As filed with the U.S. Securities and Exchange Commission on June 27, 2022
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AVALON GLOBOCARE CORP.
(Exact name of Registrant as specified in its charter)
| Delaware | 47-1685128 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification Number) |
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
732-780-4400
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
David Jin, MD, PhD
Chief Executive Officer
Avalon GloboCare Corp.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
732-780-4400
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Approximate date of commencement of proposed sale to the public: From time to time, after the effective date of this registration statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
The information in this prospectus is not complete and may be changed. Neither we nor the selling shareholders may sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
Subject to completion, dated June 27, 2022
PROSPECTUS

$50,000,000
Common Stock
Preferred Stock
Warrants
Units
and 6,198,237 Shares of Common Stock
We may offer, from time to time, in one or more offerings, common stock, preferred stock, warrants or units, which we collectively refer to as the “securities”. The aggregate initial offering price of the securities that we may offer and sell under this prospectus will not exceed $50,000,000. We may offer and sell any combination of the securities described in this prospectus in different series, at times, in amounts, at prices and on terms to be determined at, or prior to, the time of each offering. This prospectus describes the general terms of these securities and the general manner in which these securities will be offered. We will provide the specific terms of these securities in supplements to this prospectus. The prospectus supplements will also describe the specific manner in which these securities will be offered and may also supplement, update or amend information contained in this prospectus. This prospectus may not be used to consummate a sale of securities unless accompanied by the applicable prospectus supplement. You should read this prospectus and any applicable prospectus supplement before you invest.
In addition, the selling shareholder named in this prospectus may use this prospectus to offer and resell from time to time up to 6,198,237 shares of our common stock, par value $0.0001 per share, which are comprised of (i) 4,958,590 shares (the “Private Placement Note Shares”) of our common stock issuable upon conversion of a convertible note (“Convertible Note”) issued in a private placement in April 2022 (the “Private Placement”), pursuant to that certain Securities Purchase Agreement by and among us and the investor (the “Purchaser”) dated as of March 28, 2022 (the “Securities Purchase Agreement”) and (ii) 1,239,647 shares (the “Private Placement Warrant Shares” and together with the Private Placement Note Shares, the “Private Placement Shares”) of our common stock issuable upon the exercise of the warrants (the “Warrants”) issued in the Private Placement pursuant to the Securities Purchase Agreement.
We are registering the offer and resale of the Private Placement Note Shares and Private Placement Warrant Shares to satisfy the provisions of that certain Securities Purchase Agreement pursuant to which we agreed to register the resale of the Private Placement Note Shares and the Private Placement Warrant Shares.
We will not receive any of the proceeds from the sale of Private Placement Shares by the selling shareholder. We will, however, receive the net proceeds of any Warrants exercised for cash.
This prospectus describes some of the general terms that may apply to these securities. Each time we or a selling shareholder sell securities, to the extent required by applicable law, we will provide a supplement to this prospectus that contains specific information about the offering and the terms of the securities being offered. The supplement may also add, update or change information contained in this prospectus.
The selling shareholders will bear all commissions and discounts, if any, attributable to the sale or disposition of the Private Placement Shares, or interests therein and all costs, expenses and fees in connection with the registration of the Private Placement Shares. We will not be paying any underwriting discounts or commissions in this offering or costs, expenses, and fees in connection with the registration of the Private Placement Shares of common stock described in this prospectus. We will pay the expenses of registering the Private Placement Shares.
Our common stock is traded on The Nasdaq Capital Market under the symbol “AVCO.” On June 22, 2022, the last reported sale price of our common stock was $0.41 per share.
We may amend or supplement this prospectus from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements carefully before you make your investment decision.
You should carefully read this prospectus, all prospectus supplements and all other documents incorporated by reference in this prospectus before you invest in our securities.
An investment in our common stock involves a high degree of risk. See “Risk Factors” on page 17 of this prospectus for more information on these risks.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2022.
TABLE OF CONTENTS
You should rely only on the information provided in this prospectus, as well as the information incorporated by reference into this prospectus and any applicable prospectus supplement. Neither we nor the selling shareholders have authorized anyone to provide you with different information. Neither we nor the selling shareholders are making an offer of these securities in any jurisdiction where the offer is not permitted. You should not assume that the information in this prospectus, any applicable prospectus supplement or any documents incorporated by reference is accurate as of any date other than the date of the applicable document. Since the respective dates of this prospectus and the documents incorporated by reference into this prospectus, our business, financial condition, results of operations and prospects may have changed.
i
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission using a “shelf” registration process. Using this process, we may sell any combination of the securities described in this prospectus in one or more offerings up to a total dollar amount of $50,000,000 and the selling shareholders referred to in this prospectus and identified in supplements to the prospectus may also offer and sell our shares of common stock under this prospectus.
This prospectus provides you with a general description of the securities that we may offer. Each time we use this prospectus to offer securities, we will provide a prospectus supplement that will describe the specific terms of the offering. The prospectus supplement may also add to or update other information contained in this prospectus.
In making your investment decision, you should rely only on the information contained or incorporated by reference in this prospectus and any prospectus supplement we may authorize to be delivered to you. This prospectus incorporates important business and financial information about us that is not included in or delivered with this prospectus. You may obtain a copy of this information, without charge, as described in the “Where You Can Find More Information” section. We have not authorized anyone to provide you with any other information. If you receive any other information, you should not rely on it.
You should not assume that the information appearing in this prospectus is accurate as of any date other than the date on the front cover of this prospectus. You should not assume that the information contained in the documents incorporated by reference in this prospectus is accurate as of any date other than the respective dates of those documents. Our business, financial condition, results of operations, reserves and prospects may have changed since that date.
We encourage you to read this entire prospectus together with the documents incorporated by reference into this prospectus before making a decision whether to invest in our securities.
Unless the context otherwise requires or as otherwise noted, we use the terms “Avalon,” “company,” “we,” “us” and “our” in this prospectus to refer to Avalon GloboCare Corp. and its directly and indirectly owned subsidiaries taken as a whole.
ii
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference into this prospectus and any applicable prospectus supplement may contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (“Exchange Act”), about us and our subsidiaries. These forward-looking statements are intended to be covered by the safe harbor for forward-looking statements provided by the Private Securities Litigation Reform Act of 1995. Forward-looking statements are not statements of historical fact, and can be identified by the use of forward-looking terminology such as “believes,” “expects,” “may,” “will,” “could,” “should,” “projects,” “plans,” “goal,” “targets,” “potential,” “estimates,” “pro forma,” “seeks,” “intends,” or “anticipates” or the negative thereof or comparable terminology. Forward-looking statements include, among other things, statements about:
General Operating and Business Risks
| ● | Our business is subject to risks arising from epidemic diseases, such as the recent outbreak of the COVID-19 illness. |
| ● | Our limited operating history makes it difficult for us to evaluate our future business prospects and make decisions based on those estimates of our future performance. |
| ● | Our results of operations have not resulted in profitability and we may not be able to achieve profitability going forward. |
| ● | We depend upon key personnel and need additional personnel. |
| ● | Currently, we have several consulting contracts with related parties in China. The loss of such customers could adversely impact our financial condition and results of operations. |
| ● | Our auditors have issued an audit opinion which raises substantial doubt about our ability to continue as a going concern. |
| ● | We must effectively manage the growth of our operations, or our company will suffer. |
| ● | Our business requires substantial capital, and if we are unable to maintain adequate financing sources our profitability and financial condition will suffer and jeopardize our ability to continue operations. |
| ● | Our revenue and results of operations may suffer if we are unable to attract new clients, continue to engage existing clients, or sell additional products and services. |
| ● | Our prospects will suffer if we are not able to hire, train, motivate, manage, and retain a significant number of highly skilled employees. |
| ● | Potential liability claims may adversely affect our business. |
| ● | In accordance with our strategic development policy, we may invest in companies for strategic reasons and may not realize a return on our investments. |
| ● | Our growing operations in the PRC could expose us to risks that could have an adverse effect on our costs of operations. |
| ● | We face intense competition which could cause us to lose market share. |
| ● | If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business. |
| ● | We may face uncertainty and difficulty in obtaining and enforcing our patents and other proprietary rights. |
| ● | We may not be able to protect our intellectual property rights throughout the world. |
| ● | Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time. |
| ● | Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and any patent protection we may obtain in the future could be reduced or eliminated for non-compliance with these requirements. |
iii
| ● | It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection. If we fail to protect or enforce our intellectual property rights adequately or secure rights to patents of others, the value of our intellectual property rights would diminish. |
| ● | We may be subject to claims challenging the inventorship of patents and other intellectual property. |
| ● | If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer. |
| ● | We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to protect our rights to, or use of, our technology. |
| ● | Breaches or compromises of our information security systems or our information technology systems or infrastructure could result in exposure of private information, disruption of our business and damage to our reputation, which could harm our business, results of operation and financial condition. |
| ● | We may be exposed to liabilities under the Foreign Corrupt Practices Act, and any determination that we violated the Foreign Corrupt Practices Act or Chinese anti-corruption law could have a material adverse effect on our business. |
Risk Factors Related to Clinical and Commercialization Activity
| ● | We may not be able to file INDs to commence additional clinical trials on the timelines we expect, and even if we are able to do so, the FDA may not permit us to proceed. |
| ● | We have limited experience in conducting clinical trials. |
| ● | Delays in the commencement, enrollment, and completion of clinical testing could result in increased costs to us and delay or limit our ability to obtain regulatory approval for our product candidates. |
| ● | Our success depends upon the viability of our product candidates and we cannot be certain any of them will receive regulatory approval to be commercialized. |
| ● | As the results of earlier pre-clinical studies or clinical trials are not necessarily predictive of future results, any product candidate we advance into clinical trials may not have favorable results in later clinical trials or receive regulatory approval. |
| ● | Our business faces significant government regulation, and there is no guarantee that our product candidates will receive regulatory approval. |
| ● | Even if our product candidates receive regulatory approval, we may still face future development and regulatory difficulties. |
| ● | If we or current or future collaborators, manufacturers, or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions and substantial penalties, which could affect our ability to develop, market and sell our products and may harm our reputation. |
| ● | Any cell based therapies we develop may become subject to unfavorable pricing regulations, third party coverage and reimbursement practices or healthcare reform initiatives, thereby harming our business. |
| ● | The healthcare industry is heavily regulated in the U.S. at the federal, state, and local levels, and our failure to comply with applicable requirements may subject us to penalties and negatively affect our financial condition. |
| ● | Our ability to obtain reimbursement or funding from the federal government may be impacted by possible reductions in federal spending. |
iv
Risks Related to Doing Business in China
| ● | Trading in Avalon’s securities may be restricted under the Holding Foreign Companies Accountable Act if the PCAOB determines that it cannot inspect or fully investigate Avalon’s auditors, and as a result, U.S. national securities exchanges, such as Nasdaq, may determine to delist Avalon’s securities. | |
| ● | Our business might be subject to various evolving PRC laws and regulations regarding data privacy and cybersecurity. Failure of cybersecurity and data security compliance could subject us to penalties, damage our reputation and brand and harm our business and results of operations. | |
| ● | If we become directly subject to the recent scrutiny, criticism and negative publicity involving certain U.S.-listed Chinese companies, we may have to expend significant resources to investigate and resolve the matter which could harm our business operations, stock price and reputation and could result in a loss of your investment in our stock, especially if such matter cannot be addressed and resolved quickly. |
| ● | Adverse changes in political and economic policies of the PRC government could impede the overall economic growth of China, which could reduce the demand for our products and damage our business. |
| ● | Uncertainties with respect to the PRC legal system could limit the legal protections available to you and us. |
| ● | The PRC government exerts substantial influence over the manner in which Avalon must conduct its business activities and Avalon may face the risk that the future regulatory actions by the PRC government could significantly limit or completely hinder Avalon’s ability to offer future securities to investors. | |
| ● | Under the current Enterprise Income Tax, or EIT, law, we may be classified as a “resident enterprise” of China. Such classification will likely result in unfavorable tax consequences to us and our non- PRC stockholders. | |
| ● | We may be unable to complete a business combination transaction efficiently or on favorable terms due to complicated merger and acquisition regulations implemented on September 8, 2006. |
| ● | We may be subject to fines and legal sanctions if we or our Chinese employees fail to comply with PRC regulations relating to employee stock options granted by overseas listed companies to PRC citizens. |
| ● | The new M&A Rules establish more complex procedures for some acquisitions of Chinese companies by foreign investor which could make it more difficult for us to pursue growth through acquisitions in China. |
| ● | Government control of currency conversion and future movements in exchange rates may adversely affect our operations and financial results. |
Risks Related to Our Securities
| ● | If we are unable to maintain listing of our securities on the Nasdaq Capital Market or another reputable stock exchange, it may be more difficult for our stockholders to sell their securities. | |
| ● | The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our stockholders. |
| ● | Future sales of our common stock or securities convertible or exchangeable for our common stock may cause our stock price to decline. |
| ● | You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock. |
| ● | The ability of our Board of Directors to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger. |
| ● | We are a “smaller reporting company,” and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors. |
| ● | If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline. |
| ● | Our officers, directors and principal stockholders own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval. |
| ● | We do not anticipate paying dividends on our common stock, and investors may lose the entire amount of their investment. |
v
| ● | Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of our business and our ability to obtain or retain listing of our common stock on a national securities exchange. |
| ● | If we cannot satisfy, or continue to satisfy, the initial listing requirements and other rules of the Nasdaq Capital Market, our securities may be delisted, which could negatively impact the price of our securities and your ability to sell them. |
| ● | We could be subject to securities class action litigation. |
| ● | The novel coronavirus (COVID-19) outbreak has disrupted and is expected to continue to disrupt our business, which has and could continue to materially affect our operations, financial condition and results of operations for an extended period of time. |
Any one or more of these uncertainties, risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information, future events or otherwise.
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
We caution our shareholders and other readers not to place undue reliance on such statements.
You should read this prospectus and the documents incorporated by reference completely and with the understanding that our actual future results may be materially different from what we currently expect. Our business and operations are and will be subject to a variety of risks, uncertainties and other factors. Consequently, actual results and experience may materially differ from those contained in any forward-looking statements. Such risks, uncertainties and other factors that could cause actual results and experience to differ from those projected include, but are not limited to, the risk factors set forth herein under the title “Risk Factors,” in our Annual Report on Form 10-K for the year ended December 31, 2021, and any updates described in our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and elsewhere in the documents incorporated by reference into this prospectus and any applicable prospectus supplement.
You should assume that the information appearing in this prospectus and any document incorporated herein by reference is accurate as of its date only. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which the statement is made. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. All written or oral forward-looking statements attributable to us or any person acting on our behalf made after the date of this prospectus and any applicable prospectus supplement are expressly qualified in their entirety by the risk factors and cautionary statements contained in and incorporated by reference into this prospectus and any applicable prospectus supplement. Unless legally required, we do not undertake any obligation to release publicly any revisions to such forward-looking statements to reflect events or circumstances after the date of this prospectus and any applicable prospectus supplement or to reflect the occurrence of unanticipated events.
vi
The following summary highlights some information from this prospectus. It is not complete and does not contain all of the information that you should consider before making an investment decision. You should read this entire prospectus, including the “Risk Factors” section on page 17, the financial statements and related notes and the other more detailed information appearing elsewhere or incorporated by reference into this prospectus and any applicable prospectus supplement.
Overview
The Company is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients’ growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and COVID-19 related vaccine and therapeutics.
Avalon achieves and fosters seamless integration of unique verticals to bridge and accelerate innovative research, bio-process development, clinical programs and product commercialization. Avalon’s upstream innovative research includes:
| ● | Development of Avalon Clinical-grade Tissue-specific Exosome (“ACTEX™”) |
| ● | Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of a hemofiltration device to treat Cytokine Storm. |
| ● | Co-development of next generation, transposon-based, multi-target CAR-T, CAR-NK and other immune effector cell therapeutic modalities with Arbele Limited. |
| ● | Strategic partnership with the University of Natural Resources and Life Sciences (BOKU) in Vienna, Austria to develop an S-layer vaccine that can be administered by an intranasal or oral route against SARS-CoV-2, the novel coronavirus that causes COVID-19 disease. |
Avalon’s midstream bio-processing and bio-production facility is located in Nanjing, China with state-of-the-art, automated GMP and QC/QA infrastructure for standardized bio-manufacturing of clinical-grade cellular products involved in our clinical programs in immune effector cell therapy, regenerative therapeutics, as well as bio-banking.
Avalon’s downstream medical team and facility consists of top-rated affiliated hospital network and experts specialized in hematology, oncology, cellular immunotherapy, hematopoietic stem/progenitor cell transplant, as well as regenerative therapeutics. Our major clinical programs include:
| ● | AVA-001: Avalon has initiated its first-in-human clinical trial of CD19 CAR-T candidate, AVA-001 in August 2019 at the Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital in China (the world’s single largest CAR-T treatment network with over 600 patients being treated with CAR-T) for the indication of relapsed/refractory B-cell acute lymphoblastic leukemia and non-Hodgkin Lymphoma. The AVA-001 candidate (co-developed with China Immunotech Co. Ltd) is characterized by the utilization of 4-1BB (CD137) co-stimulatory signaling pathway, conferring a strong anti-cancer activity during pre-clinical study. It also features a shorter bio-manufacturing time which leads to the advantage of prompt treatment to patients where timing is important related hematologic malignancies. Avalon has successfully completed the first-in-human clinical trial of its AVA-001 anti-CD19 CAR-T cell therapy as a bridge to allogeneic bone marrow transplantation for patients with relapsed/refractory B-cell acute lymphoblastic leukemia at the Lu Daopei Hospital (registered clinical trial number NCT03952923) with excellent efficacy (90% complete remission rate) and minimal adverse side effects. Avalon is currently expanding the patient recruitment for AVA-001 to include relapsed/refractory non-Hodgkin lymphoma patients. |
1
| ● | AVA-011 and FLASH-CAR™: The Company advanced its next generation immune cell therapy using RNA-based, non-viral FLASH-CAR™ technology co-developed with the Company’s strategic partner Arbele Limited. The adaptable FLASH-CAR™ platform can be used to create personalized cell therapy from a patient’s own cells, as well as off-the-shelf cell therapy from a universal donor. Our leading candidate, AVA-011, is currently at process development stage to generate clinical-grade cell-therapy products for subsequent clinical studies. On July 8, 2021, the Company and the University of Pittsburgh of the Commonwealth System of Higher Education (the “University”) entered into a Corporate Research Agreement (the “University Agreement”). Pursuant to the University Agreement, for a term of two years the University agreed to use its reasonable efforts to perform academic research funded by the Company in connection with the development of point-of-care modular autonomous processing system to generate clinical-grade AVA-011, a RNA-based chimeric antigen receptor (CAR) T-cell therapy candidate (the “Project”) subject to the appointment of Dr. Yen Michael S. Hsu as Principal Investigator. During the term, the Company agreed to make eight payments of $125,000 to the University. As of March 31, 2022, the Company did not make any payment. The Company and the University shall each own an undivided, one half interest in any intellectual property rights jointly developed by both parties. The Company has been granted a worldwide, irrevocable, non-exclusive, royalty free, fully paid-up, perpetual right to use intellectual property developed by the University in connection with the Project for commercial purposes research activities and other purposes. Further, the Company will have an exclusive right of first offer to an exclusive royalty-bearing license to intellectual property developed by the University or co-developed by the Company and the University in connection with the Project. | |
| ● | ACTEX™: Stem cell-derived Avalon Clinical-grade Tissue-specific Exosomes (ACTEX™) is one of the core technology platforms that has been co-developed by Avalon GloboCare and the University of Pittsburgh Medical Center. The Company formed a strategic partnership with HydroPeptide, LLC, a leading epigenetics skin care company, to engage in co-development and commercialization of a series of clinical-grade, exosome-based cosmeceutical and orthopedic products. As part of this agreement, the Company signed a three-way Material Transfer Agreement between Avalon GloboCare, HydroPeptide and the University of Pittsburgh Medical Center. | |
| ● | AVA-Trap™: Avalon’s AVA-Trap™ therapeutic program plans to enter animal model testing followed by expedited clinical studies with the goal of providing an effective therapeutic option to combat COVID-19 and other life-threatening conditions involving cytokine storms. The Company initiated a sponsored research and co-development project with Massachusetts Institute of Technology (MIT) led by Professor Shuguang Zhang as Principal Investigator in May 2019. Using the unique QTY code protein design platform, six water-soluble variant cytokine receptors have been successfully designed and tested to show binding affinity to the respective cytokines. |
For the year ended December 31, 2021 we generated revenue by providing medical related consulting services in advanced areas of immunotherapy and second opinion/referral services through our wholly-owned subsidiary Avalon (Shanghai) Healthcare Technology Co., Ltd., or Avalon Shanghai. We also own and operate rental commercial real property in New Jersey, where we are headquartered.
For the three months ended March 31, 2022, we did not generate any medical related consulting services revenue.
COVID-19 has not significantly impacted Company operations or the work performed as part of our clinical trials in China. The clinical trials are being conducted at Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital. Both hospitals are considered primarily hematology specialty hospitals and experienced minor disruption as part of the pandemic.
While Avalon is not a People’s Republic of China (the “PRC”) operating company, certain of its subsidiaries are PRC operating companies and through them Avalon currently has operations in PRC, which involves unique risks. See “China Operations” below, and “Risk Factors—Risks Related to Doing Business in China.”
Corporate Information/Company History
We were incorporated under the laws of the State of Delaware on July 28, 2014 under the name Global Technologies Corp.
We own 100% of the capital stock of Avalon Healthcare Systems, Inc., a Delaware corporation, or AHS, which we acquired on October 19, 2016. AHS was incorporated on May 18, 2015 under the laws of the State of Delaware. In addition, we own through AHS 100% of the capital stock of Avalon (Shanghai) Healthcare Technology Co., Ltd., or Avalon Shanghai, which is a wholly foreign-owned enterprise, or WOFE, organized under the laws of the People’s Republic of China, or PRC or China. Avalon Shanghai was incorporated on April 29, 2016 and is engaged in medical related consulting services for customers. On January 23, 2017, we incorporated Avalon (BVI) Ltd, a British Virgin Islands company (dormant and in process of being dissolved). On February 7, 2017, we formed Avalon RT 9 Properties, LLC, a New Jersey limited liability company. In July 2017, we formed Genexosome Technologies Inc., a Nevada corporation, or Genexosome. Effective October 25, 2017, Genexosome owns 100% of the capital stock of Beijing Jieteng (Genexosome) Biotech Co., Ltd., a corporation incorporated in the People’s Republic of China on August 7, 2015 (“Beijing Genexosome”), and the Company holds 60% of Genexosome and Dr. Yu Zhou holds 40% of Genexosome. Both Genexosome and Beijing Genexosome are inactive now.
2
On May 29, 2018, Avalon Shanghai entered into a Joint Venture Agreement with Jiangsu Unicorn Biological Technology Co., Ltd., or Unicorn, pursuant to which a company named Epicon Biotech Co., Ltd. (“Epicon”) was formed on August 14, 2018. Epicon is owned 60% by Unicorn and 40% by Avalon Shanghai. Within five years of execution of the Joint Venture Agreement, Unicorn shall invest cash into Epicon in an amount not less than RMB 8,000,000 (approximately $1.2 million) and the premises of the laboratories of Nanjing Hospital of Chinese Medicine for exclusive operation by Epicon, and Avalon Shanghai shall invest cash into Epicon in an amount not less than RMB 10,000,000 (approximately $1.5 million). The board of directors of Epicon shall consist of five members with Unicorn appointing three members and Avalon Shanghai appointing two members. As of March 31, 2022, Unicorn has invested the premises of the laboratories of Nanjing BENQ hospital as GMP level research and manufacture facility and Avalon Shanghai has contributed RMB 4,760,000 (approximately $0.7 million). Epicon is focused on cell preparation, third party testing, biological sample repository for commercial and scientific research purposes and the clinical transformation of scientific achievements.
On July 18, 2018, we formed Avactis Biosciences Inc. (“Avactis”), a Nevada corporation, as a wholly owned subsidiary. On October 23, 2018, Avactis and Arbele Limited (“Arbele”) agreed to the establishment of AVAR BioTherapeutics (China) Co. Ltd. (“AVAR”), a Sino-foreign equity joint venture, pursuant to an Equity Joint Venture Agreement (the “AVAR Agreement”), which was to be owned 60% by Avactis and 40% by Arbele. On April 6, 2022, the Company, Acactis, Arbele and Arbele Biotherapeutics Limited (“Arbele Biotherapeutics”), a wholly owned subsidiary of Arbele, entered into an Amendment No. 1 to the Equity Joint Venture Agreement pursuant to which Arbele Biotherapeutics acquired 40% of Avactis for the purpose of the Company and Arbele establishing a joint venture in the United States and the parties agreed that they would no longer pursue AVAR as a joint venture. Further, all rights and obligations under the AVAR Agreement were assigned by Avactis to Avalon and by Arbele to Arbele Biotherapeutics. Avactis established Avactis Nanjing Biosciences Ltd., a wholly owned foreign entity in the PRC. Further, the parties agreed that the Exclusive Patent License Agreement dated January 3, 2019 entered between Arbele, as licensor, and AVAR, as licensee (the “Arbele License Agreement”), was assigned to Avactis and Avalon and Arbele agreed to enter into a new Arbele License Agreement with Avactis on the same/similar terms as the Arbele License Agreement. Further, Dr. Anthony Chan was appointed to the Board of Directors of Avactis and as the Chief Scientific Officer of Avactis. Avactis purpose and business scope is to research, research, develop, produce, sell, distribute and generally commercialize CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy globally including in the PRC. Avactis is required to contribute $10 million (or equivalent in RMB) in cash and/or services, which shall be contributed in tranches based on milestones to be determined jointly by AVAR and Avactis in writing subject to Avactis’ cash reserves. Within 30 days, Arbele shall make contribution of $6.66 million in the form of entering into a License Agreement with AVAR granting AVAR with an exclusive right and license in China to its technology and intellectual property pertaining to CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy technology and any additional technology developed in the future with terms and conditions to be mutually agreed upon Avactis and AVAR and services. As of the date hereof, the License Agreement has not been finalized.
The following diagram illustrates our corporate structure:
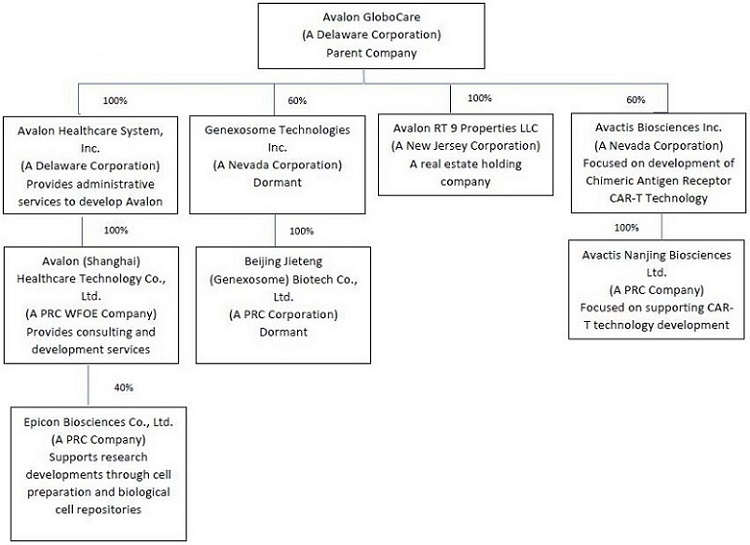
3
On June 13, 2021, the Company entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang BVI”), the holders of the share capital of Sen Lang BVI (the “Sen Lang BVI Shareholders”), the ultimate beneficial owners of the Sen Lang BVI Shareholders (the “Sen Lang BVI Beneficial Shareholders” and, together with the Sen Lang BVI Shareholders, the “Sen Lang BVI Owners”) and a representative of the Sen Lang BVI Owners (the “Sen Lang BVI Representative”). On January 1, 2022, the Company, on the one hand, and Sen Lang BVI, the Sen Lang Shareholders, the Sen Lang Beneficial Shareholders and Ding Wei, in his capacity as the Sen Lang Representative, on the other hand, terminated the Purchase Agreement.
China Operations
Certain of Avalon’s subsidiaries are PRC operating companies, and through them Avalon currently has operations in the People’s Republic of China, which involves unique risks.
The method by which cash is transferred in Avalon’s organization, in light of its PRC subsidiaries, is complex. The payment and amount of any future dividend of the PRC subsidiaries to Avalon will be restricted by PRC laws and regulations regarding dividends and PRC foreign exchange regulations. PRC laws require that dividends be paid only out of the profit for the year calculated according to PRC accounting principles. PRC laws also require foreign-invested enterprises to set aside at least 10% of their after-tax profits as the statutory common reserve fund until the cumulative amount of the statutory common reserve fund reaches 50% or more of such enterprises’ registered capital, if any, to fund its statutory common reserves, which are not available for distribution as cash dividends. Avalon and, ultimately, Avalon stockholders will receive the economic benefit of its PRC subsidiaries by way of dividends, which are subject to restrictions under current United States (“U.S.”) laws and regulations regarding dividends. Furthermore, under applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities within ten years after the taxable year when the transactions are conducted. Avalon and its subsidiaries may face material and adverse tax consequences if the PRC tax authorities determine that the contractual arrangements were not entered into on an arm’s length basis.
Pursuant to the PRC Enterprise Income Tax Law, a withholding tax rate of 10% currently applies to dividends paid by a PRC resident enterprise to a foreign enterprise investor, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for preferential tax treatment. Avalon currently believes that its PRC subsidiaries’ distribution of dividends to Avalon, if any, shall be subject to a withholding tax rate of 10%, unless a reduced rate under a tax treaty is applicable. Avalon reported net losses and had negative net cash flows from operations in 2021. No net income will be generated from Avalon’s PRC subsidiaries’ operations in the foreseeable future and therefore no dividends or distributions will be paid by such subsidiaries to Avalon and its stockholders in the foreseeable future. However, if such subsidiaries do make distributions of cash or property to Avalon, absent a distribution by Avalon to the U.S. holders of Avalon common stock, there would be no flow-through of such income to the U.S. holders of Avalon common stock for U.S. federal income tax purposes. As of the date of this report, no transfers, dividends or distributions from our PRC subsidiaries to Avalon have been made to date.
As described below under “Holding Foreign Companies Accountable Act Compliance,” the Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. According to the HFCA Act, if the SEC determines that Avalon has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in 2021, the SEC will prohibit Avalon’s securities from being traded on a national securities exchange or in the over-the-counter trading market in the United States. Avalon’s auditor is Marcum LLP (“Marcum”), based in New York, New York. Marcum is registered with the PCAOB and is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. Since Marcum is located in the United States, the PCAOB has been able to conduct inspections of Marcum. In addition, Marcum is not among the PCAOB registered public accounting firms registered in mainland China or Hong Kong that are subject to PCAOB’s determination on December 16, 2021. Although Avalon is currently not subject to the HFCA Act, any uncertainty of its applicability to Avalon, for example if Avalon switched to using a PRC-based auditing firm, could cause the market price of Avalon’s securities to be materially and adversely affected and could cause Avalon’s securities to be delisted or prohibited from being traded “over-the-counter”. If Avalon’s securities are unable to be listed on another securities exchange, such a delisting would substantially impair your ability to sell or purchase Avalon’s securities when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of Avalon’s securities.
4
Moreover, Avalon’s business operations in the PRC are governed by PRC laws, rules and regulations. The associated legal and operational risks could result in a material change in the business operations of Avalon’s PRC subsidiaries and could negatively impact the value of Avalon’s common stock or could even cause the value of such securities to significantly decline or be worthless. The PRC government has recently announced its plans to enhance its regulatory oversight of Chinese companies listing overseas, and there is some uncertainty with respect to the interpretation and implementation of such plans. The PRC government has also issued statements and has undertaken regulatory actions related to the use of variable interest entities, data security and anti-monopoly concerns. The PRC government may promulgate relevant laws, internal rules and regulations that may impose additional and significant obligations and liabilities on overseas listed Chinese companies regarding data security, cross-border data flow, compliance with PRC securities laws and anti-monopoly laws. These laws and regulations can be complex and stringent, and can be subjected to change and uncertain interpretation, which could limit Avalon’s ability to conduct its business and accept foreign investments, or could significantly impact its operating results and stock price. However, because Avalon is the issuer of the common stock listed on Nasdaq and is a Delaware operating and holding company, no approval or permission is required under current applicable PRC laws and regulations for any future issuances of Avalon securities to non-PRC investors. Nevertheless, PRC laws, regulations and/or their interpretations may change in the future, such that they may have an extraterritorial effect, whereby Avalon may be required to obtain such approval or permission under PRC laws and regulations. In such event, Avalon may face the risk that these future regulatory actions by the PRC government could significantly limit or completely hinder Avalon’s ability to offer future securities to investors. Under this scenario, Avalon’s ability to raise capital and thereby execute its business plan would be significantly limited or completely hindered, which would likely result in a material change in Avalon’s operations and the value of Avalon’s common stock, including that it could cause the value of such securities to significantly decline or become worthless. In addition, Avalon faces the risk that Avalon may not currently ascertain, and therefore may not actually have, all requisite permissions to offer securities, which would likely result in a material change in Avalon’s operations and/or value of Avalon’s common stock, including that it could cause the value of such securities to significantly decline or become worthless. See “Risk Factors—The PRC government exerts substantial influence over the manner in which Avalon must conduct its business activities and Avalon may face the risk that the future regulatory actions by the PRC government could significantly limit or completely hinder Avalon’s ability to offer future securities to investors.
Sales and Marketing
We seek to develop new business through relationships driven by our senior management, which have extensive contacts throughout the healthcare system. Our senior management will be seeking opportunities for joint ventures, strategic relationships and acquisitions in consulting, biomedical innovations, laboratory, and medical device companies.
Services
We currently generate revenue from related party strategic relationships through Avalon Shanghai that provide consultative services in advanced areas of immunotherapy and second opinion/referral services. In addition, our services are targeted at serving our clients and using our insights and deep expertise to produce tangible and significant results. Our services include research studies, executive education, daily online executive briefings, tailored expert advisory services, and consulting and management services. Through our services, we attempt to have our clients focus on important problems by providing an analysis of the evolving healthcare industry and the methods prevalent in the industry to solve those problems through counsel, business planning and support. We tailor these solutions to the client’s specific strategic challenges, operational issues, and management concerns.
Strategic Partnerships and Acquisitions
We are actively seeking potential strategic partnerships in our area of focus. In addition, we are actively seeking target acquisitions that add accretive value to our strategic plan. There is no guarantee that we will be able to successfully sign a definitive agreement, close or implement such business arrangement.
Markets
We focus on the following markets in developing our core business:
| ● | Cellular Immunotherapy in Oncology: Regarded as the future of medicine, we believe cell-based technologies and therapeutics will replace pharmaceuticals as a more effective and functional modality in certain unmet medical areas. We are actively engaging in this revolutionary trend and positioning to take a leading role in immune effector cell therapies in the immuno-oncology domain, particularly related to the development of Chimeric Antigen Receptor (CAR) T cell and CAR-NK cell therapies against hematologic malignancies. CAR-T cell therapy is considered as a “living drug” which involves isolation of a patient’s peripheral T cells and re-engineering these T cells with CAR molecules equipped with a weapon attacking a specific target on tumor cells. Our leading candidate is “AVA-001”, an anti-CD19 CAR-T which has successfully completed first-in-human clinical trial for relapsed/refractory (R/R) B-cell lymphoblastic leukemia (B-ALL); we are in the process of expanding patient recruitment to include R/R non-Hodgkin’s lymphoma. We are also developing a RNA-based “FASH-CARTM” cell therapy platform, which may potentially reduce manufacturing time and cost. The lead candidate, “AVA-011”, has completed pre-clinical laboratory studies and currently undergoing IND-enabling process development stage to generate cGMP-grade AVA-011 CAR-T cells for upcoming clinical trials. |
| ● | Regenerative Medicine: Avalon Clinical-grade Tissue-specific Exosome (“ACTEX™”) is a technology platform to generate clinical-grade exosomes from stem/progenitor cells, with potential regenerative applications in skin care and orthopedic joint repair. |
5
| ● | QTY-Code Protein Design: Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of a hemofiltration device to treat Cytokine Storm (aka Cytokine Release Syndrome). QTY-code can be applied to generate water-soluble, antibody-like molecular variants of native membrane-bound receptors, which may expand the repertoire of therapeutic targets in CAR-T cell therapies. |
| ● | S-Layer based Vaccine Development: Strategic partnership with the University of Natural Resources and Life Sciences (BOKU) in Vienna, Austria to develop an S-layer based vaccine that can be administered by an intranasal or oral route against SARS-CoV-2 (the novel coronavirus that causes COVID-19), Influenza A/B and other respiratory pathogens. |
Revenue
Avalon RT 9 Properties, LLC
In May 2017, we acquired commercial property located in Freehold, New Jersey. This property is now our corporate headquarters and contains several commercial tenants that generate revenue through rental income.
Avalon Shanghai
We currently generate revenue by providing medical related consulting services in advanced areas of immunotherapy and second opinion/referral services through Avalon (Shanghai) Healthcare Technology Co., Ltd., or Avalon Shanghai. Our medical related consulting services include research studies, executive education, daily online executive briefings, tailored expert advisory services, and consulting and management services. Through our services we attempt to have our clients focus on important problems by providing an analysis of the evolving healthcare industry and the methods prevalent in the industry to solve those problems through counsel, business planning and support. The revenue generated from our related parties in China is managed by our employees residing in China and contactors who are retained as needed. Consulting services have been provided by Avalon Shanghai under the contract include:
| ● | providing scientific research consulting services; | |
| ● | integrating experts, medical institutions and other resources in the United States in support of scientific research; | |
| ● | providing technical education and training; and | |
| ● | assisting in publication of academic papers. |
Strategic Development
We intend to pursue the acquisition and development of healthcare related technologies for cell related diagnostics and therapeutics through acquisition, licensing or joint ventures with major universities and biotech companies. We will also consider a third avenue of investing in certain technologies for cell related diagnostics and therapeutics and are seeking laboratory or medical device acquisitions.
Intellectual Property
Our goal is to obtain, maintain and enforce patent rights for our products, formulations, processes, methods of use and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and abroad. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product candidates and any future product candidates, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the United States and abroad. Even patent protection, however, may not always afford us with complete protection against competitors who seek to circumvent our patents. If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish. To this end, we require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure and use of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions relevant to our technologies and important to our business.
6
Competition
Avalon Shanghai
In our current consulting business in the People’s Republic of China, or PRC or China, we compete with a number of advisory firms offering similar service including consulting and strategy firms; market research, data, benchmarking, and forecasting providers; technology vendors and services firms; healthcare information technology firms; technology advisory firms; outsourcing firms; and specialized providers of educational and training services. Other organizations, such as state and national trade associations, group purchasing organizations, non-profit think-tanks, and database companies, also may offer research, consulting, tools, and education services to health care and education organizations.
We believe that the principal competitive factors in our market include quality and timeliness of our services, strength and depth of relationships with our clients, ability to meet the changing needs of current and prospective clients, measurable returns on customer investment, and service and affordability.
As our business develops and we expand through joint ventures, acquisitions and strategic partnerships in the U.S. and PRC, we will have competition with other direct service providers, emerging technologies and medical communication platforms. We will seek to maintain a competitive advantage through intellectual property, superior quality management and cutting-edge technology.
Avalon RT 9 Properties LLC
Our executive commercial building in Freehold, New Jersey is located on a major highway and is one of the largest buildings in the surrounding areas. It is centrally located and maintains high occupancy. There are other commercial properties in the vicinity that offer similar amenities. However, premier executive offices are limited and as such we expect to continue to maintain high occupancy in the near term.
Employees
As of June 27, 2022, we employed six employees, five of which are full time employees. None of our employees are represented by a collective bargaining arrangement.
Government Regulation
Overview
The healthcare industry in the PRC and U.S. is highly regulated and subject to changing political, legislative, regulatory, and other influences. Further, the healthcare industry is currently undergoing rapid change. We are uncertain how, when or in what context these new changes will be adopted or implemented. These new regulations could create unexpected liabilities for us, could cause us or our members to incur additional costs and could restrict our or our clients’ operations. Many of the laws are complex and their application to us, our clients, or the specific services and relationships we have with our members are not always clear. Our failure to anticipate accurately the application of these laws and regulations, or our other failure to comply, could create liability for us, result in adverse publicity, and otherwise negatively affect our business.
PRC Regulation
Despite efforts to develop its legal system over the past several decades, including but not limited to legislation dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade, the PRC continues to lack a comprehensive system of laws. Further, the laws that do exist in the PRC are often vague, ambiguous and difficult to enforce, which could negatively affect our ability to do business in China and compete with other companies in our segments.
In September 2006, the Ministry of Commerce, or MOFCOM, promulgated the Regulations on Foreign Investors’ Mergers and Acquisitions of Domestic Enterprises, or the M&A Regulations, in an effort to better regulate foreign investment in the PRC. The M&A Regulations were adopted in part as a needed codification of certain joint venture formation and operating practices, and also in response to the government’s increasing concern about protecting domestic companies in perceived key industries and those associated with national security, as well as the outflow of well-known trademarks, including traditional Chinese brands.
7
As a U.S. based company doing business in the PRC, we seek to comply with all PRC laws, rules and regulations and pronouncements, and endeavor to obtain all necessary approvals from applicable PRC regulatory agencies such as the MOFCOM, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration for Industry and Commerce, the China Securities Regulatory Commission, and the State Administration of Foreign Exchange, or SAFE.
Our PRC subsidiary, Avalon Shanghai, provides outsourced and customized healthcare services to the rapidly changing health care industry. Currently, our PRC subsidiary, Beijing Genexosome, is dormant. These subsidiaries have obtained their respective business licenses, which permit each of them to operate its business in the PRC. No other special permission is required for our PRC subsidiaries to conduct their respective current business under applicable PRC regulations and laws. Additionally, the operation of Avalon and its PRC subsidiaries are not covered by permissions requirements of the China Securities Regulatory Commission (CSRC) or the Cyberspace Administration of China (CAC).
Because Avalon is the issuer of the common stock listed on Nasdaq and is a Delaware operating and holding company, no approval or permission is required under current applicable PRC laws and regulations for any future issuances of Avalon securities to non-PRC investors. Nevertheless, according to the Opinions of the General Office of the CPC Central Committee and the General Office of the State Council on Strictly Cracking Down on Illegal Securities Activities in accordance with the Law (“Opinions”), the PRC intends to establish and improve the system of extraterritorial application of the PRC securities laws. Although the details of the extraterritorial application of the PRC securities laws are still scarce as of the date of this report, PRC laws, regulations and/or their interpretations may change in the future, such that they have may an extraterritorial effect, whereby Avalon may be required to obtain such approval or permission under PRC laws and regulations. In such event, Avalon may face the risk that these future regulatory actions by the PRC government could significantly limit or completely hinder Avalon’s ability to offer future securities to investors. Under this scenario, Avalon’s ability to raise capital and thereby execute its business plan would be significantly limited or completely hindered, which would likely result in a material change in Avalon’s operations and the value of Avalon’s common stock, including that it could cause the value of such securities to significantly decline or become worthless. In addition, Avalon faces the risk that Avalon may not currently ascertain, and therefore may not actually have, all requisite permissions to offer securities, which would likely result in a material change in Avalon’s operations and/or value of Avalon’s common stock, including that it could cause the value of such securities to significantly decline or become worthless.
The Flow of Economic Benefits from PRC Subsidiaries
The payment and amount of any future dividend of Avalon’s PRC subsidiaries to Avalon will be restricted by PRC laws and regulations regarding dividends and PRC foreign exchange regulations. PRC laws require that dividends be paid only out of the profit for the year calculated according to PRC accounting principles, which differ in certain respects from the generally accepted accounting principles in other jurisdictions, including accounting principles generally accepted in the United States of America, or US GAAP, and international financial reporting standards as issued by the International Accounting Standards Board, or IFRS. PRC laws also require foreign-invested enterprises to set aside at least 10% of their after-tax profits as the statutory common reserve fund until the cumulative amount of the statutory common reserve fund reaches 50% or more of such enterprises’ registered capital, if any, to fund its statutory common reserves, which are not available for distribution as cash dividends. Furthermore, under applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities within ten years after the taxable year when the transactions are conducted.
Pursuant to the PRC Enterprise Income Tax Law, a withholding tax rate of 10% currently applies to dividends paid by a PRC resident enterprise to a foreign enterprise investor, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for preferential tax treatment. Furthermore, the Announcement of State Taxation Administration on Promulgation of the Administrative Measures on Non-Resident Taxpayers Enjoying Treaty Benefits, issued on October 14, 2019 by the PRC State Taxation Administration, which became effective from January 1, 2020, requires non-resident enterprises to determine whether they are qualified to enjoy the preferential tax treatment under the tax treaties and make appropriate filings with the competent tax authorities. In addition, based on the Notice on Issues concerning Beneficial Owner in Tax Treaties, or Circular 9, issued on February 3, 2018 by the PRC State Taxation Administration, which became effective from April 1, 2018, when determining the applicant’s “beneficial owner” status regarding tax treatments in connection with dividends, interests or royalties in the tax treaties, several factors, including, without limitation, whether the applicant is obligated to pay more than 50% of the applicant’s income for twelve months to residents in a third country or region, whether the business operated by the applicant constitutes the actual business activities, and whether the counterparty country or region to the tax treaties does not levy any tax or grant tax exemption on relevant incomes or levy tax at an extremely low rate, will be taken into account, and it will be analyzed according to the actual circumstances of the specific cases. There are also other conditions for enjoying the reduced withholding tax rate according to other relevant tax rules and regulations. Therefore, Avalon currently believes that dividends from its PRC subsidiaries to Avalon, if any, shall be subject to a withholding tax rate of 10%, unless a reduced rate under a tax treaty is applicable. Avalon reported net losses and had negative net cash flows from operations in 2021. No net income will be generated from Avalon’s PRC subsidiaries’ operations in the foreseeable future and therefore no dividends or distributions will be paid by such subsidiaries to Avalon and its stockholders in the foreseeable future. However, if such subsidiaries do make distributions of cash or property to Avalon, absent a distribution by Avalon to the U.S. holders of Avalon common stock, there would be no flow-through of such income to the U.S. holders of Avalon common stock for U.S. federal income tax purposes.
As of the date of this report, no transfers, dividends or distributions from our PRC subsidiaries to Avalon have been made to date.
8
Restrictions on Foreign Exchange and Avalon’s Ability to Transfer Cash Across Borders
The PRC government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. Under existing PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, approval from or registration with appropriate governmental authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses. As a result, SAFE approval may need to be obtained to use cash generated from the operations of Avalon’s PRC subsidiaries. Any failure to comply with applicable foreign exchange regulations may subject us to administrative fines.
Holding Foreign Companies Accountable Act Compliance
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. According to the HFCA Act, if the SEC determines that Avalon has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in 2021, the SEC will prohibit Avalon’s securities from being traded on a national securities exchange or in the over-the-counter trading market in the United States.
On December 16, 2021, the PCAOB issued a Determination Report which reported that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China, because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region of the PRC, because of a position taken by one or more authorities in Hong Kong.
Avalon’s auditor is Marcum LLP (“Marcum”), based in New York, New York. Marcum is registered with the PCAOB and is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. Since Marcum is located in the United States, the PCAOB has been able to conduct inspections of Marcum. In addition, Marcum is not among the PCAOB registered public accounting firms registered in mainland China or Hong Kong that are subject to PCAOB’s determination on December 16, 2021.
Although the audit reports of Avalon are prepared by U.S. auditors that are subject to inspection by the PCAOB, the PCAOB is currently unable to conduct inspections over the audit work of Avalon’s independent registered public accounting firms with respect to Avalon’s operations in mainland China without the approval of certain Chinese authorities. Also, there is no guarantee that future audit reports will be prepared by auditors that are completely inspected by the PCAOB and, as such, future investors may be deprived of such inspections, which could result in limitations or restrictions to Avalon’s access of the U.S. capital markets.
9
Inspections of certain other firms that the PCAOB has conducted outside of China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future audit quality. However, the PCAOB is currently unable to inspect an auditor’s audit work related to a company’s operations in China where such documentation of the audit work is located in China. As a result, Avalon’s investors may be deprived of the benefits of the PCAOB’s oversight of auditors that are located in China through such inspections.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCA Act. Avalon will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCA Act, including the listing and trading prohibition requirements described above.
On June 22, 2021, the U.S. Senate passed a bill which, if passed by the U.S. House of Representatives and signed into law, would reduce the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two, which would shorten the timeframe before Avalon’s share may be delisted and before the trading in Avalon’s shares is prohibited.
On November 5, 2021, the SEC approved Rule 6100 adopted by the PCAOB to determine its inability to inspect or investigate registered firms completely under the HFCA Act. This rule establishes the framework for the PCAOB to make these required determinations. The trading in Avalon’s securities may be prohibited under the HFCA Act if the PCAOB subsequently determines Avalon’s audit work is performed by auditors that the PCAOB is unable to inspect or investigate completely pursuant to Rule 6100, and as a result, U.S. national securities exchanges, such as Nasdaq, may determine to delist Avalon’s securities. Such a delisting would likely cause the value of such securities to significantly decline or become worthless.
The SEC may propose additional regulatory or legislative requirements or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCA Act. However, some of the recommendations were more stringent than the HFCA Act. For example, if a company was not subject to PCAOB inspection, the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCA Act and to address the recommendations in the PWG report. It is unclear when the SEC will complete its rulemaking and when such rules will become effective and what, if any, of the PWG recommendations will be adopted. The implications of this possible regulation in addition to the requirements of the HFCA Act are uncertain. Although Avalon is currently not subject to the HFCA Act, any uncertainty of its applicability to Avalon, for example if Avalon switched to using a PRC-based auditing firm, could cause the market price of Avalon’s securities to be materially and adversely affected and could cause Avalon’s securities to be delisted or prohibited from being traded “over-the-counter”. If Avalon’s securities are unable to be listed on another securities exchange, such a delisting would substantially impair your ability to sell or purchase Avalon’s securities when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of Avalon’s securities. See “Risk Factors— Trading in Avalon’s securities may be restricted under the Holding Foreign Companies Accountable Act if the PCAOB determines that it cannot inspect or fully investigate Avalon’s auditors, and as a result, U.S. national securities exchanges, such as Nasdaq, may determine to delist Avalon’s securities.
Drug Approval Process
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our product candidates are extensively regulated by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or the FDCA, and its implementing regulations. Failure to comply with the applicable U.S. requirements may subject us to administrative or judicial sanctions, such as the FDA’s refusal to approve a pending new drug application, or NDA, or a pending biologics license application, or BLA, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution.
10
Pharmaceutical products such as ours may not be commercially marketed without prior approval from the FDA and comparable regulatory agencies in other countries. In the United States, the process to receiving such approval is long, expensive and risky, and includes the following steps:
| ● | pre-clinical laboratory tests, animal studies, and formulation studies; | |
| ● | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug for each indication; | |
| ● | submission to the FDA of an NDA or BLA; | |
| ● | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current good manufacturing practices, or cGMPs; | |
| ● | a potential FDA audit of the preclinical and clinical trial sites that generated the data in support of the NDA or BLA; | |
| ● | the ability to obtain clearance or approval of companion diagnostic tests, if required, on a timely basis, or at all; and | |
| ● | FDA review and approval of the NDA or BLA. |
Regulation by U.S. and foreign governmental authorities is a significant factor affecting our ability to commercialize any of our products, as well as the timing of such commercialization and our ongoing research and development activities. The commercialization of drug products requires regulatory approval by governmental agencies prior to commercialization. Various laws and regulations govern or influence the research and development, non-clinical and clinical testing, manufacturing, processing, packing, validation, safety, labeling, storage, record keeping, registration, listing, distribution, advertising, sale, marketing and post-marketing commitments of our products. The lengthy process of seeking these approvals, and the subsequent compliance with applicable laws and regulations, require expending substantial resources.
The results of pre-clinical testing, which include laboratory evaluation of product chemistry and formulation, animal studies to assess the potential safety and efficacy of the product and its formulations, details concerning the drug manufacturing process and its controls, and a proposed clinical trial protocol and other information must be submitted to the FDA as part of an IND that must be reviewed and become effective before clinical testing can begin. The study protocol and informed consent information for patients in clinical trials must also be submitted to an independent Institutional Review Board, or IRB, for approval covering each institution at which the clinical trial will be conducted. Once a sponsor submits an IND, the sponsor must wait 30 calendar days before initiating any clinical trials. If the FDA has comments or questions within this 30-day period, the issue(s) must be resolved to the satisfaction of the FDA before clinical trials can begin. In addition, the FDA, an IRB or the company may impose a clinical hold on ongoing clinical trials due to safety concerns. If the FDA imposes a clinical hold, clinical trials can only proceed under terms authorized by the FDA. Our pre-clinical and clinical studies must conform to the FDA’s Good Laboratory Practice, or GLP, and Good Clinical Practice, or GCP, requirements, respectively, which are designed to ensure the quality and integrity of submitted data and protect the rights and well-being of study patients. Information for certain clinical trials also must be publicly disclosed within certain time limits on the clinical trial registry and results databank maintained by the NIH.
Typically, clinical testing involves a three-phase process; however, the phases may overlap or be combined:
| ● | Phase I clinical trials typically are conducted in a small number of volunteers or patients to assess the early tolerability and safety profile, and the pattern of drug absorption, distribution and metabolism; | |
| ● | Phase II clinical trials typically are conducted in a limited patient population with a specific disease in order to assess appropriate dosages and dose regimens, expand evidence of the safety profile and evaluate preliminary efficacy; and | |
| ● | Phase III clinical trials typically are larger scale, multicenter, well-controlled trials conducted on patients with a specific disease to generate enough data to statistically evaluate the efficacy and safety of the product, to establish the overall benefit-risk relationship of the drug and to provide adequate information for the registration of the drug. |
A therapeutic product candidate being studied in clinical trials may be made available for treatment of individual patients, in certain circumstances. Pursuant to the 21st Century Cures Act (Cures Act), which was signed into law in December 2016. The manufacturer of an investigational product for a serious disease or condition is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for individual patient access to such investigational product.
The results of the pre-clinical and clinical testing, chemistry, manufacturing and control information, proposed labeling and other information are then submitted to the FDA in the form of either an NDA or BLA for review and potential approval to begin commercial sales. In responding to an NDA or BLA, the FDA may grant marketing approval, request additional information in a Complete Response Letter, or CRL, or deny the approval if it determines that the NDA or BLA does not provide an adequate basis for approval. A CRL generally contains a statement of specific conditions that must be met in order to secure final approval of an NDA or BLA and may require additional testing. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter, which authorizes commercial marketing of the product with specific prescribing information for specific indications, and sometimes with specified post-marketing commitments and/or distribution and use restrictions imposed under a Risk Evaluation and Mitigation Strategy program. Any approval required from the FDA might not be obtained on a timely basis, if at all.
11
Among the conditions for an NDA or BLA approval is the requirement that the manufacturing operations conform on an ongoing basis with cGMPs. In complying with cGMPs, we must expend time, money and effort in the areas of training, production and quality control within our own organization and at our contract manufacturing facilities. A successful inspection of the manufacturing facility by the FDA is usually a prerequisite for final approval of a pharmaceutical product. Following approval of the NDA or BLA, we and our manufacturers will remain subject to periodic inspections by the FDA to assess compliance with cGMPs requirements and the conditions of approval. We will also face similar inspections coordinated by foreign regulatory authorities.
Disclosure of Clinical Trial Information
Sponsors of certain clinical trials of FDA-regulated products are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical trial are then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product. Unique to a Fast Track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Under the Breakthrough Therapy program, products intended to treat a serious or life-threatening disease or condition may be eligible for the benefits of the Fast Track program when preliminary clinical evidence demonstrates that such product may have substantial improvement on one or more clinically significant endpoints over existing therapies. Additionally, FDA will seek to ensure the sponsor of a breakthrough therapy product receives timely advice and interactive communications to help the sponsor design and conduct a development program as efficiently as possible. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies. In addition, the FDA currently requires as a condition for accelerated approval the pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track designation, Breakthrough Therapy designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Regenerative Medicine Advanced Therapies (RMAT) Designation
The FDA has established a Regenerative Medicine Advanced Therapy, or RMAT, designation as part of its implementation of the 21st Century Cures Act, or Cures Act. The RMAT designation program is intended to fulfill the Cures Act requirement that the FDA facilitate an efficient development program for, and expedite review of, any drug that meets the following criteria: (1) it qualifies as a RMAT, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (2) it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and (3) preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such a disease or condition. Like breakthrough therapy designation, RMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate, and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. RMAT-designated products that receive accelerated approval may, as appropriate, fulfill their post-approval requirements through the submission of clinical evidence, clinical studies, patient registries, or other sources of real world evidence (such as electronic health records); through the collection of larger confirmatory data sets; or via post-approval monitoring of all patients treated with such therapy prior to approval of the therapy.
Post-Approval Requirements
Oftentimes, even after a drug has been approved by the FDA for sale, the FDA may require that certain post-approval requirements be satisfied, including the conduct of additional clinical studies. If such post-approval requirements are not satisfied, the FDA may withdraw its approval of the drug. In addition, holders of an approved NDA or BLA are required to report certain adverse reactions to the FDA, comply with certain requirements concerning advertising and promotional labeling for their products, and continue to have quality control and manufacturing procedures conform to cGMPs after approval. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities; this latter effort includes assessment of compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMPs compliance.
12
Other Healthcare Fraud and Abuse Laws
In the U.S., our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare and Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services (such as the Office of Inspector General and the Health Resources and Service Administration), the U.S. Department of Justice, or the DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, sales, marketing and scientific/educational grant programs may have to comply with the anti-fraud and abuse provisions of the Social Security Act, the false claims laws, the privacy and security provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws, each as amended, as applicable.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between therapeutic product manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Additionally, the intent standard under the Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act, or FCA.
The federal false claims and civil monetary penalty laws, including the FCA, which imposes significant penalties and can be enforced by private citizens through civil qui tam actions, prohibit any person or entity from, among other things, knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to, or approval by, the federal healthcare programs, including Medicare and Medicaid, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. For instance, historically, pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, off-label, and thus generally non-reimbursable, uses.
HIPAA created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, the ACA amended the intent standard for certain healthcare fraud statutes under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Many states have similar, and typically more prohibitive, fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Additionally, to the extent that our product candidates may in the future be sold in a foreign country, we may be subject to similar foreign laws.
We may be subject to data privacy and security regulations by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors, or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, many state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways, are often not pre-empted by HIPAA, and may have a more prohibitive effect than HIPAA, thus complicating compliance efforts.
13
We expect our product, after approval, may be eligible for coverage under Medicare, the federal health care program that provides health care benefits to the aged and disabled, and covers outpatient services and supplies, including certain pharmaceutical products, that are medically necessary to treat a beneficiary’s health condition. In addition, the product may be covered and reimbursed under other government programs, such as Medicaid and the 340B Drug Pricing Program. The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Under the 340B Drug Pricing Program, the manufacturer must extend discounts to entities that participate in the program. As part of the requirements to participate in certain government programs, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average manufacturer price, or AMP, and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely.
Additionally, the federal Physician Payments Sunshine Act, or the Sunshine Act, within the ACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) report annually to CMS information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to report accurately could result in penalties. In addition, many states also govern the reporting of payments or other transfers of value, many of which differ from each other in significant ways, are often not pre-empted, and may have a more prohibitive effect than the Sunshine Act, thus further complicating compliance efforts.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that my significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted or whether FDA regulations, guidance, policies or interpretations will be changed or what the effect of such changes, if any, may be.
Legal Proceedings
On October 25, 2017, Genexosome entered into and closed a Stock Purchase Agreement with Beijing Genexosome and Yu Zhou, MD, PhD, the sole shareholder of Beijing Genexosome, pursuant to which Genexosome acquired all of the issued and outstanding securities of Beijing Genexosome in consideration of a cash payment in the amount of $450,000, of which $100,000 is still owed. Further, on October 25, 2017, Genexosome entered into and closed an Asset Purchase Agreement with Dr. Zhou, pursuant to which the Company acquired all assets, including all intellectual property and exosome separation systems, held by Dr. Zhou pertaining to the business of researching, developing and commercializing exosome technologies. In consideration of the assets, Genexosome paid Dr. Zhou $876,087 in cash, transferred 500,000 shares of common stock of the Company to Dr. Zhou and issued Dr. Zhou 400 shares of common stock of Genexosome. Further, the Company had not been able to realize the financial projections provided by Dr. Zhou at the time of the acquisition and has decided to impair the intangible asset associated with this acquisition to zero. Dr. Zhou was terminated as Co-CEO of Genexosome on August 14, 2019. Further, on October 28, 2019, Research Institute at Nationwide Children’s Hospital (“Research Institute”) filed a Complaint in the United States District Court for the Southern District of Ohio Eastern Division against Dr. Zhou, Li Chen, the Company and Genexosome with various claims against the Company and Genexosome. The criminal proceedings against Dr. Zhou and Li Chen have been concluded. The Company, Genexosome and the Research Institute entered into a Settlement Agreement dated June 7, 2022 (the “Settlement Date”) whereby the Company agreed to pay the Research Institute $450,000 on each of the sixty-day, one year and two-year anniversaries of the Settlement Date. In addition, the Company agreed to pay the Research Institute 30% of the Company’s initial pre-tax profit of $3,333,333, 20% of the Company’s second pre-tax profit of $3,333,333 and 10% of the Company’s third pre-tax profit of $3,333,333. The parties provided a mutual release as well.
Company Information
Our principal executive office is located at 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728. Our telephone number is 732-780-4400. Our website address is www.avalon-globocare.com. Information contained in, or accessible through, our website does not constitute a part of this prospectus or any prospectus supplement.
14
PRIVATE PLACEMENT OF CONVERTIBLE NOTES AND WARRANTS
On March 28, 2022, the Company entered into Securities Purchase Agreement with an accredited investor, which was amended on June 8, 2022, providing for the sale by the Company to the investor of a Convertible Note in the amount of $3,718,942.74 (the “2022 Convertible Note”). In addition to the 2022 Convertible Note, the investor will also receive a Stock Purchase Warrant (the “2022 Warrant”) to acquire an aggregate of 1,239,647 shares of common stock. The 2022 Warrants will be exercisable for five years at an exercise price of $1.25. The financing closed with respect to:
| ● | $2,669,521.60 of the financing on April 15, 2022, |
| ● | $659,580.64 of the financing on April 29, 2022, |
| ● | $199,840.50 of the financing on May 18, 2022 and |
| ● | $190,000 of the financing on May 25, 2022. |
The Convertible Note bears interest at 1% per annum payable at maturity and matures ten years from issuance. The Purchaser may elect to convert all or part of the Convertible Note, plus accrued interest, at any time into Note Shares at a conversion price equal to 95% of the average of the highest three trading prices for the common stock during the 20-trading day period ending one trading day prior to the conversion date but in no event will the conversion price be lower than $0.75 per share.
The Purchaser agreed to restrict its ability to convert the Convertible Note and exercise the Warrants and receive shares of common stock such that the number of shares of common stock held by the investor after such conversion or exercise does not exceed 4.99% of the then issued and outstanding shares of common stock. Further, the Purchaser agreed to not sell or transfer any or all of the Note Shares underlying the Convertible Note or the Warrant Shares underlying the Warrants for a period of 90 days beginning on the closing date (the “Lock-Up Period”). Following the expiration of the Lock-Up Period, the investor has agreed to limit its sale or transfer of such shares of common stock to a maximum monthly amount equal to 20% of the shares of common stock issuable upon conversion of the Convertible Note. We agreed to use our reasonable best efforts to file a registration statement on Form S-3 (or other appropriate form) providing for the resale by the Purchaser of the Private Placement Note Shares and the Private Placement Warrant Shares.
The securities issued pursuant to the Securities Purchase Agreement were issued pursuant to an exemption from registration under Section 4(a)(2) and/or Rule 506 of Regulation D, which is promulgated under the Securities Act of 1933, as amended (the “Securities Act”). The Company relied on this exemption from registration based in part on representations made by the parties to such agreements.
15
Pursuant to this prospectus, the Selling Stockholder is offering on a resale basis an aggregate of 6,198,237 shares of our common stock, par value $0.0001 per share, which are comprised of (i) 4,958,590 Private Placement Note Shares issuable upon conversion of a convertible note issued in the Private Placement pursuant to that certain Securities Purchase Agreement and (ii) 1,239,647 Private Placement Warrant Shares issuable upon the exercise of the Warrants issued in the Private Placement pursuant to the Securities Purchase Agreement we issued to the Purchaser.
| Common Stock to be offered by the Selling Stockholder | 6,198,237 Shares including 4,958,590 Private Placement Note Shares issuable upon conversion of the Convertible Note and 1,239,647 Private Placement Warrant Shares issuable upon the exercise of the Warrants issued to the Selling Stockholder. | |
| Common Stock outstanding prior to this offering | 89,034,766 Shares. | |
| Common Stock to be outstanding after this offering | 95,233,003 Shares (assuming the full conversion of the Convertible Note and exercise of the Warrants). | |
| Use of Proceeds | We will not receive any of the proceeds from the sale by the Selling Stockholder of the Common Stock. Upon any exercise of the Warrants Shares by payment of cash, however, we will receive the exercise price of the Warrants. See “Use of Proceeds” on page 18 of this prospectus. | |
| Risk Factors | You should read the “Risk Factors” section beginning on page 17 of this prospectus for a discussion of factors to consider carefully before deciding to invest in our securities. | |
| Nasdaq Capital Market symbol | Our Common Stock is listed on The Nasdaq Capital Market under the symbol “AVCO”. We do not intend to apply for listing of the Warrants on any securities exchange or nationally recognized trading system | |
16
Investing in our securities involves a high degree of risk. Before investing in our securities, you should carefully consider the risks, uncertainties and assumptions contained in this prospectus and discussed under the heading “Risk Factors” included in our Annual Report on Form 10-K for the year ended December 31, 2021 and subsequent Quarterly Reports on Form 10-Q, which are on file with the SEC and are incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future, including any accompanying prospectus supplement and the documents incorporated by reference therein. Our business, financial condition, results of operations and future growth prospects could be materially and adversely affected by any of these risks. In these circumstances, the market price of our Common Stock could decline, and you may lose all or part of your investment.
17
The net proceeds from any disposition of the Private Placement Shares covered hereby will be received by the selling shareholder. We will not receive any of the proceeds from any such shares of common stock offered by this prospectus. We will, however, receive the net proceeds of any Warrants exercised for cash. We expect to use the proceeds received from the exercise of the Warrants, if any, for the development of our product candidates and general working capital purposes. We intend to use the net proceeds from the sale of the securities as set forth in the applicable prospectus supplement.
The common stock being offered by the selling shareholder are those issuable to the selling shareholder upon conversion of the Convertible Note and upon exercise of the Warrants. For additional information regarding the issuances of those shares of common stock, the Convertible Notes and Warrants, see “Private Placement of Convertible Notes and Warrants” above. We are registering the shares of common stock in order to permit the selling shareholder to offer the shares for resale from time to time. Except for the ownership of the shares of common stock issuable upon conversion of the Convertible Notes and the Warrants, the selling shareholder has not had any material relationship with us within the past three years.
The table below lists the selling shareholder and other information regarding the beneficial ownership of the shares of common stock by the selling shareholder. The second column lists the number of shares of common stock beneficially owned by the selling shareholder, based on its ownership of the shares of common stock issuable upon conversion of the Convertible Note and exercise of the Warrants, as of June 27, 2022, assuming conversion of the Convertible Note and exercise of the warrants held by the selling shareholder on that date, without regard to any limitations on exercises.
The third column lists the shares of common stock being offered by this prospectus by the selling shareholder.
In accordance with the terms of the Securities Purchase Agreement with the selling shareholder, this prospectus generally covers the resale of the sum of (i) the number of shares of common stock issuable to the selling shareholder upon conversion of the Convertible Note in the “Private Placement of Convertible Notes and Warrants” described above and (ii) the maximum number of shares of common stock issuable upon exercise of the related Warrants, determined as if the outstanding Warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment as provided in the Securities Purchase Agreement, without regard to any limitations on the conversion of the Convertible Notes and exercise of the warrants. The fourth column assumes the sale of all of the shares offered by the selling shareholder pursuant to this prospectus.
Under the terms of the Convertible Note and Warrants held by the selling shareholder, a selling shareholder may not convert such Convertible Note or exercise any such warrants to the extent such exercise would cause such selling shareholder, together with its affiliates and attribution parties, to beneficially own a number of shares of common stock which would exceed 4.99% of our then outstanding common stock following such conversion or exercise. The number of shares in the second and fourth columns do not reflect this limitation. The selling shareholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Shareholder | Number of shares of Common Stock Owned Prior to Offering (1) | Maximum Number of shares of Common Stock to be Sold Pursuant to this Prospectus | Number of shares of Common Stock Owned After Offering | |||||||||
| Fsunshine Trading Pte. Ltd. (2) | 4,676,176 | 6,198,237 | — | |||||||||
| (1) | Beneficial ownership as reflected in the Selling Stockholder table reflects the total number of shares potentially issuable underlying warrants and does not give effect to these beneficial ownership limitations. Accordingly, actual beneficial ownership, as calculated in accordance with Section 13(d) and Rule 13d-3 thereunder may be lower than as reflected in the table. Beneficial ownership is determined in accordance with Rule 13d-3 under the Exchange Act. In computing the number of shares beneficially owned by a person and the percentage ownership of the Selling Stockholder, securities that are currently convertible into or exercisable into shares of our common stock, or convertible or exercisable into shares of our common stock within 60 days of the date hereof are deemed outstanding. There is a beneficial ownership limitation on the Convertible Notes and warrants owned by the holder that limits beneficial ownership of the holder to 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares of common stock issuable upon conversion of the Convertible Notes and exercise of the warrant at any time. |
| (2) | Includes 4,958,590 Private Placement Note Shares issuable upon conversion of the Convertible Note and 1,239,647 Private Placement Warrant Shares issuable upon the exercise of the Warrants. Yulin Sun has discretionary authority to vote and dispose of the shares held by the selling stockholder and may be deemed to be the beneficial owner of these shares. |
18
The following is a summary description of the material terms of our common stock as provided in our Articles of Incorporation, as amended (“Articles of Incorporation”) and Bylaws (“Bylaws”), copies of which are incorporated by reference as exhibits to the registration statement of which this prospectus forms a part. The following discussion is only a summary and may not contain all the information that is important to you or that you should consider before investing in our stock, and is qualified in its entirety by reference to the complete text of the Articles of Incorporation and Bylaws. For a more detailed description of these securities, you should read the applicable provisions of Delaware law, our Articles of Incorporation, our Bylaws and the reports that we file with the SEC, which are incorporated herein by reference.
General
As of the date of this prospectus, our authorized capital stock consisted of 490,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. As of the date of this prospectus, there were 89,034,766 shares of our common stock issued and outstanding and no shares of preferred stock issued and outstanding.
Common Stock
All outstanding shares of common stock are of the same class and have equal rights and attributes. The holders of common stock are entitled to one vote per share on all matters submitted to a vote of stockholders of the company. All stockholders are entitled to share equally in dividends, if any, as may be declared from time to time by the Board of Directors out of funds legally available. In the event of liquidation, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities. The stockholders do not have cumulative or preemptive rights.
Preferred Stock
Our Certificate of Incorporation authorizes the issuance of up to 10,000,000 shares of preferred stock with designations, rights and preferences determined from time to time by our Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the common stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company, which is sometimes referred to in corporate parlance as a “poison pill”.
Stockholder Action by Written Consent
Any action required or permitted to be taken at any annual or special meetings of the stockholders of the company may be taken without a meeting, without prior notice and without a vote, by a consent or consents in writing, setting forth the action so taken, (a) signed by stockholders of the company holding not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all the shares of the company entitled to vote thereon were present and voted and (b) delivered to the company in accordance with Section 228 of the DGCL.
Anti-Takeover Effects of Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law
Some provisions of Delaware law, our certificate of incorporation and our bylaws contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions that might result in a premium over the price of our common stock.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which regulates corporate takeovers. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, such as discouraging takeover attempts that might result in a premium over the price of our common stock.
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management.
Transfer Agent
The stock transfer agent for our securities is Vstock Transfer, LLC, 18 Lafayette Place, Woodmere, NY 11598, (212) 828-8436.
Nasdaq Capital Market Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “AVCO”.
19
We may issue warrants for the purchase of shares of our common stock or preferred stock. We may issue warrants independently or together with other securities, and the warrants may be attached to or separate from any offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and the investors or a warrant agent. The following summary of material provisions of the warrants and warrant agreements are subject to, and qualified in their entirety by reference to, all the provisions of the warrant agreement and warrant certificate applicable to a particular series of warrants. The terms of any warrants offered under a prospectus supplement may differ from the terms described below. We urge you to read the applicable prospectus supplement and any related free writing prospectus, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants.
The particular terms of any issue of warrants will be described in the prospectus supplement relating to the issue. Those terms may include:
| ● | the number of shares of common stock or preferred stock purchasable upon the exercise of warrants to purchase such shares and the price at which such number of shares may be purchased upon such exercise; |
| ● | the designation, stated value and terms (including, without limitation, liquidation, dividend, conversion and voting rights) of the series of preferred stock purchasable upon exercise of warrants to purchase preferred stock; |
| ● | the date, if any, on and after which the warrants, preferred stock or common stock will be separately transferable; |
| ● | the terms of any rights to redeem or call the warrants; |
| ● | the date on which the right to exercise the warrants will commence and the date on which the right will expire; |
| ● | United States federal income tax consequences applicable to the warrants; and |
| ● | any additional terms of the warrants, including terms, procedures, and limitations relating to the exchange, exercise and settlement of the warrants. |
Holders of equity warrants will not be entitled to:
| ● | vote, consent or receive dividends; |
| ● | receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter; or |
| ● | exercise any rights as stockholders of Avalon Globocare. |
Each warrant will entitle its holder to purchase the principal amount of the number of shares of preferred stock or common stock at the exercise price set forth in, or calculable as set forth in, the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
A holder of warrant certificates may exchange them for new warrant certificates of different denominations, present them for registration of transfer and exercise them at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement. Until any warrants to purchase common stock or preferred stock are exercised, the holders of the warrants will not have any rights of holders of the underlying common stock or preferred stock, including any rights to receive dividends or payments upon any liquidation, dissolution or winding up on the common stock or preferred stock, if any.
20
We may issue units consisting of any combination of the other types of securities offered under this prospectus in one or more series. We may evidence each series of units by unit certificates that we will issue under a separate agreement. We may enter into unit agreements with a unit agent. Each unit agent will be a bank or trust company that we select. We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
The following description, together with the additional information included in any applicable prospectus supplement, summarizes the general features of the units that we may offer under this prospectus. You should read any prospectus supplement and any free writing prospectus that we may authorize to be provided to you related to the series of units being offered, as well as the complete unit agreements that contain the terms of the units. Specific unit agreements will contain additional important terms and provisions and we will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from another report that we file with the SEC, the form of each unit agreement relating to units offered under this prospectus.
If we offer any units, certain terms of that series of units will be described in the applicable prospectus supplement, including, without limitation, the following, as applicable:
| ● | the title of the series of units; |
| ● | identification and description of the separate constituent securities comprising the units; |
| ● | the price or prices at which the units will be issued; |
| ● | the date, if any, on and after which the constituent securities comprising the units will be separately transferable; |
| ● | a discussion of certain United States federal income tax considerations applicable to the units; and |
| ● | any other terms of the units and their constituent securities. |
21
PLAN OF DISTRIBUTION
We and the selling shareholders may sell securities in and outside the United States through underwriters or dealers, directly to purchasers or through agents or in ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers. To the extent required by applicable law, a prospectus supplement will include the following information:
| ● | the terms of the offering; | |
| ● | the names of any underwriters or agents; | |
| ● | the names of the selling stockholders ; | |
| ● | the purchase price of the securities; | |
| ● | the net proceeds to us from the sale of the securities; | |
| ● | any delayed delivery arrangements; | |
| ● | any underwriting discounts, commissions and other items constituting underwriters’ compensation; | |
| ● | the initial public offering price; | |
| ● | any discounts or concessions allowed or reallowed or paid to dealers; and | |
| ● | any commissions paid to agents. |
Sale Through Underwriters or Dealers
If we use underwriters in the sale of securities, the underwriters will acquire the securities for their own account. The underwriters may resell the securities from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Underwriters may offer securities to the public either through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. Unless we inform you otherwise in the prospectus supplement, the obligations of the underwriters to purchase the securities will be subject to conditions, and the underwriters will be obligated to purchase all the securities if they purchase any securities. The underwriters may change from time to time any initial public offering price and any discounts or concessions allowed or reallowed or paid to dealers.
During and after an offering through underwriters, the underwriters may purchase and sell the securities in the open market. These transactions may include over allotment and stabilizing transactions and purchases to cover syndicate short positions created in connection with the offering. The underwriters may also impose a penalty bid, whereby selling concessions allowed to syndicate members or other broker-dealers for the offered securities sold for their account may be reclaimed by the syndicate if such offered securities are repurchased by the syndicate in stabilizing or covering transactions. These activities may stabilize, maintain or otherwise affect the market price of the offered securities, which may be higher than the price that might otherwise prevail in the open market. If commenced, these activities may be discontinued at any time.
If we or the selling shareholders use dealers in the sale of securities, we or the selling shareholders will sell the securities to them as principals. They may then resell the securities to the public at varying prices determined by the dealers at the time of resale. The dealers participating in any sale of the securities may be deemed to be underwriters within the meaning of the Securities Act with respect to any sale of such securities. We will include in any prospectus supplement the names of the dealers and the terms of the transactions.
We will bear costs relating to all of the securities being registered under this registration statement of which this prospectus forms a part.
Any broker-dealers or other persons acting on our behalf or on behalf of the selling shareholder that participate with us in the distribution of the securities may be deemed to be underwriters and any commissions received or profit realized by them on the resale of the securities may be deemed to be underwriting discounts and commissions under the Securities Act. As of the date of this prospectus, we are not a party to any agreement, arrangement or understanding between any broker or dealer and us with respect to the offer or sale of the securities pursuant to this prospectus.
Pursuant to a requirement by the Financial Industry Regulatory Authority, or FINRA, the maximum commission or discount to be received by any FINRA member or independent broker/dealer may not be greater than eight percent (8%) of the gross proceeds received by us for the sale of any securities being registered pursuant to SEC Rule 415 under the Securities Act. If more than 5% of the net proceeds of any offering of securities made under this prospectus will be received by a FINRA member participating in the offering or its affiliates or associated persons of such FINRA member, the offering will be conducted in accordance with FINRA Conduct Rule 5121.
22
Direct Sales and Sales Through Agents
We and the selling shareholders may sell the securities directly. In that event, no underwriters or agents would be involved. We and the selling shareholders may also sell the securities through agents we designate from time to time. In the prospectus supplement, we will name any agent involved in the offer or sale of the securities, and we will describe any commissions payable by us to the agent. Unless we inform you otherwise in the prospectus supplement, any agent will agree to use its reasonable best efforts to solicit purchases for the period of its appointment.
We may sell the securities directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect to any sale of the securities. We will describe the terms of any such sales in the prospectus supplement.
Delayed Delivery Contracts
If we so indicate in the prospectus supplement, we may authorize agents, underwriters or dealers to solicit offers from certain types of institutions to purchase securities from us at the public offering price under delayed delivery contracts. These contracts would provide for payment and delivery on a specified date in the future. The contracts would be subject only to those conditions described in the prospectus supplement. The prospectus supplement will describe the commission payable for solicitation of those contracts.
Subscription Offerings
We may also make direct sales through subscription rights distributed to our existing stockholders on a pro rata basis, which may or may not be transferable. In any distribution of subscription rights to our stockholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the unsubscribed securities to third parties.
General Information
We may have agreements with the agents, dealers and underwriters to indemnify them against civil liabilities, including liabilities under the Securities Act, or to contribute with respect to payments that the agents, dealers or underwriters may be required to make. Agents, dealers and underwriters may engage in transactions with us or perform services for us in the ordinary course of their businesses.
LEGAL MATTERS
The validity of the issuance of the securities offered hereby will be passed upon for us by Fleming PLLC, New York, New York. Additional legal matters may be passed upon for us or any underwriters, dealers, or agents by counsel that we will name in the applicable prospectus supplement.
Marcum LLP, our independent registered public accounting firm, has audited our consolidated financial statements at December 31, 2021 and 2020 as set forth in their report. The consolidated financial statements have been incorporated by reference in the prospectus and in the registration statement in reliance on Marcum LLP’s report which includes an explanatory paragraph about the existence of substantial doubt concerning our ability to continue as a going concern, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities the Selling Stockholders are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus or incorporated by reference in this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered by this prospectus.
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public from commercial document retrieval services and over the Internet at the SEC’s website at http://www.sec.gov.
You can also obtain copies of materials we file with the SEC from our website found at www.avalon-globocare.com. Information on our website does not constitute a part of, nor is it incorporated in any way, into this prospectus and should not be relied upon in connection with making an investment decision.
23
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference information into this document. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act made subsequent to the date of this prospectus until the termination of the offering of the securities described in this prospectus (other than information in such filings that was “furnished,” under applicable SEC rules, rather than “filed”).
We incorporate by reference the following documents or information that we have filed with the SEC:
| ● | our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the SEC on March 30, 2022; |
| ● | our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2022, filed with the SEC on May 11, 2022; |
| ● | our Current Reports on Form 8-K (other than Current Reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) filed with the SEC on January 4, 2022, February 14, 2022, April 18, 2022, April 29, 2022, May 2, 2022, May 23, 2022, and June 8, 2022; |
| ● | the description of our common stock contained in our Registration Statement on Form 8-A filed with the SEC on November 2, 2018, including any amendments or reports filed for the purpose of updating that description (File No. 001-38728), including any amendments or reports filed with the SEC for the purposes of updating such description. |
Any statement contained in this prospectus or contained in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded to the extent that a statement contained in this prospectus or any subsequently filed supplement to this prospectus, or document deemed to be incorporated by reference into this prospectus, modifies or supersedes such statement.
You may request a copy of these filings at no cost, by writing or telephoning us at the following address:
Avalon GloboCare Corp.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
732-780-4400
You should rely only on the information incorporated by reference or provided in this prospectus or in any prospectus supplement. We have not authorized anyone else to provide you with different or additional information. An offer of these securities is not being made in any jurisdiction where the offer or sale is not permitted. You should not assume that the information in this prospectus or any prospectus supplement is accurate as of any date other than the date on the front of those documents.
24
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following table sets forth an estimate of the fees and expenses relating to the issuance and distribution of the securities being registered hereby, other than underwriting discounts and commissions, all of which shall be borne by the selling stockholders. All of such fees and expenses, except for the SEC Registration Fee, are estimated:
| SEC registration fee | $ | 4,967.10 | ||
| Legal fees and expenses | $ | 25,000.00 | ||
| Printing fees and expenses | $ | 2,500.00 | ||
| Accounting fees and expenses | $ | 15,000.00 | ||
| Miscellaneous fees and expenses | $ | 2,000.00 | ||
| Total | $ | 49,467.10 |
Item 15. Indemnification of Officers and Directors.
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law provides that a Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, provided that the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was illegal. A Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit provided the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the corporation’s best interests, except that no indemnification is permitted without judicial approval if the person is adjudged to be liable to the corporation. Where a present or former director or officer is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify the person against the expenses that such person has actually and reasonably incurred. Our certificate of incorporation provides for indemnification of our directors, officers, employees and other agents to the maximum extent permitted by the Delaware General Corporation Law.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| ● | transaction from which the director derives an improper personal benefit; |
| ● | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| ● | unlawful payment of dividends, unlawful stock purchase or redemption of shares; or |
| ● | breach of a director’s duty of loyalty to the corporation or its stockholders. |
Our amended and restated certificate of incorporation includes such a provision.
The right to indemnification provided in our certificate of incorporation includes the right to be paid by us the expenses (including, without limitation, attorneys’ fees and expenses) incurred in defending any action referred to above in advance of its final disposition, provided, however, that, if the Delaware General Corporation Law so requires, such an advancement of expenses incurred by a person in the person’s capacity as a director or officer (and not in any other capacity in which service was or is rendered by the person, including, without limitation, service to an employee benefit plan) will be made only upon delivery to us of an undertaking, by or on behalf of the person, to repay all amounts so advanced if it is ultimately determined by final judicial decision from which there is no further right to appeal that the person is not entitled to be indemnified by us.
II-1
Section 174 of the Delaware General Corporation Law provides, among other things, that a director who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption may be held liable for such actions. A director who was either absent when the unlawful action was approved, or dissented at the time, may avoid liability by causing his or her dissent to such action to be entered in the books containing minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful act.
As permitted by the Delaware General Corporation Law, we have entered into indemnity agreements with each of our directors and officers that require us to indemnify such persons against any and all expenses (including attorneys’ fees), witness fees, judgments, fines, settlements and other amounts incurred (including expenses of a derivative action) in connection with any action, suit or proceeding or alternative dispute resolution mechanism, inquiry hearing or investigation, whether threatened, pending or completed, to which any such person may be made a party by reason of the fact that such person is or was a director, an officer or an employee of our company, provided that such person’s conduct did not constitute a breach of his or her duty of loyalty to us or our stockholders, and was not an act or omission not in good faith or which involved intentional misconduct or a knowing violation of laws.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act of 1933, as amended (the “Securities Act”), or otherwise.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 16. Exhibits.
a) Exhibits.
| * | Filed herewith. |
| + | To be filed, if necessary, after effectiveness of this registration statement by an amendment to the registration statement or incorporated by reference to a Current Report on Form 8-K filed in connection with an underwritten offering of the securities offered hereunder. |
II-2
Item 17. Undertakings.
| (1) | The undersigned registrant hereby undertakes: |
| a. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”); |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that the undertaking set forth in paragraphs (1)(a)(i), (1)(a)(ii) and (1)(a)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement;
| b. | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; and |
| c. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| d. | That, for the purpose of determining liability under the Securities Act to any purchaser: |
| i. | Each prospectus filed by a registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| ii. | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which the prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
| (2) | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the undersigned registrant, pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the undersigned registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Freehold, State of New Jersey, on June 27, 2022.
| By: | /s/ David Jin, MD, PhD | |
| David Jin, MD, PhD | ||
| Chief Executive Officer |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints David Jin and Luisa Ingargiola, and each of them acting individually, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this Registration Statement, including post-effective amendments or any abbreviated registration statement and any amendments thereto filed pursuant to Rule 462(b) under the Securities Act of 1933, and to file the same, with all exhibits thereto and other documents in connection therewith, with the SEC, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his, her or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | ||
| /s/ David K. Jin | Chief Executive Officer, President and Director | June 27, 2022 | ||
| David K. Jin | (Principal Executive Officer) | |||
| /s/ Luisa Ingargolia | Chief Financial Officer | June 27, 2022 | ||
| Steve Schrader | (Principal Financial and Accounting Officer) | |||
| /s/ Wenzhao Lu | Chairman of the Board of Directors | June 27, 2022 | ||
| Wenzhao Lu | ||||
| /s/ Meng Li | Chief Operating Officer, Secretary and Director | June 27, 2022 | ||
| Meng Li | ||||
| /s/ Steven A. Sanders | Director | June 27, 2022 | ||
| Steven A. Sanders | ||||
| /s/ Yancen Lu | Director | June 27, 2022 | ||
| Yancen Lu | ||||
| /s/ Wilbert J. Tauzin II | Director | June 27, 2022 | ||
| Wilbert J. Tauzin II | ||||
| /s/ William B. Stilley III | Director | June 27, 2022 | ||
| William B. Stilley III | ||||
| /s/ Tevi Troy | Director | June 27, 2022 | ||
| Tevi Troy | ||||
| /s/ Yue “Charles” Li | Director | June 27, 2022 | ||
| Yue “Charles” Li |
II-4