Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic or biosimilar products.
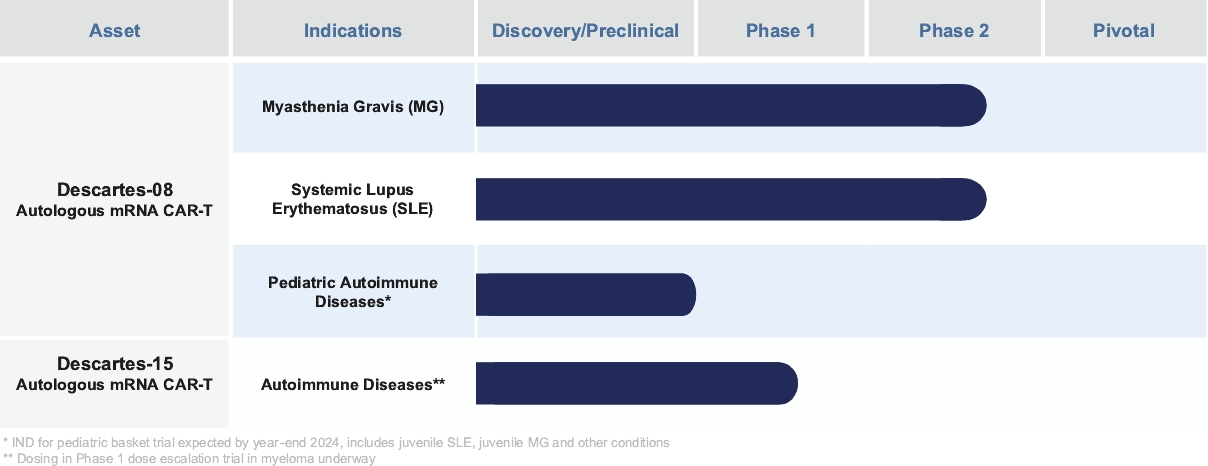
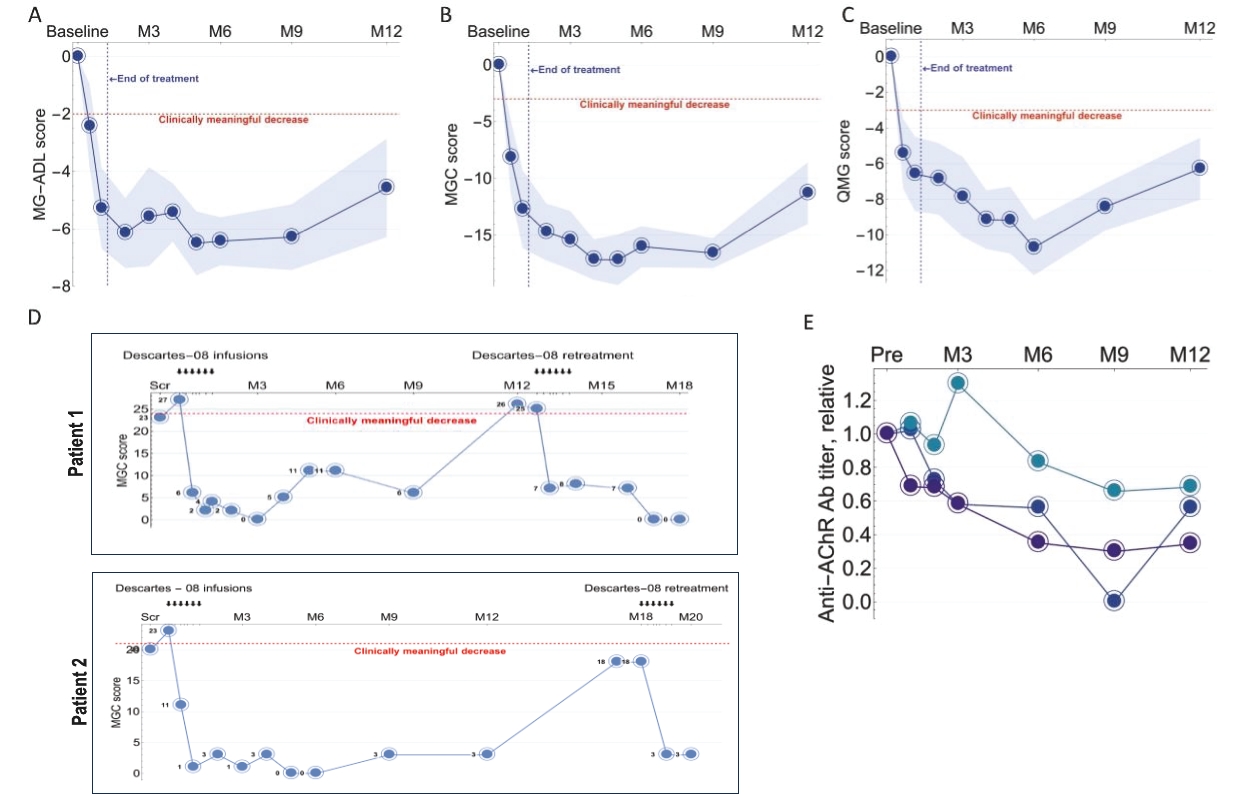
Descartes-08 may compete with products of other companies in the MG market, including Argenx SE, UCB S.A., Johnson & Johnson, Alexion Pharmaceuticals, Inc. and Cabaletta Bio, Inc.
Other companies developing CAR-T therapies include large, fully integrated pharmaceutical companies such as Novartis AG, Gilead Sciences, Inc., through its Kite Pharma, Inc. subsidiary, Bristol-Myers Squibb Company, AstraZeneca PLC and Janssen Pharmaceuticals, Inc. and biopharmaceutical companies such as Kyverna Therapeutics, Inc. and Cabaletta Bio, Inc.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing.
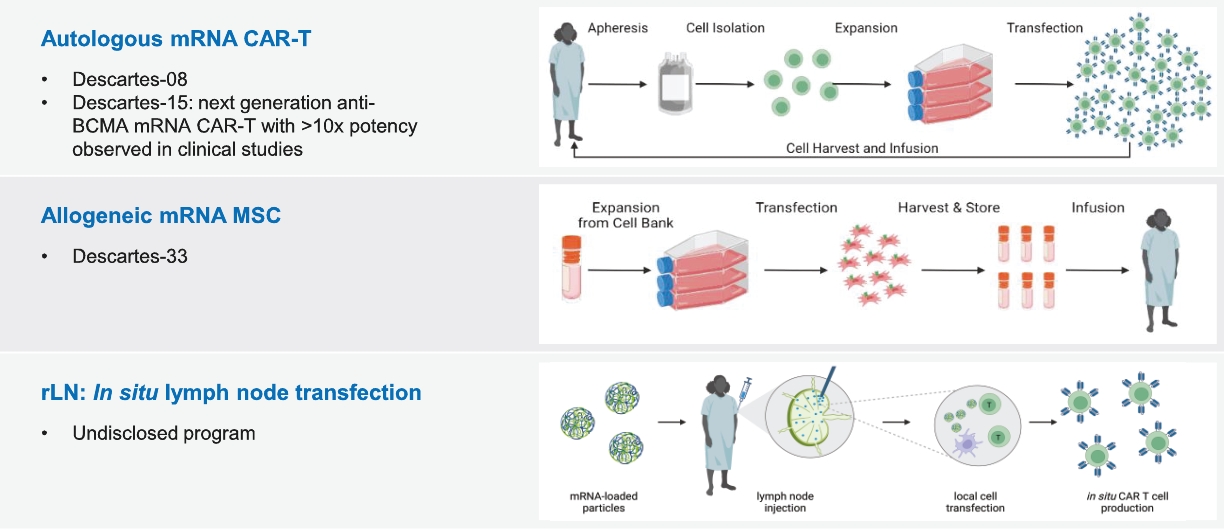
We believe our cell therapy product candidates are subject to regulation in the United States as “biologics” or “biological products.” We expect to seek approval of Descartes-08 through a single BLA reviewed by FDA’s Center for Biologics Evaluation and Research (“CBER”).
Biological products are subject to regulation under the Federal Food, Drug, and Cosmetic Act (“FD&C Act”) and the Public Health Service Act (“PHS Act”), and other federal, state, local and foreign statutes and regulations. Descartes-08 and any other product candidates that we develop must be approved by the FDA before they may be legally marketed in the United States and by the appropriate foreign regulatory agency before they may be legally marketed in foreign countries.
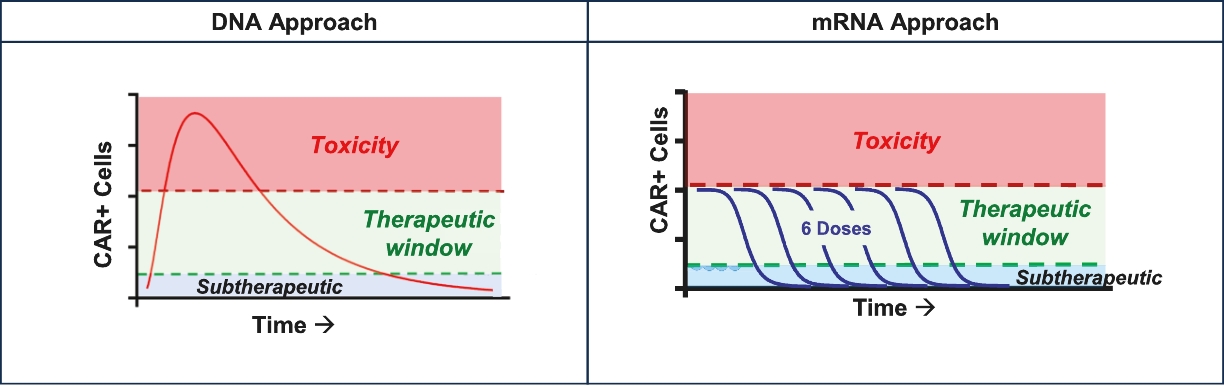
We regard our mRNA-modified products as cell therapy products and not as genetic engineering or gene therapy products, because mRNA modifications are not embodied in DNA or incorporated into a genome. However, it is possible that in some jurisdictions, regulations on genetic engineering or genetic therapy may intentionally or unintentionally apply to our technology. This could create additional regulatory burden.
U.S. Biological Products Development Process
The process required by the FDA before a biologic, including a cell therapy, may be marketed in the United States is summarized below.
Biological product candidates are preclinically tested before any testing is done in humans. These tests, or non-clinical studies, include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the product candidate. The conduct of the preclinical tests must comply with federal requirements including good laboratory practices (“GLPs”).
The clinical study sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND which must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA places the clinical study on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. The FDA may also impose clinical holds on a biological product candidate at any time before or during clinical trials due to safety concerns, non-compliance with regulatory requirements, or other issues. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA. In addition to these requirements, biological product candidates may also require evaluation and assessment by an institutional biosafety committee (“IBC”), that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at an institution participating in a clinical trial.
Clinical trials are conducted under protocols detailing the objectives of the clinical study, dosing procedures, patient selection and exclusion criteria, and the parameters to be used to monitor patient safety. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted and