velocity at twelve months. Key secondary and additional endpoints include body proportionality and change in BMI, both evaluated after twelve months of weekly TransCon CNP treatment, and patient reported outcome measures. In December 2021, we announced that enrollment in ACcomplisH Trial was completed. All patients in the open-label extension portion of the ACcomplisH Trial continue on drug at the 100 microgram per kilogram dose.
We are planning regulatory submissions during the fourth quarter of 2022 for a new global randomized, double-blind, placebo-controlled Phase 2b trial in achondroplasia patients down to 2 years of age. In addition, we are assessing additional trials evaluating the safety and efficacy of TransCon CNP in achondroplasia.
In collaboration with VISEN, we are sponsoring the ACcomplisH China Trial, a randomized, double-blind, placebo-controlled, Phase 2 dose expansion trial to evaluate the safety and efficacy of TransCon CNP in subjects with achondroplasia. The primary endpoint is to evaluate the safety of treatment and its effect on
12-month
annualized height velocity. In January 2021, China Center for Drug Evaluation of National Medical Products Administration approved VISEN’s IND application to conduct the ACcomplisH China Trial.
In July 2020, we received orphan designation from the EC for TransCon CNP for treatment of achondroplasia.
In February 2019, we were granted orphan drug designation by the FDA for TransCon CNP for the treatment of achondroplasia.
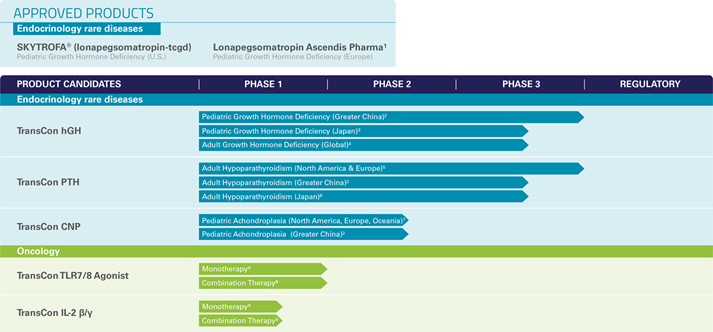
TransCon Products Candidates – Oncology
In January 2019, we established oncology as our second independent therapeutic area of focus for our TransCon technologies. Our goal is to improve treatment efficacy while limiting or reducing toxicity by applying TransCon technologies to clinically validated drugs, using our unique algorithm for product innovation.
We are currently investigating two clinical-stage product candidates designed to activate the patients’ own immune system to eradicate malignant cells. We believe our approach, if successfully developed, has the potential to optimize the efficacy of systemically administered, clinically validated therapies while limiting adverse effects.
Our TransCon product candidates in oncology are designed to provide sustained systemic or intratumoral administration, which we believe could provide potent and durable anti-tumor efficacy. Our nonclinical studies have showed sustained activation of cytotoxic immune cells that resulted in robust anti-tumor responses by TransCon product candidates using infrequent administration.
TransCon TLR7/8 Agonist is an investigational long-acting prodrug, designed for sustained release of resiquimod, a small molecule agonist of TLR 7 and 8. It is designed to provide sustained and potent activation of the innate immune system in the tumor and tumor draining lymph node and to have a low risk of systemic toxicity for weeks or months following a single intratumoral injection. Enrollment continues in the Phase 1/2
transcendIT-101
Trial for which we submitted an IND in 2020.
TransCon
IL-2
ß/
g
is an investigational long-acting prodrug designed to improve cancer immunotherapy through sustained release of an
IL-2
variant that selectively activates the
IL-2Rß/
g
, with minimal binding to
IL-2Rα.
The Phase 1/2
IL-ßelie
g
e Trial evaluating TransCon
IL-2
ß/
g
monotherapy in patients with advanced cancer is enrolling patients in dose escalation cohorts. During the second quarter of 2022, we dosed the first patient in the combination dose escalation cohort for TransCon
IL-2
ß/
g
and checkpoint inhibitor, in the
IL-ßelie
g
e Trial.
We are evaluating additional TransCon product candidates in nonclinical research studies with potential to enhance anti-tumor immune responses for the treatment of multiple tumor types. We are exploring product candidates using both systemic and intratumoral administration as monotherapies and as components of combination regimens. We believe these programs have the potential to make a positive impact to the lives of many patients with cancer.