AXSOME THERAPEUTICS, INC.
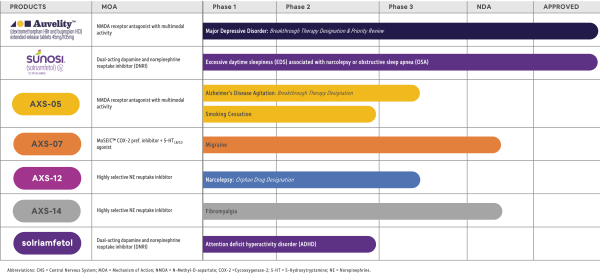
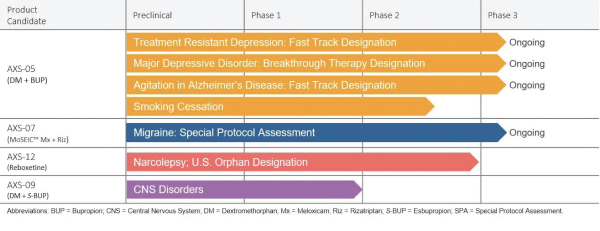
We are a clinical-stage biopharmaceutical company developing novel therapies for the management of central nervous system, or CNS, disorders for which there are limited treatment options. By focusing on this therapeutic area, we are addressing significant and growing markets where current treatment options are limited or inadequate. Our core CNS portfolio includes four CNS product candidates, AXS-05, AXS-07, AXS-09, and AXS-12, which are being developed for multiple indications. AXS-05 is currently in a Phase 3 trial in treatment resistant depression, or TRD, which we refer to as the STRIDE-1 study, a Phase 3 trial in major depressive disorder, or MDD, which we refer to as the GEMINI study, a Phase 3 open-label safety trial in patients with TRD and MDD; and a Phase 2/3 trial in agitation associated with Alzheimer’s disease, or AD, which we refer to as the ADVANCE-1 study. We have completed a Phase 2 trial in MDD, which we refer to as the ASCEND study and a Phase 2 trial in smoking cessation. AXS-07 is currently in two Phase 3 trials for the acute treatment of migraine, which we refer to as the MOMENTUM and the INTERCEPT studies, and a Phase 3 open-label safety trial in patients with migraine. AXS-12 is being developed for the treatment of narcolepsy. We have completed a Phase 2 trial, which we refer to as the CONCERT study. The Axsome Pain and Primary Care business unit, or Axsome PPC, houses our pain and primary care assets, including AXS-02 and AXS-06, and intellectual property which covers these and related product candidates and molecules being developed by us and others. AXS-02 is being developed for the treatment of knee osteoarthritis, or OA, associated with bone marrow lesions, or BMLs. AXS-06 is being developed for the treatment of osteoarthritis and rheumatoid arthritis and for the reduction of the risk of nonsteroidal anti-inflammatory drug, or NSAID, associated gastrointestinal ulcers. Additionally, we are currently evaluating other product candidates, which we intend to develop for CNS disorders. We aim to become a fully integrated biopharmaceutical company that develops and commercializes differentiated therapies that expand the treatment options available to caregivers and improve the lives of patients living with CNS disorders.
AXS-05 is a novel, oral, investigational NMDA receptor antagonist with multimodal activity under development for the treatment of CNS disorders. AXS-05 consists of bupropion and dextromethorphan, or DM, and utilizes our metabolic inhibition technology. We are developing AXS-05 initially for the following four indications: TRD, agitation associated with AD, MDD, and as an aid to smoking cessation. The DM component of AXS-05 is a non-competitive N-methyl-D-aspartate, or NMDA, receptor antagonist, also known as a glutamate receptor modulator. The DM component of AXS-05 is also a sigma-1 receptor agonist, nicotinic acetylcholine receptor antagonist, and inhibitor of the serotonin and norepinephrine transporters. The bupropion component of AXS-05 serves to increase the bioavailability of dextromethorphan, and is a norepinephrine and dopamine reuptake inhibitor, and a nicotinic acetylcholine receptor antagonist. We intend to seek U.S. Food and Drug Administration, or FDA, approval for AXS-05 utilizing the 505(b)(2) regulatory development pathway.
AXS-07 is a novel, oral, investigational medicine with distinct dual mechanisms of action consisting of MoSEIC™, or Molecular Solubility Enhanced Inclusion Complex, meloxicam and rizatriptan. We are developing AXS-07 initially for the acute treatment of migraine. Meloxicam is a long-acting nonsteroidal anti-inflammatory drug, or NSAID, with COX-2, an enzyme involved in inflammation and pain pathways, preferential inhibition and potent pain-relieving effects. However, standard meloxicam has an extended time to maximum plasma concentration, or Tmax, which delays its onset of action. AXS-07 utilizes our proprietary MoSEIC™ technology to substantially increase the solubility and speed the absorption of meloxicam while potentially maintaining durability of action. Meloxicam is a new molecular entity for migraine enabled by our MoSEIC™ technology. Rizatriptan is a 5-HT1B/D agonist that inhibits calcitonin gene-related peptide, or CGRP-mediated vasodilation, has been shown to have central trigeminal antinociceptive activity, and may reduce the release of inflammatory mediators from trigeminal nerves. Rizatriptan is approved as a single agent for the acute treatment of migraine. We intend to seek FDA approval for AXS-07 utilizing the 505(b)(2) regulatory development pathway.
AXS-09 is a novel, oral, investigational medicine consisting of esbupropion and DM, which is being developed for the treatment of CNS disorders. AXS-09 contains esbupropion, the chirally pure S-enantiomer of bupropion, as compared to the company’s first generation product candidate AXS-05 which contains racemic bupropion,
2