UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2024
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____________ to _____________
Commission file number 001-37568
PDS Biotechnology Corporation | ||
| (Exact name of registrant as specified in its charter) |
| Delaware | 26-4231384 | |
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) |
| 303A College Road East, Princeton, NJ 08540 | ||
| (Address of principal executive offices) |
| (800) 208-3343 | ||
| (Registrant’s telephone number) |
| (Former name, former address and former fiscal year, if changed since last report) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.00033 per share | PDSB | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☒ | Smaller Reporting Company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of shares of the registrant’s Common Stock, par value $0.00033 per share, outstanding as of May 8, 2024 was 36,679,275.
PDS BIOTECHNOLOGY CORPORATION
FORM 10-Q FOR THE QUARTER ENDED March 31, 2024
| Page | |||
Part I — Financial Information | |||
| Item 1. | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| Item 2. | 17 | ||
| Item 3. | 30 | ||
| Item 4. | 30 | ||
| 31 | |||
| Item 1. | 31 | ||
| Item 1A. | 31 | ||
| Item 2. | 31 | ||
| Item 3. | 31 | ||
| Item 4. | 31 | ||
| Item 5. | 31 | ||
| Item 6. | 31 | ||
| 32 | |||
| 33 | |||
| PART 1. | FINANCIAL INFORMATION |
PDS BIOTECHNOLOGY CORPORATION AND SUBSIDIARY
| March 31, 2024 | December 31, 2023 | |||||||
| ASSETS | (unaudited) | |||||||
| Current assets: | ||||||||
Cash and cash equivalents | $ | 66,634,417 | $ | 56,560,517 | ||||
| Prepaid expenses and other assets | 2,051,348 | 2,494,558 | ||||||
| Total current assets | 68,685,765 | 59,055,075 | ||||||
| Property and equipment, net | 129,398 | 134,132 | ||||||
Financing lease right-to-use assets | 191,203 | 200,873 | ||||||
| Total assets | $ | 69,006,366 | $ | 59,390,080 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 6,000,831 | $ | 6,982,824 | ||||
| Accrued expenses | 1,714,111 | 2,424,692 | ||||||
| Note payable - short term | 7,291,667 | 4,166,667 | ||||||
| Financing lease obligation-short term | 57,081 | 55,794 | ||||||
| Total current liabilities | 15,063,690 | 13,629,977 | ||||||
Noncurrent liabilities: | ||||||||
| Note payable, net of debt discount | 16,651,420 | 19,506,183 | ||||||
| Financing lease obligation-long term | 108,211 | 122,973 | ||||||
Total liabilities: | $ | 31,823,321 | $ | 33,259,133 | ||||
| STOCKHOLDERS’ EQUITY | ||||||||
Common stock, $0.00033 par value, 75,000,000 shares authorized at March 31, 2024 and December 31, 2023, 36,679,275 shares and 33,094,521 shares issued and outstanding at March 31, 2024 and December 31, 2023, respectively | 12,104 | 10,921 | ||||||
| Additional paid-in capital | 192,275,033 | 170,620,641 | ||||||
| Accumulated deficit | (155,104,092 | ) | (144,500,615 | ) | ||||
| Total stockholders’ equity | 37,183,045 | 26,130,947 | ||||||
| Total liabilities and stockholders’ equity | $ | 69,006,366 | $ | 59,390,080 | ||||
See accompanying notes to the condensed consolidated financial statements.
PDS BIOTECHNOLOGY CORPORATION AND SUBSIDIARY
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| Operating expenses: | ||||||||
Research and development expenses | $ | 6,704,164 | $ | 5,843,686 | ||||
| General and administrative expenses | 3,393,463 | 3,578,728 | ||||||
| Total operating expenses | 10,097,627 | 9,422,414 | ||||||
| Loss from operations | (10,097,627 | ) | (9,422,414 | ) | ||||
| Interest income (expense), net | ||||||||
| Interest income | 668,895 | 729,341 | ||||||
| Interest expense | (1,174,745 | ) | (966,845 | ) | ||||
Interest income (expense), net | (505,850 | ) | (237,504 | ) | ||||
| Net loss and comprehensive loss | (10,603,477 | ) | (9,659,918 | ) | ||||
| Per share information: | ||||||||
| Net loss per share, basic and diluted | $ | (0.30 | ) | $ | (0.32 | ) | ||
| Weighted average common shares outstanding basic and diluted | 34,815,870 | 30,428,053 | ||||||
See accompanying notes to the condensed consolidated financial statements.
PDS BIOTECHNOLOGY CORPORATION AND SUBSIDIARY
(Unaudited)
| Common Stock | ||||||||||||||||||||
Shares Issued | Amount | Additional Paid-in Capital | Accumulated Deficit | Total Equity | ||||||||||||||||
| January 1, 2023 | 30,170,317 | $ | 9,956 | $ | 145,550,491 | $ | (101,558,417 | ) | $ | 44,002,030 | ||||||||||
| Stock-based compensation expense | – | – | 2,080,319 | – | 2,080,319 | |||||||||||||||
| Issuances of common stock from the Sales Agreement, net | 553,293 | 183 | 4,588,339 | – | 4,588,522 | |||||||||||||||
| Net loss | – | – | – | (9,659,918 | ) | (9,659,918 | ) | |||||||||||||
| Balance - March 31, 2023 | 30,723,610 | $ | 10,139 | $ | 152,219,149 | $ | (111,218,335 | ) | $ | 41,010,953 | ||||||||||
| Common Stock | ||||||||||||||||||||
Shares Issued | Amount | Additional Paid-in Capital | Accumulated Deficit | Total Equity | ||||||||||||||||
| January 1, 2024 | 33,094,521 | $ | 10,921 | $ | 170,620,641 | $ | (144,500,615 | ) | $ | 26,130,947 | ||||||||||
| Stock-based compensation expense | – | – | 1,630,011 | – | 1,630,011 | |||||||||||||||
Issuances of common stock from the Sales Agreement, net | 3,428,681 | 1,131 | 19,493,342 | – | 19,494,473 | |||||||||||||||
| Issuances of common stock, from exercise of stock options | 156,073 | 52 | 531,039 | – | 531,091 | |||||||||||||||
| Net loss | – | – | – | (10,603,477 | ) | (10,603,477 | ) | |||||||||||||
| Balance - March 31, 2024 | 36,679,275 | $ | 12,104 | $ | 192,275,033 | $ | (155,104,092 | ) | $ | 37,183,045 | ||||||||||
See accompanying notes to the condensed consolidated financial statements.
PDS BIOTECHNOLOGY CORPORATION AND SUBSIDIARY
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| Cash flows from operating activities: | ||||||||
Net loss | $ | (10,603,477 | ) | $ | (9,659,918 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation expense | 1,630,011 | 2,080,319 | ||||||
Amortization of debt discount | 270,236 | 121,997 | ||||||
| Depreciation expense | 4,735 | 3,155 | ||||||
| Operating lease expense | – | 60,257 | ||||||
| Finance lease depreciation expense | 9,670 | 10,957 | ||||||
| Changes in assets and liabilities: | ||||||||
| Prepaid expenses and other assets | 443,210 | (113,758 | ) | |||||
Accounts payable | (981,993 | ) | 852,774 | |||||
| Accrued expenses | (710,581 | ) | (6,455,317 | ) | ||||
| Operating lease liabilities | – | (89,102 | ) | |||||
| Net cash used in operating activities | (9,938,189 | ) | (13,188,636 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from exercise of stock options | 531,091 | – | ||||||
| Payments of finance lease obligations | (13,475 | ) | (20,667 | ) | ||||
| Proceeds from issuance of common stock, net of issuance costs | 19,494,473 | 4,588,522 | ||||||
| Net cash provided by financing activities | 20,012,089 | 4,567,855 | ||||||
| Net increase in cash and cash equivalents | 10,073,900 | (8,620,781 | ) | |||||
| Cash and cash equivalents at beginning of period | 56,560,517 | 73,820,160 | ||||||
| Cash and cash equivalents at the end of period | $ | 66,634,417 | $ | 65,199,379 | ||||
Supplemental information of cash and non-cash transactions: | ||||||||
Cash paid for interest | $ | 1,174,745 | $ | 966,845 | ||||
See accompanying notes to the condensed consolidated financial statements.
PDS BIOTECHNOLOGY CORPORATION AND SUBSIDIARY
Note 1 – Nature of Operations
PDS Biotechnology Corporation, a Delaware corporation (the “Company” or “PDS Biotech”), is a clinical-stage immunotherapy company developing a growing pipeline of molecularly targeted immunotherapies designed to overcome the limitations of current immunotherapy and vaccine technologies. The Company develops proprietary platforms designed to train and enable the immune system to attack and destroy disease; Versamune®, and Versamune® in combination with PDS01ADC for treatments in oncology and Infectimune® for treatments in infectious diseases. When paired with an antigen, which is a disease-related protein that is recognizable by the immune system, Versamune® and Infectimune® have both been shown to induce, in vivo, large quantities of high-quality, highly potent polyfunctional CD4 helper and CD8 killer T cells, a specific sub-type of T cell that is more effective at killing infected or target cells. PDS01ADC is a novel investigational tumor-targeting fusion protein of Interleukin 12 that enhances the proliferation, potency, infiltration and longevity of T cells in the tumor microenvironment and is therefore designed to overcome the limitations of cytokine therapy which today has resulted in high toxicity and limited therapeutic potential. Infectimune® is also designed to promote the induction of disease-specific neutralizing antibodies. The Company’s immuno-oncology product candidates are of potential interest for use as a component of combination product candidates (for example, in combination with other leading technologies such as immune checkpoint inhibitors) to provide more effective treatments across a range of advanced and/or refractory cancers. The Company is also evaluating its immunotherapies as monotherapies in early-stage disease. The Company is developing targeted product candidates to treat several cancers, including Human Papillomavirus (HPV) associated cancers, melanoma, colorectal, lung, breast and prostate cancers. The Company’s infectious disease candidate is of potential interest for use in universal influenza vaccines.
Note 2 – Summary of Significant Accounting Policies
| (A) | Unaudited interim financial statements: |
The unaudited financial statements for all periods presented are referred to as “Condensed Consolidated Financial Statements”, and have been prepared by the Company in United States (“U.S.”) dollars and in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial reporting and pursuant to the rules and regulations for reporting on Form 10-Q, which do not conform in all respects to the requirements of U.S. GAAP for annual financial statements. Accordingly, certain information and disclosures required by U.S. GAAP for complete consolidated financial statements are not included herein. Accordingly, these notes to the unaudited condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements prepared in accordance with U.S. GAAP that are contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (“SEC”) on March 28, 2024. The unaudited condensed consolidated financial statements have been prepared using accounting policies that are consistent with the policies used in preparing the Company’s audited consolidated financial statements for the year ended December 31, 2023. The unaudited Condensed Consolidated Financial Statements reflect all normal and recurring adjustments necessary for a fair statement of the Company’s financial position and results of operations for the interim periods. The operating results for the interim periods presented are not necessarily indicative of the results expected for the full year.
| (B) | Use of estimates: |
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the reported amounts of expenses at the date of the condensed consolidated financial statements and during the reporting periods, and to disclose contingent assets and liabilities at the date of the condensed consolidated financial statements. Actual results could differ from those estimates. The most significant estimate relates to the fair value of securities underlying stock-based compensation.
| (C) | Significant risks and uncertainties: |
The Company’s operations are subject to a number of factors that may affect its operating results and financial condition. Such factors include, but are not limited to: the Company’s ability to complete clinical trials necessary to obtain regulatory product licenses, the regulatory approvals needed to pursue development of its products, the Company’s adherence to covenants under its debt agreement, the Company’s ability to preserve its cash resources, the Company’s ability to add product candidates to its pipeline, the Company’s intellectual property, competition from products manufactured and sold or being developed by other companies, the price of, and demand for, Company products if approved for sale, the Company’s ability to negotiate favorable licensing or other manufacturing and marketing agreements for its products, and the Company’s ability to raise capital.
The Company currently has no commercially approved products. As such, there can be no assurance that the Company’s future research and development programs will be successfully commercialized. Developing and commercializing a product requires significant time and capital and is subject to regulatory review and approval as well as competition from other biotechnology and pharmaceutical companies. The Company operates in an environment of rapid change and is dependent upon the continued services of its employees and consultants and obtaining and protecting its intellectual property.
| (D) | Cash equivalents and concentration of cash balance: |
The Company considers all highly liquid securities with a maturity of less than three months to be cash equivalents. The Company’s cash and cash equivalents in bank deposit accounts, at times, may exceed federally insured limits.
| (E) | Research and development: |
Costs incurred in connection with research and development activities are expensed as incurred. These costs include licensing fees to use certain technology in the Company’s research and development projects as well as fees paid to consultants and entities that perform certain research and testing on behalf of the Company.
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data, such as patient enrollment, clinical site activations or information provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred.
| (F) | Patent costs: |
The Company expenses patent costs as incurred and classifies such costs as general and administrative expenses in the accompanying condensed consolidated statements of operations and comprehensive loss.
| (G) | Stock-based compensation: |
The Company accounts for its stock-based compensation in accordance with ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees, directors and non-employees to be recognized as expense in the condensed consolidated statements of operations and comprehensive loss based on their grant date fair values. In order to determine the fair value of stock options on the date of grant, the Company uses the Black-Scholes option-pricing model. Inherent in this model are assumptions related to expected stock-price volatility, option term, risk-free interest rate and dividend yield. While the risk-free interest rate and dividend yield are less subjective assumptions that are based on factual data derived from public sources, the expected stock-price volatility and option term assumptions require a greater level of judgment. The Company expenses the fair value of its stock-based compensation awards to employees and directors on a straight-line basis over the requisite service period, which is generally the vesting period. The Company recognizes forfeitures as they occur.
| (H) | Net loss per common share: |
Basic and diluted net loss per common share is determined by dividing net loss attributable to common stockholders by the weighted average common shares outstanding during the period. For all periods presented, the common shares underlying the stock options and warrants have been excluded from the calculation because their effect would be anti-dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted loss per common share is the same.
The potentially dilutive securities excluded from the determination of diluted loss per share as their effect is antidilutive, are as follows:
| As of March 31, | ||||||||
2024 | 2023 | |||||||
| Stock options to purchase Common Stock | 5,314,661 | 5,295,911 | ||||||
| Warrants to purchase Common Stock | 466,112 | 506,229 | ||||||
| Total | 5,780,773 | 5,802,140 | ||||||
| (I) | Income taxes: |
The Company provides for deferred income taxes under the asset and liability method, which requires deferred tax assets and liabilities to be recognized for the future tax consequences attributable to net operating loss carryforwards and for differences between the financial statement carrying amounts and the respective tax bases of assets and liabilities. Deferred tax assets are reduced if necessary, by a valuation allowance if it is more likely than not that some portion or all of the deferred tax assets will not be realized.
| (J) | Fair value of financial instruments: |
FASB ASC 820, Fair Value Measurement, specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
| ● | Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. |
| ● | Level 2 — Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). Level 2 includes financial instruments that are valued using models or other valuation methodologies. |
| ● | Level 3 — Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable. |
| (K) | Leases: |
The Company determines if an arrangement is a lease at inception and recognizes the lease in accordance with ASC 842, Leases (“ASC 842”). Both financing and operating leases are included in right-of-use (“ROU”) assets, lease obligation-short term and lease obligation-long term in the Company’s condensed consolidated balance sheets. ROU assets represent the right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. The ROU assets and lease liabilities are recognized at the lease commencement date based on the present value of the lease payments over the lease term. The Company determines the portion of the lease liability that is current as the difference between the calculated lease liability at the end of the current period and the lease liability that is projected 12 months from the current period.
| (L) | New accounting standards: |
Recently Adopted Accounting Pronouncements
Recently issued accounting pronouncements did not, or are not believed by management to, have a material effect on our present or future condensed consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which improves the disclosures required for reportable segments in the Company’s annual and interim financial statements, primarily through enhanced disclosures about significant segment expenses. ASU 2023-07 is effective for annual periods beginning after December 15, 2023 and interim periods within fiscal years beginning after December 15, 2024. Adoption of this ASU should be applied retrospectively to all prior periods presented in the financial statements. Early adoption is permitted. The Company is currently evaluating the impact that adopting this standard will have on the consolidated financial statements and disclosures and given the Company has one reportable segment, this policy is not expected to have a material impact on the consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Improvements to Income Tax Disclosures, which requires public entities, on an annual basis, to provide disclosures of specific categories in the rate reconciliation, additional information for reconciling items that meet a quantitative threshold and income taxes paid disaggregated by jurisdiction. ASU 2023-09 is effective for annual periods beginning after December 15, 2024. Early adoption is permitted. The Company is currently evaluating the impact that adopting this standard will have on the consolidated financial statements and disclosures.
Note 3 – Liquidity and Capital Resources
As of March 31, 2024, the Company had $66.6 million of cash and cash equivalents. The Company’s primary use of cash is to fund operating expenses, primarily research and development expenditures. Cash used to fund operating expenses is impacted by the level of activities undertaken, as well as the timing of when the Company pays these expenses, as reflected in the change to the Company’s outstanding accounts payable and accrued expenses. Since inception, the Company has experienced net losses and negative cash flows from operations each fiscal year. The Company has no revenues and expects to continue to incur operating losses for the foreseeable future and may never become profitable. In addition, the Loan and Security Agreement allows for the lenders to call the outstanding balance of the term loans if the minimum cash balances outlined in the Loans and Security Agreement are not maintained.
The Company funds its operations through equity and/or debt financings such as the following:
In August 2022, the Company filed a shelf registration statement, or the 2022 Shelf Registration Statement, with the SEC for the issuance of common stock, preferred stock, warrants, rights, debt securities, and units, up to an aggregate amount of $150 million, $50 million of which covers the offer, issuance and sale by the Company of its common stock under the Sales Agreement (as discussed below). The 2022 Shelf Registration Statement was declared effective on September 2, 2022.
In August 2022, the Company entered into an At Market Issuance Sales Agreement, or the Sales Agreement, with B. Riley Securities, Inc. and BTIG, LLC, each an Agent and collectively the Agents, with respect to an at-the-market offering program under which the Company may offer and sell, from time to time at its sole discretion, shares of its common stock, having an aggregate offering price of up to $50 million, or the Placement Shares, through or to the Agents, as sales agents or principals. Upon delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, the Agents may sell the Placement Shares by any method permitted by law deemed to be an “at the market” offering as defined in Rule 415 of the Securities Act of 1933, as amended, including, without limitation, sales made through The Nasdaq Capital Market or on any other existing trading market for the Company’s common stock. The Agents will use commercially reasonable efforts to sell the Placement Shares from time to time, based upon the Company’s instructions (including any price, time or size limits or other customary parameters or conditions the Company may impose). The Company will pay the Agents a commission equal to three percent (3%) of the gross sales proceeds of any Placement Shares sold through the Agents under the Sales Agreement, and the Company has also provided the Agents with customary indemnification and contribution rights. The Company is not obligated to make any sales of its common stock under the Sales Agreement. The offering of Placement Shares pursuant to the Sales Agreement will terminate upon the earlier of (i) the sale of all Placement Shares subject to the Sales Agreement or (ii) termination of the Sales Agreement in accordance with its terms. For the year ended December 31, 2023, the Company sold 2,642,269 shares of common stock for a net value of $16.1 million pursuant to the Sales Agreement. During the quarter ended March 31, 2024 and 2023, the Company sold 3,428,681 and 553,293 shares of common stock for a net value of $19.5 and $4.6 million, respectively, pursuant to the Sales Agreement.
In August 2022, the Company entered into a venture loan and security agreement, or the Loan and Security Agreement, with Horizon Technology Finance Corporation, as lender and collateral agent for itself and the other lenders. The Loan and Security Agreement provides for the following 6 separate and independent term loans: (a) a term loan in the amount of $7,500,000, or Loan A, (b) a term loan in the amount of $10,000,000, or Loan B, (c) a term loan in the amount of $3,750,000, or Loan C, (d) a term loan in the amount of $3,750,000, or Loan D, (e) a term loan in the amount of $5,000,000, or Loan E, and (f) a term loan in the amount of $5,000,000, or Loan F, (with each of Loan A, Loan B, Loan C, Loan D, Loan E, and Loan F, individually a Loan and, collectively, the Loans). Loan A, Loan B, Loan C, and Loan D were delivered to the Company on August 24, 2022. In total, the Company received $24.6 million in net proceeds. Loan E and Loan F were uncommitted Loans that could have been advanced by the lenders upon the parties agreement prior to July 31, 2023 upon the satisfaction of certain conditions. At this time the option to advance Loan E and Loan F has expired and Loan E and Loan F are no longer available to the Company under the Loan and Security Agreement. The Company may only use the proceeds of the Loans for working capital or general corporate purposes. Each Loan matures on the 48-month anniversary following the applicable funding date unless accelerated pursuant to certain events of default. Payments on the principal balance begin on October 1, 2024 and are paid monthly in the succeeding 24 months. The principal balance of each Loan bears a floating interest. The interest rate is calculated initially and, thereafter, each calendar month as the sum of (a) the per annum rate of interest from time to time published in The Wall Street Journal as contemplated by the Loan and Security Agreement, or any successor publication thereto, as the “prime rate” then in effect, plus (b) 5.75%; provided that, in the event such rate of interest is less than 4.00%, such rate shall be deemed to be 4.00% for purposes of calculating the interest rate. Interest is payable on a monthly basis based on each Loan principal amount outstanding the preceding month. The Company, at its option upon at least ten (10) business days’ written notice to the lenders, may prepay all (and not less than all) of the outstanding Loan by simultaneously paying to each lender an amount equal to (i) any accrued and unpaid interest on the outstanding principal balance of the Loans; plus (ii) an amount equal to (A) if such Loan is prepaid on or before the Loan Amortization Date (as defined in the Loan and Security Agreement) applicable to such Loan, 3% of the then outstanding principal balance of such Loan, (B) if such Loan is prepaid after the Loan Amortization Date applicable to such Loan, but on or before the date that is 12 months after such Loan Amortization Date, 2% of the then outstanding principal balance of such Loan, or (C) if such Loan is prepaid more than 12 months after the Loan Amortization Date but prior to the stated maturity date applicable to such Loan, 1% of the then outstanding principal balance of such Loan; plus (iii) the outstanding principal balance of such Loan; plus (iv) all other sums, if any, that shall have become due and payable thereunder. No prepayment premium will be applied to any outstanding balance of any Loan paid on the stated maturity date.
The Loan and Security Agreement contains customary representations, warranties and covenants, including maintenance of minimum cash balances as well as covenants by the Company limiting additional indebtedness, liens, including on intellectual property, guaranties, mergers and consolidations, substantial asset sales, investments and loans, certain corporate changes, transactions with affiliates, and fundamental changes.
In April 2023, the Company received approximately $1.4 million from the net sale of tax benefits to an unrelated, profitable New Jersey corporation pursuant to the Company’s participation in the New Jersey Technology Business Tax Certificate Transfer NOL program for tax year 2021.
In April 2024, the Company received approximately $0.9 million from the net sale of tax benefits to an unrelated, profitable New Jersey corporation pursuant to its participation in the New Jersey Technology Business Tax Certificate Transfer of Net Operating Loss (NOL) program for tax year 2022.
Going Concern
The Company evaluated whether there are any conditions and events, considered in the aggregate, that raise substantial doubt about its ability to continue as a going concern within one year after the filing of this Quarterly Report on Form 10-Q in accordance with ASC Subtopic 205-40, Going Concern . Since inception, the Company has experienced net losses and negative cash flows from operations each fiscal year. The Company has no revenues and expects to continue to incur operating losses for the foreseeable future and may never become profitable. In addition, the Loan and Security Agreement allows for the lenders to call the outstanding balance of the term loans if the minimum cash balances outlined in the Loan and Security Agreement are not maintained.
The Company’s budgeted cash requirements in 2024 and beyond include expenses related to continuing development and clinical trials as well as payments on its debt. The Company plans to execute its operating plan by obtaining additional capital, principally through entering into collaborations, strategic alliances, or license agreements with third parties and/or additional public or private debt and equity financing. However, there is no assurance that additional capital and/or financing will be available to the Company, and even if available, whether it will be on terms acceptable to the Company or its existing shareholders or in the amounts required. The Company may also enter into government funding programs and consider selectively partnering for clinical development and commercialization. The sale of additional equity would result in additional dilution to the Company’s stockholders. Incurring debt financing would result in debt service obligations, and the instruments governing such debt could provide for operating and financing covenants that would restrict its operations. If the Company is unsuccessful in securing sufficient financing, it may need to delay, reduce, or eliminate its research and development programs, which could adversely affect its business prospects, grant rights to third parties to develop and market immunotherapies that the Company would otherwise prefer to develop and market itself or cease operations. Any of these actions could harm its business, results of operations and prospects. Failure to obtain adequate financing also may adversely affect the Company’s ability to operate as a going concern.
As a result of these uncertainties, and as its plans are outside of management’s control, the Company has concluded that substantial doubt exists about the Company’s ability to continue as a going concern for a period of at least 12 months from the date of the issuance of these unaudited condensed consolidated financial statements. The unaudited condensed consolidated financial statements do not include any adjustments to the carrying amounts and classifications of assets and liabilities that would result if the Company was unable to continue as a going concern.
Note 4 – Fair Value of Financial Instruments
There were no transfers among Levels 1, 2, or 3 during the three month ended March 31, 2024 or 2023.
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Total | Quoted Prices in Active Markets (Level 1) | Quoted Prices in Inactive Markets (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| As of March 31, 2024: (unaudited) | ||||||||||||||||
Cash and cash equivalents | $ | 66,634,417 | $ | 66,634,417 | $ | – | $ | – | ||||||||
| As of December 31, 2023 | ||||||||||||||||
| Cash and cash equivalents | $ | 56,560,517 | $ | 56,560,517 | $ | – | $ | – | ||||||||
The carrying value of the Loan and Security Agreement approximated its fair value as of March 31, 2024 due to its variable rate.
Note 5 – Leases
Operating Lease:
Effective March 5, 2020, the Company entered into a sublease for approximately 11,200 square feet of office space located at 25B Vreeland Road, Suite 300, Florham Park, NJ. The sublease commenced on May 1, 2020 with a term of forty (40) months with an option to renew through October 31, 2027. The sublease term expired on August 31, 2023, and was not renewed. Upon inception of the sublease, the Company recognized approximately $0.7 million of ROU assets and operating lease liabilities. The discount rate used to measure the operating lease liability as of May 1, 2020 was 9.15%. Throughout the period described above, the Company has maintained, and continues to maintain, a month-to-month lease for its research facilities at the Princeton Innovation Center BioLabs located at 303A College Road E, Princeton NJ, 08540.
Supplemental cash flow information related to operating leases is as follows:
| As of March 31, | ||||||||
| 2024 | 2023 | |||||||
| Cash paid for operating lease liabilities | $ | – | $ | 89,102 | ||||
Financing Lease:
The Company has financed certain laboratory equipment as follows:
| As of March 31, | ||||||||
| 2024 | 2023 | |||||||
| Cash paid for finance lease liabilities | $ | 17,463 | $ | 20,667 | ||||
Maturity of the Company’s financing lease liabilities is as follows:
| Year ended December 31, | ||||
| 2024 | $ | 52,387 | ||
| 2025 | 69,850 | |||
| 2026 | 40,108 | |||
| 2027 | | 26,721 | ||
| 2028 and after | | 1 | ||
| Total future minimum lease payments | 189,067 | |||
| Less imputed interest | (23,775 | ) | ||
| Remaining lease liability | $ | 165,292 | ||
The Company entered into four financing leases for laboratory equipment with a total cost of $251,959 with four to five-year terms and a capitalized interest rate of 9.15%. Each of the lease agreements include a bargain purchase option to acquire the equipment at the end of the lease term. The aggregate monthly payments are approximately $6,000. During the year ended December 31, 2023, the Company exercised the bargain purchase option, which resulted in recognition of property and equipment of $151,490.
Note 6 – Accrued Expenses
Accrued expenses consist of the following:
As of March 31, 2024 | As of December 31, 2023 | |||||||
Accrued research and development | $ | 457,229 | $ | - | ||||
| Accrued professional fees | 531,294 | 827,863 | ||||||
| Accrued compensation | 418,449 | 1,289,690 | ||||||
| Accrued interest on debt | 306,771 | 306,771 | ||||||
| Accrued rent | 368 | 368 | ||||||
| Total | $ | 1,714,111 | $ | 2,424,692 | ||||
Note 7 – Stock-Based Compensation
In 2014, the Company’s stockholders approved the 2014 Equity Incentive Plan (the “Original Plan”) pursuant to which the Company may grant up to 91,367 shares as ISOs, NQs and restricted stock units (“RSUs”), subject to increases as hereafter described (the “Plan Limit”). In addition, on January 1, 2015, and each January 1 thereafter and prior to the termination of the 2014 Equity Incentive Plan, pursuant to the terms of the Original Plan, the Plan Limit was and shall be increased by the lesser of (x) 4% of the number of shares of Common Stock outstanding as of the immediately preceding December 31 and (y) such lesser number as the Board of Directors (“Board”) may determine in its discretion. In March 2019, the Board adopted and the Company’s stockholders approved the Amended and Restated PDS Biotechnology Corporation 2014 Equity Incentive Plan (the “Prior Plan”) which amended and restated the Original Plan in order to remove the annual increase component and was limited to 826,292 shares.
On December 8, 2020, the Board adopted and on June 17, 2021, the stockholders approved, the Second Amended and Restated PDS Biotechnology Corporation 2014 Equity Incentive Plan (the “Restated Plan”), which amended and restated the Prior Plan. The Restated Plan is identical to the Prior Plan in all material respects, except (a) the number of shares of Common Stock authorized for issuance under the Restated Plan was increased from 826,292 shares to 4,165,535 shares, plus the total number of shares that remained available for issuance, that were not covered by outstanding awards issued under the Prior Plan, immediately prior to December 8, 2020; and (b) the Prior Plan was amended to terminate on December 7, 2030, unless earlier terminated. On May 19, 2023, the Board adopted, subject to stockholder approval, the Third Amended and Restated PDS Biotechnology Corporation 2014 Equity Incentive Plan (the “Third Restated Plan”). At the 2023 annual meeting of stockholders held on July 14, 2023, the stockholders approved the Third Restated Plan, which amended and restated the Restated Plan to increase the total amount of shares authorized for issuance thereunder. The Third Restated Plan is identical to the Restated Plan in all material respects, except, the number of shares of Common Stock authorized for issuance under the Third Restated Plan increased from 4,165,535 to 6,565,535. As of March 31, 2024, there were 2,645,723 shares available for grant under the Third Restated Plan.
Pursuant to the terms of the Third Restated Plan, stock options have a term of ten years from the date of grant or such shorter term as may be provided in the option agreement. Unless specified otherwise in an individual option agreement, ISOs generally vest over a four-year period.
On June 17, 2019, the Board adopted the 2019 Inducement Plan (the “Inducement Plan”). On December 8, 2020, the Company amended the Inducement Plan solely to increase the total number of shares of common stock reserved for issuance under the Inducement Plan from 200,000 shares to 500,000 shares. On May 17, 2022, the Company further amended the Inducement Plan solely to increase the total number of shares of Common Stock reserved for issuance under the Inducement Plan from 500,000 shares to 1,100,000 shares. On January 22, 2024, the Company further amended the Inducement Plan solely to increase the total number of shares of Common Stock reserved for issuance under the Inducement Plan from 1,100,000 shares to 2,100,000 shares. The Inducement Plan provides for the grant of non-qualified stock options. The Inducement Plan, and each amendment thereto, was recommended for approval by the Compensation Committee of the Board and subsequently approved and adopted by the Board without stockholder approval pursuant to Rule 5635(c)(4) of the Nasdaq Listing Rules.
The Inducement Plan is administered by the Compensation Committee of the Board. In accordance with Rule 5635(c)(4) of the Nasdaq Listing Rules, non-qualified stock options under the Inducement Plan may only be made to an employee who has not previously been an employee of the Company or member of the Board of Directors of the Company (or any parent or subsidiary of the Company), if he or she is granted such non-qualified stock options in connection with his or her commencement of employment with the Company or a subsidiary and such grant is an inducement material to his or her entering into employment with the Company or such subsidiary. As of March 31, 2024, there were 1,232,200 shares available for grant under the Inducement Plan.
The following table summarizes the components of stock-based compensation expense in the condensed consolidated statements of operations and comprehensive loss for the three months ended March 31, 2024 and 2023:
| Three Months Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| (unaudited) | ||||||||
| Research and development | $ | 551,918 | $ | 800,764 | ||||
| General and administrative | 1,078,093 | 1,279,555 | ||||||
| Total | $ | 1,630,011 | $ | 2,080,319 | ||||
There were 938,648 and 1,124,600 options granted during the three months ended March 31, 2024 and 2023, respectively. The fair value of options granted during the three months ended March 31, 2024 and 2023 was estimated using the Black-Scholes option valuation model utilizing the following assumptions:
| Three Months Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
| Weighted Average | Weighted Average | |||||||
| (unaudited) | ||||||||
Volatility | 145.41 | % | 142.02 | % | ||||
| Risk-Free Interest Rate | 3.98 | % | 4.06 | % | ||||
| Expected Term in Years | 6.08 | 6.08 | ||||||
| Dividend Rate | – | – | ||||||
| Fair Value of Option on Grant Date | $ | 5.22 | $ | 10.79 | ||||
The following table summarizes the number of options outstanding and the weighted average exercise price:
Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life in Years | Aggregate Intrinsic Value | |||||||||||||
Options outstanding at December 31, 2023 | 5,029,345 | $ | 6.43 | 7.42 | $ | 4,395,227 | ||||||||||
| Granted | 938,648 | 5.58 | 9.90 | – | ||||||||||||
| Exercised | (156,073 | ) | 3.40 | |||||||||||||
| Forfeited and expired | (497,259 | ) | 8.64 | |||||||||||||
| Options outstanding at March 31, 2024 | 5,314,661 | $ | 6.16 | 7.60 | $ | 2,327,199 | ||||||||||
Vested and expected to vest at March 31, 2024 | 5,314,661 | $ | 6.16 | 7.60 | $ | 2,327,199 | ||||||||||
| Exercisable at March 31, 2024 | 2,858,485 | $ | 5.80 | 6.42 | $ | 1,971,346 | ||||||||||
As of March 31, 2024 there was approximately $15,111,713 of unamortized stock option compensation expense, which is expected to be recognized over a remaining average vesting period of 2.95 years.
Note 8 – Income Taxes
The Company records a valuation allowance against its deferred tax assets to reduce the net carrying value to an amount that it believes is more likely than not to be realized. In assessing the realizability of the net deferred tax assets, the Company considers all relevant positive and negative evidence to determine whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The realization of the gross deferred tax assets is dependent on several factors, including the generation of sufficient taxable income prior to the expiration of the net operating loss carryforwards. The Company expects to have a loss for 2024 and therefore there will be no current income tax expense. The Company recorded a full valuation allowance against the net deferred tax assets as of March 31, 2024 and December 31, 2023. Consequently, the Company recorded no income tax benefit due to realization uncertainties.
The Company is subject to a U.S. federal statutory income tax rate of 21%. The primary factor impacting the effective tax rate for the three months ended March 31, 2024 is the anticipated full year operating loss which will require a full valuation allowance against any associated net deferred tax assets.
Entities are required to evaluate, measure, recognize and disclose any uncertain income tax positions taken on their income tax returns. The Company has analyzed its tax positions and has concluded that as of March 31, 2024, there were no uncertain positions. The Company’s U.S. federal and state net operating losses have occurred since its inception and as such, tax years subject to potential tax examination could apply from that date because the utilization of net operating losses from prior years opens the relevant year to audit by the IRS and/or state taxing authorities. The Company did not have any unrecognized tax benefits and has not accrued any interest or penalties for the three months ended March 31, 2024 or for the year ended December 31, 2023. In April 2024, the Company received approximately $0.9 million from the sale of its New Jersey state net operating losses.
Note 9 – Commitments and Contingencies
Rent
For month-to-month arrangements not impacted by the adoption of ASC 842, rent for the three months ended March 31, 2024 and 2023 was $66,000 and $66,000, respectively.
Exclusive License Agreement
In January 2023, the Company entered into an exclusive global license agreement with Merck KGaA, Darmstadt, Germany for the tumor targeting IL 12 fused antibody drug conjugate, M9241 (the “Merck KGaA License Agreement”). Pursuant to the Merck KGaA License Agreement, the Company agreed to make (i) development and first commercial sales milestone payments totaling up to $11 million upon the achievement of certain milestones, including the dosing of the fifth patient in a Phase 3 trial of the clinical candidate and first commercial sale of the product for a first and second indication in a major market, and (ii) up to $105 million upon achieving certain aggregate sales levels of the product.
The Company also agreed to pay Merck KGaA, Darmstadt, Germany a royalty of 10% on aggregate net sales of product as specified in the Merck KGaA License Agreement on a product-by-product and country-by-country basis until the later of: (i) ten years after the first commercial sale of a product in a given country; and (ii) the expiration or invalidation of the licensed patents covering the compound or product in such country. The royalty rate is subject to reduction in the event that a product is not covered by a valid patent claim, a biosimilar to the compound or the product comes on the market in a particular country, or if the Company obtains a license to any intellectual property owned or controlled by a third-party which, but for such license would be infringed by making, using or selling the compound.
Legal Proceedings
The Company is currently not a party to, and the Company’s property is not currently the subject of, any material pending legal proceedings. The Company may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of business. Such matters are subject to many uncertainties and outcomes that may not be predictable with assurance.
Note 10 – Venture Loan and Security Agreement
In August 2022, the Company entered into a Venture Loan and Security Agreement (the “Loan and Security Agreement”) with Horizon Technology Finance Corporation, as a lender and collateral agent for itself and the other Lenders (in such capacity, the “Collateral Agent”), and the other persons party thereto from time to time as lenders (“Lenders”).
Term loan Amounts. The Loan and Security Agreement provides for the following six (6) separate and independent term loans: (a) a term loan in the amount of $7,500,000 (“Loan A”), (b) a term loan in the amount of $10,000,000 (“Loan B”), (c) a term loan in the amount of $3,750,000 (“Loan C”), (d) a term loan in the amount of $3,750,000 (“Loan D”), (e) a term loan in the amount of $5,000,000 (“Loan E”), and (f) a term loan in the amount of $5,000,000 (“Loan F”) (with each of Loan A, Loan B, Loan C, Loan D, Loan E, and Loan F, individually a “Loan” and, collectively, the “Loans”). Loan A, Loan B, Loan C, and Loan D were delivered to the Company on August 24, 2022. Loan E and Loan F were uncommitted Loans that could have been advanced by the Lenders upon the parties agreement prior to July 31, 2023 upon the satisfaction by the Company of certain agreed upon conditions. At this time the option has expired and Loan E and Loan F are no longer available to the Company under the Loan and Security Agreement. The Company may only use the proceeds of the Loans for working capital or general corporate purposes.
Maturity. Each Loan matures on the 48 month anniversary following the applicable date on which a Loan is made to or on account of the Company under the Loan and Security Agreement (the “Maturity Date”) unless accelerated pursuant to agreed upon events of default. All amounts outstanding under each Loan will be due and payable upon the earlier of the Maturity Date or the acceleration of the loans and commitments upon an event of default. Payments on the principal balance begin on October 1, 2024 and are paid monthly in the succeeding 24 months.
Interest Rate. The principal balance of each Loan bears a floating interest. The interest rate is calculated initially and, thereafter, each calendar month as the sum of (a) the per annum rate of interest from time to time published in The Wall Street Journal as contemplated by the Loan and Security Agreement, or any successor publication thereto, as the “prime rate” then in effect, plus (b) 5.75%; provided that, in the event such rate of interest is less than 4.00%, such rate shall be deemed to be 4.00% for purposes of calculating the interest rate. Interest is payable on a monthly basis based on each Loan principal amount outstanding during the preceding month.
Amortization. Each Loan shall commence amortization upon the date set forth on the promissory note executed in connection with the respective Loan, upon which the Company is required to commence making equal payments of principal plus accrued interest on the outstanding principal amount of the respective Loan (the “Loan Amortization Date”), and continuing thereafter on the first business day of each calendar month through the Maturity Date.
Prepayment Premium. The Company may, at its option upon at least ten (10) business days’ written notice to the Lenders, prepay all (and not less than all) of the outstanding Loan by simultaneously paying to each Lender an amount equal to (i) any accrued and unpaid interest on the outstanding principal balance of the Loans; plus (ii) an amount equal to (A) if such Loan is prepaid on or before the Loan Amortization Date applicable to such Loan, three percent (3%) of the then outstanding principal balance of such Loan, (B) if such Loan is prepaid after the Loan Amortization Date applicable to such Loan, but on or before the date that is twelve (12) months after such Loan Amortization Date, two percent (2%) of the then outstanding principal balance of such Loan, or (C) if such Loan is prepaid more than twelve (12) months after the Loan Amortization Date but prior to the stated Maturity Date applicable to such Loan, one percent (1%) of the then outstanding principal balance of such Loan; plus (iii) the outstanding principal balance of such Loan; plus (iv) all other sums, if any, that shall have become due and payable hereunder. No prepayment premium will be applied to any outstanding balance of any Loan paid on the stated Maturity Date.
Security. The Company’s obligations are secured by a security interest in all of the assets of the Company, subject to limited exceptions and excluding the Company’s intellectual property.
Covenants; Representations and Warranties; Other Provisions. The Loan and Security Agreement contains customary representations, warranties and covenants, including maintenance of minimum cash balances as well as covenants by the Company limiting additional indebtedness, liens, including on intellectual property, guaranties, mergers and consolidations, substantial asset sales, investments and loans, certain corporate changes, transactions with affiliates, and fundamental changes. As of March 31, 2024, the Company is in compliance with all covenants in all material respects.
Default Provisions. The Loan and Security Agreement provides for events of default customary for term loans of this type, including but not limited to non-payment, breaches or defaults in the performance of covenants, insolvency, and bankruptcy by and/or of the Company.
Warrant and Debt Discount. In connection with the Loan and Security Agreement, the Company issued Horizon Technology Finance Corporation and Powerscourt Investments XXV, LP warrants to purchase an aggregate total of 381,625 shares of the Company’s common stock at an initial exercise price of $3.6685 per share. Each warrant is classified as equity and is exercisable at any time for a period beginning on the date of grant and ending on the earlier of (A) 10 years from the date of grant, and (B) the closing of (A) (i) the sale, lease, exchange, conveyance or other disposition of all or substantially all of the Company’s property or business, or (ii) its merger into or consolidation with any other corporation (other than a wholly-owned subsidiary of the Company), or any transaction (including a merger or other reorganization) or series of related transactions, in which more than 50% of the voting power of the Company is disposed of, in each case, for cash or for marketable securities meeting certain requirements as described in the applicable warrants. The key assumptions used in the Black-Scholes option pricing model were (i) expected term of 10 years, (ii) a risk-free rate of 3.11%, (iii) expected volatility of 93.8%, (iv) and no estimated dividend yield. In addition, the Company incurred third party and lender fees of $449,329 during the nine months ended September 30, 2022. These proceeds were allocated on a basis that approximates the relative fair value method. The fair value of the warrant and fees incurred were recorded as a debt discount and are being recognized as interest expense over the life of the Loan and Security Agreement using the effective interest method. The unamortized debt discount was $1,994,412 as of March 31, 2024.
For the three months ended March 31, 2024 and 2023, the Company recognized interest expense of $1,170,758 and $961,753, respectively, of which $270,236 and $121,997, respectively, was related to the amortization of the debt discount.
Note 11 – Retirement Plan
The Company has a 401(k) defined contribution plan for the benefit of all employees and permits voluntary contributions by employees, subject to IRS-imposed limitations. The 401(k) employer contributions were $66,488 and $94,907 for the three months ended March 31, 2024 and 2023, respectively.
Note 12– Subsequent Events
In April 2024, the Company received approximately $0.9 million from the net sale of tax benefits to an unrelated, profitable New Jersey corporation pursuant to its participation in the New Jersey Technology Business Tax Certificate Transfer of Net Operating Loss (NOL) program for tax year 2022.
ITEM 2. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited interim condensed consolidated financial statements and related notes thereto appearing elsewhere in this Quarterly Report on Form 10-Q (this “Quarterly Report”) and with the audited financial statements and notes thereto of the Company as of and for the year ended December 31, 2023 on Form 10-K, filed with the Securities and Exchange Commission, or SEC, on March 28, 2024.
Cautionary Note Regarding Forward-Looking Statements
This Quarterly Report contains forward-looking statements (including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act of 1933, as amended) concerning the Company and other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the Company’s management, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “forecast,” “guidance”, “outlook” and other similar expressions among others. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation:
| ● | the Company’s ability to protect its intellectual property rights; |
| ● | the Company’s anticipated capital requirements, including the Company’s anticipated cash runway and the Company’s current expectations regarding its plans for future equity financings; |
| ● | the Company’s dependence on additional financing to fund its operations and complete the development and commercialization of its clinical candidates, and the risks that raising such additional capital may restrict the Company’s operations or require the Company to relinquish rights to the Company’s technologies or clinical candidates; |
| ● | the Company’s limited operating history in the Company’s current line of business, which makes it difficult to evaluate the Company’s prospects, the Company’s business plan or the likelihood of the Company’s successful implementation of such business plan; |
| ● | the timing for the Company or its partners to initiate the planned clinical trials for its Versamune® products, including PDS0101, PDS0103, and others, alone or in combination with PDS01ADC, as well as Infectimune® based clinical candidates and the future success of such trials; |
| ● | the successful implementation of the Company’s research and development programs and collaborations, including any collaboration trials concerning the Company’s Versamune®, PDS01ADC and Infectimune® based clinical candidates and the Company’s interpretation of the results and findings of such programs and collaborations and whether such results are sufficient to support the future success of the Company’s clinical candidates; |
| ● | the success, timing and cost of the Company’s ongoing clinical trials and anticipated clinical trials for the Company’s current clinical candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim results (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of the Company’s ongoing clinical trials; |
| ● | expectations for the clinical and preclinical development, manufacturing, regulatory approval, and commercialization of the Company’s clinical candidates; |
| ● | any Company statements about its understanding of clinical candidates’ mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs and any collaboration trials; the acceptance by the market of the Company’s clinical candidates, if approved; |
| ● | the timing of and the Company’s ability to obtain and maintain U.S. Food and Drug Administration or other regulatory authority approval of, or other action with respect to, the Company’s clinical candidates; and |
| ● | other factors, including legislative, regulatory, political and economic developments not within the Company’s control, including unforeseen circumstances or other disruptions to normal business operations arising from or related to those listed under Part II, Item 1A. Risk Factors. |
Any forward-looking statements in this Quarterly Report reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, whether as a result of new information, future events or otherwise.
In this Quarterly Report, unless otherwise stated or the context otherwise indicates, references to “PDS Biotech,” “the Company,” “we,” “us,” “our” and similar references refer to PDS Biotechnology Corporation, a Delaware corporation.
Company Overview
We are a clinical-stage immunotherapy company developing a growing pipeline of targeted cancer and infectious disease immunotherapies based on our Versamune® T cell activator and Versamune® in combination with our interleukin 12 (IL-12) fused anti-body drug conjugate (ADC) PDS01ADC. In addition, we are developing the Infectimune® T cell-activator in infectious diseases.
We believe our investigational targeted immunotherapies have the potential to overcome the limitations of current immunotherapy approaches through effective conversion of the immune suppressive tumor to an immunogenic microenvironment in addition to the induction of the right type, potency and quantity of tumor-targeting killer (CD8) T cells. Our Versamune® immunotherapies and Versamune® in combination with PDS01ADC, are utilized for treatments in oncology, and Infectimune® is utilized for preventive vaccines against infectious agents. When paired with an antigen, which is a disease-related protein that is recognizable by the immune system, Versamune® and Infectimune® have both been shown to induce, in vivo, large quantities of high-quality, highly potent polyfunctional disease-specific CD4 helper and CD8 killer T cells, a specific sub-type of T cell that has shown potential to be more effective at killing infected or target cells. Infectimune® is also designed to promote the induction of disease-specific neutralizing antibodies. PDS01ADC is an investigational tumor targeting IL-12 that we believe may enhance the proliferation, potency and longevity of T cells in the tumor microenvironment and reduces the prevalence of immune suppressive cells and components within the tumor. We believe that our proprietary combination of Versamune® and PDS01ADC may enhance the proliferation, potency and longevity of antigen specific multifunctional CD8 T cells in the tumor microenvironment and work synergistically to inhibit or treat cancer.
Recent Developments
In December 2022, we executed an exclusive global license agreement with Merck KGaA, Darmstadt, Germany for the tumor targeting IL-12 fused antibody drug conjugate, M9241, which joined our pipeline as PDS01ADC. PDS01ADC is a novel investigational tumor-targeting fusion protein of Interleukin 12 that enhances the proliferation, potency, infiltration and longevity of T cells in the tumor microenvironment and is therefore designed to overcome the limitations of cytokine therapy which today have resulted in high toxicity and limited therapeutic potential. The proprietary combination of Versamune® and PDS01ADC is designed to overcome tumor immune suppression utilizing a different mechanism from immune checkpoint inhibitors (ICI). The combination of Versamune® and PDS01ADC to overcome immune suppression is patented by us, and we believe our ownership of both assets will streamline the clinical development, registrational process and their potential therapeutic use. In a Phase 2 National Cancer Institute (NCI)-led clinical trial in ICI resistant patients, the combination of PDS0101 and PDS01ADC administered with an investigational bi-functional ICI resulted in a median overall survival of approximately 20 months. The historical median survival reported in ICI resistant HPV-positive cancers when treated with ICIs is 3-4 months, and best reported median survival to date with systemic therapy is 8.2 months in ICI resistant head and neck cancer.
In February 2023, we announced a successful completion of a Type B meeting with the FDA for the triple combination of PDS0101 and PDS01ADC with an FDA-approved immune checkpoint inhibitor for the treatment of recurrent/metastatic, ICI resistant head and neck cancer that is positive for the HPV type 16. In recent interactions with the FDA, we confirmed the required contents of a clinical protocol for the potential registrational trial.
In June 2023, an abstract was presented at the 2023 American Society of Clinical Oncology: Abstract number 6012, Safety and Efficacy of Immune Checkpoint Inhibitor (ICI) Naïve Cohort from Study of PDS0101 and Pembrolizumab in HPV16-Positive Head and Neck Squamous Cell Carcinoma (HNSCC). The abstract was also selected as one of the featured posters reviewed by an expert panel in the Head and Neck Cancer discussion session.
In September 2023, data on our investigational universal flu vaccine, PDS0202, was presented at the 9th European Scientific Working Group on Influenza (ESWI) conference. This data demonstrated broad neutralization across multiple influenza strains in animals and provided protection against infection after challenging animals not previously exposed to flu with lethal doses of the pandemic H1N1 flu virus.
In October 2023, data demonstrating PDS0101 in combination with standard-of-care (SOC) chemoradiotherapy was associated with a rapid decline in human papillomavirus circulating cell-free DNA (ctHPV-DNA), a potential predictive biomarker of treatment response. The data from the IMMUNOCERV Phase 2 clinical trial were featured in an oral presentation at the American Society for Radiation Oncology Annual Meeting.
In October 2023, updated interim data based on an August 2, 2023 cut off from our VERSATILE-002 Phase 2 clinical trial evaluating the combination of PDS0101 in combination with Merck’s anti-PD-1 therapy, Keytruda® (pembrolizumab) which is the FDA-approved standard of care for first-line treatment of recurrent/metastatic head and neck cancer was presented at a key opinion leader roundtable that we sponsored.
In October 2023, interim safety and immune response data was presented for the first-in-human Phase1/2 clinical trial evaluating PDS01ADC in combination with current SOC chemotherapy, docetaxel, to treat metastatic castration sensitive and castration resistant prostate cancer. The data was featured in an oral presentation at the 11th Annual Meeting of the International Cytokine & Interferon Society.
In October 2023, immune response data from a preliminary analysis of a subset of patients in our VERSATILE-002 Phase 2 clinical trial was presented at the European Society for Medical Oncology Congress 2023.
In November 2023, we announced updated survival data from our NCI-led Phase 2 trial investigating the triple combination of PDS0101, PDS01ADC and an investigational immune checkpoint inhibitor (ICI) in two groups of advanced cancer patients with various types of human papillomavirus (HPV) 16-positive cancers. The data showed 75% Survival of ICI naïve patients at 36 months.
In November 2023, preclinical data from our NCI-led trial including PDS0101, PDS01ADC and an HDAC inhibitor in ICI-resistant HPV-16 positive cancer was presented during a poster presentation at the Society for Immunotherapy of Cancer 38th Annual Meeting.
Clinical Candidate Pipeline
VERSATILE-002: PDS0101 + Keytruda®
In November 2020, our VERSATILE-002 Phase 2 clinical trial evaluating the combination of PDS0101 in combination with Merck’s anti-PD-1 therapy, Keytruda® (pembrolizumab) which is the FDA-approved standard of care for first-line treatment of recurrent/metastatic head and neck cancer commenced. Enrollment in stage 2 of 2 for the ICI naïve arm and the ICI resistant arms are complete. The clinical trial will evaluate the efficacy and safety of this therapeutic combination as a first and second line treatment in patients with recurrent or metastatic head and neck cancer and high-risk human papillomavirus-16 (HPV16) infection.
In this trial sponsored by PDS Biotech, patients whose cancer has returned following initial treatment or spread will be treated with the combination of PDS0101 and Keytruda® to evaluate if the addition of PDS0101 might improve the efficacy reported in published studies of Keytruda® alone. Patients in the trial will receive a total of 5 cycles of combination therapy in the context of standard of care Keytruda® therapy administered every three weeks until disease progression. The primary endpoint of VERSATILE-002 is the objective response rate, or ORR, at six months following initiation of treatment. There are two cohorts in the trial. Cohort 1 is for patients who have yet to be treated with an immune checkpoint inhibitor (ICI naïve) and cohort 2 which consists of patients who have failed immune checkpoint inhibitor therapy (ICI resistant).
In February 2022, we achieved the preliminary efficacy milestone of at least four or more objective responses of the first 17 patients in the ICI naïve arm that allowed that arm to proceed to full enrollment. We also announced detailed preliminary safety data which showed that the combination is well tolerated without evidence of enhanced or significant toxicity in the first 18 patients in the ICI naïve arm. We have completed enrollment in Stage 1 of the ICI resistant arm and we are waiting for sufficient follow up to conduct the futility analysis.
In June 2022, we presented additional preliminary efficacy and safety data from this trial at the ASCO Annual Meeting (Weiss J et al. J Clin Oncol 40, 2022 (suppl 16; abstr 6041)). The abstract provided preliminary data on 19 patients (safety) with available imaging data for 17 of the 19 patients (efficacy). Data on 17 patients was presented. Highlights from the abstract were as follows:
| ● | Confirmed and unconfirmed response rates thus far (tumor shrinkage greater than 30%) seen in 7/17 (41.2%) patients in comparison to the published results of approximately 19% for approved ICIs, used as monotherapy for recurrent or metastatic head and neck cancer, with 2 of the 7 having complete responses (CR) |
| ● | Stable disease (SD) was reported in 6/17 (35.3%) patients, with 4 of the 6 (67%) experiencing tumor shrinkage of less than 30% |
| ● | Clinical efficacy (ORR + SD) was seen in 13/17 (76.5%) patients |
| ● | Progressive/ongoing disease was reported in 4/17 (23.5%) patients |
| ● | Patients had received a median of 4/5 doses of PDS0101 (range 1-5) and 9/35 doses of Keytruda® (range 1-18) |
| ● | There were no treatment-related adverse events greater than or equal to Grade 3 (N=19) |
| ● | No patients required dose interruption or reduction on the combination treatment |
| ● | No patients discontinued the combination treatment |
| ● | At 9 months of follow up (median not yet achieved): |
| ● | Progression free survival (PFS) rate was 55.2% |
| ● | Overall survival (OS) rate was 87.2% |
| ● | no control or comparative studies have been conducted between ICIs and PDS0101 |
In May 2022, we expanded this trial into Europe and in June 2022, as described above, we received Fast Track designation from the FDA for PDS0101 in combination with Keytruda®.
In August 2022, our independent Data Monitoring Committee (DMC) met and evaluated data from 43 patients and noted there were no Grade 3 or greater treatment-related adverse events attributed to the combination. The DMC recommended continuing the trial with no modifications.
In October 2022, we announced the results of an end-of-phase 2 meeting with the FDA for PDS0101 in combination with Keytruda®. We have completed the plan for a potential Phase 3 clinical program that will support the submission of a BLA for PDS0101 and have submitted our plan to the FDA.
In In May 2023, we completed enrollment in the ICI naïve arm. We filed our amended IND with the FDA in the third quarter of 2023. In October 2023, we received feedback from the FDA on the amended IND.
In June 2023, an abstract was presented at the 2023 American Society of Clinical Oncology: Abstract number 6012, Safety and Efficacy of Immune Checkpoint Inhibitor (ICI) Naïve Cohort from Study of PDS0101 and Pembrolizumab in HPV16-Positive Head and Neck Squamous Cell Carcinoma (HNSCC). The abstract was also selected as one of the featured posters to be reviewed by an expert panel in the Head and Neck Cancer discussion session. Data on 34 patients was presented. The data from the abstract is as follows:
| ● | Estimated 12-month overall survival rate was 87.1%. Published results are 36-50% with approved ICIs used alone. |
| ● | Median progression-free survival was 10.4 months (95% CI 4.2, 15.3). Published results are median PFS of 2-3 months for approved ICIs when used as monotherapy in patients with similar PD-L1 levels. |
| ● | A disease control rate (disease stabilization or tumor shrinkage) of 70.6% (24/34) |
| ● | Confirmed and unconfirmed objective response rate is 41.2% (14/34 patients), which is identical to the preliminary response rate data PDS Biotech previously reported at ASCO 2022 (7/17 patients). To date these responses have been confirmed in nine of the 34 patients (26.5%), including one complete response. |
| ● | 15/34 patients (44.1%) had stable disease. |
| ● | 9/34 patients (26.5%) had progressive disease. |
| ● | 4/48 (8.3%) of patients had a Grade 3 treatment-related adverse event (TRAE). No Grade 4 or higher TRAEs were observed. |
In October 2023, at a key opinion roundtable updated interim data was presented based on an August 2, 2023 cut-off from our VERSATILE-002 Phase 2 clinical trial evaluating the combination of PDS0101 in combination with Merck’s anti-PD-1 therapy, Keytruda® (pembrolizumab) which is an FDA-approved standard of care for first-line treatment of recurrent/metastatic head and neck cancer. Data on 52 patients was presented. The data from the roundtable based on investigator assessment was as follows:
Highlights from the ICI naïve cohort include:
| ● | 24-month overall survival (OS) rate is 74%; published 24-month survival rate of less than 30% for approved ICI. |
| ● | 12-month OS rate is 80%; published results of 30-50% with approved ICIs. |
| ● | Tumor shrinkage seen in 60% (31/52) of patients. |
| ● | Confirmed overall response rate ORR is 27% (14/52) to date. |
| ● | Median progression-free survival (PFS) is 8.1 months to date; published results of 2-3 months PFS with approved ICIs. |
| ● | 13% (8/62) of patients experienced Grade 3 treatment-related adverse events (TRAE) and 0% (0/62) experienced Grade 4 or 5 TRAE; published results report 13-17% Grade 3-5 TRAE with approved ICI monotherapy. |
| ● | 60% (33/55) of patients have CPS score of 1-19 (who generally have a weaker response to Keytruda®), and 40% (22/55) have CPS score >20 (who generally have a higher response to Keytruda®). |
Highlights from the ICI refractory cohort include:
| ● | The 12-month OS rate is 56%. The published median 12-month OS rate is 17% with no salvage chemotherapy following tumor progression on ICI (ICI Resistant). |
| ● | 0% (0/21) confirmed ORR suggests that PDS0101’s impact on survival does not appear to be dependent on tumor shrinkage. |
| ● | 4% (1/25) of patients experienced Grade 3 TRAE and 0% (0/21) patients experienced Grade 4 and 5 TRAE. |
In May 2024, at a virtual key opinion leader event, updated interim data was presented based on a February 15, 2024 cut-off from our VERSATILE-002 Phase 2 clinical trial evaluating the combination of PDS0101 in combination with Merck’s anti-PD-1 therapy, Keytruda® (pembrolizumab) which is an FDA-approved standard of care for first-line treatment of recurrent/metastatic head and neck cancer. Data on 53 patients was presented. The data from the event based on investigator assessment was as follows:
Highlights from the ICI naïve cohort with CPS > 1include:
| ● | Median overall survival of 30 months; published results for ICIs are 7-18 months. |
| ● | Confirmed overall response rate ORR is 34% (18/53) to date; published results for comparable patients receiving treatment with ICIs are less than 20%. |
| ● | Confirmed complete responses, partial responses and stable disease according to RECIST v1.1 were seen in 75.5% of patients. |
| ● | Median progression-free survival (PFS) is 6.3 months to date; published results of 2-3 months PFS with approved ICIs. |
| ● | The combination of PDS0101 and Keytruda® appeared to be well tolerated with 11% (7/62) of patients experienced Grade 3 treatment-related adverse events (TRAE) and 2% (1/62) experienced Grade 4 or 5 TRAE; published results report 13-17% Grade 3-5 TRAE with approved ICI monotherapy. |
| ● | 60% (32/53) of patients have CPS score of 1-19 (who generally have a weaker response to Keytruda®), and 40% (21/53) have CPS score >20 (who generally have a higher response to Keytruda®). |
During the May 2024 event, we also announced an updated clinical strategy with a two-part registrational trial focused on the triple combination of Versamune® HPV + PDS01ADC + pembrolizumab as a first line treatment in HPV16-positive recurrent/metastatic HNSCC.
National Cancer Institute: PDS0101+ M9241 (now PDS01ADC) +Bintrafusp Alfa
In June 2020, the first patient was dosed under a PDS0101 Cooperative Research and Development Agreement (CRADA), in the NCI led Phase 2 investigator-initiated trial evaluating PDS0101 with an IL-12 ADC now PDS01ADC, and M7824 (Bintrafusp alfa), which is owned by EMD Serono (Merck KGaA) in patients with advanced HPV-positive cancers who have failed prior treatment. In February 2021, the NCI’s Phase 2 clinical trial of PDS0101 for the treatment of advanced HPV-positive cancers had achieved its preliminary objective response target in patients naïve to check point inhibitors which allowed for full enrollment of approximately 20 patients in this group. In addition, based on promising results in the ICI naïve arm, the trial was amended to allow enrollment of a separate cohort of IC -resistant patients for assessment of safety and activity of the triple combination. The trial has been closed for enrollment. Preliminary efficacy assessment of the triple combination in this added group of 29 ICI resistant patients has been completed and evaluation of long-term patient survival is ongoing.
Preclinical study results arising from this CRADA were published in the Journal for ImmunoTherapy of Cancer, Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine (Journal for ImmunoTherapy of Cancer2020;8:e000612. Doi:10.1136/ jitc-2020-000612), and indicate that PDS0101 generated both HPV-specific T cells and an associated antitumor response when used as a monotherapy. When PDS0101 was combined with the two other novel clinical-stage anti-cancer agents, Bintrafusp Alfa and M9241 (which is now owned by us and referred to as PDS01ADC), the preclinical data suggested that all three therapeutic agents worked synergistically to provide superior tumor T cell responses and subsequent tumor regression when compared to any of the agents alone or the 2-component combinations. The published preclinical data demonstrating powerful activity of the triple combination appears to be corroborated in the Phase 2 trial, and this triple combination could form the basis of a unique platform providing improved cancer treatments across multiple cancers.
In June 2022, at the 2022 ASCO Annual Meeting, the NCI provided an update to the preliminary data presented at the 2021 meeting (Strauss J et al. J Clin Oncol 40, 2022 [suppl 16; abstr 2518]). This included data from 30 HPV16-positive patients and highlights were as follows:
| ● | Objective response (OR = >30% tumor reduction) was seen in 88% (7/8) of patients with ICI naive disease; 4/7 (57%) patients’ responses are ongoing (median 17 months). |
| ● | With ICI resistant patients: PDS01ADC dosing appears to affect response rates, with 5/8 (63%) patients receiving PDS01ADC at 16.8 mcg/kg achieving an OR compared to 1/14 (7%) patients who received PDS01ADC at 8 mcg/kg achieving an OR; 4/6 (67%) patients’ responses are ongoing (median 12 months). |
| ● | Tumor reduction was seen in 45% (10/22) of patients with ICI resistant disease, including patients receiving high or low dose PDS01ADC. |
| ● | In ICI resistant patients treated with high or low dose PDS01ADC, survival outcomes were similar (p=0.96 by Kaplan Meier analysis). At a median of 12 months of follow up 17/22 (77%) of patients were alive. |
| ● | In ICI naïve patients 6/8 (75%) were alive at median 17 months of follow up. |
| ● | Similar OR and survival were seen across all types of HPV16-positive cancers. |
| ● | Preliminary safety data: 13/30 (43%) of patients experienced Grade 3 treatment-related adverse events (AEs), and 2/30 patients (7%) experienced Grade 4 AEs. There were no grade 5 treatment-related AEs. |
We believe the trial results to date strongly suggest, in agreement with the published preclinical studies, that all 3 drugs contribute to the clinical outcomes.
In September 2022, we determined, in agreement with the NCI, to select the ICI resistant patients as the preferred treatment group in the on-going PDS0101-based triple combination therapy in advanced HPV-positive cancers and the trial was closed to further enrollment given the ICI resistant arm had been fully recruited.
In October 2022, we presented additional interim data as follows:
| ● | Survival data: 66% (19/29) of HPV16-positive ICI resistant patients in the cohort were alive at a median follow up of 16 months. |
| ● | Safety profile: 48% (24/50) patients experienced Grade 3 treatment-related adverse events (AEs), and 4% (2/50) patients experienced Grade 4 AEs. There were no Grade 5 treatment-related AEs. |
| ● | HPV16-positive ICI naïve patients: 75% (6/8) were alive at a median follow up of 25 months and 38% (3/8) of responders had a complete response. |
In December 2022, we presented interim data as follows:
| ● | Median OS was 21 months in 29 checkpoint inhibitor resistant patients who received the triple combination. The reported historical median OS in patients with ICI resistant disease is 3-4 months seen with checkpoint inhibitors and best reported median survival to date with systemic therapy of 8.2 months in ICI resistant head and neck cancer. |
| ● | In ICI naïve subjects, 75% remain alive at a median follow-up of 27 months. As a result, median OS had not yet been reached. Historically median OS for similar patients with platinum experienced ICI naïve disease is 7-11 months. |
| ● | Objective response rate (ORR) in ICI resistant patients who received the optimal dose of the triple combination is 63% (5/8). In current approaches ORR is reported to be less than 10%. |
| ● | ORR in ICI naïve patients with the triple combination is 88%. In current approaches ORR is reported to be less than 25% with FDA-approved ICIs in HPV-positive cancers. |
| ● | Safety data had not changed since October’s update. 48% (24/50) of patients experienced Grade 3 (moderate) treatment-related adverse events (AEs), and 4% (2/50) patients experienced Grade 4 (severe) AEs, compared with approximately 70% of patients receiving the combination of ICIs and chemotherapy reporting Grade 3 and higher treatment-related AEs.. |
In February 2023, we announced the successful completion of a Type B meeting with the FDA for the combination therapy of PDS0101, PDS01ADC, and an FDA-approved immune checkpoint inhibitor for the treatment of recurrent/metastatic HPV-positive ICI-resistant head and neck cancer. We confirmed the required contents of the trial design for a potential registrational trial of the combination.
In November 2023, we released updated interim survival data as follows:
| ● | 75% of immune checkpoint inhibitor (ICI) naïve patients remain alive at 36 months; published median overall survival (OS) in similar patients is 7-11 months |
| ● | 12-month survival rate in (ICI) resistant patients of 72% |
| ● | Median OS in ICI resistant HPV-positive patients is approximately 20 months; published median OS is 3.4 months |
MD Anderson Cancer Center (IMMUNOCERV): PDS0101+ Chemoradiotherapy
In October 2020, a PDS0101 Phase 2 IIT was initiated with The University of Texas MD Anderson Cancer Center and is actively recruiting patients. This clinical trial is investigating the safety and anti-tumor efficacy of PDS0101 in combination with standard-of-care chemo-radiotherapy, or CRT, and their correlation with critical immunological biomarkers in patients with locally advanced cervical cancer. We believe that Versamune® has strong T cell induction with the potential to enhance efficacy of the current standard of care CRT treatment in this indication with the FDA at this meeting.
In November 2022, data from this trial was included in a poster presentation at the 2022 SITC Annual Meeting which included the following:
| ● | 9 of the 17 patients had completed a Day 170 post-treatment Positron Emission Tomography, Computed Tomography (PET CT) scan to assess the status of the cancer. This included 78% (7/9) of treated patients with advanced cervical cancer (FIGO stage III or IV). |
| ● | 100% (9/9) of patients treated with the combination of PDS0101 and CRT had an objective response. |
| ● | 89% (8/9) of patients treated with the combination of PDS0101 and CRT demonstrated a complete response (CR) on Day 170 by PET CT. One patient who received 3 of the 5 scheduled doses of PDS0101 showed signs of residual disease. One patient who had a CR died from an event unrelated to either their underlying disease or treatment. |
| ● | 1-year disease-free survival and 1-year overall survival of 89% (8/9) in patients treated with the combination of PDS0101 and CRT. |
| ● | As previously reported, data confirm PDS0101 treatment activates HPV16-specific CD8 T cells. This increase was not seen in patients who did not receive PDS0101. The increase in HPV16-specific T cells generated by the treatment is positively correlated with tumor cell death, suggesting cytotoxic CD8 T cells are important mediators of antigen-specific immunity. |
| ● | The data affirms that PDS0101 activates Type 1 interferon pathway in humans, mimicking the mechanism previously demonstrated in preclinical studies in animal models. |
| ● | Toxicity of PDS0101 remains limited to low-grade local injection site reactions. |
In October 2023, data demonstrating PDS0101 in combination with standard-of-care (SOC) chemoradiotherapy was associated with a rapid decline in human papillomavirus circulating cell-free DNA (ctHPV-DNA), a potential predictive biomarker of treatment response. The data from the IMMUNOCERV Phase 2 clinical trial was featured in an oral presentation at the American Society for Radiation Oncology Annual Meeting which included the following:
| ● | Earlier and greater proportion of ctDNA clearance with PDS0101 plus chemoradiation (CRT) vs. SOC CRT alone (81.3% clearance after 3 weeks vs. 30.3% with SOC (p=0.0018), and 91.7% of clearance at 5 weeks vs. 53.1% with SOC (p=0.0179). |
| ● | Baseline ctDNA levels correlated with the International Federation of Gynecology and Obstetrics (FIGO) stage and lymph node involvement; 100% of patients treated with PDS0101 had cancer that had spread to the lymph nodes. |
Mayo Clinic: PDS0101 Monotherapy and in combination with Keytruda®
In February 2022, we initiated an Investigator-Initiated Trial (ITT), MC200710, for PDS0101 alone or in combination with the immune checkpoint inhibitor, Keytruda®, in patients with HPV-positive oropharyngeal cancer (HPV(+)OPSCC) at high risk of recurrence. The trial is being led by Drs. David Routman, Katharine Price, Kathryn Van Abel, and Ashish Chintakuntlawar at Mayo Clinic, a nationally and internationally recognized center of excellence for the treatment of head and neck cancers. We believe that this trial not only broadens our addressable patient population of those affected by the increasing incidence of HPV(+)OPSCC, but also allows us to better understand the activity of PDS0101 alone or in combination with Keytruda® in earlier stages of disease. This trial is currently open for enrollment.
In this trial, treatment will be administered before patients proceed to transoral robotic surgery (TORS) with curative intent. Treatment in this setting is referred to as neoadjuvant treatment. PDS0101 has been shown to induce killer T cells that target and kill HPV-positive cancers, either alone or in combination with ICIs in preclinical studies, and in combination in clinical studies of patients with advanced recurrent/metastatic HPV-positive cancers. This trial will explore whether PDS0101 with or without checkpoint inhibition may increase HPV-specific anti-tumor responses, potentially resulting in tumor shrinkage, pathologic regression, and decreases in circulating tumor DNA (ctDNA).
PDS0102
PDS0102 is an investigational immunotherapy utilizing tumor-associated and immunologically active T cell receptor gamma alternate reading framed protein (TARP) from the NCI. PDS0102 is designed to treat TARP-associated cancers including, acute myeloid leukemia (AML), prostate and breast cancer. In our preclinical work, in the administration of PDS0102, the Versamune®+TARP antigen combination led to the induction of large numbers of tumor targeted killer T cells. In addition, the TARP tumor antigen alone has already been studied at the NCI in men with prostate cancer and has been shown to be safe, and immunogenic with slowing tumor growth rates (NCT00972309).
PDS0103
In April 2020, the above mentioned CRADA between PDS Biotech and the NCI was expanded beyond PDS0101 to include clinical and preclinical development of PDS0103. PDS0103 is an investigational immune therapy owned by PDS Biotech and designed to treat cancers associated with the mucin-1, or MUC1, oncogenic protein. These include cancers such as ovarian, breast, colorectal and lung cancers. PDS0103 combines Versamune® with novel highly immunogenic agonist epitopes of MUC1 developed by the NCI and licensed by PDS Biotech. PDS0103 is currently in the tech transfer, clinical scale up and manufacturing stage.
MUC1 is highly expressed in several types of cancer and has been shown to be associated with drug resistance and poor disease prognosis in breast, colorectal, lung and ovarian cancers, for which PDS0103 is being developed. Expression of MUC1 is often associated with poor disease prognosis, due in part to drug resistance. In preclinical studies, and similarly to PDS0101, PDS0103 demonstrated the ability to generate powerful MUC1-specific CD8 killer T cells.
In the first quarter of 2022, we held a pre-IND meeting with the FDA on PDS0103 and we are prepared to submit our IND package by the end of 2024. However, the actual submission date may potentially be impacted by the allocation of resources to initiate a pivotal trial for PDS0101 in combination with PDS01ADC.
IL-12 Oncology Immunocytokine Pipeline
PDS01ADC is a novel investigational IL-12 fused antibody drug conjugate (IgG1), tumor-targeting interleukin 12 (IL-12) immune-cytokine that enhances the proliferation, potency and longevity of T cells in the tumor microenvironment. Together with Versamune® based immunotherapies, PDS01ADC works synergistically to overcome tumor immune suppression and to promote a targeted T cell attack against cancers. As with Versamune®, PDS01ADC is given by a simple subcutaneous injection. Clinical data suggests the addition of PDS01ADC to Versamune® based immunotherapies may demonstrate significant disease control in advanced cancer patients by shrinking tumors and/or prolonging life.
With the exclusive global license agreement with Merck KGaA, Darmstadt, Germany for PDS01ADC, we believe we have simplified our registrational pathway for the NCI-led triple combination by owning both PDS0101 and PDS01ADC and combining these agents with an FDA approved ICI. PDS01ADC has been designed to overcome the limitations of cytokine therapy as explained above, and based on extensive preclinical studies performed at the NCI evaluating PDS01ADC as a monotherapy and also in combinations with established standard of care treatments for cancer, we believe that PDS01ADC has significant potential as a cytokine therapy independent of Versamune®. Based on the informative preclinical studies, a number of ITT Phase 2 trials are currently in progress at the NCI, some of which are outlined below:
| ● | Phase II Study Evaluating ICI Naïve and Resistant Patients with HPV-positive malignancies treated with PDS01ADC, PDS0101 and bintrafusp alfa. |
| ● | A Phase II Study Evaluating T-Cell Clonality After Stereotactic Body Radiation Therapy Alone and in Combination with the Immunocytokine PDS01ADC in Localized High and Intermediate Risk Prostate Cancer Treated with Androgen Deprivation Therapy |
| ● | A Phase I/II Study of PDS01ADC in Combination with Docetaxel in Adults with Metastatic Castration Sensitive and Castration Resistant Prostate Cancer |
| ● | Phase I/II of PDS01ADC going forward as a Monotherapy in Advanced Kaposi Sarcoma |
| ● | Phase I/II of PDS01ADC in Combination of with a Histone Deacetylase (HDAC) Inhibitor in ICI resistant MUC1-positive colon and bladder cancers among others |
In October 2023, interim safety and immune response data was presented for the first-in-human Phase 1/2 clinical trial evaluating PDS01ADC in combination with current SOC chemotherapy, docetaxel, to treat metastatic castration sensitive and castration resistant prostate cancer. The data was featured in an oral presentation at the 11th Annual Meeting of the International Cytokine & Interferon Society. The data presented included the following:
| ● | Decrease in PSA levels was seen in all patients at all three tested doses of PDS01ADC and 61% of patients had at least a 60% decrease in PSA levels. |
| ● | All doses of the combination were well-tolerated with one patient experiencing Grade 4 neutropenia. |
| ● | Administration of the combination was associated with decreases in T reg cells and increases in activated natural killer (NK) cells, memory CD8 T cells, proliferating CD4 and CD8 T cells and cytokines INF-γ and Interleukin 10 (IL-10). |
| ● | The changes in immune responses with the combination were independent of the PDS01ADC dose. |
We are working closely with the NCI to determine the best pathway forward for the prioritized PDS01ADC studies, as well as evaluating the use of PDS01ADC in combination with other Versamune® based clinical candidates.
Infectimune® Development Strategy
We believe that the key differentiating attributes of the Infectimune® platform technology are strong induction of CD8 and CD4 T cells as well as antibodies which can be leveraged to improve treatment and preventive options in several infectious disease indications. In January 2022, we presented preclinical data on our universal flu program sponsored by the National Institute of Allergy and Infectious Disease (NIAID) demonstrating the potential of the Infectimune® technology with computationally designed influenza proteins developed by the laboratory of Dr. Ted Ross at the University of Georgia to generate broadly protective anti-influenza immune responses across multiple strains of influenza. This data has provided a unique opportunity to highlight Infectimune®’s potentially transformative utility in the development of more broadly effective and longer lasting protective vaccines. Current preventive and prophylactic vaccine approaches and technologies predominantly focus on creating strong induction of antibody responses. However, the induction of T cell responses, in addition to antibody responses, provides more durable and broad protection against infectious diseases.
Based on the preclinical data with the universal seasonal flu vaccine and the current focus of the NIAID in developing more effective flu vaccines, we have decided to focus our near-term infectious disease activities to align with the interests of the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs) program. This will involve development of a universal seasonal flu vaccine and the potential development of a universal pandemic influenza vaccine based on similar computationally designed antigens as have shown promise with Infectimune®.
In July 2022, universal flu vaccine preclinical data for PDS0202 at the 41st American Society of Virology meeting: Abstract number 3733830, Infectimune® enhances antibodies elicited by COBRA hemagglutinin influenza vaccine. We are evaluating the next steps in the clinical development and funding for PDS0202.
The preclinical results for Infectimune® based vaccines were published in two separate articles in the peer reviewed journal Viruses in February 2023: 1. preclinical studies demonstrating complete protection against sickness after lethal challenge with live SARS-CoV-2 or influenza viruses (Gandhapudi SK et al. Viruses 2023, 15, 432) and 2. Dramatically enhanced CD4 T cell responses to recombinant influenza proteins compared to leading commercial vaccine adjuvants (Henson TR et al. Viruses 2023, 15, 538).
In September 2023, preclinical data on our investigational universal flu vaccine, PDS0202, was presented at the 9th European Scientific Working Group on Influenza (ESWI) conference. This data demonstrated active neutralization across multiple influenza viruses in animals and provided protection against infection and weight loss after challenging with high doses of H1N1 viruses when they were not previously exposed to flu.
Our current clinical pipeline of Versamune®, PDS01ADC and Infectimune® based therapies is as follows:
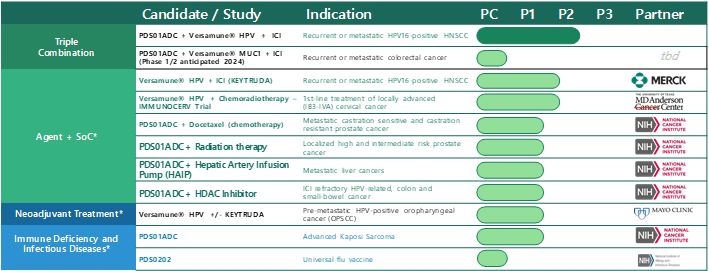
We have never been profitable and have incurred net losses in each year since inception. Our net losses were $10.6 million, and $9.7 million for the three months ended March 31, 2024 and 2023, respectively. As of March 31, 2024, we had an accumulated deficit of $155.1 million. Substantially all of our net losses have resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with these operations.
As of March 31, 2024, we had $66.6 million in cash and cash equivalents.
Our future funding requirements will depend on many factors, including the following:
| ● | the timing and costs of our planned clinical trials; |
| ● | the timing and costs of our planned preclinical studies of our Versamune® platform; |
| ● | the outcome, timing and costs of seeking regulatory approvals; |
| ● | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may enter into; |
| ● | the amount and timing of any payments we may be required to make in connection with the licensing, filing, prosecution, maintenance, defense and enforcement of any patents or patent applications or other intellectual property rights; and |
| ● | the extent to which we license or acquire other products and technologies. |
SELECTED FINANCIAL OPERATIONS OVERVIEW
Revenue
We have not generated any revenues from commercial product sales and do not expect to generate any such revenue in the near future. We may generate revenue in the future from a combination of research and development payments, license fees and other upfront payments or milestone payments.
Research and Development Expenses
Research and development expenses include employee-related expenses, costs to acquire license rights to use certain technology in our research and development projects, costs of acquiring, developing and manufacturing clinical trial materials, as well as fees paid to consultants and various entities that perform certain research and testing on our behalf. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations or information provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the condensed consolidated financial statements as prepaid or accrued expenses. Costs incurred in connection with research and development activities are expensed as incurred.
We expect that our research and development expenses will increase significantly over the next several years as we advance our Versamune® and PDS01ADC product candidates into and through clinical trials, pursue regulatory approval of our Versamune® product and PDS01ADC candidates and prepare for a possible commercial launch, all of which will also require a significant investment in contract research services, manufacturing process validation and inventory related costs.
The process of conducting human clinical trials necessary to obtain regulatory approval is costly and time consuming. We may never succeed in achieving marketing approval for our clinical product candidates. The probability of successful commercialization of our product candidates may be affected by numerous factors, including clinical data obtained in future trials, competition, manufacturing capability and commercial viability. As a result, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of any of our product candidates.
Results of Operations
The following table summarizes the results of our operations for the three months ended March 31, 2024 and 2023:
Three Months Ended March 31, | Increase | |||||||||||||||
| 2024 | 2023 | $ Amount | % | |||||||||||||
| (in thousands) | ||||||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development expenses | $ | 6,704 | $ | 5,844 | $ | 860 | 15 | % | ||||||||
| General and administrative expenses | 3,393 | 3,579 | (186 | ) | (5 | )% | ||||||||||
| Total operating expenses | 10,097 | 9,423 | 674 | 7 | % | |||||||||||
| Loss from operations | (10,097 | ) | (9,423 | ) | (674 | ) | 7 | % | ||||||||
| Interest income (expense), net | (506 | ) | (237 | ) | (269 | ) | 114 | % | ||||||||
| Net loss and comprehensive loss | $ | (10,603 | ) | $ | (9,660 | ) | $ | (943 | ) | 10 | % | |||||
Research and Development Expenses
Research and development (R&D) expenses increased to $6.7 million for the three months ended March 31, 2024 from $5.8 million for the three months ended March 31, 2023. The increase of $0.9 million in 2024 was primarily attributable to an increase of $1.2 million in clinical studies and medical affairs offset by a decrease of $0.1 million in personnel costs, $0.1 million in professional fees and $0.1 million in manufacturing costs.
General and Administrative Expenses
General and administrative expenses decreased to $3.4 million for the three months ended March 31, 2024 from $3.6 million for the three months ended March 31, 2023. The decrease of $0.2 million was primarily attributable to a decrease of $0.5 million in personnel costs offset by an increase of $0.3 million in professional fees.
Liquidity and Capital Resources
In August 2022, we filed a shelf registration statement, or the 2022 Shelf Registration Statement, with the SEC for the issuance of common stock, preferred stock, warrants, rights, debt securities, and units, up to an aggregate amount of $150 million, $50 million of which covers the offer, issuance and sale by us of our common stock under the Sales Agreement (as discussed below). The 2022 Shelf Registration Statement was declared effective on September 2, 2022.
In August 2022, we entered into an At Market Issuance Sales Agreement, or the Sales Agreement, with B. Riley Securities, Inc. and BTIG, LLC, each an Agent and collectively the Agents, with respect to an at-the-market offering program under which we may offer and sell, from time to time at our sole discretion, shares of our common stock, having an aggregate offering price of up to $50 million, or the Placement Shares, through or to the Agents, as sales agents or principals. Upon delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, the Agents may sell the Placement Shares by any method permitted by law deemed to be an “at the market” offering as defined in Rule 415 of the Securities Act of 1933, as amended, including, without limitation, sales made through The Nasdaq Capital Market or on any other existing trading market for our common stock. The Agents will use commercially reasonable efforts to sell the Placement Shares from time to time, based upon our instructions (including any price, time or size limits or other customary parameters or conditions we may impose). We will pay the Agents a commission equal to three percent (3%) of the gross sales proceeds of any Placement Shares sold through the Agents under the Sales Agreement, and we have also provided the Agents with customary indemnification and contribution rights. We are not obligated to make any sales of our common stock under the Sales Agreement. The offering of Placement Shares pursuant to the Sales Agreement will terminate upon the earlier of (i) the sale of all Placement Shares subject to the Sales Agreement or (ii) the termination of the Sales Agreement in accordance with its terms. For the year ended December 31, 2023, we sold 2,642,269 shares of common stock with a net value of $16.1 million pursuant to the Sales Agreement. During the quarter ended March 31, 2024 and 2023, we sold 3,428,681 and 553,293 shares of common stock with a net value of $19.5 and $4.6 million, respectively, pursuant to the Sales Agreement.
In August 2022, we entered into a venture loan and security agreement, or the Loan and Security Agreement, with Horizon Technology Finance Corporation, as lender and collateral agent for itself and the other lenders. The Loan and Security Agreement provides for the following 6 separate and independent term loans: (a) a term loan in the amount of $7,500,000, or Loan A, (b) a term loan in the amount of $10,000,000, or Loan B, (c) a term loan in the amount of $3,750,000, or Loan C, (d) a term loan in the amount of $3,750,000, or Loan D, (e) a term loan in the amount of $5,000,000, or Loan E, and (f) a term loan in the amount of $5,000,000, or Loan F, (with each of Loan A, Loan B, Loan C, Loan D, Loan E, and Loan F, individually a Loan and, collectively, the Loans). Loan A, Loan B, Loan C, and Loan D were delivered to us on August 24, 2022. Loan E and Loan F were uncommitted Loans that could have been advanced by the Lenders upon the parties agreement prior to July 31, 2023 upon the satisfaction by the Company of certain agreed upon conditions. At this time the option has expired and Loan E and Loan F are no longer available to the Company under the Loan and Security Agreement. We may only use the proceeds of the Loans for working capital or general corporate purposes.
Each Loan matures on the 48-month anniversary following the applicable funding date unless accelerated pursuant to agreed upon events of default. Payments on the principal balance begin on October 1, 2024 and are paid monthly in the succeeding 24 months. The principal balance of each Loan bears a floating interest. The interest rate is calculated initially and, thereafter, each calendar month as the sum of (a) the per annum rate of interest from time to time published in The Wall Street Journal as contemplated by the Loan and Security Agreement, or any successor publication thereto, as the “prime rate” then in effect, plus (b) 5.75%; provided that, in the event such rate of interest is less than 4.00%, such rate shall be deemed to be 4.00% for purposes of calculating the interest rate.
Interest is payable on a monthly basis based on each Loan principal amount outstanding the preceding month. We, at our option upon at least ten (10) business days’ written notice to the lenders, may prepay all (and not less than all) of the outstanding Loan by simultaneously paying to each lender an amount equal to (i) any accrued and unpaid interest on the outstanding principal balance of the Loans; plus (ii) an amount equal to (A) if such Loan is prepaid on or before the Loan Amortization Date (as defined in the Loan and Security Agreement) applicable to such Loan, 3% of the then outstanding principal balance of such Loan, (B) if such Loan is prepaid after the Loan Amortization Date applicable to such Loan, but on or before the date that is 12 months after such Loan Amortization Date, 2% of the then outstanding principal balance of such Loan, or (C) if such Loan is prepaid more than 12 months after the Loan Amortization Date but prior to the stated maturity date applicable to such Loan, 1% of the then outstanding principal balance of such Loan; plus (iii) the outstanding principal balance of such Loan; plus (iv) all other sums, if any, that shall have become due and payable thereunder. No prepayment premium will be applied to any outstanding balance of any Loan paid on the stated maturity date.
In connection with the Loan and Security Agreement, we issued Horizon Technology Finance Corporation and Powerscourt Investments XXV, LP warrants to purchase an aggregate total of 381,625 shares of our common stock at an initial exercise price of $3.6685 per share. Each warrant is classified as equity and is exercisable at any time for a period beginning on the date of grant and ending on the earlier of (A) 10 years from the date of grant, and (B) the closing of (A) (i) the sale, lease, exchange, conveyance or other disposition of all or substantially all of the our property or business, or (ii) its merger into or consolidation with any other corporation (other than a wholly-owned subsidiary of the Company), or any transaction (including a merger or other reorganization) or series of related transactions, in which more than 50% of the voting power of the Company is disposed of, in each case, for cash or for marketable securities meeting certain requirements as described in the applicable warrants. The key assumptions used in Black-Scholes option pricing model were (i) expected term of 10 years, (ii) a risk-free rate of 3.11%, (iii) expected volatility of 93.8%, and (iv) no estimated dividend yield.
In April 2023, we received approximately $1.4 million from the net sale of tax benefits to an unrelated, profitable New Jersey corporation pursuant our participation in the New Jersey Technology Business Tax Certificate Transfer NOL program for tax year 2021.
In April 2024, we received approximately $0.9 million from the net sale of tax benefits to an unrelated, profitable New Jersey corporation pursuant to its participation in the New Jersey Technology Business Tax Certificate Transfer of Net Operating Loss (NOL) program for tax year 2022.
As of March 31, 2024, we had $66.6 million in cash and cash equivalents. Our primary uses of cash are to fund operating expenses, primarily research and development expenditures. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
We evaluated whether there are any conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year beyond the filing of this Quarterly Report on Form 10-Q. Our budgeted cash requirements in 2024 and beyond include expenses related to continuing development and clinical studies as well as payments on our debt.
We plan to continue to fund our operations and capital funding needs through existing cash and additional equity and/or debt financing. However, we cannot be certain that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or our existing stockholders. We may also enter into government funding programs and consider selectively partnering for clinical development and commercialization. The sale of additional equity would result in additional dilution to our stockholders. Incurring debt financing would result in debt service obligations, and the instruments governing such debt could provide for operating and financing covenants that would restrict our operations. If we are unable to raise additional capital in sufficient amounts or on acceptable terms, we may be required to delay, limit, reduce, or terminate our product development or future commercialization efforts or grant rights to develop and market immunotherapies that we would otherwise prefer to develop and market ourselves. In addition, the Loan and Security Agreement allows for the lenders to call the outstanding balance of the term loans if the minimum cash balances outlined in the Loans and Security Agreement are not maintained. Any of these actions could harm our business, results of operations and prospects. Failure to obtain adequate financing also may adversely affect our ability to operate as a going concern.
As a result of these uncertainties, and as its plans are outside of management’s control, we have concluded that substantial doubt exists about our ability to continue as a going concern for a period of at least 12 months from the date of the issuance of these unaudited condensed consolidated financial statements. The unaudited condensed consolidated financial statements do not include any adjustments to the carrying amounts and classifications of assets and liabilities that would result if were unable to continue as a going concern.
Cash Flows
The following table shows a summary of our cash flows for each of the periods indicated (in thousands):
| Three Months Ended March 31, | ||||||||
| 2024 | 2023 | |||||||
Net cash used in operating activities | $ | (9,938 | ) | $ | (13,189 | ) | ||
| Net cash provided by financing activities | 20,012 | 4,568 | ||||||
| Net increase (decrease) in cash and cash equivalents | $ | 10,074 | $ | (8,621 | ) | |||
Net Cash Used in Operating Activities
Net cash used in operating activities was $9.9 million and $13.2 million for the three months ended March 31, 2024 and 2023, respectively. The decrease in net cash used in operating activities of $3.3 million was primarily due to a decrease in the non-cash stock-based compensation expense of $0.5 million offset by an increase in net loss of $1.0 million and changes in the timing of working capital requirements, including changes in prepaid expenses and other assets, accrued expenses and accounts payable.
Net Cash Provided by Financing Activities
Net cash provided by financing activities for the three months ended March 31, 2024 and 2023 was primarily due to the receipt of net proceeds of $19.5 million and $ 4.6 million, respectively, from the sale of common stock under the Sales Agreement.
Operating Capital Requirements
To date, we have not generated any product revenue. We do not know when, or if, we will generate any product revenue and we do not expect to generate significant product revenue unless and until we obtain regulatory approval and commercialize one of our current or future product candidates. We anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of, and seek regulatory approvals for, our product candidates, and begin to commercialize any approved products. We are subject to all of the risks incident to the development of new products, and may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may harm our business. We expect to incur additional costs associated with operating as a public company and anticipate that we will need substantial additional funding in connection with our continuing operations.
We evaluated whether there are any conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the filing of this Quarterly Report. Our budgeted cash requirements in 2024 and beyond include expenses related to continuing development and clinical studies as well as payments on our debt. Until we can generate significant cash from our operations, we expect to continue to fund our operations with available financial resources. These financial resources may not be adequate to sustain our operations. While we intend to finance our cash needs principally through collaborations, strategic alliances, or license agreements with third parties and/or debt or equity financings, there is no assurance that new financing will be available to us on commercially acceptable terms or in the amounts required, if at all. In addition, the Loan and Security Agreement allows for the lenders to call the outstanding balance of the term loans if the minimum cash balances outlined in the Loans and Security Agreement are not maintained. We have concluded that substantial doubt exists about our ability to continue as a going concern for a period of at least 12 months from the date of the issuance of these unaudited condensed consolidated financial statements.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| ● | the initiation, progress, timing, costs and results of our planned clinical trials; |
| ● | the effects of health epidemics, pandemics, or outbreaks of infectious diseases, on our business operations, financial condition, results of operations and cash flows; |
| ● | the outcome, timing and cost of meeting regulatory requirements established by the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, and other comparable foreign regulatory authorities; |
| ● | the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
| ● | the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us now or in the future; |
| ● | the effect of competing technological and market developments; |
| ● | the cost of establishing sales, marketing and distribution capabilities in regions where we choose to commercialize our products on our own; and |
| ● | the initiation, progress, timing and results of our commercialization of our clinical candidates, if approved, for commercial sale. |
Please see the section titled “Risk Factors” elsewhere in the Quarterly Report and Annual Report for additional risks associated with our operations.
Purchase Commitments
We have no material non-cancelable purchase commitments with service providers as we have generally contracted on a cancelable, purchase order basis.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. GAAP. Our accounting policies are more fully described in Note 2 to the condensed consolidated financial statements included in this Quarterly Report on Form 10-Q. As described in Note 2, the preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Estimates are assessed each period and updated to reflect current information. Actual results may differ from these estimates under different assumptions or conditions. We believe that the discussion in our management’s discussion and analysis addresses our most critical accounting policies, which are those that are most important to the portrayal of our financial condition and results of operations and require management’s most difficult, subjective and complex judgments.
There have been no material changes to our critical accounting policies and estimates during the three months ended March 31, 2024 from those disclosed in our Annual Report on Form 10-K for the year ended December 31, 2023.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Smaller Reporting Company
As of January 1, 2021, we were no longer an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. However, we remain a “smaller reporting company,” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended. We will cease to be a smaller reporting company if we have a non-affiliate public float in excess of $250 million and annual revenues in excess of $100 million, or a non-affiliate public float in excess of $700 million, determined on an annual basis. As a smaller reporting company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not smaller reporting companies. We will continue to take advantage of some or all of the available exemptions.
Interest Rate Risk
We are exposed to market risk related changes in interest rates. As of March 31, 2024, our cash equivalents consisted of bank deposits and money market accounts. Additionally, the principal balance of our Loan and Security agreement with Horizon Technology Finance Corporation bears a floating interest pegged to the prime rate. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Historically, the net impact of fluctuations in interest rates have not been material to us.
Inflation Risk
Inflation generally affects us by increasing our cost of labor and pricing of contracts. We do not believe that inflation has had a material effect on our business, financial condition, or results of operations during the three months ended March 31, 2024.
| ITEM 4: | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
An evaluation was carried out, under the supervision of and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15 (e)) under the Securities Exchange Act of 1934, or the Exchange Act, as of the end of the period covered by this report. Based on the evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that the information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the Exchange Act) identified in connection with the evaluation identified above that occurred during the quarter ended March 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 1. | LEGAL PROCEEDINGS |
The information in Note 9 to the condensed consolidated financial statements contained in Part I, Item 1 of this Quarterly Report on Form 10-Q is incorporated herein by reference. There are no matters which constitute material pending legal proceedings to which we are a party other than those incorporated into this item by reference from Note 9 to our condensed consolidated financial statements for the quarter ended March 31, 2024 contained in this Quarterly Report on Form 10-Q.
| ITEM 1A. | RISK FACTORS |
There have been no material changes from our risk factors as previously reported in our Annual Report on Form 10-K for the year ended December 31, 2023. However, any investment in our business involves a high degree of risk. Before making an investment decision, you should carefully consider the information we include in this Quarterly Report on Form 10-Q, including our unaudited interim condensed consolidated financial statements and accompanying notes, our Annual Report on Form 10-K for the year ended December 31, 2023 filed on March 28, 2024, including our financial statements and related notes contained therein, and the additional information in the other reports we file with the Securities and Exchange Commission. These risks may result in material harm to our business and our financial condition and results of operations. In this event, the market price of our common stock may decline and you could lose part or all of your investment. Additional risks that we currently believe are immaterial may also impair our business operations. Our business, financial conditions and future prospects and the trading price of our common stock could be harmed as a result of any of these risks.
| ITEM 2. | UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS |
There were no unregistered sales of the Company’s equity securities during the three months ended March 31, 2024.
| ITEM 3. | DEFAULTS UPON SENIOR SECURITIES |
None.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| ITEM 5. | OTHER INFORMATION |
None.
| ITEM 6. | EXHIBITS |
Exhibit Number | Exhibit Description | |
10.1+ | Executive Employment Agreement by and between Kirk V. Shephard, M.D. and PDS Biotechnology Corporation, effective as of January 22, 2024 (incorporated by reference to Exhibit 10.27 to the Registrant’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 28, 2024). | |
10.2+ | PDS Biotechnology Corporation 2019 Inducement Plan, as amended (filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on January 22, 2024, and incorporated by reference herein). | |
10.3*+ | Executive Employment Agreement by and between Stephan F. Toutain and PDS Biotechnology Corporation, effective as of May 1, 2024. | |
| Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
| Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
| Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (furnished herewith). | ||
| Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (furnished herewith). | ||
| 101.INS* | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |
| 101.SCH* | Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101). |
* Filed herewith (unless otherwise noted as being furnished herewith)
+ Indicates management compensatory plan or arrangement.
Pursuant to the requirements of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
PDS Biotechnology Corporation | ||
May 15, 2024 | By: | /s/ Frank Bedu-Addo |
Frank Bedu-Addo, Ph.D. | ||
President and Chief Executive Officer | ||
(Principal Executive Officer) | ||
May 15, 2024 | By: | /s/ Lars Boesgaard |
| Lars Boesgaard | ||
Chief Financial Officer | ||
(Principal Financial and Accounting Officer) | ||
33