UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
OR
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-33708
PHILIP MORRIS INTERNATIONAL INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Virginia | | 13-3435103 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| |
677 Washington Blvd, Suite 1100
| | |
| Stamford | | |
| Connecticut | | 06901 |
| (Address of principal executive offices) | | (Zip Code) |
203-905-2410
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, no par value | | PM | | New York Stock Exchange |
| 2.750% Notes due 2025 | | PM25 | | New York Stock Exchange |
| 3.375% Notes due 2025 | | PM25A | | New York Stock Exchange |
| 2.750% Notes due 2026 | | PM26A | | New York Stock Exchange |
| 2.875% Notes due 2026 | | PM26 | | New York Stock Exchange |
| 0.125% Notes due 2026 | | PM26B | | New York Stock Exchange |
| 3.125% Notes due 2027 | | PM27 | | New York Stock Exchange |
| 3.125% Notes due 2028 | | PM28 | | New York Stock Exchange |
| 2.875% Notes due 2029 | | PM29 | | New York Stock Exchange |
| 3.375% Notes due 2029 | | PM29A | | New York Stock Exchange |
| 3.750% Notes due 2031 | | PM31B | | New York Stock Exchange |
| 0.800% Notes due 2031 | | PM31 | | New York Stock Exchange |
| 3.125% Notes due 2033 | | PM33 | | New York Stock Exchange |
| 2.000% Notes due 2036 | | PM36 | | New York Stock Exchange |
| 1.875% Notes due 2037 | | PM37A | | New York Stock Exchange |
| 6.375% Notes due 2038 | | PM38 | | New York Stock Exchange |
| 1.450% Notes due 2039 | | PM39 | | New York Stock Exchange |
| 4.375% Notes due 2041 | | PM41 | | New York Stock Exchange |
| 4.500% Notes due 2042 | | PM42 | | New York Stock Exchange |
| 3.875% Notes due 2042 | | PM42A | | New York Stock Exchange |
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| 4.125% Notes due 2043 | | PM43 | | New York Stock Exchange |
| 4.875% Notes due 2043 | | PM43A | | New York Stock Exchange |
| 4.250% Notes due 2044 | | PM44 | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☑ Accelerated filer ☐
Non-accelerated filer ☐ Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
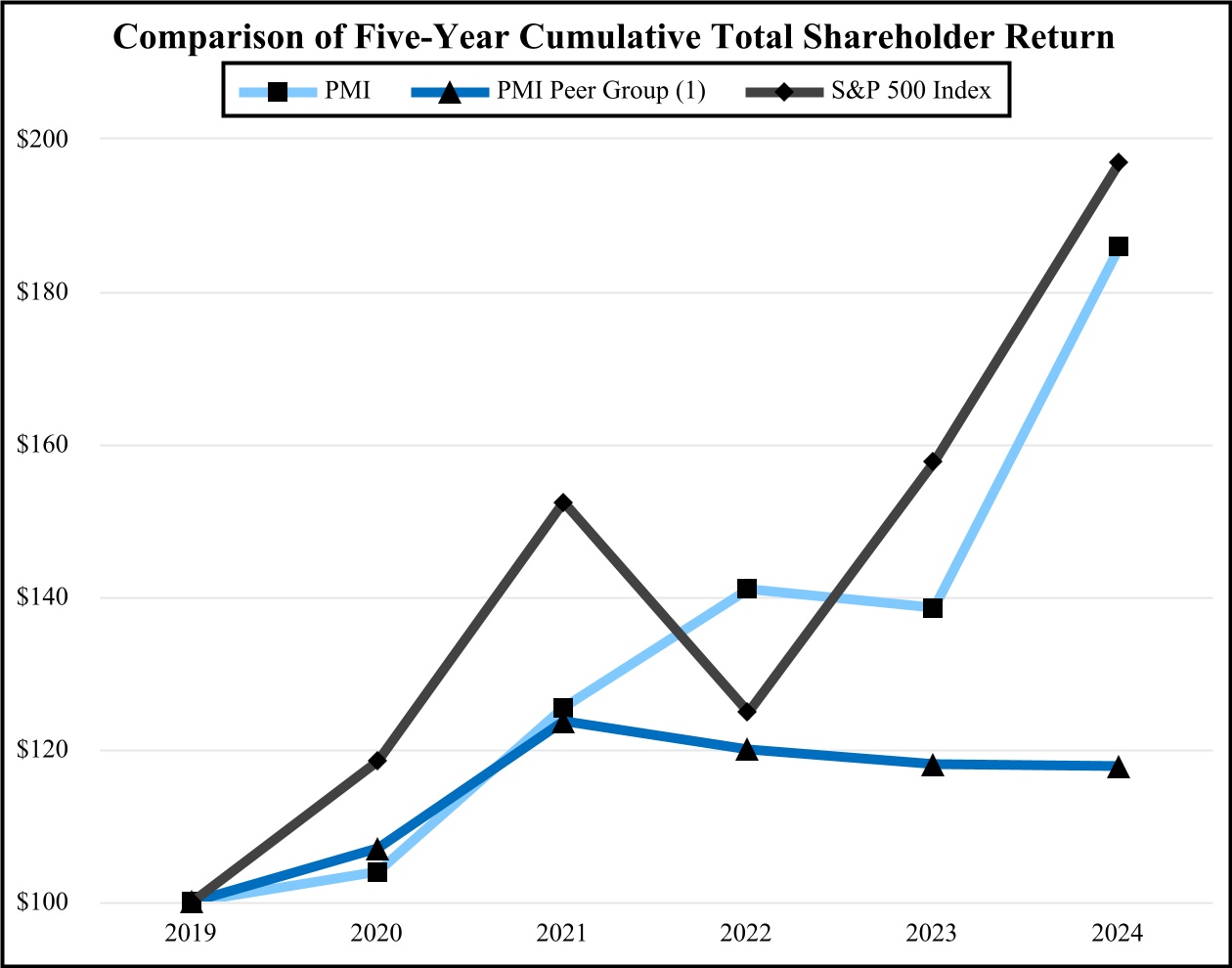
As of June 30, 2024, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $158 billion based on the closing sale price of the common stock as reported on the New York Stock Exchange.
| | | | | | | | | | | |
| Class | | Outstanding at | January 31, 2025 |
Common Stock,
no par value | | 1,554,857,221 | | shares |
DOCUMENTS INCORPORATED BY REFERENCE
| | | | | |
| Parts Into Which Incorporated |
| Portions of the registrant’s definitive proxy statement for use in connection with its annual meeting of shareholders to be held on May 7, 2025, to be filed with the Securities and Exchange Commission on or about March 27, 2025. | Part III |
TABLE OF CONTENTS
| | | | | | | | | | | |
| | | | Page |
| PART I | |
| Item 1. | | Business | |
| Item 1A. | | Risk Factors | |
| Item 1B. | | Unresolved Staff Comments | |
| Item 1C. | | Cybersecurity | |
| Item 2. | | Properties | |
| Item 3. | | Legal Proceedings | |
| Item 4. | | Mine Safety Disclosures | |
| | | |
| PART II | |
| Item 5. | | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
| Item 6. | | | |
| Item 7. | | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |
| Item 7A. | | Quantitative and Qualitative Disclosures About Market Risk | |
| Item 8. | | Financial Statements and Supplementary Data | |
| Item 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |
| Item 9A. | | Controls and Procedures | |
| Item 9B. | | Other Information | |
| Item 9C. | | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | |
| | | |
| PART III | |
| Item 10. | | Directors, Executive Officers and Corporate Governance | |
| Item 11. | | Executive Compensation | |
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence | |
| Item 14. | | Principal Accounting Fees and Services | |
| | | |
| PART IV |
| Item 15. | | Exhibits and Financial Statement Schedules | |
| | | |
| Signatures | |
In this report, “PMI,” “we,” “us” and “our” refers to Philip Morris International Inc. and its subsidiaries.
Trademarks and service marks in this report are the registered property of, or licensed by, the subsidiaries of Philip Morris International Inc. and are italicized.
PART I
Item 1.Business.
General Development of Business
General
Philip Morris International Inc. is a Virginia holding company incorporated in 1987. In March 2008, we became a U.S. public company listed on the New York Stock Exchange and subject to the rules of the U.S. Securities and Exchange Commission (the "SEC"). We are a leading international tobacco company, actively delivering a smoke-free future and evolving our portfolio for the long term to include products outside of the tobacco and nicotine sector. Our current product portfolio primarily consists of cigarettes and smoke-free products. Since 2008, we have invested over $14 billion to develop, scientifically substantiate and commercialize innovative smoke-free products for adults who would otherwise continue to smoke, with the goal of completely ending the sale of cigarettes. This investment includes the building of world-class scientific assessment capabilities, notably in the areas of pre-clinical systems toxicology, clinical and behavioral research, as well as post-market studies. In November 2022, we acquired Swedish Match AB ("Swedish Match") – a leader in oral nicotine delivery – creating a global smoke-free combination led by the companies’ IQOS and ZYN brands. Following a robust science-based review, the U.S. Food and Drug Administration (the "FDA") has authorized the marketing of Swedish Match’s General snus and ZYN nicotine pouches and versions of PMI’s IQOS devices and consumables - the first-ever such authorizations in their respective categories. Versions of IQOS devices and consumables and General snus also obtained the first-ever Modified Risk Tobacco Product ("MRTP") authorizations from the FDA. We describe the MRTP orders in more detail in the "Business Environment" section of Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Through our acquisition of Swedish Match in 2022, with its leading nicotine pouch franchise in the U.S. under the ZYN brand name, we acquired a market leader in oral nicotine delivery with a significant presence in the United States market. The Swedish Match acquisition has been a key milestone in PMI’s transformation to becoming a smoke-free company. The Swedish Match product portfolio is complementary to our existing portfolio, permitting us to bring together a leading oral nicotine product with the leading heat-not-burn product. By joining forces with Swedish Match, we expect to accelerate the achievement of our joint smoke-free ambitions, switching more adults who would otherwise continue to smoke cigarettes to better alternatives faster than either company could achieve separately.
In 2022, we reached an agreement with Altria Group, Inc. to end our commercial relationship in the U.S. covering IQOS as of April 30, 2024. PMI now holds the full rights to commercialize IQOS in the U.S. For further details, see Item 8, Note 3. Acquisitions and Divestitures.
In 2021, we began our long-term growth ambitions beyond nicotine in wellness and healthcare given our strong foundation and significant expertise in life sciences. Our Wellness and Healthcare business strategy currently focuses on developing and commercializing oral and inhaled consumer health and wellness offerings and inhaled prescription products for therapy areas that include pain management and cardiovascular emergencies. This includes medical and pharmaceutical cannabinoids, and non-recreational cannabinoid products (including CBD), in line with applicable regulatory requirements, though any revenue related to cannabinoids is expected to be negligible in the near to medium term.
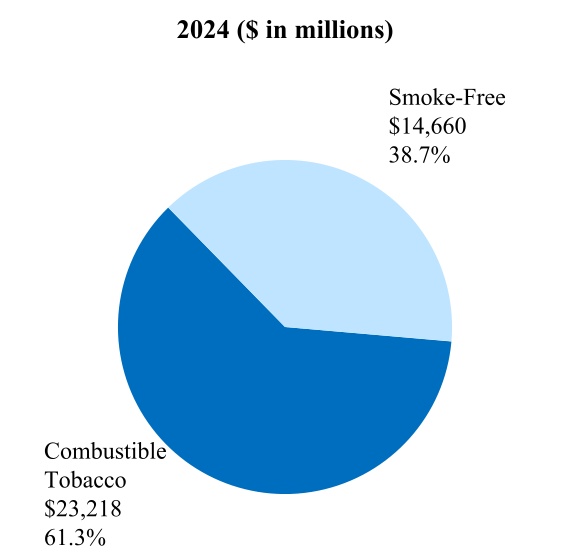
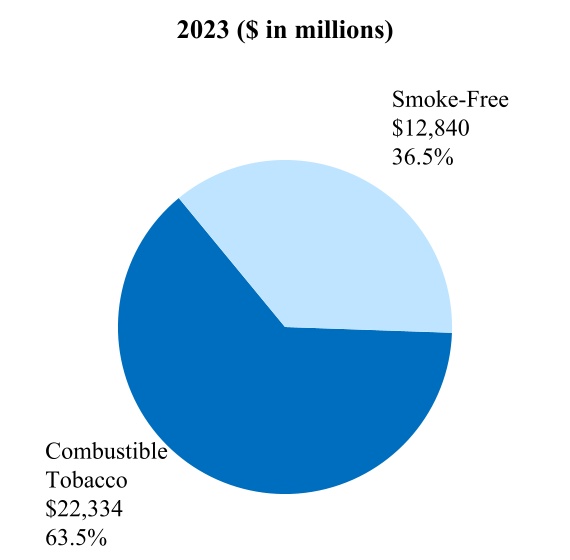
Smoke-Free Business ("SFB”) is the term PMI uses to refer to all of its smoke-free products. SFB also includes wellness and healthcare products, as well as consumer accessories, such as lighters and matches.
Smoke-free products (also referred to herein as "SFPs") is the term PMI uses to refer to all of its products that provide nicotine without combusting tobacco, such as heat-not-burn, e-vapor, and oral smokeless, and that therefore generate far lower levels of harmful chemicals. As such, these products have the potential to present less risk of harm versus continued smoking.
We have a range of SFPs in various stages of development, scientific assessment and commercialization. Our smoke-free products are designed for, and directed toward, current adult smokers and adult users of nicotine-containing products. We put significant effort to restrict access of our products from non-smokers and youth. We believe regulation must include measures designed to prevent youth initiation; and we also support and engage with relevant authorities to seek sensible regulation of flavors, mandated health warnings and minimum age laws.
Our IQOS smoke-free product brand portfolio includes heated tobacco and nicotine-containing vapor products. It uses a precisely controlled heating device into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol. Heated tobacco units ("HTUs") is the term we use to refer to heated tobacco consumables, which include our BLENDS, DELIA, HEETS, HEETS Creations (defined collectively as "HEETS"), SENTIA, TEREA, TEREA CRAFTED and TEREA Dimensions, as well
as the KT&G-licensed brands, Fiit and Miix (outside of South Korea). HTUs also include zero tobacco heat-not-burn consumables (LEVIA). IQOS was first introduced in Nagoya, Japan, in 2014.
IQOS and ZYN are the leading brands in our SFPs portfolio. As of December 31, 2024, our smoke-free products were available for sale in 95 markets. With regard to nicotine pouches, we increased our presence to 37 markets.
Our cigarettes are sold in approximately 170 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands and is led by Marlboro, the world’s best-selling international cigarette, which accounted for approximately 40% of our total 2024 cigarette shipment volume. Marlboro is complemented in the premium-price category by Parliament. Our other leading international cigarette brands are Chesterfield, L&M, and Philip Morris. These five international cigarette brands contributed 80% of our cigarette shipment volume in 2024. We also own a number of important local cigarette brands, such as Dji Sam Soe and Sampoerna A in Indonesia, and Fortune and Jackpot in the Philippines.
Source of Funds — Dividends
We are a legal entity separate and distinct from our direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions that are otherwise compliant with law, including governmental capital and foreign currency exchange controls.
Description of Business
Following the combination and the progress in 2023 toward the integration of the Swedish Match business into PMI's existing regional structure, PMI updated in January 2024 its segment reporting by including the former Swedish Match segment results into the four existing geographical segments. Our four geographical segments are as follows:
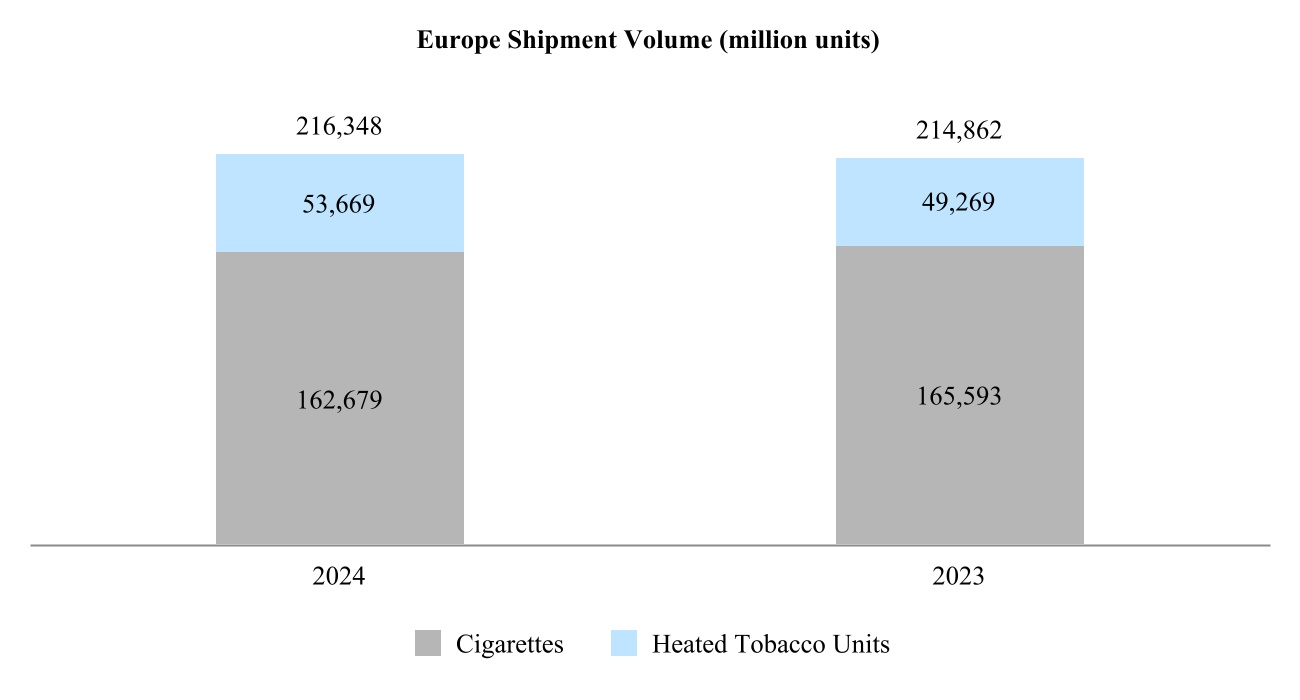
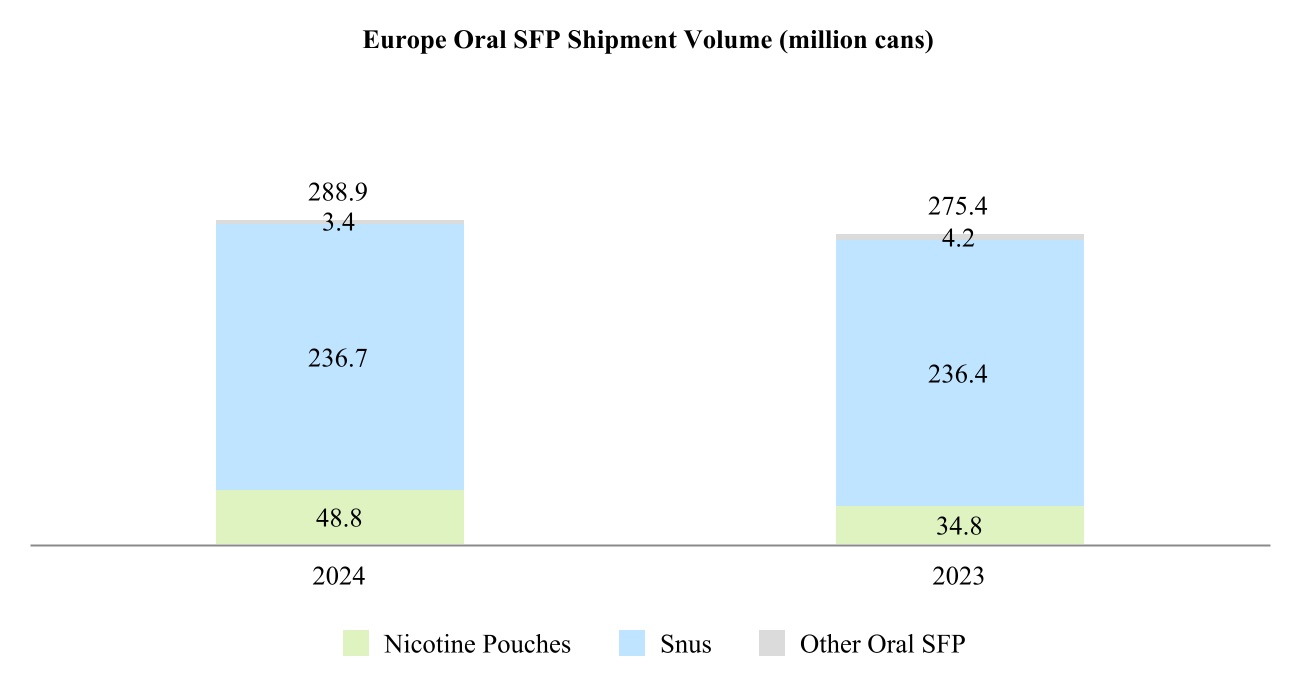
•Europe Region is headquartered in Lausanne, Switzerland, and covers all the European Union countries, Switzerland, the United Kingdom, and also Ukraine, Moldova and Southeast Europe;
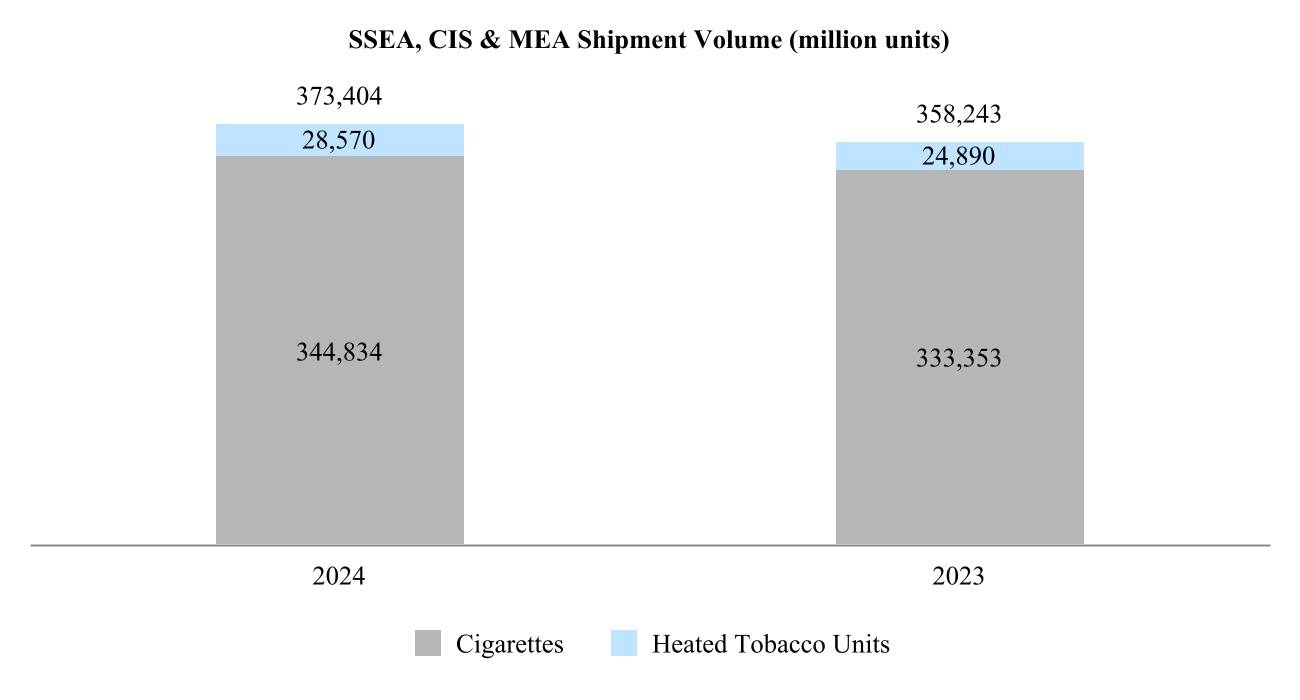
•South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA") is headquartered in Dubai, United Arab Emirates. It covers South and Southeast Asia, the African continent, the Middle East, Turkey, as well as Israel, Central Asia, Caucasus and Russia;
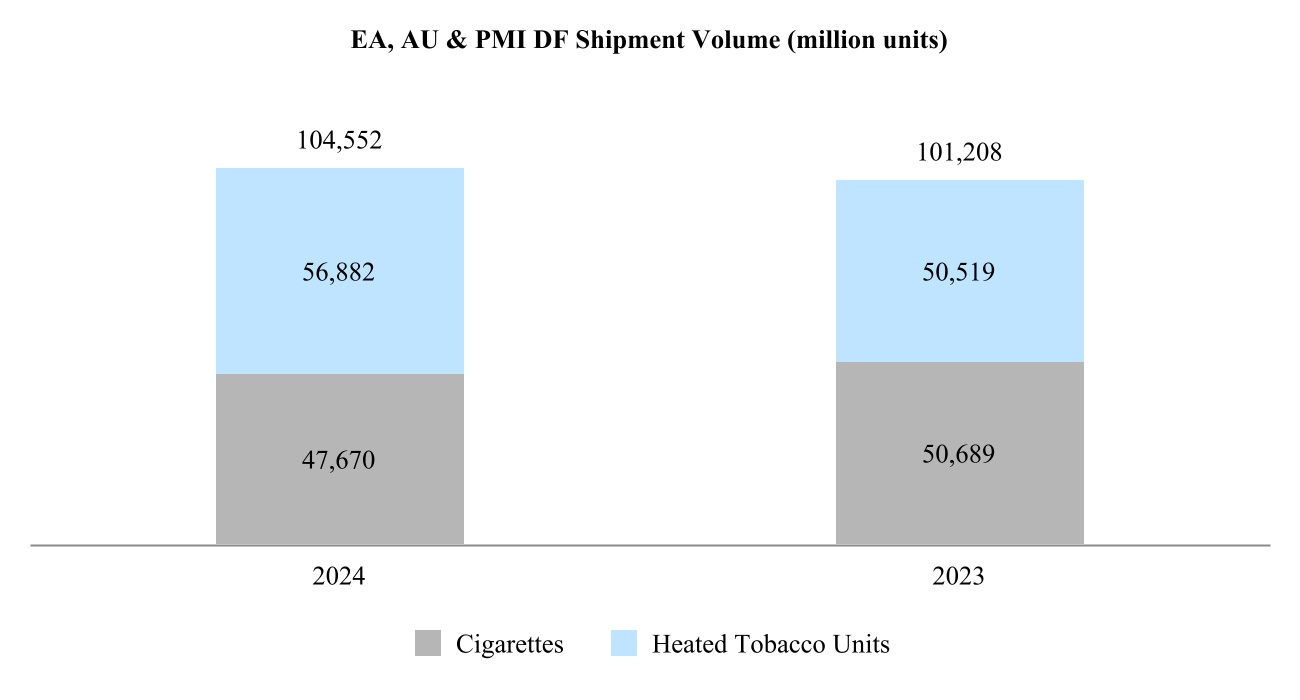
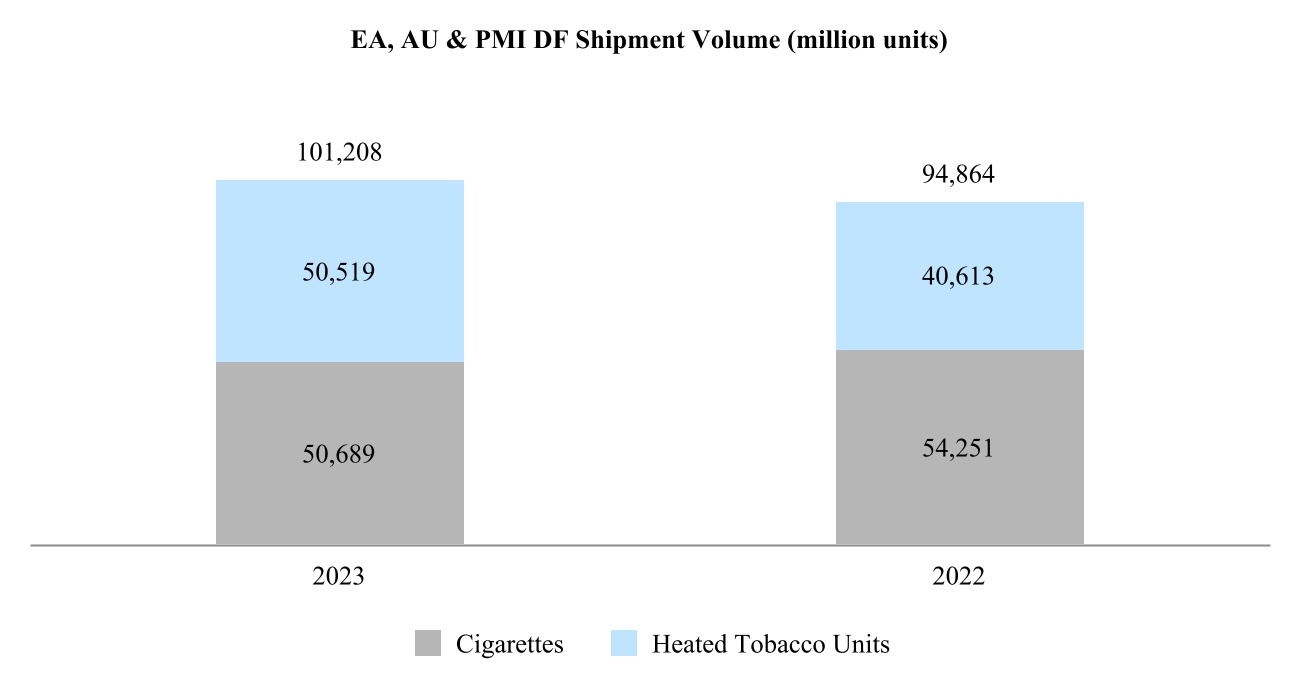
•East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF") is headquartered in Hong Kong, and includes the consolidation of our international duty free business with East Asia & Australia; and
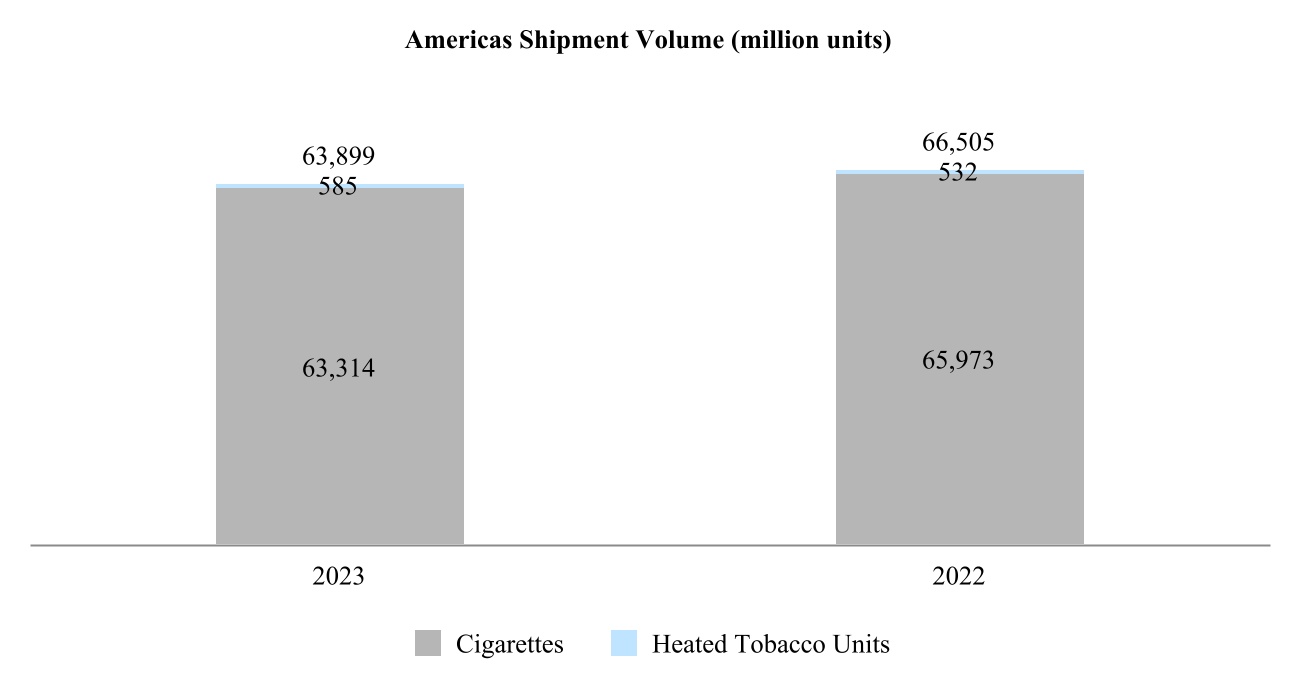
•Americas Region is headquartered in Stamford, Connecticut, and covers the United States, Canada and Latin America.
Our Wellness and Healthcare ("W&H") segment, which includes the operating results of our Wellness and Healthcare business, remained unchanged in 2024.
Following the sale of Vectura Group Ltd. on December 31, 2024, we will update our segment reporting by including the remaining Wellness & Healthcare results in the Europe segment. In addition, we will be renaming our “PMI Duty Free” business to “PMI Global Travel Retail” effective in the first quarter of 2025. As a result of this change, PMI's segment that includes our duty free business will be renamed East Asia, Australia & PMI Global Travel Retail (“EA, AU & PMI GTR”). As of the first quarter of 2025, our reporting will reflect these changes.
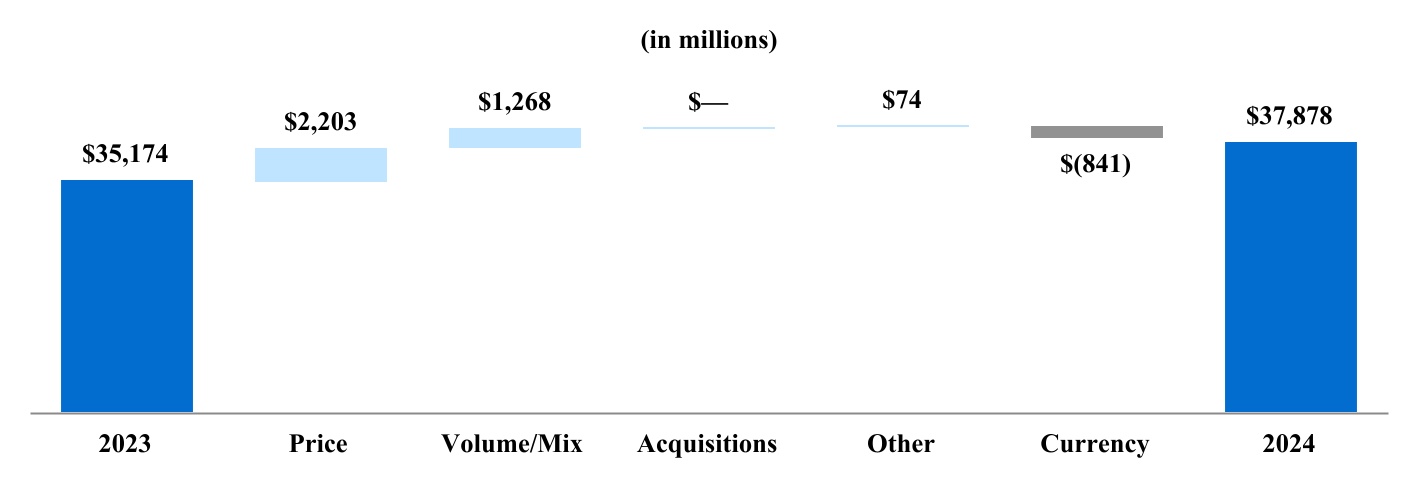
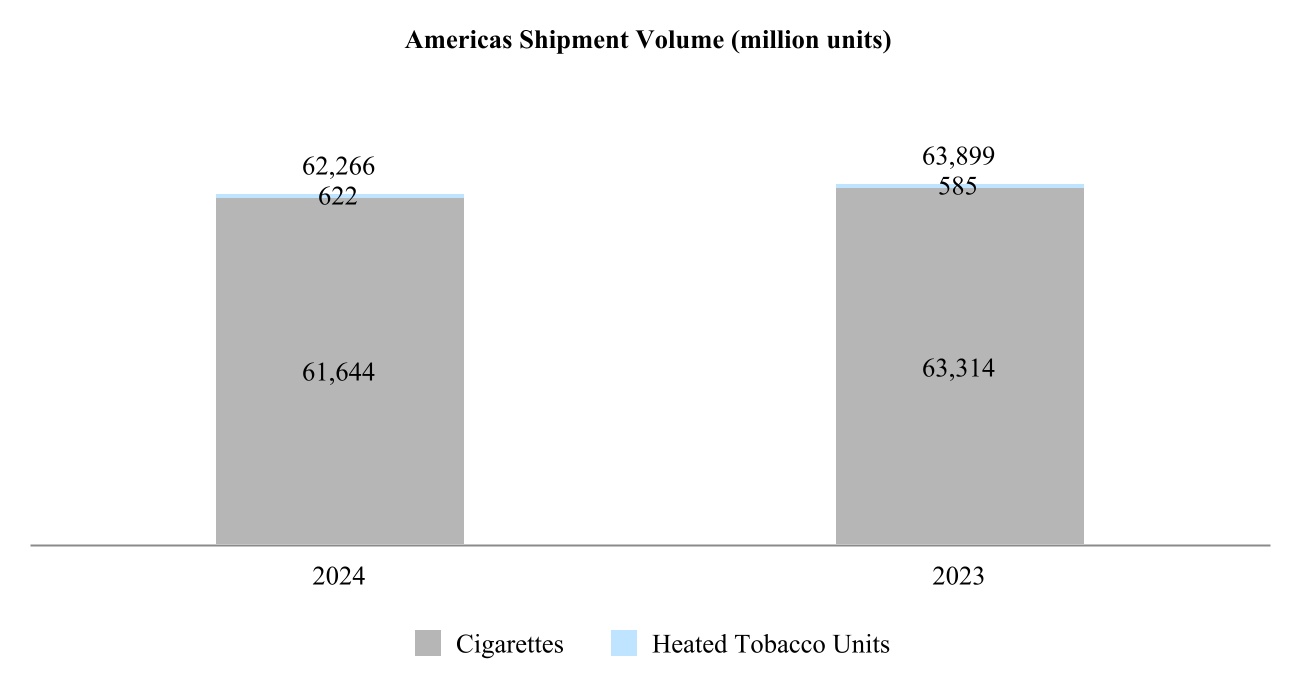
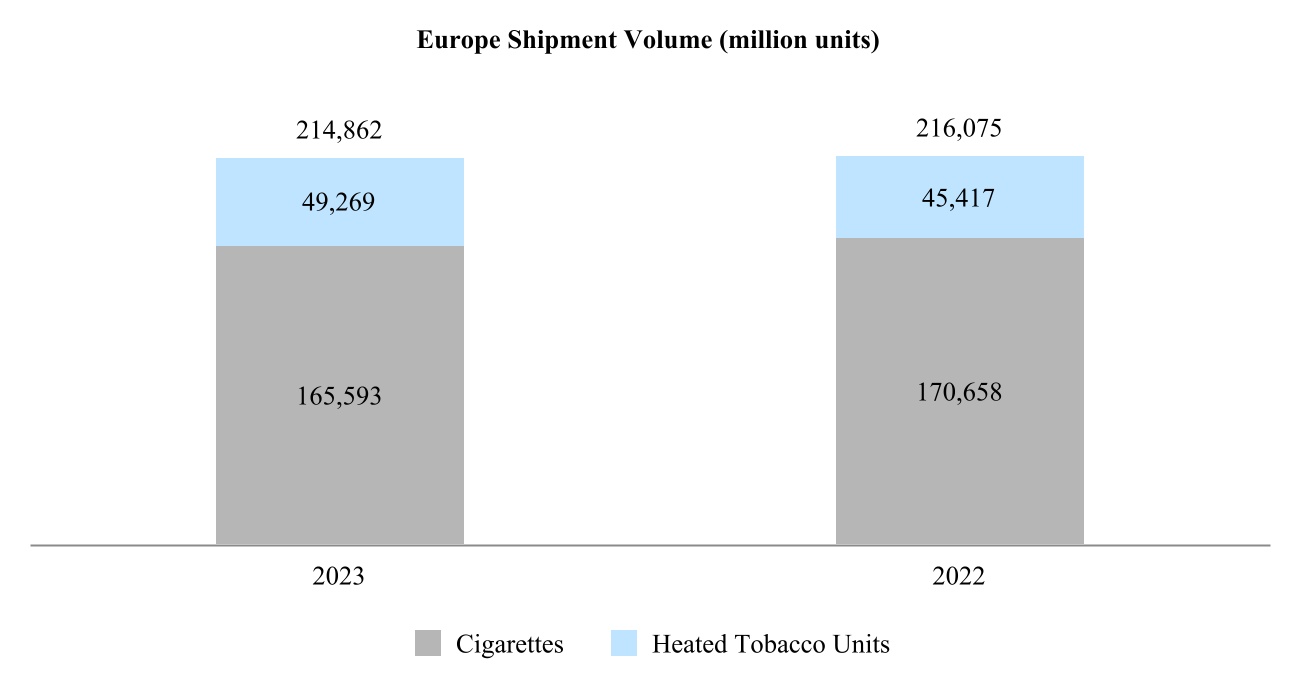
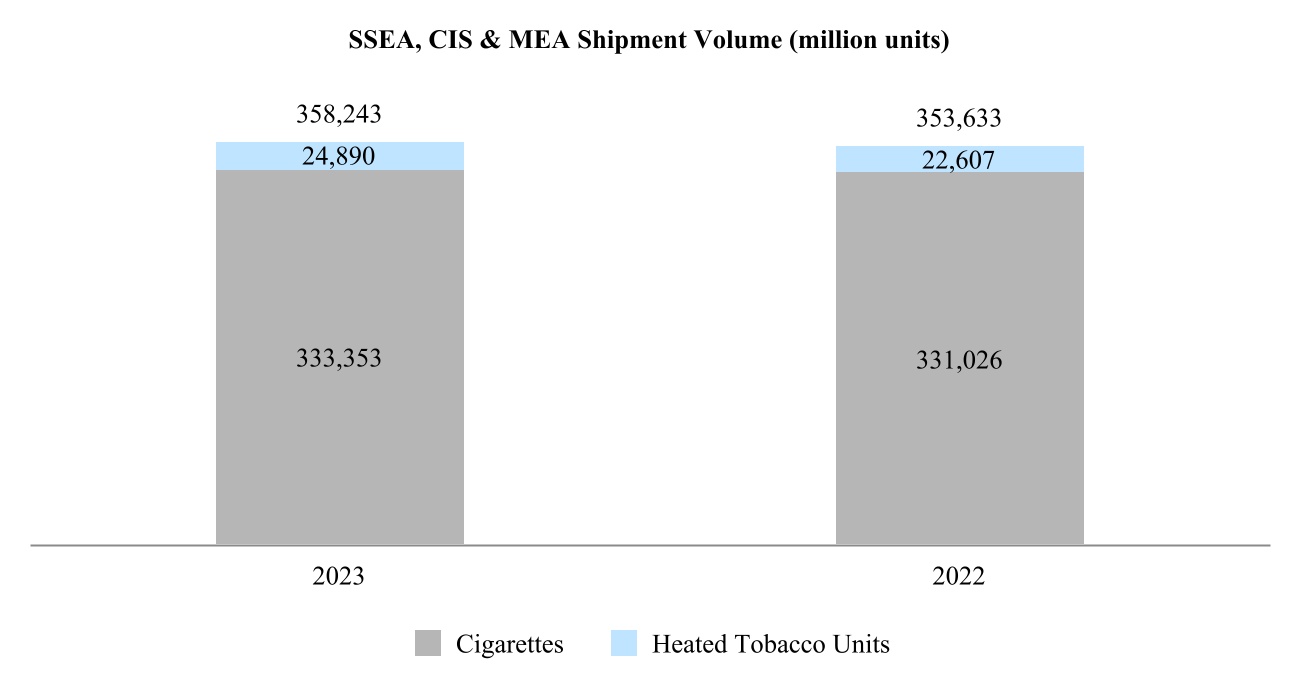
Our total shipment volume, including cigarettes and heated tobacco units, increased by 2.5% in 2024 to 756.6 billion units, with shipment volume of heated tobacco units reaching 139.7 billion units in 2024, up from 125.3 billion units in 2023. Shipment volume of our principal cigarette brand, Marlboro, increased by 3.7% in 2024.
References in this Form 10-K to total international market, defined as worldwide cigarette and heated tobacco unit volume, excluding the United States, total industry (or total market) and market shares, are our estimates for tax-paid products based on data from a number of internal and external sources, and may, in defined instances, exclude China. Past reported periods may be updated to ensure comparability and to incorporate the most current information for industry and market share reporting.
Unless otherwise stated, references to total industry (or total market), our shipment volume and our market share performance reflect cigarettes and heated tobacco units.
Key data regarding total market and market share were as follows:
| | | | | | | | | | | |
| 2024 | 2023 | 2022 |
| Total Market, billion units (excluding China and the U.S.) | 2,618 | 2,579 | 2,621 |
| | | |
Total International Market Share (1) | 28.7% | 28.3% | 27.7% |
| Cigarettes | 23.5% | 23.7% | 23.6% |
| HTU | 5.2% | 4.7% | 4.1% |
| | | |
PMI Cigarette over Cigarette Market Share (2) | 25.3% | 25.2% | 25.0% |
Marlboro Cigarette over Cigarette Market Share (3) | 10.1% | 9.8% | 9.8% |
| | | |
| | | |
| (1) Defined as PMI's cigarette and heated tobacco unit in-market sales volume as a percentage of total industry cigarette and heated tobacco unit sales volume, excluding China and the U.S., including cigarillos in Japan |
| (2) Defined as PMI's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
(3) Defined as Marlboro's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
| Note: Sum of share of market by product categories might not foot to total due to roundings |
We have a market share of at least 15% in approximately 100 markets, including Algeria, Argentina, Australia, Austria, Belgium, Brazil, the Czech Republic, Egypt, France, Germany, Greece, Hong Kong, Hungary, Indonesia, Israel, Italy, Japan, Kazakhstan, Kuwait, Mexico, the Netherlands, the Philippines, Poland, Portugal, Romania, Russia, Saudi Arabia, the Slovak Republic, South Korea, Spain, Switzerland, Turkey and Ukraine.
Distribution & Sales
Our main types of distribution and sales are tailored to the characteristics of each market and are often used simultaneously:
•Direct sales and distribution, where we sell directly to the retailers;
•Distribution through independent distributors that often distribute other fast-moving consumer goods and are responsible for distribution in a particular market;
•Exclusive zonified distribution, where the dedicated multicategory product distributors are assigned to exclusive territories within a market;
•Distribution through national or regional wholesalers that then supply the retail trade;
•Our own e-commerce infrastructure for product sales to trade partners and to consumers; and
•Our own brand retail infrastructure for our SFPs and accessories for sales to consumers.
Competition
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, R&D, innovation, packaging, customer service, marketing, advertising and retail price.
The competitive environment and our competitive position can be significantly influenced by weak economic conditions; erosion of consumer confidence; competitors' introduction of lower-price products or innovative products; adult smoker willingness to convert to our SFPs; higher product taxes; higher absolute prices and larger gaps between retail price categories; and product regulation that diminishes the ability to differentiate tobacco products, restricts adult consumer access to truthful and non-misleading information about our SFPs, or disproportionately impacts the commercialization of our products in relation to our competitors.
Competitors in our industry include Altria Group, Inc., British American Tobacco plc, Japan Tobacco Inc., Imperial Brands plc, new market entrants, particularly with respect to innovative products, several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, China, Taiwan, Thailand and Vietnam. Some competitors have different profit, volume and regulatory objectives, and some international competitors may be less susceptible than PMI to changes in currency exchange rates. Certain new market entrants in the non-combustible product category may alienate consumers from innovative products through inappropriate marketing campaigns, messaging and inferior product satisfaction, and without scientific substantiation based on appropriate R&D protocols and standards. The growing use of digital media could increase the speed and extent of the dissemination of inaccurate and misleading information about our SFPs, all of which could have a material adverse effect on our profitability and results of operations.
Procurement and Raw Materials
We purchase tobacco leaf of various types, grades and styles throughout the world, mostly through independent international tobacco suppliers. In 2024, we also contracted directly with farmers in several countries, including Argentina, Brazil, Italy, Pakistan and Poland. In 2024, direct sourcing from farmers represented approximately 18% of PMI’s global leaf requirements. The largest supplies of tobacco leaf are sourced from Argentina, Brazil, China, India, Italy, Indonesia (mostly for domestic use in kretek products), Malawi, Mozambique, the Philippines, Turkey and the United States. We believe that there is a sufficient supply of tobacco leaf in the world markets to satisfy our current and anticipated production requirements.
Given the global reach of our value chain, properly managing land and water resources and utilizing a geographically diversified sourcing strategy for agricultural products are priorities as we seek to increase the resilience of our production systems and minimize operational risks. We conduct a global water risk assessment annually in tobacco-growing regions to identify potential hotspots for physical water risks that require adaptation measures. Our water stewardship strategy includes guidance for applying a landscape approach to water optimization projects, protecting natural resources and recharge areas, and improving the efficiency of irrigation systems to integrate better farm water management. These business practices are intended to mitigate the risk that climate change could influence weather patterns in ways that negatively impact the quality or cost of the agricultural products used to manufacture our products.
In addition to tobacco leaf, we purchase a wide variety of direct materials from a total of approximately 500 suppliers. In 2024, our top ten suppliers of direct materials combined represented approximately 60% of our total direct materials purchases. The four most significant direct materials that we purchase are printed paper board used in packaging, acetate tow used in filter making, fine paper used in the manufacturing of cigarettes and heated tobacco units, and susceptors used for TEREA and other consumables. In addition, the adequate supply and procurement of cloves are of particular importance to our Indonesian business. For our oral smoke-free products, direct materials include plastic cans and lids for nicotine pouches and traditional snus products, nicotine salt or nicotine premix for nicotine pouches, pouch material for individual pouchmaking and additives. Our devices are made by various electronic manufacturing services providers ("EMS"). The components used in the assembly of these devices can be made by the EMS or purchased from other suppliers. These components include mechanical parts, electrical and electromechanical components, batteries, semiconductors, and packaging materials.
We discuss the details of our supply chain for our SFPs in Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report on Form 10-K (“Item 7”) in Business Environment—Reduced-Risk Products.
Business Environment
Information called for by this Item is hereby incorporated by reference to the paragraphs in Item 7, Business Environment to this Annual Report on Form 10-K.
Other Matters
Customers
As described in more detail in “Distribution & Sales” above, in many of our markets we sell our products to distributors. In 2024, sales to a distributor in the Europe Region and a distributor in the EA, AU & PMI DF Region each amounted to 10 percent or more of our consolidated net revenues. See Item 8, Note 13. Segment Reporting for more information. We believe that none of our business segments is dependent upon a single customer or a few customers, the loss of which would have a material adverse effect on our consolidated results of operations. In some of our markets, particularly in the Europe, SSEA, CIS & MEA, and EA, AU & PMI DF Regions, a loss of a distributor may result in a temporary market disruption.
Human Capital
Our Workforce. At December 31, 2024, we employed approximately 83,100 people worldwide, including full-time, temporary and part-time staff. Our businesses are subject to a number of laws and regulations relating to our relationship with our employees. Generally, these laws and regulations are specific to the location of each business. We engage with legally recognized employee representative bodies and we have collective bargaining agreements in several of the countries in which we operate. In addition, in accordance with E.U. requirements, we have established a European Works Council composed of management and elected members of our workforce. We believe we maintain good relations with our employees and their representative organizations.
Our Internal Transformation. To be successful in our transformation to a smoke-free future, we must continue transforming our culture and ways of working, align our talent with our business needs, successfully integrate acquired businesses and innovate to become a truly consumer-centric business. To achieve our strategic goals, we need to attract, retain, develop and motivate the best global talent, talent that is diverse and has the right degree of experience, competencies and skills. Our compensation and benefit programs are set at the levels that we believe are necessary to attract the best talent and remain competitive with other consumer product companies.
Oversight and Management. Our Board of Directors (the "Board") provides oversight of various matters pertaining to our workforce. The Compensation and Leadership Development Committee of the Board is responsible for executive compensation matters and oversight of the risks and programs related to human capital management. Our Code of Conduct and Corporate Governance Guidelines highlight our commitment to ethical business conduct and honesty, respect, and fairness.
Collaborative Culture. We are proud of the global reach of our company, which operates in approximately 170 markets with employees representing more than 130 nationalities. We reflect the demographics of the countries, communities, and consumers we serve, which is key to the variety of thought, innovation, and consumer-centric approach that enables us to deliver a smoke-free future.
Our focus is on fostering a performance-driven and collaborative culture, recognizing the value of our global workforce and their contributions. We remain committed to the impact, growth, and well-being of our employees, providing them with the right environment, support, and resources for their careers and professional development.
These principles are codified in our cultural values, which we call the PMI DNA, and we embed these principles throughout our people and business guidelines, practices, and systems.
As the first multinational company to receive a global EQUAL-SALARY certification from the EQUAL-SALARY Foundation in 2019, we completed another year of market level reviews with success and maintained our global certification. We believe this certification is a valuable component in our reputation as an employer.
Government Regulation
As a company with global operations in a heavily regulated industry, we are subject to multiple laws and regulations of the jurisdictions in which we operate. We discuss our regulatory environment in Item 7, Business Environment.
The regulatory landscape related to sustainability matters is rapidly evolving. We closely monitor these developments and implement initiatives addressing PMI’s priorities in line with our sustainability strategy. In particular, we are subject to international, national and local environmental laws and regulations in the countries in which we do business. We have specific programs across our business units designed to meet applicable environmental compliance requirements and reduce our carbon footprint, wastage, as well as water and energy consumption. We report externally about our climate change mitigation strategy, together with associated targets and results in reducing our carbon footprint, through CDP (formerly known as the Carbon Disclosure Project), the leading international non-governmental organization assessing the work of thousands of companies worldwide in the area of environmental impact, including climate change.
Our environmental and occupational health and safety management program includes policies, standard practices and procedures at all our manufacturing centers. Furthermore, we have engaged an external certification body to validate the effectiveness of this management program at our manufacturing centers around the world, in accordance with internationally recognized standards ISO 14001 and ISO 45001 for safety and environmental management. Furthermore, we progressively certify our manufacturing centers against AWS (Alliance for Water Stewardship) and Carbon Neutrality, requiring improvement actions year on year. Our subsidiaries expect to continue to make investments in order to drive improved performance and maintain compliance with environmental laws and regulations. We assess and report to our management the compliance status of all our legal entities on a regular basis. Based on current regulations, the management and controls we have in place and our review of climate change risks (both physical and regulatory), environmental expenditures have not had, and are not expected to have, a material adverse effect on our consolidated results of operations, capital expenditures, financial position, earnings or competitive position.
As discussed in more detail in Item 1A. Risk Factors, our financial results could be significantly affected by regulatory initiatives that could result in a significant decrease in demand for our brands or by climate-related regulations that increase our cost of operation. More specifically, any regulatory requirements that lead to a commoditization of tobacco products or impede adult consumers' ability to convert to our SFPs, as well as any significant increase in the cost of complying with new regulatory requirements could have a material adverse effect on our financial results. Further, tightened climate-related regulation may lead to additional carbon taxation or energy price increases impacting our cost of operation. These shifts in regulation and other market trends could, amongst others, impact current deforestation rates. Availability of deforestation-free materials could be impacted by increased demand for alternative energy sources and low-carbon fuels, such as biomass, which could result in increased sourcing costs.
We discuss additional information regarding regulatory matters relating to climate change in Item 7, Climate Change Laws and Regulations.
Information About Our Executive Officers
The disclosure regarding executive officers is hereby incorporated by reference to the discussion under the heading “Information about our Executive Officers as of February 6, 2025” in Part III, Item 10. Directors, Executive Officers and Corporate Governance of this Annual Report on Form 10-K (“Item 10”).
Intellectual Property
Our trademarks are valuable assets, and their protection and reputation are essential to us. We own the trademark rights to all of our principal brands, including Marlboro (outside of the U.S.), HEETS, IQOS, IQOS ILUMA, TEREA, VEEV and ZYN, or have the right to use them in all countries in which these brands are advertised or sold.
In addition, we have a large number of granted patents and pending patent applications worldwide. Our patent portfolio, as a whole, is material to our business. However, no one patent, or group of related patents, is material to us. We also have registered industrial designs, as well as unregistered proprietary trade secrets, technology, know-how, processes and other unregistered intellectual property rights.
Effective January 1, 2008, PMI entered into an Intellectual Property Agreement with Philip Morris USA Inc., a wholly owned subsidiary of Altria Group, Inc. (“PM USA”). The Intellectual Property Agreement allocates ownership of jointly funded intellectual property as follows:
•PMI owns all rights to jointly funded intellectual property outside the United States, its territories and possessions; and
•PM USA owns all rights to jointly funded intellectual property in the United States, its territories and possessions.
The parties agreed to submit disputes under the Intellectual Property Agreement first to negotiation between senior executives and then to binding arbitration.
An agreement was reached with PM USA in 2022 relating to IQOS commercialization rights in the U.S. including, among other things, an agreement relating to intellectual property rights consistent with the commercialization rights for relevant IQOS products.
On February 1, 2024, Philip Morris Products S.A., an indirect, wholly-owned subsidiary of PMI, and Nicoventures Trading Limited, an indirect, wholly-owned subsidiary of British American Tobacco p.l.c., entered into a settlement agreement (the “Settlement Agreement”) for an eight-year term, under which, among other things: (i) certain pending legal proceedings (the “Proceedings”) between them concerning certain of their respective products were dismissed with prejudice, subject to certain limited exceptions, and
without admission of liability; (ii) the Limited Exclusion Order and Cease and Desist Order issued by the International Trade Commission on September 29, 2021, prohibiting the importation of certain heat-not-burn products by PMI and its affiliates into the United States was rescinded; and (iii) any injunctions granted to either party in the Proceedings were fully and finally discharged, without admission of liability.
The parties have also agreed to certain covenants not to sue on a perpetual, royalty-free basis or on a royalty-bearing basis, subject to the terms and conditions set forth in the Settlement Agreement, and, among other things, both parties may introduce certain heat-not-burn and vapor products under the Settlement Agreement (including certain evolutions of the existing heat-not-burn and e-vapor products, and certain variants and product line extensions of heat-not-burn and e-vapor products), which products may be royalty-bearing or royalty-free depending on the patents of the other party that may have been used in the development thereof.
Seasonality
Our business segments are not significantly affected by seasonality, although in certain markets cigarette consumption may be lower during the winter months due to the cold weather and may rise during the summer months due to outdoor use, longer daylight, and tourism. However, we typically experience higher SFP adult user growth in the first half of each year versus the second half of each year due to seasonal influences.
Available Information
We are required to file with the SEC annual, quarterly and current reports, proxy statements and other information required by the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The SEC maintains an Internet website at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, from which investors can electronically access our SEC filings.
We make available free of charge on, or through, our website at www.pmi.com our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Investors can access our filings with the SEC by visiting www.pmi.com.
The information on our website is not, and shall not be deemed to be, a part of this report or incorporated into any other filings we make with the SEC.
Item 1A. Risk Factors.
The following risk factors should be read carefully in connection with evaluating our business and the forward-looking statements contained in this Annual Report on Form 10-K. Any of the following risks could materially adversely affect our business, our operating results, our financial condition and the actual outcome of matters as to which forward-looking statements are made in this Annual Report on Form 10-K.
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in this Annual Report on Form 10-K and other filings with the SEC, in reports to investors and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "aspires," "estimates," "intends," "projects," "aims," "goals," "targets," "forecasts" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Our SFPs constitute a relatively new product category that is less predictable than our mature cigarette business. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in Item 7, Business Environment. You should understand that it is not possible to predict or identify all risk factors. Consequently, you
should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Overall Business Risks
We may be unsuccessful in our attempts to introduce, commercialize, and grow smoke-free products in existing and new markets, and regulators may prohibit or significantly restrict the commercialization of these products or the communication of scientifically substantiated information and claims.
Our key strategic priorities are to: (i) continue developing and commercializing products that present less risk of harm to adult smokers who switch to smoke-free products versus continued cigarette smoking; and (ii) encourage and educate current adult smokers who would otherwise continue to smoke cigarettes to switch to those products. For our efforts to be successful, we must:
| | | | | |
| • | develop SFPs that adult smokers who would otherwise continue to smoke cigarettes find to be satisfying alternatives to smoking; |
| • | for those adult smokers, our goal is to develop and offer SFPs with a scientifically substantiated risk-reduction profile that approaches as closely as possible the risk-reduction profile associated with smoking cessation; |
| • | substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on scientific evidence of the highest standard that is made available for scrutiny and review by external independent scientists and relevant regulatory bodies; and |
| • | advocate for the development of science-based regulatory frameworks for the development and commercialization of SFPs, including the communication of scientifically substantiated information to enable adult smokers to make better choices. |
We might not succeed in our effort to introduce, commercialize, and grow our SFPs in existing and new markets. If we do not succeed, but others do, or if heat-not-burn products are inequitably regulated compared to other SFP categories without regard to the totality of the scientific evidence available for such products, we may be at a competitive disadvantage. In addition, actions of some market participants, such as the inappropriate marketing of e-vapor products to youth, as well as alleged health consequences associated with the use of certain e-vapor products, may unfavorably impact public opinion and/or mischaracterize the health consequences of all e-vapor products or other SFPs to consumers, regulators and policy makers without regard to the totality of scientific evidence available for specific products. This may impede our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of SFPs. We cannot predict the extent to which regulators will permit the sale and/or marketing of SFPs. Regulatory restrictions could limit the success of our SFPs.
The World Health Organization (the "WHO") study group on tobacco product regulation published their ninth report on the scientific basis of tobacco product regulation in August 2023. The report is based on a review of scientific evidence related to novel and emerging nicotine and tobacco products, such as electronic nicotine delivery systems ("ENDS"), electronic non-nicotine delivery systems and HTPs. The report concludes by making a number of policy recommendations on HTPs and ENDS that, if implemented, could restrict both the availability of these products and the access to accurate information about them. In August 2021, the Framework Convention on Tobacco Control (the "FCTC") Secretariat published two reports on novel and emerging tobacco products to the Ninth Session of the CoP of the FCTC, which are not materially different from the WHO study group report. Substantive decisions based on these reports were deferred to the Tenth Session of the CoP ("CoP 10"). CoP 10 to the FCTC took place in February 2024. According to reports and decisions published, neither new decisions nor new policy recommendations on novel and emerging tobacco products were adopted. Specific Guidelines were adopted to address cross-border Tobacco Advertising, Promotion, and Sponsorship ("TAPS") and the depiction of tobacco in entertainment media. The Eleventh Session of the CoP is currently scheduled to take place in November 2025.
Reports issued by the WHO and other FCTC guidelines or recommendations are not binding on the WHO Member States or on parties to the FCTC, and so it is not possible to predict the extent to which any proposals it adopts will be implemented. However, the WHO proposals could lead to restrictions on the availability of certain of our SFPs and access to accurate information about them in one or more of our markets, which could have a material adverse effect on our results of operations.
Additionally, any claims, regardless of merit, challenging our research and clinical data available to date, may impact the development of science-based regulatory frameworks for the commercialization of the SFP category and the commercialization of the SFP category in general.
Our SFPs and commercial activities for these products are designed for, and directed toward, current adult smokers and adult users of nicotine-containing products. We put significant effort to restrict access of our products from non-smokers and youth. Despite our efforts, technological, operational, regulatory and/or commercial developments might impact the implementation or effectiveness of youth access prevention mechanisms and surrounding infrastructure. If there is significant usage, whether actual or perceived, of our
products or competitive products among youth or non-smokers, even in situations over which we have no control, our reputation and credibility may suffer, the regulatory approach to our products may become more restrictive, and our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of SFPs may be significantly impacted.
The FDA’s premarket tobacco product and modified risk tobacco product authorizations of two versions of our IQOS product as well as the premarket tobacco authorizations of 20 varieties of ZYN pouches are subject to strict marketing, reporting and other requirements. Although we have received these authorizations from the FDA, there is no guarantee that the products will remain authorized for sale in the U.S., or that new versions of IQOS or other ZYN products will receive necessary authorizations, particularly if there is a significant uptake in youth or non-smoker initiation.
Moreover, we also submitted additional premarket tobacco applications for other ZYN products after the September 9, 2020 deadline, and we are unable to market these products until the FDA authorizes such applications. In April 2024, we also submitted MRTPAs for ZYN products currently marketed in the U.S. and requested authorization of the modified risk claim. There is no guarantee that the ZYN products will receive the necessary authorizations from the FDA.
The commercialization of our products in the United States is dependent on successfully managing compliance with federal, state, and local laws, regulations, legal agreements, and related interpretations. Failure to successfully manage compliance and to resolve any disputes that may arise regarding the application of legal and administrative requirements to our products could negatively impact the timing, manner, or success of our SFP commercialization in the United States, which could in turn have a material adverse effect on our results of operations, revenues, cash flows, or profitability.
The financial and business performance of our smoke-free products is less predictable than our cigarette business.
Our SFPs are novel products in a relatively new category, and the pace at which adult smokers adopt them may vary, depending on the competitive, regulatory, fiscal and cultural environment, and other factors in a specific market. There may be periods of accelerated growth and periods of slower growth for these products, the timing and drivers of which may be more difficult for us to predict versus our mature cigarette business. The impact of this lower predictability on our projected results for a specific period may be significant, due to geopolitical or macroeconomic events that negatively impact SFP availability or adoption, which in turn may have a material adverse effect on our results of operations.
We may be unsuccessful in our efforts to differentiate smoke-free products and cigarettes with respect to taxation.
To date, we have been largely successful in demonstrating to regulators that our SFPs are not cigarettes due to the absence of combustion, and accordingly they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Nevertheless, we are unable to predict whether regulators will be issuing new regulations under which SFPs will be equally taxed in line with other tobacco products such as conventional cigarettes. If we cease to be successful in these efforts, SFP unit margins may be materially adversely affected, which in turn may have a material adverse effect on our results of operations, revenues, cash flows, and profitability.
Consumption of tax-paid cigarettes continues to decline in many of our markets.
This decline is due to multiple factors, including increased taxes and pricing, governmental actions, the diminishing social acceptance of smoking, health concerns, competition, continuing economic and geopolitical uncertainty, and the continuing prevalence of illicit products. These factors and their potential consequences are discussed more fully below and in Item 7, Business Environment. A continuous decline in the consumption of cigarettes could have a material adverse effect on our revenues, cash flows and profitability, which in turn may have a material adverse effect on our ability to fund our smoke-free transformation.
Cigarettes are subject to substantial taxes. Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions. These tax increases may disproportionately affect our profitability and make us less competitive versus certain of our competitors.
Tax regimes, including excise taxes, sales taxes and import duties, can disproportionately affect the retail price of cigarettes versus other combustible tobacco products, or disproportionately affect the relative retail price of our cigarette brands versus cigarette brands manufactured by certain of our competitors. Because our portfolio is weighted toward the premium-price cigarette category, tax regimes based on sales price can place us at a competitive disadvantage in certain markets. Furthermore, our volume and profitability may be adversely affected in these markets.
In addition, increases in cigarette taxes are expected to continue to have an adverse impact on our sales of cigarettes, due to resulting lower consumption levels, a shift in sales from manufactured cigarettes to other combustible tobacco products and from the premium-price to the mid-price or low-price cigarette categories, where we may be under-represented, from local sales to cross-border purchases of lower price products, or to illicit products such as contraband, counterfeit and other non-compliant or otherwise illicit products.
Each of these risks could have a material adverse effect on our business, operations, results of operations, revenues, cash flows and profitability.
Our business faces significant governmental action aimed at increasing regulatory requirements with the goal of reducing or preventing the use of tobacco or nicotine-containing products.
Governmental actions, combined with the diminishing social acceptance of smoking and private actions to restrict smoking, have resulted in reduced industry volumes for our products in many of our markets, and we expect that such factors will continue to reduce consumption levels and will increase down-trading and the risk of counterfeiting, contraband, illicit trade and cross-border purchases. Significant regulatory developments will continue to take place over the next few years in most of our markets, driven principally by the Framework Convention on Tobacco Control (the "FCTC"). Since it came into force in 2005, the FCTC has led to increased efforts by tobacco control advocates and public health organizations to promote increasingly restrictive regulatory measures on the marketing and sale of tobacco and nicotine-containing products to adult nicotine users. Regulatory initiatives that have been proposed, introduced or enacted by governmental authorities in various jurisdictions include:
| | | | | |
| • | restrictions on or licensing of outlets permitted to sell tobacco or nicotine-containing products; |
| • | the levying of substantial and increasing tax and duty charges; |
| • | restrictions or bans on advertising, marketing and sponsorship; |
| • | the display of larger health warnings, graphic health warnings and other labeling requirements; |
| • | restrictions on packaging design, including the use of colors, and mandating plain packaging; |
| • | restrictions on packaging and cigarette formats and dimensions; |
| • | restrictions or bans on the display of product packaging at the point of sale and restrictions or bans on vending machines; |
| • | generation sales bans, under which the sale of certain tobacco or nicotine-containing products to people born after a certain year would be prohibited; |
| • | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and/or other smoke or product constituents; |
| • | disclosure, restrictions, or bans of tobacco product ingredients, including bans on the flavors of certain tobacco and nicotine-containing products; |
| • | increased restrictions on smoking and use of tobacco and nicotine-containing products in public and work places and, in some instances, in private places and outdoors; |
| • | restrictions or prohibitions of novel tobacco or nicotine-containing products or related devices; |
| • | elimination of duty free sales and duty free allowances for travelers; |
| • | restrictions in terms of importing or exporting our products impacting our logistics activities and ability to ship our products; |
| • | encouraging litigation against tobacco companies; and |
| • | excluding tobacco companies from transparent public dialogue regarding public health and other policy matters. |
Our financial results could be materially affected by regulatory initiatives resulting in a significant decrease in demand for our brands. More specifically, requirements that lead to a commoditization of tobacco products or impede adult consumers' ability to convert to our SFPs, as well as any significant increase in the cost of complying with new regulatory requirements could have a material adverse effect on our financial results.
Changes in the earnings mix and changes in tax laws may result in significant variability in our effective tax rates. Our ability to receive payments from foreign subsidiaries or to repatriate royalties and dividends could be restricted by local country currency exchange controls and other regulations.
We are subject to income tax laws in the United States and numerous foreign jurisdictions. Changes in the tax laws of foreign jurisdictions could arise as a result of the base erosion and profit shifting project undertaken by the Organisation for Economic Co-operation and Development (the "OECD"), which recommended changes to numerous long-standing tax principles, and could have a material adverse impact on our effective tax rate thereby reducing our net earnings. Such changes, as well as changes in taxing
jurisdictions’ administrative interpretations, decisions, policies, or positions, could also have a material adverse impact on our effective tax rate thereby reducing our net earnings. Currently, many countries have enacted or taken actions to align with the OECD’s framework on a global minimum tax (referred to as “Pillar Two”), effective for taxable years beginning after December 31, 2023. We will continue to evaluate and monitor as additional guidance and clarification becomes available. In future periods, our ability to recover deferred tax assets could be subject to additional uncertainty as a result of such developments. Furthermore, changes in the earnings mix or applicable foreign tax laws may result in significant variability in our effective tax rates.
As a result of Russia’s invasion of Ukraine, certain taxing jurisdictions, including the U.S., have proposed punitive tax legislation applicable to companies doing business in Russia, which could also have a material adverse impact on our effective tax rate if enacted thereby reducing our net earnings.
Because we are a U.S. holding company, our most significant source of funds is distributions from our non-U.S. subsidiaries. Certain countries in which we operate have adopted or could institute currency exchange controls and other regulations or policies that limit or prohibit our local subsidiaries' ability to convert local currency into U.S. dollars or to make payments outside the country. This could subject us to the risks of local currency devaluation and business disruption.
Disruptions in the credit markets or changes to our credit ratings may adversely affect our business.
We currently generate significant cash flows from ongoing operations and have access to global credit markets through our various short- and long- term financing activities. Our financial performance, credit ratings, interest rates, the stability of financial institutions with which we partner, geopolitical or national developments, the stability and liquidity of the credit markets and the state of the global economy could affect the availability and cost of financing.
Disruption in the credit markets, limitations on our ability to borrow, slower than anticipated debt deleveraging, or a downgrade of our current credit rating could increase our future borrowing costs which could materially and adversely affect our financial condition and results of operations. In addition, tighter or more volatile credit markets may lead to business disruptions for certain of our suppliers, contract manufacturers or trade customers which could, in turn, adversely impact our business, results of operations, cash flows and financial condition.
We could decide, or be required to, recall products, which could have a material adverse effect on our business, reputation, results of operations, cash flows or financial position.
We could decide - or laws, regulations, or judicial administrative action could require us - to recall products due to the failure, or alleged failure, to meet quality or safety standards or specifications, suspected or confirmed and deliberate or unintentional product contamination, manufacturing defects, or other product safety concerns, adulteration, misbranding or tampering. A product recall or a product liability or other claim (even if unsuccessful or without merit) could generate negative publicity about us and our products, and our Company’s reputation or that of our brands may be adversely affected. In addition, if another company recalls or experiences negative publicity related to a product in a category in which we compete, adult nicotine consumers might reduce their overall consumption of products in that product category. Any of these events could have a material adverse effect on our business, reputation, results of operations, cash flows or financial position.
We may be required to write down assets due to impairment, which could have a material adverse effect on our results of operations or financial position.
We continuously monitor the values of our long-lived assets, reporting units, intangible assets, as well as investments in equity securities, to determine whether events or changes in circumstances indicate that an impairment exists. Additionally, we test goodwill and non-amortizable intangible assets for impairment annually. The values of these assets may be affected by several factors, including general macroeconomic and geopolitical conditions; regulatory and legal developments; changes in product volume growth rates; changes in pricing strategies and costs bases; discount rates; success of planned new product expansions; competitive activity; and income and excise taxes. If an impairment is determined to exist, we will incur impairment losses, which could have a material adverse effect on our results of operations or financial position. See Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Estimates for additional information concerning impairment determination and calculation.
Our management uses certain key business metrics to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections and make strategic decisions and such metrics may not accurately reflect all of the aspects of our business needed to make such evaluations and decisions, in particular as our business continues to evolve.
In addition to our consolidated financial results, our management regularly reviews a number of operating and financial metrics, including various revenue, user and sales metrics (such as market shares, in-market sales, adjusted in-market sales, and SFP users) to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections and make strategic decisions. We believe that these metrics are representative of our current business; however, these metrics may not accurately reflect all aspects of our business and we anticipate that these metrics may change or may be substituted for additional or different metrics as our business evolves. Furthermore, in some instances the metrics are based upon a number of assumptions and estimates
that, while presented with numerical specificity, are inherently subject to significant uncertainties and contingencies. If our management fails to account for other relevant information or substitute the key business metrics they review as our business changes or if the assumptions or estimates underlying the metrics are inaccurate, their ability to accurately formulate financial projections and make strategic decisions may be compromised and our business, financial results and future growth prospects may be adversely impacted.
Risks Related to the Impact of the War in Ukraine on our Business
Our business, results of operations, cash flows and financial position may be adversely impacted by the continuation and consequences of the war in Ukraine.
In 2024, Russia accounted for around 9% of our total cigarette and heated tobacco unit shipment volume, and around 6% of our total net revenues. Ukraine accounted for around 2% of our total cigarette and heated tobacco unit shipment volume, and around 1% of our total net revenues. Historically, we also produced finished goods in Ukraine for export and manufactured products in Russia. In 2022, as a result of Russia’s invasion of Ukraine, we suspended planned investments and scaled down our manufacturing operations in Russia.
The full implications of the Russian invasion of Ukraine for our operations in those countries are impossible to predict at this time. The likelihood of retaliatory action by the Russian government against companies, including PMI, as a result of actions and statements made in response to the Russian invasion or otherwise, including the possibility of legal action against us or our employees; the deprivation of rights in, or access to, our Russian or Russia-related assets; or nationalization of foreign businesses or assets (including cash reserves held in Russia and intangible assets such as trademarks), is impossible to predict. We are continuously assessing the evolving situation in Russia, including regulatory constraints in the market entailing very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. In the event of a divestment, our ability to fully realize the value of the business would likely be subject to material impairment. The deprivation of rights in, or access to, our Russian or Russia-related assets could also result in a material impairment and could cause the deconsolidation of our Russian business. In Ukraine, there is no way to know when and to what extent we will be able to fully normalize our operations or to what extent our workforce, facilities, inventory, and other assets will remain intact. These developments have and will continue to have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in further impairment charges.
The conflict also continues to elevate the likelihood of supply chain disruptions, both in the region and globally, and may inhibit our ability to timely source materials and services needed to make and sell our products. For example, historically we sourced certain finished goods, production materials and components from both Russia and Ukraine, including printed materials and filters, and the invasion has, and may continue to, disrupt the availability of and impact our supply chain for these materials. These disruptions, to the extent we are unable to find alternative sources or otherwise address these supply constraints, may impact the availability and cost of our products in other markets, which would adversely impact our business, results of operations, cash flows and financial position, and may result in impairment charges. Furthermore, the imposition of various restrictions on transactions with parties from certain jurisdictions, the ban on exports of various products, and other economic and financial restrictions may adversely affect certain third parties with which we do business in Russia, such as customers, suppliers, intermediaries, service providers and banks.
The broader consequences of the invasion are also impossible to predict, but could include reputational consequences, further sanctions, financial or currency restrictions, punitive tax law changes, embargoes, regional instability, and geopolitical shifts as well as adverse effects on macroeconomic conditions, security conditions, currency exchange rates, and financial markets. Given the nature of our business and global operations, such geo-political instability and uncertainty could increase the costs of our materials and operations; reduce demand for our products; have a negative impact on our supply chains, manufacturing capabilities, or distribution capabilities; increase our exposure to currency fluctuations; constrain our liquidity or our ability to access capital markets; create staffing or operations difficulties; or subject us to increased cyber-attacks. While we will continue to monitor this fluid situation and develop contingency plans as necessary to address any disruptions to our business operations as they develop, the extent of the conflict’s effect on our business and results of operations as well as the global economy, cannot be predicted.
The conflict may also heighten many other risks disclosed in this Form 10-K, any of which could adversely affect our business, results of operations, cash flows or financial position. Such risks could affect, without limitation, the achievement of our strategic priorities, including achievement of our smoke-free business growth targets; the availability of third-party manufacturing resources; the availability of attractive acquisition and strategic business opportunities and our ability to fully realize the benefits of these transactions; our ability to attract, motivate, and retain the best global talent; and our loss of revenue from counterfeiting and similar illicit activities.
Risks Related to Sourcing and Distribution of Products, Services and Materials
Use of third-parties may negatively impact the distribution, quality, and availability of our products and services, and we may be required to replace third-party contract distributors, manufacturers or service providers.
We increasingly rely on third-parties and their subcontractors/suppliers, sometimes concentrated in a specific geographic area, for product distribution and to manufacture some of our products and product parts (particularly, the electronic devices and accessories), as well as to provide services, including to support our finance, commercialization and information technology processes. While many of these arrangements improve efficiencies and decrease our operating costs, they also diminish our direct control. Such diminished control may lead to disruption in the distribution of our products and may have a material adverse effect on the quality and availability of products or services, our supply chain, and the speed and flexibility in our response to changing market conditions and adult consumer preferences, all of which may place us at a competitive disadvantage. In addition, we may be unable to renew these agreements on satisfactory terms for numerous reasons, including government regulations, and the distribution of our products may be disrupted in certain markets or our costs may increase significantly if we must replace such third parties with other partners or our own resources.
The effects of climate change, other environmental issues, and related legal or regulatory responses may have a negative impact on our business and results of operations.
While we seek to mitigate our business risks associated with environmental issues, such as climate change, by establishing environmental goals and standards and seeking business partners, including within our supply chain, that are committed to operating in ways that protect the environment or mitigate environmental impacts, we recognize that there are inherent environmental-related risks, including climate change-related risks, wherever business is conducted. Among other potential impacts, climate change could influence the quality and volume of the agricultural products we rely on, including tobacco, due to several factors beyond our control, including more frequent variations in weather patterns, extreme weather events causing unexpected downtime and inventory losses, other adverse weather conditions, and governmental restrictions on trade, all of which may lead to disruption of operations at factories, warehouses and other premises.
Furthermore, nature-related risks, including those related to natural ecosystems degradation, decreased agricultural productivity in certain regions of the world, biodiversity loss, water resource depletion and deforestation, which are partially driven or exacerbated by climate change, may negatively impact the resilience of, or otherwise disrupt, our business operations or those of our suppliers and business partners.
There is an increased focus by foreign, federal, state and local regulatory and legislative bodies on environmental policies, including those relating to climate change. New environmental-related legal or regulatory requirements may lead to additional carbon taxation, raw or other materials taxation, energy price increases, new compliance costs, increased distribution and supply chain costs, and other expenses impacting our cost of operations. Moreover, given that the regulatory framework in this regard is highly dynamic, additional uncertainties may be driven by further upcoming regulatory changes on which we might have limited visibility or limited time to implement, which could have an impact on several elements of our business, including elevating the cost or complexity of our operations. Even if we make changes to align ourselves with legal or regulatory requirements, we may still be subject to significant penalties if such laws or regulations are interpreted and applied in a manner inconsistent with our practices. Additionally, government authorities, non-governmental organizations or external stakeholders are increasingly filing lawsuits or initiating regulatory actions, alleging that public statements regarding sustainability-related matters and practices are misleading or false.
Government mandated prices, production control programs, and shifts in crops driven by economic conditions may increase the cost or reduce the quality of the tobacco and other agricultural products used to manufacture our products.
As with other agricultural commodities, the price of tobacco leaf and cloves can be influenced by imbalances in supply and demand and the impacts of natural disasters and pandemics such as COVID-19. Tobacco production in certain countries is subject to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for agricultural products could cause farmers to produce less tobacco or cloves. Any significant change in tobacco leaf and clove prices, quality and quantity could affect our profitability and our business.
A prolonged disruption of our production facilities could have a material adverse effect on our business, financial condition and results of operations.
A prolonged disruption at or shut-down of one or more of the facilities where our products are produced, especially our ZYN production facility in Kentucky, U.S., which currently supplies substantially all of our capacity for ZYN sales in the U.S., due to natural- or man-made disasters or other events outside of our control, such as equipment malfunction or widespread outbreaks of acute illness, including COVID-19, supply chain constraints, or for any other reason, could limit our capacity to meet customer demands. Such an event could disrupt our operations; delay production, shipments and revenue; and result in significant expense to repair or replace our affected facilities. As a result, we could forgo revenue opportunities and potentially lose market share, which could materially and adversely affect our business, financial condition and results of operations.
Risks Related to our International Operations
Because we have operations in numerous countries, our results may be adversely impacted by economic, regulatory and political developments, natural disasters, pandemics or conflicts.
Some of the countries in which we operate face the threat of civil unrest and can be subject to regime changes. In others, nationalization, terrorism, conflict and the threats of war or acts of war may have a significant impact on the business environment. Factors beyond our control, such as, without limitation, natural disasters, extreme weather events, pandemics (including COVID-19), economic, political, regulatory, acts of war or threats of war or other developments could disrupt or increase the expenses related to our supply chain, manufacturing capabilities, distribution capabilities, or the energy and other utility services required to operate our factories, warehouses, and other premises. Our business continuity plans and other safeguards might not always be effective to fully mitigate their impact. For example, the global pandemic outbreak of the COVID-19 virus in 2020 created significant societal and economic disruption and the closure of stores, factories and offices, restrictions on manufacturing, distribution and travel, and supply chain disruptions, among other impacts. Additionally, while the supply chains our operations rely on are generally self-contained within their respective trade regions and have limited inflexible trade connections to markets that represent a high tariff risk, a broader increase in tariffs could disrupt our supply chains and increase our costs. Such developments – including the impact of geopolitical disruptions resulting from the conflict in the Middle East and the impact on energy prices and availability in the EU and elsewhere resulting from the invasion of Ukraine by Russia – could cause significant volume declines in our duty-free business and certain other key markets; disrupt or delay our distribution, manufacturing or supply chain; increase currency volatility; increase costs of our materials and operations and lead to loss of property or equipment that are critical to our business in certain markets and difficulty in staffing and managing our operations, all of which could have a material adverse effect on our business, operations, volumes, revenues, cash flows, financial position, net earnings and profitability. We discuss additional risks associated with Russia's invasion of Ukraine and climate change, above.
In certain markets, we are dependent on governmental approvals of various actions such as price changes, and failure to obtain such approvals could impair growth of our profitability.
In addition, despite our high ethical standards and rigorous controls and compliance policies aimed at preventing and detecting unlawful conduct, given the breadth and scope of our international operations, we may not be able to detect all potential improper or unlawful conduct by our employees and partners. Such improper or unlawful conduct (actual or alleged) could lead to litigation and regulatory action, cause damage to our reputation and that of our brands, and result in substantial costs.
Our reported results could be adversely affected by unfavorable currency exchange rates and currency fluctuations could impair our competitiveness. Our results could also be adversely affected by capital controls or by foreign currency exchange constraints or devaluations.
We conduct our business primarily in local currency and, for purposes of financial reporting, the local currency results are translated into U.S. dollars based on average exchange rates prevailing during a reporting period. Foreign currencies may fluctuate significantly against the U.S. dollar, reducing our net revenues, operating income and EPS. Our primary local currency cost bases may be different from our primary currency revenue markets, and U.S. dollar fluctuations against various currencies may have disproportionate negative impact on cash flows and on net revenues as compared to our gross profit and operating income margins.
Capital controls and/or foreign currency exchange constraints may affect the ability of our subsidiaries in impacted jurisdictions to settle foreign currency denominated imports of goods and services and/or to pay dividends and royalties. These factors may also increase foreign currency devaluation risks, which may have a negative impact on our net assets and results of operations in these jurisdictions. All of which could have a material adverse effect on our financial condition, including our leverage ratios, cash flows, net earnings, and profitability.
A sustained period of elevated inflation across the markets in which we operate could result in higher operating and financing costs and lead to reduced demand for our products.
Increasing inflationary pressures have and may continue to result in significant increases to our expenses, including direct materials, wages, energy, and transportation costs. While we take actions, wherever possible, to reduce the impact of the effects of inflation, in cases of sustained and elevated inflation across several of our major markets, it may be difficult to effectively control the increases to our costs. In recent periods, increased inflation has and may continue to lead to growing pressures on the cost of certain direct materials, wages, energy, transportation, and logistics as well as an increased cost of capital due to interest rate increases driven by the response to increased inflation. Inflationary pressures may also negatively impact consumer purchasing power, which could result in reduced demand for our products. We expect a moderate inflationary increase in 2025. If we are unable to increase our prices sufficiently or take other actions to mitigate the effect of inflationary pressures, our profitability and financial position could be negatively impacted.
Risks Related to Legal Challenges and Investigations
Litigation related to tobacco products and nicotine products could substantially reduce our profitability and could severely impair our liquidity.
There is litigation related to tobacco products and/or nicotine products pending in certain jurisdictions in which we operate. Damages claimed in some tobacco-related litigation are significant and, in certain cases, range into the billions of U.S. dollars. As of March 2024, we began facing litigation related to our oral nicotine products before certain courts in the United States. We anticipate that new cases will continue to be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our consolidated results of operations, cash flows or financial position could be materially adversely affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. We face various administrative and legal challenges related to certain SFP activities, including allegations concerning product classification, advertising and distribution restrictions, corporate communications, product coach activities, scientific substantiation, product liability, antitrust, and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize SFPs and to communicate with the public. The outcomes of these matters may affect our SFP commercialization and public communication activities and performance in one or more markets. Also see Item 8, Note 18. Contingencies to our consolidated financial statements for a discussion of pending litigation.
From time to time, we are subject to governmental investigations on a range of matters.
Investigations include allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within certain markets, allegations of underpayment of income taxes, customs duties and/or excise taxes, allegations of false and misleading usage of descriptors, allegations of unlawful advertising or distribution, and allegations of unlawful labor practices. We cannot predict the outcome of those investigations or whether additional investigations may be commenced, and it is possible that our business could be materially adversely affected by an unfavorable outcome of pending or future investigations. See Item 8, Note 18. Contingencies—Other Litigation and Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations—Operating Results by Business Segment—Business Environment—Governmental Investigations for a description of certain governmental investigations to which we are subject.
We may be unable to adequately protect our intellectual property rights, and disputes relating to intellectual property rights could harm our business.
Our intellectual property rights are valuable assets, their protection is important to our business, and that protection may not be equally available in every country in which we operate or in which our products are sold. If the steps we take to protect our intellectual property rights globally, including through applying for, prosecuting, maintaining and enforcing, where relevant, a combination of trademark, design, copyright, patent, trade secrets and other intellectual property rights, are inadequate, or if others infringe or misappropriate our intellectual property rights, notwithstanding legal protection, our business, financial condition, and results of operations could be adversely impacted. Moreover, failing to manage our existing and/or future intellectual property may place us at a competitive disadvantage. Intellectual property rights of third parties may limit our ability to develop, manufacture and/or commercialize our products in one or more markets. Competitors or other third parties may claim that we infringe their intellectual property rights. Any such claims, regardless of merit, could divert management’s attention, be costly, disruptive, time-consuming and unpredictable and expose us to significant litigation costs and damages, and may impede our ability to develop, manufacture and/or commercialize new or existing SFPs and improve our products, and thus have a material adverse effect on our revenues and our profitability. In addition, if, as a result, we are unable to manufacture or sell our SFPs or improve their quality in one or more markets, our ability to convert adult smokers to our SFPs in such markets would be adversely affected. See Item 8, Note 18. Contingencies—Other Litigation to our consolidated financial statements for a description of certain intellectual property proceedings.
The research, development, and commercialization of non-recreational cannabinoid products subjects the Company to legal, regulatory, reputational and other risks.
Our Wellness and Healthcare business is researching, developing, and exploring the commercialization of medical and pharmaceutical cannabinoids and non-recreational cannabinoid products (including CBD). Our Wellness and Healthcare business currently anticipates pursuing these activities in select non-U.S. markets. While we will undertake the activities in a manner consistent with all applicable requirements, successful commercialization is dependent on compliance with a constantly evolving legal and regulatory environment, and subject us to various legal, reputational and regulatory risks, which could have a material, adverse effect on our business and results of operations. A failure by our Wellness and Healthcare business to comply with applicable laws could result in criminal, civil, or tax liability.
Risks Related to our Competitive Environment
We face intense competition, and our failure to compete effectively could have a material adverse effect on our profitability and results of operations.
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, R&D, innovation, packaging, customer service, marketing, advertising and retail price. The competitive environment and our competitive position can be significantly influenced by weak economic conditions; erosion of consumer confidence; competitors' introduction of lower-price products or innovative products; adult smoker willingness to convert to our SFPs; higher product taxes; higher absolute prices and larger gaps between retail price categories; unfair competition; and product regulation that diminishes the ability to differentiate tobacco products, restricts adult consumer access to truthful and non-misleading information about our SFPs, or disproportionately impacts the commercialization of our products in relation to our competitors.
Competitors in our industry include Altria Group, Inc., British American Tobacco plc, Japan Tobacco Inc., Imperial Brands plc, new market entrants, particularly with respect to innovative products, several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, China, Taiwan, Thailand and Vietnam. Some competitors have different profit, volume and regulatory objectives, some international competitors may be less susceptible than PMI to changes in currency exchange rates, and some competitors may sell products in circumvention of applicable regulations that compete directly with our products. Certain new market entrants in the non-combustible product category may alienate consumers from innovative products through inappropriate marketing campaigns, messaging and inferior product satisfaction, and without scientific substantiation based on appropriate R&D protocols and standards. The growing use of digital media could increase the speed and extent of the dissemination of inaccurate and misleading information about our SFPs, all of which could have a material adverse effect on our profitability and results of operations. See Item 1, Business—Competition for a description of the competitive environment in which we operate.
We may be unable to anticipate changes in adult consumer preferences.
Our business is subject to changes in adult consumer preferences, which may be influenced by local economic conditions, accessibility to our products and availability of accurate information related to our products.
To be successful, we must: | | | | | |
| • | promote brand equity successfully; |
| • | anticipate and respond to new adult consumer trends; |
| • | ensure that our products meet our quality standards; |
| • | develop new products and markets and broaden brand portfolios; |
| • | improve productivity; |
| • | educate and encourage adult smokers to convert to our SFPs; |
| • | ensure effective adult consumer engagement, including communication about product characteristics and usage of SFPs; |
| • | mitigate the impact of developments that cause damage to our reputation and that of our brands; |
| • | provide excellent customer care; |
| • | ensure adequate production capacity to meet demand for our products; and |
| • | be able to protect or enhance margins through price increases. |
In periods of economic uncertainty, adult consumers may tend to purchase low-price brands, and the volume of our premium-price and mid-price brands and our profitability could be materially adversely impacted as a result. Such down-trading trends may be reinforced by regulation that limits branding, communication and product differentiation. In addition to economic uncertainty (including recessions and inflation) unusual weather events and global or local epidemics, endemics or pandemics (such as COVID-19) has and may change the preferences of our adult consumers and lower demand for our products, particularly for our mid-price or premium-price brands.
Our ability to grow profitability may be limited by our inability to introduce new products, enter new markets, maintain sufficient production capacity, or improve our margins through higher pricing and improvements in our brand and geographic mix.
Our profit growth may be materially adversely impacted if we are unable to introduce new products or enter new markets successfully, to meet the demand for our products with increased production capacity, to raise prices, or to improve the proportion of our sales of higher margin products and in higher margin geographies.
We may be unable to expand our brand portfolio through acquisitions or the development of strategic business relationships, and the intended benefits from our investments may not materialize.
One element of our growth strategy is to expand our brand portfolio and market positions through selective acquisitions and the development of strategic business relationships. Acquisition and strategic business development opportunities are limited and present risks of failing to achieve efficient and effective integration, strategic objectives and/or anticipated revenue improvements and cost savings. There is no assurance that we will be able to acquire attractive businesses or enter into strategic business relationships on favorable terms ahead of our competitors, or that such acquisitions or strategic business development relationships will be accretive to earnings or improve our competitive position. In addition, we may not have a controlling position in certain strategic investments or relationships, which could impact the extent to which the intended financial growth and other benefits from these investments or relationships may ultimately materialize.
Our ability to achieve our strategic goals may be impaired if we fail to attract, motivate and retain the best global talent and effectively align our organizational design with the goals of our transformation.
To be successful, we must continue transforming our culture and ways of working, align our talent and organizational design with our increasingly complex business needs, and innovate and transform to a consumer-centric business. We compete for talent, including in areas that are relatively new to us such as digital, information technology, and life sciences, with companies in the consumer products, technology, pharmaceutical and other sectors that enjoy greater societal acceptance. As a result, we may be unable to attract, motivate and retain the best global talent with the right degree of diversity, experience and skills to achieve our strategic goals.
Risks Related to Illicit Trade
Our revenues may be materially adversely affected as a result of counterfeiting, contraband, cross-border purchases, illicit products, non-tax-paid volume produced by local manufacturers and other non-compliant or illicit cigarettes or smoke-free products.
Large quantities of counterfeit cigarettes are sold in the international market. We believe that Marlboro is the most heavily counterfeited international cigarette brand, although we cannot quantify the revenues we lose as a result of this activity. Counterfeits of our smoke-free products are not subject to our scientific validation procedures, are unlikely to meet our product quality standards, and may materially adversely affect the reputation of our smoke-free products with consumers, regulators, and other stakeholders. In addition, our revenues may be materially adversely affected by counterfeiting, contraband, cross-border purchases, non-tax-paid volume produced by local manufacturers and other non-compliant or illicit cigarettes or smoke-free products.
Risks Related to Cybersecurity and Data Governance
We are significantly dependent on our and third-party information technology networks and systems, and a cybersecurity incident or attack against those networks or systems may adversely impact our business and operations.
We and our business partners heavily rely on information technology networks and systems, including those connected to the Internet, to help manage business processes and operations, including the collection, storage, interpretation, and processing of confidential, sensitive, personal and other data; internal and external communications; marketing and e-commerce activities; the manufacture, sale, and distribution of our products; management of third-party business relationships; engagement with governmental authorities; innovation through research and development; and other activities necessary for business operations. Some of these information systems and networks are developed, supplied, or managed by third-party service providers that may make us vulnerable to “supply chain” style cyberattacks. Additionally, some information technology systems may be supported by artificial intelligence capabilities that may not function as intended, posing cybersecurity and data protection risks. The failure or disruption of our information technology networks and systems, or those managed by third-party service providers or owned by our business partners and used in furtherance of PMI’s business, due to cybersecurity attacks; unauthorized attempts to corrupt or extract data; security vulnerabilities; misconfigurations; human error; or failure or inability by us, third-parties, or our business partners to adhere to cybersecurity industry best practices, could place us at a competitive disadvantage, cause reputational damage, impact our operations, result in data breaches, significant business disruption, litigation, regulatory action including significant fines or penalties, financial impact, loss of revenue or assets including our intellectual property, personal, confidential, or sensitive data.
Cyberattacks, security incidents and vulnerabilities impacting PMI, newly acquired companies, our business partners, or our third-party providers, continue to dynamically evolve in sophistication and volume, making it difficult for us to predict probability, frequency, and impact severity of security incidents. Further, it may be inherently difficult to detect vulnerabilities during due diligence, for long periods of time, or soon enough to mitigate exploitation. There can be no assurance that such security incidents or vulnerabilities will not have a material adverse effect on us in the future. While PMI works to mitigate these risks by implementing a cybersecurity risk program and a third-party cybersecurity risk management program, there can be no assurance that these programs are comprehensive or accurately identify and sufficiently mitigate all cybersecurity risks.
We continue to make investments in administrative, technical, and physical safeguards to maintain information security protections in line with industry standards and best practices. We evaluate the adequacy of preventative actions to reduce security incidents on an ongoing basis.
Cyberattacks, security incidents and vulnerabilities have impacted, and we expect will continue to impact, PMI, our business partners, and our third-party providers. Cyberattacks continue to dynamically evolve in sophistication and volume, making it difficult for us to predict probability, frequency, and impact severity of security incidents on the Company. We also have, and continue to face, immaterial third-party information security breaches. While these types of incidents have occurred frequently within the last three years, none have been material to our business, financial condition, or results.
Our safeguards may not, however, be effective in mitigating the impact of service disruptions or other failures of these information technology networks and systems. Failure to timely respond and mitigate security incidents, could result in wide-ranging business interruptions. Such security incidents could place us at a competitive disadvantage; result in financial impacts, a loss of revenue, assets, including our intellectual property, personal or other sensitive data; result in litigation and regulatory action including significant fines or penalties; impact our operations; cause damage to our reputation and that of our brands; and result in significant remediation and other costs. See Item 1C. Cybersecurity for a description of our cybersecurity risk management and strategy and governance.
Our or our business partners’ failure or inability to adhere to privacy, data, artificial intelligence and information security laws could result in business disruption, loss of reputation and consumer trust, litigation, regulatory action including significant fines or penalties, financial impact, and loss of revenue, assets or personal, confidential, or sensitive data.
An actual or alleged failure to comply with complex and changing privacy, data, artificial intelligence and information security laws and regulations under the EU General Data Protection Regulation, various U.S. state and federal laws, and other similar privacy and information security laws across the jurisdictions in which PMI operates, such as the failure to protect personal data; implement appropriate technological and reasonable security measures; implement and maintain appropriate safeguards for personal data being transferred internationally; respect the privacy rights of data subjects; provide sufficient detailed notices of personal data processing; retrieve consent and provide opt-outs; meet stringent timeframe requirements for incident reporting to regulatory authorities; comply with artificial intelligence regulations, and others, could have a material adverse effect on us, subject us to substantial fines and/or legal challenges, and/or harm our business, reputation, financial condition, or operating results. Such laws and regulations across the jurisdictions in which PMI operates may vary, resulting in inconsistent or conflicting legal obligations. Although we maintain a cyber
liability insurance policy to address many of these risks, such policy may not be sufficient to prevent a cybersecurity incident or attack from resulting in a material adverse effect on our business, reputation, financial condition, or operating results.
Risks Related to Acquisitions and Divestitures
We may not successfully identify, complete, or realize the benefits from strategic acquisitions, divestitures, joint ventures, or investments.
From time to time, we evaluate acquisition candidates, joint ventures, or investments that may strategically fit our business objectives. As a result of some of these evaluations, we have acquired and may acquire in the future certain businesses (or parts of businesses) or assets. We have also divested and may divest businesses from time to time. These activities may present financial, managerial, and operational risks including, but not limited to, diversion of management’s attention from existing core businesses; difficulties in integrating, or inability to successfully integrate, acquired businesses, including integrating or separating personnel, information technology, financial and other systems; inability to effectively and immediately implement control environment processes across a diverse employee population; adverse effects on existing or acquired customer and supplier business relationships; potential disputes with buyers, sellers, or partners, as well as other unanticipated problems or liabilities, such as contingent liabilities and litigation. Activities in such areas are regulated by numerous antitrust and competition laws in the United States, the European Union, the United Kingdom, and elsewhere. We have in the past and may in the future be required to obtain approval of these transactions by competition or other regulatory authorities or to satisfy certain legal requirements, and we may be unable to obtain such approvals or satisfy such requirements, each of which may result in additional costs, delays, or our inability to complete such transactions. Any of these factors could prevent us from realizing the anticipated benefits of any such transaction and/or could materially and adversely affect our financial condition and operating results.
We may face additional risks related to divestitures. For example, risks related to our ability to find appropriate buyers, execute transactions on favorable terms, separate divested business operations with minimal impact to our remaining operations, and effectively manage any transitional or long-term service arrangements. Further, our divestiture activities may require us to recognize impairment charges. Any of these factors could materially and adversely affect our financial condition and operating results.
Accounting adjustments related to acquisitions could adversely affect our financial results.
Given the nature of assets acquired through acquisitions, we may not be able to avoid future impairments of those assets, which may also have a material adverse impact on our future operating results and financial position.
Item 1B.Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
PMI relies heavily on the availability, reliability, and security of our information systems, networks, data, and intellectual property to, among other things, help manage our business processes and operations, collect and interpret data, and communicate internally and externally with employees, suppliers, consumers and customers, and business partners. We have a cross-functional cybersecurity risk program developed using standard industry practices, which monitors and manages cybersecurity threats to our business and information systems. We invest in administrative, technical, and physical safeguards, including continuity planning, to enhance resilience on our core processes, to maintain information security protections of our data and to safeguard the privacy of consumers, customers, employees and business partners. As of the date of this Form 10-K, cybersecurity threats have not materially affected our business, financial condition, or operating results.
Risk Management and Strategy
Our cybersecurity risk program, managed by our Chief Information Security Officer (“CISO”) and the information security team, is conducted under our enterprise risk management framework and operates on a risk-based approach in assessing risks from cybersecurity threats, as follows:
•Cybersecurity Threat Scenarios. Our cybersecurity risk assessment process consists of identifying and compiling a catalogue of top cybersecurity threat scenarios relevant to PMI, which facilitates risk assessments with our IT and business stakeholders.
•Cybersecurity Maturity Assessment. Our risk exposure from relevant cybersecurity threat scenarios is mitigated by evaluating existing cybersecurity capabilities and corresponding maturity to identify and address areas for improvement.
•Cybersecurity Threat Assessment. To establish PMI’s current and target cybersecurity risk exposure, residual risk exposure from the most relevant cybersecurity threat scenarios across IT platforms and regions is evaluated and measured based upon the cybersecurity maturity assessments.
•Cybersecurity Risk Program. PMI has a cybersecurity risk program to enhance its ability to identify, prevent, mitigate, respond and recover from disruptive cybersecurity threats and incidents and to reduce cybersecurity risk exposure. Improvements in our cybersecurity defense capabilities are prioritized based upon the results of cybersecurity threat assessments and cybersecurity maturity assessments. Identified issues from these assessments form the improvement initiatives under our cybersecurity risk program. As discussed in more detail below under “Governance,” the program’s key improvement initiatives, their implementation status, and the overall progression in our cybersecurity capability maturity are regularly presented to the applicable governing body within PMI. In addition, our cybersecurity risk program operates in coordination with the following:
Cyber Defense. Our dedicated cyber defense team provides services to identify, help prevent, detect and respond against cybersecurity threats and intrusions and collaborates with internal and external stakeholders to help protect PMI’s information, mitigate operational disruptions and maintain business continuity. The cyber defense team’s controls and procedures identify and enable escalation of cybersecurity incidents to the applicable governing body within PMI, as appropriate, to meet disclosure and reporting requirements for such incidents.
Third-Party Cyber Risk Management. Some of our information systems and networks are developed, supplied, or managed by third-party service providers. Our third-party cyber risk management process analyzes and seeks to control risks associated with outsourcing products or services, such as “supply chain” style cyberattacks, and identifies preventative and detective controls to mitigate third-party vendor and service provider cybersecurity risks that could adversely impact our business and operations.
Education and Awareness. PMI regularly and annually provides its in scope workforce with mandatory cybersecurity awareness education and training addressing information security related tasks in line with our evolving information security policies, standards, procedures, and practice as well as supplemental role-based training and awareness programs.
We engage external assessors, auditors and other third parties to independently evaluate our cybersecurity risk management process and related controls, including the relevance to PMI of identified cybersecurity scenarios and the results of cybersecurity maturity assessments. The outcome of such evaluations, audits or reviews are reported to the Corporate Risk Governance Committee and to the Audit & Risk Committee, and our cybersecurity policies, standards and processes are adjusted, as necessary.
PMI follows a risk evaluation process for issues identified through internal audits, security assessments, third-party cybersecurity risk assessments, or self-assessment disclosures, and resulting information technology risks are recorded for risk remediation, transfer, avoidance, or acceptance as appropriate. Some of our information systems are managed by specialist third-party service providers, and we work with internal specialists to protect systems and data from unauthorized access and other cybersecurity threats.
Governance
The Audit and Risk Committee of our Board of Directors oversees our policies and practices with respect to risk assessment and risk management, including a review, in coordination with our management, of PMI’s management of cybersecurity. Our CISO presents reports to the Audit and Risk Committee or to the full Board of Directors at least quarterly, which reports include cybersecurity risk status along with key performance indicators and key risk response strategies and plans.
The Corporate Risk Governance Committee receives quarterly reports on the Company’s overall cybersecurity risk exposure including the individual top cybersecurity threat scenario residual risk ratings and the plan and status of the cybersecurity risk program, to facilitate calibration with other enterprise risk domains and validation of the risk response plans. The Corporate Risk Governance Committee includes our Chief Executive Officer (“CEO”), Chief Financial Officer (“CFO”), General Counsel (“GC”), Senior Vice President Operations, and our Chief Digital & Information Officer (“CDIO”).
Cybersecurity incidents that have been determined to meet established SEC reporting consideration thresholds are promptly communicated to the Disclosure Committee, which is responsible for evaluating the potential materiality of such incidents and ensuring the accuracy, timeliness and completeness of related disclosures under applicable reporting obligations, and other relevant
communications or presentations. The Disclosure Committee’s membership includes the following executives: the Corporate Secretary; the GC; the CFO; the Controller & Principal Accounting Officer; the Chief Risk Assurance Officer; and the Vice President, Investor Relations. In addition, the CISO serves as an advisor to the Disclosure Committee.
The CISO has served in various roles in information technology and information security for over 25 years, including in the telecommunications and management consultancy sectors and serving as the Chief Information Security Officer of two large public companies. The CDIO holds an engineering degree and has served in various senior positions in information technology for over 20 years, including serving as Senior Vice President, IT Sales, and Global Chief Information Officer at a public company. The CEO has served in various positions in finance and general management at PMI for over 30 years, including as Chief Financial Officer and Chief Operating Officer, and holds a master’s degree in economics. The CFO has over 15 years of experience in finance and management, having held several executive positions in charge of finance, legal affairs information systems and industry administration at various companies. The GC has served at PMI for 18 years in several positions within the Legal & Compliance department, including as Vice President and Associate General Counsel of various regions, and holds two master’s degrees having studied law, management and finance.
As of the date of this Annual Report on Form 10-K, PMI is not aware of any risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, that have materially affected or are reasonably likely to materially affect PMI, its business strategy, results of operations or financial condition. For additional information concerning PMI’s risks related to cybersecurity, see Item 1.A. Risk Factors.
Item 2. Properties.
We own or lease various manufacturing, office and research and development facilities in locations around the world. We own properties in Switzerland where our operations center and state-of-the-art research and development facility are located.
At December 31, 2024, we operated and owned a total of 51 manufacturing facilities across our segments. Among them, 9 factories produced heated tobacco units and 7 factories produced oral nicotine products.
In 2024, certain of our facilities each manufactured over 30 billion units (cigarettes and heated tobacco units combined). Our largest manufacturing facilities, in terms of cigarette and heated tobacco unit volume, are located in Turkey (SSEA, CIS & MEA), Russia (SSEA, CIS & MEA), Indonesia (SSEA, CIS & MEA), Poland (Europe), Italy (Europe), Czech Republic (Europe) and Lithuania (Europe). Our largest nicotine pouch manufacturing facility is located in the United States. As part of our global operating model, products manufactured in a particular manufacturing facility are not necessarily distributed in the operating segment where the facility is located.
We have integrated the production of our heated tobacco units into a number of our existing manufacturing facilities, and we are progressing with our plans to build manufacturing capacity for our smoke-free products. We will continue to optimize our manufacturing infrastructure.
We believe the properties owned or leased by our subsidiaries are maintained in good condition and are believed to be suitable and adequate for our present needs.
Item 3.Legal Proceedings.
The information called for by this Item is incorporated herein by reference to Item 8, Note 18. Contingencies.
Item 4.Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The principal stock exchange on which our common stock (no par value) is listed is the New York Stock Exchange (ticker symbol "PM"). At January 31, 2025, there were approximately 38,800 holders of record of our common stock.
Information regarding equity-based compensation plans required by Regulation S-K Item 201(d) is provided in Item 12 of this 10-K.
Performance Graph
The graph below compares the cumulative total shareholder return on PMI's common stock with the cumulative total return for the same period of PMI's Peer Group and the S&P 500 Index. The graph assumes the investment of $100 as of December 31, 2019, in PMI common stock (at prices quoted on the New York Stock Exchange), and each of the indices as of the market close and reinvestment of dividends on a quarterly basis.
| | | | | | | | | | | | | | | | | | | | | | | |
| Date | | PMI | | | PMI Peer Group (1) | | S&P 500 Index |
| December 31, 2019 | | $100.00 | | | $100.00 | | $100.00 |
| December 31, 2020 | | $103.90 | | | $107.00 | | $118.40 |
| December 31, 2021 | | $125.50 | | | $123.70 | | $152.40 |
| December 31, 2022 | | $141.00 | | | $119.90 | | $124.80 |
| December 31, 2023 | | $138.50 | | | $118.00 | | $157.60 |
| December 31, 2024 | | $186.00 | | | $117.80 | | $197.00 |
(1) The PMI Peer Group presented in this graph is the same as that used in the prior year. The PMI Peer Group was established based on a review of four characteristics: global presence; a focus on consumer products; and net revenues and a market capitalization of a similar size to those of PMI. The review also considered the primary international tobacco companies. As a result of this review, the following companies constitute the PMI Peer Group: Altria Group, Inc., Anheuser-Busch InBev SA/NV, British American Tobacco p.l.c., The Coca-Cola Company, Colgate-Palmolive Co., Diageo plc, Heineken N.V., Imperial Brands PLC, Japan Tobacco Inc., Johnson & Johnson, Kimberly-Clark Corporation, The Kraft-Heinz Company, McDonald's Corp., Mondelēz International, Inc., Nestlé S.A., PepsiCo, Inc., The Procter & Gamble Company, Roche Holding AG, and Unilever NV and PLC.
Note: Figures are rounded to the nearest $0.10.
Issuer Purchases of Equity Securities During the Quarter Ended December 31, 2024
Our share repurchase activity for each of the three months in the quarter ended December 31, 2024, was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period | | Total
Number of
Shares
Repurchased | | Average
Price Paid
per Share | | Total Number
of Shares
Purchased as
Part of Publicly
Announced
Plans or
Programs | | Approximate
Dollar Value
of Shares that
May Yet be
Purchased
Under the Plans
or Programs |
October 1, 2024 –
October 31, 2024 (1) | | | | $ | — | | | — | | | $ | — | |
November 1, 2024 –
November 30, 2024 (1) | | | | $ | — | | | — | | | $ | — | |
December 1, 2024 –
December 31, 2024 (1) | | | | $ | — | | | — | | | $ | — | |
Pursuant to Publicly Announced Plans or Programs | | — | | | $ | — | | | | | |
October 1, 2024 –
October 31, 2024 (2) | | 1,346 | | | $ | 121.24 | | | | | |
November 1, 2024 –
November 30, 2024 (2) | | 4,173 | | | $ | 132.92 | | | | | |
December 1, 2024 –
December 31, 2024 (2) | | 857 | | | $ | 130.20 | | | | | |
For the Quarter Ended
December 31, 2024 | | 6,376 | | | $ | 130.09 | | | | | |
(1)On June 11, 2021, our Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period that commenced in July 2021. These share repurchases have been made pursuant to the $7 billion program. On May 11, 2022, we announced the suspension of our three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. Our three-year share repurchase program expired on July 21, 2024.
(2)Shares repurchased represent shares tendered to us by employees who vested in restricted and performance share unit awards and used shares to pay all, or a portion of, the related taxes.
Item 6. [Reserved].
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion should be read in conjunction with the other sections of this Annual Report on Form 10-K, including the consolidated financial statements and related notes contained in Item 8, and the discussion of risks and cautionary factors that may affect future results in Item 1A. Risk Factors.
Description of Our Company
We are a leading international tobacco company, actively delivering a smoke-free future. We are evolving our portfolio for the long term to include products outside of the tobacco and nicotine sector. Our current product portfolio primarily consists of cigarettes and smoke-free products. Since 2008, we have invested over $14 billion to develop, scientifically substantiate and commercialize innovative smoke-free products for adults who would otherwise continue to smoke, with the goal of completely ending the sale of cigarettes. This investment includes the building of world-class scientific assessment capabilities, notably in the areas of pre-clinical systems toxicology, clinical and behavioral research, as well as post-market studies. In November 2022, we acquired Swedish Match AB ("Swedish Match") – a leader in oral nicotine delivery – creating a global smoke-free combination led by the companies’ IQOS and ZYN brands. Following a robust science-based review, the U.S. Food and Drug Administration (the "FDA") has authorized the marketing of Swedish Match’s General snus and ZYN nicotine pouches and versions of PMI’s IQOS devices and consumables - the first-ever such authorizations in their respective categories. Versions of IQOS devices and consumables and General snus also obtained the first-ever Modified Risk Tobacco Product ("MRTP") authorizations from the FDA. We describe the MRTP orders in more detail in the "Business Environment" section of this Item 7.
Following the combination and the progress in 2023 toward the integration of the Swedish Match business into PMI's existing regional structure, PMI updated in January 2024 its segment reporting by including the former Swedish Match segment results into the four existing geographical segments. Our four geographical segments are as follows:
•Europe Region;
•South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA");
•East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF"); and
•Americas Region.
Our Wellness and Healthcare segment ("W&H") remained unchanged in 2024.
Following the sale of Vectura Group Ltd. on December 31, 2024, we will update our segment reporting by including the remaining Wellness & Healthcare results in the Europe segment. In addition, we will be renaming our “PMI Duty Free” business to “PMI Global Travel Retail” effective in the first quarter of 2025. As a result of this change, PMI's segment that includes our duty free business will be renamed East Asia, Australia & PMI Global Travel Retail (“EA, AU & PMI GTR”). As of the first quarter of 2025, our reporting will reflect these changes.
Our cigarettes are sold in approximately 170 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands.
Smoke-Free Business ("SFB”) is the term PMI uses to refer to all of its smoke-free products. SFB also includes wellness and healthcare products, as well as consumer accessories, such as lighters and matches.
Smoke-free products (also referred to herein as "SFPs") is the term PMI uses to refer to all of its products that provide nicotine without combusting tobacco, such as heat-not-burn, e-vapor, and oral smokeless, and that therefore generate far lower levels of harmful chemicals. As such, these products have the potential to present less risk of harm versus continued smoking.
IQOS and ZYN are the leading brands in our SFPs portfolio. As of December 31, 2024, our smoke-free products were available for sale in 95 markets.
Our Wellness and Healthcare business strategy focuses on developing and commercializing oral and inhaled consumer health and wellness offerings and inhaled prescription products for therapy areas that include pain management and cardiovascular emergencies. This includes medical and pharmaceutical cannabinoids, and non-recreational cannabinoid products (including CBD), in line with applicable regulatory requirements, though any revenue related to cannabinoids is expected to be negligible in the near to medium term.
In 2022, we acquired Swedish Match AB, a market leader in oral nicotine delivery with a significant presence in the United States market. The Swedish Match acquisition was a key milestone in PMI’s transformation to becoming a smoke-free company. The Swedish Match product portfolio is complementary to our portfolio, permitting us to bring together a leading oral nicotine product with the leading heat-not-burn product.
In 2022, we reached an agreement with Altria Group, Inc. to end our commercial relationship in the U.S. covering IQOS as of April 30, 2024. PMI now holds the full rights to commercialize IQOS in the U.S.
For further details of our 2022 acquisition of Swedish Match, as well as the agreement with Altria Group, Inc. discussed above, see Item 8, Note 3. Acquisitions and Divestitures and the "Business Environment" section of this Item 7.
We use the term net revenues to refer to our operating revenues from the sale of our products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. Our net revenues and operating income are affected by various factors, including the volume of products we sell, the price of our products, changes in currency exchange rates and the mix of products we sell. Mix is a term used to refer to the proportionate value of premium-price brands to mid-price or low-price brands in any given market (product mix). Mix can also refer to the proportion of shipment volume in more profitable markets versus shipment volume in less profitable markets (geographic mix).
Our cost of sales consists principally of: tobacco leaf, non-tobacco raw materials, labor and manufacturing costs; shipping and handling costs; and the cost of devices produced by third-party electronics manufacturing service providers. Estimated costs associated with device warranty programs are generally provided for in cost of sales in the period the related revenues are recognized.
Our marketing, administration and research costs include the costs of marketing and selling our products, other costs generally not related to the manufacture of our products (including general corporate expenses), and costs incurred to develop new products. The most significant components of our marketing, administration and research costs are marketing and sales expenses and general and administrative expenses.
Philip Morris International Inc. is a legal entity separate and distinct from its direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions that are otherwise compliant with law, including governmental capital and foreign currency exchange controls.
Executive Summary
The following executive summary provides the business update and significant highlights from the Discussion and Analysis that follows.
Impairment Related to the Rothmans, Benson & Hedges Equity Investment
On October 17, 2024, the court-appointed mediator and monitor in the CCAA proceedings filed a proposed plan of compromise and arrangement (“Proposed Plan”) setting forth, among other things, certain terms of a proposed comprehensive resolution of Canadian tobacco claims and related litigation. Under the resolution contemplated by the Proposed Plan, RBH, Imperial Tobacco Canada Limited ("ITL") and JTI Macdonald Corp ("JTIM") would pay an aggregate global settlement amount of CAD 32.5 billion (approximately $22.3 billion). A significant determinative factor in the analysis of impairment indicators was the issue of allocation of CAD 32.5 billion aggregate settlement amount among RBH, ITL, and JTIM which remained unresolved at the time of filing. On January 24, 2025, RBH filed an objection to approval of the Proposed Plan with the CCAA court (for further details, see Item 8, Note 18. Contingencies). Developments, including the positions taken by RBH in this objection and the positions taken by other parties in related filings narrowed the range of possible outcomes with respect to the allocation of the aggregate settlement amount of CAD 32.5 billion among RBH, ITL, and JTIM, which was determined to be an indicator that PMI’s investment in RBH may be impaired. Although there remains some uncertainty as to the final terms of the Proposed plan, PMI evaluated its investment in RBH for potential impairment and concluded that the estimated fair value of its investment in RBH was lower than its carrying value. As a result, PMI performed a quantitative valuation of its investment in RBH as of December 31, 2024, and recorded a non-cash impairment charge of $2,316 million (representing a diluted EPS charge of $1.49 per share) in the consolidated statement of earnings for the year ended December 31, 2024, as a recognized subsequent event. For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Consolidated Operating Results
•Net Revenues – Net revenues of $37.9 billion for the year ended December 31, 2024, increased by $2.7 billion, or 7.7%, from the comparable 2023 amount. The change in our net revenues from the comparable 2023 amount was driven by the following (variances not to scale):
Net revenues increased by 7.7%. Net revenues, excluding currency and acquisitions, increased by 10.1%, mainly reflecting: a favorable pricing variance, primarily driven by higher combustible tobacco pricing; and favorable volume/mix, driven by higher smoke-free products volume, partly offset by unfavorable cigarette mix, as well as a favorable comparison to 2023 reflecting a charge in the first quarter of 2023 of $80 million following the termination of a distribution arrangement in the Middle East, shown in "Other." The termination of a distribution arrangement in the Middle East is further described in the following "Diluted Earnings Per Share" discussion.
Net revenues by product category for the years ended December 31, 2024 and 2023, are shown below:
•Diluted Earnings Per Share – The changes in our reported diluted earnings per share (“diluted EPS”) for the year ended December 31, 2024, from the comparable 2023 amounts, were as follows:
| | | | | | | | |
| Diluted EPS | % Change |
| For the year ended December 31, 2023 | $ | 5.02 | | |
| | |
| 2023 Charges related to the war in Ukraine | 0.03 | | |
| 2023 Restructuring charges | 0.06 | | |
| 2023 South Korea indirect tax charge | 0.11 | | |
| 2023 Termination of agreement with Foundation for a Smoke-Free World | 0.07 | | |
| 2023 Fair value adjustment for equity security investments | (0.02) | | |
| 2023 Amortization of intangibles | 0.25 | | |
| 2023 Impairment of goodwill and other intangibles | 0.44 | | |
| 2023 Termination of distribution arrangement in the Middle East | 0.04 | | |
| 2023 Swedish Match AB acquisition accounting related items | 0.01 | | |
| 2023 Income tax impact associated with Swedish Match AB financing | (0.11) | | |
| 2023 Tax items | 0.11 | | |
| Subtotal of 2023 items | 0.99 | | |
| | |
| 2024 Restructuring charges | (0.10) | | |
| 2024 Impairment of other intangibles | (0.01) | | |
| 2024 Fair value adjustment for equity security investments | 0.27 | | |
| 2024 Impairment related to RBH equity investment | (1.49) | | |
| 2024 Amortization of intangibles | (0.40) | | |
| 2024 Loss on sale of Vectura Group | (0.13) | | |
| 2024 Egypt sales tax charge | (0.03) | | |
| 2024 Megapolis localization tax impact | (0.05) | | |
| 2024 Income tax impact associated with Swedish Match AB financing | (0.14) | | |
| 2024 Tax items | 0.03 | | |
| Subtotal of 2024 items | (2.05) | | |
| | |
| Currency | (0.38) | | |
| Interest | (0.03) | | |
| Change in tax rate | (0.07) | | |
| Operations | 1.04 | | |
| For the year ended December 31, 2024 | $ | 4.52 | | (10.0) | % |
Charges related to the war in Ukraine – During 2023, we recorded a pre-tax charge of $53 million (representing $43 million net of income tax and a diluted EPS charge of $0.03 per share), related to circumstances driven by the war, including the cost of PMI’s humanitarian efforts, severance payments, as well as an impairment of certain long-lived assets. For further details, see Item 8, Note 4. War in Ukraine.
Restructuring charges – During 2023, we recorded pre-tax restructuring charges of $109 million (representing $96 million net of income tax and a diluted EPS charge of $0.06 per share), related to a project to fully outsource and restructure the manufacturing of e-vapor devices and consumables. During 2024, we recorded pre-tax restructuring charges of $180 million (representing $150 million net of income tax and a diluted EPS charge of $0.10 per share), related to the restructuring of the sourcing of IQOS products to be commercialized in the U.S., and the cessation of our operations in Venezuela. For further details, see Item 8, Note 20. Restructuring Activities.
South Korea indirect tax charge – On July 13, 2023, our South Korean subsidiary, PM Korea, received an adverse ruling from the Supreme Court of South Korea related to cases alleging underpayment of excise taxes in connection with a 2015 excise tax increase and subsequent audit by the South Korean Board of Audit and Inspection. The Supreme Court ruling reversed previous decisions that were in PM Korea’s favor at the trial and appellate levels. As a result of the ruling, we concluded that an adverse outcome was probable. Consequently, we recorded a non-cash pre-tax charge of $204 million (representing $174 million net of income tax or $0.11 per share decrease in diluted EPS) in the second quarter results of 2023, reflecting the full amount previously paid by PM Korea.
Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the Agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million (representing $111 million net of income tax or $0.07 per share decrease in diluted EPS) commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings during the year ended December 31, 2023. For further details, see "Other Developments" within the Business Environment section of this Item 7.
Fair value adjustment for equity security investments – During 2023, we recorded a favorable fair value adjustment for our equity security investments in India and Sri Lanka of $38 million after tax (or $0.02 per share increase in diluted EPS). During 2024, we recorded a favorable fair value adjustment for our equity security investments in India and Sri Lanka of $418 million after tax (or $0.27 per share increase in diluted EPS). For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Amortization of intangibles – During 2023 and 2024, we recorded amortization of intangible expense of $497 million (representing $389 million net of income tax or $0.25 per share decrease in diluted EPS) and $835 million (representing $629 million net of income tax or $0.40 per share decrease in diluted EPS), respectively. The higher amortization expense in 2024 included the reacquired rights recorded as other intangible assets, net following the reacquisition of IQOS commercialization rights in the U.S. from Altria Group, Inc. For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Impairment of goodwill and other intangibles – During the second quarter of 2023, as a result of the completion of our annual review of goodwill and non-amortizable intangible assets for potential impairment, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value. Consequently, we recorded a total non-cash impairment charge of $680 million (representing a $0.44 per share decrease in diluted EPS) consisting of a goodwill impairment charge of $665 million and a non-amortizable intangible asset pre-tax impairment charge of $15 million for an in-process research and development project related to one of our 2021 acquisitions. The impairment charge was recorded in impairment of goodwill ($665 million) and marketing, administration and research costs ($15 million) in the consolidated statements of earnings during the year ended December 31, 2023 and was included in the Wellness and Healthcare segment results. During the first quarter of 2024, we recorded an impairment charge of $27 million (representing $20 million net of income tax or $0.01 per share decrease in diluted EPS), primarily reflecting the impairment of non-amortizable intangible assets related to an in-process research and development project in the Wellness and Healthcare segment. The impairment charge of $27 million was recorded in marketing, administration and research costs in the consolidated statements of earnings during the year ended December 31, 2024. For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Termination of distribution arrangement in the Middle East – Following the termination of a distribution arrangement in the Middle East, we recorded a pre-tax charge of $80 million in the first quarter of 2023 (representing $70 million net of income tax and a diluted EPS charge of $0.04 per share). The pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings for the year ended December 31, 2023 and was included in the SSEA, CIS & MEA segment results.
Swedish Match AB acquisition accounting related items – During the first quarter of 2023, we recorded pre-tax purchase accounting adjustments of $18 million related to the sale of acquired inventories stepped up to fair value (representing $13 million net of income tax and a diluted EPS charge of $0.01 per share). These pre-tax adjustments were recorded in cost of sales in the consolidated statements of earnings for the year ended December 31, 2023. For further details, see Item 8, Note 3. Acquisitions and Divestitures.
Loss on sale of Vectura Group – In September 2024, we announced the execution of a definitive agreement to sell Vectura to Molex Asia Holdings Ltd. On December 31, 2024, we completed the sale. The sale resulted in a pre-tax loss of $199 million (representing $206 million including the tax costs or a diluted EPS charge of $0.13 per share). This pre-tax loss was recorded in marketing, administration and research costs in PMI’s consolidated statements of earnings for the year ended December 31, 2024,
and was included in the Wellness and Healthcare segment results. For further details, see Item 8, Note 3. Acquisitions and Divestitures.
Egypt sales tax charge – In the third quarter of 2024, following a ruling issued by the Higher Administrative Court in Egypt and subsequent evaluation of available remedies, we concluded that an adverse outcome was probable and recorded a pre-tax charge of $45 million (representing $39 million net of income tax and a diluted EPS charge of $0.03 per share) in relation to tax assessments for general sales tax deducted on imported cutfiller for the years 2014 to 2016. This pre-tax charge was recorded in marketing, administration and research costs in the consolidated statement of earnings for the year ended December 31, 2024, and was included in the SSEA, CIS & MEA segment results.
Megapolis localization tax impact – PMI holds a 23% equity interest in Megapolis Distribution B.V. ("MDBV"), which was the holding company of JSC TK Megapolis (formerly CJSC TK Megapolis), pursuant to Dutch law, PMI's distributor in Russia. In June 2024, the Russian government included JSC TK Megapolis in the list of economically significant organizations that may be subject to forced localization under applicable Russian law, which refers to the mandatory removal of a foreign holding company from the shareholding structure. On August 8, 2024, the Arbitrazh Court of the Moscow Region granted the forced localization of MDBV as requested by the Ministry of Industry and Trade on July 18, 2024. As a result, MDBV’s shares in JSC TK Megapolis were transferred to JSC TK Megapolis and subsequently transferred to the Russian subsidiary of its indirect shareholders during the fourth quarter of 2024. As a result of the transfer of shares, PMI recorded a tax charge of $77 million (representing a diluted EPS charge of $0.05 per share). This charge primarily reflected additional deferred withholding taxes related to the JSC TK Megapolis pre-localization earnings and other adjustments of accumulated earnings of the Russian subsidiary. For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Impairment related to the RBH equity investment – On October 17, 2024, the court-appointed mediator and monitor in the CCAA proceedings filed a proposed plan of compromise and arrangement (“Proposed Plan”) setting forth, among other things, certain terms of a proposed comprehensive resolution of Canadian tobacco claims and related litigation. Under the resolution contemplated by the Proposed Plan, RBH, Imperial Tobacco Canada Limited ("ITL") and JTI Macdonald Corp ("JTIM") would pay an aggregate global settlement amount of CAD 32.5 billion (approximately $22.3 billion). A significant determinative factor in the analysis of impairment indicators was the issue of allocation of CAD 32.5 billion aggregate settlement amount among RBH, ITL, and JTIM which remained unresolved at the time of filing. On January 24, 2025, RBH filed an objection to approval of the Proposed Plan with the CCAA court (for further details, see Item 8, Note 18. Contingencies). Developments, including the positions taken by RBH in this objection and the positions taken by other parties in related filings narrowed the range of possible outcomes with respect to the allocation of the aggregate settlement amount of CAD 32.5 billion among RBH, ITL, and JTIM, which was determined to be an indicator that PMI’s investment in RBH may be impaired. Although there remains some uncertainty as to the final terms of the Proposed plan, PMI evaluated its investment in RBH for potential impairment and concluded that the estimated fair value of its investment in RBH was lower than its carrying value. As a result, PMI performed a quantitative valuation of its investment in RBH as of December 31, 2024, and recorded a non-cash impairment charge of $2,316 million (representing a diluted EPS charge of $1.49 per share) in the consolidated statement of earnings for the year ended December 31, 2024, as a recognized subsequent event. For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Income taxes – The Income tax impact associated with Swedish Match AB financing that increased our 2023 diluted EPS by $0.11 per share and decreased our 2024 diluted EPS by $0.14 per share in the table above was due to a deferred tax impact for unrealized foreign currency gains and losses on intercompany loans related to the Swedish Match acquisition financing reflected in the consolidated statements of earnings, while the underlying pre-tax foreign currency movements fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in the consolidated statements of stockholders' (deficit) equity.
The 2023 tax items that decreased our 2023 diluted EPS by $0.11 per share in the table above were due to an increase in deferred tax liabilities related to the unremitted earnings of PMI's Russian subsidiaries due to the unilateral suspension of certain Russian double tax treaties by the Russian authorities on August 8, 2023, with respect to certain payments including dividends. The 2024 tax items that increased our 2024 diluted EPS by $0.03 per share in the table above were due to a U.S. tax benefit for a worthless stock deduction under section 165(g) of the Internal Revenue Code related to PMI’s investment in C.A. Tabacalera Nacional, a wholly owned foreign corporation incorporated in Venezuela.
The change in the tax rate that decreased our diluted EPS by $0.07 per share in the table above was primarily due to increases in U.S. state tax expense, repatriation cost differences, and changes in earnings mix by taxing jurisdiction.
Currency – The unfavorable impact of $0.38 per share during the reporting period primarily results from the fluctuations of the U.S. dollar, especially against the Egyptian pound, Euro, Japanese yen and Russian ruble. This unfavorable currency movement has impacted our profitability across our primary revenue markets and local currency cost bases.
Interest – The unfavorable impact of $0.03 per share from interest in the table above was due primarily to higher average debt levels and higher average interest rates on debt, partially offset by higher interest income.
Operations – The increase in diluted EPS of $1.04 per share from our operations in the table above was due primarily to the following segments:
•SSEA, CIS & MEA: Favorable pricing and favorable volume/mix, partly offset by higher manufacturing costs and higher marketing, administration and research costs;
•Europe: Favorable pricing and favorable volume/mix, partly offset by higher marketing, administration and research costs;
•EA, AU & PMI DF: Favorable pricing, favorable volume/mix and lower manufacturing costs, partly offset by higher marketing, administration and research costs; and
•Americas: Favorable volume/mix and favorable pricing, partly offset by higher marketing, administration and research costs.
For further details, see the Consolidated Operating Results and Operating Results by Business Segment sections of the following Discussion and Analysis.
Discussion and Analysis
Critical Accounting Estimates
Item 8, Note 2. Summary of Significant Accounting Policies to our consolidated financial statements includes a summary of the significant accounting policies and methods used in the preparation of our consolidated financial statements. In most instances, we must use a particular accounting policy or method because it is the only one that is permitted under U.S. GAAP.
The preparation of financial statements requires that we use estimates and assumptions that affect the reported amounts of our assets, liabilities, net revenues and expenses, as well as our disclosure of contingencies. If actual amounts differ from previous estimates, we include the revisions in our consolidated results of operations in the period during which we know the actual amounts. Historically, aggregate differences, if any, between our estimates and actual amounts in any year have not had a significant impact on our consolidated financial statements.
The selection and disclosure of our critical accounting estimates have been discussed with our Audit & Risk Committee. The following is a discussion of the more significant assumptions, estimates, accounting policies and methods used in the preparation of our consolidated financial statements:
Acquisitions - PMI accounts for business combinations using the acquisition method of accounting. PMI allocates the purchase price of an acquired business to the assets acquired and liabilities assumed based upon their estimated fair values at the acquisition date with the excess recorded as Goodwill. The fair value of the applicable assets acquired and liabilities assumed is determined through established valuation techniques, such as the income, cost or market approach. PMI may utilize third-party valuation experts to assist in the fair value determination of certain assets acquired and liabilities assumed. The determination of fair value requires management to make judgements and may involve the use of significant estimates, including assumptions with respect to estimated projected revenue growth, future cash flows, terminal growth rates, useful economic lives of intangible assets acquired, discount rates, royalty rates and other factors. Certain acquired intangibles are expected to have indefinite lives based on their history and PMI’s intent to continue to support and build the intangible.
Although PMI believes its estimates of fair value are reasonable, actual financial results could differ from those estimates. Changes in assumptions related to future financial results or other underlying assumptions could have a significant impact on the determination of the fair value of the intangible assets acquired.
See Item 8, Note 3. Acquisitions and Divestitures to our consolidated financial statements for details of the critical accounting estimates relevant to the business combinations in the periods presented in this Form 10-K.
Revenue Recognition - We recognize revenue as performance obligations are satisfied. Our primary performance obligation is the distribution and sales of cigarettes and smoke-free products, including heat-not-burn, e-vapor and oral nicotine products. Our performance obligations are typically satisfied upon shipment or delivery to our customers. PMI estimates the cost of sales returns based on historical experience, and these estimates are immaterial. Estimated costs associated with warranty programs for IQOS devices are generally provided for in cost of sales in the period the related revenues are recognized, based on a number of factors, including historical experience, product failure rates and warranty policies. The transaction price is typically based on the amount billed to the customer and includes estimated variable consideration where applicable. Such variable consideration is typically not constrained and is estimated based on the most likely amount that PMI expects to be entitled to under the terms of the contracts with customers, historical experience of discount or rebate redemption, where relevant, and the terms of any underlying discount or rebate programs, which may change from time to time as the business and product categories evolve.
Goodwill and Non-Amortizable Intangible Assets Valuation - We test goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review, using either a qualitative or quantitative assessment for each of our reporting units. Where a qualitative assessment is followed, we determine whether it is more likely than not that an impairment exists based on qualitative factors such as macroeconomic conditions, industry and competitive conditions, legal and regulatory environment, historical financial performance and significant changes within the reporting unit. If the qualitative assessment indicates that it is more likely than not that an impairment exists, then a quantitative assessment is performed. The quantitative impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired. To determine the fair value of a reporting unit, we use the market approach using earnings multiples of comparable global companies within the tobacco industry and a discounted cash flow model. To determine the fair value of non-amortizable intangible assets, we primarily use a discounted cash flow model applying the relief-from-royalty method. These discounted cash flow models include management assumptions relevant for forecasting operating cash flows, which are subject to changes in business conditions, such as volumes and prices, costs to produce, discount rates and estimated capital needs. Management considers historical experience and all available information at the time the fair values are estimated, and we believe these assumptions are consistent with the assumptions a hypothetical marketplace participant would use.
During the second quarter of 2023, PMI completed its annual review of goodwill and non-amortizable intangible assets for potential impairment. We performed a quantitative impairment assessment for all of our reporting units and non-amortizable intangible assets with the exception of the three reporting units and non-amortizable intangible asset related to the Swedish Match segment for which we have performed a qualitative assessment. Based on this review, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value. Consequently, PMI recorded a goodwill impairment charge of $665 million. Additionally, as a result of the impairment test of non-amortizable intangible assets, PMI recorded a pre-tax impairment charge of $15 million. There was no impairment charge of goodwill and non-amortizable intangible assets for our other reporting units. For additional information, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
During the second quarter of 2024, PMI completed its annual review of goodwill and non-amortizable intangible assets for potential impairment using a quantitative assessment for all of its reporting units and non-amortizable intangible assets. As a result of this review, no impairment charges were required.
At December 31, 2024, the carrying value of our goodwill was $16.6 billion, which is related to eleven geographical reporting units, each consisting of a group of markets with similar operating and economic characteristics, and the Wellness and Healthcare business. The estimated fair value of each of our twelve reporting units and non-amortizable intangible assets exceeded the carrying value as of December 31, 2024.
Investment in non-marketable equity securities – For further details, see Item 8, Note 6. Related Parties – Equity Investments and Other to our consolidated financial statements
Marketing Costs - We incur certain costs to support our products through programs that include advertising, marketing, consumer engagement and trade promotions. The costs of our advertising and marketing programs are expensed in accordance with U.S. GAAP. Recognition of the cost related to our consumer engagement and trade promotion programs contain uncertainties due to the judgment required in estimating the potential performance and compliance for each program. For volume-based incentives provided to customers, management continually assesses and estimates, by customer, the likelihood of the customer's achieving the specified targets, and records the reduction of revenue as the sales are made. For other trade promotions, management relies on estimated utilization rates that have been developed from historical experience. Changes in the assumptions used in estimating the cost of any individual marketing program would not result in a material change in our financial position, results of operations or operating cash flows.
Employee Benefit Plans - As discussed in Item 8, Note 14. Benefit Plans to our consolidated financial statements, we provide a range of benefits to our employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). We record annual amounts relating to these plans based on calculations specified by U.S. GAAP. These calculations include various actuarial assumptions, such as discount rates, assumed rates of return on plan assets, compensation increases, mortality, turnover rates and health care cost trend rates. We review actuarial assumptions on an annual basis and make modifications to the assumptions based on current rates and trends when it is deemed appropriate to do so. As permitted by U.S. GAAP, any effect of the modifications is generally amortized over future periods. We believe that the assumptions utilized in calculating our obligations under these plans are reasonable based upon our historical experience and advice from our actuaries.
Weighted-average discount rate assumptions for pension and postretirement plan obligations at December 31, 2024 and 2023 are as follows:
| | | | | | | | |
| 2024 | 2023 |
| Pension plans | 2.07% | 2.28% |
| Postretirement plans | 5.35% | 5.19% |
We anticipate that assumption changes will increase 2025 pre-tax pension and postretirement expense to approximately $166 million as compared with approximately $158 million in 2024, excluding amounts related to employee severance and early retirement programs. The anticipated increase is primarily due to higher amortization of unrecognized actuarial losses of $45 million, coupled with higher service cost of $17 million, partially offset by higher expected return on assets of $20 million, coupled with lower interest cost of $30 million and other movements of $4 million.
Weighted-average expected rate of return and discount rate assumptions have a significant effect on the amount of expense reported for the employee benefit plans. A fifty-basis-point decrease in our discount rate would increase our 2025 pension and postretirement expense by approximately $56 million, and a fifty-basis-point increase in our discount rate would decrease our 2025 pension and postretirement expense by approximately $46 million. Similarly, a fifty-basis-point decrease (increase) in the expected return on plan assets would increase (decrease) our 2025 pension expense by approximately $44 million.
Income Taxes - Income tax provisions for jurisdictions outside the United States, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in our consolidated balance sheets.
The extent of our operations involves dealing with uncertainties and judgments in the application of complex tax regulations in a multitude of jurisdictions. The final taxes paid are dependent upon many factors, including negotiations with taxing authorities in various jurisdictions and resolution of disputes arising from federal, state, and international tax audits. In accordance with the authoritative guidance for income taxes, we evaluate potential tax exposures and record tax liabilities for anticipated tax audit issues based on our estimate of whether, and the extent to which, additional taxes will be due. We adjust these reserves in light of changing facts and circumstances; however, due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the tax liabilities. If our estimate of tax liabilities proves to be less than the ultimate assessment, an additional charge to expense would generally result. If payment of these amounts ultimately proves to be less than the recorded amounts, the reversal of the liabilities would result in tax benefits being recognized in the period when we determine the liabilities are no longer necessary.
We are required to assess the likelihood of recovering deferred tax assets against future sources of taxable income. If we determine, using all available evidence, that we do not reach the more likely than not threshold for recovery, a valuation allowance is recorded. Significant judgment is required in determining the need for and amount of valuation allowances for deferred tax assets including estimates of future taxable income in the applicable jurisdictions and the feasibility of on-going tax planning strategies, as applicable.
The effective tax rates used for interim reporting are based on our full-year geographic earnings mix projections. Changes in currency exchange rates, earnings mix by taxing jurisdiction or future regulatory developments may have an impact on the effective tax rates. Significant judgment is required in determining income tax provisions and in evaluating tax positions.
For further details, see Item 8, Note 12. Income Taxes to our consolidated financial statements.
Hedging - As discussed below in “Market Risk,” we use derivative financial instruments principally to reduce exposures to market risks resulting from fluctuations in foreign currency exchange and interest rates by creating offsetting exposures. For derivative contracts that are designated and qualify as fair value hedges the gain or loss on the derivative, as well as the offsetting gain or loss on the hedged items attributable to the hedged risk, is recognized in the consolidated statement of earnings. For our other derivatives to which we have elected to apply hedge accounting, gains and losses on these derivatives are initially deferred in accumulated other
comprehensive losses on the consolidated balance sheet and recognized in the consolidated statement of earnings into the same line item as the impact of the underlying transaction and in the periods when the related hedged transactions are also recognized in operating results. Gain (losses) related to derivatives contracts for which hedge accounting provisions have not been elected are recognized in the consolidated statement of earnings.
Contingencies - As discussed in Item 8, Note 18. Contingencies, to our consolidated financial statements, legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. At the present time, except as stated otherwise in Item 8, Note 18. Contingencies, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it: (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
Consolidated Operating Results
Net revenues, significant expenses, and operating income (loss) by segment were as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | Europe | SSEA, CIS & MEA | EA, AU & PMI DF | Americas | Wellness & Healthcare | Total |
For the Year Ended December 31, 2024 | | | | | | |
| Net revenues | $ | 15,357 | | $ | 11,261 | | $ | 6,393 | | $ | 4,534 | | $ | 333 | | $ | 37,878 | |
| Less: | | | | | | |
| Cost of sales | 4,206 | | 5,313 | | 2,011 | | 1,531 | | 268 | | 13,329 | |
| Marketing, administration and research costs | 4,213 | | 2,519 | | 1,504 | | 2,455 | | 456 | | 11,147 | |
| Operating income (loss) | $ | 6,938 | | $ | 3,429 | | $ | 2,878 | | $ | 548 | | $ | (391) | | $ | 13,402 | |
For the Year Ended December 31, 2023 | | | | | | |
| Net revenues | $ | 14,231 | | $ | 10,629 | | $ | 6,201 | | $ | 3,807 | | $ | 306 | | $ | 35,174 | |
| Less: | | | | | | |
| Cost of sales | 4,045 | | 5,109 | | 1,978 | | 1,485 | | 276 | | 12,893 | |
| Marketing, administration and research costs | 4,017 | | 2,384 | | 1,684 | | 1,740 | | 235 | | 10,060 | |
| Impairment of goodwill | — | | — | | — | | — | | 665 | | 665 | |
| Operating income (loss) | $ | 6,169 | | $ | 3,136 | | $ | 2,539 | | $ | 582 | | $ | (870) | | $ | 11,556 | |
For the Year Ended December 31, 2022 | | | | | | |
| Net revenues | $ | 12,972 | | $ | 10,467 | | $ | 5,936 | | $ | 2,116 | | $ | 271 | | $ | 31,762 | |
| Less: | | | | | | |
| Cost of sales | 3,656 | | 4,343 | | 2,026 | | 1,036 | | 341 | | 11,402 | |
| Marketing, administration and research costs | 3,540 | | 2,260 | | 1,486 | | 640 | | 188 | | 8,114 | |
| Operating income (loss) | $ | 5,776 | | $ | 3,864 | | $ | 2,424 | | $ | 440 | | $ | (258) | | $ | 12,246 | |
Items affecting the comparability of results from operations were as follows:
•Egypt sales tax charge – In the third quarter of 2024, following a ruling issued by the Higher Administrative Court in Egypt and subsequent evaluation of available remedies, PMI concluded that an adverse outcome was probable and recorded a pre-tax charge of $45 million in relation to tax assessments for general sales tax deducted on imported cutfiller for the years 2014 to 2016. This pre-tax charge was recorded in marketing, administration and research costs in the consolidated statement of earnings for the year ended December 31, 2024, and was included in the SSEA, CIS & MEA segment results.
•Loss on sale of Vectura Group – In September 2024, PMI announced the execution of a definitive agreement to sell Vectura to Molex Asia Holdings Ltd. On December 31, 2024, we completed the sale. The sale resulted in a pre-tax loss of $199 million. This pre-tax loss was recorded in marketing, administration and research costs in the consolidated statement of earnings for the year ended December 31, 2024, and was included in the Wellness and Healthcare segment results. For further details, see Item 8, Note 3. Acquisitions and Divestitures.
•Restructuring charges – See Item 8, Note 20. Restructuring Activities for details of the $180 million and $109 million pre-tax charges for the year ended December 31, 2024 and 2023, respectively, as well as a breakdown of these costs by segment.
•Termination of distribution arrangement in the Middle East – In the first quarter of 2023, PMI recorded a pre-tax charge of $80 million following the termination of a distribution arrangement in the Middle East. This pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings, and was included in the SSEA, CIS & MEA segment results for the year ended December 31, 2023.
•Impairment of goodwill and other intangibles – For the year ended December 31, 2023, PMI recorded $680 million of goodwill and non-amortizable intangible assets impairment charges that was included in the Wellness and Healthcare segment. For the year ended December 31, 2022, PMI recorded an impairment charge related to definite-lived intangible assets of $112 million. This charge was included in the Wellness and Healthcare segment. For further details, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
•South Korea indirect tax charge – On July 13, 2023, PMI's South Korean subsidiary, PM Korea, received an adverse ruling from the Supreme Court of South Korea related to cases alleging underpayment of excise taxes in connection with a 2015 excise tax increase and subsequent audit by the South Korean Board of Audit and Inspection. The Supreme Court ruling reversed previous decisions that were in PM Korea’s favor at the trial and appellate levels. As a result of the ruling, we concluded that an adverse outcome was probable. Consequently, we recorded a non-cash pre-tax charge of $204 million in marketing, administration and research costs in the consolidated statements of earning, reflecting the full amount previously paid by PM Korea, which was included in the EA, AU & PMI DF segment for the year ended December 31, 2023.
•Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023 and was included in the operating results of the following segments: Europe ($60 million); SSEA, CIS & MEA ($41 million); EA, AU & PMI DF ($24 million); and Americas ($15 million).
•Charges related to the war in Ukraine – See Item 8, Note 4. War in Ukraine for details of the $53 million and $151 million pre-tax charges in the Europe segment for the years ended December 31, 2023 and 2022, respectively.
•Swedish Match AB acquisition accounting related items – See Item 8, Note 3. Acquisitions and Divestitures for details of the $18 million and $125 million pre-tax purchase accounting adjustments related to the sale of acquired inventories stepped up to fair value. These pre-tax purchase accounting adjustments were included in the Americas segment ($18 million in 2023 and $77 million in 2022) and the Europe segment ($48 million in 2022).
Our net revenues by product category were as follows:
| | | | | | | | | | | |
| PMI Net Revenues by Product Category |
| (in millions) | 2024 | 2023 | 2022 |
| Combustible tobacco products | | | |
| Europe | $ | 8,599 | | $ | 8,037 | | $ | 7,694 | |
| SSEA, CIS & MEA | 9,848 | | 9,321 | | 9,173 | |
| EA, AU & PMI DF | 2,516 | | 2,676 | | 2,831 | |
| Americas | 2,255 | | 2,299 | | 1,874 | |
| Total combustible tobacco products | 23,218 | | 22,334 | | 21,572 | |
| Smoke-free products | | | |
| Smoke-free excluding Wellness and Healthcare: | | | |
| Europe | 6,758 | | 6,194 | | 5,278 | |
| SSEA, CIS & MEA | 1,413 | | 1,308 | | 1,294 | |
| EA, AU & PMI DF | 3,877 | | 3,525 | | 3,105 | |
| Americas | 2,279 | | 1,508 | | 242 | |
| Total Smoke-free excluding Wellness and Healthcare | 14,327 | | 12,534 | | 9,919 | |
| Wellness and Healthcare | 333 | | 306 | | 271 | |
| Total Smoke-free | 14,660 | | 12,840 | | 10,190 | |
| | | |
| Total PMI net revenues | $ | 37,878 | | $ | 35,174 | | $ | 31,762 | |
Note: Sum of product categories or Regions might not foot to total PMI due to rounding.
Net revenues related to combustible tobacco refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of our cigarettes and other tobacco products that are combusted. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos and do not include smoke-free products.
Net revenues related to smoke-free, excluding wellness and healthcare, refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes, if applicable. These net revenue amounts consist of the sale our products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral products, as well as consumer accessories.
Net revenues related to wellness and healthcare consist of operating revenues generated from the sale of products primarily associated with inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of our Wellness and Healthcare business.
PMI's heat-not-burn products include licensed KT&G heat-not-burn products.
References to "Cost/Other" in the Consolidated Financial Summary table of total PMI and the five segments throughout this "Discussion and Analysis" reflects the currency and acquisition-neutral variances of: cost of sales (excluding the volume/mix cost component); marketing, administration and research costs (including restructuring charges); and amortization and impairment of intangibles. “Cost/Other” also includes the currency and acquisition-neutral net revenue variance, unrelated to volume/mix and price components, attributable to: fees for certain distribution rights billed to customers in certain markets in the SSEA, CIS & MEA Region, the revenue adjustment for the termination of a distribution arrangement in the Middle East.
Our consolidated shipment volume is shown in the table below:
| | | | | | | | | | | |
| Consolidated Shipment Volume |
| Cigarettes and Heated Tobacco Units (million units) | 2024 | 2023 | 2022 |
| Cigarettes | 616,827 | | 612,949 | | 621,908 | |
| Heated Tobacco Units | 139,743 | | 125,263 | | 109,169 | |
| Total Cigarettes and Heated Tobacco Units | 756,570 | | 738,212 | | 731,077 | |
| | | |
Oral SFP Volume (million cans) (1) | | | |
| Nicotine Pouches | 644.0 | | 421.1 | | 42.5 | |
| Snus | 239.6 | | 240.4 | | 54.8 | |
| Moist Snuff | 134.6 | | 133.7 | | 16.0 | |
Other Oral SFP (2) | 3.4 | | 4.2 | | — | |
| Total Oral Products | 1,021.6 | | 799.3 | | 113.2 | |
(1) Excluding snuff, snuff leaf and U.S. chew | | | |
(2) Includes chew bags and tobacco bits | | | |
Note: Sum may not foot due to rounding
Following the deconsolidation of our Canadian subsidiary, we continue to report the volume and corresponding royalty revenues of brands sold by RBH for which other PMI subsidiaries are the trademark owners. These include HEETS, Next, Philip Morris and Rooftop. The volume and corresponding royalty revenues of these brands sold by RBH were not material to PMI for all periods presented.
Heated tobacco units ("HTUs") is the term we use to refer to heated tobacco consumables, which include our BLENDS, DELIA, HEETS, HEETS Creations (defined collectively as HEETS), SENTIA, TEREA, TEREA CRAFTED and TEREA Dimensions, as well as the KT&G-licensed brands, Fiit and Miix (outside of South Korea). HTUs also include zero tobacco heat-not-burn consumables (LEVIA).
Oral smoke-free product volume excludes snuff, snuff leaf and U.S. chew and is measured in cans or, for the purposes of total shipment volumes, in pouches or pouch equivalents.
Oral smoke-free products conversion: (i) nicotine pouches: 15 pouches per can in the U.S. and approximately 20 pouches per can outside the U.S.; (ii) snus products: weighted average 21 pouches equivalent per can; (iii) moist snuff products: weighted average 17 pouches equivalent per can; (iv) tobacco bits products: weighted average 30 pouches equivalent per can; (v) chew bags products: weighted average 20 pouches per can.
Unless otherwise stated, market share for HTUs is defined as the in-market sales volume for HTUs as a percentage of the total estimated industry sales volume for cigarettes and HTUs.
References to total industry (or total market), our shipment volume and our market share performance reflect cigarettes and heated tobacco units, unless otherwise stated.
Total industry volume, PMI in-market sales volume and PMI market share for the following geographies include the cigarillo category in Japan: the total international market, EA, AU & PMI DF Region, and Japanese domestic market.
In-market sales ("IMS") is defined as sales to the trade channels, which serve the end legal age nicotine users. Depending on the market and distribution model, IMS may represent an estimate. Consequently, past reported periods may be updated to ensure comparability and to incorporate the most current information.
Adjusted market share for HTUs is defined as the total in-market sales volume for PMI HTUs as a percentage of the total estimated sales volume for cigarettes and HTUs, excluding the impact of estimated distributor and wholesaler inventory movements.
References to total international market, defined as worldwide cigarette and heated tobacco unit volume excluding the United States, total industry (or total market) and market shares throughout this "Discussion and Analysis" are our estimates for tax-paid products based on data from a number of internal and external sources and may, in defined instances, exclude China. Past reported periods may be updated to ensure comparability and to incorporate the most current information for industry and market share reporting.
From time to time, PMI’s shipment volumes and IMS are subject to the impact of distributor inventory movements (or wholesaler inventory movements in certain markets where PMI does not sell to distributors), and estimated total industry/market volumes are subject to the impact of inventory movements in various trade channels that include estimated trade inventory movements of PMI’s competitors arising from market-specific factors that significantly distort reported volume disclosures. Such factors may include changes to the manufacturing supply chain, shipment methods, consumer demand, timing of excise tax increases or other influences that may affect the timing of sales to customers. In such instances, in addition to reviewing PMI shipment volumes, IMS, certain estimated total industry/market volumes and estimated market shares on a reported basis, management reviews these measures on an adjusted basis that excludes the impact of distributor and/or estimated trade inventory movements. Management also believes that disclosing PMI's shipment volumes, IMS, estimated total industry/market volumes and estimated market shares in such circumstances on a basis that excludes the impact of distributor and/or estimated trade inventory movements improves the comparability of performance and trends for these measures over different reporting periods.
2024 compared with 2023
The following discussion compares our consolidated operating results for the year ended December 31, 2024, with the year ended December 31, 2023.
Total Market
Estimated international industry volume (excluding China and the U.S.) for cigarettes and HTUs increased by 1.5% to 2.6 trillion units.
For the full year 2025, we currently expect an estimated total international industry volume decline of around 1% for cigarettes and HTUs, excluding China and the U.S.
Shipment Volume
Our total cigarette and HTU shipment volume increased by 2.5% (HTU shipments increased by 11.6%, while cigarette shipments increased by 0.6%) with increases across all regions except the Americas.
Our total oral product shipment volume in cans increased by 27.8%, primarily reflecting growth in nicotine pouches.
For the full year 2025, we currently expect total cigarette and smoke-free product shipment volume growth for PMI of up to 2% driven by smoke-free products volume growth of 12% to 14%.
For the full year 2025, we also expect nicotine pouch shipment volume in the U.S. of 780 to 820 million cans.
International Share of Market - Cigarette and HTUs (Excluding China and the United States)
| | | | | | | | | | | |
| Full-Year |
| 2024 | 2023 | Change (pp) |
Total International Market Share (1) | 28.7 | % | 28.3 | % | 0.4 | |
| Cigarettes | 23.5 | % | 23.7 | % | (0.2) | |
| HTU | 5.2 | % | 4.7 | % | 0.5 | |
| | | |
Cigarette over Cigarette Market Share (2) | 25.3 | % | 25.2 | % | 0.1 | |
| | | |
| (1) Defined as PMI's cigarette and heated tobacco unit in-market sales volume as a percentage of total industry cigarette and heated tobacco unit sales volume, excluding China and the U.S., including cigarillos in Japan |
| (2) Defined as PMI's cigarette in-market sales volume as a percentage of total industry cigarette sales volume, excluding China and the U.S., including cigarillos in Japan |
| Note: Sum of share of market by product categories might not foot to total due to rounding |
Key Market Data
Key market data regarding total market size, our shipments and market share of cigarettes and heated tobacco units were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | PMI Shipments (billion units) | | PMI Market Share (%)(2) |
| Market | | Total Market
(billion units) | | Total | | Cigarette | | Heated Tobacco Unit | | Total | | Heated Tobacco Unit |
| | 2024 | 2023 | | 2024 | 2023 | | 2024 | 2023 | | 2024 | 2023 | | 2024 | 2023 | | 2024 | 2023 |
Total (1) (2) | | 2,617.9 | 2,579.4 | | 756.6 | 738.2 | | 616.8 | 612.9 | | 139.7 | 125.3 | | 28.7 | 28.3 | | 5.2 | 4.7 |
| Europe | | | | | | | | | | | | | | | | |
| France | | 26.1 | 29.8 | | 10.7 | 13.0 | | 10.5 | 12.8 | | 0.2 | 0.2 | | 41.2 | 42.5 | | 0.6 | 0.7 |
Germany (3) | | 69.2 | 69.0 | | 26.7 | 26.5 | | 22.4 | 23.3 | | 4.3 | 3.1 | | 38.7 | 39.0 | | 6.2 | 5.3 |
Italy (3) | | 73.6 | 73.3 | | 39.1 | 39.7 | | 27.3 | 27.3 | | 11.8 | 12.4 | | 53.6 | 53.6 | | 16.9 | 16.8 |
Poland (3) | | 58.0 | 56.7 | | 25.6 | 23.7 | | 20.1 | 18.7 | | 5.4 | 5.0 | | 43.9 | 41.9 | | 9.1 | 9.0 |
| Spain | | 44.3 | 43.6 | | 12.7 | 12.9 | | 11.4 | 11.8 | | 1.3 | 1.1 | | 29.4 | 29.3 | | 2.9 | 2.3 |
| SSEA, CIS & MEA | | | | | | | | | | | | | | | | |
| Egypt | | 82.5 | 74.2 | | 23.9 | 24.3 | | 22.3 | 23.0 | | 1.6 | 1.3 | | 28.9 | 32.8 | | 1.8 | 1.7 |
Indonesia (4) | | 295.5 | 292.2 | | 80.8 | 84.0 | | 79.6 | 83.4 | | 1.2 | 0.6 | | 27.4 | 28.7 | | 0.4 | 0.2 |
| Philippines | | 41.0 | 42.9 | | 21.1 | 23.8 | | 20.8 | 23.5 | | 0.3 | 0.2 | | 51.3 | 55.4 | | 0.7 | 0.5 |
| Russia | | 216.5 | 203.4 | | 69.9 | 64.8 | | 51.4 | 47.9 | | 18.5 | 16.9 | | 32.3 | 31.8 | | 8.6 | 8.0 |
| Turkey | | 150.5 | 137.4 | | 78.2 | 69.0 | | 78.2 | 69.0 | | — | — | | 52.0 | 50.2 | | — | — |
| EA, AU & PMI DF | | | | | | | | | | | | | | | | |
| Australia | | 5.1 | 7.2 | | 1.8 | 2.5 | | 1.8 | 2.5 | | — | — | | 34.8 | 34.8 | | — | — |
Japan (2) | | 151.1 | 149.0 | | 64.8 | 60.9 | | 16.5 | 17.9 | | 48.3 | 43.0 | | 41.3 | 39.6 | | 29.8 | 26.7 |
| South Korea | | 70.5 | 72.0 | | 14.0 | 14.0 | | 8.3 | 8.9 | | 5.7 | 5.1 | | 19.9 | 19.5 | | 8.1 | 7.1 |
| Americas | | | | | | | | | | | | | | | |
| Argentina | | 26.4 | 28.8 | | 16.4 | 17.8 | | 16.4 | 17.8 | | — | — | | 62.1 | 61.9 | | — | — |
| Mexico | | 29.5 | 30.0 | | 18.5 | 18.9 | | 18.3 | 18.8 | | 0.2 | 0.1 | | 62.6 | 63.0 | | 0.7 | 0.5 |
|
| (1) Market share estimates are calculated using IMS data, unless otherwise stated |
| (2) Total market and market share estimates include cigarillos in Japan |
| (3) PMI market share reflects estimated adjusted IMS volume share; Total Market is based on reported IMS |
| (4) 2024 includes 0.6 billion units of cigarettes shipment volume under an arrangement where PMI acts as brand management and fulfillment services agent |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary |
Financial Summary -
Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/Other |
| (in millions) | | | |
Net Revenues (1) | | $ | 37,878 | | $ | 35,174 | | | 7.7 | % | 10.1 | % | | $ | 2,704 | | $ | (841) | | $ | — | | $ | 2,203 | | $ | 1,268 | | $ | 74 | |
Cost of Sales (2) | | (13,329) | | (12,893) | | | (3.4) | % | (4.5) | % | | (436) | | 101 | | 47 | | — | | (504) | | (80) | |
Marketing, Administration and Research Costs (3) | | (11,147) | | (10,060) | | | (10.8) | % | (11.4) | % | | (1,087) | | 58 | | (1) | | — | | — | | (1,144) | |
Impairment of Goodwill (4) | | — | | (665) | | | +100% | +100% | | 665 | | — | | — | | — | | — | | 665 | |
| Operating Income | | $ | 13,402 | | $ | 11,556 | | | 16.0 | % | 21.5 | % | | $ | 1,846 | | $ | (682) | | $ | 46 | | $ | 2,203 | | $ | 764 | | $ | (485) | |
(1) Cost/Other variance includes charges in 2023 of $80 million following the termination of a distribution arrangement in the Middle East.
(2) Cost/Other variance includes charges in 2024 of $51 million related to amortization of intangibles and in 2023 of $15 million related to the war in Ukraine, $58 million related to amortization of intangibles and $18 million related to the Swedish Match AB acquisition accounting related items.
(3) Cost/Other variance includes charges in 2024 of $180 million related to restructuring charges, $27 million related to impairment of other intangibles, $784 million related to amortization of intangibles, $199 million related to the loss on sale of Vectura Group, and $45 million related to the Egypt sales tax charge, partly offset by charges in 2023 of $109 million related to restructuring charges, $204 million related to the South Korea indirect tax charge, $140 million related to the termination of a pledge agreement with the Foundation for a Smoke-Free World, $439 million related to amortization of intangibles, $38 million related to the war in Ukraine and $15 million related to the impairment of other intangibles. For more details, see Item 8, Note 3. Acquisitions and Divestitures, Note 4. War in Ukraine, Note 5. Goodwill and Other Intangible Assets, net., Note 13. Segment Reporting and Note 20. Restructuring Activities.
(4) For details on the impairment of goodwill recorded in the second quarter of 2023, see Item 8, Note 5. Goodwill and Other Intangible Assets, net.
Net revenues increased by 7.7%. Net revenues, excluding currency and acquisitions, increased by 10.1%, mainly reflecting: a favorable pricing variance, primarily driven by higher combustible tobacco pricing; favorable volume/mix, driven by higher smoke-free products volume, partly offset by unfavorable cigarette mix; and a favorable comparison to 2023 reflecting a charge in the first quarter of 2023 of $80 million (recognized as a reduction of net revenues in 2023) following the termination of a distribution arrangement in the Middle East, shown in "Other."
The unfavorable currency impact in net revenues was due primarily to the Egyptian pound, Indonesian rupiah, Japanese yen and Russian ruble.
Net revenues include $14.7 billion in 2024 and $12.8 billion in 2023 related to smoke-free.
Operating income increased by 16.0%. Operating income, excluding currency and acquisitions, increased by 21.5%, mainly reflecting: the same factors as for net revenues, as well as a favorable comparison to 2023 reflecting the impairment of goodwill of $665 million recorded in the second quarter of 2023, the South Korea indirect tax charge of $204 million in 2023, the termination of a pledge agreement with the Foundation for a Smoke-Free World of $140 million and $53 million related to the war in Ukraine. The increase was partly offset by higher amortization of intangibles in 2024, higher restructuring charges in 2024, a charge of $199 million related to the loss on sale of Vectura Group and the Egypt sales tax charge of $45 million in 2024, as well as higher marketing, administration and research costs (primarily due to inflationary impacts, notably related to wages, and higher commercial investments), as well as higher manufacturing costs, notably related to tobacco leaf and the impact of the EU single-use plastics directive, partly offset by productivity.
Amortization expense on a pre-tax basis for each of the next five years is estimated to be approximately $1.0 billion or less, assuming no additional transactions occur that require the amortization of intangible assets. This amount includes the reacquired rights recorded as other intangible assets, net, in May 2024 following the reacquisition of IQOS commercialization rights in the U.S. from Altria Group, Inc. (See Item 8, Note 3. Acquisitions and Divestitures, Note 5. Goodwill and Other Intangible Assets, net. and the "Business Environment" section of this MD&A for details).
Interest expense, net, of $1.1 billion increased by $82 million (7.7%), primarily due to higher average debt levels and higher average interest rates on debt, partially offset by higher interest income.
Our effective tax rate increased by 2.3 percentage points to 24.7%. We estimate that our 2025 effective tax rate will be approximately 22.5% to 23.5%, excluding discrete tax events. For further details, see Item 8, Note 12. Income Taxes.
We recorded an impairment charge of $2,316 million related to our RBH equity investment in the consolidated statement of earnings for the year ended December 31, 2024. For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other and Item 8, Note 18. Contingencies.
Income from equity investments and securities, net, increased by $480 million or over 100%, primarily driven by a favorable fair value adjustment for our equity security investments in India and Sri Lanka. For further details, see Item 8, Note 6. Related Parties - Equity Investments and Other.
Net earnings attributable to PMI of $7.1 billion decreased by $756 million or 9.7%. This decrease was due primarily to the impairment related to the RBH equity investment and a higher effective tax rate, partly offset by higher operating income and higher income from equity investments and securities. Basic earnings per share of $4.53 decreased by 9.8%. Diluted EPS of $4.52 decreased by 10.0%. Excluding an unfavorable currency impact of $0.38, diluted EPS decreased by 2.4%.
2023 compared with 2022
For a discussion comparing our consolidated operating results for the year ended December 31, 2023, with the year ended December 31, 2022, refer to Part II, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation - Discussion and Analysis - Consolidated Operating Results in our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the U.S. Securities and Exchange Commission on February 8, 2024. This section is incorporated by reference into this Annual Report on Form 10-K for the year ended December 31, 2024.
Operating Results by Business Segment
2024 compared with 2023
The following discussion compares operating results within each of our segments for 2024 with 2023.
Europe:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 15,357 | | $ | 14,231 | | | 7.9 | % | 7.1 | % | | $ | 1,126 | | $ | 116 | | $ | — | | $ | 798 | | $ | 212 | | $ | — | |
| Operating Income | | $ | 6,938 | | $ | 6,169 | | | 12.5 | % | 12.4 | % | | $ | 769 | | $ | 6 | | $ | — | | $ | 798 | | $ | 90 | | $ | (125) | |
Net revenues increased by 7.9%. Net revenues, excluding currency and acquisitions, increased by 7.1%, reflecting a favorable pricing variance, mainly driven by higher combustible tobacco pricing; and favorable volume/mix, primarily driven by higher HTU volume, partly offset by lower cigarette volume and adverse cigarette mix.
The pricing variance for 2023 and 2024 was negatively impacted by the supplemental tax surcharge on heated tobacco products ("HTPs") in Germany, which went into effect in 2022. On March 14, 2024, the Court of Justice of the European Union (the "CJEU") ruled that the German fiscal regulation imposing an additional excise tax on HTPs does not contravene EU law. On May 15, 2024, following the decision issued by CJEU, the Fiscal Court in Dusseldorf (the "FCD") also ruled that the German fiscal regulation imposing an additional excise tax on HTPs does not contravene EU law. The FCD admitted an appeal to the Federal Tax Court. On June 19, 2024, PMI submitted an appeal. The negative impact will continue, at least until the appeal ruling on the legality of the surcharge is concluded. PMI currently accounts for the surcharge as a reduction in net revenues, with amounts withdrawn before May 15, 2024, currently under a payment suspension. Despite this payment suspension and, in order to avoid the future addition of interest, PMI elected, on January 14, 2025, to pay the amount outstanding of EUR 721 million (approximately $751 million), excluding accrued interest. An unfavorable outcome to the appeal would negatively impact PMI’s future cash provided by operating activities for the amounts of unpaid interest. A favorable outcome to the appeal would positively impact future PMI’s operating results and future cash provided by operating activities for the amounts paid in January 2025.
Operating income increased by 12.5%. Operating income, excluding currency and acquisitions, increased by 12.4%, primarily reflecting: the same factors as for net revenues, as well as a favorable comparison to 2023 reflecting the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World of $60 million, $53 million charge related to the war in Ukraine in 2023 and $47 million related to restructuring charges in 2023. The positive impact of this favorable comparison was partly offset by higher marketing, administration and research costs.
Europe - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region was broadly stable (541.9 billion units), reflecting a 1.4% decrease for cigarettes and continued HTU growth. Notable decreases in the estimated total market in France (down by 12.5%), and the United Kingdom (down by 12.6%) were largely offset by Ukraine (up by 4.3%), Bulgaria (up by 7.3%), and Poland (up by 2.3%).
| | | | | | | | | | | |
| | | |
| Europe Key Data | Full-Year |
| | | Change |
| 2024 | 2023 | % / pp |
| PMI Market Share | | | |
| Cigarettes | 30.0 | % | 30.3 | % | (0.3) | |
| Heated Tobacco Units | 10.0 | % | 9.1 | % | 0.9 | |
| Total Europe | 40.0 | % | 39.4 | % | 0.6 | |
Note: Sum may not foot due to rounding.
Our total cigarette and HTU shipment volume in the Region increased by 0.7% to 216.3 billion units. Total cigarette and HTU shipment volume increased notably in Poland (up by 7.9%) and Ukraine (up by 9.4%), and decreased notably in France (down by 17.3%), as well as the Netherlands (down by 20.9%).
Our estimated HTU adjusted in-market sales volume in the Region increased by 9.4%, reflecting continued growth momentum for IQOS, partly offset by the impact from the EU characterizing flavor ban. HTU shipments increased by 8.9%.
Our HTU share of the total cigarette and HTU market in the Region increased by 0.9 pp on an adjusted basis.
Other Oral SFP includes chew bags and tobacco bits
Note: Sum may not foot due to rounding.
Our oral SFP shipments increased by 4.9%, driven by growth of nicotine pouches (up by 40.2%).
SSEA, CIS & MEA:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 11,261 | | $ | 10,629 | | | 5.9 | % | 11.7 | % | | $ | 632 | | $ | (610) | | $ | — | | $ | 821 | | $ | 343 | | $ | 78 | |
| Operating Income | | $ | 3,429 | | $ | 3,136 | | | 9.3 | % | 26.8 | % | | $ | 293 | | $ | (592) | | $ | 46 | | $ | 821 | | $ | 145 | | $ | (127) | |
Net revenues increased by 5.9%. Net revenues, excluding currency and acquisitions, increased by 11.7%, primarily reflecting: a favorable pricing variance, predominantly driven by higher combustible tobacco pricing; favorable volume/mix, driven by higher cigarette and HTU volume, coupled with favorable mix; and a favorable comparison to 2023 due to the charge of $80 million following the termination of a distribution arrangement in the Middle East (recognized as a reduction of net revenues in 2023).
Operating income increased by 9.3%. Operating income, excluding currency and acquisitions, increased by 26.8%, primarily reflecting: a favorable pricing variance, predominantly driven by higher combustible tobacco pricing; and favorable volume/mix, driven by higher cigarette and HTU volume; a favorable comparison to 2023 reflecting a 2023 charge of $80 million following the termination of a distribution arrangement in the Middle East, a 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World of $41 million and $32 million related to restructuring charges in 2023. These increases were partly offset by higher manufacturing costs (primarily due to higher cost of tobacco leaf), higher marketing, administration and research costs, and the Egypt sales tax charge of $45 million in 2024.
SSEA, CIS & MEA - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region increased by 2.8% to 1,571.9 billion units. The increase in the estimated total market was mainly due to Turkey (up by 9.5%), and Egypt (up by 11.3%), partly offset by Bangladesh (down by 5.0%), and Thailand (down by 13.7%).
Our Regional market share increased by 0.4 points to 23.8%.
Our total cigarette and HTU shipment volume in the Region increased by 4.2% to 373.4 billion units, mainly driven by Turkey (up by 13.4%), partly offset by Indonesia (down by 3.7%). Our estimated HTU adjusted in-market sales volume increased by approximately 15.6%, with about 15% HTU shipment volume growth.
EA, AU & PMI DF:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 6,393 | | $ | 6,201 | | | 3.1 | % | 8.6 | % | | $ | 192 | | $ | (341) | | $ | — | | $ | 325 | | $ | 208 | | $ | — | |
| Operating Income | | $ | 2,878 | | $ | 2,539 | | | 13.4 | % | 25.1 | % | | $ | 339 | | $ | (298) | | $ | — | | $ | 325 | | $ | 59 | | $ | 253 | |
Net revenues increased by 3.1%. Net revenues, excluding currency and acquisitions, increased by 8.6%, reflecting: a favorable pricing variance and favorable volume/mix, mainly driven by higher HTU volume, partly offset by lower cigarette volume.
Operating income increased by 13.4%. Operating income, excluding currency and acquisitions, increased by 25.1%, mainly reflecting
a favorable pricing variance and favorable volume/mix, mainly driven by higher HTU volume, partly offset by lower cigarette volume, while lower manufacturing costs were largely offset by higher marketing, administration and research costs, as well as a favorable comparison to 2023 reflecting the South Korea indirect tax charge of $204 million in 2023.
EA, AU & PMI DF - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding China, was broadly stable (318.9 billion units), with a decrease in cigarettes, largely offset by HTU growth. The decrease in the estimated total market in Australia (down by 28.5%) and Korea (down by 2.2%) was offset by International Duty Free (up by 11.3%) and Japan (up by 1.4%).
Our Regional market share increased by 1.3 points to 31.3%.
Our total cigarette and HTU shipment volume in the Region increased by 3.3% to 104.6 billion units, driven by Japan (up by 6.4%), partly offset by Australia (down by 28.5%).
Our estimated HTU adjusted in-market sales volume in the Region increased by 14.5%. HTU shipments increased by 12.6%.
Americas:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 4,534 | | $ | 3,807 | | | 19.1 | % | 19.3 | % | | $ | 727 | | $ | (9) | | $ | — | | $ | 233 | | $ | 505 | | $ | (2) | |
| Operating Income | | $ | 548 | | $ | 582 | | | (5.8) | % | (41.1) | % | | $ | (34) | | $ | 205 | | $ | — | | $ | 233 | | $ | 470 | | $ | (942) | |
Net revenues increased by 19.1%. Net revenues, excluding currency and acquisitions, increased by 19.3%, primarily reflecting: favorable volume/mix, mainly due to growth of ZYN nicotine pouches in the U.S., partly offset by cigarette volume declines outside of the U.S.; and favorable cigarette and ZYN pricing.
Operating income decreased by 5.8%. Operating income, excluding currency and acquisitions, decreased by 41.1%, mainly reflecting: higher marketing, administration and research costs (including incremental investment in the U.S.), higher restructuring charges of $169 million and higher amortization of intangibles of $355 million. These decreases were partly offset by favorable volume/mix and a favorable price variance, mainly due to the same factors as for net revenues.
Americas - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding the U.S., decreased by 1.9% to 185.1 billion units, primarily reflecting a decline for cigarettes. The decrease in the estimated total market was mainly due to Argentina (down by 8.4%) and Canada (down by 12.3%), partly offset by Brazil (up by 6.8%).
Our Regional market share, excluding the U.S., was flat at 33.7%.
Our total cigarette and HTU shipment volume in the Region decreased by 2.6% to 62.3 billion units, mainly due to Argentina (down by 8.1%), partly offset by Brazil (up by 8.5%).
(1) Excluding U.S. chew
Note: Volumes of other oral SFP introduced in Q3 2024 are not material. Sum may not foot due to rounding.
Oral products shipments increased by 37.6%, predominantly driven by ZYN nicotine pouches in the U.S.
Cigar shipment volume decreased by 11%, while gross profit grew mid-single digits.
Wellness and Healthcare:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2024 | 2023 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 333 | | $ | 306 | | | 8.8 | % | 7.8 | % | | $ | 27 | | $ | 3 | | $ | — | | $ | 26 | | $ | — | | $ | (2) | |
| Operating Income / (Loss) | | $ | (391) | | $ | (870) | | | 55.1 | % | 55.4 | % | | $ | 479 | | $ | (3) | | $ | — | | $ | 26 | | $ | — | | $ | 456 | |
Net revenues increased by 8.8%. Net revenues, excluding currency and acquisitions, increased by 7.8%.
The operating loss of $391 million in 2024 was primarily due to a charge of $199 million related to the loss on sale of Vectura Group and amortization of acquired intangibles, as well as research and development, and administration costs. The operating loss of $870 million in 2023 was primarily due to an impairment charge for goodwill and other intangibles of $680 million in the second quarter, as well as commercial investments and higher administration costs and the amortization of acquired intangibles. For further details, see Item 8, Note 3. Acquisitions and Divestitures and Note 5. Goodwill and Other Intangible Assets, net.
2023 compared with 2022
As previously disclosed in the Description of Our Company section of this Item 7, in January 2024, we updated our segment reporting by including the former Swedish Match segment results into the four existing geographical segments. The following discussion compares operating results within each of our segments for 2023 with 2022 under this operating segment structure.
Europe:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2023 | 2022 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 14,231 | | $ | 12,972 | | | 9.7 | % | 3.6 | % | | $ | 1,259 | | $ | 249 | | $ | 546 | | $ | 535 | | $ | (71) | | $ | — | |
| Operating Income | | $ | 6,169 | | $ | 5,776 | | | 6.8 | % | 2.3 | % | | $ | 393 | | $ | 191 | | $ | 67 | | $ | 535 | | $ | (87) | | $ | (313) | |
Net revenues increased by 9.7%. Net revenues, excluding currency and acquisitions, increased by 3.6%, reflecting: a favorable pricing variance, mainly driven by higher combustible tobacco pricing; partially offset by unfavorable volume/mix, mainly due to lower cigarette volume, as well as unfavorable cigarette mix, partly offset by higher HTU volume.
Operating income increased by 6.8%. Operating income, excluding currency and acquisitions, increased by 2.3%, primarily reflecting: lower charges in 2023 related to the war in Ukraine ($98 million), a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer ($53 million) and a favorable pricing variance. The increase was partly offset by the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World ($60 million), the 2023 charges for restructuring charges ($47 million), higher marketing, administration and research costs (mainly due to inflationary impacts and lower commercial investments in the prior year period); higher manufacturing costs (primarily due to inflationary impacts); and unfavorable volume/mix, mainly due to the same factors as for net revenues.
Europe - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by 1.3% to 542.6 billion units, reflecting a 3.0% decline for cigarettes, partly offset by a 15.4% increase for HTUs. The decrease in the estimated total market was predominantly due to the
United Kingdom (down by 15.4%), France (down by 8.1%), Germany (down by 1.8%) and Spain (down by 2.4%), partly offset by Poland (up by 1.8%).
| | | | | | | | | | | | | | |
| Europe Key Data | | Full-Year |
| | | | Change |
| | 2023 | 2022 | % / pp |
| | | | |
| | | | |
| PMI Market Share | | | | |
| Cigarettes | | 30.3 | % | 31.1 | % | (0.8) | |
| Heated Tobacco Units | | 9.1 | % | 7.8 | % | 1.3 | |
| Total Europe | | 39.4 | % | 38.9 | % | 0.5 | |
Note: Sum may not foot due to rounding
Our total cigarette and HTU shipment volume in the Region decreased by 0.6% to 214.9 billion units, mainly due to Germany (down by 6.0%), Italy (down by 2.8%; or up by 0.4% excluding the net unfavorable impact of estimated distributor inventory movements) and France (down by 7.3%), partly offset by Poland (up by 9.4%).
Our estimated HTU adjusted in-market sales volume in the Region increased by 17.8%, including growth in Germany and Italy of 29.7% and 16.8%, respectively.
Our HTU share of the total cigarette and HTU market in the Region increased by 1.3 points, or by 1.5 points on an adjusted basis.
In 2023, our oral SFP shipments reached 275.4 million cans in the Region.
SSEA, CIS & MEA:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2023 | 2022 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 10,629 | | $ | 10,467 | | | 1.5 | % | 11.7 | % | | $ | 162 | | $ | (1,060) | | $ | — | | $ | 1,008 | | $ | 400 | | $ | (186) | |
| Operating Income | | $ | 3,136 | | $ | 3,864 | | | (18.8) | % | (2.0) | % | | $ | (728) | | $ | (649) | | $ | — | | $ | 1,008 | | $ | (237) | | $ | (850) | |
Net revenues increased by 1.5%. Net revenues, excluding currency and acquisitions, increased by 11.7%, primarily reflecting: a favorable pricing variance, mainly driven by higher combustible tobacco pricing, with HTU pricing also higher; and favorable volume/mix, primarily driven by favorable cigarette mix, as well as higher volume for HTUs, partly offset by an unfavorable cigarette volume impact. This increase was partially offset by the 2023 termination of a distribution arrangement in the Middle East of $80 million and lower fees for certain distribution rights billed to customers in certain markets, both included in "Cost/Other" in the table above.
Operating income decreased by 18.8%. Operating income, excluding currency and acquisitions, decreased by 2.0%, primarily reflecting: higher marketing, administration and research costs; higher manufacturing costs (primarily due to inflationary impacts); unfavorable volume/mix, mainly due to an unfavorable cigarette volume impact and unfavorable cigarette mix, partly offset by higher HTU volume; and the termination of a distribution arrangement, coupled with the impact of lower fees for certain distribution rights, as noted for net revenues; as well as the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World of $41 million and the 2023 charges for restructuring charges of $32 million. The decrease was partially offset by the favorable pricing variance and a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer of $33 million.
SSEA, CIS & MEA - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region decreased by approximately 2% to 1,528.6 billion units, due to a decline for cigarettes. The decrease in the estimated total market was predominantly due to Egypt (down by 20.8%) and Pakistan (down by 35.1%), partly offset by Turkey (up by 17.7%).
Our Regional market share increased by 0.8 points to 23.4%.
Our total cigarette and HTU shipment volume in the Region increased by 1.3% to 358.2 billion units, mainly driven by Turkey (up by 23.0%), partly offset by the Philippines (down by 26.2%). Our estimated HTU adjusted in-market sales volume increased by 8.2% including limited growth in Russia.
EA, AU & PMI DF:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2023 | 2022 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 6,201 | | $ | 5,936 | | | 4.5 | % | 11.2 | % | | $ | 265 | | $ | (400) | | $ | — | | $ | 206 | | $ | 459 | | $ | — | |
| Operating Income | | $ | 2,539 | | $ | 2,424 | | | 4.7 | % | 21.0 | % | | $ | 115 | | $ | (394) | | $ | — | | $ | 206 | | $ | 326 | | $ | (23) | |
Net revenues increased by 4.5%. Net revenues, excluding currency and acquisitions, increased by 11.2%, reflecting: favorable volume/mix, mainly driven by higher HTU volume, partly offset by lower cigarette volume and unfavorable smoke-free mix (for HTUs and devices); and a favorable pricing variance, driven by higher combustible tobacco and device pricing, partly offset by lower HTU (net) pricing (primarily related to Japan).
Operating income increased by 4.7%. Operating income, excluding currency and acquisitions, increased by 21.0%, mainly reflecting favorable volume/mix, primarily driven by higher HTU volume, partly offset by lower cigarette volume and unfavorable HTU mix; the favorable pricing variance; lower supply chain costs (primarily related to Japan); and a favorable comparison to 2022 related to costs associated with the Swedish Match AB offer ($24 million). The increase was partly offset by the 2023 charge related to the South Korea indirect tax ($204 million), the 2023 charge related to the termination of a pledge agreement with the Foundation for a Smoke-Free World ($24 million) and the 2023 charges for restructuring charges ($19 million).
EA, AU & PMI DF - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding China, increased by 1% to 319.4 billion units, reflecting growth for HTUs, partly offset by a decline for cigarettes. The increase in the estimated total market was mainly driven by International Duty Free (up by 34.4%), partly offset by Taiwan (down by 7.4%) and Australia (down by 19.4%).
Our Regional market share increased by 1.3 points to 30.0%.
Our total cigarette and HTU shipment volume in the Region increased by 6.7% to 101.2 billion units, mainly driven by Japan (up by 9.7%) and International Duty Free (up by 14.5%).
PMI's estimated HTU adjusted in-market sales volume in the Region increased by 15.8%, including growth in Japan of 14.5%.
Americas:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2023 | 2022 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 3,807 | | $ | 2,116 | | | 79.9 | % | 1.3 | % | | $ | 1,691 | | $ | 96 | | $ | 1,567 | | $ | 158 | | $ | (124) | | $ | (6) | |
| Operating Income | | $ | 582 | | $ | 440 | | | 32.3 | % | (61.4) | % | | $ | 142 | | $ | (215) | | $ | 627 | | $ | 158 | | $ | (93) | | $ | (335) | |
Net revenues increased by 79.9%. Net revenues, excluding currency and the impact of the Swedish Match acquisition, increased by 1.3%, primarily reflecting: a favorable pricing variance, driven by higher combustible tobacco pricing, partly offset by unfavorable volume/mix was mainly due to lower cigarette volume and unfavorable cigarette mix outside of the U.S.
Operating income increased by 32.3%. Operating income, excluding currency and the impact of the Swedish Match acquisition, decreased by 61.4%, mainly reflecting: higher marketing, administration and research costs (including incremental investments in the U.S. in preparation for smoke-free commercialization); higher amortization of intangibles; and unfavorable volume/mix, mainly due to the same factors as for net revenues; partly offset by the favorable pricing variance and a favorable comparison to 2022 related to the Swedish Match AB acquisition related items.
Americas - Total Market, PMI Shipment Volume and Market Share Commentaries
The estimated total market for cigarettes and HTUs in the Region, excluding the U.S., decreased by 1% to 188.8 billion units, driven by a decline for cigarettes. The decrease in the estimated total market was mainly due to Mexico (down by 6.9%), Canada (down by 12.6%) and Argentina (down by 5.0%), partly offset by Brazil (up by 10.1%).
Our Regional market share, excluding the U.S., decreased by 1.2 points to 33.7%.
Our total cigarette and HTU shipment volume in the Region decreased by 3.9% to 63.9 billion units, mainly due to Mexico (down by 9.8%) and Argentina (down by 7.9%), partly offset by Brazil (up by 12.8%).
In 2023, our oral SFP shipments reached 522.5 million cans in the Region, driven by ZYN nicotine pouches in the U.S.
Wellness and Healthcare:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Financial Summary - Years Ended December 31, | | | | | Change
Fav./(Unfav.) | | Variance
Fav./(Unfav.) |
| 2023 | 2022 | | Total | Excl.
Curr. & Acquis. | | Total | Cur-
rency | Acqui-sitions | Price | Vol/
Mix | Cost/
Other |
| (in millions) | | | |
| Net Revenues | | $ | 306 | | $ | 271 | | | 12.9 | % | 11.8 | % | | $ | 35 | | $ | 3 | | $ | — | | $ | 33 | | $ | — | | $ | (1) | |
| Operating Income / (Loss) | | $ | (870) | | $ | (258) | | | -(100)% | -(100)% | | $ | (612) | | $ | (6) | | $ | — | | $ | 33 | | $ | — | | $ | (639) | |
Net revenues increased by 12.9%. Net revenues, excluding currency and acquisitions, increased by 11.8%, notably reflecting the higher net revenues for smoking cessation products and select inhalation products.
The operating loss of $870 million in 2023 was primarily due to an impairment charge for goodwill and other intangibles of $680 million in the second quarter, as well as commercial investments and higher administration costs and the amortization of acquired intangibles. The operating loss of $258 million in 2022 included a charge for impairment of other intangible assets of $112 million and the amortization of acquired intangibles.
Business Environment
The tobacco industry and our company face a number of challenges that may adversely affect our business, product sales volume, results of operations, cash flows, and financial position. These challenges, which are discussed below and in Part II, Item 1A. Risk Factors — Cautionary Factors That May Affect Future Results in this report, include:
•regulatory restrictions on our products, including restrictions on the packaging, marketing, and sale of tobacco or other nicotine-containing products or related devices that could reduce our competitiveness, eliminate our ability to communicate with adult consumers, or ban certain of our products;
•fiscal challenges, such as excessive excise tax increases and discriminatory tax structures;
•illicit trade in cigarettes and other tobacco and nicotine-containing products, including counterfeit, contraband and other non-compliant or otherwise illicit products;
•intense competition, including unfair competition from non-tax paid volume by certain manufacturers; and
•legal challenges, including the pending and threatened litigation discussed in Part II, Item 8, Note 18. Contingencies in this report.
Smoke-Free Products (SFPs)
Our Approach to SFPs
We recognize that smoking cigarettes causes serious diseases and that the best way to avoid the harm of smoking is to never start or to quit. Nevertheless, according to WHO estimates, there are approximately 1 billion smokers globally. This number has not meaningfully changed in decades and, based on current trends, is not expected to significantly change in the near future.
Cigarettes burn tobacco, which produces smoke. As a result of the combustion process, the smoker inhales high levels of various toxic substances. In contrast, while SFPs contain nicotine, which is addictive and not risk-free, SFPs do not burn tobacco and therefore contain significantly lower levels of harmful and potentially harmful constituents ("HPHCs") than found in cigarette smoke.
Our SFPs and commercial activities for these products are designed for, and directed toward, current adult smokers and adult users of nicotine-containing products. We put in significant effort to restrict access to our products from non-smokers and youth.
For adult smokers who would otherwise continue to smoke cigarettes, we believe that SFPs, while not risk-free, offer a much better choice. Accordingly, our key strategic priorities are to: (i) continue developing and commercializing products that have the potential to present less risk of harm to adult smokers who switch to such products versus continued cigarette smoking; and (ii) educate and encourage current adult smokers who would otherwise continue to smoke cigarettes to switch to those products.
We recognize that this transformation from cigarettes to SFPs will take time and that the rate of transformation will depend in part upon factors beyond our control, such as the willingness of governments, regulators, and other policy groups to embrace SFPs as a desirable alternative to continued cigarette smoking. As a leading international cigarette manufacturer, we will continue to accelerate this transformation by using our extensive commercial and distribution infrastructure as an effective platform for the commercialization of our SFPs, and communication with adult smokers and trade partners about the substantiated benefits of switching to our SFPs. As long as a significant number of adult smokers continue to smoke cigarettes, responsible leadership of the category is critical. We aim to maintain our competitive position in the cigarette market through selective investment. We are judiciously reallocating resources from cigarettes to SFPs and are streamlining our cigarette portfolio.
We have a range of SFPs in various stages of development, scientific assessment, and commercialization. We are committed to conducting rigorous scientific assessments of our SFPs to substantiate that they reduce exposure to HPHCs and, ultimately, that these products present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to SFPs versus continued cigarette smoking. We draw upon a team of expert scientists and engineers from a broad spectrum of scientific disciplines and our extensive learnings of adult consumer preferences to further develop and assess our SFPs. Our efforts are guided by the following key objectives:
•to develop SFPs as satisfying alternatives to smoking for adult smokers who would otherwise continue to smoke cigarettes;
•for those adult smokers, our goal is to develop and offer SFPs with a scientifically substantiated risk-reduction profile that approaches as closely as possible the risk-reduction profile associated with smoking cessation;
•to substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on scientific evidence of the highest standard that is made available for scrutiny and review by external independent scientists and relevant regulatory bodies; and
•to advocate for the development of science-based regulatory frameworks for the development and commercialization of SFPs, including the communication of scientifically substantiated information to enable adult smokers to make better choices.
Our SFPs
Our product development is based on the elimination of combustion via tobacco heating and other innovative systems, as well as through oral tobacco and nicotine products, which we believe are the most promising paths to providing a better consumer choice for those who would otherwise continue to smoke cigarettes. We recognize that no single product will appeal to all adult smokers. Therefore, we are developing and commercializing a portfolio of products intended to appeal to a variety of distinct adult consumer preferences and achieve population harm reduction, including:
•Heat-not-burn products, which use a precisely controlled heating device incorporating our IQOS HeatControl technology, into which a specially designed and proprietary tobacco unit is inserted in a holder and heated to generate an aerosol. We have conducted a series of clinical studies for this platform, the results of which were included in our submissions to the U.S. Food and Drug Administration (“FDA”). In addition to the original version of IQOS, which relies on a heating technology using a blade, a newer version of IQOS uses induction heating. Most of the studies referenced above were conducted with the blade version of IQOS and additional research was conducted with the induction technology. We believe that there is full comparability between the bladed versions of IQOS and the subsequent induction versions of IQOS, and that the data from the studies conducted with the blade version of IQOS remain valid and applicable to the newer and adjacent versions of IQOS. In 2022, we also began the initial launch of a heat-not-burn product that utilizes external resistive heating technology and is commercialized under the BONDS by IQOS brand.
•Oral tobacco and nicotine products, which include snus and modern oral nicotine pouches. Snus refers to (a) dried loose tobacco, or snuff, which is consumed by sniffing the product through the nose; (b) moist loose tobacco which is put in the mouth between the lower or upper lip and gum; and (c) snus pouches which contain ground tobacco, water, salt and flavors. Modern oral nicotine pouches consist of white pre-conditioned pouches containing nicotine derived from tobacco, and they primarily contain nicotine, flavors, and cellulose substrate. Users place a pouch between the upper lip and gum and leave it there while the nicotine and flavor are being released. Like snus, nicotine pouches are inherently smoke-free as they are consumed orally, and no combustion process occurs during use. The pouches contain pharmaceutical-grade tobacco-derived nicotine like the nicotine used in medicinal products, such as nicotine-containing gums and inhalers, and flavors approved for use in food in accordance with the product quality standards for nicotine pouches developed by the Swedish Institute for Standards and the British Standards Institute. In 2022, we significantly expanded our oral smokeless products portfolio with the acquisition of Swedish Match. Swedish Match's ZYN is the leading nicotine pouch brand in the U.S. market.
•e-Vapor products, which are battery-powered devices that produce an aerosol by vaporizing a tobacco-free liquid solution. We have developed e-liquids for our e-Vapor products designed to deliver an authentic tobacco taste. Using patented technology, flavors and nicotine are extracted directly from the tobacco leaves and captured in a tobacco-free liquid solution, without having to add flavoring ingredients.
Data show that, in a stable regulatory environment, only a very small percentage of adult smokers who convert to IQOS switch back to cigarettes.
We aim to continue to develop and expand our SFP brand portfolio and market positions. In addition, we continue to use our expertise, technology and capabilities to explore new growth opportunities beyond our current business, including products that do not contain nicotine or tobacco.
Commercialization of SFPs
We are continuing to develop a multiplatform approach and tailoring our commercialization strategy to the characteristics of each specific market. We focus our commercialization efforts on consumer retail experience, guided consumer trials and customer care, and increasingly, digital communication programs and e-commerce. In order to accelerate switching to our SFPs, our initial market introductions typically entail one-on-one consumer engagement (in person or by digital means) and device discounts. These initial commercialization efforts require substantial investment, which we believe will moderate over time and further benefit from the increased use of digital engagement capabilities. PMI has, and continues to, invest in digital consumer engagement.
In 2014, we introduced IQOS in pilot city launches in Nagoya, Japan, and in Milan, Italy. Since then, we have continuously expanded our commercialization activities.
As of December 31, 2024, PMI's smoke-free products were available for sale in 95 markets.
We have integrated the production of our heated tobacco units into several of our existing manufacturing facilities, are progressing with our plans to build manufacturing capacity for our other SFPs, and continue to optimize our manufacturing infrastructure and expand our commercialization activities for new products and markets. We discuss certain risks related to the commercialization and supply of our SFP portfolio in Part I, Item 1A. Risk Factors—Cautionary Factors That May Affect Future Results in this report.
We discuss product warranties in more detail in Part II, Item 8, Financial Statements—Note 7. Product Warranty in this report. The significance of warranty claims depends on a number of factors, including device version mix, product failure rates, logistics and service delivery costs, and warranty policies, and may increase with the number of devices sold.
On October 20, 2022, PMI announced that it had reached an agreement with Altria Group, Inc., ("Altria") to end the companies' commercial relationship as of April 30, 2024, with respect to IQOS in the U.S. (the “Altria Agreement”). Under the Altria Agreement, PMI now holds the full rights to commercialize IQOS in the United States - the world’s largest smoke-free market. The Altria Agreement provides a clear path to expanding IQOS’s international success in a market where over 28 million adults continue to smoke cigarettes.
In January 2020, we announced an agreement with KT&G, a leading tobacco and nicotine company in South Korea, for the commercialization of KT&G’s smoke-free products outside of South Korea on an exclusive basis. On January 30, 2023, we announced a renewal and extension of this arrangement. For more information, see Acquisitions, Divestitures and Other Business Arrangements below.
Our commercialization efforts for the other PMI-developed SFPs are as follows:
•In late 2022, we began commercializing our BONDS product in the Philippines and Colombia.
•Since August 2020, we have launched and expanded our portfolio of vaping products (branded VEEV) in 40 markets.
•Following our acquisition of Swedish Match, we have access to a strong portfolio of Swedish Match brands in both the snus and nicotine pouch categories. Nicotine pouches are currently available in 38 markets.
Legislation, Regulation, Taxation and Other Matters Regarding the Manufacture, Marketing, Sale and Use of Tobacco and Other Nicotine-Containing Products
Fiscal Challenges
Excessive and disruptive excise, sales and other tax increases and discriminatory tax structures are expected to continue to have an adverse impact on our profitability, due to lower consumption and consumer down-trading to non-premium, discount, other low-price or low-taxed products such as fine cut tobacco, illicit cigarettes or illicit SFPs. In addition, in certain jurisdictions, some of our products are subject to tax structures that discriminate against premium-price products and manufactured cigarettes. We believe that such tax policies undermine public health by encouraging consumers to turn to illicit trade, and ultimately undercut government revenue objectives, disrupt the competitive environment, and encourage criminal activity. Other jurisdictions have imposed, or are seeking to impose, levies or other taxes specifically on tobacco companies, such as taxes on revenues and/or profits.
On March 14, 2024, the Court of Justice of the European Union (the "CJEU") ruled that the German fiscal regulation imposing an additional excise tax on HTPs does not contravene EU law. Fiscal Court in Dusseldorf (the "FCD") had previously referred that question to the CJEU. On May 21, 2024, the FCD delivered its judgement and dismissed our local affiliate's, f6 Cigarettenfabrik GmbH & Co.KG, ("PM Germany") claim. PM Germany filed a notice of appeal to the Federal Fiscal Court on June 19, 2024.
Legislative and Regulatory Environment
The tobacco industry operates in a highly regulated environment. The well-known risks of smoking have led regulators to impose significant restrictions and high excise taxes on cigarettes. The regulatory landscape for novel nicotine-containing products is inconsistent and evolving but is, in some instances, even more restrictive.
Much of the regulation that shapes the business environment in which we operate is driven by the World Health Organization's (the "WHO") Framework Convention on Tobacco Control (the "FCTC"), which entered into force in 2005. The main objective of the FCTC is to establish a global agenda for tobacco regulation, with the purpose of reducing tobacco use. To date, 182 countries and the European Union ("EU") are Parties to the FCTC. The treaty requires Parties to have in place various tobacco control measures and recommends others. The FCTC governing body, the Conference of the Parties (“CoP”), has also adopted non-binding guidelines and policy recommendations related to certain articles of the FCTC that go beyond the text of the treaty. In October 2018, the CoP recognized the need for more scientific assessment and improved reporting to define policy on heated tobacco products. Similar to its previous policy recommendations on e-cigarettes, the CoP invited countries to regulate, restrict or prohibit heated tobacco products, as appropriate under their national laws.
The WHO study group on tobacco product regulation published their ninth report on the scientific basis of tobacco product regulation in August 2023. The report is based on a review of scientific evidence related to novel and emerging nicotine and tobacco products, such as electronic nicotine delivery systems ("ENDS"), electronic non-nicotine delivery systems and HTPs. The report concludes by making a number of policy recommendations on HTPs and ENDS that, if implemented, could restrict both the availability of these products and the access to accurate information about them.
The Tenth Session of the CoP took place in February 2024. No new decisions or policy recommendations on novel and emerging tobacco products were adopted. Specific Guidelines were adopted to address cross-border Tobacco Advertising, Promotion, and Sponsorship (“TAPS”) and the depiction of tobacco in entertainment media. The Eleventh session of the CoP is currently scheduled to take place in November 2025. The WHO’s reports and other FCTC guidelines or recommendations are not binding on the WHO Member States or on parties to the FCTC.
We believe that when better alternatives to cigarettes exist, the discussion should not be whether these alternatives should be made available to the more than one billion people who smoke cigarettes today, but how fast, and within what regulatory framework to maximize their adoption by adult smokers while minimizing unintended use. Therefore, we advocate for regulatory frameworks that are based on a continuum of risk where non-combustible products fall below combustible cigarettes. And we believe that regulation and taxation should differentiate between cigarettes and products that present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to these products versus continued smoking.
Product regulation should include measures that encourage and accelerate switching to non-combustible products, for example, by allowing adult consumers who would otherwise continue smoking cigarettes to receive truthful and non-misleading information about such alternatives to enable them to make informed decisions and by applying uniform product standards to enable manufacturers to demonstrate the reduction in harmful and potentially harmful constituents, as well as the absence of combustion.
Regulation should also include specific rules for ingredients, labeling and consumer communication, and should ensure that the public is informed about the health risks of all combustible and non-combustible tobacco and nicotine-containing products. Importantly, regulation must include measures designed to prevent initiation by youth and non-smokers. We support mandated accurate and factual health warnings on packaging, minimum age laws, restrictions on advertising, and smoking restrictions in public spaces. We also support regulatory measures that help reduce illicit trade. At the same time, we oppose excessive or prohibitive regulations that may prevent adult smokers, who would otherwise continue smoking, from accessing and switching to SFPs or trigger unintended consequences such as illicit trade.
Regulatory Restrictions
SFP Sales Prohibitions: Some governments have banned, are seeking to ban, or severely restrict emerging tobacco and nicotine-containing products such as our SFPs, and communication of truthful and non-misleading information about such products. Significant markets that have prohibited or severely restricted the sale of one or more category of SFP include Argentina, Brazil, Canada, India, Mexico, Turkey, Australia, Thailand, and Vietnam. In the U.S., some states and municipalities have introduced stringent restrictions on the sale of certain SFPs, including those authorized by the FDA.
These regulations might foreclose or unreasonably restrict adult consumer access to products that might be a better consumer choice than continuing to smoke cigarettes. We oppose blanket bans and unreasonable restrictions of products that have the potential to present less risk of harm compared to continued cigarette smoking. By contrast, we support regulation that sets clear standards for all SFP categories and propels innovation to benefit adult smokers who would otherwise continue to smoke cigarettes.
EU Tobacco Products Directive ("TPD"): In April 2014, the EU adopted a significantly revised TPD, which came into force in May 2016. All EU Member States have adopted laws transposing the TPD. The TPD sets forth a comprehensive set of regulatory requirements for tobacco products, including:
•health warnings covering 65% of the front and back panels of cigarette packs, with an option for Member States to further standardize tobacco packaging, including the introduction of plain packaging;
•a ban on characterizing flavors in some tobacco products;
•security features and tracking and tracing measures; and
•a framework for the regulation of novel tobacco products and e-cigarettes, including requirements for health warnings and information leaflets, a prohibition on product packaging text related to reduced risk, and the introduction of notification requirements or authorization procedures in advance of commercialization.
In February 2024, the European Commission published an updated implementation roadmap to Europe's Beating Cancer Plan (the "Plan"). Further developments concerning a revision of TPD are expected during the mandate of the European Commission that took office in December 2024.
In May 2024, the EU-wide systems of traceability and security features for tobacco products were extended to include tobacco products other than cigarettes and roll-your-own tobacco products. As such, all tobacco products are covered by the traceability system.
EU Tobacco Excise Directive ("TED"): The EU Commission is preparing a legislative proposal for the revision of the 2011 EU Tobacco Excise Directive that may include definitions and tax treatment for novel tobacco and nicotine-containing products, including heated tobacco products, e-cigarettes and nicotine pouches. The timeline for the revision has not been announced. Any final amendments to TED require unanimous agreement by all EU Member States, followed by transposition of TED into national legislation.
Plain Packaging and Other Packaging Restrictions: Plain packaging legislation bans the use of branding, logos and colors on packaging other than the brand name and variant that may be printed only in specified locations and in a uniform font. To date, plain packaging laws have been adopted in certain markets in all of our operating segments, including the key markets of Australia, France, Saudi Arabia and Turkey. Some countries, such as Canada, Denmark and Israel, adopted plain packaging regulations that apply to all tobacco products, including SFPs. Other countries are also considering plain packaging legislation.
Some countries have adopted, or are considering adopting, packaging restrictions that could have an impact similar to plain packaging. Examples of such restrictions include standardizing the shape and size of packages, prohibiting certain colors or the use of certain descriptive phrases on packaging, and requiring very large graphic health warnings that leave little space for branding.
Restrictions and Bans on the Use of Ingredients: The WHO and others in the public health community have recommended restrictions or total bans on the use of some or all ingredients in both combustible and SFPs, including menthol. Broad restrictions and ingredient bans would require us to reformulate our American blend tobacco products, which could reduce our ability to differentiate these products in the market in the long term. In many countries, menthol or flavor bans would eliminate entire product categories.
The EU banned cigarettes and roll-your-own tobacco products with characterizing flavors. Other tobacco products are currently exempted under EU TPD from this characterizing flavor ban. This was also the case for heated tobacco products until November 23, 2022, when the EU Commission published a delegated directive that eliminated this exemption. All EU Member States were required to apply the delegated directive as of October 23, 2023, which bans the use of characterizing flavors in heated tobacco products, impacting a significant proportion of our SFP products currently sold in the EU. Currently, all but six EU Member States have transposed this directive, withdrawing the heated tobacco product exemption from the characterizing flavor ban into national law. Based on high consumer switching to non-flavored products in reaction to past bans on flavors in other categories and markets, we anticipated that, while short-term volatility would be possible, the ban’s impact on our shipment volumes in the EU would be relatively limited in the near term. To date, our experience is consistent with this expectation. There has been some short-term disruption in countries that have implemented the ban, but the impact has generally been limited in time and magnitude. Nevertheless, it remains possible that the impact in countries that have yet to implement the ban could be more significant in duration or magnitude. But our fundamental view remains that we do not expect a meaningful long-term change in the structural growth of the category. We will continue to actively monitor relevant developments in the EU market, including from an illicit trade standpoint.
Other countries may follow the EU’s approach toward tobacco product ingredients. Turkey banned menthol as of May 2020. Broader ingredient bans have been adopted by Brazil and Canada. In the U.S., certain states and localities have adopted flavor bans that apply to SFPs, including certain ZYN varieties.
Bans on Display of Tobacco Products at Retail: In a number of our markets, including, but not limited to, Australia, Canada and Russia, governments have banned the display of tobacco products at the point of sale. Other countries are considering similar bans.
Bans and Restrictions on Advertising, Marketing, Promotions and Sponsorships: For many years, the FCTC has called for, and countries have imposed, partial or total bans on tobacco advertising, marketing, promotions and sponsorships, including bans and restrictions on advertising on radio and television, in print and on the Internet. The FCTC's non-binding guidelines recommend that governments prohibit all forms of communication with adult smokers.
Product Standards and Restrictions on Product Design: In some countries, including in the EU, cigarettes are subject to testing, disclosure and mandatory emissions limits for tar, nicotine, carbon monoxide and other smoke constituents.
Some members of the public health community are calling for the further standardization of tobacco products by requiring, for example, that cigarettes have a certain minimum diameter, which would result in a ban on slim cigarettes, or requiring the use of standardized filter and cigarette paper designs. In addition, at its meeting in November 2016, the CoP adopted non-binding guidelines recommending that countries regulate product design features that increase the attractiveness of tobacco products, such as the diameter of cigarettes and the use of flavor capsules.
Currently, national standards in certain countries set minimum quality and safety requirements for heat-not-burn products with technical heat-not-burn specifications and/or methods for demonstrating the absence of combustion. These standards are mandatory in Armenia, Bahrain, Egypt, Jordan, Lebanon, the Philippines, Saudi Arabia, Tajikistan, Tunisia, the UAE and Uzbekistan, and voluntary in Algeria, Azerbaijan, Colombia, Costa Rica, Dominican Republic, Indonesia, Kazakhstan, Kyrgyzstan, Morocco, Russia, South Africa, Vietnam, the U.K. and Ukraine. In Japan, a voluntary standard sets minimum safety requirements for tobacco heating devices.
For e-Vapor products, national standards setting minimum quality and safety requirements have been adopted in several markets. These standards are mandatory in Armenia, Bahrain, China, Egypt, Jordan, New Zealand, the Philippines, Russia, United Arab Emirates, and Saudi Arabia and Tajikistan, and voluntary in Azerbaijan, Costa Rica, Dominican Republic, France, Indonesia, Kazakhstan, the Philippines, Russia, the U.K., and Ukraine.
Currently, industry standards setting minimum quality and safety requirements for modern oral nicotine pouches have been adopted in Armenia, Bahrain, Costa Rica, Pakistan, Sweden, the U.K., and Ukraine. These standards are voluntary.
We expect other governments to consider similar product standards for all novel tobacco and nicotine-containing products and encourage making them mandatory.
Restrictions on Public Smoking and Use of Nicotine-Containing Products in Public: The pace and scope of restrictions on the use of our products have increased significantly in most of our markets. Many countries around the world have adopted, or are likely to adopt, regulations that restrict or ban smoking and use of certain nicotine-containing products in public and/or workplaces, restaurants, bars and nightclubs. Some public health groups have called for, and some countries, regional governments and municipalities have adopted or proposed, bans on smoking in outdoor places, as well as bans on smoking in cars (typically, when minors are present) and
private homes. On December 3, 2024, the EU Council adopted its legally non-binding recommendation on smoke- and aerosol-free environments. While the recommendations recognize scientifically proven differences between smoke-free and combustible products, they nevertheless encourage EU member states to restrict usage of both conventional and novel tobacco products in indoor public spaces and some outdoor areas. Each member state is to decide whether or not to implement these recommendations.
Generation Bans: Certain regulators are considering generation sales bans, which prohibit the sale of certain tobacco or nicotine products to people born after a certain year. In December 2022, New Zealand adopted regulatory measures restricting the sale and supply of smoked tobacco products, including prohibiting the sale of smoked tobacco products to anyone born on or after January 1, 2009. These measures were limited to smoked tobacco products and did not apply to heated tobacco products and e-cigarettes. The New Zealand parliament repealed the measures in February 2024, before they were implemented. On November 5, 2024, the UK government introduced a bill to the parliament, which if adopted, would ban the sale of tobacco products, including HTPs, herbal smoking products and cigarette papers to those born on or after January 1, 2009.
Other Regulatory Issues: Some regulators are considering, or in some cases have adopted, regulatory measures designed to reduce the supply of tobacco products. These include regulations intended to reduce the number of retailers selling tobacco products by, for example, reducing the overall number of tobacco retail licenses available or banning the sale of tobacco products within specified distances of certain public facilities. In a limited number of markets, most notably Japan, we are dependent on governmental approvals that may limit our pricing flexibility.
The EU Single-Use Plastics Directive, which will require tobacco manufacturers and importers to cover the costs of public collection systems for tobacco product filters, under Extended Producer Responsibility ("EPR") schemes, came into force on July 2, 2019. As of December 31, 2024, the majority of EU Member States transposed the directive into national legislation and brought into force mandatory EPR schemes and related EPR costs for tobacco manufacturers and importers. We currently expect further adoption of similar laws in other jurisdictions, and we are monitoring developments in this area. We do not estimate a material impact to our business in the EU as a result of compliance with these mandatory EPR schemes.
SFP Commercialization and Risk Statement Authorizations
Certain markets have instituted regulatory authorization processes that govern the commercialization of SFPs or the use of statements addressing SFP harm-reduction.
FDA Approval Process and Status of PMI SFPs: In the United States, an established regulatory framework for assessing “Modified Risk Tobacco Products” ("MRTP") and “New Tobacco Products” exists under the jurisdiction of the FDA. FDA actions may influence the regulatory approach of other governments and international regulatory agencies.
FDA Review of IQOS: We submitted to the FDA a Modified Risk Tobacco Product Application (“MRTPA”) for IQOS in December 2016, and a Premarket Tobacco Product Application (“PMTA”) for IQOS in March 2017.
On April 30, 2019, the FDA determined that a version of IQOS, namely, IQOS 2.4 and three related consumables, is appropriate for the protection of public health and authorized it for sale in the United States. The FDA’s decision followed its comprehensive assessment of our PMTA. On December 7, 2020, the FDA reached the same determination for the IQOS 3 device and authorized that version of IQOS for sale in the United States.
On July 7, 2020, the FDA determined that the available scientific evidence demonstrates that the issuance of an exposure modification order would be appropriate for the promotion of public health and authorized the marketing of a version of IQOS, namely IQOS 2.4 and three related consumables, as an MRTP with reduced exposure claims. On March 11, 2022, the FDA reached the same determination for the IQOS 3 device. The FDA authorized the marketing of this product in the U.S. with the following information:
"AVAILABLE EVIDENCE TO DATE:
•the IQOS system heats tobacco but does not burn it.
•this significantly reduces the production of harmful and potentially harmful chemicals.
•scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals."
The FDA may issue two types of MRTP orders: a “risk modification” order or an “exposure modification” order. We had requested both types of orders for IQOS 2.4 and an initial selection of three related consumables' variants. After review, the FDA determined that the evidence did not support issuing a "risk modification" order at that time, but that it did support issuing an "exposure modification" order for the product. This determination included a finding that issuance of the exposure modification order is expected to benefit the health of the population as a whole. We also received an exposure modification order for IQOS 3 in March 2022. We look forward to working with the FDA to provide any additional information they may require to market SFPs with reduced risk claims.
The FDA’s PMTA and MRTP orders do not mean that the agency “approved” IQOS. These authorizations are subject to strict marketing, reporting, and other requirements, and are not a guarantee that the product will remain authorized, particularly if there is a significant uptake in youth or non-smoker initiation. The FDA will monitor the marketing of the product.
On January 26, 2023, the FDA authorized the marketing of two new tobacco-flavored consumables (Marlboro Sienna HeatSticks and Marlboro Bronze HeatSticks) and a modified version of the authorized Marlboro Amber HeatSticks. These products are line extensions and/or modified versions of the tobacco-flavored consumables for which the FDA had previously issued a marketing granted order. In its assessment, the FDA determined that the three variants of HeatSticks were comparable to the previously authorized tobacco-flavored consumables.
On July 5, 2023, we submitted renewal applications to the FDA requesting re-authorization to continue to market those IQOS products that previously received an exposure modification order with a modified exposure claim in the United States. These renewal requests were received by the FDA 360 days prior to the stated July 2024 expiration date of the original exposure modification orders, as requested by the FDA in the original orders. On May 9, 2024, the FDA filed for scientific review of our MRTP renewal applications for IQOS products and posted materials from these applications. As our applications proceed through the review process, we have responded to FDA requests for additional information. The FDA may make further information requests or conduct subsequent inspections to verify the information we submitted. The FDA did not issue a decision on our MRTP renewal applications prior to the stated July 7, 2024, expiration date of the original exposure modification orders. The MRTP renewal applications were timely filed in accordance with FDA direction, and we believe that we should be permitted to continue to use the modified exposure claim with respect to those products that received exposure modification orders until the FDA decides on our MRTP renewal applications.
On October 20, 2023, we submitted bundled PMTAs for our IQOS ILUMA THS products together with MRTPAs requesting authorization of the exposure reduction marketing order previously granted for IQOS blade versions. We submitted these applications at the same time in order for the FDA to evaluate the PMTAs and MRTPAs concurrently. In March 2024, the FDA formally accepted our bundled PMTAs and MRTPAs. As our applications proceed through the review process, the FDA may request additional information or conduct subsequent inspections to verify the information we submitted.
On January 19, 2024, the FDA completed its review of our Requests for Exemption from Substantial Equivalence (the “EX REQs”) for the five submitted IQOS consumables and determined that these tobacco products were exempt from the requirements outlined for substantial equivalence (a regulatory pathway that can be used to introduce new tobacco products which have the same characteristics as a product previously authorized by the FDA). These submissions were made in November 2022 (for the three initial IQOS consumables) and February 2023 (for the two new IQOS consumables) to enable domestic manufacturing of IQOS consumables utilizing materials purchased from vendors operating in the United States.
On April 29, 2024, we submitted the Annual Report for the IQOS Tobacco Heating System ("THS") to the FDA. The report included a systematic review of the literature covering publications related to the IQOS THS between March 1, 2023, and February 29, 2024. The report included publications in various scientific fields including aerosol chemistry and physics, standard and systems toxicology, clinical studies on exposure reduction to HPHCs, and observational studies. Overall, the review continues to support the finding that IQOS THS is "appropriate for the promotion of public health."
FDA Review of Swedish Match Products: Premarket tobacco applications for 20 varieties of ZYN nicotine pouches were submitted in March 2020. In the same month, these applications were filed for scientific review and in July 2020 the FDA issued a deficiency letter, which Swedish Match USA, Inc. addressed in September 2020. On January 16, 2025, the FDA determined that all 20 ZYN nicotine pouch varieties currently marketed in the U.S. met the applicable public health standard and were appropriate for the protection of public health, and therefore authorized them for sale in the United States. In their assessment, the FDA concluded that “among several key considerations, the agency’s evaluation showed that, due to substantially lower amounts of harmful constituents than cigarettes and most smokeless tobacco products, such as moist snuff and snus, the authorized products pose lower risk of cancer and other serious health conditions than such products.” Prior to the authorization these 20 varieties of ZYN pouches were marketed in the U.S. consistent with the FDA's practice not to take enforcement action to prevent products with respect to which applications were filed
prior to a September 9, 2020 deadline from being marketed. After the September 9, 2020 deadline, we also submitted additional applications for authorization to market other ZYN products. We are unable to market these products until the FDA authorizes such applications. In April 2024, we submitted MRTPAs for ZYN products currently marketed in the U.S. and requested authorization of the modified risk claim. As these applications proceed through the review process, the FDA may request additional information or conduct subsequent inspections to verify the submitted information.
On July 17, 2023, Swedish Match USA, Inc. submitted a renewal application to the FDA requesting re-authorization to continue to market its eight snus smokeless tobacco products (sold under the General snus brand name) with a modified risk claim. General snus products received modified risk orders on October 22, 2019. Swedish Match USA, Inc. was authorized to market these products with the claim, “Using General snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.” On November 7, 2024, the FDA renewed the General snus modified risk orders, with a stated expiration date in November 2032.
Other Commercialization and Risk Statement Authorization Frameworks: On March 22, 2023, a bill amending the Tobacco Hazards Prevention and Control Act in Taiwan went into effect. It regulates heated tobacco products and bans e-cigarettes. The amendment particularly specifies that designated tobacco products (including heated tobacco products) that are not cigarettes, cut tobacco, cigars, snuff nor chewing tobacco, must undergo a health risk assessment as part of an authorization system. We have filed an authorization request to commercialize IQOS in Taiwan pursuant to this Act, but this authorization is currently still pending authorization by the Ministry of Health of Taiwan.
On March 23, 2023, the Greek Ministry of Health authorized a claim for IQOS with HEETS AMBER to inform Greek IQOS users about reduction in emissions of toxicants when using such product compared to cigarette smoking. The decision authorized the following claim: “The concentration of chemical substances with recognized toxicity produced when using IQOS with HEETS AMBER tobacco sticks is lower compared to conventional smoking. A reduction in the concentration of chemical substances with recognized toxicity does not mean a corresponding reduction in risk for health. The aerosol of this tobacco product contains nicotine and other hazardous chemicals. This tobacco product harms your health and is addictive. The best choice is to quit tobacco and nicotine use altogether.” With this authorization, Greece is the second country officially recognizing the reduction in level of toxicants in the IQOS aerosol compared to cigarette smoke.
SFP Scientific Findings
We make our scientific findings publicly available for scrutiny and peer review through several channels, including our websites. From time to time, adult consumers, competitors, members of the scientific community, and others inquire into our scientific methodologies, challenge our scientific conclusions or request further study of certain aspects of our SFPs and their health effects. We are committed to a robust and open scientific debate and believe that such debate should be based on accurate and reliable scientific information. We seek to provide accurate and reliable scientific information about our SFPs; nonetheless, we may not be able to prevent third-party dissemination of false, misleading or unsubstantiated information about these products. The dissemination of scientifically unsubstantiated information or studies with a strong confirmation bias by third parties may cause confusion among adult smokers and affect their decision to switch from continued smoking to better alternatives, such as our SFPs.
To date, we have been largely successful in demonstrating to regulators that our heated tobacco units are not cigarettes due to the absence of combustion, and as such, they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Although we believe that this is sensible from the public health perspective, there is no guarantee regulators will continue this approach. Further, there can be no assurance that we will succeed in our efforts to replace cigarettes with SFPs or that regulation will allow us to commercialize SFPs in all markets, to communicate about our SFPs, including making scientifically substantiated risk-reduction claims, or to treat SFPs differently from cigarettes.
To date, several governmental agencies have published their scientific findings that analyze the harm-reduction potential of certain SFPs versus continuing to smoke cigarettes, including:
In December 2017, at the request of the U.K. Department of Health and Public Health England, the U.K. Committee on Toxicity published its assessment of the risk of heat-not-burn products relative to cigarette smoking. This assessment included analysis of scientific data for two heat-not-burn products, one of which was IQOS. The assessment concluded that, while still harmful to health, compared with the known risks from cigarettes, heat-not-burn products are probably less harmful. Subsequently, in February 2018, Public Health England published a report stating that the available evidence suggests that heat-not-burn products may be considerably less harmful than cigarettes but more harmful than e-cigarettes.
In May 2018, the German Federal Institute for Risk Assessment (“BfR”) published a study on IQOS aerosol relative to cigarette smoke using the Health Canada Intense Smoking Regimen. BfR found reductions in selected HPHCs in a range of 80-99%. This publication indicates that significant reductions in the levels of selected toxicants are likely to reduce toxicant exposure, which BfR stated might be regarded as a discrete benefit compared to combustible cigarettes.
In May 2018, the Dutch National Institute for Public Health and Environment (“RIVM”) published a factsheet on novel tobacco products that heat rather than burn tobacco, focusing on IQOS. RIVM analyzed the aerosol generated by our IQOS product and concluded that the use of this product, while still harmful to health, is probably less harmful than continuing to smoke cigarettes.
In June 2018, the Korean Food and Drug Administration (“KFDA”) issued a statement on products that heat rather than burn tobacco. The KFDA tested three heat-not-burn products, one of which was IQOS. The KFDA confirmed that the levels of the nine HPHCs tested in the aerosol of these products were on average approximately 90% lower compared to those measured in the cigarette smoke of the top five cigarette brands in South Korea. However, the KFDA stated that it could not establish that the tested heat-not-burn products are less harmful than cigarettes. In October 2018, our Korean subsidiary filed a request with a local court seeking information underlying KFDA’s analysis, conclusions and public statements. In May 2020, the court ordered KFDA to produce certain records. Subsequent to that decision, and after exchanges between the parties, the case was closed.
In August 2018, the Science & Technology Committee of the U.K. House of Commons published a report of its inquiry into e-cigarettes and heat-not-burn products. The report concluded that e-cigarettes are significantly less harmful to health than smoking tobacco. The report also observed that for those smokers who do not accept e-cigarettes, heat-not-burn products may offer a public health benefit despite their relative risk. The report called for a risk-proportionate regulatory environment for both e-cigarettes and heat-not-burn products and noted that e-cigarettes should remain the least taxed, cigarettes the most taxed, with heat-not-burn products falling between the two. The U.K. Committee on Advertising Practice announced the removal of a prohibition of health claims in the advertising of e-cigarettes in the U.K., effective November 2018.
In November 2018, the Eurasian Economic Commission (regulatory body of the Eurasian Union consisting of Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) published the results of its commissioned study on novel nicotine-containing products, including IQOS. The study confirms significantly lower levels of HPHCs in the aerosol generated by this product compared to cigarette smoke.
In January 2019, scientific media published the results of the study of the China National Tobacco Quality Supervision and Test Centre (“CNTQST”) comparing the aerosol generated by IQOS with cigarette smoke. The CNTQST found that the former contained fewer, and lower levels of, harmful constituents than the latter and concluded that the lower temperature of heating tobacco in IQOS contributed to the difference. The CNTQST stated that the reduction in emissions of harmful constituents cannot be interpreted as a harm/risk reduction for cigarette smokers in the same proportion.
In April 2020, the Superior Health Council of Belgium (“SHC”) published results of its inquiry into heat-not-burn products. The SHC concluded that heat-not-burn products, while not safe, have a more favorable toxicity profile than cigarettes. However, in light of the uncertainty of such products’ short and long-term impacts, the toxic effects of the dual use with cigarettes, and the existence of approved smoking cessation tools, the SHC recommended that current regulations for cigarettes should apply to heat-not-burn products.
In June 2022, the SHC published new advice on e-cigarettes in which they confirm that e-cigarettes are substantially less harmful than smoking cigarettes and, therefore, a better alternative for smokers. The SHC underlines that the vast majority of the risks of tobacco smoking are not caused by nicotine, but by the harmful substances that are released by the combustion of tobacco. Based on the cited science, the SHC calls for legislation that makes a clear distinction between cigarettes and e-cigarettes by focusing on better informing smokers about the benefits of the lower-risk (but not risk-free) alternative, as well as on protecting non-smokers and young people.
The foregoing scientific findings of government agencies may not be indicative of the measures that the relevant government authorities could take in regulating our products.
Legal Challenges to SFPs
We face various administrative and legal challenges related to certain SFP activities, including allegations concerning product classification; advertising, distribution and sales restrictions; corporate communications; product coach activities; scientific substantiation; product liability; and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize SFPs and to communicate with the public. The outcomes of these matters may affect our SFP commercialization and public communication activities and performance in one or more markets.
On June 11, 2024, Swedish Match North America LLC ("SMNA") received a subpoena from the office of the Attorney General of the District of Columbia (“D.C.”). The subpoena requested, among other things, information concerning SMNA’s compliance with D.C.’s licensing regulations, age-verification requirements, and prohibition on the sale of flavored tobacco products, including with respect to ZYN nicotine pouches. SMNA cooperated with this investigation, and on December 12, 2024, reached an agreement to resolve the D.C. Attorney General’s investigation without admitting liability or wrongdoing. Pursuant to the agreement, SMNA paid $1.2 million to D.C. and agreed to conduct certain sales monitoring activities in D.C.
As part of a previously announced sales and supply chain review, SMNA has discontinued sales through the e-commerce shop associated with ZYN.com and direct sales to dedicated online retailers in the United States indefinitely. U.S. e-commerce has represented a small fraction of sales of SMNA in the United States, and the current network of approximately 170,000 brick-and-mortar retailers will remain SMNA’s focus.
Illicit Trade
Illicit trade creates a cheap and unregulated supply of tobacco and nicotine-containing products, undermines efforts to reduce smoking prevalence, especially among youth, damages legitimate businesses and intellectual property rights, stimulates organized crime, increases corruption and reduces government tax revenue. We generally estimate that, excluding China and the U.S., illicit trade may account for as much as 14% of global cigarette consumption; this includes counterfeit, contraband and the persistent problem of “illicit whites,” which are cigarettes legally purchased in one jurisdiction for the sole purpose of being exported and illegally sold in another jurisdiction where they have no legitimate market. Currently, we estimate that illicit trade in the EU accounted for approximately 8% of total cigarette consumption in 2023. Illicit trade also increasingly targets SFPs.
We devote substantial resources to help prevent illicit trade in combustible tobacco products and SFPs. We engage with governments, our business partners and other stakeholders to implement effective measures to combat illicit trade. Where effective and appropriate, we pursue legal remedies to protect our intellectual property rights from counterfeiting or to counter the illicit diversion of our products. We also cooperate with governmental authorities to combat fraudulent imports of non-compliant or unauthorized tobacco and nicotine-containing products.
As an example, the recent commercial success of the nicotine pouch category makes it more prone to be affected by illicit trade. Our ongoing anti-illicit initiatives for nicotine pouches include PMI’s ‘know-your-customer’ and ‘anti-diversion’ governance and other measures, such as volume monitoring, tracking and tracing, product security, as well as internal and external awareness training and communications. We are also expanding our market monitoring -both online and offline- and illicit trade research program to nicotine pouches. PMI affiliates and Swedish Match affiliates, are taking appropriate actions to address the illicit resale of certain of our oral products including nicotine pouches outside their initial intended market of retail, such as Scandinavia, the U.S. and other markets. Such actions include awareness communications to trade partners, cease-and-desist letters to those involved in illicit trade of products bearing our brands and limiting and/or terminating sales to certain customers in both the online and traditional trade.
A number of jurisdictions are considering actions to prevent illicit trade. In November 2012, the FCTC adopted the Protocol to Eliminate Illicit Trade in Tobacco Products (the “Protocol”), which includes supply chain control measures, such as licensing of manufacturers and distributors, enforcement of these control measures in free trade zones, controls on duty free and Internet channels and the implementation of tracking and tracing technologies. To date, 69 Parties, including the EU, have ratified it. The Protocol came into force in September 2018. Since then, implementation in national legislations has been ongoing. In February 2024, the third Meeting of the Parties to the Protocol took place. No additional restrictive measures were adopted, and a mandate to conduct further work will be considered at the next session, currently scheduled to take place in November 2025.
The tracking and tracing regulations for cigarettes and roll-your-own products manufactured or destined for the EU were extended to include tobacco products other than cigarettes, including some of our SFPs, as of May 20, 2024.
Other Developments
In September 2017, we announced our support of the Foundation for a Smoke-Free World (the "Foundation"). The Foundation is an independent, nonprofit organization dedicated to reducing the health impacts of smoking as set out in its Articles of Incorporation and its Bylaws. In September 2020, our pledge agreement with the Foundation was amended. We contributed $45 million in 2020, $40 million in 2021, $17.5 million in 2022, and had expected to contribute up to $35 million annually from 2023 through 2029, as specified in the amended pledge agreement. In 2023, the Foundation and PMI agreed to terminate the existing pledge agreement and PMI has made final grant payments totaling $140 million, commensurate with the early termination of the pledge agreement.
Governmental Investigations
From time to time, we are subject to governmental investigations on a range of matters, including tax, customs, antitrust, advertising, and labor practices. We describe certain pending matters in Item 8, Note 18. Contingencies.
In November 2010, a World Trade Organization ("WTO") panel issued its decision in a dispute between the Philippines and Thailand, concerning a series of Thai customs and tax measures affecting cigarettes imported by Philip Morris (Thailand) Limited ("PM Thailand") into Thailand. The decision concluded that Thailand had no basis to find that PM Thailand's declared customs values and taxes paid were too low, as alleged by the Thai government and created obligations for Thailand to revise its laws, regulations, or practices affecting the customs valuation and tax treatment of future cigarette imports. Thailand agreed to fully comply with the decision, but the Philippines asserts that to date Thailand has not fully complied with the WTO panel decision and commenced challenges at the WTO Appellate Body. The WTO Appellate Body is not operational, and the appeals by Thailand are suspended indefinitely. In December 2020, the Philippines and Thailand agreed to pursue facilitator-assisted discussions aimed at progressing and resolving outstanding issues and the countries have since agreed to seek the establishment of a bilateral consultative mechanism, with the goal of reaching a comprehensive settlement of their dispute, consistent with their rights and obligations under the WTO Agreements, as well as the recommendations and rulings of the WTO Dispute Settlement Body.
War in Ukraine
In Ukraine, our main priority remains the safety and security of our employees and their families in the country. We continue commercial activities in select locations where safety allows, in order to provide product availability and service to adult consumers, and supplies the market from production centers outside Ukraine, as well as through a contract manufacturing arrangement. Production at our factory in Kharkiv remains suspended. On June 20, 2023, we announced the investment of $30 million in a new production facility in the Lviv region, in Western Ukraine. Preparatory work for the facility began in July 2023. The new production facility was completed at the end of the first quarter of 2024 and local production commenced in April 2024. As of December 31, 2024, our Ukrainian operations had approximately $0.6 billion in total assets, excluding intercompany balances.
In Russia, we are continuously assessing the evolving situation in the country. This includes regulatory constraints in the market entailing very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. In the event of a divestment, our ability to fully realize the value of the business would likely be subject to material impairment. As of December 31, 2024, our Russian operations had approximately $2.7 billion in total assets, excluding intercompany balances, of which approximately $1.0 billion consisted of cash and cash equivalents held mostly in local currency (Russian rubles).
Additionally, we hold a 23% equity interest in Megapolis Distribution B.V., which was the holding company of JSC TK Megapolis (formerly CJSC TK Megapolis), pursuant to Dutch law, PMI's distributor in Russia. On July 18, 2024, the Ministry of Industry and Trade filed a petition before the Arbitrazh Court of Moscow seeking the forced localization of JSC TK Megapolis. On December 5, 2024, JSC TK Megapolis registered the transfer of this equity interest to PMI affiliate ZAO Philip Morris Izhora. For further details, see Item 8, Note 6. Related Parties – Equity Investments and Other and Item 8, Note 18. Contingencies.
These developments above have or may have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in impairment charges.
For further details, see Item 1A. Risk Factors, Item 8, Note 4. War in Ukraine and the "Trade Policy" section of this Item 7.
Impact of Inflation on Our Business and Mitigation Efforts
Like many other global companies, we have experienced inflationary pressures in 2022, 2023 and 2024, including: growing pressures on the cost of certain direct materials, wages, energy, transportation, and logistics as well as an increased cost of capital due to interest rate increases driven by the response to increased inflation. For the year ended December 31, 2023, the impact on cost of sales was approximately $580 million. In 2024, the impact on cost of sales was approximately 30% of the 2023 level, benefiting from lower prices for direct materials and utilities, despite continued pressure on tobacco leaf costs. In 2025, we expect certain inflationary elements such as direct materials and utilities to stabilize, with a moderate overall increase in inflationary pressures driven by tobacco leaf costs.
Inflationary impacts driven by higher wages have resulted from merit increases that reflect local inflation as we continuously evaluate our compensation and benefit offerings to be competitive with the current market. Increased transportation costs resulted from increased shipping rates for all modes of transportation (air, ocean and inland) due to ocean and air capacity constraints. Increases in
cost of sales resulted from higher cost of direct materials due to the pass on of energy, transportation, labor and commodity price increases from suppliers as well as increases in utility costs, including gas and electricity prices, primarily in Europe resulting from the war in Ukraine. Raw materials such as tobacco leaf have longer inventory durations which resulted in insignificant inflationary impacts to our cost of sales in 2022; however tobacco leaf purchases in 2022, 2023 and 2024 were at higher prices due to inflationary impacts on fertilizer prices and labor costs, thus resulting in increases in the cost of inventory with corresponding impacts on our financial results in 2023, 2024 and 2025. In addition, our cash flow from operating activities, particularly in 2023, was impacted by the net working capital investment related to the procurement of tobacco leaf inventory and higher cost of direct materials.
We have taken several actions to mitigate these inflationary pressures. Mitigation efforts have included (i) indexation clauses related to commodity costs and energy pricing within contracts, (ii) tactical inventory purchases, (iii) identification of new suppliers in different geographical locations for incremental sourcing, (iv) increasing tobacco leaf inventory durations to secure additional volumes at favorable prices, (v) optimizing the mix of tobacco leaf origins and suppliers, (vi) continuous evaluation of shipping routes and methods of shipment, (vii) supplier negotiations, (viii) variable contract durations for energy costs, (ix) hedging strategies, and (x) other pricing, productivity and procurement initiatives.
Research and Development Expense
The research and development expense for our smoke-free portfolio accounted for approximately 100% of our total research and development expense for the year ended December 31, 2024 and approximately 99% for each of the two years ended December 31, 2023 and 2022. The research and development expense for the years ended December 31, 2024, 2023 and 2022, is set forth in Item 8, Note 15. Additional Information to the consolidated financial statements.
Restructuring Activities
We discuss restructuring activities in Item 8, Note 20. Restructuring Activities to our consolidated financial statements.
U.S. GAAP Treatment of Highly Inflationary Economies
We apply highly inflationary accounting to the results of operations of our subsidiaries in Argentina, Egypt, Turkey and Lebanon as the cumulative inflation rate in these economies for a three-year period meets or exceeds 100%, in accordance with U.S. GAAP. As a result, monetary assets and liabilities denominated in local currencies are remeasured to the U.S. Dollar at each balance sheet date, with remeasurement gains and losses recognized in consolidated statement of earnings.
This impact of currency fluctuations could negatively impact our financial condition and results of operations. Following the categorization of Egypt by the International Practices Task Force of the Center for Audit Quality as a country with a three-year cumulative inflation rate greater than 100%, the country is considered highly inflationary in accordance with U.S. GAAP. Consequently, PMI has begun to account for the operations of its Egyptian subsidiaries as highly inflationary, and treat the U.S. dollar as the functional currency of the subsidiaries, effective October 1, 2024. For the years ended December 31, 2024, 2023 and 2022, we recognized exchange gains (losses) of $(46) million, $(194) million, and $11 million, respectively, resulting from remeasurement adjustments related to highly inflationary accounting.
Climate Change Laws and Regulations
While, to date, the effect of climate-related laws and regulations on PMI has not been material to our business, results of operations or financial condition, consideration of environmental and climate-related laws and regulations is an integral aspect of PMI’s climate-related risk assessment process. To this end, we actively monitor the existing and potential impact on PMI of significant pending or existing climate change-related legislation, regulations, international accords, reporting frameworks, standards, principles, and other forms of guidance. Examples include, but are not limited to, the EU Emissions Trading System, the 2015 Paris Climate Agreement, the work of the International Financial Reporting Standards Foundation, including the International Sustainability Standards Board proposed climate standard and the recommendations of the Task Force on Climate-related Financial Disclosures, the SEC’s rules regarding climate-related disclosures, the California Climate Corporate Accountability Act, the California Greenhouse Gases: Climate-Related Financial Risk Act, the Task Force on Nature-related Financial Disclosures, the EU Corporate Sustainability Reporting Directive, the EU Taxonomy Regulation, the EU Deforestation Regulation, the EU Corporate Sustainability Due Diligence Directive, CDP, the GHG Protocol, and carbon tax programs in Europe and Canada.
Acquisitions, Divestitures and Other Business Arrangements
We discuss our acquisitions and divestitures in Item 8, Note 3. Acquisitions and Divestitures to our consolidated financial statements.
KT&G
On January 30, 2023, PMI announced a long-term collaboration with KT&G, South Korea’s leading tobacco and nicotine manufacturer, to continue to commercialize KT&G’s innovative smoke-free devices and consumables on an exclusive, worldwide basis (excluding South Korea).
The agreement covers fifteen years, to January 29, 2038, with performance-review cycles and associated commitments, based on volume, to be confirmed for each three-year period, to allow flexibility for evolving market conditions.
The agreement gives PMI continued exclusive access to KT&G’s smoke-free brands and product-innovation pipeline, including offerings for low- and middle-income markets, that will enhance PMI’s existing portfolio of smoke-free products.
Products sold under the agreement will be subject to assessment to ensure they meet the regulatory requirements in the markets where they are launched, as well as PMI’s high standards of quality and scientific substantiation. PMI and KT&G will seek any necessary regulatory approvals that may be required on a market-by-market basis.
On July 30, 2024, PMI announced a memorandum of understanding with KT&G. This non-binding memorandum establishes the parties’ intent to collaborate on regulatory submissions for those new KT&G heat-not-burn products that PMI selects to commercialize in the U.S. KT&G’s new platform products are expected to be launched first outside the U.S. Thereafter, the partners plan to work on a PMTA submission for review by the U.S. FDA, in accordance with the memorandum.
Equity Investments
We discuss our equity investments in Item 8, Note 6. Related Parties - Equity Investments and Other to our consolidated financial statements.
Trade Policy
PMI complies with all applicable trade restrictions and requirements, including sanctions, in the markets in which it operates. We have taken appropriate actions in response to the latest sanctions to ensure full compliance with the relevant restrictions.
We are subject to various trade restrictions imposed by the U.S., the EU, Switzerland, the U.K., and other jurisdictions in which we do business (“Trade Sanctions”), including the trade and economic sanctions administered by the U.S. Department of the Treasury's Office of Foreign Assets Control (“OFAC”) and the U.S. Department of State. It is our policy to comply fully with these Trade Sanctions.
Pursuant to specific exemptions or licenses, or where sanctions do not apply to our business, PMI may make sales in countries subject to Trade Sanctions.
We do not do business or sell products in Belarus, Iran, North Korea or Syria.
We sell cigarettes in Cuba under a distribution agreement. These sales are permitted by U.S. law under a License Exception for Agricultural Commodities, issued by the U.S. Department of Commerce (Bureau of Industry and Security), and specifically granted to our distributor.
Certain states within the U.S. have enacted legislation permitting or requiring state pension funds to divest or abstain from future investment in stocks of companies that do business with certain countries that are sanctioned by the U.S. Because we do business in certain of these countries, consistent with our policy to fully comply with Trade Sanctions and as described above, these state pension funds may have divested of our stock or may not invest in our stock. We do not believe such legislation has had a material effect on the price of our shares.
Following the start of the conflict in Ukraine on February 24, 2022, the U.S., the EU, the U.K., Switzerland, Canada, Australia, New Zealand, Singapore, South Korea, Japan and other countries introduced extensive economic sanctions and export controls in relation
to Russia. While the introduced sanctions vary from jurisdiction to jurisdiction, they are largely aligned. The restrictions target, among others, the Russian financial, banking, oil, military, aviation and marine sectors. The U.S. has also introduced a prohibition on new investment in the Russian Federation by a U.S. person, wherever located, and authorized the imposition of blocking sanctions on anyone operating in the Russian manufacturing sector. Among sanctions targets are Russian political figures and military personnel, certain oligarchs and journalists, and companies operating in the above-mentioned sectors. Export to Russia of certain luxury goods, and goods and technology which might contribute to Russia’s technological enhancement was banned. Seven non-EU countries (Norway, Iceland, Liechtenstein, North Macedonia, Bosnia and Herzegovina, Montenegro, and Albania) announced that they “aligned themselves” with the majority of the EU sanctions. The U.S., the EU, Switzerland and Japan introduced additional trade restrictions banning, among many other goods, the export of certain non-tobacco materials used to produce cigarettes and heated tobacco consumables in Russia. The EU, Switzerland and the U.K. also prohibited technical assistance and other services related to restricted goods. The EU, Switzerland and the U.K. prohibited import into their territories of certain goods, including cigarettes, among others, which might generate significant revenues for Russia if they originate in Russia or are exported from Russia. The EU and Switzerland prohibited transfer and licensing of intellectual property rights in relation to restricted goods. Additionally, the EU, the U.S., the U.K., Switzerland, Canada, Australia, New Zealand and Ukraine imposed sanctions on Mr. Igor Kesaev, a non-majority shareholder of Megapolis Distribution B.V.
The U.S., the U.K., Switzerland and the EU banned the export of electric accumulators, static converters and electronic cigarettes and similar personal electric vaporizing devices, including IQOS devices, to Russia. Certain countries have also banned the delivery of services to Russia, such as information technology consultancy services, accounting and business and management consulting services, or require licenses to continue delivering these services to Russian persons or entities. We are working to mitigate any potential impacts from these restrictions.
Russia introduced certain countermeasures aimed at reducing the effect of Western sanctions. Countermeasures include restrictions on export of certain goods from Russia, including tobacco-related production equipment, restrictions on lending to foreign borrowers, repatriation of dividends and transactions with securities and real estate involving companies from “hostile” countries (i.e., those which introduced sanctions in relation to Russia).
PMI continues to monitor the development of new sanctions and ensure full compliance.
Financial Review
| | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | 2023 | 2022 |
| Net cash provided by (used in) operating activities | $ | 12,217 | | $ | 9,204 | | $ | 10,803 | |
| Net cash provided by (used in) investing activities | (1,092) | | (3,598) | | (15,679) | |
| Net cash provided by (used in) financing activities | (9,481) | | (5,582) | | 3,806 | |
2024 compared with 2023
•Net Cash Provided by Operating Activities
Net cash provided by operating activities of $12.2 billion for the year ended December 31, 2024 increased by $3.0 billion from the comparable 2023 period. Excluding unfavorable currency movements of $0.4 billion, the favorable variance of $3.4 billion was due primarily to lower working capital requirements of $1.5 billion and higher currency-neutral net earnings, excluding non-cash depreciation and amortization expense, impairment charges of goodwill and other intangibles, the loss on the sale of Vectura Group and the impairment charge related to the RBH equity investment.
The unfavorable currency movements primarily related to the currency impact on net earnings and represented the fluctuations of the U.S. dollar, especially against the Egyptian pound, Euro, Japanese yen and Russian ruble.
The lower working capital requirements in 2024 as compared with 2023 were primarily due to more cash provided by inventories, coupled with more cash provided by accrued liabilities and other current assets, mainly reflecting the timing of excise tax-paid inventory movements primarily related to excise tax increases and the timing of the corresponding excise tax payments and less cash used in accounts payable in 2024 primarily due to comparison with 2023 payment for higher IQOS ILUMA device purchases in the fourth quarter of 2022 to meet the needs of ILUMA launches. The favorable 2024 working capital comparison to 2023 is also impacted by higher 2023 working capital requirements following the higher cost of tobacco leaf and other direct materials due to inflationary pressures (for further details, see “Impact of Inflation on Our Business and Mitigation Efforts” section of this MD&A), as well as tactical stock increases for certain direct materials. These changes in the working capital requirements were partly offset by more cash used in accounts receivable mainly reflecting lower usage of our factoring arrangements to sell trade receivables, as well as the timing of sales and cash collections. For further detail on our factoring arrangements, see Item 8, Note 19. Sale of Accounts Receivable.
For the full year 2025, we currently expect net cash provided by operating activities of around $11 billion at prevailing exchange rates, subject to year-end working capital requirements. This takes into account non-recurring payments relating to the German tax surcharge and the U.S. Tax Cuts and Jobs Act, which amount to approximately $1 billion.
•Net Cash Used in Investing Activities
Net cash used in investing activities of $1.1 billion for the year ended December 31, 2024 decreased by $2.5 billion from the comparable 2023 period. This decrease in net cash used was primarily due to the remaining cash payment (including interest) to Altria Group, Inc. in July 2023 of $1.8 billion for PMI to reacquire the IQOS commercialization rights in the U.S., and higher cash collateral received for derivative instruments, partially offset by higher capital expenditures.
Our capital expenditures were $1.4 billion in 2024 and $1.3 billion in 2023. The 2024 expenditures were primarily related to our ongoing investments in smoke-free product manufacturing capacity. We expect total capital expenditures in 2025 of approximately $1.5 billion, including further investments in ZYN capacity in the U.S.
•Net Cash Provided by (Used in) Financing Activities
Net cash used in financing activities of $9.5 billion for the year ended December 31, 2024 increased by $3.9 billion from the comparable 2023 period. The increase in net cash used was primarily due to lower net borrowings in 2024, reflecting higher repayments on long-term debt, lower long-term debt proceeds and higher repayments on short-term borrowings (primarily commercial paper). The increase was partly offset by favorable comparisons to 2023 mainly reflecting repayment on a credit facility in 2023 related to the Swedish Match acquisition, and payments in 2023 to acquire the remaining issued and outstanding shares in Swedish Match, as well as higher cash collateral received for derivative instruments.
Dividends paid in 2024 and 2023 were $8.2 billion and $8.0 billion, respectively.
2023 compared with 2022
For a discussion comparing our net cash activities (operating, investing and financing) for the year ended December 31, 2023, with the year ended December 31, 2022, refer to Part II, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation - Financial Review in our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the U.S. Securities and Exchange Commission on February 8, 2024. This section is incorporated by reference into this Annual Report on Form 10-K for the year ended December 31, 2024.
•Debt and Liquidity
We define cash and cash equivalents as short-term, highly liquid investments, readily convertible to known amounts of cash that mature within a maximum of three months and have an insignificant risk of change in value due to interest rate or credit risk changes. As a policy, we do not hold any investments in structured or equity-linked products. Our cash and cash equivalents are mostly held with institutions that have investment-grade long-term credit rating.
In a number of jurisdictions, including Argentina and Russia, we are impacted by various capital controls and/or foreign currency exchange constraints that affect the ability of our subsidiaries in these jurisdictions to settle foreign currency denominated imports of goods and services and/or to pay dividends. These factors increase foreign currency devaluation risks, which may have a negative impact on our financial condition, net assets and results of operations in these jurisdictions.
We utilize long-term and short-term debt financing, including a commercial paper program that is regularly used to finance ongoing liquidity requirements, as part of our overall cash management strategy. Our ability to access the capital and credit markets as well as overall dynamics of these markets may impact borrowing costs. We expect that the combination of our long-term and short-term debt financing, the commercial paper program and the committed credit facilities, coupled with our operating cash flows, will enable us to meet our liquidity requirements.
Credit Ratings – The cost and terms of our financing arrangements as well as our access to commercial paper markets may be affected by applicable credit ratings. At February 6, 2025, our credit ratings and outlook by major credit rating agencies were as follows:
| | | | | | | | | | | |
| Short-term | Long-term | Outlook |
| Moody’s | P-1 | A2 | Stable |
| Standard & Poor’s | A-2 | A- | Stable |
| Fitch | F1 | A | Negative |
Revolving Credit Facilities
On December 17, 2024, we entered into a credit agreement, effective as of January 29, 2025, relating to a senior unsecured revolving credit facility with borrowings up to an aggregate principal amount of €1.5 billion, (approximately $1.6 billion) expiring on January 29, 2028. Concurrently, we did not request an extension of the maturity date of the existing 364-day revolving credit facility and the facility matured on January 28, 2025.
At February 6, 2025, our committed revolving credit facilities were as follows:
| | | | | | | | | | |
Type (in billions) | | Committed Revolving Credit Facilities | | |
Multi-year $2.0 billion revolving credit, expiring February 10, 2026 (1) | | $ | 2.0 | | | |
Multi-year $2.5 billion revolving credit, expiring September 29, 2026 (2) (3) | | 2.5 | | | |
| Multi-year €1.5 billion revolving credit, expiring January 29, 2028 | | 1.6 | | | |
| Total facilities | | $ | 6.1 | | | |
| | | | |
(1) On January 28, 2022, we entered into an agreement, effective February 10, 2022, to amend and extend the term of our $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(2) Includes business transformation-linked pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets based on its business transformation goals.
(3) On September 20, 2022, we entered into an agreement, effective September 29, 2022, to amend and extend the term of our $2.5 billion multi-year revolving credit facility, for an additional year covering the period September 30, 2026 to September 29, 2027, in the amount of $2.3 billion. On September 20, 2023, PMI entered into an agreement, effective September 29, 2023, to amend and further extend the term to September 29, 2028.
At February 6, 2025, there were no borrowings under the committed revolving credit facilities, and the entire committed amounts were available for borrowing.
All banks participating in our committed revolving credit facilities have an investment-grade long-term credit rating from the credit rating agencies. We continuously monitor the credit quality of our banking group, and at this time we are not aware of any potential non-performing credit provider.
These committed revolving credit facilities do not include any credit rating triggers, material adverse change clauses or any provisions that could require us to post collateral. We expect to continue to meet our covenants.
In addition to the committed revolving credit facilities discussed above, PMI maintains certain short-term credit arrangements, including uncommitted credit lines, to primarily meet working capital needs. These credit arrangements amounted to approximately $2.1 billion at December 31, 2024 and approximately $2.7 billion at December 31, 2023. Borrowings under these arrangements and other bank loans amounted to $137 million at December 31, 2024, and $283 million at December 31, 2023.
Term Loan Facility related to the Financing of the Swedish Match Acquisition – On June 23, 2022, PMI entered into a €5.5 billion (approximately $5.8 billion at the date of signing) senior unsecured term loan credit agreement consisting of a €3.0 billion (approximately $3.2 billion at the date of signing) tranche expiring three years after the occurrence of certain events and a €2.5 billion (approximately $2.6 billion at the date of signing) tranche expiring on June 23, 2027.
On November 7, 2022, PMI delivered notices of borrowing for advances totaling €5.5 billion under the term loan facility, of which €3.0 billion would become due on November 9, 2025, and €2.5 billion would become due on June 23, 2027, unless prepaid pursuant to the terms of the credit agreement.
On November 21, 2024, PMI prepaid approximately €3 billion (approximately $3.2 billion), including outstanding principal and accrued interest, representing all borrowings outstanding under the 3-year tranche of the senior unsecured term loan facility. As of December 31, 2024, borrowings in the amount of €2.5 billion (approximately $2.6 billion) under the 5-year tranche of the term loan facility remained outstanding.
Commercial Paper Program – We continue to have access to liquidity in the commercial paper market through programs in place in the U.S. and in Europe having an aggregate issuance capacity of $8.0 billion. At December 31, 2024, we had no commercial paper
outstanding. At December 31, 2023, we had $1.7 billion of commercial paper outstanding. The average commercial paper balance outstanding during 2024 and 2023 was $1.3 billion and $3.6 billion, respectively.
Sale of Accounts Receivable – To mitigate credit risk and enhance cash and liquidity management, we sell trade receivables to unaffiliated financial institutions. For further details, see Item 8, Note 19. Sale of Accounts Receivable to our consolidated financial statements.
Supply Chain Financing – We engage with unaffiliated global financial institutions that offer a voluntary supply chain financing program to some of our suppliers. For further details, see Item 8, Note 22. Supply Chain Financing to our consolidated financial statements.
Debt – Our total debt was $45.7 billion at December 31, 2024, and $47.9 billion at December 31, 2023. Our total debt is primarily fixed rate in nature. The weighted-average all-in financing cost of our total debt was 3.5% in 2024 and 3.3% in 2023. For further details, including the fair value of our debt, see Item 8, Note 8. Indebtedness. The amount of debt that we can issue is subject to approval by our Board of Directors.
On February 10, 2023, we filed a shelf registration statement with the U.S. Securities and Exchange Commission, under which we may from time to time sell debt securities and/or warrants to purchase debt securities over a three-year period.
Our debt issuances in 2024 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | | | | | | | |
| Type | | Face Value | | Interest Rate | | Issuance | | Maturity |
| U.S. dollar notes | (a) | $750 | | 4.750% | | February 2024 | | February 2027 |
| U.S. dollar notes | (a) | $1,000 | | 4.875% | | February 2024 | | February 2029 |
| U.S. dollar notes | (a) | $1,250 | | 5.125% | | February 2024 | | February 2031 |
| U.S. dollar notes | (a) | $1,750 | | 5.250% | | February 2024 | | February 2034 |
| Euro notes | (b) (c) | €500 (approximately $543) | | 3.750% | | June 2024 | | January 2031 |
| U.S. dollar notes | (d) | $750 | | 4.375% | | November 2024 | | November 2027 |
| U.S. dollar notes | (d) | $750 | | 4.625% | | November 2024 | | November 2029 |
| U.S. dollar notes | (d) | $750 | | 4.750% | | November 2024 | | November 2031 |
| U.S. dollar notes | (d) | $750 | | 4.900% | | November 2024 | | November 2034 |
| | | | | | | | |
| | | | | | | | |
(a) Interest is payable semi-annually, commencing in August 2024
(b) Interest is payable annually, commencing in January 2025
(c) USD equivalents for foreign currency notes were calculated based on exchange rates on the date of issuance.
(d) Interest is payable semi-annually, commencing in May 2025
The net proceeds from the sale of the securities listed in the table above were primarily used for general corporate purposes, including working capital requirements, repayment of commercial paper and to refinance certain of our outstanding notes. On November 21, 2024, PMI financed the prepayment of the 3-year tranche of the senior unsecured term loan facility with the proceeds of the November 2024 bond issuances and cash on hand.
The weighted-average time to maturity of our long-term debt was approximately 7 years at the end of 2024 and 2023.
Cash Requirements – At December 31, 2024, our material short-term and long-term cash requirements for various contractual obligations and commitments primarily consisted of the following:
•principal payments related to long-term debt and the associated interest payments. For further details, see Item 8, Note 8. Indebtedness to our consolidated financial statements;
•accounts payable and accrued liabilities on our consolidated balance sheet (primarily short-term in nature);
•purchase obligations for inventory and production costs to be utilized in the normal course of business such as raw materials, electronic devices, indirect materials and supplies, packaging, co-manufacturing arrangements, storage and distribution, as well as capital expenditures. These purchase obligations are expected to be approximately $3.0 billion in 2025 and approximately $1.7 billion for years beyond; and
•operating lease liabilities, on an undiscounted basis, which were included in our consolidated balance sheets. For further details, see Item 8, Note 21. Leases to our consolidated financial statements.
•Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including special purpose entities, other than guarantees, and cash requirements discussed above.
Guarantees – At December 31, 2024, we have guarantees of our own performance, which are primarily related to excise taxes on the shipment of our products. There is no liability in the consolidated financial statements associated with these guarantees. These guarantees have not had, and are not expected to have, a significant impact on PMI’s liquidity.
In August 2024, PMI entered into a guarantee agreement for an equity investee. For further details, see Item 8, Note 6. Related Parties – Equity Investments and Other.
Swedish Match Notes Consent Solicitation and PMI Guarantee – On June 15, 2023, our wholly owned subsidiary, Swedish Match AB ("Swedish Match"), initiated a public consent solicitation of eligible holders of certain outstanding series of its notes to amend certain terms and conditions of these respective notes. The eligible noteholders provided the requisite irrevocable consent instructions voting in favor of the amendments, which were subsequently passed by way of extraordinary resolution at the noteholders’ meeting held on July 28, 2023. As a result of the passage of the extraordinary resolution, Philip Morris International Inc. entered into a guarantee, which guarantees unconditionally and irrevocably to the noteholders the punctual payment when due, whether at stated maturity, by acceleration or otherwise, of the principal, premium, if any, and interest on the notes.
Equity and Dividends
We discuss our stock awards as of December 31, 2024, in Item 8, Note 10. Stock Plans to our consolidated financial statements.
On June 11, 2021, our Board of Directors authorized a share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period. On July 22, 2021, we began repurchasing shares under this share repurchase program. On May 11, 2022, we announced the suspension of our three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. We did not make any share repurchases in 2023 and 2024. Our three-year share repurchase program expired on July 21, 2024.
Dividends paid in 2024 were $8.2 billion. During the third quarter of 2024, our Board of Directors approved a 3.8% increase in the quarterly dividend to $1.35 per common share. As a result, the present annualized dividend rate is $5.40 per common share.
Market Risk
Counterparty Risk - We predominantly work with financial institutions with strong short- and long-term credit ratings as assigned by Standard & Poor’s and Moody’s. These banks are also part of a defined group of relationship banks. Non-investment grade institutions are only used in certain emerging markets to the extent required by local business needs. We have a conservative approach when it comes to choosing financial counterparties and financial instruments. As such we do not invest or hold investments in any structured or equity-linked products. The majority of our cash and cash equivalents is currently invested with maturities of less than 30 days.
We continuously monitor and assess the credit worthiness of all our counterparties.
Derivative Financial Instruments - We operate globally with manufacturing and sales facilities in various locations around the world. Consequently, we use certain financial instruments to manage our foreign currency and interest rate exposure. We use derivative financial instruments principally to reduce our exposure to market risks resulting from fluctuations in foreign exchange and interest rates by creating offsetting exposures. We are not a party to leveraged derivatives and, by policy, do not use derivative financial instruments for speculative purposes.
See Item 8, Note 16. Financial Instruments to our consolidated financial statements for further details on our derivative financial instruments and the related collateral arrangements.
Value at Risk - We use a value at risk computation to estimate the potential one-day loss in the fair value of our interest-rate-sensitive and foreign currency price-sensitive derivative financial instruments, representing the majority of our derivative financial instruments exposure. This computation includes our debt and foreign currency forwards, swaps and options. Anticipated transactions, foreign currency trade payables and receivables, and net investments in foreign subsidiaries, which the foregoing instruments are intended to hedge, were excluded from the computation.
The computation estimates were made assuming normal market conditions, using a 95% confidence interval and a one-day holding period using a "parametric delta-gamma" approximation technique to determine the observed interrelationships between movements in interest rates and various currencies and in calculating the risk of the underlying positions in the portfolio. These interrelationships were determined by observing interest rate and forward currency rate movements primarily over the preceding quarter for determining value at risk at December 31, 2024 and 2023, and primarily over each of the four preceding quarters for the calculation of average, high and low value at risk amounts during each year.
| | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Impact |
| (in millions) | At December 31, 2024 | | Average | | High | | Low |
| Instruments sensitive to: | | | | | | | |
| Foreign currency rates | $130 | | $92 | | $130 | | $69 |
| | | | | | | |
| Interest rates | $221 | | $233 | | $272 | | $200 |
| | | | | | | |
| Fair Value Impact |
| (in millions) | At December 31, 2023 | | Average | | High | | Low |
| Instruments sensitive to: | | | | | | | |
| Foreign currency rates | $77 | | $74 | | $82 | | $66 |
| | | | | | | |
| Interest rates | $297 | | $332 | | $505 | | $219 |
Year-over-year increases of the impact of foreign currency rates and decreases of the impact of interest rates on the value at risk computation above was primarily due to higher foreign currency volatility and lower interest rate volatility in 2024 compared to 2023.
The value at risk computation is a risk analysis tool designed to statistically estimate the maximum probable daily loss from adverse movements in interest and foreign currency rates under normal market conditions. The computation does not purport to represent actual losses in fair value or earnings to be incurred by us, nor does it consider the effect of favorable changes in market rates. We cannot predict actual future movements in such market rates and do not present these results to be indicative of future movements in market rates or to be representative of any actual impact that future changes in market rates may have on our future results of operations or financial position.
Contingencies
See Item 3 and Item 8, Note 18. Contingencies to our consolidated financial statements for a discussion of contingencies.
Cautionary Factors That May Affect Future Results
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "aspires," "estimates," "intends," "projects," "aims," "goals," "targets," "forecasts" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Our SFPs constitute a relatively new product category that is less predictable than our mature cigarette business.
Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in Item 1A. Risk Factors and Business Environment of this section. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk.
The information called for by this Item is included in Item 7, Market Risk.
Item 8.Financial Statements and Supplementary Data.
Consolidated Statements of Earnings
(in millions of dollars, except per share data)
| | | | | | | | | | | | | | | | | |
| for the years ended December 31, | 2024 | | 2023 | | 2022 |
| | | | | |
| | | | | |
Net revenues 1 & 2 (Notes 6 & 13) | $ | 37,878 | | | $ | 35,174 | | | $ | 31,762 | |
| Cost of sales (Notes 4 & 5) | 13,329 | | | 12,893 | | | 11,402 | |
| Gross profit | 24,549 | | | 22,281 | | | 20,360 | |
| Marketing, administration and research costs (Notes 3, 4, 5, 13 & 20) | 11,147 | | | 10,060 | | | 8,114 | |
| Impairment of goodwill (Note 5) | — | | | 665 | | | — | |
| Operating income | 13,402 | | | 11,556 | | | 12,246 | |
| Interest expense, net (Note 15) | 1,143 | | | 1,061 | | | 588 | |
| Pension and other employee benefit costs (Note 14) | 60 | | | 45 | | | 24 | |
| Earnings before income taxes | 12,199 | | | 10,450 | | | 11,634 | |
| Provision for income taxes (Note 12) | 3,017 | | | 2,339 | | | 2,244 | |
| Impairment related to the RBH equity investment (Note 6) | 2,316 | | | — | | | — | |
| Equity investments and securities (income)/loss, net | (637) | | | (157) | | | (137) | |
| Net earnings | 7,503 | | | 8,268 | | | 9,527 | |
| Net earnings attributable to noncontrolling interests | 446 | | | 455 | | | 479 | |
| Net earnings attributable to PMI | $ | 7,057 | | | $ | 7,813 | | | $ | 9,048 | |
| Per share data (Note 11): | | | | | |
| Basic earnings per share | $ | 4.53 | | | $ | 5.02 | | | $ | 5.82 | |
| Diluted earnings per share | $ | 4.52 | | | $ | 5.02 | | | $ | 5.81 | |
(1) Includes net revenues from related parties of $3,876 million, $3,553 million and $3,658 million for the years ended December 31, 2024, 2023 and 2022, respectively
(2) Net revenues are shown net of excise tax on products. For the years ended December 31, 2024, 2023 and 2022, excise tax on products was $51,563 million, $49,404 million and $48,958 million, respectively
See notes to consolidated financial statements.
Consolidated Statements of Comprehensive Earnings
(in millions of dollars)
| | | | | | | | | | | | | | | | | |
| for the years ended December 31, | 2024 | | 2023 | | 2022 |
| Net earnings | $ | 7,503 | | | $ | 8,268 | | | $ | 9,527 | |
| Other comprehensive earnings (losses), net of income taxes: | | | | | |
| Change in currency translation adjustments: | | | | | |
| Unrealized gains (losses), net of income taxes of $(208) in 2024, $156 in 2023 and $(169) in 2022 | (122) | | | (1,643) | | | (1,268) | |
| (Gains)/losses transferred to earnings, net of income taxes of $7 in 2024, $0 in 2023 and 2022 (Notes 3, 17 & 20) | 171 | | | 12 | | | — | |
| | | | | |
| Change in net loss and prior service cost: | | | | | |
| Net gains (losses) and prior service costs, net of income taxes of $8 in 2024, $182 in 2023 and $(132) in 2022 | (10) | | | (861) | | | 843 | |
Amortization of net losses, prior service costs and net transition costs, net of income taxes of $(36) in 2024, $(28) in 2023 and $(49) in 2022 | 135 | | | 87 | | | 217 | |
| | | | | |
| Change in fair value of derivatives accounted for as hedges: | | | | | |
Gains (losses) recognized, net of income taxes of $(88) in 2024, $(30) in 2023 and $(99) in 2022 | 439 | | | 195 | | | 481 | |
(Gains) losses transferred to earnings, net of income taxes of $45 in 2024, $32 in 2023 and $35 in 2022 | (213) | | | (220) | | | (219) | |
| | | | | |
| Total other comprehensive earnings (losses) | 400 | | | (2,430) | | | 54 | |
| Total comprehensive earnings | 7,903 | | | 5,838 | | | 9,581 | |
| Less comprehensive earnings attributable to: | | | | | |
| Noncontrolling interests | 345 | | | 281 | | | 515 | |
| | | | | |
| Comprehensive earnings attributable to PMI | $ | 7,558 | | | $ | 5,557 | | | $ | 9,066 | |
See notes to consolidated financial statements.
Consolidated Balance Sheets
(in millions of dollars, except share data)
| | | | | | | | | | | |
| at December 31, | 2024 | | 2023 |
| Assets | | | |
| Cash and cash equivalents | $ | 4,216 | | | $ | 3,060 | |
Trade receivables (less allowances of $47 in 2024 and $79 in 2023) (1) | 3,789 | | | 3,461 | |
| Other receivables (less allowances of $22 in 2024 and $35 in 2023) | 886 | | | 930 | |
| Inventories: | | | |
| Leaf tobacco | 2,080 | | | 1,942 | |
| Other raw materials | 2,261 | | | 2,293 | |
| Finished product | 5,112 | | | 6,539 | |
| 9,453 | | | 10,774 | |
| | | |
| Other current assets | 1,826 | | | 1,530 | |
| Total current assets | 20,170 | | | 19,755 | |
| Property, plant and equipment, at cost: | | | |
| Land and land improvements | 581 | | | 550 | |
| Buildings and building equipment | 4,391 | | | 4,617 | |
| Machinery and equipment | 10,632 | | | 10,713 | |
| Construction in progress | 1,081 | | | 1,200 | |
| 16,685 | | | 17,080 | |
| Less: accumulated depreciation | 9,375 | | | 9,564 | |
| 7,310 | | | 7,516 | |
| Goodwill (Note 5) | 16,600 | | | 16,779 | |
| Other intangible assets, net (Note 5) | 11,327 | | | 9,864 | |
| Equity investments (Note 6) | 2,654 | | | 4,929 | |
| Deferred income taxes | 940 | | | 814 | |
| Other assets (less allowances of $26 in 2024 and $25 in 2023) (Note 3) | 2,783 | | | 5,647 | |
| Total Assets | $ | 61,784 | | | $ | 65,304 | |
(1) Includes trade receivables from related parties of $691 million and $710 million as of December 31, 2024, and 2023, respectively. For further details, see Note 6. Related Parties - Equity Investments and Other.
See notes to consolidated financial statements.
| | | | | | | | | | | |
| at December 31, | 2024 | | 2023 |
| Liabilities | | | |
| Short-term borrowings (Note 8) | $ | 137 | | | $ | 1,968 | |
| Current portion of long-term debt (Note 8) | 3,392 | | | 4,698 | |
| Accounts payable | 3,952 | | | 4,143 | |
| Accrued liabilities: | | | |
| Marketing and selling | 1,015 | | | 862 | |
| Taxes, except income taxes | 6,904 | | | 7,514 | |
| Employment costs | 1,305 | | | 1,262 | |
| Dividends payable | 2,120 | | | 2,041 | |
| Other | 2,832 | | | 2,737 | |
| Income taxes | 1,258 | | | 1,158 | |
| | | |
| Total current liabilities | 22,915 | | | 26,383 | |
| Long-term debt (Note 8) | 42,166 | | | 41,243 | |
| Deferred income taxes | 2,517 | | | 2,335 | |
| Employment costs | 2,940 | | | 3,046 | |
| Income taxes and other liabilities (Note 12) | 1,116 | | | 1,743 | |
| Total liabilities | 71,654 | | | 74,750 | |
| Contingencies (Note 18) | | | |
Stockholders’ (Deficit) Equity | | | |
| Common stock, no par value (2,109,316,331 shares issued in 2024 and 2023) (Note 9) | — | | | — | |
| Additional paid-in capital | 2,335 | | | 2,285 | |
| Earnings reinvested in the business | 32,869 | | | 34,090 | |
| Accumulated other comprehensive losses (Note 17) | (11,314) | | | (11,815) | |
| 23,890 | | | 24,560 | |
| Less: cost of repurchased stock (554,470,731 and 556,891,800 shares in 2024 and 2023, respectively) | 35,640 | | | 35,785 | |
| Total PMI stockholders’ deficit | (11,750) | | | (11,225) | |
| Noncontrolling interests | 1,880 | | | 1,779 | |
| Total stockholders’ deficit | (9,870) | | | (9,446) | |
| Total Liabilities and Stockholders’ (Deficit) Equity | $ | 61,784 | | | $ | 65,304 | |
See notes to consolidated financial statements.
Consolidated Statements of Cash Flows
(in millions of dollars)
| | | | | | | | | | | | | | | | | | |
| for the years ended December 31, | 2024 | | 2023 | | 2022 | |
| CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | | | | | | |
| Net earnings | $ | 7,503 | | | $ | 8,268 | | | $ | 9,527 | | |
| Adjustments to reconcile net earnings to operating cash flows: | | | | | | |
| Depreciation and amortization expense | 1,787 | | | 1,398 | | | 1,077 | | |
| Impairment of goodwill and other intangibles (Note 5) | 27 | | | 680 | | | 112 | | |
| Loss on sale of Vectura Group (Note 3) | 206 | | | — | | | — | | |
| Impairment related to the RBH equity investment (Note 6) | 2,316 | | | — | | | — | | |
| Deferred income tax (benefit) provision | (123) | | | (330) | | | (234) | | |
| Restructuring charges, net of cash paid (Note 20) | 122 | | | 30 | | | (93) | | |
| Cash effects of changes, net of the effects from acquired and divested companies: | | | | | | |
| Receivables, net | (738) | | | 314 | | | (871) | | |
| Inventories | 552 | | | (862) | | | (1,287) | | |
| Accounts payable | 297 | | | (288) | | | 719 | | |
| Accrued liabilities and other current assets | 628 | | | (232) | | | 1,862 | | |
| Income taxes | (62) | | | (232) | | | (261) | | |
| Pension plan contributions, net of refunds (Note 14) | (110) | | | (21) | | | 3 | | |
| Other | (188) | | | 479 | | | 249 | | |
| Net cash provided (used in) by operating activities | 12,217 | | | 9,204 | | | 10,803 | | |
| CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | | | | | | |
| Capital expenditures | (1,444) | | | (1,321) | | | (1,077) | | |
| Acquisition of Swedish Match AB, net of acquired cash (Note 3) | — | | | — | | | (13,976) | | |
| Other acquisitions, net of acquired cash (Note 3) | 43 | | | — | | | — | | |
| Altria Group, Inc. agreement (Note 3) | — | | | (1,775) | | | (1,002) | | |
| Proceeds from sale of business, net of cash disposed (Note 3) | 136 | | | 191 | | | — | | |
| Equity investments | (124) | | | (111) | | | (20) | | |
| | | | | | |
| | | | | | |
| Collateral posted/settlements for derivatives, (paid)/returned (Note 16) | 351 | | | (660) | | | 284 | | |
| Other | (54) | | | 78 | | | 112 | | |
| Net cash provided by (used in) investing activities | (1,092) | | | (3,598) | | | (15,679) | | |
See notes to consolidated financial statements.
| | | | | | | | | | | | | | | | | |
| for the years ended December 31, | 2024 | | 2023 | | 2022 |
| CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | | | | | |
| Short-term borrowing activity by original maturity: | | | | | |
| Net issuances (repayments) - maturities of 90 days or less | $ | (1,461) | | | $ | 530 | | | $ | 876 | |
| Issuances - maturities longer than 90 days | 100 | | | 1,366 | | | 934 | |
| Repayments - maturities longer than 90 days | (433) | | | (1,172) | | | (795) | |
| Borrowings under credit facilities related to Swedish Match AB acquisition | — | | | — | | | 13,920 | |
| Repayments under credit facilities related to Swedish Match AB acquisition | (3,168) | | | (4,430) | | | (4,000) | |
| Long-term debt proceeds | 8,142 | | | 9,959 | | | 5,965 | |
| Long-term debt repaid | (4,803) | | | (2,551) | | | (2,724) | |
| Repurchases of common stock | — | | | — | | | (209) | |
| | | | | |
| Dividends paid | (8,197) | | | (7,964) | | | (7,812) | |
| | | | | |
| Collateral received/settlements for derivatives, received/(returned) | 828 | | | (62) | | | 27 | |
| Payments to acquire Swedish Match AB noncontrolling interests (Note 3) | — | | | (883) | | | (1,495) | |
| Noncontrolling interests activity and Other (Note 3) | (489) | | | (375) | | | (881) | |
| Net cash provided by (used in) financing activities | (9,481) | | | (5,582) | | | 3,806 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (536) | | | (95) | | | (213) | |
Cash, cash equivalents and restricted cash(1): | | | | | |
| Increase (Decrease) | 1,108 | | | (71) | | | (1,283) | |
| Balance at beginning of year | 3,146 | | | 3,217 | | | 4,500 | |
| Balance at end of year | $ | 4,254 | | | $ | 3,146 | | | $ | 3,217 | |
| | | | | |
| Cash Paid: | | | | | |
| Interest | $ | 1,559 | | | $ | 1,342 | | | $ | 717 | |
| Income taxes | $ | 3,178 | | | $ | 2,952 | | | $ | 2,751 | |
(1) The amounts for cash, cash equivalents and restricted cash shown above include restricted cash of $38 million, $86 million and $10 million as of December 31, 2024, 2023 and 2022, respectively, which were included in other current assets in the consolidated balance sheets.
See notes to consolidated financial statements.
Consolidated Statements of Stockholders' (Deficit) Equity
(in millions of dollars, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| PMI Stockholders’ (Deficit) Equity | | | | | | |
| Common
Stock | | Additional
Paid-in
Capital | | Earnings Reinvested
in the Business | | Accumulated Other
Comprehensive
Losses | | Cost of
Repurchased
Stock | | Noncontrolling
Interests | | | Total | |
| Balances, January 1, 2022 | $ | — | | | $ | 2,225 | | | $ | 33,082 | | | $ | (9,577) | | | $ | (35,836) | | | $ | 1,898 | | | | $ | (8,208) | | |
| Net earnings | | | | | 9,048 | | | | | | | 479 | | | | 9,527 | | |
| Other comprehensive earnings (losses), net of income taxes | | | | | | | 189 | | | | | (135) | | | | 54 | | |
| Issuance of stock awards (Note 10) | | | 37 | | | | | | | 118 | | | | | | 155 | | |
| Dividends declared ($5.04 per share) | | | | | (7,841) | | | | | | | | | | (7,841) | | |
| Dividends paid to noncontrolling interests | | | | | | | | | | | (472) | | | | (472) | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Common stock repurchased | | | | | | | | | (199) | | | | | | (199) | | |
| Acquisitions (Note 3) | | | | | | | | | | | 2,379 | | | | 2,379 | | |
| Purchases of shares from noncontrolling interests (Note 3) | | | (32) | | | | | (171) | | | | | (1,503) | | | | (1,706) | | |
| Balances, December 31, 2022 | — | | | 2,230 | | | 34,289 | | | (9,559) | | | (35,917) | | | 2,646 | | | | (6,311) | | |
| Net earnings | | | | | 7,813 | | | | | | | 455 | | | | 8,268 | | |
| Other comprehensive earnings (losses), net of income taxes | | | | | | | (2,436) | | | | | 6 | | | | (2,430) | | |
| Issuance of stock awards (Note 10) | | | 61 | | | | | | | 132 | | | | | | 193 | | |
| Dividends declared ($5.14 per share) | | | | | (8,012) | | | | | | | | | | (8,012) | | |
| Dividends paid to noncontrolling interests | | | | | | | | | | | (497) | | | | (497) | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Sale (purchases) of subsidiary shares to/(from) noncontrolling interests (Note 3) | | | (6) | | | | | 180 | | | | | (831) | | | | (657) | | |
| Balances, December 31, 2023 | — | | | 2,285 | | | 34,090 | | | (11,815) | | | (35,785) | | | 1,779 | | | | (9,446) | | |
| | | | | | | | | | | | | | | |
| Net earnings | | | | | 7,057 | | | | | | | 446 | | | | 7,503 | | |
| Other comprehensive earnings (losses), net of income taxes | | | | | | | 501 | | | | | (101) | | | | 400 | | |
| Issuance of stock awards (Note 10) | | | 50 | | | | | | | 145 | | | | | | 195 | | |
| Dividends declared ($5.30 per share) | | | | | (8,278) | | | | | | | | | | (8,278) | | |
| Dividends paid to noncontrolling interests | | | | | | | | | | | (494) | | | | (494) | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Acquisitions (Note 3) | | | | | | | | | | | 160 | | | | 160 | | |
| Sale (purchase) of subsidiary shares to/(from) noncontrolling interests | | | | | | | | | | | 90 | | | | 90 | | |
| Balances, December 31, 2024 | $ | — | | | $ | 2,335 | | | $ | 32,869 | | | $ | (11,314) | | | $ | (35,640) | | | $ | 1,880 | | | | $ | (9,870) | | |
See notes to consolidated financial statements.
Notes to Consolidated Financial Statements
Background and Basis of Presentation:
Background
Philip Morris International Inc. is a holding company incorporated in Virginia, U.S.A. (also referred to herein as the U.S., the United States or the United States of America), whose subsidiaries and affiliates and their licensees are primarily engaged in the manufacture and sale of cigarettes and smoke-free products. Throughout these financial statements, the term "PMI" refers to Philip Morris International Inc. and its subsidiaries.
Smoke-Free Business ("SFB”) is the term PMI uses to refer to all of its smoke-free products. SFB also includes wellness and healthcare products, as well as consumer accessories, such as lighters and matches.
Smoke-free products (also referred to herein as "SFPs") is the term PMI uses to refer to all of its products that provide nicotine without combusting tobacco, such as heat-not-burn, e-vapor, and oral smokeless, and that therefore generate far lower levels of harmful chemicals. As such, these products have the potential to present less risk of harm versus continued smoking.
On September 17, 2024, PMI announced the execution of a definitive agreement pursuant to which PMI’s direct, wholly-owned subsidiary, Vectura Fertin Pharma Inc., agreed to sell Vectura Group Ltd. (formerly, Vectura Group plc, and hereinafter referred to as “Vectura” or "Vectura Group") to Molex Asia Holdings Ltd. The transaction was completed on December 31, 2024. For further details, see Note 3. Acquisitions and Divestitures.
Basis of presentation
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the dates of the financial statements and the reported amounts of net revenues and expenses during the reporting periods. Significant estimates and assumptions include, among other things: pension and benefit plan assumptions; useful lives and valuation assumptions of goodwill and other intangible assets; valuation assumptions for non-marketable equity securities; marketing programs, and income taxes. Actual results could differ from those estimates.
The consolidated financial statements include PMI, as well as its wholly owned and majority-owned subsidiaries. Investments in which PMI exercises significant influence (generally 20%-50% ownership interest) are accounted for under the equity method of accounting. Investments not accounted for under the equity method of accounting are measured at fair value, if it is readily determinable, with changes in fair value recognized in net income. Investments without readily determinable fair values, non-marketable equity securities, are measured and recorded using a measurement alternative that values the security at cost minus any impairment. All intercompany transactions and balances have been eliminated.
Following the combination and the progress in 2023 toward the integration of the Swedish Match business into PMI's existing regional structure, PMI updated in January 2024 its segment reporting by including the former Swedish Match segment results into the four existing geographical segments. The four existing geographical segments are as follows: Europe Region; South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA"); East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF"); and Americas Region. The Wellness and Healthcare ("W&H") segment remained unchanged.
Certain prior years' amounts have been reclassified to conform with the current year's presentation as a result of the new segment structure discussed above. For further details, see Note 5. Goodwill and Other Intangible Assets, net, Note 13. Segment Reporting and Note 20. Restructuring Activities. These reclassifications did not impact PMI's consolidated financial position, results of operations or cash flows in any of the periods presented.
Summary of Significant Accounting Policies:
Acquisitions
PMI uses the acquisition method of accounting for acquired businesses. Under the acquisition method, PMI’s consolidated financial statements reflect the operations of an acquired business starting from the closing date of the acquisition. PMI allocates the purchase price to the tangible and identifiable intangible assets acquired and liabilities assumed based on the estimated fair values as of the acquisition date. Any residual purchase price is recorded as goodwill. The fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value during a period of time not to exceed 12 months from the acquisition date. Contingent consideration liabilities are recognized at the estimated fair value on the acquisition date. Subsequent changes to the fair value of contingent consideration are recognized in marketing, administration and research costs in the consolidated statement of earnings. Transaction costs are expensed as incurred.
If PMI determines that assets acquired do not meet the definition of a business, the transaction will be accounted for as an acquisition of assets rather than a business combination and, therefore, no goodwill will be recorded. In an asset acquisition, acquired in-process research and development ("IPR&D") with no alternative future use is charged to expense.
Cash and cash equivalents
Cash equivalents include demand deposits with banks and all highly liquid investments with original maturities of three months or less.
Depreciation and Amortization
Property, plant and equipment are stated at historical cost and depreciated primarily using the straight-line method over the estimated useful lives of the assets. Machinery and equipment are depreciated primarily over periods ranging from 3 to 15 years, and buildings and building improvements primarily over periods up to 40 years.
Definite-lived intangible assets are amortized over their useful lives. For further details, see Note 5. Goodwill and Other Intangible Assets, net.
Employee benefit plans
PMI provides a range of benefits to its employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). PMI records annual amounts relating to these plans based on calculations specified under U.S. GAAP. PMI recognizes the funded status of its defined pension and postretirement plans on the consolidated balance sheets. The funded status is measured as the difference between the fair value of the plans assets and the benefit obligation. PMI measures the plan assets and liabilities at the end of the fiscal year. For defined benefit pension plans, the benefit obligation is the projected benefit obligation. For the postretirement health care plans, the benefit obligation is the accumulated postretirement benefit obligation. Any plan with an overfunded status is recognized as an asset, and any plan with an underfunded status is recognized as a liability. Any gains or losses and prior service costs or credits that have not been recognized as a component of net periodic benefit costs are recorded as a component of other comprehensive earnings (losses), net of deferred taxes. PMI elects to recognize actuarial gains/(losses) using the corridor approach.
Fair value measurements
PMI follows ASC 820, Fair Value Measurements and Disclosures with respect to assets and liabilities that are measured at fair value. The guidance defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The guidance also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The guidance describes three levels of input that may be used to measure fair value. Level 1 inputs are quoted prices in active markets for identical assets or liabilities. Level 2 inputs include quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Level 3 are unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Foreign currency translation
PMI translates the results of operations of its subsidiaries and affiliates, except those operating in highly inflationary economies, using average exchange rates during each period, whereas balance sheet accounts are translated using exchange rates at the end of each period. Currency translation adjustments are recorded as a component of stockholders’ (deficit) equity. In addition, some of PMI’s subsidiaries have assets and liabilities denominated in currencies other than their functional currencies, and to the extent those are not designated as net investment hedges, these assets and liabilities generate transaction gains and losses when translated into their respective functional currencies.
PMI applies highly inflationary accounting if the cumulative inflation rate in an economy for a three-year period meets or exceeds 100%. Subsidiaries operating in highly inflationary economies use the U.S. dollar as the functional currency. Monetary assets and liabilities are translated at exchange rates in effect at the balance sheet date while non-monetary assets and liabilities are translated at historical exchange rates. Exchange gains and losses resulting from remeasurement adjustments are recorded within marketing, administration and research costs in the consolidated statements of earnings.
Goodwill and non-amortizable intangible assets valuation
PMI tests goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review. PMI performs its annual impairment analysis in the second quarter of each year. The impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired.
Hedging instruments
Derivative financial instruments are recorded at fair value on the consolidated balance sheets as either assets or liabilities. Changes in the fair value of derivatives are recorded each period either in accumulated other comprehensive losses on the consolidated balance sheet or in earnings, depending on whether a derivative is designated and effective as part of a hedge transaction and, if it is, the type of hedge transaction. Gains and losses on derivative instruments reported in accumulated other comprehensive losses are reclassified to the consolidated statements of earnings, into the same line item as the impact of the underlying transaction, in the periods in which operating results are affected by the hedged item. Cash flows from hedging instruments are classified in the same manner as the affected hedged item in the consolidated statements of cash flows.
Impairment of long-lived assets
PMI reviews long-lived assets, including amortizable intangible assets, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. PMI performs undiscounted operating cash flow analyses to determine if an impairment exists. For purposes of recognition and measurement of an impairment for assets held for use, PMI groups assets and liabilities at the lowest level for which cash flows are separately identifiable. If an impairment is determined to exist, any related impairment loss is calculated based on fair value. Impairment losses on assets to be disposed of, if any, are based on the lower of carrying value or estimated proceeds to be received less costs of disposal.
Investment in non-marketable equity securities
Non-marketable equity securities are subject to periodic impairment reviews during which PMI considers both qualitative and quantitative factors that may have a significant impact on the investees' fair value. Upon determining that an impairment may exist, the security’s fair value is calculated and compared to its carrying value, and an impairment is recognized immediately if the carrying value exceeds the fair value. For further details, see Note 6. Related Parties - Equity Investments and Other.
Impairment of equity method investments
Equity method investments are evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of the investments may not be recoverable. An impairment loss would be recorded whenever a decline in value of an equity investment below its carrying amount is determined to be other than temporary. PMI determines whether a loss is other than temporary by considering the length of time and extent to which the fair value of the equity investment has been less than the carrying amount, the financial condition of the equity investment, and the intent to retain the investment for a period of time is sufficient to allow for any anticipated recovery in market value.
Income taxes
Income taxes are provided on all earnings for jurisdictions outside the United States. These provisions, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in PMI’s consolidated balance sheets. Significant judgment is required in determining income tax provisions and in evaluating tax positions. PMI recognizes accrued interest and penalties associated with uncertain tax positions as part of the provision for income taxes on the consolidated statements of earnings. PMI recognizes income taxes associated with Global Intangible Low-Taxed Income ("GILTI") taxes as current period expense rather than including these amounts in the measurement of deferred taxes.
Inventories
Inventories are stated at the lower of cost or net realizable value. The first-in, first-out and average cost methods are used to cost substantially all inventories. It is a generally recognized industry practice to classify leaf tobacco inventory as a current asset, although part of such inventory, because of the duration of the aging process, ordinarily would not be utilized within one year.
Leases
PMI determines that a contract contains a lease if the contract conveys a right to control the use of the identified asset for a period of time in exchange for consideration. Operating lease expense is recognized on a straight-line basis over the lease term. Finance lease expense is amortized based on production activity or the lease term. Lease expense is recorded in cost of sales or marketing, administration and research costs depending on the nature of the leased item. At lease commencement, PMI recognizes lease liabilities and the corresponding right-of-use assets (at the present value of future payments) for predominately all of its leases. The recognition of the right-of-use asset and lease liability includes renewal options when it is reasonably certain that they will be exercised. Certain of PMI’s leases include payments that are based on changes to an index or on actual usage. These lease payments are adjusted periodically and are included within variable lease costs. PMI accounts for lease and nonlease components as a single-lease component with the exception of its vehicle leases, of which PMI accounts for the lease components separately from the nonlease components. Additionally, leases with an initial term of 12 months or less are not included in the right-of-use asset or lease liability on the consolidated statement of financial position.
Marketing costs
PMI supports its products with advertising, adult consumer engagement and trade promotions. Such programs include, but are not limited to, discounts, rebates, in-store display incentives, e-commerce, mobile and other digital platforms, adult consumer activation and promotion activities, as well as costs associated with adult consumer experience outlets and other adult consumer touchpoints and volume-based incentives. Advertising, as well as certain consumer engagement and trade activities costs, are expensed as incurred. Trade promotions are recorded as a reduction of revenues based on amounts estimated as being due to customers at the end of a period, based principally on historical utilization. For interim reporting purposes, advertising and certain consumer engagement expenses are charged to earnings based on estimated sales and related expenses for the full year.
Revenue recognition
PMI recognizes revenue primarily through the manufacture and sale of cigarettes and smoke-free products, including heat-not-burn, vapor and oral nicotine products. The majority of PMI revenues are generated by sales through direct and indirect distribution networks with short-term payment conditions and where control is typically transferred to the customer either upon shipment or delivery of goods. PMI evaluates the transfer of control through evidence of the customer’s receipt and acceptance, transfer of title, PMI’s right to payment for those products and the customer’s ability to direct the use of those products upon receipt. Typically, PMI’s performance obligations are satisfied and revenue is recognized either upon shipment or delivery of goods.
In certain instances, PMI facilitates shipping and handling activities after control has transferred to the customer. PMI has elected to record all shipping and handling activities as costs to fulfill a contract. The shipping and handling costs that have not been incurred at the time revenue is recognized are accrued. PMI is a principal for majority of its arrangements and recognizes revenue at the transaction price, with the associated costs recorded in cost of sales. The transaction price is typically based on the amount billed to the customer and includes estimated variable consideration, where applicable. Such variable consideration is typically not constrained and is estimated based on the most likely amount that PMI expects to be entitled to under the terms of the contracts with customers, historical experience of discount or rebate redemption, where relevant, and the terms of any underlying discount or rebate programs, which may change from time to time as the business and product categories evolve. For arrangements where PMI acts as an agent, the net commission earned is recognized as revenue. PMI has elected to exclude excise taxes collected from customers from the measurement of the transaction price, thereby presenting revenues net of excise taxes. Estimated costs associated with warranty programs are generally provided for in cost of sales in the period the related revenues are recognized.
Research and Development and Acquired In-Process Research and Development ("IPR&D")
Research and development costs are expensed as incurred.
In a business combination, the fair value of IPR&D acquired is initially capitalized and accounted for as indefinite-lived intangible assets until completion or abandonment of the projects. Upon completion, a determination as to the useful life is performed and the intangible asset is accounted for as a definite-lived intangible asset. Both the indefinite and definite-lived intangible assets are subject to impairment testing annually or more frequently if indicators exist. In an asset acquisition, the initial cost to acquire the IPR&D is expensed in the consolidated statements of earnings when the project has no alternative future use. PMI records these costs within marketing, administration and research costs in its consolidated statements of earnings.
Stock-based compensation
PMI measures compensation cost for all stock-based awards at fair value on date of grant and recognizes the compensation costs over the service periods for awards expected to vest. PMI’s accounting policy is to estimate the number of awards expected to be forfeited and adjust the expense when it is no longer probable that the employee will fulfill the service condition. For further details, see Note 10. Stock Plans.
Acquisitions and Divestitures:
Sale of Vectura Group Ltd.
On September 17, 2024, PMI announced the execution of a definitive agreement pursuant to which PMI’s direct, wholly-owned subsidiary, Vectura Fertin Pharma Inc., agreed to sell Vectura Group Ltd. (formerly, Vectura Group plc, and hereinafter referred to as “Vectura” or "Vectura Group") to Molex Asia Holdings Ltd. ("Molex"), subject to customary regulatory approval and other completion conditions. On December 31, 2024, PMI completed the sale of Vectura for an upfront cash consideration of GBP 152 million (approximately $191 million) and a short-term receivable of GBP 24 million (approximately $30 million), reflecting certain customary completion account adjustments, with additional deferred payments of up to GBP 148 million (approximately $186 million), contingent on achievement of certain milestones over periods up to and through 2039. In addition, PMI agreed to indemnify Molex for certain claims related to the pre-completion period. For the year ended December 31, 2024, no liability has been recorded in relation to the indemnity.
As of September 17, 2024, and through the completion date, Vectura's net assets and liabilities were classified as held-for-sale in PMI’s consolidated balance sheet. The sale resulted in a pre-tax loss of $199 million ($206 million including the tax costs), of which $198 million of loss related to the impairment charge recognized in the third quarter of 2024, to record the net assets held-for-sale at the lower of their carrying value or fair value less costs to sell. This amount also included reclassification of currency translation losses from other comprehensive losses of $16 million. The loss on sale of Vectura has been recorded in marketing, administration and research costs under the Wellness and Healthcare segment in PMI’s consolidated statement of earnings for the year ended December 31, 2024.
Altria Group, Inc. Agreement
On October 20, 2022, PMI announced that it had reached an agreement with Altria Group, Inc. ("Altria") to end the companies' relationship regarding the IQOS commercialization rights in the U.S. as of April 30, 2024. As a result of PMI reacquiring these rights, effective May 1, 2024 ("acquisition date"), PMI holds the full rights to commercialize IQOS in the U.S. As part of the agreement, PMI agreed to pay a total cash consideration of $2.8 billion, including interest, of which $1.0 billion was paid at the inception of the agreement and the remaining $1.8 billion was paid on July 14, 2023. The cash consideration paid was accounted for within Other assets in PMI's consolidated balance sheets as of December 31, 2023.
On the acquisition date and as of December 31, 2024, the reacquired rights were classified as Other intangible assets, net in PMI's consolidated balance sheets, and will be amortized over their useful life of 5 years.
Business Combinations
United Tobacco Company – In April 2023, PMI acquired 66.73% of Egyptian Investment Holding (“EIH”), a United Arab Emirates based company and as a result, acquired an approximate economic interest of 25% in United Tobacco Company ("UTC"), which was
accounted for using the equity method of accounting. In May 2024, PMI increased its indirect economic interest and acquired a controlling interest of 54.25% in UTC. UTC is an entity incorporated in Egypt and manufactures products under license for Philip Morris Misr LLC (“PMM”), PMI’s Egyptian subsidiary. The acquisition builds on PMI’s existing investments in Egypt and increases the manufacturing synergies between PMM and UTC.
As a result of PMI obtaining control over UTC, PMI’s previously held 25% economic interest in UTC was remeasured to its fair value by applying the guideline transaction method adjusted for a discount for lack of control. The difference between the book value of $312 million, including related cumulative translation losses balance of $112 million, which was reclassified from accumulated other comprehensive losses and the fair value of PMI’s previously held interest in UTC was not material.
The total purchase price for the incremental equity interest of $316 million included cash consideration of $31 million, contingent consideration of $22 million and $263 million of assumed bank loan liabilities. During the third quarter of 2024, PMI paid the contingent consideration of $22 million and $240 million of assumed bank loans.
The following table summarizes the preliminary purchase price allocation for the fair value of assets acquired and liabilities assumed as of the date of the acquisition, which includes previously held interest:
| | | | | |
| (in millions) | |
| Cash and cash equivalent | $ | 74 | |
| Current assets, including receivables and inventories | 11 | |
| Other intangible assets - Tobacco manufacturing license | 211 | |
| Other non-current assets, including property, plant and equipment | 16 | |
| Current liabilities | (8) | |
| Identifiable net assets acquired | 304 | |
| Noncontrolling interest | (160) | |
| Goodwill | 512 | |
| Acquisition fair value | $ | 656 | |
Goodwill is primarily attributable to future growth opportunities, anticipated synergies in the manufacturing processes and intangible assets that did not qualify for separate recognition. The fair value of the noncontrolling interest was estimated based on the enterprise value of UTC, adjusted for a discount for lack of control. The manufacturing license, which relates to the manufacturing of both smoke-free and combustible tobacco products, was valued using the multi-period excess earnings method and has been determined to have an indefinite life.
The purchase price allocation is preliminary and continues to be subject to refinement. PMI is evaluating the deductibility of goodwill for income tax purposes. UTC's results of operations from May 16, 2024, through December 31, 2024, were included in PMI's consolidated statements of earnings and were not material. Pro forma results of operations for the business combination have not been presented as the aggregate impact is not material to PMI's consolidated statements of earnings.
Swedish Match AB – On November 11, 2022 (the acquisition date), Philip Morris Holland Holdings B.V. (“PMHH”), a wholly owned subsidiary of PMI, acquired a controlling interest of 85.87% of the total issued shares in Swedish Match AB (“Swedish Match”) and acquired 94.81% of its outstanding shares as of December 31, 2022. The shares were acquired through acceptances of the tender offer and a series of open market and over-the-counter purchases. PMI funded the acquisition through cash on-hand and debt proceeds, as described in Note 8. Indebtedness. The aggregate cash paid as of the acquisition date was $14,460 million (or $13,976 million net of cash acquired), which was included in investing activities in the consolidated statements of cash flows for the year ended December 31, 2022. The cash paid in connection with the additional purchases of the noncontrolling interests after the acquisition date and through December 31, 2022 amounted to $1,495 million and was included in financing activities in the consolidated statements of cash flows for the year ended December 31, 2022.
In accordance with the Swedish Companies Act, PMI subsequently exercised its right to initiate arbitral proceedings to compulsorily redeem the remaining shares for which acceptances were not received and obtained legal title to 100% of the shares in Swedish Match on February 17, 2023. Cash paid in connection with such legal title, together with an immaterial amount attributable to open market purchases that were executed in December 2022 but settled in January 2023, amounted to $883 million and was included in financing activities in the consolidated statements of cash flows for the year ended December 31, 2023. While PMI paid the referenced amounts and acquired legal title to the shares, under the Swedish Companies Act the redemption process was not complete until the final redemption price was determined by an arbitral tribunal. On September 12, 2023, the arbitral tribunal determined the final redemption price to be Swedish krona (SEK) 115.07, unchanged from the SEK 115.07 that PMI paid per share in connection with obtaining legal title to the shares. This process was completed in the fourth quarter of 2023 when the opportunity to appeal the arbitral tribunal determination ended.
Swedish Match is a market leader in oral nicotine delivery with a significant presence in the United States market. The acquisition is accelerating PMI’s transformation to become a smoke-free company with a comprehensive global smoke-free portfolio with leadership positions in heat-not-burn and the fastest growing category of oral nicotine.
In November 2023, PMI finalized all measurement period adjustments related to the Swedish Match acquisition. The table below summarizes the final purchase price allocation for the fair value of assets acquired and liabilities assumed as of the acquisition date:
| | | | | | | |
| (in millions) | | | Final Purchase Price Allocation Recognized as of
the acquisition date |
| Cash and cash equivalents | | | $ | 484 | |
| Trade receivables | | | 135 | |
| Other receivables | | | 53 | |
| Inventories | | | 437 | |
| Other current assets | | | 415 | |
| Property, plant and equipment | | | 677 | |
| Other intangible assets | | | 7,868 | |
| Other non-current assets | | | 216 | |
| Current portion of long-term debt | | | 224 | |
| Accounts payable | | | 120 | |
| Other current liabilities | | | 532 | |
| Income taxes | | | 14 | |
| Long-term debt | | | 1,121 | |
| Deferred income taxes | | | 1,970 | |
| Other non-current liabilities | | | 196 | |
| Identifiable net assets acquired | | | 6,108 | |
| Noncontrolling interest | | | 2,379 | |
| Goodwill | | | 10,731 | |
| Total consideration transferred | | | $ | 14,460 | |
The total fair value step-up adjustment for inventories was $146 million, of which $125 million was recognized in cost of sales in the fourth quarter of 2022 and the remaining balance in the first quarter of 2023.
The fair value of long-term debt was primarily determined using readily available market prices as of the acquisition date and the total purchase price adjustment of $(107) million is being amortized as an increase to interest expense, net over the lives of the related debt.
Goodwill is primarily attributable to future growth opportunities, anticipated synergies in the U.S. and intangible assets that did not qualify for separate recognition. The goodwill is not deductible for income tax purposes.
Identifiable intangible assets of Swedish Match consist of:
| | | | | | | | | | | |
| Type | Useful Life | Estimated Fair Value (in millions) |
| Trademarks | Non-amortizable | | $ | 3,133 | |
| Trademarks | Amortizable | 20 - 30 years | 1,067 | |
| Developed technology, including patents | | 10 years | 113 | |
| Customer relationships | | 6 - 15 years | 3,555 | |
| | | |
| Total identifiable intangible assets | | | $ | 7,868 | |
The significant assumptions used in determining the fair values of the identifiable intangible assets included royalty rates, revenue growth rates, profit margins, customer attrition rates and discount rates.
Trademarks primarily relate to $3,133 million for the ZYN trademark, which has been determined to have an indefinite life due to the fast growth and the leading position of the brand in the U.S. market. All other trademarks have been determined to have a useful life ranging between 20 - 30 years. The trademarks have been valued using the relief from royalty method supported by revenue growth rate assumptions and royalty rates disaggregated at the individual trademark level.
Developed technology, including patents, relates to the nicotine pouch technology of $113 million. These patents have been assigned a useful life of 10 years, which is in line with their protection period and have been valued using the comparable transactions and income methods.
Customer relationships have been valued by categories of customers and geographic locations, namely the U.S. market, Scandinavia, and other markets using the multiple periods excess earnings method. The significant assumptions included customer attrition rates disaggregated at the customer category level, the revenue growth rates, as well as profit margins.
PMI consolidated statements of earnings for the year ended December 31, 2022, include $316 million of net revenues and $(26) million of net losses associated with the results of operations of Swedish Match from the acquisition date to December 31, 2022. The operating results of Swedish Match are included in the Europe and Americas segments.
Acquisition related transaction costs, which were comprised primarily of regulatory, financial advisory and legal fees, totaled $59 million for the year ended December 31, 2022, and were included in marketing, administration and research costs in the consolidated statements of earnings. Bridge and term loan credit agreement related fees associated with the issuance of debt amounted to $54 million, of which $37 million were capitalized at the acquisition date. The fair value of the noncontrolling interest was based on the tender offer as of the acquisition date.
Under the EU Merger Regulation, approval by the European Commission of PMI's acquisition of Swedish Match was conditional on PMHH's divestiture of Swedish Match's subsidiary, SMD Logistics AB ("SMDL"), following the completion of the offer to tender all shares in Swedish Match to PMHH. As a result, these assets were accounted for as assets held for sale and included within other current assets and other accrued liabilities in PMI’s consolidated balance sheets at March 31, 2023 and December 31, 2022. PMI subsequently sold SMDL on June 30, 2023 and the transaction did not have a material impact on the consolidated statements of earnings for the year ended December 31, 2023.
The unaudited pro forma combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of PMI and Swedish Match. In order to reflect the occurrence of the acquisition on January 1, 2021, as required, the unaudited pro forma financial information includes adjustments to reflect the following:
•incremental amortization expense to be incurred based on the current fair values of the identifiable intangible assets acquired;
•incremental cost of products sold related to the fair value adjustments associated with acquisition date inventory;
•additional interest expense associated with the issuance of debt to finance the acquisition, including the effects of the related derivative financial instruments designated to hedge interest rate risks as well as economic hedges;
•reclassification of non-recurring acquisition-related costs incurred during the year ended December 31, 2022, to the year ended December 31, 2021;
•impact of a deferred tax cost of $430 million in 2022 and $321 million in 2021 related to the theoretical unrealized foreign currency gains on intercompany loans related to the acquisition financing. These theoretical unrealized pre-tax foreign currency movements were fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in
PMI's consolidated statements of stockholders' (deficit) equity, while the corresponding deferred tax impacts were reflected in PMI's consolidated statements of earnings; and
•other immaterial items (i.e., the alignment of accounting policies from IFRS to US GAAP.)
The unaudited pro forma financial information is not necessarily indicative of what the consolidated results of operations would have been had the acquisition been completed on January 1, 2021. In addition, the unaudited pro forma financial information is not a projection of future results of operations of the combined company, nor does it reflect the expected realization of any synergies or cost savings associated with the acquisition.
The unaudited pro forma financial information for the year ended December 31, 2022 is as follows:
| | | | | | |
| (in millions) | 2022 | |
| Net revenues | $ | 33,579 | | |
| Net earnings attributable to PMI | $ | 8,779 | | |
Transactions With Noncontrolling Interests
Turkey – In the first quarter of 2022, PMI acquired the remaining 25% stake of its holding in Philip Morris Tütün Mamulleri Sanayi ve Ticaret A.Ş. ("PMTM") (formerly Philsa Philip Morris Sabanci Sigara ve Tütüncülük Sanayi ve Ticaret A.Ş.) and 24.75% stake in Philip Morris Pazarlama ve Satiş A.Ş. ("PMPS") (formerly Philip Morris SA, Philip Morris Sabanci Pazarlama ve Satiş A.Ş.) from its Turkish partners, Sabanci Holding for a total acquisition price including transaction costs and remaining dividend entitlements of approximately $223 million. As a result of this acquisition, PMI owned 100% of these Turkish subsidiaries as of December 31, 2022. The purchase of the remaining stakes in these holdings resulted in a decrease to PMI's additional paid-in capital of $30 million and an increase to accumulated other comprehensive losses of $171 million primarily following the reclassification of accumulated currency translation losses from noncontrolling interests to PMI’s accumulated other comprehensive losses during the first quarter of 2022.
In January 2023, PMI sold the acquired stakes of its holdings in PMTM and PMPS to Pioneers Tutun Yatirim Anonim Sirketi (“Pioneers”) for a consideration of approximately $258 million, including transaction costs and dividend entitlements. The sale resulted in an increase to PMI's additional paid-in capital of $36 million and a decrease to accumulated other comprehensive losses of $179 million, following the reclassification of accumulated other comprehensive losses from PMI’s accumulated other comprehensive losses to noncontrolling interests.
War in Ukraine:
Since the onset of the war in Ukraine in February 2022, PMI's main priority has been the safety and security of its employees and their families in the country.
Ukraine
PMI temporarily suspended its commercial and manufacturing operations in Ukraine, including the closing of its factory in Kharkiv at the end of February 2022, in order to preserve the safety of its employees. PMI subsequently resumed commercial activities in select locations where safety allowed, in order to provide product availability and service to adult consumers, and began to supply the market from production centers outside Ukraine, as well as through a contract manufacturing arrangement. Production at the factory in Kharkiv remains suspended. PMI is not aware of any major damage to its production facilities, inventories or other assets in Ukraine. On June 20, 2023, PMI announced the investment of $30 million in a new production facility in the Lviv region, in Western Ukraine. In the fourth quarter of 2023, as a result of the completion of certain preparatory work for this new production facility, PMI recorded impairment of certain long-lived assets. The new production facility was completed at the end of the first quarter of 2024 and local production commenced in April 2024. As of December 31, 2024, PMI’s Ukrainian operations had approximately $562 million in total assets, excluding intercompany balances. These total assets included $105 million and $396 million in receivables and inventories, respectively.
Russia
PMI has suspended its planned investments in the Russian Federation including all new product launches and commercial, innovation, and manufacturing investments. PMI has also taken steps to scale down its manufacturing operations in Russia amid ongoing supply chain disruptions and the evolving regulatory environment. PMI is continuously assessing the evolving situation in Russia. This includes regulatory constraints in the market entailing very complex terms and conditions that must be met for any divestment transaction to be granted approval by the authorities, and restrictions resulting from international regulations. In the event of a divestment, PMI's ability to fully realize the value of the business would likely be subject to material impairment. As a result of PMI continuing operations within Russia in 2023 and 2024, it has not recorded an impairment of long-lived and other assets. However, PMI recorded specific asset write downs in 2022 as referred to in the table below. PMI’s Russian operations as of December 31, 2024 had approximately $2.7 billion in total assets, excluding intercompany balances. These total assets included $1,038 million, $426 million, $789 million, $219 million and $154 million in cash (primarily held in local currency), receivables, inventories, property, plant and equipment and goodwill, respectively. In addition, there was approximately $1,585 million of cumulative foreign currency translation losses reflected in accumulated other comprehensive losses in the consolidated statement of stockholders’ equity as of December 31, 2024. Additionally, we hold a 23% equity interest in JSC TK Megapolis, PMI's distributor in Russia. For further details, see Note 6. Related Parties – Equity Investments and Other.
For the year ended December 31, 2024, PMI did not incur charges related to circumstances driven by the war. For the years ended December 31, 2023 and 2022, PMI recorded in its consolidated statements of earnings pre-tax charges related to circumstances driven by the war as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 | | |
| Cost of sales | Marketing, administration and research costs | Total | | Cost of sales | Marketing, administration and research costs | Total | | | | |
Ukraine 1 | $ | 15 | | $ | 38 | | $ | 53 | | | $ | 42 | | $ | 36 | | $ | 78 | | | | | |
Russia 2 | — | | — | | — | | | 20 | | 53 | | 73 | | | | | |
| Total | $ | 15 | | $ | 38 | | $ | 53 | | | $ | 62 | | $ | 89 | | $ | 151 | | | | | |
1 The 2023 pre-tax charges were primarily due to the cost of PMI’s humanitarian efforts, which includes salary continuation for its employees, severance payments, as well as an impairment of certain long-lived assets in the fourth quarter of 2023. The 2022 pre-tax charges were primarily due to an inventory write-down, additional allowance for receivables and the cost of PMI’s humanitarian efforts, which includes salary continuation for its employees.
2 The 2022 pre-tax charges were primarily due to machinery and inventory write downs related to the commercial decisions noted above.
PMI will continue to monitor the situation as it evolves and will determine if further charges are needed.
Goodwill and Other Intangible Assets, net:
Goodwill
The movements in goodwill were as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | Europe | SSEA, CIS & MEA | EA, AU & PMI DF | Americas | Wellness & Healthcare | Total |
| Balances at January 1, 2023 | $ | 4,774 | | $ | 2,869 | | $ | 493 | | $ | 10,507 | | $ | 1,012 | | $ | 19,655 | |
| Changes due to: | | | | | | |
| | | | | | |
| Impairment | — | | — | | — | | — | | (665) | | (665) | |
| Currency | 220 | | 8 | | (1) | | 89 | | 43 | | 359 | |
| Measurement period adjustments | (821) | | — | | — | | (1,749) | | — | | (2,570) | |
| Balances, December 31, 2023 | 4,173 | | 2,877 | | 492 | | 8,847 | | 390 | | 16,779 | |
| Changes due to: | | | | | | |
| Acquisitions and divestitures | — | | 512 | | — | | — | | (65) | | 447 | |
| | | | | | |
| Currency | (360) | | (138) | | (22) | | (98) | | (8) | | (626) | |
| | | | | | |
| | | | | | |
| Balances, December 31, 2024 | $ | 3,813 | | $ | 3,251 | | $ | 470 | | $ | 8,749 | | $ | 317 | | $ | 16,600 | |
As discussed in Note 1. Background and Basis of Presentation, PMI updated in January 2024 its segment reporting by including the former Swedish Match segment results into its existing geographical segments. As a result, the January 1, 2023 and December 31, 2023 goodwill balances in the table above included the reclassifications of the former Swedish Match segment to the Europe and Americas segments.
The decrease in goodwill in 2023 was primarily due to the measurement period adjustments to the Swedish Match final purchase price allocation (see Note 3, Acquisitions and Divestitures), coupled with the impairment discussed below and partially offset by currency movements.
The decrease in goodwill in 2024 was due to currency movements and goodwill allocated to disposal group in relation to Vectura Group' sale, partially offset by the preliminary purchase price allocation of PMI's acquisition in Egypt of United Tobacco Company in the second quarter of 2024. For further details on the acquisition in Egypt and Vectura Group's sale, see Note 3. Acquisitions and Divestitures.
At December 31, 2024, goodwill primarily reflects PMI’s acquisitions of Swedish Match AB and Fertin Pharma A/S, as well as acquisitions in Egypt, Greece, Indonesia, Mexico, the Philippines and Serbia.
Other Intangible Assets
Details of other intangible assets were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2024 | | December 31, 2023 |
| (in millions) | Weighted-Average Remaining Useful Life | Gross Carrying Amount | Accumulated Amortization | Net | | Gross Carrying Amount | Accumulated Amortization | Net |
| Non-amortizable intangible assets | | $ | 4,446 | | | $ | 4,446 | | | $ | 4,543 | | | $ | 4,543 | |
| Amortizable intangible assets: | | | | | | | | |
| Trademarks | 15 years | 2,134 | | $ | 850 | | 1,284 | | | 2,267 | | $ | 784 | | 1,483 | |
Reacquired commercialization rights for IQOS in the U.S. | 4 years | 2,777 | | 370 | | 2,407 | | | — | | — | | — | |
| | | | | | | | |
| Developed technology, including patents | 7 years | 320 | | 121 | | 199 | | | 774 | | 329 | | 445 | |
| Customer relationships and other | 11 years | 3,712 | | 721 | | 2,991 | | | 3,843 | | 450 | | 3,393 | |
| Total other intangible assets | | $ | 13,389 | | $ | 2,062 | | $ | 11,327 | | | $ | 11,427 | | $ | 1,563 | | $ | 9,864 | |
Non-amortizable intangible assets substantially consist of the ZYN trademarks and other trademarks related to acquisitions in Indonesia and Mexico, as well as the tobacco manufacturing license associated with the preliminary purchase price allocation of PMI's acquisition in Egypt in the second quarter of 2024 (see Note 3. Acquisitions and Divestitures for further details). The decrease since December 31, 2023, was mainly due to currency movements of $276 million and a pre-tax impairment charge in the first quarter of 2024 of $27 million primarily for an in-process research and development project in the Wellness and Healthcare segment. The pre-tax impairment charge of $27 million was recorded in marketing, administration and research costs on PMI's consolidated statements of earnings for the year ended December 31, 2024. The decrease was partially offset by the recognition of the Egyptian tobacco manufacturing license.
The increase in the gross carrying amount of amortizable intangible assets from December 31, 2023, was primarily due to the classification of the IQOS commercialization rights in the U.S. on the acquisition date (May 1, 2024) as Other intangible assets, net (see Note 3. Acquisitions and Divestitures), partially offset by developed technology assets of $454 million allocated to disposal group in relation to Vectura Group's sale (see Note 3. Acquisitions and Divestitures) and currency movements of $269 million.
The change in the accumulated amortization from December 31, 2023, was mainly due to the 2024 amortization of $835 million, partially offset by accumulated amortization of developed technology assets of $267 million allocated to disposal group in relation to Vectura Group's sale and currency movements of $69 million. The amortization of intangibles for the year ended December 31, 2024 was recorded in cost of sales ($51 million) and in marketing, administration and research costs ($784 million) on PMI's consolidated statements of earnings.
Amortization expense on a pre-tax basis for each of the next five years is estimated to be approximately $991 million or less, assuming no additional transactions occur that require the amortization of intangible assets. This amount includes amortization of IQOS commercialization rights in the U.S. (see Note 3, Acquisitions and Divestitures).
2024 Annual impairment review of goodwill and non-amortizable intangible assets
During the second quarter of 2024, PMI completed its annual review of goodwill and non-amortizable intangible assets for potential impairment using a quantitative assessment for all of its reporting units and non-amortizable intangible assets. As a result of this review, no impairment charges were required. Each of PMI's reporting units had fair values substantially in excess of their carrying values with the exception of the Wellness & Healthcare reporting unit, which had less than 20% excess of fair value over its carrying value. PMI will continue to monitor this reporting unit as any changes in assumptions and estimates, unfavorable clinical trial results, failure to obtain regulatory approvals or other market factors could result in additional future goodwill and other intangible asset impairments. In addition, there are still risks related to PMI’s Russian reporting unit’s assets as the fair value of these assets is difficult to predict due to the current economic, political, regulatory and social conditions as well as the foreign currency volatility. As of December 31, 2024, our Russian operations had approximately $2.7 billion in total assets, excluding intercompany balances, of which approximately $1.0 billion consisted of cash and cash equivalents held mostly in local currency (Russian rubles). Additionally, we hold a 23% equity interest in JSC TK Megapolis, PMI's distributor in Russia. For further details, see Note 4. War in Ukraine, Note 6. Related Parties – Equity Investments and Other, and Note 18. Contingencies.
2023 Annual impairment review of goodwill and non-amortizable intangible assets
During the second quarter of 2023, as a result of the completion of PMI's annual review of goodwill and non-amortizable intangible assets for potential impairment, it was determined that the estimated fair value of the Wellness and Healthcare reporting unit was lower than its carrying value. Consequently, PMI recorded a goodwill impairment charge of $665 million in the consolidated statements of earnings for the year ended December 31, 2023, reflecting the impact of reduced estimated future cash flows, which were primarily attributable to unfavorable clinical trial results that became available in June 2023 for an inhalable aspirin product being developed by the Wellness and Healthcare business. Additionally, as a result of the impairment test of non-amortizable intangible assets, PMI recorded a pre-tax impairment charge of $15 million for an in-process research and development project related to one of PMI's 2021 acquisitions. This pre-tax impairment charge of $15 million was recorded within marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023.
Related Parties - Equity Investments and Other:
Equity Method Investments:
At December 31, 2024 and 2023, PMI had total equity method investments of $1,005 million and $1,309 million, respectively. Equity method investments are initially recorded at cost. Under the equity method of accounting, the investment is adjusted for PMI's proportionate share of earnings or losses, dividends, capital contributions, changes in ownership interests and movements in currency translation adjustments. The carrying value of our equity method investments at December 31, 2024 and 2023, exceeded our share of the investees' book value by $1,060 million and $907 million, respectively. The difference between the investment carrying value and the amount of underlying equity in net assets is mainly attributable to equity method goodwill, convertible debt instruments, and definite-lived intangible assets and other assets. The difference related to the definite-lived intangibles and other assets at December 31, 2024 and 2023 of $152 million and $31 million, respectively, is amortized on a straight-line basis and is included in Equity investments and securities (income)/loss, net on the consolidated statements of earnings. At December 31, 2024 and 2023, PMI received year-to-date dividends from equity method investees of $151 million and $57 million, respectively.
PMI holds a 23% equity interest in Megapolis Distribution B.V. ("MDBV"), which was the holding company of JSC TK Megapolis (formerly CJSC TK Megapolis), pursuant to Dutch law, PMI's distributor in Russia (SSEA, CIS & MEA segment), which as of December 31, 2024 had a carrying value of $264 million. Additionally, there was approximately $633 million of cumulative foreign currency translation losses associated with MDBV reflected in accumulated other comprehensive losses in the consolidated statement of stockholders’ equity as of December 31, 2024. In June 2024, the Russian government included JSC TK Megapolis in the list of economically significant organizations that may be subject to forced localization under applicable Russian law, which refers to the mandatory removal of a foreign holding company from the shareholding structure. On August 8, 2024, the Arbitrazh Court of the Moscow Region granted the forced localization of MDBV as requested by the Ministry of Industry and Trade on July 18, 2024. As a result, MDBV’s shares in JSC TK Megapolis were transferred to JSC TK Megapolis and subsequently transferred to the Russian subsidiary of its indirect shareholders during the fourth quarter of 2024. As a result of the transfer of shares, PMI recorded a tax charge of $77 million, primarily reflecting additional deferred withholding taxes related to the JSC TK Megapolis pre-localization earnings and other adjustments of accumulated earnings of the Russian subsidiary. As of December 31, 2024, there are risks related to this investment as the fair value of these assets with their associated rights is difficult to predict due to the current economic, political, regulatory, legal and social conditions as well as the foreign currency volatility.
PMI holds a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) (“EITA”). PMI holds an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie (“STAEM”), an Algerian joint venture that is 51% owned by EITA and 49% by the Algerian state-owned enterprise Management et Développement des Actifs et des Ressources Holding ("MADAR Holding"), which manufactures and distributes under license some of PMI’s brands (SSEA, CIS & MEA segment).
In April 2023, PMI acquired an approximate economic interest of 25% in United Tobacco Company ("UTC"). UTC is an entity incorporated in Egypt which manufactures products under license for PMI’s Egyptian subsidiary. On May 16, 2024, PMI acquired a controlling interest in UTC. For further details, see Note 3. Acquisitions and Divestitures.
In May 2024, PMI acquired an indirect economic interest of 14.7% in Eastern Company (“Eastern"), Egypt’s largest cigarette manufacturer which also includes cigars and pipe tobacco, among others, in its portfolio. PMI accounted for its investment in Eastern under the equity method of accounting as it has the indirect ability to participate in Eastern's policy making processes. In relation to the acquisition, PMI subsequently entered into an agreement in August 2024 to guarantee certain credit facilities and repayment of certain bank loan liabilities. The maximum amount of these guarantee obligations is $385 million and they will be in effect until 2034. As of December 31, 2024, PMI has not finalized the basis difference allocation resulting from the investment.
The initial investments in Megapolis Distribution BV, EITA, Eastern and UTC (up to the acquisition of controlling interest in UTC on May 16, 2024) have been recorded at cost and are included in equity investments on the consolidated balance sheets. Transactions between these equity method investees and PMI subsidiaries are considered to be related-party transactions and are included in the tables below.
Equity securities:
On March 22, 2019, PMI’s wholly owned subsidiary in Canada, Rothmans, Benson & Hedges Inc. (“RBH”) obtained an initial order from the Ontario Superior Court of Justice granting it protection under the Companies’ Creditors Arrangement Act ("CCAA"), which is a Canadian federal law that permits a Canadian business to restructure its affairs while carrying on its business in the ordinary course with minimal disruption to its customers, suppliers and employees. The administration of the CCAA process, principally relating to the powers provided to the court under the CCAA and the oversight provided by the court appointed monitor, removes certain elements of control of the business from both PMI and RBH. As a result, PMI determined that it no longer had a controlling financial interest over RBH as defined in ASC 810 (Consolidation), and deconsolidated RBH as of the date of the CCAA filing.
Since the deconsolidation of RBH on March 22, 2019, PMI has accounted for its continuing investment in RBH in accordance with ASC 321 (Investments-Equity Securities) as an equity security, without readily determinable fair value, and recorded its continuing investment in RBH at fair value of $3,280 million, which included the estimated settlement amount at the date of deconsolidation, within equity investments.
On October 17, 2024, the court-appointed mediator and monitor in the CCAA proceedings filed a proposed plan of compromise and arrangement (“Proposed Plan”) setting forth, among other things, certain terms of a proposed comprehensive resolution of Canadian tobacco claims and related litigation. Under the resolution contemplated by the Proposed Plan, RBH, Imperial Tobacco Canada Limited ("ITL") and JTI Macdonald Corp ("JTIM") would pay an aggregate global settlement amount of CAD 32.5 billion (approximately $22.3 billion). A significant determinative factor in the analysis of impairment indicators was the issue of allocation of CAD 32.5 billion aggregate settlement amount among RBH, ITL, and JTIM which remained unresolved at the time of filing.
There has been no agreed allocation under the Proposed Plan and there has been no ruling from the CCAA court on the matter. On January 24, 2025, RBH filed an objection to approval of the Proposed Plan with the CCAA court (for further details, see Note 18. Contingencies). Developments, including the positions taken by RBH in this objection and the positions taken by other parties in related filings narrowed the range of possible outcomes with respect to the allocation of the aggregate settlement amount of CAD 32.5 billion among RBH, ITL, and JTIM, which was determined to be an indicator that PMI’s investment in RBH may be impaired. Although there remains some uncertainty as to the final terms of the Proposed plan, PMI evaluated its investment in RBH for potential impairment and concluded that the estimated fair value of its investment in RBH was lower than its carrying value. As a result, PMI performed a quantitative valuation of its investment in RBH as of December 31, 2024, and recorded a non-cash impairment charge of $2,316 million in the consolidated statement of earnings for the year ended December 31, 2024, as a recognized subsequent event. The fair value of PMI’s continuing investment in RBH of $714 million represented the estimated fair value of the underlying business, net of PMI’s best estimate of the share of the aggregate global settlement amount that could be allocated to RBH, and was determined based on an income approach using a discounted cash flow analysis.
In determining the fair value of PMI’s investment in RBH, PMI made various judgements, estimates and assumptions, the most significant of which were the discount rate, sales volumes and operating margins related to the fair value of the combustible tobacco product business in Canada. In addition, significant estimates were made with respect to the allocation amount of the aggregate global
settlement amount among RBH, ITL and JTIM, as well as the deductibility of the settlement amount payment for income tax purposes in Canada. All significant inputs used in the valuation are classified in Level 3 of fair value hierarchy. Transactions between PMI and RBH are considered to be related-party transactions from the date of deconsolidation and are included in the tables below.
The fair value of PMI’s other equity securities, which have been classified within Level 1, was $921 million and $375 million for the years ended December 31, 2024 and 2023, respectively. Unrealized pre-tax gains (losses) of $546 million and $49 million ($418 million and $38 million net of tax) on these equity securities were recorded in equity investments and securities (income)/loss, net on the consolidated statements of earnings for the years ended December 31, 2024 and 2023, respectively. For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
Other related parties:
United Arab Emirates-based Trans-Emirates Trading and Investments (FZC) ("TTI") holds a 33% non-controlling interest in Philip Morris Misr LLC ("PMM"), an entity incorporated in Egypt which is consolidated in PMI’s financial statements in the SSEA, CIS & MEA segment. PMM sells, under license, PMI brands in Egypt through an exclusive distribution agreement with a local entity that is also controlled by TTI.
Godfrey Phillips India Ltd ("GPI") is one of the non-controlling interest holders in IPM India, which is a 56.3% owned PMI consolidated subsidiary in the SSEA, CIS & MEA segment. GPI also acts as contract manufacturer and distributor for IPM India.
Financial activity with the above related parties:
PMI’s net revenues and expenses with the above related parties were as follows:
| | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| (in millions) | | 2024 | 2023 | 2022 |
| Net revenues: | | | | |
| Megapolis Group | | $ | 2,393 | | $ | 2,267 | | $ | 2,485 | |
| Other | | 1,483 | | 1,286 | | 1,173 | |
Net revenues (a) | | $ | 3,876 | | $ | 3,553 | | $ | 3,658 | |
| | | | |
| Expenses: | | | | |
| Other | | $ | 101 | | $ | 186 | | $ | 119 | |
| Expenses | | $ | 101 | | $ | 186 | | $ | 119 | |
(a) Net revenues exclude excise taxes and VAT billed to customers.
PMI’s balance sheet activity with the above related parties was as follows:
| | | | | | | | | | | |
| | At December 31, |
| (in millions) | | 2024 | 2023 |
| Receivables: | | | |
| Megapolis Group | | $ | 405 | | $ | 474 | |
| Other | | 286 | | 236 | |
| Receivables | | $ | 691 | | $ | 710 | |
| | | |
| Payables: | | | |
| Other | | $ | 60 | | $ | 18 | |
| Payables | | $ | 60 | | $ | 18 | |
| | | |
| | | |
The activities with the above related parties are in the ordinary course of business, and are primarily for distribution, service fees, contract manufacturing and license agreements. PMI eliminated its respective share of all significant intercompany transactions with the equity method investees.
Product Warranty:
PMI's heat-not-burn devices and e-vapor products are subject to standard product warranties generally for a period of 12 months from the date of purchase or such other periods as required by law. PMI generally provides in cost of sales for the estimated cost of warranty in the period the related revenue is recognized. PMI assesses the adequacy of its accrued product warranties and adjusts the amounts as necessary based on actual experience and changes in future estimates. Factors that affect product warranties may vary across markets but typically include device version mix, product failure rates, logistics and service delivery costs, and warranty policies. PMI accounts for its product warranties within other accrued liabilities. At December 31, 2024 and December 31, 2023, these amounts were as follows:
| | | | | | | | |
| At December 31, |
| (in millions) | 2024 | 2023 |
| Balance at beginning of period | $ | 80 | | $ | 104 | |
| Changes due to: | | |
| Warranties issued | 76 | | 60 | |
| Settlements | (77) | | (83) | |
| Currency/Other | (3) | | (1) | |
| Balance at end of period | $ | 76 | | $ | 80 | |
Indebtedness:
Short-Term Borrowings
At December 31, 2024 and 2023, PMI’s short-term borrowings and related average interest rates consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2024 | | December 31, 2023 |
| (in millions) | Amount Outstanding | | Average Year-End Rate | | Amount Outstanding | | Average Year-End Rate |
| Commercial paper | $ | — | | | — | % | | $ | 1,685 | | | 5.6 | % |
| Bank loans | 137 | | | 8.6 | | | 283 | | | 8.9 | |
| | | | | | | |
| $ | 137 | | | | | $ | 1,968 | | | |
Given the mix of PMI's legal entities and their respective local economic environments, the average interest rate for bank loans above can vary significantly from day to day and country to country.
The fair values of PMI’s short-term borrowings at December 31, 2024 and 2023, based on current market interest rates, approximate carrying value.
Long-Term Debt
At December 31, 2024 and 2023, PMI’s long-term debt consisted of the following:
| | | | | | | | | | | |
| December 31, |
| (in millions) | 2024 | | 2023 |
| U.S. dollar notes, 0.875% to 6.375% (average interest rate 4.592%), due through 2044 | $ | 35,297 | | | $ | 30,272 | |
| Foreign currency obligations: | | | |
| Euro notes, 0.125% to 3.750% (average interest rate 2.062%), due through 2039 | 7,082 | | | 8,526 | |
| Swiss franc note, 1.625%, due 2024 | — | | | 299 | |
| Euro credit facility borrowings related to Swedish Match AB acquisition, (average interest rate 3.445%), due through 2027 | 2,610 | | | 6,121 | |
| Swedish krona notes, 1.395% to 2.710% (average interest rate 2.016%), due through 2029 | 218 | | | 236 | |
Other (average interest rate 5.378%), due through 2032 (a) | 351 | | | 487 | |
| Carrying value of long-term debt | 45,558 | | | 45,941 | |
| Less current portion of long-term debt | 3,392 | | | 4,698 | |
| $ | 42,166 | | | $ | 41,243 | |
(a) Includes long-term bank loans at subsidiaries, as well as $67 million and $53 million in finance leases at December 31, 2024 and 2023, respectively.
The fair value of PMI’s outstanding long-term debt, which is utilized solely for disclosure purposes, is determined using quotes and market interest rates currently available to PMI for issuances of debt with similar terms and remaining maturities. At December 31, 2024 and 2023 the fair value of PMI's outstanding long-term debt, excluding the aforementioned finance leases, was as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, |
| (in millions) | 2024 | | 2023 |
| Level 1 | $ | 41,431 | | | $ | 38,259 | |
| Level 2 | 3,011 | | | 6,687 | |
For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
Term Loan Facility related to the Financing of the Swedish Match Acquisition
On June 23, 2022, PMI entered into a €5.5 billion (approximately $5.8 billion at the date of signing) senior unsecured term loan credit agreement consisting of a €3.0 billion (approximately $3.2 billion at the date of signing) tranche expiring three years after the occurrence of certain events and a €2.5 billion (approximately $2.6 billion at the date of signing) tranche expiring on June 23, 2027.
On November 7, 2022, PMI delivered notices of borrowing for advances totaling €5.5 billion under the term loan facility, of which €3.0 billion would become due on November 9, 2025, and €2.5 billion would become due on June 23, 2027, unless prepaid pursuant to the terms of the credit agreement.
On November 21, 2024, PMI prepaid approximately €3 billion (approximately $3.2 billion), including outstanding principal and accrued interest, representing all borrowings outstanding under the 3-year tranche of the senior unsecured term loan facility. As of December 31, 2024, borrowings in the amount of €2.5 billion (approximately $2.6 billion) under the 5-year tranche of the term loan facility remained outstanding.
Notes Outstanding:
PMI’s notes outstanding at December 31, 2024, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | | | | | | | |
| Type | | Face Value | | Interest
Rate | | Issuance | | Maturity |
| U.S. dollar notes | | $750 | | 1.500% | | May 2020 | | May 2025 |
| U.S. dollar notes | | $750 | | 3.375% | | August 2015 | | August 2025 |
| U.S. dollar notes | | $750 | | 5.000% | | November 2022 | | November 2025 |
| U.S. dollar notes | | $750 | | 2.750% | | February 2016 | | February 2026 |
| U.S. dollar notes | | $1,250 | | 4.875% | | February 2023 | | February 2026 |
| U.S. dollar notes | (a) | $450 | | 4.875% | | May 2023 | | February 2026 |
| U.S. dollar notes | | $750 | | 0.875% | | November 2020 | | May 2026 |
| U.S. dollar notes | | $750 | | 4.750% | | February 2024 | | February 2027 |
| U.S. dollar notes | | $500 | | 3.125% | | August 2017 | | August 2027 |
| U.S. dollar notes | | $750 | | 4.375% | | November 2024 | | November 2027 |
| U.S. dollar notes | | $1,500 | | 5.125% | | November 2022 | | November 2027 |
| U.S. dollar notes | | $1,000 | | 4.875% | | February 2023 | | February 2028 |
| U.S. dollar notes | (b) | $550 | | 4.875% | | May 2023 | | February 2028 |
| U.S. dollar notes | | $500 | | 3.125% | | November 2017 | | March 2028 |
| U.S. dollar notes | (c) | $50 | | 4.000% | | May 2013 | | May 2028 |
| U.S. dollar notes | | $650 | | 5.250% | | September 2023 | | September 2028 |
| U.S. dollar notes | | $1,000 | | 4.875% | | February 2024 | | February 2029 |
| U.S. dollar notes | | $750 | | 3.375% | | May 2019 | | August 2029 |
| U.S. dollar notes | | $750 | | 4.625% | | November 2024 | | November 2029 |
| U.S. dollar notes | | $1,250 | | 5.625% | | November 2022 | | November 2029 |
| U.S. dollar notes | | $1,500 | | 5.125% | | February 2023 | | February 2030 |
| U.S. dollar notes | (d) | $700 | | 5.125% | | May 2023 | | February 2030 |
| U.S. dollar notes | | $750 | | 2.100% | | May 2020 | | May 2030 |
| U.S. dollar notes | | $700 | | 5.500% | | September 2023 | | September 2030 |
| U.S. dollar notes | | $750 | | 1.750% | | November 2020 | | November 2030 |
| U.S. dollar notes | | $750 | | 4.750% | | November 2024 | | November 2031 |
| U.S. dollar notes | | $1,500 | | 5.750% | | November 2022 | | November 2032 |
| U.S. dollar notes | | $1,250 | | 5.125% | | February 2024 | | February 2031 |
| U.S. dollar notes | | $1,500 | | 5.375% | | February 2023 | | February 2033 |
| U.S. dollar notes | (e) | $750 | | 5.375% | | May 2023 | | February 2033 |
| U.S. dollar notes | | $1,000 | | 5.625% | | September 2023 | | September 2033 |
| U.S. dollar notes | | $1,750 | | 5.250% | | February 2024 | | February 2034 |
| U.S. dollar notes | | $750 | | 4.900% | | November 2024 | | November 2034 |
| U.S. dollar notes | | $1,500 | | 6.375% | | May 2008 | | May 2038 |
| U.S. dollar notes | | $750 | | 4.375% | | November 2011 | | November 2041 |
| U.S. dollar notes | | $700 | | 4.500% | | March 2012 | | March 2042 |
| U.S. dollar notes | | $750 | | 3.875% | | August 2012 | | August 2042 |
| U.S. dollar notes | | $850 | | 4.125% | | March 2013 | | March 2043 |
| U.S. dollar notes | | $750 | | 4.875% | | November 2013 | | November 2043 |
| U.S. dollar notes | | $750 | | 4.250% | | November 2014 | | November 2044 |
| U.S. dollar notes | (f) | $500 | | 4.250% | | May 2016 | | November 2044 |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | | | | | | | |
| Type | | Face Value | | Interest
Rate | | Issuance | | Maturity |
| EURO notes | (g) | €750 (approximately $972) | | 2.750% | | March 2013 | | March 2025 |
| EURO notes | (c) | €200 (approximately $205) | | 1.200% | | November 2017 | | November 2025 |
| EURO notes | (c) | €50 (approximately $51) | | 1.200% | | December 2020 | | November 2025 |
| EURO notes | (c) | €50 (approximately $51) | | 1.200% | | June 2021 | | November 2025 |
| EURO notes | (g) | €1,000 (approximately $1,372) | | 2.875% | | March 2014 | | March 2026 |
| EURO notes | (g) | €500 (approximately $557) | | 0.125% | | August 2019 | | August 2026 |
| EURO notes | (c) | €300 (approximately $308) | | 0.875% | | February 2020 | | February 2027 |
| EURO notes | (g) | €500 (approximately $697) | | 2.875% | | May 2014 | | May 2029 |
| EURO notes | (g) | €500 (approximately $543) | | 3.750% | | June 2024 | | January 2031 |
| EURO notes | (g) | €750 (approximately $835) | | 0.800% | | August 2019 | | August 2031 |
| EURO notes | (g) | €500 (approximately $648) | | 3.125% | | June 2013 | | June 2033 |
| EURO notes | (g) | €500 (approximately $578) | | 2.000% | | May 2016 | | May 2036 |
| EURO notes | (g) | €500 (approximately $582) | | 1.875% | | November 2017 | | November 2037 |
| EURO notes | (g) | €750 (approximately $835) | | 1.450% | | August 2019 | | August 2039 |
| Swedish krona notes | (c) | SEK1,000 (approximately $95) | | 2.710% | | January 2019 | | January 2026 |
| Swedish krona notes | (c) | SEK700 (approximately $67) | | 1.395% | | February 2021 | | February 2026 |
| Swedish krona notes | (c) | SEK100 (approximately $10) | | 1.395% | | March 2021 | | February 2026 |
| Swedish krona notes | (c) | SEK200 (approximately $19) | | 1.395% | | September 2021 | | February 2026 |
| Swedish krona notes | (c) | SEK200 (approximately $19) | | 1.395% | | January 2022 | | February 2026 |
| Swedish krona notes | (c) | SEK300 (approximately $29) | | 2.190% | | April 2021 | | April 2029 |
(a) These notes are a further issuance of the 4.875% notes issued in February 2023.
(b) These notes are a further issuance of the 4.875% notes issued in February 2023.
(c) Notes issued by Swedish Match AB. USD equivalents for foreign currency notes were calculated based on exchange rates on the date of acquisition.
(d) These notes are a further issuance of the 5.125% notes issued in February 2023.
(e) These notes are a further issuance of the 5.375% notes issued in February 2023.
(f) These notes are a further issuance of the 4.250% notes issued by PMI in November 2014.
(g) USD equivalents for foreign currency notes were calculated based on exchange rates on the date of issuance.
The net proceeds from the sale of the securities listed in the table above were primarily used for general corporate purposes, including working capital requirements, repayment of commercial paper, and to refinance certain of our outstanding notes. On November 21, 2024, PMI financed the prepayment of the 3-year tranche of the senior unsecured term loan facility with the proceeds of the November 2024 bond issuances and cash on hand.
Aggregate maturities:
Aggregate maturities of long-term debt are as follows:
| | | | | |
| (in millions) | |
| 2025 | $ | 3,404 | |
| 2026 | 5,000 | |
| 2027 | 6,451 | |
| 2028 | 2,777 | |
| 2029 | 4,324 | |
| 2030-2034 | 15,656 | |
| 2035-2039 | 3,327 | |
| Thereafter | 5,050 | |
| 45,989 | |
| Debt discounts and fair value adjustments | (431) | |
| Total long-term debt | $ | 45,558 | |
Revolving Credit Facilities
At December 31, 2024, PMI’s total committed revolving credit facilities were as follows:
| | | | | | | | | |
Type
(in billions) | Committed Revolving Credit Facilities | | |
| 364-day revolving credit, expiring January 28, 2025 | $ | 1.7 | | | |
Multi-year revolving credit, expiring February 10, 2026 (1) | 2.0 | | | |
Multi-year revolving credit, expiring September 29, 2026 (2) (3) | 2.5 | | | |
| Total facilities | $ | 6.2 | | | |
| | | |
(1) On January 28, 2022, PMI entered into an agreement, effective February 10, 2022, to amend and extend the term of its $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(2) Includes pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets.
(3) On September 20, 2022, PMI entered into an agreement, effective September 29, 2022, to amend and extend the term of its $2.5 billion multi-year revolving credit facility, for an additional year covering the period September 30, 2026 to September 29, 2027, in the amount of $2.3 billion. On September 20, 2023, PMI entered into an agreement, effective September 29, 2023, to amend and further extend the term to September 29, 2028.
On December 17, 2024, PMI entered into a credit agreement, effective January 29, 2025, relating to a senior unsecured revolving credit facility with borrowings up to an aggregate principal amount of €1.5 billion, (approximately $1.6 billion) expiring on January 29, 2028. Concurrently, PMI did not request an extension of the maturity date of the existing 364-day revolving credit facility and the facility matured on January 28, 2025.
At December 31, 2024, there were no borrowings under these committed revolving credit facilities, and the entire committed amounts were available for borrowing.
In addition to the committed revolving credit facilities discussed above, PMI maintains certain short-term credit arrangements, including uncommitted credit lines, to primarily meet working capital needs. These credit arrangements amounted to approximately $2.1 billion at December 31, 2024, and approximately $2.7 billion at December 31, 2023. Borrowings under these arrangements and other bank loans amounted to $137 million at December 31, 2024, and $283 million at December 31, 2023.
Capital Stock:
Shares of authorized common stock are 6.0 billion; issued, repurchased and outstanding shares were as follows:
| | | | | | | | | | | | | | | | | |
| Shares Issued | | Shares
Repurchased | | Shares
Outstanding |
| Balances, January 1, 2022 | 2,109,316,331 | | | (559,146,338) | | | 1,550,169,993 | |
| Repurchase of shares | | | (1,966,730) | | | (1,966,730) | |
| Issuance of stock awards | | | 2,014,448 | | | 2,014,448 | |
| Balances, December 31, 2022 | 2,109,316,331 | | | (559,098,620) | | | 1,550,217,711 | |
| | | | | |
| Issuance of stock awards | | | 2,206,820 | | | 2,206,820 | |
| Balances, December 31, 2023 | 2,109,316,331 | | | (556,891,800) | | | 1,552,424,531 | |
| | | | | |
| Issuance of stock awards | | | 2,421,069 | | | 2,421,069 | |
| Balances, December 31, 2024 | 2,109,316,331 | | | (554,470,731) | | | 1,554,845,600 | |
On June 11, 2021, PMI's Board of Directors authorized a share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period that commenced in July 2021. From July 22, 2021 through March 31, 2022, PMI repurchased 10.5 million shares of its common stock at a cost of approximately $1.0 billion. During the first three months of 2022, PMI repurchased 2.0 million shares of its common stock at a cost of $199 million. On May 11, 2022, we announced the suspension of the three-year share repurchase program following the recommended public offer to acquire the outstanding shares of Swedish Match from its shareholders. For further details, see Note 3. Acquisitions and Divestitures. Prior to the suspension of the program, PMI made no share repurchases during the second quarter of 2022. The three-year share repurchase program expired on July 21, 2024.
At December 31, 2024, 27,288,770 shares of common stock were reserved for stock awards under PMI’s stock plans, and 250 million shares of preferred stock, without par value, were authorized but unissued. PMI currently has no plans to issue any shares of preferred stock.
Stock Plans:
In May 2022, PMI’s shareholders approved the Philip Morris International Inc. 2022 Performance Incentive Plan (the “2022 Plan”). Under the 2022 Plan, PMI may grant to eligible employees restricted shares and restricted share units, performance-based cash incentive awards and performance-based equity awards. Up to 25 million shares of PMI’s common stock may be issued under the 2022 Plan. At December 31, 2024, shares available for grant under the 2022 Plan were 19,189,876.
In May 2017, PMI’s shareholders approved the Philip Morris International Inc. 2017 Stock Compensation Plan for Non-Employee Directors (the “2017 Non-Employee Directors Plan”). A non-employee director is defined as a member of the PMI Board of Directors who is not a full-time employee of PMI or of any corporation in which PMI owns, directly or indirectly, stock possessing at least 50% of the total combined voting power of all classes of stock entitled to vote in the election of directors in such corporation. Up to 1 million shares of PMI common stock may be awarded under the 2017 Non-Employee Directors Plan. At December 31, 2024, shares available for grant under the plan were 855,920.
Restricted share unit (RSU) awards
PMI may grant RSU awards to eligible employees; recipients may not sell, assign, pledge or otherwise encumber such awards. Such awards are subject to forfeiture if certain employment conditions are not met. RSU awards generally vest on the third anniversary of the grant date. RSU awards do not carry voting rights, although they do earn dividend equivalents.
During 2024, the activity for RSU awards was as follows:
| | | | | | | | |
| Number of
Shares | Weighted-
Average Grant
Date Fair Value
Per Share |
| Balance at January 1, 2024 | 4,603,321 | | $ | 96.38 | |
| Granted | 2,013,350 | | 89.71 | |
| Vested | (1,849,088) | | 85.57 | |
| Forfeited | (224,929) | | 97.43 | |
| Balance at December 31, 2024 | 4,542,654 | | $ | 97.78 | |
During the years ended December 31, 2024, 2023 and 2022, the grant date fair value of the RSU awards granted to PMI employees and the recorded compensation expense related to RSU awards were as follows:
| | | | | | | | | | | | | | |
| (in millions, except per RSU award granted) | Total Grant Date Fair Value of RSU Awards Granted | | Weighted-Average Grant Date Fair Value Per RSU Award Granted | Compensation Expense related to RSU Awards |
| 2024 | $ | 181 | | | $ | 89.71 | | $ | 157 | |
| 2023 | $ | 179 | | | $ | 101.96 | | $ | 153 | |
| 2022 | $ | 174 | | | $ | 104.75 | | $ | 135 | |
The fair value of the RSU awards at the date of grant is amortized to expense over the restriction period, typically three years after the date of the award, or upon death, disability or reaching the age of 58. As of December 31, 2024, PMI had $154 million of total unrecognized compensation costs related to non-vested RSU awards. These costs are expected to be recognized over a weighted-average period of approximately seventeen months, or upon death, disability or reaching the age of 58.
During the years ended December 31, 2024, 2023 and 2022, share and fair value information for PMI RSU awards that vested were as follows:
| | | | | | | | | | | | | | |
| (dollars in millions) | Shares of RSU Awards that Vested | | Grant Date Fair Value of Vested Shares of RSU Awards | Total Fair Value of RSU Awards that Vested |
| 2024 | 1,849,088 | | | $ | 158 | | $ | 171 | |
| 2023 | 1,483,356 | | | $ | 129 | | $ | 148 | |
| 2022 | 1,603,571 | | | $ | 126 | | $ | 174 | |
Performance share unit (PSU) awards
PMI may grant PSU awards to certain executives; recipients may not sell, assign, pledge or otherwise encumber such awards. The PSU awards require the achievement of certain performance metrics, which are predetermined at the time of grant, typically over a three-year performance cycle. The performance metrics for such PSU's granted during 2023 and 2022 consisted of PMI's Total Shareholder Return ("TSR") relative to a predetermined peer group and on an absolute basis (40% weight), PMI’s currency-neutral compound annual adjusted diluted earnings per share growth rate (30% weight), and a Sustainability Index, which consists of two drivers:
•Product Sustainability (20% weight) measuring progress primarily on PMI's efforts to maximize the benefits of smoke-free products, purposefully phase out cigarettes, and reduce post-consumer waste; and
•Operational Sustainability (10% weight) measuring progress on PMI's efforts to benefit PMI and its stakeholders by tackling climate change, preserving nature, improving the quality of life of people in its supply chain, and fostering an empowered, and inclusive workplace.
The performance metrics, targets and relative weights for the PSU’s granted during 2024 were the same as the PSU’s granted during 2023 and 2022, with the exception of adjustments made to certain components of the Sustainability Index intended to address PMI's developing sustainability strategy and reporting.
The PSU performance metrics may be adjusted if appropriate to reflect the impact of unusual or infrequently occurring events, including, to the extent significant, corporate transactions, accounting or tax law changes, asset write-downs, litigation or claim adjustments, foreign exchange gains and losses, unbudgeted capital expenditures and other such events.
The aggregate of the weighted performance factors for the three metrics in each such PSU award determines the percentage of PSUs that will vest at the end of the three-year performance cycle. The minimum percentage of such PSUs that can vest is zero, with a target percentage of 100 and a maximum percentage of 200. Each such vested PSU entitles the participant to one share of common stock. An aggregate weighted PSU performance factor of 100 will result in the targeted number of PSUs being vested. At the end of the performance cycle, participants are entitled to an amount equivalent to the accumulated dividends paid on common stock during the performance cycle for the number of shares earned. PSU awards do not carry voting rights.
During 2024, the activity for PSU awards was as follows:
| | | | | | | | | | | | | | |
| Number of
Shares | | Weighted-
Average PSU Grant Date
Fair Value Subject to Other Performance Factors | Weighted-
Average PSU Grant Date
Fair Value Subject to TSR Performance Factors |
| | (Per Share) | (Per Share) |
| Balance at January 1, 2024 | 1,427,280 | | | $ | 95.45 | | $ | 126.86 | |
| Granted | 543,560 | | | 89.01 | | 85.72 | |
| Vested | (916,452) | | | 82.28 | | 107.69 | |
| Adjustments for performance achievement | 374,402 | | | 82.17 | | 107.70 | |
| Forfeited | (78,630) | | | 97.42 | | 117.24 | |
| Balance at December 31, 2024 | 1,350,160 | | | $ | 98.00 | | $ | 118.55 | |
During the years ended December 31, 2024, 2023 and 2022, the grant date fair value of the PSU awards granted to PMI employees and the recorded compensation expense related to PSU awards were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions, except per PSU award granted) | Weighted-
Average PSU Grant Date
Fair Value Subject to Other Performance Factors | | Weighted-
Average PSU Grant Date
Fair Value Subject to TSR Performance Factors | | Compensation Expense related to PSU Awards |
| Total | Per PSU Award | | Total | Per PSU Award | | Total |
| 2024 | $ | 29 | | $ | 89.01 | | | $ | 19 | | $ | 85.72 | | | $ | 70 | |
| 2023 | $ | 29 | | $ | 102.02 | | | $ | 26 | | $ | 133.54 | | | $ | 59 | |
| 2022 | $ | 30 | | $ | 104.92 | | | $ | 27 | | $ | 143.89 | | | $ | 48 | |
The grant date fair value of the PSU awards subject to the other performance factors was determined by using the market price of PMI’s stock on the date of the grant. The grant date fair value of the PSU market-based awards subject to the TSR performance factor was determined by using the Monte Carlo simulation model. The following assumptions were used to determine the grant date fair value of the PSU awards subject to the TSR performance factor for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2024 | | 2023 | | 2022 |
Average risk-free interest rate (a) | 4.2 | % | | 4.1 | % | | 1.7 | % |
Average expected volatility (b) | 19.9 | % | | 24.3 | % | | 28.3 | % |
(a) Based on the U.S. Treasury yield curve.
(b) Determined using the observed historical volatility.
The fair value of the PSU award at the date of grant is amortized to expense over the performance period, which is typically three years after the date of the award, or upon death, disability or reaching the age of 58. As of December 31, 2024, PMI had $31 million of total unrecognized compensation cost related to non-vested PSU awards. This cost is recognized over a weighted-average performance cycle period of approximately seventeen months, or upon death, disability or reaching the age of 58.
During the years ended December 31, 2024, 2023 and 2022, share and fair value information for PMI PSU awards that vested were as follows:
| | | | | | | | | | | | | | |
| (dollars in millions) | Shares of PSU Awards that Vested | | Grant Date Fair Value of Vested Shares of PSU Awards | Total Fair Value of PSU Awards that Vested |
| 2024 | 916,452 | | | $ | 86 | | $ | 83 | |
| 2023 | 902,232 | | | $ | 83 | | $ | 91 | |
| 2022 | 669,960 | | | $ | 54 | | $ | 74 | |
Earnings per Share:
Unvested share-based payment awards that contain non-forfeitable rights to dividends or dividend equivalents are participating securities and therefore are included in PMI’s earnings per share calculation pursuant to the two-class method.
Basic and diluted earnings per share (“EPS”) were calculated using the following:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Net earnings attributable to PMI | $ | 7,057 | | | $ | 7,813 | | | $ | 9,048 | |
Less distributed and undistributed earnings attributable to share-based payment awards (1) | 23 | | | 22 | | | 24 | |
| Net earnings for basic and diluted EPS | $ | 7,034 | | | $ | 7,791 | | | $ | 9,024 | |
| Weighted-average shares for basic EPS | 1,554 | | | 1,552 | | | 1,550 | |
Plus contingently issuable performance stock units (PSUs) (1) | 2 | | | 1 | | | 2 | |
| Weighted-average shares for diluted EPS | 1,556 | | | 1,553 | | | 1,552 | |
(1) Including rounding adjustment
For the 2024, 2023 and 2022 computations, there were no antidilutive stock awards.
Income Taxes:
Earnings before income taxes and provision for income taxes consisted of the following for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2024 | | 2023 | | 2022 |
| Earnings before income taxes | $ | 12,199 | | | $ | 10,450 | | | $ | 11,634 | |
| Provision for income taxes: | | | | | |
| United States federal and state: | | | | | |
| Current | $ | 465 | | | $ | 201 | | | $ | (75) | |
| Deferred | (81) | | | (368) | | | (139) | |
| Total United States | 384 | | | (167) | | | (214) | |
| Outside United States: | | | | | |
| Current | 2,668 | | | 2,468 | | | 2,553 | |
| Deferred | (35) | | | 38 | | | (95) | |
| Total outside United States | 2,633 | | | 2,506 | | | 2,458 | |
| Total provision for income taxes | $ | 3,017 | | | $ | 2,339 | | | $ | 2,244 | |
Changes in the tax laws of foreign jurisdictions could arise as a result of the Base Erosion and Profit Shifting project undertaken by the Organisation for Economic Co-operation and Development (“OECD”), which recommended changes to numerous long-standing tax principles. Many countries have enacted the OECD’s framework on a global minimum tax (referred to as “Pillar Two”), effective for taxable years beginning after December 31, 2023. PMI has determined that Pillar Two did not have a material impact on its 2024 consolidated financial statements.
At December 31, 2017, PMI recorded a one-time transition tax liability on its accumulated foreign earnings, which is payable over an eight-year period beginning in 2018. At December 31, 2023, $0.3 billion of PMI's remaining long-term portion of transition tax liability was recorded in "income taxes and other liabilities" on PMI's consolidated balance sheets. At December 31, 2024, PMI had no remaining long-term portion of transition tax liability as the final transition tax payment is anticipated to be made in the second quarter of 2025.
At December 31, 2024 and 2023, applicable U.S. federal income taxes have not been provided on approximately $1.6 billion and $0.6 billion, respectively, of accumulated earnings of Swedish Match U.S. subsidiaries that are expected to be permanently reinvested. PMI does not foresee a need to repatriate these earnings since its U.S. cash requirements are supported by distributions of earnings from PMI foreign entities that have not been designated as permanently reinvested and existing credit facilities. At December 31, 2024 and 2023, PMI has determined the amount of deferred tax liabilities related to these unremitted Swedish Match U.S. earnings was approximately $192 million and $71 million, respectively.
At December 31, 2024 and 2023, U.S. federal and foreign deferred income taxes have been provided on all accumulated earnings of PMI's foreign subsidiaries.
PMI is regularly examined by tax authorities around the world and is currently under examination in a number of jurisdictions. The U.S. federal statute of limitations on assessment remains open for the years 2019 and onward. Foreign and U.S. state jurisdictions have statutes of limitations generally ranging from 3 to 5 years after the filing of a return. Years still open to examination by foreign tax authorities in major jurisdictions include Germany (2018 onward), Indonesia (2019 onward), Italy (2019 onward), Russia (2021 onward) and Switzerland (2020 onward).
Subsidiaries of PMI in Indonesia, principally PT Hanjaya Mandala Sampoerna Tbk ("HMS"), have recorded income tax receivables in the amount of 4.0 trillion Indonesian rupiah (approximately $249 million) relating to corporate income tax assessments paid to avoid potential penalties, primarily for domestic and other intercompany transactions for the years 2015 to 2021. Objection letters have been filed with the Tax Office and these assessments are being challenged at various levels in court. These income tax receivables are included in other assets in PMI’s consolidated balance sheets at December 31, 2024 and 2023.
It is reasonably possible that within the next 12 months certain tax examinations will close, which could result in a change in unrecognized tax benefits along with related interest and penalties. An estimate of any possible change cannot be made at this time.
A reconciliation of the beginning and ending amount of unrecognized tax benefits was as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2024 | | 2023 | | 2022 |
| Balance at January 1, | $ | 55 | | | $ | 72 | | | $ | 89 | |
| Additions based on tax positions related to the current year | 6 | | | 7 | | | 12 | |
| Additions for tax positions of previous years | 1 | | | 1 | | | 2 | |
| Reductions for tax positions of prior years | — | | | (23) | | | (18) | |
| Reductions due to lapse of statute of limitations | (2) | | | (3) | | | (6) | |
| Settlements | — | | | — | | | (4) | |
| Other | (4) | | | 1 | | | (3) | |
| Balance at December 31, | $ | 56 | | | $ | 55 | | | $ | 72 | |
Unrecognized tax benefits and PMI’s liability for contingent income taxes, interest and penalties were as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | December 31, 2024 | | December 31, 2023 | | December 31, 2022 |
| Unrecognized tax benefits | $ | 56 | | | $ | 55 | | | $ | 72 | |
| Accrued interest and penalties | 11 | | | 9 | | | 13 | |
| Tax credits and other indirect benefits | (1) | | | (1) | | | (3) | |
| Liability for tax contingencies | $ | 66 | | | $ | 63 | | | $ | 82 | |
The amount of unrecognized tax benefits that, if recognized, would impact the effective tax rate was $56 million at December 31, 2024. The remainder, if recognized, would principally affect deferred taxes.
For the years ended December 31, 2024, 2023 and 2022, PMI recognized income (expense) in its consolidated statements of earnings of $(2) million, $5 million and $2 million, respectively, related to interest and penalties associated with uncertain tax positions.
The effective income tax rate on pre-tax earnings differed from the U.S. federal statutory rate for the following reasons for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| U.S. federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Increase (decrease) resulting from: | | | | | |
| Foreign rate differences | (1.6) | | | (1.0) | | | (0.5) | |
| Dividend repatriation cost | 0.6 | | | 0.7 | | | 0.6 | |
| | | | | |
| Global intangible low-taxed income | 1.7 | | | 2.0 | | | 1.0 | |
| U.S. state taxes | 0.6 | | | (0.1) | | | 0.1 | |
| Foreign derived intangible income | (0.7) | | | (0.9) | | | (0.8) | |
| Foreign exchange | 1.7 | | | (1.6) | | | (1.7) | |
| Non-deductible goodwill impairment | — | | | 1.3 | | | — | |
| Unremitted earnings of Russian subsidiaries | 0.6 | | | 1.7 | | | — | |
| Fair value adjustment of equity securities | 1.1 | | | 0.1 | | | 0.1 | |
| Other | (0.3) | | | (0.8) | | | (0.5) | |
| Effective tax rate | 24.7 | % | | 22.4 | % | | 19.3 | % |
The 2024 effective tax rate increased 2.3 percentage points to 24.7%. The change in the effective tax rate for 2024, as compared to 2023, was unfavorably impacted by: (i) an increase in deferred tax liabilities related to the fair value adjustment of equity securities held by PMI; (ii) U.S. state taxes; and (iii) a deferred tax charge for unrealized foreign currency losses on intercompany loans related to the Swedish Match acquisition financing reflected in the consolidated statements of earnings, while the underlying pre-tax foreign currency movements fully offset in the consolidated statements of earnings and were reflected as currency translation adjustments in its consolidated statements of stockholders' (deficit) equity, partially offset by: (i) a lower deferred tax charge in 2024 related to the unremitted earnings of PMI's Russian subsidiaries as compared to the 2023 charge following the suspension of certain double tax treaties; (ii) the non-deductible Wellness and Healthcare goodwill impairment charge recorded in 2023; and (iii) a U.S. tax benefit for a worthless stock deduction under section 165(g) of the Internal Revenue Code related to PMI's investment in C.A. Tabacalera Nacional, a wholly owned foreign corporation incorporated in Venezuela. For further details on PMI's ceased operations in Venezuela and the impairment loss related to the sale of Vectura Group, see Note 20. Restructuring Activities and Note 3. Acquisitions and Divestitures, respectively.
The 2023 effective tax rate increased 3.1 percentage points to 22.4%. The change in the effective tax rate for 2023, as compared to 2022, was unfavorably impacted by: (i) an increase in deferred tax liabilities related to the unremitted earnings of PMI's Russian subsidiaries due to the unilateral suspension of certain Russian double tax treaties by the Russian authorities on August 8, 2023, with respect to certain payments including dividends; (ii) the non-deductible Wellness and Healthcare goodwill impairment charge and (iii) an increase in foreign tax credit limitation related to GILTI, partially offset by changes in earnings mix by taxing jurisdiction.
The tax effects of temporary differences that gave rise to deferred income tax assets and liabilities consisted of the following:
| | | | | | | | | | | |
| At December 31, |
| (in millions) | 2024 | | 2023 |
| Deferred income tax assets: | | | |
| Accrued postretirement and postemployment benefits | $ | 208 | | | $ | 223 | |
| Accrued pension costs | 384 | | | 450 | |
| Inventory | 40 | | | 27 | |
| Accrued liabilities | 213 | | | 191 | |
| Net operating loss, tax credit, and other carryforwards | 912 | | | 501 | |
| Investments in equity interests | 507 | | | 80 | |
| Foreign exchange | — | | | 149 | |
| Other | 62 | | | 19 | |
| Total deferred income tax assets | 2,326 | | | 1,640 | |
| Less: valuation allowance | (1,130) | | | (369) | |
| Deferred income tax assets, net of valuation allowance | 1,196 | | | 1,271 | |
| Deferred income tax liabilities: | | | |
| Intangible assets | (1,862) | | | (2,136) | |
| Property, plant and equipment | (152) | | | (218) | |
| Unremitted earnings | (495) | | | (438) | |
| Foreign exchange | (264) | | | — | |
| Other | — | | | — | |
| Total deferred income tax liabilities | (2,773) | | | (2,792) | |
| Net deferred income tax assets (liabilities) | $ | (1,577) | | | $ | (1,521) | |
At December 31, 2024, PMI recorded deferred tax assets for net operating loss, tax credit, and other carryforwards of $912 million, with varying dates of expiration, primarily after 2029, including $381 million with an unlimited carryforward period. At December 31, 2024, PMI has recorded a valuation allowance of $1,130 million against deferred tax assets that do not meet the more-likely-than not recognition threshold.
At December 31, 2023, PMI recorded deferred tax assets for net operating loss, tax credit, and other carryforwards of $501 million, with varying dates of expiration, primarily after 2028, including $274 million with an unlimited carryforward period. At December 31, 2023, PMI has recorded a valuation allowance of $369 million against deferred tax assets that do not meet the more-likely-than-not recognition threshold.
Segment Reporting:
PMI’s subsidiaries and affiliates are primarily engaged in the manufacture and sale of cigarettes and smoke-free products, including heat-not-burn, e-vapor and oral nicotine products. Excluding the Wellness and Healthcare segment, PMI's segments are generally organized by geographic region and managed by segment managers who are responsible for the operating and financial results of the regions inclusive of combustible tobacco and smoke-free product categories sold in the region. As discussed in Note 1. Background and Basis of Presentation, in January 2024, PMI updated its segment reporting by including the former Swedish Match segment results into the four existing geographical segments. The four geographical segments are as follows: Europe Region; South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region ("SSEA, CIS & MEA"); East Asia, Australia, and PMI Duty Free Region ("EA, AU & PMI DF"); and Americas Region. The Wellness and Healthcare segment remains unchanged.
PMI’s Chief Executive Officer, who is the chief operating decision maker ("CODM") evaluates geographical segment performance based on the regional operating income, which includes results from all product categories sold in each region, excluding Wellness and Healthcare products. Business operations in the Wellness and Healthcare segment are evaluated separately. The CODM reviews short-term and long-term trends, forecasts, and budget-to-actual variances to assess geographical segment performance and to allocate resources. Interest expense, net, and provision for income taxes are centrally managed and, accordingly, such items are not presented
by segment since they are excluded from the measure of segment profitability reviewed by management. Information about total assets by segment is not disclosed because such information is not reported to or used by PMI’s CODM. Segment goodwill and other intangible assets, net, are disclosed in Note 5. Goodwill and Other Intangible Assets, net. The accounting policies of the segments are the same as those described in Note 2. Summary of Significant Accounting Policies.
PMI disaggregates its net revenues from contracts with customers by product category for each of PMI's four geographical segments, except the Wellness and Healthcare business. PMI believes this best depicts how the nature, amount, timing and uncertainty of its revenue and cash flows are affected by economic factors.
On November 27, 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update ASU 2023-07, “Improvements to Reportable Segment Disclosures” (“ASU 2023-07”). ASU 2023-07 improves reportable segment disclosures, primarily through enhanced disclosures about significant segment expenses that impact segment profit or loss, regularly provided to the chief operating decision maker. For further details, see Note 23. New Accounting Standards.
Net revenues, significant expenses, and operating income (loss) by segment were as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | Europe | SSEA, CIS & MEA | EA, AU & PMI DF | Americas | Wellness & Healthcare | Total |
For the Year Ended December 31, 2024 | | | | | | |
| Net revenues | $ | 15,357 | | $ | 11,261 | | $ | 6,393 | | $ | 4,534 | | $ | 333 | | $ | 37,878 | |
| Less: | | | | | | |
| Cost of sales | 4,206 | | 5,313 | | 2,011 | | 1,531 | | 268 | | 13,329 | |
| Marketing, administration and research costs | 4,213 | | 2,519 | | 1,504 | | 2,455 | | 456 | | 11,147 | |
| Operating income (loss) | $ | 6,938 | | $ | 3,429 | | $ | 2,878 | | $ | 548 | | $ | (391) | | $ | 13,402 | |
For the Year Ended December 31, 2023 | | | | | | |
| Net revenues | $ | 14,231 | | $ | 10,629 | | $ | 6,201 | | $ | 3,807 | | $ | 306 | | $ | 35,174 | |
| Less: | | | | | | |
| Cost of sales | 4,045 | | 5,109 | | 1,978 | | 1,485 | | 276 | | 12,893 | |
| Marketing, administration and research costs | 4,017 | | 2,384 | | 1,684 | | 1,740 | | 235 | | 10,060 | |
| Impairment of goodwill | — | | — | | — | | — | | 665 | | 665 | |
| Operating income (loss) | $ | 6,169 | | $ | 3,136 | | $ | 2,539 | | $ | 582 | | $ | (870) | | $ | 11,556 | |
For the Year Ended December 31, 2022 | | | | | | |
| Net revenues | $ | 12,972 | | $ | 10,467 | | $ | 5,936 | | $ | 2,116 | | $ | 271 | | $ | 31,762 | |
| Less: | | | | | | |
| Cost of sales | 3,656 | | 4,343 | | 2,026 | | 1,036 | | 341 | | 11,402 | |
| Marketing, administration and research costs | 3,540 | | 2,260 | | 1,486 | | 640 | | 188 | | 8,114 | |
| Operating income (loss) | $ | 5,776 | | $ | 3,864 | | $ | 2,424 | | $ | 440 | | $ | (258) | | $ | 12,246 | |
Total net revenues attributable to customers located in Japan, PMI's largest market in terms of net revenues, were $4.1 billion, $3.9 billion and $3.9 billion in 2024, 2023 and 2022, respectively. PMI had one customer in the EA, AU & PMI DF segment that accounted for 11%, 11% and 12% of PMI’s consolidated net revenues, and one customer in the Europe segment that accounted for 11%, 12% and 13% of PMI’s consolidated net revenues in 2024, 2023 and 2022, respectively.
PMI's net revenues by product category were as follows: | | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Combustible tobacco: | | | | | |
| Europe | $ | 8,599 | | | $ | 8,037 | | | $ | 7,694 | |
| SSEA, CIS & MEA | 9,848 | | | 9,321 | | | 9,173 | |
| EA, AU & PMI DF | 2,516 | | | 2,676 | | | 2,831 | |
| Americas | 2,255 | | | 2,299 | | | 1,874 | |
| Total combustible tobacco | 23,218 | | | 22,334 | | | 21,572 | |
| Smoke-free: | | | | | |
| Smoke-free excluding Wellness and Healthcare: | | | | | |
| Europe | 6,758 | | | 6,194 | | | 5,278 | |
| SSEA, CIS & MEA | 1,413 | | | 1,308 | | | 1,294 | |
| EA, AU & PMI DF | 3,877 | | | 3,525 | | | 3,105 | |
| Americas | 2,279 | | | 1,508 | | | 242 | |
| Total Smoke-free excluding Wellness and Healthcare | 14,327 | | | 12,534 | | | 9,919 | |
| Wellness and Healthcare | 333 | | | 306 | | | 271 | |
| Total Smoke-free | 14,660 | | | 12,840 | | | 10,190 | |
| | | | | |
| Total PMI net revenues | $ | 37,878 | | | $ | 35,174 | | | $ | 31,762 | |
Note: Sum of product categories or Regions might not foot to total PMI due to rounding.
Net revenues related to combustible tobacco refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of PMI's cigarettes and other tobacco products that are combusted. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos and do not include smoke-free products.
Net revenues related to smoke-free, excluding wellness and healthcare, refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes, if applicable. These net revenue amounts consist of the sale of PMI's products that are not combustible tobacco products, such as heat-not-burn, e-vapor, and oral products, as well as consumer accessories.
Net revenues related to wellness and healthcare consist of operating revenues generated from the sale of products primarily associated with inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of PMI's Wellness and Healthcare business.
Items affecting the comparability of results from operations were as follows:
•Egypt sales tax charge – In the third quarter of 2024, following a ruling issued by the Higher Administrative Court in Egypt and subsequent evaluation of available remedies, PMI concluded that an adverse outcome was probable and recorded a pre-tax charge of $45 million in relation to tax assessments for general sales tax deducted on imported cutfiller for the years 2014 to 2016. This pre-tax charge was recorded in marketing, administration and research costs in the consolidated statement of earnings for the year ended December 31, 2024, and was included in the SSEA, CIS & MEA segment results.
•Loss on sale of Vectura Group – In September 2024, PMI announced the execution of a definitive agreement to sell Vectura to Molex Asia Holdings Ltd. On December 31, 2024, we completed the sale. The sale resulted in a pre-tax loss of $199 million. This pre-tax loss was recorded in marketing, administration and research costs in the consolidated statement of earnings for the year ended December 31, 2024, and was included in the Wellness and Healthcare segment results. For further details, see Note 3. Acquisitions and Divestitures.
•Restructuring charges - See Note 20. Restructuring Activities for details of the $180 million and $109 million pre-tax charges for the year ended December 31, 2024 and 2023, respectively, as well as a breakdown of these costs by segment.
•Termination of distribution arrangement in the Middle East – In the first quarter of 2023, PMI recorded a pre-tax charge of $80 million following the termination of a distribution arrangement in the Middle East. This pre-tax charge was recorded as a reduction of net revenues in the consolidated statements of earnings, and was included in the SSEA, CIS & MEA segment results for the year ended December 31, 2023.
•Impairment of goodwill and other intangibles – For the year ended December 31, 2023, PMI recorded $680 million of goodwill and non-amortizable intangible assets impairment charges that was included in the Wellness and Healthcare segment. For the year ended December 31, 2022, PMI recorded an impairment charge related to definite-lived intangible assets of $112 million. This charge was included in the Wellness and Healthcare segment. For further details, see Note 5. Goodwill and Other Intangible Assets, net.
•South Korea indirect tax charge – On July 13, 2023, PMI's South Korean subsidiary, PM Korea, received an adverse ruling from the Supreme Court of South Korea related to cases alleging underpayment of excise taxes in connection with a 2015 excise tax increase and subsequent audit by the South Korean Board of Audit and Inspection. The Supreme Court ruling reversed previous decisions that were in PM Korea’s favor at the trial and appellate levels. As a result of the ruling, we concluded that an adverse outcome was probable. Consequently, we recorded a non-cash pre-tax charge of $204 million in marketing, administration and research costs in the consolidated statements of earning, reflecting the full amount previously paid by PM Korea, which was included in the EA, AU & PMI DF segment for the year ended December 31, 2023.
•Termination of agreement with Foundation for a Smoke-Free World – On September 29, 2023, PMI and the Foundation for a Smoke-Free World (the "Foundation") entered into the Final Grant Agreement and Termination of the Second Amended and Restated Pledge Agreement ("Agreement"). Under the terms of the agreement, PMI paid $140 million in the third quarter of 2023 in return for the termination of the pledge agreement between the parties. As a result, in the third quarter of 2023, PMI recorded a pre-tax charge of $140 million commensurate with the early termination of the pledge agreement. The pre-tax charge was recorded in marketing, administration and research costs in the consolidated statements of earnings for the year ended December 31, 2023 and was included in the operating results of the following segments: Europe ($60 million); SSEA, CIS & MEA ($41 million); EA, AU & PMI DF ($24 million); and Americas ($15 million).
•Charges related to the war in Ukraine – See Note 4. War in Ukraine for details of the $53 million and $151 million pre-tax charges in the Europe segment for the years ended December 31, 2023 and 2022, respectively.
•Swedish Match AB acquisition accounting related items – See Note 3. Acquisitions and Divestitures for details of the $18 million and $125 million pre-tax purchase accounting adjustments related to the sale of acquired inventories stepped up to fair value. These pre-tax purchase accounting adjustments were included in the Americas segment ($18 million in 2023 and $77 million in 2022) and the Europe segment ($48 million in 2022).
Other segment data were as follows:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Depreciation and amortization expense: | | | | | |
| Europe | $ | 492 | | | $ | 479 | | | $ | 388 | |
| SSEA, CIS & MEA | 304 | | | 304 | | | 340 | |
| EA, AU & PMI DF | 173 | | | 144 | | | 167 | |
| Americas | 748 | | | 387 | | | 97 | |
| Wellness & Healthcare | 70 | | | 84 | | | 85 | |
| Total depreciation and amortization expense | $ | 1,787 | | | $ | 1,398 | | | $ | 1,077 | |
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Capital expenditures: | | | | | |
| Europe | $ | 783 | | | $ | 905 | | | $ | 657 | |
| SSEA, CIS & MEA | 306 | | | 287 | | | 258 | |
| EA, AU & PMI DF | 22 | | | 38 | | | 25 | |
| Americas | 280 | | | 57 | | | 92 | |
| Wellness & Healthcare | 53 | | | 34 | | | 45 | |
| Total capital expenditures | $ | 1,444 | | | $ | 1,321 | | | $ | 1,077 | |
PMI’s total property, plant and equipment, net and other assets by geographic area were:
| | | | | | | | | | | | | | | | | |
| At December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Long-lived assets: | | | | | |
| Europe | $ | 5,540 | | | $ | 5,697 | | | $ | 5,179 | |
| SSEA, CIS & MEA | 2,160 | | | 2,197 | | | 2,047 | |
| East Asia and Australia | 378 | | | 481 | | | 675 | |
| Americas | 1,559 | | | 1,310 | | | 1,282 | |
| Total long-lived assets | 9,637 | | | 9,685 | | | 9,183 | |
| Altria Group, Inc. agreement | — | | | 2,777 | | | 1,002 | |
| Financial instruments | 456 | | | 701 | | | 456 | |
| Total property, plant and equipment, net and Other assets | $ | 10,093 | | | $ | 13,163 | | | $ | 10,641 | |
Long-lived assets consist of non-current assets other than goodwill; other intangible assets, net; deferred tax assets, equity investments, financial instruments and payment under the agreement with Altria Group, Inc., see Note 3, Acquisitions and Divestitures and Note 18, Contingencies. PMI's largest markets in terms of long-lived assets are Switzerland, Indonesia and Italy. Total long-lived assets located in Switzerland, which is reflected in the Europe segment above, were $1.4 billion, $1.6 billion and $1.4 billion at December 31, 2024, 2023 and 2022, respectively. Total long-lived assets located in Indonesia, which is reflected in the SSEA, CIS & MEA segment above, were $1.0 billion, $1.1 billion and $0.9 billion at December 31, 2024, 2023 and 2022, respectively. Total long-lived assets located in Italy, which is reflected in the Europe segment above, were $1.0 billion, $1.0 billion and $0.9 billion at December 31, 2024, 2023 and 2022, respectively.
Benefit Plans:
Pension coverage for employees of PMI’s subsidiaries is provided, to the extent deemed appropriate, through separate plans, many of which are governed by local statutory requirements. In addition, PMI provides health care and other benefits to certain U.S. retired employees and certain non-U.S. retired employees. In general, health care benefits for non-U.S. retired employees are covered through local government plans.
Pension and other employee benefit costs per the consolidated statements of earnings consisted of the following for December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2024 | | 2023 | | 2022 |
| Net pension costs (income) | $ | (76) | | | $ | (84) | | | $ | (93) | |
| Net postemployment costs | 123 | | | 117 | | | 107 | |
| Net postretirement costs | 13 | | | 12 | | | 10 | |
| Total pension and other employee benefit costs | $ | 60 | | | $ | 45 | | | $ | 24 | |
Pension and Postretirement Benefit Plans
Obligations and Funded Status
The projected benefit obligations, plan assets and funded status of PMI’s pension plans, and the accumulated benefit obligation, plan assets and net amount accrued for PMI's postretirement health care plans, at December 31, 2024 and 2023, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Pension(1) | | Postretirement |
| (in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Benefit obligation at January 1 | $ | 10,567 | | | $ | 8,606 | | | $ | 246 | | | $ | 229 | |
| Service cost | 218 | | | 174 | | | 3 | | | 4 | |
| Interest cost | 234 | | | 258 | | | 13 | | | 12 | |
| Benefits paid | (434) | | | (520) | | | (14) | | | (13) | |
| Employee contributions | 157 | | | 145 | | | — | | | — | |
| Settlement, curtailment and plan amendment | (12) | | | (17) | | | (1) | | | — | |
| | | | | | | |
| Actuarial losses (gains) | 460 | | | 1,209 | | | 2 | | | 24 | |
| Currency | (708) | | | 763 | | | 5 | | | (4) | |
| | | | | | | |
| Other | (26) | | | (51) | | | (6) | | | (6) | |
| Benefit obligation at December 31, | 10,456 | | | 10,567 | | | 248 | | | 246 | |
| Fair value of plan assets at January 1, | 8,851 | | | 7,939 | | | 3 | | | 3 | |
| Actual return on plan assets | 973 | | | 643 | | | — | | | — | |
| Employer contributions, net of refunds | 110 | | | 21 | | | 14 | | | 13 | |
| Employee contributions | 157 | | | 145 | | | — | | | — | |
| Benefits paid | (434) | | | (520) | | | (14) | | | (13) | |
| Settlement | (10) | | | (17) | | | (1) | | | — | |
| Currency | (600) | | | 639 | | | 1 | | | — | |
| | | | | | | |
| Other | (17) | | | 1 | | | — | | | — | |
| Fair value of plan assets at December 31, | 9,030 | | | 8,851 | | | 3 | | | 3 | |
| Net pension and postretirement liability recognized at December 31, | $ | (1,426) | | | $ | (1,716) | | | $ | (245) | | | $ | (243) | |
(1) Primarily non-U.S. based defined benefit retirement plans.
At December 31, 2024, actuarial losses (gains) consisted primarily of losses for assumption changes related to lower discount rates year-over-year for the Swiss plan. At December 31, 2023, actuarial losses (gains) consisted primarily of losses for assumption changes related to lower discount rates year-over-year for the Swiss, German and Dutch plans.
At December 31, 2024 and 2023, the Swiss pension plan represented 69% and 67% of the benefit obligation, respectively, and approximately 63% and 62% of the fair value of plan assets at December 31, 2024 and 2023, respectively. At December 31, 2024 and 2023, the U.S. pension plans represented 6% and 6% of the benefit obligation, respectively, and approximately 6% and 6% of the fair value of plan assets at December 31, 2024 and 2023, respectively.
At December 31, 2024 and 2023, the amounts recognized on PMI's consolidated balance sheets for the pension and postretirement plans were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Pension | | Postretirement |
| (in millions) | 2024 | | 2023 | | 2024 | | 2023 |
| Other assets | $ | 490 | | | $ | 294 | | | | | |
| Accrued liabilities — employment costs | (33) | | | (31) | | | $ | (14) | | | $ | (12) | |
| Long-term employment costs | (1,883) | | | (1,979) | | | (231) | | | (231) | |
| $ | (1,426) | | | $ | (1,716) | | | $ | (245) | | | $ | (243) | |
The accumulated benefit obligation, which represents benefits earned to date, for the pension plans was $9.9 billion and $10.0 billion at December 31, 2024 and 2023, respectively.
For pension plans with accumulated benefit obligations in excess of plan assets, the accumulated benefit obligation and fair value of plan assets were $7.7 billion and $6.2 billion, respectively, as of December 31, 2024. The accumulated benefit obligation and fair value of plan assets were $8.8 billion and $7.2 billion, respectively, as of December 31, 2023.
For pension plans with projected benefit obligations in excess of plan assets, the projected benefit obligation and fair value of plan assets were $8.2 billion and $6.2 billion, respectively, as of December 31, 2024. The projected benefit obligation and fair value of plan assets were $9.2 billion and $7.2 billion, respectively, as of December 31, 2023.
The following weighted-average assumptions were used to determine PMI’s pension and postretirement benefit obligations at December 31:
| | | | | | | | | | | | | | | | | | | | | | | |
| Pension | | Postretirement |
| 2024 | | 2023 | | 2024 | | 2023 |
| Discount rate | 2.07 | % | | 2.28 | % | | 5.35 | % | | 5.19 | % |
| Rate of compensation increase | 1.89 | | | 2.05 | | | | | |
| Interest crediting rate | 3.05 | | | 2.99 | | | | | |
| Health care cost trend rate assumed for next year | | | | | 6.82 | | | 6.54 | |
| Ultimate trend rate | | | | | 4.71 | | | 4.49 | |
| Year that rate reaches the ultimate trend rate | | | | | 2048 | | 2047 |
The discount rate for the largest pension plans is based on a yield curve constructed from a portfolio of high quality corporate bonds that produces a cash flow pattern equivalent to each plan’s expected benefit payments. The discount rate for the remaining plans is developed from local bond indices that match local benefit obligations as closely as possible.
Components of Net Periodic Benefit Cost
Net periodic pension and postretirement health care costs consisted of the following for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Pension | | Postretirement |
| (in millions) | 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Service cost | $ | 218 | | | $ | 174 | | | $ | 233 | | | $ | 3 | | | $ | 4 | | | $ | 2 | |
| Interest cost | 234 | | | 258 | | | 78 | | | 13 | | | 12 | | | 6 | |
| Expected return on plan assets | (403) | | | (365) | | | (352) | | | — | | | — | | | — | |
| Amortization: | | | | | | | | | | | |
| Net losses | 93 | | | 18 | | | 181 | | | (1) | | | (1) | | | 2 | |
| Prior service cost (credit) | (2) | | | (2) | | | (2) | | | — | | | — | | | — | |
| Net transition obligation | — | | | — | | | — | | | — | | | — | | | — | |
| Settlement and curtailment | 2 | | | 7 | | | 2 | | | 1 | | | 1 | | | 2 | |
| Net periodic pension and postretirement costs | $ | 142 | | | $ | 90 | | | $ | 140 | | | $ | 16 | | | $ | 16 | | | $ | 12 | |
Settlement and curtailment charges were due primarily to employee severance and early retirement programs.
All of the amounts in the table above, other than service cost, are recognized in pension and other employee benefit costs in the consolidated statement of earnings.
The following weighted-average assumptions were used to determine PMI’s net pension and postretirement health care costs:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Pension | | Postretirement |
| 2024 | | 2023 | | 2022 | | 2024 | | 2023 | | 2022 |
| Discount rate - service cost | 2.68 | % | | 3.27 | % | | 1.03 | % | | 5.19 | % | | 5.89 | % | | 3.08 | % |
| Discount rate - interest cost | 2.34 | | | 3.03 | | | 0.71 | | | 5.19 | | | 5.89 | | | 3.08 | |
| Expected rate of return on plan assets | 4.63 | | | 4.42 | | | 4.17 | | | | | | | |
| Rate of compensation increase | 2.05 | | | 1.98 | | | 1.77 | | | | | | | |
| Interest crediting rate | 2.99 | | | 2.97 | | | 3.15 | | | | | | | |
| Health care cost trend rate | | | | | | | 6.54 | | | 6.14 | | | 6.27 | |
PMI’s expected rate of return on pension plan assets is determined by the plan assets’ historical long-term investment performance, current asset allocation and estimates of future long-term returns by asset class.
PMI and certain of its subsidiaries sponsor defined contribution plans. Amounts charged to expense for defined contribution plans totaled $123 million, $111 million and $82 million for the years ended December 31, 2024, 2023 and 2022, respectively.
Plan Assets
PMI’s investment strategy for pension plans is based on an expectation that equity securities will outperform debt securities over the long term. Accordingly, the target allocation of PMI’s plan assets is broadly characterized as approximately 55% in equity securities and approximately 45% in debt securities and other assets. The strategy primarily utilizes indexed U.S. equity securities, international equity securities and investment-grade debt securities. PMI attempts to mitigate investment risk by rebalancing between equity and debt asset classes once a year or as PMI’s contributions and benefit payments are made.
The fair value of PMI’s pension plan assets at December 31, 2024 and 2023, by asset category was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Asset Category
(in millions) | At December 31, 2024 | | Quoted Prices
In Active
Markets for
Identical
Assets/Liabilities
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
| Cash and cash equivalents | $ | 83 | | | $ | 83 | | | | | | |
| Equity securities: | | | | | | | | |
| U.S. securities | 144 | | | 144 | | | | | | |
| International securities | 525 | | | 525 | | | | | | |
Investment funds(a) | 7,317 | | | 5,245 | | | $ | 2,072 | | | | |
| Government bonds | 238 | | | 166 | | | 72 | | | | |
| Corporate bonds | 409 | | | 409 | | | | | | |
| Other | 31 | | | — | | | 3 | | | 28 | | (c) |
| Total assets in the fair value hierarchy | $ | 8,747 | | | $ | 6,572 | | | $ | 2,147 | | | $ | 28 | | |
Investment funds measured at net asset value(b) | 283 | | | | | | | | |
| Total assets | $ | 9,030 | | | | | | | | |
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI — Europe, Switzerland, North America, Asia Pacific, Japan, Emerging Markets and Small Cap for equities / FTSE EMU, FTSE Non-EGBI EuroBIG, SBI AAA-BBB and JP Morgan EMBI for bonds / SXI Real Estate and KGAST for real estate) , primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 57% are invested in U.S. and international equities; 13% are invested in U.S. and international government bonds; 14% are invested in corporate bonds and 16% are invested in real estate.
(b) In accordance with FASB ASC Subtopic 820-10, certain investments measured at fair value using the net asset value per share practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented in the statement of financial position.
(c) Amount relates to annuity policies of which the fair value is calculated using an actuarial model.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Asset Category
(in millions) | At December 31, 2023 | | Quoted Prices
In Active
Markets for
Identical
Assets/Liabilities
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
| Cash and cash equivalents | $ | 117 | | | $ | 117 | | | | | | |
| Equity securities: | | | | | | | | |
| U.S. securities | 158 | | | 158 | | | | | | |
| International securities | 569 | | | 569 | | | | | | |
Investment funds(a) | 7,123 | | | 5,366 | | | $ | 1,757 | | | | |
| Government bonds | 255 | | | 183 | | | 72 | | | | |
| Corporate bonds | 320 | | | 320 | | | | | | |
| Other | 37 | | | — | | | 5 | | | 32 | | (c) |
| Total assets in the fair value hierarchy | $ | 8,579 | | | $ | 6,713 | | | $ | 1,834 | | | $ | 32 | | |
Investment funds measured at net asset value(b) | 272 | | | | | | | | |
| Total assets | $ | 8,851 | | | | | | | | |
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI — Europe, Switzerland, North America, Asia Pacific, Japan, Emerging Markets for equities, and FTSE EMU, FTSE Non-EGBI EuroBIG, SBI AAA-BBB and JP Morgan EMBI for bonds), primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 57% were invested in U.S. and international equities; 15% were invested in U.S. and international government bonds; 15% were invested in corporate bonds, and 13% were invested in real estate.
(b) In accordance with FASB ASC Subtopic 820-10, certain investments measured at fair value using the net asset value per share practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented in the statement of financial position.
(c) Amount relates to annuity policies of which the fair value is calculated using an actuarial model.
For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Note 2. Summary of Significant Accounting Policies.
PMI makes, and plans to make, contributions, to the extent that they are tax deductible and meet specific funding requirements of its funded pension plans. Currently, PMI anticipates making contributions of approximately $161 million in 2025 to its pension plans, based on current tax and benefit laws. However, this estimate is subject to change as a result of changes in tax and other benefit laws, as well as asset performance significantly above or below the assumed long-term rate of return on pension assets, or changes in interest and currency rates.
The estimated future benefit payments from PMI pension plans at December 31, 2024, are as follows:
| | | | | |
| (in millions) | |
| 2025 | $ | 444 | |
| 2026 | 452 | |
| 2027 | 459 | |
| 2028 | 466 | |
| 2029 | 479 | |
| 2030 - 2034 | 2,630 | |
PMI's expected future annual benefit payments for its postretirement health care plans are estimated to be not material through 2034.
Postemployment Benefit Plans
PMI and certain of its subsidiaries sponsor postemployment benefit plans covering certain designated salaried and hourly employees. The cost of these plans is charged to expense over the working life of the covered employees. Net postemployment costs were $238 million, $213 million and $184 million for the years ended December 31, 2024, 2023 and 2022, respectively.
The amounts recognized in accrued postemployment costs net of plan assets on PMI's consolidated balance sheets at December 31, 2024 and 2023, were $929 million and $915 million, respectively.
The accrued postemployment costs were determined using a weighted-average discount rate of 4.9% and 4.3% in 2024 and 2023, respectively; an assumed ultimate annual weighted-average turnover rate of 2.9% and 2.8% in 2024 and 2023, respectively; assumed compensation cost increases of 2.3% in 2024 and 2.4% in 2023, and assumed benefits as defined in the respective plans. In accordance with local regulations, certain postemployment plans are funded. As a result, the accrued postemployment costs disclosed above are presented net of the related assets of $30 million and $33 million at December 31, 2024 and 2023, respectively. Postemployment costs arising from actions that offer employees benefits in excess of those specified in the respective plans are charged to expense when incurred.
Comprehensive Earnings (Losses)
The amounts recorded in accumulated other comprehensive losses at December 31, 2024, consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Net (losses) gains | $ | (2,122) | | | $ | (36) | | | $ | (815) | | | $ | (2,973) | |
| Prior service (cost) credit | 75 | | | 1 | | | (21) | | | 55 | |
| Net transition (obligation) asset | (3) | | | — | | | — | | | (3) | |
| Deferred income taxes | 246 | | | 19 | | | 195 | | | 460 | |
| Losses to be amortized | $ | (1,804) | | | $ | (16) | | | $ | (641) | | | $ | (2,461) | |
The amounts recorded in accumulated other comprehensive losses at December 31, 2023, consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Net (losses) gains | $ | (2,325) | | | $ | (36) | | | $ | (770) | | | $ | (3,131) | |
| Prior service (cost) credit | 77 | | | 1 | | | (21) | | | 57 | |
| Net transition (obligation) asset | (3) | | | — | | | — | | | (3) | |
| Deferred income taxes | 283 | | | 19 | | | 186 | | | 488 | |
| Losses to be amortized | $ | (1,968) | | | $ | (16) | | | $ | (605) | | | $ | (2,589) | |
The amounts recorded in accumulated other comprehensive losses at December 31, 2022, consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Net (losses) gains | $ | (1,437) | | | $ | (14) | | | $ | (753) | | | $ | (2,204) | |
| Prior service (cost) credit | 70 | | | 1 | | | (21) | | | 50 | |
| Net transition (obligation) asset | (3) | | | — | | | — | | | (3) | |
| Deferred income taxes | 138 | | | 14 | | | 183 | | | 335 | |
| Losses to be amortized | $ | (1,232) | | | $ | 1 | | | $ | (591) | | | $ | (1,822) | |
The movements in other comprehensive earnings (losses) during the year ended December 31, 2024, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Amounts transferred to earnings: | | | | | | | |
| Amortization: | | | | | | | |
| Net losses (gains) | $ | 88 | | | $ | 1 | | | $ | 86 | | | $ | 175 | |
| Prior service cost (credit) | (6) | | | — | | | — | | | (6) | |
| Net transition obligation (asset) | — | | | — | | | — | | | — | |
| Other income/expense: | | | | | | | |
| Net losses (gains) | 1 | | | 1 | | | — | | | 2 | |
| Prior service cost (credit) | — | | | — | | | — | | | — | |
| Deferred income taxes | (13) | | | (2) | | | (21) | | | (36) | |
| 70 | | | — | | | 65 | | | 135 | |
| Other movements during the year: | | | | | | | |
| Net (losses) gains | 114 | | | (2) | | | (131) | | | (19) | |
| Prior service (cost) credit | 4 | | | — | | | — | | | 4 | |
| | | | | | | |
| | | | | | | |
| Deferred income taxes | (24) | | | 2 | | | 30 | | | 8 | |
| 94 | | | — | | | (101) | | | (7) | |
| Total movements in other comprehensive earnings (losses) | $ | 164 | | | $ | — | | | $ | (36) | | | $ | 128 | |
The movements in other comprehensive earnings (losses) during the year ended December 31, 2023, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Amounts transferred to earnings: | | | | | | | |
| Amortization: | | | | | | | |
| Net losses (gains) | $ | 19 | | | $ | 1 | | | $ | 76 | | | $ | 96 | |
| Prior service cost (credit) | 7 | | | — | | | — | | | 7 | |
| Net transition obligation (asset) | — | | | — | | | — | | | — | |
| Other income/expense: | | | | | | | |
| Net losses (gains) | 11 | | | 1 | | | — | | | 12 | |
| Prior service cost (credit) | — | | | — | | | — | | | — | |
| Deferred income taxes | (9) | | | (1) | | | (18) | | | (28) | |
| 28 | | | 1 | | | 58 | | | 87 | |
| Other movements during the year: | | | | | | | |
| Net (losses) gains | (918) | | | (24) | | | (93) | | | (1,035) | |
| Prior service (cost) credit | — | | | — | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| Deferred income taxes | 154 | | | 6 | | | 21 | | | 181 | |
| (764) | | | (18) | | | (72) | | | (854) | |
| Total movements in other comprehensive earnings (losses) | $ | (736) | | | $ | (17) | | | $ | (14) | | | $ | (767) | |
The movements in other comprehensive earnings (losses) during the year ended December 31, 2022, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Pension | | Post-
retirement | | Post-
employment | | Total |
| Amounts transferred to earnings: | | | | | | | |
| Amortization: | | | | | | | |
| Net losses (gains) | $ | 178 | | | $ | 3 | | | $ | 85 | | | $ | 266 | |
| Prior service cost (credit) | (4) | | | — | | | — | | | (4) | |
| | | | | | | |
| Other income/expense: | | | | | | | |
| Net losses (gains) | 2 | | | 1 | | | — | | | 3 | |
| Prior service cost (credit) | — | | | — | | | 1 | | | 1 | |
| Deferred income taxes | (28) | | | (1) | | | (20) | | | (49) | |
| 148 | | | 3 | | | 66 | | | 217 | |
| Other movements during the year: | | | | | | | |
| Net (losses) gains | 878 | | | 46 | | | 46 | | | 970 | |
| Prior service (cost) credit | 3 | | | — | | | — | | | 3 | |
| | | | | | | |
| | | | | | | |
| Deferred income taxes | (112) | | | (9) | | | (11) | | | (132) | |
| 769 | | | 37 | | | 35 | | | 841 | |
| Total movements in other comprehensive earnings (losses) | $ | 917 | | | $ | 40 | | | $ | 101 | | | $ | 1,058 | |
Additional Information:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Depreciation expense | $ | 952 | | | $ | 901 | | | $ | 918 | |
| Research and development expense | $ | 759 | | | $ | 709 | | | $ | 642 | |
| Advertising expense | $ | 1,069 | | | $ | 965 | | | $ | 777 | |
| Foreign currency net transaction (gains)/losses | $ | 302 | | | $ | 305 | | | $ | 199 | |
| Interest expense | $ | 1,763 | | | $ | 1,526 | | | $ | 768 | |
| Interest income | (620) | | | (465) | | | (180) | |
| Interest expense, net | $ | 1,143 | | | $ | 1,061 | | | $ | 588 | |
Financial Instruments:
Overview
PMI operates globally with manufacturing and sales facilities in various locations around the world and is exposed to risks such as changes in foreign currency exchange rates and interest rates. As a result, PMI uses deliverable and non-deliverable forward foreign exchange contracts, foreign currency swaps and foreign currency options, (collectively referred to as "foreign exchange contracts"), and interest rate contracts to mitigate its exposure to changes in foreign currency exchange and interest rates related to net investments in foreign operations, third-party and intercompany actual and forecasted transactions. The primary currencies to which PMI is exposed include the Euro, Egyptian pound, Indonesian rupiah, Japanese yen, Mexican peso, Philippine peso, Polish zloty, Russian ruble and Swiss franc.
Additionally, certain materials that PMI uses in the manufacturing of its products are exposed to market price risks. PMI uses commodity derivative contracts (“commodity contracts") to manage its exposure to the market price volatility of certain commodity components of these materials.
These foreign exchange contracts, interest rate contracts and commodity contracts are collectively referred to as "derivative contracts". PMI is not a party to leveraged derivatives and, by policy, does not use derivative financial instruments for speculative purposes. Substantially all of PMI's derivative financial instruments are subject to master netting arrangements, whereby the right to offset occurs in the event of default by a participating party. While these contracts contain the enforceable right to offset through close-out netting rights, PMI elects to present them on a gross basis in the consolidated balance sheets. Collateral associated with these arrangements is in the form of cash and is unrestricted. Changes in collateral posted are included in cash flows from investing activities and changes in collateral received are included in cash flows from financing activities. Financial instruments qualifying for hedge accounting must maintain a specified level of effectiveness between the hedging instrument and the item being hedged, both at inception and throughout the hedged period. PMI formally documents the nature and relationships between the hedging instruments and hedged items, as well as its risk-management objectives, strategies for undertaking the various hedge transactions and method of assessing hedge effectiveness. Additionally, for hedges of forecasted transactions, the significant characteristics and expected terms of the forecasted transaction must be specifically identified, and it must be probable that each forecasted transaction will occur. If it were deemed probable that the forecasted transaction would not occur, the gain or loss would be recognized in earnings.
The gross notional amounts for outstanding derivatives as of December 31, 2024 and 2023, were as follows:
| | | | | | | | |
| (in millions) | 2024 | 2023 |
| Derivative contracts designated as hedging instruments: | | |
| Foreign exchange contracts | $ | 25,149 | | $ | 21,987 | |
| Interest rate contracts | 3,000 | | 3,600 | |
| Commodity contracts | 9 | | 20 | |
| | |
| Derivative contracts not designated as hedging instruments: | | |
| Foreign exchange contracts | 15,942 | | 17,658 | |
| Total | $ | 44,100 | | $ | 43,265 | |
The fair value of PMI’s derivative contracts included in the consolidated balance sheets as of December 31, 2024 and 2023, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Derivative Assets | | Derivative Liabilities |
| | | Fair Value | | | | Fair Value |
| (in millions) | Balance Sheet Classification | | 2024 | | 2023 | | Balance Sheet Classification | | 2024 | | 2023 |
| Derivative contracts designated as hedging instruments: | | | | | | | | | | | |
| Foreign exchange contracts | Other current assets | | $ | 772 | | | $ | 345 | | | Other accrued liabilities | | $ | 7 | | | $ | 249 | |
| Other assets | | 299 | | | 153 | | | Income taxes and other liabilities | | 60 | | | 449 | |
| Interest rate contracts | Other current assets | | — | | | 1 | | | Other accrued liabilities | | 50 | | | 78 | |
| Other assets | | 4 | | | — | | | Income taxes and other liabilities | | 3 | | | 18 | |
| Commodity contracts | Other current assets | | — | | | — | | | Other accrued liabilities | | — | | | 5 | |
| Other assets | | — | | | — | | | Income taxes and other liabilities | | — | | | 1 | |
| Derivative contracts not designated as hedging instruments: | | | | | | | | | | | |
| Foreign exchange contracts | Other current assets | | 331 | | | 85 | | | Other accrued liabilities | | 69 | | | 425 | |
| Other assets | | 12 | | | — | | | Income taxes and other liabilities | | 58 | | | 143 | |
| Total gross amount derivatives contracts presented in the consolidated balance sheets | | | $ | 1,418 | | | $ | 584 | | | | | $ | 247 | | | $ | 1,368 | |
| Gross amounts not offset in the consolidated balance sheets | | | | | | | | | | | |
| Financial instruments | | | (218) | | | (374) | | | | | (218) | | | (374) | |
| Cash collateral received/pledged | | | (863) | | | (109) | | | | | (18) | | | (551) | |
| Net amount | | | $ | 337 | | | $ | 101 | | | | | $ | 11 | | | $ | 443 | |
PMI assesses the fair value of its derivative contracts using standard valuation models that use, as their basis, readily observable market inputs. The fair value of PMI’s foreign exchange forward contracts, foreign currency swaps and interest rate contracts is determined by using the prevailing foreign exchange spot rates and interest rate differentials, and the respective maturity dates of the instruments. The fair value of PMI’s currency options is determined by using a Black-Scholes methodology based on foreign
exchange spot rates and interest rate differentials, currency volatilities and maturity dates. The fair value of PMI’s commodity contracts is determined by using the prevailing market spot and futures prices and the respective maturity dates of the instruments. PMI’s derivative contracts have been classified within Level 2 at December 31, 2024 and 2023.
For the years ended December 31, 2024, 2023 and 2022, PMI's derivative contracts impacted the consolidated statements of earnings and comprehensive earnings as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (pre-tax, in millions) | For the Years Ended December 31, |
| Amount of Gain/(Loss) Recognized in Other Comprehensive Earnings/(Losses) on Derivatives | | Statement of Earnings
Classification of Gain/(Loss)
on Derivatives | | Amount of Gain/(Loss) Reclassified from Other Comprehensive Earnings/(Losses) into Earnings | | Amount of Gain/(Loss) Recognized in Earnings | |
| 2024 | 2023 | 2022 | | | | 2024 | 2023 | 2022 | | 2024 | 2023 | 2022 | |
| Derivative contracts designated as hedging instruments: | | | | | | | | | | | | | | |
| Cash flow hedges: | | | | | | | | | | | | | | |
| Foreign exchange contracts | $ | 433 | | $ | 195 | | $ | 288 | | | | | | | | | | | | |
| | | | | Net revenues | | $ | 186 | | $ | 194 | | $ | 233 | | | | | | |
| | | | | Cost of sales | | — | | — | | — | | | | | | |
| | | | | Marketing, administration and research costs | | 20 | | 27 | | 30 | | | | | | |
| | | | | Interest expense, net | | 2 | | (15) | | (7) | | | | | | |
| Interest rate contracts | 95 | | 37 | | 292 | | | Interest expense, net | | 51 | | 46 | | (2) | | | | | | |
| Commodity contracts | (1) | | (7) | | — | | | Cost of sales | | (1) | | — | | — | | | | | | |
| Fair value hedges: | | | | | | | | | | | | | | |
| Interest rate contracts | | | | | Interest expense, net (a) | | | | | | $ | (32) | | $ | (14) | | $ | (83) | | |
Net investment hedges (b): | | | | | | | | | | | | | | |
| Foreign exchange contracts | 867 | | (788) | | 300 | | | Interest expense, net (c) | | | | | | 293 | | 268 | | 181 | | |
| | | | | Marketing, administration and research costs | | 35 | | — | | — | | | | | | |
| Derivative contracts not designated as hedging instruments: | | | | | | | | | | | | | | |
| Foreign exchange contracts | | | | | Interest expense, net | | | | | | 219 | | 301 | | 112 | | |
| | | | | Marketing, administration and research costs (d) | | | | | | 523 | | (575) | | (169) | | |
| Total | $ | 1,394 | | $ | (563) | | $ | 880 | | | | | $ | 293 | | $ | 252 | | $ | 254 | | | $ | 1,003 | | $ | (20) | | $ | 41 | | |
(a) The gains (losses) from these contracts are offset by the changes in the fair value of the hedged item
(b) Amount of gains (losses) on hedges of net investments principally related to changes in foreign currency exchange and interest rates between the Euro and U.S. dollar
(c) Represent the gains for amounts excluded from the effectiveness testing
(d) The gains (losses) from these contracts attributable to changes in foreign currency exchange rates are partially offset by the (losses) and gains generated by the underlying intercompany and third-party loans being hedged
Cash Flow Hedges
PMI has entered into derivative contracts to hedge the foreign currency exchange, interest rate and commodity price risks related to certain forecasted transactions. Gains and losses associated with qualifying cash flow hedge contracts are deferred as components of accumulated other comprehensive losses until the underlying hedged transactions are reported in PMI’s consolidated statements of earnings. As of December 31, 2024, PMI has hedged forecasted transactions with derivative contracts expiring at various dates through May 2028. Premiums paid for, and settlements of, the derivative contracts designated as cash flow hedges are included primarily in cash flows from operating activities on PMI’s consolidated statements of cash flows.
Fair Value Hedges
PMI has entered into fixed-to-floating interest rate contracts, designated as fair value hedges to minimize exposure to changes in the fair value of fixed rate U.S. dollar-denominated debt that results from fluctuations in benchmark interest rates. For derivative contracts that are designated and qualify as fair value hedges the gain or loss on the derivative, as well as the offsetting gain or loss on the hedged items attributable to the hedged risk, is recognized in current earnings. The carrying amount of the debt hedged, which includes the cumulative adjustment for fair value gains/losses, as of December 31, 2024 was $2,941 million, of which $346 million was recorded in current portion of long-term debt and $2,595 million was recorded in long-term debt in the consolidated balance sheets. The cumulative amount of fair value gains/(losses) included in the carrying amount of the debt hedged was $42 million as of December 31, 2024.
Hedges of Net Investments in Foreign Operations
PMI designates derivative contracts and certain foreign currency denominated debt and other financial instruments as net investment hedges, primarily of its Euro net assets. The amount of pre-tax gain/(loss) related to the non-derivative financial instruments, that was reported as a component of accumulated other comprehensive losses within currency translation adjustments, was $99 million, $48 million and $521 million, for the years ended December 31, 2024, 2023 and 2022, respectively. Settlements of the derivative contracts designated as net investment hedges are included in cash flows from investing activities on PMI’s consolidated statements of cash flows.
Other Derivatives
PMI has entered into derivative contracts to hedge the foreign currency exchange and interest rate risks related to intercompany loans between certain subsidiaries, and third-party loans. While effective as economic hedges, no hedge accounting is applied for these contracts; therefore, the gains (losses) relating to these contracts are reported in PMI’s consolidated statements of earnings. Settlements of other derivative contracts are included primarily in cash flows from investing activities on PMI's consolidated statements of cash flows.
Qualifying Hedging Activities Reported in Accumulated Other Comprehensive Losses
Derivative gains or losses reported in accumulated other comprehensive losses are a result of qualifying hedging activity. Transfers of these gains or losses to earnings are offset by the corresponding gains or losses on the underlying hedged item. Hedging activity affected accumulated other comprehensive losses, net of income taxes, as follows:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Gain/(loss) as of January 1, | $ | 241 | | | $ | 266 | | | $ | 4 | |
| Derivative (gains)/losses transferred to earnings | (213) | | | (220) | | | (219) | |
| Change in fair value | 439 | | | 195 | | | 481 | |
| Gain/(loss) as of December 31, | $ | 467 | | | $ | 241 | | | $ | 266 | |
At December 31, 2024, PMI expects $260 million of derivative gains that are included in accumulated other comprehensive losses to be reclassified to the consolidated statement of earnings within the next 12 months. These gains are expected to be substantially offset by the statement of earnings impact of the respective hedged transactions.
Contingent Features
PMI’s derivative instruments do not contain contingent features.
Credit Exposure and Credit Risk
PMI is exposed to credit loss in the event of non-performance by counterparties. While PMI does not anticipate non-performance, its risk is limited to the fair value of the financial instruments less any cash collateral received or pledged. PMI actively monitors its exposure to credit risk through the use of credit approvals and credit limits and by selecting and continuously monitoring a diverse group of major international banks and financial institutions as counterparties.
Other Investments
Certain PMI investments, which are comprised of held-to-maturity U.S. dollar denominated bonds in Argentina, have been classified within Level 1 and had a fair value of $125 million at December 31, 2024. For the year ended December 31, 2024, the gross unrecognized holding losses on these investments were $7 million.
Accumulated Other Comprehensive Losses:
PMI's accumulated other comprehensive losses, net of taxes, consisted of the following:
| | | | | | | | | | | | | | | | | |
| (Losses) Earnings | At December 31, |
| (in millions) | 2024 | | 2023 | | 2022 |
| Currency translation adjustments | $ | (9,320) | | | $ | (9,467) | | | $ | (8,003) | |
| Pension and other benefits | (2,461) | | | (2,589) | | | (1,822) | |
| Derivatives accounted for as hedges | 467 | | | 241 | | | 266 | |
| | | | | |
| Total accumulated other comprehensive losses | $ | (11,314) | | | $ | (11,815) | | | $ | (9,559) | |
Reclassifications from Other Comprehensive Earnings
The movements in accumulated other comprehensive losses and the related tax impact, for each of the components above, that are due to current period activity and reclassifications to the income statement are shown on the consolidated statements of comprehensive earnings for the years ended December 31, 2024, 2023, and 2022. For additional information, see Note 3. Acquisitions and Divestitures and Note 20 Restructuring Activities for disclosures related to the reclassification of accumulated foreign currency translation losses from other comprehensive losses, Note 14. Benefit Plans for disclosures related to PMI's pension, and other benefits and Note 16. Financial Instruments for disclosures related to derivative financial instruments.
Contingencies:
Tobacco and/or Nicotine-Related Litigation
Legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. Our indemnitees include distributors, licensees, and others that have been named as parties in certain cases and that we have agreed to defend, as well as to pay costs and some or all of judgments, if any, that may be entered against them. Pursuant to the terms of the Distribution Agreement between Altria Group, Inc. ("Altria") and PMI, PMI will indemnify Altria and Philip Morris USA Inc. ("PM USA"), a U.S. tobacco subsidiary of Altria, for tobacco product claims based in substantial part on products manufactured by PMI or contract manufactured for PMI by PM USA, and PM USA will indemnify PMI for tobacco product claims based in substantial part on products manufactured by PM USA, excluding tobacco products contract manufactured for PMI.
It is possible that there could be adverse developments in pending cases against us and our subsidiaries. An unfavorable outcome or settlement of pending tobacco or nicotine-related litigation could encourage the commencement of additional litigation.
Damages claimed in some of the tobacco-related litigation are significant and, in the case of the "Health Care Cost Recovery
Litigation" described below, could range into the billions of U.S. dollars. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. While, as discussed below, we have to date been largely successful in defending tobacco-related litigation, litigation is subject to uncertainty. Additionally, as reported further below, beginning in March 2024, litigation related to oral nicotine products was filed against us and our subsidiary before certain courts in the United States.
We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. At the present time, except as stated otherwise in this Note 18. Contingencies, it is reasonably possible that an unfavorable outcome in a case may occur. Legal defense costs are expensed as incurred.
It is possible that our consolidated financial statements, including our results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. Nevertheless, although litigation is subject to uncertainty, we and each of our subsidiaries named as a defendant believe, and each has been so advised by counsel handling the respective cases, that we have valid defenses to the litigation pending against us, as well as valid bases for appeal of adverse verdicts. All such cases are, and will continue to be, vigorously defended. However, we and our subsidiaries may enter into settlement discussions in particular cases if we believe it is in our best interests to do so.
After assessing the information available to it, except as stated otherwise in this Note 18. Contingencies, (i) management has not concluded that it is probable that a loss has been incurred in any of the pending combustible tobacco product-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending combustible tobacco product-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any.
CCAA Proceedings and Stay of Combustible Tobacco Product-Related Cases Pending in Canada
As a result of the Court of Appeal of Quebec’s decision in both the Létourneau and Blais cases described below, our subsidiary, Rothmans, Benson & Hedges Inc. (“RBH”), and the other defendants, JTI Macdonald Corp. ("JTIM"), and Imperial Tobacco Canada Limited ("ITL"), sought protection in the Ontario Superior Court of Justice under the Companies’ Creditors Arrangement Act (“CCAA”) on March 22, March 8, and March 12, 2019, respectively. CCAA is a Canadian federal law that permits a Canadian business to restructure its affairs while carrying on its business in the ordinary course. The initial CCAA order made by the Ontario Superior Court on March 22, 2019 authorizes RBH to pay all expenses incurred in carrying on its business in the ordinary course after the CCAA filing, including obligations to employees, vendors, and suppliers. RBH's financial results have been deconsolidated from our consolidated financial statements since March 22, 2019.
As part of the CCAA proceedings, there is currently a comprehensive stay up to and including March 3, 2025 or the date on which the CCAA court issues an order on the Sanction Motion (discussed below) of all combustible tobacco product-related litigation pending in Canada against RBH and the other defendants, including PMI and our indemnitees (PM USA and Altria), namely, the smoking and health class actions filed in various Canadian provinces and health care cost recovery actions. These proceedings are presented below under the caption “Stayed Litigation — Canada.” Ernst & Young Inc. has been appointed as monitor of RBH in the CCAA proceedings. On April 17, 2019, the Ontario Superior Court ruled that RBH and the other defendants will not be allowed to file an application to the Supreme Court of Canada for leave to appeal the Court of Appeal’s decision in the Létourneau and the Blais cases so long as the comprehensive stay of all combustible tobacco product-related litigation in Canada remains in effect and that the time period to file the application would be extended by the stay period.
While RBH believes that the findings of liability and damages in both Létourneau and the Blais cases were incorrect, the CCAA proceedings provide a forum for RBH to seek resolution through a plan of arrangement or compromise of all combustible tobacco product-related litigation pending in Canada. On October 17, 2024, the court-appointed mediator and monitor in the CCAA proceedings filed a proposed plan of compromise and arrangement (“Proposed Plan”) setting forth, among other things, certain terms of a proposed comprehensive resolution of Canadian tobacco claims and related litigation against RBH, its affiliates, and its affiliates’ indemnitees. The court-appointed mediator and monitors also filed substantially similar proposed plans for ITL and JTIM.
Under the resolution contemplated by the Proposed Plan, RBH, ITL and JTIM (together, the “Companies”) would pay an aggregate global settlement amount of CAD 32.5 billion (approximately $22.3 billion). This amount would be funded by an upfront payment equal to the companies’ cash and cash equivalents on hand plus court deposits (subject to an aggregate withholding of CAD 750 million (approximately $514 million) of working capital) and annual payments based on a percentage of the Companies’ aggregate net income after taxes (excluding that generated by alternative products such as heat-not-burn, nicotine pouches, and e-vapor) until the global settlement amount is paid in full. As stated in the Proposed Plan, the issue of allocation of the CAD 32.5 billion aggregate settlement amount among RBH, ITL, and JTIM ("Allocation Issue") remains unresolved. RBH and its affiliates, including PMI and its indemnitees, would obtain a release of claims relating to the manufacture, marketing, sale, or use of or exposure
to, RBH’s combustible and traditional smokeless tobacco products based on conduct prior to the effective date of the Proposed Plan; related litigation would also be dismissed—including those actions described in the section below entitled “Stayed Litigation – Canada.” Alternative product businesses (including heat-not-burn, e-vapor, and nicotine pouches) would be transferred to an RBH affiliate or otherwise maintained separately from RBH's combustible business. The Proposed Plan also contains a number of operating covenants that would govern RBH’s business going forward until the settlement amount has been paid.
On January 15, 2025, RBH’s court-appointed monitor filed a motion seeking an order by the CCAA court approving and sanctioning the Proposed Plan and authorizing and directing the monitor, among others, to take all steps and actions necessary and appropriate to implement the Proposed Plan (“Sanction Motion” and the requested order, the “Proposed Sanction Order”). On January 24, 2025, RBH filed an objection to the Sanction Motion (“RBH Objection”). The RBH Objection argues that the Proposed Plan cannot be approved because it fails to resolve the Allocation Issue. The RBH Objection also states that, without an appropriate, fair and reasonable resolution of the Allocation Issue, RBH cannot presently consent to implementation of the Proposed Plan. To address the Allocation Issue, the RBH Objection seeks to amend the Proposed Sanction Order to include provisions (the “Proposed Allocation Provisions”) requiring that ITL and JTIM make payments to RBH from their retained working capital and net income after taxes over a period of years. ITL, JTIM, and certain claimant groups have opposed the Proposed Allocation Provisions set forth in the RBH Objection.
A judicial hearing to consider approval of the Proposed Plan, including the Sanction Motion, the RBH Objection and the Proposed Allocation Provisions, was held from January 29 through January 31, 2025. Further hearing dates may be scheduled. A decision from the CCAA court on whether and what form to sanction the Proposed Plan is expected in the first quarter. If ultimately approved by the CCAA court and, among other things, not subject to appeal, implementation of the Proposed Plan is expected in 2025.
For additional information concerning the fair value of PMI’s continuing investment in RBH and the impairment charge recorded in the Company’s consolidated statement of earnings for the year ended December 31, 2024, as a recognized subsequent event, see Item 8, Note 6. Related Parties – Equity Investments and Other.
Stayed Litigation — Canada
Smoking and Health Litigation — Canada
In the first class action pending in Canada, Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais v. Imperial Tobacco Canada Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in November 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants (the "Blais Class Action"). The plaintiffs, an anti-smoking organization and an individual smoker, sought compensatory and punitive damages for each member of the class who suffers allegedly from certain smoking-related diseases. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and found that the class members’ compensatory damages totaled approximately CAD 15.5 billion (approximately $10.6 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion (approximately $2.1 billion) including pre-judgment interest). In addition, the trial court awarded CAD 90,000 (approximately $62,000) in punitive damages, allocating CAD 30,000 (approximately $21,000) to RBH. The trial court estimated the disease class at 99,957 members. RBH appealed to the Court of Appeal of Quebec. In October 2015, the Court of Appeal ordered RBH to furnish security totaling CAD 226 million (approximately $155 million) to cover both the Létourneau and Blais cases, which RBH has paid in installments through March 2017. The Court of Appeal ordered Imperial Tobacco Canada Ltd. to furnish security totaling CAD 758 million (approximately $520 million) in installments through June 2017. JTI Macdonald Corp. was not required to furnish security in accordance with plaintiffs’ motion. The Court of Appeal ordered that the security is payable upon a final judgment of the Court of Appeal affirming the trial court’s judgment or upon further order of the Court of Appeal.
On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the compensatory and punitive damages award while reducing the total amount of compensatory damages to approximately CAD 13.5 billion (approximately $9.3 billion), including interest due to the trial court’s error in the calculation of interest. The compensatory damages award is on a joint and several basis with an allocation of 20% to RBH (approximately CAD 2.7 billion (approximately $1.9 billion), including pre-judgment interest). The Court of Appeal upheld the trial court’s findings that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking and by conspiring to prevent consumers from learning of the dangers of smoking. The Court of Appeal further held that the plaintiffs either need not prove, or had adequately proven, that these faults were a cause of the class members’ injuries. In accordance with the judgment, defendants were required to deposit their respective portions of the damages awarded in both the Létourneau case described below and the Blais case, approximately CAD 1.1 billion (approximately $754 million), into trust accounts within 60 days. RBH’s share of the deposit was approximately CAD 257 million (approximately $194 million). PMI recorded a pre-tax charge of $194 million in its consolidated results, representing $142 million net of tax, as tobacco litigation-related
expense, in the first quarter of 2019. The charge reflects PMI’s assessment of the portion of the judgment that represents probable and estimable loss prior to the deconsolidation of RBH and corresponds to the trust account deposit required by the judgment.
In the second class action pending in Canada, Cecilia Létourneau v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in September 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants (the "Létourneau Class Action"). The plaintiff, an individual smoker, sought compensatory and punitive damages for each member of the class who is deemed addicted to smoking. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and awarded a total of CAD 131 million (approximately $90 million) in punitive damages, allocating CAD 46 million (approximately $32 million) to RBH. The trial court estimated the size of the addiction class at 918,000 members but declined to award compensatory damages to the addiction class because the evidence did not establish the claims with sufficient accuracy. The trial court found that a claims process to allocate the awarded punitive damages to individual class members would be too expensive and difficult to administer. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the total amount of punitive damages awarded allocating CAD 57 million (approximately $39 million), including interest to RBH. See the Blais description above for further detail concerning the security order pertaining to both Létourneau and Blais cases and the impact of the decision on PMI’s financial statements.
RBH and PMI believe the findings of liability and damages in both Létourneau and the Blais cases were incorrect and in contravention of applicable law on several grounds including, the following: (i) defendants had no obligation to warn class members who knew, or should have known, of the risks of smoking; (ii) defendants cannot be liable to class members who would have smoked regardless of what warnings were given; and (iii) defendants cannot be liable to all class members given the individual differences among class members.
In the third class action pending in Canada, Kunta v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Winnipeg, Canada, filed June 12, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic obstructive pulmonary disease (“COPD”), severe asthma, and mild reversible lung disease resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the fourth class action pending in Canada, Adams v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Saskatchewan, Canada, filed July 10, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, emphysema, heart disease, or cancer, as well as restitution of profits.
In the fifth class action pending in Canada, Semple v. Canadian Tobacco Manufacturers' Council, et al., The Supreme Court (trial court), Nova Scotia, Canada, filed June 18, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and COPD resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the sixth class action pending in Canada, Dorion v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Alberta, Canada, filed June 15, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic bronchitis and severe sinus infections resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. To date, we, our subsidiaries, and our indemnitees have not been properly served with the complaint.
In the seventh class action pending in Canada, McDermid v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and heart disease resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from heart disease allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed.
In the eighth class action pending in Canada, Bourassa v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants.
The plaintiff, the heir to a deceased smoker, alleges that the decedent was addicted to tobacco products and suffered from emphysema resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from chronic respiratory diseases allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed. In December 2014, plaintiff filed an amended statement of claim.
In the ninth class action pending in Canada, Suzanne Jacklin v. Canadian Tobacco Manufacturers' Council, et al., Ontario Superior Court of Justice, filed June 20, 2012, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, heart disease, or cancer, as well as restitution of profits.
Health Care Cost Recovery Litigation — Canada
In the first health care cost recovery case pending in Canada, Her Majesty the Queen in Right of British Columbia v. Imperial Tobacco Limited, et al., Supreme Court, British Columbia, Vancouver Registry, Canada, filed January 24, 2001, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The plaintiff, the government of the province of British Columbia, brought a claim based upon legislation enacted by the province authorizing the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, resulting from a “tobacco related wrong.”
In the second health care cost recovery case filed in Canada, Her Majesty the Queen in Right of New Brunswick v. Rothmans Inc., et al., Court of Queen's Bench of New Brunswick, Trial Court, New Brunswick, Fredericton, Canada, filed March 13, 2008, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of New Brunswick based on legislation enacted in the province. This legislation is similar to the law introduced in British Columbia that authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the third health care cost recovery case filed in Canada, Her Majesty the Queen in Right of Ontario v. Rothmans Inc., et al., Ontario Superior Court of Justice, Toronto, Canada, filed September 29, 2009, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Ontario based on legislation enacted in the province. This legislation is similar to the laws introduced in British Columbia and New Brunswick that authorize the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fourth health care cost recovery case filed in Canada, Attorney General of Newfoundland and Labrador v. Rothmans Inc., et al., Supreme Court of Newfoundland and Labrador, St. Johns, Canada, filed February 8, 2011, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Newfoundland and Labrador based on legislation enacted in the province that is similar to the laws introduced in British Columbia, New Brunswick and Ontario. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fifth health care cost recovery case filed in Canada, Attorney General of Quebec v. Imperial Tobacco Limited, et al., Superior Court of Quebec, Canada, filed June 8, 2012, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The claim was filed by the government of the province of Quebec based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the sixth health care cost recovery case filed in Canada, Her Majesty in Right of Alberta v. Altria Group, Inc., et al., Supreme Court of Queen's Bench Alberta, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Alberta based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the seventh health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Manitoba v. Rothmans, Benson & Hedges, Inc., et al., The Queen's Bench, Winnipeg Judicial Centre, Canada, filed May 31, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Manitoba based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the eighth health care cost recovery case filed in Canada, The Government of Saskatchewan v. Rothmans, Benson & Hedges Inc., et al., Queen's Bench, Judicial Centre of Saskatchewan, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Saskatchewan based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the ninth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Prince Edward Island v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Prince Edward Island (General Section), Canada, filed September 10, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Prince Edward Island based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the tenth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Nova Scotia v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Nova Scotia, Canada, filed January 2, 2015, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Nova Scotia based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
Combustible tobacco products litigation
Since 1995, more than 600 combustible tobacco product-related cases, including smoking and health, label-related, health care cost recovery, and public civil actions, have been filed by governmental entities or individual plaintiffs, or on behalf of a class or purported class of individual plaintiffs against a PMI entity. All resolved cases have been terminated in our favor and only a small number of cases remain pending. The pending cases include nine proposed class actions, 17 health care cost recovery cases, one public civil action, and individual cases. The amounts at issue in the pending individual cases would not have a material adverse effect on our consolidated financial statements, including our results of operations, cash flows, or financial position. Of the pending combustible tobacco product-related cases, four were initially decided in favor of plaintiffs and remain on appeal, or are subject to an appeal. These four cases include the Blais Class Action and the Létourneau Class Action, described above under the caption "Smoking and Health Litigation — Canada," and two individual cases where final resolution in the amount of the verdict would not have a material adverse effect on our consolidated financial statements, including our results of operations, cash flows, or financial position.
Pending claims related to combustible tobacco products generally fall within the following categories:
Smoking and Health Proposed Class Actions: These cases primarily allege personal injury and are brought by individual plaintiffs on behalf of a class or purported class of individual plaintiffs. Plaintiffs' allegations of liability in these cases are based on various theories of recovery, including negligence, gross negligence, strict liability, fraud, misrepresentation, design defect, failure to warn, breach of express and implied warranties, violations of deceptive trade practice laws and consumer protection statutes. Plaintiffs in these cases seek various forms of relief, including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include licit activity, failure to state a claim, lack of defect, lack of proximate cause, assumption of the risk, contributory negligence, and statute of limitations.
As of December 31, 2024, there were nine cases brought on behalf of classes of individual plaintiffs pending against us, our subsidiaries or indemnitees, compared with nine such cases on December 31, 2023, and nine such cases on December 31, 2022, and such cases are described above under the caption “Smoking and Health Litigation — Canada.”
Health Care Cost Recovery Litigation: These cases, brought by governmental and non-governmental plaintiffs, seek reimbursement of health care cost expenditures allegedly caused by tobacco products. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including unjust enrichment, negligence, negligent design, strict liability, breach of express and implied warranties, violation of a voluntary undertaking or special duty, fraud, negligent misrepresentation, conspiracy, public nuisance, defective product, failure to warn, sale of cigarettes to minors, and claims under statutes governing competition and deceptive trade practices. Plaintiffs in these cases seek various forms of relief including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include lack of proximate cause, remoteness of injury, failure to state a claim, adequate remedy at law, “unclean hands” (namely, that plaintiffs cannot obtain equitable relief because they participated in, and benefited from, the sale of cigarettes), and statute of limitations.
As of December 31, 2024, there were 17 health care cost recovery cases pending against us, our subsidiaries or indemnitees in Brazil (1), Canada (10), Korea (1) and Nigeria (5), compared with 17 such cases on December 31, 2023 and 17 such cases on December 31, 2022.
The health care cost recovery actions pending in Canada are described above under the caption “Health Care Cost Recovery Litigation — Canada.”
In the health care cost recovery case in Brazil, The Attorney General of Brazil v. Souza Cruz Ltda., et al., Federal Trial Court, Porto Alegre, Rio Grande do Sul, Brazil, filed May 21, 2019, we, our subsidiaries, and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases in certain prior years, payment of anticipated costs of treating future alleged smoking-related diseases, and moral damages. Defendants filed answers to the complaint in May 2020.
In the first health care cost recovery case in Nigeria, The Attorney General of Lagos State v. British American Tobacco (Nigeria) Limited, et al., High Court of Lagos State, Lagos, Nigeria, filed March 13, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the second health care cost recovery case in Nigeria, The Attorney General of Kano State v. British American Tobacco (Nigeria) Limited, et al., High Court of Kano State, Kano, Nigeria, filed May 9, 2007, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of challenging the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the third health care cost recovery case in Nigeria, The Attorney General of Gombe State v. British American Tobacco (Nigeria) Limited, et al., High Court of Gombe State, Gombe, Nigeria, filed October 17, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In February 2011, the court ruled that the plaintiff had not complied with the procedural steps necessary to serve us. As a result of this ruling, plaintiff must re-serve its claim. We have not yet been re-served.
In the fourth health care cost recovery case in Nigeria, The Attorney General of Oyo State, et al., v. British American Tobacco (Nigeria) Limited, et al., High Court of Oyo State, Ibadan, Nigeria, filed May 25, 2007, we and other members of the industry are defendants. Plaintiffs seek reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We challenged service as improper. In June 2010, the court ruled that plaintiffs did not have leave to serve the writ of summons on the defendants and that they must re-serve the writ. We have not yet been re-served.
In the fifth health care cost recovery case in Nigeria, The Attorney General of Ogun State v. British American Tobacco (Nigeria) Limited, et al., High Court of Ogun State, Abeokuta, Nigeria, filed February 26, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In May 2010, the trial court rejected our objections to the court's jurisdiction. We have appealed. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the health care cost recovery case in Korea, the National Health Insurance Service v. KT&G, et. al., filed April 14, 2014, our subsidiary and other Korean manufacturers are defendants. Plaintiff alleges, among other things, that defendants concealed the health hazards of smoking, marketed to youth, added ingredients to make their products more harmful and addictive, and misled consumers into believing that Lights cigarettes are safer than regular cigarettes. The National Health Insurance Service seeks to recover damages allegedly incurred in treating 3,484 patients with small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer from 2003 to 2012. The trial court dismissed the case in its entirety on November 20, 2020. The Appellate court granted the Plaintiff a de novo appeal in 2021 and determined that the appellate proceedings will take place in stages: wrongful conduct/product defect allegations first, then causation and finally issues such as standing/direct action. The plaintiff's appeal remains pending.
Public Civil Actions: Claims have been filed either by an individual, or a public or private entity, seeking to protect collective or individual rights, such as the right to health, the right to information or the right to safety. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including product defect, concealment, and misrepresentation. Plaintiffs in these cases seek various forms of relief including injunctive relief such as banning cigarettes, descriptors, smoking in certain places and advertising, as well as implementing communication campaigns and reimbursement of medical expenses incurred by public or private institutions.
As of December 31, 2024, there was one public civil action pending against our subsidiary in Venezuela (1), compared with one such case on December 31, 2023, and one such case on December 31, 2022.
In a public civil action in Venezuela, Federation of Consumers and Users Associations (“FEVACU”), et al. v. National Assembly of Venezuela and the Venezuelan Ministry of Health, Constitutional Chamber of the Venezuelan Supreme Court, filed April 29, 2008, we were not named as a defendant, but the plaintiffs published a notice pursuant to court order, notifying all interested parties to appear in the case. In January 2009, our subsidiary appeared in the case in response to this notice. The plaintiffs purport to represent the right to health of the citizens of Venezuela and claim that the government failed to protect adequately its citizens' right to health. The claim asks the court to order the government to enact stricter regulations on the manufacture and sale of tobacco products. In addition, the plaintiffs ask the court to order companies involved in the tobacco industry to allocate a percentage of their “sales or benefits” to establish a fund to pay for the health care costs of treating smoking-related diseases. In October 2008, the court ruled that plaintiffs have standing to file the claim and that the claim meets the threshold admissibility requirements. In December 2012, the court admitted our subsidiary and a subsidiary of British American Tobacco plc as interested third parties. In February 2013, our subsidiary answered the complaint. On February 27, 2024, the Attorney General of Venezuela filed, on behalf of defendants, a motion to dismiss the case for lack of prosecution.
Smoke-Free Products-Related Litigation
Claims have been filed against PMI and one or more subsidiaries related to ZYN nicotine pouches. These cases were filed either on behalf of an individual plaintiff, or on behalf of a purported class of individuals. Plaintiffs assert a variety of common law and statutory claims, and seek various forms of relief, including monetary and equitable relief.
In the first case, a putative class action, Kelly v. Philip Morris International Inc., et al., filed on March 19, 2024, before United States District Court for the Southern District of Florida, plaintiff alleges, among other things, addiction to nicotine resulting from the use of ZYN nicotine pouches (the "Kelly class action"). The complaint named PMI and Swedish Match North America LLC as defendants. Plaintiff purports to represent classes comprised of (i) all persons who purchased ZYN products in the United States, (ii) all residents of Florida who purchased ZYN products, and (iii) all residents of Florida who, at the time of their use of ZYN products, were under the age of 21, and who procured and used ZYN products. Plaintiff alleges, among other things, that defendants defectively designed ZYN products and sold them in an unreasonably unsafe and dangerous condition, marketed ZYN products to minors, and misrepresented or failed to warn consumers about information related to ZYN products, including information about health risks associated with these products. Plaintiff asserts strict liability design defect and failure to warn claims, as well as negligence and fraud claims and is seeking compensatory and punitive damages, attorney’s fees and costs, interest, and medical monitoring. On May 6, 2024, PMI and Swedish Match North America LLC filed motions to dismiss the complaint with prejudice. On August 20, 2024, the court granted Swedish Match North America LLC’s motion to dismiss the fraud claim and plaintiff’s request for medical monitoring, but denied the motion to dismiss other claims, denied PMI’s motion to dismiss without prejudice, and granted plaintiff’s request to conduct jurisdictional discovery. On December 4, 2024, plaintiff filed an amended complaint against PMI and Swedish Match North America LLC and added three additional entities as named defendants: Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc. On December 18, 2024, PMI, Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc., filed motions to dismiss the amended complaint with prejudice, and Swedish Match North America LLC filed a motion to dismiss the fraud claim. At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
In the second case, a putative class action, Bates-Ferreira v. Philip Morris International Inc., et al., filed March 29, 2024, before United States District Court for the Eastern District of California, plaintiff alleges, among other things, addiction to nicotine resulting from the use of ZYN nicotine pouches. The complaint named PMI and Swedish Match North America LLC as defendants. Plaintiff purports to represent classes comprised of (i) all persons who used ZYN products in the United States, (ii) all persons who used ZYN products in the United States while under the age of 18, (iii) all residents of California who used ZYN products, and (iv) all residents of California who used ZYN products while under the age of 18. Plaintiff alleges, among other things, that defendants made misrepresentations about ZYN products in their advertising and marketing, marketed ZYN products to minors, and misrepresented or failed to disclose to consumers information about ZYN products, including information about health risks associated with these products. Plaintiff asserts fraud, unjust enrichment, breach of implied warranty, and breach of consumer protection, unfair competition and advertising statutes claims and is seeking compensatory and punitive damages, disgorgement of profits, attorney’s fees and expenses, interest and other applicable injunctive relief. On June 7, 2024, PMI and Swedish Match North America LLC filed motions
to dismiss the complaint with prejudice, and Swedish Match North America LLC also filed a motion to stay the proceedings pending resolution of the Kelly class action. On August 5, 2024, plaintiff voluntarily dismissed his claim against PMI without prejudice. At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
In the third case, an individual complaint, Palmer v. Philip Morris International Inc., et al., filed April 3, 2024, before United States District Court for the Southern District of Florida, plaintiff alleges, among other things, addiction to nicotine resulting from the use of ZYN nicotine pouches. The complaint named PMI and Swedish Match North America LLC as defendants. He alleges, among other things, that defendants defectively designed ZYN products and sold them in an unreasonably unsafe and dangerous condition, marketed ZYN products to minors, and misrepresented or failed to warn consumers about information related to ZYN products, including information about health risks associated with these products. Plaintiff asserts strict liability design defect and failure to warn claims, as well as negligence and fraud claims, and is seeking compensatory and punitive damages, attorney’s fees and costs, interest, and medical monitoring. On June 3, 2024, PMI and Swedish Match North America LLC filed motions to dismiss the complaint with prejudice. On August 20, 2024, the court granted Swedish Match North America LLC’s motion to dismiss the fraud claim and plaintiff’s request for medical monitoring, but denied the motion to dismiss other claims, denied PMI’s motion to dismiss without prejudice, and granted plaintiff’s request to conduct jurisdictional discovery. On December 4, 2024, plaintiff filed an amended complaint against PMI and Swedish Match North America LLC and added three additional entities as named defendants: Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc. On December 18, 2024, PMI, Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc., filed motions to dismiss the amended complaint with prejudice, and Swedish Match North America LLC filed a motion to dismiss the fraud claim. At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
In the fourth case, an individual complaint, Lendinara v. Philip Morris International Inc., et al., filed July 30, 2024, before United States District Court for the Southern District of Florida, plaintiff alleges, among other things, addiction to nicotine resulting from the use of ZYN nicotine pouches. The complaint named PMI and Swedish Match North America LLC as defendants. He alleges, among other things, that defendants defectively designed ZYN products and sold them in an unreasonably unsafe and dangerous condition, marketed ZYN products to minors, and misrepresented or failed to warn consumers about information related to ZYN products, including information about health risks associated with these products. Plaintiff asserts strict liability design defect and failure to warn claims, as well as negligence and fraud claims, and is seeking compensatory and punitive damages, attorney’s fees and costs, interest, and medical monitoring. On September 19, 2024, the court granted the parties’ joint motion to apply its decisions on the motions to dismiss in Palmer to the Lendinara matter, including granting plaintiff’s request to conduct jurisdictional discovery and setting the same timeline for plaintiff to amend his complaint. On December 4, 2024, plaintiff filed an amended complaint against PMI and Swedish Match North America LLC and added three additional entities as named defendants: Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc. On December 18, 2024, PMI, Swedish Match USA Inc., PMI Global Services Inc., and Philip Morris Global Brands Inc., filed motions to dismiss the amended complaint with prejudice, and Swedish Match North America LLC filed a motion to dismiss the fraud claim. At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
In the fifth case, a putative class action, Norris v. Philip Morris International Inc., et al., filed July 30, 2024, before United States District Court for the District of Connecticut, plaintiff alleges, among other things, addiction to nicotine resulting from the use of ZYN nicotine pouches. The complaint named PMI and Swedish Match North America LLC as defendants. Plaintiff purports to represent classes comprised of (i) all persons who used ZYN products in the United States, (ii) all persons who used ZYN products in the United States while under the age of 18, (iii) all residents of Florida who used ZYN products, and (iv) all residents of Florida who used ZYN products while under the age of 18. Plaintiff alleges, among other things, that defendants made misrepresentations about ZYN products in their advertising and marketing, marketed ZYN products to minors, and misrepresented or failed to disclose to consumers information about ZYN products, including information about health risks associated with these products. Plaintiff asserts unjust enrichment, and breach of consumer protection, unfair trade and advertising statutes claims and is seeking compensatory and punitive damages, disgorgement of profits, attorney’s fees and expenses, interest and other applicable injunctive relief. On September 24, PMI and Swedish Match North America LLC filed motions to dismiss the complaint with prejudice, and a motion to stay discovery. On October 2, 2024, Plaintiff filed a notice of voluntary dismissal without prejudice as to Swedish Match, which the Court ordered on October 3, 2024. At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
District of Columbia Attorney General Investigation: On June 11, 2024, Swedish Match North America LLC received a subpoena from the office of the Attorney General of the District of Columbia (“D.C.”). The subpoena requested, among other things, information concerning Swedish Match North America LLC’s compliance with D.C.’s licensing regulations, age-verification requirements, and prohibition on the sale of flavored tobacco products, including with respect to ZYN nicotine pouches. Swedish Match North America LLC cooperated with the investigation, and on December 12, 2024, it executed an assurance of voluntary compliance with the office of the Attorney General to resolve the Attorney General's investigation, without admitting liability or
wrongdoing. Pursuant to the assurance of voluntary compliance, Swedish Match North America LLC paid $1,200,000 to D.C. and agreed to undertake certain limited conduct obligations moving forward.
Other Litigation
On November 18, 2024, a putative class action, Neumark v. Swedish Match North America LLC, was filed before United States District Court for the Eastern District of Virginia. The complaint named Swedish Match North America LLC as the defendant. Plaintiff alleges that Swedish Match North America LLC violated federal and state antitrust laws by, among other things, driving a competitor from the market through purportedly baseless litigation and entering into an allegedly anticompetitive agreement with PMI whereby PMI, through an indirect subsidiary, acquired Swedish Match North America LLC and eliminated itself as a competitor in the U.S. nicotine pouch market. Plaintiff asserts claims under the Sherman Antitrust Act, the Clayton Antitrust Act, state antitrust law, and for unjust enrichment. Plaintiff seeks to represent (i) all natural persons, businesses, entities, and corporations in the United States who purchased ZYN at retail during the class period; and (ii) all natural persons, businesses, entities, and corporations in the United States who live in states that have certain antitrust statutes who purchase ZYN at retail during the class period. Plaintiff is seeking damages (including treble damages as available under antitrust laws), costs, attorneys’ fees, disgorgement of profit, pre- and post-judgment interest, and declaratory and injunctive relief (including a declaration that the acquisition of Swedish Match North America LLC by PMI is unlawful and must result in divestiture or be enjoined). On January 15, 2025, Swedish Match North America LLC filed a motion to dismiss the complaint with prejudice. Subsequently thereto, Plaintiff informed Swedish Match North America LLC that he will file an amended complaint, which is due on February 10, 2025 At this time, no estimated loss has been accrued in the consolidated financial statements for this proceeding and we cannot determine the likelihood of loss, or reasonably estimate a range of loss, if any, from this proceeding.
The Department of Special Investigations of the government of Thailand ("DSI") conducted an investigation into alleged underpayment by Philip Morris (Thailand) Limited ("PM Thailand") of customs duties and excise taxes relating to imports from Indonesia covering the period 2000-2003. On January 26, 2017, the Public Prosecutor filed charges against PM Thailand and its former Thai employee in the Bangkok Criminal Court alleging that PM Thailand and its former employee jointly and with the intention to defraud the Thai government under-declared import prices of cigarettes to avoid full payment of taxes and duties in connection with import entries during the period from January 2002 to July 2003. The government sought a fine of approximately THB 19.8 billion (approximately $584 million). In May 2017, Thailand enacted a new customs act. The new act, which took effect in November 2017, substantially limits the amount of fines that Thailand could seek in these proceedings. PM Thailand believes that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law, and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and a Thai court. Trial in the case began in November 2018 and concluded in December 2019. In March 2020, the trial court found our subsidiary guilty of under-declaration of the prices and imposed a fine of approximately THB 130 million (approximately $3.9 million). The trial court dismissed all charges against the individual defendant. In April 2020, as required by Thai law, our subsidiary paid the fine. This payment is included in other assets on the consolidated balance sheets and negatively impacted net cash provided by operating activities in the consolidated statements of cash flows in the period of payment. Our subsidiary filed an appeal of the trial court's decision. In addition, the Public Prosecutor filed an appeal of the trial court's decision challenging the dismissal of charges against the individual defendant and the amount of the fine imposed. The appellate court issued its decision on the appeals on January 31, 2023. The appellate court affirmed the findings of under-declaration of import prices of cigarettes but reduced the fine imposed by the trial court. The appellate court directed the Public Prosecutor to coordinate with customs officials to calculate such reduced fine in accordance with the appellate court’s decision. The appellate court affirmed the acquittal of the individual defendant. Our subsidiary has appealed the decision to the Supreme Court of Thailand. The Public Prosecutor has filed an appeal to the Supreme Court of Thailand challenging the dismissal of charges against the individual defendant and the amount of the fine. Thailand is required to refund any payment made by our subsidiary in excess of any fine assessed by the courts.
In July 2020, the Public Prosecutor’s office of Rome, Italy, notified our Italian subsidiary, Philip Morris Italia S.r.l. (“PM Italia”), as well as three former or current employees and a former external consultant of PM Italia in July and March 2020, respectively, that it concluded a preliminary investigation against them for alleged contravention of anti-corruption laws and related disruption of trade freedom. The Public Prosecutor alleges that the individuals involved promised certain personal favors to government officials from January to July of 2018 in exchange for favorable treatment for PM Italia, and that PM Italia lacked appropriate organizational controls to prevent the alleged actions by the individuals. On September 21, 2020, the Public Prosecutor issued his indictment and referred the matter to the court. At the preliminary hearing held on May 11, 2021, the judge decided to refer all charges/defendants (including our affiliate) to trial. The first trial hearing took place on September 22, 2021. BAT has filed a civil claim against PM Italia claiming vicarious liability for the alleged wrongdoings of its former or current employees and seeking EUR50 million (approximately $51 million) in damages. After various postponements, the trial started on September 25, 2023, and is expected to continue in 2025 through a series of evidentiary hearings. PM Italia believes it has strong defenses to the charges against it and will defend them vigorously.
The Ministry of Industry and Trade of the Russian Federation filed a petition before the Arbitrazh Court of the Moscow Region seeking the suspension of corporate rights that Megapolis Distribution B.V. (“MDBV”), a legal entity incorporated in the Netherlands, held, pursuant to Dutch law, in JSC TK Megapolis (formerly CJSC TK Megapolis), which is the distributor of PMI’s products in Russia. On July 18, 2024, the court admitted the petition. On August 8, 2024, the Arbitrazh Court of the Moscow Region granted the forced localization, as requested by the Ministry of Industry and Trade. As a result, MDBV's interest and corporate rights in JSC TK Megapolis were transferred to JSC TK Megapolis. On December 5, 2024, JSC TK Megapolis registered the subsequent transfer of such interest and corporate rights to a PMI affiliate, ZAO Philip Morris Izhora. For additional information, see Note 6. Related Parties – Equity Investments and Other.
Following an October 2020 final decision by the highest court in Brazil in tax litigation pertaining to overpayments of certain indirect taxes, our affiliate modified the methodology for calculation of the deduction applicable to the indirect taxes at issue. The Brazilian Tax Authority objected to such methodology and, on December 3, 2024, served our affiliate with notice of an assessment alleging underpayments of these indirect taxes during the 2020 fiscal year, for approximately BRL 137 million ($24 million). Our affiliate believes it is probable that the Brazilian Tax Authority will issue assessments alleging underpayment of indirect taxes for subsequent fiscal years. We disagree with the position of the Brazilian Tax Authority, and will defend vigorously.
On December 21, 2023, we were informed that Future Technology K.K. (“FTKK”) filed an application with Tokyo Customs against Sojitz Corporation (“Sojitz”), Philip Morris Japan Limited’s (“PMJL”) importer and distributor, due to alleged infringement of JP7299432. FTKK sought an order stopping the importation of TEREA consumables. FTKK did not in its application seek any monetary damages or costs. PMJL entered an appearance in the proceeding as an interested party and filed its response to FTKK's application on January 31, 2024. The Customs hearing was held on May 28, 2024. On June 27, 2024 expert advisors to Customs provided their opinion that the patent at issue was not infringed. On June 28, 2024, FTKK withdrew its Customs application. The proceeding is now concluded. On January 26, 2024, PMJL filed a declaratory judgment action in Tokyo District Court seeking a declaration that JP7299432 is invalid and/or not infringed. The declaratory judgment action has now concluded following FTKK's waiver of its right to seek relief from PMJL for infringement of JP7299432, which effectively resolved PMJL's request for a declaration of no liability for infringement of that patent.
In July and August 2024, respectively, FTKK filed two patent infringement actions against Sojitz, PMJL’s importer and distributor, for alleged infringement of two patents by TEREA consumables. FTKK asserts a claim for damages. PMJL is obligated to indemnify Sojitz for damages and intervened in the matters. Merits briefing in the matters commenced in December 2024. In November and December 2024, FTKK filed seven additional patent infringement actions against Sojitz for alleged infringement of seven new FTKK patents by TEREA and SENTIA consumables. FTKK asserts a claim for damages in these actions. PMJL is obligated to indemnify Sojitz for damages and intervened in the matters. Merits briefing in these matters is expected to commence in the first half of 2025. On November 27, 2024, we were informed that FTKK filed a new application with Tokyo Customs against Sojitz, PMJL’s importer and distributor, on the basis of alleged infringement of another FTKK patent. FTKK is seeking an order stopping the importation of TEREA and SENTIA consumables. At this time, FTKK is not seeking any monetary damages or costs. PMJL has entered an appearance in the proceeding as an interested party and filed its opposition to FTKK’s application on January 9, 2025. On January 9, 2025, PMJL and Sojitz filed a declaratory judgment action against FTKK in Tokyo District Court with respect to the patent at issue in the pending Tokyo Customs matter on the basis that the relevant patent is not infringed or is invalid. PMJL intends to vigorously defend the matters commenced by FTKK and take steps to mitigate disruption, if any, that could result from FTKK’s claims.
Other patent challenges are pending in various jurisdictions.
We are also involved in additional litigation arising in the ordinary course of our business. While the outcomes of these proceedings are uncertain, management does not expect that the ultimate outcomes of other litigation, including any reasonably possible losses in excess of current accruals, will have a material adverse effect on our consolidated results of operations, cash flows or financial position.
Sale of Accounts Receivable:
To mitigate risk and enhance cash and liquidity management PMI sells trade receivables to unaffiliated financial institutions. These arrangements allow PMI to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the consolidated balance sheets. PMI sells trade receivables under two types of arrangements, servicing and non-servicing. For servicing arrangements, PMI continues to service the sold trade receivables on an administrative basis and does not act on behalf of the unaffiliated financial institutions. When applicable, a servicing liability is recorded for the estimated fair value of the servicing. The amounts associated with the servicing liability were not material for the
years ended December 31, 2024 and 2023. Under the non-servicing arrangements, PMI does not provide any administrative support or servicing after the trade receivables have been sold to the unaffiliated financial institutions.
Cumulative trade receivables sold, including excise taxes, for the years ended December 31, 2024 and 2023, were $11.9 billion and $13.3 billion, respectively. PMI’s operating cash flows were positively impacted by the amount of the trade receivables sold and derecognized from the consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of December 31, 2024, 2023 and 2022, were $0.9 billion, $1.6 billion and $1.0 billion, respectively. The net proceeds received are included in cash provided by operating activities in the consolidated statements of cash flows. The difference between the carrying amount of the trade receivables sold and the sum of the cash received is recorded as a loss on sale of trade receivables within marketing, administration and research costs in the consolidated statements of earnings. For the years ended December 31, 2024, 2023 and 2022 the loss on sale of trade receivables was $42 million, $49 million and $26 million, respectively.
Restructuring Activities:
For the years ended December 31, 2024 and 2023, PMI recorded total pre-tax restructuring charges of $180 million and $109 million, respectively. These pre-tax charges were included in marketing, administration and research costs on the consolidated statements of earnings. For the year ended December 31, 2022, PMI did not record any charges related to restructuring activities.
IQOS products sourcing for the U.S. market
On February 1, 2024, a subsidiary of PMI entered into a settlement agreement (the “Settlement Agreement”) with Nicoventures Trading Limited (“NTV”), an affiliate of British American Tobacco p.l.c. (“BAT”). In accordance with its terms, the parties to the Settlement Agreement filed a joint motion to rescind the limited exclusion order and the cease-and-desist order issued by the International Trade Commission (“ITC”) on September 29, 2021, which was granted on March 11, 2024. Prior to their rescission, the orders prohibited the importation and sales of imported IQOS products to the United States of America (for further details of the Settlement Agreement, ITC order and its rescission, see Note 18. Contingencies). As a result, PMI has initiated a project in the first quarter of 2024 to restructure the sourcing of IQOS products to commercialize them in the United States. For further details on IQOS commercialization in the U.S. and the related agreement with Altria Group, Inc (“Altria”), see Note 3. Acquisitions and Divestitures.
In 2024, PMI recorded pre-tax restructuring charges of $133 million related to this restructuring activity. This amount included contract termination costs with suppliers of $73 million, including prepaid commitments of $20 million. The amount also included asset impairment costs of $60 million, primarily related to machinery and equipment and other assets, which were non-cash charges.
Venezuela
In the first quarter of 2024, PMI ceased its operations in Venezuela and as a result, recorded pre-tax restructuring charges of $47 million. The amount primarily included non-cash charges related to the reclassification of accumulated foreign currency translation losses from other comprehensive losses of $38 million and asset impairment charge of $5 million related to land and buildings. This amount also included contract termination, severance and other related costs of $4 million, which were paid in cash.
For details on the income tax impact of the transaction, see Note 12. Income Taxes.
Manufacturing Footprint Optimization - Germany
As a result of declining demand for cigarettes and other tobacco products in Europe, two of PMI’s German subsidiaries, Philip Morris Manufacturing GmbH and F6 Cigarettenfabrik GmbH & Co. KG, initiated consultations with employee representatives on October 29, 2024, on a proposal to end production in the factories located in Berlin and in Dresden by the end of the second quarter of 2025, and to seek to agree on fair solutions for any impacted employees. Until the consultation process is concluded, the closure is not considered probable, and the total potential costs associated with this contemplated proposal cannot be determined and, as a result, no related costs were recorded in the fourth quarter of 2024.
E-Vapor Products Manufacturing Optimization
In the first quarter of 2023, PMI initiated a project to fully outsource and restructure the manufacturing of e-vapor devices and consumables. As a result, PMI recorded pre-tax restructuring charges of $109 million. This amount included contract termination costs for suppliers of $78 million, including $21 million of embedded finance lease terminations, payable in cash. This amount also included asset impairment costs of $31 million, primarily related to machinery and equipment, which were non-cash charges.
Restructuring charges by Segment
During 2024 and 2023, PMI recorded the following pre-tax restructuring charges by segment:
| | | | | | | | | | | | | |
(in millions) | 2024 | | 2023 | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Reclassification of accumulated foreign currency translation losses from other comprehensive losses: | | | | | |
| Americas | $ | 38 | | | $ | — | | |
| Total reclassification of accumulated foreign currency translation losses from other comprehensive losses | 38 | | | — | | |
Contract termination charges: (1) | | | | | |
| Europe | — | | 34 | | |
| SSEA, CIS & MEA | — | | 23 | | |
| EA, AU & PMI DF | — | | 14 | | |
| Americas | 77 | | | 7 | | |
| | | | | |
| Total contract termination charges | 77 | | | 78 | | |
Asset impairment charges (1) | | | | | |
| Europe | — | | 13 | | |
| SSEA, CIS & MEA | — | | 9 | | |
| EA, AU & PMI DF | — | | 5 | | |
| Americas | 65 | | | 4 | | |
| | | | | |
| Total asset impairment charges | 65 | | | 31 | | |
| Restructuring charges | $ | 180 | | | 109 | | |
(1) E-vapor products manufacturing optimization charges in 2023 were allocated across all geographical segments.
Movement in Restructuring Related Liabilities
The movement in restructuring related liabilities for the year ended December 31, 2024 was as follows:
| | | | | |
| (in millions) | |
| Liability balance, January 1, 2024 | $ | 29 | |
| Charges, net | 77 | |
| Cash spent | (58) | |
| Prepaid commitments | (20) | |
| Currency/other | — | |
| Liability balance, December 31, 2024 | $ | 28 | |
Future cash payments for restructuring activities incurred to date are anticipated to be substantially paid by the end of 2025.
Leases:
PMI has operating and finance leases that are principally for real estate (office space, warehouses and retail store space), machinery and equipment, and vehicles. Lease terms range from 1 year to 69 years, some of which include options to renew, which are reasonably certain to be renewed. Lease terms may also include options to terminate the lease. The exercise of a lease renewal or termination option is at PMI’s discretion.
PMI’s operating and finance leases at December 31, 2024 and 2023, were as follows:
| | | | | | | | | | | | | | |
| At December 31, |
| (in millions) | 2024 | 2023 |
| Operating Leases | Finance Leases | Operating Leases | Finance Leases |
| Assets: | | | | |
| Machinery and equipment | $ | — | | $ | 126 | | $ | — | | $ | 111 | |
| Other assets | 585 | | — | | 631 | | — | |
| Total lease assets | $ | 585 | | $ | 126 | | $ | 631 | | $ | 111 | |
| | | | |
| Liabilities: | | | | |
| Current | | | | |
| Current portion of long-term debt | $ | — | | $ | 37 | | $ | — | | $ | 30 | |
| Accrued liabilities - Other | 177 | | — | | 197 | | — | |
| Noncurrent | | | | |
| Long-term debt | — | | 30 | | — | | 23 | |
| Income taxes and other liabilities | 427 | | — | | 456 | | — | |
| Total lease liabilities | $ | 604 | | $ | 67 | | $ | 653 | | $ | 53 | |
The components of PMI’s lease cost were as follows for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | |
| For the Years Ended December 31, | |
| (in millions) | 2024 | 2023 | 2022 | |
| Operating lease cost | $ | 283 | | $ | 266 | | $ | 248 | | |
| Finance lease cost: | | | | |
| Amortization of right-of-use assets | 72 | | 49 | | 83 | | |
| Interest on lease liabilities | 2 | | 1 | | 1 | | |
| Short-term lease cost | 63 | | 59 | | 59 | | |
| Variable lease cost | 28 | | 28 | | 23 | | |
| Total lease cost | $ | 448 | | $ | 403 | | $ | 414 | | |
Maturity of PMI’s lease liabilities, on an undiscounted basis, as of December 31, 2024, were as follows:
| | | | | | | | |
| (in millions) | Operating Leases | Finance Leases |
| 2025 | $ | 207 | | $ | 39 | |
| 2026 | 147 | | 17 | |
| 2027 | 102 | | 8 | |
| 2028 | 66 | | 5 | |
| 2029 | 35 | | 2 | |
| Thereafter | 175 | | — | |
| Total lease payments | 732 | | 71 | |
| Less: Interest | 128 | | 4 | |
| Present value of lease liabilities | $ | 604 | | $ | 67 | |
Other information related to PMI’s leases was as follows for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | |
| December 31, |
| (in millions) | 2024 | 2023 | 2022 |
| Operating Leases | Finance Leases | Operating Leases | Finance Leases | Operating Leases | Finance Leases |
Cash paid for amounts included in the measurement of lease liabilities in operating cash flows (1) | $ | 281 | | $ | — | | $ | 265 | | $ | — | | $ | 243 | | $ | — | |
| Cash paid for amounts included in the measurement of lease liabilities in financing cash flows | $ | — | | $ | 29 | | $ | — | | $ | 27 | | $ | — | | $ | 76 | |
| Leased assets obtained in exchange for new lease liabilities | $ | 214 | | $ | 73 | | $ | 205 | | $ | 55 | | $ | 255 | | $ | 100 | |
| Weighted-average remaining lease term (years) | 10.4 | 2.6 | 10.2 | 2.6 | 10.3 | 2.1 |
Weighted-average discount rate(2) (3) | 5.9 | % | 4.4 | % | 5.1 | % | 4.9 | % | 3.4 | % | 4.4 | % |
(1) Cash paid included in the operating cash flows for finance leases is not material.
(2) PMI’s weighted-average discount rate for operating leases is based on its estimated pre-tax cost of debt adjusted for country-specific risk.
(3) PMI’s weighted-average discount rate for finance leases, excluding embedded leases, is based on its estimated pre-tax cost of debt adjusted for country-specific risk and where applicable the interest rate explicit in lease contracts.
Supply Chain Financing:
PMI has engaged with unaffiliated global financial institutions that offer a voluntary supply chain financing ("SCF") program to some of our suppliers. Under the SCF program, the suppliers may elect, at their sole discretion, to sell PMI's payment obligations to these financial institutions. The suppliers independently negotiate the sale arrangements directly with these financial institutions. PMI does not participate in these negotiations, nor does it have any economic interest in these agreements, or in the designated suppliers’ voluntary decision to sell PMI's payment obligations to these financial institutions. No guarantees or securities are provided by PMI or any of its subsidiaries under the SCF programs. PMI's obligations to its suppliers, including amounts due and scheduled payment terms are not impacted by the suppliers’ decision to sell amounts under the SCF program. The payment terms of PMI’s suppliers generally do not exceed 120 days. All outstanding payable amounts related to suppliers that are participating in the SCF program are recorded in accounts payable in PMI's consolidated balance sheets. The associated payments are included in cash flows from operating activities within PMI's consolidated statement of cash flows.
The rollforward of PMI's outstanding obligations under its SCF program for the year ended December 31, 2024 were as follows:
| | | | | | |
| (in millions) | 2024 | |
| Amount due to suppliers participating in the SCF program as of January 1, 2024 | $ | 864 | | |
| Invoices added during the year | 3,405 | | |
| Invoices paid during the year | (3,207) | | |
| Currency/Other | (48) | | |
| Amount due to suppliers participating in the SCF program as of December 31, 2024 | $ | 1,014 | | |
New Accounting Standards:
Recently adopted
On November 27, 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update ASU 2023-07, “Improvements to Reportable Segment Disclosures” (“ASU 2023-07”). ASU 2023-07 improves reportable segment disclosures, primarily through enhanced disclosures about significant segment expenses that impact segment profit or loss, regularly provided to the chief operating decision maker. The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024, on a retrospective basis, with early adoption permitted. PMI adopted the additional disclosure requirements on the specified effective date within its annual reporting for the year ended December 31, 2024, and its interim reporting starting for the quarter ending March 31, 2025.
Recently issued
On December 14, 2023, the FASB issued Accounting Standards Update ASU 2023-09, “Improvements to Income Tax Disclosures” (“ASU 2023-09”). ASU 2023-09 enhances the transparency of income tax disclosures, primarily by requiring public business entities to disclose specific categories in the rate reconciliation tabular presentation, as well as by providing additional information for reconciling items that meet a quantitative threshold. ASU 2023-09 also requires disaggregated disclosures of federal, state and foreign income tax taxes paid. ASU 2023-09 is effective for annual periods beginning after December 15, 2024, and early adoption is permitted. The amendments are applicable on a prospective basis, although retrospective basis is also permitted. PMI is currently evaluating the impact of ASU 2023-09 on its disclosures.
On November 4, 2024, the FASB issued Accounting Standards Update ASU 2024-03, “Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses” (“ASU 2024-03”). ASU 2024-03 requires disclosure of more detailed information about certain costs and expenses in the notes to the financial statements at interim and annual reporting periods. ASU 2024-03 is effective for annual reporting periods beginning after December 15, 2026, and for interim periods within annual reporting periods beginning after December 15, 2027, with early adoption permitted. PMI is currently evaluating the impact of ASU 2024-03 on its disclosures.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Philip Morris International Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Philip Morris International Inc. and its subsidiaries (the “Company”) as of December 31, 2024 and 2023, and the related consolidated statements of earnings, comprehensive earnings, stockholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2024, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Management on Internal Control Over Financial Reporting. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Tobacco-Related Litigation for Smoking and Health Class Actions and Health Care Cost Recovery Cases
As described in Note 18 to the consolidated financial statements, the Company has nine smoking and health class actions and 17 health care cost recovery cases pending. The Company records provisions in the consolidated financial statements for pending litigation when management determines that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. Except as stated otherwise in Note 18, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available, (i) management has not concluded that it is probable that a loss has been incurred in any of the pending smoking and health class actions and health care cost recovery cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending smoking and health class actions and health care cost recovery cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any.
The principal considerations for our determination that performing procedures relating to tobacco-related litigation for smoking and health class actions and health care cost recovery actions is a critical audit matter are (i) the significant judgment by management when assessing the probability of a loss being incurred and determining whether the amount or range of the potential loss for each case can be reasonably estimated and (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating audit evidence related to management’s assessment of the loss contingencies associated with smoking and health class actions and health care cost recovery actions.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s assessment of smoking and health class actions and health care cost recovery cases, including controls over determining whether the probability and range of loss can be reasonably estimated, as well as controls over financial statement disclosures. These procedures also included, among others (i) confirming with external and internal legal counsel the possibility or probability of an unfavorable outcome and the extent to which the loss or range of loss is reasonably estimable; (ii) evaluating the reasonableness of management’s assessment regarding whether an unfavorable outcome is reasonably possible or probable and reasonably estimable; and (iii) evaluating the sufficiency of the Company’s smoking and health class actions and health care cost recovery cases contingencies disclosures.
Impairment Related to the Rothmans, Benson & Hedges Equity Investment
As described in Note 6 to the consolidated financial statements, since the 2019 deconsolidation of Rothmans, Benson and Hedges Inc. (RBH), the Company has accounted for its continuing investment in the business as an equity security, without readily determinable fair value. On January 24, 2025, RBH filed an objection to approval of the proposed plan of compromise and arrangement (Proposed Plan) with the CCAA court. Developments, including the positions taken by RBH in this objection and the positions taken by other parties in related filings narrowed the range of possible outcomes with respect to the allocation of the aggregate settlement, which was determined to be an indicator that the Company’s investment in RBH may be impaired. Although there remains some uncertainty as to the final terms of the Proposed Plan, management evaluated its investment in RBH for potential impairment and concluded that the estimated fair value of its investment in RBH was lower than its carrying value. As a result, management performed a quantitative valuation of its investment in RBH as of December 31, 2024, and recorded a non-cash impairment charge of $2,316 million. The fair value of the continuing investment in RBH of $714 million represented the estimated fair value of the underlying business, net of management’s best estimate of the share of the aggregate global settlement amount that could be allocated to RBH, and was determined based on an income approach using a discounted cash flow analysis. In determining the fair value of the investment in RBH, management made various judgments, estimates and assumptions, the most significant of which were discount rate, sales volumes and operating margins related to the fair value of the combustible tobacco product business in Canada. In addition, significant estimates were made with respect to the allocation amount of the aggregate global settlement amount, as well as the deductibility of the settlement amount payment for income tax purposes in Canada.
The principal considerations for our determination that performing procedures relating to the impairment of the equity investment in RBH is a critical audit matter are (i) the significant judgment by management when developing the fair value estimate of the Company’s investment in RBH; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to the discount rate, sales volumes and operating margins related to the fair value of the combustible tobacco product business in Canada and the estimate of the allocation amount of the aggregate global settlement amount as well as the deductibility of the settlement amount payment for income tax purposes in Canada; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s equity investment impairment assessment, including controls over the valuation of the Company’s investment in RBH. These procedures also included, among others (i) testing management’s process for developing the fair value estimate of the investment in RBH; (ii) evaluating the appropriateness of the discounted cash flow analysis; (iii) testing completeness and accuracy of underlying data used in the discounted cash flow analysis; (iv) evaluating the reasonableness of significant assumptions used by management related to the discount rate, sales volumes and operating margins related to the valuation of the combustible cigarette business and the estimate of the allocation amount of the aggregate global settlement amount; and (v) evaluating the deductibility of the settlement amount payment for income tax purposes in Canada. Evaluating management’s assumptions related to sales volumes and operating margins related to the valuation of the combustible tobacco product business in Canada and the estimate of the allocation amount of the aggregate global settlement amount involved evaluating whether the assumptions used by management were reasonable considering (i) the current economic conditions and recent operating results of RBH; (ii) the consistency with external market and industry data; and (iii) whether the assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the discounted cash flow analysis; (ii) the reasonableness of the discount rate assumption related to the valuation of the combustible tobacco product business in Canada; and (iii) the deductibility of the settlement amount payment for income tax purposes in Canada.
| | | | | | | | |
| /S/ PRICEWATERHOUSECOOPERS SA | | |
| PricewaterhouseCoopers SA | | |
| | |
| | |
| | |
| | |
| | |
| Lausanne, Switzerland | | |
| February 6, 2025 | | |
We have served as the Company’s auditor since 2008.
Report of Management on Internal Control Over Financial Reporting
Management of Philip Morris International Inc. (“PMI” or "we") is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. PMI’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes those written policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of PMI;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America;
•provide reasonable assurance that receipts and expenditures of PMI are being made only in accordance with the authorization of management and directors of PMI; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements.
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of PMI’s internal control over financial reporting as of December 31, 2024. Management based this assessment on criteria for effective internal control over financial reporting described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included an evaluation of the design of PMI’s internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. Management reviewed the results of its assessment with the Audit & Risk Committee of our Board of Directors.
Based on this assessment, management determined that, as of December 31, 2024, PMI maintained effective internal control over financial reporting.
PricewaterhouseCoopers SA, an independent registered public accounting firm, who audited and reported on the consolidated financial statements of PMI included in this report, has audited the effectiveness of PMI’s internal control over financial reporting as of December 31, 2024, as stated in their report herein.
February 6, 2025
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A.Controls and Procedures.
PMI carried out an evaluation, with the participation of PMI’s management, including PMI’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of PMI’s disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based upon that evaluation, PMI’s Chief Executive Officer and Chief Financial Officer concluded that PMI’s disclosure controls and procedures are effective. There have been no changes in PMI’s internal control over financial reporting during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, PMI’s internal control over financial reporting.
The Report of Management on Internal Control over Financial Reporting and the Report of Independent Registered Public Accounting Firm are included in Item 8.
Item 9B.Other Information.
On February 4, 2025, Juan José Daboub informed PMI's board of directors (the “Board”) that he will not stand for re-election to the Board at our 2025 annual meeting of shareholders. Dr. Daboub’s decision not to stand for re-election to the Board was not a result of any disagreement with the Company.
During the three months ended December 31, 2024, no director or officer of PMI adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as such terms are defined in Item 408(a) of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Except for the information relating to the executive officers set forth in Item 10 and the information relating to equity compensation plans set forth in Item 12, the information called for by Items 10-14 is hereby incorporated by reference to PMI’s definitive proxy statement for use in connection with its annual meeting of stockholders to be held on May 7, 2025, that will be filed with the SEC on or about March 27, 2025 (the “proxy statement”), and, except as indicated therein, made a part hereof.
Item 10.Directors, Executive Officers and Corporate Governance.
Information About Our Executive Officers as of February 6, 2025:
| | | | | | | | | | | | | | | | | |
| Name | | Office | | Age | |
| Jacek Olczak | | Chief Executive Officer | | 60 | | |
| Massimo Andolina | | President, Europe Region | | 56 | | |
| Emmanuel Babeau | | Chief Financial Officer | | 57 | | |
| Werner Barth | | President, Combustibles Category & Global Combustibles Marketing | | 60 | | |
| Lars Dahlgren | | President, Smoke-Free Oral Products & Chief Executive Officer Swedish Match | | 54 | | |
| Frederic de Wilde | | President, South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Region | | 57 | | |
| Reginaldo Dobrowolski | | Vice President and Controller | | 50 | | |
| Vassilis Gkatzelis | | President, East Asia, Australia, and PMI Duty Free Region | | 47 | |
| Yann Guérin | | Senior Vice President and General Counsel | | 48 | | |
| Stacey Kennedy | | President, Americas Region & CEO of PMI's U.S. Business | | 52 | | |
| Stefano Volpetti | | President, Smoke-Free Inhalable Products & Chief Consumer Officer | | 53 | | |
Jacek Olczak – Age 60
Mr. Olczak was appointed as our Chief Executive Officer in May 2021. From January 2018 until May 2021, Mr. Olczak has served as our Chief Operating Officer, and from August 2012 until December 31, 2017, he served as our Chief Financial Officer. He joined PMI’s Polish affiliate in 1993 and progressed through various roles in finance and general management positions across Europe, including as Managing Director of PMI’s markets in Poland and Germany and as President of the European Union Region, before being appointed Chief Financial Officer. Prior to joining PMI, Mr. Olczak worked for BDO, an international network of public accounting, tax, consulting and business advisory firms.
Massimo Andolina – Age 56
Mr. Andolina was appointed as our President, Europe Region in January 2023, prior to which he served as our Senior Vice President, Operations since January 2018. He joined PMI in 2008 as Director, Operations Planning, and has held several various roles at PMI, including Vice President, Operations of Latin America & Canada Region from December 2010 to July 2013; Vice President, EU Operations, from August 2013 to June 2016; and Vice President, PMI Transformation from July 2016 to December 2017. Prior to joining PMI, Mr. Andolina held a variety of international positions in strategic marketing and general management for Tetra Pak International and in operations for R.J. Reynolds International.
Emmanuel Babeau – Age 57
Mr. Babeau was appointed as our Chief Financial Officer in May 2020. Prior to joining PMI in May 2020, Mr. Babeau served as the Deputy Chief Executive Officer of Schneider Electric, an energy and automation digital solutions company. In this position, he was in charge of Finance and Legal Affairs. Mr. Babeau joined Schneider Electric in 2009 as Executive Vice President Finance and a member of the Management Board. Mr. Babeau also served on the board of Sanofi S.A., a French multinational healthcare company, from 2018 to 2020. Mr. Babeau started his career in 1990 at Arthur Andersen, and from 1993 to 2009, he progressed through various positions at Pernod Ricard, a beverage company, the latest being Chief Financial Officer and Group Deputy Managing Director. Mr. Babeau also served as a non-executive director at Sodexo, a French food services and facilities management company, from January 2016 until December 2021. He currently sits on the board of Davide Campari-Milano N.V.
Werner Barth – Age 60
Mr. Barth was appointed as our President, Combustibles Category & Global Combustibles Marketing in November 2021. Mr. Barth joined PMI in 1990 as Marketing Trainee at Philip Morris Germany and throughout his career he progressed through various roles at PMI in marketing, product management, brand supervision and general management. Prior to his current position, from 2015, Mr. Barth held the role of Senior Vice President, Marketing & Sales, and from 2018, he held the role of Senior Vice President, Commercial.
Lars Dahlgren – Age 54
Mr. Dahlgren was appointed as our President Smoke-Free Oral Products and CEO Swedish Match in January 2023. Prior to PMI’s acquisition of Swedish Match, he served as President and Chief Executive Officer of Swedish Match since June 2008, and as its Chief Financial Officer and Senior Vice President from July 2004 until June 2008. Prior to that, from April 2004 to July 2004, he was Acting Chief Financial Officer and Vice President of Finance at Swedish Match. Mr. Dahlgren joined Swedish Match in 1996 and has been a member of its Group Management Team since 2004.
Frederic de Wilde – Age 57
Mr. de Wilde was appointed as our President, South and Southeast Asia, Commonwealth of Independent States, Middle East and Africa Regions in January 2023, prior to which he served as President, European Union Region from July 2015. From July 2011 until July 2015, Mr. de Wilde held the role of Senior Vice President, Marketing & Sales. Mr. de Wilde joined PMI in 1992 as Brand Manager L&M at Philip Morris Belgium, and throughout his career, he progressed through various roles at PMI in marketing, sales and general management.
Reginaldo Dobrowolski – Age 50
Mr. Dobrowolski was appointed as our Vice President and Controller in August 2021. From May 2019 until August 2021, Mr. Dobrowolski was our Vice President, Corporate Financial Planning, Data & Reporting. Prior to that, Mr. Dobrowolski held various roles in our Finance department, including Director Corporate Financial Planning & Reporting from October 2014 until May 2019.
Vassilis Gkatzelis – Age 47
Mr. Gkatzelis was appointed as our President, East Asia, Australia, and PMI Duty Free Region in May 2024. Previously, he served as our President Director, PT HM Sampoerna Tbk, PMI’s affiliate listed on the Indonesia Stock Exchange, overseeing the smoke-free and combustible business since 2022. Mr. Gkatzelis joined PMI in 2003 as a management trainee at Philip Morris Greece during the acquisition and integration with Papastratos. He has held roles of increasing responsibilities in marketing, sales, and general management across markets in Asia, Europe, Middle East & Africa, and in the Global Operations Center in Switzerland, where he served as Director of Commercial Approach Strategy & Deployment. Since 2014, he assumed roles in general management, including General Manager, Morocco and Managing Director, Egypt & Levant, where he and his team built the smoke-free product category across all markets of the cluster. Before joining PMI, he worked at L’Oréal.
Yann Guérin - Age 48
Mr. Guérin was appointed as Senior Vice President and General Counsel in July 2023, having served as Senior Vice President and Global Head of Law and Compliance for June 2023. Previously, he served as Vice President and Associate General Counsel, Corporate from July 2021 to May 2023; as Vice President and Associate General Counsel, South & Southern Asia from November 2019 to June 2021; and as Vice President and Associate General Counsel, Middle-East, Africa & Global Duty Free from January 2018 to October 2019. Prior to that, since joining PMI in 2006, Mr. Guérin held a variety of legal roles across the company’s businesses, regions and functions. Before joining PMI, he was an attorney at Skadden Arps.
Stacey Kennedy – Age 52
Ms. Kennedy was appointed as our President, Americas Region & CEO of PMI's U.S. Business in January 2023. Previously, she served as our President, South and Southeast Asia Region from January 2018. From 2015 until 2018, Ms. Kennedy served as Managing Director for Germany, Austria, Croatia, and Slovenia. Ms. Kennedy began her career with Philip Morris USA in 1995 as a Territory Sales Manager. Throughout her career, she held a number of positions of increasing responsibility in commercial and general management.
Stefano Volpetti – Age 53
Mr. Volpetti was appointed as our President Smoke-Free Inhalable Products & Chief Consumer Officer in January 2023, having served as President Smoke-Free Products Category & Chief Consumer Officer from November 2021. Mr. Volpetti joined PMI in June 2019 as Chief Consumer Officer. From February 2016 until May 2019, Mr. Volpetti served as the Vice President & Brand Franchise Leader of a multi-functional, global business unit at Procter & Gamble, a multinational consumer goods company. Mr. Volpetti spent 22 years at Procter & Gamble, progressing through various roles with increasing responsibility locally in Italy and Mexico, and on a regional level for the European market. Mr. Volpetti also served as Chief Marketing Officer at Luxottica Group S.p.A, an Italian eyewear conglomerate, in 2015.
Insider Trading Policies and Procedures
We have adopted an insider trading policy that governs the purchase, sale, and/or other dispositions of our securities that applies to our directors, officers, employees, and other covered persons and entities, that we believe is reasonably designed to promote compliance with insider trading laws, rules and regulations and applicable listing standards. A copy of our insider trading policy is filed with this Annual Report on Form 10-K as Exhibit 19.
Codes of Ethics and Corporate Governance
We have adopted a code of ethics, which we call the Code of Conduct. The Code of Conduct complies with requirements set forth in Item 406 of Regulation S-K, applies to our Board of Directors and to all of our employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, and persons performing similar functions. The Code of Conduct is available free of charge on our website at www.pmi.com.
In addition, we have adopted corporate governance guidelines and charters for our Audit and Risk, Compensation and Leadership Development, Science and Technology and Nominating and Corporate Governance committees of the Board of Directors. All of these documents are available free of charge on our website at www.pmi.com. Any waiver granted by Philip Morris International Inc. to its principal executive officer, principal financial officer or controller, or any person performing similar functions under our code of ethics, or certain amendments to the code of ethics, will be disclosed on our website at www.pmi.com.
The information on our website is not, and shall not be deemed to be, a part of this Report or incorporated into any other filings made with the SEC.
Also refer to Board Operations and Governance—Committees of the Board, Election of Directors—Process for Nominating Directors, Election of Directors—Director Nominees, Stock Ownership Information and Availability of Reports, Other Matters and 2026 Annual Meeting—2026 Annual Meeting sections of the proxy statement.
Item 11.Executive Compensation.
Refer to Compensation Discussion and Analysis, Compensation Tables, Compensation of Directors, and Pay Ratio sections of the proxy statement.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The number of shares to be issued upon exercise or vesting and the number of shares remaining available for future issuance under PMI’s equity compensation plans at December 31, 2024, were as follows:
| | | | | | | | | | | | | | | | | | | | |
| Number of Securities to be Issued upon Exercise of Outstanding Options and Vesting of RSUs and PSUs (a) | | Weighted Average Exercise Price of Outstanding Options (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding Securities reflected in column (a)) (c) | |
Equity compensation plans
approved by stockholders | 7,242,974 | | 1 | $ | — | | | 20,045,796 | | |
1 Represents 4,542,654 shares of common stock that may be issued upon vesting of the restricted share units and 2,700,320 shares that may be issued upon vesting of the performance share units if maximum performance targets are achieved for each performance cycle. PMI has not granted options since the spin-off from Altria on March 28, 2008.
Also refer to Stock Ownership Information—Ownership of Equity Securities section of the proxy statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Refer to Related Person Transactions and Code of Conduct and Election of Directors—Independence of Nominees sections of the proxy statement.
Item 14.Principal Accounting Fees and Services.
Refer to Audit and Risk Committee Matters section of the proxy statement.
PART IV
Item 15.Exhibits and Financial Statement Schedules.
(a) Index to Consolidated Financial Statements and Schedules
| | | | | |
| Page |
| Consolidated Statements of Earnings for the years ended December 31, 2024, 2023 and 2022 | |
Consolidated Statements of Comprehensive Earnings for the years ended December 31, 2024, 2023 and 2022 | |
| Consolidated Balance Sheets at December 31, 2024 and 2023 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2024, 2023 and 2022 | |
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2024, 2023 and 2022 | |
| Notes to Consolidated Financial Statements | |
| Report of Independent Registered Public Accounting Firm (PCAOB ID 1358) | |
| Report of Management on Internal Control Over Financial Reporting | |
Schedules have been omitted either because such schedules are not required or are not applicable.
(b) The following exhibits are filed as part of this Report:
| | | | | | | | | | | | | | |
| 2.1 | | — | | |
| 2.2 | | — | | |
| 3.1 | | — | | |
| 3.2 | | — | | |
| 4.1 | | — | | |
| 4.2 | | — | | |
| 4.3 | | — | | |
| 4.4 | | — | | |
| | | | | | | | | | | | | | | | | |
| 10.4 | | __ | | Extension Agreement, effective February 7, 2017, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders party thereto, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 30, 2017). | |
| 10.5 | | __ | | Extension Agreement, effective January 31, 2014, to Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders party thereto and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2014). | |
| 10.6 | | __ | | Extension Agreement, effective as of February 10, 2015, to Credit Agreement dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2015). | |
| 10.7 | | __ | | Amendment No. 1, dated as of July 20, 2015, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, The Royal Bank of Scotland plc, as resigning administrative agent, and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as successor administrative agent (incorporated by reference to Exhibit 10.52 to the Annual Report on Form 10-K for the year ended December 31, 2015). | |
| 10.8 | | — | | Credit Agreement, dated as of October 1, 2015, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed October 5, 2015). | |
| 10.9 | | —
| |
| |
| 10.10 | | —
| | Extension Agreement, effective as of October 1, 2016, to the Credit Agreement dated as of October 1, 2015, among Philip Morris International Inc., lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 31, 2016). | |
| 10.11 | | — | | Extension Agreement, effective as of October 1, 2017, to the Credit Agreement, dated as of October 1, 2015, among Philip Morris International Inc., the lenders party thereto and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 29, 2017). | |
| 10.12 | | —
| | Extension Agreement, effective as of February 6, 2018, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2018). | |
| 10.13 | | —
| | Extension Agreement, effective as of February 5, 2019, to the Credit Agreement dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2019). | |
| 10.14 | | — | | Amendment and Extension Agreement, effective February 4, 2020, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., each lender named therein and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 3, 2020). | |
| 10.15 | | — | | Credit Agreement, dated as of February 10, 2020, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch, as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 11, 2020). | |
| 10.16 | | — | | Amendment and Extension Agreement, effective February 2, 2021, to the Credit Agreement, dated as of February 12, 2013, among PMI, the lenders named therein and Citibank Europe PLC, UK Branch (legal successor to Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 2, 2021). | |
| | | | | | | | | | | | | | | | | |
| 10.17 | | — | | Amendment and Extension Agreement, effective February 10, 2021, to the Credit Agreement, dated as of February 10, 2020, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed February 2, 2021). | |
| 10.18 | | — | | Credit Agreement, dated as of September 29, 2021, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1to the Current Report on Form 8-K filed September 30, 2021). | |
| 10.19 | | — | | Amendment and Extension Agreement, effective February 1, 2022, to the Credit Agreement, dated as of February 12, 2013, among PMI, the lenders named therein and Citibank Europe PLC, UK Branch (legal successor to Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 1, 2022). | |
| 10.20 | | — | | Amendment and Extension Agreement, effective February 10, 2022, to the Credit Agreement, dated as of February 10, 2020, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed February 1, 2022). | |
| 10.21 | | — | | | |
| 10.22 | | — | | | |
| 10.23 | | — | | | |
| 10.24 | | — | | | |
| 10.25 | | — | | Amendment and Extension Agreement, dated as of September 20, 2022, to the Credit Agreement, dated as of September 29, 2021, among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed September 23, 2022). | |
| 10.26 | | — | | | |
| 10.27 | | — | | Amendment and Extension Agreement, dated as of September 20, 2023 among PMI, the lenders named therein, Citibank Europe PLC, UK Branch, as facility agent, and Citibank, N.A., as swingline agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed September 20, 2023). | |
| 10.28 | | — | | | |
| 10.29 | | — | | | |
| 10.30 | | — | | | |
| 10.31 | | — | | Terms Agreement, dated October 30, 2024, among PMI and BBVA Securities Inc., BofA Securities, Inc., Deutsche Bank Securities Inc., Goldman Sachs & Co. LLC, Wells Fargo Securities, LLC, Barclays Capital Inc., Citigroup Global Markets Inc., Mizuho Securities USA LLC and UBS Securities LLC, as representatives of the several underwriters named therein (incorporated by reference to Exhibit 1.2 to the Current Report on Form 8-K filed November 1, 2024). | |
| | | | | | | | | | | | | | |
| 10.35 | | — | | |
| 10.36 | | — | | |
| 10.37 | | — | | |
| 10.38 | | — | | |
| 10.39 | | — | | |
| 10.40 | | — | | |
| 10.41 | | — | | |
| 10.42 | | — | | |
| 10.43 | | — | | |
| 10.44 | | — | | |
| 10.45 | | — | | |
| 10.46 | | — | | |
| 10.47 | | — | | |
| 10.48 | | — | | |
| 10.49 | | — | | |
| 10.50 | | — | | |
| 10.51 | | — | | |
| 10.52 | | — | | |
| 10.53 | | — | | |
| 10.54 | | — | | |
| | | | | | | | | | | | | | |
| 10.55 | | — | | |
| 10.56 | | — | | |
| 10.57 | | — | | |
| 10.58 | | — | | |
| 10.59 | | — | | |
| 10.60 | | — | | |
| 10.61 | | — | | |
| 10.62 | | — | | |
| 10.63 | | — | | |
| 10.64 | | — | | |
| 10.65 | | — | | |
| 10.66 | | — | | |
| 10.67 | | — | | |
| 10.68 | | — | | |
| 10.69 | | — | | |
| 10.70 | | — | | |
| 10.71 | | — | | |
| 10.72 | | — | | |
| 10.73 | | — | | |
| 10.74 | | — | | |
| | | | | | | | | | | | | | |
| 10.75 | | — | | |
| 10.76 | | — | | |
| 10.77 | | — | | |
| 10.78 | | — | | |
| 10.79 | | — | | |
| 10.80 | | — | | |
| 10.81 | | — | | |
| 10.82 | | — | | |
| 10.83 | | — | | |
| 10.84 | | — | | |
| 10.85 | | — | | |
| 10.86 | | — | | |
| 10.87 | | — | | |
| 10.88 | | — | | |
| 10.89 | | — | | |
| 10.90 | | — | | |
| 10.91 | | — | | |
| 10.92 | | — | | |
| 10.93 | | — | | |
| 10.94 | | — | | |
| 10.95 | | — | | |
| 10.96 | | — | | |
| | | | | | | | | | | | | | |
| 19 | | — | | |
| 21 | | — | | |
| 23 | | — | | |
| 31.1 | | — | | |
| 31.2 | | — | | |
| 32.1 | | — | | |
| 32.2 | | — | | |
| 97 | | — | | |
| 101.INS | | — | | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document.
|
| 101.SCH | | — | | XBRL Taxonomy Extension Schema. |
| | | | | | | | | | | | | | |
| 101.CAL | | — | | XBRL Taxonomy Extension Calculation Linkbase. |
| 101.DEF | | — | | XBRL Taxonomy Extension Definition Linkbase. |
| 101.LAB | | — | | XBRL Taxonomy Extension Label Linkbase. |
| 101.PRE | | — | | XBRL Taxonomy Extension Presentation Linkbase. |
| 104 | | — | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
________
* Denotes management contract or compensatory plan or arrangement in which directors or executive officers are eligible to participate.
** Schedules and certain portions of this exhibit have been omitted pursuant to Item 601(a)(5) and Item 601(b)(10)(iv) of Regulation S-K.
x Denotes exhibits filed herewith.
The exhibits filed herewith do not include certain instruments with respect to long-term debt of PMI, inasmuch as the total amount of debt authorized under any such instrument does not exceed 10 percent of the total assets of PMI on a consolidated basis. PMI agrees, pursuant to Item 601(b)(4)(iii) of Regulation S-K, that it will furnish a copy of any such instrument to the SEC upon request.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| PHILIP MORRIS INTERNATIONAL INC. |
| |
| By: | /s/ JACEK OLCZAK |
| (Jacek Olczak
Chief Executive Officer) |
Date: February 6, 2025
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jacek Olczak, Emmanuel Babeau, and Darlene Quashie Henry and each of them, acting individually, as his or her true and lawful attorney-in-fact, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K for the year ended December 31, 2024, and other documents in connection herewith and therewith, and to file the same, with all exhibits thereto, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection herewith and therewith and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated:
| | | | | | | | |
| Signature | Title | Date |
| | |
| /s/ JACEK OLCZAK | Chief Executive Officer and Director | February 6, 2025 |
| (Jacek Olczak) |
| /s/ EMMANUEL BABEAU | Chief Financial Officer | February 6, 2025 |
| (Emmanuel Babeau) |
| /s/ REGINALDO DOBROWOLSKI | Vice President and Controller | February 6, 2025 |
| (Reginaldo Dobrowolski) |
| /s/ ANDRÉ CALANTZOPOULOS | Non-Executive Chairman | February 6, 2025 |
| (André Calantzopoulos) |
| /s/ BONIN BOUGH | Director | February 6, 2025 |
| (Bonin Bough) |
| /s/ MICHEL COMBES | Director | February 6, 2025 |
| (Michel Combes) |
| /s/ DR. JUAN JOSÉ DABOUB | Director | February 6, 2025 |
| (Juan José Daboub) |
| | | | | | | | |
| /s/ WERNER GEISSLER | Director | February 6, 2025 |
| (Werner Geissler) |
| /s/ VICTORIA HARKER | Director | February 6, 2025 |
| (Victoria Harker) |
| /s/ LISA A. HOOK | Director | February 6, 2025 |
| (Lisa A. Hook) |
| /s/ KALPANA MORPARIA | Director | February 6, 2025 |
| (Kalpana Morparia) |
| /s/ ROBERT B. POLET | Director | February 6, 2025 |
| (Robert B. Polet) |
| /s/ DESSISLAVA TEMPERLEY | Director | February 6, 2025 |
| (Dessislava Temperley) |
| /s/ SHLOMO YANAI | Director | February 6, 2025 |
| (Shlomo Yanai) |