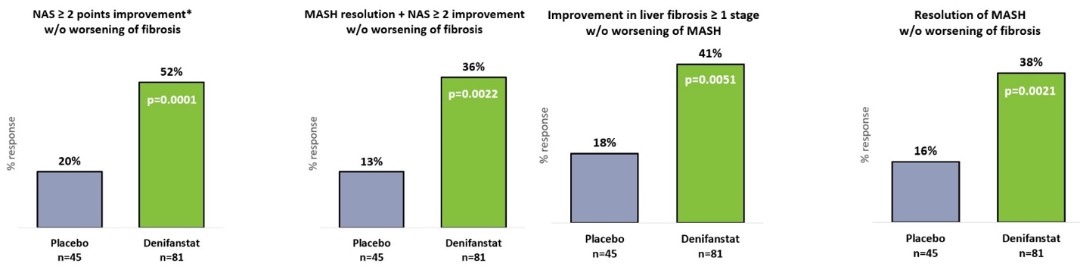
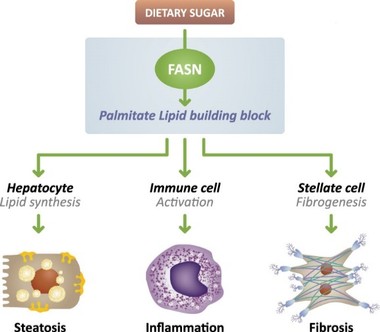
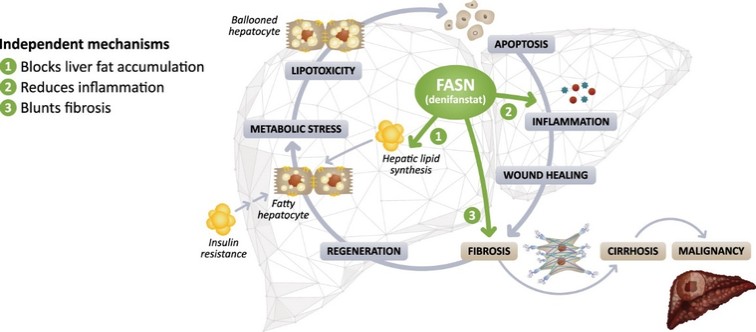
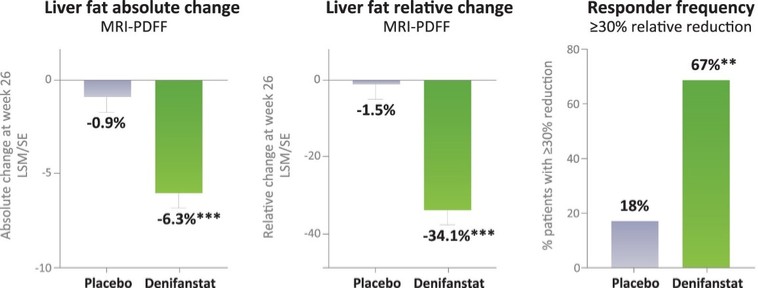
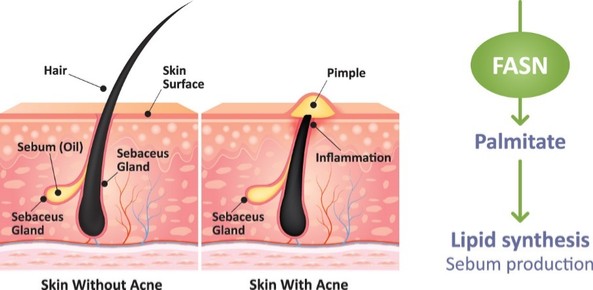
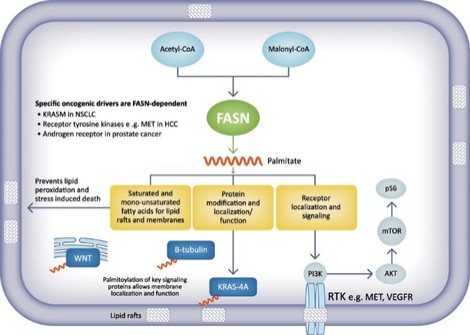
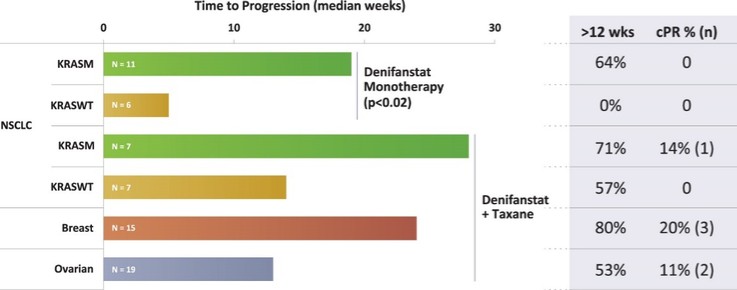
There are extensive regulations that govern the manufacturing of biopharmaceutical products, and the third-party manufacturing organizations we work with are required to adhere to these. Our CMOs are required to manufacture our drug candidates under cGMP requirements, alongside other applicable laws and regulations.
Intellectual property
We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover the composition of matter of our drug candidates, for example, denifanstat and TVB-3567, their methods of use, related technologies and other inventions that are important to our business. In addition to patent protection, we also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
Our commercial success depends in part upon our ability to obtain and maintain patent and other proprietary protection for our drug candidates and other commercially important technologies, inventions and know-how related to our business, defend and enforce our intellectual property rights, in particular, our patent rights, preserve the confidentiality of our trade secrets and operate without infringing valid and enforceable intellectual property rights of others.
The patent positions for biotechnology and pharmaceutical companies like us are generally uncertain and can involve complex legal, scientific and factual issues. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued, and its scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our drug candidates will be protected or remain protectable by enforceable patents. Moreover, any patents that we hold may be challenged, circumvented or invalidated by third parties. For more information regarding the risks related to our intellectual property see “Risk Factors—Risks related to our intellectual property.”
As of December 5, 2023, we owned and/or had control of 12 U.S. patents, 143 issued foreign patents, which includes European patents that have been validated in various European countries, two pending non-provisional U.S. patent applications, one pending U.S. provisional patent application, one pending international PCT application, and 16 pending foreign patent applications.
With regard to denifanstat, as of December 5, 2023, we owned one issued U.S. patent with composition of matter and pharmaceutical composition claims directed to denifanstat. The issued U.S. patent is expected to expire in 2032, without taking any potential patent term extension (PTE) into account. In addition, we own and/or have control of patents that have been granted in various jurisdictions including Australia, Argentina, Brazil, countries across Europe, Japan, China, South Korea, India, Israel, Macau, Mexico, New Zealand, Taiwan, and South Africa, which are expected to expire in 2032, without taking potential PTEs or other forms of extension into account. We also own three issued U.S. patents with claims directed to methods of using denifanstat and combinations of denifanstat with additional agents. The issued U.S. patents are expected to expire in 2035 and 2036, without taking a potential PTE into account. Specifically, U.S. Patent No. 10,363,249, which is expected to expire in 2035, issued with claims directed to a method of treating a taxane-resistant tumor or cancer comprising administering a combination of denifanstat and a taxane. U.S. Patent No. 10,189,822, which is expected to expire in 2036, issued with claims directed to a method of treating various types of cancers (mantle cell lymphoma, chronic myelogenous leukemia, sarcoma; endometrial tumors, non-small cell lung carcinoma, gastric carcinomas, hepatocellular tumors, and head and neck cancer) comprising administering denifanstat, or a combination of denifanstat with additional agents. U.S. Patent No. 11,034,690, which is expected to expire in 2036, issued with claims directed to methods of treating MASH, formerly referred to as NASH, MASLD, formerly referred to as NAFLD, liver cirrhosis and liver fibrosis comprising administering denifanstat. In addition we own and/or have control of patents with claims directed to methods of using denifanstat, and/or methods of using combinations of denifanstat with additional agents, in China, Japan, various countries across Europe, South Korea, Israel, New Zealand, and Russia, which are expected to expire in 2035, 2036 and/or 2037. We also own and/or have control of at least 12 pending applications in jurisdictions including Australia, China, Canada, Europe, Japan, South Korea, Singapore, and South Africa, which, if issued, are expected to expire in 2036 and/or 2037, without taking potential PTEs into account.
With regard to TVB-3567, as of December 5, 2023, we owned one issued U.S. patent with composition of matter claims, as well as claims directed to methods of using TVB-3567 to treat various types of cancer. The issued U.S. Patent No. 9,994,550 is expected to expire in 2035, without taking a potential PTE into account. In addition, we own and/or have control of patents that have been granted in Australia, Brazil, Canada, South Africa, Japan, South Korea, China, Hong Kong, Macau, Israel, India, Singapore, New Zealand, Russia, Mexico, and various countries across Europe, which are expected to expire in 2035, without taking potential term extensions into account. Furthermore, we own one pending application in Singapore which, if issued, is also expected to expire in 2035, without