| ● | seize or detain the product or otherwise require the withdrawal of the product from the market; |
| ● | refuse to permit the import or export of products; or |
| ● | refuse to allow us to enter into supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and adversely affect our business, financial condition, results of operations and prospects.
In addition, FDA policies, and those of equivalent foreign regulatory agencies, may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would harm our business, financial condition, results of operations and prospects.
We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business and financial condition and our ability to successfully market or commercialize our product candidates.
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies. Any product candidates that we successfully develop into products and commercialize may compete with existing therapies and new therapies that may become available in the future. While we believe that our gene therapy platform, vectorized antibody platform, product programs, product candidates and scientific expertise in the fields of gene therapy and neuroscience provide us with competitive advantages, we face potential competition from various sources, including larger and better-funded pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions, governmental agencies and public and private research institutions.
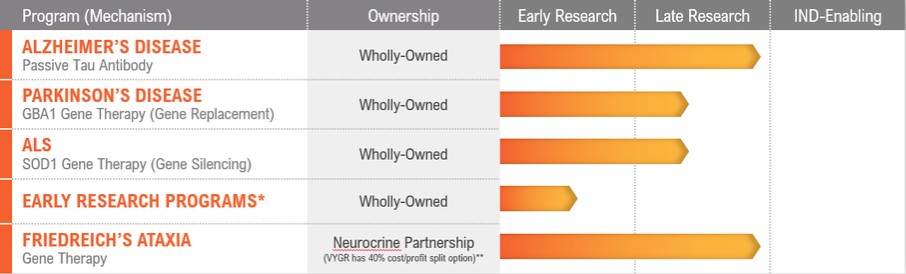
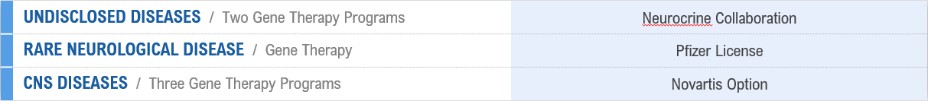
We are aware of several companies focused on developing AAV gene therapies in various indications, including AavantiBio, Inc. (merger with Solid Biosciences, Inc., or Solid, announced), Abeona Therapeutics, Inc., Adverum Biotechnologies, Inc., Aevitas Therapeutics, Inc., Akouos, Inc. (acquisition by Eli Lilly and Company, or Eli Lilly, announced), Alcyone Therapeutics, Inc., Amicus Therapeutics, Inc., Apic Bio, Inc., Applied Genetic Technologies Corporation (acquisition by Syncona Limited announced), Asklepios BioPharmaceutical, Inc., or AskBio (acquired by Bayer), Audentes Therapeutics, Inc. (acquired by Astellas Pharma Inc.), Biogen, Inc., or Biogen, Brain Neurotherapy Bio, Inc. (merged with AskBio), BioMarin, Encoded Therapeutics, Inc., GenSight Biologics SA, Homology Medicines, Inc., LEXEO Therapeutics, Inc., LogicBio Therapeutics, Inc. (acquisition by AstraZeneca announced), Lysogene SA, MeiraGTx Ltd., or MeiraGTx, Neurogene, Inc., Novartis Gene Therapies, Inc. (formerly AveXis, Inc.), Passage Bio, Inc., Pfizer, Prevail Therapeutics Inc. (acquired by Eli Lilly), PTC, REGENXBio Inc., Sarepta Therapeutics, Inc., Sio Gene Therapies, Inc., Solid, Spark, StrideBio, Inc., Taysha Gene Therapies, Inc. and uniQure, as well as several companies addressing other methods for modifying genes and regulating gene expression. Any advances in gene therapy technology made by a competitor may be used to develop therapies that could compete against any of our product candidates.
We expect that our TRACER platform preclinical programs will compete with a variety of technologies and therapies in development, including:
| ● | Our TRACER discovery platform will potentially compete with a variety of companies developing AAV capsids, including: 4D Molecular Therapeutics, Inc., Affinia Therapeutics Inc., Apertura Gene Therapy, |