UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) | | | | | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the fiscal year ended | |
| December 31, 2023 | |
| or | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the transition period from __________ to __________ | |
Commission File Number: 001-35797 | | |
| Zoetis Inc. |
| (Exact name of registrant as specified in its charter) |
| | | | | | | | | | | | | | |
| Delaware | | 46-0696167 |
| (State or other jurisdiction of | | (I.R.S. Employer Identification No.) |
| incorporation or organization) | | |
| 10 Sylvan Way, | Parsippany, | New Jersey | | 07054 |
| (Address of principal executive offices) | | (Zip Code) |
(973)-822-7000
| | |
| (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | | ZTS | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | x | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No x
The aggregate market value of the voting stock held by nonaffiliates of the registrant as of June 30, 2023, the last business day of the registrant's most recently completed second fiscal quarter, was $79,346 million. The registrant has no non-voting common stock.
The number of shares outstanding of the registrant's common stock as of February 9, 2024 was 457,867,115 shares.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement for the 2024 Annual Meeting of Shareholders (hereinafter referred to as the “2024 Proxy Statement”) are incorporated into Part III of this Form 10-K.
TABLE OF CONTENTS | | | | | | | | | | | | | | |
| | | | Page |
| Item 1. | | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| Item 1A. | | | | |
| Item 1B. | | | | |
| Item 1C. | | | | |
| Item 2. | | | | |
| Item 3. | | | | |
| Item 4. | | | | |
| | | | |
| Item 5. | | | | |
| Item 7. | | | | |
| Item 7A. | | | | |
| Item 8. | | | | |
| Item 9. | | | | |
| Item 9A. | | | | |
| Item 9B. | | | | |
| Item 9C. | | | | |
| | | | |
| Item 10. | | | | |
| Item 11. | | | | |
| Item 12. | | | | |
| Item 13. | | | | |
| Item 14. | | | | |
| | | | |
| Item 15. | | | | |
| Item 16. | | | | |
| | |
| | |
PART I
Item 1. Business.
Overview
Zoetis Inc. is a global leader in the animal health industry, focused on the discovery, development, manufacture and commercialization of medicines, vaccines, diagnostic products and services, biodevices, genetic tests and precision animal health. We have a diversified business, commercializing products across eight core species: dogs, cats and horses (collectively, companion animals) and cattle, swine, poultry, fish and sheep (collectively, livestock); and within seven major product categories: parasiticides, vaccines, dermatology, other pharmaceutical, anti-infectives, animal health diagnostics and medicated feed additives. For over 70 years, we have been innovating ways to predict, prevent, detect, and treat animal illness, and continue to stand by those raising and caring for animals worldwide - from veterinarians and pet owners to livestock farmers and ranchers.
We were incorporated in Delaware in July 2012 and prior to that the company was a business unit of Pfizer Inc. (Pfizer). The address of our principal executive offices is 10 Sylvan Way, Parsippany, New Jersey 07054. Unless the context requires otherwise, references to “Zoetis,” “the company,” “we,” “us” or “our” in this Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (2023 Annual Report) refer to Zoetis Inc., a Delaware corporation, and its subsidiaries. In addition, unless the context requires otherwise, references to “Pfizer” in this 2023 Annual Report refer to Pfizer Inc., a Delaware corporation, and its subsidiaries.
Operating Segments
The animal health medicines, vaccines and diagnostics market is characterized by meaningful differences in customer needs across different regions. This is due to a variety of factors, including:
•economic differences, such as standards of living in developed markets as compared to emerging markets;
•cultural differences, such as dietary preferences for different animal proteins, pet ownership preferences and pet care standards;
•epidemiological differences, such as the prevalence of certain bacterial and viral strains and disease dynamics;
•treatment differences, such as utilization of different types of medicines and vaccines, as well as the pace of adoption of new technologies;
•environmental differences, such as seasonality, climate and the availability of arable land and fresh water; and
•regulatory differences, such as standards for product approval and manufacturing.
As a result of these differences, among other things, we organize and operate our business in two segments:
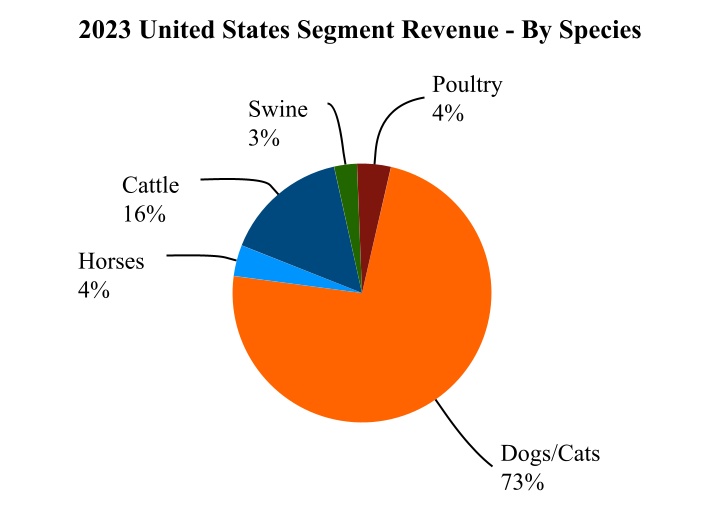
•United States (U.S.) with revenue of $4,555 million, or 53% of total revenue for the year ended December 31, 2023; and
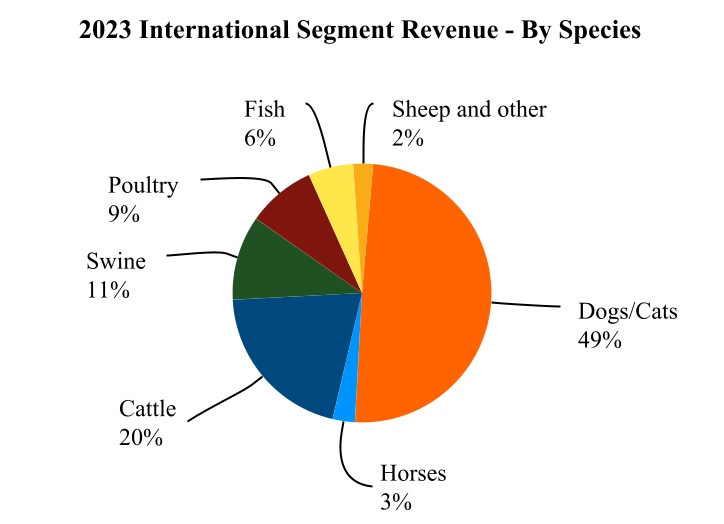
•International with revenue of $3,911 million, or 46% of total revenue for the year ended December 31, 2023.
Within each of these operating segments, we offer a diversified product portfolio for both companion animal and livestock customers so that we can capitalize on local trends and customer needs.
In addition, our Client Supply Services (CSS) organization which provides contract manufacturing services to third parties, and our human health products, together represented approximately 1% of our total revenue for the year ended December 31, 2023.
Our 2023 revenue for the U.S. and key international markets, together with the percentage of revenue attributable to companion animal and livestock products in those markets, is as follows: | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | Revenue | Companion Animal | Livestock |
| United States | $4,555 | 77% | 23% |
| Total International | $3,911 | 52% | 48% |
| Australia | $323 | 50% | 50% |
| Brazil | $393 | 38% | 62% |
| Canada | $255 | 62% | 38% |
| Chile | $140 | 17% | 83% |
| China | $320 | 63% | 37% |
| France | $142 | 62% | 38% |
| Germany | $202 | 77% | 23% |
| Italy | $121 | 77% | 23% |
| Japan | $158 | 70% | 30% |
| Mexico | $162 | 34% | 66% |
| Spain | $122 | 62% | 38% |
| United Kingdom | $277 | 76% | 24% |
| Other Developed | $512 | 50% | 50% |
| Other Emerging | $784 | 39% | 61% |
For additional information regarding our performance in each of our operating segments and the impact of foreign exchange rates, see Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations and Item 8. Financial Statements and Supplementary Data: Notes to Consolidated Financial Statements—Note 4. Revenue and Note 19. Segment Information. Our 2023 reported revenue for each segment, by species, is as follows:
Products
Over the course of our history, we have focused on developing a diverse portfolio of animal health products that deliver solutions across the continuum of care. We refer to all different brands of a particular product, or its dosage forms for all species, as a product line. We have approximately 300 comprehensive product lines, including products for both companion animals and livestock within each of our major product categories.
Our companion animal products help extend and improve the quality of life for pets; increase convenience and compliance for pet owners; and help veterinarians improve the quality of their care and the efficiency of their businesses. Growth in the companion animal medicines, vaccines and diagnostics sectors is driven by economic development; related increases in disposable income; and increases in pet ownership and spending on pet care. Companion animals are also living longer, deepening the human-animal bond, receiving increased medical treatment and benefiting from advances in animal health medicines, vaccines and diagnostics. Companion animal products represented approximately 65% of our revenue for the year ended December 31, 2023.
Our livestock products focus on predicting, preventing, detecting and treating diseases, with the understanding that veterinarians and farmers must have the tools at their disposal to treat disease expeditiously, which in turn enables the sustainable production of safe, high-quality animal protein. Human population growth and increased standards of living continue to drive demand for increased production of quality animal protein. Population growth leads to greater consumption of natural resources, driving a need for innovative solutions to increase sustainability and productivity. As global food security takes on heightened importance, producers are tasked with facilitating a high quality, safe and reliable food supply. Livestock products represented approximately 34% of our revenue for the year ended December 31, 2023.
In addition, our CSS organization, which provides contract manufacturing services to third parties, and our human health products, together represented approximately 1% of our total revenue for the year ended December 31, 2023.
Our major product categories are:
•parasiticides: products that prevent or eliminate external and internal parasites such as fleas, ticks and worms;
•vaccines: biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response;
•dermatology: products that relieve itch associated with allergic conditions and atopic dermatitis;
•other pharmaceutical: pain and sedation, antiemetic, reproductive, and oncology products;
•anti-infectives: products that kill or slow the growth of bacteria, fungi or protozoa;
•animal health diagnostics: testing and analysis of blood, urine and other animal samples and related products and services, including point-of-care diagnostic products, instruments and reagents, rapid immunoassay tests, reference laboratory kits and services and blood glucose monitors; and
•medicated feed additives: products added to animal feed that provide medicines to livestock.
Our remaining revenue is derived from other non-pharmaceutical product categories, such as nutritionals, as well as products and services in biodevices, genetic tests and precision animal health.
As part of our growth strategy, we focus on the discovery and development of new chemical, biopharmaceutical and biological entities, as well as product lifecycle innovation, primarily through our research and development (R&D) group. Historically, a substantial portion of our products and revenue has been the result of product lifecycle innovation where we actively work to broaden the value of existing products by developing claims in additional species, more convenient formulations and combinations, and by expanding usage into more countries. For example, the first product in our ceftiofur line was an anti-infective approved for treating bovine respiratory disease (BRD) in cattle that was administered via intramuscular injection. Through follow-on studies and reformulations, we have expanded the product line into additional cattle claims and administration routes, as well as other species and regions. The ceftiofur product line currently includes the brands Excede®, Excenel®, Naxcel® and Spectramast®.
The following are examples of our first-in-class and/or best-in-class products that we have launched in recent years and products that we believe may represent platforms for future product lifecycle innovation (listed alphabetically):
•Apoquel®, the first Janus kinase inhibitor for use in veterinary medicine, was approved in 2013 for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs at least 12 months of age. Since January 2014, we have launched Apoquel in many key markets globally. In 2021, a chewable version of Apoquel was approved in the European Union (EU) and the United Kingdom (U.K.), and has since been approved in other key markets globally, including the U.S. in 2023;
•Core EQ Innovator®, the first and only vaccine for horses to contain all five core equine disease antigens - West Nile, Eastern and Western Equine encephalomyelitis, tetanus and rabies - in one combination, was approved in the U.S. in 2018 and in Canada in 2019;
•Cytopoint®, the first canine monoclonal antibody (mAb) to help reduce the clinical signs of atopic dermatitis (such as itching) in dogs of any age, was licensed in the U.S. in 2016 (and was later granted an expanded indication to treat allergic dermatitis in 2018). The product has since been approved in many key markets globally. An injection given once every four to eight weeks, Cytopoint neutralizes interleukin-31, a protein that has been demonstrated to trigger itching in dogs;
•Fostera® PCV MH was introduced in November 2013 in the U.S. and has since been approved in many key markets globally. It was developed to help protect pigs from porcine circovirus-associated disease (PCVAD) and enzootic pneumonia caused by M. hyopneumoniae (M. hyo). Fostera Gold PCV MH, the only vaccine to contain two PCV2 genotypes and long-lasting M. hyo coverage, was approved in the U.S. and Canada in 2018 and has since been approved in many key markets globally. The Fostera franchise also includes Fostera/Suvaxyn® PRRS, which was approved in the U.S. in 2015 and has since been approved in many key markets globally. This vaccine offers protection against both the respiratory and reproductive forms of disease caused by porcine reproductive and respiratory syndrome (PRRS) virus;
•Librela™ (bedinvetmab), the first and only injectable mAb therapy for monthly alleviation of osteoarthritis (OA) pain in dogs, was approved in the EU in 2020 and has since been approved in other key markets globally, including the U.S. in 2023;
•Poulvac® Procerta® HVT-ND, our first vector vaccine that helps protect against Marek’s disease and Newcastle disease, highly contagious viral infections affecting poultry, was approved in the U.S. in 2019. In 2020, we expanded our line of recombinant vector vaccines with the launch of Poulvac Procerta HVT-IBD, which helps protect against Marek's disease and provides early protection against the contemporary infectious bursal disease (IBD) viruses. We have since expanded Poulvac Procerta HVT-ND into other key markets globally. In 2022, we further expanded our line of recombinant vector vaccines with the launch of Poulvac Procerta HVT-IBD-ND in the U.S., which is an advanced trivalent vector vaccine that delivers powerful early protection against Marek's disease, infectious bursal disease and Newcastle disease in one dose;
•ProHeart® 12 (moxidectin), a once-yearly injection to prevent heartworm disease in dogs 12 months of age and older, was approved in the U.S. in 2019;
•Protivity®, a modified-live bacterial vaccine that is effective in protecting healthy beef and dairy cattle against respiratory disease caused by Mycoplasma bovis (M. bovis), was approved in the U.S. and Canada in 2022 and in the EU and Mexico in 2023;
•Simparica® (sarolaner) Chewables, a monthly chewable tablet for dogs to control fleas and ticks, was approved in the EU in 2015 and has since been approved in other key markets globally, including the U.S. Simparica Trio®, a triple combination parasiticide for dogs, was approved in the EU and Canada in 2019 and has since been approved in other key markets globally, including the U.S., and received approval in 2023 in other key markets globally for new claims related to the prevention of eyeworms, Otodectes cynotis, flea tapeworms and efficacy against sarcoptic and demodectic manges. This product is a key internal lifecycle innovation that combines flea and tick treatment (sarolaner) with the prevention of heartworm disease and treatment of gastrointestinal parasites;
•Solensia® (frunevetmab), the first injectable mAb therapy for monthly alleviation of OA pain in cats, was approved in Switzerland in 2020 and has since been approved in other key markets globally, including the EU and the U.S.;
•Stronghold Plus® (selamectin/sarolaner), also referred to as Revolution® Plus in certain jurisdictions, a topical combination product that treats ticks, fleas, ear mites, lice and gastrointestinal worms and prevents heartworm disease in cats, received EU approval in 2017 and has since been approved in other key markets globally, including the U.S.; and
•Vanguard®/Versican® is a market leading vaccine line for dogs intended to help prevent a range of diseases. Since 2016, Zoetis has added new and innovative enhancements to its Vanguard line in the U.S. with Vanguard crLyme, Vanguard Rapid Resp Intranasal, Vanguard B Oral, Vanguard CIV H3N2/H3N8 and Versican Plus Bb Oral.
We pursue the development of new vaccines for emerging infectious diseases, with an operating philosophy of “first to know and fast to market.” Examples of the successful execution of this strategy include the first SARS-CoV-2 (COVID-19) vaccine to help protect the health and well-being of more than 300 mammalian species living in zoos, aquariums, conservatories and other animal organizations around the world; the first equine vaccine for West Nile virus in the U.S. and EU; the first swine vaccine for pandemic H1N1 influenza virus in the U.S.; the first licensed vaccine against the pandemic H5N1 bird flu in the U.S. and EU, which we provided to the U.S. Department of Agriculture when it recommended our vaccine be used by the U.S. Fish and Wildlife Service to help protect California condors; a conditionally licensed vaccine to help fight porcine epidemic diarrhea virus (PEDv) in the U.S.; and the first conditionally licensed vaccine to help prevent the H3N2 type of canine influenza that emerged in the U.S. In 2019, Zoetis established a research facility with Texas A&M University to develop vaccines for transboundary and emerging diseases in animals, including Foot-and-Mouth Disease (FMD), a virus that can cause serious illness in cattle, pigs, and sheep. In 2020, the company opened a research lab at Colorado State University in a partnership to increase our understanding of the potential use of immunomodulators in livestock that could reduce the need for antibiotics, as well as advance our understanding of the biology of key diseases affecting companion animals which could lead to new therapies that can treat chronic health conditions in pets.
Additionally, the Pharmaq business of Zoetis is the global leader in vaccines and innovation for aquatic health products, with its leading Alpha Ject® vaccine line, Alpha Flux®, a parasiticide that helps salmon farmers in Chile control sea lice infestations, one of the major challenges in the aquatic health industry and Alpha ERM Salar, a water-based injectable vaccine that helps protect salmon from red mouth, a common bacterial infection.
Zoetis enhanced the portfolio of its diagnostic products with the acquisition in 2018 of Abaxis, Inc. (Abaxis), a leading provider of veterinary point-of-care diagnostic instruments. With this acquisition came the VetScan® portfolio of benchtop and handheld diagnostic instruments and consumables, which serves a large customer base of veterinary practices both in the U.S. and international markets. In 2019, the company acquired Phoenix Central Laboratory for Veterinarians, Inc. and ZNLabs, LLC marking its entry into reference laboratory services and building on a strategy to develop a more comprehensive diagnostics offering with enhanced value for veterinarians. In 2020, the company acquired a third veterinary reference lab business, Ethos Diagnostic Science. The Zoetis diagnostic portfolio also includes the Witness®, Serelisa® and ProFlok® lines of immunodiagnostic kits, which provide disease detection capabilities for various species, including dogs, cats, cattle, swine and poultry. In 2020, the company launched Vetscan Imagyst®, which uses a combination of image recognition technology, algorithms and cloud-based artificial intelligence (AI) to deliver rapid testing results to veterinary clinics. In 2021, the company added digital cytology testing to the Vetscan Imagyst platform and in 2022 the company added AI blood smear testing to the Vetscan Imagyst platform. In 2023, the company added AI dermatology and AI fecal for equine to the Vetscan Imagyst platform. As Zoetis continues to develop additional innovative applications for Vetscan Imagyst, it plans to seamlessly integrate even more new capabilities into the platform, helping veterinarians provide the best possible care for animals. Also in 2023, the company added Vetscan Mastigram+ for rapid, on-farm mastitis diagnosis for dairy cows.
Zoetis also entered the field of animal nutritionals with the acquisition of Platinum Performance in 2019. The acquisition brings us premium nutritional product formulas and a unique approach to the field of scientific wellness for horses, dogs and cats.
In 2022, the company completed the acquisition of Jurox, an animal health company based in Australia, which brings the company a range of companion animal and livestock products and provides the company with future growth opportunities, manufacturing capacity and increased capabilities in Australia. Also in 2022, the company acquired Basepaws, a petcare genetics company that provides pet owners with genetic tests, analytics and early health risk assessments, which help pet owners and veterinarians understand an individual pet’s risk for disease and helps Zoetis identify solutions to complex diseases by informing our research and innovation.
In 2023, the company completed the acquisition of PetMedix Ltd, a privately held research and development stage animal health biopharmaceutical company based in the U.K., which develops antibody-based therapeutics for companion animals. Also in 2023, the company completed the acquisition of adivo GmbH, a privately held research and development stage animal health biopharmaceutical company based in Germany.
In 2023, our two top-selling products and product lines, Simparica/Simparica Trio and Apoquel/Apoquel Chewable, contributed approximately 13% and 10%, respectively, of our revenue. Combined with our next three top-selling products and product lines, Cytopoint, Revolution/Revolution Plus/Stronghold and ceftiofur line, these five products and product lines contributed approximately 37% of our revenue. In 2023, our ten top-selling products and product lines contributed approximately 49% of our revenue. In 2023, we had 15 products and product lines with revenues of $100 million or more.
Our products and product lines that represented approximately 1% or more of our revenue in 2023, which comprise approximately 62% of our total revenue, are as follows (listed alphabetically by product category):
Companion animal products | | | | | | | | | | | | | | |
| | | | |
| Product / product line | | Description | | Primary species |
| | | | |
| Vaccines | | | | |
Vanguard® L4 (4-way Lepto) / Vanguard® line | | Helps protect against leptospirosis caused by Leptospira canicola, L. grippotyphosa, L. icterohaemorrhagiae and L. pomona. Aids in preventing canine distemper caused by canine distemper virus; infectious canine hepatitis caused by canine adenovirus type 1; respiratory disease caused by canine adenovirus type 2; canine parainfluenza caused by canine parainfluenza virus; canine parvoviral enteritis caused by canine parvovirus; Lyme disease and subclinical arthritis associated with Borrelia burgdorferi, the causative agent of Lyme disease. Vanguard Rapid Resp is a group of three vaccines combating infections in dogs caused by Bordetella bronchiseptica, canine parainfluenza and canine adenovirus; canine influenza vaccines; and an oral vaccine for Bordatella bronchiseptica | | Dogs |
| | | | |
| Anti-infectives | | | | |
Clavamox® / Synulox® | | A broad-spectrum antibiotic and the first potentiated penicillin approved for use in dogs and cats | | Cats, dogs |
Convenia® | | Anti-infective for the treatment of common bacterial skin infections that provides a course of treatment in a single injection | | Cats, dogs |
| | | | |
| Parasiticides | | | | |
ProHeart® | | Prevents heartworm infestation; also for treatment of existing larval and adult hookworm infections | | Dogs |
Revolution® / Revolution® Plus / Stronghold® line | | An antiparasitic for protection against fleas, heartworm disease and ear mites in cats and dogs; sarcoptic mites and American dog tick in dogs and roundworms and hookworms for cats | | Cats, dogs |
Simparica®/ Simparica Trio® | | A monthly chewable tablet for dogs to control fleas and ticks; Simparica Trio, also a monthly chewable tablet, is a triple combination parasiticide that delivers all-in-one protection from fleas and ticks, as well as heartworm disease, roundworms and hookworms | | Dogs |
| | |
| Other Pharmaceutical | | |
Cerenia®
| | A medication that prevents and treats acute vomiting in dogs, treats acute vomiting in cats and prevents vomiting due to motion sickness in dogs | | Cats, dogs |
LibrelaTM | | An injectable monthly antibody therapy to alleviate osteoarthritis pain in dogs | | Dogs |
Rimadyl® | | For the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries | | Dogs |
Solensia® | | An injectable monthly antibody therapy to alleviate osteoarthritis pain in cats | | Cats |
| | | | |
| Dermatology | | | | |
Apoquel® / Apoquel® Chewable | | A selective inhibitor of the Janus Kinase 1 enzyme that controls pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age | | Dogs |
Cytopoint® | | An injectable to help reduce the clinical signs such as itching of atopic dermatitis in dogs of any age | | Dogs |
| | |
| Animal Health Diagnostics | | | | |
VetScan® | | A portfolio of benchtop and handheld diagnostic instruments, rapid tests and associated consumables | | Cats, dogs |
Livestock products | | | | | | | | | | | | | | |
| | | | |
| Product / product line | | Description | | Primary species |
| | | | |
| Vaccines | | | | |
Improvac® / Improvest® / Vivax® | | Reduces boar taint, as an alternative to surgical castration and suppression of estrus in gilts | | Swine |
Rispoval® / Bovishield® line | | Aids in preventing three key viruses involved with pneumonia in cattle (BRSV, PI3 virus and BVD), as well as other respiratory diseases, depending on formulation | | Cattle |
Suvaxyn® / Fostera® | | Aids in preventing or controlling diseases associated with major pig pathogens such as porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSv) and Mycoplasma hyopneumoniae (M. hyo), depending on formulation | | Swine |
| | | | |
| Anti-infectives | | | | |
| Ceftiofur injectable line | | Broad-spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria, including ß-lactamase-producing strains, with some formulations producing a single course of therapy in one injection | | Cattle, sheep, swine |
Draxxin® / Draxxin KP | | Single-dose low-volume antibiotic for the treatment and prevention of bovine and swine respiratory disease, infectious bovine keratoconjunctivitis and bovine foot rot. This franchise also includes Draxxin KP/Draxxin Plus, an injectable for the treatment of bovine respiratory disease that combines the antimicrobial properties of Draxxin with the anti-inflammatory, analgesic and antipyretic properties of the non-steroidal Ketoprofen to rapidly reduce fever in a single dose | | Cattle, sheep, swine |
Spectramast® | | Treatment of subclinical or clinical mastitis in dry or lactating dairy cattle, delivered via intramammary infusion; same active ingredient as the ceftiofur line | | Cattle |
| | | | |
| Parasiticides | | | | |
Dectomax® | | Injectable or pour-on endectocide, characterized by extended duration of activity, for the treatment and control of internal and external parasite infections | | Cattle, swine |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | |
| | | | |
| | | | |
International Operations
We directly market our products in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America, and our products are sold in more than 100 countries. Operations outside the U.S. accounted for 46% of our total revenue for the year ended December 31, 2023. Through our efforts to establish an early and direct presence in many emerging markets, such as Brazil, Chile, China and Mexico, emerging markets contributed 21% of our total revenue for the year ended December 31, 2023.
Our international businesses are subject, in varying degrees, to a number of risks inherent in carrying on business in other countries. These include, among other things, currency fluctuations, capital and exchange control regulations, expropriation and other restrictive government actions. See Item 1A. Risk Factors— Risks related to operating in foreign jurisdictions.
Sales and Marketing
Our sales organization includes sales representatives and technical and veterinary operations specialists. We also contract with distributors that provide logistics and sales and marketing support for many of our products. In regions where we do not maintain a direct commercial presence, we rely solely on distributors for these services.
Our sales representatives visit our customers, including veterinarians and livestock producers, to provide information and to promote and sell our products and services. Our technical and veterinary operations specialists, who generally have advanced veterinary medicine degrees, provide scientific consulting focused on disease management and herd management, training and education on diverse topics, including responsible product use. These direct relationships with customers allow us to understand the needs of our customers. Additionally, our sales representatives and technical and veterinary operations specialists partner with customers to provide training and support in areas of disease awareness and treatment protocols, including the use of our products. As a result of these relationships, our sales and consulting visits are typically longer, more meaningful and provide us with better access to customer decision makers as compared to those in human health. In certain markets, including the U.S., pet owners are taking a more active role in product purchasing decisions, and as a result we are increasingly investing in direct-to-consumer marketing efforts. As of December 31, 2023, our sales organization consisted of approximately 4,100 employees.
Our companion animal and livestock products are primarily available by prescription through a veterinarian. On a more limited basis, in certain markets, we sell certain products through retail and e-commerce outlets. We also market our products by advertising to veterinarians, livestock producers and pet owners.
Customers
We primarily sell our companion animal products to veterinarians or to third-party veterinary distributors that typically then sell our products to veterinarians, and in each case veterinarians then typically sell our products to pet owners. In certain markets, we also sell certain companion animal products through retail and e-commerce outlets. We sell our livestock products primarily to veterinarians and livestock producers, including beef and dairy farmers as well as pork and poultry operators, in addition to third-party veterinary distributors and retail outlets who then typically sell the products to livestock producers. Sales to our largest customer, a U.S. veterinary distributor, represented approximately 15% of total revenue for 2023.
Research and Development
Our R&D operations are comprised of a dedicated veterinary medicine R&D organization, external alliances and other operations focused on the development, registration and regulatory maintenance of our products. In addition, we have R&D operations focused on diagnostics, devices, data, digital and other technological innovation. We incurred R&D expenses of $614 million in 2023, $539 million in 2022 and $508 million in 2021.
Our R&D efforts are comprised of more than 300 programs and reflect our commitment to develop solutions for unmet needs and advance the current standards of care. We create new insights for predicting, preventing, detecting and treating health conditions that result in the development of new platforms of knowledge which become the basis for continuous innovation. Leveraging internal discoveries, complemented by diverse external research collaborations, results in the delivery of novel vaccine, pharmaceutical, biopharmaceutical, biodevice and diagnostic products and services to help our customers face their toughest challenges. While the development of new chemical, biopharmaceutical and biological entities through new product R&D plays a critical role in our growth strategies, a significant share of our R&D investment (including regulatory functions) is focused on product lifecycle innovation. A commitment to continuous innovation, based on customer need, ensures we actively work to broaden the value of existing products by developing claims in additional species, more convenient formulations, routes of administration and combinations, and by expanding usage into more countries. We also create opportunities by integrating product offerings to optimize solutions based on the totality of customer need.
We prioritize our R&D spending on an annual basis with the goal of aligning our research and business objectives, and do not disaggregate our R&D operations by research stage or by therapeutic area for purposes of managing our business. We make our strategic investments in R&D based on four criteria: strategic fit and importance to our current portfolio; technical feasibility of development and manufacture; return on investment; and the needs of customers and the market. A centralized portfolio management function links development plans with financial systems to build a comprehensive view of the status of project progression and spend. This view facilitates our ability to set targets for project timing and goals for investment efficiency. The allocation of our R&D investment between product lifecycle innovation and new product development, in addition to our ability to leverage the discoveries of our existing R&D and other industries, supports a cost-effective, efficient, sustainable and relatively predictable R&D process.
We regularly enter into agreements with external parties that enable us to collaborate on research programs or gain access to substrates and technologies (such as new devices). Some of our external partnerships involve funding from a non-governmental organization or a government grant. We are generally responsible for providing technical direction and supplemental expertise for, as well as investment in, such external partnerships. Depending on the nature of the agreement, we may act as the commercialization partner for discoveries that originate during the period of collaborative research, or we may own or have exclusive rights to any intellectual property that enables the development of proprietary products or models.
As of December 31, 2023, we employed approximately 1,600 employees in our global R&D operations. Our R&D headquarters is located in Kalamazoo, Michigan. We have R&D operations co-located with manufacturing sites in Rutherford, Australia; Louvain-la-Neuve, Belgium; Campinas, Brazil; Suzhou, China; Farum, Denmark; Olot, Spain; and in the following U.S. locations: Union City, California; Charles City, Iowa; Kalamazoo, Michigan; Durham, North Carolina; and Lincoln, Nebraska. We co-locate R&D operations with manufacturing sites to facilitate the efficient transfer of production processes from our laboratories to manufacturing. In addition, we maintain R&D operations in Sydney, Australia; Zaventem, Belgium; São Paulo, Brazil; Beijing, China; Puchheim, Germany; Thane, India; Oslo, Norway; Hong Ngu, Vietnam; Con Tho, Vietnam; Cambridge, U.K.; Torrance, California, Fort Collins, Colorado and College Station, Texas, U.S. Each site is designed to meet the regulatory requirements for working with chemical or infectious disease agents, as appropriate.
Manufacturing and Supply Chain
Our products are manufactured at sites operated by us and sites operated by third-party contract manufacturing organizations, which we refer to as CMOs. We have a global manufacturing network of 29 sites operated by us.
Our global manufacturing network is comprised of the following sites: | | | | | | | | | | | | | | | | | | | | |
| Site | | Location | | Site | | Location |
| Buellton | | California, U.S. | | Overhalla | | Norway |
| Campinas | | Brazil | | Rathdrum | | Ireland |
| Catania | | Italy | | Rutherford | | Australia |
| Charles City | | Iowa, U.S. | | Salisbury | | Maryland, U.S. |
| Chicago Heights | | Illinois, U.S. | | San Diego | | California, U.S. |
| Durham | | North Carolina, U.S. | | Suzhou (Bio) | | China |
| Eagle Grove | | Iowa, U.S. | | Suzhou (MFA) | | China |
| Farum | | Denmark | | Tallaght | | Ireland |
| Kalamazoo | | Michigan, U.S. | | Tullamore | | Ireland |
| Klofta | | Norway | | Union City | | California, U.S. |
| Lincoln | | Nebraska, U.S. | | Weibern | | Austria |
| Louvain-la-Neuve | | Belgium | | Wellington | | New Zealand |
| Medolla | | Italy | | White Hall | | Illinois, U.S. |
| Melbourne | | Australia | | Willow Island | | West Virginia, U.S. |
| Olot | | Spain | | | | |
We own the majority of these sites, with the exception of our facilities in Buellton, California (U.S.), Durham, North Carolina (U.S.), Klofta (Norway), Medolla (Italy), Melbourne (Australia), San Diego, California (U.S.), Union City, California (U.S.), Weibern (Austria) and Willow Island, West Virginia (U.S.), which are leased sites. In addition, a portion of our facilities in Tullamore (Ireland) are leased.
We regularly evaluate the adequacy of our manufacturing capabilities. We are currently in the process of expanding these capabilities at certain existing sites. Additionally, the company purchased a manufacturing site outside Atlanta, Georgia in 2023, where we plan to begin commercial production in the future.
Our global manufacturing and supply chain is supported by a network of CMOs. As of December 31, 2023, this network was comprised of 109 CMOs, including those centrally-managed as well as locally-managed CMOs.
We select CMOs based on several factors: (i) their ability to reliably supply products or materials that meet our quality standards at an optimized cost; (ii) their access to niche products and technologies; (iii) capacity; and (iv) financial efficiency analyses. Our regional and global manufacturing teams seek to ensure that all of the CMOs we use adhere to our standards of manufacturing quality.
We purchase certain raw materials necessary for the commercial production of our products from a variety of third-party suppliers. We utilize logistics service providers as a part of our global supply chain, primarily for shipping and logistics support.
We intend to continue our efficiency improvement programs in our manufacturing and supply chain organization, including Six Sigma and Lean capabilities, which are processes intended to improve manufacturing efficiency. We have strong globally managed and coordinated quality control and quality assurance programs in place at our global manufacturing network sites, and we regularly inspect and audit our global manufacturing network and CMO sites.
Competition
Although our business is the largest based on revenue in the animal health industry (which includes medicines, vaccines and diagnostics), we face competition in the regions in which we compete. Principal drivers of competition vary depending on the particular region, species, product category and individual product, and include new product development, quality, price, service and promotion to veterinary professionals, pet owners and livestock producers.
Our primary competitors include animal health medicines, vaccines and diagnostic companies such as Boehringer Ingelheim Animal Health Inc., the animal health division of Boehringer Ingelheim GmbH; Merck Animal Health, the animal health division of Merck & Co., Inc.; Elanco Animal Health; and IDEXX Laboratories. There are also several new start-up companies working in the animal health area. In addition, we compete with hundreds of other producers of animal health products throughout the world.
The level of competition from generic products varies from market to market. Unlike in the human health market, there is no large, well-capitalized company focused on generic animal health products that exists as a global competitor in the industry. Historically, the reasons for this include the relatively smaller average market size of each product opportunity, the importance of direct distribution and education to veterinarians and livestock producers and the primarily self-pay nature of the business. In addition, companion animal health products are often directly prescribed and dispensed by veterinarians. Nonetheless, we continue to face increased competition from generic products and lower cost alternatives. For more information regarding the generic competition we have and expect to encounter as patents on certain of our key products expire, see Item 1. Business - Intellectual Property.
The importance of quality and safety concerns to pet owners, veterinarians and livestock producers also contributes to animal health brand loyalty. As a result, we believe that significant brand loyalty to products often continues after the loss of patent-based and regulatory exclusivity.
Intellectual Property
Our technology, brands and other intellectual property are important elements of our business. We rely on patent, trademark, copyright, design and trade secret laws, as well as regulatory exclusivity periods and non-disclosure agreements to protect our intellectual property rights. Our policy is to vigorously protect, enforce and defend our rights to our intellectual property, as appropriate.
Our product portfolio enjoys the protection of approximately 5,880 granted patents and 1,490 pending patent applications, filed in more than 50 countries, with a focus on our major markets, including Australia, Brazil, Canada, China, Europe, Japan and the U.S., as well as other countries with strong patent systems. Many of the patents and patent applications in our portfolio are the result of our in-house research and development, while other patents and patent applications in our portfolio were wholly or partially developed by third parties and are licensed to Zoetis.
Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the countries where such patents are obtained. Below is a summary of our recent and upcoming key patent expirations.
•Patents relating to the active ingredient (tulathromycin) and formulation of Draxxin have expired in the majority of markets. Exceptions are the formulation patent that expires in November 2025 in Japan, and formulation and active ingredient patents in Brazil which expire in July and December 2025, respectively. Generic or other competing tulathromycin products are now marketed in most major markets as well as in many smaller markets. Additional marketing authorizations for generic tulathromycin products may be granted in various markets in the future. Sales of Draxxin have been negatively affected by generic competition in the markets where the patents have expired.
•All patents relating to the active ingredient of Excede/Naxcel (ceftiofur crystalline free acid) have expired. The patents covering the commercial formulation of Excede in the U.S., Japan and Brazil extend to July 2024, September 2026 and August 2027, respectively, but the corresponding patents in Europe, Canada and Australia expired in September 2021. The commercial method of administration patent relevant to the product line expired in March 2023 in the U.S., Europe and Australia, and will expire in March 2028 in Japan. Generic versions of Excede have entered the market in Brazil, Mexico and Russia. At this time, the market entry of a generic version of Excede in the U.S. is not anticipated before July 2024.
•All patents relating to Revolution/Stronghold containing selamectin as the sole active ingredient have expired. Generic versions of selamectin are now sold in markets including the U.S., Europe, Australia and Canada. Selamectin is one of the active ingredients in our combination parasiticide product, Revolution Plus/Stronghold Plus, which is separately patent protected.
•The patent for the active ingredient of Convenia (cefovecin sodium) has expired in all countries, the patents covering the commercial formulation expired in all countries in November 2022, except for the U.S., which expired in October 2023 and Brazil which will expire in November 2025.
•The patent for the active ingredient of Cerenia has expired in all countries and the formulation patents relevant to the injectable product line expire between 2025 and 2028. Generic versions of Cerenia injectable have been registered and marketed in Europe, Canada and Australia and we are aware that regulatory approval of at least one generic version of Cerenia injectable is currently being pursued in the U.S.
Zoetis typically enforces its patents vigorously as appropriate globally, including by filing infringement claims against other parties.
Additionally, many of our vaccine products are based on proprietary master seeds and proprietary or patented adjuvant formulations. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by seeking to require our employees, consultants, advisors and partners to enter into confidentiality agreements and other arrangements upon the commencement of their employment or engagement.
We seek to file and maintain trademarks around the world based on commercial activities in most regions where we have, or desire to have, a business presence for a particular product or service. We currently maintain more than 10,500 trademark applications and registrations in our market countries, identifying products and services dedicated to the care of companion animals and livestock.
Regulatory
The sale of animal health products is governed by the laws and regulations specific to each country in which we market our products. To maintain compliance with these regulatory requirements, we have established processes, systems, and dedicated resources with end-to-end involvement from product concept to launch and maintenance in the market. Our regulatory function actively engages in dialogue with various global agencies regarding their policies that relate to animal health products.
United States
United States Food and Drug Administration (FDA). The regulatory body that is responsible for the regulation of animal health pharmaceuticals in the U.S. is the Center for Veterinary Medicine (CVM), housed within the FDA. All manufacturers of animal health pharmaceuticals must show their products to be safe, effective and produced by a consistent method of manufacture as defined under the Federal Food, Drug and Cosmetic Act. The FDA's basis for approving a drug application is documented in a Freedom of Information Summary. Post-approval monitoring of products is required by law, with reports being provided to CVM's Office of Surveillance and Compliance. Reports of product quality defects, adverse events or unexpected results are submitted in accordance with the legal and agency requirements.
As a result of our acquisition of Abaxis, our product portfolio includes human medical devices, which are subject to regulation in the U.S. by the FDA under the Federal Food, Drug, and Cosmetic Act, including the 1976 Medical Device Amendments and the Quality System Regulation, and the Clinical Laboratory Improvement Amendments of 1988, and by the Department of Health and Human Services Office for Civil Rights under the Health Insurance Portability and Accountability Act of 1996. Post-market surveillance is required, with reports provided to the FDA in accordance with agency requirements.
United States Department of Agriculture (USDA). The regulatory body in the U.S. for veterinary vaccines is the USDA. The USDA's Center for Veterinary Biologics is responsible for the regulation of animal health vaccines, including certain immunotherapeutics and certain diagnostic products. All manufacturers of animal health biologicals must show their products to be pure, safe, effective and produced by a consistent method of
manufacture as defined under the Virus Serum Toxin Act. Post-approval monitoring of products is required. Reports of product quality defects, adverse events or unexpected results are submitted in accordance with the agency requirements.
Environmental Protection Agency (EPA). The main regulatory body in the U.S. for veterinary pesticides is the EPA. The EPA's Office of Pesticide Programs is responsible for the regulation of pesticide products applied to animals. All manufacturers of animal health pesticides must show their products, when used according to specifications, will not cause “unreasonable adverse effects to man or the environment” as stated in the Federal Insecticide, Fungicide, and Rodenticide Act. Within the U.S., pesticide products that are approved by the EPA must also be approved by individual state pesticide authorities before distribution in that state. Post-approval monitoring of products is required, with reports provided to the EPA and some state regulatory agencies.
Foreign Trade Controls. In addition, the U.S. Foreign Corrupt Practices Act (FCPA) prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Zoetis is also subject to foreign trade controls administered by certain U.S. government agencies, including the Bureau of Industry and Security within the Commerce Department, Customs and Border Protection within the Department of Homeland Security and the U.S. Department of the Treasury’s Office of Foreign Assets Control (OFAC). As a global animal health company, we conduct business in multiple jurisdictions throughout the world. This includes supplying medicines and medical products for use in Iran and shipment of products to Iran, and conducting related activities, in accordance with a general license issued by OFAC and in line with our corporate policies. As previously disclosed, we acquired Platinum Performance (Platinum) in August 2019. During the integration process, after the closing of the acquisition, we discovered that Platinum had initiated certain transactions involving sales of food, medicine or devices to individuals or entities who may have been resident in or had ties to Iran. These sales were not conducted pursuant to a general license from OFAC and potentially violated the Iranian Transactions and Sanctions Regulations (ITSR) administered by OFAC. We submitted an initial voluntary disclosure to OFAC in February 2020 while our internal investigation was ongoing. After concluding our internal investigation, in December 2020, we submitted a final voluntary disclosure to OFAC and the U.S. Department of Justice regarding these transactions. In July 2023, OFAC provided a No Action letter confirming a final determination that no further action would be taken in the matter. To date, the Department of Justice has not responded. In addition, we are subject to a wide variety of state level regulations in the United States covering topics such as the environment, animal welfare and privacy.
Outside the United States
EU. The European Medicines Agency (EMA) is the centralized regulatory agency of the EU. The agency is responsible for the scientific evaluation of medicines developed by healthcare companies seeking centralized approval for use in the EU. The agency has a veterinary review section distinct from the medical review section. The Committee for Veterinary Medicinal Products (CVMP) is responsible for scientific and technical review of the submissions for innovative pharmaceuticals, biopharmaceuticals and vaccines. After the CVMP issues a positive opinion on the approvability of a product, the EU commission reviews the opinion and, if they agree with the CVMP, they grant the product market authorization. Once granted by the European Commission, a centralized marketing authorization is valid in all EU and European Economic Area-European Free Trade Association states. The centralized procedure is mandatory for certain types of products, such as those developed by means of recombinant DNA technology or novel therapeutic veterinary medicinal products. Products (other than those covered by the mandatory scope of the centralized procedure) can also be registered in the EU via the decentralized or the mutual recognition routes under the supervision of the Co-ordination Group for Mutual Recognition and Decentralized Procedures for Veterinary Medicinal Products (CMDv). This co-ordination group is composed of one representative per member state from each national regulatory agency, including Norway, Iceland and Liechtenstein. A series of Regulations, Directives, Guidelines and EU Pharmacopeia Monographs provide the requirements for product approval in the EU. In general, these requirements are similar to those in the U.S., requiring demonstrated evidence of, safety, efficacy, and quality/consistency of manufacturing processes. We are also subject to the EU General Data Protection Regulation (GDPR) that requires us to meet enhanced requirements regarding the handling of personal data, including its use, protection and the rights of data subjects to request correction or deletion of their personal data.
United Kingdom. The making, updating and enforcing of U.K. legislation for Veterinary Medicinal products is the responsibility of the U.K.’s Veterinary Medicine Directorate (VMD) agency.
China. The Ministry of Agriculture and Rural Affairs (MARA), a ministerial-level component of the State Council, drafts and implements policies related to agriculture, rural areas and rural residents, and regulates crop farming, animal husbandry, fisheries, agriculture mechanization and the quality of agriculture products. MARA is also the regulatory body responsible for the regulation and control of pharmaceuticals, biologicals and medicated feed additives for animal use. Regulatory requirements in China have become increasingly stringent, with MARA recently issuing new regulations and changes to the regulatory review process.
Brazil. The Ministry of Agriculture, Livestock and Food Supply (MAPA) is the regulatory body in Brazil that is responsible for the regulation and control of pharmaceuticals, biologicals and medicated feed additives for animal use. MAPA's regulatory activities are conducted through the Secretary of Agricultural Defense and its Livestock Products Inspection Department. In addition, regulatory activities are conducted at a local level through the Federal Agriculture Superintendence. These activities include the inspection and licensing of both manufacturing and commercial establishments for veterinary products, as well as the submission, review and approval of pharmaceuticals, biologicals, and medicated feed additives. MAPA is one of the most active regulatory agencies in Latin America, having permanent seats at several international animal health forums, such as Codex Alimentarius, World Organization for Animal Health and Committee of Veterinary Medicines for the Americas. MAPA was also invited to be a Latin American representative at meetings of the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). Several normative instructions issued by MAPA have set regulatory trends in Latin America.
Australia. The Australian Pesticides and Veterinary Medicines Authority (APVMA) is an Australian government statutory authority established in 1993 to centralize the registration of all agricultural and veterinary products for the Australian marketplace. Previously each State and Territory government had its own system of registration. The APVMA assesses applications from companies and individuals seeking registration or a permit so they can supply their product to the marketplace. Applications undergo rigorous assessment using the expertise of the APVMA's scientific staff
and drawing on the technical knowledge of other relevant scientific organizations, Commonwealth government departments and state agriculture departments. If the product works as intended and the scientific data confirms that when used as directed on the product label it will have no harmful or unintended effects on people, animals, the environment or international trade, the APVMA will register the product. As well as registering new agricultural and veterinary products, the APVMA receives and reviews adverse event information which may be submitted by registrants or members of the public. The APVMA also reviews registered products when particular concerns are raised about their safety and effectiveness. The review of a product may result in confirmation of its registration, or it may see registration continue with some changes to the way the product can be used. In some cases the review may result in the registration of a product being canceled and the product taken off the market.
Rest of world. Country-specific regulatory laws have provisions that include requirements for certain labeling, safety, efficacy, and manufacturers' quality control procedures (to assure the consistency of the products), as well as company records and reports. With the exception of the EU, most other countries' regulatory agencies will generally refer to the FDA, USDA, EU and other international animal health entities, including the World Organization for Animal Health and Codex Alimentarius, in establishing standards and regulations for veterinary pharmaceuticals, immunotherapies and vaccines.
Global policy and guidance
Joint FAO/WHO Expert Committee on Food Additives. The Joint FAO/WHO Expert Committee on Food Additives is an international expert scientific committee that is administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO). They provide a risk assessment/safety evaluation of residues of veterinary drugs in animal products, exposure and residue definition and maximum residue limit proposals for veterinary drugs. We work with them to establish acceptable safe levels of residual product in food-producing animals after treatment. This in turn enables the calculation of appropriate withdrawal times for our products prior to an animal entering the food chain.
Advertising and promotion review. Promotion of regulated animal health products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and endorsed by the applicable agency. We conduct a review of promotion materials for compliance with the local and regional requirements in the markets where we sell animal health products.
Food Safety Inspection Service/generally recognized as safe. The FDA is authorized to determine the safety of substances (including “generally recognized as safe” substances, food additives and color additives), as well as prescribing safe conditions of use. However, although the FDA has the responsibility for determining the safety of substances, the Food Safety and Inspection Service, the public health agency in the USDA, still retains, under the tenets of the Federal Meat Inspection Act and the Poultry Products Inspection Act and their implementing regulations, the authority to determine whether new substances and new uses of previously approved substances are suitable for use in meat and poultry products.
International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). VICH is a trilateral (EU-Japan-USA) program aimed at harmonizing technical requirements for veterinary product registration. The objectives of the VICH are as follows:
•Establish and implement harmonized technical requirements for the registration of veterinary medicinal products in the VICH regions, which meet high quality, safety and efficacy standards and minimize the use of test animals and costs of product development.
•Provide a basis for wider international harmonization of registration requirements through the VICH Outreach Forum.
•Monitor and maintain existing VICH guidelines, taking particular note of the VICH work program and, where necessary, update these VICH guidelines.
•Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following the implementation of VICH guidelines.
•By means of a constructive dialogue between regulatory authorities and industry, provide technical guidance enabling response to significant emerging global issues and science that impact on regulatory requirements within the VICH regions.
Human Capital Management
As of December 31, 2023, we had approximately 14,100 employees worldwide, which included approximately 6,900 employees in the U.S. and approximately 7,200 in other jurisdictions. We view the strength of our leadership team and our talented colleagues around the world as critical components of our past and future success. We remain committed to being a company our colleagues can be proud of and one where they can thrive. We achieve this by attracting, retaining and developing the best talent in the industry through our focus on workplace culture and engagement, diversity, equity and inclusion (DE&I), talent recruitment, development and retention, benefits and compensation, and employee well-being, health and safety. The Human Resources Committee of our Board of Directors is responsible for overseeing talent development, DE&I, and employee engagement programs and policies, and the Quality and Innovation Committee regularly reviews employee health and safety metrics.
Some of our employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements in particular jurisdictions, including a small number of employees in the U.S.
Workplace Culture and Employee Engagement
We have established the following Core Beliefs that are the foundation of the commitments we make to each other, our customers and our stakeholders every day:
•Our Colleagues Make the Difference
•Always Do the Right Thing
•Customer Obsessed
•Run It Like You Own It
•We are One Zoetis
We value responsibility and integrity. Our Code of Conduct contains general guidelines for conducting business with the highest ethical standards. We are committed to an environment where open, honest communications are the expectation, not the exception. We have an Open Door Policy where colleagues are encouraged to present ideas, concerns, questions, issues or suggestions directly to any level of leadership within Zoetis, without fear of retaliation.
We assess colleague engagement and key drivers enabling organizational performance by regularly conducting employee engagement surveys. Our engagement rate, as measured by favorable responses to qualitative questions about alignment with Zoetis objectives and employment with Zoetis, was 86% in 2023. We are proud to continue to have an engagement rate in the high eighties for the last three years. Insights from the Company’s engagement survey are used to develop both company-wide and business function level organizational and talent development plans.
Diversity, Equity and Inclusion (DE&I)
We strive to create an environment where colleagues feel valued and cared for and understand the important role we play in embracing diversity to improve the quality of our innovation, collaboration and relationships. Our Chief Talent, Diversity, Equity & Inclusion Officer, reporting to our Chief Human Resources Officer, oversees a team dedicated to executing on our diversity, equity and inclusion strategy, which is reviewed with our executive leadership team and Board of Directors throughout each year.
Our DE&I focus and commitment begins with our leadership team of diverse backgrounds, experiences and ethnicities (for example, 64% of the executive team, including our Chief Executive Officer, are women), and it is demonstrated in our support of our colleagues and industry. We are committed to accelerating inclusion, equity and more diverse representation across the company. In May 2021, we released our first Sustainability Report, which included aspirations for change to make Zoetis and our industry more inclusive, including the following specific aspirations focused on increasing the representation of underrepresented groups within our company by the end of 2025 (as compared to 2020):
•Increase representation of women at the director level and above globally from 32% to 40%;
•Increase overall representation among people of color in the U.S. from 21% to 25%;
•Increase representation of Black colleagues in the U.S. from 4% to 5%; and
•Increase representation of Latinx colleagues in the U.S. from 5% to 6%.
We continue to make great strides towards achieving these aspirations. In addition, we offer nine Colleague Resource Groups, which are an important catalyst to foster a diverse, inclusive environment, while positively impacting our business and community. In 2023, we continued to offer DE&I training globally to all our employees.
Talent Recruitment, Development and Retention
We employ a variety of career development, employee benefits, policies and compensation programs designed to attract, develop and retain our colleagues. Employee benefits and policies are designed for diverse needs including generous parental leave policies and expanded adoption, fertility and surrogacy benefits for all colleagues equitably. We have internal programs designed to develop and retain talent, including a talent development portal, mentoring programs, career planning resources, leadership development programs, and performance management and training programs. In particular, our R&D team recruits scientists and research and development personnel from universities and scientific associations and forums and leverages a variety of R&D-specific talent tools. Our global voluntary attrition rate decreased from 10% in 2022 to 8% in 2023.
Compensation and Benefits
We strive to support our colleagues’ well-being and enable them to achieve their best. Our compensation and benefits programs are designed to support colleague well-being including physical and mental health, financial wellness, and family and lifestyle resources. We recognize the diverse needs of our colleagues around the world and have developed comprehensive programs that vary by country and region to best address them. In the U.S., these benefits include flexible work arrangements, educational assistance, mental health support, and inclusive family-friendly benefits like fully paid parental leave, including for adoptions, parents of all genders and same sex partners, as well as fertility and surrogacy benefits. To support colleague well-being and work life balance, we continue to offer enhanced childcare benefits and flexible work arrangements to aid our colleagues in managing their work and family responsibilities.
We are proud of our continuing record as a top employer, as recognized by esteemed publications and organizations around the world.
Employee Health and Safety
We are committed to ensuring a safe working environment for our employees and our Global Environmental Health and Safety (EHS) Policy standards define EHS performance requirements for all sites, procedures and recommended practices. Our sites have injury prevention programs, and we strive to build a best-in-class safety culture. Our procedures emphasize the importance of investigating causes of injuries and action plans to be implemented to mitigate potential recurrence.
We track health and safety performance metrics including total injury rate (TIR), lost time injury rate (LTIR), restricted work injuries and medical treatment injuries on a monthly basis for all manufacturing and research and development sites, as well as for U.S. offices, field force, fleet and logistics. Since 2018, we have tracked TIR and LTIR for all our operations worldwide. Our safety programs have resulted in strong safety performance, with TIR and LTIR rates being lower than the industry averages.
Information about our Executive Officers
Kristin C. Peck
Age 52
Chief Executive Officer and Director
Ms. Peck has served as our Chief Executive Officer since January 2020 and as a director since October 2019. Prior to becoming CEO, Ms. Peck was Executive Vice President and Group President, U.S. Operations, Business Development and Strategy at Zoetis from March 2018 to December 2019. Ms. Peck previously served as our Executive Vice President and President, U.S. Operations from May 2015 to February 2018 and Executive Vice President and Group President from October 2012 through April 2015. In these roles, Ms. Peck helped usher Zoetis through its Initial Public Offering in 2013 and has been a driving force of change in areas including Global Manufacturing and Supply, Global Poultry, Global Diagnostics, Corporate Development, and New Product Marketing and Global Market Research. Ms. Peck joined Pfizer in 2004 and held various positions, including Executive Vice President, Worldwide Business Development and Innovation and as a member of Pfizer's Executive Leadership Team.
Wetteny Joseph
Age 51
Executive Vice President and Chief Financial Officer
Mr. Joseph has served as our Executive Vice President and Chief Financial Officer since June 2021. Mr. Joseph joined Zoetis from Catalent, where he served for 13 years, most recently as Senior Vice President and Chief Financial Officer of Catalent from February 2018 to May 2021. Mr. Joseph joined Catalent in September 2008 where he served as Corporate Controller until October 2012, then as Vice President Finance across multiple business units until October 2015, when he was named President, Clinical Supply Services, one of the company's principal business units. Before joining Catalent, Mr. Joseph held a variety of senior financial positions at the industrial distribution company HD Supply, including as CFO of its plumbing and HVAC business unit. He also served as Corporate Controller for Hughes Supply, a Fortune 500, NYSE-listed company that was acquired by Home Depot and became part of HD Supply. In his early career, Mr. Joseph spent six years at PricewaterhouseCoopers as an auditor and strategic financial advisor across a variety of industries.
Nick Ashton
Age 51
Executive Vice President and President, Global Manufacturing and Supply
Mr. Ashton has served as our Executive Vice President and President, Global Manufacturing and Supply since May 2022. Mr. Ashton joined Zoetis in 2020 as Head of Global External Supply, where he led all aspects of the company’s global external manufacturing network, overseeing CMOs to keep pace with customer demand. Mr. Ashton brings more than 25 years of global experience in areas of production, supply chain, external manufacturing, procurement, network strategy and execution for major international companies including GSK, Babcock International and Merck.
Ester Banque
Age 54
Executive Vice President and President, U.S. Operations
Ms. Banque has served as our Executive Vice President and President, U.S. Operations since July 2023. Ms. Banque joined Zoetis from Bristol-Meyers Squibb (BMS) where she most recently served as Senior Vice President and General Manager of U.S. Hematology and Cell Therapy. Prior to that she was Senior Vice President and Head of International Markets. Before joining BMS, Ms. Banque spent 25 years at Novartis where she held a variety of roles with increasing responsibility.
Jamie Brannan
Age 51
Executive Vice President and Group President International Operations, Aquaculture and Global Diagnostics
Mr. Brannan has served as our Executive Vice President and Group President International Operations, Aquaculture and Global Diagnostics since November 2022. He served as our President of International Operations from June 2021 to November 2022. He served as our Senior Vice President of the U.K., Ireland and Nordics Cluster from 2016 to 2021. Mr. Brannan joined Zoetis from Mölnlycke Health Care where he most recently served as the Surgical Business Director and General Manager for the U.K. and Ireland.
Heidi C. Chen
Age 57
Executive Vice President, General Counsel and Corporate Secretary; Business Lead of Human Health Diagnostics
Ms. Chen has served as our Executive Vice President and General Counsel since October 2012, and as our Corporate Secretary since July 2012. Since January 2020, Ms. Chen has had oversight responsibility for our Human Health Diagnostics business. Ms. Chen joined Pfizer in 1998 and held various legal and compliance positions of increasing responsibility, including Vice President and Chief Counsel of Pfizer Animal Health, our predecessor company, and lead counsel for Pfizer’s Established Products (generics) business.
Rimma Driscoll
Age 51
Executive Vice President and Head of Global Strategy, Commercial and Business Development, and Global BioDevices
Ms. Driscoll has served as our Executive Vice President and Head of Global Strategy, Commercial and Business Development since November 2022. She also has had oversight for the company’s Global BioDevices business since February 2023. Previously, she served as our Senior Vice President, Business Development from January 2020 to November 2022. She served as our Vice President, Business Development and Commercial Alliances from 2016 to January 2020. Ms. Driscoll joined Zoetis in February 2016, from Procter & Gamble Company, where she worked for more than 20 years running Global Business Development, Mergers & Acquisitions and Alliance organizations for their consumer healthcare and pharmaceuticals businesses.
Jeannette Ferran Astorga
Age 49
Executive Vice President, Corporate Affairs, Communications and Chief Sustainability Officer
Ms. Ferran Astorga has served as our Executive Vice President, Corporate Affairs, Communications and Chief Sustainability Officer since January 2022 and also serves as President of the Zoetis Foundation. She joined Zoetis in September 2020 as Vice President, Head of Sustainability. Prior to joining Zoetis, Ms. Ferran Astorga was Vice President of Corporate Responsibility at the Ascena Retail Group where she served since 2015 leading the global team responsible for strategic enterprise initiatives, including supply chain compliance, sustainability, diversity and inclusion, and corporate philanthropy. Ms. Ferran Astorga held a variety of leadership roles with increasing responsibility at ANN INC., a women’s fashion retail company that was acquired by Ascena Retail Group.
Roxanne Lagano
Age 59
Executive Vice President, Chief Human Resources Officer and Global Operations
Ms. Lagano has served as our Executive Vice President and Chief Human Resources Officer since November 2012 and in January 2020 she took on responsibility for Global Operations and Security functions. She previously had oversight of the company’s Corporate Communications function from 2015 to 2019. Ms. Lagano joined Pfizer in 1997 and held various positions, including Senior Vice President, Global Compensation, Benefits and Wellness and Senior Director, Business Transactions, Pfizer Worldwide Human Resources.
Wafaa Mamilli
Age 56
Executive Vice President, Chief Digital & Technology Officer and Group President for China, Brazil and Precision Animal Health
Ms. Mamilli has served as our Executive Vice President, Chief Digital & Technology Officer and Group President for China, Brazil and Precision Animal Health since November 2022. She served as our Executive Vice President and Chief Information and Digital Officer from January 2020 to October 2022. Ms. Mamilli joined Zoetis from Eli Lilly and Company where she most recently served as Global Chief Information Officer for business units from January 2019 to January 2020. Prior to that, she was Eli Lilly’s Chief Information Security Officer from March 2016 to March 2019 and Information Officer for the Diabetes Business Unit & Real World Evidence from May 2014 to March 2016.
Robert J. Polzer
Age 55
Executive Vice President and President, Research and Development
Dr. Polzer has served as our Executive Vice President and President, Research and Development since January 2022. Dr. Polzer joined Zoetis in 2015 as the Head of Global Therapeutics. Prior to Zoetis, Dr. Polzer spent over 20 years with Pfizer in drug metabolism research with roles of increasing organizational impact including global leadership of Pharmcokinetics, Dynamics, and Metabolism.
Environmental, Health and Safety
We are subject to various federal, state, local and foreign environmental, health and safety laws and regulations. These laws and regulations govern matters such as the emission and discharge of hazardous materials into the ground, air or water; the generation, use, storage, handling, treatment, packaging, transportation, exposure to, and disposal of hazardous and biological materials, including recordkeeping, reporting and registration requirements; and the health and safety of our employees. Due to our operations, these laws and regulations also require us to obtain, and comply with, permits, registrations or other authorizations issued by governmental authorities. These authorities can modify or revoke our permits, registrations or other authorizations and can enforce compliance through fines and injunctions.
Certain environmental laws, such as the U.S. Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (CERCLA), impose joint and several liability, without regard to fault, for cleanup costs on persons who disposed of or released hazardous substances into the environment, including at third-party sites or offsite disposal locations, or those who currently own or operate (or formerly owned or operated) sites where such a release occurred. In addition to clean-up actions brought by federal, state, local and foreign governmental entities, private parties could raise personal injury or other claims against us due to the presence of, or exposure to, hazardous materials on, from or otherwise relating to such a property.
We have made, and intend to continue to make, necessary expenditures for compliance with applicable environmental, health and safety laws and regulations. We are also a party to proceedings in which the primary relief sought is the cost of past and/or future remediation, or remedial measures to mitigate or remediate pollution. In connection with such proceedings, and otherwise, we are investigating and cleaning up environmental contamination from past industrial activity at certain sites, or financing other parties' completion of such activities. As a result, we incurred capital and operational expenditures in 2023 for environmental compliance purposes and for the clean-up of certain past industrial activities as follows:
•environmental-related capital expenditures - approximately $15 million; and
•other environmental-related expenditures - approximately $19 million.
However, we may not have identified all of the potential environmental liabilities relating to our current and former properties, or those liabilities associated with off-site disposal locations. Such liability could have a material adverse effect on our operating results and financial condition. Furthermore, regulatory agencies are showing increasing concern over the impact of animal health products and livestock operations on the environment. This increased regulatory scrutiny may necessitate that additional time and resources be spent to address these concerns in both new and existing products.
In connection with past acquisitions and divestitures, we have undertaken certain indemnification obligations that require us, or may require us in the future, to conduct or finance environmental cleanups at sites that we no longer own or operate. We have also entered into indemnification agreements in which we are being indemnified for various environmental cleanups; however, such indemnities are limited in both time and scope and may be further limited in the presence of new information, or may not be available at all.
While we cannot predict with certainty our future capital expenditures or operating costs for environmental compliance or remediation of contaminated sites, we currently have no reason to believe that they will have a material adverse effect on our operating results or financial condition.
Available Information
The company's internet website address is www.zoetis.com. On our website, the company makes available, free of charge, its annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after the company electronically files such material with, or furnishes such material to, the Securities and Exchange Commission (SEC). The SEC maintains an internet website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at www.sec.gov.
Also available on our website is information relating to corporate governance at Zoetis and our Board of Directors, including as follows: our Corporate Governance Principles; Zoetis Code of Conduct (for all of our directors and employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer, and Controller); Board Committees membership and Committee Charters; and ways to communicate by email with our Directors. We will provide any of the foregoing information without charge upon written request to our Corporate Secretary, Zoetis Inc., 10 Sylvan Way, Parsippany, New Jersey 07054. Information relating to shareholder services is also available on our website. We will disclose any future amendments to, or waivers from, provisions of these ethics policies and standards affecting our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer, and Controller on our website as promptly as practicable, as may be required under applicable SEC and New York Stock Exchange (NYSE) rules.
We use our website (www.zoetis.com) as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. These disclosures are included in the “Investor Relations” and “News & Insights” sections of our website. Accordingly, investors should monitor these portions of our website, in addition to following our press releases, SEC filings and public conference calls and webcasts.
The information contained on our website does not constitute, and shall not be deemed to constitute, a part of this 2023 Annual Report, or any other report we file with, or furnish to, the SEC. Our references to the URLs for websites are intended to be inactive textual references only.
Item 1A. Risk Factors.
In addition to the other information set forth in this 2023 Annual Report, any of the factors described below could materially adversely affect our operating results, financial condition and liquidity, which could cause the trading price of our securities to decline.
This report contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect our current views with respect to, among other things, future events and performance. We generally identify forward-looking statements by words such as “anticipate,” “estimate,” “could,” “expect,” “intend,” “project,” “plan,” “predict,” “believe,” “seek,” “continue,” “outlook,” "objective," "target," “may,” “might,” “will,” “should,” “can have,” “likely” or the negative version of these words or comparable words or by using future dates in connection with any discussion of future performance, actions or events. Forward-looking statements are based on beliefs and assumptions made by management using currently available information. These statements are not guarantees of future performance, actions or events.
In particular, forward-looking statements include statements relating to our future actions, business plans or prospects, prospective products, product approvals or products under development, product and supply chain disruptions, R&D costs, timing and likelihood of success, future operating or financial performance, future results of current and anticipated products and services, strategies, sales efforts, expenses, production efficiencies, production margins, anticipated timing of generic market entries, integration of acquired businesses, interest rates, tax rates, changes in tax regimes and laws, foreign exchange rates, growth in emerging markets, the outcome of contingencies, such as legal proceedings, plans related to share repurchases and dividends, government regulation and financial results. Forward-looking statements are subject to risks and uncertainties, many of which are beyond our control, and potentially inaccurate assumptions. However, there may also be other risks that we are unable to predict at this time. If one or more of these risks or uncertainties materialize, or if management's underlying beliefs and assumptions prove to be incorrect, actual results may differ materially from those contemplated by a forward-looking statement. You should not put undue reliance on forward-looking statements. Forward-looking statements speak only as of the date on which they are made.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects in our Form 10-Q and 8-K reports and our other filings with the SEC. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
Risks related to our business and the animal health industry
The animal health industry is highly competitive.
We believe many of our competitors are conducting R&D activities in areas served by our products and in areas in which we are developing products. Our competitors include standalone animal health businesses, start-up companies working in the animal health area and the animal health businesses of large pharmaceutical companies. These competitors may have access to greater financial, marketing, technical and other resources or have significant market share in particular areas. As a result, they may be able to devote more resources to developing, manufacturing, marketing and selling their products, initiating or withstanding substantial price competition or more readily taking advantage of acquisitions or other opportunities. In recent years, there has been an increase in consolidation in the animal health industry, which could result in existing competitors realizing additional efficiencies or improving portfolio bundling opportunities, thereby potentially increasing their market share and pricing power, which could lead to a decrease in our revenue and profitability and an increase in competition.
To the extent that any of our competitors are more successful with respect to any key competitive factor or we are forced to reduce, or are unable to raise, the price of any of our products in order to remain competitive, our operating results and financial condition could be materially adversely affected.
Our results of operations are dependent upon the success of our top-selling products.
If any of our top-selling products or product lines experience issues, such as loss of patent protection, material product liability litigation, new or unexpected side effects, manufacturing or supply chain disruptions, regulatory proceedings, labeling changes, negative publicity, changes to veterinarian or customer preferences, and/or disruptive innovations or the introduction of competing and/or more effective products, our revenues could be negatively impacted, perhaps significantly. For example, our five top-selling products and product lines, Simparica/Simparica Trio, Apoquel/Apoquel Chewable, Cytopoint, Revolution/Revolution Plus/Stronghold and ceftiofur line, contributed approximately 37% of our revenue in 2023, and any issues with these top-selling products and product lines would have a more significant impact to our results of operations.
Our products are subject to unanticipated safety, quality or efficacy concerns.
Unanticipated safety, quality or efficacy concerns can arise with respect to our products, whether or not scientifically or clinically supported, which can lead to product recalls, withdrawals or suspended or declining sales, as well as product liability and other claims.
Regulatory actions based on these types of safety, quality or efficacy concerns could impact all or a significant portion of a product’s sales and could, depending on the circumstances, materially adversely affect our operating results.
In addition, since we depend on positive perceptions of the safety, quality and efficacy of our products, and animal health products generally, by our customers, veterinarians and end-users, any concerns as to the safety, quality or efficacy of our products, whether actual or perceived, may harm our reputation or materially adversely affect our operating results and financial condition, regardless of whether such concerns are accurate.
Generic and other products may be viewed as more cost-effective than our products.
We face competition from products produced by other companies, including generic alternatives to our products. We depend on patents and regulatory data exclusivity periods to provide us with exclusive marketing rights for some of our products. Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the countries where such patents are obtained. The extent of protection afforded by our patents varies from country to country and is limited by the scope of the claimed
subject matter of our patents, the term of the patent and the availability and enforcement of legal remedies in the applicable country. As a result, we face competition from lower-priced generic alternatives to many of our products that no longer have patent protection. In certain circumstances, we have been forced to lower our prices and provide discounts or rebates in order to compete with generic products. Generic competitors are becoming more aggressive in terms of launching at risk before patent rights expire and, because of their pricing, are an increasing percentage of overall animal health sales in certain regions. For example, several companies have launched generic versions of our Rimadyl chewable and Draxxin products. In the years since the start of generic and other competition, sales of our Rimadyl chewable and Draxxin products have declined in the U.S., the largest market for these products, by 33% and 47%, respectively, and additional declines are expected in subsequent years.
If animal health customers increase their use of new or existing generic products, our operating results and financial condition could be materially adversely affected.
Our business is subject to risk based on global economic and political conditions.
Macroeconomic, business, political and financial disruptions, including public health crises or pandemics, such as COVID-19, could have a material adverse effect on our operating results, financial condition and liquidity. Certain of our customers and suppliers may be affected directly by economic downturns and could face credit issues or cash flow problems that could give rise to payment delays, increased credit risk, bankruptcies and other financial hardships that could decrease the demand for our products or hinder our ability to collect amounts due from customers or goods from our suppliers. If one or more of our large customers, including distributors, discontinue their relationship with us as a result of economic conditions, public health conditions, sanctions or otherwise, our operating results and financial condition may be materially adversely affected. In addition, economic concerns and geopolitical instability may cause shortages in veterinary healthcare workers or some pet owners to forgo or defer visits to veterinary practices or could reduce their willingness to treat pet health conditions or even to continue to own a pet. Moreover, customers may seek lower price alternatives to our products if they are negatively impacted by the current poor economic conditions. Russia’s invasion of Ukraine, the conflict between Israel and Hamas (including any escalation or expansion), economic weakness in China, the COVID-19 pandemic, as well as inflation, are examples of recent global economic conditions that could have an adverse effect on our operating results, financial condition and liquidity.
Infectious disease outbreaks, pandemics, sanctions, geopolitical instability and widespread fear of spreading disease through human contact can cause disruptions to or negatively impact our customers’ and our distributors’ business operations, which could materially adversely affect our operating results. Furthermore, our exposure to credit and collectability risk and cybersecurity risk is higher in certain international markets, our ability to mitigate such risks may be limited. While we have procedures to monitor and limit exposure to credit and collectability risk and have defensive measures in place to prevent and mitigate cyberattacks, there can be no assurances that such procedures and measures will effectively limit such risks and avoid losses.
Consolidation of our customers and distributors could negatively affect the pricing of our products.
Veterinarians and livestock producers are our primary customers. In recent years, there has been a trend towards the concentration of veterinarians in large clinics and hospitals. In addition, livestock producers, particularly swine and poultry producers, and our distributors, have seen consolidation in their industries. Furthermore, we have seen the expansion of larger cross-border corporate customers and an increase in the consolidation of buying groups (cooperatives of veterinary practices that leverage volume to pursue discounts from manufacturers). If these trends towards consolidation continue, these customers and distributors could attempt to improve their profitability by leveraging their buying power to obtain favorable pricing. The resulting decrease in our prices could have a material adverse effect on our operating results and financial condition.
Changes in distribution channels for companion animal products could negatively impact our market share, margins and distribution of our products.
In most markets, companion animal owners typically purchase their animal health products directly from veterinarians. Companion animal owners increasingly have the option to purchase animal health products and, in some cases, veterinary services from sources other than veterinarians, such as internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the significant shift away from the veterinarian distribution channel in the sale of flea and tick products in recent years and has been accelerated by the increase in e-commerce during the COVID-19 pandemic. Companion animal owners also could decrease their reliance on, and visits to, veterinarians as they rely more on internet-based animal health information. Because we primarily market our companion animal products through the veterinarian distribution channel, any decrease in visits to veterinarians by companion animal owners could reduce our market share for such products and materially adversely affect our operating results and financial condition. In addition, companion animal owners may substitute human health products for animal health products if human health products are deemed to be lower-cost alternatives.
In the U.S. and certain other markets, these and other competitive conditions have increased, and may continue to increase, our reliance on internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels to sell our companion animal products. Over time we may be unable to sustain our current margins due to the increased purchasing power of such retailers as compared to traditional veterinary practices. Any of these events could materially adversely affect our operating results and financial condition.
Disruptive innovations and advances in medical practices and technologies could negatively affect the market for our products.
The market for our products could be impacted negatively by the introduction and/or broad market acceptance of newly-developed or alternative products that address the diseases and conditions for which we sell products, including “green” or “holistic” health products or specially bred disease-resistant animals. In addition, technological breakthroughs by others may obviate our technology and reduce or eliminate the market for our products. Introduction or acceptance of such products or technologies could materially adversely affect our operating results and financial condition.
An outbreak of infectious disease carried by animals could negatively affect the sale and production of our products.
Sales of our livestock products could be materially adversely affected by the outbreak of disease carried by animals, which could lead to the widespread death or precautionary destruction of animals as well as the reduced consumption and demand for animal protein. In addition, outbreaks of disease carried by animals may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products due to reduced herd or flock sizes. In recent years, outbreaks of various diseases, including African Swine Fever, avian influenza, foot-and-mouth disease, bovine spongiform
encephalopathy (otherwise known as BSE or mad cow disease) and porcine epidemic diarrhea virus (otherwise known as PEDv), have impacted the animal health business. The discovery of additional cases of any of these, or new diseases may result in additional restrictions on animal proteins, reduced herd sizes, or reduced demand for animal protein, which may have a material adverse effect on our operating results and financial condition. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere.
Implementing new business lines or offering new products and services may subject us to additional risks.
From time to time, we may implement new business lines or offer new products and services within existing lines of business. There may be substantial risks and uncertainties associated with these efforts. We may invest significant time and resources in developing, marketing, or acquiring new lines of business and/or offering new products and services. Initial timetables for the introduction and development or acquisition of new lines of business and/or the offering of new products or services may not be achieved, and price and profitability targets may prove to be unachievable. Our lack of experience or knowledge, as well as external factors, such as compliance with regulations, competitive alternatives and shifting market preferences, may also impact the success of an acquisition or the implementation of a new line of business or a new product or service. New business lines or new products and services within existing lines of business could affect the sales and profitability of existing lines of business or products and services. Failure to successfully manage these risks in the implementation or acquisition of new lines of business or the offering of new products or services could have a material adverse effect on our reputation, business, results of operations, and financial condition.
Restrictions and bans on the use of and consumer preferences regarding antibacterials in food-producing animals may become more prevalent.
The issue of the potential transfer of increased antibacterial resistance in bacteria from food-producing animals to human pathogens, and the causality of that transfer, continue to be the subject of global scientific and regulatory discussion. Antibacterials refer to small molecules that can be used to treat or prevent bacterial infections and are a sub-categorization of the products that make up our anti-infectives and medicated feed additives portfolios. In some countries, this issue has led to government restrictions and bans on the use of specific antibacterials in some food-producing animals, regardless of the route of administration (in feed or injectable). These restrictions are more prevalent in countries where animal protein is plentiful and governments are willing to take action even when there is scientific uncertainty. Our total revenue attributable to antibacterials for livestock was less than $950 million for the year ended December 31, 2023.
For example, regulations regarding antibiotic usage in animals have been introduced in certain markets, including the U.S., the EU, China, France, Germany, and Vietnam. In addition, certain jurisdictions like Italy have implemented the use of electronic prescriptions, which has caused more disciplined use of antibiotics and decreased the demand for our antibacterial products. Also, in certain markets, there has been an increase in consumer preference towards proteins produced without the use of antibiotics.
We cannot predict whether antibacterial resistance concerns will result in additional restrictions or bans, expanded regulations, public pressure to discontinue or reduce use of antibacterials in food-producing animals or increased consumer preference for antibiotic-free protein, any of which could materially adversely affect our operating results and financial condition.
Perceived adverse effects linked to the consumption of food derived from animals that utilize our products or animals generally could cause a decline in the sales of such products.
Our livestock business depends heavily on a healthy and growing livestock industry. If the public perceives a risk to human health from the consumption of the food derived from animals that utilize our products, there may be a decline in the production of such food products and, in turn, demand for our products. Furthermore, changing consumer preferences and increasing consumer interest in alternatives to animal-based protein and dairy products has driven the growth of plant-based substitutes. Any reputational harm to the livestock industry may also extend to companies in related industries, including our company. Adverse consumer views related to the use of one or more of our products in livestock also may result in a decrease in the use of such products and could materially adversely affect our operating results and financial condition.
Increased regulation or decreased governmental financial support relating to the raising, processing or consumption of food-producing animals could reduce demand for our livestock products.
Companies in the livestock industries are subject to extensive and increasingly stringent regulations. If livestock producers are adversely affected by new regulations or changes to existing regulations, they may reduce herd sizes or become less profitable and, as a result, they may reduce their use of our products, which may materially adversely affect our operating results and financial condition. Also, many food-producing companies, including livestock producers, benefit from governmental subsidies, and if such subsidies were to be reduced or eliminated, these companies may become less profitable and, as a result, may reduce their use of our products. Furthermore, new or more stringent regulations could, directly or indirectly, impact the use of one or more of our products. More stringent regulation of the livestock industry or our products could have a material adverse effect on our operating results and financial condition.
We may not successfully acquire and integrate other businesses, license rights to technologies or products, form and manage alliances or divest businesses.
We pursue acquisitions, technology licensing arrangements, strategic alliances or divestitures of some of our businesses as part of our business strategy. We may not complete these transactions in a timely manner, on a cost-effective basis, or at all, due to regulatory constraints or limitations or other unforeseen factors that prevent us from realizing the expected benefits. We may be unable to integrate acquisitions successfully into our existing business, and we may be unable to achieve expected sales, gross margin improvements or efficiencies. Our reported results of operations could be negatively affected by acquisition or disposition-related charges, amortization of expenses related to intangibles and charges for impairment of long-term assets. We may be subject to litigation or government investigations in connection with, or as a result of, acquisitions, dispositions, licenses or other alliances. While our evaluation of any potential transaction includes business, legal and financial due diligence with the goal of identifying and evaluating the material risks involved, our due diligence reviews may not identify all of the issues necessary to accurately estimate the cost and potential loss contingencies of a particular transaction, including potential exposure to regulatory sanctions or fines resulting from an acquisition target's previous activities, inadequate controls, or costs associated with any quality issues with an acquisition target’s legacy products. Any of these events could cause us to incur significant expenses and could materially adversely affect our operating results and financial condition.
Our business may be negatively affected by weather conditions, natural disasters and the availability of natural resources.
Adverse weather events and natural disasters may also interfere with and negatively impact operations at our manufacturing sites, research and development facilities and office buildings, which could have a material adverse effect on our operating results and financial condition, especially if such interruptions to regular operations are frequent or prolonged.
Weather conditions, including excessive cold or heat, natural disasters, floods, droughts and other events, could negatively impact our livestock customers by impairing the health or growth of their animals or the production or availability of feed. Such events could also interfere with our livestock customers’ operations due to power outages, fuel shortages, damage to their farms or facilities or disruption of transportation channels, among other things. For example, severe droughts can lead to a decrease in harvested corn and higher corn prices, which may impact the profitability of livestock producers of cattle, pork and poultry. Higher corn prices may contribute to reductions in herd or flock sizes that may result in reduced spending on animal health products. Adverse weather conditions and natural disasters may also have a material adverse impact on the aquaculture business. In the event of a natural disaster, adverse weather conditions, or a shortage of fresh water, veterinarians or livestock producers may purchase less of our products and our operating results and financial condition could be materially adversely affected.
In addition, veterinary hospitals and practitioners depend on visits from and access to animals under their care. Veterinarians’ patient volume and ability to operate could be adversely affected if they experience natural disasters or adverse weather conditions, including floods, fires, earthquakes and hurricanes or other storms, or prolonged snow or ice, particularly in regions not accustomed to sustained inclement weather.
Climate change could have a material adverse impact on our and our customers’ businesses.
We operate in many regions, countries and communities around the world where our businesses, and our activities and the activities of our customers and suppliers, could be disrupted by climate change. Potential physical risks from climate change may include altered distribution and intensity of rainfall, prolonged droughts or flooding, increased frequency of wildfires and other natural disasters, rising sea levels, and a rising heat index, any of which could cause negative impacts to our and our customers’ and suppliers’ businesses. Increased temperatures and rising water levels may negatively impact our livestock customers by increasing the prevalence of parasites and diseases that affect food animals. In addition, changes in water temperatures could affect the timing of reproduction and growth of various fish species, and trigger the outbreak of certain water borne diseases. The physical changes caused by climate change may also prompt changes in regulations or consumer preferences which in turn could have negative consequences for our and our customers’ businesses. Climate change may negatively impact our customers’ operations, particularly those in the livestock industry, through climate-related impacts such as increased air and water temperatures, rising water levels and increased incidence of disease in livestock. In addition, concerns regarding greenhouse gas emissions and other potential environmental impacts of livestock production have led to some consumers opting to limit or avoid consuming animal products. If such events affect our customers’ businesses, they may purchase fewer Zoetis products, and our revenues may be negatively impacted. Such climate driven changes could have a material adverse impact on the financial performance of our business, and on our customers.
The impacts from climate change may also impact Zoetis’ and our suppliers’ manufacturing processes. For example, ample amounts of clean water are needed to produce our products, and the effects from climate change could result in water supply interruptions and low water quality. In addition, increased frequency of natural disasters and adverse weather conditions as a result of climate change may disrupt our manufacturing processes or our supply chain. These disruptions may have a material adverse effect on our business, financial condition, results of operations and/or cash flows.
Modification of foreign trade policy by the U.S. or other countries or the imposition of tariffs on imported goods may harm our business.
Changes in trade laws, agreements and policies governing trade in and out of the territories and countries where our customers do business could negatively impact such customers’ businesses and adversely affect our operating results. A number of our customers, particularly U.S.-based livestock producers, benefit from free trade agreements. As well, international trade agreements or policies could harm our business and customers, and, as a result, negatively impact our financial condition and results of operations.
Additionally, in response to U.S. tariffs affecting exports, some governments, including China, have instituted and may in the future institute tariffs on certain U.S. goods. While the scope and duration of these and any future tariffs remains uncertain, tariffs imposed by the U.S. or other governments on our products or the active pharmaceutical ingredients or other components thereof could negatively impact our financial condition and results of operations.
Our business is subject to risk based on customer exposure to rising costs and reduced customer income.
Feed, fuel and transportation and other key costs for livestock producers may increase or animal protein prices or sales may decrease. Either of these trends could cause deterioration in the financial condition of our livestock product customers, potentially inhibiting their ability to purchase our products or pay us for products delivered. Our livestock product customers may offset rising costs by reducing spending on our products, including by switching to lower-cost alternatives to our products. In addition, concerns about the financial resources of pet owners also could cause veterinarians to alter their treatment recommendations in favor of lower-cost alternatives to our products. These shifts could result in a decrease in sales of our companion animal products, especially in developed countries where there is a higher rate of pet ownership.
Our business could be adversely affected by labor disputes, strikes or work stoppages.
Some of our employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements in certain jurisdictions, including the U.S. As a result, we are subject to the risk of labor disputes, strikes, work stoppages and other labor-relations matters. We may be unable to negotiate new collective bargaining agreements on similar or more favorable terms and may experience work stoppages or other labor problems in the future at our sites. We could experience a disruption of our operations or higher ongoing labor costs, which could have a material adverse effect on our operating results and financial condition, potentially resulting in canceled orders by customers, unanticipated inventory accumulation or shortages and reduced revenue and net income. We may also experience difficulty or delays in implementing changes to our workforce in certain markets. In addition, labor problems at our suppliers, CMOs or other service providers could have a material adverse effect on our operating results and financial condition.
Our business may be harmed if we are unable to retain and hire executive officers or other key personnel.
We depend on the efforts of our executive officers and certain key personnel, including research, technical, sales, security, marketing, manufacturing and administrative personnel. Our ability to recruit and retain such talent will depend on a number of factors, including compensation and benefits, work location and work environment. From time to time there may be shortages of skilled labor, which may make it more difficult for us to attract and retain qualified employees or lead to increased labor costs. In addition, we generally do not enter into employment agreements with our executive officers and other key personnel. If we cannot effectively recruit and retain qualified executives and employees, we may not be able to maintain or expand our operations, or our business could be otherwise adversely affected and could, at least temporarily, have a material adverse effect on our operating results and financial condition.
We may be required to write down goodwill or identifiable intangible assets.
Under accounting principles generally accepted in the United States of America (U.S. GAAP), if we determine goodwill or identifiable intangible assets are impaired, we will be required to write down these assets and record a non-cash impairment charge. As of December 31, 2023, we had goodwill of $2.8 billion and identifiable intangible assets, less accumulated amortization, of $1.3 billion. Identifiable intangible assets consist primarily of developed technology rights, brands, trademarks, license agreements, patents, acquired customer relationships and in-process R&D.
Determining whether an impairment exists and the amount of the potential impairment involves quantitative data and qualitative criteria that are based on estimates and assumptions requiring significant management judgment. Future events or new information may change management's valuation of an intangible asset in a short amount of time. The timing and amount of impairment charges recorded in our Consolidated Statements of Income and write-downs recorded in our Consolidated Balance Sheets could vary if management's conclusions change. Any impairment of goodwill or identifiable intangible assets could have a material adverse effect on our operating results and financial position.
Risks related to manufacturing and supply
Manufacturing problems and capacity imbalances may cause product launch delays, inventory shortages, recalls or unanticipated costs.
In order to sell our products, we must be able to produce and ship our products in sufficient quantities. On December 31, 2023, we had a global manufacturing network consisting of 29 manufacturing sites located in 12 countries. We also employ a network of 109 third-party CMOs. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites.
Minor deviations in our or our suppliers' manufacturing or logistical processes, such as temperature excursions or improper package sealing, could result in delays, inventory shortages, unanticipated costs, product recalls, product liability and/or regulatory action. In addition, a number of factors could cause production interruptions, including:
•the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality assurance guidelines, including any changes to Good Manufacturing Practices (GMP);
•the failure to accurately forecast demand for our products;
•mislabeling;
•construction delays;
•equipment malfunctions;
•shortages of materials;
•labor problems, including any COVID-related impacts;
•delays in receiving any required governmental authorizations or regulatory approvals, including as a result of any prolonged shutdown of the U.S. government;
•natural disasters and adverse weather conditions;
•power outages;
•criminal and terrorist activities;
•changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; and
•the outbreak of any highly contagious diseases at or near our production sites.
These interruptions could result in launch delays, inventory shortages, recalls, unanticipated costs or issues with our agreements under which we supply third parties, which may adversely affect our operating results and financial condition. For example, during the COVID-19 pandemic we have experienced challenges in manufacturing certain products including Simparica Trio, and the component parts of certain products including Librela and Solensia, that have impacted our ability to meet customer demand. As a result, we have had to take certain measures including placing limits on the amounts of product veterinarians could purchase and delayed the launch of the product in certain markets.
Our manufacturing network, including our CMOs, may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites, and shifting customer demand (including as a result of market conditions or entry of branded or generic competition) increase the potential for capacity imbalances. In addition, construction of sites is expensive, and our ability to recover costs will depend on the market acceptance and success of the products produced at the new sites, which is uncertain.
We rely on third parties to provide us with products, materials and services, and are subject to increased labor and material costs and potential disruptions in supply.
The materials used to manufacture our products may be subject to availability constraints and price volatility caused by changes in demand, weather conditions, supply conditions, government regulations, economic climate and other factors, including any impacts caused by the COVID-19 pandemic. In addition, labor costs may be subject to volatility caused by the supply of labor, governmental regulations, economic climate and other
factors. Increases in the demand for, availability or the price of, materials used to manufacture our products and increases in labor costs could increase the costs to manufacture our products, result in product delivery delays or shortages, and impact our ability to launch new products on a timely basis or at all. We may not be able to pass all or a material portion of any higher product, material, transportation or labor costs on to our customers, which could materially adversely affect our operating results and financial condition.
Certain third-party suppliers are the sole or exclusive source of certain products, materials and services necessary for production of our products. We may be unable to meet demand for certain of our products if any of our third-party suppliers cease or interrupt operations, fail to renew contracts with us or otherwise fail to meet their obligations to us.
There may be delays and additional costs due to changes to our existing manufacturing facilities and the construction of new manufacturing plants.
As part of our supply network strategy, we have invested and will continue to invest in improvements to our existing manufacturing facilities and in new manufacturing plants. In addition, certain of our existing manufacturing facilities are in the process of being upgraded. These types of projects are subject to risks of delay or cost overruns inherent in any large construction project, and require licensure by various regulatory authorities. Significant cost overruns or delays in completing these projects could have an adverse effect on the Company’s return on investment.
Risks related to our research and development
Our R&D, acquisition and licensing efforts may fail to generate new products and product lifecycle innovations.
Our future success depends on both our existing product portfolio and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. We commit substantial effort, funds and other resources to R&D, both through our own dedicated resources and through collaborations with third parties.
We may be unable to determine with accuracy when or whether any of our products now under development will be approved or launched, or we may be unable to develop, license or otherwise acquire product candidates or products. In addition, we cannot predict whether any products, once launched, will be commercially successful or will achieve sales and revenue that are consistent with our expectations. The animal health industry is subject to regional and local trends and regulations and, as a result, products that are successful in some of our markets may not achieve similar success when introduced into new markets. Furthermore, the timing and cost of our R&D may increase, and our R&D may become less predictable. For example, changes in regulations applicable to our industry may make it more time-consuming and/or costly to research, develop and register products. If we are unable to generate new products or expand the use of our existing products, our business, financial condition and results of operations will be materially adversely affected.
New product R&D leverages discoveries of pharmaceutical and biotechnology R&D. We have and expect to continue to enter into collaboration or licensing arrangements with third parties to provide us with access to molecules, compounds and other technology for purposes of our business. Such agreements are typically complex and require time to negotiate and implement. If we enter into these arrangements, we may not be able to maintain these relationships or establish new ones in the future on acceptable terms or at all. In addition, any collaboration that we enter into may not be successful, and the success may depend on the efforts and actions of our collaborators, which we may not be able to control. If we are unable to access these technologies to conduct R&D on cost-effective terms, our ability to develop some types of new products could be limited.
We may experience difficulties or delays in the development and commercialization of new products.
New products may appear promising in development but fail to reach the market within the expected or optimal timeframe, or at all. In addition, product extensions or additional indications may not be approved. Developing and commercializing new products subjects us to inherent risks and uncertainties, including (i) delayed or denied regulatory approvals, (ii) delays or challenges with producing products in accordance with regulatory requirements, on a commercial scale and at a reasonable cost; (iii) failure to accurately predict the market for new products; and (iv) efficacy and safety concerns. In addition, a failure to continue to identify and develop products, both internally and through external sources, could impact our future success. Once necessary regulatory approvals are obtained, the commercial success of any new product depends upon, among other things, its acceptance by veterinarians and end customers, and on our ability to successfully manufacture, market, and distribute products in sufficient quantities to meet actual demand. The inability to successfully bring a product to market could negatively impact our revenues and earnings.
Our R&D relies on evaluations in animals, which may become subject to bans or additional restrictive regulations.
The evaluation of our existing and new medicines and vaccines for animals is required in order to develop and commercialize them. Animal testing in certain countries has been the subject of increased regulation, controversy and adverse publicity. Our licenses or permits for animal testing could be revoked or put on hold due to animal welfare events that may occur. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations applicable to animal testing and animal welfare. To the extent that the activities of such organizations and individuals are successful, our R&D, and by extension our operating results and financial condition, could be materially adversely affected. In addition, negative publicity about us or our industry could impact R&D projected timelines or harm our reputation or the reputation of our contract research organizations.
Risks related to legal matters and regulation
Our business is subject to substantial regulation.
As a global company, we are subject to various state, federal and international laws and regulations, including regulations relating to the development, quality assurance, manufacturing, importation, distribution, marketing and sale of our products. In addition, our manufacturing facilities are subject to periodic inspections by regulatory agencies. Our failure, or the failure of third parties we rely on, including CMOs, to comply with these regulatory requirements, allegations of such non-compliance or the discovery of previously unknown problems with a product or manufacturer could result in, among other things, inspection observation notices, warning letters or similar regulatory correspondence, fines, a partial or total shutdown of production in one or more of our facilities while an alleged violation is remediated, withdrawals or suspensions of current
products from the market, and civil or criminal prosecution, as well as decreased sales as a result of negative publicity and product liability claims. Any one of these consequences could materially adversely affect our operating results and financial condition.
In addition, we will not be able to market new products unless and until we have obtained all required regulatory approvals in each jurisdiction where we propose to market those products. Even after a product reaches market, it may be subject to re-review and may lose its approvals. We have changed, and may in the future change, the locations of where certain of our products are manufactured and, because of these changes, we may be required to obtain new regulatory approvals. Our failure to obtain approvals, delays in the approval process, including any delays resulting from any prolonged shutdown of the U.S. government, or our failure to maintain approvals in any jurisdiction, may prevent us from selling products in that jurisdiction until approval or reapproval is obtained, if ever.
The OFAC at the U.S. Treasury Department and the Bureau of Industry and Security at the U.S. Department of Commerce (BIS), and similar agencies in other countries and territories outside the U.S., administer certain laws and regulations that restrict its persons and, in some instances, extraterritorial persons, in conducting activities, transacting business with or making investments in certain countries, governments, entities and individuals subject to economic sanctions. Our international operations subject us to these laws and regulations, which are complex, restrict our business dealings with certain countries, governments, entities, and individuals, and are constantly changing. For example, we sell limited humanitarian animal health products, including medicines, diagnostics and vaccines, to Russia and Iran, in compliance with economic sanctions affecting these countries. Violations of sanctions regulations may be punishable by civil penalties, including fines, denial of export privileges, injunctions, asset seizures, debarment from government contracts and revocations or restrictions of licenses, as well as criminal fines and imprisonment, which could adversely affect our reputation, business, financial condition, results of operations and cash flows. In addition, our internal control policies and procedures may not protect us from reckless or criminal acts committed by our employees and agents. For example, in December 2020, we submitted a final voluntary disclosure to OFAC and the U.S. Department of Justice regarding certain transactions involving sales of food, medicine or devices to individuals or entities who may have been resident in or had ties to Iran potentially in violation of the ITSR administered by OFAC. The sales were made by our Platinum Performance business, which we acquired in August 2019. In July 2023, OFAC provided a No Action letter confirming a final determination that no further action would be taken in the matter. The U.S. Department of Justice has not responded to date.
A failure to comply with the environmental, health and safety laws and regulations to which we are subject, including any permits issued thereunder, may result in environmental remediation costs, loss of permits, fines, penalties or other adverse governmental or private actions, including regulatory or judicial orders enjoining or curtailing operations or requiring corrective measures, installation of pollution control equipment or remedial measures. We cannot assure you that our costs of complying with current and future environmental, health and safety laws, and our liabilities arising from past or future releases of, or exposure to, hazardous materials will not materially adversely affect our business, results of operations or financial condition.
There has been a broad range of proposed and promulgated state, national and international regulation aimed at reducing the effects of climate change. Such regulations apply or could apply in countries where we have interests or could have interests in the future. The EU recently adopted the European Sustainability Reporting Standards (ESRS) and the Corporate Sustainability Reporting Directive (CSRD) that will require disclosure by EU entities, including certain EU subsidiaries of non-EU entities, regarding the risks and opportunities arising from environmental, social and corporate governance issues, and on the impact of companies' activities on people and the environment. Similarly, the State of California recently passed the Climate Corporate Data Accountability Act and the Climate-Related Financial Risk Act that will impose broad climate-related disclosure obligations on certain companies doing business in California, including us, starting in 2026. In the U.S., there is a significant possibility that some form of regulation will be enacted at the federal level to address the effects of climate change, including the climate change disclosure rules from the SEC that are expected to be finalized in 2024. Such regulation could take several forms that could result in additional costs in the form of investments of capital to maintain compliance with laws and regulations and taxes. Climate change regulation continues to evolve, and it is not possible to accurately estimate either a timetable for implementation or our future compliance costs relating to implementation.
We are also subject to chemical regulation in the United States and internationally. For example, governmental authorities in the United States are increasingly focused on preventing environmental contamination from per and polyfluoroalkyl substances (PFAS), which may be contained in certain of our products. For example, the state of Maine requires reporting of intentionally added PFAS in products, and the sale in Maine of any products containing intentionally added PFAS will be prohibited after January 1, 2030 (subject to certain exceptions to be promulgated by the Maine Department of Environmental Protection). In addition, federal and state governments and agencies are in various stages of considering and/or implementing laws and regulations requiring the reporting, restriction and/or phase-out of PFAS products.
Furthermore, we cannot predict the nature of future laws, regulations, or changes in tax laws and tariffs, nor can we determine the effect that additional laws or regulations or changes in existing laws or regulations could have on our business when and if promulgated. Changes in applicable federal, state, local and foreign laws and regulations could have a material adverse effect on our operating results and financial condition.
We may incur substantial costs and receive adverse outcomes in litigation and other legal matters.
Our operating results, financial condition and liquidity could be materially adversely affected by unfavorable results in pending or future litigation matters. In addition, changes in the interpretations of laws and regulations to which we are subject, or in legal standards in one or more of the jurisdictions in which we operate, could increase our exposure to liability. For example, in the U.S., attempts have been made to allow damages for emotional distress and pain and suffering in connection with the loss of, or injury to, a companion animal. If such attempts were successful, our exposure with respect to product liability claims could increase materially. We also sell certain nutritional and diagnostic products used in human health that could increase the scope of our liability.
Litigation matters, regardless of their merits or their ultimate outcomes, are costly, divert management's attention and may materially adversely affect our reputation and demand for our products. We cannot predict with certainty the eventual outcome of pending or future litigation matters. An adverse outcome of litigation or legal matters could result in our being responsible for significant damages. Any of these negative effects resulting from litigation matters could materially adversely affect our operating results and financial condition.
The illegal distribution and sale by third parties of counterfeit or illegally compounded versions of our products or of stolen, diverted or relabeled products could have a negative impact on our reputation and business.
Third parties may illegally distribute and sell counterfeit or illegally compounded versions of our products that do not meet the exacting standards of our development, manufacturing and distribution processes. Counterfeit or illegally compounded medicines pose a significant risk to animal health and safety because of the conditions under which they are manufactured and the lack of regulation of their contents. Our reputation and business could suffer harm as a result of counterfeit or illegally compounded products which are alleged to be equivalent and/or which are sold under our brand name. We are aware of at least one pharmacy in Brazil that may be engaged in the practice of illegally compounding oclacitinib, the active pharmaceutical ingredient in our Apoquel product. We are also aware of some counterfeit versions of our Simparica product in Brazil and are coordinating with the local authorities. In addition, products stolen or unlawfully diverted from inventory, warehouses, plants or while in transit, which are not properly stored or which have an expired shelf life and which have been repackaged or relabeled and which are sold through unauthorized channels, could adversely impact animal health and safety, our reputation and our business. Public loss of confidence in the integrity of vaccines and/or pharmaceutical products as a result of counterfeiting, illegally compounding or theft could have a material adverse effect on our product sales, business and results of operations.
The misuse or off-label use of our products may harm our reputation or result in financial or other damages.
Our products have been approved for use under specific circumstances for the treatment of certain diseases and conditions in specific species. There may be increased risk of product liability claims and other liability if veterinarians, livestock producers, pet owners or others attempt to use our products off-label, including the use of our products in species (including humans) for which they have not been approved. In addition, certain of our products are regulated by the U.S. Drug Enforcement Administration as controlled substances because of their potential to be misused or abused by humans, which could expose us to liability. For example, Ketamine, the active pharmaceutical ingredient in our Ketaset product (a nonnarcotic, nonbarbiturate agent for anesthetic use in cats), is classified as a Schedule III drug under the Controlled Substances Act due to its moderate to low potential for physical and psychological dependence. Furthermore, the use of our products for indications other than those indications for which our products have been approved may not be effective, which could harm our reputation and lead to an increased risk of litigation. If we are deemed by a governmental or regulatory agency to have engaged in the promotion of any of our products for off-label use, such agency could request that we modify our training or promotional materials and practices and we could be subject to significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. Additionally, if we fail to maintain effective controls against diversion of our products that are classified as controlled substances, we could be subject to significant fines and penalties as well as reputational damage. Any of these events could materially adversely affect our operating results and financial condition.
Our operations and reputation may be impacted if we do not comply with continually changing laws and regulations regarding data privacy.
We collect and use personal data of our customers, employees and suppliers, including health information in our human health business, in a variety of ways. In addition, we have been investing in data and digital capabilities and have expanded our diagnostics portfolio. As a result, we possess and process an increasing amount of personal data. Our customers, employees and suppliers expect that we will adequately protect their data.
Our collection, use, retention, storage, and sharing of personal data is subject to a variety of data privacy laws and regulations in the United States and other regions where we operate. The legal environment surrounding data privacy is demanding with the frequent imposition of new and changing regulatory requirements.
As a global company, we are faced with the challenge of how to manage a diverse patchwork of laws, rules, regulations and industry standards, including, but not limited to, the California Consumer Privacy Act, the EU's General Data Protection Regulation, the U.K.'s General Data Protection Regulation, the Brazilian General Data Protection Law, and China’s Personal Information Protection Law. These laws and regulations vary across countries, are complex and can be subject to significant change. Any actual or perceived failure to comply with these current and future laws could result in significant consequences for Zoetis. This includes substantial fines and penalties, regulatory investigations, and civil lawsuits with damages, all of which could have a material adverse effect on our reputation and our business. In addition, the costs associated with compliance with these legal and regulatory requirements are significant and likely to increase in the future.
Our aspirations, goals and disclosures related to environmental, social and governance (“ESG”) matters expose us to numerous risks, including risks to our reputation.
Our Driven to Care sustainability program includes various ESG aspirations and goals. These goal statements reflect our current plans and aspirations and are not guarantees that we will be able to achieve them. Our efforts to accomplish and accurately report on these goals and objectives present numerous operational, reputational, financial, legal and other risks, any of which could have a material negative impact, including on our reputation. Our ability to achieve any goal or objective, including with respect to environmental and diversity initiatives, is subject to numerous risks, many of which are outside of our control. Examples of such risks include: (1) the availability and cost of low- or non-carbon-based energy sources and technologies, (2) evolving regulatory requirements and rulings affecting ESG and diversity standards or disclosures, (3) our ability to recruit, develop and retain diverse talent in our labor markets, and (4) the impact of our organic growth and acquisitions or dispositions of businesses or operations.
The standards for tracking and reporting on ESG matters and continue to evolve. Our processes and controls may not always align with evolving standards for identifying, measuring and reporting ESG metrics, our interpretation of reporting standards that may be required by the SEC, European Union and other regulators may differ from those of others and such standards may change over time, any of which could result in significant revisions to our goals or reported progress in achieving such goals. If our ESG practices do not meet evolving investor or other stakeholder expectations and standards, then our reputation, our ability to attract or retain employees and our attractiveness as an investment, business partner or acquirer could be negatively impacted. Similarly, our failure or perceived failure to pursue or fulfill our goals, targets and objectives or to satisfy various reporting standards within the timelines we announce, or at all, could also have similar negative impacts and expose us to government enforcement actions and private litigation.
Risks related to operating in foreign jurisdictions
A significant portion of our operations are conducted in foreign jurisdictions, including jurisdictions presenting a high risk of bribery and corruption, and are subject to the economic, political, legal and business environments of the countries in which we do business.
Our international operations could be limited or disrupted by any of the following:
•volatility in the international financial markets;
•difficulties enforcing contractual and intellectual property rights;
•theft or compromise of technology, data and intellectual property;
•parallel trade in our products (importation of our products from EU countries where our products are sold at lower prices into EU countries where the products are sold at higher prices);
•compliance with a wide variety of potentially changing and conflicting laws and regulations, such as the FCPA, the U.K. Bribery Act of 2010 and similar anti-bribery and corruption-related laws globally, including labor laws, tax laws, tariffs and those relating to environmental, health and safety requirements;
•political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts;
•trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by OFAC and the EU, in relation to our products or the products of farmers and other customers (e.g., restrictions on the importation of agricultural products from the EU to Russia);
•government limitations on foreign ownership or government takeover or nationalization of our business;
•imposition of anti-dumping and countervailing duties or other trade-related sanctions;
•costs and difficulties and compliance risks in staffing, managing and monitoring international operations, including the use of overseas third-party goods and service providers;
•longer payment cycles and increased exposure to counterparty risk; and
•additional limitations on transferring personal information between countries or other restrictions on the processing of personal information.
While the impact of these factors is difficult to predict, any of them could materially adversely affect our operating results and financial condition. Changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings.
Foreign exchange rate fluctuations and potential currency controls affect our results of operations, as reported in our financial statements.
We conduct operations in many areas of the world, involving transactions denominated in a variety of currencies. In 2023, we generated approximately 43% of our revenue in currencies other than the U.S. dollar, principally the euro, Brazilian real, Australian dollar, Chinese renminbi, British pound and Canadian dollar. We are subject to currency exchange rate risk to the extent that our costs are denominated in currencies other than those in which we earn revenue. In addition, because our financial statements are reported in U.S. dollars, changes in currency exchange rates, including changes in countries with highly inflationary economies, between the U.S. dollar and other currencies have had, and will continue to have, an impact on our results of operations.
We also face risks arising from currency devaluations and the imposition of cash repatriation restrictions and exchange controls. Currency devaluations result in a diminished value of funds denominated in the currency of the country instituting the devaluation. Cash repatriation restrictions and exchange controls may limit our ability to convert foreign currencies into U.S. dollars or to remit dividends and other payments by our foreign subsidiaries or businesses located in or conducted within a country imposing restrictions or controls. We currently have substantial operations in countries that have cash repatriation restrictions or exchange controls in place, including China, and, if we were to need to repatriate or convert such cash, these controls and restrictions may have a material adverse effect on our operating results and financial condition.
We may not be able to realize the expected benefits of our investments in emerging markets and are subject to certain risks due to our presence in emerging markets, including political or economic instability and failure to adequately comply with legal and regulatory requirements.
We have been taking steps to increase our presence in emerging markets. Failure to continue to maintain and expand our business in emerging markets could materially adversely affect our operating results and financial condition.
Some countries within emerging markets may be especially vulnerable to periods of local, regional or global economic, political or social instability or crisis. For example, our sales in certain emerging markets have suffered from extended periods of disruption due to natural disasters and adverse weather conditions. Furthermore, we have also experienced lower than expected sales in certain emerging markets due to local, regional and global restrictions on banking and commercial activities in those countries. In addition, certain emerging markets have currencies that fluctuate substantially, which may impact our financial performance. For example, in the past, our revenue in certain emerging markets in Latin America has been adversely impacted by currency fluctuations and devaluations.
In addition, certain emerging markets have legal systems that are less developed or familiar to us. Compliance with diverse legal requirements is costly and time-consuming and requires significant resources. In the event we believe or have reason to believe our employees have or may have violated applicable laws or regulations, we may be subject to investigation costs, potential penalties and other related costs which in turn could negatively affect our reputation and our results of operations.
Risks related to tax matters
The Company could be subject to changes in its tax rates, the adoption of new U.S. or foreign tax legislation or exposure to additional tax liabilities.
The multinational nature of our business subjects us to taxation in the U.S. and numerous foreign jurisdictions. Due to economic and political conditions, tax rates in various jurisdictions may be subject to significant change. The company’s future effective tax rates could be affected by changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, or changes in tax laws or their interpretation.
For example, the Organisation for Economic Co-operation and Development (OECD) is continuing to work on fundamental changes in the allocation of profits among tax jurisdictions in which companies do business (Pillar One), as well as the implementation of a global minimum tax (Pillar Two). Global minimum tax legislation has been proposed and/or enacted in various jurisdictions. These two pillars combined represent a significant change in the international tax regime, and there is risk of an adverse impact to our effective tax rate, but the amount of such impact remains uncertain at this time.
In addition, our effective tax rate is subject to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional tax, adversely impacting our effective tax rate and our tax liability. The company is also subject to the examination of its tax positions, tax returns and other tax matters by the Internal Revenue Service and other tax authorities and governmental bodies. There can be no assurance as to the outcome of these examinations. If the company’s effective tax rates were to increase, particularly in the U.S. or other material foreign jurisdictions, or if the company's tax positions are either not sustained upon examination or only partially sustained, the company’s operating results, cash flows and financial condition could be adversely affected.
Risks related to intellectual property
The alleged intellectual property rights of third parties may negatively affect our business.
A third party may sue us, our distributors or licensors, or otherwise make a claim, alleging infringement or other violation of the third-party's patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights. The costs of defending an intellectual property action are often substantial and could materially adversely affect our operating results and financial condition, even if we successfully defend such action. Moreover, even if we believe that we do not infringe a validly existing third-party patent, we may choose to license such patent, which would result in associated costs and obligations. We may also incur costs in connection with an obligation to compensate a distributor, licensor or other third party. The intellectual property positions of animal health medicines and vaccines businesses frequently involve complex legal and factual questions, and an issued patent does not provide the right to practice the patented technology or to develop, manufacture or commercialize the patented product. We cannot guarantee that a competitor or other third party does not have or will not obtain rights to intellectual property that, in the absence of a license, may prevent us from manufacturing, developing or marketing certain of our products, regardless of whether we believe such intellectual property rights are valid and enforceable, which may harm our operating results and financial condition.
If our intellectual property rights are challenged or circumvented, competitors may be able to take advantage of our research and development efforts.
Our long-term success largely depends on our ability to market technologically competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret, data protection, and domain name protection laws, as well as confidentiality and license agreements with our employees and others, to protect our intellectual property and proprietary rights. In addition, many of our vaccine products and other products are based on or incorporate proprietary information, including proprietary master seeds and proprietary or patented adjuvant formulations.
If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from using our proprietary technologies or from marketing products that are very similar or identical to ours. The validity, enforceability, scope and effective term of our intellectual property can be highly uncertain and often involve complex legal and factual questions and proceedings that differ between jurisdictions. Our ability to enforce our intellectual property rights also depends on the laws of individual countries and each country's practice with respect to enforcement of intellectual property rights. In addition, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, including because such agreements expire or are terminated, our operating results and financial condition could be adversely affected.
We are regularly party to patent litigation and other intellectual property rights claims that are expensive and time consuming, and if resolved adversely, could have a significant impact on our business and financial condition.
Risks related to information technology
We may be unable to adequately protect our information technology systems from cyberattacks, breaches of security or misappropriation of data, which could result in the disclosure of confidential information, damage our reputation, and subject us to significant financial and legal exposure.
Our reputation as a global leader in animal health and our reliance on complex information systems and digital solutions make us inherently vulnerable to malicious cyber intrusion and attack. In addition, we have been investing in data and digital capabilities and have expanded our diagnostics portfolio, and as a result, there could be an increased likelihood of a cyberattack or breach of security that could negatively impact us or our customers. Cyberattacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyberattacks could include wrongful conduct by hostile foreign governments, industrial espionage, the deployment of harmful malware, ransomware, denial-of-service attacks, and other means to threaten data confidentiality, integrity and availability. In addition, despite our efforts to protect sensitive, confidential or personal data or information, we (or our third party partners) may be vulnerable to material security breaches, theft, misplaced or lost data, programming errors, employee errors and/or malfeasance that could potentially lead to the compromise of sensitive, confidential or personal data or information, improper use of our systems or networks, unauthorized access, use, disclosure, modification or
destruction of information (including confidential business information, trade secrets, intellectual property and corporate strategic plans), defective products, production downtimes and operational disruptions.
In support of our flexible work environment, many of our workforce work either part-time or full-time remotely, which could increase risks associated with cybersecurity, information technology and systems which could have a material adverse effect on our business.
The costs imposed on us as a result of a cyberattack or network disruption could be significant. Among others, such costs could include increased expenditures on cybersecurity measures, litigation, regulatory investigations, fines, and sanctions, lost revenues from business interruption, damage to the public’s perception regarding our ability to keep our information secure and significant remediation costs. As a result, a cyberattack or network disruption could have a material adverse effect on our business, financial condition, and operating results.
We depend on sophisticated information technology and infrastructure.
We rely on the efficient and uninterrupted operation of complex information technology systems to manage our operations, to process, transmit and store electronic and financial information, and to comply with regulatory, legal and tax requirements. We also depend on our information technology infrastructure for digital marketing activities and for electronic communications among our personnel, customers and suppliers around the world. System failures or outages could compromise our ability to perform these functions in a timely manner, which could harm our ability to conduct business, hurt our relationships with our customers, or delay our financial reporting. Such failures could materially adversely affect our operating results and financial condition.
In addition, we depend on third parties and applications on virtualized (cloud) infrastructure to operate and support our information systems. These third parties include large established vendors, as well as many small, privately owned companies. Failure by these providers to adequately support our operations or a change in control or insolvency of these providers could have an adverse effect on our business, which in turn may materially adversely affect our operating results and financial condition.
We may be unable to successfully manage our online ordering sites.
In many markets around the world, such as the U.S. and Brazil, we provide online ordering sites to customers, often relying on third parties to host and support the application. The operation of our online business depends on our ability to maintain the efficient and uninterrupted operation of our online order-taking and fulfillment operations. Risks associated with our online business include: disruptions in telephone or internet service or power outages; failures of the information systems that support our website, including inadequate system capacity, computer viruses, human error, changes in programming, security breaches, system upgrades or migration of these services to new systems; reliance on third parties for computer hardware and software as well as delivery of merchandise to our customers; rapid technology changes; credit card fraud; natural disasters or adverse weather conditions; power and network outages; changes in applicable federal and state regulations; liability for online content; and consumer privacy concerns. Problems in any one or more of these areas could have a material adverse effect on our operating results and financial condition and could damage our reputation.
Risks related to our indebtedness
We have substantial indebtedness.
We have a significant amount of indebtedness, which could materially adversely affect our operating results, financial condition and liquidity. As of December 31, 2023, we had approximately $6.7 billion of total unsecured indebtedness outstanding. In addition, we currently have agreements for a multi-year revolving credit facility and a commercial paper program, each with a capacity of up to $1.0 billion. While we currently do not have any amounts drawn under the credit facility nor any commercial paper issued under the commercial paper program, we may incur indebtedness under these arrangements in the future.
We may incur substantial additional debt from time to time to finance working capital, capital expenditures, investments or acquisitions, or for other purposes. If we do so, the risks related to our high level of debt could intensify. Specifically, our high level of debt could have important consequences, including:
•making it more difficult for us to satisfy our obligations with respect to our debt;
•limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general corporate requirements, including dividends;
•increasing our vulnerability to general adverse economic and industry conditions;
•exposing us to the risk of increased interest rates as certain of our borrowings may in the future be at variable rates of interest;
•limiting our flexibility in planning for and reacting to changes in the animal health industry;
•placing us at a competitive disadvantage to other, less leveraged competitors;
•impacting our effective tax rate; and
•increasing our cost of borrowing.
In addition, the instruments governing our indebtedness contain restrictive covenants that will limit our ability to engage in activities that may be in our long-term best interest. Our failure to comply with such covenants could result in an event of default, which could result in the acceleration of all our debt.
Our credit ratings may not reflect all risks of an investment in our senior notes. Actual or anticipated changes or downgrades in our credit ratings, including any announcement that our ratings are under further review for a downgrade, could affect the market prices of our securities and increase our borrowing costs.
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or refinance our debt obligations depends on our financial condition and operating performance, which are subject to prevailing economic and competitive conditions and to certain financial, business, legislative, regulatory and other factors beyond our control. We may be unable to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we could face substantial liquidity problems and could be forced to reduce or delay investments and capital expenditures, or to dispose of material assets or operations, alter our dividend policy, seek additional debt or equity capital or restructure or refinance our indebtedness. We may not be able to effect any such alternative measures on commercially reasonable terms or at all and, even if successful, those alternative actions may not allow us to meet our scheduled debt service obligations.
In addition, we conduct our operations through our subsidiaries. Accordingly, repayment of our indebtedness will depend on the generation of cash flow by our subsidiaries, including certain international subsidiaries, and their ability to make such cash available to us, by dividend, debt repayment or otherwise. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness.
Our inability to generate sufficient cash flows to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, may materially adversely affect our operating results, financial condition and liquidity and our ability to satisfy our obligations under our indebtedness or pay dividends on our common stock.
We may not have the funds necessary to finance the change of control offer required by the indenture governing our senior notes.
Upon the occurrence of a change of control of Zoetis and a downgrade below investment grade by Moody's Investor Services, Inc. and S&P Global Ratings, a division of S&P Global Inc., we will be required to offer to repurchase all of our outstanding senior notes. However, we may not have sufficient funds available at the time of the change of control to finance the required change of control offer or restrictions in our then-existing debt instruments will not allow such repurchases. Our failure to purchase the senior notes as required under the indenture would result in a default under the indenture, which could have material adverse consequences for us and the holders of the senior notes.
Risks related to our relationship with Pfizer
Pfizer's rights as licensor under the patent and know-how license could limit our ability to develop and commercialize certain products.
Under the Patent and Know-How License Agreement (Pfizer as licensor) Pfizer licenses to us certain of its intellectual property. If we fail to comply with our obligations under this license agreement and Pfizer exercises its right to terminate it, our ability to continue to research, develop and commercialize products incorporating that intellectual property will be limited. In addition, in circumstances where Pfizer has an interest in the licensed intellectual property in connection with its human health development programs, our rights to use the licensed intellectual property are restricted and/or, in limited instances, subject to Pfizer's right to terminate such license at will. These limitations and termination rights may make it more difficult, time-consuming or expensive for us to develop and commercialize certain new products, or may result in our products being later to market than those of our competitors.
We are dependent on Pfizer to prosecute, maintain and enforce certain intellectual property.
Under the Patent and Know-How License Agreement, Pfizer is responsible for filing, prosecuting and maintaining patents that Pfizer licenses to us. In the animal health field, Pfizer has the first right, and in some cases the sole right, to enforce such licensed patents, and in the human health field, subject to certain exceptions, Pfizer has the sole right to enforce the licensed patents. If Pfizer fails to fulfill its obligations or chooses to not enforce the licensed patents under this agreement, we may not be able to prevent competitors from making, using and selling competitive products, which could have an adverse effect on our business.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
As a global leader in animal health, we are reliant on complex information systems and digital solutions that make us inherently vulnerable to malicious cyber intrusion and attack. In addition, we have been expanding our data and digital capabilities including in our diagnostics portfolio, and as a result, there could be an increased likelihood of a cyberattack or breach of security that could negatively impact us or our customers. Despite the presence of these risks, to date, the identified risks of cybersecurity threats (including as a result of any previous cybersecurity incidents) have not materially affected, and are not reasonably likely to materially affect, us or our business strategy, results of operations, or financial condition. For a description of the risks from cybersecurity threats that may materially affect us and how they may do so, see our risk factors under Part 1. Item 1A. Risk Factors in this Annual Report on Form 10-K.
Cybersecurity Program
As part of our risk management processes, we have an enterprise-wide cybersecurity program aligned to the NIST Cybersecurity Framework. Our program is a risk-based program designed to protect our information systems through multiple defenses and layers of security, commonly referred to as a “Defense in Depth” approach. Key elements of our program include:
Independent Third-Party Assessments
We engage an independent third party to conduct assessments of our cybersecurity program approximately every 18 months. This independent third-party assessment includes an evaluation of our cybersecurity controls based on the NIST Cybersecurity Framework.
Training
We have an information security training program that includes: monthly awareness articles, a phishing training program (with reports reviewed by the Executive Team), a Security Ambassador program for additional training and awareness for individuals in high risk roles, required and optional training modules in our Learning Management System, and quarterly security-focused podcasts.
Incident Response Procedure
We have a 24/7 managed Security Operations Center (SOC) for escalation of any critical events, including cybersecurity incidents. In the event of an incident, we use an Incident Response procedure leveraging NIST Standard 800-61 standards that we have customized for Zoetis. Additionally, we have in place disaster recovery and business continuity practices designed to provide for continuous business operations for our customers in the event of a cybersecurity incident. While we maintain cybersecurity insurance, the costs related to cybersecurity threats or disruptions may not be fully insured.
Third Party Onboarding
We depend on third parties and applications on virtualized (cloud) infrastructure to operate and support our information systems and have a third-party risk management program and assessment process for onboarding third parties.
Management’s Role in Risk Oversight
Our information security team includes our Executive Vice President, Chief Digital & Technology Officer; our Vice President, Chief Information Officer; and our Head of Technology Risk, Compliance and Chief Information Security Officer. Our Executive Vice President, Chief Digital and Technology Officer has over 20 years of information technology experience. She was the Chief Information Officer for key business units at an S&P 500 healthcare company, and was that company’s first Chief Information Security Officer, where she led the strategy and execution to secure products, devices, manufacturing systems and information across businesses. She holds a master’s degree in computer science and a master’s degree in business applications of information and technology. Our Vice President, Chief Information Officer has over 20 years of experience in technology and digital leadership roles at large public companies and holds a bachelor’s degree in information systems. Our Head of Technology Risk, Compliance and Chief Information Security Officer has over 20 years of experience in information security, and holds a bachelor’s degrees in computer science and biology.
We have established a cybersecurity governance program with clear roles for the executive management team as well as oversight by the Board of Directors and the Audit Committee. The Zoetis information security team provides regular cyber threat intelligence briefings to management and provides updates to our senior executives on the status of the Company’s security measures and our efforts to identify and mitigate risks from cybersecurity threats. The Zoetis information security team also works closely with the Zoetis Legal team, including the Chief Privacy Officer, to further enhance incident response procedures. For example, we have a corporate crisis management plan in place to govern our response to corporate crises, which could include cyber incidents, and we conduct periodic simulated programs to ensure readiness. This plan also includes a standard framework for categorization of incidents based on risk level and severity, and requires escalation to Zoetis senior management and/or the Audit Committee of the Board of Directors if certain severity levels are met.
Role of the Board of Directors and Committees
The Board of Directors maintains an active role in the oversight of material risks. The Board of Directors utilizes its various Committees to oversee certain key risks, and has delegated responsibility to the Audit Committee for oversight of the Company’s enterprise risk management process and information security risk management program. Management, with oversight from the Zoetis Board of Directors, is responsible for the Company’s assessment and management of exposure to risk. The Audit Committee of the Board of Directors is also responsible for oversight of compliance with disclosure requirements under applicable laws and regulations, and would be consulted prior to the disclosure of any material cybersecurity incident.
The Zoetis information security team regularly provides an information security dashboard to the Audit Committee, covering the most active and relevant threats to Zoetis, relevant trends, and any notable events. At least twice annually, the Zoetis information security team presents updates to the Audit Committee with respect to the information security program, including the status of our security measures and our efforts to identify and mitigate information security risks. The Audit Committee also regularly reviews certain data privacy and cybersecurity metrics as part of the compliance update presented to the Audit Committee.
In addition, the Chief Information Security Officer presents updates at least annually to the Board of Directors with respect to the information security program, including the results of our independent, third-party assessment. The Board of Directors also participates in annual table-top exercises involving simulated data security incidents and the Company’s responses to those incidents.
Item 2. Properties.
We have approximately 190 owned and leased properties, amounting to approximately 13.5 million square feet, around the world for sales and marketing, customer service, regulatory compliance, R&D, manufacturing and distribution, and administrative support functions. In many locations, operations are co-located to achieve synergies and operational efficiencies. Our largest R&D facility is our owned U.S. research and development site located in Kalamazoo, Michigan, which represents approximately 1.6 million square feet. None of our other non-manufacturing sites are more than 0.3 million square feet. The largest manufacturing site in our global manufacturing network is our manufacturing site located in Kalamazoo, Michigan, which represents approximately 0.6 million square feet. No other site in our global manufacturing network is more than 0.6 million square feet. In addition, our global manufacturing network continues to be supplemented by 109 CMOs.
Our corporate headquarters are located at 10 Sylvan Way, Parsippany, New Jersey 07054. Our operations extend internationally to 57 countries.
We believe that our existing properties, as supplemented by sites operated by CMOs, are adequate for our current requirements and for our operations in the foreseeable future.
Item 3. Legal Proceedings.
We are from time to time subject to claims and litigation arising in the ordinary course of business. These claims and litigation may include, among other things, allegations of violation of U.S. and foreign competition law, labor laws, consumer protection laws, and environmental laws and regulations, as well as claims or litigation relating to product liability, intellectual property, securities, breach of contract and tort. We operate in multiple jurisdictions and, as a result, a claim in one jurisdiction may lead to claims or regulatory penalties in other jurisdictions. We intend to defend vigorously against any pending or future claims and litigation.
At this time, in the opinion of management, the likelihood is remote that the impact of such proceedings, either individually or in the aggregate, would have a material adverse effect on our consolidated results of operations, financial condition or cash flows. However, one or more unfavorable outcomes in any claim or litigation against us could have a material adverse effect for the period in which they are resolved. In addition, regardless of their merits or their ultimate outcomes, such matters are costly, divert management's attention and may materially adversely affect our reputation, even if resolved in our favor.
Certain legal proceedings in which we are involved are discussed in Notes to Consolidated Financial Statements— Note 18. Commitments and Contingencies, and are incorporated by reference from such discussion.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our shares of common stock have been listed on the NYSE (symbol ZTS) since February 1, 2013. Prior to that time, there was no public market for our stock.
As of February 9, 2024, there were 457,867,115 shares of our common stock outstanding, held by 1,574 shareholders of record.
Additional information relating to our common stock is included in this Annual Report on Form 10-K in Notes to Consolidated Financial Statements— Note 16. Stockholders' Equity.
Purchases of Equity Securities by the Issuer
On December 7, 2021, our Board of Directors authorized a multi-year share repurchase program of up to $3.5 billion of our outstanding common stock. As of December 31, 2023, there was $1.5 billion remaining under this authorization.
The program does not have a stated expiration date. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs. We repurchase shares pursuant to Rules 10b5-1 and 10b-18 under the Securities Exchange Act of 1934, as amended (Exchange Act), through repurchase agreements established with several brokers.
Issuer purchases of equity securities for the three months ended December 31, 2023 were as follows: | | | | | | | | | | | | | | |
| Issuer Purchases of Equity Securities(b) |
| Total Number of Shares Purchased(a) | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Programs | Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs |
| October 1 - October 31, 2023 | 621,480 | $167.90 | 619,842 | $1,626,212,036 |
| November 1 - November 30, 2023 | 312,415 | $165.78 | 311,950 | $1,574,494,738 |
| December 1 - December 31, 2023 | 419,971 | $189.76 | 418,966 | $1,494,864,468 |
| Total | 1,353,866 | $174.19 | 1,350,758 | $1,494,864,468 |
(a) The company repurchased 3,108 shares during the three-month period ended December 31, 2023, that were not part of the publicly announced share repurchase authorization. These shares were purchased from employees to satisfy tax withholding requirements on the vesting of restricted shares from equity-based awards.
(b) Amounts exclude the impact of excise tax on net share repurchases.
Dividend Policy, Declaration and Payment
The declaration and payment of dividends to holders of our common stock will be at the discretion of our Board of Directors in accordance with applicable law after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, cash flows available in the U.S., impact on our effective tax rate, indebtedness, legal requirements and other factors that our Board of Directors deems relevant. In addition, the instruments governing our indebtedness may limit our ability to pay dividends. Therefore, no assurance is given that we will pay any dividends to our common stockholders or as to the amount of any such dividends if our Board of Directors determines to do so.
Because we are a holding company, our ability to pay cash dividends on our common stock will depend on the receipt of dividends or other distributions from certain of our subsidiaries.
Stock Performance Graph(a)
The graph below compares the cumulative total shareholder return on an investment in our common stock, the S&P 500 Index and the S&P 500 Pharmaceuticals Index for the five fiscal years beginning with the close of trading on December 31, 2018 and ending December 31, 2023. The shareholder return shown on the graph is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future shareholder returns.
The graph assumes an investment of $100 on December 31, 2018, in our common stock, the S&P 500 Index and the S&P 500 Pharmaceuticals Index and assumes dividends, if any, were reinvested.
COMPARISON OF CUMULATIVE TOTAL RETURN
Among Zoetis Inc., the S&P 500 Index and the S&P 500 Pharmaceuticals Index
| | | | | | | | | | | | | | | | | | | | |
| December 31, 2018 | December 31, 2019 | December 31, 2020 | December 31, 2021 | December 31, 2022 | December 31, 2023 |
| Zoetis Inc. | $100 | $155.71 | $195.83 | $290.34 | $175.64 | $238.70 |
| S&P 500 Index | $100 | $131.49 | $155.68 | $200.37 | $164.08 | $207.21 |
| S&P 500 Pharmaceuticals Index | $100 | $115.09 | $123.75 | $155.62 | $168.77 | $169.33 |
(a) This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of Zoetis under the Securities Act of 1933, as amended, or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language contained in any such filing.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Introduction
Our management’s discussion and analysis of financial condition and results of operations (MD&A) is provided to assist readers in understanding our performance, as reflected in the results of our operations, our financial condition and our cash flows. This MD&A should be read in conjunction with our consolidated financial statements and notes to consolidated financial statements included in Item 8. Financial Statements and Supplementary Data. The discussion in this MD&A contains forward-looking statements that involve substantial risks and uncertainties. Our objective is to also provide discussion of material events and uncertainties known to management that are reasonably likely to cause reported financial information not to be indicative of future results, which could differ materially from historical performance and from those anticipated in the forward-looking statements as a result of various factors such as those discussed in Item 1A. Risk Factors and Forward-looking statements and factors that may affect future results sections of this MD&A.
A discussion regarding our financial condition and results of operations for fiscal 2023 compared to fiscal 2022 is presented below. A discussion regarding our financial condition and results of operations for fiscal 2022 compared to fiscal 2021 can be found under Item 7 of Part II of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 14, 2023 (our “2022 Annual Report”), which is available free of charge on the SEC’s website at www.sec.gov.
Overview of our business
We are a global leader in the animal health industry, focused on the discovery, development, manufacture and commercialization of medicines, vaccines, diagnostic products and services, biodevices, genetic tests and precision animal health. For over 70 years, we have been innovating ways to predict, prevent, detect, and treat animal illness, and continue to stand by those raising and caring for animals worldwide - from veterinarians and pet owners to livestock farmers and ranchers.
We manage our operations through two geographic operating segments: the United States (U.S.) and International. Within each of these operating segments, we offer a diversified product portfolio for both companion animals and livestock customers in order to capitalize on local and regional trends and customer needs. See Notes to Consolidated Financial Statements—Note 19. Segment Information.
We directly market our products to veterinarians and livestock producers located in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America, and are a market leader in nearly all of the major regions in which we operate. Through our efforts to establish an early and direct presence in many emerging markets, such as Brazil, Chile, China and Mexico, we believe we are one of the largest animal health medicines and vaccines businesses as measured by revenue across emerging markets as a whole. In markets where we do not have a direct commercial presence, we generally contract with distributors that provide logistics and sales and marketing support for our products.
We believe our investments in one of the industry’s largest sales organizations, including our extensive network of technical and veterinary operations specialists, our high-quality manufacturing and reliability of supply, and our long track record of developing products that meet customer needs, has led to enduring and valued relationships with our customers. Our research and development (R&D) efforts enable us to deliver innovative products to address unmet needs and evolve our product lines so that they remain relevant for our customers.
We have approximately 300 product lines that we sell in over 100 countries for the prediction, prevention, detection and treatment of diseases and conditions that affect various companion animal and livestock species. The diversity of our product portfolio and our global operations provides stability to our overall business. For instance, in livestock, impacts on our revenue that may result from disease outbreaks or weather conditions in a particular market or region are often offset by increased sales in other regions from exports and other species as consumers shift to other animal proteins.
A summary of our 2023 performance compared with the comparable 2022 and 2021 periods follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | | | 6 | | | 4 | |
| Net income attributable to Zoetis | | 2,344 | | | 2,114 | | | 2,037 | | | 11 | | | 4 | |
Adjusted net income(a) | | 2,457 | | | 2,297 | | | 2,240 | | | 7 | | | 3 | |
(a) Adjusted net income is a non-GAAP financial measure. See the Non-GAAP financial measures and Adjusted net income sections of this MD&A for more information.
Our operating environment
Industry
The animal health industry, which focuses on both companion animals and livestock, is a growing industry that impacts billions of people worldwide. The primary companion animal species are dogs, cats and horses. Factors influencing growth in demand for companion animal medicines, vaccines and diagnostics include:
•economic development and related increases in disposable income, particularly in many emerging markets;
•increasing pet ownership and pet owners’ commitment to the health and well-being of their pets;
•companion animals living longer;
•increasing medical treatment of companion animals; and
•advances in companion animal medicines, vaccines and diagnostics.
The primary livestock species for the production of animal protein are cattle (both beef and dairy), swine, poultry, fish and sheep. Livestock health
and production are essential to meeting the growing demand for animal protein of a global population. Factors influencing growth in demand for livestock medicines and vaccines include:
•human population growth and increasing standards of living, particularly in many emerging markets;
•increasing demand for improved nutrition, particularly animal protein;
•natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, resulting in fewer resources that will be available to meet an increasing demand for animal protein;
•increasing urbanization; and
•increased focus on food safety and food security.
The overall economic environment
In addition to industry-specific factors, we, like other businesses, face challenges related to global economic conditions, an economic downturn and high inflation. Growth in both the livestock and companion animal sectors is driven by overall economic development and related growth, particularly in many emerging markets. In the past, certain of our customers and suppliers have been affected directly by shortages in veterinary healthcare workers and economic downturns or inflation, which decreased the demand for our products and, in some cases, hindered our ability to collect amounts due from customers.
The cost of medicines and vaccines to our livestock producer customers is small relative to other production costs, including feed, and the use of these products is intended to improve livestock producers’ economic outcomes. As a result, demand for our products has historically been more stable than demand for other production inputs. Similarly, industry sources have reported that pet owners indicated a preference for reducing spending on other aspects of their lifestyle, including entertainment, clothing and household goods, before reducing spending on pet care. Each of these factors, plus our broad and innovative portfolio, contributes to our ability to incorporate inflationary challenges into our product pricing and mitigate the impact on our results. While these factors have mitigated the impact of prior downturns in the global economy, economic challenges, including an economic downturn and inflation, could increase cost sensitivity among our customers, which may result in reduced demand for our products, which could have a material adverse effect on our operating results and financial condition.
Competition
The animal health industry is highly competitive. Although our business is the largest based on revenue in the animal health industry (which includes medicines, vaccines and diagnostics), we face competition in the regions in which we operate. Principal methods of competition vary depending on the particular region, species, product category or individual product. Some of these methods include new product development, quality, price, service and promotion to veterinary professionals, pet owners and livestock producers. Our competitors include standalone animal health businesses and the animal health businesses of large pharmaceutical companies. In recent years, there has been an increase in consolidation in the animal health industry. There are also many start-up companies working in the animal health area. In addition to competition from established market participants, there could be new entrants to the animal health medicines, vaccines and diagnostics industry in the future. We also compete with companies that produce generic products, following our products’ loss of exclusivity in a given market. For example, Draxxin currently competes with generic products in key markets including the U.S., Europe, Canada, Mexico and Australia. Since 2021, the first year of generic competition, sales of Draxxin declined by 47% in the U.S., its largest market, and additional declines are expected in subsequent years. For more information regarding the generic competition we expect to encounter as patents on certain of our key products expire, see Item 1. Business – Intellectual Property.
Manufacturing and supply
In order to sell our products, we must be able to produce and ship our products in sufficient quantities. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites. Minor deviations in our manufacturing or logistical processes, such as temperature excursions or improper package sealing, could result in delays, inventory shortages, unanticipated costs, product recalls, product liability and/or regulatory action. In addition, a number of factors could cause production interruptions that could result in launch delays, inventory shortages, recalls, unanticipated costs or issues with our supply agreements with third parties.
Our manufacturing network may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites, and shifting customer demand increase the potential for capacity imbalances.
Product development initiatives
Our future success depends on both our existing product portfolio and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. We believe we are an industry leader in animal health R&D, with a track record of generating new products and product lifecycle innovation. The majority of our R&D programs focus on new products. However, we also continue to invest in lifecycle innovation, which is defined as R&D programs that leverage existing animal health products by adding new species or claims, achieving approvals in new markets or creating new combinations and reformulations. In addition to traditional medicines and vaccines, we develop products across additional categories to address the needs of veterinarians and producers to predict, prevent, detect and treat conditions in both companion animals and livestock, including products and services in diagnostics, genetics, precision animal health and digital and data analytics.
Changing distribution channels for companion animal products
In most markets, companion animal owners typically purchase their animal health products directly from veterinarians. However, in the U.S. and certain other markets, companion animal owners increasingly have the option to purchase animal health products from sources other than veterinarians, such as internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the shift away from the veterinarian distribution channel in the sale of flea and tick products in recent years and has been accelerated by the increase in e-commerce during the COVID-19 pandemic. We believe the ability of pet owners to purchase our products online and from retail
stores may increase pet owner compliance and result in increased sales. However, over time, we may be unable to sustain our current margins due to the increased purchasing power of such retailers as compared to traditional veterinary practices.
In addition, this trend could negatively impact the sales of products we primarily sell through the veterinarian distribution channel, as any decrease in visits to veterinarians by companion animal owners could reduce our market share and sales of such products. A reduction in the number of pet owners who purchase our products directly from their veterinarian could also lead to increased use of generic alternatives to our products or the increased substitution of our products with other animal health products or human health products if such other products are deemed to be lower-cost alternatives.
Perceptions of product quality, safety and reliability
We believe that animal health customers value high-quality manufacturing and reliability of supply. The importance of quality and safety concerns to pet owners, veterinarians and livestock producers also contributes to animal health brand loyalty, which often continues after the loss of patent-based and regulatory exclusivity. We depend on positive perceptions of the safety and quality of our products by our customers, veterinarians and end-users.
In addition, negative beliefs about animal health products generally could impact demand for our products. For example, the issue of the potential transfer of increased antibacterial resistance in bacteria from food-producing animals to human pathogens, and the causality of that transfer, continue to be the subject of global scientific and regulatory discussion. Antibacterials refer to small molecules that can be used to treat or prevent bacterial infections and are a sub-categorization of the products that make up our anti-infectives and medicated feed additives portfolios. In some countries, this issue has led to government restrictions and bans on the use of specific antibacterials in some food-producing animals, regardless of the route of administration (in feed or injectable). These restrictions are more prevalent in countries where animal protein is plentiful and governments are willing to take action even when there is scientific uncertainty. In addition, consumer preferences in some markets have impacted the use of antibacterials in food producing animals. Such restrictions and consumer preferences in some cases may negatively impact sales of our antibacterial products, but in other instances may increase sales of our products that can be used as antibacterial alternatives. Our total revenue attributable to antibacterials for livestock was less than $950 million for the year ended December 31, 2023.
Similarly, concerns regarding greenhouse gas emissions and other potential environmental impacts of livestock production have led to some consumers opting to limit or avoid consuming animal products. However, we believe the impact of this trend is limited as the livestock industry is still expected to continue to grow in order to feed a growing global population.
Disease Outbreaks
Sales of our livestock products have in the past, and may in the future be, adversely affected by the outbreak of disease carried by animals. Outbreaks of disease may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere. Alternatively, sales of products that treat specific disease outbreaks may increase.
Weather conditions, climate change and the availability of natural resources
The animal health industry and demand for many of our animal health products in a particular region are affected by weather conditions, as usage of our products follows varying weather patterns and weather-related pressures from pests, such as ticks. As a result, we may experience regional and seasonal fluctuations in our results of operations.
In addition, veterinary hospitals and practitioners depend on visits from and access to the animals under their care. Veterinarians’ patient volume and ability to operate could be adversely affected if they experience prolonged snow, ice or other severe weather conditions, particularly in regions not accustomed to sustained inclement weather. Furthermore, weather conditions, including excessive cold or heat, natural disasters and other events, could negatively impact our livestock customers by impairing the health or growth of their animals or the production or availability of feed, as well as disrupting their normal operations. For example, livestock producers depend on the availability of natural resources, including large supplies of fresh water. Their animals’ health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth, climate change or floods, droughts or other weather conditions. In the event of adverse weather conditions, climate-change related impacts or a shortage of fresh water, veterinarians and livestock producers may purchase less of our products.
For example, drought conditions could negatively impact, among other things, the supply of corn and the availability of grazing pastures. A decrease in harvested corn results in higher corn prices, which could negatively impact the profitability of livestock producers of cattle, pork and poultry. Higher corn prices and reduced availability of grazing pastures contribute to reductions in herd or flock sizes that in turn result in less spending on animal health products. As such, a prolonged drought could have an adverse impact on our operating results and financial condition.
Adverse weather conditions, natural disasters and climate change may also impact the aquaculture business. Changes in water temperatures could affect the timing of reproduction and growth of various fish species, as well as trigger the outbreak of certain waterborne diseases.
Foreign exchange rates
Significant portions of our revenue and costs are exposed to changes in foreign exchange rates. Our products are sold in more than 100 countries and, as a result, our revenue is influenced by changes in foreign exchange rates. For the year ended December 31, 2023, approximately 43% of our revenue was denominated in foreign currencies. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. As we operate in multiple foreign currencies, including the Australian dollar, Brazilian real, British pound, Canadian dollar, Chinese renminbi, euro and other currencies, changes in those currencies relative to the U.S. dollar will impact our revenue, cost of goods and expenses, and consequently, net income. Exchange rate fluctuations may also have an impact beyond our reported financial results and directly impact operations. These fluctuations may affect the ability to buy and sell our goods and services between markets impacted by significant exchange rate variances. For the year ended December 31, 2023, approximately 57% of our total revenue was in U.S. dollars. Our year-over-year total revenue growth was unfavorably impacted by 1% from changes in foreign currency values relative to the U.S. dollar.
Our strategic pillars
Our vision is to be the most trusted and valued animal health company, shaping the future of animal care through our innovation, customer obsession and purpose-driven colleagues. We have a global presence in both developed and emerging markets and across eight core species. We intend to grow our business by pursuing the following core strategies:
•Lead through innovation across our diverse portfolio - We seek to define the future of animal health by delivering new products and solutions as well as lifecycle innovations across the continuum of care that spans from disease prediction and prevention to detection and treatment. We are focused on innovating across vaccines, pharmaceuticals, diagnostics, genetics, biodevices, and other product segments, and across all core species. Our internal R&D capabilities are differentiators, but we also collaborate across both academia and industry to ensure we are bringing the best possible innovations to our customers;
•Deliver an exceptional experience to delight our customers - We believe that our customers' success is our success and that the best way to realize that success is by enabling veterinarians, livestock producers and pet owners to provide the best possible care for animals. We are focused on providing greater value to our customers through the integration and connectedness of our portfolio and by reducing frictions in the way they engage with us and our products and solutions;
•Power our business through digital solutions and data insights - We believe that digital and data have surpassed the stage of enablement and are now core decision drivers for the future of animal health. We are leaders in the translation of digital solutions and data insights to positive outcomes for our customers and for the animals they care for, whether for better identification of health care solutions in pets or improved production capabilities in livestock;
•Support a workplace where our colleagues can thrive - We view the strength of our leadership team and our talented colleagues around the world as a critical component of our past and future success. We believe that by focusing on colleague well-being and inclusion and providing all of our colleagues with supportive tools, training and environment that we are best positioned to succeed. With that, our colleagues are committed to our purpose, our customers and each other. We are committed to maintaining a workplace culture that attracts, retains and develops the best talent in the industry;
•Advance sustainability in animal health for a better future - As the world’s leading animal health company, our business purpose is well aligned with our social purpose. We strive to make a meaningful difference in society through the three pillars of our sustainability approach: (1) by caring and collaborating with our customers, colleagues, and communities, and the animals that depend on them by improving access to care for animals and by supporting the veterinary profession; (2) by leveraging our innovation capabilities to develop solutions that improve productivity, keep animals healthy, and fight emerging infectious diseases; and (3) by taking actions to protect our planet that reduce our footprint on the environment; and
•Perform with excellence and agility - We recognize the increasing uncertainty in our industry and more broadly across the world. While we aim to improve our ability to predict, we more importantly aim to be proactive in how effectively we manage our resources and how quickly we can redeploy focus to emerging themes or priorities. We actively: (1) review and manage our resource; (2) continuously improve key operational processes; and (3) ensure strategic focus on our core business.
Components of revenue and costs and expenses
Our revenue, costs and expenses are reported for the year ended December 31 for each year presented, except for operations outside the U.S., for which the financial information is included in our consolidated financial statements for the fiscal year ended November 30 for each year presented.
Revenue
Our revenue is primarily derived from our diversified product portfolio of medicines, vaccines and diagnostic products and services used to treat and protect companion animals and livestock. Generally, our products are promoted to veterinarians and livestock producers by our sales organization which includes sales representatives and technical and veterinary operations specialists, and then sold directly by us or through distributors, retailers or e-commerce outlets. The depth of our product portfolio enables us to address the varying needs of customers in different species and geographies. In 2023, our two top-selling products and product lines, Simparica/Simparica Trio and Apoquel/Apoquel Chewable, each contributed approximately 13% and 10% of our revenue, respectively, and combined with our next three top-selling products and product lines, Cytopoint, Revolution/Revolution Plus/Stronghold and ceftiofur line, these five products and product lines contributed approximately 37% of our revenue. Our ten top-selling products and product lines contributed 49% of our revenue. For additional information regarding our products, including descriptions of our product lines that each represented approximately 1% or more of our revenue in 2023, see Item 1. Business—Products.
Costs and expenses
Costs of sales consist primarily of cost of materials, facilities and other infrastructure used to manufacture our medicine and vaccine products, as well as costs to operate our reference labs and royalty expenses associated with the intellectual property of our products, when relevant.
Selling, general and administrative (SG&A) expenses consist of, among other things, the internal and external costs of marketing, promotion, advertising and shipping and handling as well as certain costs related to business technology, facilities, legal, finance, human resources, business development, public affairs and procurement.
Research and development (R&D) expenses consist primarily of project costs specific to new product R&D and product lifecycle innovation, overhead costs associated with R&D operations and investments that support local market clinical trials for approved indications and expenses related to regulatory approvals for our products. We do not disaggregate R&D expenses by research stage or by therapeutic area for purposes of managing our business.
Amortization of intangible assets consists primarily of the amortization expense for identifiable finite-lived intangible assets that have been acquired through business combinations. These assets consist of, but are not limited to, developed technology, brands and trademarks.
Restructuring charges and certain acquisition-related costs consist of all restructuring charges (those associated with cost reduction/productivity initiatives and those associated with acquisition activity), as well as costs associated with acquiring and integrating businesses. Restructuring charges
are associated with employees, assets and activities that will not continue in the company. Acquisition-related costs are associated with acquiring and integrating acquired businesses and may include transaction costs and expenditures for consulting and the integration of systems and processes.
Other (income)/deductions—net consists of various items, primarily net (gains)/losses on business sales and divestitures, as well as asset disposals, interest income, royalty-related income, foreign exchange translation (gains)/losses and certain asset impairment charges.
Significant accounting policies and application of critical accounting estimates
In presenting our financial statements in conformity with U.S. GAAP, we are required to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs and expenses and related disclosures. For a description of our significant accounting policies, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies.
We believe that the following accounting policies are critical to an understanding of our consolidated financial statements as they require the application of the most difficult, subjective and complex judgments and, therefore, could have the greatest impact on our financial statements: (i) fair value; (ii) revenue; (iii) asset impairment reviews; and (iv) contingencies.
Below are some of our more critical accounting estimates. See also Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Estimates and Assumptions for a discussion about the risks associated with estimates and assumptions.
Fair value
For a discussion about the application of fair value to our long-term debt and financial instruments, see Notes to Consolidated Financial Statements—Note 9. Financial Instruments.
For a discussion about the application of fair value to our business combinations, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Fair Value.
For a discussion about the application of fair value to our asset impairment reviews, see Asset impairment reviews below.
Revenue
Our gross product revenue is subject to deductions that are generally estimated and recorded in the same period that the revenue is recognized and primarily represents sales returns and revenue incentives. For example:
•for sales returns, we perform calculations in each market that incorporate the following, as appropriate: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, product recalls, discontinuation of products or a changing competitive environment; and
•for revenue incentives, we use our historical experience with similar incentives programs to estimate the impact of such programs on revenue.
If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate predictors of our future experience, our results could be materially affected. Although the amounts recorded for these revenue deductions are dependent on estimates and assumptions, historically our adjustments to actual results have not been material. The sensitivity of our estimates can vary by program, type of customer and geographic location.
Amounts recorded for revenue deductions can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For further information about the risks associated with estimates and assumptions, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Estimates and Assumptions.
Asset impairment reviews
We review all of our long-lived assets for impairment indicators throughout the year and we perform detailed testing whenever impairment indicators are present. In addition, we perform impairment testing for goodwill and indefinite-lived intangible assets at least annually. When necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets.
Our impairment review processes are described below and in Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets and, for deferred tax assets, in Note 3. Significant Accounting Policies: Deferred Tax Assets and Liabilities and Income Tax Contingencies.
Examples of events or circumstances that may be indicative of impairment include:
•a significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the regulatory authorities could affect our ability to manufacture or sell a product; and
•a projection or forecast that demonstrates losses or reduced profits associated with an asset. This could result, for example, from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously projected revenue growth, or from the lack of acceptance of a product by customers.
For finite-lived identifiable intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate the remaining useful lives of the assets and modify them, as appropriate.
Our impairment reviews of most of our long-lived assets depend on the determination of fair value, as defined by U.S. GAAP, and these judgments can materially impact our results of operations. A single estimate of fair value can result from a complex series of judgments about future events and
uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Estimates and Assumptions.
Intangible assets other than goodwill
We test indefinite-lived intangible assets for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized. Impairments of identifiable intangible assets other than goodwill, are recorded in Restructuring charges and certain acquisition-related costs and Other (income)/deductions—net, as applicable. We did not have any material intangible asset impairment charges for the years ended December 31, 2023, 2022 and 2021.
When we are required to determine the fair value of intangible assets other than goodwill, we use an income approach, specifically the multi-period excess earnings method, also known as the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the asset, which includes the application of a terminal value for indefinite-lived assets, and then we apply an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the projections, the impact of technological risk associated with IPR&D assets, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the risks inherent in the projected cash flows; foreign currency fluctuations; and the effective tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
While all identifiable intangible assets can be impacted by events and thus lead to impairment, in general, identifiable intangible assets that are at the highest risk of impairment include IPR&D assets ($141 million as of December 31, 2023). IPR&D assets are higher-risk assets given the uncertain nature of R&D activity.
For a description of our accounting policy, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Goodwill
Goodwill represents the excess of the consideration transferred over the fair value of net assets of businesses purchased and is assigned to reporting units. We test goodwill for impairment on at least an annual basis, or more frequently if necessary, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount or by performing a periodic quantitative assessment.
Factors considered in the qualitative assessment include general macroeconomic conditions, conditions specific to the industry and market, cost factors which could have a significant effect on earnings or cash flows, the overall financial performance of the reporting unit and whether there have been sustained declines in our share price. Additionally, we evaluate the extent to which the fair value exceeded the carrying value of the reporting unit at the date of the last quantitative assessment performed.
When performing a quantitative assessment to test for goodwill impairment we utilize the income approach, which is forward-looking, and relies primarily on internal forecasts. Within the income approach, the method that we use is the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the reporting unit, which includes the application of a terminal value, and then apply a reporting unit-specific discount rate to arrive at a net present value. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of technological risk and competitive, legal and/or regulatory forces on the projections, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the effective tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
We test goodwill for impairment on at least an annual basis, or more frequently if necessary, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount or by performing a periodic quantitative assessment. In 2023, we performed a qualitative impairment assessment as of September 30, 2023, which did not result in the impairment of goodwill associated with any of our reporting units. In 2022, we performed a periodic quantitative impairment assessment as of September 30, 2022, which did not result in the impairment of goodwill associated with any of our reporting units.
For all of our reporting units, there are a number of future events and factors that may impact future results and that could potentially have an impact on the outcome of subsequent goodwill impairment testing. For a list of these factors, see Forward-looking statements and factors that may affect future results.
For a description of our accounting policy, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Contingencies
For a discussion about income tax contingencies, see Notes to Consolidated Financial Statements—Note 8D. Tax Matters: Tax Contingencies.
For a discussion about legal contingencies, guarantees and indemnifications, see Notes to Consolidated Financial Statement—Note 18. Commitments and Contingencies.
Non-GAAP financial measures
We report information in accordance with U.S. generally accepted accounting principles (GAAP). Management also measures performance using non-GAAP financial measures that may exclude certain amounts from the most directly comparable GAAP measure. Despite the importance of these measures to management in goal setting and performance measurement, non-GAAP financial measures have no standardized meaning prescribed by U.S. GAAP and, therefore, have limits in their usefulness to investors and may not be comparable to the calculation of similar measures of other
companies. We present certain identified non-GAAP measures solely to provide investors with useful information to more fully understand how management assesses performance.
Operational Growth
We believe that it is important to not only understand overall revenue and earnings growth, but also “operational growth.” Operational growth is a non-GAAP financial measure defined as revenue or earnings growth excluding the impact of foreign exchange. This measure provides information on the change in revenue and earnings as if foreign currency exchange rates had not changed between the current and prior periods to facilitate a period-to-period comparison. We believe this non-GAAP measure provides a useful comparison to previous periods for the company and investors, but should not be viewed as a substitute for U.S. GAAP reported growth.
Adjusted Net Income and Adjusted Earnings Per Share
Adjusted net income and the corresponding adjusted earnings per share (EPS) are non-GAAP financial measures of performance used by management. We believe these financial measures are useful supplemental information to investors when considered together with our U.S. GAAP financial measures. We report adjusted net income to portray the results of our major operations, and the discovery, development, manufacture and commercialization of our products, prior to considering certain income statement elements. We define adjusted net income and adjusted EPS as net income attributable to Zoetis and EPS before the impact of purchase accounting adjustments, acquisition-related costs and certain significant items.
We recognize that, as an internal measure of performance, the adjusted net income and adjusted EPS measures have limitations, and we do not restrict our performance management process solely to these metrics. A limitation of the adjusted net income and adjusted EPS measures is that they provide a view of our operations without including all events during a period, such as the effects of an acquisition or amortization of purchased intangibles, and do not provide a comparable view of our performance to other companies. The adjusted net income and adjusted EPS measures are not, and should not be viewed as, a substitute for U.S. GAAP reported net income attributable to Zoetis and reported EPS. See the Adjusted Net Income section below for more information.
Analysis of the Consolidated Statements of Income
The following discussion and analysis of our Consolidated Statements of Income should be read along with our consolidated financial statements, and the notes thereto. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | | | 6 | | | 4 | |
| Costs and expenses: | | | | | | | | | | |
Cost of sales(a) | | 2,561 | | | 2,454 | | | 2,303 | | | 4 | | | 7 | |
| % of revenue | | 30.0 | % | | 30.4 | % | | 29.6 | % | | | | |
Selling, general and administrative expenses(a) | | 2,151 | | | 2,009 | | | 2,001 | | | 7 | | | — | |
| % of revenue | | 25 | % | | 25 | % | | 26 | % | | | | |
Research and development expenses(a) | | 614 | | | 539 | | | 508 | | | 14 | | | 6 | |
| % of revenue | | 7 | % | | 7 | % | | 7 | % | | | | |
Amortization of intangible assets(a) | | 149 | | | 150 | | | 161 | | | (1) | | | (7) | |
| Restructuring charges and certain acquisition-related costs | | 53 | | | 11 | | | 43 | | | * | | (74) | |
| Interest expense, net of capitalized interest | | 239 | | | 221 | | | 224 | | | 8 | | | (1) | |
| Other (income)/deductions—net | | (159) | | | 40 | | | 48 | | | * | | (17) | |
| Income before provision for taxes on income | | 2,936 | | | 2,656 | | | 2,488 | | | 11 | | | 7 | |
| % of revenue | | 34 | % | | 33 | % | | 32 | % | | | | |
| Provision for taxes on income | | 596 | | | 545 | | | 454 | | | 9 | | | 20 | |
| Effective tax rate | | 20.3 | % | | 20.5 | % | | 18.2 | % | | | | |
| Net income before allocation to noncontrolling interests | | 2,340 | | | 2,111 | | | 2,034 | | | 11 | | | 4 | |
| Less: Net loss attributable to noncontrolling interests | | (4) | | | (3) | | | (3) | | | 33 | | | — | |
| Net income attributable to Zoetis | | $ | 2,344 | | | $ | 2,114 | | | $ | 2,037 | | | 11 | | | 4 | |
| % of revenue | | 27 | % | | 26 | % | | 26 | % | | | | |
* Calculation not meaningful.
(a) Amortization expense related to finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property is included in Amortization of intangible assets as these intangible assets benefit multiple business functions. Amortization expense related to finite-lived acquired intangible assets that are associated with a single function is included in Cost of sales, Selling, general and administrative expenses or Research and development expenses, as appropriate.
Revenue
Total revenue by operating segment was as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| U.S. | | $ | 4,555 | | | $ | 4,313 | | | $ | 4,042 | | | 6 | | | 7 | |
| International | | 3,911 | | | 3,681 | | | 3,652 | | | 6 | | | 1 | |
| Total operating segments | | 8,466 | | | 7,994 | | | 7,694 | | | 6 | | | 4 | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | | | (9) | | | 5 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | | | 6 | | | 4 | |
On a global basis, the mix of revenue between companion animal and livestock products was as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Companion animal | | $ | 5,576 | | | $ | 5,203 | | | $ | 4,689 | | | 7 | | | 11 | |
| Livestock | | 2,890 | | | 2,791 | | | 3,005 | | | 4 | | | (7) | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | | | (9) | | | 5 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | | | 6 | | | 4 | |
2023 vs. 2022
Total revenue increased by $464 million, or 6%, in 2023 compared with 2022 reflecting operational revenue growth of $579 million, or 7%. Operational revenue growth was primarily due to the following:
•price growth of approximately 5%; and
•volume growth from new products of approximately 2%.
Foreign exchange decreased our reported revenue growth by approximately 1%.
Costs and Expenses
Cost of sales | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Cost of sales | | $ | 2,561 | | | $ | 2,454 | | | $ | 2,303 | | | 4 | | | 7 | |
| % of revenue | | 30.0 | % | | 30.4 | % | | 29.6 | % | | | | |
2023 vs. 2022
Cost of sales as a percentage of revenue was 30.0% in 2023, compared with 30.4% in 2022. The decrease was primarily as a result of:
•price increases;
•lower freight costs; and
•favorable foreign exchange,
partially offset by:
•unfavorable manufacturing and other costs;
•inventory obsolescence, scrap and other charges; and
•unfavorable product mix.
Selling, general and administrative expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Selling, general and administrative expenses | | $ | 2,151 | | | $ | 2,009 | | | $ | 2,001 | | | 7 | | | — | |
| % of revenue | | 25 | % | | 25 | % | | 26 | % | | | | |
2023 vs. 2022
SG&A expenses increased by $142 million, or 7%, in 2023 compared with 2022, primarily as a result of:
•compensation-related costs and the resulting increases in office expenses and travel and entertainment expenses;
•higher charitable contributions;
•higher freight and logistics costs due to increased sales volume; and
•technology project investments,
partially offset by:
•favorable foreign exchange; and
•the reduced impact of purchase accounting adjustments.
Research and development expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Research and development expenses | | $ | 614 | | | $ | 539 | | | $ | 508 | | | 14 | | | 6 | |
| % of revenue | | 7 | % | | 7 | % | | 7 | % | | | | |
2023 vs. 2022
R&D expenses increased by $75 million, or 14%, in 2023 compared with 2022, primarily as a result of:
•an increase in certain compensation-related costs to support innovation and portfolio progression;
•higher other operating costs; and
•higher spend in project investments.
Amortization of intangible assets | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Amortization of intangible assets | | $ | 149 | | | $ | 150 | | | $ | 161 | | | (1) | | | (7) | |
2023 vs. 2022
Amortization of intangible assets decreased in 2023 compared with 2022 primarily due to asset impairments taken in 2023 and 2022, as well as assets that became fully amortized in the current year, partially offset by intangible assets acquired during 2023 and 2022.
Restructuring charges and certain acquisition-related costs | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Restructuring charges and certain acquisition-related costs | | $ | 53 | | | $ | 11 | | | $ | 43 | | | * | | (74) | |
* Calculation not meaningful.
2023 vs. 2022
Restructuring charges and certain acquisition-related costs were $53 million and $11 million in 2023 and 2022, respectively. Restructuring charges and certain acquisition-related costs in 2023 primarily consisted of employee termination and exit costs related to organizational structure refinements and other cost-reduction and productivity initiatives, as well as costs related to recent acquisitions. Restructuring charges and certain acquisition-related costs in 2022 primarily consisted of integration and acquisition costs related to recent acquisitions, employee termination and exit costs associated with cost-reduction and productivity initiatives in certain international markets and asset impairment charges related to the consolidation of manufacturing sites in China.
For additional information regarding restructuring charges and acquisition-related costs, see Notes to Consolidated Financial Statements— Note 6. Restructuring Charges and Other Costs Associated with Acquisitions, Cost-Reduction and Productivity Initiatives.
Interest expense, net of capitalized interest | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Interest expense, net of capitalized interest | | $ | 239 | | | $ | 221 | | | $ | 224 | | | 8 | | | (1) | |
2023 vs. 2022
Interest expense, net of capitalized interest, increased by $18 million, or 8%, in 2023 compared with 2022, primarily as a result of higher interest rates on the $1.35 billion aggregate principal amount of our 2022 senior notes issued in November 2022 as compared to the $1.35 billion aggregate principal amount of our 2013 senior notes redeemed in February 2023, upon maturity, partially offset by lower average debt balances in 2023 compared to 2022, as well as an increase in capitalized interest and higher gains on foreign exchange derivative instruments.
Other (income)/deductions—net | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Other (income)/deductions—net | | $ | (159) | | | $ | 40 | | | $ | 48 | | | * | | (17) | |
* Calculation not meaningful.
2023 vs. 2022
The change in Other (income)/deductions—net is primarily as a result of higher interest income in the current period due to higher interest rates on cash balances denominated in the U.S. dollar, a gain on the sale of a majority interest in our pet insurance business and royalty-related income that was predominantly associated with a settlement received from a third-party for underpayment of royalties related to prior periods, partially offset by higher foreign currency losses and certain asset impairment charges primarily related to our precision animal health and diagnostics businesses.
Provision for taxes on income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Provision for taxes on income | | $ | 596 | | | $ | 545 | | | $ | 454 | | | 9 | | | 20 | |
| Effective tax rate | | 20.3 | % | | 20.5 | % | | 18.2 | % | | | | |
The income tax provision in the Consolidated Statements of Income includes tax costs and benefits, such as uncertain tax positions, repatriation decisions and audit settlements, among others.
2023 vs. 2022
Our effective tax rate was 20.3% for 2023, compared with 20.5% for 2022. The lower effective tax rate for 2023 was primarily attributable to a higher benefit in the U.S. related to foreign-derived intangible income, a more favorable jurisdictional mix of earnings (which includes the impact of the location of earnings and repatriation costs), partially offset by a higher net discrete tax expense in 2023, mainly related to changes to prior years' tax positions. Jurisdictional mix of earnings can vary depending on repatriation decisions, operating fluctuations in the normal course of business and the impact of non-deductible items and non-taxable items.
Operating Segment Results
The mix of revenue between companion animal and livestock products was as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | % Change |
| | | | | 23/22 | | 22/21 |
| | | | | Related to | | | | Related to |
| Year Ended December 31, | | | | Foreign Exchange | | | | | | Foreign Exchange | | |
| (MILLIONS OF DOLLARS) | 2023 | 2022 | 2021 | | Total | | | Operational | | Total | | | Operational |
| U.S. | | | | | | | | | | | | | | | |
| Companion animal | $ | 3,529 | | $ | 3,341 | | $ | 2,990 | | | 6 | | | — | | | 6 | | | 12 | | | — | | | 12 | |
| Livestock | 1,026 | | 972 | | 1,052 | | | 6 | | | — | | | 6 | | | (8) | | | — | | | (8) | |
| 4,555 | | 4,313 | | 4,042 | | | 6 | | | — | | | 6 | | | 7 | | | — | | | 7 | |
| International | | | | | | | | | | | | | | | |
| Companion animal | 2,047 | | 1,862 | | 1,699 | | | 10 | | | (2) | | | 12 | | | 10 | | | (9) | | | 19 | |
| Livestock | 1,864 | | 1,819 | | 1,953 | | | 2 | | | (5) | | | 7 | | | (7) | | | (7) | | | — | |
| 3,911 | | 3,681 | | 3,652 | | | 6 | | | (3) | | | 9 | | | 1 | | | (8) | | | 9 | |
| Total | | | | | | | | | | | | | | | |
| Companion animal | 5,576 | | 5,203 | | 4,689 | | | 7 | | | (1) | | | 8 | | | 11 | | | (3) | | | 14 | |
| Livestock | 2,890 | | 2,791 | | 3,005 | | | 4 | | | (2) | | | 6 | | | (7) | | | (5) | | | (2) | |
| Contract manufacturing & human health | 78 | | 86 | | 82 | | | (9) | | | 1 | | | (10) | | | 5 | | | (2) | | | 7 | |
| $ | 8,544 | | $ | 8,080 | | $ | 7,776 | | | 6 | | | (1) | | | 7 | | | 4 | | | (4) | | | 8 | |
Earnings by segment and the operational and foreign exchange changes versus the comparable prior year period were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | % Change |
| | | | 23/22 | | 22/21 |
| | | | Related to | | | Related to |
| Year Ended December 31, | | | Foreign Exchange | | | | Foreign Exchange | |
| (MILLIONS OF DOLLARS) | 2023 | 2022 | 2021 | | Total | Operational | | Total | Operational |
| U.S. | $ | 2,863 | | $ | 2,763 | | $ | 2,569 | | | 4 | | — | | 4 | | | 8 | | — | | 8 | |
| International | 2,037 | | 1,990 | | 1,948 | | | 2 | | (2) | | 4 | | | 2 | | (10) | | 12 | |
| Total reportable segments | 4,900 | | 4,753 | | 4,517 | | | 3 | | (1) | | 4 | | | 5 | | (5) | | 10 | |
| Other business activities | (496) | | (424) | | (406) | | | 17 | | | | | 4 | | | |
| Reconciling Items: | | | | | | | | | | | |
| Corporate | (1,042) | | (1,073) | | (1,052) | | | (3) | | | | | 2 | | | |
| Purchase accounting adjustments | (159) | | (160) | | (175) | | | (1) | | | | | (9) | | | |
| Acquisition-related costs | (9) | | (5) | | (12) | | | 80 | | | | | (58) | | | |
| Certain significant items | 33 | | (56) | | (73) | | | * | | | | (23) | | | |
| Other unallocated | (291) | | (379) | | (311) | | | (23) | | | | | 22 | | | |
| Income before income taxes | $ | 2,936 | | $ | 2,656 | | $ | 2,488 | | | 11 | | | | | 7 | | | |
* Calculation not meaningful.
2023 vs. 2022
U.S. operating segment
U.S. segment revenue increased by $242 million, or 6%, in 2023 compared with 2022, of which $188 million resulted from growth in companion animal products and $54 million growth in livestock products.
•Companion animal revenue growth was primarily due to our mAb products for OA pain, Librela and Solensia, as well as increased sales of key dermatology, small animal parasiticides, small animal vaccines and small animal diagnostics, partially offset by lower sales of anti-infective products. Strong growth in the second, third and fourth quarters was partially offset by the first quarter impacts of distributor de-stocking across the portfolio, as well as purchases in the fourth quarter of 2022 ahead of expected price increases and promotional activities.
•Livestock revenue growth was due to cattle and poultry, partially offset by a decline in swine. Sales of cattle products grew due to increased sales of cattle implants, higher volume of anti-infective products and improved supply of key products. Sales of products in our poultry portfolio grew due to increases in vaccines, medicated feed additives and biodevices. Sales of swine products declined due to decreased disease prevalence.
U.S. segment earnings increased by $100 million, or 4%, in 2023 compared with 2022, primarily due to higher revenue, partially offset by higher cost of sales, operating expense growth and certain asset impairment charges related to acquired intangible assets.
International operating segment
International segment revenue increased by $230 million, or 6%, in 2023 compared with 2022. Operational revenue growth was $346 million, or 9%, reflecting growth of $228 million in companion animal products and $118 million in livestock products.
•Companion animal operational revenue growth was primarily due to increased sales of our mAb products for OA pain, Librela and Solensia, as well as small animal parasiticides and key dermatology, partially offset by lower sales of vaccine products.
•Livestock operational revenue growth was due to increased sales of cattle, poultry, fish and sheep products. Sales of cattle products grew due to price and improved supply of key products. Sales of poultry products grew due to market growth, demand generation efforts and price in key poultry markets. Growth in our fish portfolio was primarily the result of increased sales of vaccines across key salmon markets, primarily Norway, partially offset by lower sales in Chile. Sales of sheep products grew primarily as a result of the acquisition of Jurox.
•Additionally, International segment revenue was unfavorably impacted by foreign exchange which decreased revenue by approximately $116 million, or 3%, primarily driven by fluctuations in the Argentine peso, Chinese renminbi, Australian dollar, Turkish lira and Japanese yen.
International segment earnings increased by $47 million, or 2%, in 2023 compared with 2022. Operational earnings growth was $88 million, or 4%, primarily due to higher revenue, partially offset by higher cost of sales and operating expenses.
Other business activities
Other business activities includes our Client Supply Services contract manufacturing results, our human health business and expenses associated with our dedicated veterinary medicine research and development organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the International segment.
2023 vs. 2022
Other business activities net loss increased by $72 million, or 17%, in 2023 compared with 2022, reflecting an increase in R&D costs due to an increase in certain compensation-related costs to support innovation, an increase in operating costs and higher project investments, as well as lower earnings in our human health business, partially offset by favorable foreign exchange.
Reconciling items
Reconciling items include certain costs that are not allocated to our operating segments results, such as costs associated with the following:
•Corporate, which includes certain costs associated with information technology, facilities, legal, finance, human resources, business development, certain diagnostics costs and communications, among others. These costs also include certain compensation costs, certain procurement costs, and other miscellaneous operating expenses that are not charged to our operating segments, as well as interest income and expense;
•Certain transactions and events such as Purchase accounting adjustments, Acquisition-related activities and Certain significant items, which are defined below; and
•Other unallocated, which includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with finance that specifically support our global manufacturing operations; (iii) certain supply chain and global logistics costs; and (iv) certain procurement costs.
2023 vs. 2022
Corporate expenses decreased by $31 million, or 3%, in 2023 compared with 2022, primarily associated with favorable foreign exchange, higher interest income and a settlement received from a third-party for underpayment of royalties related to prior periods, partially offset by higher compensation-related costs, investments in information technology and higher interest expense.
Other unallocated expenses decreased by $88 million, or 23%, in 2023 compared with 2022, primarily due to lower manufacturing costs and freight charges, partially offset by unfavorable foreign exchange, as well as inventory obsolescence, scrap and other charges.
See Notes to Consolidated Financial Statements— Note 19. Segment Information for further information.
Adjusted net income
General description of adjusted net income (a non-GAAP financial measure)
Adjusted net income is an alternative view of performance used by management, and we believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. The adjusted net income measure is an important internal measurement for us. Additionally, we measure our overall performance on this basis in conjunction with other performance metrics. The following are examples of how the adjusted net income measure is utilized:
•senior management receives a monthly analysis of our operating results that is prepared on an adjusted net income basis;
•our annual budgets are prepared on an adjusted net income basis; and
•other goal setting and performance measurements.
Purchase accounting adjustments
Adjusted net income is calculated prior to considering certain significant purchase accounting impacts that result from business combinations and net asset acquisitions. These impacts, primarily associated with certain acquisitions, include amortization related to the increase in fair value of the acquired finite-lived intangible assets and depreciation related to the increase/decrease to fair value of the acquired fixed assets. Therefore, the adjusted net income measure includes the revenue earned upon the sale of the acquired products without considering the aforementioned significant charges.
While certain purchase accounting adjustments can occur through 20 or more years, this presentation provides an alternative view of our performance that is used by management to internally assess business performance. We believe the elimination of amortization attributable to acquired intangible assets provides management and investors an alternative view of our business results by providing a degree of parity to internally developed intangible assets for which R&D costs previously have been expensed.
A completely accurate comparison of internally developed intangible assets and acquired intangible assets cannot be achieved through adjusted net income. These components of adjusted net income are derived solely from the impact of the items listed above. We have not factored in the impact of any other differences in experience that might have occurred if we had discovered and developed those intangible assets on our own, and this approach does not intend to be representative of the results that would have occurred in those circumstances. For example, our R&D costs in total, and in the periods presented, may have been different; our speed to commercialization and resulting revenue, if any, may have been different; or our costs to manufacture may have been different. In addition, our marketing efforts may have been received differently by our customers. As such, in total, there can be no assurance that our adjusted net income amounts would have been the same as presented had we discovered and developed the acquired intangible assets.
Acquisition-related costs
Adjusted net income is calculated prior to considering transaction and integration costs associated with significant business combinations or net asset acquisitions because these costs are unique to each transaction and represent costs that were incurred to acquire and integrate certain businesses as a result of the acquisition decision. We have made no adjustments for the resulting synergies.
We believe that viewing income prior to considering these charges provides investors with a useful additional perspective because the significant costs incurred in a business combination result primarily from the need to eliminate duplicate assets, activities or employees––a natural result of acquiring a fully integrated set of activities. For this reason, we believe that the costs incurred to convert disparate systems, to close duplicative facilities or to eliminate duplicate positions (for example, in the context of a business combination) can be viewed differently from those costs incurred in the ordinary course of business.
The integration costs associated with a business combination may occur over several years, with the more significant impacts generally ending within three years of the transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy. For example, due to the regulated nature of the animal health medicines, vaccines and diagnostic business, the closure of excess facilities can take several years, as all manufacturing changes are subject to extensive validation and testing and must be approved by the FDA and/or other regulatory authorities.
Certain significant items
Adjusted net income is calculated excluding certain significant items. Certain significant items represent substantive, unusual items that are evaluated on an individual basis. Such evaluation considers both the quantitative and the qualitative aspect of their unusual nature. Unusual, in this context, may represent items that are not part of our ongoing business; items that, either as a result of their nature or size, we would not expect to occur as part of our normal business on a regular basis; items that would be nonrecurring; or items that relate to products that we no longer sell. While not all-inclusive, examples of items that could be included as certain significant items would be costs related to a major non-acquisition-related restructuring charge and associated implementation costs for a program that is specific in nature with a defined term, such as those related to our non-acquisition-related cost-reduction and productivity initiatives; amounts related to disposals of products or facilities that do not qualify as discontinued operations as defined by U.S. GAAP; certain asset impairment charges; adjustments related to the resolution of certain tax positions; significant currency devaluation; the impact of adopting certain significant, event-driven tax legislation; or charges related to legal matters. See Notes to Consolidated Financial Statements— Note 18. Commitments and Contingencies. Our normal, ongoing defense costs or settlements of and accruals on legal matters made in the normal course of our business would not be considered certain significant items.
Reconciliation
A reconciliation of net income attributable to Zoetis, as reported under U.S. GAAP, to adjusted net income follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| GAAP reported net income attributable to Zoetis | | $ | 2,344 | | | $ | 2,114 | | | $ | 2,037 | | | 11 | | | 4 | |
| Purchase accounting adjustments—net of tax | | 127 | | | 120 | | | 136 | | | 6 | | | (12) | |
| Acquisition-related costs—net of tax | | 7 | | | 4 | | | 10 | | | 75 | | | (60) | |
| Certain significant items—net of tax | | (21) | | | 59 | | | 57 | | | * | | 4 | |
Non-GAAP adjusted net income(a) | | $ | 2,457 | | | $ | 2,297 | | | $ | 2,240 | | | 7 | | | 3 | |
* Calculation not meaningful.
(a) The effective tax rate on adjusted pretax income was 20.1%, 20.3% and 18.6% in 2023, 2022 and 2021, respectively.
The lower effective tax rate for 2023, compared with 2022, was primarily attributable to a higher benefit in the U.S. related to foreign-derived intangible income, a more favorable jurisdictional mix of earnings (which includes the impact of the location of earnings and repatriation costs), partially offset by a higher net discrete tax expense in 2023, mainly related to changes to prior years’ tax positions. Jurisdictional mix of earnings can vary depending on repatriation decisions, operating fluctuations in the normal course of business and the impact of non-deductible and non-taxable items.
The higher effective tax rate for 2022, compared with 2021, was attributable to a less favorable jurisdictional mix of earnings (which includes the impact of the location of earnings and repatriation costs), a lower benefit in the U.S. related to foreign-derived intangible income and lower net discrete tax benefits in 2022. Jurisdictional mix of earnings can vary depending on repatriation decisions, operating fluctuations in the normal course of business and the impact of non-deductible and non-taxable items.
A reconciliation of reported diluted earnings per share (EPS), as reported under U.S. GAAP, to non-GAAP adjusted diluted EPS follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | % Change |
| | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
Earnings per share—diluted(a): | | | | | | | | | | |
| GAAP reported EPS attributable to Zoetis—diluted | | $ | 5.07 | | | $ | 4.49 | | | $ | 4.27 | | | 13 | | | 5 | |
| Purchase accounting adjustments—net of tax | | 0.28 | | | 0.26 | | | 0.29 | | | 8 | | | (10) | |
| Acquisition-related costs—net of tax | | 0.02 | | | 0.01 | | | 0.02 | | | * | | (50) | |
| Certain significant items—net of tax | | (0.05) | | | 0.12 | | | 0.12 | | | * | | — | |
| Non-GAAP adjusted EPS—diluted | | $ | 5.32 | | | $ | 4.88 | | | $ | 4.70 | | | 9 | | | 4 | |
* Calculation not meaningful.
(a) Diluted earnings per share was computed using the weighted-average common shares outstanding during the period plus the common stock equivalents related to stock options, restricted stock units, performance-vesting restricted stock units and deferred stock units.
Adjusted net income includes the following for each of the periods presented: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Interest expense, net of capitalized interest | | $ | 239 | | | $ | 221 | | | $ | 224 | |
| Interest income | | (103) | | | (50) | | | (6) | |
| Income taxes | | 618 | | | 583 | | | 511 | |
| Depreciation | | 302 | | | 266 | | | 236 | |
| Amortization | | 36 | | | 40 | | | 37 | |
Adjusted net income, as shown above, excludes the following items: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Purchase accounting adjustments: | | | | | | |
| Amortization and depreciation | | $ | 153 | | | $ | 160 | | | $ | 175 | |
| Cost of sales | | 6 | | | — | | | — | |
| Total purchase accounting adjustments—pre-tax | | 159 | | | 160 | | | 175 | |
Income taxes(a) | | 32 | | | 40 | | | 39 | |
| Total purchase accounting adjustments—net of tax | | 127 | | | 120 | | | 136 | |
| Acquisition-related costs: | | | | | | |
| Integration costs | | 3 | | | 4 | | | 10 | |
| Transaction costs | | 4 | | | 1 | | | — | |
| Restructuring costs | | 2 | | | — | | | 2 | |
| | | | | | |
| Total acquisition-related costs—pre-tax | | 9 | | | 5 | | | 12 | |
Income taxes(a) | | 2 | | | 1 | | | 2 | |
| Total acquisition-related costs—net of tax | | 7 | | | 4 | | | 10 | |
| Certain significant items: | | | | | | |
| | | | | | |
| | | | | | |
Other restructuring charges and cost-reduction/productivity initiatives(b) | | 44 | | | 8 | | | 24 | |
| | | | | | |
Certain asset impairment charges(c) | | 24 | | | 47 | | | 46 | |
| Net loss on sale of assets | | — | | | — | | | 3 | |
| | | | | | |
Net gain on sale of businesses(d) | | (101) | | | — | | | — | |
| | | | | | |
| Other | | — | | | 1 | | | — | |
| Total certain significant items—pre-tax | | (33) | | | 56 | | | 73 | |
Income taxes(a) | | (12) | | | (3) | | | 16 | |
| Total certain significant items—net of tax | | (21) | | | 59 | | | 57 | |
| Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax | | $ | 113 | | | $ | 183 | | | $ | 203 | |
(a) Income taxes include the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction's applicable tax rate.
Income taxes in Purchase accounting adjustments also includes:
•For 2022, tax benefits related to a deferred adjustment as a result of a change in tax basis.
Income taxes in Certain significant items also includes:
•For 2023, a benefit from the tax loss on the divestiture of Performance Livestock Analytics, partially offset by a tax expense related to changes to prior years’ tax positions with regard to the one-time mandatory deemed repatriation tax under the Tax Cuts and Jobs Act.
•For 2022, a tax expense related to changes in valuation allowances related to impairments of certain assets and changes in uncertain tax positions.
(b) For 2023, primarily represents employee termination and exit costs related to organizational structure refinements and other cost-reduction and productivity initiatives.
For 2022, primarily represents employee termination and exit costs associated with cost-reduction and productivity initiatives in certain international markets, as well as product transfer costs.
For 2021, primarily represents employee termination costs associated with the realignment of our international operations and other costs associated with cost-reduction and productivity initiatives.
(c) For 2023, primarily represents certain asset impairment charges related to our precision animal health and diagnostics businesses included in Other (income)/deductions-net, Cost of sales and Restructuring charges and certain acquisition related costs.
For 2022, primarily represents asset impairment charges related to:
•Customer relationships, developed technology rights and property, plant and equipment in our diagnostics, poultry, cattle and swine businesses included in Other (income)/deductions-net; and
•Inventory and other charges related to the consolidation of manufacturing sites in China, included in Cost of sales and Restructuring charges and certain acquisition related costs.
For 2021, primarily represents asset impairment charges related to:
•Developed technology rights and trademarks in our dairy cattle, diagnostics and aquatic health businesses, included in Other (income)/deductions-net;
•The consolidation of manufacturing sites in China, included in Restructuring charges and certain acquisition related costs; and
•Property, plant and equipment and inventory related to a dairy product termination included in Other (income)/deductions-net and Cost of sales.
(d) Primarily represents a net gain on the sale of a majority interest in our pet insurance business.
The classification of the above items excluded from adjusted net income are as follows: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Cost of sales: | | | | | | |
| Purchase accounting adjustments | | $ | 10 | | | $ | 6 | | | $ | 6 | |
| Inventory write-offs | | 2 | | | 4 | | | 2 | |
| | | | | | |
| Other | | 1 | | | 4 | | | 6 | |
| Total Cost of sales | | 13 | | | 14 | | | 14 | |
| | | | | | |
| Selling, general & administrative expenses: | | | | | | |
| Purchase accounting adjustments | | 21 | | | 29 | | | 30 | |
| | | | | | |
| | | | | | |
| | | | | | |
| Total Selling, general & administrative expenses | | 21 | | | 29 | | | 30 | |
| | | | | | |
| Research & development expenses: | | | | | | |
| Purchase accounting adjustments | | 1 | | | 1 | | | 1 | |
| | | | | | |
| Total Research & development expenses | | 1 | | | 1 | | | 1 | |
| | | | | | |
| Amortization of intangible assets: | | | | | | |
| Purchase accounting adjustments | | 127 | | | 124 | | | 138 | |
| Total Amortization of intangible assets | | 127 | | | 124 | | | 138 | |
| | | | | | |
| Restructuring charges and certain acquisition-related costs: | | | | | | |
| Integration costs | | 3 | | | 4 | | | 10 | |
| Transaction costs | | 4 | | | 1 | | | — | |
| Employee termination costs | | 41 | | | 3 | | | 17 | |
| Asset impairments | | 1 | | | 2 | | | 13 | |
| Exit costs | | 4 | | | 1 | | | 3 | |
| Total Restructuring charges and certain acquisition-related costs | | 53 | | | 11 | | | 43 | |
| | | | | | |
| Other (income)/deductions—net: | | | | | | |
| Net loss on sale of assets | | — | | | — | | | 3 | |
| | | | | | |
| Asset impairments | | 21 | | | 41 | | | 31 | |
| | | | | | |
| Net gain on sale of businesses | | (101) | | | — | | | — | |
| Other | | — | | | 1 | | | — | |
| Total Other (income)/deductions—net | | (80) | | | 42 | | | 34 | |
| | | | | | |
| Provision for taxes on income | | 22 | | | 38 | | | 57 | |
| | | | | | |
| Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax | | $ | 113 | | | $ | 183 | | | $ | 203 | |
Analysis of the Consolidated Statements of Comprehensive Income
Changes in other comprehensive income for the periods presented are primarily related to foreign currency translation adjustments and unrealized gains/(losses) on derivative instruments. The foreign currency translation adjustment changes result from the strengthening or weakening of the U.S. dollar as compared to the currencies in the countries in which we do business. Unrealized gains/(losses) on the changes in the fair value of derivative instruments are recorded within Accumulated other comprehensive income/(loss) and reclassified into earnings depending on the nature and purpose of the financial instrument, as described in Note 9. Financial Instruments of the Notes to Consolidated Financial Statements.
Analysis of the Consolidated Balance Sheets
December 31, 2023 vs. December 31, 2022
For a discussion about the changes in Cash and cash equivalents, Short-term borrowings, Current portion of long-term debt and Long-term debt, net of discount and issuance costs, see “Analysis of financial condition, liquidity and capital resources” below.
Accounts Receivable, less allowance for doubtful accounts increased primarily as a result of higher net sales in the period, partially offset by the timing of customer payments.
Inventories increased primarily as a result of inventory build-up to support production for the forecasted demand of certain products, including new product launches. See Notes to Consolidated Financial Statements - Note 11. Inventories.
Other current assets increased primarily due to the recognition of the deferral of a prepaid tax benefit in connection with a prepayment from a related foreign entity in Belgium, the jurisdictional netting of income taxes receivable and income taxes payable, as well as an increase in collateral posted related to derivative contracts, partially offset by the mark-to-market adjustment of derivative instruments.
Property, plant and equipment less accumulated depreciation increased primarily as a result of capital spending, partially offset by depreciation expense. See Notes to Consolidated Financial Statements - Note 12. Property, Plant and Equipment
The net changes in Noncurrent deferred tax assets, Noncurrent deferred tax liabilities, Income taxes payable and Other taxes payable primarily reflect adjustments to the accrual for the income tax provision, the timing of income tax payments, the tax impact of various acquisitions and the impact of the remeasurement of deferred tax assets and liabilities as a result of changes in tax rates.
Other noncurrent assets increased primarily due to the retained noncontrolling investment following the sale of a majority interest in our pet insurance business, capitalized cloud computing arrangements implementation costs and the addition of a financing lease.
Dividends payable increased as a result of an increase in the dividend rate for the first quarter 2024 dividend, which was declared on December 7, 2023.
Accrued compensation and related items��increased primarily due to the accrual of 2023 annual incentive bonuses, sales incentive bonuses and savings plan contributions to eligible employees, as well as the timing of the bi-weekly payroll, partially offset by the payments of 2022 annual incentive bonuses and sales incentive bonuses, as well as savings plan contributions to eligible employees.
Other noncurrent liabilities increased primarily due to the net increase in purchase price liabilities associated with acquisitions and the addition of a financing lease obligation, partially offset by the mark-to-market adjustment of derivative instruments.
For an analysis of the changes in Total Equity, see the Consolidated Statements of Equity and Notes to Consolidated Financial Statements— Note 16. Stockholders' Equity.
Analysis of the Consolidated Statements of Cash Flows | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | $ Change |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Net cash provided by (used in): | | | | | | | | | | |
| Operating activities | | $ | 2,353 | | | $ | 1,912 | | | $ | 2,213 | | | $ | 441 | | | $ | (301) | |
| Investing activities | | (777) | | | (883) | | | (458) | | | 106 | | | (425) | |
| Financing activities | | (3,109) | | | (904) | | | (1,862) | | | (2,205) | | | 958 | |
| Effect of exchange-rate changes on cash and cash equivalents | | (7) | | | (29) | | | (12) | | | 22 | | | (17) | |
| Net (decrease)/increase in cash and cash equivalents | | $ | (1,540) | | | $ | 96 | | | $ | (119) | | | $ | (1,636) | | | $ | 215 | |
Operating activities
2023 vs. 2022
Net cash provided by operating activities was $2,353 million in 2023 compared with $1,912 million in 2022. The increase in operating cash flows was primarily attributable to higher net income as adjusted by non-cash items, higher inventory build-up of certain products in the prior year period for increased demand and to mitigate potential supply constraints and the timing of receipts and payments in the ordinary course of business.
Investing activities
2023 vs. 2022
Net cash used in investing activities was $777 million in 2023 compared with $883 million in 2022. The net cash used in investing activities for 2023 was primarily due to capital expenditures and acquisitions, partially offset by net proceeds on the sale of a majority interest in our pet insurance business and net proceeds from derivative instrument activity. The net cash used in investing activities for 2022 was primarily attributable to capital expenditures and acquisitions partially offset by net proceeds from derivative instrument activity.
Financing activities
2023 vs. 2022
Net cash used in financing activities was $3,109 million in 2023 compared with $904 million in 2022. The net cash used in financing activities for 2023 was primarily attributable to the repayment at maturity of the $1.35 billion aggregate principal amount of our 2013 senior notes due 2023 in February 2023, the purchase of treasury shares, the payment of dividends and taxes paid on withholding shares, partially offset by proceeds in connection with the issuance of common stock under our equity incentive plan. The net cash used in financing activities for 2022 was primarily attributable to the purchase of treasury shares, the payment of dividends and taxes paid on withholding shares, partially offset by proceeds from the issuance of senior notes in November 2022 and proceeds in connection with the issuance of common stock under our equity incentive plan.
Analysis of financial condition, liquidity and capital resources
While we believe our cash and cash equivalents on hand, our operating cash flows and our existing financing arrangements will be sufficient to support our cash needs for the next twelve months and beyond, this may be subject to the environment in which we operate. Risks to our meeting future funding requirements are described in Global economic conditions below.
Selected measures of liquidity and capital resources
Certain relevant measures of our liquidity and capital resources follow: | | | | | | | | | | | |
| December 31, |
| (MILLIONS OF DOLLARS) | 2023 | | 2022 |
| Cash and cash equivalents | $ | 2,041 | | | $ | 3,581 | |
Accounts receivable, net(a) | 1,304 | | | 1,215 | |
| Short-term borrowings | 3 | | | 2 | |
| Current portion of long-term debt | — | | | 1,350 | |
| Long-term debt | 6,564 | | | 6,552 | |
| Working capital | 4,454 | | | 4,339 | |
| Ratio of current assets to current liabilities | 3.36:1 | | 2.37:1 |
| | | |
(a) Accounts receivable are usually collected over a period of 45 to 75 days. For the years ended December 31, 2023 and 2022, the number of days that accounts receivables were outstanding have remained within this range. We regularly monitor our accounts receivable for collectability, particularly in markets where economic conditions remain uncertain. We believe that our allowance for doubtful accounts is appropriate. Our assessment is based on such factors as past due aging, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment.
For additional information about the sources and uses of our funds, see the Analysis of the Consolidated Balance Sheets and Analysis of the Consolidated Statements of Cash Flows sections of this MD&A.
Credit facility and other lines of credit
In December 2022, we entered into an amended and restated revolving credit agreement with a syndicate of banks providing for a multi-year $1.0 billion senior unsecured revolving credit facility (the credit facility), which expires in December 2027. Subject to certain conditions, we have the right to increase the credit facility up to $1.5 billion. The credit facility contains a financial covenant requiring us to not exceed a maximum total leverage ratio (the ratio of consolidated net debt as of the end of the period to consolidated Earnings Before Interest, Income Taxes, Depreciation and Amortization (EBITDA) for such period) of 3.50:1. Upon entering into a material acquisition, the maximum total leverage ratio increases to 4.00:1, and extends until the fourth full consecutive fiscal quarter ended immediately following the consummation of a material acquisition. In addition, the credit facility contains other customary covenants.
We were in compliance with all financial covenants as of December 31, 2023 and December 31, 2022. There were no amounts drawn under the credit facility as of December 31, 2023 or December 31, 2022.
We have additional lines of credit and other credit arrangements with a group of banks and other financial intermediaries for general corporate purposes. We maintain cash and cash equivalent balances in excess of our outstanding short-term borrowings. As of December 31, 2023, we had access to $56 million of lines of credit which expire at various times and are generally renewed annually. There were $3 million of borrowings outstanding related to these facilities as of December 31, 2023 and $2 million borrowings outstanding related to these facilities as of December 31, 2022.
Domestic and international short-term funds
Many of our operations are conducted outside the U.S. The amount of funds held in the U.S. will fluctuate due to the timing of receipts and payments in the ordinary course of business and due to other reasons, such as business development activities. As part of our ongoing liquidity assessments, we regularly monitor the mix of U.S. and international cash flows (both inflows and outflows). Actual repatriation of overseas funds can result in additional U.S. and local income taxes, such as U.S. state income taxes, local withholding taxes, and taxes on currency gains and losses. See Notes to Consolidated Financial Statements—Note 8. Tax Matters.
Global economic conditions
Challenging economic conditions in recent years have not had, nor do we anticipate that it will have, a significant impact on our liquidity. Due to our operating cash flows, financial assets, access to capital markets and available lines of credit and revolving credit agreements, we continue to believe that we have the ability to meet our liquidity needs for the foreseeable future. As markets change, we continue to monitor our liquidity position. There can be no assurance that a challenging economic environment or an economic downturn would not impact our ability to obtain financing in the future.
Contractual obligations
In the normal course of business, we enter into contracts and commitments that obligate us to make payments in the future. These obligations include long-term debt, including interest obligations, purchase obligations, lease commitments, other liabilities, benefit plan obligations and uncertain tax positions. See Notes to Consolidated Financial Statements—Note 9. Financial Instruments, Note 18. Commitments and Contingencies, Note 10. Leases, Note 6. Restructuring Charges and Other Costs Associated with Acquisitions, Cost-Reduction and Productivity Initiatives, Note 14. Benefit Plans and Note 8. Tax Matters for further information on material cash requirements from known contractual and other obligations.
Debt securities
On November 8, 2022, we issued $1.35 billion aggregate principal amount of our senior notes (2022 senior notes), with an original issue discount of $2 million. These notes are comprised of $600 million aggregate principal amount of 5.400% senior notes due 2025 and $750 million aggregate principal amount of 5.600% senior notes due 2032. On February 1, 2023, the net proceeds were used to redeem in full, upon maturity, the $1.35 billion aggregate principal amount of our 3.250% 2013 senior notes due 2023.
Our senior notes are governed by an indenture and supplemental indentures (collectively, the indenture) between us and Deutsche Bank Trust Company Americas, as trustee. The indenture contains certain covenants, including limitations on our and certain of our subsidiaries' ability to incur liens or engage in sale lease-back transactions. The indenture also contains restrictions on our ability to consolidate, merge or sell substantially all of our assets. In addition, the indenture contains other customary terms, including certain events of default, upon the occurrence of which, the senior notes may be declared immediately due and payable.
Pursuant to the indenture, we are able to redeem the senior notes of any series, in whole or in part, at any time by paying a “make whole” premium, plus accrued and unpaid interest to, but excluding, the date of redemption. Upon the occurrence of a change of control of us and a downgrade of the senior notes below an investment grade rating by each of Moody's Investors Service, Inc. and Standard & Poor's Ratings Services, we are, in certain circumstances, required to make an offer to repurchase all of the outstanding senior notes at a price equal to 101% of the aggregate principal amount of the senior notes together with accrued and unpaid interest to, but excluding, the date of repurchase.
Our outstanding debt securities are as follows:
| | | | | | | | | | | |
| Description | Principal Amount | Interest Rate | Terms |
| | | |
| 2015 Senior Notes due 2025 | $750 million | 4.500% | Interest due semi annually, not subject to amortization, aggregate principal due on November 13, 2025 |
| 2022 Senior Notes due 2025 | $600 million | 5.400% | Interest due semi annually, not subject to amortization, aggregate principal due on November 14, 2025 |
| 2017 Senior Notes due 2027 | $750 million | 3.000% | Interest due semi annually, not subject to amortization, aggregate principal due on September 12, 2027 |
| 2018 Senior Notes due 2028 | $500 million | 3.900% | Interest due semi annually, not subject to amortization, aggregate principal due on August 20, 2028 |
| 2020 Senior Notes due 2030 | $750 million | 2.000% | Interest due semi annually, not subject to amortization, aggregate principal due on May 15, 2030 |
| 2022 Senior Notes due 2032 | $750 million | 5.600% | Interest due semi annually, not subject to amortization, aggregate principal due on November 16, 2032 |
| 2013 Senior Notes due 2043 | $1,150 million | 4.700% | Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2043 |
| 2017 Senior Notes due 2047 | $500 million | 3.950% | Interest due semi annually, not subject to amortization, aggregate principal due on September 12, 2047 |
| 2018 Senior Notes due 2048 | $400 million | 4.450% | Interest due semi annually, not subject to amortization, aggregate principal due on August 20, 2048 |
| 2020 Senior Notes due 2050 | $500 million | 3.000% | Interest due semi annually, not subject to amortization, aggregate principal due on May 15, 2050 |
Credit ratings
Two major corporate debt-rating organizations, Moody's and S&P, assign ratings to our short-term and long-term debt. A security rating is not a recommendation to buy, sell or hold securities and the rating is subject to revision or withdrawal at any time by the rating organization. Each rating should be evaluated independently of any other rating.
The following table provides the current ratings assigned by these rating agencies to our commercial paper and senior unsecured non-credit-enhanced long-term debt: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Commercial Paper | | Long-term Debt | | Date of Last Action |
| Name of Rating Agency | | Rating | | Rating | | Outlook | |
| Moody’s | | P-2 | | Baa1 | | Stable | | August 2017 |
| S&P | | A-2 | | BBB | | Stable | | December 2016 |
Pension obligations
Our employees ceased to participate in the Pfizer U.S. qualified defined benefit and U.S. retiree medical plans effective December 31, 2012, and liabilities associated with our employees under these plans were retained by Pfizer. As part of the separation from Pfizer, Pfizer continued to credit certain employees' service with Zoetis generally through December 31, 2017 (or termination of employment from Zoetis, if earlier), for certain early retirement benefits with respect to Pfizer's U.S. defined benefit pension and retiree medical plans. In connection with the employee matters agreement, Zoetis was responsible for payment of three-fifths of the total cost of the service credit continuation ($38 million) for these plans. The amount of the service cost continuation payment to be paid by Zoetis to Pfizer was determined and fixed based on an actuarial assessment of the value of the grow-in benefits and will be paid in equal installments over a period of 10 years. As of December 31, 2023, there are no remaining payments due to Pfizer.
As part of the separation from Pfizer, Pfizer transferred to us the net pension obligations associated with certain international defined benefit plans. We expect to contribute a total of $7 million to these plans in 2024.
As of December 31, 2023, the supplemental savings plan liability was $51 million.
For additional information, see Notes to Consolidated Financial Statements— Note 14. Benefit Plans.
Share repurchase program
In December 2021, our Board of Directors authorized a $3.5 billion share repurchase program. As of December 31, 2023, there was $1.5 billion remaining under this authorization. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs. Share repurchases may be executed through various means, including open market or privately negotiated transactions. During 2023, 6.3 million shares were repurchased for $1.1 billion, which excludes a $10 million accrual for excise tax on net share repurchases.
Off-balance sheet arrangements
In the ordinary course of business and in connection with the sale of assets and businesses, we may indemnify our counterparties against certain liabilities that may arise in connection with a transaction or that are related to activities prior to a transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters, and patent-infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2023 and 2022, recorded amounts for the estimated fair value of these indemnifications are not material.
New accounting standards
See Note 3. Significant Accounting Policies in the Notes to Consolidated Financial Statements for discussion of recent accounting pronouncements, including the respective dates of adoption or expected adoption and effects or expected effects on our consolidated financial position, results of operations and cash flows.
Forward-looking statements and factors that may affect future results
This report contains “forward-looking” statements. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. We generally identify forward-looking statements by using words such as “anticipate,” “estimate,” “could,” “expect,” “intend,” “project,” “plan,” “predict,” “believe,” “seek,” “continue,” “outlook,” “objective,” “target,” “may,” “might,” “will,” “should,” “can have,” “likely” or the negative version of these words or comparable words or by using future dates in connection with any discussion of future performance, actions or events.
In particular, forward-looking statements include statements relating to our future actions, business plans or prospects, prospective products, product approvals or products under development, product and supply chain disruptions, R&D costs, timing and likelihood of success, future operating or financial performance, future results of current and anticipated products and services, strategies, sales efforts, expenses, production efficiencies, production margins, anticipated timing of generic market entries, integration of acquired businesses, interest rates, tax rates, changes in tax regimes and laws, foreign exchange rates, growth in emerging markets, the outcome of contingencies, such as legal proceedings, plans related to share repurchases and dividends, government regulation and financial results. These statements are not guarantees of future performance, actions or events. Forward-looking statements are subject to risks and uncertainties, many of which are beyond our control, and are based on assumptions that could prove to be inaccurate. Among the factors that could cause actual results to differ materially from past results and future plans and projected future results are the following:
•the possible impact and timing of competing products, including generic alternatives, on our products and our ability to compete against such products;
•unanticipated safety, quality or efficacy concerns or issues about our products;
•the decline in global economic conditions, including the conflict between Israel and Hamas (including any escalation or expansion), economic weakness in China and inflation;
•the economic, political, legal and business environment of the foreign jurisdictions in which we do business;
•consolidation of our customers and distributors;
•changes in the distribution channel for companion animal products;
•disruptive innovations and advances in medical practices and technologies;
•an outbreak of infectious disease carried by animals;
•failure to successfully acquire businesses, license rights or products, integrate businesses, form and manage alliances or divest businesses;
•restrictions and bans on the use of and consumer preferences regarding antibacterials in food-producing animals;
•perceived adverse effects linked to the consumption of food derived from animals that utilize our products or animals generally;
•increased regulation or decreased governmental support relating to the raising, processing or consumption of food-producing animals;
•adverse weather conditions and the availability of natural resources;
•the impact of climate change on our activities and the activities of our customers and suppliers, including, for example, altered distribution and intensity of rainfall, prolonged droughts or flooding, increased frequency of wildfires and other natural disasters, rising sea levels, and rising heat index;
•product launch delays, inventory shortages, recalls or unanticipated costs caused by manufacturing problems and capacity imbalances;
•failure of our R&D, acquisition and licensing efforts to generate new products and product lifecycle innovations;
•difficulties or delays in the development or commercialization of new products;
•illegal distribution and/or sale of our products or the misuse or off-label use of our products;
•legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns, laws and regulations regarding data privacy, commercial disputes and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products;
•fluctuations in foreign exchange rates and potential currency controls;
•governmental laws and regulations affecting domestic and foreign operations, including without limitation, tax obligations and changes affecting the tax treatment by the U.S. of income earned outside the U.S. that may result from pending or possible future proposals;
•failure to protect our intellectual property rights or to operate our business without infringing the intellectual property rights of others;
•a cyberattack, information security breach or other misappropriation of our data;
•failure to generate sufficient cash to service our substantial indebtedness; and
•the other factors set forth under “Risk Factors” in Item 1A of Part I of this 2023 Annual Report.
However, there may also be other risks that we are unable to predict at this time. These risks or uncertainties may cause actual results to differ materially from those contemplated by a forward-looking statement. You should not put undue reliance on forward-looking statements. Forward-looking statements speak only as of the date on which they are made. We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects in our Form 10-Q and 8-K reports and our other filings with the SEC. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the above to be a complete discussion of all potential risks or uncertainties.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
A significant portion of our revenue and costs are exposed to changes in foreign exchange rates. In addition, our outstanding borrowings may be subject to risk from changes in interest rates and foreign exchange rates. The overall objective of our financial risk management program is to seek to manage the impact of foreign exchange rate movements and interest rate movements on our earnings. We manage these financial exposures through operational means and by using certain financial instruments. These practices may change as economic conditions change.
Foreign exchange risk
Our primary net foreign currency translation exposures are the Australian dollar, Brazilian real, British pound, Canadian dollar, Chinese renminbi and euro. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities.
We use foreign exchange derivative instruments designated as net investment hedges to hedge the foreign currency risks related to our investment in foreign subsidiaries. These foreign exchange derivative instruments serve to offset the foreign currency translation risk from certain of our foreign operations.
Our foreign exchange derivative instruments at December 31, 2023 were analyzed to determine their sensitivity to foreign exchange rate changes. If the U.S. dollar were to strengthen or weaken against all other currencies by 10%, the amount recorded in cumulative translation adjustment (CTA) within Accumulated other comprehensive loss related to our net investment hedge would increase or decrease, respectively, by approximately $82 million. The change in value recorded to CTA would be expected to offset a corresponding foreign currency translation gain or loss from our investment in foreign subsidiaries. See Notes to Consolidated Financial Statements—Note 9. Financial Instruments: B. Derivative Financial Instruments.
Foreign exchange risk is also managed through the use of foreign currency forward-exchange contracts. These contracts are used to offset the potential earnings effects from mostly intercompany short-term foreign currency assets and liabilities that arise from operations but are not designated as hedges.
Our foreign currency forward-exchange contracts at December 31, 2023 were analyzed to determine their sensitivity to foreign exchange rate changes. If the U.S. dollar were to strengthen or weaken against all other currencies by 10%, the fair value of these contracts would decrease or increase, respectively, by $11 million. The foreign currency gains and losses on the assets and liabilities are recorded in Other (income)/deductions-net. See Notes to Consolidated Financial Statements—Note 9. Financial Instruments: B. Derivative Financial Instruments.
Interest rate risk
Our outstanding debt balances are predominantly fixed rate debt. While changes in interest rates will have no impact on the interest we pay on our fixed rate debt, interest on our commercial paper and revolving credit facility will be exposed to interest rate fluctuations. Additionally, as of December 31, 2023, because we held certain interest rate swap agreements that have the economic effect of modifying the fixed-interest obligations associated with our 3.900% Senior Notes due 2028 and our 2.00% Senior Notes due 2030, a portion of the fixed-rate interest payable on these senior notes effectively became variable based on SOFR. At December 31, 2023, there were no commercial paper borrowings outstanding and no outstanding principal balance under our revolving credit facility.
By entering into the aforementioned swap arrangements, we have assumed risks associated with variable interest rates based upon SOFR. Changes in the overall level of interest rates affect the interest expense that we recognize in our Consolidated Statements of Income. An interest rate risk sensitivity analysis is used to measure interest rate risk by computing estimated changes in cash flows as a result of assumed changes in market interest rates. As of December 31, 2023, if SOFR-based interest rates would have been higher by 100 basis points, the change would have increased our interest expense annually by approximately $3 million, as it relates to our fixed to floating interest rate swap agreements. See Notes to Consolidated Financial Statements—Note 9. Financial Instruments.
In anticipation of issuing fixed-rate debt, we may use forward-starting interest rate swaps that are designated as cash flow hedges to hedge against changes in interest rates that could impact expected future issuances of debt. A 100-basis point increase or (decrease) in SOFR-based interest rates would have resulted in a increase or (decrease) in the fair value of our forward-starting interest rate swaps by $6 million and $(7) million, respectively at December 31, 2023.
At December 31, 2023, our cash equivalents were primarily invested in money market funds. Interest paid on such funds fluctuates with the prevailing interest rate.
Item 8. Financial Statements and Supplementary Data.
INDEX TO FINANCIAL STATEMENTS | | | | | |
| Page |
| Audited Consolidated Financial Statements of Zoetis Inc. and Subsidiaries: | |
| Reports of Independent Registered Public Accounting Firm | |
| Consolidated Statements of Income for the Years Ended December 31, 2023, 2022 and 2021 | |
| Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2023, 2022 and 2021 | |
| Consolidated Balance Sheets as of December 31, 2023 and 2022 | |
| Consolidated Statements of Equity for the Years Ended December 31, 2023, 2022 and 2021 | |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2023, 2022 and 2021 | |
| Notes to Consolidated Financial Statements | |
| Schedule II—Valuation and Qualifying Accounts | |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Zoetis Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Zoetis Inc. and subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2023, and the related notes and financial statement Schedule II - Valuation and Qualifying Accounts (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 13, 2024 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of gross unrecognized tax benefits
As discussed in Notes 3 and 8D in the consolidated financial statements, the Company’s tax positions are subject to examination by local taxing authorities in each respective tax jurisdiction, and the resolution of such examinations may span multiple years. Since tax law is complex and often subject to varied interpretations and judgments, it is uncertain whether some of the Company’s tax positions will be sustained upon examination. The Company accounts for income tax contingencies using a benefit recognition model. If a tax position is more likely than not (more than a 50% likelihood) to be sustained upon examination, based solely on the technical merits of the position, a benefit is recognized. As of December 31, 2023, the Company has recorded gross unrecognized tax benefits of $209 million.
We identified the evaluation of gross unrecognized tax benefits as a critical audit matter. Complex auditor judgment, including the involvement of tax professionals with specialized skills and knowledge was required to assess the valuation of a tax position, which included interpretation of relevant tax law, identification of relevant tax elements, the estimate of the more likely than not assessment of tax positions being sustained under examination and the estimate of the amount of the gross unrecognized tax benefit.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s unrecognized tax benefit process. This included controls related to (a) interpretation of relevant tax law, (b) evaluation of which of the Company’s tax positions may not be sustained upon examination, and (c) estimation of the gross unrecognized tax benefits. We involved tax professionals, with specialized skills and knowledge, who assisted in:
■evaluating the Company’s interpretation of relevant tax laws and the potential impact on the unrecognized tax benefits by developing an independent assessment of the tax position’s more likely than not to be sustained under examination determination as well as the estimate of the amount of the gross unrecognized tax benefit, if any, based on our understanding and interpretation of tax laws,
■reading and evaluating the tax opinion, and
■assessing the Company’s transfer pricing policies for compliance with applicable laws and regulations.
Deductions from revenue related to the rebates accrual for the U.S. segment
As discussed in Note 3 to the consolidated financial statements, the Company records an accrual for estimated rebates as a deduction from revenue when the related revenue is recognized. Included in the rebates accrual are estimated revenue deductions for future payments to distributors, clinics, veterinarians and pet owners under various programs offered by the Company. The volume of varied rebate programs offered, each with varying terms and conditions covering how the rebate is measured and earned, requires the Company to estimate the accrual using a number of inputs from multiple sources. Accruals for deductions from revenue are recorded as either a reduction in accounts receivable or within accrued expenses, depending on the nature of the contract and method of expected payment. Amounts recorded as a reduction in accounts receivable as of December 31, 2023 are approximately $301 million and accruals for deductions from revenue included in accrued expenses are approximately $323 million. These amounts include rebate accruals for both the U.S. and international segments.
We identified assessing deductions from revenue related to the rebates accrual for the U.S. segment as a critical audit matter. Because of the variety of programs offered by the Company in the U.S., the size of the U.S. market, and the length of time between when a sale is made and when the related rebate is settled by the Company, challenging auditor judgment was required in assessing the estimate of the required rebates accrual. In particular, the identification of which revenue transactions were subject to a rebate and the relevance and reliability of information used to estimate an individual rebate programs’ accrual required challenging auditor judgment.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s rebate accrual process for the U.S. segment. This included controls related to the identification of revenue transactions subject to a rebate and the relevance and reliability of information used in the estimated rebates accrual. We identified and considered the relevance, reliability and sufficiency of sources of data used by the Company in developing the estimate. We tested the estimate of the rebates accrual for a sample of U.S. programs, using a combination of Company internal data, historical information, executed contracts, and third-party data and compared our estimate to the amount recorded by the Company. We evaluated the historical accuracy of the Company’s U.S. rebates accrual by comparing the previously recorded accrual as of December 31, 2022 to the actual amount that ultimately was paid by the Company during 2023.
/s/ KPMG LLP
We have served as the Company’s auditor since 2011.
Short Hills, New Jersey
February 13, 2024
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Zoetis Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Zoetis Inc. and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2023 and 2022, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2023, and the related notes and financial statement Schedule II - Valuation and Qualifying Accounts (collectively, the consolidated financial statements), and our report dated February 13, 2024 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Short Hills, New Jersey
February 13, 2024
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA) | | 2023 | | 2022 | | 2021 |
| Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | |
| Costs and expenses: | | | | | | |
Cost of sales(a) | | 2,561 | | | 2,454 | | | 2,303 | |
Selling, general and administrative expenses(a) | | 2,151 | | | 2,009 | | | 2,001 | |
Research and development expenses(a) | | 614 | | | 539 | | | 508 | |
| Amortization of intangible assets | | 149 | | | 150 | | | 161 | |
| Restructuring charges and certain acquisition-related costs | | 53 | | | 11 | | | 43 | |
| Interest expense, net of capitalized interest | | 239 | | | 221 | | | 224 | |
| Other (income)/deductions––net | | (159) | | | 40 | | | 48 | |
| Income before provision for taxes on income | | 2,936 | | | 2,656 | | | 2,488 | |
| Provision for taxes on income | | 596 | | | 545 | | | 454 | |
| Net income before allocation to noncontrolling interests | | 2,340 | | | 2,111 | | | 2,034 | |
| Less: Net loss attributable to noncontrolling interests | | (4) | | | (3) | | | (3) | |
| Net income attributable to Zoetis Inc. | | $ | 2,344 | | | $ | 2,114 | | | $ | 2,037 | |
| Earnings per share attributable to Zoetis Inc. stockholders: | | | | | | |
| Basic | | $ | 5.08 | | | $ | 4.51 | | | $ | 4.29 | |
| Diluted | | $ | 5.07 | | | $ | 4.49 | | | $ | 4.27 | |
| Weighted-average common shares outstanding: | | | | | | |
| Basic | | 461.172 | | | 468.891 | | | 474.348 | |
| Diluted | | 462.269 | | | 470.385 | | | 476.717 | |
| Dividends declared per common share | | $ | 1.557 | | | $ | 1.350 | | | $ | 1.075 | |
(a) Exclusive of amortization of intangible assets, except as disclosed in Note 3. Significant Accounting Policies—Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Net income before allocation to noncontrolling interests | | $ | 2,340 | | | $ | 2,111 | | | $ | 2,034 | |
Other comprehensive loss, net of tax(a): | | | | | | |
| Unrealized (losses)/gains on derivatives for cash flow hedges, net of tax of $(2), $26 and $5 for the years ended December 31, 2023, 2022 and 2021, respectively | | (5) | | | 86 | | | 19 | |
| Unrealized (losses)/gains on derivatives for net investment hedges, net of tax of $(7), $11 and $13 for the years ended December 31, 2023, 2022 and 2021, respectively | | (23) | | | 36 | | | 42 | |
| Foreign currency translation adjustments, net | | — | | | (188) | | | (101) | |
| Benefit plans: Actuarial gains, net of tax of $2, $6 and $3 for the years ended December 31, 2023, 2022 and 2021, respectively | | 6 | | | 13 | | | 6 | |
| | | | | | |
| Total other comprehensive loss, net of tax | | (22) | | | (53) | | | (34) | |
| Comprehensive income before allocation to noncontrolling interests | | 2,318 | | | 2,058 | | | 2,000 | |
| Less: Comprehensive loss attributable to noncontrolling interests | | (4) | | | (3) | | | (3) | |
| Comprehensive income attributable to Zoetis Inc. | | $ | 2,322 | | | $ | 2,061 | | | $ | 2,003 | |
(a) Presented net of reclassification adjustments, which are not material in any period presented. Reclassification adjustments related to benefit plans are generally reclassified, as part of net periodic pension cost, into Cost of sales, Selling, general and administrative expenses, and/or Research and development expenses, as appropriate, in the Consolidated Statements of Income.
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | | | | | | |
| | December 31, | | December 31, |
| (MILLIONS OF DOLLARS, EXCEPT PER SHARE DATA) | | 2023 | | 2022 |
| Assets | | | | |
Cash and cash equivalents(a) | | $ | 2,041 | | | $ | 3,581 | |
| | | | |
| Accounts receivable, less allowance for doubtful accounts of $18 in 2023 and $19 in 2022 | | 1,304 | | | 1,215 | |
| Inventories | | 2,564 | | | 2,345 | |
| Other current assets | | 434 | | | 365 | |
| Total current assets | | 6,343 | | | 7,506 | |
| Property, plant and equipment, less accumulated depreciation of $2,594 in 2023 and $2,297 in 2022 | | 3,204 | | | 2,753 | |
| Operating lease right of use assets | | 230 | | | 220 | |
| Goodwill | | 2,759 | | | 2,746 | |
| Identifiable intangible assets, less accumulated amortization | | 1,338 | | | 1,380 | |
| Noncurrent deferred tax assets | | 206 | | | 173 | |
| Other noncurrent assets | | 206 | | | 147 | |
| Total assets | | $ | 14,286 | | | $ | 14,925 | |
| | | | |
| Liabilities and Equity | | | | |
| Short-term borrowings | | $ | 3 | | | $ | 2 | |
| Current portion of long-term debt | | — | | | 1,350 | |
| Accounts payable | | 411 | | | 405 | |
| Dividends payable | | 198 | | | 174 | |
| Accrued expenses | | 683 | | | 682 | |
| Accrued compensation and related items | | 382 | | | 300 | |
| Income taxes payable | | 110 | | | 157 | |
| Other current liabilities | | 102 | | | 97 | |
| Total current liabilities | | 1,889 | | | 3,167 | |
| Long-term debt, net of discount and issuance costs | | 6,564 | | | 6,552 | |
| Noncurrent deferred tax liabilities | | 146 | | | 142 | |
| Operating lease liabilities | | 188 | | | 186 | |
| Other taxes payable | | 271 | | | 258 | |
| Other noncurrent liabilities | | 237 | | | 217 | |
| Total liabilities | | 9,295 | | | 10,522 | |
| Commitments and contingencies (Note 18) | | | | |
| Stockholders' equity: | | | | |
| Common stock, $0.01 par value: 6,000,000,000 authorized, 501,891,243 and 501,891,243 shares issued; 458,367,358 and 463,808,059 shares outstanding at December 31, 2023 and 2022, respectively | | 5 | | | 5 | |
| Treasury stock, at cost, 43,523,885 and 38,083,184 shares of common stock at December 31, 2023 and 2022, respectively | | (5,597) | | | (4,539) | |
| Additional paid-in capital | | 1,133 | | | 1,088 | |
| Retained earnings | | 10,295 | | | 8,668 | |
| Accumulated other comprehensive loss | | (839) | | | (817) | |
| Total Zoetis Inc. equity | | 4,997 | | | 4,405 | |
| Equity attributable to noncontrolling interests | | (6) | | | (2) | |
| Total equity | | 4,991 | | | 4,403 | |
| Total liabilities and equity | | $ | 14,286 | | | $ | 14,925 | |
(a) As of December 31, 2023 and 2022, includes $2 million and $4 million, respectively, of restricted cash.
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Zoetis | | | | |
| | | | | | | | | | | | | | Accumulated | | Equity | | |
| | | | | | | | | | Additional | | | | Other | | Attributable to | | |
| (MILLIONS OF DOLLARS, EXCEPT SHARE DATA) | | Common Stock | | Treasury Stock | | Paid-in | | Retained | | Comprehensive | | Noncontrolling | | Total |
| Shares | | Amount | | Shares | | Amount | | Capital | | Earnings | | Loss | | Interests | | Equity |
| Balance, December 31, 2020 | | 501.9 | | | $ | 5 | | | 26.6 | | | $ | (2,230) | | | $ | 1,065 | | | $ | 5,659 | | | $ | (730) | | | $ | 4 | | | $ | 3,773 | |
| Net income/(loss) | | — | | | — | | | — | | | — | | | — | | | 2,037 | | | — | | | (3) | | | 2,034 | |
| Other comprehensive loss | | — | | | — | | | — | | | — | | | — | | | — | | | (34) | | | — | | | (34) | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
Share-based compensation awards(a) | | — | | | — | | | (1.3) | | | 21 | | | — | | | (1) | | | — | | | — | | | 20 | |
Treasury stock acquired(b) | | — | | | — | | | 4.0 | | | (743) | | | — | | | — | | | — | | | — | | | (743) | |
Employee benefit plan contribution from Pfizer Inc.(c) | | — | | | — | | | — | | | — | | | 3 | | | — | | | — | | | — | | | 3 | |
| Dividends declared | | — | | | — | | | — | | | — | | | — | | | (509) | | | — | | | — | | | (509) | |
| Balance, December 31, 2021 | | 501.9 | | | $ | 5 | | | 29.3 | | | $ | (2,952) | | | $ | 1,068 | | | $ | 7,186 | | | $ | (764) | | | $ | 1 | | | $ | 4,544 | |
| Net income/(loss) | | — | | | — | | | — | | | | | | | 2,114 | | | — | | | (3) | | | 2,111 | |
| Other comprehensive loss | | — | | | — | | | — | | | | | — | | | — | | | (53) | | | — | | | (53) | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
Share-based compensation awards(a) | | — | | | — | | | (0.7) | | | 7 | | | 17 | | | (1) | | | — | | | — | | | 23 | |
Treasury stock acquired(b) | | — | | | — | | | 9.5 | | | (1,594) | | | — | | | — | | | — | | | — | | | (1,594) | |
Employee benefit plan contribution from Pfizer Inc.(c) | | — | | | — | | | — | | | | | 3 | | | — | | | — | | | — | | | 3 | |
| Dividends declared | | — | | | — | | | — | | | | | — | | | (631) | | | — | | | — | | | (631) | |
| Balance, December 31, 2022 | | 501.9 | | | $ | 5 | | | 38.1 | | | $ | (4,539) | | | $ | 1,088 | | | $ | 8,668 | | | $ | (817) | | | $ | (2) | | | $ | 4,403 | |
| Net income/(loss) | | — | | | — | | | — | | | — | | | — | | | 2,344 | | | — | | | (4) | | | 2,340 | |
| Other comprehensive loss | | — | | | — | | | — | | | — | | | — | | | — | | | (22) | | | — | | | (22) | |
| | | | | | | | | | | | | | | | | | |
Share-based compensation awards(a) | | — | | | — | | | (0.9) | | | 44 | | | 45 | | | (1) | | | — | | | — | | | 88 | |
Treasury stock acquired(b) | | — | | | — | | | 6.3 | | | (1,102) | | | — | | | — | | | — | | | — | | | (1,102) | |
| | | | | | | | | | | | | | | | | | |
| Dividends declared | | — | | | — | | | — | | | — | | | — | | | (716) | | | — | | | — | | | (716) | |
| Balance, December 31, 2023 | | 501.9 | | | $ | 5 | | | 43.5 | | | $ | (5,597) | | | $ | 1,133 | | | $ | 10,295 | | | $ | (839) | | | $ | (6) | | | $ | 4,991 | |
Shares may not add due to rounding.
(a) Includes the issuance of shares of Zoetis Inc. common stock and the reacquisition of shares of treasury stock associated with exercises of employee share-based awards. Also includes the reacquisition of shares of treasury stock associated with the vesting of employee share-based awards to satisfy tax withholding requirements. For additional information, see Note 15. Share-based Payments and Note 16. Stockholders' Equity.
(b) Reflects the acquisition of treasury shares in connection with the share repurchase program. For 2023, includes excise tax accrued on net share repurchases. For additional information, see Note 16. Stockholders' Equity.
(c) Represents contributed capital from Pfizer Inc. associated with service credit continuation for certain Zoetis Inc. employees in Pfizer Inc.'s U.S. qualified defined benefit and U.S. retiree medical plans. See Note 14. Benefit Plans.
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Operating Activities | | | | | | |
| Net income before allocation to noncontrolling interests | | $ | 2,340 | | | $ | 2,111 | | | $ | 2,034 | |
| Adjustments to reconcile net income before noncontrolling interests to net cash provided by operating activities: | | | | | | |
| Depreciation and amortization expense | | 491 | | | 465 | | | 448 | |
| Share-based compensation expense | | 60 | | | 62 | | | 58 | |
| Asset write-offs and asset impairments | | 46 | | | 53 | | | 47 | |
| | | | | | |
| Net gain on sale of businesses, excluding transaction costs | | (118) | | | — | | | — | |
| Provision for losses on inventory | | 115 | | | 76 | | | 46 | |
| Deferred taxes | | (61) | | | (286) | | | (80) | |
| Settlement of derivative contracts | | — | | | 114 | | | — | |
| Employee benefit plan contribution from Pfizer Inc. | | — | | | 3 | | | 3 | |
| Other non-cash adjustments | | (8) | | | 13 | | | — | |
| Other changes in assets and liabilities, net of acquisitions and divestitures: | | | | | | |
| Accounts receivable | | (102) | | | (137) | | | (155) | |
| Inventories | | (361) | | | (486) | | | (366) | |
| Other assets | | (95) | | | 35 | | | (7) | |
| Accounts payable | | 13 | | | (29) | | | (17) | |
| Other liabilities | | 67 | | | (180) | | | 227 | |
| Other tax accounts, net | | (34) | | | 98 | | | (25) | |
| Net cash provided by operating activities | | 2,353 | | | 1,912 | | | 2,213 | |
| Investing Activities | | | | | | |
| Capital expenditures | | (732) | | | (586) | | | (477) | |
| | | | | | |
| Acquisitions, net of cash acquired | | (155) | | | (312) | | | (14) | |
| Purchase of investments | | (4) | | | (9) | | | (12) | |
| | | | | | |
| Proceeds from derivative instrument activity, net | | 12 | | | 23 | | | 44 | |
| Proceeds from sale of businesses, net of cash sold | | 96 | | | — | | | — | |
| Net proceeds from sale of assets | | 4 | | | 1 | | | 2 | |
| Other investing activities | | 2 | | | — | | | (1) | |
| Net cash used in investing activities | | (777) | | | (883) | | | (458) | |
| Financing Activities | | | | | | |
| Increase/(decrease) in short-term borrowings, net | | 1 | | | 2 | | | (4) | |
| Principal payments on long-term debt | | (1,350) | | | — | | | (600) | |
| Proceeds from issuance of long-term debt—senior notes, net of discount | | — | | | 1,348 | | | — | |
| Payment of debt issuance costs | | — | | | (10) | | | — | |
| Payment of consideration related to previous acquisitions | | (3) | | | (1) | | | (6) | |
| Share-based compensation-related proceeds, net of taxes paid on withholding shares | | 27 | | | (38) | | | (35) | |
| Purchases of treasury stock | | (1,092) | | | (1,594) | | | (743) | |
| Cash dividends paid | | (692) | | | (611) | | | (474) | |
| | | | | | |
| Net cash used in financing activities | | (3,109) | | | (904) | | | (1,862) | |
| Effect of exchange-rate changes on cash and cash equivalents | | (7) | | | (29) | | | (12) | |
| Net (decrease)/increase in cash and cash equivalents | | (1,540) | | | 96 | | | (119) | |
| Cash and cash equivalents at beginning of period | | 3,581 | | | 3,485 | | | 3,604 | |
| Cash and cash equivalents at end of period | | $ | 2,041 | | | $ | 3,581 | | | $ | 3,485 | |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| Supplemental cash flow information | | | | | | |
| Cash paid during the period for: | | | | | | |
| Income taxes | | $ | 754 | | | $ | 638 | | | $ | 548 | |
| Interest, net of capitalized interest | | 295 | | | 242 | | | 253 | |
| Non-cash transactions: | | | | | | |
| Capital expenditures | | $ | 2 | | | $ | 3 | | | $ | 6 | |
| | | | | | |
| Dividends declared, not paid | | 198 | | | 174 | | | 154 | |
| Excise tax accrued on net share repurchases, not paid | | 10 | | | — | | | — | |
ZOETIS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Description
Zoetis Inc. (including its subsidiaries, collectively, Zoetis, the company, we, us or our) is a global leader in the animal health industry, focused on the discovery, development, manufacture and commercialization of medicines, vaccines, diagnostic products and services, biodevices, genetic tests and precision animal health. We organize and operate our business in two geographic regions: the United States (U.S.) and International.
We directly market our products in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America. Our products are sold in more than 100 countries, including developed markets and emerging markets. We have a diversified business, marketing products across eight core species: dogs, cats and horses (collectively, companion animals) and cattle, swine, poultry, fish and sheep (collectively, livestock); and within seven major product categories: parasiticides, vaccines, dermatology, other pharmaceutical, anti-infectives, animal health diagnostics and medicated feed additives.
We were incorporated in Delaware in July 2012 and prior to that the company was a business unit of Pfizer Inc. (Pfizer).
2. Basis of Presentation
The consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP). For subsidiaries operating outside the United States, the consolidated financial information is included as of and for the fiscal year ended November 30 for each year presented. All significant intercompany balances and transactions between the legal entities that comprise Zoetis have been eliminated. For those subsidiaries included in these consolidated financial statements where our ownership is less than 100%, including a variable interest entity consolidated by Zoetis as the primary beneficiary, the noncontrolling interests have been shown in equity as Equity attributable to noncontrolling interests.
3. Significant Accounting Policies
Recently Adopted Accounting Standards
In March 2020, the FASB issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting. In January 2021 and December 2022, it issued subsequent amendments to the initial guidance: ASU No. 2021-01 and ASU No. 2022-06, Reference Rate Reform (Topic 848). The new guidance provides temporary optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions that reference the London Interbank Offered Rate (LIBOR) or another reference rate expected to be discontinued because of reference rate reform. Adoption of the guidance is optional and effective as of March 12, 2020, but only available through December 31, 2024. During the first quarter of 2023, we adopted the guidance by executing amendments to our affected contracts that referenced LIBOR. The adoption did not have a material impact on our consolidated financial statements or related disclosures.
Estimates and Assumptions
In preparing the consolidated financial statements, we use certain estimates and assumptions that affect reported amounts and disclosures, including amounts recorded in connection with acquisitions. These estimates and underlying assumptions can impact all elements of our consolidated financial statements. For example, in the Consolidated Statements of Income, estimates are used when accounting for deductions from revenue (such as rebates, sales allowances, product returns and discounts), determining cost of sales, allocating cost in the form of depreciation and amortization, and estimating restructuring charges and the impact of contingencies. On the Consolidated Balance Sheets, estimates are used in determining the valuation and recoverability of assets, such as accounts receivables, inventories, fixed assets, goodwill and other identifiable intangible assets, and estimates are used in determining the reported amounts of liabilities, such as taxes payable, uncertain tax positions, benefit obligations, the impact of contingencies, deductions from revenue and restructuring reserves, all of which also impact the Consolidated Statements of Income.
Our estimates are often based on complex judgments, probabilities and assumptions that we believe to be reasonable but that can be inherently uncertain and unpredictable. If our estimates and assumptions are not representative of actual outcomes, our results could be materially impacted.
As future events and their effects cannot be determined with precision, our estimates and assumptions may prove to be incomplete or inaccurate, or unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions. We are subject to risks and uncertainties that may cause actual results to differ from estimated amounts, such as changes in competition, litigation, legislation and regulations. We regularly evaluate our estimates and assumptions using historical experience and expectations about the future. We adjust our estimates and assumptions when facts and circumstances indicate the need for change. Those changes generally will be reflected in our consolidated financial statements on a prospective basis unless they are required to be treated retrospectively under relevant accounting standards. It is possible that others, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts.
Acquisitions
Our consolidated financial statements include the operations of acquired businesses from the date of acquisition. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired in-process research and development (IPR&D) be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired is recorded as goodwill. When we acquire net assets that do not constitute a business as defined in U.S. GAAP, no goodwill is recognized.
Amounts recorded for acquisitions can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Leases
We determine if a contract contains a lease at inception. Leases are recorded as a right of use asset, as of the lease commencement date, in an amount equal to the present value of future payments over the lease term. A corresponding lease liability is also recorded. We have elected not to recognize right of use assets and lease liabilities for short-term leases of vehicles and equipment with a lease term of twelve months or less.
Options to extend or terminate a lease are considered in the lease term to the extent that the option is reasonably certain of exercise. The present value of future payments is discounted using the rate implicit in the lease, when available. When the implicit rate is not available, as is frequently the case with our lease portfolio, the present value is calculated using our incremental borrowing rate, which is determined on the commencement date. The incremental borrowing rate represents the rate of interest that we would expect to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms. As we do not borrow on a collateralized basis, our non-collateralized borrowing rate is used as an input in deriving the incremental borrowing rate.
Our lease portfolio primarily consists of operating leases, in which fixed lease payments are recognized on a straight-line basis over the lease term. Operating lease assets are recorded within Operating lease right of use assets with the corresponding operating lease liabilities recorded within Other current liabilities and Operating lease liabilities on the Consolidated Balance Sheets. Finance lease assets are recorded within Other noncurrent assets with the corresponding finance lease liabilities recorded within Other current liabilities and Other noncurrent liabilities on the Consolidated Balance Sheets. Variable payments are recognized in the period incurred. Variable lease payments include real estate taxes and charges for other non-lease services due to lessors that are not dependent on an index or rate and utilization based charges associated with fleet vehicles.
Our real estate and fleet lease contracts may include fixed consideration attributable to both lease and non-lease components, including non-lease services provided by the vendor, which are accounted for as a single fixed minimum payment. For leases of certain classes of machinery and equipment, contract consideration is allocated to lease and non-lease components on the basis of relative standalone price.
Foreign Currency Translation
For most of our international operations, local currencies have been determined to be the functional currencies. We translate functional currency assets and liabilities to their U.S. dollar equivalents at exchange rates in effect at the balance sheet date and we translate functional currency income and expense amounts to their U.S. dollar equivalents at average exchange rates for the period. The U.S. dollar effects that arise from changing translation rates are recorded in Other comprehensive income/(loss), net of tax. The effects of converting non-functional currency assets and liabilities into the functional currency are recorded in Other (income)/deductions––net. For operations in highly inflationary economies, we translate monetary items at rates in effect at the balance sheet date, with translation adjustments recorded in Other (income)/deductions––net, and we translate non-monetary items at historical rates.
Revenue, Deductions from Revenue and the Allowance for Doubtful Accounts
We recognize revenue from product sales when control of the goods has transferred to the customer, which is typically once the goods have shipped and the customer has assumed title. Revenue reflects the total consideration to which we expect to be entitled (i.e., the transaction price), in exchange for products sold, after considering various types of variable consideration including rebates, sales allowances, product returns and discounts.
Variable consideration is estimated and recorded at the time that related revenue is recognized. Our estimates reflect the amount by which we expect variable consideration to impact revenue recognized and are generally based on contractual terms or historical experience, adjusted as necessary to reflect our expectations about the future. Our customer payment terms generally range from 30 to 90 days.
Estimates of variable consideration utilize a complex series of judgments and assumptions to determine the amount by which we expect revenue to be reduced, for example;
•for sales returns, we perform calculations in each market that incorporate the following, as appropriate: local returns policies and practices; historic returns as a percentage of revenue; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, product recalls, discontinuation of products or a changing competitive environment; and
•for revenue incentives, we use our historical experience with similar incentives programs to estimate the impact of such programs on revenue for the current period.
Although the amounts recorded for these deductions from revenue are dependent on estimates and assumptions, historically our adjustments to actual results have not been material. The sensitivity of our estimates can vary by program, type of customer and geographic location.
Accruals for deductions from revenue are recorded as either a reduction in Accounts receivable or within Accrued expenses, depending on the nature of the contract and method of expected payment. Amounts recorded as a reduction in Accounts receivable as of December 31, 2023 and 2022 are approximately $301 million and $295 million, respectively. As of December 31, 2023, and 2022, accruals for deductions from revenue included in Accrued expenses are approximately $323 million and $285 million, respectively.
A deferral of revenue may be required in the event that we have not satisfied all customer obligations for which we have been compensated. The transaction price is allocated to the individual performance obligations on the basis of relative stand-alone selling price, which is typically based on actual sales prices. Revenue associated with unsatisfied performance obligations are contract liabilities is recorded within Other current liabilities and Other noncurrent liabilities. Recognition of revenue occurs once control of the underlying products has transferred to the customer. Contract liabilities reflected within Other current liabilities as of December 31, 2022 and subsequently recognized as revenue during 2023 were approximately $5 million. Contract liabilities as of December 31, 2023 were approximately $11 million.
We do not disclose the transaction price allocated to unsatisfied performance obligations related to contracts with an original expected duration of one year or less, or for contracts for which we recognize revenue in line with our right to invoice the customer. Estimated future revenue expected to be generated from long-term contracts with unsatisfied performance obligations as of December 31, 2023 is not material.
Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from Revenue. Shipping and handling costs incurred after control of the purchased product has transferred to the customer are accounted for as a fulfillment cost, within Selling, general and administrative expenses.
We also record estimates for bad debts. We periodically assess the adequacy of the allowance for doubtful accounts by evaluating the collectability of outstanding receivables based on factors such as past due history, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment.
Amounts recorded for sales deductions and bad debts can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Cost of Sales and Inventories
Inventories are carried at the lower of cost or net realizable value. The cost of finished goods, work-in-process and raw materials is determined using average actual cost. We regularly review our inventories for impairment and adjustments are recorded when necessary.
Selling, General and Administrative Expenses
Selling, general and administrative costs are expensed as incurred. Among other things, these expenses include the internal and external costs of marketing, advertising, and shipping and handling as well as certain costs related to business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, among others.
Advertising expenses relating to production costs are expensed as incurred, and the costs of space in publications are expensed when the related advertising occurs. Advertising and promotion expenses totaled approximately $281 million in 2023, $287 million in 2022 and $292 million in 2021.
Shipping and handling costs totaled approximately $101 million in 2023, $82 million in 2022 and $80 million in 2021.
Research and Development Expenses
Research and development (R&D) costs are expensed as incurred. Research is the effort associated with the discovery of new knowledge that will be useful in developing a new product or in significantly improving an existing product. Development is the implementation of the research findings. Before a compound receives regulatory approval, we record upfront and milestone payments made by us to third parties under licensing arrangements as expense. Upfront payments are recorded when incurred, and milestone payments are recorded when the specific milestone has been achieved. Once a compound receives regulatory approval in a major market, we record any milestone payments in Identifiable intangible assets, less accumulated amortization and, unless the assets are determined to have an indefinite life, we amortize them on a straight-line basis over the remaining agreement term or the expected product life cycle, whichever is shorter.
Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
• Goodwill—goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized.
• Identifiable intangible assets, less accumulated amortization—these acquired assets are recorded at our cost. Identifiable intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Identifiable intangible assets with indefinite lives that are associated with marketed products are not amortized until a useful life can be determined. Identifiable intangible assets associated with IPR&D projects are not amortized until regulatory approval is obtained. The useful life of an amortizing asset generally is determined by identifying the period in which substantially all of the cash flows are expected to be generated.
• Property, plant and equipment, less accumulated depreciation––these assets are recorded at our cost and are increased by the cost of any significant improvements after purchase. Property, plant and equipment assets, other than land and construction-in-progress, are depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws.
Amortization expense related to finite-lived identifiable intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property are included in Amortization of intangible assets as they benefit multiple business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of property, plant and equipment are included in Cost of sales, Selling, general and administrative expenses and Research and development expenses, as appropriate.
We review all of our long-lived assets for impairment indicators throughout the year and we perform detailed testing whenever impairment indicators are present. In addition, we perform impairment testing for goodwill and indefinite-lived assets at least annually. When necessary, we record charges for impairments. Specifically:
• For finite-lived identifiable intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate the remaining useful lives of the assets and modify them, as appropriate.
• For indefinite-lived identifiable intangible assets, such as brands and IPR&D assets, we test for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized. We record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate.
• For goodwill, we test for impairment on at least an annual basis, or more frequently if necessary, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, or by performing a periodic quantitative assessment. If we choose to perform a qualitative analysis and conclude it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. We determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net assets other than goodwill from the fair value of the reporting unit and record an impairment loss for the excess, if any, of book value of goodwill over the implied fair value. In 2023 we performed a qualitative impairment assessment as of September 30, 2023, which did not result in the impairment of goodwill associated with any of our reporting units. In 2022, we performed a periodic quantitative impairment assessment as of September 30, 2022, which did not result in the impairment of goodwill associated with any of our reporting units.
Impairment reviews can involve a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Software Capitalization and Depreciation
We capitalize certain costs incurred in connection with obtaining or developing internal-use software, including payroll and payroll-related costs for employees who are directly associated with the internal-use software project, external direct costs of materials and services and interest costs while developing the software. Capitalized software costs are included in Property, plant and equipment and are amortized using the straight-line method over the estimated useful life of 5 to 10 years. Capitalization of such costs ceases when the project is substantially complete and ready for its intended purpose. Costs incurred during the preliminary project and post-implementation stages, as well as software maintenance and training costs, are expensed in the period in which they are incurred. The company capitalized $35 million and $57 million of internal-use software for the years ended December 31, 2023 and 2022, respectively. Depreciation expense for capitalized software was $75 million in 2023, $69 million in 2022 and $52 million in 2021.
In addition, we capitalize qualifying implementation costs under cloud computing arrangements (“CCA”). The capitalized CCA implementation costs are allocated between Other current assets and Other noncurrent assets on the accompanying Consolidated Balance Sheets based on the expected period that amortization will be recognized. CCA implementation costs are amortized using the straight-line method over the expected term of the related service contract.
Restructuring Charges and Certain Acquisition-Related Costs
We may incur restructuring charges in connection with acquisitions when we implement plans to restructure and integrate the acquired operations or in connection with cost-reduction and productivity initiatives. Included in Restructuring charges and certain acquisition-related costs are all restructuring charges and certain costs associated with acquiring and integrating an acquired business. Transaction costs and integration costs are expensed as incurred. Termination costs are a significant component of restructuring charges and are generally recorded when the actions are probable and estimable.
Amounts recorded for restructuring charges and other associated costs can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Earnings per Share
Basic earnings per share is computed by dividing net income attributable to Zoetis by the weighted-average number of common shares outstanding during the period. Diluted earnings per share adjusts the weighted-average number of common shares outstanding for the potential dilution that could occur if common stock equivalents (stock options, restricted stock units, and performance-vesting restricted stock units) were exercised or converted into common stock, calculated using the treasury stock method.
Cash Equivalents
Cash equivalents include items almost as liquid as cash, such as money market funds, certificates of deposit and time deposits with maturity periods of three months or less when purchased.
Fair Value
Certain assets and liabilities are required to be measured at fair value, either upon initial recognition or for subsequent accounting or reporting. For example, we use fair value extensively in the initial recognition of net assets acquired in a business combination. Fair value is estimated using an exit price approach, which requires, among other things, that we determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants, considering the highest and best use of assets and, for liabilities, assuming that the risk of non-performance will be the same before and after the transfer.
When estimating fair value, depending on the nature and complexity of the asset or liability, we may use one or all of the following approaches:
• Income approach, which is based on the present value of a future stream of net cash flows.
• Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
• Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence.
These fair value methodologies depend on the following types of inputs:
• Quoted prices for identical assets or liabilities in active markets (Level 1 inputs).
• Quoted prices for similar assets or liabilities in active markets or quoted prices for identical or similar assets or liabilities in markets that are not active or are directly or indirectly observable (Level 2 inputs).
• Unobservable inputs that reflect estimates and assumptions (Level 3 inputs).
A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Accounts Receivable
The recorded amounts of accounts receivable approximate fair value because of their relatively short-term nature. As of December 31, 2023 and 2022, Accounts receivable, less allowance for doubtful accounts, of $1,304 million and $1,215 million, respectively, includes approximately $58 million and $71 million, respectively, of other receivables, such as trade notes receivable and royalty receivables, among others.
Deferred Tax Assets and Liabilities and Income Tax Contingencies
Deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates and laws. We provide a valuation allowance when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax planning strategies.
We account for income tax contingencies using a benefit recognition model. If we consider that a tax position is more likely than not to be sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the appropriate taxing authority that has full knowledge of all relevant information. Under the benefit recognition model, if the initial assessment fails to result in the recognition of a tax benefit, we regularly monitor our position and subsequently recognize the tax benefit: (i) if there are changes in tax law, analogous case law or there is new information that sufficiently raise the likelihood of prevailing on the technical merits of the position to more likely than not; (ii) if the statute of limitations expires; or (iii) if there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. We regularly re-evaluate our tax positions based on the results of audits of federal, state and foreign income tax filings, statute of limitations expirations, changes in tax law or receipt of new information that would either increase or decrease the technical merits of a position relative to the “more-likely-than-not” standard. Liabilities associated with uncertain tax positions are classified as current only when we expect to pay cash within the next 12 months. Interest and penalties, if any, are recorded in Provision for taxes on income and are classified on our Consolidated Balance Sheets with the related tax liability.
Amounts recorded for valuation allowances and income tax contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Benefit Plans
All dedicated benefit plans are pension plans. For our dedicated benefit plans, we recognize the overfunded or underfunded status of defined benefit plans as an asset or liability on the Consolidated Balance Sheets and the obligations generally are measured at the actuarial present value of all benefits attributable to employee service rendered, as provided by the applicable benefit formula. Pension obligations may include assumptions such as long-term rate of return on plan assets, expected employee turnover, participant mortality, and future compensation levels. Plan assets are measured at fair value. Net periodic benefit costs are recognized, as required, into Cost of sales, Selling, general and administrative expenses and Research and development expenses, as appropriate.
Amounts recorded for benefit plans can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Asset Retirement Obligations
We record accruals for the legal obligations associated with the retirement of tangible long-lived assets, including obligations under the doctrine of promissory estoppel and those that are conditioned upon the occurrence of future events. These obligations generally result from the acquisition, construction, development and/or normal operation of long-lived assets. We recognize the fair value of these obligations in the period in which they are incurred by increasing the carrying amount of the related asset. Over time, we recognize expense for the accretion of the liability and for the amortization of the asset.
As of December 31, 2023 and 2022, accruals for asset retirement obligations are $28 million and $25 million, respectively, and are included in Other noncurrent liabilities.
Amounts recorded for asset retirement obligations can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Legal and Environmental Contingencies
We are subject to numerous contingencies arising in the ordinary course of business, such as product liability and other product-related litigation, commercial litigation, patent litigation, environmental claims and proceedings, government investigations and guarantees and indemnifications. We record accruals for these contingencies to the extent that we conclude that a loss is both probable and reasonably estimable. If some amount within a range of loss appears to be a better estimate than any other amount within the range, we accrue that amount. Alternatively, when no amount within a range of loss appears to be a better estimate than any other amount, we accrue the lowest amount in the range. We record anticipated recoveries under existing insurance contracts when recovery is assured.
Amounts recorded for contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Share-Based Payments
Our compensation programs can include share-based payment plans. All grants under share-based payment programs are accounted for at fair value and such amounts generally are amortized on a straight-line basis over the vesting term to Cost of sales, Selling, general and administrative expenses, and Research and development expenses, as appropriate. We include the impact of estimated forfeitures when determining share-based compensation expense.
Amounts recorded for share-based compensation can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
4. Revenue
A. Revenue from Product Sales
We offer a diversified portfolio of products which allows us to capitalize on local and regional customer needs. Generally, our products are promoted to veterinarians and livestock producers by our sales organization which includes sales representatives and technical and veterinary operations specialists, and then sold directly by us or through distributors, retailers or e-commerce outlets. The depth of our product portfolio enables us to address the varying needs of customers in different species and geographies. Many of our top-selling product lines are distributed across both of our operating segments, leveraging our R&D operations and manufacturing and supply chain network.
Over the course of our history, we have focused on developing a diverse portfolio of animal health products, including medicines, vaccines and diagnostics, complemented by biodevices, genetic tests and a range of services. We refer to all different brands of a particular product, or its dosage forms for all species, as a product line. We have approximately 300 comprehensive product lines, including products for both companion animals and livestock within each of our major product categories.
Our major product categories are:
•parasiticides: products that prevent or eliminate external and internal parasites such as fleas, ticks and worms;
•vaccines: biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response;
•dermatology: products that relieve itch associated with allergic conditions and atopic dermatitis;
•other pharmaceutical: pain and sedation, antiemetic, reproductive, and oncology products;
•anti-infectives: products that kill or slow the growth of bacteria, fungi or protozoa;
•animal health diagnostics: testing and analysis of blood, urine and other animal samples and related products and services, including point-of-care diagnostic products, instruments and reagents, rapid immunoassay tests, reference laboratory kits and services and blood glucose monitors; and
•medicated feed additives: products added to animal feed that provide medicines to livestock.
Our remaining revenue is derived from other non-pharmaceutical product categories, such as nutritionals, as well as products and services in biodevices, genetic tests and precision animal health.
Our companion animal products help extend and improve the quality of life for pets; increase convenience and compliance for pet owners; and help veterinarians improve the quality of their care and the efficiency of their businesses. Growth in the companion animal medicines, vaccines and diagnostics sector is driven by economic development, related increases in disposable income and increases in pet ownership and spending on pet care. Companion animals are also living longer, deepening the human-animal bond, receiving increased medical treatment and benefiting from advances in animal health medicine, vaccines and diagnostics.
Our livestock products primarily help prevent or treat diseases and conditions to allow veterinarians and producers to care for their animals and to enable the cost-effective production of safe, high-quality animal protein. Human population growth and increasing standards of living are important long-term growth drivers for our livestock products in three major ways. First, population growth and increasing standards of living drive demand for improved nutrition, particularly through increased consumption of animal protein. Second, population growth leads to greater natural resource constraints driving a need for enhanced productivity. Finally, as standards of living improve and the global chain faces increased scrutiny, there is more focus on food quality, safety, and reliability of supply.
The following tables present our revenue disaggregated by geographic area, species, and major product category:
Revenue by geographic area | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| United States | | $ | 4,555 | | | $ | 4,313 | | | $ | 4,042 | |
| Australia | | 323 | | | 289 | | | 259 | |
| Brazil | | 393 | | | 330 | | | 312 | |
| Canada | | 255 | | | 238 | | | 232 | |
| Chile | | 140 | | | 141 | | | 136 | |
| China | | 320 | | | 382 | | | 357 | |
| France | | 142 | | | 126 | | | 132 | |
| Germany | | 202 | | | 176 | | | 183 | |
| Italy | | 121 | | | 111 | | | 115 | |
| Japan | | 158 | | | 173 | | | 186 | |
| Mexico | | 162 | | | 136 | | | 133 | |
| Spain | | 122 | | | 118 | | | 128 | |
| United Kingdom | | 277 | | | 235 | | | 234 | |
| Other developed markets | | 512 | | | 468 | | | 467 | |
| Other emerging markets | | 784 | | | 758 | | | 778 | |
| | 8,466 | | | 7,994 | | | 7,694 | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | |
Revenue exceeded $100 million in twelve countries outside the U.S. in 2023, 2022 and 2021. The U.S. was the only country to contribute more than 10% of total revenue in each year.
Revenue by major species | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| U.S. | | | | | | |
| Companion animal | | $ | 3,529 | | | $ | 3,341 | | | $ | 2,990 | |
| Livestock | | 1,026 | | | 972 | | | 1,052 | |
| | 4,555 | | | 4,313 | | | 4,042 | |
| International | | | | | | |
| Companion animal | | 2,047 | | | 1,862 | | | 1,699 | |
| Livestock | | 1,864 | | | 1,819 | | | 1,953 | |
| | 3,911 | | | 3,681 | | | 3,652 | |
| Total | | | | | | |
| Companion animal | | 5,576 | | | 5,203 | | | 4,689 | |
| Livestock | | 2,890 | | | 2,791 | | | 3,005 | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | |
Revenue by species | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Companion Animal: | | | | | | |
| Dogs and Cats | | $ | 5,291 | | | $ | 4,939 | | | $ | 4,426 | |
| Horses | | 285 | | | 264 | | | 263 | |
| | 5,576 | | | 5,203 | | | 4,689 | |
| Livestock: | | | | | | |
| Cattle | | 1,503 | | | 1,440 | | | 1,557 | |
| Swine | | 543 | | | 565 | | | 659 | |
| Poultry | | 524 | | | 476 | | | 507 | |
| Fish | | 220 | | | 212 | | | 187 | |
| Sheep and other | | 100 | | | 98 | | | 95 | |
| | 2,890 | | | 2,791 | | | 3,005 | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | |
Revenue by product category | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Parasiticides | | $ | 1,947 | | | $ | 1,860 | | | $ | 1,635 | |
| Vaccines | | 1,771 | | | 1,718 | | | 1,673 | |
| Dermatology | | 1,427 | | | 1,329 | | | 1,180 | |
| Other pharmaceuticals | | 1,280 | | | 1,043 | | | 966 | |
| Anti-infectives | | 1,057 | | | 1,081 | | | 1,215 | |
| Animal health diagnostics | | 376 | | | 353 | | | 374 | |
| Medicated feed additives | | 354 | | | 360 | | | 420 | |
| Other non-pharmaceuticals | | 254 | | | 250 | | | 231 | |
| | 8,466 | | | 7,994 | | | 7,694 | |
| Contract manufacturing & human health | | 78 | | | 86 | | | 82 | |
| Total Revenue | | $ | 8,544 | | | $ | 8,080 | | | $ | 7,776 | |
B. Other Revenue Information
Significant Customers
We primarily sell our companion animal products to veterinarians or to third-party veterinary distributors that typically then sell our products to veterinarians, and in each case, veterinarians then typically sell our products to pet owners. In certain markets, we also sell certain companion animal products through retail and e-commerce outlets. We sell our livestock products primarily to veterinarians and livestock producers, including beef and dairy farmers as well as pork and poultry operations, in addition to third-party veterinary distributors and retail outlets who then typically sell the products to livestock producers. Sales to our largest customer, a U.S. veterinary distributor, represented approximately 15% of total revenue for 2023, and 14% of total revenue for 2022 and 2021.
5. Acquisitions and Divestitures
A. Acquisitions
During 2023, we acquired 100% of the issued share capital of PetMedix Ltd (PetMedix), a privately held research and development stage animal health biopharmaceutical company based in the U.K., which develops antibody-based therapeutics for companion animals. The purchase price included upfront cash consideration of $111 million, excluding $19 million of cash acquired, $5 million in cash withheld for customary post-closing adjustments, and contingent consideration up to $100 million based on the achievement of certain milestones. There are additional contingent payments to be made to the seller upon receipt of payments from a third party related to a preexisting collaboration arrangement between PetMedix and the third party. The initial fair value assessment of the contingent consideration and additional contingent payments is not material and the transaction did not have a material impact on our consolidated financial statements. We also completed the acquisition of adivo GmbH (adivo), a privately held research and development stage animal health biopharmaceutical company based in Germany. The transaction did not have a material impact on our consolidated financial statements.
During 2022, we completed the acquisition of Basepaws, a privately held petcare genetics company based in the U.S., which provides pet owners with genetic tests, analytics and early health risk assessments that can help manage the health, wellness and quality of care for their pets and helps Zoetis identify solutions to complex diseases by informing our research and innovation. We also completed the acquisition of NewMetrica, a privately held company based in Scotland, that provides scientifically-developed instruments to measure quality of life in companion animals. These transactions did not have a material impact on our consolidated financial statements.
During 2021, we entered into an agreement to acquire Jurox, a privately held animal health company based in Australia, which develops, manufactures and markets a wide range of veterinary medicines for treating companion animals and livestock. On September 30, 2022, after satisfying all customary closing conditions, including clearance from the Australian Competition and Consumer Commission, we completed the acquisition of Jurox. We acquired 100% of the outstanding shares for an aggregate cash purchase price of $226 million, which was adjusted to $240 million for cash and working capital and other adjustments as of the closing date. Net cash consideration transferred to the seller was $215 million during 2022 and $5 million during 2023. The transaction was accounted for as a business combination, with the assets acquired and liabilities assumed measured at their respective acquisition date fair values. The valuation was finalized during 2023. The table below presents the final fair values allocated to the assets and liabilities of Jurox as of the acquisition date:
| | | | | |
| (MILLIONS OF DOLLARS) | Amounts |
| Cash and cash equivalents | $ | 20 | |
| Accounts receivable | 8 | |
Inventories(a) | 21 | |
| Other current assets | 1 | |
Property, plant and equipment(b) | 25 | |
Identifiable intangible assets(c) | 135 | |
| Other noncurrent assets | 7 | |
| Accounts payable | 2 | |
| |
| Other current liabilities | 12 | |
| Other noncurrent liabilities | 1 | |
| |
| Total net assets acquired | 202 | |
Goodwill(d) | 38 | |
| Total consideration | $ | 240 | |
(a) Acquired inventory is comprised of finished goods, work in process and raw materials. The fair value of finished goods was determined based on net realizable value adjusted for the costs of the selling effort, a reasonable profit allowance for the selling effort, and estimated holding costs. The fair value of work in process was determined based on net realizable value adjusted for costs to complete the manufacturing process, costs of the selling effort, a reasonable profit allowance for the remaining manufacturing and selling effort, and an estimate of holding costs. The fair value of raw materials was determined to approximate book value.
(b) Property, plant and equipment is comprised of buildings, machinery and equipment, land, construction in progress and furniture and fixtures. The fair value was primarily determined using a reproduction/replacement cost approach which measures the value of an asset by estimating the cost to acquire or construct comparable assets adjusted for age and condition of the asset.
(c) Identifiable intangible assets consist of developed technology rights. The fair value of identifiable intangible assets was determined using the income approach, which includes a forecast of expected future cash flows. For additional information regarding identifiable intangible assets, see Note 13. Goodwill and Other Intangible Assets.
(d) Goodwill represents the excess of consideration transferred over the fair values of the assets acquired and liabilities assumed. It is allocated to our International segment and is primarily attributable to cost and revenue synergies including market share capture, elimination of cost redundancies and gain of cost efficiencies, and intangible assets such as assembled workforce which are not separately recognizable. The primary strategic purpose of the acquisition was to enhance the company’s existing product portfolio.
In 2021, we also acquired certain assets to expand our portfolio of equine care products, which did not have a material impact on our consolidated financial statements.
B. Divestitures
During 2023, we received net cash proceeds of $93 million ($99 million sales proceeds, net of cash sold of $6 million) for the sale of a majority interest in our pet insurance business, Pumpkin Insurance Services. We recorded a net pre-tax gain of $101 million within Other (income)/deductions—net, which includes $24 million related to the remeasurement of our retained noncontrolling investment to fair value. We also completed the divestiture of Performance Livestock Analytics, part of our precision animal health business. This transaction did not have a material impact on our consolidated financial statements.
6. Restructuring Charges and Other Costs Associated with Acquisitions, Cost-Reduction and Productivity Initiatives
In connection with our cost-reduction/productivity initiatives, we typically incur costs and charges associated with workforce reductions and site closings. In connection with our acquisition activity, we typically incur costs and charges associated with executing the transactions, integrating the acquired operations, which may include expenditures for consulting and the integration of systems and processes, product transfers and restructuring the company, which may include charges related to employees, assets and activities that will not continue in the company. All operating functions can be impacted by these actions, including sales and marketing, manufacturing and R&D, as well as functions such as business technology, shared services and corporate operations.
The components of costs incurred in connection with restructuring initiatives, acquisitions and cost-reduction/productivity initiatives are as follows: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Restructuring charges and certain acquisition-related costs: | | | | | | |
Integration costs(a) | | $ | 3 | | | $ | 4 | | | $ | 10 | |
Transaction costs(b) | | 4 | | | 1 | | | — | |
Restructuring charges(c)(d): | | | | | | |
| Employee termination costs | | 41 | | | 3 | | | 17 | |
| Asset impairment charges | | 1 | | | 2 | | | 13 | |
| Exit costs | | 4 | | | 1 | | | 3 | |
Total Restructuring charges and certain acquisition-related costs | | $ | 53 | | | $ | 11 | | | $ | 43 | |
(a) Integration costs represent external, incremental costs directly related to integrating acquired businesses and primarily include expenditures for consulting and the integration of systems and processes, as well as product transfer costs.
(b) Transaction costs represent external costs directly related to acquiring businesses and primarily includes expenditures for banking, legal, accounting and other similar services.
(c) The restructuring charges for the year ended December 31, 2023 primarily relates to employee termination and exit costs related to organizational structure refinements and other cost-reduction and productivity initiatives.
The restructuring charges for the year ended December 31, 2022 primarily relates to employee termination and exit costs associated with cost-reduction and productivity initiatives in certain international markets, as well as asset impairment charges primarily related to the consolidation of manufacturing sites in China.
The restructuring charges for the year ended December 31, 2021 primarily relates to the realignment of our international operations and other cost-reduction and productivity initiatives.
(d) The restructuring charges are associated with the following:
•For the year ended December 31, 2023, Manufacturing/research/corporate of $22 million, U.S. of $3 million and International of $21 million.
•For the year ended December 31, 2022, Manufacturing/research/corporate of $2 million and International of $4 million.
•For the year ended December 31, 2021, Manufacturing/research/corporate of $21 million and International of $12 million.
The components of, and changes in, our restructuring accruals are as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Employee | | Asset | | | | |
| | Termination | | Impairment | | Exit | | |
| (MILLIONS OF DOLLARS) | | Costs | | Charges | | Costs | | Accrual |
| Balance, December 31, 2020 | | $ | 21 | | | $ | — | | | $ | — | | | $ | 21 | |
| Provision | | 17 | | | 13 | | | 3 | | | 33 | |
| Non-cash activity | | — | | | (13) | | | — | | | (13) | |
Utilization and other(a) | | (15) | | | — | | | (1) | | | (16) | |
| Balance, December 31, 2021 | | $ | 23 | | | $ | — | | | $ | 2 | | | $ | 25 | |
| Provision | | 3 | | | 2 | | | 1 | | | 6 | |
| Non-cash activity | | — | | | (2) | | | — | | | (2) | |
Utilization and other(a) | | (12) | | | — | | | (2) | | | (14) | |
Balance, December 31, 2022(b) | | $ | 14 | | | $ | — | | | $ | 1 | | | $ | 15 | |
| Provision | | 41 | | | 1 | | | 4 | | | 46 | |
| Non-cash activity | | — | | | (1) | | | — | | | (1) | |
Utilization and other(a) | | (23) | | | — | | | (2) | | | (25) | |
Balance, December 31, 2023(b)(c) | | $ | 32 | | | $ | — | | | $ | 3 | | | $ | 35 | |
(a) Includes adjustments for foreign currency translation.
(b) At December 31, 2023 and 2022, included in Accrued Expenses ($26 million and $5 million, respectively) and Other noncurrent liabilities ($9 million and $10 million, respectively).
(c) Includes contractual obligations of $26 million, of which payments are expected to be approximately $25 million in 2024 and $1 million thereafter.
7. Other (Income)/Deductions—Net
The components of Other (income)/deductions—net follow: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
Royalty-related income(a) | | $ | (37) | | | $ | (4) | | | $ | (10) | |
| Interest income | | (105) | | | (50) | | | (6) | |
Identifiable intangible asset impairment charges(b) | | 35 | | | 39 | | | 27 | |
| Other asset impairment charges | | 1 | | | 7 | | | 3 | |
Net gain on sale of businesses(c) | | (101) | | | — | | | — | |
| Net loss on sale of assets | | — | | | — | | | 3 | |
| | | | | | |
| | | | | | |
Foreign currency loss(d) | | 47 | | | 62 | | | 27 | |
| Other, net | | 1 | | | (14) | | | 4 | |
| Other (income)/deductions—net | | $ | (159) | | | $ | 40 | | | $ | 48 | |
(a) For 2023, predominantly associated with a settlement received from a third-party for underpayment of royalties related to prior periods.
(b) For 2023, primarily represents certain asset impairment charges related to our precision animal health and diagnostics businesses. For 2022, primarily represents asset impairment charges related to customer relationships and developed technology rights in our diagnostics, poultry, cattle and swine businesses. For 2021, primarily represents asset impairment charges related to developed technology rights and trademarks in our dairy cattle, diagnostics and aquatic health businesses.
(c) Primarily relates to the gain on sale of a majority interest in our pet insurance business.
(d) Primarily driven by costs related to hedging and exposures to certain developed and emerging market currencies.
8. Tax Matters
A. Taxes on Income
The income tax provision in the Consolidated Statements of Income includes tax costs and benefits, such as uncertain tax positions, repatriation decisions and audit settlements, among others.
The components of Income before provision for taxes on income follow: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| United States | | $ | 1,636 | | | $ | 1,645 | | | $ | 1,308 | |
| International | | 1,300 | | | 1,011 | | | 1,180 | |
| Income before provision for taxes on income | | $ | 2,936 | | | $ | 2,656 | | | $ | 2,488 | |
The components of Provision for taxes on income based on the location of the taxing authorities follow: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| United States: | | | | | | |
| Current income taxes: | | | | | | |
| Federal | | $ | 341 | | | $ | 514 | | | $ | 311 | |
| State and local | | 35 | | | 81 | | | 35 | |
| Deferred income taxes: | | | | | | |
| Federal | | (40) | | | (198) | | | (84) | |
| State and local | | 25 | | | (49) | | | (10) | |
| Total U.S. tax provision | | 361 | | | 348 | | | 252 | |
| International: | | | | | | |
| Current income taxes | | 281 | | | 235 | | | 188 | |
| Deferred income taxes | | (46) | | | (38) | | | 14 | |
| Total international tax provision | | 235 | | | 197 | | | 202 | |
| Provision for taxes on income | | $ | 596 | | | $ | 545 | | | $ | 454 | |
Tax Rate Reconciliation
The reconciliation of the U.S. statutory income tax rate to our effective tax rate follows: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| U.S. statutory income tax rate | | 21 | % | | 21 | % | | 21 | % |
| State and local taxes, net of federal benefits | | 1.6 | | | 0.9 | | | 0.8 | |
Unrecognized tax benefits and tax settlements and resolution of certain tax positions(a) | | 0.9 | | | 0.1 | | | 0.1 | |
| | | | | | |
| | | | | | |
| Foreign Derived Intangible Income | | (0.7) | | | (0.2) | | | (1.1) | |
| U.S. Research and Development Tax Credit | | (0.7) | | | (0.7) | | | (0.6) | |
| Share-based payments | | (0.3) | | | (0.6) | | | (0.9) | |
| Non-deductible / non-taxable items | | 0.2 | | | 0.1 | | | 0.3 | |
| Taxation of non-U.S. operations | | (0.8) | | | (0.4) | | | (1.3) | |
| All other—net | | (0.9) | | | 0.3 | | | (0.1) | |
| Effective tax rate | | 20.3 | % | | 20.5 | % | | 18.2 | % |
(a) For a discussion about unrecognized tax benefits and tax settlements and resolution of certain tax positions, see D. Tax Contingencies.
Our effective income tax rate was 20.3%, 20.5% and 18.2% in 2023, 2022 and 2021, respectively.
The lower effective tax rate for 2023, compared with 2022, was primarily attributable to a higher benefit in the U.S. related to foreign-derived intangible income, a more favorable jurisdictional mix of earning (which includes the impact of the location of earnings and repatriation costs), partially offset by a higher net discrete tax expense in 2023, mainly related to changes to prior years’ tax positions. Jurisdictional mix of earnings can vary depending on repatriation decisions, operating fluctuations in the normal course of business and the impact of non-deductible items and non-taxable items.
The higher effective tax rate for 2022, compared with 2021, was attributable to a less favorable jurisdictional mix of earnings (which includes the impact of the location of earnings and repatriation costs), a lower benefit in the U.S. related to foreign-derived intangible income and lower net discrete tax benefits in 2022. Jurisdictional mix of earnings can vary depending on repatriation decisions, operating fluctuations in the normal course of business and the impact of non-deductible and non-taxable items.
In 2022, the company implemented an initiative to maximize its cash position in the U.S. This initiative resulted in a tax benefit in the U.S. in connection with a prepayment from a related foreign entity in Belgium which qualifies as foreign-derived intangible income; however, this income tax benefit was deferred to 2023 and 2024. A portion of this benefit was recognized during 2023. The remaining deferred benefit is included in Other current assets on our Consolidated Balance Sheets as of December 31, 2023 in the amount of $12 million.
B. Tax Matters Agreement
In connection with the separation from Pfizer in 2013, we entered into a tax matters agreement with Pfizer that governs the parties' respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes.
In general, under the agreement:
•Pfizer will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments and including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Pfizer or any of its subsidiaries (and us and/or any of our subsidiaries) for any periods or portions thereof ending on or prior to December 31, 2012. We will be responsible for the portion of any such taxes for periods or portions thereof beginning on or after January 1, 2013, as would be applicable to us if we filed the relevant tax returns on a standalone basis.
•We will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments) that are reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the completion of the separation from Pfizer.
•Pfizer will be responsible for certain specified foreign taxes directly resulting from certain aspects of the separation from Pfizer.
We will not generally be entitled to receive payment from Pfizer in respect of any of our tax attributes or tax benefits or any reduction of taxes of Pfizer. Neither party's obligations under the agreement will be limited in amount or subject to any cap. The agreement also assigns responsibilities for administrative matters, such as the filing of returns, payment of taxes due, retention of records and conduct of audits, examinations or similar proceedings. In addition, the agreement provides for cooperation and information sharing with respect to tax matters.
Pfizer is primarily responsible for preparing and filing any tax return with respect to the Pfizer affiliated group for U.S. federal income tax purposes and with respect to any consolidated, combined, unitary or similar group for U.S. state or local or foreign income tax purposes or U.S. state or local non-income tax purposes that includes Pfizer or any of its subsidiaries, including those that also include us and/or any of our subsidiaries. We are generally responsible for preparing and filing any tax returns that include only us and/or any of our subsidiaries.
The party responsible for preparing and filing a given tax return will generally have exclusive authority to control tax contests related to any such tax return.
C. Deferred Taxes
Deferred taxes arise as a result of basis differentials between financial statement accounting and tax amounts.
The components of our deferred tax assets and liabilities follow: | | | | | | | | | | | | | | |
| | As of December 31, |
| | 2023 | | 2022 |
| (MILLIONS OF DOLLARS) | | Assets (Liabilities) |
| Prepaid/deferred items | | $ | 72 | | | $ | 192 | |
| Inventories | | 30 | | | 22 | |
| Capitalized R&D for tax | | 224 | | | 111 | |
| Identifiable intangible assets | | (154) | | | (154) | |
| Property, plant and equipment | | (199) | | | (204) | |
| Employee benefits | | 62 | | | 61 | |
| Restructuring and other charges | | (1) | | | 1 | |
| Legal and product liability reserves | | 12 | | | 14 | |
| Net operating loss/credit carryforwards | | 133 | | | 112 | |
| Unremitted earnings | | (4) | | | (4) | |
| All other | | 16 | | | 9 | |
| Subtotal | | 191 | | | 160 | |
| Valuation allowance | | (131) | | | (129) | |
Net deferred tax asset/(liability)(a)(b) | | $ | 60 | | | $ | 31 | |
(a) The change in the total net deferred tax asset/(liability) from December 31, 2022 to December 31, 2023 is primarily attributable to an increase in deferred tax assets related to the capitalization and amortization of research & development costs for U.S. tax purposes and an increase in net operating loss/credit carryforwards, partially offset by a decrease in deferred tax assets related to prepaid/deferred items as a result of a prepayment from a related foreign entity in Belgium.
(b) In 2023, included in Noncurrent deferred tax assets ($206 million) and Noncurrent deferred tax liabilities ($146 million). In 2022, included in Noncurrent deferred tax assets ($173 million) and Noncurrent deferred tax liabilities ($142 million).
We have carryforwards, primarily related to net operating losses, which are available to reduce future foreign, U.S. federal, and U.S. state income taxes payable with either an indefinite life or expiring at various times from 2024 to 2043.
Valuation allowances are provided when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax planning strategies. On the basis of this evaluation, as of December 31, 2023 and 2022, a valuation allowance of $131 million and $129 million, respectively, has been recorded to reflect only the portion of the deferred tax asset that is more likely than not to be realized. The amount of the deferred tax asset considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period are reduced or increased or if objective negative evidence in the form of cumulative losses is no longer present and additional weight may be given to subjective evidence such as projections for growth.
In general, it is our practice and intention to permanently reinvest the majority of the earnings of the company’s non-U.S. subsidiaries. As of December 31, 2023, the cumulative amount of such undistributed earnings was approximately $7.6 billion, for which we have not provided U.S. and local income taxes, such as U.S. state income taxes, local withholding taxes, and taxes on currency gains and losses. Since these earnings are intended to be indefinitely reinvested overseas as of December 31, 2023, we cannot predict the time or manner of a potential repatriation. As such, other than the deferred tax liability associated with the one-time mandatory deemed repatriation tax on such undistributed earnings imposed by the Tax Cuts and Jobs Act of 2017, it is not practicable to estimate the additional deferred tax liability associated with the potential repatriation of the unremitted earnings.
D. Tax Contingencies
We are subject to income tax in many jurisdictions, and a certain degree of estimation is required in recording the assets and liabilities related to income taxes. All of our tax positions are subject to audit by the local taxing authorities in each tax jurisdiction. These tax audits can involve complex issues, interpretations and judgments and the resolution of matters may span multiple years, particularly if subject to negotiation or litigation. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statute of limitations expire. We treat these events as discrete items in the period of resolution.
For a description of our accounting policies associated with accounting for income tax contingencies, see Note 3. Significant Accounting Policies: Deferred Tax Assets and Liabilities and Income Tax Contingencies. For a description of the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies: Estimates and Assumptions.
Uncertain Tax Positions
As tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon audit. As of December 31, 2023, 2022 and 2021, we had approximately $209 million, $192 million and $188 million, respectively, in net liabilities associated with uncertain tax positions, excluding associated interest and penalties. As of December 31, 2023, 2022 and 2021, we had approximately $0 million,
$11 million and $3 million, respectively, in assets associated with uncertain tax benefits recorded in Noncurrent deferred tax assets and Other noncurrent assets.
•Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate.
The reconciliation of the beginning and ending amounts of gross unrecognized tax benefits follows: | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Balance, January 1 | | $ | (194) | | | $ | (189) | | | $ | (188) | |
Increases based on tax positions taken during a prior period(a) | | (27) | | | (20) | | | (1) | |
Decreases based on tax positions taken during a prior period(a) | | 20 | | | 9 | | | 7 | |
Increases based on tax positions taken during the current period(a) | | (13) | | | (4) | | | (9) | |
| Settlements | | — | | | 7 | | | — | |
Lapse in statute of limitations(a) | | 5 | | | 3 | | | 2 | |
Balance, December 31(b) | | $ | (209) | | | $ | (194) | | | $ | (189) | |
(a) Primarily included in Provision for taxes on income.
(b) In 2023, included in Other taxes payable ($209 million). In 2022, included in Noncurrent deferred tax assets and Other noncurrent assets ($2 million) and Other taxes payable ($192 million). In 2021, included in Noncurrent deferred tax assets and Other noncurrent assets ($1 million) and Other taxes payable ($188 million).
•Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded in Provision for taxes on income in our Consolidated Statements of Income. We recorded net interest expense of $10 million, $4 million and $1 million in 2023, 2022 and 2021, respectively. Gross accrued interest totaled $26 million, $16 million and $12 million as of December 31, 2023, 2022 and 2021, respectively, and were included in Other taxes payable. As of December 31, 2023, 2022 and 2021, gross accrued penalties totaled $1 million, $3 million and $3 million, respectively, and were included in Other taxes payable.
Status of Tax Audits and Potential Impact on Accruals for Uncertain Tax Positions
We are subject to taxation in the U.S. including various states, and foreign jurisdictions. The U.S. is one of our major tax jurisdictions, and we are currently under audit for tax years 2017 through 2018. For U.S. state tax purposes, tax years 2014 through 2023 are open for examination (see B. Tax Matters Agreement for years prior to 2013).
In addition to the open audit years in the U.S., we have open audit years in other major foreign tax jurisdictions, such as Canada (2021-2023), Asia-Pacific (2015-2023, primarily reflecting Australia, China and Japan), Europe (2012-2023, primarily reflecting France, Germany, Italy, Spain and the U.K.) and Latin America (2016-2023, primarily reflecting Brazil and Mexico).
Any settlements or statute of limitations expirations could result in a significant decrease in our uncertain tax positions. We do not expect that within the next twelve months any of our gross unrecognized tax benefits, exclusive of interest, could significantly decrease as a result of settlements with taxing authorities or the expiration of the statutes of limitations. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and any variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings, and, as a result, it is difficult to estimate the timing and range of possible change related to our uncertain tax positions, and such changes could be significant.
9. Financial Instruments
A. Debt
Credit Facilities
In December 2022, we entered into an amended and restated revolving credit agreement with a syndicate of banks providing for a multi-year $1.0 billion senior unsecured revolving credit facility (the credit facility), which expires in December 2027. Subject to certain conditions, we have the right to increase the credit facility up to $1.5 billion. The credit facility contains a financial covenant requiring us to not exceed a maximum total leverage ratio (the ratio of consolidated net debt as of the end of the period to consolidated Earnings Before Interest, Income Taxes, Depreciation and Amortization (EBITDA) for such period) of 3.50:1. Upon entering into a material acquisition, the maximum total leverage ratio increases to 4.00:1, and extends until the fourth full consecutive fiscal quarter ended immediately following the consummation of a material acquisition. In addition, the credit facility contains other customary covenants.
We were in compliance with all financial covenants as of December 31, 2023 and 2022. There were no amounts drawn under the credit facility as of December 31, 2023 and 2022.
We have additional lines of credit and other credit arrangements with a group of banks and other financial intermediaries for general corporate purposes. We maintain cash and cash equivalent balances in excess of our outstanding short-term borrowings. As of December 31, 2023, we had access to $56 million of lines of credit which expire at various times and are generally renewed annually. As of December 31, 2023 we had $3 million of borrowings outstanding related to these facilities and $2 million borrowings outstanding related to these facilities as of December 31, 2022.
Commercial Paper Program
In February 2013, we entered into a commercial paper program with a capacity of up to $1.0 billion. As of December 31, 2023 and 2022, there was no commercial paper outstanding under this program.
Senior Notes and Other Long-Term Debt
On November 8, 2022, we issued $1.35 billion aggregate principal amount of our senior notes (2022 senior notes), with an original issue discount of $2 million. These notes are comprised of $600 million aggregate principal amount of 5.400% senior notes due 2025 and $750 million aggregate principal amount of 5.600% senior notes due 2032. On February 1, 2023, the net proceeds were used to redeem in full, upon maturity, the $1.35 billion aggregate principal amount of our 3.250% 2013 senior notes due 2023.
Our senior notes are governed by an indenture and supplemental indentures (collectively, the indenture) between us and Deutsche Bank Trust Company Americas, as trustee. The indenture contains certain covenants, including limitations on our and certain of our subsidiaries' ability to incur liens or engage in sale lease-back transactions. The indenture also contains restrictions on our ability to consolidate, merge or sell substantially all of our assets. In addition, the indenture contains other customary terms, including certain events of default, upon the occurrence of which the senior notes may be declared immediately due and payable.
Pursuant to the indenture, we are able to redeem the senior notes of any series, in whole or in part, at any time by paying a “make whole” premium, plus accrued and unpaid interest to, but excluding, the date of redemption. Upon the occurrence of a change of control of us and a downgrade of the senior notes below an investment grade rating by each of Moody's Investors Service, Inc. and Standard & Poor's Ratings Services, we are, in certain circumstances, required to make an offer to repurchase all of the outstanding senior notes at a price equal to 101% of the aggregate principal amount of the senior notes together with accrued and unpaid interest to, but excluding, the date of repurchase.
The components of our long-term debt are as follows: | | | | | | | | | | | | | | |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| | | | |
| | | | |
| | | | |
| | | | |
| 3.250% 2013 senior notes due 2023 | | $ | — | | | $ | 1,350 | |
| 4.500% 2015 senior notes due 2025 | | 750 | | | 750 | |
| 5.400% 2022 senior notes due 2025 | | 600 | | | 600 | |
| 3.000% 2017 senior notes due 2027 | | 750 | | | 750 | |
| 3.900% 2018 senior notes due 2028 | | 500 | | | 500 | |
| 2.000% 2020 senior notes due 2030 | | 750 | | | 750 | |
| 5.600% 2022 senior notes due 2032 | | 750 | | | 750 | |
| 4.700% 2013 senior notes due 2043 | | 1,150 | | | 1,150 | |
| 3.950% 2017 senior notes due 2047 | | 500 | | | 500 | |
| 4.450% 2018 senior notes due 2048 | | 400 | | | 400 | |
| 3.000% 2020 senior notes due 2050 | | 500 | | | 500 | |
| | 6,650 | | | 8,000 | |
| Unamortized debt discount / debt issuance costs | | (60) | | | (66) | |
| Less current portion of long-term debt | | — | | | 1,350 | |
| Cumulative fair value adjustment for interest rate swap contracts | | (26) | | | (32) | |
| Long-term debt, net of discount and issuance costs | | $ | 6,564 | | | $ | 6,552 | |
The fair value of our long-term debt was $6,319 million and $6,108 million as of December 31, 2023 and 2022, respectively, and has been determined using a third-party model that uses significant inputs derived from, or corroborated by, observable market data and Zoetis’ credit rating (Level 2 inputs). See Note 3. Significant Accounting Policies— Fair Value.
The following table provides the principal amount of debt outstanding as of December 31, 2023 by scheduled maturity date. The table also provides the expected interest payments on these borrowings as of December 31, 2023. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | After | | |
| (MILLIONS OF DOLLARS) | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | 2028 | | Total |
| Maturities | | $ | — | | | $ | 1,350 | | | $ | — | | | $ | 750 | | | $ | 500 | | | $ | 4,050 | | | $ | 6,650 | |
| Interest payments | | $ | 272 | | | $ | 272 | | | $ | 206 | | | $ | 206 | | | $ | 183 | | | $ | 2,027 | | | $ | 3,166 | |
Interest Expense
Interest expense, net of capitalized interest, was $239 million, $221 million and $224 million for 2023, 2022 and 2021, respectively. Capitalized interest expense was $27 million, $21 million and $20 million for 2023, 2022 and 2021, respectively.
B. Derivative Financial Instruments
Foreign Exchange Risk
A significant portion of our revenue, earnings and net investment in foreign affiliates is exposed to changes in foreign exchange rates. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. Depending on market conditions, foreign exchange risk is also managed through the use of various derivative financial instruments. These derivative financial instruments serve to manage the exposure of our net investment in certain foreign operations to changes in foreign exchange rates and protect net income against the impact of translation into U.S. dollars of certain foreign exchange-denominated transactions.
All derivative financial instruments used to manage foreign currency risk are measured at fair value and are reported as assets or liabilities on the Consolidated Balance Sheets. The derivative financial instruments primarily offset exposures in the Australian dollar, British pound, Canadian dollar, Chinese renminbi, euro and Norwegian krone. Changes in fair value are reported in earnings or in Accumulated other comprehensive loss, depending on the nature and purpose of the financial instrument, as follows:
•For foreign currency forward-exchange contracts not designated as hedging instruments, we recognize the gains and losses that are used to offset the same foreign currency assets or liabilities immediately into earnings along with the earnings impact of the items they generally offset. These contracts essentially take the opposite currency position of that reflected in the month-end balance sheet to counterbalance the effect of any currency movement. The vast majority of the foreign currency forward-exchange contracts mature within 60 days and all mature within four years.
•For foreign exchange derivative instruments that are designated as hedging instruments against our net investment in foreign operations, changes in the fair value are recorded as a component of cumulative translation adjustment within Accumulated other comprehensive loss and reclassified into earnings when the foreign investment is sold or substantially liquidated. These instruments include cross-currency interest rate swaps and foreign currency forward-exchange contracts. Gains and losses excluded from the assessment of hedge effectiveness are recognized in earnings (Interest expense—net of capitalized interest). The cash flows from these contracts are reflected within the investing section of our Consolidated Statements of Cash Flows. These contracts have varying maturities of up to 2 years.
Interest Rate Risk
The company may use interest rate swap contracts on certain investing and borrowing transactions to manage its net exposure to interest rates and to reduce its overall cost of borrowing.
•In anticipation of issuing fixed-rate debt, we may use forward-starting interest rate swaps that are designated as cash flow hedges to hedge against changes in interest rates that could impact expected future issuances of debt. Unrealized gains or losses on the forward-starting interest rate swaps are reported in Accumulated other comprehensive loss and are recognized in earnings over the life of the future fixed rate notes. When the company discontinues hedge accounting because it is no longer probable that an anticipated transaction will occur within the originally expected period of execution, or within an additional two-month period thereafter, changes to fair value accumulated in other comprehensive income are recognized immediately in earnings.
•During the period from 2019 to 2022, we entered into forward-starting interest rate swaps with an aggregate notional value of $650 million. We designated these swaps as cash flow hedges against interest rate exposure related principally to the issuance of fixed-rate debt to refinance our 3.250% 2013 senior notes due 2023. Upon issuance of our 2022 senior notes, we terminated these contracts and received $114 million in cash from the counterparties for settlement, included in Net cash provided by operating activities in the Consolidated Statements of Cash Flows. The settlement amount, which represented the fair value of the contracts at the time of termination, was recorded in Accumulated other comprehensive loss, and will be amortized into income over the life of the 5.600% 2022 senior notes due 2032.
•As of December 31, 2023, we had outstanding forward-starting interest rate swaps, having an effective date and mandatory termination date in March 2026, to hedge against interest rate exposure related principally to the anticipated future issuance of fixed-rate debt to be used primarily to refinance our 4.500% 2015 senior notes due 2025.
•We may use fixed-to-floating interest rate swaps that are designated as fair value hedges to hedge against changes in the fair value of certain fixed-rate debt attributable to changes in the benchmark the Secured Overnight Financing Rate (SOFR). These derivative instruments effectively convert a portion of the company’s long-term debt from fixed-rate to floating-rate debt based on the daily SOFR rate plus a spread. Gains or losses on the fixed-to-floating interest rate swaps due to changes in SOFR are recorded in Interest expense, net of capitalized interest. Changes in the fair value of the fixed-to-floating interest rate swaps are offset by changes in the fair value of the underlying fixed-rate debt. As of December 31, 2023, we had outstanding fixed-to-floating interest rate swaps that correspond to a portion of the 3.900% 2018 senior notes due 2028 and the 2.000% 2020 senior notes due 2030. The amounts recorded during 2023 for changes in the fair value of these hedges are not material to our consolidated financial statements.
During the first quarter of 2023, we executed amendments to certain of our interest rate swap contract, which changed the floating rate index from LIBOR to SOFR. These amendments did not have a material impact on our consolidated financial statements.
Outstanding Positions
The aggregate notional amount of derivative instruments are as follows: | | | | | | | | | | | | | | |
| | Notional |
| | As of December 31, |
| (MILLIONS) | | 2023 | | 2022 |
| Derivatives not Designated as Hedging Instruments | | | | |
| Foreign currency forward-exchange contracts | | $ | 1,948 | | | $ | 1,753 | |
| | | | |
| Derivatives Designated as Hedging Instruments | | | | |
| Foreign exchange derivative instruments (in foreign currency): | | | | |
| Euro | | 650 | | | 650 | |
| Danish krone | | 600 | | | 600 | |
| Swiss franc | | 25 | | | 25 | |
| | | | |
| Forward-starting interest rate swaps | | $ | 100 | | | $ | 50 | |
| | | | |
| Fixed-to-floating interest rate swap contracts | | $ | 250 | | | $ | 250 | |
Fair Value of Derivative Instruments
The classification and fair values of derivative instruments are as follows: | | | | | | | | | | | | | | | | | | | | |
| | | | Fair Value of Derivatives |
| | | | As of December 31, |
| (MILLIONS OF DOLLARS) | | Balance Sheet Location | | 2023 | | 2022 |
| Derivatives Not Designated as Hedging Instruments: | | | | | | |
| Foreign currency forward-exchange contracts | | Other current assets | | $ | 11 | | | $ | 22 | |
| Foreign currency forward-exchange contracts | | Other current liabilities | | (11) | | | (21) | |
| Total derivatives not designated as hedging instruments | | | | — | | | 1 | |
| Derivatives Designated as Hedging Instruments: | | | | | | |
| Forward-starting interest rate swap contracts | | Other non-current assets | | $ | 12 | | | $ | 10 | |
| | | | | | |
| Foreign exchange derivative instruments | | Other current assets | | 5 | | | 21 | |
| Foreign exchange derivative instruments | | Other non-current assets | | 11 | | | 19 | |
| Foreign exchange derivative instruments | | Other current liabilities | | (20) | | | (9) | |
| Foreign exchange derivative instruments | | Other non-current liabilities | | (1) | | | (4) | |
| | | | | | |
| Fixed-to-floating interest rate swap contracts | | Other non-current liabilities | | (26) | | | (32) | |
| Total derivatives designated as hedging instruments | | | | (19) | | | 5 | |
| Total derivatives | | | | $ | (19) | | | $ | 6 | |
| | | | | | |
The company’s derivative transactions are subject to master netting agreements that mitigate credit risk by permitting net settlement of transactions with the same counterparty. The company also has collateral security agreements with certain of its counterparties. Under these collateral security agreements either party is required to post cash collateral when the net fair value of derivative instruments covered by the collateral agreement exceeds contractually established thresholds. At December 31, 2023, there was $13 million of collateral received and $33 million posted related to derivative instruments recorded in Other current liabilities and Other current assets, respectively. At December 31, 2022, there was $8 million of collateral received and $21 million of collateral posted related to derivative instruments recorded in Other current liabilities and Other current assets, respectively.
We use a market approach in valuing financial instruments on a recurring basis. Our derivative financial instruments are measured at fair value on a recurring basis using Level 2 inputs in the calculation of fair value. See Note 3. Significant Accounting Policies— Fair Value.
The amounts of net losses on derivative instruments not designated as hedging instruments, recorded in Other (income)/deductions - net, are as follows: | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Foreign currency forward-exchange contracts | | $ | (25) | | | $ | (58) | |
These amounts were substantially offset in Other (income)/deductions—net by the effect of changing exchange rates on the underlying foreign currency exposures.
The amounts of unrecognized net gains/(losses) on interest rate swap contracts, recorded, net of tax, in Accumulated other comprehensive loss, are as follows: | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Forward starting interest rate swap contracts | | $ | 2 | | | $ | (2) | |
| Foreign exchange derivative instruments | | $ | (23) | | | $ | 36 | |
Gains on interest rate swap contracts, recognized within Interest expense, net of capitalized interest, are as follows: | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Foreign exchange derivative instruments | | $ | 19 | | | $ | 16 | |
The net amount of deferred losses related to derivative instruments designated as cash flow hedges that is expected to be reclassified from Accumulated other comprehensive loss into earnings over the next 12 months is not material.
10. Leases
We have facilities and vehicles under various non-cancellable operating leases with third parties and an equipment finance lease with a third party. The operating leases generally have remaining terms ranging from 1 to 15 years, inclusive of renewal options that are reasonably certain of exercise. The finance lease has a remaining term of 30 years.
Supplemental information for our lease portfolio is as follows: | | | | | | | | | | | | | | | | | | | | |
| | | | As of December 31, |
| (MILLIONS OF DOLLARS, EXCEPT LEASE TERM AND DISCOUNT RATE AMOUNTS) | | | | 2023 | | 2022 |
| Supplemental Balance Sheet information: | | | | | | |
| Operating lease right of use assets | | | | $ | 230 | | | $ | 220 | |
Finance lease right of use assets (in Other noncurrent assets) | | | | 9 | | | — | |
| Total lease assets | | | | $ | 239 | | | $ | 220 | |
| | | | | | |
| Lease liabilities: | | | | | | |
Operating lease liabilities - current (in Other current liabilities) | | | | $ | 48 | | | $ | 43 | |
Finance lease liabilities - current (in Other current liabilities) | | | | 1 | | | — | |
| Operating lease liabilities - noncurrent | | | | 188 | | | 186 | |
Finance lease liabilities - noncurrent (in Other noncurrent liabilities) | | | | 8 | | | — | |
| Total lease liabilities | | | | $ | 245 | | | $ | 229 | |
| | | | | | |
| Weighted-average remaining lease term—operating leases (years) | | | | 7.26 | | 7.72 |
| Weighted-average remaining lease term—finance leases (years) | | | | 30.27 | | 0.00 |
| Weighted-average discount rate—operating leases | | | | 3.41 | % | | 2.78 | % |
| Weighted-average discount rate—finance leases | | | | 4.99 | % | | — | % |
| | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Supplemental Income Statement information for operating leases: | | | | | | |
| Operating lease expense | | $ | 56 | | | $ | 51 | | | $ | 50 | |
| Variable lease costs | | 20 | | | 12 | | | 19 | |
| Short-term lease costs not included in the measurement of lease liabilities | | 11 | | | 12 | | | 9 | |
| | | | | | |
| Supplemental Income Statement information for finance leases: | | | | | | |
| Amortization of right-of-use assets | | 1 | | | — | | | — | |
| | | | | | |
| Total lease costs | | $ | 88 | | | $ | 75 | | | $ | 78 | |
| | | | | | |
| Supplemental Cash Flow information for leases | | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | |
| Operating cash flows – operating leases | | $ | 57 | | | $ | 51 | | | $ | 47 | |
| | | | | | |
| | | | | | |
| Lease obligations obtained in exchange for right-of-use assets - operating (non-cash) | | 73 | | | 99 | | | 39 | |
| Lease obligations obtained in exchange for right-of-use assets – finance (non-cash) | | 9 | | | — | | | — | |
Future minimum lease payments under non-cancellable lease contracts as of December 31, 2023 are as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | Total | | Less: | | |
| | | | | | | | | | | | After | | Lease | | Imputed | | |
| (MILLIONS OF DOLLARS) | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | 2028 | | Payments | | Interest | | Total |
| Operating leases | | $ | 57 | | | $ | 48 | | | $ | 38 | | | $ | 30 | | | $ | 25 | | | $ | 73 | | | $ | 271 | | | $ | (35) | | | $ | 236 | |
| Finance leases | | $ | 2 | | | $ | 1 | | | $ | 1 | | | $ | 1 | | | $ | 1 | | | $ | 6 | | | $ | 12 | | | $ | (3) | | | $ | 9 | |
11. Inventories
The components of inventory follow: | | | | | | | | | | | | | | |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Finished goods | | $ | 1,147 | | | $ | 1,090 | |
| Work-in-process | | 966 | | | 825 | |
| Raw materials and supplies | | 451 | | | 430 | |
| Inventories | | $ | 2,564 | | | $ | 2,345 | |
12. Property, Plant and Equipment
The components of property, plant and equipment follow: | | | | | | | | | | | | | | | | | | | | |
| | Useful Lives | | As of December 31, |
| (MILLIONS OF DOLLARS) | | (Years) | | 2023 | | 2022 |
| Land | | — | | $ | 25 | | | $ | 26 | |
| Buildings | | 33.3 - 50 | | 1,316 | | | 1,197 | |
| Machinery, equipment and fixtures | | 3 - 20 | | 3,372 | | | 3,013 | |
| Construction-in-progress | | — | | 1,085 | | | 814 | |
| | | 5,798 | | | 5,050 | |
| Less: Accumulated depreciation | | | 2,594 | | | 2,297 | |
| Property, plant and equipment | | | $ | 3,204 | | | $ | 2,753 | |
Depreciation expense was $306 million in 2023, $272 million in 2022 and $244 million in 2021.
13. Goodwill and Other Intangible Assets
A. Goodwill
The components of, and changes in, the carrying amount of goodwill follow: | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | | U.S. | | International | | Total |
| Balance, December 31, 2021 | | $ | 1,424 | | | $ | 1,258 | | | $ | 2,682 | |
Additions(a) | | 61 | | | 44 | | | 105 | |
| | | | | | |
Other(c) | | — | | | (41) | | | (41) | |
| Balance, December 31, 2022 | | $ | 1,485 | | | $ | 1,261 | | | $ | 2,746 | |
Additions(a) | | 24 | | | 14 | | | 38 | |
Transfers/Adjustments(b) | | 25 | | | (34) | | | (9) | |
Other(c) | | (2) | | | (14) | | | (16) | |
| Balance, December 31, 2023 | | $ | 1,532 | | | $ | 1,227 | | | $ | 2,759 | |
(a) For 2023, primarily relates to the acquisitions of PetMedix and adivo. See Note 5. Acquisitions and Divestitures.
For 2022, primarily relates to the acquisitions of Basepaws and Jurox. See Note 5. Acquisitions and Divestitures.
(b) For 2023, primarily relates to asset transfers from international markets to the U.S.
(c) Includes adjustments for foreign currency translation. In 2023, also includes the sale of a majority interest in our pet insurance business within our U.S. segment.
The gross goodwill balance was $3,295 million as of December 31, 2023 and $3,282 million as of December 31, 2022. Accumulated goodwill impairment losses (generated entirely in fiscal 2002) were $536 million as of December 31, 2023 and 2022.
B. Other Intangible Assets
The components of identifiable intangible assets follow: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | As of December 31, 2023 | | As of December 31, 2022 |
| | | | | | Identifiable | | | | | | Identifiable |
| | Gross | | | | Intangible Assets, | | Gross | | | | Intangible Assets, |
| | Carrying | | Accumulated | | Less Accumulated | | Carrying | | Accumulated | | Less Accumulated |
| (MILLIONS OF DOLLARS) | | Amount | | Amortization | | Amortization | | Amount | | Amortization | | Amortization |
| Finite-lived intangible assets: | | | | | | | | | | | | |
Developed technology rights(a) | | $ | 1,986 | | | $ | (1,101) | | | $ | 885 | | | $ | 1,918 | | | $ | (975) | | | $ | 943 | |
| Brands and tradenames | | 383 | | | (246) | | | 137 | | | 395 | | | (237) | | | 158 | |
| Other | | 270 | | | (190) | | | 80 | | | 337 | | | (233) | | | 104 | |
| Total finite-lived intangible assets | | 2,639 | | | (1,537) | | | 1,102 | | | 2,650 | | | (1,445) | | | 1,205 | |
| Indefinite-lived intangible assets: | | | | | | | | | | | | |
| Brands and tradenames | | 88 | | | — | | | 88 | | | 91 | | | — | | | 91 | |
In-process research and development(a)(b) | | 141 | | | — | | | 141 | | | 77 | | | — | | | 77 | |
| Product rights | | 7 | | | — | | | 7 | | | 7 | | | — | | | 7 | |
| Total indefinite-lived intangible assets | | 236 | | | — | | | 236 | | | 175 | | | — | | | 175 | |
| Identifiable intangible assets | | $ | 2,875 | | | $ | (1,537) | | | $ | 1,338 | | | $ | 2,825 | | | $ | (1,445) | | | $ | 1,380 | |
(a) During 2023, certain intangible assets related to the acquisition of an Irish biologic therapeutics company, acquired in 2017, were placed into service.
(b) As of December 31, 2023, primarily includes intangible assets related to the acquisitions of PetMedix and adivo in 2023.
Developed Technology Rights
Developed technology rights represent the amortized cost associated with developed technology, which has been acquired from third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. These assets include technologies related to the care and treatment of dogs, cats, horses, cattle, swine, poultry, fish and sheep.
Brands and Tradenames
Brands and tradenames represent the amortized or unamortized cost associated with product name recognition, as the products themselves do not receive patent protection. The most significant finite-lived brands and tradenames are related to Abaxis, Platinum Performance, and Lutalyse. The most significant indefinite-lived brands and tradenames were acquired from SmithKlineBeecham and the Linco family of products.
In-Process Research and Development
IPR&D assets represent R&D assets that have not yet received regulatory approval in a major market. The majority of these IPR&D assets were acquired in connection with our acquisition of two research and development stage animal health biopharmaceutical companies, PetMedix and adivo.
IPR&D assets are required to be classified as indefinite-lived assets until the successful completion or abandonment of the associated R&D effort. Accordingly, during the development period after the date of acquisition, these assets will not be amortized until approval is obtained in a major market, typically either the U.S., U.K. or the EU, or in a series of other countries, subject to certain specified conditions and management judgment. At that time, we will determine the useful life of the asset, reclassify the asset out of IPR&D and begin amortization. If the associated R&D effort is abandoned, the related IPR&D assets will be written-off and we will record an impairment charge.
There can be no certainty that IPR&D assets ultimately will yield a successful product.
Product Rights
Product rights represent product registration and application rights that were acquired from Pfizer in 2014.
C. Amortization
The weighted average life of our total finite-lived intangible assets is approximately 9 years. Total amortization expense for finite-lived intangible assets was $185 million in 2023, $193 million in 2022 and $204 million in 2021.
The annual amortization expense expected for the years 2024 through 2028 is as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 |
| Amortization expense | | $ | 171 | | | $ | 159 | | | $ | 153 | | | $ | 148 | | | $ | 119 | |
14. Benefit Plans
Our employees ceased to participate in the Pfizer U.S. qualified defined benefit and U.S. retiree medical plans effective December 31, 2012 and liabilities associated with our employees under these plans were retained by Pfizer. Pfizer continued to credit certain employees' service with Zoetis generally through December 31, 2017 (or termination of employment from Zoetis, if earlier) for certain early retirement benefits with respect to Pfizer's U.S. defined benefit pension and retiree medical plans. In connection with an employee matters agreement between Pfizer and Zoetis, Zoetis is responsible for payment of three-fifths of the total cost of the service credit continuation ($38 million) for these plans and Pfizer is responsible for the remaining two-fifths of the total cost ($25 million). The $25 million capital contribution from Pfizer and corresponding contra-equity account (which is being reduced as the service credit continuation is incurred) is included in Employee benefit plan contribution from Pfizer Inc. in the Consolidated Statements of Equity. The balance in the contra-equity account was $0 million as of December 31, 2023 and 2022. The amount of the service cost continuation payment to be paid by Zoetis to Pfizer was determined and fixed based on an actuarial assessment of the value of the grow-in benefits and was being paid in equal installments over a period of 10 years. As of December 31, 2023, there are no remaining payments due to Pfizer. Pension and postretirement benefit expense associated with the extended service for certain employees in the U.S. plans totaled $0 million in 2023 and $6 million in 2022.
Pension expense associated with the U.S. and certain significant international locations (inclusive of service cost grow-in benefits discussed above) totaled $6 million in 2023, $12 million in 2022 and $14 million in 2021.
A. International Pension Plans
Information about the dedicated pension plans, including the plans transferred to us as part of the separation from Pfizer, is provided in the tables below.
Obligations and Funded Status––Dedicated Plans
The following table provides an analysis of the changes in the benefit obligations, plan assets and funded status of our dedicated pension plans (including those transferred to us): | | | | | | | | | | | | | | |
| | As of and for the |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Change in benefit obligation: | | | | |
| Projected benefit obligation, beginning | | $ | 122 | | | $ | 159 | |
| Service cost | | 5 | | | 6 | |
| Interest cost | | 5 | | | 2 | |
| | | | |
| Changes in actuarial assumptions and other | | (4) | | | (27) | |
| Settlements and curtailments | | — | | | (3) | |
| Benefits paid | | (3) | | | (1) | |
| Adjustments for foreign currency translation | | 5 | | | (13) | |
| Other––net | | (1) | | | (1) | |
| Benefit obligation, ending | | 129 | | | 122 | |
| Change in plan assets: | | | | |
| Fair value of plan assets, beginning | | 78 | | | 92 | |
| | | | |
| Actual return on plan assets | | 3 | | | (4) | |
| Company contributions | | 6 | | | 4 | |
| Settlements and curtailments | | — | | | (3) | |
| Benefits paid | | (3) | | | (1) | |
| Adjustments for foreign currency translation | | 2 | | | (9) | |
| Other––net | | — | | | (1) | |
| Fair value of plan assets, ending | | 86 | | | 78 | |
Funded status—Projected benefit obligation in excess of plan assets at end of year(a) | | $ | (43) | | | $ | (44) | |
(a) Included in Other noncurrent liabilities.
Changes in the benefit obligation resulted in a net gain of $4 million in 2023 and $27 million in 2022.
Actuarial gains were $4 million ($4 million, net of tax) at December 31, 2023 and $6 million ($3 million, net of tax) at December 31, 2022. The actuarial gains and losses primarily represent the cumulative difference between the actuarial assumptions and actual return on plan assets, changes in discount rates and changes in other assumptions used in measuring the benefit obligations. These actuarial gains and losses are recognized in Accumulated other comprehensive loss. The actuarial losses will be amortized into net periodic benefit costs over an average period of 10.1 years.
Information related to the funded status of selected plans follows: | | | | | | | | | | | | | | |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Pension plans with an accumulated benefit obligation in excess of plan assets: | | | | |
| Fair value of plan assets | | $ | 8 | | | $ | 7 | |
| Accumulated benefit obligation | | 45 | | | 40 | |
| Pension plans with a projected benefit obligation in excess of plan assets: | | | | |
| Fair value of plan assets | | 64 | | | 58 | |
| Projected benefit obligation | | 109 | | | 103 | |
Net Periodic Benefit Costs––Dedicated Plans
The following table provides the net periodic benefit cost associated with dedicated pension plans (including those transferred to us): | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Service cost | | $ | 5 | | | $ | 6 | | | $ | 8 | |
| Interest cost | | 5 | | | 2 | | | 2 | |
| Expected return on plan assets | | (4) | | | (3) | | | (3) | |
| Amortization of net losses | | — | | | 1 | | | 1 | |
| | | | | | |
| | | | | | |
| Net periodic benefit cost | | $ | 6 | | | $ | 6 | | | $ | 8 | |
Actuarial Assumptions––Dedicated Plans
The following table provides the weighted average actuarial assumptions for the dedicated pension plans (including those transferred to us): | | | | | | | | | | | | | | | | | | | | |
| | As of December 31, |
| (PERCENTAGES) | | 2023 | | 2022 | | 2021 |
| Weighted average assumptions used to determine benefit obligations: | | | | | | |
| Discount rate | | 4.2 | % | | 3.7 | % | | 1.4 | % |
| Rate of compensation increase | | 3.6 | % | | 3.5 | % | | 3.4 | % |
| Cash balance credit interest rate | | 1.6 | % | | 1.7 | % | | 1.5 | % |
| Weighted average assumptions used to determine net benefit cost for the year ended December 31: | | | | | | |
| Discount rate | | 3.7 | % | | 1.4 | % | | 1.2 | % |
| Expected return on plan assets | | 4.7 | % | | 3.3 | % | | 3.8 | % |
| Rate of compensation increase | | 3.5 | % | | 3.4 | % | | 3.1 | % |
| Cash balance credit interest rate | | 1.7 | % | | 1.5 | % | | 1.5 | % |
The assumptions above are used to develop the benefit obligations at the end of the year and to develop the net periodic benefit cost for the following year. Therefore, the assumptions used to determine the net periodic benefit cost for each year are established at the end of each previous year, while the assumptions used to determine the benefit obligations are established at each year-end. The net periodic benefit cost and the benefit obligations are based on actuarial assumptions that are reviewed on an annual basis. The assumptions are revised based on an annual evaluation of long-term trends, as well as market conditions that may have an impact on the cost of providing retirement benefits.
Actuarial and other assumptions for pension plans can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For a description of the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies—Estimates and Assumptions.
Plan Assets—Dedicated Plans
The components of plan assets follow: | | | | | | | | | | | | | | |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| Cash and cash equivalents | | $ | 1 | | | $ | 2 | |
| Equity securities: Equity commingled funds | | 34 | | | 29 | |
| Debt securities: Government bonds | | 40 | | | 38 | |
| Other investments | | 11 | | | 9 | |
Total(a) | | $ | 86 | | | $ | 78 | |
(a) Fair values are determined based on valuation inputs categorized as Level 1, 2 or 3 (see Note 3. Significant Accounting Policies—Fair Value). Investment plan assets are valued using Level 1 or Level 2 inputs.
A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies—Estimates and Assumptions.
Specifically, the following methods and assumptions were used to estimate the fair value of our pension assets:
• Equity commingled funds––observable market prices (Level 1).
• Government bonds and other investments––principally observable market prices (Level 2).
The long-term target asset allocations and the percentage of the fair value of plans assets for dedicated benefit plans follow: | | | | | | | | | | | | | | | | | | | | |
| | As of December 31, |
| | Target allocation | | | | |
| | percentage | | Percentage of Plan Assets |
| (PERCENTAGES) | | 2023 | | 2023 | | 2022 |
| Cash and cash equivalents | | 0-10% | | 1.7 | % | | 2.3 | % |
| Equity securities | | 0-60% | | 39.4 | % | | 37.7 | % |
| Debt securities | | 15-100% | | 46.9 | % | | 48.0 | % |
| Other investments | | 0-100% | | 12.0 | % | | 12.0 | % |
| Total | | 100% | | 100 | % | | 100 | % |
Zoetis utilizes long-term asset allocation ranges in the management of our plans’ invested assets. Long-term return expectations are developed with input from outside investment consultants based on the company’s investment strategy, which takes into account historical experience, as well as the impact of portfolio diversification, active portfolio management, and the investment consultant’s view of current and future economic and financial market conditions. As market conditions and other factors change, the targets may be adjusted accordingly and actual asset allocations may vary from the target allocations.
The long-term asset allocation ranges reflect the asset class return expectations and tolerance for investment risk within the context of the respective plans’ long-term benefit obligations. These ranges are supported by an analysis that incorporates historical and expected returns by asset class, as well as volatilities and correlations across asset classes and our liability profile. This analysis, referred to as an asset-liability analysis, also provides an estimate of expected returns on plan assets, as well as a forecast of potential future asset and liability balances.
The investment consultants review investment performance with Zoetis on a quarterly basis in total, as well as by asset class, relative to one or more benchmarks.
Cash Flows—Dedicated Plans
Our plans are generally funded in amounts that are at least sufficient to meet the minimum requirements set forth in applicable employee benefit laws and local tax and other laws.
We expect to contribute approximately $7 million to our dedicated pension plans in 2024. Benefit payments are expected to be approximately $7 million for 2024, $6 million for 2025, $11 million for 2026, $7 million for 2027 and $7 million for 2028. Benefit payments are expected to be approximately $53 million in the aggregate for the five years thereafter. These expected benefit payments reflect the future plan benefits subsequent to 2024 projected to be paid from the plans or from the general assets of Zoetis entities under the current actuarial assumptions used for the calculation of the projected benefit obligation and, therefore, actual benefit payments may differ from projected benefit payments.
B. Postretirement Plans
Postretirement benefit expense associated with these U.S. retiree medical plans totaled $0 million in 2023, and $4 million per year in 2022 and 2021 (inclusive of service cost grow-in benefits discussed above).
C. Defined Contribution Plans
Zoetis has a voluntary defined contribution plan, the Zoetis Savings Plan (ZSP) that allows participation by substantially all U.S. employees. Zoetis matches 100% of employee contributions, up to a maximum of 5% of each employee’s eligible compensation. The ZSP also includes a profit-sharing feature that provides for an additional contribution ranging between 0 and 8 percent of each employee’s eligible compensation. All eligible employees receive the profit-sharing contribution regardless of the amount they choose to contribute to the ZSP. The profit-sharing contribution is a discretionary amount provided by Zoetis and is determined on an annual basis. Employees can direct their contributions and the company's matching and profit-sharing contributions into any of the funds offered. These funds provide participants with a cross section of investing options, including the Zoetis stock fund. The matching and profit-sharing contributions are cash funded.
Employees are permitted to diversify all or any portion of their company matching or profit-sharing contribution. Once the contributions have been paid, Zoetis has no further payment obligations. Contribution expense, associated with the ZSP, totaled $69 million in 2023, $57 million in 2022 and $54 million in 2021.
Employees in the U.S. who meet certain eligibility requirements participate in a supplemental (non-qualified) savings plan sponsored by Zoetis. The cost/(benefit) of the supplemental savings plan was $11 million in 2023, $(9) million in 2022 and $12 million in 2021. Benefit payments for this plan are expected to be approximately $5 million in 2024 and $46 million thereafter.
15. Share-based Payments
The Zoetis 2013 Equity and Incentive Plan, Amended and Restated as of May 19, 2022 (Equity Plan), provides long-term incentives to our employees and non-employee directors. The principal types of share-based awards available under the Equity Plan may include, but are not limited to, stock options, restricted stock and restricted stock units (RSUs), deferred stock units (DSUs), performance-vesting restricted stock units (PSUs), and other equity-based or cash-based awards.
Thirty million shares of stock were approved and registered with the Securities and Exchange Commission for grants to participants under the Equity Plan. The shares reserved may be used for any type of award under the Equity Plan. At December 31, 2023, the aggregate number of remaining shares available for future grant under the Equity Plan was approximately 14 million shares.
A. Share-Based Compensation Expense
The components of share-based compensation expense follow: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 |
| Stock options / stock appreciation rights | | $ | 8 | | | $ | 10 | | | $ | 9 | |
| RSUs / DSUs | | 37 | | | 34 | | | 33 | |
| PSUs | | 15 | | | 18 | | | 16 | |
Share-based compensation expense—total(a) | | $ | 60 | | | $ | 62 | | | $ | 58 | |
| Tax benefit for share-based compensation expense | | (8) | | | (8) | | | (7) | |
| Share-based compensation expense, net of tax | | $ | 52 | | | $ | 54 | | | $ | 51 | |
(a) For each of the years ended December 31, 2023, 2022 and 2021, we capitalized up to $1 million of share-based compensation expense to inventory.
B. Stock Options
Stock options represent the right to purchase shares of our common stock within a specified period of time at a specified price. The exercise price for a stock option will be not less than 100% of the fair market value of the common stock as of the NYSE market close on the date of grant. Stock options granted may include those intended to be “incentive stock options” within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986 (the Code).
Stock options are accounted for using a fair-value-based method at the date of grant in the Consolidated Statements of Income. The values determined through this fair-value-based method generally are amortized on a straight-line basis over the vesting term.
Eligible employees may receive Zoetis stock option awards. Zoetis stock option awards granted prior to 2023 generally vest after three years of continuous service from the date of grant and have a contractual term of 10 years while stock option awards granted in 2023 are subject to graded vesting over three years from the date of grant and have a contractual term of 10 years.
The fair-value-based method for valuing each Zoetis stock option grant on the grant date uses the Black-Scholes-Merton option-pricing model, which incorporates a number of valuation assumptions noted in the following table, shown at their weighted-average values: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2023 | | 2022 | | 2021 |
Expected dividend yield(a) | | 0.92 | % | | 0.64 | % | | 0.62 | % |
Risk-free interest rate(b) | | 3.84 | % | | 1.81 | % | | 0.53 | % |
Expected stock price volatility(c) | | 28.63 | % | | 27.64 | % | | 27.94 | % |
Expected term(d) (years) | | 4.2 | | 4.9 | | 5.0 |
(a) Determined using a constant dividend yield during the expected term of the Zoetis stock option.
(b) Determined using the interpolated yield on U.S. Treasury zero-coupon issues.
(c) Determined using an equal weighting between historical volatility of the Zoetis stock price and implied volatility. The selection of the blended historical and implied volatility approach was based on our assessment that this calculation of expected volatility is more representative of future stock price trends.
(d) Determined using expected exercise and post-vesting termination patterns.
The following table provides an analysis of stock option activity for the year ended December 31, 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Weighted-Average | | |
| | | | | | Remaining | | Aggregate |
| | | | Weighted-Average | | Contractual Term | | Intrinsic Value(a) |
| | Shares | | Exercise Price | | (Years) | | (Millions) |
| Outstanding, December 31, 2022 | | 2,067,606 | | | $ | 95.32 | | | | | |
| Granted | | 271,150 | | | 162.12 | | | | | |
| Exercised | | (695,987) | | | 59.89 | | | | | |
| Forfeited | | (116,267) | | | 150.66 | | | | | |
| Outstanding, December 31, 2023 | | 1,526,502 | | | $ | 119.13 | | | 5.5 | | $ | 120 | |
| | | | | | | | |
| Exercisable, December 31, 2023 | | 858,314 | | | $ | 77.31 | | | 3.5 | | $ | 103 | |
(a) Market price of underlying Zoetis common stock less exercise price.
As of December 31, 2023, there was approximately $10 million of unrecognized compensation costs related to nonvested stock options, which will be recognized over an expected remaining weighted-average period of nine months.
The following table summarizes data related to stock option activity: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended/As of December 31, |
| (MILLIONS OF DOLLARS, EXCEPT PER STOCK OPTION AMOUNTS) | | 2023 | | 2022 | | 2021 |
| Weighted-average grant date fair value per stock option | | $ | 43.56 | | | $ | 51.13 | | | $ | 37.81 | |
| Aggregate intrinsic value on exercise | | 81 | | | 31 | | | 87 | |
| Cash received upon exercise | | 42 | | | 15 | | | 36 | |
| Tax benefits realized related to exercise | | 17 | | | 19 | | | 34 | |
C. Restricted Stock Units (RSUs)
Restricted stock units represent the right to receive a share of our common stock that is subject to a risk of forfeiture until the restrictions lapse at the end of the vesting period subject to the recipient's continued employment. RSUs accrue dividend equivalent units and are paid in shares of our common stock upon vesting (or cash determined by reference to the value of our common stock).
RSUs are accounted for using a fair-value-based method that utilizes the closing price of Zoetis common stock on the date of grant. Zoetis RSUs granted prior to 2023 generally vest after three years of continuous service from the grant date while RSUs granted in 2023 are subject to graded vesting over three years. These values are amortized on a straight-line basis over the vest terms.
The following table provides an analysis of RSU activity for the year ended December 31, 2023: | | | | | | | | | | | | | | |
| | | | Weighted-Average |
| | RSUs | | Grant Date Fair Value |
| Nonvested, December 31, 2022 | | 620,598 | | | $ | 169.24 | |
| Granted | | 275,664 | | | 162.63 | |
| | | | |
| Vested | | (201,011) | | | 145.80 | |
| Reinvested dividend equivalents | | 6,036 | | | 171.21 | |
| Forfeited | | (45,436) | | | 171.72 | |
| Nonvested, December 31, 2023 | | 655,851 | | | $ | 173.49 | |
As of December 31, 2023, there was approximately $45 million of unrecognized compensation costs related to nonvested RSUs, which will be recognized over an expected remaining weighted-average period of ten months.
D. Deferred Stock Units (DSUs)
Deferred stock units, which were granted to non-employee compensated Directors in 2013 and 2014, represent the right to receive shares of our common stock at a future date. The DSU awards will be automatically settled and paid in shares within 60 days following the Director’s separation from service on the Board of Directors.
DSUs are accounted for using a fair-value-based method that utilizes the closing price of Zoetis common stock on the date of grant. DSUs vested immediately as of the grant date and the values were expensed at the time of grant into Selling, general and administrative expenses.
For the years ended December 31, 2023 and 2022, there were no DSUs granted. As of December 31, 2023 and 2022, there were 65,654 and 65,071 DSUs outstanding, respectively, including dividend equivalents.
E. Performance-Vesting Restricted Stock Units (PSUs)
Performance-vesting restricted stock units, which are granted to eligible senior management, represent the right to receive a share of our common stock that is subject to a risk of forfeiture until the restrictions lapse, which include continued employment through the end of the vesting period and the attainment of performance goals. PSUs represent the right to receive shares of our common stock in the future (or cash determined by reference to the value of our common stock).
PSUs are accounted for using a Monte Carlo simulation model. The units underlying the PSUs will be earned and vested over a three-year performance period, based upon the total shareholder return of the company in comparison to the total shareholder return of the companies comprising the S&P 500 index at the start of the performance period, excluding companies that during the performance period are acquired or are no longer publicly traded (Relative TSR). The weighted-average fair value was estimated based on volatility assumptions of Zoetis common stock and an average of peer companies, which were 38.1% and 40.9%, respectively, in 2023, and 28.4% and 38.1%, respectively, in 2022. Depending on the company’s Relative TSR performance at the end of the performance period, the recipient may earn between 0% and 200% of the target number of units. Vested units, including dividend equivalent units, are paid in shares of the company’s common stock. PSU values are amortized on a straight-line basis over the vesting term.
The following table provides an analysis of PSU activity for the year ended December 31, 2023: | | | | | | | | | | | | | | |
| | | | |
| | | | Weighted-Average |
| | PSUs | | Grant Date Fair Value |
| Nonvested, December 31, 2022 | | 253,044 | | | $ | 221.59 | |
| Granted | | 99,626 | | | 238.24 | |
| Vested | | (65,578) | | | 217.66 | |
| Reinvested dividend equivalents | | 2,392 | | | 226.48 | |
| Forfeited | | (37,704) | | | 223.30 | |
| Nonvested, December 31, 2023 | | 251,780 | | | $ | 228.99 | |
| Shares issued, December 31, 2023 | | 64,673 | | | $ | 217.49 | |
As of December 31, 2023, there was approximately $25 million of unrecognized compensation costs related to nonvested PSUs, which will be recognized over an expected remaining weighted-average period of 1.2 years.
F. Other Equity-Based or Cash-Based Awards
Our Compensation Committee is authorized to grant awards in the form of other equity-based awards or other cash-based awards, as deemed to be consistent with the purposes of the Equity Plan.
16. Stockholders' Equity
Zoetis is authorized to issue 6 billion shares of common stock and 1 billion shares of preferred stock.
In December 2021, our Board of Directors authorized a $3.5 billion share repurchase program. As of December 31, 2023, there was $1.5 billion remaining under this authorization. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs.
Accumulated other comprehensive loss
Changes, net of tax, in accumulated other comprehensive loss, excluding noncontrolling interest, follow: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Currency Translation Adjustments | | Benefit Plans | | Accumulated Other |
| | Cash Flow | | Net Investment | | Other Currency | | Actuarial | | Comprehensive |
| (MILLIONS OF DOLLARS) | | Hedges | | Hedges | | Translation Adj | | (Losses)/Gains | | Loss |
| Balance, December 31, 2020 | | $ | (15) | | | $ | (37) | | | $ | (655) | | | $ | (23) | | | $ | (730) | |
| Other comprehensive gain/(loss), net of tax | | 19 | | | 42 | | | (101) | | | 6 | |
| (34) | |
| | | | | | | | | | |
| | | | | | | | | | |
| Balance, December 31, 2021 | | 4 | | | 5 | | | (756) | | | (17) | | | (764) | |
| Other comprehensive gain/(loss), net of tax | | 86 | | | 36 | | | (188) | | | 13 | | | (53) | |
| | | | | | | | | | |
| Balance, December 31, 2022 | | 90 | | | 41 | | | (944) | | | (4) | | | (817) | |
| Other comprehensive (loss)/gain, net of tax | | (5) | | | (23) | | | — | | | 6 | |
| (22) | |
| Balance, December 31, 2023 | | $ | 85 | | | $ | 18 | | | $ | (944) | | | $ | 2 | | | $ | (839) | |
17. Earnings per Share
The following table presents the calculation of basic and diluted earnings per share: | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA) | | 2023 | | 2022 | | 2021 |
| Numerator | | | | | | |
| Net income before allocation to noncontrolling interests | | $ | 2,340 | | | $ | 2,111 | | | $ | 2,034 | |
| Less: net loss attributable to noncontrolling interests | | (4) | | | (3) | | | (3) | |
| Net income attributable to Zoetis Inc. | | $ | 2,344 | | | $ | 2,114 | | | $ | 2,037 | |
| Denominator | | | | | | |
| Weighted-average common shares outstanding | | 461.172 | | | 468.891 | | | 474.348 | |
| Common stock equivalents: stock options, RSUs, DSUs and PSUs | | 1.097 | | | 1.494 | | | 2.369 | |
| Weighted-average common and potential dilutive shares outstanding | | 462.269 | | | 470.385 | | | 476.717 | |
| Earnings per share attributable to Zoetis Inc. stockholders—basic | | $ | 5.08 | | | $ | 4.51 | | | $ | 4.29 | |
| Earnings per share attributable to Zoetis Inc. stockholders—diluted | | $ | 5.07 | | | $ | 4.49 | | | $ | 4.27 | |
The number of stock options outstanding under the company's Equity Plan that were excluded from the computation of diluted earnings per share, as the effect would have been antidilutive, were not material for the years ended December 31, 2023, 2022 and 2021.
18. Commitments and Contingencies
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business. For a discussion of our tax contingencies, see Note 8. Tax Matters.
A. Legal Proceedings
Our non-tax contingencies include, among others, the following:
• Product liability and other product-related litigation, which can include injury, consumer, off-label promotion, antitrust and breach of contract claims.
• Commercial and other matters, which can include product-pricing claims and environmental claims and proceedings.
• Patent litigation, which typically involves challenges to the coverage and/or validity of our patents or those of third parties on various products or processes.
• Government investigations, which can involve regulation by national, state and local government agencies in the U.S. and in other countries.
Certain of these contingencies could result in losses, including damages, fines and/or civil penalties, and/or criminal charges, which could be substantial.
We believe that we have strong defenses in these types of matters, but litigation is inherently unpredictable and excessive verdicts do occur. We do not believe that any of these matters will have a material adverse effect on our financial position. However, we could incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations or cash flows in the period in which the amounts are paid.
We have accrued for losses that are both probable and reasonably estimable. Substantially all of these contingencies are subject to significant uncertainties and, therefore, determining the likelihood of a loss and/or the measurement of any loss can be complex. Consequently, we are unable to estimate the range of reasonably possible loss in excess of amounts accrued. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but the assessment process relies on estimates and assumptions that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions.
Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions.
The principal matters to which we are a party are discussed below. In determining whether a pending matter is significant for financial reporting and disclosure purposes, we consider both quantitative and qualitative factors in order to assess materiality, such as, among other things, the amount of damages and the nature of any other relief sought in the proceeding, if such damages and other relief are specified; our view of the merits of the claims and of the strength of our defenses; whether the action purports to be a class action and our view of the likelihood that a class will be certified by the court; the jurisdiction in which the proceeding is pending; any experience that we or, to our knowledge, other companies have had in similar proceedings; whether disclosure of the action would be important to a reader of our financial statements, including whether disclosure might change a reader’s judgment about our financial statements in light of all of the information about the company that is available to the reader; the potential impact of the proceeding on our reputation; and the extent of public interest in the matter. In addition, with respect to patent matters, we consider, among other things, the financial significance of the product protected by the patent.
Ulianopolis, Brazil
In February 2012, the Municipality of Ulianopolis (State of Para, Brazil) filed a complaint against Fort Dodge Saúde Animal Ltda. (FDSAL), a Zoetis entity, and five other large companies alleging that waste sent to a local waste incineration facility for destruction, but that was not ultimately destroyed as the facility lost its operating permit, caused environmental impacts requiring cleanup.
The Municipality is seeking recovery of cleanup costs purportedly related to FDSAL's share of all waste accumulated at the incineration facility awaiting destruction, and compensatory damages to be allocated among the six defendants. We believe we have strong arguments against the claim, including defense strategies against any claim of joint and several liability.
At the request of the Municipal prosecutor, in April 2012, the lawsuit was suspended for one year. Since that time, the prosecutor has initiated investigations into the Municipality's actions in the matter as well as the efforts undertaken by the six defendants to remove and dispose of their individual waste from the incineration facility. On October 3, 2014, the Municipal prosecutor announced that the investigation remained ongoing and outlined the terms of a proposed Term of Reference (a document that establishes the minimum elements to be addressed in the preparation of an Environmental Impact Assessment), under which the companies would be liable to withdraw the waste and remediate the area.
On March 5, 2015, we presented our response to the prosecutor’s proposed Term of Reference, arguing that the proposed terms were overly general in nature and expressing our interest in discussing alternatives to address the matter. The prosecutor agreed to consider our request to engage a technical consultant to conduct an environmental diagnostic of the contaminated area. On May 29, 2015, we, in conjunction with the other defendant companies, submitted a draft cooperation agreement to the prosecutor, which outlined the proposed terms and conditions for the engagement of a technical consultant to conduct the environmental diagnostic. On August 19, 2016, the parties and the prosecutor agreed to engage the services of a third-party consultant to conduct a limited environmental assessment of the site. The site assessment was conducted during June 2017, and a written report summarizing the results of the assessment was provided to the parties and the prosecutor in November 2017. The report noted that waste is still present on the site and that further (Phase II) environmental assessments are needed before a plan to manage that remaining waste can be prepared. On April 1, 2019, the defendants met with the Prosecutor to discuss the conclusions set forth in the written report. Following that discussion, on April 10, 2019, the Prosecutor issued a procedural order requesting that the defendants prepare and submit a technical proposal outlining the steps needed to conduct the additional Phase II environmental assessments. The defendants presented the technical proposal to the Prosecutor on October 21, 2019. On March 3, 2020, the Prosecutor notified the defendants that he submitted the proposal to the Ministry of the Environment for its review and consideration by the Prosecutor. On July 15, 2020, the Prosecutor recommended certain amendments to the proposal for the Phase II testing. On September 28, 2020, the parties and the Prosecutor agreed to the final terms and conditions concerning the cooperation agreement with respect to the Phase II testing. Due to the ongoing issues presented by the COVID-19 pandemic, the parties have been unable to secure a start date for the Phase II testing and have no timeline at this point when testing will begin.
Belgium Excess Profit Tax Regime
On February 14, 2019, the General Court of the European Union (General Court) annulled the January 11, 2016 decision of the European Commission (EC) that selective tax advantages granted by Belgium under its “excess profit” tax scheme constitute illegal state aid. As a result of the 2016 decision, the company recorded a net tax charge of approximately $35 million in the first half of 2016. After several appeals and judgements, on September 20, 2023, the General Court confirmed the decision of the EC that the Belgium excess profit tax scheme constitutes illegal state aid. We have filed an appeal of the General Court’s decision. The company has not reflected any potential benefits in its consolidated financial statements as of December 31, 2023 as a result of the 2019 annulment. We will continue to monitor the developments of the appeal and its ultimate resolution.
B. Guarantees and Indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses, we indemnify our counterparties against certain liabilities that may arise in connection with the transaction or related to activities prior to the transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters and patent-infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2023, recorded amounts for the estimated fair value of these indemnifications were not material.
C. Purchase Commitments
As of December 31, 2023, we have agreements totaling $336 million to purchase goods and services, as well as commitments for capital expenditures, that are enforceable and legally binding and include amounts relating to contract manufacturing, information technology services and site acquisitions deemed reasonably likely to occur. Payments for these obligations are expected to be approximately $200 million in 2024 and $136 million thereafter.
19. Segment Information
A. Segment Information
We manage our operations through two geographic regions. Each operating segment has responsibility for its commercial activities. Within each of these operating segments, we offer a diversified product portfolio, including parasiticides, vaccines, dermatology, other pharmaceutical, anti-infectives, animal health diagnostics and medicated feed additives, for both companion animal and livestock customers.
Operating Segments
Our operating segments are the U.S. and International. Our chief operating decision maker uses the revenue and earnings of the two operating segments, among other factors, for performance evaluation and resource allocation.
Other Costs and Business Activities
Certain costs are not allocated to our operating segment results, such as costs associated with the following:
• Other business activities, includes our CSS contract manufacturing results, our human health business, and expenses associated with our dedicated veterinary medicine research and development organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the international commercial segment.
• Corporate, includes enabling functions such as information technology, facilities, legal, finance, human resources, business development, certain diagnostic costs and communications, among others. These costs also include certain compensation costs, certain procurement costs and other miscellaneous operating expenses not charged to our operating segments, as well as interest income and expense.
• Certain transactions and events such as (i) Purchase accounting adjustments, where we incur expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii) Acquisition-related activities, where we incur costs associated with acquiring and integrating newly acquired businesses, such as transaction costs and integration costs; and (iii) Certain significant items, which comprise substantive, unusual items that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis, such as restructuring charges and implementation costs associated with our cost-reduction/productivity initiatives that are not associated with an acquisition, certain asset impairment charges, certain legal and commercial settlements and the impact of divestiture-related gains and losses.
•Other unallocated includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with finance that specifically support our global manufacturing operations; (iii) certain supply chain and global logistics costs; and (iv) certain procurement costs.
Segment Assets
We manage our assets on a total company basis, not by operating segment. Therefore, our chief operating decision maker does not regularly review any asset information by operating segment and, accordingly, we do not report asset information by operating segment. Total assets were approximately $14.3 billion and $14.9 billion at December 31, 2023 and 2022, respectively.
Selected Statement of Income Information | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Earnings | | Depreciation and Amortization(a) |
| | Year Ended December 31, | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| U.S. | | | | | | | | | | | | |
| Revenue | | $ | 4,555 | | | $ | 4,313 | | | $ | 4,042 | | | | | | | |
| Cost of Sales | | 900 | | | 803 | | | 788 | | | | | | | |
| Gross Profit | | 3,655 | | | 3,510 | | | 3,254 | | | | | | | |
| Gross Margin | | 80.2 | % | | 81.4 | % | | 80.5 | % | | | | | | |
| Operating Expenses | | 786 | | | 765 | | | 681 | | | | | | | |
| Other (income)/deductions-net | | 6 | | | (18) | | | 4 | | | | | | | |
| U.S. Earnings | | 2,863 | | | 2,763 | | | 2,569 | | | $ | 80 | | | $ | 55 | | | $ | 54 | |
| International | | | | | | | | | | | | |
Revenue(b) | | 3,911 | | | 3,681 | | | 3,652 | | | | | | | |
| Cost of Sales | | 1,234 | | | 1,083 | | | 1,106 | | | | | | | |
| Gross Profit | | 2,677 | | | 2,598 | | | 2,546 | | | | | | | |
| Gross Margin | | 68.4 | % | | 70.6 | % | | 69.7 | % | | | | | | |
| Operating Expenses | | 638 | | | 611 | | | 602 | | | | | | | |
| Other (income)/deductions-net | | 2 | | | (3) | | | (4) | | | | | | | |
| International Earnings | | 2,037 | | | 1,990 | | | 1,948 | | | 92 | | | 86 | | | 74 | |
| Total operating segments | | 4,900 | | | 4,753 | | | 4,517 | | | 172 | | | 141 | | | 128 | |
| Other business activities | | (496) | | | (424) | | | (406) | | | 33 | | | 28 | | | 28 | |
| Reconciling Items: | | | | | | | | | | | | |
| Corporate | | (1,042) | | | (1,073) | | | (1,052) | | | 128 | | | 132 | | | 115 | |
| Purchase accounting adjustments | | (159) | | | (160) | | | (175) | | | 153 | | | 159 | | | 175 | |
| Acquisition-related costs | | (9) | | | (5) | | | (12) | | | — | | | — | | | — | |
Certain significant items(c) | | 33 | | | (56) | | | (73) | | | — | | | — | | | — | |
| Other unallocated | | (291) | | | (379) | | | (311) | | | 5 | | | 5 | | | 2 | |
Total Earnings(d) | | $ | 2,936 | | | $ | 2,656 | | | $ | 2,488 | | | $ | 491 | | | $ | 465 | | | $ | 448 | |
(a) Certain production facilities are shared. Depreciation and amortization is allocated to the reportable operating segments based on estimates of where the benefits of the related assets are realized.
(b) Revenue denominated in euros was $853 million in 2023, $774 million in 2022 and $814 million in 2021.
(c) For 2023, certain significant items primarily consisted of a gain on the sale of a majority interest in our pet insurance business of $101 million, partially offset by employee termination and exit costs related to organizational structure refinements of $43 million and certain asset impairment charges primarily related to our precision animal health and diagnostics businesses of $24 million.
For 2022, certain significant items primarily represents inventory and asset impairment charges related to customer relationships, developed technology rights and property, plant and equipment in our diagnostics, poultry, cattle and swine businesses and the consolidation of manufacturing sites in China of $47 million, as well as employee termination and exit costs associated with cost-reduction and productivity initiatives in certain international markets of $4 million.
For 2021, certain significant items primarily included certain asset impairment charges of $46 million, as well as employee termination costs associated with our international operations and other costs associated with cost-reduction and productivity initiatives of $24 million.
(d) Defined as income before provision for taxes on income.
B. Geographic Information
Property, plant and equipment, less accumulated depreciation, by geographic region follow: | | | | | | | | | | | | | | |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2023 | | 2022 |
| U.S. | | $ | 2,092 | | | $ | 1,820 | |
| International | | 1,112 | | | 933 | |
| Property, plant and equipment, less accumulated depreciation | | $ | 3,204 | | | $ | 2,753 | |
Zoetis Inc. and Subsidiaries
Schedule II—Valuation and Qualifying Accounts | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance, | | | | | | Balance, |
| | Beginning of | | | | | | End of |
| (MILLIONS OF DOLLARS) | | Period | | Additions | | Deductions | | Period |
| Year Ended December 31, 2023 | | | | | | | | |
| Allowance for doubtful accounts | | $ | 19 | | | $ | — | | | $ | (1) | | | $ | 18 | |
| | | | | | | | |
| Year Ended December 31, 2022 | | | | | | | | |
| Allowance for doubtful accounts | | $ | 17 | | | $ | 4 | | | $ | (2) | | | $ | 19 | |
| | | | | | | | |
| Year Ended December 31, 2021 | | | | | | | | |
| Allowance for doubtful accounts | | $ | 20 | | | $ | 1 | | | $ | (4) | | | $ | 17 | |
| | | | | | | | |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
An evaluation was carried out under the supervision and with the participation of the company's management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934). Based upon that evaluation as of December 31, 2023, the company's Chief Executive Officer and Chief Financial Officer concluded that the company's disclosure controls and procedures are effective at a reasonable level of assurance in alerting them in a timely manner to material information required to be disclosed in our periodic reports filed with the SEC.
Management's Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined under Rule 13a-15(f) and 15d-15(f) of the Securities Exchange Act of 1934. Under the supervision and with the participation of management, including the company's Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2023. The effectiveness of our internal control over financial reporting as of December 31, 2023, has been audited by KPMG LLP, an independent registered public accounting firm, as stated in its report included herein.
Changes in Internal Control over Financial Reporting
During our most recent fiscal quarter, there has not been any change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
Rule 10b5-1 Trading Arrangements
Heidi Chen, Executive Vice President, General Counsel and Corporate Secretary; Business Lead of Human Health Diagnostics, adopted a pre-arranged trading plan on December 15, 2023, that is intended to satisfy the affirmative defense of Rule 10b5-1(c) under the Securities Exchange Act of 1934, as amended. Ms. Chen's plan provides for the sale of up to 8,310 shares of Zoetis common stock between March 15, 2024 and March 13, 2026.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information about our directors is incorporated by reference from the discussion under the heading Item 1-Election of Directors in our 2024 Proxy Statement. Information regarding our executive officers is presented in Part I, Item 1 of this report under the heading Information about our Executive Officers. Information about compliance with Section 16(a) of the Exchange Act is incorporated by reference from the discussion under the heading Delinquent Section 16(a) Reports in our 2024 Proxy Statement. Information about the Zoetis Code of Conduct governing our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer and Controller, and our Board of Directors, is incorporated by reference from the discussions under the heading Corporate Governance at Zoetis in our 2024 Proxy Statement. If we make any substantive amendments to the Code of Conduct or grant any waiver from a provision of the Code of Conduct to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website. The full text of our Code of Conduct is available at the Investor Relations section of our website at www.zoetis.com. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be part of this Annual Report on Form 10-K. Information regarding the procedures by which our stockholders may recommend nominees to our Board of Directors is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2024 Proxy Statement. Information about our Audit Committee, including the members of the Committee, and our Audit Committee financial experts, is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2024 Proxy Statement.
Item 11. Executive Compensation.
Information about director compensation is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2024 Proxy Statement. Information about executive compensation is incorporated by reference from the discussion under the heading Executive Compensation in our 2024 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required by this item is incorporated by reference from the discussion under the heading Ownership of Our Common Stock in our 2024 Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information about certain relationships and transactions with related parties and our policies and procedures in relation to such transactions is incorporated by reference from the discussion under the heading Transactions with Related Persons in our 2024 Proxy Statement. Information about director independence is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis-Corporate Governance Principles and Practices-Director Independence in our 2024 Proxy Statement.
Item 14. Principal Accountant Fees and Services.
Our independent registered public accounting firm is KPMG LLP, Short Hills, NJ, Auditor Firm ID: 185.
Information about the fees for professional services rendered by our independent registered public accounting firm in 2023 and 2022, is incorporated by reference from the discussion under the heading Item 3—Ratification of Appointment of KPMG as our Independent Registered Public Accounting Firm for 2024 in our 2024 Proxy Statement. Our Audit Committee's policy on pre-approval of audit and permissible non-audit services of our independent registered public accounting firm is incorporated by reference from the discussion under the heading Item 3—Ratification of Appointment of KPMG as our Independent Registered Public Accounting Firm for 2024 in our 2024 Proxy Statement.
PART IV
Item 15. Exhibit and Financial Statement Schedules.
The following entire exhibits are included:
(1) The financial statements and notes to financial statements are filed as part of this report in Item 8. Financial Statements and Supplementary Data.
(2) The financial statement schedule is listed in the Index to Financial Statements.
(3) The exhibits are listed in the Index to Exhibits.
Item 16. Form 10-K Summary.
None.
EXHIBITS
The exhibits listed below and designated with a † are filed with this report. The exhibits listed below and not so designated are incorporated by reference to the documents following the descriptions of the exhibits. | | | | | | | | |
| | Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to Zoetis Inc.'s Current |
| | Report on Form 8-K filed on May 29, 2023 (File No. 001-35797)) |
| | Amended and Restated By-laws of the Registrant, (incorporated by reference to Exhibit 3.2 to Zoetis Inc.'s Current |
| | Report on Form 8-K filed on May 19, 2023 (File No. 001-35797)) |
| | Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to Zoetis Inc.'s Annual Report on Form 10-K |
| | filed on February 15, 2022 (File No. 001-35797)) |
| | Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company Americas, as trustee |
| | (incorporated by reference to Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254)) |
| | First Supplemental Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company |
| | Americas, as trustee (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on Form S-1 |
| | (File No. 333-183254)) |
| | Second Supplemental Indenture, dated November 13, 2015, between Zoetis Inc. and Deutsche Bank Trust Company |
| | Americas, as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on |
| | November 13, 2015 (File No. 001-35797)) |
| | Third Supplemental Indenture, dated September 12, 2017, between Zoetis Inc. and Deutsche Bank Trust Company Americas, |
| | as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on September 12, 2017 |
| | (File No. 001-35797)) |
| | Fourth Supplemental Indenture, dated August 20, 2018, between Zoetis Inc. and Deutsche Bank Trust Company Americas, |
| | as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on August 20, 2018 |
| | (File No. 001-35797)) |
| | Fifth Supplemental Indenture, dated May 12, 2020, between Zoetis Inc. and Deutsche Bank Trust Company Americas, |
| | as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.'s Current Report on Form 8-K filed on May 12, 2020 |
| | (File No. 001-35797)) |
| | Sixth Supplemental Indenture, dated November 16, 2022, between Zoetis Inc. and Deutsche Bank Trust Company Americas, |
| | as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on November 16, 2022 |
| | (File No. 001-35797)) |
| | Form of 4.500% Senior Notes due 2025 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on |
| | Form 8-K filed on November 13, 2015 (File No. 001-35797)) |
| | Form of 4.700% Senior Notes due 2043 (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on |
| | Form S-1 (File No. 333-183254)) |
| | Form of 3.000% Senior Notes due 2027 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on September 12, 2017 (File No. 001-35797)) |
| | Form of 3.950% Senior Notes due 2027 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on September 12, 2017 (File No. 001-35797)) |
| | Form of 3.900% Senior Notes due 2028 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on August 20, 2018 (File No. 001-35797)) |
| | Form of 4.450% Senior Notes due 2048 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on August 20, 2018 (File No. 001-35797)) |
| | Form of 2.000% Senior Notes due 2030 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on May 12, 2020 (File No. 001-35797)) |
| | Form of 3.000% Senior Notes due 2050 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on May 12, 2020 (File No. 001-35797)) |
| | Form of 5.400% Senior Notes due 2025 (incorporated by reference to Exhibit 4.3 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on November 16, 2022 (File No. 001-35797)) |
| | | | | | | | |
| | Form of 5.600% Senior Notes due 2032 (incorporated by reference to Exhibit 4.4 to Zoetis Inc.’s Current Report on Form 8-K |
| | filed on November 16, 2022 (File No. 001-35797)) |
| | Description of the Registrant’s Securities (incorporated by reference to Exhibit 4.20 to Zoetis Inc.'s Annual Report on Form 10-K |
| | filed on February 14, 2023 (File No. 001-35797))† |
| | Global Separation Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to |
| | Exhibit 10.1 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797)) |
| | Research and Development Collaboration and License Agreement, dated February 6, 2013, by and between Zoetis Inc. |
| | and Pfizer Inc. (incorporated by reference to Exhibit 10.4 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on |
| | March 28, 2013 (File No. 001-35797)) |
| | Pfizer Inc. 2004 Stock Plan, as amended and restated (incorporated by reference to Exhibit 10.6 of Zoetis Inc.'s registration |
| | statement on Form S-1 (File No. 333-183254))* |
| | Patent and Know-How License Agreement (Zoetis as licensor), dated February 6, 2013, by and between Zoetis Inc. and |
| | Pfizer Inc. (incorporated by reference to Exhibit 10.8 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on |
| | March 28, 2013 (File No. 001-35797)) |
| | Patent and Know-How License Agreement (Pfizer as licensor), dated February 6, 2013, by and between Zoetis Inc. and |
| | Pfizer Inc. (incorporated by reference to Exhibit 10.9 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on |
| | March 28, 2013 (File No. 001-35797)) |
| | Trademark and Copyright License Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. |
| | (incorporated by reference to Exhibit 10.10 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 |
| | (File No. 001-35797)) |
| | Environmental Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by |
| | reference to Exhibit 10.13 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013) (File No. 001-35797)) |
| | Zoetis Inc. 2013 Equity and Incentive Plan, as amended and restated as of May 19, 2022 (incorporated by reference to Exhibit |
| | 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on August 4, 2022 (File No. 001-35797))* |
| | Revolving Credit Agreement, dated as of December 21, 2022, among Zoetis Inc., the lenders party thereto and JPMorgan |
| | Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 of Zoetis Inc.'s Current Report |
| | on Form 8-K filed on December 21, 2022 (File No. 001-35797)) |
| | Form of Indemnification Agreement for directors and officers (incorporated by reference to Exhibit 10.19 of Zoetis Inc's |
| | registration statement on Form S-1 (File No. 333-183254)) |
| | Form of Restricted Stock Unit Award agreement (incorporated by reference to Exhibit 10.21 to Zoetis Inc.’s 2012 Annual |
| | Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))* |
| | Form of Stock Option Award agreement (incorporated by reference to Exhibit 10.22 to Zoetis Inc.’s 2012 Annual Report |
| | on Form 10-K filed on March 28, 2013 (File No. 001-35797))* |
| | Form of Non-Employee Director Deferred Stock Unit Award agreement (incorporated by reference to Exhibit 10.23 |
| | on Form 10-K filed on March 28, 2013 (File No. 001-35797))* |
| | Form of Cash Award agreement (incorporated by reference to Exhibit 10.24 to Zoetis Inc.’s 2012 Annual Report on |
| | Form 10-K filed on March 28, 2013 (File No. 001-35797))* |
| | Form of Performance Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by |
| | reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* |
| | Form of Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by reference to |
| | Exhibit 99.2 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* |
| | Form of Stock Option Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.3 |
| | to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* |
| | Form of Cash Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.4 to |
| | Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* |
| | Zoetis Amended and Restated Non-Employee Director Deferred Compensation Plan (incorporated by reference to |
| | Exhibit 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 1, 2018 (File No. 001-35797))* |
| | Zoetis Executive Severance Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q |
| | filed on August 14, 2013 (File No. 001-35797))* |
| | | | | | | | |
| | Zoetis Supplemental Savings Plan, as amended and restated, effective September 15, 2014 (incorporated by reference to |
| | Exhibit 10.4 to Zoetis Inc.'s Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))* |
| | Amendment No. 1 to Zoetis Supplemental Savings Plan effective December 21, 2020 (incorporated by reference to Exhibit 10.24 |
| | to Zoetis Inc.'s 2020 Annual Report on Form 10-K filed on February 16, 2021 (File No. 001-35797))* |
| | Zoetis Equity Deferral Plan, effective November 1, 2014 (incorporated by reference to Exhibit 10.5 to Zoetis Inc.’s |
| | Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))* |
| | Amendment No. 1 to Zoetis Equity Deferral Plan effective December 21, 2020 (incorporated by reference to Exhibit 10.26 |
| | to Zoetis Inc.'s 2020 Annual Report on Form 10-K filed on February 16, 2021 (File No. 001-35797))* |
| | Letter Agreement dated as of October 2, 2019, by and between Juan Ramón Alaix and Zoetis Inc. (incorporated by |
| | reference to Exhibit 10.1 to Zoetis Inc.'s Current Report on Form 8-K filed on October 3, 2019 (File No. 001-35797)) |
| | Letter Agreement dated as of December 9, 2019, by and between Clinton A. Lewis, Jr. and Zoetis Inc. (incorporated by |
| | reference to Exhibit 10.1 to Zoetis Inc.'s Current Report on Form 8-K filed on December 12, 2019 (File No. 001-35797)) |
| | Offer Letter, dated as of May 6, 2021, by and between Wetteny Joseph and Zoetis Inc. (incorporated by reference to Exhibit 10.1 |
| | to Zoetis Inc.'s Current Report on Form 8-K filed on May 11, 2021 (File No. 001-35797))* |
| | Amendment No. 2 to Zoetis Supplemental Savings Plan effective May 15, 2021 (incorporated by reference to Exhibit 10.1 to |
| | Zoetis Inc.'s Quarterly Report on Form 10-Q filed on August 5, 2021 (File No. 001-35797))* |
| | Form of Restricted Stock Unit Award Agreement, effective as of July 27, 2022 (incorporated by reference to Exhibit 10.1 to |
| | Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 3, 2022 (File No 001-35797))* |
| | Form of Stock Option Award Agreement, effective as of July 27, 2022 (incorporated by reference to Exhibit 10.2 to Zoetis Inc.’s |
| | Quarterly Report on Form 10-Q filed on November 3, 2022 (File No 001-35797))* |
| | Form of Performance Restricted Stock Unit Award Agreement, effective as of July 27, 2022 (incorporated by reference to Exhibit |
| | 10.3 to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 3, 2022 (File No 001-35797))* |
| | Form of Cash Award Agreement, effective as of July 27, 2022 (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s |
| | Quarterly Report on Form 10-Q filed on November 3, 2022 (File No 001-35797))* |
| | Form of Non-Employee Director Restricted Stock Unit Award Agreement, effective as of July 27, 2022 (incorporated by |
| | reference to Exhibit 10.5 to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 3, 2022 (File No 001-35797))* |
| | Form of Restricted Stock Unit Award Agreement, effective as of December 8, 2022 (incorporated by reference to Exhibit 10.36 |
| | to Zoetis Inc.'s Annual Report on Form 10-K filed on February 14, 2023 (File No 001-35797))* |
| | Form of Stock Option Award Agreement, effective as of December 8, 2022 (incorporated by reference to Exhibit 10.37 |
| | to Zoetis Inc.'s Annual Report on Form 10-K filed on February 14, 2023 (File No 001-35797))* |
| | Form of Performance Restricted Stock Unit Award Agreement, effective as of December 8, 2022 (incorporated by reference to |
| | Exhibit 10.38 to Zoetis Inc.'s Annual Report on Form 10-K filed on February 14, 2023 (File No 001-35797))* |
| | Form of Non-Employee Director Restricted Stock Unit Award Agreement, effective as of February 8, 2023 (incorporated by |
| | reference to Exhibit 10.1 to Zoetis Inc.'s Quarterly Report on Form 10Q filed on May 4, 2023 (File No 001-35797))* |
| | Form of Cash Restricted Stock Unit Award Agreement, effective as of February 8, 2023 (incorporated by reference to |
| | Exhibit 10.2 to Zoetis Inc.'s Quarterly Report on Form 10Q filed on May 4, 2023 (File No 001-35797))* |
| | Subsidiaries of the Registrant † |
| | Consent of KPMG LLP † |
| | Power of Attorney (included as part of signature page) † |
| | Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 † |
| | Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 † |
| | Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the |
| | Sarbanes-Oxley Act of 2002 †† |
| | Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the |
| | Sarbanes-Oxley Act of 2002 †† |
| | Zoetis Inc. Compensation Recovery Policy † |
| EX-101.INS | | Inline XBRL INSTANCE DOCUMENT |
| EX-101.SCH | | Inline XBRL TAXONOMY EXTENSION SCHEMA DOCUMENT |
| | | | | | | | |
| EX-101.CAL | | Inline XBRL TAXONOMY EXTENSION CALCULATION LINKBASE DOCUMENT |
| EX-101.LAB | | Inline XBRL TAXONOMY EXTENSION LABEL LINKBASE DOCUMENT |
| EX-101.PRE | | Inline XBRL TAXONOMY EXTENSION PRESENTATION LINKBASE DOCUMENT |
| EX-101.DEF | | Inline XBRL TAXONOMY EXTENSION DEFINITION LINKBASE DOCUMENT |
| EX-104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
| | | | | |
| † | Filed herewith |
| †† | Furnished herewith |
| * | Management contracts or compensatory plans or arrangements |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | Zoetis Inc. | |
| Dated: February 13, 2024 | | By: | /S/ KRISTIN C. PECK | |
| | | Kristin C. Peck Chief Executive Officer and Director | |
We, the undersigned directors and officers of Zoetis Inc., hereby severally constitute Kristin C. Peck and Heidi C. Chen, and each of them singly, our true and lawful attorneys with full power to them and each of them to sign for us, in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K filed with the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. | | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | |
| /S/ KRISTIN C. PECK | | Chief Executive Officer and Director | | February 13, 2024 |
| Kristin C. Peck | (Principal Executive Officer) | |
| | | | |
| /S/ WETTENY JOSEPH | | Executive Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | | February 13, 2024 |
| Wetteny Joseph | | |
| | | | |
| /S/ MICHAEL B. MCCALLISTER | | Chairman and Director | | February 13, 2024 |
| Michael B. McCallister | | | | |
| | | | |
| /S/ PAUL M. BISARO | | Director | | February 13, 2024 |
| Paul M. Bisaro | | | | |
| | | | |
| /S/ VANESSA BROADHURST | | Director | | February 13, 2024 |
| Vanessa Broadhurst | | | | |
| | | | |
| /S/ FRANK A. D'AMELIO | | Director | | February 13, 2024 |
| Frank A. D’Amelio | | | |
| | | | |
| /S/ SANJAY KHOSLA | | Director | | February 13, 2024 |
| Sanjay Khosla | | | |
| | | | |
| /S/ ANTOINETTE R. LEATHERBERRY | | Director | | February 13, 2024 |
| Antoinette R. Leatherberry | | | | |
| | | | |
| /S/ GREGORY NORDEN | | Director | | February 13, 2024 |
| Gregory Norden | | | |
| | | | |
| /S/ LOUISE M. PARENT | | Director | | February 13, 2024 |
| Louise M. Parent | | | |
| | | | |
| /S/ WILLIE M. REED | | Director | | February 13, 2024 |
| Willie M. Reed | | | | |
| | | | |
| /S/ LINDA RHODES | | Director | | February 13, 2024 |
| Linda Rhodes | | | | |
| | | | |
| /S/ ROBERT W. SCULLY | | Director | | February 13, 2024 |
| Robert W. Scully | | | | |
| | | | |
| | | | |