benefits, advertising and promotion expense, and other selling, general and administrative expenses, increased $16.7 million, or 9.7%, to $189.9 million during Fiscal 2010, compared to $173.1 million for Fiscal 2009. The components of selling, general and administrative expenses are explained below. Selling, general and administrative expenses as a percentage of net sales for Fiscal 2010 decreased to 25.3% compared to 25.7% for Fiscal 2009.
Operating payroll and related benefits increased $6.7 million, or 10.0%, to $73.9 million for Fiscal 2010 compared to $67.2 million for Fiscal 2009. The increase is due mainly to our increase in retail locations throughout Fiscal 2010. Operating payroll and related benefits expenses as a percentage of net sales decreased to 9.8% during Fiscal 2010 compared to 10.0% for Fiscal 2009. The decrease as a percentage of net sales was primarily due to greater sales per hour for Fiscal 2010, as compared to Fiscal 2009, due to the maturation of our newer stores.
Advertising and promotion expenses increased $0.2 million, or 1.9%, to $13.0 million for Fiscal 2010 compared to $12.8 million for Fiscal 2009. Advertising and promotion expenses as a percentage of net sales decreased to 1.7% during Fiscal 2010 compared to 1.9% for Fiscal 2009. The decrease as a percentage of net sales was due primarily to a decrease in new store promotions expense during Fiscal 2010 as compared to Fiscal 2009.
Other selling, general and administrative expenses, which include depreciation and amortization expense, increased $9.8 million, or 10.5%, to $103.0 million in Fiscal 2010 compared to $93.2 million for Fiscal 2009. The dollar increase was due primarily to increases in the following: $4.8 million increase in corporate payroll expense which was primarily due to an increase in corporate personnel, incentive compensation and health care costs during Fiscal 2010; credit card fees of $1.6 million, due to the increase in sales; professional fees of $0.9 million, and stock-based compensation expense of approximately $1.0 million, due to additional grants issued in Fiscal 2010. In addition we incurred fees associated with our two secondary common stock offerings during Fiscal 2010 of approximately $0.8 million, as well as a charge for impairment of fixed assets of $1.3 million, related to three of our stores still in operation. These increases were offset in part by a $1.4 million increase in credit card fee rebates received during Fiscal 2010. Other selling, general and administrative expenses as a percentage of net sales decreased to 13.7% during Fiscal 2010 compared to 13.8% for Fiscal 2009. The decrease as a percentage of sales was largely the result of experiencing overall economies of scale with regards to these expenses relative to the increase in sales for Fiscal 2010, as compared to Fiscal 2009.
Related Party Expenses
Related party expenses of $2.4 million during Fiscal 2009, represents management fees paid to IPC Manager II, LLC for three fiscal quarters of Fiscal 2009 as well as a one time termination fee of our management service agreement paid to IPC Manager II,
29
LLC, of approximately $0.8 million (for a detailed presentation of related party expenses, see Note 11 to our consolidated financial statements). As a result of the termination of the management services agreement, there were no related party expenses during Fiscal 2010.
Income from Operations
As a result of the foregoing, income from operations increased $18.3 million, or 44.3%, to $59.7 million for Fiscal 2010 compared to $41.3 million for Fiscal 2009. Income from operations as a percentage of net sales increased to 7.9% during Fiscal 2010 as compared to 6.1% for Fiscal 2009.
Retail
Income from operations for the retail segment increased $23.8 million, or 25.2%, to $118.3 million for Fiscal 2010 compared to $94.5 million for Fiscal 2009. Income from operations as a percentage of net sales for the retail segment increased to 17.7% for Fiscal 2010 compared to 15.8% for Fiscal 2009. This increase as a percentage of sales is largely due to a decrease in product costs of 0.9% and occupancy costs of 0.4% as percentages of sales. The decrease in product costs as a percentage of sales is largely due to greater efficiency in the managing of our inventory as well as a decrease in net promotional activity during Fiscal 2010, as compared to Fiscal 2009. The decrease in occupancy costs as a percentage of sales is largely attributable to the maturation of our newer stores as the increase in comparable sales during the Fiscal year ended December 25, 2010, more than offsets the increase in our store operation costs.
Direct
Income from operations for the direct segment decreased $0.3 million, or 1.7%, to $14.9 million for Fiscal 2010 compared to $15.1 million for Fiscal 2009. Income from operations as a percentage of net sales for the direct segment decreased to 17.8% for Fiscal 2010 compared to 19.3% for Fiscal 2009. This decrease as a percentage of net sales was primarily due to an increase in product costs as a percentage of net sales which was primarily due to increases in shipping costs and promotional pricing, as well as a shift in product mix towards lower margin items during Fiscal 2010 as compared to Fiscal 2009.
Corporate Costs
Corporate costs increased $5.2 million, or 7.7%, to $73.5 million during Fiscal 2010 compared to $68.3 million for Fiscal 2009. Corporate costs as a percentage of net sales decreased to 9.8% for Fiscal 2010 compared to 10.1% for Fiscal 2009. This dollar increase was due primarily to an increase in corporate payroll costs of approximately $4.8 million, primarily due to an increase to our corporate staff, incentive compensation and health care costs during Fiscal 2010; and an increase in stock compensation expense of $1.0 million, due to additional grants issued in Fiscal 2010, as well as a charge for impairment of fixed assets of $1.3 million, related to three of our stores still in operation. These increases were offset in part by an increase in our credit card fee rebates received of approximately $1.4 million during Fiscal 2010 as compared to Fiscal 2009.
Loss on extinguishment of debt
Loss on extinguishment of debt of $1.3 million during Fiscal 2010 represents the write-off of portions of deferred financing fees and the unrecognized loss of our interest rate swap of $0.9 million and $0.4 million, respectively, related to the repurchase of $65.0 million of our Notes during Fiscal 2010. Loss on extinguishment of debt was $2.0 million during Fiscal 2009, which included the following: $0.4 million for the premium on the repurchase of approximately $44.9 million of our Notes; the write-off of the related portions of deferred financing fees and a portion of the unrecognized loss of our interest rate swap as a result of the aforementioned repurchase, of $0.7 million and $0.6 million, respectively; and a $0.3 million write-off of deferred financing fees related to the repayment of our former revolving credit facility which was terminated in September 2009.
Interest Expense, net
Interest expense, net decreased $9.1 million, or 48.9%, to $9.5 million in Fiscal 2010 compared to $18.6 million for Fiscal 2009. The decrease in interest expense was primarily due to the decrease in our outstanding Notes as a result of the redemption of approximately $109.9 million in aggregate principal from December 2009 through November 2010, offset in part by an increase in average borrowings from our Revolving Credit Line of $10.9 million during Fiscal 2010, which bears a significantly lower interest rate than our Notes.
Provision for Income Taxes
We recognized $19.6 million of income tax expense during Fiscal 2010 as compared to $8.0 million in Fiscal 2009. The effective tax rate for Fiscal 2010 was 40.1%, compared to 38.8% for Fiscal 2009. The 1.3% increase in the effective tax rate is
30
primarily due to favorable developments during Fiscal 2009, for certain outstanding income tax matters related to previously identified uncertain tax positions, as compared to Fiscal 2010.
Net Income
As a result of the foregoing, we generated net income of $29.2 million in Fiscal 2010 compared to net income of $12.7 million in Fiscal 2009.
Comparison of Fiscal 2009 with Fiscal 2008 Net Sales
Net sales increased $73.0 million, or 12.1%, to $674.5 million for Fiscal 2009 compared to $601.5 million for Fiscal 2008. The increase was the result of an increase in our comparable store sales, as well as sales from our new non-comparable stores.
Retail
Net sales from our retail stores increased $73.7 million, or 14.1%, to $596.3 million for Fiscal 2009 compared to $522.5 million for Fiscal 2008. We operated 438 stores as of December 26, 2009 compared to 401 stores as of December 27, 2008. Our overall store sales increased due to non-comparable store sales of $46.7 million, as well as an increase in comparable store sales growth of $27.0 million, or 5.2% (Comparable store sales include only those stores open more than 410 days and align with Fiscal 2008). Our overall sales increased primarily in the categories of specialty supplements, which increased $16.0 million; vitamins and minerals, which increased $13.0 million; and sports nutrition, which increased $24.0 million. These increases were offset in part by a decrease in our weight management category of $2.5 million, which was largely due to a recall of a non-core product which began in the second Fiscal quarter and continued in the third Fiscal quarter of 2009.
Product sales in the specialty supplements category were among our fastest growing categories as we continue to experience significant growth in sales of essential fatty acids, or EFAs, as well as experiencing growth in other products during the Fiscal year, such as Ubiquinol (CoQ10) and probiotics for digestive health. Sales in our vitamin and minerals category increased at a rate greater than the overall increase in net sales due to the introduction of new special formulations for men and women as well as an increase in sales of Vitamin D. Product sales in the sports nutrition category continues to be among our fastest growing categories and has been so for over three consecutive fiscal years. We expect this trend to continue based on the growth of the fitness-conscious market.
Direct
Net sales to our direct customers decreased $0.8 million, or 1.0%, to $78.2 million for Fiscal 2009 compared to $79.0 million for Fiscal 2008. The $0.8 million decrease in direct sales during Fiscal 2009 was comprised of an increase in our internet sales of $3.4 million, offset by a decrease in our catalog sales. The increase in our web-based sales was primarily due to a greater influx of customers this Fiscal year as compared to Fiscal 2008, as a result of our prior web-based marketing initiatives. We have reduced our catalog circulation and catalog customer prospecting as we believe catalog purchasing in general is declining in popularity as a purchasing medium, especially in the wake of the growth of online shopping. In addition, as we continue to open more stores in new markets, some catalog customers choose to shop at our retail locations.
Cost of Goods Sold
Cost of goods sold, which includes product, warehouse and distribution and occupancy costs, increased $51.9 million, or 12.8%, to $457.6 million for Fiscal 2009 compared to $405.7 million for Fiscal 2008. The increase was primarily due to an increase in product costs and occupancy costs for the year ended December 26, 2009, as compared to the year ended December 27, 2008. Cost of goods sold as a percentage of net sales increased to 67.8% for the year ended December 26, 2009, compared to 67.4% for the year ended December 27, 2008. The increase of cost of goods sold as a percentage of net sales was due to increases in both occupancy costs of 0.5%, primarily attributable to the impact of new (non-comparable) stores commencing operations in the last quarter of Fiscal 2008, and product costs of 0.2% as a percentage of sales, due to the impact of promotional coupons issued during the third quarter of Fiscal 2009. These increases were offset by a decrease in distribution costs of 0.3% as a percentage of sales.
Gross Profit
As a result of the foregoing, gross profit increased $21.0 million, or 10.7%, to $216.9 million for Fiscal 2009 compared to $195.9 million for Fiscal 2008.
Selling, General and Administrative Expenses
Selling, general and administrative expenses, including operating payroll and related benefits, advertising and promotion expense, and other selling, general and administrative expenses, increased $14.4 million, or 9.1%, to $173.1 million during Fiscal 2009, compared to $158.7 million for Fiscal 2008. The components of selling, general and administrative expenses are explained
31
below. Selling, general and administrative expenses as a percentage of net sales for Fiscal 2009 decreased to 25.7% compared to 26.4% for Fiscal 2008.
Operating payroll and related benefits increased $8.1 million, or 13.8%, to $67.2 million for Fiscal 2009 compared to $59.0 million for Fiscal 2008. The increase is due mainly to our increase in retail locations throughout Fiscal 2009. Operating payroll and related benefits expenses as a percentage of net sales increased to 10.0% during Fiscal 2009 compared to 9.8% for Fiscal 2008. The increase as a percentage of sales was due to lower sales per hour for Fiscal 2009, as compared to Fiscal 2008, due to a greater number of new (non-comparable) stores commencing operations in the last quarter of Fiscal 2008, as well increases in health benefits expense experienced during Fiscal 2009.
Advertising and promotion expenses decreased $0.4 million, or 3.3%, to $12.8 million for Fiscal 2009 compared to $13.2 million for Fiscal 2008. Advertising and promotion expenses as a percentage of net sales decreased to 1.9% during Fiscal 2009 compared to 2.2% for Fiscal 2008. The decrease as a percentage of net sales was due primarily to a continued reduction in our catalog advertising and prospecting efforts, as well as a decrease in store grand opening promotions, as we opened 23 fewer stores in Fiscal 2009 as compared to Fiscal 2008.
Other selling, general and administrative expenses, which include depreciation and amortization expense, increased $6.7 million, or 7.8%, to $93.2 million in Fiscal 2009 compared to $86.5 million for Fiscal 2008. The increase was due primarily to an increase in depreciation and amortization of approximately $3.6 million, reflecting our new stores; $3.0 million for corporate payroll expense which was primarily due to an increase in incentive compensation and health care costs during Fiscal 2009; and stock-based compensation expense of approximately $0.7 million, due to additional grants issued in Fiscal 2009. These increases in expense were offset by a $1.2 million decrease in store pre-opening costs due to the decrease in the number of new stores we opened during Fiscal 2009. Other selling, general and administrative expenses as a percentage of net sales decreased to 13.8% during Fiscal 2009 compared to 14.4% for Fiscal 2008. The decrease as a percentage of sales was largely the result of experiencing overall economies of scale with regards to these expenses relative to the increase in sales for Fiscal 2009, as compared to Fiscal 2008.
Related Party Expenses
Related party expenses increased $0.9 million, or 60.6%, to $2.4 million during Fiscal 2009, as compared to $1.5 million for Fiscal 2008. The increase is due to fees driven from increased sales during the first three fiscal quarters of Fiscal 2009 as well as a one time termination fee of our management service agreement paid to IPC Manager II, LLC, of approximately $0.8 million (for a detailed presentation of related party expenses, see Note 12 to our consolidated financial statements). As a result of the termination of the management services agreement, there will be no further related party fees associated with this agreement in future periods.
Income from Operations
As a result of the foregoing, income from operations increased $5.7 million, or 16.0%, to $41.3 million for Fiscal 2009 compared to $35.6 million for Fiscal 2008. Income from operations as a percentage of net sales increased to 6.1% during Fiscal 2009 as compared to 5.9% for Fiscal 2008.
Retail
Income from operations for the retail segment increased $14.1 million, or 17.5%, to $94.5 million for Fiscal 2009 compared to $80.4 million for Fiscal 2008. Income from operations as a percentage of net sales for the retail segment increased to 15.8% for Fiscal 2009 compared to 15.4% for Fiscal 2008. This increase as a percentage of sales is largely due to a decrease in store pre-opening costs of 0.2% as a percent of sales and a decrease in retail store computer equipment of 0.2% as a percent of sales.
Direct
Income from operations for the direct segment increased $0.2 million, or 1.6%, to $15.1 million for Fiscal 2009 compared to $14.9 million for Fiscal 2008. Income from operations as a percentage of net sales for the direct segment increased to 19.3% for Fiscal 2009 compared to 18.8% for Fiscal 2008. This increase as a percentage of net sales was primarily due to a decrease in advertising expense of 1.3% as a percentage of sales, due to the decrease in catalog mailings, offset in part by an increase in product costs of 0.7% as a percentage of sales, due to greater price promotions for our direct products, during Fiscal 2009 as compared to Fiscal 2008.
Corporate Costs
Corporate costs increased $8.6 million, or 14.5%, to $68.3 million during Fiscal 2009 compared to $59.7 million for Fiscal 2008. Corporate costs as a percentage of net sales increased to 10.1% for Fiscal 2009 compared to 9.9% for Fiscal 2008. This dollar increase was due primarily to the increase in depreciation and amortization expense of $3.6 million, reflecting our new stores; an increase in corporate payroll costs of approximately $3.0 million, primarily due to an increase in incentive compensation and health care costs during Fiscal 2009; and an increase in stock compensation expense of $0.7 million, due to additional grants issued in Fiscal 2009.
32
Loss on extinguishment of debt
Loss on extinguishment of debt was $2.0 million during Fiscal 2009, which included the following: $0.4 million for the premium on the repurchase of approximately $44.9 million of our Notes; the write-off of the related portions of deferred financing fees and a portion of the unrecognized loss of our interest rate swap as a result of the aforementioned repurchase, of $0.7 million and $0.6 million, respectively; and a $0.3 million write-off of deferred financing fees related to the repayment of our former revolving credit facility which was terminated in September 2009.
Interest Expense, net
Interest expense, net decreased $2.5 million, or 13.4%, to $18.6 million in Fiscal 2009 compared to $21.1 million for Fiscal 2008. The decrease was attributable to a decrease in interest rates in Fiscal 2009 compared to Fiscal 2008.
Provision for Income Taxes
We recognized $8.0 million of income tax expense during Fiscal 2009 compared to $6.3 million in Fiscal 2008. The effective tax rate was 38.8%, compared to 43.7% for Fiscal 2008. The 4.9% decrease in the effective tax rate is primarily due to favorable developments on certain outstanding income tax matters related to previously identified uncertain tax positions during Fiscal 2009, as compared to a net increase in tax expense related to uncertain tax positions in Fiscal 2008.
Net Income
As a result of the foregoing, we generated net income of $12.7 million in Fiscal 2009 compared to net income of $8.2 million in Fiscal 2008.
Key Indicators of Liquidity and Capital Resources
The following table sets forth key indicators of our liquidity and capital resources (in thousands):
| | | | | | | | | | | | |
| | | As of | | | | |
| | | December 25,
2010 | | | December 26,
2009 | | | | |
Balance Sheet Data: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 25,968 | | | $ | 8,797 | | | | | |
Working capital | | | 75,959 | | | | 50,416 | | | | | |
Total assets | | | 485,717 | | | | 469,257 | | | | | |
Total debt, including capital lease obligations | | | 75,794 | | | | 123,946 | | | | | |
| |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Other Information: | | | | | | | | | | | | |
Depreciation and amortization (1) | | $ | 21,112 | | | $ | 21,095 | | | $ | 17,483 | |
| | | |
Cash Flows Provided By (Used In): | | | | | | | | | | | | |
Operating activities | | $ | 55,184 | | | $ | 43,434 | | | $ | 19,588 | |
Investing activities | | | (18,448 | ) | | | (21,281 | ) | | | (35,389 | ) |
Financing activities | | | (19,565 | ) | | | (14,979 | ) | | | 15,971 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | $ | 17,171 | | | $ | 7,174 | | | $ | 170 | |
| | | | | | | | | | | | |
| (1) | Excludes amortization of deferred financing fees. |
Liquidity and Capital Resources
Our primary uses of cash are to fund working capital, operating expenses, debt service and capital expenditures related primarily to the construction of new stores. Historically, we have financed these requirements predominately through internally generated cash flow, supplemented with short-term financing. We believe that the cash generated by operations and cash and cash equivalents,
33
together with the borrowing availability under our revolving credit facility, will be sufficient to meet our working capital needs for the next twelve months, including investments made and expenses incurred in connection with our store growth plans, systems development and store improvements.
During Fiscal 2010 we spent approximately $15.7 million, out of the $18.4 million of total capital expenditures, in connection with our store growth and improvement plans. We expect to spend approximately $23.0 million on capital expenditures in Fiscal 2011, most of which will pertain to approximately 48 new stores we anticipate opening throughout the year. We opened 47 new stores during Fiscal 2010, and closed one store. Our working capital requirements for merchandise inventory will continue to increase as we continue to open additional stores. Currently, our practice is to establish an inventory level of $165,000 to $185,000 at cost for each of our stores, a portion of which is vendor-financed based upon agreed credit terms. Giving consideration to both our revolving credit facility and cash generated from our operations, we feel we will have sufficient liquidity through the next fiscal year to fund our capital requirements and operations. Additionally, 30 day payment terms have been extended to us by some of our suppliers allowing us to effectively manage our inventory and working capital.
During April 2010, we increased our credit facility by $20.0 million giving us up to $70.0 million in borrowing availability. In addition, during the first quarter of fiscal 2011 we extended the credit facility another two years resulting in a maturity date of September 25, 2015. Also, in connection with the revised revolving credit agreement, we entered into a term loan agreement providing us with an additional $25.0 million in financing which was used along with existing cash and available borrowing under our revolving credit facility for the redemption of the remaining portion of our Notes during February 2011.
We were in compliance with all debt covenants as of December 25, 2010. We expect to be in compliance with these same debt covenants during Fiscal 2011 as well.
Cash Provided by Operating Activities
Cash provided by operating activities was $55.2 million and $43.4 million during Fiscal 2010 and Fiscal 2009, respectively. The $11.8 million increase in cash flows from operating activities is primarily due to an increase in our net income, offset in part by an increase in expenditures on our inventory and a reduction in our accounts payable during Fiscal 2010, as compared to Fiscal 2009. In addition, there was a one time payment of approximately $2.6 million which occurred during September 2009, for the termination of our interest rate swap. The increase in expenditures on our inventory increased as a result of the increase to our net sales, and increased at a rate of less than 50% than the increase in net sales. The reduction in on our accounts payable is attributable to both faster payments to our suppliers to take advantage of certain payment terms, offset by the increase in our inventory expenditures.
Cash provided by operating activities was $43.4 million and $19.6 million during Fiscal 2009 and Fiscal 2008, respectively. The $23.8 million increase in cash flows from operating activities is primarily due to an increase in our net income, as well as a decrease in expenditures on inventory, due to effective management of inventory in our stores along with more efficient levels of replenishment; and a decrease in expenditures on our accounts payable during Fiscal 2009, related in part to the decrease in inventory expenditures, as compared to Fiscal 2008. This increase in cash flows from operating activities was offset in part by a $2.6 million cash payment, for the termination of our interest rate swap during September 2009.
Cash Used in Investing Activities
Net cash used in investing activities was $18.4 million during Fiscal 2010 as compared to $21.3 million during Fiscal 2009. The decrease in cash used in investing activities of $2.8 million during Fiscal 2010, as compared to Fiscal 2009, is primarily the result of a lower construction cost per store and a decrease in refurbishing of older stores. The lower cost per store reflects less work required to ready a store for operations based on its existing condition during the inception of the lease.
Net cash used in investing activities during Fiscal 2009 and Fiscal 2008 was $21.3 million and $35.4 million, respectively. The decrease in cash used in investing activities of $14.1 million was primarily due to the opening of 23 more stores during Fiscal 2008 as compared to Fiscal 2009, as well as the acquisition of $3.5 million of intangible assets (as discussed in Note 4 to our Financial Statements) during Fiscal 2008.
Cash (Used in) Provided by Financing Activities
Net cash used in financing activities was $19.6 million in Fiscal 2010, compared to net cash used in financing activities of $15.0 million during Fiscal 2009. The $4.6 million increase in net cash used in financing activities, which includes the non cash impact of tax benefits on exercises of stock options of $12.0 million, was primarily due to the redemption of an additional $20.1 million of our Notes during Fiscal 2010, as compared to Fiscal 2009, which was more than offset by the net change in borrowings under our revolving credit agreement of $35.0 million and an increase in proceeds received from the exercise of stock options of $16.1 million during Fiscal 2010, as compared to Fiscal 2009. In addition, excluding the $44.9 million redemption of our Notes, Fiscal 2009 included net proceeds of approximately $47.0 million received from our IPO.
34
Net cash used in financing activities was $15.0 million in Fiscal 2009, compared to net cash provided by financing activities of $16.0 million during Fiscal 2008. The decrease in net cash provided by financing activities was primarily due to net repayments of $17.0 million on our revolving credit facility during Fiscal 2009, compared to net borrowings of $17.0 million from our revolving credit facility during Fiscal 2008.
2005 Senior Notes
On November 7, 2005, we completed our Second Priority Senior Secured Floating Rate Notes (the “Notes”) offering for $165.0 million. During Fiscal 2009 and Fiscal 2010, we redeemed approximately $109.9 million of our Notes leaving $55.1 million of the original $165.0 million outstanding at December 25, 2010. During January 2011, we announced our intent to redeem the remaining balance of $55.1 million of our Notes. The redemption was completed on February 22, 2011, after which there were no Notes outstanding.
Revolving Credit Facilities
On November 15, 2005, Vitamin Shoppe Industries, Inc. entered into a $50.0 million senior secured revolving credit facility, which was terminated on September 25, 2009, resulting in a loss on extinguishment of debt of approximately $0.3 million. There was no balance under this line of credit at the time of its termination on September 25, 2009. The largest amount borrowed at any given point during the period ended September 25, 2009 was $17.0 million.
2009 Revolving Credit Facility
On September 25, 2009, we entered into a new revolving credit facility (the “2009 Revolving Credit Facility”), and simultaneously terminated our existing credit facility that was entered into on November 15, 2005. We entered into the 2009 Revolving Credit Facility to obtain an additional two years of liquidity beyond the termination date of our previous facility. In doing so, we incurred an incremental borrowing rate of 1% as compared to the former revolving credit facility. The terms of the 2009 Revolving Credit Facility extend through September 2013, and allow us to borrow up to $70.0 million subject to the terms of the facility. Similar to our previous credit facility, the availability under the 2009 Revolving Credit Facility is subject to a borrowing base calculated on the value of certain accounts receivable from credit card companies as well as the inventory of Vitamin Shoppe Industries, Inc. (“Industries”) and VS Direct Inc. (“Direct”). The obligations thereunder are secured by a security interest in substantially all of the assets of Vitamin Shoppe, Inc. (“VSI”), Industries and Direct. Direct and VSI, provided guarantees in respect of our obligations under the 2009 Revolving Credit Facility, and Industries and VSI, Inc., have provided guarantees in respect of Direct’s obligations under the 2009 Revolving Credit Facility. The 2009 Revolving Credit Facility provides for affirmative and negative covenants affecting Industries, VSI and Direct. The 2009 Revolving Credit Facility restricts, among other things, our ability to incur indebtedness, create or permit liens on our assets, declare or pay dividends and make certain other restricted payments, consolidate, merge or recapitalize, acquire or sell assets, make certain investments, loans or other advances, enter into transactions with affiliates, change our line of business, and restricts the types of hedging activities we can enter into. The largest amount borrowed at any given point during Fiscal 2010 was $38.0 million. The unused available line of credit under the 2009 Revolving Credit Facility at December 25, 2010 was $41.7 million.
Contractual Obligations and Commercial Commitments
As of December 25, 2010, our lease commitments and contractual obligations are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | |
Fiscal year ending | | Total | | | Operating
Leases (1) | | | Capital Lease
Obligations
including Interest | | | Long-Term
Debt (2) | | | Interest
Payments (3) | | | Credit
Facility (4) | |
2011 | | $ | 83,760 | | | $ | 77,577 | | | $ | 1,846 | | | $ | — | | | $ | 4,337 | | | $ | — | |
2012 | | | 135,922 | | | | 76,098 | | | | 985 | | | | 55,106 | | | | 3,733 | | | | — | |
2013 | | | 88,251 | | | | 70,251 | | | | — | | | | — | | | | — | | | | 18,000 | |
2014 | | | 60,326 | | | | 60,326 | | | | — | | | | — | | | | — | | | | — | |
2015 | | | 48,436 | | | | 48,436 | | | | — | | | | — | | | | — | | | | — | |
Thereafter | | | 117,653 | | | | 117,653 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 534,348 | | | $ | 450,341 | | | $ | 2,831 | | | $ | 55,106 | | | $ | 8,070 | | | $ | 18,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | The operating leases included in the above table do not include contingent rent based upon sales volume, which represented less than 1% of our minimum lease obligations during Fiscal 2010. In addition, the operating leases do not include common area |
35
| | maintenance costs or real estate taxes that are paid to the landlord during the year, which combined represented approximately 16.1% of our minimum lease obligations during Fiscal 2010. |
| (2) | The $55.1 million balance of our Notes is classified as long-term per the above table as the tender to redeem the Notes in their entirety occurred during January 2011 subsequent to our fiscal year end. In addition, our term loan entered into during January 2011 of $25.0 million is not included in the table, as it was not a component of our consolidated balance sheet as of December 25, 2010. |
| (3) | Interest payments are estimated based upon the prevailing interest rates at December 25, 2010. |
| (4) | The credit facility does not reflect the amendment to the agreement which extended its maturity date to September 25, 2015. |
Severance. As of December 25, 2010, we have an aggregate contingent liability of up to $2.0 million related to potential severance payments for five executives pursuant to their respective employment agreements. These potential severance payments are not reflected in the table above.
Excluded from the above commitments is $4.6 million of long-term liabilities related to uncertain tax positions, due to the uncertainty of the time and nature of resolution.
Off-Balance Sheet Arrangements
We have not created, and are not party to, any special-purpose or off-balance sheet entities for the purpose of raising capital, incurring debt or operating our business. We do not have any off-balance sheet arrangements or relationships with entities that are not consolidated into our financial statements that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources.
Effects of Inflation
We do not believe that our sales or operating results have been materially impacted by inflation during the periods presented in our financial statements. There can be no assurance, however, that our sales or operating results will not be impacted by inflation in the future.
Recent Accounting Pronouncements
We have considered all new accounting pronouncements and have concluded that there are no new pronouncements that have a material impact on results of operations, financial condition, or cash flows, based on current information.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Our market risks relate primarily to changes in interest rates. Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows. In the ordinary course of business, we are primarily exposed to interest rate risks. Other than on our Notes, which carry a floating interest rate, we have not used derivative financial instruments in connection with these market risks.
Our Revolving Credit Facility and Notes carry floating interest rates that are tied to LIBOR and the prime rate and, therefore, our statements of operations and our cash flows are exposed to changes in interest rates. A one percentage point increase in LIBOR would cause an increase to the interest expense on our Notes of approximately $0.5 million. A one point percentage increase in Libor would cause an increase to the interest expense on our Revolving Credit Facility of approximately $0.2 million based on the balance in our Revolving Credit Facility at December 25, 2010. The balance of our Revolving Credit Facility at December 25, 2010, is not indicative of future balances which may be subject to fluctuations in interest rates.
| Item 8. | Financial Statements and Supplementary Data |
The response to this item is provided in this Annual Report on Form 10-K, commencing on page 43, which is incorporated herein by reference.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
We carried out an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, our principal executive officer and principal financial officer, respectively, of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a (e) and 15d—15(e) under the
36
Securities Exchange Act of 1934, as amended (the Exchange Act”), to provide reasonable assurance of achieving the control objectives as of the end of the period covered by this report. Based upon that evaluation, the Principal Executive Officer and Principal Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report in providing a reasonable level of assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods in Securities and Exchange commission rules and forms, including providing a reasonable level of assurance that information required to be disclosed by us in such reports is accumulated and communicated to our management, including our Principal Executive Officer and our Principal Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
See Item 15 “Exhibits and Financial Statement Schedules” appearing at the end of this Annual Report on Form 10-K for Management’s Report on Internal Control Over Financial Reporting.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control structure, policies and procedures for the quarter ended December 25, 2010, that could significantly affect our internal control over financial reporting, or be reasonably likely to materially affect our internal control over financial reporting.
| Item 9B. | Other Information |
None.
37
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
Information with respect to this Item is included in the Company’s Proxy Statement to be filed in April 2011, which is incorporated herein by reference.
| Item 11. | Executive Compensation |
Information with respect to this Item is included in the Company’s Proxy Statement to be filed in April 2011, which is incorporated herein by reference, under the captions, “Compensation of Directors”, “Executive Compensation” and “Compensation Discussion and Analysis”; provided, however, that the subsection entitled “Executive Compensation—Report of the Executive Compensation Committee of the Board of Directors on Executive Compensation” shall not be deemed to be incorporated by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information with respect to this Item is included in the Company’s Proxy Statement to be filed in April 2011, which is incorporated herein by reference.
| Item 13. | Certain Relationships, Related Transactions and Director Independence |
Information with respect to this Item is included in the Company’s Proxy Statement to be filed in April 2011, which is incorporated herein by reference, under the captions “Securityholders Agreement,” “Advisory Services Agreement,” “Transactions with Management,” and “Board of Directors.”
| Item 14. | Principal Accountant Fees and Services |
Information with respect to this Item is included in the Company’s Proxy Statement to be filed in April 2011, which is incorporated herein by reference.
| Item 15. | Exhibits and Financial Statement Schedules |
| | (a) | The following documents are filed as part of this annual report on Form 10-K: |
| | 1. | The following consolidated financial statements listed below are filed as a separate section of this annual report on Form 10-K commencing on page 44: |
Management’s Reports and Reports of Independent Registered Public Accounting Firm—Deloitte & Touche LLP.
Consolidated Balance Sheets as of December 25, 2010 and December 26, 2009.
Consolidated Statements of Operations for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008.
Consolidated Statements of Stockholders’ Equity for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008.
Consolidated Statements of Cash Flows for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008.
Notes to Consolidated Financial Statements for the Fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008.
38
| | |
EXHIBIT NO. | | DESCRIPTION |
| 2.1 | | Agreement and Plan of Merger by and between VS Holdings, Inc. and VS Parent, Inc., dated as of October 27, 2009. ‡ |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation of Vitamin Shoppe, Inc. ‡‡ |
| |
| 3.2 | | Second Amended and Restated By-Laws of Vitamin Shoppe, Inc. ‡‡ |
| |
| 4.1 | | Indenture dated as of November 15, 2005, by and among Vitamin Shoppe Industries Inc., VS Holdings, Inc. and VS Direct Inc., as Guarantors, and Wilmington Trust Company, as Trustee. ‡‡‡ |
| |
| 4.2 | | Form of Second Priority Senior Secured Floating Rate Note due 2012. ‡‡‡ |
| |
| 10.1 | | Securityholders Agreement, by and among Vitamin Shoppe, Inc. and its securityholders, dated October 27, 2009. ‡ |
| |
| 10.2 | | Amended and Restated Loan and Security Agreement, dated as of September 25, 2009, as amended January 20, 2011, by and among Vitamin Shoppe Industries Inc. and VS Direct Inc. as borrowers, Vitamin Shoppe, Inc. as Guarantor, the Lenders and Issuing Bank from time to time party thereto, and JPMorgan Chase Bank, N.A. as Administrative Agent. |
| |
| 10.3 | | Intercreditor Agreement Joinder, dated as of September 25, 2009, by JPMorgan Chase Bank, N.A. ‡‡‡‡ |
| |
| 10.4 | | Intellectual Property Security Agreement, dated as of September 25, 2009, by and among Vitamin Shoppe Industries Inc., VS Direct Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and JPMorgan Chase Bank, N.A., as Administrative Agent for the Lenders. ‡‡‡‡ |
| |
| 10.5 | | Stock Pledge Agreement, dated September 25, 2009 by and between Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) as Pledgor and JPMorgan Chase Bank, N.A. as Pledgee. ‡‡‡‡ |
| |
| 10.6 | | Stock Pledge Agreement, dated September 25, 2009 by and between Vitamin Shoppe Industries Inc. as Pledgor and JPMorgan Chase Bank, N.A. as Pledgee. ‡‡‡‡ |
| |
| 10.7 | | Guarantee of Vitamin Shoppe Industries Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.), dated September 25, 2009, of obligations of VS Direct Inc. under the Loan and Security Agreement. ‡‡‡‡ |
| |
| 10.8 | | Guarantee of VS Direct Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.), dated September 25, 2009, of obligations of Vitamin Shoppe Industries Inc. under the Loan and Security Agreement. ‡‡‡‡ |
| |
| 10.9 | | Lease Agreement, dated as of May 2, 2002, between Hartz Mountain Industries, Inc. and Vitamin Shoppe Industries Inc. ‡‡‡ |
| |
| 10.10 | | Purchase Agreement, dated as of November 1, 2004, between Natures Value, Inc. and Vitamin Shoppe Industries Inc. ‡‡‡ |
| |
| 10.11 | | Form of Employment Agreement by and among executive officer, VS Parent, Inc., Vitamin Shoppe Industries Inc. and VS Holdings, Inc. § ‡‡‡ |
39
| | |
| |
| 10.12 | | Form of Indemnification Agreement by and among executive officer, VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § ††† |
| |
| 10.13 | | Form of Indemnification Agreement by and among director, VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § ††† |
| |
| 10.14 | | VS Parent, Inc. 2006 Stock Option Plan. § † |
| |
| 10.15 | | 2009 Vitamin Shoppe Equity Incentive Plan, effective as of September 2, 2009. § †† |
| |
| 10.16 | | Vitamin Shoppe 2010 Employee Stock Purchase Plan, effective December 16, 2009.v |
| |
| 10.17 | | Employment and Non-Competition Agreement, dated as of September 9, 2009, among Richard Markee, VS Parent, Inc., VS Direct, Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. †† |
| |
| 10.18 | | Amended and Restated Employment and Non-Competition Agreement, dated as of June 12, 2006, by and among Anthony Truesdale, VS Parent, Inc., VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § ‡‡‡ |
| |
| 10.19 | | Amendment to Amended and Restated Employment and Non-Competition Agreement, dated as of December 28, 2007, by and among Anthony Truesdale, VS Parent, Inc., VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § * |
| |
| 10.20 | | Amendment No. 2 to Employment and Non-Competition Agreement, dated as of September 25, 2009 by and among Anthony Truesdale, VS Parent, Inc., Vitamin Shoppe Industries Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.). ‡‡‡‡ |
| |
| 10.21 | | Employment and Non-Competition Agreement, dated as of April 16, 2007, by and among Michael G. Archbold, VS Parent, Inc., VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § *** |
| |
| 10.22 | | Amendment to Employment and Non-Competition Agreement, dated as of December 28, 2007, by and among Michael G. Archbold, VS Parent, Inc. and VS Holdings, Inc. and Vitamin Shoppe Industries Inc. § ‡‡‡‡ |
| |
| 10.23 | | Amendment No. 2 to Employment and Non-Competition Agreement, dated as of September 25, 2009 by and among Michael G. Archbold, VS Parent, Inc., Vitamin Shoppe Industries Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.). ‡‡‡‡ |
| |
| 10.24 | | Employment and Non-Competition Agreement, dated as of January 15, 2007, by and among Louis H. Weiss, VS Parent, Inc., VS Holdings, Inc., VS Direct, Inc., and Vitamin Shoppe Industries, Inc. § ** |
| |
| 10.25 | | Amendment to Employment and Non-Competition Agreement, dated as of December 28, 2007, by and among Louis H. Weiss, VS Parent, Inc., VS Holdings, Inc., VS Direct, Inc. and Vitamin Shoppe Industries Inc. § * |
| |
| 10.26 | | Amendment to Employment Agreement, dated as of June 12, 2006, by and among Cosmo La Forgia, VS Parent, Inc., Vitamin Shoppe Industries Inc. and VS Holdings, Inc. § ‡‡‡ |
| |
| 10.27 | | Second Amendment to Employment Agreement, dated as of December 28, 2007, by and among Cosmo La Forgia, VS Parent, Inc., Vitamin Shoppe Industries Inc. and VS Holdings, Inc. § * |
| |
| 10.28 | | Third Amendment to Employment Agreement, dated as of March 6, 2008, by and among Cosmo La Forgia, VS Parent, Inc., Vitamin Shoppe Industries Inc. and VS Holdings, Inc. § * |
| |
| 10.29 | | Letter Agreement, dated as of February 10, 2011, by and between Brenda Galgano and Vitamin Shoppe Industries, Inc. |
40
| | | | |
| | 10.30 | | | Amendment No. 3 to Employment and Non-Competition Agreement, dated as of February 24, 2011, by and between Michael Archbold and Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. |
| |
| | 10.31 | | | Amendment No. 3 to Employment and Non-Competition Agreement, dated as of February 24, 2011, by and between Anthony Truesdale and Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. |
| |
| | 10.32 | | | Amendment No. 1 to Employment and Non-Competition Agreement, dated as of February 24, 2011, by and between Richard Markee and Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. |
| |
| | 21.1 | | | Subsidiaries of the Registrant. ‡‡‡ |
| |
| | 23.1 | | | Consent of Independent Registered Public Accounting Firm. |
| |
| | 31.1 | | | Certification of Richard L. Markee pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| | 31.2 | | | Certification of Michael G. Archbold pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| | 32.1 | | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the |
| |
| | | | Sarbanes-Oxley Act of 2002—Chief Executive Officer. |
| |
| | 32.2 | | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002— Chief Financial Officer. |
| | | | | |
| |
| | v | | | Incorporated by reference to our Form 10-K, filed on March 17, 2010 (File No. 001-34507). |
| | ‡ | | | Incorporated by reference to our Current Report on Form 8-K, filed on November 2, 2009 (File No. 001-34507). |
| | ‡‡ | | | Incorporated by reference to our Form 10-Q/A, filed on November 13, 2009 (File No. 001-34507). |
| | ‡‡‡ | | | Incorporated by reference to Registration Statement No. 333-134983 on Form S-4 filed on June 13, 2006, as amended (File No. 333-134983-2). |
| | ‡‡‡‡ | | | Incorporated by reference to our Current Report on Form 8-K, filed on September 30, 2009 (File No. 001-34507). |
| | * | | | Incorporated by reference to our Annual report on Form 10-K for the Fiscal year ended December 29, 2007, filed on March 28, 2008. |
| | ** | | | Incorporated by reference to our Current Report on Form 8-K, filed on January 16, 2007. (File No. 001-34507). |
| | *** | | | Incorporated by reference to our Current Report on Form 8-K, filed on April 19, 2007. (File No. 001-34507). |
| | † | | | Incorporated by reference to Amendment No. 5 to our Registration Statement on Form S-1, filed on October 22, 2009 (File No. 333-160756). |
| | †† | | | Incorporated by reference to Amendment No. 2 to our Registration Statement on Form S-1, filed on September 22, 2009 (File No. 333-160756). |
| | ††† | | | Incorporated by reference to Amendment No. 4 to our Registration Statement on Form S-1, filed on October 14, 2009 (File No. 333-160756). |
| | § | | | Management contract or compensation plan or arrangement. |
41
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 9, 2011.
| | |
| VITAMIN SHOPPE, INC. |
| |
| By: | | /S/ RICHARD L. MARKEE |
| | Richard L. Markee Chief Executive Officer and
Chairman of the Board |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | |
| | | Name | | Title | | Date |
| | | |
By:
| | /S/ RICHARD L. MARKEE Richard L. Markee | | Chief Executive Officer, Chairman of the Board, Director (Principal Executive Officer) | | March 9, 2011 |
| | | |
| By: | | /S/ MICHAEL G. ARCHBOLD Michael G. Archbold | | Chief Financial Officer and Chief Operating Officer (Principal Financial and Accounting Officer) | | March 9, 2011 |
| | | |
| By: | | /S/ B. MICHAEL BECKER B. Michael Becker | | Director | | March 9, 2011 |
| | | |
| By: | | /S/ CATHERINE BUGGELN Catherine Buggeln | | Director | | March 9, 2011 |
| | | |
| By: | | /S/ DAVID H. EDWAB David H. Edwab | | Director | | March 9, 2011 |
| | | |
| By: | | /S/ JOHN H. EDMONDSON John H. Edmondson | | Director | | March 9, 2011 |
| | | |
| By: | | /S/ DOUGLAS R. KORN Douglas R. Korn | | Director | | March 9, 2011 |
| | | |
| By: | | /S/ RICHARD L. PERKAL Richard L. Perkal | | Director | | March 9, 2011 |
| | | |
| By: | | Beth M. Pritchard | | Director | | |
| | | |
| By: | | Katherine Savitt-Lennon | | Director | | |
42
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined under the Exchange Act) to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States (“GAAP”). Such internal control includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets; (ii) provide reasonable assurance (A) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors; and (B) regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Our management assessed the effectiveness of our internal control over financial reporting as of December 25, 2010. In making this assessment, it used the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, management has determined that, as of December 25, 2010, our internal control over financial reporting is effective based on those criteria.
The Company’s internal control over financial reporting as of December 25, 2010 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their attestation report which appears herein.
March 9, 2011
| | |
/S/ RICHARD L. MARKEE | | /S/ MICHAEL G. ARCHBOLD |
Richard L. Markee | | Michael G. Archbold |
Chief Executive Officer, Chairman of the Board | | Chief Financial Officer and Chief Operating Officer |
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL STATEMENTS
The management of Vitamin Shoppe, Inc. is responsible for the preparation, objectivity and integrity of the consolidated financial statements and other information contained in this Annual Report on Form 10-K. The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America and include some amounts that are based on management’s informed judgments and best estimates.
Deloitte & Touche LLP, an independent registered public accounting firm, has audited these consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States) and has expressed herein their unqualified opinion on those financial statements.
The Audit Committee of the Board of Directors, which oversees all of the Company’s financial reporting process on behalf of the Board of Directors, consists solely of independent directors, meets with the independent registered public accounting firm, internal auditors and management periodically to review their respective activities and the discharge of their respective responsibilities. Both the independent registered public accounting firm and the internal auditors have unrestricted access to the Audit Committee, with or without management, to discuss the scope and results of their audits and any recommendations regarding the system of internal controls.
March 9, 2011
| | |
/S/ RICHARD L. MARKEE | | /S/ MICHAEL G. ARCHBOLD |
Richard L. Markee | | Michael G. Archbold |
Chief Executive Officer, Chairman of the Board | | Chief Financial Officer and Chief Operating Officer |
43
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Vitamin Shoppe, Inc.
North Bergen, New Jersey
We have audited the internal control over financial reporting of Vitamin Shoppe, Inc. and Subsidiary (the “Company”) as of December 25, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 25, 2010, based on the criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the fiscal year ended December 25, 2010 of the Company and our report dated March 9, 2011 expressed an unqualified opinion on those financial statements.
/s/ Deloitte & Touche LLP
New York, New York
March 9, 2011
44
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Vitamin Shoppe, Inc.
North Bergen, New Jersey
We have audited the accompanying consolidated balance sheets of Vitamin Shoppe, Inc. and Subsidiary (the “Company”) as of December 25, 2010 and December 26, 2009, and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the three fiscal years in the period ended December 25, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Vitamin Shoppe, Inc. and Subsidiary as of December 25, 2010 and December 26, 2009, and the results of their operations and their cash flows for each of the three fiscal years in the period ended December 25, 2010, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 25, 2010, based on criteria establishedin Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 9, 2011 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
New York, New York
March 9, 2011
45
PART I. FINANCIAL INFORMATION
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
| | | | | | | | |
| | | December 25,
2010 | | | December 26,
2009 | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 25,968 | | | $ | 8,797 | |
Inventories | | | 111,305 | | | | 106,091 | |
Prepaid expenses and other current assets | | | 13,612 | | | | 13,401 | |
Deferred income taxes | | | 4,033 | | | | 5,145 | |
| | | | | | | | |
Total current assets | | | 154,918 | | | | 133,434 | |
Property and equipment, net | | | 80,949 | | | | 83,960 | |
Goodwill | | | 177,248 | | | | 177,248 | |
Other intangibles, net | | | 69,718 | | | | 70,356 | |
Other assets: | | | | | | | | |
Deferred financing fees, net of accumulated amortization of $1,961 and $2,856, respectively | | | 816 | | | | 2,384 | |
Other | | | 2,068 | | | | 1,875 | |
| | | | | | | | |
Total other assets | | | 2,884 | | | | 4,259 | |
| | | | | | | | |
Total assets | | $ | 485,717 | | | $ | 469,257 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Current portion of long-term debt | | $ | — | | | $ | 20,000 | |
Current portion of capital lease obligations | | | 1,711 | | | | 1,537 | |
Revolving credit facility | | | 18,000 | | | | — | |
Accounts payable | | | 18,994 | | | | 25,075 | |
Deferred sales | | | 15,929 | | | | 14,386 | |
Accrued salaries and related expenses | | | 9,573 | | | | 7,551 | |
Other accrued expenses | | | 14,752 | | | | 14,469 | |
| | | | | | | | |
Total current liabilities | | | 78,959 | | | | 83,018 | |
Long-term debt | | | 55,106 | | | | 100,106 | |
Capital lease obligations, net of current portion | | | 977 | | | | 2,303 | |
Deferred income taxes | | | 20,595 | | | | 19,945 | |
Other long-term liabilities | | | 5,304 | | | | 4,766 | |
Deferred rent | | | 27,080 | | | | 24,768 | |
| | |
Commitments and contingencies | | | | | | | | |
| | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, $0.01 par value; 250,000,000 shares authorized and no shares issued and outstanding at December 25, 2010 and December 26, 2009 | | | — | | | | — | |
Common stock, $0.01 par value; 400,000,000 shares authorized, 28,627,897 shares issued and outstanding at December 25, 2010, and 26,750,423 shares outstanding at December 26, 2009 | | | 286 | | | | 268 | |
Additional paid-in capital | | | 243,558 | | | | 210,359 | |
Accumulated other comprehensive loss | | | — | | | | (882 | ) |
Retained earnings | | | 53,852 | | | | 24,606 | |
| | | | | | | | |
Total stockholders’ equity | | | 297,696 | | | | 234,351 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 485,717 | | | $ | 469,257 | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
46
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except share and per share data)
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Net sales | | $ | 751,482 | | | $ | 674,495 | | | $ | 601,540 | |
Cost of goods sold | | | 501,948 | | | | 457,573 | | | | 405,659 | |
| | | | | | | | | | | | |
Gross profit | | | 249,534 | | | | 216,922 | | | | 195,881 | |
Selling, general and administrative expenses | | | 189,872 | | | | 173,144 | | | | 158,713 | |
Related party expenses | | | — | | | | 2,446 | | | | 1,523 | |
| | | | | | | | | | | | |
Income from operations | | | 59,662 | | | | 41,332 | | | | 35,645 | |
Loss on extinguishment of debt | | | 1,349 | | | | 2,016 | | | | — | |
Interest expense, net | | | 9,517 | | | | 18,636 | | | | 21,137 | |
| | | | | | | | | | | | |
Income before provision for income taxes | | | 48,796 | | | | 20,680 | | | | 14,508 | |
Provision for income taxes | | | 19,550 | | | | 8,014 | | | | 6,341 | |
| | | | | | | | | | | | |
Net income | | | 29,246 | | | | 12,666 | | | | 8,167 | |
Preferred stock dividends in arrears | | | — | | | | 7,692 | | | | 9,279 | |
| | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | 29,246 | | | $ | 4,974 | | | $ | (1,112 | ) |
| | | | | | | | | | | | |
Weighted average common shares outstanding | | | | | | | | | | | | |
Basic | | | 27,390,419 | | | | 16,238,338 | | | | 14,175,906 | |
Diluted | | | 28,338,788 | | | | 17,748,371 | | | | 14,175,906 | |
Net income (loss) per common share | | | | | | | | | | | | |
Basic | | $ | 1.07 | | | $ | 0.31 | | | $ | (0.08 | ) |
Diluted | | $ | 1.03 | | | $ | 0.28 | | | $ | (0.08 | ) |
See accompanying notes to consolidated financial statements.
47
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Series A | | | Common Stock | | | Additional
Paid-In
Capital | | | Warrants | | | Note
Receivable
Due from
Officer | | | Accumulated
Other
Comprehensive
(Loss) Income | | | Retained
Earnings | | | Total | |
| | | Shares | | | Amounts | | | Shares | | | Amounts | | | | | | | |
Balance at December 29, 2007 | | | 79,860 | | | $ | 1 | | | | 14,175,906 | | | $ | 142 | | | $ | 153,057 | | | $ | 5,666 | | | $ | (1,500 | ) | | $ | (1,350 | ) | | $ | 3,773 | | | $ | 159,789 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 8,167 | | | | 8,167 | |
Interest rate swap, net of taxes of $0.8 million | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,264 | ) | | | — | | | | (1,264 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Comprehensive Income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 6,903 | |
Equity compensation | | | — | | | | — | | | | — | | | | — | | | | 2,352 | | | | — | | | | — | | | | — | | | | — | | | | 2,352 | |
Redemption of preferred shares | | | (358 | ) | | | — | | | | — | | | | — | | | | (561 | ) | | | — | | | | — | | | | — | | | | — | | | | (561 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 27, 2008 | | | 79,502 | | | | 1 | | | | 14,175,906 | | | | 142 | | | | 154,848 | | | | 5,666 | | | | (1,500 | ) | | | (2,614 | ) | | | 11,940 | | | | 168,483 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 12,666 | | | | 12,666 | |
Interest rate swap, net of taxes of $1.2 million | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,732 | | | | — | | | | 1,732 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Comprehensive Income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 14,398 | |
Extinguishment of officers’ note | | | (634 | ) | | | — | | | | (140,507 | ) | | | — | | | | (1,837 | ) | | | — | | | | 1,500 | | | | — | | | | — | | | | (337 | ) |
Equity compensation | | | — | | | | — | | | | — | | | | — | | | | 3,040 | | | | — | | | | — | | | | — | | | | — | | | | 3,040 | |
Issuance of restricted shares | | | — | | | | — | | | | 90,557 | | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock | | | — | | | | — | | | | 49,950 | | | | — | | | | 755 | | | | — | | | | — | | | | — | | | | — | | | | 755 | |
Issuance of common stock during offering, net* | | | (36,969 | ) | | | — | | | | 12,486,920 | | | | 124 | | | | 125,051 | | | | (5,666 | ) | | | — | | | | — | | | | — | | | | 119,509 | |
Redemption of preferred shares | | | (41,899 | ) | | | (1 | ) | | | — | | | | — | | | | (72,535 | ) | | | — | | | | — | | | | — | | | | — | | | | (72,535 | ) |
Exercise of stock options | | | — | | | | — | | | | 87,597 | | | | 1 | | | | 685 | | | | — | | | | — | | | | — | | | | — | | | | 686 | |
Tax benefits on exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | 352 | | | | — | | | | — | | | | — | | | | — | | | | 352 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 26, 2009 | | | — | | | | — | | | | 26,750,423 | | | | 268 | | | | 210,359 | | | | — | | | | — | | | | (882 | ) | | | 24,606 | | | | 234,351 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 29,246 | | | | 29,246 | |
Interest rate swap, net of taxes of $0.6 million | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 882 | | | | — | | | | 882 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Comprehensive Income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 30,128 | |
Expenses relating to the Initial Public Offering | | | — | | | | — | | | | — | | | | — | | | | (87 | ) | | | — | | | | — | | | | — | | | | — | | | | (87 | ) |
Equity compensation | | | — | | | | — | | | | — | | | | — | | | | 4,076 | | | | — | | | | — | | | | — | | | | — | | | | 4,076 | |
Issuance of restricted shares | | | — | | | | — | | | | 67,813 | | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1 | |
Issuance of shares under employee stock purchase plan | | | — | | | | — | | | | 17,138 | | | | — | | | | 359 | | | | — | | | | — | | | | — | | | | — | | | | 359 | |
Exercise of stock options | | | — | | | | — | | | | 1,792,523 | | | | 17 | | | | 16,849 | | | | — | | | | — | | | | — | | | | — | | | | 16,866 | |
Tax benefits on exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | 12,002 | | | | — | | | | — | | | | — | | | | — | | | | 12,002 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 25, 2010 | | | — | | | $ | — | | | | 28,627,897 | | | $ | 286 | | | $ | 243,558 | | | $ | — | | | $ | — | | | $ | — | | | $ | 53,852 | | | $ | 297,696 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| * | Includes conversion of preferred shares and warrants. |
See accompanying notes to consolidated financial statements.
48
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income | | $ | 29,246 | | | $ | 12,666 | | | $ | 8,167 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization of fixed and intangible assets | | | 21,112 | | | | 21,095 | | | | 17,483 | |
Impairment charge on fixed assets | | | 1,326 | | | | — | | | | — | |
Amortization of deferred financing fees | | | 740 | | | | 1,227 | | | | 1,168 | |
Loss on extinguishment of debt, net of premium on Note redemption | | | 1,349 | | | | 1,568 | | | | — | |
Loss on disposal of fixed assets | | | 187 | | | | 130 | | | | 79 | |
Amortization of unrealized loss on terminated swap | | | 1,079 | | | | 565 | | | | — | |
Deferred income taxes | | | 1,166 | | | | (4,995 | ) | | | 695 | |
Deferred rent | | | 1,528 | | | | 3,041 | | | | 3,447 | |
Equity compensation expense | | | 4,076 | | | | 3,040 | | | | 2,352 | |
Tax benefits on exercises of stock options | | | (12,002 | ) | | | (352 | ) | | | | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Inventories | | | (5,214 | ) | | | 800 | | | | (9,082 | ) |
Prepaid expenses and other current assets | | | 12,547 | | | | 800 | | | | 74 | |
Other non-current assets | | | (193 | ) | | | (217 | ) | | | (95 | ) |
Accounts payable | | | (6,204 | ) | | | 1,336 | | | | (10,908 | ) |
Accrued expenses and other current liabilities | | | 3,903 | | | | 4,900 | | | | 5,644 | |
Other long-term liabilities | | | 538 | | | | (2,170 | ) | | | 564 | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 55,184 | | | | 43,434 | | | | 19,588 | |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Capital expenditures | | | (18,356 | ) | | | (21,244 | ) | | | (31,895 | ) |
Trademarks and other intangible assets | | | (92 | ) | | | (37 | ) | | | (3,494 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (18,448 | ) | | | (21,281 | ) | | | (35,389 | ) |
Cash flows from financing activities: | | | | | | | | | | | | |
Borrowings under revolving credit agreement | | | 38,000 | | | | 8,594 | | | | 20,000 | |
Repayment of borrowings under revolving credit agreement | | | (20,000 | ) | | | (25,594 | ) | | | (3,000 | ) |
Payments of capital lease obligation | | | (1,611 | ) | | | (1,334 | ) | | | (468 | ) |
Redemption of long term debt (Notes) | | | (65,000 | ) | | | (44,894 | ) | | | — | |
Redemption of preferred shares | | | — | | | | (72,535 | ) | | | (561 | ) |
Proceeds from issuance of common stock | | | — | | | | 755 | | | | — | |
Proceeds from issuance of common stock during offering, net | | | — | | | | 121,209 | | | | — | |
Payments for expenses related to the offering | | | (87 | ) | | | (1,700 | ) | | | — | |
Proceeds from exercises of common stock options | | | 16,866 | | | | 686 | | | | — | |
Issuance of shares under employee stock purchase plan | | | 359 | | | | — | | | | — | |
Tax benefits on exercises of stock options | | | 12,002 | | | | 352 | | | | — | |
Deferred financing fees | | | (94 | ) | | | (518 | ) | | | — | |
| | | | | | | | | | | | |
Net cash (used in) provided by financing activities | | | (19,565 | ) | | | (14,979 | ) | | | 15,971 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | 17,171 | | | | 7,174 | | | | 170 | |
Cash and cash equivalents beginning of year | | | 8,797 | | | | 1,623 | | | | 1,453 | |
| | | | | | | | | | | | |
Cash and cash equivalents end of year | | $ | 25,968 | | | $ | 8,797 | | | $ | 1,623 | |
| | | | | | | | | | | | |
Supplemental disclosures of cash flow information: | | | | | | | | | | | | |
Interest paid | | $ | 7,628 | | | $ | 17,279 | | | $ | 20,386 | |
Income taxes paid | | $ | 6,732 | | | $ | 11,258 | | | $ | 5,919 | |
Supplemental disclosures of non-cash investing activities: | | | | | | | | | | | | |
Accrued purchases of property and equipment | | $ | 1,648 | | | $ | 1,525 | | | $ | 2,134 | |
Assets acquired under capital lease | | $ | 459 | | | $ | 792 | | | $ | 4,850 | |
See accompanying notes to consolidated financial statements.
49
VITAMIN SHOPPE, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Basis of Presentation
Vitamin Shoppe, Inc. (“VSI”), formerly VS Holdings, Inc., is incorporated in the State of Delaware, and through its wholly-owned subsidiary, Vitamin Shoppe Industries Inc. (“Subsidiary” or “Industries”) and Industries’ wholly-owned subsidiary, VS Direct Inc. (“Direct,” and, together with Industries and VSI, the “Company”), is a leading specialty retailer and direct marketer of nutritional products. Sales of both national brands and proprietary brands of vitamins, minerals, nutritional supplements, herbs, sports nutrition formulas, homeopathic remedies and other health and beauty aids are made through VSI-owned retail stores, the Internet and mail order catalogs to customers located primarily in the United States. VSI operates from its headquarters in North Bergen, New Jersey.
For all periods presented, share and per share information in these consolidated financial statements and the notes hereto have been adjusted to reflect the Company’s approximately 1.8611-for-one stock split effective on October 27, 2009, described in Note 2- Reorganization and Initial Public Offering, below. In addition, as the merger discussed below was between entities under common control, the consolidated financial statements for all years presented reflect the activity and balances of the merged company described in Note 2, as if the merger had occurred prior to December 29, 2007.
The consolidated financial statements for the fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008 include the accounts of VSI, Industries and Direct. All significant intercompany transactions have been eliminated.
The Company’s fiscal year ends on the last Saturday in December. As used herein, the term “Fiscal Year” or “Fiscal” refers to the 52-week period ending the last Saturday in December. Fiscal 2010 is a 52-week period ended December 25, 2010, Fiscal 2009 is a 52-week period ended December 26, 2009, and Fiscal 2008 is a 52-week period ended December 27, 2008.
2. Reorganization and Initial Public Offering
On October 27, 2009, VS Parent, Inc. merged into VS Holdings, Inc., with VS Holdings being renamed, as Vitamin Shoppe, Inc (the “Merger”). All common shares and warrants previously issued by VS Parent, Inc., were converted to common shares and warrants of Vitamin Shoppe, Inc., at approximately a 1.8611-for-one split, resulting in 14,085,349 common shares and 1,055,540 warrants issued and outstanding at October 27, 2009. In addition 78,868 preferred shares were converted to preferred shares of Vitamin Shoppe, Inc. Also in connection with the Merger, a note receivable of $1.5 million from the Company’s former chief executive officer, which was accounted for as a separate component of VS Parent, Inc.’s stockholders’ equity, along with accrued interest of $0.3 million, was extinguished. As consideration for extinguishment of the note and accrued interest, 140,507 common shares (after taking into effect the stock split) and 634 preferred shares of VS Parent, Inc., which were held by the Company’s former chief executive officer to whom the note was extended, were surrendered. The common shares were surrendered at their acquisition cost and the preferred shares were surrendered in satisfaction of the remaining balance on the note.
As a result of the Merger, the following balance sheet items of VS Parent, Inc., at October 27, 2009, were combined into Vitamin Shoppe, Inc: accrued expenses of $16,000, additional paid-in capital of $773,000, and an accumulated deficit of $154,000. In addition, due from/to affiliate balances were combined in and reclassified to intercompany accounts where they were eliminated upon consolidation.
On November 2, 2009, the Company completed an initial public offering (“IPO”), issuing 7,666,667 new common shares in connection with the IPO, at a price of $17 per share, resulting in net proceeds from the offering of approximately $121.2 million, net of underwriters commissions. Other fees associated with the IPO amounted to approximately $1.7 million, which were offset against the proceeds of the IPO. In connection with the IPO, 36,969 preferred shares previously held by VS Parent Inc., along with accumulated dividends in arrears, were converted into 3,764,720 common shares of Vitamin Shoppe, Inc., with the remaining 41,899 preferred shares being redeemed for cash of approximately $72.5 million.
In addition, certain designated proceeds of the IPO were used to redeem $44.9 million in aggregate principal of the Company’s Second Priority Senior Secured Floating Rate Notes due 2012 (the “Notes”) along with a premium on the Notes of approximately $0.4 million, which reduced the outstanding balance of the Notes from $165.0 million to approximately $120.1 million. In connection with the redemption of the Notes, approximately $0.7 million of deferred financing fees and $0.6 million of unrecognized losses related to a terminated interest rate swap along with the aforementioned premium were expensed in the fourth fiscal quarter of 2009.
On November 2, 2009, in connection with the IPO, the Company’s management agreement with IPC Manager II, LLC (the Company’s former controlling shareholder) was terminated. A termination fee of approximately $0.8 million was paid and expensed in the fourth fiscal quarter of 2009. There were no obligations remaining under the management agreement as of November 2, 2009.
50
3. Summary of Significant Accounting Policies
Use of Estimates—The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosures of contingent assets and liabilities at the date of the financial statements, and revenue and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents—All highly liquid investments with original maturities of three months or less are considered to be cash equivalents.
Inventories—Inventories, which are comprised solely of finished goods, are stated at the lower of cost or market value. Cost is determined using the moving weighted average method. Finished goods inventory includes the cost of labor and overhead required to package products. In addition, the cost of inventory is reduced by purchase discounts and allowances received from certain of our vendors. The Company estimates losses for excess and/or expiring inventory and the net realizable value of inventory based on when a product is close to expiration and not expected to be sold, when a product has reached its expiration date, or when a product is not expected to be saleable. In determining the reserves for these products consideration is given to such factors as the amount of inventory on hand, the remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand. The following table details the activity and balances for the Company’s reserve for expiring inventory for the years ended December 25, 2010, December 26, 2009, and December 27, 2008 (in thousands):
| | | | | | | | | | | | | | | | |
| | | Balance
Beginning
of Year | | | Amounts
Charged to
Cost of
Goods Sold | | | Write-Offs
Against
Reserves | | | Balance
at End
of Year | |
Obsolescence Reserves: | | | | | | | | | | | | | | | | |
Year Ended December 25, 2010 | | $ | 1,366.7 | | | $ | 2,045.0 | | | $ | (1,611.0 | ) | | $ | 1,800.7 | |
Year Ended December 26, 2009 | | | 1,389.1 | | | | 1,985.6 | | | | (2,008.0 | ) | | | 1,366.7 | |
Year Ended December 27, 2008 | | | 1,252.8 | | | | 1,929.3 | | | | (1,793.0 | ) | | | 1,389.1 | |
51
Property and Equipment—Property and equipment is stated at cost less accumulated depreciation and amortization. Depreciation and amortization are provided for on a straight-line basis over the estimated useful lives of the related assets. Furniture, fixtures and equipment are depreciated over seven years. Leasehold improvements are amortized over the shorter of their useful lives or related lease terms. The direct internal and external costs associated with the development of the features and functionality of the Company’s website, transaction processing systems, telecommunications infrastructure and network operations, are capitalized and are amortized on a straight line basis over the estimated useful lives of five years. Capitalization of costs begin when the preliminary project stage is completed and management authorizes and commits to funding the computer software project and that it is probable that the project will be completed and the software will be used to perform the function intended. Depreciation of the assets commence when they are put into use. Expenditures for repairs and maintenance are expensed as incurred and expenditures for major renovations and improvements are capitalized. Upon retirement or disposition of property and equipment, the applicable cost and accumulated depreciation are removed from the accounts and any resulting gains or losses are included in the results of operations.
Impairment of Long-Lived Assets—The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets held and used is measured by a comparison of the carrying amount of an asset to undiscounted pre-tax future net cash flows expected to be generated by that asset. If the undiscounted future cash flows are not adequate to recover the carrying value of the asset, an impairment loss is recognized for the amount by which the carrying amount of the assets exceeds the fair value of the assets. During Fiscal 2010, the Company recognized an impairment charge of approximately $1.3 million (which was charged to selling, general and administrative expenses) on fixed assets related to three underperforming retail locations currently still in use. There were no charges related to the impairment of fixed assets for the remaining periods presented.
Goodwill and Other Intangibles—Goodwill is not amortized but is reviewed for impairment at least annually, in the fourth quarter of each year, or whenever impairment indicators exist. Judgments regarding the existence of impairment indicators are based on market conditions and operational performance of the business. Goodwill is tested for impairment at the reporting unit level (the Company’s operating segments). Impairment tests involve calculating the fair value of both reporting units using the discounted cash flow analysis method along with the market multiples method which is used for additional validation of the fair value calculated. This valuation method requires certain assumptions and estimates be made by the Company regarding certain industry trends and future profitability. It is the Company’s policy to conduct goodwill impairment testing from information based on the most current business projections, which include projected future revenues and cash flows. The cash flows utilized in the discounted cash flow analysis are based on five-year financial forecasts developed internally by management. Cash flows for each reporting unit are discounted using an internally derived weighted average cost of capital which reflects the costs of borrowing for the funding of each unit as well as the risk associated with the units themselves. The Company also conducts the test using a 10% decrease in its revenue projections as an additional sensitivity test to ensure the business unit’s fair value is greater than its carrying value should events in the future be less favorable than anticipated. If the carrying amount of a reporting unit exceeds its fair value, the Company would compare the implied fair value of the reporting unit goodwill with its carrying value. To compute the implied fair value, the Company would assign the fair value of the reporting unit to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination. The excess of the fair value of a reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill. To the extent that the implied fair value associated with the goodwill and indefinite-lived intangible assets is less than the recorded value, this would result in a write down of the carrying value of the asset. Impairment tests between annual tests may be undertaken if an event occurs or circumstances change that would reduce the fair value of a reporting unit below its carrying value. The valuation of the goodwill and indefinite-lived intangible assets is affected by, among other things, the Company’s projections for the future and estimated results of future operations. Changes in the business plan or operating results that are different than the estimates used to develop the valuation of the assets may impact these valuations. Intangible assets with indefinite lives are not amortized but are reviewed for impairment annually or more frequently if circumstances indicate a possible impairment may have occurred. For those intangible assets which have definite lives, the Company amortizes their cost on a straight-line basis over their estimated useful lives, the periods of which vary based on their particular contractual terms.
Insurance Liabilities—Based on the Company’s assessment of risk and cost efficiency, the Company purchases insurance policies to provide for workers’ compensation, general liability, and property losses, as well as director’s and officer’s liability. The Company self insures its employee medical benefits, up to a certain limit on individual claims. At December 25, 2010 and December 26, 2009, the accruals for claims incurred but not reported amounted to $1.3 million and $1.2 million, respectively.
Rent Expenses, Deferred Rent and Landlord Construction Allowances—Rent expense and rent incentives, including landlord construction allowances, are recognized on a straight-line basis over the lease term. The Company records rent expense for stores and the distribution center as a component of cost of goods sold. The Company accounts for landlord construction allowances as lease incentives and records them as a component of deferred rent, which is recognized in cost of goods sold over the lease term.
Deferred Financing Fees—The Company capitalizes costs directly associated with acquiring third party financing. Deferred financing fees are included in other assets and are amortized as interest expense over the term of the related indebtedness.
52
Revenue Recognition—The Company recognizes revenue, net of sales returns and deferred sales, when merchandise is sold “over-the-counter” in retail stores or upon delivery to a direct customer. In addition, shipping fees billed to customers are classified as sales. Amount recognized as shipping revenue during the years ended December 25, 2010, December 26, 2009, and December 27, 2008, were $4.4 million, $3.8 million, and $4.3 million, respectively. To arrive at net sales, gross sales are reduced by actual customer returns and a provision for estimated future customer returns, which is based on management’s review of historical and current customer returns. The following table details the activity and balances of the sales return reserves for the years ended December 25, 2010, December 26, 2009 and December 27, 2008 (in thousands):
| | | | | | | | | | | | | | | | |
| | | Balance
Beginning
of Year | | | Amounts
Charged to
Sales | | | Write-Offs/
Recoveries
Against
Reserves | | | Balance
at End
of Year | |
Sales return reserves: | | | | | | | | | | | | | | | | |
Year Ended December 25, 2010 | | $ | 125.0 | | | $ | 13,299.0 | | | $ | (13,289.0 | ) | | $ | 135.0 | |
Year Ended December 26, 2009 | | | 102.8 | | | | 12,602.2 | | | | (12,580.0 | ) | | | 125.0 | |
Year Ended December 27, 2008 | | | 119.9 | | | | 10,739.0 | | | | (10,756.1 | ) | | | 102.8 | |
Cost of Goods Sold—The Company includes the cost of inventory sold, costs of warehousing and distribution and store occupancy costs in cost of goods sold. Warehousing and distribution costs include freight on internally transferred merchandise as well as for shipments to direct customers, rent for the distribution center and costs associated with our buying department and distribution facility, including payroll, which are capitalized into inventory and then expensed as merchandise is sold. Store occupancy costs include rent, common area maintenance, real estate taxes, repairs and maintenance, insurance and utilities.
Frequent Buyer Program—The Company has a frequent buyer program (“Healthy Awards Program”), whereby customers earn points toward free merchandise based on the volume of purchases. Points are earned each calendar year and must be redeemed within the first three months of the following year or they expire. Sales are deferred at the time points are earned based on the value of points that are projected to be redeemed, which are based on historical redemption data. The Company records a liability for points earned within the current period. This is reported as a reduction of sales with a liability recorded as “deferred sales” on the consolidated balance sheet.
Store Pre-opening Costs—Costs associated with the opening of new retail stores and start up activities are expensed as incurred.
Advertising Costs—Costs associated with the production and distribution of the Company’s monthly and quarterly catalogs are expensed as incurred. The costs of advertising for online marketing arrangements, magazines, television and radio are expensed the first time the advertising takes place. Advertising expense was $13.0 million, $12.8 million and $13.2 million for Fiscal 2010, Fiscal 2009 and Fiscal 2008, respectively.
Online Marketing Arrangements—The Company has entered into online marketing arrangements with various online companies. These agreements are established for periods of 24 months, 12 months or, in some cases, a lesser period and generally provide for compensation based on revenue sharing upon the attainment of stipulated revenue amounts, a percentage of the media expenditure managed by the online partner, or based on the number of visitors that the online company refers to the Company. The Company had no fixed payment commitments during Fiscal 2010, Fiscal 2009 and Fiscal 2008.
Research and Development Costs—Research and development costs are expensed as incurred and recorded in selling, general and administrative expenses in the consolidated statements of operations. The Company incurred $2.1 million, $1.5 million, and $1.4 million of research and development expense for the fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008, respectively.
Income Taxes—Deferred income tax assets and liabilities are recorded in accordance with the liability method. Deferred income taxes have been provided for temporary differences between the tax bases and financial reporting bases of the Company’s assets and liabilities using the tax rates and laws in effect for the periods in which the differences are expected to reverse.
The Company accounts for tax positions based on the provisions of the accounting literature related to accounting for uncertainty in income tax positions. Such literature provides guidance for the recognition threshold and measurement attribute for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This requires that companies recognize the largest amount that would more likely than not be unsustainable upon audit by the relevant taxing authority for the impact of an uncertain income tax position on our income tax return. An uncertain income tax position will be recognized if it has less than a 50% likelihood of being sustained. The tax positions are analyzed periodically (at least quarterly) and adjustments are made as events occur that warrant adjustments for those positions. The Company records interest expense and penalties payable to relevant tax authorities as income tax expense.
Comprehensive Income—Comprehensive income represents net income plus the results of certain non-stockholders’ equity changes not reflected in the statement of operations. The amounts recorded in accumulated other comprehensive loss at December 26, 2009,
53
represent the unamortized residual value of the interest rate swap discussed in detail below, which represents the fair value of the swap at its termination during September 2009, net of amortization.
Financial Instruments Policy—The Company has used interest rate swaps as cash flow hedges to manage exposure to fluctuating interest rates on the Company’s debt. In accordance with hedge accounting derivative instruments are reported in the consolidated financial statements at fair value. Changes in the fair value of derivatives are to be recorded each period in earnings in other comprehensive income (loss), depending on whether the derivative is designated as a hedge and if so whether it is effective as a hedge. Gains or losses on derivative instruments reported in other comprehensive income (loss) must be reclassified as earnings in the period in which earnings are affected by the underlying hedged item, and the ineffective portion of all hedges must be recognized in earnings in the current period.
On the date a derivative contract is entered into, a qualifying derivative is required to be designated as (1) a hedge of a recognized asset or liability or an unrecognized firm commitment (a fair value hedge), or (2) a hedge of a forecasted transaction or the variability of cash flows to be received or paid related to the asset or liability (cash flow hedge). At the inception of the hedging relationship, the Company documents its hedge relationships, including identification of the hedging instruments and the hedged items, as well as its risk management objectives and strategies for undertaking the hedge transaction. Derivatives are recorded in the consolidated balance sheet at fair value in other long-term assets or other long-term liabilities. Both at inception of the hedge and quarterly thereafter, the Company has performed an assessment to determine whether the derivatives that were used in hedging transactions were highly effective in offsetting changes in the cash flows of the hedged item.
Prior to the fiscal year ended December 25, 2010, the effective portion of the changes in fair value of the Company’s now terminated interest rate swap, which was designated as a cash flow hedge, was recorded in accumulated other comprehensive income (loss), net of tax. The ineffective portion of the change in fair value was recorded as a component of interest expense. Changes in fair value were estimated by management quarterly, based on dealer quotes.
The aforementioned terminated interest rate swap was entered into during December 2005 to hedge a portion of the Company’s Notes. The interest rate swap had a maturity date of November 2010, and was terminated on September 25, 2009, at a cost of $2.6 million (its then fair market value). The unamortized residual unrecognized loss of the interest rate swap resulting from the termination was recorded in accumulated other comprehensive loss in the amount of $0.9 million along with related deferred taxes of $0.6 million at December 26, 2009. The amounts in both accumulated other comprehensive loss and deferred tax assets relating to the unrecognized loss were fully amortized during Fiscal 2010 and were charged as a component of interest expense. The Company had no contracts related to derivative instruments during Fiscal 2010 and at December 25, 2010.
Concentrations of Credit Risk—The Company’s customers are consumers who purchase products at the Company’s retail stores, and through the Company’s websites and mail-order services. Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of accounts receivable from credit card processors. As of December 25, 2010, there were no significant concentrations of accounts receivable, or related credit risks. Accounts receivable from credit card processors, included in prepaid expenses and other current assets on the consolidated balance sheets, totaled $3.6 million at December 25, 2010 and $4.5 million at December 26, 2009.
Optimum Nutrition is the only supplier from whom the Company purchased at least 5% of its merchandise during Fiscal 2010, and Nature’s Value, Inc. was the only supplier from whom the Company purchased at least 5% of its merchandise during Fiscal 2009 and 2008. The Company purchased approximately 6% of its total merchandise from Optimum Nutrition during Fiscal 2010, and 6% and 7% of its total merchandise from Nature’s Value, Inc. during Fiscal 2009 and 2008, respectively.
Stock-Based Compensation—Stock-based compensation cost is measured at the grant date based on the fair value of awards and is recognized as expense over the vesting period, net of anticipated forfeitures. With the exception of restricted shares, determining the fair value of stock-based awards at the grant date requires considerable judgment, including estimating expected volatility, expected term and risk-free rate. The expected volatility is derived from the average volatility of similar actively traded companies over our expected holding periods. Generally, the expected holding period of non performance based options is calculated using the simplified method using the vesting term of 4 years and the contractual term of 10 years, resulting in a holding period of 6.25 years. Certain limited grants have contractual terms of 7.5 years, and as such have a calculated holding period of 4.81 years. The Company’s performance based grants vest annually over four years depending on a particular year’s attainment of certain internal financial performance metrics. For accounting purposes, performance based grants are measured, and expense is calculated and recorded, subsequent to the determination that the achievement of the pre-established performance targets are probable, over the relevant service period. The target metrics underlying the vesting of performance based options are established each year. The vesting requirements for performance-based options permit a catch-up of vesting should the target not be achieved in a fiscal year but achieved in a subsequent calendar year, over the four year vesting period. Accordingly, the holding period for performance based options is calculated using the vesting term of 1 year and the remainder of the contractual term of 10 years, depending on which year of the four year grant is currently vesting; e.g. 25% of the grant vesting in year two of the grant would have a holding period calculated using 1 year and the
54
remaining 9 years of the contractual term. The resulting holding period for the performance options expensed during Fiscal 2010, was 5.5 years. The simplified method was chosen as a means to determine the Company’s holding periods as prior to November 2009 there was no historical option exercise experience due to the Company being privately held. As of December 25, 2010 there is insufficient information for purposes of determining a Company specific holding period due to the Company being a relatively new publicly owned company. The risk-free interest rate is derived from the average yields of zero-coupon U.S. Treasury Strips for the expected holding period of each of the Company’s stock option grants. Compensation expense resulting from the granting of restricted shares is based on the grant date fair value of those common shares and is recognized generally over a four year vesting period.
Expense related to shares purchased under the Company’s Employee Stock Purchase Plan (“ESPP”) is accounted for based on fair value recognition requirements similar to stock options. ESPP participation occurs quarterly (the “Participation Period”) and the expense of which is subject to employee participation in the plan. Under the ESPP, participating employees are allowed to purchase shares at 85% of the lower of the market price of the Company’s common stock at either the first or last trading day of the Participation Period. Compensation expense related to the ESPP is based on the estimated fair value of the discount and purchase price offered on the estimated shares to be purchased under the ESPP. Expense is calculated quarterly, based on the employee contributions made over three-month Participation Period, using volatility and risk free rates applicable to that three month period.
Compensation expense attributable to stock-based compensation for Fiscal 2010 was approximately $4.1 million, for Fiscal 2009 was approximately $3.0 million and for Fiscal 2008 was approximately $2.4 million. The weighted average grant date fair value for grants was $11.91, $7.70 and $7.92 for Fiscal 2010, Fiscal 2009 and Fiscal 2008, respectively. As of December 25, 2010, the remaining unrecognized stock-based compensation expense for non-vested stock options and restricted shares to be expensed in future periods is $7.7 million, and the related weighted-average period over which it is expected to be recognized is 2.7 years. There were 1,362,218 vested options and 890,835 non-vested outstanding options at December 25, 2010, and 126,446 unvested restricted shares at December 25, 2010. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company estimates forfeitures based on its historical forfeiture rate since the plan inception in Fiscal 2002. The estimated value of future forfeitures for equity grants as of December 25, 2010 is approximately $0.4 million.
The Company previously accounted for stock options under Accounting Principles Bulletin (“APB”) No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”), using the intrinsic value method. The FASB permits companies to adopt its requirements using various methods. The Company adopted the prospective method for all stock option grants issued prior to December 31, 2005. Subsequent to December 31, 2005, under the prospective method, those nonpublic companies that used the minimum value method of measuring equity share options and similar instruments for either recognition or pro forma disclosure purposes applied the new fair value measurement requirements prospectively to new awards and to awards modified, repurchased, or cancelled after the required effective date. The Company continues to account for any portion of awards outstanding at the date of initial application using the accounting principles originally applied to those awards as allowed by the prospective method. As such, no stock-based compensation costs were reflected in net income for those stock option grants issued prior to the adoption of the provisions of fair value accounting for equity shares, as the Company was not required to do so under the previous guidance nor under the new guidance.
The following table represents assumptions used to estimate the fair value of options:
| | | | | | | | | | | | |
| | | Fiscal Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Expected dividend yield | | | 0.0% | | | | 0.0% | | | | 0.0% | |
Volatility factor | | | 48.4% | | | | 49.1% | | | | 47.9% | |
Weighted average risk-free interest rate | | | 2.9% | | | | 2.8% | | | | 3.2% | |
Expected life of option | | | 6.19 years | | | | 5.37 years | | | | 6.25 years | |
Net Income Per Share—The Company’s basic net income per share excludes the dilutive effect of stock options, warrants and unvested restricted shares. It is based upon the weighted average number of common shares outstanding during the period divided into net income (loss) after deducting accumulated dividends on the Company’s Series A Preferred Stock, up until such time the preferred shares were either liquidated or converted to common shares.
Diluted net income per share reflects the potential dilution that would occur if securities or other contracts to issue common stock were exercised or converted into common stock. Stock options, warrants and unvested restricted shares are included as potential dilutive securities for the periods applicable.
55
For the purposes of basic and diluted net income per share, as a result of the merger on October 27, 2009, weighted average shares outstanding for purposes of presenting net income per share on a comparative basis were retroactively restated for all periods presented based on a approximately a 1.8611-for-one split at the time of the merger.
The computation of basic net income per share is based on the weighted average number of common shares outstanding during the period. The computation of diluted net income per share assumes the foregoing and the exercise of stock options and warrants, as well as vesting of restricted shares, using the treasury stock method to the extent dilutive.
The components of the calculation of basic net income per common share and diluted net income per common share are as follows (in thousands except share and per share data):
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Numerator: | | | | | | | | | | | | |
Net income (loss) available to common stockholders | | $ | 29,246 | | | $ | 4,974 | | | $ | (1,112 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Basic weighted average common shares outstanding | | | 27,390,419 | | | | 16,238,338 | | | | 14,175,906 | |
Diluted weighted average common shares outstanding | | | 28,338,788 | | | | 17,748,371 | | | | 14,175,906 | |
| | | | | | | | | | | | |
Basic net income (loss) per common share | | $ | 1.07 | | | $ | 0.31 | | | $ | (0.08 | ) |
| | | | | | | | | | | | |
Diluted net income (loss) per common share | | $ | 1.03 | | | $ | 0.28 | | | $ | (0.08 | ) |
| | | | | | | | | | | | |
Stock options for the fiscal years ended December 25, 2010 and December 26, 2009 for 275,134 and 522,363 shares, respectively, have been excluded from the above calculation as they were anti-dilutive. Stock options and warrants for the fiscal year ended December 27, 2008 have been excluded from the above calculation as they were anti-dilutive.
Recent Accounting Pronouncements—The Company has considered all new accounting pronouncements and has concluded that there are no new pronouncements that have a material impact on results of operations, financial condition, or cash flows, based on current information.
4. Goodwill and Intangible Assets
The Company acquired $88.0 million of intangible assets and recorded $177.2 million of goodwill in connection with an acquisition completed in Fiscal 2002. Other intangible asset relate to asset purchases which occurred in Fiscal 2008.
The following table discloses the carrying value of all intangible assets (in thousands):
56
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 25, 2010 | | | December 26, 2009 | |
| | | Gross
Carrying
Amount | | | Accumulated
Amortization | | | Net | | | Gross
Carrying
Amount | | | Accumulated
Amortization | | | Net | |
Intangible assets: | | | | | | | | | | | | | | | | | | | | | | | | |
Intangibles related to asset purchases | | $ | 3,000 | | | $ | 2,127 | | | $ | 873 | | | $ | 3,000 | | | $ | 1,398 | | | $ | 1,602 | |
Tradenames | | | 68,845 | | | | — | | | | 68,845 | | | | 68,754 | | | | — | | | | 68,754 | |
Goodwill | | | 177,248 | | | | — | | | | 177,248 | | | | 177,248 | | | | — | | | | 177,248 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 249,093 | | | $ | 2,127 | | | $ | 246,966 | | | $ | 249,002 | | | $ | 1,398 | | | $ | 247,604 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Intangible amortization expense for Fiscal 2010, Fiscal 2009 and Fiscal 2008 was $0.7 million, $0.8 million and $0.6 million, respectively. Tradenames are not amortized, as they are determined to be intangible assets with indefinite lives. The annual impairment tests for Goodwill and Tradenames were performed during the fourth quarter of Fiscal 2010 and neither asset was found to be impaired. In addition, there has been no impairment charges related to Goodwill or Tradenames in previous periods.
The useful lives of the Company’s definite-lived intangible assets is between 1 to 7 years. The expected future amortization expense on definite-lived intangible assets on the Company’s consolidated balance sheet at December 25, 2010, is as follows (in thousands):
| | | | |
Fiscal 2011 | | $ | 542 | |
Fiscal 2012 | | | 125 | |
Fiscal 2013 | | | 125 | |
Fiscal 2014 | | | 81 | |
| | | | |
| | $ | 873 | |
| | | | |
5. Property and Equipment
Property and equipment consists of the following (in thousands):
| | | | | | | | |
| | | As of | |
| | | December 25,
2010 | | | December 26,
2009 | |
Furniture, fixtures and equipment | | $ | 108,155 | | | $ | 99,215 | |
Leasehold improvements | | | 103,875 | | | | 95,897 | |
Website development costs | | | 11,014 | | | | 11,014 | |
| | | | | | | | |
| | | 223,044 | | | | 206,126 | |
Less: accumulated depreciation and amortization | | | (143,794 | ) | | | (123,123 | ) |
| | | | | | | | |
Subtotal | | | 79,250 | | | | 83,003 | |
Construction in progress | | | 1,699 | | | | 957 | |
| | | | | | | | |
| | $ | 80,949 | | | $ | 83,960 | |
| | | | | | | | |
Depreciation and amortization expense on property and equipment for the fiscal years ended December 25, 2010, December 26, 2009, and December 27, 2008 was approximately $20.4 million, $20.3 million and $16.9 million, respectively. Depreciation and amortization expense is included in selling, general and administrative expense in the Company’s consolidated statements of operations. Assets held under capital leases, which are classified under furniture, fixtures and equipment, were $3.4 million, net of accumulated amortization of $4.1 million, at December 25, 2010, and $4.2 million, net of accumulated amortization of $2.9 million, at December 26, 2009.
57
6. Credit Arrangements
Debt consists of the following (in thousands):
| | | | | | | | |
| | | As of | |
| | | December 25,
2010 | | | December 26
2009 | |
Revolving Credit Facility | | $ | 18,000 | | | $ | — | |
| | | | | | | | |
Second Priority Senior Secured Floating Rate Notes (the “Notes”) | | $ | 55,106 | | | $ | 120,106 | |
| | | | | | | | |
2005 Second Priority Senior Secured Floating Rate Notes
During Fiscal 2009 through Fiscal 2010, the Company repurchased approximately $109.9 million of its Notes. Interest on the Notes is set at a per annum rate equal to a three month LIBOR plus 7.5%, which is reset quarterly on February 15, May 15, August 15 and November 15 of each year. The combined weighted average interest rate before the impact of our hedging activities for Fiscal 2010 was 7.81%, and for Fiscal 2009 was 8.45% . The Notes will mature on November 15, 2012. Interest on overdue principal and interest and liquidated damages, if any, will accrue at a rate that is 1% higher than the applicable interest rate on the Notes. If Industries cannot make payments on the Notes when they are due, VSI and Industries’ only subsidiary, Direct (collectively, the “Guarantors”), have guaranteed the Notes and must make payments instead. The Notes and the guarantees are secured by a second priority security interest in substantially all of Industries’ and the Guarantors’ assets that secure Industries’ new first priority senior secured credit facility. The Notes and the guarantees are Industries’, and the Guarantors’ second priority senior secured obligations, and rank equally in right of payment with all of Industries’ and the Guarantors’ existing and future senior indebtedness and senior to all of Industries’ and the Guarantors’ existing and future subordinated indebtedness. The Notes and the guarantees are effectively subordinated to all of Industries’ and the Guarantors’ first priority senior secured indebtedness, including Industries’ new first priority senior secured credit facility, to the extent of the collateral securing such indebtedness. If Industries sells certain assets, issues equity or experiences specific kinds of changes in control, Industries must offer to repurchase the Notes. Since November 15, 2007, Industries has had the option to redeem some or all of the Notes. Industries used the proceeds from the sale of the Notes to repay all of its and VSI’s existing indebtedness and to pay related fees and expenses.
Revolving Credit Facility
On November 15, 2005, the Company entered into a $50.0 million senior secured revolving credit facility, which was terminated on September 25, 2009.
2009 Revolving Credit Facility
On September 25, 2009, the Company entered into a new revolving credit facility (the “2009 Revolving Credit Facility”), and simultaneously terminated its existing credit facility. The terms of the 2009 Revolving Credit Facility extend through September, 2013, and allow the Company to borrow up to $70.0 million (as amended) subject to the terms of the facility. Similar to the Company’s previous credit facility, the availability under the 2009 Revolving Credit Facility is subject to a borrowing base calculated on the value of certain accounts receivable from credit card companies as well as the inventory of Vitamin Shoppe Industries Inc. and VS Direct Inc. The obligations thereunder are secured by a security interest in substantially all of the assets of Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and VS Direct Inc. VS Direct Inc. and Vitamin Shoppe, Inc. provided guarantees in respect of the Company’s obligations under the 2009 Revolving Credit Facility, and Vitamin Shoppe Industries Inc. and Vitamin Shoppe, Inc. have provided guarantees in respect of VS Direct Inc.’s obligations under the 2009 Revolving Credit Facility. The 2009 Revolving Credit Facility provides for affirmative and negative covenants affecting Vitamin Shoppe Industries Inc., Vitamin Shoppe, Inc. and VS Direct Inc. The 2009 Revolving Credit Facility restricts, among other things, the Company’s ability to incur indebtedness, create or permit liens on the Company’s assets, declare or pay dividends and make certain other restricted payments, consolidate, merge or recapitalize, acquire or sell assets, make certain investments, loans or other advances, enter into transactions with affiliates, change the line of business, and restricts the types of hedging activities can be entered into. The 2009 Revolving Credit Facility has a maturity date of September 2013. The largest amount borrowed at any given point during Fiscal 2010 was $38.0 million. The unused available line of credit under the 2009 Revolving Credit Facility at December 25, 2010 was $41.7 million. As of February 22, 2011, the available balance was decreased by $12.0 million due to the funding of the redemption of a portion of the Notes.
58
The borrowings under our 2009 Revolving Credit Facility accrue interest, at the Company’s option, at the rate per annum announced from time to time by the agent as its “prime rate,” or at a per annum rate equal to 2.50% above the adjusted Eurodollar rate. The weighted average interest rate for the 2009 Revolving Credit Facility for Fiscal 2010 was 2.89%.
The borrowings under the terminated revolving credit facility accrued interest through September 25, 2009, at a per annum rate equal to between 1.25% and 1.75% (depending on excess availability) above the adjusted Eurodollar rate. The combined weighted average interest rate for both the 2009 and terminated revolving credit lines for 2009 was 2.51%.
Scheduled maturities of borrowings as of December 25, 2010, are as follows (in thousands):
| | | | | | | | |
Year | | The Notes | | | Revolving
Credit Facility | |
2011 | | $ | — | | | $ | — | |
2012 | | | 55,106 | | | | — | |
2013 | | | — | | | | 18,000 | |
| | | | | | | | |
| | $ | 55,106 | | | $ | 18,000 | |
| | | | | | | | |
See Note 15, Subsequent Events, for information regarding an amendment to the above Revolving Credit Facility as well as the redemption of the Notes subsequent to December 25, 2010.
59
Net interest expense for Fiscal 2010, 2009 and 2008 consists of the following (in thousands):
| | | | | | | | | | | | |
| | | Fiscal Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Interest on the Notes | | $ | 7,717 | | | $ | 16,681 | | | $ | 19,404 | |
Amortization of deferred financing fees | | | 740 | | | | 1,227 | | | | 1,168 | |
Interest on revolving credit facilities and other | | | 1,110 | | | | 771 | | | | 681 | |
Interest income | | | (50 | ) | | | (43 | ) | | | (116 | ) |
| | | | | | | | | | | | |
| | $ | 9,517 | | | $ | 18,636 | | | $ | 21,137 | |
| | | | | | | | | | | | |
Capital Leases
The Company leases certain computer equipment under capital leases, which expire in Fiscal 2011 and Fiscal 2012. The following is a schedule of the future minimum lease payments under capital leases as of December 25, 2010 (in thousands):
| | | | |
Fiscal 2011 | | $ | 1,846 | |
Fiscal 2012 | | | 985 | |
| | | | |
Total | | | 2,831 | |
Less amount representing interest | | | 143 | |
| | | | |
Present value of minimum lease payments | | | 2,688 | |
Less current portion of capital lease obligation | | | 1,711 | |
| | | | |
| | $ | 977 | |
| | | | |
7. Income Taxes
The provision for income taxes for Fiscal 2010, Fiscal 2009 and Fiscal 2008 consists of the following (in thousands):
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Current: | | | | | | | | | | | | |
Federal | | $ | 14,674 | | | $ | 10,469 | | | $ | 3,297 | |
State | | | 3,710 | | | | 2,540 | | | | 2,349 | |
| | | | | | | | | | | | |
Total current | | | 18,384 | | | | 13,009 | | | | 5,646 | |
| | | | | | | | | | | | |
Deferred: | | | | | | | | | | | | |
Federal | | | 1,345 | | | | (4,051 | ) | | | 1,003 | |
State | | | (179 | ) | | | (944 | ) | | | (308 | ) |
| | | | | | | | | | | | |
Total deferred | | | 1,166 | | | | (4,995 | ) | | | 695 | |
| | | | | | | | | | | | |
Provision for income taxes | | $ | 19,550 | | | $ | 8,014 | | | $ | 6,341 | |
| | | | | | | | | | | | |
60
A reconciliation of the statutory Federal income tax rate and effective rate of the provision for income taxes is as follows:
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Federal statutory rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
State income taxes, net of Federal income tax benefit | | | 4.6 | % | | | 4.1 | % | | | 2.6 | % |
Adjustments for uncertain tax positions | | | 0.2 | % | | | (1.6 | )% | | | 3.1 | % |
Other | | | 0.3 | % | | | 1.3 | % | | | 2.9 | % |
| | | | | | | | | | | | |
Effective tax rate | | | 40.1 | % | | | 38.8 | % | | | 43.6 | % |
| | | | | | | | | | | | |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying value of assets and liabilities for financial reporting purposes and amounts used for income tax purposes. The temporary differences and carryforwards that give rise to deferred tax assets and liabilities at December 25, 2010 and December 26, 2009 are as follows (in thousands):
| | | | | | | | |
| | | As of | |
| | | December 25,
2010 | | | December 26,
2009 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforward | | $ | 883 | | | $ | 883 | |
Deferred rent | | | 9,521 | | | | 8,715 | |
Tenant allowance | | | 1,091 | | | | 996 | |
Deferred sales | | | 3,352 | | | | 3,115 | |
Organizational costs | | | 31 | | | | 31 | |
Inventory | | | 3,071 | | | | 2,511 | |
Other comprehensive income | | | — | | | | 555 | |
Equity compensation expense | | | 2,758 | | | | 3,279 | |
Other | | | 2,632 | | | | 1,877 | |
| | | | | | | | |
| | | 23,339 | | | | 21,962 | |
Valuation allowance | | | (883 | ) | | | (883 | ) |
| | | | | | | | |
Deferred tax assets | | | 22,456 | | | | 21,079 | |
| | | | | | | | |
Deferred tax liabilities: | | | | | | | | |
Trade name | | | (27,678 | ) | | | (27,696 | ) |
Accumulated depreciation | | | (10,178 | ) | | | (6,981 | ) |
Prepaid expenses | | | (1,162 | ) | | | (1,202 | ) |
| | | | | | | | |
Deferred tax liabilities | | | (39,018 | ) | | | (35,879 | ) |
| | | | | | | | |
Net deferred tax liability | | $ | (16,562 | ) | | $ | (14,800 | ) |
| | | | | | | | |
Amounts recognized in the consolidated balance sheets consist of: | | | | | | | | |
Deferred tax assets—current | | $ | 4,033 | | | $ | 5,145 | |
Deferred tax liabilities—long term | | | (20,595 | ) | | | (19,945 | ) |
| | | | | | | | |
Net deferred tax liability | | $ | (16,562 | ) | | $ | (14,800 | ) |
| | | | | | | | |
Management periodically assesses whether the Company is more likely than not to realize some or all of its deferred tax assets. As of December 25, 2010, with the exception of $883,000 of deferred tax assets arising from a state net operating loss carryforward for which there is a valuation allowance against (see above table), management determined that the Company is more likely than not to realize the deferred tax assets detailed above.
As of December 25, 2010, with the exception of the above net operating loss related to the valuation allowance, the Company has no net operating loss carryforwards for which there are expectations for utilization in future periods. Realization of deferred tax assets associated with the state net operating loss carryforwards is dependent upon generating sufficient taxable income prior to their expiration by tax jurisdiction. The Company believes that it is more likely than not that their remaining state net operating loss (held at
61
the holding company level) may expire unused and, accordingly, has established the aforementioned valuation allowance against it. There was no change in the valuation allowance during Fiscal 2010.
The Company and its subsidiaries file income tax returns in the U.S. federal jurisdiction, and various state jurisdictions. As of December 25, 2010, the Company has accrued a liability of approximately $4.3 million related to uncertain tax positions, which is included in other long-term liabilities in the consolidated balance sheet. The Company expects a net decrease of approximately $1.0 million related to its accrual for uncertain tax positions to occur in the next twelve months.
The total amount of unrecognized tax benefits that, if recognized, would impact the effective tax rate was approximately $3.7 million at December 27, 2008, $3.1 million at December 26, 2009, and the amount at December 25, 2010 was $3.2 million. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| | | | |
Balance at December 29, 2007 | | $ | 3,496 | |
Additions based on tax positions related to the current year | | | 380 | |
Additions for tax positions of prior years | | | 231 | |
| | | | |
Balance at December 27, 2008 | | | 4,107 | |
Additions based on tax positions related to the current year | | | 92 | |
Decreases for tax positions of prior years | | | (190 | ) |
Additions for tax positions of prior years | | | 226 | |
| | | | |
Balance at December 26, 2009 | | | 4,235 | |
Additions based on tax positions related to the current year | | | 113 | |
Decreases for tax positions of prior years | | | (158 | ) |
Additions for tax positions of prior years | | | 72 | |
| | | | |
Balance at December 25, 2010 | | $ | 4,262 | |
| | | | |
The Company recognizes interest related to uncertain tax positions in income tax expense. At December 25, 2010, the Company has recorded approximately $0.1 million of accrued interest. Interest recognized through the consolidated statements of operations for Fiscal 2009 and Fiscal 2008 was approximately $0.2 million and $0.1 million, respectively. The Company is no longer subject to U.S. federal examinations by tax authorities for years before 2005 and for state examinations before 2005. However, the tax authorities still have the ability to review the relevance of net operating loss carryforwards created in closed years if such tax attributes are utilized in open years (subsequent to 2005).
8. Stock Based Compensation
During fiscal 2002, the Company adopted a stock option plan (the “2002 Plan”) for certain directors, officers, consultants and employees of the Company. The 2002 Plan authorized the issuance of up to 2,046,041 shares of common stock. In June 2006, the 2002 Plan was amended and assigned to VS Parent, Inc. (the Company’s former parent) where it was adopted as the VS Parent, Inc. 2006 Stock Option Plan (the “2006 Plan”), converting all grants on a one-to-one basis for the right to receive a common share of VS parent upon exercise. In connection with the Merger, the 2006 Plan was assigned to Vitamin Shoppe, Inc. where it was converted at approximately a 1.8611-for-one share split resulting in up to 3,807,862 common shares authorized for issuance. In addition to the 2006 Plan, prior to the Merger, VS Parent, Inc. adopted the Vitamin Shoppe 2009 Equity Incentive Plan (the “2009 Plan”) on September 2, 2009, which authorized the issuance of up to 1,395,816 (after taking into consideration the approximately 1.8611-for-one share split) common shares for issuance of both stock options (as well as performance based stock options, of which 59,375 were granted during Fiscal 2010) and restricted stock shares for certain employees of the Company. At December 25, 2010, there were 916,305 shares available for issuance under both plans.
Stock Options—Stock options are generally exercisable at no less than the fair market value on the date of grant. Generally, options awarded shall become vested in four equal increments (with the exception of performance based grants which require both time and the attainment of a performance target) on each of the first, second, third and fourth anniversaries of the date on which such options were awarded. The stock options generally have a maximum term of 10 years. The following table summarizes the activity for both the 2006 Plan and 2009 Plan for Fiscal 2010, and information about options outstanding at December 25, 2010:
62
| | | | | | | | | | | | | | | | |
| | | Number of
Options | | | Weighted
Average
Exercise Price | | | Weighted
Average
Remaining
Contractual
Life (years) | | | Aggregate
Intrinsic
Value
(in thousands) | |
Outstanding at December 26, 2009 | | | 3,868,980 | | | $ | 11.93 | | | | | | | | | |
Granted | | | 212,612 | | | $ | 23.78 | | | | | | | | | |
Exercised | | | (1,792,523 | ) | | $ | 9.41 | | | | | | | | | |
Canceled/forfeited | | | (36,016 | ) | | $ | 17.68 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 25, 2010 | | | 2,253,053 | | | $ | 14.96 | | | | 6.08 | | | $ | 42,307 | |
| | | | | | | | | | | | | | | | |
Vested or expected to vest at December 25, 2010 | | | 2,140,400 | | | $ | 14.96 | | | | 6.08 | | | | | |
| | | | | | | | | | | | | | | | |
Vested and exercisable at December 25, 2010 | | | 1,362,218 | | | $ | 13.11 | | | | 5.32 | | | $ | 28,097 | |
| | | | | | | | | | | | | | | | |
The total intrinsic value of options exercised during Fiscal 2010 and Fiscal 2009 was $28.9 million and $1.0 million, respectively. The cash received from options exercised during Fiscal 2010 and Fiscal 2009 was $16.9 million and $0.7 million, respectively. There were no exercises during Fiscal 2008.
Restricted Shares—Restricted shares are issued at a value no less than the fair market value of the common shares on the date of the grant, and vest over a four year period after date on which such shares were issued. The following table summarizes restricted shares for the 2009 Plan as of December 25, 2010 and changes during the fiscal year then ended:
| | | | | | | | |
| | | Number of
Unvested
Restricted
Shares | | | Weighted
Average
Grant Date
Fair Value | |
Unvested at December 26, 2009 | | | 84,897 | | | $ | 15.11 | |
Granted | | | 67,813 | | | $ | 23.24 | |
Vested | | | (24,094 | ) | | $ | 15.59 | |
Canceled/forfeited | | | (2,170 | ) | | $ | 23.00 | |
| | | | | | | | |
Unvested at December 25, 2010 | | | 126,446 | | | $ | 19.24 | |
| | | | | | | | |
Employee Stock Purchase Plan—The Vitamin Shoppe 2010 Employee Stock Purchase Plan (the “ESPP”) was approved by the Company’s shareholders during June 2010. Pursuant to the plan, shares of common stock were issued beginning on June 30, 2010, and will continue to be issued quarterly (the “Participation Period”) thereafter subject to employee participation in the plan. Under the ESPP, participating employees are allowed to purchase shares at 85% of the lower of the market price of the Company’s common stock at either the first or last trading day of the Participation Period. Compensation expense related to the ESPP is based on the estimated fair value of the discount and purchase price offered on the estimated shares to be purchased under the ESPP. There were 17,138 shares purchased under the ESPP during Fiscal 2010 resulting in employee payroll deductions for purposes of ESPP purchases of approximately $0.4 million. As of December 25, 2010, there was approximately $0.2 million of employee payroll deductions available under the ESPP for purchasing common shares on the December 31, 2010 purchase date.
63
9. Lease Commitments
The Company has non-cancelable operating leases, which expire through 2029. The leases generally contain renewal options for periods ranging from 1 to 10 years and require the Company to pay costs such as real estate taxes and common area maintenance. Contingent rentals are paid based on a percentage of gross sales as defined by lease agreements. The following table provides the net rental expense for all operating leases (in thousands):
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Minimum rentals | | $ | 72,802 | | | $ | 65,249 | | | $ | 55,602 | |
Contingent rentals | | | 180 | | | | 135 | | | | 101 | |
| | | | | | | | | | | | |
| | | 72,982 | | | | 65,384 | | | | 55,703 | |
Less: Sublease rentals | | | (224 | ) | | | (170 | ) | | | (122 | ) |
| | | | | | | | | | | | |
Net rental expense | | $ | 72,758 | | | $ | 65,214 | | | $ | 55,581 | |
| | | | | | | | | | | | |
As of December 25, 2010, the Company’s lease commitments are as follows (in thousands):
| | | | |
Fiscal year | | Total
Operating
Leases (1) | |
2011 | | $ | 77,577 | |
2012 | | | 76,098 | |
2013 | | | 70,251 | |
2014 | | | 60,326 | |
2015 | | | 48,436 | |
Thereafter | | | 117,653 | |
| | | | |
| | $ | 450,341 | |
| | | | |
| (1) | The operating leases included in the above table do not include contingent rent based upon sales volume, which represented less than 1% of our minimum lease obligations during Fiscal 2010. In addition, the operating leases do not include common area maintenance costs or real estate taxes that are paid to the landlord during the year, which combined represented approximately 16.1% of our minimum lease obligations during Fiscal 2010. |
10. Legal Proceedings
Dwight Thompson v. The Vitamin Shoppe and Consolidated Actions. On February 25, 2005, a former store manager in California, Dwight Thompson, filed suit against the Vitamin Shoppe in the Superior Court of the State of California for the County of Orange (Case No. 05CC00048) on behalf of himself and other “similarly situated” current and former California store managers and assistant store managers, who were employed by the Company on or after April 14, 2006 alleging causes of action for alleged wage and hour violations. Almost one year later, on July 7, 2006, the same group of plaintiffs’ attorneys who were representing Thompson filed another wage and hour lawsuit against The Vitamin Shoppe based on substantively identical allegations in the Orange County Superior Court, entitled Estel v. The Vitamin Shoppe Industries Inc. (Case No. 06CC07852) (the “Estel Action”). In January 2008, the Court consolidated the Thompson and Estel actions. On October 6, 2010, the Company reached agreement with Plaintiffs to settle and resolve all individual and class claims for amounts, in the aggregate, not material to the Company. The Court gave preliminarily
64
approval on December 20, 2010, and final approval of the class settlement is set for March 11, 2011. All settlement amounts were fully accrued in Fiscal 2010 and paid to the disbursing agent during February 2011.
California District Attorney’s Letter. On May 17, 2007, the Company received a letter from the Napa County (California) District Attorney alleging that six of the Company’s private label products contain levels of lead that, pursuant to California’s Proposition 65, Cal. Health & Safety Code section 25249.5 et seq., (“Proposition 65”) require the products to bear a warning when sold in California. The letter claims that 12 other public prosecutors in California, including the California Attorney General, “are involved in a joint investigation of dietary supplements containing lead in amounts that expose users to lead in excess of 0.50 micrograms (ug) per day.” The letter demands that the Company immediately cease all sales of these products in California unless it provides a warning to consumers. It also notes that Proposition 65 provides for civil penalties of up to $2,500 per violation per day. The Company has met with the California Attorney General and certain District Attorneys, and is investigating these allegations and consulting with its third party suppliers of these products. The Company has withdrawn certain named products from the California market and has provided warnings with respect to other products still available in California pending discussions with the public prosecutors. The Napa County District Attorney has expressed concerns on several occasions as to the method of warning employed by the Company and the completeness of its implementation. The Company has revised its warnings and reviewed its procedures for implementing warnings. The Company has responded to numerous requests for information and has met in person with representatives of the Napa County District Attorney and the California Attorney General to attempt to resolve this matter. As of December 25, 2010, the Company does not believe the financial statement impact of this matter will be material.
The People of the State of California v. 21st Century Healthcare, Inc. On October 22, 2008, a private enforcer named Vicky Hamilton sent over 70 manufacturers and retailers of multivitamin products, including the Company, various Sixty-Day Notices of Violation of Proposition 65, Cal. Health & Safety Code section 25249.5 et seq. alleging that certain products contain lead and lead compounds and were sold in California without a Proposition 65 warning threatening litigation pertaining to two of the Company’s multivitamin products. On December 23, 2008, the California Attorney General and nine California District Attorneys filed a complaint on behalf of the People of the State of California against a number of companies who received notices of violation from Ms. Hamilton, including the Company in Alameda County Superior Court. The action alleges violations of both Proposition 65 and the UCL and supplants the litigation Ms. Hamilton sought to bring against the Company on the claims stated in her Notice of Violation. Penalties under Proposition 65 may be assessed at the maximum rate of $2,500 per violation per day. Penalties under the UCL may be assessed at the same rate and are cumulative to those available under Proposition 65. Injunctive relief and attorneys fees are also available. The Company is investigating the claims in the action and has been discussing them with the California Attorney General and District Attorneys. At this time it is premature to determine the extent of any potential loss. Accordingly, as of December 25, 2010, the Company has not accrued any liabilities related to this litigation.
J.C. Romero v. ErgoPharm Inc., Proviant Technologies Inc., VS Holdings Inc, d/b/a Vitamin Shoppe, and General Nutrition Centers Inc. On April 27, 2009, plaintiff, a professional baseball player, filed a complaint against us, among others, in Superior Court of New Jersey (Law Division/Camden County). Plaintiff alleges that he purchased from one of our stores and consumed 6-OXO Extreme, which was manufactured by a third party, and in August 2008, allegedly tested positive for a banned substance. Plaintiff served a 50 game suspension imposed by Major League Baseball. The seven count complaint asserts, among other things, claims for negligence, strict liability, misrepresentation, breach of implied warranty and violations of the New Jersey Consumer Fraud Act, and seeks unspecified monetary damages, including lost income during the suspension. The Company denies any and all liability and intends to vigorously defend these claims. Any liabilities that may arise from this matter are not probable or reasonably estimable at this time. Accordingly, as of December 25, 2010, the Company has not accrued any liabilities related to this litigation.
The Company is party to various lawsuits arising from time to time in the normal course of business, many of which are covered by insurance. Except as described above, as of December 25, 2010, the Company was not party to any material legal proceedings. Although the impact of the final resolution of these matters on the Company’s financial condition, results of operations or cash flows is not known, management does not believe that the resolution of these lawsuits will have a material adverse effect on the financial condition, results of operations or liquidity of the Company.
11. Related Party Transactions
The Company had a management agreement with IPC Manager II, LLC (formerly Bear Stearns Merchant Manager II, LLC), which terminated on November 2, 2009, as a result of the IPO. This agreement provided for a quarterly fee of the greater of $187,500 or 0.25% of gross sales for the preceding fiscal quarter for advisory and consulting services. In addition, per the agreement a one-time termination fee of approximately $0.8 million was charged to expense during the fourth Fiscal quarter of 2009 in connection with the IPO. Amounts paid for the fiscal years ended December 26, 2009 and December 27, 2008 were approximately $2.4 million and $1.5 million, respectively. There were no amounts paid during Fiscal 2010.
65
The Company loaned $1.5 million to the Company’s former Chief Executive Officer as part of a purchase of the Company’s common stock, of which the Company had recourse on $375,000. The note incurred interest at 3.06% annually. In connection with the Merger on October 27, 2009, this $1.5 million note receivable along with a related accrued interest receivable of approximately $0.3 million, which was held by VS Parent, Inc. prior to the Merger, was extinguished in consideration of the surrender of 140,507 common shares and 634 preferred shares of VS Parent.
12. Segment Data
The Company currently operates two business segments, retail and direct. The operating segments are segments of the Company for which separate financial information is available and for which operating results are evaluated regularly by executive management in deciding how to allocate resources and in assessing performance. The Company’s management evaluates segment operating results based on several indicators. The primary key performance indicators are sales and operating income for each segment. The table below represents key financial information for each of the Company’s business segments, retail and direct, as well as corporate costs. The retail segment includes the Company’s retail stores. The retail segment generates revenue primarily through the sale of third party branded and proprietary branded vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products through retail stores throughout the United States. The direct segment generates revenue through the sale of third party branded and proprietary branded vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products through the Company’s Websites and its catalog. A catalog is mailed each month to customers in the Company’s Healthy Awards Program database, and the Company’s website atwww.vitaminshoppe.com offers its customers online access to a full assortment of over 20,000 SKUs. Corporate costs represent the Company’s administrative expenses which include, but are not limited to human resources, legal, finance, information technology, depreciation and amortization, and various other corporate level activity related expenses. There are no inter-segment sales transactions.
The Company’s segments are designed to allocate resources internally and provide a framework to determine management responsibility. The Company has allocated $131.9 million and $45.3 million of its recorded goodwill to the retail and direct segments, respectively. The Company does not have identifiable assets separated by segment.
The following table contains key financial information of the Company’s business segments (in thousands):
| | | | | | | | | | | | |
| | | Year Ended | |
| | | December 25,
2010 | | | December 26,
2009 | | | December 27,
2008 | |
Net Sales: | | | | | | | | | | | | |
Retail | | $ | 668,008 | | | $ | 596,253 | | | $ | 522,541 | |
Direct | | | 83,474 | | | | 78,242 | | | | 78,999 | |
| | | | | | | | | | | | |
Total net sales | | | 751,482 | | | | 674,495 | | | | 601,540 | |
| | | |
Income from operations: | | | | | | | | | | | | |
Retail | | | 118,319 | | | | 94,494 | | | | 80,422 | |
Direct | | | 14,863 | | | | 15,126 | | | | 14,884 | |
Corporate costs | | | (73,520 | ) | | | (68,288 | ) | | | (59,661 | ) |
| | | | | | | | | | | | |
Income from operations | | $ | 59,662 | | | $ | 41,332 | | | $ | 35,645 | |
| | | | | | | | | | | | |
66
13. Fair Value of Financial Instruments
The fair value of the Company’s Notes have been determined by the Company using quoted market prices. The following table sets forth the carrying amounts and fair values of the Company’s Notes at December 25, 2010 and December 26, 2009 (in thousands):
| | | | | | | | | | | | | | | | |
| | | December 25, 2010 | | | December 26, 2009 | |
| | | Carrying
Amount | | | Fair Value | | | Carrying
Amount | | | Fair Value | |
Second Priority Senior Secured Floating Rate Notes | | $ | 55,106 | | | $ | 55,519 | | | $ | 120,106 | | | $ | 120,669 | |
The fair value for December 25, 2010, is based on the last trade closest to that date which is December 30, 2010. The fair value for December 26, 2009, is based on the last trade closest to that date which is December 23, 2009.
Prior to its termination, the interest rate swap, as described in Note 6, was utilized to offset fluctuations related to the variable interest rate payments on a portion of the Company’s Notes. Prior to December 25, 2010, the unrecognized loss related to the interest rate swap was included in accumulated other comprehensive loss in the consolidated balance sheets. The swap was previously categorized within Level 2 in the fair value hierarchy. For the fiscal year ended December 25, 2010, approximately $0.6 million, net of taxes, was reclassified from accumulated other comprehensive loss to earnings (as a component of interest expense). For the fiscal year ended December 26, 2009, approximately $1.4 million, net of taxes, was reclassified from accumulated other comprehensive loss to earnings. At December 25, 2010, the unrecognized loss was fully amortized and charged to earnings.
14. Secondary Stock Offering
The Company completed two secondary public offerings of 7,171,768 and 6,303,006 shares of its common stock on May 29, 2010, and December 2, 2010, respectively. All of the shares of common stock were sold by certain stockholders of Vitamin Shoppe, Inc. The Company did not receive any proceeds from the sale of shares in either offering. As a result of the offerings, $0.8 million in fees were incurred and charged to selling, general and administrative expenses during Fiscal 2010.
15. Subsequent Events
On January 20, 2011 the Company amended its 2009 Revolving Credit Facility agreement providing for a term loan of $25.0 million maturing on January 2015, for purposes of the redemption of the outstanding balance of its Notes. The term loan is payable in quarterly installments over a two year period and will bear a variable interest rate of LIBOR plus 3.75% . In addition the amendment extended the maturity date on the revolving credit facility an additional two years, resulting in a maturity date of September 25, 2015. On February 22, 2011, the Company completed its tender offer for the redemption of $55.1 million of its outstanding Notes, using proceeds from the above term loan along with existing cash and availability on the revolving credit line, which will result in a charge to extinguishment of debt of approximately $0.6 million in the first fiscal quarter of 2011.
16. Supplemental Guarantor Information
The payment obligations of Industries under the Senior Notes due 2012 are jointly and severally and fully and unconditionally guaranteed on a senior basis by: VSI, the parent company; Direct, the only subsidiary; and all of the Industries’ future restricted domestic subsidiaries. The Notes and the guarantees will be VSI’s, Industries’ and Direct’s second priority senior secured obligations. They rank equally with all of the Company’s existing and future senior indebtedness and rank senior to all of the Company’s existing and future subordinated indebtedness. The Notes and the guarantees are effectively subordinated to all of the Company’s existing first priority senior secured indebtedness, to the extent of the collateral securing such indebtedness, including indebtedness under the Credit Facility.
The indenture governing the Notes restrict the ability of Industries and Direct to incur additional debt, pay dividends and make distributions, make certain investments, repurchase stock, incur liens, enter into transactions with affiliates, enter into sale and lease back transactions, merge, or consolidate or transfer or sell assets.
The following supplemental financial information sets forth, on a consolidating basis, balance sheets, statements of operations, and statements of cash flows for Vitamin Shoppe, Inc. and the Company’s guarantor subsidiary.
67
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING BALANCE SHEET AS OF DECEMBER 25, 2010
(In thousands, except share data)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
ASSETS | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 1,669 | | | $ | 801 | | | $ | 23,498 | | | $ | — | | | $ | 25,968 | |
Inventories | | | — | | | | 17,510 | | | | 93,795 | | | | — | | | | 111,305 | |
Prepaid expenses and other current assets | | | — | | | | 223 | | | | 13,389 | | | | | | | | 13,612 | |
Intercompany receivable | | | 74,634 | | | | 306,490 | | | | 221,211 | | | | (602,335 | ) | | | — | |
Deferred income taxes | | | 971 | | | | 727 | | | | 2,335 | | | | — | | | | 4,033 | |
| | | | | | | | | | | | | | | | | | | | |
Total current assets | | | 77,274 | | | | 325,751 | | | | 354,228 | | | | (602,335 | ) | | | 154,918 | |
Property and equipment, net | | | — | | | | 18,099 | | | | 62,850 | | | | — | | | | 80,949 | |
Goodwill | | | — | | | | — | | | | 177,248 | | | | — | | | | 177,248 | |
Other intangibles, net | | | — | | | | — | | | | 69,718 | | | | — | | | | 69,718 | |
Other assets: | | | | | | | | | | | | | | | | | | | | |
Deferred financing fees, net of accumulated amortization of $1,961 | | | — | | | | — | | | | 816 | | | | — | | | | 816 | |
Other | | | — | | | | 2 | | | | 2,066 | | | | — | | | | 2,068 | |
Deferred income tax asset | | | 6,054 | | | | 1,866 | | | | 14,112 | | | | (22,032 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total other assets | | | 6,054 | | | | 1,868 | | | | 16,994 | | | | (22,032 | ) | | | 2,884 | |
Investment in Subsidiary | | | 233,807 | | | | — | | | | 65,070 | | | | (298,877 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 317,135 | | | $ | 345,718 | | | $ | 746,108 | | | $ | (923,244 | ) | | $ | 485,717 | |
| | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Current portion of capital lease obligation | | $ | — | | | $ | — | | | $ | 1,711 | | | $ | — | | | $ | 1,711 | |
Revolving credit facility | | | — | | | | — | | | | 18,000 | | | | — | | | | 18,000 | |
Intercompany payable | | | 17,400 | | | | 269,225 | | | | 315,710 | | | | (602,335 | ) | | | — | |
Accounts payable | | | — | | | | 156 | | | | 18,838 | | | | — | | | | 18,994 | |
Deferred sales | | | — | | | | 2,908 | | | | 13,021 | | | | — | | | | 15,929 | |
Accrued salaries and related expenses | | | — | | | | 1,063 | | | | 8,510 | | | | — | | | | 9,573 | |
Other accrued expenses | | | — | | | | 726 | | | | 14,026 | | | | — | | | | 14,752 | |
| | | | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 17,400 | | | | 274,078 | | | | 389,816 | | | | (602,335 | ) | | | 78,959 | |
Long-term debt | | | — | | | | — | | | | 55,106 | | | | — | | | | 55,106 | |
Capital lease obligation, net of current portion | | | — | | | | — | | | | 977 | | | | — | | | | 977 | |
Deferred income taxes | | | 2,039 | | | | 2,111 | | | | 38,477 | | | | (22,032 | ) | | | 20,595 | |
Other long term liabilities | | | — | | | | 9 | | | | 5,295 | | | | — | | | | 5,304 | |
Deferred rent | | | — | | | | 4,450 | | | | 22,630 | | | | — | | | | 27,080 | |
| | | | | |
Commitments and contingencies | | | | | | | | | | | | | | | | | | | | |
| | | | | |
Stockholders’ equity: | | | | | | | | | | | | | | | | | | | | |
Common stock, $0.01 par value, 400,000,000 shares authorized, 28,627,897 shares issued and outstanding | | | 286 | | | | — | | | | — | | | | — | | | | 286 | |
Additional paid-in capital | | | 243,558 | | | | 20,165 | | | | 166,791 | | | | (186,956 | ) | | | 243,558 | |
Retained earnings | | | 53,852 | | | | 44,905 | | | | 67,016 | | | | (111,921 | ) | | | 53,852 | |
| | | | | | | | | | | | | | | | | | | | |
Total stockholders’ equity | | | 297,696 | | | | 65,070 | | | | 233,807 | | | | (298,877 | ) | | | 297,696 | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 317,135 | | | $ | 345,718 | | | $ | 746,108 | | | $ | (923,244 | ) | | $ | 485,717 | |
| | | | | | | | | | | | | | | | | | | | |
68
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING BALANCE SHEET AS OF DECEMBER 26, 2009
(In thousands, except share data)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
ASSETS | | | | | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 1,767 | | | $ | 917 | | | $ | 6,113 | | | $ | — | | | $ | 8,797 | |
Inventories | | | — | | | | 17,510 | | | | 88,581 | | | | — | | | | 106,091 | |
Prepaid expenses and other current assets | | | — | | | | 208 | | | | 13,193 | | | | | | | | 13,401 | |
Intercompany receivable | | | 47,444 | | | | 292,145 | | | | 262,745 | | | | (602,334 | ) | | | — | |
Deferred income taxes | | | — | | | | 723 | | | | 4,422 | | | | — | | | | 5,145 | |
| | | | | | | | | | | | | | | | | | | | |
Total current assets | | | 49,211 | | | | 311,503 | | | | 375,054 | | | | (602,334 | ) | | | 133,434 | |
Property and equipment, net | | | — | | | | 21,869 | | | | 62,091 | | | | — | | | | 83,960 | |
Goodwill | | | — | | | | — | | | | 177,248 | | | | — | | | | 177,248 | |
Other intangibles, net | | | — | | | | — | | | | 70,356 | | | | — | | | | 70,356 | |
Other assets: | | | | | | | | | | | | | | | | | | | | |
Deferred financing fees, net of accumulated amortization of $2,856 | | | — | | | | — | | | | 2,384 | | | | — | | | | 2,384 | |
Other | | | — | | | | 2 | | | | 1,873 | | | | — | | | | 1,875 | |
Deferred income tax asset | | | 3,741 | | | | 1,969 | | | | 15,844 | | | | (21,554 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total other assets | | | 3,741 | | | | 1,971 | | | | 20,101 | | | | (21,554 | ) | | | 4,259 | |
Investment in Subsidiary | | | 200,051 | | | | — | | | | 54,533 | | | | (254,584 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 253,003 | | | $ | 335,343 | | | $ | 759,383 | | | $ | (878,472 | ) | | $ | 469,257 | |
| | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | | | | | |
Current portion of long-term debt | | $ | — | | | $ | — | | | $ | 20,000 | | | $ | — | | | $ | 20,000 | |
Current portion of capital lease obligation | | | — | | | | — | | | | 1,537 | | | | — | | | | 1,537 | |
Intercompany payable | | | 17,400 | | | | 269,225 | | | | 315,709 | | | | (602,334 | ) | | | — | |
Accounts payable | | | — | | | | 166 | | | | 24,909 | | | | — | | | | 25,075 | |
Deferred sales | | | — | | | | 2,596 | | | | 11,790 | | | | — | | | | 14,386 | |
Accrued salaries and related expenses | | | — | | | | 716 | | | | 6,835 | | | | — | | | | 7,551 | |
Other accrued expenses | | | 803 | | | | 953 | | | | 12,713 | | | | — | | | | 14,469 | |
| | | | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 18,203 | | | | 273,656 | | | | 393,493 | | | | (602,334 | ) | | | 83,018 | |
Long-term debt | | | — | | | | — | | | | 100,106 | | | | — | | | | 100,106 | |
Capital lease obligation, net of current portion | | | — | | | | — | | | | 2,303 | | | | — | | | | 2,303 | |
Deferred income taxes | | | 449 | | | | 3,020 | | | | 38,030 | | | | (21,554 | ) | | | 19,945 | |
Other long term liabilities | | | — | | | | 4 | | | | 4,762 | | | | — | | | | 4,766 | |
Deferred rent | | | — | | | | 4,130 | | | | 20,638 | | | | — | | | | 24,768 | |
| | | | | |
Commitments and contingencies | | | | | | | | | | | | | | | | | | | | |
| | | | | |
Stockholders’ equity: | | | | | | | | | | | | | | | | | | | | |
Common stock, $0.01 par value, 400,000,000 shares authorized, 26,750,423 shares issued and outstanding | | | 268 | | | | — | | | | — | | | | — | | | | 268 | |
Additional paid-in capital | | | 210,359 | | | | 20,165 | | | | 166,791 | | | | (186,956 | ) | | | 210,359 | |
Accumulated other comprehensive loss | | | (882 | ) | | | — | | | | (882 | ) | | | 882 | | | | (882 | ) |
Retained earnings | | | 24,606 | | | | 34,368 | | | | 34,142 | | | | (68,510 | ) | | | 24,606 | |
| | | | | | | | | | | | | | | | | | | | |
Total stockholders’ equity | | | 234,351 | | | | 54,533 | | | | 200,051 | | | | (254,584 | ) | | | 234,351 | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 253,003 | | | $ | 335,343 | | | $ | 759,383 | | | $ | (878,472 | ) | | $ | 469,257 | |
| | | | | | | | | | | | | | | | | | | | |
69
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF OPERATIONS FOR THE YEAR ENDED DECEMBER 25, 2010
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Net sales | | $ | — | | | $ | 152,052 | | | $ | 599,430 | | | $ | — | | | $ | 751,482 | |
Commissions | | | — | | | | 19,305 | | | | 8,481 | | | | (27,786 | ) | | | — | |
Cost of goods sold | | | — | | | | 106,645 | | | | 401,276 | | | | (5,973 | ) | | | 501,948 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | — | | | | 64,712 | | | | 206,635 | | | | (21,813 | ) | | | 249,534 | |
Selling, general and administrative expenses | | | 4,076 | | | | 47,433 | | | | 160,176 | | | | (21,813 | ) | | | 189,872 | |
(Loss) income from operations | | | (4,076 | ) | | | 17,279 | | | | 46,459 | | | | — | | | | 59,662 | |
Loss on extinguishment of debt | | | — | | | | — | | | | 1,349 | | | | — | | | | 1,349 | |
Interest expense, net | | | — | | | | 1 | | | | 9,516 | | | | — | | | | 9,517 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before provision for income taxes | | | (4,076 | ) | | | 17,278 | | | | 35,594 | | | | — | | | | 48,796 | |
Provision from income taxes | | | (448 | ) | | | 6,741 | | | | 13,257 | | | | — | | | | 19,550 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before equity in net earnings of subsidiary | | | (3,628 | ) | | | 10,537 | | | | 22,337 | | | | — | | | | 29,246 | |
Equity in net earnings of subsidiary | | | 32,874 | | | | — | | | | 10,537 | | | | (43,411 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 29,246 | | | $ | 10,537 | | | $ | 32,874 | | | $ | (43,411 | ) | | $ | 29,246 | |
| | | | | | | | | | | | | | | | | | | | |
70
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF OPERATIONS FOR THE YEAR ENDED DECEMBER 26, 2009
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Net sales | | $ | — | | | $ | 133,216 | | | $ | 541,279 | | | $ | — | | | $ | 674,495 | |
Commissions | | | — | | | | 18,622 | | | | 7,723 | | | | (26,345 | ) | | | — | |
Cost of goods sold | | | — | | | | 96,079 | | | | 366,759 | | | | (5,265 | ) | | | 457,573 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | — | | | | 55,759 | | | | 182,243 | | | | (21,080 | ) | | | 216,922 | |
Selling, general and administrative expenses | | | 3,052 | | | | 43,994 | | | | 147,178 | | | | (21,080 | ) | | | 173,144 | |
Related party expenses | | | — | | | | — | | | | 2,446 | | | | — | | | | 2,446 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income from operations | | | (3,052 | ) | | | 11,765 | | | | 32,619 | | | | — | | | | 41,332 | |
Loss on extinguishment of debt | | | — | | | | — | | | | 2,016 | | | | — | | | | 2,016 | |
Interest expense, net | | | (39 | ) | | | 1,101 | | | | 17,574 | | | | — | | | | 18,636 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before (benefit) provision for income taxes | | | (3,013 | ) | | | 10,664 | | | | 13,029 | | | | — | | | | 20,680 | |
(Benefit) provision from income taxes | | | (1,333 | ) | | | 3,759 | | | | 5,588 | | | | — | | | | 8,014 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before equity in net earnings of subsidiary | | | (1,680 | ) | | | 6,905 | | | | 7,441 | | | | — | | | | 12,666 | |
Equity in net earnings of subsidiary | | | 14,346 | | | | — | | | | 6,905 | | | | (21,251 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 12,666 | | | $ | 6,905 | | | $ | 14,346 | | | $ | (21,251 | ) | | $ | 12,666 | |
| | | | | | | | | | | | | | | | | | | | |
71
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF OPERATIONS FOR THE YEAR ENDED DECEMBER 27, 2008
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Net sales | | $ | — | | | $ | 112,864 | | | $ | 488,676 | | | $ | — | | | $ | 601,540 | |
Commissions | | | — | | | | 25,776 | | | | 6,924 | | | | (32,700 | ) | | | — | |
Cost of goods sold | | | — | | | | 80,890 | | | | 329,368 | | | | (4,599 | ) | | | 405,659 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | — | | | | 57,750 | | | | 166,232 | | | | (28,101 | ) | | | 195,881 | |
Selling, general and administrative expenses | | | 2,448 | | | | 40,257 | | | | 144,109 | | | | (28,101 | ) | | | 158,713 | |
Related party expenses | | | — | | | | — | | | | 1,523 | | | | | | | | 1,523 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income from operations | | | (2,448 | ) | | | 17,493 | | | | 20,600 | | | | — | | | | 35,645 | |
Interest expense, net | | | (54 | ) | | | 3,239 | | | | 17,952 | | | | — | | | | 21,137 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before (benefit) provision for income taxes | | | (2,394 | ) | | | 14,254 | | | | 2,648 | | | | — | | | | 14,508 | |
(Benefit) provision from income taxes | | | (1,048 | ) | | | 5,331 | | | | 2,058 | | | | — | | | | 6,341 | |
| | | | | | | | | | | | | | | | | | | | |
(Loss) income before equity in net earnings of subsidiary | | | (1,346 | ) | | | 8,923 | | | | 590 | | | | — | | | | 8,167 | |
Equity in net earnings of subsidiary | | | 9,513 | | | | — | | | | 8,923 | | | | (18,436 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 8,167 | | | $ | 8,923 | | | $ | 9,513 | | | $ | (18,436 | ) | | $ | 8,167 | |
| | | | | | | | | | | | | | | | | | | | |
72
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF CASH FLOWS FOR THE YEAR ENDED DECEMBER 25, 2010
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 29,246 | | | $ | 10,537 | | | $ | 32,874 | | | $ | (43,411 | ) | | $ | 29,246 | |
Adjustments to reconcile net income to net cash (used in) provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Depreciation and amortization of fixed and intangible assets | | | — | | | | 5,988 | | | | 15,124 | | | | — | | | | 21,112 | |
Impairment charge on fixed assets | | | — | | | | — | | | | 1,326 | | | | | | | | 1,326 | |
Amortization of deferred financing fees | | | — | | | | — | | | | 740 | | | | — | | | | 740 | |
Loss on extinguishment of debt, net of premium on Note redemption | | | — | | | | — | | | | 1,349 | | | | | | | | 1,349 | |
Loss on disposal of fixed assets | | | — | | | | — | | | | 187 | | | | — | | | | 187 | |
Amortization of unrealized loss on terminated swap | | | — | | | | — | | | | 1,079 | | | | | | | | 1,079 | |
Deferred income taxes | | | (1,694 | ) | | | (810 | ) | | | 3,670 | | | | — | | | | 1,166 | |
Deferred rent | | | — | | | | 320 | | | | 1,208 | | | | — | | | | 1,528 | |
Equity compensation expense | | | 4,076 | | | | — | | | | — | | | | — | | | | 4,076 | |
Tax benefits on exercise of stock options | | | — | | | | — | | | | (12,002 | ) | | | — | | | | (12,002 | ) |
Equity in earnings of subsidiary | | | (32,874 | ) | | | — | | | | (10,537 | ) | | | 43,411 | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | | |
Inventories | | | — | | | | — | | | | (5,214 | ) | | | — | | | | (5,214 | ) |
Prepaid expenses and other current assets | | | — | | | | (15 | ) | | | 12,562 | | | | — | | | | 12,547 | |
Intercompany | | | (14,827 | ) | | | (14,345 | ) | | | 29,172 | | | | — | | | | — | |
Other non-current assets | | | — | | | | — | | | | (193 | ) | | | — | | | | (193 | ) |
Accounts payable | | | — | | | | (10 | ) | | | (6,194 | ) | | | — | | | | (6,204 | ) |
Accrued expenses and other current liabilities | | | (804 | ) | | | 432 | | | | 4,275 | | | | | | | | 3,903 | |
Other long-term liabilities | | | — | | | | 5 | | | | 533 | | | | — | | | | 538 | |
| | | | | | | | | | | | | | | | | | | | |
Net cash (used in) provided by operating activities | | | (16,877 | ) | | | 2,102 | | | | 69,959 | | | | — | | | | 55,184 | |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | — | | | | (2,218 | ) | | | (16,138 | ) | | | — | | | | (18,356 | ) |
Trademarks and other intangible assets | | | — | | | | — | | | | (92 | ) | | | — | | | | (92 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | — | | | | (2,218 | ) | | | (16,230 | ) | | | — | | | | (18,448 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | |
Borrowings under revolving credit agreement | | | — | | | | — | | | | 38,000 | | | | — | | | | 38,000 | |
Repayment of borrowings under revolving credit agreement | | | — | | | | — | | | | (20,000 | ) | | | — | | | | (20,000 | ) |
Payments of capital lease obligation | | | — | | | | — | | | | (1,611 | ) | | | — | | | | (1,611 | ) |
Redemption of long term debt (Notes) | | | — | | | | — | | | | (65,000 | ) | | | — | | | | (65,000 | ) |
Payments for expenses related to the offering | | | (87 | ) | | | — | | | | — | | | | — | | | | (87 | ) |
Proceeds from exercises of common stock options | | | 16,866 | | | | — | | | | — | | | | — | | | | 16,866 | |
Issuance of shares under employee stock purchase plan | | | 359 | | | | — | | | | — | | | | — | | | | 359 | |
Tax benefits on exercise of stock options | | | — | | | | — | | | | 12,002 | | | | — | | | | 12,002 | |
Deferred financing fees | | | — | | | | — | | | | (94 | ) | | | — | | | | (94 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 17,138 | | | | — | | | | (36,703 | ) | | | — | | | | (19,565 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents | | | 261 | | | | (116 | ) | | | 17,026 | | | | — | | | | 17,171 | |
Cash and cash equivalents beginning of year | | | 1,767 | | | | 917 | | | | 6,113 | | | | — | | | | 8,797 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents end of year | | $ | 2,028 | | | $ | 801 | | | $ | 23,139 | | | $ | — | | | $ | 25,968 | |
| | | | | | | | | | | | | | | | | | | | |
73
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF CASH FLOWS FOR THE YEAR ENDED DECEMBER 26, 2009
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS
Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 12,666 | | | $ | 6,905 | | | $ | 14,346 | | | $ | (21,251 | ) | | $ | 12,666 | |
Adjustments to reconcile net income to net cash (used in) provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Depreciation and amortization of fixed and intangible assets | | | — | | | | 4,767 | | | | 16,328 | | | | — | | | | 21,095 | |
Amortization of deferred financing fees | | | — | | | | — | | | | 1,227 | | | | — | | | | 1,227 | |
Loss on extinguishment of debt, net of premium on Note redemption | | | — | | | | — | | | | 1,568 | | | | | | | | 1,568 | |
Loss on disposal of fixed assets | | | — | | | | 9 | | | | 121 | | | | — | | | | 130 | |
Amortization of unrealized loss on terminated swap | | | — | | | | — | | | | 565 | | | | | | | | 565 | |
Deferred income taxes | | | (1,307 | ) | | | (301 | ) | | | (3,387 | ) | | | — | | | | (4,995 | ) |
Deferred rent | | | — | | | | 354 | | | | 2,687 | | | | — | | | | 3,041 | |
Equity compensation expense | | | 3,040 | | | | — | | | | — | | | | — | | | | 3,040 | |
Tax benefits on exercise of stock options | | | — | | | | — | | | | (352 | ) | | | — | | | | (352 | ) |
Equity in earnings of subsidiary | | | (14,346 | ) | | | — | | | | (6,905 | ) | | | 21,251 | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | | |
Inventories | | | — | | | | 37 | | | | 763 | | | | — | | | | 800 | |
Prepaid expenses and other current assets | | | — | | | | (10 | ) | | | 810 | | | | — | | | | 800 | |
Intercompany | | | (47,442 | ) | | | (7,382 | ) | | | 54,824 | | | | — | | | | — | |
Other non-current assets | | | — | | | | (2 | ) | | | (215 | ) | | | — | | | | (217 | ) |
Accounts payable | | | — | | | | 64 | | | | 1,272 | | | | — | | | | 1,336 | |
Accrued expenses and other current liabilities | | | 389 | | | | 877 | | | | 3,634 | | | | | | | | 4,900 | |
Other long-term liabilities | | | — | | | | 4 | | | | (2,174 | ) | | | — | | | | (2,170 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash (used in) provided by operating activities | | | (47,000 | ) | | | 5,322 | | | | 85,112 | | | | — | | | | 43,434 | |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | — | | | | (5,246 | ) | | | (15,998 | ) | | | — | | | | (21,244 | ) |
Trademarks and other intangible assets | | | — | | | | — | | | | (37 | ) | | | — | | | | (37 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | — | | | | (5,246 | ) | | | (16,035 | ) | | | — | | | | (21,281 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | |
Borrowings under revolving credit agreement | | | — | | | | — | | | | 8,594 | | | | — | | | | 8,594 | |
Repayment of borrowings under revolving credit agreement | | | — | | | | — | | | | (25,594 | ) | | | — | | | | (25,594 | ) |
Payments of capital lease obligation | | | — | | | | — | | | | (1,334 | ) | | | — | | | | (1,334 | ) |
Redemption of long term debt (Notes) | | | — | | | | | | | | (44,894 | ) | | | — | | | | (44,894 | ) |
Redemption of preferred shares | | | (72,535 | ) | | | — | | | | — | | | | — | | | | (72,535 | ) |
Proceeds from issuance of common stock | | | 755 | | | | — | | | | — | | | | — | | | | 755 | |
Proceeds from issuance of common stock during offering, net | | | 121,209 | | | | — | | | | — | | | | — | | | | 121,209 | |
Payments for expenses related to the offering | | | (1,700 | ) | | | — | | | | — | | | | — | | | | (1,700 | ) |
Proceeds from exercises of common stock options | | | 686 | | | | — | | | | — | | | | — | | | | 686 | |
Tax benefits on exercise of stock options | | | 352 | | | | — | | | | — | | | | — | | | | 352 | |
Deferred financing fees | | | — | | | | — | | | | (518 | ) | | | — | | | | (518 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 48,767 | | | | — | | | | (63,746 | ) | | | — | | | | (14,979 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | 1,767 | | | | 76 | | | | 5,331 | | | | — | | | | 7,174 | |
Cash and cash equivalents beginning of year | | | — | | | | 841 | | | | 782 | | | | — | | | | 1,623 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents end of year | | $ | 1,767 | | | $ | 917 | | | $ | 6,113 | | | $ | — | | | $ | 8,797 | |
| | | | | | | | | | | | | | | | | | | | |
74
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATING STATEMENT OF CASH FLOWS FOR THE YEAR ENDED DECEMBER 27, 2008
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Vitamin
Shoppe, Inc. | | | VS Direct | | | Vitamin
Shoppe
Industries
Inc. | | | Eliminations | | | Consolidated | |
Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | |
Net income | | $ | 8,167 | | | $ | 8,923 | | | $ | 9,513 | | | $ | (18,436 | ) | | $ | 8,167 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | | | | | | | | | |
Depreciation and amortization of fixed and intangible assets | | | — | | | | 3,651 | | | | 13,832 | | | | — | | | | 17,483 | |
Amortization of deferred financing fees | | | — | | | | — | | | | 1,168 | | | | | | | | 1,168 | |
Loss on disposal of fixed assets | | | — | | | | 7 | | | | 72 | | | | — | | | | 79 | |
Deferred income taxes | | | (1,047 | ) | | | 638 | | | | 1,104 | | | | — | | | | 695 | |
Deferred rent | | | — | | | | 1,217 | | | | 2,230 | | | | — | | | | 3,447 | |
Equity compensation expense | | | 2,352 | | | | — | | | | — | | | | — | | | | 2,352 | |
Equity in earnings of subsidiary | | | (9,513 | ) | | | — | | | | (8,923 | ) | | | 18,436 | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | | |
Inventories | | | — | | | | (1,296 | ) | | | (7,786 | ) | | | — | | | | (9,082 | ) |
Prepaid expenses and other current assets | | | — | | | | (110 | ) | | | 184 | | | | — | | | | 74 | |
Intercompany | | | 83 | | | | (3,448 | ) | | | 3,365 | | | | — | | | | — | |
Other non-current assets | | | (54 | ) | | | 20 | | | | (61 | ) | | | — | | | | (95 | ) |
Accounts payable | | | — | | | | (124 | ) | | | (10,784 | ) | | | — | | | | (10,908 | ) |
Accrued expenses and other current liabilities | | | 12 | | | | 868 | | | | 4,764 | | | | | | | | 5,644 | |
Other long-term liabilities | | | — | | | | — | | | | 564 | | | | — | | | | 564 | |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by operating activities | | | — | | | | 10,346 | | | | 9,242 | | | | — | | | | 19,588 | |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | |
Capital expenditures | | | — | | | | (10,072 | ) | | | (21,823 | ) | | | — | | | | (31,895 | ) |
Trademarks and other intangible assets | | | — | | | | — | | | | (3,494 | ) | | | — | | | | (3,494 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | — | | | | (10,072 | ) | | | (25,317 | ) | | | — | | | | (35,389 | ) |
| | | | | | | | | | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | |
Borrowings under revolving credit agreement | | | — | | | | — | | | | 20,000 | | | | — | | | | 20,000 | |
Repayment of borrowings under revolving credit agreement | | | — | | | | — | | | | (3,000 | ) | | | — | | | | (3,000 | ) |
Redemption of preferred shares | | | — | | | | — | | | | (561 | ) | | | — | | | | (561 | ) |
Payments of capital lease obligation | | | — | | | | — | | | | (468 | ) | | | — | | | | (468 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by financing activities | | | — | | | | — | | | | 15,971 | | | | — | | | | 15,971 | |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | — | | | | 274 | | | | (104 | ) | | | — | | | | 170 | |
Cash and cash equivalents beginning of year | | | — | | | | 567 | | | | 886 | | | | — | | | | 1,453 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents end of year | | $ | — | | | $ | 841 | | | $ | 782 | | | $ | — | | | $ | 1,623 | |
| | | | | | | | | | | | | | | | | | | | |
75
16. Selected Quarterly Financial Information (unaudited)
The following table summarizes the 2010 and 2009 quarterly results:
| | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
| | | March | | | June | | | September | | | December | |
Year Ended December 25, 2010 | | | | | | | | | | | | | | | | |
Net sales | | $ | 191,613 | | | $ | 192,234 | | | $ | 187,359 | | | $ | 180,276 | |
Gross profit | | | 65,014 | | | | 63,693 | | | | 61,169 | | | | 59,658 | |
Income from operations | | | 18,072 | | | | 15,447 | | | | 13,853 | | | | 12,290 | |
Net income | | | 8,726 | | | | 7,309 | | | | 7,249 | | | | 5,962 | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | |
Basic | | | 26,692,983 | | | | 27,130,809 | | | | 27,710,913 | | | | 28,026,972 | |
Diluted | | | 27,708,463 | | | | 28,159,448 | | | | 28,597,381 | | | | 28,889,861 | |
| | | | |
Year Ended December 26, 2009 | | | | | | | | | | | | | | | | |
Net sales | | $ | 172,555 | | | $ | 171,143 | | | $ | 168,400 | | | $ | 162,397 | |
Gross profit | | | 57,012 | | | | 55,762 | | | | 52,389 | | | | 51,759 | |
Income from operations | | | 12,701 | | | | 12,144 | | | | 8,506 | | | | 7,981 | |
Net income | | | 4,562 | | | | 4,206 | | | | 2,035 | | | | 1,863 | |
Weighted average shares outstanding: | | | | | | | | | | | | | | | | |
Basic | | | 14,175,906 | | | | 14,175,906 | | | | 14,175,906 | | | | 22,455,694 | |
Diluted | | | 15,969,484 | | | | 15,670,533 | | | | 15,789,680 | | | | 23,607,922 | |
The following table details certain items, net of income taxes where applicable, which positively (negatively) impacted net income for the quarters presented:
| | | | | | | | | | | | | | | | |
| | | March | | | June | | | September | | | December | |
Year Ended December 25, 2010 | | | | | | | | | | | | | | | | |
Extinguishment of debt | | $ | (331 | ) | | $ | (341 | ) | | $ | — | | | $ | (137 | ) |
Impairment of fixed assets | | $ | — | | | $ | — | | | $ | (810 | ) | | $ | — | |
Fees associated with offerings | | $ | — | | | $ | (500 | ) | | $ | — | | | $ | (271 | ) |
Credit card fee rebate | | $ | — | | | $ | — | | | $ | 711 | | | $ | 120 | |
| | | | |
Year Ended December 26, 2009 | | | | | | | | | | | | | | | | |
Extinguishment of debt | | $ | — | | | $ | — | | | $ | — | | | $ | (1,020 | ) |
Management termination fee | | $ | — | | | $ | — | | | $ | — | | | $ | (480 | ) |
Tax benefit related to uncertain tax positions | | $ | — | | | $ | — | | | $ | — | | | $ | 500 | |
76
