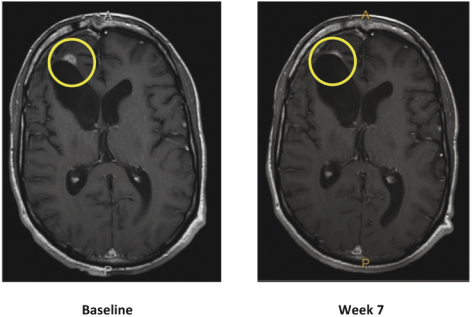
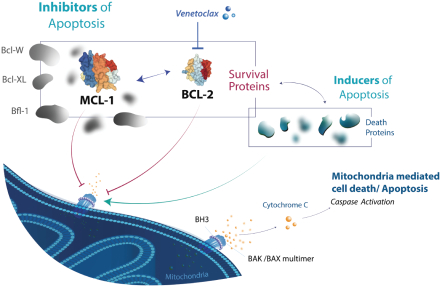
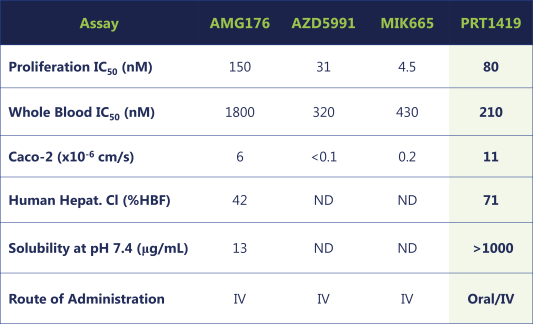
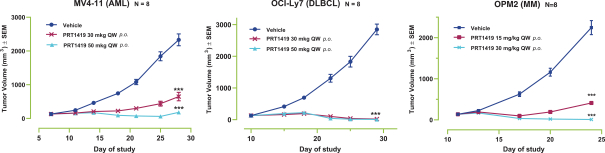
compounds that degrade SMARCA2. A U.S. patent directed to PRT543 has issued and is expected to expire no earlier than August 9, 2038. In addition, a U.S. patent directed to PRT811 has issued and is expected to expire no earlier than March 14, 2039.
In addition to our filings in the United States, we own patent applications that are pending in Australia, Brazil, Canada, China, Eurasia, Europe, Israel, Hong Kong, India, Japan, Korea, Mexico, New Zealand, Ukraine, and South Africa. Included in these applications are claims directed to the PRT543 composition and methods of using the same therapeutically. The patents from these applications, if issued, are expected to expire in August 2038, subject to any disclaimers or extensions.
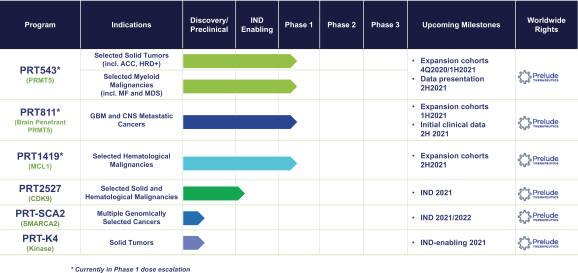
The patent portfolios for our most advanced programs are summarized below.
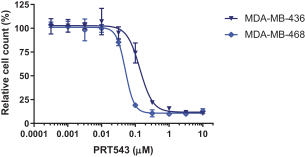
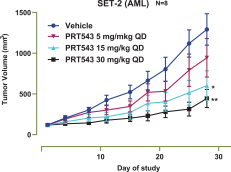
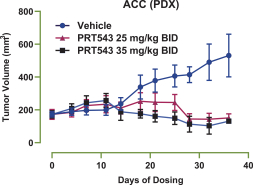
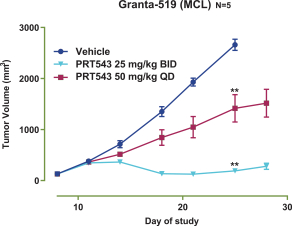
PRT543
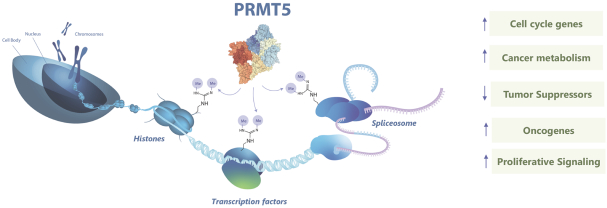
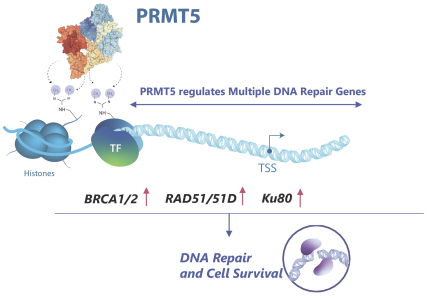
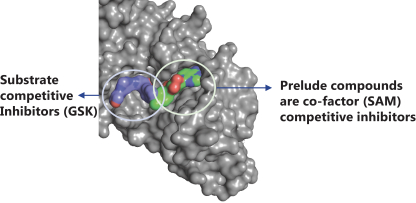
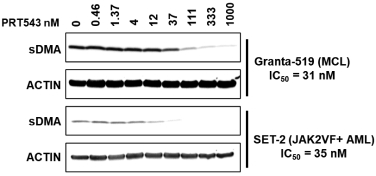
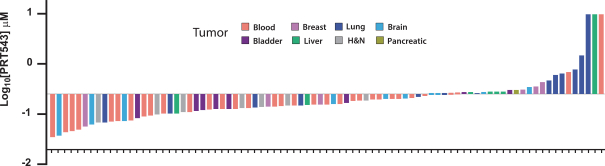
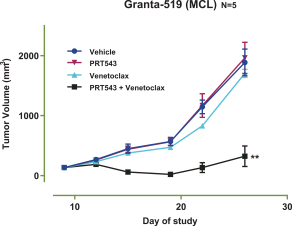
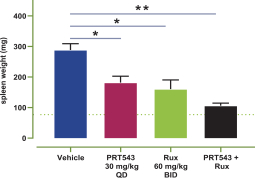
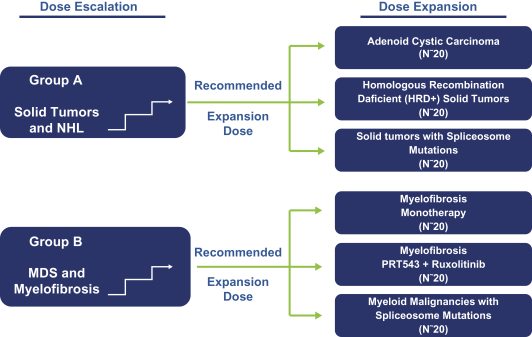
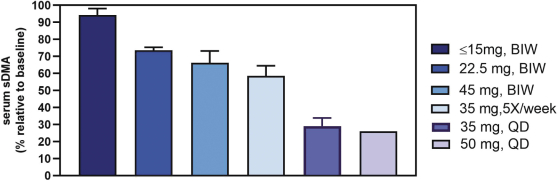
Our PRT543 patent portfolio is wholly owned by us. The portfolio includes one issued U.S. patent, which claims, among other things, PRT543, pharmaceutical compositions comprising PRT543, methods of inhibiting PRMT5 using PRT543, and methods of treating certain cancers, including breast and ovarian cancers, using PRT543. This U.S. patent is expected to expire no earlier than August 9, 2038, subject to any disclaimers or extensions available under the Hatch-Waxman Act. Corresponding patent applications are pending in several other countries and regions, including Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, Ukraine, and South Africa. Any patents resulting from these patent applications, if issued, are also expected to expire no earlier than August 9, 2038, subject to any disclaimers or extensions.
The PRT543 patent portfolio also includes three pending U.S. and two pending PCT patent applications, which claim, among other things, a genus of compounds that encompass PRT543, PRT543 salts and crystalline forms, methods of preparing PRT543, and additional methods of treatment using PRT543. Any U.S. patents issuing from these applications would be expected to expire no earlier than August 9, 2038, February 13, 2040, April 3, 2040, and December 10, 2041 respectively, subject to any disclaimers or extensions.
PRT811
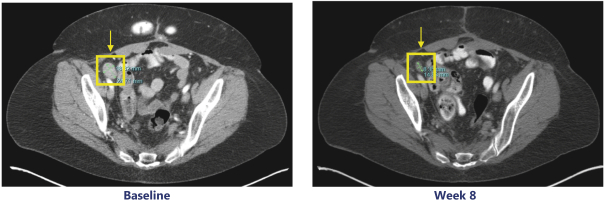
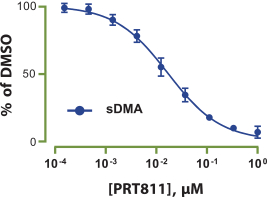
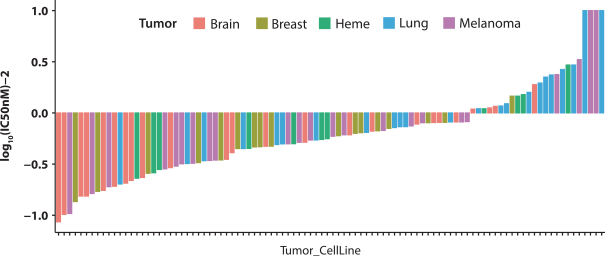
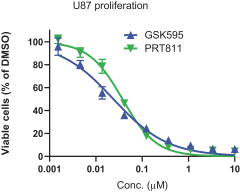
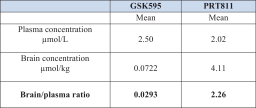
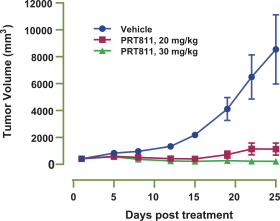
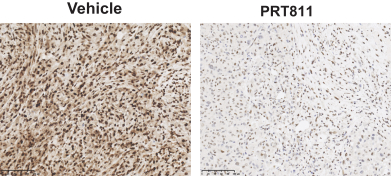
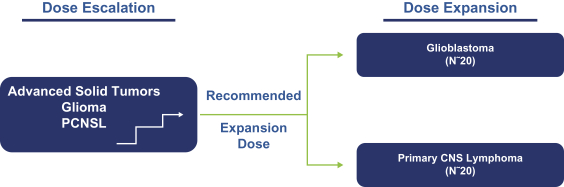
Our PRT811 patent portfolio is wholly owned by us. The portfolio includes one issued U.S. patent, which claims, among other things, PRT811, pharmaceutical compositions comprising PRT811, methods of inhibiting PRMT5 using PRT811, and methods of treating certain cancers, including glioblastoma, using PRT811. The patent is expected to expire no earlier than March 14, 2039, subject to any disclaimers or extensions available under the Hatch-Waxman Act. A related PCT application was filed, and corresponding national phase applications were filed in Australia, Brazil, Canada, China, Eurasia, Europe, India, Israel, Japan, Korea, Mexico, New Zealand, Ukraine and South Africa. Any patents resulting from these national patent applications, if issued, are expected to expire no earlier than March 14, 2039, subject to any disclaimers or extensions.
The PRT811 patent portfolio also includes two pending U.S. non-provisional applications, a first PCT application that claims compositions of matter, and a second PCT application that claims methods of treatment. Any patents issuing from the two pending U.S. non-provisional applications would be expected to expire in 2039, and any patents issuing from the two PCT applications would be expected to expire in 2040, subject to any disclaimers or extensions.
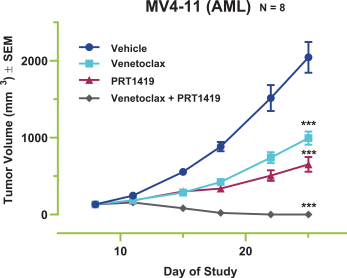
PRT1419
Our PRT1419 patent portfolio, which is wholly owned by us, includes pending U.S. patent applications claiming, among other things, PRT1419 and other compounds, pharmaceutical compositions comprising PRT1419, and methods of using PRT1419. Any patents issued from this application would be expected to expire
137