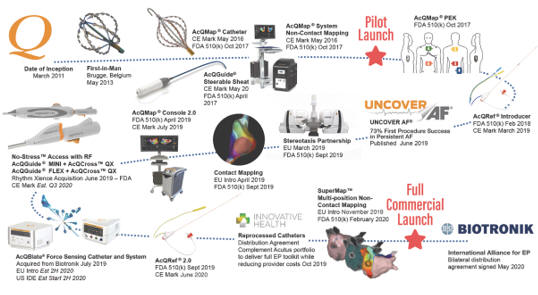
. Beginning in early March 2020, the
COVID-19
pandemic and the measures imposed to contain this pandemic disrupted and are expected to continue to impact our business. For example, on March 19, 2020, the Executive Department of the State of California issued Executive Order
ordering all individuals in the State of California to stay home or at their place of residence except as needed to maintain continuity of operations of the federal critical infrastructure sectors. Our primary operations are located in Carlsbad, California. As a result of such order, the majority of our employees have telecommuted, which may impact certain of our operations over the near term and long term. Moreover, beginning in March 2020, access to hospitals and other customer sites was restricted to essential personnel, which negatively impacted our ability to install our AcQMap consoles and workstations in new accounts and for our sales representatives and mappers to promote the use of our products with physicians. Moreover, hospitals and other therapeutic centers suspended many elective procedures, resulting in a significantly reduced volume of procedures using our products. In addition, all clinical trials in Europe were suspended with
follow-ups
for clinical trials done via telecom, and we believe enrollment timing in our planned clinical trials will be slowed due to
COVID-19
driven delayed access to enrollment sites. As a result of the interruptions to our business due to
COVID-19,
we enacted a cash conservation program, which included delaying certain
non-critical
capital expenditures and other projects and implementing a hiring freeze, headcount reductions and temporary compensation reductions (through August 2020). Although the effects of the pandemic began to decrease in late April 2020 as electrophysiology labs began reopening and procedure volumes began increasing as compared to
COVID-19
related low points in March 2020, the magnitude of the impact of the
COVID-19
pandemic on our productivity, results of operations and financial position, and its disruption to our business and our clinical programs and timelines, will depend, in part, on the length and severity of these restrictions and on our ability to conduct business in the ordinary course. Quarantines,
and similar government orders have also impacted, and may continue to impact, our third-party manufacturers and suppliers, and could in turn adversely impact the availability or cost of materials, which could disrupt our supply chain.