Brazil, India, Israel, Mexico, New Zealand, Singapore, South Africa, and Hong Kong. The 20 year term of patents in this family runs through June 2037, absent any available patent term adjustments or extensions.
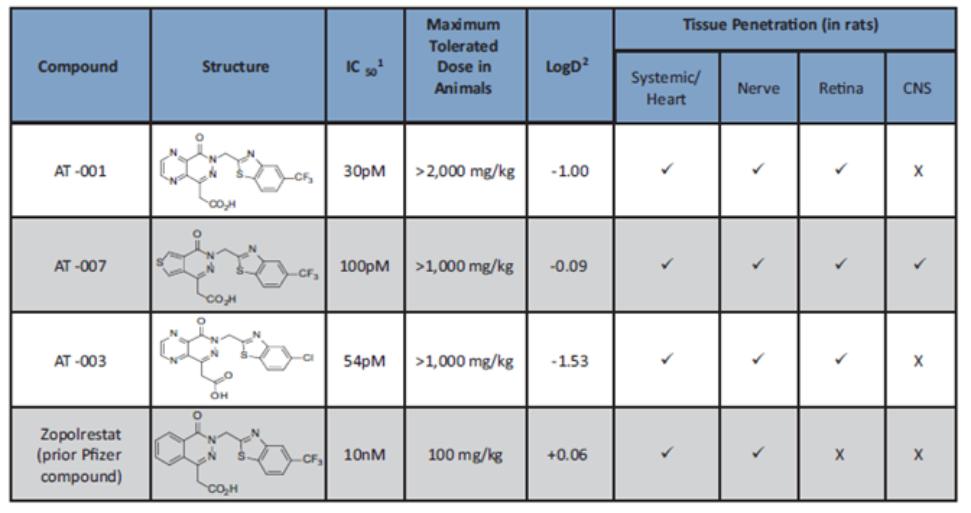
AT-001 and AT-003. As of February 9, 2021, we have exclusively licensed from Columbia University a patent family that includes four issued patents in the United States, 44 issued patents in Europe, Japan, Canada, and Australia, two pending applications in the United States and a pending application in Europe that claim the composition of matter of and certain methods of use with respect to AT-001 and AT-003. The 20-year term of the patents in this family runs through July 2031, absent any available patent term adjustments or extensions.
AT-104. We have exclusively licensed an early-stage patent family from Columbia University that currently includes patent applications in the United States, Europe, Australia, Brazil, Canada, China, India, Israel, Japan, Mexico, New Zealand, Russia, Singapore, South Africa, and Hong Kong, that claims the composition of matter of and certain methods of use with respect to AT-104. No patents have issued to date, but we expect that the 20-year term of any patents in this family that may issue will run through July 2038, absent any available patent term adjustments or extensions.
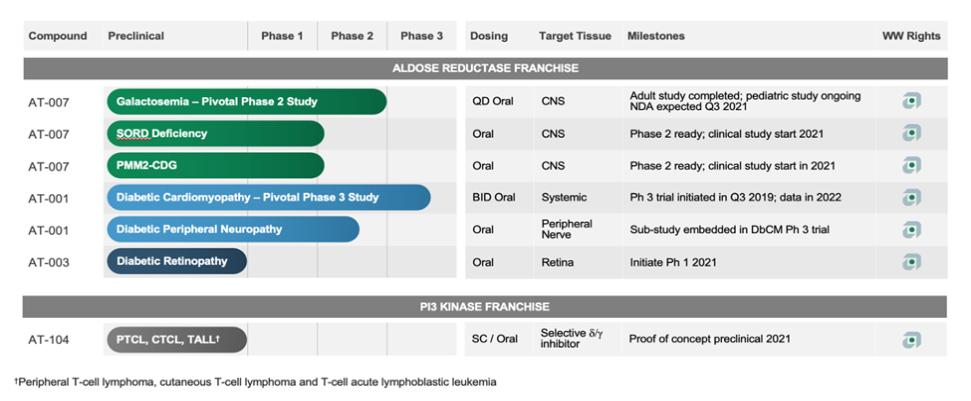
Methods for Treating Galactosemia
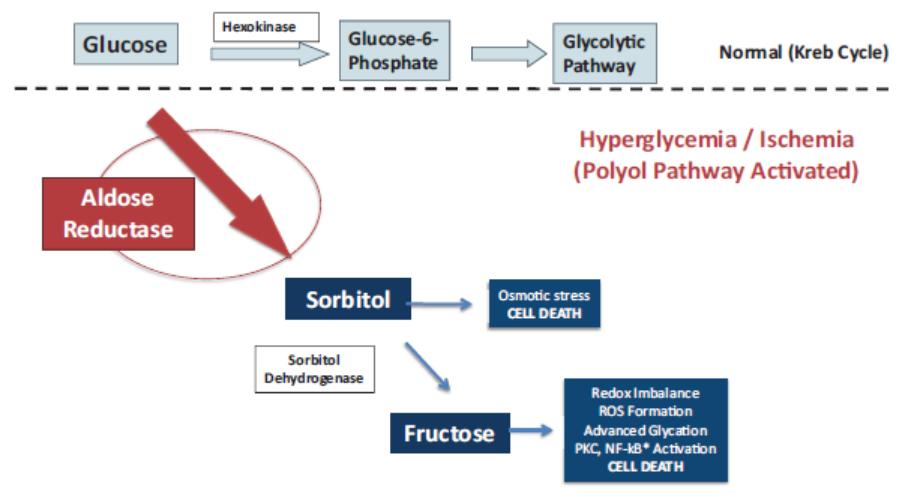
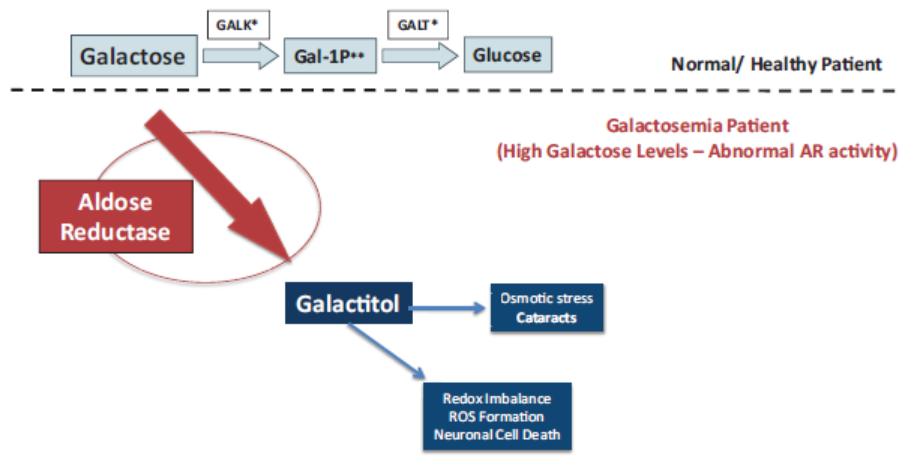
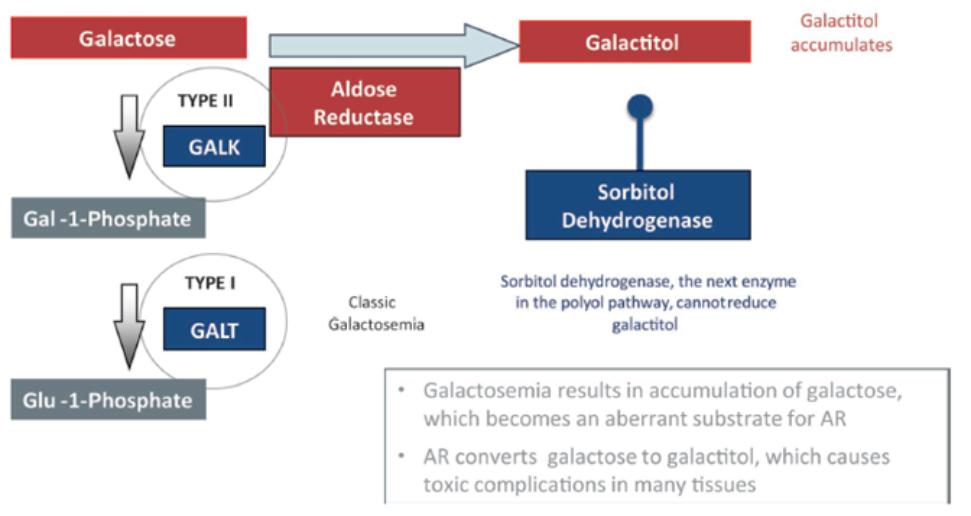
We own a family of patent applications that claims methods for treating galactosemia and preventing complications associated with galactosemia using AT-007 and other inhibitors of AR. This family currently includes pending patent application in the United States, Europe, Japan, Australia, Brazil, Canada, China, India, Israel, Mexico, New Zealand, Russia, Singapore, South Africa, and Hong Kong. No patents have issued to date, but we expect that the 20-year term of patents that do issue in this family will run through July 2038, absent any available patent term adjustments or extensions.
Methods for Treating SORD Deficiencies
We own a pending provisional application that claims methods for treating SORD deficiency using AT-007 and other inhibitors of AR. We plan to file an international patent application under the Patent Cooperation Treaty, or PCT, based on this provisional application before the applicable deadline. The 20-year term of any patents in this family that may issue will run through 2041, absent any available patent term adjustments or extensions.
Methods for Treating PMM2-CDG
We own a pending PCT patent application that claim methods for treating PMM2-CDG using AT-007 and other inhibitors of AR. We plan to file national stage applications in the United States, Europe, and other jurisdictions before the deadlines to file such applications. The 20-year term of any patents in this family that may issue will run through 2040, absent any available patent term adjustments or extensions.
We expect to file future patent applications on innovations that are developed in the course of advancing our pipeline through preclinical and clinical development.
Patent Term and Term Extensions
Individual patents have terms for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, the term of a U.S. patent can be extended to recapture a portion of the United States Patent and Trademark Office, or the USPTO, delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the restoration period cannot extend the patent term beyond 14 years from FDA approval. In addition, only one patent applicable to an approved drug is eligible for the extension, and only those claims covering the approved drug, a method for using it, or a method of manufacturing may be extended. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is