The following summary highlights selected information contained elsewhere in this prospectus and does not contain all of the information that you need to consider in making your investment decision. We urge you to read this entire prospectus, including the more detailed consolidated financial statements, notes to the consolidated financial statements and other information incorporated by reference from our other filings with the SEC, or included in any applicable prospectus supplement. Investing in our securities involves risks. Therefore, carefully consider the risk factors set forth in any prospectus supplements and in our most recent filings with the SEC including our Annual Reports on Form 20-F and reports on Form 6-K, as well as other information in this prospectus and any prospectus supplements and the documents incorporated by reference herein or therein, before purchasing our securities. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
Company Overview
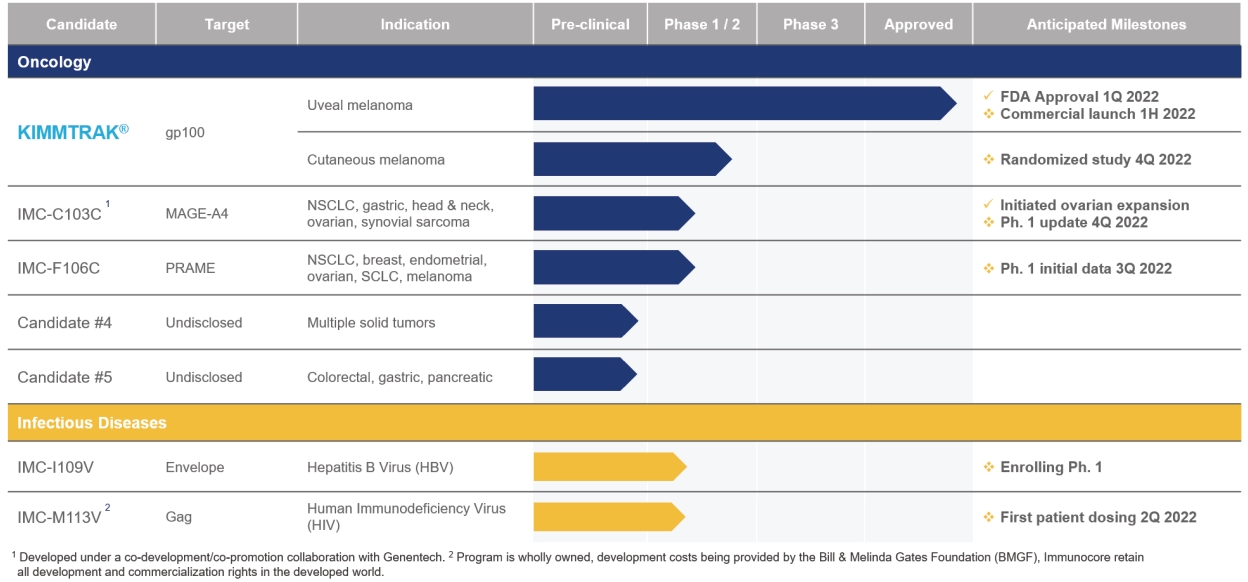
We are a commercial-stage biotechnology company pioneering the development of a novel class of TCR bispecific immunotherapies called ImmTAX - Immune mobilizing monoclonal TCRs Against X disease - designed to treat a broad range of diseases, including cancer, infectious and autoimmune. Leveraging our proprietary, flexible, off-the-shelf ImmTAX platform, we are developing a deep pipeline in multiple therapeutic areas, including five clinical stage programs in oncology and infectious disease, advanced pre-clinical programs in autoimmune disease and multiple earlier pre-clinical programs. Our lead product, KIMMTRAK, is a first-in-class TCR therapeutic approved for the treatment of patients with unresectable or metastatic uveal melanoma, or mUM, in the United States.
We have three clinical stage programs within our ImmTAC (Immune mobilizing monoclonal TCRs Against Cancer) platform, including KIMMTRAK. Our clinical programs are being conducted with patients with a broad range of cancers including melanoma, lung, gastric, head and neck and ovarian, among others. As of December 31, 2021, we have dosed over 700 cancer patients with our ImmTAX product candidates, which we believe is the largest clinical data set of any bispecific in a solid tumor and any TCR therapeutic. Our other ImmTAX product candidates have the potential to address other tumor types with larger addressable patient bispecific therapeutic populations and significant unmet need, and we are studying the application of our ImmTAX platform to infectious diseases and autoimmune conditions.
Our Lead Product - KIMMTRAK
On January 26, 2022, we announced the U.S. Food and Drug Administration, or the FDA, approved KIMMTRAK (tebentafusp-tebn) for the treatment of patients with unresectable or mUM. KIMMTRAK is the first TCR therapeutic to receive regulatory approval from the FDA, the first bispecific T cell engager to receive regulatory approval from the FDA to treat a solid tumor, and the first and only therapy for the treatment of unresectable or mUM to be approved by the FDA.
On April 1, 2022 the European Commission approved KIMMTRAK for the treatment of HLA-A*02:01-positive adult patients with unresectable or mUM. The European Commission approval follows a positive opinion by the Committee for Medicinal Products for Human Use, or CHMP, in February 2022. The CHMP recommendation of KIMMTRAK is based on the results of Immunocore’s Phase 3 IMCgp100-202 clinical trial. The European Commission has the authority to approve medicines for use throughout the European Union, and in Iceland, Liechtenstein, and Norway following completion of related national procedures. We anticipate launching KIMMTRAK in Europe in the second quarter of 2022. We also intend to continue to support patients through our global early access program, which allows us to offer KIMMTRAK as a treatment to mUM patients as regulatory approval is sought in the European Union and their respective European countries. As of December 31, 2021, there were over 200 mUM patients enrolled in the program.
In addition, the United Kingdom’s Medicines and Healthcare Regulatory Agency, or MHRA, Health Canada, and Australia’s Department of Health Therapeutic Goods Administration, or TGA, have each accepted the submission of our Marketing Authorisation Application, or MAA.
KIMMTRAK is manufactured at facilities located in Denmark and Germany. We are supporting the appropriate use of KIMMTRAK in the United States through a well-equipped and fit-for-purpose commercial team that includes medical, sales, and value access team members. We utilize a hybrid model that includes in-house and