Issuer Free Writing Prospectus
Filed Pursuant to Rule 433 of the Securities Act of 1933
Relating to Preliminary Prospectus Supplement dated April 15, 2020
Registration StatementNo. 333-235945
Free Writing Prospectus Published or Distributed by Media
This free writing prospectus relates to the proposed public offering of common stock (the “Offering”) of Athersys, Inc. (“Athersys” or the “Company”), pursuant to a shelf registration statement filed on FormS-3 (FileNo. 333-235945) (the “Registration Statement”), that Athersys filed with the Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended, and was declared effective on January 29, 2020.
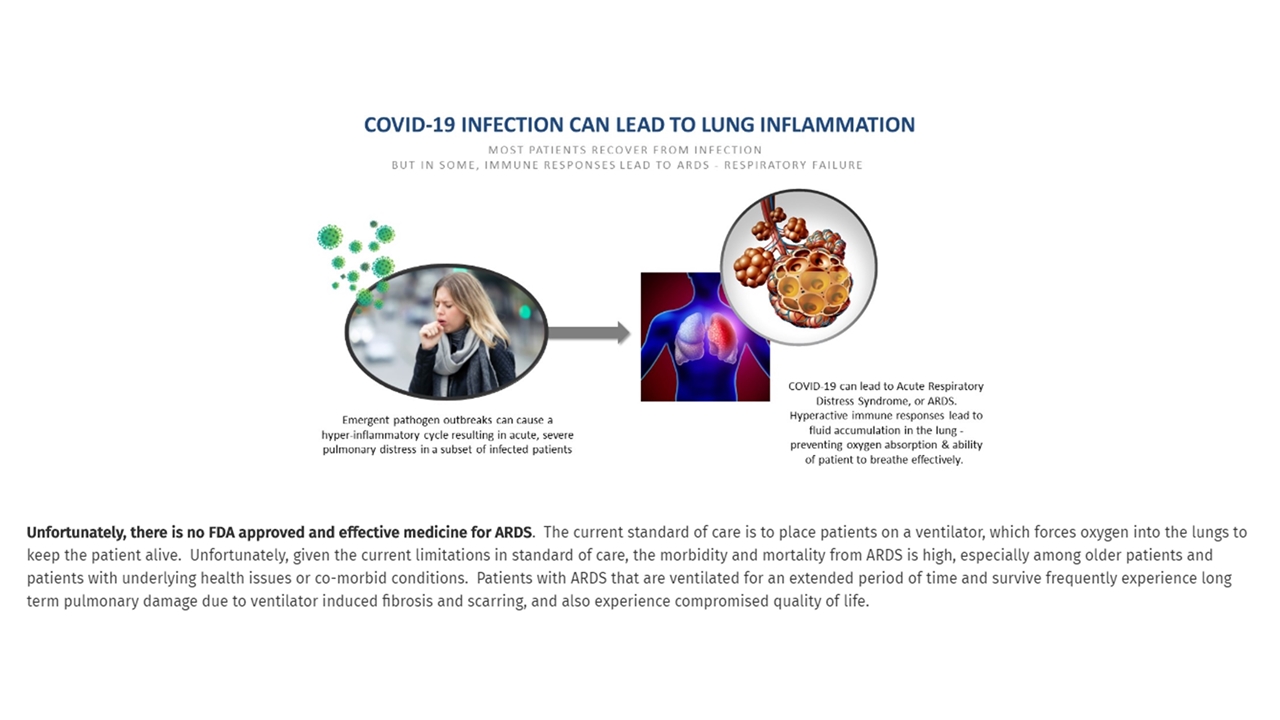
On April 14, 2020, STAT News published an article after Athersys issued a press release on April 13, 2020 announcing that the U.S. Food and Drug Administration authorized the Company to initiate a Phase 2/3 pivotal study to assess the safety and efficacy of MultiStem therapy in subjects with moderate to severe acute respiratory distress syndrome induced by the novel coronavirus disease.
The article was not prepared by or reviewed by the Company prior to publication. The publisher of the article is not affiliated with the Company. The Company made no payment and gave no consideration to the publisher in connection with the publication of the article or any other articles published by the publisher concerning the Company.
In reliance on Rule 433(f)(2)(iii) of the Securities Act of 1933, the Company, in lieu of filing the actual article, is providing in this free writing prospectus the information provided to the author of the article, which consisted solely of thee-mail response below in response to the questions posed by the author of the article.
You should consider statements in this free writing prospectus only after carefully evaluating all of the information in the Company’s preliminary prospectus supplement, dated as of April 15, 2020, and accompanying prospectus forming a part of the Registration Statement as filed with the SEC, including the Company’s other public filings with the SEC that are incorporated therein by reference.
Hi Adam,
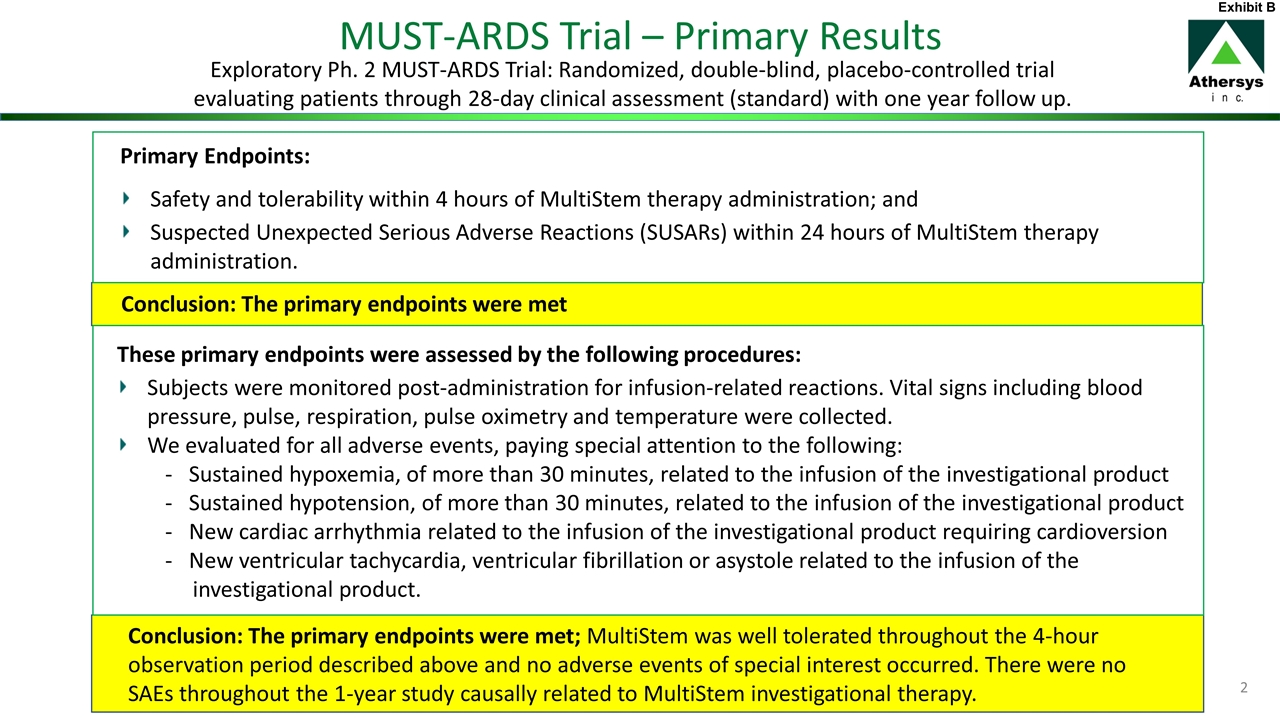
Theone-year data from the MUST-ARDS study was described in our press release [see Exhibit A to this free writing prospectus], and a manuscript to a peer-reviewed journal is in progress where the full data set will be disclosed. The FDA analyzed the data and granted the ARDS program Fast Track designation based on the results from this study. This study was an exploratory study primary meant to evaluate safety but also to evaluate efficacy parameters. There were two primary endpoints from this study (both for safety and tolerability), taken directly from our trial design and clinical study report. They are outlined on slide 19 in our corporate slide deck on our website [see Exhibit B to this free writing prospectus], and on our ARDS page [see Exhibit C to this free writing prospectus].
| | • | | Safety and tolerability within 4 hours of MultiStem therapy administration; and |
| | • | | Suspected Unexpected Serious Adverse Reactions (SUSARs) within 24 hours of MultiStem therapy administration. |
Safety and tolerability within 4 hours was assessed by several factors, such as infusion-related reactions, but also hypoxemia and hypotension as you mention. The study fully met both primary endpoints. MultiStem was well tolerated throughout the4-hour observation period described above and no adverse events of special interest occurred. Importantly, there were no SAEs throughout the1-year study causally related to MultiStem investigational therapy.
We stand by the consistency of the efficacy results, for the study overall and particularly in the prospectively defined study of patients with more severe ARDS, which was very well balanced – so any suggestion that our results are due to an imbalance are unwarranted. We also stand by the biomarker data that shows mechanistically that MultiStem was working the way that we predicted, as we have seen in other studies. We also stand by the FDA’s review of all of the data, and subsequent decision to bestow Fast Track designation for the program based on these promising results. Finally, we are particularly pleased with the dramatic improvement in pulmonary function (with 45% of MultiStem treated patients being off the ventilator within 7 days or less, vs only 20% for placebo) and the one year follow up which showed that there was a meaningful difference in improvement in patient independence and self-care, and