UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
______________________
FORM 10-K
______________________
|
| |
x
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended December 31, 2019 |
| | |
o
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the transition period from _____ to ______ |
Commission file number: 001-36225
KINDRED BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
___________________
|
| | |
| Delaware | | 46-1160142 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification Number) |
| | 1555 Bayshore Highway, Suite 200 Burlingame, California 94010 | |
| | (Address of principal executive offices) | |
| | (650) 701-7901 | |
| | Registrant’s telephone number: | |
Securities registered pursuant to Section 12(b) of the Act: |
| | | | |
| Title of each class | | Trading Symbol | | Name of each exchange on which registered |
| Common Stock, $0.0001 par value | | KIN | | The NASDAQ Stock Market LLC |
| Preferred Stock Purchase Rights | | KIN | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and, (2) has been subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
| | | | | |
Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o | Smaller reporting company þ |
| | | | Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes o No þ
As of June 28, 2019, (the last business day of the registrant’s most recently completed second fiscal quarter since June 30, 2019 was a Sunday ), the aggregate market value of the common stock of the registrant held by non-affiliates of the registrant was approximately $249.5 million.
The outstanding number of shares of the registrant’s common stock as of February 28, 2020 was 39,289,624.
Certain portions of the registrant’s Proxy Statement for the 2020 annual meeting of stockholders, to be filed with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the close of the registrant’s fiscal year, are incorporated by reference into Part III of this Form 10-K.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this Annual Report that do not relate to matters of historical fact should be considered forward-looking statements, including, but not limited to, statements regarding our expectations about the trials, regulatory approval, manufacturing, distribution and commercialization of our current and future products and product candidates, and statements regarding our anticipated revenues, expenses, margins, profits and use of cash. These forward-looking statements are based on our current expectations. The words “anticipates,” “believes,” “expects,” “intends,” “future,” “could,” “estimates,” “plans,” “would,” “should,” “potential,” “continues” and similar words or expressions (as well as other words or expressions referencing future events, conditions or circumstances) often identify forward-looking statements. These statements are not promises or guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results to be materially different from any future results expressed or implied by the forward-looking statements. These risks include, but are not limited to, the following: our limited operating history and expectations of losses for the foreseeable future; the absence of significant revenue from our products and product candidates for the foreseeable future; the likelihood that our revenue will vary from quarter to quarter; our potential inability to obtain any necessary additional financing; our substantial dependence on the success of our products and our lead product candidates, which may not be successfully commercialized even if they are approved for marketing; the effect of competition; our potential inability to obtain regulatory approval for our existing or future product candidates; our dependence on third parties to conduct some of our development activities; our dependence upon third-party manufacturers for supplies of our products and product candidates and the potential inability of these manufacturers to deliver a sufficient amount of supplies on a timely basis, including by reason of the coronavirus disease (COVID-19) currently impacting multiple jurisdictions worldwide; uncertainties regarding the outcomes of trials regarding our product candidates; our potential failure to attract and retain senior management and key scientific personnel; uncertainty about our ability to enter into satisfactory agreements with third-party licensees of our biologic products or to develop a satisfactory sales organization for our equine small molecule products; our significant costs of operating as a public company; potential cyber-attacks on our information technology systems or on our third-party providers’ information technology systems, which could disrupt our operations; our potential inability to repay the secured indebtedness that we have incurred from third-party lenders, and the restrictions on our business activities that are contained in our loan agreement with these lenders; the risk that our 2020 strategic realignment plan will result in unanticipated costs or revenue shortfalls; the risk that our sale of Mirataz to Dechra Pharmaceuticals PLC will not be completed because one or more of the closing conditions in the sale agreement are not satisfied and uncertainty about the amount of royalties that we will receive if the sale is completed; our potential inability to obtain and maintain patent protection and other intellectual property protection for our products and product candidates; potential claims by third parties alleging our infringement of their patents and other intellectual property rights; our potential failure to comply with regulatory requirements, which are subject to change on an ongoing basis; the potential volatility of our stock price; and the significant control over our business by our principal stockholders and management.
For a further description of these risks and uncertainties and other risks and uncertainties that we face, please see the risk factors described in Item 1A of this Annual Report under the caption “Risk Factors” and any subsequent updates that may be contained in our Quarterly Reports on Form 10-Q and other documents we file with the Securities and Exchange Commission (the “SEC”). As a result of these risks and uncertainties, actual results may differ materially from those indicated by the forward-looking statements made in this Annual Report. Forward-looking statements contained in this Annual Report speak only as of the date of this Annual Report, and we undertake no obligation to update or revise these statements except as may be required by law.
PART I.
Overview
We are a biopharmaceutical company developing innovative biologics focused on saving and improving the lives of pets. Our mission is to bring to our pets the same kinds of safe and effective medicines that our human family members enjoy. Our core strategy is to identify targets that have already demonstrated safety and efficacy in humans and to develop therapeutics based on these validated targets for dogs and cats. We believe that this approach will lead to shorter development times and higher approval rates than pursuing new, non-validated targets. Our current portfolio includes two approved products and over 20 product candidates in development, predominantly biologics. We also have state-of-the-art biologics manufacturing capabilities and a broad intellectual property portfolio.
On March 16, 2020, we announced we will further prioritize biologics programs for dogs and cats and rely primarily on a partnership-focused commercialization model, which is expected to significantly reduce the amount of additional dilutive capital the company will require. We believe monoclonal antibodies are the future of veterinary medicine, and represent the greatest opportunity for value creation, given large potential markets for our programs and our competitive advantage in biologics. As part of the strategic realignment, we will substantially reduce our commercial footprint and discontinue development of canine and feline small molecule programs. This business model is expected to achieve a better return for our shareholders.
We were incorporated in Delaware in September 2012. The address of our principal executive offices is 1555 Bayshore Highway, Suite 200, Burlingame, CA 94010. Unless the context requires otherwise, references to “KindredBio,” “the Company,” “we,” “us” or “our” in this Annual Report on Form 10-K for the fiscal year ended December 31, 2019 (the “2019 Annual Report”) refer to Kindred Biosciences, Inc., a Delaware corporation, and its subsidiaries.
Product Highlights

On March 16, 2020, we announced that we signed an agreement to sell Mirataz® (mirtazapine transdermal ointment), our transdermal drug for the management of weight loss in cats, to Dechra Pharmaceuticals PLC for a cash purchase price of $43 million, of which $38.7 million will be paid on the closing date and $4.3 million will be paid out of escrow beginning in 12 months assuming no escrow claims, alongside an ongoing royalty on global net sales. The acquisition comprises worldwide marketing rights, intellectual property rights, marketing authorizations and associated regulatory documentation, third party supply contracts related to raw material and manufacture of the finished product, and certain product inventory. With commercial sales and marketing teams in 25 countries, and distributor relationships in an additional 68 countries, Dechra is strongly positioned to market Mirataz in the United States, Europe, and globally. Their focus on the sale of technical and value-added specialty pharmaceuticals has led to the development of market-leading brands in the specialty veterinarian sector, particularly within the field of chronic disease management. With a complementary feline product portfolio targeting diseases linked to feline weight loss, Mirataz will represent an important cross-promotional product for Dechra worldwide.
Mirataz is the first and only FDA approved transdermal medication specifically developed for the management of weight loss in cats. Unintended weight loss is a serious unmet medical need in cats, and may be caused by multiple factors, including chronic illness, like chronic kidney disease, or behavioral issues, such as stress. If untreated, it may lead to hepatic lipidosis, which can be a life-threatening condition.
Weight loss in cats represents a leading cause of visits to the veterinarian for cats, and a veterinarian will see on average 7 or more cats per week with this condition. Our research estimates that as many as 9,000,000 cats each year are diagnosed with unintended weight loss caused by varying underlying conditions, such as chronic kidney disease, cancer or diabetes, and prior to Mirataz’s launch, approximately 3,000,000 cats being treated for unintended weight loss each year. Mirataz, which is formulated with our proprietary Accusorb™ technology, is applied topically to the cat’s inner ear (pinna) once a day, providing a more attractive application route compared to oral administration. 74% of veterinarians report that ease of administering medication is a primary factor in selecting medication for feline weight loss. The product is classified as a weight gain drug and can be used in cats with various underlying diseases associated with unintended weight loss.
Clinical Data
The pivotal field study, KB105, was a multicenter, randomized, double-blind, placebo-controlled pivotal field study that enrolled 230 cats to assess the effectiveness of Mirataz in managing weight loss in cats. The primary endpoint was percentage change in body weight from Day 1 to Week 2. At Week 2, the mean percent increase in body weight from Day 1 was 3.94% in the Mirataz group (n=83), versus 0.41% in the placebo group (n=94) (p<0.0001). In the target animal safety study, Mirataz was generally well-tolerated and no significant safety concerns were identified. At the label dose, topical administration of mirtazapine ointment was associated with mild, reversible skin changes at the site of dose application (ear).
Commercialization and Distribution
Prior to Mirataz’s sale to Dechra on March 16, 2020, the product was marketed through KindredBio’s commercial team in conjunction with third-party national and regional distributors, who in turn warehouse, ship, market, and sell Mirataz to veterinarians. Consistent with our adoption of a partnership-focused commercialization model, we intend to substantially reduce our companion animal commercial infrastructure, thereby reducing operating expenditures.
Combined sales to three large distributors, namely MWI, Covetrus, and Patterson, each accounted for more than 10% of total revenues for the year ended December 31, 2019. In total, these distributors accounted for approximately 85% of our product sales in 2019, with regional, home delivery partners, and e-commerce partners making up the remainder.
As of December 31, 2019, we recorded $4.1 million in Mirataz net product revenues compared to $2.0 million in 2018, when the product was launched in July of that year. Approximately 55% of the around 25,000 veterinary clinics in the U.S. purchased Mirataz in 2019. Since launch, approximately 71% of veterinary clinics placed re-orders.
On December 12, 2019, KindredBio announced that the European Commission granted marketing authorization of Mirataz for bodyweight gain in cats experiencing poor appetite and weight loss resulting from chronic medical conditions. Mirataz is the first and only medication approved in the EU to induce bodyweight gain
in cats experiencing poor appetite and weight loss resulting from chronic medical conditions. Europe represents the second largest market for veterinary therapeutics internationally. The authorization is valid in all 28-member states of the European Union, together with Iceland, Liechtenstein, and Norway, and including the UK. Dechra, which is based in the United Kingdom, plans to launch Mirataz in the UK and the European Union, and intends to conduct the necessary regulatory activities to achieve approvals in other key international markets.
ZimetaTM (dipyrone injection) (Zimeta IV)
On November 25, 2019 KindredBio announced that the U.S. Food and Drug Administration approved Zimeta™ (dipyrone injection) for the control of pyrexia in horses. Pyrexia, or fever, is associated with a number of underlying diseases and can result in significant negative outcomes, including dehydration, laminitis, muscle wasting, weight loss, and in some cases death. Among performance horses, fever can also lead to loss of training and competition days. There are more than eight million horses in the United States, and over one million are seen by a veterinarian for fever annually.
As part of the strategic restructuring announcement on March 16, 2020, the company’s equine assets will be segregated into the KindredBio Equine subsidiary, and a strategic review process will commence, which may result in a potential spin-out or divestiture of assets. The subsidiary’s assets will comprise of Zimeta, among several equine pipeline assets, as disclosed in the press release of the same date. Equine is an attractive market, with high willingness to spend and low commercialization costs
Zimeta is the first injectable dipyrone product to receive FDA approval for use in horses. Dipyrone, the active ingredient in Zimeta, is a member of the non-steroidal anti-inflammatory drug (NSAID) class and has a centrally acting mechanism of action on the hypothalamus where fever originates and is regulated, and is widely used both for horses and humans as an antipyretic outside of the United States. Outside of the US, other dipyrone products may have label indications for other indications, including use as an anti-spasmodic that can be used in horses without masking the surgical signs of colic.
In humans, the active ingredient in Zimeta can, in very rare cases, cause bone marrow suppression. In some countries, it is still available as prescription or over the counter medication, while in other countries, it has been withdrawn from market. However, the side effects have not been seen in horses, and the product is widely used outside the U.S. by many equine veterinarians.
The active ingredient in Zimeta IV is available as GMP-grade material, and we believe our current manufacturer will be able to provide sufficient quantities for market demand. In addition, we executed a commercial manufacturing agreement with Corden Pharma SPA for the manufacture of Zimeta IV. The agreement provides for production to supply KindredBio’s initial launch and future commercial campaigns, with capabilities to meet excess demand.
We launched Zimeta IV in December 2019 and recorded $127,000 in net product revenues. Additionally, an application for Zimeta IV was made in Canada in November, with anticipated approval in the second quarter of 2020.
Clinical Data
The pivotal field study was a multicenter, randomized, blinded, placebo-controlled pivotal study that enrolled 138 horses to assess the effectiveness of Zimeta. The primary endpoint was improvement (a 2°F or greater decrease in temperature from baseline) or resolution of fever (a return to normothermia (≤101.0°F)) at hour 6 following treatment. The success rate was approximately 75% in the Zimeta group vs. approximately 20% in the placebo group (p < 0.0001).
Biologic Product Candidates
On March 16, 2020, we announced we will further prioritize our biologics programs for dogs and cats, which we view as our highest potential assets.
KIND-016
In October 2018, we announced positive topline results from our pilot laboratory effectiveness study of KIND-016, a fully caninized, high-affinity monoclonal antibody targeting interleukin-31 (IL-31), for the treatment of atopic dermatitis in dogs. In addition, we announced that the U.S. Patent and Trademark Office has issued a patent (Patent No. 10,093,731) for KindredBio's anti-IL31 antibody.
The study was a randomized, blinded, placebo-controlled, pilot laboratory study that enrolled 32 dogs to assess the effectiveness of KIND-016 at three doses. A single dose of KIND-016 was administered on day 0 and itching was induced at weeks 1, 2, 3, 4, 6, and 8 with an injection of canine IL-31.
KindredBio's IL-31 antibody resulted in statistically significant reductions in pruritus (p<0.0001 to p<0.05) across all dose groups and was sustained for 6 to 8 weeks, with a clear dose response. The reduction in the itching score was as high as 86.1%. Based on a preliminary review of the safety data, the drug appears to be well tolerated.
In July 2019, we reported positive topline results from a pilot field effectiveness study for our IL-31 antibody that confirmed the results from our pilot laboratory study. As announced on March 16, 2020, the scale up process is proceeding as planned, and the pivotal effectiveness study is expected to commence in the second half of 2020.
Canine atopic dermatitis (CAD) is an immune-mediated inflammatory skin condition in dogs and is the leading reason owners take their dog to the veterinarian. Atopic dermatitis is a large market, with the leading two products on the market selling over $700 million per year. We are pursuing a multi-pronged approach toward atopic dermatitis, with a portfolio of promising biologics. Our market research tells us there is strong demand for new biological treatments for pruritic dogs, with 70% of veterinarians, and a higher percentage of dermatologists, expressing a need for alternatives to current therapies.
KIND-025
As announced on March 16, 2020, the in-life portion of the pilot effectiveness study of KIND-025, a fully caninized, high-affinity fusion protein targeting interleukin-4/13, for the treatment of atopic dermatitis in dogs, is complete. We are completing development of our PK assays and expect to read out the study over the coming weeks. The interleukin-4 and interleukin-13 pathways are key drivers of the inflammation that underlies atopic dermatitis and other allergic diseases.
KIND-032
In December 2019 we announced the outcome of a positive pilot laboratory study of KIND-032, a fully caninized monoclonal antibody targeting interleukin-4 receptor, for the treatment of atopic dermatitis in dogs. In the study, 14 laboratory dogs with clinical signs consistent with atopic dermatitis were dosed with placebo or with KIND-032 at two different doses. The CADESI scores were assessed by board-certified veterinary dermatologists who were blinded to treatment assignments. The study demonstrated that KindredBio's antibody was well-tolerated. Although the study was a single-dose study designed primarily to assess safety and pharmacokinetics, evidence of positive efficacy and dose response was observed at Week 1, as measured by CADESI-04. A second pilot study to further assess efficacy and dosing is planned for 2020.
The IL-4 pathway is a key driver of the inflammation that underlies atopic dermatitis and several other allergic diseases. Unlike KIND-025, which binds to IL-4 and IL-13 circulating in blood, KIND-032 binds to the IL-4 receptor on the surface of immune cells.
KIND-510a
In January 2019, we announced positive topline results from our pilot field effectiveness study of KIND-510a, a long-acting feline recombinant erythropoietin that is being developed for the management of anemia in cats. We completed our cGMP fill & finish for feline recombinant erythropoietin at our Elwood, Kansas biologics manufacturing facility in the third quarter of 2019, and the pivotal efficacy study was initiated in the fourth quarter of 2019, with enrollment ongoing.
Anemia is a common condition that is estimated to affect millions of older cats. It is often associated with chronic kidney disease, because kidneys produce erythropoietin and chronic kidney disease leads to decreased levels of endogenous erythropoietin. Chronic kidney disease affects approximately half of older cats, making it a leading cause of feline mortality. Human erythropoietins, which are multi-billion-dollar products in the human market, are immunogenic in cats.
KIND-510a is a recombinant feline erythropoietin that has been engineered by KindredBio to have a prolonged half-life. Erythropoietin is an endogenous protein that regulates and stimulates production of red blood cells.
In the study, which enrolled 23 cats with anemia, KIND-510a rapidly increased mean hematocrit (a measure of red blood cell count), with statistically significant improvement seen as early as Week 1 (p<0.0001). The effect was sustained, with continued statistically significant improvement at Weeks 2, 3, 4, 5, and 6 (p<0.0001 at each visit). Compared to baseline, the mean peak improvement in hematocrit was 55.4%.
In addition, 95.5% of the 22 evaluable patients achieved treatment success over the 6-week treatment period, defined prospectively as either a 30% increase in hematocrit value over baseline or the hematocrit value reaching normal range. Furthermore, cats treated with KIND-510a demonstrated statistically significant improvements over baseline (p<0.01 to p<0.05) across all three health-related quality of life (QoL) domains, Vitality, Comfort, and Emotional Wellbeing, as measured by a validated QoL instrument. Based on a preliminary review of the safety data, the drug appears to be well tolerated.
KIND-030
In August 2019, we announced positive results from our pilot efficacy study of KIND-030, a chimeric, high-affinity monoclonal antibody targeting canine parvovirus (CPV). This was a 12-dog study, of which 4 dogs were treated prophylactically and 2 dogs were treated after establishment of the infection. All treated dogs survived, compared to none in the applicable placebo group. The effect was seen in both prophylaxis setting, as well as in a treatment setting after establishment of infection. Pivotal studies for this molecule are expected to be completed in 2020. Approval is anticipated by late 2020 or early 2021.
CPV is the most significant cause of viral enteritis in dogs, especially puppies, with over 90% mortality rate if untreated. Banfield Medical records report that at least 250,000 dogs are infected with parvoviruses each year, excluding emergency rooms, specialty hospitals, shelters, or undiagnosed cases. This does not include puppies that have potentially been exposed to the virus where the prophylaxis product candidate could be used after lisensure if demonstrated effective. While there are vaccines available for CPV, they have to be administered multiple times and many puppies don't receive the vaccine at all, or don't get the complete series. This will not replace the need for vaccination; it may just change the timing of the vaccination post administration. There are currently no approved or unapproved treatments for CPV. Currently, owners spend up to thousands of dollars for supportive care for dogs infected with CPV.
KIND-509
The pilot field efficacy study for our anti-TNF antibody for canine inflammatory bowel disease (IBD) has been initiated with completion expected to be in the first half of 2020. IBD is a chronic disease of the gastrointestinal tract and can affect dogs at any age, but is more common in middle-aged and older dogs.
The majority of canine IBD cases involve chronic states of diarrhea, vomiting, gastroenteritis, inappetence, and other symptoms, certain of which are cited as among the most frequent disorders impacting dogs. For certain dog breeds, the prevalence of diarrhea exceeds 5%. Existing treatments can have significant drawbacks, including limited diets and excessive antibiotic use, which can lead to owner frustration, lapses in treatment adherence, or poor quality of life for the affected animal.
KIND-511
As disclosed in the press release dated March 16, 2020, KIND-511 will be included in KindredBio Equine, for which there is a strategic review process underway. KIND-511 is an anti-Tumor Necrosis Factor (“anti-TNF”) treatment for newborn foals. Sick newborn foals, defined as sepsis score ≥ 11 or positive blood culture, are challenging, and difficult to treat and result in approximately 50% mortality. We have completed a pilot field study in sick or septic foals to assess safety and efficacy of anti-TNF monoclonal antibody, with positive results. By Kaplan-Meier analysis, the difference in survival between the control and placebo groups was statistically significant (p=0.0293). We intend to continue field studies during the 2021 foaling season following discussion with the FDA regarding the pivotal study design for KIND-511. There is currently no FDA-approved therapy.
We have promising new antibody candidates under development, which will be disclosed at a later stage of development and as lead programs further advance.
Small Molecule Product Candidates
As disclosed in our press release dated March 16, 2020, KindredBio is discontinuing development of canine and feline small molecule candidates as we prioritize our pipeline of innovative biologic candidates. Equine small molecule candidates will be housed in KindredBio Equine, for which there is a strategic review process underway. Pending that review process, development of certain equine candidates may be put on hold.
KIND-012 (dipyrone oral gel)
We have completed the pivotal field effectiveness study of KIND-012 (dipyrone oral gel) for the treatment of fever in horses and announced positive topline results in December 2017. This study was a multicenter, randomized, blinded, placebo-controlled pivotal study that enrolled 139 horses to assess the effectiveness of dipyrone oral. The primary endpoint was improvement or resolution of fever 6 hours after treatment. The success rate was approximately 78% in the dipyrone oral group vs. approximately 18% in the placebo group (p = 0.0026). The target animal safety study is also complete, and dipyrone oral was found to be well-tolerated. The FDA has determined the study to be acceptable pending the outcome of additional bridging studies. We have transferred the product to the commercial manufacturer and have agreement with the FDA regarding the data required to show relative bioequivalence to the previously manufactured product. Scientific advice has also been received from the EMA on dossier requirements. Dipyrone oral, which is a proprietary oral gel, is expected to expand use of the drug and build upon the success of Zimeta IV. If approved, this would be the first oral dipyrone product approved in the US and EU.
KIND-014
KIND-014 is a small molecule product candidate we are developing for treatment of equine gastric ulcers in horses. In May 2018, we announced positive results from our pilot field effectiveness study of KIND-014 for the treatment of gastric ulcers in horses. This study was a randomized, single-blind, placebo-controlled, dose-ranging study that enrolled 53 horses (40 horses in three KIND-014 groups with different doses and dosing schedules, 13 horses in the placebo group). The objective was to determine the effective dose of KIND-014 for the treatment of gastric ulcers in horses. At Week 3, the gastric ulcer resolution (gastric ulcer score=0) rates in all three KIND-014 groups were statistically significantly higher than the placebo group (p-values < 0.05). The pivotal field efficacy study for KIND-014 for the treatment of gastric ulcers in horses was initiated in December 2019.
Equine gastric ulcer syndrome (EGUS) is a common condition in horses which encompasses primary and secondary erosive and ulcerative diseases of both the squamous and glandular parts of the stomach. It affects approximately half of all recreational horses, more than 60% of sport horses, and approximately 90% of racing horses. A variety of clinical signs are associated with EGUS, including poor appetite, poor condition, colic, and behavioral issues.
Market Overview
We believe there are significant unmet medical needs for pets, and that the pet therapeutics segment of the animal health industry is likely to grow substantially as new therapeutics are identified, developed and marketed specifically for pets. We plan to commercialize our feline and canine biologics in the United States through commercial partnerships, and in the European Union (the “EU”) primarily through commercial partnerships, distributors and other third parties.
Relative to human drug development, the development of pet therapeutics is generally faster and less expensive, since it requires fewer clinical studies involving fewer subjects and can be conducted directly in the target species. For example, studies that are typically required for approval of human drugs such as QTC studies, which detect cardiac irregularities, elderly patient studies, renal impairment studies, hepatic impairment studies or costly, long-term genotoxicity studies are not required for pet therapeutics. Based on our progress since inception in September 2012, we believe we can develop biologics in around six years at an average cost of approximately $8 million. The lower cost associated with the development of pet therapeutics permits us to pursue multiple product candidates simultaneously and avoid the binary outcome associated with the development of a single lead therapy by some human biotechnology companies. Because our strategy is to identify targets that have already demonstrated safety and efficacy in humans and to develop therapeutics based on these validated targets, we can often advance our programs more rapidly than if we were pursuing unvalidated targets. Biologics could be submitted under USDA, FDA or EMA regulatory authorities for approval.
We estimate that the total U.S. market for veterinary care was approximately $95.7 billion in 2019, of which veterinary care and product sales comprised $29.3 billion. In 2018, 67% of households owned a pet, which translates to an estimated 97.0 million dogs and 76.0 million cats currently living in the United States. We believe there are many unmet or underserved medical needs and that the pet therapeutics portion of the market can grow significantly as new, safe and effective therapeutics are identified, developed and marketed. We expect continued market growth as new pet therapeutics are developed and owners grow more familiar with the treatment of pets with such therapeutics.
The equine sector shares many similarities with the orphan human market. There are fewer horses than dogs or cats, but the willingness to pay is substantially higher. In addition, the cost of building a commercial infrastructure is much less for the equine market. We believe that as few as three to five representatives are sufficient to launch and support multiple equine products nationally, in conjunction with distribution.
Management Team
Our small molecule approvals at both the FDA and EMA jurisdiction and the progress of the biologicals portfolio are testament to the management team’s extensive experience in both human and animal drug development.
Richard Chin, M.D., our Chief Executive Officer, was previously Head of Clinical Research for the Biotherapeutics Unit at Genentech, Inc., where he oversaw Phase I through Phase IV clinical programs for all products except oncology. Denise Bevers, our President and Chief Operating Officer, has over 20 years of experience in clinical operations and medical affairs. She previously held leadership positions at Elan Pharmaceuticals, Scripps Clinic and Research Foundation, Quintiles, and SkyePharma. Wendy Wee, our Chief Financial Officer, has over 30 years of experience and most recently was Vice President of Finance and Principal Accounting Officer at Telik, Inc. Hangjun Zhan, Ph.D., our Chief Scientific Officer, is a well-established protein biochemist and biophysicist with over 20 years of drug discovery experience in the biotechnology industry.
Product Pipeline
As of March 16, 2020, our product pipeline is focused on innovative biologics for dogs and cats.
The US Department of Agriculture’s (the “USDA”) Center for Veterinary Biologics and the FDA’s Center for Veterinary Medicine have a memorandum of understanding under which animal products are to be regulated by the USDA as biologics, if they are intended for use to diagnose, cure, mitigate, treat, or prevent disease in animals and they work primarily through an immune process, or by the FDA as drugs, if they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of animal disease if the primary mechanism of action is not immunological or is undefined. Although we believe that most of our current animal biologics will be regulated by the USDA based on their mechanisms of action, certain of our animal biologics will be regulated by the FDA instead of the USDA.
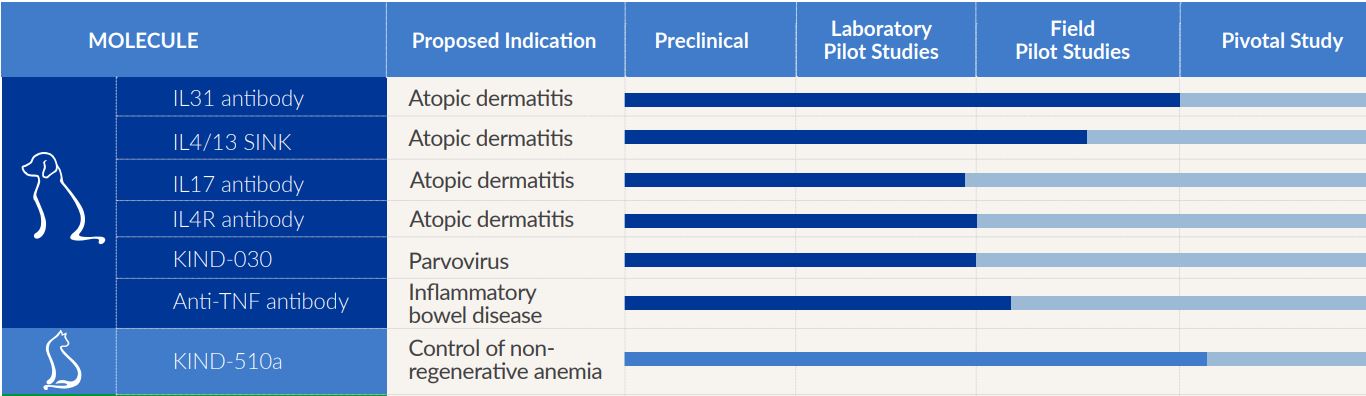
We are currently pursuing over 20 indications with a focus on biologics product candidates. The following table illustrates some of the product candidates that we are developing.
Not all programs are listed. Some are not disclosed for competitive reasons.
Product Selection and Development
We utilize a rigorous screening and review process to identify targets that have demonstrated safety and efficacy in humans and address unmet medical needs in veterinary medicine. In some cases, we identify a chemical or functional equivalent of a validated human drug that addresses the same biological target or pathway. We review these targets with a view to differentiating them from existing treatments, including human products used extra-label in animals, based on ease of administration, method of delivery, dosing regimen, and other similar factors.
Biologic therapies are typically derived from living organisms. A biologic can be defined as a large complex molecule (nucleic acid and protein platforms) produced from or extracted from a biological or living system. They are made by genetically engineering living cells, and a high level of precision is required in the manufacturing process to produce a consistent biologic product each time. A biologic product can be a monoclonal antibody, a vaccine, a tissue, or various proteins such as cytokines, enzymes, fusion proteins, whole cells, and viral and non-viral gene therapies. Our biologic product candidates are usually based on therapies and targets for which products have been successfully commercialized for humans. Human antibody therapies are expensive and are often ineffective in other species since they are usually immunogenic or recognized as foreign bodies and rejected by the immune systems of dogs, cats, horses, and other animals. We identify or create biologics, including antibodies, that are fully or mostly canine, feline, or equine. We generally intend to seek composition-of-matter and other patents for these new biologics.
In January 2020 we announced that we developed a technology to extend canine antibody half-life by up to three-fold. A patent application for this technology has been filed.
The study comprised 12 dogs, including four groups with various modifications incorporating KindredBio technology and one wild type canine antibody as control. Half-life extension was observed in all dogs across all
groups other than the wild type, with the magnitude of extension over native antibody ranging from two to three-fold. Additional studies to further differentiate between lead molecules and expand sample size are planned for 2020.
Half-life extension technologies have the potential to improve therapeutic performance in numerous ways. Reduced dosing frequency and/or amount of dosing can lead to improved patient convenience and compliance. It can also substantially lower the cost of goods and enhance profitability and market positioning. In addition, higher drug concentration using the same dose and dosing interval as the parent antibody can result in extended drug exposure and potentially improved efficacy.
In addition, KIND-Bodies, a unique biologics scaffold with certain advantages over traditional monoclonal antibodies, including bi-specific binding, is under development. We have also developed Fc engineering technologies that can improve affinity of canine antibodies to protein A, which is important for manufacturing of antibodies, and other technologies to modify the immune function of antibodies, and we have filed for IP on those technologies.
We have an in-house laboratory capable of protein engineering, cell line development, analytics, and other activities necessary for advancing a world-class biologics pipeline. We believe that we have one of the best biologics teams in the pharmaceutical industry, drawn from some of the top biotechnology companies.
We have constructed a state-of-the-art manufacturing plant in Burlingame, California for our initial biologic product candidates, which we believe is one of the first GMP biologics plants for veterinary products. We started GMP manufacturing in January 2018 and believe that the plant will position us as a leader in the veterinary biologics field, and potentially afford us an advantage in cost of goods for our products. We acquired a second manufacturing plant in August 2017 in Elwood, Kansas and construction to support initial production lines on our biologics manufacturing is complete. The bioreactors and fill & finish equipment are installed and fully commissioned. The Elwood facility includes approximately 180,000 square feet with clean rooms, utility, equipment, and related quality documentation suitable for small molecule and biologics manufacturing.
Our biologic product candidates are not expected to face generic competition in the United States as there is no pathway for approval of a generic veterinary biologic regulated by the USDA.
Business Strategy
Key elements of our business strategy are as follows:
Continue to focus on the development of our pipeline
In addition to our focus on patient enrollment for our pivotal field study of KIND-510a and KIND-014, we expect to initiate pivotal field studies for KIND-030 and KIND-016 in 2020. We are currently developing KIND-509 for inflammatory bowel disease in dogs. Additionally, we are also developing multiple other products. In all, we have over 20 programs for various indications.
Continue to focus on cost-effective research and development execution
In order to execute our studies rapidly and efficiently, we have built an experienced team drawn from both the veterinary and human pharmaceutical industries. We rely primarily on our own personnel or independent contractors, rather than on contract research organizations (“CROs”), for many business-critical tasks, including protocol designs, regulatory interactions, statistics, data management and clinical operations. By doing so, we believe we can maintain higher quality, achieve lower costs and seek regulatory approval more quickly. Since our inception in September 2012, we have been able to quickly and efficiently build and advance our pipeline.
Leverage our antibody and biologics experience
Members of our team have extensive experience developing biologics such as antibodies. We are leveraging their expertise to identify and develop antibody-based therapies for pets based on approved human therapies, and to identify appropriate manufacturing technologies for these product candidates.
Leverage our current product pipeline in additional animal species
We intend to develop our product candidates primarily for approval in one or more indications in dogs, cats, and horses. We may consider the development of our current or future product candidates for additional species in the future, but our pipeline currently is focused on dogs, cats and horses only.
Expand our pipeline with additional product candidates
We actively seek to identify biologic therapeutics, or in some cases therapeutic targets, that have demonstrated safety and efficacy in humans, focusing on small molecules that are already marketed for humans or biologics for which there are no animal counterparts. These therapeutics typically have been tested in animals such as dogs as part of standard toxicology studies in human clinical development. We have identified a number of additional product candidates in the pre-INAD stage that we may potentially pursue. We will seek to protect our product candidates through a combination of patents, know-how and other customary means. Importantly, there is no biosimilar pathway for biologics.
Commercialize our canine and feline products through commercial partners in the United States and other regions
As disclosed in our March 16, 2020 press release, KindredBio is transitioning to a partnership-focused commercialization model whereby pipeline assets are out-licensed to larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. Accordingly, the companion animal commercial infrastructure sill be substantially reduced.
Commercialize our equine products with a small direct sales force in the United States and with partners in other regions
We intend to utilize a small equine-specific direct sales force to commercialize Zimeta and future equine products. In addition, select distributor relationships will be used to market equine products directly to veterinarians in the United States. For our equine products, we believe we can accomplish this with a sales force of 3 to 5 sales representatives, once there are multiple products, and reach most of the prescribing equine veterinarians in the United States. We also intend to establish collaborations with distributors or licensing partners to commercialize any of our products that may be approved by the EMA. As noted in our press release dated March 16, 2020, a strategic review process is underway for KindredBio Equine, which may result in a spin-out or divestiture of assets.
Pet Therapeutics Market
Overview
U.S. consumers spent an estimated $95.7 billion on their pets in 2019, according to the American Pet Products Association (APPA). The veterinary care segment has been among the fastest growing segments of the overall U.S. pet market. This segment accounted for an estimated $29.3 billion spent on veterinary care in 2019.
We believe several factors will contribute to an increase in spending on pet therapeutics. Pets are generally living longer, with the average lifespan for dogs increasing by nearly a year to 12 years and 13.1 years for cats between 2012 and 2016 according to a study by Banfield Pet Hospitals. As a result, pets are increasingly exhibiting many of the same diseases associated with aging in humans such as cardiovascular disease, arthritis, and diabetes. For example, the incidence of diabetes in dogs has increased by 79.7% since 2006, while in felines, the prevalence of diabetes in cats has increased 18.1% over the same timeframe. The incidence of osteoarthritis in dogs has increased by 82% since 2006 according to the same study. As it is with human health, obesity is a growing concern for pets. The Association for Pet Obesity Prevention estimates that in 2017, 56% of dogs and 60% of cats are overweight or obese, which translates to 50.2 million dogs and 65.5 million cats. According to a 2016 study, in the
past 10 years Banfield Pet Hospitals witnessed a 169 percent increase in overweight cats and a 158 percent increase in overweight dogs. Banfield further reports that obesity in cats and dogs has been linked to more than 20 ailments. Not surprisingly then, Banfield’s records indicate dog owners spend 17% more on healthcare costs and nearly 25% more on medications versus owners of healthy-weight dogs. In addition, pet ownership numbers may increase as more people become aware of the myriad health benefits of pet ownership. According to the Human Animal Bond Research Institute, studies show that some of the benefits of having a dog include helping to lower your blood pressure, decrease your risk of heart disease, and preventing allergies in children.
Among pet owners, there is growing familiarity in treating these pet diseases with medications. According to the APPA, approximately 77% of U.S. dog owners treated their dogs with medications in 2015, an increase of over 50% from the level reported in 2004. In a 2010 poll by the Associated Press, 35% of pet owners are willing to spend $2,000 to treat their pet for a serious medical condition. More recently, a 2017 Harris Poll by the American Institute of Certified Public Accountants indicated that 76% of the U.S. adults (1,004, of which 526 identified as pet owners) surveyed would make financial sacrifices for their pets to pay for an emergency expense such as medical care. Additionally, 79% said they would stop eating at restaurants and 67% would give up a vacation to pay for pet-related expenses if they were in a difficult financial situation. Respondents also indicated that they would cancel cable and TV streaming services (61%), sacrifice contributions to their retirement account (37%), cancel a cell phone plan (35%), or forego paying a credit card bill (27%) to pay for their pet’s expenses. We expect pet owners to spend more on their pets’ health and welfare as new therapeutics are developed specifically for pets, particularly as 95% of pet owners considered their pet to be a member of their family, according to a 2015 survey by the Harris Poll of Harris Interactive.
Pet Therapeutics Market Dynamics
The respective businesses of developing and commercializing therapeutics for pets and for humans share a number of characteristics, including the need to demonstrate safety and efficacy in clinical trials, obtain FDA or other regulatory approval for marketing, manufacture the therapeutics in facilities compliant with GMP requirements and market the therapeutics only for their intended indication based on claims permitted in the product label, and not for other uses, which is referred to as extra-label use.
Despite their similarities, there are a number of important differences between the pet therapeutics and human therapeutics businesses, including:
| |
| • | Faster, less expensive and more predictable development. The development of pet therapeutics requires fewer clinical studies in fewer subject animals than the development of human therapeutics and, unlike human therapeutics, is conducted directly in the target animals. We believe our strategy of selecting compounds and targets with demonstrated efficacy and safety in humans enhances the predictability of results and probability of success of our pivotal trials relative to compounds and targets that have not been previously validated. |
| |
| • | Role and incentives for veterinary practices. In the United States, veterinarians generally serve the dual role of doctor and pharmacist, and pet owners typically purchase medicines directly from their veterinarians. Therapeutics specifically developed for pets enable veterinarians to provide potentially superior treatment options, while also increasing revenue from the sale of these therapeutics. |
| |
| • | Primarily private-pay nature of veterinary market. Pet owners in the United States generally pay for pet therapeutics out-of-pocket, and 10% of dog owners and 5% of cat owners have health insurance for their pets. As a result, pet owners must make decisions primarily on their veterinarians’ advice regarding available treatment options, rather than on the treatment options’ eligibility for reimbursement by insurance companies or government payers. We believe this results in less pricing pressure than in human healthcare, although the limited adoption of insurance may also reduce pet owners’ ability to pay for therapeutics recommended by their veterinarians. |
| |
| • | Less generic competition and strong brand loyalty. There is less generic competition in the pet therapeutics industry than in the human healthcare industry. Approximately 14% of veterinary drugs face generic competition, and the percentage of generic prescriptions in the veterinary space is only 7% as compared to approximately 81% for human drugs. For example, Rimadyl, the leading U.S. pet NSAID, |
lost regulatory exclusivity in 2001, but its sales continued to grow since generic competition was introduced in 2005. We believe that stronger brand loyalty and lack of mandatory generic drug substitution, as in human pharmaceuticals, partially explains the low penetration of generics in veterinary medicine.
Unmet Medical Needs in the Pet Therapeutics Market
Despite the growing market for pet therapeutics, there are relatively few treatment options approved for use in pets as compared to human therapeutic treatments. As a result, veterinarians often must resort to prescribing products approved for use in humans but not approved, formulated or even formally studied in pets. Veterinarians must then rely upon trial and error or untested rules of thumb to assess the proper dosage needed to be effective in the particular species without undue risk of side effects. The veterinarian must also find a way to administer the human product in animals and determine the appropriate dose to treat the disease in the species, which are important and potentially overlooked practical considerations in the treatment of pets.
Even in disease categories with approved pet therapeutics, significant unmet medical needs remain. For example, the NSAID class of products, commonly prescribed for pain, have potentially serious side effects in dogs that limit their long-term use and may require ongoing monitoring by veterinarians. The treatment of pain in cats is further complicated as a result of their differing biology, which makes NSAIDs toxic.
Animal health companies have been relatively slow to develop new therapeutics for pets and have tended to focus primarily on the larger market for the treatment of livestock and other farm animals. In 2018 in the United States, for every 19 NDAs approved for human therapeutics, one NADA was approved for animal therapeutics. In 2019 Zimeta was the only approved FDA product for horses. Human pharmaceutical companies received FDA approval for 59 novel drugs (non-generic), while pet therapeutic companies received only three novel FDA drug approvals. In the EU, human pharmaceutical companies received EMA approval for 42 novel drugs in 2018, compared to only 3 novel drug approvals for pet therapeutic companies.
We believe that therapeutics specifically developed for pets can extend and improve pets’ quality of life, help veterinarians achieve improved medical outcomes and make the process of administering therapeutics to pets much more convenient. Advances in human medicines have created new therapeutics for managing chronic diseases associated with aging, such as osteoarthritis, cancer, diabetes and cardiovascular diseases. Pets often suffer from the same disease as humans, including diabetes, arthritis, cancer, Alzheimer’s disease (canine cognitive dysfunction), lupus, Crohn’s disease, Lou Gehrig’s disease (degenerative myelopathy) and others. In most cases, the biologies of the diseases in pets are very similar to those in humans. Because of the similarity of the diseases, many human drugs, when formulated properly and administered in proper doses, are effective in pets. However, most human drugs are neither formulated nor approved for animals.
Commercialization
As stated in our press release dated March 16, 2020, we are substantially reducing our companion animal commercial infrastructure and relaying primarily on a partnership-focused commercialization model, whereby pipeline assets are out-licensed to larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. We believe this strategy will maximize the value of our pipeline, generate attractive commercial terms, and rely less on dilutive capital.
According to industry sources, approximately one-third of pet veterinary practice revenue comes from prescription drug sales, vaccinations and non-prescription medicines. We believe veterinarians are self-motivated to prescribe innovative therapeutics that are safe, effective, and supported by reliable clinical data and regulatory approval in order to improve the health of pets, while also generating additional revenue.
Manufacturing
For biologics, we have established our own GMP manufacturing capabilities in Burlingame, California and proceeded to GMP manufacturing in January 2018. In August 2017, we acquired a manufacturing facility in Elwood, Kansas and have completed construction and commissioning. The Elwood facility includes approximately
180,000 square feet with clean rooms, utility, equipment, and related quality documentation suitable for biologics and small molecule manufacturing. The USDA regulates the manufacture of pet biologics under standards that are less stringent than those for human biologics, which may reduce the cost of goods of our biologic product candidates relative to human biologics.
Competition
While there are fewer competitors in the pet therapeutics industry than in the human pharmaceutical industry, the development and commercialization of new animal health medicines is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies.
Our potential competitors include large animal health companies, which currently derive the majority of their revenue from livestock medications. For example, in 2019 livestock accounted for 48%, and pets 50%, of sales for Zoetis, a large company focused on animal health. Within the pet therapeutics market, vaccines and parasiticides are currently the greatest sources of revenue.
Large animal health companies include Merck Animal Health, the animal health division of Merck & Co., Inc.; Boehringer Ingelheim Animal Health, the animal health division of Boehringer Ingelheim GmbH; Elanco Animal Health Incorporated; and Zoetis, Inc. We will also compete against several animal health companies, such as the Virbac Group, Ceva Animal Health and Dechra Pharmaceuticals PLC. We are also aware of smaller companies that are developing products for use in the pet therapeutics market, including Zomedica, Scout Bio, and Anivive Lifesciences. Zoetis and Elanco both recently announced collaborations with Colorado State University and Purdue University, respectively.
At the product level, we will face competition for Zimeta from Flunixin and phenylbutazone, even though they are not approved for control of fever in horses. In addition, we may face competition from various products including additional products in development. Our products may also face competition from generic medicines and products approved for use in humans that are used extra-label for pets. Some of our other products also may face competition from their human generic equivalents in countries where such equivalents are available.
Many of our competitors and potential competitors have substantially more financial, technical and human resources than we do. Many also have far more experience than we have in the development, manufacture, regulation and worldwide commercialization of animal health medicines, including pet therapeutics. In addition, these and other potential competing products may benefit from greater brand recognition and brand loyalty than any that our product candidates may achieve. Accordingly, there is no assurance that we and our products can compete effectively.
Intellectual Property
We intend to rely primarily upon a combination of regulatory exclusivity, patents, trade secret protection, proprietary know-how, license agreements, and confidentiality agreements to protect our product formulations, biologics, processes, therapeutic methods and other technologies, and to operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. We currently have numerous provisional, nonprovisional, and international patent applications pending, and two issued patents, for our IL-31 antibody compositions-of-matter and corresponding methods of use. Because our first approved product and our other small molecule product candidates are based on generic human drugs, there is little, if any, composition-of-matter patent protection available for the API in such product candidates. We have filed patent applications on many of our biologic products. However, even intellectual property protection, if available to us, may not afford us with complete protection against competitors. See “Risk Factors-Risks Related to Intellectual Property.”
We depend upon the skills, knowledge and experience of our management personnel, as well as that of our other employees, advisors, consultants and contractors, none of which are patentable. To help protect our know-how, and any inventions for which patents may be difficult to obtain or enforce, we require all of our employees, consultants, advisors and other contractors to enter into customary confidentiality and inventions agreements that
prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business.
Regulatory
The development, approval and sale of animal health products are governed by the laws and regulations of each country in which we intend to sell our products. To comply with these regulatory requirements, we have established processes and resources to provide oversight of the development and launch of our products and their maintenance in the market.
United States
Three federal regulatory agencies regulate the health aspects of animal health products in the United States: the FDA; the USDA; and the Environmental Protection Agency (the “EPA”). In addition, the Drug Enforcement Administration (the “DEA”) regulates animal therapeutics that are classified as controlled substances.
The FDA Center for Veterinary Medicine (the “CVM”), regulates animal pharmaceuticals under the Federal Food, Drug and Cosmetic Act. The USDA Center for Veterinary Biologics (the “CVB”), regulates veterinary vaccines and certain biologics pursuant to the Virus, Serum, Toxin Act. The EPA Office of Pesticide Programs (the “OPP”) regulates veterinary pesticides under the Federal Insecticide, Fungicide and Rodenticide Act. Many topical products used for treatment of flea and tick infestations are regulated by the EPA.
All of our current product candidates are animal pharmaceuticals or biologics regulated by the CVM or the CVB, respectively. Manufacturers of animal health pharmaceuticals and biologics, including us, must show their products to be safe, effective and produced by a consistent method of manufacture. We are also required to conduct post-approval monitoring of products and to submit reports of product quality defects, adverse events or unexpected results, and are subject to regulatory inspection from time to time. In addition, for our controlled substance product candidates, we are required to comply with the Controlled Substances Act (the “CSA”) and related state laws regarding manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal.
Requirements for Approval of Veterinary Pharmaceuticals for Pets
As a condition to regulatory approval for the sale of animal products, regulatory agencies worldwide generally require that a product to be used for pets be demonstrated to:
| |
| • | be safe for the intended use in the intended species; |
| |
| • | have substantial evidence of effectiveness for the intended use; |
| |
| • | have a defined manufacturing process that ensures the product can be made with high quality consistency; and |
| |
| • | be safe for humans handling the product and for the environment. |
Safety. To determine that a new veterinary drug is safe for use, most regulatory authorities will require us to provide data from a safety study generated in laboratory cats, dogs, and horses tested at doses higher than the intended label dose, over a period of time determined by the intended length of dosing of the product. In the case of the FDA, the design and review of the safety study and the study protocol can be completed prior to initiation of the study to help assure that the data generated will meet FDA requirements. These studies are conducted under rigorous quality control, including GLP, to assure integrity of the data. They are designed to clearly define a safety margin, identify any potential safety concerns, and establish a safe dose for the product. In addition, safety data from pivotal field studies conducted under GCP standards are evaluated to assure that the product will be safe in the target population. Furthermore, because safety and effectiveness studies must conform to VICH guidelines, which are established under an international program aimed at harmonizing technical requirements for veterinary product registration, they can be utilized by regulatory bodies in the European Union, Japan, Canada, New Zealand and Australia.
Effectiveness. Early pilot studies may be conducted in laboratory cats, dogs, or horses to establish effectiveness and the dose range for each product. Data on how well the drug is absorbed when dosed by different routes of administration and the relationship of the dose to the effectiveness are studied. When an effective dose is established, a study protocol to test the product in real world conditions is developed prior to beginning the study. In the case of the FDA, the pivotal effectiveness field study protocol can be submitted for review and concurrence prior to study initiation, to help assure that the data generated will meet requirements.
The pivotal field effectiveness study must be conducted with the formulation of the product that is intended to be commercialized, and is a multi-site, randomized, controlled study, generally with a placebo control. To reduce bias in the study, individuals doing the assessment are not told whether the subject is in the group receiving the treatment being tested or the placebo group. In both the United States and the European Union, the number of subjects enrolled in pivotal field effectiveness studies is required to be approximately 100 to 150 animal subjects treated with the test product and a comparable number of subjects in the control group that receive the placebo. In many cases, a pivotal field study may be designed with clinical sites in both the European Union and the United States, and this single study may satisfy regulatory requirements in both jurisdictions.
Chemistry, Manufacturing and Controls ("CMC"). To assure that the product can be manufactured consistently, regulatory agencies will require us to provide documentation of the process by which the API is made and the controls applicable to that process that assure the API and the formulation of the final commercial product meet certain criteria, including quality, purity and stability. After a product is approved, we will be required to communicate with the regulatory bodies any changes in the procedures or manufacturing site. Both API and commercial formulations are required to be manufactured at facilities that practice pharmaceutical GMP.
Environmental and Human Safety. We will not be required under United States law to provide an environmental impact statement for products currently in development if the products are given at the home of the pet’s owner or in a veterinary hospital. If products might result in some type of environmental exposure or release, the environmental impact must be assessed. For approval in the EU, a risk assessment for potential human exposure will be required.
Labeling, All Other Information, and Freedom of Information Summary. We also will be required to submit the intended label for the product, and also any information regarding additional research that has been conducted with the drug, to the CVM and other regulatory bodies for review. We will draft, and submit for regulatory review, the Freedom of Information Summary for use in the United States. This summary outlines the studies and provides substantial information that the FDA uses to assess the drug’s safety and effectiveness and then publishes on its website.
Regulatory Process at the FDA
To begin the development process for products in the United States, we must file an Investigational New Animal Drug (INAD) submission with the FDA. We will then usually hold a pre-development meeting with the FDA to reach a general agreement on the plans for providing the data necessary to fulfill requirements for an NADA. We evaluate if drug candidates can benefit from approval under the minor use minor species (MUMS) or expanded conditional approval programs. During development, we will usually submit pivotal protocols to the FDA for review and concurrence prior to conducting the required studies. We will gather and submit data on manufacturing, safety and effectiveness to the FDA for review, and this review will be conducted according to timelines specified in the Animal Drug User Fee Act. These are called technical sections, which collectively form the basis of the NADA. Once all data have been submitted and reviewed for each technical section - safety, effectiveness and CMC - the FDA will issue us a technical section complete letter as each section review is completed, and when the three letters have been issued, we will compile a draft of the Freedom of Information Summary, the proposed labeling, and all other relevant information, and submit these for FDA review. An administrative NADA is a NADA that is submitted after all of the technical sections that fulfill the requirements for the approval of the new animal drug have been reviewed by the CVM and the CVM has issued a technical section complete letter for each of those technical sections. Although this process is not required and submission of a non-administrative NADA is also acceptable, we plan to take advantage of the administrative NADA process to obtain a more timely, phased review. Because the CVM has already reviewed the individual technical sections before the
administrative NADA is filed, the CVM is committed under its user fee agreements to reviewing and acting on 90% of administrative NADAs within 60 days after submission. The CVM user fee goal is to review and act on 90% of non-administrative NADAs within 180 days after submission. After approval, we will be required to collect reports of adverse events and submit them on a regular basis to the FDA.
Regulatory Process at the USDA
To begin the development process for veterinary biologics products in the United States, we typically file an Application for United States Veterinary Biological Product License with the USDA. For the biologics products that we develop, we may then meet with the USDA to reach a general agreement on the plans for providing the data necessary to fulfill requirements for an approval. During development, we gather and submit data on manufacturing, purity and potency to the USDA for review. Once all data have been submitted and reviewed, the USDA will issue its decision. Unlike the FDA, there are no timelines specified by law for the USDA’s review.
In some cases, it may be unclear whether our product candidates meet the definition of a biological product subject to regulation by the USDA or a drug subject to regulation by the FDA. The USDA’s Center for Veterinary Biologics and the FDA’s Center for Veterinary Medicine have a memorandum of understanding concerning their joint responsibilities for resolving jurisdictional issues over products of this nature. Under the memorandum of understanding, animal products are to be regulated by the USDA as biologics if they are intended for use to diagnose, cure, mitigate, treat, or prevent disease in animals and they work primarily through an immune process, or by the FDA as drugs, if they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of animal disease if the primary mechanism of action is not immunological or is undefined. There is a process to gain a jurisdiction decision.
Regulatory Process at the EMA
The EMA is responsible for coordinating scientific evaluation of applications for marketing approval for pet therapeutics in the EU. Its veterinary review section is distinct from the review section for human drugs. To perform these evaluations the EMA established a specific scientific committee, the Committee for Medicinal Products for Veterinary Use ("CVMP"). The CVMP considers applications submitted by companies for the marketing approval of individual pet therapeutics and evaluates whether or not the medicines meet the necessary quality, safety and efficacy requirements. Assessments conducted by the CVMP are based on scientific criteria and are intended to ensure that pet therapeutics reaching the marketplace have a positive benefit-risk balance in the pet population for which they are intended. Based on the CVMP’s recommendation, a centralized marketing authorization is granted by the EMA, which allows the product to be marketed in any of the EU states, Norway, Lichtenstein and Iceland. The CVMP is also responsible for various post-authorization and maintenance activities, including the assessment of modifications or extensions to an existing marketing authorization.
To obtain authorization from the EMA, we must submit a marketing authorization application called a dossier. The dossier is the EMA’s equivalent of the FDA’s NADA and includes data from studies showing the quality, safety and efficacy of the product. The CVMP reviews and evaluates the dossier. For any dossier, a rapporteur and co-rapporteur are appointed from the members of the CVMP. Their role is to lead the scientific evaluation and prepare the assessment report. The rapporteur can utilize experts to assist it in performing its assessment. The report is critiqued by the co-rapporteur and other members of the CVMP before the CVMP makes its determination. The final opinion of the CVMP is generally given within 210 days of the submission of a dossier, but the EMA makes the final decision on the approval of products. In general, the requirements for regulatory approval of an animal health product in the EU are similar to those in the United States, requiring demonstrated evidence of purity, safety, efficacy and consistency of manufacturing processes.
Alternatively, product approval applications may be submitted directly to the regulatory authority in each country rather than by centralized approval by the EMA.
Regulatory Processes at the DEA
The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may not be marketed or sold in the United States. An animal
drug may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Certain of our product candidates are likely to be scheduled as controlled substances under the CSA. Consequently, their manufacture, shipment, storage, sale and use will be subject to a high degree of regulation.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Required security measures include background checks on employees and physical control of inventory through measures such as cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and periodic reports must be made to the DEA, for example, distribution reports for Schedule II controlled substances, Schedule III substances that are narcotics, and other designated substances. Reports must also be made for thefts or losses of any controlled substance, and to obtain authorization to destroy any controlled substance. In addition, special authorization and notification requirements apply to imports and exports.
In addition, a DEA quota system controls and limits the availability and production of controlled substances in Schedule I or II. Distributions of any Schedule I or II controlled substance must also be accompanied by special order forms, with copies provided to the DEA. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments.
Other Regulatory Considerations
Regulatory rules relating to human food safety, food additives, or drug residues in food will not apply to the products we currently are developing because our products are not intended for use in food animals or food production animals, with the exception of horses, which qualify as food animals in Europe and Canada.
Advertising and promotion of animal health products is controlled by regulations in the United States and other countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and authorized by the applicable agency. We will conduct a review of advertising and promotional material for compliance with local and regional requirements in the markets where we sell pet therapeutics.
While small molecule product drugs may eventually face generic competition in the United States, there is no pathway for approval of a generic veterinary biologic regulated by the USDA.
Employees
As of December 31, 2019, we had 156 employees, including 29 employees with D.V.M., M.D. or Ph.D. degrees. Of our employees, 73, including Dr. Chin and Ms. Bevers, are engaged in one or more aspects of our research and development activities. Dr. Chin and Ms. Bevers also are engaged in corporate and administrative activities. None of our employees are represented by labor unions or covered by collective bargaining agreements.
On March 16, 2020, KindredBio announced a reduction in our workforce of approximately 53 people as we further prioritizes biologics programs for dogs and cats, and substantially reduces its commercial footprint to rely primarily on a partnership focused commercialization model. The eliminated positions primarily relate to the companion animal sales force and research and development for small molecule programs.
Available Information
We file annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements and other documents with the Securities and Exchange Commission (the “SEC”). All of our filings with
the SEC (including documents that we “furnish” with the SEC rather than “file” with the SEC) are available on the SEC’s website at www.sec.gov. Our filings with the SEC are also available free of charge on our website at www.kindredbio.com as soon as reasonably practicable after each document is electronically filed with or furnished to the SEC. The information contained in, or accessible through, our website is not a part of this Annual Report on Form 10-K and is not incorporated by reference into any other filings that we make with the SEC.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information contained in or incorporated by reference into our other public filings with the Securities and Exchange Commission, before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Related to Our Business
We have a limited operating history, are not profitable and may never become profitable.
We are a commercial stage biopharmaceutical company. Since our formation in September 2012 and until June 2018, our operations were limited to the identification of product candidates and research and development of our product candidates, including our lead product candidates, Mirataz and Zimeta. Mirataz became commercially available in July 2018 and Zimeta in December 2019. As a result, we have limited historical operations upon which to evaluate our business and prospects, we have demonstrated an ability to obtain marketing approval for only two of our product candidates and have not yet successfully overcome the risks and uncertainties frequently encountered by companies in emerging fields such as the pet therapeutics industry. We have only generated revenue on Mirataz for six quarters and one month for Zimeta and will continue to incur significant research and development and other expenses. As of December 31, 2019, we had an accumulated deficit of $223.1 million. For the foreseeable future, we expect to continue to incur losses, which will increase significantly from historical levels as we expand our product development activities, seek regulatory approvals for our product candidates and begin to commercialize them if they are approved by the Center for Veterinary Medicine branch of the FDA, the USDA or the EMA. Even if we succeed in developing and commercializing one or more product candidates, we expect to continue to incur losses for the foreseeable future, and we may never become profitable. If we fail to achieve or maintain profitability, it would adversely affect the value of our common stock.
We may need to raise additional capital to achieve our goals.
Our small molecule product candidates will require from three to five years of further development at a cost of an average of approximately $5 million per product candidate, and our biologics candidates will require four to six years of further development at an average cost of approximately $8 million per product candidate before we expect to be able to apply for marketing approval in the United States. We also are actively involved in identifying additional human therapeutics for development and commercialization as pet therapeutics and will continue to expend substantial resources for the foreseeable future to develop our current product candidates and any other product candidates we may develop or acquire. These expenditures will include: costs of identifying additional potential product candidates; costs associated with drug formulation; costs associated with conducting pilot, pivotal, and toxicology studies; costs associated with completing other research and development activities; costs associated with payments to technology licensors and maintaining other intellectual property; costs of obtaining regulatory approvals; costs associated with establishing commercial manufacturing and supply capabilities; and costs associated with marketing and selling any of our products approved for sale, either directly by us or through licensees. We also may incur unanticipated costs. Because the outcome of these activities is inherently uncertain, the actual amounts necessary to successfully complete the development and commercialization of our current or future product candidates may be greater or less than we anticipate.
We believe we have sufficient cash and cash equivalents to fund our operating plan for approximately another 36 months. However, we may seek additional funds through public or private equity or debt financings or other sources such as strategic collaborations. Additionally, we do not expect our existing cash and cash equivalents to be sufficient to complete the development of all of our current product candidates, or of any additional product candidates that we may identify, and we may need to raise additional capital to fund these activities. Even if we believe we have sufficient funds on hand for our current or planned future business and operations, we may seek
from time to time to raise additional capital based upon favorable market conditions or strategic considerations such as potential acquisitions.
Our future capital requirements depend on many factors, including, but not limited to:
| |
| • | the scope, progress, results and costs of researching and developing our current or future product candidates; |
| |
| • | the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates; |
| |
| • | the number and characteristics of the product candidates we pursue; |
| |
| • | the cost of manufacturing our current and future product candidates and any products we successfully commercialize; |
| |
| • | the cost of commercialization activities for equine products and any other products that we elect to sell directly rather than through licensees, including marketing, sales and distribution costs; |
| |
| • | the amount of licensing and royalty payments that we receive with respect to our products that are out-licensed to commercial partners or are sold to other companies; |
| |
| • | the expenses needed to attract and retain skilled personnel; |
| |
| • | the costs associated with being a public company; |
| |
| • | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; and |
| |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing current and future patents, including litigation costs and the outcome of any such litigation. |
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate one or more of our product development programs or any future commercialization efforts.
We are substantially dependent on the success of the product candidates in our pipeline and cannot be certain that any of our product candidates will be approved for marketing or successfully commercialized even if approved.
A substantial portion of our efforts over the foreseeable future will be focused on the long-term commercial success of our product candidates that are in our pipeline. Accordingly, our prospects, including our ability to generate material product revenue, obtain any new financing if needed to fund our business and operations, or enter into potential strategic transactions, will depend heavily on the successful development and commercialization of our lead product candidates, which in turn will depend on a number of factors, including the following:
| |
| • | the successful completion of the pivotal trials and toxicology studies of one or more of our current product candidates, which may take significantly longer than we currently anticipate and will depend, in part, upon the satisfactory performance of third-party contractors; |
| |
| • | our ability to demonstrate to the satisfaction of the FDA, the USDA and the EMA the safety and efficacy of our product candidates and to obtain regulatory approvals; |
| |
| • | our ability or the ability of our third-party manufacturers to manufacture satisfactory supplies of our products and product candidates and to develop, validate and maintain commercially viable |
manufacturing processes that are compliant with GMP and to manufacture them at an acceptable cost as well as the ability to sell them at an acceptable price with reasonable margins;
| |
| • | our ability to successfully launch commercial sales of our current product candidates, assuming marketing approval is obtained, whether through our own efforts or through licensing agreements with commercial partners; |
| |
| • | the effectiveness of the commercialization efforts for our products and product candidates, including the effectiveness of marketing, sales and distribution strategy and operations, whether performed by us or by our commercial partners through licensing agreements. |
| |
| • | the availability, perceived advantages, relative cost, relative safety and relative efficacy of our products and product candidates compared to alternative and competing treatments; |
| |
| • | the acceptance of our products and product candidates as safe and effective by veterinarians, pet owners and the animal health community; |
| |
| • | the prevalence and severity of adverse side effects and our ability to maintain a continued acceptable safety profile of a product following approval; |
| |
| • | our ability to obtain supplemental indications for our products and product candidates; |
| |
| • | any product liability claim or lawsuit we may be involved in from time to time with regards to our products and product candidates; |
| |
| • | our ability to achieve and maintain compliance with all regulatory requirements applicable to our business; and |
| |
| • | our ability to obtain and enforce our intellectual property rights and obtain marketing exclusivity for our products and product candidates, and avoid or prevail in any third-party patent interference, patent infringement claims or administrative patent proceedings initiated by third parties or the U.S. Patent and Trademark Office, or USPTO. |
Many of these factors are beyond our control.
Most of our current and future equine small molecule product candidates are or will be based on generic human drugs, and other companies may develop substantially similar products that may compete with our products.
Most of the equine small molecule product candidates we are currently developing or expect to develop are based on generic human drugs. We do not engage in early-stage research or discovery with respect to these small molecule product candidates, but focus primarily on product candidates whose active pharmaceutical ingredient has been successfully commercialized or demonstrated to be safe and/or effective in human trials, which we sometimes refer to as validated. Our equine small molecule product candidates may face competition from their human generic equivalents in countries where such equivalents are available and used in unapproved animal indications, which is known as extra-label use.
In cases where there is a human generic available there is no assurance that the eventual prices of our products will be lower than or competitive with the prices of human generic equivalents used extra-label, or that a palatable, easy-to-administer formulation such as the chewable, beef-flavored formulation that we utilize will be sufficient to differentiate them from their human equivalents.
In most cases, we target equine small molecule product candidates for which the active ingredients have not been previously approved for use in animals. If we are the first to gain approval for the use of such active ingredients in animals, our small molecule products will enjoy five years of marketing exclusivity in the United States and ten years in the EU for the approved indication. We also plan to differentiate our products where possible with specific formulations, including flavors, methods of administration, and other strategies, but we cannot assure
you that we will be able to prevent competitors from developing substantially similar products and bringing those products to market earlier than we can. In addition, while we expect to have composition of matter patent applications for most of our biologic product candidates, those applications may not ultimately result in granted patents. Although there are no generic regulatory approval pathways for animal biologics in the United States and European Economic Area ("EEA"), our competitors may develop similar biologics that do not infringe any patents we may obtain. For example, competitors may develop different antibodies that bind to the same target as our antibodies. Thus, our competitors may be able to develop and market competing products if they are willing and able to conduct the full set of required studies, file a NADA with the FDA, or Application for United States Veterinary Biological Product License with the USDA, which is also called a Product License Application ("PLA"), and obtain marketing approval. If such competing products achieve regulatory approval and commercialization prior to our product candidates, or if our intellectual property protection and efforts to obtain regulatory exclusivity fail to provide us with exclusive marketing rights for some of our products, then our business and prospects could be materially adversely affected.
If our product candidates are approved, they may face significant competition and may be unable to compete effectively.
The development and commercialization of pet therapeutics is highly competitive and our success will depend on our ability to compete effectively with other products in the market. We expect to compete with animal health divisions of major pharmaceutical and biotechnology companies such as Merck Animal Health, Zoetis, Elanco, and Boehringer Ingelheim Animal Health, as well as specialty animal health medicines companies such as Virbac Group, Ceva Animal Health and Dechra Pharmaceuticals PLC. Additionally, we are aware of smaller companies that are developing products for use in the pet therapeutics market, including Zomedica, Scout Bio, and Anivive Lifesciences. Zoetis and Elanco both recently announced collaborations with Colorado State University and Purdue University, respectively. We also expect to compete with academic institutions, governmental agencies and private organizations that are conducting research in the field of animal health medicines.
Many of our competitors and potential competitors have substantially more financial, technical and human resources than we do. Many also have far more experience than we have in the development, manufacture, regulation and worldwide commercialization of animal health medicines, including pet therapeutics.
For these reasons, there is no assurance that we and any of our approved products can compete effectively.
The development of our biologic product candidates is dependent upon relatively novel technologies and uncertain regulatory pathways.
We have developed and plan to continue to develop biologics, including animal antibodies, for pets. Identification, optimization, and manufacture of therapeutic animal biologics is a relatively new field in which unanticipated difficulties or challenges could arise, and we expect the discovery, development, manufacturing and sale of biologic products to be a long, expensive and uncertain process. While many biologics have been approved for use in humans, apart from vaccines, relatively few recombinant proteins or antibodies have been approved for use in animals. There are unique risks and uncertainties with biologics, the development, manufacturing, and sale of which are subject to regulations that are often more complex and extensive than the regulations applicable to other small molecule products. We may be unable to identify biologics suitable for development or to achieve the potency and stability required for use in pets. In particular, canine and feline antibodies represent new types of product candidates that may be difficult to develop successfully.
In some cases, it may be unclear whether our product candidates meet the definition of a biological product subject to regulation by the USDA or a drug subject to regulation by the FDA. The USDA’s Center for Veterinary Biologics and the FDA’s Center for Veterinary Medicine have a memorandum of understanding concerning their joint responsibilities for resolving jurisdictional issues over products of this nature. Under the memorandum of understanding, animal products are to be regulated by the USDA as biologics, if they are intended for use to diagnose, cure, mitigate, treat, or prevent disease in animals and they work primarily through an immune process, or by the FDA as drugs, if they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of animal disease if the primary mechanism of action is not immunological or is undefined.
Although we believe that most of our current animal biologics will be regulated by the USDA based on their mechanisms of action, the USDA and the FDA may not agree with our assessment, or disputes may arise between the USDA and the FDA over regulatory jurisdiction for one or more of such biologics. If so, the development of our biologics may be delayed while any such disputes are adjudicated by the agencies. Furthermore, if the agencies were to determine that one or more of our animal biologics will be regulated by the FDA instead of the USDA, the time and cost of developing such biologics may be longer and more expensive than we currently anticipate, and we may determine to discontinue development of such biologics. It is also possible that the USDA’s regulatory standards for novel biologics may be more difficult to satisfy than we anticipate. The current trend indicates that the FDA is attempting to exert jurisdiction over more biologics.
We believe that some veterinarians prefer to see further efficacy data before making a new biologic product prescribing decision. Accordingly, we may also find it necessary to conduct additional studies of our biologic product candidates in order to achieve commercial success.
The results of earlier studies may not be predictive of the results of our pivotal trials, and we may be unable to obtain regulatory approval for our existing or future product candidates under applicable regulatory requirements. The denial or delay of any regulatory approval would prevent or delay our commercialization efforts and adversely affect our potential to generate material product revenue and our financial condition and results of operations.
The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of pet therapeutics are subject to extensive regulation. We are usually not permitted to market our products in the United States until we receive approval of an NADA from the FDA or a PLA from the USDA, or in the EU or in other EEA countries until we receive marketing approval from the EMA. To gain approval to market a pet therapeutic for a particular species, we must provide the FDA, the USDA and the EMA, as applicable, with efficacy data from pivotal trials that adequately demonstrate that our product candidates are safe and effective in the target species (e.g., dogs, cats or horses) for the intended indications. In addition, we must provide manufacturing data. For the FDA and EMA, we must provide data from toxicology studies, also called target animal safety studies, and in some cases environmental impact data. We are conducting the pivotal trial of our compounds internally without significant outsourcing, but we rely on contract research organizations ("CROs") and other third parties to conduct our toxicology studies and for certain other development activities. The results of toxicology studies and other initial development activities, and of any previous studies in humans or animals conducted by us or third parties, may not be predictive of future results of pivotal trials or other future studies, and failure can occur at any time during the conduct of pivotal trials and other development activities by us or our CROs. Our pivotal trial may fail to show the desired safety or efficacy of our product candidates despite promising initial data or the results in previous human or animal studies conducted by others, and success of a product candidate in prior animal studies, or in the treatment of human beings, does not ensure success in subsequent studies. Clinical trials in humans and pivotal trials in animals sometimes fail to show a benefit even for drugs that are effective, because of statistical limitations in the design of the trials or other statistical anomalies. Therefore, even if our studies and other development activities are completed as planned, the results may not be sufficient to obtain regulatory approval for our product candidates.
The FDA, USDA or EMA can delay, limit or deny approval of any of our product candidates for many reasons, including:
| |
| • | if the FDA, USDA or EMA disagrees with our interpretation of data from our pivotal studies or other development efforts; |
| |
| • | if we are unable to demonstrate to the satisfaction of the FDA, USDA or EMA that the product candidate is safe and effective for the target indication; |
| |
| • | if the FDA, USDA or EMA requires additional studies or changes its approval policies or regulations; |
| |
| • | if the FDA, USDA or EMA does not approve of the formulation, labeling or the specifications of our current and future product candidates; and |
| |
| • | if the FDA, USDA or EMA fails to approve the manufacturing processes of our third-party contract manufacturers. |
Further, even if we receive approval of our product candidates, such approval may be for a more limited indication than we originally requested, and the FDA, USDA or EMA may not approve the labeling that we believe is necessary or desirable for the successful commercialization of our product candidates.
Any delay or failure in obtaining applicable regulatory approval for the intended indications of our product candidates would delay or prevent commercialization of such product candidates and would materially adversely impact our business and prospects.
Our Protocol Concurrences with the FDA for our pivotal studies do not guarantee marketing approval in the United States.
We may conduct pivotal trials under Protocol Concurrences with the FDA. A Protocol Concurrence in animal drug development is analogous to a Special Protocol Assessment in human drug development, and means that the FDA agrees that the design and analyses proposed in a protocol are acceptable to support regulatory approval of the product candidate with respect to effectiveness of the indication studied and will not change its view of these matters, unless public or animal health concerns arise that were not recognized at the time of protocol concurrence or we change the protocol. Even under a Protocol Concurrence, approval of a NADA by the FDA is not guaranteed, because a final determination that the agreed-upon protocol satisfies a specific objective, such as the demonstration of efficacy, or supports an approval decision, will be based on a complete review of all the data submitted to the FDA.
Development of pet therapeutics is inherently expensive, time-consuming and uncertain, and any delay or discontinuance of our current or future pivotal trials would significantly harm our business and prospects.
Development of pet therapeutics remains an inherently lengthy, expensive and uncertain process, and there is no assurance that our development activities will be successful. We do not know whether the trials of our current or future product candidates will begin or conclude on time, and they may be delayed or discontinued for a variety of reasons, including if we are unable to:
| |
| • | address any safety concerns that arise during the course of the studies; |
| |
| • | complete the studies due to deviations from the study protocols or the occurrence of adverse events; |
| |
| • | address any conflicts with new or existing laws or regulations; or |
| |
| • | reach agreement on acceptable terms with study sites, which can be subject to extensive negotiation and may vary significantly among different sites. |
Any delays in completing our development efforts will increase our costs, delay our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenue. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, factors that may cause a delay in the commencement or completion of our development efforts may also ultimately lead to the denial of regulatory approval of our product candidates which, as described above, would materially, adversely impact our business and prospects.
We currently rely on third parties to conduct some of our development activities and may rely more heavily on such third parties in the future. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize our current or future product candidates as planned.
We currently plan to conduct our own pivotal trials, but we rely upon CROs to conduct our toxicology studies and for other development activities. We also may rely on CROs in the future to conduct one or more pivotal trials. These CROs are not our employees, and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they devote to our programs or manage the risks associated with their activities on our behalf. We are responsible to regulatory authorities for ensuring that each of our studies is conducted in accordance with the development plans and trial protocols, and any failure by our CROs to do so may adversely affect our ability to obtain regulatory approvals, subject us to penalties, or harm our credibility with regulators. The FDA and foreign regulatory authorities also require us and our CROs to comply with regulations and standards, commonly referred to as good clinical practices ("GCPs"), or good laboratory practices ("GLPs"), for conducting, monitoring, recording and reporting the results of our studies to ensure that the data and results are scientifically credible and accurate.
Our agreements with CROs may allow termination by the CROs in certain circumstances with little or no advance notice to us. These agreements generally will require our CROs to reasonably cooperate with us at our expense for an orderly winding down of the CROs’ services under the agreements. If the CROs conducting our studies do not comply with their contractual duties or obligations to us, or if they experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our development protocols or GCPs or for any other reason, we may need to secure new arrangements with alternative CROs, which could be difficult and costly. In such event, our studies also may need to be extended, delayed or terminated as a result, or may need to be repeated. If any of the foregoing were to occur, regulatory approval and commercialization of our product candidates may be delayed and we may be required to expend substantial additional resources.
Even if we obtain regulatory approval of one or more of our current or future product candidates, they may never achieve market acceptance or commercial success.
If we obtain FDA, USDA or EMA approvals for one or more of our current or future product candidates, they may not achieve market acceptance among veterinarians and pet owners and may not be commercially successful. Market acceptance of any of our current or future product candidates for which we may receive approval depends on a number of factors, including:
| |
| • | the indications for which our products are approved; |
| |
| • | the potential and perceived advantages of our product candidates over alternative treatments, including generic medicines and competing products currently prescribed by veterinarians, and products approved for use in humans that are used extra-label in animals; |
| |
| • | the cost of treatment in relation to alternative treatments and willingness on the part of veterinarians and pet owners to pay for our products, including other discretionary items, especially during economically challenging times; |
| |
| • | the prevalence and severity of any adverse side effects of our products; |
| |
| • | the relative convenience and ease of administration of our products; |
| |
| • | the effectiveness of our sales and marketing efforts; and |
| |
| • | the proper training and administration of our products by veterinarians and acceptance by veterinarians and pet owners of our products as safe and effective. |
Any failure by our product candidates that obtain regulatory approval to achieve market acceptance or commercial success would adversely affect our financial condition and results of operations.
Pet therapeutics, like human therapeutics, are subject to unanticipated post-approval safety or efficacy concerns, which may harm our business and reputation.
The success of our commercialization efforts will depend upon the perceived safety and effectiveness of pet therapeutics, in general, and of our products, in particular. Unanticipated safety or efficacy concerns can arise with respect to approved pet therapeutics after they enter into commerce, which may result in product recalls or withdrawals or suspension of sales, as well as product liability and other claims. Because reliable detection of rare events might require exposure of millions of animals, it is not possible to rule out the risk until well after the launch of the product.
It is also possible that the occurrence of significant adverse side effects in approved human generic compounds upon which our product candidates are based could impact our products. Any safety or efficacy concerns, or recalls, withdrawals or suspensions of sales of our products or other pet therapeutics, or of their human equivalents, could harm our reputation, in particular, or pet therapeutics, generally, and materially, adversely affect our business and prospects or the potential growth of the pet therapeutics industry, regardless of whether such concerns or actions are justified.
Future federal and state legislation may result in increased exposure to product liability claims, which could result in substantial losses to us.
Under current federal and state laws, pets are generally considered to be personal property of their pet owners and, as such, pet owners’ recovery for product liability claims involving their pets may be limited to the replacement value of the pets. Pet owners and their advocates, however, have filed lawsuits from time to time seeking non-economic damages such as pain and suffering and emotional distress for harm to their pets based on theories applicable to personal injuries to humans. If new legislation is passed to allow recovery for such non-economic damages, or if precedents are set allowing for such recovery, we could be exposed to increased product liability claims that could result in substantial losses to us if successful. In addition, some horses can be worth millions of dollars or more, and product liability for horses may be very high.
It is also possible that our product liability insurance will not be sufficient to cover any future product liability claims against us.
If we fail to retain current members of our senior management, or to attract and keep additional key personnel, our business and prospects could be materially adversely impacted.
Our success depends on our continued ability to attract, retain and motivate highly qualified management and scientific personnel. We are highly dependent upon our senior management, particularly Richard Chin, M.D., our Chief Executive Officer, Denise Bevers, our President and Chief Operating Officer, Wendy Wee, our Chief Financial Officer and Hangjun Zhan, Ph.D., our Chief Scientific Officer. The loss of services of any of our key personnel could adversely affect our ability to successfully develop our current or future product pipeline and commercialize our product candidates. Although we have entered into employment agreements with these key members of senior management, such agreements generally do not prohibit them from leaving our employ at any time. We currently do not maintain “key man” life insurance on any of our senior management team. The loss of Dr. Chin or other members of our current senior management could adversely affect the timing or outcomes of our current and planned studies, as well as longer-term prospects for commercializing our product candidates.
In addition, competition for qualified personnel in the animal health fields is intense, because there is a limited number of individuals who are trained or experienced in the field. We will need to hire additional personnel as we expand our biologics product development activities, and we may not be able to attract and retain qualified personnel on acceptable terms, or at all.
We are dependent upon third-party manufacturers for supplies of our products and our current product candidates.
We currently have no internal capability to manufacture our products or product candidates and will be dependent upon third-party manufacturers for such supplies. We and our contract manufacturers have historically
been able to obtain supplies of the API for development of our small molecule product candidates, but neither we nor our contract manufacturers have long-term supply agreements with the API manufacturers. We also have no agreements for commercial-scale supply of our products or any of our product candidates. As a result, we and our contract manufacturers may be unable to procure API in a timely manner on commercially reasonable terms, or at all. Any delay in identifying and contracting with third-party contract manufacturers on commercially reasonable terms would have an adverse impact upon our current product development activities and current and future commercialization efforts.
The facilities used by our contract manufacturers to manufacture the drugs are subject to inspections by the FDA, USDA, and the EMA, and we depend on our contract manufacturers to comply with GMP or other applicable regulatory standards. If our contract manufacturers cannot successfully manufacture material in compliance with these strict regulatory requirements, we and they will not be able to secure or maintain regulatory approval for their manufacturing facilities. In some cases, we also are dependent on our contract manufacturers to produce supplies in conformity to our specifications and maintain quality control and quality assurance practices and not to employ disqualified personnel. If the FDA or a comparable foreign regulatory authority does not approve the manufacturing facilities of our contract manufacturers, or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which could result in delays in, or adversely affect our ability to, develop or commercialize our product candidates. We and our contract manufacturers also may be subject to penalties and sanctions from the FDA and other regulatory authorities for any violations of applicable regulatory requirements. The USDA and EMA employ different regulatory standards than the FDA, so we may require multiple manufacturing processes and facilities for the same product candidate or any approved product.
The commercialization of our products and product candidates could be adversely affected if we are unable to secure sufficient quantities and quality of drug products in a timely manner, including by reason of a public health epidemic such as the COVID-19 outbreak.
The raw materials used to manufacture our products and our equine small molecule product candidates are generally readily available in commercial quantities from multiple suppliers, but we will be dependent upon our contract manufacturers to obtain these raw materials. If manufacturers are unable to do so as and when they are needed to supply our development and commercial needs, we will have no other means of producing our products and equine small molecule product candidates until they are able to do so or we or they procure alternative supplies of the API. If our third-party manufacturers suffer damage or destruction to their facilities or equipment, we may experience disruptions in supplies, or be unable to obtain supplies of our products or product candidates on a timely basis. Any inability to secure sufficient quantities and quality of the API or other raw materials in our products and product candidates would adversely impact our development activities and commercialization efforts. In some cases, contract manufacturers may be reluctant to manufacture the API in pet therapeutics, because of regulatory or other concerns. This may make it more difficult for us to identify manufacturers needed to supply sufficient quantities of our products and product candidates for development.
Our contract manufacturers purchase raw materials from suppliers located in China and India in order to manufacture our products and product candidates. The December 2019 outbreak of the novel strain of coronavirus (COVID-19) in China and India and other countries with which we do business may result in the full or partial shutdown of manufacturers or other businesses that are affected by the coronavirus, which may adversely impact both our ability to obtain sufficient and timely supplies of our products and other product candidates and our revenue from those products.
Public health epidemics or other widespread outbreaks of contagious diseases such as COVID-19 could adversely impact our business.
In addition to adversely affecting our ability to obtain sufficient and timely supplies of products and product candidates from suppliers, any outbreak of contagious diseases, such as the recent novel strain of coronavirus (COVID-19) that is affecting the global community, could adversely affect our business and operations in other ways, many of which cannot currently be determined or quantified. These uncertain factors, including the duration of the outbreak, the severity of the disease and the actions to contain or treat its impact, could impair our operations including, among others, employee mobility and productivity, availability of facilities, conduct of our clinical trials, manufacturing and supply capacity, and availability and productivity of third party service suppliers.
Biologics manufacturing is difficult and costly and may not be commercially viable.
There are no established sources of the active ingredients in our biologic product candidates, so we or our collaborators will be required to develop the manufacturing process, perform validation and in some cases establish new facilities to manufacture pet biologics. Manufacturing of pet biologics, apart from vaccines, is a relatively new field in which unanticipated difficulties or challenges could arise. Small changes in the manufacturing process can have significant impact on product quality, consistency and yield. Manufacturing biologics, especially in large quantities, is complex and may require the use of innovative technologies that we may need to develop ourselves or in conjunction with third-party collaborators. Such manufacturing requires facilities specifically designed and validated for this purpose and sophisticated quality assurance and quality control procedures. Biologics are also usually costly to manufacture, because production usually requires the use of living organisms. Factors such as these may make it more technically challenging, time-consuming and expensive than we anticipate to manufacture biologics. Animal antibodies also must be manufactured at a sufficiently low cost that they are economically viable for us and for our customers. We built a manufacturing plant for biologics manufacturing and if it is not utilized to full capacity, it may result in increased annual costs and increase in cost of goods. There is no assurance that we will be able to manufacture biologics at an economical cost, if at all.
Our facilities used to manufacture the biologics are subject to inspections by the FDA, USDA and the EMA. If we cannot successfully manufacture material in compliance with these strict regulatory requirements, we will not be able to secure or maintain regulatory approval for the manufacturing facility. If the FDA or a comparable foreign regulatory authority does not approve the manufacturing facilities, or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which could result in delays in, or adversely affect our ability to, develop or commercialize our product candidates. We also may be subject to penalties and sanctions from the FDA and other regulatory authorities for any violations of applicable regulatory requirements. The USDA and EMA employ different regulatory standards than the FDA, so we may require multiple manufacturing processes and facilities for the same product candidate or any approved product.
There is no assurance that our strategic restructuring transition to a partnership-based model for the commercialization of our biologic products will be successful.
As we announced on March 16, 2020, our strategic restructuring involves a transition to a partnership-based model for commercialization in which our canine and feline biologic products will be out-licensed to larger commercial partners in return for upfront payments, contingent milestone payments and royalties on future sales. The strategic restructuring involves discontinuing our canine and feline commercial infrastructure.
There is no assurance that our transition to a partnership-based model for commercialization of our canine and feline biologic products will be successful or that it will result in greater profits for us than if we directly sold these biologic products. We may be unable to enter into licensing agreements that contain favorable upfront payments, contingent milestone payments and royalties. In addition, there is no assurance that the parties to which we out-license our biologic products will fulfill the terms of their agreements or be successful in their efforts to sell our products at favorable prices and in satisfactory amounts.
There is no assurance that our strategic restructuring decision to use a small equine-specific direct sales force to sell Zimeta and future equine small molecule products will be successful
As we announced on March 16, 2020, our strategic restructuring involves a decision to discontinue development of canine and feline small molecule product candidates and to use a small equine-specific direct sales force, with select distributor relationships, to sell Zimeta and future equine small molecule products in the United States. We intend to establish collaborations with distributors or licensing partners to commercialize any of these products that may be approved by the EMA. Our current intention is to sell these products through our subsidiary, KindredBio Equine.
There is no assurance that this strategic restructuring decision will be successful. Among other things, we may be unable to enter into satisfactory agreements with distributors or licensing partners, or they may unsuccessful in their efforts to sell our equine products at favorable prices and in satisfactory amounts. The members of our direct
sales force may also be unsuccessful in their sales efforts, and we may be unable to hire, retain and motivate qualified individuals.
Even if we successfully identify product candidates, they may be unsuccessfully commercialized for many reasons.
Even if we successfully identify product candidates, they may be unsuccessfully commercialized for many reasons, including the following:
| |
| • | a product candidate may be covered by third parties’ patents or other exclusive rights unknown to us; |
| |
| • | a product candidate may on further study be shown to have harmful side effects in pets or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; |
| |
| • | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; |
| |
| • | a product candidate may not be accepted as safe and effective by veterinarians, pet owners and the pet therapeutic community; and |
| |
| • | competitors may develop alternatives that render our product candidates obsolete. |
Failure to identify further product candidates ultimately suitable for development and commercialization would have an adverse impact on our growth strategy and future business prospects.
Changes in distribution channels for pet therapeutics may make it more difficult or expensive to distribute our products.
In the United States, pet owners typically purchase their pet therapeutics from their local veterinarians who also prescribe such therapeutics. There is a trend, however, toward increased purchases of pet therapeutics from Internet-based retailers, “big-box” retail stores and other over-the-counter distribution channels, which follows a significant shift in recent years away from the traditional veterinarian distribution channel in the sale of parasiticides and vaccines. It is also possible that pet owners may come to rely increasingly on internet-based animal health information rather than on their veterinarians. We currently expect that our pet therapeutics will be marketed directly to veterinarians, so any reduced reliance on veterinarians by pet owners could materially adversely affect our business and prospects. Pet owners also may substitute human health products for pet therapeutics if the human health products are less expensive or more readily available, which substitution also could adversely affect our business.
Legislation has been or may be proposed in the United States or abroad that would require veterinarians to provide pet owners with written prescriptions and disclosures that the pet owner has the right to fill the prescriptions through other means. If enacted, such legislation could lead to a reduction in the number of pet owners who purchase their pet therapeutics directly from veterinarians, which also could adversely affect our business.
Consolidation of our customers could negatively affect the pricing of our products.
Veterinarians will be our primary customers for any approved products. In recent years, there has been a trend towards the consolidation of veterinary clinics and animal hospitals. If this trend continues, these large clinics and hospitals could attempt to leverage their buying power to obtain favorable pricing from us and other pet therapeutics companies. Any resulting downward pressure on the prices of any of our approved products could have a material adverse effect on our results of operations and financial condition.
Our research and development relies on evaluations in animals, which is controversial and may become subject to bans or additional regulations.
The evaluation of our product candidates in target animals is required to develop and commercialize our product candidates. Although our animal testing will be subject to GLP and GCP requirements, as applicable, animal testing in the human pharmaceutical industry and in other industries has been the subject of controversy and adverse publicity. Some organizations and individuals have sought to ban animal testing or encourage the adoption of additional regulations applicable to animal testing. To the extent that such bans or regulations are imposed, our research and development activities, and by extension our operating results and financial condition, could be materially adversely affected. In addition, negative publicity about animal practices by us or in our industry could harm our reputation among potential customers for our products.
Our products and, if approved, our product candidates may be marketed in the United States only in the target animals and for the indications for which they are approved, and if we want to expand the approved animals or indications, we will need to obtain additional FDA or USDA approvals, which may not be granted.
We and our licensees may market or advertise our products and any of our product candidates that are approved only in the specific species and for treatment of the specific indications for which they were approved, which could limit use of the products by veterinarians and pet owners.
Use of a drug outside its cleared or approved indications in the animal context is known as extra-label use. Under the Animal Medicinal Drug Use Clarification Act of 1994 (the "AMDUCA"), veterinarians are permitted to prescribe extra-label uses of certain approved animal drugs and approved human drugs for animals under certain conditions. Thus, although veterinarians may in the future prescribe and use human-approved products or our products for extra-label uses, we may not promote our products for extra-label uses. If the FDA determines that any of our marketing activities constitute promotion of an extra-label use, it could subject us to regulatory enforcement, which could have an adverse impact on our reputation and potential liability to us.
The commercial potential of a product candidate in development is difficult to predict. The market for our product candidates, or for the pet therapeutics industry as a whole, is uncertain and may be smaller than we anticipate, which could significantly and negatively impact our revenue, results of operations and financial condition.
It is very difficult to estimate the commercial potential of any of our product candidates because of the emerging nature of our industry as a whole. The pet therapeutics market continues to evolve, and it is difficult to predict the market potential for what we believe to be the unmet medical needs of pets. The market will depend on important factors such as safety and efficacy compared to other available treatments, including potential human generic therapeutic alternatives with similar efficacy profiles, changing standards of care, preferences of veterinarians, the willingness of pet owners to pay for such products, and the availability of competitive alternatives that may emerge either during the product development process or after commercial introduction. If the market potential for our product candidates is less than we anticipate due to one or more of these factors, it could negatively impact our business, financial condition and results of operations. Further, the willingness of pet owners to pay for our product candidates, if approved, may be less than we anticipate, and may be negatively affected by overall economic conditions. The current penetration of pet insurance in the United States is low, pet owners are likely to have to pay for our products, if at all, out-of-pocket, and pet owners may not be willing or able to pay for any approved products of ours.
We may acquire other businesses or form joint ventures that may be unsuccessful and could adversely dilute your ownership of our company.
As part of our business strategy, we may pursue acquisitions of other complementary assets and businesses and may also pursue strategic alliances. Our company has no experience in acquiring other assets or businesses and has limited experience in forming such alliances. We may not be able to successfully integrate any acquisitions into our existing business, and we could assume unknown or contingent liabilities or become subject to possible stockholder claims in connection with any related-party or third-party acquisitions or other transactions. We also could experience adverse effects on our reported results of operations from acquisition-related charges, amortization of acquired technology and other intangibles and impairment charges relating to write-offs of goodwill and other intangible assets from time to time following an acquisition. Integration of an acquired company requires
management resources that otherwise would be available for ongoing development of our existing business. We may not realize the anticipated benefits of any acquisition, technology license or strategic alliance.
To finance future acquisitions, we may choose to issue shares of our common stock as consideration, which would dilute your ownership interest in us. Alternatively, it may be necessary for us to raise additional funds through public or private financings. Additional funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders.
Our information technology systems and the information technology systems of third parties with whom we do business are vulnerable to cyber-attacks, breaches of security and misappropriation of data, which could result in substantial damage to our business and operations.
Our internal computer systems and those of our current and future employees and third-party vendors, manufacturers, licensees and consultants are vulnerable to damage from unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. The secure processing, maintenance and transmission of electronic information, including customer, employee and company data, is critical to our operations and the legal environment surrounding information security, storage, use, processing, disclosure and privacy is demanding with the frequent imposition of new and changing requirements. We also store certain information with third parties, and we utilize third-party service providers to process, manage or transmit data, which may also increase our risk. Our information systems and those of third parties with whom we do business are subjected to computer viruses or other malicious codes, cyber- or phishing-attacks and also are vulnerable to an increasing threat of continually evolving cybersecurity risks and external hazards, as well as improper or inadvertent employee behavior, all of which could expose confidential company and personal data systems and information to security breaches. Any system failure or security breach by employees or others may pose a risk that sensitive data, including data from our target animal studies, intellectual property, trade secrets, confidential information or personal information belonging to us may be exposed to unauthorized persons or to the public. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of data from completed or future studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our therapeutics and therapeutic candidates, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, the further development of our therapeutic candidates and commercialization of our therapeutics could be delayed, and the trading price of our common stock could be adversely affected. To date, we have not experienced any material impact to our business or operations resulting from security breaches, including from information or cybersecurity attacks; however, because of the frequently changing attack techniques, along with the increased volume and sophistication of the attacks, there is the potential for us to be adversely impacted.
Our secured loan agreement contains restrictions that limit our flexibility in operating our business.
On September 30, 2019, we entered into a Loan and Security Agreement (the “Loan Agreement”) with Solar Capital Ltd., as collateral agent and lender, and the other lenders named in the Loan Agreement (Solar Capital Ltd. and the other lenders collectively, the “Lenders”). The Lenders have agreed to make available to KindredBio an aggregate principal amount of up to $50.0 million under the Loan Agreement. The Loan Agreement provides for a term loan commitment of $50.0 million in three tranches: (1) a $20.0 million term A loan that was funded on September 30, 2019; (2) a $15.0 million term B loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, no later than December 31, 2020; and (3) a $15.0 million term C loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, on or before June 30, 2021. Each term loan has a maturity date of September 30, 2024. Each term loan bears interest at a floating per annum rate equal to the one-month LIBOR rate (with a floor of 2.17%) plus 6.75%. We are permitted to make interest-only payments on each term loan through October 31, 2021. The interest-only period can be extended by six months upon our satisfaction of the minimum liquidity requirements described in the Loan Agreement.
The Loan Agreement contains numerous negative covenants that prohibit us from taking certain actions in connection with the operation of our business without obtaining the prior written consent of the Lenders. For example, without the prior written consent of the Lenders, we are not permitted to (1) sell, license or otherwise
transfer specified assets or portions of our business, (2) engage in specified new businesses, (3) issue more than a specified amount of securities, (4) merge with another entity, (5) acquire another business, (6) incur additional indebtedness or encumber assets, subject to certain exceptions, (7) pay cash dividends, (8) enter into specified material contracts, or (9) permit third parties to acquire ownership interests in our subsidiaries. There is no assurance that the Lenders will provide their written consent to any of these actions even if the actions are in the best interests of our stockholders.
We may be unable to repay the outstanding principal and accrued interest under the Loan Agreement, in which event the Lenders could exercise their default remedies under the Loan Agreement.
Our obligations under the Loan Agreement are secured by a first-priority security interest in substantially all of our assets, including our intellectual property, and a lien on our real property. The Loan Agreement contains customary representations, warranties and covenants and also includes customary events of default, including payment defaults, breaches of covenants, and a default upon the occurrence of a material adverse change affecting us. Upon the occurrence of an event of default, a default interest rate of an additional 5.00% per annum may be applied to the outstanding loan balance, and the Lenders may declare all outstanding obligations immediately due and payable and exercise all of their rights and remedies as set forth in the Loan Agreement and under applicable law. Among other things, the Lenders could attempt to take possession of and sell substantially all of our assets, which would have a material adverse effect on the market value of our common stock.
There is no assurance that we will be able to repay all outstanding principal and accrued interest under the Loan Agreement. In order to attempt to prevent the occurrence of an event of default under the Loan Agreement, we might be required to take actions that might not be in our long-term best interests such as (1) dedicating a substantial portion of our cash flow from operations to the payment of principal and accrued interest under the Loan Agreement, thereby reducing funds available to us for other purposes, (2) divesting valuable assets in order to raise funds with which to repay the principal and accrued interest under the Loan Agreement, and (3) delaying capital expenditures, new product candidate initiatives and acquisitions of other businesses. The existence of the Loan Agreement and the obligations under the Loan Agreement might also limit our ability to obtain additional equity or debt funding from third parties.
There is no assurance that our sale of Mirataz to Dechra will be completed, and there is no assurance as to the amount of royalties that we will receive from Dechra after Mirataz is sold to Dechra.
Dechra’s obligation to purchase Mirataz from us pursuant to the sale agreement that we have entered into with Dechra is subject to the satisfaction of specified closing conditions. There is no assurance that all of these conditions will be satisfied and that the sale will be completed. If the sale is completed, a portion of the consideration to us payable by Dechra will be based on royalties from sales of Mirataz by Dechra following the completion of the sale. The amount of such sales and royalties cannot be predicted by us with any degree of certainty, and the total purchase price that we will receive from Dechra under the sale agreement therefore is uncertain.
Risks Related to Intellectual Property
Our commercial success will depend, in part, on obtaining and maintaining patent protection for our products.
In so far as our business strategy is to develop successful human drugs and biologics for veterinary use, our ability to obtain a proprietary intellectual property position for our products is uncertain. We have two issued patents for our IL-31 antibody, and a patent will be soon issued for Mirataz, but we do not have an issued patent for Zimeta or any of our other lead product candidates at this time. However, we have filed patent applications covering various aspects of our products and product candidates, and the U.S. Patent and Trademark Office (the "USPTO") has issued patents for our anti IL-31 antibody (U.S. Patent No. 10,093,731) and for methods of its use (U.S. Patent No. 10,150,810). Our other patent applications may never result in the issuance of patents, and/or patents issued to us may be dominated by the patents of third parties, including, for example, patents issued to analogous human drug or biological compositions and their usages. Furthermore, even if they are unchallenged by third parties, our current or future patents, if issued, may not adequately protect our intellectual property or prevent others from designing around their claims. In order to commercialize our products and product candidates in one or more species, we could be required to enter into third party licenses or, if a license is not available on terms that we consider reasonable, we could be required to cease development or commercialization of one or more of our drug or biologic
products or product candidates. Thus, if we cannot obtain ownership of issued patents covering our products and product candidates, our business and prospects would be adversely affected.
It is possible that no patents will issue to us to cover an approved product, and/ or that we will have little to no commercial protection against competing products. In such cases, we would then rely solely on other forms of exclusivity, such as regulatory exclusivity provided by the Federal Food, Drug and Cosmetic Act, if available, which may provide less protection to our competitive position.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any patents that issue. On September 16, 2011, the Leahy-Smith America Invents Act (the "Leahy-Smith Act") was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation, and switch the U.S. patent system from a “first-to-invent” system to a “first-to-file” system. Under a “first-to-file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first-to-file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any patents that issue, all of which could have a material adverse effect on our business and financial condition.
We may become subject to third parties’ claims alleging infringement of patents and proprietary rights or priority of invention, which would be costly, time-consuming and, if successfully asserted against us, delay or prevent the development and commercialization of our current or future product candidates.
There has been substantial litigation regarding patents and other intellectual property rights in the field of therapeutics, as well as patent challenge proceedings, including interference and administrative law proceedings before the USPTO, and oppositions and other comparable proceedings in foreign jurisdictions. Under U.S. patent reform laws, new procedures, including inter partes review and post-grant review, were implemented as of March 16, 2013, and the implementation of such reform laws presents uncertainty regarding the outcome of any challenges to our patents. We are aware of a European patent which claims antibodies that bind to dog IL-31 with certain functional characteristics, and the patent is currently opposed by multiple parties. The patent may or may not be relevant to one of our product candidates in Europe. There may be issued patents and pending patent applications with claims directed to long-acting or extended-release pharmaceutical formulations and uses of the same small molecules as in some of our small molecule product candidates, and other patents and pending patent applications with claims directed to pharmaceutical formulations and use of biologics conceptually similar to some of our biologics product candidates. There also may be other patents already issued of which we are unaware that might be infringed by one of our current or future product candidates. Because patent applications can take many years to issue and may be confidential for eighteen months or more after filing, there may be applications now pending of which we are unaware and which may later result in issued patents that may be infringed by our current or future product candidates. There is no assurance that our current or future product candidates will not infringe these or other existing or future third-party patents. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
To the extent we become subject to future third-party claims against us or our collaborators, we could incur substantial expenses and, if any such claims are successful, we could be liable to pay substantial damages, including treble damages and attorney’s fees if we or our collaborators are found to be willfully infringing a third-party’s patents. If a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of our product or product candidate that is the subject of the suit. Even if we are successful in defending such claims, infringement and other intellectual property claims can be expensive and time-consuming to litigate and divert management’s attention from our business and operations. As a result of or in order to avoid potential patent infringement claims, we or our collaborators may be compelled to seek
a license from a third party for which we would be required to pay license fees or royalties, or both. Moreover, these licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain such a license, the rights may be nonexclusive, which could allow our competitors access to the same intellectual property. Any of these events could harm our business and prospects.
In addition to possible infringement claims against us, we may be subject to third-party preissuance submission of prior art to the USPTO, or become involved in opposition, interference, derivation, reexamination, inter partes review, post-grant review, or other patent office proceedings or litigation in the United States or elsewhere, challenging our patent rights or the patent rights of others. We may also become involved in similar opposition proceedings in the European Patent Office or similar offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our current or future patent rights, if any, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
If our efforts to protect the proprietary nature of the intellectual property related to our products or any of our current or future product candidates are not adequate, we may not be able to compete effectively in our market.
We intend to rely upon a combination of regulatory exclusivity periods, patents, trade secret protection, confidentiality agreements, and license agreements to protect the intellectual property related to our p roducts and our product candidates and our development programs.
Composition-of-matter patents on the active pharmaceutical ingredients in pharmaceutical products, including pet therapeutics, are generally considered to be the strongest form of intellectual property protection, since such patents provide protection without regard to any particular method of use or manufacture. Because all of our current small molecule product candidates are based on generic human drugs, and because there is little, if any, composition of matter patent protection still available for the API of such drugs, we do not seek to obtain such composition-of-matter patents for the API in our small molecule product candidates. In addition, we cannot be certain that all of the composition-of-matter claims in our patent applications for our biologics product candidates will be considered patentable by the USPTO and courts in the United States, or by the patent offices and courts in foreign countries.
Method-of-use patents protect the use of a product for the claimed method. This type of patent does not prevent a competitor from developing or marketing an identical product for an indication that is outside the scope of the issued claims. Moreover, even if competitors do not actively promote their product for our targeted indications for which we may obtain patents, veterinarians may recommend that pet owners use these products extra-label, or pet owners may do so themselves. Although extra-label use may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute.
If the breadth or strength of protection provided by any patent applications or patents we may own, in-license, or pursue with respect to any of our current or future product candidates is threatened, it could threaten our ability to commercialize any of our current or future product candidates. Further, if we encounter delays in our development efforts, the period of time during which we could market any of our current or future product candidates under any patent protection we obtain would be reduced.
Even where laws provide protection or we are able to obtain patents, costly and time-consuming litigation may be necessary to enforce and determine the scope of our proprietary rights, and the outcome of such litigation would be uncertain. Moreover, any actions we may bring to enforce our intellectual property against our competitors could provoke them to bring counterclaims against us, and some of our competitors have substantially greater intellectual property portfolios than we have.
We also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or for which we have not filed patent applications, processes for which patents are difficult to enforce and other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we require all of our employees to assign their inventions to us, and endeavor to execute confidentiality agreements with all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology, we cannot be certain that we
have executed such agreements with all parties who may have helped to develop our intellectual property or had access to our proprietary information, or that our agreements will not be breached. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
We may be involved in lawsuits to protect or enforce any patents issued to us, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe any patents that issue to us, or any patents that we may license. To counter infringement or unauthorized use of any patents we may obtain, we may be required to file infringement claims, which can be expensive and time-consuming to litigate. In addition, if we or one of our future collaborators were to initiate legal proceedings against a third party to enforce a patent covering our current product candidates, or one of our future products, the defendant could counterclaim that the patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar claims before the USPTO, even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any current or future patent protection on our current or future product candidates. Such a loss of patent protection could have a material adverse impact on our business.
Litigation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be unsuccessful, it could have an adverse effect on the price of our common stock. Finally, we may not be able to prevent, alone or with the support of our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and implemented wide-ranging patent reform legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product and product candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents, if any, or marketing of competing products in violation of our proprietary rights generally. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. Proceedings to enforce our future patent rights, if any, in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We have the following 41 Registered Trademarks and 19 pending trademark applications:
We have two registered US trademarks (KINDLET and MIRATAZ), and nine pending US trademark applications for which we have received Notices of Allowance (ACCUSORB [IC016], ACCUSORB [IC042], EPOCAT, ZOLEREX, ZOLEREX K9, ZOLEREX FELINE, REFORMIN, ZIMETA, and BIOKIN). We are required to provide proof of use for the nine pending US trademarks prior to receiving Certificates of Registration. Petitions for Extension of Time have been filed in each of these pending US trademark applications.
International Trademark Applications under the Madrid Protocol have been filed for the following trademarks: ZOLEREX, ZOLEREX FELINE, ZOLEREX K9, MIRATAZ, REFORMIN, and ZIMETA. National Registrations have been received for ZOLEREX, ZOLEREX K9, and ZOLEREX FELINE in China, United Kingdom, Japan, Morocco, New Zealand, United Arab Emirates, and Saudi Arabia. A National Registration has been received for ZOLEREX K9 in Australia. National Registrations have been received for MIRATAZ in Canada, European Union, Liechtenstein, and Norway. National Registrations have been received for REFORMIN in China, United Kingdom, Japan, Morocco, United Arab Emirates, and Saudi Arabia. National Registration have been received for ZIMETA in Australia, Canada, European Union, New Zealand, and United Kingdom. We have two Registered Community Trade Marks (i.e., European Union Trade Marks) for KINDLET.
The European Union Trademark applications for ZOLEREX, ZOLEREX K9, and ZOLEREX FELINE have been opposed by a third-party. However we are in the process of negotiating a Coexistence Agreement wherein the third-party has agreed to withdraw the opposition on conditions that we will not use these marks in connection with products or services for dogs and/or cats. Accordingly, our use of the ZOLEREX brand in Europe will be limited animals other than dogs and cats.
Canadian trademark applications are pending for ZOLEREX, ZOLEREX K9, and ZOLEREX FELINE, for which we have received Notices of Allowance. We are required to provide proof of use for these pending Canadian trademarks prior to receiving Certificates of Registration.
Egyptian trademark applications are pending for ZOLEREX, ZOLEREX K9, ZOLEREX FELINE, and REFORMIN. The Refusal Period for these marks has ended without opposition; we are waiting to receive the Egyptian National Registrations.
The Opposition Period for the European Union and the New Zealand ZIMETA National Registrations is still active, and therefore it is possible that a third-party opposition may be filed.
We have no registered trademarks for our company name or for various of our current product candidates in the United States or any other countries, and failure to obtain those registrations could adversely affect our business.
While various of our trademarks have been registered, and some of our pending trademark applications have been allowed but not yet registered, various other of our pending trademark applications have neither been allowed nor registered. During trademark registration proceedings, we may receive rejections. If so, we will have an opportunity to respond, but we may be unable to overcome such rejections. In addition, USPTO and comparable agencies in many foreign jurisdictions may permit third parties to oppose pending trademark applications and to seek to cancel registered trademarks. Such opposition proceedings have been filed and are currently pending for three European trademark applications. Current and future opposition or cancellation proceedings, if any, filed against any of our trademark applications or any registered trademarks may result in cancellation of these trademarks. Moreover, any name we propose to use with our product candidates in the United States must be approved by the FDA or the USDA, regardless of whether we have registered or applied to register as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA or the USDA objects to any of our
proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA or USDA.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at other biotechnology, pharmaceutical or animal health companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise improperly used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against any such claims. Even if we are successful in defending against any such claims, such litigation could result in substantial cost and be a distraction to our management and employees.
Risks Related to Government Regulation
Even after receiving regulatory approval of Mirataz and Zimeta, and even if we receive regulatory approval for any of our current or future product candidates, we will be subject to ongoing FDA, USDA, and EMA obligations and continued regulatory review, which may result in significant additional expense. Additionally, Mirataz, Zimeta and any product candidates, if approved, will be subject to labeling and manufacturing requirements and could be subject to other restrictions. Failure to comply with these regulatory requirements or the occurrence of unanticipated problems with our products could result in significant penalties.
For Mirataz, Zimeta and for any of our current or future product candidates approved by the FDA, USDA, or EMA, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for Mirataz, Zimeta and every other approved product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, establishment registration, and product listing, as well as continued compliance with GMP, GLP and GCP for any studies that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| |
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary product recalls; |
| |
| • | fines, warning letters or holds on target animal studies; |
| |
| • | refusal by the FDA, USDA, or EMA to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals; |
| |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA, USDA, or EMA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business.
Any of our current or future approved products may cause or contribute to adverse medical events that we are required to report to regulatory authorities and, if we fail to do so, we could be subject to sanctions that would materially harm our business.
Certain regulatory authorities will require that we report certain information about adverse medical events regarding an approved product if the product may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our product. If we fail to comply with our reporting obligations, the regulatory authorities could take action including criminal prosecution, seizure of our product or delay in approval or clearance of future products.
Legislative or regulatory reforms with respect to pet therapeutics may make it more difficult and costly for us to obtain regulatory clearance or approval of any of our current or future product candidates and to produce, market, and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress or EU that could significantly change the statutory provisions governing the testing, regulatory clearance or approval, manufacture, and marketing of regulated products. In addition, FDA and USDA regulations and guidance are often revised or reinterpreted by the FDA and USDA in ways that may significantly affect our business and our products. Similar changes in laws or regulations can occur in other countries. Any new regulations or revisions or reinterpretations of existing regulations in the United States or in other countries may impose additional costs or lengthen review times of any of our current or future product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| |
| • | changes to manufacturing methods; |
| |
| • | recall, replacement, or discontinuance of certain products; and |
| |
| • | additional record keeping. |
Each of these would likely entail substantial time and cost and could materially harm our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition, and results of operations.
Certain of our product candidates currently in development may be classified as controlled substances, the manufacture, use, sale, importation, exportation, and distribution of which are subject to additional regulation by state, federal, and foreign law enforcement and other regulatory agencies.
Certain of our product candidates may be subject to regulation as controlled substances under the federal Controlled Substances Act of 1970, or CSA, and regulations of the U.S. Drug Enforcement Administration, or DEA. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may not be marketed or sold in the United States. An animal drug product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances.
Various states also independently regulate controlled substances. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule drugs as well. While some states automatically schedule a drug when the DEA does so, in other states there must be rulemaking or a legislative action. State scheduling may delay commercial sale of any controlled substance drug product for which we obtain federal regulatory approval and adverse scheduling could impair the commercial attractiveness of such product. We would also be required to obtain separate state registrations in order to be able to obtain, handle and distribute controlled substances for target animal studies, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
For any of our product candidates classified as controlled substances, we and our suppliers, manufacturers, contractors, customers and distributors will be required to obtain and maintain applicable registrations from state, federal and foreign law enforcement and regulatory agencies and comply with state, federal and foreign laws and regulations regarding the manufacture, use, sale, importation, exportation and distribution of controlled substances. There is a risk that DEA regulations may limit the supply of the compounds used in pivotal trials of our product candidates, and, in the future, the ability to produce and distribute our products in the volume needed to meet commercial demand.
Regulations associated with controlled substances govern manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal. These regulations increase the personnel needs and the expense associated with development and commercialization of product candidates containing controlled substances. The DEA and some states conduct periodic inspections of registered establishments that handle controlled substances. Failure to obtain and maintain required registrations or comply with any applicable regulations could delay or preclude us from developing and commercializing our product candidates containing controlled substances and subject us to enforcement action. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to revoke those registrations. In some circumstances, violations could lead to criminal proceedings. Because of their restrictive nature, these regulations could limit commercialization of any of our product candidates that are classified as controlled substances.
Risks Related to Our Common Stock
The price of our common stock could be subject to volatility related or unrelated to our operations.
Our stock prices and the market prices for securities of biotechnology companies in general have been highly volatile, with recent significant price and volume fluctuations, and may continue to be highly volatile in the future. Since our initial public offering in December 2013, the trading price of our common stock has ranged from a low of $2.90 to a high of $26.99. The trading price may continue to be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include those discussed previously in this “Risk Factors” section of this Annual Report on Form 10-K and others, such as:
| |
| • | any delays in, or suspension or failure of, our current and future studies; |
| |
| • | announcements of regulatory approval or disapproval of any of our current or future product candidates or of regulatory actions affecting us or our industry; |
| |
| • | delays in the commercialization of our products or product candidates; |
| |
| • | manufacturing and supply issues related to our development programs and commercialization of our product or product candidates; |
| |
| • | quarterly variations in our results of operations or those of our competitors; |
| |
| • | changes in our earnings estimates or recommendations by securities analysts or adverse publicity regarding us or products or product candidates; |
| |
| • | announcements by us or our competitors of new product candidates, significant contracts, commercial relationships, acquisitions or capital commitments; |
| |
| • | announcements relating to future development or license agreements including termination of such agreements; |
| |
| • | adverse developments with respect to our intellectual property rights or those of our principal collaborators; |
| |
| • | commencement of litigation involving us or our competitors; |
| |
| • | any major changes in our board of directors or management; |
| |
| • | new legislation in the United States relating to the prescription, sale, distribution or pricing of pet therapeutics; |
| |
| • | product liability claims, other litigation or public concern about the safety of our products or product candidates; |
| |
| • | market conditions in the animal health industry, in general, or in the pet therapeutics sector, in particular, including performance of our competitors; and |
| |
| • | general economic conditions in the United States and abroad. |
In addition, the stock market, in general, or the market for stocks in our industry, in particular, may experience broad market fluctuations, which may adversely affect the market price or liquidity of our common stock. Any sudden decline in the market price of our common stock could trigger securities class-action lawsuits against us. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the time and attention of our management would be diverted from our business and operations. We also could be subject to damages claims if we are found to be at fault in connection with a decline in our stock price.
If securities or industry analysts do not publish research or reports about our company, or if they issue adverse or misleading opinions regarding us or our stock, our stock price and trading volume could decline.
Although we have research coverage by securities and industry analysts, if coverage is not maintained, the market price for our common stock may be adversely affected. Our stock price also may decline if any analyst who covers us issues an adverse or erroneous opinion regarding us, our business model, our intellectual property or our stock performance, or if our target animal studies and operating results fail to meet analysts’ expectations. If one or more analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline and possibly adversely affect our ability to engage in future financings.
Our quarterly and annual operating results may be volatile and may vary significantly from the estimates and expectations of investors and third parties, including securities or industry analysts, which could cause the market price of our common stock to decline.
It has been our practice not to provide forward-looking sales, revenue or earnings guidance and not to endorse any third party’s sales, revenue or earnings estimates, including the estimates of securities or industry analysts. As a result, our actual operating results may be below the expectations of our investors and third parties, including securities or industry analysts. Investors should not rely on any estimates, research or reports published
by third parties, including analysts. Furthermore, many factors could cause our revenues and operating results to vary significantly in the future, including, but not limited to, those set out in the “Risk Factors” section of this Annual Report on Form 10-K. Accordingly, we believe that quarter-to-quarter comparisons of our operating results are not necessarily meaningful. Investors should not rely on the results of one quarter as an indication of our future performance.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of December 31, 2019, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates beneficially own in the aggregate approximately 29.5% of our outstanding shares of common stock, excluding shares they may acquire upon exercise of stock options or upon the vesting or restricted stock units they hold. As a result of their stock ownership, these stockholders may have the ability to influence our management and policies and will be able to significantly affect the outcome of matters requiring stockholder approval such as elections of directors, amendments of our organizational documents or approvals of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), the Sarbanes-Oxley Act of 2002, as amended (the "Sarbanes-Oxley Act"), and the listing standards of the NASDAQ Stock Market. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time consuming and costly, and place significant strain on our personnel, systems and resources.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we will file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers. We are also continuing to improve our internal control over financial reporting. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, we have expended, and anticipate that we will continue to expend, significant resources, including accounting-related costs and significant management oversight.
Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls or our internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls, or any difficulties encountered in their implementation or improvement, could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of management evaluations of our internal control over financial reporting that we are required to include in our periodic reports that we file with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on the NASDAQ Stock Market.
Any failure to maintain effective disclosure controls and internal control over financial reporting could have a material and adverse effect on our business and operating results and cause a decline in the price of our common stock.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent changes in control or changes in our management without the consent of our board of directors. These provisions include the following:
| |
| • | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; |
| |
| • | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| |
| • | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| |
| • | the ability of our board of directors to authorize the issuance of shares of preferred stock and to determine the terms of those shares, including preferences and voting rights, without stockholder approval, which could adversely affect the rights of our common stockholders or be used to deter a possible acquisition of our company; |
| |
| • | the ability of our board of directors to amend our bylaws without obtaining stockholder approval; |
| |
| • | the required approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors to adopt, amend or repeal our bylaws or repeal the provisions of our amended and restated certificate of incorporation regarding the election and removal of directors; |
| |
| • | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
| |
| • | the requirement that a special meeting of stockholders may be called only by the chairman of the board of directors, the chief executive officer, the president or the board of directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and |
| |
| • | advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us. |
These provisions could inhibit or prevent possible transactions that some stockholders may consider attractive.
We are also subject to the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law. Under Section 203, a corporation generally may not engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the board of directors has approved the transaction.
Our stockholder rights agreement could discourage a takeover that stockholders may consider favorable and may lead to an entrenchment of management.
On May 19, 2017, our board of directors approved and adopted a rights agreement with American Stock Transfer & Trust Company, LLC, as rights agent, and, on July 24, 2017, our stockholders approved the adoption of the rights agreement. The rights agreement is intended to protect our stockholders from coercive or otherwise unfair proposals to acquire control of KindredBio by significantly diluting the ownership interest of any person who acquires at least 20% of our outstanding common stock by providing all other stockholders with the right to acquire additional shares of our preferred stock or common stock at a significant discount. Although the rights agreement is intended to encourage an acquiring person to negotiate a proposed merger or other business combination with our
board of directors and management, it could discourage a takeover transaction that stockholders may consider favorable and may lead to an entrenchment of management.
Our amended and restated by-laws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our amended and restated by-laws provide that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee to us or our stockholders, (3) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, or (4) any action asserting a claim that is governed by the internal affairs doctrine. Any person purchasing or otherwise acquiring any interest in any shares of our capital stock shall be deemed to have notice of and to have consented to this provision of our amended and restated by-laws. This choice-of-forum provision may limit our stockholders’ ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits. Alternatively, if a court were to find this provision of our amended and restated by-laws inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
We do not intend to pay dividends on our common stock, and the ability of investors in our common stock to achieve a return on their investment will depend on appreciation in the market price of our common stock.
We currently intend to invest any future earnings to fund our growth and not to pay any cash dividends on our common stock. Since we do not intend to pay dividends, the ability of investors in our common stock to receive a return on their investment will depend on any appreciation in the market price of our common stock. There is no assurance that our common stock will appreciate in price.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes (such as research and development tax credits) to offset its post-change income and taxes may be limited. In general, an “ownership change” occurs if there is a cumulative change in our ownership by “5% shareholders” that exceeds 50 percentage points over a rolling three-year period. Similar rules may apply under state tax laws. If we experience one or more ownership changes as a result of future transactions in our stock, we may be limited in our ability to use our net operating loss carryforwards and other tax assets to reduce taxes owed on the net taxable income that we earn. Any such limitations on the ability to use our net operating loss carryforwards and other tax assets could potentially result in increased future tax liability to us.
| |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
None.
We have non-cancelable operating leases for laboratory space in Burlingame, California with several amendments to expand the facility. We have a non-cancelable operating lease for the entire existing laboratory space of a total 11,476 square feet that expires in May 2025. In August 2015, we entered into a non-cancelable operating lease for 3,126 square feet of office space in San Diego, California and in June 2019, renewed the lease through February 2025. Our headquarters office lease for 8,090 square feet of office space in Burlingame, California expires November 30, 2020. In October 2018, we entered into a short-term sublease agreement for an additional 5,613 square feet of laboratory space next to our current laboratory facility and terminated the lease in June 2019. In April 2019, we signed a short-term lease in Burlingame ("April 2019 lease"), consisting of 1,979 square feet of space through April 2020. In May 2019, we signed another lease in Burlingame ("May 2019 lease"), consisting of 1,346 square feet of space through April 2022. In addition, we have four equipment leases expiring through 2023.
On June 21, 2017, we entered into a purchase agreement with Strategic Veterinary Pharmaceuticals, Inc. ("SVP") for the purchase of an approximately 180,000 sq. ft. biologics plant (the "Plant") with clean rooms, utility, equipment, and related quality documentation suitable for small molecule and biologics manufacturing, that is located in Elwood, Kansas. The purchase was finalized on August 7, 2017 upon completion of the diligence period and satisfaction of the conditions of escrow. The Plant was purchased for $3,750,000, which includes approximately eight acres of land located at 1411 Oak Street, Elwood, Kansas, all improvements located at the Plant, and all personal property and intangible property owned by SVP and located at the Plant or used in connection with the operation of the Plant.
| |
| ITEM 3. | LEGAL PROCEEDINGS. |
We are not currently a party to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
PART II
| |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Since December 12, 2013, our common stock has been traded on The NASDAQ Capital Market under the symbol “KIN.” Prior to December 12, 2013, there was no public trading market for our common stock.
Common Stock Information
As of February 28, 2020, there were 39,289,624 outstanding shares of our common stock outstanding held of record by approximately 26 holders.
Dividends
We have never declared or paid any cash dividends on our capital stock. We intend to retain any future earnings and do not expect to pay any dividends in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to applicable laws, and will depend on a number of factors, including our financial condition, results of operations, capital requirements, contractual restrictions, general business conditions and other factors that our board of directors may deem relevant.
Equity Compensation Plan Information
The following table sets forth certain information as of December 31, 2019 regarding securities authorized for issuance under our equity compensation plans:
|
| | | | | | | | | |
| Plan Category | | Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights | | Weighted-average
exercise price of
outstanding options,
warrants and rights | | Number of securities
remaining available
for future issuance
under equity
compensation plans
(excluding securities
reflected in column (a)) |
| | | (a) | | (b) | | (c) |
| Equity compensation plans approved by stockholders: | | | | | | |
| 2012 Equity Incentive Plan (terminated) | | 2,740,842 |
| | $6.74 | | — |
|
| 2016 Equity Incentive Plan (terminated) | | 2,134,701 |
| | $7.71 | | — |
|
| 2018 Equity Incentive Plan | | 1,477,827 |
| | $10.49 | | 1,258,098 |
|
| Equity compensation plans not approved by stockholders | | — |
| | — |
| | — |
|
| Total | | 6,353,370 |
| | $7.94 | | 1,258,098 |
|
Stock Performance Graph
The following line graph presentation compares cumulative total stockholder returns of Kindred Biosciences, Inc. with The NASDAQ Stock Market Index and The NASDAQ Biotechnology Index (the “Peer Index”) for the period from December 12, 2013 (the date that our common stock commenced trading on the NASDAQ Capital Market) to December 31, 2019. The graph and table assume that $100 was invested in each of our common stock, The NASDAQ Stock Market Index and the Peer Index on December 12, 2013, and that all dividends were reinvested. The returns shown are based on historical results and are not intended to suggest future
performance. The graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings of under the Securities Act of 1933, as amended, or the Exchange Act.
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 12/31/14 | | 6/30/15 | | 12/31/15 | | 6/30/16 | | 12/31/16 | | 6/30/17 | | 12/31/17 | | 6/30/18 | | 12/31/18 | | 6/30/19 | | 12/31/19 |
| Kindred Biosciences, Inc. | 100.00 |
| | 91.54 |
| | 45.64 |
| | 47.52 |
| | 57.05 |
| | 115.44 |
| | 126.85 |
| | 142.95 |
| | 146.98 |
| | 111.81 |
| | 113.82 |
|
| NASDAQ Composite-Total Returns | 100.00 |
| | 105.90 |
| | 106.96 |
| | 104.12 |
| | 116.45 |
| | 133.58 |
| | 150.96 |
| | 165.11 |
| | 146.67 |
| | 177.95 |
| | 200.49 |
|
| NASDAQ Biotechnology Index | 100.00 |
| | 121.79 |
| | 111.77 |
| | 85.22 |
| | 87.91 |
| | 103.18 |
| | 106.95 |
| | 110.31 |
| | 97.47 |
| | 110.09 |
| | 121.94 |
|
Unregistered Sales of Securities
As previously reported, on July 8, 2019, we issued 7,500 shares of our common stock to a service provider in exchange for our receipt of advisory and consulting services. The issuance of the shares was exempt from the registration requirements of the Securities Act of 1933, as amended, pursuant to Section 4(2) of the act applicable to a transaction by an issuer not involving a public offering of securities. No underwriter was involved in the issuance of the shares. During the fiscal year ended December 31, 2019, we did not sell any other securities that were not registered under the Securities Act of 1933, as amended.
Repurchase of Shares
We did not repurchase any of our shares of capital stock during the fiscal year ended December 31, 2019.
| |
| ITEM 6. | SELECTED FINANCIAL DATA. |
The following selected historical information has been derived from the audited consolidated financial statements of Kindred Biosciences and should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Item 8. Financial Statements and Supplementary Data” included elsewhere in this Annual Report on Form 10-K. The Statement of Operations
and Comprehensive Loss Data and the Balance Sheet Data are derived from the audited consolidated financial statements which are included in the Form 10-K. The historical results are not necessarily indicative of the results of operations to be expected in the future.
|
| | | | | | | | | | | | | | | | | | | |
| (In thousands, except per share amounts) | Years ended December 31, |
| | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Statement of Operations and Comprehensive Loss Data: | | | | | | | | | |
| | | | | | | | | | |
| Net product revenues | $ | 4,256 |
| | $ | 1,966 |
| | $ | — |
| | $ | — |
| | $ | — |
|
| | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | |
| Cost of product sales | 587 |
| | 324 |
| | — |
| | — |
| | — |
|
| Research and development | 28,310 |
| | 26,399 |
| | 17,665 |
| | 13,861 |
| | 19,412 |
|
| General and administrative | 37,926 |
| | 26,499 |
| | 13,988 |
| | 8,308 |
| | 7,850 |
|
| Restructuring costs | — |
| | — |
| | — |
| | 655 |
| | — |
|
| Total operating costs and expenses | 66,823 |
| | 53,222 |
| | 31,653 |
| | 22,824 |
| | 27,262 |
|
| | | | | | | | | | |
| Loss from operations | (62,567 | ) | | (51,256 | ) | | (31,653 | ) | | (22,824 | ) | | (27,262 | ) |
| Interest and other income, net | 1,178 |
| | 1,566 |
| | 774 |
| | 325 |
| | 132 |
|
| Net loss | (61,389 | ) | | (49,690 | ) | | (30,879 | ) | | (22,499 | ) | | (27,130 | ) |
| Change in unrealized gains or losses on available-for-sale securities | 24 |
| | 20 |
| | — |
| | 19 |
| | (23 | ) |
| Comprehensive loss | $ | (61,365 | ) | | $ | (49,670 | ) | | $ | (30,879 | ) | | $ | (22,480 | ) | | $ | (27,153 | ) |
| | | | | | | | | | |
Net loss per share, basic and diluted (1) | $ | (1.59 | ) | | $ | (1.60 | ) | | $ | (1.23 | ) | | $ | (1.13 | ) | | $ | (1.37 | ) |
| | | | | | | | | | |
| Weighted-average number of common shares outstanding, basic and diluted | 38,657 |
| | 31,001 |
| | 25,084 |
| | 19,873 |
| | 19,773 |
|
|
| | | | | | | | | | | | | | | | | | | |
| (In thousands) | As of December 31, |
| | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Balance Sheet Data: | | | | | | | | | |
| Cash and cash equivalents | $ | 15,986 |
| | $ | 56,302 |
| | $ | 34,813 |
| | $ | 6,687 |
| | $ | 19,992 |
|
| Short-term investments | 55,723 |
| | 17,630 |
| | 46,207 |
| | 50,068 |
| | 53,051 |
|
| Long-term investments | 1,837 |
| | — |
| | 1,499 |
| | 1,052 |
| | 4,590 |
|
| Working capital | 69,121 |
| | 64,888 |
| | 75,790 |
| | 54,170 |
| | 70,547 |
|
| Total assets | 114,024 |
| | 106,482 |
| | 90,822 |
| | 61,576 |
| | 79,619 |
|
| Total liabilities | 32,103 |
| | 15,275 |
| | 6,142 |
| | 3,896 |
| | 3,248 |
|
| Accumulated deficit | (223,059 | ) | | (161,670 | ) | | (111,980 | ) | | (81,101 | ) | | (58,602 | ) |
| Total stockholders' equity | 81,921 |
| | 91,207 |
| | 84,680 |
| | 57,680 |
| | 76,371 |
|
(1) See Note 14 of the notes to consolidated financial statements included elsewhere in this annual report for an explanation of the method used to calculate the basic and diluted net loss per share attributable to common stockholders and the number of shares used in the computation of the per share amounts.
| |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this Annual Report for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis or elsewhere in this Annual Report.
Overview
We are a commercial-stage biopharmaceutical company focused on saving and improving the lives of pets. Our mission is to bring to our pets the same kinds of safe and effective medicines that our human family members enjoy. Our core strategy is to identify compounds and targets that have already demonstrated safety and efficacy in humans and to develop therapeutics based on these validated compounds and targets for pets, primarily dogs, cats and horses. We believe this approach will lead to shorter development times and higher approval rates than pursuing new, non-validated compounds and targets. Our current portfolio includes over 20 product candidates in development consisting of both small molecule pharmaceuticals and biologics.
Our first product, Mirataz® was approved in May 2018 and became commercial availability to veterinarians in the United States in July 2018. In November 2019, our second product, Zimeta™ (dipyrone injection) for the control of fever in horses was approved by the FDA and became commercially available in December 2019. In addition, we have multiple other product candidates, including several biologics, in various stages of development. We believe there are significant unmet medical needs for pets, and that the pet therapeutics segment of the animal health industry is likely to grow substantially as new therapeutics are identified, developed and marketed specifically for pets.
Mirataz is the first and only transdermal medication specifically developed and FDA-approved for the management of weight loss in cats. Weight loss is a serious and potentially fatal condition that represents the leading cause of visits to the veterinarian for cats. Mirataz, which is formulated with our proprietary Accusorb™ technology, is applied topically to the cat’s inner ear (pinna) once a day, providing a more attractive application route compared to oral administration. The product is classified as a weight gain drug and can be used in cats with various underlying diseases associated with unintended weight loss.
Zimeta is the first injectable dipyrone product to receive FDA approval for use in horses. Dipyrone, the active ingredient in Zimeta, is a member of the non-steroidal anti-inflammatory drug (NSAID) class and has a centrally acting mechanism of action on the hypothalamus where fever originates and is regulated, and is widely used both for horses and humans as an antipyretic outside of the United States.
On March 16, 2020, we announced a strategic realignment of our business model whereby KindredBio becomes a biologics-only company focused on accelerating our deep pipeline of late-stage biologics candidates in canine and feline markets, while discontinuing small molecule development for these species. We believe monoclonal antibodies are the future of veterinary medicine, and represent the greatest opportunity for value creation, given large potential markets for our programs and our competitive advantage in biologics. We plan to rely more on a partnership-based model for commercialization strategy similar to the traditional human biotech commercialization strategy whereby pipeline assets are partnered with larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. Accordingly, the companion animal commercial infrastructure will be substantially reduced. In connection with this strategic shift, Kindredbio is eliminating approximately 53 positions, representing about one-third of our current workforce. The eliminated positions primarily relate to the companion animal sales force and research and
development for small molecule programs. Restructuring expenses and retirement costs related to severance and health care benefits are expected to be approximately $1.7 million, exclusive of stock compensation.
In addition, we entered into a transaction for the sale of Mirataz to Dechra for an upfront payment of $43 million and royalties on worldwide sales. The acquisition comprises worldwide marketing rights, intellectual property rights, marketing authorizations and associated regulatory documentation, third party supply contracts related to raw material and manufacture of the finished product, and certain product inventory. Completion is expected before the end of June 2020, following satisfactory completion of certain deliverables. Dechra, which is based in the United Kingdom, plans to launch Mirataz in the UK and the European Union, and intends to conduct the necessary regulatory activities to achieve approvals in other key international markets. With commercial sales and marketing teams in 25 countries, and distributor relationships in an additional 68 countries, Dechra is strongly positioned to market Mirataz in the United States, Europe, and globally. Proceeds from the transaction, alongside the reduction in the company’s workforce and operations, will extend cash runway for approximately another 36 months, while maintaining a focused research engine dedicated to the development of KindredBio’s biologics pipeline.
In addition, we are overseeing a strategic review process of our KindredBio Equine subsidiary, including a potential spin-out or divestiture of assets. The KindredBio Equine asset portfolio will include Zimeta™ (dipyrone injection) for the control of pyrexia in horses, KIND-012 (dipyrone oral gel), KIND-014 for equine gastric ulcers, KIND-015 for metabolic syndrome, and anti-TNF antibody for sick newborn foals, alongside undisclosed equine product candidates.
Business Updates
In November 2019, the FDA approved Zimeta, the first and only medication, for the control of pyrexia in horses and in December 2019, Zimeta became commercially available to veterinarians in the United States through our network of national and regional distributors.
Pyrexia, or fever, is associated with a number of underlying diseases and can result in significant negative outcomes, including dehydration, laminitis, muscle wasting, weight loss, and in some cases death. Among performance horses, fever can also lead to loss of training and competition days. There are more than eight million horses in the United States, and over one million are seen by a veterinarian for fever annually.
Zimeta, which is classified as a nonsteroidal anti-inflammatory drug, targets fever centrally in the brain, where it originates. In a clinical study, Zimeta demonstrated rapid and effective fever reduction in horses with naturally occurring fever. The most common cause of fever in horses is respiratory disease, both viral and bacterial, but fever also can occur with other infections or inflammation of any body system. Zimeta is administered intravenously at 30 mg/kg once or twice daily, at 12-hour intervals, for up to three days. The overall number of doses and duration of treatment is dependent on the response observed (fever reduction). Zimeta may be re-administered based on recurrence of fever for up to three days.
In December 2019, the European Medicines Agency (EMA) granted marketing authorization of Mirataz for bodyweight gain in cats experiencing poor appetite and weight loss resulting from chronic medical conditions. Europe represents the second largest market for veterinary therapeutics internationally. Regulations restrict use of human medicine once an approved therapeutic is available. The authorization is valid in all 28-member states of the European Union, together with Iceland, Liechtenstein, and Norway.The approval of Mirataz in the European Union is based on results from both clinical and non-clinical studies.
Product Development Updates
Biologics
In August 2019, we announced positive results from our pilot efficacy study of KIND-030, a chimeric, high-affinity monoclonal antibody targeting canine parvovirus (CPV). This was a 12-dog study, of which 4 dogs were treated prophylactically and 2 dogs were treated after establishment of the infection. All treated dogs survived, compared to none in the applicable placebo group. The effect was seen in both prophylaxis setting, as well as in a
treatment setting after establishment of infection. Pivotal studies for this molecule are expected to be completed in 2020. Approval is anticipated by late 2020 or early 2021.
CPV is the most significant cause of viral enteritis in dogs, especially puppies, with over 90% mortality rate if untreated.There are currently no approved or unapproved treatments for CPV.
In July 2019, we reported positive topline results from our pilot field effectiveness study of KIND-016, a fully caninized, high-affinity monoclonal antibody targeting interleukin-31, for the treatment of atopic dermatitis in dogs. The scale up process is proceeding as planned, and the pivotal effectiveness study is expected to start in the second half of 2020.
In December 2019 we announced the outcome of a positive pilot laboratory study of KIND-032, a fully caninized monoclonal antibody targeting interleukin-4 receptor, for the treatment of atopic dermatitis in dogs. In the study, 14 laboratory dogs with clinical signs consistent with atopic dermatitis were dosed with placebo or with KIND-032 at two different doses. The CADESI scores were assessed by board-certified veterinary dermatologists who were blinded to treatment assignments. The study demonstrated that KindredBio's antibody was well-tolerated. Although the study was a single-dose study designed primarily to assess safety and pharmacokinetics, evidence of positive efficacy and dose response was observed at Week 1, as measured by CADESI-04. A second pilot study to further assess efficacy and dosing is planned for 2020.
The IL-4 pathway is a key driver of the inflammation that underlies atopic dermatitis and several other allergic diseases. Unlike KIND-025, which binds to IL-4 and IL-13 circulating in blood, KIND-032 binds to the IL-4 receptor on the surface of immune cells.
The pilot effectiveness study of KIND-025, a fully caninized, high-affinity fusion protein targeting interleukin-4/13, for the treatment of atopic dermatitis in dogs, is fully enrolled. We are completing development of our PK assays and expect topline results from the study in the coming weeks. The interleukin-4 and interleukin-13 pathways are key drivers of the inflammation that underlies atopic dermatitis and other allergic diseases.
Canine atopic dermatitis (CAD) is an immune-mediated inflammatory skin condition in dogs. It is the leading reason owners take their dog to the veterinarian, and the current market size is more than $700 million annually and growing. KindredBio is pursuing a multi-pronged approach toward atopic dermatitis, with a portfolio of promising biologics.
The pilot field efficacy study of KIND-509, our anti-TNF antibody for canine inflammatory bowel disease (IBD) is underway, with completion expected to be in the first half of 2020. IBD is a chronic disease of the gastrointestinal tract and can affect dogs at any age, but is more common in middle-aged and older dogs.
The majority of canine IBD cases involve chronic states of diarrhea, vomiting, gastroenteritis, inappetence, and other symptoms, certain of which are cited as among the most frequent disorders impacting dogs. For certain dog breeds, the prevalence of diarrhea exceeds 5%. Existing treatments can have significant drawbacks, including limited diets and excessive antibiotic use, which can lead to owner frustration, lapses in treatment adherence, or poor quality of life for the affected animal.
In January 2019, we announced positive topline results from our pilot field effectiveness study of KIND-510a, a long-acting feline recombinant erythropoietin that is being developed for the management of anemia in cats. We completed our cGMP fill & finish for feline recombinant erythropoietin at our Elwood, Kansas biologics manufacturing facility in the third quarter of 2019, and the pivotal efficacy study was initiated in the fourth quarter of 2019, with enrollment ongoing.
Anemia is a common condition in older cats which is often associated with chronic kidney disease resulting in decreased levels of endogenous erythropoietin. epoCat is a recombinant feline erythropoietin that has been specially engineered by us with a prolonged half-life, intended to be administered once-monthly. Erythropoietin is an endogenous protein that regulates and stimulates production of red blood cells. In the study, which enrolled 23 cats with anemia secondary to chronic kidney disease, epoCat rapidly increased mean hematocrit, with statistically significant improvement as early as Week 1 (p<0.0001). The effect was sustained, with continued
statistically significant improvement at Weeks 2, 3, 4, 5, and 6 (p<0.0001 at each visit). Compared to baseline, the mean of peak percent improvement in hematocrit by Week 6 was 55.4%.
In addition, 95.5% of the 22 evaluable patients achieved treatment success over the 6-week treatment period, defined prospectively as either a 30% increase in hematocrit value over baseline or the hematocrit value reaching normal range. Furthermore, epoCat resulted in statistically significant improvements over baseline (p<0.01 to p<0.05) across all three health-related quality of life (QoL) domains, namely Vitality, Comfort, and Emotional Wellbeing, as measured by a validated QoL instrument. Based on a preliminary review of the safety data, the drug appears to be well tolerated.
KIND-511 is an anti-Tumor Necrosis Factor (“anti-TNF”) treatment for newborn foals. Sick newborn foals, defined as sepsis score ≥ 11 or positive blood culture, are challenging, and difficult to treat and result in approximately 50% mortality. We have completed a pilot field study in sick or septic foals to assess safety and efficacy of anti-TNF monoclonal antibody, with positive results. By Kaplan-Meier analysis, the difference in survival between the control and placebo groups was statistically significant (p=0.0293). We intend to continue field studies during the 2021 foaling season following discussion with the FDA regarding the pivotal study design for KIND-511. There is currently no FDA-approved therapy
Small Molecules
The pivotal field effectiveness study for KIND-012 (dipyrone oral gel) for the treatment of fever in horses has been completed with positive results. The target animal safety study is also complete, and KIND-012 was found to be well-tolerated. KIND-012, which is a proprietary oral gel, is expected to expand use of the drug and build upon the success of Zimeta. We have transferred the product to the commercial manufacturer and are in discussions with the FDA and EMA regarding the data required to show bioequivalence to the previously manufactured product. Scientific advice has also been received from the EMA on further data required on dossier requirements.
We are also developing KIND-014 for the treatment of equine gastric ulcers in horses. In May 2018, we announced positive results from its pilot field effectiveness study of KIND-014 for the treatment of gastric ulcers in horses. This study was a randomized, single-blind, placebo-controlled, dose-ranging study that enrolled 53 horses (40 horses in three KIND-014 groups with different doses and dosing schedules, 13 horses in the placebo group). The objective was to determine the effective dose of KIND-014 for the treatment of gastric ulcers in horses. At Week 3, the gastric ulcer resolution (gastric ulcer score=0) rates in all three KIND-014 groups were statistically significantly higher than the placebo group (p-values < 0.05). The pivotal field efficacy study for KIND-014 for the treatment of gastric ulcers in horses was initiated in December 2019.
In addition to the product candidates discussed above, we are in the early stages of development for multiple additional indications, including interleukin antibodies and canine checkpoint inhibitors, with the potential to attain approval for one or more products annually for several years. In all, we have over 20 programs for various indications for dogs, cats, and horses.
We have constructed a Good Manufacturing Practice, or GMP, biologics manufacturing plant in Burlingame, CA which is fully commissioned. We have proceeded to GMP manufacturing of our feline erythropoietin product candidate in January 2018. In addition, construction and commissioning of our biologics manufacturing lines in our manufacturing plant in Elwood, Kansas have been completed. The Elwood facility includes approximately 180,000 square feet with clean rooms, utility, equipment, and related quality documentation suitable for biologics and small molecule manufacturing.
We are a commercial-stage company with two products just recently approved for marketing and sale. We have incurred significant net losses since our inception. We incurred cumulative net losses of $223.1 million through December 31, 2019. These losses have resulted principally from costs incurred in connection with investigating and developing our product candidates, research and development activities and general and administrative costs associated with our operations.
Historically, our funding has been a combination of private and public offerings. From our initial public offering in December 2013 through December 2018, we raised approximately $226.4 million in net proceeds, after
deducting underwriting discounts and commissions and offering expenses. On January 23, 2019, we completed a public offering of 4,847,250 shares of our common stock, which included the exercise in full by the underwriters of their option to purchase 632,250 additional shares of our common stock, at a public offering price of $9.50 per share for total gross proceeds of approximately $46 million. Net proceeds, after deducting underwriting discounts and commissions and offering expenses were approximately $43.1 million. As of December 31, 2019, we had cash, cash equivalents and investments in available-for-sale securities of $73.5 million.
For the foreseeable future, we expect to continue to incur losses, which will increase significantly from historical levels as we expand our product development activities, seek regulatory approvals for our product candidates and begin to commercialize them if they are approved by the Center for Veterinary Medicine branch, or CVM, of the FDA, the U.S. Department of Agriculture, or USDA, or the European Medicines Agency, or EMA. If we are required to further fund our operations, we expect to do so through public or private equity offerings, debt financings, corporate collaborations and licensing arrangements. We cannot assure you that such funds will be available on terms favorable to us, if at all. Arrangements with collaborators or others may require us to relinquish rights to certain of our technologies or product candidates. In addition, we may never successfully complete development of, obtain adequate patent protection for, obtain necessary regulatory approval, or achieve commercial viability for any of our biologic product candidates. If we are not able to raise additional capital on terms acceptable to us, or at all, as and when needed, we may be required to curtail our operations, and we may be unable to continue as a going concern.
Financial Overview
Revenues
Our revenues consist of net sales of our two products, Mirataz and Zimeta.
Cost of Product Revenues
Cost of product revenues consists primarily of the cost of direct materials, direct labor and overhead costs associated with manufacturing, inbound shipping and other third-party logistics costs.
Research and Development Expense
All costs of research and development are expensed in the period incurred. Research and development costs primarily consist of contracted development costs, manufacturing costs, salaries and related expenses for personnel, stock-based compensation expense, regulatory, outside service providers, professional and consulting services, travel costs and materials used in clinical trials and research and development.
We are currently pursuing over 10 indications. We typically use our employee and infrastructure resources across multiple development programs. We track outsourced development costs by development compound but do not allocate personnel or other internal costs related to development to specific programs or development compounds as these expenses are included in personnel costs and other internal costs.
Selling, General and Administrative Expense
Selling, general and administrative expense consists primarily of personnel costs, including salaries, related benefits and stock-based compensation for employees, consultants and directors. Selling, general and administrative expenses also include rent and other facilities costs, conference and sponsorship activities, travel costs, professional fees for legal, accounting and tax, information technology services, business development activities, costs associated with being a public company and other general and commercial business services.
As disclosed in our announced on March 16, 2020, KindredBio will transition to a partnership-focused commercialization strategy similar to the traditional human biotech commercialization strategy whereby pipeline assets are partnered with larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. Furthermore, the divestiture of Mirataz will result in our companion animal commercial infrastructure to be substantially reduced.
Interest and Other Income, Net
Consist of interest earned on our cash, cash equivalents and short-term investments, interest expenses on our long-term loan and asset disposals.
Income Taxes
As of December 31, 2019, we had net operating loss carryforwards for federal and state income tax purposes of $194,548,000 and $113,110,000, respectively, which will begin to expire in fiscal year 2032. The Federal NOL generated after 2017 of $101,436,000 will carryforward indefinitely and be available to offset up to 80% of future taxable income each year. Our management has evaluated the factors bearing upon the realizability of our deferred tax assets, which are comprised principally of net operating loss carryforwards. Our management concluded that, due to the uncertainty of realizing any tax benefits as of December 31, 2019, a valuation allowance was necessary to fully offset our deferred tax assets.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, and revenue, costs and expenses and related disclosures during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 of the notes to our consolidated financial statements appearing elsewhere in this document, we believe that the estimates and assumptions involved in the following accounting policies may have the greatest potential impact on our consolidated financial statements.
Revenue Recognition
Our revenues consist of product revenues resulting from the sale of Mirataz and Zimeta. We account for a contract with a customer when there is a legally enforceable contract between us and our customer, the rights of the parties are identified, the contract has commercial substance, and collectability of the contract consideration is probable. Our customers could either be distributors who subsequently resell our products to third parties such as veterinarians, clinics or animal hospitals or the third parties themselves.
In accordance with ASC 606, we applied the following steps to recognize revenue for the sale of Mirataz and Zimeta that reflects the consideration to which we expect to be entitled to receive in exchange for the promised goods (See Note 2):
1. Identify the contract with a customer
2. Identify the performance obligations in the contract
3. Determine the transaction price
4. Allocate the transaction price to the performance obligations
5. Determine the satisfaction of performance obligation
Product Returns
Consistent with the industry practice, we generally offer customers a limited right of return of damaged or expired product that has been purchased directly from us. Our return policy generally allows customers to receive
credit for expired products within 90 days after the product’s expiration date. We estimate the amount of our product revenues that may be returned by our customers and record these estimates as a reduction of product revenues in the period the related product revenues are recognized, as well as within accrued liabilities, in the consolidated balance sheets.
Fair Value Measurements
We invest our cash in money market funds, cash deposits and debt instruments of the U.S. government agency securities. In the current market environment, the assessment of the fair value of the debt securities can be difficult and subjective. Accounting Standards Codification, or ASC, 820, “Fair Value Measurements and Disclosure" standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
|
| | | | |
| Level 1 | | Quoted prices in active markets for identical assets or liabilities; |
| Level 2 | | Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and |
| Level 3 | | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The determination of fair value for Level 3 instruments requires the most management judgment and subjectivity. |
Inventories
We value inventory at the lower of cost or net realizable value. We determine the cost of inventory using the standard-cost method, which approximates actual cost based on a first-in, first-out method. We analyze our inventory levels quarterly and write down inventory subject to expire in excess of expected requirements, or that has a cost basis in excess of its expected net realizable value. These inventory related costs are recognized as cost of product revenues on the accompanying consolidated statements of operations. Currently our inventory consists of finished goods only.
Research and Development
As part of the process of preparing our consolidated financial statements, we are required to estimate accrued research and development expenses. Examples of estimated accrued expenses include fees paid to vendors and clinical sites in connection with our pivotal studies, to CROs in connection with our toxicology studies, and to contract manufacturers in connection with the production of API and formulated drug.
We review new and open contracts and communicate with applicable internal and vendor personnel to identify services that have been performed on our behalf and estimate the level of service performed and the associated costs incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost for accrued expenses. The majority of our service providers invoice us monthly in arrears for services performed or as milestones are achieved in relation to our contract manufacturers. We make estimates of our accrued expenses as of each balance sheet date.
We base our accrued expenses related to pivotal studies on our estimates of the services received and efforts expended pursuant to contracts with vendors, our internal resources, and payments to clinical sites based on enrollment projections. The financial terms of the vendor agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of animals and the completion of development milestones. We estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the related expense accrual accordingly on a prospective basis. If we do not identify costs that have been incurred or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not made any material adjustments to our estimates of accrued research and development expenses or the level of services performed in any reporting period presented.
Stock-Based Compensation
We measure stock-based awards granted to employees and directors at fair value on the date of grant and recognize the corresponding compensation expense of the awards, net of estimated forfeitures, over the requisite service periods, which correspond to the vesting periods of the awards. Generally, we issue stock-based awards with only service-based vesting conditions, and record compensation expense for these awards using the straight-line method. Our intention is to grant stock-based awards with exercise prices equivalent to the fair value of our common stock as of the date of grant.
Our accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows Financial Accounting Standards Board ("FASB") guidance. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s performance is complete or the date at which a commitment for performance is reached. Non-employee grants of stock-based compensation, except those grants to non-employee, shareholder-approved Board Members, were previously fair valued under ASC 505-50 “Equity-Based Payments to Non-employees”. In Q2 2018, we adopted ASU 2018-07 “Compensation - Stock Compensation (Topic 718) - Improvements to Non-Employee Share-Based Payment Accounting and non-employee grants are now fair valued in the same manner as employee grants.
The fair value of each stock-based award is estimated using the Black-Scholes option-pricing model. The expected volatility is based on the historic volatility of our own stock. The expected terms of our awards have been determined utilizing the “simplified” method, since our historical experience for option grants is not relevant to our expectations for recent grants. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is zero, based on the fact that we have never paid cash dividends and do not expect to pay any cash dividends in the foreseeable future. See Note 10 in Notes to Consolidated Financial Statements for further information.
Results of Operations
The following table summarizes the results of our operations for the periods indicated:
|
| | | | | | | | | | | |
| (In thousands, except per share amounts) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| | | | | | |
| Net product revenues | $ | 4,256 |
| | $ | 1,966 |
| | $ | — |
|
| | | | | | |
| Operating costs and expenses: | | | | | |
| Cost of product revenues | 587 |
| | 324 |
| | — |
|
| Research and development | 28,310 |
| | 26,399 |
| | 17,665 |
|
| General and administrative | 37,926 |
| | 26,499 |
| | 13,988 |
|
| Total operating costs and expenses | 66,823 |
| | 53,222 |
| | 31,653 |
|
| | | | | | |
| Loss from operations | (62,567 | ) | | (51,256 | ) | | (31,653 | ) |
| Interest and other income, net | 1,178 |
| | 1,566 |
| | 774 |
|
| Net loss | (61,389 | ) | | (49,690 | ) | | (30,879 | ) |
| Change in unrealized gains or losses on available-for-sale securities | 24 |
| | 20 |
| | — |
|
| Comprehensive loss | $ | (61,365 | ) | | $ | (49,670 | ) | | $ | (30,879 | ) |
| | | | | | |
| Net loss per share attributable to common stockholders, basic and diluted | $ | (1.59 | ) | | $ | (1.60 | ) | | $ | (1.23 | ) |
| | | | | | |
| Weighted-average number of common shares outstanding, basic and diluted | 38,657 |
| | 31,001 |
| | 25,084 |
|
Revenues
We adopted ASC 606 in the first quarter of our fiscal year that began on January 1, 2018. This new standard replaced the previous revenue recognition guidance in U.S. GAAP. No prior period adjustments were needed as our first commercial shipments began in July 2018. Our revenues consist of product revenue resulting from the sale of Mirataz for the management of weight loss in cats and Zimeta for the treatment of fever in horses. We account for a contract with a customer when there is a legally enforceable contract between us and our customer, the rights of the parties are identified, the contract has commercial substance, and collectability of the contract consideration is probable. Our revenues are measured based on the consideration specified in the contract with each customer, net of product returns, discounts and allowances.
The following table presents revenues and cost of product revenues for the years ended December 31, 2019, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2019 | | 2018 | | 2017 |
| | | | | | | |
| Gross product revenues | | | | | | |
| Mirataz | | $ | 4,151 |
| | $ | 2,027 |
| | $ | — |
|
| Zimeta | | 131 |
| | — |
| | — |
|
| Total gross product revenues | | 4,282 |
| | 2,027 |
| | — |
|
| Less allowance for product returns | | | | | | — |
|
| Mirataz | | (22 | ) | | (61 | ) | | — |
|
| Zimeta | | (4 | ) | | — |
| | — |
|
| Total allowance for product returns | | (26 | ) | | (61 | ) | | — |
|
| Net product revenues | | | | | | — |
|
| Mirataz | | 4,129 |
| | 1,966 |
| | — |
|
| Zimeta | | 127 |
| | — |
| | — |
|
| Total net product revenues | | 4,256 |
| | 1,966 |
| | |
| Cost of product revenues | | | | | | — |
|
| Mirataz | | (554 | ) | | (324 | ) | | — |
|
| Zimeta | | (33 | ) | | — |
| | — |
|
| Total cost of product revenues | | (587 | ) | | (324 | ) | | — |
|
| Gross profit | | | | | | |
| Mirataz | | 3,575 |
| | 1,642 |
| | — |
|
| Zimeta | | 94 |
| | — |
| | — |
|
| Total gross profit | | $ | 3,669 |
| | $ | 1,642 |
| | $ | — |
|
Concentrations of credit risk
Our revenue was generated entirely from sales within the United States. Our combined sales to three large distributors, namely MWI, Covetrus, and Patterson, each accounted for more than 10% of total revenues for the year ended December 31, 2019. In total, these distributors accounted for approximately 85% of our product sales in 2019, with regional, home delivery partners, and e-commerce partners making up the remainder.
Four large distributors, namely MWI, Covetrus, Patterson and Midwest, each accounted for more than 10% of our total product sales for the year ended December 31, 2018. On a combined basis, in 2018, these distributors accounted for approximately 91% of our product sales in the United States.
Product returns
Our return policy generally allows customers to receive credit for expired products within 90 days after the product’s expiration date. We currently estimate product return liabilities of 2% for Mirtaza and 3% for Zimeta of gross revenue using probability-weighted available industry data and data provided by our distributors such as the inventories remaining in the distribution channel. Adjustments will be made in the future if actual results vary from our estimates.
Accounts receivable and allowance for doubtful accounts
Accounts receivable are stated at their carrying values, net of any allowances for doubtful accounts. Accounts receivable consist primarily of amounts due from distributors, for which collection is probable based on the customer's intent and ability to pay. Receivables are evaluated for collection probability on a regular basis and an allowance for doubtful accounts is recorded, if necessary. We have no allowance for doubtful accounts as of December 31, 2019 as our analysis did not uncover any collection risks.
Research and Development Expense
All costs of research and development are expensed in the period incurred. Research and development costs consist primarily of salaries and related expenses for personnel, stock-based compensation expense, fees paid to consultants, outside service providers, professional services, travel costs and materials used in clinical trials and research and development. We are currently pursuing multiple product candidates for over 20 indications. We typically use our employee and infrastructure resources across multiple development programs.
Research and development expense was as follows for the periods indicated:
|
| | | | | | | | | | | | | | | | | |
| (In thousands except percentages) | Years Ended December 31, | | Annual percent change |
| | 2019 | | 2018 | | 2017 | | 2019/2018 | | 2018/2017 |
| Payroll and related | $ | 12,646 |
| | $ | 10,204 |
| | $ | 6,517 |
| | 24 | % | | 57 | % |
| Consulting | 2,406 |
| | 2,360 |
| | 1,669 |
| | 2 | % | | 41 | % |
| Field trial costs, including materials | 4,015 |
| | 3,915 |
| | 3,675 |
| | 3 | % | | 7 | % |
| Biologics development and supplies | 2,526 |
| | 3,890 |
| | 1,418 |
| | (35 | )% | | 174 | % |
| Stock-based compensation | 1,848 |
| | 1,746 |
| | 1,650 |
| | 6 | % | | 6 | % |
| Other | 4,869 |
| | 4,284 |
| | 2,736 |
| | 14 | % | | 57 | % |
| | $ | 28,310 |
| | $ | 26,399 |
| | $ | 17,665 |
| | 7 | % | | 49 | % |
During the year ended December 31, 2019, research and development expense related primarily to advancing the development of KIND-014, KIND-012, CAD programs (KIND-016, 025 and 032), KIND-510a and other early stage programs. In addition, we have increased the headcount of our in-house team to focus on the GMP manufacturing process for our potential biologic candidates.
Research and development expenses for the year ended December 31, 2019 increased by 7% to $28.3 million compared with $26.4 million for the same period in 2018. Payroll and related expenses increased by $2.4 million due to increase in headcount as we advance our biologics development and manufacturing programs. Higher regulatory and research fees, consulting, depreciation, rent as a result of expanded lab spaces and other facility costs also contributed to the increase in expenses, offset by lower non-GMP production and testing expenses. Outsourced research and development expense related to KIND-014, KIND-012, CAD programs, KIND-510a and other product development programs for the year ended December 31, 2019 were $1.7 million, $0.6 million, $0.5 million, $0.4 million and $1.3 million, respectively.
Research and development expenses for the year ended December 31, 2018 increased by 49% to $26.4 million compared with $17.7 million for the same period in 2017. Payroll and related expenses increased by $3.7 million due to increase in headcount as we advance our development programs and our biologics manufacturing for epoCat and KIND-016, studies including lab supplies increased by $2.5 million. Higher regulatory and research fees, consulting, depreciation, rent as a result of expanded lab spaces and other facility costs as well as field trial costs also contributed to the increase in expenses. Outsourced research and development expense related to our Zimeta (IV and Oral), KIND-014, Mirataz and other product development programs for the year ended December 31, 2018 were $1.4 million, $0.7 million, $0.3 million and $2.0 million, respectively.
We expect research and development expense to increase for the foreseeable future as we continue to further develop our biologics programs. In addition, we expect biologics manufacturing expenses to increase due to the manufacture of KIND-016 and KIND-030 antibodies for our pivotal field studies. Due to the inherently unpredictable nature of our development, we cannot reasonably estimate or predict the nature, specific timing or estimated costs of the efforts that will be necessary to complete the development of our product candidates.
Selling, General and Administrative Expense
The composition of general and administrative expense was as follows for the periods indicated:
|
| | | | | | | | | | | | | | | | | |
| (In thousands except percentages) | Years Ended December 31, | | Annual percent change |
| | 2019 | | 2018 | | 2017 | | 2019/2018 | | 2018/2017 |
| Payroll and related | $ | 15,385 |
| | $ | 9,498 |
| | $ | 4,391 |
| | 62 | % | | 116 | % |
| Consulting, professional and legal fees | 3,523 |
| | 3,561 |
| | 2,011 |
| | (1 | )% | | 77 | % |
| Stock-based compensation | 5,509 |
| | 4,531 |
| | 3,557 |
| | 22 | % | | 27 | % |
| Corporate and marketing expenses | 5,022 |
| | 4,420 |
| | 2,084 |
| | 14 | % | | 112 | % |
| Other | 8,487 |
| | 4,489 |
| | 1,945 |
| | 89 | % | | 131 | % |
| | $ | 37,926 |
| | $ | 26,499 |
| | $ | 13,988 |
| | 43 | % | | 89 | % |
Selling, general and administrative expenses for the year ended December 31, 2019 increased by 43% to $37.9 million compared with $26.5 million for the same period in 2018. Headcount increase was due to the expansion of our commercial organization and administrative personnel to support the company's growth. Sales and marketing expenses account for a big component of the increase. Higher stock-based compensation expense also contributed to the increase.
General and administrative expenses for the year ended December 31, 2018 increased by 89% to $26.5 million compared with $14.0 million for the same period in 2017. The overall increase is primarily the result of KindredBio's transition from a development stage to a commercial stage company. Headcount increase was due to the expansion of our commercial organization and administrative personnel to support the company's growth. Sales and marketing expenses account for a big component of the increase due to the launch of Mirataz. Consulting, legal and professional fees as well as facilities costs increased as we became a commercial stage organization. Higher stock-based compensation expense also contributed to the increase.
We expect selling, general and administrative expense to decrease going forward as we transition to a partnership-focused commercialization strategy whereby pipeline assets are out-licensed to larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. Furthermore, the divestiture of Mirataz will result in our companion animal commercial infrastructure to be substantially reduced.
Interest and Other Income, Net
|
| | | | | | | | | | | |
| (In thousands) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Interest and other income, net | $ | 1,178 |
| | $ | 1,566 |
| | $ | 774 |
|
The decrease of approximately $388,000 in 2019 compared to 2018 was mainly caused by the borrowing on a loan, which incurred approximately $461,000 of interest expenses. In addition, the change was further impacted by disposals of fixed assets, which was $212,000 and offset by an increase in interest income of $339,000, which resulted from our investments. The $792,000 increase in 2018 compared to 2017 was due to higher interest income from higher cash balances as a result of the completion of our ATM financings and follow-on public offerings of common stock in 2018 and 2017.
Liquidity and Capital Resources
We have incurred losses and negative cash flows from operations since our inception in September 2012. We incurred net losses of $61.4 million, $49.7 million and $30.9 million for the years ended December 31, 2019, 2018, and 2017, respectively. These losses have resulted primarily from costs incurred in research and development activities and selling, general and administrative costs associated with our operations. As of December 31, 2019, we had an accumulated deficit of $223.1 million.
From our initial public offering in December 2013 through December 2018, we raised approximately $226.4 million in net proceeds, after deducting underwriting discounts and commissions and offering expenses. In
January 2019, we completed a public offering of 4,847,250 shares of our common stock, which includes the exercise in full by the underwriters of their option to purchase 632,250 additional shares of the Company’s common stock, at a public offering price of $9.50 per share for total gross proceeds of approximately $46.0 million. Net proceeds, after deducting underwriting discounts and commissions and offering expenses were approximately $43.1 million. As of December 31, 2019, we had cash, cash equivalents and investments in available-for-sale securities of approximately $73.5 million, and including the $43 million from divestiture of our Mirataz asset, we believe are sufficient to fund our planned operations for approximately 36 months.
Cash Flows
The following table shows a summary of our cash flows for the periods set forth below: |
| | | | | | | | | | | |
| (In thousands) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Cash flows used in operating activities | $ | (56,342 | ) | | $ | (45,035 | ) | | $ | (21,878 | ) |
| Cash flows provided by (used in) investing activities | $ | (47,816 | ) | | $ | 16,604 |
| | $ | (2,668 | ) |
| Cash flows provided by financing activities | $ | 63,842 |
| | $ | 49,920 |
| | $ | 52,672 |
|
Net cash used in operating activities
During the year ended December 31, 2019, net cash used in operating activities was $56,342,000. Our net loss of $61,389,000 included non-cash charges primarily in the form of share-based compensation of $7,357,000, depreciation expense of $2,539,000, amortization of the debt discount of long-term loan of $84,000, shares issued for consulting services of $61,000 and loss on disposal of property and equipment of $212,000, partially offset by discounts and amortization of premiums on investments of $513,000. The non-cash charges were partly impacted by changes in operating assets and liabilities that resulted in approximately $4,693,000 of cash used in operating activities.
During the year ended December 31, 2018, net cash used in operating activities was $45,035,000. Our net loss of $49,690,000 included non-cash charges primarily in the form of share-based compensation of $6,277,000, depreciation expense of $805,000 and loss on disposal of property and equipment of $34,000, partially offset by discounts and amortization of premiums on investments of $179,000. The non-cash charges were partly impacted by changes in operating assets and liabilities that resulted in approximately $2,282,000 of cash provided by operating activities.
During the year ended December 31, 2017, net cash used in operating activities was $21,878,000. Our net loss of $30,879,000 included non-cash charges primarily in the form of share-based compensation of $5,207,000, depreciation expense of $475,000, loss on disposal of property and equipment of $27,000 and accretion of discounts and amortization of premiums on investments of $162,000. The non-cash charges were partly offset by changes in operating assets and liabilities that resulted in $3,130,000 of cash used in operating activities.
Net cash provided by (used in) investing activities
During the year ended December 31, 2019, net cash used in investing activities was $47,816,000, which resulted from $39,393,000 related to the purchases of investments, net of proceeds of sales and maturities of investments, further impacted by $9,725,000 in purchases of property and equipment, of which $1,297,000 is included in accounts payable and accrued liabilities at December 31, 2019 , also positively affected by sale of equipment of $5,000.
During the year ended December 31, 2018, net cash provided in investing activities was $16,604,000, which resulted from $30,275,000 related to the proceeds of sales and maturities of investments, net of purchases of investments, offset by $20,124,000 in purchases of property and equipment, of which $6,205,000 is included in accounts payable and accrued liabilities at December 31, 2018, also positively affected by sale of equipment of $248,000.
During the year ended December 31, 2017, net cash used by investing activities was $2,668,000, which resulted from $3,252,000 related to the maturities of investments, net of purchases of investments, partially offset by $6,186,000 in purchases of property and equipment, of which $266,000 is included in accounts payable and accrued liabilities at December 31, 2017.
Net cash provided by financing activities
During the year ended December 31, 2019, net cash provided by financing activities consisted of approximately $43,125,000 in net proceeds from the sale of common stock through a follow-on public offering, net proceeds of $19,181,000 from a loan agreement and approximately $2,029,000 from the exercise of stock options and purchase of ESPP shares, offset by payment of $493,000 related to restricted stock awards tax liability on net settlement.
During the year ended December 31, 2018, net cash provided by financing activities consisted of approximately $49,180,000 in net proceeds from the sale of common stock through an ATM and follow-on public offering, and approximately $987,000 from the exercise of stock options and purchase of ESPP shares, offset by payment of $247,000 related to restricted stock awards tax liability on net settlement.
During the year ended December 31, 2017, net cash provided by net cash provided by financing activities consisted of approximately $52,160,000 in net proceeds from the sale of common stock through an ATM and follow-on public offering, and approximately $512,000 from stock option exercises and purchase of ESPP shares.
Future Funding Requirements
We anticipate that we will continue to incur losses for the next several years due to expenses relating to:
| |
| • | pivotal trials of our product candidates; |
| |
| • | toxicology studies for our product candidates; and |
| |
| • | biologics manufacturing. |
We believe our existing cash, cash equivalents and investments in available-for-sale securities will be sufficient to fund our operating plan for approximately 36 months. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to stockholders, imposition of debt covenants and repayment obligations or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements depend on many factors, including, but not limited to:
| |
| • | the scope, progress, results and costs of researching and developing our current or future product candidates; |
| |
| • | the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates; |
| |
| • | the number and characteristics of the product candidates we pursue; |
| |
| • | the cost of manufacturing our current and future product candidates and any products we successfully out-license, including cost of building internal biologics manufacturing capacity; |
| |
| • | the expenses needed to attract and retain skilled personnel; |
| |
| • | the costs associated with being a public company; |
| |
| • | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; and |
| |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing current and future patents, including litigation costs and the outcome of any such litigation. |
Contractual Obligations
We have non-cancelable operating leases for two office spaces and expanded laboratory space under which we are obligated to make minimum lease payments totaling $3.8 million through May 2025, the timing of which is described in more detail in the notes to the consolidated financial statements. In addition, we have four operating leases for equipment under which we are obligated to make minimum lease payments totaling $39,000 through 2023.
Off-Balance Sheet Arrangements
Since inception, we have not engaged in the use of any off-balance sheet arrangements, such as structured finance entities, special purpose entities or variable interest entities.
Recently Issued Accounting Pronouncements
In February 2016, Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-02, "Leases (Topic 842)", requiring organizations that lease assets—referred to as “lessees”—to recognize on the consolidated balance sheet the assets and liabilities for the rights and obligations created by those leases. Under the new guidance, a lessee will be required to recognize assets and liabilities for leases with lease terms of more than 12 months. The ASU on leases took effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. We adopted this new standard on January 1, 2019, using the alternative modified transition method, that means using a cumulative-effect adjustment, if any, to the opening balance of retained earnings to be recognized on the date of adoption with prior periods not restated. Upon adoption, on January 1, 2019, we recorded a $1,941,000 increase in operating lease right-of-use assets, a $69,000 decrease in other current assets, a $115,000 decrease in other liabilities and a $1,985,000 increase in operating lease obligations. There was no cumulative-effect adjustment needed on January 1, 2019. The new standard provides a number of optional practical expedients in transition. We elected the following "package of practical expedients" when assessing the transition impact as the lessee as of January 1, 2019: (1) not to reassess whether any expired or existing contracts, contain leases; (2) not to reassess the lease classification for any expired or existing leases; and (3) not to reassess initial direct costs for any existing leases. Leases with an initial term of 12 months or less are not recorded on the balance sheet as we recognize lease expense for these leases on a straight-line basis over the lease term. The major impact for KindredBio was the balance sheet recognition of right-of-use ("ROU") assets and lease liabilities for operating leases as a lessee. We determine if an agreement contains a lease at inception. For agreements where we are the lessee, operating lease are included in operating lease right-of-use assets, current portion of operating lease liabilities and long-term portion operating lease liabilities on the condensed consolidated balance sheet as of December 31, 2019. Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. ROU assets also include any initial direct costs incurred and any lease payments made at or before the lease commencement date, less lease incentive received. We use our own incremental borrowing rate based on the information available at the commencement date in determining the lease liabilities as our leases generally do not provide an implicit rate. Lease terms don't include options to extend or terminate when we are reasonably certain that the option will not be exercised. Lease expense is recognized on a straight-line basis over the lease term.
In August 2018, the FASB issued ASU No. 2018-13, "Fair Value Measurement (Topic 820)", which changes to disclosure requirements for fair value measurement. The amendments of this update modify the disclosure requirements on fair value measurements about Topic 820. It applies to all reporting entities within the scope of the affected accounting guidance. It will take effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. We are currently evaluating the new guidance and have not determined the impact this standard may have on our financial statements.
In December 2019, the FASB issued ASU No. 2019-12, "Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes". The amendments of this update simplify the accounting for income taxes by removing several exceptions. One of the exceptions may affect our company is the following: exception to the incremental approach for intra-period tax allocation when there is a loss from continuing operations and income or a gain from other items (say, other comprehensive income). It applies to all reporting entities within the scope of the affected accounting guidance. It will take effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. Early adoption is permitted. We are currently evaluating the new guidance and have not determined the impact this standard may have on our financial statements.
We do not believe there are any other recently issued standards not yet effective that will have a material impact on our consolidated financial statements when the standards become effective.
| |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Fluctuation Risk
The primary objective of our investment activities is to preserve capital. We do not utilize hedging contracts or similar instruments.
We are exposed to certain market risks relating primarily to (1) interest rate risk on our cash and cash equivalents, (2) market price risk on our short-term investments, and (3) risks relating to the financial viability of the institutions which hold our capital and through which we have invested our funds. We manage such risks by investing in short-term, liquid, highly-rated instruments. As of December 31, 2019, our cash equivalents, short-term and long-term investments are invested in money market funds, U.S. treasury bills, U.S. treasury bonds, U.S. government agencies, commercial paper and high-grade corporate notes. We do not believe we have any material exposure to interest rate risk due to the extremely low interest rate environment, the short duration of the securities we hold and our ability to hold our investments to maturity if necessary. Declines in interest rates would reduce investment income, but would not have a material effect on our financial condition or results of operations.
We do not currently have exposure to foreign currency risk.
| |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
Our consolidated financial statements appear commencing on page F-1 of this Annual Report on Form 10-K, which information is incorporated herein by reference.
| |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| |
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) that are designed to assure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer (the “Certifying Officers"), as appropriate, to allow timely decisions regarding required disclosures.
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide reasonable assurance only of achieving the desired control objectives, and management necessarily is required to apply its judgment in weighing the costs and benefits of possible new or different controls and procedures. Limitations are inherent in all control systems, so no evaluation of controls can provide absolute assurance that all control issues and any fraud within the company have been detected.
As required by Exchange Act Rule 13a-15(b), as of the end of the period covered by this Annual Report on Form 10-K, management, under the supervision and with the participation of our Certifying Officers, evaluated the effectiveness of our disclosure controls and procedures. Based on this evaluation, the Certifying Officers have concluded that, as of the end of the period covered by this Annual Report on Form 10-K, our disclosure controls and procedures were effective at a reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations (COSO). Based on such evaluation, our management concluded that our internal control over financial reporting was effective at a reasonable assurance level as of December 31, 2019.
The effectiveness of our internal control over financial reporting as of December 31, 2019 has been audited by an independent registered public accounting firm, as stated in their attestation report, which is included in their annual report under “Item 8. Financial Statements and Supplementary Data” of this Annual Report.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| |
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required to be disclosed by this item will be contained in our Definitive Proxy Statement for the Annual Meeting of Stockholders to be filed with the SEC no later than April 30, 2020 and is incorporated herein by reference.
| |
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required to be disclosed by this item will be contained in our Definitive Proxy Statement for the Annual Meeting of Stockholders to be filed with the SEC no later than April 30, 2020 and is incorporated herein by reference.
| |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required to be disclosed by this item will be contained in our Definitive Proxy Statement for the Annual Meeting of Stockholders to be filed with the SEC no later than April 30, 2020 and is incorporated herein by reference.
| |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required to be disclosed by this item will be contained in our Definitive Proxy Statement for the Annual Meeting of Stockholders to be filed with the SEC no later than April 30, 2020 and is incorporated herein by reference.
| |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required to be disclosed by this item will be contained in our Definitive Proxy Statement for the Annual Meeting of Stockholders to be filed with the SEC no later than April 30, 2020 and is incorporated herein by reference.
PART IV
| |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
Our financial statements and related notes thereto are listed and included in this Annual Report on Form 10-K beginning on page F-1. The following exhibits are filed with, or are incorporated by reference into, this Annual Report:
EXHIBIT INDEX
|
| | |
| Exhibit No. | | Description |
| 2.1 | | |
| 3.1 | | |
| 3.2 | | |
| 3.3 | | |
| 4.1 | | |
| 4.2 | | |
| 4.3 | | |
| 10.1 | | |
| 10.2 | | |
| 10.3 | | |
| 10.4 | | |
| 10.5 | | |
| 10.6 | | |
| 10.7 | | |
| 10.8 | | |
| 10.9 | | |
| 10.10 | | |
| 10.11 | | |
| 10.12 | | |
| 10.13 | | |
| 10.14 | | |
| 10.15 | | |
|
| | |
| Exhibit No. | | Description |
| 10.16 | | |
| 10.17 | |
|
| 10.18 | | |
| 10.19 | | |
| 10.20 | | |
| 10.21 | | |
| 10.22 | | |
| 10.23 | | |
| 10.24 | | |
| 10.25 | | |
| 10.26 | | |
| 10.27 | | |
| 10.28 | | |
| 10.29 | | |
| 10.30 | | |
| 10.31 | | |
| 10.32 | | |
| 10.33 | | |
| 10.34 | | |
| 10.35 | | |
| 10.36 | | |
| 10.37 | |
|
| 10.38 | | First Amendment to Loan and Security Agreement dated as of March 16, 2020 among Kindred Biosciences, Inc., KindredBio Equine, Inc., Centaur Biopharmaceutical Services, Inc., Solar Capital, Ltd., as collateral agent and lender, and the other lenders named therein (27)
|
| 21.1 | |
|
| 23.1 | | |
|
| | |
| Exhibit No. | | Description |
| 31.1 | | |
| 31.2 | | |
| 32.1 | | |
| 101.INS | | Inline XBRL Instance Document |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB | | Iinline XBRL Taxonomy Extension Labels Linkbase Document |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| |
| * | Filed with this Annual Report on Form 10-K. |
| |
| † | Indicates a management contract or compensatory plan or arrangement. |
| |
| (1) | Previously filed on December 17, 2013 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference. |
| |
| (2) | Previously filed on December 2, 2013 as an exhibit to Registrant’s Amendment No. 4 to Registration Statement on Form S‑1 (File No. 333‑192242) and incorporated herein by reference. |
| |
| (3) | Previously filed on November 8, 2013 as an exhibit to Registrant’s Registration Statement on Form S-1 (File No. 333-192242) and incorporated herein by reference. |
| |
| (4) | Previously filed on November 13, 2013 as an exhibit to Registrant’s Amendment No. 1 to Registration Statement on Form S-1 (File No. 333-192242) and incorporated herein by reference. |
(5) Previously filed on May 14, 2014 as an exhibit to Registrant’s Quarterly Report on Form 10-Q and incorporated herein by reference.
(6) Previously filed on August 13, 2014 as an exhibit to Registrant’s Quarterly Report on Form 10-Q and incorporated herein by reference.
(7) Previously filed on October 14, 2014 as an appendix to Registrant’s Definitive Proxy Statement on Schedule 14A and incorporated herein by reference.
| |
| (8) | Previously filed on March 13, 2015 as an exhibit to Registrant’s Annual Report on Form 10-K and incorporated herein by reference. |
| |
| (9) | Previously filed on June 4, 2015 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference. |
| |
| (10) | Previously filed on April 8, 2016 as Appendix A to the Registrant’s Definitive Proxy Statement on Schedule 14A, and incorporated herein by reference. |
(11) Previously filed on June 3, 2016 as an exhibit to Registrant’s Report to Registration Statement on Form S‑8 (File No. 333‑211839) and incorporated herein by reference.
(12) Previously filed on May 24, 2017 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference.
(13) Previously filed on June 26, 2017 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference.
(14) Previously filed on July 28, 2017 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference.
(15) Previously filed on November 7, 2017 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference.
(16) Previously filed on March 1, 2017 as an exhibit to Registrant’s Annual Report on Form 10-K and incorporated herein by reference.
(17) Previously filed on March 1, 2018 as an exhibit to Registrant’s Annual Report on Form 10-K and incorporated herein by reference.
(18) Previously filed on April 25, 2018 as Appendix A to the Registrant’s Definitive Proxy Statement on Schedule 14A, and incorporated herein by reference.
(19) Previously filed on April 25, 2018 as Appendix B to the Registrant’s Definitive Proxy Statement on Schedule 14A, and incorporated herein by reference.
(20) Previously filed on May 8, 2018 as an exhibit to Registrant’s Quarterly Report on Form 10-Q and incorporated herein by reference.
(21) Previously filed on May 29, 2018 as an exhibit to Registrant’s Report on Form 8-K and incorporated herein by reference.
(22) Previously filed on July 24, 2018 as an exhibit to Registrant’s Registration Statement on Form S‑8 (File No. 333‑226321) and incorporated herein by reference.
(23) Previously filed on August 9, 2018 as an exhibit to Registrant’s Quarterly Report on Form 10-Q and incorporated herein by reference.
(24) Previously filed on November 7, 2018 as an exhibit to Registrant’s Quarterly Report on Form 10-Q and incorporated herein by reference.
| |
| (25) | Previously filed on October 2, 2019 as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K and incorporated herein by reference. |
(26) Previously filed on March 16, 2020 as Exhibit 2.1 to the Registrant's Current Report on Form 8-K and incorporated herein by reference.
(27) Previously filed on March 16, 2020 as Exhibit 10.1 to the Registrant's Current Report on Form 8-K and incorporated herein by reference.
| |
| ITEM 16. | FORM 10-K SUMMARY |
None
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | |
| | KINDRED BIOSCIENCES, INC. |
| | | |
| Date: March 16, 2020 | By: | /s/ Wendy Wee |
| | | Wendy Wee |
| | | Chief Financial Officer and Principal Financial and Accounting Officer |
Pursuant to the requirements of Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
| | |
| Signature | Title | Date |
| /s/ Richard Chin | Chief Executive Officer and Director | March 16, 2020 |
| Richard Chin, M.D. | (Principal Executive Officer) | |
| | | |
| /s/ Wendy Wee | Chief Financial Officer | March 16, 2020 |
| Wendy Wee | (Principal Financial and Accounting Officer) | |
| | | |
| /s/ Denise Bevers | President, Chief Operating Officer and Director | March 16, 2020 |
| Denise Bevers | | |
| | | |
| /s/ Ernest Mario | Director | March 16, 2020 |
| Ernest Mario, Ph.D. | | |
| | | |
| /s/ Joseph McCracken | Director | March 16, 2020 |
| Joseph McCracken, DVM | | |
| | | |
| /s/ Herbert Montgomery | Director | March 16, 2020 |
| Herbert Montgomery | | |
| | | |
| /s/ Raymond Townsend | Director | March 16, 2020 |
| Raymond Townsend, Pharm.D. | | |
| | | |
| /s/ Ervin Veszprémi | Director | March 16, 2020 |
| Ervin Veszprémi | | |
Index to Consolidated Financial Statements
|
| |
| | Page |
| Financial Statements: | |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
| | |
| |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Kindred Biosciences, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Kindred Biosciences, Inc. and subsidiaries (the “Company”) as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”), and our report dated March 16, 2020 expressed an unqualified opinion on the Company’s internal control over financial reporting.
Adoption of New Accounting Standard
As discussed in Note 2 to the consolidated financial statements, the Company changed its method of accounting for leases as of January 1, 2019 due to the adoption of ASU No. 2016-02, Leases (Topic 842).
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KMJ Corbin & Company LLP
We have served as the Company's auditor since 2013.
Costa Mesa, California
March 16, 2020
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Kindred Biosciences, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Kindred Biosciences, Inc. and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”), and our report dated March 16, 2020 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting included in Item 9A. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect
misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KMJ Corbin & Company LLP
Costa Mesa, California
March 16, 2020
Kindred Biosciences, Inc.
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
|
| | | | | | | |
| | December 31, |
| | 2019 | | 2018 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 15,986 |
| | $ | 56,302 |
|
| Short-term investments | 55,723 |
| | 17,630 |
|
| Accounts receivable | 923 |
| | 903 |
|
| Inventories | 4,218 |
| | 3,570 |
|
| Prepaid expenses and other | 2,495 |
| | 1,664 |
|
| Total current assets | 79,345 |
| | 80,069 |
|
| Property and equipment, net | 29,777 |
| | 26,343 |
|
| Long-term investments | 1,837 |
| | — |
|
| Operating lease right-of-use assets | 3,001 |
| | — |
|
| Other assets | 64 |
| | 70 |
|
| Total assets | $ | 114,024 |
| | $ | 106,482 |
|
| | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 1,256 |
| | $ | 3,576 |
|
| Accrued compensation | 4,193 |
| | 3,436 |
|
| Accrued liabilities | 4,131 |
| | 8,169 |
|
| Current portion of operating lease liabilities | 644 |
| | — |
|
| Total current liabilities | 10,224 |
| | 15,181 |
|
| Long-term liability | | | |
| Long-term operating lease liabilities | 2,614 |
| | — |
|
| Long-term deferred rent | — |
| | 94 |
|
| Long-term loan payable, net of debt discount | 19,265 |
| | — |
|
| Total liabilities | 32,103 |
| | 15,275 |
|
| | | | |
| Commitments and contingencies (Note 12) |
| |
|
| Stockholders’ equity: | | | |
| Common stock; $0.0001 par value; 100,000,000 shares authorized; 39,203,533 shares and 33,948,254 shares issued and outstanding at December 31, 2019 and 2018, respectively | 4 |
| | 3 |
|
| Additional paid-in capital | 304,963 |
| | 252,885 |
|
| Accumulated other comprehensive gain (loss) | 13 |
| | (11 | ) |
| Accumulated deficit | (223,059 | ) | | (161,670 | ) |
| Total stockholders’ equity | 81,921 |
| | 91,207 |
|
| Total liabilities and stockholders’ equity | $ | 114,024 |
| | $ | 106,482 |
|
The accompanying notes are integral part of these consolidated financial statements.
Kindred Biosciences, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except per share amounts)
|
| | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2019 | | 2018 | | 2017 |
| Net product revenues | | $ | 4,256 |
| | $ | 1,966 |
| | $ | — |
|
| | | | | | | |
| Operating costs and expenses: | | | | | | |
| Cost of product revenues | | 587 |
| | 324 |
| | — |
|
| Research and development | | 28,310 |
| | 26,399 |
| | 17,665 |
|
| General and administrative | | 37,926 |
| | 26,499 |
| | 13,988 |
|
| Total operating costs and expenses | | 66,823 |
| | 53,222 |
| | 31,653 |
|
| | | | | | | |
| Loss from operations | | (62,567 | ) | | (51,256 | ) | | (31,653 | ) |
| Interest and other income, net | | 1,178 |
| | 1,566 |
| | 774 |
|
| Net loss | | (61,389 | ) | | (49,690 | ) | | (30,879 | ) |
| Change in unrealized gains on available-for-sale securities | | 24 |
| | 20 |
| | — |
|
| Comprehensive loss | | $ | (61,365 | ) | | $ | (49,670 | ) | | $ | (30,879 | ) |
| | | | | | | |
| Net loss per share, basic and diluted | | $ | (1.59 | ) | | $ | (1.60 | ) | | $ | (1.23 | ) |
| | | | | | | |
| Weighted-average number of common shares outstanding, basic and diluted | | 38,657 |
| | 31,001 |
| | 25,084 |
|
The accompanying notes are integral part of these consolidated financial statements.
Kindred Biosciences, Inc.
Consolidated Statements of Changes in Stockholders' Equity
(In thousands)
|
| | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive income (Loss) | | Accumulated Deficit | | Total Stockholders' Equity |
| | Shares | | Amount | | | | |
| Balance at December 31, 2016 | 19,916 |
| | $ | 2 |
| | $ | 138,810 |
| | $ | (31 | ) | | $ | (81,101 | ) | | $ | 57,680 |
|
| Comprehensive loss | | | | | | | | | | | |
| Net loss | — |
| | — |
| | — |
| | — |
| | (30,879 | ) | | (30,879 | ) |
| Change in unrealized losses on available-for-sale securities | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Total comprehensive loss | | | | | | | | | | | (30,879 | ) |
| Restricted stock awards, unvested | 250 |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Stock-based compensation | — |
| | — |
| | 5,207 |
| | — |
| | — |
| | 5,207 |
|
| Exercise of common stock options | 157 |
| | — |
| | 311 |
| | — |
| | — |
| | 311 |
|
| At-the-Market issuance of common stock, net of $1,038 of issuance costs | 4,502 |
| | 1 |
| | 28,961 |
| | — |
| | — |
| | 28,962 |
|
| Public offering of common stock, net of $1,657 of offering costs | 3,314 |
| | — |
| | 23,198 |
| | — |
| | — |
| | 23,198 |
|
| Common stock issued under ESPP | 44 |
| | — |
| | 201 |
| | — |
| | — |
| | 201 |
|
| Balance at December 31, 2017 | 28,183 |
| | 3 |
| | 196,688 |
| | (31 | ) | | (111,980 | ) | | 84,680 |
|
| Comprehensive loss | | | | | | | | | | | |
| Net loss | — |
| | — |
| | — |
| | — |
| | (49,690 | ) | | (49,690 | ) |
| Change in unrealized gains on available for sale securities | — |
| | — |
| | — |
| | 20 |
| | — |
| | 20 |
|
| Total comprehensive loss | | | | | | | | | | | (49,670 | ) |
| Shares withheld related to net share settlement of equity awards | (27 | ) | | — |
| | (247 | ) | | — |
| | — |
| | (247 | ) |
| Stock-based compensation | — |
| | — |
| | 6,277 |
| | — |
| | — |
| | 6,277 |
|
| Exercise of common stock options | 231 |
| | — |
| | 635 |
| | — |
| | — |
| | 635 |
|
| At-the-Market issuance of common stock, net of $145 of issuance costs | 188 |
| | — |
| | 1,758 |
| | — |
| | — |
| | 1,758 |
|
| Public offering of common stock, net of $3,178 of offering costs | 5,326 |
| | — |
| | 47,422 |
| | — |
| | — |
| | 47,422 |
|
| Common stock issued under ESPP | 47 |
| | — |
| | 352 |
| | — |
| | — |
| | 352 |
|
| Balance at December 31, 2018 | 33,948 |
| | 3 |
| | 252,885 |
| | (11 | ) | | (161,670 | ) | | 91,207 |
|
| Comprehensive loss | | | | | | | | | | |
|
| Net loss | — |
| | — |
| | — |
| | — |
| | (61,389 | ) | | (61,389 | ) |
| Change in unrealized gains on available for sale securities | — |
| | — |
| | — |
| | 24 |
| | — |
| | 24 |
|
| Total comprehensive loss | | | | | | | | | | | (61,365 | ) |
| Shares withheld related to net share settlement of equity awards | (21 | ) | | — |
| | (214 | ) | | — |
| | — |
| | (214 | ) |
| RSU Issuance of shares when vested | 51 |
| | — |
| | (279 | ) | | — |
| | — |
| | (279 | ) |
| Stock-based compensation | — |
| | — |
| | 7,357 |
| | — |
| | — |
| | 7,357 |
|
| Shares issued for consulting services | 8 |
| | — |
| | 61 |
| | — |
| | — |
| | 61 |
|
| Exercise of common stock options | 306 |
| | — |
| | 1,591 |
| | — |
| | — |
| | 1,591 |
|
| Public offering of common stock, net of $2,924 of offering costs | 4,847 |
| | 1 |
| | 43,124 |
| | — |
| | — |
| | 43,125 |
|
| Common stock issued under ESPP | 65 |
| | — |
| | 438 |
| | — |
| | — |
| | 438 |
|
| Balance at December 31, 2019 | 39,204 |
| | $ | 4 |
| | $ | 304,963 |
| | $ | 13 |
| | $ | (223,059 | ) | | $ | 81,921 |
|
The accompanying notes are integral part of these consolidated financial statements.
Kindred Biosciences, Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
| | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2019 | | 2018 | | 2017 |
| Cash Flows from Operating Activities | | | | | | |
| Net loss | | $ | (61,389 | ) | | $ | (49,690 | ) | | $ | (30,879 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
| Stock-based compensation expense | | 7,357 |
| | 6,277 |
| | 5,207 |
|
| Shares issued for consulting services | | 61 |
| | — |
| | — |
|
| Depreciation and amortization expense | | 2,539 |
| | 805 |
| | 475 |
|
| Loss on disposal of property and equipment | | 212 |
| | 34 |
| | 27 |
|
| Amortization of (discount) premium on marketable securities | | (513 | ) | | (179 | ) | | 162 |
|
| Amortization of debt discount of long-term loan | | 84 |
| | — |
| | — |
|
| Changes in operating assets and liabilities: | | | | | | |
| Accounts receivable | | (20 | ) | | (903 | ) | | — |
|
| Inventories | | (648 | ) | | (3,570 | ) | | — |
|
| Prepaid expenses and other | | (825 | ) | | (867 | ) | | 485 |
|
| Other assets | | — |
| | (21 | ) | | (3 | ) |
| Accounts payable | | 1,838 |
| | 277 |
| | 1,280 |
|
| Accrued liabilities and accrued compensation | | (5,038 | ) | | 2,802 |
| | 1,368 |
|
| Net cash used in operating activities | | (56,342 | ) | | (45,035 | ) | | (21,878 | ) |
| | | | | | | |
| Cash Flows from Investing Activities | | | | | | |
| Purchases of investments | | (125,020 | ) | | (25,100 | ) | | (70,110 | ) |
| Sales of investments | | 2,999 |
| | 800 |
| | 4,897 |
|
| Maturities of investments | | 82,628 |
| | 54,575 |
| | 68,465 |
|
| Purchases of property and equipment | | (8,428 | ) | | (13,919 | ) | | (5,920 | ) |
| Proceeds from sale of property and equipment | | 5 |
| | 248 |
| | — |
|
| Net cash provided by (used in) investing activities | | (47,816 | ) | | 16,604 |
| | (2,668 | ) |
| | | | | | | |
| Cash Flows from Financing Activities | | | | | | |
| Exercise of stock options and purchase of ESPP shares | | 2,029 |
| | 987 |
| | 512 |
|
| Proceeds from loan payable, net of issuance costs | | 19,181 |
| | — |
| | — |
|
| Payment of restricted stock awards tax liability on net settlement | | (493 | ) | | (247 | ) | | — |
|
| Net proceeds from sales of common stock | | 43,125 |
| | 49,180 |
| | 52,160 |
|
| Net cash provided by financing activities | | 63,842 |
| | 49,920 |
| | 52,672 |
|
| Net change in cash and cash equivalents | | (40,316 | ) | | 21,489 |
| | 28,126 |
|
| Cash and cash equivalents at beginning of year | | 56,302 |
| | 34,813 |
| | 6,687 |
|
| Cash and cash equivalents at end of year | | $ | 15,986 |
| | $ | 56,302 |
| | $ | 34,813 |
|
| | | | | | | |
| Supplemental disclosure of non-cash financing activities: | | | | | | |
| Purchases of property and equipment included in accounts payable and accrued liabilities | | $ | 1,297 |
| | $ | 6,205 |
| | $ | 266 |
|
The accompanying notes are integral part of these consolidated financial statements.
Kindred Biosciences, Inc
Notes to Consolidated Financial Statements
1. Organization and Description of Business
Kindred Biosciences, Inc. (“we”, "us" or "our") was incorporated on September 25, 2012 (inception) in the State of Delaware. On April 25, 2016, we filed a Certificate of Incorporation with the State of Delaware for a wholly owned subsidiary, KindredBio Equine, Inc. ("KindredBio Equine"). KindredBio Equine has one class of capital stock which is designated common stock, $0.0001 par value per share. The authorized number of shares of common stock for KindredBio Equine is 1,000. On February 1, 2019, we filed a Certificate of Incorporation with the State of Delaware for a wholly owned subsidiary, Centaur Biopharmaceutical Services, Inc. ("Centaur Biopharmaceutical Services"). Centaur Biopharmaceutical Services has one class of capital stock which is designated common stock, $0.0001 par value per share. The authorized number of shares of common stock for Centaur Biopharmaceutical Services is 1,000.
We are a commercial-stage biopharmaceutical company focused on saving and improving the lives of pets. Our activities since inception have consisted principally of raising capital, establishing facilities, recruiting management and technical staff and performing research and development and advancing our product candidates seeking regulatory approval. Our headquarters are in Burlingame, California.
We are subject to risks common to companies in the biotechnology and pharmaceutical industries. There can be no assurance that our research and development will be successfully completed, that adequate protection for our technology will be obtained, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. We operate in an environment of substantial competition from other animal health companies. In addition, we are dependent upon the services of our employees and consultants, as well as third-party contract research organizations and manufacturers.
Liquidity
We have incurred losses and negative cash flows from operations and had an accumulated deficit of $223.1 million as of December 31, 2019. We expect to continue to incur losses and negative cash flows, which will increase significantly from historical levels as we expand our product development activities, seek regulatory approvals for our product candidates, establish a biologics manufacturing capability, and commercialize approved products. We might require additional capital until such time as we can generate operating revenues in excess of operating expenses. To date, we have been funded primarily through sales of convertible preferred stock and sales of our common stock. From our initial public offering in December 2013 through December 2018, we raised approximately $226.4 million in net proceeds, after deducting underwriting discounts and commissions and offering expenses. In January 2019, we completed a public offering of 4,847,250 shares of common stock, which includes the exercise in full by the underwriters' option to purchase 632,250 additional shares of common stock, at a public offering price of $9.50 per share for total gross proceeds of approximately $46.0 million. Net proceeds, after deducting underwriting discounts and commissions and offering expenses were approximately $43.1 million. As of December 31, 2019, we believe our cash, cash equivalents and investments in available-for-sale securities of approximately $73.5 million, along with the $43.0 million from the divestiture of Mirataz, are sufficient to fund our planned operations for approximately the next 36 months.
If we require additional funding for operations, we may seek such funding through public or private equity or debt financings or other sources, such as corporate collaborations and licensing arrangements. We may not be able to obtain financing on acceptable terms, or at all, and we may not be able to enter into corporate collaborations or licensing arrangements. The terms of any financing may result in dilution or otherwise adversely affect the holdings or the rights of our stockholders. If we are unable to obtain funding, we could be forced to delay, reduce or eliminate our research and development programs or commercialization efforts, which could adversely affect our business prospects.
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and include the accounts of the Company and its wholly owned subsidiaries (the "Company"). All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements and related disclosures in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting periods. Estimates are based historical experiences or on forecasts, including information received from third parties and other assumptions that the Company believes are reasonable under the circumstances. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Actual results could differ from those estimates.
Cash, Cash Equivalents and Investments
We consider all highly liquid investments purchased with an original maturity of three months or less at the date of acquisition to be cash equivalents. Debt securities with original maturities greater than three months and remaining maturities less than one year are classified as short-term investments. We classify all investments as available-for-sale. Available-for-sale securities are carried at estimated fair value, with accumulated unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) in the accompanying consolidated balance sheets.
Realized gains or losses on the sale of investments are determined on a specific identification method, and such gains and losses are reflected as a component of interest and other income, net in the accompanying consolidated statements of operations and comprehensive loss.
Marketable securities investments are evaluated periodically for impairment. We take into account general market conditions, changes in the economic environment as well as specific investment attributes, such as credit downgrade or illiquidity for each investment, the expected cash flows from the securities, our intent to sell the securities and whether or not we will be required to sell the securities before the recovery of their amortized cost, to estimate the fair value of our investments and to determine whether impairment is other than temporary. If it is determined that a decline in fair value of any investment is other than temporary, then the unrealized loss related to credit risk would be included in interest and other income, net.
Borrowings
On September 30, 2019, we entered into a Loan and Security Agreement (the “Loan Agreement”) with Solar Capital Ltd., as collateral agent and lender, and the other lenders named in the Loan Agreement (Solar Capital Ltd. and the other lenders collectively, the “Lenders”). The Lenders have agreed to make available to KindredBio an aggregate principal amount of up to $50.0 million under the Loan Agreement. We plan to use the loan proceeds to support the development and commercialization of our products and product candidates as well as for working capital and general corporate purposes. The Loan Agreement provides for a term loan commitment of $50.0 million in three tranches: (1) a $20.0 million term A loan that was funded on September 30, 2019; (2) a $15.0 million term B loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, no later than December 31, 2020; and (3) a $15.0
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
million term C loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, on or before June 30, 2021. Each term loan has a maturity date of September 30, 2024. Each term loan bears interest at a floating per annum rate equal to the one-month LIBOR rate (with a floor of 2.17%) plus 6.75%. We are permitted to make interest-only payments on each term loan through October 31, 2021. The interest-only period can be extended by six months upon our satisfaction of the minimum liquidity requirements described in the Loan Agreement. See Note 6. Based on the borrowing rates currently available for loans with similar terms, we believe the fair value of long-term debt approximates its carrying value.
Concentration of Credit Risk and of Significant Suppliers and Customers
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash, cash equivalents and investments. From time to time, we maintain cash and cash equivalent balances in excess of amounts insured by the Federal Deposit Insurance Corporation (“FDIC”) and the Securities Investor Protection Corporation ("SIPC"). Primarily all of our cash, cash equivalents and investments at December 31, 2019 were in excess of amounts insured by the FDIC and SIPC. We do not believe that we are subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
We are dependent on third-party manufacturers to supply products for research and development activities in our programs. In particular, we rely on a small number of manufacturers to supply us with our requirements for the active pharmaceutical ingredients, or API, and formulated drugs related to some of these programs. These programs would be adversely affected by a significant interruption in the supply of API.
We are also dependent on a combination of national and regional distributors for our product sales of Mirataz and Zimeta. See Note 3.
Fair Value Measurements
We use the provisions of Accounting Standards Codification ("ASC") 820, “Fair Value Measurements and Disclosure", to determine the fair values of our financial and nonfinancial assets and liabilities where applicable. ASC 820 defines fair value, establishes a framework for measuring fair value in U.S. GAAP and expands disclosure about fair value measurements. The objective of fair value measurement is to determine the price that would be received to sell the asset or paid to transfer the liability (an exit price) in an orderly transaction between market participants at the measurement date. ASC 820 emphasizes that fair value is a market-based measurement, not an entity-specific measurement, and that market participant assumptions include assumptions about risk and effect of a restriction on the sale or use of an asset. To increase consistency and comparability in fair value measurement and related disclosures, ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels: (1) Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date; (2) Level 2 inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly through corroboration with observable market data; and (3) Level 3 inputs are unobservable inputs for the asset or liability that reflect the reporting entity’s own assumptions about risk and the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances.
Government agency notes, corporate notes and commercial papers are recorded at their estimated fair value. Since these available-for-sale securities generally have market prices from multiple sources and it can be difficult to select the best individual price directly from the quoted prices in the active markets, we use Level 2 inputs for the valuation of these securities. Using the Level 2 inputs, a “consensus price” or a weighted average price for each of these securities can be derived from a distribution-curve-based algorithm which
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
includes market prices obtained from a variety of industrial standard data providers (e.g. Bloomberg), security master files from large financial institutions, and other third-party sources.
The carrying amount of financial instruments, including cash, accounts receivable, accounts payable and accrued liabilities approximate fair value due to the short maturities of these financial instruments. Financial assets, which consist of money market funds and available-for-sale securities, are measured at fair value on a recurring basis. (see Note 4). Based on the borrowing rates currently available for loans with similar terms, we believe the fair value of long-term debt approximates its carrying value.
Property and Equipment
On June 21, 2017, we entered into a purchase agreement with Strategic Veterinary Pharmaceuticals, Inc. ("SVP") for the purchase of an approximately 180,000 sq. ft. biologics plant ("the Plant") with clean rooms, utility, equipment, and related quality documentation suitable for small molecule and biologics manufacturing, that is located in Elwood, Kansas. The purchase was finalized on August 7, 2017 upon completion of the diligence period and satisfaction of the conditions of escrow. The Plant was purchased for $3,750,000, which includes approximately eight acres of land located at 1411 Oak Street, Elwood, Kansas, all improvements located at the Plant, and all personal property and intangible property owned by SVP and located at the Plant or used in connection with the operation of the Plant.
Property and equipment are stated at cost less accumulated depreciation and amortization. We calculate depreciation using the straight-line method over the estimated useful lives of the assets, which range from two to five years for furniture, fixtures, lab and computer equipment and software, and fifteen to thirty-nine years for land improvements and real property. Land and assets held within construction in progress are not depreciated. Construction in progress is related to the construction or development of property and equipment that have not yet been placed in service for their intended use. Expenditures for repairs and maintenance of assets are charged to expense as incurred. We amortize leasehold improvements using the straight-line method over the estimated useful lives of the respective assets or the lease term, whichever is shorter. Upon retirement or sale, the cost and related accumulated depreciation and amortization of assets disposed of are removed from the accounts and any resulting gain or loss is included in other income/expense.
Licenses
The costs incurred for the rights to use licensed technologies in the research and development process, including licensing fees and milestone payments, are charged to research and development expense as incurred in situations where we have not identified an alternative future use for the acquired rights, and are capitalized in situations where we have identified an alternative future use. No costs associated with the use of licensed technologies have been capitalized to date.
Impairment of Long-Lived Assets
We review long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Factors that we consider in deciding when to perform an impairment review include significant underperformance of the business in relation to expectations, significant negative industry or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate a long-lived asset for recoverability, we compare forecasts of undiscounted cash flows expected to result from the use and eventual disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would be based on the excess of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows. To date, we have not
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
recorded any impairment losses on long-lived assets.
Revenue Recognition
We adopted ASC Topic 606 (“ASC 606”), "Revenue from Contracts with Customers" in the first quarter of our fiscal year that began on January 1, 2018. This new standard replaced the previous revenue recognition guidance in U.S. GAAP. No prior period adjustments were needed as our first commercial shipments began in July 2018. Our revenue consists of product revenue resulting from the sale of Mirataz and Zimeta. We account for a contract with a customer when there is a legally enforceable contract between us and our customer, the rights of the parties are identified, the contract has commercial substance, and collectability of the contract consideration is probable. Our customers could either be distributors who subsequently resell our products to third parties such as veterinarians, clinics or animal hospitals or the third parties themselves.
We use contract manufacturers to produce Mirataz and Zimeta, and a third-party logistics vendor to process and fulfill orders. We concluded we are the principal in our sales because we control access to services rendered by both vendors and direct their activities. We have no significant obligations to distributors to generate pull-through sales.
In accordance with ASC 606, we applied the following steps to recognize revenue for the sale of Mirataz and Zimeta that reflects the consideration to which we expect to be entitled to receive in exchange for the promised goods:
1. Identify the contract with a customer
A contract with a customer exists when we enter into an enforceable contract with a customer. These contracts define each party's rights, payment terms and other contractual terms and conditions of the sale. We apply judgment in determining the customer’s ability and intention to pay, which is based on published credit and financial information pertaining to the customer.
2. Identify the performance obligations in the contract
Our product in a given purchase order is delivered at the same time and we do not separate an individual order into separate performance obligations. We have concluded the sale of finished goods and related shipping and handling are accounted for as a single performance obligation as there are no other promises to deliver goods beyond what is specified in each accepted customer order.
3. Determine the transaction price
The transaction price is determined based on the consideration to which we will be entitled to receive in exchange for transferring goods to the customer, typically a fixed consideration in our contractual agreements.
4. Allocate the transaction price to the performance obligations
The transaction price is allocated entirely to the performance obligation to provide pharmaceutical products. The nature of the promises/obligations under our contracts is to transfer a distinct good. Accordingly, because a single performance obligation exists, no allocation of the transaction price is necessary.
5. Determine the satisfaction of performance obligation
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
Revenue is recognized when control of the finished goods is transferred to the customer, net of applicable reserves for variable consideration. Control of the finished goods is transferred at a point in time, upon delivery to the customer.
Reserves for Variable Consideration
Revenues from product sales are recorded at the net sales price, which includes estimates of variable consideration for which reserves are established. Components of variable consideration include product returns, allowances and discounts. These estimates take into consideration a range of possible outcomes for the expected value (probability-weighted estimate) or relevant factors such as current contractual and statutory requirements, specific known market events and trends, industry data, and forecasted customer buying and payment patterns. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the respective underlying contracts. The amount of variable consideration included in the transaction price may be constrained and is included in the net sales price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized where the contract will not occur in a future period. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from our estimates, we will adjust these estimates, which would affect net product revenues and earnings in the period such variances become known.
Product Returns
Consistent with the industry practice, we generally offer customers a limited right of return of damaged or expired product that has been purchased directly from us. Our return policy generally allows customers to receive credit for expired products within 90 days after the product’s expiration date. We estimate the amount of our product revenues that may be returned by our customers and record these estimates as a reduction of product revenues in the period the related product revenues are recognized, as well as within accrued liabilities, in the consolidated balance sheets. We currently estimate product return liabilities using probability-weighted available industry data and data provided by the our distributors such as the inventories remaining in the distribution channel. To-date, we have no returns and believe that returns of our product in future periods will be minimal. We do not record a return asset associated with the returned damaged or expired goods due to such asset is deemed to be fully impaired at the time of product return.
Sales Discounts and Allowances
We compensate our distributors for sales order management, data and distribution and other services through sales discounts and allowances. However, such services are not distinct from our sale of products to distributors and, therefore, these discounts and allowances are recorded as a reduction of product revenues in the statements of operations, as well as a reduction to accounts receivable in the consolidated balance sheets.
Practical Expedients and Exemptions
We generally expense sales commissions when incurred because the amortization period would have been one year or less. These costs are recorded within sales and marketing expenses.
Cost of Product Revenues
Cost of product revenues consists primarily of the cost of direct materials, direct labor and overhead costs associated with manufacturing, inbound shipping and other third-party logistics costs.
Inventories
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
We value inventory at the lower of cost or net realizable value. We determine the cost of inventory using the standard-cost method, which approximates actual cost based on a first-in, first-out method. We analyze our inventory levels quarterly and write down inventory subject to expire in excess of expected requirements, or that has a cost basis in excess of its expected net realizable value. These inventory related costs are recognized as cost of product revenues on the accompanying Consolidated Statements of Operations and Comprehensive Loss. Currently our inventory consists of finished goods only.
Research and Development Costs
All costs of research and development are expensed in the period incurred. Research and development costs primarily consist of salaries and related expenses for personnel, stock-based compensation expense, fees paid to consultants, outside service providers, professional services, travel costs and materials used in clinical trials and research and development.
Patent Costs
All patent-related costs incurred in connection with filing patent applications are recorded in research and development expenses when incurred, as recoverability of such expenditures is uncertain.
Stock-Based Compensation
Our stock-based compensation plan (see Note 10) provides for the grant of stock options, restricted common stock, restricted stock units and stock appreciation rights. The estimated fair values of employee stock option grants are determined as of the date of grant using the Black-Scholes option pricing model. This method incorporates the fair value of our common stock at the date of each grant and various assumptions such as the risk-free interest rate, expected volatility based on historic volatility of Kindred Biosciences own stock prices, and expected dividend yield, and expected term of the options. The estimated fair values of restricted stock awards are determined based on the fair value of our common stock on the date of grant. The estimated fair values of stock-based awards, including the effect of estimated forfeitures, are expensed over the requisite service period, which is generally the awards’ vesting period. We classify stock-based compensation expense in the consolidated statements of operations and comprehensive loss in the same manner in which the award recipient’s payroll costs are classified.
Our accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows Financial Accounting Standards Board ("FASB") guidance. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s performance is complete or the date at which a commitment for performance is reached. Non-employee grants of stock-based compensation, except those grants to non-employee, shareholder-approved Board Members, were previously valued under ASC 505-50 "Equity-Based Payments to Non-Employees". In Q2 2018, we adopted ASU 2018-07 "Compensation - Stock Compensation (Topic 718) - Improvements to Non-Employee Share-Based Payment Accounting" and non-employee grants are now valued in the same manner as employee grants.
Income Taxes
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in our tax returns. Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
in the years in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. We assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe, based upon the weight of available evidence, that it is more likely than not that all or a portion of deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
We account for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Comprehensive Loss
Our comprehensive loss includes the change in unrealized gains or losses on available-for-sale securities. The cumulative amount of gains or losses is reflected as a separate component of stockholders' equity in the accompanying consolidated balance sheets as accumulated other comprehensive loss.
Segment Data
We manage our operations as a single segment for the purposes of assessing performance and making operating decisions. We are a veterinary biotechnology company focusing on developing therapies for pets. Our chief operating decision maker is our Chief Executive Officer. All assets are held in the United States.
Basic and Diluted Net Loss Per Common Share
Basic net loss per common share is computed by dividing net loss attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders for the period by the weighted average number of common shares, including potential dilutive shares of common stock assuming the dilutive effect of potentially dilutive securities. For periods in which we have reported a net loss, diluted net loss per common share is the same as basic net loss per common share, since the impact of the potentially dilutive securities would be anti-dilutive to the calculation of net loss per common share (see Note 14).
Recently Issued Accounting Pronouncements
In February 2016, Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-02, "Leases (Topic 842)", requiring organizations that lease assets—referred to as “lessees”—to recognize on the consolidated balance sheet the assets and liabilities for the rights and obligations created by those leases. Under the new guidance, a lessee will be required to recognize assets and liabilities for leases with lease terms of more than 12 months. The ASU on leases took effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. We adopted this new standard on January 1, 2019, using the alternative modified transition method, that means using a cumulative-effect adjustment, if any, to the opening balance of retained earnings to be recognized on the date of adoption with prior periods not restated. Upon adoption, on January 1, 2019, we
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
recorded a $1,941,000 increase in operating lease right-of-use assets, a $69,000 decrease in other current assets, a $115,000 decrease in other liabilities and a $1,985,000 increase in operating lease obligations. There was no cumulative-effect adjustment needed on January 1, 2019. The new standard provides a number of optional practical expedients in transition. We elected the following "package of practical expedients" when assessing the transition impact as the lessee as of January 1, 2019: (1) not to reassess whether any expired or existing contracts, contain leases; (2) not to reassess the lease classification for any expired or existing leases; and (3) not to reassess initial direct costs for any existing leases. Leases with an initial term of 12 months or less are not recorded on the balance sheet as we recognize lease expense for these leases on a straight-line basis over the lease term. The major impact for KindredBio was the balance sheet recognition of right-of-use ("ROU") assets and lease liabilities for operating leases as a lessee. We determine if an agreement contains a lease at inception. For agreements where we are the lessee, operating lease are included in operating lease right-of-use assets, current portion of operating lease liabilities and long-term portion operating lease liabilities on the condensed consolidated balance sheet as of December 31, 2019. Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. ROU assets also include any initial direct costs incurred and any lease payments made at or before the lease commencement date, less lease incentive received. We use our own incremental borrowing rate based on the information available at the commencement date in determining the lease liabilities as our leases generally do not provide an implicit rate. Lease terms don't include options to extend or terminate when we are reasonably certain that the option will not be exercised. Lease expense is recognized on a straight-line basis over the lease term.
In August 2018, the FASB issued ASU No. 2018-13, "Fair Value Measurement (Topic 820)", which changes to disclosure requirements for fair value measurement. The amendments of this update modify the disclosure requirements on fair value measurements about Topic 820. It applies to all reporting entities within the scope of the affected accounting guidance. It will take effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. We are currently evaluating the new guidance and have not determined the impact this standard may have on our consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, "Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes". The amendments of this update simplify the accounting for income taxes by removing several exceptions. One of the exceptions may affect our company is the following: exception to the incremental approach for intraperiod tax allocation when there is a loss from continuing operations and income or a gain from other items (say, other comprehensive income). It applies to all reporting entities within the scope of the affected accounting guidance. It will take effect for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. Early adoption is permitted. We are currently evaluating the new guidance and have not determined the impact this standard may have on our financial statements.
We do not believe there are any other recently issued standards not yet effective that will have a material impact on our consolidated financial statements when the standards become effective.
3. Revenues and Cost of Product Revenues
We adopted ASC 606 in the first quarter of our fiscal year that began on January 1, 2018. This new standard replaced the previous revenue recognition guidance in U.S. GAAP. No prior period adjustments were needed as our first commercial shipments began in July 2018. Our revenues consist of product revenue resulting from the sale of Mirataz for the management of weight loss in cats and Zimeta for the treatment of fever in horses. We account for a contract with a customer when there is a legally enforceable contract between us and our customer, the rights of the parties are identified, the contract has commercial substance, and
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
collectability of the contract consideration is probable. Our revenues are measured based on the consideration specified in the contract with each customer, net of product returns, discounts and allowances.
The following table presents revenues and cost of product revenues for the year ended December 31, 2019, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2019 | | 2018 | | 2017 |
| | | | | | | |
| Gross product revenues | | | | | | |
| Mirataz | | $ | 4,151 |
| | $ | 2,027 |
| | $ | — |
|
| Zimeta | | 131 |
| | — |
| | — |
|
| Total gross product revenues | | 4,282 |
| | 2,027 |
| | — |
|
| Less allowance for product returns | | | | | | |
| Mirataz | | (22 | ) | | (61 | ) | | — |
|
| Zimeta | | (4 | ) | | — |
| | — |
|
| Total allowance for product returns | | (26 | ) | | (61 | ) | | — |
|
| Net product revenues | | | | | | |
| Mirataz | | 4,129 |
| | 1,966 |
| | — |
|
| Zimeta | | 127 |
| | — |
| | — |
|
| Total net product revenues | | 4,256 |
| | 1,966 |
| | — |
|
| Cost of product revenues | | | | | | |
| Mirataz | | (554 | ) | | (324 | ) | | — |
|
| Zimeta | | (33 | ) | | — |
| | — |
|
| Total cost of product revenues | | (587 | ) | | (324 | ) | | — |
|
| Gross Profit | | | | | | |
| Mirataz | | 3,575 |
| | 1,642 |
| | — |
|
| Zimeta | | 94 |
| | — |
| | — |
|
| Total gross profit | | $ | 3,669 |
| | $ | 1,642 |
| | $ | — |
|
Concentrations of credit risk
Our revenue was generated entirely from sales within the United States. Our product sales to three large distributors, namely Henry Schein (now Covetrus), MWI and Patterson each accounted for more than 10% of total revenues for the year ended December 31, 2019. On a combined basis, in 2019, these distributors accounted for approximately 85% of our product sales in the United States.
Our product sales to four large distributors, namely MWI, Henry Schein (now Covetrus), Patterson and Midwest each accounted for more than 10% of total revenues for the year ended December 31, 2018. On a combined basis, in 2018, these distributors accounted for approximately 91% of our product sales in the United States.
Product returns
Our return policy generally allows customers to receive credit for expired products within 90 days after the product’s expiration date. We currently estimate product return liabilities of 2% for Mirataz and 3% for Zimeta of gross revenue using probability-weighted available industry data and data provided by our
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
distributors such as the inventories remaining in the distribution channel. Adjustments will be made in the future if actual results vary from our estimates.
Accounts receivable and allowance for doubtful accounts
Accounts receivable are stated at their carrying values, net of any allowances for doubtful accounts. Accounts receivable consist primarily of amounts due from distributors, for which collection is probable based on the customer's intent and ability to pay. Receivables are evaluated for collection probability on a regular basis and an allowance for doubtful accounts is recorded, if necessary. We have no allowance for doubtful accounts as of December 31, 2019 and 2018, as our analysis did not uncover any collection risks.
4. Fair Value Measurements
We measure certain financial assets at fair value on a recurring basis, including cash equivalents and available-for-sale securities. The fair value of these financial assets was determined based on a three-tier fair value hierarchy as described in Note 2, which prioritizes the inputs used in measuring fair value.
The following table presents information about our financial assets that are measured at fair value on a recurring basis as of December 31, 2019 and indicates the fair value hierarchy of the valuation techniques utilized to determine such fair value:
|
| | | | | | | | | | | | | | | | |
| (In thousands) | | Fair Value Measurements as of December 31, 2019 |
| Description | | Total | | Quoted Prices in Active Markets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Unobservable Inputs (Level 3) |
| Cash equivalents: | | | | | | | | |
| Money market funds | | $ | 1,592 |
| | $ | 1,592 |
| | $ | — |
| | $ | — |
|
| Commercial paper | | 13,580 |
| | — |
| | 13,580 |
| | — |
|
| Short-term investments: | | | | | | | | |
| U.S. treasury bills | | 8,524 |
| | 8,524 |
| | — |
| | — |
|
| Commercial paper | | 25,573 |
| | — |
| | 25,573 |
| | — |
|
| U.S. government agency notes | | 11,461 |
| | — |
| | 11,461 |
| | — |
|
| Corporate notes | | 10,165 |
| | — |
| | 10,165 |
| | — |
|
| Long-term investments: | | | | | | | | |
| US Government agency notes | | 801 |
| | — |
| | 801 |
| | — |
|
| Corporate notes | | 1,036 |
| | — |
| | 1,036 |
| | — |
|
| Total | | $ | 72,732 |
| | $ | 10,116 |
| | $ | 62,616 |
| | $ | — |
|
The following table presents information about our financial assets that are measured at fair value on a recurring basis as of December 31, 2018 and indicates the fair value hierarchy of the valuation techniques utilized to determine such fair value:
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | | | | | | | | | | | | | |
| (In thousands) | | Fair Value Measurements as of December 31, 2018 |
| Description | | Total | | Quoted Prices in Active Markets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Unobservable Inputs (Level 3) |
| Cash equivalents: | | | | | | | | |
| Money market funds | | $ | 1,276 |
| | $ | 1,276 |
| | $ | — |
| | $ | — |
|
| U.S. treasury bills | | 500 |
| | 500 |
| | — |
| | — |
|
| Commercial paper | | 45,332 |
| | — |
| | 45,332 |
| | — |
|
| U.S. treasury bonds and notes | | 7,949 |
| | — |
| | 7,949 |
| | — |
|
| Short-term investments: | | | | | | | | — |
|
| Commercial paper | | 5,353 |
| | — |
| | 5,353 |
| | — |
|
| Corporate notes | | 12,277 |
| | — |
| | 12,277 |
| | — |
|
| Total | | $ | 72,687 |
| | $ | 1,776 |
| | $ | 70,911 |
| | $ | — |
|
There were no other transfers of assets between Level 1, Level 2 or Level 3 of the fair value hierarchy during the years ended December 31, 2019 and 2018.
At December 31, 2019 and 2018, we did not have any financial liabilities which were measured at fair value on a recurring basis.
5. Investments
The following tables summarize our investments in available-for-sale securities by significant investment category reported as short-term or long-term investments as of December 31, 2019 and 2018 (in thousands):
|
| | | | | | | | | | | | | | | | |
| | | December 31, 2019 |
| | | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| Short-term investments: | | | | | | | | |
| U.S. treasury bills | | $ | 8,517 |
| | $ | 7 |
| | $ | — |
| | $ | 8,524 |
|
| Commercial paper | | 25,576 |
| | 3 |
| | (6 | ) | | 25,573 |
|
| �� U.S. government agency notes | | 11,460 |
| | 2 |
| | (1 | ) | | 11,461 |
|
| Corporate notes | | 10,157 |
| | 8 |
| | — |
| | 10,165 |
|
| | | 55,710 |
| | 20 |
| | (7 | ) | | 55,723 |
|
| Long-term investments: | | | | | | | | |
| U.S. government agency notes | | 801 |
| | — |
| | — |
| | 801 |
|
| Corporate notes | | 1,036 |
| | — |
| | — |
| | 1,036 |
|
| | | 1,837 |
| | — |
| | — |
| | 1,837 |
|
| Total available-for-sale investments | | $ | 57,547 |
| | $ | 20 |
| | $ | (7 | ) | | $ | 57,560 |
|
|
| | | | | | | | | | | | | | | | |
| | | December 31, 2018 |
| | | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| Short-term investments: | | | | | | | | |
| Commercial paper | | $ | 5,353 |
| | $ | — |
| | $ | — |
| | $ | 5,353 |
|
| Corporate notes | | 12,288 |
| | — |
| | (11 | ) | | 12,277 |
|
| Total available-for-sale investments | | $ | 17,641 |
| | $ | — |
| | $ | (11 | ) | | $ | 17,630 |
|
The following table summarizes the contractual maturities of our available-for-sale securities at December 31, 2019 (in thousands):
|
| | | | | | | |
| | Amortized Cost | | Fair Value |
| Mature in less than one year | $ | 55,710 |
| | $ | 55,723 |
|
| Mature in one year or more | $ | 1,837 |
| | $ | 1,837 |
|
6. Borrowings
On September 30, 2019, we entered into the Loan Agreement with the Lenders. The Lenders have agreed to make available to KindredBio an aggregate principal amount of up to $50.0 million under the Loan Agreement. We plan to use the loan proceeds to support the development and commercialization of our products and product candidates as well as for working capital and general corporate purposes.The Loan Agreement provides for a term loan commitment of $50.0 million in three tranches: (1) a $20.0 million term A loan that was funded on September 30, 2019; (2) a $15.0 million term B loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, no later than December 31, 2020; and (3) a $15.0 million term C loan that is to be funded at our request, subject to certain conditions described in the Loan Agreement being satisfied, on or before June 30, 2021. Each term loan has a maturity date of September 30, 2024. Each term loan bears interest at a floating per annum rate equal to the one-month LIBOR rate (with a floor of 2.17%) plus 6.75%. We are permitted to make interest-only payments on each term loan through October 31, 2021. The interest-only period can be extended by six months upon our satisfaction of the minimum liquidity requirements described in the Loan Agreement. We have agreed to maintain cash at all times equal to at least $5.0 million prior to the funding of the term B loan, at least $10.0 million after the funding of the term B loan and at least $15.0 million after the funding of the term C loan, plus in each case the amount of our accounts payable that have not been paid within 90 days from the invoice date subject to certain exceptions. Equal monthly payments of principal will be due and payable commencing at the end of the interest-only period of the term loans. In connection with the term loan, we incurred closing costs of $819,000, which are shown net of the proceeds and will be amortized over the term of the loan using the effective interest method. We are obligated to pay a facility fee in the amount of 0.50% of each term loan that is funded and a non-utilization fee in the amount of 0.25% of each term B loan and term C loan to the extent that such loans are not funded. We are obligated to pay a final fee equal to 3.60% of the aggregate amount of the term loans funded (or 4.35% of such funded loans if the interest-only period is extended as described above), such final fee to be due and payable upon the earliest to occur of (1) the maturity date, (2) the
acceleration of the term loans, and (3) the prepayment of the term loans. This final fee is being accrued over the term of the loan agreement. We have the option to prepay all, but not less than all, of the outstanding principal balance of the term loans under the Loan Agreement. If we prepay the term loans prior to the maturity date, we must pay the Lenders a prepayment premium fee based on a percentage of the outstanding principal balance, equal to 3.0% if the payment occurs on or before September 30, 2020, 2.0% if the prepayment occurs after September 30, 2020 but on or before September 30, 2021, or 1.0% if the prepayment
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
occurs after September 30, 2021. Our obligations under the Loan Agreement are secured by a first-priority security interest in substantially all of KindredBio’s assets, including our intellectual
property, and a lien on our real property. The Loan Agreement contains customary representations, warranties and covenants and also includes customary events of default, including payment defaults, breaches of covenants, and a default upon the occurrence of a material adverse change affecting us. Upon the occurrence of an event of default, a default interest rate of an additional 5.00% per annum may be applied to the outstanding loan balance, and the Lenders may declare all outstanding obligations immediately due and payable and exercise all their rights and remedies as set forth in the Loan Agreement and under applicable law. We were in compliance with all covenants as of December 31, 2019.
As of December 31, 2019, assuming the principal payments start on November 1, 2021, our future debt payment obligations towards the principal and final fee, excluding interest payments and exit fee, for the respective fiscal years are as follows (in thousands):
|
| | | | |
| 2020 | | $ | — |
|
| 2021 | | 1,111 |
|
| 2022 | | 6,667 |
|
| 2023 | | 6,667 |
|
| 2024 | | 6,275 |
|
| Total principal and final fee payments | | 20,720 |
|
| Less: unamortized debt issuance costs | | (771 | ) |
| Less: unaccreted value of final fee | | (684 | ) |
| Loan payable, long term | | $ | 19,265 |
|
7. Property and Equipment, Net
Property and equipment consisted of the following: |
| | | | | | | |
| | As of December 31, |
| (In thousands) | 2019 | | 2018 |
| Computer and lab equipment | $ | 10,188 |
| | $ | 4,923 |
|
| Furniture and fixtures | 143 |
| | 65 |
|
| Leasehold improvements | 958 |
| | 930 |
|
| Building | 9,520 |
| | — |
|
| Building improvements | 1,238 |
| | — |
|
| Land | 85 |
| | — |
|
| Land improvement | 166 |
| | — |
|
| Construction-in-process | 10,932 |
| | 21,999 |
|
| Total | 33,230 |
| | 27,917 |
|
| Less accumulated depreciation and amortization | (3,453 | ) | | (1,574) |
|
| Property and equipment, net | $ | 29,777 |
| | $ | 26,343 |
|
We constructed a Good Manufacturing Practice, or GMP, biologics manufacturing plant in Burlingame, CA which is fully commissioned. We have successfully completed cGMP manufacturing of our feline erythropoietin drug substance. In addition, construction to support initial production lines on our biologics manufacturing facility in Elwood, Kansas is completed. The fill finish line has been installed and fully commissioned. cGMP fill finish of our feline erythropoietin drug substance has been completed at our
Elwood, Kansas facility and is currently on test for quality release. The facility includes approximately 180,000 square feet with clean rooms, utility, equipment and related quality documentation suitable for small molecule and biologics manufacturing. Construction-in-process is comprised of equipment that have not been put into service for their intended use as of December 31, 2019. As disclosed in Note 2, the Kansas Plant was purchased for $3,750,000, which includes approximately eight acres of land, all improvements located at the Plant, and all personal property and intangible property located at the Plant or used in connection with the operation of the Plant.
Depreciation and amortization expense was $1,880,000, $805,000 and $475,000 for the years ended December 31, 2019, 2018 and 2017, respectively.
8. Accrued Liabilities
Accrued liabilities consisted of the following as of December 31, 2019 and 2018:
|
| | | | | | | |
| (In thousands) | As of December 31, |
| | 2019 | | 2018 |
| Accrued consulting | $ | 589 |
| | $ | 627 |
|
| Accrued research and development costs | 1,336 |
| | 2,509 |
|
| Accrued other | 2,206 |
| | 5,012 |
|
| Deferred rent | — |
| | 115 |
|
| | 4,131 |
| | 8,263 |
|
| Less current portion | (4,131 | ) | | (8,169 | ) |
| Long-term liability (deferred rent) | $ | — |
| | $ | 94 |
|
9. Stockholders' Equity
Preferred Stock
Our Certificate of Incorporation, as amended, authorizes us to issue 10,000,000 shares of $0.0001 par value preferred stock. At December 31, 2019, 100,000 unissued shares of our preferred stock are designated as Series A Preferred Stock, and the remaining 9,900,000 unissued shares of our preferred stock are undesignated.
Common Stock
Our Certificate of Incorporation, as amended and restated, authorizes us to issue 100,000,000 shares of $0.0001 par value common stock.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of our stockholders, provided, however, that, except as otherwise required by law, holders of common stock shall not be entitled to vote on any amendment to the Certificate of Incorporation that relates solely to the terms of one or more outstanding shares of preferred stock if the holders of such affected series are entitled, either separately or together with the holders of one or more other series, to vote thereon pursuant to the Certificate of Incorporation or pursuant to the Delaware General Corporation Law.
In 2017, we issued 156,927 shares of common stock upon exercise of stock options for total proceeds of $311,000. In addition, we issued 43,561 shares of common stock to employees in connection with our employee stock purchase program for total proceeds of $201,000.
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
In 2018, we issued 231,407 shares of common stock upon exercise of stock options for total proceeds of $635,000. In addition, we issued 46,850 shares of common stock to employees in connection with our employee stock purchase program for total proceeds of $352,000.
In 2019, we issued 305,801 shares of common stock upon exercise of stock options for total proceeds of $1,591,000. In addition, we issued 65,078 shares of common stock to employees in connection with our employee stock purchase program for total proceeds of $438,000.
As of December 31, 2019, we had 39,203,533 shares of common stock outstanding.
Stock Offerings
In January 2015, we filed a shelf registration statement on Form S-3 to offer and sell, from time to time, equity and debt securities in one or more offerings up to a total dollar amount of $150.0 million. On December 19, 2016, we entered into an At Market Issuance Sales Agreement with FBR Capital Markets & Co., or FBR, pursuant to which we were able to issue and sell shares of our common stock having an aggregate offering price up to $30.0 million, through FBR as our sales agent. In conjunction with the sales agreement, FBR received compensation based on an aggregate of 3% of the gross proceeds on the sale price per share of our common stock. Any sales made pursuant to the sales agreement were deemed an “at-the-market” offering and were made pursuant to the shelf registration statement on Form S-3. During the year ended December 31, 2017, we completed the sale of 4,501,985 shares of common stock under the Sales Agreement. Net proceeds, after deducting approximately $906,000 in commissions and fees and approximately $132,000 in offering costs, were approximately $28,962,000. On July 12, 2017, we completed an underwritten public offering of 3,000,000 shares of common stock at an offering price of $7.50 per share for total gross proceeds of $22,500,000. On August 11, 2017, we completed the closing of the exercise of the underwriter's option to purchase an additional 314,000 shares of common stock at the public offering price of $7.50 per share, resulting in additional gross proceeds of $2,355,000. After giving effect to the exercise of the over-allotment option, the total number of shares sold by us in the public offering increased to 3,314,000 shares and gross proceeds increased to $24,855,000. Net proceeds, after deducting underwriting commission and offering costs, were approximately $23,198,000.
In January 2018, we filed a new shelf registration statement on Form S-3 to offer and sell, from time to time, equity and debt securities in one or more offerings up to a total dollar amount of $150.0 million due to the expiration of our January 2015 shelf registration. In May 2018, we entered into an At Market Issuance Sales Agreement, or the Sales Agreement, with B. Riley FBR, Inc., and Oppenheimer & Co. Inc. acting as our distribution agents, relating to the sale of up to $50,000,000 of our common stock from time to time. We terminated the Sales Agreement in June 2018 after having sold 188,100 shares, representing gross proceeds of approximately $1,903,000. Net proceeds, after deducting commission, fees and offering costs, were approximately $1,758,000. On June 20, 2018, we entered into an underwriting agreement with Cantor Fitzgerald & Co., as representative of the underwriters, and on June 22, 2018 we completed a public offering of 5,326,314 shares of common stock, which included the underwriters' option to purchase additional shares, at a public offering price of $9.50 per share for total gross proceeds of approximately $50,600,000. Net proceeds, after deducting underwriting discounts and commissions and offering expenses were approximately$47,422,000.
On January 18, 2019, we entered into an underwriting agreement with Barclays Capital Inc. and Stifel, Nicolaus & Company, Incorporated, as representatives of the underwriters, and on January 23, 2019, we completed a public offering of 4,847,250 shares of our common stock, which included the exercise in full by the underwriters of their option to purchase 632,250 additional shares of the Company’s common stock, at a public offering price of $9.50 per share for total gross proceeds of approximately $46,049,000. Net proceeds,
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
after deducting underwriting discounts and commissions and offering expenses were approximately $43,125,000.
10. Stock-Based Awards and Benefit Plan
On November 4, 2012, our board of directors adopted the Kindred Biosciences, Inc. 2012 Equity Incentive Plan (the “2012 Plan"). The 2012 Plan provided for our board of directors to grant incentive stock options or non-qualified stock options for the purchase of common stock, to issue or sell shares of restricted common stock and to grant stock appreciation rights (“SARs”) to our employees, directors, consultants and advisers of the Company. Pursuant to the terms of the 2012 Plan, no options or SARs shall be granted under the 2012 Plan after 10 years from the date of adoption of the 2012 Plan. We reserved 4,000,000 shares of our common stock for issuance under the 2012 Plan. The 2012 Plan terminated in May 2016 and 2,740,842 stock option shares which had been granted prior to the plan’s expiration remaining outstanding as of December 31, 2019.
In May 2016, we adopted the 2016 Equity Incentive Plan (the “2016 Plan”), and reserved 3,000,000 shares of our common stock for issuance under the 2016 Plan. The 2016 Plan was the successor to our 2012 Plan and all awards made under the 2012 Plan remained subject to the terms of that plan. Options granted under the 2016 Plan were either incentive stock options or nonstatutory stock options. The 2016 Plan also provided for the grant of stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, performance cash awards and other stock awards. The exercise price of a stock option was not less than 100% of the closing price of our common stock on the date of the grant. If, at any time we granted an option, and the optionee directly or by attribution owned stock possessing more than 10% of the total combined voting power of all classes of our stock, the option price was at least 110% of the fair value and was not exercisable more than five years after the date of grant. Options generally vested over a period of one or four years from the date of grant. Options granted under the 2016 Plan expired no later than 10 years from the date of grant. As of December 31, 2019, there were 2,134,701 option shares outstanding, 125,000 restricted stock awards issued but unvested, and 236,250 restricted stock units granted but unvested, and no shares are available for future grants under the 2016 Plan since it was retired in June 2018.
In June 2018, we adopted the 2018 Equity Incentive Plan (the “2018 Plan”), and reserved 3,000,000 shares of our common stock for issuance under the 2018 Plan. The 2018 Plan is the successor to our 2016 Plan. All awards made under the 2016 and 2012 Plans shall remain subject to the terms of these plans. Options granted under the 2018 Plan may be either incentive stock options or nonstatutory stock options. The 2018 Plan also provides for the grant of stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, performance cash awards and other stock awards. The exercise price of a stock option may not be less than 100% of the closing price of our common stock on the date of the grant. If, at any time we grant an incentive stock option, and the optionee directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of our stock, the option price shall be at least 110% of the fair value and shall not be exercisable more than five years after the date of grant. Options generally vest over a period of one or four years from the date of grant. Options granted under the 2018 Plan expire no later than 10 years from the date of grant. As of December 31, 2019, there were 1,477,827 option shares outstanding, 264,075 restricted stock units granted but unvested, and 1,258,098 shares available for future grants under the 2018 Plan.
2014 Employee Stock Purchase Plan
In December 2014, our board of directors adopted the Kindred Biosciences, Inc. 2014 Employee Stock Purchase Plan (the “Purchase Plan”). A total of 200,000 shares of our common stock are authorized for issuance under the Purchase Plan. At the Annual Meeting of Stockholders of Kindred Biosciences, Inc. held on June 22, 2018, our stockholders approved an amendment to increase the number of shares that may be issued
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
under the ESPP from 200,000 shares to 500,000 shares. The Purchase Plan permits eligible employees to purchase common stock at a discount through payroll deductions during defined six months consecutive offering periods beginning on December 1st. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock on the first day of the offering or 85% of the fair market value of our common stock on the purchase date. A participant may purchase a maximum of 2,000 shares of common stock during each offering period, not to exceed $25,000 worth of common stock on the offering date during each calendar year. We use the Black-Scholes option pricing model, in combination with discounted employee price, in determining the value of the Purchase Plan expense to be recognized during each offering period. The weighted-average grant date fair value per share using the Black-Scholes option pricing model was $2.62 during the year ended December 31, 2019.
As of December 31, 2019, there were 249,470 shares of common stock issued under the Purchase Plan and 250,530 shares available for future issuance under the Purchase Plan. At December 31, 2019 and 2018, we had an outstanding liability of $40,000 and $47,000, respectively, which is included in accrued compensation on the consolidated balance sheets, for employee contributions to the Purchase Plan for shares pending issuance at the end of the next offering period.
Reserved Shares
At December 31, 2019, shares of common stock reserved for future issuance inclusive of outstanding option shares are as follows:
|
| | |
| 2018 Equity Incentive Plan | 1,258,098 |
|
| 2014 Employee Stock Purchase Plan | 250,530 |
|
| | 1,508,628 |
|
Stock Option Plan Activity Summary
A summary of activity under our stock option plans is as follows:
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | | | | | | | | |
| | Shares Available For Grant | | Shares Issuable Under Options | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term (In Years) | | Aggregate Intrinsic Value |
| Balance, December 31, 2016 | 2,927,650 |
| | 3,568,329 |
| | $6.36 | | 7.5 | | $4,353,000 |
| 2012 Plan true up retired shares (a) | (26,977 | ) | | — |
| | — | | | | |
| 2016 Plan issued RSA shares (b) | (250,000 | ) | | — |
| | — | | | | |
| Granted | (1,208,200 | ) | | 1,208,200 |
| | $6.62 | | | | |
| Exercised | — |
| | (156,927 | ) | | $1.98 | | | | |
| Expired | 7,267 |
| | (7,267 | ) | | $14.54 | | | | |
| Forfeited - stock options | 38,710 |
| | (38,710 | ) | | $6.60 | | | | |
| Balance, December 31, 2017 | 1,488,450 |
| | 4,573,625 |
| | $6.57 | | 7.2 | | $18,745,000 |
| 2012 Plan true up retired shares (c) | (56,953 | ) | | — |
| | — | | | | |
| 2016 Plan RSA forfeited on 1/23/18 (d) | 26,980 |
| | — |
| | — | | | | |
| 2016 Plan RSU issued on 1/22/18 (e) | (315,000 | ) | | — |
| | — | | | | |
| 2016 Plan true up retired shares (f) | (56,636 | ) | | — |
| | — | | | | |
| 2018 Incentive Plan (g) | 3,000,000 |
| | — |
| | — | | | | |
| Granted | (1,607,193 | ) | | 1,607,193 |
| | $9.78 | | | | |
| Exercised | — |
| | (242,031 | ) | | $3.19 | | | | |
| Expired | 36,791 |
| | (36,791 | ) | | $13.05 | | | | |
| Forfeited - stock options | 80,068 |
| | (80,068 | ) | | $7.40 | | | | |
| Balance, December 31, 2018 | 2,596,507 |
| | 5,821,928 |
| | $7.54 | | 7.1 | | $24,780,000 |
| 2012 Plan true up retired shares (h) | (94,700 | ) | | — |
| | — | | | | |
| 2016 Plan RSA forfeited on 1/23/19 (i) | 21,562 |
| | — |
| | — | | | | |
| 2016 Plan RSU forfeited on 1/22/19 (j) | 27,538 |
| | — |
| | — | | | | |
| 2016 Plan true up retired shares (k) | (191,491 | ) | | — |
| | — | | | | |
| 2018 Plan RSU Issued in Q1/2019 (l) | (264,075 | ) | | — |
| | — | | | | |
| Granted | (1,107,500 | ) | | 1,107,500 |
| | $9.77 | | | | |
| Exercised | — |
| | (305,801 | ) | | $5.21 | | | | |
| Expired | 104,710 |
| | (104,710 | ) | | $13.26 | | | | |
| Forfeited - stock options | 165,547 |
| | (165,547 | ) | | $7.97 | | | | |
| Balance, December 31, 2019 | 1,258,098 | | 6,353,370 |
| | $7.94 | | 6.6 | | $12,516,000 |
| Options vested and expected to vest, December 31, 2019 | | | 6,353,370 |
| | $7.94 | | 6.6 | | $12,516,000 |
| Options exercisable, December 31, 2019 | | | 4,504,720 |
| | $7.33 | | 5.8 | | $11,878,000 |
(a) The 2012 Equity Incentive Plan terminated in May 2016. All shares available for grant under this Plan expired.
True up all expired shares available for grant under the 2012 Equity Incentive Plan.
(b) Issued 250,000 RSA shares on January 23, 2017 under the 2016 Equity Incentive Plan.
(c) 2012 Equity Incentive Plan retired in May 2016. True up retirement in 2018.
(d) Vested 62,500 RSA shares on January 23, 2018. 26,980 shares were forfeited to cover tax liability.
(e) Issued 315,000 RSU units on January 22, 2018 under the 2016 Equity Incentive Plan.
(f) The 2016 Equity Incentive Plan terminated in June 2018. All shares available for grant under this Plan expired.
(g) The 2018 Equity Incentive Plan was adopted and approved by stockholders in June 2018.
(h) 2012 Equity Incentive Plan retired in May 2016. True up retirement in 2019.
(i) Vested 62,500 RSA shares on January 23, 2019. 21,562 shares were forfeited to cover tax liability.
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
(j) Vested 78,750 RSU units on January 22, 2019, 27,538 units were forfeited to cover tax liability.
(k) The 2016 Equity Incentive Plan terminated in June 2018. True up retirement in 2019.
(l) Issued 264,075 RSU units in Q1 2019 under the 2018 Equity Incentive Plan.
The aggregate intrinsic value of options is calculated as the difference between the exercise price of options and the fair value of our common stock for those options that had exercise prices lower than the fair value of our common stock on December 31, 2019, 2018 and 2017.
The aggregate intrinsic value of stock options exercised during the years ended December 31, 2019, 2018 and 2017 was $1,046,000, $2,261,000 and $911,000, respectively.
We received proceeds of $1,591,000, $635,000 and $311,000 from the exercise of common stock options during the years ended December 31, 2019, 2018 and 2017, respectively.
The weighted-average grant date fair value of options granted during the years ended December 31, 2019, 2018 and 2017 was $5.34, $5.58 and $4.21 per share, respectively.
We had an aggregate of approximately $8,616,000 of unrecognized stock-based compensation expense for unvested stock options and employee stock purchases as of December 31, 2019, which is expected to be recognized over a weighted average period of 2.5 years.
Restricted Stock
On January 23, 2017, we granted 250,000 shares of restricted stock awards to four employees. Shares will vest 25% on each one year anniversary of the grant date provided that the employee is in the employment of the Company on such vesting date. The total stock-based compensation expense related to these awards is $1,600,000. On January 22, 2018, we granted 315,000 shares of restricted stock units to four employees. Shares will vest 25% on each one year anniversary of the grant date provided that the employee is in the employment of the Company on such vesting date. The total stock-based compensation expense related to these units is $2,756,000. In Q1 2019, we granted 300,775 shares of restricted stock units to most of our current employees. Shares will vest 25% on each one year anniversary of the grant date provided that the employee is in the employment of the Company on such vesting date. The total stock-based compensation expense related to these units is $3,156,000. As of December 31, 2019, we have an aggregate of approximately $3,995,000 unrecognized stock-based compensation expense for unvested restricted stock awards and units which is expected to be recognized over a weighted-average period of 2.5 years.
Restricted stock activity for the year ended December 31, 2019, was as follows:
|
| | | | | |
| Restricted Stock Award / Restricted Stock Units | | Shares | | Weighted Average Grant Date Fair Value |
| Unvested balance at December 31, 2018 | | 502,500 |
| | $7.87 |
| Granted | | 300,775 |
| | 10.49 |
| Vested | | (141,250 | ) | | 7.71 |
| Forfeited | | (36,700 | ) | | 10.61 |
| Unvested balance at December 31, 2019 | | 625,325 |
| | $9.01 |
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
Stock-Based Compensation
We recognize stock-based compensation expense for only the portion of awards that are expected to vest. In developing a forfeiture rate estimate, we have considered our historical experience to estimate pre-vesting forfeitures for service-based awards. The impact of a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual forfeiture rate is materially different from our estimate, we may be required to record adjustments to stock-based compensation expense in future periods.
The fair value of each stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model. Due to insufficient company-specific historical and implied volatility information, we used to estimate the expected stock price volatility based on the historical volatility of publicly traded peer companies. During the quarter ended March 31, 2018, an analysis was completed to compare our own stock volatility to the volatility calculated using peer analysis. The conclusion was that Kindred Biosciences has enough history to provide a reliable expected volatility. As a result, we use the volatility of our own stock. The expected term of our common stock options has been determined utilizing the “simplified” method as we have insufficient historical experience for options grants overall, rendering existing historical experience irrelevant to expectations for current grants. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that we have never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
Total stock-based compensation expense, related to all of our share-based payment awards, is comprised of the following:
|
| | | | | | | | |
| (In thousands) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Research and development | $1,848 | | $1,746 | | $1,650 |
| General and administrative | 5,509 |
| | 4,531 |
| | 3,557 |
|
| | $7,357 | | $6,277 | | $5,207 |
Total stock-based compensation expense includes stock options, restricted stock awards, restricted stock units, and expense from the Purchase Plan for the years ended December 31, 2019, 2018, and 2017.
Valuation assumptions
The relevant data used to determine the fair value of stock-based awards is as follows:
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | | |
| | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Stock options: | | | | | |
| Weighted average risk-free interest rate | 2.49% | | 2.62% | | 1.98% |
| Weighted average expected term (in years) | 5.9 | | 5.9 | | 6.0 |
| Weighted average expected volatility | 56.9% | | 59.2% | | 70.4% |
| Weighted average expected dividend yield | — | | — | | — |
| Fair value at grant date | $5.34 | | $5.58 | | $4.21 |
| | | | | | |
| Employee stock purchase plan: | | | | | |
| Weighted average risk-free interest rate | 2.17% | | 2.02% | | 1.04% |
| Weighted average expected term (in years) | 0.5 | | 0.5 | | 0.5 |
| Weighted average expected volatility | 47.5% | | 43.7% | | 56.7% |
| Weighted average expected dividend yield | — | | — | | — |
| Fair value at grant date | $2.62 | | $2.72 | | $1.73 |
| | | | | | |
| Restricted stock awards: | | | | | |
| Fair value at grant date | $0.00 | | $0.00 | | $6.40 |
| | | | | | |
| Restricted stock units: | | | | | |
| Fair value at grant date | $10.49 | | $8.75 | | $0.00 |
11. Leases
We have non-cancelable operating leases for laboratory space in Burlingame, California with several amendments to expand the facility. We have a non-cancellable operating lease for the entire existing laboratory space of 11,476 square feet that expires in May 2025. In August 2015, we entered into a new non-cancelable operating lease for 3,126 square feet of office space in San Diego, California and in June 2019, renewed the lease through February 2025. Our headquarters office lease for 8,090 square feet of office space in Burlingame, California expires November 30, 2020. In October 2018, we entered into a short-term sublease agreement for an additional 5,613 square feet of laboratory space next to our current laboratory facility and terminated the sublease in June 2019. In April 2019, we signed a short-term lease in Burlingame ("April 2019 lease"), consisting of 1,979 square feet of space through April 2020. In May 2019, we signed another lease in Burlingame ("May 2019 lease"), consisting of 1,346 square feet of space through April 2022. In addition, we have four equipment leases expiring through 2023.
Operating lease expense was $1,204,000, $814,000 and $679,000 for the years ended December 31, 2019, 2018 and 2017, respectively.
The following tables below do not include the April 2019 lease since it is not more than 12 months. The following tables below also do not include the February 2020 lease ("February 2020 lease") of the additional 2,260 square feet of laboratory space commencing on May 1, 2020, mentioned in Note 17, since it has not started yet.
Supplemental cash flow information for the year ended December 31, 2019, related to operating leases was as follows (in thousands):
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | |
| Amortization of operating lease | | $ | 659 |
|
| Cash paid within operating cash flows | | $ | 846 |
|
| Right-of-use assets obtained in exchange for new or modified lease liabilities | | $ | 1,807 |
|
Supplemental balance sheet information, as of December 31, 2019, related to operating leases was as follows (in thousands, except lease term and discount rate):
|
| | | | |
| Reported as: | | |
| Operating lease right-of-use assets | | $ | 3,001 |
|
| | | |
| Current portion of operating lease liabilities | | $ | 644 |
|
| Long-term operating lease liabilities | | 2,614 |
|
| Total lease liabilities | | $ | 3,258 |
|
| | | |
| Weighted average remaining lease term (years) | | 4.9 years |
| Weighted average discount rate | | 5.5% |
As of December 31, 2019, we are obligated to make minimum lease payments under non-cancelable operating leases, as follows (in thousands):
|
| | | | |
| Year ending December 31, | | Lease Payments |
| 2020 | | $ | 807 |
|
| 2021 | | 648 |
|
| 2022 | | 675 |
|
| 2023 | | 701 |
|
| 2024 | | 719 |
|
| Thereafter | | 205 |
|
| Total lease payments | | 3,755 |
|
| Less: imputed interest | | (497 | ) |
| Total lease liabilities | | $ | 3,258 |
|
ASC 840 Disclosures
The Company elected the alternative modified transition method and is required to present previously disclosed information under the prior accounting standards for leases.
As of December 31, 2018, we are obligated to make minimum lease payments under all of our operating leases as follows (in thousands):
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | |
| Year ending December 31, | | Lease Payments |
| 2019 | | $ | 835 |
|
| 2020 | | 726 |
|
| 2021 | | 459 |
|
| 2022 | | 194 |
|
| Thereafter | | — |
|
| Total | | $ | 2,214 |
|
12. Commitments and Contingencies
Purchase Commitments
In June 2018, we entered into a Strategic Supply Agreement (the “Agreement”), with Pall Corporation (“Pall”) for purchase of equipment and consumables to be used in support of our manufacturing requirements, including, but not limited to, the Plant. Pursuant to the Agreement, we will purchase certain pharmaceutical manufacturing equipment and related services in the aggregate amount of $3.8 million with a seven-year consumable purchase obligation in the aggregate amount of approximately $16.5 million. The Agreement is subject to customary undertakings, covenants, obligations, rights and conditions. As of December 31, 2019, we have incurred the full $3.8 million in equipment purchase costs and are obligated to make consumable purchases as follows (in thousands):
|
| | | | |
| Year ending December 31, | | Consumable commitments |
| 2020 | | $ | 1,650 |
|
| 2021 | | 3,300 |
|
| 2022 | | 3,625 |
|
| 2023 | | 3,625 |
|
| 2024 | | 4,285 |
|
| Total | | $ | 16,485 |
|
Indemnities and Guarantees
We have made certain indemnities and guarantees, under which we may be required to make payments to a guaranteed or indemnified party, in relation to certain transactions. We indemnify our officers and directors to the maximum extent permitted under the laws of the State of Delaware. The duration of these indemnities and guarantees varies and, in certain cases, is indefinite. These indemnities and guarantees do not provide for any limitation of the maximum potential future payments we could be obligated to make. Historically, we have not been obligated to make any payments for these obligations and no liabilities have been recorded for these indemnities and guarantees in the accompanying consolidated balance sheets.
Legal Matters
In the ordinary course of business, we may face various claims brought by third parties and may, from time to time, make claims or take legal actions to assert our rights, including intellectual property disputes, contractual disputes and other commercial disputes. Any of these claims could subject us to litigation. Management believes there are currently no claims that are likely to have a material effect on our consolidated financial position and results of operations.
13. Income Taxes
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
There is no provision for income taxes because we have historically incurred operating losses and we maintain a full valuation allowance against our net deferred tax assets.
Differences between the provision (benefit) for income taxes and income taxes at the statutory federal income tax rate are as follows:
|
| | | | | | | | | | | | | | | | | | | | |
| (In thousands, except percentages) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Income tax expense (benefit) at statutory federal rate | $ | (12,893 | ) | | 21.0 | % | | $ | (10,436 | ) | | 21.0 | % | | $ | (10,499 | ) | | 34.0 | % |
| State income tax, net of federal benefit | (3,392 | ) | | 5.5 |
| | (3,748 | ) | | 7.5 |
| | (1,880 | ) | | 6.1 |
|
| Permanent items | 198 |
| | (0.3 | ) | | 13 |
| | — |
| | 63 |
| | (0.2 | ) |
| Research and development credits | (1,913 | ) | | 3.1 |
| | (1,909 | ) | | 3.8 |
| | (1,172 | ) | | 3.8 |
|
| Stock-based compensation | 637 |
| | (1.0 | ) | | (237 | ) | | 0.5 |
| | (122 | ) | | 0.4 |
|
| Reserve for uncertain tax positions | 861 |
| | (1.4 | ) | | 864 |
| | (1.7 | ) | | 469 |
| | (1.5 | ) |
| Change in valuation allowance | 15,382 |
| | (25.1 | ) | | 14,949 |
| | (30.1 | ) | | (128 | ) | | 0.4 |
|
| Tax Cuts and Jobs Act | — |
| | — |
| | — |
| | — |
| | 13,092 |
| | (42.4 | ) |
| Other | 1,120 |
| | (1.8 | ) | | 504 |
| | (1.0 | ) | | 177 |
| | (0.6 | ) |
| Provision (benefit) for income taxes | $ | — |
| | — | % | | $ | — |
| | — | % | | $ | — |
| | — | % |
Deferred tax assets are recognized for temporary differences that will result in deductible amounts in future periods. The components of the deferred tax assets are as follows at December 31, 2019 and 2018:
|
| | | | | | | |
| | December 31, |
| (In thousands) | 2019 | | 2018 |
| Deferred tax assets: |
|
| | |
| Net operating loss carryforwards | $ | 53,247 |
| | $ | 39,579 |
|
| Research and development credits | 4,682 |
| | 3,589 |
|
| Accrued expenses | 1,020 |
| | 893 |
|
| Amortization and depreciation | (222 | ) | | (393 | ) |
| Stock-based compensation | 6,412 |
| | 6,173 |
|
| ROU Lease - Liabilities | 872 |
| | — |
|
| ROU Lease - Assets | (804 | ) | | — |
|
| Other | 20 |
| | 10 |
|
| | 65,227 |
| | 49,851 |
|
| Valuation Allowance | (65,227 | ) | | (49,851 | ) |
| Net current deferred tax assets | $ | — |
| | $ | — |
|
At December 31, 2019, we had net deferred tax assets of $65,227,000. Due to uncertainties surrounding our ability to generate future taxable income to realize these assets, a full valuation allowance has been established to offset the net deferred tax asset.
Additionally, the future utilization of our net operating loss and research and development tax credits carryforwards is subject to annual limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, and similar state tax provisions due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes limit the amount of the net operating loss and research and development tax credit carryforward and other deferred tax assets that can be utilized to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Sections 382 and
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
383, results from transactions increasing ownership of certain stockholders or public groups in the stock of the corporation by more than 50 percent points over a three-year period. We believe we incurred ownership changes since April 2014, however, we have not completed an analysis yet to determine the impact of our ability to use net operating losses and research and development credits as of December 31, 2019.
At December 31, 2019, we had federal and California net operating loss carryovers of $194,548,000 and $113,110,000, respectively. The federal and California net loss carryforwards will begin to expire in 2032. The Federal NOL generated after 2017 of $101,436,000 will carryforward indefinitely and be available to offset up to 80% of future taxable income each year.
At December 31, 2019, we had federal and state research and development tax credit carryovers of approximately $4,815,000 and $4,625,000, respectively. The federal research and development tax credit carryforwards will begin to expire in 2033. The California research and development credit carryforwards are available indefinitely.
The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. There were no unrecognized tax benefits recorded by us as of the date of adoption. As a result of the implementation, we did not recognize an increase in the liability for unrecognized tax benefits.
A rollforward of changes in our unrecognized tax benefits is shown below.
|
| | | | | | | | | | | |
| (In thousands) | December 31, |
| | 2019 | | 2018 | | 2017 |
| Balance at beginning of year | $ | 3,196 |
| | $ | 2,252 |
| | $ | 1,698 |
|
| Additions based on tax positions related to the current year | 942 |
| | 944 |
| | 554 |
|
| Additions for tax positions of prior years | 37 |
| | — |
| | — |
|
| Balance at end of year | $ | 4,175 |
| | $ | 3,196 |
| | $ | 2,252 |
|
The amount of unrecognized tax benefits that would impact the effective tax rate if recognized and realized is $3,787,000.
Our practice is to recognize interest and/or penalties related to income tax matters as income tax expense. We had no accrual for interest or penalties on our accompanying consolidated balance sheets at December 31, 2019, 2018 and 2017, and have not recognized interest and/or penalties in our consolidated statements of operations and comprehensive loss for the years ended December 31, 2019, 2018 and 2017.
We do not anticipate a significant change to our unrecognized tax benefits during the next twelve months.
We file tax returns as prescribed by tax laws of the jurisdictions in which we operate. In the normal course of business, we are subject to examination by federal and state jurisdictions, where applicable. There are currently no pending tax examinations. Our federal and state tax returns are still open under statute from 2014 to present.
14. Net Loss Per Share
Basic and diluted net loss per share was calculated as follows for the years ended December 31, 2019, 2018 and 2017:
|
| | | | | | | | | | | |
| (In thousands, except per share amounts) | Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Basic and diluted net loss per share attributable to common stockholders: | | | | | |
| Numerator: | | | | | |
| Net loss attributable to common stockholders | $ | (61,389 | ) | | $ | (49,690 | ) | | $ | (30,879 | ) |
| Denominator: | | | | | |
| Weighted-average number of common shares outstanding, basic and diluted | 38,657 |
| | 31,001 |
| | 25,084 |
|
| Net loss per common share attributable to common stockholders, basic and diluted | $ | (1.59 | ) | | $ | (1.60 | ) | | $ | (1.23 | ) |
There was no difference between our net loss and the net loss attributable to common stockholders for all periods presented.
Stock options to purchase 6,353,370 shares, 5,821,928 shares and 4,573,625 shares of common stock as of December 31, 2019, 2018 and 2017, respectively, were excluded from the computation of diluted net loss per share attributable to common stockholders because their effect was anti-dilutive. 125,000 shares, 187,500 shares, 250,000 shares of unvested restricted stock award as of December 31, 2019, 2018 and 2017, respectively, were also excluded from the computation of diluted net loss per share calculations because their effect was anti-dilutive. 500,325 and 315,000 units of granted but unvested restricted stock units as of December 31, 2019 and 2018, respectively, were also excluded from the computation of diluted net loss per share calculations because their effect was anti-dilutive.
15. Employee Savings Plan
We have established an employee savings plan pursuant to Section 401(k) of the Internal Revenue Code, effective May 1, 2014. The plan allows participating employees to deposit into tax deferred investment accounts up to 90% of their salary, subject to annual limits. We make contributions to the plan in an amount equal to 50% on the first 6% for a maximum of 3% of the participant’s compensation which is deferred. We contributed approximately $452,000, $292,000 and $149,000 to the plan during the years ended December 31, 2019, 2018 and 2017, respectively.
16. Selected Quarterly Financial Information (unaudited)
The following table presents selected unaudited quarterly financial data for each of the quarters in the years ended December 31, 2019 and 2018.
Kindred Biosciences, Inc.
Notes to Consolidated Financial Statements (continued)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands, except per share amounts) | 2019 | | 2018 |
| Quarter ended | Dec. 31 | | Sep. 30 | | Jun. 30 | | Mar. 31 | | Dec. 31 | | Sep. 30 | | Jun. 30 | | Mar. 31 |
| | | | | | | | | | | | | | | | |
| Net product revenues | $ | 1,401 |
| | $ | 1,104 |
| | $ | 1,236 |
| | $ | 515 |
| | $ | 1,326 |
| | $ | 640 |
| | $ | — |
| | $ | — |
|
| | | | | | | | | | | | | | | | |
| Operating costs and expenses | | | | | | | | | | | | | | | |
| Cost of product revenues | 187 |
| | 139 |
| | 169 |
| | 92 |
| | 214 |
| | 110 |
| | — |
| | — |
|
| Research and development | 7,134 |
| | 7,290 |
| | 6,734 |
| | 7,152 |
| | 7,756 |
| | 7,477 |
| | 5,820 |
| | 5,346 |
|
| General and administrative | 9,578 |
| | 9,382 |
| | 9,065 |
| | 9,901 |
| | 9,219 |
| | 6,608 |
| | 5,770 |
| | 4,902 |
|
| Total operating cost and expenses | 16,899 |
| | 16,811 |
| | 15,968 |
| | 17,145 |
| | 17,189 |
| | 14,195 |
| | 11,590 |
| | 10,248 |
|
| | | | | | | | | | | | | | | | |
| Loss from operations | (15,498 | ) | | (15,707 | ) | | (14,732 | ) | | (16,630 | ) | | (15,863 | ) | | (13,555 | ) | | (11,590 | ) | | (10,248 | ) |
| Interest and other income, net | (236 | ) | | 414 |
| | 425 |
| | 575 |
| | 422 |
| | 518 |
| | 349 |
| | 277 |
|
| Net loss | $ | (15,734 | ) | | $ | (15,293 | ) | | $ | (14,307 | ) | | $ | (16,055 | ) | | $ | (15,441 | ) | | $ | (13,037 | ) | | $ | (11,241 | ) | | $ | (9,971 | ) |
| | | | | | | | | | | | | | | | |
| Net loss per share, basic and diluted (1) | $ | (0.40 | ) | | $ | (0.39 | ) | | $ | (0.37 | ) | | $ | (0.42 | ) | | $ | (0.46 | ) | | $ | (0.39 | ) | | $ | (0.39 | ) | | $ | (0.36 | ) |
| Weighted average shares used in computing net loss per share, basic and diluted | 38,999 |
| | 38,940 |
| | 38,887 |
| | 37,786 |
| | 33,708 |
| | 33,601 |
| | 28,619 |
| | 27,986 |
|
| (1) Net loss per share for each quarter is calculated as a discrete period; the sum of four quarters may not equal the calculated full year amount. |
17. Subsequent Events
In February 2020, we further amended non-cancelable operating leases for laboratory space in Burlingame, California for an expansion of an additional 2,260 square feet of laboratory space commencing on May 1, 2020 and expires on May 31, 2025. The total non-cancellable operating lease for the entire existing laboratory space is 13,736 square feet, expiring May 31, 2025.
On March 16, 2020, we announced an agreement to sell Mirataz to Dechra for a cash purchase price of $43 million, of which $38.7 million will be paid on the closing date and the balance $4.3 million will be paid out of escrow beginning in 12 months assuming no escrow claims, alongside an ongoing royalty on global net sales. The acquisition comprises worldwide marketing rights, intellectual property rights, marketing authorizations and associated regulatory documentation, third party supply contracts related to raw material and manufacture of the finished product, and certain product inventory. Completion is expected before the end of June 2020, following satisfactory completion of certain deliverables.
In addition, we announced a strategic realignment of our business model whereby Kindredbio becomes a biologics-only company focused on accelerating our deep pipeline of late-stage biologics candidates in canine and feline markets, while discontinuing small molecule development for these species. We plan to rely more on a partnership-based model for commercialization strategy whereby pipeline assets are partnered with larger commercial partners that can maximize product opportunity in return for upfront payment, contingent milestones, and royalties on future sales. Accordingly, the companion animal commercial infrastructure will be substantially reduced. In connection with this strategic shift, Kindredbio is eliminating approximately 53 positions, representing about one-third of our current workforce. The eliminated positions primarily relate to the companion animal sales force and research and development for small molecule programs. Restructuring expenses and retirement costs related to severance and health care benefits are expected to be approximately $1.7 million, exclusive of stock compensation.