UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14C INFORMATION
INFORMATION STATEMENT PURSUANT TO SECTION 14(C) OF THE
SECURITIES EXCHANGE ACT OF 1934
Check the appropriate box:
| ☒ | Preliminary Information Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14c-5(d)(2)) |
| ☐ | Definitive Information Statement |
FINDIT, INC.
(Name of Registrant As Specified In Its Charter)
Payment of Filing Fee (Check the appropriate box):
☐ No fee required
☒ Fee computed on table below per Exchange Act Rules 14c-5(g) and 0-11
| (1) | Title of each class of securities to which transaction applies: |
Common Stock, par value $0.001 per share, of Findit, Inc.
Series A Preferred Stock, par value $0.001per share, of Findit, Inc.
| (2) | Aggregate number of securities to which transaction applies: |
(a) 337,793,959 shares of Common Stock
(b) 836,048 shares of Series A Preferred Stock
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
Solely for purposes of calculating this filing fee, the underlying value of the transaction was determined based upon the average closing price for the month of November 2022 ( (i) 337,793,959 shares of Common Stock, multiplied by $0.01495 per share (the average of the close price of such Common Stock for the month of November, as reported on the OTC Market Pinks), totaling $5,050,020; and (ii) 836,048 shares of Series A Preferred Stock, par value $0.001, totaling $836.
In accordance with Section 14(g) of the Securities Exchange Act of 1934, as amended, the filing fee was determined by multiplying 0.0001162 by the sum of the preceding sentence.
(4) Proposed maximum aggregate value of transaction:
$5,050,856
(5) Total fee paid:
$586.91
☐ Fee paid previously with preliminary materials.
☐ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing.
(1) Amount Previously Paid:
(2) Form, Schedule or Registration Statement No.:
| (3) | Filing party: |
| (4) | Date Filed: |
FINDIT, INC.
5051 Peachtree Corners Circle, #200
Peachtree Corners, GA 30092
NOTICE OF ACTION BY WRITTEN CONSENT AND INFORMATION STATEMENT
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY.
*, 2023
Dear Stockholder:
This notice of action by written consent and the accompanying information statement (this “Information Statement”) is being furnished by the Board of Directors (the “Board”) of Findit, Inc., a Nevada corporation (“Findit”, the “Company”, “we”, “us” or “our”) to the holders of record at the close of business on January 4, 2023 of the outstanding shares of our common stock, $0.001 par value, Series A preferred stock, $0.001 par value and Series B preferred stock, $0.001 par value (together, the “Voting Securities”), pursuant to Rule 14c-2 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
The purpose of this Information Statement is to inform the Company’s stockholders that on December 29, 2022, holders of approximately 78.14% of the outstanding Voting Securities acted by written consent (the “Written Consent”) in lieu of a special meeting of stockholders to approve (i) an amendment to the Company’s Articles of Incorporation (the “Charter Amendment”) to increase the number of authorized shares of Common Stock from 500,000,000 to 4,000,000,000 (the “Share Increase”) and change the name of the Company to BioRegenx, Inc., (ii) the Agreement and Plan of Merger dated as of December 29, 2022, by and among the Company and BioRegenx, Inc. (“BioRegenx”), and subject to the satisfaction or waiver of specified conditions, the merger of BioRegenx with and into the Company, with the Company existing as the surviving corporation (the “Merger”), pursuant to which the Common Stock will, upon consummation of the Merger, be converted into the right to receive not more than (x) 337,793,959 shares of Common Stock (subject to the terms and conditions of the Merger Agreement) (such shares of Common Stock, the “Common Stock Consideration”) and (y) 836,048 shares of Series A Preferred Stock (the “Preferred Stock Consideration” and, together with the Common Stock Consideration, the “Merger Consideration”). Concurrently, holder(s) of the Company’s Series A and Series B preferred shares shall retire their Series A and Series B preferred shares back into the treasury. Additionally, the stockholder consent approved, as soon as practicable, the implementation of up to a 1 for 25 reverse split of the Company’s common and preferred stock to improve the Company’s ability to attract institutional investors and analysts as well as to graduate to a senior exchange (OTCQB, NASDAQ).
Simultaneously with the close of the proposed transaction and as a condition precedent to said closing, all of the current assets and liabilities of FDIT, shall be transferred into a wholly owned subsidiary. Both parties agrees that the wholly owned subsidiary may be subsequently spun out upon yet to be determined terms. The parties acknowledge that, subsequent to the spin out, all assets and liabilities currently outstanding in FDIT, including the $150,000 SBA loan, will follow the subsidiary and will not be the responsibility of the merged entity post-closing of this transaction. Also, simultaneously with the close of the proposed transaction and as a condition precedent to said closing, the principals of the Company shall execute an option agreement with the Company relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger. After said closing of the merger with BioRegenx and subsequently upon exercising the option of the acquisition of CHNO, the Company’s board of directors agree to support and cause to be placed on the ballot at each election of the Company’s directors one name chosen by the current principals of CHNO which shall be a nominee to the Company’s board of directors.
Copies of the Merger Agreement and the Amendment to the Articles of Incorporation have been included as Annex A and Annex B, respectively. Approval of the Charter Amendment, the Merger Agreement, the Merger and the Share Issuances are referred to herein collectively as the “Shareholder Approved Actions.” The transactions contemplated by the Merger Agreement, are referred to herein collectively as the “Transactions.”
Under Nevada law and the Company’s Articles of Incorporation and Bylaws, adoption of the Charter Amendment, Merger Agreement and Merger, and the transactions contemplated thereby, by the Company’s stockholders required the affirmative vote or written consent of the holders of a majority of the voting power of all outstanding shares of Common Stock.
Effective December 29, 2022, Firth Family Trust (“Firth”) HVA Family Trust (“HVA Family”) and Wooeb, Inc. (Wooeb”), (together, the “Principal Stockholders”) delivered to the corporate secretary of the Company an irrevocable written consent approving the Shareholder Approved Actions. Effective December 29, 2022, the Principal Stockholders held Voting Securities representing approximately 78.14% of the voting power of all outstanding shares of Common Stock. Accordingly, the adoption of the Shareholder Approved Actions by the Company’s stockholders was affected in accordance with Section 78.390 of the Nevada Revised Statutes as of December 29, 2022. No further approval of the stockholders of the Company under the Nevada Revised Statutes is required to complete the Shareholder Approved Actions. As a result, the Company has not solicited and will not be soliciting your vote for the Shareholder Approved Actions and does not intend to call a meeting of stockholders for purposes of voting on the adoption of either matter.
Pursuant to Rule 14c-2 of the Exchange Act, the actions contemplated by written consent may not be taken until *, 2023, which is twenty (20) calendar days following the date we first mail this Information Statement to our stockholders. The Charter Amendment will not become effective until it is filed with the Secretary of State of the State of Nevada, which will occur only if the other conditions under the Merger Agreement are satisfied.
The Board carefully reviewed and considered the terms and conditions of the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement. The Board (i)(a) determined that the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement, are fair to, and in the best interests of, the Company and its stockholders, (b) approved and declared advisable the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement, in the Information Statement, recommended that the holders of Common Stock and Preferred Stock vote in favor of adopting the Merger Agreement and (ii) directed that the Shareholder Approved Actions be submitted to the holders of the Common Stock for their adoption.
This notice of action by written consent and the Information Statement shall constitute notice to you from the Company that the Shareholder Approved Actions have been adopted by the holders of a majority of the voting power of the Common Stock by written consent in lieu of a meeting in accordance with Section 78.390 of the Nevada Revised Statutes.
FINDIT IS NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND FINDIT A PROXY.
PLEASE NOTE THAT THE PRINCIPAL STOCKHOLDERS OF FINDIT HAVE VOTED TO APPROVE AND ADOPT THE SHAREHOLDER APPROVED ACTIONS. THE NUMBER OF VOTES HELD BY THE PRINCIPAL STOCKHOLDERS IS SUFFICIENT TO SATISFY THE STOCKHOLDER VOTE REQUIREMENT UNDER THE NEVADA REVISED STATUTES FOR THESE ACTIONS AND CONSEQUENTLY, NO ADDITIONAL VOTES WILL BE NEEDED TO APPROVE THESE TRANSACTIONS. THE CORPORATE ACTIONS DESCRIBED IN THIS INFORMATION STATEMENT REQUIRED SHAREHOLDER APPROVAL FROM THE HOLDERS OF OUR OUTSTANDING COMMON STOCK BECAUSE OF THE REQUIREMENTS OF NEVADA LAW.
The Information Statement accompanying this letter provides you with more specific information concerning the Charter Amendment, the Merger Agreement and the other transactions contemplated by the Merger Agreement. We encourage you to carefully read this Information Statement and the copy of the Merger Agreement included as Annex A to the Information Statement.
By order of the Board of Directors
Thomas Powers
Chief Executive Officer
The Merger has not been approved or disapproved by the U.S. Securities and Exchange Commission or any state securities or other regulatory agency. Neither the U.S. Securities and Exchange Commission nor any state securities or other regulatory agency has passed upon the merits or fairness of the Merger or upon the adequacy or accuracy of the information contained in this document or the Information Statement. Any representation to the contrary is a criminal offense.
The Information Statement is dated February 3, 2023 and is first being mailed to our stockholders on or about February ___, 2023.
TABLE OF CONTENTS
| SUMMARY | 9 | |||||
| The Parties | 9 | |||||
| The Merger | 9 | |||||
| Recommendations of the Board; Reasons for the Merger | 10 | |||||
| Approval of the Merger; Record Date, Action by Stockholder Consent | 10 | |||||
| Certain Effects of the Merger | 10 | |||||
| Governance Matters After the Merger | 11 | |||||
| Effects on the Company if the Merger is Not Completed | 11 | |||||
| Conditions to the Merger | 11 | |||||
| Termination | 13 | |||||
| Amendment to the Articles of Incorporation | 14 | |||||
| Material U.S. Federal Income Tax Consequences | 14 | |||||
| Accounting Treatment of the Merger | 14 | |||||
| Risk Factors | 14 | |||||
| Additional Information | 14 | |||||
| Selected Historical Consolidated Financial Data of Findit | 15 | |||||
| Selected Historical Financial Data of BioRegenx | 16 | |||||
| Summary Selected Unaudited Pro Forma Combined Financial Information | 18 | |||||
| QUESTIONS AND ANSWERS ABOUT THE MERGER AND THE CHARTER AMENDMENT | 19 | |||||
| CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS | 21 | |||||
| RISK FACTORS | 22 | |||||
| THE PARTIES | 25 | |||||
| THE MERGER | 25 | |||||
| Overview | 25 | |||||
| Background of the Merger | 26 | |||||
| Reasons for the Merger | 27 | |||||
| Required Approval of the Merger; Record Date; Action by Stockholder Consent | 28 | |||||
| Recommendation of the Board | 28 | |||||
| Certain Effects of the Merger | 28 | |||||
| Governance Matters After the Merger | 29 | |||||
| Effects on the Company if the Merger is Not Completed | 32 | |||||
| Merger Consideration | 32 | |||||
| Interests of the Company’s Directors and Executive Officers in the Merger | 33 | |||||
| Material U.S. Federal Income Tax Consequences of the Merger | 33 | |||||
| Accounting Treatment of the Merger | 35 | |||||
| Dividend Policy | 35 | |||||
| THE MERGER AGREEMENT | 35 | |||||
| The Merger | 36 | |||||
| Closing and Effective Time of the Merger | 36 | |||||
| Consideration to be Received in the Merger | 36 | |||||
| Representations and Warranties | 37 | |||||
| Operating Covenants | 39 | |||||
| Action by Stockholder Consent | 39 | |||||
| Reasonable Best Efforts and Certain Pre-Closing Obligations | 40 | |||||
| Employment Matters | 40 | |||||
| Additional Agreements | 40 | |||||
| Conditions to the Merger | 40 | |||||
| Termination | 41 | |||||
| Effect of Termination | 42 | |||||
| Expenses | 42 | |||||
| Amendment; Extension; Waiver | 42 | |||||
| Governing Law | 42 | |||||
| FINDIT UNAUDITED PRO FORMA COMBINED FINANCIAL STATEMENTS | 43 | |||||
| AMENDMENT TO THE ARTICLES OF INCORPORATION | 46 | ||||
| Description of the Proposed Amendment | 46 | ||||
| Purpose and Reasons for the Share Increase | 46 | ||||
| Effect of the Share Increase | 46 | ||||
| DESCRIPTION OF CAPITAL STOCK | 47 | ||||
| General | 47 | ||||
| Common Stock | 47 | ||||
| Anti-Takeover Provisions | 48 | ||||
| Combination with Interested Stockholders Statute | 48 | ||||
| Acquisition of Controlling Interest Statute | 49 | ||||
| Our Transfer Agent | 50 | ||||
| Listing of Common Stock | 50 | ||||
| NO DISSENTER’S RIGHTS | 50 | ||||
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 50 | ||||
| INFORMATION ABOUT BIOREGENX | 51 | ||||
| Operating Subsidiaries | 52 | ||||
| Proposed Acquisition | 54 | ||||
| Market Size | 54 | ||||
| Market Strategy | 55 | ||||
| Revenues | 56 | ||||
| Competition | 56 | ||||
| Competitive Advantages | 56 | ||||
| Facilities | 57 | ||||
| Employees | 57 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF BIOREGENX | 58 | ||||
| HOUSEHOLDING | 61 | ||||
| WHERE YOU CAN FIND ADDITIONAL INFORMATION | 61 | ||||
| INFORMATION INCORPORATED BY REFERENCE | 61 | ||||
| INDEX TO FINANCIAL STATEMENTS | 63 | ||||
| ANNEX A: AGREEMENT AND PLAN OF MERGER | 105 | ||||
| ANNEX B: FORM OF AMENDMENT TO THE ARTICLES OF INCORPORATION | 109 | ||||
FINDIT, INC.
5051 Peachtree Corners Circle, #200
Peachtree Corners, GA 30092
INFORMATION STATEMENT
This information statement and notice of action by written consent (collectively, this “Information Statement”) contains information relating to (i) an amendment to the Company’s Articles of Incorporation (the “Charter Amendment”) to increase the number of authorized shares of Common Stock from 500,000,000 to 4,000,000,000 (the “Share Increase”) and change the name of the Company to BioRegenx, Inc., (ii) the Agreement and Plan of Merger effective December 29, 2022 (the “Merger Agreement”), by and among the Company and BioRegenx, Inc. (“BioRegenx”), and subject to the satisfaction or waiver of specified conditions, the merger of BioRegenx with and into the Company, with the Company existing as the surviving corporation (the “Merger”), pursuant to which the Common Stock will, upon consummation of the Merger, be converted into the right to receive not more than (x) 337,793,959 shares of Common Stock (subject to the terms and conditions of the Merger Agreement) (such shares of Common Stock, the “Common Stock Consideration”) and (y) 836,048 shares of Series A Preferred Stock (the “Preferred Stock Consideration” and, together with the Common Stock Consideration, the “Merger Consideration”). Concurrently, holder(s) of the Company’s Series A and Series B preferred shares shall retire their Series A and Series B preferred shares back into the treasury. Additionally, the stockholder consent approved, as soon as practicable, the implementation of up to a 1 for 25 reverse split of the Company’s common and preferred stock to improve the Company’s ability to attract institutional investors and analysts as well as to graduate to a senior exchange (OTCQB, NASDAQ).
Simultaneously with the close of the proposed transaction and as a condition precedent to said closing, all of the current assets and liabilities of FDIT, shall be transferred into a wholly owned subsidiary. Both parties agrees that the wholly owned subsidiary may be subsequently spun out upon yet to be determined terms. The parties acknowledge that, subsequent to the spin out, all assets and liabilities currently outstanding in FDIT, including the $150,000 SBA loan, will follow the subsidiary and will not be the responsibility of the merged entity post-closing of this transaction. Also, simultaneously with the close of the proposed transaction and as a condition precedent to said closing, the principals of the Company shall execute an option agreement with the Company relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger. After said closing of the merger with BioRegenx and subsequently upon exercising the option of the acquisition of CHNO, the Company’s board of directors agree to support and cause to be placed on the ballot at each election of the Company’s directors one name chosen by the current principals of CHNO which shall be a nominee to the Company’s board of directors.
The Charter Amendment, the Merger Agreement and the transactions contemplated thereby and the Share Issuances are referred to herein collectively as the “Shareholder Approved Actions.” The Merger Agreement, the Subscription Agreement, the Shareholders Agreement, the Charter Amendment, the Certificate of Designations and the Registration Rights Agreement (as further described below) are referred to herein collectively as the “Transaction Documents” and, the transactions contemplated thereby, including the Share Issuances, the “Transactions.” We are furnishing this Information Statement to stockholders of the Company pursuant to applicable provisions of Nevada law and certain securities regulations. The Information Statement is dated February 3, 2023 and is first being mailed to our stockholders on or about February ___, 2023.
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY.
SUMMARY
This summary highlights selected information in this Information Statement and may not contain all of the information about the Transactions that is important to you. We have included page references in parentheses to direct you to more complete descriptions of the topics presented in this summary. You should carefully read this Information Statement in its entirety, including the annexes hereto and the other documents to which we have referred you, for a more complete understanding of the Transactions. You may obtain, without charge, copies of documents incorporated by reference into this Information Statement by following the instructions under the section of this Information Statement entitled “Where You Can Find Additional Information” beginning on page 61.
The Parties
Findit, Inc. owns Findit.com and the Findit App that is available in Android and IOS through the Google Play Store and the Apple App store. Findit.com and the Findit App operate as a Social Media Content Management Platform, that includes its own interactive search engine. Members can utilize Findit search to submit URLs they want Findit to index in Findit search results. Nonmembers can come to Findit and enter search queries, Findit returns matching search results from content posted in Findit along with outside web pages that have been submitted.
BioRegenx, Inc. was created to integrate leading-edge companies into one synergistic platform offering 360-degree solutions for health improvement. BioRegenx provides single-source development, marketing, and education, and offers multiple sales channel deployment. Integrated data is compiled in a secure cloud-based platform and is focused on comprehensive microcirculation, epigenetic, and metabolic profiles. These profiles generate both actionable and comprehensive test result dashboards for the physician. Individual patients have access to personalized mobile apps that are integrated to track improvements and measurable results from their personalized action plan. The result: improved vitality and longevity. This data allows researchers, governments, insurance plans and employers access to real data to improve employee health and reduce the costs.
Please see the section of this Information Statement entitled “The Parties” beginning on page 25.
The Merger
The Company and BioRegenx entered into the Merger Agreement effective December 29, 2022. A copy of the Merger Agreement is included as Annex A to this Information Statement. Under the terms of the Merger Agreement, subject to the satisfaction or waiver of specified conditions, BioRegenx will be merged with and into the Company (the “Merger”) in accordance with the applicable provisions of the Nevada Revised Statutes. As a result of the Merger, the separate existence of BioRegenx shall cease and the Company shall continue its existence under the laws of the State of Nevada as the surviving entity (in such capacity, the Company is sometimes referred to herein as the “surviving corporation”).
At the effective time of the Merger (defined below under “The Merger Agreement—Closing and Effective Time of the Merger”), all of the equity of BioRegenx (the “BioRegenx Equity”) that is issued and outstanding immediately prior to the effective time of the Merger, will be converted into the right to receive not more than (x) 337,793,959 shares of Common Stock (subject to the terms and conditions of the Merger Agreement) (such shares of Common Stock, the “Common Stock Consideration”) and (y) 836,048 shares of Series A Preferred Stock (the “Preferred Stock Consideration” and, together with the Common Stock Consideration, the “Merger Consideration”). Please see the section of this Information Statement entitled “The Merger” beginning on page 25.
The Series A Preferred Stock and the Series B Preferred Stock will be returned to the treasury as more particularly described in the section of this Information Statement entitled “The Merger” beginning on page 25.
Recommendation of the Board; Reasons for the Merger
After careful consideration, the Company’s Board of Directors (the “Board”) approved the Merger Agreement and recommended the approval of the Shareholder Approved Actions by the Company’s stockholders.
The Board believes that the Merger Agreement and the Merger are advisable and in the best interests of the Company and its stockholders. For a discussion of the material factors that the Board considered in determining to approve the Merger Agreement, please see the section of this Information Statement entitled “The Merger—Reasons for the Merger” beginning on page 27.
Approval of the Merger; Record Date; Action by Stockholder Consent
Effective December 29, 2022, Firth Family Trust (“Firth”) HVA Family Trust (“HVA Family”) and Wooeb, Inc. (Wooeb”), (together, the “Principal Stockholders”), delivered to the corporate secretary of the Company an irrevocable written consent approving the Shareholder Approved Actions (the “Written Consent”). As of December 29, 2022, the Principal Stockholders held voting securities representing approximately 78.14% of the voting power of all outstanding shares of Common Stock, Series A and Series B Preferred Stock. Accordingly, the approval of the Shareholder Approved Actions by the Company’s stockholders was affected in accordance with the Nevada Revised Statutes effective December 29, 2022. No further approval of the stockholders of the Company is required to approve the Shareholder Approved Actions. As a result, the Company has not solicited and will not be soliciting your vote for the approval of the Shareholder Approved Actions and does not intend to call a meeting of stockholders for purposes of voting on the approval of the Shareholder Approved Actions. If the Merger Agreement is terminated in accordance with its terms, the Written Consent will be of no further force and effect.
Federal securities laws state that the Shareholder Approved Actions may not be completed until twenty (20) days after the date of mailing of this Information Statement to the Company’s stockholders. Therefore, notwithstanding the execution and delivery of the Written Consent (which was obtained concurrently with the execution of the Merger Agreement), the Shareholder Approved Actions will not occur until that time has elapsed. We currently expect the Merger to be completed before the end of the first quarter of 2023, subject to certain government regulatory reviews and approvals and the satisfaction of the other conditions to closing in the Merger Agreement. However, there can be no assurance that the Merger will be completed on or prior to that time, or at all.
This Information Statement shall constitute notice to you from the Company that the Shareholder Approved Actions have been adopted by the holders of a majority of the voting power of the Common Stock and Preferred Stock by written consent in lieu of a meeting in accordance with the Nevada Revised Statutes.
Please see the section of this Information Statement entitled “The Merger Agreement—Required Approval of the Merger; Record Date; Action by Stockholder Consent” beginning on page 28.
Certain Effects of the Merger
Upon the consummation of the Merger, BioRegenx will be merged with and into the Company, and the Company shall continue its existence under the laws of the State of Nevada as the surviving corporation. At the effective time of the Merger, all of the property, rights, privileges, powers, and franchises of the Company and BioRegenx will vest in the Company, as the surviving corporation, and all debts liabilities, obligations, restrictions, disabilities, and duties of the Company and BioRegenx shall become the debts, liabilities, obligations, restrictions, disabilities, and duties of the Company, as the surviving corporation.
Please see the section of this Information Statement entitled “The Merger—Certain Effects of the Merger” beginning on page 28.
Governance Matters After the Merger
Following the effective time of the Merger, Ray Firth shall resign as an officer and director and Thomas Powers shall resign as an officer of the Company, the directors of BioRegenx shall be the directors of the Company along with Thomas Powers, and William Resides, Gary Hennerberg, Dan Cortes and Hitesh Juneja shall be the officers of the Company after the effective time of the Merger.
Please see the section of this Information Statement entitled “The Merger—Governance Matters After the Merger” beginning on page 29.
Effects on the Company if the Merger is Not Completed
If the Merger is not completed for any reason, the Company and BioRegenx will remain separate, independent companies and the holder of the BioRegenx Equity will continue to own the BioRegenx Equity.
Please see the section of this Information Statement entitled “The Merger—Effects on the Company if the Merger is Not Completed” beginning on page 32.
Conditions to the Merger
Mutual Conditions
Pursuant to the Merger Agreement each party’s obligation to effect the Merger is subject to the satisfaction or waiver (if permissible under applicable law), on or prior to the closing date of the Merger, which we refer to as the “Closing Date”, of the following conditions:
| · | no governmental authority having jurisdiction over any party shall have issued any order, decree, ruling, injunction or other action that is in effect (whether temporary, preliminary or permanent) restraining, enjoining or otherwise prohibiting the consummation of the Merger and no law shall have been adopted that makes consummation of the Merger illegal or otherwise prohibited; |
| · | this Information Statement shall have been mailed to the Company’s stockholders at least twenty (20) days prior to the Closing Date and the issuance of the Merger Consideration shall be permitted by Regulation 14C of the Exchange Act (including Rule 14c-2 promulgated under the Exchange Act); |
| · | all of the current assets and liabilities of FDIT, shall be transferred into a wholly owned subsidiary. Both parties agrees that the wholly owned subsidiary may be subsequently spun out upon yet to be determined terms, except that the parties agree that no spin out shall occur until the outstanding $150,000 EIDL loan is repaid in full. The parties acknowledge that, subsequent to the spin out, all assets and liabilities currently outstanding in FDIT will follow the subsidiary and will not be the responsibility of the merged entity post-closing of this transaction. |
| · | the Principals of FDIT shall execute an option agreement with FDIT relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger. The option shall be on terms comparable to those agreed to in this Agreement. After said closing of merger with FDIT and subsequently upon exercising the option of the acquisition of ClassWorx, the Board of Directors agree to support and cause to be placed on the ballot at each election of Directors one name chosen by the current principals of Classworx which shall be a nominee to the current Board of Directors of the Company. |
Additional Conditions of the Obligations of Findit
(a) Accuracy of Representations. The representations of Findit in the MergerAgreement or in any Exhibit hereto shall be true and accurate in all material respects at and as of Closing with the same effect as if made at and as of Closing, except as affected by the transactions contemplated hereby.
(b) Performance of Agreements. Findit shall have performed all obligations and agreements and complied with all covenants in this Agreement to be performed and complied with by it at or before Closing.
(c) Retirement of Series A and Series B preferred shares. Findit shall have retired the issued and outstanding Series A and Series B preferred shares, in accordance with the authorization from the Series A and Series B preferred shareholders.
(d) Receipt of Findit Common Stock. Findit shall have delivered to BioRegenx at Closing, certificates (or book entry) representing not more than three hundred thirty seven million, seven hundred ninety three thousand nine hundred fifty nine (337,793,959) common shares and eight hundred thirty six thousand forty eight (836,048) Series A preferred shares issued in the name of the shareholders of BioRegenx.
(e) Officer's Certificate. BioRegenx shall have received a certificate executed by an executive officer of Findit, dated as of Closing, reasonably satisfactory in form and substance to BioRegenx certifying that the conditions stated in the Merger Agreement have been satisfied.
(f) Legal Proceedings. There shall be no Legal Requirement, and no judgment shall have been entered and not vacated by any governmental authority of competent jurisdiction and no litigation shall be pending which restrains, makes illegal or prohibits consummation of the transactions contemplated hereby.
(g) Consents. BioRegenx shall have obtained evidence, in form and substance satisfactory to it, that there has been obtained all consents, approvals and authorizations required by the Merger Agreement.
(h) Resignation of Officers and Directors. Each of the officers and directors of Findit whose written resignation BioRegenx has requested shall have delivered to BioRegenx effective as of the Closing.
(i) Legal Matters Satisfactory to BioRegenx's Counsel. All actions, proceedings, instruments and documents required to carry out the transactions contemplated by this Agreement or incidental thereto and all related legal matters shall be reasonably satisfactory to and approved by BioRegenx's counsel, and such counsel shall have been furnished with such certified copies of actions and proceedings and such other instruments and documents as it shall have reasonably requested. Additionally, the principals of Findit, Inc. shall have executed an option agreement with Findit, Inc. relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger.
Additional Conditions of the Obligations of BioRegenx
(a) Accuracy of Representations. The representations of BioRegenx in the Merger Agreement or in any Exhibit thereto shall be true and accurate (in all material respects) at and as of Closing with the same effect as if they were made at and as of Closing, except as affected by the transactions contemplated hereby.
(b) Performance of Agreements. BioRegenx shall have performed all obligations and agreements and complied with all covenants in the Merger Agreement or in any Transaction Document to which it is a party to be performed and complied with by it at or before Closing.
(a) Delivery of BioRegenx Stock. BioRegenx shall have delivered at Closing, certificate/book entry representing all of its outstanding common shares and Series A preferred shares.
(d) Officer's Certificate. Findit shall have received a certificate executed by an executive officer of BioRegenx, dated as of Closing, reasonably satisfactory in form and substance to Findit, certifying that the conditions stated in the Merger have been satisfied.
(e) Legal Proceedings. There shall be no Legal Requirement, and no judgment shall have been entered and not created by any governmental authority of competent jurisdiction and no litigation shall be pending which,
| (i) | restrains, make illegal or prohibits consummation of the transactions contemplated hereby, or |
| (ii) | could have a material adverse effect upon the operations or financial condition of BioRegenx. |
(f) Consents. Findit shall have received evidence, in form and substance satisfactory to it, that there have been obtained all consents, approvals, and authorizations required by the Merger Agreement.
(g) Legal Matters Satisfactory to Findit and its Representatives. All actions, proceedings, instruments and documents required to carry out the transactions contemplated by this Agreement or incidental thereto and all related legal matters shall be reasonably satisfactory to and approved by Findit's counsel, and such counsel shall have been furnished with such certified copies of actions and proceedings and such other instruments and documents as it shall have reasonably requested.
Please see the section of this Information Statement entitled “The Merger Agreement—Conditions to the Merger” beginning on page 40.
As further discussed under the section titled “Risk Factors,” the Company cannot be certain when, or if, the conditions of the Merger will be satisfied or waived, or that the Merger will be completed.
Termination
The Merger Agreement may be terminated and the Merger may be abandoned at any time prior to the effective time in the following circumstances:
• by mutual written consent of the Company and BioRegenx;
• by either the Company or BioRegenx:
| o | if any governmental authority having jurisdiction over any party shall have issued any order, decree, ruling or injunction or taken any other action permanently restraining, enjoining or otherwise prohibiting the consummation of the Merger and such order, decree, ruling or injunction or other action shall have become final and non-appealable,or if there shall be adopted any law that permanently makes consummation of the Merger illegal or otherwise permanently prohibited; provided that the right to terminate the Merger Agreement will not be available to any party whose failure to fulfill any material covenants or agreement has caused such legal restraint; or |
| o | if the Merger has not been consummated on or before 5:00 p.m. time, March 31, 2023 (such date being the “End Date”); provided that the right to so terminate the Merger Agreement will not be available to a party whose material breach of any provision of the Merger Agreement results in the failure of the Merger to be consummated on or before the End Date; provided, further, that either the Company or BioRegenx shall have the unilateral right to extend the End Date by up to 30 additional days in the event that as of the End Date (prior to such extension) all conditions (other than conditions that by their nature are to be satisfied at the Closing) under certain circumstances have been satisfied or waived and the closing shall not have occurred due solely or in part to the failure of the Company to mail the Information Statement to its stockholders at least 20 days prior to the Closing Date. |
| • | by the Company: |
| o | in the event of certain breaches of the Merger Agreement by BioRegenx; provided that the Company is not then in material breach of any representation, warranty or covenant contained in the Merger Agreement; provided, further, that said breach by BioRegenx cannot be or has not been cured pursuant to the terms of the Merger Agreement. |
• by BioRegenx:
| o | in the event of certain breaches of the Merger Agreement by the Company; provided that BioRegenx is not then in material breach of any representation, warranty or covenant contained in the Merger Agreement provided, further, that said breach by the Company cannot be or has not been cured pursuant to the terms of the Merger Agreement; or |
| o | upon written notice to the Company stating that all conditions to closing of the transactions contemplated by the Merger Agreement shall have been satisfied or waived (other than those conditions that by their nature are to be satisfied at the closing) and that BioRegenx is ready willing and able to consummate the transactions contemplated under the Merger Agreement and, following receipt of such written notice, that the Company fails to consummate the transactions contemplated under the Merger Agreement within five (5)business days. |
Please see the section of this Information Statement entitled “The Merger Agreement—Termination” beginning on page 41.
Amendment to Articles of Incorporation
Prior to the Closing Date of the Merger, the Company will file the Charter Amendment with the Secretary of State of the State of Nevada to increase the number of authorized shares of Common Stock from 500,000,000 to 4,000,000,000 in order to ensure there are enough shares of Common Stock to provide for the Share Issuances.
Please see the section of this Information Statement entitled “Amendment to the Articles of Incorporation” beginning on page 46.
Material U.S. Federal Income Tax Consequences
For U.S. federal income tax purposes, the exchange of property for Common Stock and Series A Preferred Stock, effected pursuant to the Merger are intended to qualify together as an “exchange” described in Section 351 of the Internal Revenue Code of 1986, as amended (the “Code”). If the Exchanges, taken together, qualify as an “exchange” within the meaning of Code Section 351(a), then holders of Common Stock receiving Common Stock and Series A Preferred Stock in the Merger, if any, (“Exchangor(s)”) should not recognize any gain or loss for U.S. federal income tax purposes as a result of the Merger.
For a more detailed discussion, see “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” - page 33.
Tax matters can be complicated and the tax consequences of the Merger to any particular holder of Common Stock and Series A Preferred Stock will depend on such holder’s particular facts and circumstances. Holders of Common Stock and Series A Preferred Stock should consult their own tax advisors to determine the tax consequences of the Merger to them, including the effects of U.S. federal, state, local and foreign tax laws.
Please see the section of this Information Statement entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” beginning on page 33 for a more complete discussion of the material U.S. federal income tax consequences of the Merger.
Accounting Treatment of the Merger
The Company prepares its financial statements in accordance with GAAP and its pro forma financial statements as required by the SEC Article 11 of Regulation S-X. The Merger will be accounted for as an acquisition under Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 805, Business Combinations, with BioRegenx being considered the acquirer of the Company for accounting purposes.
Please see the section of this Information Statement entitled “The Merger—Accounting Treatment of the Merger” beginning on page 35.
Risk Factors
An investment in the Company, as the surviving corporation, may include different risks than an investment in the Company on a stand-alone basis. You should carefully review the risks described in the section entitled “Risk Factors” beginning on page 22 together with all of the other information included in this Information Statement.
Additional Information
You can find more information about the Company in the periodic reports and other information we file with the U.S. Securities and Exchange Commission (the “SEC”). The information is available at the website maintained by the SEC at www.sec.gov.
Please see the section of this Information Statement entitled “Where You Can Find Additional Information” beginning on page 61.
Selected Historical Consolidated Financial Data of Findit
The historical financial data presented in the table below sets forth the Company’s selected historical financial information that has been derived from (i) the Company’s financial statements as of and for each of the two years ended December 31, 2021 and 2020, and (ii) the Company’s unaudited condensed financial statements as of and for the nine months ended September 30, 2022 and 2021. The financial results are not necessarily indicative of our future operations or future financial results. The data presented below is only a summary and is not necessarily indicative of our future operations nor does it include the effects of the Merger. You should read this financial information in conjunction with our financial statements, the notes thereto, and the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Company’s Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, all which are incorporated into this Information Statement by reference.
Statement of Operations Data:
| Years Ended December 31, | Nine Months Ended September 30, | |||
| 2021 | 2020 | 2022 | 2021 | |
| Revenues: | ||||
| Findit Services | $197,447 | $363,306 | 33,245 | 185,597 |
| Findit Services – Related Party | - | 35,868 | - | - |
| Sales of Essential Oils | 1,137 | 5,218 | - | 1,137 |
| Total Revenues | 198,584 | 404,392 | ||
| Cost of Goods Sold: | ||||
| Purchases of Materials & Supplies | 554 | 3,229 | - | 554 |
| Cost of Goods Sold – related party | - | 35,868 | - | - |
| Total Cost of Goods Sold | 554 | 39,097 | - | 554 |
| Gross Margin | 198,030 | 365,295 | 33,245 | 186,180 |
| Operating Expenses: | ||||
| Advertising, Marketing & Press Release Expenses | 7,383 | 260,870 | 6,043 | 5,884 |
| Amortization Expense | 4,449 | 5,932 | 4,449 | 4,449 |
| Consulting Services | 6,350 | 21,408 | - | 6,350 |
| Content Writing | 43,100 | 88,649 | 21,750 | 40,300 |
| General and Administrative Expense | 81,115 | 71,488 | 20,884 | 72,654 |
| Professional Fees | 60,231 | 39,738 | 27,613 | 45,651 |
| Programming Fees | 111,270 | 47,483 | 9,150 | 79,470 |
| Web Design and Hosting Expense | 49,250 | 45,375 | 16,129 | 38,408 |
| Total Operating Expenses | 363,148 | 580,943 | (72,773) | (106,986) |
| Loss from Operations | (165,118) | (215,648) | - | 68,245 |
| Other Income: | ||||
| Other Income | 68,245 | - | - | 68,245 |
| Loss on Impairment of Investment | - | (58,088) | - | - |
| Total Other Income | 68,245 | (58,088) | - | 68,245 |
| Loss Before Provision for Income Tax | (96,873) | (273,736) | (72,773) | (38,741) |
| Provision for Income Tax | - | - | - | - |
| Net Loss | (96,873) | (273,736) | (72,773) | (38,741) |
| Other Comprehensive Income (Loss) | ||||
| Unrealized (loss) gain on available-for-sale securities | (165,000) | 189,132 | (39,000) | 475,000 |
| Total Comprehensive Loss | $(261,873) | $(84,604) | (111,773) | 436,259 |
| Loss Per Share, Basic and Diluted | $(0.00) | $(0.00) | $(0.00) | 0.00 |
| Weighted Average Shares Outstanding, | ||||
| Basic and Diluted | 269,745,006 | 267,963,583 | 269,745,006 | 269,745,006 |
Balance Sheet Data:
| December 31, 2021 | December 31, 2020 | September 30, 2022 | September 30, 2021 | |
| Cash and cash equivalents | $ 24,358 | $ 93,875 | $1,541 | 94,725 |
| Accounts Receivable | - | 971 | - | 3,700 |
| Domain Name & Website, net of amortization of $45,577 and $51,399, respectively |
43,404 |
47,854 |
38,955 |
43,404 |
| Investment in Chill N Out Chryotherapy – related party |
60,000 |
225,000 |
21,000 |
700,000 |
| Total assets | $ 127,762 | $ 367,700 | $ 61,496 | $ 841,829 |
| Total debt | $ 197,839 | $ 148,511 | $ 257,503 | $ 227,931 |
| Stockholders’ equity (deficit) |
$ (84,234) |
$ 177,639 |
$ (196,007) |
$ 613,898 |
Cash Flow Data:
| December 31 | Nine Months Ended | Nine Months Ended | ||
| 2021 | 2020 | September 30, 2022 | September 30, 2021 | |
| Cash flows used for operating activities | (119,517) | (94,100) | (60,288) | (49,150) |
| Cash flows used for investing activities | - | - | - | - |
| Cash flows provided by financing activities | 50,000 | 150,000 | 37,471 | 50,000 |
Selected Historical Financial Data of BioRegenx
The selected historical financial data of BioRegenx as of December 31, 2021 and 2020 were derived from the audited historical consolidated financial statements of BioRegenx, Inc. included elsewhere in this Information Statement. The selected historical financial data of BioRegenx, Inc. as of and for the nine months ended September 30, 2022 and 2021 are derived from the unaudited interim financial statements of BioRegenx included elsewhere in this Information Statement.
The information set forth below is only a summary and is not necessarily indicative of the results of future operations nor does it include the effects of the Merger. You should read the following selected data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations of BioRegenx” and BioRegenx’s historical financial statements and related notes included elsewhere in this Information Statement.
Statement of Operations Data:
| For the Years Ended December 31, | For the Nine Months Ended September 30, | |||
| 2021 | 2020 | 2022 | 2021 | |
| Revenues: | ||||
| Net sales | $2,990,772 | $7,787,092 | $1,932,512 | $2,281,713 |
| Cost of Sales | 1,072,878 | 2,063,206 | 689,116 | 1,016,702 |
| Gross Profit | 1,917,894 | 5,723, | 1,236,404 | 1,265,011 |
| Operating Expenses: | ||||
| Distributors incentives | 479,816 | 2,218,66 | 166,706 | 216,502 |
| Selling, general and administrative |
3,885,839 |
4,067,567 |
2,530,926 |
2,050,785 |
| Impairment expense | 830,763 | - | - | - |
| Total Operating Expenses | 5,196,418 | 6,286,229 | 2,697,632 | 2,267,287 |
| Loss from Operations | (3,278,524) | (562,343) | (1,461,632) | (1,002,276) |
| Other Income: | ||||
| Interest income | 25,538 | 28,288 | 148 | 20,929 |
| Interest expense and financing costs |
(341,037) |
(127,561) |
(317,842) |
(30,281) |
| Gain (loss) on sale of investments |
390 |
(120,055) |
- |
- |
| Loan forgiveness PPP | 53,387 | - | - | - |
| Total Other Expenses | (261,722) | (219,328) | (317,694) | (9,352) |
| Income (Loss) Before Provision for Income Tax | (3,540,246)) | (781,671) | (1,778,922) | (1,011,238) |
| Provision for Income Tax | - | - | - | - |
| Net Loss | $(3,540,246) | $(781,671) | $(1,776,922) | $(1,011,238) |
| Loss Per Share, Basic and Diluted |
$(0.02) |
$(0.05) |
$(0.05) |
$(0.03) |
| Weighted Average Shares Outstanding, - Basic and Diluted |
35,266,678 |
34,912,500 |
37,876,930 |
35,266,678 |
Balance Sheet Data:
| December 31, 2021 | December 31, 2020 | September 30, 2022 | September 30, 2021 | |
| Cash and cash equivalents | $ 882,112 | $ 592,973 | $ 218,418 | $1,655,681 |
| Accounts Receivable | 985 | - | - | 141,059 |
| Inventories | 653,291 | 850,312 | 390,654 | 688.817 |
| Prepaid expenses and other current assets |
349,281 |
426,798 |
566,173 |
1,751,048 |
| Total assets | $ 1,919,964 | $1,880,718 | $1,194,935 | $4,216,605 |
| Total debt | $ 5,180,176 | $3,885,284 | $ 2,621,177 | $4,947,810 |
| Stockholders’ equity (deficit) | $ (1,419,401) | $(2,004,566) | $ (1,426,242) | $(731,205) |
Cash Flow Data:
| December 31 | Nine Months Ended | Nine Months Ended | ||
| 2021 | 2020 | September 30, 2022 | September 30, 2021 | |
| Cash flows used for operating activities |
(3,726,316) |
(1,840,390) |
(2,374,865) |
(2,241,453) |
| Cash flows used for investing activities |
(23,600) |
- |
- |
(830,762) |
| Cash flows provided by financing activities |
4,039,115 |
122,229 |
1,711,171 |
4,134,923 |
Summary Selected Unaudited Pro Forma Combined Financial Information
The following selected unaudited pro forma combined consolidated statements have been prepared to reflect the effects of the Merger on the financial statements of the Company. The unaudited pro forma combined statements of operations for the nine months ended September 30, 2022 and for the year ended December 31, 2021, are presented as if the Merger had been completed on January 1, 2021. The unaudited pro forma combined balance sheet is presented as if the Merger had been completed on September 30, 2022.
The following selected unaudited pro forma combined consolidated financial information is not necessarily indicative of the results that might have occurred had the Merger taken place on December 31, 2020 for statement of operations purposes or on September 30, 2022 for balance sheet purposes, and is not intended to be a projection of future results. Future results may vary significantly from the results reflected because of various factors, including those discussed in the section entitled “Risk Factors.” The following selected unaudited pro forma combined consolidated financial information should be read in conjunction with the section entitled “Unaudited Pro Forma Combined Financial Statements” and related notes included in this Information Statement.
| Statement of Operations (Unaudited) | Nine Months Ended September 30, 2022 | Year Ended December 31, 2021 | |||
| Revenue | |||||
| Net Sales | $ 1,965,757 | $ 3,189,356 | |||
| Costs of Goods Sold | 696,107 | 1,073,432 | |||
| Gross Profit | 1,269,650 | 2,115,924 | |||
| Costs and Operating Expenses: | |||||
| All selling, general and administrative | 2,790,112 | 4,728,804 | |||
| Impairment expense | - | 830,762 | |||
| Total Costs and Operating Expenses | 2,790,112 | 5,559,566 | |||
| Loss from Operations | (1,520,462) | (3,443,642) | |||
| Other expense and comprehensive losses, net | (356,694) | (358,477) | |||
| Total Comprehensive Loss | $ (1,877,156) | $ (3,802,119) | |||
| Net loss per common share, basic and diluted | $ (0.01) | $ (0.01) | |||
| Weighted average shares outstanding, basic and diluted | 269,745,006 | 269,745,006 | |||
| Balance Sheet (Unaudited) | Nine Months Ended September 30, 2022 | ||||
| Assets | |||||
| Cash and cash equivalents | $ 19,959 | ||||
| Accounts receivable | - | ||||
| Inventories | 390,654 | ||||
| Prepaid and other current assets | 566,173 | ||||
| Other assets | 79,645 | ||||
| Total Assets | $ 1,256,431 | ||||
| Liabilities and stockholders' deficit | |||||
| Total Debt | $ 2,878,680 | ||||
| Total Stockholders' Deficit | (1,622,249) | ||||
| Total Liabilities and Stockholders' Deficit | $ 1,256,431 | ||||
QUESTIONS AND ANSWERS ABOUT THE MERGER AND THE CHARTER AMENDMENT
The following questions and answers are intended to briefly address some commonly asked questions regarding the Merger and Charter Amendment. These questions and answers may not address all questions that may be important to you as a stockholder. You should read the more detailed information contained elsewhere in this Information Statement, the annexes to this Information Statement and the documents referred to or incorporated by reference in this Information Statement.
Q: Why am I receiving this Information Statement?
A: Effective December 29, 2022, the Company entered into the Merger Agreement with BioRegenx, and the Principal Stockholders approved the Shareholder Approved Actions. Prior to the Closing Date, the Company will need to amend its Articles of Incorporation to increase the number of authorized shares of Common Stock in order to provide for the Share Issuances. The filing of the Charter Amendment with the Secretary of State of the State of Nevada is being undertaken solely for the purposes of effecting the Merger Agreement. Applicable provisions of Nevada law and certain securities regulations require us to provide you with information regarding the Shareholder Approved Actions, even though your vote or consent is neither required nor requested to adopt the Charter Amendment or Merger Agreement or to complete the Shareholder Approved Actions.
Q: If the Merger occurs, what happens to my shares of Common Stock in the Company?
A: Your ownership of shares of Common Stock will not change as a result of the Closing. There will be neither any exchange of nor any new consideration received for your shares of Common Stock. All of the preferences, relative rights and other rights you have in your shares of Common Stock will be unaffected.
Q: When is the Merger expected to close?
A: The closing of the Merger cannot occur until 20 days after the Company mails this Information Statement to its stockholders, assuming all of the other closing conditions specified in the Merger Agreement have been satisfied. We currently expect the Transactions to be completed before the end of the first quarter of 2023, subject to certain government regulatory reviews and approvals and the satisfaction of the other conditions to closing in the Transaction Documents. However, there can be no assurance that the Transactions will be completed on or prior to that time, or at all.
Q: What happens if the Merger does not close?
A: If the Merger does not close for any reason, the holder of the BioRegenx Equity will not receive any payment in connection with the Merger. Instead, the Company and BioRegenx will remain separate, independent companies. In addition, if the closing of the Merger does not occur, the Charter Amendment will not be filed with the Secretary of State of the State of Nevada and will not become effective.
Q: Why did the Board approve the Merger and the Merger Agreement?
A: After careful consideration and evaluation of the Merger, our Board approved and declared advisable the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement.
For a discussion of the factors that the Board considered in determining to approve the Merger Agreement, please see the section of this Information Statement entitled “The Merger—Reasons for the Merger.”
Q: What factors did the Board consider in evaluating the Merger Agreement and the Merger?
A: In evaluating the Merger, the Board consulted with Findit’s management, as well as Findit’s legal and financial advisors, and considered a number of factors, weighing both perceived benefits of the Merger as well as potential risks of the Merger. In the course of its deliberations, the Board considered a variety of factors and information that it believes support its determinations and recommendations, including the following (which are not all-inclusive and not necessarily presented in order of relative importance):
| • | The Company’s expectation that the Merger will provide significant EBITDAX accretion as a result of enhanced capital efficiency and operating margins. |
| • | The Company’s expectation that the combination will materially enhance the Company’s financial, operational, and credit metrics. |
| • | The attractiveness of the Merger to the Company in comparison to other acquisition opportunities reasonably available to the Company, including BioRegenx’s desirable asset quality, potential synergies between the companies, accretive cash flow and the immediate actionability of the BioRegenx acquisition opportunity. |
| • | The recommendation of the Merger by the Company’s management team. |
| • | That the Company’s Board believes the restrictions imposed on the Company’s business and operations during the pendency of the Merger are reasonable and not unduly burdensome. |
| • | The likelihood of consummation of the Merger and the Board’s evaluation of the likely time period necessary to close the Merger. |
| • | That the addition of the seven directors to the Company’s Board in connection with the Merger will add further valuable expertise and experience and in-depth familiarity with BioRegenx to the Company’s Board, which will enhance the likelihood of realizing the strategic benefits that the Company expects to derive from the Merger. |
The foregoing factors are some of the principal factors considered by the Board. The Board did not assign relative weights to the factors it considered or determine that any factor was of particular importance. For a discussion of the factors that the Board considered in determining to approve the Merger Agreement, please see the section of this Information Statement entitled “The Merger—Reasons for the Merger.”
Q: Is the approval of stockholders necessary to adopt the Merger Agreement and Charter Amendment? Why am I not being asked to vote on the Merger Agreement and Charter Amendment?
A: Under Nevada law, the approval of the Merger Agreement, the Merger and the Charter Amendments requires approval by the Board and a majority of the outstanding shares of the Common Stock voting as a single class. Per the Nevada Revised Statutes, stockholder approval may be had at a stockholders’ meeting or by written consent in lieu of a stockholders’ meeting. The stockholder approval was obtained effective December 29, 2022, the date on which the Principal Stockholders delivered to the corporate secretary of the Company the Written Consent adopting the Shareholder Approved Actions. As of December 29, 2022, the Principal Stockholders held approximately 78.14% of the voting power of all outstanding shares of Common Stock. Accordingly, the approval of the Shareholder Approved Actions by the Company’s stockholders was effected in accordance with the Nevada Revised Statutes.
No further approval of the stockholders of the Company is required to adopt the Shareholder Approved Actions. As a result, the Company has not solicited and will not be soliciting your vote for the approval of the Shareholder Approved Actions and does not intend to call a meeting of stockholders for purposes of voting on the approval of the Shareholder Approved Actions.
Q: Am I entitled to appraisal rights in connection with the Merger?
A: As no further stockholder approval is required for the Shareholder Approved Actions, dissenter’s appraisal rights or similar rights are inapplicable. Dissenters to the other Shareholder Approved Actions do not have any appraisal rights or similar rights.
Q: What are the U.S. federal income tax consequences?
A: For U.S. federal income tax purposes, the Exchanges are intended to qualify together as an “exchange” described in Section 351 of the Code. If the Exchanges, taken together, qualify as an “exchange” within the meaning of Code Section 351(a), then the Exchangors should not recognize any gain or loss for U.S. federal income tax purposes as a result of the Merger.
For all holders of Common Stock that are not Exchangors, the Merger should not have U.S. federal income tax consequences for such holders.
For a more detailed discussion, see “The Merger—Material U.S. Federal Income Tax Consequences of the Merger.” Tax matters can be complicated and the tax consequences of the Merger to any particular holder of Common Stock will depend on such holder’s particular facts and circumstances. Holders of Common Stock should consult their own tax advisors to determine the tax consequences of the Merger to them, including the effects of U.S. federal, state, local and foreign tax laws.
Q: What is householding and how does it affect me?
A: The SEC permits companies to send a single set of certain disclosure documents to stockholders who share the same address and have the same last name, unless contrary instructions have been received, but only if the applicable company provides advance notice and follows certain procedures. In such cases, each stockholder continues to receive a separate set of disclosure documents. This practice, known as “householding”, is designed to reduce duplicate mailings and save significant printing and postage costs as well as natural resources.
If you received a householded mailing and you would like to have additional copies of this Information Statement mailed to you, or you would like to opt out of this practice for future mailings, please submit your request to the Company by phone at (404) 443-3224 or by mail to Findit, Inc., 5051 Peachtree Corners Circle, 3200, Peachtree Corners, GA 30092. We will promptly send additional copies of this Information Statement upon receipt of such request.
Q: Who can help answer my questions?
A: If you have questions about the Merger after reading this Information Statement, please contact the Company by phone at (404) 443-3224 or by mail to Findit, Inc., 5051 Peachtree Corners Circle, #200, Peachtree Corners, GA 30092.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Any statements in this Information Statement regarding the Charter Amendment, the Merger, the expected timetable for completing the Merger, future financial and operating results, future capital structure and liquidity, benefits and synergies of the Merger, future opportunities for the combined company, general business outlook and any other statements about the future expectations, beliefs, goals, plans or prospects of the Board or management of the Company constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words “expects,” “intends,” “anticipates,” “estimates,” “predicts,” “believes,” “should,” “potential,” “may,” “forecast,” “objective,” “plan,” or “targets,” and their variants and other similar expressions) are intended to identify forward-looking statements.
There are a number of factors that could cause actual results or events to differ materially from those indicated by such forward-looking statements, including:
| • | risks and uncertainties relating to the Merger, including the possibility that the Merger does not close when expected or at all because conditions to closing are not satisfied on a timely basis or at all; |
| • | potential adverse reactions or changes to business or employee relationships, including those resulting from the announcement or completion of the Merger; |
| o | timing of the Merger; |
| o | the possibility that the anticipated benefits of the Merger are not realized when expected or at all, including as a result of the impact of, or problems arising from, the integration of the two companies; |
| o | the possibility that the Merger may be more expensive to complete than anticipated, including as a result of unexpected factors or events; |
| o | diversion of management’s attention from ongoing business operations and opportunities; |
| • | changes in operational and capital plans; |
| • | the potential for production decline rates to be greater than expected; |
| • | delays, |
| • | higher than expected costs and expenses, including the availability and cost of services and material and the surviving corporation’s potential inability to achieve expected cost savings; |
| • | unexpected future capital expenditures; |
| • | economic and competitive conditions; |
| • | debt and equity market conditions, including the availability and costs of financing to fund the surviving corporation’s operations; |
| • | the ability to obtain industry partners to jointly explore certain prospects, and the willingness and ability of those partners to meet capital obligations when requested; |
| • | compliance with environmental and other regulations; |
| • | the success of the surviving corporation’s risk management activities; |
| • | title to properties, including, if any, those to be acquired in the Merger; |
| • | litigation, including litigation concerning the Merger; |
| • | the other factors and financial, operational and legal risks or uncertainties described in the Company’s public filings with the SEC, including its Annual Report on Form 10-K for the year ended December 31, 2022 and subsequent reports on Form 10-Q and 8-K filed with the SEC, all of which are or may in the future be incorporated by reference into this Information Statement. |
The Company disclaims any intention or obligation to update or revise any forward-looking statements as a result of developments occurring after the date of this document except as required by law.
RISK FACTORS
An investment in the surviving corporation may include different risks than an investment in the Company on a stand-alone basis. Moreover, by reason of the pending Merger, and the potential change in the business of the surviving corporation as compared to the Company’s business as currently conducted, the nature of the investment by each stockholder of the Company will change. In addition to the other information contained in or incorporated by reference in this Information Statement, including the risk factors contained in the Company’s annual report on Form 10-K for the year ended December 31, 2021, as updated by subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, which are all incorporated by reference in this Information Statement, you should carefully review the risks described below together with all of the other information included in this Information Statement. Additional risks and uncertainties not presently known to the Company or BioRegenx, or that are not currently believed to be important to you, if they materialize, may also adversely affect the Merger and the Company as the surviving corporation. See “Where You Can Find Additional Information” for the location of information incorporated by reference into this Information Statement.
Risks Relating to the Merger
The Company and BioRegenx may fail to complete the Merger if certain required conditions, many of which are outside the companies’ control, are not satisfied.
Completion of the Merger is subject to various customary closing conditions, including, but not limited to, (i) the absence of any order of injunction prohibiting the consummation of the Merger, (ii) no material adverse effect occurring with respect to the Company or BioRegenx, (iii) subject to certain exceptions and materiality and material adverse effect standards, the accuracy of the representations and warranties of the parties to the Merger Agreement and (iv) performance and compliance by the parties to the Merger Agreement in all material respects with the agreements and covenants contained in the Merger Agreement. Despite the companies’ best efforts, they may not be able to satisfy or receive the various closing conditions and obtain the necessary approvals in a timely fashion or at all.
Failure to complete the Merger could negatively impact the Company’s stock price, future business and financial results.
If the Merger is not completed, the Company will be subject to several risks, including the following:
| • | payment for certain costs relating to the Merger, whether or not the Merger is completed, such as legal, accounting and printing fees; |
| • | negative reactions from the financial markets, including declines in the price of the Common Stock due to the fact that current prices may reflect a market assumption that the Merger will be completed; |
| • | diverted attention of Company management to the Merger rather than to the Company’s operations and pursuit of other opportunities that could have been beneficial to it; and |
| • | negative impact on the Company’s future growth plan, including with regard to potential acquisitions, for which the Company is likely to provide a stronger foundation. |
The Company will be subject to various uncertainties and contractual restrictions while the Merger is pending that could adversely affect its business and operations.
Uncertainty about the effect of the Merger on customers, suppliers and vendors may have an adverse effect on the Company’s business, financial condition and results of operations. It is possible that some customers, suppliers and other persons with whom the Company has business relationships may delay or defer certain business decisions, or might decide to seek to terminate, change or renegotiate their relationship with the Company as a result of the Merger, which could negatively affect the Company’s financial results, as well as the market price of the Common Stock, regardless of whether the Merger is completed.
Additionally, under the terms of the Merger Agreement, the Company is subject to certain restrictions on the conduct of its business prior to the consummation of the Merger, which may adversely affect its ability to execute certain aspects of its business strategies. These restrictions may, among other matters, prevent the Company from pursuing otherwise attractive business opportunities, selling assets, incurring indebtedness, engaging in significant capital expenditures in excess of certain limits set forth in the Merger Agreement, entering into other transactions or making other changes to the Company’s business prior to consummation of the Merger or termination of the Merger Agreement. Such limitations could negatively affect the Company’s businesses and operations prior to the completion of the Merger.
The Company may have difficulty attracting, motivating and retaining executives and other employees in light of the Merger.
Uncertainty about the effect of the Merger on the Company’s employees may impair its ability to attract, retain and motivate personnel until the Merger is completed. Employee retention may be particularly challenging during the pendency of the Merger, as employees may feel uncertain about their future roles with the Company. In addition, the Company may have to provide additional compensation in order to retain employees. If employees depart because of issues relating to the uncertainty and difficulty of integration or a desire not to become employees of the Company, the Company’s ability to realize the anticipated benefits of the Merger could be adversely affected.
In connection with the Merger, the Company may be required to take write-downs or write-offs, restructuring and impairment or other charges that could negatively affect the business, assets, liabilities, prospects, outlook, financial condition and results of operations of the Company.
Although the Company has conducted extensive due diligence in connection with the Merger, it cannot assure you that this diligence revealed all material issues that may be present, that it would be possible to uncover all material issues through a customary amount of due diligence, or that factors outside of the Company’s control will not later arise. Even if the Company’s due diligence successfully identifies certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistent with the Company’s preliminary risk analysis. Further, as a result of the Merger, purchase accounting, and the proposed operation of the Company going forward, the Company may be required to take write-offs or write-downs, restructuring and impairment or other charges. As a result, the Company may be forced to write-down or write-off assets, restructure its operations, or incur impairment or other charges that could negatively affect the business, assets, liabilities, prospects, outlook, financial condition and results of operations of the Company.
The market price of the Common Stock may be volatile, and holders of the Common Stock could lose a significant portion of their investment due to drops in the market price of the Common Stock following completion of the Merger.
The market price of the Common Stock may be volatile, including changes in price caused by factors unrelated to the Company’s operating performance or prospects.
Specific factors that may have a significant effect on the market price for the Common Stock include, among others, the following:
| • | changes in stock market analyst recommendations or earnings estimates regarding the Common Stock, other companies comparable to it or companies in the industries they serve; |
| • | actual or anticipated fluctuations in the Company’s operating results of future prospects; |
| • | reaction to public announcements by the Company; |
| • | strategic actions taken by the Company or its competitors, such as the intended business separations, acquisitions or restructurings; |
| • | failure of the Company to achieve the perceived benefits of the Merger, including financial results and anticipated synergies, as rapidly as or to the extent anticipated by financial or industry analysts; |
| • | adverse conditions in the financial market or general U.S. or international economic conditions, including those resulting from war, incidents of terrorism and responses to such events; and |
| • | sales of Common Stock by the Company, members of its management team or significant stockholders. |
Risks Relating to the Business of the Company upon Completion of the Merger
The Company may fail to realize the anticipated benefits of the Merger and may assume unanticipated liabilities.
The success of the Merger will depend on, among other things, the Company’s ability to combine the Company’s and BioRegenx’s businesses in a manner that realizes the various benefits, growth opportunities and synergies identified by the companies. Achieving the anticipated benefits of the transaction is subject to a number of risks and uncertainties. It is uncertain whether the Company’s and BioRegenx’s existing operations and the acquired properties and assets can be integrated in an efficient and effective manner.
The pro forma financial statements presented for illustrative purposes may not be indicative of the Company’s financial condition or results of operations following the Merger.
The pro forma financial statements contained in this Information Statement are presented for illustrative purposes only and may not be indicative of the Company’s financial condition or results of operations following the Merger for several reasons. The pro forma financial statements have been derived from the Company’s and BioRegenx’s historical financial statements, and certain adjustments and assumptions have been made regarding the Company after giving effect to the Merger. The information upon which these adjustments and assumptions have been made is preliminary, and these kinds of adjustments and assumptions are difficult to make with complete accuracy. Moreover, the pro forma financial statements do not reflect all costs that are expected to be incurred by the Company in connection with the Merger. For example, the impact of any incremental costs incurred in integrating the two companies is not reflected in the pro forma financial statements. As a result, the actual financial condition and results of operations of the Company following the Merger may not be consistent with, or evident from, these pro forma financial statements.
The assumptions used in preparing the pro forma financial information may not prove to be accurate, and other factors may affect the Company’s financial condition or results of operations following the Merger. Any potential decline in the Company’s financial condition or results of operations could cause the stock price of the Company to decline.
Combining the businesses of the Company and BioRegenx may be more difficult, costly and time-consuming than expected, which may adversely affect the Company’s results and negatively affect the value of the Common Stock following the Merger.
The Company and BioRegenx have entered into the Merger Agreement because each believes that combining the businesses of the Company and BioRegenx will produce benefits and cost savings. However, the Company and BioRegenx have historically operated as independent companies and will continue to do so until the completion of the Merger. Following the completion of the Merger, the Company’s management will need to integrate the Company’s and BioRegenx’s respective business. The combination of two independent businesses is a complex, costly and time-consuming process and the Company’s management may face significant challenges in implementing such integration, many of which may be beyond the control of management, including, without limitation:
| • | difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects; |
| • | the possibility of faulty assumptions underlying expectations regarding the integration process, including with respect to the intended tax efficient transactions; |
| • | unanticipated changes in applicable laws and regulations; and |
| • | unforeseen expenses or delays associated with the Merger. |
Some of these factors will be outside of the control of the Company and any one of them could result in increased costs and diversion of management’s time and energy, as well as decreases in the amount of expected revenue that could materially impact the business, financial conditions and results of operations of the Company. The integration process and other disruptions resulting from the Merger may also adversely affect the Company’s relationships with employees, suppliers, customers, distributors, licensors and others with whom the Company and BioRegenx have business or other dealings, and difficulties in integrating the businesses of the Company and BioRegenx could harm the reputation of the Company.
If the Company is not able to successfully combine the businesses of the Company and BioRegenx in an efficient, cost-effective and timely manner, the anticipated benefits and cost savings of the Merger may not be realized fully, or at all, or may take longer to realize than expected, and the value of the Common Stock, the revenues, levels of expenses and results of operations of the Company may be affected adversely. If the Company is not able to adequately address integration challenges, the Company may be unable to successfully integrate the Company’s and BioRegenx’s operations or realize the anticipated benefits of the Merger.
The Share Issuances will dilute the percentage ownership interests of the Company’s stockholders.
If the Merger is completed, the Company will issue to BioRegenx, not more than 337,793,959 shares of Common Stock, and 836,048 Series A preferred shares representing approximately 90% of the Company’s outstanding voting securities.
THE PARTIES
Findit, Inc. owns Findit.com and the Findit App that is available in Android and IOS through the Google Play Store and the Apple App store. Findit.com and the Findit App operate as a Social Media Content Management Platform, that includes its own interactive search engine. Members can utilize Findit search to submit URLs they want Findit to index in Findit search results. Nonmembers can come to Findit and enter search queries, Findit returns matching search results from content posted in Findit along with outside web pages that have been submitted.
BioRegenx, Inc. was created to integrate leading-edge companies into one synergistic platform offering 360-degree solutions for health improvement. BioRegenx provides single-source development, marketing, and education, and offers multiple sales channel deployment. Integrated data is compiled in a secure cloud-based platform and is focused on comprehensive microcirculation, epigenetic, and metabolic profiles. These profiles generate both actionable and comprehensive test result dashboards for the physician. Individual patients have access to personalized mobile apps that are integrated to track improvements and measurable results from their personalized action plan. The result: improved vitality and longevity. This data allows researchers, governments, insurance plans and employers access to real data to improve employee health and reduce the costs.
THE MERGER
Overview
Under the terms of the Merger Agreement, subject to the satisfaction or waiver (if permissible under applicable law) of specified conditions, BioRegenx will be merged with and into the Company, with the Company surviving the Merger as the surviving corporation. The Board approved the Merger Agreement effective December 29, 2022 and the Principal Stockholders, among other things, approved and adopted the Shareholder Approved Actions effective December 29, 2022. This section of the Information Statement describes material aspects of the Merger, including the Merger Agreement and other transactions that are being entered into in connection with the Merger. While the Company believes that the description covers the material terms of the Merger, the Merger Agreement, and the other
documents in connection thereto, this summary may not contain all of the information that is important to you. You should read carefully this entire document and the other documents to which the Company refers, including the Merger Agreement attached as Annex A, for a more complete understanding of the Merger and the Merger Agreement.
Background of the Merger
BioRegenx has had a long-term strategic plan to go public. In the first quarter of 2021, BioRegenxs management team began exploring potential merger candidates to achieve this goal. During the 1st quarter of 2021, BioRegenx was made aware that a company named Blue Water Ventures International held potential as a merger candidate. Bill Resides and Bob Doran, officers and directors of BioRegenx then reached out to Keith Webb, the founder of Blue Water. After some talks, Mr. Webb, with his many years of mergers and acquisitions experience, proceeded to share with us that Blue Water was not a good reverse merge candidate for us. Following extensive deliberation, the parties determined that Mr. Webb should be engaged as a paid consultant to investigate the feasibility of a direct listing to take BioRegenx public, as well as to provide guidance and identify potential merger opportunities, attorneys, consultants, and other resources to facilitate the process of going public.
Following a thorough assessment of numerous potential merger candidates, BioRegenx was encouraged to come across the Company in December 2021 as a viable option. The Company became a potential acquisition/merger candidate after the unfortunate and unexpected death of one of its principals due to COVID. During December 2021 and January 2022, Messrs. Firth and Powers discussed the potential acquisition of BioRegenx with Messrs. Resides, Doran who indicated that they would be interested in evaluating such a transaction.
Through a series of telecommunications in the 4th quarter of 2021, Messrs. Firth and Powers along with Holly Andrews, a principal shareholder discussed materials that laid out possible transaction structures and objectives for the transaction, to ultimately be shared with Messrs. Resides and Doran.
Through a series of telecommunications in the 4th quarter of 2021, Messrs. Firth and Powers along with Holly Andrews met to discuss the financial model of a combined company and to discuss the potential merger and potential terms of such merger.
During the 4th quarter of 2021, Findit and BioRegenx agreed to exchange technical data of their respective businesses and began the initial steps for due diligence and set forth the terms of the potential merger.
After conducting due diligence and evaluating the potential benefits of the merger, BioRegenx and Findit, Inc. entered into a Letter of Intent on January 07, 2022, that set out the agreed upon terms of the potential merger.
During the end of the first quarter, the Company’s BOD held meetings where it discussed the potential merger with BioRegenx. The Company’s BOD reviewed BioRegenx’s corporate presentation and discussed the term sheet that was given to BioRegenx under which the Company would complete a merger. The Company’s principal equity holder, Holly Andrews, was still considering the proposal.
On January 28, 2022, Holly Andrews and Tom Powers traveled to Tennessee to meet with Messrs. Resides to discuss the terms of the letter of intent.
On February 2, 2022, Holly Andrews through an email connected the Company’s attorney, Thomas Cook and accountant Doug Erwin, with BioRegenx attorney, Jody Walker regarding a checklist to get things moving forward. Due to the delay in the preparation of the audit and the material terms of the initial Letter of Intent, the Company and BioRegenx met via telecommunication to reassess the potential merger and discuss potential terms of such merger.
On March 3, 2022, the Company and BioRegenx amended the original letter of intent that set out the revised agreed upon terms of the potential merger and extended the term of the letter of intent.
On March 23, 2022, the parties’ management teams met via telecommunication to discuss the closing agenda & checklist.
On April 22, 2022, Findit and BioRegenx management teams met via telecommunication to discuss the following items: 1) Agreement and Plan of Reorganization (signed by board of directors of both companies, kept in corporate records relating to tax-free status) 2) Agreement and Plan of Merger (Exhibit to Agreement and Plan of Reorganization – sent to shareholders with 14C) 3) Articles of Merger (filed with the State of Nevada 20 days after filing DEF 14C and mailing DEF 14C to shareholders).
During the remainder of the 2nd quarter of 2022, the management teams met on numerous occasions to discuss the Merger. Each of parties conducted extensive due diligence.
Continued delays in the completion of the BioRegenx audit resulted in an Amended and Restated Letter of Intent on July 1, 2022 and an addendum to the Amended and Restated Letter of Intent that same day.
Over the course of the 3rd and 4th quarters, the parties finalized negotiation of the transaction documentation.
In December 2020, the Company’s board of directors held a telephonic meeting. Thomas Powers gave an updated presentation summarizing the proposed terms of the Merger Agreement. The Company’s management then recommended that the Board approve the transactions. After considering the proposed terms of the Merger and the terms of the Merger Agreement and transactions contemplated by the Merger Agreement, the Company’s board (i)(A) determined that the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement, are fair to, and in the best interests of, the Company and its stockholders and (B) approved and declared advisable the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement, recommended that the holders of the common shares, Series A preferred shares and the Series B preferred shares vote in favor of adopting the Merger Agreement and (ii) directed that the Merger Agreement be submitted to the holders of the voting securities for their adoption.
Effective December 29, 2022, the Company and BioRegenx executed and delivered the Merger Agreement and related transaction agreements. On December 29, 2022, the Company and BioRegenx issued a joint press release announcing entry into the Merger Agreement.
Reasons for the Merger
In evaluating the Merger, the Company’s Board consulted with the Company’s management, as well as its legal and financial advisors, and considered a number of factors, weighing both perceived benefits of the Merger as well as potential risks of the Merger. In the course of its deliberations, the Board considered a variety of factors and information that it believes support its determinations and recommendations, including the following (which are not all-inclusive and not necessarily presented in order of relative importance):
| • | The Company’s expectation that the Merger will provide significant EBITDAX accretion as a result of enhanced capital efficiency and operating margins. |
| • | The Company’s expectation that the combination will materially enhance the Company’s financial, operational, and credit metrics. |
| • | The attractiveness of the Merger to the Company in comparison to other acquisition opportunities reasonably available to the Company, including BioRegenx’s desirable asset quality, potential synergies between the companies, accretive cash flow and the immediate actionability of the BioRegenx acquisition opportunity. |
| • | The recommendation of the Merger by the Company’s management team. |
| • | That the Company’s Board believes the restrictions imposed on the Company’s business and operations during the pendency of the Merger are reasonable and not unduly burdensome. |
| • | The likelihood of consummation of the Merger and the Board’s evaluation of the likely time period necessary to close the Merger. |
| • | That the addition of the seven directors to the Company’s Board in connection with the Merger will add further valuable expertise and experience and in-depth familiarity with BioRegenx to the Company’s Board, which will enhance the likelihood of realizing the strategic benefits that the Company expects to derive from the Merger. |
In the course of its deliberations, the Company’s Board also considered a variety of risks, uncertainties and other potentially negative factors, including the following (which are not necessarily all-inclusive and not presented in order of relative importance):
| • | That the Merger may not be completed in a timely manner or at all and the potential consequences of non-completion or delays in completion. |
| • | The effect that the length of time from announcement of the Merger until completion of the Merger could have on the market price of the Company’s Common Stock, its operating results and the relationship with the Company’s shareholders, customers, suppliers, regulators and others who do business with the Company. |
| • | That the integration of BioRegenx and the Company may not be as successful as expected and that the anticipated benefits of the Merger may not be realized in full or in part, including the risk that synergies and cost-savings may not be achieved or not achieved in the expected time frame. |
| • | That the attention of the Company’s management team may be diverted from other strategic priorities to implement the Merger and make arrangements for the integration of BioRegenx’s and the Company’s operations, assets and employees following the Merger. |
| • | The transaction costs to be incurred by the Company in connection with the Merger. |
| • | The risks associated with the occurrence of events that may materially and adversely affect the financial condition, properties, assets, liabilities, business or results of operations of BioRegenx and its subsidiaries but that will not entitle the Company to terminate the Merger Agreement. |
| • | The potential impact on the market price of the Company’s Common Stock as a result of the issuance of the Merger Consideration to BioRegenx stockholders. |
| • | Various other risks described in the section entitled “Risk Factors” beginning on page 22. |
The Company’s Board considered all of these factors as a whole and unanimously concluded that they supported a determination to approve the Merger Agreement and the transactions contemplated thereby, including the issuance of shares of the Company’s Common Stock and Preferred Stock in connection with the Merger. The foregoing discussion of the information and factors considered by the Board is not exhaustive. In view of the wide variety of factors considered by the Board in connection with its evaluation of the Merger and the complexity of these matters, the Board did not consider it practical to, nor did it attempt to, quantify, rank or otherwise assign relative weights to the specific factors that it considered in reaching its decision. In considering the factors described above and any other factors, individual members of the Board may have viewed factors differently or given different weight or merit to different factors.
Required Approval of the Shareholder Approved Actions; Record Date; Action by Stockholder Consent
Under Nevada law and the Company’s Articles of Incorporation and Bylaws, the Shareholder Approved Actions required the affirmative vote or written consent of the holders of a majority of the voting power of all outstanding shares of Common Stock. On January 4, 2023, the record date, there were 269,745,006 shares of Common Stock outstanding and entitled to vote.
Effective December 29, 2022, the Principal Stockholders delivered to the corporate secretary of the Company the Written Consent in respect of shares of Common Stock representing approximately 78.14% of the voting power of all outstanding shares of Common Stock. Such Written Consent constituted approval of the Shareholder Approved Actions by the holders of the requisite number of shares of Common Stock in accordance with the Nevada Revised Statutes and, accordingly, the approval of the Shareholder Approved Actions by the Company’s stockholders was effective December 29, 2022. No further approval of the stockholders of the Company is required to approve the Shareholder Approved Actions. As a result, the Company has not solicited and will not be soliciting your vote for the approval of the Shareholder Approved Actions and does not intend to call a meeting of stockholders for purposes of voting on the approval of the Shareholder Approved Actions.
Federal securities laws state that the Shareholder Approved Actions may not be completed until 20 days after the date of mailing of this Information Statement to the Company’s stockholders. Therefore, notwithstanding the execution and delivery of the Written Consent (which was obtained concurrently with the execution of the Merger Agreement), the Merger will not occur until that time has elapsed. We currently expect the Merger to be completed on or around
March 31, 2023, subject to certain government regulatory reviews and approvals and the satisfaction of the other conditions to closing in the Merger Agreement. However, there can be no assurance that the Merger will be completed on or prior to that time, or at all.
Recommendation of the Board
At the meeting of the Company’s Board on December 29, 2022, after careful consideration, including detailed discussions with the Company’s management and its legal and financial advisors, the Board:
| • | determined that the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement, are fair to, and in the best interests of, the Company and its stockholders; |
| • | approved, adopted, declared advisable and authorized the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement, on the terms and subject to the conditions set forth in the Merger Agreement; |
| • | subject to certain provisions of the Merger Agreement described below under “The Merger Agreement—No Solicitation; Board Recommendation,” recommended that the holders of Common Shares vote in favor of adopting the Merger Agreement; and |
| • | directed that the Merger Agreement be submitted to the holders of the Common Shares, Series A Preferred Shares and the Series B Preferred Shares for their adoption. |
Certain Effects of the Merger
Upon the consummation of the Merger, BioRegenx will be merged with and into the Company, and the Company shall continue its existence under the laws of the State of Nevada as the surviving corporation. At the effective time of the Merger, all of the property, rights, privileges, powers, and franchises of the Company and BioRegenx will vest in the Company, as the surviving corporation, and all debts, liabilities, obligations, restrictions, disabilities, and duties of the Company and BioRegenx shall become the debts, liabilities, obligations, restrictions, disabilities, and duties of the Company, as the surviving corporation.
At the effective time, the organizational documents of the Company in effect immediately prior to the effective time shall continue to be the organizational documents of the Company, as the surviving corporation. Subject to the terms and conditions of the Shareholders Agreement, the directors and officers of the Company immediately prior to the effective time of the Merger shall continue to be the directors and officers of the Company, as the surviving corporation.
Governance Matters After the Merger
Following the consummation of the Merger and the transactions contemplated in connection with the Merger Agreement, the Board will consist of eight directors, of whom one will have been a director of the Company prior to the consummation of the Merger and seven of whom will be persons appointed to the Board in accordance with the terms of the Merger Agreement.
The following is a list of the directors and executive officers of the Company following the Merger.
Name | Age | Position | |||
| William Resides | 55 | Chief Executive Officer, Director | |||
| Robert M. Long | 69 | Chairman of the Board | |||
| Hans Vink | 56 | Director | |||
| Robert Doran | 59 | Director | |||
| Gary Hennerberg | 67 | Secretary, Director | |||
| Dan Cortes | 57 | Chief Financial Officer | |||
| Hitesh Juneja | 35 | Chief Technology Officer | |||
| Suzanne Bird | 59 | Director | |||
| Jody Walker | 63 | Director | |||
| Thomas Powers | 68 | Director |
Officers and Directors
Biographical information for the Company’s current director who will serve on the Board is incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2021. The following is the biographical information for the officers and directors to be in effect after the Merger.
William Resides
An experienced sales executive and passionate entrepreneur, Mr. Resides is the Co-founder and Chief Executive Officer of NuLife where he leads a global team of Brand Partners on a mission of redefining health utilizing the latest in technologies and top of class products in the health and wellness sector. He has served in that role since January 2, 2019. From October 2012 to January 1, 2019, Mr. Resides has been a managing member of NuLife Ventures LLC. From March 2016 to present, Mr. Resides has been a managing member of Resides Enterprises, LLC., a management consulting company. In addition, William has served as Vice President of Business Development for Loop Media Inc, a public company, as well as the Vice President of Business Development for Crypto Insiders LLC, and Money Clip 360 Inc., developing innovation agendas and digital strategies to move their portfolio of products and services into online “Hy-bred Direct Sales” model. Before NuLife, William was in Chief Executive roles at AllTech Systems LLC, Allwired Technologies Inc. and Audio Visions Inc., throughout the ’90s until 2011. From 1986 to 1988, Mr. Resides attended Carson Newman College.
Robert M. Long
Since June 2014, Mr. Long has been the Chief Executive Officer and Manager of MVHS, a research and development and nutraceutical company. He is the co-inventor of Endocalyx Pro™ on patents in multiple countries. Since October 2013, Mr. Long has been the Chief Executive Officer and Manager of GlycoCheck™, a research and development, medical device company specializing in the research of endothelial glycocalyx. Since May 2015, Mr. Long has been Chief Executive Officer and Manager of MyBodyRx™, a research and development company. Since November 2008, Mr. Long has been the Managing Partner of RNL Lone Peak Holdings. Mr. Long attended Brigham Young University from 1972-1973. He was Managing Director for Northwestern Mutual from 1980 to 2004. Served as a member of a variety of Northwestern Mutual company committees, including compliance, claims, disability, products, life products, annuities, and compensation marketing. In 2003, served as the President of the Managing Directors Association representing 355 managing directors and the interest of their agents, representing about 45 percent of the company’s sales force. Spoke regularly at the Marriott School of Business at Brigham Young University for accounting and business management classes. Topics included risk management, life insurance, annuities, and investments. Also spoke on recruiting and training effective salespeople. Mr. Long sold his insurance and financial planning group in 2004. He then became Vice President of Sales of Vantage Controls, an early-market leader in lighting control home automation. In 2006, Mr. Long was involved in the negotiations and sale of Vantage Controls to Legrand, the largest electrical device company in the world.
Hans Vink, PhD.
Dr. Vink is a pioneer in the study of the endothelial glycocalyx. He is one of the first researchers to study the glycocalyx when he focused his expertise on medical imaging. As a result, Dr. Vink and his team were one of the first research groups to capture realistic images of the glycocalyx and focus on its significance. He had been studying the microvascular system since the 1980s. It wasn’t until the mid-1990s that technology advancements enabled peering deep inside the capillaries to take pictures. The discovery of a very thick glycocalyx, and development of new techniques to take the early pictures, has led to ongoing research to determine how a compromised glycocalyx is linked to diseases and conditions. In September 1989, Dr. Vink obtained a M.Sc. Physics from the University of Amsterdam. In June 1994, Dr. Vink obtained a Ph.D. Medical Physics from the University of Amsterdam. He continued his research as a postdoctoral fellow at the University of Virginia and returned to Amsterdam in 1997 to continue his work. He was awarded a Research Fellowship from the Royal Netherlands Academy of Arts and Sciences for his study of the glycocalyx from 2000 through 2005. In 2005 he became an Established Investigator of the Netherlands Heart Foundation. From 2006 to 2021, Dr. Vink was an Associate Professor at Maastricht University. From 2010 to present, Dr. Vink has been Chief Science Officer and Director of GlycoCheck™ where he conducts research and
development relating to glycocalyx science. He is the co-inventor of the GlycoCheck™ methods patent and Endocalyx Pro™ on patents in multiple countries. From 2014 to present, Dr Vink has been Chief Science Officer of MVHS where he conducts validation, testing and science relating to the development of the Endocalyx Pro™ supplement.
Robert Doran
Mr. Doran has been the CMO and Co-Founder of NuLife since December 2018 to December 2019 and the Executive Vice President and Co-Founder of NuLife since January 2020. From March 1995 to present Mr. Doran has been the Co-Founder of LifePower, Inc., a management company. From June 1999 to present, Mr. Doran has been the Co-Founder of Energy Network, LLC, a marketing consulting company From January 2020 to present, Mr. Doran has been the Co-Founder of Traction X39 LLC, a real estate holdings company. Mr. Doran is a traditional business guy that started in Cable TV at age 14... then graduated into Real Estate sales and Investing. In 1999, after a few years of Mentorship, he and his wife launched their own Magazine, House & Home Magazine, that they grew to over 500,000 readers, exiting in 2012 with a sale to a large media company. Mr. Doran has 28 years in Direct Sales, on the Ownership side as well as the Master Distributor and Rep sides. He received this first direct sales experience with Rexall Showcase International and his first direct sales ownership experience was with AmeriStar, a company that had an agreement with DISH Network. Mr. Doran helped grow a customer base of 250,000 in a third-party Direct Sales energy company before helping the company to a successful exit with a valuation of over 100 million dollars.
Gary Hennerberg
Since May 1, 2016 to present, Mr. Hennerberg was the Chief Marketing Officer for MVHS. From 2012 to April 30, 2016, Mr. Hennerberg was a marketing consultant for MVHS. In 1992, Mr. Hennerberg founded Hennerberg Group, Inc., an independent marketing consulting entity providing consulting analytics and sales copywriting serves to over 100 clients nationwide including AAA, CitiFinancial, Collin Street Bakery, Dow Chemical, Sprint, and dozens more. Mr. Hennerberg’s process begins with analysis and interpretation of data and numbers. He develops custom formulas, analyses and models based on 30-plus years of working in traditional direct response marketing. These models are foundational for every organization to calculate long-term value, allowable marketing costs, and budget forecasts. He is considered a full-stack marketer with experience in a broad array of marketing responsibilities. Mr. Hennerberg is the author of “Crack the Customer Mind Code / Seven Pathways from Head to Heart to YES!” (Morgan James Publishing) 2016; Author of “Direct Marketing Quantified: The Knowledge is in the Numbers” (Target Marketing Publishing) 2005. Columnist and webinar presenter for Target Marketing Magazine and Today @ Target Marketing 1993 – 2016, and a speaker at numerous events. He has taught copywriting courses for American Writers and Artists since 2004. Prior to 1992, he was Vice President of U.S. Communications Marketing Agency, Vice President of Direct Marketing for CMF&Z, a Young and Rubicam Advertising Agency, and on the client-side Mr. Hennerberg has worked as a Product Manager and Marketing Manager. Mr. Hennerberg obtained a B.A. Communications from Fort Hays State University in 1978.
Dan Cortes
Mr. Cortes is a Certified Public Accountant and has over 25 years of financial management experience in various capacities. He started his career with KPMG in their Phoenix Office. After spending nine years at KPMG, Mr. Cortes moved into financial management positions in the private industry. Mr. Cortes has a broad range of experience in finance, operations, accounting, international business and taxation and has worked in the direct sales, wholesale, manufacturing and real estate industries. Mr. Cortes has held various CFO positions including with multi-million dollar, multinational Direct Company. He has experience with developing and implementing accounting and tax systems for startups to more mature companies, staff development, reporting insurance and claims, operational procedures, legal support and international operations. Mr. Cortes graduated with honors from Arizona Statement University with a Bachelor of Science in Accounting.
Hitesh Juneja
Mr. Juneja is an entrepreneur who started his first business at the age of 14. From June 2020 to present, Mr. Juneja has been Strategy and Senior Product Manager for Solar Oil Project Manager, developing the first blockchain based exchange platform for tracking and tokenizing commodity production from carbon-neutral recycled oil production. ,From September 2017 to September 2021, Mr. Juneja worked for BlockCommerce as Chief Strategy and Product Manager, developing products and related key markets where BlockCommerce’s payment and asset management suit could be adapted to solve critical issues for the customers. From January 2018 to present, Mr. Juneja has been the co-found of SPECTRE/Skilled Gaming developing trust less systems with the world’s first brokerless speculative trading platform built on the blockchain based DALP. From November 2015 to December 2020, Mr. Juneja was the founder and head of all business development, marketing and overall business strategy for Infinii Group, which engaged in customer-facing web application and 3-learning products. From July 2008 to present, Mr. Juneja co-founded J & H Business Institute Corp, LLC where he managed multiple marketing and development projects in both B2B and B2C industries. Mr. Juneja attended the University of Northern Iowa from 2005-2008 where he studied biotechnology.
Suzanne Bird
From 2006 to present, she has been the Financial Administrator of the Fertility Center with offices located in Chattanooga and Knoxville, Tennessee. Mrs Bird wrote and supported accounting software for the rental/retail industry from 1986 to 1991. In December 1985, Mrs. Bird received her Bachelor of Science in Computer Science from Southern Adventist University, Collegedale, Tennessee. She is the wife of Joseph S. Bird, Jr., M.D., co-founder of the Company and NuLife.
Jody Walker
From 1989 to present, Mrs. Walker has been in private practice as a securities and corporate attorney. Her practice focuses primarily on regulatory compliance, mergers and acquisitions, public and private offerings with debt and equity securities, blue sky filings and general corporate matters. Mrs. Walker earned her Juris Doctorate from Hamline University in 1985 focusing on securities and taxation. She earned a Bachelor of Individualized Studies majoring in international business, french and government from the University of Minnesota in 1982.
Effects on the Company if the Merger is not Completed
If the Merger is not completed for any reason, the Company and BioRegenx will remain separate, independent companies and the current BioRegenx shareholders will continue to own the BioRegenx Equity.
If the Merger is not completed, there is no assurance as to the effect of these risks and opportunities on the future value of your shares of Common Stock, including the risk that the market price of the Common Stock may decline to the extent that the current market price of the Common Stock reflects a market assumption that the Merger will be completed. If the Merger is not completed, there is no assurance that any other transaction acceptable to the Company will be offered or that the business, operations, financial condition, earnings or prospects of the Company will not be adversely impacted. Pursuant to the Merger Agreement, under certain circumstances the Company and BioRegenx are permitted to terminate the Merger Agreement. Please see the section of this Information Statement entitled “The Merger Agreement—Termination.”
Merger Consideration
At the effective time of the Merger, and subject to the terms and conditions of the Merger Agreement, all of the BioRegenx Equity that is issued and outstanding immediately prior to the effective time will be converted into the right to receive the Stock Consideration, which shall consist of not more than 337,793,959 shares of Common Stock and 836,048 shares of Series A Preferred Stock. Following the consummation of the Merger it is expected that Holdings will own approximately 90% of the outstanding FDIT voting securities.
Series A Preferred Stock
The following is a summary of the material rights, preferences and privileges of the Series A Preferred Stock.. This summary is qualified in its entirety by reference to the complete Certificate of Designations included as part of Annex C to this Information Statement and incorporated by reference herein.
Series A Preferred Shares consist of 50,000,000 authorized shares, par value $0.001. There are 5,000,000 Series A Preferred Shares issued and outstanding as of December 31, 2020. The holders of shares of Series A Preferred Stock shall not be entitled to receive any dividends and shall not have any liquidation rights. The holders of Series A Preferred Stock shall be entitled to (a) notice of any meeting of the shareholders of the Corporation; and (b) have the power to vote each share at any shareholder meeting, where each share of Series A Preferred Stock carries the weight of two thousand five hundred (2,500) votes for each share of common stock.
Interests of the Company’s Directors and Executive Officers in the Merger
Certain of the Company’s directors and executive officers may have interests in the Merger that may be different from, or in addition to, the interests of the Company’s stockholders generally. These interests may present actual or potential conflicts of interest and you should be aware of these interests. The members of the Board were aware of and considered these interests in reaching the determination to approve the Merger Agreement and deem the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement to be fair to, and in the best interests of, the Company and its stockholders.
Material U.S. Federal Income Tax Consequences of the Merger
The following is a general discussion of the material United States federal income tax consequences of the Merger to holders of Common Stock. This discussion is limited to U.S. holders and non-U.S. holders that hold their shares of the Common Stock as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion is based upon the Code, the Treasury Regulations, judicial authorities and published positions of the Internal Revenue Service (“IRS”) all as currently in effect, and all of which are subject to change or differing interpretations possibly with retroactive effect, and any such change or differing interpretation could affect the accuracy of the statements and conclusions set forth herein.
This discussion is for general information purposes only and does not address all of the U.S. federal income tax consequences and considerations that may be relevant to a particular holder in light of such holder’s particular facts and circumstances and does not apply to holders that are subject to special treatment under U.S. federal income tax laws, such as, for example, banks or other financial institutions; insurance companies, regulated investment companies, real estate investment trusts or mutual funds; holders liable for the alternative minimum tax; certain former citizens or former long-term residents of the United States; U.S. holders having a “functional currency” other than the U.S. dollar; tax-exempt organizations; holders that exercise dissenters’ rights; dealers in securities or currencies; entities or arrangements treated as partnerships for U.S. federal income tax purposes or other flow-through entities (or investors therein); subchapter S corporations, retirement plans, individual retirement accounts or other tax-deferred accounts; traders in securities that elect to use a mark to market method of accounting; holders that hold (or that held, directly or constructively, at any time during the five year period ending on the date of the Merger) 5% or more of the equity exchanged, directly or constructively; holders that hold stock of the Company as part of a straddle, hedge, constructive sale, or conversion transaction or other integrated or risk reduction transaction; “controlled foreign corporations”; “passive foreign investment companies”; and holders that acquired the equity exchanged through the exercise of an employee stock option or otherwise as compensation or through a tax-qualified retirement plan.
This discussion does not address any tax consequences of the Merger under U.S. federal tax laws other than those pertaining to the income tax, nor does it address any considerations under any state, local or foreign tax laws or under the unearned income Medicare contribution tax pursuant to the Health Care and Education Reconciliation Act of 2010. This discussion also does not address any withholding considerations under the Foreign Account Tax Compliance Act of 2010 (including the Treasury Regulations issued thereunder and intergovernmental agreements entered into pursuant thereto or in connection therewith). No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax consequences set forth below.
If a partnership or other entity or arrangement treated as a partnership for U.S. federal income tax purposes holds shares of the Common Stock, the tax treatment of a person treated as a partner in such partnership for U.S. federal income tax purposes generally will depend upon the status of the partner and the activities of the partnership. Such partnerships and partners in such partnerships should consult their tax advisors about the tax consequences of the Merger to them.
All holders of the Common Stock are urged to consult with their tax advisors as to the specific tax consequences of the Merger to them in light of their particular facts and circumstances, including the applicability and effect of any U.S. federal, state, local, foreign or other tax laws.
For purposes of this discussion, the term “U.S. holder” means a beneficial owner of the Common Stock that for U.S. federal income tax purposes is:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust, (1) if a court within the United States is able to exercise primary supervision over the trust’s administration and U.S. persons have the authority to control all substantial decisions of the trust or (2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes. |
For purposes of this discussion, the term “non-U.S. holder” means a beneficial owner of the Common Stock that is neither a U.S. holder nor a partnership for U.S. federal income tax purposes.
Tax Consequences of the Merger Generally
For U.S. federal income tax purposes, together with the rest of the Exchanges, the Company is intended to qualify as a transaction described in Section 351 of the Code. The Company has not sought and will not seek any opinion of counsel with respect to the U.S. federal income tax treatment of the Exchanges. The Company has not sought and will not seek any ruling from the IRS as to the U.S. federal income tax consequences of the Exchanges. Consequently, no assurance can be given that the IRS will not assert, or that a court would not sustain, a position contrary to any of the conclusions set forth below.
The remainder of this discussion proceeds on the basis that the Exchanges, taken together, will qualify as a transaction described in Section 351 of the Code.
Tax Consequences to U.S. Holders
The U.S. federal income tax consequences of the Merger to U.S. holders that are Exchangors (“U.S. Exchangors”) generally are as follows:
| • | a U.S. Exchangor will not recognize gain or loss in connection with the Merger if such Exchangor only receives Common Stock pursuant to the Merger; |
| • | the aggregate tax basis of the Common Stock received by such U.S. Exchangor will be equal to the aggregate tax basis of the equity exchanged therefor (decreased by any cash received and increased by any gain recognized in connection with the Merger) plus the amount of any cash contributed; and |
| • | the holding period of the Common Stock received by a U.S. Exchangor pursuant to the Merger will include such U.S. Exchangor’s holding period of the equity surrendered in exchange therefor. |
U.S. Exchangors who acquired different blocks of the equity being surrendered at different times or different prices should consult their tax advisors as to the determination of the tax bases and holding periods of the Common Stock received in the Merger.
The Merger should not have U.S. federal income tax consequences for a U.S. Holder that is not an Exchangor.
Non-U.S. Holders
The U.S. federal income tax consequences of the Merger to non-U.S. holders that are Exchangors (“Non-U.S. Exchangors”) generally will be the same as those described above for U.S. Exchangors, except that a Non-U.S. Exchangor generally will not be subject to U.S. federal income tax or withholding tax on any gain realized in connection with the Merger unless:
| • | such gain is effectively connected with such Non-U.S. Exchangor’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment of such Non-U.S. Exchangor in the United States); |
| • | Such Non-U.S. Exchangor is an individual who is present in the United States for 183 days or more in the taxable year in which the gain is realized and certain other conditions are met; or |
| • | the Common Stock constitutes a U.S. real property interest by reason of the Company’s status as a “U.S. real property holding corporation”, as defined in the Code, at any time within the five-year period preceding the Merger or the Non-U.S. Exchangor’s holding period, whichever is shorter. We believe that we currently are, and expect to be for the foreseeable future, a U.S. real property holding corporation. However, so long as the Common Stock is regularly traded on an established securities market, the Common Stock will be treated as a U.S. real property interest for these purposes only if the Non-U.S. Exchangor actually or constructively held more than five percent (5%) of the Common Stock at any time during the five year (or shorter) period that is described above. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates in the same manner as if such Non-U.S. Exchangor were a U.S. person. A Non-U.S. Exchangor that is a corporation also may be subject to an additional branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty) on its “effectively connected earnings and profits” for the taxable year, subject to certain adjustments.
Gain described in the second bullet point above will be subject to U.S. federal income tax at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty), but may be offset by U.S.-source capital losses, if any, of the Non-U.S.Exchangor.
The Merger should not have U.S. federal income tax consequences for a non-U.S. holder that is not an Exchangor.
The preceding discussion is intended only as a general discussion of the material U.S. federal income tax consequences of the Merger. It is not a complete analysis or discussion of all potential tax effects that may be important to particular holders. Holders of Common Shares and Series A Preferred Shares should consult their own tax advisors as to the particular tax consequences to them of the Merger, including tax return reporting requirements, the applicability and effect of federal, state, local and other tax laws and the effect of any proposed changes in the tax laws.
Accounting Treatment of the Merger
The Company prepares its financial statements in accordance with GAAP and its pro forma financial statements as required by the SEC Article 11 of Regulation S-X. The Merger will be accounted for as an acquisition under FASB ASC Topic 805, Business Combinations, with BioRegenx being considered the acquirer of the Company for accounting purposes. This means that BioRegenx will allocate the purchase price to the fair value of the Company’s tangible and intangible assets and liabilities at the acquisition date, with the excess purchase price, if any, being recorded as goodwill. Under the acquisition method of accounting, goodwill is not amortized but is tested for impairment at least annually.
Dividend Policy
Since inception, the Company has not paid any cash dividends. Because the Company anticipates that all earnings will be retained for the development of its business, the Company does not expect that any cash dividends will be paid on the Common Stock for the foreseeable future.
Subject to limited exceptions, the Merger Agreement prohibits the Company (unless consented to in writing in advance by BioRegenx) from paying dividends to holders of Common Stock until the earlier of the effective time and the termination of the Merger Agreement in accordance with its terms.
For additional information on the Company’s dividend policy, see “Description of Capital Stock.” For additional information on the treatment of dividends under the Merger Agreement, see “The Merger Agreement—Operating Covenants.”
THE MERGER AGREEMENT
The following summary of the material provisions of the Merger Agreement, and such summary and the description of the Merger Agreement elsewhere in this Information Statement are qualified in their entirety by reference to the complete text of the Merger Agreement, a copy of which is included as Annex A to this Information Statement and is incorporated into this Information Statement by reference. This summary does not purport to be complete and may
not contain all of the information about the Merger Agreement that is important to you. We urge you to carefully read this entire Information Statement, including the annexes and the other documents to which we have referred you. You should also review the section entitled “Where You Can Find Additional Information.”
The Merger Agreement has been included for your convenience to provide you with information regarding its terms and we recommend that you read it in its entirety. The Merger Agreement is not intended to be a source of factual, business or operational information about the Company or BioRegenx and the following summary of the Merger Agreement and the copy thereof included as Annex A to this Information Statement are not intended to modify or supplement any factual disclosure about the Company in any documents it publicly files with the SEC. The Merger Agreement is a contractual document that establishes and governs the legal relations between the Company and BioRegenx and allocates risks between the parties, with respect to the Merger. The Merger Agreement contains representations and warranties made by the Company, on the one hand, and BioRegenx, on the other hand, that are qualified in several important respects, which you should consider as you read them in the Merger Agreement.
The representations and warranties are qualified by certain information of the Company filed with the SEC prior to the date of the Merger Agreement. Certain of the representations and warranties made by the Company, on the one hand, and BioRegenx on the other hand, were also made as of a specified date, may be subject to a contractual standard of materiality different from what might be viewed as material to stockholders, and may have been used for the purpose of allocating risk between the parties to the Merger Agreement rather than as establishing matters as facts. Moreover, information concerning the subject matter of the representations and warranties, which do not purport to be accurate as of the date of this Information Statement, may have changed since the date of the Merger Agreement, and subsequent developments or new information qualifying a representation or warranty may or may not be fully reflected in this Information Statement or the Company’s public disclosures. Accordingly, you should not rely on the representations and warranties as being accurate or complete or characterizations of the actual state of facts as of any specified date.
The Merger
At the effective time of the Merger, upon the terms and subject to the satisfaction or waiver of the conditions of the Merger Agreement and in accordance with the Nevada Revised Statutes, BioRegenx will merge with and into the Company. The separate corporate existence of BioRegenx will cease and the Company will be the surviving corporation of the Merger. The Articles of Incorporation and Bylaws of the Company as in effect immediately prior to the effective time will be the Articles of Incorporation and the Bylaws of the surviving corporation. Subject to the appointment of additional directors at the effective time of the Merger (see “Agreements Related to the Merger—The Shareholders Agreement”), the directors and officers of the Company immediately prior to the effective time of the Merger will, from and after the effective time of the Merger, be the directors and officers of the surviving corporation until their respective successors have been duly elected or appointed and qualified, or until their earlier death, resignation or removal.
Closing and Effective Time of the Merger
The closing of the Merger will take place at 9:00 a.m., Chattanooga, Tennessee time, on a date that is two (2) business days following the satisfaction or (to the extent permitted by applicable law) waiver (in accordance with the Merger Agreement) of all of the conditions described in the section below entitled “The Merger Agreement—Conditions to the Merger” (other than any such conditions that by their nature cannot be satisfied until the closing of the Merger, but subject to satisfaction of any such condition), unless the Company and BioRegenx agree to another time in writing.
The Merger will become effective upon the filing and acceptance of the articles of merger with the offices of the Secretaries of State of Nevada and Delaware, or at such later time as shall be agreed upon in writing by the Company and BioRegenx and specified in the articles of merger, which is referred to as the “effective time” of the Merger.
Consideration to be Received in the Merger
The Merger Agreement provides that, at the effective time of the Merger, all of the issued and outstanding BioRegenx Equity outstanding immediately prior to the effective time of the Merger, shall be converted into the right to receive from the Company not more than three hundred thirty seven million, seven hundred ninety three thousand nine hundred fifty nine (337,793,959) common shares and eight hundred thirty six thousand forty eight (836,048) Series A preferred shares. These issuances will represent 90% of the voting securities of the newly merged company.
Representations and Warranties
The Merger Agreement contains a number of representations and warranties made by the Company, including representations and warranties relating to:
| • | corporate organization, good standing and similar matters; |
| • | corporate power and authority to execute and deliver the Merger Agreement and to consummate the transactions contemplated by the Merger Agreement; |
| • | authorization of the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement and enforceability of the Merger Agreement against the Company; |
| • | required governmental filings and consents, applicable expiration or termination of the HSR Act waiting period, and absence of violation of applicable laws in connection with the execution, delivery and performance of the Merger Agreement and the consummation of the Merger and the other transactions contemplated by the Merger Agreement; |
| • | absence of conflicts with, violation or breach of, defaults under, the Company’s organizational documents and certain contracts and permits in connection with the execution, delivery and performance of the Merger Agreement and the consummation of the Merger and the other transactions contemplated by the Merger Agreement; |
| • | brokers’, finders’ and similar fees or commissions payable in connection with the Merger and the other transactions contemplated by the Merger Agreement; |
| • | the absence of any current or pending bankruptcy, insolvency, or reorganization proceedings; |
| • | legal proceedings |
| • | the accuracy and sufficiency of reports and financial statements filed with the SEC; |
| • | the absence of undisclosed liabilities; |
| • | internal controls over financial reporting; |
| • | ownership of the Common Stock; |
| • | accuracy of the information in this Information Statement; |
| • | taxes; |
| • | material contracts; |
| • | relationships with related parties and affiliates; |
| • | financing matters; |
| • | compliance with applicable laws, court orders and certain regulatory matters; and |
The Merger Agreement also contains a number of representations and warranties made by BioRegenx, including representations and warranties relating to:
| • | corporate organization, good standing and similar matters; |
| • | corporate power and authority to execute and deliver the Merger Agreement and to consummate the transactions contemplated by the Merger Agreement; |
| • | authorization of the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement and enforceability of the Merger Agreement against BioRegenx; |
| • | capital structure and equity securities; |
| • | absence of conflicts with, violation or breach of, defaults under, BioRegenx’s organizational documents and certain contracts and permits in connection with the execution, delivery and performance of the Merger Agreement and the consummation of the Merger and the other transactions contemplated by the Merger Agreement; |
| • | financial statement matters; |
| • | the absence of undisclosed liabilities; |
| • | absence of certain changes or events and the conduct of business in the ordinary course of business; |
| • | the absence of any current or pending bankruptcy, insolvency, or reorganization proceedings; |
| • | legal proceedings; |
| • | taxes; |
| • | compliance with applicable laws, court orders and certain regulatory matters; |
| • | permits; |
| • | real property; |
| • | employee compensation and benefits matters and matters relating to the Employee Retirement Income Securities Act of 1974, as amended; |
| • | labor and employment matters; |
| • | material contracts; |
| • | insurance; |
| • | intellectual property; |
| • | environmental matters and compliance with environmental laws; |
| • | the existence of any preferential rights and consents that will be triggered by the Merger; |
| • | rights-of-way, easements, and other surface or sub-surface use, ingress, or egress right; |
| • | brokers’, finders’ and similar fees or commissions payable in connection with the Merger and the other transactions contemplated by the Merger Agreement; |
| • | maintenance of books and records; |
| • | ownership of the BioRegenx Equity; |
| • | accuracy of certain information in this Information Statement; |
Significant portions of the representations and warranties of each of the Company, BioRegenx are qualified as to “materiality” or “material adverse effect.”
Under the Merger Agreement, a Company material adverse effect means a material adverse effect on (a) the business, assets, condition (financial or otherwise) or results of operations of the Company and its affiliates, taken as a whole, or (b) the Company’s ability to perform its obligations under the Merger Agreement (or the other transaction documents contemplated therein) or to consummate the transactions contemplated by the Merger Agreement (or the other transaction documents contemplated therein); provided, however, that the following shall not be considered when determining whether a Company material adverse effect has occurred or would reasonably be expected to occur:
| · | general economic, financial, credit, or political conditions and general changes in markets, including changes generally in supply, demand, price levels or interest or exchange rates; |
| · | political conditions (or changes in such conditions) or acts of war, sabotage or terrorism (including any escalation or general worsening of any such acts of war, sabotage or terrorism); |
| · | changes in laws or GAAP or the interpretation thereof; |
| · | any effect resulting from any action taken by BioRegenx or any of its affiliates; |
| · | any effect resulting from any action taken by the Company or any affiliates of the Company that is expressly required hereunder or failure of the Company or any affiliate of the Company to take any action that is prohibited under the Merger Agreement; |
| · | any failure to meet any projections, budgets, forecasts, estimates, plans predictions, performance metrics or operating statistics or the inputs into such items; or |
| · | any effects or changes resulting from entering into the Merger Agreement or the announcement of the transactions contemplated thereby, except to the extent and then only to the extent any of the events, changes or circumstances referred to in (i) through (v) above disproportionately affect the Company as compared to other participants in the industries and areas in which the Company operates. |
Under the Merger Agreement, a BioRegenx material adverse effect means a material adverse effect on (a) the business, assets, condition (financial or otherwise) or results of operations of BioRegenx or any of its affiliates, taken as a whole, or (b) BioRegenx’s or any of its affiliates’ ability to perform their respective obligations under Merger Agreement or to consummate the transactions contemplated by the Merger Agreement; provided, however, that the following shall not be considered in determining whether a BioRegenx material adverse effect has occurred or would be reasonably expected to occur:
| · | general economic, financial, credit, or political conditions and general changes in markets, including changes generally in supply, demand, price levels or interest or exchange rates; |
| · | political conditions (or changes in such conditions) or acts of war, sabotage or terrorism (including any escalation or general worsening of any such acts of war, sabotage or terrorism); |
| · | changes in laws or GAAP or the interpretation thereof; |
| · | any effect resulting from any action taken by the Company or any affiliate of the Company; |
| · | any effect resulting from any action taken by BioRegenx or any affiliate of BioRegenx that is expressly required under the Merger Agreement or any failure of BioRegenx or any affiliate of BioRegenx to take any action that is prohibited under the Merger Agreement; |
| · | any failure to meet any projections, budgets, forecasts, estimates, plans predictions, performance metrics or operating statistics or the inputs into such items; or |
| · | any effects or changes resulting from entering into the Merger Agreement or the announcement of the transactions contemplated thereby, except to the extent and then only to the extent any of the events, changes or circumstances referred to above disproportionately affect BioRegenx or any of its affiliates as compared to other participants in the industries and areas in which BioRegenx operates. |
Operating Covenants
The Company has agreed, with certain exceptions or with the prior written consent of BioRegenx, that during the period from the date of the Merger Agreement until the closing of the Merger (or the termination of the Merger Agreement, as applicable):
| · | the Company will conduct its business in the ordinary course consistent with past practice; |
| · | neither the Company will (i) declare, set aside or pay any dividends on, or make any other distributions (whether in cash, stock or property) in respect of, the Equity Securities (as defined in the Merger Agreement) of the Company or any subsidiary, (ii) purchase, redeem or otherwise acquire, or offer to purchase redeem or otherwise acquire, Equity Securities of the Company or any subsidiary or any options, warrants, or rights to acquire any Equity Securities in the Company or any subsidiary or (iii) split, combine, reclassify or otherwise amend the terms of the Equity Securities of the Company or any subsidiary; |
| · | the Company will amend or otherwise change, or authorize or propose to amend or otherwise changes, its organizational documents; |
| · | the Company will (i) incur, create, assume or otherwise become liable for, or prepay, any indebtedness, or amend, modify or refinance any indebtedness, other than borrowings under the Company’s revolving credit facility in the ordinary course of business or (ii) make any loans, advances or capital contributions to, or investments in, any other person, other than to a subsidiary of the Company; |
| · | the Company will consummate or adopt a plan of complete or partial liquidation, dissolution, recapitalization or other reorganization, or merge, consolidate, combine or amalgamate with any other person or entity; |
| · | the Company will change its financial or tax accounting methods, principles or practices, except insofar as may have been required by a change in GAAP or applicable law; |
| · | the Company will take any action that would or would reasonably be expected to prevent or materially delay the closing and the consummation of the transactions contemplated by the Merger Agreement or the other transaction documents contemplated therein; and |
| · | the Company will authorize any of, or commit, resolve or agree to take any of, the foregoing actions. |
The Merger Agreement also contains pre-closing covenants by BioRegenx similar to those, and in certain instances, beyond those by which the Company is bound, during the period from the date of the Merger Agreement until the closing of the Merger (or termination of the Merger Agreement, as applicable).
Action by Stockholder Consent
Contemporaneously with the execution of the Merger Agreement and in lieu of calling a meeting of the Company’s stockholders, the Company submitted a form of irrevocable written consent approving and adopting the Charter Amendment in connection with the Share Increase and Merger Agreement. No further approval of the stockholders of the Company is required to adopt the Merger Agreement. As a result, the Company has not solicited and will not be soliciting your vote for the adoption of the Merger Agreement and does not intend to call a meeting of stockholders for purposes of voting on the adoption of the Merger Agreement.
Reasonable Best Efforts and Certain Pre-Closing Obligations
The Merger Agreement requires the parties to use reasonable best efforts to take, or cause to be taken, all actions, and do, or cause to be done, and to assist and cooperate with the other parties in doing, all things necessary, proper or advisable to consummate and make effective, as promptly as practicable, the Merger and the other transactions contemplated by the Merger Agreement, including:
| • | the obtaining of all necessary actions or non-actions, waivers and consents from, the making of all necessary registrations, declarations and filings with and the taking of all reasonable steps as may be necessary to avoid a proceeding by any governmental entity with respect to the Merger Agreement or the transactions contemplated by the Merger Agreement; and |
| • | the execution and delivery of any additional instruments necessary to consummate the transactions contemplated by the Merger Agreement and to fully carry out the purposes of the Merger Agreement. |
In addition, the Company and BioRegenx have agreed to consult and cooperate with each other in connection with regulatory filings.
Employment Matters
The Company has no employees.
Additional Agreements
The Merger Agreement contains additional agreements between the Company and BioRegenx relating to, among other things:
| • | the filing of this Information Statement; |
| • | consultations regarding public announcements; |
| • | stockholder litigation relating to the transactions contemplated by the Merger Agreement; |
| • | non-solicitation; and |
| • | notification of certain matters. |
Conditions to the Merger
The obligation of each party to the Merger Agreement to effect the Merger is subject to the satisfaction or waiver (if permissible under applicable law), on or prior to the closing of the Merger, of the following conditions.
| • | the absence of any judgment issued by any governmental entity or other legal restraint or prohibition (in each case, with respect to any competition, merger control, antitrust or similar law, solely with respect to the required regulatory approvals) preventing or prohibiting the consummation of the Merger; and |
| • | this Information Statement shall have been mailed to the Company’s stockholders at least 20 days prior to the Closing Date in accordance with the terms and conditions of the Merger Agreement, and the issuance of the Stock Consideration shall be permitted by Regulation 14C of the Exchange Act. |
| · | all of the current assets and liabilities of FDIT, shall be transferred into a wholly owned subsidiary. Both parties agrees that the wholly owned subsidiary may be subsequently spun out upon yet to be determined terms. The parties acknowledge that, subsequent to the spin out, all assets and liabilities currently outstanding in FDIT, including the $150,000 SBA loan, will follow the subsidiary and will not be the responsibility of the merged entity post-closing of this transaction. |
| · | the principals of the Company shall execute an option agreement with the Company relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger. After said closing of the merger with BioRegenx and subsequently upon exercising the option of the acquisition of CHNO, the Company’s board of directors agree to support and cause to be placed on the ballot at each election of the Company’s directors one name chosen by the current principals of CHNO which shall be a nominee to the Company’s board of directors. |
The obligation of the Company to effect the Merger is further subject to the satisfaction, or waiver by the Company (if permissible under applicable law), on or prior to the Closing Date of the Merger, of the following conditions:
| • | subject to certain materiality qualifiers, accuracy as of the date of the Merger Agreement and as of the Closing of the Merger of the representations and warranties made by BioRegenx to the extent specified in the Merger Agreement; |
| • | performance of or compliance in all material respects with the covenants and agreements contained in the Merger Agreement to be performed or complied with by BioRegenx prior to or on the Closing Date to the extent specified in the Merger Agreement; |
| • | receipt of a certificate, dated as of the Closing Date, executed by an executive officer of BioRegenx confirming the satisfaction of certain conditions required to be performed and accuracy of representations and warranties made under the Merger Agreement; and |
| • | there shall not have been, since the date of the Merger Agreement, any event, change, effect, or development that, individually or in the aggregate, would reasonably be expected to have a material adverse effect; |
The obligation of BioRegenx to effect the Merger is further subject to the satisfaction, or waiver by BioRegenx (if permissible under applicable law), on or prior to the closing date of the Merger, of the following conditions:
| • | subject to certain materiality qualifiers, accuracy as of the date of the Merger Agreement and as of the closing of the Merger of the representations and warranties made by the Company to the extent specified in the Merger Agreement; |
| • | performance of or compliance in all material respects with the covenants and agreements of the Company contained in the Merger Agreement to be performed or complied with by it prior to or on the closing date to the extent specified in the Merger Agreement; |
| • | receipt of a certificate, dated as of the Closing Date, executed by an executive officer of the Company confirming the satisfaction of certain conditions required to be performed and accuracy of representations and warranties made under the Merger Agreement; |
| • | the Company shall have executed and filed the Charter Amendment with the Secretary of State of the State of Nevada and the Charter Amendment shall have been accepted by the Secretary of State of the State of Nevada; and |
| • | there shall not have been, since the date of the Merger Agreement, any event, change, effect, or development that, individually or in the aggregate, would reasonably be expected to have a material adverse effect; |
The Company and BioRegenx can provide no assurance that all of the conditions precedent to the Merger will be satisfied or waived by the party permitted to do so.
Termination
The Merger Agreement may be terminated at any time prior to the closing of the Merger in the following circumstances:
| • | by mutual written consent of the Company and BioRegenx; |
| • | by either the Company and BioRegenx if the other is in material breach or default of its respective covenants, agreements or other obligations hereunder or if any of its representations and warranties herein are not true and accurate in all material respects when made or when otherwise required by this Agreement to be true and accurate; or |
by the Company:
| • | if any of the conditions to its obligations set forth in Section 6.1 of the Merger Agreement shall not have been satisfied as of Closing, unless satisfaction shall have been frustrated or made impossible by an act or failure to act of BioRegenx. |
by BioRegenx:
| • | if any of the conditions to its obligations set forth in Section 6.2 under the Merger Agreement shall not have been satisfied as of Closing, unless satisfaction shall have been frustrated or made impossible by an act or failure to act of the Company; or |
Effect of Termination
If the Merger Agreement shall be terminated, all obligations of the parties hereunder shall terminate, except for the obligations set forth in Section 5.6 and 5.7 of the Merger Agreement. In such event, BioRegenx shall return any and all Company Common Stock received hereunder and both parties shall file the necessary documents in the state of Nevada, to complete the transfer of any and all BioRegenx shares of stock received by the Company hereunder to the Principal Shareholders.
Expenses
Subject to certain exceptions, each party shall pay its own expenses incident to preparing for, entering into and carrying out the Merger Agreement and the transactions contemplated thereby, whether or not the Merger will be consummated.
Amendment; Extension; Waiver
No amendment, modification or alteration of the terms or provisions of this Agreement shall be binding unless the same shall be in writing and duly executed by the parties hereto, except that any of the terms or provisions of this Agreement may be waived in writing at any time by the party which is entitled to the benefits of such waived terms or provisions. No waiver of any of the provisions of this Agreement shall be deemed to or shall constitute a waiver of any other provision hereof (whether or not similar). No delay on the part of any party in exercising any right, power or privilege hereunder shall operate as a waiver thereof.
Governing Law
The Merger Agreement is governed by the laws of the State of Tennessee.
FINDIT, INC.
UNAUDITED PRO FORMA COMBINED FINANCIAL STATEMENTS
Introduction
Following are the unaudited pro forma combined financial statements and accompanying notes as of and for the nine months ended September 30, 2022 and for the year ended December 31, 2021, which have been prepared by the Company’s management and are derived from, and should be read in conjunction with, (a) the Company’s audited consolidated financial statements as of and for the year ended December 31, 2021 included in the Company’s Annual Report on Form 10-K; (b) the Company’s unaudited consolidated financial statements as of and for the nine months ended September 30, 2022 included in the Company’s Quarterly Report on Form 10-Q for the period then ended; (c) the audited consolidated financial statements of BioRegenx for the year ended December 31, 2021; and (d) the unaudited consolidated financial statements of BioRegenx as of and for the nine months ended September 30, 2022, which are included herein. Certain of BioRegenx’s historical amounts have been reclassified to conform to the Company’s financial statement presentation. The unaudited pro forma balance sheet as of September 30, 2022 gives effect to the Merger and related transactions as if these transactions had been completed on September 30, 2022. The unaudited pro forma combined statements of operations for the year ended December 31, 2021 and the nine months ended September 30, 2022 give effect to the Merger and related transactions as if these transactions had been completed on January 1, 2021.
On December 29 2022, the Company entered into the Merger Agreement under which subject to certain conditions BioRegenx will merge with and into the Company with the Company as the surviving entity. The unaudited pro forma combined financial information presented gives effect to the transactions contemplated in the Merger Agreement, including the issuance of additional shares of Common Stock and the issuance of Preferred Stock.
The pro forma financial statements have been prepared in accordance with SEC Article 11 of Regulation S-X. In addition, the acquisition method of accounting was used per ASC 805, Business Combinations, with the BioRegenx treated as the acquirer. The acquisition method of accounting is dependent upon certain valuations and other studies that have yet to commence or progress to a stage where there is sufficient information for a definitive measure. Accordingly, the pro forma adjustments are preliminary, have been made solely for the purpose of providing pro forma financial statements, and are subject to revision based on a final determination of fair value as of the date of acquisition. Differences between these preliminary estimates and the final acquisition accounting may have a material impact on the accompanying pro forma financial statements and the combined company’s future results of operations and financial position.
The pro forma financial statements do not purport to represent the financial position or results of operations of Findit which would have occurred had the Merger been consummated on the dates indicated or the Company’s financial position or results of operations for any future date or period. The pro forma statements of operations are not necessarily indicative of the Company’s operations going forward.
| FINDIT, INC | |||||
| Pro Forma Condensed Consolidated Statement of Operations | |||||
| For the nine months ended September 30, 2022 | |||||
| (Unaudited) | |||||
| Historical Consolidated | Transaction Accounting Adjustments | Notes | Proforma | ||
| Total Revenue | $ 1,965,757 | $ 1,965,757 | |||
| Costs and Operating Expense | |||||
| Costs of Goods Sold | 696,107 | 696,107 | |||
| Selling, General and Administrative | 2,803,651 | (4,389) | a | 2,799,262 | |
| Total Costs and Operating Expenses | 3,499,758 | (4,389) | 3,495,369 | ||
| Loss from operations | (1,534,001) | 4,389 | $ (1,529,612) | ||
| Other expense, net | |||||
| Interest income | 148 | 148 | |||
| interest expense | (317,842) | (317,842) | |||
| Total other expense, net | (317,694) | - | (317,694) | ||
| Loss before income taxes | (1,851,695) | 4,389 | (1,847,306) | ||
| Income tax benefit | - | - | - | ||
| Other Comprehensive Income | (39,000) | - | (39,000) | ||
| Total Comprehensive Loss | $ (1,890,695) | $ 4,389 | $ (1,886,306) | ||
| a The Unaudited pro forma condensed consolidated statement of operations reflects the following adjustments: | |||||
| Adjustment for duplicate or avoidable costs as if the proposed transaction was consummated as of January 1, 2021. The adjustments remove legal fees related to the transaction and adjust administrative costs to reflect administration of the combined companies. No amortization is included in the pro forma statements. | |||||
| FINDIT, INC | |||||
| Pro Forma Condensed Consolidated Statement of Operations | |||||
| For the three months ended September 30, 2022 | |||||
| (Unaudited) | |||||
| Historical Consolidated | Transaction Accounting Adjustments | Notes | Proforma | ||
| Total Revenue | $ 539,309 | $ - | $ 539,309 | ||
| Costs and Operating Expense | |||||
| Costs of Goods Sold | 104,496 | 104,496 | |||
| Selling, General and Administrative | 798,174 | (6,061) | a | 792,113 | |
| Total Costs and Operating Expenses | 902,670 | (6,061) | 896,609 | ||
| Loss from operations | (363,361) | 6,061 | $ (357,300) | ||
| Other expense, net | |||||
| Interest income | 79 | 79 | |||
| interest expense | (72,340) | (72,340) | |||
| Total other expense, net | (72,261) | - | (72,261) | ||
| Loss before income taxes | (435,622) | 6,061 | (429,561) | ||
| Income tax benefit | - | - | - | ||
| Other Comprehensive Income | 20,400 | - | 20,400 | ||
| Total Comprehensive Loss | $ (415,222) | $ 6,061 | $ (409,161) | ||
| a - The Unaudited pro forma condensed consolidated statement of operations reflects the following adjustments: | |||||
| Adjustment for duplicate or avoidable costs as if the proposed transaction was consummated as of January 1, 2021. The adjustments adjust administrative costs to reflect administration of the combined companies. No amortization is included in the pro forma statements. | |||||
AMENDMENT TO THE ARTICLES OF INCORPORATION
Description of the Proposed Amendment
Effective December 29, 2022, the Company’s Board unanimously approved the Merger Agreement, the Merger, and the transactions contemplated therein, including the amendment to the Company’s Articles of Incorporation, subject to required stockholder approval and the requirements of Regulation 14C, to increase the number of authorized shares of Common Stock from 500,000,000 to 4,000,000,000. The Charter Amendment will be effected upon a filing with the Secretary of State of the State of Nevada. The full text of the proposed Charter Amendment is set out in Annex E to this Information Statement. The text of the proposed Charter Amendment is subject to modification to include such changes as may be required by the office of the Secretary of State of the State of Nevada or as the Company’s Board deems necessary and advisable to effect the Share Increase.
Following approval of the Charter Amendment by the Company’s Board, action was taken without a meeting of the stockholders effective December 29, 2022 to approve the Charter Amendment by written consent signed by a majority of the voting power of the stockholders as required by Nevada law. As of the record date of January 4, 2023, there were 269,745,006 common shares issued and outstanding of which each common share was entitled to one vote, 5,000,000 Series A preferred shares issued and outstanding of which each Series A preferred share was entitled to 2,500 votes and 4,900,000 Series B preferred shares issued and outstanding of which each Series B preferred share was entitled to 1,000 votes. Effective December 29, 2022, the Principal Stockholders, who collectively own common shares, Series A preferred shares and Series B preferred shares representing approximately 78.14% of the voting power of all the outstanding voting securities, delivered to the corporate secretary of the Company an irrevocable written consent adopting the Charter Amendment.
Therefore, the Charter Amendment has been approved by the Company’s Board of Directors and a majority of the stockholders but will not become effective until the Charter Amendment is filed with the Secretary of State of the State of Nevada. No filing of the Charter Amendment can be made with the Secretary of State of the State of Nevada until at least twenty (20) calendar days following the filing of this Information Statement with the SEC and the transmission of this Information Statement to all holders of record of the Company’s Common Stock as of the record date of January 4, 2023.
Purpose and Reasons for the Share Increase
The Board determined to increase the number of authorized shares of Common Stock because the current number of authorized shares of Common Stock is insufficient to issue the Common Stock Consideration to the BioRegenx Equity holders as well as have shares available for future issuances. Currently BioRegenx has 375,000,000 shares of Common Stock authorized, and 41,670,688 shares of Common Stock issued and outstanding. Additionally, BioRegenx has 95,000 shares of Series A Preferred Stock issued and outstanding. On the Closing Date of the Merger, the BioRegenx Equity holders will receive not more than 337,793,959 shares of Common Stock and 836,048 shares of Series A Preferred Stock, which shall represent the Stock Consideration under the Merger Agreement. Furthermore, the Company Board believes that the current number is insufficient for existing and future corporate purposes, and that the increase is needed to provide flexibility for issuances of Common Stock to raise additional capital, to support strategic business opportunities that may be presented from time to time, and to reserve and make available shares of Common Stock for issuance under any equity incentive plan that the Company Board may approve and adopt.
Therefore, the Company Board has determined that it is in the best interests of the Company and its stockholders to increase the number of authorized shares of Common Stock from 500,000,000 to 4,000,000,000. The increase would become effective upon filing the proposed Charter Amendment with the Secretary of State of the State of Nevada.
Effect of the Share Increase
The Share Increase will not change the number of outstanding shares of Common Stock but will provide the Company Board with the ability to issue additional shares of Common Stock as the Board determines to be for proper corporate purposes and in the best interests of the Company.
With the exception of the number of authorized shares of Common Stock, the rights and preferences of the shares of Common Stock prior and subsequent to the Share Increase will remain the same. After the effectiveness of the Share Increase, it is not anticipated that the financial condition of the Company, the percentage ownership of management, the number of the Company’s stockholders or any aspect of the Company’s business would materially change solely as a result of the Share Increase.
The Common Stock is currently registered under Section 12(g) of the Exchange Act, and, as a result, the Company is subject to the periodic reporting and other requirements of the Exchange Act. The Share Increase will not affect the registration of the Common Stock under the Exchange Act. If the proposed Amendments are implemented, the Company’s Common Stock will continue to be reported on the OTC Markets Pink under the symbol “FDIT.”
DESCRIPTION OF CAPITAL STOCK
The following description of our Common Stock is intended as a summary only and therefore is not complete. This description is based upon, and is qualified by reference to, our Articles of Incorporation and Bylaws, each as amended from time to time, and by applicable provisions of the common law of the State of Nevada. For the complete terms of the Common Stock, please refer to our Articles of Incorporation and Bylaws, which are incorporated by reference into this Information Statement.
General
We are a company incorporated under the laws of the State of Nevada and our affairs are governed by our Articles of Incorporation (which includes all amendments thereto), our Bylaws, and the common law of the State of Nevada. Our authorized capital stock consists of 500,000,000 shares of Common Stock, par value $0.001 per share as well as 50,000,000 Series A preferred shares, par value $0.001 per Series A preferred share and 5,000,000 Series B preferred shares, par value $0.001. As of January 21, 2023, there were 269,745,006 common shares issued and outstanding, 5,000,000 Series A preferred shares issued and outstanding and 4,900,000 Series B preferred shares issued and outstanding. The following description summarizes certain terms of our shares as set out more particularly in our Articles of Incorporation and Bylaws. Because it is only a summary, it may not contain all the information that is important to you.
Common Stock
Each holder of Common Stock is entitled to one vote per share. Subject to the rights, if any, of the holders of any series of preferred stock pursuant to applicable law or the provision of the certificate of designation creating that series, all voting rights are vested in the holders of shares of Common Stock. Holders of shares of Common Stock have no right to cumulate votes in the election of directors, thus, the holders of a majority of the shares of Common Stock can elect all of the members of the board of directors standing for election. All outstanding shares of Common Stock are fully paid and non-assessable.
Dividends may be paid to the holders of Common Stock when, as, and if declared by the Board out of funds legally available for their payment, subject to the rights of the holders of preferred stock, if any. To date, no dividends have been paid. Any future determination as to the payment of dividends will depend upon the results of our operations, capital requirements, our financial condition and such other factors as our Board may deem relevant.
In the event of our voluntary or involuntary liquidation, dissolution, or winding up, the holders of Common Stock will be entitled to share equally, in proportion to the number of shares of Common Stock held by them, in any of our assets available for distribution after the payment in full of all debts and distributions and after the holders of all series of outstanding preferred stock, if any, have received their liquidation preferences in full. Holders of Common Stock are not entitled to preemptive purchase rights in future offerings of our Common Stock. Although our Articles of Incorporation states that the Company elects to have preemptive rights, pursuant to Nevada law, our stockholders do not have preemptive rights with respect to shares that are registered under Section 12 of the Exchange Act and our Common Stock is so registered.
Anti-Takeover Provisions
Our Articles of Incorporation, our Bylaws, and Nevada common law include certain provisions which may have the effect of delaying or deterring a change in control or in our management or encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include authorized blank check preferred stock, restrictions on business combinations, and the availability of authorized but unissued Common Stock.
Combination with Interested Stockholders Statute
Sections 78.411 to 78.444 of the Nevada Revised Statutes, which apply to any Nevada corporation subject to the reporting requirements of Section 12 of the Exchange Act, including us, prohibits an “interested stockholder” from entering into a “combination” with the corporation for two years, unless certain conditions are met. A “combination” includes:
| · | any merger of the corporation or any subsidiary of the corporation with an “interested stockholder,” or any other entity, whether or not itself an “interested stockholder,” which is, or after and as a result of the merger would be, an affiliate or associate of an “interested stockholder;” |
| · | any sale, lease, exchange, mortgage, pledge, transfer, or other disposition in one transaction, or a series of transactions, to or with an “interested stockholder” or any affiliate or associate of an “interested stockholder,” of assets of the corporation or any subsidiary: |
| o | having an aggregate market value equal to more than 5% of the aggregate market value of the corporation’s assets, determined on a consolidated basis; |
| o | having an aggregate market value equal to more than 5% of the aggregate market value of all outstanding voting shares of the corporation; or |
| o | representing more than 10% of the earning power or net income, determined on a consolidated basis, of the corporation; |
| · | the issuance or transfer by the corporation or any subsidiary, of any shares of the corporation or any subsidiary to an “interested stockholder” or any affiliate or associate of an “interested stockholder,” having an aggregate market value equal to 5% or more of the aggregate market value of all of the outstanding voting shares of the corporation, except under the exercise of warrants or rights to purchase shares offered, or a dividend or distribution paid or made, pro rata to all stockholders of the resident domestic corporation; |
| · | the adoption of any plan, or proposal for the liquidation or dissolution of the corporation, under any agreement, arrangement or understanding, with the “interested stockholder,” or any affiliate or associate of the “interested stockholder;” |
| · | if any of the following actions occurs: |
| o | a reclassification of the corporation’s securities, including, without limitation, any splitting of shares, share dividend, or other distribution of shares with respect to other shares, or any issuance of new shares in exchange for a proportionately greater number of old shares; |
| o | recapitalization of the corporation; |
| o | merger or consolidation of the corporation with any subsidiary; |
| o | or any other transaction, whether or not with or into or otherwise involving the interested stockholder, |
| o | under any agreement, arrangement or understanding, whether or not in writing, with the interested stockholder or any affiliate or associate of the interested stockholder, which has the immediate and proximate effect of increasing the proportionate share of the outstanding shares of any class or series of voting shares or securities convertible into voting shares of the corporation or any subsidiary of the corporation which is beneficially owned by the interested stockholder or any affiliate or associate of the interested stockholder, except as a result of immaterial changes because of adjustments of fractional shares. |
| · | any receipt by an “interested stockholder” or any affiliate or associate of an “interested stockholder,” except proportionately as a stockholder of the corporation, of the benefit of any loan, advance, guarantee, pledge or other financial assistance or any tax credit or other tax advantage provided by or through the corporation. |
An “interested stockholder” is a person who is:
| · | directly or indirectly, the beneficial owner of 10% or more of the voting power of the outstanding voting shares of the corporation; or |
| · | an affiliate or associate of the corporation, which at any time within two years immediately before the date in question was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then outstanding shares of the corporation. |
A corporation to which the Combinations with Interested Stockholders Statute applies may not engage in a “combination” within two years after the interested stockholder first became an interested stockholder, unless the combination meets all of the requirements of the corporation’s articles of incorporation and (i) the combination or the transaction by which the person first became an interested stockholder is approved by the board of directors before the person first became an interested stockholder, or (ii)(a) the combination is approved by the board of directors and (b) at or after that time, the combination is approved at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of the stockholders representing at least sixty percent (60%) of the outstanding voting power of the corporation not beneficially owned by the interested stockholder or the affiliates or associates of the interested stockholder. If this approval is not obtained, the combination may be consummated after the two year period expires if either (a) the combination or transaction by which the person first became an interested stockholder is approved by the board of directors before such person first became an interested stockholder, (b) the combination is approved by a majority of the outstanding voting power of the corporation not beneficially owned by the interested stockholder or any affiliate or associate of the interested stockholder, or (c) the combination otherwise meets the requirements of the Combination with Interested Stockholders statute. Alternatively, a combination with an interested stockholder engaged in more than 2 years after the date the person first became an interested stockholder may be permissible if the aggregate amount of cash and the market value of consideration other than cash to be received by holders of shares of Common Stock and holders of any other class or series of shares meets the minimum requirements set forth in the statue, and prior to the completion of the combination, except in limited circumstances, the interested stockholder has not become the beneficial owner of additional voting shares of the corporation.
Acquisition of Controlling Interest Statute
In addition, Nevada’s “Acquisition of Controlling Interest Statute,” prohibits an acquiror, under certain circumstances, from voting shares of a target corporation’s stock after crossing certain threshold ownership percentages, unless the acquiror obtains the approval of the target corporation’s stockholders. Sections 78.378 to 78.3793 of the Nevada Revised Statutes only apply to Nevada corporations with at least 200 stockholders, including at least 100 record stockholders who are Nevada residents, that do business directly or indirectly in Nevada and whose articles of incorporation or bylaws in effect ten (10) days following the acquisition of a controlling interest by an acquiror do not prohibit its application.
We do not intend to “do business” in Nevada within the meaning of the Acquisition of Controlling Interest Statute. Therefore, we believe it is unlikely that this statute will apply to us. The statute specifies three thresholds that constitute a controlling interest:
• at least one-fifth but less than one-third;
• at least one-third but less than a majority; and
• a majority or more, of the outstanding voting power.
Once an acquiror crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold (or within ninety days preceding the date thereof) become “control shares” which could be deprived of the right to vote until a majority of the disinterested stockholders restore that right.
A special stockholders’ meeting may be called at the request of the acquiror to consider the voting rights of the acquiror’s shares. If the acquiror requests a special meeting and gives an undertaking to pay the expenses of said meeting, then the meeting must take place no earlier than 30 days (unless the acquiror requests that the meeting be held sooner) and no more than 50 days (unless the acquiror agrees to a later date) after the delivery by the acquiror to
the corporation of an information statement which sets forth the range of voting power that the acquiror has acquired or proposes to acquire and certain other information concerning the acquiror and the proposed control share acquisition.
If no such request for a stockholders’ meeting is made, consideration of the voting rights of the acquiror’s shares must be taken at the next special or annual stockholders’ meeting. If the stockholders fail to restore voting rights to the acquiror, or if the acquiror fails to timely deliver an information statement to the corporation, then the corporation may, if so provided in its articles of incorporation or bylaws, call certain of the acquiror’s shares for redemption at the average price paid for the control shares by the acquiror.
Our Articles of Incorporation and Bylaws do not currently permit us to redeem an acquiror’s shares under these circumstances. The Acquisition of Controlling Interest Statute also provides that in the event the stockholders restore full voting rights to a holder of control shares that owns a majority of the voting stock, then all other stockholders who do not vote in favor of restoring voting rights to the control shares may demand payment for the “fair value” of their shares as determined by a court in dissenters rights proceeding pursuant to Chapter 92A of the Nevada Revised Statutes.
Our Transfer Agent
Clear Trust, LLC is transfer agent and registrar for our Common Stock.
Listing of Common Stock
Our Common Stock trades on the OTC Markets Pink under the symbol “FDIT.”
NO DISSENTER’S RIGHTS
Under the Nevada Revised Statutes, as well as the Articles of Incorporation and Bylaws of the Company, the stockholders of the Company are not entitled to dissenters’ rights in connection with the Charter Amendment, the Merger and the other transactions contemplated under the Merger Agreement.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table, which was prepared on the basis of information furnished by the persons described, shows ownership of Common Stock issued and outstanding as of January 21, 2023, by the Chief Executive Officer, by the Chief Financial Officer, by each of the other named executive officers, by each director, and by the directors and executive officers of the Company as a group. The following table also lists the stockholders (other than our directors and executive officers) known to have been the beneficial owners of more than 5% of our common stock.
The percentage of beneficial ownership of Common Stock indicated in the following table is based on 269,745,006 shares outstanding. Except as indicated in footnotes to this table, each of the individuals named in this table are known to the Company to have sole voting and investment power with respect to all shares of Common Stock shown as beneficially owned by such person, subject to community property laws where applicable. Shares shown as beneficially owned by any person have been determined in accordance with the requirements of Rule 13d-3 promulgated under the Exchange Act.
Unless otherwise indicated, the address for each of the Company’s directors and named executive officers and each beneficial owner of more than five percent of Common Stock listed in the tables below is 5051 Peachtree Corners Circle, #200, Peachtree Corners, GA 30092.
Name |
Title of Class | Amount and Nature of Beneficial Ownership |
Percent of Class |
Voting Power Percent |
| Raymond Firth | Common | 98,674,081/indirect(1) | 36.0% | .56% |
| Series A Preferred | 2,500,000/indirect(1) | 50.0% | 35.36% | |
| Series B Preferred | 950,000/indirect(1)(2) | 19.4% | 5.38% |
| Thomas Powers | Common | 0 | 0% | 0% |
| Series A Preferred | 0 | 0% | 0% | |
| Series B Preferred | 0 | 0% | 0% | |
| Officers and Directors, as a group |
Common |
98,674,081/indirect(1) |
36.0% |
.56% |
| Series A Preferred | 2,500,000/indirect(1) | 50.0% | 35.36% | |
| Series B Preferred | 950,000/indirect(1)(2) | 19.4% | 5.38% | |
| Other 5% Shareholders |
| |||
| HVA Family Trust(3) | Common | 98,674,081/direct | 36.0% | .56% |
| Series A Preferred | 2,500,000/direct | 50.0% | 35.36% | |
| Series B Preferred | 50,000/direct | 0.1% | 0% |
(1) The Common Shares and Series A preferred shares are held by Firth Family Trust, Raymond Firth is the trustee
(2) The 50,000 Series A preferred shares are held by the Firth Family Trust and the 900,000 Series B preferred shares are held by Wooeb, Inc., Raymond Firth is the Chief Executive Officer
(3)Holly Andrews is the trustee of the HVA Family Trust
INFORMATION ABOUT BIOREGENX
BioRegenx was incorporated in the State of Nevada on January 29, 2021. BioRegenx is a health intelligence company empowering proactive healthcare and enabling a life better lived. BioRegenx is also a biotech holdings company, focused on growing and acquiring companies on the cutting edge of anti-aging and longevity.
The organizational structure for BioRegenx and its subsidiaries generally is as set forth below.
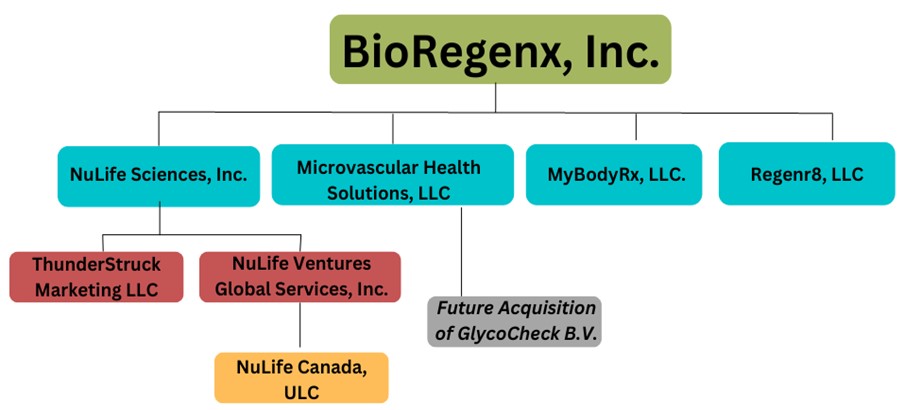
Operating Subsidiaries
NuLife Sciences™, Inc.
NuLife is a marketing and distribution company whose mission is to harness advanced technologies to redefine health and longevity. We achieve this by increasing understanding of the biology that controls lifespan and anti-aging in the human body. NuLife exclusively markets the MyBodyRx supplement line. NuLife will also distribute GlycoCheck™, along with other products.
NuLife also exclusively markets the products developed and manufactured by Sedona Wellness Ltd. The Company has entered into a Vendor/Reseller Agreement which grants the exclusive license to market, promote, distribute and sell its products worldwide except for the United Arab Emirates. The products include SEDONA Pulsed Electro-Magnetic Field (PEMF) and StimaWELL Electronic Muscle Stimulation (EMS, e-stim) therapies.
Through NuLife Sciences network of Independent Brand Partners, practitioners and customers, NuLife Sciences intend to be an industry leader with our SEDONA & StimaWELL therapy devices both in clinical settings and for at-home use to anyone seeking improvement of their overall wellbeing. Therapeutic SEDONA Pulsed Electro-Magnetic Field (PEMF) product line currently includes the Face Mask to now include SEDONA Pro and SEDONA Pro PLUS+.
SEDONA Pro and SEDONA Pro PLUS+ include an easy-to-use comprehensive control unit with pre-programmed and customizable settings. In addition to one connector mat for full body use and one connector pillow for local use. Both sets offer variable and flexible ranges for targeted, customized treatments delivering a wide range of beneficial frequencies from 1-15,000 Hz and intensities from 1-100 Gauss to support dozens of specific health indications anywhere from muscle soreness to diabetes and more. This design allows for two devices to be used simultaneously either on one patient, or two different patients. No action or physical movement is needed, the patient simply relaxes for a suggested time of 30 minutes, twice a day for cellular metabolism healing to take place for as long as it is in use, whether that be for days, weeks, months, or years. Making this an attractive alternative healing option for both patients and practitioners.
In addition to SEDONA, NuLife Sciences has partnered with StimaWELL, which is an Electronic Muscle Stimulation (EMS, e-stim) device, offering a new type of therapy available through NuLife Sciences. StimaWELL is an FDA Cleared Class II Medical Device & European (CE) Approved Medical Device with a patented BackUP! System specially developed for the alleviation of back pain, a health issue that affects about 80% of adults at least once in their lives. This machine provides four modalities in one device - pain therapy (TENS impulses), muscle therapy (EMS impulses), dynamic tissue massage and heat therapy - offering a wide range of health benefits. The patient simply lies on the ergonomically-shaped 12-channel stimulation mat with 24 electrodes and receives EMS pulses (and heat if desired) for healing treatment. Additional accessories such as remote control, power cord, etc are provided in the package for an elevated user experience.
After abundant in-house market research, practitioner focus groups, and financial consideration, the SEDONA Pro and Pro PLUS+ sets are competitively priced at US$4,990 and US$5,990, respectively, with StimaWELL at US$16,990. This creates a 26% average margin for NuLife Sciences after payout of all commissions for this suite of products.
These PEMF and EMS therapies also align with the NuLife Sciences mission of redefining health from the inside out and are complementary to existing NuLife Science products. When all of NuLife Sciences products are used in combination, an elevated overall feeling of wellbeing is experienced for healthier aging.
NuLife currently has a network of over 1,637 Independent Brand Partners in addition to 5,491 customers.
Microvascular Health Solutions™ LLC
MVHS is a research and product development, sales, education, and marketing company that has developed the patented and clinically tested Endocalyx Pro™ dietary supplement to improve the health of the endothelial glycocalyx. In addition, MVHS manufactures, exclusively sells and distributes the patented GlycoCheck™ BV software and Class 1 medical device.
Patents
Material Contracts. Endocalyx Pro™ patents:
U.S. Patent Number: 9943572
Canada Patent Pending Number: CA3020277A1
European Patent Pending Number: EP3280423A4
Japan Patent Number: 6518796
South Korea Patent Number: 10-1972691
China Patent Number: CN 107771080
Endocalyx Pro™ is produced by a fully accredited and certified manufacturing facility for over 30 years. Our source has provided outstanding contract manufacturing, formulation expertise, and custom finishing and packaging to the dietary supplement industry. They specialize in encapsulation, tablet compression, powder blending and filling, and product development.
Currently, there are ten double blind placebo studies using Endocalyx Pro being conducted by academic research hospitals in various locations throughout the world. The studies are funded by grants from government organizations in the United States and the European Union. Areas of interest being studied include sepsis, Covid-19, long-haul Covid, psoriasis, cardiovascular, hypertension, diabetes and anti-aging.
MVHS’ facility, which features an in-house laboratory, complies with the ordinance of current Good Manufacturing Practices (cGMP's) set by the Food and Drug Administration (FDA). The company is constantly inspected to ensure continuous compliance in the areas of Personnel, Manufacturing Plant, Grounds, Sanitation, Equipment, Quality Operations, Production and Process Controls, Warehouse, Distribution, and Post-Production Practices
Distribution is handled at NuLife Sciences in Chattanooga, TN and Midstates Fulfillment, Aberdeen, SD.
Material Contracts
MVHS has an agreement with Omni Imaging, a camera development company, to jointly develop and produce the CapiVision™ cameras for GlycoCheck™. MVHS has filed two patents relating to the camera which are pending. MVHS has the worldwide exclusive license to sell GlycoCheck™ systems and will own the GlycoCheck™ method patent after pending purchase of GlycoCheck™ from Maastricht University. Medical grade computers are designed and produced for the GlycoCheck™ system by a leader in specialized computers for the Healthcare, Industrial and Government markets. These purpose-built computers are specifically engineered for industries and applications not served by traditional computer manufacturers. Products include Medical Grade and Industrial Grade.
MyBodyRx™ LLC
MyBodyRx™ is a manufacturing, sales and product development company that produces dietary supplements that complement and work synergistically with the patented and clinically tested Endocalyx Pro™ product to support and improve healthy aging and increase longevity.
MyBodyRx™ is produced by a fully accredited and certified manufacturing facility for over 30 years. Our source has provided outstanding contract manufacturing, formulation expertise, and custom finishing and packaging to the dietary supplement industry. They specialize in encapsulation, tablet compression, powder blending and filling, and product development.
The company's facility, which features an in-house laboratory, complies with the ordinance of current Good Manufacturing Practices (cGMP's) set by the Food and Drug Administration (FDA). The company is constantly inspected to ensure continuous compliance in the areas of personnel, manufacturing plant, grounds, sanitation, equipment, quality operations, production and process controls, warehouse, distribution, and post-production practices.
Proposed Acquisition
GlycoCheck B.V acquisition
GlycoCheck™ is an affiliated entity controlled by Robert Long and Hans Vink, officers and directors of BioRegenx. GlycoCheck™ is a research and development company dedicated to the study the endothelial glycocalyx and vascular function. GlycoCheck™ develops medical software that measures microvascular and glycocalyx function in healthy subjects and patients. In addition, GlycoCheck™ assesses and monitors the beneficial therapeutic effects of specific glycocalyx dietary supplements and pharmaceutical applications. From the funds used to purchase GlycoCheckTM, Hans Vink will receive $81,762 from the sale of his GlycoCheckTM shares and $316,501 for accrued management fees. Robert Long will receive $61,322 from the sale of his GlycoCheckTM shares and $393,391 for accrued management fees.
To date, BioRegenx has been unable to raise sufficient funds to complete the proposed acquisition and is continuing to pursue the necessary funds utilizing debt and equity financing.
Market Size
Anti-aging market
The global anti-aging market reached a value of US$ 58.5 Billion in 2020. Aging is brought about by a cycle of biochemical processes which cause the body to degenerate over a period of time, impacting the health, fitness and physical appearance of the individual. Anti-aging refers to the process of limiting or retarding these changes through various products and services. Nowadays, good physical personality has become a necessity and determines the success of an individual in different areas of life. The growing consciousness among both the young and old consumers regarding their physical appearance has fostered the demand for anti-aging products and devices. Looking forward, IMARC Group expects the global anti-aging market to reach a value of US$ 88.30 Billion by 2026, exhibiting a CAGR of 7.10% during 2021-2026. Source: IMARC Group.
Health and longevity market
The number of people affected by the diseases that are linked to a compromised glycocalyx and microvascular dysfunction are immense. Over 80% of people of all ages are exposed to risks associated with microvascular dysfunction.
| • | Diabetes affects over 34 million Americans (CDC.gov); 425 million globally |
| • | Cardiovascular disease kills 665,000 in the U.S. annually, one every 36 seconds (CDC.gov) |
| • | Kidney disease affects 37 million Americans, one out of every nine (CDC.gov) |
| • | Dementia impacts over 5 million in the U.S. over age 65 (CDC.gov) |
| • | 45% of American adults have hypertension (CDC.gov) |
| • | Each year, at least 1.7 million adults in America develop sepsis, and nearly 270,000 Americans die. 1 in 3 patients who dies in a hospital has sepsis. (CDC.gov). (reference NOSTRADAMUS study https://ccforum.biomedcentral.com/articles/10.1186/s13054-021-03520-w). |
| • | COVID-19 patients have severe damage to microcirculation and the endothelial glycocalyx, losing as much as 95% of the tiniest of capillaries (reference COVID-19 Angiogenesis https://link.springer.com/article/10.1007/s10456-020-09753-7) |
Pain Management and Recovery
Sales of the Pulse Electromagnetic Field (PEMF) Therapy Devices Market in 2021 had reached at level of US$ 416.6 Mn and are projected to reach a revenue of US$ one billion. Unlike the smaller consumer market, SEDONA has a consumer friendly and a professional offering with the SEDONA PRO through our network of chiropractors and regenerative health care practitioners.
The United States remains the most dominant country in the Pulse Electromagnetic Field Therapy Market having the expected largest market share of US$ 358.3 Million by 2032. The market in the U.K. is projected to reach a valuation of US$ 48 Million by 2032. In Japan, the market is expected to reach US$ 46 Million by 2032. December 20, 2022 21:30 ET | Source: Future Market Insights Global and Consulting Pvt. Ltd.
According to the International Osteoporosis Foundation, osteoporosis results in 8.9 million fractures annually. Additionally, there are 1.6 million hip fractures worldwide each year, and between 4.5 and 6.3 million hip fractures are anticipated by 2050. As a result, as the incidence of osteoporosis increases, so will the demand for PEMF therapy devices.
Increased fractures from falls, accidents, and sports injuries, as well as poor road conditions in various nations, are some of the causes driving the market's expansion. A significant portion of people worldwide are affected by fractures brought on by sports-related injuries, accidents, falls from a particular height, and symptoms including arthritis, knee discomfort, and joint pain.
The global pain management devices market size is projected to reach USD 3.3 billion by 2026, growing at a CAGR of 8,6%. Growth of this market includes expanding patient population, growing demand for long-term pain management among the geriatric population, and increasing preference for pain management devices owing to the adverse effects of pain medications on patients. "Pain Management Devices Market by Type (Neurostimulation, SCS, TENS, RF Ablation, Infusion Pumps), Application (Neuropathy, Cancer, Facial, MSK, Migraine), Mode of Purchase (OTC, Prescription-based) & Region (NA, Europe, APAC).
Market Strategy
BioRegenx and its wholly owned subsidiaries use an omni-channel approach that includes both business-to-business (B2B) and business-to-consumer (B2C) marketing strategies.
MVHS Marketing Strategy
There are three primary markets for GlycoCheck™ and Endocalyx Pro™:
University Hospitals and Researchers. GlycoCheck™ is currently used in over 140 academic research hospitals worldwide. These professionals provide cutting-edge research, validation, and credibility in multiple clinical studies across a wide range of diseases that support the science. Over 99 peer-reviewed clinical studies using GlycoCheck™ have been published. These studies represent millions of dollars in ongoing research.
Medical Professionals. GlycoCheck™ measurement testing is only available from trained and certified medical professionals. This includes medical doctors and naturopathic physicians and wellness-based chiropractors. Marketing initiatives will drive patients to their practices and support them with a system for recurring revenue from testing and supplement sales. There are about 950,000 active doctors in the U.S. Seventy-six percent of medical doctors, naturopathic physicians, chiropractors, and nutritionists sell supplements from their offices.
The medical devices market 2020 size reached nearly $456.9 billion in 2019, having increased at a compound annual growth rate (CAGR) of 4.4% since 2015. The market is expected to decline from $456.9 billion in 2019 to $442.5 billion in 2020 at a rate of -3.2%. The decline is mainly due to lockdowns imposed by the governments across the world that hindered the supply chain in the medical devices manufacturing industry. However, there is an exceptional increase in the manufacturing of the ventilators that are used to treat COVID-19 patients. The medical devices market is expected to recover and grow at a CAGR of 6.1% from 2021 and reach $603.5 billion in 2023. This is because as mentioned, beyond 2020, an increase in the incidences of infectious and chronic diseases will drive the market growth. Analysis of the medical device market by country shows that North America accounts for about 39%, the largest share in the global market. Source: October 27, 2020, The Business Research Company.
Direct-to-Consumer. Marketing initiatives will include paid and online media, video, social media, pay-per-click, affiliate partnerships, print, direct mail and broadcasting. The dietary supplements market is expected to grow at a compound annual growth rate of 8.6% from 2021 to 2028 to reach USD $272.44 billion by 2028. Source: January 19, 2021, Facts & Factors.
NuLife Market Strategy
NuLife will leverage agreements and relationships with a network of over 1,637 Independent Brand Partners (IBPs)/Customers in addition to marketing to over 5,500 customers. Since NuLife markets the MyBody supplement line, distributes GlycoCheck™, and markets additional products in the anti-aging category. IBPs are motivated to be first in the direct sales channel market to offer the patented GlycoCheck™ and patented Endocalyx Pro™ dietary supplements, as well as the line of MyBodyRx™ products that are exclusive to NuLife.
NuLife’s compensation plan in the direct selling industry today is focused on recruiting, training, and create the right behaviors that sustain recurring revenue and stability. Another advantage is state-of-the-art industry leading technology, a back office which provides replicated websites for each brand partner, sales, and commission tracking engine. NuLife utilizes an industry leading social sharing app tool which shares compliant marketing videos, fact sheets, and tracks customer engagement for the sharing of products in today’s digital gig economy.
Revenues
BioRegenx
Revenue is derived from dividend and interest from our subsidiaries. Income could also be derived from Patents, Rights, and Royalties.
NuLife
Revenue is generated from the marketing, promotion, distribution and sale of goods and services to doctors, healthcare practitioners, patients, and consumers.
MVHS
Sales of GlycoCheck™ systems to academic research hospitals and groups, private medical practices, pharmacies, dentists, and veterinarians. Income is also derived from transfer sales of GlycoCheck™ with NuLife Sciences™. Endocalyx Pro™ dietary supplements are sold to doctors, patients, and consumers. In addition, revenue is derived from Endocalyx Pro™ private labeled to specialty ecommerce medical resellers and clinics. Endocalyx Pro™ is produced and sold at transfer pricing as an intercompany transaction with NuLife Sciences™.
MyBodyRx™
Proprietary formulas that are designed to be synergistic with Endocalyx ProTM are produced and sold at transfer pricing as an intercompany transaction with NuLife SciencesTM.
GlycoCheckTM
Currently, we receive income from royalties from sales of GlycoCheckTMsoftware by MVHS. Once the GlycoCheck B.V. acquisition is complete, GlycoCheck B.V. will become a research, education, and development company.
Competition
There is huge competition in the anti-aging market, as previously stated in the Risk Factors section, due to the presence of many domestic and international market players. Most of the market players are adopting various growth strategies, such as acquisitions, partnerships, and new product launches, in order to secure their position in the market. (https://www.mordorintelligence.com/industry-reports/anti-aging-market)
All aspects of the health, nutritional, supplements and medical devices business are highly competitive. The firms that our subsidiaries compete with include large well-known firms who have substantially greater financial and personnel resources. Our subsidiaries compete for business on the basis of our experience in the industry, their ability to execute business transactions and the strength of our relationships with their clients. Intense competition could negatively affect their operations.
Competitive Advantages
NuLife
NuLife has an educated and passionate existing team and network of over 1,637 independent brand partners who are focused on and understand. microcirculation and its important role in improving anti-aging, health, and longevity. NuLife’s corporate team has over 30 years of combined experience and success in the direct sales space. NuLife’s compensation plan in the direct selling industry today is focused on recruiting, training, and create the right behaviors that sustain recurring revenue and stability. Another advantage is state-of-the-art industry leading technology, a back
office which provides replicated websites for each brand partner, sales, and commission tracking engine. NuLife utilizes an industry leading social sharing app tool which shares compliant marketing videos, fact sheets, and tracks customer engagement for the sharing of products in today’s digital gig economy.
MVHS
Hans Vink, PhD is a pioneer and a leader in endothelial glycocalyx science and microcirculation. He is co-founder and chief science officer of MVHS. GlycoCheck™, developed by Dr. Vink, has been used in 100 peer-reviewed papers and his work has been cited in over 14,333 scholarly articles. Dr. Vink has contributed as a co-author to about 100 papers in studies on the glycocalyx (some with, some without the GlycoCheck device). MVHS is the exclusive worldwide distributor and manufacture of the GlycoCheck™ system. GlycoCheck™ is the only system in the world with patented clinically validated software to assess, measure, and analyze endothelial glycocalyx function. Based on in-house research, we believe Endocalyx Pro™ is the only internationally patented nutraceutical focused on the restoration, regeneration, and protection of the endothelial glycocalyx. Endocalyx Pro™ has been shown in studies to improve and repair glycocalyx and microvascular function in aging, cardiovascular, diabetes, kidney disease, inflammation, hypertension, wound healing, and sepsis. Endocalyx Pro™ is in multiple double-blind placebo studies worldwide studying its effectiveness on COVID-19, aging, diabetes, kidney disease, psoriasis, heart disease and sepsis. To date, thousands of people worldwide have taken Endocalyx Pro™ with no reported negative side effects or conflicts with other medications.
MyBodyRx™
Access to the GlycoCheck™ system to test for synergistic effects of supplements on improving the health of the glycocalyx, which in turn supports the entire body including the blood, brain, heart, lungs, kidneys—all organs—muscles, and tissues. In addition to the patented Endocalyx Pro™ formula, MyBodyRx™ have proprietary formulas that use the protected method of nano-chelation for absorption and processing all essential minerals.
GlycoCheck™
The GlycoCheck™ system is the only medical device worldwide to assess the status of the endothelial glycocalyx and monitor the health of the microvascular system. It has been in use globally since 2011 in over 180 medical centers and academic research hospitals. The GlycoCheck™ method to assess the status of the glycocalyx is patented internationally. A total of 100 peer-reviewed clinical research studies have been published using GlycoCheck™. Recent studies have linked traditional risk markers to the MicroVascular Health Score™ as determined by the GlycoCheck™ system such as hypertension, diabetes, aging, smoking, obesity, cardiovascular, kidney disease, Covid-19 and gender health and disease differences.
Facilities
BioRegenx is a global company that takes advantage of the ability to work remotely from many locations utilizing the latest video technology that has proven to be effective for businesses during the Covid-19 pandemic. We were effectively working remotely for several years prior to the pandemic and consequently weren’t affected by stay-at-home orders since early 2020.
BioRegenx is currently located at 7407 Ziegler Rd., Chattanooga, Tennessee 37421 with a second office at 175 West Canyon Crest, Suite 306, Alpine, UT 84004. BioRegenx’s shipping/warehousing is located at 16420 Midway Road, Addison TX 75001. BioRegenx shares the Chattanooga offices with NuLife. The offices consist of 1,000 sq ft. leased on a month to month basis with a monthly lease rate of $1,275. The lease payment is paid by NuLife.
MVHS’ office is currently located in Alpine, UT. The office consists of 1,100 sq ft leased from an unrelated party under a twelve-month extension to the original lease with a monthly lease rate of $1,140.
GlycoCheck™’ office is currently located in Schimmert, The Netherlands. The home office consists of 200 sq ft. Dr. Vink has had a home office since March 2020 due to Covid-19 lockdowns in The Netherlands.
Employees
In addition to its respective officers, BioRegenx and its subsidiaries currently employ eight individuals.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS OF BIOREGENX
The following discussion and analysis of BioRegenx’s financial condition and results of operations should be read in conjunction with the “Selected Historical Financial Data” and the accompanying financial statements and related notes included elsewhere in this Information Statement. The following discussion contains forward-looking statements that reflect BioRegenx’s future plans, estimates, beliefs and expected performance. The forward-looking statements are dependent upon events, risks and uncertainties that may be outside BioRegenx’s control. BioRegenx’s actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, natural gas prices, production volumes, estimates of proved reserves, capital expenditures, economic and competitive conditions, regulatory changes and other uncertainties, as well as those factors discussed below and elsewhere in this information statement, particularly in “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements,” all of which are difficult to predict. In light of these risks, uncertainties and assumptions, the forward-looking events discussed may not occur. BioRegenx does not undertake any obligation to publicly update any forward-looking statements except as otherwise required by applicable law.
Trends and Uncertainties
BioRegenx has entered into a Definitive Agreement to be acquired by Findit, Inc., a publicly traded company. The closing of the merger had been delayed while waiting for the completion of our audit. BioRegenx has finally completed its audit. However, there can be no assurance that the extended delay along with the require regulatory requirements will not negatively affect our ability to meet the terms of the Definitive Agreement.
The COVID-19 pandemic is still impacting countries, communities, supply chains and markets as well as the global financial markets. Governments have imposed laws requiring social distancing, travel bans and quarantine, and these laws may limit access to BioRegenx’s facilities, customers, management, support staff and professional advisors. These factors, in turn, have not only impacted BioRegenx’s operations, financial condition and demand for BioRegenx’s goods and services, but BioRegenx’s overall ability to react timely to mitigate the impact of this event. Depending on the severity and longevity of the COVID-19 pandemic, BioRegenx’s business and stockholders may continue to experience a significant negative impact.
There are no other trends, events or uncertainties that have had or are reasonably expected to have a material impact on the net sales or revenues or income from continuing operations. There are no significant elements of income or loss that do not arise from our continuing operations.
Liquidity and Capital Resources
BioRegenx was incorporated on January 29, 2021 and subsequently acquired its operating subsidiaries. The below discussion presents the information for the year ended December 31, 2020 utilizing the historical information of its operating subsidiaries as if consolidated with BioRegenx.
For the nine months ended September 30, 2022
For the nine months ended September 30, 2022, BioRegenx had a net loss of $1,778,922. For the nine months ended September 30, 2022, BioRegenx incurred depreciation and amortization of $3,580, had a decrease in accounts receivable of $17,985, inventory of $262,637, an increase in trade note receivable of $176,600, a decrease of $17,496 in prepaid expenses and other assets, a decrease of $83,200 in accounts payable, a decrease of $635,059 in accrued expenses and other liabilities, and an increase to deferred revenues of $32,210. As a result, BioRegenx had net cash used in operating activities of $2,374,865 for the nine months ended September 30, 2022.
For the nine months ended September 30, 2022, BioRegenx did not pursue any investing activities. As a result, BioRegenx had net cash used in investing activities of $0.00 for the nine months ended September 30, 2022.
For the nine months ended September 30, 2022, BioRegenx had an increase in SBA loans of $149,900, a decrease in note and loan payments of $819,175, a decrease in note and loan balances of $1,232,446, an increase in equity for debt conversion of $2,998,892 and proceeds from the issuance of common stock of $614,000. As a result, BioRegenx had net cash provided by financing activities of $1,711,171 for the nine months ended September 30, 2022.
For the nine months ended September 30, 2021
For the nine months ended September 30, 2021, BioRegenx had a net loss of $1,011238. For the nine months ended September 30, 2021, BioRegenx incurred depreciation and amortization of $0, issued common stock for services valued at $482,544, had an increase in accounts receivable of $16,785, decrease in inventory of $181,495, a decrease in trade note receivable of $29,157, an increase of $60,000 in prepaid expenses and other assets, a decrease of $1,672,780 in accounts payable, a increase of $198,446 in accrued expenses and other liabilities, and an increase to deferred revenues of $2,348. As a result, BioRegenx had net cash used in operating activities of $2,241,453 for the nine months ended September 30, 2021.
For the nine months ended September 30, 2021, BioRegenx acquired $830,762 in intangible assets. As a result, BioRegenx had net cash used in investing activities of $830,762 for the nine months ended September 30, 2021.
For the nine months ended September 30, 2021, BioRegenx had an increase in SBA loans of $105,542, an increase in note and loan balances of $2,227,325 an increase in equity for acquisition of $691,200 and proceeds from the issuance of common stock of $1,110,856. As a result, BioRegenx had net cash provided by financing activities of $4,134,923 for the nine months ended September 30, 2021.
For the year ended December 31, 2021
For the year ended December 31, 2021, BioRegenx had a net loss of $3,540,246. For the year ended December 31, 2021, BioRegenx incurred depreciation and amortization of $597, issued common stock for services valued at $482,544, an increase in accounts receivable of $1,909, purchased inventory of $197,021, an increase in trade note receivable of $263,040, a decrease in prepaid expenses and other liabilities of $105,900, a decrease in accounts payable of $1,292,262, an increase in accrued expenses and other liabilities of $197,981, and an increase to deferred revenues of $69,000. As a result, BioRegenx had net cash used in operating activities of $3,726,316 for the year ended December 31, 2021.
For the year ended December 31, 2021, BioRegenx acquired property and equipment of $23,660. As a result, BioRegenx had net cash used in investing activities of $23,660 for the year ended December 31, 2021.
For the year ended December 31, 2021, BioRegenx had an increase in SBA loans of $188,258, a decrease in note and loan payments of $159,449, an increase in note and loan balances of $2,208,250, an increase in equity for acquisition of $691,200 and proceeds from the issuance of common stock of $1,110,856. As a result, BioRegenx had net cash provided by financing activities of $4,039,115 for the year ended December 31, 2021.
For the year ended December 31, 2020
For the year ended December 31, 2020, BioRegenx had a net loss of $781,671. For the year ended December 31, 2020, BioRegenx issued common stock for services valued at $417,199, an increase in accounts receivable of $48,610, purchased inventory of $814,138, an increase in trade note receivable of $31,940, an increase in prepaid expenses and other liabilities of $154,688, a decrease in accounts payable of $660,652, an increase in accrued expenses and other liabilities of $152,818, and a decrease in deferred revenues of $83,548. As a result, BioRegenx had net cash used in operating activities of $1,840,390 for the year ended December 31, 2020.
For the year ended December 31, 2020, BioRegenx did not pursue any investing activities. As a result, BioRegenx had net cash used in investing activities of $0.00 for the year ended December 31, 2020.
For the year ended December 31, 2020, BioRegenx had an increase in SBA loans of $160,642 and a decrease in note and loan payments of $36,913 and distributions to shareholders of $1,500. As a result, BioRegenx had net cash provided by financing activities of $122,229 for the year ended December 31, 2020.
Results of Operations
For the three months ended September 30, 2022
For the three months ended September 30, 2022, BioRegenx had total sales of $528,026, cost of goods sold of $104,495, resulting in gross profit of $423,530. For the three months ended September 30, 2022, BioRegenx had employee expenses of $102,783 and operating expenses of $681,918. Operating expenses consisted of advertising and marketing of $48,531, software development costs of $226,446, commissions of $37,001, bank charges and fees of $7,305, contract labor of $61,684, management fees of $86,100, legal and accounting of $56,636, professional services of $55,889, insurance of $2,116, taxes and licenses of $10,631, postage and freight of $3,944, rent & lease of $14,419, travel of $7,280 and miscellaneous expenses of $63,936. Additionally, BioRegenx had interest income of $79 and interest expense of $72,340 resulting in net loss of $433,431 for the three months ended September 30, 2022.
For the three months ended September 30, 2021
For the three months ended September 30, 2021, BioRegenx had total sales of $762,330, cost of goods sold of $305,038, resulting in gross profit of $457,292. For the three months ended September 30, 2021, BioRegenx had employee expenses of $88,859 and operating expenses of $525,372. Operating expenses consisted of advertising and marketing of $30,430, software development costs of $11,704, product development of $7,430, commissions of $7,054, bank charges and fees of $7,152, contract labor of $38,813, management fees of $121,900, legal and accounting of $193,793, professional services of $24,670, insurance of $8,229, taxes and licenses of $7,146, postage and freight of $4,913, rent & lease of $8,626, travel of $11,762 and miscellaneous expenses of $41,750. Additionally, BioRegens had interest income of $9,022, other loss of $130 and interest expense of $11,528 resulting in net loss of $159,575 for the three months ended September 30, 2021.
For the nine months ended September 30, 2022
For the nine months ended September 30, 2022, BioRegenx had total sales of $1,932,512, cost of goods sold of $696,107 resulting in gross profit of $1,236,404. For the nine months ended September 30, 2022, BioRegenx paid distributors’ incentives of $166,706, selling, general and administrative expenses of $2,530,926 primarily consisting of employee expenses of $372,042 and operating expenses of $2,158,884. Operating expenses consisted of advertising and marketing of $145,128, software development costs of $920,885, product development of $10,565, bank charges and fees of $36,659, contract labor of $161,647, management fees of $184,600, legal and accounting of $170,993, professional services of $208,665, insurance of $8,664, taxes and licenses of $16,436, postage and freight of $37,664, rent & lease of $37,467, travel of $39,670 and miscellaneous expenses of $179,841. Additionally, BioRegenx had interest income of $148, interest expense and financial costs of $317,842 resulting in net loss of $1,778,922 for the nine months ended September 30, 2022.
For the nine months ended September 30, 2021
For the nine months ended September 30, 2021, BioRegenx had total sales of $2,281,713, cost of goods sold of $1,016,702 resulting in gross profit of $1,265,011. For the nine months ended September 30, 2021, BioRegenx paid distributors’ incentives of $216,502, selling, general and administrative expenses of $2,050,785 primarily consisting of employee expenses of $200,756 and operating expenses of $1,850,029. Operating expenses consisted of advertising and marketing of $129,203, software development costs of $34,078, product development of $39,527, bank charges and fees of $36,061, contract labor of $108,379, management fees of $426,756, legal and accounting of $384,758, professional services of $440,468, insurance of $22,021, taxes and licenses of $13,323, postage and freight of $12,364, rent & lease of $24,796, travel of $28,482 and miscellaneous expenses of $149,813. Additionally, BioRegenx had interest income of $20,929, other income of $390, interest expense and financial costs of $30,281 resulting in net loss of $1,011,238 for the nine months ended September 30, 2021.
For the year ended December 31, 2021
For the year ended December 31, 2021, BioRegenx had total sales of $2,990,772, cost of goods sold of $1,072,878, resulting in gross profit of $1,917,894. For the year ended December 31, 2021, BioRegenx paid distributors’ incentives of $479,816, incurred impairment expense of $830,762, paid selling, general and administrative expenses of $3,885,839 primarily consisting of employee expenses of $334,303 and operating expenses of $3,551,536. Operating expenses advertising and marketing of $185,575, software development costs of $631,979, product development of $253,378, bank charges and fees of $51,001, contract labor of $175,252, management fees of $571,374, legal and accounting of $498,574 professional services of $473,458, insurance of $31,456, taxes and licenses of $16,871, postage and freight of $40,166, rent & lease of $35,954, travel of $41,742 and miscellaneous expenses of $544,756. Additionally, BioRegenx had interest income of $25,538, interest expense and financial costs of $341,037, gain on sale of investments of $390 and received loan forgiveness PPP of $53,387 resulting in net loss of $3,540,246 for the year ended December 31, 2021.
For the year ended December 31, 2020
For the year ended December 31, 2020, BioRegenx had total sales of $7,787,092, cost of goods sold of $2,063,206, resulting in gross profit of $5,723,886. For the year ended December 31, 2020, BioRegenx paid distributors’ incentives of $2,218,662, paid selling, general and administrative expenses of $4,067,567 primarily consisting of employee expenses of $204,692 and operating expenses of $3,862,876. Operating expenses consisted advertising and marketing of $124,279, software development costs of $224,037 product development of $93,561, bank charges and fees of $214,811, contract labor of $630,653, management fees of $276,884, legal and accounting of $343,699, professional services of $429,586, insurance of $40,447, taxes and licenses of $4,733, conference-event of $94,275, postage and freight of $98,215, rent & lease of $24,529, travel of $71,493 and miscellaneous expenses of $1,191,674. Additionally, BioRegenx had interest income of $28,288, interest expense and financial costs of $(127,561) and loss on sale of investments of $(120,055) resulting in net loss of $(781,671) for the year ended December 31, 2020.
Recent Accounting Pronouncements
Recent accounting pronouncements issued by the FASB and the SEC did not have, are not believed by management to have, a material impact, or are currently evaluating the potential impact of updated authoritative guidance on the Company’s present or future consolidated financial statements.
HOUSEHOLDING
As permitted under the Exchange Act, in those instances where we are mailing a printed copy of this Information Statement, only one copy of this Information Statement is being delivered to stockholders that reside at the same address and share the same last name, unless such stockholders have notified the Company of their desire to receive multiple copies of this Information Statement. This practice, known as “householding”, is designed to reduce duplicate mailings and save significant printing and postage costs as well as natural resources.
The Company will promptly deliver, upon oral or written request, a separate copy of this Information Statement to any stockholder residing at an address to which only one copy was mailed. Requests for additional copies should be directed to the Company by phone at (404) 443-3224 or by mail to Findit, Inc., 5051 Peachtree Corners Circle, 3200, Peachtree Corners, GA 30092. Stockholders residing at the same address and currently receiving multiple copies of this Information Statement may contact the Company at the address or telephone number above to request that only a single copy of an information statement be mailed in the future.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
The Company is subject to the informational requirements of the Exchange Act. We file reports, proxy statements and other information with the SEC. The SEC also maintains an internet site that contains our reports, proxy and information statements and other information at www.sec.gov.
The Company will make available a copy of the documents we file with the SEC on the “Investors” section of our website at http://crkfrisco.com as soon as reasonably practicable after filing these materials with the SEC. The information provided on our website is not part of this Information Statement, and therefore is not incorporated by reference. Copies of any of these documents may be obtained free of charge on our website.
This Information Statement incorporates important business and financial information about the Company from documents that are not attached to this Information Statement. This information is available to you without charge upon your request. You can obtain the documents incorporated by reference into this Information Statement free of charge by requesting them in writing or by telephone from the Company at the following addresses and telephone number:
Findit, Inc.
Attention: Raymond Firth, Chief Executive Officer
5051 Peachtree Corners Circle, #200
Peachtree Corners, GA 30092
Telephone number: (404) 443-3224
INFORMATION INCORPORATED BY REFERENCE
Statements contained in this Information Statement, or in any document incorporated in this Information Statement by reference, regarding the contents of any contract or other document, are not necessarily complete and each such statement is qualified in its entirety by reference to that contract or other document filed as an exhibit with the SEC. The SEC allows us to “incorporate by reference” information into this Information Statement. This means that we can disclose important information by referring to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this Information Statement. This Information Statement and the information that we later file with the SEC may update and supersede the information incorporated by reference. Similarly, the information that we later file with the SEC may update and supersede the information in this Information Statement. We also incorporate by reference into this Information Statement the following documents filed by us with the SEC under the Exchange Act and any documents filed by us pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this Information Statement (provide that we are not incorporating by reference any information furnished to, but not filed with, the SEC):
• our Annual Report on Form 10-K for the fiscal year ended December 31, 2021;
• our Quarterly Report on Form 10-Q for the period ended September 30, 2022;
The information contained in this Information Statement speaks only as of the date indicated on the cover of this Information Statement unless the information specifically indicates that another date applies.
We have not authorized anyone to give you any information or to make any representation about the proposed merger or the Company that is different from or adds to the information contained in this Information Statement or in the documents we have publicly filed with the SEC. Therefore, if anyone does give you any different or additional information, you should not rely on it.
INDEX TO FINANCIAL STATEMENTS
| Audited Financial Statements of BioRegenx | ||||
| Report of Independent Accounting Firm | 65 | |||
| Consolidated Balance Sheets as of December 31, 2021 and 2020 | 66 | |||
| Consolidated Statements of Operations for the Years Ended December 31, 2021 and 2020 | 67 | |||
| Consolidated Statements of Stockholders’ Deficit for the Years ended December 31, 2021 and 2020 | 68 | |||
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2021 and 2020 | 69 | |||
| Notes to the Consolidated Financial Statements | 71 | |||
| Unaudited Financial Statements of BioRegenx | ||||
| Condensed Consolidated Balance Sheets as of September 30, 2022 and 2021 | 90 | |||
Condensed Consolidated Statements of Operations for the Three and Nine Months Ended September 30, 2022 and 2021 | 91 | |||
Condensed Consolidated Statements of Stockholders’ Equity for the Three and Nine Months Ended September 30, 2022 and 2021 | 92 | |||
| Condensed Consolidated of Cash Flows for the Nine Months Ended September 30, 2022 and 2021 | 93 | |||
| Notes to the Condensed Consolidated Financial Statements | 94 | |||

Financial Report for BioRegenx, Inc. and Subsidiaries for the Year ended December 31, 2021 and the combined financial statements for Microvascular Health Solutions, LLC, My Body Rx, LLC and NuLife Sciences, Inc. for the year ended December 31, 2020.



Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of Bioregenx, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Bioregenx, Inc. as of December 31, 2021 and 2020, the related statements of operations, stockholders' equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note A to the financial statements, the Company has suffered recurring losses from operations and has a significant accumulated deficit. In addition, the Company continues to experience negative cash flows from operations. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note A. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/S/ BF Borgers CPA PC
BF Borgers CPA PC (PCAOB ID 5041)
We have served as the Company's auditor since 2021
Lakewood, CO
January 18, 2023
BIOREGENX, INC. AND SUBSIDIARIES
BALANCE SHEETS
| December 31, | December 31, | ||
| 2021 Consolidated | 2020 Combined | ||
| ASSETS | |||
| Current Assets: | |||
| Cash and cash equivalents | $ 882,112 | $ 592,973 | |
| Accounts receivable | 985 | 0 | |
| Inventories | 653,291 | 850,312 | |
| Prepaid expenses and other current assets | 349,281 | 426,798 | |
| Total current assets | 1,858,669 | 1,870,083 | |
| Property and equipment (Net) | 23,270 | 0 | |
| Intangible assets (Net) | 0 | 0 | |
| Other assets | 11,025 | 10,635 | |
| TOTAL ASSETS | $ 1,919,964 | $ 1,880,718 | |
| LIABILITIES AND STOCKHOLDERS' DEFICIT | |||
| Current Liabilities: | |||
| Accounts payable | $ 610,024 | $ 1,894,596 | |
| Accrued expenses | 995,234 | 719,979 | |
| Promissory notes payable and loans | 1,316,758 | 496,231 | |
| Deferred revenue | 69,000 | 0 | |
| Total current liabilities | 2,991,016 | 3,110,806 | |
| Notes payable | 2,189,160 | 774,478 | |
| TOTAL LIABILITIES | 5,180,176 | 3,885,284 | |
| Stockholders’ Deficit: | |||
| BioRegenx, Inc. common stock, $0.0001 par value; 375,000,000 __shares authorized; 36,169,936 issued and outstanding | 3,616 | 6,400 | |
| BioRegenx Inc. series a preferred stock, non-dividend, 10 votes per -__share, $0.0001 par value, 150,000 authorized; issued and __outstanding 95,000 | 10 | 0 | |
| Additional paid-in capital | 2,698,172 | 410,799 | |
| Member equity deficit | - | (1,863,692) | |
| Accumulated deficit | (5,962,010) | (558,073) | |
| TOTAL STOCKHOLDERS' DEFICIT | (3,260,212) | (2,004,566) | |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ 1,919,964 | $ 1,880,718 | |
The accompanying notes are an integral part of these consolidated financial statements
BIOREGENX, INC. AND SUBSIDIARIES
STATEMENTS OF OPERATIONS
| Years Ended December 31, | |||||
| 2021 Consolidated | 2020 Combined | ||||
| Revenues: | |||||
| Net sales | $ 2,990,772 | $ 7,787,092 | |||
| Cost of sales | 1,072,878 | 2,063,206 | |||
| Gross profit | 1,917,894 | 5,723,886 | |||
| Operating expenses: | |||||
| Distributors incentives | 479,816 | 2,218,662 | |||
| Selling, general and administrative | 3,885.840 | 4,067,567 | |||
| Impairment Expense | 830.762 | 0 | |||
| Total operating expenses | 5,196,418 | 6,286,229 | |||
| Loss from operations | (3,278,524) | (562,343) | |||
| Other income (expense): | |||||
| Interest income | 25,538 | 28,288 | |||
| Interest expense and financing costs | (341,037) | (127,561) | |||
| Gain (loss) on sale of investments | 390 | (120,055) | |||
| Loan forgiveness PPP | 53,387 | 0 | |||
| Total other expenses | (261,722) | (219,328) | |||
| Income (loss) before provision for taxes | (3,540,246) | (781,671) | |||
| Provision for income taxes | - | - | |||
| Net loss | $ (3,540,246) | $ (781,671) | |||
| Weighted average shares outstanding - basic and diluted | 35,266,678 | 34,912,500 | |||
| Earnings (loss) per share - basic and diluted | $ (0.10) | $ (0.02) | |||
The accompanying notes are an integral part of these consolidated financial statements.
MICROVASCULAR HEALTH SOLUTIONS, LLC, MY BODY RX, LLC AND NULIFE SCIENCES, INC.
COMBINED STATEMENTS OF OWNERS’ DEFICIT
| Common Stock | Additional | Members’ | Stockholders’ | Total Members’ / | |||
| Shares | Amount | Paid-in Capital | Equity Deficit | Accumulated Deficit | Stockholders’ Deficit | ||
| Balance, December 31, 2019 | 0 | 0 | 0 | 0 | (1,577,923) | (60,671) | (1,638,594) |
| Issuance of subsidiary common stock | 6,400,000 | 6,400 | 0 | 410,799 | 0 | 0 | 417,199 |
| Net loss | (285,769) | (495,902) | (781,671) | ||||
| Distribution | (1,500) | (1,500) | |||||
| Balance, December 31, 2020 | 6,400,000 | 6,400 | 0 | 410,799 | (1,863,692) | (558,073) | (2,004,566) |
| Issuance of subsidiary equity for services | 1,600,000 | 1,600 | 0 | 369,588 | 111,356 | 0 | 482,544 |
| Net loss | (267,617) | (691,664) | (959,281) | ||||
| Balance, April 6th, 2021 | 8,000,000 | $ 8,000 | 0 | $780,387 | $(2,019,953) | $(1,249,737) |
$(2,481,303) |
BIOREGENX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
| Common Stock | Preferred Stock | Additional Paid-in | Accumulated | Total Stockholders' | |||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | |
| Balance, April 6, 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Issuance of common stock for merger. | 34,912,500 | 3,491 | 95,000 | 10 | 896,241 | (3,381,045) | (2,481,303) |
| Issuance of common share in acquisition of Regenr8, LLC | 480,000 | 48 | 0 | 0 | 691,152 | 0 | 691,200 |
| Issuance of common shares in private placement | 777,436 | 77 | 0 | 0 | 1,110,779 | 0 | 1,110,856 |
| Net loss | (2,580,965) | (2,580,765) | |||||
| Balance, December 31, 2021 | 36,169,936 | $ 3,616 | 95,000 | $10 | $2,698,172 | $(5,962,010) |
$(3,260,212) |
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
STATEMENTS OF CASH FLOWS
| Years Ended December 31, | |||||||
| 2021 Consolidated | 2020 Combined | ||||||
| OPERATING ACTIVITIES: | |||||||
| Net loss | $ (3,540,246) | $ (781,671) | |||||
| Adjustments to reconcile net income (loss) to | |||||||
| net cash used in operating activities: | |||||||
| Depreciation and amortization | 597 | 0 | |||||
| PPP Loan Forgiveness | (53,387) | 0 | |||||
| Common stock issued for services | 482,544 | 417,199 | |||||
| Impairment expense | 830,762 | 0 | |||||
| Change in operating assets and liabilities (net of amounts acquired): | |||||||
| Accounts receivable | 1,909 | 48,610 | |||||
| Inventories | 197,021 | (814,138) | |||||
| Trade note receivable | 263,040 | 31,940 | |||||
| Prepaid expenses and other assets | (105,900) | 154,688 | |||||
| Accounts payable | (1,292,262) | (660,652) | |||||
| Accrued expenses and other liabilities | 197,981 | (152,818) | |||||
| Deferred Revenue | 69,000 | (83,548) | |||||
| Net cash used in operating activities | (2,948,941) | (1,840,390) | |||||
| INVESTING ACTIVITIES: | |||||||
| Purchases of property and equipment | (23,660) | 0 | |||||
| Net cash used in investing activities | (23,660) | 0 | |||||
| FINANCING ACTIVITIES: | |||||||
| Increase in SBA loans | 102,083 | 160,642 | |||||
| Note and loan payments | (159,449) | (36,913) | |||||
| Increase in note and loan balances | 2,208,250 | 0 | |||||
| Proceeds from the issuance of common stock | 1,110,856 | 0 | |||||
| Distributions | 0 | (1,500) | |||||
| Net cash provided by financing activities | 3,261,740 | 122,229 | |||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 289,139 | (1,718,161) | |||||
| CASH AND CASH EQUIVALENTS, BEGINNING BALANCE | 592,973 | 2,311,134 | |||||
| CASH AND CASH EQUIVALENTS, ENDING BALANCE | 882,112 | 592,973 | |||||
| CASH PAID FOR: | |||||||
| Interest | $ 41,486 | $ 45,671 | |||||
| Income taxes | $ - | $ - | |||||
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
STATEMENTS OF CASH FLOWS
(CONTINUED)
| Years Ended December 31, | |||||||
| 2021 Consolidated | 2020 Combined | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | |||||||
| Conversion of accounts payable to notes | 1,789,277 | 0 | |||||
| Net non-cash activities | 1,789,277 | 0 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING
Organization and Business:
The Company Bioregenx, Inc. develops and manufactures medical test equipment and high quality, science-based nutritional products that are sold nationally through a direct selling channel, to health professionals and research organizations.
On April 6th, 2021 Bioregenx entered into a combination agreement with Microvascular Health Services, LLC., My Body Rx, LLC and Nulife Sciences, Inc. that resulted in the addition of three subsidiary companies to the group. Due to the ownership structure the combination is accounted for as a combination of entities under common control under the Financial Accounting Standards Board’s Accounting Standard Codification (ASC) Topic 805. The activities of the subsidiaries are included in the financial statements for the entire reporting period with assets and liabilities stated at the historical carrying value.
On September 15, 2021, the Company acquired all the interest in Regenr8, LLC in exchange for shares of the Company’s stock. This acquired company is accounted for as an acquisition and the activities of the acquired company are included in the consolidated financial statements starting with the acquisition. Assets are liabilities are reported at the purchase price allocated to the relative fair market value.
The Consolidated Financial Statements (the “Financial Statements”) include the accounts and operations of the Company, and its controlled subsidiaries. All inter-company accounts and transactions have been eliminated in consolidation. The accounting and reporting policies of the Company conform with accounting principles generally accepted in the United States of America (“US GAAP”).
Going Concern
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplates the continuation of the Company as a going concern. The Company has generated recurring losses from operations and cash flow deficits from its operations since inception and has had to raise funds through equity offerings or borrowings to continue operating. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The continued operations of the Company are dependent upon its ability to raise additional capital, obtain additional financing and develop a business that generates sufficient positive cash flows from operations. The Company has secured a commitment for financing from a private equity fund as detailed in Note O and continues to raise funds from the issuance of additional common stock issuances. In December of 2022, the company began sales of the second-generation medical testing machine and other additions to its product lines that Management believes will result in profits and positive case flow.. Management believes that the Company should be able to continue as a going concern based on their ability to raise additional funds and generate cash from operations.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue as a going concern.
Use of Estimates
The preparation of Consolidated Financial Statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amounts of revenues and expenses during the reporting period. These estimates may be adjusted as more current information becomes available, and any adjustment could be significant.
Fiscal Year
The Company operates on a calendar year ending on December 31.
Fair Value Measurements
As of December 31, 2021 and December 31, 2020, the Company’s financial instruments include cash equivalents. The cost values of cash equivalents and restricted cash approximate their fair values, based on their short-term nature.
Translation of Foreign Currencies
The Company’s foreign sales activities are conducted in US Dollars and no foreign currency translations are recorded in these financial statements.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash equivalents. Cash equivalents consisted primarily of bank balances and amounts receivable from credit card processors. Amounts receivable from credit card processors and other forms of electronic payment are considered cash equivalents because they are both short-term and highly liquid in nature and are typically converted to cash within three days of the sales transaction.
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method. Net realizable value is determined using various assumptions with regard to excess or slow-moving inventories, non-conforming inventories, expiration dates, current and future product
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
demand, and market conditions. A change in any of these variables could result in an adjustment to inventory.
Accounts Receivable
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company establishes an allowance for doubtful accounts for estimated losses inherent in its accounts receivable as determined by management. In establishing the required allowance, management considers historical losses adjusted to take into account current market conditions and our customers’ financial condition, the amount of the receivables in dispute, and the current receivables aging and current payment patterns. The Company reviews its allowance for doubtful accounts regularly. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of the differences between the financial statement assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates that are expected to apply to taxable income in the year in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax law is recognized in income in the period that includes the enactment date. Deferred tax expense or benefit is the result of changes in deferred tax assets and liabilities. The Company evaluates the probability of realizing the future benefits of its deferred tax assets and provides a valuation allowance for the portion of any deferred tax assets where the likelihood of realizing an income tax benefit in the future does not meet the “more-likely-than-not” criteria for recognition. The Company recognizes tax benefits from uncertain tax positions only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the Financial Statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution.
Property and Equipment
Property and equipment are recorded at cost. Maintenance, repairs, and renewals, which neither materially add to the value of the property nor appreciably prolong its life, are charged to expense as incurred. Depreciation is provided in amounts sufficient to relate the cost of depreciable assets to operations over the estimated useful lives of the related assets. The straight-line method of depreciation and amortization is followed for financial statement purposes. Property and equipment are reviewed for impairment whenever events or changes in circumstances exist that indicate the carrying amount of an asset may not be recoverable. When property and equipment are retired or otherwise disposed of, the
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the results of operations for the respective period.
Leases
The Company has adopted ASC Topic 842 for lease accounting. This standard requires that right of use assets and liabilities are measured and recorded on the balance sheet. ASC Topic 842 provides an exception for short term leases that are for 12 months or less. The Company reports short term leases under the exception to the standard. Leases for a period of 12 months or less are recorded as expense on a ratable basis throughout the term of the lease.
The Company leases two office spaces, its headquarters in Chattanooga Tennessee and a satellite office in Alpine Utah, both are short term leases.
The headquarters is leased from a related party on a month-to-month basis for $1,275.00 per month
The satellite office is leased from an unrelated party under a twelve-month extension to the original lease at $1,140.00 per month.
Intangible Assets
Intangible assets were acquired in the purchase of Regenr8, LLC on September 15th, 2021. See note G Intangibles include product intangibles, formulations and customer-based intangibles. Amortized intangible assets are amortized over their related useful lives, using a straight-line or accelerated method consistent with the underlying expected future cash flows related to the specific intangible asset. Amortized intangible assets ae reviewed for impairment whenever events or changes in circumstances exist that indicate the carrying amount of an asset may not be recoverable. When indicators of impairment exist, an estimate of undiscounted net cash flows is used in measuring whether the carrying amount of the asset or related asset group is recoverable. Measurement of the amount of impairment, if any, is based upon the difference between the asset or asset group’s carrying value and fair value. Fair value is determined through various valuation techniques, including market and income approaches as considered necessary.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
Revenue Recognition
The Company applies ASC Topic 606, Revenue from Contracts with Customers ("ASC 606") for all periods presented in the consolidated financial statements. To determine the appropriate amount of revenue to be recognized in accordance with ASC 606, the Company follows a five-step model as follows:
1 – Identification of the contract with a customer
2 – Identification of the performance obligation in the contract
3 – Determination of the transaction price
4 – Allocation of the transaction price to the performance obligation in the contract
5 – Recognition of revenue when, or as, a performance obligation is satisfied
Sales contract with the independent business partners and customers
The Company through its subsidiaries provides a medical testing machine, consumables for the testing process and nutritional supplements to its Independent Brand Partners (IBP) and customers. IBPs pay an annual membership fee to maintain the IBP status which is a requirement to participate in the compensation plan. Prior to the merger April 4th, 2021, a medical device was also sold directly to health professionals and customers but this product has been discontinued. The products are distributed through a network of IBPs. During the periods presented, the testing machines were primarily sold to educational institutions directly by the Company. The medical devices and the supplements sold to individuals by the independent brand partners. The medical device was exclusively sold through the network of independent brand partners. The Company assesses the contract term as the period in which the parties to the contract have enforceable rights and obligations. The contract term may differ from the stated term in contracts with certain termination or renewal rights, depending on whether there are substantive penalties associated with those rights. Customer contracts are generally standardized and noncancellable for the duration of the stated contract term. Consumption taxes collected and remitted to tax authorities are excluded from revenue. The Company may use third-party vendors to provide certain goods or services to its customers. The Company evaluates those relationships to determine whether revenue should be reported gross or net. The Company recognizes revenue on a gross basis where it acts as principal and controls the goods and services used to fulfill the performance obligations to the customer and on a net basis where it acts as an agent. The Company has not acted as an agent during the years ended December 31, 2021 and 2020.
Performance Obligation
The counterparties contract to obtain goods and meet the requirements to be customers whether termed customers or independent brand partners. The Company’s sales to educational institutions are of a tangible product. The company requires payment prior to shipping, with only a few exceptions. Sales to educational institutions are not shipped until payment is received. Once an order is paid the invoice is sent to an outsourced fulfillment house for shipping. Shipping typically occurs in 24 hours of the payment. Sales are booked upon shipment when the performance obligation is satisfied.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
Sales to health professional and individual consumers, other than annual memberships, are of tangible goods. The company requires payment prior to shipping, with only a few exceptions. Sales to health professionals, other IBPs and consumer are also not shipped until payment is received, typically via credit card payments. Once an order is paid the invoice is sent to an outsourced fulfillment house for shipping. Shipping typically occurs in 24 hours of the payment. Sales are booked upon shipment when the performance obligation is satisfied.
All counterparties to the contract may return the product pursuant to the Company’s return policy. Annual Membership of IBPs, which entitle the IBP to distributor status and allow them to participate in the compensation plan, are non-refundable.
Other Revenue
Other types of revenue include fees, which are paid by the customer at the beginning of the service period, for access to online customer service applications. Such fees are amortized over the period to which they relate, typically 12 months.
Transaction Price
The transaction price is the amount received or expected to be received for transferring goods and services to its customers. Each order entered has a fixed price listed in the company’s distributor portal or on a price list at the time the sale is originated.
Payments from the customers are typically made at the time of the order before satisfaction of the performance obligation. The Company in rare instances grants 30-day terms to select long term customers. In such instances the revenue recognition occurs when the performance obligation is satisfied. The Company has elected the practical expedient that permits an entity not to recognize a significant financing component if the time between the transfer of a good or service and payment is one year or less.
Allocations
The Company as a matter of ordinary operations allocated the purchase price against the performance obligation on an order-by-order basis for the entire order. In the event an order has not been fulfilled or partially filled revenues are not recognized for the portion of the order for which the performance obligation has not been satisfied. There has been no transaction price allocated to performance obligations that have not been fully satisfied the years ended December 31, 2021 and 2020.
Recognition
Revenue is recognized when the performance obligation is satisfied, primarily upon passage of title, for tangible goods. The majority of collections are through online orders paid through credit cards. There are few exceptions where terms are given to well-known customers, primarily in the academic market. The
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
company’s distributors’ have the option to apply their earned commissions to their orders resulting in an internal entry to record their payment.
The company has not engaged in financing related to its products. When third party finance companies are used by the customer the order is considered paid when funds are received from the finance company.
There are no contracts that extend beyond the performance of the original sales obligation.
Product Return Policy
All product orders that are unused and returned within the first 30 days following purchase are refunded at 100% of the sales price.
Independent Business Partners Incentives
Incentives expenses include all forms of commissions, and other incentives paid to our Independent Business Partners (IBAs).
Selling, General and Administrative
Selling, general and administrative expenses include wages and benefits, depreciation and amortization, rents and utilities, Associate event costs, advertising and professional fees, marketing, and research and development expenses.
Equity-Based Compensation
The Company records compensation expense in the Financial Statements for equity-based awards based on the fair value on the date of transfer. Fair value was determined by valuations of the separate subsidiaries before the merger based on a variety of factors including expected cash flow, comparable industry values and tangible and intangible asset values. . For the period presented, all equity-based compensation consisted of direct transfers of equity interests for services provided in prior periods and were fully vested upon the award. There was no formal vesting period for the awards and the awards were recognized upon the grant.
Advertising
Advertising costs are charged to expense as incurred and are presented as part of the “Selling, general and administrative” line item.
Research and Development
Research and development costs are expensed as incurred per ASC Topic 730. None of the activities of the Company in the periods presented qualified for exceptions to the general guidance of ASC Topic 730.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
Earnings Per Share
Basic earnings per common share ("EPS") are based on the weighted-average number of common shares that were outstanding during each period. For the weighted-average number of common shares outstanding for combined period ended December 31, 2020 the initial shares issued in the merger on April 6th, 2021 were used as the divisor. Before the merger, the equity of the combined group consisted of both limited liability company equity and corporate equity making the comparison of shares outstanding of the subsidiaries to the consolidated group inaccurate. Diluted EPS is not applicable during the periods presented, as there were no convertible securities or options outstanding.
Accounting for debt-to-equity conversions
In order to simplify, and provide less confusion, on accounting for debt with conversion options, FASB release ASU 2020-06 in August 2020 has been adopted by the Company. For the years ended December 31, 2021and December 31, 2020 there were no convertible debt instruments outstanding. See Subsequent Event Note for description of debt-to-equity conversion transactions that occurred March 31, 2022.
ASU 2020-06 simplifies the accounting for convertible instruments. Therefore, the embedded conversion features no longer are separated from the debt with conversion features that are not required to be accounted for as derivatives under or that do not result in substantial premiums accounted for as paid-in capital. Consequently, a convertible debt instrument will be accounted for as a single liability measured at its amortized cost and therefor will be accounted for as a single equity instrument measured at its historical cost.
NOTE B—INVENTORIES
12/31/2021 12/31/2020
Finished goods..................................................................... $653,291.… $850,312
S
upplement Inventories are purchased in finished form with labels purchased separately in an amount to support the production run. Medical testing equipment was purchase and assembled once orders were received during the financial statement period.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE C—PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consisted of the following:
12/31/2021 12/31/2020
Prepaid commissions................................................................................$.....243,381……...$...163,759
Supplier advances….................................................................................$................0……...$...263,039
Other current assets .................................................................................$.....105,900.........$...........-0-
Total ……………………….....................................................................$......349,281........$...426,798
NOTE D—INCOME TAXES
Consolidated earnings before income taxes consists of the following:
12/31/2021 12/31/2020
Consolidated Net Loss before income Taxes.....................................($3,540,245).($781,671)...
Income tax expense (benefit) included in income from continuing operations consists of the following:
12/31/2021 12/31/2020
Year Ended 2021 and 2020 Current and Deferred .................................................$-0-………$-0-
During the financial statement period before the combination date of April 6th, 2021, each subsidiary filed separate income tax returns. Each subsidiary other than NuLife Sciences, Inc filed as flow through entities for tax purposes and the owner’s reported income and loss on their tax returns for the 2020 and short period ended April 6, 2021 period. The Company reported net taxable loss for the short period beginning April 6th and ending December 31. 2021. As of January 1, 2022, the Company expect to have net operating loss carryovers and research tax credit carryovers. The Company believes the utilization of the carryforwards cannot be determined with reasonable accuracy at this time due to provisions in the tax code that could act to limit the utilization of the carryovers and uncertainty in the determination of the periods that the carryovers may be utilized against future taxable income. The Company maintains a full valuation allowance on its carryforwards. Valuation allowances are determined using a more-likely-than-not realization criteria and are based upon all facts and circumstances. The Company files income tax returns in the United States and multiple states. In general, the Company's and predecessor’s tax filings are subject to examination for years ending on or after December 31, 2018, however, the statutes of limitations in certain instances may be longer.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE E—PROPERTY AND EQUIPMENT
Cost of property and equipment and their estimated useful lives is as follows:
12/31/2021 12/31/2020
Computer equipment and software .......................................$23,270…......$......-0-
Estimated useful lives for computer equipment and software is 5 years.
Depreciation of property and equipment was $597 and $-0- for the years ended 2021 and 2020, respectively.
NOTE F—OPERATING LEASES
The following table presents supplemental lease information:
12/31/2021 12/31/2020
Operating Lease Cost…………………… ...............................................$35,954.......$24,529
Operating lease cost were related to the Company’s office space, see related party disclosures below.
NOTE G—INTANGIBLE ASSETS
The Company performed its annual indefinite-lived intangible asset impairment test during 2021. The Company performed a qualitative assessment of the intangible assets and based on a delay in implementing the acquired assets determined that it was more-likely-than-not that the fair value of any indefinite-lived intangible asset was less than the carrying amount. The Company determined that the fair market value of the acquired intangibles was not determinable as of 12/31/2021. As a result, an impairment expense was recognized for the amount acquired.
Intangible assets consist of the following:
Amortized intangible assets
12/31/2021 12/31/2020
Acquired intangibles – Regenr8
Acquisition………….………….………………..................................$...........-0-.................-0-
Impairment Expense (in operating expense).....................................................$..830,762..................-0-
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE H—OTHER ASSETS
Other Assets consisted of the following:
12/31/2021 12/31/2020
Investment in Thunder Struck .............................................$..11,025…$..10,635
Investment in Thunder Struck represents a marketing arrangement entered into to develop the health practitioner distribution channel.
NOTE I—COMMITMENTS AND CONTINGENCIES
Unconditional Purchase Obligations
The Company is involved in various lawsuits, claims, and other legal matters from time to time that arise in the ordinary course of conducting business, including matters involving its products, intellectual property, supplier relationships, distributors, competitor relationships, employees and other matters. The Company records a liability when a particular contingency is probable and estimable. The Company faces contingencies that are reasonably possible to occur; however, they cannot currently be estimated. While complete assurance cannot be given as to the outcome of these proceedings, management does not currently believe that any of these matters, individually or in the aggregate, will have a material adverse effect on the Company’s financial condition, liquidity or results of operations. It is reasonably possible that a change in the contingencies could result in a change in the amount recorded by the Company in the future.
NOTE J—ACQUISITIONS
Formation
On April 6th, 2021 the Company’s consolidated group was formed by the contribution of 100% of the equity interests of three companies, Microvascular Health Services, LLC, My Body Rx, LLC and NuLife Sciences, Inc. in exchange for newly issued common and preferred stock representing all the issued and outstanding shares of Bioregenx. After the contribution of the Microvascular Health Services, LLC interest it was discovered that certain prior arrangements may have impaired the transferability of 10% of the interest. Authorized share corresponding to this amount were placed in reserve pending resolution. The combination is expected to product synergies between companies with the production activities and the distribution network of the marketing company.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE J—ACQUISITIONS (Continued)Regenr8
On September 15, 2021, 100% of the equity of Regenr8, LLC (Regenr8) was acquired in exchange of newly issued common shares of the Company. The Regenr8 acquisition added a consumable testing product and a nutritional supplement that complements and expands the Company’s product line.
Goodwill was not recorded as part of the acquisition.
The table that follows summarizes the consideration paid, the assets acquired and liabilities assumed recognized at the acquisition date.
| At September 15, 2021 | ||||||
| $ Amount | ||||||
| Consideration | ||||||
| Bioregenx common stock | 480,000 shares | 691,200 | ||||
| Fair value of total consideration transferred | $ 691,200 | |||||
Recognized amounts of identifiable assets acquired and liabilities assumed | ||||||
| Cash | 109,021 | |||||
| Food product formulas | 46,250 | |||||
| Genetic testing product | 738,262 | |||||
| Customer lists | 46,250 | |||||
| Accounts payable | (14,083) | |||||
| Notes payable | (234,500) | |||||
| Total identifiable net assets | $ 691,200 | |||||
| Acquisition related costs | ||||||
| (Included in selling, general and administrative costs in the Company's | ||||||
| income statement for the year ended December 31, 2021) | $ 8,322 | |||||
The fair market value of the 480,000 shares issued as consideration paid for Regenr8 of $691,000 was determined by the share value of the private placement offering that was open concurrent to the acquisition period.
The financial assets acquired consisted solely of a cash account and it was valued at the ending bank balance on the date of the acquisition.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE K—EXECUTIVE COMPENSATION
Executive compensation for the periods presented are shown in the table below.
| Name and principal position | Year | Salary | Bonus | Stock awards | Option Awards | All other compensation | Total |
| ($) | ($) | ($) | ($) | ($) | ($) | ||
| William Resides, Chief Executive | 2020 | 0 | 0 | 207,796 | 0 | 337,747 | $ 545,543 |
| Officer and Chief Financial Officer | 2021 | 0 | 0 | 0 | 0 | 180,000 | $ 180,000 |
| Robert M. Long, Chief Operations | 2020 | 0 | 0 | 0 | 0 | 33,225 | $ 33,225 |
| Officer | 2021 | 100,385 | 0 | 0 | 0 | 22,178 | $ 122,563 |
| Hans Vink, Chief Science Officer | 2020 | 0 | 0 | 0 | 0 | 55,000 | $ 55,000 |
| 2021 | 0 | 0 | 0 | 0 | 191,800 | $ 191,800 | |
| Joseph S Bird, Chief Medical Officer | 2020 | 0 | 0 | 208,603 | 0 | 0 | $ 208,603 |
| and Treasurer | 2021 | 0 | 0 | 0 | 0 | 67,606 | $ 67,606 |
| Robert Doran, Executive Vice | 2020 | 0 | 0 | 0 | 0 | 55,000 | $ 55,000 |
| President | 2021 | 0 | 0 | 371,188 | 0 | 60,000 | $ 431,188 |
| Gary Hennerberg, Chief Marketing | 2020 | 0 | 0 | 0 | 0 | 48,000 | $ 48,000 |
| Officer and Secretary | 2021 | 0 | 0 | 111,356 | 0 | 67,000 | $ 178,356 |
The Company does not currently sponsor a retirement plan of any type and does not have a non-equity incentive plan.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE K—OFFICERS’ COMPENSATION (Continued)
Equity based compensation
Total equity-based compensation expense was $482,544 and $417,199 for years 2021 and 2020, respectively. The equity compensation was granted to key employees for past services rendered. The equity was paid in the form of direct equity interests of the member companies prior to the combination on April 6, 2021. There was not formal vesting period for the grants.
The amounts granted were as follows:
12/31/2021 12/31/2020
Joseph Bird…………………………...................................................................$.........-0-……$208,603
William Resides……………………....................................................................$.........-0-…....$207,796
Robert Doran..……………………......................................................................$.371,188…....$.........-0-
Gary Hennerberg……………………..................................................................$.111,356…....$..........-0-
Total………………….…………………….........................................................$.482,544…….$.416,399
NOTE L—STOCK AWARDS PLAN
On May 31, 2021, the Board of Directors adopted a Stock Awards Plan (“Plan”). The purpose of the Plan is to attract, retain and motivate employees, directors and persons affiliated with the Company and to provide such participants with additional incentive and reward opportunities. The awards may be in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock awards, phantom stock awards, or any combination of the foregoing. The total number of shares of stock reserved for issuance under the Plan is 5,600,000. No awards have been granted under the plan to date.
NOTE M—SHARES SOLD
In June 2021, the Company Issued a private placement memorandum which offered for sale shares of its common stock to qualified accredited investors. The Shares were valued at $1.44 per common share. The offering was closed September of 2021. The results are summarized in the following table.
Number Of Shareholders Number of Shares Funds Raised
39 771,436 $1,110,856
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE N—RELATED-PARTY TRANSACTIONS
Loans
Bioregenx and its subsidiaries have financed past activities, in part, with borrowing from certain related parties. The amount of debt from related parties is summarized in the following table:
| Related Party | Balance | 12/31/2021 | Balance | 12/31/2020 | |
| Libertas Trust | $180,000 | -0- | A | ||
| Libertas Trust | $400,664 | $316,865 | A | ||
| Wilshire Holding Trust | $420,000 | -0- | A | ||
| Wilshire Holding Trust | $934,882 | $739,353 | A | ||
| Resco Enterprises Trust | $157,747 | $157,747 | A | ||
| Avis Trust | $67,606 | $67,606 | A | ||
| Lone Peak Holdings, LLC | $3,500 | $3,500 | B | ||
| Robert Long | $64,746 | $65,792 | C | ||
| Robert Long | $4,082 | $6,078 | C | ||
| Michael Long | $7,495 | $7,495 | D | ||
| Richard Long | $60,863 | $60,862 | D | ||
| Gail Long | $44,735 | $44,735 | D | ||
| Diane Long | $547,678 | $613,839 | D |
A Stockholder and related to current officer
B Entity controlled by current officer
C Stockholder and officer
D Relative of current officer
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE N—RELATED-PARTY TRANSACTIONS (Continued)
The Company has made advances to related parties as follows.
12/31/2021 12/31/2020
GlycoCheck B.V. $ 24,194 $ 16,500
Robert Long $ 149,003 $ -0-
Total $ 173,197 $ 16,500
Total accrued interest on related party debts was $727,935 and $444,615 for 12/31/2021 and 12/31/2020, respectively. Related party balances have been reserved to administrative expense.
Rental
The Company rents its home office from BBD Holdings, LLC which is wholly controlled by Joseph Bird, an officer and director. The rental is on the month-to-month basis and is at a rate of $1,275.00 per month is no more than the prevailing rate for the Chattanooga, TN market.
Royalties
The Company sells a product subject to a royalty agreement with the VHS Pool that was set up by a predecessor entity, through two if its subsidiaries. An officer and director – Robert Long through a related entity – Lone Peak Innovative Holdings, LLC, has a creditor interest in the VHS Pool. Lone Peak Innovative Holdings, LLC has to date not received payments from the VHS Pool and the likelihood of future payments are not ascertainable.
The royalty agreement calls for the payment of 1% of the gross sales of the subject product(s). The royalty applies to any product designed to support a healthy Endothelium Glycocalyx, such as the company’s Endocalyx. The royalty agreement also calls for the payment of 1% of the proceeds, after taxes, on a liquidity event. A liquidity event is defined as the subsidiary entering an arms-length transaction with a third party or making an initial public offering. Should a liquidity even occur, the agreement requires a minimum payment to raise the pool amount to $7,500,000. The pool ceiling is $15,000,000 and the Company may have two subsidiaries subject to the agreement.
Royalties paid during the periods presented were:
VHS Pool Royalties
2021 $5,950
2020 $2,579
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE N—RELATED-PARTY TRANSACTIONS (Continued)
Distribution Agreement
The Company has a worldwide distribution agreement with GlycoCheck B.V. for the GycoCheck machine. GlycoCheck B.V. is 26.6% owned by an officer and board member of the Company – Hans Vink.
Accrued Expenses and Reimbursements:
The Company accrued payments to entities related to the owners in 2021 and 2020 before the merger. The balance of the amounts accrued were $60,597 and $1,056,218 for 2021 and 2020, respectively. During 2021 in anticipation of the merger, the unpaid balances of the accruals and other loans were converted into notes payable as follows:
Wilshire Holdings Trust $1,094,747
Libertas Trust $ 469,177
Resco Enterprises Trust $ 157,747
Avis Trust $ 67,606
Total $1,789,277
The Company reimburses certain officers for approved company expenses paid through individual credit cards.
NOTE O—SUBSEQUENT EVENTS:
In March of 2022, certain related party debt, including accrued interest to date, was converted into common Stock of the Company.
The table below shows the number of shares issued for the debt retired.
| Amount | Shares | |
| Loan Peak Holdings | $12,882 | 8,947 |
| Robert Long Trust | $80,935 | 56,205 |
| Connie W Wilson | ||
| Revocable Trust | $232,663 | 161,572 |
| Gail Long | $68,683 | 47,697 |
| David R and Diane H Long | ||
| Living Trust | $1,056,949 | 733,993 |
| Wilshire Holdings Trust | $1,070,005 | 743,059 |
| Libertas Trust | $458,573 | 318,454 |
| Total | $2,980,690 | 2,069,927 |
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE O—SUBSEQUENT EVENTS (Continued)
Findit Commitment
The Company executed an amended and restated letter of intent with Findit, Inc (FDIT) on July 1, 2022. The agreement provides for a merger of Bioregenx into Findit Inc, with all BioRegenx Common and Preferred Shares being exchanged for 90% of the FDIT common and preferred shares outstanding after the completion of the terms of the exchange. The letter of intent is valid for a 90 day period, unless mutually extended by the parties. The letter of intent was extended again in September 2022 without a set expiration date.
Immediately after the merger Bioregenx will trade under the FDIT symbol on the OTC Pink Markets. As soon as practicable after the merger the parties will implement an up to 25 to 1 reverse split to improve the ability to attract institutional investors and qualify for a senior exchange.
Simultaneously with the close of the propose transaction, all the current assets and liabilities of FDIT, shall be transferred to a newly formed subsidiary. The parties agree that the newly formed subsidiary may be spun out on yet to be determined terms
As a condition precedent to closing, the principals of FDIT shall execute an option agreement with FDIT relating to its acquisition of all or a majority of the shares of ClassWorx (CHNO) subsequent to the merger. After closing of the merger with FDIT and subsequently, upon exercising the option of the acquisition of ClassWorx, the Board of Directors agree to support and cause to be placed on the ballot, at each election of Directors, a designated nominee to the Board of Directors of the Company.
On January 6th, 2023 Findit, Inc filed form 8-K material event reporting the definitive merger agreement with BioRegenx, Inc.
PFV Fund I, Ltd
The Company secured a term sheet for a Senior Secured Debt Financing and Equity proposal in February of 2022. The Terms provides for a total funding package of up to $10,000,000.00 Senior Secured Loan and $15,000,000 in Equity Contributions for common shares, preferred shares and warrants.
The proceeds of the debt are designated for purchases of controlling interests in Board approved acquisitions. The proceeds of the equity funds are designated for expanded production, product marketing and working capital.
The term sheet does not bind the parties to the agreement until a definitive loan agreement, security agreement and stock purchase agreement are executed by the parties. The Company Management believes that funding is likely and further facilitated by the merger transaction described in the previous note to the financial statements - FIndit Commitment.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2021 and 2020
NOTE O—SUBSEQUENT EVENTS (Continued)
Private Placement Memorandum
The Company is preparing to issue a Private Placement Memorandum to raise additional funds from accredited investors in the first quarter of 2023. The Private Placement Memorandum will offer up to a maximum of 1,200,000 common shares of the Company at $3.00 per share. The offering will have a dilutive effect on the holdings of the existing shareholders. The offering will terminate on or before the merger with Findit is finalized or earlier at the option of management.
| BIOREGENX, INC. AND SUBSIDIARIES | |||||
| CONSOLIDATED BALANCE SHEET | |||||
| September 30 | December 31, | ||||
| 2022 | 2021 | ||||
| ASSETS | |||||
| Current Assets: | |||||
| Cash and cash equivalents | $ 218,418 | $ 882,112 | |||
| Accounts receivable | 0 | 985 | |||
| Inventories | 390,654 | 653,291 | |||
| Prepaid expenses and other current assets | 566,173 | 349,281 | |||
| Total current assets | 1,175,245 | 1,858,669 | |||
| Property and equipment (Net) | 19,690 | 23,270 | |||
| Intangible assets (Net) | 0 | 0 | |||
| Other assets | 0 | 11,025 | |||
| TOTAL ASSETS | $1,194,935 | $1,919,964 | |||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | |||||
| Current Liabilities: | |||||
| Accounts payable | $ 511,916 | $ 610,024 | |||
| Accrued expenses | 403,855 | 995,234 | |||
| Promissory notes payable and loans | 175,943 | 1,316,758 | |||
| Deferred revenue | 101,210 | 69,000 | |||
| Total current liabilities | 1,192,924 | 2,991,016 | |||
| Notes payable | 1,428,253 | 2,189,160 | |||
| TOTAL LIABILITIES | 2,621,177 | 5,180,176 | |||
| STOCKHOLDERS' DEFICIT | |||||
| Common stock, $0.0001 par value; 375,000,000 shares authorized; 36,169,936 issued and outstanding | 3,854 | 3,616 | |||
| Series A preferred stock, non-dividend, 100 votes per -share, $0.0001 par value, 150,000 authorized; issued and outstanding 95,000 | 10 | 10 | |||
| Additional paid-in capital | 6,310,826 | 2,698,172 | |||
| Accumulated deficit | (7,740,932) | (5,962,010) | |||
| Total stockholders' deficit | (1,426,242) | (3,260,212) | |||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $1,194,935 | $1,919,964 | |||
The accompanying notes are an integral part of these consolidated financial statements
BIOREGENX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE AND NINE MONTHS
ENDED SEPTEMBER 30, 2022 AND 2021
Three Months ended September 30, | Nine Months ended September 30, | |||
| 2022 | 2021 | 2022 | 2021 | |
| Revenues: | ||||
| Net sales | $ 528,026 | $ 762,330 | $1,932,512 | $2,281,713 |
| Cost of sales | 104,496 | 305,038 | 696,107 | 1,016,702 |
| Gross profit | 423,530 | 457,292 | 1,236,404 | 1,265,011 |
| Operating expenses: | ||||
| Distributors incentives | 37,001 | 7,054 | 166,706 | 216,502 |
| Selling, general and administrative | 747,699 | 607,178 | 2,530,926 | 2,050,785 |
| Impairment Expense | - | - | - | |
| Total operating expenses | 784,700 | 614,232 | 2,697,632 | 2,267,287 |
| Loss from operations | (361,170) | (156,940) | (1,461,228) | (1,002,276) |
| Other income (expense): | ||||
| Interest income | 79 | 9,022 | 148 | 20,929 |
| Other loss | - | (130) | - | |
| Interest expense and financing costs | (72,340) | (11,528) | (317,842) | (30,281) |
| Total other expenses | (72,261) | (2,636) | (317,694) | (9,352) |
| Income (loss) before provision for taxes | (433,431) | (159,576) | (1,778,922) | (1,011,238) |
| Provision for income taxes | - | - | - | - |
| Net loss | (433,431) | (159,576) | ($1,778,992) | (1,011,238) |
| Weighted average shares outstanding – basic and diluted | 37,876,930 | 35,266,678 | 37,876,930 | 35,266,678 |
| Earnings (loss) per share – basic and diluted | $(0.01) | $(0.00) | $(0.05) | $(0.03) |
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDER’ DEFICIT
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022
| Common Stock | Preferred Stock | Additional Paid-in | Member Equity/ Accumulated | Total Stockholders' | |||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | |
| Balance, April 6, 2021 | - | - | - | - | - | - | |
| Issuance of common stock for acquisitions. | 34,912,500 | 3,491 | 95,000 | 10 | 896,241 | (3,381,045) | (2,481,303) |
| Issuance of common share in acquisition of Regenr8, LLC | 480,000 | 48 | - | - | -691,152 | - | 691,200 |
| Issuance of common shares in private placement | 777,436 | 77 | - | - | 1,110,779 | - | 1,110,856 |
| Net loss | (2,580,965) | (2,580,765) | |||||
| Balance, December 31, 2021 | 36,169,936 | $ 3,616 | 95,000 | $10 | $2,698,172 | $(5,962,010) |
$(3,260,212) |
| Issuance of common stock for debt conversion | 2,069,927 | 207 | - | - | 2,998,685 | - | 2,998,892 |
| Issuance of common stock in private placements | 307,000 | 31 | - | - | 613,969 | - | 614,000 |
| Net loss | - | - | - | - | - | (2,580,965) | (1,772,081) |
| Balance, September 30, 2022 | 38,546,863 | $3,854 | 95,000 | $10 | $6,310,826 | (8,542,975) | $(1,419,401 |
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 AND 2021
Nine Months Ended September 30, | |||
| 2022 | 2021 | ||
| Operating Activities: | |||
| Net loss | $(1,778,992) | $(1,011,238) | |
| Adjustments to reconcile net income (loss) to | |||
| net cash used in operating activities: | |||
| Depreciation and amortization | 3,580 | - | |
| Common stock issued for services | - | 482,544 | |
| Change in operating assets and liabilities: | |||
| Accounts receivable | 17,985 | (16,785) | |
| Inventories | 262,637 | 181,495 | |
| Trade note receivable | (176,600) | 29,157 | |
| Prepaid expenses and other assets | (17,496) | (434,640) | |
| Accounts payable | (83,200) | (1,672,780) | |
| Accrued expenses and other liabilities | (635,059) | 198,446 | |
| Deferred Revenue | 32,210 | 2,348 | |
| Net cash used in operating activities | (2,374,865) | (2,241,453) | |
| Investing Activities: | |||
| Acquisition of intangible assets | - | (830,762) | |
| Net cash used in investing activities | - | (830,762) | |
| Financing Activities: | |||
| Increase in SBA loans | 149,900 | 105,542 | |
| Note and loan payments | (819,175) | (95,796) | |
| Increase in note and loan balances | (1,232,446) | 2,323,121 | |
| Increase in equity for acquisition | - | 691,200 | |
| Increase in equity for Debt conversion | 2,980,723 | - | |
| Proceeds from the issuance of common stock | 632,169 | 1,110,856 | |
| Net cash provided by financing activities | 1,711,171 | 4,134,923 | |
| Net Increase (decrease) in Cash and Cash Equivalents | (663,694) | 1,062,708 | |
| Cash and Cash Equivalents, Beginning Balance | 882,112 | 592,973 | |
| Cash and Cash Equivalents, Ending Balance | 218,418 | 1,655,681 | |
The accompanying notes are an integral part of these consolidated financial statements.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 AND 2021
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING
Organization and Business:
The Company Bioregenx, Inc. develops and manufactures medical test equipment and high quality, science-based nutritional products that are sold nationally through a direct selling channel, to health professionals and research organizations.
On April 6th, 2021 Bioregenx entered into a combination agreement with Microvascular Health Services, LLC., My Body Rx, LLC and Nulife Sciences, Inc. that resulted in the addition of three subsidiary companies to the group. Due to the ownership structure the combination is accounted for as a combination of entities under common control under the Financial Accounting Standards Board’s Accounting Standard Codification (ASC) Topic 805. The activities of the subsidiaries are included in the financial statements for the entire reporting period with assets and liabilities stated at the historical carrying value.
On September 15, 2021, the Company acquired all the interest in Regenr8, LLC in exchange for shares of the Company’s stock. This acquired company is accounted for as an acquisition and the activities of the acquired company are included in the consolidated financial statements starting with the acquisition. Assets are liabilities are reported at the purchase price allocated to the relative fair market value.
The Consolidated Financial Statements (the “Financial Statements”) include the accounts and operations of the Company, and its controlled subsidiaries. All inter-company accounts and transactions have been eliminated in consolidation. The accounting and reporting policies of the Company conform with accounting principles generally accepted in the United States of America (“US GAAP”).
Use of Estimates
The preparation of Consolidated Financial Statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amounts of revenues and expenses during the reporting period. These estimates may be adjusted as more current information becomes available, and any adjustment could be significant.
Fiscal Year
The Company operates on a calendar year ending on December 31.
Fair Value Measurements
As of December 31, 2021 and December 31, 2020, the Company’s financial instruments include cash equivalents. The cost values of cash equivalents and restricted cash approximate their fair values, based on their short-term nature.
Translation of Foreign Currencies
The Company’s foreign sales activities are conducted in US Dollars and no foreign currency translations are recorded in these financial statements.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash equivalents. Cash equivalents consisted primarily of bank balances and amounts receivable from credit card processors. Amounts receivable from credit card processors and other forms of electronic payment are considered cash equivalents because they are both short-term and highly liquid in nature and are typically converted to cash within three days of the sales transaction.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 AND 2021
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out method. Net realizable value is determined using various assumptions with regard to excess or slow-moving inventories, non-conforming inventories, expiration dates, current and future product demand, and market conditions. A change in any of these variables could result in an adjustment to inventory.
Accounts Receivable
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company establishes an allowance for doubtful accounts for estimated losses inherent in its accounts receivable as determined by management. In establishing the required allowance, management considers historical losses adjusted to take into account current market conditions and our customers’ financial condition, the amount of the receivables in dispute, and the current receivables aging and current payment patterns. The Company reviews its allowance for doubtful accounts regularly. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of the differences between the financial statement assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates that are expected to apply to taxable income in the year in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax law is recognized in income in the period that includes the enactment date. Deferred tax expense or benefit is the result of changes in deferred tax assets and liabilities. The Company evaluates the probability of realizing the future benefits of its deferred tax assets and provides a valuation allowance for the portion of any deferred tax assets where the likelihood of realizing an income tax benefit in the future does not meet the “more-likely-than-not” criteria for recognition. The Company recognizes tax benefits from uncertain tax positions only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the Financial Statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution.
Property and Equipment
Property and equipment are recorded at cost. Maintenance, repairs, and renewals, which neither materially add to the value of the property nor appreciably prolong its life, are charged to expense as incurred. Depreciation is provided in amounts sufficient to relate the cost of depreciable assets to operations over the estimated useful lives of the related assets. The straight-line method of depreciation and amortization is followed for financial statement purposes. Property and equipment are reviewed for impairment whenever events or changes in circumstances exist that indicate the carrying amount of an asset may not be recoverable. When property and equipment are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the results of operations for the respective period.
Leases
The Company has adopted ASC Topic 842 for lease accounting. This standard requires that right of use assets and liabilities are measured and recorded on the balance sheet. ASC Topic 842 provides an exception for short term leases that are for 12 months or less. The Company reports short term leases under the exception to the standard. Leases for a period of 12 months or less are recorded as expense on a ratable basis throughout the term of the lease.
The Company leases two office spaces, its headquarters in Chattanooga Tennessee and a satellite office in Alpine Utah, both are short term leases.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
The headquarters is leased from a related party on a month-to-month basis for $1,725.00 per month
The satellite office is leased from an unrelated party under a twelve-month extension to the original lease at $1,300.00 per month.
Intangible Assets
Intangible assets represent intangibles acquired in the purchase of Regenr8, LLC. Intangibles include product intangibles, formulations and customer-based intangibles. Amortized intangible assets are amortized over their related useful lives, using a straight-line or accelerated method consistent with the underlying expected future cash flows related to the specific intangible asset. Amortized intangible assets are reviewed for impairment whenever events or changes in circumstances exist that indicate the carrying amount of an asset may not be recoverable. When indicators of impairment exist, an estimate of undiscounted net cash flows is used in measuring whether the carrying amount of the asset or related asset group is recoverable. Measurement of the amount of impairment, if any, is based upon the difference between the asset or asset group’s carrying value and fair value. Fair value is determined through various valuation techniques, including market and income approaches as considered necessary.
Goodwill
Goodwill represents the excess of the purchase price over the fair market value of identifiable net assets of acquired companies. Goodwill is not amortized, but rather is tested at the reporting unit level at least annually for impairment or more frequently if triggering events or changes in circumstances indicate impairment. Initially, qualitative factors are considered to determine whether it is more likely than not that the fair value of a reporting unit is less than it’s carrying amount. Some of these qualitative factors may include macroeconomic conditions, industry and market considerations, a change in financial performance, entity-specific events, a sustained decrease in share price, and consideration of the difference between the fair value and carrying amount of a reporting unit as determined in the most recent quantitative assessment. If, through this qualitative assessment, the conclusion is made that it is more likely than not that a reporting unit’s fair value is less than it’s carrying amount, a quantitative impairment analysis is performed. This analysis involves estimating the fair value of a reporting unit using widely accepted valuation methodologies including the income and market approaches, which requires the use of estimates and assumptions. These estimates and assumptions include revenue growth rates, discounts rates, and determination of appropriate market comparable. If the fair value of the reporting unit is less than it’s carrying amount, an impairment loss is recognized in an amount equal to the excess of the carrying amount over the fair value of the reporting unit, not to exceed the carrying amount of the goodwill. The Company does not have recorded goodwill.
Revenue Recognition
The Company applies ASC Topic 606, Revenue from Contracts with Customers ("ASC 606") for all periods presented in the consolidated financial statements. To determine the appropriate amount of revenue to be recognized in accordance with ASC 606, the Company follows a five-step model as follows:
1 – Identification of the contract with a customer
2 – Identification of the performance obligation in the contract
3 – Determination of the transaction price
4 – Allocation of the transaction price to the performance obligation in the contract
5 – Recognition of revenue when, or as, a performance obligation is satisfied
Contract with the customer
The Company through its subsidiaries provides a medical testing machine, consumables for the testing process and nutritional supplements to its Independent Brand Partners (IBP) and customers. IBPs pay an annual Membership fee. Prior to the merger 04/06/2021, a medical device was also sold but this product has been discontinued. The products are distributed through a network of IBPs. During the audit period the testing machines were primarily sold to educational institutions directly by the company. The medical devices and the supplements sold to individuals by the
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
independent brand partners. The medical device was exclusively sold through the network of independent brand partners. The Company assesses the contract term as the period in which the parties to the contract have enforceable rights and obligations. The contract term may differ from the stated term in contracts with certain termination or renewal rights, depending on whether there are substantive penalties associated with those rights. Customer contracts are generally standardized and noncancellable for the duration of the stated contract term. Consumption taxes collected and remitted to tax authorities are excluded from revenue. The Company may use third-party vendors to provide certain goods or services to its customers. The Company evaluates those relationships to determine whether revenue should be reported gross or net. The Company recognizes revenue on a gross basis where it acts as principal and controls the goods and services used to fulfill the performance obligations to the customer and on a net basis where it acts as an agent. The Company has not acted as an agent during the years ended December 31, 2021 and 2020.
Performance Obligation
The counterparties contract to obtain goods and meet the requirements to be customers whether termed customers or independent brand partners. The Company’s sales to educational institutions are of a tangible product. The company requires payment prior to shipping, with only a few exceptions. Sales to educational institutions are not shipped until payment is received. Once an order is paid the invoice is sent to an outsourced fulfillment house for shipping. Shipping typically occurs in 24 hours of the payment. Sales are booked upon shipment when the performance obligation is satisfied.
Sales to health professional and individual consumers, other than annual memberships, are of tangible goods. The company requires payment prior to shipping, with only a few exceptions. Sales to health professionals, other IBPs and consumer are also not shipped until payment is received, typically via credit card payments. Once an order is paid the invoice is sent to an outsourced fulfillment house for shipping. Shipping typically occurs in 24 hours of the payment. Sales are booked upon shipment when the performance obligation is satisfied.
All counterparties to the contract may return the product pursuant to the Company’s return policy. Annual Membership of IBPs are non-refundable.
Other Revenue
Other types of revenue include fees, which are paid by the customer at the beginning of the service period, for access to online customer service applications and annual account renewal fees for Associates.
Transaction Price
The transaction price is the amount received or expected to be received for transferring goods and services to its customers. Each order entered has a fixed price listed in the company’s distributor portal or on a price list at the time the sale is originated.
Payments from the customers are typically made at the time of the order before satisfaction of the performance obligation. The Company in rare instances grants 30-day terms to select long term customers. In such instances the revenue recognition occurs when the performance obligation is satisfied. The Company has elected the practical expedient that permits an entity not to recognize a significant financing component if the time between the transfer of a good or service and payment is one year or less.
Allocations
The Company, as a matter of ordinary operations, allocated the purchase price against the performance obligation on an order-by-order basis for the entire order. In the event an order has not been fulfilled or partially filled revenues are not recognized for the portion of the order for which the performance obligation has not been satisfied. There has been no transaction price allocated to performance obligations that have not been fully satisfied the years ended December 31, 2021 and 2020.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE A—SUMMARY OF SIGNIFICANT ACCOUNTING (Continued)
Recognition
Revenue is recognized when the performance obligation is satisfied, primarily upon shipment for tangible goods. The majority of collections are through online orders paid through credit cards. There are few exceptions where terms are given to well-known customers, primarily in the academic market. The company’s distributors’ have the option to apply their earned commissions to their orders resulting in an internal entry to record their payment.
The company has not engaged in financing related to its products. When third party finance companies are used by the customer the order is considered paid when funds are received from the finance company.
There are no contracts that extend beyond the performance of the original sales obligation.
Product Return Policy
All product orders that are unused and returned within the first 30 days following purchase are refunded at 100% of the sales price.
Independent Business Partners Incentives
Incentives expenses include all forms of commissions, and other incentives paid to our Independent Business Partners (IBAs).
Selling, General and Administrative Selling, general and administrative expenses include wages and benefits, depreciation and amortization, rents and utilities, Associate event costs, advertising and professional fees, marketing, and research and development expenses.
Equity-Based Compensation
The Company records compensation expense in the Financial Statements for equity-based awards based on the fair value on the date of transfer. For the financial statement period all equity-based compensation consisted of direct transfers of equity interests.
Advertising
Advertising costs are charged to expense as incurred and are presented as part of the “Selling, general and administrative” line item.
Research and Development
Research and development costs are expensed as incurred per ASC Topic 730. None of the activities of the Company in the audit period qualified for exceptions to the general guidance of ASC Topic 730.
Earnings Per Share
Basic earnings per common share ("EPS") are based on the weighted-average number of common shares that were outstanding during each period.
Diluted EPS is not applicable during the audit period
09/30/2022 12/31/2021
Finished goods $390,654 $653,291
Supplement Inventories are purchased in finished form with labels purchased separately in an amount to support the production run. Medical testing equipment was purchase and assembled once orders were received during the financial statement period.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE B—PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consisted of the following:
09/30/2022 12/31/2021
Prepaid commissions $...275,881 $...243,381
Other current assets $...290,292 $...105,900
Total $...566,173 $...349,281
NOTE C - INCOME TAXES
Consolidated earnings before income taxes consists of the following:
09/30/2022 12/31/2021
Consolidated Net Loss before income Taxes ($1,772,081) $3,540,245)
Income tax expense (benefit) included in income from continuing operations consists of the following:
09/30/2022 12/31/2021
Year Ended 2021 and 2020 Current and Deferred $ - $ -
During the financial statement period before the combination date of April 6th, 2021, each subsidiary filed separate income tax returns. Each subsidiary other than NuLife Sciences, Inc filed as flow through entities for tax purposes and the owner’s reported income and loss on their tax returns for the 2020 and short period ended April 6, 2021 period. The Company reported net taxable loss for the short period beginning April 6th and ending December 31. 2021. As of January 1, 2022, the Company expect to have net operating loss carryovers and research tax credit carryovers. The Company believes the utilization of the carryforwards cannot be determined with reasonable accuracy at this time due to provisions in the tax code that could act to limit the utilization of the carryovers and uncertainty in the determination of the periods that the carryovers may be utilized against future taxable income. The Company maintains a full valuation allowance on its carryforwards. Valuation allowances are determined using a more-likely-than-not realization criteria and are based upon all facts and circumstances. The Company files income tax returns in the United States and multiple states. In general, the Company's and predecessor’s tax filings are subject to examination for years ending on or after December 31, 2018, however, the statutes of limitations in certain instances may be longer.
NOTE C – PROPERTY AND EQUIPMENT
Cost of property and equipment and their estimated useful lives is as follows:
09/30/2022 12/31/2021
Computer equipment and software ....................................................$19,690…..........$...23,270
Depreciation of property and equipment was $3,580 and $597- for the year to date period year ended 2021, respectively.
NOTE D – PROPERTY AND EQUIPMENT
The following table presents supplemental lease information:
09/30/2022 12/31/2021
Operating Lease Cost $37,467 $ 35,954
Operating lease cost were related to the Company’s office space, see related party disclosures below.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE E - INTANGIBLE ASSETS
The Company performed its annual indefinite-lived intangible asset impairment test during 2021. The Company performed a qualitative assessment of the indefinite-lived intangible assets and based on a delay in implementing the acquired assets determined that it was more-likely-than-not that the fair value of any indefinite-lived intangible asset was less than the carrying amount. The Company determined that the fair market value of the acquired intangibles was not determinable as of 12/31/2021. As a result, an impairment expense was recognized for the amount acquired.
Intangible assets consist of the following:
Amortized intangible assets 09/30/2022 12/31/2021
Acquired intangibles – Regenr8
Acquisition $ - $ -
Impairment Expense $ - $830,762
NOTE F – OTHER ASSETS
Other Assets consisted of the following:
09/30/2022 12/31/2021
Investment in Thunder Struck $ - $11,025
Investment in Thunder Struck represents a marketing arrangement entered into to develop the health practitioner distribution channel.
NOTE G - COMMITMENTS AND CONTINGENCIES
Unconditional Purchase Obligations
The Company is involved in various lawsuits, claims, and other legal matters from time to time that arise in the ordinary course of conducting business, including matters involving its products, intellectual property, supplier relationships, distributors, competitor relationships, employees and other matters. The Company records a liability when a particular contingency is probable and estimable. The Company faces contingencies that are reasonably possible to occur; however, they cannot currently be estimated. While complete assurance cannot be given as to the outcome of these proceedings, management does not currently believe that any of these matters, individually or in the aggregate, will have a material adverse effect on the Company’s financial condition, liquidity or results of operations. It is reasonably possible that a change in the contingencies could result in a change in the amount recorded by the Company in the future.
NOTE H - ACQUISITIONS
On April 6th, 2021 the Company’s consolidated group was formed by the contribution of 100% of the equity interests of three companies, Microvascular Health Services, LLC, My Body Rx, LLC and NuLife Sciences, Inc. in exchange for newly issued common and preferred stock representing all the issued and outstanding shares of Bioregenx. After the contribution of the Microvascular Health Services, LLC interest it was discovered that certain prior arrangements may have impaired the transferability of 10% of the interest. Authorized share corresponding to this amount were placed in reserve pending resolution. The combination is expected to product synergies between companies with the production activities and the distribution network of the marketing company.
On September 15, 2021, 100% of the equity of Regen8, LLC was acquired in exchange of newly issued common shares of the Company. The Regen8, LLC acquisition added a consumable testing product and a nutritional supplement to the Company’s product line.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE I - SHARES SOLD
In June 2021, the Company Issued a private placement memorandum which offered for sale shares of its common stock to qualified accredited investors. The Shares were valued at $1.44 per common share. The offering was closed September of 2021. The results are summarized in the following table.
| Number Of Shareholders | Number of Shares | Funds Raised |
| 39 | 771,436 | $1,110,856 |
An individual investor purchased a total of 307,000 shares. The first purchase of 125,000 shares was on April 18, 2022. Two purchases were made in August. 120,000 shares on August 19, 2022 and 62,000 shares on August 29th. All shares were purchased at $2.00 per share.
NOTE J - DEBT CONVERTED TO EQUITY
In March 2022, debtholders of the Company’s subsidiaries were offered the ability to convert their debt into the Company’s common shares. On March 31, 2022, 2,069,927 shares were issued at a value of $1.44.
The table below shows the number of shares issued for the debt retired.
| Amount | Shares | |
| Loan Peak Holdings | $12,882 | 8,947 |
| Robert Long Trust | $80,935 | 56,205 |
| Connie W Wilson | ||
| Revocable Trust | $232,663 | 161,572 |
| Gail Long | $68,683 | 47,697 |
| David R and Diane H Long | ||
| Living Trust | $1,056,949 | 733,993 |
| Wilshire Holdings Trust | $1,070,005 | 743,059 |
| Libertas Trust | $458,573 | 318,454 |
| Total | $2,980,690 | 2,069,927 |
NOTE K - EQUITY-BASED COMPENSATION
Total equity-based compensation expense was $482,544 and $417,199 for years 2021 and 2020, respectively. The equity compensation was granted to key employees for past services rendered. The equity was paid in the form of direct equity interests of the member companies prior to the combination on April 6, 2021.
The amounts granted were as follows:
09/30/2022 12/31/2021
Robert Doran $ - $371,188
Gary Hennerberg - $111,356
Total $ - $482,544
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE L - RELATED-PARTY TRANSACTIONS
Loans
Bioregenx and its subsidiaries have financed past activities, in part, with borrowing from certain related parties. The amount of debt from related parties is summarized in the following table:
| Balance | Balance | ||||
| Related Party | 9/30/2022 | 12/31/2021 | |||
| Libertas Trust | $180,000 | $180,000 | |||
| Libertas Trust | 0 | $400,664 | |||
| Wilshire Holding Trust | $673,000 | $420,000 | |||
| Wilshire Holding Trust | $0 | $934,882 | |||
| Resco Enterprises Trust | $157,747 | $157,747 | |||
| Avis Trust | $67,606 | $67,606 | |||
| Lone Peak Holdings, LLC | $0 | $3,500 | |||
| Robert Long | $0 | $64,746 | |||
| Robert Long | $0 | $4,082 | |||
| Michael Long | $0 | $7,495 | |||
| Richard Long | $52,862 | $60,863 | |||
| Gail Long | $0 | $44,735 | |||
| Diane Long | $0 | $547,678 | |||
The Company has made advances to related parties as follows:
09/30/2022 12/31/2021
GlycoCheck B.V. $ - $ 24.194
Robert Long $ 18,392 $ 149,003
Total $ 18,392 $ 173,197
Total accrued interest on related party debts was $_________ and $727,935 for 9/30/20 and 12/31/21, respectively. Related party balances have been reserved to administrative expense.
Rental
The Company rents its home office from BBD Holdings, LLC which is wholly controlled by Joseph Bird, an officer and director. The rental is on the month-to-month basis and is at a rate of $1,275.00 per month is no more than the prevailing rate for the Chattanooga, TN market.
Royalties
The Company sells a product subject to a royalty agreement with the VHS Pool that was set up by a predecessor entity, through two if its subsidiaries. An officer and director – Robert Long through a related entity – Lone Peak Innovative Holdings, LLC, has a creditor interest in the VHS Pool. Lone Peak Innovative Holdings, LLC has to date not received payments from the VHS Pool and the likelihood of future payments are not ascertainable.
The royalty agreement calls for the payment of 1% of the gross sales of the subject product(s). The royalty applies to any product designed to support a healthy Endothelium Glycocalyx, such as the company’s Endocalyx. The royalty agreement also calls for the payment of 1% of the proceeds, after taxes, on a liquidity event. A liquidity event is
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE L – RELATED PARTY TRANSACTIONS (Continued)
defined as the subsidiary entering an arms-length transaction with a third party or making an initial public offering. Should a liquidity even occur, the agreement requires a minimum payment to raise the pool amount to $7,500,000. The pool ceiling is $15,000,000 and the Company may have two subsidiaries subject to the agreement.
Royalties paid during the period were:
VHS Pool Royalties
09/2022 $6,617
09/2021 $5,950
Distribution Agreement:
The Company has a worldwide distribution agreement with GlycoCheck B.V. for the GycoCheck machine. GlycoCheck B.V. is 26.6% owned by an officer and board member of the Company – Hans Vink.
Accrued Expenses and Reimbursements:
The Company accrued payments to entities related to the owners in 2021 and 2020 before the merger. The balance of the amounts accrued were $60,597 and $1,056,218 for 2021 and 2020, respectively. During 2021 in anticipation of the merger, the unpaid balances of the accruals and other loans were converted into notes payable as follows:
Wilshire Holdings Trust $1,094,747
Libertas Trust 469,177
Resco Enterprises Trust 157,747
Avis Trust 67,606
Total $1,789,277
The Company reimburses certain officers for approved company expenses paid through individual credit cards.
NOTE M - SUBSEQUENT EVENTS:
Findit Commitment
The Company executed an amended and restated letter of intent with Findit, Inc (FDIT) on July 1, 2022. The agreement provides for a merger of Bioregenx into Findit Inc, with all BioRegenx Common and Preferred Shares being exchanged for 90% of the FDIT common and preferred shares outstanding after the completion of the terms of the exchange. The letter of intent is valid for a 90 day period, unless mutually extended by the parties. The letter of intent was extended again in September 2022 without a set expiration date.
Immediately after the merger Bioregenx will trade under the FDIT symbol on the OTC Pink Markets. As soon as practicable after the merger the parties will implement an up to 25 to 1 reverse split to improve the ability to attract institutional investors and qualify for a senior exchange.
Simultaneously with the close of the propose transaction, all the current assets and liabilities of FDIT, shall be transferred to a newly formed subsidiary. The parties agree that the newly formed subsidiary may be spun out on yet to be determined terms
As a condition precedent to closing, the principals of FDIT shall execute an option agreement with FDIT relating to its acquisition of all or a majority of the shares of ClassWorx (CHNO) subsequent to the merger. After closing of the merger with FDIT and subsequently, upon exercising the option of the acquisition of ClassWorx, the Board of Directors agree to support and cause to be placed on the ballot, at each election of Directors, a designated nominee to the Board of Directors of the Company.
BIOREGENX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022 and 2021
NOTE M - SUBSEQUENT EVENTS: (Continued)
PFV Fund I, Ltd
The Company secured a term sheet for a Senior Secured Debt Financing and Equity proposal in February of 2022. The Terms provides for a total funding package of up to $10,000,000.00 Senior Secured Loan and $15,000,000 in Equity Contributions for common shares, preferred shares and warrants.
The proceeds of the debt are designated for purchases of controlling interests in Board approved acquisitions. The proceeds of the equity funds are designated for expanded production, product marketing and working capital.
The term sheet does not bind the parties to the agreement until a definitive loan agreement, security agreement and stock purchase agreement are executed by the parties. The Company Management believes that funding is likely and further facilitated by the merger transaction described in the previous note to the financial statements - Findit Commitment.
Annex A
Agreement and Plan of Merger
AGREEMENT AND PLAN OF MERGER
AGREEMENT AND PLAN OF MERGER (this "Merger Agreement") made and entered into effective December 29, 2022 by and between BioRegenx, Inc., a Nevada corporation ("BioRegenx") and Findit, Inc., a Nevada corporation ("Findit").
WITNESSETH:
WHEREAS, BioRegenx is a corporation duly organized and existing under the laws of the state of Nevada;
WHEREAS, Findit is a corporation duly organized and existing under the laws of the state of Nevada;
WHEREAS, on the date of this Merger Agreement, BioRegenx has the authority to issue three hundred seventy five million (375,000,000) shares of common stock, $.0001 par value per share, of which thirty eight million, five hundred eighty eight thousand, one hundred seventy three (39,588,173) common shares are validly issued and outstanding, fully paid and non-assessable and twenty five million (25,000,000) preferred shares, $.001 par value. BioRegenx has designated one hundred fifty thousand (150,000) Series A preferred shares, of which ninety five thousand (95,000) Series A preferred shares are validly issued and outstanding.;
WHEREAS, on the date of this Merger Agreement, Findit has authority to issue five hundred million (500,000,000) authorized shares of common stock $.001 par value per share of which two hundred sixty nine million, seven hundred forty five thousand, six (269,745,006) common shares are issued and outstanding, fifty million (50,000,000) authorized Series A preferred shares of which five million (5,000,000) Series A preferred shares are issued and outstanding and five million (5,000,000) authorized Series B preferred shares, of preferred shares of which four million nine hundred thousand (4,900,000) Series B preferred shares are issued and outstanding.
WHEREAS, the respective Boards of Directors of BioRegenx and Findit have determined that it is advisable and to the advantage of said two corporations that BioRegenx merge into Findit (hereinafter also referred to as the “Surviving Corporation” upon the terms and conditions herein provided; and
WHEREAS, the respective Boards of Directors of BioRegenx and Findit have approved this Merger Agreement and the Boards of Directors of BioRegenx and Findit have directed that this Merger Agreement be submitted to a vote of their shareholders, if required by state law.
NOW, THEREFORE, in consideration of the mutual agreements and covenants set forth herein, BioRegenx and Findit hereby agree to merge as follows:
(1) Name Change. The name of the Surviving Corporation shall be amended to be BioRegenx, Inc.
(2) Increase in Authorized Common Shares. The authorized common shares shall be increased to four billion (4,000,000,000).
(3) Mechanics for Closing Merger. Prior to Closing, each party shall execute and deliver, or cause to be executed and delivered to Jody M. Walker, Attorney At Law as escrow agent, all common stock, Series A preferred stock, documents and instruments, in form and substance satisfactory as reasonably required to carry out or evidence the terms of this Agreement.
Upon the approval of the respective shareholders, the executed Articles of Merger shall be filed with the Nevada Secretary of State.
(4) Further Assurances. At or after Closing, BioRegenx, at the request of Findit, shall promptly execute and deliver, or cause to be executed and delivered, to Findit all such documents and instruments, in form and substance satisfactory to Findit, as Findit reasonably may request in order to carry out or evidence the terms of this Agreement.
(5) Retirement of Series A and Series B preferred shares. Findit shall have retired its issued and outstanding Series A and Series B preferred shares, in accordance with the authorization from the Series A and Series B preferred shareholders.
(6) Reverse stock split. As soon as practicable, both Parties agree to the implementation of up to a 1 for 25 reverse split of FDIT’s common and preferred stock to improve FDIT’s ability to attract institutional investors and analysts as well as to graduate to a senior exchange (OTCQB, NASDAQ).
(7) Potential Spinoff. Simultaneously with the close of the proposed transaction, all of the current assets and liabilities of FDIT, shall be transferred into a wholly owned subsidiary. Both parties agrees that the wholly owned subsidiary may be subsequently spun out upon yet to be determined terms, except that the parties agree that no spin out shall occur until the outstanding $150,000 EIDL loan is repaid in full. The parties acknowledge that, subsequent to the spin out, all assets and liabilities currently outstanding in FDIT will follow the subsidiary and will not be the responsibility of the merged entity post-closing of this transaction.
(8) Classworx Option. Simultaneously with the close of the proposed transaction and as a condition precedent to said closing, the Principals of FDIT shall execute an option agreement with FDIT relating to its acquisition of all of their shares in Classworx (CHNO), subsequent to the merger. The option shall be on terms comparable to those agreed to in this Agreement. After said closing of merger with FDIT and subsequently upon exercising the option of the acquisition of ClassWorx, the Board of Directors agree to support and cause to be placed on the ballot at each election of Directors one name chosen by the current principals of Classworx which shall be a nominee to the current Board of Directors of the Company.
(9) Stock of BioRegenx. On and after the Effective Date, all of the outstanding certificates that prior to that time represented shares of BioRegenx shall be recalled and canceled and not more than three hundred thirty seven thousand, seven hundred ninety three thousand, nine hundred fifty nine (337,793,959) common shares and eight hundred thirty six thousand, forty eight (836,048) Series A preferred shares Findit Common and Preferred Shares shall be issued in proportion to their ownership percentage. Said merger consideration will represent not more than ninety (90) percent of the total voting securities of the newly merged company.
The registered owner on the books and records of BioRegenx or its transfer agents of any outstanding certificate shall, until such certificate shall have been surrendered for transfer or otherwise accounted for to Findit or its transfer agents, have and be entitled to exercise any voting and other rights with respect to and to receive any dividend and other distributions upon the shares of Findit Common Stock evidenced by such outstanding certificate as above provided.
(10) Resignation of Officers and Directors. Each of the current officers and directors of Findit whose written resignation BioRegenx has requested shall have delivered to BioRegenx written resignations effective as of the effective date of the Merger (the “Effective Date”).
(11) Book Entries. As of the Effective Date, entries shall be made upon the books of Findit in accordance with the following.
(a) The assets and liabilities of BioRegenx shall be recorded at the amounts at which they were carried on the books of BioRegenx immediately prior to the Effective Date.
(b) There shall be credited to the common stock account of Findit the aggregate amount of the total paid-in capital of all shares of Findit Common Stock resulting from the conversion of the outstanding BioRegenx Common Stock pursuant to the merger.
(c) There shall be credited to the retained earnings account of Findit the aggregate of the amount carried in the retained earnings account of BioRegenx immediately prior to the Effective Date.
(12) Access to Documentation. Prior to the merger, Findit and BioRegenx shall provide each other full access to their books and records, and shall furnish financial and operating data and such other information with respect to their business and assets as may reasonably be requested from time to time. If the proposed transaction is not consummated, all parties shall keep confidential any information (unless ascertainable from public filings or published information) obtained concerning each others operations, assets and business.
(13) Abandonment. At any time before the Effective Date, the Agreement and Plan of Merger and the Articles of Merger may be terminated and the Merger may be abandoned by the Board of Directors of either Findit or BioRegenx or both, notwithstanding approval of the Merger Agreement by the shareholders of Findit or the shareholders of BioRegenx or both.
(14) Counterparts. In order to facilitate the filing and recording of this Merger Agreement the same may be executed in any number of counterparts, each of which shall be deemed to be an original.
IN WITNESS WHEREOF, this Merger Agreement, having first been duly approved by resolution of the Boards of Directors of BioRegenx and Findit, is hereby executed on behalf of each of said two corporations by their respective officers thereunto duly authorized.
BioRegenx, Inc.
A Nevada corporation
/s/William Resides
William Resides, Chief Executive Officer
Findit, Inc.
A Nevada corporation
/s/Raymond Firth
Raymond Firth, President
Annex B
Form of Amendment to Articles of Incorporation
[Seal of the State of Nevada]
FRANCISCO V. AGUILAR
Secretary of State
202 North Carson Street
Carson City, Nevada 89701-4201
(775) 684-5708
Website: www.nvsos.gov
Profit Corporation:
Certificate of Amendment (PURSUANT TO NRS 78.380 & 78.385/78.390)
Certificate to Accompany Restated Articles or Amended and Restated Articles (PURSUANT TO NRS 78.403)
Restated Articles (PURSUANT TO NRS 78.403)
Officer’s Statement (PURSUANT TO NRS 80.030)
TYPE OR PRINT - USE DARK INK ONLY - DO NOT HIGHLIGHT
| 1. | Entity Information: Name of entity as on file with the Nevada Secretary of State: |
Findit, Inc.
Entity or Nevada Business Identification Number (NVID): C30222-1998
| 2. | Restated or Amended and Restated Articles: (Select one) (If amending and restating only, complete section 1,2,3,5 and 6) |
[ ] Certificate to Accompany Restated Articles or Amended and Restated Articles
[ ] Restated Articles - No amendments; articles are restated only and are signed by an officer of the corporation who has been authorized to execute the certificate by resolution of the board of directors adopted on: ______. The certificate correctly sets forth the text of the articles or certificate as amended to the date of the certificate.
[ ] Amended and Restated Articles
* Restated or Amended and Restated Articles must be included with this filing type.
| 3. | Type of Amendment Filing Being Completed: (Select only one box) (If amending complete section 1,3,5 and 6.) |
[ ] Certificate of Amendment to the Articles of Incorporation (Pursuant to NRS 78.380 - Before Issuance of Stock)
The undersigned declare that they constitute at least two-thirds of the following:
(Check only one box) [ ] incorporators [ ] board of directors
The undersigned affirmatively declare that to the date of this certificate, no stock of the corporation has been issued.
[X] Certificate of Amendment to Articles of Incorporation (Pursuant to NRS 78.385 and 78.390 - After Issuance of Stock)
The vote by which the stockholders holding shares in the corporation entitling them to exercise at least a majority of the voting power, or such greater proportion of the voting power as may be required in the case of a vote by classes or series, or as may be required by the provisions of the articles of incorporation* have voted in favor of the amendment is: 78.14%
[ ] Officer’s Statement (foreign qualified entities only)-
Name in home state, if using a modified name in Nevada: _____
Jurisdiction of formation: _____
Changes to takes the following effect:
[ ] The entity name has been amended. [ ] Dissolution
[ ] The purpose of the entity has been amended. [ ] Merger
[ ] The authorized shares have been amended. [ ] Conversion
[ ] Other: (specify changes) _____
* Officer’s statement must be submitted with either a certified copy of or a certificate evidencing the filing of any document, amendatory or otherwise, relating to the original articles in the place of the corporations creation.
| 4. | Effective Date and Time: (Optional) Date: _____ Time: _____ |
| 5. | Information Being Changed: (Domestic corporations only) |
Changes to takes the following effect:
[ ] The entity name has been amended.
[ ] The registered agent has been changed. (attached Certificate of Acceptance from new registered agent)
[ ] The purpose of the entity has been amended.
[X] The authorized shares have been amended.
[ ] The directors, managers or general partners have been amended.
[ ] IRS tax language has been added.
[ ] Articles have been added.
[ ] Articles have been deleted.
[ ] Other.
The articles have been amended as follows: (provide article numbers, if available)
Article IV Capital Stock has been amended to read as follows: see attached
(attach additional page(s) if necessary)
| 6. | Signature: (Required) X /s/ Raymond Firth President |
Signature of Officer or Title
Authorized Signer
*If any proposed amendment would alter or change any preference or any relative or other right given to any class or series of outstanding shares, then the amendment must be approved by the vote, in addition to the affirmative vote otherwise required, of the holders of shares representing a majority of the voting power of each class or series affected by the amendment regardless to limitations or restrictions on the voting power thereof.
Please include any required or optional information in space below: (attach additional page(s) if necessary)
FINDIT, INC.
AMENDMENT TO THE ARTICLES OF INCORPORATION
ATTACHMENT
ARTICLE IV Capital Stock
The aggregate number of shares that the corporation shall have authority to issue is:
4,000,000,000 shares of Common Stock, par value $0.001
50,000,000 shares of Series A Preferred Stock, par value $0.001; and
5,000,000 shares of Series B Preferred Stock, par value $0.001; and
The stock may be issued from time to time without any action by the stockholder for such consideration as may be fixed from time to time by the Board of Directors and shares so issued, the full consideration of which has been paid or delivered, shall be deemed the full paid up stock, and the bolder of such share shall not be liable for any further payment for such shares. Such stock shall not be subject to assessment to pay the debts of the corporation, and no. paid up stock and no stock issued as fully paid shall ever be assessed or be assessable by the corporation The holders of such stock shall not be individually responsible for the debts, contracts, or liabilities of the corporation and shall not be liable for assessments to restore impairments in the capital of the