As filed with the Securities and Exchange Commission on November 22, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
APTEVO THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
| | |
Delaware | | 81-1567056 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification Number) |
2401 4th Avenue, Suite 1050
Seattle, WA 98121
(206) 838-0500
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Marvin L. White
President and Chief Executive Officer
Aptevo Therapeutics Inc.
2401 4th Avenue, Suite 1050
Seattle, WA 98121
(206) 838-0500
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| |
Sean M. Donahue Paul Hastings LLP 2050 M Street, NW Washington, DC 20036 (202) 551-1704 | SoYoung Kwon Senior Vice President and General Counsel Aptevo Therapeutics Inc. 2401 4th Avenue, Suite 1050 Seattle, Washington, 98121 (206) 838-0500 |
Approximate date of commencement of proposed sale to the public: From time to time after this Registration Statement becomes effective.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box: ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | |
Large accelerated filer | ☐ | | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until this registration statement shall become effective on such date as the SEC, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This registration statement contains a prospectus covering the resale by the holders named herein, from time to time, of up to 32,026,068 shares of our Common Stock underlying common warrants issuable in connection with the Warrant Inducement Agreement, dated November 9, 2023 (the “Warrant Inducement Agreement”) with certain holders of our Series A and Series B common warrants (the “Existing Warrants”). The Existing Warrants were issued as part of our August 4, 2023 public offering.
The information contained in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, Dated November 22, 2023
PRELIMINARY PROSPECTUS

Up to 32,026,068 Shares of Common Stock Issuable Upon the Exercise of Common Warrants
This prospectus relates to the resale from time to time by certain selling stockholders named herein (the “Holders”) of up to 32,026,068 shares of our Common Stock par value $0.001 per share (“Common Stock”) of Aptevo Therapeutics Inc. (“Company,” “us” or “we”), issuable upon exercise of Series A-1 Warrants (the “Series A-1 Warrants”), Series A-2 Warrants (the “Series A-2 Warrants,” together with the Series A-1 Warrants the “New Series A Warrants”), Series B-1 Warrants (the “Series B-1 Warrants”) and Series B-2 Warrants (the “Series B-2 Warrants,” together with the Series B-1 Warrants, the “New Series B Warrants,” together with the New Series A Warrants the “New Warrants”). The New Warrants were or may be issued pursuant to that certain Warrant Inducement Agreement, dated as of November 9, 2023, by and between the Company and the Holders (the “Warrant Inducement Agreement”). Such shares of Common Stock underlying the New Warrants are collectively referred to herein as the “Resale Shares.” We are registering the Resale Shares on behalf of the Holders, to be offered and sold from time to time, to satisfy certain registration rights that we have granted to the Holders pursuant to the Warrant Inducement Agreement.
The Holders may resell or dispose of the Resale Shares to or through underwriters, broker-dealers, agents, or through any other means described in the section of this prospectus entitled “Plan of Distribution.” The Holders will bear the commissions and discounts, if any, attributable to the sale or disposition of the Resale Shares. We will bear all costs, expenses and fees in connection with the registration of the Resale Shares. We will not receive any of the proceeds from the sale of the Resale Shares by the Holders.
Our Common Stock is listed on the Nasdaq Capital Market under the symbol “APVO.” On November 21, 2023, the last reported sale price of our Common Stock on the Nasdaq Capital Market was $0.1959 per share.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 13 of this prospectus, any free writing prospectuses we have authorized for use in connection with a specific offering and in the documents incorporated by reference herein and therein.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 22, 2023. .
TABLE OF CONTENTS
About this Prospectus
This prospectus is part of a resale registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC.
If information in this prospectus is inconsistent with any document incorporated by reference that was filed with the SEC before the date of this prospectus, you should rely on this prospectus. This prospectus and the documents incorporated by reference include important information about us, the securities being offered and other information you should know before investing in our securities. You should also read and consider information in the documents we have referred you to in the sections of this prospectus entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference.”
You should rely only on the information contained in, or incorporated by reference into, this prospectus (as supplemented and amended), along with the information contained in any free writing prospectuses. We have not authorized anyone to provide you with different information. We take no responsibility for, and can provide no assurances as to the reliability of, any other information that others may give you. The information contained in this prospectus (and in any supplement or amendment to this prospectus) or any related free writing prospectus, and the documents incorporated by reference herein and therein, are accurate only as of their respective dates, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus, or any sale of a security. We urge you to read carefully this prospectus (as supplemented and amended), together with the information incorporated herein by reference as described under the heading “Incorporation of Certain Information by Reference” before deciding whether to invest in any of the Common Stock being offered.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in this prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Aptevo,” “the Company,” “we,” “us,” “our” and similar references refer to Aptevo Therapeutics Inc., a corporation organized under the laws of the State of Delaware, and its subsidiaries on a consolidated basis.
We are not making an offer or sale of our Common Stock in any jurisdiction where such offer or sale is not permitted. Neither we nor any of our representatives are making any representation to you regarding the legality of an investment in our Common Stock by you under applicable laws. We urge you to consult with your own advisors as to legal, tax, business, financial and related aspects of an investment in our Common Stock.
This prospectus and the information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus and the information incorporated herein by reference are the property of their respective owners.
i
Forward-Looking Statements
This prospectus, any applicable prospectus supplement and any free writing prospectus, including the documents we incorporate by reference herein and therein, contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and involve substantial risks and uncertainties. All statements other than statements of historical fact are forward-looking statements. These statements include, but are not limited to, statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates and any future product candidates, our intellectual property position, the degree of clinical utility of our product candidates, particularly in specific patient populations, our ability to develop and commercialize any product candidates, expectations regarding clinical trial data, statements regarding potential milestone payments, potential partnerships and collaborations, the advancement of our clinical and preclinical trials, our goals and milestones, our expectations regarding the size of the patient populations for our product candidates if approved for commercial use, our expectations regarding the effectiveness of our ADAPTIR and ADAPTIR-FLEX platforms, our ability to utilize any net operating losses, our results of operations, cash needs, financial condition, liquidity, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “might,” “approximately,” “expect,” “predict,” “could,” “potentially” or the negative of these terms or other similar expressions, but the absence of these words does not mean that a statement is not forward looking.
These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or results of operations to differ materially from those expressed or implied by these forward-looking statements. These statements reflect our views with respect to future events as of the time they were made and are based on assumptions and subject to risks and uncertainties. You should read the matters described in “Risk Factors” in this prospectus, in our Annual Report on Form 10-K and in our Quarterly Reports on Form 10-Q which is incorporated by reference into this prospectus and the other cautionary statements made in this prospectus as being applicable to all related forward-looking statements wherever they appear in this prospectus or the documents incorporated by reference into this prospectus. In addition to factors identified under the section titled “Risk Factors” in this prospectus, factors that may impact such forward-looking statements include:
•our plans to develop and commercialize our drug candidates;
•our ability to become profitable;
•our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•our ability to maintain and establish collaborations or obtain additional funding;
•our ability to obtain regulatory approval of current and future drug candidates;
•our expectations regarding our ability to fund operating expenses and capital expenditure requirements with our existing cash and cash equivalents, and future expenses and expenditures;
•our ability to secure sufficient funding and alternative source of funding to support when needed and on terms favorable to us to support our business objective, product development, other operations or commercialization efforts;
•the success of our clinical development activities, clinical trials and research and development programs;
•our ability to retain key employees, consultants and advisors;
•our ability to obtain, maintain, protect and enforce sufficient intellectual property rights for our candidates and technology;
•our anticipated strategies and our ability to manage our business operations effectively;
•the impact of legislative, regulatory or policy changes;
1
•the possibility that we may be adversely impacted by other economic, business, and/or competitive factors; and
•our ability to continue as a going concern.
Should one or more of the risks or uncertainties described in this prospectus occur, additional material risks and uncertainties not currently known to us arise or should our underlying assumptions prove incorrect, actual results and plans could differ materially from those expressed in any forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
2
PROSPECTUS Summary
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the “Risk Factors” section in this prospectus, in our most recent Annual Report on Form 10-K, in any subsequent Quarterly Reports on Form 10-Q and in our other reports filed from time to time with the SEC, as well as our historical financial statements and the notes thereto and the other documents that are incorporated by reference in this prospectus.
Business Overview
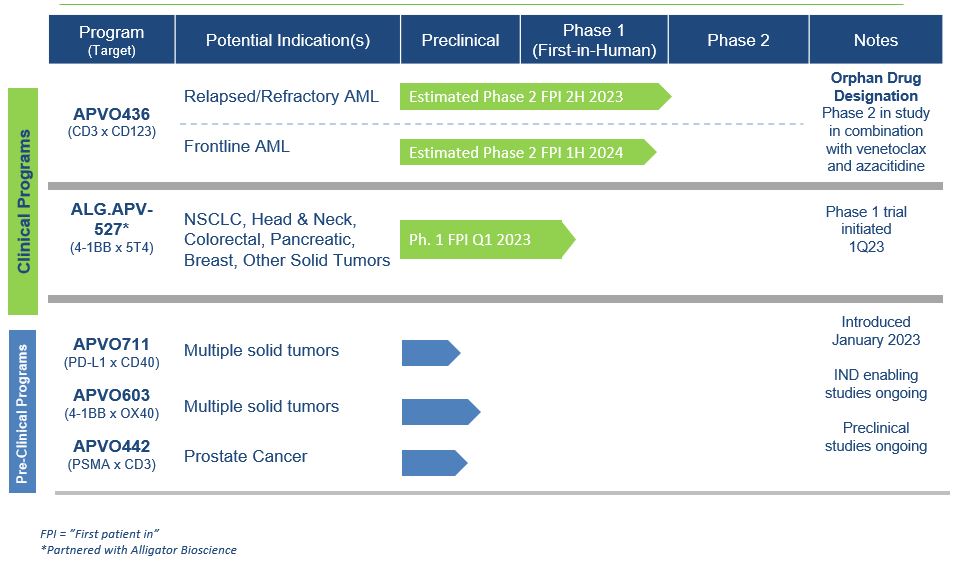
We are a clinical-stage, research and development biotechnology company focused on developing novel immuno-oncology candidates for the treatment of different forms of cancer. We have developed two versatile and enabling platform technologies for rational design of precision immune modulatory drugs. Our lead clinical candidates, APVO436 and ALG.APV-527, and preclinical candidates, APVO603 and APVO711, were developed using our ADAPTIR™ modular protein technology platform. Our preclinical candidate APVO442 was developed using our ADAPTIR-FLEX™ modular protein technology platform.
Our ADAPTIR and ADAPTIR-FLEX platforms are designed to generate monospecific, bispecific, and multi-specific antibody candidates capable of enhancing the human immune system against cancer cells. ADAPTIR and ADAPTIR-FLEX are both modular platforms, which gives us the flexibility to potentially generate immuno-oncology candidates with a variety of mechanisms of action. This flexibility in design allows us to generate novel therapeutic candidates that may provide effective strategies against difficult to treat, as well as advanced forms of cancer. We have successfully designed and constructed numerous investigational-stage product candidates based on our ADAPTIR platform. The ADAPTIR platform technology is designed to generate monospecific and bispecific immuno-oncology proteins that specifically bind to one or more targets, for example, bispecific therapeutic molecules, which may have structural and functional advantages over monoclonal antibodies. The structural differences of ADAPTIR molecules over monoclonal antibodies allow for the development of ADAPTIR immuno-oncology therapies that are designed to engage immune effector cells and disease targets to produce signaling responses that modulate the immune system to kill tumor cells.
We believe we are skilled at candidate generation, validation, and subsequent preclinical and clinical development using the ADAPTIR platform and the ADAPTIR-FLEX platform to generate multi-specific candidates or other candidates to our platform capabilities. We have developed a preclinical candidate based on the ADAPTIR-FLEX platform which is advancing in our pipeline. We are developing our ADAPTIR and ADAPTIR-FLEX molecules using our protein engineering, preclinical development, process development, and clinical development capabilities.
Our Strategy
We seek to grow our business by, among other things:
Advancing our lead clinical stage candidate, APVO436, through clinical development to evaluate its therapeutic potential alone and in combination with other therapies. Based on the positive results from our Phase 1 dose escalation and dose expansion study, we plan to initiate a Phase 2 clinical trial to continue to assess efficacy of APVO436 in combination with Venetoclax and Azacitidine for the treatment of acute myelogenous leukemia (AML). APVO436 is designed to engage CD3 and CD123 to redirect T-cells to destroy leukemia cells expressing the target CD123 molecule on their surface.
Advancing our solid tumor candidate, ALG.APV-527, developed in partnership with Alligator Bioscience AB (Alligator), further in the clinic. Aptevo and Alligator continue to investigate ALG.APV-527 for the treatment of multiple 5T4-tumor expressing antigens in multiple solid tumor indications in a first-in-human Phase I clinical study that started in the first quarter of 2023. We are currently enrolling new patients. ALG.APV-527 targets the 4-1BB co-stimulatory receptor (on T lymphocytes and NK cells) and 5T4 (solid tumor antigen) and is designed to promote anti-tumor immunity.
Continued development and advancement of our preclinical candidates, APVO603 (targeting 4-1BB (CD137) and OX40 (CD134), both members of the TNF-receptor family), APVO442 (targeting Prostate Specific Membrane Antigen (PSMA), a tumor antigen that is highly expressed on prostate cancer cells and CD3), and APVO711 (an anti-PD-L1 x anti-CD40 compound). We continue to advance APVO603 and APVO442 through preclinical and
3
IND-enabling studies. In January 2023, we filed a provisional patent with the U.S. Patent and Trademark Office (USPTO) pertaining to APVO711, with the potential to treat a range of solid malignancies such as head and neck cancer. APVO711 is a dual mechanism bispecific antibody candidate that is designed to provide synergistic stimulation of CD40 on antigen presenting cells while simultaneously blocking the PD-1/PD-L1 inhibitory pathway to potentially promote a robust anti-tumor response. Preclinical studies are planned to further evaluate the mechanism of action and efficacy of APVO711.
Development of novel bispecific and multi-specific proteins for the treatment of cancer using our ADAPTIR and ADAPTIR-FLEX platforms. We have expertise in molecular and cellular biology, immunology, oncology, pharmacology, translational sciences, antibody engineering and the development of protein therapeutics. This includes target validation, preclinical proof of concept, cell line development, protein purification, bioassay and process development and analytical characterization. We focus on product development using our ADAPTIR and ADAPTIR-FLEX platforms. We plan to generate additional monospecific, bispecific, and multi-specific protein immunotherapies for development, potentially with other collaborative partners, to exploit the potential of the ADAPTIR and ADAPTIR-FLEX platforms. We will select novel candidates that have the potential to demonstrate proof of concept early in development. We expect to continue to expand the ADAPTIR and ADAPTIR-FLEX product pipelines to address areas of unmet medical need.
Establishing collaborative partnerships to broaden our pipeline and provide funding for research and development. We intend to pursue collaborations with other biotechnology and pharmaceutical companies, academia, and non-governmental organizations to advance our product portfolio.
Platform Technology and Product Candidates
| | |
Characteristics | Technology |
ADAPTIR | ADAPTIR-FLEX |
Drug Targeting | Bind up to two targets | Bind up to four targets |
Genetic and Structural Format | Single gene that assembles into a homodimer based on an antibody backbone | Two genes that assemble into a heterodimer based on a mutated antibody backbone |
Half-life | Contains Immunoglobulin Gamma 1 Fc Demonstrated antibody-like half-life in mice |
Effector Function | Fc mutations may be utilized to eliminate binding to Fc Gamma Receptors or to enhance effector function |
Manufacturing | Antibody-like manufacturing processes |
Current Pipeline Candidates | APVO436 (CD123 x CD3) ALG.APV-527 (41BB x 5T4) APVO603 (41BB x OX40) APVO711 (PD-L1 x CD40) | APVO442 (PSMA x CD3) |
4
Product Portfolio
Our current product candidate pipeline is summarized in the table below:
Smaller Reporting Company
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. To the extent we qualify as a smaller reporting company, we may continue to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies, including, among other things, providing only two years of audited financial statements and we are also permitted to elect to incorporate by reference information filed after the effective date of the S-3 registration statement of which this prospectus forms a part. We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our shares of Common Stock held by non-affiliates exceeds $250 million as of the prior June 30, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our shares of Common Stock held by non-affiliates exceeds $700 million as of the prior June 30.
Corporate Information
On August 6, 2015, Emergent BioSolutions Inc. (“Emergent”), announced a plan to separate into two independent publicly traded companies. To accomplish this separation, Emergent created Aptevo Therapeutics Inc. (“Aptevo”), to be the parent company for the development-based biotechnology business focused on novel oncology and hematology therapeutics. Aptevo was incorporated in Delaware in February 2016 as a wholly owned subsidiary of Emergent. To effect the separation, Emergent made a pro rata distribution of Aptevo’s Common Stock to Emergent’s stockholders on August 1, 2016.
Our Common Stock currently trades on the Nasdaq under the symbol “APVO.” Our primary executive offices are located at 2401 4th Avenue, Suite 1050, Seattle, Washington and our telephone number is (206) 838-0500. Our website address is www.aptevotherapeutics.com. The information contained in, or that can be accessed through, our website is not a part of or incorporated by reference in this prospectus, and you should not consider it part of this prospectus or of any prospectus supplement. We have included our website address in this prospectus solely as an inactive textual reference.
5
Risks Associated with our Business
Our business is subject to numerous risks, as described under the heading “Risk Factors” and under similar headings in any applicable prospectus supplement, any related free writing prospectus and the documents incorporated by reference herein and therein.
6
The Offering
| |
Shares of Common Stock Offered By the Holders: | Up to 32,026,068 Resale Shares |
| |
Common Stock outstanding prior to this offering | 15,657,772 |
| |
Terms of the offering: | The Holders will determine when and how they will dispose of any shares of Common Stock registered under this prospectus for resale. |
| |
Use of proceeds: | We will not receive any proceeds from the sale of shares of Common Stock by the Holders. |
| |
Risk factors: | Investing in our Common Stock involves significant risks. Before deciding whether to invest in our Common Stock, please read the information contained and incorporated by reference in this prospectus, including under the heading “Risk Factors” on page 13 of this prospectus and under similar headings in any related free writing prospectus and the documents incorporated by reference herein and therein. |
| |
Nasdaq Capital Market Symbol: | “APVO” |
Unless otherwise indicated, the number of shares of Common Stock to be outstanding after this offering is based on 15,657,772 shares of Common Stock outstanding as of November 9, 2023. The number of shares of Common Stock outstanding after this offering excludes:
•269,460 shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $15.12 per share;
•281,534 shares of Common Stock issuable upon the vesting of outstanding restricted stock units at a weighted average fair value per unit of $4.78 per share;
•131,015 shares of Common Stock reserved for future grants of equity-based awards under our equity incentive plans;
•350,589 shares of Common Stock reserved for issuance upon exercise of common warrants outstanding related to our 2019 public raise at a weighted average exercise price of $18.20 per share;
•16,013,034 shares of Common Stock reserved for issuance upon exercise of Series A and Series B Existing Warrants outstanding related to our November 2023 warrant Inducement Agreement at an exercise price of $0.233 per share;
•116,000 shares of Common Stock reserved for issuance upon exercise of Series A and Series B Existing Warrants outstanding related to our August 2023 public offering at an exercise price of $0.62 per share.
7
Description of Capital Stock
As of the date of this prospectus, our certificate of incorporation, authorizes us to issue up to 500,000,000 shares of Common Stock, $0.001 par value per share, and 15,000,000 shares of preferred stock, $0.001 par value per share. Our Common Stock is registered under Section 12(b) of the Exchange Act and is listed on the Nasdaq under the trading symbol “APVO.” As of November 9, 2023, 15,657,772 shares of Common Stock were outstanding and no shares of preferred stock were outstanding.
The following summary describes the material terms of our capital stock. The summary is qualified in its entirety by reference to our certificate of incorporation and our bylaws.
Common Stock
Voting Rights.
Each holder of our Common Stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Under our amended and restated certificate of incorporation and amended and restated bylaws, our stockholders do not have cumulative voting rights. Because of this, the holders of a majority of the shares of Common Stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends.
Subject to preferences that may be applicable to any then-outstanding shares of preferred stock, holders of Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation.
In the event of our liquidation, dissolution or winding up, holders of Common Stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Rights and Preferences.
Each share of Common Stock includes an associated right pursuant to and as set forth in the Rights Agreement that we entered into with Broadridge Corporate Issuer Solutions, Inc. on November 8, 2020 (the “rights agreement”). Each right initially represents the right to purchase from us one one-thousandth of a share of our Series A Junior Participating Preferred Stock, par value $0.001 per share. This right is not exercisable until the occurrence of certain events specified in such rights agreement. The value attributable to these rights, if any, is reflected in the value of our Common Stock. The rights agreement and the rights granted thereunder will expire upon the earliest to occur of (i) the date on which all of such rights are redeemed, (ii) the date on which such rights are exchanged, and (iii) the close of business on November 4, 2023.
Fully Paid and Nonassessable.
All of our outstanding shares of Common Stock are fully paid and nonassessable.
Preferred Stock
Under our certificate of incorporation, our board of directors is authorized by resolution to divide the preferred stock into one or more series and, with respect to each series, to determine the designations, powers, preferences, rights, qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights, redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors can, without stockholder approval but subject to the terms of our
8
certificate of incorporation, issue preferred stock with voting and other rights that could adversely affect the voting power of the holders of our Common Stock and which could have certain anti-takeover effects. Before we may issue any series of preferred stock, our board of directors will be required to adopt resolutions creating and designating such series of preferred stock.
The following summary of terms of our preferred stock is not complete. You should refer to the provisions of our certificate of incorporation, our bylaws and the resolutions containing the terms of each series of preferred stock, which have been or will be filed with the SEC at or prior to the time of issuance of such series and described in the applicable prospectus supplement. The applicable prospectus supplement may also state that any of the terms set forth herein are inapplicable to such series of preferred stock, provided that the information set forth in such prospectus supplement does not constitute a material change to the information herein such that it alters the nature of the offering or the securities being offered.
We will fix the designations, voting powers, preferences and rights of each series of preferred stock that we issue under this prospectus, as well as the qualifications, limitations or restrictions thereof, in the certificate of designation relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference herein from reports that we file with the SEC, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering. We will describe in the applicable prospectus supplement the terms of the series of preferred stock being offered, including, to the extent applicable:
| |
• | the title and stated value; |
• | the number of shares we are offering; |
• | the liquidation preference per share; |
• | the purchase price; |
• | the dividend rate, period and payment date and method of calculation for dividends; |
• | whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
• | the procedures for any auction and remarketing; |
• | the provisions for a sinking fund; |
• | the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
• | any listing of the preferred stock on any securities exchange or market; |
• | whether the preferred stock will be convertible into our Common Stock, and, if applicable, the conversion price, or how it will be calculated, and the conversion period; |
• | whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price, or how it will be calculated, and the exchange period; |
• | voting rights of the preferred stock; |
• | preemptive rights; |
• | restrictions on transfer, sale or other assignment; |
• | whether interests in the preferred stock will be represented by depositary shares; |
• | a discussion of material United States federal income tax considerations applicable to the preferred stock; |
• | the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; |
• | any limitations on the issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and |
• | any other specific terms, preferences, rights or limitations of, or restrictions on, the preferred stock. |
If we issue shares of preferred stock under this prospectus, the shares will be fully paid and non-assessable.
The issuance of preferred stock could adversely affect the voting power of holders of Common Stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation. The issuance could have the effect of decreasing the market price of the Common Stock. The issuance of preferred stock also could have the effect of delaying, deterring or preventing a change in control of us.
9
Outstanding Options, Restricted Stock Units, and Warrants
Unless otherwise indicated, the number of shares of Common Stock to be outstanding after this offering is based on 15,657,772 shares of Common Stock outstanding as of November 9, 2023. The number of shares of Common Stock outstanding after this offering excludes:
•269,460 shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $15.12 per share;
•281,534 shares of Common Stock issuable upon the vesting of outstanding restricted stock units at a weighted average fair value per unit of $4.78 per share;
•131,015 shares of Common Stock reserved for future grants of equity-based awards under our equity incentive plans;
•350,589 shares of Common Stock reserved for issuance upon exercise of common warrants outstanding related to our 2019 public raise at a weighted average exercise price of $18.20 per share.
•16,013,034 shares of Common Stock reserved for issuance upon exercise of Series A and Series B Existing Warrants outstanding related to our November 2023 warrant Inducement Agreement at an exercise price of $0.233 per share;
•116,000 shares of Common Stock reserved for issuance upon exercise of Series A and Series B Existing Warrants outstanding related to our August 2023 public offering at an exercise price of $0.62 per share.
Warrants
Warrants 2019 – As of November 9, 2023, we have issued and outstanding common warrants to purchase 350,589 shares of our Common Stock at an exercise price of $18.20 per share issued as part of our March 2019 public offering. These warrants are immediately exercisable and expire on March 11, 2024. The exercise price and the number of shares of Common Stock purchasable upon the exercise of the warrants are subject to adjustment upon the occurrence of specific events, including sales of additional shares of Common Stock, stock dividends, stock splits, reclassifications and combinations of our Common Stock. If, at any time warrants are outstanding, any fundamental transaction occurs, as described in the warrants, the successor entity must assume the obligations to the warrant holders. Additionally, in the event of a fundamental transaction, other than one in which a successor entity that is a publicly traded corporation assumes the warrants, each holder will have the right to require us, or our successor, to repurchase the warrants for an amount of cash equal to the Black-Scholes value of the remaining unexercised portion of such warrants. Holders of the warrants do not have the rights or privileges of holders of our common shares, including any voting rights, until they exercise their warrants, with exceptions for participation in rights offerings or extraordinary distributions.
Existing Series A and Series B Common Warrants - As of November 9, 2023, we have issued and outstanding Series A common warrants to purchase 8,006,517 shares of our Common Stock and Series B common warrants to purchase 8,006,517 shares of our Common Stock at an exercise price of $0.233 per share as well as Series A common warrants to purchase 58,000 shares of our Common Stock and Series B common warrants to purchase 58,000 shares of our Common Stock at an exercise price of $0.62 per share. Each Series A Common Warrant may be exercised at any time following the date of issuance and from time to time thereafter through and including the five year anniversary of the initial exercise date. Each Series B Common Warrant may be exercised at any time following the date of issuance and from time to time thereafter through and including the eighteen month anniversary of the initial exercise date.
The exercise price and the number of shares of Common Stock purchasable upon the exercise of the warrants are subject to adjustment upon the occurrence of specific events, including sales of additional shares of Common Stock, stock dividends, stock splits, reclassifications and combinations of our Common Stock. If, at any time warrants are outstanding, any fundamental transaction occurs, as described in the warrants, the successor entity must assume the obligations to the warrant holders. Additionally, in the event of a fundamental transaction, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
10
Holders of the warrants do not have the rights or privileges of holders of our common shares, including any voting rights, until they exercise their warrants, with exceptions for participation in rights offerings or extraordinary distributions.
Certain Anti-Takeover Provisions of Our Certificate of Incorporation, Our Bylaws, the DGCL and our Rights Plan
Delaware Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| | |
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (1) by persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines a “business combination” to include the following:
| | |
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Staggered Board; Removal of Directors. Our amended and restated certificate of incorporation provides for our board of directors to be divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, stockholders holding a majority of the shares of Common Stock outstanding are able to elect all of our directors. Our certificate of incorporation and our bylaws also provide that directors may be removed by the stockholders only for cause upon the vote of 75% of our outstanding Common Stock. Furthermore, the authorized number of directors may be changed only by resolution of the board of directors, and vacancies and newly created directorships on the board of directors may, except as otherwise required by law or determined by the board, only be filled by a majority vote of the directors then serving on the board, even though less than a quorum.
Stockholder Action by Written Consent. Our amended and restated certificate of incorporation and amended and restated bylaws also provide that all stockholder actions must be effected at a duly called meeting of stockholders and eliminates the right of stockholders to act by written consent without a meeting. Our amended and restated bylaws also provide that only our chairman of the board, chief executive officer or the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors may call a special meeting of stockholders.
11
Requirements for Advance Notification of Stockholder Nominations, Proposals and Amendments. Our amended and restated bylaws also provide that stockholders seeking to present proposals before a meeting of stockholders to nominate candidates for election as directors at a meeting of stockholders must provide timely advance notice in writing, and specify requirements as to the form and content of a stockholder’s notice. Our certificate of incorporation and bylaws provide that the stockholders cannot amend many of the provisions described above except by a vote of 75% or more of our outstanding Common Stock.
Shareholder Rights Plan. On November 8, 2020, our board of directors adopted a rights plan pursuant to our rights agreement. The rights plan works by causing substantial dilution to any person or group that acquires beneficial ownership of ten percent (10%) or more of our Common Stock without the approval of our board of directors. As a result, the overall effect of the rights plan and the issuance of the rights pursuant to the rights plan may be to render more difficult or discourage a merger, tender or exchange offer or other business combination involving the Company that is not approved by our board of directors. The rights plan is not intended to interfere with any merger, tender or exchange offer or other business combination approved by our board of directors. The rights plan also does not prevent our board of directors from considering any offer that it considers to be in the best interest of our stockholders. On November 2, 2023, we entered into Amendment No. 3 to the Rights Agreement and extended the expiration of such agreement to November 4, 2024 and changed the exercise price to $2.02 per one one-thousandth of a Series A Junior Participating Preferred Share, subject to adjustment.
Certain Anti-Takeover Effects. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Broadridge, which can be contacted at 51 Mercedes Way, Edgewood, NY 11717, shareholder@broadridge.com, or +1 (720) 378-5591.
Listing on the Nasdaq Capital Market
Our Common Stock is listed on the Nasdaq Capital Market under the symbol “APVO.”
12
Risk Factors
An investment in our securities involves a significant degree of risk. You should carefully consider the risk factors and all of the other information included in this prospectus and the documents we have incorporated by reference into this prospectus, including those in “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and our Quarterly Report on Form 10-Q for the periods ended March 31, 2023, June 30, 2023 and September 30, 2023, incorporated herein by reference, before making an investment decision. Any of these risks and uncertainties could have a material adverse effect on our business, financial condition, cash flows and results of operations. If that occurs, the trading price of our Common Stock could decline materially, and you could lose all or part of your investment.
The risks included in this prospectus and the documents we have incorporated by reference into this prospectus are not the only risks we face. We may experience additional risks and uncertainties not currently known to us, or as a result of developments occurring in the future. Conditions that we currently deem to be immaterial may also materially and adversely affect our business, financial condition, cash flows and results of operations, and our ability to pay distributions to stockholders. Please also read carefully the section above entitled “Forward-Looking Statements.”
Risks Related to This Offering
The number of shares being registered for resale is significant in relation to the number of our outstanding shares of Common Stock.
We have filed a registration statement of which this prospectus is a part to register the shares offered hereunder for sale into the public market by the selling stockholder. These shares represent a large number of shares of our Common Stock, and if sold in the market all at once or at about the same time, could depress the market price of our Common Stock during the period the registration statement remains effective and could also affect our ability to raise equity capital.
You may also experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional common shares or other securities convertible into or exchangeable for our common shares that could result in further dilution to the investors purchasing our common shares in this offering or result in downward pressure on the price of our common shares. We may sell our common shares or other securities in any other offering at prices that are higher or lower than the prices paid by the investors in this offering, and the investors purchasing shares or other securities in the future could have rights superior to existing shareholders. Moreover, to the extent that we issue subscription rights, options or warrants to purchase, or securities convertible into or exchangeable for, our common shares in the future and those subscription rights, options, warrants or other securities are exercised, converted or exchanged, stockholders may experience further dilution.
13
USE OF PROCEEDS
We will not receive any of the proceeds from the sale or other disposition of the Resale Shares held by the Holders pursuant to this prospectus.
SELLING STOCKHOLDERS
We have prepared this prospectus to allow the Holders to sell or otherwise dispose of, from time to time, up to 32,026,068 Resale Shares.
On November 9, 2023, we entered into the Warrant Inducement Agreement with the Holders, who are the holders of certain Existing Warrants. Pursuant to the Warrant Inducement Agreement, the selling stockholder agreed to exercise for cash their Existing Warrants to purchase up to 16,013,034 shares of our Common Stock at an exercise price of $0.233 per share, the exercise price per share of the Existing Warrants, during the period from the date of the Warrant Inducement Agreement until 7:30 a.m., Eastern Time, on December 9, 2023.
In consideration of the Holders’ agreement to exercise the Existing Warrants in accordance with the Warrant Inducement Agreement, we agreed to issue the New Warrants to purchase shares of Common Stock equal to 200% of the number of shares of Common Stock issued upon exercise of the Existing Warrants. We agreed in the Warrant Inducement Agreement to register for resale by the Holders the Resale Shares underlying the New Warrants and have filed with the SEC a registration statement on Form S-3, of which this prospectus forms a part, with respect to the resale or other disposition of the securities offered from time to time by the Holders under this prospectus. We will not receive any of the proceeds of sales by the holder of any of the Resale Shares covered by this prospectus.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to shares of our Common Stock. Unless otherwise indicated below, to our knowledge, the Holders named in the table below have sole voting and investment power with respect to the shares of Common Stock beneficially owned by them. The number of shares of Common Stock beneficially owned prior to the offering for each Holder includes (i) all shares of our Common Stock held by such Holder prior to the transactions contemplated by the Warrant Inducement Agreement plus (ii) all shares of our Common Stock (or pre-funded warrants in lieu of) issued to such Holder pursuant to our August 4, 2023 public offering, as well as (iii) all shares of our Common Stock that may be received by such Holder pursuant to the transactions contemplated by the Warrant Inducement Agreement and being offered pursuant to the prospectus. The inclusion of any shares in this table does not constitute an admission of beneficial ownership by the person named below.
The beneficial ownership information presented in the table below is as of November 9, 2023. The Holders may have sold or transferred, in transactions exempt from the registration requirements of the Securities Act, some or all of their shares of Common Stock since the date on which the information in the table below is presented. Information about the Holders may change over time.
| | | | | | | | | | | | | | | | |
Name of Holder | | Number of Common Shares Beneficially Owned Prior to Offering (1) | | | Maximum Number of Common Shares to be Sold Pursuant to this Prospectus (2) | | | Number of Common Shares Beneficially Owned After Offering (3) | | | Percentage Beneficially Owned After Offering (3) | |
Armistice Capital, LLC (4) | | | 9,218,870 | | | | 15,967,740 | | | | - | | | | - | |
Sabby Management, LLC (5) | | | 9,783,265 | | | | 14,516,128 | | | | - | | | | - | |
Brio Capital Master Fund, Ltd.(6) | | | 1,329,313 | | | | 1,542,200 | | | | - | | | | - | |
(1)The beneficial ownership prior to this offering number for each investor includes the following:
a.Armistice Capital, LLC: 1,235,000 Common Stock held by the Holder as of September 30, 2023, per the Holder's Form 13F filed on November 14, 2023, and 7,983,870 shares of Common Stock underlying the Existing Warrants issued as part of our August 4, 2023, public offering.
14
b.Sabby Management, LLC: 1,334,169 Common Stock held by the Holder as of September 30, 2023, per the Holder's Form 13F filed on November 14, 2023, 1,191,032 remaining pre-funded warrants (with 9.99% blocker) and 7,258,064 shares of Common Stock underlying the Existing Warrants issued as part of our August 4, 2023 public offering.
c.Brio Capital Master Fund, Ltd.: 558,213 Common Stock held by the Holder, including shares of our Common Stock issued to the Holder as part of our August 4, 2023 public offering and 771,100 shares of Common Stock underlying the Existing Warrants.
(2) The warrants, including the Resale Shares, are subject to a beneficial ownership limitation of 4.99% or 9.99%, as applicable, which such limitation restricts the Holders from exercising that portion of the warrants that would result in the Holders and its affiliates owning, after exercise, a number of shares of common stock in excess of the beneficial ownership limitation.
(3) Because the Selling Stockholders may sell, transfer or otherwise dispose of all, some or none of the common shares covered by this prospectus, we cannot determine the number of such common shares that will be sold, transferred or otherwise disposed of by the Selling Stockholders, or the amount or percentage of our common shares that will be held by the Selling Stockholders upon completion of this offering. For purposes of this table, we have assumed that the Selling Stockholders will sell all their common shares covered by this Prospectus, including common shares issuable upon exercise of the Existing Warrants and the remaining pre-funded warrants issued in connection to our August 4, 2023, public offering.
(4) The securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial ownership limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of the warrants that would result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of common stock in excess of the beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. is c/o Armistice Capital, LLC, 510 Madison Avenue, 7th Floor, New York, NY 10022.
(5) Sabby Management, LLC is the investment manager of Sabby Volatility Warrant Master Fund, Ltd. and shares voting and investment power with respect to these shares in this capacity. As manager of Sabby Management, LLC, Hal Mintz also shares voting and investment power on behalf of Sabby Volatility Warrant Master Fund, Ltd. Each of Sabby Management, LLC and Hal Mintz disclaims beneficial ownership over the securities listed except to the extent of their pecuniary interest therein.
(6) Shaye Hirsch has voting and investment control over the securities held by Brio Capital Master Fund, Ltd. Brio Capital Master Fund, Ltd.’s address is 100 Merrick Road, Suite 401W, Rockville Centre, NY 11570.
Certain Relationships and Related Party Transactions
On November 9, 2023, we entered into the Warrant Inducement Agreement with the Holders, who are the holders of the Existing Warrants. Pursuant to the Warrant Inducement Agreement, the Holders agreed to exercise for cash their Existing Warrants to purchase up to 16,013,034 shares of our Common Stock at an exercise price of $0.233 per share, the exercise price per share of the Existing Warrants, during the period from the date of the Warrant Inducement Agreement until 7:30 a.m., Eastern Time, on December 9, 2023.
The aggregate gross proceeds to be received by us will depend on the number of Existing Warrants actually exercised by the Holders. If all of the Existing Warrants are exercised in connection with the Warrant Inducement Agreement, we would anticipate receiving aggregate gross proceeds of up to approximately $3.7 million from the exercise of the Existing Warrants before deducting financial advisory fees and other expenses payable by us. There is, however, no guarantee that all of the Existing Warrants will be exercised by the Holders in accordance with the Warrant Inducement Agreement. Upon the exercise of the Existing Warrants by the Holders in accordance with the Warrant Inducement Agreement, we agreed to issue the New Warrants to purchase shares of Common Stock equal to 200% of the number
15
of shares of Common Stock issued upon exercise of the Existing Warrants. We would anticipate receiving aggregate gross proceeds of up to approximately $7.5 million if the New Warrants are issued and exercised.
Pursuant to the Warrant Inducement Agreement, we agreed to file a registration statement on Form S-3 (or other appropriate form if the Company is not eligible to use Form S-3) to register the resale of the Resale Shares upon exercise of the New Warrants by November 27, 2023, and to use commercially reasonable efforts to have such registration statement declared effective by the SEC within ninety (90) days following the date of filing the registration statement and to keep the registration statement effective at all times until no holder of the New Warrants owns any New Warrants or Resale Shares. In the event that the Company fails to timely deliver to the holder the Resale Shares without restrictive legends, the Company agreed to pay certain liquidated damages to the Holder. We are registering the shares to be sold by the Holders under the registration statement of which this prospectus is a part to satisfy our obligation under the Warrant Inducement Agreement.
On August 1, 2023, we entered into a Securities Purchase Agreement (the “August 2023 Purchase Agreement”) with the Holders, pursuant to which we issued and sold to the Holders (i) 2,163,550 shares of Common Stock, (ii) pre-funded warrants to purchase up to an aggregate of 5,842,967 shares of Common Stock (the “August 2023 Pre-funded Warrants”), and (iii) Series A and Series B common warrants to purchase up to an aggregate of 16,129,034 shares of Common Stock (the “August 2023 Common Warrants” and together with the Pre-funded Warrants, the “August 2023 Warrants”). The Existing Warrants that are subject to the Warrant Inducement Agreement consist of the August 2023 Warrants.
16
Plan Of Distribution
The Holders, and its pledgees, donees, transferees or other successors in interest, may from time to time offer and sell, separately or together, some or all of the Resale Shares covered by this prospectus. Registration of the Resale Shares covered by this prospectus does not mean, however, that those Resale Shares necessarily will be offered or sold.
The Resale Shares covered by this prospectus may be sold from time to time, at market prices prevailing at the time of sale, at prices related to market prices, at a fixed price or prices subject to change or at negotiated prices, by a variety of methods including the following:
| | |
| • | in the Nasdaq Capital Market; |
| | |
| • | in privately negotiated transactions; |
| | |
| • | through broker-dealers, who may act as agents or principals; |
| | |
| • | through one or more underwriters on a firm commitment or best-efforts basis; |
| | |
| • | in a block trade in which a broker-dealer will attempt to sell a block of securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| | |
| • | directly to one or more purchasers; |
| | |
| • | in any combination of the above. |
In effecting sales, brokers or dealers engaged by the Holders may arrange for other brokers or dealers to participate. Broker-dealer transactions may include:
| | |
| • | purchases of the Resale Shares by a broker-dealer as principal and resales of the Resale Shares by the broker-dealer for its account pursuant to this prospectus; |
| | |
| • | ordinary brokerage transactions; or |
| | |
| • | transactions in which the broker-dealer solicits purchasers on a best efforts basis. |
To our knowledge, the Holders have not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the sale of the Resale Shares covered by this prospectus. At any time a particular offer of the securities covered by this prospectus is made, a revised prospectus or prospectus supplement, if required, will be distributed which will set forth the aggregate amount of Resale Shares covered by this prospectus being offered and the terms of the offering, including the name or names of any underwriters, dealers, brokers or agents. In addition, to the extent required, any discounts, commissions, concessions and other items constituting underwriters’ or agents’ compensation, as well as any discounts, commissions or concessions allowed or reallowed or paid to dealers, will be set forth in such revised prospectus supplement. Any such required prospectus supplement, and, if necessary, a post-effective amendment to the registration statement of which this prospectus is a part, will be filed with the SEC to reflect the disclosure of additional information with respect to the distribution of the securities covered by this prospectus.
17
Legal Matters
The validity of the securities being offered by this prospectus will be passed upon by Paul Hastings LLP, Washington, DC.
Experts
Our consolidated financial statements as of December 31, 2022 and 2021, and for the years then ended, incorporated in this prospectus by reference and included in our Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Moss Adams LLP, an independent registered public accounting firm, as set forth in their report therein. Such consolidated financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
Where You Can Find More Information
This prospectus is part of a registration statement on Form S-3 we filed with the SEC under the Securities Act and does not contain all the information set forth in the registration statement. Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated by reference therein. For further information with respect to us and the common stock we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. Neither we nor any agent, underwriter or dealer has authorized any person to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered by this prospectus.
We are subject to the informational requirements of the Securities Exchange Act and are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. Any information we file with the SEC, including the documents incorporated by reference into this prospectus, is also available on the SEC’s website at www.sec.gov. We also make these documents publicly available, free of charge, on our website at www.aptevotherapeutics.com as soon as reasonably practicable after filing such documents with the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
18
Incorporation of Certain Information by Reference
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring you to those documents instead of having to repeat the information in this prospectus. The information incorporated by reference is considered to be part of this prospectus and later information that we file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings (including those made after the initial filing of the registration statement of which this prospectus is a part and prior to the effectiveness of such registration statement) we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act until the termination of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
| | |
| • | our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on March 30, 2023; |
| • | our Quarterly Reports on Form 10-Q filed with the SEC on May 11, 2023, August 10, 2023 and November 14, 2023; |
| • | our definitive proxy statement on Schedule 14A, filed with the SEC on April 18, 2023; |
| • | our Current Reports on Form 8-K filed with the SEC on January 9, 2023, February 13, 2023, March 7, 2023, March 30, 2023, May 11, 2023, June 2, 2023, July 18, 2023, August 7, 2023, September 15, 2023, November 3, 2023, and November 9, 2023; and |
| • | the description of our Common Stock contained in Exhibit 4.5 to our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on March 30, 2023, including any amendment or report filed for the purpose of updating such description. |
We also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, after the date of the initial filing of the registration statement of which this prospectus is a part and before the effective date of the registration statement and after the date of this prospectus until the termination of the offering. Any statements contained in a previously filed document incorporated by reference into this prospectus is deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, or in a subsequently filed document also incorporated by reference herein, modifies or supersedes that statement.
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, at no cost, upon written or oral request, a copy of any or all of the reports or documents that have been incorporated by reference in the prospectus contained in the registration statement but not delivered with the prospectus. You should direct requests for documents to:
Aptevo Therapeutics Inc.
2401 4th Avenue, Suite 1050
Seattle, WA 98121
Attn: General Counsel
(206) 838-0500
19
Up to 32,026,068 Shares of Common Stock Issuable Upon the Exercise of Common Warrants

________________________
Preliminary Prospectus
________________________
November 22, 2023
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following sets forth the estimated costs and expenses, all of which shall be borne by the registrant, in connection with the offering of the securities pursuant to this Registration Statement:
| | | | |
SEC registration fee | $ | | 950 | |
Legal fees and expenses (1) | | | 25,000 | |
Accounting Fees (1) | | | 10,000 | |
Printing and Miscellaneous Fees (1) | | | 2,000 | |
Total | $ | | 37,950 | |
|
(1) The fees in the above table are estimated as permitted under Item 511 of Regulation S-K. |
Item 15. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law, or the DGCL, authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act.
The registrant’s certificate of incorporation and bylaws each provide for indemnification of the registrant’s directors, officers, employees and other agents to the maximum extent permitted by the DGCL.
The registrant has entered into indemnification agreements with its directors and officers whereby it has agreed to indemnify its directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of the registrant, provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interest of the registrant. At present, there is no pending litigation or proceeding involving a director or officer of the registrant regarding which indemnification is sought, nor is the registrant aware of any threatened litigation that may result in claims for indemnification.
The registrant maintains insurance policies that indemnify its directors and officers against various liabilities arising under the Securities Act and the Securities Exchange Act of 1934, as amended, that might be incurred by any director or officer in his or her capacity as such.
Item 16. Exhibits.
(a)
Exhibit Index
| | | | | | | |
| | | | | | | |
Exhibit Number | | Description | Form | Exhibit | Filing Date | File No. | Filed Herewith |
| | | | | | | |
3.1 | | Amended and Restated Certificate of Incorporation of Aptevo Therapeutics Inc. | 8-K | 3.1 | August 2, 2016 | 001-37746 | |
| | | | | | | |
3.2 | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of Aptevo Therapeutics Inc. | 8-K | 3.1 | March 27, 2020 | 001-37746 | |
| | | | | | | |
3.3 | | Certificate of Designation of Series A Junior Participating Preferred Stock of Aptevo Therapeutics Inc. | 8-K | 3.1 | November 9, 2020 | 001-37746 | |
| | | | | | | |
3.4 | | Amended and Restated Bylaws of Aptevo Therapeutics Inc. | 10-Q | 3.1 | November 10, 2022 | 001-37746 | |
| | | | | | | |
4.1 | | Form of Common Stock Certificate | 10 | 4.1 | June 29, 2016 | 001-37746 | |
| | | | | | | |
4.2 | | Rights Agreement, dated as of November 8, 2020, by and between Aptevo Therapeutics Inc. and Broadridge Corporate Issuer Solutions, Inc., as rights agent | 8-K | 4.1 | November 9, 2020 | 001-37746 | |
| | | | | | | |
4.3 | | Amendment No. 1 to Right Agreement, dated as of November 5, 2021, between the Company and Broadridge Corporate Issuer Solutions, Inc., as Rights Agent | 8-K | 4.1 | November 5, 2021 | 001-37746 | |
| | | | | | | |
4.4 | | Amendment No. 2 to Rights Agreement, dated as of November 4, 2022, between the Company and Broadridge Corporate Issuer Solutions, Inc., as Rights Agent. | 8-K | 4.1 | November 4, 2022 | 001-37746 | |
| | | | | | | |
4.5 | | Amendment No. 3 to Rights Agreement, dated as of November 2, 2023, between the Company and Broadridge Corporate Issuer Solutions, Inc., as Rights Agent | 8-K | 4.1 | November 3, 2023 | 001-37746 | |
| | | | | | | |
4.6 | | Description of Capital Stock of Aptevo Therapeutics | | 4.5 | March 31, 2021 | 001-37746 | |
| | | | | | | |
4.7 | | Form of Pre-Funded Warrant | S-1/A | 4.9 | July 14, 2023 | 333-37746 | |
| | | | | | | |
4.8 | | Form of Common Warrant | S-1/A | 4.10 | July 14, 2023 | 333-37746 | |
4.9 | | Form of New Series A-1 Warrant | 8-K | 4.1 | November 9, 2023 | 001-37746 | |
4.10 | | Form of New Series A-2 Warrant | 8-K | 4.2 | November 9, 2023 | 001-37746 | |
4.11 | | Form of New Series B-1 Warrant | 8-K | 4.3 | November 9, 2023 | 001-37746 | |
4.12 | | Form of New Series B-2 Warrant | 8-K | 4.4 | November 9, 2023 | 001-37746 | |
4.13 | | Form of Warrant Inducement Agreement, by and between the Company and each Holder | 8-K | 10.1 | November 9, 2023 | 001-37746 | |
4.14 | | Financial Advisory Agreement, dated as of November 9, 2023, between A.G.P./Alliance Global Partners and the Company | 8-K | 10.2 | November 9, 2023 | 001-37746 | |
| | | | | | | |
5.1 | | Opinion of Paul Hastings LLP | | | | | X |
| | | | | | | |
Item 17. Undertakings
The undersigned registrant hereby undertakes:
(a) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (i), (ii) and (iii) of this section do not apply if the registration statement is on Form S-3 or Form F-3 and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(b) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(e) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following
communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(f) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(g) That, for purposes of determining any liability under the Securities Act, (i) the information omitted from the form of prospectus filed as part of the registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(l) or (4) or 497(h) under the Securities Act shall be deemed to be a part of the registration statement as of the time it was declared effective; and (ii) each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Seattle, Washington, on November 22, 2023.
| | |
| Aptevo Therapeutics Inc. |
| | |
| By: | /s/ Marvin L. White |
| | Marvin L. White |
| | President and Chief Executive Officer |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Marvin L. White and Daphne Taylor, his or her true and lawful agent, proxy and attorney-in-fact, each acting alone, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to (i) act on, sign, and file with the SEC any and all amendments (including post-effective amendments) to this registration statement together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act, and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
| | | | |
Signatures | | Title | | Date |
| | | | |
/s/ Marvin L. White | | President, Chief Executive Officer and Director (Principal Executive Officer) | | November 22, 2023 |
Marvin L. White | | | | |
| | | | |
/s/ Daphne Taylor | | Chief Financial Officer (Principal Financial and Accounting Officer) | | November 22, 2023 |
Daphne Taylor | | | | |
| | | | |
/s/ John E. Niederhuber, M.D. | | Chairman of the Board of Directors | | November 22, 2023 |
John E. Niederhuber, M.D. | | | | |
| | | | |
/s/ Daniel J. Abdun-Nabi | | Director | | November 22, 2023 |
Daniel J. Abdun-Nabi | | | | |
| | | | |
/s/ Grady Grant, III | | Director | | November 22, 2023 |
Grady Grant, III | | | | |
| | | | |
/s/ Zsolt Harsanyi, Ph. D. | | Director | | November 22, 2023 |
Zsolt Harsanyi, Ph. D. | | | | |
| | | | |
/s/ Barbara Lopez Kunz | | Director | | November 22, 2023 |
Barbara Lopez Kunz | | | | |


