As filed with the Securities and Exchange Commission on June 15, 2022
Registration No. 333-_______
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
RemSleep Holdings, Inc.
(Exact name of registrant as specified in its charter)
| | Nevada | | 7200 | | 47-5386867 | |
| | (State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) | |
14175 Icot Boulevard, Suite 300
Clearwater, Florida 33760
(813) 367-3855
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Thomas J. Wood
Chief Executive Officer
RemSleep Holdings, Inc.
14175 Icot Boulevard, Suite 300
Clearwater, Florida 33760
(813) 367-3855
(Name, address and telephone number of agent for service)
With copies to:
Eric Newlan, Esq.
Newlan Law Firm, PLLC
2201 Long Prairie Road, Suite 107-762
Flower Mound, Texas 75022
Phone: (940) 367-6154
Approximate date of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act of 1933, check the following box and list the Securities Act of 1933 registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act of 1933, check the following box and list the Securities Act of 1933 registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an “emerging growth company”. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | Large accelerated filer | ☐ | Accelerated Filer | ☐ |
| | Non-accelerated filer | ☒ | Smaller reporting company Emerging growth company | ☒ ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act of 1933. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to Section 8(a) may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until this registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Registration No. 333-________
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION, DATED JUNE 15, 2022 |

500,000,000 Shares of Common Stock
REMSLEEP HOLDINGS, INC.
This Prospectus relates to the offer and sale of up to 500,000,000 shares of common stock (the “Shares”) of RemSleep Holdings, Inc., a Nevada corporation, by JanBella Group, LLC (the “Selling Stockholder”). See “Selling Stockholder.”
The resale of the 500,000,000 Shares by the Selling Stockholder pursuant to this Prospectus is referred to as the “Offering.”
The Selling Stockholder, or its transferees, pledgees, donees or other successors-in-interest, may sell their Shares from time to time at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Stockholder may sell any, all or none of the Shares offered by this Prospectus, and we do not know when or in what amount the Selling Stockholder may sell its Shares hereunder following the effective date of the Registration Statement of which this Prospectus forms a part (the “Registration Statement”).
The Shares are issuable under an equity line (the “Equity Line”) established by the Purchase Agreement (the “JanBella Agreement”) entered into on May 10, 2022, between our company and JanBella Group, LLC (“JanBella” or the “Selling Stockholder”). We may draw on the Equity Line from time to time, as and when we determine appropriate, in accordance with the terms and conditions of the JanBella Agreement. For a more complete discussion of the terms and conditions of the Equity Line, see “Prospectus Summary—Equity Line” and “Plan of Distribution.”
We are not selling any securities under this Prospectus and will not receive any of the proceeds from the sale of the Shares by the Selling Stockholder.
The Selling Stockholder is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act. The Selling Stockholder may sell the Shares described in this Prospectus in a number of different ways and at varying prices. See “Plan of Distribution” for more information about how the Selling Stockholder may sell the Shares being offered pursuant to this Prospectus.
We will pay the expenses incurred in registering the Shares, including legal and accounting fees. See “Plan of Distribution.”
Our common stock is currently quoted on the OTC Market Group, Inc.’s OTC PINK tier under the symbol “RMSL.” On June 10, 2022, the last reported sale price of our Common Stock was $0.0187.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 6 of this Prospectus.
Neither the Securities and Exchange Commission (the “SEC”) nor any state securities commission has approved or disapproved of these securities or determined if this Prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus is ________, 2022
TABLE OF CONTENTS
You should rely only on the information contained in this Prospectus. We have not authorized anyone to provide you with information that is different from that contained in this Prospectus. This Prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted. The information in this Prospectus is complete and accurate only as of the date on the front cover regardless of the time of delivery of this Prospectus or of any sale of our securities.
PROSPECTUS SUMMARY
This summary highlights material information concerning our business and this offering. This summary does not contain all of the information that you should consider before making your investment decision. You should carefully read the entire prospectus and the information incorporated by reference into this prospectus, including the information presented under the section entitled “Risk Factors” and the financial data and related notes, before making an investment decision. This summary contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from future results contemplated in the forward-looking statements as a result of factors such as those set forth in “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements.”
In this Prospectus, unless the context indicates otherwise, the terms “RemSleep,” “Company,” “we,” “us,” “our,” and “ours” refer and relate to RemSleep Holdings, Inc., a Nevada corporation, including its wholly-owned subsidiary, REMSleep LLC.
Overview
We were incorporated in the State of Nevada on June 6, 2007. On August 2, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC, in exchange for 50,000,000 shares of our company’s common stock, at which time REMSleep LLC became our wholly-owned subsidiary; we, then, adopted the business plan of REMSleep LLC, that of developing and distributing sleep apnea products. On January 5, 2015, we changed our name to RemSleep Holdings, Inc. to reflect our new business model.
We are a medical technology company focused on the development and commercialization of innovative and minimally invasive solutions for patients with obstructive sleep apnea. Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our goal is to develop sleep products that achieve optimum compliance and comfort for CPAP patients.
In May 2017, we applied for a patent with the US Patent and Trademark Office for our proprietary DeltaWave CPAP interface (“DeltaWave”), a new, innovative sleep apnea product to act as an interface for the delivery of CPAP therapy and other respiratory needs. DeltaWave is a nasal-pillow type interface designed to offer better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally.
Existing CPAP systems use nasal pillow interfaces that are round. These pillow interfaces cause discomfort when worn as the nasal septum is typically more sensitive to pressure and is typically relatively flat in areas of the nasal openings. Round cross-sectional shapes do not interface well with flat walls and, as an accommodation, prior pillow interfaces are typically made of a soft material that flattens when inserted. This flattening reduces the cross-sectional area of these prior nasal pillows, resulting in an acceleration of the air velocity and noise. The increase in air flow velocity leads to dryness, a sudden burning sensation in the nostrils, and stuffiness in the sinuses. Higher air flow velocity and lower volume of incoming air is less efficient at correcting apnea and often interrupts normal breathing patterns. DeltaWave is designed to deliver more air volume with less driving pressure. Therefore, the patient gets more air coming into the upper airways at a slower air velocity. Also, the pillows that fit into the nostrils are designed to better fit the shape of the nostrils for better comfort and better airtight seal.
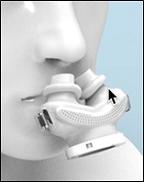 | | 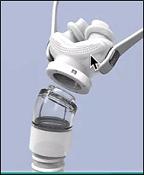 |
A complete discussion concerning our DeltaWave CPAP interface device, including a discussion concerning our market opportunity, is presented under “Business” herein.
Risk Factors
There are numerous risks and uncertainties associated with an investment in our Common Stock, including those presented under “Risk Factors” herein. These risks and uncertainties include, but are not limited to, the following:
| | ● | our history of losses; |
| | | |
| | ● | our need for substantial additional capital to fund our ongoing operations and to fund the marketing and sales efforts relating to our DeltaWave CPAP device, once approved; |
| | | |
| | ● | we currently have only patent-pending protection for our DeltaWave CPAP device; |
| | | |
| | ● | the medical technology industry is highly competitive; |
| | | |
| | ● | we have limited experience marketing and selling our DeltaWave CPAP device; |
| | | |
| | ● | we face the risk of product liability claims; |
| | | |
| | ● | we are highly dependent upon our current management team; |
| | | |
| | ● | our products are subject to extensive government regulation and oversight both in the United States and abroad; |
| | | |
| | ● | we may seek capital that would result in shareholder dilution or that would result in our issuing securities having rights senior to those of our Common Stock; and |
| | | |
| | ● | our Common Stock is a “penny stock,” which may impair trading liquidity. |
In addition, the report of our independent registered public accounting firm for the years ended December 31, 2021 and 2020, contains a statement with respect to substantial doubt as to our ability to continue as a going concern as a result of our accumulated deficit, net losses, and negative cash flows from operations.
Equity Line
On May 10, 2022, we entered into a Purchase Agreement (the JanBella Agreement) with JanBella Group, LLC (JanBella or the Selling Stockholder), pursuant to which, and upon the terms and subject to the conditions thereof, JanBella is committed to purchase, on an unconditional basis, up to 500,000,000 shares of our common stock (the “Purchase Shares”) over the course of its term. The term of the JanBella Agreement will end on the earlier of (a) the date on which the Selling Stockholder has purchased all of the 500,000,000 Shares pursuant to the JanBella Agreement, (b) May 10, 2025, (c) written notice of termination by us, (d) the date the Registration Statement is no longer effective, or (e) the date that, pursuant to or within the meaning of any Bankruptcy Law, we commence a voluntary case or any person commences a proceeding against us, a custodian is appointed for us or for all or substantially all of our property or we make a general assignment for the benefit of creditors.
From time to time over the term of the JanBella Agreement, commencing on the date the Registration Statement registering the Shares becomes effective, we may, in our sole discretion, provide JanBella with a purchase notice (each a “Purchase Notice”) to purchase a specified number of Purchase Shares (each a “Purchase Amount Requested”) subject to the limitations discussed below and contained in the JanBella Agreement. Upon delivery of a Purchase Notice, we must deliver the Purchase Amount Requested as Deposit Withdrawal at Custodian (DWAC) shares to the Selling Stockholder within one trading day.
The actual amount of proceeds we receive pursuant to each Purchase Notice (each, the “Purchase Amount”) is determined by multiplying the Purchase Amount Requested by the applicable purchase price. The Purchase Price for each of the Put Shares equals 75% of the lowest traded price of our common stock during the Valuation Period. The Valuation Period is the ten (10) consecutive business days immediately preceding, but not including, the date a Purchase Notice is delivered. Should we uplist our common stock on The Nasdaq Stock Market or an equivalent national exchange, the Purchase Price shall be set at 80% of the lowest traded price of the our common stock during the Valuation Period, subject to a floor of $0.01, per share (subject to adjustments for stock splits, dividends, and similar occurrences), below which we shall not deliver a Purchase Notice. JanBella will deliver the Purchase Amount to us on the Settlement Date. The Settlement Date is the date on which the Purchase Shares are confirmed as being received by JanBella’s broker, against the payment of the Purchase Price by JanBella, which date will be five (5) business days following the Valuation Period.
The Purchase Amount requested pursuant to any Purchase Notice must have an aggregate value of at least $20,000 and cannot exceed the lesser of (1) $150,000 or (2) 100% of the average daily volume traded for our common stock during the relevant Valuation Period (subject to adjustments for stock splits, dividends, and similar occurrences).
In order to deliver a Purchase Notice, certain conditions set forth in the JanBella Agreement must be met. In addition, we are prohibited from delivering a Purchase Notice if the sale of Purchase Shares pursuant to such Purchase Notice would cause us to issue and sell to the Selling Stockholder, or the Selling Stockholder to acquire or purchase, a number of shares of our common stock that would result in Selling Stockholder beneficially owning more than 4.99% of the issued and outstanding shares of our common stock.
By the terms of the JanBella Agreement, we agreed to file a registration statement to register the resale of the Purchase Shares. We agreed to (A) file the Registration Statement, (B) use reasonable efforts to cause the Registration Statement to be declared effective under the Securities Act of 1933, as amended, as promptly as possible after the filing thereof, and (C) use our reasonable efforts to keep the Registration Statement continuously effective under the Securities Act until all of the Shares have been sold thereunder or pursuant to Rule 144.
Use of Proceeds
We intend to use the proceeds, if any, from the Equity Line for marketing and advertising, lab studies/testing, research and development, regulatory compliance, inventory, professional fees, general corporate purposes and working capital requirements. (See “Use of Proceeds”).
In the future, we intend to raise additional capital through equity and debt financings as needed, though there cannot be any assurance that such funds will be available to us on acceptable terms, on an acceptable schedule, or at all.
Corporate Information
Our principal executive offices are located at 14175 Icot Boulevard, Suite 300, Clearwater, Florida 33760; our telephone number is (813) 367-3855; our corporate website is located at www.remsleep.com. No information found on, or connected to, our company’s website is incorporated by reference into, any you must not consider the information to a part of, this Prospectus.
The Offering
| Securities Offered by the Selling Shareholder | | 500,000,000 shares of common stock (the Shares), all of which are issuable pursuant to the JanBella Agreement. |
| | | |
| Common Stock Outstanding Before Offering | | 1,461,616,601 shares of common stock. |
| | | |
| Common Stock Outstanding After Offering | | 1,961,616,601shares of common stock, assuming all 500,000,000 Shares are sold to JanBella under the Equity Line. |
| | | |
| Use of Proceeds | | We will not receive any of the proceeds from the sale of the Common Stock registered hereunder. We will, however, receive proceeds from our sales of Common Stock to the Selling Stockholder under the Equity Line. We intend to use such proceeds, if any, for marketing and advertising, lab studies/testing, research and development, regulatory compliance, inventory, professional fees, general corporate purposes and working capital requirements. |
| | | |
| Risk Factors | | An investment in our Common Stock involves a high degree of risk and could result in a loss of your entire investment. Further, the issuance to, or sale by, the Selling Stockholder of a significant amount of shares being registered in the Registration Statement of which this Prospectus forms a part at any given time could cause the market price of our Common Stock to decline and to be highly volatile and we do not have the right to control the timing and amount of any sales by the Selling Stockholder of such shares. Prior to making an investment decision, you should carefully consider all of the information in this Prospectus and, in particular, you should evaluate the risk factors set forth under the caption “Risk Factors” beginning on page 6. |
| | | |
| Trading Symbol | | RMSL |
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
We have made some statements in this Prospectus, including some under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere, which constitute forward-looking statements. These statements may discuss our future expectations or contain projections of our results of operations or financial condition or expected benefits to us resulting from acquisitions or transactions and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any results, levels of activity, performance or achievements expressed or implied by any forward-looking statements. These factors include, among other things, those listed under “Risk Factors” and elsewhere in this Prospectus. In some cases, forward-looking statements can be identified by terminology such as “may,” “should,” “could,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other comparable terminology. Although we believe that the expectations reflected in forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
RISK FACTORS
An investment in our securities involves a high degree of risk. Before you invest in our securities, you should give careful consideration to the following risk factors, in addition to the other information included in this prospectus, including our financial statements and related notes, before deciding whether to invest in our securities. The occurrence of any of the adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operations or prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business
The report of our independent auditors on our financial statements for the year ended December 31, 2021, indicates uncertainty concerning our ability to continue as a going concern and this may impair our ability to raise capital to fund our business. The report of our independent auditors indicates uncertainty concerning our ability to continue as a going concern and this may impair our ability to raise capital to fund our business. In its opinion on our financial statements for the year ended December 31, 2021, our independent auditors raised substantial doubt about our ability to continue as a going concern. We cannot assure you that this will not impair our ability to raise capital on attractive terms. Additionally, we cannot assure you that we will ever achieve significant revenues and therefore remain a going concern. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our company had an accumulated deficit of $10,707,914 (unaudited) at March 31, 2022, had a net loss of $316,299 (unaudited) and net cash used in operating activities of $199,085 (unaudited) for the three months ended March 31, 2022. Our ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of our contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for us to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about our ability to continue as a going concern.
If we are unable to raise enough capital in this offering or obtain additional financing, we may not be able to fulfill our business plan. At March 31, 2022, we had $4,014,578 (unaudited) cash on hand. Our entire business plan, including our ability to obtain FDA approval of our DeltaWave CPAP device and to conduct manufacturing, marketing, generate sales and further develop products, are entirely dependent upon adequate financing. Should we fail to obtain adequate financing: (a) our financial condition will be negatively affected; (b) we will be unable to conduct the essential aspects of our business plan, including marketing; (c) investments in our common stock will be negatively impacted; (d) we will be forced to liquidate our business and file for bankruptcy protection.
We currently have only patent-pending protection for our DeltaWave device. We have filed a patent application for our DeltaWave device with the USPTO; however, as yet, it has not granted yet and there can be no assurance that one will be issued.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements. Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent and, in some jurisdictions, during the pendency of a patent application. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products. Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
If patients or physicians are not willing to change current practices to adopt our DeltaWave device to treat moderate to severe sleep apnea, our DeltaWave device may fail to gain increased market acceptance, and our business will be adversely affected. Our primary strategy to generate and grow our revenue is to drive the adoption of our DeltaWave device to treat patients with moderate to severe sleep apnea who are unable to use or get consistent benefit from their current CPAP masks. Physicians and users may choose not to use our DeltaWave device for a number of reasons, including:
| | ● | lack of availability of adequate third-party payor coverage or reimbursement; |
| | | |
| | ● | lack of experience with our products; |
| | | |
| | ● | our inability to convince key opinion leaders to provide recommendations regarding our DeltaWave device, or to convince physicians, patients and healthcare payors that our DeltaWave device is an attractive alternative to other treatment devices; |
| | | |
| | ● | perceived inadequacy of evidence supporting benefits of our DeltaWave device over existing alternatives; |
| | | |
| | ● | liability risks generally associated with the use of new products and procedures; and |
| | | |
| | ● | the training required to use new products. |
If additional physicians or other medical professionals do not adopt our DeltaWave device for any reason, including those listed above, our ability to execute our growth strategy will be impaired, and our business may be adversely affected. In addition, patients may choose not to use our DeltaWave device if, among other potential reasons, their airway anatomy would not allow for effective treatment with DeltaWave device, they are worried about potential adverse effects of our DeltaWave device, such as infection, discomfort or they are unable to obtain adequate third-party coverage or reimbursement.
We currently compete and will in the future continue to compete against other companies, some of which have longer operating histories, more established products or greater resources than we do, which may prevent us from achieving increased market penetration and improved operating results. The medical technology industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Our competitors have historically dedicated and will continue to dedicate significant resources to promoting their products or developing new products or methods to treat moderate to severe sleep apnea. We also believe other emerging businesses are in the early stages of developing devices designed to treat sleep apnea. In addition, we also compete, both within and outside of the United States with invasive surgical treatment options which are primarily used in the treatment of mild to moderate sleep apnea.
In addition, our DeltaWave device is designed in the treatment of sleep apnea in patients who cannot use or obtain consistent benefit from current CPAP system devices. If one or more CPAP device manufacturers successfully develop a CPAP device that is more effective, better tolerated or otherwise results in better compliance by patients, or if improvements in other devices make them more effective, cost effective, easier to use or otherwise more attractive than our DeltaWave device, sales of our DeltaWave device could be significantly and adversely affected, which could have a material adverse effect on our business, financial condition and results of operations. In addition, if other companies are successful in developing devices that are approved for a broader range of indications than our DeltaWave device, we will be at a further competitive disadvantage, which could also affect our business, financial condition and results of operations.
Many of the companies against which we compete may have competitive advantages with respect to primary competitive factors in the sleep apnea treatment market, including:
| | ● | greater company, product and brand recognition; |
| | | |
| | ● | superior product safety, reliability and durability; |
| | | |
| | ● | more effective marketing to and education of patients, physicians and sleep centers; |
| | | |
| | ● | greater product ease of use and patient comfort; |
| | | |
| | ● | more sales force experience and greater market access |
| | | |
| | ● | better product support and service; |
| | | |
| | ● | more advanced technological innovation, product enhancements and speed of innovation; |
| | | |
| | ● | more effective pricing and revenue strategies; and |
| | | |
| | ● | more effective sales teams and strategies. |
We also compete with other medical technology companies to recruit and retain qualified sales, training and other personnel.
In addition, though there are currently no pharmacologic therapies approved to treat sleep apnea, we may in the future face competition from pharmaceutical companies that develop such therapies. We also expect to experience increased competition in the future as other companies develop and commercialize competing devices. Any of these companies may also have the competitive advantages described above.
Our long-term growth depends on our ability to enhance our DeltaWave device, expand our indications and develop and commercialize additional products. It is important to our business that we continue to enhance our DeltaWave device and develop and introduce new products. Developing products is expensive and time-consuming and could divert management’s attention away from our core business. The success of any new product offering or product enhancements to our DeltaWave device will depend on several factors, including our ability to:
| | ● | properly identify and anticipate physician and patient needs; |
| | | |
| | ● | develop and introduce new products and product enhancements in a timely manner; |
| | | |
| | ● | avoid infringing upon the intellectual property rights of third-parties; |
| | | |
| | ● | demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials; |
| | | |
| | ● | obtain the necessary regulatory clearances or approvals for expanded indications, new products or product modifications; |
| | | |
| | ● | be fully FDA-compliant with marketing of new devices or modified products; |
| | | |
| | ● | provide adequate training to potential users of our products; |
| | | |
| | ● | receive adequate coverage and reimbursement for procedures performed with our products; and |
| | | |
| | ● | develop an effective and dedicated sales and marketing team. |
If we are not successful in expanding our indications and developing and commercializing new products and product enhancements, our ability to increase our revenue may be impaired, which could have a material adverse effect on our business, financial condition and results of operations. Our financial results may fluctuate significantly and may not fully reflect the underlying performance of our business. Our quarterly and annual results of operations may vary significantly in the future, and period-to-period comparisons of our operating results may not be meaningful. Accordingly, the results of any one quarter or period should not be relied upon as an indication of future performance. Our quarterly and annual financial results may fluctuate as a result of a variety of factors, many of which are outside our control and, as a result, may not fully reflect the underlying performance of our business. One such factor includes seasonal variations in our sales.
Other factors that may cause fluctuations in our quarterly and annual results include:
| | ● | patient and physician adoption of our DeltaWave device; |
| | | |
| | ● | changes in coverage policies by third-party payors that affect the reimbursement of procedures using our products; |
| | | |
| | ● | timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; |
| | | |
| | ● | unanticipated pricing pressure; |
| | | |
| | ● | the hiring, retention and continued productivity of our sales representatives; |
| | | |
| | ● | our ability to expand the geographic reach of our sales and marketing efforts; |
| | | |
| | ● | our ability to obtain regulatory clearance or approval for any products in development or for our current products for additional indications or in additional countries outside the United States; |
| | | |
| | ● | results of clinical research and trials on our existing products and products in development; |
| | | |
| | ● | delays in receipt of anticipated purchase orders; |
| | | |
| | ● | delays in, or failure of, component and raw material deliveries by our suppliers; and |
| | | |
| | ● | positive or negative coverage in the media or clinical publications of our products or products of our competitors or our industry. |
Because our quarterly and annual results may fluctuate, period-to-period comparisons may not be the best indication of the underlying results of our business and should only be relied upon as one factor in determining how our business is performing. These fluctuations may also increase the likelihood that we will not meet our forecasted performance, which could negatively affect the market price for our common stock. Our results of operations could be materially harmed if we are unable to accurately forecast customer demand for our DeltaWave device and manage our inventory. To ensure adequate inventory supply, we must forecast inventory needs and place orders with our suppliers based on our estimates of future demand for our DeltaWave device. Our ability to accurately forecast demand for our DeltaWave device could be negatively affected by many factors, including our failure to accurately manage our expansion strategy, product introductions by competitors, an increase or decrease in customer demand for our DeltaWave device or for products of our competitors, our failure to accurately forecast customer acceptance of new products, unanticipated changes in general market conditions or regulatory matters and weakening of economic conditions or consumer confidence in future economic conditions. Inventory levels in excess of customer demand may result in inventory write-downs or write-offs, which would cause our gross margin to be adversely affected and could impair the strength of our brand. Conversely, if we underestimate customer demand for our DeltaWave device, our third-party contract manufacturers may not be able to deliver products to meet our requirements, and this could result in damage to our reputation and customer relationships. In addition, if we experience a significant increase in demand, additional supplies of raw materials or additional manufacturing capacity may not be available when required on terms that are acceptable to us, or at all, or suppliers or our third-party manufacturers may not be able to allocate sufficient capacity in order to meet our increased requirements, which could have an adverse effect on our ability to meet customer demand for our DeltaWave device and our results of operations.
We seek to maintain sufficient levels of inventory in order to protect ourselves from supply interruptions. As a result, we are subject to the risk that a portion of our inventory will become obsolete or expire, which could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
We will rely on a limited number of third-party suppliers and contract manufacturers for the manufacture and assembly of our products, and a loss or degradation in performance of these suppliers and contract manufacturers could have a material adverse effect on our business, financial condition and results of operations. We will rely on third-party suppliers and contract manufacturers for the raw materials and components used in our DeltaWave device and to manufacture and assemble our products. Any of our suppliers or our third-party contract manufacturers may be unwilling or unable to supply the necessary materials and components or manufacture and assemble our products reliably and at the levels we anticipate or that are required by the market. Our ability to supply our products commercially and to develop any future products depends, in part, on our ability to obtain these materials, components and products in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. Our manufacturers may decide in the future to discontinue or reduce the level of business they conduct with us. If we are required to change contract manufacturers due to any change in or termination of our relationships with these third parties, or if our manufacturers are unable to obtain the materials they need to produce our products at consistent prices or at all, we may lose sales, experience manufacturing or other delays, incur increased costs or otherwise experience impairment to our customer relationships. We cannot guarantee that we will be able to establish alternative relationships on similar terms, without delay or at all.
Establishing additional or replacement suppliers for any of these materials, components or services, if required, could be time-consuming and expensive, may result in interruptions in our operations and product delivery, may affect the performance specifications of our DeltaWave device or could require that we modify its design. Even if we are able to find replacement suppliers or third-party contract manufacturers, we will be required to verify that the new supplier or third-party manufacturer maintains facilities, procedures and operations that comply with our quality expectations and applicable regulatory requirements. Furthermore, our contract manufacturers could require us to move to another one of their production facilities or use alternative materials or components. Any of these events could require that we obtain a new regulatory authority approval before we implement the change, which could result in further delay and which may not be obtained at all. While we seek to maintain sufficient levels of inventory as discussed above, those inventories may not fully protect us from supply interruptions.
If our third-party suppliers fail to deliver the required commercial quantities of materials on a timely basis and at commercially reasonable prices, and we are unable to find one or more replacement suppliers capable of production at a substantially equivalent cost in substantially equivalent volumes and quality on a timely basis, the continued commercialization of our DeltaWave device, the supply of our products to customers and the development of any future products will be delayed, limited or prevented, which could have material adverse effect on our business, financial condition and results of operations.
Performance issues, service interruptions or price increases by our shipping carriers could adversely affect our business and harm our reputation and ability to provide our services on a timely basis. Expedited, reliable shipping will be essential to our operations. We will rely heavily on providers of transport services for reliable and secure point-to-point transport of our DeltaWave device to our customers and for tracking of these shipments. Should a carrier encounter delivery performance issues such as loss, damage or destruction of any systems, it would be costly to replace such systems in a timely manner and such occurrences may damage our reputation and lead to decreased demand for our DeltaWave device and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions affecting delivery services we use would adversely affect our ability to process orders for our DeltaWave device on a timely basis.
Consolidation in the healthcare industry or group purchasing organizations could lead to demands for price concessions, which may affect our ability to sell our products at prices necessary to support our current business strategies. Healthcare costs have risen significantly over the past decade, which has resulted in or led to numerous cost reform initiatives by legislators, regulators and third-party payors. Cost reform has triggered a consolidation trend in the healthcare industry to aggregate purchasing power, which may create more requests for pricing concessions in the future. Additionally, group purchasing organizations, independent delivery networks and large single accounts may continue to use their market power to consolidate purchasing decisions for hospitals and ASCs. We expect that market demand, government regulation, third-party coverage and reimbursement policies and societal pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products.
We have limited experience marketing and selling our DeltaWave device, and if we are unable to expand, manage and maintain our direct sales and marketing organization we may not be able to generate revenue growth. We have not commenced sales of our DeltaWave device and as a result, we have no experience in marketing and selling our DeltaWave device. We intend to sell our DeltaWave device through a direct sales force that targets ENT physicians and sleep centers in the United States and Europe, and also utilize various direct-to-patient marketing initiatives, including paid search, radio, social media and online videos. Our operating results will be directly dependent upon the efforts of these employees. If our direct sales force fails to adequately promote, market and sell our DeltaWave device and obtain reimbursement for it, our revenue may be adversely affected.
In order to generate revenue, we plan to engage a direct sales organization. This may require us to split or adjust sales territories, which may adversely affect our ability to retain customers in those territories. Additionally, our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled sales and reimbursement personnel with significant industry experience and technical knowledge of sleep apnea devices and related products. Because the competition for their services is high, we cannot assure you we will be able to hire and retain additional personnel on favorable or commercially reasonable terms, if at all. Failure to hire or retain qualified sales and reimbursement personnel would prevent us from expanding our business and generating revenue. If we are unable to expand our sales and marketing capabilities, e be able to effectively commercialize our DeltaWave device, which could have an adverse effect on our business, financial condition and results of operations.
Our ability to maintain our competitive position depends on our ability to attract and retain senior management and other highly qualified personnel. Our success will depend in part on our ability to attract, retain and motivate highly qualified management and other personnel. We are highly dependent upon our management team, Thomas J. Wood, our Chief Executive Officer, and Russell F. Bird, our President. The replacement of any of our management team likely would involve significant time and costs and may significantly delay or prevent the achievement of our business objectives and could therefore have an adverse effect on our business. In addition, we do not carry any “key person” insurance policies that could offset potential loss of service under applicable circumstances.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance. Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. This risk exists even if a device is cleared or approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our DeltaWave device is designed to affect, and any future products will be designed to affect, important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our DeltaWave device could result in patient injury or death. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot offer any assurance that we will not face product liability suits. We may be subject to product liability claims if our DeltaWave device causes, or merely appears to have caused, patient injury or death. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us. Product liability claims may be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with our DeltaWave device, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| | ● | costs of litigation; |
| | | |
| | ● | distraction of management’s attention from our primary business; |
| | | |
| | ● | the inability to commercialize our DeltaWave device or new products; |
| | | |
| | ● | decreased demand for our DeltaWave device; |
| | | |
| | ● | damage to our business reputation; |
| | | |
| | ● | product recalls or withdrawals from the market; |
| | | |
| | ● | withdrawal of clinical trial participants; |
| | | |
| | ● | substantial monetary awards to patients or other claimants; or |
| | | |
| | ● | loss of sales. |
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we would be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
If the quality of our DeltaWave device does not meet the expectations of physicians or patients, then our brand and reputation or our business could be adversely affected. In the course of conducting our business, we must adequately address quality issues that may arise with our DeltaWave device, including defects in third-party components included in our DeltaWave device. In addition, even in the absence of quality issues, we may be subject to claims and liability if the performance of our DeltaWave device does not live up to the expectations of physicians or patients as a result of the patient’s use of the product. If the quality of our DeltaWave device does not meet the expectations of physicians or patients, then our brand and reputation with those physicians or patients, or our business, financial condition and results of operations, could be adversely affected.
If we choose to acquire new and complementary businesses, products or technologies, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner. Our success depends, in part, on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and advances in technologies. Accordingly, although we have no current commitments with respect to any acquisition or investment, we may in the future pursue the acquisition of, or joint ventures relating to, complementary businesses, products or technologies instead of developing them ourselves. We do not know if we will be able to successfully complete any future acquisitions or joint ventures, or whether we will be able to successfully integrate any acquired business, product or technology or retain any key employees related thereto. Integrating any business, product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business and distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business will be adversely affected. In addition, any amortization or charges resulting from the costs of acquisitions could increase our expenses.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations. Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the global financial crisis, could result in a variety of risks to our business, including weakened demand for our DeltaWave device, and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain our manufacturers or suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our services. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the economic climate and financial market conditions could adversely affect our business.
Failure of a key information technology system, process or site could have an adverse effect on our business. We will rely extensively on information technology systems to conduct our business. These systems affect, among other things, ordering and managing materials from suppliers, shipping products to customers, processing transactions, summarizing and reporting results of operations, complying with regulatory, legal or tax requirements, data security and other processes necessary to manage our business. If our systems are damaged or cease to function properly due to any number of causes, ranging from catastrophic events to power outages to security breaches, and our business continuity plans do not effectively compensate on a timely basis, we may experience interruptions in our operations, which could have an adverse effect on our business.
In addition, we accept payments for many of our sales through credit and debit card transactions, which are handled through a third-party payment processor. As a result, we are subject to a number of risks related to credit and debit card payments. As a result of these transactions, we pay interchange and other fees, which may increase over time and could require us to either increase the prices we charge for our DeltaWave device or experience an increase in our costs and expenses. In addition, as part of the payment processing process, we transmit our customers’ credit and debit card information to our third-party payment processor. We may in the future become subject to lawsuits or other proceedings for purportedly fraudulent transactions arising out of the actual or alleged theft of our customers’ credit or debit card information if the security of our third-party credit card payment processor is breached. We and our third-party credit card payment processor are also subject to payment card association operating rules, certification requirements and rules governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we or our third-party credit card payment processor fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our customers, and there may be an adverse effect on our business.
We are subject to anti-bribery, anti-corruption, and anti-money laundering laws, including the U.S. Foreign Corrupt Practices Act, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
As we grow our international presence and global operations, we will have increasing obligations to comply with trade and economic sanctions and other restrictions imposed by the United States, the European Union and other governments and organizations. The U.S. Departments of Justice, Commerce, State and Treasury and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the U.S. Foreign Corrupt Practices Act, or the FCPA, and other federal statutes and regulations, including those established by the Office of Foreign Assets Control, or OFAC. In addition, the U.K. Bribery Act of 2010, or the Bribery Act, prohibits both domestic and international bribery, as well as bribery across both private and public sectors. An organization that “fails to prevent bribery” by anyone associated with the organization can be charged under the Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. Under these laws and regulations, as well as other anti-corruption laws, anti-money laundering laws, export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities and modifications to compliance programs, which may increase compliance costs, and may subject us to fines, penalties and other sanctions. A violation of these laws or regulations would negatively affect our business, financial condition and results of operations.
We bear the risk of warranty claims on our DeltaWave device. We bear the risk of warranty claims on our DeltaWave device. We may not be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer or that any recovery from such vendor or supplier would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in costs to us.
We may need substantial additional funding beyond our existing cash resources and the proceeds from this offering and may be unable to raise capital when needed, which could force us to delay or reduce our commercialization efforts or product development programs. We believe that the net proceeds from this offering will be sufficient to meet our capital requirements and fund our operations for at least 12 months. However, we have based these estimates on assumptions that may prove to be incorrect, and we could spend our available financial resources much faster than we currently expect. Any future funding requirements will depend on many factors, including:
| | ● | patient, physician and market acceptance of our DeltaWave device; |
| | | |
| | ● | the cost of filing and prosecuting patent applications and defending and enforcing our patent or other intellectual property rights; |
| | | |
| | ● | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| | | |
| | ● | the cost and timing of additional regulatory clearances or approvals; |
| | | |
| | ● | the cost and timing of establishing additional sales and marketing capabilities; |
| | | |
| | ● | costs associated with any product recall that may occur; |
| | | |
| | ● | the effect of competing technological and market developments; |
| | | |
| | ● | the extent to which we acquire or invest in products, technologies and businesses, although we currently have no commitments or agreements relating to any of these types of transactions; and |
| | | |
| | ● | the costs of operating as a public company. |
Any additional equity or debt financing that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds by selling additional shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock after this offering (including through the exercise by the underwriters of their option to purchase additional shares of our common stock), the issuance of such securities will result in dilution to our stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible into or exercisable or exchangeable for shares of our common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering. Furthermore, investors purchasing any securities we may issue in the future may have rights superior to your rights as a holder of our common stock.
In addition, any future debt financing into which we enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. If we raise additional funds through collaboration and licensing arrangements with third-parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us.
Furthermore, we cannot be certain that additional funding will be available on acceptable terms, if at all. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third-parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations. Any of these factors could harm our business, financial condition and results of operations.
If we fail to successfully manage our new product development or if we fail to anticipate the issues associated with such development or expansion, our business may suffer. We have one patent pending for DeltaWave. Our ability to anticipate and manage a variety of issues associated with any new product development or market expansion, such as:
| | ● | market acceptance; and |
| | | |
| | ● | effective management of our applications and other products |
Our business would suffer if we fail to successfully anticipate and manage these issues associated with product development publishing and you may lose all or part of your investment.
If we cannot attract customers, we will not generate revenues and our business will fail. As of the date of this Prospectus, we have not generated any revenue. If our business fails, you will lose all or part of your investment.
We may encounter difficulties managing our planned growth, which would adversely affect our business and could result in increasing costs as well as a decrease in our stock price. We intend to establish a customer base and develop new products for them. To manage our anticipated growth, we must continue to improve our operational and financial systems and expand, train, retain and manage our employee base to meet new opportunities. Because of the registration of our securities, we are subject to reporting and disclosure obligations, and we anticipate that we will hire additional finance and administrative personnel to address these obligations. In addition, the anticipated growth of our business will place a significant strain on our existing managerial and financial resources. If we cannot effectively manage our growth, our business may be harmed.
Because we do not have an audit committee, shareholders will have to rely on the directors, who are not independent, to perform these functions. We do not have an audit or compensation committee comprised of independent directors. These functions are performed by the board of directors as a whole. The members of the Board of Directors are not independent directors. Thus, there is a potential conflict in that the board members are also engaged in management and participate in decisions concerning management compensation and audit issues that may affect management performance.
Failure to protect our proprietary technology and intellectual property rights could substantially harm our business and results of operations. Our success depends to a significant degree on our ability to protect our proprietary technology, methodologies, know-how and our brand. We will rely on a combination of contractual restrictions, and other intellectual property laws and confidentiality procedures to establish and protect our proprietary rights. However, the steps we will take to protect our intellectual property may be inadequate. We will not be able to protect our intellectual property if we are unable to enforce our rights or if we do not detect unauthorized use of our intellectual property. If we fail to protect our intellectual property rights adequately, our competitors may gain access to our technology and our business may be harmed. In addition, defending our intellectual property rights might entail significant expense. We may be unable to prevent third parties from acquiring domain names or trademarks that are similar to, infringe upon, or diminish the value of our trademarks and other proprietary rights.
As we grow our business, our plan is to enter into confidentiality and invention assignment agreements with our employees and consultants and enter into confidentiality agreements with other parties. No assurance can be given that these agreements will be effective in controlling access to and distribution of our proprietary information. Further, these agreements may not prevent our competitors from independently developing technologies that are substantially equivalent or superior to our products.
In order to protect our intellectual property rights, we may be required to spend significant resources to monitor and protect our intellectual property rights. Litigation may be necessary in the future to enforce our intellectual property rights and to protect our trade secrets. Litigation brought to protect and enforce our intellectual property rights could be costly, time-consuming, and distracting to management, and could result in the impairment or loss of portions of our intellectual property. Further, our efforts to enforce our intellectual property rights may be met with defenses, counterclaims, and countersuits attacking the validity and enforceability of our intellectual property rights. Our inability to protect our proprietary technology against unauthorized copying or use, as well as any costly litigation or diversion of our management’s attention and resources, could delay further sales or the implementation of our products, impair the functionality of our products, delay introductions of new products, result in our substituting inferior or more costly technologies into our products, or injure our reputation.
We could incur substantial costs as a result of any claim of infringement of another party’s intellectual property rights. We do not currently have a patent portfolio, which could prevent us from deterring patent infringement claims through our own patent portfolio, and our competitors and others may now and in the future have significantly larger and more mature patent portfolios than we have. The risk of patent litigation has been amplified by the increase in the number of a type of patent holder, which we refer to as a non-practicing entity, whose sole business is to assert such claims and against whom our own intellectual property portfolio may provide little deterrent value. We could incur substantial costs in prosecuting or defending any intellectual property litigation. If we sue to enforce our rights or are sued by a third party that claims that our solution infringes its rights, the litigation could be expensive and could divert our management resources. As of the date of this Prospectus, we have not received any written notice of an infringement claim, invitation to license, or other intellectual property infringement action.
Any intellectual property litigation to which we might become a party, or for which we are required to provide indemnification, may require us to do one or more of the following:
| | ● | Cease selling or using products that incorporate the intellectual property that we allegedly infringe; |
| | | |
| | ● | Make substantial payments for legal fees, settlement payments or other costs or damages; |
| | | |
| | ● | Obtain a license, which may not be available on reasonable terms or at all, to sell or use the relevant technology; or |
| | | |
| | ● | Redesign the allegedly infringing products to avoid infringement, which could be costly, time-consuming or impossible. |
If we are required to make substantial payments or undertake any of the other actions noted above as a result of any intellectual property infringement claims against us or any obligation to indemnify our customers for such claims, such payments or actions could harm our business.
Our Articles of Incorporation and Bylaws and certain provisions of Nevada law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest. Our Articles of Incorporation and Bylaws and certain provisions of Nevada State law could have the effect of making it more difficult or more expensive for a third party to acquire, or from discouraging a third party from attempting to acquire, control of the Company, even when these attempts may be in the best interests of our stockholders. In general, a public Nevada corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales or other transactions resulting in a financial benefit to the stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years, did own, fifteen percent (15%) or more of the corporation’s outstanding voting stock. These provisions may have the effect of delaying, deferring or preventing a change in our control.
Limitations of director liability and indemnification of directors, officers and employees. Our Articles of Incorporation limit the liability of directors to the maximum extent permitted by Nevada law. Nevada law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| | ● | breach of their duty of loyalty to us or our stockholders; |
| | | |
| | ● | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| | | |
| | ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in the Nevada Revised Statutes; or |
| | | |
| | ● | transactions for which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission. Our corporate bylaws provide that we will indemnify our directors, officers and employees to the fullest extent permitted by law. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding. We believe that these bylaw provisions are necessary to attract and retain qualified persons as directors and officers. The limitation of liability in our Articles of Incorporation and bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Risks Related to Government Regulation
Our products are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business. We and our products are subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance and approval; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; post-market approval studies; and product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA enforces these regulatory requirements through periodic unannounced inspections. We do not know whether we will pass any future FDA inspections. Failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as: warning letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances or approvals; withdrawals or suspensions of current approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal penalties.
We may not receive the necessary clearances or approvals for our future products or expanded indications, and failure to timely obtain necessary clearances or approvals for our future products or expanded indications would adversely affect our ability to grow our business. An element of our strategy is to continue to upgrade our products, add new features and expand the indications and uses for our current products. In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA, or approval of a pre-market approval application, or PMA, from the FDA, unless an exemption applies. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that a proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally on the U.S. market pursuant to an approved PMA and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the process of obtaining PMA approval, which was required for our DeltaWave device, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k) may require a new 510(k) clearance. Both the PMA approval and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is filed with the FDA. In addition, a PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses for the device, which may limit the market for the device.
In the United States, we obtained approval of our DeltaWave device through the PMA pathway in June 2008. Any modification to the DeltaWave device that has not been previously approved may require us to submit a new PMA or PMA supplement and obtain FDA approval prior to implementing the change. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
| | ● | our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or notified body that our products are safe or effective for their intended uses; |
| | | |
| | ● | the disagreement of the FDA or the applicable foreign regulatory body with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials; |
| | | |
| | ● | serious and unexpected adverse device effects experienced by participants in our clinical trials; |
| | | |
| | ● | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; |
| | | |
| | ● | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; |
| | | |
| | ● | the manufacturing process or facilities we use may not meet applicable requirements; and |
| | | |
| | ● | the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain new approvals, increase the costs of compliance or restrict our ability to maintain our current approval. For example, as part of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance and approval. Some of these proposals and reforms could impose additional regulatory requirements upon us that could delay our ability to obtain new approvals, increase the costs of compliance or restrict our ability to maintain our current approval.
In order to sell our products in member countries of the EEA our products must comply with the essential requirements of the European Union Medical Devices Directive (Council Directive 93/42/EEC) and the Active Implantable Medical Devices Directive (Council Directive 90/385/EEC). Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene, or CE, mark to our products, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the European Union Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a member state of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. If we fail to remain in compliance with applicable European laws and directives, we would be unable to continue to affix the CE mark to our products, which would prevent us from selling them within the EEA.
Modifications to our products may require us to obtain new PMA approvals or approvals of a PMA supplement, and if we market modified products without obtaining necessary approvals, we may be required to cease marketing or recall the modified products until required approvals are obtained. Certain modifications to a PMA-approved device may require approval of a new PMA or a PMA supplement, or alternatively a notification or other submission to the FDA. The FDA may not agree with our decisions regarding whether a new PMA or PMA supplement is necessary. We may make modifications to our approved devices in the future that we believe do not require approval of a new PMA or PMA supplement. If the FDA disagrees with our determination and requires us to submit a new PMA or PMA supplement for modifications to our previously approved products, we may be required to cease marketing or to recall the modified product until we obtain approval, and we may be subject to significant regulatory fines or penalties. In addition, the FDA may not approve our products for the indications that are necessary or desirable for successful commercialization or could require clinical trials to support any modifications. Any delay or failure in obtaining required approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market. Even though we have obtained approval for the DeltaWave device, we are subject to ongoing and pervasive regulatory requirements governing, among other things, the manufacture, marketing, advertising, medical device reporting, sale, promotion, registration, and listing of devices. For example, we must submit periodic reports to the FDA as a condition of PMA approval. These reports include safety and effectiveness information about the device after its approval. Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA. Following its review of the periodic reports, the FDA might ask for additional information or initiate further investigation.
In addition, the PMA approval for our DeltaWave device was subject to several conditions of approval, including a post-market long-term study and extended follow-up of the pre-market study cohort. Though we believe we have complied with these conditions to date, any failure to comply with the conditions of approval could result in the withdrawal of PMA approval and the inability to continue to market the device. Failure to conduct the required studies in accordance with institutional review board, or IRB, and informed consent requirements, or adverse findings in these studies, could also be grounds for withdrawal of approval of the PMA.
The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs, or lower than anticipated sales. Even after we have obtained the proper regulatory approval to market a device, we have ongoing responsibilities under FDA regulations and applicable foreign laws and regulations. The FDA, state and foreign regulatory authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state or foreign regulatory authorities, which may include any of the following sanctions:
| | ● | untitled letters or warning letters; |
| | | |
| | ● | fines, injunctions, consent decrees and civil penalties; |
| | | |
| | ● | recalls, termination of distribution, administrative detention, or seizure of our products; |
| | | |
| | ● | customer notifications or repair, replacement or refunds; |
| | | |
| | ● | operating restrictions or partial suspension or total shutdown of production; |
| | | |
| | ● | delays in or refusal to grant our requests for future PMA approvals or foreign regulatory approvals of new products, new intended uses, or modifications to existing products; |
| | | |
| | ● | withdrawals or suspensions of our current PMA or foreign regulatory approvals, resulting in prohibitions on sales of our products; |
| | | |
| | ● | FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; and |
| | | |
| | ● | criminal prosecution. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, financial condition and results of operations.
Our products must be manufactured in accordance with federal and state regulations, and we or any of our suppliers or third-party manufacturers could be forced to recall our installed systems or terminate production if we fail to comply with these regulations. The methods used in, and the facilities used for, the manufacture of our products must comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of medical devices. Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. Our products are also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
Our third-party manufacturers may not take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of our products. In addition, failure to comply with applicable FDA requirements or later discovery of previously unknown problems with our products or manufacturing processes could result in, among other things: warning letters or untitled letters; fines, injunctions or civil penalties; suspension or withdrawal of approvals; seizures or recalls of our products; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s refusal to grant pending or future clearances or approvals for our products; clinical holds; refusal to permit the import or export of our products; and criminal prosecution of us or our employees.
Any of these actions could significantly and negatively affect supply of our products. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and experience reduced sales and increased costs.
If treatment guidelines for sleep apnea change or the standard of care evolves, we may need to redesign and seek new marketing authorization from the FDA for one or more of our products. If treatment guidelines for sleep apnea changes or the standard of care for this condition evolves, we may need to redesign the applicable product and seek new approvals from the FDA. Our PMA approvals from the FDA will be based on the then current treatment guidelines. If treatment guidelines change so that different treatments become desirable, the clinical utility of one or more of products could be reduced or eliminated.
Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us. We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of the product. If we fail to comply with our reporting obligations, the FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device approval, seizure of our products or delay in clearance or approval of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals for the device before we may market or distribute the corrected device. Seeking such approvals may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If we do not obtain and maintain international regulatory registrations or approvals for our products, we will be unable to market and sell our products outside of the United States. Sales of our products outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the United States. While the regulations of some countries may not impose barriers to marketing and selling our products or only require notification, others require that we obtain the approval of a specified regulatory body. Complying with foreign regulatory requirements, including obtaining registrations or approvals, can be expensive and time-consuming, and we may not receive regulatory approvals in each country in which we plan to market our products, or we may be unable to do so on a timely basis. The time required to obtain registrations or approvals, if required by other countries, may be longer than that required for FDA approval, and requirements for such registrations, clearances or approvals may significantly differ from FDA requirements. If we modify our products, we may need to apply for additional regulatory approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we have received. If we are unable to maintain our authorizations in a particular country, we will no longer be able to sell the applicable product in that country.
Regulatory approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, and registration, clearance or approval by one or more foreign regulatory authorities does not ensure registration, clearance or approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining registration or regulatory clearance or approval in one country may have a negative effect on the regulatory process in others.
Legislative or regulatory reforms in the United States or the European Union, or EU, may make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market or distribute our products after clearance or approval is obtained. From time to time, legislation is drafted and introduced in the United States Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
On April 5, 2017, the European Parliament passed the Medical Devices Regulation (Regulation 2017/745), which repeals and replaces the EU Medical Devices Directive and the Active Implantable Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable, i.e., without the need for adoption of EEA member state laws implementing them, in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation.
The Medical Devices Regulation will become applicable three years after publication (in 2020). Once applicable, the new regulations will among other things:
| | ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; |
| | | |
| | ● | establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| | | |
| | ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| | | |
| | ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; |
| | | |
| | ● | strengthened rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an effect on the way we conduct our business in the EEA.
We are subject to certain federal, state and foreign fraud and abuse laws, health information privacy and security laws and transparency laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
There are numerous U.S. federal and state, as well as foreign, laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims and physician transparency laws. Our business practices and relationships with providers are subject to scrutiny under these laws. We may also be subject to privacy and security regulation related to patient, customer, employee and other third-party information by both the federal government and the states and foreign jurisdictions in which we conduct our business. The healthcare laws and regulations that may affect our ability to operate include, but are not limited to:
| ● | The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual or furnishing or arranging for a good or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. The U.S. government has interpreted this law broadly to apply to the marketing and sales activities of manufacturers. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Violations of the federal Anti-Kickback Statute may result in civil monetary penalties up to $100,000 for each violation, plus up to three times the remuneration involved. Civil penalties for such conduct can further be assessed under the federal False Claims Act. Violations can also result in criminal penalties, including criminal fines of up to $100,000 and imprisonment of up to 10 years. Similarly, violations can result in exclusion from participation in government healthcare programs, including Medicare and Medicaid; |
| ● | The federal civil and criminal false claims laws and civil monetary penalties laws, including the federal civil False Claims Act, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other federal healthcare programs that are false or fraudulent. These laws can apply to manufacturers who provide information on coverage, coding, and reimbursement of their products to persons who bill third-party payers. Private individuals can bring False Claims Act “qui tam” actions, on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in amounts paid by the entity to the government in fines or settlement. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties ranging from $11,181 to $22,363 for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs; |
| ● | The federal Civil Monetary Penalties Law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier; |
| ● | The Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| ● | The federal Physician Sunshine Act under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable Care Act, which require certain applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, or CHIP, to report annually to the DHHS Centers for Medicare and Medicaid Services, or CMS, information related to payments and other transfers of value to physicians, certain other healthcare providers, and teaching hospitals, and applicable manufacturers and group purchasing organizations, to report annually ownership and investment interests held by physicians and their immediate family members. Applicable manufacturers are required to submit annual reports to CMS. Failure to submit required information may result in civil monetary penalties of $11,052 per failure up to an aggregate of $165,786 per year (or up to an aggregate of $1.105 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission, and may result in liability under other federal laws or regulations; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH Act, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their business associates that perform services for them that involve individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization, including mandatory contractual terms as well as directly applicable privacy and security standards and requirements. Failure to comply with the HIPAA privacy and security standards can result in civil monetary penalties up to $55,910 per violation, not to exceed $1.68 million per calendar year for non-compliance of an identical provision, and, in certain circumstances, criminal penalties with fines up to $250,000 per violation and/or imprisonment. State attorneys general can also bring a civil action to enjoin a HIPAA violation or to obtain statutory damages on behalf of residents of his or her state; and |
| ● | Analogous state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers or patients; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm customers, foreign and state laws, including the EU General Data Protection Regulation, or GDPR, governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; and state laws related to insurance fraud in the case of claims involving private insurers. |
These laws and regulations, among other things, constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of our products. Due to the breadth of these laws, the narrowness of statutory exceptions and regulatory safe harbors available, and the range of interpretations to which they are subject, it is possible that some of our current or future practices might be challenged under one or more of these laws.
To enforce compliance with the healthcare regulatory laws, certain enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Additionally, as a result of these investigations, healthcare providers and entities may have to agree to additional compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity and be costly to respond to. If our operations are found to be in violation of any of the healthcare laws or regulations described above or any other healthcare regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, contractual damages, reputational harm, disgorgement and the curtailment or restructuring of our operations.
We may be subject to, or may in the future become subject to, U.S. federal, state, and foreign laws and regulations imposing obligations on how we collect, store and process personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue. In the conduct of our business, we may at times process personal data, including health-related personal data. The U.S. federal government and various states have adopted or proposed laws, regulations, guidelines and rules for the collection, distribution, use and storage of personal information of individuals. We may also be subject to U.S. federal rules, regulations and guidance concerning data security for medical devices, including guidance from the FDA. State privacy and security laws vary from state to state and, in some cases, can impose more restrictive requirements than U.S. federal law. Where state laws are more protective, we must comply with the stricter provisions. In addition to fines and penalties that may be imposed for failure to comply with state law, some states also provide for private rights of action to individuals for misuse of personal information.
The EU also has laws and regulations dealing with the collection, use and processing of personal data obtained from individuals in the EU, which are often more restrictive than those in the United States and which restrict transfers of personal data to the United States unless certain requirements are met. These obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict with other requirements or our practices. In addition, these rules are constantly under scrutiny. For example, following a decision of the Court of Justice of the European Union in October 2015, transferring personal data to U.S. companies that had certified as members of the U.S. Safe Harbor Scheme was declared invalid. In July 2016 the European Commission adopted the U.S.-EU Privacy Shield Framework which replaces the Safe Harbor Scheme. However, this Framework is under review and there is currently litigation challenging other EU mechanisms for adequate data transfers (i.e., the standard contractual clauses). It is uncertain whether the Privacy Shield Framework and/or the standard contractual clauses will be similarly invalidated by the European courts. We rely on a mixture of mechanisms to transfer personal data from our EU business to the United States and could be impacted by changes in law as a result of a future review of these transfer mechanisms by European regulators under the GDPR, as well as current challenges to these mechanisms in the European courts.
Any actual or perceived failure by us or the third parties with whom we work to comply with privacy or security laws, policies, legal obligations or industry standards, or any security incident that results in the unauthorized release or transfer of personally identifiable information, may result in governmental enforcement actions and investigations including by European Data Protection Authorities and U.S. federal and state regulatory authorities, fines and penalties, litigation and/or adverse publicity, including by consumer advocacy groups, and could cause our customers, their patients and other healthcare professionals to lose trust in us, which could harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could harm our business, financial condition and results of operations. In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. In March 2010, the Affordable Care Act was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other ways in which it may affect our business, the Affordable Care Act:
| | ● | imposed an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions (described in more detail below), although the effective rate paid may be lower. Through a series of legislative amendments, the tax was suspended for 2016 through 2019. Absent further legislative action, the device excise tax will be reinstated on medical device sales starting January 1, 2020; |
| | | |
| | ● | established a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| | | |
| | ● | implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| | | |
| | ● | expanded the eligibility criteria for Medicaid programs. |
We do not yet know the full impact that the Affordable Care Act will have on our business. The taxes imposed by the Affordable Care Act and the expansion in the government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursement by payors for our DeltaWave device, and/or reduced medical procedure volumes, all of which may have a material adverse effect on our business, financial condition and results of operations. The Trump Administration and the U.S. Congress may take further action regarding the Affordable Care Act, including, but not limited to, repeal or replacement. Most recently, the Tax Cuts and Jobs Act of 2017 was enacted, which, among other things, removes penalties for not complying with the individual mandate to carry health insurance. Additionally, all or a portion of the Affordable Care Act and related subsequent legislation may be modified, repealed or otherwise invalidated through judicial challenge, which could result in lower numbers of insured individuals, reduced coverage for insured individuals and adversely affect our business.
We expect additional state and federal healthcare policies and reform measures to be adopted in the future, any of which could limit reimbursement for healthcare products and services or otherwise result in reduced demand for our DeltaWave device or additional pricing pressure and have a material adverse effect on our industry generally and on our customers. Any changes of, or uncertainty with respect to, future coverage or reimbursement rates could affect demand for our DeltaWave device, which in turn could impact our ability to successfully commercialize our DeltaWave device and could have a material adverse effect on our business, financial condition and results of operations.
The clinical trial process required to obtain regulatory approvals is lengthy and expensive with uncertain outcomes. If clinical studies of our future products do not produce results necessary to support regulatory clearance or approval in the United States or, with respect to our current or future products, elsewhere, we will be unable to expand the indications for or commercialize these products and may incur additional costs or experience delays in completing, or ultimately be unable to complete, the commercialization of those products.
We will need to obtain PMA approval for any future other products we develop. In order to obtain PMA approval for a device, the sponsor must conduct well-controlled clinical trials designed to assess the safety and efficacy of the product candidate. Conducting clinical trials is a complex and expensive process, can take many years, and outcomes are inherently uncertain. We incur substantial expense for, and devote significant time to, clinical trials but cannot be certain that the trials will ever result in commercial revenue. We may experience significant setbacks in clinical trials, even after earlier clinical trials showed promising results, and failure can occur at any time during the clinical trial process. Any of our products may malfunction or may produce undesirable adverse effects that could cause us or regulatory authorities to interrupt, delay or halt clinical trials. We, the FDA, or another regulatory authority may suspend or terminate clinical trials at any time to avoid exposing trial participants to unacceptable health risks.
Risks Related to Our Organization and Structure
Our holding company structure makes us dependent on our subsidiaries for our cash flow and could serve to subordinate the rights of our shareholders to the rights of creditors of our subsidiaries, in the event of an insolvency or liquidation of any such subsidiary. Our company acts as a holding company and, accordingly, substantially all of our operations are conducted through our subsidiaries. Such subsidiaries will be separate and distinct legal entities. As a result, substantially all of our cash flow will depend upon the earnings of our subsidiaries. In addition, we will depend on the distribution of earnings, loans or other payments by our subsidiaries. No subsidiary will have any obligation to provide our company with funds for our payment obligations. If there is an insolvency, liquidation or other reorganization of any of our subsidiaries, our shareholders will have no right to proceed against their assets. Creditors of those subsidiaries will be entitled to payment in full from the sale or other disposal of the assets of those subsidiaries before our company, as a shareholder, would be entitled to receive any distribution from that sale or disposal.
Risks Related to a Purchase of Our Common Stock
We may seek capital that may result in shareholder dilution or that may have rights senior to those of our common stock. From time to time, we may seek to obtain additional capital, either through equity, equity-linked or debt securities. The decision to obtain additional capital will depend on, among other factors, our business plans, operating performance and condition of the capital markets. If we raise additional funds through the issuance of equity, equity-linked or debt securities, those securities may have rights, preferences or privileges senior to the rights of our common stock, which could negatively affect the market price of our common stock or cause our shareholders to experience dilution.
Future issuances of debt securities and equity securities could negatively affect the market price of shares of our common stock and, in the case of equity securities, may be dilutive to existing shareholders. In the future, we may issue debt or equity securities or incur other financial obligations, including stock dividends. Upon liquidation, it is possible that holders of our debt securities and other loans and preferred stock would receive a distribution of our available assets before common shareholders. We are not required to offer any such additional debt or equity securities to existing shareholders on a preemptive basis. Therefore, additional common stock issuances, directly or through convertible or exchangeable securities, warrants or options, would dilute the holdings of our existing common shareholders and such issuances, or the perception of such issuances, could reduce the market price of shares of our common stock.
We do not intend to pay dividends on our common stock. We intend to retain earnings, if any, to provide funds for the implementation of our business strategy. We do not intend to declare or pay any dividends in the foreseeable future. Therefore, there can be no assurance that holders of our common stock will receive cash, stock or other dividends on their shares of our common stock, until we have funds which our Board of Directors determines can be allocated to dividends.
Because our common stock is considered a “penny stock,” any investment in our common stock is considered to be a high-risk investment and is subject to restrictions on marketability. Our common stock is considered a “penny stock” because it is quoted on the OTC PINK and it trades for less than $5.00 per share. The OTC PINK is generally regarded as a less efficient trading market than the NASDAQ Capital or Global Markets or the New York Stock Exchange. The SEC has rules that regulate broker-dealer practices in connection with transactions in “penny stocks.” Penny stocks generally are equity securities with a price of less than $5.00 per share (other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document prepared by the SEC, which specifies information about penny stocks and the nature and significance of risks of the penny stock market. The broker-dealer also must provide the customer with bid and offer quotations for the penny stock, the compensation of the broker-dealer and any salesperson in the transaction, and monthly account statements indicating the market value of each penny stock held in the customer’s account. In addition, the penny stock rules require that, prior to effecting a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock. Since our common stock is subject to the regulations applicable to penny stocks, the market liquidity for our common stock could be adversely affected because the regulations on penny stocks could limit the ability of broker-dealers to sell our common stock and thus your ability to sell our common stock in the secondary market in the future. We can provide no assurance that our common stock will be quoted or listed on any trading platform of higher quality than the OTC PINK, including the OTCQB, NASDAQ or any exchange, even if eligible, in the future.
It is possible that our common stock will continue to experience volatility in its trading volume and its market price. Our common stock is quoted in the over-the-counter market under the symbol “RMSL” on the OTC PINK marketplace. For over the past five years, our common stock has experienced both volume and price volatility. The market for low-priced securities is generally less liquid and more volatile than securities traded on national stock markets. Wide fluctuations in market prices are not uncommon.
The price of our common stock may be subject to wide fluctuations in response to factors such as the following, some of which are beyond our control:
| | ● | quarterly variations in our operating results; |
| | | |
| | ● | operating results that vary from the expectations of investors; |
| | | |
| | ● | changes in expectations as to our future financial performance, including financial estimates by investors; |
| | | |
| | ● | reaction to our periodic filings, or presentations by executives at investor and industry conferences; |
| | | |
| | ● | changes in our capital structure; |
| | | |
| | ● | announcements of innovations or new products by us or our competitors; |
| | | |
| | ● | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| | | |
| | ● | lack of success in the expansion of our business operations; |
| | | |
| | ● | third-party announcements of claims or proceedings against us or adverse developments in pending proceedings; |
| | | |
| | ● | additions or departures of key personnel; |
| | | |
| | ● | asset impairment; |
| | | |
| | ● | temporary or permanent inability to offer products or services; and |
| | | |
| | ● | rumors or public speculation about any of the above factors. |
Our future results may vary significantly which may adversely affect the price of our common stock. It is possible that our quarterly revenues and operating results may vary significantly in the future and that period-to-period comparisons of our revenues and operating results are not necessarily meaningful indicators of the future. You should not rely on the results of one quarter as an indication of our future performance. It is also possible that in some future quarters, our revenues and operating results will fall below our expectations or the expectations of market analysts and investors. If we do not meet these expectations, the price of our common stock may decline significantly.
Our internal controls may be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public. Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of our company; |
| | | |
| | ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of our company are being made only in accordance with authorizations of management and/or directors of our company; and |
| | | |
| | ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our company’s assets that could have a material effect on the financial statements. |
Our internal controls may be inadequate or ineffective, which could cause financial reporting to be unreliable and lead to misinformation being disseminated to the public. Investors relying upon this misinformation may make an uninformed investment decision.
Failure to achieve and maintain an effective internal control environment could cause us to face regulatory action and also cause investors to lose confidence in our reported financial information, either of which could have a material adverse effect on the Company’s business, financial condition, results of operations and future prospects.
However, our auditors will not be required to formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 until we are no longer an “emerging growth company” as defined in the JOBS Act if we take advantage of the exemptions available to us through the JOBS Act.
The costs of being a public company could result in us being unable to continue as a going concern. As a public company, we are required to comply with numerous financial reporting and legal requirements, including those pertaining to audits and internal control. The costs of maintaining public company reporting requirements could be significant and may preclude us from seeking financing or equity investment on terms acceptable to us and our shareholders. We estimate these costs to be approximately $75,000 per year and may be higher if our business volume or business activity increases significantly.
If our revenues are insufficient or non-existent and/or we cannot satisfy many of these costs through the issuance of equity or debt securities, we may be unable to satisfy these costs in the normal course of business. This would certainly result in our being unable to continue as a going concern.
Future sales of our common stock, or the perception in the public markets that these sales may occur, could reduce the market price of our common stock. Our current shareholders, including our management, hold shares of our restricted common stock, but will be able to sell their shares in the market. In general, our officers and directors and 10% shareholders, as affiliates, under Rule 144 may not sell more than one percent of the total issued and outstanding shares in any 90-day period, and must resell the shares in an unsolicited brokerage transaction at the market price. The availability for sale of substantial amounts of our common stock under Rule 144 or otherwise could reduce prevailing market prices for our common stock.
As of the date of this Prospectus, there is a total of 333,853,334 shares of our common stock reserved for issuance upon the exercise of outstanding warrants. All such shares constitute an overhang on the market for our common stock and, if and when issued, will be issued without transfer restrictions, pursuant to certain exemptions from registration, and could reduce prevailing market prices for our common stock. Also, in the future, we may also issue securities in connection with our obtaining needed capital or an acquisition transaction. The amount of shares of our common stock issued in connection with any such transaction could constitute a material portion of our then-outstanding shares of common stock.
The outstanding shares of our Series A Preferred Stock and Series B Preferred Stock could have the effect of precluding current and future owners of our common stock from influencing any corporate decision. Two of our officers, Thomas J. Wood and Russell F. Bird, own, collectively, 100% of the outstanding shares of our Series A Preferred Stock and Series B Preferred Stock. Each share of the Series A Preferred Stock has the vote equivalency of 25 shares of our common stock on all matters submitted to the holders of our common stock and votes together with the holders of our common stock as a single class; each share of the Series B Preferred Stock has the vote equivalency of 100 shares of our common stock on all matters submitted to the holders of our common stock and votes together with the holders of our common stock as a single class. Messrs. Wood and Bird could, therefore, be able effectively to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. This control of the outstanding Series A Preferred Stock and Series B Preferred Stock may also delay or prevent a future change of control of our company at a premium price, if Messrs. Wood and Bird oppose it.
As an issuer of penny stock, the protection provided by the federal securities laws relating to forward-looking statements does not apply to us. Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection, in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
Risks Relating to This Offering
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our common stock. The Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our Common Stock, which may have the effect of reducing the level of trading activity in our common stock. As a result, fewer broker-dealers may be willing to make a market in our common stock, reducing a stockholder’s ability to resell shares of our common stock.
State securities laws may limit secondary trading, which may restrict the states in which you can sell the shares offered by this Prospectus. If you purchase shares of our common stock sold in this Offering, you may not be able to resell the shares in any state unless and until the shares of our common stock are qualified for secondary trading under the applicable securities laws of such state or there is confirmation that an exemption, such as listing in certain recognized securities manuals, is available for secondary trading in such state. There can be no assurance that we will be successful in registering or qualifying our common stock for secondary trading, or identifying an available exemption for secondary trading in our common stock in every state. If we fail to register or qualify, or to obtain or verify an exemption for the secondary trading of, our common stock in any particular state, our common stock could not be offered or sold to, or purchased by, a resident of that state. In the event that a significant number of states refuse to permit secondary trading in our common stock, the market for our common stock will be limited which could drive down the market price of our common stock and reduce the liquidity of the shares of our common stock and a stockholder’s ability to resell shares of our common stock at all or at current market prices, which could increase a stockholder’s risk of losing some or all of his, her or its investment.
USE OF PROCEEDS
This Prospectus relates to up to the 500,000,000 Shares that may be offered and sold from time to time by the Selling Stockholder. We will receive no proceeds from the sale of the Shares by the Selling Stockholder in this Offering. The proceeds from the sales will belong to the Selling Stockholder. However, we will receive proceeds from the sale of the Purchase Shares to the Selling Stockholder pursuant to the JanBella Agreement.
We intend to use such proceeds, if any, for marketing and advertising, lab studies/testing, research and development, regulatory compliance, inventory, professional fees, general corporate purposes and working capital requirements. There can be no assurance that we will sell any of the Purchase Shares.
We cannot provide any assurance that we will be able to sell any of the Purchase Shares, such that the proceeds received would be a source of financing for us.
We intend to raise additional capital through equity and debt financing, as needed, though there cannot be any assurance that such funds will be available to us on acceptable terms, on an acceptable schedule, or at all.
DETERMINATION OF THE OFFERING PRICE
The Selling Stockholder will offer the Shares at the prevailing market prices or privately negotiated prices. The offering price of the Shares does not necessarily bear any relationship to our book value, assets, past operating results, financial condition or any other established criteria of value. Our common stock may not trade at the market prices in excess of the offering prices for the Shares in any public market, will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for our common stock.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock is currently quoted on the OTC PINK tier of the OTC Markets under the symbol “RMSL.” Trading in OTC PINK stocks can be volatile, sporadic and risky, as thinly traded stocks tend to move more rapidly in price than more liquid securities. Such trading may also depress the market price of our common stock and make it difficult for our stockholders to resell their common stock.
The following table reflects the high and low closing price for our common stock for the periods indicated. The information was obtained from the OTC Markets Group, Inc. and reflects inter-dealer prices, without retail mark-up, markdown or commission, and may not necessarily represent actual transactions.
| Year Ending December 31, 2022 | | High | | | Low | |
| March 31, 2022 | | $ | 0.0267 | | | $ | 0.012 | |
| | | | | | | | | |
| Year Ended December 31, 2021 | | | | | | | | |
| March 31, 2021 | | $ | 0.015 | | | $ | 0.004 | |
| June 30, 2021 | | $ | 0.047 | | | $ | 0.007 | |
| September 30, 2021 | | $ | 0.028 | | | $ | 0.009 | |
| December 31, 2021 | | $ | 0.033 | | | $ | 0.01 | |
| | | | | | | | | |
| Year Ended December 31, 2020 | | | | | | | | |
| March 31, 2020 | | $ | 0.034 | | | $ | 0.006 | |
| June 30, 2020 | | $ | 0.009 | | | $ | 0.007 | |
| September 30, 2020 | | $ | 0.009 | | | $ | 0.003 | |
| December 31, 2020 | | $ | 0.005 | | | $ | 0.001 | |
On June 10, 2022, the closing price of our Common Stock was $0.0187.
Shareholders of Record
As of June 10, 2022, we had 1,461,616,601outstanding shares of common stock and there were approximately 4,162 record holders of our common stock. The number of record holders does not include persons who hold our common stock in nominee or “street name” accounts through brokers.
Dividends
We have never declared or paid a cash dividend. At this time, we do not anticipate paying dividends in the future. We are under no legal or contractual obligation to declare or to pay dividends, and the timing and amount of any future cash dividends and distributions is at the discretion of our Board of Directors and will depend, among other things, on our future after-tax earnings, operations, capital requirements, borrowing capacity, financial condition and general business conditions. We plan to retain any earnings for use in the operation of our business and to fund future growth. You should not purchase our common stock on the expectation of future dividends.
SELLING STOCKHOLDER
On May 10, 2022, we entered into a Purchase Agreement (the JanBella Agreement) with JanBella Group, LLC (JanBella or the Selling Stockholder), pursuant to which, and upon the terms and subject to the conditions thereof, JanBella is committed to purchase, on an unconditional basis, up to 500,000,000 shares of our common stock (the “Purchase Shares”) over the course of its term. The term of the JanBella Agreement will end on the earlier of (a) the date on which the Selling Stockholder has purchased all of the 500,000,000 Shares pursuant to the JanBella Agreement, (b) May 10, 2025, (c) written notice of termination by us, (d) the date the Registration Statement is no longer effective, or (e) the date that, pursuant to or within the meaning of any Bankruptcy Law, we commence a voluntary case or any person commences a proceeding against us, a custodian is appointed for us or for all or substantially all of our property or we make a general assignment for the benefit of creditors.
From time to time over the term of the JanBella Agreement, commencing on the date the Registration Statement registering the Shares becomes effective, we may, in our sole discretion, provide JanBella with a purchase notice (each a “Purchase Notice”) to purchase a specified number of Purchase Shares (each a “Purchase Amount Requested”) subject to the limitations discussed below and contained in the JanBella Agreement. Upon delivery of a Purchase Notice, we must deliver the Purchase Amount Requested as Deposit Withdrawal at Custodian (DWAC) shares to the Selling Stockholder within one trading day.
The actual amount of proceeds we receive pursuant to each Purchase Notice (each, the “Purchase Amount”) is determined by multiplying the Purchase Amount Requested by the applicable purchase price. The Purchase Price for each of the Put Shares equals 75% of the lowest traded price of our common stock during the Valuation Period. The Valuation Period is the ten (10) consecutive business days immediately preceding, but not including, the date a Purchase Notice is delivered. Should we uplist our common stock on The Nasdaq Stock Market or an equivalent national exchange, the Purchase Price shall be set at 80% of the lowest traded price of the our common stock during the Valuation Period, subject to a floor of $0.01, per share (subject to adjustments for stock splits, dividends, and similar occurrences), below which we shall not deliver a Purchase Notice. JanBella will deliver the Purchase Amount to us on the Settlement Date. The Settlement Date is the date on which the Purchase Shares are confirmed as being received by JanBella’s broker, against the payment of the Purchase Price by JanBella, which date will be five (5) business days following the Valuation Period.
The Purchase Amount requested pursuant to any Purchase Notice must have an aggregate value of at least $20,000 and cannot exceed the lesser of (1) $150,000 or (2) 100% of the average daily volume traded for our common stock during the relevant Valuation Period (subject to adjustments for stock splits, dividends, and similar occurrences).
In order to deliver a Purchase Notice, certain conditions set forth in the JanBella Agreement must be met. In addition, we are prohibited from delivering a Purchase Notice if the sale of Purchase Shares pursuant to such Purchase Notice would cause us to issue and sell to the Selling Stockholder, or the Selling Stockholder to acquire or purchase, a number of shares of our common stock that would result in Selling Stockholder beneficially owning more than 4.99% of the issued and outstanding shares of our common stock.
By the terms of the JanBella Agreement, we agreed to file a registration statement to register the resale of the Purchase Shares. We agreed to (A) file the Registration Statement, (B) use reasonable efforts to cause the Registration Statement to be declared effective under the Securities Act of 1933, as amended, as promptly as possible after the filing thereof, and (C) use our reasonable efforts to keep the Registration Statement continuously effective under the Securities Act until all of the Shares have been sold thereunder or pursuant to Rule 144.
J.H. Darbie & Co., a FINRA-registered broker, acted as a finder in connection with the execution and delivery of the JanBella Agreement. We will pay J.H. Darbie & Co. a finder’s fee equal to 3% of the gross proceeds from sales of Purchase Shares under the JanBella Agreement.
The issuance and sale of the Shares by us to JanBella under the JanBella Agreement was made without registration under the Securities Act of 1933, as amended (the “Act”), or the securities laws of the applicable state, in reliance on the exemptions provided by Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder, and in reliance on similar exemptions under applicable state law, based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, the representations of JanBella to us that, among others, it was an accredited investor (as that term is defined in Rule 501(a) of Regulation D), and that it was purchasing the shares for its own account and without a view to distribute them.
The Selling Stockholder may dispose of the Shares covered by this Prospectus from time to time at such prices as it may choose. The following table provides as of the date of this Prospectus, information regarding the beneficial ownership of our common stock held by the Selling Stockholder and the percentage owned by the Selling Stockholder. Assuming all of the Shares are sold by the Selling Stockholder, the Selling Stockholder will not own one percent or more or our common stock.
Selling Stockholder | | Beneficial Ownership Before the Offering(1) | | | Number of Shares Being
Offered | | | Beneficial Ownership After the Offering | | | Percentage of Ownership After the Offering | |
| JanBella Group, LLC(2) | | | 0 | | | | 500,000,000 | (3) | | | 0 | (4) | | | 0 | % |
| (1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to shares of common stock. Shares of common stock subject to options, warrants and convertible debentures currently exercisable or convertible, or exercisable or convertible within 60 days, are counted as outstanding. The actual number of shares of common stock issuable upon the conversion of the convertible debentures is subject to adjustment depending on, among other factors, the future market price of our common stock, and could be materially less or more than the number estimated in the table. |
| (2) | William Alessi, the managing member of JanBella Group, LLC, has sole voting and dispositive power over the shares of our common stock held by, or issuable to, JanBella Group, LLC. The principal business address of JanBella Group, LLC is 20311 Chartwell Center Drive, Unit 1469, Cornelius, North Carolina 28031. |
| (3) | Represents 500,000,000 shares of our common stock to be sold by the Selling Stockholder pursuant to the JanBella Agreement. |
| (4) | Because the Selling Stockholder may offer and sell all or only some portion of the 500,000,000 shares of our common stock being offered pursuant to this prospectus and may acquire additional shares of our common stock in the future, we can only estimate the number and percentage of shares of our common stock that the selling stockholder will hold upon termination of the offering. |
The Selling Stockholder has not had a material relationship with us or any of our affiliates, other than as a stockholder, at any time within the past three years.
PLAN OF DISTRIBUTION
The Selling Stockholder and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their Shares covered hereby on any trading market, stock exchange or other trading facility on which our common stock is traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholder may use any one or more of the following methods when selling securities:
| | ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| | | |
| | ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| | | |
| | ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| | | |
| | ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| | | |
| | ● | privately negotiated transactions; |
| | | |
| | ● | settlement of short sales; |
| | | |
| | ● | in transactions through broker-dealers that agree with the Selling Stockholder to sell a specified number of such securities at a stipulated price per security; |
| | | |
| | ● | through the writing or settlement of options or other hedging transactions, whether trough an options exchange or otherwise; |
| | | |
| | ● | a combination of any such methods of sale; or |
| | | |
| | ● | any other method permitted pursuant to applicable law. |
The Selling Stockholder may also sell securities under Rule 144 under the Securities Act, if available, rather than under this Prospectus.
Broker-dealers engaged by the Selling Stockholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the Shares covered hereby, the Selling Stockholder may enter into hedging transactions with broker-dealers or other financial institutions, which may, in turn, engage in short sales of our common stock in the course of hedging the positions they assume. The Selling Stockholder may also sell our common stock short and deliver the Shares to close out its short positions, or loan or pledge the securities to broker-dealers that, in turn, may sell these Shares. The Selling Stockholder may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of the Shares offered by this Prospectus, which Shares such broker-dealer or other financial institution may resell pursuant to this Prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholder and any broker-dealers or agents that are involved in selling the Shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the Shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. We are requesting that the Selling Stockholder inform us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the Shares. We will pay certain fees and expenses incurred by us incident to the registration of the Shares.
Because the Selling Stockholder may be deemed to be an “underwriter” within the meaning of the Securities Act, it will be subject to the prospectus delivery requirements of the Securities Act, including Rule 172 thereunder. In addition, any of the Shares covered by this Prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this Prospectus. We are requesting that the Selling Stockholder confirm that there is no underwriter or coordinating broker acting in connection with the proposed sale of the resale Shares by the Selling Stockholder.
We intend to keep this Prospectus effective until the earlier of (a) the date on which the Shares may be resold by the Selling Stockholder without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for us to be in compliance with the current public information requirement under Rule 144 under the Securities Act or any other rule of similar effect or (b) all of the Shares have been sold pursuant to this Prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale Shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale Shares covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale Shares may not simultaneously engage in market making activities with respect to our common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholder will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Shares by the Selling Stockholder or any other person. We will make copies of this Prospectus available to the Selling Stockholder and are informing the Selling Stockholder of the need to deliver a copy of this Prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL PROCEEDINGS
General
Except as discussed, below, there are no pending legal proceedings to which our company is a party or in which any director, officer or affiliate of our company, any owner of record or beneficially of more than 5% of any class of voting securities of our company, or shareholder is a party adverse to us or has a material interest adverse to us.
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. While the Company was under previous management, the loans were removed from the books in first quarter of 2015. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. The $50,000 plus $7,341 was booked to retained earnings in 2016 as a correction of an error. As of March 31, 2022, there is $45,000 and $22,140 of principal and interest due on this loan, respectively.
BUSINESS
Background
We were incorporated in the State of Nevada on June 6, 2007. On August 2, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC in exchange for 50,000,000 common shares of REMSleep Holdings, Inc.’s stock, at which time REMSleep LLC became our wholly-owned subsidiary and adopted their business of developing and distributing our sleep apnea products. On January 5, 2015, we changed our name to REMSleep Holdings, Inc. to reflect our new business model.
Our principal executive offices are located at 14175 Icot Boulevard, Suite 300, Clearwater, Florida 33760; our telephone number is (813) 367-3855; our corporate website is located at www.remsleep.com. No information found on, or connected to, our company’s website is incorporated by reference into, any you must not consider the information to a part of, this Prospectus.
Overview
In May 2017, we applied for a patent with the US Patent and Trademark Office for our proprietary DeltaWave CPAP interface (“DeltaWave”), a new, innovative sleep apnea product to act as an interface for the delivery of CPAP therapy and other respiratory needs. DeltaWave is a nasal-pillow type interface designed to offer better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally.
Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our officers invented the DeltaWave as an innovative new device to treat patients with sleep apnea. The patent-pending DeltaWave device is a nasal-pillows type interface that will result in better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally.
A survey that appeared in DME Business found that 89% of patients stated that mask-interface comfort was their primary concern. The primary issue that we have addressed with the DeltaWave is the “work of breathing” component. We believe that our DeltaWave is designed to effectively address the stubborn issues that continue to affect a patient’s ability to comply with treatment, as follows:
| | ● | does not disrupt normal breathing mechanics; |
| | | |
| | ● | is not claustrophobic; |
| | | |
| | ● | causes zero work of breathing (WOB); |
| | | |
| | ● | minimizes or eliminates drying of the sinuses; |
| | | |
| | ● | uses less driving pressure; and |
| | | |
| | ● | allows users to feel safe and secure while sleeping. |
Clinical Trials
We have, on an informal basis, done extensive testing of our DeltaWave CPAP device. However, to date, we have not conducted any formal clinical trials. We intend to apply a portion of the proceeds from the sale of the Purchase Shares to the Selling Stockholder, if any, to establishing and conducting clinical trials of our DeltaWave CPAP device.
Market Opportunity
Sleep apnea is a serious and chronic disease that negatively impacts a patient’s sleep, health and quality of life. According to the World Health Organization, sleep apnea affects more than 100 million people worldwide. Left untreated, sleep apnea increases the risk of high blood pressure, hypertension, heart failure, stroke, coronary artery disease and other life-threatening diseases.
Ventilation is commonly used in the treatment of respiratory conditions such as sleep apnea. On such form of ventilation is a continuous positive air pressure system often referred to as a CPAP system which typically includes a mask that interfaces with a user’s mouth or nasal passages, supplying air pressure from an air flow generator that is typically located in the proximity of the user. The mask directs the flow of air into the breathing passage of the user while allowing the user to exhale.
If a user suffers from claustrophobia, a mask that fits over the mouth might be upsetting. Also, men with facial hair may have trouble getting a seal with a standard CPAP mask that fits over the nose or around the mouth. Nasal pillows eliminate these concerns by applying CPAP pressure directly into the user’s nostrils. Nasal pillows are a type of CPAP mask consisting of plastic inserts that look like headphone earbuds that slip directly into the nostrils. The prescribed pressure used to keep the airway open is delivered through this mask. Furthermore, some people prefer nasal pillows because they do not leave marks on the face from either a mask interface or the straps needed to keep the mask in place.
Many people find nasal pillows to be a favorable option for the administration of CPAP to treat their sleep apnea. However, it is important that nasal pillows are properly sized. If they are too small, air may leak out around them and reduce the effectiveness of treatment. Conversely, if the nasal pillows are too large, they may uncomfortably stretch the nostrils. Proper fitting will address most of the issues.
For the treatment of sleep apnea, the CPAP system is worn while the user sleeps. In CPAP systems where the mask interfaces with the user’s nostrils, the nostril interface need be held in place and maintain a seal so that, as the user moves during sleep, the mask/nostril interface remains intact and sealed to provide positive airway pressure. The mask/nostril interface is often referred to as pillow interfaces that are inserted partially into each nostril, maintaining an air-tight seal. As the pillow interfaces are worn while the user sleeps, it is important that the pillow interfaces be as comfortable as possible. If the seal is not air-tight, less positive airway pressure is delivered and, the break in the seal will often create noise that may impact the user’s sleep.
Another issue with continuous positive air pressure system is the flow velocity of the positive airway pressure. In providing an adequate volume of air for each breath the patient takes (inhalation), the CPAP system must provide a minimum volume per time period. Tradeoffs are made between providing greater volumes by using larger orifices or by using smaller orifices and increasing the velocity of the provided air. Unfortunately, when the velocity of the provided air is increased, several user issues occur including burning sensations in the user’s sinuses, drying of the user’s sinuses, a compromised therapeutic index, and increased noise that is undesirable when the user is trying to sleep.
Existing CPAP systems use nasal pillow interfaces that are round. These pillow interfaces cause discomfort when worn as the nasal septum is typically more sensitive to pressure and is typically relatively flat in areas of the nasal openings. Round cross-sectional shapes do not interface well with flat walls and, as an accommodation, prior pillow interfaces are typically made of a soft material that flattens when inserted. This flattening reduces the cross-sectional area of these prior nasal pillows, resulting in an acceleration of the air velocity and noise. The increase in air flow velocity leads to dryness, a sudden burning sensation in the nostrils, and stuffiness in the sinuses. Higher air flow velocity and lower volume of incoming air is less efficient at correcting apnea and often interrupts normal breathing patterns. DeltaWave is designed to deliver more air volume with less driving pressure. Therefore, the patient gets more air coming into the upper airways at a slower air velocity. Also, the pillows that fit into the nostrils are designed to better fit the shape of the nostrils for better comfort and better airtight seal.
Further, most prior interface pillow designs included turning points for the incoming air, some as much as 90 degrees, resulting in turbulence, pressure drop, and a decrease in the therapeutic index of the treatment. As a result of this, more pressure is typically required to deliver the required increased flow rate which partially compensates for inadequate air volume needed for treatment. This increase in air velocity increases unwanted side effects. An increase in air flow velocity is not preferred over supplying adequate air volume. Another issue with existing CPAP systems is disposal of extra pressure, either from the air flow generator or from the user’s exhalation. Many existing CPAP systems have inadequate exit venting or exit venting that is directed toward the user’s body, creating discomfort as the user exhales. DeltaWave is designed to deliver air flow to the nostrils without using any sharp turning points for the incoming air. DeltaWave is also designed to avoid any choke points or areas where the air flow would be restricted, which would cause pressure drop in the system. Avoiding air flow turning points and avoiding restriction (choke) points create and assure laminar flow.
Our DeltaWave CPAP system is designed to provide interface pillows that seal within nostrils while retaining comfort and proper air flow.
Industry Background
The market for sleep treatment and equipment was $7.96 billion in 2011 and will reach a projected $19.72 billion by 2017, with North America accounting for a majority of the market. More than 8 million CPAP interfaces are sold annually in the U.S., with another 2.5 million globally. There are also an estimated 80 million people with undiagnosed sleep apnea. Sleep apnea is a condition that affects millions of people in the United States alone. An increasingly sedentary lifestyle and bad working habits has led to obesity and otherwise poor cardiac and aerobic health. This has led to a fast-growing epidemic of obstructive sleep apnea (sleep apnea), which greatly reduces the quality of sleep one gets and can ultimately result in hypertension, heart failure, stroke, and at the least, reduced performance in everyday life. Sleep apnea results in numerous afflictions that affect people’s day-to-day lives and can eventually contribute to serious health conditions. While people’s knowledge of this affliction has grown strongly in recent years, and the market is expanding fast nationwide, up to 80% of people with sleep apnea may be undiagnosed (Note 1) – a market of millions of new potential users. Even those who are tested and prescribed a sleep apnea machine often give up after a short time due to discomfort or what is called the “work of breathing” with traditional machines. In fact, over 50% of patients give up on using CPAP therapy after 6 months. This is a major waste of resources and a very telling statistic.
A major challenge in the current market is not only to get more patients diagnosed but to also increase CPAP compliance. According to market analyst Frost & Sullivan, “The development of finer and ergonomic CPAP devices will help increase patient ability to adhere to sleep therapy. The market is also seeing a rise in newer technologies that replace elaborate practices, target patient comfort to improve compliance, and help drive acceptance of sleep monitoring devices.”
A growing knowledge of sleep apnea and its treatment has helped to increase awareness with the public. In addition to making the use of a CPAP or related device less intimidating, a move toward affordable and prescription-based technology can greatly expand the market “Evolving technologies will also influence patient preferences for products, treatment modalities, and diagnostic locations,” states Frost & Sullivan (Note 2). “As such, the global sleep apnea treatment market is expected to shift to home-based diagnostics for early identification and treatment of patients as well as portable devices that can reduce sleep apnea with minimal inconvenience.”
Sleep apnea causes breathing interruptions of between 10 to 20 seconds that can occur hundreds of times during a night, disrupting the natural sleep rhythm and depriving people of the restorative sleep they need to be energetic, mentally sharp, and productive the next day. CPAP can be a very effective method used to treat sleep apnea, but as noted, noncompliance remains a stubborn issue for both physicians and patients. CPAP technology therefore is constantly being updated and improved, and the new CPAP devices are lighter, quieter, and more comfortable.
Health care spending continues to grow rapidly on an annual basis in the United States. Spending was $2.7 trillion in 2011 and, in 2013, it reached over $3.6 trillion. By 2022, spending is projected to reach $5 trillion, or around 20% of GDP, according to the Centers for Medicare and Medicaid Services (Note 3). Growing alongside this market is the U.S. life science industry, which will grow an estimated 2.2% in 2014 to $93 billion. This includes R&D spending, with growth primarily from smaller biopharmaceutical innovators and medical device manufacturers.
Within this market, sleep apnea products have experienced rapid growth. In the past couple of decades there has been a rapid increase in the technological developments in the field of sleep apnea diagnosis and treatment. The result has been strong growth for sleep apnea devices globally. Demand for new and innovative treatment methodologies is driving growth, helping to provide patients with a healthy lifestyle. “Obstructive sleep apnea is destroying the health of millions of Americans, and the problem has only gotten worse over the last two decades,” according to American Academy of Sleep Medicine President Dr. Timothy Morgenthaler (Note 4). “The effective treatment of sleep apnea is one of the keys to success as our nation attempts to reduce health care spending and improve chronic disease management.”
Sleep problems are considered a “global epidemic,” with sleep apnea as a major contributor to the disorder. An estimated 100 million people worldwide have sleep apnea, though more than 80% of these people are undiagnosed. The market for sleep apnea diagnostic and therapeutic devices on a global level was $7.96 billion in 2011 and will reach a projected $19.72 billion by 2017, according to a study from Markets & Markets (Note 5). Nationwide in the U.S., there are more than 1,600 businesses in the Sleep Disorder Clinics market, according to research firm IBISWorld. These businesses have combined annual revenue of $7 billion and have maintained a combined annual growth rate (CAGR) of 9.8% from 2008 to 2013. “Sleep clinics have gained exposure during the period due to the rising number of sleep disorders,” states IBISWorld. “Moreover, health insurance policies are increasingly covering all or at least part of the costs of tests and, as more patients have been able to gain greater access to specialized sleep clinics, industry revenue grows.”
There are also more than 972,000 physicians and 365,000 doctors’ offices, as well as nearly 5,800 hospitals. In addition, the market for U.S. home healthcare is served by about 30,000 businesses with combined annual revenue of $59 billion. The market includes medical and skilled nursing services; medical equipment, supplies, and medication services; personal care; and therapeutic services (like physical and respiratory therapy).
| Sources: | |
| | | |
| | Note 1. | Markets & Markets. “Global Sleep Apnea Diagnostics & Therapeutic Devices Market.” |
| | | http://www.marketsandmarkets.com/PressReleases/sleep-apnea-devices.asp |
| | | |
| | Note 2. | Frost & Sullivan. “Sleep apnea market is in need of finer, ergonomic treatments.” June 4, 2014. |
| | | http://www.frost.com/prod/servlet/press-release.pag?docid=290951848 |
| | | |
| | Note 3. | Forbes. “Annual U.S. Healthcare Spending Hits $3.8 Trillion.” Feb. 2, 2014. |
| | | http://www.forbes.com/sites/danmunro/2014/02/02/annual-u-s-healthcare-spending-hits-3-8-trillion/ |
| | | |
| | Note 4. | American Academy of Sleep Medicine. “Rising prevalence of sleep apnea in U.S. threatens public health.” Sept. 2014. |
| | | http://www.aasmnet.org/articles.aspx?id=5043 |
| | | |
| | Note 5. | Markets & Markets. “Global Sleep Apnea Diagnostics & Therapeutic Devices Market.” |
| | | http://www.marketsandmarkets.com/PressReleases/sleep-apnea-devices.asp |
Marketing
We plan to market the DeltaWave device in the U.S., as follows:
| | ● | Submit manufacture orders to our manufacturer according to market demand; |
| | | |
| | ● | Negotiate and secure agreements with industry distributor partners; |
| | | |
| | ● | Secure agreements with Internet retailers for online sales; |
| | | |
| | ● | Market DeltaWave at respiratory trade shows, social media, press releases; |
| | | |
| | ● | Market and generate online sales through our website supplemented by search engine optimization; |
| | | |
| | ● | Disseminate press releases to media outlets and publications that reach sleep medical practices and DME managers/ distributors, including trade publications like Sleep Medicine, Sleep Review, Sleep, The Sleep Magazine; and |
| | | |
| | ● | Attend sleep and healthcare, respiratory industry trade shows. |
Our ability to engage in the foregoing activities is contingent upon adequate financing, of which there is no assurance.
Target Market
Our target market includes:
| | ● | Sleep product distributors that will distribute our product; |
| | | |
| | ● | Home care dealers; |
| | | |
| | ● | Private sleep labs; |
| | | |
| | ● | Internet providers; |
| | | |
| | ● | Product end users; |
| | | |
| | ● | Physicians, particularly sleep physicians; |
| | | |
| | ● | Medical groups; |
| | | |
| | ● | Hospitals; and |
| | | |
| | ● | Medical associations, such as the American Academy of Sleep Medicine and the American Sleep Association. |
We expect that most of our revenues will be in the home care dealers and hospital target market.
Manufacturing
Our product will be manufactured by mold makers. We presently have molds for prototypes made in China; however, we are considering relocating the manufacturing of our molds to the United States.
Operations Contingent Upon Adequate Financing
Our entire business plan, including our ability to conduct manufacturing, marketing, generate sales and further develop products, are entirely dependent upon adequate financing. Should we fail to obtain adequate financing: (a) our financial condition will be negatively affected; (b) we will be unable to conduct the essential aspects of our business plan, including marketing as reflected above; (c) investments in our common stock will be negatively impacted; (d) we will be forced to liquidate our business and file for bankruptcy protection.
Competition
The sleep apnea devices market is highly consolidated, with primary competitors being ResMed, Philips Respironics, Naus Medical, Fisher & Paykel Healthcare, DeVilbiss Healthcare, CareFusion, InnoMed, DeVilbiss and TAP. ResMed is the market leader (45% of market share), followed by Philips (30%), and Fisher/Paykel (12%). Our competitors offer a full range of sleep products.
Our competitors have greater financial, operational and personnel resources than we do. We will attempt to overcome our competitors’ competitive advantages by emphasizing the advantages of our Delta Wave product.
Government Regulations
FDA. Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil and administrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter supply contracts, and criminal prosecution.
Unless an exemption applies, the FDA requires that a manufacturer introducing a new medical device or a new indication for use of an existing medical device obtain either a Section 510(k) premarket notification clearance or a premarket approval, or PMA, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness.
Our products currently marketed in the United States are marketed in reliance on 510(k) pre-marketing clearances as either Class I or Class II devices. The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and often clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to a device that was on the market before 1976 or to a device that has been found by the FDA to be “substantially equivalent” to such a pre-1976 device, a predecessor device is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In addition, in some cases, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices or those that are used to support or sustain human life, may take several years and requires the submission of extensive performance and clinical information.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties. The FDA is currently reviewing its guidance describing when it believes a manufacturer is obligated to submit a new 510(k) for modifications or changes to a previously cleared device. The FDA is expected to issue revised guidance to assist device manufacturers in making this determination. It is unclear whether the FDA’s approach in this new guidance will result in substantive changes to existing policy and practice regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices.
Any devices we manufacture and distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions. As a medical device manufacturer, our manufacturing facilities are subject to inspection on a routine basis by the FDA. We are required to adhere to applicable regulations setting forth detailed cGMP requirements, as set forth in the QSR, which require, manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process. Noncompliance with these standards can result in, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, refusal of the government to grant 510(k) clearance or PMA approval of devices, withdrawal of marketing approvals and criminal prosecutions. We believe that our design, manufacturing and quality control procedures are in compliance with the FDA’s regulatory requirements.
We must also comply with post-market surveillance regulations, including medical device reporting, or MDR, requirements which require that we review and report to the FDA any incident in which our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Medical devices approved or cleared by the FDA may not be promoted for unapproved or un-cleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Other Healthcare Laws. Even though we do not submit claims or bill governmental programs and other third-party payers directly for reimbursement for our products sold in the United States, we are still subject to laws and regulations that may restrict our business practices, including, without limitation, anti-kickback, false claims, physician payment transparency and data privacy and security laws. The government has interpreted these laws broadly to apply to the marketing and sales activities of manufacturers and distributors like us.
The federal Anti-Kickback Statute prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. The civil False Claims Act also applies to false submissions that cause the government to be paid less than the amount to which it is entitled, such as a rebate. Intent to deceive is not required to establish liability under the civil False Claims Act.
The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them to have committed a violation.
Also, many states and countries outside the U.S. have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs.
Under HIPAA, the Department of Health and Human Services, or HHS, has issued regulations to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties. In addition to federal privacy and security regulations, there are state laws governing confidentiality and security of health information that are applicable to our business. New laws governing privacy may be adopted in the future as well. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Additionally, there has been a recent trend of increased federal and state regulation of payments and transfers of value provided to healthcare professionals or entities. The Physician Payment Sunshine Act was enacted in law as part of PPACA, which imposed new annual reporting requirements on device manufacturers for payments and other transfers of value provided by them, directly or indirectly, to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties. Certain states also mandate implementation of commercial compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities.
The shifting commercial compliance environment and the need to build and maintain robust systems to comply with different compliance or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may fail to comply fully with one or more of these requirements. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Environmental Regulation
Our operations are not subject to environmental regulation.
Properties
We own no real property. We lease a small office that is adequate for our current level of operations.
Employees
We have the following employees Thomas J. Wood, Chief Executive Officer, and John Lane, Chief Technology Officer. All other services are provided by independent contractors who are primarily paid with stock-based compensation. Personnel will be added on an as-needed basis and based on available funds.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of our operations together with our consolidated financial statements and the notes thereto appearing elsewhere in this Prospectus. This discussion contains forward-looking statements reflecting our current expectations, whose actual outcomes involve risks and uncertainties. Actual results and the timing of events may differ materially from those stated in or implied by these forward-looking statements due to a number of factors, including those discussed in the sections entitled “Risk Factors,” “Cautionary Statement Regarding Forward-Looking Statements” and elsewhere in this Prospectus. Please see the notes to our Financial Statements for information about our Critical Accounting Policies and Recently Issued Accounting Pronouncements.
Forward-looking Statements
There are “forward-looking statements” contained herein. All statements that express expectations, estimates, forecasts or projections are forward-looking statements. In addition, other written or oral statements which constitute forward-looking statements may be made by us or on our behalf. Words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “estimate,” “project,” “forecast,” “may,” “should,” and variations of such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions which are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or forecasted in or suggested by such forward-looking statements. We undertake no obligation to update or revise any of the forward-looking statements after the date of this quarterly report to conform forward-looking statements to actual results. Important factors on which such statements are based are assumptions concerning uncertainties, including but not limited to, uncertainties associated with the following:
| | ● | Inadequate capital and barriers to raising the additional capital or to obtaining the financing needed to implement our business plans; |
| | | |
| | ● | Our failure to earn revenues or profits; |
| | | |
| | ● | Inadequate capital to continue business; |
| | | |
| | ● | Volatility or decline of our stock price; |
| | | |
| | ● | Potential fluctuation in quarterly results; |
| | | |
| | ● | Rapid and significant changes in markets; |
| | | |
| | ● | Litigation with or legal claims and allegations by outside parties; and |
| | | |
| | ● | Insufficient revenues to cover operating costs. |
The following discussion should be read in conjunction with the financial statements and the notes thereto which are included in this quarterly report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ substantially from those anticipated in any forward-looking statements included in this discussion as a result of various factors.
Overview
We were incorporated in the State of Nevada on June 6, 2007. On August 26, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of RemSleep LLC in exchange for 50,000,000 shares of common stock of RemSleep Holdings, Inc. at which time RemSleep LLC became our wholly-owned subsidiary and we adopted their business of developing and distributing sleep apnea products. On January 5, 2015, we changed our name to REMSleep Holdings, Inc. to reflect our new business model.
Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our officers invented our DeltaWave CPAP interface (the “DeltaWave”) as an innovative new device to treat patients with sleep apnea. The patent-pending DeltaWave product is a nasal-pillows type interface that will result in better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally. A survey that appeared in DME Business found that 89% of patients stated that mask-interface comfort was their primary concern. The primary issue that we have addressed with the DeltaWave is the “work of breathing” component. We believe that our DeltaWave is designed to effectively address the stubborn issues that continue to affect a patient’s ability to comply with treatment, as follows:
| | ● | Does not disrupt normal breathing mechanics; |
| | | |
| | ● | Is not claustrophobic; |
| | | |
| | ● | Causes zero work of breathing (WOB); |
| | | |
| | ● | Minimizes or eliminates drying of the sinuses; |
| | | |
| | ● | Uses less driving pressure; and |
| | | |
| | ● | Allows users to feel safe and secure while sleeping. |
Pending adequate financing, of which there is no assurance, we plan to conduct clinical trials to test product effectiveness.
Effects of COVID-19
The COVID-19 pandemic had a discernable short-term negative impact on the ability of our company to obtain capital needed to accelerate the development of our business. While these limitations have eased, we are unable to predict when such limitations will be entirely resolved.
Overall, our company is not of a size that required us to implement “company-wide” policies in response to the COVID-19 pandemic.
For purposes of the discussion below, except where otherwise indicated, the descriptions of our business, our strategies, our risk factors and any other forward-looking statements, including regarding us, our business and the market generally, do not reflect the potential impact of the COVID-19 pandemic or our responses thereto.
Results of Operations
Three Months Ended March 31, 2022, Compared to Three Months Ended March 31, 2021. We have not generated any revenue to date.
Professional fees were $26,000 compared to $24,598 for the three months ended March 31, 2022 and 2021, respectively, an increase of $1,402, or 5.7%. Professional fees consist mostly of accounting, audit and legal fees. The increase is attributed to an increase in accounting and audit fees.
Compensation expense was $21,000 and $21,000 for the three months ended March 31, 2022 and 2021, respectively.
Development expense related to our DeltaWave CPAP system was $25,667 and $0 for the three months ended March 31, 2022 and 2021, respectively, an increase of $25,667. Development expense increased over the prior period as we worked to bring our product to market.
General and administrative expense (“G&A”) was $81,891 and $48,666 for the three months ended March 31, 2022 and 2021, respectively, an increase of $33,225, or 68.3%. During the current period we incurred additional expense (approximately $16,500) related to the process of obtaining our 510k for DeltaWave. We also incurred additional expense involved with moving our corporate headquarters.
Total other expense for the three months ended March 31, 2022, was $161,741. Other expense includes a gain in the change of fair value of $11,907 and interest expense of $173,648 (includes $159,383 amortization of debt discount). Total other expense for the three months ended March 31, 2021, was $377,202. Other expense includes a gain in the change of fair value of $395,148, a loss on the issuance of convertible debt of $442,979, a penalty for default on convertible debt of $162,798 and interest expense of $166,573 (includes $138,622 amortization of debt discount).
Net Loss. For the three months ended March 31, 2022, we had a net loss of $316,299 as compared to a loss of $471,466 for the three months ended March 31, 2021. Our net loss decreased due to the decrease in other expense during the period.
Results of Operations for the Year Ended December 31, 2021, Compared to the Year Ended December 31, 2020. We generated no revenues during our fiscal years ending December 31, 2021 and 2020.
Operating Expenses. Professional fees were $82,043 and $41,525 for the years ended December 31, 2021 and 2020, respectively, an increase of $40,518, or 97.6%. Professional fees consist mostly of accounting, audit and legal fees. The increase is attributed to an increase in legal and audit fees associated with the filing of our Regulation A offering. We had an increase of legal fees of approximately $22,100 and an increase of audit fees of approximately $14,900.
Development expense related to our DeltaWave CPAP system was $129,311 and $72,587 for the years ended December 31, 2021 and 2020, respectively, an increase of $56,724, or 78.1%. Our development expense has increased in 2021 as we get closer to completing the DeltaWave CPAP system.
Compensation expense was $84,000 and $211,500 for the years ended December 31, 2021 and 2020, respectively. In the prior we incurred non-cash stock compensation expense of $127,500. There was no stock compensation expense incurred in the current year.
General and administrative expense was $130,334 and $144,232 for the years ended December 31, 2021 and 2020, respectively, a decrease of $13,898, or 9.6%. The decrease is largely due to a decrease of investor relation services.
Total other expense for the years ended December 31, 2021, was $3,399,985. Other expense includes a loss in the change of fair value of $1,601,016, a loss on the issuance of convertible debt of $717,592, a penalty for default on convertible debt of $162,798 and interest expense of $918,579 (includes $813,619 amortization of debt discount). Total other expense for the year ended December 31, 2020, was $705,608. Other expense includes $611,535 of interest expense (includes $561,576 amortization of debt discount), a $350,986 loss on the issuance of convertible debt, an early payment penalty of $49,162, a gain on forgiveness of debt of $226,398 and a gain in the change of fair value of derivatives of $79,677. These are all expenses related to our convertible debt.
Net Loss. For the year ended December 31, 2021, we had a net loss of $3,825,673 as compared to a net loss of $1,175,452 for the year ended December 31, 2020. The increase in net loss can be attributed to our increase in other expense as discussed above.
Liquidity and Capital Resources
March 31, 2022.
Cash Flows from Operations. Cash used in operating activities for the three months ended March 31, 2022 was $199,085 as compared to $63,182 of cash used in operating activities for the three months ended March 31, 2021. During the current period the Company used more cash for activities related to bringing its product to market.
Cash Flows from Investing. Cash used in investing activities for the purchase of equipment and tooling for the three months ended March 31, 2022 was $24,095 as compared to $17,705 of cash used in investing activities for the three months ended March 31, 2021.
Cash Flows from Financing. For the three months ended March 31, 2022, we received $855,000 from the sale of common stock. For the three months ended March 31, 2021 we received $126,400 from convertible debt loans and repaid $867 on other loans.
As of March 31, 2022, we had an outstanding balance on one convertible promissory note. The table below sets forth information with respect to our convertible promissory notes during the three months ended March 31, 2022.
| Note Holder | | Date | | Maturity Date | | Interest | | | Balance 12/31/21 | | | Additions | | | Conversions/ Repayments | | | Balance 3/31/22 | |
| Granite Global Investments Ltd. | | 4/7/2021 | | 4/7/2021 | | | 10 | % | | | 36,500 | | | | --- | | | | (36,500 | ) | | | --- | |
| Granite Global Investments Ltd. | | 4/9/2021 | | 4/9/2021 | | | 10 | % | | | 100,000 | | | | --- | | | | (100,000 | ) | | | --- | |
| Power Up Lending Group LTD | | 7/22/2021 | | 7/22/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 8/26/2021 | | 8/26/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 9/22/2021 | | 9/22/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 10/12/2021 | | 10/12/2021 | | | 10 | % | | | 86,350 | | | | --- | | | | --- | | | | 86,350 | (1) |
| | | | | | | | Total | | | $ | 399,400 | | | $ | --- | | | $ | (313,050 | ) | | $ | 86,350 | |
| (1) | This note was converted in full on April 15, 2022. |
December 31, 2021.
Cash Flows from Operations. Cash used in operating activities for the year ended December 31, 2021 was $349,995 as compared to $335,293 of cash used in operating activities for the year ended December 31, 2020.
Cash Flows from Investing. Cash used in investing activities for the purchase of equipment and tooling for the year ended December 31, 2021 was $67,252 as compared to $36,710 of cash used in investing activities for the year ended December 31, 2020.
Cash Flows from Financing. For the year ended December 31, 2021, we received $591,300 from the issuance of convertible debt and $3,103,500 from the sale of common stock. We repaid $8,212 on our auto loan. For the year ended December 31, 2020, we received $460,000 from convertible debt loans and repaid $165,000. We also received $75,000 from the sale of common stock and repaid $3,344 on our auto loan.
Going Concern
As of March 31, 2022, there is substantial doubt regarding our ability to continue as a going concern as we have not generated sufficient cash flow to fund our proposed business.
We have suffered recurring losses from operations since our inception. In addition, we have yet to generate an internal cash flow from our business operations or successfully raised the financing required to develop our proposed business. As a result of these and other factors, our independent auditor has expressed substantial doubt about our ability to continue as a going concern. Our future success and viability, therefore, are dependent upon our ability to generate capital financing. The failure to generate sufficient revenues or raise additional capital may have a material and adverse effect upon us and our shareholders.
Management’s plans with regard to these matters encompass the following actions: (i) obtaining funding from new investors to alleviate our working capital deficiency, and (ii) implementing a plan to generate sales. Our continued existence is dependent upon our ability to resolve our liquidity problems and increase profitability in our current business operations. However, the outcome of management’s plans cannot be ascertained with any degree of certainty. Our financial statements do not include any adjustments that might result from the outcome of these risks and uncertainties.
The industry in which we operate depends heavily upon our ability to obtain raw material and manufacture our product as well as the overall level of consumer and business spending. A sustained deterioration in general economic conditions (including distress in financial markets, turmoil in specific economies around the world, public health crises, and additional government intervention), particularly in the United States, may have a negative financial impact to our Company. Adverse conditions as a result of the global COVID-19 outbreak, will and may continue to impact our manufacturing processes and ultimately our ability to sell our product.
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Note 2 to the Financial Statements describes the significant accounting policies and methods used in the preparation of the Financial Statements. Estimates are used for, but not limited to, contingencies and taxes. Actual results could differ materially from those estimates. The following critical accounting policies are impacted significantly by judgments, assumptions, and estimates used in the preparation of the Financial Statements.
We are subject to various loss contingencies arising in the ordinary course of business. We consider the likelihood of loss or impairment of an asset or the incurrence of a liability, as well as our ability to reasonably estimate the amount of loss in determining loss contingencies. An estimated loss contingency is accrued when management concludes that it is probable that an asset has been impaired, or a liability has been incurred and the amount of the loss can be reasonably estimated. We regularly evaluate current information available to us to determine whether such accruals should be adjusted.
We recognize deferred tax assets (future tax benefits) and liabilities for the expected future tax consequences of temporary differences between the book carrying amounts and the tax basis of assets and liabilities. The deferred tax assets and liabilities represent the expected future tax return consequences of those differences, which are expected to be either deductible or taxable when the assets and liabilities are recovered or settled. Future tax benefits have been fully offset by a 100% valuation allowance as management is unable to determine that it is more likely than not that this deferred tax asset will be realized.
Recent Accounting Pronouncements
We have reviewed other recently issued accounting pronouncements and plan to adopt those that are applicable to us. We do not expect the adoption of any other pronouncements to have an impact on our results of operations or financial position.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
The following table sets forth certain information concerning our company’s executive management.
| Name | | Age | | Position(s) |
| Russell F. Bird | | 73 | | President, Chairman and Director |
| Thomas J. Wood | | 75 | | Chief Executive Officer, Secretary and Director |
| Jonathan B. Lane | | 61 | | Vice President and Chief Technology Officer |
Our directors serve until a successor is elected and qualified. Our officers are elected by the Board of Directors to a term of one (1) year and serves until their successor(s) is duly elected and qualified, or until they are removed from office. The Board of Directors has no nominating or compensation committees. The company’s current Audit Committee consists of our officers and directors.
There exist no family relationships between our officers and directors.
Certain information regarding the backgrounds of each of our officers and directors is set forth below.
Russell F. Bird has been our Chairman and Director since January 1, 2015, and our President since January 1, 2019. From year to year, he operated businesses that offered sleep apnea interfaces, devices, and other respiratory equipment and supplies. From May 23, 2013 to the present, he has been the Managing Member of REMSleep, LLC, an Iowa limited liability company. In 1979, he founded Medical Gases Australia, a medical manufacturing and distribution firm that specializing in respiratory and other health products. Medical Gases Australia placed the first patients on CPAP therapy. He then started Medical Industries of America in 1985, a medical manufacturing and distribution firm specializing in respiratory and other health products.
Thomas J. Wood has been our Chief Executive Officer and Director effective January 1, 2015. From May 23, 2013 to the present, he has been the Managing Member of REMSleep, LLC, an Iowa limited liability company. Thomas J. Wood has been awarded several U.S. patents in the area of sleep apnea. He is the inventor and developer of Nasal Aire, which won the 2004 Frost and Sullivan Award for Product Innovation. His US Patents also include the Nasal Aire II and Petite Nasal Aire. Tom has 25 years of experience as a respiratory therapist in the ICU at Baylor Medical Center and Parkland Memorial hospitals in Dallas, Texas. He also worked for two years with the Muscular Dystrophy Association, responsible for respiratory care for patients with Amyotrophic Lateral Sclerosis.
Jonathan B. Lane has been serving as the Chief Technology Officer of the Company since July 2018 and Vice President since January 2019. Mr. Lane has 35 years of design and engineering experience. He was the CEO/Founder of Badencorp from 1992 to 2018, and Director of Engineering/Co-founder of Searchmont Engine Company from 2006 to 2009. Jonathan worked twelve years for various fortune 500 companies including Boeing, General Dynamics and Bell Helicopter. From there, he went on to form his own company, Badencorp, which specialized in providing engineering and design services across all disciplines within Aerospace, Automotive, Biomedical, Consumer Products and Heavy Industries.
Conflicts of Interest
We do not currently foresee any conflict of interest.
Indemnification of Directors and Officers
Our Articles of Incorporation and Bylaws, both as amended to date, provide for the indemnification of our officers and directors to the fullest extent permitted by Nevada law.
Board Composition
Our Board of Directors currently consists of two members, Thomas J. Wood and Russell F. Bird. Each director of the Company serves until the next annual meeting of stockholders and until his successor is elected and duly qualified, or until his earlier death, resignation or removal. Our board is authorized to appoint persons to the offices of Chairman of the Board of Directors, President, Chief Executive Officer, one or more vice presidents, a Treasurer or Chief Financial Officer and a Secretary and such other offices as may be determined by the board.
Board Committees
Our board does not currently have a standing Audit Committee, Compensation Committee or Nominating/Corporate Governance Committee due the board’s limited size and the Company’s limited operations. The entire Board of Directors performs all functions that would otherwise be performed by committees. Given the present size of our Board, it is not practical for us to have committees other than those described above, or to have more than two directors on such committees. If we are able to grow our business and increase our operations, we intend to expand the size of our board and our committees and allocate responsibilities accordingly.
Board Leadership Structure and Risk Oversight
The Board of Directors oversees our business and considers the risks associated with our business strategy and decisions. The board currently implements its risk oversight function as a whole. Each of the board committees, when established, will provide risk oversight in respect of its areas of concentration and report material risks to the board for further consideration.
Independence of Board of Directors
No member of our Board of Directors is not independent, within the meaning of definitions established by the SEC or any self-regulatory organization. We are not currently subject to any law, rule or regulation requiring that all or any portion of our Board of Directors include independent directors.
Shareholder Communications with Our Board of Directors
Our company welcomes comments and questions from our shareholders. Shareholders should direct all communications to our Chief Executive Officer, Thomas J. Wood, at our executive offices. However, while we appreciate all comments from shareholders, we may not be able to respond individually to all communications. We will attempt to address shareholder questions and concerns in our press releases and documents filed with the SEC, so that all shareholders have access to information about us at the same time. Mr. Wood collects and evaluates all shareholder communications. All communications addressed to our directors and executive officers will be reviewed by those parties, unless the communication is clearly frivolous.
Code of Ethics
As of the date of this Prospectus, our Board of Directors has not adopted a code of ethics with respect to our directors, officers and employees.
EXECUTIVE COMPENSATION
In General
Currently, our management is unable to estimate accurately when, if ever, our company will possess sufficient capital, whether derived from sales revenues, this offering or otherwise, for the payment of increased salaries to our management.
As of the date of this Prospectus, there are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of our company, pursuant to any presently existing plan provided by, or contributed to, our company.
Compensation Summary
The following table summarizes information concerning the compensation awarded, paid to or earned by, our executive officers.
| Name and Principal Position | | Year | | Salary ($) | | | Bonus ($) | | | Stock Awards ($) | | | Option Awards ($) | | | Non-Equity Incentive Plan Compensation ($) | | | Non-qualified Deferred Compensation Earnings ($) | | | All Other Compen- sation ($) | | | Total ($) | |
| Russell F. Bird | | 2021 | | | 24,000 | | | | --- | | | | --- | | | | --- | | | | --- | | | | --- | | | | --- | | | | 24,000 | |
| Chairman | | 2020 | | | 18,000 | | | | --- | | | | 63,750 | | | | --- | | | | --- | | | | --- | | | | --- | | | | 81,750 | |
Thomas J. Wood
Chief Executive Officer | | 2021
2020 | | | 48,000 46,000 | | | | --- --- | | | | ---
63,750 | | | | --- --- | | | | --- --- | | | | --- --- | | | | --- --- | | | | 48,000 109,750 | |
Jonathan B. Lane
Vice President and Chief Technology Officer | | 2021
2020 | | | 26,000 15,000 | | | | --- --- | | | | --- --- | | | | --- --- | | | | --- --- | | | | --- --- | | | | --- --- | | | | 26,000 15,000 | |
Outstanding Option Awards
The following table provides certain information regarding unexercised options to purchase common stock, stock options that have not vested and equity-incentive plan awards outstanding as of the date of this Prospectus, for each named executive officer.
| | | | Option Awards | | | Stock Awards | |
| Name | | | Number of Securities Underlying Unexercised Options (#) Exercisable | | | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | | | | Option Exercise Price ($) | | | Option Expiration Date | | | Number of Shares or Units of Stock That Have Not Vested (#) | | | Market Value of Shares or Units of Stock That Have Not Vested ($) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |
| Russell F. Bird | | | --- | | | | --- | | | | --- | | | | --- | | | n/a | | | --- | | | n/a | | | --- | | | | --- | |
| Thomas J. Wood | | | --- | | | | --- | | | | --- | | | | --- | | | n/a | | | --- | | | n/a | | | --- | | | | --- | |
| Jonathan B. Lane | | | --- | | | | --- | | | | --- | | | | --- | | | n/a | | | --- | | | n/a | | | --- | | | | --- | |
Employment Agreements
Thomas J. Wood. The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $4,000 per month. The agreement expired on April 1, 2020 and has been renewed for two more years. As of December 31, 2021 and 2020, there is $2,000 and $2,000 of accrued compensation, respectively, due to Mr. Wood. During the years ended December 31, 2021 and 2020, cash payments of $48,000 and $46,000, respectively, were paid to Mr. Wood.
Russell Bird. The Company executed an employment agreement with its Chairman, Russell Bird, on January 1, 2019. Per the terms of the agreement, which is effective for one year, Mr. Bird is to be compensated $3,000 per month. As of December 31, 2021 and 2020, there is $45,000 and $33,000 of accrued compensation, respectively, due to Mr. Bird. Mr. Bird’s employment agreement has been renewed in 2020 for two more years. During the years ended December 31, 2021 and 2020, cash payments of $24,000 and $18,000, respectively, were paid to Mr. Bird.
Jonathan Lane. The Company has entered into an at-will consulting agreement with Jonathan Lane to serve as Chief Technology Officer. During the years ended December 31, 2021 and 2020, the Company made cash payments to Mr. Lane of $26,000 and $15,000, respectively.
Outstanding Equity Awards
Our Board of Directors has made no equity awards and no such award is pending.
Long-Term Incentive Plans
We currently have no employee incentive plans.
Director Compensation
Our directors receive no compensation for their serving as directors.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information about the beneficial ownership of our capital stock at June 10, 2022 for:
| | ● | each person known to us to be the beneficial owner of more than 5% of our common stock; |
| | | |
| | ● | each named executive officer; |
| | | |
| | ● | each of our directors; and |
| | | |
| | ● | all of our named executive officers and directors as a group. |
Unless otherwise noted below, the address for each beneficial owner listed on the table is in care of RemSleep Holdings, Inc., 14175 Icot Boulevard, Suite 300, Clearwater, Florida 33760. We have determined beneficial ownership in accordance with the rules of the SEC. We believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all shares of our common stock that they beneficially own, subject to applicable community property laws.
In computing the number of shares of our common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of our common stock subject to options, warrants, preferred stock or restricted stock units held by that person that are currently exercisable or convertible or exercisable or convertible within 60 days of June 10, 2022. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
Name of Shareholder | | Number of Shares Beneficially Owned | | | Percentage Beneficially Owned (1) | |
| Common Stock | | | | | | |
| Directors and Executive Officers | | | | | | |
| Thomas J. Wood | | | 75,969,494 | (2) | | | 4.86 | % |
| Russell F. Bird | | | 76,219,494 | (2) | | | 4.88 | % |
| Jonathan B. Lane | | | 1,000,000 | | | | * | |
| Officers and directors, as a group (3 persons) | | | 153,188,989 | (3) | | | 9.81 | % |
| | | | | | | | | |
| Series A Preferred Stock (4) | | | | | | | | |
| Thomas J. Wood | | | 2,500,000 | | | | 50.00 | % |
| Russell B. Lane | | | 2,500,000 | | | | 50.00 | % |
| | | | | | | | | |
| Series B Preferred Stock (5) | | | | | | | | |
| Thomas J. Wood | | | 500,000 | | | | 50.00 | % |
| Russell B. Lane | | | 500,000 | | | | 50.00 | % |
| * | Less than 1%. |
| (1) | We have based our calculation of the percentage of beneficial ownership of common stock on 1,461,616,601 shares outstanding as of June 10, 2022. Except in the case of the holders thereof, our calculation does not give effect to the 100,000,000 shares of our common stock issuable upon conversion of the 1,000,000 shares of Series B Convertible Preferred Stock that were issued and outstanding as of June 10, 2022. |
| (2) | Includes 50,000,000 shares of Common Stock issuable upon conversion of 500,000 shares Series B Preferred Stock. |
| (3) | Includes 100,000,000 shares of Common Stock issuable upon conversion of 1,000,000 shares Series B Preferred Stock. |
| (4) | Each share of Series A Preferred Stock is convertible into one share of Common Stock at any time after our net profits exceed $1,000,000 and has the voting power of 25 shares of Common Stock. |
| (5) | Each share of Series B Preferred Stock is convertible into 100 shares of Common Stock and has the voting power of 100 shares of Common Stock. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related-Party Advances
The Company has received support from parties related through common ownership and directorship. These loans are unsecured, and due on demand. As of December 31, 2021 and 2020, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance due accrues interest at 12.5%. As of December 31, 2021, total accrued interest is $67,505.
Employment Agreements
Thomas J. Wood. The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $4,000 per month. The agreement expired on April 1, 2020 and has been renewed for two more years. As of December 31, 2021 and 2020, there is $2,000 and $2,000 of accrued compensation, respectively, due to Mr. Wood. During the years ended December 31, 2021 and 2020, cash payments of $48,000 and $46,000, respectively, were paid to Mr. Wood.
Russell Bird. The Company executed an employment agreement with its Chairman, Russell Bird, on January 1, 2019. Per the terms of the agreement, which is effective for one year, Mr. Bird is to be compensated $3,000 per month. As of December 31, 2021 and 2020, there is $45,000 and $33,000 of accrued compensation, respectively, due to Mr. Bird. Mr. Bird’s employment agreement has been renewed in 2020 for two more years. During the years ended December 31, 2021 and 2020, cash payments of $24,000 and $18,000, respectively, were paid to Mr. Bird.
Jonathan Lane. The Company has entered into an at-will consulting agreement with Jonathan Lane to serve as Chief Technology Officer. During the years ended December 31, 2021 and 2020, the Company made cash payments to Mr. Lane of $26,000 and $15,000, respectively.
Stock Issuances
Common Stock. On June 14, 2019, we granted 25,000,000 shares of common stock to each of Mr. Wood and Mr. Bird for services rendered on behalf of our company. These shares were valued at $0.04 per share, the closing stock price on the date of grant, for total non-cash compensation expense of $2,000,000.
Preferred Stock. On June 14, 2019, we granted 500,000 shares of Series A preferred stock to Mr. Bird for services rendered on behalf of our company. The shares were valued at $0.04, the closing price of our common stock on the date of grant, for total non-cash compensation expense of $20,000. The closing price for common stock was deemed an acceptable method for valuation as one share of Series A preferred stock is convertible into one share of common stock.
On November 23, 2020, we granted 500,000 shares of Series A preferred stock to Mr. Bird for services rendered on behalf of our company. The shares were valued at $0.0025, the closing price of our common stock on the date of grant, for total non-cash compensation expense of $1,250. The closing price for common stock was deemed an acceptable method for valuation as one share of Series A preferred stock is convertible into one share of common stock.
On November 23, 2020, we granted 500,000 shares of Series A preferred stock to Mr. Wood for services rendered on behalf of our company. The shares were valued at $0.0025, the closing price of our common stock on the date of grant, for total non-cash compensation expense of $1,250. The closing price for common stock was deemed an acceptable method for valuation as one share of Series A preferred stock is convertible into one share of common stock.
On November 23, 2020, we granted 250,000 shares of Series B preferred stock to Mr. Bird for services rendered on behalf of our company. The shares were valued at $0.0025, the closing price of our common shares on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. The closing price for common stock multiplied by 100 was deemed an acceptable method for valuation as one share of Series B preferred stock is convertible into 100 shares of common stock.
On November 23, 2020, the Company granted 250,000 shares of Series B preferred stock to Mr. Wood for services rendered on behalf of our company. The shares were valued at $0.0025, the closing price of our common shares on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. The closing price for common stock multiplied by 100 was deemed an acceptable method for valuation as one share of Series B preferred stock is convertible into 100 shares of common stock.
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of: (a) 3,000,000,000 shares of common stock, $.001 par value per share; (b) 5,000,000 shares of Series A Preferred Stock, $.001 par value per share; (c) 5,000,000 shares of Series B Preferred Stock, $.001 par value per share; and (d) 5,000,000 shares of Series C Preferred Stock, $.001 par value per share.
As of the date of this Prospectus, there were 1,461,616,601 shares of our common stock issued and outstanding, held by 4,162holders of record; a total of 333,853,334 shares of common stock reserved for issuance upon the exercise of outstanding warrants; 5,000,000 shares of Series A Preferred Stock issued and outstanding, held by two of our officers; and 1,000,000 shares of Series B Preferred Stock issued and outstanding, held by two of our officers.
Common Stock
General. The holders of our common stock currently have (a) equal ratable rights to dividends from funds legally available therefor, when, as and if declared by our Board of Directors; (b) are entitled to share ratably in all of our assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of the affairs of our company; (c) do not have preemptive, subscriptive or conversion rights and there are no redemption or sinking fund provisions or rights applicable thereto; and (d) are entitled to one non-cumulative vote per share on all matters on which shareholders may vote. Our Bylaws provide that, at all meetings of the shareholders for the election of directors, a plurality of the votes cast shall be sufficient to elect. On all other matters, except as otherwise required by Nevada law or our Articles of Incorporation, as amended, a majority of the votes cast at a meeting of the shareholders shall be necessary to authorize any corporate action to be taken by vote of the shareholders.
Non-cumulative Voting. Holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares, voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in such event, the holders of the remaining shares will not be able to elect any of our directors. As of the date of this Prospectus, our officers and directors own, directly or indirectly, a total of 158,188,989 shares of our common stock (including a total of 105,000,000 shares underlying currently convertible shares of our Series A Preferred Stock and our Series B Preferred Stock), or approximately 9.77%, of our common stock, which ownership percentage would be reduced to approximately 7.46%, assuming all of the Shares are sold under the Equity Line.
Pre-emptive Rights. As of the date of this Prospectus, no holder of any shares of our common stock has pre-emptive or preferential rights to acquire or subscribe for any unissued shares of any class of our capital stock not disclosed herein.
Preferred Stock
Two of our officers, Thomas J. Wood and Russell F. Bird, own, collectively, all of the issued and outstanding shares of our Series A Preferred Stock and Series B Preferred Stock. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares” and “Security Ownership of Certain Beneficial Owners and Management”).
Series A Preferred Stock. We are authorized to issue 5,000,000 Series A Preferred Stock, par value $.001 per share. Each share of Series A Preferred Stock is convertible into one share of common stock at any time after our net profits exceed $1,000,000 and has the voting power of 25 shares of common stock. The Series A Preferred Stock ranks equal to the common stock on liquidation and pays no dividend. Two of our officers own an aggregate of 5,000,000 shares of Series A Preferred Stock. (See “Security Ownership of Certain Beneficial Owners and Management”).
Series B Preferred Stock. We are authorized to issue 5,000,000 Series B Preferred Stock, par value $.001 per share. Each share of Series B Preferred Stock is convertible into 100 shares of common stock and has the voting power of 100 shares of common stock. No dividends will be paid on the Series B Preferred Stock and, in the event of liquidation, all shares of Series B Preferred Stock will automatically convert into common stock. Tow of our officers own an aggregate of 1,000,000 shares of Series B Preferred Stock. (See “Security Ownership of Certain Beneficial Owners and Management”).
Series C Preferred Stock. We are authorized to issue 5,000,000 Series C Preferred Stock, par value $.001 per share. Each share of Series C Preferred Stock is convertible into 50 shares of common stock and has the voting power of 50 shares of common stock. No dividends will be paid on the Series C Preferred Stock and, in the event of liquidation, all shares of Series C will automatically convert into common stock. As of the date of this Prospectus, there are no shares of Series C Preferred Stock issued and outstanding.
Common Stock Purchase Warrants
The following table sets forth information regarding our outstanding common stock purchase warrants as of March 31, 2022:
| | | Number of Warrants | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contract Term | | | Aggregate Intrinsic Value | |
| Exercisable at December 31, 2021 | | | 226,500,000 | | | $ | 0.0013 | | | | 3.78 | | | $ | --- | |
| Granted | | | --- | | | $ | --- | | | | --- | | | $ | --- | |
| Expired | | | --- | | | $ | --- | | | | --- | | | $ | --- | |
| Exercised | | | (60,000,000 | ) | | $ | --- | | | | --- | | | $ | --- | |
| | | | 166,500,000 | | | $ | 0.0023 | | | | 3.89 | | | $ | 1,956,000 | |
Convertible Promissory Notes
As of March 31, 2022, we had an outstanding balance on one convertible promissory note. The table below sets forth information with respect to our convertible promissory notes during the three months ended March 31, 2022.
Note Holder | | Date | | Maturity Date | | Interest | | | Balance 12/31/21 | | | Additions | | | Conversions/ Repayments | | | Balance 3/31/22 | |
| Granite Global Investments Ltd. | | 4/7/2021 | | 4/7/2021 | | | 10 | % | | | 36,500 | | | | --- | | | | (36,500 | ) | | | --- | |
| Granite Global Investments Ltd. | | 4/9/2021 | | 4/9/2021 | | | 10 | % | | | 100,000 | | | | --- | | | | (100,000 | ) | | | --- | |
| Power Up Lending Group LTD | | 7/22/2021 | | 7/22/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 8/26/2021 | | 8/26/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 9/22/2021 | | 9/22/2021 | | | 10 | % | | | 58,850 | | | | --- | | | | (58,850 | ) | | | --- | |
| Power Up Lending Group LTD | | 10/12/2021 | | 10/12/2021 | | | 10 | % | | | 86,350 | | | | --- | | | | --- | | | | 86,350 | (1) |
| | | | | | | | Total | | | $ | 399,400 | | | $ | --- | | | $ | (313,050 | ) | | $ | 86,350 | |
(1) This note was converted in full on April 15, 2022.
Shareholder Meetings
Our bylaws provide that special meetings of shareholders may be called only by our Board of Directors, the chairman of the board, or our president, or as otherwise provided under Nevada law.
Transfer Agent
We have retained the services of Action Stock Transfer Corporation, 2469 E. Fort Union Boulevard, Suite 214, Salt Lake City, Utah 84121, as the transfer agent for our common stock. Action Stock Transfer’s website is located at: www.actionstocktransfer.com. No information found on, or connected to, Action Stock Transfer’s website is incorporated by reference into, any you must not consider the information to a part of, this Prospectus.
SHARES ELIGIBLE FOR FUTURE SALE
Market sales of shares of our common stock after this Offering from time to time, and the availability of shares of our common stock for future sale, may reduce the market price of our common stock. Sales of substantial amounts of our common stock, or the perception that these sales could occur, could adversely affect prevailing market prices for our common stock and could impair our future ability to obtain capital, especially through an offering of equity securities. After the effective date of the Registration Statement, all of the Shares will be freely tradable without restrictions or further registration under the Securities Act, unless the Shares are purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act. The balance of Shares which are not being registered will be eligible for sale pursuant to exemptions from registration. However, these shares not being registered are held by our management and other affiliates who are limited to selling only 1% of our issued and outstanding shares every 90 days.
Our common stock is considered a “penny stock” and will continue to be considered a penny stock so long as it trades below $5.00 per share and, as such, trading in our common stock is subject to the requirements of Rule 15g-9 under the Securities Exchange Act of 1934. Under this rule, broker/dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker/dealer must make an individualized written suitability determination for the purchaser and receive the purchaser’s written consent prior to the transaction.
SEC regulations also require additional disclosure in connection with any trades involving a “penny stock,” including the delivery, prior to any penny stock transaction, of a disclosure schedule explaining the penny stock market and its associated risks. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from recommending transactions in our securities, which could severely limit the liquidity of our securities and consequently adversely affect the market price for our securities. In addition, few broker or dealers are likely to undertake these compliance activities. Other risks associated with trading in penny stocks could also be price fluctuations and the lack of a liquid market. (See “Risk Factors”).
Rule 144
In general, under Rule 144, a person who has beneficially owned restricted shares for at least six months would be entitled to sell those securities provided that (1) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (2) we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale and are current in filing our periodic reports. Persons who have beneficially owned restricted shares of common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed 1% of the number of shares of common stock outstanding. Such sales by affiliates must also comply with the manner of sale and notice provisions of Rule 144 and to the availability of current public information about us.
LEGAL MATTERS
The validity of the securities offered by this Prospectus will be passed upon for us by Newlan Law Firm, PLLC, Flower Mound, Texas.
EXPERTS
Our balance sheets as of December 31, 2021 and 2020, and the related statements of operations, changes in stockholders’ equity and cash flows for the years ended December 31, 2021 and 2020, included in this Prospectus have been audited by Fruci & Associates II, PLLC, independent registered public accounting firm, as indicated in their report with respect thereto, and have been so included in reliance upon the report of such firm given on their authority as experts in accounting and auditing.
DISCLOSURE OF COMMISSION’S POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
In the opinion of the Securities and Exchange Commission, indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act and is, therefore, unenforceable. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC the Registration Statement on Form S-1 under the Securities Act with respect to the Shares being offered by this Prospectus. This Prospectus, which constitutes a part of the Registration Statement, does not contain all of the information in the Registration Statement and its exhibits. For further information with respect to our company and the Shares offered by this Prospectus, you should refer to the Registration Statement and the exhibits filed as a part thereof. Statements contained in this Prospectus as to the contents of any contract or any other document referred to are not necessarily complete and, in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the Registration Statement. Each of these statements is qualified in all respects by this reference.
We are subject to the informational requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the Registration Statement, over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing or telephoning us at: RemSleep Holdings, Inc., 14175 Icot Boulevard, Suite 300, Clearwater, Florida 33760; telephone: (813) 367-3855.
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS PROSPECTUS. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE INFORMATION DIFFERENT FROM THAT CONTAINED IN THIS PROSPECTUS. WE ARE OFFERING TO SELL, AND SEEKING OFFERS TO BUY, SHARES OF COMMON STOCK ONLY IN JURISDICTIONS WHERE OFFERS AND SALES ARE PERMITTED. THE INFORMATION CONTAINED IN THIS PROSPECTUS IS ACCURATE ONLY AS OF THE DATE OF THIS PROSPECTUS REGARDLESS OF THE TIME OF DELIVERY OF THIS PROSPECTUS, OR OF ANY SALE OF OUR COMMON STOCK.
INDEX TO FINANCIAL STATEMENTS
REMSLEEP HOLDINGS, INC.
CONDENSED BALANCE SHEETS
| | | March 31,
2022 | | | December 31,
2021 | |
| ASSETS | | (Unaudited) | | | (Audited) | |
| Current assets: | | | | | | |
| Cash | | $ | 4,014,578 | | | $ | 3,383,568 | |
| Prepaid | | | 69,494 | | | | — | |
| Total current assets | | | 4,084,072 | | | | 3,383,568 | |
| | | | | | | | | |
| Other asset | | | 10,000 | | | | 10,000 | |
| Property and equipment, net | | | 113,435 | | | | 105,061 | |
| | | | | | | | | |
| Total Assets | | $ | 4,207,507 | | | $ | 3,498,629 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable | | $ | 22,941 | | | $ | 15,505 | |
| Accrued compensation | | | 48,000 | | | | 47,000 | |
| Accrued interest | | | 26,065 | | | | 41,851 | |
| Accrued interest – related party | | | 73,151 | | | | 67,505 | |
| Convertible Notes, net of discount of $46,774 and $206,157, respectively | | | 39,576 | | | | 193,243 | |
| Derivative Liability | | | 76,423 | | | | 290,712 | |
| Loan payable – related party | | | 179,191 | | | | 179,191 | |
| Loans payable | | | 45,000 | | | | 45,000 | |
| | | | | | | | | |
| Total Liabilities | | | 510,347 | | | | 880,007 | |
| | | | | | | | | |
| Commitments and Contingencies | | | — | | | | — | |
| | | | | | | | | |
| STOCKHOLDERS’ EQUITY | | | | | | | | |
| | | | | | | | | |
| Series A preferred stock, $0.001 par value, 5,000,000 shares authorized, 4,000,000 and issued and outstanding | | | 5,000 | | | | 5,000 | |
| Series B preferred stock, $0.001 par value, 5,000,000 shares authorized, 500,000 shares issued | | | 500 | | | | 500 | |
| Series C preferred stock, $0.001 par value, 5,000,000 shares authorized, no shares issued | | | — | | | | — | |
| Common stock, $0.001 par value, 3,000,000,000 shares authorized, 1,452,936,313 and 1,234,008,735 shares issued and outstanding, respectively | | | 1,452,935 | | | | 1,234,006 | |
| Discount to common stock | | | (94,708 | ) | | | (94,708 | ) |
| Additional paid in capital | | | 13,041,347 | | | | 11,865,439 | |
| Accumulated Deficit | | | (10,707,914 | ) | | | (10,391,615 | ) |
| TOTAL STOCKHOLDERS’ EQUITY | | | 3,697,160 | | | | 2,618,622 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 4,207,507 | | | $ | 3,498,629 | |
The accompanying notes are an integral part of these unaudited condensed financial statements.
REMSLEEP HOLDINGS, INC.
CONDENSED STATEMENTS OF OPERATIONS
(UNAUDITED)
| | | For the
Three Months
Ended March 31, | |
| | | 2022 | | | 2021 | |
| Operating Expenses: | | | | | | |
| Professional fees | | $ | 26,000 | | | $ | 24,598 | |
| Compensation expense – related party | | | 21,000 | | | | 21,000 | |
| Development expense | | | 25,667 | | | | — | |
| General and administrative | | | 81,891 | | | | 48,666 | |
| | | | | | | | | |
| Total operating expenses | | | 154,558 | | | | 94,264 | |
| | | | | | | | | |
| Loss from operations | | | (154,558 | ) | | | (94,264 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest expense | | | (173,648 | ) | | | (166,573 | ) |
| Default penalty of convertible note | | | — | | | | (162,798 | ) |
| Loss on issuance of convertible debt | | | — | | | | (442,979 | ) |
| Change in fair value of derivative | | | 11,907 | | | | 395,148 | |
| Total other expense | | | (161,741 | ) | | | (377,202 | ) |
| | | | | | | | | |
| Loss before income taxes | | | (316,299 | ) | | | (471,466 | ) |
| | | | | | | | | |
| Provision for income taxes | | | — | | | | — | |
| | | | | | | | | |
| Net Loss | | $ | (316,299 | ) | | $ | (471,466 | ) |
| | | | | | | | | |
| Net loss per share, basic and diluted | | $ | (0.00 | ) | | $ | (0.00 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding, basic and diluted | | | 1,383,367,206 | | | | 406,969,823 | |
The accompanying notes are an integral part of these unaudited condensed financial statements.
REMSLEEP HOLDINGS, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE THREE MOTHS ENDED MARCH 31, 2022 AND 2021
(UNAUDITED)
| | | Series A
Preferred Stock | | | Series B
Preferred Stock | | | Common Stock | | | Discount to
Common | | | Additional
Paid-in | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Stock | | | Capital | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 5,000,000 | | | $ | 5,000 | | | | 500,000 | | | $ | 500 | | | | 1,234,008,735 | | | $ | 1,234,006 | | | $ | (94,708 | ) | | $ | 11,865,439 | | | $ | (10,391,615 | ) | | $ | 2,618,622 | |
| Common stock issued for conversion of debt | | | — | | | | — | | | | — | | | | — | | | | 34,799,374 | | | | 34,801 | | | | — | | | | 505,036 | | | | — | | | | 539,837 | |
| Common stock issued for cash | | | — | | | | — | | | | — | | | | — | | | | 114,000,000 | | | | 114,000 | | | | — | | | | 741,000 | | | | — | | | | 855,000 | |
| Warrants converted to common stock | | | — | | | | — | | | | — | | | | — | | | | 70,128,204 | | | | 70,128 | | | | — | | | | (70,128 | ) | | | — | | | | — | |
| Net Loss | | | — | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | — | | | | (316,299 | ) | | | (316,299 | ) |
| Balance, March 31, 2022 | | | 5,000,000 | | | $ | 5,000 | | | | 500,000 | | | $ | 500 | | | | 1,452,936,313 | | | $ | 1,452,935 | | | $ | (94,708 | ) | | $ | (13,041,347 | ) | | $ | (10,707,914 | ) | | $ | 3,697,160 | |
| | | Series A
PreferredStock | | | Series B
PreferredStock | | | Common Stock | | | Additional
Paid-in | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2020 | | | 5,000,000 | | | $ | 126,000 | | | | 500,000 | | | $ | 500 | | | | 368,063,606 | | | $ | 368,061 | | | $ | 5,200,885 | | | $ | (6,565,942 | ) | | $ | (870,496 | ) |
| Common stock issued for conversion of debt | | | — | | | | — | | | | — | | | | — | | | | 74,985,965 | | | | 74,986 | | | | 467,990 | | | | — | | �� | | 542,976 | |
| Warrants issued with convertible debt | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 75,070 | | | | — | | | | 75,070 | |
| Net Loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (471,466 | ) | | | (471,466 | ) |
| Balance, March 31, 2021 | | | 5,000,000 | | | $ | 126,000 | | | | 500,000 | | | $ | 500 | | | | 443,049,571 | | | $ | 443,047 | | | $ | 5,743,945 | | | $ | (7,037,408 | ) | | $ | (723,916 | ) |
The accompanying notes are an integral part of these unaudited condensed financial statements.
REMSLEEP HOLDINGS, INC.
CONDENSED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | | For the
Three Months
Ended March 31, | |
| | | 2022 | | | 2021 | |
| Cash Flows from Operating Activities: | | | | | | |
| Net loss | | $ | (316,299 | ) | | $ | (471,466 | ) |
| Adjustments to reconcile net loss to net cash used in operations: | | | | | | | | |
| Depreciation expense | | | 16,532 | | | | 12,890 | |
| Change in fair value of derivative | | | (11,907 | ) | | | (395,148 | ) |
| Discount amortization | | | 159,383 | | | | 138,622 | |
| Loss on issuance of convertible debt | | | — | | | | 442,979 | |
| Default penalty of convertible note | | | — | | | | 162,798 | |
| Changes in Operating Assets and Liabilities | | | | | | | | |
| Prepaid expenses | | | (69,494 | ) | | | — | |
| Accounts payable | | | 7,435 | | | | 13,306 | |
| Accrued officer compensation | | | 1,000 | | | | 5,000 | |
| Accrued interest | | | 8,619 | | | | 22,191 | |
| Accrued interest – related party | | | 5,646 | | | | 5,646 | |
| Net cash used in operating activities | | | (199,085 | ) | | | (63,182 | ) |
| | | | | | | | | |
| Cash Flows from Investing Activities: | | | | | | | | |
| Purchase of equipment | | | (24,905 | ) | | | (17,705 | ) |
| Net Cash used in investing activities | | | (24,905 | ) | | | (17,705 | ) |
| | | | | | | | | |
| Cash Flows from Financing Activities: | | | | | | | | |
| Repayment of loans | | | — | | | | (867 | ) |
| Proceeds from convertible notes payable | | | — | | | | 126,400 | |
| Common stock sold for cash | | | 855,000 | | | | — | |
| Net cash provided by financing activities | | | 855,000 | | | | 125,533 | |
| | | | | | | | | |
| Net change in cash | | | 631,010 | | | | 44,646 | |
| Cash at beginning of the period | | | 3,383,568 | | | | 114,227 | |
| Cash at end of the period | | $ | 4,014,578 | | | $ | 158,873 | |
| | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | |
| Interest paid in cash | | $ | — | | | $ | 114 | |
| Taxes paid | | $ | — | | | $ | — | |
| Supplemental non-cash disclosure: | | | | | | | | |
| Common stock issued for conversion of debt | | $ | 337,455 | | | $ | 86,915 | |
The accompanying notes are an integral part of these unaudited condensed financial statements.
REMSLEEP HOLDINGS, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
March 31, 2022
(Unaudited)
NOTE 1 - BACKGROUND
Business Activity
REMSleep Holdings, Inc., (the “Company”) was incorporated in the State of Nevada on June 6, 2007. On January 5, 2015 the name of the Company was changed to REMSleep Holdings, Inc. and the business model was changed to reflect the new direction of the Company; to develop and distribute products to help people affected by sleep apnea. On May 30, 2015 REMSleep LLC was formally merged into REMSleep Holdings, Inc.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and the rules and regulations of the Securities and Exchange Commission (“SEC”). These financial statements and the notes attached hereto should be read in conjunction with the financial statements and notes included in the Company’s 10-K for its fiscal year ended December 31, 2021. In the opinion of the Company, all adjustments, including normal recurring adjustments necessary to present fairly the financial position of the Company, as of March 31, 2022 and the results of its operations and cash flows for the three months then ended have been included. The results of operations for the interim period are not necessarily indicative of the results for the full year ending December 31, 2022.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
| | Level 1: | Quoted market prices available in active markets for identical assets or liabilities as of the reporting date. |
| | | |
| | Level 2: | Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date. |
| | | |
| | Level 3: | Pricing inputs that are generally unobservable inputs and not corroborated by market data. |
The carrying amount of the Company’s financial assets and liabilities, such as cash, prepaid expenses and accrued expenses approximate their fair value because of the short maturity of those instruments. The Company’s notes payable approximates the fair value of such instruments as the notes bear interest rates that are consistent with current market rates.
The following table classifies the Company’s liabilities measured at fair value on a recurring basis into the fair value hierarchy as of March 31, 2022 and December 31, 2021:
March 31, 2022:
| Description | | Level 1 | | | Level 2 | | | Level 3 | |
| Derivative | | $ | — | | | $ | — | | | $ | 76,423 | |
| Total | | $ | — | | | $ | — | | | $ | 76,423 | |
December 31, 2021:
| Description | | Level 1 | | | Level 2 | | | Level 3 | |
| Derivative | | $ | — | | | $ | — | | | $ | 290,712 | |
| Total | | $ | — | | | $ | — | | | $ | 290,712 | |
Basic and Diluted Earnings Per Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period. The weighted average number of common shares outstanding and potentially outstanding common shares assumes that the Company incorporated as of the beginning of the first period presented.
As of March 31, 2022, the Company had 8,680,288 of potentially dilutive shares of common stock from convertible debt, 139,714,286 potentially dilutive shares of common stock warrants, 5,000,000 shares from Series A preferred stock and 50,000,000 from Series B preferred stock.
As of March 31, 2021, the Company had 73,121,000 of potentially dilutive shares of common stock from convertible debt 170,974,026 potentially dilutive shares of common stock warrants, 5,000,000 shares from Series A preferred stock and 50,000,000 from Series B preferred stock.
The Company’s diluted loss per share is the same as the basic loss per share for all periods, as the inclusion of any potential shares would have had an anti-dilutive effect due to the Company generating a loss in those periods.
Recently Adopted Accounting Pronouncements
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
NOTE 3 - GOING CONCERN
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has an accumulated deficit of $10,707,914 at March 31, 2022, had a net loss of $316,299, and net cash used in operating activities of $199,085 for the three months ended March 31, 2022. The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
The Company is in the final stages of product development and plans to begin selling its product in Q2 of 2022. The Company will continue to finance its operations through debt and/or equity financing as needed.
The industry in which we operate depends heavily upon our ability to obtain raw material and manufacture our product as well as the overall level of consumer and business spending. A sustained deterioration in general economic conditions (including distress in financial markets, turmoil in specific economies around the world, public health crises, and additional government intervention), particularly in the United States, may have a negative financial impact to our Company. Adverse conditions as a result of the global COVID-19 outbreak, have and may continue to impact our manufacturing processes and ultimately our ability to sell our product.
NOTE 4 - PROPERTY & EQUIPMENT
Long lived assets, including property and equipment and certain intangible assets to be held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Impairment losses are recognized if expected future cash flows of the related assets are less than their carrying values. Measurement of an impairment loss is based on the fair value of the asset. Long-lived assets and certain identifiable intangibles to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Property and Equipment and intangible assets are first recorded at cost. Depreciation and/or amortization is computed using the straight-line method over the estimated useful lives of the various classes of assets as follows between three and five years.
Maintenance and repair expenses, as incurred, are charged to expense. Betterments and renewals are capitalized in plant and equipment accounts. Cost and accumulated depreciation applicable to items replaced or retired are eliminated from the related accounts with any gain or loss on the disposition included as income.
Property and equipment, stated at cost, less accumulated depreciation consisted of the following:
| | | March 31,
2022 | | | December 31,
2021 | |
| Furniture/fixtures | | $ | 27,310 | | | $ | 14,904 | |
| Office equipment | | | 14,522 | | | | 14,522 | |
| Automobile | | | 29,905 | | | | 29,905 | |
| Tooling/Molds | | | 189,490 | | | | 176,990 | |
| Less: accumulated depreciation | | | (147,792 | ) | | | (131,260 | ) |
| Property and equipment, net | | $ | 113,435 | | | $ | 105,061 | |
Depreciation expense
Depreciation expense for the three months ended March 31, 2022 and 2021 was $16,532 and $12,890, respectively.
NOTE 5 - LOANS PAYABLE
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by a Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. As of March 31, 2022, there is $45,000 and $22,140 of principal and interest due on this loan. As of December 31, 2021, there is $45,000 and $21,549 of principal and interest due on this loan.
NOTE 6 - CONVERTIBLE NOTES
The following table summarizes the convertible notes and related activity as of March 31, 2022:
| Note Holder | | Date | | Maturity Date | | Interest | | | Balance
December 31,
2021 | | | Additions | | | Conversions/
Repayments | | | Balance
March 31,
2022 | |
| Granite Global Investments Ltd | | 4/7/2021 | | 4/7/2022 | | | 10 | % | | | 36,500 | | | | — | | | | (36,500 | ) | | | — | |
| Granite Global Investments Ltd | | 4/9/2021 | | 4/9/2022 | | | 10 | % | | | 100,000 | | | | — | | | | (100,000 | ) | | | — | |
| Power Up Lending Group LTD | | 7/22/2021 | | 7/22/2022 | | | 10 | % | | | 58,850 | | | | — | | | | (58,850 | ) | | | — | |
| Power Up Lending Group LTD | | 8/26/2021 | | 8/26/2022 | | | 10 | % | | | 58,850 | | | | — | | | | (58,850 | ) | | | — | |
| Power Up Lending Group LTD | | 9/22/2021 | | 9/22/2022 | | | 10 | % | | | 58,850 | | | | — | | | | (58,850 | ) | | | — | |
| Power Up Lending Group LTD | | 10/12/2021 | | 10/12/2022 | | | 10 | % | | | 86,350 | | | | — | | | | — | | | | 86,350 | |
| | | | | | | | Total | | | $ | 399,400 | | | $ | — | | | $ | (313,050 | ) | | $ | 86,350 | |
| | | | | Less debt discount | | | | (206,157 | ) | | | | | | | | | | | (46,774 | ) |
| | | | | | | | | | | $ | 193,243 | | | | | | | | | | | $ | 39,576 | |
A summary of the activity of the derivative liability for the notes above is as follows:
| Balance at December 31, 2020 | | | 700,719 | |
| Increase to derivative due to new issuances | | | 1,087,302 | |
| Decrease to derivative due to conversion/repayments | | | (3,098,325 | ) |
| Derivative loss due to mark to market adjustment | | | 1,601,016 | |
| Balance at December 31, 2021 | | $ | 290,712 | |
| Decrease to derivative due to conversion/repayments | | | (202,382 | ) |
| Derivative gain due to mark to market adjustment | | | (11,907 | ) |
| Balance at March 31, 2022 | | $ | 76,423 | |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy as of March 31, 2022 is as follows:
| Inputs | | March 31, 2022 | | | Initial Valuation | |
| Stock price | | $ | .014 | | | $ | .028 | |
| Conversion price | | $ | .0092 | | | $ | 0.012 | |
| Volatility (annual) | | | 175.09 | % | | | 233.62 | % |
| Risk-free rate | | | 1.06 | % | | | .10 | % |
| Dividend rate | | | - | | | | - | |
| Years to maturity | | | .52 | | | | 1 | |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy at the time of conversion is as follows:
| Inputs | | | | |
| Stock price | | $ | .01 - .02 | |
| Conversion price | | $ | .0097 - .01 | |
| Volatility (annual) | | | 169.37% – 172.14 | % |
| Risk-free rate | | | .39% - .93 | |
| Dividend rate | | | - | |
| Years to maturity | | | .49 - .50 | |
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s management.
NOTE 7 - RELATED PARTY TRANSACTIONS
The Company has received support from parties related through common ownership and directorship. These loans are unsecured, and due on demand. As of March 31, 2022 and December 31, 2021, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance due accrues interest at 12.5%. As of March 31, 2022, total accrued interest is $73,151.
The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $4,000 per month. The agreement expired on April 1, 2020 and has been renewed for two more years. As of March 31, 2022 and December 31, 2021, there is $0 and $2,000 of accrued compensation, respectively, due to Mr. Wood. During the three months ended March 31, 2022 and 2021, cash payments of $14,000 and $16,000, respectively, were paid to Mr. Wood.
The Company executed an employment agreement with its Chairman, Russell Bird, on January 1, 2019. Per the terms of the agreement, which is effective for one year, Mr. Bird is to be compensated $3,000 per month. As of March 31, 2022 and December 31, 2021, there is $48,000 and $45,000 of accrued compensation, respectively, due to Mr. Bird. Mr. Bird’s employment agreement has been renewed in 2020 for two more years. During the three months ended March 31, 2022 and 2021, cash payments of $6,000 and $0, respectively, were paid to Mr. Bird.
The Company has entered into an at-will consulting agreement with Jonathan Lane to serve as Chief Technology Officer. During the three months ended March 31, 2022 and 2021, the Company made cash payments to Mr. Lane of $10,000 and $3,000, respectively.
During the three months ended March 31, 2022 and, 2021, the Company paid $7,500 and $7,000, respectively, to the brother of the CEO for services related to development of the Company’s product.
During the three months ended March 31, 2022 and 2021, the Company paid $4,000 and $3,000, respectively, to the son of the CEO for website design services.
NOTE 8 - COMMON STOCK
During the three months ended March 31, 2022, Granite Global Value converted $152,880 of principal and interest into 16,146,666 shares of common stock.
During the three months ended March 31, 2022, Power Up Lending Group LTD converted $184,575 of principal and interest into 18,652,708 shares of common stock.
During the three months ended March 31, 2022, the Company issued 70,128,204 shares of common stock for the conversion of warrants.
During the three months ended March 31, 2022, the Company sold 114,000,000 shares of common stock for total cash proceeds of $855,000. The shares were sold pursuant to its Tier 2 of Regulation A Offering Statement.
NOTE 9 - PREFERRED STOCK
The Company is currently authorized to issue 5,000,000 shares of Series A Preferred Stock, par value $0.001 per share value with 1:25 voting rights. The Series A Preferred Stock ranks equal to the common stock on liquidation, pays no dividend and is convertible to common stock for one share of common for one share of Series A Preferred Stock.
The Company is currently authorized to issue 5,000,000 shares of Series B Preferred Stock, par value $0.001 per share. Each share of Series B Preferred Stock has a 1:100 voting right and is convertible into 100 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are 500,000 shares of Series B Preferred Stock issued and outstanding.
The Company is currently authorized to issue 5,000,000 shares of Series C Preferred Stock, par value $0.001 per share value. Each share of Series C Preferred Stock has a 1:50 voting right and is convertible into 50 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series C will automatically convert into common stock. There are no shares of Series C Preferred Stock issued and outstanding.
NOTE 10 - WARRANTS
A summary of the status of the Company’s outstanding stock warrants and changes during the year is presented below:
| | | Number of
Warrants | | | Weighted
Average
Exercise
Price | | | Weighted
Average
Remaining
Contract
Term | | | Aggregate
Intrinsic
Value | |
| Exercisable at December 31, 2020 | | | 15,974,026 | | | $ | 0.00385 | | | | 2.06 | | | $ | — | |
| Granted | | | 201,500,000 | | | $ | 0.0029 | | | | 4.62 | | | $ | — | |
| Expired | | | — | | | $ | — | | | | — | | | $ | — | |
| Increased for adjustment(1) | | | 12,012,987 | | | $ | — | | | | — | | | $ | — | |
| Exercised | | | (2,987,013 | ) | | $ | — | | | | — | | | $ | — | |
| Exercisable at December 31, 2021 | | | 226,500,000 | | | $ | 0.0013 | | | | 3.78 | | | $ | — | |
| Granted | | | — | | | $ | — | | | | — | | | $ | — | |
| Expired | | | — | | | $ | — | | | | — | | | $ | — | |
| Exercised | | | (60,000,000 | ) | | $ | — | | | | — | | | $ | — | |
| Exercisable at March 31, 2022 | | | 166,500,000 | | | $ | 0.0023 | | | | 3.89 | | | $ | 1,956,000 | |
Range of
Exercise Prices | | Number Outstanding
3/31/2022 | | | Weighted Average
Remaining
Contractual Life | | Weighted Average
Exercise Price | |
| $ | 0.002 - 0.0137 | | | 166,500,000 | | | 3.89 years | | $ | 0.0117 | |
| (1) | Pursuant to the terms of certain warrant agreements, when the exercise price is reduced for any reason outlined in the agreement, the number of warrant shares is increased so that the aggregated exercise price is equal to the original exercise price. |
The aggregate intrinsic value represents the total pretax intrinsic value, based on warrants with an exercise price less than the Company’s stock price as of March 31, 2022, which would have been received by the warrant holder had the warrant holder exercised their warrants as of that date.
NOTE 11 – COMMITMENTS AND CONTINGENCIES
The Company has been in the process of obtaining its 510k for DeltaWave. This requires a myriad of tests to prove to the FDA that the device is safe and effective. The company has diligently carried out these tests through independent testing labs. There have been no issues aside from a negative result on a cytotoxicity test due to incorrect procedures performed by a third-party lab. This roadblock has required the company to perform a retest. The company has failed the retest due to what is believed to be a faulty analysis by the testing company. The company believes they can narrow down the exact part of the device that is failing the test and quickly resolve this matter. They have committed to a new third party lab to redo the test and provide results within the next few weeks. If the Company were to fail the next test it would re-apply for its 510K resulting in additional time and expense. The Company is reliant upon passing the required test and receiving its 510K in order to begin operations and acknowledges that there is the possibility of this not occurring.
NOTE 12 - SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) management has performed an evaluation of subsequent events through the date that the financial statements were available to be issued and has determined that it does not have any material subsequent events to disclose in these financial statements other than the following.
Subsequent to March 31, 2022 Power Up converted $90,275 of principal and interest, into 8,680,288 shares of common stock.

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of REMSleep Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of REMSleep Holdings, Inc. (“the Company”) as of December 31, 2021 and 2020, and the related statements of operations, stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020 and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has an accumulated deficit, net losses, and negative cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Accounting for Embedded Conversion Features on Notes Payable — Refer to Notes 1 and 6 to the financial statements
Critical Audit Matter Description
The Company has issued several notes payable during the year with conversion rates that are adjustable at a discounted rate to public trading prices near the conversion date. The terms allow for variable amounts of shares to be converted for a set dollar value; this and other factors require the embedded conversion feature to be accounted for as a derivative and revalued at the conversion date or each period end if still outstanding. Calculations and accounting for the notes payable and embedded conversion features require management’s judgments related to initial and subsequent recognition of the debt and related features, use of a valuation model, and value of the inputs used in the selected valuation model.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to evaluating the Company’s accounting for notes payable and related accounts included the following, among others:
| | ● | Confirmation of notes payable and related terms, including notes paid off during the year. |
| | ● | Independent assessment of the appropriate valuation model for derivatives, performing independent calculations based on the model and comparing the Company’s results to a reasonable range as determined during the audit. |
| | ● | Determining if there were unusual transactions related to notes payable and the appropriate accounting treatment for such transactions. |
| | ● | Testing of substantially all transactions related to this matter. |

We have served as the Company’s auditor since 2018. Spokane, Washington |
| March 29, 2022 | |
REMSLEEP HOLDINGS, INC.
BALANCE SHEETS
| | | December 31,
2021 | | | December 31,
2020 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 3,383,568 | | | $ | 114,227 | |
| Inventory | | | — | | | | 11,064 | |
| Total current assets | | | 3,383,568 | | | | 125,291 | |
| | | | | | | | | |
| Other asset | | | 10,000 | | | | 10,000 | |
| Property and equipment, net | | | 105,061 | | | | 95,371 | |
| | | | | | | | | |
| Total Assets | | $ | 3,498,629 | | | $ | 230,662 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable | | $ | 15,505 | | | $ | 20,235 | |
| Accrued compensation | | | 47,000 | | | | 35,000 | |
| Accrued interest | | | 41,851 | | | | 23,013 | |
| Accrued interest – related party | | | 67,505 | | | | 44,921 | |
| Convertible Notes, net of discount of $206,157 and $157,233, respectively | | | 193,243 | | | | 44,867 | |
| Derivative Liability | | | 290,712 | | | | 700,719 | |
| Loan payable – related party | | | 179,191 | | | | 179,191 | |
| Loans payable | | | 45,000 | | | | 53,212 | |
| | | | | | | | | |
| Total Liabilities | | | 880,007 | | | | 1,101,158 | |
| | | | | | | | | |
| Commitments and Contingencies | | | — | | | | — | |
| | | | | | | | | |
| STOCKHOLDERS’ EQUITY (DEFICIT): | | | | | | | | |
| | | | | | | | | |
| Series A preferred stock, $0.001 par value, 5,000,000 shares authorized, 5,000,000 and issued and outstanding | | | 5,000 | | | | 5,000 | |
| Series B preferred stock, $0.001 par value, 5,000,000 shares authorized, 500,000 shares issued | | | 500 | | | | 500 | |
| Series C preferred stock, $0.001 par value, 5,000,000 shares authorized, no shares issued | | | — | | | | — | |
| Common stock, $0.001 par value, 1,000,000,000 shares authorized, 1,234,008,735 and 368,063,606 shares issued and outstanding, respectively | | | 1,234,006 | | | | 368,061 | |
| Discount to common stock | | | (94,708 | ) | | | — | |
| Additional paid in capital | | | 11,865,439 | | | | 5,321,885 | |
| Accumulated Deficit | | | (10,391,615 | ) | | | (6,565,942 | ) |
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | | | 2,618,622 | | | | (870,496 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 3,498,629 | | | $ | 230,662 | |
The accompanying notes are an integral part of these financial statements.
REMSLEEP HOLDINGS, INC.
STATEMENTS OF OPERATIONS
| | | For the Years Ended
December 31, | |
| | | 2021 | | | 2020 | |
| Operating Expenses: | | | | | | |
| Professional fees | | $ | 82,043 | | | $ | 41,525 | |
| Development expense | | | 129,311 | | | | 72,587 | |
| Compensation – related party | | | 84,000 | | | | 211,500 | |
| General and administrative | | | 130,334 | | | | 144,232 | |
| | | | | | | | | |
| Total operating expenses | | | 425,688 | | | | 469,844 | |
| | | | | | | | | |
| Loss from operations | | | (425,688 | ) | | | (469,844 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest expense | | | (918,579 | ) | | | (611,535 | ) |
| Default penalty of convertible note | | | (162,798 | ) | | | — | |
| Early payment penalty | | | — | | | | (49,162 | ) |
| Loss on issuance of convertible debt | | | (717,592 | ) | | | (350,986 | ) |
| Gain on forgiveness of debt | | | — | | | | 226,398 | |
| Change in fair value of derivative | | | (1,601,016 | ) | | | 79,677 | |
| Total other expense | | | (3,399,985 | ) | | | (705,608 | ) |
| | | | | | | | | |
| Loss before income taxes | | | (3,825,673 | ) | | | (1,175,452 | ) |
| | | | | | | | | |
| Provision for income taxes | | | — | | | | — | |
| | | | | | | | | |
| Net Loss | | $ | (3,825,673 | ) | | $ | (1,175,452 | ) |
| | | | | | | | | |
| Net loss per share, basic and diluted | | $ | (0.01 | ) | | $ | (0.01 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding, basic and diluted | | | 700,895,412 | | | | 215,671,601 | |
The accompanying notes are an integral part of these financial statements.
REMSLEEP HOLDINGS, INC.
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2021 AND 2020
| | | Series A Preferred Stock | | | Series B Preferred Stock | | | Common Stock | | | Discount to
Common | | | Additional
Paid-in | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Stock | | | Capital | | | Deficit | | | Total | |
| Balance, December 31, 2019 | | | 4,000,000 | | | $ | 4,000 | | | | — | | | $ | — | | | | 116,269,466 | | | $ | 116,268 | | | $ | — | | | $ | 4,260,395 | | | | (5,390,490 | ) | | $ | (1,009,827 | ) |
| Common stock issued for conversion of debt | | | — | | | | — | | | | — | | | | — | | | | 198,903,759 | | | | 198,903 | | | | — | | | | 910,031 | | | | — | | | | 1,108,934 | |
| Common stock issued for cash | | | — | | | | — | | | | — | | | | — | | | | 15,000,000 | | | | 15,000 | | | | — | | | | 60,000 | | | | — | | | | 75,000 | |
| Warrants converted to common stock | | | — | | | | — | | | | — | | | | — | | | | 37,890,381 | | | | 37,890 | | | | — | | | | (37,890 | ) | | | — | | | | — | |
| Warrant down round protection | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,349 | | | | — | | | | 3,349 | |
| Preferred stock issued for services - related party | | | 1,000,000 | | | | 1,000 | | | | 500,000 | | | | 500 | | | | — | | | | — | | | | — | | | | 126,000 | | | | — | | | | 127,500 | |
| Net Loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,175,452 | ) | | | (1,175,452 | ) |
| Balance, December 31, 2020 | | | 5,000,000 | | | | 5,000 | | | | 500,000 | | | | 500 | | | | 368,063,606 | | | | 368,061 | | | | — | | | | 5,321,885 | | | | (6,565,942 | ) | | | (870,496 | ) |
| Common stock issued for conversion of debt | | | — | | | | — | | | | — | | | | — | | | | 408,666,436 | | | | 408,666 | | | | (94,708 | ) | | | 3,685,763 | | | | — | | | | 3,999,721 | |
| Common stock issued for cash | | | — | | | | — | | | | — | | | | — | | | | 413,800,000 | | | | 413,800 | | | | — | | | | 2,689,700 | | | | — | | | | 3,103,500 | |
| Warrants converted to common stock | | | — | | | | — | | | | — | | | | — | | | | 43,478,693 | | | | 43,479 | | | | — | | | | (43,479 | ) | | | — | | | | — | |
| Beneficial conversion feature | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 211,570 | | | | — | | | | 211,570 | |
| Net Loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,825,673 | ) | | | (3,825,673 | ) |
| Balance, December 31, 2021 | | | 5,000,000 | | | $ | 5,000 | | | | 500,000 | | | $ | 500 | | | | 1,234,008,735 | | | $ | 1,234,006 | | | $ | (94,708 | ) | | $ | 11,865,439 | | | $ | (10,391,615 | ) | | $ | 2,618,622 | |
The accompanying notes are an integral part of these financial statements.
REMSLEEP HOLDINGS, INC.
STATEMENTS OF CASH FLOWS
| | | For the Years Ended
December 31, | |
| | | 2021 | | | 2020 | |
| Cash Flows from Operating Activities: | | | | | | |
| Net loss | | $ | (3,825,673 | ) | | $ | (1,175,452 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation expense | | | 57,561 | | | | 49,153 | |
| Stock compensation expense – related party | | | — | | | | 127,500 | |
| Change in fair value of derivative | | | 1,601,016 | | | | (79,677 | ) |
| Discount amortization | | | 813,619 | | | | 561,576 | |
| Loss on issuance of convertible debt | | | 717,592 | | | | 350,986 | |
| Gain on forgiveness of debt | | | — | | | | (226,398 | ) |
| Default penalty of convertible note | | | 162,798 | | | | — | |
| Changes in Operating Assets and Liabilities: | | | | | | | | |
| Prepaids and other assets | | | — | | | | 7,909 | |
| Inventory | | | 11,064 | | | | (3,064 | ) |
| Accounts payable | | | (4,730 | ) | | | (11,654 | ) |
| Accrued compensation – related party | | | 12,000 | | | | 20,000 | |
| Accrued interest | | | 82,174 | | | | 21,295 | |
| Accrued interest – related party | | | 22,584 | | | | 22,533 | |
| Net cash used by operating activities | | | (349,995 | ) | | | (335,293 | ) |
| | | | | | | | | |
| Cash Flows from Investing Activities: | | | | | | | | |
| Purchase of property and equipment | | | (67,252 | ) | | | (36,710 | ) |
| Net cash used by investing activities | | | (67,252 | ) | | | (36,710 | ) |
| | | | | | | | | |
| Cash Flows from Financing Activities: | | | | | | | | |
| Repayment of loans | | | (8,212 | ) | | | (3,344 | ) |
| Proceeds from convertible notes payable | | | 591,300 | | | | 460,000 | |
| Repayment of convertible notes payable | | | — | | | | (165,000 | ) |
| Proceeds from sale of common stock | | | 3,103,500 | | | | 75,000 | |
| Net cash provided by financing activities | | | 3,686,588 | | | | 366,656 | |
| | | | | | | | | |
| Net increase (decrease) in cash | | | 3,269,341 | | | | (5,347 | ) |
| Cash at beginning of the year | | | 114,227 | | | | 119,574 | |
| Cash at end of the year | | $ | 3,383,568 | | | $ | 114,227 | |
| | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | |
| Interest paid in cash | | $ | — | | | $ | — | |
| Taxes paid | | $ | — | | | $ | — | |
| | | | | | | | | |
| Supplemental non-cash disclosure: | | | | | | | | |
Common stock issued for conversion of note payable principal
and accrued interest | | $ | 724,359 | | | $ | 452,477 | |
The accompanying notes are an integral part of these financial statements.
REMSLEEP HOLDINGS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2021
NOTE 1 - BACKGROUND
Business Activity
REMSleep Holdings, Inc., (the “Company”) was incorporated in the State of Nevada on June 6, 2007. On January 5, 2015 the name of the Company was changed to REMSleep Holdings, Inc. and the business model was changed to reflect the new direction of the Company; to develop and distribute products to help people affected by sleep apnea. On May 30, 2015 REMSleep LLC was formally merged into REMSleep Holdings, Inc.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Concentrations of Credit Risk
We maintain our cash in bank deposit accounts, the balances of which at times may exceed federally insured limits. We continually monitor our banking relationships and consequently have not experienced any losses in our accounts. At times, such deposits may be in excess of the Federal Deposit Insurance Corporation insurable amount (“FDIC”). As of December 31, 2021, the Company had $3,133,568 of cash above the FDIC’s $250,000 coverage limit.
Cash equivalents
The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. There were no cash equivalents for the years ended December 31, 2021 or 2020.
Reclassifications
Certain reclassifications have been made to the prior period financial information to conform to the presentation used in the financial statements for the year ended December 31, 2021.
Property and Equipment
Fixed assets are carried at the lower of cost or net realizable value. All fixed assets with a cost of $2,000 or greater are capitalized. Depreciation of property and equipment is calculated using the straight-line method over the estimated useful lives of the assets, which range from three to five years. Leasehold improvements are amortized over the lesser of the remaining term of the lease or the estimated useful life of the asset. Major betterments that extend the useful lives of assets are also capitalized. Normal maintenance and repairs are charged to expense as incurred. When assets are sold or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is recognized in operations.
Basic and Diluted Earnings Per Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period. The weighted average number of common shares outstanding and potentially outstanding common shares assumes that the Company incorporated as of the beginning of the first period presented.
As of December 31, 2021, the Company had approximately 46,972,920 of potentially dilutive shares of common stock from convertible debt, 190,064,171 potentially dilutive shares of common stock warrants, 5,000,000 shares from Series A preferred stock and 50,000,000 from Series B preferred stock.
As of December 31, 2020, the Company had approximately 209,383,191 of potentially dilutive shares of common stock from convertible debt, 15,974,026 potentially dilutive shares of common stock warrants, 5,000,000 shares from Series A preferred stock and 50,000,000 from Series B preferred stock.
Stock-based Compensation
In June 2018, the FASB issued ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 allows companies to account for nonemployee awards in the same manner as employee awards. The guidance is effective for fiscal years beginning after December 15, 2018, and interim periods within those annual periods.
Fair Value of Financial Instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
| Level 1: | Quoted market prices available in active markets for identical assets or liabilities as of the reporting date. |
| | |
| Level 2: | Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date. |
| | |
| Level 3: | Pricing inputs that are generally unobservable inputs and not corroborated by market data. |
The carrying amount of the Company’s financial assets and liabilities, such as cash, prepaid expenses and accrued expenses approximate their fair value because of the short maturity of those instruments. The Company’s notes payable approximates the fair value of such instruments as the notes bear interest rates that are consistent with current market rates.
The following table classifies the Company’s liabilities measured at fair value on a recurring basis into the fair value hierarchy as of December 31, 2021 and 2020:
December 31, 2021:
| Description | | Level 1 | | | Level 2 | | | Level 3 | |
| Derivative | | $ | - | | | $ | - | | | $ | 290,712 | |
| Total | | $ | - | | | $ | - | | | $ | 290,712 | |
December 31, 2020:
| Description | | Level 1 | | | Level 2 | | | Level 3 | |
| Derivative | | $ | - | | | $ | - | | | $ | 700,719 | |
| Total | | $ | - | | | $ | - | | | $ | 700,719 | |
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40)—Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. ASU 2020-06 reduces the number of accounting models for convertible debt instruments and convertible preferred stock. For convertible instruments with conversion features that are not required to be accounted for as derivatives under Topic 815, Derivatives and Hedging, or that do not result in substantial premiums accounted for as paid-in capital, the embedded conversion features no longer are separated from the host contract. ASU 2020-06 also removes certain conditions that should be considered in the derivatives scope exception evaluation under Subtopic 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, and clarify the scope and certain requirements under Subtopic 815-40. In addition, ASU 2020-06 improves the guidance related to the disclosures and earnings-per-share (EPS) for convertible instruments and contract in entity’s own equity. ASU 2020-06 is effective for public business entities that meet the definition of a Securities and Exchange Commission (SEC) filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. The Company is currently evaluating the impact this ASU will have on its financial statements.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
NOTE 3 - GOING CONCERN
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has an accumulated deficit of $10,391,615 at December 31, 2021, had a net loss of $3,825,673 (approximately $3,295,000 was non-cash other expense related to convertible debt) and net cash used in operating activities of $349,995 for the year ended December 31, 2021. The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
The Company is in the final stages of product development and plans to begin selling its product in Q2 of 2022. The Company will continue to finance its operations through debt and/or equity financing as needed.
The industry in which we operate depends heavily upon our ability to obtain raw material and manufacture our product as well as the overall level of consumer and business spending. A sustained deterioration in general economic conditions (including distress in financial markets, turmoil in specific economies around the world, public health crises, and additional government intervention), particularly in the United States, may have a negative financial impact to our Company. Adverse conditions as a result of the global COVID-19 outbreak, have and may continue to impact our manufacturing processes and ultimately our ability to sell our product.
NOTE 4 - PROPERTY & EQUIPMENT
Long lived assets, including property and equipment and certain intangible assets to be held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Impairment losses are recognized if expected future cash flows of the related assets are less than their carrying values. Measurement of an impairment loss is based on the fair value of the asset. Long-lived assets and certain identifiable intangibles to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Property and Equipment and intangible assets are first recorded at cost. Depreciation and/or amortization is computed using the straight-line method over the estimated useful lives of the various classes of assets as follows between three and five years.
Maintenance and repair expenses, as incurred, are charged to expense. Betterments and renewals are capitalized in plant and equipment accounts. Cost and accumulated depreciation applicable to items replaced or retired are eliminated from the related accounts with any gain or loss on the disposition included as income.
Assets stated at cost, less accumulated depreciation consisted of the following:
| | | December 31,
2021 | | | December 31,
2020 | |
| Furniture/fixtures | | $ | 14,904 | | | $ | 14,904 | |
| Office equipment | | | 14,522 | | | | 7,136 | |
| Automobile | | | 29,905 | | | | 17,189 | |
| Tooling/Molds | | | 176,990 | | | | 141,785 | |
| Less: accumulated depreciation | | | (131,260 | ) | | | (85,643 | ) |
| Fixed assets, net | | $ | 105,061 | | | $ | 95,371 | |
Depreciation expense
Depreciation expense for the years ended December 31, 2021 and 2020 was $57,561 and $49,153, respectively.
NOTE 5 - LOANS PAYABLE
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by a Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. As of December 31, 2021, there is $45,000 and $21,549 of principal and interest due on this loan. As of December 31, 2020, there is $45,000 and $19,355 of principal and interest due on this loan.
On March 23, 2018, the Company purchased an automobile. The purchase price was $16,963.46. The interest rate on the loan is 5.8% and matures on April 7, 2023. Payments on the loan, consisting of principal and interest, are $327 per month. The automobile was sold and the loan was paid in full in October 2021. As of December 31, 2021 and December 31, 2020 there is $0 and $8,212, respectively, due on this loan.
NOTE 6 - CONVERTIBLE NOTES
The following table summarizes the convertible notes and related activity as of December 31, 2021:
| Note Holder | | Date | | Maturity Date | | Interest | | | Balance
December 31,
2020 | | | Additions | | | Conversions/
Repayments | | | Balance
December 31,
2021 | |
| Diamond Investments II LLC | | 8/28/2020 | | 8/28/2021 | | | 8 | % | | | 110,250 | | | | — | | | | (110,250 | ) | | | — | |
| Power Up Lending Group LTD | | 12/18/2020 | | 12/18/2021 | | | 10 | % | | | 91,850 | | | | — | | | | (91,850 | ) | | | — | |
| Granite Global Investments Ltd | | 10/26/2020 | | 11/6/2021 | | | 24.5 | % | | | — | | | | 162,798 | | | | (162,798 | ) | | | — | |
| Granite Global Investments Ltd | | 1/6/2021 | | 1/6/2022 | | | 12 | % | | | — | | | | 31,000 | | | | (31,000 | ) | | | — | |
| Granite Global Investments Ltd | | 1/30/2021 | | 1/30/2022 | | | 12 | % | | | — | | | | 36,000 | | | | (36,000 | ) | | | — | |
| Power Up Lending Group LTD | | 2/22/2021 | | 2/22/2022 | | | 10 | % | | | — | | | | 84,150 | | | | (84,150 | ) | | | — | |
| Granite Global Investments Ltd | | 4/7/2021 | | 4/7/2022 | | | 10 | % | | | — | | | | 36,500 | | | | — | | | | 36,500 | |
| Granite Global Investments Ltd | | 4/7/2021 | | 4/7/2022 | | | 10 | % | | | — | | | | 91,850 | | | | (91,850 | ) | | | — | |
| Granite Global Investments Ltd | | 4/9/2021 | | 4/9/2022 | | | 10 | % | | | — | | | | 100,000 | | | | — | | | | 100,000 | |
| Granite Global Investments Ltd | | 5/3/2021 | | 5/3/2022 | | | 10 | % | | | — | | | | 53,625 | | | | (53,625 | ) | | | — | |
| Power Up Lending Group LTD | | 7/22/2021 | | 7/22/2022 | | | 10 | % | | | — | | | | 58,850 | | | | — | | | | 58,850 | |
| Power Up Lending Group LTD | | 8/26/2021 | | 8/26/2022 | | | 10 | % | | | — | | | | 58,850 | | | | — | | | | 58,850 | |
| Power Up Lending Group LTD | | 9/22/2021 | | 9/22/2022 | | | 10 | % | | | — | | | | 58,850 | | | | — | | | | 58,850 | |
| Power Up Lending Group LTD | | 10/12/2021 | | 10/12/2022 | | | 10 | % | | | — | | | | 86,350 | | | | — | | | | 86,350 | |
| | | | | | | | Total | | | $ | 202,100 | | | $ | 858,823 | | | $ | (661,523 | ) | | $ | 399,400 | |
| | | | | Less debt discount | | | | (157,233 | ) | | | | | | | | | | | (206,157 | ) |
| | | | | | | | | | | $ | 44,867 | | | | | | | | | | | $ | 193,243 | |
A summary of the activity of the derivative liability for the notes above is as follows:
| Balance at December 31, 2019 | | $ | 626,831 | |
| Increase to derivative due to new issuances | | | 808,643 | |
| Decrease to derivative due to conversion/repayments | | | (897,519 | ) |
| Derivative loss due to mark to market adjustment | | | 162,764 | |
| Balance at December 31, 2020 | | | 700,719 | |
| Increase to derivative due to new issuances | | | 1,087,302 | |
| Decrease to derivative due to conversion/repayments | | | (3,098,325 | ) |
| Derivative loss due to mark to market adjustment | | | 1,601,016 | |
| Balance at December 31, 2021 | | $ | 290,712 | |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy as of December 31, 2021 is as follows:
| Inputs | | December 31,
2021 | | | Initial
Valuation | |
| Stock price | | $ | .0022 | | | $ | .0018 - .028 | |
| Conversion price | | $ | .0094 | | | $ | .002 - .0115 | |
| Volatility (annual) | | | 234.92 – 246.30 | % | | | 203.69% - 246.30 | % |
| Risk-free rate | | | .05% - .09 | % | | | .10% - .39 | % |
| Dividend rate | | | - | | | | - | |
| Years to maturity | | | .52 – .98 | | | | .83 - 1 | |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy at the time of conversion is as follows:
| Inputs | | | | |
| Stock price | | $ | .013 - .029 | |
| Conversion price | | $ | .003 - .0096 | |
| Volatility (annual) | | | 148.35% – 293.57 | % |
| Risk-free rate | | | .04% - .06 | % |
| Dividend rate | | | - | |
| Years to maturity | | | .25 - .50 | |
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s management.
NOTE 7 - RELATED PARTY TRANSACTIONS
The Company has received support from parties related through common ownership and directorship. These loans are unsecured, and due on demand. As of December 31, 2021 and 2020, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance due accrues interest at 12.5%. As of December 31, 2021, total accrued interest is $67,505.
The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $4,000 per month. The agreement expired on April 1, 2020 and has been renewed for two more years. As of December 31, 2021 and 2020, there is $2,000 and $2,000 of accrued compensation, respectively, due to Mr. Wood. During the years ended December 31, 2021 and 2020, cash payments of $48,000 and $46,000, respectively, were paid to Mr. Wood.
The Company executed an employment agreement with its Chairman, Russell Bird, on January 1, 2019. Per the terms of the agreement, which is effective for one year, Mr. Bird is to be compensated $3,000 per month. As of December 31, 2021 and 2020, there is $45,000 and $33,000 of accrued compensation, respectively, due to Mr. Bird. Mr. Bird’s employment agreement has been renewed in 2020 for two more years. During the years ended December 31, 2021 and 2020, cash payments of $24,000 and $18,000, respectively, were paid to Mr. Bird.
The Company has entered into an at-will consulting agreement with Jonathan Lane to serve as Chief Technology Officer. During the years ended December 31, 2021 and 2020, the Company made cash payments to Mr. Lane of $26,000 and $15,000, respectively.
During the years ended December 31, 2021 and 2020, the Company paid $12,000 and $1,000, respectively, to the son of the CEO for services related to development of the Company’s product.
During the years ended December 31, 2021 and 2020, the Company paid $9,000 and $22,000, respectively, to the brother of the CEO for website design services.
On November 23, 2020, the Company granted 500,000 shares of Series A preferred stock to Mr. Bird for services rendered to the Company. The shares were valued at $0.0025, the closing stock price of the Company’s common shares on the date of grant, for total non-cash compensation expense of $1,250. The closing price for common stock was deemed an acceptable method for valuation as one share of Series A preferred stock is convertible into one share of common stock.
On November 23, 2020, the Company granted 500,000 shares of Series A preferred stock to Mr. Wood for services rendered to the Company. The shares were valued at $0.0025, the closing stock price of the Company’s common shares on the date of grant, for total non-cash compensation expense of $1,250. The closing price for common stock was deemed an acceptable method for valuation as one share of Series A preferred stock is convertible into one share of common stock.
On November 23, 2020, the Company granted 250,000 shares of Series B preferred stock to Mr. Bird for services rendered to the Company. The shares were valued at $0.0025, the closing stock price of the Company’s common shares on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. The closing price for common stock multiplied by 100 was deemed an acceptable method for valuation as one share of Series B preferred stock is convertible into 100 shares of common stock.
On November 23, 2020, the Company granted 250,000 shares of Series B preferred stock to Mr. Wood for services rendered to the Company. The shares were valued at $0.0025, the closing stock price of the Company’s common shares on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. The closing price for common stock multiplied by 100 was deemed an acceptable method for valuation as one share of Series B preferred stock is convertible into 100 shares of common stock.
NOTE 8 - COMMON STOCK
During the year ended December 31, 2021, Diamond Investments converted $110,250 of principal and $5,059 of interest, into 29,954,167 shares of common stock.
During the year ended December 31, 2021, Granite Global Value converted $229,798 and $43,164 of principal and interest, respectively, into 340,735,898 shares of common stock.
During the year ended December 31, 2021, Power Up Lending Group LTD converted $321,475 and $14,613 of principal and interest, respectively, into 37,976,371 shares of common stock.
During the year ended December 31, 2021, the Company issued 43,478,695 shares of common stock for the conversion of warrants.
During the year ended December 31, 2021, the Company sold 413,800,000 shares of common stock for total cash proceeds of $3,103,500. The shares were sold pursuant to its Tier 2 of Regulation A Offering Statement.
During the year ended December 31, 2020, Armada Capital Partners LLC converted $20,850 and $110 of principal and interest, respectively, into 5,202,346 shares of common stock. As of December 31, 2020, this loan has been fully converted.
During the year ended December 31, 2020, BHP Capital NY Inc converted $7,394 and $35 of principal and interest, respectively, into 1,919,620 shares of common stock. As of December 31, 2020, this loan has been fully converted.
During the year ended December 31, 2020, Jefferson Street Capital LLC converted $13,750 of principal and $2,205 of interest, respectively, into 3,989,090 shares of common stock. As of December 31, 2020, this loan has been fully converted.
During the year ended December 31, 2020, Odyssey Capital Funding LLC converted $35,000 of principal and $2,890 of interest, respectively, into 8,630,042 shares of common stock. As of December 31, 2020, this loan has been fully converted.
During the year ended December 31, 2020, 37,890,381 shares of common stock were issued in conversion of 50,262,343 warrants.
During the year ended December 31, 2020, Power Up Lending Group LTD converted $188,300 of principal and $7,650 of interest, respectively, into 62,639,262 shares of common stock. As of December 31, 2020, this loan has been fully converted.
During the year ended December 31, 2020, Granite Global Value converted $174,265 of principal into 116,523,399 shares of common stock.
During the year ended December 31, 2020, the Company sold 15,000,000 shares of common stock pursuant to the terms of its Form 1-A, Regulation A Offering Statement, for total cash proceeds of $75,000.
See Note 7 for stock issued to related parties.
NOTE 9 - PREFERRED STOCK
The Company is currently authorized to issue 5,000,000 shares of Series A Preferred Stock, par value $0.001 per share value with 1:25 voting rights. The Series A Preferred Stock ranks equal to the common stock on liquidation, pays no dividend and is convertible to common stock for one share of common for one share of Series A Preferred Stock.
The Company is currently authorized to issue 5,000,000 shares of Series B Preferred Stock, par value $0.001 per share. Each share of Series B Preferred Stock has a 1:100 voting right and is convertible into 100 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are 500,000 shares of Series B Preferred Stock issued and outstanding.
The Company is currently authorized to issue 5,000,000 shares of Series C Preferred Stock, par value $0.001 per share value. Each share of Series C Preferred Stock has a 1:50 voting right and is convertible into 50 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series C will automatically convert into common stock. There are no shares of Series C Preferred Stock issued and outstanding.
See Note 7 for stock issued to related parties.
NOTE 10 - INCOME TAX
Deferred taxes are provided on a liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carry forwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The Company has evaluated Staff Accounting Bulletin No. 118 regarding the impact of the decreased tax rates of the Tax Cuts & Jobs Act. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment. The U.S. federal income tax rate of 21% is being used.
The provision for Federal income tax consists of the following December 31:
| | | 2021 | | | 2020 | |
| Federal income tax benefit attributable to: | | | | | | |
| Current Operations | | $ | 803,000 | | | $ | 221,700 | |
| Less: valuation allowance | | | (803,000 | ) | | | (221,700 | ) |
| Net provision for Federal income taxes | | $ | - | | | $ | - | |
The cumulative tax effect at the expected rate of 21% of significant items comprising our net deferred tax amount is as follows:
| | | 2021 | | | 2020 | |
| Deferred tax asset attributable to: | | | | | | |
| Net operating loss carryover | | $ | 2,182,000 | | | $ | 1,343,000 | |
| Less: valuation allowance | | | (2,182,000 | ) | | | (1,343,000 | ) |
| Net deferred tax asset | | $ | - | | | $ | - | |
At December 31, 2021, the Company had net operating loss carry forwards of approximately $2,182,000 that may be offset against future taxable income. NOLs from tax years up to 2017 can be carried forward twenty years. Under the CARES Act, the Company carry forward NOLs indefinitely for NOLs generated in a tax year beginning after 2017, that remain after they are carried back to tax years in the five-year carryback period. No tax benefit has been reported in the December 31, 2021 financial statements since the potential tax benefit is offset by a valuation allowance of the same amount.
Due to the change in ownership provisions of the Tax Reform Act of 1986, net operating loss carry forwards for Federal Income tax reporting purposes are subject to annual limitations. Should a change in ownership occur, net operating loss carry forwards may be limited as to use in future years. With few exceptions, the Company is no longer subject to U.S. federal, state and local income tax examinations by tax authorities for years before 2016.
NOTE 11 - WARRANTS
On January 6, 2021, the Company issued 35,000,000 warrants to Granite Global Investments Ltd in conjunction with convertible debt. The warrants are exercisable for 5 years at $.006 per share. The warrants were evaluated for purposes of classification between liability and equity. The warrants do not contain features that would require a liability classification and are therefore considered equity.
Using the fair value calculation, the relative fair value between the debt issued and the warrants was calculated to determine the warrants recorded equity amount of $24,440, accounted for in additional paid in capital.
The Black Scholes pricing model was used to estimate the fair value of the Warrants issued with the following inputs:
| Warrants | | | 35,000,000 | |
| Share price | | $ | 0.0033 | |
| Exercise Price | | $ | 0.006 | |
| Term | | | 5 years | |
| Volatility | | | 353 | % |
| Risk Free Interest Rate | | | .43 | % |
| Dividend rate | | | - | |
On January 30, 2021, the Company issued 120,000,000 warrants to Granite Global Investments Ltd in conjunction with convertible debt. The warrants are exercisable for 5 years at $.0003 per share. The warrants were evaluated for purposes of classification between liability and equity. The warrants do not contain features that would require a liability classification and are therefore considered equity.
Using the fair value calculation, the relative fair value between the debt issued and the warrants was calculated to determine the warrants recorded equity amount of $33,652, accounted for in additional paid in capital.
The Black Scholes pricing model was used to estimate the fair value of the Warrants issued with the following inputs:
| Warrants | | | 120,000,000 | |
| Share price | | $ | 0.0043 | |
| Exercise Price | | $ | 0.0003 | |
| Term | | | 5 years | |
| Volatility | | | 352 | % |
| Risk Free Interest Rate | | | 0.45 | % |
| Dividend rate | | | - | |
On April 7, 2021, the Company issued 36,500,000 warrants to Granite Global Investments Ltd in conjunction with convertible debt. The warrants are exercisable for 5 years at $.006 per share. The warrants were evaluated for purposes of classification between liability and equity. The warrants do not contain features that would require a liability classification and are therefore considered equity.
Using the fair value calculation, the relative fair value between the debt issued and the warrants was calculated to determine the warrants recorded equity amount of $34,505, accounted for in additional paid in capital.
The Black Scholes pricing model was used to estimate the fair value of the Warrants issued with the following inputs:
| Warrants | | | 36,500,000 | |
| Share price | | $ | 0.0173 | |
| Exercise Price | | $ | 0.006 | |
| Term | | | 5 years | |
| Volatility | | | 319 | % |
| Risk Free Interest Rate | | | 0.45 | % |
| Dividend rate | | | — | |
On April 9, 2021, the Company issued 10,000,000 warrants to Granite Global Investments Ltd in conjunction with convertible debt. The warrants are exercisable for 5 years at $.012 per share. The warrants were evaluated for purposes of classification between liability and equity. The warrants do not contain features that would require a liability classification and are therefore considered equity.
Using the fair value calculation, the relative fair value between the debt issued and the warrants was calculated to determine the warrants recorded equity amount of $72,217, accounted for in additional paid in capital.
The Black Scholes pricing model was used to estimate the fair value of the Warrants issued with the following inputs:
| Warrants | | | 10,000,000 | |
| Share price | | $ | 0.026 | |
| Exercise Price | | $ | 0.012 | |
| Term | | | 5 years | |
| Volatility | | | 319 | % |
| Risk Free Interest Rate | | | 0.87 | % |
| Dividend rate | | | — | |
A summary of the status of the Company’s outstanding stock warrants and changes during the year is presented below:
| | | Number of
Warrants | | | Weighted
Average
Exercise
Price | | | Weighted
Average
Remaining
Contract
Term | | | Aggregate
Intrinsic
Value | |
| Outstanding at December 31, 2019 | | | 3,000,000 | | | $ | 0.07 | | | | 2.59 | | | $ | — | |
| Granted | | | 63,236,369 | | | $ | 0.00385 | | | | 2.56 | | | $ | — | |
| Expired | | | — | | | $ | — | | | | — | | | $ | — | |
| Exercised | | | (50,262,343 | ) | | $ | 0.00385 | | | | — | | | $ | — | |
| Exercisable at December 31, 2020 | | | 15,974,026 | | | $ | 0.00385 | | | | 2.06 | | | $ | — | |
| Granted | | | 201,500,000 | | | $ | 0.0029 | | | | 4.62 | | | $ | — | |
| Expired | | | — | | | $ | — | | | | — | | | $ | — | |
| Increased for adjustment(1) | | | 12,012,987 | | | $ | — | | | | — | | | $ | — | |
| Exercised | | | (2,987,013 | ) | | $ | — | | | | — | | | $ | — | |
| Exercisable at December 31, 2021 | | | 226,500,000 | | | $ | 0.0136 | | | | 3.78 | | | $ | 724,800 | |
| | (1) | Pursuant to the terms of certain warrant agreements, when the exercise price is reduced for any reason outlined in the agreement, the number of warrant shares is increased so that the aggregated exercise price is equal to the original exercise price. |
Range of Exercise
Prices | | Number Outstanding
12/31/2021 | | | Weighted Average
Remaining
Contractual Life | | Weighted Average
Exercise Price | |
| $0.0003 - $0.012 | | | 214,804,492 | | | 3.78 years | | $ | 0.0136 | |
The aggregate intrinsic value represents the total pretax intrinsic value, based on warrants with an exercise price less than the Company’s stock price as of December 31, 2021, which would have been received by the warrant holder had the warrant holder exercised their warrants as of that date.
NOTE 12 – COMMITMENTS AND CONTINGENCIES
The Company has been in the process of obtaining its 510k for DeltaWave. This requires a myriad of tests to prove to the FDA that the device is safe and effective. The company has diligently carried out these tests through independent testing labs. There have been no issues aside from a negative result on a cytotoxicity test due to incorrect procedures performed by a third-party lab. This roadblock has required the company to perform a retest. The company has failed the retest due to what is believed to be a faulty analysis by the testing company. The company believes they can narrow down the exact part of the device that is failing the test and quickly resolve this matter. They have committed to a new third party lab to redo the test and provide results within the next few weeks. If the Company were to fail the next test it would re-apply for its 510K resulting in additional time and expense. The Company is reliant upon passing the required test and receiving its 510K in order to begin operations and acknowledges that there is the possibility of this not occurring.
NOTE 13 - SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) management has performed an evaluation of subsequent events through the date that the financial statements were available to be issued and has determined that it does not have any material subsequent events to disclose in these financial statements other than the following.
Subsequent to December 31, 2021, the Company sold 114,000,000 shares of common stock to for total cash proceeds of $855,000. The shares were sold pursuant to its Tier 2 of Regulation A Offering Statement.
Subsequent to December 31, 2021, Power Up converted $61,525 of principal and interest, into 12,309,924 shares of common stock.
Subsequent to December 31, 2021, Granite Global converted $152,880 of principal and interest, into 16,146,666 shares of common stock.
Subsequent to December 31, 2021, the Company issued Jefferson Street Capital LLC 40,654,520 shares of common stock for the exercise of warrants.
Subsequent to December 31, 2021, the Company issued Granite Global 29,473,684 shares of common stock for the exercise of warrants.
RemSleep Holdings, Inc.
500,000,000 Shares of Common Stock
PROSPECTUS
___________, 2022
Until ____________, 2022, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses payable by us in connection with the issuance and distribution of the securities being registered hereunder. No expenses will be borne by the Selling Stockholder. All of the amounts shown are estimates, except for the SEC registration fee.
| Type | | Amount | |
| SEC registration fee | | $ | 866.75 | |
| Accounting fees and expenses* | | | 10,000 | |
| Legal fees and expenses* | | | 20,000 | |
| Printing expenses* | | | 1,500 | |
| Miscellaneous fees and expenses* | | | 1,000 | |
| Total expenses* | | | 33,366.75 | |
Item 14. Indemnification of Directors and Officers
Article Twelfth the Company’s Articles of Incorporation, as amended, provides, as follows:
“Twelfth. No Director or Officer of the corporation shall be personally liable to the corporation or any of its stockholders for damages for breach of fiduciary duty as a Director or Officer involving any act or omission of any such Director or Officer; provided, however, that the foregoing provision shall not eliminate or limit the liability of a Director or Officer for acts or omissions which involve intentional misconduct, fraud or a knowing violation of the law, or (ii) the payment of dividends in violation of Section 78.300 of the Nevada Revised Statutes. Any repeal or modification of this Article by the Stockholders of the corporation shall be prospective only, and shall not adversely affect any limitations on the personal liability of a Director or Officer of the corporation for acts or omissions prior to such repeal or modification.”
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us pursuant to the foregoing provisions or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Item 15. Recent Sales of Unregistered Securities
The following is a summary of transactions by us within the past three years involving sales or our securities that were not registered under the Securities Act.
During 2022, the Company has issued securities that were not registered under the Securities Act, as follows:
Common Stock
During 2022, a total of 43,479,662 shares of Company Common Stock have been issued in payment of indebtedness under convertible promissory notes in the total amount of $630,112. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During 2022, a total of 114,000,000 shares of Company Common Stock have been sold for cash in the total amount of $855,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Regulation A under the Securities Act.
During 2022, a total of 70,128,204 shares of Company Common Stock have been issued upon conversion of warrants. These warrants were issued on a cashless basis. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During 2021, the Company issued securities that were not registered under the Securities Act, as follows:
Convertible Promissory Notes
On January 6, 2021, the Company issued a convertible promissory note in the principal amount of $31,000 to Granite Global Investments Ltd. The note was due January 6, 2022, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On January 30, 2021, the Company issued a convertible promissory note in the principal amount of $36,000 to Granite Global Investments Ltd. The note was due January 30, 2022, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On February 22, 2021, the Company issued a convertible promissory note in the principal amount of $84,150 to Power Up Lending Group LTD. The note was due February 22, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On April 7, 2021, the Company issued a convertible promissory note in the principal amount of $36,500 to Granite Global Investments Ltd. The note was due April 7, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On April 7, 2021, the Company issued a convertible promissory note in the principal amount of $91,850 to Granite Global Investments Ltd. The note was due April 7, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On April 9, 2021, the Company issued a convertible promissory note in the principal amount of $100,000 to Granite Global Investments Ltd. The note was due April 9, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On May 3, 2021, the Company issued a convertible promissory note in the principal amount of $53,625 to Granite Global Investments Ltd. The note was due May 3, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On July 22, 2021, the Company issued a convertible promissory note in the principal amount of $58,850 to Power Up Lending Group LTD. The note was due July 22, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On August 26, 2021, the Company issued a convertible promissory note in the principal amount of $84,150 to Power Up Lending Group LTD. The note was due August 26, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On September 22, 2021, the Company issued a convertible promissory note in the principal amount of $58,850 to Power Up Lending Group LTD. The note was due September 22, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On October 12, 2021, the Company issued a convertible promissory note in the principal amount of $86,350 to Power Up Lending Group LTD. The note was due October 12, 2022, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
The issuances of the foregoing convertible promissory notes were exempt from the registration requirements of the Securities Act under Rule 506 of Regulation D and Section 4(a)(2) of the Securities Act.
Warrants
During the three months ended March 31, 2021, the Company issued 35,000,000 warrants to Granite Global Investments Ltd. in connection with a loan transaction, which warrants had an exercise period of five years at an initial exercise price of $0.006 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended March 31, 2021, the Company issued 120,000,000 warrants to Granite Global Investments Ltd. in connection with a loan transaction, which warrants had an exercise period of five years at an initial exercise price of $0.006 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2021, the Company issued 36,500,000 warrants to Granite Global Investments Ltd. in connection with a loan transaction, which warrants had an exercise period of five years at an initial exercise price of $0.006 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2021, the Company issued 10,000,000 warrants to Granite Global Investments Ltd. in connection with a loan transaction, which warrants had an exercise period of five years at an initial exercise price of $0.012 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Common Stock
During the three months ended March 31, 2021, a total of 74,985,965 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $542,976. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2021, a total of 87,252,322 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $2,201,994. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2021, a total of 43,478,695 shares of Company Common Stock were issued upon conversion of warrants. These warrants were issued on a cashless basis. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2021, a total of 12,800,000 shares of Company Common Stock were sold for cash in the total amount of $96,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Regulation A under the Securities Act.
During the three months ended September 30, 2021, a total of 97,822,249 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $898,076. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended September 30, 2021, a total of 260,000,000 shares of Company Common Stock were sold for cash in the total amount of $1,950,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Regulation A under the Securities Act.
During the three months ended December 31, 2021, a total of 148,605,900 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $356,675. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended December 31, 2021, a total of 141,000,000 shares of Company Common Stock were sold for cash in the total amount of $1,057,500. These shares of Company Common Stock were issued in reliance on the exemption provided by Regulation A under the Securities Act.
During 2020, the Company issued securities that were not registered under the Securities Act, as follows:
Convertible Promissory Notes
On January 27, 2020, the Company issued a convertible promissory note in the principal amount of $168,300 to Power Up Lending Group LTD. The note was due January 27, 2021, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On March 2, 2020, the Company issued a convertible promissory note in the principal amount of $165,800 to Power Up Lending Group LTD. The note was due March 2, 2021, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
On August 28, 2020, the Company issued a convertible promissory note in the principal amount of $110,250 to Diamond Investments II LLC. The note was due August 28, 2021, had an interest rate of 8% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On October 26, 2020, the Company issued a convertible promissory note in the principal amount of $165,800 to Granite Global Investments Ltd. The note was due November 6, 2021, had an interest rate of 24.5% per annum and was convertible into Company Common Stock at a discount of 40% of the lowest trading price in the last 20 trading days.
On December 18, 2020, the Company issued a convertible promissory note in the principal amount of $91,850 to Power Up Lending Group LTD. The note was due December 18, 2021, had an interest rate of 10% per annum and was convertible into Company Common Stock at a discount of 25% of the lowest trading price in the last 20 trading days.
The issuances of the foregoing convertible promissory notes were exempt from the registration requirements of the Securities Act under Rule 506 of Regulation D and Section 4(a)(2) of the Securities Act.
Warrants
During the three months ended March 31, 2020, the Company issued 63,236,369 warrants pursuant to round-down protection provisions in outstanding warrants. The exercise price of such warrants was thereby adjusted to $0.00385 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Preferred Stock
On November 23, 2020, the Company issued 500,000 shares of Series A preferred stock to Russell F. Bird for services rendered on behalf of the Company. The shares were valued at $0.0025, the closing price of the Company’s common stock on the date of grant, for total non-cash compensation expense of $1,250. These shares of Company Preferred Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
On November 23, 2020, the Company issued 500,000 shares of Series A preferred stock to Thomas J. Wood for services rendered on behalf of the Company. The shares were valued at $0.0025, the closing price of the Company’s common stock on the date of grant, for total non-cash compensation expense of $1,250. These shares of Company Preferred Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
On November 23, 2020, the Company issued 250,000 shares of Series B preferred stock to Russell F. Bird for services rendered on behalf of the Company. The shares were valued at $0.0025, the closing price of the Company’s common stock on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. These shares of Company Preferred Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
On November 23, 2020, the Company issued 250,000 shares of Series B preferred stock to Thomas J. Wood for services rendered on behalf of the Company. The shares were valued at $0.0025, the closing price of the Company’s common stock on the date of grant, multiplied by 100, for total non-cash compensation expense of $62,500. These shares of Company Preferred Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Common Stock
During the three months ended March 31, 2020, a total of 19,741,098 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $298,732. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2020, a total of 36,769,439 shares of Company Common Stock were issued upon conversion of warrants. These warrants were issued on a cashless basis. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended September 30, 2020, a total of 67,639,262 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $429,194. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended September 30, 2020, a total of 1,120,942 shares of Company Common Stock were issued upon conversion of warrants. These warrants were issued on a cashless basis. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended September 30, 2020, a total of 15,000,000 shares of Company Common Stock were sold for cash in the total amount of $75,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended December 31, 2020, a total of 111,523,399 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $381,008. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During 2019, the Company issued securities that were not registered under the Securities Act, as follows:
Convertible Promissory Notes
On January 23, 2019, the Company issued a convertible promissory note in the principal amount of $100,000 to ONE44 Capital LLC. The note was due January 23, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On May 3, 2019, the Company issued a convertible promissory note in the principal amount of $35,000 to Odyssey Capital Funding, LLC. The note was due May 3, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On May 30, 2019, the Company issued a convertible promissory note in the principal amount of $36,750 to Armada Investment Fund LLC. The note was due February 29, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On May 30, 2019, the Company issued a convertible promissory note in the principal amount of $36,750 to BHP Capital NY Inc. The note was due February 29, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On May 30, 2019, the Company issued a convertible promissory note in the principal amount of $36,750 to Jefferson Street Capital LLC. The note was due February 29, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On October 4, 2019, the Company issued a convertible promissory note in the principal amount of $55,000 to Armada Investment Fund LLC. The note was due July 4, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On October 4, 2019, the Company issued a convertible promissory note in the principal amount of $55,000 to BHP Capital NY Inc. The note was due July 4, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
On October 4, 2019, the Company issued a convertible promissory note in the principal amount of $55,000 to Jefferson Street Capital LLC. The note was due July 4, 2020, had an interest rate of 12% per annum and was convertible into Company Common Stock at a discount of 45% of the lowest trading price in the last 20 trading days.
The issuances of the foregoing convertible promissory notes were exempt from the registration requirements of the Securities Act under Rule 506 of Regulation D and Section 4(a)(2) of the Securities Act.
Warrants
During the three months ended June 30, 2019, the Company issued 1,500,000 warrants in connection with a loan transaction, which warrants had an exercise period of three years at an initial exercise price of $0.07 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended December 31, 2019, the Company issued 1,500,000 warrants in connection with a loan transaction, which warrants had an exercise period of three years at an initial exercise price of $0.07 per share. These warrants were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Preferred Stock
On June 14, 2019, the Company issued 500,000 shares of Series A Preferred Stock to Russell F. Bird for services rendered on behalf of the Company. The shares were valued at $0.04, the closing price of the Company’s Common Stock on the date of grant, for total non-cash compensation expense of $20,000. These shares of Company Preferred Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Common Stock
During the three months ended March 31, 2019, a total of 1,523,291 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $51,127. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2019, a total of 8,881,974 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $316,650. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2019, the Company issued 25,000,000 shares of common stock to each of Thomas J. Wood and Russell F. Bird for services rendered on behalf of the Company. These shares were valued at $0.04 per share, the closing price of the Company’s common stock on the date of grant, for total non-cash compensation expense of $2,000,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended June 30, 2019, the Company issued 1,000,000 shares of common stock to Jonathan B. Lane for services rendered on behalf of the Company. These shares were valued at $0.037 per share, the closing price of the Company’s common stock on the date of grant, for total non-cash compensation expense of $37,000. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended September 30, 2019, a total of 6,108,746 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $212,545. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
During the three months ended December 31, 2019, a total of 43,130,300 shares of Company Common Stock were issued in payment of indebtedness under convertible promissory notes in the total amount of $732,301. These shares of Company Common Stock were issued in reliance on the exemption provided by Section 4(a)(2) under the Securities Act.
Item 16. Exhibits and Financial Statement Schedules
| Exhibit No.: | | Description of Exhibit | | Incorporated by Reference to: |
| 3.1 | | Articles of Incorporation | | Exhibit 3.1 to Form SB-2 filed on October 16, 2007 |
| 3.2 | | Certificate of Amendment dated August 2, 2010 | | Exhibit 3.1 to Form 8-K filed on August 9, 2010 |
| 3.3 | | Certificate of Amendment dated December 26, 2012 | | Exhibit 3.1 to Form 8-K filed on January 30, 2013 |
| 3.4 | | Certificate of Amendment dated January 5, 2015 | | Exhibit 3.1 to Form 8-K filed on January 13, 2015 |
| 3.5 | | Certificate of Amendment dated June 26, 2017 | | Exhibit 3.1 to Form 8-K filed on June 30, 2017 |
| 3.6 | | Series A Preferred Stock Certificate of Designation | | Exhibit 4.1 to Form 8-K filed on April 24, 2012 |
| 3.7 | | Series B Preferred Stock Certificate of Designation | | To be filed. |
| 3.8 | | Bylaws | | Exhibit 3.2 to Form 8-K filed on May 2, 2017 |
| 4.1 | | Specimen Stock Certificate evidencing the shares of common stock | | Filed herewith. |
| 5.1 | | Opinion of Newlan Law Firm, PLLC | | Filed herewith. |
| 10.1 | | Exchange Agreement, dated March 31, 2015, between REMSleep Holdings, Inc. and REMSleep LLC | | Exhibit 3.2 to Form 8-K filed on April 2, 2015 |
| 10.2 | | Employment Agreement, dated January 1, 2019, between REMSleep Holdings, Inc. and Russell Bird | | Exhibit 1A-6.5 to Form 1-A filed on October 4, 2019 |
| 10.3 | | Employment Agreement, dated April 1, 2019, between REMSleep Holdings, Inc. and Tom Wood | | Exhibit 1A-6.6 to Form 1-A filed on October 4, 2019 |
| 10.4 | | Purchase Agreement dated May 10, 2022, between REMSleep Holdings, Inc. and JanBellas Group, LLC | | Filed herewith. |
| 10.5 | | Finder’s Fee Agreement between REMSleep Holdings, Inc. and J.H. Darbie, Inc. | | Filed herewith. |
| 23.1 | | Consent of Fruci & Associates II, PLLC | | Filed herewith. |
| 23.2 | | Consent of Newlan Law Firm, PLLC (included in Exhibit 5.1) | | Filed herewith. |
| 24.1 | | Power of Attorney (included on signature page hereof) | | Included on Signature Page hereof. |
| 107 | | Filing Fee Table | | Filed herewith. |
| 101.INS | | Inline XBRL Instance Document. |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document. |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
| (b) | Financial Statement Schedules. |
None.
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (a)(1)(i), (a)(1)(ii), and (a)(1)(iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) | That, insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| (c) | The undersigned registrant hereby undertakes: |
| (1) | That, for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (2) | That, for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing Form S-1 and has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Clearwater, Florida, on June 15, 2022.
| | REMSLEEP HOLDINGS, INC. |
| | |
| | By: | /s/ Thomas J. Wood |
| | | Thomas J. Wood |
| | | Chief Executive Officer |
| | | (Principal Executive Officer) |
| | | (Principal Financial and Accounting Officer) |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Thomas J. Wood, Chief Executive Officer, as his true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place and stead in any and all capacities, in connection with this Registration Statement, including to sign in the name and on behalf of the undersigned, this Registration Statement and any and all amendments thereto, including post-effective amendments and registrations filed pursuant to Rule 462 under the U.S. Securities Act of 1933, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto such attorney-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement on Form S-1 has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | | Title(s) | | Date |
| | | | | |
| /s/ Thomas J. Wood | | | | |
| Thomas J. Wood | | Chief Executive Officer (Principal Executive Officer) (Principal Financial and Accounting Officer), Secretary and Director | | June 15, 2022 |
| | | | | |
| /s/ Russell F. Bird | | | | |
| Russell F. Bird | | President, Chairman and Director | | June 15, 2022 |
II-9
iso4217:USD xbrli:shares




