With respect to CT-388, as of August 31, 2023, we wholly own three pending US patent applications, one pending international applications submitted under the Patent Cooperation Treaty, and one pending foreign patent applications in Taiwan, directed to composition of matter and methods of use. Any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2042 and 2044, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
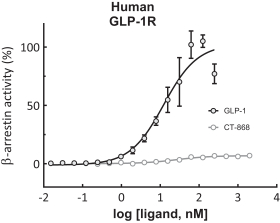
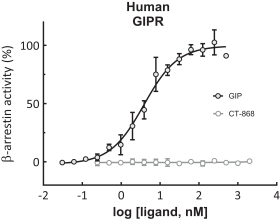
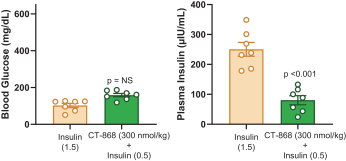
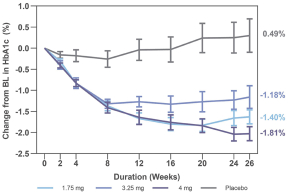
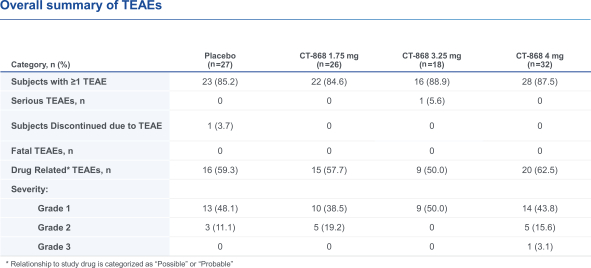
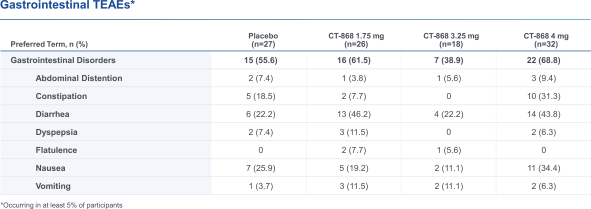
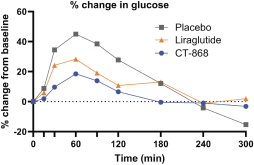
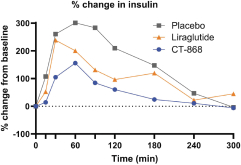
With respect to CT-868, as of August 31, 2023, we wholly own one issued US patent, five pending US patent applications, and 23 pending foreign patent applications in ARIPO, Argentina, Australia, Brazil, Canada, China, Eurasian, EPO, Hong Kong, Israel, India, Japan, Korea, Mexico, Malaysia, New Zealand, Philippines, Saudi Arabia, Singapore, South Africa, Taiwan, Ukraine, and United Arab Emirates, directed to composition of matter, methods of use, and methods of making. The patent and any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2039 and 2044, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
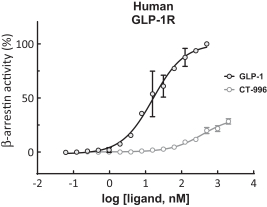
With respect to GLP-1R agonists, as of August 31, 2023, we wholly own two pending US patent applications and 31 pending foreign patent applications filed in various jurisdictions including UAE, Australia, Brazil, Canada, Chile, China, Columbia, Costa Rica, the Eurasian patent office, Ecuador, Egypt, European patent office, Georgia, Israel, India, Jordan, Japan, South Korea, Mexico, Malaysia, Nicaragua, New Zealand, Panama, Peru, Philippines, Saudi Arabia, Singapore, Taiwan, Thailand, Ukraine, and South Africa. We also have two pending international applications submitted under the Patent Cooperation Treaty. Our pending applications are directed to composition of matter GLP-1R agonists, including CT-996, and methods of use for treating a metabolic disease or condition. Any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2042 and 2043, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
Individual patents have terms of varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the earliest nonprovisional filing date. In the United States, a patent’s term may be lengthened by patent term adjustment (PTA), which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent. In addition, in certain instances, the patent term of a U.S. patent that covers an FDA-approved drug may also be eligible for extension to recapture a portion of the term effectively lost as a result of clinical trials and the FDA regulatory review period, such extension is referred to as patent term extension (PTE). The restoration period cannot be longer than five years and the total patent term, including the restoration period, must not exceed 14 years following FDA approval; however, there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug. The duration of patents outside of the United States varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest nonpriority filing date. The actual protection afforded by a patent varies on a product-by-product basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
143