UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number: 001-37906
ORGANOGENESIS HOLDINGS INC.
(Exact Name of Registrant as Specified in Its Charter)
| |
Delaware | 98-1329150 |
(State or Other Jurisdiction of | (I.R.S. Employer |
Incorporation or Organization) | Identification No.) |
85 Dan Road
Canton, MA 02021
(Address of Principal Executive Offices, Including Zip Code)
(781) 575-0775
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Class A Common Stock, $0.0001 par value | | ORGO | | Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| | | |
Large accelerated filer | ☐ | Accelerated filer | ☒ |
Non-accelerated filer | ☐ | Smaller reporting company | ☐ |
|
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting common shares held by non-affiliates of the registrant was approximately $199.9 million, computed by reference to the closing sale price of the Class A common stock as reported by The Nasdaq Capital Market on June 30, 2024, the last trading day of the registrant’s most recently completed second fiscal quarter. The Company has no non-voting common shares.
The number of shares of the registrant’s Class A common stock outstanding as of February 24, 2025 was 126,828,092.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required to be provided in Part III of this Annual Report on Form 10-K will be provided by a Definitive Proxy Statement for our 2024 Annual Meeting of Stockholders (the "Proxy Statement") to be filed with the Securities and Exchange Commission on or before April 30, 2025.
| | | | | |
Auditor Firm Id: | 49 | Auditor Name: | RSM US LLP | Auditor Location: | Boston, Massachusetts |
ORGANOGENESIS HOLDINGS INC.
ANNUAL REPORT ON FORM 10-K
FOR FISCAL YEAR ENDED December 31, 2024
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, including the sections entitled "Business," "Risk Factors" and "Management’s Discussion and Analysis of Financial Condition and Results of Operations," contains forward-looking statements. These statements may relate to, but are not limited to, expectations of our future results of operations, business strategies and operations, financing plans, potential growth opportunities, potential market opportunities and the effects of competition, as well as assumptions relating to the foregoing. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. These risks and other factors include, but are not limited to, those listed under "Risk Factors." In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "could," "expect," "plan," "anticipate," "believe," "estimate," "predict," "intend," "potential," "might," "would," "continue" or the negative of these terms or other comparable terminology. These statements are only predictions. Actual events or results may differ materially.
As used herein, except as otherwise indicated by context, references to "we," "us," "our," "the Company," "Organogenesis" and "ORGO" will refer to Organogenesis Holdings Inc. and its subsidiaries.
TRADEMARKS AND SERVICE MARKS
All trademarks, trade names, product names, graphics and logos of Organogenesis contained herein are trademarks or registered trademarks of Organogenesis Holdings Inc. or its subsidiaries, as applicable, in the United States and/or other countries. All other party trademarks, trade names, product names, graphics and logos contained herein are the property of their respective owners. The use or display of other parties’ trademarks, trade names, product names, graphics or logos is not intended to imply, and should not be construed to imply a relationship with, or endorsement or sponsorship of Organogenesis by such other party.
Solely for convenience, the trademarks, service marks and trade names referred to in this annual report are listed without the ®, (sm) and (TM) symbols, but we will assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those described in Part I, Item 1A. “Risk Factors” in this Annual Report on Form 10-K. You should carefully consider these risks and uncertainties when investing in our Class A common stock. The principal risks and uncertainties affecting our business include the following:
•Our success will depend in part on the extent to which coverage and adequate reimbursement for the costs of our products and related services will be available from government payers, private health insurers, and other third-party payers and it is uncertain whether such reimbursement will be available or, if such reimbursement is available, the rate at which it will be available. The rate of reimbursement and coverage for the use of our products has been and may continue to be unstable, unpredictable and subject to changes in government and private payer policies (including but not limited to the adoption or implementation of new or revised Medicare Local Coverage Determinations (LCDs)) that could adversely affect our business, results of operations, and financial condition. Currently, not all of our products are covered by all payers.
•Many existing and potential customers for our products are members of group purchasing organizations (GPOs) and/or integrated delivery networks (IDNs), including accountable care organizations or public-based purchasing organizations, and our business is partly dependent on major contracts with these organizations. Cost-containment efforts of our customers, GPOs, IDNs, third-party payers, and governmental organizations could adversely affect our business, results of operations, and financial condition.
•We could be subject to legal exposure if we do not comply with our reporting and payment obligations under Medicare, the Medicaid Drug Rebate Program, or any other governmental pricing programs in which our products or product candidates may participate, including through additional rebate or discount requirements, fines, sanctions, and litigation.
•We have remediated our previously reported material weaknesses as of December 31, 2024. However, we cannot guarantee that additional material weaknesses or significant deficiencies will not occur in the future. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results or prevent fraud, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price.
•We face significant and continuing competition, which could adversely affect our business, results of operations, and financial condition.
•Rapid technological change could cause our products to become obsolete, and if we do not enhance our product offerings through our research and development efforts, we may be unable to effectively compete.
•To be commercially successful, we must convince physicians that our products are safe and effective alternatives to existing treatments and that our products should be used in their procedures.
•Our failure to comply with regulatory obligations could result in negative effects on our business.
•The FDA may determine that certain of our products that are, or are derived from, human cells or tissues, such as Affinity, Novachor, and NuShield, do not qualify for regulation solely under Section 361 of the Public Health Services Act, or PHSA. To the extent that any of these products are deemed not to be HCT/Ps or Section 361 HCT/Ps, the FDA may require that we revise our labeling and marketing claims for these products or that we suspend sales of such products until FDA approval is obtained, which could adversely affect our business, results of operations, and financial condition.
•The FDA may determine that our suspension of NuCel and ReNu commercialization on May 31, 2021 was not conducted in a timely or otherwise proper manner. To the extent that our suspension of any of these products is determined not to comply with the 361 HCT/P Guidance, we may be subject to regulatory sanctions, which could adversely affect our business, results of operations, and financial condition.
•Because we depend upon a limited group of suppliers and manufacturers for our products, including Apligraf, Affinity, CYGNUS, Novachor, NuShield and PuraPly Antimicrobial products, we may incur significant product development costs or experience material delivery delays if there is an interruption in supply from any one of these suppliers or manufacturers, which could materially impact sales of our products.
•Uncertainty and adverse changes in the general economic conditions, including recent turmoil in the global banking system, may negatively affect our business.
•Our operating results may fluctuate significantly as a result of a variety of factors, many of which are outside of our control. For example, although we have reported net income for each fiscal year since the fiscal year ended December 31, 2020, we incurred significant losses in past years and we may incur losses in the future.
•Significant disruptions of our information technology systems or breaches of information security could adversely affect our business, results of operations, and financial condition.
•Our patents and other intellectual property rights may not adequately protect our products.
•We engage in transactions with related parties and the transactions present possible conflicts of interest that could have an adverse effect on our business, results of operations, and financial condition.
•Enacted or future legislation, as well as other potential regulatory reform or other healthcare reform initiatives, may result in reductions in federal funding for healthcare and/or place downward pressure on the price or reimbursement that we may receive for any approved product, which could adversely affect the operation of our business.
•The outstanding shares of our Series A Convertible Preferred Stock, par value $0.0001 (Convertible Preferred Stock) reduce the relative voting power of holders of our Class A common stock, dilute the ownership of those holders, and may adversely affect the market price of our Class A common stock.
•The holders of our Convertible Preferred Stock have special rights to exercise influence over us and our board of directors.
•Our Convertible Preferred Stock has rights, preferences, and privileges that are not held by, and are preferential to, the rights of holders of our Class A common stock, which could adversely affect our liquidity and financial condition, and may result in the interests of the Investors differing from holders of our Class A common stock.
PART I
ITEM 1. BUSINESS
Overview
Organogenesis is a leading regenerative medicine and tissue innovations company focused on empowering healing through the development, manufacturing, and sale of products for the advanced wound care, and surgical and sports medicine markets.
Our mission is to provide an integrated portfolio of healing and tissue solutions that improve lives while lowering the overall cost of health care. Several of our existing and pipeline products in our portfolio have Premarket Application (PMA) approval, or 510(k) clearance from the United States Food and Drug Administration (FDA). Our solutions address large and growing markets driven by aging demographics and increases in comorbidities such as diabetes, obesity, cardiovascular and peripheral vascular disease. We offer our differentiated products and in-house customer support to a wide range of health care customers including hospitals, wound care centers, government facilities, ambulatory surgery centers (ASCs) and physician offices.
In the Advanced Wound Care market, we focus on the development and commercialization of products for the treatment of chronic and acute wounds. We have a portfolio of regenerative medicine products capable of supporting patients from early in the wound healing process through wound closure. Our products that address the Advanced Wound Care market include Apligraf for the treatment of venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs); Dermagraft for the treatment of DFUs (manufacturing and distribution currently suspended pending transition to a new manufacturing facility or engagement of a third-party manufacturer); PuraPly AM as an antimicrobial barrier and native, cross-linked extracellular matrix scaffold for a broad variety of wound types; CYGNUS Dual as a dual-layered amniotic membrane that promotes an optimal environment for wound healing; and VIA Matrix, Affinity, Novachor, and NuShield placental allografts to address a variety of wound sizes and types as a protective barrier and extracellular matrix scaffold.
In the Surgical & Sports Medicine market, we are leveraging our broad regenerative medicine capabilities to address chronic and acute surgical wounds and tendon and ligament injuries. Our Sports Medicine products include NuShield as a surgical barrier and PuraForce as a reinforcement matrix in targeted soft tissue repairs; and Affinity, Novachor, PuraPly MZ, PuraPly AM, and PuraPly SX for management of open wounds in the surgical setting. Additionally, our Phase 3 clinical study evaluating the safety and efficacy of ReNu in symptomatic knee osteoarthritis (OA) is ongoing, and we expect to submit the biologics license application (BLA) in the second half of 2025. We also plan to evaluate the safety and efficacy of ReNu in symptomatic hip OA.
Recent Developments
License and Manufacturing Agreement with Vivex Biologics, Inc.
We enter into license and manufacturing agreements from time to time in the ordinary course of our business. In November 2023, we entered into a trademark license and manufacturing agreement with Vivex Biologics, Inc. (Vivex) to sell its CYGNUS Dual (Dual) and CYGNUS Matrix (Matrix) products, with the option to license the VIA Matrix (VIA) products. In March 2024, we exercised the option to license VIA, and accordingly in July 2024, entered into the first amendment to the trademark license and manufacturing agreement (together with the original agreement, the Vivex Agreement).
We paid an upfront licensing fee to Vivex to sell Dual and Matrix, and also agreed to pay a fixed milestone payment for Dual in the event that its average sales price (ASP) is published by certain government agencies for a specified period of time, which we remitted in December 2024. We remitted the option payment for VIA in April 2024. Additionally, we are required to pay a low double-digit royalty on the Net Sales of Dual and VIA, and a high single-digit royalty on the Net Sales of Matrix, respectively, during the royalty term, as defined in the Vivex Agreement. The royalty term is commensurate with the initial term of the contract and will continue for each subsequent renewal period. The initial term of the agreement expires on December 31, 2026 and can be renewed for up to five additional one-year terms.
Biomanufacturing facility in Smithfield, Rhode Island
In November 2024, we entered into a lease with DIV Technology Way, LLC (Davis) for a 122,000-square foot state-of-the-art biomanufacturing facility located in Smithfield, Rhode Island (Smithfield Facility). We intend to build out the Smithfield Facility for the manufacture of Dermagraft, Apligraft, and PuraPly, which we expect to commence in 2027. We are obligated under the lease to complete our build out of the Smithfield Facility within thirty-six (36) months of the lease signing, and will receive an allowance from Davis to partially offset the cost of the build out. The initial term of the lease expires in May 2041, with two ten-year renewal
options. We have a one-time right of first offer to purchase the Smithfield Facility and have a right to terminate the lease for a payment to Davis of $1.3 million, if we have not secured certain anticipated state and local tax incentives by March 31, 2025.
Series A Convertible Preferred Stock Financing and Class A Common Stock Repurchases
On November 12, 2024, we entered into a subscription agreement with Avista Healthcare Partners III, L.P. (Avista Onshore) and AHP III Orchestra Holdings, L.P. (together with Avista Onshore, the Investors, and each an Investor) pursuant to which the Investors purchased 130,000 shares of our Series A Convertible Preferred Stock, par value $0.0001 per share (Convertible Preferred Stock), for a purchase price of $1,000 per share, or aggregate gross proceeds of $130.0 million to us, prior to deduction of commissions, fees and expenses (Offering). The net proceeds will be used to fund strategic growth initiatives including, but not limited to, operating and commercial activities, clinical development programs, working capital, capital expenditures, debt repayment and for general corporate purposes. In addition, approximately $25.5 million of the net proceeds were used to fund the repurchase of an aggregate of 7,921,731 shares of Class A common stock from certain existing stockholders of the Company, including certain of its directors and their affiliates that are members of the Significant Stockholders Group. 7,421,731 of the shares of Class A common stock were repurchased at a price per share equal to $3.1597, which represented the 10-day trailing volume weighted average price of the Class A common stock as of market close on November 11, 2024 and 500,000 shares of Class A common stock were repurchased at a price per share equal to $4.057 per share, which represented the 10-day trailing volume weighted average price of the Class A common stock as of market close on November 26, 2024, pursuant to stock repurchase agreements entered into on November 12, 2024 and November 27, 2024, respectively, between us and such stockholders (Stock Repurchase Agreements, and each stock repurchase thereunder, a Repurchase).
Pursuant to the Certificate of Designations of Series A Convertible Preferred Stock (Certificate of Designation), each share of Convertible Preferred Stock is initially convertible into 263.7358 shares of Class A common stock, subject to adjustment as provided therein. The Convertible Preferred Stock ranks senior to shares of Class A common stock with respect to payment of dividends and the distribution of assets upon our liquidation, dissolution or winding up. The Convertible Preferred Stock initially has a liquidation preference of $1,000 per share; provided that the liquidation preference upon a change of control on or before November 12, 2026 will be increased to be no less than $1,500 per share. Holders of the Convertible Preferred Stock will be entitled to a regular dividend at the rate of 8.0% per annum, compounding and payable quarterly in kind or in cash, at our election, subject to the 19.99% ownership limitations described below. Any accrued but unpaid dividends will become part of the liquidation preference of such share, as set forth in the Certificate of Designation.
Until we receive stockholder approval, as contemplated by Nasdaq listing rules, with respect to the issuance of shares of Class A common stock upon conversion of the Convertible Preferred Stock in excess of the limitations imposed by such rules, holders of Convertible Preferred Stock (Preferred Stockholders) the Investors cannot convert the Convertible Preferred Stock into a number of shares of Class A common stock in excess of 26,502,042 shares, which represents 19.99% of the outstanding shares of Class A common stock at the time of signing the Subscription Agreement, or to the extent such conversion will result in a Preferred Stockholder beneficially owning greater than 19.99% of the Company’s then-outstanding shares. If, prior to receipt of the stockholder approval, a Preferred Stockholder elects to convert any Convertible Preferred Stock that would result in the issuance, when aggregated with the number of shares previously issued upon conversion of the Convertible Preferred Stock, of more than 19.99% of the outstanding shares of Class A common stock at the time of signing the Subscription Agreement, then we will, in lieu of issuing shares of Class A common stock, pay the Preferred Stockholder a cash amount equal to the product of the number of shares of Class A common stock that could not be issued due to such limitation and the 10-day trailing volume weighted average price of the Class A common stock as of the trading day immediately prior to the conversion date (Cash-in-Lieu Payments), which Cash-in-Lieu Payments shall be paid no later than November 4, 2026, together with accrued interest of 10% per annum, to the extent an earlier cash payment is prohibited pursuant to the terms of our credit agreement.
The Convertible Preferred Stock is subject to certain transfer restrictions, and contains terms regarding anti-dilution, liquidation preference, and preemptive rights, and its holders will vote together with the Class A common stock on an as-converted basis. The Convertible Preferred Stock is redeemable at the option of the Preferred Stockholders at any time after November 12, 2031, and is convertible at our option after the second anniversary of issuance if the closing price of our Class A common stock equals or exceeds 200% of the conversion price for twenty trading days out of a period of thirty consecutive trading days. The Preferred Stockholders are entitled to elect one member and one observer to our Board of Directors, subject to the terms of the Convertible Preferred Stock and applicable listing standards.
Market Overview
Advanced Wound Care Market
Wounds represent a large and growing burden on the public health as well as a significant cost to the health care system. Wounds are divided into two primary types, chronic and acute. It is estimated that approximately 27 million patients suffer from chronic and acute wounds in the United States each year, excluding surgical incisions. Chronic wounds account for most of the expenses due to their complexity and length of treatment.
Chronic wounds are wounds that have not appropriately closed after four weeks of traditional treatment such as dressings. While the underlying etiology of these chronic wounds is different, at a cellular level many of the problems that result in failed healing are the same. These include uncontrolled inflammatory processes, shortages of cell types, and reduced growth factors secreted or sensitivity to those factors by cells that are critical to healing, and that result in disrupted cell signaling pathways. Chronic wounds include:
•VLUs: wounds that occur in the lower extremities when blood does not circulate properly to the heart, caused by abnormal or damaged veins.
•DFUs: open sores or wounds that occur in patients with diabetes and are commonly located on the bottom of the foot.
•Pressure Ulcers: localized injuries to the skin and/or underlying tissues as a result of pressure or pressure in combination with shear.
•Surgical Wounds: acute wounds caused by surgical incisions that become chronic wounds if they do not heal properly.
The wound care market includes traditional dressings such as bandages, gauzes, and ointments and advanced wound care products such as mechanical devices, advanced dressings, and biologics. These advanced wound care products target chronic and acute wounds not adequately addressed by traditional therapies. Our products are primarily classified as skin substitutes, which fall within the biologics category of the Advanced Wound Care market. As of 2021, the global total addressable market for both acute and chronic wounds is estimated at approximately $20 billion.
Surgical & Sports Medicine Market
A surgical or acute wound is an injury that causes a rapid break in the skin and sometimes the underlying tissue. Acute wounds can be traumatic wounds, such as abrasions, lacerations, penetrating injuries or burns, or surgical wounds (skin grafts, dehiscences, necrotizing soft tissue infections) from surgical incisions. In contrast to chronic wounds, which would normally heal but stall due to biologic factors, acute wounds can be so severe that they overwhelm the body’s normal healing capacity. Biofilm and other infectious conditions, particularly in acute wounds with a high risk of infection such as open fractures, may also pose challenges to the healing of acute wounds.
In tendon and ligament repair, conventional surgical approaches rely on mechanical fixation to temporarily approximate damaged tissues, assuming that the natural healing process will then result in a permanent repair. Patients with impaired healing may be unable to generate the necessary tissue structures, resulting in unacceptable failure rates over time.
OA and other degenerative conditions, as well as soft tissue injuries such as tendinosis and fasciitis, are currently treated by injection with steroids or hyaluronic acid (HA). However, steroids offer pain relief for only a limited period and have been shown to further degrade some types of tissues over time, worsening the underlying condition. The evidence of HA’s efficacy has been questioned, and it is clear that a significant percentage of patients do not adequately respond to HA treatment. Patients who fail these less invasive therapies have limited options and may require surgical intervention, including total joint replacement.
Orthobiologics have been shown to be an effective alternative to traditional treatments. Due to their anti-inflammatory and pro-healing effects, they go beyond mechanical intervention to support the healing process in the damaged tissue and often result in faster healing times and shorter hospital stays. The orthobiologics market includes bone morphogenetic protein, viscosupplementation with HA, synthetic bone graft substitutes, and stem cell therapy, in addition to demineralized bone matrix (DBM) and allograft. Our current product pipeline includes Sports Medicine solutions based on placental-based technologies (ReNu). There is a rapidly growing body of clinical and scientific evidence indicating the potential of these products, particularly orthobiologics, in surgical applications, resulting in increased adoption of these products. As of 2023, the total addressable OA market is estimated at approximately $7 billion.
Our Commercial Products
We focus our efforts on medical conditions that involve difficult-to-heal wounds and musculoskeletal injuries. Healing difficulties arise from a variety of causes and in various types of tissue and anatomic areas. Impaired healing is commonly associated with an inability to move beyond the inflammatory stages of healing, resulting in a chronic wound or injury, an ongoing inflammatory cycle, and an inability to achieve normal tissue healing. Biofilm and other infectious conditions also play a key role in disrupting wound healing processes. Regenerative medicine is a collection of technologies aimed at generating tissue as close as possible to native or natural tissue, to replace damaged tissue, and to fill or replace defects. Demand for these technologies is increasing as physician understanding of the underlying wound healing processes grows and as demographic and population health trends result in the increased prevalence of systemic comorbidities that contribute to healing problems throughout the body. Our products use regenerative medicine technologies to provide solutions in the Advanced Wound Care (Chronic Wound) and Surgical (Acute Wound) & Sports Medicine markets.
Advanced Wound Care
Affinity and Novachor are fresh, amnion and chorion placental allografts, respectively, for application in the care of chronic and acute wounds as protective barriers and extracellular matrix (ECM) scaffolds. We believe both products are one of only a few placental tissue products containing viable amniotic cells, and are unique in that they undergo our proprietary AlloFresh process that hypothermically stores the products in their fresh state, never dried or frozen, which retains their native benefits and structure. Regulated as human cells, tissues, and cellular and tissue-based product, or HCT/P, under Section 361 of the Public Health Service Act (the PHSA), these products are referred to as Section 361 HCT/Ps, or simply 361 HCT/Ps. Affinity was launched in 2014 by NuTech Medical and acquired by us in 2017. Novachor was launched in December 2021.
Apligraf is a bioengineered bi-layered skin substitute that is the only product that has, to date, received PMA approval for the treatment of both VLUs and DFUs. Launched in 1998, Apligraf drives faster healing and more complete wound closure through its tissue-engineered structure, which includes an outer layer of protective skin cells (human epidermal keratinocytes), and an inner layer of cells (human dermal fibroblasts) contained within a collagen matrix. Apligraf is the leading skin substitute product for the treatment of VLUs, and its effectiveness has been established based on an extensive clinical history with over one million units shipped. We believe Apligraf is also the first and only wound-healing therapy to demonstrate in a randomized controlled trial, or RCT, a significant change in patients’ VLU wound tissue, showing a shift from a non-healing gene profile to a healing profile. Apligraf plays an active role in healing by providing the wound with living human skin cells, growth factors and other proteins produced by the cells, and a collagen matrix.
Dermagraft is a dermal substitute grown from human dermal fibroblasts and has received PMA approval for the treatment of DFUs. Launched in 2001 by Smith & Nephew and acquired by us in 2014, this product helps to restore the compromised wound bed to facilitate healing. The living cells in Dermagraft produce many of the same proteins and growth factors that support the healing response in healthy skin. In addition to an FDA-monitored RCT demonstrating its superiority to conventional therapy in the healing of DFUs, studies based on real-world evidence and Medicare data have demonstrated its superior clinical efficacy and value as compared to competitive wound care products and conventional therapy. Dermagraft can be applied weekly (up to eight times) over a twelve-week period and contains a temporary mesh fabric that is dissolvable and becomes part of the body’s own healing processes. Manufacturing of Dermagraft was suspended in the fourth quarter of 2021 and sales of Dermagraft were suspended in the second quarter of 2022. We currently plan to transition our Dermagraft manufacturing to our newly leased biomanufacturing facility in Smithfield, Rhode Island, which we expect will begin in 2027, and will result in substantial long-term cost savings. In the period when Dermagraft is not available, we expect that customers will be willing to substitute Apligraf for Dermagraft and that the suspension of Dermagraft sales will not have a material impact on our net revenue.
NuShield is a dehydrated placental allograft and surgical barrier that is topically or surgically applied to the target tissue to provide a protective barrier and ECM scaffold to support native healing. Regulated as a 361 HCT/P, NuShield is processed using our proprietary LayerLoc process, which preserves the native structure of the amnion and chorion membranes, including the intermediate or spongy layer, and their native structural and regulatory proteins. NuShield is available in multiple sizes, can be used as a protective barrier and ECM scaffold to help support native healing of chronic and acute wounds of many sizes, and can be stored at room temperature with a five-year shelf life. NuShield was launched in 2010 by NuTech Medical and acquired by us in 2017.
PuraPly Antimicrobial, or PuraPly AM, was developed to address the challenges posed by bioburden and excessive inflammation in the wound. Functioning as an antimicrobial barrier skin substitute, PuraPly AM is a purified native porcine type I collagen matrix embedded with polyhexamethylene biguanide, or PHMB, a localized broad-spectrum antimicrobial. PuraPly AM was launched in 2016 and has received 510(k) clearance for the management of multiple wound types, including partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds, trauma wounds, draining wounds, and first- and second-degree burns. The combination of PHMB with a native collagen matrix helps manage bioburden while supporting healing across a wide variety of wound types, regardless of severity or duration. Line extensions include PuraPly XT, which contains additional layers of collagen matrix and a higher level of PHMB. Extra-fenestrated (EF) versions
of the products allow for added conformability and fluid drainage. We also developed and received 510(k) clearance for PuraPly without PHMB, which we refer to as "PuraPly," including a micronized version, PuraPly MZ, for those patients who do not require an antimicrobial agent.
CYGNUS Dual is a dual-layered amniotic tissue graft used to treat chronic and acute wounds that can be stored at room temperature and has a five-year shelf life. It is manufactured in accordance with FDA regulations and American Association of Tissue Banks (AATB) standards using a methodology that helps maintain the inherent levels of key extracellular matrices, including carbohydrates, growth factors, and cytokines.
Surgical & Sports Medicine
We market our NuShield product for surgical and orthopedic applications. NuShield may be used as a surgical barrier or as an on-lay or wrap barrier to support soft tissue repairs. When used as a barrier membrane, the native biological characteristics of this placental tissue may help support the healing of soft tissue defects, particularly in difficult-to-heal locations or challenging patient populations.
We market our Affinity and Novachor products as placental allografts for acute surgical wounds and our PuraPly AM and PuraPly SX products as antimicrobial barriers for the management of open wounds in the surgical setting.
PuraForce is a bioengineered porcine collagen surgical matrix for use in soft tissue reinforcement applications. PuraPly MZ is a micronized particulate version of PuraPly that allows application in powder or gel form for the management of open wounds in the surgical setting.
Our Business Strategy
We continue to leverage our comprehensive product portfolio and relationships with key constituents to deepen our presence in the Advanced Wound Care market. We believe the breadth and flexibility of our portfolio allows and will continue to allow us to address a wide variety of wound types (chronic and acute), sizes, and reimbursement levels, offering significant new opportunities for growth. Furthermore, we believe our expanded product portfolio is enhancing the ability of our sales representatives to reach and penetrate customer accounts in various: sites of care, including, but not limited to,: operating rooms and surgical settings, physicians’ offices, wound care centers, long term care facilities, and critical access hospitals; as well as: clinical specialties, including, but not limited to, podiatry; and, various surgical categories, including: vascular, plastic, general, orthopedic, trauma, and dermatology, contributing to strong growth over time. Additionally, we believe there is significant room for expansion of the Advanced Wound Care market as a whole and our wound biologics product category in particular as more physicians and payers are educated about the benefits of regenerative medicine technologies versus traditional therapies, and as the incidence of chronic and acute wounds increases with the growing impact of societal disease states, such as diabetes, obesity, and heart disease, that cause these conditions. We continue to invest to support physician and payer education as well as preclinical and clinical trials, real-world evidence, and other research to confirm the benefits of our products. We will continue to seek expanded payer coverage for all of our products, particularly PuraPly AM/XT, Novachor, NuShield and Affinity, for which we do not yet have the broad commercial payer coverage enjoyed by Apligraf and Dermagraft.
We entered the Surgical & Sports Medicine market with the acquisition of NuTech Medical and its established and leading presence in placental-based products in 2017. We plan to continue to accelerate penetration into this market with our placental-based and collagen biomaterial products by leveraging our established commercial and operational infrastructure including our direct sales force and independent sales agencies. We also plan to continue to take advantage of significant opportunities to cross-sell within our established customer bases in both the Advanced Wound Care and Surgical & Sports Medicine markets. We believe that the Surgical & Sports Medicine market presents a strong near-term opportunity with respect to our current product portfolio as well as a significant long-term opportunity with respect to chronic inflammatory and degenerative conditions. Given our experience in the Advanced Wound Care market and regenerative medicine in general, we believe we are well positioned to capture this opportunity.
We have a robust pipeline of products in both the Advanced Wound Care and Surgical & Sports Medicine markets that we expect to launch in the next few years. We expect these products will deepen our portfolios and allow us to address additional clinical applications, such as patients requiring care for burns of varying degrees. In addition, we anticipate our ongoing efforts to complete clinical studies and publish research regarding our products will further enhance physician and payer receptiveness to our products over time. Our proven research and development capabilities and established technology platforms also support a robust and adaptable product pipeline for future applications.
We plan to continue to expand the reach and penetration of our products by optimizing our sales organization to serve the Advanced Wound Care and Surgical & Sports Medicine markets. This effort should allow us to achieve more focused and effective sales coverage for specific market categories, broaden our geographic footprint, and leverage our expanding relationships with large
hospital systems and GPOs. We also plan to increase our focus on sales outside of the United States, including the European Union and the Middle East. Currently, substantially all of our sales are in the United States.
We have demonstrated our ability to successfully identify and integrate assets that complement our strategy through the acquisitions of Dermagraft and TransCyte from Shire and our placental-based products from NuTech Medical. We continue to evaluate tuck-in acquisitions which complement our existing portfolios in both the Advanced Wound Care and Surgical & Sports Medicine markets and will leverage our established commercial and manufacturing infrastructure.
Platform Technologies
Our proven research and development capabilities and established technology platforms support a robust and adaptable product pipeline for future applications. The platform technologies in which we have deep experience include:
•Bioengineered Cultured Cellular Products: The development and production of bioengineered cultured cellular products have been a core competency since our founding. Our Apligraf, Dermagraft, and TransCyte products all draw from our expertise in this area.
•Collagen Biomaterial Technology Platform: Our porcine collagen biomaterial technology platform incorporates proprietary tissue cleaning processes and allows us to bioengineer products for specific applications by controlling thickness, strength, and remodeling rates. We currently hold 510(k) clearances for a number of products in this platform with indications ranging from tendon reinforcement to plastic surgery and general surgery applications.
•Placental-Based Products: Our placental-based products are based on significant expertise in the processing of placental tissues and fluids to yield products with desirable characteristics. We have expertise using the full array of available tissue types and multiple processing methodologies, including our proprietary AlloFresh and LayerLoc processing methods. Our proprietary AlloFresh process hypothermically stores our Affinity product in its fresh state, never dried or frozen, which retains its native benefits and structure. Our proprietary LayerLoc process preserves the native structure of the amnion and chorion membranes, optimized to provide excellent strength, flexibility, and handling.
•Antimicrobial Technology: Our PHMB antimicrobial technology provides clinical and competitive advantage for multiple wound indications. PHMB is a broad-spectrum effective antimicrobial that prevents biofilm reformation. We have developed multiple product versions incorporating PHMB that have demonstrated clinical benefit to control bioburden and support wound healing when used following wound debridement.
Product Pipeline
We have a robust pipeline of products under development for both the Advanced Wound Care and Surgical & Sports Medicine markets. We believe our pipeline efforts will deepen our comprehensive portfolio of offerings as well as allow us to address additional clinical applications.
PuraPly and PuraPlyAM Line Extensions
The PuraPly portfolio is comprised of a purified native collagen matrix. PuraPly AM and PuraPly SX are native collagen scaffolds that also provide an antimicrobial barrier utilizing a broad spectrum antimicrobial agent (PHMB). We have several line extensions in development.
Placental Portfolio Expansion
We have placental products under development. Our research and development (R&D) team continues to research and develop additional product concepts from our placental technology platform, as well as to collaborate with our Business Development team to assess additional product in-licensing or acquisition opportunities.
Apligraf and Dermagraft Line Extensions
We have two development projects underway to develop additional sizes of Apligraf and Dermagraft. The objective is to develop at least one additional smaller size of each product to optimize clinical utilization for smaller wounds such as DFUs. These types of changes to living cell-based products require significant development and validation work and will require FDA PMA Supplement approval for the changes. Therefore, we expect the duration of the development projects to be several years before commercial products will be available. Manufacturing of Dermagraft line extensions is dependent on the completion of manufacturing and supply capabilities for the product.
FortiShield
FortiShield is a biosynthetic wound matrix made from a semi-permeable silicone membrane bonded to a kitted nylon fabric and coated with collagen, to provide a flexible dressing that is designed to adhere to the application site, provide a barrier to the external environment, and allow for excess exudate drainage. FortiShield is intended for use as a temporary protective covering, and to provide a moist wound healing environment on cleanly debrided wounds after hemostasis has been established. The primary indication for the product is as a transitional wound matrix for second degree burns. There are additional chronic and acute wound applications. The product received 510(k) clearance in May 2023. Commercial launch is dependent upon the completion of manufacturing and supply capabilities for the product.
TransCyte
TransCyte is a bioengineered tissue scaffold that promotes burn healing and has received PMA approval for the treatment of deep second- and third-degree burns. TransCyte is a flexible, durable product that provides bioactive dermal components, an outer protective barrier, increased re-epithelialization and pain relief for patients suffering from burns. Full launch is dependent on the completion of manufacturing capabilities.
ReNu
ReNu is a cryopreserved suspension derived from human amniotic membrane and cells derived from amniotic fluid. The initial target indication for ReNu is for the management of symptoms associated with knee OA. We are in the planning stages for clinical studies of ReNu to support the management of symptoms associated with hip OA, and we believe ReNu may have potential as a treatment for additional OA and tissue regeneration applications, which would need to be clinically evaluated further before any such approved uses.
Ongoing Clinical Studies
We believe gathering robust and comprehensive clinical and real-world outcomes data is an essential component of developing a competitive product portfolio and driving further penetration in the markets where we compete. We continue to invest in generating clinical data for our Advanced Wound Care and Surgical & Sports Medicine products, and believe such data enhance sales efforts with physicians and reimbursement dynamics with payers over time. As used herein, p value is a measure of statistical significance. The lower the p value, the more likely it is that the results of a clinical trial or study are statistically significant rather than an experimental anomaly. Generally, to be considered statistically significant, such results must have a p value <0.05.
As noted above, we completed a phase 3 prospective, multicenter, double-blind, randomized, saline-controlled clinical trial to evaluate the efficacy of amniotic suspension allograft (ASA, ReNu) in patients with knee OA, and completed topline analysis in the second quarter of 2024. We reported results consistent with the predefined requirements for study success: statistically significant reduction in knee pain (p=0.0177) and statistically significant maintenance of function (p<0.0001) at six months.
We completed enrollment in a second phase 3 prospective, multicenter, double-blind, randomized, saline-controlled clinical trial to evaluate the efficacy of ASA in patients with OA of the knee in the second quarter of 2024. This clinical trial completed enrollment with 594 randomized subjects with Kellgren-Lawrence (KL) severity 2 to 4 knee OA. The study performed the prespecified interim analysis on 50% of the planned 474 subjects after six-months of follow up in the fourth quarter of 2024. The DMC recommended the clinical trial proceed without modification and without increase in sample size. The DMC also reviewed available safety data and found the safety data to be consistent with the known safety profile for ASA (ReNu). We expect to have all patients completing the study by the end of the second quarter of 2025, and to complete the initial statistical analysis and have top-line data results from the second phase 3 study to share publicly in September 2025. Our current timeline targets completion of the final clinical study report required for the BLA submission in the fourth quarter of 2025, and expect to submit the BLA by the end of 2025.
Commercial Infrastructure
Sales and Marketing
Our current Advanced Wound Care portfolio is sold throughout the United States via an experienced direct sales force. We use a mix of direct sales representatives and independent agencies to service the Surgical & Sports Medicine market. As of December 31, 2024, we had 256 direct sales representatives and approximately 160 independent agencies. These sales representatives are supported by teams of professionals focused on sales management, sales operations and effectiveness, ongoing training, analytics, and marketing.
Sales generated by our direct sales forces in the United States have represented, and we anticipate will continue to represent, a majority of our revenues. In addition, we have obtained marketing registrations, developed commercial and distribution capabilities, and are currently selling products in several countries outside of the United States. Our Apligraf product is currently distributed by our direct sales force in Switzerland, and through independent sales agents in Saudi Arabia and Kuwait. Our NuShield product is also distributed by our direct sales force in Switzerland, and through independent sales agents in Kuwait. We have obtained marketing registration for our Dermagraft product in Mexico, but we are not currently distributing it. Additionally, we are evaluating the regulatory pathways and market potential for our products in other major markets, including the European Union.
Customer Support Services
We offer in-house customer support services, including our reimbursement support team, our medical and technical support team, and our field-based medical science liaison team. We believe that providing these essential support services in-house creates a competitive advantage by allowing us to align our support services with our sales efforts leading to improvements in the overall customer experience.
Research and Development
Our R&D team works to design products that are intended to improve patient outcomes, simplify techniques, shorten procedures, reduce hospitalization and rehabilitation times, and, as a result, reduce costs. We conduct research and development activities at our laboratory facilities in Canton, MA, Birmingham, AL, and San Diego, CA. We have an internal team that is comprised of individuals with significant experience and training at leading colleges and universities with regenerative medicine graduate programs. In addition to our internal staff, our external network of development labs, testing labs, and expert clinicians aid us in our research and development process.
The majority of our product portfolio, including Apligraf, our PuraPly product family, our collagen biomaterial technology platform product family, and all of our placental-based products, was developed by our R&D team at our three facilities. We have proven competencies to bring products to market through a broad range of regulatory classifications.
Manufacturing and Suppliers
We manufacture our primary non-placental-based products and use third-party manufacturers for our placental-based products. We have significant expansion capabilities in our in-house manufacturing facilities and we believe that our contract manufacturers are well positioned to support future expansion.
We have robust internal compliance processes to maintain the quality and reliability of our products. We conduct annual internal audits, combined with external audits by regulatory agencies, to monitor our quality control practices. We are registered with the FDA as a medical device manufacturing establishment and a HCT/P registered establishment. We are also accredited by the AATB and licensed with several states per their tissue banks regulations. All of our contract manufacturers are registered with the FDA as HCT/P establishments and are AATB accredited.
We utilize third-party raw material suppliers to support our internal manufacturing processes. All prospective suppliers are subject to a rigorous vetting process to ensure quality and reliability. Additionally, our approved suppliers are audited at pre-determined intervals to ensure continued reliability.
The manufacture of our products is dependent on the availability of sufficient quantities of source tissue, which is the primary component of our products. Source tissue includes donated human tissue, porcine tissue, and bovine tissue. We acquire donated human tissue directly through institutional review board-approved protocols at multiple hospitals, as well as through tissue procurement firms engaged by us or by our contract manufacturers. We have two qualified porcine tissue suppliers, and currently one source of bovine tissue. Historically, we have not experienced significant difficulty locating and obtaining the suppliers or materials necessary to fulfill our production requirements.
Government Regulation
FDA Regulation of Product Registration, Manufacture, and Promotion
We market medical products in the United States that have either been approved or cleared by the FDA prior to marketing, or do not require FDA premarket review. Our marketed products that have received marketing authorization from the FDA have done so under one of the following agency pathways: 510(k) clearance for a Class II medical device or approval of a PMA for a Class III
medical device. These medical products are regulated by the FDA under the PHSA or the Federal Food, Drug, and Cosmetic Act (FDCA) along with the FDA’s implementing regulations. These federal statutes and regulations govern, among other things, the following activities that we perform or are performed on our behalf and will continue to perform or have performed on our behalf: the production, research, development, testing, manufacture, quality control, packaging, labeling, storage, approval, advertising, and promotion, distribution of our products into interstate commerce, record keeping, service and surveillance, complaint handling, repair or recall of products, adverse event reporting and other field safety corrective actions.
FDA Regulatory Review and Approval Process
With respect to the manufacture of medical devices and biologics, the FDA regulates and inspects equipment, facilities, laboratories, and processes used in the manufacturing and testing of products prior to providing approval to market products. After receiving approval from the FDA, additional regulatory review or inspection may be required if we make a material change in manufacturing equipment, location or process. Our manufacturing processes must comply with the FDA’s Quality System Regulation, or QSR, for our medical device products. The QSR requires that each device manufacturer establish and implement a quality system under which the manufacturer monitors the manufacturing process and maintains records that show compliance with FDA regulations and the manufacturer’s written specifications and procedures relating to the devices. Among other things, these regulations require that manufacturers establish performance requirements before production and follow requirements applicable to design controls, testing, record keeping, documentation, manufacturing standards, labeling, complaint handling, and management review.
Manufacturers of biologics must comply with the FDA’s applicable Current Good Manufacturing Practices (cGMP) regulations, including quality control and quality assurance and maintenance of records and documentation. Manufacturers and others involved in the manufacture and distribution of such products also must register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process. Concurrent with clinical trials, companies usually complete additional preclinical studies and must also develop additional information about the physical characteristics of the biologic product candidate, as well as finalize a process for manufacturing the product candidate in commercial quantities in accordance with cGMP requirements. To help reduce the risk of the introduction of adventitious agents or of causing other adverse events with the use of biologic products, the PHSA emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other requirements, the sponsor must develop methods for testing the identity, strength, quality, potency, and purity of the final biologic product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the biologic product candidate does not undergo unacceptable deterioration over its shelf life.
In addition, we must comply with medical device reporting regulations and corrections and removal reporting regulations. Medical device reporting regulations require that manufacturers report to the FDA if their devices may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. Corrections and removal reporting regulations require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health. The FDA may also order a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death.
Certain human cells, tissues, and cellular and tissue-based products, or HCT/Ps, are regulated under Section 361 of the PHSA and are referred to as "Section 361 HCT/Ps" or simply "361 HCT/Ps," while other HCT/Ps are subject to the FDA’s regulatory requirements for medical devices and/or biologics. A product that is regulated as a 361 HCT/P may be commercially distributed without prior FDA clearance or approval. Pursuant to 21 CFR 1271.10, in order to be regulated as a 361 HCT/P, and hence exempt from premarket review, an HCT/P must be minimally manipulated, intended for homologous use, and manufactured without being combined with another article (except for water, crystalloids, or sterilizing, preserving, or storage agents). The HCT/P must also either have no systemic effect and not be dependent upon the metabolic activity of living cells for its primary function or, if it has a systemic effect, be intended for autologous use, for allogeneic use in a first-degree or second-degree blood relative or for reproductive use. We believe that Affinity and NuShield generally fulfill the relevant criteria under 21 CFR 1271.10. In light of the 361 HCT/P Guidance, our labeling and marketing claims for Affinity and NuShield clarify that they are intended for use as protective barriers, and thus qualify as Section 361 HCT/Ps. However, the FDA could disagree with our conclusion and require premarket approval or clearance for Affinity, NuShield, or any placental-based sheet product we presently have or may have in the future market, which would disrupt the marketing of these products, potentially expose us to regulatory sanctions, and have a material adverse effect on our business, financial condition and results of operations. Section 361 HCT/Ps are subject to specific FDA regulations that include cGTPs, donor eligibility determination requirements, adverse event reporting, and advertising and labeling requirements. cGTP regulations govern the methods used in, and the facilities and controls used for, the manufacture of HCT/Ps, including but not limited to all steps in recovery, donor screening, donor testing, processing, storage, labeling, packaging, and distribution.
The FDA is authorized to expedite the review of BLAs in several ways. Under the Fast Track program, the sponsor of a biologic product candidate may request the FDA to designate the product for a specific indication as a Fast Track product concurrent with or after the filing of the BLA. Biologic products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product candidate and the specific indication for which it is being studied. In addition to other benefits, such as the ability to have greater interactions with the FDA, the FDA may initiate review of sections of a Fast Track BLA before the application is complete, a process known as rolling review. We plan to request Priority Review of the ReNu BLA.
Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as breakthrough therapy designation, regenerative medicine advance therapy designation, priority review and accelerated approval.
Post-approval Requirements
FDA regulation of biologic products continues after approval, particularly with respect to cGMP requirements, including quality control and quality assurance and maintenance of records and documentation. Other post-approval requirements applicable to biologic products include reporting of cGMP deviations that may affect the identity, potency, purity and overall safety of a distributed product, record-keeping requirements, reporting of adverse effects, reporting updated safety and efficacy information and complying with electronic record and signature requirements. Failure to comply with the applicable United States requirements at any time during the product development process, approval process or after approval, may subject an applicant or manufacturer to administrative or judicial civil or criminal actions and adverse publicity. These actions could include refusal to approve pending applications or supplemental applications, withdrawal of an approval, clinical hold, suspension or termination of a clinical trial by an Institutional Review Board (IRB), warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines or other monetary penalties, refusals of government contracts, mandated corrective advertising or communications with healthcare providers, debarment, restitution, disgorgement of profits or other civil or criminal penalties.
Advertising, marketing and promotional activities for devices and biologics are also subject to FDA oversight and must comply with the statutory standards of the FDCA, and the FDA’s implementing regulations. The FDA’s oversight authority review of marketing and promotional activities encompasses, but is not limited to, direct-to-consumer advertising, healthcare provider-directed advertising and promotion, sales representative communications to healthcare professionals, promotional programming and promotional activities involving electronic media. The FDA also regulates industry-sponsored scientific and educational activities that make representations regarding product safety or efficacy in a promotional context. A sponsor also must comply with the FDA’s advertising and promotion requirements, such as the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as off-label use). The FDA may take enforcement action against a company for promoting unapproved uses of a product or for other violations of its advertising and labeling laws and regulations. Enforcement actions may include product seizures, injunctions, civil or criminal penalties or regulatory letters, which may require corrective advertising or other corrective communications to healthcare professionals.
Reimbursement
Our customers primarily consist of hospitals, wound care centers, government facilities, ASCs, and physician offices, all of which rely on coverage and reimbursement for our products by Medicare, Medicaid, and other third-party payers. Governmental healthcare programs, such as Medicare and Medicaid, typically have published and defined coverage criteria and published reimbursement rates for medical products, services, and procedures that are established by law or regulation. Non-government payers have their own coverage criteria and often negotiate payment rates for medical products, services, and procedures. Many also require prior authorization as a prerequisite to coverage. In addition, in the United States, an increasing percentage of insured individuals are receiving their medical care through managed care programs, which monitor utilization and also may require prior authorization for the products and services that a member receives. Coverage and reimbursement from government and commercial payers are not assured and are subject to change.
Medicare, the federally funded program that provides healthcare coverage for senior citizens and people with disabilities, is the largest third-party payer in the United States. The Centers for Medicare and Medicaid Services (CMS) administers the Medicare program and, for Medicare Parts A and B (often referred to as "traditional Medicare") uses Medicare Administrative Contractors (MACs) to process claims, develop coverage policies and make payments within designated geographic jurisdictions. CMS does not currently have a national coverage determination related to skin substitutes. Coverage for our skin substitute products falls under the jurisdiction of the Part A/B MACs. Medicare coverage for these products is determined by each MAC for its specific jurisdiction; coverage by MACs can be determined either through case-by-case review of claims for medical necessity or based on local coverage determinations (LCDs). Implementation of LCDs by one or more MACs can therefore affect coverage policy for certain products or product candidates and/or certain uses of those products, depending on the scope of the LCD(s). Additionally, Medicare Advantage
(MA) Plans (Medicare Part C) are required to cover items and services that are covered by Medicare Parts A and B, and MA Plans are not required to cover items and services that are not covered by Medicare Parts A and B. MA Plans also must specify any additional benefits that they provide as supplemental benefits approved by CMS.
Private payers often, but not always, follow the lead of Medicare or other governmental payers in making coverage and reimbursement determinations. Therefore, achieving favorable Medicare coverage and reimbursement can sometimes be a significant factor in obtaining favorable coverage and reimbursement for products by private payers. While most private payers currently cover Apligraf and Dermagraft, and some cover Affinity, most of those payers provide limited coverage for our other products, such as PuraPly, PuraPly AM, NuShield and CYGNUS.
Currently, Medicare makes a separate payment for our products when used in the physician office at a payment rate based on the average sales price (ASP) methodology, including ASP plus 6% for some products. In the outpatient hospital and ASC settings, Medicare payment for all our products is currently bundled into the payment for the application procedure.
Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more of our products or product candidates, less favorable coverage policies and reimbursement rates may be implemented in the future. It is difficult to predict whether changes in Medicare and/or other third-party coverage and reimbursement policies could be implemented that would affect our products and product candidates.
Intellectual Property
Our success depends in part on our ability to protect our proprietary technology and intellectual property and operate without infringing the patents and other proprietary rights of third parties. We rely on a combination of trademark, trade secret, patents, copyright, and other measures to protect the intellectual property rights that we consider important to our business. We also rely on know-how and continuing technological innovation to develop and maintain our competitive position. Other than a license from Novartis Pharma AG for trademark and domain name rights to Apligraf and an exclusive license from RESORBA Medical GmbH, or Resorba, to a United States patent for a collagen-based wound dressing containing PHMB, we do not have any additional material licenses to any technology or intellectual property rights.
As of December 31, 2024, we owned 49 issued patents globally, of which 17 were United States patents. As of December 31, 2024, we owned 21 pending patent applications, of which 9 were patent applications pending in the United States. Many of our issued patents are currently expected to expire between 2027 and 2042. The expiration of these patents is not expected to have a material impact on our business. Additionally, we own or have rights to trademarks or trade names that are used in our business and in conjunction with the sale of our products, including 14 United States trademark registrations and 36 foreign trademark registrations, as of December 31, 2024.
Seasonality
Revenues during our fourth quarter tend to be stronger than other quarters because many hospitals increase their purchases of our products during the fourth quarter to coincide with the end of their budget cycles in the United States. Satisfaction of deductibles through the course of the year also results in increased revenues later in the year. In general, our first quarter usually has lower revenues than the preceding fourth quarter, the second and third quarters have higher revenues than the first quarter, and the fourth quarter revenues are the highest in the year.
Competition
We operate in highly competitive markets that are subject to rapid technological change. Additionally, due to lower barriers to entry in the Section 361 HCT/P regulated market, competition in the placenta-based and allograft tissue field is intense and subject to new entrants and evolving market dynamics. We are aware of several companies that compete, or are developing technologies, in our current and future product areas. Our products compete primarily with skin substitute products, placental-based technology products, orthobiologics products, other advanced wound care and traditional wound care products, among others. We also compete in the marketplace to recruit and retain qualified scientific, management and sales personnel, as well as to acquire technologies and technology licenses complementary to our products or advantageous to our business.
Success in these markets depends primarily on product efficacy, ease of product use, product price, availability of coverage and adequate third-party reimbursement, customer support services for technical, clinical, and reimbursement support, and customer
preference for, and loyalty to, the products. We believe that the demonstrated clinical efficacy of our products, the breadth of our product portfolio, our in-house customer support services, our customer relationships and reputation offer us advantages over our competitors. We also believe our success in obtaining third-party reimbursement, our strong position with group purchasing organizations, and the established clinical evidence for our products are competitive advantages. In addition, we believe we are one of the few regenerative medicine companies offering PMA approved and 510(k) cleared products in addition to our 361 HCT/Ps.
Human Capital Resources
Our success is realized through the engagement and commitment of our people. As of December 31, 2024, we had approximately 869 employees worldwide. In managing our business, we focus on a number of factors with respect to the attraction, development, and retention of our employees, including:
•We are proud to be an equal opportunity employer. We seek to attract a diverse slate of candidates, including from historically underrepresented groups. We believe that diversity and inclusion in the workplace enhance employee engagement and stimulate innovation, and that people in diverse groups work better, share information more broadly and consider a wider range of views. We pride ourselves on our diverse workforce, which we believe has been and will continue to be a major contributor to our growth and innovation, and intend to continue to make diversity and inclusion a focus of our efforts regarding our workforce.
•We aim to maintain an "open door" culture, and encourage employees to voice their concerns, questions, suggestions and comments. We strive to foster an atmosphere where employees openly share ideas and where people are treated with dignity and respect. Our goal is to provide a productive working environment based on mutual respect and the highest level of ethical and lawful conduct. We have also established a hotline for employees to report suspected violations of law and concerns related to accounting, auditing, compliance and ethical violations.
•We provide our employees a competitive wage and evaluate our compensation programs to ensure that our employees are paid fairly for the valuable work they are doing. We are also committed to achieving internal pay equity and rewarding outstanding performance. We offer our employees competitive benefits and are proud that we have not raised employee contributions to our healthcare benefits for 8 years running.
•We aim to foster a culture where learning is continuous, and we strive to promote from within. We believe in our people and their ability to accept new responsibilities and challenges and to grow with us to contribute to our success. Growth is fostered through professional development and learning programs as well as practical experience. Employees receive regular performance reviews to support their progress and development.
•We recognize the benefits of a healthy workforce and offer our employees the opportunity to participate in wellness activities and programs throughout the year. We also support the mental health of our employees by offering Mental Health and Wellness training for managers and employees. We also provide an employee assistance program for employees and their families that provides free counseling sessions and offers other resources for employees. Additionally, our healthcare benefit allows for reimbursement for fitness and weight loss programs.
•We prioritize the health and safety of our employees. Guided by an Environmental Health & Safety (EHS) manual that is regularly reviewed, we have a dedicated EHS team, who seek to prevent and reduce workplace risks and injuries through various programs, training, projects, services, and assistance, such as ergonomic evaluation, hazard reporting, risk assessment, and first aid training. We require all work-related injuries or illnesses to be reported. This information is reviewed bi-monthly by our EHS Team and Safety Committee for analysis and trending.
Available Information
Our Internet website address is http://www.organogenesis.com. Through our website, we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as well as proxy statements, and, from time to time, other documents as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or SEC. These SEC reports can be accessed through the "Investors" section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
Item 1A. Risk factors
An investment in our securities, including our common stock, involves a high degree of risk. Investors should carefully consider the risks and uncertainties described below, together with the information included elsewhere in this Annual Report on Form 10-K and other documents we file with the SEC.
Risks Related to Organogenesis and its business
Our operating results may fluctuate significantly as a result of a variety of factors, many of which are outside of our control.
We are subject to the following factors, among others, that may negatively affect our operating results:
•the announcement or introduction of new products by our competitors;
•failure of government healthcare programs and private health plans to cover our products or to timely and adequately reimburse the users of our products;
•the rate of reimbursement by government and private insurers for use of our products;
•any change in Medicare payment policy which provides a competitive advantage to our competitor’s products;
•any change in government healthcare programs’ and private health plans’ policies regarding sales and reimbursement of durable medical equipment (DME), including a prohibition on physician-owned DME supplier entities;
•our ability to upgrade and develop our systems and infrastructure to accommodate growth;
•our ability to attract and retain key personnel in a timely and cost-effective manner;
•our ability to offer our wound care and surgical products and supplies using our existing sales force and distribution network;
•the amount and timing of operating costs and capital expenditures relating to the expansion of our business, operations, and infrastructure;
•changes in, or enactment of new laws or regulations promulgated by federal, state, or local governments;
•cost containment initiatives or policies developed by government and commercial payers that create financial incentives not to use our products;
•our inability to demonstrate that our products are cost-effective or superior to competing products;
•our ability to develop new products;
•discovery of product defects during the manufacturing process;
•initiation of a government investigation into potential non-compliance with laws or regulations;
•issuance of government advisory opinions or program bulletins that could negatively affect one or more of our sales models;
•sanctions imposed by federal or state governments due to non-compliance with laws or regulations;
•recall of one or more of our products by the FDA due to noncompliance with FDA requirements; and
•general economic conditions as well as economic conditions specific to the healthcare industry.
Rapid technological change could cause our products to become obsolete, and if we do not enhance our product offerings through our research and development efforts, we may be unable to effectively compete.
The technologies underlying our products are subject to rapid and profound technological change. Competition intensifies as technical advances in each field are made and become more widely known. We can give no assurance that others will not develop services, products, or processes with significant advantages over the products, services, and processes that we offer or are seeking to develop. Any such occurrence could have a material and adverse effect on our business, results of operations, and financial condition.
We plan to enhance and broaden our product offerings in response to changing customer demands and competitive pressure and technologies, but we may not be successful. The success of any new product offering or enhancement to an existing product will depend on numerous factors, including our ability to:
•properly identify and anticipate physician and patient needs;
•develop and introduce new products or product enhancements in a timely manner;
•adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties;
•demonstrate the safety and efficacy of new products, including through the conduct of additional clinical trials;
•obtain the necessary regulatory clearances or approvals for new products or product enhancements;
•achieve adequate coverage and reimbursement for our products; and
•compete successfully against other skin substitutes and other modalities for treating wounds such as negative-pressure wound therapy and hyperbaric oxygen.
If we do not develop and, when necessary, obtain regulatory clearance or approval for new products or product enhancements in time to meet market demand, or if there is insufficient demand for these products or enhancements, our results of operations will suffer. Our research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. In addition, even if we are able to successfully develop enhancements or new generations of our products, these enhancements or new generations of products may not be covered or reimbursed by government healthcare programs such as Medicare or private health plans, may not produce sales in excess of the costs of development and/or may be quickly rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
To be commercially successful, we must convince physicians that our products are safe and effective alternatives to existing treatments and that our products should be used in their procedures.
We believe physicians will only adopt our products if they determine, based on experience, clinical data and published peer-reviewed journal articles, that the use of our products in a particular procedure is a favorable alternative to conventional methods. Physicians also are more interested in using cost-effective products and may practice in settings like Accountable Care Organizations, or ACOs, or Medical Homes, where they face considerable cost-containment pressure. In general, physicians may be slow to change their medical treatment practices and use of our products for many reasons, including but not limited to: their lack of experience using our products; pressure to contain costs; preference for other treatment modalities or our competitors’ products; perceived liability risks generally associated with the use of new products and procedures; limited availability of coverage and/or reimbursement from third-party payers; and the time that must be dedicated to training.
We believe recommendations for, and support of our products by, influential physicians are essential for market acceptance and adoption. If we do not receive this support (e.g., because we are unable to demonstrate favorable long-term clinical data), physicians and hospitals may not use our products, which would significantly reduce our ability to achieve expected revenue and would prevent us from sustaining profitability.
We face the risk of product liability claims and may not be able to obtain or maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the manufacturing, processing, investigating, and marketing of medical devices and human tissue products. We are, and may in the future be, subject to product liability claims and lawsuits, including potential class actions or mass tort claims, alleging that our products have resulted or could result in an unsafe condition or injury. Product liability claims may be made by patients and their families, healthcare providers, or others selling our products. Defending a lawsuit, regardless of merit, could be costly, divert management attention, and result in adverse publicity, which could result in the withdrawal of, or reduced acceptance of, our products in the market. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. Additionally, regardless of merit or eventual outcome, product liability claims may result in harm to our business reputation, investigations by regulators, significant defense costs, distraction of and substantial monetary awards to patients or other claimants, among other adverse consequences.
Although we have product liability insurance that we believe is adequate, this insurance is subject to deductibles and coverage limitations and we may not be able to maintain this insurance. Also, it is possible that claims could exceed the limits of our coverage or be excluded from coverage under our policy. If we are unable to maintain product liability insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect ourselves against potential product liability claims or we underestimate
the amount of insurance we need, we could be exposed to significant liabilities, which may harm our business. One or more product liability claims could cause our stock price to decline and, if our liability exceeds our insurance coverage, could adversely affect our business, results of operations, and financial condition.
Interruptions in the supply of our products or inventory loss may adversely affect our business, results of operations, and financial condition.
Our products are manufactured using technically complex processes requiring specialized facilities, highly specific raw materials, and other production constraints. The complexity of these processes, as well as strict company and government standards for the manufacture and storage of our products, subjects us to production risks.
In addition to ongoing production risks, process deviations or unanticipated effects of approved process changes may result in non-compliance with regulatory requirements including stability requirements or specifications. Most of our products must be stored and transported within a specified temperature range. For example, if environmental conditions deviate from that range, our products’ remaining shelf-lives could be impaired or their safety and efficacy could be adversely affected, making them unsuitable for use. These deviations may go undetected. The occurrence of actual or suspected production and distribution problems can lead to lost inventories, and recalls, with consequential reputational damage and the risk of product liability. The investigation and remediation of any identified problems can cause production delays and result in a loss of our market share and negatively affect our revenues and operations.
Because we depend upon a limited group of suppliers and manufacturers for our products, including Apligraf, Affinity, CYGNUS, Novachor, NuShield and PuraPly Antimicrobial products, we may incur significant product development costs or experience material delivery delays if we lose any significant supplier, which could materially impact sales of our products.
We obtain some of the components for our products from a limited group of suppliers. These suppliers must be able to provide us with these components in substantial quantities, in compliance with regulatory requirements, in accordance with agreed-upon specifications, at acceptable costs, and on a timely basis. Our efforts to maintain a continuity of supply may not be successful. Manufacturing disruptions experienced by our suppliers may jeopardize our supply of these components. Due to the stringent regulations and requirements of the FDA regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources for certain components or materials. A change in suppliers could require significant effort or investment. A reduction or interruption in manufacturing, or an inability to secure alternative sources of raw materials or components, could have a material effect on our business, results of operations, and financial condition.
In addition, one or more of our suppliers may refuse to extend us credit with respect to our purchasing or leasing equipment, supplies, products, or components, or may only agree to extend us credit on significantly less favorable terms or subject to more onerous conditions. This could significantly disrupt our ability to purchase or lease required equipment, supplies, products and components in a cost-effective and timely manner and could have a material adverse effect on our business, results of operations, and financial condition. Any casualty, natural disaster, other disruption of any of our sole-source suppliers’ operations, or any unexpected loss of any existing exclusive supply contract, could have a material adverse effect on our business, results of operations, and financial condition.
Our products are dependent on the availability of tissue from human donors, and any disruption in supply could adversely affect our business, results of operations, and financial condition.
Many of the products that we manufacture require that we obtain human tissue. The success of our business depends upon, among other factors, the availability of tissue from human donors. Any failure to obtain tissue from our sources will interfere with our ability to effectively meet the demand for our products incorporating human tissue. The processing of human tissue for our products is very labor-intensive and it is therefore difficult to maintain a steady supply stream. The availability of donated tissue could also be adversely impacted by regulatory changes, public opinion of the donor process as well as our own reputation in the industry. The challenges we may face in obtaining adequate supplies of human tissue involve several risks, including limited control over the availability, quality, and delivery schedules. In addition, any interruption in the supply of any human tissue component could materially harm our ability to manufacture our products until a new source of supply, if any, could be found. We may be unable to find a sufficient alternative supply channel in a reasonable time period or on commercially reasonable terms, if at all, which would have a material adverse effect on our business, results of operations, and financial condition.
Increased prices for, or unavailability of, raw materials used in our products could adversely affect our business, results of operations, and financial condition.
Our profitability is affected by the prices of the raw materials used in the manufacture of our products. These prices may fluctuate based on a number of factors beyond our control, including changes in supply and demand, general economic conditions, labor costs, fuel-related delivery costs, competition, import duties, excises and other indirect taxes, currency exchange rates, and government regulation. Due to the highly competitive nature of the healthcare industry and the cost containment efforts of our customers and third-party payers, we may be unable to pass along cost increases for key components or raw materials through higher prices to our customers. If the cost of key components or raw materials increases, and we are unable fully to recover these increased costs through price increases or offset these increases through other cost reductions, we could experience lower margins and profitability. Significant increases in the prices of raw materials, due to inflation or otherwise, that cannot be recovered through productivity gains, price increases or other methods could adversely affect our business, results of operations, and financial condition.
We continue to invest significant capital to maximize our sales and marketing infrastructure, and there can be no assurance that these efforts will result in significant increases in sales.
We are committed to maximizing our internal sales and marketing capabilities, including by optimizing our sales force to further support the marketing and sales of the products acquired in connection with our 2017 acquisition of NuTech Medical and our 2020 acquisition of CPN Biosciences. As a result, we continue to invest in sales and marketing resources for our products to allow us to reach new customers and potentially increase sales. These expenses impact our operating results, and there can be no assurance that we will continue to be successful in significantly increasing the sales of our products.
The impairment or termination of our relationships with independent sales agencies, whom we do not control, could materially and adversely affect our ability to generate revenues and profits. We intend to develop additional relationships with independent sales agencies in order to increase revenue from certain of our products; our inability to do so may prevent us from increasing sales.
We derive a portion of our revenues through our relationships with independent sales agencies. The impairment or termination of these relationships for any reason could materially and adversely affect our ability to generate revenues and profits. Because the independent sales agency often controls the customer relationships within its territory, there is a risk that if our relationship with the independent sales agency ends, our relationship with the customer will be lost. Also, because we do not control an independent sales agency’s field sales agents, there is a risk we will be unable to ensure that our sales processes, regulatory compliance, and other priorities will be consistently communicated and executed by the distributor. If we fail to maintain relationships with our key independent sales agencies, or fail to ensure that our independent sales agencies adhere to our sales processes, regulatory compliance, and other priorities, this could have an adverse effect on our business, results of operations, and financial condition. We may have liability for the actions of independent sales agencies in marketing our products and our lack of control over their activities impedes our ability to prevent, detect or address such non-compliance.
We intend to develop relationships and arrangements with additional independent sales agencies in order to increase our sales with respect to certain of our products. However, we may fail to develop such relationships, in which case we may not be able to increase our sales. Our success is partially dependent upon our ability to retain and motivate our independent sales agencies and their representatives to sell our products in certain territories. They may not be successful in implementing our marketing plans. Some of our independent sales agencies may not sell our products exclusively and may offer similar products from other companies. Our independent sales agencies may terminate their contracts with us, may devote insufficient sales efforts to our products, or may focus their sales efforts on other products that produce greater commissions for them, which could have an adverse effect on our business, results of operations, and financial condition. We also may not be able to find additional independent sales agencies who will agree to market and/or distribute those products on commercially reasonable terms, if at all. If we are unable to establish new independent sales agency relationships or renew current sales agency agreements on commercially acceptable terms, our business, results of operations, and financial condition could be materially and adversely affected. In addition, because we do not control these independent sales agencies as closely as our employees, while we may take steps to mitigate the risks associated with noncompliance by independent sales agencies, there remains a risk they do not comply with regulatory requirements or our requirements or our policies which could also adversely affect our business.
We will need to continue to expand our organization, and managing growth may be more difficult than expected.
Managing our growth may be more difficult than we expect. We anticipate that a period of significant expansion will be required to penetrate and service the markets for our existing and anticipated future products and to continue to develop new products. This expansion will place a significant strain on management, operational and financial resources. To manage the expected growth of
our operations, we must both modify our existing operational and financial systems, procedures and controls and implement new systems, procedures and controls. We must also expand our finance, administrative, and operations staff. Management may be unable to hire, train, retain, motivate, and manage necessary personnel or to identify, manage, and exploit existing and potential strategic relationships and market opportunities.
In addition to expanding our organization, we are expanding our manufacturing capabilities, which requires significant capital expenditures. If these capital expenditures are higher than expected, it may adversely affect our financial condition and capital resources. In addition, if the expansion of our manufacturing facilities is delayed, for regulatory or other reasons, it may limit our ability to expand the size of our organization and to meet our corporate goals. Even if we are able to expand our manufacturing facilities as we plan, we may not realize the full expected benefit of our investment.
We may expand our business through acquisitions, licenses, investments, and other commercial arrangements in other companies or technologies. Such acquisitions or commercial arrangements may entail significant risks.
We periodically evaluate strategic opportunities to acquire companies, divisions, technologies, products, and rights through licenses, distribution agreements, investments, and outright acquisitions to grow our business. Business acquisitions involve the risk of unknown liabilities associated with the acquired business, which could be material. We may not realize the increased revenues, cost savings, and synergies that we anticipate from an acquisition in the near term or at all due to many factors. Incurring unknown liabilities or the failure to realize the anticipated benefits of an acquisition could materially and adversely affect our business and we may lose our entire investment or be unable to recover our initial investment, which could include the cost of acquiring licenses or distribution rights, acquiring products, purchasing initial inventory, or investments in early-stage companies. Inability to recover our investment, or any write off of such investment, associated goodwill, or assets, could have a material and adverse effect on our business, results of operations, and financial condition.
New lines of business or new products and services may subject us to additional risks.
From time to time, we may implement or may acquire new lines of business, or we may offer new products and services within existing lines of business. There are risks and uncertainties associated with these efforts, particularly in instances where the markets are not fully developed or are evolving. In developing and marketing new lines of business and new products and services, we may invest significant time and resources. External factors, such as regulatory compliance obligations, competitive alternatives, lack of market acceptance, and shifting market preferences, may also affect the successful implementation of a new line of business or a new product or service. Failure to successfully manage these risks in the development and implementation of new lines of business or new products or services could have a material adverse effect on our business, results of operations, and financial condition.
Significant disruptions of information technology systems or breaches of information security could adversely affect our business, results of operations, and financial condition.
Our business depends on the availability, reliability, and security of our information systems, networks, data, and intellectual property. In the ordinary course of business, we collect, store, and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property). Any disruption, compromise, or breach of our systems or data due to a cybersecurity threat or incident could adversely affect our operations, customer service, product development, sales, competitive position, and privacy and confidentiality of our stakeholders. Such a breach could expose us to business interruption, lost revenue, ransom payments, remediation costs, liabilities to affected parties, cybersecurity protection costs, lost assets, litigation, regulatory scrutiny and actions, reputational harm, customer dissatisfaction, harm to our vendor relationships, or loss of market share.
Cyberattacks have become increasingly more prevalent and much harder to detect, defend against or prevent. As the frequency of cyberattacks and resulting breaches reported by other businesses and governments increases, we expect to continue to devote significant resources to improve and maintain our information technology (IT) infrastructure. We have incurred and may in the future incur significant costs in order to implement, maintain and/or update security systems we believe are necessary to protect our IT infrastructure. As the techniques used to obtain unauthorized access or to sabotage systems change frequently and are often not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventive measures. A breakdown in existing controls and procedures around our cyber-security environment may prevent us from detecting, reporting or responding to cyber incidents in a timely manner and could have a material adverse effect on our financial position and value of our stock. We cannot guarantee that our implemented processes for IT and risk mitigation measures will be effective for IT systems under our control.
We also have outsourced significant elements of our operations to third parties, including significant elements of our information technology infrastructure and, as a result, we are managing many independent vendor relationships with third parties who may or could have access to our confidential information. The size and complexity of our information technology and information
security systems, and those of our third-party vendors with whom we contract (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. While we have invested significantly in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches. For example, in August 2020, our information technology (IT) systems were exposed to a ransomware attack, which partially impaired certain IT systems for a short period of time. We finished investigating the incident, together with legal counsel and other incident response professionals. We did not experience any material losses related to the ransomware attack and were able to recover all data quickly, with only a minimal and temporary interruption to our business. While we have implemented measures to protect our data security and information technology systems, such measures may not prevent these events. Although we have cyber-insurance coverage that may cover certain events described above, this insurance is subject to deductibles and coverage limitations and we may not be able to maintain this insurance. Also, it is possible that claims could exceed the limits of our coverage.
If a breach of our measures protecting personal data covered by HIPAA, the HITECH Act, or the CCPA occurs, we may incur significant liabilities.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the HITECH Act, and the regulations that have been issued under it, impose certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of protected health information. The requirements and restrictions apply to "covered entities" (which include health care providers and insurers) as well as to their business associates that receive protected health information from them in order to provide services to or perform certain activities on their behalf. The statute and regulations also impose notification obligations on covered entities and their business associates in the event of a breach of the privacy or security of protected health information. We occasionally receive protected health information from our customers in the course of our business.
In addition, California has enacted the California Consumer Privacy Act (CCPA), which came into effect on January 1, 2020. Pursuant to the CCPA, certain businesses are required, among other things, to make certain enhanced disclosures related to California residents regarding the use or disclosure of their personal information, allow California residents to opt-out of certain uses and disclosures of their personal information without penalty, provide Californians with other choices related to personal data in our possession, and obtain opt-in consent before engaging in certain uses of personal information relating to Californians under the age of 16. The California Attorney General may seek substantial monetary penalties and injunctive relief in the event of our non-compliance with the CCPA. The CCPA also allows for private lawsuits from Californians in the event of certain data breaches. Aspects of the CCPA remain uncertain, and we may be required to make modifications to our policies or practices in order to comply. Aside from California, Texas and several other major states impose rigorous local medical privacy requirements.
It is possible the data protection laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. In addition, these privacy regulations may differ from country to country and state to state, and may vary based on whether testing is performed in the United States or in the local country. Complying with these various laws and regulations could cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business. Further, compliance with data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. We can provide no assurance that we are or will remain in compliance with diverse privacy and security requirements in all of the jurisdictions in which we do business. If we fail to comply or are deemed to have failed to comply with applicable privacy protection laws and regulations such failure could result in government enforcement actions and create liability for us, which could include substantial civil and/or criminal penalties, as well as private litigation and/or adverse publicity that could negatively affect our operating results and business.
We engage in transactions with related parties and such transactions present possible conflicts of interest that could have an adverse effect on our business, results of operations, and financial condition.
We have entered into a significant number of transactions with related parties. Related party transactions create the possibility of conflicts of interest with regard to our management, including that:
•we may enter into contracts between us, on the one hand, and related parties, on the other, that are not as a result of arm’s-length transactions;
•our executive officers and directors that hold positions of responsibility with related parties may be aware of certain business opportunities that are appropriate for presentation to us as well as to such other related parties and may present such business opportunities to such other parties; and
•our executive officers and directors that hold positions of responsibility with related parties may have significant duties with, and spend significant time serving, other entities and may have conflicts of interest in allocating time.
Such conflicts could cause an executive officer or a director to seek to advance his or her economic interests or the economic interests of certain related parties above ours. Conversely, we may not be able to enter into transactions with third parties on terms as favorable as the terms of existing transactions with related parties. Further, the appearance of conflicts of interest created by related party transactions could impair the confidence of our investors. It is possible that a conflict of interest could have a material adverse effect on our business, results of operations, and financial condition.
We incurred non-cash impairment and write down charges during 2024 which adversely affected our fiscal year 2024 operating results and we may be required to incur additional future impairment and write down charges, which could adversely affect our operating results.
Our long-term assets include property and equipment of $89.1 million and $116.2 million, of which $63.3 million and $60.8 million represents the value of improvements to our leased assets, and of which $21.9 million and $59.1 million represents construction in progress (each as described more fully in Note 8, Property and Equipment, Net, to our audited consolidated financial statements included in this Annual Report on Form 10-K), as of December 31, 2024 and 2023, respectively. During the year ended December 31, 2024, we recorded impairment of property and construction and a write-down of capitalized internal-use software costs in the amounts of $18.8 million and $4.0 million, respectively. We did not recognize any impairment charges with respect to our long-lived assets during the years ended December 31, 2023 and 2022.
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If an asset is determined to be impaired, the asset is written down to fair value, which is determined based on appraised value. Any such impairment could result in a non-cash charge equal to the full carrying value of the associated assets. Changes in our assumptions with respect to our expected use of our long-lived assets may result in additional impairment and write down charges in the future, which could adversely affect our business, results of operations, and financial condition.
We may be required to record a significant charge to earnings if our goodwill and other amortizable intangible assets, or other assets become impaired.
We are required under generally accepted accounting principles in the United States (GAAP) to test goodwill for impairment at least annually and to review our goodwill, amortizable intangible assets, and other assets acquired through merger and acquisition activity, for impairment when events or changes in circumstance indicate the carrying value may not be recoverable. Factors that could lead to impairment of goodwill, amortizable intangible assets, and other assets acquired via acquisitions include significant adverse changes in the business climate and actual or projected operating results (affecting our company as a whole or affecting any particular segment) and declines in the financial condition of our business. We may be required in the future to record additional charges to earnings if our goodwill, amortizable intangible assets, or other investments become impaired. Any such charge would adversely impact our financial results.
Our ability to use our net operating loss carryforwards may be subject to certain limitations.
As of December 31, 2024, we had state net operating loss carry-forwards of approximately $7.4 million expiring from the year ended December 31, 2027 through 2038. We had state research and development tax credits of approximately $1.1 million, expiring in the year ended December 31, 2038. It is uncertain whether and to what extent applicable federal and state tax laws will limit the
deductibility of our operating loss and credit carryforwards, though we are already subject to limitations in net operating loss utilization in certain states.
In addition, our ability to utilize our federal net operating loss carryforwards may be limited under Section 382 of the Code. In the event of an "ownership change", Section 382 imposes an annual limitation on the amount of post-ownership change taxable income that may be offset with pre-ownership change net operating losses of the loss corporation experiencing the ownership change. An "ownership change" is defined by Section 382 as a cumulative change in ownership of our company of more than 50% within a three-year period. As of December 31, 2021, we performed a study and determined that there is no limitation on our federal net operating losses. Current or future changes in our stock ownership may trigger an "ownership change," some of which may be outside our control. Accordingly, our ability to utilize our net operating loss carryforwards to offset federal taxable income, if any, could be limited by Section 382, which could potentially result in increased future tax liability to us.
We previously identified a material weakness in our internal control over financial reporting, which has now been remediated. If we fail to maintain an effective system of internal controls over financial reporting, we may not be able to report our financial results timely and accurately, which could adversely affect investor confidence in the Company, and in turn, our results of operations and our stock price.
Effective internal controls are necessary for us to provide reliable financial reports and operate successfully as a public company. Section 404 of the Sarbanes-Oxley Act of 2002 (SOX) requires that companies evaluate and report on their systems of internal control over financial reporting.
As disclosed in Item 9A of this Annual Report on Form 10-K, we previously identified a material weakness in our internal controls over financial reporting relating to the design and maintenance of effective controls over information technology general controls and proper segregation of duties to support the initiation and recording of transactions and the resulting impact on business process controls and applications that rely on such data. We completed our remediation efforts related to the material weakness by, among other things, implementing certain modules in a new company-wide enterprise resource planning (ERP) system to provide additional systematic controls and segregation of duties for our accounting processes; implementing additional controls to mitigate existing risks of proper segregation and change configurations; adding personnel to our accounting and finance team with the requisite accounting and internal controls knowledge and experience to sufficiently enhance our internal controls environment; designing and implementing new information technology general controls to ensure proper segregation of duties in our change management processes; engaging an outside firm to assist management with performing control design and operating effectiveness testing; reporting the results of control testing to the key stakeholders across our organization, including our Audit Committee, on testing progress and defined corrective actions; monitoring and reporting on the results of control remediation; and documenting and structuring the Company’s processes to meet SOX 404(b) requirements.
Although we have remediated this material weakness in our internal controls over financial reporting, any failure to maintain effective internal controls could cause a delay in compliance with our reporting obligations, SEC rules and regulations or Section 404 of the Sarbanes-Oxley Act of 2002, which could subject us to a variety of administrative sanctions, including, but not limited to, SEC enforcement action, ineligibility for short form registration, the suspension or delisting of our common stock from the stock exchange on which it is listed and the inability of registered broker-dealers to make a market in our common stock, which could adversely affect our business and the trading price of our common stock.
Risks Related to Regulation of Our Products and Other Government Regulations
Our products are subject to the Infrastructure Investment and Jobs Act and corresponding rebate obligations that took effect on January 1, 2023, and we may owe rebates, which could be material, on our Apligraf, Dermagraft, and PuraPly products and possibly other products.
Section 90004 of the Infrastructure Investment and Jobs Act, enacted in November 2021, requires manufacturers to pay a refund to the federal government if more than a certain applicable percentage of their single-use product is not administered to a patient and is discarded ("wasted") by providers. Because there is a lack of consistency and uniformity in wound sizes, it is likely that some skin substitute product is discarded with every treatment. The rebate obligation took effect January 1, 2023. In the calendar year 2024 Medicare Physician Fee Schedule (MPFS) rulemaking, CMS exempted skin substitutes from this refund requirement for calendar quarters in 2025. This exemption is based on a possibility that CMS will, in future rulemaking, stop paying for skin substitutes using the ASP methodology and bundle payment into the payment for the application of the product. It is unclear whether CMS will
continue exempting skin substitute products from this refund requirement in subsequent years and what impact any future regulatory actions may have on the ASP reimbursement landscape and our products and/or product candidates.
We may encounter substantial delays or difficulties in our clinical trials.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates. Clinical testing is expensive, time-consuming and uncertain as to the outcome. We have limited experience with clinical trials. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing.
Events that may prevent successful or timely completion of clinical development include:
•the FDA may require additional clinical trials in connection with the premarket review of product candidates;
•delays in reaching a consensus with the FDA or other regulatory authorities on trial design;
•delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites;
•delays in opening clinical trial sites or obtaining required IRB or independent ethics committee approval at each clinical trial site;
•our decision or the requirement of regulators or IRBs to suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements, a finding that the participants are being exposed to unacceptable health risks, or the imposition of a clinical hold as a result of a serious adverse event or after an inspection of our clinical trial operations or clinical trial sites;
•failure by us, any CROs we engage or any other third parties to adhere to clinical trial or regulatory requirements;
•failure by us, any CROs we engage or any other third parties to perform in accordance with Good Clinical Practice, or GCP, cGMPs, or applicable regulatory guidelines in the United States and other international markets;
•failure by physicians to adhere to delivery protocols leading to variable results;
•delays in the testing, validation, manufacturing and delivery of our product candidates to the clinical trial sites, including delays by third parties with whom we have contracted to perform certain of those functions;
•insufficient or inadequate supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates;
•delays in having patients complete participation in a clinical trial or return for post-treatment follow-up;
•clinical trial sites or patients dropping out of a clinical trial at a rate higher than we anticipate;
•enrollment of clinical trial participants that are not representative of the intended user population;
•selection of clinical endpoints that require prolonged periods of clinical observation or analysis of the resulting data;
•receipt of negative or inconclusive clinical trial results;
•occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits;
•occurrence of serious adverse events in clinical trials of the same class of agents conducted by other sponsors; and
•changes in regulatory requirements and guidance that require amending or submitting new clinical protocols.
ReNu is in Phase 3 clinical development for the management of symptoms associated with knee OA. Our anticipated timeline for these and other trials and studies on our clinical trial candidates may be subject to delays due to factors such as those discussed above.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory, development and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business, financial condition, results of operations and prospects.
Success in research and preclinical studies or early clinical trial results may not be indicative of results obtained in later trials. Likewise, preliminary, initial or interim data from clinical trials should be considered carefully and with caution since the final data may be materially different from the preliminary, initial or interim data, particularly as more patient data become available.
Results from preclinical studies or early clinical trials, including feasibility studies, or earlier conducted clinical trials are not necessarily predictive of future clinical trial results, and interim results of a clinical trial are not necessarily indicative of final results. Our clinical trial candidates, including ReNu, may fail to show the desired safety and efficacy in clinical development despite demonstrating positive results in preclinical studies or having successfully advanced through initial or earlier clinical trials or preliminary stages of clinical trials. From time to time, we have and may in the future publish or report preliminary, initial or interim data. Preliminary, initial or interim data from our clinical trials and those of our partners may not be indicative of the final results of the trial and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and/or more patient data become available. In this regard, such data may show initial evidence of clinical benefit, but as patients continue to be followed and more patient data becomes available, there is a risk that any therapeutic effects will not be durable in patients and/or will decrease over time, or cease entirely. Preliminary, initial or interim data also remain subject to audit and verification procedures that may result in the final data being materially different from such preliminary, initial or interim data. As a result, preliminary, initial or interim data should be considered carefully and with caution until the final data are available.
There is no guarantee that any of our clinical trials will be successful. In addition, there is a high failure rate for drugs, biologic products and cell therapies proceeding through clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in preclinical testing and earlier-stage clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. Any such setbacks could adversely affect our business, financial condition, results of operations and prospects.
Obtaining the necessary regulatory approvals or clearances for certain of our products will be expensive and time-consuming and may impede our ability to fully exploit our technologies or otherwise limit our ability to meet other business objectives.
As biological products and medical devices, many of the products that we market require regulatory approvals or clearances from the FDA, or from similar regulatory authorities outside of the United States, before they may legally be distributed in commerce. In particular, such products may require FDA approval of BLAs, under Section 351 of the PHSA, Premarket Approval, or PMA, submissions under Section 515 of the Federal Food, Drug, and Cosmetic Act, or FDCA, or may require clearance under Section 510(k) of the FDCA. Although we believe that we have all necessary regulatory approvals or clearances legally required for the products that we currently market, the introduction of new or modified products, or new or modified FDA regulatory rules, may require us to secure new approvals or clearances. Additionally, the FDA may take the position that some of the products that we currently market without premarket approval or clearance in fact require such approval or clearance. The process of obtaining an approved BLA or PMA requires the expenditure of substantial time, effort and financial resources and may take years to complete. Although obtaining clearance under section 510(k) is somewhat less burdensome, it is also associated with significant costs and resource commitments. The fee for filing a BLA, PMA or 510(k) notification, and the annual user fees for any establishment that manufactures biologics or medical devices, as well as product fees applicable to each approved product are substantial.
In May 2024, we announced that our Phase 3 RCT evaluating the safety and efficacy of ReNu, a cryopreserved ASA for the management of symptoms associated with knee OA, achieved its primary endpoint upon the analysis of positive top line data. There are significant costs associated with conducting clinical trials to support approvals that cannot necessarily be estimated with any accuracy until investigational plans have been developed. Moreover, data obtained from clinical activities may show a lack of safety or efficacy or may be inconclusive or susceptible to varying interpretations, any of which could delay, limit or prevent regulatory approval. Failure or delay can occur at any time during the clinical trial process. Success in preclinical testing and early clinical trials
does not ensure that later clinical trials will be successful. Even product candidates in later stages of clinical trials may fail to show the required safety profile or meet the efficacy endpoints despite having progressed through preclinical studies and initial clinical trials. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. We cannot be certain that we will not face similar setbacks. Even with positive clinical trial results, there may be other barriers to approval or clearance, and the FDA may not grant approval or clearance on a timely basis, or at all. Even if the FDA clears or approves our products, the clinical data submitted to the FDA may not be sufficient for payers to cover and/or adequately reimburse our customers for use of our products. Additionally, the FDA may limit the indications for use in an approval or clearance, or place other conditions on an approval, that could restrict the commercial application of the products.
Regenerative medicine advanced therapy, or RMAT, designation for our product candidates may not lead to faster development or regulatory processes nor does it increase the likelihood that such product candidates will receive marketing approval.
RMAT was introduced as a new designation under the 21st Century Cures Act for the development and review of certain regenerative medicine therapies. To receive RMAT designation, a regenerative medicine product candidate must be intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition with preliminary clinical evidence indicating that the drug has the potential to address the unmet medical needs. RMAT designation does not require evidence to indicate that the drug may offer a substantial improvement over available therapies, as breakthrough designation requires.
An RMAT product candidate receives intensive guidance on an efficient product development program; involvement of senior managers and experienced staff on a proactive, collaborative and cross-disciplinary review; and a rolling review. Regenerative medicine therapies that qualify for RMAT designation may also qualify for other FDA expedited programs, including fast track designation, breakthrough therapy designation, accelerated approval and priority review designation, if they meet the criteria for such programs. However, RMAT designation does not assure that marketing approval will be granted and, if granted, that the approval process would be any faster than it would have otherwise been.
In January 2021, we announced RMAT designation for ReNu for the management of symptoms associated with knee OA. However, there is no guarantee that the receipt of RMAT designation will result in a faster development process, review or approval for ReNu for the management of symptoms associated with knee OA or increase the likelihood that ReNu will be granted marketing approval for the management of symptoms associated with knee OA. Likewise, any future RMAT designation or other expedited review status such as breakthrough therapy designation for any of our other product candidates neither guarantees a faster development process, review or approval nor improves the likelihood of the grant of marketing approval by FDA for any such product candidate compared to drugs considered for approval under conventional FDA procedures. In addition, the FDA may withdraw any RMAT or other expedited review status at any time. We may seek RMAT or breakthrough therapy designation for our other product candidates, but the FDA may not grant this status to any such product candidates.
We may seek fast track designation by the FDA for one or more of our product candidates, but we might not receive such designation, and even if we do, such designation may not actually lead to a faster development or regulatory review or approval process.
If a product is intended for the treatment of a serious or life-threatening condition and the product demonstrates the potential to address unmet needs for this condition, the treatment sponsor may apply for FDA fast track designation. Even if we receive fast track designation, fast track designation does not ensure that we will receive marketing approval or that approval will be granted within any particular time frame. We may not experience a faster development, regulatory review or approval process with fast track designation compared to conventional FDA procedures. Additionally, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast track designation alone does not guarantee qualification for the FDA’s priority review procedures.
A breakthrough therapy designation by the FDA for a product candidate may not lead to a faster development or regulatory review or approval process, and it would not increase the likelihood that the product candidate will receive marketing approval.
We may seek a breakthrough therapy designation for one or more product candidates. A breakthrough therapy is defined as a product candidate that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product candidate may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For product candidates that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of
patients placed in ineffective control regimens. Product candidates designated as breakthrough therapies by the FDA are also eligible for priority review if supported by clinical data at the time of the submission of the new drug application.
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe that one of our product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review or approval compared to product candidates considered for approval under conventional FDA procedures and it would not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as breakthrough therapies, the FDA may later decide that the product candidate no longer meets the conditions for qualification or it may decide that the time period for FDA review or approval will not be shortened.
We must comply with applicable post-marketing regulatory obligations, which could include obtaining new regulatory approvals or clearances.
Following approval or clearance, some types of changes to the approved or cleared product, such as adding new indications or additional labeling claims or introducing manufacturing changes, are subject to FDA review and approval, which may require further nonclinical or clinical testing. The costs and other resource burdens associated with obtaining new regulatory approvals or clearances for existing or future products may limit the resources available to us to fully exploit our technologies or may otherwise limit our ability to carry out other business activities. Depending on the nature of the change, we may determine that the change may be carried out without obtaining premarket approval or clearance. The FDA or another regulatory body could disagree with our conclusion and require such premarket approval or clearance, which would disrupt the marketing of these products, potentially expose us to regulatory sanctions, and have a material adverse effect on our business, financial condition and results of operations.
The FDA may determine that certain of our products that are, or are derived from, human cells or tissues, such as Affinity, Novachor, and NuShield, do not qualify for regulation solely under Section 361 of the Public Health Services Act, or PHSA. To the extent that any of these products are deemed not to be HCT/Ps or Section 361 HCT/Ps, the FDA may require that we revise our labeling and marketing claims for these products or that we suspend sales of such products until FDA approval is obtained, which could adversely affect our business, results of operations, and financial condition.
Certain of the products that we manufacture, process and distribute are, or are derived from, human cells or tissues, including amniotic tissue. The FDA has specific regulations governing human cells, tissues and cellular and tissue-based products, or HCT/Ps. In particular, HCT/Ps that meet certain criteria set forth in the FDA’s regulations at 21 C.F.R. § 1271.10 are regulated solely under Section 361 of the PHSA, so-called "Section 361 HCT/Ps", and are not subject to any premarket clearance or approval requirements. They are also subject to less stringent post-market regulatory requirements than products regulated under Section 351 of the PHSA and/or under Sections 505, 510 or 515 of the FDCA. The Company has believed that certain of our HCT/Ps, including our products derived from amniotic membrane, qualify for regulation as Section 361 HCT/Ps. However, the regulatory classification of an HCT/P as a Section 361 HCT/P depends in part on the purposes for which the product is intended and in part on the processing to which an HCT/P is subject. On November 16, 2017, the FDA issued a final guidance document entitled, "Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use", or 361 HCT/P Guidance, which provides FDA’s current thinking on how to apply the existing regulatory criteria for regulation as a Section 361 HCT/P. These include, in addition to other requirements, requirements that an HCT/P be both minimally manipulated and intended for homologous use. In general, "minimal manipulation" is a standard referring to the degree to which the original characteristics of an HCT/P have been altered by processing and "homologous use" refers to the requirement that an HCT/P perform the same basic function in the donor as in the recipient. Any action by the FDA to apply the principles set forth in the 361 HCT/P Guidance to the HCT/Ps that we distribute could have adverse consequences for us and make it more difficult or expensive for us to conduct our business.
In light of the 361 HCT/P Guidance, our labeling and marketing claims for our placental-based membrane products, including our Affinity, NuShield, and Novachor products, clarify that they are intended as protective barriers, and thus meet the homologous use requirement to qualify as Section 361 HCT/Ps. However, the FDA could disagree with our conclusion and require premarket approval or clearance for Affinity, NuShield, or any placental-based sheet product we market, which would disrupt the marketing of these products, potentially expose us to regulatory sanctions, and have a material adverse effect on our business, financial condition and results of operations. Further, we believe it is necessary to obtain FDA approval of a BLA for NuCel and ReNu because those products may be deemed to be more than minimally manipulated, not for homologous use, or otherwise not regulated as Section 361 HCT/Ps. We continue to conduct clinical studies of ReNu to support FDA approval of a BLA for the management of symptoms associated with knee OA and, based on favorable feasibility studies that are subject to further evaluation, we believe ReNu has potential as a treatment for additional OA and tissue regeneration applications. We have discontinued clinical development of NuCel. If we obtain BLA approval for ReNu, compliance with applicable post-market regulatory requirements will involve significant time and substantial costs. Even for those products that remain regulated as Section 361 HCT/Ps, increasing regulatory scrutiny within the industry in which we operate could lead to heightened requirements, compliance with which could be costly. The costs and other resource burdens associated with any of these regulatory outcomes may limit the resources available to us to fully exploit our technologies or may otherwise limit our ability to carry out other business activities.
The 361 HCT/P Guidance originally indicated that the FDA was providing a 36-month enforcement grace period to allow time for distributors of HCT/Ps to make any regulatory submissions and obtain any premarket approvals necessary to comply with the guidance. In July 2020, the FDA announced that the enforcement grace period would be extended until May 31, 2021 as a result of the challenges presented by the COVID-19 public health emergency. On April 21, 2021, the FDA reaffirmed that the enforcement grace period would end on May 31, 2021, at which time we ceased commercial distribution of ReNu and NuCel. Although we believe our suspension of ReNu and NuCel commercialization was timely and proper, the FDA and other regulators may disagree with how or when such commercialization practices were conducted, which could expose us to regulatory sanctions, and have a material adverse effect on our business, financial condition and results of operations.
To the extent that the FDA may determine that certain of our products that are, or are derived from, human cells or tissues do not qualify for regulation solely under Section 361 of the PHSA, the introduction of new tissue products would become more expensive, expansion of our tissue product offerings could be significantly delayed, and we could be subject to additional post-market regulatory requirements or suspension of product sales until FDA approval is obtained.
As stated above, in light of the 361 HCT/P Guidance, the FDA may determine that the types of cell- and tissue-based products that we distribute—and in particular, products derived from allografts consisting of human skin or amniotic tissue—are subject to premarket clearance or approval requirements. Should the FDA make such a determination, products of this type, including future products that we seek to introduce, will be much more costly to commercialize, as we will likely have to carry out preclinical work in animals and/or clinical trials in humans to support approval. Such preclinical work and clinical trials are expensive and time-consuming with no guarantee of success. In addition, these products will be subject to more stringent post-market regulatory requirements than those that currently apply, including but not limited to more stringent restrictions on advertising and promotion of these products, as well as more extensive adverse event reporting. In the future, we may also wish to market our existing HCT/P products for new intended uses that may render them ineligible for regulation as Section 361 HCT/Ps and cause them to require premarket clearance or approval and comply with post-market regulations under the medical device or biological product provisions of the FDCA and/or PHSA instead. Compliance with these requirements will involve significant time and substantial costs and could limit the resources available to us to fully exploit our technologies, including limiting our ability to introduce new allograft-derived products.
We conduct a range of nonclinical, as well as clinical trials, comparative effectiveness, economic and other studies of our products. Unfavorable results from these trials or studies or from similar trials or studies conducted by others may negatively affect the use or adoption of our products by physicians, hospitals, and payers, which could have a negative impact on the market acceptance of these products and their profitability.
We conduct a variety of nonclinical and clinical trials, comparative effectiveness studies and economic and other studies of our products, including our ongoing clinical trial for ReNu, in an effort to generate comprehensive clinical and real-world outcomes data and cost-effectiveness data in order to obtain product approval and drive further penetration in the markets we serve. In the event that these trials and studies, or similar trials and studies conducted by others, yield unfavorable results, those results could negatively affect the use or adoption of our products by physicians, hospitals, and payers, thereby compromising market acceptance and profitability.
Our business is subject to continuing and evolving significant regulatory obligations by the FDA and other authorities, compliance with which is expensive and time-consuming and may impede our ability to fully exploit our technologies or otherwise limit our ability to meet other business objectives.
Aside from the obligation to obtain regulatory approvals or clearances, companies such as ours have ongoing regulatory obligations that are expensive and time-consuming to meet. In particular, the production and marketing of our products are subject to extensive regulation and review by the FDA and numerous other governmental authorities both in the United States and abroad. As noted above, some of the products that we distribute are considered Section 361 HCT/Ps. The FDA’s regulation of HCT/Ps includes requirements for registration and listing of products; donor screening and testing; processing and distribution, known as "Current Good Tissue Practices," or cGTP; labeling; record keeping and adverse-reaction reporting; and inspection and enforcement. Moreover, it is likely that the FDA’s regulation of HCT/Ps will continue to evolve in the future. Complying with any such new regulatory requirements may entail significant time delays and expense, which could have a material adverse effect on our business, results of operations, and financial condition.
Our other products are regulated as biologics and medical devices, which are subject to even more stringent regulation by the FDA. As noted above, these products are subject to rigorous premarket review processes, and an approval or clearance may place substantial restrictions on the indications for which the product may be marketed or the population for whom it may be marketed, may require warnings to accompany the product or may impose other restrictions on the sale and/or use of the product. In addition, most of our products are subject to continuing obligations to comply with other substantial regulatory requirements, including the FDA’s cGTP regulations, the FDA’s Current Good Manufacturing Practices (cGMP) regulations, adverse event reporting, FDA inspections, and the FDA’s QSR, and the regulatory expectations for these types of regulatory obligations may evolve over time. For example, on January 31, 2024, the FDA issued a final rule amending the QSR for medical devices. This final rule is intended to more closely align the FDA QSR with the international consensus standard for device quality management and will become effective on February 2, 2026. We may need to dedicate considerable resources to come into compliance with the new QSR by the final rule’s effective date. The costs and other resource burdens associated with maintaining regulatory approvals or clearances for our products and otherwise meeting our regulatory obligations may limit the resources available to us to fully exploit our technologies or may otherwise limit our ability to carry out other business activities.
In some states, the manufacture, storage, or distribution of HCT/Ps requires a license or permit to operate as a tissue bank or tissue distributor. We believe that we have all required state licenses or permits applicable to the distribution of HCT/Ps, but there is a risk that there may be state or local license or permit requirements of which we are unaware or with which we have not complied. In the event that such noncompliance exists in a given jurisdiction, we could be precluded from distributing HCT/Ps in that jurisdiction and also could be subject to fines or other penalties. If any such actions were to be instituted against us, it could adversely affect our business and/or financial condition.
The American Association of Tissue Banks, or AATB, has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an accredited tissue bank. In addition, some states have their own tissue banking regulations. In addition, procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act, or NOTA, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks, hospitals, and physicians for their services associated with the recovery, storage, and transportation of donated human tissue. Although we have independent third-party appraisals that confirm the reasonableness of the service fees we pay, if we were to be found to have violated NOTA’s prohibition on the sale or transfer of human tissue for valuable consideration, we, our officers, or employees, would potentially be subject to criminal enforcement sanctions, which could materially and adversely affect our business, results of operations, and financial condition.
Many of the products we manufacture and process are derived from human tissue and therefore have the potential for disease transmission.
The utilization of human tissue creates the potential for transmission of communicable diseases, including, but not limited to, human immunodeficiency virus, or HIV, viral hepatitis, syphilis and other viral, fungal or bacterial pathogens. We are required to comply with federal and state regulations intended to prevent communicable disease transmission.
Although we maintain strict quality controls over the procurement and processing of our tissue, there is no assurance that these quality controls will be adequate. In addition, negative publicity concerning disease transmission from other companies’ improperly processed donated tissue could have a negative impact on the demand for our products. If any of our products are implicated in the transmission of any communicable disease, our officers, employees and we could be subject to government sanctions including but not limited to recalls, and civil and criminal liability, with sanctions that include exclusion from doing business with the federal
government. We could also be exposed to product liability claims from those who used or received our products as well as loss of our reputation.
Defects, failures, or quality issues associated with our products could lead to product recalls or safety alerts, adverse regulatory actions, litigation, including product liability claims, and negative publicity that could erode our competitive advantage and market share and materially adversely affect our reputation, business, results of operations, and financial condition.
Quality is extremely important to us and our customers due to the serious and costly consequences of product failure. Quality and safety issues may occur with respect to any of our products, and our future operating results will depend on our ability to maintain an effective quality control system and effectively train and manage our workforce with respect to our quality system. The development, manufacture, and control of our products are subject to extensive and rigorous regulation by numerous government agencies, including the FDA and similar foreign agencies. Compliance with these regulatory requirements, including but not limited to the FDA’s QSR, GMPs, and adverse events/recall reporting requirements in the United States and other applicable regulations worldwide, is subject to continual review and is monitored rigorously through periodic inspections by the FDA and foreign regulatory authorities. The FDA and foreign regulatory authorities may also require post-market testing and surveillance to monitor the performance of approved products. Our manufacturing facilities and those of our suppliers and independent sales agencies are also subject to periodic regulatory inspections. If the FDA or a foreign authority were to conclude that we have failed to comply with any of these requirements, it could institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, such as product recalls or seizures, withdrawals, monetary penalties, consent decrees, injunctive actions to halt the manufacture or distribution of products, import detentions of products made outside the United States, export restrictions, restrictions on operations or other civil or criminal sanctions. Civil or criminal sanctions could be assessed against our officers, employees, or us. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively manufacturing, marketing, and selling our products.
In addition, we cannot predict the results of future legislative activity or future court decisions, any of which could increase regulatory requirements, subject us to government investigations or expose us to unexpected litigation. Any regulatory action or litigation, regardless of the merits, may result in substantial costs, divert management’s attention from other business concerns, and place additional restrictions on our sales or the use of our products. In addition, negative publicity, including regarding a quality or safety issue, could damage our reputation, reduce market acceptance of our products, cause us to lose customers, and decrease demand for our products. Any actual or perceived quality issues may also result in issuances of physician’s advisories against our products or cause us to conduct voluntary recalls. Any product defects or problems, regulatory action, litigation, negative publicity or recalls could disrupt our business and have a material adverse effect on our business, results of operations, and financial condition.
We may implement a product recall or voluntary market withdrawal, which could significantly increase our costs, damage our reputation and disrupt our business.
The manufacturing, marketing, and processing of our products involve an inherent risk that our products or processes may not meet manufacturing specifications, applicable regulatory requirements or quality standards. In that event, we may voluntarily implement a recall or market withdrawal or may be required to do so by a regulatory authority. A recall or market withdrawal of one of our products would be costly and would divert management resources. A recall or withdrawal of one of our products, or a similar product processed by another entity, also could impair sales of our products as a result of confusion concerning the scope of the recall or withdrawal, or as a result of the damage to our reputation for quality and safety.
We are subject to various governmental regulations relating to the labeling, marketing, and sale of our products.
Both before and after a product is commercially released, we have ongoing responsibilities under regulations promulgated by the FDA, the Federal Trade Commission, and similar United States and foreign regulations governing product labeling and advertising, distribution, sale, and marketing of our products.
Manufacturers of medical devices and biological products are permitted to promote products solely for the uses and indications set forth in the approved or cleared product labeling. Traditionally, many of our wound dressing products have been marketed and, in some cases, specifically cleared, for use in “wound management;” however, the FDA is currently reconsidering whether wound dressings may continue to use that term in device labeling and promotional materials. On November 30, 2023, the FDA issued a proposed rule that would prohibit wound dressings from using the term “wound management,” a generally well-understood and accepted term in the healthcare community that describes a context of use. If the rule is finalized, we will be required to update the labeling and promotional material for many of our wound dressings which may make it more difficult to distinguish our wound dressings from competing wound care products.
In addition, a number of enforcement actions have been taken against manufacturers that promote products for off-label uses (i.e., uses that are not described in the approved or cleared labeling), including actions alleging that claims submitted to government healthcare programs for reimbursement of products that were promoted for off-label uses are fraudulent in violation of the Federal False Claims Act or other federal and state statutes and that the submission of those claims was caused by off-label promotion. The failure to comply with prohibitions on off-label promotion can result in significant monetary penalties, revocation or suspension of a company’s business license, suspension of sales of certain products, product recalls, civil or criminal sanctions, exclusion from participating in federal healthcare programs, or other enforcement actions. In the United States, allegations of such wrongful conduct could also result in a corporate integrity agreement with the United States government that imposes significant administrative obligations and costs.
We and our employees and contractors are subject, directly or indirectly, to federal, state and foreign healthcare fraud and abuse laws, including false claims laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our operations are subject to various federal, state, and foreign fraud and abuse laws. These laws may constrain our operations, including the financial arrangements and relationships through which we market, sell, and distribute our products.
United States federal and state laws that affect our ability to operate include, but are not limited to:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering, or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind in return for, the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
•the federal physician self-referral law, which prohibits a physician from referring a patient to an entity with which the physician (or an immediate family member) has a financial relationship, for the furnishing of certain designated health services for which payment may be made by Medicare or Medicaid, unless an exception applies;
•federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other government payers that are false or fraudulent;
•18 U.S.C. § 1347, which created new federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or from making false or fraudulent statements to defraud any healthcare benefit program (i.e., public or private);
•federal transparency laws, including the Physician Payments Sunshine Act which requires the tracking and disclosure to the federal government by pharmaceutical and medical device manufacturers of payments and other transfers of value to physicians and teaching hospitals as well as ownership and investment interests that are held by physicians and their immediate family members; and
•state law equivalents of each of these federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical and medical device companies to comply with their industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government or otherwise restrict certain payments that may be made to healthcare providers and other potential referral sources; state laws that require drug and medical device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state laws that prohibit giving gifts to licensed healthcare professionals; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts in certain circumstances, such as specific disease states.
Activities and arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, waste, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of activities or other arrangements related to the development, marketing, or promotion of products, including pricing and discounting of products, provision of customer incentives, provision of reimbursement support, other customer support services, provision of sales commissions or other incentives to employees and independent contractors and other interactions with healthcare practitioners, other healthcare providers and patients.
Because of the breadth of these laws and the narrow scope of the statutory or regulatory exceptions and safe harbors available, our business activities could be challenged under one or more of these laws. Relationships between medical product manufacturers and health care providers are an area of heightened scrutiny by the government. We engage in various types of activities, including the
conduct of speaker programs to educate physicians, the provision of reimbursement advice and support to customers, and the provision of customer and patient support services, that have been the subject of government scrutiny and enforcement action within the medical device industry.
Government expectations and industry best practices for compliance continue to evolve and our past activities may not always be consistent with current industry best practices. Further, there is a lack of government guidance as to whether many varied industry practices comply with these laws, and government interpretations of these laws continue to evolve, all of which create compliance uncertainties. Any non-compliance could result in regulatory sanctions, criminal or civil liability, and serious harm to our reputation. Although we have a comprehensive compliance program designed to ensure that our employees’ and commercial partners’ activities and interactions with healthcare professionals and patients are appropriate, ethical, and consistent with all applicable laws, regulations, guidelines, policies, and standards, it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in preventing such conduct, mitigating risks, or reducing the chance of governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations.
If a government entity opens an investigation into possible violations of any of these laws (which may include the issuance of subpoenas or civil investigative demands), we would have to expend significant resources to defend ourselves against the allegations. Allegations that we, our officers, or our employees violated any one of these laws can be made by individuals called "whistleblowers" who may be our employees, customers, competitors, or other parties. Government policy is to encourage individuals to become whistleblowers and file a complaint in federal court alleging wrongful conduct. The government is required to investigate all of these complaints and decide whether to intervene. If the government intervenes and we are required to pay money back to the government, the whistleblower, as a reward, is awarded a percentage of the collection. If the government declines to intervene, the whistleblower may proceed on their own and, if they are successful, they will receive a percentage of any judgment or settlement amount the company is required to pay. The government may also initiate an investigation on its own. Such actions could have a significant impact on our business, including the imposition of significant fines, and other sanctions that may materially impair our ability to run a profitable business. In particular, if our operations are found to be in violation of any of the laws described above or if we agree to settle with the government without admitting to any wrongful conduct or if we are found to be in violation of any other governmental regulations that apply to us, we, our officers and employees may be subject to sanctions, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, the curtailment or restructuring of our operations and the imposition of a corporate integrity agreement, any of which could adversely affect our business, results of operations, and financial condition.
We could be subject to legal exposure if we do not comply with our reporting and payment obligations under Medicare, the Medicaid Drug Rebate Program, or any other governmental pricing programs in which our products or product candidates may participate, including through additional rebate or discount requirements, fines, sanctions, and litigation.
Our products are currently reimbursed by Medicare in physician office settings at a rate of ASP plus 6%. Beginning in April 2013, the Budget Control Act of 2011 created an automatic reduction of Medicare payments to providers of up to 2%. As a result of the COVID-19 pandemic, this reduction was temporarily suspended from May 1, 2020 through March 31, 2022, with subsequent reductions to 1% from April 1, 2022 through June 30, 2022. The 2% reduction was then reinstated and has been in effect since July 1, 2022, and will remain in effect through the first eight months in which the fiscal year 2032 sequestration order is in effect, unless additional Congressional action is taken. Sequestration applies to the government’s payment portion, which is 80% of the total payment amount. Additionally, in future years, it is possible that an up-to 4% Medicare sequestration could be ordered under Statutory Pay-As-You-GO Act of 2010 (PAYGO), which requires deficit neutrality in most laws passed by Congress. Until January 2022, we were not required to report ASP for all our skin substitute products that are paid separately as biologics because they are regulated as medical devices by the FDA, although we chose to report ASP for some of our products. However, starting with the reporting deadline for the first quarter of 2022, we have been required, and have submitted, ASP reports for all our skin substitute products that are paid separately as biologics as a result of provisions included in the Consolidated Appropriations Act of 2020. Pricing requirements and rebate/discount calculations are complex, vary among products and programs, and are often subject to interpretation by governmental or regulatory agencies and the courts. The requirements of these programs, including, by way of example, their respective terms and scope, change frequently. Responding to current and future changes may increase our costs, and the complexity of compliance will be time consuming. We are liable for errors associated with our submission of pricing data and for any overcharging of government payers. Failure to make necessary disclosures and/or to identify overpayments could result in allegations against us under the federal False Claims Act and other laws and regulations. Any required refunds to the United States government or response to a government investigation or enforcement action would be expensive and time consuming and could have an adverse effect on our business, results of operations and financial condition.
We face significant uncertainty in the industry due to government healthcare reform and other legislative action.
There have been and continue to be laws enacted by the federal government, state governments, regulators, and third-party payers to control healthcare costs, and generally, to reform the healthcare system in the United States. For example, the Affordable
Care Act of 2010 (ACA) and the Medicare Access and CHIP Reauthorization Act of 2015 substantially changed the way healthcare is delivered and financed by both governmental and private insurers. These changes included the creation of demonstration programs and other value-based purchasing initiatives that provide financial incentives for physicians and hospitals to reduce costs, including incentives for furnishing low-cost therapies for chronic wounds even if those therapies may be less effective than our products. Since its enactment, there have been several efforts to modify or repeal all or part of ACA. Additionally, tax reform legislation was passed that includes provisions that impact healthcare insurance coverage and payment such as the elimination of the tax penalty for individuals who do not maintain health insurance coverage (the so-called "individual mandate"). On June 17, 2021, the United States Supreme Court dismissed a judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the law. It is unclear how any future litigation and other healthcare reform efforts may impact the ACA.
Additionally, on August 16, 2022, Congress passed the Inflation Reduction Act (IRA), which implements substantial changes to the Medicare program, including drug pricing reforms. Among other reforms, the IRA imposes inflation rebates on drug manufacturers for certain products reimbursed under Medicare Parts B and D if the prices of those products increase faster than inflation, and, beginning in 2026, establishes a “maximum fair price” for a fixed number of pharmaceutical and biological products covered under Medicare Parts B and D following a price negotiation process with CMS. CMS has continued to take steps to implement the IRA, including: releasing the negotiated maximum prices, which will be effective in 2026, for the first ten drugs that were subject to the IRA’s negotiation process, releasing quarterly lists of Medicare Part B products that are subject to adjusted coinsurance rates based on the inflationary rebate provisions of the IRA, and announcing a list of fifteen additional drugs that will be subject to price negotiations during 2025, with maximum prices to be effective starting in 2027.
The results of the 2024 Presidential and Congressional elections, and potential subsequent developments, further increase the uncertainty related to the healthcare regulatory environment. In addition, on June 28, 2024, the United States Supreme Court issued an opinion holding that courts reviewing agency action pursuant to the Administrative Procedure Act (APA) “must exercise their independent judgment” and “may not defer to an agency interpretation of the law simply because a statute is ambiguous.” The decision will have a significant impact on how lower courts evaluate challenges to agency interpretations of law, including those by CMS and other agencies with significant oversight of the healthcare industry. The new framework is likely to increase both the frequency of such challenges and their odds of success by eliminating one way in which the government previously prevailed in such cases. As a result, significant regulatory policies may be subject to increased litigation and judicial scrutiny. Any resulting changes in regulation may result in unexpected delays, increased costs, or other negative impacts that are difficult to predict but could have a material adverse effect on our business and financial condition. For example, certain of these changes could impose additional limitations on the rates we will be able to charge for our future products or the amounts of reimbursement available for our future products from governmental agencies or third-party payers.
Inadequate funding for the FDA, the SEC and other government agencies, including from government shutdowns, or other disruptions to these agencies’ operations, could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, the ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of CMS and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new product candidates to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Our sales into foreign markets expose us to risks associated with international sales and operations.
We are currently selling into foreign markets and plan to expand such sales. Managing a global organization is difficult, time-consuming, and expensive. Conducting international operations subjects us to risks that could be different from those faced by us in the United States. The sale and shipment of our products across international borders, as well as the purchase of components and products from international sources, subject us to extensive United States and foreign governmental trade, import and export and customs regulations and laws, including but not limited to, the Export Administration Regulations and trade sanctions against
embargoed countries, which are administered by the Office of Foreign Assets Control within the Department of the Treasury, as well as the laws and regulations administered by the Department of Commerce. These regulations limit our ability to market, sell, distribute, or otherwise transfer our products or technology to prohibited countries or persons.
Compliance with these regulations and laws is costly, and failure to comply with applicable legal and regulatory obligations could adversely affect us in a variety of ways that include, but are not limited to, significant criminal, civil, and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities.
These risks may limit or disrupt our expansion, restrict the movement of funds, or result in the deprivation of contractual rights or the taking of property by nationalization or expropriation without fair compensation. Operating in international markets also requires significant management attention and financial resources.
We could be adversely affected by violations of the United States Foreign Corrupt Practices Act and similar worldwide anti-bribery laws.
The United States Foreign Corrupt Practices Act, or FCPA, the U.K. Bribery Act of 2010, and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments for the purpose of obtaining or retaining business. Our policies mandate compliance with these anti-bribery laws, including the requirements to maintain accurate information and internal controls. We operate in many parts of the world that have experienced governmental corruption to some degree and in certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices. There is no assurance that our internal control policies and procedures will protect us from acts committed by our employees or agents. If we are found to be liable for FCPA or other violations (either due to our own acts or our inadvertence, or due to the acts or inadvertence of others), we could suffer from civil and criminal penalties or other sanctions, including contract cancellations or debarment, and loss of reputation, any of which could have a material adverse impact on our business, financial condition, and results of operations.
Risks Related to Reimbursement for our Products
Coverage policies and reimbursement rates for our products by government and private insurance are subject to change.
The commercial success of any product for which we have obtained regulatory approval, or for which we may obtain regulatory approval in the future, will depend substantially on the extent to which the costs of our product or product candidates are or will be paid by third-party payers, including government health care programs and private health insurers. There is a significant trend in the health care industry by public and private payers to seek to contain or reduce their costs, including by taking the following steps, among others: decreasing the portion of costs payers will cover, ceasing to provide full payment for certain products depending on outcomes, and/or not covering certain products at all. If payers implement any of the foregoing with respect to our products, it would have an adverse impact on our revenue and results of operations.
Our success will depend in part on whether and to what extent coverage and adequate reimbursement will be available from government health administration authorities, private health insurers, and other third-party payers. Third-party coverage and reimbursement may not be available to enable us to maintain price levels sufficient to cover our costs, including research, development, manufacture, sale and distribution. For example, currently most private payers provide limited coverage for our PuraPly AM, PuraPly, Novachor, and NuShield products and, as a result, there may be limited use of these products for patients covered by private payers.
The continuing efforts of government agencies, private health plans, and other payers of healthcare services to contain or reduce costs of healthcare may adversely affect:
•the availability of our products due to restricted coverage;
•the ability of our customers to pay for our products;
•our ability to maintain pricing so as to generate revenues or achieve or maintain profitability; and
•our ability to access capital.
The implementation of cost containment measures or other healthcare reforms may have an adverse effect on our business operations. For example, the ACA, enacted in 2010, contains provisions for Medicare demonstration programs that create financial incentives to treat patients with chronic wounds conservatively and could result in decreased utilization of our products. Furthermore, CMS has historically maintained a policy in the Medicare hospital outpatient setting that bundles the administration and product costs associated with graft skin substitutes, which similarly creates incentives that may result in decreased utilization of our products in the outpatient hospital setting. On November 7, 2024, CMS rejected a recommendation by the Advisory Panel on Hospital Outpatient Payment that CMS end this bundling policy. Accordingly, even if coverage and reimbursement are provided, market acceptance of our products has been and will be adversely affected if access to coverage and/or use of our products is administratively burdensome to obtain and/or more costly than alternative treatments. In addition, CMS in recent years has considered potential payment reform for skin substitute products in the Medicare physician office setting under the Physician Fee Schedule. Future changes to Medicare reimbursement for skin substitutes in this setting also could affect utilization of our skin substitute products.
Possible reductions in, or eliminations of, coverage or reimbursement by third-party payers, or the denial of, or provision of uneconomical reimbursement for new products, as a result of changes in coverage and reimbursement, may affect our customers’ revenue and ability to purchase our products or product candidates. Any changes in the healthcare regulatory, payment, or enforcement landscape relative to our customers’ healthcare services also have the potential to significantly affect our operations and revenue.
In addition, Medicare uses regional contractors called MACs, to process claims, develop coverage policies and make payments within designated geographic jurisdictions. On April 25, 2024, seven Medicare Part A/B MACs published new proposed LCDs for skin substitute grafts/CTPs for the treatment of DFUs and VLUs in the Medicare population. These LCDs were finalized by the MACs on November 14, 2024, and were originally set to become effective on February 12, 2025. However, on January 24, 2025, the MACs announced a delay in the implementation of the LCDs until April 13, 2025. Under the new LCDs finalized in November 2024, should they take effect as scheduled, eighteen products would remain covered, including our Apligraf and Dermagraft products for DFU and VLU, and our Affinity and NuShield products for DFU; however, more than 200 products would be classified as “non-covered,” including our PuraPly, PuraPly AM, PuraPly XT, Novachor, TransCyte, Dual and Matrix products for DFU and VLU. The LCDs as finalized apply only to DFU and VLU indications for skin substitute products; other indications would remain subject to case-by-case review of medical necessity by the MACs if the LCDs take effect. It is uncertain if there will be further delays in implementing the new LCDs and/or if the new LCDs will be revised or rescinded going forward. If implemented, the LCDs could materially impact utilization of these products, our business, and our revenue. Any future changes or other developments related to these or other LCDs also could affect utilization of our products, our business, and our revenue.
While we cannot predict the outcome of current or future legislation or regulation, we anticipate, particularly given the recent focus on healthcare reform legislation and regulatory actions, that governmental authorities will continue to introduce initiatives directed at lowering the total cost of healthcare and restricting coverage and reimbursement for our products. If we are not successful in obtaining adequate reimbursement for our products from third-party payers, the market’s acceptance of our products could be adversely affected. Inadequate reimbursement levels also likely would create downward price pressure on our products. Even if we do succeed in obtaining widespread reimbursement for our products, future changes in reimbursement policies could have a negative impact on our business, financial condition and results of operations.
Cost-containment efforts of our customers, purchasing groups, third-party payers, and governmental organizations could adversely affect our business, results of operations, and financial condition.
Many existing and potential customers for our products within the United States are members of GPOs and/or IDNs, including accountable care organizations or public-based purchasing organizations, and our business is partly dependent on major contracts with these organizations. Our products can be contracted under national tenders or with larger hospital GPOs. GPOs and IDNs negotiate pricing arrangements with healthcare product manufacturers and distributors and offer the negotiated prices to affiliated hospitals and other members. GPOs and IDNs typically award contracts on a category-by-category basis through a competitive bidding process. At any given time, we are typically at various stages of responding to bids and negotiating and renewing GPO and IDN agreements, including agreements that would otherwise expire. Bids are generally solicited from multiple manufacturers or service providers with the intention of obtaining lower pricing. Due to the highly competitive nature of the bidding process and the GPO and IDN contracting processes in the United States, we may not be able to obtain or maintain contract positions with major GPOs and IDNs across our product portfolio. Failure to be included in certain of these agreements could have a material adverse effect on our business, financial condition and results of operations. In addition, while having a contract with a major purchaser, such as a GPO or IDN, for a given product category can facilitate sales, sales volumes of those products may not be maintained. For example, GPOs and IDNs are increasingly awarding contracts to multiple suppliers for the same product category. Even when we are the sole contracted supplier of a GPO or IDN for a certain product category, members of the GPO or IDN generally are free to purchase from other suppliers. Furthermore, GPO and IDN contracts typically are terminable without cause upon 60 to 90 days’ notice. The healthcare industry has been consolidating, and the consolidation among third-party payers into larger purchasing groups will increase their negotiating and
purchasing power. Such consolidation may result in greater pricing pressure on us due to pricing concessions and may further exacerbate the risks described above.
Risks Related to Our Intellectual Property
Our patents and other intellectual property rights may not adequately protect our products.
Our ability to compete effectively will depend, in part, on our ability to maintain the proprietary nature of our technology and manufacturing processes. We rely on manufacturing and other know-how, patents, trade secrets, trademarks, license agreements, and contractual provisions to establish our intellectual property rights and protect our products. These legal means, however, afford only limited protection and may not adequately protect our rights. The failure to obtain, maintain, enforce, or defend such intellectual property rights, for any reason, could allow third parties to make competing products or impact our ability to develop, manufacture and market our own products on a commercially viable basis, or at all, which could have a material adverse effect on our revenues, financial condition or results of operations.
In particular, we rely primarily on trade secrets, know-how, and other unpatented technology, which are difficult to protect. Although we seek such protection in part by entering into confidentiality agreements with our vendors, employees, consultants, and others who may have access to proprietary information, we cannot be certain that these agreements will not be breached, adequate remedies for any breach would be available or our trade secrets, know-how, and other unpatented proprietary technology will not otherwise become known to or be independently developed by our competitors. If we are unsuccessful in protecting our intellectual property rights, sales of our products may suffer and our ability to generate revenue could be severely impacted.
We have filed applications to register various trademarks for use in connection with our products in various countries and also, with respect to certain products, rely on the trademarks of third parties. These trademarks may not afford adequate protection. We or these third parties also may not have the financial resources to enforce the rights under these trademarks which may enable others to use the trademarks and dilute their value. Additionally, our marks may be found to conflict with the trademarks of third parties. In such a case, we may not be able to derive any value from such trademarks or, even, may be required to cease using the conflicting mark. The value of our trademarks may also be diminished by our own actions, such as failing to impose appropriate quality control when licensing our trademarks. Any of the foregoing could impair the value of, or ability to use, our trademarks and have an adverse effect on our business.
Most of the key patents related to our marketed products are expired. We have no patent protection covering, for example, our Apligraf, Dermagraft, or NuShield products. However, in addition to trade secrets, trademarks, know-how, and other unpatented technology, we have pursued and plan to continue to pursue patent protection where we believe that doing so offers potential commercial benefits. However, we may be incorrect in our assessments of whether or when to pursue patent protection. Moreover, patents may not issue from any of our pending patent applications. Even if we obtain or in-license issued patents, such patent rights may not provide valid patent protection sufficiently broad to prevent any third party from developing, using, or commercializing products that are similar or functionally equivalent to our products or technologies, or otherwise provide any competitive advantage. In addition, these patent rights may be challenged, revoked, invalidated, infringed, or circumvented by third parties. Laws relating to such rights may in the future be changed or withdrawn in a manner adverse to us.
Additionally, our products or the technologies or processes used to formulate or manufacture our products may now, or in the future, infringe the patent rights of third parties. It is also possible that third parties will obtain patent or other proprietary rights that might be necessary or useful for the development, manufacture, or sale of our products. In such cases, we may need or choose to obtain licenses for intellectual property rights from others and it is possible that we may not be able to obtain these licenses on commercially reasonable terms, if at all.
Pending and future intellectual property litigation could be costly and disruptive and may have an adverse effect on our business, results of operations, and financial condition.
We operate in an industry characterized by extensive intellectual property litigation. Defending intellectual property litigation is expensive and complex, takes significant time and diverts management’s attention from other business concerns, and the outcomes are difficult to predict. We have in the past been subject to claims that our products or technology violate a third party’s intellectual property rights, and we may be subject to such assertions in the future. Any pending or future intellectual property litigation may result in significant damage awards, including treble damages under certain circumstances, and injunctions that could prevent the manufacture and sale of affected products or could force us to seek a license and/or make significant royalty or other payments in order to continue selling the affected products. Such licenses may not be available on commercially reasonable terms, if at all. We
have in the past and may in the future choose to settle disputes involving third-party intellectual property by taking a license. Such licenses or other settlements may involve, for example, upfront payments, yearly maintenance fees and royalties. At any given time, we may be involved as either a plaintiff or a defendant in a number of intellectual property actions, the outcomes of which may not be known for prolonged periods of time. A successful claim of patent or other intellectual property infringement or misappropriation against us could materially adversely affect our business, results of operations, and financial condition.
We may be subject to damages resulting from claims that we, our employees, or our independent contractors have wrongfully used or disclosed alleged trade secrets, proprietary or confidential information of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Some of our employees were previously employed at other medical device, pharmaceutical, or biotechnology companies. We may also hire additional employees who are currently employed at other medical device, pharmaceutical, or biotechnology companies, including our competitors. Additionally, consultants or other independent agents with whom we may contract may be or have been in a contractual arrangement with one or more of our competitors. Although no claims are currently pending, we may be subject to claims that we, our employees, or our independent contractors have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of these former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail to defend such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. There can be no assurance that this type of litigation will not occur, and any future litigation or the threat thereof may adversely affect our ability to hire additional direct sales representatives, or other personnel. A loss of key personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming, and ultimately unsuccessful.
Competitors may infringe or misappropriate the patents or other intellectual property that we own or license. In response, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us, such as alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent that we own or license is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or conclude that there is no infringement. An adverse result in any litigation or defense proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to the patents or patent applications that we own or license. An unfavorable outcome could require us to cease using the invention or attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
If we are unable to protect the confidentiality of our trade secrets and know-how, our business and competitive position would be harmed.
We seek to protect our proprietary technology and processes, in part, by entering into confidentiality and assignment of inventions agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Despite our efforts, agreements may be breached and security measures may fail, and we may not have adequate remedies for any breach or failure. In addition, our trade secrets and know-how may otherwise become known or be independently discovered by competitors. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. Moreover, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We may be subject to claims challenging the inventorship or ownership of the patents and other intellectual property that we own or license.
We may be subject to claims that former employees, collaborators, or other third parties have an ownership interest in the patents and intellectual property that we own or license. While it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements obligating them to assign such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own; our licensors may face similar obstacles. We could be subject to ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against any claims challenging inventorship or ownership. If we fail in defending any such claims, we may have to pay monetary damages and may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property, which could adversely impact our business, results of operations, and financial condition.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and other fees on patents and patent applications will be due to be paid to the United States Patent and Trademark Office and similar foreign agencies in several stages over the lifetime of the patents and patent applications. We rely on our outside counsel to pay these fees due to foreign patent agencies. The United States Patent and Trademark Office and various foreign patent agencies require compliance with a number of procedural, documentary, fee payment, and other provisions during the patent application process. We employ law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market, which could have a material adverse effect on our business, results of operations, and financial condition.
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Success in the biopharmaceutical industry is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve both technological and legal complexity, and therefore obtaining and enforcing pharmaceutical patents is costly, time-consuming, and inherently uncertain.
Recent patent reform legislation could increase the uncertainties and costs of prosecuting patent applications and enforcing and defending patents. Enacted in 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, made significant changes to United States patent law, including provisions that affect the prosecution of patent applications and also affect patent litigation. The United States Patent and Trademark Office developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, including the first to file provisions, only became effective in March 2013. The full impact of the Leahy-Smith Act on our business is not yet clear, but it could result in increased costs and more limited patent protection, either of which could adversely affect our business, results of operations, and financial condition.
Moreover, recent United States Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty regarding our ability to obtain patents in the future, this combination of events has created uncertainty regarding the value of any patents we do obtain. Depending on decisions by the United States Congress, the federal courts, and the United States Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce any current or future patents that we may own or license.
Risks Related to Our Series A Preferred Stock
A significant number of shares of our Class A common stock may be issued and sold upon the conversion of the Company’s Series A Convertible Preferred Stock. Such issuances would reduce the relative voting power of holders of our Class A common stock, dilute the ownership of such holders, and may adversely affect the market price of our Class A common stock.
As of the closing of our Series A Convertible Preferred Stock offering in November 2024, there were approximately 34,285,653 shares of Class A common stock issuable upon conversion of outstanding Convertible Preferred Stock, subject to applicable limitations under Nasdaq rules prior to stockholder approval. Holders of Convertible Preferred Stock are entitled to a regular dividend at the rate of 8.0% per annum, subject to adjustment and payable quarterly in cash or in-kind through an increase in the liquidation preference of each share of Convertible Preferred Stock. In addition, no dividend or other distribution on the Class A common stock will be declared or paid on the Class A common stock unless, at the time of such declaration and payment, an equivalent dividend or distribution is declared and paid on the Convertible Preferred Stock.
Under various circumstances defined in the Certificate of Designation, shares of our Convertible Preferred Stock can be converted into shares of our Class A common stock. The number of shares of Class A common stock into which Convertible Preferred Stock may convert or be redeemed is based in part on the liquidation preference for the Convertible Preferred Stock, so any increase in the liquidation preference may lead to an increase in the number of deemed shares of Class A common stock held by the Investors on an “as-converted” basis.
As holders of our Convertible Preferred Stock are entitled to vote, on an as-converted basis, together with holders of our Class A common stock, on all matters submitted to a vote of the holders of our Class A common stock, the issuance of the Convertible Preferred Stock to the Investors, and any subsequent increase in the liquidation preference of those shares by a payment-in-kind of the dividends payable thereon, effectively reduces the relative voting power of the holders of our Class A common stock.
Any conversion of the Convertible Preferred Stock into shares of our Class A common stock would dilute the ownership interest of existing holders of our Class A common stock, and any sale in the public market of shares of our Class A common stock issued upon such conversion or redemption could adversely affect the market prices of our Class A common stock. We granted the Investors customary registration rights in respect of their shares of Convertible Preferred Stock and any share of our Class A common stock issued upon any conversion thereof. These registration rights would facilitate the resale of such securities into the public market, and any such resale would increase the number of shares of our Class A common stock available for public trading. Sales by the Investors of a substantial number of shares of our Class A common stock in the public market, or the perception that such sales might occur, could have a material adverse effect on the trading price of our Class A common stock.
The Investors may exercise influence over us, including through their ability to designate, and the ability of the holders of Convertible Preferred Stock to elect, a member of our board of directors.
As of December 31, 2024, the outstanding shares of our Convertible Preferred Stock represented approximately 28% of our outstanding Class A common stock, on an as-converted basis and without giving effect to limitations under applicable Nasdaq rules prior to stockholder approval. In addition, the terms of the Convertible Preferred Stock grant the Investors consent rights with respect to certain actions by us, including:
•any amendment, modification, repeal or waiver of any provision of our Certificate of Incorporation, as amended, bylaws or of the Certificate of Designation that would amend, modify or otherwise fail to give effect to the rights of the Preferred Stockholders pursuant to the Certificate of Designation;
•any increase or decrease in the number of authorized shares of Convertible Preferred Stock, except as permitted in the Certificate of Designation;
•the creation of any new class or series of equity securities (including any additional class or series of preferred stock or any debt that is convertible into equity securities of the company or equity-linked securities) that would be senior or pari passu to the Convertible Preferred Stock in respect of liquidation preference or dividend rights or that would provide any unique governance rights to holders of such securities that are not existing rights of the holders of Class A common stock;
•the declaration or payment of any dividend to holders of Class A common stock;
•any increase to the size of the Board above 12 directors prior to our 2025 annual meeting and 11 directors after such meeting;
•incurrence by us (including our subsidiaries) of aggregate indebtedness in one or a series of transactions that would result in a consolidated total net leverage ratio (as defined in the Certificate of Designation) in excess of 3.5 to 1; or
•the entry into, or amendment or waiver of, any agreement by us (including our subsidiaries) that would prevent or delay us from complying, or impair our ability to comply, with our obligations to make the Cash-in-Lieu Payments.
As a result, the Investors have the ability to influence the outcome of certain matters affecting our governance and capitalization. The Investors are in the business of making or advising on investments in companies, including businesses that may directly or indirectly compete with certain portions of our business, and they may have interests that diverge from, or even conflict with, those of our other shareholders. They may also pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us.
In addition, the terms of the Certificate of Designation grant the Investors certain rights to designate a director to serve on our board of directors, which director is elected by a separate class vote of the holders of shares of the Convertible Preferred Stock. For so long as the Investors hold outstanding shares of Convertible Preferred Stock convertible into shares of Class A common stock representing at least 5.0% of the Company’s then-outstanding shares of Class A common stock, the Investors shall have the right to designate one director for election to our board of directors. Additionally at all times the Investors hold any outstanding shares of Convertible Preferred Stock, the Investors have a right to appoint one board observer.
The director designated by the Investors is entitled to serve on committees of our board of directors, subject to applicable law and stock exchange rules. Notwithstanding the fact that all directors will be subject to fiduciary duties to us and to applicable law, the interests of the director designated by the Investors may differ from the interests of our security holders as a whole or of our other directors.
Our Convertible Preferred Stock has rights, preferences, and privileges that are not held by, and are preferential to, the rights of holders of our Class A common stock, which could adversely affect our liquidity and financial condition, and may result in the interests of the Investors differing from holders of our Class A common stock.
The holders of Convertible Preferred Stock have the right under the Certificate of Designation to receive a liquidation preference entitling them to be paid out of our assets available for distribution to stockholders before any payment may be made to holders of any other class or series of capital stock, an amount equal to the greater of (a) the liquidation preference of their preferred shares plus all accrued and unpaid dividends or (b) the amount that such holders would have been entitled to receive upon our liquidation, dissolution, and winding up if all outstanding shares of Convertible Preferred Stock had been converted into shares of our Class A common stock immediately prior to such liquidation, dissolution, or winding up. The Convertible Preferred Stock initially had a liquidation preference of $1,000 per share; provided that the liquidation preference upon a change of control on or before November 12, 2026, will be increased to be no less than $1,500 per share. In addition, regular dividends on the Convertible Preferred Stock accrue and are cumulative at the rate of 8% per annum, subject to adjustment and payable quarterly. The dividend on each share of Convertible Preferred Stock is to be paid in cash or in-kind through an increase in the liquidation preference of such share.
As described herein, the Convertible Preferred Stock is convertible into shares of Class A common stock at any time at the option of the Preferred Stockholders. However, until we receive stockholder approval (Requisite Stockholder Approval), as contemplated by Nasdaq listing rules, with respect to the issuance of shares of Class A common stock upon conversion of the Convertible Preferred Stock in excess of the limitations imposed by such rules, the Preferred Stockholders cannot convert the Convertible Preferred Stock into a number of shares of Class A common stock in excess of 26,502,042 shares, which represents 19.99% of the outstanding shares of Class A common stock at the time of signing the subscription agreement, or to the extent such conversion will result in a Preferred Stockholder beneficially owning greater than 19.99% of our then-outstanding shares (Ownership Limitations). If, prior to receipt of the Requisite Stockholder Approval, a Preferred Stockholder elects to convert any Convertible Preferred Stock that would result in the issuance, when aggregated with the number of shares previously issued upon conversion of the
Convertible Preferred Stock, of more than 19.99% of the outstanding shares of Class A common stock at the time of signing the subscription agreement, then the Company will, in lieu of issuing shares of Class A common stock, pay the Preferred Stockholder a cash amount equal to the product of the number of shares of Class A common stock that could not be issued due to such limitation and the 10-day trailing volume weighted average price of the Class A common stock as of the trading day immediately prior to the conversion date (Cash-in-Lieu Payments), which Cash-in-Lieu Payments shall be paid no later than November 5, 2026, together with accrued interest of 10% per annum, to the extent an earlier cash payment is prohibited pursuant to the terms of the 2021 Credit Agreement as amended by the 2024 Amendment.
These dividend and Cash-in-Lieu Payment obligations could adversely affect our liquidity and reduce the amount of cash available for working capital, capital expenditures, growth opportunities, acquisitions, and other general corporate purposes. Our obligations to the holders of Convertible Preferred Stock could also limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial condition. The preferential rights could also result in divergent interests between the Investors and holders of shares of our Class A common stock.
Risks Related to Our Class A Common Stock
The Significant Stockholder Group exercises significant control over us, and their interests may conflict with yours in the future.
Alan A. Ades, Albert Erani, Glenn H. Nussdorf, Dennis Erani, Starr Wisdom, Josette Ades, and certain of their respective affiliates, including Organo PFG LLC, Organo Investors LLC, Dennis Erani 2012 Issue Trust, Alan Ades as Trustee of the Alan Ades 2014 GRAT, Albert Erani Family Trust dated 12/29/2012, GN 2016 Family Trust u/a/d August 12, 2016, GN 2016 Organo 10-Year GRAT u/a/d September 30, 2016 and RED Holdings, LLC, who we refer to collectively as the Significant Stockholder Group, control a significant amount of the voting power of the outstanding Class A common stock. As of February 24, 2025, the Significant Stockholder Group collectively beneficially owns approximately 40% of the Company’s Class A common stock. As a result of this voting control, the Significant Stockholder Group collectively can effectively determine the outcome of all matters requiring stockholder approval, including, but not limited to, the election and removal of the Company’s directors (including the right to designate four of our directors pursuant to the terms of an agreement between the Company and the Significant Stockholder Group), as well as other matters of corporate or management policy (such as potential mergers or acquisitions, payment of dividends, asset sales, and amendments to the Company’s certificate of incorporation and bylaws). This concentration of ownership may delay or deter possible changes in control and limit the liquidity of the trading market for the Company’s Class A common stock, which may reduce the value of an investment in its Class A common stock. This voting control could also deprive stockholders of an opportunity to receive a premium for their shares of Class A common stock as part of a potential sale of the Company. So long as the Significant Stockholder Group and their affiliates continue to own a significant amount of the Company’s combined voting power, they may continue to be able to strongly influence or effectively control its decisions. The interests of the Significant Stockholder Group and their affiliates may not coincide with the interests of other holders of the Company Class A common stock.
In the ordinary course of their business activities, the Significant Stockholder Group and their affiliates may engage in activities where their interests conflict with our interests or those of our other stockholders. In addition, the Significant Stockholder Group may have an interest in pursuing acquisitions, divestitures, and other transactions that, in their judgment, could enhance their investment, even though such transactions might involve risks to you.
Our stock price has been, and is likely to continue to be, volatile. Fluctuations in revenue or results of operations could cause additional volatility in our stock price and thus our stockholders could incur substantial losses.
Our stock price has been volatile and could be subject to wide fluctuations in response to various factors, many of which are beyond our control. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. Any unanticipated shortfall in our revenue in any fiscal quarter could have an adverse effect on our results of operations in that quarter. The effect on our net income of such a shortfall could be exacerbated by the relatively fixed nature of most of our costs, which primarily include personnel costs as well as facilities costs. These fluctuations could cause the trading price of our stock to be negatively affected. Our quarterly operating results have varied substantially in the past and may vary substantially in the future.
The Company bylaws designate the Court of Chancery of the State of Delaware, to the fullest extent permitted by law, as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by the Company stockholders, which could limit the ability of the Company stockholders to obtain a favorable judicial forum for disputes with the Company or with directors, officers or employees of the Company and may discourage stockholders from bringing such claims.
Under the Company bylaws, unless the Company consents in writing to the selection of an alternative forum, the sole and exclusive forum will be the Court of Chancery of the State of Delaware for:
•any derivative action or proceeding brought on behalf of the Company;
•any action asserting a claim of breach of a fiduciary duty owed by, or any wrongdoing by, any director, officer or employee of the Company to the Company or the Company’s stockholders;
•any action asserting a claim arising pursuant to any provision of the DGCL, the certificate of incorporation (including as it may be amended from time to time), or the bylaws;
•any action to interpret, apply, enforce or determine the validity of the certificate of incorporation or the bylaws; or
•any action asserting a claim governed by the internal affairs doctrine, in each case, except for, (1) any action as to which the Court of Chancery determines that there is an indispensable party not subject to the personal jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten (10) days following such determination) and (2) any action asserted under the Securities Exchange Act of 1934, as amended, or the rules and regulations promulgated thereunder, for which federal courts have exclusive jurisdiction.
These provisions of the Company’s certificate of incorporation and bylaws could limit the ability of the Company stockholders to obtain a favorable judicial forum for certain disputes with the Company or with its directors, officers or other employees, which may discourage such lawsuits against the Company and its directors, officers, and employees. Alternatively, if a court were to find these provisions of the Company’s certificate of incorporation or bylaws inapplicable to, or unenforceable in respect of, one or more of the types of actions or proceedings listed above including, without limitation, any actions asserted under the Securities Act of 1933, as amended, the Company may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect its business, financial condition and results of operations. In addition, there is uncertainty as to whether a court would enforce the Company’s forum selection provision with respect to any actions asserted under the Securities Act of 1933, as amended, as investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
Provisions in the Company’s charter may inhibit a takeover of the Company, which could limit the price investors might be willing to pay in the future for the Company's Class A common stock and could entrench management.
The Company’s certificate of incorporation contains provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests. These provisions include the ability of the Board of Directors to designate the terms of and issue new series of preferred shares, which may make more difficult the removal of management and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for the Company’s securities.
General Risk Factors
We have previously been, and may in the future be, subject to securities class action litigation or other litigation that could cause us to incur significant legal expenses, divert management’s attention, and result in harm to our business.
We may be exposed to potential liabilities and reputational risk associated with securities class action litigation. We have previously been the subject of a securities class action lawsuit, and, though that lawsuit was ultimately dismissed with prejudice, we may be subject to future lawsuits, including class action or securities derivative lawsuits as well as incur additional legal fees and may face negative impacts to our stock price and reputation. In addition, we may be obligated to indemnify and advance expenses to certain individuals involved in certain of these proceedings.
Any adverse judgment in or settlement of any future litigation could result in significant payments, fines and penalties that could have a material adverse effect on our business, results of operations, financial condition and reputation. Such payments, damages or settlement costs, if any, related to these matters could be in excess of our insurance coverage. The amount of time that is required to resolve these lawsuits is unpredictable and any litigation or claims against us, even those without merit, may cause us to
incur substantial costs, divert management’s attention from the day-to-day operation of our business, and materially harm our reputation.
We face significant and continuing competition, which could adversely affect our business, results of operations, and financial condition.
We face significant and continuing competition in our business, which is characterized by rapid technological change and significant price competition. Market share can shift as a result of technological innovation and other business factors. Our customers consider many factors when selecting a product, including product reliability, clinical outcomes, economic outcomes, price, and services provided by the manufacturer. Our ability to compete depends in large part on our ability to provide compelling clinical and economic benefits to our customers and payers, develop and commercialize new products and technologies and anticipate technological advances. Product introductions or enhancements by competitors which may have advanced technology, better features, or lower pricing may make our products obsolete or less competitive. In addition, consolidation in the healthcare industry continues to lead the demand for price concessions or to the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, results of operations or financial condition. The presence of this competition in our market may lead to pricing pressure, which would make it more difficult to sell our products at a price that will make us profitable or prevent us from selling our products at all. As a result, we will be required to devote continued efforts and financial resources to bring our products under development to market, deliver cost-effective clinical outcomes, expand our geographic reach, enhance our existing products, and develop new products for the advanced wound care and soft tissue repair markets. Even if we develop cost effective and/or new products, they may not be covered or reimbursed due to cost-containment and other financial pressures from payers.
Our future capital needs are uncertain and we may need to raise funds in the future, and such funds may not be available on acceptable terms or at all.
Continued expansion of our business will be expensive and we may seek funds from stock offerings, borrowings under our existing or future credit facilities or other sources. Our capital requirements will depend on many factors, including:
•the revenues generated by sales of our products;
•the costs associated with expanding our sales and marketing efforts;
•the expenses we incur in manufacturing and selling our products;
•the costs of developing and commercializing new products or technologies;
•the cost of obtaining and maintaining regulatory approval or clearance of certain products and products in development;
•the number and timing of acquisitions and other strategic transactions such as our acquisitions of NuTech Medical and CPN Biosciences, and integration costs associated with such acquisitions;
•the costs associated with capital expenditures; and
•unanticipated general, legal, and administrative expenses.
Our operating plan may change as a result of many factors currently unknown to us and we may need additional funds sooner than planned. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. Furthermore, if we issue equity or convertible debt securities to raise capital, you may experience dilution, and the new equity or convertible debt securities may have rights, preferences, and privileges that are senior to or otherwise adversely affect your rights as a stockholder. In addition, if we raise capital through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our products, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise capital on acceptable terms, we may not be able to develop our product candidates, enhance our existing products, execute our business plan, take advantage of future opportunities, or respond to competitive pressure, changes in our supplier relationships, or unanticipated customer requirements. Any of these events could adversely affect our ability to achieve our development and commercialization goals, which could have a material adverse effect on our business, results of operations, and financial condition.
Our future success depends on our ability to retain key employees, consultants and advisors, and to attract, retain and motivate qualified personnel.
We are highly dependent on our executive officers, the loss of whose services may adversely impact the achievement of our objectives. In particular, we depend on Gary Gillheeney, our President and Chief Executive Officer. Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives and scientific personnel in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous medical device companies for individuals with similar skill sets. The inability to recruit or loss of the services of any executive, key employee, consultant or advisor may impede the progress of our research, development, and sales growth objectives.
Our ability to recruit, retain and motivate our employees and consultants will depend in part on our ability to offer attractive compensation. We may also need to increase the level of cash compensation that we pay to them, which may reduce funds available for research and development and support of our sales growth objectives. There can be no assurance that we will have sufficient cash available to offer our employees and consultants attractive compensation.
Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us. The loss of the services of any of our executive officers or other key employees and our inability to find suitable replacements could potentially harm our business, prospects, financial condition or results of operations. We do not maintain "key person" insurance policies on the lives of these individuals or any of our other employees.
Many of the companies that we compete against for qualified personnel have substantially greater financial and other resources and different risk profiles than we do. They may also provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we can offer. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can discover, develop and commercialize product candidates will be limited.
Uncertainty and adverse changes in the general economic conditions, including recent turmoil in the global banking system, may negatively affect our business.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. If general economic conditions in the United States decline, or if consumers fear that economic conditions will decline, sales of our products may decline. Adverse changes may occur as a result of adverse economic conditions, fluctuating oil prices, supply chain problems, inflation, political instability, declining consumer confidence, a continuation or worsening of the COVID-19 pandemic or another pandemic, unemployment, fluctuations in stock markets, contraction of credit availability, or other factors affecting economic conditions generally. These changes may negatively affect the sales of our existing or development of future products, increase the cost, and decrease the availability of financing, or increase costs associated with producing and distributing our products and potential product candidates.
Moreover, there has been recent turmoil in the global banking system over the past few years. On March 10, 2023, Silicon Valley Bank (SVB), was closed, followed on March 11, 2023 and May 1, 2023, by Signature Bank and First Republic Bank, respectively, and the FDIC was appointed as receiver for those banks. SVB is one of our lenders at which we maintained deposit and money market accounts prior to its closure and have since transferred all of our deposits previously held with the bank to other banking institutions, with the exception of $2.3 million which we maintain in one operating account at SVB. There have been reports of instability at other banks across the globe including Credit Suisse, which was acquired by UBS. Despite the steps taken to date by United States agencies to protect depositors and our current belief that we do not have exposure to loss as a result of SVB’s receivership, the follow-on effects of the events surrounding the SVB, Signature Bank and First Republic Bank failures and pressure on other banks are unknown and could include failures of other financial institutions or significant disruptions to our operations, financial position, and reputation. A severe or prolonged economic downturn, such as the global financial crisis of 2007-2008, could result in a variety of risks to our business, including a decrease in the demand for our products and in our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy also could strain our suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our products. We cannot anticipate all the ways in which the foregoing, and the current economic climate and financial market conditions generally, could adversely impact our business. Furthermore, our stock price may decline due in part to the volatility of the stock market and any general economic downturn.
Changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters could significantly affect our business, results of operations, and financial condition.
GAAP and related accounting pronouncements, implementation guidelines and interpretations with regard to a wide range of matters that are relevant to our business are highly complex. These matters include, but are not limited to, revenue recognition, leases, income taxes, impairment of goodwill and long-lived assets and equity-based compensation. Changes in these rules, guidelines or interpretations could significantly change our reported or expected financial performance or financial condition.
In addition, the preparation of financial statements in conformity with GAAP requires management to make assumptions, estimates and judgments that affect the amounts reported in the consolidated financial statements and accompanying notes. We base our estimates and judgments on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. The results of these estimates form the basis for making judgments about the carrying values of assets, liabilities and equity, and the amount of net revenues and expenses that are not readily apparent from other sources. Our operating results may be adversely affected if our assumptions change or if actual circumstances differ from those in our assumptions, which could cause our operating results to fall below the expectations of securities analysts and investors, resulting in a decline in our stock price.
Our failure to comply with regulatory obligations could result in negative effects on our business.
The failure by us or one of our suppliers to comply with applicable regulatory requirements could result in, among other things, the FDA or other governmental authorities:
•imposing fines and penalties on us;
•preventing us from manufacturing or selling our products;
•delaying or denying pending applications for approval or clearance of our products or of new uses or modifications to our existing products, or withdrawing or suspending current approvals or clearances;
•ordering or requesting a recall of our products;
•issuing warning letters or untitled letters;
•imposing operating restrictions, including a partial or total shutdown of production or investigation of any or all of our products;
•refusing to permit to import or export of our products;
•detaining or seizing our products;
•obtaining injunctions preventing us from manufacturing or distributing any or all of our products;
•commencing criminal prosecutions or seeking civil penalties; and
•requiring changes in our advertising and promotion practices.
Failure to comply with applicable regulatory requirements could also result in civil actions against us by private parties (e.g., under the federal Lanham Act and/or state unfair competition laws), and other unanticipated negative consequences. If any of these actions were to occur it could harm our reputation and cause our product sales to suffer and may prevent us from generating revenue.
Our officers, employees, independent contractors, principal investigators, consultants and commercial partners may engage in misconduct or activities that are improper under other laws and regulations, which would create liability for us.
We are exposed to the risk that our officers, employees, independent contractors (including contract research organizations, or CROs), principal investigators, consultants and commercial partners may engage in fraudulent conduct or other illegal activity and/or may fail to disclose unauthorized activities to us. Misconduct by these parties could include, but is not limited to, intentional, reckless and/or negligent failures to comply with:
•the laws and regulations of the FDA and its foreign counterparts requiring the reporting of true, complete and accurate information to such regulatory bodies, including but not limited to safety problems associated with the use of our products;
•laws and regulations of the FDA and its foreign counterparts concerning the conduct of clinical trials and the protection of human research subjects;
•other laws and regulations of the FDA and its foreign counterparts relating to the manufacture, processing, packing, holding, investigating or distributing in commerce of medical devices, biological products and/or HCT/Ps; or
•manufacturing standards we have established.
In particular, companies involved in the manufacture of medical products are subject to laws and regulations intended to ensure that medical products that will be used in patients are safe and effective, and specifically that they are not adulterated or contaminated, that they are properly labeled, and have the identity, strength, quality and purity that which they are represented to possess. Further, companies involved in the research and development of medical products are subject to extensive laws and regulations intended to protect research subjects and ensure the integrity of data generated from clinical trials and of the regulatory review process. Any misconduct in any of these areas — whether by our own employees or by contractors, vendors, business associates, consultants, or other entities acting as our agents — could result in regulatory sanctions, criminal or civil liability and serious harm to our reputation. Although we have a comprehensive compliance program designed to ensure that our employees’, CRO partners’, principal investigators’, consultants’, and commercial partners’ activities and interactions with healthcare professionals and patients are appropriate, ethical, and consistent with all applicable laws, regulations, guidelines, policies and standards, it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in preventing such conduct, mitigating risks, or reducing the chance of governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, those actions could have a significant impact on our business, including the imposition of significant fines, and other sanctions that may materially impair our ability to run a profitable business.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, results of operations, and financial condition.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment, manufacture and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of the Company’s income or other tax returns could adversely affect the Company’s financial condition and results of operations.
The Company is subject to income tax in the United States and Switzerland, and the Company’s domestic tax liabilities will be subject to the allocation of expenses in differing jurisdictions. The Company’s future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
•changes in the valuation of the Company’s deferred tax assets and liabilities;
•expected timing and amount of the release of any tax valuation allowances;
•tax effects of stock-based compensation;
•costs related to intercompany restructurings;
•changes in tax laws, regulations or interpretations thereof; and
•lower than anticipated future earnings in jurisdictions where the Company has lower statutory tax rates and higher than anticipated future earnings in jurisdictions where the Company has higher statutory tax rates.
In addition, the Company may be subject to audits of the Company’s income, sales and other taxes by United States federal, state, local and non-United States taxing authorities. Outcomes from these audits could have an adverse effect on the Company’s financial condition and results of operations.
A market for the Company’s securities may not continue, which would adversely affect the liquidity and price of the Company’s securities.
The price of the Company’s securities may fluctuate significantly due to general market and economic conditions. An active trading market for the Company’s securities may never develop or, if developed, it may not be sustained. In addition, the price of the Company’s securities can vary due to general economic conditions and forecasts, the Company’s general business condition and the release of the Company’s financial reports. Additionally, if the Company’s securities are not listed on, or become delisted from, Nasdaq for any reason, and are quoted on the OTC Bulletin Board, an inter-dealer automated quotation system for equity securities that is not a national securities exchange, the liquidity and price of the Company’s securities may be more limited than if the Company was quoted or listed on Nasdaq or another national securities exchange. You may be unable to sell your securities unless a market can be established or sustained.
The Company’s quarterly operating results may fluctuate significantly and could fall below the expectations of securities analysts and investors due to seasonality and other factors, some of which are beyond the Company’s control, resulting in a decline in the Company’s stock price.
The Company’s quarterly operating results may fluctuate significantly because of several factors, including:
•labor availability and costs for hourly and management personnel;
•profitability of the Company’s products, especially in new markets and due to seasonal fluctuations;
•changes in interest or exchange rates;
•impairment of long-lived assets;
•macroeconomic conditions, both nationally and locally, including changes in regulatory coverage and pricing of our products;
•negative publicity relating to our products;
•changes in consumer preferences and competitive conditions; and
•expansion to new markets.
If securities or industry analysts do not publish or cease publishing research or reports about the Company, its business, or its market, or if they change their recommendations regarding the Company Class A common stock adversely, then the price and trading volume of the Company Class A common stock could decline.
The trading market for the Company Class A common stock will be influenced by the research and reports that industry or securities analysts may publish about us, the Company’s business, the Company’s market, or the Company’s competitors. Securities and industry analysts may stop publishing research on the Company. If any analyst who covers the Company were to cease coverage of the Company or fail to regularly publish reports on it, we could lose visibility in the financial markets, which could cause the Company’s stock price or trading volume to decline. If any of the analysts who cover the Company change their recommendation regarding the Company’s stock adversely, or provide more favorable relative recommendations about the Company’s competitors, the price of the Company Class A common stock would likely decline.
Changes in laws, regulations or rules, or a failure to comply with any laws, regulations or rules, may adversely affect the Company’s business, investments and results of operations.
The Company is subject to laws, regulations and rules enacted by national, regional and local governments and Nasdaq. In particular, the Company is required to comply with certain SEC, Nasdaq and other legal or regulatory requirements. Compliance with, and monitoring of, applicable laws, regulations and rules is difficult, time-consuming and costly. Those laws, regulations or rules and their interpretation and application may also change from time to time and those changes could have a material adverse effect on the Company’s business, investments and results of operations. In addition, a failure to comply with applicable laws, regulations or rules, as interpreted and applied, could have a material adverse effect on the Company’s business and results of operations.
Our failure to meet the continued listing requirements of Nasdaq could result in a delisting of our securities.
If we fail to satisfy the continued listing requirements of Nasdaq such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to delist our securities. Such a delisting would likely have a negative effect on the price of the securities and would impair your ability to sell or purchase the securities when you wish to do so. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our securities to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our securities from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements. Additionally, if our securities are not listed on, or become delisted from, Nasdaq for any reason, trading our common stock could be conducted only in the over-the-counter (OTC) market or on an electronic bulletin board established for unlisted securities such as the OTC Bulletin Board, an inter-dealer automated quotation system for equity securities that is not a national securities exchange, the liquidity and price of our securities may be more limited than if we were quoted or listed on Nasdaq or another national securities exchange. You may be unable to sell your securities unless a market can be established or sustained.
Changes to trade policy, including tariff and customs regulations, or failure to comply with such regulations may have an adverse effect on our reputation, business, financial condition and results of operations.
Changes in United States or international social, political, regulatory and economic conditions or in laws and policies governing trade, manufacturing, development and investment in the countries where we currently conduct our business or may conduct our business in the future could adversely affect our business, reputation, financial condition and results of operations. Changes or proposed changes in United States or other countries’ trade policies may result in restrictions and economic disincentives on international trade. The United States government has recently imposed, or is currently considering imposing, tariffs on certain trade partners, including China, Mexico, and Canada. Tariffs, economic sanctions and other changes in United States trade policy have in the past and could in the future trigger retaliatory actions by affected countries, and certain foreign governments have instituted or are considering imposing retaliatory measures on certain United States goods. Further, any emerging protectionist or nationalist trends (whether regulatory- or consumer-driven) either in the United States or in other countries could affect the trade environment. Our business, like many other corporations, would be impacted by changes to the trade policies of the United States and foreign countries (including governmental action related to tariffs, international trade agreements, or economic sanctions). Such changes have the potential to adversely impact the United States economy or certain sectors thereof, the global economy, and our industry, and as a result, could have a material adverse effect on our business, financial condition and results of operations.
None.
ITEM 1C. CYBERSECURITY
Risk Management and Strategy
We recognize the importance of developing, implementing, and maintaining measures to safeguard our information systems and protect the confidentiality, integrity, and availability of our data and to address potential cybersecurity incidents that may materially affect our business.
Our information security team manages and enhances our cybersecurity infrastructure with the ultimate goal of preventing cybersecurity incidents to the extent feasible, while simultaneously increasing our system resilience in an effort to minimize the business impact should an incident occur. We utilize cybersecurity tools, including the NIST Cybersecurity Framework, in assessing the threat landscape and continuously monitoring our environment.
We face a number of cybersecurity risks in connection with our business. We have, from time to time, experienced threats to our data and systems, including malware and computer virus attacks. However, such risks and threats have not materially affected our business strategy, results of operations, or financial condition to date.
Third-party service providers and consultants
Cybersecurity partners are a key part of our cybersecurity infrastructure. We partner with cybersecurity companies and leverage their technology and expertise to better protect the Company. From time to time, we engage certain vendors to monitor our environment, which includes an outsourced security operations center. We may also from time to time engage partners for periodic penetration testing and vulnerability assessments. Our third-party service providers, suppliers, and vendors face their own risks from cybersecurity threats that could potentially impact Organogenesis. We are developing and implementing processes for overseeing and managing these risks and are committed to maintaining robust governance and oversight of these risks. Those processes include assessing the third parties’ cybersecurity practices and where applicable, requiring the third parties to implement appropriate cybersecurity controls and otherwise agree to contractual requirements designed to address cybersecurity risks in our agreements with them including conducting ongoing monitoring of their compliance with those requirements.
We have also identified the potential for cybersecurity risks stemming from the use of artificial intelligence (AI) tools developed by third parties. We have implemented policies and training programs to govern the use of AI by our employees. Additionally, the Company’s Audit Committee regularly reviews our uses of AI as part of its ongoing risk oversight responsibility.
Governance
Our cybersecurity organization, led by our Assistant Vice President of IT and our Director of Information Security, is responsible for our overall information security strategy, policy, security engineering, operations and cyber threat detection and response. Within our team, our current Director of Information Security has professional cybersecurity certifications. The Company’s Board of Directors administers risk management oversight through the Audit Committee of the Board. Our Audit Committee receives quarterly updates about the effectiveness of the Company’s cybersecurity and information security programs, vulnerability and threat detection, progress relative to the Company’s cybersecurity roadmap, and the status of projects to strengthen our information security systems. The Audit Committee discusses with Company management and the Board the Company’s processes with respect to risk assessment and risk management.
ITEM 2. PROPERTIES
Our corporate headquarters is located on our four-building campus in Canton, Massachusetts, comprising approximately 300,000 square feet of leased and purchased space devoted to manufacturing, shipping, operations, and research and development. Three of the buildings are leased. The leases were initially set to expire on December 31, 2022, and were subsequently extended to December 31, 2027 when we exercised an option to renew these leases for an additional five-year term in December 2021. We lease the buildings in Canton from entities that are controlled by Alan A. Ades, Albert Erani, Dennis Erani and Glenn H. Nussdorf, who are also our stockholders. In addition, Messrs. Ades, Erani and Nussdorf are members of the Significant Stockholder Group, which has the right to designate four members of our Board of Directors.
In Norwood, Massachusetts, we have a leased facility of approximately 43,850 square feet for office, laboratory, and manufacturing use. The lease commenced on March 13, 2019. The rent commencement date was February 1, 2020. The initial lease
term is ten years from the rent commencement date and was extended for additional five years in December 2021. We have an option to extend the term for another ten years if exercised within 16-24 months from the end of the lease term.
In November 2024, we entered into a lease for a facility in Smithfield, Rhode Island, comprising approximately 122,000 square feet of manufacturing and office space. The lease of the office space commenced at the time of the lease signing, and we expect lease commencement of the manufacturing space in 2027, upon completion of the build out of the space for commercial manufacturing purposes. The initial term of the lease expires in May 2041, with two ten-year renewal options. We have a one-time right of first offer to purchase the Smithfield Facility and have a right to terminate the lease for a payment to the landlord of $1.3 million, if we have not secured certain anticipated state and local tax incentives by March 31, 2025.
We lease smaller facilities in Alabama, California, Florida, and Massachusetts, for manufacturing, warehouse, office, and laboratory space, under agreements with varying expiration dates through 2031.
ITEM 3. LEGAL PROCEEDINGS
On January 22, 2025, the Company was served with a complaint captioned United States of America, State of Texas, ex rel. John Doe vs. Organogenesis Holdings, Inc., which was filed in the United States District Court for the Southern District of Texas. The complaint is being brought by an employee the Company terminated. The United States and the State of Texas each declined to intervene in the case in September 2024. The complaint alleges claims pursuant to the United States False Claims Act and the Texas State Medicaid Fraud Prevention Act, seeking unquantified damages as well as fines, attorneys’ fees and other costs. The Company believes the claims are without merit and intends to vigorously contest them.
We are not a party to any other material legal proceedings. From time to time, we may become involved in litigation or other legal proceedings relating to claims arising from the ordinary course of business. These matters may include intellectual property, employment and other general claims. With respect to our outstanding legal matters, based on our current knowledge, we believe that the amount or range of reasonably possible loss will not, either individually or in the aggregate, have a material adverse effect on our business, consolidated financial position, results of operations, or cash flows. However, the outcome of such legal matters is inherently unpredictable and subject to significant uncertainties.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Class A common stock is listed on the Nasdaq Capital Market under the symbol "ORGO". As of February 24, 2025, a total of 126,828,092 shares of our Class A common stock were outstanding and we had 585 holders of record of our Class A common stock. This number does not include stockholders for whom shares are held in "nominee" or "street" name.
Dividend policy
We have never declared or paid any cash dividends on our Class A common stock. We currently intend to retain all available funds and future earnings, if any, to finance the growth and development of our business. We do not expect to pay any cash dividends on our Class A common stock in the foreseeable future. In addition, the terms of our 2021 Credit Agreement, as amended by the 2024 Amendment, as well as the terms of our Convertible Preferred Stock, restrict our ability to pay cash dividends on our capital stock without the bank’s consent.
Stock Performance Graph(1)
The following graph shows a comparison from December 31, 2019 through December 31, 2024 of cumulative total return on assumed investments of $100.00 in cash in each of our Class A common stock, the NASDAQ Composite Index and the NASDAQ Biotechnology Index. Such returns are based on historical results and are not intended to suggest future performance. Data for the NASDAQ Composite Index and the NASDAQ Biotechnology Index assume reinvestment of dividends.
COMPARISON OF FIVE YEARS CUMULATIVE TOTAL RETURN
Among Organogenesis Holdings Inc., the NASDAQ Composite Index,
and the NASDAQ Biotechnology Index
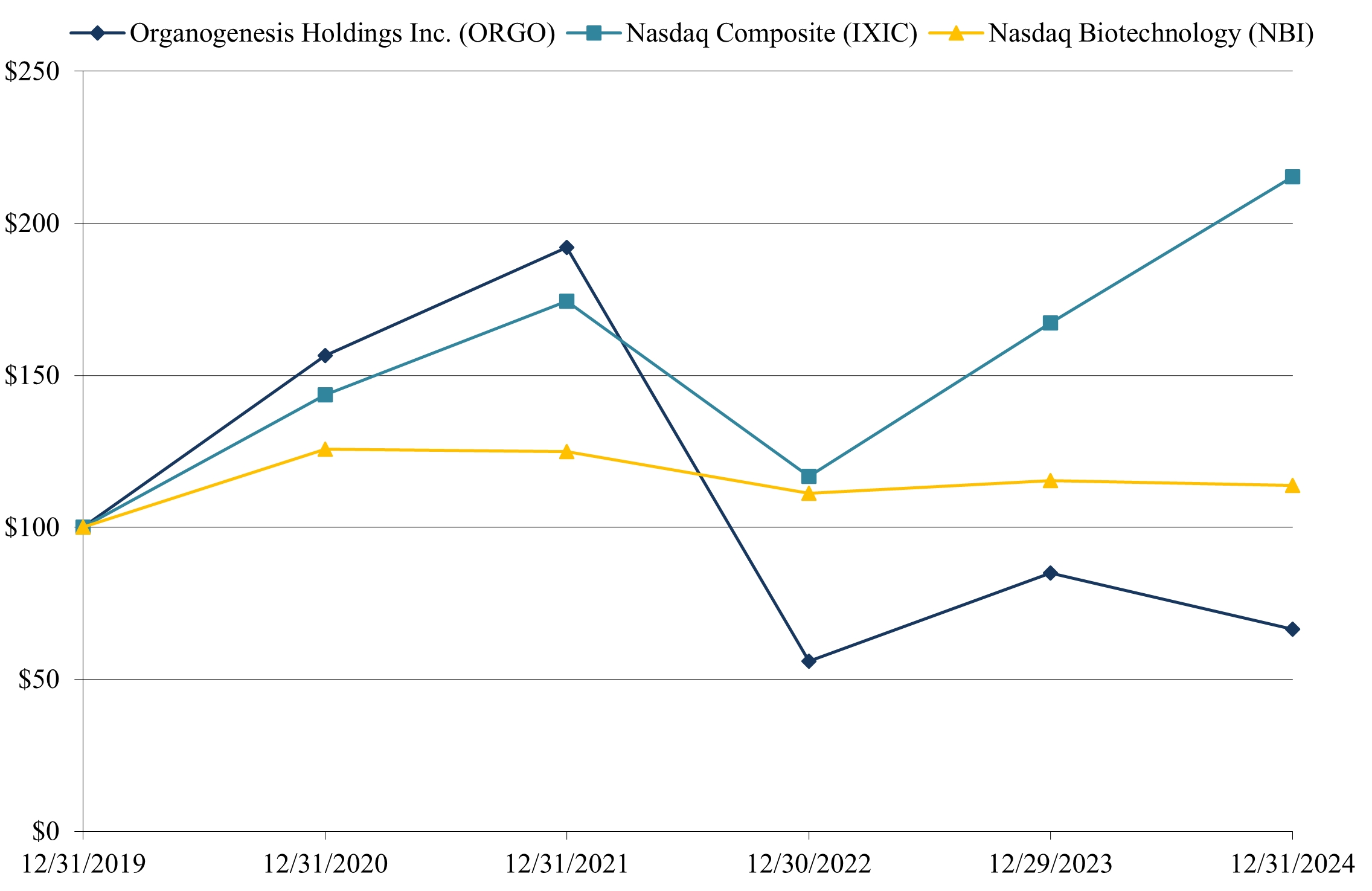
(1)This performance graph shall not be deemed to be "soliciting material" or to be "filed" with the SEC for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities under that Section, and shall not be deemed incorporated by reference into any filing of Organogenesis Holdings Inc. under the Securities Act of 1933, as amended.
Issuer Purchases of Equity Securities
The following table presents information with respect to purchases of Class A common stock we made during the three months ended December 31, 2024. As previously disclosed, in connection with our November 2024 offering of Convertible Preferred Stock, approximately $25.5 million of the net proceeds were used to fund the repurchase of an aggregate of 7,921,731 shares of Class A common stock from certain existing stockholders of the Company, including certain of its directors and their affiliates that are members of the Significant Stockholders Group.
| | | | | | | | | | |
Period | Total number of shares purchased | | Average price paid per share | Total number of shares purchased as part of publicly announced plans or programs | | Maximum number (or approximate dollar value) of shares that may yet be purchased under the plans or programs | |
October 1, 2024 | | — | | $-- | | — | | | — | |
November 1, 2024 | 7,921,7311 | | $ 3.21632 | | — | | | — | |
December 1, 2024 | | — | | $-- | | — | | | — | |
Total | | 7,921,731 | | N/A | | — | | | — | |
1. 7,421,731 shares of Class A common stock were repurchased from certain existing stockholders of the Company, including certain of its directors and their affiliates, at a price per share equal to $3.1597, which represents the 10-day trailing volume weighted average price of the Common Stock as of market close on November 11, 2024, pursuant to stock repurchase agreements entered into on November 12, 2024 between the Company and the stockholders. 500,000 shares of Class A common stock were repurchased from an affiliate of a director of the Company at a price per share equal to $4.057, which represents the 10-day trailing volume weighted average price of the Common Stock as of market close on November 26, 2024, pursuant to a stock repurchase agreement entered into on November 27, 2024 between the Company and the stockholder.
2. Represents a weighted average purchase price.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations together with our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and other parts of this Annual Report on Form 10-K contain forward-looking statements that involve risks and uncertainties, such as statements regarding our plans, objectives, expectations, intentions and projections. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the "Risk Factors" section of this Annual Report on Form 10-K.
Unless the context otherwise requires, for purposes of this section, the terms we," "us," "our," "the Company," "Organogenesis" and "ORGO" will refer to Organogenesis Holdings Inc. and its subsidiaries as they currently exist.
Overview
Organogenesis is a leading regenerative medicine and tissue innovations company focused on empowering healing through the development, manufacturing, and sale of products for the advanced wound care, and surgical and sports medicine markets. Our products have been shown through clinical and scientific studies to support and in some cases accelerate tissue healing and improve patient outcomes. We are advancing the standard of care in each phase of the healing process through multiple breakthroughs in tissue engineering and cell therapy. Our solutions address large and growing markets driven by aging demographics and increases in comorbidities such as diabetes, obesity, cardiovascular and peripheral vascular disease. We offer our differentiated products and in-house customer support to a wide range of health care customers including hospitals, wound care centers, government facilities, ASCs and physician offices. Our mission is to provide an integrated portfolio of healing and tissue solutions that improve lives while lowering the overall cost of health care.
We offer a comprehensive portfolio of products in the markets we serve that address patient needs across the continuum of care. We have and intend to continue to generate data from clinical trials, real-world outcomes and health economics research that validate the clinical efficacy and value proposition offered by our products. Several of our existing and pipeline products in our portfolio have PMA, or 510(k) clearance from the FDA. Given the extensive time and cost required to conduct clinical trials and receive FDA approvals, we believe that our data and regulatory approvals provide us with a strong competitive advantage. Our product development expertise and multiple technology platforms provide a robust product pipeline, which we believe will drive future growth.
In the Advanced Wound Care market, we focus on the development and commercialization of advanced wound care products for the treatment of chronic and acute wounds in various treatment settings. We have a comprehensive portfolio of regenerative medicine products capable of supporting patients from early in the wound healing process through wound closure regardless of wound type. Our Advanced Wound Care products include Apligraf for the treatment of VLUs and DFUs; Dermagraft for the treatment of DFUs (manufacturing and distribution currently suspended pending transition to a new manufacturing facility or engagement of a third-party manufacturer); PuraPly AM and PuraPly XT as antimicrobial barriers and native, cross-linked ECM scaffold for a broad variety of wound types; CYGNUS Dual as a dual-layered amniotic membrane that promotes an optimal environment for wound healing; and VIA Matrix, Affinity, Novachor, and NuShield placental allografts to address a variety of wound sizes and types as a protective barrier and extracellular matrix scaffold. We have a highly trained and specialized direct wound care sales force paired with comprehensive customer support services.
In the Surgical & Sports Medicine market, we are leveraging our broad regenerative medicine capabilities to address chronic and acute surgical wounds and tendon and ligament injuries. Our Sports Medicine products include NuShield for surgical applications in targeted soft tissue repairs; and Affinity, Novachor, PuraPly MZ, PuraPly AM, and PuraPly SX for management of open wounds in the surgical setting. We currently sell these products through independent agencies and our direct sales force.
Dermagraft
As previously disclosed, manufacturing of Dermagraft was suspended in the fourth quarter of 2021 and sales of Dermagraft were suspended in the second quarter of 2022. We currently plan to transition our Dermagraft manufacturing to our newly-leased biomanufacturing facility in Smithfield, Rhode Island, which we expect will begin in 2027, and will result in substantial long-term cost savings. If there are significant delays in the build out of the Smithfield Facility or in approval of the facility for manufacturing of Dermagraft, it could have an adverse effect on our consolidated net revenue and results of operations.
Local Coverage Determinations
On April 25, 2024, seven Medicare Part A/B MACs published new proposed LCDs for skin substitute grafts/CTPs for the treatment of DFUs and VLUs in the Medicare population. These LCDs were finalized by the MACs on November 14, 2024, and were originally set to become effective on February 12, 2025. However, on January 24, 2025, the MACs announced a delay in the implementation of the LCDs until April 13, 2025. Under the new LCDs finalized in November 2024, should they take effect as scheduled, eighteen products would remain covered, including our Apligraf and Dermagraft products for DFU and VLU, and our Affinity and NuShield products for DFU; however, more than 200 products would be classified as “non-covered,” including our PuraPly, PuraPly AM, PuraPly XT, Novachor, TransCyte, Dual and Matrix products for DFU and VLU. The LCDs as finalized apply only to DFU and VLU indications for skin substitute products; other indications would remain subject to case-by-case review of medical necessity by the MACs if the LCDs take effect. It is uncertain if there will be further delays in implementing the new LCDs and/or if the new LCDs will be revised or rescinded going forward. If implemented, the LCDs could materially impact utilization of these products, our business, and our revenue. Any future changes or other developments related to these or other LCDs also could affect utilization of our products, our business, and our revenue.
License And Manufacturing Agreement
We have a trademark license and manufacturing agreement with Vivex for Dual, Matrix, and VIA. We paid an upfront licensing fee to Vivex to sell Dual and Matrix, and also agreed to pay a fixed milestone payment for Dual in the event that its average sales price (ASP) is published by certain government agencies for a specified period of time, which we remitted in December 2024. Additionally, we are required to pay a low double-digit royalty on the Net Sales of Dual and VIA, and a high single-digit royalty on the Net Sales of Matrix, respectively, during the royalty term, as defined in the Vivex Agreement. The royalty term is commensurate with the initial term of the contract and will continue for each subsequent renewal period. The initial term of the agreement expires on December 31, 2026 and can be renewed for up to five additional one-year terms.
Management’s Use of Non-GAAP Measures
Our management uses financial measures that are not in accordance with GAAP (Non-GAAP), in addition to financial measures in accordance with GAAP, to evaluate our operating results. These Non-GAAP financial measures should be considered supplemental to, and not a substitute for, our reported financial results prepared in accordance with GAAP. Our management uses Adjusted EBITDA to evaluate our operating performance and trends and make planning decisions. Our management believes Adjusted EBITDA helps identify underlying trends in our business that could otherwise be masked by the effect of the items that we exclude. Accordingly, we believe that Adjusted EBITDA provides useful information to investors and others in understanding and evaluating our operating results, enhancing the overall understanding of our past performance and future prospects, and allowing for greater transparency with respect to key financial metrics used by our management in its financial and operational decision-making.
We define EBITDA as net income (loss) before depreciation and amortization, interest expense and income taxes. We define Adjusted EBITDA as EBITDA, further adjusted for the impact of certain items that we do not consider indicative of our core operating performance. These items include non-cash equity compensation, restructuring charges, write-off of the capitalized costs related to certain unfinished construction work and other long-term assets, fees paid in connection with settlement of previously disputed GPO fees, the cancellation fee for terminating certain agreements or pausing a certain construction project, legal and consulting fees associated with, as well as compensation expense related to retention for certain sales employees impacted by the published and subsequently withdrawn LCDs, impairment charges of a purchased building and associated unfinished construction work, and the write-down of costs previously capitalized in the development of internal-use software, that the Company determined have no future value. We have presented Adjusted EBITDA in this Annual Report on Form 10-K because it is a key measure used by our management and Board of Directors to understand and evaluate our operating performance, generate future operating plans and make strategic decisions regarding the allocation of capital. In particular, we believe that the exclusion of certain items in calculating Adjusted EBITDA can produce a useful measure for period-to-period comparisons of our business.
Our Adjusted EBITDA is not prepared in accordance with GAAP, and should not be considered in isolation of, or as an alternative to, measures prepared in accordance with GAAP. There are a number of limitations related to the use of Adjusted EBITDA rather than net income (loss), which is the most directly comparable financial measure calculated and presented in accordance with GAAP. Some of these limitations are:
•Although depreciation and amortization are non-cash charges, the assets that we currently depreciate and amortize will likely have to be replaced in the future, and Adjusted EBITDA does not reflect the cash required to fund such replacements;
•Adjusted EBITDA does not reflect interest expense or the cash requirements necessary to service payments on our debt;
•Adjusted EBITDA excludes stock-based compensation expense which has been, and will continue to be for the foreseeable future, a significant recurring non-cash expense for our business and an important part of our compensation strategy;
•Adjusted EBITDA does not reflect the effect of earnings or charges resulting from matters that our management does not consider to be indicative of our ongoing operations. However, some of these charges and gains (such as restructuring and impairment charges) have recurred and may recur; and
•Other companies, including companies in our industry, may calculate Adjusted EBITDA differently, which reduces its usefulness as a comparative measure.
Because of these limitations, we consider, and you should consider, Adjusted EBITDA together with other operating and financial performance measures presented in accordance with GAAP. A reconciliation of Adjusted EBITDA from net income (loss), the most directly comparable financial measure calculated in accordance with GAAP, has been included herein.
Components of Our Consolidated Results of Operations
In assessing the performance of our business, we consider a variety of performance and financial measures. We believe the items discussed below provide insight into the factors that affect these key measures.
Revenue
We derive our net revenue from our portfolio of Advanced Wound Care and Surgical & Sports Medicine products. We primarily sell our Advanced Wound Care products through direct sales representatives who manage and maintain the sales relationships with hospitals, wound care centers, government facilities, ASCs, and physician offices. We primarily sell our Surgical & Sports Medicine products through third-party agencies. As of December 31, 2024, we had 256 direct sales representatives and approximately 160 independent agencies.
We recognize revenue from sales of our Advanced Wound Care and Surgical & Sports Medicine products when the customer obtains control of our product, which occurs at a point in time and may be upon procedure date, shipment, or delivery, based on the contractual terms of a contract. We record revenue net of a reserve for returns, discounts and GPO rebates, which represent a direct reduction to the revenue we recognize.
Several factors affect our reported revenue in any period, including product, payer and geographic sales mix, operational effectiveness, pricing realization, marketing and promotional efforts, the timing of orders and shipments, regulatory actions including healthcare reimbursement scenarios, competition and business acquisitions.
Cost of goods sold and gross profit
Cost of goods sold includes personnel costs, product testing costs, quality assurance costs, raw materials and product costs, manufacturing costs, and the costs associated with our manufacturing and warehouse facilities. The changes in our cost of goods sold correspond with the changes in sales units and are also affected by product mix.
Gross profit is calculated as net revenue less cost of goods sold and generally increases as revenue increases. Our gross profit is affected by product and geographic sales mix, realized pricing of our products, the efficiency of our manufacturing operations and the costs of materials used and fees charged by third-party manufacturers to produce our products. Regulatory actions, including healthcare reimbursement scenarios, which may require costly expenditures or result in pricing pressures, may decrease our gross profit.
Selling, general and administrative expenses
Selling, general and administrative expenses generally include personnel costs for sales, marketing, sales support, customer support, and general and administrative personnel, sales commissions, incentive compensation, insurance, professional fees, depreciation, amortization, bad debt expense, royalties, information systems costs, gain or loss on disposal of long-lived assets, and costs associated with our administrative facilities. We generally expect our selling, general and administrative expenses to continue to increase due to increased investments in market development and the geographic expansion of our sales forces as we drive for continued revenue growth.
Research and development expenses
Research and development expenses include expenses for clinical trials, personnel costs for our research and development personnel, expenses related to improvements in our manufacturing processes, enhancements to our currently available products, and additional investments in our product and platform development pipeline. We expense research and development costs as incurred. We generally expect that research and development expenses will increase as we continue to conduct clinical trials on new and existing products, move products through the regulatory pathway (e.g., seek BLA approval), add personnel to support product enhancements as well as to bring new products to market, and enhance our manufacturing process and procedures.
Other expense, net
Other expense, net comprises primarily interest expense on our indebtedness that was outstanding until November 2024, including amortization of debt discount and debt issuance costs, net of interest income recognized.
Income taxes
We account for income taxes using an asset and liability approach. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Valuation allowances are provided when necessary to reduce net deferred tax assets to an amount that is more likely than not to be realized.
In determining whether a valuation allowance for deferred tax assets is necessary, we analyze both positive and negative evidence related to the realization of deferred tax assets including projected future taxable income, recent financial results and estimates of future reversals of deferred tax assets and liabilities. In addition, we consider whether it is more likely than not that the tax position will be sustained on examination by taxing authorities based on the technical merits of the position. We believe that our net U.S. deferred tax assets did not require a valuation allowance as of December 31, 2024.
Our U.S. provision for income taxes relates to current tax expense associated with taxable income that could not be offset by net operating losses or research and development credits. The utilization of our remaining federal net operating losses is subject to an 80% taxable income limitation and for certain states we have no net operating losses remaining to offset state taxable income or the utilization of the remaining state net operating losses are subject to a limitation. We have also recorded a foreign provision for income taxes related to our wholly-owned subsidiary in Switzerland.
We account for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Results of Operations
The following table sets forth, for the periods indicated, our results of operations:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Net revenue | | $ | 482,043 | | | $ | 433,140 | | | $ | 450,893 | |
Cost of goods sold | | | 115,741 | | | | 106,481 | | | | 105,019 | |
Gross profit | | | 366,302 | | | | 326,659 | | | | 345,874 | |
Operating expenses: | | | | | | | | | |
Selling, general and administrative | | | 294,513 | | | | 269,754 | | | | 283,808 | |
Research and development | | | 50,271 | | | | 44,380 | | | | 39,762 | |
Impairment of property and construction | | | 18,842 | | | | — | | | | — | |
Write down of capitalized internal-use software costs | | | 3,959 | | | | — | | | | — | |
Total operating expenses | | | 367,585 | | | | 314,134 | | | | 323,570 | |
Income (loss) from operations | | | (1,283 | ) | | | 12,525 | | | | 22,304 | |
Other expense, net: | | | | | | | | | |
Interest expense, net | | | (1,544 | ) | | | (2,190 | ) | | | (2,009 | ) |
Other income (expense), net | | | 20 | | | | 57 | | | | (13 | ) |
Total other expense, net | | | (1,524 | ) | | | (2,133 | ) | | | (2,022 | ) |
Net income (loss) before income taxes | | | (2,807 | ) | | | 10,392 | | | | 20,282 | |
Income tax (expense) benefit | | | 3,668 | | | | (5,447 | ) | | | (4,750 | ) |
Net income and comprehensive income | | | 861 | | | | 4,945 | | | | 15,532 | |
EBITDA and Adjusted EBITDA
The following table presents a reconciliation of GAAP net income to non-GAAP EBITDA and non-GAAP Adjusted EBITDA, for each of the periods presented:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
| | (in thousands) | |
Net income | | $ | 861 | | | $ | 4,945 | | | $ | 15,532 | |
Interest expense, net | | | 1,544 | | | | 2,190 | | | | 2,009 | |
Income tax expense (benefit) | | | (3,668 | ) | | | 5,447 | | | | 4,750 | |
Depreciation and amortization | | | 13,623 | | | | 10,448 | | | | 5,845 | |
Amortization of intangible assets | | | 3,403 | | | | 4,918 | | | | 4,883 | |
EBITDA | | | 15,763 | | | | 27,948 | | | | 33,019 | |
Stock-based compensation expense | | | 10,578 | | | | 8,996 | | | | 6,552 | |
Restructuring charge (1) | | | — | | | | 3,796 | | | | 2,268 | |
Write-off of certain assets (2) | | | — | | | | — | | | | 4,200 | |
Settlement fee (3) | | | — | | | | — | | | | 2,600 | |
Facility construction project pause (4) | | | — | | | | — | | | | 632 | |
Legal and consulting fees (5) | | | — | | | | 1,182 | | | | — | |
Sales retention (6) | | | — | | | | 694 | | | | — | |
Impairment of property and construction (7) | | | 18,842 | | | | — | | | | — | |
Write-down of capitalized software costs (8) | | | 3,959 | | | | — | | | | — | |
Disposal of construction in progress (9) | | | 645 | | | | | | | |
Adjusted EBITDA | | $ | 49,787 | | | $ | 42,616 | | | $ | 49,271 | |
(1)Amounts reflect employee retention and benefits as well as other exit costs associated with our restructuring activities. See Note 11, Restructuring, to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(2)Amount reflects the disposal of certain equipment related to construction in progress at one of our Canton, Massachusetts facilities. See Note 8, Property and Equipment, Net, to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(3)Amounts reflect the fee we paid to a GPO to settle previously disputed GPO fees. See Note 2, Significant Accounting Policies to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(4)Amount reflects the cancellation fees incurred in connection with the Company’s decision to pause one of its manufacturing facility construction projects. See Note 8, Property and Equipment, Net, to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(5)Amount reflects the legal and consulting fees incurred related to the published and subsequently withdrawn 2023 LCDs. See Local Coverage Determinations above.
(6)Amount reflects the compensation expenses related to retention for those sales employees impacted by the published and subsequently withdrawn 2023 LCDs. See Local Coverage Determinations above.
(7)Amount reflects the impairment of a purchased building and associated unfinished construction work. See Note 8, Property and Equipment, Net to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(8)Amount reflects the write-down of costs previously capitalized as construction in progress in the development of internal-use software, that the Company determined have no future value. See Note 8, Property and Equipment, Net to our audited consolidated financial statements included in this Annual Report on Form 10-K.
(9)Amount reflects construction in progress terminated and disposed of at one of our Canton, Massachusetts facilities, resulting from the Company’s decision to move certain operations to the Smithfield Facility.
Comparison of the Years Ended December 31, 2024, 2023, and 2022
Revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Advanced Wound Care | | $ | 453,639 | | | $ | 405,514 | | | $ | 422,231 | | | $ | 48,125 | | | | 12 | % | | $ | (16,717 | ) | | | (4 | %) |
Surgical & Sports Medicine | | | 28,404 | | | | 27,626 | | | | 28,662 | | | | 778 | | | | 3 | % | | | (1,036 | ) | | | (4 | %) |
Net revenue | | $ | 482,043 | | | $ | 433,140 | | | $ | 450,893 | | | $ | 48,903 | | | | 11 | % | | $ | (17,753 | ) | | | (4 | %) |
For the year ended December 31, 2024, net revenue from our Advanced Wound Care products increased by $48.1 million, or 12%, as compared to the year ended December 31, 2023. The increase in Advanced Wound Care net revenue was primarily attributable to an increase in sales of certain products for new and existing customers.
For the year ended December 31, 2024, net revenue from our Surgical & Sports Medicine products increased by $0.8 million, or 3%, as compared to the year ended December 31, 2023. The increase in Surgical & Sports Medicine net revenue was primarily due to growth in new customers and product mix.
For the year ended December 31, 2023, net revenue from our Advanced Wound Care products decreased by $16.7 million, or 4%, as compared to the year ended December 31, 2022. The decrease in Advanced Wound Care net revenue was primarily attributable to a decrease in sales of certain of our products due to changes in customer buying patterns as well as the impact of the 2023 withdrawn LCDs on sales of certain of our products, partially offset by an increase in sales of certain of our products to our existing and new customers.
For the year ended December 31, 2023, net revenue from our Surgical & Sports Medicine products decreased by $1.0 million, or 4%, as compared to the year ended December 31, 2022. The decrease in Surgical & Sports Medicine net revenue was primarily due to a shift in distributor focus.
Cost of Goods Sold and Gross Profit
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Cost of goods sold | | $ | 115,741 | | | $ | 106,481 | | | $ | 105,019 | | | $ | 9,260 | | | | 9 | % | | $ | 1,462 | | | | 1 | % |
Gross profit | | $ | 366,302 | | | $ | 326,659 | | | $ | 345,874 | | | $ | 39,643 | | | | 12 | % | | $ | (19,215 | ) | | | (6 | %) |
| | | | | | | | | | | | | | | | | | | | | |
For the year ended December 31, 2024, cost of goods sold increased by $9.3 million, or 9%, as compared to the year ended December 31, 2023. The increase in cost of goods sold was primarily driven by an increase in volume along with product mix, as well as the construction in progress terminated and disposed of at one of our Canton, Massachusetts facilities, resulting from the Company’s decision to move certain operations to the Smithfield Facility.
For the year ended December 31, 2024, gross profit increased by $39.6 million, or 12%, as compared to the year ended December 31, 2023. The increase in gross profit resulted primarily from an increase in volume and a shift in product mix.
For the year ended December 31, 2023, cost of goods sold increased by $1.5 million, or 1%, as compared to the year ended December 31, 2022. The increase in cost of goods sold was primarily due to product mix.
For the year ended December 31, 2023, gross profit decreased by $19.2 million, or 6%, as compared to the year ended December 31, 2022. The decrease in gross profit resulted primarily from a decrease in the pricing for certain of our products, as well as a shift in product mix.
Selling, General and Administrative Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Selling, general and administrative | | $ | 294,513 | | | $ | 269,754 | | | $ | 283,808 | | | $ | 24,759 | | | | 9 | % | | $ | (14,054 | ) | | | -5 | % |
| | | | | | | | | | | | | | | | | | | | | |
For the year ended December 31, 2024, selling, general and administrative expenses increased by $24.8 million, or 9%, as compared to the year ended December 31, 2023. The increase in selling, general and administrative expenses was primarily due to a $19.8 million increase in royalty expense; a $4.4 million increase in building and other facilities expense; and a $3.1 million increase in the allowance for expected credit losses. These increases in expenses were partially offset by a $1.0 million decrease in commissions, restructuring and other headcount-related expense; and a $1.5 million decrease in amortization expense.
For the year ended December 31, 2023, selling, general and administrative expenses decreased by $14.1 million, or 5%, as compared to the year ended December 31, 2022. The decrease in selling, general and administrative expenses was primarily due to a $6.1 million decrease in compensation and restructuring, largely related to decreased commissions paid to our sales force; a $4.4 million decrease primarily related to disposal of certain equipment related to the construction in progress in one of the Company's Canton, Massachusetts facilities; a $1.5 million decrease in royalty expenses, and a $3.3 million decrease in travel-related expenses. These expenses were partially offset by a $1.2 million increase in legal and consulting costs primarily related to efforts to convince three MACs to withdraw the final LCDs for skin substitutes for the treatment of DFUs and VLUs.
Research and Development Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Research and development | | $ | 50,271 | | | $ | 44,380 | | | $ | 39,762 | | | $ | 5,891 | | | | 13 | % | | $ | 4,618 | | | | 12 | % |
| | | | | | | | | | | | | | | | | | | | | |
For the year ended December 31, 2024, research and development expenses increased by $5.9 million, or 13%, as compared to the year ended December 31, 2023. The increase in research and development expenses was primarily driven by an increase in clinical research and consulting costs associated with our pipeline products not yet commercialized, and an increase in the clinical study and related costs necessary to seek regulatory approvals for certain of our product candidates.
For the year ended December 31, 2023, research and development expenses increased by $4.6 million, or 12%, as compared to the year ended December 31, 2022. The increase in research and development expenses was primarily driven by an increase in compensation expenses of $2.2 million, due to increased headcount associated with our existing Advanced Wound Care and Surgical & Sports Medicine products, an increase of $2.4 million in other clinical research and consulting costs associated with our pipeline products not yet commercialized, and an increase in the clinical study and related costs necessary to seek regulatory approvals for certain of our product candidates.
Impairment and Write Down Expenses
During the year ended December 31, 2024, we recorded a $4.0 million write down of costs related to internal-use software and an $18.8 million impairment of a purchased building and associated unfinished construction work. There were no such costs recorded in the year ended December 31, 2023. See Note 8, Property and Equipment, Net, to our consolidated financial statements included in this Annual Report on Form 10-K.
Other Expense, Net
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Interest expense, net | | $ | (1,544 | ) | | $ | (2,190 | ) | | $ | (2,009 | ) | | $ | 646 | | | | (29 | %) | | $ | (181 | ) | | | 9 | % |
Other income (expense), net | | | 20 | | | | 57 | | | | (13 | ) | | | (37 | ) | | | (65 | %) | | | 70 | | | | (538 | %) |
Total other expense, net | | $ | (1,524 | ) | | $ | (2,133 | ) | | $ | (2,022 | ) | | $ | 609 | | | | (29 | %) | | $ | (111 | ) | | | 5 | % |
| | | | | | | | | | | | | | | | | | | | | |
For the year ended December 31, 2024, total other expense, net, decreased by $0.6 million, or 29%, as compared to the year ended December 31, 2023. The decrease resulted primarily from a decrease in the balance of the Term Loan Facility, leading to lower interest expense in 2024.
For the year ended December 31, 2023, total other expense, net, increased by $0.1 million, or 5%, as compared to the year ended December 31, 2022. The increase resulted primarily from increases in interest rates in 2023.
Income Tax (Expense) Benefit
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | | Change | |
| | 2024 | | | 2023 | | | 2022 | | | 2024 to 2023 | | | 2023 to 2022 | |
| | (in thousands, except for percentages) | |
Income tax (expense) benefit | | $ | 3,668 | | | $ | (5,447 | ) | | $ | (4,750 | ) | | $ | 9,115 | | | | (167 | %) | | $ | (697 | ) | | | 15 | % |
For the year ended December 31, 2024, income tax benefit of $3.7 million included $7.1 million of current income taxes and ($10.7) million of deferred income taxes. The effective tax rate for 2024 was 130.5% and was computed based on the statutory rate of 21% adjusted primarily for tax benefits related to the generation of federal and state research and development tax credits, offset in part by non-deductible transaction costs.
For the year ended December 31, 2023, income tax expense of $5.4 million included $3.4 million of current income taxes and $2.0 million of deferred income taxes. The effective tax rate for 2023 was 52.4% and was computed based on the statutory rate of 21% adjusted primarily for state and local income taxes, nondeductible officer compensation and certain meals and other expenses that were fully deductible in prior years pursuant to temporary relief provisions enacted as part of the Taxpayer Certainty and Disaster Tax Relief Act for tax years 2021 and 2022, but that are now subject to a deduction limitation.
Liquidity and Capital Resources
Since our inception, we have funded our operations and capital expenditures through cash flows from product sales, loans from affiliates and entities controlled by certain of our affiliates, third-party debt and proceeds from the sale of our capital stock. In November 2024, we issued 130,000 shares of our newly-created Series A Convertible Preferred Stock, for total net proceeds of $120.6 million. We used $66.6 million of the proceeds to repay the balance on our Term Loan Facility, and $25.5 million for the repurchase of 7,921,731 shares of outstanding Class A common stock from certain members of the Significant Stockholder Group.
As of December 31, 2024, we had an accumulated deficit of $40.1 million and working capital of $208.5 million which included $135.6 million in cash and cash equivalents. We also have $125.0 million available for future revolving borrowings under our Revolving Facility (see Note 12, Long-Term Debt Obligations, to our audited consolidated financial statements included in this Annual Report on Form 10-K). For the year ended December 31, 2024, we reported $482.0 million in net revenue, $0.9 million in net income and $14.2 million of cash inflows from operating activities. We expect that our cash on hand and other components of working capital as of December 31, 2024, availability under the 2021 Credit Agreement as amended by the 2024 Amendment, plus net cash flows from product sales, will be sufficient to fund our operating expenses, capital expenditure requirements and debt service payments for at least 12 months beyond the filing date of this Annual Report on Form 10-K.
Our primary uses of cash are working capital requirements and capital expenditure. Additionally, from time to time, we may use capital for acquisitions and other investing and financing activities, such as our recent repurchase of our Class A common stock. Working capital is used principally for our personnel as well as manufacturing costs related to the production of our products. Our working capital requirements vary from period to period depending on manufacturing volumes, the timing of shipments and the payment cycles of our customers and payers. Our capital expenditures consist primarily of building improvements, manufacturing equipment, and computer hardware and software.
To the extent additional funds are necessary to meet our long-term liquidity needs as we continue to execute on our business strategy, we anticipate that they will be obtained through additional equity or debt financings, other strategic transactions or a combination of these potential sources of funds. There can be no assurance that we will be able to obtain additional funds on terms acceptable to us, on a timely basis or at all.
The following table presents our cash and outstanding debt as of the dates indicated:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
| | (in thousands) | |
Cash and cash equivalents | | $ | 135,571 | | | $ | 103,840 | |
| | | | | | |
Line of credit | | $ | — | | | $ | — | |
Term loan net of debt discount and issuance cost | | | — | | | | 66,231 | |
Finance lease obligations | | | 1,888 | | | | 2,969 | |
Total debt | | $ | 1,888 | | | $ | 69,200 | |
Under the Revolving Facility, we have up to $125.0 million available for future revolving borrowings, subject to maintaining compliance with financial and non-financial covenants.
Cash Flows
The following table summarizes our cash flows for each of the periods presented:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
| | (in thousands) | |
Net cash provided by operating activities | | $ | 14,208 | | | $ | 30,917 | | | $ | 24,859 | |
Net cash used in investing activities | | | (10,032 | ) | | | (24,364 | ) | | | (33,898 | ) |
Net cash provided by (used in) financing activities | | | 27,637 | | | | (5,505 | ) | | | (2,199 | ) |
Net increase (decrease) in cash, cash equivalents, and restricted cash | | $ | 31,813 | | | $ | 1,048 | | | $ | (11,238 | ) |
Operating Activities
During the year ended December 31, 2024, net cash provided by operating activities was $14.2 million, resulting from our net income of $0.9 million, non-cash charges of $62.2 million, partially offset by net cash used in connection with changes in our operating assets and liabilities of $48.9 million. Net cash used in changes in our operating assets and liabilities included an increase in accounts receivable of $31.8 million, an increase in inventories of $6.2 million, an increase prepaid expenses and other current and other assets of $2.5 million, a decrease in net operating lease liabilities of $14.1 million, and a decrease in accounts payable of $2.4 million; partially offset by an increase in accrued expenses and other current liabilities of $9.2 million, and a decrease in other liabilities of $1.1 million.
During the year ended December 31, 2023, net cash provided by operating activities was $30.9 million, resulting from our net income of $4.9 million, non-cash charges of $44.0 million, partially offset by net cash used in connection with changes in our operating assets and liabilities of $18.1 million. Net cash used in changes in our operating assets and liabilities included an increase in inventories and prepaid expenses of a total of $18.3 million, and a decrease in net operating lease liabilities of $8.4 million, partially offset by an increase in accounts payable, accrued expenses, and other current and noncurrent liabilities of $3.1 million, and a decrease in accounts receivable of $5.5 million.
During the year ended December 31, 2022, net cash provided by operating activities was $24.9 million, resulting from our net income of $15.5 million, non-cash charges of $43.4 million, partially offset by net cash used in connection with changes in our operating assets and liabilities of $34.1 million. Net cash used in changes in our operating assets and liabilities included an increase in accounts receivable of $8.8 million, an increase in inventory and prepaid expenses of $9.8 million, a decrease in operating lease liability of $7.0 million and a decrease of accrued expenses of $11.9 million, all of which were partially offset by an increase in accounts payable and other liabilities of $3.3 million.
Investing Activities
During the year ended December 31, 2024, we used $10.0 million of cash in investing activities solely consisting of capital expenditures.
During the year ended December 31, 2023, we used $24.4 million of cash in investing activities solely consisting of capital expenditures.
During the year ended December 31, 2022, we used $33.9 million of cash in investing activities solely consisting of capital expenditures.
Financing Activities
During the year ended December 31, 2024, net cash provided by financing activities was $27.6 million. This consisted primarily of proceeds from issuance of our Series A Convertible Preferred Stock, net of issuance costs of $120.7 million, and net payments of $0.1 million in connection with stock awards activities; partially offset by repayment of our Term Loan Facility of $66.6 million, payments for repurchases of our Class A common stock of $25.5 million, and payments on our finance lease obligations of $1.1 million.
During the year ended December 31, 2023, net cash used in financing activities was $5.5 million. This consisted primarily of principal payments on the Term Loan of $4.7 million, and on finance lease obligations of $0.5 million, and payments of $0.3 million in connection with stock awards activities.
During the year ended December 31, 2022, net cash used in financing activities was $2.2 million. This consisted primarily of the payment of term loan and finance lease obligations of $3.0 million and the payment of $0.6 million related to the CPN deferred acquisition consideration, partially offset by the net receipts of $1.4 million in connection with stock awards activities.
Indebtedness
2021 Credit Agreement
In August 2021, we and our subsidiaries entered into a credit agreement with SVB and several other lenders (the Lenders), which we refer to as the 2021 Credit Agreement. The 2021 Credit Agreement, as amended, provides for a term loan facility not to exceed $75.0 million (the Term Loan Facility) and a revolving credit facility not to exceed $125.0 million (the Revolving Facility). In November 2024, we and the Lenders amended the 2021 Credit Agreement to allow for the issuance of the Convertible Preferred Stock, and to require the repayment of the Term Loan Facility within one business day of such issuance, among other terms (the 2024 Amendment).
Advances made under the 2021 Credit Agreement were either SOFR Loans or ABR Loans, at our option. For SOFR Loans, the interest rate was a per annum interest rate equal to the Adjusted Term SOFR plus an Applicable Margin between 2.00% to 3.25% based on the Total Net Leverage Ratio. For ABR Loans, the interest rate was equal to (1) the highest of (a) the Wall Street Journal Prime Rate, (b) the Federal Funds Rate plus 0.50% and (c) the Adjusted Term SOFR rate plus 1.0%, plus (2) an Applicable Margin between 1.00% to 2.25% based on the Total Net Leverage Ratio.
The 2021 Credit Agreement required us to make consecutive quarterly installment payments equal to the following: (a) from September 30, 2021 through and including June 30, 2022, $0.5 million; (b) from September 30, 2022 through and including June 30, 2023, $0.9 million; (c) from September 30, 2023 through and including June 30, 2025, $1.4 million and (d) from September 30, 2025 and the last day of each quarter thereafter until August 6, 2026 (the Term Loan Maturity Date), $1.9 million. We prepaid the Term Loan Facility in November 2024, and amounts borrowed under the Term Loan Facility may not be re-borrowed.
We must pay in arrears, on the first day of each quarter prior to August 6, 2026 (the Revolving Termination Date) and on the Revolving Termination Date, a fee for our non-use of available funds (the Commitment Fee). The Commitment Fee rate is between 0.25% to 0.45% based on the Total Net Leverage Ratio. We may elect to reduce or terminate the Revolving Facility in its entirety at any time by repaying all outstanding principal and unpaid accrued interest.
Under the 2021 Credit Agreement as amended by the 2024 Amendment, we are required to comply with certain financial covenants including the Consolidated Fixed Charge Coverage Ratio and Consolidated Total Net Leverage Ratio, tested quarterly. In addition, we are also required to make representations and warranties and comply with certain non-financial covenants that are
customary in loan agreements of this type, including restrictions on the payment of dividends, repurchase of stock, incurrence of indebtedness, dispositions and acquisitions.
As of December 31, 2024, we were in compliance with the covenants under the 2021 Credit Agreement, as amended by the 2024 Amendment. We did not have outstanding borrowings under our Term Loan Facility or our Revolving Facility, with $125 million available for future revolving borrowings.
Critical Accounting Estimates
Our consolidated financial statements have been prepared in accordance with GAAP. The preparation of our consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, and the disclosure at the date of the consolidated financial statements, as well as revenue and expenses recorded during the reporting periods. Management bases its estimates, assumptions and judgments on historical experience and on various other factors that it believes to be reasonable under the circumstances. Different assumptions and judgments would change the estimates used in the preparation of our consolidated financial statements, which, in turn, could materially change our results from those reported. Management evaluates its estimates, assumptions and judgments on an ongoing basis. Historically, our critical accounting estimates have not differed materially from actual results. However, if our assumptions change, we may need to revise our estimates or take other corrective actions, either of which may also have a material adverse effect on our consolidated statements of operations, liquidity and financial condition.
We believe the following critical accounting estimates involve significant areas where management applies judgments and estimates in the preparation of our consolidated financial statements, and supplement our discussion in Note 2, Significant Accounting Policies, to our audited consolidated financial statements included in this Annual Report on Form 10-K.
Income Taxes
We account for income taxes using an asset and liability approach. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Valuation allowances are provided when necessary to reduce net deferred tax assets to an amount that is more likely than not to be realized.
In determining whether a valuation allowance for deferred tax assets is necessary, we analyze both positive and negative evidence related to the realization of deferred tax assets including projected future taxable income, recent financial results and estimates of future reversals of deferred tax assets and liabilities. In addition, we consider whether it is more likely than not that the tax position will be sustained on examination by taxing authorities based on the technical merits of the position.
Impairment of Long-Lived Assets
We review long-lived assets, excluding goodwill, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Factors that we consider in deciding when to perform an impairment review include, but are not limited to, significant underperformance of the business in relation to expectations, significant negative industry or economic trends and significant changes or planned changes in the use of our assets. When such an event occurs, we determine whether our asset groups are appropriate for impairment considerations, based on any changed facts and circumstances, and we then determine whether there has been impairment by comparing the anticipated undiscounted future net cash flows to the related asset group’s carrying value. If we determine an asset to be impaired, we reduce its carrying value to fair value, which is determined based on discounted cash flows or its appraised value, depending on the nature of the asset. Judgments and estimates used by management when evaluating long-lived assets for impairment include: an assessment as to whether an adverse event or circumstance has triggered the need for an impairment review; determination of asset groups, the primary asset within each group, and the primary asset's average estimated useful life; undiscounted future cash flows generated by the assets; and determination of fair value when an impairment is deemed to exist. The estimation of fair value may require significant assumptions related to estimated future costs to prepare the impaired asset for potential sale or disposal, and the discount rate applied to estimated future cash flows generated by the assets. If these estimates or their related assumptions change in the future, we may be required to record impairment charges against these assets in the reporting period in which the impairment is identified.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Recently Issued Accounting Pronouncements
For a description of recently issued accounting pronouncements, including the expected dates of adoption and the estimated effects, if any, on our consolidated financial statements, see Note 2, Significant Accounting Policies to our consolidated financial statements appearing at the end of this Annual Report on Form 10-K.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to various market risks, including variability in currency exchange rates. We have established policies, procedures and internal processes governing our management of market risk.
Foreign Currency and Market Risk
The majority of our employees and our major operations are currently located in the United States. The functional currency of our foreign subsidiary in Switzerland is the United States dollar. We have, in the normal course of business, engaged in contracts with contractors or other vendors in a currency other than the United States dollar. To date, we have had minimal exposure to fluctuations in foreign currency exchange rates as the time period from the date that transactions are initiated and the date of payment or receipt of payment is generally of short duration. Accordingly, we believe we do not have a material exposure to foreign currency risk.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Our consolidated financial statements, together with the report of our independent registered public accounting firm, appear on pages F-1 through F-28 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2024. The term "disclosure controls and procedures," as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms promulgated by the SEC. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based on that evaluation, our management, including our principal executive officer and principal financial officer, concluded with reasonable assurance that, as of December 31, 2024, our disclosure controls and procedures were effective.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, the Company’s principal executive officer and principal financial officer and effected by the Company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Management conducted the assessment of the effectiveness of the Company’s internal control over financial reporting based on criteria in the SEC guidance on conducting such assessments as of the end of the period covered by this report. Management conducted the assessment based on certain criteria established in Internal Control— Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. As a result of this assessment, management concluded that, as of December 31, 2024, our internal controls over financial reporting were effective.
Remediation of Previously Identified Material Weakness
As previously reported in our Annual Report on Form 10-K for the year ended December 31, 2023, in previous years we did not design and maintain effective controls over information technology general controls and proper segregation of duties to support the initiation and recording of transactions and the resulting impact on business process controls and applications that rely on such data.
Management has taken actions to remediate the deficiencies in its internal controls over financial reporting and implemented additional processes and controls designed to address the underlying causes associated with the above-mentioned material weakness. Management’s internal control remediation efforts included the following:
•During the second quarter of 2024, we completed the implementation of certain modules in a new company-wide enterprise resource planning (ERP) system to provide additional systematic controls and segregation of duties for our accounting processes. We have implemented additional controls to mitigate existing risks of proper segregation and change configurations.
•We added personnel to our accounting and finance team with the requisite accounting and internal controls knowledge and experience to sufficiently enhance our internal controls environment.
•We designed and implemented new information technology general controls to ensure proper segregation of duties in our change management processes.
•An outside firm continued to assist management with performing control design and operating effectiveness testing throughout the year.
•We regularly reported the results of control testing to the key stakeholders across our organization, including our Audit Committee, on testing progress and defined corrective actions, and we monitored and reported on the results of control remediation. We have strengthened our internal policies, processes, and reviews through these actions.
•We have continued working on documenting and structuring the Company’s processes to meet Sarbanes-Oxley (SOX) 404(b) requirements.
Based on the successful implementation and testing of these new and enhanced control processes, we have concluded that the material weakness described above has been remediated as of December 31, 2024.
Changes in Internal Control Over Financial Reporting
Except as noted above, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Securities Exchange Act) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting during the fourth quarter of our fiscal year ended December 31, 2024.
Attestation Report of the Registered Public Accounting Firm
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2024, has been audited by RSM US LLP, an independent registered public accounting firm, as stated in their attestation report, which appears in Item 8 above.
ITEM 9B. OTHER INFORMATION
During the three months ended December 31, 2024, no director or officer of the Company adopted or terminated a "Rule 10b5-1 trading arrangement" or "non-rule 10b5-1 trading arrangement," as each term is defined in item 408(a) of Regulation S-K.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item will be set forth in an amendment to our Annual Report on Form 10-K/A to be filed with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year (the Annual Report Amendment), and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item will be set forth in our Annual Report Amendment and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item will be set forth in our Annual Report Amendment and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item will be set forth in our Annual Report Amendment and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item will be set forth in our Annual Report Amendment and is incorporated herein by reference.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Documents filed as a part of this Report:
(1) Financial Statements —See Index to Consolidated Financial Statements and Item 8 of this Annual Report on Form 10-K.
(2) Financial Statement Schedules —Schedules are omitted because they are not applicable, or are not required, or because the information is included in the Consolidated Financial Statements and notes thereto.
(3) Index to Exhibits.
Exhibit Index
| | |
Exhibit No. | | Exhibit |
| | |
| | |
3.1 | | Certificate of Incorporation of Organogenesis Holdings Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-3/A (File No. 333-233621) filed with the SEC on September 16, 2019) |
| | |
3.2 | | Certificate of Amendment of Certificate of Incorporation of Organogenesis Holdings Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on June 27, 2022) |
| | |
3.3 | | Certificate of Designations of Series A Convertible Preferred Stock (incorporated by reference to Exhibit 3.3 to the Company’s Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on November 12, 2024) |
| | |
3.4 | | Bylaws of Organogenesis Holdings Inc. (incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement on Form S-3/A (File No. 333-233621) filed with the SEC on September 16, 2019) |
| | |
4.1* | | Description of Securities registered pursuant to Section 12 of the Securities Exchange Act of 1934 |
| | |
10.1 | | Amended and Restated Registration Rights Agreement dated as of December 10, 2018 among Organogenesis Holdings Inc., Avista Acquisition Corp., Avista Capital Partners Fund IV L.P., Avista Capital Partners Fund IV (Offshore), L.P., and certain holders of Organogenesis Common Stock (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.2 | | Lease dated as of January 1, 2013 by and between Organogenesis Inc. and 65 Dan Road SPE, LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.3 | | Lease dated as of January 1, 2013 by and between Organogenesis Inc. and 85 Dan Road Associates, LLC (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.4 | | Lease dated as of January 1, 2013 by and between Organogenesis Inc. and Dan Road Equity I, LLC (incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.5‡ | | Amended and Restated Key Employee Agreement dated as of February 1, 2007 by and between Organogenesis Inc. and Gary Gillheeney (incorporated by reference to Exhibit 10.13 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.6‡ | | Employee Letter Agreement dated as of February 14, 2017 by and between Organogenesis Inc. and Patrick Bilbo (incorporated by reference to Exhibit 10.14 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.7‡ | | Employee Letter Agreement dated as of February 14, 2017 by and between Organogenesis Inc. and Antonio Montecalvo (incorporated by reference to Exhibit 10.16 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.8‡ | | Employee Letter Agreement dated as of January 19, 2018 by and between Organogenesis Inc. and Lori Freedman (incorporated by reference to Exhibit 10.18 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
Exhibit No. | | Exhibit |
| | |
10.9‡ | | Employee Letter Agreement dated as of May 9, 2017 by and between Organogenesis Inc. and Brian Grow (incorporated by reference to Exhibit 10.19 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.10‡ | | 2003 Stock Incentive Plan, as amended (incorporated by reference to Exhibit 10.27 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.11‡ | | Form of Incentive Stock Option Agreement under the 2003 Stock Incentive Plan (incorporated by reference to Exhibit 10.28 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.12‡ | | Form of Non-Statutory Stock Option Agreement under the 2003 Stock Incentive Plan (incorporated by reference to Exhibit 10.29 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.13‡ | | 2018 Equity Incentive Plan (as amended) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on June 21, 2024) |
| | |
10.14‡ | | Form of Incentive Stock Option Agreement under the 2018 Equity Incentive Plan (incorporated by reference to Exhibit 10.31 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.15‡ | | Form of Non-Statutory Stock Option Agreement under the 2018 Equity Incentive Plan (incorporated by reference to Exhibit 10.32 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.16‡ | | Form of Restricted Stock Unit Agreement under the 2018 Equity Incentive Plan (incorporated by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on May 11, 2020) |
| | |
10.17‡ | | Form of Indemnification Agreement for Directors and Officers (incorporated by reference to Exhibit 10.33 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.18† | | Settlement and License Agreement effective as of October 25, 2017 by and among Organogenesis Inc., RESORBA Medical GmbH, and Advanced Medical Solutions Group plc (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement in Form S-4 (File No. 333-227090) filed with the SEC on October 9, 2018) |
| | |
10.19 | | Amended and Restated Code of Ethics and Conduct of ORGO adopted on December 10, 2018 (incorporated by reference to Exhibit 10.35 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.20 | | Controlling Stockholders Agreement dated as of December 10, 2018 by and among ORGO and the Controlling Entities (incorporated by reference to Exhibit 10.36 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 11, 2018) |
| | |
10.21 | | Lease dated March 13, 2019 between Organogenesis Inc., as tenant, and Bobson Norwood Commercial, LLC, as landlord (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on March 19, 2019) |
| | |
10.22‡ | | Summary of Amendment to Severance for Gary S. Gillheeney, Sr. (incorporated by reference to Exhibit 10.43 to the Company's Annual Report on Form 10-K/A (File No. 001-37906) filed with the SEC on April 29, 2020) |
| | |
10.23‡ | | Offer Letter dated January 15, 2021 between the Company and David C. Francisco (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on February 16, 2021) |
| | |
10.24‡ | | Change in Control Retention Agreement between Organogenesis Holdings Inc. and Gary S. Gillheeney, Sr. effective as of May 10, 2021 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on May 10, 2021) |
| | |
10.25‡ | | Form of Change in Control Retention Agreement (Non-CEO Executive Officers) (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on May 10, 2021) |
| | |
10.26‡ | | Form of Change in Control Retention Agreement (Independent Directors) (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on May 10, 2021) |
| | |
10.27 | | Credit Agreement dated and effective as of August 6, 2021 among Organogenesis Holdings Inc., as borrower, Organogenesis Inc. and Prime Merger Sub, LLC, as guarantors, and Silicon Valley Bank, as Administrative Agent, |
| | |
Exhibit No. | | Exhibit |
| | Lead Arranger, Bookrunner, Issuing Lender and Swingline Lender, and Silicon Valley Bank and the several other lenders from time to time party thereto, collectively as Lenders (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on August 9, 2021) |
| | |
10.28 | | First Amendment to Credit Agreement dated as of December 8, 2022 by and among Organogenesis Holdings Inc., as borrower, the several banks and other financial institutions or entities party hereto and Silicon Valley Bank, as the Administrative Agent, and as the Issuing Lender and the Swingline Lender (incorporated by reference to Exhibit 10.33 to the Company’s Annual Report on Form 10-K (File No. 001-37906) filed with the SEC on March 1, 2023) |
| | |
10.29 | | Second Amendment to Credit Agreement dated and effective as of April 17, 2023 by and among Organogenesis Holdings Inc., as borrower, the several banks and other financial institutions or entities party hereto and Silicon Valley Bank, a division of First-Citizens Bank & Trust Company (successor by purchase to the Federal Deposit Insurance Corporation as receiver for Silicon Valley Bridge Bank, N.A. (as successor to Silicon Valley Bank)), as the Administrative Agent (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q (File No. 001-37906) filed with the SEC on May 10, 2023) |
| | |
10.30 | | Purchase and Sale Agreement dated as of August 11, 2021 by and between Organogenesis Inc. and 275 Dan Road SPE, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on August 16, 2021) |
| | |
10.31 | | Subscription Agreement, dated November 12, 2024, by and among Organogenesis Holdings Inc., Avista Healthcare Partners III, L.P. and AHP III Orchestra Holdings, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on November 13, 2024) |
| | |
10.32 | | Third Amendment to Credit Agreement dated as of November 12, 2024 by and among the Company, the lenders named therein and the administrative agent (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on November 13, 2024) |
| | |
10.33 | | Form of Stock Repurchase Agreement, dated November 12, 2024 (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on November 13, 2024) |
| | |
10.34 | | Stock Repurchase Agreement, dated November 27, 2024, by and between Organogenesis Holdings Inc. and GN 2016 Family Trust u/a/d August 12, 2016 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-37906) filed with the SEC on December 2, 2024) |
| | |
10.35*+ | | Lease dated as of November 18, 2024 by and between DIV Technology Way, LLC and Organogenesis Holdings Inc. |
| | |
10.36*‡ | | Form of Performance Share Award Agreement under the 2018 Equity Incentive Plan |
| | |
10.37*‡ | | Employee Letter Agreement dated as of July 30, 2021 by and between Organogenesis Inc. and Robert Cavorsi |
| | |
19.1* | | Organogenesis Holdings Inc. Amended and Restated Insider Trading Compliance Policy |
| | |
21.1* | | Subsidiaries of Organogenesis Holdings Inc. |
| | |
23.1* | | Consent of RSM US LLP |
| | |
31.1* | | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934 |
| | |
31.2* | | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934 |
| | |
32.1* | | Certification of the Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
97.1 | | Organogenesis Holdings Inc. Compensation Recovery Policy (incorporated by reference to Exhibit 97.1 to the Company's Annual Report on Form 10-K (File No. 001-37906) filed with the SEC on February 29, 2024) |
| | |
101* | | The following materials from the Annual Report of Organogenesis Holdings Inc. on Form 10-K for the year ended December 31, 2024, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2024 and December 31, 2023 of Organogenesis Holdings Inc., (ii) Consolidated Statements of Operations and Comprehensive Income for the years ended December 31, 2024, 2023, and 2022 of Organogenesis Holdings Inc., (iii) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2024, 2023, and 2022 of Organogenesis Holdings Inc., (iv) Consolidated Statements of Cash Flows for the years ended |
| | |
Exhibit No. | | Exhibit |
| | December 31, 2024, 2023, and 2022 of Organogenesis Holdings Inc., and (v) Notes to Consolidated Financial Statements of Organogenesis Holdings Inc. |
104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
+ Certain exhibits and schedules to this Exhibit have been omitted in accordance with Regulation S-K Item 601(b)(2). The Company agrees to furnish supplementally a copy of all omitted exhibits and schedules to the Securities and Exchange Commission upon its request.
† Confidential treatment granted as to portions of this Exhibit. The confidential portions of this Exhibit have been omitted and are marked by asterisks.
‡ Management contract or compensatory plan or arrangement.
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| |
ORGANOGENESIS HOLDINGS INC. |
| |
By: | /s/ Gary S. Gillheeney, Sr. |
| Gary S. Gillheeney, Sr. Chief Executive Officer, President, and Chair of the Board of Directors |
Date: | February 27, 2025 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report has been signed below by the following persons on behalf of the Company and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | | | |
/s/ Gary S. Gillheeney, Sr. | | Chief Executive Officer, President and Chair of the Board of Directors (Principal Executive Officer) | | February 27, 2025 |
Gary S. Gillheeney, Sr. | |
| | | | |
/s/ David Francisco | | Chief Financial Officer (Principal Financial and Accounting Officer) | | February 27, 2025 |
David Francisco | | |
| | | | |
/s/ Alan A. Ades | | Director | | February 27, 2025 |
Alan A. Ades | | |
| | | | |
/s/ Robert Ades | | Director | | February 27, 2025 |
Robert Ades | | |
| | | | |
/s/ Michael J. Driscoll | | Director | | February 27, 2025 |
Michael J. Driscoll | | | | |
| | | | |
/s/ Prathyusha Duraibabu | | Director | | February 27, 2025 |
Prathyusha Duraibabu | | |
| | | | |
/s/ David Erani | | Director | | February 27, 2025 |
David Erani |
| | | | |
/s/ Jon Giacomin | | Director | | February 27, 2025
|
Jon Giacomin |
| | | | |
/s/ Michele Korfin | | Director | | February 27, 2025 |
Michele Korfin |
| | | | |
/s/ Arthur S. Leibowitz | | Director | | February 27, 2025 |
Arthur S. Leibowitz |
| | | | |
/s/ Garrett Lustig | | Director | | February 27, 2025 |
Garrett Lustig | | |
| | | | |
/s/ Glenn H. Nussdorf | | Director | | February 27, 2025 |
Glenn H. Nussdorf | |
| | | | |
/s/ Gilberto Quintero | | Director | | February 27, 2025 |
Gilberto Quintero | |
| | | | |
ORGANOGENESIS HOLDINGS INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| |
| Page |
Reports of Independent Registered Public Accounting Firm | F-2 |
Consolidated Balance Sheets as of December 31, 2024 and 2023 | F-5 |
Consolidated Statements of Operations and Comprehensive Income for the Years Ended December 31, 2024, 2023, and 2022 | F-6 |
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity for the Years Ended December 31, 2024, 2023, and 2022 | F-7 |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2024, 2023, and 2022 | F-8 |
Notes to Consolidated Financial Statements | F-9 |
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Organogenesis Holdings Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Organogenesis Holdings Inc. and its subsidiaries (the Company) as of December 31, 2024 and 2023, the related consolidated statements of operations and comprehensive income, redeemable convertible preferred stock and stockholders' equity and cash flows for each of the three years in the period ended December 31, 2024, and the related notes to the consolidated financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013, and our report dated February 27, 2025, expressed an unqualified opinion on the effectiveness of the Company's internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Determination of fair value of the impaired building and associated construction
As discussed in Notes 4 and 8 to the consolidated financial statements, during the year ended December 31, 2024, the Company recorded an impairment charge of $18,842 related to a building and associated construction located on the Company’s Canton, Massachusetts campus. When performing an impairment assessment for a long-lived asset or asset group, the Company determines whether there has been impairment by first comparing the anticipated undiscounted future net cash flows expected to be generated over the remaining life of the asset or asset group to the carrying value of the asset or asset group. If an asset or asset group is determined to be impaired, the asset or asset group is written down to its estimated fair value, which is determined based upon an income approach such as a discounted cash flow model or a market approach based upon appraised value, depending on the nature of the asset or asset group.
We identified the determination of the fair value of the impaired building and the associated construction work, along with the resulting impairment charge, as a critical audit matter due to the significant judgments made by management to estimate the fair value of the assets, specifically the accuracy of the estimate related to the costs of capital improvements to complete the buildout of the property. Auditing management’s assumptions involved a high degree of auditor judgment and an increase in audit effort, including the use of our valuation specialists, due to the impact these assumptions have on the estimate of fair value.
Our audit procedures related to the Company’s determination of the fair value of the impaired building and associated accuracy of the construction work, along with the resulting impairment charge, included the following, among others:
•We obtained an understanding and tested the design and operating effectiveness of the relevant controls related to management’s review of the impairment analysis and estimates of fair value.
•We tested the completeness and accuracy of the source data management utilized in the estimate of fair value by agreeing it to the underlying support.
•We used our valuation specialists to assist in the following procedures:
•Evaluated the appropriateness of the valuation model and method used by management to estimate the fair value of the building.
•Tested the accuracy of management’s estimated costs of capital improvements to complete the buildout of the property by agreeing such information to publicly available market data.
•Tested the clerical accuracy of the Company's discounted cash flow model.
Accounting for redeemable convertible preferred stock
As described in Note 13 to the consolidated financial statements, during the year ended December 31, 2024 the Company entered into a subscription agreement for the sale of 130,000 shares of the Company’s newly-created Series A redeemable convertible preferred stock, par value $0.0001 per share (Convertible Preferred Stock) for a purchase price of $1,000 per share, or aggregate gross proceeds of $130 million. The Convertible Preferred Stock contains embedded features, which are more fully described in Note 13. The evaluation of embedded features in a preferred stock instrument requires the application of complex accounting rules and consideration of a number of factors to determine if any of those embedded features are required to be separately recorded as a derivative liability or would otherwise impact the financial statement classification of the preferred stock instrument.
We identified the Company’s evaluation of both the accounting treatment for the embedded features and financial statement classification of the Convertible Preferred Stock as a critical audit matter because of the complexity involved in management’s evaluation of the embedded features in the Convertible Preferred Stock and management’s interpretation and application of the applicable accounting rules. Auditing management’s accounting conclusion involved a high degree of auditor judgment and an increase in audit effort, including the use of an internal accounting specialist, due to the impact management’s conclusions have on the Company’s financial statements and related disclosures.
Our audit procedures related to the Company’s evaluation of the accounting treatment for the embedded features and financial statement classification of the Convertible Preferred Stock included the following, among others:
•We obtained an understanding of the relevant controls related to management’s identification and assessment of significant nonroutine transactions and tested such controls for design and operating effectiveness.
•We tested the accuracy and completeness of the embedded terms identified by management by reading the Convertible Preferred Stock agreement and related agreements.
•With the assistance of an internal accounting specialist, we obtained management’s technical memoranda and evaluated the reasonableness of the conclusions reached by management of the accounting treatment for the embedded features as well as the classification of the Convertible Preferred Stock. Our assessment included:
•Evaluating the nature of the features of the host contract.
•Evaluating the economic characteristics and risks of each embedded feature compared to the economic characteristics and risks of the host contract.
•Determining the impact the embedded features may have on the classification of the Convertible Preferred Stock.
/s/ RSM US LLP
We have served as the Company's auditor since 2004.
Boston, Massachusetts
February 27, 2025
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Organogenesis Holdings Inc.
Opinion on the Internal Control Over Financial Reporting
We have audited Organogenesis Holdings Inc.'s (the Company) internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements of the Company and our report dated February 27, 2025, expressed an unqualified opinion.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ RSM US LLP
Boston, Massachusetts
February 27, 2025
ORGANOGENESIS HOLDINGS INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share amounts)
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Assets | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 135,571 | | | $ | 103,840 | |
Restricted cash | | | 580 | | | | 498 | |
Accounts receivable, net of allowance for credit losses of $9,576 and $6,860 | | | 109,861 | | | | 81,999 | |
Inventories, net | | | 26,219 | | | | 28,253 | |
Prepaid expenses and other current assets | | | 13,710 | | | | 10,454 | |
Total current assets | | | 285,941 | | | | 225,044 | |
Property and equipment, net | | | 89,128 | | | | 116,228 | |
Intangible assets, net | | | 12,468 | | | | 15,871 | |
Goodwill | | | 28,772 | | | | 28,772 | |
Operating lease right-of-use assets, net | | | 37,110 | | | | 40,118 | |
Deferred tax asset, net | | | 39,462 | | | | 28,002 | |
Other assets | | | 5,005 | | | | 5,990 | |
Total assets | | $ | 497,886 | | | $ | 460,025 | |
| | | | | | |
Liabilities, Redeemable Convertible Preferred Stock, and Stockholders’ Equity | | | | | | |
Current liabilities: | | | | | | |
Current portion of term loan | | $ | — | | | $ | 5,486 | |
Current portion of finance lease obligations | | | 1,170 | | | | 1,081 | |
Current portion of operating lease obligations - related party | | | 3,671 | | | | 8,413 | |
Current portion of operating lease obligations | | | 4,272 | | | | 4,731 | |
Accounts payable | | | 28,911 | | | | 30,724 | |
Accrued expenses and other current liabilities | | | 39,453 | | | | 30,074 | |
Total current liabilities | | | 77,477 | | | | 80,509 | |
Term loan, net of current portion | | | — | | | | 60,745 | |
Finance lease obligations, net of current portion | | | 718 | | | | 1,888 | |
Operating lease obligations, net of current portion - related party | | | 8,283 | | | | 11,954 | |
Operating lease obligations, net of current portion | | | 25,198 | | | | 25,053 | |
Other liabilities | | | 894 | | | | 1,213 | |
Total liabilities | | | 112,570 | | | | 181,362 | |
| | | | | | |
Commitments and contingencies (Note 20) | | | | | | |
| | | | | | |
Series A redeemable convertible preferred stock, $0.0001 par value; 130,000 and 0 shares authorized, issued and outstanding at December 31, 2024 and 2023, respectively; liquidation preference of $131,387 and $0 at December 31, 2024 and 2023, respectively. | | | 122,419 | | | | — | |
| | | | | | |
Stockholders’ equity: | | | | | | |
Preferred stock, $0.0001 par value; 870,000 and 1,000,000 shares authorized at December 31, 2024 and 2023, respectively; none issued or outstanding | | | — | | | | — | |
Common stock, $0.0001 par value; 400,000,000 shares authorized; 126,458,784 and 132,044,944 shares issued; 125,730,236 and 131,316,396 shares outstanding at December 31, 2024 and 2023, respectively | | | 13 | | | | 13 | |
Additional paid-in capital | | | 302,994 | | | | 319,621 | |
Accumulated deficit | | | (40,110 | ) | | | (40,971 | ) |
Total stockholders' equity | | | 262,897 | | | | 278,663 | |
Total liabilities, redeemable convertible preferred stock, and stockholders' equity | | $ | 497,886 | | | $ | 460,025 | |
The accompanying notes are an integral part of these consolidated financial statements
ORGANOGENESIS HOLDINGS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
(in thousands, except share and per share amounts)
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Net revenue | | $ | 482,043 | | | $ | 433,140 | | | $ | 450,893 | |
Cost of goods sold | | | 115,741 | | | | 106,481 | | | | 105,019 | |
Gross profit | | | 366,302 | | | | 326,659 | | | | 345,874 | |
Operating expenses: | | | | | | | | | |
Selling, general and administrative | | | 294,513 | | | | 269,754 | | | | 283,808 | |
Research and development | | | 50,271 | | | | 44,380 | | | | 39,762 | |
Impairment of property and construction | | | 18,842 | | | | — | | | | — | |
Write down of capitalized internal-use software costs | | | 3,959 | | | | — | | | | — | |
Total operating expenses | | | 367,585 | | | | 314,134 | | | | 323,570 | |
Income (loss) from operations | | | (1,283 | ) | | | 12,525 | | | | 22,304 | |
Other expense, net: | | | | | | | | | |
Interest expense, net | | | (1,544 | ) | | | (2,190 | ) | | | (2,009 | ) |
Other income (expense), net | | | 20 | | | | 57 | | | | (13 | ) |
Total other expense, net | | | (1,524 | ) | | | (2,133 | ) | | | (2,022 | ) |
Net income (loss) before income taxes | | | (2,807 | ) | | | 10,392 | | | | 20,282 | |
Income tax (expense) benefit | | | 3,668 | | | | (5,447 | ) | | | (4,750 | ) |
Net income and comprehensive income | | | 861 | | | | 4,945 | | | | 15,532 | |
Accretion of redeemable convertible preferred stock to redemption value | | | (412 | ) | | | — | | | | — | |
Cumulative dividend on redeemable convertible preferred stock | | | (1,386 | ) | | | — | | | | — | |
Net income (loss) attributable to common stockholders | | $ | (937 | ) | | $ | 4,945 | | | $ | 15,532 | |
Net income (loss), per share: | | | | | | | | | |
Basic | | $ | (0.01 | ) | | $ | 0.04 | | | $ | 0.12 | |
Diluted | | $ | (0.01 | ) | | $ | 0.04 | | | $ | 0.12 | |
Weighted-average common shares outstanding | | | | | | | | | |
Basic | | | 131,673,278 | | | | 131,231,317 | | | | 130,070,231 | |
Diluted | | | 131,673,278 | | | | 132,746,727 | | | | 132,383,152 | |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements
ORGANOGENESIS HOLDINGS INC.
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | Additional | | | | | | Total | |
| Redeemable Convertible Preferred Stock | | | | Common Stock | | | Paid-in | | | Accumulated | | | Stockholders’ | |
| Shares | | | Amount | | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balance as of December 31, 2021 | | — | | | $ | — | | | | | 128,680,192 | | | $ | 13 | | | $ | 302,155 | | | $ | (60,833 | ) | | $ | 241,335 | |
Exercise of stock options | | — | | | | — | | | | | 1,864,961 | | | | — | | | | 2,070 | | | | — | | | | 2,070 | |
Vesting of RSUs, net of shares surrendered to pay taxes | | — | | | | — | | | | | 170,491 | | | | — | | | | (648 | ) | | | — | | | | (648 | ) |
Issuance of common stock associated with CPN acquisition | | — | | | | — | | | | | 203,485 | | | | — | | | | 828 | | | | — | | | | 828 | |
Stock-based compensation expense | | — | | | | — | | | | | — | | | | — | | | | 6,552 | | | | — | | | | 6,552 | |
Net income | | — | | | | — | | | | | — | | | | — | | | | — | | | | 15,532 | | | | 15,532 | |
Balance as of December 31, 2022 | | — | | | | — | | | | | 130,919,129 | | | | 13 | | | | 310,957 | | | | (45,301 | ) | | | 265,669 | |
Cumulative-effect adjustment from adoption of ASU 2016-13, net of tax | | — | | | | — | | | | | — | | | | — | | | | — | | | | (615 | ) | | | (615 | ) |
Vesting of RSUs, net of shares surrendered to pay taxes | | — | | | | — | | | | | 397,267 | | | | — | | | | (332 | ) | | | — | | | | (332 | ) |
Stock-based compensation expense | | — | | | | — | | | | | — | | | | — | | | | 8,996 | | | | — | | | | 8,996 | |
Net income | | — | | | | — | | | | | — | | | | — | | | | — | | | | 4,945 | | | | 4,945 | |
Balance as of December 31, 2023 | | — | | | | — | | | | | 131,316,396 | | | | 13 | | | | 319,621 | | | | (40,971 | ) | | | 278,663 | |
Issuance of Series A redeemable convertible preferred stock, net of issuance costs of $9,379 | | 130,000 | | | | 120,621 | | | | | — | | | | — | | | | — | | | | — | | | | — | |
Accretion to redemption value and cumulative dividends on redeemable convertible preferred stock | | — | | | | 1,798 | | | | | — | | | | — | | | | (1,798 | ) | | | — | | | | (1,798 | ) |
Vesting of RSUs, net of shares surrendered to pay taxes | | — | | | | — | | | | | 1,110,136 | | | | — | | | | (1,175 | ) | | | — | | | | (1,175 | ) |
Exercise of stock options | | — | | | | — | | | | | 1,225,435 | | | | 1 | | | | 1,246 | | | | — | | | | 1,247 | |
Repurchase of common shares | | — | | | | — | | | | | (7,921,731 | ) | | | (1 | ) | | | (25,478 | ) | | | — | | | | (25,479 | ) |
Stock-based compensation expense | | — | | | | — | | | | | — | | | | — | | | | 10,578 | | | | — | | | | 10,578 | |
Net income | | — | | | | — | | | | | — | | | | — | | | | — | | | | 861 | | | | 861 | |
Balance as of December 31, 2024 | | 130,000 | | | $ | 122,419 | | | | | 125,730,236 | | | $ | 13 | | | $ | 302,994 | | | $ | (40,110 | ) | | $ | 262,897 | |
The accompanying notes are an integral part of these consolidated financial statements
ORGANOGENESIS HOLDINGS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Cash flows from operating activities: | | | | | | | | | |
Net income | | $ | 861 | | | $ | 4,945 | | | $ | 15,532 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | |
Depreciation and amortization | | | 13,623 | | | | 10,448 | | | | 5,845 | |
Amortization of intangible assets | | | 3,403 | | | | 4,918 | | | | 4,883 | |
Reduction in the carrying value of right-of-use assets | | | 8,348 | | | | 8,083 | | | | 7,303 | |
Non-cash interest expense | | | 394 | | | | 427 | | | | 434 | |
Deferred interest expense | | | 305 | | | | 490 | | | | 501 | |
Deferred tax expense (benefit) | | | (10,719 | ) | | | 2,012 | | | | 1,980 | |
Loss on disposal of property and equipment | | | 1,140 | | | | 235 | | | | 4,482 | |
Loss on lease termination | | | — | | | | 559 | | | | — | |
Loss on extinguishment of term loan | | | 215 | | | | — | | | | — | |
Provision recorded for credit losses | | | 3,938 | | | | 1,297 | | | | 1,781 | |
Adjustment for excess and obsolete inventories | | | 8,210 | | | | 6,580 | | | | 9,648 | |
Stock-based compensation | | | 10,578 | | | | 8,996 | | | | 6,552 | |
Impairment of property and construction (Note 8) | | | 18,842 | | | | — | | | | — | |
Write down of capitalized internal-use software costs (Note 8) | | | 3,959 | | | | — | | | | — | |
Changes in operating assets and liabilities: | | | | | | | | | |
Accounts receivable | | | (31,800 | ) | | | 5,539 | | | | (8,770 | ) |
Inventories | | | (6,204 | ) | | | (8,179 | ) | | | (9,410 | ) |
Prepaid expenses and other current and other assets | | | (2,549 | ) | | | (10,115 | ) | | | (378 | ) |
Operating leases | | | (14,066 | ) | | | (8,439 | ) | | | (7,006 | ) |
Accounts payable | | | (2,372 | ) | | | (108 | ) | | | 3,260 | |
Accrued expenses and other current liabilities | | | 9,164 | | | | 3,138 | | | | (11,850 | ) |
Other liabilities | | | (1,062 | ) | | | 91 | | | | 72 | |
Net cash provided by operating activities | | | 14,208 | | | | 30,917 | | | | 24,859 | |
Cash flows from investing activities: | | | | | | | | | |
Purchases of property and equipment | | | (10,032 | ) | | | (24,364 | ) | | | (33,898 | ) |
Net cash used in investing activities | | | (10,032 | ) | | | (24,364 | ) | | | (33,898 | ) |
Cash flows from financing activities: | | | | | | | | | |
Term loan repayments under the 2021 Credit Agreement | | | (66,563 | ) | | | (4,688 | ) | | | (2,813 | ) |
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs | | | 120,688 | | | | — | | | | — | |
Payments for the repurchase of common stock | | | (25,479 | ) | | | — | | | | — | |
Principal repayments of finance lease obligations | | | (1,081 | ) | | | (485 | ) | | | (200 | ) |
Proceeds from the exercise of stock options | | | 1,247 | | | | — | | | | 2,070 | |
Payments of withholding taxes in connection with RSUs vesting | | | (1,175 | ) | | | (332 | ) | | | (648 | ) |
Payments of deferred acquisition consideration | | | — | | | | — | | | | (608 | ) |
Net cash provided by (used in) financing activities | | | 27,637 | | | | (5,505 | ) | | | (2,199 | ) |
Change in cash, cash equivalents and restricted cash | | | 31,813 | | | | 1,048 | | | | (11,238 | ) |
Cash, cash equivalents, and restricted cash, beginning of year | | | 104,338 | | | | 103,290 | | | | 114,528 | |
Cash, cash equivalents, and restricted cash, end of year | | $ | 136,151 | | | $ | 104,338 | | | $ | 103,290 | |
Supplemental disclosure of cash flow information: | | | | | | | | | |
Cash paid for interest | | $ | 4,970 | | | $ | 5,436 | | | $ | 2,649 | |
Cash paid for income taxes | | $ | 6,965 | | | $ | 3,052 | | | $ | 1,201 | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | |
Cumulative effect adjustment for adoption of ASU No. 2016-13 | | $ | — | | | $ | 615 | | | $ | — | |
Deferred acquisition consideration and earnout liability recorded for business acquisition | | $ | — | | | $ | — | | | $ | 828 | |
Change in purchases of property and equipment included in accounts payable and accrued expenses and other current liabilities | | $ | (432 | ) | | $ | 841 | | | $ | 1,928 | |
Right-of-use assets obtained through operating lease obligations | | $ | 5,109 | | | $ | 5,869 | | | $ | 1,350 | |
Right-of-use assets obtained through finance lease obligations | | $ | — | | | $ | 3,454 | | | $ | — | |
Redeemable convertible preferred stock issuance costs included in accrued expenses | | $ | 67 | | | $ | — | | | $ | — | |
Prepaid rent reclassified to right-of-use assets | | $ | 230 | | | $ | — | | | $ | — | |
Accretion to redemption value and cumulative dividends on redeemable convertible preferred stock | | $ | 1,798 | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
1. Nature of Business and Basis of Presentation
Organogenesis Holdings Inc. (ORGO or the Company) is a leading regenerative medicine and tissue innovations company focused on empowering healing through the development, manufacturing, and sale of products for the advanced wound care, and surgical and sports medicine markets. Several of the existing and pipeline products in the Company’s portfolio have Premarket Application (PMA) approval, or Premarket Notification 510(k) clearance from the United States Food and Drug Administration (FDA). The Company’s customers include hospitals, wound care centers, government facilities, ambulatory surgery centers (ASCs) and physician offices. The Company has one operating and reportable segment.
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (GAAP) and include the accounts and results of operations of Organogenesis Holdings Inc., and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
2. Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported results of operations during the reporting periods. In preparing the consolidated financial statements, the estimates and assumptions that management considers to be significant and that present the greatest amount of uncertainty include: recognition and measurement of current and deferred income tax assets and liabilities; and the assessment of recoverability of long-lived assets, including impairment and write-downs. Actual results and outcomes may differ significantly from those estimates and assumptions.
Foreign Currency
The Company’s functional currency, including that of the Company’s Swiss subsidiary, Organogenesis GmbH, is the United States dollar. Foreign currency gains and losses resulting from remeasurement of assets and liabilities held in foreign currencies and transactions settled in a currency other than the functional currency are included separately as non-operating income or expense in the consolidated statements of operations and comprehensive income (loss) as a component of other expense, net. The foreign currency amounts recorded for all periods presented were insignificant.
Segment Reporting
Operating segments are defined as components of an enterprise about which discrete financial information is available that is evaluated regularly by the chief operating decision maker (CODM), or decision-making group, in making decisions on how to allocate resources and assess performance for the organization. The Company manages its operations as a single operating segment for the purposes of assessing performance and making operating decisions. The Company’s portfolio includes regenerative medicine products in various stages, ranging from preclinical to late-stage development, and commercialized advanced wound care and surgical and sports medicine products which support healing across a wide variety of wound types at many different types of facilities. The Company has determined that it has a single operating segment—regenerative medicine.
The Company’s measure of segment profit and loss is reported as consolidated net income on the accompanying consolidated statements of operations and comprehensive income, and the Company’s measure of segment assets is reported as consolidated assets on the accompanying consolidated balance sheets. The accounting policies of the regenerative medicine segment are the same as those described in this summary of significant accounting policies.
Cash and Cash Equivalents
The Company primarily maintains its cash in bank deposit accounts in the United States which, at times, may exceed the federally insured limits. The Company has not experienced losses in such accounts and believes it is not exposed to significant credit
risk on cash. The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Restricted Cash
The Company had restricted cash of $580 and $498 as of December 31, 2024 and 2023, respectively. Restricted cash represents employee deposits in connection with the Company’s health benefit plan.
Accounts Receivable, Net
Accounts receivable are stated at invoice value less estimated allowances for credit losses. The Company evaluates expected credit losses on accounts receivable considering historical experience, credit quality, age of the accounts receivable balances, geography-related risks and current and expected economic conditions that may affect a customer’s ability to pay. The Company continually monitors customer payments and in cases where there are circumstances that may impair a specific customer’s ability to meet its financial obligations, a specific allowance is recorded against amounts due, thereby reducing the net recognized receivable to the amount reasonably believed to be collectible. Accounts receivable are charged against the allowance when deemed uncollectible. Recoveries of accounts receivables previously written off are recorded when received.
Inventories
Inventories are stated at the lower of cost (determined using the first-in first-out method) or net realizable value. Work in process and finished goods include materials, labor and allocated overhead. Inventories also include cell banks and the cost of tests mandated by regulatory agencies of the materials to qualify them for production.
The Company regularly reviews inventory quantities on hand and records a provision to write down excess and obsolete inventory to its estimated net realizable value based upon management’s assumptions of future material usage, yields and obsolescence, which are based primarily on analysis of historical usage and sales information, as well as market conditions and the effective life of certain inventory items.
The Company also tests other components of its inventory for future growth projections. The Company determines the average yield of the component and compares it to projected revenue to ensure it is properly reserved.
Property and Equipment, Net
Property and equipment are stated at cost less accumulated depreciation. As of December 31, 2024 and 2023, the Company’s property and equipment consisted of leasehold improvements, building, furniture and computers, and equipment. Depreciation expense is recognized using the straight-line method over the useful lives of the assets, which are as follows:
| | |
Leasehold improvements | | Lesser of the life of the lease or the economic life of the asset |
Building | | 30 years |
Furniture and computers | | 3 - 5 years |
Equipment | | 5 - 10 years |
Construction in progress costs are capitalized when incurred until the assets are placed in service, at which time the costs will be transferred to the related property and equipment, and depreciated over their respective useful lives. Upon retirement or sale, the cost and related accumulated depreciation of assets disposed of are removed from the accounts and any resulting gain or loss is included in the consolidated statements of operations and comprehensive income. Expenditures for repairs and maintenance are charged to expense as incurred. Expenditures for major improvements that extend the useful lives of the related asset are capitalized and depreciated over their remaining estimated useful lives.
Internal Use Software
The Company capitalizes costs to purchase and develop internal-use software. These costs are capitalized from the time that the preliminary project stage is completed, and it is considered probable that the software will be used to perform the function intended, until the time the software is placed in service for its intended use. Any costs incurred during subsequent efforts to upgrade and
enhance the functionality of the software are also capitalized. Costs incurred for maintenance activities relating to the software are expensed as incurred.
When the Company places the software in service, it begins amortizing the capitalized costs over the estimated useful life of the software, generally three to five years.
Goodwill
Goodwill represents the excess of the purchase price of an acquired business over the fair value of the identifiable assets acquired and liabilities assumed. Goodwill is not amortized, but is tested for impairment at least annually (as of December 31), or more frequently if events or circumstances indicate the carrying value may no longer be recoverable and that an impairment loss may have occurred. Circumstances that could trigger an impairment test include, but are not limited to, a significant adverse change in the business climate or legal factors, an adverse action or assessment by a regulator, or unanticipated competition. The Company operates as one segment, which is considered to be the sole reporting unit, and therefore goodwill is tested for impairment at the consolidated level.
The Company may first assess qualitative factors to determine whether it is necessary to perform the quantitative goodwill impairment test, or the Company can bypass the qualitative assessment and proceed directly to the quantitative test. The quantitative goodwill impairment test requires the Company to estimate and compare the fair value of the reporting unit with its carrying value. If the fair value of the reporting unit exceeds the carrying value of the net assets, goodwill is not impaired. If the fair value of the reporting unit is less than the carrying value, the difference is recorded as an impairment loss up to the amount of goodwill. At December 31, 2024 and 2023, the Company elected to perform a quantitative analysis directly, and used its market capitalization to approximate the fair value of the reporting unit. The fair value of the reporting unit exceeded its carrying value at December 31, 2024 and 2023, and accordingly the Company did not record any impairment on its goodwill.
Intangible Assets Subject to Amortization
Intangible assets include intellectual property either owned by the Company or to which the Company has a license. Intangible assets acquired in a business combination are recognized at fair value using generally accepted valuation methods deemed appropriate for the type of intangible asset acquired. Intangible assets are reported net of accumulated amortization, separately from goodwill. Intangible assets with finite lives are amortized over their estimated useful lives. Intangible assets include developed technology and patents, trade names, trademarks, customer relationships and non-compete agreements obtained through business acquisitions. Amortization of intangible assets with finite lives is calculated on a straight-line basis or using an accelerated method based on the following estimated useful lives:
| | |
Trade names and trademarks | | 1-12 years |
Developed technology | | 6-12 years |
Customer relationships | | 10 years |
Non-compete agreements | | 5 years |
Impairment of Long-Lived Assets
Long-lived assets include property and equipment, definite-lived intangible assets, and right-of-use assets associated with the Company’s lease agreements. The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Factors that the Company considers in deciding when to perform an impairment review include, but are not limited to, significant underperformance of the business in relation to expectations, significant negative industry or economic trends, and significant changes or planned changes in the use of the assets. When such an event occurs, the Company determines whether there has been impairment by comparing the anticipated undiscounted future net cash flows to the related asset group’s carrying value. If an asset is determined to be impaired, the asset is written down to fair value, which is determined based on discounted cash flows or appraised value, depending on the nature of the asset. The Company recorded impairment of $18,842 during the year ended December 31, 2024, and did not record any impairment of long-lived assets during the years ended December 31, 2023 or 2022.
Revenue Recognition
Product Revenue
The Company generates revenue through the sale of Advanced Wound Care and Surgical & Sports Medicine products. There is a single performance obligation in all of the Company’s contracts, which is the Company’s promise to transfer the Company’s product to customers based on specific payment and shipping terms in the arrangement. The entire transaction price is allocated to this single performance obligation. Product revenue is recognized when a customer obtains control of the Company’s product which occurs at a point in time and may be upon shipment, procedure date, or delivery, based on the terms of the contract.
Reserves for Variable Consideration
Revenues from product sales are recorded net of reserves for variable consideration which includes but is not limited to product return, discounts, rebates and GPO fees that are offered within contracts between the Company and its customers relating to the Company’s sales of its products. These reserves are based on the amounts earned or to be claimed by its customers on the related sales and are recorded as a reduction of accounts receivable or an establishment of a liability. Where appropriate, these estimates take into consideration a range of possible outcomes which are probability-weighted for relevant factors such as the Company’s historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, these reserves reflect the Company’s best estimates of the amount of consideration to which it is entitled based on the terms of the contract and is included in the net sales price to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. Actual amounts of consideration ultimately paid may differ from the Company’s estimates. If actual results vary from the Company’s estimates, the Company adjusts these estimates, which would affect net product revenue and earnings in the period such variances become known.
Product Returns
Consistent with industry practice, the Company generally offers customers a limited right of return for product purchased. The Company estimates the amount of its product sales that may be returned by its customers and records this estimate as a reduction of revenue in the period in which the related product revenue is recognized. The Company currently estimates product return reserves using its historical return rates as well as factors that it becomes aware of that it believes could significantly impact its expected returns, including product recalls, pricing changes, or changes in reimbursement rates. The Company does not record an asset for the returned product as the product is discarded upon receipt.
Rebates and Allowances
The Company provides certain customers with rebates and allowances that are explicitly stated in the Company’s contracts, resulting in a reduction of revenue and the establishment of a liability that is included in accrued expenses in the accompanying consolidated balance sheets in the period the related product revenue is recognized.
GPO Fees
The Company pays fees to GPOs for administrative services that the GPOs perform in connection with the purchases of the product by the GPO members. These fees are based on a contractually-determined percentage of the Company’s applicable sales. The Company classifies these GPO fees as a reduction of revenue based on the substance of the relationship of all parties involved in the transaction.
Other Revenue Policies
Sales, value add, and other taxes collected on behalf of third parties are excluded from revenue. The Company accounts for shipping and handling activities related to contracts with customers as costs to fulfill the promise to transfer the associated products. The Company records the related costs as part of the cost of goods sold.
The Company does not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between payment by the customer and the transfer of the promised products to the customer will be one year or less, which is the case with substantially all customers. The Company recognizes the incremental costs of obtaining contracts as an expense when incurred if the amortization period of the assets that the Company otherwise would have recognized is one year or less. These costs are included in selling, general, and administrative expenses.
License and manufacturing agreement
The Company licenses the rights to sell certain of its products, which are manufactured by third parties, including the trademarks and other license rights associated with such products. Payments to the third parties under these arrangements typically include one or more of the following: non-refundable, upfront license fees; manufacturing supply services and associated purchase commitments at specified prices; milestone payments; and royalties on future product sales. The Company allocates payments in these arrangements based on the relative fair value of the goods and services received, and recognizes the expenses associated with each good or service as it receives the associated benefit.
Stock-Based Compensation
The Company measures stock-based awards granted to employees, non-employees, and directors based on the fair value of the awards on the date of grant and recognizes compensation expense for those awards over the requisite service period, which is generally the vesting period of the respective award. Generally, the Company issues stock options, restricted stock units and restricted stock awards with only service-based vesting conditions and records the expense for these awards using the straight-line method. The Company has not issued any stock-based awards with performance-based vesting conditions.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company recognizes stock-based compensation expense within selling, general and administrative expenses in the consolidated statements of operations and comprehensive income for all share-based payments based upon the estimated grant-date fair value for the awards expected to ultimately vest.
The fair value of each restricted stock unit grant is based on the fair market value of the Company’s Class A common stock on the date of grant. The fair value of each stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model. The Company has been a public company for a short period of time, has limited public float and lacks company-specific historical and implied volatility information for its Class A common stock. Therefore, it estimates its expected stock price volatility based on the historical volatility of publicly traded peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded stock price. The expected term of the Company’s stock options has been determined utilizing the "simplified" method for awards that qualify as "plain-vanilla" options. The risk-free interest rate is determined by reference to the United States Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends on its Class A common stock and does not expect to pay any cash dividends in the foreseeable future.
Advertising
Advertising costs are expensed as incurred and are included in selling, general and administrative expenses in the consolidated statements of operations and comprehensive income. Advertising costs were approximately $3,825, $5,225, and $4,812, for the years ended December 31, 2024, 2023, and 2022, respectively.
Research and Development Costs
Research and development expenses include personnel costs for the Company’s research and development personnel, expenses related to improvements in manufacturing processes, enhancements to the Company’s currently available products, and additional investments in the product and platform development pipeline. Research and development expenses also include expenses for clinical trials. The Company expenses research and development costs as incurred.
Income Taxes
The Company accounts for income taxes using the asset and liability method which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined on the basis of the differences between the consolidated financial statement and the tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company quarterly assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. In determining whether a valuation allowance for deferred tax assets is necessary, the Company analyzes both positive and negative evidence related to the realization of deferred tax assets, including projected future taxable income, recent financial results and estimates of future reversals of deferred tax assets and liabilities. In addition, the Company considers whether it is more likely than not that the tax position will be sustained on examination by taxing authorities based on the technical merits of the position.
The Company accounts for uncertain income tax positions recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Fair Value of Financial Instruments
Certain assets and liabilities of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
•Level 1—Quoted prices in active markets for identical assets or liabilities.
•Level 2—Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
•Level 3—Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
The carrying values of accounts receivable, inventories, prepaid expenses and other current assets, accounts payable and accrued expenses and other assets and liabilities approximate their fair values due to the short-term nature of these assets and liabilities. The carrying values of outstanding borrowings under the Company’s debt arrangements (see Note 12, Long-Term Debt Obligations) approximate their fair values as determined based on a discounted cash flow model, which represents a Level 3 measurement.
Nonrecurring Fair Value Measurements of Nonfinancial Assets
The Company estimates fair value to perform impairment tests on long-lived asset groups when required. The methodologies used to determine fair value in these circumstances are primarily based upon discounted cash flow models and the inputs to such models are classified within Level 3 of the fair value hierarchy. If impaired, these assets or asset groups are measured and recorded at fair value within the accompanying consolidated financial statements.
Classification and Accretion of Series A Redeemable Convertible Preferred Stock
The Company has classified the Series A Redeemable Convertible Preferred Stock (Convertible Preferred Stock) outside of stockholders’ equity on the Company’s consolidated balance sheets because the holders of such stock have certain redemption and liquidation rights that, in certain situations, are not solely within the control of the Company and would require the redemption of the then-outstanding Convertible Preferred Stock. The Convertible Preferred Stock is redeemable in an amount equal to the original issue price per share plus all declared but unpaid dividends thereon, as specified in the Convertible Preferred Stock certificate of designation. The Company records periodic accretion to the values of its outstanding Convertible Preferred Stock such that its carrying value will be equal to the redemption value at the earliest redemption date. Adjustments to the carrying value of the Convertible Preferred Stock at each reporting date reduce additional paid-in capital. See Note 13, Convertible Preferred Stock.
Earnings per Share (EPS)
The Company applies the two-class method when computing net income (loss) per share attributable to common stockholders as the Company has issued shares that meet the definition of participating securities. The two-class method determines net income (loss) per share for each class of common and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires income (loss) available to common stockholders for the period to be allocated between common and participating securities based upon their respective rights to share in the undistributed earnings as if all income (loss) for the period had been distributed. The Company considers its Convertible Preferred Stock to be participating securities
as, in the event a dividend is paid on its Class A common stock (common shares), the holders of Convertible Preferred Stock would be entitled to receive dividends on a basis consistent with the common stockholders. The holders of the Convertible Preferred Stock are also entitled to residual value in liquidation. There is no allocation required under the two-class method during periods of loss since the participating securities do not have a contractual obligation to share in the losses of the Company.
Basic net income (loss) per share attributable to common stockholders is computed by dividing the net income (loss) attributable to common stockholders by the weighted-average number of shares of common shares outstanding for the period, excluding potentially dilutive common shares. Diluted net income (loss) per share attributable to common stockholders is computed by adjusting net income (loss) attributable to common stockholders to reallocate undistributed earnings based on the potential impact of dilutive securities. Diluted net income (loss) per share attributable to common stockholders is computed by dividing the diluted net income (loss) attributable to common stockholders by the weighted-average number of common shares outstanding for the period, including potentially dilutive common shares. For purposes of this calculation, Convertible Preferred Stock, unvested RSUs and options to purchase common stock are considered common stock equivalents. In periods in which the Company reports a net loss available to common stockholders, diluted net loss per share available to common stockholders is the same as basic net loss per share available to common stockholders, since dilutive common shares are not assumed to have been issued as their effect is anti-dilutive. The Company calculates diluted net income (loss) per share using the treasury stock method which includes consideration of unrecognized compensation expense as additional proceeds.
Leases
The Company determines if an arrangement is a lease at lease inception. A contract is or contains a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company classifies leases at the lease commencement date as operating or finance leases and records a right-of-use asset and a lease liability on the consolidated balance sheets for all leases with an initial lease term of greater than 12 months. Leases with an initial term of 12 months or less are not recorded on the balance sheet, but payments are recognized as expense on a straight-line basis over the lease term. The Company has elected not to record a right-of-use asset or lease liability for leases with terms of 12 months or less.
A lease qualifies as a finance lease if any of the following criteria are met at the inception of the lease: (i) there is a transfer of ownership of the leased asset to the Company by the end of the lease term, (ii) the Company holds an option to purchase the leased asset that it is reasonably certain to exercise, (iii) the lease term is for a major part of the remaining economic life of the leased asset, (iv) the present value of the sum of lease payments equals or exceeds substantially all of the fair value of the leased asset, or (v) the nature of the leased asset is specialized to the point that it is expected to provide the lessor no alternative use at the end of the lease term.
The Company enters into contracts that contain both lease and non-lease components. Non-lease components may include maintenance, utilities, and other operating costs. The Company combines the lease and non-lease components of fixed costs in its lease arrangements as a single lease component. When a contract contains more than one lease component, the Company allocates consideration in the contract to the separate lease and associated non-lease components based on the relative standalone selling price of the lease components within the contract.
Variable costs, such as utilities, common area maintenance, and maintenance programs for leased vehicles are not included in the measurement of right-of-use assets and lease liabilities, but rather are expensed when the event determining the amount of variable consideration to be paid occurs.
The options to extend or terminate a lease are included in the lease terms when it is reasonably certain that the Company will exercise the options. Operating leases are included in operating lease right-of-use assets and operating lease obligations on the consolidated balance sheets. Finance lease right-of-use assets are included in property and equipment, net, and the related liabilities are included in finance lease obligations on the consolidated balance sheets.
Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the leases. Right-of-use assets and lease liabilities are recognized based on the present value of the fixed lease payments over the lease term at the commencement date. The right-of-use assets also include any initial direct costs incurred and lease payments made at or before the commencement date and are reduced by lease incentives. The Company uses its incremental borrowing rate as the discount rate to determine the present value of the lease payments for leases that do not have a readily determinable implicit discount rate. The Company’s incremental borrowing rate is the rate of interest that it would have to borrow on a collateralized basis over a similar term and amount in a similar economic environment. The Company determines the incremental borrowing rates for its leases by adjusting the risk-free interest rate with a credit risk premium corresponding to the Company’s credit rating, in consideration of the collateral and lease term.
The Company records rent expense for its operating leases on a straight-line basis from the lease commencement date until the end of the lease term. The Company records finance lease cost as a combination of the amortization expense for the leased assets and interest expense for the outstanding lease liabilities using the discount rate discussed above.
Recently Adopted Accounting Pronouncements
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires public entities to disclose information about their reportable segments’ significant expenses and other segment items on an interim and annual basis. Public entities with a single reportable segment are required to apply the disclosure requirements in ASU 2023-07, as well as all existing segment disclosures and reconciliation requirements in ASC 280. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The Company adopted ASU 2023-07 for the year ended December 31, 2024. See Note 15, Segment Information.
Recently Issued Accounting Pronouncements Not Yet Adopted
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires public entities to disclose specific categories in the effective tax rate reconciliation, as well as additional information for reconciling items that exceed a quantitative threshold. ASU 2023-09 also requires all entities to disclose income taxes paid disaggregated by federal, state and foreign taxes, and further disaggregated for specific jurisdictions that exceed 5% of total income taxes paid, among other expanded disclosures. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2023-09 on its consolidated financial statements and related disclosures.
In November 2024, the FASB issued ASU 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses. This standard requires entities to provide additional disclosure regarding certain expenses presented within the statements of operations, and aims to improve such disclosures and address requests from investors for more detailed information about the types of expenses incurred by public entities. The standard is effective for annual periods beginning after December 15, 2026, and interim periods beginning after December 15, 2027, with early adoption permitted. The Company is currently evaluating the impact of the adoption of ASU 2024-03 on its consolidated financial statements and related disclosures.
Correction of Immaterial Classification Error
Subsequent to the issuance of the consolidated financial statements as of and for the year ended December 31, 2023, the Company determined that as of December 31, 2023, it had incorrectly classified $5,273 of accrued but unpaid lease obligations as current portion of operating lease obligations instead of as current portion of operating lease obligations - related party. As a result, the Company also incorrectly classified $5,273 of operating lease obligations, net of current portion as operating lease obligations, net of current portion - related party. These misclassifications have been corrected in the accompanying condensed consolidated balance sheets and conform to the current period presentation of operating lease obligations. These reclassifications had no impact on reported results of operations, stockholders’ equity, cash flows, total current liabilities, or total liabilities.
3. Revenue from Contracts with Customers
The following table sets forth revenue by product category:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Advanced Wound Care | | $ | 453,639 | | | $ | 405,514 | | | $ | 422,231 | |
Surgical and Sports Medicine | | | 28,404 | | | | 27,626 | | | | 28,662 | |
Total revenue | | $ | 482,043 | | | $ | 433,140 | | | $ | 450,893 | |
For all periods presented, net revenue generated outside the United States represented less than 1% of total net revenue.
For the years ended December 31, 2024, 2023, and 2022, the Company recorded GPO fees of $6,102, $5,623 and $6,654, respectively, as a direct reduction of revenue.
4. Fair Value Measurement
During the second quarter of 2024, the Company determined that a purchased building and unfinished construction work had been impaired and recorded an impairment charge of $18,842 to record the building and unfinished construction work at its then fair value of $13,600 for impairment purposes. The Company determined the fair value of the building by estimating rental income, net of expenses to maintain the building over an anticipated lease term, as well as costs estimated to complete construction prior to commencement of the lease; these cash flows were then discounted over an anticipated lease term. The significant unobservable quantitative inputs to the fair value of the building at the time of the impairment are as follows:
| | | | |
Unobservable input | | Range | |
Discount rate | | | 8.0 | % |
Terminal capitalization rate | | | 6.5 | % |
Operating expense ratio | | 24.3% - 32.9% | |
For more information, see Note 8, Property and Equipment, Net.
5. Accounts receivable, net
Accounts receivable consisted of the following:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Accounts receivable | | $ | 119,437 | | | $ | 88,859 | |
Less - allowance for credit losses | | | (9,576 | ) | | | (6,860 | ) |
| | $ | 109,861 | | | $ | 81,999 | |
The Company’s allowance for credit losses is comprised of the following:
| | | | |
Balance as of December 31, 2022 | | $ | 6,362 | |
Cumulative-effect adjustment from adoption of ASU 2016-13, net of tax | | | 615 | |
Additions | | | 1,297 | |
Write-offs | | | (1,414 | ) |
Balance as of December 31, 2023 | | $ | 6,860 | |
Additions | | | 3,938 | |
Write-offs | | | (1,222 | ) |
Balance as of December 31, 2024 | | $ | 9,576 | |
6. Inventories
Inventories, net of related reserves for excess and obsolescence, consist of the following:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Raw materials | | $ | 13,252 | | | $ | 12,988 | |
Work in process | | | 923 | | | | 810 | |
Finished goods | | | 12,044 | | | | 14,455 | |
| | $ | 26,219 | | | $ | 28,253 | |
Raw materials include various components used in the Company’s manufacturing process. The Company’s excess and obsolete inventory review process includes analysis of historical sales as compared to inventory levels and working with operations to maximize recovery of excess inventory. During the years ended December 31, 2024, 2023, and 2022, the Company charged $8,210, $6,580, and $9,648, respectively, for inventory excess and obsolescence to cost of goods sold within the consolidated statements of operations and comprehensive income.
7. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Subscriptions | | $ | 4,866 | | | $ | 4,800 | |
Prepaid research and development expenses | | | 2,929 | | | | 1,066 | |
Prepaid licensing fee (Note 18) | | | 3,301 | | | | 2,368 | |
Conferences and marketing expenses | | | 1,477 | | | | 945 | |
Prepaid taxes | | | 445 | | | | — | |
Deposits | | | 309 | | | | 872 | |
Other | | | 228 | | | | 318 | |
Insurance | | | 155 | | | | 85 | |
| | $ | 13,710 | | | $ | 10,454 | |
Deposits are funds held by vendors which are expected to be released within twelve months and therefore they are recorded as current assets.
8. Property and Equipment, Net
Property and equipment consisted of the following:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Leasehold improvements | | $ | 63,342 | | | $ | 60,819 | |
Building | | | 13,600 | | | | 4,943 | |
Furniture, computers and equipment | | | 63,248 | | | | 64,585 | |
| | | 140,190 | | | | 130,347 | |
Accumulated depreciation | | | (72,949 | ) | | | (73,186 | ) |
Construction in progress | | | 21,887 | | | | 59,067 | |
| | $ | 89,128 | | | $ | 116,228 | |
Depreciation expense was $13,623, $10,448 and $5,845, for the years ended December 31, 2024, 2023, and 2022, respectively.
Construction in progress primarily represents improvements at the Company’s leased facilities in Canton and Norwood, Massachusetts, as well as costs incurred to implement the remaining modules of the company-wide enterprise resource planning (ERP) system.
During the second quarter of 2024, the Company placed certain modules of its ERP system into service, the costs of which had previously been capitalized as construction in progress and will be expensed over their anticipated useful life, currently estimated to be five years. At such time, the Company determined that certain other modules within the ERP system and other internal-use software had no future use, and accordingly the Company recorded a write down of $3,959 of costs related to this internal-use software.
During the second quarter of 2024, the Company decided to pursue the potential sale of a purchased building, located on the Company’s Canton, Massachusetts campus, on which it had previously paused construction work. The Company identified this change in expectation regarding the use of the building as an impairment indicator. The Company determined the asset group to be comprised of the building and associated construction, and performed the impairment assessment at the asset group level. The Company determined the impairment charge by comparing the fair value of the asset group to its book value and recorded an impairment charge of $18,842 related to the building and associated unfinished construction work, allocated to each asset class within the asset group based on its relative carrying value. See Note 4, Fair Value Measurements.
During the year ended December 31, 2023, the Company identified certain impairment triggers relating to its asset groups, which included incurred expenses in excess of planned expenses for the ERP implementation. The impairment triggers indicated that the Company’s long-lived assets might be impaired. The Company performed recoverability tests during the year ended December 31,
2023. The estimated undiscounted cash flows directly attributable to the asset group exceeded the carrying value of the asset group. Therefore, the Company did not record any impairment related to its asset group as of December 31, 2023.
During the year ended December 31, 2022, the Company recorded a charge of $4,200 for the sale and donation of some equipment related to the construction in progress in one of its Canton, Massachusetts facilities. The disposal was the result of a change in the design of the construction plan for the manufacturing facility and the determination that this equipment was no longer compatible with the ongoing design. During 2022, the Company decided to temporarily pause the construction of this manufacturing facility due to inflation and market conditions that adversely impacted construction projects across the biotechnology and life sciences industries. In connection with this decision, the Company recorded a charge of $632 as cancellation fees to various vendors. These charges were included in selling, general and administrative expenses on the consolidated statements of operations and comprehensive income for the year ended December 31, 2022.
9. Goodwill and Intangible Assets
Goodwill was $28,772 as of December 31, 2024 and 2023. There was no impairment of goodwill recorded during the years ended December 31, 2024, 2023, or 2022.
Identifiable intangible assets consisted of the following as of December 31, 2024:
| | | | | | | | | | | | |
| | Original | | | Accumulated | | | Net Book | |
| | Cost | | | Amortization | | | Value | |
Developed technology | | $ | 32,620 | | | $ | (26,708 | ) | | $ | 5,912 | |
Customer relationship | | | 10,690 | | | | (4,588 | ) | | | 6,102 | |
Patent | | | 7,623 | | | | (7,623 | ) | | | — | |
Independent sales agency network | | | 4,500 | | | | (4,500 | ) | | | — | |
Trade names and trademarks | | | 2,080 | | | | (1,733 | ) | | | 347 | |
Non-compete agreements | | | 1,010 | | | | (903 | ) | | | 107 | |
Total | | $ | 58,523 | | | $ | (46,055 | ) | | $ | 12,468 | |
Identifiable intangible assets consisted of the following as of December 31, 2023:
| | | | | | | | | | | | |
| | Original | | | Accumulated | | | Net Book | |
| | Cost | | | Amortization | | | Value | |
Developed technology | | $ | 32,620 | | | $ | (24,666 | ) | | $ | 7,954 | |
Customer relationship | | | 10,690 | | | | (3,519 | ) | | | 7,171 | |
Patent | | | 7,623 | | | | (7,623 | ) | | | — | |
Independent sales agency network | | | 4,500 | | | | (4,500 | ) | | | — | |
Trade names and trademarks | | | 2,080 | | | | (1,590 | ) | | | 490 | |
Non-compete agreements | | | 1,010 | | | | (754 | ) | | | 256 | |
Total | | $ | 58,523 | | | $ | (42,652 | ) | | $ | 15,871 | |
Amortization of intangible assets, calculated on a straight-line basis or using an accelerated method, which reflects the pattern in which the economic benefits of the intangible assets are consumed, was $3,403, $4,918 and $4,883 for the years ended December 31, 2024, 2023, and 2022, respectively. Estimated future annual amortization expense related to these intangible assets is as follows:
| | | | |
2025 | | $ | 3,323 | |
2026 | | | 3,043 | |
2027 | | | 2,283 | |
2028 | | | 1,968 | |
2029 | | | 1,094 | |
Thereafter | | | 757 | |
Total | | $ | 12,468 | |
10. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Personnel costs | | $ | 23,836 | | | $ | 18,287 | |
Royalties | | | 7,381 | | | | 3,075 | |
Accrued but unpaid lease obligations and interest (Note 17) | | | — | | | | 2,326 | |
Accrued milestone payment (Note 20) | | | 2,500 | | | | 2,500 | |
Accrued taxes | | | 4,286 | | | | 2,799 | |
Other | | | 1,450 | | | | 1,087 | |
| | $ | 39,453 | | | $ | 30,074 | |
11. Restructuring
In order to reduce the Company’s cost structure and improve operating efficiency, the Company consolidated its manufacturing operations in various locations into Massachusetts facilities.
On October 21, 2020, the Company committed to a plan to restructure the workforce and operations in its La Jolla, California facilities. The restructuring involved 65 employees and was substantially completed as of December 31, 2021, with certain facility and storage activities continuing through 2024. On March 9, 2022, the Company committed to a plan to restructure the workforce and operations in its Birmingham, Alabama facilities. The restructuring involved approximately 25 employees and was substantially completed as of December 31, 2022, with minimal expenses incurred in 2023.
On February 3, 2023, the Company committed to a plan to restructure its workforce to increase productivity and enhance profitability. The reduction in force reduced the Company’s headcount by 71 employees, or approximately 7% of all employees. The Company incurred total employee-related charges of $1,609 in connection with the restructuring, primarily consisting of severance payments. It was substantially completed as of March 31, 2023.
On October 27, 2023, the Company committed to a plan to restructure its workforce to increase productivity and enhance profitability. The reduction in force reduced the Company’s headcount by 49 employees, or approximately 5% of all employees. The Company incurred a total charge of $1,820 in the fourth quarter of 2023, primarily consisting of severance payments.
As a result of the restructuring activities, the Company incurred pre-tax charges of $0, $3,796 and $2,268 in the years ended December 31, 2024, 2023, and 2022, respectively. These charges were included in selling, general and administrative expenses in the consolidated statements of operations and comprehensive income. The liability related to the restructuring activities was $0 and $904 as of December 31, 2024 and 2023, respectively, and was included in accrued expenses and other current liabilities in the consolidated balance sheets. The following table provides a rollforward of the restructuring liability.
| | | | | | | | | | | | |
| | Employee | | | Other | | | Total | |
Liability balance as of December 31, 2022 | | | 1,010 | | | | 182 | | | | 1,192 | |
Expenses | | | 3,429 | | | | 367 | | | | 3,796 | |
Cash distributions | | | (3,535 | ) | | | (549 | ) | | | (4,084 | ) |
Liability balance as of December 31, 2023 | | | 904 | | | | — | | | | 904 | |
Cash distributions and other adjustments | | | (904 | ) | | | — | | | | (904 | ) |
Liability balance as of December 31, 2024 | | $ | — | | | $ | — | | | $ | — | |
12. Long-Term Debt Obligations
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Term loan | | | — | | | | 66,563 | |
Less debt discount and debt issuance cost | | | — | | | | (332 | ) |
Term loan, net of debt discount and debt issuance cost | | $ | — | | | $ | 66,231 | |
2021 Credit Agreement
In August 2021, the Company, as borrower, its subsidiaries, as guarantors, and Silicon Valley Bank (SVB), and the several other lenders thereto (collectively, the Lenders) entered into a credit agreement, as amended (the 2021 Credit Agreement), providing for a term loan facility not to exceed $75,000 (the Term Loan Facility) and a revolving credit facility not to exceed $125,000 (the Revolving Facility and, together with the Term Loan Facility, the Facilities). In November 2024, the Company and the Lenders amended the 2021 Credit Agreement to allow for the issuance of the Convertible Preferred Stock, and to require the repayment of the Term Loan Facility within one business day of such issuance, among other terms (the 2024 Amendment).
The Company’s obligations to the Lenders are secured by substantially all of the Company’s assets, including intellectual property. Capitalized terms used herein and not otherwise defined are defined as set forth in the 2021 Credit Agreement, as amended by the 2024 Amendment.
Advances made under the 2021 Credit Agreement were either SOFR Loans or ABR Loans, at the Company’s option. For SOFR Loans, the interest rate was a per annum interest rate equal to the Adjusted Term SOFR plus an Applicable Margin between 2.00% to 3.25% based on the Total Net Leverage Ratio. For ABR Loans, the interest rate was equal to (1) the highest of (a) the Wall Street Journal Prime Rate, (b) the Federal Funds Rate plus 0.50% and (c) the Adjusted Term SOFR rate plus 1.0%, plus (2) an Applicable Margin between 1.00% to 2.25% based on the Total Net Leverage Ratio.
The 2021 Credit Agreement required the Company to make consecutive quarterly installment payments equal to the following: (a) from September 30, 2021 through and including June 30, 2022, $469; (b) from September 30, 2022 through and including June 30, 2023, $938; (c) from September 30, 2023 through and including June 30, 2025, $1,406 and (d) from September 30, 2025 and the last day of each quarter thereafter until August 6, 2026 (the Term Loan Maturity Date), $1,875. The Company prepaid the Term Loan Facility in November 2024, and amounts borrowed under the Term Loan Facility may not be re-borrowed.
The Company must pay in arrears, on the first day of each quarter prior to August 6, 2026 (the Revolving Termination Date) and on the Revolving Termination Date, a fee for the Company’s non-use of available funds (the Commitment Fee). The Commitment Fee rate is between 0.25% to 0.45% based on the Total Net Leverage Ratio. The Company may elect to reduce or terminate the Revolving Facility in its entirety at any time by repaying all outstanding principal and unpaid accrued interest.
Under the 2021 Credit Agreement as amended by the 2024 Amendment, the Company is required to comply with certain financial covenants including the Consolidated Fixed Charge Coverage Ratio and Consolidated Total Net Leverage Ratio, tested quarterly. In addition, the Company is also required to make representations and warranties and comply with certain non-financial covenants that are customary in loan agreements of this type, including restrictions on the payment of dividends, repurchase of stock, incurrence of indebtedness, dispositions and acquisitions.
The Company recorded debt issuance costs and related fees of $604 in connection with entering into the Term Loan Facility, which were recorded as a reduction of the carrying value of the Term Loan Facility on the Company’s consolidated balance sheets, and amortized to interest expense over the expected term of the Term Loan Facility. Upon repayment of the Term Loan Facility, the remaining balance of these debt issuance costs of $215 was recorded as a loss on debt extinguishment in the consolidated statements of operations and comprehensive income. In connection with entering into the Revolving Facility, the Company recorded debt issuance costs and related fees of $1,223, which are recorded as other assets and are being amortized to interest expense through the maturity date of the Revolving Facility.
As of December 31, 2024 and 2023, the Company had outstanding borrowings of $0 and $66,563 under the Term Loan Facility, respectively, and $0 under the Revolving Facility with $125,000 available for future revolving borrowings.
13. Convertible Preferred Stock
On November 12, 2024, the Company entered into a subscription agreement (Subscription Agreement) with Avista Healthcare Partners III, L.P. (Avista Onshore) and AHP III Orchestra Holdings, L.P. (together with Avista Onshore, the Investors, and each an Investor and now related parties of the Company) pursuant to which the Investors purchased 130,000 shares of the Company’s newly-created Series A Convertible Preferred Stock, par value $0.0001 per share, for a purchase price of $1,000 per share, or aggregate gross proceeds of $130,000 to the Company, prior to deduction of commissions, fees and expenses (Offering). The net proceeds will be used to fund strategic growth initiatives including, but not limited to, operating and commercial activities, clinical development programs, working capital, capital expenditures, debt repayment and for general corporate purposes. In addition, $25,479 of the net proceeds were used to fund the repurchase of an aggregate of 7,921,731 shares of Class A common stock from certain existing stockholders of the Company. See Note 14, Stockholders’ Equity.
The holders of the Convertible Preferred Stock have the following rights and preferences:
Voting
The Convertible Preferred Stock is subject to certain transfer restrictions, and contain terms regarding anti-dilution, liquidation preference, and preemptive rights, and its holders vote together with the Class A common stock on an as-converted basis. The Preferred Stockholders are entitled to elect one member and one observer to the Company’s Board of Directors, subject to the terms of the Convertible Preferred Stock.
Dividends
Holders of the Convertible Preferred Stock are entitled to a regular dividend at the rate of 8.0% per annum, compounding and payable quarterly in kind or in cash, at the Company’s election, subject to the 19.99% ownership limitations described below. Any accrued but unpaid dividends will become part of the liquidation preference of such share, as set forth in the Certificate of Designation. As of December 31, 2024, the Company had not paid any dividends in cash, and all such dividends had been accrued and added to the liquidation preference of each share.
Conversion and Cash-In-Lieu Payments
Pursuant to the Certificate of Designations of Series A Convertible Preferred Stock (Certificate of Designation), each share of Convertible Preferred Stock is initially convertible into 263.7358 shares of Common Stock, subject to adjustment as provided therein. Until the Company receives stockholder approval, as contemplated by Nasdaq listing rules, with respect to the issuance of shares of Class A common stock upon conversion of the Convertible Preferred Stock in excess of the limitations imposed by such rules, holders of the Convertible Preferred Stock (Preferred Stockholders) cannot convert the Convertible Preferred Stock into a number of shares of Class A common stock in excess of 26,502,042 shares, which represents 19.99% of the outstanding shares of Common Stock at the time of signing the Subscription Agreement, or to the extent such conversion will result in a Preferred Stockholder beneficially owning greater than 19.99% of the Company’s then-outstanding shares. If, prior to receipt of the stockholder approval, a Preferred Stockholder elects to convert any Convertible Preferred Stock that would result in the issuance, when aggregated with the number of shares previously issued upon conversion of the Convertible Preferred Stock, of more than 19.99% of the outstanding shares of Class A common stock at the time of signing the Subscription Agreement, then the Company will, in lieu of issuing shares of Common Stock, pay the Preferred Stockholder a cash amount equal to the product of the number of shares of Common Stock that could not be issued due to such limitation and the 10-day trailing volume weighted average price of the Common Stock as of the trading day immediately prior to the conversion date (Cash-in-Lieu Payments), which Cash-in-Lieu Payments shall be paid no later than November 4, 2026, together with accrued interest of 10% per annum, to the extent an earlier cash payment is prohibited pursuant to the terms of the 2021 Credit Agreement, as amended by the 2024 Amendment.
The Convertible Preferred Stock is convertible at the option of the Company after the second anniversary of issuance if the closing price of the Company’s common stock equals or exceeds 200% of the conversion price for twenty trading days out of a period of thirty consecutive trading days.
Liquidation
The Convertible Preferred Stock ranks senior to shares of Class A common stock with respect to payment of dividends and the distribution of assets upon a liquidation, dissolution or winding up of the Company. The Convertible Preferred Stock initially had a
liquidation preference of $1,000 per share; provided that the liquidation preference upon a change of control on or before November 12, 2026 will be increased to be no less than $1,500 per share.
Redemption
The Convertible Preferred Stock is redeemable at the option of the Preferred Stockholders at any time after November 12, 2031, for the amount of the then-applicable liquidation preference per share, plus accrued but unpaid dividends.
Upon issuance of the Convertible Preferred Stock, the Company assessed the embedded conversion and liquidation features of the securities and determined that such features did not require the Company to separately account for these features. The Company also concluded that no beneficial conversion feature existed on the issuance date of the Convertible Preferred Stock.
The Company recorded the Convertible Preferred Stock at its fair value at the date of issuance, $130,000, net of issuance costs of $9,379, in the accompanying consolidated balance sheets and statements of convertible preferred stock and stockholders’ equity. The SEC’s Accounting Series Release No. 268 (ASR 268), which requires preferred securities that are redeemable for cash or other assets to be classified outside of permanent equity if they are redeemable (i) at a fixed or determinable price on a fixed or determinable date, (ii) at the option of the holder, or (iii) upon the occurrence of an event that is not solely within the control of the issuer. The Convertible Preferred Stock is redeemable at the option of the holder, and accordingly the Company classified the Convertible Preferred Stock as mezzanine equity.
The Company recognizes changes in the redemption value of the Convertible Preferred Stock, which include accretion of the associated issuance costs and accrual of unpaid dividends using the effective interest method, over the period from the issuance date to the earliest redemption date, November 12, 2031. During the year ended December 31, 2024, the Company increased the carrying value of the Convertible Preferred Stock by $1,798 which resulted in a corresponding decrease to additional paid-in-capital during the same period.
14. Stockholders’ Equity
In November 2024, the Company repurchased 7,421,731 shares of Class A common stock from certain existing stockholders of the Company, including certain of its directors and their affiliates, at a price per share equal to $3.1597, which represents the 10-day trailing volume weighted average price of the Class A common stock as of market close on November 11, 2024, pursuant to stock repurchase agreements entered into on November 12, 2024 between the Company and such stockholders (Stock Repurchase Agreements and each stock repurchase thereunder, a Repurchase). Also in November 2024, the Company repurchased 500,000 shares of Class A common stock from an existing stockholder, an entity beneficially owned by a member of the Board of Directors of the Company, at a price per share equal to $4.057, which represents the 10-day trailing volume weighted average price of the Common Stock as of market close on November 26, 2024, pursuant to a stock repurchase agreement entered into on November 27, 2024 between the Company and such stockholder (Additional Stock Repurchase Agreement).
As of December 31, 2024 and 2023, the issued shares of Class A common stock include 728,548 treasury shares that were reacquired in connection with the redemption of redeemable shares in March 2019. The 7,921,731 shares of Class A common stock repurchased in November 2024 pursuant to the Stock Repurchase Agreements and the Additional Stock Repurchase Agreement were retired and returned to authorized and unissued status.
Each share of Class A common stock entitles the holder to one vote on all matters submitted to the stockholders for a vote. Class A common stockholders are entitled to receive dividends, as may be declared by the Board of Directors to the extent permissible under the 2021 Credit Agreement as amended by the 2024 Amendment. Through December 31, 2024, no cash dividends have been declared or paid.
15. Share-Based Compensation
Stock Incentive Plans-the 2018 Plan
On November 28, 2018, the Board of Directors of the Company adopted, and on December 10, 2018, the Company’s stockholders approved, the Organogenesis 2018 Equity and Incentive Plan (the 2018 Plan). The purposes of the 2018 Plan are to provide long-term incentives and rewards to the Company’s employees, officers, directors and other key persons (including consultants), to attract and retain persons with the requisite experience and ability, and to more closely align the interests of such employees, officers, directors and other key persons with the interests of the Company’s stockholders.
The 2018 Plan authorizes the Company’s Board of Directors or a committee of not less than two independent directors (in either case, the Administrator) to grant the following types of awards: non-statutory stock options; incentive stock options; restricted stock awards; restricted stock units; stock appreciation rights; unrestricted stock awards; performance share awards; and dividend equivalent rights. The 2018 Plan is administered by the Company’s Board of Directors.
At the adoption of the 2018 Plan, a total of 9,198,996 shares of Class A common stock was authorized to be issued (subject to adjustment in the case of any stock dividend, stock split, reverse stock split, or similar change in capitalization of the Company). In June 2022, the 2018 Plan was amended to increase the number of shares of Class A common stock reserved for issuance by 7,826,970 shares. In June 2024, the 2018 Plan was amended to increase the number of shares of Class A common stock reserved for issuance by 15,900,000 shares.
Stock Incentive Plans-the 2003 Plan
The Organogenesis 2003 Stock Incentive Plan (the 2003 Plan), provided for the Company to issue restricted stock awards, or to grant incentive stock options or non-statutory stock options. Incentive stock options were granted only to the Company’s employees. Restricted stock awards and non-statutory stock options were granted to employees, members of the Board of Directors, outside advisors and consultants of the Company.
Effective December 10, 2018, no additional awards may be made under the 2003 Plan and as a result (i) any shares in respect of stock options that are expired or terminated under the 2003 Plan without having been fully exercised will not be available for future awards; (ii) any shares in respect of restricted stock that are forfeited to, or otherwise repurchased by the Company, will not be available for future awards; and (iii) any shares of Class A common stock that are tendered to the Company by a participant to exercise an award will not be available for future awards.
Stock-Based Compensation Expense
Stock options awarded under the stock incentive plans expire 10 years after the grant date and typically vest over four or five years. Restricted stock units awarded typically vest over four years.
During the years ended December 31, 2024, 2023, and 2022, the Company recorded stock-based compensation expense of $10,578, $8,996 and $6,552, respectively, within selling, general and administrative expenses on the consolidated statements of operations and comprehensive income.
Restricted Stock Units (RSUs)
During the years ended December 31, 2024 and 2023, the Company granted 2,156,874 and 3,192,372 time-based restricted stock units to its employees, executives and the Board of Directors. Each restricted stock unit represents the contingent right to receive one share of the Company’s Class A common stock. The fair value of the restricted stock units is based on the fair market value of the Company’s stock on the date of grant.
The activity of restricted stock units is set forth below:
| | | | | | | | |
| | | | | Weighted | |
| | | | | Average | |
| | Number | | | Grant Date | |
| | of Shares | | | Fair Value | |
Unvested at December 31, 2023 | | | 3,898,331 | | | $ | 3.54 | |
Granted | | | 2,156,874 | | | $ | 3.36 | |
Vested | | | (1,434,809 | ) | | $ | 3.67 | |
Canceled/Forfeited | | | (91,066 | ) | | $ | 4.16 | |
Unvested at December 31, 2024 | | | 4,529,330 | | | $ | 3.40 | |
As of December 31, 2024, the total unrecognized compensation cost related to unvested restricted stock units expected to vest was $8,316 and the weighted average remaining recognition period for unvested awards was 2.46 years.
Stock Options
The stock options granted during the years ended December 31, 2024 and 2023 were 2,640,601 and 3,554,528, respectively. The assumptions that the Company used to determine the grant-date fair value of stock options granted during these periods are as follows, presented on a weighted-average basis:
| | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2024 | | | 2023 | |
| | | | | | |
Risk-free interest rate | | | 4.27 | % | | | 4.00 | % |
Expected term (in years) | | | 6.21 | | | | 6.25 | |
Expected volatility | | | 52.24 | % | | | 51.00 | % |
Expected dividend yield | | | 0.0 | % | | | 0.0 | % |
Underlying stock price | | $ | 3.43 | | | $ | 2.47 | |
These assumptions resulted in an estimated weighted-average grant-date fair value per share of stock options granted during the years ended December 31, 2024 and 2023 of $1.89 and $1.32, respectively.
The following table summarizes the Company’s stock option activity since December 31, 2023:
| | | | | | | | | | | | | | | | |
| | | | | | | | Weighted | | | | |
| | | | | Weighted | | | Average | | | | |
| | | | | Average | | | Remaining | | | Aggregate | |
| | Number of | | | Exercise | | | Contractual | | | Intrinsic | |
| | Shares | | | Price | | | Term | | | Value | |
| | | | | | | | (in years) | | | | |
Options outstanding as of December 31, 2023 | | | 9,340,046 | | | $ | 4.60 | | | | 6.66 | | | $ | 10,267 | |
Granted | | | 2,640,601 | | | $ | 3.43 | | | | | | | |
Exercised | | | (1,225,435 | ) | | $ | 1.02 | | | | | | $ | 3,044 | |
Canceled / forfeited | | | (191,332 | ) | | $ | 3.65 | | | | | | | |
Outstanding as of December 31, 2024 | | | 10,563,880 | | | $ | 4.74 | | | | 7.26 | | | $ | 2,521 | |
Options exercisable as of December 31, 2024 | | | 4,419,875 | | | $ | 5.89 | | | | 5.68 | | | $ | 715 | |
Options vested or expected to vest as of December 31, 2024 | | | 9,709,088 | | | $ | 4.86 | | | | 7.14 | | | $ | 2,278 | |
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s Class A common stock for those stock options that have exercise prices lower than the fair value of the Company’s Class A common stock.
The total fair value of options vested during the years ended December 31, 2024 and 2023 was $4,136 and $3,117, respectively.
As of December 31, 2024, the total unrecognized stock compensation expense was $6,514 and is expected to be recognized over a weighted-average period of 2.33 years.
16. Segment Information
The Company offers a comprehensive portfolio of regenerative medicine products. The Company organizes its products into two product categories, Advanced Wound Care (AWC) and Surgical & Sports Medicine (SSM), which serve two adjacent markets. Many of the Company’s products are clinically interchangeable and certain products are categorized as both AWC and SSM products. The Company’s products all contain regenerative medicine technologies and have the same customers and target market, require similar raw materials and commercial infrastructure, and exist within the same regulatory environment.
The Company’s CODM is the Chief Executive Officer. The CODM reviews consolidated gross profit and operating results to assess the overall performance of the Company, and make decisions to allocate resources among the consolidated entity. The CODM uses both gross profit and net income (loss) for the consolidated entity in the annual budget and forecasting process, and considers budget-to-actual variances in gross profit and operating expenses on a quarterly basis when making decisions about the allocation of operating and capital resources to each predominant business activity (research and development, capital expenditure, and employee headcount and compensation).
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Net revenue | | $ | 482,043 | | | $ | 433,140 | | | $ | 450,893 | |
Less: | | | | | | | | | |
Cost of goods sold | | | 115,741 | | | | 106,481 | | | | 105,019 | |
Clinical expense | | | 23,614 | | | | 19,377 | | | | 15,156 | |
Salaries, wages, and other compensation (a) | | | 181,773 | | | | 177,396 | | | | 179,947 | |
Other segment items (b) | | | 160,054 | | | | 124,941 | | | | 135,239 | |
Segment net income | | | 861 | | | | 4,945 | | | | 15,532 | |
| | | | | | | | | |
Reconciliation of segment net income: | | | | | | | | | |
Reconciling items | | | — | | | | — | | | | — | |
Consolidated net income | | $ | 861 | | | $ | 4,945 | | | $ | 15,532 | |
(a) Salaries, wages, and other compensation includes: commissions, share-based compensation, and payroll taxes and benefits.
(b) Other segment items includes: impairment of property and construction, write down of capitalized internal-use software costs, internal technology, rent and other facilities expense, royalty expense, travel and entertainment expense, marketing expense, depreciation and amortization, and interest expense.
17. Income Taxes
The components of the income tax expense (benefit) consisted of the following for the years ended December 31, 2024, 2023, and 2022:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Income tax expense (benefit): | | | | | | | | | |
Current tax expense (benefit) | | | | | | | | | |
Federal | | $ | 5,127 | | | $ | 1,275 | | | $ | 178 | |
State | | | 1,913 | | | | 2,157 | | | | 2,575 | |
Foreign | | | 11 | | | | 3 | | | | 17 | |
Total current tax expense | | | 7,051 | | | | 3,435 | | | | 2,770 | |
Deferred tax expense (benefit) | | | | | | | | | |
Federal | | | (8,193 | ) | | | 3,311 | | | | 5,446 | |
State | | | (2,553 | ) | | | (1,312 | ) | | | (3,466 | ) |
Foreign | | | 27 | | | | 13 | | | | — | |
Total deferred tax expense (benefit) | | | (10,719 | ) | | | 2,012 | | | | 1,980 | |
Total income tax expense (benefit) | | $ | (3,668 | ) | | $ | 5,447 | | | $ | 4,750 | |
On a periodic basis, the Company reassesses the valuation allowance on its deferred income tax assets, weighing positive and negative evidence to assess the recoverability of the deferred tax assets. The Company determined that its net U.S. deferred tax assets did not require a valuation allowance as of December 31, 2024 and 2023.
As of December 31, 2024, the Company had state net operating loss carry-forwards of approximately $7,441, expiring from the year ended December 31, 2027 through 2038. The Company had state research and development tax credits of approximately $1,078, expiring in the year ended December 31, 2038.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2024 and 2023 are as follows:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Net operating loss carryforwards | | | | | | |
Federal | | $ | — | | | $ | 2,455 | |
State | | | 489 | | | | 665 | |
Foreign | | | — | | | | 4 | |
Capitalized research and development | | | 24,250 | | | | 17,608 | |
Operating leases | | | 10,326 | | | | 12,503 | |
Property and equipment | | | 4,084 | | | | — | |
Tax credit carryforwards | | | 1,078 | | | | — | |
Stock-based compensation | | | 2,661 | | | | 1,699 | |
Other | | | 7,107 | | | | 6,404 | |
Net deferred tax assets before valuation allowance | | | 49,995 | | | | 41,338 | |
Property and equipment | | | — | | | | (1,493 | ) |
Right-of-use assets | | | (9,251 | ) | | | (10,002 | ) |
Intangibles | | | (1,282 | ) | | | (1,841 | ) |
Net deferred tax assets | | $ | 39,462 | | | $ | 28,002 | |
The Company has not recorded withholding taxes on the undistributed earnings of its Swiss subsidiary because it is the Company’s intent to reinvest such earnings indefinitely.
Ownership changes, as defined in the Internal Revenue Code, may limit the amount of net operating losses and research and development tax credit carryforwards that can be utilized annually to offset future taxable income. Subsequent ownership changes could further affect the limitation in future years. The Company completed an analysis and determined that it had not experienced an ownership change during the periods 2001 through 2024.
The differences between income taxes expected at the U.S. federal statutory income tax rate of 21% and the reported consolidated income tax benefit (expense) are summarized as follows:
| | | | | | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
U.S. federal statutory income tax rate | | | 21.0 | % | | | 21.0 | % | | | 21.0 | % |
Federal valuation allowance | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
State valuation allowance | | | 0.0 | % | | | 0.0 | % | | | 0.0 | % |
Return to provision and other adjustments | | | 34.1 | % | | | (1.4 | %) | | | (1.6 | %) |
Prior period correction | | | 0.0 | % | | | 0.0 | % | | | (8.5 | %) |
Executive compensation limited by 162(m) | | | (24.8 | %) | | | 12.0 | % | | | 3.1 | % |
State and local income taxes | | | 3.8 | % | | | 8.8 | % | | | 6.8 | % |
Meals and entertainment | | | (3.8 | %) | | | 5.9 | % | | | 0.0 | % |
Nondeductible lobbying expenses | | | (6.4 | %) | | | 1.7 | % | | | 0.4 | % |
Stock-based compensation | | | 11.8 | % | | | 1.3 | % | | | 0.3 | % |
Foreign rate differential | | | 0.4 | % | | | (0.1 | %) | | | 0.1 | % |
Uncertain tax position reserves | | | 22.1 | % | | | 0.7 | % | | | 0.3 | % |
Nondeductible fringe benefits | | | (4.5 | %) | | | 1.0 | % | | | 0.4 | % |
State credits | | | 0.0 | % | | | 1.1 | % | | | 0.9 | % |
Other nondeductible expenses | | | (0.9 | %) | | | 0.4 | % | | | 0.2 | % |
Research and development credits | | | 147.9 | % | | | 0.0 | % | | | 0.0 | % |
Nondeductible transaction costs | | | (70.2 | %) | | | 0.0 | % | | | 0.0 | % |
Effective income tax rate | | | 130.5 | % | | | 52.4 | % | | | 23.4 | % |
The Company recognizes the tax benefit from an uncertain tax position only if it is more-likely-than-not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The amount of unrecognized tax benefits is $2,030, $2,837 and $2,642, as of December 31, 2024, 2023, and 2022, respectively.
A tabular roll forward of the Company’s uncertainties in its income tax provision liability is presented below:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Gross balance at beginning of year | | $ | 1,619 | | | $ | 1,632 | | | $ | 1,612 | |
Additions based on tax positions related to the current period | | | — | | | | 113 | | | | 206 | |
Reductions for tax positions of prior years | | | (746 | ) | | | (126 | ) | | | (186 | ) |
Gross balance at end of year | | $ | 873 | | | $ | 1,619 | | | $ | 1,632 | |
The Company files income tax returns in the United States federal and state jurisdictions and Switzerland. With limited exceptions, the Company is no longer subject to federal, state, local or foreign examinations for years prior to December 31, 2020. However, carryforward attributes that were generated prior to December 31, 2020 may still be adjusted upon examination by state or local tax authorities if they either have been or will be used in a future period.
The Company recognizes interest and penalty-related expenses in tax expenses. The Company recorded $11 and $500 of interest for uncertain tax positions for the years ended December 31, 2024 and 2023, respectively, which is classified in accrued expenses and other current liabilities in the consolidated balance sheets. These amounts are not reflected in the reconciliation above.
18. Earnings (Loss) per Share (EPS)
A reconciliation of the numerator and denominator used in the calculation of the basic and diluted net income (loss) attributable to the Class A common stockholders is as follows:
| | | | | | | | | | | | |
| | Year Ended December 31, | |
| | 2024 | | | 2023 | | | 2022 | |
Numerator: | | | | | | | | | |
Net income | | $ | 861 | | | $ | 4,945 | | | $ | 15,532 | |
Accretion of Convertible Preferred Stock to redemption value | | | (412 | ) | | | — | | | | — | |
Cumulative dividend on Convertible Preferred Stock | | | (1,386 | ) | | | — | | | | — | |
Income tax on above | | | — | | | | — | | | | — | |
Net income (loss) attributable to common stockholders - basic and diluted | | $ | (937 | ) | | $ | 4,945 | | | $ | 15,532 | |
Denominator: | | | | | | | | | |
Weighted average common shares outstanding —basic | | | 131,673,278 | | | | 131,231,317 | | | | 130,070,231 | |
Dilutive effect of restricted stock units | | | — | | | | 710,813 | | | | 149,215 | |
Dilutive effect of options | | | — | | | | 804,597 | | | | 2,163,706 | |
Weighted-average common shares outstanding—diluted | | | 131,673,278 | | | | 132,746,727 | | | | 132,383,152 | |
Earnings per share—basic | | $ | (0.01 | ) | | $ | 0.04 | | | $ | 0.12 | |
Earnings per share—diluted | | $ | (0.01 | ) | | $ | 0.04 | | | $ | 0.12 | |
For the years ended December 31, 2024, 2023, and 2022, outstanding stock-based awards of 15,092,510, 3,147,503 and 3,445,191, respectively, were excluded from the diluted EPS calculation as they were anti-dilutive. For the year ended December 31, 2024, 25,133,474 shares of common stock available upon conversion of Convertible Preferred Stock were excluded from the diluted EPS calculation as they were anti-dilutive.
19. Leases
The Company’s leases consist primarily of real estate, equipment and vehicle leases.
The Company leases real estate for office, lab, warehouse and production space under noncancelable leases that expire at various dates through 2041, subject to the Company’s options to terminate or renew certain leases for an additional five to ten years.
The Company leases vehicles under operating leases for certain employees and has fleet services agreements for service on these vehicles. The minimum lease term for each newly leased vehicle is 367 days with renewal options. The Company may terminate the vehicle lease after the minimum lease term upon thirty days’ prior notice.
The Company also leases other equipment under noncancelable operating leases that expire at various dates through 2025, and certain equipment required for its cleanroom facilities under finance leases that expire in 2026.
On January 1, 2013, the Company entered into finance lease arrangements with 65 Dan Road SPE, LLC, 85 Dan Road Associates, LLC, Dan Road Equity I, LLC and 275 Dan Road SPE, LLC for office and laboratory space in Canton, Massachusetts (the Related-Party Leases). 65 Dan Road SPE, LLC, 85 Dan Road Associates, LLC, Dan Road Equity I, LLC and 275 Dan Road SPE, LLC are related parties as the owners of these entities are also directors, former directors and / or stockholders of the Company.
In August 2021, the Company purchased the building (the 275 Dan Road Building) under the lease with 275 Dan Road SPE, LLC for $6,013 and the lease was terminated. The Company recorded an asset of $4,943 to buildings within property and equipment, net, on the accompanying consolidated balance sheets.
The remaining three Related-Party Leases were set to terminate on December 31, 2022 and each contained a renewal option for a five-year period with a rental rate at the greater of (i) rent for the last year of the prior term, or (ii) the then fair market value. In November 2021, the Company exercised the option to extend the leases for an additional five years, and at such time, remeasured the right of use assets and lease liabilities based on its best estimate of the market rental rate in the renewal period and reassessed the classification for these leases. As a result, these leases were reclassified from finance leases to operating leases on the consolidated balance sheets as of December 31, 2021. In December 2022, the Company and the landlord finalized the market rental rate in the renewal period for these properties, resulting in an additional $8,060 to be recorded as variable lease expenses over the renewal period.
Effective April 1, 2019, the Company agreed to accrue interest on accrued but unpaid lease obligations owed for rent in arrears to the owners of the buildings subject to the Related-Party Leases, at an interest rate equal to the rate charged under the 2019 Credit Agreement. The Company repaid the remaining accrued but unpaid lease obligations and associated accrued interest in installments throughout 2024. The accrued but unpaid lease obligations as well as the related accrued interest with respect to the remaining three Related-Party Leases are shown below:
| | | | | | | | |
| | December 31, | |
| | 2024 | | | 2023 | |
Principal portion of rent in arrears | | $ | — | | | $ | 5,273 | |
Accrued interest on accrued but unpaid lease obligations | | $ | — | | | $ | 2,326 | |
The accrued but unpaid lease obligations owed for rent in arrears was included in current portion of operating lease obligations, other than the balance related to the 275 Dan Road Building, which was included in accrued expenses and other current liabilities on the consolidated balance sheets, as of December 31, 2023. The accrued interest on the accrued but unpaid lease obligations was included in accrued expenses and other current liabilities on the consolidated balance sheets as of December 31, 2023.
In November 2024, the Company entered into a lease for a facility in Smithfield, Rhode Island, comprising manufacturing and office space (Smithfield Facility). The initial lease term is approximately sixteen years, with two ten-year renewal options, not considered probable of exercise at lease inception, and a right of first offer to purchase the Smithfield Facility in the event that its owner markets it for sale. The undiscounted minimum lease payments are $102,645, and the Company is entitled to a tenant improvement allowance of up to $18,376 for its planned build out of the manufacturing space, expected to be completed in fiscal 2027. The lease of the office space commenced at lease inception, and in connection therewith, the Company recorded a right-of-use asset and associated lease liability of $3,425. The Company has unilateral right to terminate the lease for a payment to the landlord of $1,250 in the event it does not secure certain anticipated state and local tax incentives by March 31, 2025.
During the year ended December 31, 2023, the Company terminated an existing agreement for the rental of certain medical garments. The Company recorded a loss of $559 in connection with the lease termination.
The components of lease cost were as follows:
| | | | | | | | | | |
| | Classification | | Year Ended
December 31, 2024 | | | Year Ended
December 31, 2023 | |
Finance lease | | | | | | | | |
Amortization of right-of-use assets | | COGS and SG&A | | $ | 1,151 | | | $ | 479 | |
Interest on lease liabilities | | Interest Expense | | | 197 | | | | 137 | |
Total finance lease cost | | | | | 1,348 | | | | 616 | |
Operating lease cost | | COGS, R&D, SG&A | | | 9,474 | | | | 10,052 | |
Short-term lease cost | | COGS, R&D, SG&A | | | 2,893 | | | | 2,921 | |
Variable lease cost | | COGS, R&D, SG&A | | | 6,615 | | | | 5,595 | |
Total lease cost | | | | $ | 20,330 | | | $ | 19,184 | |
Supplemental balance sheet information related to finance leases was as follows:
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2024 | | | 2023 | |
Property and equipment, gross | | $ | 3,454 | | | $ | 3,454 | |
Accumulated depreciation | | | (1,631 | ) | | | (479 | ) |
Property and equipment, net | | $ | 1,823 | | | $ | 2,975 | |
| | | | | | |
Current portion of finance lease obligations | | $ | 1,170 | | | $ | 1,081 | |
Finance lease obligations, net of current portion | | | 718 | | | | 1,888 | |
Total finance lease liabilities | | $ | 1,888 | | | $ | 2,969 | |
Supplemental cash flow information related to leases was as follows:
| | | | | | | | |
| | Year Ended
December 31, 2024 | | | Year Ended
December 31, 2023 | |
Cash paid for amounts included in the measurement of lease liabilities: | | | | | | |
Operating cash flows for operating leases | | $ | 14,962 | | | $ | 10,401 | |
Operating cash flows for finance leases | | $ | 197 | | | $ | 137 | |
Financing cash flows for finance leases | | $ | 1,081 | | | $ | 485 | |
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2024 | | | 2023 | |
Weighted-average remaining lease term | | | | | | |
Finance leases | | | 1.58 | | | | 2.58 | |
Operating leases | | | 7.04 | | | | 6.49 | |
| | | | | | |
| | December 31, | | | December 31, | |
| | 2024 | | | 2023 | |
Weighted-average discount rate | | | | | | |
Finance leases | | | 7.91 | % | | | 7.91 | % |
Operating leases | | | 4.91 | % | | | 4.71 | % |
As of December 31, 2024, the maturities of lease liabilities were as follows:
| | | | | | | | |
| | Operating leases | | | Finance leases | |
2025 | | $ | 11,119 | | | $ | 1,278 | |
2026 | | | 11,955 | | | | 737 | |
2027 | | | 13,586 | | | | — | |
2028 | | | 9,210 | | | | — | |
2029 | | | 9,475 | | | | |
Thereafter | | | 91,341 | | | | — | |
Total lease payments | | | 146,686 | | | | 2,015 | |
Less: interest | | | (8,330 | ) | | | (127 | ) |
Total lease liabilities | | $ | 138,356 | | | $ | 1,888 | |
20. Commitments and Contingencies
License and Manufacturing Agreement
In November 2023, the Company entered into a trademark license and manufacturing agreement with Vivex Biologics, Inc. (Vivex) to sell its CYGNUS Dual (Dual) and CYGNUS Matrix (Matrix) products, with the option to license the VIA Matrix (VIA) products. In March 2024, the Company exercised the option to license VIA, and accordingly in July 2024, entered into the first amendment to the trademark license and manufacturing agreement (together with the original agreement, the Vivex Agreement).
The Company paid an upfront licensing fee to Vivex to sell Dual and Matrix, and also agreed to pay a fixed milestone payment for Dual in the event that its average sales price (ASP) is published by certain government agencies for a specified period of time, which the Company determined was probable. Additionally, the Company pays a low double-digit royalty on the Net Sales of Dual and VIA, and a high single-digit royalty on the Net Sales of Matrix, respectively, during the royalty term, as defined in the Vivex Agreement. The royalty term is commensurate with the initial term of the contract and will continue for each subsequent renewal period. The initial term of the agreement expires on December 31, 2026 and can be renewed for up to five additional one-year terms.
The Company recorded $5,000 in prepaid and other current assets and other assets for the payment of the upfront licensing fee, which is recognized as expense on a straight-line basis over the estimated life of the arrangement, which the Company determined to be three years, commensurate with the initial term of the contract. In December 2023, the Company recorded $2,500 in prepaid and other current assets, other assets, and accrued expenses and other current liabilities for the milestone payment, which it remitted in
January 2025. The Company remitted the option payment of $5,000 for VIA in April 2024. As of December 31, 2024 and 2023, $3,158 and $3,158 is recorded in prepaid and other current assets and $2,368 and $4,737 is recorded in other assets, respectively, in the accompanying consolidated balance sheets for the upfront licensing fees and milestone payment.
Royalties
The Company entered into a license agreement with a university for certain patent rights related to the development, use and production of one of its advanced wound care products. Under this agreement, the Company incurred a royalty based on a percentage of net product sales, for the use of these patents until the patents expired, which was in November 2006. In December 2024, the Company no longer contractually owed the royalties of $1,187 it had accrued, and accordingly at such time recorded an adjustment of $(1,187) in selling, general and administrative expenses in the consolidated statements of operations and comprehensive income. As of December 31, 2024 and 2023, accrued royalties totaled $0 and $1,187 respectively, and were classified as part of accrued expenses and other current liabilities on the Company’s consolidated balance sheets. There was no royalty expense incurred during the years ended December 31, 2023, and 2022, related to this agreement.
In October 2017, the Company entered into a license agreement with a third party. Under the license agreement, the Company is required to pay royalties based on a percentage of net sales of the licensed product that occur, after December 31, 2017, through the expiration of the underlying patent in October 2026, subject to minimum royalty payment provisions.
The Company recorded $24,736, $5,456, and $7,279 in total royalty expense for the years ended December 31, 2024, 2023, and 2022, respectively, within selling, general and administrative expenses on the consolidated statements of operations and comprehensive income.
Legal Matters
In conducting its activities, the Company, from time to time, is subject to various claims and also has claims against others. In management’s opinion, the ultimate resolution of such claims would not have a material effect on the financial position, operating results or cash flows of the Company. The Company accrues for these claims when amounts due are probable and estimable.
Other Commitments
As of December 31, 2024, we had commitments totaling $26,303 that are legally binding and enforceable. These commitments include purchase obligations for goods and services.
21. Related Party Transactions
Lease obligations to affiliates, including accrued but unpaid lease obligations, purchase of an asset under a finance lease with an affiliate, and renewal of leases with affiliates are further described in Note 19, Leases.
In November 2024, the Company repurchased 7,921,731 shares of Class A common stock from certain existing stockholders of the Company, including certain of its directors and their affiliates. These transactions are further described in Note 14, Stockholders’ Equity.
22. Employee Benefit Plan
The Company maintains a 401(k) Savings Plan (the "Plan") for the United States employees. Under the Plan, eligible employees may contribute, subject to statutory limitations, a percentage of their salary to the Plan. Contributions made by the Company are made at the discretion of the Board of Directors and vest immediately. During the years ended December 31, 2024, 2023, and 2022, the Company made employer contributions of $6,885, $7,430 and $6,601, respectively.
