may inhibit our ability to commercialize our product candidates and adversely affect our business, financial condition, results of operations and prospects.
In addition, FDA policies, and those of equivalent foreign regulatory agencies, may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would harm our business, financial condition, results of operations and prospects.
We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business and financial condition, and our ability to successfully market or commercialize our product candidates.
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies. Any product candidates that we successfully develop into products and commercialize may compete with existing therapies and new therapies that may become available in the future. While we believe that our gene therapy platform, product programs, product candidates and scientific expertise in the fields of gene therapy and neuroscience provide us with competitive advantages, we face potential competition from various sources, including larger and better-funded pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions, governmental agencies and public and private research institutions.
We are aware of several companies focused on developing AAV gene therapies in various indications, including AAVANTIBio, Inc., Abeona Therapeutics, Inc., Adverum Biotechnologies, Inc., Aevitas Therapeutics, Inc., Alcyone Therapeutics, Inc., Amicus Therapeutics, Inc., Apic Bio, Inc., Applied Genetic Technologies Corporation, Asklepios BioPharmaceutical, Inc., or AskBio (acquired by Bayer), Audentes Therapeutics, Inc. (acquired by Astellas Pharma Inc.), Biogen, Inc., or Biogen, Brain Neurotherapy Bio, Inc. (merged with AskBio), Encoded Therapeutics, Inc., GenSight Biologics SA, Homology Medicines, Inc., LEXEO Therapeutics, Inc., LogicBio Therapeutics, Inc., Lysogene SA, MeiraGTx Ltd., or MeiraGTx, Neurogene, Inc., Novartis Gene Therapies, Inc. (formerly AveXis, Inc.), Passage Bio, Inc., Pfizer, Prevail Therapeutics, Inc. (acquired by Eli Lilly), PTC Therapeutics, Inc., REGENXBio Inc., Sarepta Therapeutics, Inc., Sio Gene Therapies, Inc., Solid Biosciences, Inc., Spark Therapeutics, Inc. (acquired by Roche), StrideBio, Inc., Taysha Gene Therapies, Inc. and uniQure, as well as several companies addressing other methods for modifying genes and regulating gene expression. Any advances in gene therapy technology made by a competitor may be used to develop therapies that could compete against any of our product candidates.
We expect that VY-AADC (NBIb-1817) will potentially compete with a variety of therapies currently marketed and in development for Parkinson’s disease, including DBS marketed by Medtronic plc, Abbott Laboratories (acquired from St. Jude Medical in 2017), and other medical device companies, DUOPA/Duodopa marketed by AbbVie, as well as other novel, non-oral forms of levodopa, including Mitsubishi Tanabe Pharma’s ND0612 (acquired from NeuroDerm in 2017), Acorda Therapeutics’ inhaled levodopa, INBRIJA, and Sunovion Pharmaceuticals’, or Sunovion’s, sublingual apomorphine, KYNMOBI. Gene therapy competition for Parkinson’s disease includes AAV2-GDNF being developed by Brain Neurotherapy Bio, Inc. and AAV-GAD being developed by MeiraGTx. Sio Gene Therapies, Inc. is developing a second generation LentiVector gene therapy, AXO-Lenti-PD (previously OXB-102, licensed from Oxford Biomedica in 2018).
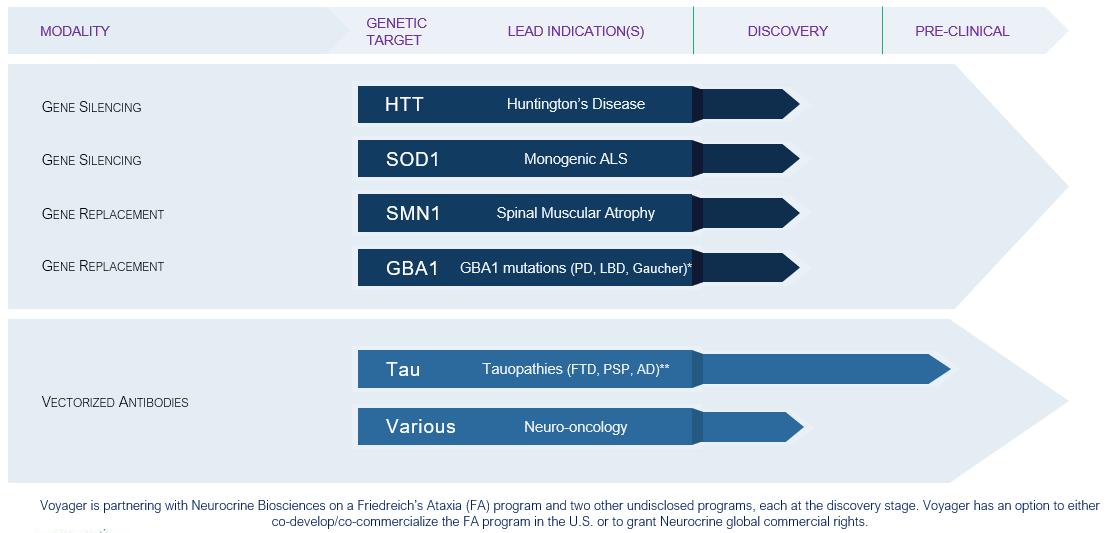
We expect that our preclinical programs will compete with a variety of therapies in development, including:
| ● | Our program for Huntington’s disease will potentially compete with TAK-686 being developed by Sangamo Therapeutics, Inc. in collaboration with Takeda, and AMT-130, an AAV gene therapy being developed by uniQure and a gene therapy being developed by Spark; |