and requiring disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business.
Our patent portfolio includes patents and patent applications that are exclusively licensed from Columbia University and patent applications that are owned by us. Our patent portfolio includes patents and patent applications that cover our product candidates AT-007, AT-001, and AT-003, and the use of these candidates for therapeutic purposes in certain territories. Our proprietary technology has been developed primarily through relationships with academic research centers and contract research organizations.
For our product candidates, we will, in general, initially pursue patent protection covering compositions of matter and methods of use. Throughout the development of our product candidates, we seek to identify additional means of obtaining patent protection that would potentially enhance commercial success, including through additional methods of use, process of making, formulation and dosing regimen-related claims.
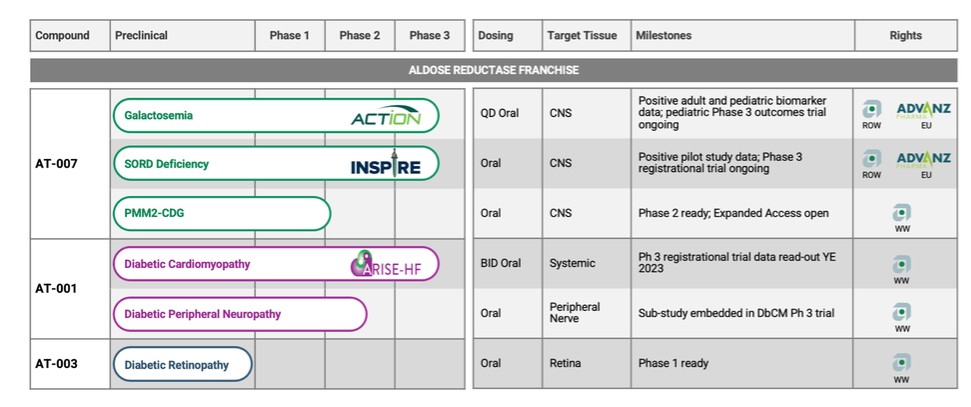
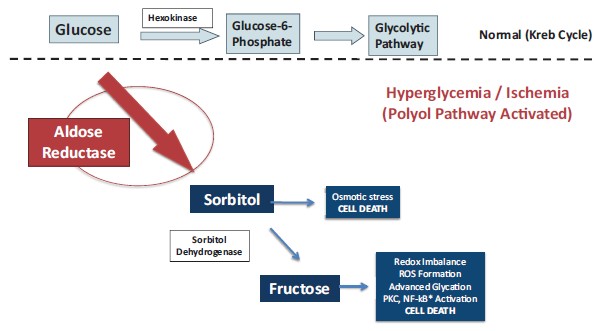
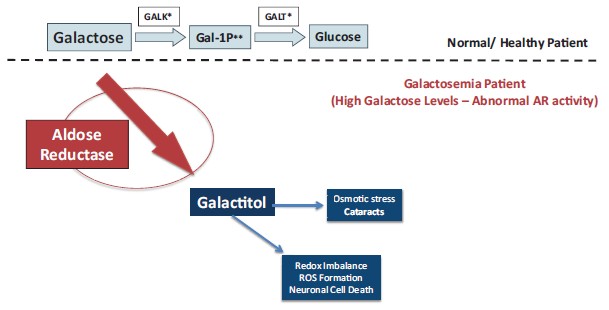
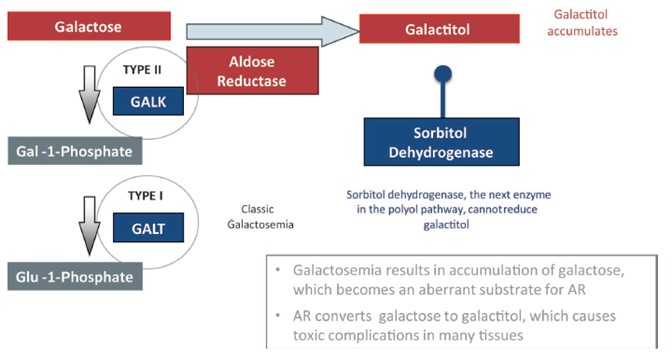
In total, our patent portfolio, including patents licensed from Columbia University and patents owned by us, comprises nine different patent families, filed in various jurisdictions worldwide, including families directed to composition of matter for AR inhibitors, and families directed to methods of treating Galactosemia and complications associated with Galactosemia, SORD Deficiency and PMM2-CDG using AR inhibitors. Our patent portfolio includes issued patents in the United States, Europe, Japan, Australia, Canada, and other jurisdictions. Our patent portfolio is outlined below:
Composition of Matter Patents
AT-007. We have exclusively licensed a patent family from Columbia University that includes two issued composition of matter patents and two issued method use of patents in the United States, and 40 issued patents in Europe, Japan, Australia, India, Israel, China, Mexico and South Africa, that claim the composition of matter of and certain methods of use with respect to AT 007. In addition, we have pending patent applications in the United States, Europe, Japan, China, Canada, Australia, Russia, Brazil, India, Israel, Mexico, New Zealand, Singapore, South Africa, and Hong Kong. The 20-year term of patents in this family runs through June 2037, absent any available patent term adjustments or extensions.
AT-001 and AT-003. We have exclusively licensed from Columbia University a patent family that includes five issued patents in the United States, 82 issued patents in Europe, Japan, Canada, and Australia, a pending application in the United States and a pending application in Europe that claim the composition of matter of and certain methods of use with respect to AT-001 and AT-003. The 20-year term of the patents in this family runs through July 2031, absent any available patent term adjustments or extensions.
Methods for Treating Galactosemia
We own a family of patent applications that claims methods for treating Galactosemia and preventing complications associated with Galactosemia using AT-007 and other inhibitors of AR. This family currently includes granted patents in the United States and Mexico, and pending patent application in the United States, Europe, Japan, Australia, Brazil, Canada, China, Israel, Mexico, New Zealand, Russia, Singapore, South Africa, and Hong Kong. The 20-year term of patents in this family run through July 2038, absent any available patent term adjustments or extensions.
Methods for Treating SORD Deficiencies
We own a family of patent applications that claims methods for treating SORD Deficiency using AT-007 and other inhibitors of AR. This family currently includes pending patent applications in the United States, Europe, Japan, Australia, Brazil, Canada, China, Israel, Mexico, New Zealand, Russia, Singapore, South Africa and South Korea. No patents have issued to date, but we expect that the 20-year term of any patents that do issue in this family will run through 2041, absent any available patent term adjustments or extensions.