As filed with the Securities and Exchange Commission on November 14, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-4
REGISTRATION STATEMENT
Under
The Securities Act of 1933
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
AgeX Therapeutics, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | | 2834 | | 82-1436829 |
(State or Other Jurisdiction of
Incorporation or Organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer Identification Number) |
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
(510) 671-8370
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Andrea E. Park
Chief Financial Officer
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
(510) 671-8370
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Please send copies of all communications to:
Robert Phillips
Harrison Tucker Chris W. Trester
Gibson, Dunn & Crutcher LLP
555 Mission Street
San Francisco, CA 94105-0921
(415) 393 8200 | | Scott Ludwig
Stephen Hinton
Bradley Arant Boult Cummings LLP
200 Clinton Avenue Huntsville Alabama 35801
(256) 517-5100 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effectiveness of this registration statement and the satisfaction or waiver of all other conditions under the Merger Agreement described herein.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, please check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13(e)-4(i) (Cross-Border Issuer Tender Offer) ☐
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ☐
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this proxy statement/prospectus/information statement is not complete and may be changed. AgeX may not sell its securities pursuant to the proposed transactions until the registration statement filed with the Securities and Exchange Commission is effective. This proxy statement/prospectus/information statement is not an offer to sell these securities, and is not soliciting an offer to buy these securities, in any state where the offer or sale is not permitted.
PRELIMINARY-SUBJECT TO COMPLETION-DATED November 14, 2023

PRELIMINARY PROSPECTUS
Common Stock of AgeX Therapeutics, Inc.
Warrants of AgeX Therapeutics, Inc.
PROPOSED MERGER
YOUR VOTE IS VERY IMPORTANT
To the Stockholders of AgeX Therapeutics, Inc. and Serina Therapeutics, Inc.:
AgeX Therapeutics, Inc., a Delaware corporation (AgeX), and Serina Therapeutics, Inc., an Alabama corporation (Serina), entered into an Agreement and Plan of Merger and Reorganization, dated as of August 29, 2023, as may be amended from time to time (the Merger Agreement), pursuant to which, among other matters and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Canaria Transaction Corporation, an Alabama corporation and wholly owned subsidiary of AgeX (Merger Sub), will merge with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the Merger). AgeX following the Merger is referred to herein as the “combined company.”
The Merger will become effective on the date and at the time the Statement of Merger has been duly filed with the Secretary of State of the State of Alabama or at such later time as may be specified in the Statement of Merger with the consent of AgeX and Serina in accordance with the Alabama Business Corporation Law of the State of Alabama (ABCL) (such date, the Closing Date, and such time, the Effective Time).
At the Effective Time, each outstanding share of common stock, $0.01 par value per share, of Serina (Serina common stock) (after giving effect to the conversion of each share of preferred stock of Serina into common stock of Serina (the Serina preferred stock conversion) and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of common stock of AgeX, $0.0001 par value per share (AgeX common stock) equal to the exchange ratio (the exchange ratio) determined in accordance with the Merger Agreement as described in more detail in the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of the accompanying proxy statement/prospectus/information statement. The exchange ratio is currently estimated to be equal to approximately 0.83217216 shares of AgeX common stock for each share of Serina common stock, which estimated exchange ratio assumes (i) the Actual Closing Price (as defined in the accompanying proxy statement/prospectus/information statement) of a share of AgeX common stock is equal to $12.00 per share (on a post-reverse stock split basis), (ii) the number of Company Outstanding Shares (as defined in the accompanying proxy statement/prospectus/information statement) is equal to 9,012,558, (iii) the number of Company Merger Shares (as defined in the accompanying proxy statement/prospectus/information statement) is equal to 7,500,000 and (iv) the implementation of the reverse stock split (as defined below) and the AgeX preferred stock conversion (as defined below) prior to the consummation of the Merger, as described in the accompanying proxy statement/prospectus/information statement. There can be no assurance that any of these assumptions will be accurate when the final exchange ratio is determined.
At the Effective Time, (i) each outstanding and unexercised option to purchase shares of Serina common stock (Serina option) immediately prior to the Effective Time under the Serina Therapeutics, Inc. 2017 Stock Option Plan, as amended (the Serina Plan) will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option, and (ii) each outstanding and unexercised warrant to purchase shares of Serina common stock (Serina warrant) immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Following the AgeX preferred stock conversion (as defined below) and prior to the closing of the Merger, AgeX will conduct a reverse stock split of AgeX common stock (the reverse stock split) at a ratio in the range from 1-for-35 to 1-for-36 with such specific ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors. Prior to the reverse stock split, all outstanding shares of preferred stock of AgeX will be converted into AgeX common stock pursuant to the Side Letter (the AgeX preferred stock conversion).
Immediately prior to the reverse stock split, each outstanding and unexercised AgeX option with an exercise price equal to or greater than $0.7751 (which, on a pre-reverse stock split basis, is referred to herein as the AgeX closing price) (each, an out of the money AgeX option) as of immediately prior to the reverse stock split, if any, will be canceled without payment. In accordance with the terms and conditions set forth in AgeX’s 2017 Equity Incentive Plan (the Incentive Plan), AgeX will notify each holder of an out of the money AgeX option that each such option will become exercisable at least 10 days prior to the reverse stock split and will thereafter be canceled if not exercised. All other outstanding and unexercised options to purchase shares of AgeX common stock will remain outstanding and exercisable in accordance with their terms immediately following the Effective Time.
Following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock as of the close of business on a business day following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger (the Warrant Dividend Record Date) three warrants (each, a post-merger warrant) for each five shares of AgeX common stock issued and outstanding held by such holder as of the Warrant Dividend Record Date (the Warrant Dividend). Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one warrant (each, an incentive warrant). Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock. Each post-merger warrant will expire on July 31, 2025, and each incentive warrant will expire on the four-year anniversary of the Closing Date. For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 179 of the accompanying proxy statement/prospectus/information statement.
Concurrently with the execution of the Merger Agreement, AgeX, Serina and Juvenescence Limited (Juvenescence) entered into a letter agreement in substantially the form attached to the Merger Agreement (the Side Letter), which will become effective immediately prior to the closing of the Merger. The Side Letter provides that:
| (i) | immediately prior to the Effective Time, Juvenescence will cancel all outstanding and unexercised AgeX warrants with an exercise price equal to or greater than the AgeX closing price (each, an out of the money AgeX warrant) held by Juvenescence; |
| | | |
| (ii) | Juvenescence will exercise all of the post-merger warrants it holds to provide the combined company approximately an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; |
| | | |
| (iii) | AgeX and Serina shall use reasonable best efforts to appoint certain directors, including Steve Ledger and Dr. J. Milton Harris, who are each affiliated with Serina, and Dr. Gregory Bailey and Dr. Richard Marshall, who are each affiliated with Juvenescence, to the board of the combined company effective as of the Effective Time; |
| | | |
| (iv) | Juvenescence will not sell any shares of AgeX Series A preferred stock or AgeX Series B preferred stock and will take all actions necessary to effect the AgeX preferred stock conversion, whereby all of such preferred stock will be converted into AgeX common stock prior to the reverse stock split; |
| | | |
| (v) | Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of certain loans to AgeX; and |
| | | |
| (vi) | Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loans agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. |
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant. For a more complete description of the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
The accompanying proxy statement/prospectus/information statement also constitutes the prospectus of AgeX with respect to (i) the issuance by AgeX of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants, (ii) the issuance by AgeX of the shares of AgeX common stock upon the exercise of the incentive warrants and (iii) the potential resale from time to time by the selling stockholder set forth therein with respect to shares of AgeX common stock issuable upon the exercise of the post-merger warrants and the incentive warrants.
The shares of AgeX common stock are currently listed on the NYSE American under the symbol “AGE.” AgeX intends to file an initial listing application for the combined company with the NYSE American. Substantially concurrently with the completion of the Merger, the combined company will be renamed “Serina Therapeutics, Inc.” and expects to trade on the NYSE American under the symbol “SER.” On , 2023, the last trading day before the date of the accompanying proxy statement/prospectus/information statement, the closing sale price of AgeX common stock was $ per share.
AgeX stockholders are cordially invited to attend the special meeting of AgeX stockholders (the AgeX special meeting). AgeX is holding the AgeX special meeting on at , unless postponed or adjourned to a later date, in order to obtain the stockholder approvals necessary to complete the Merger and related matters. The AgeX special meeting will be held entirely online. AgeX stockholders will be able to attend and participate in the AgeX special meeting online by visiting www.virtualshareholdermeeting.com/ , where they will be able to listen to the meeting live, submit questions and vote. At the AgeX special meeting, AgeX will ask its stockholders:
| 1. | To approve (i) the issuance of shares of AgeX common stock, which will represent more than 20% of the shares of AgeX common stock outstanding immediately prior to the Merger, to stockholders of Serina, pursuant to the terms of the Merger Agreement, a copy of which is attached as Annex A to the accompanying proxy statement/prospectus/information statement, and (ii) the change of control of AgeX resulting from the Merger, pursuant to Sections 712(b) and 713(b) of the NYSE American Company Guide, referred to herein as the Stock Issuance Proposal; |
| 2. | To approve an amendment to the certificate of incorporation of AgeX (the AgeX Charter) to effect a reverse stock split of AgeX common stock at a ratio within the range between 1-for-35 to 1-for-36 with such ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors prior to the effectiveness of the Merger or, if the Stock Issuance Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal is not approved, by AgeX stockholders, determined solely by the AgeX board of directors (the AgeX Board), referred to herein as the Reverse Stock Split Proposal; |
| 3. | To approve the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants, which will represent securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance, referred to herein as the Warrant Issuance Proposal; |
| 4. | To approve an amendment and restatement of the AgeX Charter (the Combined Company Charter), in the form attached as Annex B to the accompanying proxy statement/prospectus/information statement, referred to herein as the A&R Charter Proposal; |
| 5. | To approve the Serina Therapeutics, Inc. 2024 Equity Incentive Plan, in the form attached as Annex C to the accompanying proxy statement/prospectus/information statement, referred to herein as the 2024 Equity Incentive Plan Proposal; and |
| 6. | To approve a postponement or adjournment of the AgeX special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal, referred to as the Adjournment Proposal. |
Concurrently with the execution of the Merger Agreement, Juvenescence, a stockholder of AgeX holding approximately 43.3% of the outstanding shares of common stock of AgeX, 100% of the outstanding shares of Series A preferred stock of AgeX and 100% of the outstanding shares of Series B preferred stock of AgeX, in each case as of August 29, 2023, entered into a support agreement (the AgeX Support Agreement) in favor of Serina, providing, among other things, that Juvenescence will vote all of their shares of AgeX capital stock: (i) in favor of adopting the Merger Agreement and approving the Merger, the AgeX Stockholder Matters, and the other transactions and actions contemplated by the Merger Agreement, (ii) against any proposal made in opposition to, or in competition with, the Merger Agreement or the Merger and (iii) against any acquisition proposal involving a third party other than Serina.
In connection with the execution of the Merger Agreement, stockholders of Serina holding 100% of the outstanding shares of common stock of Serina, 100% of the outstanding shares of Series A preferred stock of Serina, 100% of the outstanding shares of Series A-1 preferred stock of Serina, 100% of the outstanding shares of Series A-2 preferred stock of Serina, 100% of the outstanding shares of Series A-3 preferred stock of Serina, 100% of the outstanding shares of Series A-4 preferred stock of Serina, and 100% of the outstanding shares of Series A-5 preferred stock of Serina, in each case effective as of August 29, 2023, entered into support agreements (the Serina Support Agreements) in favor of AgeX, providing, among other things, that such stockholders will vote all of their shares of Serina capital stock (or execute a written consent): (i) in favor of adopting the Merger Agreement and approving the Merger, the Company Stockholder Matters (as defined in the Merger Agreement), and the other transactions and actions contemplated by the Merger Agreement, (ii) against any proposal made in opposition to, or in competition with, the Merger Agreement or the Merger and (iii) against any acquisition proposal involving a third party other than AgeX.
After careful consideration, the AgeX Board has: (i) determined that the Merger and the transactions and actions contemplated by the Merger Agreement are fair to, advisable and in the best interests of AgeX and its stockholders; (ii) authorized, approved and declared advisable the Merger Agreement and the transactions contemplated therein, including the Merger; and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, that the stockholders of AgeX vote “FOR” each of the proposals set forth in the accompanying proxy statement/prospectus/information statement.
After careful consideration, Serina’s board of directors (Serina’s Board or the Serina Board) has (i) determined that the Merger and the transactions and actions contemplated by the Merger Agreement are fair to, advisable and in the best interests of Serina and its stockholders, (ii) authorized, and declared advisable the Merger Agreement and the transactions contemplated therein, including the Merger, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, that each Serina stockholder sign and return the written consent indicating its (a) adoption of the Merger Agreement and approval of the transactions contemplated therein, including the Merger, (b) acknowledgement that the approval given is irrevocable, (c) agreement that such stockholder is aware of its rights to demand appraisal for its shares pursuant to Article 13 of the ABCL, (d) agreement that such stockholder has received and read a copy of Article 13 of the ABCL, (e) acknowledgement that by its approval of the Merger such stockholder is not entitled to appraisal rights or Appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under the ABCL, (f) election that the consummation of the Merger is not a deemed liquidation event as defined in Serina’s certificate of incorporation and (g) election of an automatic conversion of Serina’s preferred stock to Serina common stock immediately prior to the closing of the Merger in accordance with the relevant provisions of Serina’s certificate of incorporation.
More information about AgeX, Serina, the Merger Agreement and the transactions contemplated thereby and the foregoing proposals is contained in the accompanying proxy statement/prospectus/information statement. AgeX and Serina urge you to read the accompanying proxy statement/prospectus/information statement carefully and in its entirety. In particular, you should carefully consider the matters discussed under “Risk Factors” beginning on page 25 of the accompanying proxy statement/prospectus/information statement.
AgeX and Serina are excited about the opportunities the Merger brings to AgeX’s and Serina’s stockholders, and thank you for your consideration and continued support.
Andrea E. Park
Chief Financial Officer
AgeX Therapeutics, Inc. | Steve Ledger
Chief Financial Officer
Serina Therapeutics, Inc. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of the accompanying proxy statement/prospectus/information statement. Any representation to the contrary is a criminal offense.
The accompanying proxy statement/prospectus/information statement is dated , 2023, and is first being mailed to AgeX’s and Serina’s stockholders on or about , 2023.
AGEX THERAPEUTICS, INC.
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
TO BE HELD ON
To the stockholders of AgeX Therapeutics, Inc.:
NOTICE IS HEREBY GIVEN that a virtual special meeting of stockholders (the AgeX special meeting) will be held on at , unless postponed or adjourned to a later date. The AgeX special meeting will be held entirely online. You will be able to attend and participate in the AgeX special meeting online by visiting www.virtualshareholdermeeting.com/ , where you will be able to listen to the meeting live, submit questions and vote.
AgeX is holding the AgeX special meeting to consider the following purposes:
| 1. | To approve (i) the issuance of shares of AgeX common stock, which will represent more than 20% of the shares of AgeX common stock outstanding immediately prior to the Merger, to stockholders of Serina, pursuant to the terms of the Merger Agreement, a copy of which is attached as Annex A to the accompanying proxy statement/prospectus/information statement, and (ii) the change of control of AgeX resulting from the Merger, pursuant to Sections 712(b) and 713(b) of the NYSE American Company Guide, referred to herein as the Stock Issuance Proposal; |
| 2. | To approve an amendment to the certificate of incorporation of AgeX (the AgeX Charter) to effect a reverse stock split of AgeX common stock at a ratio within the range between 1-for-35 to 1-for-36 with such ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors prior to the effectiveness of the Merger or, if the Stock Issuance Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal is not approved, by AgeX stockholders, determined solely by the AgeX board of directors (the AgeX Board), referred to herein as the Reverse Stock Split Proposal; |
| 3. | To approve the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants, which will represent securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance, referred to herein as the Warrant Issuance Proposal; |
| 4. | To approve an amendment and restatement of the AgeX Charter (the Combined Company Charter), in the form attached as Annex B to the accompanying proxy statement/prospectus/information statement, referred to herein as the A&R Charter Proposal; |
| 5. | To approve the Serina Therapeutics, Inc. 2024 Equity Incentive Plan, in the form attached as Annex C to the accompanying proxy statement/prospectus/information statement, referred to herein as the 2024 Equity Incentive Plan Proposal; and |
| 6. | To approve a postponement or adjournment of the AgeX special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of the Stock Issuance Proposal, the Reverse Stock Split Proposal, Warrant Issuance Proposal, A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal, referred to as the Adjournment Proposal. |
These proposals are referred to collectively as the AgeX Proposals.
Please read the accompanying proxy statement/prospectus/information statement for further information with respect to the business to be transacted at the AgeX special meeting. The AgeX Board has fixed as the record date for the determination of stockholders entitled to notice of, and to vote at, the AgeX special meeting and any adjournment or postponement thereof. Only holders of record of shares of AgeX common stock at the close of business on the record date are entitled to notice of, and to vote at, the AgeX special meeting. At the close of business on the record date, AgeX had shares of common stock outstanding and entitled to vote. A complete list of such stockholders entitled to vote at the AgeX special meeting will be available for examination in the Corporate Secretary’s Office at AgeX Therapeutics, Inc., 1101 Marina Village Parkway, Suite 201, Alameda, California 94501, for purposes pertaining to the AgeX special meeting, during ordinary business hours for a period of 10 days before the AgeX special meeting, and at the AgeX special meeting.
Your vote is important. Assuming a quorum is present (i) the affirmative vote of the holders of a majority of AgeX common stock, the holders of a majority of AgeX Series A preferred stock and the holders of a majority of AgeX Series B preferred stock entitled to vote at the AgeX special meeting, each voting as a separate class, is required for the approval of the A&R Charter Proposal, and (ii) the affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote on the matter is required for the approval each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the 2024 Equity Incentive Plan Proposal and the Adjournment Proposal. No AgeX Proposal is conditioned upon any other AgeX Proposal. However, each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal is a condition to the consummation of the Merger. Therefore, the Merger cannot be consummated without the approval of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal.
Even if you plan to virtually attend the AgeX special meeting, AgeX requests that you sign and return the enclosed proxy or vote by mail or online to ensure that your shares will be represented at the AgeX special meeting if you are unable to virtually attend. You may change or revoke your proxy at any time before it is voted at the AgeX special meeting.
THE AGEX BOARD HAS DETERMINED AND BELIEVES THAT EACH OF THE AGEX PROPOSALS OUTLINED ABOVE IS FAIR TO, ADVISABLE AND IN THE BEST INTERESTS OF AGEX AND ITS STOCKHOLDERS AND HAS APPROVED EACH SUCH PROPOSAL. THE AGEX BOARD RECOMMENDS THAT AGEX STOCKHOLDERS VOTE “FOR” EACH OF THE AGEX PROPOSALS.
Important Notice Regarding the Availability of Proxy Materials for the Stockholders’ Meeting to Be Held on at via the internet
The proxy statement/prospectus/information statement is available at
www.virtualshareholdermeeting.com/
By Order of the AgeX Board of Directors,
Andrea E. Park
Chief Financial Officer
Alameda, California
ABOUT THIS PROXY STATEMENT/PROSPECTUS/INFORMATION STATEMENT
This proxy statement/prospectus/information statement, which forms a part of a registration statement on Form S-4/S-1 filed with the SEC by AgeX, constitutes a prospectus of AgeX under the Securities Act of 1933, as amended (the Securities Act), with respect to (i) the issuance of the shares of AgeX common stock in exchange for each share of Serina common stock (after giving effect to the Serina preferred stock conversion (as defined below) and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares), (ii) the issuance by AgeX of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants (as defined below) and (iii) the issuance by AgeX of the shares of AgeX common stock upon the exercise of the incentive warrants (as defined below), in each case pursuant to the Agreement and Plan of Merger and Reorganization, dated August 29, 2023, as may be amended from time to time (the Merger Agreement), by and among AgeX, Merger Sub and Serina.
This proxy statement/prospectus/information statement also constitutes a prospectus of AgeX with respect to the potential resale from time to time by the selling stockholder set forth herein (the Selling Stockholder) of shares of AgeX common stock issuable upon the exercise of the post-merger warrants and the incentive warrants, which uses a “shelf” registration process. Under this shelf registration process, the Selling Stockholder may, from time to time, offer and sell the shares of AgeX common stock described herein in one or more offerings through any means described in the section entitled “Plan of Distribution.” More specific terms of any securities that the Selling Stockholder may offer and sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of AgeX common stock being offered and the terms of the offering.
A prospectus supplement may also add, update or change information included in this proxy statement/prospectus/information statement. Any statement contained herein will be deemed to be modified or superseded for purposes of this proxy statement/prospectus/information statement to the extent that a statement contained in such prospectus supplement modifies or supersedes such statement. Any statement so modified will be deemed to constitute a part of this proxy statement/prospectus/information statement only as so modified, and any statement so superseded will be deemed not to constitute a part of this document.
This proxy statement/prospectus/information statement also constitutes a notice of a meeting and a proxy statement of AgeX under Section 14(a) of the Securities Exchange Act of 1934, as amended (Exchange Act), with respect to the special meeting of AgeX stockholders (the AgeX special meeting).
No one has been authorized to provide you with information that is different from that contained in this proxy statement/prospectus/information statement. This proxy statement/prospectus/information statement is dated , and you should not assume that the information contained in this proxy statement/prospectus/information statement is accurate as of any date other than that date, unless otherwise specifically provided herein. Neither the mailing of this proxy statement/prospectus/information statement to AgeX stockholders nor the issuance by AgeX of AgeX common stock pursuant to the Merger Agreement will create any implication to the contrary.
This proxy statement/prospectus/information statement does not constitute an offer to sell, or a solicitation of an offer to buy, any securities, or the solicitation of a proxy, in any jurisdiction in which or from any person to whom it is unlawful to make any such offer or solicitation in such jurisdiction.
Information contained in this proxy statement/prospectus/information statement regarding Serina and its business, operations, management and other matters has been provided by Serina and information contained in this proxy statement/prospectus/information statement regarding AgeX and its business, operations, management and other matters has been provided by AgeX.
This document contains references to trademarks and service marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this proxy statement/prospectus/information statement may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. Neither of AgeX or Serina intend for the use or display of other companies’ trade names, trademarks or service marks contained in this document to imply a relationship with, or endorsement or sponsorship of AgeX or Serina by, any other companies.
Web links are provided throughout this proxy statement/prospectus/information statement for convenience and are inactive textual references only. The content on the referenced websites does not constitute a part of, and is not incorporated by reference into, this proxy statement/prospectus/information statement.
If you would like additional copies of this proxy statement/prospectus/information statement or if you have questions about the Merger or the proposals to be presented at the AgeX special meeting, please contact AgeX’s proxy solicitor listed below. You will not be charged for any of the documents that you request.
You may also request additional copies from AgeX using the following contact information:
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 945013
Attention: Andrea Park
Phone Number: (510) 671-8370
Email: information@agexinc.com
Subject Line: Request for Proxy Materials
To ensure timely delivery of these documents, any request should be made no later than to receive them before the AgeX special meeting.
This proxy statement/prospectus/information statement is first being mailed on or about to all AgeX’s stockholders of record entitled to vote at the AgeX special meeting.
TABLE OF CONTENTS
QUESTIONS AND ANSWERS ABOUT THE MERGER
Except where specifically noted, the following information and all other information contained in this proxy statement/prospectus/information statement does not give effect to the proposed reverse stock split of common stock of AgeX, as described in Proposal No. 2 beginning on page 182 of this proxy statement/prospectus/information statement.
The following section provides answers to frequently asked questions about the Merger and the special meeting of AgeX stockholders (the AgeX special meeting). This section, however, provides only summary information. For a more complete response to these questions and for additional information, please refer to the cross-referenced sections.
| A: | On August 29, 2023, AgeX Therapeutics, Inc., a Delaware corporation (AgeX), Canaria Transaction Corporation, an Alabama corporation and wholly owned subsidiary of AgeX (Merger Sub), and Serina Therapeutics, Inc., an Alabama corporation (Serina), entered into an Agreement and Plan of Merger and Reorganization, dated as of August 29, 2023, as may be amended from time to time (the Merger Agreement), a copy of which is attached hereto as Annex A. Pursuant to the Merger Agreement, Merger Sub will merge with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the Merger). The Merger Agreement contains the terms and conditions of the Merger. Substantially concurrently with the completion of the Merger, AgeX will be renamed “Serina Therapeutics, Inc.” AgeX following the Merger is referred to herein as the “combined company.” |
The Merger will become effective on the date and at the time the Statement of Merger has been duly filed with the Secretary of State of the State of Alabama or at such later time as may be specified in the Statement of Merger with the consent of AgeX and Serina in accordance with the Alabama Business Corporation Law of the State of Alabama (ABCL) (such date, the Closing Date, and such time, the Effective Time).
At the Effective Time, each outstanding share of common stock, $0.01 par value per share, of Serina (Serina common stock) (after giving effect to the conversion of each share of preferred stock of Serina into common stock of Serina (the Serina preferred stock conversion) and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of common stock of AgeX, $0.0001 par value per share (AgeX common stock) equal to the exchange ratio (the exchange ratio) determined in accordance with the Merger Agreement as described in more detail in the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of this proxy statement/prospectus/information statement.
At the Effective Time, (i) each outstanding and unexercised option to purchase shares of Serina common stock (Serina option) immediately prior to the Effective Time under the Serina Therapeutics, Inc. 2017 Stock Option Plan, as amended (the Serina Plan) will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option, and (ii) each outstanding and unexercised warrant to purchase shares of Serina common stock (Serina warrant) immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Immediately prior to the reverse stock split, each outstanding and unexercised AgeX option with an exercise price equal to or greater than $0.7751 (the AgeX closing price) (each, an out of the money AgeX option) as of immediately prior to the reverse stock split, if any, will be canceled without payment. In accordance with the terms and conditions set forth in AgeX’s 2017 Equity Incentive Plan (the Incentive Plan), AgeX will notify each holder of an out of the money AgeX option that each such option will become exercisable at least 10 days prior to the reverse stock split and will thereafter be canceled if not exercised. All other outstanding and unexercised options to purchase shares of AgeX common stock will remain outstanding and exercisable in accordance with their terms immediately following the Effective Time.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant (as defined below), incentive warrant (as defined below) or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant. For a more complete description of the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
| Q: | Why are the two companies proposing to merge? |
| A: | AgeX and Serina believe that combining the two companies will result in a company with a promising pipeline, a strong leadership team and greater access to capital resources, focused primarily on developing Serina’s product candidates to treat neurological diseases and pain. |
If the Merger is completed, the combined company will primarily focus on developing Serina’s product candidates, which are described on page 231 under the section titled “Description of Serina’s Business.”
For a more complete description of the reasons for the Merger, please see the sections titled “The Merger—AgeX Reasons for the Merger” and “The Merger—Serina Reasons for the Merger” beginning on pages 134 and 134, respectively, of this proxy statement/prospectus/information statement.
| Q: | What will happen to AgeX if, for any reason, the Merger does not close? |
| A: | If, for any reason, the Merger does not close, AgeX expects to continue to execute on its current business strategies as more fully described in the section titled “Description of AgeX’s Business—Business Strategy” beginning on page 209 of this proxy statement/prospectus/information statement while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs. |
Under certain circumstances, AgeX may be obligated to pay Serina a termination fee of $1,000,000 or reimburse certain expenses of Serina up to $1,000,000 as more fully described in the section titled “The Merger Agreement—Termination and Termination Fees” beginning on page 173 of this proxy statement/prospectus/information statement.
| Q: | Why am I receiving this proxy statement/prospectus/information statement? |
| A: | You are receiving this proxy statement/prospectus/information statement because you have been identified as a holder of AgeX common stock as of the record date and you are entitled to vote to approve the matters set forth herein, or you have been identified as a stockholder of Serina eligible to execute the Serina written consent. This document serves as: |
| ● | a proxy statement of AgeX used to solicit proxies for the AgeX special meeting to vote on the matters set forth herein; |
| | | |
| ● | a prospectus of AgeX used to offer shares of AgeX common stock in exchange for shares of Serina common stock in the Merger (other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares); and |
| | | |
| ● | an information statement of Serina used to solicit the written consent of its stockholders for the adoption of the Merger Agreement and the approval of the Merger and related transactions after the declaration of the effectiveness of this registration statement on Form S-4/S-1, of which this proxy statement/prospectus/information statement is a part. |
Information about the AgeX special meeting, the Merger, the Merger Agreement and the other business to be considered by AgeX stockholders at the AgeX special meeting, and for Serina stockholders to consider in determining whether to sign and return the Serina written consent, is contained in this proxy statement/prospectus/information statement. AgeX stockholders and Serina stockholders should read this information carefully and in its entirety. The enclosed voting materials allow AgeX stockholders to vote their shares by proxy without attending the AgeX special meeting.
If you are a stockholder of Serina, you are requested to sign and return the Serina written consent to (i) adopt the Merger Agreement and approve the transactions and actions contemplated by the Merger Agreement, including the Merger, (ii) acknowledge that your approval will be irrevocable, (iii) agree that you are aware of your rights to demand appraisal for your shares pursuant to Article 13 of the Alabama Business Corporation Law of the State of Alabama (ABCL), (iv) agree that you have received and read a copy of Article 13 of the ABCL, (v) acknowledge that by your approval of the Merger you are not entitled to appraisal rights with respect to your shares of Serina capital stock in connection with the Merger and thereby waive any rights to receive payment of the fair value of your capital stock under the ABCL, (vi) elect that the consummation of the Merger is not a deemed liquidation event and (vii) consent to conversion of Serina preferred stock to Serina common stock immediately prior to the closing of the Merger in accordance with the relevant provisions of Serina’s organization documents (items (i) through (vii), collectively, the Serina Stockholder Matters).
| Q: | What are the post-merger warrants and the incentive warrants? |
| A: | Following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock as of the close of business on a business day following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger (the Warrant Dividend Record Date) three warrants (each, a post-merger warrant) for each five shares of AgeX common stock issued and outstanding held by such holder as of the Warrant Dividend Record Date (the Warrant Dividend). Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one warrant (each, an incentive warrant) and will expire on July 31, 2025. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock and will expire on the four-year anniversary of the Closing Date. For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 180 of this proxy statement/prospectus/information statement. |
| Q: | What is the Side Letter? |
| A: | Concurrently with the execution of the Merger Agreement, AgeX, Serina and Juvenescence Limited (Juvenescence) entered into a letter agreement in substantially the form attached to the Merger Agreement (the Side Letter), which will become effective immediately prior to the closing of the Merger. The Side Letter provides that: |
| (i) | immediately prior to the Effective Time, Juvenescence will cancel all outstanding and unexercised AgeX warrants with an exercise price equal to or greater than the AgeX closing price (each, an out of the money AgeX warrant) held by Juvenescence; |
| (ii) | Juvenescence will exercise all of the post-merger warrants it holds to provide the combined company approximately an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; |
| (iii) | AgeX and Serina shall use reasonable best efforts to appoint certain directors, including Steve Ledger and Dr. J. Milton Harris, who are each affiliated with Serina, and Dr. Gregory Bailey and Dr. Richard Marshall, who are each affiliated with Juvenescence, to the board of the combined company effective as of the Effective Time; |
| (iv) | Juvenescence will not sell any shares of AgeX Series A preferred stock or AgeX Series B preferred stock and will take all actions necessary to effect the AgeX preferred stock conversion, whereby all of such preferred stock will be converted into AgeX common stock prior to the reverse stock split; |
| (v) | Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of certain loans to AgeX; and |
| (vi) | Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loans agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. |
| Q: | What is required to consummate the Merger? |
| A: | To consummate the Merger, AgeX’s common stockholders must approve the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal (collectively, the Required AgeX Closing Stockholder Matters) and Serina’s stockholders must approve the Serina Stockholder Matters. |
Concurrently with the execution of the Merger Agreement, Juvenescence, a stockholder of AgeX holding approximately 43.3% of the outstanding shares of common stock of AgeX, 100% of the outstanding shares of Series A preferred stock of AgeX and 100% of the outstanding shares of Series B preferred stock of AgeX, in each case as of August 29, 2023, entered into a support agreement (the AgeX Support Agreement) in favor of Serina, providing, among other things, that Juvenescence will vote all of their shares of AgeX capital stock in favor of the AgeX Stockholder Matters.
In connection with the execution of the Merger Agreement, stockholders of Serina holding 100% of the outstanding shares of common stock of Serina, 100% of the outstanding shares of Series A preferred stock of Serina, 100% of the outstanding shares of Series A-1 preferred stock of Serina, 100% of the outstanding shares of Series A-2 preferred stock of Serina, 100% of the outstanding shares of Series A-3 preferred stock of Serina, 100% of the outstanding shares of Series A-4 preferred stock of Serina, and 100% of the outstanding shares of Series A-5 preferred stock of Serina, in each case effective as of August 29, 2023, entered into support agreements (the Serina Support Agreements and, together with the AgeX Support Agreement, the Support Agreements) in favor of AgeX, providing, among other things, that such stockholders will vote all of their shares of Serina capital stock (or execute a written consent) in favor of Serina Stockholder Matters.
In addition to the requirement of obtaining stockholder approval of the Required AgeX Closing Stockholder Matters and the Serina Stockholder Matters, each of the other closing conditions set forth in the Merger Agreement must be satisfied or waived. For a more complete description of the closing conditions under the Merger Agreement, refer to the section titled “The Merger Agreement—Conditions to the Completion of the Merger” beginning on page 153 of this proxy statement/prospectus/information statement.
| Q: | What will Serina’s stockholders, option holders and warrant holders receive in the Merger? |
| A: | At the Effective Time, each outstanding share of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of AgeX common stock equal to the exchange ratio determined in accordance with the Merger Agreement as described in more detail in the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of the proxy statement/prospectus/information statement. |
At the Effective Time, (i) each outstanding and unexercised Serina option immediately prior to the Effective Time under the Serina Plan will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and the exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option, and (ii) each outstanding and unexercised option to purchase shares of Serina common stock immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
For a more complete description of what Serina’s stockholders and option holders will receive in the Merger, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of this proxy statement/prospectus/information statement.
| Q: | What will AgeX’s stockholders, option holders and warrant holders receive in the Merger? |
| A: | At the Effective Time, AgeX’s stockholders will continue to own and hold their existing shares, options and warrants to purchase shares of AgeX common stock. However, immediately prior to the reverse stock split, each out of the money AgeX option as of immediately prior to the reverse stock split, if any, will be canceled without payment. In accordance with the terms and conditions set forth in the Incentive Plan, AgeX will notify each holder of an out of the money AgeX option that each such option will become exercisable at least 10 days prior to the reverse stock split and will thereafter be canceled if not exercised. |
| Q: | Will the common stock of the combined company trade on an exchange? |
| A: | Shares of AgeX common stock are currently listed on the NYSE American under the symbol “AGE.” AgeX intends to file an initial listing application for the combined company with the NYSE American. It is a condition of the closing of the Merger that the initial listing application with NYSE American shall have been approved. If such condition is not satisfied, the Merger may not be consummated. Each of AgeX and Serina may waive this condition as set forth in the Merger Agreement. NYSE American’s determination is not expected to be known at the time that you are asked to vote on the AgeX Proposals at the AgeX special meeting. Substantially concurrently with the completion of the Merger, AgeX will be renamed “Serina Therapeutics, Inc.,” and the combined company expects to trade on the NYSE American under the symbol “SER.” On , 2023, the last trading day before the date of this proxy statement/prospectus/information statement, the closing sale price of AgeX common stock was $ per share. |
| Q: | Who will be the directors of AgeX following the Merger? |
| A: | At the Effective Time, the combined company is expected to initially have a seven-member board of directors (the Combined Company Board), comprising (a) Remy Gross, designated by both AgeX and Serina, (b) J. Milton Harris and Steven Ledger, each as a Serina designee, (c) Gregory Bailey and Richard Marshall, each as an AgeX designee, and (d) and , each as an independent director, until their respective successors are duly elected or appointed and qualified or their earlier death, resignation or removal. The Combined Company Board will have an audit committee, a compensation committee and a nominating and corporate governance committee, in accordance with the rules of the NYSE American. All of AgeX’s current directors other than Gregory Bailey are expected to resign from their positions as directors of AgeX, effective upon the Effective Time. |
| Q: | Who will be the executive officers of AgeX following the Merger? |
| A: | Immediately following the Merger, the executive management team of the combined company is expected to comprise the following individuals with such additional officers as may be added by Serina or the combined company: |
| Name | | Position with the Combined Company | | Current Position at AgeX / Serina |
| Steve Ledger | | Interim Chief Executive Officer | | Chief Financial Officer at Serina |
| Andrea Park | | Interim Chief Financial Officer and Chief Accounting Officer | | Chief Financial Officer at AgeX |
| Randall Moreadith, M.D., Ph.D. | | Chief Scientific Officer | | President and Chief Executive Officer at Serina |
| Tacey Viegas | | Chief Operating Officer and Secretary | | Chief Operating Officer at Serina |
| Q: | As a stockholder of AgeX, how does the AgeX Board recommend that I vote? |
| A: | After careful consideration, the AgeX board of directors (the AgeX Board) recommends that the holders of AgeX common stock vote: |
| ● | “FOR” Proposal No. 1—the Stock Issuance Proposal; |
| ● | “FOR” Proposal No. 2—the Reverse Stock Split Proposal; |
| ● | “FOR” Proposal No. 3—the Warrant Issuance Proposal; |
| ● | “FOR” Proposal No. 4—the A&R Charter Proposal; |
| ● | “FOR” Proposal No. 5—the 2024 Equity Incentive Plan Proposal; and |
| ● | “FOR” Proposal No. 6—the Adjournment Proposal. |
After careful consideration, the AgeX Board recommends that the holders of AgeX Series A preferred stock and AgeX Series B preferred stock vote:
| ● | “FOR” Proposal No. 4—the A&R Charter Proposal. |
For more information on each proposal and the AgeX Board’s recommendations, please see the section titled “Matters Being Submitted to a Vote of AgeX’s Stockholders” beginning on page 181 of this proxy statement/prospectus/information statement.
| Q: | How many votes are needed to approve each proposal? |
| A: | Assuming a quorum is present, the affirmative vote of the holders of a majority of AgeX common stock, the holders of a majority of AgeX Series A preferred stock and the holders of a majority of AgeX Series B preferred stock entitled to vote at the AgeX special meeting, each voting as a separate class, is required for the approval of the A&R Charter Proposal. |
Assuming a quorum is present, the affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote on the matter is required for the approval of each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the 2024 Equity Incentive Plan Proposal and the Adjournment Proposal.
Votes will be counted by the inspector of election appointed for the AgeX special meeting, who will separately count “FOR” and “AGAINST” votes, abstentions and broker non-votes, if any, as applicable to each proposal. Abstentions and broker non-votes, if any, will also be treated as shares present for the purpose of determining the presence of a quorum for the transaction of business at the AgeX special meeting. Abstentions and broker non-votes, if any, will be counted as “shares entitled to vote” for the A&R Charter Proposal and therefore will have the same effect of a vote “AGAINST” the A&R Charter Proposal. Abstentions and broker non-votes, if any, will not be counted as “votes cast” and will therefore have no effect on the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the 2024 Equity Incentive Plan Proposal and the Adjournment Proposal.
| Q: | As a stockholder of Serina, how does the board of directors of Serina recommend that I vote? |
| A: | After careful consideration, Serina’s board of directors (Serina’s Board or the Serina Board) recommends that the Serina stockholders execute the written consent stating their vote in favor of the Serina Stockholder Matters. |
| Q: | What risks should I consider in deciding whether to vote in favor of the AgeX Proposals or to execute and return the written consent, as applicable? |
| A: | You should carefully review the section titled “Risk Factors” beginning on page 25 of this proxy statement/prospectus/information statement, which sets forth certain risks and uncertainties related to the Merger, risks and uncertainties to which the combined company’s business will be subject, and risks and uncertainties to which each of AgeX and Serina, as an independent company, is subject. |
| Q: | When do you expect the Merger to be consummated? |
| A: | We anticipate that the Merger will be consummated during the first quarter of 2024, soon after the AgeX special meeting to be held on , but we cannot predict the exact timing. For more information, please see the section titled “The Merger Agreement—Conditions to the Completion of the Merger” beginning on page 157 of this proxy statement/prospectus/information statement. |
| Q: | What are the material U.S. federal income tax consequences of the Merger to U.S. holders of Serina shares? |
| A: | AgeX and Serina intend the Merger to qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (Code), as described in the section titled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” beginning on page 148 of this proxy statement/prospectus/information statement. Assuming the Merger so qualifies, Serina stockholders who are U.S. holders (as defined in the section titled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” beginning on page 148 of this proxy statement/prospectus/information statement) generally will not recognize gain or loss for U.S. federal income tax purposes on the receipt of shares of AgeX common stock issued in connection with the Merger. |
If the Merger does not qualify as a reorganization within the meaning of Section 368(a) of the Code, then each Serina stockholder who is a U.S. holder will generally recognize capital gain or loss for U.S. federal income tax purposes on the receipt of shares of AgeX common stock issued in connection with the Merger. The tax consequences to each Serina stockholder will depend on that stockholder’s particular circumstances. Each Serina stockholder should consult with his, her or its tax advisor for a full understanding of the tax consequences of the Merger to that Serina stockholder.
| Q: | What are the material U.S. federal income tax consequences of the reverse stock split to U.S. holders of AgeX shares? |
| A: | AgeX intends to treat the reverse stock split as a “recapitalization” within the meaning of Section 368(a)(1)(E) of the Code for U.S. federal income tax purposes. Assuming it so qualifies, an AgeX stockholder who is a U.S. holder (as defined in the section titled “Matters Being Submitted to a Vote of AgeX’s Stockholders—Proposal No. 2: Approval of an Amendment to the Certificate of Incorporation of AgeX Effecting the Reverse Stock Split—Material U.S. Federal Income Tax Consequences of the Reverse Stock Split” beginning on page 187 of this proxy statement/prospectus/information statement) should not recognize gain or loss upon the reverse stock split, except with respect to cash received in lieu of a fractional share of AgeX common stock. |
Please review the information in the section titled “Matters Being Submitted to a Vote of AgeX’s Stockholders—Proposal No. 2: Approval of an Amendment to the Certificate of Incorporation of AgeX Effecting the Reverse Stock Split—Material U.S. Federal Income Tax Consequences of the Reverse Stock Split” beginning on page 187 of this proxy statement/prospectus/information statement for a more complete description of the material U.S. federal income tax consequences of the reverse stock split to AgeX U.S. holders.
| Q: | What do I need to do now? |
| A: | AgeX and Serina urge you to read this proxy statement/prospectus/information statement carefully, including its annexes and information incorporated herein, and to consider how the Merger affects you. |
If you are a holder of AgeX common stock, please vote your shares as soon as possible so that your shares will be represented at the AgeX special meeting. Please follow the instructions set forth on the enclosed AgeX proxy card or on the voting instruction form provided by the record holder of your shares if your shares are held in the name of your bank, broker or other nominee.
If you are a stockholder of Serina, you may execute and return your written consent to Serina in accordance with the instructions provided by Serina once this Registration Statement is declared effective by the SEC.
| Q: | When and where is the AgeX special meeting? What must I do to attend the AgeX special meeting? |
| A: | The AgeX special meeting will be held exclusively online via live audio-only webcast on , 2023 at . AgeX encourages you to allow ample time for the online check-in procedures. Please note that you will not be able to attend the AgeX special meeting in person. |
You or your authorized proxy may attend the AgeX special meeting if you were a registered or beneficial stockholder of AgeX common stock as of the record date.
You will be able to vote your shares and submit questions during the AgeX special meeting webcast by logging in to the website listed above using the 16-digit control number included in your proxy card. If your shares are held in “street name,” you should contact your bank, broker or other nominee if you did not receive a 16 digit control number. If you wish to submit a question during the AgeX special meeting, log into the AgeX special meeting platform at www.virtualshareholdermeeting.com/ , type your question into the “Ask a Question” field, and click “Submit.” AgeX will respond to as many properly submitted questions during the relevant portion of the AgeX special meeting agenda as time allows.
If AgeX experiences technical difficulties during the AgeX special meeting (e.g., a temporary or prolonged power outage), AgeX will determine whether the AgeX special meeting can be promptly reconvened (if the technical difficulty is temporary) or whether the AgeX special meeting will need to be reconvened on a later day (if the technical difficulty is more prolonged). In any situation, AgeX will promptly notify stockholders of the decision via .
If you own shares in street name through an account with a bank, broker or other nominee, please refer to the instructions provided by your bank, brokerage firm or other nominee to see which of the above choices are available to you. Please note that if you hold shares in street name and wish to vote virtually at the AgeX special meeting, you must obtain a legal proxy from your bank, brokerage firm or other nominee.
If your shares are registered in your name with AgeX’s stock registrar and transfer agent, Equiniti Trust Company, LLC (Equiniti), no proof of ownership is necessary for you to vote virtually at the AgeX special meeting because AgeX can verify your ownership.
| A: | Votes will be counted by the inspector of elections appointed for the AgeX special meeting, who will separately count votes “FOR” and “AGAINST,” abstentions and, if applicable, broker non-votes. |
AgeX does not expect that any matters other than the AgeX Proposals will be brought before the AgeX special meeting.
| Q: | What are “broker non-votes”? |
| A: | The term “broker non-votes” refers to shares held by a broker, bank or other nominee (for the benefit of its client) that are represented at the AgeX special meeting, but with respect to which such broker, bank or nominee is not instructed to vote on a particular proposal and does not have discretionary authority to vote on that proposal. Broker non-votes, if any, will be treated as shares that are present at the AgeX special meeting for purposes of determining whether a quorum exists but will not have any effect for the purpose of voting on Proposal No. 1 (Stock Issuance Proposal), Proposal No. 2 (Reverse Stock Split Proposal), Proposal No. 3 (Warrant Issuance Proposal) , Proposal No. 5 (2024 Equity Incentive Plan Proposal) and Proposal No. 6 (Adjournment Proposal). Broker non-votes, if any, will have the same effect as “AGAINST” votes for the Proposal No. 4 (A&R Charter Proposal). |
| Q: | What will happen if I return my proxy form without indicating how to vote? |
| A: | If you submit your proxy form without indicating how to vote your shares on any particular proposal, the common stock represented by your proxy will be voted as recommended by the AgeX Board with respect to that proposal. |
| Q: | May I change my vote after I have submitted a proxy or voting instruction form? |
| A: | AgeX’s common stockholders as of the record date, other than those AgeX stockholders who are parties to support agreements, may change their vote at any time before their proxy is voted at the AgeX special meeting in one of following ways: |
| ● | By sending a written notice to the Secretary of AgeX stating that it would like to revoke its proxy; |
| ● | By duly executing a subsequently dated proxy relating to the same shares of AgeX common stock and returning it in the postage-paid envelope provided or similar means, which subsequent proxy is received before the prior proxy is exercised at the AgeX special meeting; |
| ● | Duly submitting a subsequently dated proxy relating to the same shares of common stock by telephone or via the internet (i.e., your most recent duly submitted voting instructions will be followed) before 11:59 p.m. Eastern time on ; and |
| ● | By attending the AgeX special meeting and voting such shares during the AgeX special meeting. |
If a stockholder who owns AgeX shares in “street name” has instructed a broker to vote its shares of AgeX common stock, the stockholder must follow directions received from its broker to change those instructions.
| Q: | Who is paying for this proxy solicitation? |
| A: | AgeX will pay for the cost of printing and filing of this proxy statement/prospectus/information statement and the proxy card. Arrangements will also be made with brokerage firms and other custodians, nominees and fiduciaries who are record holders of AgeX common stock for the forwarding of solicitation materials to the beneficial owners of AgeX common stock. AgeX will reimburse these brokers, custodians, nominees and fiduciaries for the reasonable out-of-pocket expenses they incur in connection with the forwarding of solicitation materials. Fees paid to the SEC in connection with filing the statement/prospectus/information statement, and any amendments and supplements thereto, with the SEC will be paid by AgeX. |
| Q: | What is the quorum requirement? |
| A: | A quorum of stockholders is necessary to hold a valid meeting. The presence at the AgeX special meeting, present in person or represented by proxy, of the holders of a majority of the voting power of the stock issued, outstanding and entitled to vote thereat, as of the record date, will constitute a quorum for the transaction of business at the AgeX special meeting. |
Your shares will be counted towards the quorum if you submit a valid proxy (or one is submitted on your behalf by your broker, bank or other nominee) or if you attend the AgeX special meeting and vote your shares during the AgeX special meeting. Abstentions and broker non-votes, if applicable, will also be counted towards the quorum requirement. If there is no quorum, the holders of a majority of shares present at the AgeX special meeting or represented by proxy may adjourn the meeting to another date.
| Q: | Should AgeX’s stockholders send in their stock certificates now, to the extent they have any? |
| A: | AgeX stockholders should not send in their stock certificates, if any. For more information on the procedures for exchanging stock certificates, please see the section titled “The Merger—Procedures for Exchange Stock Certificates” beginning on page 147 of this proxy statement/prospectus/information statement. |
| Q: | Who can help answer my questions? |
| A: | If you are a stockholder of AgeX and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the Merger or related matters, including the procedures for voting your shares, you should contact: |
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 945013
Attention: Andrea Park
Phone Number: (510) 671-8370
Email: information@agexinc.com
Subject Line: Request for Proxy Materials
If you are a stockholder of Serina, and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the Merger or related matters, including the procedures for voting your shares, you should contact:
601 Genome Way, Suite 2001,
Huntsville, Alabama 35806
(256) 327-9630
investor.relations@serinatherapeutics.com
Attention: Chief Financial Officer
PROSPECTUS SUMMARY
This summary highlights selected information from this proxy statement/prospectus/information statement and may not contain all of the information that is important to you. To better understand the Merger, the proposals being considered at the AgeX special meeting and Serina’s stockholder actions that are the subject of the written consent, you should read this entire proxy statement/prospectus/information statement carefully, including the Merger Agreement attached as Annex A and the other annexes to which you are referred herein. For more information, please see the section titled “Where You Can Find More Information” beginning on page 379 of this proxy statement/prospectus/information statement.
The Companies
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
(510) 671-8370
AgeX is a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases. AgeX’s mission is to apply its comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions. AgeX believes that demand for therapeutics addressing such conditions is on the rise, commensurate with the demographic shift of aging in the United States and many other industrialized countries.
AgeX’s proprietary technology, based on telomerase-mediated cellular immortality and regenerative biology, allows it to utilize telomerase-expressing regenerative pluripotent stem cell (PSCs) for the manufacture of cell-based therapies to regenerate tissues afflicted with age-related chronic degenerative disease. AgeX owns or has licenses to a number of patents and patent applications used in the generation of these product candidates, including intellectual property related to PSC-derived clonal embryonic progenitor cell lines (PureStem® technology). AgeX’s technology platform also includes UniverCyte™ which uses the Human Leukocyte Antigen-G (HLA-G) gene to potentially confer low immune observability to cells, so as to suppress rejection of transplanted cells and tissues. AgeX plans to use or license the use of this patented technology to produce genetically-modified master cell banks of pluripotent stem cells that can then be differentiated into any young cell type of the human body that now express the immune tolerogenic molecule.
AgeX’s product candidates in the discovery stage include two cell-based therapies derived from telomerase-positive PSCs and two product candidates derived from its proprietary induced tissue regeneration (iTRTM) technology. AgeX has also sponsored a research program to derive neural stem cells from PSCs to treat degenerative diseases such as Huntington’s Disease. AgeX will need to conduct or sponsor research and development work, or license our technology to other biotechnology or pharmaceutical companies interested in furthering research and development in order to develop these cell- and drug-based therapies, each targeting large unmet needs in age-related medicine.
On August 29, 2023, AgeX entered into the Merger Agreement with Serina and Merger Sub, pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into Serina, with Serina surviving as our wholly owned subsidiary. For additional information, see the sections titled “The Merger” and “The Merger Agreement” beginning on pages 134 and 153 of this proxy statement/prospectus/information statement, respectively.
If the Merger is completed, the combined company will primarily focus on developing Serina’s product candidates, which are described on page 231 under the section titled “Description of Serina’s Business.” If the Merger is not completed, AgeX expects to continue to execute on its current business strategies as more fully described in the section titled “Description of AgeX’s Business—Business Strategy” beginning on page 207 of this proxy statement/prospectus/information statement while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs.
Serina Therapeutics, Inc.
601 Genome Way, Suite 2001,
Huntsville, Alabama 35806
(256) 327-9630
Serina is a clinical-stage biotechnology company developing a pipeline of wholly-owned drug product candidates to treat neurological diseases and pain. Serina’s POZ PlatformTM (POZ) drug delivery technology is designed to enable certain existing drugs and novel drug candidates to be modified in a way that may provide an increase in efficacy and safety of the resulting polymeric drug conjugate. Serina’s proprietary POZ technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection.
The therapeutic agents in Serina’s product candidates are typically well-understood and marketed drugs that are effective but are limited by pharmacokinetic profiles that can include toxicity, side effects and short half-life. Serina believes that by using POZ technology, drugs with narrow therapeutic windows can be designed to maintain more desirable and stable levels in the blood. Serina believes that POZ technology can be applied to small molecules, proteins, antibody drug conjugates, and other classes of molecules.
Serina’s business is largely focused on the development of a wholly-owned pipeline of POZ-enabled drug candidates for central nervous system (CNS) indications, including Parkinson’s disease, epilepsy, and pain. Serina’s lead product candidate, SER 252 (POZ-apomorphine), is a POZ conjugate of the potent dopamine agonist apomorphine being developed for the treatment of Parkinson’s disease and is in preclinical development. SER 252 is designed to provide continuous dopaminergic stimulation (CDS) via a subcutaneous injection delivered one to two times per week. CDS is a long-sought clinical strategy for Parkinson’s disease that currently approved therapies fall short of delivering. Other POZ-conjugate candidates, including SER 227 (POZ-buprenorphine) for long-acting pain relief and SER 228 (POZ-cannabidiol) for epilepsy, are in preclinical development. Serina intends to develop other potential applications of the POZ technology, such as therapeutics delivered through antibody drug conjugates (ADCs) and lipid-nanoparticle (LNP), through partnerships. Serina’s SER 214, a POZ conjugate of rotigotine for the treatment of early Parkinson’s and Restless Leg Syndrome, was Serina’s first product candidate advanced into human study and has completed a Phase Ia study in 19 subjects. Serina has not internally advanced SER 214 beyond the Phase Ia study and will seek to partner on any further development of this product candidate.
Canaria Transaction Corporation
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
(510) 671-8370
Merger Sub is a wholly owned subsidiary of AgeX and was formed solely for the purposes of carrying out the Merger.
The Merger (see page 134)
If the Merger is completed, Merger Sub will merge with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX. The surviving corporation following the Merger is referred to herein as the “combined company.” Substantially concurrently with the completion of the Merger, the combined company will be renamed “Serina Therapeutics, Inc.”
Following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock as of the Warrant Dividend Record Date three post-merger warrants for each five shares of AgeX common stock issued and outstanding held by such holder as of the Warrant Dividend Record Date. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one incentive warrant and will expire on July 31, 2025. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock and will expire on the four-year anniversary of the Closing Date. For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 180 of this proxy statement/prospectus/information statement.
AgeX and Serina expect the Merger to be consummated during the , subject to satisfaction or waiver of certain conditions to the closing of the Merger, including, among other things, the Required AgeX Closing Stockholder Matters.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant. For a more complete description of the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
Reasons for the Merger (see pages 134 and 134)
The AgeX Board considered various reasons for the Merger, as described later in this proxy statement under the section titled “The Merger—AgeX Reasons for the Merger.”
The Serina Board considered various reasons for the Merger, as described later in this proxy statement under the section titled “The Merger—Serina Reasons for the Merger.”
Merger Consideration and Exchange Ratio (see page 146)
Pursuant to the Merger Agreement, at the Effective Time, each outstanding share of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of AgeX common stock equal to the exchange ratio.
No fractional shares of AgeX common stock will be issued in connection with the Merger, and no certificates or scrip for any such fractional shares will be issued. Any holder of Serina capital stock who would otherwise be entitled to receive a fraction of a share of AgeX common stock (after aggregating all fractional shares of AgeX common stock issuable to such holder) will receive from AgeX, in lieu of such fraction of a share and upon such holder’s surrender of a letter of transmittal in accordance with the Merger Agreement and any accompanying documentation required therein, (i) one share of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina capital stock would otherwise be entitled to is equal to or exceeds 0.50 or (ii) no shares of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina capital stock would otherwise be entitled to is less than 0.50.
The exchange ratio formula is based upon a fixed percentage of the post-Merger outstanding shares of AgeX common stock, expressed on a fully-diluted and as-converted basis, subject to certain adjustments and exclusions. For a more complete description of the Merger, the potential adjustments in the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
Treatment of Serina Stock Options (see page 156)
Under the terms of the Merger Agreement, at the Effective Time, each outstanding and unexercised Serina option immediately prior to the Effective Time under the Serina Plan will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option.
Treatment of Serina Warrants (see page 156)
Under the terms of the Merger Agreement, at the Effective Time, each outstanding and unexercised Serina warrant immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Treatment of AgeX Stock Options (see page 157)
Immediately prior to the reverse stock split, each outstanding and unexercised out of the money AgeX option as of immediately prior to the reverse stock split, if any, will be canceled without payment. In accordance with the terms and conditions set forth in the Incentive Plan, AgeX will notify each holder of an out of the money AgeX option that each such option will become exercisable at least 10 days prior to the reverse stock split and will thereafter be canceled if not exercised. All other outstanding and unexercised options to purchase shares of AgeX common stock will remain outstanding and exercisable in accordance with their terms immediately following the Effective Time.
Conditions to the Completion of the Merger (see page 157)
The obligations to consummate the Merger and the other transactions and actions contemplated by the Merger Agreement (collectively, the Contemplated Transactions) are subject to the satisfaction or waiver, on or prior to the Effective Time, of the conditions set forth in the section titled “The Merger Agreement—Conditions to the Completion of the Merger” below.
Non-Solicitation (see page 163)
AgeX and Serina and their respective subsidiaries are prohibited by the terms of the Merger Agreement, other than, in the case of AgeX, with respect to any Asset Disposition (as defined below), from, directly or indirectly, (i) soliciting, initiating or knowingly encouraging, inducing or facilitating the communication, making, submission or announcement of any Acquisition Proposal (as defined below) or Acquisition Inquiry (as defined below) or taking any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnishing any non-public information regarding itself or its respective subsidiaries to any person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engaging in discussions or negotiations with any person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approving, endorsing or recommending any Acquisition Proposal (subject to, in the case of AgeX, AgeX’s special meeting and the AgeX Proposals); (v) executing or entering into any letter of intent or any contract contemplating or otherwise relating to any Acquisition Transaction (as defined below) (other than, in the case of AgeX, a confidentiality agreement permitted as described below); or (vi) publicly proposing to do any of the foregoing.
Subject to certain restrictions and prior to obtaining the approval of the AgeX Stockholder Matters by the Required AgeX Stockholder Vote (as defined below), AgeX and its subsidiaries may furnish non-public information regarding AgeX or any of its subsidiaries to, and enter into discussions or negotiations with, any person in response to a bona fide Acquisition Proposal by such person, which the AgeX Board determines in good faith, after consultation with its outside financial advisor and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (as defined below) (and is not withdrawn) if: (A) none of AgeX, any of its subsidiaries or any of their respective representatives have breached the non-solicitation restrictions in the Merger Agreement in any material respect, (B) the AgeX Board concludes in good faith, based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of the AgeX Board under applicable law; (C) substantially contemporaneously with furnishing any such non-public information to such person, AgeX gives Serina notice of AgeX’s intention to furnish non-public information to, or enter into discussions with, such person and furnishes such nonpublic information to Serina (to the extent such information has not been previously furnished to Serina), and (D) AgeX receives from such person an executed confidentiality agreement containing provisions (including nondisclosure provisions, use restrictions, non-solicitation provisions, no hire and “standstill” provisions), in the aggregate, at least as favorable to AgeX as those contained in the confidentiality agreement entered into between AgeX and Serina in connection with the Merger.
For a more complete description of the non-solicitation provisions, please see the section titled “The Merger Agreement—Non-Solicitation.”
Termination and Termination Fees (see page 173)
The Merger Agreement contains certain customary termination rights, including the right of AgeX to terminate the Merger Agreement if the Serina Stockholder Written Consent (as defined below) has not been obtained within three business days of the date of the registration statement becoming effective, the right of AgeX or Serina to terminate the Merger Agreement if AgeX’s stockholders fail to adopt and approve the AgeX Stockholder Matters, the right of Serina to terminate the Merger Agreement if the AgeX Board changes or withdraws its recommendation in favor of the AgeX Stockholder Matters or approves or enters into an agreement relating to an Acquisition Proposal, the right of AgeX to terminate the Merger Agreement under certain circumstances to enter into an agreement relating to a Superior Offer and the right of Serina to terminate if certain closing conditions are not satisfied or waived prior to Closing.
Upon termination of the Merger Agreement by Serina or AgeX in certain circumstances, a nonrefundable termination fee of $1,000,000 may be payable by Serina to AgeX or by AgeX to Serina. AgeX has agreed to reimburse Serina for up to $1,000,000 in expenses if the Merger Agreement is terminated due to the failure to obtain the approval of the AgeX Stockholder Matters from AgeX’s stockholders or in the event of the failure of Serina to consummate the Contemplated Transactions solely as a result of an AgeX Material Adverse Effect (as defined below). Serina has agreed to reimburse AgeX for up to $1,000,000 in expenses if the Merger Agreement is terminated due to a breach or inaccuracy of any representation, warranty, covenant or agreement by Serina or in the event of the failure of AgeX to consummate the Contemplated Transactions solely as a result of a Serina Material Adverse Effect. AgeX or Serina may receive either the termination fee or the expense reimbursement, but not both.
For a more complete description of the termination provisions and termination fees, please see the section titled “The Merger Agreement—Termination and Termination Fees.”
Support Agreements (see page 179)
Concurrently with the execution of the Merger Agreement, Juvenescence, a stockholder of AgeX holding approximately 43.3% of the outstanding shares of common stock of AgeX, 100% of the outstanding shares of Series A preferred stock of AgeX and 100% of the outstanding shares of Series B preferred stock of AgeX, in each case as of August 29, 2023, entered into the AgeX Support Agreement in favor of Serina, providing, among other things, that Juvenescence will vote all of its shares of AgeX capital stock in favor of the AgeX Stockholder Matters.
In connection with the execution of the Merger Agreement, stockholders of Serina holding 100% of the outstanding shares of common stock of Serina, 100% of the outstanding shares of Series A preferred stock of Serina, 100% of the outstanding shares of Series A-1 preferred stock of Serina, 100% of the outstanding shares of Series A-2 preferred stock of Serina, 100% of the outstanding shares of Series A-3 preferred stock of Serina, 100% of the outstanding shares of Series A-4 preferred stock of Serina, and 100% of the outstanding shares of Series A-5 preferred stock of Serina, in each case effective as of August 29, 2023, entered into Serina Support Agreements in favor of AgeX, providing, among other things, that such stockholders will vote all of their shares of Serina capital stock (or execute a written consent) in favor of Serina Stockholder Matters.
Lock-Up Agreements (see page 179)
Concurrently with the execution of the Merger Agreement, (i) certain executive officers, directors, and stockholders of Serina and (ii) certain stockholders of AgeX, entered into lock-up agreements (Lock-Up Agreements), pursuant to which such persons accepted certain restrictions on transfers of the shares of AgeX common stock held by such persons for the 180-day period following the Effective Time.
Appraisal Rights (see page 151)
AgeX stockholders are not entitled to appraisal rights in connection with the Merger. Unless consented to by the Serina stockholder, holders of Serina capital stock are entitled to appraisal rights in connection with the Merger under Article 13 of the ABCL. For more information about such rights, please see the provisions of Article 13 of the ABCL attached as Annex D and the section titled “The Merger—Appraisal Rights” beginning on page 151 of this proxy statement/prospectus/information statement.
Management Following the Merger (see page 130)
Immediately following the Merger, the executive management team of the combined company is expected to comprise the following individuals with such additional officers as may be added by Serina or the combined company:
| Name | | Position with the Combined Company | | Current Position at AgeX / Serina |
| Steve Ledger | | Interim Chief Executive Officer | | Chief Financial Officer at Serina |
| Andrea Park | | Interim Chief Financial Officer and Chief Accounting Officer | | Chief Financial Officer at AgeX |
| Randall Moreadith, M.D., Ph.D. | | Chief Scientific Officer | | President and Chief Executive Officer at Serina |
| Tacey Viegas | | Chief Operating Officer and Secretary | | Chief Operating Officer at Serina |
Directors of the Combined Company Following the Merger
At the Effective Time, the combined company is expected to initially have a seven-member board of directors, comprised of (a) Remy Gross, designated by both AgeX and Serina, (b) J. Milton Harris and Steve Ledger, each as a Serina designee, (c) Gregory Bailey and Richard Marshall, each as an AgeX designee, and (d) and , each as an independent director, until their respective successors are duly elected or appointed and qualified or their earlier death, resignation or removal.
The Combined Company Board will have an audit committee, a compensation committee and a nominating and corporate governance committee, in accordance with NYSE American rules. All of AgeX’s current directors, other than Gregory Bailey, are expected to resign from their positions as directors of AgeX, effective as of the Effective Time.
Interests of the AgeX Directors and Executive Officers in the Merger (see page 143)
In considering the recommendation of the AgeX Board with respect to the issuance of AgeX common stock pursuant to the Merger Agreement and the other matters to be acted upon by AgeX’s stockholders at the AgeX special meeting, AgeX’s stockholders should be aware that certain members of the AgeX Board and current and former executive officers of AgeX have interests in the Merger that may be different from, or in addition to, interests they have as AgeX’s stockholders. These interests may present them with actual or potential conflicts of interest. These interests include the following:
| ● | Dr. Bailey will continue as a director of the combined company after the Effective Time, and, following the closing of the Merger, will be eligible to be compensated as a non-employee director of the combined company pursuant to the non-employee director compensation policy in place following the Effective Time; |
| ● | Ms. Park will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the combined company following the Effective Time; |
| ● | under the Merger Agreement, AgeX’s directors and executive officers are entitled to continued indemnification, expense advancement and insurance coverage; and |
| ● | Juvenescence, an affiliated stockholder of AgeX, entered into the AgeX Support Agreement in favor of Serina, providing, among other things, that Juvenescence will vote all of its shares of AgeX capital stock in favor of the AgeX Stockholder Matters. The Support Agreements are discussed in greater detail in the section titled “Agreements Related to the Merger” beginning on page 179 of this proxy statement/prospectus/information statement. |
The interests of AgeX’s officers and directors in the Merger are discussed in greater detail in the section titled “The Merger—Interests of the AgeX Directors and Executive Officers in the Merger” beginning on page 141 of this proxy statement/prospectus/information statement.
Interests of the Serina Directors and Executive Officers in the Merger (see page 141)
In considering the recommendation of the Serina Board with respect to adopting the Merger Agreement, Serina’s stockholders should be aware that members of the Serina Board and the executive officers of Serina may have interests in the Merger that may be different from, or in addition to, the interests of Serina’s stockholders. The Serina Board was aware of these potential conflicts of interest and considered them, among other matters, in reaching its decision to approve the Merger Agreement, the Merger, and the Contemplated Transactions. These interests may present them with actual or potential conflicts of interest. These interests include the following:
| ● | as described elsewhere in this proxy statement/prospectus/information statement, including in the section titled “The Merger—Management Following the Merger,” certain of Serina’s directors and executive officers are expected to become the directors and executive officers of the combined company upon the closing of the Merger; |
| ● | in connection with the Merger, each outstanding and unexercised Serina option immediately prior to the Effective Time under the Serina Plan held by Serina’s executive officers and directors will be converted into and become an option to purchase AgeX common stock; |
| ● | each of Serina’s directors and executive officers are entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement; and |
| ● | Serina’s executive officers, directors and certain affiliated stockholders have entered into the Support Agreements, pursuant to which such directors, officers and certain stockholders, respectively, have agreed, solely in their capacity as stockholders of Serina, to vote all of their shares of Serina capital stock in favor of, among other things, the adoption or approval, respectively, of the Merger Agreement and the transactions contemplated therein in connection with the Merger. The Support Agreements are discussed in greater detail in the section titled “Agreements Related to the Merger” beginning on page 179 of this proxy statement/prospectus/information statement. |
Risk Factors and Risk Factor Summary (see page 25)
Both AgeX and Serina are subject to various risks associated with their businesses and their industries. In addition, the Merger, including the possibility that the Merger may not be completed, poses a number of risks to each company and its respective stockholders. You should carefully read this proxy statement/prospectus/information statement, including the annexes, and especially consider the material risks discussed below and these and other risks discussed in greater detail under the section titled “Risk Factors” beginning on page 25 of this proxy statement/prospectus/information statement. AgeX and Serina encourage you to read and consider all of these risks carefully.
Risks Related to the Merger
| ● | The relative proportion of the combined company that AgeX stockholders will own immediately following the closing of the Merger is not adjustable based on the market price of AgeX common stock unless the Actual Closing Price of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis). If the Actual Closing Price of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis), AgeX has the option to issue additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum, which, in such circumstances, could proportionally decrease the amount of the combined company that AgeX stockholders would own immediately following the closing of the Merger. Accordingly, the Merger consideration at the closing of the Merger may have a greater or lesser value than at the time the Merger Agreement was signed; |
| ● | Failure to complete the Merger may result in AgeX or Serina paying a termination fee or reimbursement of expenses to the other party and could harm the common stock price of AgeX and future business and operations of each company; |
| ● | The issuance of AgeX common stock to Serina stockholders pursuant to the Merger Agreement and the resulting change in control of AgeX from the Merger must be approved by AgeX stockholders, and the Merger Agreement and transactions contemplated thereby must be approved by the Serina stockholders. Failure to obtain these approvals would prevent the closing of the Merger; |
| ● | If the conditions to the closing of the Merger are not satisfied or waived, the Merger may not occur or the closing of the Merger could be delayed; |
| ● | The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and/or other causes; |
| ● | Some executive officers and directors of AgeX and Serina have interests in the Merger that are different from the respective stockholders of AgeX and Serina and that may influence them to support or approve the Merger without regard to the interests of the respective stockholders of AgeX and Serina; |
| ● | Juvenescence owns a significant majority of AgeX capital stock and will be able to substantially influence AgeX and exert control over the AgeX Proposals; |
| ● | AgeX stockholders and Serina stockholders may not realize a benefit from the Merger commensurate with the ownership interest dilution they will experience in connection with the Merger; |
| ● | If the Merger is not completed, the market price of AgeX common stock may decline significantly; |
| ● | The market price of the combined company’s common stock following the Merger may decline as a result of the Merger; |
| ● | AgeX and Serina securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the combined company following the closing of the Merger as compared to their current ownership and voting interest in the respective companies; |
| ● | During the pendency of the Merger Agreement, each of AgeX and Serina may be limited in its ability to enter into a business combination with another party on more favorable terms because of restrictions in the Merger Agreement, which could adversely affect their respective business prospects; |
| ● | Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the transactions contemplated by the Merger Agreement; |
| ● | Because the lack of a public market for Serina’s capital stock makes it difficult to evaluate the fair market value of Serina’s capital stock, the value of the AgeX common stock to be issued to Serina stockholders may be more or less than the fair market value of Serina common stock; |
| ● | The Merger may fail to qualify as a reorganization for U.S. federal income tax purposes, resulting in recognition of taxable gain or loss by Serina stockholders in respect of their Serina capital stock; and |
| ● | Litigation could arise in connection with the Merger, against AgeX, the AgeX Board, Serina or the Serina Board, which could be costly, prevent the consummation of the Merger, divert management’s attention and otherwise materially harm AgeX’s, Serina’s or the combined company’s business. |
Risks Related to the Reverse Stock Split
| ● | The reverse stock split may not increase the combined company’s stock price over the long term; |
| ● | The reverse stock split may decrease the liquidity of AgeX common stock or the combined company’s common stock; and |
| ● | The reverse stock split may lead to a decrease in the combined company’s overall market capitalization. |
Risks Related to AgeX
| ● | AgeX needs additional financing to execute its operating plan and continue to operate; |
| ● | As a discovery-stage development company with incurred operating losses and limited capital resources, AgeX anticipates that it will incur continued losses for the foreseeable future and will need to continue to raise capital to finance our operations and is unable to predict whether it will achieve or sustain profitability; |
| ● | AgeX is highly leveraged, carrying a significant amount of indebtedness, including indebtedness secured by its assets, that will become due and payable over the next three years and there is no assurance that AgeX will be able to refinance those obligations as they become due; |
| | ● | The terms of our loans from Juvenescence and the Security Agreement could make it more difficult for us to raise additional capital from other sources; |
| | ● | Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates; |
| ● | Unless AgeX common stock continues to be listed on a national securities exchange, it will become subject to “penny stock” rules that impose restrictive sales practice requirements; |
| ● | Delays in, or failing to complete the Merger could materially and adversely affect AgeX’s results of operations, business, financial condition or stock price; |
| | ● | If the Merger is not approved or does not occur, AgeX may not be successful in the execution of its current business strategies or identifying and implementing any strategic alternatives with respect to its assets and development programs, and any future strategic alternatives could have negative consequences; |
| ● | As a major stockholder and creditor of AgeX, Juvenescence, will be able to substantially influence AgeX and exert control over matters subject to stockholder approval; and |
| ● | Conflicts of interest may arise from AgeX’s relationship with Juvenescence, which owns a significant percentage of AgeX common stock as well as shares of AgeX preferred stock and is a significant creditor and will be able to substantially influence AgeX and exert control over matters subject to stockholder approval, including the Merger and other AgeX Proposals. |
Risks Related to Serina
| ● | Serina has a history of operating losses that are expected to continue for the foreseeable future, and it is unable to predict the extent of future losses, or whether it will generate significant revenues or achieve or sustain profitability; |
| ● | Serina will need additional financing to execute its operating plan and continue to operate as a going concern. |
| ● | Serina has never generated revenue from product sales and may never become profitable; |
| ● | Serina’s product candidates are at an early stage of development and may not be successfully developed or commercialized; |
| ● | Failure of Serina’s technology would significantly harm its business, results of operations, and prospects; |
| ● | The FDA regulatory approval process is lengthy, time-consuming, and inherently unpredictable, and Serina may experience significant delays in the clinical development and regulatory approval, if any, of its product candidates; |
| ● | If any product candidate that Serina successfully develops does not achieve broad market acceptance among physicians, patients, health care payors and the medical community, the revenues that it generates from their sales will be limited; and |
| ● | Serina success will depend upon intellectual property and proprietary technologies, and it may be unable to protect its intellectual property. |
Risks Related to the Combined Company
| ● | The combined company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms or at all; |
| ● | The market price of the combined company’s common stock is expected to be volatile, and the market price of the common stock may drop following the Merger; |
| ● | The combined company will incur costs and demands upon management as a result of complying with the laws, rules and regulations affecting public companies; |
| ● | Anti-takeover provisions in the combined company’s governance documents and under Delaware law could make an acquisition of the combined company more difficult and may prevent attempts by the combined company stockholders to replace or remove the combined company management; and |
| ● | If the combined company fails to attract and retain management and other key personnel, it may be unable to continue to successfully develop or commercialize its product candidates or otherwise implement its business plan. |
Regulatory Approvals (see page 147)
In the United States, AgeX must comply with applicable federal and state securities laws and the rules and regulations of the NYSE American in connection with the issuance of shares of AgeX common stock to Serina’s stockholders in connection with the Contemplated Transactions and the filing of this proxy statement/prospectus/information statement with the SEC. Neither AgeX nor Serina intend to seek any regulatory approval from antitrust authorities to consummate the Contemplated Transactions.
Resale by the Selling Stockholder (see page 374)
This proxy statement/prospectus/information statement additionally provides for the registration and resale of shares of AgeX common stock issuable upon the exercise of the post-merger warrants and the incentive warrants (the Resale Shares) received by the selling stockholder of shares of AgeX common stock issuable upon the exercise of the post-merger warrants and the incentive warrants (the Selling Stockholder) pursuant to the terms of the Merger Agreement upon consummation of the Contemplated Transactions. AgeX will not receive any proceeds from the sale of the Resale Shares by the Selling Stockholder pursuant to this proxy statement/prospectus/information statement. See the sections titled “Use of Proceeds” and “Selling Stockholder” beginning on pages 372 and 374 of this proxy statement/prospectus/information statement for further information.
The Selling Stockholder may sell all or a portion of the Resale Shares owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. Registration of the Resale Shares covered by this proxy statement/prospectus/information statement does not mean, however, that such shares necessarily will be offered or sold. See the section titled “Plan of Distribution” beginning on page 375 of this proxy statement/prospectus/information statement for further information.
NYSE American Listing (see page 150)
Shares of AgeX common stock are currently listed on the NYSE American market under the symbol “AGE.” AgeX intends to file an initial listing application for the combined company with the NYSE American. If such application is accepted, AgeX anticipates that the common stock of the combined company will be listed on NYSE American following the closing of the Merger under the trading symbol “SER.”
Anticipated Accounting Treatment (see page 151)
The Merger will be treated by AgeX as a reverse recapitalization under U.S. generally accepted accounting principles (GAAP). For accounting purposes, Serina is considered to be the accounting acquirer in the Merger.
Comparison of Rights of Stockholders of AgeX, Serina and the combined company (see page 359)
AgeX is incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of AgeX currently, and will continue to be, governed by the DGCL. Serina is incorporated under the laws of the State of Alabama and, accordingly, the rights of the stockholders of Serina are currently governed by the ABCL.
If the Merger is completed, the AgeX stockholders and Serina stockholders will become the stockholders of the combined company. After giving effect to the Merger, the rights of stockholders of the combined company and the relative powers of the Combined Company Board will be governed by an amendment and restatement of the AgeX Charter (the Combined Company Charter), the bylaws of the combined company (the Combined Company Bylaws) and by the DGCL. Each share of the common stock of the combined company will be issued pursuant to, and will carry with it the rights and obligations set forth in, the Combined Company Charter. As a result of differences between the respective organizational documents of AgeX, Serina and the combined company, and applicable governing law, there will be material differences between the rights of AgeX stockholders and Serina stockholders before consummation of the Merger and the rights of the combined company stockholders after consummation of the Merger. For a full description of these differences, see the section titled “Comparison of Rights of Stockholders of AgeX, Serina and the Combined Company” beginning on page 359 of this proxy statement/prospectus/information statement.
AgeX Stockholder Meeting (see page 165)
The AgeX special meeting will be held exclusively online via audio-only webcast on , 2024 at , unless postponed or adjourned to a later date. The AgeX special meeting can be accessed by visiting www.virtualshareholdermeeting.com/ , where you will be able to vote your shares and submit questions during the AgeX special meeting webcast by logging in to the website listed above using the 16-digit control number included in your proxy card. AgeX encourages you to allow ample time for the online check-in procedures. Please note that you will not be able to attend the AgeX special meeting in person. For more information on the AgeX special meeting, see the section titled “The Special Meeting of AgeX’s Stockholders” beginning on page 126 of this proxy statement/prospectus/information statement.
Market Price and Dividend Information
AgeX common stock is currently listed on the NYSE American under the symbol “AGE.” Serina is a private company and its common stock and preferred stock are not publicly traded.
AgeX Common Stock
The closing price of AgeX common stock on August 28, 2023, the last trading day prior to the public announcement of the Merger on August 29, 2023, as reported on the NYSE American, was $0.75 per share. The closing price of AgeX common stock on , 2023, the last practicable date before the date of this proxy statement/prospectus/information statement, as reported on the NYSE American, was $ per share.
Because the market price of AgeX common stock is subject to fluctuation, the market value of the shares of AgeX common stock that Serina stockholders will be entitled to receive in the Merger may increase or decrease.
Assuming successful application for initial listing with the NYSE American, following the consummation of the Merger, AgeX anticipates that the common stock of the combined company will trade under the new name “Serina Therapeutics, Inc.” and the new trading symbol “SER” on the NYSE American.
As of , 2023, the record date for the AgeX special meeting, there were approximately holders of record of the AgeX common stock.
Dividends
AgeX has never declared or paid any cash dividends on the AgeX common stock and does not anticipate paying cash dividends on AgeX common stock for the foreseeable future. Notwithstanding the foregoing, any determination to pay cash dividends subsequent to the Merger will be at the discretion of the combined organization’s then-current board of directors and will depend upon a number of factors, including the combined organization’s results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the then-current board of directors deems relevant.
Serina has $10,826,718 of accrued and unpaid dividends on its Series A ($2,050,373), Series A-1 ($1,868,125), and Series A-2 ($6,908,220), preferred stock, which is part of the liquidation preference of those series of Serina’s preferred stock. The stockholders of Serina voted on June 27, 2019 to amend Serina’s certificate of incorporation to cease the accrual of dividends on those series of Serina’s preferred stock effective as of June 30, 2019. The certificate of amendment was recorded on June 28, 2019. Serina’s Series A-3, Series A-4, and Series A-5 preferred stock do not have any accrued dividends. Serina has never declared or paid cash dividends on any of Serina’s capital stock. Any future determination to declare cash dividends on shares of the combined company’s common stock will be made at the discretion of the combined company’s board of directors, subject to applicable law and contractual restrictions, and will depend on its financial condition, results of operations, capital requirements, general business conditions and other factors that its board of directors may deem relevant.
RISK FACTORS
The combined company will be faced with a market environment that cannot be predicted and that involves significant risks, many of which will be beyond its control. In addition to the other information contained in this proxy statement/prospectus/ information statement, you should carefully consider the material risks described below before deciding how to vote your shares of AgeX common stock. You should also read and consider the other information in this proxy statement/prospectus/information statement. Realization of any of the risks described below or any of the uncertainties described under “Cautionary Statement Regarding Forward-Looking Statements” could have a material adverse effect on AgeX’s, Serina’s or the combined company’s businesses, financial condition, cash flows and results of operations. Please see the section titled “Where You Can Find More Information” beginning on page 379 of this proxy statement/prospectus/information statement for further information.
Risks Related to the Merger
The relative proportion of the combined company that AgeX stockholders will own immediately following the closing of the Merger is not adjustable based on the market price of AgeX common stock unless the Actual Closing Price of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis). If the Actual Closing Price of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis), AgeX has the option to issue additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum, which, in such circumstances, could proportionally decrease the amount of the combined company that AgeX stockholders would own immediately following the closing of the Merger. Accordingly, the Merger consideration at the closing of the Merger may have a greater or lesser value than at the time the Merger Agreement was signed.
The relative proportion of the combined company that AgeX stockholders will own immediately following the closing of the Merger is based on the relative valuations of AgeX and Serina as negotiated by the parties and as specified in the Merger Agreement. At the Effective Time, outstanding shares of Serina common stock will be converted into shares of AgeX common stock. Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of the common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions. It is a closing condition of the Merger that the Actual Closing Price of a share of AgeX common stock is not less than $12.00 per share (on a post-reverse stock split basis). However, if the Actual Closing Price of a share of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis), AgeX has the option to satisfy such closing condition by issuing additional AgeX common stock to Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum (as defined below), which is equal to $12.00 multiplied by the anticipated aggregate number of shares of AgeX common stock to be issued (approximately 7,500,000 on a post-reverse stock split basis prior to any such adjustment), and, in such circumstances, AgeX stockholders immediately prior to the closing of the Merger could own a smaller percentage, and Serina stockholders immediately prior to the closing of the Merger could own a larger percentage, of the combined company following the closing of the Merger. The exercise of this option would also affect the exchange ratio.
In addition, changes in the market price of AgeX common stock before the completion of the Merger may not affect the number of shares Serina stockholders will be entitled to receive pursuant to the Merger Agreement so long as the Actual Closing Price of AgeX common stock is equal to or greater than $12.00 per share (on a post-reverse stock split basis). If before the completion of the Merger, the market price of AgeX common stock increases to be greater than $12.00 per share (on a post-reverse stock split basis), then Serina stockholders could receive merger consideration with substantially more value for their shares of Serina capital stock than the parties had negotiated when they established the relative ownership proportions of the combined company immediately following the closing of the Merger and the exchange ratio.
For a more complete description of the potential adjustments to and exclusions from the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of this proxy statement/prospectus/information statement.
Failure to complete the Merger may result in AgeX or Serina paying a termination fee or reimbursement of expenses to the other party and could harm the common stock price of AgeX and future business and operations of each company.
If the Merger is not completed, each of AgeX and Serina is subject to the following risks:
| ● | upon termination of the Merger Agreement, AgeX may be required to pay Serina a termination fee of $1,000,000 million or up to $1,000,000 in expense reimbursements; or Serina may be required to pay AgeX a termination fee of $1,000,000 million or up to $1,000,000 in expense reimbursements; |
| ● | the parties will have incurred significant expenses related to the Merger, such as legal and accounting fees, which must be paid even if the Merger is not completed; |
| ● | the price of AgeX’s common stock may decline and remain volatile; and |
| ● | the reputation of AgeX or Serina may be adversely impacted. |
In addition, if the Merger Agreement is terminated and the AgeX Board or Serina Board determines to seek another business combination, there can be no assurance that either AgeX or Serina will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided by each party in the Merger or any partner at all.
The issuance of AgeX common stock to Serina stockholders pursuant to the Merger Agreement and the resulting change in control of AgeX from the Merger must be approved by AgeX stockholders, and the Merger Agreement and transactions contemplated thereby must be approved by the Serina stockholders. Failure to obtain these approvals would prevent the closing of the Merger.
Before the Merger can be completed, AgeX stockholders must approve, among other things, the issuance of AgeX common stock to Serina stockholders pursuant to the Merger Agreement and the resulting change in control of AgeX from the Merger, and Serina stockholders must adopt the Merger Agreement and approve the Merger and the related transactions contemplated thereby. Failure to obtain the required stockholder approvals may result in a material delay in, or the abandonment of, the Merger. Any delay in completing the Merger may materially adversely affect the timing and benefits that are expected to be achieved from the Merger or the ability of AgeX or Serina to complete the Merger in accordance with the Merger Agreement.
If the conditions to the closing of the Merger are not satisfied or waived, the Merger may not occur or the closing of the Merger could be delayed.
Even if each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal is approved by the stockholders of AgeX, specified conditions must be satisfied or, to the extent permitted by applicable law, waived to complete the Merger. These conditions are set forth in the Merger Agreement and described in the section titled “The Merger Agreement—Conditions to the Completion of the Merger” beginning on page 157 of this proxy statement/prospectus/information statement. AgeX and Serina cannot assure you that all of the conditions will be satisfied or waived. If the conditions are not satisfied or waived, the Merger may not occur or could be delayed, and AgeX and Serina each may lose some or all the intended benefits of the Merger.
The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and/or other causes.
In general, either AgeX or Serina can refuse to complete the Merger if there is a material adverse effect affecting the other party between August 29, 2023, the date of the Merger Agreement, and the closing of the Merger. However, certain types of effects are excluded from the concept of a “material adverse effect” and do not permit the parties to refuse to complete the Merger, even if such change could be said to have a material adverse effect on AgeX or Serina. Such exclusions included, but are not limited to:
| ● | general business, political or economic conditions generally affecting the industry in which Serina or AgeX operate; |
| ● | acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions; |
| ● | changes in financial, banking or securities markets; |
| ● | any change in the stock price or trading volume of AgeX common stock; |
| ● | any failure by AgeX to meet internal or analysts’ expectations or projections or the results of operations of AgeX; |
| ● | any change in or affecting clinical trial programs or studies conducted by or on behalf of AgeX or its subsidiaries; |
| ● | any change from continued losses from operations or decreases in cash balances of Serina or any of its subsidiaries or on a consolidated basis among Serina and its subsidiaries; |
| ● | any change in, or any compliance with or action taken for the purpose of complying with, any law or GAAP (or interpretations of any law or GAAP); or |
| ● | any change resulting from the announcement of the Merger Agreement or the pendency of the Contemplated Transactions; |
| ● | any change resulting from the Asset Dispositions (as defined below); |
| ● | the taking of any action required to be taken by the Merger Agreement; or |
| ● | any reduction in the amount of AgeX’s cash and cash equivalents as a result of winding down its activities associated with the termination of its research and development activities. |
If adverse changes occur and AgeX and Serina still complete the Merger, the stock price of the combined company following the closing of the Merger may suffer. This in turn may reduce the value of the Merger to the stockholders of AgeX, Serina or both.
Some executive officers and directors of AgeX and Serina have interests in the Merger that are different from the respective stockholders of AgeX and Serina and that may influence them to support or approve the Merger without regard to the interests of the respective stockholders of AgeX and Serina.
Directors and executive officers of AgeX and Serina may have interests in the Merger that are different from, or in addition to, the interests of other stockholders of AgeX and Serina, respectively. These interests with respect to AgeX’s directors and executive officers include, but are not limited to, the expected continued service of one of the directors of AgeX as a director of the combined company following the closing of the Merger, the expected continued employment of one of the executive officers of AgeX as an executive officer of the combined company following the closing of the Merger, severance benefits if employment is terminated in a qualifying termination in connection with the Merger and rights to continued indemnification, expense advancement and insurance coverage. These interests with respect to Serina’s directors and executive officers include, but are not limited to, the expected continued service of two of the directors of Serina as directors of the combined company following the closing of the Merger, the expected continued employment of three of the executive officers of Serina as executive officers of the combined company following the closing of the Merger and rights to continued indemnification and expense advancement.
The AgeX Board and Serina Board were aware of and considered such interests, among other matters, in reaching their decisions to approve and adopt the Merger Agreement, approve the Merger and recommend the approval of the Merger Agreement to AgeX and Serina stockholders. These interests, among other factors, may have influenced the directors and executive officers of AgeX and Serina to support or approve the Merger.
For more information regarding the interests of the AgeX and Serina directors and executive officers in the Merger, see the sections titled “The Merger—Interests of the AgeX Directors and Executive Officers in the Merger” and “The Merger—Interests of the Serina Directors and Executive Officers in the Merger” of this proxy statement/prospectus/information statement.
Juvenescence owns a significant majority of AgeX capital stock and will be able to substantially influence AgeX and exert control over the AgeX Proposals.
As of August 29, 2023, Juvenescence owned approximately 43% of the outstanding shares of AgeX common stock. In addition, Juvenescence indicated that it beneficially owned approximately 78.6% of AgeX common stock in its most recent amendment of their Schedule 13D filed with the SEC on August 24, 2023. A member of the AgeX Board is also the executive chairman and co-founder of Juvenescence. Accordingly, Juvenescence is able to substantially influence AgeX and exert control over the AgeX Proposals. This may prevent or discourage unsolicited acquisition proposals or offers for AgeX common stock that an AgeX stockholder may feel is in their best interest. The interests of Juvenescence may not always coincide with the interests of other AgeX stockholders, and Juvenescence may act in a manner that advances their best interests and not necessarily those of other stockholders. As a result, the market price of AgeX common stock could decline or stockholders might not receive a premium over the then-current market price of AgeX common stock upon a change in control.
For more information on the risks related to AgeX’s relationship with Juvenescence, see the section titled “—Risks Related to AgeX—Risks Related to Our Relationship with Juvenescence” below.
AgeX stockholders and Serina stockholders may not realize a benefit from the Merger commensurate with the ownership interest dilution they will experience in connection with the Merger.
If the combined company is unable to realize the full strategic and financial benefits currently anticipated from the Merger, AgeX stockholders and Serina stockholders will have experienced substantial dilution of their ownership interests without receiving any commensurate benefit, or only receiving part of the commensurate benefit to the extent the combined company is able to realize only part of the strategic and financial benefits currently anticipated from the Merger.
If the Merger is not completed, the market price of AgeX common stock may decline significantly.
The market price of AgeX common stock is subject to significant fluctuations. Market prices for securities of pharmaceutical, biotechnology and other life science companies have historically been particularly volatile. In addition, the market price of AgeX common stock will likely be volatile based on whether stockholders and other investors believe that AgeX can complete the Merger. The volatility of the market price of AgeX common stock has been and may be exacerbated by low trading volume. Additional factors that may cause the market price of AgeX common stock to fluctuate include, but are not limited to:
| ● | the entry into, or termination of, key agreements, including commercial partner agreements; |
| ● | announcements by commercial partners or competitors of new commercial products, clinical progress or lack thereof, significant contracts, commercial relationships or capital commitments; |
| ● | the loss of key employees; |
| ● | future sales of its common stock; |
| ● | general and industry-specific economic conditions that may affect its research and development expenditures; |
| ● | the failure to meet industry analyst expectations; and |
| ● | period-to-period fluctuations in financial results. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of AgeX common stock. In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against such companies.
The market price of the combined company’s common stock following the Merger may decline as a result of the Merger.
The market price of the combined company’s common stock may decline as a result of the Merger for a number of reasons, including if:
| ● | investors react negatively to the prospects of the combined company’s product candidates, business and financial condition following the closing of the Merger; |
| ● | the effect of the Merger on the combined company’s business and prospects following the closing of the Merger is not consistent with the expectations of financial or industry analysts; or |
| ● | the combined company does not achieve the perceived benefits of the Merger as rapidly or to the extent anticipated by stockholders or financial or industry analysts. |
For additional information relating to the risks and uncertainties regarding the market price of the combined company’s common stock following the Merger, see “Risks Related to the Combined Company—The market price of the combined company’s common stock is expected to be volatile, and the market price of the common stock may drop following the Merger.”
AgeX and Serina securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the combined company following the closing of the Merger as compared to their current ownership and voting interest in the respective companies.
Immediately following the closing of the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own, excluding the impact of the exercise of any post-merger warrant or incentive warrant, approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a fully diluted basis, subject to certain assumptions, including giving effect to the reverse stock split and the AgeX preferred stock conversion. The exchange ratio formula is based upon a fixed percentage of the post-Merger outstanding shares of the combined common stock, expressed on a fully-diluted and as-converted basis, subject to certain adjustments and exclusions. For a more complete description of the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio” beginning on page 146 of this proxy statement/prospectus/information statement.
In addition, the seven-member board of directors of the combined company will initially consist of three individuals with prior affiliations with Serina, two individuals with prior affiliation with AgeX and two of such members designated by mutual agreement of Serina and AgeX. The Chief Financial Officer, the Chief Executive Officer and the Chief Operating Officer of Serina will serve as the Interim Chief Executive Officer, Chief Scientific Officer and Chief Operating Officer and Secretary of the combined company, respectively, following the closing of the Merger. The Chief Financial Officer of AgeX will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the combined company, respectively, following the closing of the Merger.
Consequently, following the closing of the Merger, the pre-Merger equity holders of AgeX and Serina will own a smaller percentage of the combined company than their ownership of their respective companies prior to the Merger and will be able to exercise less influence over the management and policies of the combined company than they currently exercise over the management and policies of their respective companies.
During the pendency of the Merger Agreement, each of AgeX and Serina may be limited in its ability to enter into a business combination with another party on more favorable terms because of restrictions in the Merger Agreement, which could adversely affect their respective business prospects.
Covenants in the Merger Agreement impede the ability of AgeX and Serina to make acquisitions during the pendency of the Merger, subject to specified exceptions. As a result, if the Merger is not completed, the parties may be at a disadvantage to their competitors during that period. In addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating or knowingly encouraging, inducing or facilitating the communication, making, submission or announcement of any acquisition proposal or acquisition inquiry or taking any action that could reasonably be expected to lead to an acquisition proposal or acquisition inquiry regarding transactions involving a third party, including a merger, sale of assets or other business combination, subject to specified exceptions. Any such transactions could be favorable to such party’s stockholders, but the parties may be unable to pursue them. For more information, see the section titled “The Merger Agreement—Non-Solicitation” beginning on page 163 of this proxy statement/prospectus/information statement.
Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the transactions contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of AgeX and Serina from soliciting competing proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances as described in further detail in the section titled “The Merger Agreement—Non-Solicitation” beginning on page 163 of this proxy statement/prospectus/information statement. In addition, if AgeX or Serina terminate the Merger Agreement under specified circumstances, AgeX may be required to pay Serina a termination fee of $1,000,000 or up to $1,000,000 in expense reimbursements or Serina may be required to pay AgeX a termination fee of $1,000,000 or up to $1,000,000 in expense reimbursements; provided that neither party will be responsible for payment of more than $1,000,000 in the aggregate. The termination fee or expense reimbursements may discourage third parties from submitting competing proposals to AgeX, Serina or their respective stockholders and may cause the AgeX Board or Serina Board to be less inclined to recommend a competing proposal. For a more complete description of the termination provisions, please see the section titled “The Merger Agreement—Termination and Termination Fees” beginning on page 173 of this proxy statement/prospectus/information statement.
Because the lack of a public market for Serina’s capital stock makes it difficult to evaluate the fair market value of Serina’s capital stock, the value of the AgeX common stock to be issued to Serina stockholders may be more or less than the fair market value of Serina common stock.
The outstanding capital stock of Serina is privately held and is not traded in any public market. The lack of a public market makes it difficult to determine the fair market value of Serina’s capital stock. Because the percentage of AgeX equity to be issued to Serina stockholders was determined based on negotiations between the parties, it is possible that the value of the AgeX common stock to be received by Serina stockholders will be more or less than the fair market value of Serina’s capital stock.
The Merger may fail to qualify as a reorganization for U.S. federal income tax purposes, resulting in recognition of taxable gain or loss by Serina stockholders in respect of their Serina capital stock.
AgeX and Serina intend for the Merger to qualify as a reorganization within the meaning of Section 368(a) of the Code, as described in the section titled “The Merger-Material U.S. Federal Income Tax Consequences of the Merger” in this proxy statement/prospectus/information statement. In the event that the Merger does not qualify as a reorganization, the Merger would result in taxable gain or loss for each Serina stockholder, with the amount of such gain or loss determined by the amount that each Serina stockholder’s adjusted tax basis in the Serina capital stock surrendered is less or more than the fair market value of the AgeX common stock received in exchange therefor. Each holder of Serina capital stock is urged to consult with his, her or its own tax advisor with respect to the tax consequences of the Merger.
Litigation could arise in connection with the Merger, against AgeX, the AgeX Board, Serina or the Serina Board, which could be costly, prevent the consummation of the Merger, divert management’s attention and otherwise materially harm AgeX’s, Serina’s or the combined company’s business.
In the past, securities class action or shareholder derivative litigation has often followed certain significant business transactions, such as the sale of a company or announcement of a merger or any other strategic transaction. AgeX, the AgeX Board, Serina, the Serina Board or the combined company may be exposed to such litigation even if no wrongdoing occurred. Litigation is usually expensive and diverts management’s attention and resources, which could adversely affect the business and cash resources or either party and the ability of either party to consummate the or the ultimate value the AgeX or Serina stockholders receive in any such transaction.
If the Merger is not consummated for any reason, litigation could be filed in connection with the failure to consummate the Merger. Any litigation related to the Merger may result in negative publicity or an unfavorable impression of AgeX, Serina or the combined company, which could adversely affect the price of AgeX common stock, Serina common stock or the combined company’s common stock, impair the ability of AgeX, Serina or the combined company to recruit or retain employees, damage relationships with customers, suppliers, and other business partners, or otherwise materially harm of AgeX’s, Serina’s or the combined company’s operations and financial performance.
Risks Related to the Reverse Stock Split
The reverse stock split may not increase the combined company’s stock price over the long-term.
While it is expected that the reduction in the number of outstanding shares of common stock as a result of the reverse stock split will proportionally increase the market price of AgeX common stock, it cannot be assured that the reverse stock split will increase the market price of AgeX common stock by a multiple of the reverse stock split ratio or result in any permanent or sustained increase in the market price of the combined company’s common stock, which is dependent upon many factors, including the combined company’s business and financial performance, general market conditions and prospects for future success. Thus, it cannot be assured that the reverse stock split will accomplish any increase in the per-share market price of the combined company’s common stock for any meaningful period of time.
The reverse stock split may decrease the liquidity of AgeX common stock or the combined company’s common stock.
Although the AgeX Board believes that the anticipated increase in the market price of the combined company’s common stock resulting from the proposed reverse stock split could encourage interest in its common stock and possibly promote greater liquidity for its stockholders, such liquidity could also be adversely affected by the reduced number of shares outstanding after the reverse stock split. The reduction in the number of outstanding shares may lead to reduced trading in and a smaller number of market makers for the combined company’s common stock.
The reverse stock split may lead to a decrease in the combined company’s overall market capitalization.
Should the market price of the combined company’s common stock decline after the reverse stock split, the percentage decline may be greater, due to the smaller number of shares outstanding, than it would have been prior to the reverse stock split. A reverse stock split is often viewed negatively by the market and, consequently, can lead to a decrease in the combined company’s overall market capitalization. If the per-share market price does not increase in proportion to the reverse stock split ratio, then the value of the combined company, as measured by its stock capitalization, will be reduced. In some cases, the per-share stock price of companies that have effected reverse stock splits subsequently declined back to pre-reverse split levels, and accordingly, it cannot be assured that the total market value of the combined company’s common stock will remain the same after the reverse stock split is effected, or that the reverse stock split will not have an adverse effect on the combined company’s stock price due to the reduced number of shares outstanding after the reverse stock split.
Risks Related to AgeX
If the Merger is not completed, we expect to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs, and, accordingly, our business will be subject to the risks described in this subsection titled “—Risks Related to AgeX.” References to “we,” “us” or “our” in this subsection generally refer to AgeX.
Risks Related to Our Current Business, Financial Condition and Capital Resources
We need additional financing to execute our operating plan and continue to operate as a going concern.
As required under Accounting Standards Update 2014-15, Presentation of Financial Statements-Going Concern (ASC 205-40), we have the responsibility to evaluate whether conditions and/or events raise substantial doubt about our ability to meet our future financial obligations as they become due within one year after the date the financial statements are issued. Based on our most recent projected cash flows, we believe that our cash and cash equivalents, even with the increased amount of credit available through the Fifth Amendment of the 2022 Secured Note with Juvenescence, and the proceeds we may receive from the sale of additional shares of AgeX common stock in “at-the-market” transactions through a Sales Agreement with Capital Markets, LLC (Chardan) as a sales agent, would not be sufficient to satisfy our anticipated operating and other funding requirements for the next twelve months from the date of filing of this proxy statement/prospectus/information statement. These factors raise substantial doubt regarding our ability to continue as a going concern and the report of our independent registered public accountants accompanying our audited consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement contains a qualification to such effect.
We have incurred operating losses and negative cash flows since inception and had an accumulated deficit of $122.2 million as of June 30, 2023. We expect to continue to incur operating losses and negative cash flows. Because we will continue to experience net operating losses, our ability to continue as a going concern is subject to our ability to obtain necessary capital from outside sources, including obtaining additional capital from the sale of AgeX common stock or other equity securities or assets, obtaining additional loans from financial institutions or investors, and entering into collaborative research and development arrangements or licensing some or all of our patents and know-how to third parties while retaining a royalty and other contingent payment rights related to the development and commercialization of products covered by the licenses. Our continued net operating losses, the amount of our debt obligations to Juvenescence and the provisions of our indebtedness agreements with them, including restrictions on the use of loan funds and the security interest they hold in our assets and assets of certain of our subsidiaries, Juvenescence’s ownership of shares of AgeX Preferred Stock, the risks associated with the development of our product candidates and technologies, and our deferral of in-house development of our product candidates and technologies in connection with our reductions in staffing and the closing of our research laboratory facilities, will increase the difficulty in obtaining such capital, and there can be no assurances that we will be able to obtain such capital on favorable terms or at all. If we are unable to raise capital when needed, we may be forced to delay, reduce or eliminate our research and development activities, or ultimately not be able to continue as a going concern.
We are a discovery-stage development company with limited capital resources and have incurred operating losses since our inception. We anticipate that we will incur continued losses for the foreseeable future and will need to continue to raise capital to finance our operations, and we do not know if we will ever attain profitability.
We are a discovery-stage therapeutics company with a limited operating history and limited capital resources. Since our inception in August 2017, we have incurred operating losses and negative cash flows and we expect to continue to incur losses and negative cash flow in the future. Our net operating losses from continuing operations were $10.5 million and $8.6 million for the years ended December 31, 2022 and 2021, respectively, and we had an accumulated deficit of approximately $116.2 million as of December 31, 2022. Our net operating losses from operations were $4.0 million and $3.6 million for the six months ended June 30, 2023 and 2022, respectively, and we had an accumulated deficit of approximately $122.2 million as of June 30, 2023.
These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. We expect to continue to incur significant additional operating losses for the foreseeable future and will need to continuously raise additional capital to fund our operations. The amount of our expenses and anticipated losses will depend on our capital resources and whether we license out product development to third parties or participate ourselves directly or financially with collaborators in research, development and commercialization efforts. Although we do not expect to advance our product candidates through clinical trials, seek regulatory approval, or commercialize our product candidates ourselves, our capital needs would increase greatly if we were to change plans and determine to do so.
The amount of our future net losses will depend, in part, on the rate of future growth of our expenses, our ability to raise the capital needed to continue our operations, and our ability to generate revenues. If we or any licensees or collaborators are unable to develop and commercialize one or more of our product candidates, or if revenues from any product candidates that receive marketing approval are insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability. If we are unable to achieve and then maintain profitability, the value of our equity securities will be materially and adversely affected.
We are highly leveraged, carrying a significant amount of indebtedness, including indebtedness secured by our assets, that will become due and payable over the next three years and there is no assurance that we will be able to refinance those obligations as they become due.
As of November 1, 2023, our borrowings from Juvenescence under various loans and accrued origination fees totaled approximately $3.4 million. The outstanding balances of those loans will become due and payable as follows: approximately $2.7 million on February 14, 2024 under the 2022 Secured Note and $692,800 on March 13, 2026 under the 2023 Secured Note. During November 2023, we entered into an amendment of the 2022 Secured Note pursuant to which we may borrow up to an additional $4,400,000 from Juvenescence. Our obligations under the 2022 Secured Note and the 2023 Secured Note are collateralized by all of our assets, including the shares of common stock we hold in subsidiaries ReCyte Therapeutics, and Reverse Bioengineering Inc. (Reverse Bio), and UniverXome Bioengineering, Inc., under the terms of an Amended and Restated Security Agreement (the Security Agreement), and those subsidiaries have guaranteed our obligations under the Secured Note, as amended, and have granted a security interest in their respective assets to Juvenescence pursuant to the Security Agreement to secure those obligations. If an Event of Default, as defined in the 2022 Secured Note or the 2023 Secured Note, were to occur Juvenescence could foreclose on its security interest and sell our assets and the assets of those subsidiaries to satisfy the unpaid principal balances of those loans plus certain loan origination fees and costs incurred in connection with the Event of Default and the foreclosure and sale of the assets. As a result, we and those subsidiaries could lose some or all of our respective assets, leaving few if any assets available for the operation of our business or the businesses of our subsidiaries, or for sale for the benefit of our stockholders through a winding up of our affairs and liquidation of our assets.
The terms of our loans from Juvenescence and the Security Agreement could make it more difficult for us to raise additional capital from other sources.
The terms of our loans from Juvenescence include certain covenants that among other matters such as financial reporting: (i) impose financial restrictions on AgeX while the loans remain unpaid, including restrictions on the incurrence of additional indebtedness by AgeX and its subsidiaries, except that Reverse Bio will be permitted to incur debt convertible into equity not guaranteed or secured by the assets of AgeX or any other AgeX subsidiary, and the restrictions on the incurrence of indebtedness applicable to Reverse Bio will end if it raises more than $15 million in debt or equity financing by October 31, 2023; (ii) require that AgeX use loan proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, for general working capital, and for repayment of all or a portion of AgeX’s indebtedness to Juvenescence; and (iii) prohibit AgeX from making additional investments in subsidiaries, unless AgeX obtains the written consent of Juvenescence to a transaction that otherwise would be prohibited or restricted. Further, our obligations under the 2022 Secured Note and 2023 Secured Note are collateralized by all of our assets under the terms of the Security Agreement. Accordingly, the terms of our loans from Juvenescence, and the grant of a security interest in our assets pursuant to the Security Agreement to secure our obligations for the repayment of the 2022 Secured Note and 2023 Secured Note with applicable loan fees that become due, could make AgeX less attractive to new equity investors and could impair our ability to finance our operations or the operations of our subsidiaries from sources other than Juvenescence.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial revenue, we may finance our cash needs through a combination of equity offerings, debt financings, strategic alliances and licensing arrangements. We do not currently have any committed external source of funds. We also have a Sales Agreement with Chardan pursuant to which we may sell shares of AgeX common stock in “at the market” sales, provided that the actual market value of common shares that we may sell through that Sales Agreement any 12 month period will be limited to one-third of aggregate market value of AgeX common stock held by stockholders that would not be considered “affiliates” of AgeX, determined in accordance with applicable SEC rules. We will need to seek additional capital regardless of market conditions and the terms of any financings that may be available to us.
To the extent that we raise additional capital through the sale of shares of AgeX common stock or other equity securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Similarly, if Reverse Bio or NeuroAirmid raise capital through the sale of equity securities or convertible debt securities AgeX’s interest in those subsidiaries will be diluted and the terms of equity securities issued by those subsidiaries may include liquidation or other preferences that adversely affect our rights as a common stockholder of the subsidiary.
Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we or any of our subsidiaries raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we or our subsidiaries may be required to relinquish valuable rights to key technologies, future revenue streams, or product candidates, and any such licenses may be granted on terms that may not be favorable to us. If we or our subsidiaries are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Unless AgeX common stock continues to be listed on a national securities exchange, AgeX will become subject to the so-called “penny stock” rules that impose restrictive sales practice requirements.
On November 17, 2021, we received a letter (the Deficiency Letter) from the staff of the NYSE American indicating that AgeX did not meet certain of the NYSE American continued listing standards as set forth in Section 1003(a)(i) of the NYSE American Company Guide in that we had stockholders’ equity of less than $2,000,000 and had incurred losses from continuing operations and/or net losses during our two most recent fiscal years. Pursuant to Section 1009 of the NYSE American Company Guide and as provided in the Deficiency Letter provided, we provided the NYSE American staff with a plan (the Compliance Plan) advising the NYSE American staff of action we had taken and will take that would bring AgeX into compliance with the NYSE American continued listing standards. The NYSE American staff accepted our Compliance Plan but later required us to revise the Compliance Plan as we remained out of listing compliance. On November 22, 2022, we received a notification from the staff of the NYSE American indicating that the NYSE American accepted our revised listing Compliance Plan and granted us an extension of time to regain compliance with the NYSE American continued listing standards as set forth in Section 1003(a)(i) and (ii) of the NYSE American Company Guide by increasing our stockholders equity to not less than $4,000,000. On May 17, 2023, we received a notice from the staff of the NYSE American indicating that they intend to commence proceedings to delist AgeX common stock from the NYSE American based upon AgeX’s non-compliance with the stockholders’ equity requirements set forth in Sections 1003(a)(i), (ii) and (iii) of the NYSE American Company Guide by the end of a compliance plan period that expired on May 17, 2023. Specifically, AgeX did not meet the continued listing standards because it had stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and had incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and had reported losses from continuing operations and/or net losses in its five most recent fiscal years. On May 24, 2023, AgeX filed a request for a review of the delisting determination by a committee of the board of directors of the NYSE American. On May 31, 2023, AgeX received a notice from the staff of the NYSE American which scheduled a hearing for July 25, 2023. On July 24, 2023, AgeX issued shares of AgeX Series A preferred stock and AgeX Series B preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence with the intent of adding $36 million to stockholders equity and returning AgeX to compliance with the NYSE American continued listing standards. However, upon subsequent consideration in consultation with our registered independent public accountants it was determined that, in accordance with applicable guidance to GAAP, the deemed liquidation preference provisions of the Preferred Stock could be considered contingent redemption provisions that are not solely within AgeX’s control, requiring that the Preferred Stock be presented outside of permanent equity in the mezzanine section of our condensed consolidated balance sheets for the three and nine months ended September 30, 2023 included in this Report. To address this issue, the applicable deemed liquidation provision of the Preferred Stock has been amended so that the Preferred Stock will now qualify as permanent equity. See Note 12-Subsequent Events. We have informed the NYSE American of the accounting issue and the remedy that we have implemented and are awaiting their confirmation whether we are now in compliance with their continued listing standards.
If we are unable to maintain the listing of AgeX common stock on the NYSE American or another national securities exchange, AgeX common stock could become subject to the so-called “penny stock” rules if the shares have a market value of less than $5.00 per share. The SEC has adopted regulations that define a penny stock to include any stock that has a market price of less than $5.00 per share, subject to certain exceptions, including an exception for stock traded on a national securities exchange. The SEC regulations impose restrictive sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. An accredited investor generally is a person whose individual annual income exceeded $200,000, or whose joint annual income with a spouse exceeded $300,000 during the past two years and who expects their annual income to exceed the applicable level during the current year, or a person with net worth in excess of $1.0 million, not including the value of the investor’s principal residence and excluding mortgage debt secured by the investor’s principal residence up to the estimated fair market value of the home, except that any mortgage debt incurred by the investor within 60 days prior to the date of the transaction shall not be excluded from the determination of the investor’s net worth unless the mortgage debt was incurred to acquire the residence. For transactions covered by this rule, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. This means that if we are unable maintain the listing of AgeX common stock on a national securities exchange, the ability of stockholders to sell their AgeX common stock in the secondary market could be adversely affected.
If a transaction involving a penny stock is not exempt from the SEC’s rule, a broker-dealer must deliver a disclosure schedule relating to the penny stock market to each investor prior to a transaction. The broker-dealer also must disclose the commissions payable to both the broker-dealer and its registered representative, current quotations for the penny stock, and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the customer’s account and information on the limited market in penny stocks.
Risks Related to Our Planned Restructuring
As part of our corporate restructuring plans, we are pursuing the Merger with Serina through which AgeX would be able to enter the field of drug delivery polymer technology, but there is no assurance that the Merger will be consummated. Failure to complete, or delays in completing, the Merger with Serina could materially and adversely affect our results of operations, business, financial condition or stock price.
We have been formulating corporate restructuring plans that include the Merger through which AgeX would be able to enter the field of drug delivery polymer technology. If the Merger is completed, the combined company will primarily focus on developing Serina’s product candidates, which are described on page 231 under the section titled “Description of Serina’s Business.” Any failure to satisfy a required condition to closing may prevent, delay or otherwise materially and adversely affect the completion of the Merger, which could materially and adversely affect AgeX’s ability to enter the field of drug delivery polymer technology and could materially and adversely affect AgeX’s results of operations, business, financial condition or stock price. AgeX cannot predict with certainty whether or when any of the required closing conditions will be satisfied and whether AgeX will be able to successfully consummate the Merger as currently contemplated under the Merger Agreement or at all. Accordingly, there is no assurance that the Merger will be consummated.
AgeX’s efforts to complete the Merger could cause substantial disruptions in, and create uncertainty surrounding, AgeX’s business, which may materially adversely affect AgeX’s results of operations and business. Uncertainty as to whether the Merger will be completed may affect AgeX’s ability to retain and motivate existing employees or recruit prospective employees if vacancies in staffing need to be filled. Employee retention may be particularly challenging while the Merger is pending because employees may experience uncertainty about their roles following the transaction. A substantial amount of AgeX’s management’s and employees’ attention is being directed toward the completion of the transaction and thus is being diverted from AgeX’s day-to-day operations.
Risks related to the failure to consummate, or delay in consummating, the Merger include, but are not limited to, the following:
| ● | AgeX would not realize any or all of the potential benefits of the Merger, which could have a negative effect on AgeX’s results of operations, business, financial condition or stock price; |
| ● | under some circumstances, AgeX may be required to pay a termination fee to Serina of $1,000,000 or expense reimbursement of up to $1,000,000; |
| ● | AgeX would remain liable for significant transaction costs, including legal, accounting and other costs relating to the merger regardless of whether the Merger is consummated; |
| ● | the trading price of AgeX’s common stock may decline to the extent that the current market price for AgeX’s stock reflects a market assumption that the Merger will be completed; |
| ● | the attention of AgeX’s management and employees may have been diverted to the Merger rather than to AgeX’s operations and the pursuit of other opportunities that could have been beneficial to AgeX; |
| ● | AgeX could be subject to litigation related to any failure to complete the Merger; |
| ● | AgeX could potentially lose key personnel during the pendency of the Merger as employees may experience uncertainty about their future roles with AgeX following completion of the Merger and seek employment opportunities elsewhere; and |
| ● | under the Merger Agreement, AgeX is subject to certain customary restrictions on the conduct of AgeX’s business prior to completing the Merger, which restrictions could adversely affect AgeX’s ability to conduct AgeX’s business as AgeX otherwise would have done if AgeX was not subject to these restrictions. |
The occurrence of any of these events individually or in combination could materially and adversely affect AgeX’s results of operations, business, financial condition and stock price.
If the Merger is not approved or does not occur, we may not be successful in the execution of our current business strategies or identifying and implementing any strategic alternatives with respect to our assets and development programs, and any future strategic alternatives could have negative consequences.
If the Merger is not approved or does not occur, we expect to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs, which may include a merger, business combination, investment into AgeX, asset sale or other strategic transaction, and we may not be successful in executing such strategies or identifying or implementing any such strategic alternatives.
In addition, the process of seeking out and evaluating a potential strategic alternative with respect to our assets and development programs is costly, time-consuming and complex, and we have incurred, and may in the future incur, significant costs related to the Merger and our other ongoing restructuring plans, such as legal and accounting fees and expenses and other related charges. We may also incur additional unanticipated expenses in connection with this process. A considerable portion of these costs will be incurred regardless of whether any such course of action is implemented or transaction is completed, decreasing the remaining cash available for use in our business.
The development and any potential commercialization of our product candidates will require substantial additional cash to fund the costs associated with conducting the necessary preclinical and clinical testing and obtaining regulatory approval. Consequently, any potential counterparty in a strategic transaction involving AgeX may be reluctant to spend additional resources required to pursue development of AgeX’s product candidates and may attribute little or no value to AgeX product assets in such a transaction.
There can be no assurance that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, lead to increased stockholder value, or achieve the anticipated results.
Our goal of spinning off Reverse Bio to our stockholders is subject to uncertainties and risks, including risks that could result in the spinoff not being consummated, income tax risks, liquidity and valuation risks pertaining to Reverse Bio stock, and Reverse Bio business risks.
The definitive terms of a spinoff of Reverse Bio have not been determined. Before a spinoff can be completed, Reverse Bio will need to raise equity capital through a financing from private investors, and there is no certainty that it will be able to raise sufficient capital to initiate operations as a separate company, independent of AgeX. There is a possibility that AgeX and Reverse Bio and any investors that may contemplate providing financing to Reverse Bio will not reach agreement as to the terms of the spinoff. Accordingly, the contemplated spinoff could be abandoned or a condition to consummating the Reverse Bio spinoff may not be satisfied. Reverse Bio might not be able to satisfy the listing qualifications to list its shares of common stock on the NYSE American or any other national stock exchange and if Reverse Bio shares are not listed on a national securities exchange the liquidity and market value of Reveres Bio common stock could be adversely affected. The Reverse Bio spinoff might not achieve the desired tax treatment, resulting in unanticipated taxes to AgeX and AgeX stockholders. The number of shares of Reverse Bio common stock that AgeX may distribute to its stockholders may be limited, or a distribution of such shares may be prohibited, by provisions of the DGCL that generally limit Delaware corporations, including AgeX, from distributing assets to their stockholders in an amount that exceeds the excess of their assets over liabilities. The other risks pertaining to AgeX’s business, operations, and future capital needs, other than financial obligations to Juvenescence, discussed in this Risk Factors section will also be applicable to the business, operations, and future capital needs of Reverse Bio.
Risks Related to Our Relationship with Juvenescence
Conflicts of interest may arise from our relationship with Juvenescence, which owns a significant percentage of AgeX common stock as well as shares of AgeX preferred stock and is a significant creditor and will be able to substantially influence us and exert control over matters subject to stockholder approval, including the Merger and other AgeX Proposals.
As of August 29, 2023, Juvenescence owned approximately 43% of the outstanding shares of AgeX common stock. In addition, Juvenescence indicated that it beneficially owned approximately 78.6% of AgeX common stock in its most recent amendment of their Schedule 13D filed with the SEC on August 24, 2023 taking into consideration shares of AgeX common stock that may be acquired through the conversion of convertible debt, the conversion of shares of AgeX preferred stock, and the exercise of AgeX common stock purchase warrants. It is expected that Juvenescence will convert its shares of preferred stock into shares of AgeX common stock before the Merger, as required by the Merger Agreement, which will increase Juvenescence’s ownership and voting power to more than 50% of the outstanding shares of common stock, enabling Juvenescence to approve or disapprove the Merger and other AgeX Proposals without regard to the vote of other AgeX stockholders.
A member of the AgeX Board is also the Executive Chairman and Co-Founder of Juvenescence, and based on Juvenescence’s ownership of shares of AgeX common stock and preferred stock, if the Merger is not consummated Juvenescence will continue to substantially influence us and exert control over matters subject to stockholder approval, the elections of directors, approval of our equity incentive plans, amendments to our organizational documents, or approval of any merger, amalgamation, sale of assets or other major corporate transaction. Furthermore, upon Juvenescence holding more than 50% outstanding AgeX common stock, AgeX would qualify as a “controlled company” as defined by the NYSE American Company Guide. Being a “controlled company” would entitle AgeX to exempt itself from the requirement that a majority of its directors be “independent” directors as defined in the NYSE American Company Guide, and that the Compensation Committee and the Nominating & Corporate Governance Committee be comprised entirely of independent directors.
As of October 31, 2023, Juvenescence has also loaned AgeX approximately $2,500,000 under the 2022 Secured Note line of credit with them and, on November 9, 2023, we entered into an amendment of the 2022 Secured Note to increase the amount of the line of credit available to AgeX from Juvenescence by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. Juvenescence also has controlling stakes and minority investments in several other companies engaged in various aspects of the aging industry, which companies may propose collaborations with AgeX.
Juvenescence’s interests may not always coincide with our corporate interests or the interests of other stockholders, and it may exercise its voting and other rights, including rights as a creditor, in a manner with which other stockholders may not agree or that may not be in the best interests of AgeX or stockholders other than Juvenescence. So long as Juvenescence continues to own a significant amount of our equity and remains a significant creditor, it will continue to be able to strongly influence and effectively control our decisions. While the directors elected by Juvenescence will be obligated to act in accordance with their fiduciary duty, they may have equity or other interests in Juvenescence and, accordingly, their interests may be aligned with Juvenescence’s interests, which may not always coincide with our corporate interests or the interests of our other stockholders.
Our Chief Operating Officer is not a fulltime AgeX employee.
Our Chief Operating Officer is an employee of Juvenescence and is expected to devote 85% of his time to our affairs and the balance of his time to the affairs of Juvenescence and accordingly we may compete with Juvenescence for his time and attention.
Risks Related to Our Business Operations
Due to our limited financial resources, we have reduced our staffing, eliminated our research laboratory facilities, and eliminated in-house research and product development work. We will seek opportunities to outsource or license product development and commercialization but there is no assurance that we will be able to do so successfully.
During April 2020, we implemented a plan to reduce spending on employee salaries and consulting fees that resulted in large staff reductions, including the elimination of most of our research personnel and certain management and administrative personnel. We then subleased most of our former laboratory facility space and we did not renew the laboratory facility lease, or lease other laboratory facility space, after our lease expired at the end of 2020. As a result, we do not have a research laboratory facility or a research staff, and we have curtailed development of our product candidates and technologies except for certain research and development work that is being conducted under sponsored research agreements with certain universities and a limited amount of work contracted out to third-party service providers. We also may license technologies or product development to, or enter into collaborative arrangements with, other companies in the cell therapy or biopharma industry to conduct research and development, manufacturing, and marketing for AgeX for particular product candidates, but there is no assurance that we will be able to enter into any such agreements on terms acceptable to us.
We may expend our limited resources to pursue one or more particular product candidates or indications and fail to pursue product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we may focus on research programs and product candidates that we identify for specific indications and we may seek to develop those product candidates through out-sourcing or out-licensing to third parties if we are able to make such arrangements. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to timely capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
We have not tested any of our product candidates in clinical trials. Success in early development and preclinical studies or clinical trials may not be indicative of results obtained in later preclinical studies and clinical trials.
Our product candidates have never been evaluated in human clinical trials, and we may experience unexpected or adverse results in the future if any human clinical trials of any of our product candidates are conducted. Typically, it takes about six to ten years to develop a new drug from the time it enters Phase 1 clinical trials to when it is approved for treating patients, but in many cases it may take longer, and the costs of advancing product candidates through clinical trials will be substantial and will tend to increase significantly with each successive clinical trial phase.
Adequate and well-controlled clinical trials will need to demonstrate that our product candidates are safe and effective, with a favorable benefit-risk profile, for use in their target indications before regulatory approvals can be sought for their commercial sale. Any positive results that may be observed for product candidates in preclinical animal models may not be predictive of future clinical trials in humans. Our product candidates may also fail to show the desired safety and efficacy in later stages of clinical development even if they successfully advance through initial clinical trials. Further, some or all of our cell-based therapies under development may require the genetic modification of the pluripotent master cell banks such that the resulting cells can escape immune rejection by the intended patient. There is no certainty that a genetic modification will provide a long-term solution to transplant rejection, or that the modified cells will not cause unanticipated health risks to the patient that could delay or even halt the development of the products.
Many companies in the biotechnology industry have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development and there is a high failure rate for product candidates proceeding through clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. Even if a clinical trial demonstrates statistical significance, regulatory agencies may not accept the use of the historical control. Regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development. We cannot be certain that any clinical trials and applications for marketing approval will not face similar setbacks.
Our choice of product candidates and our development plans for our product candidates are subject to change based on a variety of factors, and if we abandon development of a product candidate we may not be able to develop or acquire a replacement product candidate.
We may determine to abandon the development of one or more of our product candidates, or we may change the prioritization of the development of certain product candidates, or we may select or acquire and prioritize the development of new product candidates. Our choice and prioritization of product candidates for development will be influenced by a variety of factors, including but not limited to:
| ● | the amount of capital that we will have for our development programs and our projected costs for those programs; |
| ● | ability to enter into licensing or collaborative arrangements with other biotechnology or biopharma companies or universities with their own laboratory facilities and research staffs to conduct research and development of one or more product candidates; |
| ● | competitors may develop alternatives that render our potential product candidates obsolete or less attractive; |
| ● | potential product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights; |
| ● | potential product candidates may not be effective in treating their targeted diseases; |
| ● | potential product candidates may, on further study, be shown to have harmful side effects, toxicities or other characteristics that indicate that they are unlikely to be products that will receive marketing approval and achieve market acceptance; |
| ● | our analysis of market demand and market prices for the products we plan to develop could lead us to conclude that market conditions are not favorable for receiving an adequate return on our investment in product development and commercialization; |
| ● | a potential product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; or |
| ● | the regulatory pathway for a potential product candidate is too complex and difficult to navigate successfully or economically. |
We may determine to expand our organization and obtain laboratory facilities if we are able to raise sufficient capital to do so, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of September 30, 2023, we had nine employees. If we are able to obtain sufficient capital and determine to reinstitute our internal research and development efforts, we may have difficulty locating, leasing, and equipping a new laboratory facility and identifying, hiring and integrating new scientific and laboratory personnel. Many of the biotechnology companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do and are better positioned to attract and retain personnel and consultants. If we are unable to continue to attract and retain high-quality personnel and consultants, the rate and success at which we can discover and develop product candidates and operate our business will be limited.
Future growth would impose significant additional responsibilities on our management, including the need to identify, recruit, maintain, motivate and integrate additional employees, consultants and contractors. Also, our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Any growth of administrative resources could require significant capital expenditures and may divert financial resources from other projects, such as the development of product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
The commercial success of any of our current or future product candidates will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
Even with approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our products will depend in part on the health care providers, patients, and third-party payors accepting our product candidates as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors and other health care providers. The clinical development, commercialization, and marketing of cell therapies are at an early-stage, substantially research-oriented, and financially speculative. To date, very few companies have been successful in their efforts to develop and commercialize cell therapies. In general, cell therapies may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, potentially prohibitive costs or other characteristics that may prevent or limit their approval or commercial use. Furthermore, the number of people who may use cell- or tissue-based therapies is difficult to forecast with accuracy. Our future success is dependent on the establishment of a large global market for cell therapies and our ability to capture a share of this market with our product candidates.
Even if we, a collaborator, or a licensee of our technology successfully develop and obtain regulatory approval for our product candidates, the market may not understand or accept them. Our product candidates represent novel treatments and are expected to compete with a number of more conventional products and therapies manufactured and marketed by others, including major pharmaceutical and biotechnology companies. The degree of market acceptance of any of our products will depend on a number of factors, including without limitation:
| ● | the efficacy of the product as demonstrated in clinical studies and potential advantages over competing treatments; |
| ● | the prevalence and severity of the disease and any side effects; |
| ● | the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling; |
| ● | the convenience and ease of administration; |
| ● | the cost of treatment, particular as additive to existing treatments; |
| ● | the willingness of the patients and physicians to accept and use these therapies and the perception of efficacy and safety of our approved products by such parties; |
| ● | the marketing, sales and distribution support for the products; |
| ● | the publicity and ethical, social and legal concerns regarding the use of embryonic stem cells for our products or competing products and treatments; |
| ● | government regulations restricting or prohibiting our research or manufacturing processes for stem cells due to ethical, social and legal concerns regarding their use in medical research and treatment; and |
| ● | the pricing and availability of third-party insurance coverage and reimbursement. |
Even if a product displays a favorable efficacy and safety profile upon approval, market acceptance of the product will initially remain uncertain. Efforts to educate the medical community and third-party payors on the benefits of the products may require significant investment and resources and may never be successful. If our products fail to achieve an adequate level of acceptance by physicians, patients, third-party payors, and other health care providers, we will not be able to generate sufficient revenue to become or remain profitable.
If the market opportunities for our product candidates are smaller than we believe they are, we may not meet our revenue expectations and, even assuming approval of a product candidate, our business may suffer.
Our projections of the number of potential users of our product candidates in the markets we are attempting to address are based on our beliefs and estimates and include several key assumptions based on our industry knowledge, industry publications, third-party research reports and other surveys. You should bear in mind the following:
| ● | Our estimates have been derived from a variety of sources, including publications and scientific literature or market research estimating the total number of patients and currently approved or used therapies, as well as certain assumptions regarding the potential size of the market assuming broad regulatory approval or potential usage by physicians beyond the approved label, any of which may prove to be incorrect. |
| ● | The scope of approval and potential use may be significantly narrower, and the number of patients may turn out to be lower than expected. |
| ● | Competitive products or approaches may be approved or come into use by medical providers and the potentially addressable patient population for each of our product candidates may be limited or may not be amenable to treatment with our product candidates, and new patients may become increasingly difficult to identify or gain access to, any which could adversely affect our results of operations and our business. |
If the actual market for any of our product candidates is smaller than we expect, our revenue may be limited and it may be more difficult for us to achieve or maintain profitability.
We will face risks related to the manufacture of medical products for any product candidates that we develop.
The manufacture of medical products, and in particular biologics, is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, none of which we presently have. Unless we are able to raise the capital required to construct our own manufacturing facilities and are able to develop the expertise to manage and operate a manufacturing facility of our own, which we do not expect to undertake, we would need to rely on third-party manufacturers to manufacture any products that we develop. There is no assurance that we will be able to identify manufacturers on acceptable terms or at all. Regardless of whether we do our own manufacturing or rely on third parties to manufacture products for us, we will face all risks related to the manufacture of therapeutic products for use in medicine including the following risks:
| ● | We or any third-party manufacturers might be unable to timely formulate and manufacture our products or produce the quantity and quality required to meet our clinical and commercial needs, if any. |
| ● | We or any third-party manufacturers may not be able to execute our manufacturing procedures appropriately. |
| ● | Any third-party manufacturers we engage may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products on a commercial scale. |
| ● | We or any third-party manufacturers will be subject to ongoing periodic unannounced inspection by the FDA, and corresponding state agencies to ensure strict compliance with current good manufacturing practices (cGMP) and other government regulations and corresponding foreign standards. We will not have control over third-party manufacturers’ compliance with applicable regulations and standards. |
| ● | We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates. |
| ● | Third-party manufacturers could breach or terminate their agreements with us. |
| ● | We or third-party manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. |
In addition, we may rely on third parties to perform release testing on our product candidates prior to delivery to patients. If these tests are not appropriately conducted and test data are not reliable, patients could be put at risk of serious harm which could result in product liability suits.
If we or any third-party manufacturers that we may engage were to encounter any of these difficulties, our ability to provide our product candidates to patients in clinical trials or to the medical marketplace would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, could require us to either commence new clinical trials at additional expense or terminate clinical trials completely.
The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
Further, our product candidates are manufactured by starting with established master cell banks of human embryonic cells and other cells that are cryopreserved. We will be required to expand the numbers of the pluripotent stem cell master cell banks for future use, as well as produce working cell banks from which the product will be manufactured for clinical trials, produce the relevant product under cGMP conditions, expand the number of relevant cells and cryopreserve them under cGMP conditions. We may not be able to expand the numbers of the pluripotent stem cell master cell banks to provide sufficient cells for clinical trial or for commercial scale production. We may not be able to manufacture product that meets release criteria due to sterility, identity or potency issues. We may not have access or be able to make the reagents necessary to manufacture the cells and we may not have access to adequate supply channels to transport and distribute the products. There are also risks that the cells may be destroyed by interruption in their cryopreservation by means of natural disasters such as earthquakes, power outages, or other unexpected events, or the cells may be determined to be unacceptable as a source of human cellular therapies for reasons we cannot envision. We cannot assure you that any stability or other issues relating to the manufacture of any of our product candidates or products will not occur in the future. If any of our master cell banks are lost or destroyed, including due to systems failure in the cryopreservation processes, our planned clinical trials would be severely delayed, and we would incur significant costs associated with obtaining new supply of cell banks. Accordingly, failures or difficulties faced at any level of our supply chain could adversely affect our business and delay or impede the development and commercialization of any of our product candidates or products and could have an adverse effect on our business, prospects, financial condition and results of operations.
Any therapies that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing cell-based products for us. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval.
Each of these risks could delay our clinical trials, any approval of our product candidates by the FDA, or the commercialization of our product candidates, and could result in higher costs or deprive us of potential product revenue.
Any cell-based products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale.
Pluripotent stem cell and progenitor cell derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for wide scale commercialization. If we are successful in developing products that consist of cells or compounds derived from pluripotent stem cells or progenitor cells, we will need to develop facilities, processes, and technology for the commercial production of those products. Pluripotent stem cell or progenitor cell based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The higher cost of manufacturing a product will require that we charge our customers a higher price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be reluctant to purchase our products and we may not be able to sell our products in sufficient volumes to recover our costs or to earn a profit.
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends.
Our business will depend on several critical technologies that we have licensed or sublicensed from Lineage or certain Lineage subsidiaries. The license and sublicense agreements impose obligations on us, including payment obligations and obligations to pursue development and commercialization of products and technologies under the licensed patents or technology. If the licensor or sublicensor believes that we have failed to meet our obligations under a license or sublicense agreement, they could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, our loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential new products or technologies, and our ability to raise any capital that we might then need, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed or sublicensed technology in our business.
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
Our industry has experienced a high rate of turnover of management personnel in recent years. We are highly dependent on our executive officers, including our Chief Executive Officer and our Chief Financial Officer. Although we have employment agreements with our executive officers, these agreements do not prevent them from terminating their employment with us at any time. In addition, because we will rely on Juvenescence to provide the services of certain administrative and management personnel, we will not have the benefit of the full time and effort of those Juvenescence employees in the management and development of our business.
If we lose one or more of our executive officers or key employees, our ability to implement the Merger and the other Contemplated Transactions or, if the Merger is not completed, our current business strategies successfully could be seriously harmed. Furthermore, replacing executive officers and key employees may be difficult, in particular during the pendency of the Merger, and may take an extended period of time because of the uncertainty about such persons roles following the Merger and the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize product candidates successfully. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also will experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we will rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be engaged by entities other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to implement the Merger and the other Contemplated Transactions or, if the Merger is not completed, to continue to execute on our current business strategies while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs will be limited.
Our business and operations could suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters including earthquakes and tsunamis, terrorism, war, and telecommunication and electrical failures. A loss of or damage to our data, a disruption in access to our data, or inappropriate disclosure of confidential or proprietary information, could disrupt our operations, delay or otherwise adversely affect the development of our product candidates, significantly increase our costs, or result in delays in any future regulatory filings we may make.
In addition, our product candidates are manufactured using cells that are stored in a cryopreserved master cell bank. While we believe we have adequate backup should any cell bank be lost in a catastrophic event, it is possible that we or our third-party suppliers and manufacturers could lose multiple cell banks and have our manufacturing severely impacted by the need to replace the cell banks. We cannot assure you that any stability or other issues relating to the manufacture of any of our product candidates or products will not occur in the future. Any delay or interruption in the supply of clinical trial supplies could delay the completion of planned clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Any adverse developments affecting clinical or commercial manufacturing of our product candidates or products may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls or other interruptions in the supply of our product candidates or products. Accordingly, failures or difficulties faced at any level of our supply chain could adversely affect our business and delay or impede the development and commercialization of any of our product candidates or products and could have an adverse effect on our business, prospects, financial condition and results of operations.
Security breaches and other disruptions could compromise our information and expose us to liability, and could cause our business and reputation to suffer.
In the ordinary course of business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of the licensors and licensees of the patents and other intellectual property we use, and personally identifiable information of employees and consultants. The secure processing, maintenance, and transmission of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance, or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost, or stolen. Any such access, disclosure, theft, or other loss of information could result in legal claims or proceedings or liability under laws that protect the privacy of personal information, and could disrupt our operations and damage our reputation. Even if we do not incur an interruption of or our operations, fines, penalties, or financial liability to third parties from a security breach, we could suffer a loss of confidence in our services, which could adversely affect our business and competitive position.
Failure of our internal control over financial reporting could harm our business and financial results.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with U.S. GAAP. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our consolidated financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use, or disposition of our assets that could have a material effect on the consolidated financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our consolidated financial statements would be prevented or detected. Because we are an emerging growth company and a smaller reporting issuer, we are exempt from the requirement of having our internal controls over financial reporting audited by our independent registered public accountants, which means that material weaknesses or significant deficiencies in our internal controls that might be detected by an audit may not be detected and remedied. If we are successful in developing new medical products and technologies, the commercialization of those products and technologies will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud. Our accounting and other management systems and resources may not be adequately prepared to meet the financial reporting and other requirements to which we will be subject, and failure to achieve and maintain effective internal controls could have a material adverse effect on our business.
We are subject to laws and regulations governing corruption, which will require us to develop, maintain, and implement costly compliance programs.
We must comply with a wide range of laws and regulations to prevent corruption, bribery, and other unethical business practices, including the Foreign Corrupt Practices Act (FCPA), anti-bribery and anti-corruption laws in other countries. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
Anti-bribery laws prohibit us, our employees, and some of our agents or representatives from offering or providing any personal benefit to covered government officials to influence their performance of their duties or induce them to serve interests other than the missions of the public organizations in which they serve. Certain commercial bribery rules also prohibit offering or providing any personal benefit to employees and representatives of commercial companies to influence their performance of their duties or induce them to serve interests other than their employers. The FCPA also obligates companies whose securities are listed in the U.S. to comply with certain accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and devise and maintain an adequate system of internal accounting controls for international operations. The anti-bribery provisions of the FCPA are enforced primarily by the United States Department of Justice. The SEC is involved with enforcement of the books and records provisions of the FCPA.
Compliance with these anti-bribery laws is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the anti-bribery laws present particular challenges in the medical industry because in many countries including China, hospitals are state-owned or operated by the government, and doctors and other hospital employees are considered foreign government officials. Furthermore, in certain countries (China in particular), hospitals and clinics are permitted to sell pharmaceuticals to their patients and are primary or significant distributors of pharmaceuticals. Certain payments to hospitals in connection with clinical studies, procurement of pharmaceuticals and other work have been deemed to be improper payments to government officials that have led to vigorous anti-bribery law enforcement actions and heavy fines in multiple jurisdictions, particularly in the U.S. and China.
It is not always possible to identify and deter violations, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations.
In the medical industry, corrupt practices include, among others, acceptance of kickbacks, bribes or other illegal gains or benefits by the hospitals and medical practitioners from manufacturers of pharmaceutical or other products, distributors or their third party agents in connection with the prescription of certain pharmaceuticals or sale of products. If our employees, affiliates, distributors or third party marketing firms violate these laws or otherwise engage in illegal practices with respect to their sales or marketing of our products or other activities involving our products, we could be required to pay damages or heavy fines by multiple jurisdictions where we operate, which could materially and adversely affect our financial condition and results of operations. There have been recent occurrences in which certain hospitals have denied access to sales representatives from pharmaceutical companies because the hospitals wanted to avoid the perception of corruption. If this attitude becomes widespread among our potential customers, our ability to promote our products to hospitals may be adversely affected.
If we and our subsidiaries expand operations internationally, we will need to increase the scope of our compliance programs to address the risks relating to the potential for violations of the FCPA and other anti-bribery and anti-corruption laws. Our compliance programs will need to include policies addressing not only the FCPA, but also the provisions of a variety of anti-bribery and anti-corruption laws in multiple foreign jurisdictions, provisions relating to books and records that apply to us as a public company, and include effective training for our personnel throughout our organization. The creation and implementation of anti-corruption compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required. Violation of the FCPA and other anti-corruption laws can result in significant administrative and criminal penalties for us and our employees, including substantial fines, suspension or debarment from government contracting, prison sentences, or even the death penalty in extremely serious cases in certain countries. The SEC also may suspend or bar us from trading securities on U.S. exchanges for violation of the FCPA’s accounting provisions. Even if we are not ultimately punished by government authorities, the costs of investigation and review, distraction of our personnel, legal defense costs, and harm to our reputation could be substantial and could limit our profitability or our ability to develop or commercialize our product candidates. In addition, if any of our competitors are not subject to the FCPA, they may engage in practices that will lead to their receipt of preferential treatment from foreign hospitals and enable them to secure business from foreign hospitals in ways that are unavailable to us.
Risks Related to Our Industry
We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may harm our business and financial condition, and our ability to successfully market or commercialize our product candidates.
The biotechnology and pharmaceutical industries are characterized by rapidly changing technologies, competition and a strong emphasis on intellectual property. We may face competition from other companies focused on therapeutics for age-related disease, which is a highly competitive environment. There are numerous biotechnology companies developing therapeutics for human aging, with each company often focusing on a specific molecular pathway within cells. For example, ResTORbio, Inc. is developing modulators of the mechanistic target of rapamycin (mTOR) pathway to treat immunological and cardiovascular disorders. Calico Life Sciences LLC is a Google-founded research and development company aimed at identifying molecular pathways that control animal lifespan and translating these insights into novel therapeutics designed to increase human healthspan. Unity Biotechnology, Inc. focuses on cellular senescence, in particular, the use of agents that can target senescent cells for selective ablation (senolysis). Unity’s stated targeted age-related diseases include osteoarthritis as well as other ophthalmological and pulmonary diseases. Our therapeutic products in development are likely to face competition from a large number of companies and technological strategies including therapeutics intended to address our lead indications. See “Description of AgeX’s Business—Competition.”
We may also face competition from large and specialty pharmaceutical and biotechnology companies, academic research institutions, government agencies and public and private research institutions. Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology, and gene therapy industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any products that we may develop or that would render any products that we may develop obsolete or non-competitive. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In particular, the Ministry of Labor Health and Welfare in Japan may grant SAKIGAKE designation to a competing product candidate, which is designed to provide for faster review and approval for any such product candidate as compared to the conventional process. If any competing product candidate receives SAKIGAKE designation in Japan, it may be commercialized more quickly in Japan than any of our product candidates. Additionally, technologies developed by our competitors may render our potential product candidates uneconomic or obsolete, and we may not be successful in marketing any product candidates we may develop against competitors.
Any product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
The Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the ACA) includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full a biologics license application (BLA) for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. While it is uncertain when such processes intended to implement BPCIA may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
There is a risk that any of our product candidates approved as a biological product under a BLA would not qualify for the 12-year period of exclusivity or that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that any other product candidates we may seek to develop in the future will ever obtain regulatory approval. Neither we nor any future collaborator or licensee is permitted to market any of our product candidates in the United States until we receive regulatory approval of a BLA from the FDA. It is possible that the FDA may refuse to accept for substantive review any BLAs that we submit for our product candidates or may conclude after review of our data that our application is insufficient to obtain marketing approval of our product candidates.
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, we or our collaborators or licensees will need to demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or foreign regulatory agencies, that the product candidate is safe and effective for the intended uses. Results from nonclinical studies and clinical trials can be interpreted in different ways. Even if we believe the nonclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. The FDA may also require us to conduct additional preclinical studies or clinical trials for our product candidates either prior to or post-approval, or it may object to elements of our clinical development program. Depending on the extent of these or any other FDA-required studies, approval of any BLA or application that we submit may be delayed by several years or may require us to expend significantly more resources than we have available.
Any therapeutic products that we and our subsidiaries may develop cannot be sold until the FDA and corresponding foreign regulatory authorities approve the products for medical use. The need to obtain regulatory approval to market a new product means that:
| ● | Expensive and time-consuming clinical trials of new products will need to be conducted. The full cost of conducting and completing clinical trials necessary to obtain FDA and foreign regulatory approval of a new product cannot be presently determined but could exceed our financial resources or could discourage any future licensees or collaborators from pursuing FDA approval of our product candidates. |
| ● | Clinical trials and the regulatory approval process for a pharmaceutical or cell-based product can take several years to complete. As a result, we will face expenses and delays inherent in seeking FDA and foreign regulatory approval of new products, even if the results of clinical trials are favorable. |
| ● | Data obtained from preclinical and clinical studies is susceptible to varying interpretations and regulatory changes that could delay, limit, or prevent regulatory agency approvals. |
| ● | Because the therapeutic products we plan to develop with pluripotent stem cell technology or progenitor cell technology involve the application of new technologies and approaches to medicine, the FDA or foreign regulatory agencies may subject those products to additional or more stringent review than drugs or biologicals derived from other technologies. |
| ● | A product that is approved may be subject to restrictions on use. |
| ● | The FDA can recall or withdraw approval of a product, if it deems necessary. |
| ● | We will face similar regulatory issues in foreign countries. |
Approval of our product candidates may be delayed or refused for many reasons, including the following:
| ● | The FDA or comparable foreign regulatory authorities may disagree with the design or implementation of the applicable clinical trial; |
| ● | A clinical trial might not demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that our product candidates are safe and effective for any of their proposed indications; |
| ● | The results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| ● | A clinical trial fail to demonstrate that our product candidates’ clinical and other benefits outweigh their safety risks; |
| ● | The FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical programs or clinical trials; |
| ● | The data collected from clinical trials of our product candidates may not be sufficient to support the submission of a BLA or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| ● | The facilities of the any third-party manufacturers with which we may contract may not be adequate to support approval of our product candidates (for example, regulatory approval of cell- and tissue-based products require high standards of quality control); and |
| ● | The approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
Of the large number of potential products in development, only a small percentage successfully complete the FDA or foreign regulatory approval processes and are commercialized. The lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations and prospects.
Ethical, social and legal concerns about research regarding stem cells, could result in regulations restricting or prohibiting the processes we may use. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. More restrictive regulations or claims that our products are unsafe or pose a hazard could prevent us from commercializing any products. New government requirements may be established that could delay or prevent regulatory approval of our product candidates under development. It is impossible to predict whether legislative changes will be enacted, regulations, policies or guidance changed, or interpretations by agencies or courts changed, or what the impact of such changes, if any, may be.
Regulatory requirements governing gene and cell therapy products have changed frequently and may continue to change in the future. The FDA has established the Office of Tissues and Advanced Therapies within its Center for Biologics Evaluation and Research, or CBER, to consolidate the review of gene therapy and related products, and has established the Cellular, Tissue and Gene Therapies Advisory Committee to advise the CBER in its review. Gene therapy clinical trials conducted at institutions that receive funding for recombinant DNA research from the National Institute of Health (NIH) also are potentially subject to review by the NIH Office of Science Policy’s Recombinant DNA Advisory Committee, or the RAC, in limited circumstances. Although the FDA decides whether individual gene therapy protocols may proceed, the RAC public review process, if undertaken, can delay the initiation of a clinical trial, even if the FDA has reviewed the trial design and details and authorized its initiation. Conversely, the FDA can put an investigational new drug application, or IND, on clinical hold even if the RAC has provided a favorable review or an exemption from in-depth, public review. If we were to engage an NIH-funded institution to conduct a clinical trial, that institution’s institutional biosafety committee, or IBC, as well as its institutional review board, or IRB, would need to review the proposed clinical trial to assess the safety of the trial and may determine that RAC review is needed. In addition, adverse developments in clinical trials of gene therapy products conducted by others may cause the FDA or other oversight bodies to change the requirements for approval of any of our product candidates. Similarly, foreign regulatory authorities may issue new guidelines concerning the development and marketing authorization for gene therapy medicinal products and require that we comply with these new guidelines.
Some of our future products may be viewed by the FDA as combination products and the review of combination products is often more complex and more time consuming than the review of other types of products.
Our future products may be regulated by the FDA as combination products. For a combination product, the FDA must determine which center or centers within the FDA will review the product candidate and under what legal authority the product candidate will be reviewed. The process of obtaining FDA marketing clearance or approval is lengthy, expensive, and uncertain, and we cannot be sure that any of our combination products, or any other products, will be cleared or approved in a timely fashion, or at all. In addition, the review of combination products is often more complex and more time consuming than the review of a product candidate under the jurisdiction of only one center within the FDA. We cannot be sure that the FDA will not select to have our combination products reviewed and regulated by only one FDA center and/or different legal authority, in which case the path to regulatory approval would be different and could be more lengthy and costly. If the FDA does not approve or clear our products in a timely fashion, or at all, our business and financial condition will be adversely affected.
If we encounter difficulties enrolling patients in clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of clinical trials of our product candidates, and even once enrolled we may be unable to retain a sufficient number of patients to complete the trials. The enrollment of patients depends on many factors, including:
| ● | the patient eligibility criteria defined in the protocol; |
| ● | the size of the patient population required for analysis of the trial’s primary endpoints; |
| ● | the proximity of patients to study sites; |
| ● | the design of the trial; |
| ● | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| ● | clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating; |
| ● | our ability to obtain and maintain patient consents; and |
| ● | the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
In addition, clinical trials of our product candidates will compete with other clinical trials for product candidates of other companies that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in trials of our product candidates may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we expect that clinical trials or our product candidates may be conducted at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials in such clinical trial site.
Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our product candidates or could render further development impossible.
Even if we obtain FDA approval for any of our product candidates in the United States, we may never obtain approval for or commercialize it in any other jurisdiction, which would limit our ability to realize its full market potential.
In order to market any products in any particular jurisdiction, we or a licensee or collaborator will need to establish and comply with numerous and varying regulatory requirements on a country-by-country basis regarding safety and efficacy. Approval by the FDA in the United States does not ensure approval by regulatory authorities in other countries or jurisdictions. However, the failure to obtain approval in one jurisdiction may negatively impact our ability to obtain approval elsewhere. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country.
Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and increased costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our products in those countries. We do not have any product candidates approved for sale in any jurisdiction, including in international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of any product we develop will be unrealized.
Clinical studies are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Clinical development is expensive, time consuming and involves significant risk. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of development. Events that may prevent successful or timely completion of clinical development include but are not limited to:
| ● | inability to generate satisfactory preclinical, toxicology, or other in vivo or in vitro data to support the initiation or continuation of clinical studies necessary for product approval; |
| ● | delays in reaching agreement on acceptable terms with clinical research organizations (CROs) and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites; |
| ● | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical study site; |
| ● | failure to permit the conduct of a study by regulatory authorities, after review of an investigational new drug, or IND, or equivalent foreign application or amendment; |
| ● | delays in recruiting qualified patients in our clinical studies; |
| ● | failure by clinical sites or our CROs or other third parties to adhere to clinical study requirements or report complete findings; |
| ● | failure to perform the clinical studies in accordance with the FDA’s good clinical practices requirements, or applicable foreign regulatory guidelines; |
| ● | patients dropping out of our clinical studies; |
| ● | occurrence of adverse events associated with our product candidates; |
| ● | inability to use clinical trial results from foreign jurisdictions in support of U.S. regulatory approval; |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| ● | the cost of clinical studies of our product candidates; |
| ● | negative or inconclusive results from our clinical trials which may result in our deciding, or regulators requiring us, to conduct additional clinical studies or abandon development programs for a product candidate; and |
| ● | delays in reaching agreement on acceptable terms with third-party manufacturers, or delays in the manufacture of sufficient quantities of our product candidates for use in clinical studies. |
Any inability to successfully complete clinical development and obtain regulatory approval could result in additional costs to us or impair our ability to generate revenue. Clinical study delays could also shorten any periods during which our products have patent protection and may allow competitors to develop and bring products to market before we do and may harm our business and results of operations.
Even if a product candidate receives regulatory approval, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any product candidate that receives marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, packaging, distribution, adverse event reporting, storage, recordkeeping, export, import, advertising and promotional activities for such product, among other things, will be subject to extensive and ongoing requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, establishment registration and drug listing requirements, continued compliance with cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping and Good Clinical Practice (GCP) requirements for any clinical trials that we conduct post-approval.
The FDA closely regulates the post-approval marketing and promotion of genetic medicines to ensure they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we market our products for uses beyond their approved indications, we may be subject to enforcement action for off-label marketing. Violations of the U.S. federal Food, Drug, and Cosmetic Act, or FDCA, relating to the promotion of prescription drugs may lead to FDA enforcement actions and investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on manufacturing such products; |
| ● | restrictions on the labeling or marketing of a product; |
| ● | restrictions on product distribution or use; |
| ● | requirements to conduct post-marketing studies or clinical trials; |
| ● | warning letters or holds on clinical trials; |
| ● | withdrawal of the products from the market; |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| ● | fines, restitution or disgorgement of profits or revenues; |
| ● | suspension or withdrawal of marketing approvals; |
| ● | refusal to permit the import or export of our products; |
| ● | product seizure or detention; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
The FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of any of our product candidates. For example, in December 2016, the 21st Century Cures Act, or Cures Act, was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and biologics and spur innovation, but its ultimate implementation is unclear. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained which would adversely affect our business, prospects and ability to achieve or sustain profitability. We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad.
Our product candidates may cause serious adverse events or undesirable side effects or have other properties which may delay or prevent their regulatory approval, limit the commercial profile of an approved label, or, result in significant negative consequences following marketing approval, if any.
Serious adverse events or undesirable side effects caused by our product candidates could cause an interruption, delay or halt of clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. Results of clinical trials could reveal a high and unacceptable severity and prevalence of side effects, toxicities or unexpected characteristics, including death.
For example, there have been significant adverse side effects in cell therapy treatments in the past, including reported cases of certain cancers. In addition to side effects that may be caused by our product candidates, the conditioning, administration process or related procedures also can cause adverse side effects, including compromise of a patient’s immune system. If unacceptable side effects arise in the development of our product candidates, we, the FDA, the IRBs at the institutions in which our studies are conducted or Data Safety Monitoring Board, or DSMB, could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Any of these occurrences may harm our business, financial condition and prospects significantly.
If any of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by any such product, including during any long-term follow-up observation period recommended or required for patients who receive treatment using our products, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw approvals of such product; |
| ● | we may be required to recall a product or change the way such product is administered to patients; |
| ● | additional restrictions may be imposed on the marketing of the particular product or the manufacturing processes for the product; |
| ● | regulatory authorities may require additional warnings on the label, such as a “black box” warning or contraindication; |
| ● | we may be required to implement a Risk Evaluation and Mitigation Strategy, or REMS, or create a medication guide outlining the risks of such side effects for distribution to patients; |
| ● | the product could become less competitive; |
| ● | we could be sued and held liable for harm caused to patients; and |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our product candidates harm patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or could otherwise be negatively impacted, and we could be subject to costly and damaging product liability claims.
The use or misuse of any product candidates in clinical trials and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies, or others selling or otherwise coming into contact with our products. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | impairment of our business reputation; |
| ● | initiation of investigations by regulators; |
| ● | withdrawal of clinical trial participants; |
| ● | costs due to related litigation; |
| ● | distraction of management’s attention from our primary business; |
| ● | substantial monetary awards to patients or other claimants; |
| ● | the inability to commercialize our product candidates; |
| ● | product recalls, withdrawals or labeling, and marketing or promotional restrictions; |
| ● | decreased demand for our product candidates, if approved for commercial sale. |
We may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we commence clinical trials or obtain marketing approval for any product candidates, we intend to increase our insurance coverage to include clinical use or the sale of commercial products, as applicable; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on drugs or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, employment practices liability, property, auto, workers’ compensation, umbrella, and directors’ and officers’ insurance.
Any additional product liability insurance coverage we acquire in the future, may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any of our product candidates, we intend to acquire insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on commercially reasonable terms or in adequate amounts. A successful product liability claim or series of claims brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business, including preventing or limiting the commercialization of any product candidates we develop.
As a public company, it can be difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on the AgeX Board, our board committees or as executive officers. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
Misconduct by our employees and independent contractors, including principal investigators, contract research organizations, or CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization, could include intentional, reckless or negligent conduct or unauthorized activities that violate: (i) the laws and regulations of the FDA, European Medical Agency (EMA) rules and regulations and other similar regulatory requirements, including those laws that require the reporting of true, complete and accurate information to such authorities; (ii) manufacturing standards; (iii) data privacy, security, fraud and abuse and other healthcare laws and regulations; or (iv) laws that require the reporting of true, complete and accurate financial information and data. Specifically, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, creation of fraudulent data in preclinical studies or clinical trials or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgements, possible exclusion from participation in Medicare, Medicaid, other U.S. federal healthcare programs or healthcare programs in other jurisdictions, individual imprisonment, other sanctions, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations.
Government-imposed bans or restrictions and religious, moral, and ethical concerns about the use of human embryonic stem cells could prevent us from developing and successfully marketing stem cell products.
Government-imposed bans or restrictions on the use of embryos or human embryonic stem cells (hES cells), in research and development in the United States and abroad could generally constrain stem cell research, thereby limiting the market and demand for our products.
California law requires that stem cell research be conducted under the oversight of a Stem Cell Research Oversight (SCRO) Committee. Many kinds of stem cell research, including the derivation of new hES cell lines, may only be conducted in California with the prior written approval of the SCRO Committee. A SCRO Committee could prohibit or impose restrictions on the research that we plan to do. An adverse decision by a SCRO Committee, or their imposition of restrictions on a research program could adversely affect our ability to enter into co-development or licensing arrangements for the development of a product candidate.
The use of hES cells may give rise to religious, moral, and ethical issues. These considerations could lead to more restrictive government regulations or could generally constrain stem cell research, thereby limiting the market and demand for our products.
Adverse publicity regarding cell-based therapies could impact our business.
Adverse publicity due to the ethical and social controversies surrounding the use of embryonic stem cells or any adverse reported side effects from any stem cell or other cell therapy clinical trials or to the failure of such trials to demonstrate that these therapies are efficacious could materially and adversely affect our ability to raise capital, conduct and complete clinical trials and achieve market acceptance of such products, if approved. For example, research institutions, including those who may be our collaborators, may from time to time publish findings or studies regarding the human genome (such as the Human Genome Project) that adversely implicate our product candidates, including findings of cancer dependencies in cell lines used in our cell-based therapies.
The price and sale of any product candidates that we may develop may be limited by health insurance coverage and government regulation.
Success in selling any pharmaceutical and cell-based products and medical devices that we may develop may depend in part on the extent to which health insurance companies, health maintenance organizations (HMOs), and government health administration authorities such as Medicare and Medicaid will pay for the cost of the products and related treatment. Until a new product is introduced into the medical marketplace, we will not know with certainty whether adequate health insurance, HMO, and government coverage will be available to permit the product to be sold at a price high enough for us to generate a profit. In some foreign countries, pricing or profitability of health care products is subject to government control, which may result in low prices for our products. Provisions of the Inflation Reduction Act of 2022 may impact the prices of drug products that are sold in the United States, particularly through Medicare programs. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. There have been a number of other federal and state proposals to implement similar government controls, and new proposals are likely to be made in the future. We cannot be sure that coverage and reimbursement in the United States, the EU or elsewhere will be available for our product candidates or any product that we may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs or biologics when an equivalent generic drug, biosimilar or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidates as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidates, pricing of existing third-party therapeutics may limit the amount we will be able to charge for our product candidates. These payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in our product candidates. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on our product candidates.
There is significant uncertainty related to the insurance coverage and reimbursement of newly-approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models in the United States for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. We cannot predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
No uniform policy for coverage and reimbursement for products exists among third-party payors in the United States. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases on short notice, and we believe that changes in these rules and regulations are likely.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe and other countries have and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our product candidates may be reduced compared with the United States and may be insufficient to generate commercially-reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. While it is not possible to predict or model the insurance landscape at the time any of our product candidates may receive regulatory approval, we expect to experience pricing pressures in connection with the sale of our product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and biologics and surgical procedures and other treatments, has become intense. As a result, increasingly high barriers are being erected to the entry of new products.
Enacted and future healthcare legislation, including the ACA, may increase the difficulty and cost of obtaining marketing approval and commercializing our product candidates and may affect the prices we may set.
In the United States, the EU and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. As a result of the adoption of the ACA in the United States, substantial changes have been made to the system for paying for healthcare in the United States. Certain provisions related to cost-savings and reimbursement measures could adversely affect our future financial performance. For example, among the provisions of the ACA, those of greatest importance to the biopharmaceutical industry includes the following:
| ● | an annual, non-deductible fee payable by any entity that manufactures or imports certain branded prescription drugs and biologic agents (other than those designated as orphan drugs), which is apportioned among these entities according to their market share in certain government healthcare programs; |
| ● | new requirements to report certain financial arrangements with physicians and teaching hospitals, including reporting “transfers of value” made or distributed to prescribers and other healthcare providers and reporting investment interests held by physicians and their immediate family members; |
| ● | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; |
| ● | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
| ● | a licensure framework for follow on biologic products; |
| ● | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and |
| ● | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services, or CMS, to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
The ACA has been subject to revision and to judicial, congressional, and executive challenges. As a result of tax reform legislation passed in December 2017, the requirement that all individuals maintain health insurance coverage or pay a penalty, referred to as the “individual mandate” was eliminated effective January 1, 2019. According to the Congressional Budget Office, the repeal of the individual mandate will cause 13 million fewer Americans to be insured in 2027 and premiums in insurance markets may rise.
The costs of prescription pharmaceuticals in the United States have also been the subject of considerable debate, and new legislative and administrative measures could be implemented to address such costs. To date, there have been several recent U.S. congressional inquiries and proposed state and federal legislation designed to, among other things, improve transparency in drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare, and reform government program reimbursement methodologies for drug products. Under recent legislation, starting in 2023 a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. The pricing of prescription pharmaceuticals is also subject to governmental control outside the United States. In these other countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost effectiveness of our product candidates to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitable could be impaired.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for approved products. In addition, there have been several recent Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare and reform government program reimbursement methodologies for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent labeling and post-marketing testing and other requirements.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally-mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates or put pressure on our product pricing.
In the EU, similar political, economic and regulatory developments may affect our ability to profitably commercialize our product candidates, if approved. In addition to continuing pressure on prices and cost containment measures, legislative developments at the EU or member state level may result in significant additional requirements or obstacles that may increase our operating costs. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize our product candidates, if approved.
In markets outside of the United States and EU, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action in the United States, the EU or any other jurisdiction. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability.
If we fail to comply with the extensive legal and regulatory requirements affecting the health care industry, we could face increased costs, penalties and a loss of business.
Our activities, and the activities of any collaborators, distributors and other third-party providers that we may engage in the future, will be subject to extensive government regulation and oversight both in the U.S. and in foreign jurisdictions. The FDA and comparable agencies in other jurisdictions will directly regulate many of our most critical business activities, including the conduct of preclinical and clinical studies, product manufacturing, advertising and promotion, product distribution, adverse event reporting, and product risk management. Our interactions in the United States or abroad with physicians and other health care providers that prescribe or purchase our products will also be subject to government regulation designed to prevent fraud and abuse in the sale and use of the products and place greater restrictions on the marketing practices of health care companies. Health care companies such as ours are facing heightened scrutiny of their relationships with health care providers from anti-corruption enforcement officials. In addition, health care companies such as ours have been the target of lawsuits and investigations alleging violations of government regulation, including claims asserting submission of incorrect pricing information, impermissible off-label promotion of pharmaceutical products, payments intended to influence the referral of health care business, submission of false claims for government reimbursement, antitrust violations, and violations related to environmental matters. Risks relating to compliance with laws and regulations may be heightened if we operate globally.
Regulations governing the health care industry are subject to change, with possibly retroactive effect, including:
| ● | new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements; |
| ● | changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity; |
| ● | requirements that provide for increased transparency of clinical trial results and quality data, such as the EMA’s clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception, or legal action which could harm our business; and |
| ● | changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products, or otherwise adversely affect the market for our products. |
Violations of governmental regulation may be punishable by criminal and civil sanctions against us, including fines and civil monetary penalties and exclusion from participation in government programs, including Medicare and Medicaid, as well as sanctions against executives overseeing our business. In addition to penalties for violation of laws and regulations, we could be required to repay amounts we received from government payors or pay additional rebates and interest if we are found to have miscalculated the pricing information we have submitted to the government. We cannot ensure that our compliance controls, policies and procedures will in every instance protect us from acts committed by our employees, collaborators, partners or third-party providers that would violate the laws or regulations of the jurisdictions in which we operate. Whether or not we have complied with the law, an investigation into alleged unlawful conduct could increase our expenses, damage our reputation, divert management time and attention, and adversely affect our business.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws, and if we are unable to comply with such laws, we could face substantial penalties.
If the FDA grants marketing approval for any of our product candidates or technologies and commercializing those products or technologies begins in the United States, our operations may be subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, product sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| ● | HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and implementing regulations, which impose certain requirements relating to the privacy, security, and transmission of individually identifiable health information; |
| ● | the Physician Payments Sunshine Act which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; |
| ● | the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices; |
| ● | the U.S. Public Health Service Act, which prohibits, among other things, the introduction into interstate commerce of a biological product unless a biologics license is in effect for that product; and |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payors, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or that otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. Further, state laws differ from each other and from federal law in significant ways, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Risks Related to our Dependence on Third Parties
We may become dependent on future collaborations to develop and commercialize our product candidates and to provide the regulatory compliance, sales, marketing, and distribution capabilities required for the success of our business.
We may enter into various kinds of collaborative research and development and product marketing agreements to develop and commercialize our products. The expected future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our products, but there are risks associated with entering into collaboration arrangements.
The process of establishing and maintaining collaborative relationships is difficult, time-consuming and involves significant uncertainty, such as:
| ● | a collaboration partner may shift its priorities and resources away from our product candidates due to a change in business strategies, or a merger, acquisition, sale or downsizing; |
| ● | a collaboration partner may seek to renegotiate or terminate their relationships with us due to unsatisfactory clinical results, manufacturing issues, a change in business strategy, a change of control or other reasons; |
| ● | a collaboration partner may cease development in therapeutic areas which are the subject of our strategic collaboration; |
| ● | a collaboration partner may not devote sufficient capital or resources towards our product candidates; |
| ● | a collaboration partner may change the success criteria for a product candidate thereby delaying or ceasing development of such candidate; |
| ● | a significant delay in initiation of certain development activities by a collaboration partner will also delay payment of milestones tied to such activities, thereby impacting our ability to fund our own activities; |
| ● | a collaboration partner could develop a product that competes, either directly or indirectly, with our product candidate; |
| ● | a collaboration partner with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product; |
| ● | a collaboration partner with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements; |
| ● | a collaboration partner may terminate a strategic alliance; |
| ● | a dispute may arise between us and a partner concerning the research, development or commercialization of a product candidate resulting in a delay in milestones, royalty payments or termination of an alliance and possibly resulting in costly litigation or arbitration which may divert management attention and resources; and |
| ● | a partner may use our products or technology in such a way as to invite litigation from a third party. |
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its product development, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more product candidates could be delayed, curtailed, or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
We have no marketing, sales, or distribution resources for the commercialization of any products or technologies that we might successfully develop.
We do not have any infrastructure for the sales, marketing or distribution of our products, and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. There are significant expenses and risks involved with establishing our own sales, marketing and distribution capabilities, including our ability to hire, retain and appropriately incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities could delay any product launch, which would adversely impact the commercialization of any approved product candidate.
If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. As a result, our gross profit from product sales may be lower than it would be if we were to sell our products directly to end users at retail prices through our own sales force. There can be no assurance we will be able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations.
If we are unable to build our own sales force or negotiate a collaborative relationship for the commercialization of our product candidates, we may be forced to delay the potential commercialization of such candidates or reduce the scope of our sales or marketing activities for them. If we elect to increase our expenditures to fund commercialization activities ourselves, we will need to obtain additional capital, which may not be available to us on acceptable terms, or at all. We could enter into arrangements with collaborative partners at an earlier stage than otherwise would be ideal and we may be required to relinquish rights to our product candidates or otherwise agree to terms unfavorable to us, any of which may have an adverse effect on our business, operating results and prospects.
If we are unable to establish adequate sales, marketing and distribution capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates and may not become profitable and may incur significant additional losses. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our product candidates and intend to rely on third parties to conduct, supervise and monitor our clinical trials.
We will need to rely on third parties, such as contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our product candidates. We may also rely on third parties to assist with our preclinical development of product candidates.
If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. However, we will remain responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our third-party contractors will be required to comply with the good laboratory practices (GLPs) and GCPs, which are regulations and guidelines enforced by the FDA and are also required by the competent authorities of the member states of the European Economic Area (EEA) and comparable foreign regulatory authorities in the form of International Conference on Harmonization guidelines for any of our product candidates that are in preclinical and clinical development. The regulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. If we or our third-party contractors fail to comply with GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. Accordingly, if our third-party contractors fail to comply with these regulations or fail to recruit a sufficient number of subjects, we may be required to repeat clinical trials, which would delay the regulatory approval process.
Our third-party contractors will not be our employees, and we will not control whether or not they devote sufficient time and resources to our future clinical and nonclinical programs. These third-party contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other product development activities which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by third-party contractors, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our third-party contractors do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenues could be delayed.
If our relationship with any third-party contractors terminates, we may not be able to enter into arrangements with alternative third-party contractors or do so on commercially reasonable terms. Switching or adding additional third-party contractors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our third-party contractors, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition and prospects.
Risks Related to Intellectual Property
If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling our products.
| ● | Our success will depend in part on our ability to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining and enforcing patents, our competitors could use our technology and create products or technologies that compete with our products and technologies, without paying license fees or royalties to us. |
| ● | The preparation, filing, and prosecution of patent applications can be costly and time consuming. Our limited financial resources may not permit us to pursue patent protection of all of our technology and products throughout the world. |
| ● | Even if we are able to obtain issued patents covering our technology or products, we may have to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and products from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret rights. |
There is no certainty that our pending or future patent applications will result in the issuance of patents.
We acquired rights to patent applications for technology that Lineage has developed, and we may file additional new patent applications in the future seeking patent protection for new technology or products that we develop ourselves or jointly with others. However, there is no assurance that any of our licensed patent applications, or any patent applications that we may file in the future in the United States or abroad, will result in the issuance of patents.
The process of applying for and obtaining patents can be expensive and slow.
| ● | The preparation and filing of patent applications, and the maintenance of patents that are issued, may require substantial time and money. |
| ● | A patent interference proceeding may be instituted with the U.S. Patent and Trademark Office (the USPTO) when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. |
| ● | A derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor. |
| ● | Post Grant Review under the new Leahy Smith America Invents Act (America Invents Act) will make available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. |
| ● | Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application. |
Intellectual property we may develop using grants received from the federal government are subject to rights maintained by the government.
Research and development we perform that is funded by grants from the federal government, and any intellectual property that we create using those grants, is subject to the rights maintained by the federal government.
Our patents may not protect our technologies or products from competition.
| ● | We might not be able to obtain any patents beyond those we already own or have licensed or sublicensed, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful patent protection. |
| ● | There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability of any patent issued to us. |
| ● | In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party. Our patents may be subject to inter partes review (replacing the reexamination proceeding), a proceeding in which a third party can challenge the validity of one of our patents to have the patent invalidated. This means that patents owned or licensed by us may be subject to reexamination and may be lost if the outcome of the reexamination is unfavorable to us. |
| ● | The patents to which we have licenses to in overlapping fields of use. AgeX, through our subsidiary ReCyte Therapeutics, is a sublicensee under a cross-license between Lineage and Asterias, which creates another potential risk of Asterias and AgeX creating competing products. |
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents, if issued, on our technology and product candidates in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly in developing countries. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. The products offered by foreign competitors may compete with our products in jurisdictions where we do not have any issued or licensed patents or where any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing with us.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the United States and the EU do not afford intellectual property protection to the same extent as the laws of the United States and the EU. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, including India, China and certain developing countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and the EU. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, if our ability to enforce our patents to stop infringing activities is inadequate. These products may compete with our products, and our patents, if issued, or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business. Furthermore, while we intend to protect our intellectual property rights in major markets, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market products or license our patented technologies. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our technologies or products, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us.
The success of our business depends significantly on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing research, development, and commercialization of technologies and products that rely on that technology, unless we are able to obtain a license to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a technology or product with which our technologies or products would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to alternative technologies, which could prove costly and could cause delays in developing our technologies or products, or we could be forced to discontinue the development or marketing of any technologies and products that were developed using the technology covered by the patent.
Risks Related to AgeX Common Stock
There is a limited history to the public trading of AgeX common stock and there is no assurance that a market for AgeX common stock will be sustained.
Public trading of AgeX common stock on the NYSE American began on November 29, 2018. Accordingly, there is only a limited history of the public trading of AgeX common stock and there can be no assurance that an active market for AgeX common stock will be sustained.
We cannot predict the prices at which AgeX common stock may trade. The market price of AgeX common stock may fluctuate significantly, depending upon many factors, some of which may be beyond our control, including, but not limited to:
| ● | a shift in our investor base; |
| ● | our quarterly or annual earnings, or those of comparable companies; |
| ● | actual or anticipated fluctuations in our operating results; |
| ● | our ability to obtain financing as needed; |
| ● | changes in laws and regulations affecting our business; |
| ● | changes in accounting standards, policies, guidance, interpretations or principles; |
| ● | announcements by us or our competitors of significant investments, acquisitions or dispositions; |
| ● | the failure of securities analysts to cover AgeX common stock; |
| ● | changes in earnings estimates by securities analysts or our ability to meet those estimates; |
| ● | the operating performance and stock price of comparable companies; |
| ● | overall market fluctuations; and |
| ● | general economic conditions and other external factors. |
Because we are engaged in the development of pharmaceutical and cell therapy products, the price of shares of AgeX common stock may rise and fall rapidly.
The price of AgeX common stock may rise rapidly in response to certain events, such as the commencement of clinical trials of an experimental new therapy, even though the outcome of those trials and the likelihood of ultimate FDA approval of a therapeutic product remain uncertain. Similarly, prices of AgeX common stock may fall rapidly in response to certain events such as unfavorable results of clinical trials or a delay or failure to obtain FDA approval. Further, the failure of our earnings to meet analysts’ expectations could result in a significant rapid decline in the market price of AgeX common stock.
Because we do not pay dividends, AgeX common stock may not be a suitable investment for anyone who needs to earn dividend income.
We do not have current plans to pay any cash dividends on AgeX common stock. The declaration, amount and payment of any future dividends on shares of common stock will be at the sole discretion of the AgeX Board. The AgeX Board may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions and implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as the AgeX Board may deem relevant. For the foreseeable future we anticipate that any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our stockholders. This means that our stock may not be a suitable investment for anyone who needs to earn income from their investments.
Securities analysts may not initiate coverage or continue to cover AgeX common stock, and this may have a negative impact on the market price of our shares.
The market price and liquidity of AgeX common stock will depend, in part, on the research and reports that securities analysts publish about our business and AgeX common stock. We do not have any control over these analysts. There is no guarantee that securities analysts will cover AgeX common stock. If securities analysts do not cover AgeX common stock, the lack of research coverage may adversely affect the market price of those shares. If securities analysts do cover our shares, they could issue reports or recommendations that are unfavorable to the price of our shares, and they could downgrade a previously favorable report or recommendation, and in either case our share price could decline as a result of the report. If one or more of these analysts ceases to cover our shares or fails to publish regular reports on our business, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
You may experience dilution of your ownership interests if we issue additional shares of common stock or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are currently authorized to issue an aggregate of 205,000,000 shares of capital stock consisting of 200,000,000 shares of common stock and 5,000,000 “blank check” shares of preferred stock. As of November 3, 2023, there were 37,951,261 shares of AgeX common stock issued and outstanding, 211,600 shares of AgeX Series A preferred stock issued and outstanding and 148,400 shares of AgeX Series B preferred stock issued and outstanding, and 3,286,800 shares of AgeX common stock reserved for issuance upon the exercise of outstanding stock options or other stock-based awards under our 2017 Equity Incentive Plan, 12,127,583 shares of AgeX common stock reserved for issuance upon the exercise of outstanding warrants held by Juvenescence, 29,388,888 shares of AgeX common stock reserved for issuance upon the conversion of 211,600 shares of AgeX Series A preferred stock held by Juvenescence and 20,611,111 shares of AgeX common stock reserved for issuance upon the conversion of 148,400 shares of AgeX Series B preferred stock held by Juvenescence.
We may issue additional AgeX common stock or other securities that are convertible into or exercisable for AgeX common stock in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or medical products or for other business purposes. The future issuance of any additional shares of AgeX common stock or other securities may create downward pressure on the trading price of AgeX common stock.
We may also issue additional preferred stock, and such preferred stock may have rights, preferences, and privileges senior to the rights of AgeX common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into common stock on terms that would be dilutive to holders of common stock.
Our subsidiaries may finance a portion of their operations by selling shares of their capital stock or debt securities convertible into shares of their capital stock to private investors. Sales of subsidiary shares would reduce our ownership interest in the subsidiaries, and correspondingly dilute our shareholder’s ownership interests in our consolidated enterprise. Reverse Bio has, and other AgeX subsidiaries could also have, their own stock option plans and the exercise of subsidiary stock options or the sale of restricted stock under those plans would also reduce our ownership interest in the subsidiaries, with a resulting dilutive effect on the ownership interest of our shareholders in our consolidated enterprise. Subsidiaries might also issue preferred stock having rights, preferences, and privileges senior to the rights of the subsidiary common stock we hold with respect to dividends, rights to share in distributions of our assets if the subsidiary is liquidated, or voting rights. Any subsidiary preferred stock may also be convertible into common stock on terms that would be dilutive to us as a holder of subsidiary common stock.
We are an “emerging growth company,” and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies, which could make AgeX common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find AgeX common stock less attractive because we may rely on these exemptions. If some investors find AgeX common stock less attractive as a result, there may be a less active trading market for AgeX common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the Securities Act); (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended (the Exchange Act).
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biopharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Provisions in the AgeX Charter and the AgeX Bylaws and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in the certificate of incorporation of AgeX (the AgeX Charter) and our bylaws (the AgeX Bylaws) may discourage, delay or prevent a merger, acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of AgeX common stock, thereby depressing the market price of AgeX common stock. In addition, because the AgeX Board is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of the AgeX Board. Among other things, these provisions include those establishing:
| ● | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| ● | the ability of the AgeX Board to authorize the issuance of shares of preferred stock and to determine the terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer; and |
| ● | the ability of the AgeX Board to alter our bylaws without obtaining stockholder approval. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the DGCL, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Risks Related to Serina
Risks Related to Serina’s Operating History, Financial Position and Capital Requirements
Serina has a history of operating losses that are expected to continue for the foreseeable future, and it is unable to predict the extent of future losses, or whether it will generate significant revenues or achieve or sustain profitability.
Serina has focused on research and product development and has not generated any revenues to date from product sales. Additionally, it expects to continue to incur operating losses for the foreseeable future. These operating losses have adversely affected and are likely to continue to adversely affect Serina’s working capital, total assets, and stockholders’ deficit.
Because Serina is an early stage-company, its prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations.
Serina expects to make substantial expenditures and incur increasing operating costs in the future and its accumulated deficit will increase significantly as it expands development and clinical trial activities for its product candidates. Because of the risks and uncertainties associated with product development, Serina is unable to predict the extent of any future losses, whether it will ever generate significant revenues or if it will ever achieve or sustain profitability.
Serina is dependent on obtaining, and will continue to pursue, necessary funding from outside sources, including obtaining additional funding from the issuance of securities in order to continue its operations. Without adequate funding, it may not be able to meet its financial obligations.
Serina has not demonstrated an ability to perform the functions necessary for the successful commercialization of any products. The successful commercialization of any of its products will require it to perform a variety of functions, including:
| ● | continuing to undertake preclinical and clinical development; |
| ● | engaging in the development of product candidate formulations and manufacturing processes; |
| ● | interacting with the applicable regulatory authorities and pursuing other required steps for regulatory approval; |
| ● | engaging with payors and other pricing and reimbursement authorities; |
| ● | submitting marketing applications to and receiving approval from the applicable regulatory authorities; and |
| ● | manufacturing the applicable products and product candidates in accordance with regulatory requirements and, if ultimately approved, conducting sales and marketing activities in accordance with health care, FDA and similar foreign regulatory authority laws and regulations. |
Serina has a limited operating history and it expects a number of factors to cause its operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict its future performance.
Serina’s operations to date have been primarily limited to organizing and staffing its company, developing and securing its proprietary technology and preclinical and clinical development of its product candidates. It has not yet successfully completed all clinical trial phases for any of its product candidates, manufactured its product candidates at commercial scale or conducted sales and marketing activities that will be necessary to successfully commercialize its product candidates, if approved.
Serina’s financial condition has varied significantly in the past and will continue to fluctuate from quarter to quarter or year to year due to a variety of factors, many of which are beyond its control. Factors relating to its business that may contribute to these fluctuations include other factors described elsewhere in this proxy statement/prospectus/information statement and also include, among other things:
| ● | Serina’s ability to obtain additional funding to develop its product candidates; |
| ● | Serina’s ability to conduct and complete nonclinical studies and clinical trials, |
| ● | delays in the commencement, enrollment, and timing of clinical trials; |
| ● | the success of Serina’s nonclinical studies and clinical trials through all phases of development; |
| ● | any delays in regulatory review and approval of product candidates in clinical development; |
| ● | Serina’s ability to obtain and maintain regulatory approval for its product candidates in the United States and foreign jurisdictions; |
| ● | potential toxicity and/or side effects of Serina’s product candidates that could delay or prevent commercialization, limit the indications for any approved products, require the establishment of risk evaluation and mitigation strategies, or cause an approved drug to be taken off the market; |
| ● | Serina’s ability to establish or maintain partnerships, collaborations, licensing or other arrangements; |
| ● | market acceptance of Serina’s product candidates, if approved; |
| ● | competition from existing products, new products or new therapeutic approaches that may emerge; |
| ● | the ability of patients or health care providers to obtain coverage of or sufficient reimbursement for
Serina’s products; |
| ● | Serina’s ability to leverage its proprietary POZ technology platform to discover and develop additional product candidates; |
| ● | Serina’s ability and its licensors’ abilities to successfully obtain, maintain, defend and enforce intellectual property rights important to its business; and |
| ● | potential product liability claims. |
Accordingly, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
We need additional financing to execute our operating plan and continue to operate as a going concern.
As required under Accounting Standards Update 2014-15, Presentation of Financial Statements-Going Concern (ASC 205-40), we have the responsibility to evaluate whether conditions and/or events raise substantial doubt about our ability to meet our future financial obligations as they become due within one year after the date the financial statements are issued. Based on our most recent projected cash flows, we believe that our cash and cash equivalents would not be sufficient to satisfy our anticipated operating and other funding requirements for the next twelve months from the June 30, 2023. These factors raise substantial doubt regarding our ability to continue as a going concern. .. In addition, the report of our independent registered public accountants accompanying our audited consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement contains a qualification to such effect.
We have incurred operating losses and negative cash flows since inception and had an accumulated deficit of approximately $35,979,888 as of June 30, 2023. We expect to continue to incur operating losses and negative cash flows. Serina’s operations have consumed substantial amounts of cash. Serina will require substantial additional funds to support its continued research and development activities, including the anticipated costs of nonclinical studies and clinical trials, regulatory approvals, and potential commercialization. Additionally, its estimates on future financial needs may be based on assumptions that prove to be wrong, and it may spend its available financial resources much faster than it expects. Because we expect to continue to experience net operating losses, our ability to continue as a going concern is subject to our ability to obtain necessary capital from outside sources, including obtaining additional capital from the sale of Serina capital stock or other equity securities or assets, obtaining additional loans from financial institutions or investors, and entering into collaborative research and development arrangements or licensing some or all of our patents and know-how to third parties while retaining a royalty and other contingent payment rights related to the development and commercialization of products covered by the licenses. Our continued net operating losses and the risks associated with the development of our product candidates and technologies, has increased the difficulty in obtaining such capital, and there can be no assurances that we will be able to obtain such capital on favorable terms or at all. Furthermore, geopolitical instability, including the ongoing military conflicts between Russia and Ukraine and Israel and Hamas, as well as the impact of inflationary pressures and resulting rise in interest rates, on global financial markets could make the terms of any available financing less attractive to Serina and more dilutive to its existing stockholders. If it is unable to raise additional capital, it will have to delay, curtail, or eliminate one or more of its research and development programs or ultimately not be able to continue as a going concern. Additionally, raising additional capital may cause dilution to its stockholders.
Serina has never generated revenue from product sales and may never become profitable.
Serina’s ability to generate product sales and achieve profitability depends on its ability, alone or with collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, its current and future product candidates. It does not anticipate generating product sales for the next several years, if ever.
Serina’s product candidates will require additional clinical, manufacturing, and non-clinical development, regulatory approval, commercial manufacturing arrangements, establishment of a commercial organization, significant marketing efforts, and further investment before it generates any product sales. It cannot guarantee that it will meet its timelines for its development programs, which may be delayed or not completed for a number of reasons. Serina’s ability to generate future revenues from product sales depends heavily on its, or its collaborators’, ability to successfully:
| ● | complete research and obtain favorable results from nonclinical and clinical development of Serina’s current and future product candidates, including addressing any clinical holds that may be placed on its development activities by regulatory authorities; |
| ● | seek and obtain regulatory and marketing approvals for any of Serina’s product candidates for which it completes clinical trials, as well as their manufacturing facilities; |
| ● | launch and commercialize any of Serina’s product candidates for which it obtains regulatory and marketing approval by establishing a sales force, marketing, and distribution infrastructure or, alternatively, collaborating with a commercialization partner; |
| ● | qualify for coverage and establish adequate reimbursement by government and third-party payors for any of Serina’s product candidates for which it obtains regulatory and marketing approval; |
| ● | develop, maintain, and enhance a sustainable, scalable, reproducible, and transferable manufacturing process for the product candidates Serina may develop; |
| ● | establish and maintain supply and manufacturing capabilities internally or with third parties that can provide adequate, in both amount and quality, products, and services to support clinical development and the market demand for any of Serina’s product candidates for which it obtains regulatory and marketing approval; |
| ● | obtain market acceptance of current or any future product candidates as viable treatment options and effectively compete with other therapies to establish market share; |
| ● | maintain a continued acceptable safety and efficacy profile of Serina’s product candidates following launch; |
| ● | address competing technological and market developments; |
| ● | implement internal systems and infrastructure, as needed; |
| ● | negotiate favorable terms in any collaboration, licensing, or other arrangements into which Serina may enter and perform its obligations in such collaborations; |
| ● | maintain, protect, enforce, defend, and expand Serina’s portfolio of intellectual property rights, including patents, trade secrets, and know how; |
| ● | avoid and defend against third party interference, infringement, and other intellectual property claims; and |
| ● | attract, hire, and retain qualified personnel. |
Even if one or more of Serina’s current and future product candidates are approved for commercial sale, it anticipates incurring significant costs associated with commercializing any approved product candidate. Its expenses could increase beyond its expectations if it is required by the FDA or other regulatory authorities to perform clinical and other studies in addition to those that it currently anticipates. If it is required to conduct additional clinical trials or other testing of its product candidates that it develops beyond those that it currently expects, if it is unable to successfully complete clinical trials of its product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive, or if there are safety concerns, Serina may be delayed in obtaining marketing approval for its product candidates, not obtain marketing approval at all, or obtain more limited approvals. Even if it is able to generate revenues from the sale of any approved product candidates, it may not become profitable and may need to obtain additional funding to continue operations.
Risks Related to the Development of Serina’s Products
Serina’s product candidates are at an early stage of development and may not be successfully developed or commercialized.
All of Serina’s current product candidates are in preclinical development and will require substantial further capital expenditures, development, testing, and regulatory approval prior to commercialization. Serina has limited experience designing clinical trials and has not yet filed or supported a marketing application. It may be unable to design and execute a clinical trial that ultimately supports marketing approval.
The time required to obtain approval from the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of regulatory authorities. The outcome of studies is also inherently uncertain. Of the large number of drugs in development, only a small percentage successfully complete the regulatory approval process and are commercialized. The results of nonclinical studies, interim or top line studies, and early clinical trials of Serina’s product candidates may not be predictive of the results of later stage clinical trials. Failure can occur at any time during the clinical trial process. Product candidates in later stages of clinical trials may fail to show the desired safety, purity, and potency traits despite having progressed through nonclinical studies and initial clinical trials. Nonclinical and early clinical studies may also reveal unfavorable product candidate characteristics, including safety concerns. A number of companies have suffered significant setbacks in advanced clinical trials, notwithstanding promising results in earlier trials. In some instances, there can be significant variability in results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols, and the rate of dropout among clinical trial participants.
Accordingly, even if Serina is able to obtain the requisite financing to fund its development programs, it cannot assure you that its product candidates will be successfully developed or commercialized. Its failure to develop, manufacture or receive regulatory approval for or successfully commercialize any of its product candidates could result in the failure of its business and a loss of all of its stockholders’ investment.
Serina’s product candidates may fail at any stage of preclinical or clinical development, and may also reveal unfavorable product candidate characteristics, including safety concerns or the failure to demonstrate efficacy in initial clinical trials. Further, its product candidates may not receive regulatory approval even if they are successful in clinical trials. Although Serina anticipates completing the preclinical development, including toxicology testing and clinical supply manufacturing development, necessary to file an IND for SER 252 and additional INDs for other product candidates in the future, it may experience numerous unforeseen events before, during, or as a result of clinical trials that could delay or prevent its ability to commence or complete development, commence or complete clinical trials, receive marketing approval or commercialize its product candidates, including:
| ● | Serina may be unable to generate sufficient nonclinical, toxicology, or other in vivo or in vitro data to support the initiation of clinical trials; |
| ● | regulators or IRBs or Independent Ethics Committees (IECs) may not authorize Serina or its investigators to commence or continue a clinical trial, conduct a clinical trial at a prospective trial site, or amend trial protocols, or may require that it modifies or amends its clinical trial protocols; |
| ● | Serina, regulators, independent data safety monitoring committees, IRBs or IECs, or its data monitoring committee(s) may recommend or require the suspension or termination of clinical research for various reasons, including non-compliance with regulatory requirements or a finding that participants are being exposed to unacceptable health risks, undesirable side effects, or a failure of the product candidate to demonstrate any benefit to subjects, or other unexpected characteristics (alone or in combination with other products) of the product candidate, or due to findings of undesirable effects caused by a chemically or mechanistically similar therapeutic or therapeutic candidate; |
| ● | new information may emerge regarding Serina’s product candidates or technology platform that result in continued development of some or all of its product candidates being deemed undesirable; |
| ● | Serina may have delays identifying, recruiting, and training suitable clinical investigators or investigators may withdraw from its studies; |
| ● | Serina may experience delays in reaching, or failing to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites or CROs. Contractual terms can be subject to extensive negotiation, may be subject to modification from time to time and may vary significantly among different CROs and trial sites; |
| ● | Serina may have delays in adding new investigators or clinical trial sites, or it may experience a withdrawal of clinical trial sites; |
| ● | the number of patients required for clinical trials of Serina’s product candidates may be larger than it anticipates, enrollment in these clinical trials may be slower than it anticipates, or participants may drop out of these clinical trials or be lost to follow up at a higher rate than it anticipates for a number of reasons, such as adverse events, an inadequate treatment response, fatigue with the clinical trial process or personal issues; |
| ● | patients who enroll in Serina’s studies may misrepresent their eligibility or may otherwise not comply with clinical trial protocols, resulting in the need to drop those patients from those studies, increase the needed enrollment size for those studies, or extend the duration of those studies; |
| ● | there may be flaws in Serina’s study design, which may not become apparent until a study is well advanced; |
| ● | Serina’s contractors may fail to comply with regulatory requirements or clinical trial protocols, or meet their contractual obligations to it in a timely manner, or at all, or it may be required to engage in additional clinical trial site monitoring; |
| ● | regulatory authorities or IRBs/IECs may disagree with the design, including endpoints, scope, or implementation of Serina’s clinical trials, or regulatory authorities may disagree with its intended indications; |
| ● | regulatory authorities may disagree with the formulation for Serina’s product candidates, or its product candidate dose or dosing schedule; |
| ● | Serina may be unable to demonstrate to the satisfaction of regulatory authorities that a product candidate is safe, pure, and potent for any indication; |
| ● | regulatory authorities may not accept, or Serina or its clinical trials may not meet the criteria required to submit, clinical data from trials which are conducted outside of their jurisdictions; |
| ● | the results of clinical trials may be negative or inconclusive, may not meet the level of statistical significance required for, or may not otherwise be sufficient to support marketing approval, and Serina may decide, or regulatory authorities may require it, to conduct additional clinical trials, analyses, reports, data, or nonclinical studies, or abandon product development programs; |
| ● | Serina’s product candidates may have undesirable or unintended side effects, toxicities, or other characteristics that preclude marketing approval or prevent or limit commercial use; |
| ● | Serina may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks or otherwise provide an advantage over current standard of care or current or future competitive therapies in development; |
| ● | the standard of care for the indications Serina is investigating may change, which changes could impact the meaningfulness of its resulting study data, or which may necessitate changes to its studies; |
| ● | regulatory authorities may disagree with Serina’s scope, design, including endpoints, implementation, or its interpretation of data from nonclinical studies or clinical trials; |
| ● | regulatory authorities may require Serina to amend its studies, perform additional or unanticipated clinical trials or nonclinical studies or manufacturing development work to obtain approval or initiate clinical trials, or it may decide to do so or abandon product development programs; |
| ● | regulatory authorities may find that Serina or its third-party manufacturers do not satisfy regulatory requirements and standards for the facilities and operations used in the manufacture of its product candidates; |
| ● | the cost of clinical trials of Serina’s product candidates may be greater than it anticipates, or it may have insufficient funds for a clinical trial or to pay the substantial user fees required by the FDA or other regulatory authorities upon the filing of a marketing application; |
| ● | the supply or quality of Serina’s product candidates or other materials necessary to conduct clinical trials of its product candidates may be insufficient or inadequate; |
| ● | regulatory authorities may take longer than Serina anticipates to make a decision on its product candidates; or |
| ● | changes in or the enactment of the approval policies, statutes, or regulations of the applicable regulatory authorities may significantly change in a manner rendering Serina’s nonclinical or clinical data insufficient for approval. |
Furthermore, Serina expects to rely on CROs and clinical trial sites to ensure the proper and timely conduct of its clinical trials and, while it expects to enter into agreements governing their committed activities, it has limited influence over their actual performance.
A clinical trial may be suspended or terminated by Serina, its partners, the IRBs of the institutions in which such trials are being conducted, the DSMB for such trial or by the FDA or other regulatory authorities due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or Serina’s clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug or therapeutic biologic, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If Serina experiences delays in the completion of, or termination of, any clinical trial of any of its potential future product candidates, the commercial prospects of such product candidate will be harmed, and its ability to generate product revenue from such product candidates will be delayed. In addition, any delays in completing its clinical trials will increase its costs, slow its product development and approval process, and jeopardize its ability to commence product sales and generate revenue, and it may not have the financial resources to continue development of the product candidate that is affected or any of its other product candidates. It may also lose, or be unable to enter into, collaborative arrangements for the affected product candidate and for other product candidates that it is developing. Any of these occurrences may materially and adversely affect Serina’s business, financial condition, results of operations and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of its potential future product candidates.
Preliminary results from Serina’s nonclinical studies and clinical trials that it announces or publishes from time to time may change as more patient data becomes available and as the data undergoes audit and verification procedures.
From time to time, Serina may publish interim, topline, or preliminary results from its nonclinical studies and clinical trials. Preliminary and interim results from its clinical trials are not necessarily predictive of final results and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary, interim, and topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data it previously published. As a result, preliminary, interim, and topline data should be viewed with caution until the final data are available. Material adverse changes in the final data compared to the preliminary, interim, or topline data could significantly harm Serina’s business prospects.
Further, others, including regulatory agencies, may not accept or agree with Serina’s assumptions, estimates, calculations, conclusions, or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or therapeutic product, if any, and Serina in general. In addition, the information it chooses to publicly disclose regarding a particular nonclinical study or clinical trial is based on what is typically extensive information, and you or others may not agree with what it determines is the material or otherwise appropriate information to include in its disclosure, and any information it determines not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular therapeutic product, if any, product candidate or its business. If the preliminary, interim, and topline data that it reports differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, its ability to obtain approval for, and commercialize, its product candidates may be harmed, which could harm its business, operating results, prospects, or financial condition.
The FDA or comparable foreign regulatory authorities may disagree with Serina’s regulatory plans, and it may fail to obtain regulatory approval of its product candidates.
The FDA standard for approval of a drug or biologic generally requires two adequate, well-controlled clinical trials, each convincingly demonstrating the product candidate’s safety and effectiveness, or one large and robust, well-controlled trial providing substantial evidence that the product candidate is safe and effective for its proposed indication. Phase 3 clinical trials typically involve hundreds of patients, have significant costs and take years to complete. Product candidates studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the product candidate has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA usually requires a sponsor of a drug or biologic receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the drug or biologic may be subject to withdrawal procedures by the FDA that are more accelerated than those available for regular approvals.
Serina’s clinical trial results may not support either accelerated or regular approval. The results of nonclinical studies and clinical trials may not be predictive of the results of later-stage clinical trials, and product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through nonclinical studies and initial clinical trials. In addition, Serina’s product candidates could fail to receive regulatory approval for many reasons, including the following:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of Serina’s clinical trials; |
| ● | the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which Serina seeks approval; |
| ● | Serina may be unable to demonstrate that its product candidates’ risk-benefit ratios for their proposed indications are acceptable; |
| ● | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| ● | Serina may be unable to demonstrate that the clinical and other benefits of its product candidates outweigh their safety risks; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with Serina’s interpretation of data from nonclinical studies or clinical trials; |
| ● | the data collected from clinical trials of Serina’s product candidates may not be sufficient to the satisfaction of the FDA or comparable foreign regulatory authorities to support the submission of a NDA or BLA or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes, Serina’s own manufacturing facilities, or a third-party manufacturer’s facilities with which it contracts for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering Serina’s clinical data insufficient for approval. |
Failure to obtain regulatory approval to market any of Serina’s product candidates would significantly harm its business, results of operations, and prospects.
Failure of Serina’s technology would significantly harm its business, results of operations, and prospects.
Serina may not be successful in its efforts to use and expand its discovery engine to build a pipeline of product candidates.
A key element of Serina’s strategy is to use and expand its POZ Platform drug delivery technology to build a pipeline of product candidates and progress these product candidates through preclinical and clinical development for the treatment of various diseases. Although its research efforts to date suggest that the POZ Platform technology can deliver payloads including small molecules, proteins, and nucleic acids, this hypothesis may prove incorrect, or Serina may not be able to identify a product candidate that is safe or effective as a treatment for various diseases. Further, the scientific evidence to support the feasibility of developing viable product candidates based on its platform has not been established.
Even if Serina is successful in building its pipeline of product candidates, the potential product candidates that it identifies may not be suitable for clinical development or generate acceptable clinical data, including as a result of being shown to have unacceptable toxicity or other characteristics that indicate that they are unlikely to be products that will receive marketing approval from the FDA or other regulatory authorities or achieve market acceptance. Investment in biopharmaceutical product development involves significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval, and become commercially viable. Serina cannot provide any assurance that it will be able to successfully advance any of these additional product candidates through the development process. Its research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development or commercialization for many reasons, including the following:
| ● | Serina’s platform may not be successful in identifying additional product candidates; |
| ● | Serina may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; |
| ● | Serina’s product candidates may not succeed in nonclinical or clinical testing; |
| ● | a product candidate may upon further study demonstrate harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; |
| ● | competitors may develop alternatives that render Serina’s product candidates obsolete or less attractive; |
| ● | product candidates Serina develops may nevertheless be covered by third parties’ patents or other exclusive rights; |
| ● | the market for a product candidate may change during Serina’s program so that the continued development of that product candidate is no longer reasonable; |
| ● | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
| ● | a product candidate may not be accepted as safe and effective by patients, the medical community, or third-party payors, if applicable. |
If any of these events occur, Serina may be forced to abandon its development efforts for a program or programs, or it may not be able to identify, discover, develop, or commercialize additional product candidates, which would have a material adverse effect on its business and could potentially cause it to cease operations. Even if it receives FDA approval to market additional product candidates, it cannot assure you that any such product candidates will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives.
Serina may seek designations under FDA programs designed to facilitate and potentially expedite product candidate development, such as fast track or breakthrough therapy designation. Its product candidates may not receive any such designations or if they do receive such designations they may not lead to faster development or regulatory review, or approval and it does not increase the likelihood that its product candidates will receive marketing approval.
Serina may seek designations under the FDA’s expedited programs for serious conditions, such as fast track or breakthrough therapy designation, which are intended to facilitate and expedite the development or regulatory review or approval process for product candidates.
The granting of fast track or breakthrough therapy designation to an investigational product is entirely within the FDA’s discretion. Accordingly, even if Serina believes one of its product candidates meets the criteria for a designation, the FDA may disagree and instead determine not to grant such designation. In any event, the receipt of a fast track or breakthrough therapy designation for a product candidate may not result in a faster development process, review, or approval compared to product candidates considered for approval under conventional FDA procedures and does not assure ultimate marketing approval by the FDA. In addition, the FDA may later decide that the product candidate no longer meets the designation conditions, in which case any granted designations may be revoked, or the agency may decide that the time period for review or approval of the product candidate will not be shortened.
If Serina is unable to obtain approval via the accelerated approval pathway, it may be required to conduct additional nonclinical studies or clinical trials. Even if it receives accelerated approval from the FDA, the FDA may seek to withdraw accelerated approval.
Serina may seek an accelerated approval development pathway for its product candidates.
If Serina chooses to pursue accelerated approval, it intends to seek feedback from the FDA or will otherwise evaluate its ability to seek and receive such accelerated approval. After Serina’s evaluation of the feedback from the FDA or other factors, it may decide not to pursue or submit a NDA for accelerated approval or any other form of expedited development, review, or approval. Furthermore, if it submits an application for accelerated approval, there can be no assurance that such application will be accepted or that approval will be granted on a timely basis, or at all. The FDA also could require Serina to conduct further studies or trials prior to considering its application or granting approval of any type and may require it to have a confirmatory trial to verify the clinical benefit of the product underway and partially or fully enrolled before granting approval. Serina might not be able to fulfill the FDA’s requirements in a timely manner, which would cause delays, or approval might not be granted because its submission is deemed incomplete by the FDA.
Even if Serina receives accelerated approval from the FDA, it will be subject to rigorous post marketing requirements, including the completion of confirmatory post market clinical trials, submission to the FDA of periodic progress reports on confirmatory trials, and submission to the FDA of all promotional materials prior to their dissemination. The FDA could seek to withdraw accelerated approval for multiple reasons, including if Serina fails to conduct any required post market study with due diligence; a post market study does not confirm the predicted clinical benefit; other evidence shows that the product is not safe or effective under the conditions of use; or Serina disseminates promotional materials that are found by the FDA to be false and misleading. Under the Consolidated Appropriations Act for 2023, the FDA may use expedited procedures to withdraw any product for which Serina receives accelerated approval if its confirmatory trials fail to verify the purported clinical benefits.
A failure to obtain accelerated approval or any other form of expedited development, review, or approval for a product candidate that Serina may choose to develop would delay its commercialization of such product candidate, could increase the cost of development of such product candidate and could harm its competitive position in the marketplace.
If Serina applies for orphan drug designation from the FDA, there is no guarantee that it will be able to obtain or maintain this designation, receive this designation for any of its other product candidates, or receive or maintain any corresponding benefits, including periods of exclusivity.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a NDA. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user fee waivers. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a NDA, to market the same drug for the same indication for seven years, except in limited circumstances such as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. As a result, even if one of Serina’s drug candidates receives orphan exclusivity, the FDA can still approve other drugs that have a different active ingredient for use in treating the same indication or disease. Furthermore, the FDA can waive orphan exclusivity if Serina is unable to manufacture sufficient supply of its product.
Serina may expend its limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because Serina has limited financial and managerial resources, it focuses on research programs and product candidates that it identifies for specific indications. As a result, it may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. its resource allocation decisions may cause it to fail to capitalize on viable commercial products or profitable market opportunities. Its spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If Serina does not accurately evaluate the commercial potential or target market for a particular product candidate, it may relinquish valuable rights to that product candidate through collaboration, licensing, or other royalty arrangements in cases in which it would have been more advantageous for it to retain sole development and commercialization rights to such product candidate.
If Serina encounters difficulties enrolling patients in its clinical trials, its clinical development activities could be delayed or otherwise adversely affected.
Serina may experience difficulties in patient enrollment in its clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their protocols depends, among other things, on Serina’s ability to enroll a sufficient number of subjects who remain in the trial until its conclusion. Serina may not be able to initiate or continue conducting clinical trials for its product candidates if it is unable to locate and enroll a sufficient number of eligible subjects to participate in these trials. The enrollment of patients depends on many factors, including:
| ● | the number of clinical trials for other product candidates in the same therapeutic area that are currently in clinical development, and Serina’s ability to compete with such trials for subjects and clinical trial sites; |
| ● | the severity of the disease under investigation and the existence of current treatments; |
| ● | the perceived risks and benefits of the product candidate, including the potential advantages or disadvantages of the product candidate being studied in relation to other available therapies; |
| ● | the subject eligibility criteria defined in the protocol, as well as Serina’s ability to compensate subjects for their time and effort; |
| ● | the size and nature of the patient population; |
| ● | the proximity and availability of clinical trial sites for prospective subjects; |
| ● | the design of the trial, including factors such as frequency of required assessments, length of the study and ongoing monitoring requirements; |
| ● | subjects’ and investigators’ ability to comply with the specific instructions related to the trial protocol, proper documentation, and use of the product candidate; |
| ● | Serina’s ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| ● | patient referral practices of physicians and the effectiveness of publicity created by clinical trials sites regarding the trial; |
| ● | the ability to adequately monitor subjects during and after treatment and compensate them for their time and effort; |
| ● | the ability of Serina’s clinical study sites, CROs, and other applicable third parties to facilitate timely enrollment; |
| ● | the ability of clinical trial sites to enroll subjects that meet all inclusion criteria and any patient exclusion due to erroneous enrollment; |
| ● | Serina’s ability to obtain and maintain subject informed consents; and |
| ● | the risk that subjects enrolled in clinical trials will drop out of the trials before completion of the study or not return for post study follow up, especially subjects in control groups. |
In addition, Serina’s clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as its product candidates, and this competition will reduce the number and types of patients available to it, because some patients who might have opted to enroll in Serina’s trials may instead opt to enroll in a trial being conducted by one of its competitors. Because the number of qualified clinical investigators is limited, Serina may conduct some of its clinical trials at the same clinical trial sites that some of its competitors use, which will reduce the number of patients who are available for its clinical trials at such clinical trial sites. Moreover, because its product candidates represent a departure from more commonly used methods for disease treatment, potential patients and their doctors may be inclined to use conventional therapies, rather than enroll patients in any future clinical trial.
Serina’s inability to enroll a sufficient number of subjects for its clinical trials would result in significant delays and could require it to abandon one or more clinical trials altogether. Moreover, a significant number of withdrawn subjects would compromise the quality of its data. Enrollment delays in its clinical trials may result in increased development costs for its product candidates, or the inability to complete development of its product candidates, which could cause the value of its company to decline, limit its ability to obtain additional financing, and materially impair its ability to generate revenues.
Any product candidate Serina advances into clinical trials may cause unacceptable adverse events or have other properties that may delay or prevent its regulatory approval or commercialization or limit its commercial potential.
As with most new drugs, use of Serina’s product candidates could be associated with side effects or adverse events, which can vary in severity from minor reactions to death and in frequency from infrequent to prevalent. Undesirable side effects caused by any potential future product candidate could cause regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities. Serina has not yet completed all clinical trial phases for any of its current product candidates, and it is possible that there will be side effects associated with their use. Results of its clinical trials could reveal a high and unacceptable severity and prevalence of these side effects. In such an event, its trials could be suspended or terminated, and the FDA or other regulatory authorities could order it to cease further development of or deny approval of a product candidate for any or all targeted indications. Such side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may materially and adversely affect Serina’s business and financial condition and impair its ability to generate revenues.
Further, clinical trials by their nature utilize a sample of the potential patient population. With a limited number of patients and limited duration of exposure, rare and severe side effects of a product candidate may only be uncovered when a significantly larger number of patients are exposed to the product candidate or when patients are exposed for a longer period of time.
If one or more of Serina’s product candidates receives marketing approval, and Serina or others later identify undesirable side effects caused by such products, including during any long term follow up observation period recommended or required for patients who receive treatment using Serina’s products, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw or limit their approvals of such products; |
| ● | regulatory authorities may require the addition of labeling statements, specific warnings or contraindications; |
| ● | Serina may be required to create a REMS plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for health care providers, and/or other elements to assure safe use; |
| ● | Serina may be required to change the way such products are distributed or administered, or change the labeling of the products; |
| ● | the FDA or a comparable foreign regulatory authority may require Serina to conduct additional clinical trials or costly post marketing testing and surveillance to monitor the safety and efficacy of the products; |
| ● | Serina may decide to recall such products from the marketplace after they are approved; |
| ● | Serina could be sued and held liable for harm caused to individuals exposed to or taking its products; and |
| ● | Serina’s reputation may suffer. |
In addition, adverse side effects caused by any therapeutics that may be similar in nature to Serina’s product candidates could delay or prevent regulatory approval of its product candidates, limit the commercial profile of an approved label for its product candidates, or result in significant negative consequences for its product candidates following marketing approval.
Any of these events could prevent Serina from achieving or maintaining market acceptance of the affected product candidates and could substantially increase the costs of commercializing its product candidates, if approved, and significantly impact its ability to successfully commercialize its product candidates and generate revenues.
Serina may form or seek strategic partnerships or enter into additional licensing arrangements in the future, and it may not realize the benefits of such alliances or licensing arrangements.
From time to time, Serina may form or seek strategic partnerships or collaborations or enter into additional licensing arrangements with third parties that it believes will complement or augment its development and commercialization efforts with respect to its product candidates and any future product candidates that it may develop. Any such relationships may require it to incur non-recurring and other charges, increase its near and long term expenditures, issue securities that dilute its existing stockholders or disrupt its management and business. These relationships also may result in a delay in the development of its product candidates if it becomes dependent upon the other party and such other party does not prioritize the development of Serina’s product candidates relative to its other development activities. Additionally, any collaborations or licensing arrangements would be subject to the same product candidate development and compliance risks and obligations as Serina would be if it were to develop the product candidate on its own. Should any third party with which it enters into any of these arrangements not comply with the applicable regulatory requirements, it or they may be subject to regulatory enforcement action and it or they may be delayed or prevented from obtaining marketing approval for the applicable product candidate.
In addition, Serina faces significant competition in seeking appropriate strategic partners and the negotiation process is time consuming and complex. Moreover, Serina may not be successful in its efforts to establish a strategic partnership or other alternative arrangement for its product candidates because they may be deemed to beat too early of a stage of development for collaborative effort, and third parties may not view its product candidates as having the requisite potential to demonstrate safety and efficacy. If Serina licenses products, it may not be able to realize the benefit of such transactions if it is unable to successfully integrate them with its existing operations and company culture. Any licensed products may also subject Serina to the risk of regulatory enforcement should the product or business not be compliant with applicable regulatory requirements. Serina cannot be certain that, following a strategic transaction or licensing arrangement, it will achieve the revenue or specific net income that justifies such a transaction.
Serina relies on contract manufacturing organizations to manufacture its nonclinical and clinical pharmaceutical supplies and expects to continue to rely on CMOs to produce commercial supplies of any approved product candidate, and its dependence on CMOs could adversely impact its business.
Serina relies on contract manufacturing organizations (CMOs) for the manufacture of nonclinical and clinical supplies for its product candidates and plans to continue to do so for commercial supplies should it receive marketing approval for any of its product candidates. This reliance also results in its reduced control over the manufacture of its product candidates and the protection of its trade secrets and know-how from misappropriation or inadvertent disclosure, which may adversely affect its future business prospects. Nevertheless, as the developer of the product candidates and sponsor of clinical trials involving such product candidates, it continues to have regulatory obligations to maintain oversight of the CMOs to ensure compliance with, among other things, contractual obligations, specifications, and cGMP.
In complying with the manufacturing regulations of the FDA and other comparable foreign regulatory authorities, Serina and its third-party suppliers must spend significant time, money, and effort in the areas of design and development, testing, production, record keeping and quality control to assure that the products meet applicable specifications and other regulatory requirements. Although its agreements with its CMOs require them to perform according to certain cGMP such as those relating to quality control, quality assurance and qualified personnel, Serina cannot control the conduct of its CMOs to implement and maintain these standards. If its CMOs do not successfully carry out their contractual duties, meet expected deadlines or manufacture its product candidates in accordance with regulatory requirements, if there are disagreements between Serina and such parties, or if such parties are unable to support the commercialization of any of its product candidates for which it obtains marketing approval, it may not be able to produce, or may be delayed in producing sufficient product to meet its supply requirements. Any delays in obtaining adequate supplies on adequate terms with respect to Serina’s product candidates and components, due to manufacturing issues, global trade policies, or for other reasons, may delay the development, approval, or commercialization of its product candidates.
Serina may not succeed in its efforts to establish manufacturing relationships on commercially reasonable terms. Its product candidates may compete with other products and product candidates for access to manufacturing facilities, of which there are a limited number that operate under cGMP conditions and that are both capable of manufacturing its product candidates and willing to do so. Even if it does establish such collaborations or arrangements, its CMOs may breach, terminate, or not renew these agreements. These facilities may also be affected by the ongoing COVID 19 pandemic, natural disasters such as floods, fires, explosions or such facilities could face manufacturing issues, such as contamination or adverse regulatory findings following a regulatory inspection. Further, Serina’s CMOs may be temporarily unable to manufacture its product candidates due to government restrictions, requirements, or limitations. If its CMOs cease to manufacture its product candidates for any reason, Serina would experience delays in obtaining sufficient quantities of its product for it to meet commercial demand if it receives marketing approval or in advancing its development programs while it identifies and qualifies replacement suppliers. Serina could also incur added costs and delays in identifying and qualifying any such replacements and transferring any necessary technology and processes. The terms of a new arrangement may also be less favorable than any prior arrangements if it is able to negotiate a new arrangement at all. The addition of a new or alternative CMO may also require FDA approval and may have a material adverse effect on its business.
Serina or its CMOs may also encounter shortages in the raw materials or substances necessary to produce its product candidates in the quantities and at the quality needed for its nonclinical studies and clinical trials or, if any of its product candidates are approved for commercialization, to produce its products on a commercial scale, meet an increase in demand, or compete effectively. Such shortages may occur for a variety of reasons, including capacity constraints, delays or disruptions in the market, and shortages caused by the purchase of such materials by its competitors or others. Serina’s or its third-party manufacturers’ failure to obtain the raw materials or substances necessary to manufacture sufficient quantities of its product candidates may have a material adverse effect on its business.
Moreover, any problems or delays Serina experiences in preparing for commercial scale manufacturing of a product candidate or component, including manufacturing validation, may result in a delay in a future marketing approval, if any, or commercial launch of any of its product candidates, should they receive regulatory approval, or may impair its ability to manufacture commercial quantities or manufacture such quantities at an acceptable cost, which could result in the delay, prevention, or impairment of commercialization of its product candidates, if approved, and could adversely affect its business. Furthermore, if the future manufacturers of the commercial supplies of its products, if approved, fail to deliver the required commercial quantities of its product candidates on a timely basis and at commercially reasonable prices, it would likely be unable to meet demand for its products and it could lose potential revenues. The manufacture of drugs requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of drugs often encounter difficulties in production, particularly in scaling up initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing, shortages of qualified personnel, and compliance with strictly enforced federal, state, and foreign regulations. If Serina’s manufacturers were to encounter any of these difficulties and were unable to perform as agreed, its ability to provide its product candidates for use in nonclinical studies or its current and planned clinical trials, or, if any of its product candidates are approved, its ability to produce its product for commercial use, could be jeopardized.
In addition, all manufacturers of Serina’s product candidates used in clinical trials and of its products for commercial supply, should any of Serina’s product candidates receive regulatory approval, must comply with cGMP regulations promulgated by the FDA and equivalent foreign regulatory authorities that are applicable to both finished products and their active components used both for clinical and commercial supply. Regulatory authorities enforce these requirements through facility inspections. CMO facilities must be satisfactory to the FDA and equivalent foreign regulatory authorities as determined by inspections that will be conducted after Serina submits its marketing applications to the appropriate agencies and prior to product approval and commercialization. Its CMOs will also be subject to continuing, periodic regulatory authority inspections should its product candidates receive marketing approval. Further, Serina, in cooperation with its CMOs, must supply all necessary chemistry, manufacturing, and control documentation to the FDA and equivalent foreign regulatory authorities in support of a marketing application on a timely basis.
The cGMP include quality control, quality assurance, and the maintenance of records and documentation. Manufacturers of Serina’s product candidates may be unable to comply with its specifications, cGMP or with other applicable regulatory requirements. Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of a product candidate that may not be detectable in final product testing. If its CMOs cannot successfully manufacture material that conforms to its specifications and the applicable regulatory requirements, they may not be able to secure or maintain regulatory acceptance of their manufacturing facilities for the purpose of producing Serina’s product candidates.
Deviations from manufacturing requirements may also require reporting and remedial measures that may be costly and/or time consuming for Serina or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales, if any of its product candidates receives regulatory approval, or the temporary or permanent closure of a facility. Any such remedial measure could materially harm its business. Any delay in obtaining products or product candidates that comply with the applicable regulatory requirements may result in delays to nonclinical studies and clinical trials, or potential product approvals or commercialization. Any such delay may also require that Serina conducts additional studies.
While Serina is ultimately responsible for the manufacture and regulatory compliance of its products and product candidates, it has little control over its manufacturers’ compliance with these regulations and standards other than through its contractual arrangements. If the FDA or a comparable foreign regulatory authority does not find these facilities satisfactory for the manufacture of its products, if approved, or product candidates or if such authorities find such facilities to be noncompliant in the future, Serina may need to find alternative manufacturing facilities, which would significantly impact its ability to develop, obtain and maintain regulatory approval for or market its product candidates, if approved. Any new manufacturers would need to either obtain or develop the necessary manufacturing know-how, and obtain the necessary equipment and materials, which may take substantial time and investment. Serina must also receive FDA or other relevant comparable regulatory authority approval for the use of any new manufacturers for commercial supply.
Serina’s failure, or the failure of its third party manufacturers, to comply with applicable regulatory requirements may result in regulatory enforcement actions against its manufacturers or itself, including fines and civil and criminal penalties, including suspension of or restrictions on production, injunctions, delay, withdrawal or denial of product approval or supplements to approved products, clinical holds or termination of clinical studies, warning or untitled letters, regulatory authority communications warning the public about safety issues with a product, refusal to permit the import or export of a product, product seizure, detention, or recall, operating restrictions, civil penalties, criminal prosecution, corporate integrity agreements, or consent decrees and equivalent foreign sanctions. Depending on the severity of any potential regulatory action, supplies of its product candidates or products, if approved, could be interrupted or limited, which could have a material adverse effect on its business.
Serina relies on third parties to conduct some of its nonclinical studies and all of its clinical trials. If these third parties do not meet its deadlines or otherwise conduct the trials as required, its development programs could be delayed or unsuccessful and it may not be able to obtain regulatory approval for or commercialize its product candidates when expected or at all.
Serina does not have the ability to conduct all aspects of its clinical trials itself and does not currently plan to independently conduct clinical trials. It uses third parties, such as CROs, to conduct, supervise, and monitor the clinical trials and will rely upon such CROs, as well as medical institutions, investigators, and consultants, to conduct any future clinical trials that it may conduct in accordance with its protocols and applicable laws and regulations. In addition, it occasionally uses third parties to conduct its nonclinical studies. Serina’s CROs, investigators and other service providers play a significant role in the conduct of clinical trials and the subsequent collection and analysis of data from such trials.
Serina’s service providers are not its employees and, except for remedies available to it under its agreements with such third parties, as a result it will have less control over the timing, quality and other aspects of such nonclinical studies and clinical trials than it would have if it were to conduct them on its own. If these third parties do not successfully carry out their contractual duties to Serina, meet Serina’s expected timelines or conduct its nonclinical studies or clinical trials in accordance with regulatory requirements or its stated protocols, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to Serina’s protocols or applicable regulatory requirements or for other reasons, Serina’s trials may need to be repeated, extended, delayed, or terminated. Further, Serina may not be able to obtain, or may be delayed in obtaining, marketing approvals for its product candidates, it may fail or be delayed in its efforts to successfully commercialize its product candidates, if approved. Such failures may also subject Serina or its third-party service providers to regulatory enforcement actions. As a result, Serina’s results of operations and the commercial prospects for its product candidates could be harmed, its costs could increase and its ability to generate revenues could be delayed. To the extent it is unable to successfully identify and manage the performance of service providers in the future, its business may be materially and adversely affected. Serina’s third-party service providers may also have relationships with other entities, some of which may be its competitors, for whom they may also be conducting trials or other therapeutic development activities that could harm its competitive position.
Agreements with third parties conducting or otherwise assisting with Serina’s nonclinical studies or clinical trials might terminate for a variety of reasons, including a failure to perform by such parties. If any of its relationships with these third parties terminate, it may not be able to enter into arrangements with suitable alternative providers or do so on commercially reasonable terms. Switching or adding third parties involves additional cost and requires management time and focus. There is also a natural transition period when a new third party commences work. As a result, if Serina needs to enter into alternative arrangements, it could delay its product development activities and adversely affect its business. Although it carefully manages its relationships with its third parties, there can be no assurance that it will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on its business, financial condition and prospects, and results of operations.
Serina’s reliance on third parties for development activities will reduce its control over these activities. Nevertheless, Serina is responsible for ensuring that each of its studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and its reliance on third parties does not relieve it of its oversight and regulatory responsibilities. For example, it will remain responsible for ensuring that each of its trials is conducted in accordance with the general investigational plan and protocols for that trial. Serina must also ensure that its nonclinical studies are conducted in accordance with GLP requirements, as appropriate. Moreover, the FDA and comparable foreign regulatory authorities require it to comply with established GCP standards for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. In addition, Serina’s clinical trials must be conducted with product candidates that were produced under cGMP conditions. Regulatory authorities enforce these requirements through periodic inspections of trial sponsors, clinical and nonclinical investigators, manufacturers, and trial sites. If Serina or any of its third-party service providers fails to comply with applicable regulatory requirements, it or they may be subject to enforcement or other legal actions, the data generated in its trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require it to perform additional studies, which may significantly delay its clinical development plans and the regulatory approval process. Serina cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that Serina, its third-party service providers, or clinical trial sites is in substantial compliance with the applicable regulatory requirements.
In addition, Serina will be required to report certain financial interests of its third-party investigators if these relationships exceed certain financial thresholds or meet other criteria. The FDA or comparable foreign regulatory authorities may question the integrity of the data from those clinical trials conducted by investigators who may have conflicts of interest. Serina is also required to register certain clinical trials and post the results of certain completed clinical trials on a government sponsored database, clinicaltrials.gov, within specified timeframes. Failure to do so can result in enforcement actions and adverse publicity.
Serina relies on other third parties to store and distribute its product candidates for nonclinical studies and clinical trials that it conducts.
Serina also relies on other third parties to store and distribute its product candidates for the nonclinical studies and clinical trials that it is conducting or plan to conduct. Any performance failure, or failure to comply with applicable regulations, on the part of its distributors could delay development, the regulatory approval process, or potential commercialization of its product candidates, producing additional losses and depriving it of potential product revenue.
Serina may incur substantial product liability or indemnification claims relating to the clinical testing of its product candidates.
Serina faces an inherent risk of product liability exposure related to the testing of its product candidates in human clinical trials, and claims could be brought against it if the use or misuse of one of its product candidates causes, or merely appears to have caused, personal injury or death. Serina will face an even greater risk of product liability if it receives marketing approval for and commercialize any of its product candidates. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, or a breach of warranties. Claims could also be asserted under state consumer protection acts. Product liability claims might be brought against it by consumers, health care providers or others using, administering, or selling its products.
Any claims against Serina, regardless of their merit, could severely harm its financial condition, strain its management and other resources, or destroy the prospects for commercialization of the product which is the subject of any such claim. For instance, product liability claims may result in:
| ● | loss of revenue from decreased demand for Serina’s products and/or product candidates; |
| ● | impairment of Serina’s business reputation or financial stability; |
| ● | incurred costs and time of related litigation; |
| ● | substantial monetary awards to patients or other claimants, and loss of revenue; |
| ● | diversion of management attention; |
| ● | withdrawal of clinical trial participants and potential termination of clinical trial sites or entire clinical programs; |
| ● | the inability to commercialize Serina’s product candidates; |
| ● | significant negative media attention; |
| ● | decrease in the value of Serina; |
| ● | initiation of investigations, and enforcement actions by regulators; and/or |
| ● | product recalls, withdrawals, revocation of approvals, or labeling, marketing or promotional restrictions. |
If Serina cannot successfully defend itself against these claims, it will incur substantial liabilities or be required to limit development or commercialization of its products or product candidates. Although it maintains product liability and clinical trial insurance coverage, it may be inadequate to cover all liabilities that it may incur. Serina anticipates that it will need to increase its insurance coverage as it continues clinical development of its product candidates and if it successfully commercializes any medicine. Insurance coverage is increasingly expensive. It may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Serina’s Business, Industry and Future Commercialization
If any product candidate that Serina successfully develops does not achieve broad market acceptance among physicians, patients, health care payors and the medical community, the revenues that it generates from their sales will be limited.
Even if Serina’s product candidates receive regulatory approval, they may not gain market acceptance among physicians, patients, health care payors and the medical community. Market acceptance of its products by the medical community, patients, and third-party payors will depend on a number of factors, some of which are beyond its control, including:
| ● | the efficacy of Serina’s products and the prevalence and severity of any adverse events; |
| ● | any potential advantages or disadvantages when compared to alternative treatments; |
| ● | interactions of Serina’s products with other medicines patients are taking and any restrictions on the use of its products together with other medications; |
| ● | the clinical indications for which the products are approved and the approved claims that Serina may make for the products; |
| ● | limitations or warnings contained in the product’s FDA approved labeling, including potential limitations or warnings for such products that may be more restrictive than other competitive products; |
| ● | changes in the standard of care for the targeted indications for such product candidates, which could reduce the marketing impact of any claims that Serina could make following approval, if obtained; |
| ● | the safety, efficacy, and other potential advantages over alternative treatments, such as relative convenience and ease of administration of such products, and the availability of alternative treatments already used or that may later be approved; |
| ● | cost of treatment versus economic and clinical benefit in relation to alternative treatments or therapies; |
| ● | the availability of formulary coverage and adequate coverage or reimbursement by third parties, such as insurance companies and other health care payors, and by U.S. and international government health care programs, including Medicaid and Medicare; |
| ● | the price concessions required by third party payors and government health care programs to obtain coverage and payment; |
| ● | the extent and strength of Serina’s marketing and distribution of such products; |
| ● | distribution and use restrictions imposed by the FDA and equivalent foreign regulatory authorities with respect to such products or to which Serina agrees, for instance, as part of a REMS or voluntary risk management plan; |
| ● | the timing of market introduction of such products, as well as competitive products; |
| ● | Serina’s ability to offer such products for sale at competitive prices; |
| ● | Serina’s ability to offer programs to facilitate market acceptance and insurance coverage from public and private insurance companies, provide patient assistance, and transition patient coverage; |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| ● | the extent and strength of Serina’s third-party manufacturer and supplier support; |
| ● | the approval of other new products, including biosimilar products that may be priced at a substantially lower price than Serina expects to offer its product candidates for, if approved; |
| ● | adverse publicity about the product or favorable publicity about competitive products; |
| ● | the success of any efforts to educate the medical community and third-party payors regarding Serina’s products, which efforts may require significant resources and may not be successful; and |
| ● | potential product liability claims. |
If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, hospitals, health care payors and patients, Serina may not generate sufficient revenue from these products and may not become or remain profitable.
If Serina is unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any product candidates it may develop, it may not be successful in commercializing those product candidates if and when they are approved.
Serina does not have a sales or marketing infrastructure and it has limited experience in the sale, marketing, or distribution of pharmaceutical products. To achieve commercial success for any approved medicine for which it retains sales and marketing responsibilities, it must either develop a sales and marketing organization or outsource these functions to third parties. In the future, Serina may choose to build a focused sales, marketing, and commercial support infrastructure to sell, or participate in sales activities with its collaborators for, some of its current and future product candidates if and when they are approved.
There are risks involved with both establishing and managing Serina’s own commercial capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force or reimbursement specialists is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which Serina recruits a sales force and establish marketing and other commercialization capabilities is delayed or does not occur for any reason, Serina would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and Serina’s investment would be lost if it cannot retain or reposition its commercialization personnel.
Factors that may inhibit Serina’s efforts to commercialize product candidates on its own include:
| ● | Serina’s inability to recruit and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs, and other support personnel; |
| ● | the inability of sales personnel to obtain access to physicians to discuss Serina’s products; |
| ● | the inability of reimbursement professionals to negotiate arrangements for formulary access, reimbursement, and other acceptance by payors, and to secure adequate coverage; |
| ● | reduced realization on government sales from mandatory discounts, rebates and fees, and from price concessions to private health plans and pharmacy benefit managers necessitated by competition for access to managed formularies; |
| ● | the clinical indications for which the products are approved and the claims that Serina may make for the products, as well as any limitations on use or warnings; |
| ● | the costs associated with training sales and marketing personnel on legal and regulatory compliance matters and monitoring their actions, and any liability for sales or marketing personnel who fail to comply with the applicable legal and regulatory requirements; |
| ● | restricted or closed distribution channels that make it difficult to distribute Serina’s products to different segments of the patient population; |
| ● | the lack of complementary medicines to be offered by sales personnel, which may put Serina at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an independent commercialization organization. |
If Serina enters into arrangements with third parties to perform sales, marketing, commercial support, and distribution services, its product revenues, or the profitability of these product revenues to it may be lower than if it were to market and sell any product it may develop itself. In addition, Serina may not be successful in entering into arrangements with third parties to commercialize its products or may be unable to do so on terms that are favorable to it. Serina may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market its products effectively. If Serina does not establish commercialization capabilities successfully, either on its own or in collaboration with third parties, it will not be successful in commercializing any products it may develop.
Serina faces significant competition in an environment of rapid technological change, and there is a possibility that its competitors may achieve regulatory approval before it or develop therapies that are safer or more advanced or effective than Serina’s, which may harm its financial condition and its ability to successfully market or commercialize any product candidates it may develop.
The development and commercialization of new drugs is highly competitive. Moreover, the CNS and drug delivery fields are characterized by rapidly changing technologies, significant competition, and a strong emphasis on intellectual property. Serina will likely face competition with respect to any product candidates that it may seek to develop or commercialize in the future from numerous pharmaceutical and biotechnology organizations, as well as from academic institutions, government agencies and other public and private research organizations for its current and future product candidates. Serina’s commercial success will be reduced or eliminated if its competitors develop products that are safer, more effective, or less costly than Serina’s.
A number of well-resourced pharmaceutical and biotechnology are developing products anticipated to be in competition with Serina’s product candidates and its drug delivery platform technology. These products and technologies, as well as marketing campaigns by competitors and clinical trial results with competitive products, could significantly diminish Serina’s ability to market and sell its future products.
Many of Serina’s current or potential competitors, either alone or with their collaboration partners, may have significantly greater financial resources and expertise in research and development, manufacturing, nonclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than Serina does. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of Serina’s competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with Serina in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, Serina’s programs. Serina’s commercial opportunity could be reduced or eliminated if its competitors develop and commercialize product candidates that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than the product candidates it may develop or that would render any of its product candidates obsolete or non-competitive. Serina’s competitors also may obtain FDA or other regulatory approval for their product candidates more rapidly than it may obtain approval for its product candidates, which could result in its competitors establishing a strong market position before Serina is able to enter the market.
Serina’s commercial opportunity may also be reduced or limited if it or its partners are unable to scale up the manufacture of its product candidates to meet clinical or commercial requirements. The compositions Serina seeks to develop may exhibit poor pharmaceutical properties, and formulation, purification and stable storage could be challenging.
In addition, Serina could face litigation with respect to the validity and/or scope of patents relating to its competitors’ products. The availability of competitive products could limit the demand and the price it is able to charge for its products. Further, intellectual property protection for Serina’s POZ platform technology and Serina’s product candidates is dynamic and rapidly evolving. The scope of intellectual property protection for its POZ platform may be limited, and its commercial opportunity may be reduced or limited if its competitors are able to acquire or develop the same or similar technologies.
Corporate and academic collaborators may take actions to delay, prevent, or undermine the success of Serina’s products.
Serina’s operating and financial strategy for the development, clinical testing, manufacture, and commercialization of product candidates is heavily dependent on Serina’s entering into collaborations with corporations, academic institutions, licensors, licensees, and other parties and it may not be successful in establishing such collaborations. Some of its existing collaborations are, and future collaborations may be, terminable at the sole discretion of the collaborator. Replacement collaborators might not be available on attractive terms, or at all. The activities of any collaborator will not be within Serina’s control and may not be within its power to influence. Any collaborators may not perform their obligations to Serina’s satisfaction, or at all, it may not derive any revenue or profits from such collaborations, and any collaborators may ultimately compete with it. If any collaboration is not pursued, Serina may require substantially greater capital to undertake development and marketing of its proposed products and may not be able to develop and market such products effectively, if at all. In addition, a lack of development and marketing collaborations may lead to significant delays in introducing proposed products into certain markets and/or reduced sales of proposed products in such markets.
Data provided by collaborators and others upon which Serina relies that has not been independently verified could turn out to be false, misleading, or incomplete.
Serina relies on third party vendors, scientists, and collaborators to provide it with significant data and other information related to its projects, clinical trials and its business. If such third parties provide inaccurate, misleading, or incomplete data, Serina’s business, prospects, and results of operations could be materially adversely affected.
Even if Serina is able to commercialize any product candidates, such products may become subject to unfavorable pricing regulations, reimbursement practices, or health care reform initiatives, which would harm its business.
The regulations that govern pricing, and reimbursement for new medicines vary widely from country to country, and current and future legislation may change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Outside the United States, some countries require approval of the sale price of a medicine before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, Serina might obtain marketing approval for a product candidate in a particular country, but then be subject to price regulations that delay or might even prevent its commercial launch of the product candidate, possibly for lengthy time periods, and negatively impact the revenues it is able to generate from the sale of the product candidate in that country. Adverse pricing limitations may hinder its ability to recoup its investment in one or more product candidates it may develop, even if any such product candidates obtain marketing approval.
Serina’s ability to commercialize any product candidates successfully also will depend in part on the extent to which reimbursement for these product candidates and related treatments will be available from government authorities or health care programs, private health plans, and other organizations. Even if Serina succeeds in bringing one or more products to the market, these products may not be considered medically necessary and/or cost effective, and the amount reimbursed for any products may be insufficient to allow it to sell its products on a competitive basis. At this time, Serina is unable to determine their cost effectiveness or the likely level or method of reimbursement for its product candidates. Government authorities and third-party payors, such as private health plans, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. health care industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third party payors are challenging the prices charged for medical products and requiring that biopharmaceutical companies provide them with predetermined discounts from list prices. Novel medical products, if covered at all, may be subject to enhanced utilization management controls designed to ensure that the products are used only when medically necessary. Such utilization management controls may discourage the prescription or use of a medical product by increasing the administrative burden associated with its prescription or creating coverage uncertainties for prescribers and patients. Serina cannot be sure that reimbursement will be available for any product candidate that it commercializes and, if reimbursement is available, that the level of reimbursement will be adequate. Reimbursement may impact the demand for, or the price of, any product candidate for which it obtains marketing approval. If reimbursement is not available or is available only to limited levels, it may not be able to successfully commercialize any product candidate for which it obtains marketing approval.
Serina currently expects that any drugs it develops may need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain therapeutic products that are not usually self-administered (such as most injectable drugs and biologics) may be eligible for coverage under the Medicare Part B program if:
| ● | they are incident to a physician’s services; |
| ● | they are reasonable and necessary for the diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and |
| ● | they have been approved by the FDA and meet other requirements of the statute. |
There may be significant delays in obtaining reimbursement for newly approved product candidates, and coverage may be more limited than the purposes for which the product candidate is approved by the FDA or other regulatory authorities. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third party payors to pay all or part of the costs associated with their prescription medications. Patients are unlikely to use Serina’s products unless coverage is provided, and payment is adequate to cover all or a significant portion of the cost of its products. Therefore, coverage and adequate payment is critical to new product acceptance. Coverage decisions may depend upon clinical and economic standards that disfavor new products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Moreover, eligibility for reimbursement does not imply that any product candidate will be paid for in all cases or at a rate that covers Serina’s costs, including research, development, manufacture, sale, and distribution. Interim reimbursement levels for new product candidates, if applicable, may also not be sufficient to cover Serina’s costs and may not be made permanent. Reimbursement rates may vary according to the use of the product candidate and reimbursement in the clinical setting in which it is used may be based on reimbursement levels already set for lower cost therapies or medicines and may be incorporated into existing payments for other services. Net prices for product candidates may be reduced by mandatory discounts or rebates required by government health care programs or private payors and by any future relaxation of laws that presently restrict imports of medicines from countries where they may be sold at lower prices than in the United States. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement rates. However, no uniform policy requirement for coverage and reimbursement for drug products exists among third party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time consuming and costly process that will require Serina to provide scientific and clinical support for the use of its products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Serina’s inability to promptly obtain coverage and profitable payment rates from both government funded and private payors for any approved product candidates it may develop could have a material adverse effect on its operating results, its ability to raise capital needed to commercialize medicines, and its overall financial condition.
This Merger may limit Serina’s ability to use some or all of its net operating loss carryforwards in the future.
The ultimate realization of Serina’s deferred income tax assets is dependent upon generating future taxable income. Serina has recorded a full valuation allowance against its deferred income tax assets. The valuation allowance may fluctuate as conditions change. Serina’s ability to utilize net operating loss carryforwards to offset its future taxable income and/or to recover previously paid taxes would be limited if it were to undergo an “ownership change” within the meaning of Section 382 of the Code. In general, an “ownership change” occurs whenever the percentage of the stock of a corporation owned by “5 percent stockholders” (within the meaning of Section 382 of the Code) increases by more than 50 percentage points over the lowest percentage of the stock of such corporation owned by such “5 percent stockholders” at any time over the testing period. An ownership change under Section 382 of the Code would establish an annual limitation to the amount of NOLs Serina could utilize to offset its taxable income in any single year. The application of these limitations might prevent full utilization of the deferred tax assets attributable to its net operating loss carryforwards.
Serina has not yet formally determined the amount of the cumulative change in its ownership resulting from this Merger or other transactions, or any resulting limitations on its ability to utilize its NOL carryforwards and other tax attributes. As a result, if Serina earns net taxable income, its ability to use its pre-Merger net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to it. If an ownership change occurs and Serina’s ability to use its net operating loss carryforwards is materially limited, it could harm Serina’s future operating results by effectively increasing its future tax obligations.
Serina’s insurance policies are expensive and protect it only from some business risks, which will leave it exposed to significant uninsured liabilities.
Serina carries insurance for most categories of risk that its business may encounter; however, it may not have adequate levels of coverage. Serina currently maintains general liability, property, workers’ compensation, products liability and directors’ and officers’ insurance, along with an umbrella policy. It may not be able to maintain existing insurance at current or adequate levels of coverage. Any significant uninsured liability may require it to pay substantial amounts, which would adversely affect its cash position and results of operations.
Risks Related to Serina’s Intellectual Property
Serina’s success is largely based upon its intellectual property and proprietary technologies, and it may be unable to protect and/or enforce its intellectual property.
Serina’s success will depend, in large part, on obtaining and maintaining patent protection and trade secret protection for its product candidates and their formulations and uses, as well as successfully enforcing its patents against third party infringers and/or defending these patents against third party challenges. If Serina (or its licensees should such licensees be granted the right to prosecute or enforce certain patents within Serina’s portfolio) fails to appropriately prosecute or is unable to obtain and maintain patent protection for its product candidates (or aspects thereof), its ability to develop, license and/or commercialize these product candidates may be adversely affected and it may not be able to prevent competitors from making, using, selling or importing competing products. This failure or inability to properly or adequately protect the intellectual property rights relating to these product candidates could have a material adverse effect on Serina’s financial condition and results of operations.
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that Serina or its partners will be successful in protecting its product candidates by obtaining, enforcing and defending patents. These risks and uncertainties include the following:
| ● | patent applications may not result in any patent being issued; |
| ● | patents that may be issued may not include claims that cover broad enough scope to prevent design around solutions by competitors; |
| ● | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable, or otherwise may not provide adequate barriers to entry or any competitive advantage; |
| ● | because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that before a potential product can be commercialized, any related patent may expire, or remain in existence for only a short period following commercialization, reducing, or eliminating any advantage of the patent; |
| ● | Serina’s competitors, many of which have substantially greater resources than it or its partners do, and many of which have made significant investments in competing technologies, may seek, or may already have sought or obtained, patents that will limit, interfere with, or eliminate Serina’s ability to make, use, and sell its potential products; |
| ● | there may be significant pressure on the U.S. government and other international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful as a matter of public policy regarding worldwide health concerns; |
| ● | countries other than the United States may have patent laws less favorable to patentees than those upheld by United States courts, allowing foreign competitors a better opportunity to create, develop, and market competing products; and |
| ● | Serina may be involved in lawsuits to protect or enforce its patents or the patents of its licensors, which could be expensive, time consuming and/or unsuccessful. |
In addition to patents, Serina also relies on trade secrets and proprietary know-how. Although it has taken steps to protect its trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and assignment agreements with employees, consultants, and advisors, there exists the potential that third parties may still somehow obtain this information or arrive at the same or similar information independently, which would reduce or eliminate Serina’s competitive advantage. Moreover, Serina may become subject to claims that it directly or indirectly (through its consultants, advisors, or independent contractors that it may engage to assist it in developing its product candidates) has wrongfully or inadvertently disclosed, acquired or used trade secrets or other proprietary information of third parties.
Serina may be forced to litigate to enforce or defend its intellectual property rights, and/or the intellectual property rights of its licensors.
Serina may be forced to litigate to enforce or defend its intellectual property rights against infringement by competitors, and to protect its trade secrets against unauthorized use. In so doing, it may place its intellectual property at risk of being invalidated, rendered unenforceable, or limited or narrowed in scope such that it may no longer be used to adequately prevent the manufacture, sale or import of competitive product. Further, an adverse result in any litigation or other proceedings before government agencies such as the USPTO, may place pending applications at risk of non-issuance or limitations in scope. Further, derivation proceedings, entitlement proceedings, ex parte reexamination, inter partes review, post grant review, and opposition proceedings provoked by third parties or brought by the USPTO or any foreign patent authority may be used to challenge the inventorship, ownership, claim scope, or validity of Serina’s patents. Additionally, because of the substantial amount of discovery typically required in connection with intellectual property litigation, there is a risk that some of Serina s confidential and proprietary information or trade secrets could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the value of Serina. Such litigation or proceedings could substantially increase Serina’s operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. Serina may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of Serina’s competitors may be able to sustain the costs of such litigation or proceedings more effectively than it can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on Serina’s ability to compete in the marketplace.
If Serina enters into future arrangements involving government funding, and it makes inventions as a result of such funding, its intellectual property rights to such discoveries may be subject to the applicable provisions of the Bayh Dole Act of 1980. To the extent any of its current or future intellectual property is generated through the use of U.S. government funding, the provisions of the Bayh Dole Act may similarly apply. Any exercise by the government of certain of its rights could harm Serina’s competitive position, business, financial condition, results of operations and prospects.
If Serina or its partners are sued for infringing on the intellectual property rights of third parties, it could be costly and time consuming, and an unfavorable outcome in any such litigation could have a material adverse effect on its business.
Serina’s success also depends upon its ability and the ability of any of its future collaborators to develop, manufacture, market and sell its product candidates without infringing on the proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which Serina is developing products, some of which may be directed at claims that overlap with the subject matter of its intellectual property. Because patent applications can take many years to issue, there may be currently pending applications, now unknown to Serina, which may later result in issued patents that its product candidates or proprietary technologies may be alleged to infringe upon. Similarly, there may be issued patents relevant to Serina product candidates of which it is not aware.
In addition, third parties may sue Serina alleging that it infringes on their patents. Even if Serina is successful in defending any claims of infringement, the defense of such claims may be costly and present a time consuming distraction. In the event of a successful claim of infringement against Serina, it may be required to:
| ● | pay substantial damages and/or ongoing royalty; |
| ● | stop using some or all of its technologies and methods; |
| ● | stop certain research and development efforts; |
| ● | develop non infringing products or methods (i.e., develop or design around); and/or |
| ● | obtain one or more licenses from third parties for an upfront lump sum, an ongoing royalty, or a combination thereof. |
If required, Serina cannot assure you that it will be able to obtain such licenses on acceptable terms, or at all. If it is sued for infringement, it could encounter substantial delays in the development, manufacture, and commercialization of its product candidates. Any litigation, whether to enforce its patent rights or to defend against allegations that it allegedly infringes on third party rights, could be costly, time consuming, and may distract management from other important tasks.
As is commonplace in the biotechnology and pharmaceutical industry, Serina employs individuals who were previously employed at other biotechnology or pharmaceutical companies, including its competitors or potential competitors. To the extent Serina’s employees are involved in research endeavors that are similar to those which they were involved in at their former place of employment, Serina may be subject to claims that such employees and/or it has inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of such former employers. Litigation may be necessary to defend against such claims, which could result in substantial costs, be a distraction to management and ultimately have a material adverse effect on Serina, even if it is successful in defending such claims.
The biotechnology and pharmaceutical industries have experienced substantial litigation and other proceedings concerning intellectual property rights, and third parties may initiate legal proceedings alleging that Serina is infringing, misappropriating, or otherwise violating their intellectual property rights, the outcome of which could be uncertain and may prevent, delay, or otherwise interfere with Serina’s product discovery and development efforts. Serina’s commercial success depends upon its ability and the ability of its collaborators and licensors to develop, manufacture, market, and sell its products. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights as well as administrative proceedings for challenging patents, including derivation, inter partes review, post grant review, and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. Serina may be subject to and may in the future become party to, or threatened with, adversarial proceedings or litigation concerning intellectual property rights with respect to its POZ platform and any product candidates it may develop, including interference proceedings, post grant review, inter partes review, and derivation proceedings before the USPTO and similar proceedings in foreign jurisdictions such as oppositions before the European Patent Office (EPO). Numerous U.S. and foreign issued patents and pending patent applications that are owned by third parties exist in the fields in which Serina is developing its product candidates and infringement claims may be asserted against it or its partners based on existing patents or patents that may be granted in the future, regardless of their merit.
As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that Serina’s POZ platform and product candidates may give rise to claims of infringement of the patent rights of others. Moreover, it is not always clear to industry participants, including Serina, which patents cover various types of therapies, products or their methods of use or manufacture. Moreover, as with many technology-based products, there may be third party patent applications that, if issued, may include the claims that could be or are construed to cover components of Serina’s POZ platform and product candidates. There may also be third party patents of which Serina is currently unaware with claims to its technologies, compositions, methods of manufacture or methods of use.
Serina’s ability to commercialize its product candidates in the United States and abroad may be adversely affected if it cannot successfully defend against infringement claims or obtain a license on commercially reasonable terms to relevant third party patents that cover its product candidates. Even if Serina has a strong defense and/or believes that third party intellectual property claims are without merit, there can be no assurance that a court would find in its favor on questions of infringement, validity, enforceability, and/or priority. A court of competent jurisdiction could hold that these third party patents are valid and enforceable and have been infringed upon, which could materially and adversely affect Serina’s ability to commercialize its product candidates or technologies covered by the asserted third party patents. In order to successfully challenge the validity of any such U.S. patent in federal court, Serina would need to overcome a presumption of validity. As this burden is a high one requiring Serina to present clear and convincing evidence as to the invalidity of any such U.S. patent claims, there is no assurance that a court of competent jurisdiction would invalidate the asserted claims of any such U.S. patent. If Serina is found to be infringing on a third party’s intellectual property rights, and it is unsuccessful in demonstrating that any such patents are invalid or unenforceable, it could be required to pay damages and/or an ongoing royalty or obtain a license from such third party to continue developing, manufacturing, and marketing its product candidates and its technologies. However, Serina may not be able to obtain any required license on commercially reasonable terms or at all. Even if Serina were able to obtain such a license, it could be non-exclusive, thereby giving its competitors and other third parties access to the same technologies licensed to it, and it could require Serina to pay substantial licensing fees and/or make ongoing royalty payments. If Serina is unable to obtain a necessary license to a third party patent on commercially reasonable terms, it may be unable to commercialize its POZ platform or product candidates or such commercialization efforts may be significantly delayed, which could in turn significantly harm its business. While less likely given the high bar required for injunction, Serina also could be temporarily or permanently forced, including by court order, to cease developing, manufacturing, and commercializing the infringing technology or product candidates. In addition, Serina could be found liable for significant monetary damages, including treble damages and attorneys’ fees, if it is found to have willfully infringed on a patent or other intellectual property right. Claims that it has misappropriated the confidential information or trade secrets of third parties could have a similar material adverse effect on its business, financial condition, results of operations, and prospects.
The defense of third party claims of alleged infringement, misappropriation, or violation of intellectual property rights often involves substantial litigation expense and could also be a substantial diversion of management and employee time and resources from Serina’s business. Some third parties may be able to sustain the costs of complex patent litigation more effectively than Serina can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on Serina’s ability to raise the funds necessary to continue its operations or could otherwise have a material adverse effect on its business, financial condition, results of operations and prospects. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, this could have a substantial adverse effect on the price of its common stock.
Obtaining and maintaining Serina’s patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and its patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and applications are due to be paid to the USPTO and foreign patent agencies outside of the United States over the lifetime of Serina’s owned or licensed patents and applications. In the case of any in-licensed patent rights, Serina generally relies on its licensors to pay these fees due to U.S. and non U.S. patent agencies. For patent rights owned by Serina, it relies on its outside patent counsel and/or annuity services in the United States and foreign countries to monitor these deadlines and to pay these fees when so instructed by Serina.
The USPTO and foreign patent agencies require compliance with procedural, documentary, fee payment, and other similar provisions, such as the requirement to disclose known prior art, during the patent application process. In the case of any in-licensed patent rights, Serina generally depends on its licensors to take the necessary action to comply with these requirements with respect to its licensed intellectual property, and for its owned patent applications, it engages counsel and other professionals to help it comply with these requirements. While certain inadvertent lapses can be cured by payment of a late fee, by petition, or by other means in accordance with the applicable rules, there are situations in which non-compliance can result in a partial or complete loss of patent rights in the relevant jurisdiction. In the unlikely event that a non-compliance event were to occur, Serina s competitors might be able to enter the market with similar or identical products or technology given the partial or complete loss of patent rights by Serina, which could have a material adverse effect on its business, financial condition, results of operations, and prospects.
If Serina does not obtain patent term extension for its drug candidates, Serina’s business may be materially harmed.
Depending upon the timing, duration, and specifics of FDA regulatory approval of Serina’s drug candidates, one or more patents issued from U.S. patent applications that Serina files or those of Serina’s future licensors may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Amendments). The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during the FDA regulatory review process based on the first regulatory approval for a particular drug or biologic. A maximum of one patent may be extended per FDA-approved drug as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of drug approval, and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended. Patent term extension may also be available in certain foreign countries upon regulatory approval of Serina’s drug candidates.
However, Serina may not be granted an extension for which it applies in the United States or any other jurisdiction because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time or the scope of patent protection afforded could be less than Serina requests.
If Serina are unable to obtain patent term extension or restoration, or the foreign equivalent, or the term of any such extension is less than Serina request, Serina’s competitors or other third parties may obtain approval of competing drugs following Serina’s patent expiration, and Serina’s revenue could be reduced, possibly materially. Further, if this occurs, Serina’s competitors or other third parties may take advantage of Serina’s investment in development and trials by referencing Serina’s clinical and preclinical data and launch their drug earlier than might otherwise be the case. Any of the foregoing could materially harm Serina’s business, financial condition, results of operations and prospects.
Changes in patent law in the United States and in non U.S. jurisdictions could diminish the value of patents in general, thereby impairing Serina’s ability to protect its technologies and product candidates.
As is the case with other biotech and pharmaceutical companies, Serina’s success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and are therefore costly, time consuming and inherently uncertain. Changes in either the patent laws or interpretation of the patent laws could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of Serina’s issued patents. For example, in March 2013, under the America Invents Act the United States transitioned from a “first to invent” to a “first to file” patent system. Under a “first to file” system, assuming that other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to a patent on an invention regardless of whether another inventor had made the invention earlier. A third party that filed a patent application in the USPTO after March 2013, but before Serina could therefore be awarded a patent covering an invention of Serina’s even if Serina had made the invention before it was made by such third party. This requires Serina to be cognizant of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, Serina cannot be certain that it or any licensors were the first to either file any patent application related to its technologies or product candidates or invent any of the inventions claimed in its or its licensor’s patents or patent applications. The America Invents Act also includes a number of other significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted, allowing third party submission of prior art, and establishing a new post grant review system, including post grant review, inter partes review, and derivation proceedings. Because of the lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use USPTO proceedings to invalidate Serina’s patent claims even though the claim would not have been invalidated if first challenged by the third party in a district court action.
In addition, U.S. Supreme Court rulings over the past decade have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to Serina’s ability to obtain patents in the future, this combination of events has created uncertainty with respect to the validity and enforceability of issued patents. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken Serina’s ability to obtain new patents or to enforce and/or defend its existing patents and patents that it might obtain in the future.
Patent terms may be inadequate to protect Serina’s competitive position on its product candidates for an adequate amount of time.
Patents have a limited lifespan. The terms of individual patents depend upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest non provisional filing date in the applicable country. However, the actual protection afforded by a patent varies from country to country, and also depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent, and whether a portion of the patent term has been terminally disclaimed based on other patents. Various extensions including patent term extension (PTE) and patent term adjustment (PTA) may be available, but the lives of such extensions, and the protections they afford, are limited. Even if patents covering Serina’s product candidates are obtained, once the patent life has expired, Serina may be open to competition from competitive products, including generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting Serina’s product candidates might expire before or shortly after Serina or its partners commercialize those candidates. As a result, Serina’s owned and/or licensed patent portfolio may not provide Serina with sufficient rights to exclude others from commercializing products similar or identical to Serina’s for an adequate time period.
If Serina is unable to protect the confidentiality of its trade secrets, its business and competitive position could be harmed.
In addition to seeking patents for its technologies and product candidates, Serina also relies on trade secret protection, as well as confidentiality agreements, non-disclosure agreements and assignment agreements with its employees, consultants and third parties, to protect its know-how and other confidential and proprietary information, especially where it does not believe patent protection is appropriate or obtainable.
It is Serina’s policy to require its employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties to execute confidentiality agreements upon the commencement of employment or consulting relationships with it. These agreements generally provide that all confidential information concerning Serina’s business or financial affairs developed by or made known to an individual or entity during the course of that party’s relationship with Serina is to be kept confidential and not disclosed to third parties, except in certain specified circumstances. In the case of employees, the agreements also provide that all inventions conceived by the individual, and that are related to Serina’s current or planned business or research and development or made during normal working hours, on Serina’s premises or using Serina’s equipment or proprietary information, are Serina’s exclusive property. In the case of consultants and other third party service providers, the agreements provide Serina with certain rights to all inventions arising from the services provided to it by those individuals or entities. However, Serina cannot guarantee that it has entered into such agreements with each party that may have or have had access to its trade secrets or proprietary technologies and processes. Additionally, the assignment of intellectual property rights may not be self-executing, or assignment agreements may be breached, and Serina may be forced to bring claims against third parties, or defend claims that they may bring against it, to determine the ownership of what Serina regards as its intellectual property. Serina may not be able to obtain adequate remedies for any breaches of such agreements. Ultimately, enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time consuming, and the outcome is unpredictable.
In addition to contractual measures, Serina protects the confidential nature of its proprietary information through other appropriate precautions, such as physical and technological security measures. However, trade secrets and know-how can be difficult to protect despite these precautions. These measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for Serina’s proprietary information. Serina’s security measures may not prevent an employee or consultant from misappropriating its trade secrets and providing them to a competitor, and any recourse it might take against this type of misconduct may not provide an adequate remedy to protect its interests fully. In addition, Serina’s trade secrets may be independently developed by others in a manner that could prevent it from receiving legal recourse. If any of Serina’s confidential or proprietary information, such as its trade secrets, were to be disclosed or misappropriated, or if any of that information was independently developed by a competitor, its competitive position could be harmed.
If Serina chooses to go to court to stop a third party from using any of Serina’s trade secrets, it may incur substantial costs. In addition, courts inside and outside the United States are sometimes less willing or unwilling to protect trade secrets. Even if Serina is successful, these types of lawsuits may consume, in addition to substantial costs, significant amounts of its time and other resources. Any of the foregoing could have a material adverse effect on its business, financial condition, results of operations and prospects.
Third parties may assert that Serina’s employees, consultants, or advisors have wrongfully used or disclosed confidential information or misappropriated trade secrets.
As is common in the biotechnology and pharmaceutical industries, Serina employs individuals that are currently or were previously employed at universities, research institutions or other biotechnology or pharmaceutical companies, including Serina’s competitors or potential competitors. Although Serina employs measures to ensure that its employees, consultants, and advisors do not use the proprietary information or know how of others in their work for it, Serina may be subject to claims that it or these individuals have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Serina may then be directly or indirectly involved in litigation proceedings to defend against these claims. If it fails in defending against any such claims, in addition to potentially paying monetary damages, it may lose valuable intellectual property rights and/or personnel. Even if it is successful in defending against such claims, litigation could result in substantial costs and distract its technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and, if securities analysts or investors perceive these results to be negative, that perception could have a substantial adverse effect on the price of Serina’s common stock. Ultimately, any such litigation could substantially increase Serina’s operating losses and reduce its resources available for development activities, and it may not have sufficient financial or other resources to adequately engage in such litigation. For example, some of Serina’s competitors may be able to sustain the costs of such litigation more effectively than it can because of their substantially greater financial resources. In any case, uncertainties resulting from the initiation and continuation of intellectual property litigation or other intellectual property related proceedings could adversely affect Serina’s ability to compete in the marketplace.
Any trademarks Serina may obtain may be infringed or successfully challenged, resulting in harm to its business.
Serina expects to rely on trademarks as one means to distinguish any of its product candidates that are approved for marketing from the products of its competitors. However, Serina’s trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. Serina may not be able to protect its rights to these trademarks and trade names, which it benefits from to build name recognition among potential partners or customers in its markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to Serina’s, thereby impeding its ability to build brand identity and possibly leading to market confusion. In addition, there could be allegations of trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of Serina’s registered or unregistered trademarks or trade names. Over the long term, if Serina is unable to establish name recognition based on its trademarks and trade names, then it may not be able to compete effectively, and its business may be adversely affected. Serina’s efforts to enforce or protect its proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversions of resources and could adversely affect Serina’s business, financial condition, results of operations and growth prospects.
In addition, any proprietary name Serina proposes to use with any product candidate in the United States must be approved by the FDA, regardless of whether it have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. If the FDA objects to any of Serina’s proposed proprietary product names, Serina may be required to expend significant additional resources in an effort to identify a suitable proprietary product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties, and be acceptable to the FDA.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by Serina’s intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect Serina’s business or permit it to maintain its competitive advantage. For example:
| ● | any of Serina’s current and future product candidates, if approved, may eventually become commercially available in generic or biosimilar product forms; |
| ● | others may be able to make immunotherapies that are similar to any of Serina’s current and future product candidates or utilize lymph node targeting technology but that are not covered by the claims of the patents that Serina licenses or may own in the future; |
| ● | Serina, or its licensors or current or future collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that it licenses or may own in the future, potentially resulting in the invalidation of such patents or refusal of such applications; |
| ● | Serina, or its licensors or current or future collaborators, might not have been the first to file patent applications covering certain of its or their inventions; |
| ● | Serina, or its licensors or current or future collaborators, may fail to meet its obligations to the U.S. government regarding any in licensed patents and patent applications funded by U.S. government grants, leading to the loss or unenforceability of patent rights; |
| ● | others may independently develop similar or alternative technologies or duplicate any of Serina’s technologies without infringing on its owned or licensed intellectual property rights; |
| ● | it is possible that Serina’s pending, owned or licensed patent applications or those that it may own or license in the future will not lead to issued patents; |
| ● | it is possible that there are prior public disclosures that could invalidate Serina’s owned or in licensed patents, or parts of its owned or in licensed patents; |
| ● | it is possible that there are unpublished patent applications that may later issue with claims covering Serina’s product candidates or technology similar to Serina’s; |
| ● | it is possible that Serina’s owned or in-licensed patents or patent applications omit individual(s) that should be listed as inventor(s) or include individual(s) that should not be listed as inventor(s), which may cause these patents or patents issuing from these patent applications to be held invalid or unenforceable or result in a change in ownership; |
| ● | issued patents to which Serina holds rights may be held invalid, unenforceable, or narrowed in scope, including as a result of legal challenges by its competitors; |
| ● | the claims of Serina’s owned or in licensed issued patents or patent applications, if and when issued, may not cover its product candidates or narrowly cover its product candidates in such a way that competitors may be able to design around to avoid infringement allegations; |
| ● | the laws of foreign countries may not protect Serina’s proprietary rights or the proprietary rights of its licensors or current or future collaborators to the same extent as the laws of the United States; |
| ● | the inventors of Serina’s owned or in licensed patents or patent applications may become involved with competitors, develop products or processes that design around its patents, or become hostile to it or the patents or patent applications on which they are named as inventors; |
| ● | Serina’s competitors might conduct research and development activities in countries where Serina does not have patent rights and then use the information learned from such activities to develop competitive products for sale in its major commercial markets; |
| ● | Serina has engaged in scientific collaborations in the past and it intends to continue to do so in the future, and its collaborators may develop adjacent or competing products that are outside the scope of its patents; |
| ● | Serina may not develop additional proprietary technologies that are patentable; |
| ● | any product candidates Serina develops may be covered by third party patents or other exclusive rights; |
| ● | the patents of others may prohibit or otherwise harm Serina’s business; or |
| ● | Serina may choose not to file a patent in order to maintain certain trade secrets or know how, and a third party may subsequently commercialize the technology and/or file a patent covering such intellectual property. |
Should any of these events occur, they could have a material adverse effect on Serina’s business, financial condition, results of operations, and prospects.
Risks Related to Regulatory and Compliance Matters
The FDA regulatory approval process is lengthy, time consuming, and inherently unpredictable, and Serina may experience significant delays in the clinical development and regulatory approval, if any, of its product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, adverse event reporting, record keeping, advertising, promotion, and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States. Serina is not permitted to market any drug product in the United States until it receives approval from the FDA. It has not previously submitted a NDA to the FDA, or similar approval filings to comparable foreign authorities. A NDA must include extensive nonclinical and clinical data and supporting information to establish that the product candidate is safe, pure, potent, and effective for each desired indication. The NDA must also include significant information regarding the chemistry, manufacturing, and controls for the product, and the manufacturing facilities must complete a successful pre license inspection. The FDA may also require a panel of experts, referred to as an Advisory Committee, to deliberate on the adequacy of the safety and efficacy data to support approval. The opinion of the Advisory Committee, although not binding, may have a significant impact on Serina’s ability to obtain licensure of the product candidates based on the completed clinical trials. Accordingly, the regulatory approval pathway for Serina’s product candidates may be uncertain, complex, expensive, and lengthy, and approval may not be obtained.
Even if Serina receives regulatory approval of its product candidates, it will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense, and it may be subject to penalties if it fails to comply with regulatory requirements.
If Serina’s product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record keeping, conduct of post marketing studies, and submission of safety, efficacy, and other post market information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities must comply with extensive FDA, and comparable foreign regulatory authority, requirements, including ensuring that quality control and manufacturing procedures conform to cGMP regulations. As such, Serina and its contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any NDA, other marketing applications, and previous responses to inspection observations. Accordingly, Serina and others with whom it works must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control.
Any regulatory approvals that Serina receives for its product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a REMS program as a condition of approval of Serina’s product candidates, which could entail requirements for long term patient follow up, a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves Serina’s product candidates, it will have to comply with requirements including submissions of safety and other post marketing information and reports, registration, as well as continued compliance with cGMP and GCP for any clinical trials that it conducts post approval.
The FDA strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. Drugs and biologics may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off label uses may be subject to significant liability.
Failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters or other enforcement related letters or clinical holds on post approval clinical trials; |
| ● | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; |
| ● | injunctions or the imposition of civil or criminal penalties; and |
| ● | consent decrees, corporate integrity agreements, debarment, or exclusion from federal health care programs; or mandated modification of promotional materials and labeling and the issuance of corrective information. |
The policies of the FDA and of other regulatory authorities may change and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of Serina’s product candidates. Serina cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If Serina is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if Serina is not able to maintain regulatory compliance, Serina may lose any marketing approval that it may have obtained and it may not achieve or sustain profitability.
Additional regulatory burdens and other risks and uncertainties in foreign markets may limit Serina’s growth.
Serina’s future growth may depend, in part, on its ability to develop and commercialize product candidates in foreign markets for which it may rely on strategic partnership with third parties. Serina will not be permitted to market or promote any product candidate before it receives regulatory approval from the applicable regulatory authority in a foreign market, and it may never receive such regulatory approval. To obtain separate regulatory approval in foreign countries, Serina generally must comply with numerous and varying regulatory requirements of such countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales, pricing, and distribution of a product candidate, and it cannot predict success in these jurisdictions. If Serina obtains approval of any of its potential future product candidates and ultimately commercialize any such product candidate in foreign markets, Serina would be subject to risks and uncertainties, including the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements and the reduced protection of intellectual property rights in some foreign countries.
In addition, obtaining and maintaining regulatory approval of Serina’s product candidates in one jurisdiction does not guarantee that it will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing, and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional nonclinical studies or clinical trials as trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that Serina intends to charge for its products is also subject to approval.
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for Serina and could delay or prevent the introduction of its products in certain countries. If Serina fails to comply with the regulatory requirements in international markets and/or to receive applicable marketing approvals, its target market will be reduced and its ability to realize the full market potential of its product candidates will be harmed.
Health care and other reform legislation may increase the difficulty and cost for Serina and any collaborators it may have to obtain marketing approval of and commercialize its product candidates and affect the prices it, or they, may obtain.
All aspects of Serina’s business, including research and development, manufacturing, marketing, pricing, sales, litigation, and intellectual property rights, are subject to extensive legislation and regulation. Changes in applicable U.S. federal and state laws and agency regulation, as well as foreign laws and regulations, could have a materially negative impact on Serina’s business. In the United States and in some other jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the health care system that could prevent or delay marketing approval of Serina’s product candidates or any potential future product candidates of Serina’s, restrict or regulate post approval activities, or affect Serina’s ability to profitably sell any product candidates for which it obtains marketing approval. Increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject Serina to more stringent product labeling and post marketing testing and other requirements. Congress also must reauthorize the FDA’s user fee programs every five years and often makes changes to those programs in addition to policy or procedural changes that may be negotiated between the FDA and industry stakeholders as part of this periodic reauthorization process. Congress most recently reauthorized the user fee programs in September 2022 without any substantive policy changes.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in health care systems with the stated goals of containing health care costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, Congress passed the ACA, which substantially changed the way health care is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry.
There remain judicial and Congressional challenges to certain aspects of the ACA, and as a result certain sections of the ACA have not been fully implemented or effectively repealed. However, following several years of litigation in the federal courts, in June 2021, the U.S. Supreme Court upheld the ACA when it dismissed a legal challenge to the law’s constitutionality. Further legislative and regulatory changes under the ACA remain possible. It is unknown what form any such changes or any law would take, and how or whether it may affect the pharmaceutical industry as a whole or Serina’s business in the future. Serina expects that changes or additions to the ACA, the Medicare and Medicaid programs, and changes stemming from other health care reform measures, especially with regard to health care access, financing, or other legislation in individual states, could have a material adverse effect on the health care industry in the United States.
The uncertainty around the future of the ACA, and in particular the impact to reimbursement levels, may lead to uncertainty or delay in the purchasing decisions of Serina’s customers, which may in turn negatively impact Serina’s product sales. If there are not adequate reimbursement levels, Serina’s business and results of operations could be adversely affected.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and was extended by the Consolidated Appropriations Act for 2023 and will remain in effect through 2032 unless additional Congressional action is taken.
In addition, the Drug Supply Chain Security Act enacted in 2013 imposed obligations on manufacturers of pharmaceutical products related to product tracking and tracing, and in February 2022, FDA released proposed regulations to amend the national standards for licensing of wholesale drug distributors by the states; establish new minimum standards for state licensing third party logistics providers; and create a federal system for licensure for use in the absence of a State program, each of which is mandated by the Drug Supply Chain Security (DSCSA). As another example, on December 20, 2019, President Trump signed the Further Consolidated Appropriations Act for 2020 into law (P.L. 116 94) that includes a piece of bipartisan legislation called the CREATES Act. The CREATES Act aims to address the concern articulated by both the FDA and others in the industry that some brand manufacturers have improperly restricted the distribution of their products, including by invoking the existence of a REMS for certain products, to deny generic and biosimilar product developers access to samples of brand products. The CREATES Act establishes a private cause of action that permits a generic or biosimilar product developer to sue the brand manufacturer to compel it to furnish the necessary samples on “commercially reasonable, market based terms.” Whether and how generic and biosimilar product developments will use this new pathway, as well as the likely outcome of any legal challenges to provisions of the CREATES Act, remain highly uncertain and its potential effects on Serina’s future commercial products are unknown. Other legislative and regulatory proposals have been made to expand post approval requirements and restrict sales and promotional activities for pharmaceutical products. Serina is unsure whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations will be changed, or whether such changes will have any impact on its business.
Additionally, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices considering the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. For example, state legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In December 2020, the U.S. Supreme Court held unanimously that federal law does not preempt the states’ ability to regulate pharmaceutical benefit managers (PBMs) and other members of the health care and pharmaceutical supply chain, an important decision that may lead to further and more aggressive efforts by states in this area.
At the federal level, the Department of Health and Human Services (HHS) has solicited feedback on various measures intended to lower drug prices and reduce the out of pocket costs of drugs and has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage plans the option to use step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019.
Most recently, in August 2022, President Biden signed into the law the Inflation Reduction Act of 2022 (IRA). Among other things, the IRA has multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease.
Many EEA countries periodically review their reimbursement procedures for medicinal products, which could have an adverse impact on reimbursement status. We expect that legislators, policymakers and healthcare insurance funds in the EEA countries will continue to propose and implement cost-containing measures, such as lower maximum prices, lower or lack of reimbursement coverage and incentives to use cheaper, usually generic, products as an alternative to branded products, and/or branded products available through parallel import to keep healthcare costs down. Moreover, in order to obtain reimbursement for Serina’s products in some European countries, including some EEA countries, we may be required to compile additional data comparing the cost-effectiveness of our products to other available therapies. Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EEA countries, including those representing the larger markets. The HTA process is the procedure to assess therapeutic, economic and societal impact of a given medicinal product in the national healthcare systems of the individual country. The outcome of an HTA will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EEA countries. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product currently varies between EEA countries.
In December 2021, Regulation No 2021/2282 on HTA amending Directive 2011/24/EU, was adopted in the EU. This Regulation, which entered into force in January 2022 and will apply as of January 2025, is intended to boost cooperation among EEA countries in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EEA level for joint clinical assessments in these areas. The Regulation foresees a three-year transitional period and will permit EEA countries to use common HTA tools, methodologies, and procedures across the EEA, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EEA countries will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement. If we are unable to maintain favorable pricing and reimbursement status in EEA countries for drug candidates that we may successfully develop and for which we may obtain regulatory approval, any anticipated revenue from and growth prospects for those products in the EEA could be negatively affected.
Legislators, policymakers and healthcare insurance funds in the EEA may continue to propose and implement cost-containing measures to keep healthcare costs down; particularly due to the financial strain that the COVID-19 pandemic placed on national healthcare systems of the EEA countries. These measures could include limitations on the prices we would be able to charge for drug candidates that we may successfully develop and for which we may obtain regulatory approval or the level of reimbursement available for these products from governmental authorities or third-party payors. Further, an increasing number of EEA and other foreign countries use prices for medicinal products established in other countries as “reference prices” to help determine the price of the product in their own territory. Consequently, a downward trend in prices of medicinal products in some countries could contribute to similar downward trends elsewhere.
Serina cannot predict whether future healthcare initiatives will be implemented at the federal or state level, supranational or national level, or how any future legislation or regulation may affect us, any of which may have a materially adverse effect on our business, financial condition, results of operations and prospects.
Any additional federal or state health care reform measures could limit the amounts that third party payers will pay for future health care products and services, and, in turn, could significantly reduce the projected value of certain development projects and reduce Serina’s profitability.
Serina’s employees, principal investigators, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
Serina is exposed to the risk of fraud or other misconduct by its employees, consultants, and commercial partners, and its principal investigators. Misconduct by these parties could include intentional failures to comply with FDA regulations or the regulations applicable in the European Union and other jurisdictions, provide accurate information to the FDA or other regulatory authorities, comply with health care fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately, or disclose unauthorized activities to Serina. In particular, sales, marketing, and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct also could involve the improper use of information obtained in the course of clinical trials or interactions with the FDA or other regulatory authorities, which could result in regulatory sanctions and cause serious harm to Serina’s reputation. It is not always possible to identify and deter employee misconduct, and the precautions Serina takes to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting Serina from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against Serina, and it is not successful in defending itself or asserting its rights, those actions could have a significant impact on its business, financial condition, results of operations, and prospects, including the imposition of significant fines or other sanctions.
Laws and regulations governing any international operations Serina may have in the future may preclude it from developing, manufacturing, and selling certain product candidates outside of the United States and require it to develop and implement costly compliance programs.
Serina is subject to numerous laws and regulations in each jurisdiction outside the United States in which it operates. The creation, implementation and maintenance of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
The FCPA prohibits any U.S. individual or business from paying, offering, authorizing payment, or offering of anything of value, directly or indirectly, to any foreign official, political party, or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. The anti-bribery provisions of the FCPA are enforced primarily by the U.S. Department of Justice. The SEC is involved with enforcement of the books and records provisions of the FCPA.
Similarly, the U.K. Bribery Act 2010 has extra territorial effect for companies and individuals having a connection with the United Kingdom. The U.K. Bribery Act prohibits inducements both to public officials and private individuals and organizations. Compliance with the FCPA and the U.K. Bribery Act is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. If Serina expands its business outside of the United States, it will be required to dedicate additional resources to comply with these laws, and these laws may preclude it from developing, manufacturing, or selling certain products and product candidates outside of the United States, which could limit Serina’s growth potential and increase its development costs. The failure to comply with laws governing international business practices may result in substantial penalties, including suspension or debarment from government contracting. Violations of the FCPA can result in significant civil and criminal penalties. Indictment alone under the FCPA can lead to suspension of the right to do business with the U.S. government until the pending claims are resolved. A conviction under the FCPA can result in long term disqualification as a government contractor. The termination of a government contract or relationship as a result of Serina’s failure to satisfy any of its obligations under laws governing international business practices could have a negative impact on its operations and harm its reputation and ability to procure government contracts. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions.
Risks Related to Employee and Operations Matters, Managing Growth and Information Technology
A pandemic, epidemic, or outbreak of an infectious disease, such as the COVID 19 pandemic, may materially and adversely affect Serina’s business and its financial results and could cause a disruption to the development of product candidates.
Public health crises such as pandemics or similar outbreaks could adversely impact Serina’s business. The extent to which an outbreak impacts Serina’s operations or those of its collaborators will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information that will emerge concerning the severity of the virus and the actions to contain it or treat its impact, among others.
The spread of any outbreak globally could adversely impact any preclinical or clinical trial operations in the United States and Europe, including Serina’s ability to recruit and retain patients and principal investigators and site staff who, as health care providers, may have heightened exposure to an infectious disease if an outbreak occurs in their geography. For example, similar to other biotechnology companies, Serina has experienced, and may in the future experience, delays in initiating IND enabling studies, delays in manufacturing, protocol deviations, enrolling patients in clinical trials and dosing patients in clinical trials, as well as in activating trial sites. An outbreak may also result in the need to suspend enrollment in clinical studies, subject withdrawals, postponement of planned clinical or nonclinical studies, redirection of site resources from studies, study modification, suspension, or termination, the introduction of remote study procedures and modified informed consent procedures, study site changes, direct delivery of investigational products to patient homes requiring state licensing, study deviations or noncompliance, and changes or delays in site monitoring. The foregoing may require that Serina consult with relevant review and ethics committees, IRBs, and the FDA. The foregoing may also impact the integrity of Serina’s study data. The effects of an outbreak may also increase the need for clinical trial patient monitoring and regulatory reporting of adverse effects. The pandemic could further impact Serina’s ability to interact with the FDA or other regulatory authorities and may result in delays in the conduct of inspections or review of pending applications or submissions. For example, the FDA may delay pre approval inspections. Although the FDA lifted restrictions relating to COVID 19 and affecting its inspection and other compliance operations in July 2022, the agency currently faces a significant backlog on compliance monitoring and enforcement activities for both domestic and foreign manufacturers, which may affect the scheduling of necessary pre approval inspections of manufacturing facilities for drug and biological product candidates.
In addition, the patient populations that Serina product candidates target may be particularly susceptible to COVID 19, which may make it more difficult for Serina to identify patients able to enroll in its current and future clinical trials and may impact the ability of enrolled patients to complete any such trials. Any negative impact the COVID 19 pandemic has on patient enrollment or treatment, or the execution of any product candidates could cause costly delays to clinical trial activities, which could adversely affect Serina’s ability to obtain regulatory approval for and to commercialize its product candidates, increase its operating expenses and have a material adverse effect on its financial results. Additionally, timely enrollment in planned clinical trials is dependent upon clinical trial sites which could be adversely affected by global health matters, such as pandemics. The COVID 19 pandemic has also impacted, and may continue to impact, Serina’s third party suppliers and manufacturers, including through the effects of facility closures, reductions in operating hours, staggered shifts and other social distancing efforts, labor shortages, decreased productivity and the unavailability of materials or components. While Serina maintains an inventory of materials used to conduct Serina’s research and development activities, a prolonged pandemic could lead to shortages in the raw materials necessary to manufacture Serina’s product candidates. If any of these third parties are adversely impacted by the COVID 19 pandemic or the restrictions resulting from the outbreak, if they cannot obtain the necessary supplies, or if such third parties need to prioritize other products or customers over Serina, including under the Defense Production Act, Serina may experience additional delays or disruptions in its supply chain, which could have a material and adverse impact on its business. CMOs may also need to implement measures and changes or deviate from typical requirements because of the COVID 19 pandemic that may otherwise adversely impact Serina’s supply chains or the quality of the resulting products or supplies. Depending on the change, Serina may need to obtain FDA pre approval or otherwise provide the FDA with a notification of the change.
An outbreak may additionally result in changes in laws and regulations. By example, in March 2020, the U.S. Congress passed the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) which includes various provisions regarding FDA drug shortage reporting requirements, as well as provisions regarding supply chain security, such as risk management plan requirements, and the promotion of supply chain redundancy and domestic manufacturing. This and any future changes in law may require that Serina changes its internal processes and procedures to ensure continued compliance.
Even after an outbreak or pandemic subsides, Serina may continue to experience an adverse impact to its business as a result of its global economic impact, including from increased inflation and the prospect that policy responses to inflation could delay economic recovery or lead to another recession.
Any of these factors, and other factors related to any such disruptions that are unforeseen, could have a material adverse effect on Serina’s business, financial condition, results of operation or prospects. Further, uncertainty around these and related issues could continue to adversely impact the economies of the United States and other countries, which could impact Serina’s ability to raise the necessary capital needed to develop and commercialize its product candidates.
Serina’s future success depends on its ability to recruit and retain its executive team and key scientists and to attract, retain, and motivate qualified personnel.
Serina is highly dependent on the principal members of its management and scientific teams. These principal members are employed “at will,” meaning Serina or they may terminate the employment relationship at any time. The loss of the services of any of these persons could impede the achievement of Serina’s research, development, and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing, business development, general and administrative and sales and marketing personnel will also be critical to Serina’s success. Serina may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. Serina also experiences competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, Serina relies on consultants and advisors, including scientific and clinical advisors, to assist it in formulating its research and development and commercialization strategy. Serina’s consultants and advisors may be employed by employers other than Serina and may have commitments under consulting or advisory contracts with other entities that may limit their availability to Serina. In addition, inflation has had, and Serina expects that it will continue to have, an impact on the costs that it incurs to attract and retain qualified personnel and may make it more difficult for it to attract and retain such personnel. The inability to recruit, or loss of services of certain executives, key employees, consultants, or advisors, may impede the progress of Serina’s research, development, and commercialization objectives and have a material adverse effect on its business, financial condition, results of operations, and prospects.
If Serina is unable to hire additional qualified personnel, its ability to grow its business may be harmed.
Over time Serina will need to hire additional qualified personnel with expertise in drug development, product registration, clinical, preclinical, and nonclinical research, quality compliance, government regulation, formulation and manufacturing, financial matters and sales and marketing. Serina competes for qualified individuals with numerous biopharmaceutical companies, universities, and other research institutions. Competition for such individuals is intense, and Serina’s search for such personnel may not be successful. Attracting and retaining qualified personnel will be critical to Serina’s success.
Serina expects to expand its development, regulatory, and future sales and marketing capabilities, and as a result, it may encounter difficulties in managing its growth, which could disrupt its operations.
As of November 9, 2023, Serina had 10 full time employees and, in connection with the growth and advancement of its pipeline and becoming a public company, it expects to increase the number of its employees and the scope of its operations, particularly in the areas of product development, business development, regulatory affairs, and sales and marketing. To manage its anticipated future growth, Serina must continue to implement and improve its managerial, operational, and financial systems, expand its facilities, and continue to recruit and train additional qualified personnel. Due to its limited financial resources and the limited experience of its management team in managing a company with such anticipated growth, Serina may not be able to effectively manage the expected expansion of its operations or recruit and train additional qualified personnel. Moreover, the expected physical expansion of its operations may lead to significant costs and may divert its management and business development resources. Any inability to manage growth could delay the execution of its business plans or disrupt its operations.
As a growing biotechnology company, Serina is actively pursuing new product candidates in many therapeutic areas and across a wide range of diseases. Successfully developing product candidates for and fully understanding the regulatory and manufacturing pathways to all of these therapeutic areas and disease states requires a significant depth of talent, resources, and corporate processes in order to allow simultaneous execution across multiple areas. Due to its limited resources, Serina may not be able to effectively manage this simultaneous execution and the expansion of its operations or recruit and train additional qualified personnel. This may result in weaknesses in its infrastructure, give rise to operational mistakes, legal or regulatory compliance failures, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of Serina’s operations may lead to significant costs and may divert financial resources from other projects, such as the development of its product candidates. If Serina’s management is unable to effectively manage its expected development and expansion, its expenses may increase more than expected, its ability to generate or increase its revenue could be reduced, and it may not be able to implement its business strategy. Serina’s future financial performance and its ability to compete effectively and commercialize its product candidates, if approved, will depend in part on its ability to effectively manage the future development and expansion of its company.
Serina’s internal computer systems, or those of its vendors, collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of its product development programs, compromise sensitive information related to its business or prevent it from accessing critical information, potentially exposing it to liability or otherwise adversely affecting its business.
Serina’s internal computer systems and those of its current and any future third party vendors, collaborators and other contractors or consultants are vulnerable to damage, interruption or data theft from computer viruses, computer hackers, malicious code, employee theft or misuse, ransomware, social engineering (including phishing attacks), denial of service attacks, sophisticated nation state and nation state supported actors, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Cybersecurity incidents, which may not be immediately or ever detected, are increasing in frequency and evolving in nature. Additionally, due to geopolitical tensions related to Russia’s invasion of Ukraine, the risk of cyber-attacks may be elevated.
While Serina seeks to protect its information technology systems from system failure, accident, and security breach, if such an event were to occur and cause interruptions in its operations, it could result in a disruption of Serina’s development programs and its business operations, whether due to a loss of its trade secrets or other proprietary information or other disruptions. For example, the loss of clinical trial data from future clinical trials could result in delays in Serina’s regulatory approval efforts and significantly increase its costs to recover or reproduce the data. If Serina were to experience a significant cybersecurity breach of its information systems or data, the costs associated with the investigation, remediation, and potential notification of the breach to counterparties and data subjects could be material. In addition, Serina’s remediation efforts may not be successful. If it does not allocate and effectively manage the resources necessary to build and sustain the proper technology and cybersecurity infrastructure, it could suffer significant business disruption, including transaction errors, supply chain or manufacturing interruptions, processing inefficiencies, data loss or the loss of or damage to intellectual property or other proprietary information. In addition, in response to the ongoing COVID 19 pandemic, some of Serina’s workforce began to work remotely. This has continued and is now considered normal business. This could increase Serina’s cyber security risk, create data accessibility concerns, and make Serina more susceptible to communication disruption.
To the extent that any disruption or security breach were to result in a loss of, or damage to, Serina’s or its third party vendors’, collaborators’ or other contractors’ or consultants’ data or applications, or inappropriate disclosure of confidential or proprietary information, Serina could incur liability including litigation exposure, penalties and fines, Serina could become the subject of regulatory actions or investigations, its competitive position could be harmed and the further development and commercialization of its product candidates could be delayed. Any of the above could have a material adverse effect on Serina’s business, financial condition, results of operations or prospects. While Serina maintains cyber liability insurance (covering security and privacy matters), such insurance may not be adequate to cover any losses experienced as a result of a cybersecurity incident.
If Serina fails to maintain proper and effective internal controls, its ability to produce accurate financial statements on a timely basis could be impaired.
Serina has material weaknesses in its internal control systems over financial reporting and will need to hire additional personnel and design and implement proper and effective internal controls over financial reporting commensurate with the accounting and reporting requirements of a public company Serina may identify additional material weaknesses in the future that may cause us to fail to meet its reporting obligations or result in material misstatements in our financial statements. If Serina fails to remediate its material weaknesses, Serina may not be able to report its financial results accurately or to prevent fraud. Serina’s management is responsible for establishing and maintaining internal control over financial reporting, disclosure controls, and compliance with the other requirements of the Sarbanes-Oxley Act and the rules promulgated by the SEC thereunder.
General Risk Factors
Unfavorable global economic conditions could adversely affect Serina’s business, financial condition, or results of operations.
Serina’s results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. For example, in 2008, the global financial crisis caused extreme volatility and disruptions in the capital and credit markets and the current COVID 19 pandemic has caused significant volatility and uncertainty in U.S. and international markets. See “Risks Related to Employee and Operations Matters, Managing Growth and Information Technology.” A pandemic, epidemic, or outbreak of an infectious disease, such as the COVID 19 pandemic, may materially and adversely affect Serina’s business and its financial results and could cause a disruption to the development of Serina’s product candidates. A severe or prolonged economic downturn, or additional global financial crises, could result in a variety of risks to Serina’s business, including weakened demand for its product candidates, if approved, or its ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain Serina’s suppliers, possibly resulting in supply disruption. Any of the foregoing could harm Serina’s business and it cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact its business.
U.S. federal income tax reform could adversely affect Serina’s business and financial condition.
The rules dealing with U.S. federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect Serina or holders of its common stock. In recent years, many such changes have been made and changes are likely to continue to occur in the future. For example, on March 27, 2020, former President Trump signed into law the CARES Act which included certain changes in tax law intended to stimulate the U.S. economy in light of the COVID 19 coronavirus outbreak, including temporary beneficial changes to the treatment of net operating losses, interest deductibility limitations and payroll tax matters. Additionally, on December 22, 2017, former President Trump signed into law the Tax Cuts and Jobs Act of 2017 (TCJA), which significantly reformed the Code. The TCJA included significant changes to corporate and individual taxation, some of which could adversely impact an investment in Serina’s common stock. Under the TCJA, in general, NOLs generated in taxable years beginning after December 31, 2017 may offset no more than 80 percent of such year’s taxable income and there is no ability for such NOLs to be carried back to a prior taxable year. The CARES Act modifies the TCJA with respect to the TCJA’s limitation on the deduction of NOLs and provides that NOLs arising in taxable years beginning after December 31, 2017 and before January 1, 2021, may be carried back to each of the five taxable years preceding the tax year of such loss, but NOLs arising in taxable years beginning after December 31, 2020 may not be carried back. In addition, the CARES Act eliminates the limitation on the deduction of NOLs to 80 percent of current year taxable income for taxable years beginning before January 1, 2021. As a result of such limitation, Serina may be required to pay federal income tax in some future year notwithstanding that it had a net loss for all years in the aggregate. Future changes in tax laws could have a material adverse effect on Serina’s business, cash flow, financial condition, or results of operations. Serina urges investors to consult with their legal and tax advisers regarding the implications of potential changes in tax laws on an investment in Serina’s common stock.
Serina faces risks associated with increased political uncertainty.
The invasion of Ukraine by Russia and the sanctions, bans and other measures taken by governments, organizations and companies against Russia and certain Russian citizens in response thereto has increased the political uncertainty in Europe and has strained the relations between Russia and a significant number of governments, including the U.S. Any retaliatory actions taken by Russia, the duration and outcome of this conflict and the conflict among Israel, Hamas and Hezbollah, and the impact on regional or global economies is unknown but could have a material adverse effect on Serina’s business, financial condition and results of its operations.
Changes in U.S. and international trade policies, particularly with respect to China, may adversely impact Serina’s business and operating results.
The U.S. government has made statements and taken actions in recent years that have led to certain changes and may lead to additional changes to U.S. and international trade policies, including imposing several rounds of tariffs affecting certain products manufactured in China. In March 2018, the Trump administration announced the imposition of tariffs on steel and aluminum entering the United States and in June 2018 announced further tariffs targeting goods imported from China. Both China and the United States have each imposed tariffs indicating the potential for further trade barriers. It is unknown whether and to what extent new tariffs (or other new laws or regulations) will be adopted, or the effect that any such actions would have on us or our industry, and it is unclear whether the Biden administration will work to reverse these measures or pursue similar policy initiatives. Any unfavorable government policies on international trade, such as export controls, capital controls or tariffs, may affect the use of testing facilities in China that we use, including pursuant to Serina’s testing arrangements with WuXi AppTec (HongKong) Limited. If any new tariffs, export controls, legislation and/or regulations are implemented, or if existing trade agreements are renegotiated or, in particular, if the U.S. government takes retaliatory trade actions due to the recent U.S.-China trade tension, such changes could have an adverse effect on our business, financial condition and results of operations.
Risks Related to the Combined Company
In determining whether you should vote approve the proposals contained in this proxy statement/prospectus/information statement, you should carefully read the following risk factors in addition to the risks described above.
The combined company will need to raise additional financing in the future to fund its operations, which may not be available to it on favorable terms or at all.
The combined company will require substantial additional funds to support its continued research and development activities, including the anticipated costs of nonclinical studies and clinical trials, regulatory approvals, and potential commercialization. The combined company’s future capital requirements will depend upon a number of factors, including: the number and timing of future product candidates in the pipeline; progress with and results from preclinical testing and clinical trials; the ability to manufacture sufficient drug supplies to complete preclinical and clinical trials; the costs involved in preparing, filing, acquiring, prosecuting, maintaining and enforcing patent and other intellectual property claims; and the time and costs involved in obtaining regulatory approvals and favorable reimbursement or formulary acceptance. Raising additional capital may be costly or difficult to obtain and could significantly dilute stockholders’ ownership interests or inhibit the combined company’s ability to achieve its business objectives. If the combined company raises additional funds through public or private equity offerings, the terms of these securities may include liquidation or other preferences that adversely affect the rights of its common stockholders. Further, to the extent that the combined company raises additional capital through the sale of common stock or securities convertible or exchangeable into common stock, its stockholder’s ownership interest in the combined company will be diluted. In addition, any debt financing may subject the combined company to fixed payment obligations and covenants limiting or restricting its ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If the combined company raises additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, the combined company may have to relinquish certain valuable intellectual property or other rights to its product candidates, technologies, future revenue streams or research programs or grant licenses on terms that may not be favorable to it. Even if the combined company were to obtain sufficient funding, there can be no assurance that it will be available on terms acceptable to the combined company or its stockholders.
The market price of the combined company’s common stock is expected to be volatile, and the market price of the common stock may drop following the Merger.
The market price of the combined company’s common stock following the Merger could be subject to significant fluctuations. Market prices for securities of early-stage pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile. Some of the factors that may cause the market price of the combined company’s common stock to fluctuate include:
| ● | the ability of the combined company to obtain regulatory approvals for its product candidates, and delays or failures to obtain such approvals; |
| ● | failure of any of the combined company’s product candidates, if approved, to achieve commercial success; |
| ● | failure by the combined company to maintain its existing third-party license and supply agreements; |
| ● | failure by the combined company or its licensors to prosecute, maintain, or enforce its intellectual property rights; |
| ● | changes in laws or regulations applicable to the combined company’s product candidates; |
| ● | any inability to obtain adequate supply of the combined company’s product candidates or the inability to do so at acceptable prices; |
| ● | adverse regulatory authority decisions; |
| ● | introduction of new products, services or technologies by the combined company’s competitors; |
| ● | failure to meet or exceed financial and development projections the combined company may provide to the public; |
| ● | failure to meet or exceed the financial and development projections of the investment community; |
| ● | the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; |
| ● | announcements of significant acquisitions, strategic collaborations, joint ventures or capital commitments by the combined company or its competitors; |
| ● | disputes or other developments relating to proprietary rights, including patents, litigation matters, and the combined company’s ability to obtain patent protection for its technologies; |
| ● | additions or departures of key personnel; |
| ● | significant lawsuits, including patent or stockholder litigation; |
| ● | if securities or industry analysts do not publish research or reports about the combined company’s business, or if they issue an adverse or misleading opinion regarding its business and stock; |
| ● | changes in the market valuations of similar companies; |
| ● | general market or macroeconomic conditions; |
| ● | sales of its common stock by the combined company or its stockholders in the future; |
| ● | trading volume of the combined company’s common stock; |
| ● | failure to maintain compliance with the listing requirements of the NYSE American; |
| ● | announcements by commercial partners or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships or capital commitments; |
| ● | adverse publicity generally, including with respect to other products and potential products in such markets; |
| ● | the introduction of technological innovations or new therapies that compete with potential products of the combined company; |
| ● | changes in the structure of health care payment systems; and |
| ● | period-to-period fluctuations in the combined company’s financial results. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of the combined company’s common stock.
In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm the combined company’s profitability and reputation.
Additionally, a decrease in the stock price of the combined company may cause the combined company’s common stock to no longer satisfy the continued listing standards of the NYSE American. If the combined company is not able to maintain the requirements for listing on the NYSE American, it could be delisted, which could have a materially adverse effect on its ability to raise additional funds as well as the price and liquidity of its common stock.
The combined company will incur costs and demands upon management as a result of complying with the laws, rules and regulations affecting public companies.
The combined company will incur significant legal, accounting and other expenses that Serina did not incur as a private company, including costs associated with public company reporting requirements. The combined company will also incur costs associated with corporate governance requirements, including requirements under the laws, rules and regulations of the SEC as well as the NYSE American rules. These laws, rules and regulations are expected to increase the combined company’s legal and financial compliance costs and to make some activities more time consuming and costly. These executive officers and other personnel will need to devote substantial time to gaining expertise regarding operations as a public company and compliance with applicable laws and regulations. These laws, rules and regulations also may make it difficult and expensive for the combined company to obtain directors’ and officers’ liability insurance. As a result, it may be more difficult for the combined company to attract and retain qualified individuals to serve on the Combined Company Board or as executive officers of the combined company, which may adversely affect investor confidence in the combined company and could cause the combined company’s business or stock price to suffer.
Anti-takeover provisions in the Combined Company governance documents and under Delaware law could make an acquisition of the combined company more difficult and may prevent attempts by the combined company stockholders to replace or remove the combined company management.
Provisions in the Combined Company Charter and the Combined Company Bylaws may delay or prevent an acquisition or a change in management. In addition, because the combined company will be incorporated in Delaware, it is governed by the provisions of Section 203 of the DGCL, which prohibits stockholders owning in excess of 15% of the outstanding combined company voting stock from merging or combining with the combined company. Although AgeX and Serina believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with the Combined Company Board, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by the combined company’s stockholders to replace or remove then current management by making it more difficult for stockholders to replace members of the board of directors, which is responsible for appointing the members of management.
The Combined Company Charter will provide that the Court of Chancery of the State of Delaware is the exclusive forum for substantially all disputes between the combined company and its stockholders, which could limit its stockholders’ ability to obtain a favorable judicial forum for disputes with the combined company or its directors, officers or other employees.
The Combined Company Charter and the Combined Company Bylaws provide, among other things, that that the Court of Chancery of the State of Delaware (or, in the event that the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) is the exclusive forum for any derivative action or proceeding brought on the combined company’s behalf, any action asserting a claim of breach of fiduciary duty, any action asserting a claim against the combined company arising pursuant to the DGCL, the Combined Company Charter or the Combined Company Bylaws, or any action asserting a claim against the combined company that is governed by the internal affairs doctrine; provided that, the exclusive forum provision will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction; and provided further that, if and only if the Court of Chancery of the State of Delaware dismisses any such action for lack of subject matter jurisdiction, such action may be brought in another state or federal court sitting in the State of Delaware. The Combined Company Charter and the Combined Company Bylaws will also provide the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action against the combined company or any of its directors, officers, employees, or agents and arising under the Securities Act. Nothing in the Combined Company Charter or the Combined Company Bylaws will preclude stockholders that assert claims under the Exchange Act from bringing such claims in state or federal court, subject to applicable law.
AgeX and Serina do not anticipate that the combined company will pay any cash dividends in the foreseeable future.
The current expectation is that the combined company will retain its future earnings, if any, to fund the development and growth of the combined company’s business. As a result, capital appreciation, if any, of the common stock of the combined company will be its stockholders’ sole source of gain, if any, for the foreseeable future.
An active trading market for the combined company’s common stock may not develop and its stockholders may not be able to resell their shares of common stock for a profit, if at all.
Prior to the Merger, there had been no public market for Serina’s common stock. An active trading market for the combined company’s shares of common stock may never develop or be sustained. If an active market for its common stock does not develop or is not sustained, it may be difficult for its stockholders to sell their shares at an attractive price or at all.
Future sales of shares by existing stockholders could cause the combined company’s stock price to decline.
If existing stockholders of AgeX and Serina sell, or indicate an intention to sell, substantial amounts of the combined company’s common stock in the public market after legal restrictions on resale discussed in this proxy statement/prospectus/information statement lapse, the trading price of the common stock of the combined company could decline. Neither AgeX nor Serina is able to predict the effect that sales may have on the prevailing market price of the combined company’s common stock.
If equity research analysts do not publish research or reports, or publish unfavorable research or reports, about the combined company, its business or its market, its stock price and trading volume could decline.
The trading market for the combined company’s common stock will be influenced by the research and reports that equity research analysts publish about it and its business. Equity research analysts may elect not to provide research coverage of the combined company’s common stock after the completion of the Merger, and such lack of research coverage may adversely affect the market price of its common stock. In the event it does have equity research analyst coverage, the combined company will not have any control over the analysts, or the content and opinions included in their reports. The price of the combined company’s common stock could decline if one or more equity research analysts downgrade its stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of the combined company or fails to publish reports on it regularly, demand for its common stock could decrease, which in turn could cause its stock price or trading volume to decline.
The pro forma condensed combined financial statements included in this proxy statement/prospectus/information statement are presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the completion of the Merger.
The pro forma condensed combined financial statements contained in this proxy statement/prospectus/information statement are presented for illustrative purposes only and may not be an indication of the combined company’s financial condition or results of operations following the Merger for several reasons. The pro forma condensed combined financial statements have been derived from the historical audited financial statements of AgeX and Serina and certain adjustments and assumptions have been made regarding the combined company after giving effect to the Merger. The information upon which these adjustments and assumptions have been made is preliminary, and these kinds of adjustments and assumptions are difficult to make with accuracy. Moreover, the pro forma condensed combined financial statements do not reflect all costs that are expected to be incurred by the combined company in connection with the Merger. For example, the impact of any incremental costs incurred in integrating the two companies is not reflected in the pro forma condensed combined financial statements. As a result, the actual financial condition of the combined company following the Merger may not be consistent with, or evident from, these pro forma condensed combined financial statements. The assumptions used in preparing the pro forma condensed combined financial statements may not prove to be accurate, and other factors may affect the combined company’s financial condition following the Merger. For more information, please see the section titled “Unaudited Pro Forma Condensed Combined Financial Statements” in this proxy statement/prospectus/information statement.
If the combined company fails to maintain proper and effective internal controls, its ability to produce accurate financial statements on a timely basis could be impaired.
The combined company will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of the NYSE American. The Sarbanes-Oxley Act requires, among other things, that the combined company maintain effective disclosure controls and procedures and internal control over financial reporting. The combined company must perform system and process evaluation and testing of its internal control over financial reporting to allow management to report on the effectiveness of its internal controls over financial reporting in its Annual Report on Form 10-K filing for that year, as required by Section 404 of the Sarbanes-Oxley Act. As a private company, Serina has never been required to test its internal controls within a specified period. This will require that the combined company incur substantial professional fees and internal costs to expand its accounting and finance functions and that it expends significant management efforts. The combined company may experience difficulty in meeting these reporting requirements in a timely manner.
The combined company may discover weaknesses in its system of internal financial and accounting controls and procedures that could result in a material misstatement of its financial statements. The combined company’s internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
If the combined company is not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act, or if it is unable to maintain proper and effective internal controls, the combined company may not be able to produce timely and accurate financial statements. If that were to happen, the market price of its common stock could decline and it could be subject to sanctions or investigations by the NYSE American, the SEC or other regulatory authorities.
If the combined company fails to attract and retain management and other key personnel, it may be unable to continue to successfully develop or commercialize its product candidates or otherwise implement its business plan.
The combined company’s ability to compete in the highly competitive pharmaceuticals industry depends on its ability to attract and retain highly qualified managerial, scientific, medical, legal, sales and marketing and other personnel. The combined company will be highly dependent on its management and scientific personnel. The loss of the services of any of these individuals could impede, delay, or prevent the successful development of the combined company’s product pipeline, completion of its planned clinical trials, commercialization of its product candidates or in-licensing or acquisition of new assets and could impact negatively its ability to implement successfully its business plan. If the combined company loses the services of any of these individuals, it might not be able to find suitable replacements on a timely basis or at all, and its business could be harmed as a result. The combined company might not be able to attract or retain qualified management and other key personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses.
The combined company is expected to take advantage of reduced disclosure and governance requirements applicable to smaller reporting companies and emerging growth companies, which could result in its common stock being less attractive to investors.
Following the Merger, the combined company is expected to have a public float of less than $250 million and therefore will qualify as a smaller reporting company under the rules of the SEC. As a smaller reporting company, the combined company will be able to take advantage of reduced disclosure requirements, such as simplified executive compensation disclosures and reduced financial statement disclosure requirements in its SEC filings. Decreased disclosures in the combined company’s SEC filings due to its status as a smaller reporting company may make it harder for investors to analyze its results of operations and financial prospects. AgeX and Serina cannot predict if investors will find the combined company’s common stock less attractive if it relies on these exemptions. If some investors find its common stock less attractive as a result, there may be a less active trading market for its common stock and its stock price may be more volatile. The combined company may take advantage of the reporting exemptions applicable to a smaller reporting company until it is no longer a smaller reporting company, which status would end once it has a public float greater than $250 million. In that event, the combined company could still be a smaller reporting company if its annual revenues were below $100 million and it has a public float of less than $700 million.
Following the Merger, the combined company is expected to be an emerging growth company (EGC), as defined in the Jumpstart Our Business Startups Act of 2012, as amended. For as long as the combined company continues to be an EGC, it may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not EGCs, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in this proxy statement/prospectus/information statement and its periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. The combined company may remain an EGC until the earlier of (a) December 31, 2026, (b) the last day of the fiscal year in which the combined company has total annual gross revenue of at least $1.235 billion or more, (c) the date the combined company is deemed to be a large accelerated filer, which requires the market value of its common stock that is held by non-affiliates to exceed $700.0 million as of the prior June 30th, and (d) the date on which the combined company has issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Changes in tax laws may materially adversely affect the combined company’s business, prospects, financial condition and operating results.
New tax laws, statutes, rules, regulations or ordinances could be enacted at any time, which could adversely affect the combined company’s business, prospects, financial condition and operating results. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to the combined company. For example, the Tax Act, the CARES Act, and the IRA enacted many significant changes to the U.S. tax laws. Future guidance from the Internal Revenue Service and other tax authorities with respect to such legislation may affect the combined company, and certain aspects of such legislation could be repealed or modified in future legislation. Such tax law changes could have a material adverse impact on the combined company. In addition, it is uncertain if and to what extent various states will conform to newly enacted federal tax legislation. While it is too early to assess the overall impact of these changes, as these and other tax laws and related regulations are revised, enacted, and implemented, the combined company’s financial condition, results of operations, and cash flows could be materially adversely impacted.
The combined company’s ability to use net operating loss (NOL) carryforwards and other tax attributes may be limited, including as a result of the Merger.
Each of AgeX and Serina has incurred losses during its history, and the combined company does not expect to become profitable in the near future and may never achieve profitability. To the extent that the combined company continues to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire, if at all. As of December 31, 2022, AgeX had U.S. federal NOL carryforwards of $52.8 million, which includes $24.9 million that will expire at various dates between 2028 and 2037 and $27.9 million that have an unlimited carryforward period. Additionally, for state income tax purposes, AgeX had NOLs of$14.9 million that will expire at various dates between 2028 and 2042. As of December 31, 2022, Serina had U.S. federal NOL carryforwards of $35.2 million, which includes $23.2 million that will expire at various dates beginning in 2027 and $12.0 million that have an unlimited carryforward period. Additionally, for state income tax purposes, Serina had NOLs of $35.5 million that will expire at various dates beginning in 2023. Under current law, U.S. federal NOL carryforwards generated in taxable periods beginning after December 31, 2017, may be carried forward indefinitely, but the deductibility of such NOL carryforwards is limited to 80% of taxable income. It is uncertain if and to what extent various states will conform to federal law. In addition, under Sections 382 and 383 of the Code, federal NOL carryforwards and other tax attributes may become subject to an annual limitation in the event of certain cumulative changes in ownership. An “ownership change” pursuant to Section 382 of the Code generally occurs if one or more stockholders or groups of stockholders who own at least 5% of a company’s stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. The combined company’s ability to utilize its NOL carryforwards and other tax attributes to offset future taxable income or tax liabilities may be limited as a result of ownership changes, including potential changes in connection with the Merger or other transactions. Similar rules may apply under state tax laws. If the combined company earns taxable income, such limitations could result in increased future income tax liability to the combined company, and the combined company’s future cash flows could be adversely affected.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement/prospectus/information statement contains forward-looking statements. These forward-looking statements are based on current expectations and beliefs and involve numerous risks and uncertainties that could cause actual results to differ materially from expectations. These forward-looking statements should not be relied upon as predictions of future events and neither AgeX nor Serina can assure you that the events or circumstances reflected in these statements will be achieved or will occur. You can identify forward-looking statements by the use of forward-looking terminology including “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “pro forma,” “should,” “would” or the negative of these words and phrases or other variations of these words and phrases or comparable terminology. For example, forward-looking statements include, but are not limited to, statements about:
| ● | the strategies, prospects, plans, expectations and objectives of management of AgeX or Serina for future operations of the combined company following the closing of the Merger; |
| ● | the progress, scope or duration of the development of product candidates or programs; |
| ● | the benefits that may be derived from, or the commercial or market opportunity of, the product candidates of AgeX, Serina and the combined company; |
| ● | the ability of AgeX and Serina to protect their intellectual property rights; |
| ● | the ability of AgeX and the combined company to maintain compliance with NYSE American listing standards; |
| ● | the level of net cash held by the combined company at the closing of the Merger; |
| ● | the anticipated operations, financial position, losses, costs or expenses of AgeX, Serina or the combined company following the closing of the Merger; |
| ● | statements regarding future economic conditions or performance; |
| ● | statements concerning proposed products or product candidates; |
| ● | the approval and closing of the Merger, including the timing of the Merger, the ability of AgeX to obtain a sufficient number of proxies to approve the Merger, other conditions to the completion of the Merger, the exchange ratio, and relative ownership levels as of the closing of the Merger; |
| ● | the expected benefits of and potential value created by the Merger for the stockholders of AgeX; and |
| ● | statements of belief and any statement of assumptions underlying any of the foregoing. |
For a discussion of the factors that may cause AgeX, Serina or the combined company’s actual results, performance or achievements following closing of the Merger to differ materially from any future results, performance or achievements expressed or implied in such forward-looking statements, and for a discussion of risk associated with the ability of AgeX and Serina to complete the Merger and the effect of the Merger on the business of AgeX, Serina and the combined company following the completion of the Merger, see “Risk Factors” beginning on page 25 of this proxy statement/prospectus/information statement. Additional factors that could cause actual results to differ materially from those expressed in the forward-looking statements are discussed in reports filed with the SEC by AgeX. See “Where You Can Find More Information” beginning on page 379 of this proxy statement/prospectus/information statement. There can be no assurance that the Merger will be completed, or if it is completed, that it will close within the anticipated time period or that the expected benefits of the Merger will be realized.
If any of these risks or uncertainties materializes or any of these assumptions proves incorrect, the results of AgeX, Serina or the combined company following completion of the Merger could differ materially from the forward-looking statements. All forward-looking statements in this proxy statement/prospectus/information statement are current only as of the date on which the statements were made. AgeX and Serina do not undertake any obligation (and expressly disclaim any such obligation) to publicly update any forward-looking statement to reflect events or circumstances after the date on which any statement is made or to reflect the occurrence of unanticipated events, except as required by applicable law.
In addition, statements that “AgeX believes” or “Serina believes” and similar statements reflect AgeX’s or Serina’s beliefs and opinions on the relevant subject. These statements are based upon information available to AgeX or Serina, as the case may be, as of the date of this proxy statement/prospectus/information statement, and while AgeX or Serina, as the case may be, believes such information forms a reasonable basis for such statements, such information may be limited or incomplete, and such statements should not be read to indicate that such party has conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
THE SPECIAL MEETING OF AGEX’S STOCKHOLDERS
Date, Time and Place
The AgeX special meeting will be held on , commencing at , unless postponed or adjourned to a later date. The AgeX special meeting will be held entirely online. AgeX is sending this proxy statement/prospectus/information statement to its stockholders in connection with the solicitation of proxies by the AgeX Board for use at the AgeX special meeting and any adjournments or postponements of the AgeX special meeting . This proxy statement/prospectus/information statement is first being mailed to AgeX stockholders on or about .
Purpose of the AgeX Special Meeting
The purpose of the AgeX special meeting is:
| 1. | Proposal No. 1 (Stock Issuance Proposal)—To approve (i) the issuance of shares of AgeX common stock, which will represent more than 20% of the shares of AgeX common stock outstanding immediately prior to the Merger, to stockholders of Serina, pursuant to the terms of the Merger Agreement, a copy of which is attached as Annex A to this proxy statement/prospectus/information statement, and (ii) the change of control of AgeX resulting from the Merger, pursuant to Sections 712(b) and 713(b) of the NYSE American Company Guide; |
| 2. | Proposal No. 2 (Reverse Stock Split Proposal)—To approve an amendment to the AgeX Charter to effect a reverse stock split of AgeX common stock at a ratio within the range between 1-for-35 to 1-for-36 with such ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors prior to the effectiveness of the Merger or, if the Stock Issuance Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal is not approved, by AgeX stockholders, determined solely by the AgeX Board; |
| 3. | Proposal No. 3 (Warrant Issuance Proposal)—To approve the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants, which will represent securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance, referred to herein as the Warrant Issuance Proposal; |
| 4. | Proposal No. 4 (A&R Charter Proposal)—To approve the Combined Company Charter, in the form attached as Annex B to this proxy statement/prospectus/information statement; |
| 4. | Proposal No. 5 (2024 Equity Incentive Plan Proposal)—To approve the Serina Therapeutics, Inc. 2024 Equity Incentive Plan, in the form attached as Annex C to this proxy statement/prospectus/information statement; and |
| 6. | Proposal No. 6 (Adjournment Proposal)—To approve a postponement or adjournment of the AgeX special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal, referred to as the Adjournment Proposal. |
These proposals are referred to collectively as the AgeX Proposals. No AgeX Proposal is conditioned upon any other AgeX Proposal. However, each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal is a condition to the consummation of the Merger. Therefore, the Merger cannot be consummated without the approval of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal.
Recommendation of the AgeX Board
| ● | The AgeX Board has determined and believes that the issuance of shares of AgeX common stock pursuant to the Merger Agreement is in the best interests of AgeX and its stockholders and has approved such proposal. The AgeX Board recommends that AgeX stockholders vote “FOR” the Stock Issuance Proposal as described in this proxy statement/prospectus/information statement. |
| ● | The AgeX Board has determined and believes that it is advisable to, and in the best interests of, AgeX and its stockholders to approve the amendment to the AgeX Charter effecting a reverse stock split at a ratio in the range from 1-for-35 to 1-for-36, with such specific ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors or, if the Stock Issuance Proposal is not approved by AgeX stockholders, determined solely by the AgeX Board following the AgeX special meeting as described in this proxy statement/prospectus/information statement. The AgeX Board recommends that AgeX stockholders vote “FOR” the Reverse Stock Split Proposal as described in this proxy statement/prospectus/information statement. |
| ● | The AgeX Board has determined and believes that the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants is in the best interests of AgeX and its stockholders and has approved such proposal. The AgeX Board recommends that AgeX stockholders vote “FOR” the Warrant Issuance Proposal as described in this proxy statement/prospectus/information statement. |
| ● | The AgeX Board has determined and believes that the adoption of the Combined Company Charter at the Effective Time is in the best interests of AgeX and its stockholders and has approved such proposal. The AgeX Board recommends that AgeX stockholders vote “FOR” the A&R Charter Proposal as described in this proxy statement/prospectus/information statement. |
| ● | The AgeX Board has determined and believes that the adoption of the Serina Therapeutics, Inc. 2024 Equity Incentive Plan is in the best interests of AgeX and its stockholders and has approved such proposal. The AgeX Board recommends that AgeX stockholders vote “FOR” the 2024 Equity Incentive Plan Proposal as described in this proxy statement/prospectus/information statement. |
| ● | The AgeX Board has determined and believes that adjourning the AgeX special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal is advisable to, and in the best interests of, AgeX and its stockholders and has approved and adopted the proposal. The AgeX Board recommends that AgeX stockholders vote “FOR” the Adjournment Proposal as described in this proxy statement/prospectus/information statement. |
Record Date and Voting Power
Only holders of record of AgeX common stock at the close of business on the record date, , 2023, are entitled to notice of, and to vote at, the AgeX special meeting. There were approximately holders of record of AgeX common stock at the close of business on the record date. At the close of business on the record date, shares of AgeX common stock were issued and outstanding. Each share of AgeX common stock entitles the holder thereof to one vote on each matter submitted for stockholder approval. See the section titled “Principal Stockholders of AgeX” beginning on page 366 of this proxy statement/prospectus/information statement for information regarding persons known to AgeX’s management to be the beneficial owners of more than 5% of the outstanding shares of AgeX common stock.
Voting and Revocation of Proxies
The proxy accompanying this proxy statement/prospectus/information statement is solicited on behalf of the AgeX Board for use at the AgeX special meeting.
If you are a stockholder of record of AgeX as of the record date referred to above, you may vote in person at the AgeX special meeting or vote by proxy on the internet, by telephone, or using the enclosed proxy card. Whether or not you plan to attend the AgeX special meeting, AgeX urges you to vote by proxy to ensure your vote is counted. You may still attend the AgeX special meeting and vote in person if you have already voted by proxy. As a stockholder of record, you are entitled:
| ● | to vote at the AgeX special meeting, attend the AgeX special meeting online and follow the instructions posted at www.virtualshareholdermeeting.com/ ; |
| ● | to vote using the proxy card, simply mark, sign and date your proxy card and return it promptly in the postage-paid envelope provided. If you return your signed proxy card to AgeX before the AgeX special meeting, AgeX will vote your shares in accordance with the proxy card; |
| ● | to vote on the internet, go to the website on the proxy card or voting instruction form to complete an electronic proxy card. You will be asked to provide the company number and control number from the enclosed proxy card. Your vote must be received by 11:59 p.m. Eastern Time on , 2024 to be counted; and |
| ● | to vote by telephone, go to the website on the proxy card or voting instruction form to complete an electronic proxy card, or call the toll-free number on the proxy card or voting instruction form to vote. You will be asked to provide the company number and control number from the enclosed proxy card. Your vote must be received by 11:59 p.m. Eastern Time on , 2024 to be counted. |
If your AgeX shares are held by your broker as your nominee, that is, in “street name,” the enclosed voting instruction card is sent by the institution that holds your shares. Please follow the instructions included on that proxy card regarding how to instruct your broker to vote your AgeX shares.
If you do not give instructions to your broker, bank or other nominee, the question of whether your broker, bank or other nominee will still be able to vote your shares depends on whether the NYSE American deems the particular proposal to be a “routine” matter and how your broker, bank or other nominee exercises any discretion they may have in the voting of the shares that you beneficially own. Brokers, banks and other nominees can use their discretion to vote “uninstructed” shares with respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. Whether a proposal is considered “routine” or “non-routine” is subject to stock exchange rules and final determination by the stock exchange.
For any AgeX Proposal that is considered a “routine” matter, your broker, bank or other nominee may vote your shares in its discretion either for or against the proposal even in the absence of your instruction. For any AgeX Proposal that is considered a “non-routine” matter for which you do not give your broker, bank or other nominee instructions, the AgeX shares will be treated as broker non-votes. Broker non-votes occur when a beneficial owner of shares held in street name does not give instructions to the broker, bank or other nominee holding the shares as to how to vote on matters deemed “non-routine.”
All properly executed proxies that are not revoked will be voted at the AgeX special meeting and at any adjournments or postponements of the AgeX special meeting in accordance with the instructions contained in the proxy. If a holder of AgeX common stock executes and returns a proxy and does not specify otherwise, the shares represented by that proxy will be voted:
| ● | “FOR” the Stock Issuance Proposal to approve the issuance of shares of AgeX common stock pursuant to the Merger Agreement; |
| ● | “FOR” the Reverse Stock Split Proposal to approve the amendment to the AgeX Charter effecting a reverse stock split at a ratio in the range from 1-for-35 to 1-for-36 with such specific ratio resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors or, if the Stock Issuance Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal is not approved by AgeX stockholders, determined solely by the AgeX Board following the AgeX special meeting; |
| ● | “FOR” the Warrant Issuance Proposal to approve the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants; |
| ● | “FOR” the A&R Charter Proposal to approve the adoption of the Combined Company Charter at the Effective Time; |
| ● | “FOR” the 2024 Equity Incentive Plan Proposal to approve the adoption of the Serina Therapeutics, Inc. 2024 Equity Incentive Plan; and |
| ● | “FOR” the Adjournment Proposal to adjourn the AgeX special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal. |
AgeX stockholders as of the record date, other than the AgeX stockholder that executed the AgeX Support Agreement, may change their vote at any time before their proxy is voted at the AgeX special meeting in one of three ways. You can revoke your proxy at any time before it is exercised by delivering a properly executed, later-dated proxy (including a proxy submitted by internet or telephone), by delivering a written revocation before the AgeX special meeting or by voting at the AgeX special meeting. Executing your proxy in advance will not limit your right to vote at the AgeX special meeting if you decide to attend the AgeX special meeting. However, if your shares are held in the name of a broker, bank or other holder of record, you cannot vote at the AgeX special meeting unless you have a legal proxy, executed in your favor, from the holder of record. If an AgeX stockholder of record or a stockholder who owns AgeX shares in “street name” has instructed a broker to vote its shares of AgeX common stock, the stockholder must follow directions received from its broker to change those instructions.
A complete list of AgeX stockholders entitled to vote at the AgeX special meeting will be available for examination by any AgeX stockholder in the Corporate Secretary’s Office at AgeX Therapeutics, Inc., 1101 Marina Village Parkway, Suite 201, Alameda, California 94501, for purposes pertaining to the AgeX special meeting, during ordinary business hours for a period of 10 days before the AgeX special meeting, and at the AgeX special meeting. A complete list of AgeX stockholders entitled to vote at the AgeX special meeting will also be available for inspection during the AgeX special meeting at www.virtualshareholdermeeting.com/ by logging in with your 16-digit control number(s).
Required Vote
The presence, in person or represented by proxy, at the AgeX special meeting of the holders of a majority of the shares of AgeX common stock outstanding and entitled to vote at the AgeX special meeting is necessary to constitute a quorum at the meeting. Abstentions and broker non-votes, if any, will be counted towards a quorum. Assuming a quorum is present, the required vote for each of the AgeX Proposals is as follows:
| Proposal Number | | Proposal Description | | Vote Required for Approval | | Effect of Abstentions | | Effect of Broker Non-Votes |
| | | | | | | | | |
| 1 | | Stock Issuance Proposal | | FOR votes from the holders of a majority of shares voting affirmatively or negatively (excluding abstentions and broker non-votes) on the matter | | None | | None |
| | | | | | | | | |
| 2 | | Reverse Stock Split Proposal | | FOR votes from the holders of a majority of shares voting affirmatively or negatively (excluding abstentions and broker non-votes) on the matter | | None | | None |
| | | | | | | | | |
| 3 | | Warrant Issuance Proposal | | FOR votes from the holders of a majority of shares voting affirmatively or negatively (excluding abstentions and broker non-votes) on the matter | | None | | None |
| | | | | | | | | |
| 4 | | A&R Charter Proposal | | FOR votes from the holders of a majority of outstanding shares AgeX common stock, AgeX Series A preferred stock and AgeX Series B preferred stock, each voting as a separate class | | Against | | Against |
| | | | | | | | | |
| 5 | | 2024 Equity Incentive Plan Proposal | | FOR votes from the holders of a majority of shares voting affirmatively or negatively (excluding abstentions and broker non-votes) on the matter | | None | | None |
| | | | | | | | | |
| 5 | | Adjournment Proposal | | FOR votes from the holders of a majority of shares voting affirmatively or negatively (excluding abstentions and broker non-votes) on the matter | | None | | None |
The information in the preceding table with respect to the effect of broker non-votes may be incorrect or change before the AgeX special meeting. Therefore, if you are a beneficial owner and want to ensure that shares you beneficially own are voted in favor or against any or all of the AgeX Proposals, the only way you can do so is to give your broker or nominee specific instructions as to how the shares are to be voted.
If on the date of the AgeX special meeting, or a date preceding the date on which the AgeX special meeting is scheduled, AgeX reasonably believes that (i) it will not receive proxies sufficient to obtain the required vote to approve the AgeX Proposals, whether or not a quorum would be present, or (ii) it will not have sufficient shares of AgeX common stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the AgeX special meeting, AgeX may postpone or adjourn, or make one or more successive postponements or adjournments of, the AgeX special meeting as long as the date of the AgeX special meeting is not postponed or adjourned more than an aggregate of 30 calendar days in connection with any postponements or adjournments.
No AgeX Proposal is conditioned upon any other AgeX Proposal. However, each of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal is a condition to the consummation of the Merger. Therefore, the Merger cannot be consummated without the approval of the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal and the 2024 Equity Incentive Plan Proposal.
Concurrently with the execution of the Merger Agreement, Juvenescence, a stockholder of AgeX holding approximately 43.3% of the outstanding shares of AgeX common stock, 100% of the outstanding shares of Series A preferred stock of AgeX and 100% of the outstanding shares of Series B preferred stock of AgeX, in each case as of August 29, 2023, entered into the AgeX Support Agreement, which provides, among other things, that Juvenescence will vote all of its shares of AgeX capital stock in favor of the AgeX Proposals.
Attendance at the AgeX Special Meeting and Voting at the AgeX Special Meeting
You or your authorized proxy may attend the AgeX special meeting virtually if you were a registered or beneficial stockholder of AgeX common stock as of the AgeX record date.
To participate in the AgeX special meeting, visit www.virtualshareholdermeeting.com/ and enter the 16-digit control number included on your proxy card or on the instructions that accompanied your proxy materials. If you wish to submit a question during the AgeX special meeting, log into the virtual meeting platform at www.virtualshareholdermeeting.com/ , type your question into the “Ask a Question” field, and click “Submit.” AgeX will respond to as many properly submitted questions during the relevant portion of the AgeX special meeting agenda as time allows.
If AgeX experiences technical difficulties during the AgeX special meeting (e.g., a temporary or prolonged power outage), it will determine whether the AgeX special meeting can be promptly reconvened (if the technical difficulty is temporary) or whether the AgeX special meeting will need to be reconvened on a later day (if the technical difficulty is more prolonged). In any situation, AgeX will promptly notify stockholders of the decision via . AgeX will have technicians ready to assist you with any technical difficulties you may have accessing the virtual meeting website. If you encounter any difficulties accessing the virtual meeting website during the check-in or meeting time, please call the technical support number that will be posted on the virtual meeting website log-in page at www.virtualshareholdermeeting.com/ .
Please note that you will not be able to attend the AgeX special meeting in person.
The AgeX special meeting will be held virtually conducted via live audio webcast. You will be able to attend the AgeX special meeting by visiting www.virtualshareholdermeeting.com/ and entering your 16-digit control number as further explained in this proxy statement/prospectus/information statement. AgeX recommends that you log in at least 15 minutes before the AgeX special meeting to ensure you are logged in when the meeting starts.
If you own shares in street name through an account with a bank, broker or other nominee, please send proof of your AgeX share ownership as of the AgeX record date (for example, a brokerage firm account statement or a “legal proxy” from your intermediary) along with your registration request. If you are not sure what proof to send, check with your intermediary.
If your shares are registered in your name with AgeX’s stock registrar and transfer agent, Equiniti, no proof of ownership is necessary because AgeX can verify your ownership.
Solicitation of Proxies; Expenses of Solicitation
The AgeX Board is soliciting proxies for the AgeX special meeting from its stockholders. AgeX will bear a portion of the cost of the solicitation of proxies, including preparation, assembly and delivery, as applicable, of this joint proxy statement/prospectus/information statement, the AgeX proxy card and any additional materials furnished to AgeX stockholders. Proxies may be solicited by directors, officers and a small number of AgeX’s regular employees by mail, email, text message, in person and by telephone, but such persons will not receive any additional compensation for these activities.
Tabulation of Votes
AgeX will appoint Equiniti to serve as the Inspector of Election for the AgeX special meeting. Equiniti will independently tabulate affirmative and negative votes and abstentions.
Other Matters
As of the date of this proxy statement/prospectus/information statement, the AgeX Board does not know of any business to be presented at the AgeX special meeting other than as set forth in the notice accompanying this proxy statement/prospectus/information statement. If any other matters should properly come before the AgeX special meeting, it is intended that the shares represented by proxies will be voted with respect to such matters in accordance with the judgment of the persons voting the proxies.
Your vote is very important regardless of the number of shares of AgeX common stock that you own, and the matters to be considered at the AgeX special meeting are of great importance to the stockholders of AgeX. Accordingly, you are urged to read and carefully consider the information contained in this proxy statement/prospectus/information statement and promptly submit your proxy via the internet or by telephone or complete, date, sign and promptly return the enclosed AgeX proxy card or voting instruction form in the enclosed postage-paid envelope. If you submit your proxy via the internet or by telephone, you do not need to return the enclosed AgeX proxy card.
Please vote your shares via the internet or by telephone, or sign, date and return an AgeX proxy card or voting instruction form promptly to ensure that your shares can be represented, even if you otherwise plan to attend the AgeX special meeting.
THE MERGER
Background of the Merger
In an effort to enhance stockholder value, the AgeX Board and AgeX management regularly review and discuss AgeX’s business, performance, financial condition, near and long-term operations and strategic priorities. These reviews and discussions have included, among other things, the risks associated with AgeX’s product candidates, current and anticipated business and industry trends, the competitive landscape, regulatory conditions, the financial markets and macroeconomic environment (including the financing challenges associated with the broad weakness in the biopharmaceutical market), opportunities for strategic relationships, collaborations and other potential long-term strategic options to strengthen AgeX’s balance sheet, strategies around obtaining financing to support the clinical development of AgeX’s product candidates and product discovery and development efforts, and other potential strategic transactions, in each case with the goal of maximizing stockholder value.
AgeX was incorporated during 2017 as a subsidiary of Lineage Cell Therapeutics, Inc. (Lineage), formerly known as BioTime, Inc. On August 17, 2017, AgeX entered into an Asset Contribution and Separation Agreement with Lineage pursuant to which Lineage contributed certain assets and cash to AgeX in exchange for 28,800,000 shares of AgeX common stock. Concurrently with the acquisition of assets and licensing of certain intellectual property from Lineage, AgeX sold 4,950,000 shares of common stock for $10.0 million in cash primarily to investors other than Lineage. At the close of the financing, Lineage owned 85.4% of AgeX’s issued and outstanding shares of common stock. On June 7, 2018, AgeX sold 2,000,000 shares of common stock for $2.50 per share to Juvenescence for aggregate cash proceeds to AgeX of $5.0 million.
On August 30, 2018, Lineage sold 14,400,000 of its shares of AgeX common stock to Juvenescence in exchange for an aggregate purchase price of $43.2 million. Upon completion of the transaction, Lineage’s ownership in AgeX was reduced from 80.4% to 40.2%, and Juvenescence’s ownership in AgeX was increased from 5.6% to 45.8%. On November 28, 2018, Lineage distributed to its shareholders, on a pro rata basis, 12,697,028 shares of the AgeX common stock it then held (the Distribution). Immediately after the Distribution, Lineage retained 1,718,972 shares of AgeX common stock, representing approximately 4.8% of the AgeX common stock. Following the Distribution, AgeX common stock began publicly trading on the NYSE American under the symbol “AGE.”
On September 4, 2019, AgeX published a paper describing its iTR technology and announced the formation of a wholly owned subsidiary, Reverse Bio that would be wholly focused on the development of iTR as a therapeutic modality.
In April 2020, based on a strategic review of AgeX’s operations, giving consideration to the status of its product development programs, human resources, capital needs and resources, and current conditions in the capital markets, the AgeX Board and management modified its corporate strategy to focus on non-dilutive measures to explore the value of the AgeX platform technology through partnerships and prioritized further development of mature assets such as AgeX-BAT1 and VASC1 over the basic science being done by AgeX in-house.
In May 2020, AgeX conducted layoffs that impacted half of its employees. Of the 11 employees impacted, 10 were employed in the research and development department. The remaining research employee’s employment was changed to be on a part-time basis instead of full-time. There have been no new hires in research and development since the layoffs.
In lieu of internal research and development, AgeX concentrated on partnering with external collaborators that could provide validation of core AgeX technologies. AgeX has sponsored research and development programs at the University of California, Irvine for the use of neural stem cells in the potential treatment of Huntington’s Disease and the use of exosomes and other extracellular vesicles for the potential treatment of adverse neurocognitive effects of cancer chemotherapy and radiation therapy on brain function. AgeX has also sponsored research at Ohio State University to investigate the potential of brown adipocytes to treat metabolic conditions. Finally, AgeX entered into research collaboration agreements with a certain Japanese biopharmaceutical company and Sernova Corp., each which ultimately expired with limited research completed due to the COVID-19 pandemic.
During the second half of 2020, Reverse Bio retained Oliver Summerfield as Chief Executive Officer. AgeX encouraged Dr. Summerfield to raise an independent financing for Reverse Bio in order to develop its lead candidate, AGEX-iTR1547. After just over one year passed without a successful financing, Dr. Summerfield resigned as Chief Executive Officer of Reverse Bio on or about October 13, 2021.
During March 2021, AgeX disposed of its interest in LifeMap Sciences, Inc. (LifeMap Sciences), a subsidiary that operated an on-line data base for use by researchers in the stem cell field. During the course of AgeX’s ownership of LifeMap Sciences, the subsidiary was unable to grow its revenue base and operated at a financial loss.
On March 2, 2021, AgeX announced that it would negotiate a merger with a Juvenescence-affiliated privately-held biotechnology company that developed cell therapies that enable organ regeneration. At the closing of the proposed merger, AgeX would have issued to the privately-held biotechnology company’s stockholders shares of AgeX common stock representing two-thirds of the total number of shares of AgeX common stock to be outstanding immediately following the consummation of the proposed merger on a fully-diluted basis, and then it would issue additional shares in a capital raise. However, on July 23, 2021, AgeX and the privately-held company announced that negotiations for the merger were discontinued. The AgeX Board and the board of directors of the potential target each determined that it was in the best interest of their respective shareholders not to proceed with the merger given the diversity of the companies’ technology platforms and product development focus.
On July 23, 2021, AgeX additionally announced it would continue to consider new strategic opportunities as they arise and remained committed to creating shareholder value through third-party collaborations and licensing agreements for its core technologies UniverCyte™ and PureStem®, and its cell therapy programs, including AGEX-VASC1 and AGEX-BAT1. AgeX also announced that it was exploring financing alternatives for its iTR™ technology and a potential spinout of Reverse Bio.
From July 2021 through the signing of the Merger Agreement, AgeX continued to incur operating losses and negative cash flows despite reducing operating expenses by out-sourcing research and development and seeking licensing arrangements for AgeX technologies. This approach made it more difficult to make progress in developing target product candidates and technologies, which in turn made it more difficult to raise capital.
As a result, AgeX has had to borrow a total of $37.2 million from Juvenescence (Juvenescence loans), as of October 31, 2023, to continue to finance operations. The Juvenescence loans included a grant of a first lien security interest on AgeX’s assets (subject to limited exceptions) as well as negative covenants which, among other things, prohibits AgeX and its subsidiaries from borrowing funds from other lenders while the Juvenescence loans remain outstanding, with exceptions to permit Reverse Bio to borrow funds through convertible debt. In addition, the prohibition on AgeX and its subsidiaries incurring other debt would no longer apply once Reverse Bio raised $15,000,000 in debt or equity financing by February 14, 2023, which date was subsequently extended to June 20, 2023, and then to October 31, 2023. The Juvenescence loans did not limit equity offerings so long as the proceeds of any stock issuance were applied for research and development work (including through third-party contractors), professional and administrative expenses, and for general working capital only in accordance with a budget approved by the AgeX Board and provided to Juvenescence, or to repay the Juvenescence loans.
On November 17, 2021, AgeX received a letter from the staff of the NYSE American indicating that AgeX did not meet certain of the NYSE American’s continued listing standards. On January 28, 2022, the NYSE American accepted a plan of action for AgeX to bring itself into compliance by May 17, 2023 through a successful financing of Reverse Bio. On November 22, 2022, AgeX received a notification from the NYSE American accepting a revised listing compliance plan; however, in such notification, the NYSE American warned that if AgeX was not in compliance with the continued listing standards by May 17, 2023, or if AgeX did not make progress consistent with the plan during the plan period, the NYSE American would initiate delisting proceedings.
In early September, 2022, Mr. Alexander Pickett, Managing Director at Juvenescence, spoke with Remy Gross III, the Vice President, Business Development at the Buck Institute for Research on Aging. Mr. Gross, an advisor to Serina, spoke to Mr. Pickett about Serina’s interest in financing and provided a non-confidential update on work done by the Serina team on its lipid nanoparticle formulations of mRNA therapeutics. Mr. Gross indicated that Serina had made major progress and that it was looking to raise a substantial investment in order to properly exploit its new platform. After an initial call with Serina’s management team, Mr. Pickett and colleagues visited Serina’s site at Huntsville Alabama on September 12, 2022. After this meeting, Mr. Pickett proposed to Dr. Gregory Bailey, then CEO of Juvenescence and a Director of AgeX, the idea of merging Serina into AgeX. Dr. Bailey viewed the idea favorably and began to socialize it with other AgeX Directors on September 22, 2022.
On October 7, 2022, Mr. Pickett had a preliminary call to assess deal terms with Serina’s management team, including Dr. Milton Harris, Dr. Randall Moreadith, and Mr. Steve Ledger, during which the parties discussed Serina’s need for additional funding in connection with the deal. Shortly thereafter, Mr. Pickett prepared a draft nonbinding term sheet for the transactions, encompassing a convertible loan note and merger terms between AgeX and Serina.
On October 18, 2022, Mr. Pickett had individual calls with AgeX Directors Dr. Michael May and Dr. Mike West to provide background on Serina and to describe the possibility of a business combination transaction.
On October 19, 2022, the AgeX Board met and Mr. Pickett was invited to present the term sheet. During this meeting, the AgeX Board was advised of its fiduciary duties, informed that Juvenescence did not have any conflicts with Serina, and discussed the risks of the structure of the deal. The AgeX Board formed a Special Committee comprised of Dr. Joanne Hackett, the Chair of the AgeX Board, and Dr. Gregory Bailey to manage the transaction process. The Special Committee requested that Mr. Pickett work with AgeX’s external transaction counsel, Gibson, Dunn & Crutcher LLP (Gibson Dunn), to revise the term sheet, provide support in negotiating the deal, and arrange due diligence.
On October 21, 2022, Dr. Hackett sent the initial term sheet to Serina’s management. Later that day, Mr. Pickett spoke with Serina’s management team to further explain the term sheet. The term sheet set a valuation for Serina of $100M and a valuation of AgeX at $50M.
On October 24, 2022, Serina’s management sent a letter to AgeX expressing that Serina believed that a merger agreement without a guarantee that AgeX would provide material financing to Serina would be difficult and that, without material financing from AgeX, Serina desired to re-open discussions with AgeX in Q1 2023 after finishing negotiations with a third-party licensee. Further discussion between Mr. Pickett and Mr. Ledger revealed that Serina viewed the proposed valuation as a key sticking point, particularly in light of ongoing license negotiations between Serina and a third party. Mr. Pickett and Mr. Ledger continued negotiations that led to a revised term sheet prepared by Mr. Pickett, Susan Bergin, another employee of Juvenescence, and Goodwin Proctor LLP (Goodwin), counsel to Juvenescence, that valued Serina at $105M, the same value as its last private financing, while AgeX was valued at $45M. Dr. Hackett sent a revised version of the term sheet incorporating this proposal on November 2, 2022. On November 4, 2022, Mr. Ledger sent a redline of the November 2nd term sheet to Mr. Pickett. This redline of the term sheet included specific language exempting two ongoing negotiations with potential third party licensees from the exclusivity provisions of the term sheet. Discussions from this point shifted from discussion of absolute valuations to relative values (70:30 rather than $105M:$45M).
On November 3, 2022, Serina’s management met to discuss the term sheet.
On November 9, 2022, the AgeX Board held a meeting, at which Dr. Bailey resigned as a member of the Special Committee. Dr. Bailey was replaced by Dr. May. Dr. Hackett sent a revised version of the term sheet to Serina’s management, which included security requirements on the convertible loan note, the removal of receiving a fairness opinion as a condition precedent to closing, and adding a consent requirement to the exclusivity clause that prohibited Serina from engaging in discussions for a competing transaction without the prior written consent of AgeX.
On November 14, 2022, Serina’s management team met to discuss the term sheet.
On November 15, 2022, Mr. Ledger provided Mr. Pickett feedback from Serina’s management team—namely that Serina’s management team objected to the proposed valuation on the basis that Serina’s ongoing license discussions provided an alternative, non-dilutive source of capital. Mr. Pickett and Mr. Ledger negotiated a change in relative values to 75:25 alongside the creation of a set of out-of-money warrants that would issue to existing AgeX shareholders upon consummation of the transaction. The warrants were arranged in three tranches of partial warrants that summed to 75% warrant coverage for AgeX Shareholders at multiples of the closing share price (150%, 200%, and 250%). This term sheet also spelled out a carveout of certain AgeX assets intended to be placed into Reverse Bio upon its successful financing. A revision of the term sheet incorporating these changes of terms was sent to Dr. Hackett on November 18, 2022. On November 20, 2022, the AgeX Special Committee met to consider the revised deal.
On November 23, 2022 Dr. Hackett sent a revised term sheet to Serina management. This version of the term sheet expanded warrant coverage to 100%. Warrants were divided into funding warrants with an 18-month term and 125% exercise price and long-dated incentive warrants priced at 175% and 225% of the closing price that were issued after the exercise of the incentive warrants. Furthermore, AgeX was entitled to participate in the ongoing third-party license negotiations and had the right to approve deal terms for the duration of the exclusivity period.
On November 28, 2022, the AgeX Special Committee met to further discuss the term sheet.
Following further discussion between Mr. Ledger and Mr. Pickett, Mr. Pickett sent a revised term sheet to Dr. Hackett on December 2, 2022, which provided a definition for incentive warrants and clarified that no fractional shares would be issued upon the exercise of the warrants.
The AgeX Special Committee met on December 5, 2022 and approved the term sheet, subject to revisions from Gibson Dunn.
On December 5, 2022, Dr. Hackett sent the term sheet to Serina’s management.
On December 8, 2022, the Serina Board met to discuss the term sheet and authorized Serina’s chief executive officer, Dr. Moreadith, to execute the term sheet.
On December 12, 2022, AgeX provided its countersignature to the term sheet. The term sheet provided for a $10 million convertible note to be issued by Serina to AgeX with certain conditions outlined therein. In addition, the term sheet outlined a proposed merger between Serina and AgeX, which included, among other things, that if the merger occurred, (i) AgeX would issue to Serina the number of shares of AgeX as would bring Serina’s aggregate shareholding of AgeX to 75% of the fully-diluted company, (ii) the combined company would consider strategic alternatives for the business of Reverse Bio, and (iii) during the duration of the term sheet, Serina would not solicit or enter into any competing transactions to the ones contemplated by the term sheet with AgeX, but AgeX was not subject to any exclusivity provisions with Serina under the term sheet or at any time prior to entering into the Merger Agreement.
On December 16, 2022, Ms. Bergin provided the AgeX Board with an update on the proposed transaction with Serina and informed the AgeX Board that due diligence had begun.
On December 20, 2022, AgeX, Gibson Dunn, transaction counsel to AgeX, Thompson, Welch, Soroko & Gilbert LLP (Thompson Welch), corporate counsel to AgeX, Serina, and Bradley Arant Boult Cummings LLP (Bradley), counsel to Serina, participated in a video conference to discuss the timeline to execution of definitive documentation, allocation of drafting responsibilities, AgeX’s due diligence review of Serina, Serina’s due diligence of AgeX and certain other topics.
Over the next few weeks, AgeX formally engaged the following advisors to conduct formal due diligence: Albeto J Espay, MD, the Professor and Endowed Chair of the James J. and Joan A. Gardner Center for Parkinson’s Disease at the University of Cincinnati on January 24, 2023 for review of SER-252; Robert Hauser, MD, MBA, the Professor of Neurology, Pharmacology and Experimental Therapeutics at University of South Florida Director at Parkinson’s Disease and Movement Disorders Center on February 23, 2023 for review of SER-252; Andy McElroy, PhD, a former Pfizer medicinal chemist and the CEO and founder of The Research Network, Ltd. on February 24, 2023 for review of the breadth and depth of the Serina chemistry platform; Glenn Prestwich, the Presidential Professor of Medicinal Chemistry at the University of Utah and manager of Clear Solutions Biomedical on January 24, 2023 for review of the breadth and depth of the Serina chemistry platform; Peter Bungay PhD of Sympetrus, Ltd. on February 15, 2023 for review of the drug metabolism and pharmacokinetic properties of SER-252 in particular and the Serina platform technology in general; S. Moein Moghimi, PhD, Professor of Pharmaceutics and Nanomedicine at the School of Pharmacy and the Translational and Clinical Research Institute at Newcastle University on February 12, 2023 for review of Serina’s lipid nanoparticle technology; Gaurav Sahay, PhD, Professor in the Department of Pharmaceutical Sciences, College of Pharmacy at Oregon State University on February 10, 2023 for review of Serina’s lipid nanoparticle technology; Yizhou Dong, PhD, a Professor at the Icahn School of Medicine at Mount Sinai on February 9, 2023 for review of Serina’s lipid nanoparticle technology; and Justin Stebbing MD, a Professor of Biomedical Sciences at ARU, Cambridge on February 15, 2022 for commercial perspectives on mRNA vaccines and lipid nanoparticle technology.
AgeX retained Finnegan, Henderson, Farabow, Garrett & Dunner LLP in relation to intellectual property diligence. Furthermore, AgeX engaged Mr. Pickett and Ms. Bergin, to support the AgeX management team in relation to scientific and IP diligence pursuant to an intracompany services agreement. These advisors worked in cooperation with AgeX management and Gibson Dunn when appropriate, to conduct due diligence on Serina’s business and report to the AgeX Board.
From the execution of the term sheet through March 13, 2023, AgeX, Serina and certain of their respective advisors, including advisors from Juvenescence, participated in a number of telephone calls and video conferences to discuss the issuance of a $10 million loan from AgeX to Serina (the AgeX-Serina Loan) in order to provide financing to Serina in contemplation of a potential merger between AgeX and Serina, the terms and conditions proposed in the AgeX-Serina Loan transaction documents, due diligence matters and also exchanged due diligence materials. To provide the capital to fund the AgeX-Serina Loan, the parties discussed the issuance of a $10 million loan from Juvenescence to AgeX (the Juvenescence March Loan).
On January 11, 2023, Gibson Dunn sent the AgeX Board an initial draft of the AgeX-Serina Loan transaction documents. Between January 11, 2023 and January 20, 2023, Gibson Dunn, Messr. May, who at the time was a director of AgeX, and Messr. Pickett and Ms. Bergin, each of whom are employed by Juvenescence, discussed the AgeX-Serina Loan and Juvenescence loan transaction documents, which were revised through rounds of review and comment. The initial draft of the AgeX-Serina Loan transaction documents did not contain any restrictions on AgeX’s ability to enter into any strategic transactions with a party other than Serina.
On January 20, 2023, the AgeX-Serina Loan transaction documents were circulated to Bradley.
During February 2023, Bradley, Gibson Dunn, and Goodwin circulated proposed revisions on the AgeX-Serina Loan transaction documents.
On February 3, 2023, the AgeX Board met and discussed the status of the negotiations with Serina.
On February 27, 2023, Goodwin sent Thompson Welch an initial draft of the Juvenescence March Loan, which was based on the existing note between AgeX and Juvenescence. Between February 27, 2023 and March 13, 2023, Thompson Welch and Goodwin traded several drafts of the Juvenescence March Loan with AgeX’s primary issue being an ability to assign the AgeX-Serina Loan to Juvenescence in order to settle the Juvenescence March Loan if a potential merger between AgeX and Serina was not consummated.
On February 28, 2023 and periodically through August 2023, management of AgeX and certain of their advisors met to review diligence and timelines associated with the potential merger with Serina.
On March 2, 2023, Bradley sent Gibson Dunn a revised draft of each of the AgeX-Serina Loan transaction documents.
On March 3, 2023, Bradley, Gibson Dunn and Goodwin discussed on a video conference an issues list from Serina regarding the Serina convertible note, one of the primary AgeX-Serina Loan transaction documents, prepared by Serina’s management.
On March 5, 2023, Gibson Dunn sent revised drafts of the AgeX-Serina Loan transaction documents to members of AgeX management, which in turn shared it with the AgeX Board. After review by members of AgeX management, Gibson Dunn sent revised drafts of each of the AgeX-Serina Loan transaction documents to Bradley.
On March 7, 2023, members of AgeX management and Serina management met to discuss the AgeX-Serina Loan transaction documents.
On March 7, 2023, the AgeX Board sent Dr. West, the Chief Executive Officer of AgeX at the time, an amendment to his employment agreement and a new employment agreement in his contemplated role as Chief Executive Officer of Reverse Bio, each in preparation for the potential Reverse Bio financing. On April 3, 2023, Dr. West sent comments to such agreements to the AgeX Board. Between April 2023 and August 2023, the AgeX Board and Dr. West discussed revisions to the agreements on multiple occasions.
On March 8, 2023, Juvenescence informed AgeX that Juvenescence’s board of directors had approved the issuance of the Juvenescence March Loan to AgeX so that AgeX could use the proceeds thereof to issue the AgeX-Serina Loan.
On March 8, 2023, Bradley sent Gibson Dunn revised AgeX-Serina Loan transaction documents that were consistent with the management discussions on March 7, 2023, and a disclosure schedule for the Serina representations and warranties. AgeX management and Juvenescence noted to Gibson Dunn that they had no more comments on the AgeX-Serina Loan transaction documents, which in turn Gibson Dunn communicated to Bradley. The final draft of the AgeX-Serina Loan transaction documents did not have any restrictions on AgeX’s ability to enter into any strategic transactions with a party other than Serina.
On March 9, 2023, Dr. Hackett and Dr. May had separate, one-on-one calls with Mr. Pickett to review the outcome of scientific diligence on Serina. Later that day, Dr. West resigned from the AgeX Board, but informed AgeX that he would continue to serve as AgeX’s Chief Executive Officer.
On March 10, 2023, Dr. May met with Gibson Dunn to discuss the material terms of the AgeX-Serina Loan transaction documents.
On March 10, 2023, the AgeX Board and the Special Committee met to discuss the results of its due diligence efforts regarding Serina, including information concerning the financial condition, capitalization, technologies and intellectual property, research and development programs, and prospects of Serina, including Serina’s plans to advance certain therapy related products into clinical trials. The information considered by the AgeX Board included the findings of a panel of independent advisers who reviewed and evaluated the products being developed by Serina, including Serina’s POZ-apomorphine program SER-252, and a diligence summary of patentability of Serina technologies. The AgeX Board considered and discussed (i) the expected benefits and risks of the proposed merger, including a potential timeline related thereto, between AgeX and Serina on terms outlined the term sheet; (ii) the terms of the AgeX-Serina Loan documents; and (iii) the expected benefits and risks of making the $10 million loan to Serina on terms presented to the directors.
The AgeX Board considered the objectives of AgeX in obtaining the Juvenescence March Loan and providing the AgeX-Serina Loan, the financial risks associated with the Juvenescence March Loan and the AgeX-Serina Loan, and the potential impact of the Investment Company Act of 1940 on AgeX that might arise from the acquisition and holding of the AgeX-Serina Loan by means of the AgeX-Serina Note or shares of Serina capital stock. After the discussion, the AgeX Board determined that to mitigate those risks the Juvenescence March Loan should include the right of AgeX to pay off the financial obligations under the Juvenescence March Loan by tendering to Juvenescence the AgeX-Serina Note and any shares of Serina capital stock issued upon conversion of the AgeX-Serina Note into Serina capital stock. The AgeX Board instructed AgeX management to negotiate with Juvenescence a right of AgeX to assign the AgeX-Serina Loan to Juvenescence in full satisfaction of the Juvenescence March Loan by executing the 2023 Secured Note on the same day.
On March 10, 2023, the Serina Board met to consider and discuss (i) the terms of the AgeX-Serina Loan documents and (ii) the expected benefits and risks of the $10 million loan from AgeX. After discussion and deliberation, the Serina Board authorized Dr. Moreadith to execute the AgeX-Serina Loan documents with such additional changes as Dr. Moreadith deemed appropriate.
On March 13, 2023, Juvenescence agreed to the assignment of the AgeX-Serina Loan and, on March 14, 2023, the AgeX Board confirmed over electronic communication consent to the revised Juvenescence March Loan. Juvenescence and AgeX entered into the Juvenescence March Loan on the same day.
On March 15, 2023, AgeX and Serina entered into the AgeX-Serina Loan by executing the AgeX-Serina Note. In accordance with the AgeX-Serina Note, on March 15, 2023, Serina appointed Dr. Bailey, a director of the AgeX Board, to Serina’s Board. In the Form 8-K announcing the execution of the AgeX-Serina Note, AgeX noted that it agreed to borrow $10 million from Juvenescence pursuant to the 2023 Secured Note and to lend the loan proceeds of the 2023 Secured Note to Serina in order to provide financing to Serina in contemplation of AgeX’s corporate restructuring plans that include a potential merger between AgeX and Serina in which AgeX would be the surviving company. The Form 8-K specifically noted that “[n]o definitive agreement regarding a merger between AgeX and Serina has been negotiated or executed nor has the merger been approved by the respective boards of directors of AgeX and Serina. Further, a merger cannot be consummated unless approved by the stockholders of AgeX and Serina.” The Form 8-K also disclosed, in a section entitled “Restrictive Covenants,” that Serina was prohibited from soliciting and entering into any “material sale or transfer transactions outside the ordinary course of business, other than in a merger between AgeX and Serina,” but AgeX was not subject to any similar exclusivity provision with Serina in the AgeX-Serina Loan transaction documents or at any time prior to entering into the Merger Agreement. Additionally, the Form 8-K noted that AgeX’s restructuring plans also included a potential spinoff of Reverse Bio through a distribution of some or all of the shares of capital stock of Reverse Bio held by AgeX to AgeX stockholders which was contingent upon the successful completion of a potential financing of Reverse Bio through the sale of shares of Reverse Bio common stock to private investors.
On March 20, 2023, Gibson Dunn sent the AgeX Board an initial draft of the Merger Agreement and on March 21, 2023, Gibson Dunn sent the AgeX Board an initial draft of the Support Agreements, which are between Serina and certain AgeX stockholders in one form and AgeX and certain Serina stockholders on the other. Pursuant to each form, the AgeX stockholders and Serina stockholders would agree to, among other things, vote their shares to take all action necessary to consummate the Merger. Between March 20, 2023 and March 27, 2023, Gibson Dunn and members of AgeX management and the AgeX Board discussed the Merger Agreement and Support Agreements, which were revised through rounds of review and comment.
On March 27, 2023, Gibson Dunn sent Bradley an initial draft of the Merger Agreement that was largely consistent with the terms set forth in the term sheet. The initial draft of the Merger Agreement contemplated, among other items: (i) the Merger, (ii) no survival of the representations and warranties post-closing, (iii) listing of the shares of AgeX common stock issued to Serina equityholders as merger consideration on a national securities exchange following completion of the Merger, (iv) certain director designation rights, (v) the entry into certain ancillary agreements concurrently with the execution of the Merger Agreement, including the Support Agreements, (vi) issuance of the post-merger warrants and incentive warrants to pre-Closing AgeX stockholders, (vii) the potential transfer and restructuring of certain assets of AgeX pre-closing of the Merger, (viii) termination and reverse termination fees, and (ix) representations, warranties and other covenants customary for transactions of this type.
From the March 27, 2023 through the execution of the Merger Agreement, AgeX, Serina and certain of their respective advisors participated in a number of telephone calls and video conferences to discuss the Merger, the terms and conditions proposed in the Merger Agreement and ancillary agreements, due diligence matters and also exchanged due diligence materials.
On March 29, 2023, the AgeX Board, with Gibson Dunn present, discussed matters related to the Merger Agreement.
The directors also authorized the Special Committee to negotiate transaction termination fee provisions for the Merger Agreement and discussed the expected range of the amount of a termination fee as a percentage of deal value. The directors also discussed requesting Support Agreements and stock Lock-Up Agreements from the officers, directors, and largest stockholders of AgeX and from the officers, directors, and principal stockholders of Serina.
On April 6, 2023, Gibson Dunn sent Bradley initial drafts of the Support Agreements and Lock-Up Agreement, each of which had representations, warranties and other covenants customary for transactions of this type.
On April 11, 2023, AgeX and Serina executed an extension of AgeX’s exclusivity period, which was set to expire on April 11, 2023 pursuant to the term sheet, to negotiate the Merger with Serina through May 31, 2023.
On April 19, 2023, Ms. Hackett, Ms. Bergin and Gibson Dunn met to discuss the potential loss of financing for Reverse Bio as communicated to the AgeX Board by Dr. West and the impact on the NYSE American deadline of May 17, 2023 to cure certain listing deficiencies.
On April 20, 2023, AgeX received a letter (the 2023 Deficiency Letter) from the staff of the NYSE American indicating that AgeX did not meet certain of the NYSE American’s continued listing standards as set forth in Sections 1003(a)(i), (ii), and (iii) of the NYSE American Company Guide because AgeX had stockholders equity of less than (A) $2 million and had incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4 million and had incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6 million or more and had reported losses from continuing operations and/or net losses in its five most recent fiscal years. The 2023 Deficiency Letter informed AgeX that it remained subject to the conditions set forth in prior deficiency letters from the NYSE American and stated that if AgeX was not in compliance with the NYSE American’s stockholders equity standards, or did not make progress consistent with an agreed-upon plan to bring AgeX into compliance with the NYSE American’s continued listing standards, by May 17, 2023, then NYSE American would initiate delisting proceedings as appropriate.
On May 3, 2023, Ms. Bergin and Gibson Dunn met to discuss Dr. West’s progress towards the potential Reverse Bio financing in anticipation of the May 17, 2023 deadline for compliance by the NYSE American. AgeX corresponded with the NYSE American on several occasions from April 2023 through August 2023 regarding potential remediations to regain compliance under the continued listing standards.
On May 11, 2023, Bradley sent Gibson Dunn a revised draft of the Merger Agreement that proposed various revisions, most notably the notion that certain post-merger warrants held by major shareholders of the merged company would be callable at the company’s election. This was intended to provide financial runway for the newly merged company.
On May 17, 2023, AgeX received a notice from the NYSE American indicating that they intended to commence proceedings to delist AgeX common stock from the NYSE American.
On May 19, 2023, the AgeX Board authorized AgeX to request an appeal to the NYSE American. Thereafter, AgeX notified a committee of the board of directors of the NYSE American that it requested an appeal of such decision, and such request stayed any further action by the NYSE American pending a decision on the appeal. The appeal was scheduled for July 25, 2023.
On May 18, 2023, AgeX management, Juvenescence and certain of their advisors met via videoconference to discuss the timing of a potential reverse stock split. Initially, AgeX and Serina had planned to conduct a reverse stock split shortly after the merger with the intent of achieving a target trading price range for the post-merger AgeX common stock. However, Serina communicated that it believed that this approach would make it difficult to communicate the economics of the transaction to its shareholders and instead proposed to AgeX that it consider an approach of effecting a reverse stock split prior to the merger close based on a target share count of approximately 2.5 million shares of common stock outstanding immediately prior to the Merger. After the videoconference, AgeX management met with Serina management to confirm that AgeX would be willing to agree in principle to such reverse stock split.
On May 20, 2023, Ms. Hackett met with Dr. West to inform him that the AgeX Board was prepared to terminate his employment as Chief Executive Officer; however, the AgeX Board was willing to let Dr. West transition into the role of Chief Executive Officer of Reverse Bio if he could close the potential financing of Reverse Bio by June 20, 2023. The AgeX Board agreed to this solution in part because, once the potential Reverse Bio financing was complete, Reverse Bio would be spun off from AgeX into a separate entity, which Serina understood and agreed with. Ms. Hackett followed up the conversation with a written notice to Dr. West confirming their conversation.
On May 24, 2023, AgeX filed a request for a review of the delisting determination by a committee of the board of directors of the NYSE American. On May 31, 2023, AgeX received notice that a hearing with the board of directors of the NYSE American had been scheduled for July 25, 2023.
On May 31, 2023, AgeX and Serina executed an extension of AgeX’s exclusivity period to negotiate the Merger with Serina through June 30, 2023.
On June 7, 2023, Gibson Dunn sent Bradley a revised draft of the Merger Agreement that proposed various revisions, including revisions to the representations and warranties of AgeX and Serina, foregoing the two classes of post-merger warrants (callable and non-callable post-merger warrants) and instead introduced the Side Letter, pursuant to which (i) AgeX would have an option to call the post-merger warrants held by Juvenescence and (ii) Juvenescence would agree to cancel all out-of-the-money warrants prior to the Merger.
Between June 7, 2023 and June 16, 2023, AgeX and Serina management discussed updates to the transaction model, including revising the structure for incentive warrants and creating a side-agreement between Juvenescence and Serina that entitled Serina to call post-merger warrants held by Juvenescence. On June 13, 2023, Bradley called Gibson Dunn to discuss some of these changes.
On June 15, 2023, certain of AgeX’s advisors discussed potential plans for remediation of the listing standards; however, it was agreed that the Merger Agreement was unlikely to be executed prior to the June 22, 2023 deadline for submitting written responses for the appeal.
On June 16, 2023, Bradley sent Gibson Dunn a revised draft of the Merger Agreement which was further updated by a draft sent by Bradley to Gibson Dunn on June 19, 2023 that proposed various revisions, including revisions to the representations and warranties of AgeX and Serina, adding the reverse stock split as a closing condition, adding that the closing price of AgeX immediately prior to the Merger must be at least $12.00 as a closing condition in order to make the effect of anti-dilution privileges attached to some of Serina’s existing preferred shares predictable, adding that Juvenescence must hold at least one million post-merger warrants as a closing condition, adding termination provisions if the new closing conditions are not met and proposing $1 million in the aggregate as a termination fee, reverse termination fee and third party expense reimbursement.
On June 20, 2023, the AgeX Board met and agreed to extend the date for termination of Dr. West’s employment in order to close a potential financing of Reverse Bio. The deadline for termination of Dr. West’s employment was subsequently extended until October 31, 2023. Also during this meeting, Gibson Dunn provided an update on the status of the negotiations with Serina.
On June 22, 2023, Gibson Dunn sent Bradley an initial draft of the Side Letter. After delivery of the Side Letter, Mr. Pickett and Mr. Ledger discussed changes to the warrant structure over the next few weeks, including a tranche concept for the callable post-merger warrants, whereby the post-merger warrants held by Juvenescence must be exercised in a series of tranches, and revisions to the post-merger warrants and incentive warrants coverage and pricing that were favorable to AgeX shareholders.
On June 30, 2023, AgeX and Serina executed an extension of AgeX’s exclusivity period to negotiate the Merger with Serina through July 31, 2023.
On July 1, 2023, Gibson Dunn sent Bradley a revised draft of the Merger Agreement that proposed various revisions, including revisions to the representations and warranties of AgeX and Serina, changing the amount of post-merger warrants received by an AgeX stockholder from one post-merger warrants for each two shares to three post-merger warrants for each five shares, agreeing that Juvenescence will convert certain Juvenescence loans into shares of AgeX common stock prior to the reverse split in accordance with the Side Letter, adding that the terms of the AgeX-Serina Loan pursuant to the AgeX-Serina Note will be amended such that it converts into shares of AgeX common stock immediately prior to the Merger, changing the terms of the incentive warrants such that each incentive warrant is exercisable for one share of AgeX common stock at a price equal to 1.5 multiplied by the lower of the volume weighted average price of a share of AgeX common stock for the 60 day period following the closing of the Merger or the actual closing price as of the Closing Date.
On July 7, 2023, Bradley sent Gibson Dunn a revised draft of the Merger Agreement that proposed various revisions, including that Juvenescence will convert all the AgeX notes set forth in the Side Letter, adding the Combined Company Charter to be approved by the AgeX stockholders, adding amended and restated AgeX bylaws, increasing the amount of post-merger warrants held by Juvenescence as a closing condition, excluding Serina warrants from calculation of exchange ratio, and reducing the post-merger warrant multiple from 1.25 to 1.1. Additionally, Bradley noted that before Serina was willing to sign the Merger Agreement, Serina wanted to discuss further the structuring of the remaining assets in AgeX prior to closing of the Merger.
On July 7, 2023, Bradley sent Gibson Dunn a revised draft of the Side Letter that proposed various revisions, including having Juvenescence’s post-merger warrants automatically exercised on a certain schedule, conversion of Juvenescence loans to AgeX, and requiring unanimous approval of the AgeX Board to amend the Side Letter following the closing of the Merger. Gibson Dunn sent the Side Letter to Goodwin for Juvenescence’s review.
On July 8, 2023, the Serina Board called a special meeting of the Serina stockholders to be held on July 19, 2023.
On July 10, 2023, Gibson Dunn and Bradley met via videoconference to discuss treatment of certain compensation and benefits plans impacting all employees in the Merger and the structuring of the remaining assets in AgeX prior to the closing of the Merger. On July 12, 2023, Bradley sent Gibson Dunn a revised draft of the Merger Agreement addressing the compensation and benefits plans items the parties discussed.
On July 14, 2023, the Serina Board met to discuss the status of the negotiations of the Merger Agreement and the ancillary documents. Later that day, Bradley sent Gibson Dunn a proposed version of the Combined Company Charter.
On July 18, 2023, representatives of Juvenescence and Serina met to discuss the Side Letter, including amending the tranches for mandatory exercise of the warrants. Additionally, Bradley sent comments to AgeX’s disclosure schedules and included a due diligence request list.
Between July 17, 2023 and July 21, 2023, Thompson Welch, on behalf of AgeX, and Goodwin, on behalf of Juvenescence, negotiated an exchange agreement (or the 2023 Exchange Agreement) pursuant to which AgeX agreed to issue to Juvenescence 211,600 shares of a newly authorized AgeX Series A preferred stock and 148,400 shares of a newly authorized AgeX Series B preferred stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of certain loans made by Juvenescence to AgeX and certain loan origination fees accrued with respect to those loans. The exchange of the indebtedness for shares of AgeX Series A preferred stock and AgeX Series B preferred stock was implemented for the purpose of bringing AgeX common stock back into compliance with the continued listing requirements of the NYSE American that require AgeX to have at least $6 million of stockholders equity; however the continued listing remained dependent upon a determination by the NYSE American that AgeX has regained compliance with their listing standards.
On July 19, 2023, the stockholders of Serina met for an informational meeting regarding the status of the negotiations of the potential merger.
On July 20, 2023, AgeX management and its advisors met to discuss the appeal of the NYSE American delisting which was to occur on July 25, 2023. The meeting included discussions on whether it would be possible to sign the Merger Agreement prior to the appeal as an executed deal would help support the appeal. Gibson Dunn informed the group that the execution of the Merger Agreement on this timeline was unlikely to occur because Bradley had communicated that it would take several weeks to obtain the necessary Support Agreement signatures from Serina’s stockholders and Serina would not execute the Merger Agreement until these signatures were obtained. Additionally, Serina planned on seeking Support Agreements from its stockholders once the Merger Agreement was in final form. Nonetheless, the parties agreed that signing the Merger Agreement is an important step to showing long term compliance with NYSE American standards.
On July 20, 2023, Juvenescence sent Serina a revised draft of the Side Letter that proposed various revisions, including having Juvenescence’s post-merger warrants callable but not automatically exercised, revising the call right from two equal tranches to three equal tranches, and removing conversion of Juvenescence’s loans as a requirement since Juvenescence was entering into the 2023 Exchange Agreement and would be exchanging loans for AgeX preferred stock. Between July 20, 2023 and July 31, 2023, representatives of Juvenescence and Serina continued to discuss the changes to the Side Letter to reach agreement on the call feature of the warrants and the tranches.
On July 21, 2023, Bradley sent to Gibson Dunn a proposed amended and restated bylaws for the post-closing company.
On July 24, 2023, AgeX issued the AgeX preferred stock to Juvenescence in exchange for the cancellation of indebtedness as contemplated by the 2023 Exchange Agreement. Shortly thereafter, the NYSE American staff withdrew its delisting determination and cancelled the scheduled hearing of AgeX’s appeal.
On July 25, 2023, Gibson Dunn sent to the AgeX Board a draft of Dr. West’s transition and separation agreement, which included a requirement to secure financing for Reverse Bio by October 31, 2023 or his employment with Reverse Bio would terminate. On July 27, 2023, Ms. Hackett sent a draft of the transition and separation agreement to Dr. West. Dr. West and members of the AgeX Board negotiated the terms of transition and separation agreement through August 9, 2023.
On July 25, 2023, the Serina board met to approve the conversion of the outstanding convertible notes held by Serina stockholders in exchange for Serina’s Series A-5 preferred stock and a warrant to purchase additional share of Series A-5 preferred stock in an amount equal to the number of Series A-5 preferred stock received in the conversion.
On July, 27, 2023, all of the convertible notes held by Serina stockholders were converted into Serina’s Series A-5 preferred stock and warrants as approved by the Serina Board on July 25, 2023.
On July 27, 2023, Goodwin sent Gibson Dunn comments to the Merger Agreement, including removing the Juvenescence loan conversion covenant and updating the third party beneficiary language to address certain Juvenescence rights. Additionally, on July 27, 2023, Serina’s management team sent certain written questions on corporate restructuring plans to the AgeX management team.
On July 31, 2023, AgeX and Serina executed an extension of AgeX’s exclusivity period to negotiate the Merger with Serina through August 31, 2023.
On July 31, 2023, Goodwin sent Gibson Dunn comments to the Side Letter, including a provision automatically exercising the tranches of post-merger warrants and a covenant that the parties use reasonable best efforts to elect certain board designees that had been agreed upon between Serina, AgeX and Juvenescence. On July 31, 2023, Gibson Dunn sent Bradley Goodwin’s comments to the Side Letter, Merger Agreement and the Combined Company Charter. Additionally, Gibson Dunn sent Bradley additional comments on the Merger Agreement, including that Serina will use commercial reasonable efforts to obtain elections from each convertible note holder to convert such notes into share of Serina capital stock prior to the Merger, a required vote to remove the cap restricting conversion of the AgeX Series B preferred stock, revising the amount of post-merger warrants to be held by Juvenescence at closing to 1,000,000 warrants, and updating the expiration of the post-merger warrants to July 31, 2025.
On August 2, 2023, AgeX, Serina and Bradley had a call to discuss AgeX’s current operations and corporate restructuring plans. In addition, on August 3, 2023, AgeX responded to Serina’s written questions on such corporate restructuring plans. Bradley sent follow up questions on August 4, 2023, which Gibson Dunn responded to on August 5, 2023.
On August 3, 2023, Goodwin sent Gibson Dunn comments to the amended and restated bylaws to remove a provision that vacancies and newly created directorships will be filled exclusively by the board, and to add provisions providing that (i) a majority of voting stock can also fill vacant or newly created directorships, (ii) directors may be removed with or without cause, (iii) stockholders holding 10% or more of the voting shares can call special meetings, and (iv) removing supermajority vote requirements. Gibson Dunn sent these comments to Bradley on the same day.
On August 3, 2023, Goodwin and Bradley met by telephone to discuss the Combined Company Charter, the amended and restated bylaws, and the Side Letter.
On August 4, 2023, Gibson Dunn sent Bradley minor comments on the Lock-Up Agreement and Support Agreements. On August 5, 2023, Bradley confirmed that these agreements were in final form.
On August 5, 2023, Bradley sent Gibson Dunn comments to the Merger Agreement, Side Letter, Combined Company Charter and amended and restated bylaws. Bradley’s comments to the Combined Company Charter and amended and restated bylaws mainly restored Bradley’s prior comments. Bradley’s comments on the Merger Agreement include the creation of a new entity (or Newco) that will assume all liabilities of AgeX in existence as of the Effective Time, Juvenescence converting all AgeX preferred stock that it owns into AgeX common stock prior to the reverse stock split, adopting certain amendments to AgeX’s and Serina’s existing equity award stock plans, reserving 1,750,000 shares of AgeX common stock for the 2024 Equity Incentive Plan, revising the amount of post-merger warrants to be held by Juvenescence at closing to 1,113,308, and including a new closing condition and termination right that required AgeX to have $500,000 of nonrestricted cash immediately prior to the Effective Time. Bradley’s comments on the Side Letter included Juvenescence agreeing to convert AgeX preferred stock into AgeX common stock prior to the reverse stock split, and Juvenescence releasing security interests in all collateral other than upon the assets transferred to Newco. Gibson Dunn forwarded all the comments and revised documents to Goodwin.
On August 7, 2023, representatives of AgeX and Serina met to discuss the outstanding issues raised by recent transaction document drafts and timing of executing the Merger Agreement.
On August 8, 2023, Goodwin sent comments to Gibson Dunn on the Merger Agreement, Side Letter, Combined Company Charter and amended and restated bylaws. Goodwin’s comments on the Merger Agreement included reducing the closing condition and termination right related to nonrestricted cash in an amount equal to $500,000. Goodwin’s comments on the Side Letter included removing release language and updating the assignment language to permit a transfer of the rights and obligations in the Side Letter to an affiliate. Goodwin’s comments on the Combined Company Charter and amended and restated bylaws mainly restored Goodwin’s prior comments.
On August 9, 2023, the AgeX Board met and approved a Transition Services and Separation Agreement pursuant to which Dr. West stepped down as Chief Executive Officer of AgeX but agreed to continue to serve as Chief Executive Officer and as a director of Reverse Bio during a transition period. Following the board meeting, Dr. West and AgeX entered into that agreement. To address the vacancy in the office of Chief Executive Officer, the AgeX Board appointed Joanne Hackett as interim Chief Executive Officer and approved a compensation agreement with her for services in that role. Ms. Hackett stepped down from the Audit Committee and Compensation Committee. The directors, desiring to fill the vacancy on the board created when Dr. West resigned from the board earlier in the year, considered the qualifications of Jean-Christophe Renondin and the recommendation of the Nominating & Corporate Governance Committee and appointed Dr. Renondin to serve as a member of the AgeX Board.
Also at the August 9, 2023 AgeX Board meeting, Gibson Dunn provided an update on the status of the negotiations with Serina. The AgeX Board determined that no “majority of the minority” vote would be required to approve a merger transaction with Serina because Juvenescence has no equity interest in Serina. Additionally, Dr. Bailey decided to abstain from voting as a director on approval matters related to the Merger because of his position as a director of the Serina Board.
On August 9, 2023, Gibson Dunn sent comments to Bradley on the Merger Agreement, Side Letter, Combined Company Charter and amended and restated bylaws. Gibson Dunn’s comments on the Merger Agreement included minor edits to the representation and warranties in addition to Goodwin’s comments. Additionally, on the same date, Gibson Dunn sent over to Bradley a revised draft of the AgeX disclosure schedule and responses to diligence requests.
On August 10, 2023, Bradley and Goodwin met to discuss the outstanding issues raised by recent transaction document drafts. Reflecting discussions among the parties and their respective counsel, the parties agreed that the final form of the Combined Company Charter and amended and restated bylaws would be the version Bradley delivered on August 5, 2023, and the final form of the Side Letter would be the version Gibson Dunn sent on August 9, 2023. On the same day, Bradley sent comments to Gibson Dunn on the Merger Agreement including decreasing the reserve shares of AgeX common stock for the 2024 Equity Incentive Plan from 1,750,000 to 1,725,000, adding a solicitation covenant for Serina pursuant to Internal Revenue Code section 280G, and clarifying that the closing cash condition shall be net of AgeX’s transaction expenses.
On August 11, 2023, Bradley sent clean-up comments to the Merger Agreement and Side Letter fixing minor non-substantive issues identified by Gibson Dunn and Goodwin. On the same day, Bradley sent over a revised Serina disclosure schedule addressing most of Gibson Dunn’s prior comments.
On August 11, 2023, the Serina Board was provided with the draft Merger Agreement and ancillary agreements. On that same date, a board meeting was called for August 15, 2023, to review the Merger Agreement and the ancillary agreements.
On August 14, 2023, Gibson Dunn sent Bradley minor comments to the Serina disclosure schedules.
On August 15, 2023, Bradley sent Gibson Dunn comments on the schedules to the Merger Agreement and the Serina disclosure schedules.
On August 15, 2023, the Serina Board met to discuss the Merger Agreement and all ancillary agreements. Serina’s management and Bradley were also in attendance. While Dr. Bailey received notice of the meeting and all documents provided to the Serina Board in advance thereof, Dr. Bailey determined that it was in the best interest of the Serina Board, Serina, and the stockholders of Serina, to recuse himself from the Serina Board meeting so that the Serina Board could independently review and vote upon the proposed Merger Agreement, the ancillary documents thereto, and the various transactions contemplated thereby. Representatives of Bradley then reminded the Serina Board of its fiduciary duties under Alabama law in connection with a merger, which had been discussed with the Serina Board throughout the process. After further discussion and after reviewing and considering the Merger Agreement, the ancillary agreements and the transactions contemplated by each of those documents and after consulting with management and counsel, based on the factors cited in “—Serina Reasons for the Merger,” the Serina Board (other than Dr. Bailey who had voluntarily recused himself): (i) determined that the Merger Agreement, the ancillary agreements, and the contemplated transactions, including the Merger, are fair to, advisable and in the best interests of Serina and its stockholders; (ii) authorized, approved and declared advisable the Merger Agreement and the contemplated transactions, including the Merger; (iii) recommended, upon the terms and subject to the conditions set forth in the Merger Agreement, that the stockholders of Serina vote or consent to approve the matters required to be approved by the Merger Agreement; (iv) approved each of the ancillary agreements and the transaction contemplated thereby; and (v) authorized the officers to make such additional changes as they deemed appropriate in consultation with counsel. Also at that meeting, the Serina Board called a special meeting of the stockholders of Serina for August 25, 2023, to provide additional information to Serina’s stockholders regarding the Merger and the required documents to be executed in connection therewith.
On August 17, 2023, Gibson Dunn sent Bradley comments on the Serina disclosure schedules. Later in the day, Bradley sent Gibson Dunn comments on the Serina and AgeX disclosure schedules.
On August 18, 2023, Bradley sent Gibson Dunn additional comments and corrections to the Merger Agreement. Later in the day, Gibson Dunn and Bradley met to discuss the status of the transaction documents and timing of the Merger. Based on the timing of expected signatures and board approvals, Bradley and Gibson Dunn agreed to target August 28, 2023 to sign and announce the Merger Agreement.
On August 22, 2023, Gibson Dunn and AgeX met to discuss any remaining diligence and substantive issues in the transaction documents. Following the meeting, Gibson Dunn sent to Bradley comments on the Merger Agreement, the post-closing director and officer list, and the AgeX disclosure schedule. Gibson Dunn’s comments to the Merger Agreement included updating AgeX’s capitalization representation and non-substantive clean up edits. Bradley and Gibson Dunn confirmed to each other that all the transaction documents were in final form.
On August 24, 2023, the AgeX Board held a meeting by videoconference, at which members of AgeX management and representatives of Gibson Dunn were present. After addressing other unrelated matters, Dr. Bailey informed the other members of the AgeX Board that he was recusing himself from the remainder of the meeting as a result of being a director of Serina and left the videoconference. Representatives of Gibson Dunn communicated that the Merger Agreement, the Side Letter and all other ancillary documents associated with the proposed Merger with Serina were in final form, with no material changes to any of the previously communicated terms. Representatives of Gibson Dunn then reminded the AgeX Board of its fiduciary duties under Delaware law in connection with a merger. After further discussion, based on the factors cited in “—AgeX Reasons for the Merger,” the AgeX Board (other than Dr. Bailey): (i) determined that the Merger and the related transactions contemplated by the Merger Agreement are fair to, advisable and in the best interests of AgeX and its stockholders; (ii) approved and declared advisable the Merger Agreement and the related transactions contemplated by the Merger Agreement, including the issuance of shares of AgeX common stock in connection with the Merger; and (iii) recommended that AgeX’s stockholders vote in favor of the Merger Agreement and related AgeX Proposals.
On August 25, 2023, Serina held its stockholders meeting at which Serina’s management provided its stockholders with additional information regarding the Merger Agreement and the ancillary documents and answered questions from stockholders. After the meeting, the Serina management team began the process of collecting the Support Agreements and Lock-up Agreements from Serina’s stockholders.
Subsequently, on August 29, 2023, AgeX and Serina entered into the Merger Agreement. On August 30, 2023, before opening of the NYSE American for trading that day, AgeX and Serina issued a joint press release announcing the execution of the Merger Agreement. AgeX also filed a current report on Form 8-K with the SEC announcing, among other things, the execution of the Merger Agreement.
AgeX Reasons for the Merger
The AgeX Board, in consultation with its advisors, evaluated the terms of the Merger Agreement and the related transactions contemplated thereby and (i) determined that the Merger and the other related transactions contemplated by the Merger Agreement (collectively, the Contemplated Transactions) are fair to, advisable and in the best interests of AgeX and its stockholders; (ii) approved and declared advisable the Merger Agreement and the Contemplated Transactions, including the issuance of shares of AgeX common stock to the stockholders of Serina pursuant to the terms of the Merger Agreement; and (iii) recommends that the stockholders of AgeX vote to approve the AgeX Proposals.
In reaching its conclusion to approve the Merger Agreement, the Merger and the other Contemplated Transactions, and to recommend that the stockholders of AgeX vote to approve the AgeX Proposals, the AgeX Board considered a number of factors, including, but not limited to, the following (all of which the AgeX Board viewed as supporting its decision to approve the Merger with Serina), which are not necessarily presented in order of their relative importance to the AgeX Board:
| ● | the AgeX Board undertook a process of reviewing and analyzing potential strategic options, including remaining a standalone company pursuing AgeX’s pipeline of product candidates and technology platforms by exploring potential partnerships and seeking to develop more mature assets, seeking to raise independent financing for certain product candidates and disposing of certain assets, strategic mergers and acquisitions, including through a reverse merger, to identify the opportunity that would, in the AgeX Board’s opinion, create the most value for AgeX’s stockholders, and the AgeX Board’s belief, after such review of strategic alternatives and discussions with its advisors, that the Merger is more favorable to AgeX’s stockholders than the potential value that might have resulted from other strategic options available to AgeX; |
| ● | the prospects of and risks associated with the other strategic options that the AgeX Board had reviewed and analyzed with its advisors; |
| ● | the AgeX Board’s belief, as a result of arm’s length negotiations with Serina, that AgeX and its representatives negotiated the most favorable exchange ratio for AgeX’s stockholders to which Serina was willing to agree and that the terms of the Merger Agreement include the most favorable terms to AgeX in the aggregate to which Serina was willing to agree; |
| ● | the AgeX Board’s belief, following its review with its advisors and Serina management, that Serina’s product candidates have the potential to create meaningful value for stockholders of the combined company and an opportunity for AgeX’s pre-Merger stockholders to participate in the growth of the combined company based on the business, scientific, regulatory, intellectual property, financial, accounting and legal due diligence conducted by its advisors (which included numerous diligence calls and a comprehensive review of Serina’s due diligence materials) regarding: |
| ○ | the regulatory pathway for, and market opportunity of, Serina’s product candidates, including the stage of development of Serina’s product candidates; |
| ○ | the quality and scope of the clinical and preclinical results for Serina’s product candidates; |
| ○ | Serina’s plans for, among other things, advancing Serina’s lead drug candidate, SER-252 (POZ-apomorphine), for the treatment of advanced Parkinson’s Disease through preclinical studies towards the goal of an IND submission to the FDA for the initiation of a Phase I clinical trial; and |
| ○ | Serina having additional product candidates in preclinical studies, providing possible additional pathways to regulatory approval; and |
| ● | the AgeX Board’s view, following its review with its advisors and Serina management, of Serina’s current development and clinical trial plans, the expected operations, management structure, operating plans and cash burn rate of the combined company, and the expected cash resources of the combined company, including the impact of the additional capital expected to be provided to the combined company by Juvenescence through its exercise of post-merger warrants pursuant to the Side Letter, and the ability of such expected cash resources to support the combined company’s current and planned preclinical and clinical trials and operations; |
| ● | the AgeX Board’s consideration of the potential benefits of the Warrant Dividend to AgeX stockholders, including the potential for AgeX stockholders that receive post-merger warrants to increase their relative ownership of the combined company upon exercise of the post-merger warrants and incentive warrants; |
| ● | the AgeX Board’s consideration of the possibility that the combined company would be able to take advantage of the potential benefits resulting from becoming a public reporting company listed on NYSE American with Serina’s business to raise additional funds in the future; |
| ● | the AgeX Board’s view that the combined company will be led by (i) a management team with operational and drug development experience, and (ii) a board of directors with representation from each of the current AgeX Board and Serina Board; |
| ● | the financial condition and prospects of AgeX, including: |
| ○ | the risks associated with AgeX remaining a standalone company pursuing its pipeline of product candidates, preclinical programs and technology platforms, including the stage of development of AgeX’s assets, which, in the future, may not be successfully developed into products that are marketed and sold; and |
| ○ | the risks associated with the need to obtain substantial amounts of financing to continue its operations and to continue the development of its current programs if AgeX were to remain a standalone company. |
The AgeX Board also reviewed the terms and conditions of the Merger Agreement and the Contemplated Transactions, as well as the safeguards and protective provisions included therein intended to mitigate risks, including those described below, and concluded that the terms of the Merger Agreement and the Contemplated Transactions, in the aggregate, were reasonable and in the best interests of AgeX and its stockholders under the circumstances:
| ● | the calculation of the exchange ratio and the estimated number of shares of AgeX common stock to be issued in the Merger; |
| ● | the number and nature of the conditions to Serina’s and AgeX’ respective obligations to consummate the Merger, as more fully described below under the caption “The Merger Agreement—Conditions to the Completion of the Merger,” beginning on page 157 in this proxy statement/prospectus/information statement, and the risk of non-satisfaction of such conditions as well as the likelihood that the Merger will be consummated on a timely basis; |
| ● | the respective rights of, and limitations on, AgeX and Serina under the Merger Agreement to consider and engage in discussions regarding unsolicited acquisition proposals under certain circumstances should AgeX or Serina receive a superior offer, and the limitations on the board of directors of each party to change its recommendation in favor of the Merger, as more fully described below under the caption “The Merger Agreement—Non-Solicitation,” beginning on page 163 in this proxy statement/prospectus/information statement; |
| ● | the $1,000,000 termination fee, payable by AgeX to Serina upon the termination of the Merger Agreement in certain circumstances, the $1,000,000 termination fee payable by Serina to AgeX upon the termination of the Merger Agreement in certain circumstances and the up to $1,000,000 in expense reimbursement payable by AgeX to Serina or by Serina to AgeX, as applicable, in the event of a termination of the Merger Agreement in certain circumstances, as more fully described below under the caption “The Merger Agreement—Termination and Termination Fees” and “—Expenses,” beginning on pages 173 and 178, respectively, in this proxy statement/prospectus/information statement; |
| ● | the Support Agreements, pursuant to which, certain stockholders of AgeX and Serina have agreed, solely in their capacity as stockholders of AgeX and Serina, respectively, to vote all of their shares of AgeX common stock or Serina capital stock (or execute a written consent) in favor of the proposals submitted to them in connection with the Merger Agreement and the Contemplated Transactions, as more fully described below under the caption “Agreements Related to the Merger — Support Agreements,” beginning on page 179 in this proxy statement/prospectus/information statement; |
| ● | the agreement of Serina to mail the written consent of Serina’s stockholders necessary to adopt the Merger Agreement thereby approving the Merger and the other Contemplated Transactions within three business days of the registration statement becoming effective; |
| ● | the expectation that the Merger will qualify as a reorganization within the meaning of Section 368(a) of the Code, and will constitute a “plan of reorganization” within the meaning of Treasury Regulations Section 1.368-2(g), with the result that Serina stockholders will generally not recognize taxable gain or loss for U.S. federal income tax purposes upon the exchange of Serina common stock for AgeX common stock pursuant to the Merger Agreement, as more fully described below under the caption “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” beginning on page 148 in this proxy statement/prospectus/information statement; and |
| ● | the belief that the terms of the Merger Agreement, including the parties’ representations, warranties and covenants, and the conditions to their respective obligations, are reasonable and in the best interests of AgeX and its stockholders under the circumstances. |
In the course of its deliberations, the AgeX Board also considered a variety of risks and other countervailing factors related to entering into the Merger, which are not necessarily presented in order of their relative importance to the AgeX Board, including:
| ● | the $1,000,000 termination fee, payable by AgeX to Serina upon the termination of the Merger Agreement in certain circumstances, and the up to $1,000,000 in expense reimbursement payable by AgeX to Serina in the event of a termination of the Merger Agreement in certain circumstances, and the potential effect of the fees in deterring other potential acquirers from proposing an alternative transaction that may be more advantageous to AgeX’s stockholders; |
| ● | the prohibition on AgeX to solicit alternative acquisition proposals during the pendency of the Merger; |
| ● | the substantial expenses to be incurred in connection with the Merger, including the costs associated with any related litigation; |
| ● | the likelihood of disruptive stockholder litigation following announcement of the Merger; |
| ● | the possible volatility of the trading price of AgeX common stock resulting from the announcement, pendency or completion of the Merger; |
| ● | the risk that the Merger might not be consummated in a timely manner, or at all, and the potential adverse effect of the public announcement of the Merger or delay or failure to complete the Merger on the reputation of AgeX; |
| ● | the likely detrimental effect on AgeX’s cash position, stock price and ability to pursue other business and operating plans or to initiate another process and to successfully complete an alternative transaction should the Merger not be completed; |
| ● | the risk to AgeX’s business, operations and financial results in the event that the Merger is not consummated, including the diminution of AgeX’s cash and the significant challenges associated with the need to raise additional capital through the public or private sale of equity securities; |
| ● | the risk that the potential benefits of the Merger may not be fully achieved or may not be achieved within the expected timeframe; |
| ● | the early stage of development of Serina’s product candidates, which, in the future, may not be successfully developed into products that are marketed and sold; |
| ● | the scientific, technical, regulatory and other risks and uncertainties associated with development and commercialization of AgeX’s product candidates; |
| ● | the scientific, technical, regulatory and other risks and uncertainties associated with development and commercialization of Serina’s product candidates; |
| ● | various risks impacting the financial condition, results of operations and prospects for AgeX, including: |
| ○ | the risks and challenges associated with pursuing any strategic alternative to the Merger available to AgeX, including the discussions that its advisors and the AgeX Board previously conducted with other potential transaction partners, including discontinued negotiations of a reverse merger transaction in 2021, and the time to negotiate and complete an alternative strategic transaction and anticipated cash burn; |
| ○ | the risks associated with AgeX remaining a standalone company pursuing its pipeline of product candidates, preclinical programs and technology platforms, including the stage of development of AgeX’s assets, which, in the future, may not be successfully developed into products that are marketed and sold; |
| ○ | the risks associated with the need to obtain substantial amounts of financing to continue its operations and to continue the development of its current programs if AgeX were to remain a standalone company; and |
| ● | various other risks associated with the combined company and the Merger, including those described in the section titled “Risk Factors” and “Cautionary Statement Concerning Forward-Looking Statements” beginning on pages 25 and 125, respectively, of this proxy statement/prospectus/information statement. |
The foregoing information and factors considered by the AgeX Board are not intended to be exhaustive but are believed to include all of the material factors considered by the AgeX Board. In view of the wide variety of factors considered in connection with its evaluation of the Merger and the complexity of these matters, the AgeX Board did not find it useful, and did not attempt, to quantify, rank or otherwise assign relative weights to these factors. In considering the factors described above, individual members of the AgeX Board may have given different weight to different factors. The AgeX Board conducted an overall analysis of the factors described above, including thorough discussions with, and questioning of, it advisors, and considered the factors overall to be favorable to, and to support, its determination. In arriving at their respective recommendations, the members of the AgeX Board considered the interests of AgeX’s executive officers and directors as described under the caption “The Merger—Interests of the AgeX Directors and Executive Officers in the Merger.”
Serina Reasons for the Merger
The following discussion sets forth material factors considered by the Serina Board in reaching its determination to approve the terms and authorize the execution of the Merger Agreement for the purpose of implementing the Merger; however, it may not include all of the factors considered by the Serina Board. In light of the number and wide variety of factors considered in connection with its evaluation of the Merger Agreement, the Serina Board did not consider it practicable to, and did not attempt to, quantify or otherwise assign relative weights to the specific factors it considered in reaching its determination. The Serina Board viewed its position and determinations as being based on all of the information available and the factors presented to and considered by it. In addition, individual directors may have given different weight to different factors.
In the course of reaching its decision to approve the terms and authorize the execution of the Merger Agreement for the purpose of consummating the Merger, the Serina Board consulted with Serina’s senior management and legal counsel, reviewed a significant amount of information and considered a number of factors, including, among others:
| ● | historical and current information concerning Serina’s business, including its financial performance and condition, operations, management and competitive position; |
| ● | Serina’s prospects if it were to remain an independent privately held company, including its need to obtain additional financing and the terms on which it would be able to obtain such financing, if at all; |
| ● | the Serina Board’s belief that no alternatives to the Merger were reasonably likely to create greater value for Serina stockholders, after reviewing the various financing and other strategic options to enhance stockholder value that were considered by the Serina Board; |
| ● | the cash resources of the combined company expected to be available at the closing of the Merger and the anticipated burn rate of the combined organization; |
| ● | the broader range of investors to support the development of Serina’s product candidates than it could otherwise obtain if it continued to operate as a privately held company; |
| ● | the potential to provide its current stockholders with greater liquidity by owning stock in a public company; |
| ● | the expectation that the Merger with AgeX would be a more time- and cost-effective means to access capital than other options considered; |
| ● | the expectation that substantially all of Serina’s employees, particularly its management, will serve in similar roles at the combined organization; |
| ● | the fact that shares of AgeX common stock issued to Serina stockholders will be registered on a Form S-4 registration statement and will become freely tradable for Serina stockholders who are not affiliates of Serina and who are not parties to the Lock-Up Agreements; |
| ● | the Support Agreements, pursuant to which certain directors, officers and stockholders of AgeX and Serina, respectively, have agreed, solely in their capacity as stockholders of AgeX and Serina, respectively, to vote all of their shares of Serina capital stock or AgeX common stock in favor of the adoption or approval, respectively, of the Merger Agreement; |
| ● | the ability to obtain an NYSE American listing and comply with the NYSE American listing requirements; |
| ● | the terms and conditions of the Merger Agreement, including, without limitation, the following: |
| ○ | the expected relative percentage ownership of AgeX securityholders and Serina securityholders in the combined organization initially at the closing of the Merger and the implied valuation of Serina; |
| ○ | the parties’ representations, warranties and covenants and the conditions to their respective obligations; |
| ○ | the number and nature of the conditions of the obligation of AgeX to consummate the Merger; and |
| ○ | the likelihood that the Merger will be consummated on a timely basis. |
Serina’s Board also considered a number of uncertainties and risks in its deliberations concerning the Merger and the other transactions contemplated by the Merger Agreement, including the following:
| ● | the risk that the potential benefits of the Merger Agreement may not be realized; |
| ● | the risk that future sales of common stock by existing AgeX stockholders may cause the price of AgeX common stock to fall, thus reducing the value of the consideration received by Serina stockholders in the Merger; |
| ● | the termination fee of $1,000,000 and/or expense reimbursements of up to $1,000,000, payable by Serina to AgeX upon the occurrence of certain events, and the |
| ● | the price volatility of AgeX’s common stock, which may reduce the value of AgeX common stock that Serina stockholders will receive upon the closing of the Merger; |
| ● | the possibility that AgeX could under certain circumstances consider unsolicited acquisition proposals if superior to the Merger; |
| ● | the possibility that the Merger might not be completed for a variety of reasons, such as the failure of AgeX to obtain the required stockholder vote, and the potential adverse effect on the reputation of Serina and the ability of Serina to obtain financing in the future in the event the Merger is not completed; |
| ● | the risk that the Merger might not be consummated in a timely manner or at all; |
| ● | the expenses to be incurred in connection with the Merger and related administrative challenges associated with combining the organizations; |
| ● | the additional expenses that Serina’s business will be subject to as a public company following the closing of the Merger to which it has not previously been subject; and |
| ● | various other risks associated with the combined organization and the Merger, including the risks described in the section titled “Risk Factors” beginning on page 25. |
The Serina Board weighed the benefits, advantages and opportunities of a potential transaction against the uncertainties and risks described above, as well as the possible diversion of management attention for an extended period of time. After taking into account these and other factors, the Serina Board approved the terms and authorized execution of the Merger Agreement for the purpose of implementing the Merger.
Interests of the AgeX Directors and Executive Officers in the Merger
In considering the recommendation of the AgeX Board with respect to the issuance of AgeX common stock pursuant to the Merger Agreement and the other matters to be acted upon by AgeX’s stockholders at the AgeX special meeting, AgeX’s stockholders should be aware that certain members of the AgeX Board and current and former executive officers of AgeX have interests in the Merger that may be different from, or in addition to, interests they have as AgeX’s stockholders. These interests may present them with actual or potential conflicts of interest.
Treatment of Equity Awards
Each of AgeX’s named executive officers and non-employee directors holds vested and unvested equity awards. For information regarding the treatment of AgeX’s named executive officers and non-employee directors stock options, see the section titled “The Merger Agreement—Treatment of AgeX Stock Options” beginning on page 156 of this proxy statement/prospectus/information statement.
Employment Agreement and Change of Control Provisions
AgeX is party to an employment agreement with Andrea E. Park that provides change of control and severance benefits to Ms. Park upon termination of her employment by AgeX without “cause” or by Ms. Park for “good reason” (each as defined in her employment agreement) following a change of control. If, within 12 months following a change of control of AgeX (which would include the Merger), Ms. Park’s employment is terminated by AgeX or Serina without “cause” or Ms. Park resigns for “good reason,” then Ms. Park would be entitled to receive, subject to signing and not revoking a release:
| ● | full payment of Ms. Park’s pro-rated target bonus due for such year; |
| ● | severance in an amount equal to nine months of Ms. Park’s base salary; |
| ● | for a period of six months, all benefits under any health insurance plan of AgeX under which Ms. Park participated in prior to termination; |
| ● | all of Ms. Park’s unvested options and restricted stock units, if any, will become fully vested and exercisable immediately; and |
| ● | with respect to any outstanding vested but unexercised options, the exercise period following termination or resignation will be extended to the earlier of (A) 12 months after termination or (B) the natural expiration date of the applicable option. |
Under the Transition Agreement, described below in the section titled “AgeX Executive and Director Compensation—Employment Agreements and Change of Control Provisions” beginning on page 141 of this proxy statement/prospectus/information statement, Michael D. West is no longer eligible to receive severance payments or other benefits in connection with a change in control under his employment agreement.
On June 4, 2021, the Compensation Committee of the AgeX Board approved by way of a meeting (the June 4, 2021 Compensation Committee Meeting) certain acceleration rights of the equity awards held by Nafees N. Malik that mirrors the acceleration rights of Ms. Park in connection with a change of control. Like Ms. Park, if Mr. Malik is terminated by AgeX without “cause” or resigns for “good reason” (each as defined in the June 4, 2021 Compensation Committee Meeting) within 12 months following a change of control of AgeX (which would include the Merger), then Mr. Malik would be entitled to, subject to signing and not revoking a release, the full vesting of all of Mr. Malik’s unvested options and restricted stock units (if any), which will become exercisable immediately. Further, with respect to any outstanding vested but unexercised options held by Mr. Malik, the exercise period following termination or resignation will be extended to the earlier of (A) 12 months after termination or (B) the natural expiration date of the applicable option.
The AgeX Board was aware of these potential conflicts of interest and considered them, among other matters, in reaching their respective decisions to approve the Merger Agreement and the Merger, and to recommend, as applicable, that AgeX’s stockholders approve the proposals to be presented to AgeX’s stockholders for consideration at the AgeX special meeting as contemplated by this proxy statement/prospectus/information statement.
Ownership Interests
As of September 12, 2023, AgeX’s non-employee directors and executive officers beneficially owned, in the aggregate, approximately 16,489,966 shares of AgeX common stock, which for purposes of this subsection (i) excludes any shares of AgeX common stock issuable upon exercise or settlement of AgeX options held by such individual and (ii) includes shares of AgeX common stock held by an affiliate of a non-employee director other than shares of AgeX common stock issuable upon exercise, settlement or conversion of AgeX warrants, outstanding loans or preferred stock held by such affiliate. As of September 12, 2023, Juvenescence, an AgeX stockholder affiliated with one of AgeX’s non-employee directors, beneficially owned 211,600 of the shares of AgeX Series A preferred stock and 148,400 of the shares of AgeX Series B preferred stock.
The affirmative vote of the holders of a majority of AgeX common stock, the holders of a majority of AgeX Series A preferred stock and the holders of a majority of AgeX Series B preferred stock entitled to vote at the AgeX special meeting, each voting as a separate class, is required for the approval of Proposal No. 4 (the A&R Charter Proposal). The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote on the matter is required for the approval each of Proposal Nos. 1, 2, 3, 5 and 6 (the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the 2024 Equity Incentive Plan Proposal and the Adjournment Proposal).
As of August 29, 2023, Juvenescence, an AgeX stockholder affiliated with one of AgeX’s non-employee directors holding approximately 43.3% of the outstanding shares AgeX common stock, 100% of the outstanding shares of AgeX Series A preferred stock and 100% of the outstanding shares of AgeX Series B preferred stock, has entered into the AgeX Support Agreement in connection with the Merger. For a more detailed discussion of the Support Agreements, please see the section titled “Agreements Related to the Merger” beginning on page 181 of this proxy statement/prospectus/information statement.
AgeX Stock Options
As of September 12, 2023, AgeX’s named executive officers and directors collectively owned unvested stock options to purchase 200,614 shares of AgeX common stock and vested stock options to purchase 2,451,741 shares of AgeX common stock, for a total of options to purchase 2,652,355 shares of AgeX common stock. All outstanding and unexercised options to purchase shares of AgeX common stock will remain effective and outstanding.
The following table presents certain information concerning the outstanding AgeX stock options held by each of AgeX’s named executive officers and directors as of September 12, 2023:
| Name | | Grant Date | | Option Expiration Date | | Option Exercise Price | | | Number of Vested Shares of Common Stock Underlying Options as of September 12, 2023 | | | Number of Unvested Shares of Common Stock Underlying Options as of September 12, 2023 | |
| Michael D. West | | 6/4/2021 | | 6/3/2031 | | $ | 1.45 | | | | 67,500 | | | | 52,500 | |
| | | 3/11/2019 | | 3/10/2029 | | $ | 4.28 | | | | 100,000 | | | | - | |
| | | 10/18/2018 | | 10/17/2028 | | $ | 3.00 | | | | 500,000 | | | | - | |
| | | 10/10/2017 | | 10/9/2027 | | $ | 2.00 | | | | 660,000 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Andrea E. Park | | 6/4/2021 | | 6/3/2031 | | $ | 1.45 | | | | 42,187 | | | | 32,813 | |
| | | 5/21/2020 | | 5/20/2030 | | $ | 0.738 | | | | 243,750 | | | | 56,250 | |
| | | 10/1/2019 | | 9/30/2029 | | $ | 1.77 | | | | 19,583 | | | | 417 | |
| | | | | | | | | | | | | | | | | |
| Nafees N. Malik | | 6/4/2021 | | 6/3/2031 | | $ | 1.45 | | | | 42,187 | | | | 32,813 | |
| | | 3/11/2019 | | 3/10/2029 | | $ | 4.28 | | | | 70,000 | | | | - | |
| | | 10/18/2018 | | 10/17/2028 | | $ | 3.00 | | | | 350,000 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Gregory H. Bailey | | 6/21/2021 | | 6/20/2031 | | $ | 1.48 | | | | 65,000 | | | | - | |
| | | 3/11/2019 | | 3/10/2029 | | $ | 4.28 | | | | 100,000 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Joanne M. Hackett | | 2/2/2022 | | 2/1/2032 | | $ | 0.8336 | | | | 65,000 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Michael H. May | | 6/21/2021 | | 6/20/2031 | | $ | 1.48 | | | | 100,000 | | | | - | |
| | | 8/5/2019 | | 8/4/2029 | | $ | 2.40 | | | | 26,534 | | | | - | |
| | | | | | | | | | | | | | | | | |
| Jean-Christophe Renondin | | 8/9/2023 | | 8/8/2033 | | $ | 0.76 | | | | - | | | | 25,821 | |
Indemnification and Insurance
For a discussion of the indemnification and insurance provisions related to the AgeX directors and officers under the Merger Agreement, please see the section titled, see the section titled “The Merger Agreement—Other Agreements—Indemnification and Insurance.”
Management Following the Merger
As described elsewhere in this proxy statement/prospectus/information statement, including in the section titled “Management Following the Merger,” upon the closing of the Merger, Gregory H. Bailey, M.D., a member of the AgeX Board, will serve on the Combined Company Board and Andrea E. Park, AgeX’s Chief Financial Officer, will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the combined company.
Interests of the Serina Directors and Executive Officers in the Merger
In considering the recommendation of the Serina Board with respect to approving the Merger, Serina stockholders should be aware that certain members of the Serina Board and executive officers of Serina have interests in the Merger that may be different from, or in addition to, interests they have as Serina stockholders. All of Serina’s executive officers and directors have options to purchase shares of Serina capital stock that will be converted into and become options to purchase shares of AgeX common stock. Certain of Serina’s directors and executive officers are expected to become directors and executive officers of the combined organization upon the closing of the Merger and all of Serina’s directors and executive officers are entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement.
Ownership Interests
Certain of Serina’s directors and executive officers currently hold shares of Serina capital stock. The table below sets forth the anticipated ownership of Serina capital stock by Serina’s directors and executive officers immediately prior to the closing of the Merger based on their ownership of Serina’s capital stock as of November 5, 2023.
| Directors and Executive Officers | | Number of Shares of Serina Capital Stock Held Immediately Prior to Closing(1) | |
| Randall Moreadith | | | — | |
| Michael D. Bentley | | | 500,000 | |
| Barbara M. Fisk | | | 509,993 | |
| Tacey Viegas | | | 60,000 | |
| James R. Hudson, Jr. | | | 212,000 | |
| Steve Ledger | | | — | |
| J. Milton Harris | | | — | |
| Gregory Bailey | | | — | |
| (1) | The number of shares of Serina capital stock (i) excludes any shares of Serina common stock issuable upon exercise or settlement of Serina options held by such individual, (ii) includes shares of Serina common stock held by an affiliate of a non-employee director other than shares of Serina common stock issuable upon exercise, settlement or conversion of Serina warrants and (iii) assumes a 1 to 1 conversion of Serina preferred stock to Serina common stock. The actual number of shares of Serina common stock that a holder of Serina preferred stock may receive upon conversion the conversion of a share of Serina preferred stock may be different. |
Serina Stock Options
Under the terms of the Merger Agreement, at the Effective Time, each outstanding and unexercised Serina option immediately prior to the Effective Time under the Serina Plan will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option.
The following table presents certain information concerning the outstanding Serina options held by Serina’s directors and current executive officers, as of November 5, 2023:
| Optionholder Name | | Grant Date | | Expiration Date | | Exercise Price | | | Number of Shares of Serina Common Stock Underlying Option as of November 5, 2023 | | | Number of Shares of Serina Common Stock Underlying Option Vested as of November 5, 2023 | |
| Randall Moreadith | | 5/6/2021 | | 5/6/2031 | | $ | 0.06 | | | | 533,550 | | | | 533,550 | |
| | | 7/29/2021 | | 7/29/2021 | | $ | 0.06 | | | | 96,000 | | | | 96,000 | |
| Michael D. Bentley | | 5/6/2021 | | 5/6/2031 | | $ | 0.06 | | | | 36,000 | | | | 36,000 | |
| | | 7/29/2021 | | 7/29/2021 | | $ | 0.06 | | | | 36,000 | | | | 36,000 | |
| Tacey Viegas | | 5/6/2021 | | 5/6/2031 | | $ | 0.06 | | | | 208,392 | | | | 208,392 | |
| | | 7/29/2021 | | 7/29/2021 | | $ | 0.06 | | | | 141,608 | | | | 141,608 | |
| Steve Ledger | | 7/29/2021 | | 7/29/2031 | | $ | 0.06 | | | | 116,666 | | | | 116,666 | |
| J. Milton Harris | | 5/6/2021 | | 5/6/2031 | | $ | 0.06 | | | | 36,000 | | | | 36,000 | |
| | | 7/29/2021 | | 7/29/2021 | | $ | 0.06 | | | | 36,000 | | | | 36,000 | |
Serina Warrants
As of November 5, 2023, Serina’s named executive officers and directors and such directors affiliates held warrants exercisable into 34,616 shares of Serina common stock. Under the terms of the Merger Agreement, at the Effective Time, each outstanding and unexercised Serina warrant immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Management Following the Merger
As described elsewhere in this proxy statement/prospectus/information statement, including in the section captioned “Management Following the Merger,” certain of Serina’s directors and executive officers are expected to become the directors and executive officers of the combined company upon the closing of the Merger.
Indemnification and Insurance
For a discussion of the indemnification and insurance provisions related to the Serina directors and officers under the Merger Agreement, please see the section titled, see the section titled “The Merger Agreement—Other Agreements—Indemnification and Insurance.”
Form of Merger
Subject to the terms and conditions of the Merger Agreement, and in accordance with Alabama law, at the completion of the Merger, Merger Sub, an Alabama corporation and wholly owned subsidiary of AgeX, will merge with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX.
Merger Consideration and Exchange Ratio
Pursuant to the Merger Agreement, at the Effective Time, each outstanding share of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of AgeX common stock equal to the exchange ratio. The exchange ratio is currently estimated to be equal to approximately 0.83217216 shares of AgeX common stock for each share of Serina common stock, which estimated exchange ratio assumes (i) the Actual Closing Price of a share of AgeX common stock is equal to $12.00 per share (on a post-reverse stock split basis), (ii) the number of Company Outstanding Shares (as defined below) is equal to 9,012,558, (iii) the number of Company Merger Shares (as defined below) is equal to 7,500,000 and (iv) the implementation of the reverse stock split and the AgeX preferred stock conversion prior to the consummation of the Merger, as described elsewhere in this proxy statement/prospectus/information statement. There can be no assurance any of these assumptions will be accurate when the final exchange ratio is determined.
No fractional shares of AgeX common stock will be issued in connection with the Merger, and no certificates or scrip for any such fractional shares will be issued. Any holder of Serina capital stock who would otherwise be entitled to receive a fraction of a share of AgeX common stock (after aggregating all fractional shares of AgeX common stock issuable to such holder) will receive from AgeX, in lieu of such fraction of a share and upon such holder’s surrender of a letter of transmittal in accordance with the Merger Agreement and any accompanying documentation required therein, (i) one share of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina capital stock would otherwise be entitled to is equal to or exceeds 0.50 or (ii) no shares of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina capital stock would otherwise be entitled to is less than 0.50.
The exchange ratio formula is based upon a fixed percentage of the post-Merger outstanding shares of AgeX common stock, expressed on a fully-diluted and as-converted basis, subject to certain adjustments and exclusions.
For a more complete description of the Merger, the potential adjustments in the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
Procedures for Exchanging Stock Certificates
Prior to the Closing Date, AgeX and Serina will select an exchange agent and, at the Effective Time, AgeX will deposit with the exchange agent evidence of book-entry shares representing the shares of AgeX common stock issuable pursuant to the terms of the Merger Agreement in exchange for shares of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares).
Promptly after the Effective Time, the exchange agent will mail to each record holder of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) (i) a letter of transmittal and (ii) instructions for effecting the surrender of the record holder’s stock certificates or uncertificated shares of capital stock in exchange for the merger consideration. Upon delivery to the exchange agent of a duly executed letter of transmittal in accordance with the exchange agent’s instructions and the declaration for tax withholding purposes, the surrender of the record holder’s stock certificates or other reasonable evidence of the ownership of uncertificated capital stock and delivery to the exchange agent of such other documents as may be reasonably required by the exchange agent or AgeX, the record holder of such stock certificates or book-entry shares, as applicable, will be entitled to receive in exchange therefor book-entry shares representing the number of whole shares of AgeX common stock issuable to such holder pursuant to the Merger Agreement. The surrendered certificates representing shares of Serina capital stock will be canceled.
After the Effective Time, each certificate representing Serina capital stock that has not been surrendered will represent only the right to receive shares of AgeX common stock issuable pursuant to the Merger Agreement to which the holder of any such certificate is entitled.
Effective Time of the Merger
The Merger will become effective on the date and at the time the Statement of Merger has been duly filed with the Secretary of State of the State of Alabama or at such later time as may be specified in the Statement of Merger with the consent of AgeX and Serina in accordance with the ABCL.
Regulatory Approvals
In the United States, AgeX must comply with applicable federal and state securities laws and the rules and regulations of the NYSE American in connection with the issuance of shares of AgeX common stock to Serina’s stockholders in connection with the Contemplated Transactions and the filing of this proxy statement/prospectus/information statement with the SEC. AgeX does not intend to seek any regulatory approval from antitrust authorities to consummate the Contemplated Transactions.
Material U.S. Federal Income Tax Consequences of the Merger
The following discussion summarizes material U.S. federal income tax consequences of the Merger that are generally expected to be applicable to Serina stockholders who are U.S. holders (as defined below), assuming the Merger is consummated as contemplated herein. This summary is based on current law, including the provisions of the Code, Treasury Regulations promulgated thereunder, judicial authority, and administrative rulings and practice, each as in effect as of the date of this proxy statement/prospectus/information statement and all of which are subject to change, possibly with retroactive effect.
This summary is not a complete description of all tax consequences of the Merger and does not address U.S. federal income tax consequences that may be relevant to particular Serina stockholders in light of their personal circumstances or to Serina stockholders who are subject to special treatment under U.S. federal income tax laws, such as Serina stockholders who: do not hold their Serina capital stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment); are banks, insurance companies, tax-exempt entities, mutual funds, financial institutions, real estate investment trusts, regulated investment companies, government entities or broker-dealers; hold their Serina capital stock as “qualified small business stock” under Section 1202 of the Code or as “Section 1244 stock” under Section 1244 of the Code; hold their Serina capital stock as part of a hedging, “straddle,” conversion or other integrated transaction or are treated as having sold their Serina stock pursuant to the constructive sale provisions of the Code; are not U.S. holders; acquired their Serina capital stock pursuant to the exercise of compensatory options, or in other compensatory transactions; acquired their Serina capital stock pursuant to the exercise of warrants or conversion rights under convertible instruments; are subject to special tax accounting rules under Section 451(b) of the Code; hold their Serina capital stock through individual retirement or other tax-deferred accounts; acquired their Serina capital stock in a transaction subject to the gain rollover provisions of Section 1045 of the Code; have a functional currency other than the U.S. dollar; or are partnerships or entities or arrangements classified as partnerships or disregarded entities for U.S. federal income tax purposes, S corporations, or other pass-through entities (including hybrid entities) and investors therein.
In addition, this summary does not address (i) the tax consequences of the Merger under U.S. federal non-income tax law (including estate, gift, or other non-income taxes), (ii) the tax consequences of the Merger under state, local or non-U.S. tax laws, (iii) the impact of the alternative minimum tax provisions of the Code (including the 15% minimum tax applicable to the adjusted financial statement income of certain corporations) or the Medicare contribution tax on net investment income, (iv) the tax consequences of transactions effectuated before, subsequent to or concurrently with the Merger (whether or not any such transactions are consummated in connection with the Merger), including, without limitation, any transaction in which shares of Serina capital stock are acquired, (v) the tax consequences to holders of Serina stock options, Serina warrants or similar rights to acquire Serina capital stock, or (vi) the tax consequences of the Merger to Serina stockholders who exercise appraisal or appraisal rights.
If a partnership or other entity or arrangement taxable as a partnership for U.S. federal income tax purposes holds Serina capital stock, the tax treatment of a partner in such partnership will generally depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partner should consult its tax advisors as to the tax consequences of the Merger.
This summary is limited to beneficial owners of Serina stockholders who are U.S. holders. A U.S. holder is a beneficial owner of Serina capital stock that is for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
| ● | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| ● | a trust with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of its substantial decisions, or an electing trust that was in existence on August 19, 1996 and was treated as a domestic trust on that date. |
No opinions of counsel or rulings from the Internal Revenue Service (IRS) have been requested or obtained in connection with the Merger. Accordingly, all Serina stockholders should consult their own tax advisors as to the specific tax consequences to them of the Merger, including applicable tax reporting requirements.
IN VIEW OF THE FOREGOING AND BECAUSE THE FOLLOWING DISCUSSION IS INTENDED AS A GENERAL SUMMARY ONLY, EACH SERINA STOCKHOLDER SHOULD CONSULT SUCH STOCKHOLDER’S OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES, INCLUDING THE APPLICABLE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES, AND ANY TAX REPORTING REQUIREMENTS OF THE MERGER AND RELATED TRANSACTIONS IN LIGHT OF SUCH STOCKHOLDER’S OWN TAX SITUATION.
Treatment of U.S. Holders in the Merger
Each of AgeX and Serina intends the Merger to qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Assuming the Merger so qualifies, U.S. holders will not recognize gain or loss upon the exchange of their Serina capital stock for AgeX common stock. U.S. holders will obtain a basis in the AgeX common stock they receive in the Merger equal to their basis in the Serina capital stock exchanged therefor. The holding period of the shares of AgeX common stock received by a U.S. holder in the Merger will include the holding period of the shares of Serina capital stock surrendered in exchange therefor.
If the Merger does not qualify as a reorganization within the meaning of Section 368(a) of the Code, then each U.S. holder would be treated as exchanging its Serina capital stock in a fully taxable transaction in exchange for AgeX common stock. U.S. holders generally would recognize capital gain or loss in such exchange equal to the difference between (i) the fair market value of the AgeX common stock received in the Merger and (ii) such holder’s tax basis in the Serina capital stock surrendered in the Merger. The aggregate tax basis of a U.S. holder in the AgeX common stock received in the Merger would equal the fair market value at the Effective Time of the U.S. holder’s Serina capital stock exchanged in the Merger, and the holding period of AgeX common stock received in the Merger would begin on the day after the Effective Time of the Merger.
For purposes of the above discussion of the bases and holding periods for shares of Serina capital stock acquired by U.S. holders at different times for different prices, such U.S. holders must calculate their gains and losses and holding periods separately for each identifiable block of such stock exchanged in the Merger.
U.S. holders are urged to consult their tax advisors regarding the U.S. federal income tax consequences of the Merger in light of their personal circumstances and the consequences to them under state, local and non-U.S. tax laws and other federal tax laws.
Information Reporting
Each U.S. holder who receives shares of AgeX common stock in the Merger is required to retain permanent records pertaining to the Merger and make such records available to any authorized IRS officers and employees. Such records should specifically include information regarding the amount, basis, and fair market value of all transferred property, and relevant facts regarding any liabilities assumed or extinguished as part of such reorganization. U.S. holders who owned immediately before the Merger at least one percent (by vote or value) of the total outstanding stock of Serina are required to attach a statement to their tax returns for the year in which the Merger is consummated that contains the information listed in Treasury Regulation Section 1.368-3(b). Such statement must include the U.S. holder’s tax basis in such U.S. holder’s Serina capital stock surrendered in the Merger, the fair market value of such Serina capital stock, the date of the Merger and the name and employer identification number of each of Serina and AgeX. U.S. holders are urged to consult with their tax advisors to comply with these rules.
This summary does not take into account your particular circumstances and does not address consequences that may be particular to you. Therefore, you should consult your tax advisor regarding the particular consequences of the Merger to you.
NYSE American Listing
AgeX common stock currently is listed on the NYSE American under the symbol “AGE.” AgeX has agreed to use commercially reasonable efforts (i) to maintain its existing listing on the NYSE American until the Effective Time and obtain approval of the listing of the combined company on the NYSE American, (ii) to the extent required by the rules and regulations of the NYSE American, to prepare and submit to the NYSE American market a notification for the listing of the shares of AgeX common stock to be issued in connection with the Merger, and to cause such shares to be approved for listing (subject to official notice of issuance), (iii) to effect the reverse stock split, and (iv) to the extent required by the NYSE American market rules, to file an initial listing application for the AgeX common stock on the NYSE American market and to cause the listing application to be conditionally approved prior to the Effective Time.
In addition, under the Merger Agreement, each of Serina’s and AgeX’s obligation to complete the Merger is subject to the satisfaction or waiver by each of the parties, at or prior to the Merger, of various conditions, including that the shares of AgeX common stock to be issued in the Merger have been approved for listing on the NYSE American market as of the closing of the Merger. Both AgeX and Serina have agreed to use commercially reasonable efforts to coordinate with respect to compliance with the NYSE American rules and regulations. AgeX has agreed to pay all fees associated with the listing application. Serina has agreed to cooperate with AgeX as reasonably requested by AgeX with respect to the listing application and promptly furnish AgeX all information concerning Serina and its members that may be required or reasonably requested in connection with any action contemplated by the listing agreement.
If the NYSE American Listing Application is accepted, AgeX anticipates that the common stock of the combined company will be listed on the NYSE American following the closing of the Merger under the trading symbol “SER.”
Anticipated Accounting Treatment
The Merger will be treated by AgeX as a reverse recapitalization under GAAP. For accounting purposes, Serina is considered to be the accounting acquirer in this transaction.
Appraisal Rights
AgeX
Under the DGCL, AgeX stockholders are not entitled to appraisal rights in connection with the Merger.
Serina
Holders of Serina capital stock as of the record date are entitled to appraisal rights under the ABCL. Pursuant to Section 10A-2A-13.02 of the ABCL, a Serina stockholder who does not wish to accept the merger consideration to be received pursuant to the terms of the merger agreement may elect to receive the fair value of such stockholder’s Serina capital stock (as determined immediately prior to the consummation of the merger), excluding any appreciation or depreciation in anticipation of the merger unless exclusion would be inequitable, but including interest from the effective date of the merger until the date of payment. Under the terms of the merger agreement, if 5% or more of the outstanding shares of Serina capital stock validly exercise their appraisal rights, then AgeX will not be obligated to complete the merger.
In order to exercise appraisal rights, a Serina stockholder must strictly comply with the statutory procedures of Sections 10A-2A-13.01 through 10A-2A-13.40 of the ABCL, which are summarized below. A copy of the full text of those Sections is included as Annex D to this proxy statement/prospectus/information statement. Serina stockholders are urged to read Annex D in its entirety and to consult with their legal advisors. Each Serina stockholder who desires to assert appraisal rights is cautioned that failure to adhere strictly to the requirements of Alabama law in any regard will cause a forfeiture of any appraisal rights. Serina stockholders who do not properly follow appraisal rights procedures will receive the merger consideration if the merger is effected.
Procedures for Exercising Rights of Appraisal. The following summary of Alabama law is qualified in its entirety by reference to the full text of the applicable provisions of the ABCL, a copy of which is included as Annex D to this proxy statement/prospectus/information statement.
A holder of Serina capital stock electing to exercise its appraisal rights shall not sign and return the written consent adopting the Merger Agreement approve the transactions and actions contemplated by the Merger Agreement, including the Merger.
No later than ten days after the merger, Serina, as the continuing corporation of the merger, will send a written notice to each stockholder exercising its appraisal rights who did not vote in favor of the merger and who duly filed a written notice of intent to demand payment in accordance with the foregoing procedures. The appraisal rights notice will specify, among other things, the deadline by which time Serina must receive a payment demand from such stockholders, the deadline for depositing the stockholders’ shares and the location for such deposit, the deadline by which time such stockholder may withdraw from the appraisal process, and Serina’s estimate of the fair value of the stock, and will include a form for demanding payment. The deadline for demanding payment will be no fewer than 40 days and no more than 60 days after the date the appraisal rights notice is delivered. It is the obligation of any stockholder electing to exercise appraisal rights to initiate all necessary action to perfect such stockholder’s appraisal rights within the time periods prescribed in Article 13 of the ABCL and the appraisal rights notice. If no payment demand is timely received from a stockholder exercising appraisal rights, all appraisal rights of said stockholder will be lost, notwithstanding any previously submitted written notice of intent to demand payment. Each stockholder exercising appraisal rights who demands payment retains all other rights of a stockholder unless and until those rights are cancelled or modified by the merger. A stockholder exercising appraisal rights who demands payment in accordance with the foregoing and does not withdraw from the appraisal process before the deadline set forth in the appraisal rights notice provided by Serina may not thereafter withdraw that demand and accept the terms offered under the merger agreement unless Serina consents thereto.
On or before the stock certificate delivery deadline, which may not be before the deadline for demanding formal payment, a stockholder exercising appraisal rights who has made a demand must submit such stockholder’s stock certificate or certificates to Serina. A stockholder’s failure to submit shares of capital stock for notation will, at Serina’s option, terminate the holder’s appraisal rights, unless a court of competent jurisdiction determines otherwise.
Promptly after the merger, or upon receipt of a payment demand, Serina shall offer to pay each stockholder exercising appraisal rights who complied with Article 13 of the ABCL the amount Serina estimates to be the fair value of such stockholder’s shares of capital stock plus accrued interest. Each stockholder exercising appraisal rights who agrees to accept the offer of payment in full satisfaction of such stockholder’s demand must surrender to Serina the certificate or certificates representing such shares of capital stock in accordance with the terms of the appraisal rights notice. Upon receiving the certificate or certificates, Serina will pay each stockholder exercising appraisal rights the fair value of such stockholder’s shares of capital stock, plus accrued interest. Upon receiving payment, each stockholder exercising appraisal rights ceases to have any interest in the shares.
Each stockholder exercising appraisal rights who has made a payment demand may notify Serina in writing such stockholder’s own estimate of the fair value of such stockholder’s shares of capital stock plus interest, and demand payment of such estimate, or reject the offer made to such stockholder as described above and demand payment of the fair value of such stockholder’s shares of capital stock and interest due, if: (1) the stockholder exercising appraisal rights believes that the amount offered is less than the fair value of the shares of capital stock or that the interest due is incorrectly calculated; or (2) Serina fails to make an offer as required by Article 13 of the ABCL within 60 days after the date set for demanding payment; provided, however, that a stockholder exercising appraisal rights waives the right to demand payment different from that offered unless such stockholder notifies Serina of its demand in writing within 30 days after Serina offered payment for the shares of capital stock.
If a demand for payment remains unsettled, Serina will commence a proceeding within 60 days after receiving the payment demand and petition the court to determine the fair value of the shares of capital stock and accrued interest. If the proceeding is not commenced within the 60-day period, each stockholder exercising appraisal rights whose demand remains unsettled shall be entitled to receive the amount demanded plus interest. Such a proceeding will be filed in the Circuit Court of Madison County, Alabama. Each stockholder exercising appraisal rights made a party to the proceeding is entitled to judgment for the amount the court finds to be the fair value of the shares of capital stock, plus accrued interest. The court’s finding may set a value above or below the value the stockholder believes is appropriate. Upon payment of the judgment and surrender to Serina of the certificate or certificates representing the judicially appraised shares of capital stock, a stockholder exercising appraisal rights will cease to have any interest in the shares of capital stock. The Court may assess costs incurred in such a proceeding against Serina or may assess the costs against all or some of the stockholders exercising appraisal rights, in amounts the court finds equitable, to the extent the Court finds that such stockholders acted arbitrarily, vexatiously or not in good faith in demanding payment different from that initially offered by Serina. The Court may also assess the reasonable fees and expenses of counsel and experts against Serina, if the Court finds that it did not substantially comply with its requirements regarding providing notice of appraisal rights and the procedures associated therewith under Article 13 of the ABCL or against either Serina or all or some of the dissenting stockholders if the Court finds that the party against whom the fees and expenses are assessed acted arbitrarily, vexatiously or not in good faith with respect to the rights provided in Article 13 of the ABCL. If the Court finds that services of counsel for any stockholder exercising appraisal rights were of substantial benefit to other similarly situated stockholders, and that fees for such services should not be assessed against Serina, then the Court may award reasonable fees to such counsel that will be paid out of the amounts awarded to stockholders exercising appraisal rights who benefited from such services.
BECAUSE OF THE COMPLEXITY OF THE PROVISIONS OF ALABAMA LAW RELATING TO APPRAISAL RIGHTS, STOCKHOLDERS WHO ARE CONSIDERING EXERCISING APPRAISAL RIGHTS WITH RESPECT TO THE MERGER ARE URGED TO CONSULT THEIR OWN LEGAL ADVISORS.
THE MERGER AGREEMENT
The following is a summary of the material terms of the Merger Agreement. A copy of the Merger Agreement is attached as Annex A to this proxy statement/prospectus/information statement and is incorporated by reference herein. The Merger Agreement has been attached to this proxy statement/prospectus/information statement to provide you with information regarding its terms. It is not intended to provide any other factual information about AgeX, Serina or Merger Sub. The following description does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement. You should refer to the full text of the Merger Agreement for details of the Merger and the terms and conditions of the Merger Agreement.
The Merger Agreement contains representations and warranties that AgeX and Merger Sub, on the one hand, and Serina, on the other hand, have made to one another as of specific dates. These representations and warranties have been made for the benefit of the other parties to the Merger Agreement and may be intended not as statements of fact but rather as a way of allocating the risk to one of the parties if such statements made in the representations and warranties prove to be incorrect. In addition, the assertions made in the representations and warranties are qualified by the information in confidential disclosure schedules exchanged by the parties in connection with the signing of the Merger Agreement. While AgeX and Serina do not believe that these disclosure schedules contain information required to be publicly disclosed under the applicable securities laws, other than information that has already been so disclosed, the disclosure schedules contain information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the Merger Agreement. Accordingly, you should not rely on the representations and warranties as current characterizations of factual information about AgeX, Serina or Merger Sub, because they were made as of specific dates, may be intended merely as a risk allocation mechanism between AgeX and Merger Sub on the one hand, and Serina on the other hand, and are modified by the disclosure schedules.
Structure
Subject to the terms and conditions of the Merger Agreement, and in accordance with Alabama law, at the completion of the Merger, Merger Sub, an Alabama corporation and wholly owned subsidiary of AgeX, will merge with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX.
Completion and Effectiveness of the Merger
The Merger will be completed as promptly as practicable (but no later than the second business day) after all of the conditions to the closing of the Merger are satisfied or waived, including the adoption of the Merger Agreement by the Serina stockholders and the approval by the AgeX stockholders of the issuance of AgeX common stock and the other transactions proposed under the Merger Agreement, other than those conditions that by their nature are to be satisfied at the closing of the Merger, but subject to the satisfaction or waiver of each of such conditions, or at such other time, date and place as AgeX and Serina may mutually agree in writing. The Merger will become effective on the date and at the time the Statement of Merger has been duly filed with the Secretary of State of the State of Alabama or at such later time as may be specified in the Statement of Merger with the consent of AgeX and Serina in accordance with the ABCL (such date, the Closing Date, and such time, the Effective Time).
Merger Consideration and Exchange Ratio
Merger Consideration
At the Effective Time, each outstanding share of Serina common stock (after giving effect to the Serina preferred stock conversion and including all such shares that are so converted, other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of validly issued, fully paid and nonassessable shares of AgeX common stock equal to exchange ratio as more fully described below.
No fractional shares of AgeX common stock will be issued in connection with the Merger, and no certificates or scrip for any such fractional shares will be issued. Any holder of Serina common stock who would otherwise be entitled to receive a fraction of a share of AgeX common stock (after aggregating all fractional shares of AgeX common stock issuable to such holder) will, in lieu of such fraction of a share and upon such holder’s surrender of a letter of transmittal in accordance with the Merger Agreement and any accompanying documentation required therein, receive (i) one share of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina common stock would otherwise be entitled to is equal to or exceeds 0.50; or (ii) no shares of AgeX common stock if the aggregate amount of fractional shares of AgeX common stock such holder of Serina common stock would otherwise be entitled to is less than 0.50.
Exchange Ratio
The exchange ratio formula is based upon a fixed percentage of the post-Merger outstanding shares of AgeX common stock, expressed on a fully-diluted and as-converted basis, subject to certain adjustments and exclusions. Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions. The exchange ratio is currently estimated to be equal to approximately 0.83217216 shares of AgeX common stock for each share of Serina common stock, which estimated exchange ratio assumes (i) the Actual Closing Price of a share of AgeX common stock is equal to $12.00 per share (on a post-reverse stock split basis), (ii) the number of Company Outstanding Shares is equal to 9,012,558, (iii) the number of Company Merger Shares is equal to 7,500,000 and (iv) the implementation of the reverse stock split and the AgeX preferred stock conversion prior to the consummation of the Merger, as described elsewhere in this proxy statement/prospectus/information statement. There can be no assurance any of these assumptions will be accurate when the final exchange ratio is determined.
While the final exchange ratio is subject to adjustment prior to the closing of the Merger based upon the Actual Closing Price of a share of AgeX common stock determined in accordance with the Merger Agreement, the fixed percentage of the post-Merger outstanding shares of AgeX common stock is subject to adjustment prior to closing of the Merger only if the Actual Closing Price of a share of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis) as more fully described under the sections titled “—Conditions to the Completion of the Merger” and “—Termination and Termination Fees” below, and, in such circumstances, AgeX stockholders immediately prior to the closing of the Merger could own a smaller percentage, and Serina stockholders immediately prior to the closing of the Merger could own a larger percentage, of the combined company following the closing of the Merger. Accordingly, while the final exchange ratio may be higher or lower than the estimated exchange ratio depending on the Actual Closing Price when the final exchange ratio is determined, the allocation of outstanding shares of the common stock of the combined company to AgeX stockholders and Serina stockholders pursuant to the exchange ratio formula may only be adjusted if the Actual Closing Price of a share of AgeX common stock is less than $12.00 per share (on a post-reverse stock split basis).
The exchange ratio is the quotient obtained by dividing (a) the Company Merger Shares (defined below) by (b) the Company Outstanding Shares (defined below), in which:
“Company Allocation Percentage” means 75.00%.
“Company Merger Shares” means the product (rounded to the nearest whole share) obtained by multiplying (a) Post-Closing Parent Shares by (b) the Company Allocation Percentage.
“Company Outstanding Shares” means the total number of shares of Serina capital stock outstanding immediately prior to the Effective Time after giving effect to the Serina preferred stock conversion, expressed on a fully-diluted and as-converted to Serina common stock basis assuming, without limitation or duplication, (i) the exercise of all Serina options outstanding as of immediately prior to the Effective Time, (ii) the issuance of shares of Serina capital stock upon the conversion of all Serina convertible notes (but excluding any Serina convertible notes issued to AgeX) and (iii) the issuance of shares of Serina capital stock in respect of all other outstanding options, restricted stock awards, restricted stock units or rights to receive such shares, whether conditional or unconditional and including any outstanding options, restricted stock awards, restricted stock units or rights triggered by or associated with the consummation of the Merger (but excluding any shares of Serina capital stock reserved for issuance other than with respect to outstanding Serina options under the Serina incentive plan as of immediately prior to the Effective Time). No Serina warrants will be included in the total number of shares of Serina common stock for purposes of determining the Company Outstanding Shares.
“Parent Outstanding Shares” means, subject to, among other things, the reverse stock split and the immediately following sentence, the total number of shares of AgeX common stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as-converted to AgeX common stock basis and using the treasury stock method (and shall include, for the avoidance of doubt, all in the money AgeX options and warrants, shares to be received upon the AgeX preferred stock conversion and shares issuable upon the conversion of AgeX’s convertible note with Juvenescence), but assuming, without limitation or duplication, the issuance of shares of AgeX common stock in respect of all AgeX options, AgeX RSUs, AgeX warrants and other outstanding options, warrants or rights to receive such shares, in each case, outstanding as of immediately prior to the Effective Time (assuming cashless exercise using the AgeX closing price), whether conditional or unconditional and including any outstanding options, warrants or rights triggered by or associated with the consummation of the Merger (but excluding any shares of AgeX common stock reserved for issuance other than with respect to outstanding AgeX options, AgeX RSUs and AgeX warrants as of immediately prior to the Effective Time and as set forth above). No (i) post-merger warrants, or (ii) incentive warrants, in each case, shall be included in the total number of shares of AgeX common stock outstanding for purposes of determining the Parent Outstanding Shares.
“Post-Closing Parent Shares” means the quotient obtained by dividing (a) the Parent Outstanding Shares by (b)(i) 1.00 minus (ii) the Company Allocation Percentage.
Treatment of Serina Stock Options
Under the terms of the Merger Agreement, at the Effective Time, each Serina option that is outstanding and unexercised immediately prior to the Effective Time under the Serina Plan, whether or not vested, will be converted into and become an option to purchase shares of AgeX common stock, and AgeX will assume the Serina Plan (if necessary) and each such Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option (but with changes to such documents as AgeX in good faith determines are appropriate to reflect the substitution of the Serina options by AgeX to purchase shares of AgeX common stock). All rights, terms, and restrictions with respect to Serina common stock under Serina options assumed by AgeX will be converted into rights with respect to AgeX common stock.
Accordingly, from and after the Effective Time: (i) each Serina option assumed by AgeX may be exercised solely for shares of AgeX common stock; (ii) the number of shares of AgeX common stock subject to each Serina option assumed by AgeX will be determined by multiplying (A) the number of shares of Serina common stock that were subject to such Serina option assumed by AgeX, by (B) the exchange ratio, as determined in accordance with the allocation certificate to be delivered by Serina pursuant to the Merger Agreement; (iii) the per share exercise price for the AgeX common stock issuable upon exercise of each Serina option assumed by AgeX will be determined by dividing (A) the per share exercise price of Serina common stock subject to such Serina option, by (B) the exchange ratio, as determined in accordance with the allocation certificate to be delivered by Serina pursuant to the Merger Agreement; and (iv) any restriction on the exercise of any Serina option assumed by AgeX will continue in full force and effect and the term, exercisability, vesting schedule and other provisions of such Serina option will otherwise remain unchanged; provided, however, that (A) the determination in the allocation certificate of the number of shares of AgeX common stock subject to each Serina option assumed by AgeX will be based on the exchange ratio as that applied to the Serina common stock to determine the number of shares of AgeX common stock each holder of Serina common stock will receive, (B) the determination in the allocation certificate of the per share exercise price for the AgeX common stock issuable upon exercise of each Serina option assumed by AgeX will be adjusted equitably to reflect the exchange ratio, (C) to the extent provided under the terms of the respective stock option agreements governing the Serina option and the Serina Plan, AgeX may amend the terms of the Serina options and the Serina Plan, in accordance with the terms thereof, to reflect AgeX’s substitution of the Serina options with options to purchase AgeX common stock (such as by making any change in control or similar definition relate to AgeX and having any provision that provides for the adjustment of Serina options upon the occurrence of certain corporate events relate to corporate events that relate to AgeX and/or AgeX common stock), and such Serina options will be subject to further adjustment as appropriate and necessary to reflect any stock split, division or subdivision of shares, stock dividend, reverse stock split, consolidation of shares, reclassification, recapitalization, or other similar transaction with respect to AgeX common stock subsequent to the Effective Time, and (D) the AgeX Board or a committee thereof will succeed to the authority and responsibility of the Serina Board or any committee thereof with respect to each Serina option assumed by AgeX.
Treatment of Serina Warrants
Under the terms of the Merger Agreement, at the Effective Time, each Serina warrant that is outstanding and unexercised immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase shares of AgeX common stock, and AgeX will assume each such Serina warrant in accordance with its terms. All rights with respect to Serina common stock under Serina warrants assumed by AgeX will be converted into rights with respect to AgeX common stock.
Accordingly, from and after the Effective Time: (i) each Serina warrant assumed by AgeX may be exercised solely for shares of AgeX common stock; (ii) the number of shares of AgeX common stock subject to each Serina warrant assumed by AgeX will be determined by multiplying (A) the number of shares of Serina capital stock that were subject to such Serina warrant assumed by AgeX, by (B) the exchange ratio, as determined in accordance with the allocation certificate to be delivered by Serina pursuant to the Merger Agreement; (iii) the per share exercise price for the AgeX common stock issuable upon exercise of each Serina warrant assumed by AgeX will be determined by dividing (A) the per share exercise price of AgeX common stock subject to such Serina warrant, by (B) the exchange ratio, as determined in accordance with the allocation certificate to be delivered by Serina pursuant to the Merger Agreement; and (iv) any restriction on any Serina warrant assumed by AgeX will continue in full force and effect and the term and other provisions of such Serina warrant will otherwise remain unchanged; provided, however, that: (A) the determination in the allocation certificate of the number of shares of AgeX common stock subject to each Serina warrant assumed by AgeX will be based on the exchange ratio as that applied to the Serina common stock to determine the number of shares of AgeX common stock each holder of Serina common stock will receive; and (B) the determination in the allocation certificate of the per share exercise price for the AgeX common stock issuable upon exercise of each Serina warrant assumed by AgeX be adjusted equitably to reflect the exchange ratio.
Treatment of AgeX Stock Options
Immediately prior to the reverse stock split, each outstanding and unexercised out of the money AgeX option as of immediately prior to the reverse stock split, if any, will be canceled without payment. In accordance with the terms and conditions set forth in the Incentive Plan, AgeX will notify each holder of an out of the money AgeX option that each such option will become exercisable at least 10 days prior to the reverse stock split and will thereafter be canceled if not exercised. All other outstanding and unexercised options to purchase shares of AgeX common stock will remain outstanding and exercisable in accordance with their terms immediately following the Effective Time.
Directors and Executive Officers of the Combined Company Following the Merger
The Merger Agreement provides that the parties will use reasonable best efforts and take all necessary action so that effective as of the Effective Time, the Combined Company Board is comprised of seven members, with two of such members designated by Serina, two of such members designated by AgeX, two of such members designated by mutual agreement of Serina and AgeX that meet the director independence requirements of the NYSE American and one member designated by mutual agreement of Serina and AgeX. Steve Ledger, Chief Financial Officer of Serina, will serve as the Interim Chief Executive Officer of the combined company, Andrea Park, Chief Financial Officer of AgeX will serve as Interim Chief Financial Officer and Chief Accounting Officer of the combined company, Randall Moreadith, M.D., Ph.D., President and Chief Executive Officer of Serina, will serve as Chief Scientific Officer of the combined company and Tacey Viegas, Ph.D., Chief Operating Officer of Serina, will serve as Chief Operating Officer and Secretary of the combined company.
Conditions to the Completion of the Merger
The obligations of each party to consummate the Merger and the Contemplated Transactions are subject to the satisfaction or, to the extent permitted by applicable law, the written waiver by each of the parties, at or prior to the closing of the Merger, of the following conditions:
| ● | there must not have been any temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the Contemplated Transactions issued by any court of competent jurisdiction or other governmental body of competent jurisdiction and that remains in effect, and there must not be any law that has the effect of making the consummation of the Contemplated Transactions illegal; |
| ● | the AgeX stockholders must have approved the AgeX Stockholder Matters; |
| ● | Serina must have delivered an action by written consent (Serina Stockholder Written Consent) executed by the holders of (a) 60% of the then outstanding shares of Series A Preferred Stock voting as a separate class, (b) 60% of the then outstanding shares of Series A-1 Preferred Stock voting as a separate class, (c) 60% of the then outstanding shares of Series A-2 Preferred Stock voting as a separate class, (d) 60% of the then outstanding shares of Series A-3 Preferred Stock voting as a separate class, (e) 60% of the then outstanding shares of Series A-4 Preferred Stock voting as a separate class, (f) 60% of the then outstanding shares of Series A-5 Preferred Stock voting as a separate class, and (g) a majority of the then outstanding shares of the capital stock of the Company on an as-converted to Company Common Stock basis and as a single class (collectively, the Required Serina Stockholder Vote): (i) adopting and approving the Merger Agreement and the Contemplated Transactions, (ii) acknowledging that the approval given thereby is irrevocable, (iii) agreeing that such stockholder is aware of its rights to demand appraisal, or assert any dissenters’ rights, for its shares pursuant to ABCL Article 13, (iv) agreeing that such stockholder has received and read a copy of ABCL Article 13, (v) acknowledging that by such stockholder’s approval of the Merger such stockholder is not entitled to appraisal rights and thereby waives any right to receive payment of the fair value of its shares of Serina capital stock under the ABCL, (vi) electing that the consummation of the Merger is not a deemed liquidation event, and (vii) electing an automatic conversion of each share of Serina preferred stock into shares of Serina common stock immediately prior to the Effective Time in accordance with the relevant provisions of Serina’s organizational documents. |
| ● | the existing shares of AgeX common stock will have been continually listed on New York Stock Exchange (NYSE) (including the NYSE American) as of and from the date of the Merger Agreement through the Closing Date, and the shares of AgeX common stock to be issued in the Merger and the other Contemplated Transactions pursuant to the Merger Agreement will have been approved for listing (subject to official notice of issuance) on NYSE (including the NYSE American) as of the closing of the Merger; |
| ● | the registration statement will have become effective in accordance with the Securities Act and will not be subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the registration statement that has not been withdrawn; |
| ● | AgeX, Serina and Juvenescence will have entered into the Side Letter, and Juvenescence will have converted all of its shares of AgeX Series A preferred stock and AgeX Series B preferred stock into shares of AgeX common stock prior to the reverse stock split; |
| ● | The reverse stock split will have occurred and the shares of AgeX common stock outstanding immediately prior to the Effective Time shall be approximately 2,500,000. |
In addition, the obligation of AgeX and Merger Sub to consummate the Merger and otherwise consummate the Contemplated Transactions is subject to the satisfaction or waiver by AgeX, at or prior to the closing of the Merger, of the following conditions:
| ● | the representations and warranties of Serina set forth in the Merger Agreement under Sections 2.1 (Due Organization; Subsidiaries), 2.3 (Authority; Binding Nature of Agreement), 2.4 (Vote Required), 2.6(a) and (c) (Capitalization) and 2.20 (No Financial Advisors) must have been true and correct in all material respects as of the date of the Merger Agreement and must be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties must have been true and correct in all material respects as of such date); |
| ● | the representations and warranties of Serina set forth in the Merger Agreement (other than the Serina representations and warranties listed above) must have been true and correct as of the date of the Merger Agreement and must be true and correct on and as of the Closing Date with the same force and effect as if made on and as of the Closing Date except (a) in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Serina Material Adverse Effect (as defined below) (without giving effect to any references therein to any Serina Material Adverse Effect or other materiality qualifications) or (b) for those representations and warranties which address matters only as of a particular date (which representations must have been true and correct, subject to the qualifications set forth in the preceding clause (a), as of such particular date); |
| ● | Serina must have performed or complied with in all material respects all agreements and covenants required to be performed or complied with by it under the Merger Agreement at or prior to the Effective Time; |
| ● | AgeX must have received from Serina (i) an officer’s certificate certifying (x) that certain conditions set forth in the Merger Agreement have been duly satisfied and (y) that the information set forth in the allocation certificate delivered by Serina containing information regarding Serina’s capitalization is true and accurate in all respects as of the Closing Date; and (ii) a copy of such allocation certificate; |
| ● | since the date of the Merger Agreement, there must not have occurred a Serina Material Adverse Effect that is continuing; |
| ● | certain of Serina’s investor agreements must have been terminated (or will be terminated as of the closing of the Merger); |
| ● | AgeX must have received duly executed copies of the required Lock-Up Agreements from certain stockholders of Serina and each executive officer and director of Serina who is elected or appointed, as applicable, as an executive officer or director of AgeX as of immediately following the closing of the Merger, each of which must be in full force and effect as of immediately following the Effective Time; |
| ● | the Serina Stockholder Written Consent executed by the required stockholders of Serina must be in full force and effect; |
| ● | the holders of no more than 5% of the shares of Serina capital stock will have exercised statutory appraisal rights pursuant to ABCL Article 13 with respect to their shares of Serina capital stock; and |
| ● | AgeX must have received (i) an original signed statement from Serina that Serina is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury Regulations Section 1.1445-2(c)(3) and 1.897-2(h), and (ii) an original signed notice from Serina to be delivered to the IRS in accordance with the provisions of Treasury Regulations Section 1.897-2(h)(2), together with written authorization for AgeX to deliver such notice to the IRS on behalf of Serina following the closing of the Merger, each dated as of the Closing Date, duly executed by an authorized officer of Serina, and in form and substance reasonably acceptable to AgeX; and |
| ● | the holders of Serina’s convertible promissory notes, other than any convertible promissory note held by AgeX, will have elected to convert such notes into shares of Serina common stock prior to the Effective Time. |
In addition, the obligation of Serina to consummate the Merger and otherwise consummate the Contemplated Transactions is subject to the satisfaction or waiver by Serina, at or prior to the closing of the Merger, of the following conditions:
| ● | the representations and warranties of AgeX and Merger Sub set forth in the Merger Agreement under Sections 3.1 (Due Organization; Subsidiaries), 3.3 (Authority; Binding Nature of Agreement), 3.4 (Vote Required), 3.6(a) and (c) (Capitalization) and 3.21 (No Financial Advisors) must have been true and correct in all material respects as of the date of the Merger Agreement and must be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties must have been true and correct in all material respects as of such date); |
| ● | the representations and warranties of AgeX and Merger Sub set forth in the Merger Agreement (other than the AgeX and Merger Sub representations and warranties listed above) must have been true and correct as of the date of the Merger Agreement and must be true and correct on and as of the Closing Date with the same force and effect as if made on and as of the Closing Date except (a) in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have an AgeX Material Adverse Effect (as defined below) (without giving effect to any references therein to any AgeX Material Adverse Effect or other materiality qualifications) or (b) for those representations and warranties which address matters only as of a particular date (which representations must have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date); |
| ● | AgeX and Merger Sub must have performed or complied with in all material respects all of their agreements and covenants required to be performed or complied with by each of them under the Merger Agreement at or prior to the Effective Time; |
| ● | Serina must have received from AgeX (i) an officer’s certificate confirming that certain conditions of the Merger Agreement have been duly satisfied; (ii) a certificate containing information regarding AgeX’s capitalization; and (iii) a reasonably satisfactory written resignation dated as of the Closing Date and effective as of the closing of the Merger executed by the directors of AgeX who will not continue as directors of AgeX after closing of the Merger; |
| ● | since the date of the Merger Agreement, there must not have occurred an AgeX Material Adverse Effect that is continuing; |
| ● | Serina must have received satisfactory evidence that (a) specified AgeX contracts have been terminated, assigned or fully performed by AgeX, and (b) all obligations of AgeX thereunder have been fully satisfied, waived or otherwise discharged; |
| ● | the Lock-Up Agreements executed by certain stockholders of AgeX must be in full force and effect as of immediately following the Effective Time; |
| ● | the Actual Closing Price of a share of AgeX common stock must not be less than $12.00 and Juvenescence must hold at least 1,133,308 post-merger warrants upon AgeX’s issuance of the post-merger warrants; and |
| ● | immediately prior to the closing of the Merger, AgeX must have on hand at least $500,000 of immediately spendable nonrestricted cash, net of all payable and other liabilities (including Transaction Expenses (as defined in “—Expenses”). |
“AgeX Material Adverse Effect” means any effect, change, event, circumstance or development (collectively, Effect) that, considered together with all other Effects, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of AgeX or its subsidiaries, taken as a whole; provided, however, that Effects arising or resulting from the following will not be taken into account in determining whether there has been an AgeX Material Adverse Effect: (a) general business, political or economic conditions generally affecting the industry in which AgeX and its subsidiaries operate, (b) acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions, (c) changes in financial, banking or securities markets, (d) any change in the stock price or trading volume of AgeX common stock (it being understood, however, that any Effect causing or contributing to any change in stock price or trading volume of AgeX common stock may be taken into account in determining whether an AgeX Material Adverse Effect has occurred, unless such Effects are otherwise excepted from this definition), (e) the failure of AgeX to meet internal or analysts’ expectations or projections or the results of operations of AgeX, (f) any changes in or affecting clinical trial programs or studies conducted by or on behalf of AgeX or its subsidiaries, including any adverse data, event or outcome arising out of or related to any such programs or studies, (g) any change in, or any compliance with or action taken for the purpose of complying with, any law or GAAP (or interpretations of any law or GAAP), (h) the announcement of the Merger Agreement or the pendency of the Contemplated Transactions, (i) the Asset Dispositions (as defined below), (j) any reduction in the amount of AgeX’s cash and cash equivalents as a result of expenditures made by AgeX related to wind down activities of AgeX associated with the termination of its research and development activities (including the termination of ongoing contractual obligations relating to AgeX current products or product candidates), (k) resulting from the taking of any action required to be taken by the Merger Agreement, or (l) as set forth in Section B of AgeX’s disclosure schedule, except in each case, with respect to clauses (a) through (c), to the extent disproportionately affecting AgeX and its subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which AgeX and its subsidiaries operate.
“Serina Material Adverse Effect” means any Effect that, considered together with all other Effects, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of Serina or its subsidiaries, taken as a whole; provided, however, that Effects arising or resulting from the following will not be taken into account in determining whether there has been a Serina Material Adverse Effect: (a) general business, political or economic conditions generally affecting the industry in which Serina and its subsidiaries operate, (b) acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions, (c) changes in financial, banking or securities markets, (d) any change in, or any compliance with or action taken for the purpose of complying with, any law or GAAP (or interpretations of any law or GAAP), (e) the announcement of the Merger Agreement or the pendency of the Contemplated Transactions, (f) the taking of any action required to be taken by the Merger Agreement, or (g) continued losses from operations or decreases in cash balances of Serina or any of its subsidiaries or on a consolidated basis among Serina and its subsidiaries; except, in each case, with respect to clauses (a) through (c), to the extent disproportionately affecting Serina and its subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which Serina and its subsidiaries operate.
“Actual Closing Price” means the volume weighted average closing trading price of a share of the AgeX common stock as reported on the NYSE American for the 10 trading days preceding the date that is the fifth business day preceding the Effective Time.
Post-Merger Warrants; Incentive Warrants
Prior to the consummation of the Merger, AgeX will issue to each holder of AgeX common stock three post-merger warrants for each five shares of AgeX common stock held by such holder. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one incentive warrant and will expire on July 31, 2025. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock and will expire on the four-year anniversary of the Closing Date. For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 180 of this proxy statement/prospectus/information statement.
Potential Asset Disposition; Legacy Assets
AgeX is entitled, but under no obligation, to sell, transfer, license, assign or otherwise divest certain assets to the extent relating exclusively to the business of Reverse Bioengineering, Inc. (Potentially Transferrable Assets) to one or more third parties in one or a series of transactions prior to or substantially concurrently with the closing of the Merger (Asset Dispositions); provided, that any such Asset Disposition will require, to the extent consistent with applicable laws, the written consent of Serina, not to be unreasonably withheld, conditioned or delayed, if such Asset Disposition would create any post-disposition material liabilities for AgeX following the closing of the Merger. If the Asset Dispositions are not completed prior to, concurrently with, or immediately following the closing of the Merger, the Potentially Transferrable Assets will be retained by AgeX.
Prior to the closing of the Merger, any assets of AgeX that remain after the Asset Dispositions other than certain assets as agreed between AgeX and Serina, assets related to NeuroAirmid and cash required to satisfy the applicable closing condition under the Merger Agreement as described above (Legacy Assets) shall be sold, transferred, licensed, assigned or conveyed into a newly formed subsidiary of AgeX (Newco). In consideration of the transfer of such assets, Newco will assume (i) any indebtedness of AgeX issued to Juvenescence that has not been previously converted into AgeX Series A preferred stock or AgeX Series B preferred stock, which will be secured by the Legacy Assets and (ii) all other liabilities of AgeX in existence as of the Effective Time (other than Transaction Expenses).
AgeX Preferred Stock
Subject to requisite AgeX stockholder approval and in accordance with the Side Letter, Juvenescence will convert all of the shares of AgeX Series A preferred stock and AgeX Series B preferred stock owned by Juvenescence or any affiliate thereof into shares of AgeX common stock prior to the reverse stock split.
Convertible Note
Any convertible note issued by Serina to AgeX will be amended prior to the closing of the Merger so that it automatically converts immediately prior to the Merger into shares of Serina capital stock, which shares will be canceled for no consideration in connection with the Merger.
Equity Plans
AgeX and Serina will amend their existing equity incentive plans to make certain administrative changes agreed upon between the parties.
Representations and Warranties
The Merger Agreement contains customary representations and warranties of Serina, AgeX and Merger Sub for a transaction of this type relating to, among other things:
| ● | Due Organization; Subsidiaries |
| ● | Organizational Documents |
| ● | Authority; Binding Nature of Agreement |
| ● | Non-Contravention; Consents |
| ● | Financial Statements and, with respect to AgeX and Merger Sub, SEC Filings |
| ● | Absence of Undisclosed Liabilities |
| ● | Real Property; Leasehold |
| ● | Agreements, Contracts and Commitments |
| ● | Compliance; Permits; Restrictions |
| ● | Legal Proceedings; Orders |
| ● | Employee and Labor Matters; Benefit Plans |
| ● | Transactions with Affiliates |
| ● | with respect to AgeX and Merger Sub, Valid Issuance |
| ● | Disclaimer of Other Representations and Warranties |
The representations and warranties of Serina, AgeX and Merger Sub contained in the Merger Agreement or any certificate or instrument delivered pursuant to the Merger Agreement will terminate at the Effective Time.
Non-Solicitation
AgeX, Serina and their respective subsidiaries are prohibited by the terms of the Merger Agreement, other than, in the case of AgeX, with respect to any Asset Disposition, from, directly or indirectly, (i) soliciting, initiating or knowingly encouraging, inducing or facilitating the communication, making, submission or announcement of any Acquisition Proposal (as defined below) or Acquisition Inquiry (as defined below) or taking any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnishing any non-public information regarding itself or its respective subsidiaries to any person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engaging in discussions (other than to inform any person of the existence of these prohibitions) or negotiations with any person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approving, endorsing or recommending any Acquisition Proposal (subject to, in the case of AgeX, AgeX’s special meeting and the AgeX Proposals); (v) executing or entering into any letter of intent or any contract contemplating or otherwise relating to any Acquisition Transaction (as defined below) (other than, in the case of AgeX, a confidentiality agreement permitted as described below); or (vi) publicly proposing to do any of the foregoing.
Pursuant to the terms of the Merger Agreement, each of AgeX and Serina agreed to immediately cease and cause to be terminated any existing discussions, negotiations and communications with any person relating to any Acquisition Proposal or Acquisition Inquiry (other than, in the case of AgeX, any Asset Dispositions) as of the date of the Merger Agreement and request the destruction or return of any of AgeX’s or Serina’s non-public information, as applicable, provided to such person as soon as practicable after the date of the Merger Agreement.
Subject to certain restrictions and prior to obtaining the approval of the AgeX Stockholder Matters by the Required AgeX Stockholder Vote, AgeX and its subsidiaries may furnish non-public information regarding AgeX or any of its subsidiaries to, and enter into discussions or negotiations with, any person in response to a bona fide Acquisition Proposal by such person, which the AgeX Board determines in good faith, after consultation with its outside financial advisor and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (as defined below) (and is not withdrawn) if: (A) none of AgeX, any of its subsidiaries or any of their respective representatives have breached the non-solicitation restrictions in the Merger Agreement in any material respect, (B) the AgeX Board concludes in good faith based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of the AgeX Board under applicable law; (C) substantially contemporaneously with furnishing any such non-public information to such person, AgeX gives Serina notice of AgeX’s intention to furnish non-public information to, or enter into discussions with, such person and furnishes such nonpublic information to Serina (to the extent such information has not been previously furnished by AgeX to Serina), and (D) AgeX receives from such person an executed confidentiality agreement containing provisions (including nondisclosure provisions, use restrictions, non-solicitation provisions, no hire and “standstill” provisions), in the aggregate, at least as favorable to AgeX as those contained in the confidentiality agreement entered into between AgeX and Serina in connection with the Merger.
If AgeX, Serina or any of their respective subsidiaries or representatives receives an Acquisition Proposal or Acquisition Inquiry during the period following the date of the Merger Agreement and continuing until the earlier of the termination of the Merger Agreement or the Effective Time, then such party will promptly (and in no event later than one business day after such party becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise the other party orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the person making or submitting such Acquisition Proposal or Acquisition Inquiry and the material terms thereof). Each party will keep the other party reasonably informed with respect to the status and material terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or proposed material modification thereto.
“Acquisition Inquiry” means, with respect to a party, an inquiry, indication of interest or request for information (other than an inquiry, an indication of interest or request for information made or submitted by Serina, on the one hand, or AgeX, on the other hand, to the other party) that could reasonably be expected to lead to an Acquisition Proposal; provided, however, that the term Acquisition Inquiry does not include the Merger, the Contemplated Transactions or any transactions related to the Asset Dispositions.
“Acquisition Proposal” means, with respect to a party, any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of Serina or any of its affiliates, on the one hand, or by or on behalf of AgeX or any of its affiliates, on the other hand, to the other party) contemplating or otherwise relating to any Acquisition Transaction with such party, other than the Asset Dispositions.
“Acquisition Transaction” means any transaction or series of related transactions (other than the Asset Dispositions) involving:
| ● | any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which a party is a constituent entity; (ii) in which a person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of a party or any of its subsidiaries; or (iii) in which a party or any of its subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such party or any of its subsidiaries; or |
| ● | any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of a party and its subsidiaries, taken as a whole. |
“Superior Offer” means an unsolicited bona fide written Acquisition Proposal (with all references to 20% in the definition of Acquisition Transaction being treated as references to greater than 50% for these purposes) that: (a) was not obtained or made as a direct or indirect result of a breach of (or in violation of) the Merger Agreement; and (b) is on terms and conditions that the AgeX Board determines in good faith, based on such matters that it deems relevant (including the likelihood of consummation thereof and the financing terms thereof), as well as any written offer by the other party to the Merger Agreement to amend the terms of the Merger Agreement, and following consultation with its outside legal counsel and outside financial advisors, if any, are more favorable, from a financial point of view, to AgeX’s stockholders than the terms of the Contemplated Transactions and is not subject to any financing condition (and if financing is required, such financing is then fully committed to the third party).
AgeX Stockholder Meeting
Promptly after the registration statement has been declared effective by the SEC under the Securities Act, AgeX will take all action necessary under applicable law to call, give notice of and hold a meeting of the holders of AgeX common stock for the purpose of seeking approval of the Merger Agreement and the Contemplated Transactions, including: (i) the amendment of AgeX’s certificate of incorporation to effect the reverse stock split, (ii) if applicable, the consummation of the Asset Dispositions, (iii) the issuance of AgeX common stock or other securities of AgeX that represent (or are convertible into) more than 20% of the shares of AgeX common stock outstanding immediately prior to the Merger to the holders of Serina capital stock, Serina stock options and Serina warrants in connection with the Contemplated Transactions and the change of control of AgeX resulting from the Contemplated Transactions, in each case pursuant to the NYSE American rules, (iv) the issuance of the post-merger warrants to the holders of AgeX common stock, (v) the amendment and restatement of AgeX’s certificate of incorporation, (vi) the adoption of the Serina Therapeutics, Inc. 2024 Equity Incentive Plan (the 2024 Equity Incentive Plan), along with the reservation of 1,725,000 shares of AgeX common stock under the 2024 Equity Incentive Plan, (vii) if not previously approved by AgeX stockholders, the issuance of shares of AgeX common stock that represent more than 20% of the shares of AgeX common stock outstanding immediately prior to conversion of the AgeX Series B preferred stock in connection with the AgeX preferred stock conversion and (viii) any other proposals the parties deem necessary or desirable to consummate the Contemplated Transactions (together with the proposals set forth in the foregoing clauses (i) through (viii), the AgeX Stockholder Matters).
The AgeX special meeting will be held as promptly as practicable after the registration statement is declared effective under the Securities Act and, in any event, no later than 45 calendar days after the effective date of the registration statement. AgeX will take reasonable measures to ensure that all proxies solicited in connection with the AgeX special meeting are solicited in compliance with all applicable laws. If, on or before the date of the AgeX special meeting, AgeX reasonably believes that it (i) will not receive proxies sufficient to obtain the required approvals of the AgeX Stockholder Matters in accordance with the Merger Agreement (Required AgeX Stockholder Vote), whether or not a quorum would be present or (ii) will not have sufficient shares of AgeX common stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the AgeX special meeting, AgeX may postpone or adjourn, or make one or more successive postponements or adjournments of, the AgeX special meeting as long as the date of the AgeX special meeting is not postponed or adjourned more than an aggregate of 60 calendar days in connection with any postponements or adjournments.
AgeX agreed that, subject to certain exceptions in the Merger Agreement: (i) the AgeX Board will recommend that the holders of AgeX common stock vote to approve the AgeX Stockholder Matters and will use commercially reasonable efforts to solicit such approval within the timeframe set forth above, (ii) this proxy statement/prospectus/information statement will include a statement to the effect that the AgeX Board recommends that AgeX’s stockholders vote to approve the AgeX Stockholder Matters (the recommendation of the AgeX Board with respect to the AgeX Stockholder Matters being referred to as the AgeX Board Recommendation); and (iii) the AgeX Board Recommendation will not be withheld, amended, withdrawn or modified (and the AgeX Board will not publicly propose to withhold, amend, withdraw or modify the AgeX Board Recommendation) in a manner adverse to Serina, and no resolution by the AgeX Board or any committee thereof to withdraw or modify the AgeX Board Recommendation in a manner adverse to Serina or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal shall be adopted or proposed (the actions set forth in the foregoing clause (iii), collectively, an AgeX Board Adverse Recommendation Change).
The terms of the Merger Agreement provide that, subject to the limitations set forth in the Merger Agreement, if at any time prior to the approval of the AgeX Stockholder Matters at the AgeX special meeting by the Required AgeX Stockholder Vote, AgeX receives a bona fide Acquisition Proposal (which did not arise out of a material breach of the non-solicitation provisions of the Merger Agreement) from any person that has not been withdrawn and after consultation with outside legal counsel, the AgeX Board determines, in good faith, that such Acquisition Proposal is a Superior Offer, the AgeX Board may make an AgeX Board Adverse Recommendation Change or AgeX may terminate the Merger Agreement to enter into a definitive agreement with respect to such Superior Offer, if and only if: (A) the AgeX Board determines in good faith, after consultation with AgeX’s outside legal counsel, that the failure to do so would be reasonably likely to be inconsistent with the fiduciary duties of the AgeX Board to AgeX’s stockholders under applicable law; (B) AgeX has given Serina prior written notice of its intention to consider making an AgeX Board Adverse Recommendation Change or terminate the Merger Agreement to enter into a definitive agreement with respect to such Superior Offer at least four business days prior to making any such AgeX Board Adverse Recommendation Change or termination (an AgeX Determination Notice, and such period, the AgeX Notice Period) (which notice will not constitute an AgeX Board Adverse Recommendation Change); and (C)(1) AgeX provided to Serina a summary of the material terms and conditions of the Acquisition Proposal in accordance with the Merger Agreement, (2) AgeX has and has caused its representatives to, during the AgeX Notice Period, negotiate in good faith with Serina (to the extent Serina desires to negotiate) to enable Serina to propose in writing an offer binding on Serina to effect such adjustments to the terms and conditions of the Merger Agreement so that such Acquisition Proposal no longer constitutes a Superior Offer, and (3) after considering the results of such negotiations and giving effect to the proposals made by Serina, if any, after consultation with outside legal counsel, the AgeX Board determines, in good faith, that such Acquisition Proposal is a Superior Offer and that the failure to make the AgeX Board Adverse Recommendation Change or terminate the Merger Agreement to enter into a definitive agreement with respect to such Superior Offer would be inconsistent with the fiduciary duties of the AgeX Board to AgeX’s stockholders under applicable law; provided that (x) Serina receives written notice from AgeX confirming that the AgeX Board has determined to change its recommendation during the AgeX Notice Period, which includes a description in reasonable detail of the reasons for such AgeX Board Adverse Recommendation Change and written copies of any relevant proposed transaction agreements with any party making a potential Superior Offer or to terminate the Merger Agreement pursuant to the terms set forth therein during the AgeX Notice Period; (y) during any AgeX Notice Period, Serina shall be entitled to deliver to AgeX one or more counterproposals to such Acquisition Proposal and AgeX will, and will cause its representatives to, negotiate with Serina in good faith (to the extent Serina desires to negotiate) to enable Serina to propose in writing an offer binding on Serina to effect such adjustments to the terms and conditions of the Merger Agreement so that the applicable Acquisition Proposal ceases to constitute a Superior Offer; and (z) in the event of any material amendment to any Superior Offer (including any revision in price or percentage of the combined company that AgeX’s stockholders would receive), AgeX must provide Serina with notice of such material amendment and the AgeX Notice Period will be extended, if applicable, to ensure that at least two business days remain in the AgeX Notice Period (it being understood that there may be multiple extensions).
The terms of the Merger Agreement also provide that, other than in connection with an Acquisition Proposal, the AgeX Board may make an AgeX Board Adverse Recommendation Change in response to an AgeX Change in Circumstance (as defined below), if and only if: (A) the AgeX Board determines in good faith, after consultation with AgeX’s outside legal counsel, that the failure to do so would be reasonably likely to be inconsistent with the fiduciary duties of the AgeX Board to AgeX’s stockholders under applicable law; (B) AgeX has given Serina an AgeX Determination Notice at least four business days prior to making any such AgeX Board Adverse Recommendation Change; and (C) (1) AgeX has specified the AgeX Change in Circumstance in reasonable detail, including the material facts and circumstances related to the applicable AgeX Change in Circumstance, (2) AgeX has given Serina four business days after the AgeX Determination Notice to propose revisions to the terms of the Merger Agreement or make another proposal, and has made its representatives reasonably available to negotiate in good faith with Serina (to the extent Serina desires to do so) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by Serina, if any, after consultation with outside legal counsel, the AgeX Board determines, in good faith, that the failure to make the AgeX Board Adverse Recommendation Change in response to such AgeX Change in Circumstance or terminate the Merger Agreement pursuant to the terms set forth therein would be inconsistent with the fiduciary duties of the AgeX Board to AgeX’s stockholders under applicable law. The provisions of the Merger Agreement described in this paragraph also apply to any material change to the facts and circumstances relating to such AgeX Change in Circumstance and require a new AgeX Determination Notice, except that the references to four business days will be deemed to be two business days.
“AgeX Change in Circumstance” means a material development or change in circumstances (other than any such event, development or change to the extent related to (A) any Acquisition Proposal, Acquisition Inquiry or the consequences thereof or (B) the fact, in and of itself, that AgeX meets or exceeds internal budgets, plans or forecasts of its revenues, earnings or other financial performance or results of operations) that affects the business, assets or operations of AgeX that occurs or arises after the date of the Merger Agreement.
Serina Stockholder Action by Written Consent
The Merger Agreement contemplates that, promptly after the registration statement is declared effective under the Securities Act, and in any event no later than three business days thereafter, Serina will prepare, with the cooperation of AgeX, and cause to be mailed to its stockholders an information statement, which shall include a copy of this proxy statement/prospectus/information statement to solicit the approval by written consent from Serina’s stockholders sufficient for the Required Serina Stockholder Vote in lieu of a meeting pursuant to the ABCL, for purposes of approving the Serina Stockholder Matters.
Serina agreed that: (i) the Serina Board will recommend that the Serina stockholders vote to approve the Serina Stockholder Matters and will use commercially reasonable efforts to solicit such approval from certain stockholders of Serina within the timeframe set forth above (the recommendation of the Serina Board that Serina’s stockholders vote to adopt and approve the Serina Stockholder Matters being referred to as the Serina Board Recommendation); and (ii) the Serina Board Recommendation will not be withdrawn or modified (and the Serina Board will not publicly propose to withdraw or modify the Serina Board Recommendation) in a manner adverse to AgeX, and no resolution by the Serina Board or any committee thereof to withdraw or modify the Serina Board Recommendation in a manner adverse to AgeX or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal will be adopted or proposed.
Appraisal Rights
Under the DGCL, AgeX stockholders are not entitled to appraisal rights in connection with the Merger.
Holders of Serina capital stock as of the record date are entitled to appraisal rights under the ABCL. Pursuant to Section 10A-2A-13.02 of the ABCL, a Serina stockholder who does not wish to accept the merger consideration to be received pursuant to the terms of the merger agreement may elect to receive the fair value of such stockholder’s Serina capital stock (as determined immediately prior to the consummation of the merger), excluding any appreciation or depreciation in anticipation of the merger unless exclusion would be inequitable, but including interest from the effective date of the merger until the date of payment. Under the terms of the merger agreement, if 5% or more of the outstanding shares of Serina capital stock validly exercise their appraisal rights, then AgeX will not be obligated to complete the merger.
In order to exercise appraisal rights, a Serina stockholder must strictly comply with the statutory procedures of Sections 10A-2A-13.01 through 10A-2A-13.40 of the ABCL, which are summarized below. A copy of the full text of those Sections is included as Annex D to this proxy statement/prospectus/information statement. Serina stockholders are urged to read Annex D in its entirety and to consult with their legal advisors. Each Serina stockholder who desires to assert appraisal rights is cautioned that failure to adhere strictly to the requirements of Alabama law in any regard will cause a forfeiture of any appraisal rights. Serina stockholders who do not properly follow appraisal rights procedures will receive the merger consideration if the merger is effected.
Procedures for Exercising Rights of Appraisal. The following summary of Alabama law is qualified in its entirety by reference to the full text of the applicable provisions of the ABCL, a copy of which is included as Annex D to this proxy statement/prospectus/information statement.
A holder of Serina capital stock electing to exercise its appraisal rights shall not sign and return the written consent adopting the Merger Agreement approve the transactions and actions contemplated by the Merger Agreement, including the Merger.
No later than ten days after the merger, Serina, as the continuing corporation of the merger, will send a written notice to each stockholder exercising its appraisal rights who did not vote in favor of the merger and who duly filed a written notice of intent to demand payment in accordance with the foregoing procedures. The appraisal rights notice will specify, among other things, the deadline by which time Serina must receive a payment demand from such stockholders, the deadline for depositing the stockholders’ shares and the location for such deposit, the deadline by which time such stockholder may withdraw from the appraisal process, and Serina’s estimate of the fair value of the stock, and will include a form for demanding payment. The deadline for demanding payment will be no fewer than 40 days and no more than 60 days after the date the appraisal rights notice is delivered. It is the obligation of any stockholder electing to exercise appraisal rights to initiate all necessary action to perfect such stockholder’s appraisal rights within the time periods prescribed in Article 13 of the ABCL and the appraisal rights notice. If no payment demand is timely received from a stockholder exercising appraisal rights, all appraisal rights of said stockholder will be lost, notwithstanding any previously submitted written notice of intent to demand payment. Each stockholder exercising appraisal rights who demands payment retains all other rights of a stockholder unless and until those rights are cancelled or modified by the merger. A stockholder exercising appraisal rights who demands payment in accordance with the foregoing and does not withdraw from the appraisal process before the deadline set forth in the appraisal rights notice provided by Serina may not thereafter withdraw that demand and accept the terms offered under the merger agreement unless Serina consents thereto.
On or before the stock certificate delivery deadline, which may not be before the deadline for demanding formal payment, a stockholder exercising appraisal rights who has made a demand must submit such stockholder’s stock certificate or certificates to Serina. A stockholder’s failure to submit shares of capital stock for notation will, at Serina’s option, terminate the holder’s appraisal rights, unless a court of competent jurisdiction determines otherwise.
Promptly after the merger, or upon receipt of a payment demand, Serina shall offer to pay each stockholder exercising appraisal rights who complied with Article 13 of the ABCL the amount Serina estimates to be the fair value of such stockholder’s shares of capital stock plus accrued interest. Each stockholder exercising appraisal rights who agrees to accept the offer of payment in full satisfaction of such stockholder’s demand must surrender to Serina the certificate or certificates representing such shares of capital stock in accordance with the terms of the appraisal rights notice. Upon receiving the certificate or certificates, Serina will pay each stockholder exercising appraisal rights the fair value of such stockholder’s shares of capital stock, plus accrued interest. Upon receiving payment, each stockholder exercising appraisal rights ceases to have any interest in the shares.
Each stockholder exercising appraisal rights who has made a payment demand may notify Serina in writing such stockholder’s own estimate of the fair value of such stockholder’s shares of capital stock plus interest, and demand payment of such estimate, or reject the offer made to such stockholder as described above and demand payment of the fair value of such stockholder’s shares of capital stock and interest due, if: (1) the stockholder exercising appraisal rights believes that the amount offered is less than the fair value of the shares of capital stock or that the interest due is incorrectly calculated; or (2) Serina fails to make an offer as required by Article 13 of the ABCL within 60 days after the date set for demanding payment; provided, however, that a stockholder exercising appraisal rights waives the right to demand payment different from that offered unless such stockholder notifies Serina of its demand in writing within 30 days after Serina offered payment for the shares of capital stock.
If a demand for payment remains unsettled, Serina will commence a proceeding within 60 days after receiving the payment demand and petition the court to determine the fair value of the shares of capital stock and accrued interest. If the proceeding is not commenced within the 60-day period, each stockholder exercising appraisal rights whose demand remains unsettled shall be entitled to receive the amount demanded plus interest. Such a proceeding will be filed in the Circuit Court of Madison County, Alabama. Each stockholder exercising appraisal rights made a party to the proceeding is entitled to judgment for the amount the court finds to be the fair value of the shares of capital stock, plus accrued interest. The court’s finding may set a value above or below the value the stockholder believes is appropriate. Upon payment of the judgment and surrender to Serina of the certificate or certificates representing the judicially appraised shares of capital stock, a stockholder exercising appraisal rights will cease to have any interest in the shares of capital stock. The Court may assess costs incurred in such a proceeding against Serina or may assess the costs against all or some of the stockholders exercising appraisal rights, in amounts the court finds equitable, to the extent the Court finds that such stockholders acted arbitrarily, vexatiously or not in good faith in demanding payment different from that initially offered by Serina. The Court may also assess the reasonable fees and expenses of counsel and experts against Serina, if the Court finds that it did not substantially comply with its requirements regarding providing notice of appraisal rights and the procedures associated therewith under Article 13 of the ABCL or against either Serina or all or some of the dissenting stockholders if the Court finds that the party against whom the fees and expenses are assessed acted arbitrarily, vexatiously or not in good faith with respect to the rights provided in Article 13 of the ABCL. If the Court finds that services of counsel for any stockholder exercising appraisal rights were of substantial benefit to other similarly situated stockholders, and that fees for such services should not be assessed against Serina, then the Court may award reasonable fees to such counsel that will be paid out of the amounts awarded to stockholders exercising appraisal rights who benefited from such services.
Covenants; Operation of Business Pending the Merger
During the period from the date of the Merger Agreement and continuing until the earlier of the termination of the Merger Agreement or the Effective Time, except (i) as set forth in AgeX’s disclosure schedule, (ii) as expressly permitted by or required in accordance with the Merger Agreement, (iii) as required by applicable law or (iv) as may be consented to in writing by Serina (not to be unreasonably withheld, delayed or conditioned), each of AgeX and its subsidiaries has agreed to (A) conduct its business and operations in the ordinary course of business (which includes actions required to effect the Asset Dispositions or effect the winding down of AgeX’s prior research and development activities, including the termination of ongoing contractual obligations relating to AgeX’s current products or product candidates) and in compliance in all material respects with all applicable laws and the requirements of all of its material contracts and (B) continue to pay material outstanding accounts payable and other material current liabilities (including payroll) when due and payable, and will not, and will not cause or permit any of its subsidiaries to:
| ● | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except repurchases of shares of AgeX common stock from terminated employees, directors or consultants of AgeX or in connection with the payment of the exercise price and/or withholding taxes incurred upon the exercise, settlement or vesting of any award or purchase rights granted under the Incentive Plan in accordance with the terms of such award in effect on the date of the Merger Agreement); |
| ● | sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of AgeX or any of its subsidiaries (except for AgeX common stock issued upon the valid exercise of outstanding AgeX options or AgeX warrants or upon settlement of AgeX restricted stock units); (B) any option, warrant or right to acquire any capital stock or any other security other than AgeX stock options or restricted stock unit awards granted to directors, employees and service providers in the ordinary course of business which are included in the calculation of the AgeX Outstanding Shares; or (C) any instrument convertible into or exchangeable for any capital stock or other security of AgeX or any of its subsidiaries; |
| ● | except as required to give effect to anything in contemplation of the closing of the Merger, amend any of its or its subsidiaries’ organizational documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions; |
| ● | form any subsidiary or acquire any equity interest or other interest in any other entity or enter into a joint venture with any other entity; |
| ● | (A) lend money to any person (except for the advancement of reasonable and customary expenses to employees, directors and consultants in the ordinary course of business), (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, (D) other than the incurrence or payment of Transaction Expenses, make any capital expenditure in excess of $100,000 of the budgeted capital expenditure amounts set forth in AgeX’s operating budget delivered to Serina concurrently with the execution of the Merger Agreement (AgeX Budget), or (E) forgive any loans to any persons, including AgeX’s employees, officers, directors or affiliates; |
| ● | other than as required by applicable law or the terms of any AgeX benefit plan as in effect on the date of the Merger Agreement: (A) adopt, terminate, establish or enter into any AgeX benefit plan; (B) cause or permit any AgeX benefit plan to be amended; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or bonus or other compensation or remuneration payable to, any of its directors, officers, consultants or employees other than increases in base salary and annual cash bonus opportunities and payments made, in each case, in the ordinary course of business; (D) hire any officer or employee; or (E) increase the severance or change of control benefits offered to any current or new employees, directors or consultants; |
| ● | recognize any labor union or labor organization, except as otherwise required by applicable law and after prior written consent of Serina (not to be unreasonably withheld, conditioned or delayed); |
| ● | enter into any material transaction other than in the ordinary course of business; |
| ● | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any encumbrance with respect to such assets or properties, except in the ordinary course of business; |
| ● | sell, assign, transfer, license, sublicense or otherwise dispose of any material AgeX intellectual property (other than pursuant to non-exclusive licenses in the ordinary course of business); |
| ● | make, change or revoke any material tax election, file any amendment making any material change to any tax return, settle or compromise any income or other material tax liability or submit any voluntary disclosure application, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than seven months), or adopt or change any material accounting method in respect of taxes; |
| ● | enter into, materially amend or terminate any AgeX material contract; |
| ● | except as otherwise set forth in the AgeX Budget and the incurrence or payment of any Transaction Expenses, make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, in amounts that exceed the aggregate amount of the AgeX Budget by $100,000; |
| ● | other than as required by law or GAAP, take any action to change accounting policies or procedures; |
| ● | initiate or settle any legal proceeding; or other claim or dispute involving or against AgeX or any of its subsidiaries; |
| ● | enter into or amend a material contract that would reasonably be expected to prevent or materially impede, interfere with, hinder or delay the consummation of the Contemplated Transactions; or |
| ● | agree, resolve or commit to do any of the foregoing. |
During the period from the date of the Merger Agreement and continuing until the earlier of the termination of the Merger Agreement or the Effective Time, except (i) as set forth in Serina’s disclosure schedule, (ii) as expressly permitted by or required in accordance with the Merger Agreement, (iii) as required by applicable law or (iv) as may be consented to in writing by AgeX (not to be unreasonably withheld, delayed or conditioned), each of Serina and its subsidiaries has agreed to (A) conduct its business and operations in the ordinary course of business and in compliance in all material respects with all applicable laws and the requirements of all of its material contracts and (B) continue to pay material outstanding accounts payable and other material current liabilities (including payroll) when due and payable, and will not, and will not cause or permit any of its subsidiaries to:
| ● | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except repurchases of shares of Serina common stock from terminated employees, directors or consultants of AgeX or in connection with the payment of the exercise price and/or withholding taxes incurred upon the exercise, settlement or vesting of any award or purchase rights granted under the Serina Plan in accordance with the terms of such award in effect on the date of the Merger Agreement); |
| ● | sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of Serina or any of its subsidiaries (except for shares of Serina common stock issued upon the valid exercise of outstanding Serina stock options or Serina warrants); (B) any option, warrant or right to acquire any capital stock or any other security other than Serina stock options or restricted stock unit awards granted to directors, employees and service providers in the ordinary course of business which are included in the calculation of the Company Outstanding Shares; or (C) any instrument convertible into or exchangeable for any capital stock or other security of Serina or any of its subsidiaries; |
| ● | except as required to give effect to anything in contemplation of the closing of the Merger, amend any of its or its subsidiaries’ organizational documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions; |
| ● | form a subsidiary or acquire any equity interest or other interest in any other entity or enter into a joint venture with any other entity; |
| ● | (A) lend money to any person (except for the advancement of reasonable and customary expenses to employees, directors and consultants in the ordinary course of business), (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, (D) other than the incurrence or payment of Transaction Expenses, make any capital expenditure in excess of $100,000 of the budgeted capital expenditure amounts set forth in Serina’s operating budget delivered to AgeX concurrently with the execution of the Merger Agreement (Serina Budget) or (E) forgive any loans to any persons, including Serina’s employees, officers, directors or affiliates; |
| ● | other than as required by applicable law or the terms of any Serina benefit plan as in effect on the date of the Merger Agreement: (A) adopt, terminate, establish or enter into any Serina benefit plan; (B) cause or permit any Serina benefit plan to be amended; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or bonus or other compensation or remuneration payable to, any of its directors, officers, consultants or employees other than increases in base salary and annual cash bonus opportunities and payments made, in each case, in the ordinary course of business; (D) increase the severance or change of control benefits offered to any current or new employees, directors or consultants; or (E) terminate or give notice to any officer other than for cause; |
| ● | recognize any labor union or labor organization, except as otherwise required by applicable law and after prior written consent of AgeX (not to be unreasonably withheld, conditioned or delayed); |
| ● | enter into any material transaction other than in the ordinary course of business; |
| ● | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any encumbrance with respect to such assets or properties, except in the ordinary course of business; |
| ● | sell, assign, transfer, license, sublicense or otherwise dispose of any Serina intellectual property (other than pursuant to non-exclusive licenses in the ordinary course of business); |
| ● | make, change or revoke any material tax election, file any amendment making any material change to any tax return, settle or compromise any income or other material tax liability or submit any voluntary disclosure application, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than seven months), or adopt or change any material accounting method in respect of taxes; |
| ● | enter into, materially amend or terminate any Serina material contract (other than statements of work entered into or amended in the ordinary course of business); |
| ● | except as otherwise set forth in the Serina Budget and the incurrence or payment of any Transaction Expenses, make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, in amounts that exceed the aggregate amount of the Serina Budget by $150,000; |
| ● | other than as required by law or GAAP, take any action to change accounting policies or procedures; |
| ● | initiate or settle any legal proceeding or other claim or dispute involving or against Serina or any of its subsidiaries; |
| ● | enter into or amend a contract that would reasonably be expected to prevent or materially impede, interfere with, hinder or delay the consummation of the Contemplated Transactions; or |
| ● | agree, resolve or commit to do any of the foregoing. |
Termination and Termination Fees
The Merger Agreement may be terminated prior to the Effective Time (whether before or after the required stockholder approvals to consummate the Merger have been obtained, unless otherwise specified below):
| (a) | by mutual written consent of AgeX and Serina; |
| | | |
| (b) | by either AgeX or Serina if the Contemplated Transactions have not been consummated by February 29, 2024 (subject to possible extension as provided in this paragraph, the End Date); provided, however, that the right to terminate the Merger Agreement under this paragraph will not be available to a party if such party’s (or, in the case of AgeX, Merger Sub’s) action or failure to act has been a principal cause of the failure of the Contemplated Transactions to occur on or before the End Date and such action or failure to act constitutes a breach of the Merger Agreement; provided, further, however, that, in the event that a request for additional information has been made by any governmental body or in the event that the SEC has not declared the registration statement effective under the Securities Act by the date which is 30 calendar days prior to the End Date, then AgeX will be entitled to extend the End Date for an additional 60 calendar days by written notice to Serina; |
| (c) | by either AgeX or Serina if a court of competent jurisdiction or other governmental body has issued a final and non-appealable order, decree or ruling, or has taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the Contemplated Transactions; |
| | | |
| (d) | by AgeX if the Serina Stockholder Written Consent has not been obtained within three business days of the date of the registration statement becoming effective in accordance with the provisions of the Securities Act; provided, however, that once the Serina Stockholder Written Consent has been obtained, AgeX may not terminate the Merger Agreement pursuant to this paragraph; |
| | | |
| (e) | by either AgeX or Serina if (i) the AgeX special meeting (including any adjournments and postponements thereof) was held and completed and (ii) the AgeX Stockholder Matters were not approved at such AgeX special meeting by the Required AgeX Stockholder Vote; provided, however, that the right to terminate the Merger Agreement pursuant to this paragraph will not be available to AgeX where the failure to obtain the Required AgeX Stockholder Vote was caused by the action or failure to act of AgeX and such action or failure to act constitutes a material breach by AgeX of the Merger Agreement; |
| | | |
| (f) | by Serina (at any time prior to the approval of the AgeX Stockholder Matters by the Required AgeX Stockholder Vote) if an AgeX Triggering Event (as defined below) has occurred; |
| | | |
| (g) | by AgeX (at any time prior to the Required Serina Stockholder Vote being obtained) if a Serina Triggering Event (as defined below) has occurred; |
| | | |
| (h) | by Serina, upon a breach of any representation, warranty, covenant or agreement set forth in the Merger Agreement by AgeX or Merger Sub or if any representation or warranty of AgeX or Merger Sub has become inaccurate, in either case, such that certain closing conditions set forth in the Merger Agreement would not be satisfied as of the time of such breach or as of the time such representation or warranty has become inaccurate; provided that Serina is not then in material breach of any representation, warranty, covenant or agreement under the Merger Agreement; provided, further, that if such inaccuracy in AgeX’s or Merger Sub’s representations and warranties or breach by AgeX or Merger Sub is curable by the End Date by AgeX or Merger Sub, then the Merger Agreement will not terminate pursuant to this paragraph as a result of such particular breach or inaccuracy until the earlier of (i) the End Date and (ii) the expiration of a 30 calendar day period commencing upon delivery of written notice from Serina to AgeX or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to this paragraph (it being understood that the Merger Agreement will not terminate pursuant to this paragraph as a result of such particular breach or inaccuracy if such breach by AgeX or Merger Sub is cured prior to such termination becoming effective); |
| | | |
| (i) | by AgeX, upon a breach of any representation, warranty, covenant or agreement set forth in the Merger Agreement by Serina or if any representation or warranty of Serina has become inaccurate, in either case, such that certain closing conditions set forth in the Merger Agreement would not be satisfied as of the time of such breach or as of the time such representation or warranty has become inaccurate; provided that AgeX is not then in material breach of any representation, warranty, covenant or agreement under the Merger Agreement; provided, further, that if such inaccuracy in Serina’s representations and warranties or breach by Serina is curable by the End Date by Serina then the Merger Agreement will not terminate pursuant to this paragraph as a result of such particular breach or inaccuracy until the earlier of (i) the End Date and (ii) the expiration of a 30 calendar day period commencing upon delivery of written notice from AgeX to Serina of such breach or inaccuracy and its intention to terminate pursuant to this paragraph (it being understood that the Merger Agreement will not terminate pursuant to this paragraph as a result of such particular breach or inaccuracy if such breach by Serina is cured prior to such termination becoming effective); |
| (j) | by AgeX, at any time prior to the approval of the AgeX Stockholder Matters by the Required AgeX Stockholder Vote, if (i) AgeX has received a Superior Offer, (ii) AgeX has complied with its obligations under the non-solicitation and AgeX Board Adverse Recommendation Change provisions of the Merger Agreement, (iii) the AgeX Board has determined in good faith, after consultation with its outside legal counsel, that the failure to terminate the Merger Agreement would be reasonably likely to be inconsistent with its fiduciary duties under applicable law, (iv) AgeX concurrently terminates the Merger Agreement and enters into a definitive agreement with respect to such Superior Offer and (v) AgeX concurrently pays to Serina the applicable termination fee; |
| | | |
| (k) | by AgeX, if Serina has not provided to AgeX the audited consolidated financial statements for the fiscal year ended 2022 in accordance with the Merger Agreement; |
| | | |
| (l) | by Serina, if the closing condition related to the Actual Closing Price being not less than $12.00 is not satisfied or waived; provided, however, that if Serina exercises its termination rights under this paragraph then Serina will provide AgeX with written notice five days prior to such termination and AgeX will have the option to within five business days of receipt by AgeX of such notice to provide Serina with a written offer to adjust the exchange ratio such that additional shares of AgeX common stock will be issued to the Serina stockholders in the amount necessary to equal the Target Merger Consideration Minimum Value (as defined below), and if AgeX provides that written offer, then Serina may not exercise its termination right under this paragraph and the related closing condition will be deemed to have been satisfied; |
| | | |
| (m) | by Serina, if the closing condition related to Juvenescence holding at least 1,133,308 post-merger warrants upon issuance is not satisfied or waived; provided, however, that if Serina exercises its termination rights under this paragraph then Serina will provide AgeX with written notice five days prior to such termination and AgeX will have the option to within five business days of receipt by AgeX of such notice to provide a sufficient number of post-merger warrants to Juvenescence such that the aggregate number of post-merger warrants subject to the Side Letter is at least 1,133,308, and if AgeX provides that additional post-merger warrants to Juvenescence, then Serina may not exercise its termination right under this paragraph and the related closing condition will be deemed to have been satisfied; or |
| | | |
| (n) | by Serina, if the closing condition related to AgeX holding at least $500,000 in unrestricted cash is not satisfied or waived; provided, however, that if Serina exercises its termination rights under this paragraph then Serina will provide AgeX with written notice prior to such termination and AgeX will have the option to within five business days of receipt by AgeX of such notice to obtain sufficient net funds, and if AgeX obtains such sufficient net funds to satisfy the contemplated closing condition, then Serina may not exercise its termination right under this paragraph and the related closing condition will be deemed to have been satisfied. |
The party desiring to terminate the Merger Agreement will give the other party written notice of such termination, specifying the provisions of the Merger Agreement pursuant to which such termination is made and the basis therefor described in reasonable detail.
“Serina Triggering Event” will be deemed to have occurred if: (a) the Serina Board has made a Serina Board Adverse Recommendation Change; (b) the Serina Board or any committee thereof has publicly approved, endorsed or recommended any Acquisition Proposal; or (c) Serina has entered into any letter of intent or similar document or any contract relating to any Acquisition Proposal.
“AgeX Triggering Event” will be deemed to have occurred if: (a) AgeX has failed to include in the proxy statement/prospectus/information statement the AgeX Board Recommendation or has made an AgeX Board Adverse Recommendation Change; (b) the AgeX Board or any committee thereof has approved, endorsed or recommended any Acquisition Proposal; (c) AgeX has entered into any letter of intent or similar document or any contract relating to any Acquisition Proposal (other than a confidentiality agreement permitted pursuant to the Merger Agreement); or (d) AgeX or any director or officer of AgeX has willfully and intentionally breached the provisions set forth in the non-solicitation or AgeX stockholders’ meeting provisions of the Merger Agreement.
“Target Merger Consideration Minimum Value” means an amount equal to $12.00 multiplied by the anticipated aggregate number of shares of AgeX common stock to be issued (approximately 7,500,000 on a post-reverse stock split basis prior to any adjustment described in “—Termination and Termination Fees”).
AgeX must pay Serina a nonrefundable termination fee of $1,000,000 if (A) the Merger Agreement is terminated by Serina pursuant to clause (b), (e) or (h) above, (B) an Acquisition Proposal with respect to AgeX has been publicly announced, disclosed or otherwise communicated to AgeX or the AgeX Board at any time after the date of the Merger Agreement but prior to the termination of the Merger Agreement (which has not been withdrawn) and (C) within 12 months after the date of such termination, AgeX enters into a definitive agreement with respect to a subsequent transaction or consummates a subsequent transaction in respect of such Acquisition Proposal. AgeX must pay Serina a nonrefundable termination fee of $1,000,000 if the Merger Agreement is terminated by Serina pursuant to clause (f) above (or at the time the Merger Agreement is terminated, Serina has the right to terminate the Merger Agreement pursuant to clause (f) above) or the Merger Agreement is terminated by AgeX pursuant to clause (j) above.
Serina must pay AgeX a nonrefundable termination fee of $1,000,000 if (i) (A) the Merger Agreement is terminated by AgeX pursuant to clause (b), (d) or (i) above, (B) an Acquisition Proposal with respect to Serina has been publicly announced, disclosed or otherwise communicated to Serina or the Serina Board at any time after the date of the Merger Agreement but prior to the termination of the Merger Agreement (which has not been withdrawn) and (C) within 12 months after the date of such termination, Serina enters into a definitive agreement with respect to a subsequent transaction or consummates a subsequent transaction in respect of such Acquisition Proposal; or (ii) the Merger Agreement is terminated by AgeX pursuant to clause (g) above (or at the time the Merger Agreement is terminated, AgeX has the right to terminate the Merger Agreement pursuant to clause (g) above).
If the Merger Agreement is terminated by Serina pursuant to clause (h) above or in the event of the failure of Serina to consummate the transactions to be contemplated at the closing of the Merger solely as a result of an AgeX Material Adverse Effect as set forth in the Merger Agreement (provided, that at such time all other conditions precedent to AgeX’s obligation to close set forth in the Merger Agreement have been satisfied by Serina, are capable of being satisfied by Serina or have been waived by AgeX), then AgeX will reimburse Serina for all reasonable out-of-pocket fees and expenses incurred by Serina in connection with the Merger Agreement and the Contemplated Transactions, up to a maximum of $1,000,000, by wire transfer of same-day funds within ten business days following the date on which Serina submits to AgeX true and correct copies of reasonable documentation supporting such expenses; provided, however, that such expenses shall not include any amounts paid to Juvenescence or any affiliate thereof or any amounts for financial advisors to Serina except for reasonably documented out-of-pocket expenses otherwise reimbursable by Serina to such financial advisors pursuant to the terms of Serina’s engagement letter or similar arrangement with such financial advisors.
If the Merger Agreement is terminated by AgeX pursuant to clause (i) or in the event of the failure of AgeX to consummate the transactions to be consummated to the closing of the Merger solely as a result of a Serina Material Adverse Effect as set forth in the Merger Agreement (provided, that at such time all other conditions precedent to Serina’s obligation to close set forth in the Merger Agreement have been satisfied by AgeX, are capable of being satisfied by AgeX or have been waived by Serina), then Serina will reimburse AgeX for all reasonable out-of-pocket fees and expenses incurred by AgeX in connection with the Merger Agreement and the Contemplated Transactions, up to a maximum of $1,000,000, by wire transfer of same-day funds within ten business days following the date on which AgeX submits to Serina true and correct copies of reasonable documentation supporting such expenses; provided, however, that such expenses shall not include any amounts paid to Juvenescence or any affiliate thereof or any amounts for financial advisors to AgeX except for reasonably documented out-of-pocket expenses otherwise reimbursable by AgeX to such financial advisors pursuant to the terms of AgeX’s engagement letter or similar arrangement with such financial advisors.
Both AgeX and Serina shall be entitled only to either the termination fee or the reimbursement of expenses, but not both.
Other Agreements
Indemnification and Insurance
The Merger Agreement provides that, subject to certain limitations as set forth in the Merger Agreement, from the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, AgeX and the combined company will indemnify each person who is, has been at any time prior to the date of the Merger Agreement, or who becomes prior to the Effective Time, a director, officer, fiduciary or agent of AgeX or Serina or their respective subsidiaries.
The Merger Agreement also provides that the provisions relating to the indemnification, advancement of expenses and exculpation of present and former directors and officers of AgeX or any of its subsidiaries set forth in the organizational documents of AgeX or any of its subsidiaries will not be amended, modified or repealed for a period of six years from the Effective Time in any manner that would adversely affect the rights of individuals who, at or prior to the Effective Time, were officers or directors of AgeX or any of its subsidiaries, unless required by applicable law. After the closing of the Merger, the organizational documents of the combined company will contain provisions at least as favorable as the provisions relating to the indemnification, advancement of expenses and exculpation of present and former directors and officers presently set forth in AgeX’s organizational documents as of the date of the Merger Agreement. AgeX has agreed to purchase a six year “tail policy” with an effective date as of the Closing Date for the non-cancellable extension of AgeX’s existing directors’ and officers’ liability insurance policies and AgeX’s existing fiduciary liability insurance policies (if any).
Listing
AgeX common stock currently is listed on the NYSE American market under the symbol “AGEX.” AgeX has agreed to use commercially reasonable efforts (i) to maintain its existing listing on NYSE American until the Closing Date and to obtain approval of the listing of the combined company on NYSE American, (ii) without derogating from the requirements of the foregoing clause (i) and to the extent required by the rules and regulations of NYSE American, to prepare and submit to NYSE American a notification form for the listing of the shares of AgeX common stock to be issued in connection with the Contemplated Transactions, and to cause such shares to be approved for listing (subject to official notice of issuance), (iii) to effect the reverse stock split and (iv) to the extent required by Section 101 of the NYSE American Company Guide, to file an initial NYSE American listing application for the AgeX common stock on NYSE American and to cause such listing application to be conditionally approved prior to the Effective Time.
The parties will use commercially reasonable efforts to coordinate with respect to compliance with NYSE American rules and regulations. Serina will cooperate with AgeX as reasonably requested by AgeX with respect to the NYSE American listing application and promptly furnish to AgeX all information concerning Serina and its stockholders that may be required or reasonably requested in connection with any action contemplated by the foregoing paragraph.
Expenses
Pursuant to the Merger Agreement, all the Transaction Expenses (as defined below) will be paid by the party incurring such expense, whether or not the Merger is consummated, except as described above in the section titled “—Termination and Termination Fees” beginning on page 173 of this proxy statement/prospectus/information statement.
“Transaction Expenses” means, with respect to each party, all fees and expenses incurred by such party at or prior to the Effective Time in connection with the Contemplated Transactions and the Merger Agreement, including (a) any fees and expenses of legal counsel and accountants and the maximum amount of fees and expenses payable to financial advisors, investment bankers, brokers, consultants, and other advisors of such party in connection with the negotiation, preparation and execution of the Merger Agreement and the consummation of the Contemplated Transactions (including in connection with any stockholder litigation relating to the Merger Agreement or any of the Contemplated Transactions), including finders’ fees; (b) only with respect to AgeX, fees paid to the SEC in connection with filing the Registration Statement, the proxy statement/prospectus/information statement, and any amendments and supplements hereto or thereto, with the SEC; (c) only with respect to AgeX, any fees and expenses in connection with the printing, mailing and distribution of the Registration Statement and any amendments and supplements hereto; (d) 50% of the fees and expenses payable to NYSE American in connection with the NYSE American listing application; (e) any bonus, severance, change-in-control or retention payments or similar payment obligations (including payments with “single-trigger” provisions triggered at and as of the closing of the Merger) that become due or payable to any director, officer, employee or consultant of AgeX or any of its subsidiaries in connection with the consummation of the Contemplated Transactions; (f) only with respect to AgeX, any fees and expenses incurred in connection with the directors’ and officers’ tail policy described above; (g) only with respect to AgeX, any notice payments, change-of-control payments, fines or other payments to be made by Parent in connection with terminating any existing contract to which AgeX is a party or winding down any of AgeX’s clinical trial obligations; and (i) only with respect to AgeX, any wind-down costs of AgeX associated with discontinued lab, research and development and related operations.
Amendment of Merger Agreement
The Merger Agreement may be amended by the parties at any time with the written approval of the respective boards of directors of Serina, Merger Sub and AgeX, except that after the Merger Agreement has been adopted and approved by a party’s stockholders, no amendment which by law requires further approval by the stockholders of that party will be made without such further stockholder approval.
AGREEMENTS RELATED TO THE MERGER
Support Agreements
Concurrently with the execution of the Merger Agreement, Juvenescence, a stockholder of AgeX holding approximately 43.3% of the outstanding shares of common stock of AgeX, 100% of the outstanding shares of Series A preferred stock of AgeX and 100% of the outstanding shares of Series B preferred stock of AgeX, in each case as of August 29, 2023, entered into the AgeX Support Agreement in favor of Serina, providing, among other things, that Juvenescence will vote all of its shares of AgeX capital stock: (i) in favor of the AgeX Stockholder Matters, (ii) against any proposal made in opposition to, or in competition with, the Merger Agreement or the Merger and (iii) against any acquisition proposal involving a third party other than Serina
In connection with the execution of the Merger Agreement, stockholders of Serina holding 100% of the outstanding shares of common stock of Serina, 100% of the outstanding shares of Series A preferred stock of Serina, 100% of the outstanding shares of Series A-1 preferred stock of Serina, 100% of the outstanding shares of Series A-2 preferred stock of Serina, 100% of the outstanding shares of Series A-3 preferred stock of Serina, 100% of the outstanding shares of Series A-4 preferred stock of Serina, and 100% of the outstanding shares of Series A-5 preferred stock of Serina, in each case effective as of August 29, 2023, entered into the Serina Support Agreements in favor of AgeX, providing, among other things, that such stockholders will vote all of their shares of Serina capital stock (or execute a written consent): (i) in favor of Serina Stockholder Matters, (ii) against any proposal made in opposition to, or in competition with, the Merger Agreement or the Merger and (iii) against any acquisition proposal involving a third party other than AgeX.
Lock-Up Agreements
Concurrently with the execution of the Merger Agreement, (i) certain executive officers, directors, and stockholders of Serina and (ii) certain stockholders of AgeX, entered into the Lock-Up Agreements, pursuant to which such persons accepted certain restrictions on transfers of the shares of AgeX common stock held by such persons for the 180-day period following the Effective Time.
Side Letter
Concurrently with the execution of the Merger Agreement, AgeX, Serina and Juvenescence entered into the Side Letter, which will become effective immediately prior to the closing of the Merger. The Side Letter provides that:
| (vii) | immediately prior to the Effective Time, Juvenescence will cancel all out of the money AgeX warrants held by Juvenescence; |
| (viii) | Juvenescence will exercise all of the post-merger warrants it holds to provide the combined company approximately an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; |
| (ix) | AgeX and Serina shall use reasonable best efforts to appoint certain directors, including Steve Ledger and Dr. J. Milton Harris, who are each affiliated with Serina, and Dr. Gregory Bailey and Dr. Richard Marshall, who are each affiliated with Juvenescence, to the Combined Company Board effective as of the Effective Time; |
| (x) | Juvenescence will not sell any shares of AgeX Series A preferred stock or AgeX Series B preferred stock and will take all actions necessary to effect the AgeX preferred stock conversion, whereby all of such preferred stock will be converted into AgeX common stock prior to the reverse stock split; |
| (xi) | Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of certain loans to AgeX; and |
| (xii) | Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loans agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. |
Warrant Agreement
In connection with the closing of the Merger, AgeX will enter into a warrant agreement (the warrant agreement) with Equiniti (the warrant agent). Pursuant to the terms of the warrant agreement, AgeX will issue to each holder of AgeX common stock three post-merger warrants for each five shares of AgeX common stock held by such holder. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one incentive warrant. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock. Each post-merger warrant will expire on July 31, 2025, and each incentive warrant will expire on the four-year anniversary of the Closing Date. Each post-merger warrant and incentive warrant will be issued pursuant to the terms of a warrant agreement to be entered into by AgeX and a warrant agent in connection with the closing of the Merger. The terms of the warrant agreement remain subject to agreement between AgeX and Serina.
MATTERS BEING SUBMITTED TO A VOTE OF AGEX’S STOCKHOLDERS
PROPOSAL NO. 1 (THE STOCK ISSUANCE PROPOSAL):
APPROVAL OF THE ISSUANCE OF SHARES OF AGEX COMMON STOCK PURSUANT TO THE MERGER AGREEMENT
General
At the AgeX special meeting, AgeX’s common stockholders will be asked to approve the issuance of AgeX common stock to Serina’s stockholders pursuant to the Merger Agreement, which shares of AgeX common stock to be issued pursuant to the Merger will represent more than 20% of the shares of AgeX common stock outstanding immediately prior to the Merger in connection with the Merger and result in a change of control resulting from the Merger, pursuant to Sections 712(b) and 713(b) of the NYSE American Company Guide.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant.
The terms of, reasons for and other aspects of the Merger Agreement, the Merger, the issuance of AgeX common stock pursuant to the Merger Agreement are described in more detail in the other sections in this proxy statement/prospectus/information statement.
Reason for the Proposal
Under Section 712(b) of the NYSE American Company Guide, stockholder approval is required prior to the issuance of common stock, or of securities convertible into common stock, in which the sales, issuance or potential issuance is equal to or in excess of 20% of the number of shares of common stock outstanding before the issuance of the common stock or securities convertible into common stock. AgeX will issue to Serina’s equityholders shares representing 20% or more of the number of shares of AgeX common stock outstanding immediately prior to the Merger pursuant to the Merger Agreement. Accordingly, in order to ensure compliance with Section 712(b) of the NYSE American Company Guide, AgeX is asking for the approval of AgeX’s stockholders for the issuance of these shares of AgeX common stock.
Under Section 713(b) of the NYSE American Company Guide, stockholder approval is required prior to the issuance or potential issuance of additional shares which will result in a change of control, including in connection with a reverse merger as defined in the NYSE American rules. It is expected that NYSE American will determine that the Merger constitutes a “change of control” of AgeX. Accordingly, in order to ensure compliance with Section 713(b) of the NYSE American Company Guide, AgeX is asking for the approval of AgeX’s stockholders of the change of control of AgeX resulting from the issuance of such shares of AgeX common stock in connection with the Merger.
Required Vote; Recommendation of the AgeX Board
The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote is required to approve Proposal No. 1. Abstentions and broker non-votes, if any, will have no effect on the outcome of this proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX’S COMMON STOCKHOLDERS VOTE “FOR” THE STOCK ISSUANCE PROPOSAL. THE APPROVAL OF EACH OF PROPOSAL NOS. 1, 2, 3, 4 AND 5 IS REQUIRED TO CONSUMMATE THE MERGER.
PROPOSAL NO. 2 (THE REVERSE STOCK SPLIT PROPOSAL):
APPROVAL OF AN AMENDMENT TO THE AGEX CERTIFICATE OF
INCORPORATION EFFECTING THE REVERSE STOCK SPLIT AT A RATIO IN THE RANGE FROM 1-FOR-35 TO 1-FOR-36
General
At the AgeX special meeting, AgeX’s common stockholders will be asked to approve an amendment to the AgeX Charter, attached to this proxy statement/prospectus/information statement as Annex E, to effect the reverse stock split of AgeX common stock at a ratio anywhere in the range between one new share for every 35 shares and one new share for every 36 shares outstanding. Prior to the effectiveness of the Merger, AgeX and Serina will mutually agree upon the exact reverse split ratio within such range. Upon the effectiveness of the amendment to the AgeX Charter effecting the reverse stock split, or the split effective time, the issued shares of AgeX common stock immediately prior to the split effective time will be reclassified into a smaller number of shares within the specified range, such that a stockholder of AgeX will own one new share of AgeX common stock for the specified number of shares of issued common stock held by that stockholder immediately prior to the split effective time.
If Proposal No. 2 is approved, the reverse stock split would become effective prior to the effectiveness of the Merger. AgeX may effect only one reverse stock split in connection with this Proposal No. 2. AgeX and Serina’s mutual decision will be based on a number of factors, including market conditions, existing and expected trading prices for AgeX common stock and the listing requirements of the NYSE American.
If Proposals No. 1, 3, 4 or 5 are not approved and the Merger is not effected, the AgeX Board may still choose to implement the reverse stock split in order to effectively increase the per share price of AgeX common stock.
The form of the amendment to the AgeX Charter to effect the reverse stock split, as more fully described below, will effect the reverse stock split but will not change the number of authorized shares of common stock or preferred stock, or the par value of AgeX common stock or preferred stock.
Purpose
The AgeX Board approved the proposal approving the amendment to the AgeX Charter for the purpose of effecting the reverse stock split pursuant to the terms of the Merger Agreement.
NYSE American Requirements for Listing on the NYSE American
AgeX common stock is listed on the NYSE American under the symbol “AGE.” AgeX intends to file an initial listing application with the NYSE American, as described below, to seek a listing upon the closing of the Merger. According to the NYSE American rules, an issuer must, in a case such as this, submit an initial listing application for a post-transaction entity prior to the consummation of a transaction whereby the issuer combines with a non-NYSE American entity, resulting in a change of control of the issuer and potentially allowing the non-NYSE American entity to obtain an NYSE American listing. Accordingly, the listing standards of the NYSE American will require AgeX to have, among other things, a $ per share minimum bid price upon the closing of the Merger. Therefore, the reverse stock split may be necessary in order to consummate the Merger.
One of the effects of the reverse stock split will be to effectively increase the proportion of authorized shares which are unissued relative to those which are issued. This could result in AgeX’s management being able to issue more shares without further stockholder approval. For example, before the reverse stock split, as of , 2023, AgeX’s authorized shares of common stock immediately prior to the closing of the Merger is 100,000,000 compared to shares issued and outstanding of . If AgeX effects the reverse stock split using a :1 ratio, its authorized shares of common stock immediately prior to the closing of the Merger would still be 100,000,000 compared to shares issued and outstanding of . The reverse stock split will not affect the number of authorized shares of AgeX common stock which will continue to be authorized pursuant to the certificate of incorporation of AgeX.
Potential Increased Investor Interest
On , 2023, AgeX common stock closed at $ per share. An investment in AgeX common stock may not appeal to brokerage firms that are reluctant to recommend lower priced securities to their clients. Investors may also be dissuaded from purchasing lower priced stocks because the brokerage commissions, as a percentage of the total transaction, tend to be higher for such stocks. Moreover, the analysts at many brokerage firms do not monitor the trading activity or otherwise provide coverage of lower priced stocks. Also, the AgeX Board believes that most investment funds are reluctant to invest in lower priced stocks.
There are risks associated with the reverse stock split, including that the reverse stock split may not result in an increase in the per share price of AgeX common stock.
AgeX cannot predict whether the reverse stock split will increase the market price for AgeX common stock in the future. The history of similar stock split combinations for companies in like circumstances is varied. There is no assurance that:
| ● | the market price per share of AgeX common stock after the reverse stock split will rise in proportion to the reduction in the number of shares of AgeX common stock outstanding before the reverse stock split; |
| ● | the reverse stock split will result in a per share price that will attract brokers and investors who do not trade in lower priced stocks; |
| ● | the reverse stock split will result in a per share price that will increase the ability of AgeX to attract and retain employees; and |
| ● | AgeX will continue to meet the listing standards of the NYSE American. |
The market price of AgeX common stock will also be based on performance of AgeX and other factors, some of which are unrelated to the number of shares outstanding. If the reverse stock split is effected and the market price of AgeX common stock declines, the percentage decline as an absolute number and as a percentage of the overall market capitalization of AgeX may be greater than would occur in the absence of a reverse stock split. Furthermore, the liquidity of AgeX common stock could be adversely affected by the reduced number of shares that would be outstanding after the reverse stock split.
Criteria to be Used for Determining Which Reverse Stock Split Ratio to Implement
In determining which reverse stock split ratio to implement, if any, following receipt of stockholder approval of the Reverse Stock Split Proposal, AgeX and/or Serina may consider the ratio that results in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time.
If Proposals No. 1, 3, 4 or 5 are not approved and the Merger is not effected, then, in determining which reverse stock split ratio to implement, if any, following receipt of stockholder approval of the Reverse Stock Split Proposal, the AgeX Board may consider, among other things, various factors, such as:
| ● | the historical trading price and trading volume of AgeX common stock; |
| ● | the then-prevailing trading price and trading volume of AgeX common stock and the expected impact of the reverse stock split on the trading market for AgeX common stock in the short- and long-term; |
| ● | the ability of the combined company to satisfy the NYSE American initial listing requirements for its common stock on the NYSE American; |
| ● | which reverse stock split ratio would result in the least administrative cost to AgeX; and |
| ● | prevailing general market and economic conditions. |
Principal Effects of the Reverse Stock Split
The amendment to the AgeX Charter effecting the reverse stock split is set forth in Annex E to this proxy statement/prospectus/information statement.
The reverse stock split will be effected simultaneously for all outstanding shares of AgeX common stock. The reverse stock split will affect all of AgeX’s stockholders uniformly and will not affect any stockholder’s percentage ownership interest in AgeX, except to the extent that the reverse stock split results in any of AgeX’s stockholders owning a fractional share. Shares of AgeX common stock issued pursuant to the reverse stock split will remain fully paid and nonassessable. The reverse stock split does not affect the total proportionate ownership of AgeX following the Merger. The reverse stock split will not affect AgeX continuing to be subject to the periodic reporting requirements of the Exchange Act.
As an example, the following table illustrates the effects of a 1-for-35 to 1-for-36 reverse stock split (without giving effect to the treatment of fractional shares):
| | | Prior to Reverse Stock Split | | | After 1-for-35 Reverse Stock Split | | | After 1-for-36 Reverse Stock Split | |
| Common stock outstanding | | | | | | | | | | | | |
| Common stock issuable pursuant to outstanding equity awards(1) | | | | | | | | | | | | |
| (1) | Substantially all such options have an exercise price higher than $ per share, the closing price of AgeX common stock on , 2023. |
In addition, if the reverse stock split is implemented, it will increase the number of AgeX stockholders who own “odd lots” of fewer than 100 shares of common stock. Brokerage commission and other costs of transactions in odd lots are generally higher than the costs of transactions of more than 100 shares of common stock. Accordingly, the reverse stock split may not achieve the desired results of increasing marketability and liquidity of AgeX common stock that have been described above.
After the effective date of the reverse stock split, AgeX common stock would have a new committee on uniform securities identification procedures (CUSIP number), a number used to identify AgeX common stock.
AgeX common stock is currently registered under Section 12(b) of the Exchange Act, and AgeX is subject to the periodic reporting and other requirements of the Exchange Act. The proposed reverse stock split will not affect the registration of AgeX common stock under the Exchange Act.
Procedure for Effecting the Reverse Stock Split and Exchange of Stock Certificates
If AgeX’s common stockholders approve the amendment to the AgeX Charter effecting the reverse stock split, and if the AgeX Board still believes that a reverse stock split is in the best interests of AgeX and its stockholders, AgeX will file the amendment to the AgeX Charter with the Secretary of State of the State of Delaware at such time as the AgeX Board has determined to be the appropriate split effective time. The AgeX Board may delay effecting the reverse stock split without resoliciting stockholder approval. Beginning at the split effective time, each certificate representing pre-split shares will be deemed for all corporate purposes to evidence ownership of post-split shares.
Beneficial Owners of Common Stock. Upon the implementation of the reverse stock split, AgeX intends to treat shares held by stockholders in “street name” (i.e., through a bank, broker, custodian or other nominee), in the same manner as registered stockholders whose shares are registered in their names. Banks, brokers, custodians or other nominees will be instructed to effect the reverse stock split for their beneficial holders holding AgeX common stock in street name. However, these banks, brokers, custodians or other nominees may have different procedures than registered stockholders for processing the reverse stock split and making payment for fractional shares. If a stockholder holds shares of AgeX common stock with a bank, broker, custodian or other nominee and has any questions in this regard, stockholders are encouraged to contact their bank, broker, custodian or other nominee.
Registered Holders of Common Stock in Book-Entry Form. Certain of AgeX’s registered holders of common stock hold some or all of their shares electronically in book-entry form with AgeX’s transfer agent, Equiniti. These stockholders do not hold physical stock certificates evidencing their ownership of AgeX common stock. However, they are provided with a statement reflecting the number of shares of AgeX common stock registered in their accounts. If a stockholder holds registered shares in book-entry form with AgeX’s transfer agent, no action needs to be taken to receive post-reverse stock split shares or payment in lieu of fractional shares, if applicable. If a stockholder is entitled to post-reverse stock split shares, a transaction statement will automatically be sent to the stockholder’s address of record indicating the number of shares of AgeX common stock held following the reverse stock split.
Registered Holders of Common Stock in Certificate Form. As soon as practicable after the split effective time, AgeX’s stockholders will be notified that the reverse stock split has been effected. AgeX expects that the AgeX transfer agent will act as exchange agent for purposes of implementing the exchange of stock certificates. Holders of pre-split shares will be asked to surrender to the exchange agent certificates representing pre-split shares held in certificated form in exchange for certificates representing post-split shares in accordance with the procedures to be set forth in a letter of transmittal to be sent by AgeX. No new certificates will be issued to a stockholder until such stockholder has surrendered such stockholder’s outstanding certificate(s) together with the properly completed and executed letter of transmittal to the exchange agent. Any pre-split shares submitted for transfer, whether pursuant to a sale or other disposition, or otherwise, will automatically be exchanged for post-split shares. Stockholders should not destroy any stock certificate(s) and should not submit any certificate(s) unless and until requested to do so.
Fractional Shares
No fractional shares will be issued in connection with the reverse stock split. Stockholders of record who otherwise would be entitled to receive fractional shares because they hold a number of pre-split shares not evenly divisible by the number of pre-split shares for which each post-split share is to be reclassified, will be entitled, upon surrender to the exchange agent of certificates representing such shares, to a cash payment in lieu thereof at a price equal to the fraction to which the stockholder would otherwise be entitled multiplied by the closing price of the common stock on the NYSE American on the date immediately preceding the split effective time. The ownership of a fractional interest will not give the holder thereof any voting, dividend, or other rights except to receive payment therefor as described herein.
By approving the amendment to the AgeX Charter effecting the reverse stock split, stockholders will be approving the combination of a whole number of shares of AgeX common stock between 35 to 36 into one share of AgeX common stock, with the actual ratio to be mutually agreed upon by the respective AgeX and Serina boards of directors prior to the effectiveness of the Merger.
Stockholders should be aware that, under the escheat laws of the various jurisdictions where stockholders reside, where AgeX is domiciled, and where the funds will be deposited, sums due for fractional interests that are not timely claimed after the effective date of the split may be required to be paid to the designated agent for each such jurisdiction, unless correspondence has been received by AgeX or the exchange agent concerning ownership of such funds within the time permitted in such jurisdiction. Thereafter, stockholders otherwise entitled to receive such funds will have to seek to obtain them directly from the state to which they were paid.
Accounting Consequences
The par value per share of AgeX common stock will remain unchanged at $0.0001 per share after the reverse stock split. As a result, at the reverse stock split effective time, the stated capital on AgeX’s balance sheet attributable to AgeX common stock will be reduced proportionately based on the reverse stock split ratio, from its present amount, and the additional paid-in capital account will be increased for the amount by which the stated capital is reduced. After the reverse stock split (and disregarding the impact of shares of AgeX common stock issued in the merger), net income or loss per share, and other per share amounts will be increased because there will be fewer shares of AgeX common stock outstanding. In future financial statements, net income or loss per share and other per share amounts for periods ending before the reverse stock split will be recast to give retroactive effect to the reverse stock split.
Potential Anti-Takeover Effect
Although the increased proportion of unissued authorized shares to issued shares could, under certain circumstances, have an anti-takeover effect, for example, by permitting issuances that would dilute the stock ownership of a person seeking to effect a change in the composition of the AgeX Board or contemplating a tender offer or other transaction for the combination of AgeX with another company, the Reverse Stock Split Proposal is not being proposed in response to any effort of which AgeX is aware to accumulate shares of AgeX common stock or obtain control of AgeX, other than in connection with the Merger, nor is it part of a plan by management to recommend a series of similar amendments to the AgeX Board and stockholders. Other than the proposals being submitted to AgeX’s common stockholders for their consideration at the AgeX special meeting, the AgeX Board does not currently contemplate recommending the adoption of any other actions that could be construed to affect the ability of third parties to take over or change control of AgeX.
Material U.S. Federal Income Tax Consequences of the Reverse Stock Split
The following is a discussion of material U.S. federal income tax consequences of the reverse stock split that are applicable to U.S. holders (as defined below) of AgeX common stock, but does not purport to be a complete analysis of all potential tax effects. This summary is based upon current provisions of the Code, existing Treasury regulations, judicial decisions, and published rulings and administrative pronouncements of the IRS, all in effect as of the date hereof and all of which are subject to differing interpretations or change. Any such change or differing interpretation, which may be retroactive, could alter the tax consequences to AgeX stockholders as described in this summary.
This summary is not a complete description of all tax consequences of the reverse stock split and does not address U.S. federal income tax consequences that may be relevant to particular AgeX stockholders in light of their personal circumstances or to AgeX stockholders who are subject to special treatment under U.S. federal income tax laws, such as AgeX stockholders who: do not hold their AgeX common stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment); are banks, insurance companies, tax-exempt entities, mutual funds, financial institutions, real estate investment trusts, regulated investment companies, government entities or broker-dealers; hold their AgeX common stock as “qualified small business stock” under Section 1202 of the Code or as “Section 1244 stock” under Section 1244 of the Code; hold their AgeX common stock as part of a hedging, “straddle,” conversion or other integrated transaction or are treated as having sold their AgeX common stock pursuant to the constructive sale provisions of the Code; are not U.S. holders (as defined below); acquired their AgeX common stock pursuant to the exercise of compensatory options, or in other compensatory transactions; acquired their AgeX common stock pursuant to the exercise of warrants or conversion rights under convertible instruments; are subject to special tax accounting rules under Section 451(b) of the Code; hold their AgeX common stock through individual retirement or other tax-deferred accounts; acquired their AgeX common stock in a transaction subject to the gain rollover provisions of Section 1045 of the Code; have a functional currency other than the U.S. dollar; or are partnerships or entities or arrangements classified as partnerships or disregarded entities for U.S. federal income tax purposes, S corporations, or other pass-through entities (including hybrid entities) and investors therein.
In addition, the following discussion does not address: (a) the tax consequences of transactions effectuated before, after or at the same time as the reverse stock split, whether or not they are in connection with the reverse stock split; (b) any U.S. federal non-income tax consequences of the reverse stock split, including estate, gift or other tax consequences; (c) any state, local or non-U.S. tax consequences of the reverse stock split; or (d) the Medicare contribution tax on net investment income. No ruling from the IRS or opinion of counsel, has been or will be requested in connection with the reverse stock split. AgeX stockholders should be aware that the IRS could adopt a position which could be sustained by a court contrary to that set forth in this discussion.
If an entity that is treated as a partnership for U.S. federal income tax purposes holds AgeX common stock prior to the reverse stock split, the tax treatment of a partner in such partnership will generally depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partner should consult its tax advisors as to the tax consequences of the reverse stock split.
No opinions of counsel or rulings from the IRS have been requested or obtained in connection with the reverse stock split. Accordingly, all AgeX stockholders should consult their own tax advisors as to the specific tax consequences to them of the reverse stock split.
IN VIEW OF THE FOREGOING AND BECAUSE THE FOLLOWING DISCUSSION IS INTENDED AS A GENERAL SUMMARY ONLY, EACH AGEX STOCKHOLDER SHOULD CONSULT SUCH STOCKHOLDER’S TAX ADVISOR REGARDING THE TAX CONSEQUENCES, INCLUDING THE APPLICABLE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES, AND ANY TAX REPORTING REQUIREMENTS OF THE REVERSE STOCK SPLIT IN LIGHT OF SUCH STOCKHOLDER’S OWN TAX SITUATION.
Definition of “U.S. Holder”
For purposes of this discussion, a “U.S. holder” is a beneficial owner of AgeX common stock that is, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation or any other entity taxable as a corporation created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
| ● | a trust if either (i) a court within the United States is able to exercise primary supervision over the administration of such trust, and one or more United States persons (within the meaning of Section 7701(a)(30) of the Code) are authorized or have the authority to control all substantial decisions of such trust, or (ii) the trust was in existence on August 20, 1996 and has a valid election in effect under applicable Treasury Regulations to be treated as a United States person for U.S. federal income tax purposes; or |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source. |
Treatment of U.S. Holders in the Reverse Stock Split
AgeX intends to treat the reverse stock split as a “recapitalization” for U.S. federal income tax purposes within the meaning of Section 368(a) of the Code. Assuming the reverse stock split qualifies as a recapitalization within the meaning of Section 368(a) of the Code, a U.S. holder will not recognize gain or loss upon the reverse stock split, except with respect to cash received in lieu of a fractional share of AgeX common stock (which fractional share will be treated as received and then exchanged for such cash). A U.S. holder’s aggregate tax basis in the shares of AgeX common stock received pursuant to the reverse stock split will equal the aggregate tax basis of the shares of the AgeX common stock surrendered (excluding any portion of such basis that is allocated to any fractional share of AgeX common stock), and such U.S. holder’s holding period in the shares of AgeX common stock received will include the holding period in the shares of AgeX common stock surrendered. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the shares of AgeX common stock surrendered to the shares of AgeX common stock received in a recapitalization pursuant to the reverse stock split. U.S. holders of shares of AgeX common stock acquired on different dates and at different prices should consult their tax advisors regarding the allocation of the tax basis and holding period of such shares.
A U.S. holder that receives cash in lieu of a fractional share of AgeX common stock pursuant to the reverse stock split will generally recognize capital gain or loss in an amount equal to the difference between the amount of cash received and the U.S. holder’s tax basis in the shares of AgeX common stock surrendered that is allocated to such fractional share of AgeX common stock. Any such gain or loss will be long-term capital gain or loss if, as of the effective time of the reverse stock split, the U.S. holder’s holding period for such fractional share exceeds one year. Long-term capital gains of certain non-corporate taxpayers, including individuals, are generally taxed at preferential rates. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
If the reverse stock split qualifies as a recapitalization within the meaning of Section 368(a) of the Code, each U.S. holder who receives shares of AgeX common stock in the reverse stock split is required to retain permanent records pertaining to the reverse stock split, and make such records available to any authorized IRS officers and employees. Such records should specifically include information regarding the amount, basis, and fair market value of all transferred property, and relevant facts regarding any liabilities assumed or extinguished as part of such reorganization. U.S. holders who owned immediately before the reverse stock split at least five percent (by vote or value) of the total outstanding stock of AgeX is required to attach a statement to their tax returns for the year in which the reverse stock split is consummated that contains the information listed in Treasury Regulation Section 1.368-3(b). Such statement must include the U.S. holder’s tax basis in such holder’s AgeX common stock surrendered in the reverse stock split, the fair market value of such stock, the date of the reverse stock split and the name and employer identification number of AgeX. U.S. holders are urged to consult with their tax advisors to comply with these rules.
A U.S. holder may be subject to information reporting and backup withholding for U.S. federal income tax purposes on cash paid in lieu of fractional shares in connection with the reverse stock split. Backup withholding will not apply, however, to a U.S. holder who (i) furnishes a correct taxpayer identification number and certifies the holder is not subject to backup withholding on IRS Form W-9 or a substantially similar form, or (ii) certifies the holder is otherwise exempt from backup withholding. If a U.S. holder does not provide a correct taxpayer identification number on IRS Form W-9 or other proper certification, the stockholder may be subject to penalties imposed by the IRS. Any amounts withheld under the backup withholding rules may be refunded or allowed as a credit against the federal income tax liability of a U.S. holder of AgeX capital stock, if any, provided the required information is timely furnished to the IRS. U.S. holders should consult their tax advisors regarding their qualification for an exemption from backup withholding, the procedures for obtaining such an exemption, and in the event backup withholding is applied, to determine if any tax credit, tax refund or other tax benefit may be obtained.
The foregoing summary is of a general nature only and is not intended to be, and should not be construed to be, legal, business or tax advice to any particular AgeX stockholder. This summary does not take into account your particular circumstances and does not address consequences that may be particular to you. Therefore, you should consult your tax advisor regarding the particular consequences of the reverse stock split to you.
Vote Required; Recommendation of the AgeX Board
The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote is required to approve Proposal No. 2. Abstentions and broker non-votes, if any, will have no effect on the outcome of this proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX’S COMMON STOCKHOLDERS VOTE “FOR” THE REVERSE STOCK SPLIT PROPOSAL. THE APPROVAL OF EACH OF PROPOSAL NOS. 1, 2, 3, 4 AND 5 IS REQUIRED TO CONSUMMATE THE MERGER.
PROPOSAL NO. 3 (THE WARRANT ISSUANCE PROPOSAL):
APPROVAL OF THE ISSUANCE OF POST-MERGER WARRANTS AND INCENTIVE WARRANTS PURSUANT TO THE MERGER aGREEMENT
General
At the AgeX special meeting, AgeX’s common stockholders will be asked to approve the issuance of the post-merger warrants to the pre-Merger holders of AgeX common stock pursuant to the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the post-merger incentive warrants, which will represent an issuance by AgeX of securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance.
Following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock as of the Warrant Dividend Record Date three post-merger warrants for each five shares of AgeX common stock held by such holder as of the Warrant Dividend Record Date, or the Warrant Dividend. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one incentive warrant. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock. Each post-merger warrant will expire on July 31, 2025, and each incentive warrant will expire on the four-year anniversary of the Closing Date. Each post-merger warrant and incentive warrant will be issued pursuant to the terms of the warrant agreement to be entered into by AgeX and a warrant agent in connection with the closing of the Merger.
The terms of, reasons for and other aspects of the Merger Agreement, the Merger and the Warrant Dividend are described in more detail in the other sections in this proxy statement/prospectus/information statement.
Reason for the Proposal
Under Section 712(b) of the NYSE American Company Guide, stockholder approval is required prior to the issuance of common stock, or of securities convertible into common stock, in which the sales, issuance or potential issuance is equal to or in excess of 20% of the number of shares of common stock outstanding before the issuance of the common stock or securities convertible into common stock. AgeX will issue the Warrant Dividend to pre-Merger holders of AgeX common stock, which will represent an issuance by AgeX of securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance. Accordingly, in order to ensure compliance with Section 712(b) of the NYSE American Company Guide, AgeX is asking for the approval of AgeX stockholders for the issuance of post-merger warrants to the pre-Merger holders of AgeX common stock pursuant to the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants.
Under Section 713(a) of the NYSE American Company Guide, stockholder approval is required prior to the issuance or potential issuance of additional shares when such shares will be issued in connection with a transaction involving the sale, issuance, or potential issuance by the issuer of common stock (or securities convertible into or exercisable for common stock) at a price less than the greater of book or market value which together with sales by officers, directors or principal shareholders of the issuer equals 20% or more of presently outstanding common stock, or the sale, issuance, or potential issuance by the issuer of common stock (or securities convertible into or exercisable for common stock) equal to 20% or more of presently outstanding stock for less than the greater of book or market value of the stock. AgeX will issue the Warrant Dividend to pre-Merger holders of AgeX common stock, which will represent an issuance by AgeX of securities convertible into or exercisable for AgeX common stock of more than 20% of the shares of AgeX common stock outstanding at the time of issuance. Accordingly, in order to ensure compliance with Section 713(a) of the NYSE American Company Guide, AgeX is asking for the approval of AgeX stockholders of the issuance of the post-merger warrants to holders of AgeX common stock pursuant to the terms of the Merger Agreement, the potential issuance of the incentive warrants and the shares of AgeX common stock upon the exercise of the post-merger warrants and the potential issuance of shares of AgeX common stock upon the exercise of the incentive warrants.
Material U.S. Federal Income Tax Consequences of the Issuance of Post-Merger Warrants and Incentive Warrants Pursuant To The Merger Agreement
The following discussion summarizes material U.S. federal income tax consequences of (1) the issuance of post-merger warrants (such issuance, the Warrant Distribution) and the ownership, exercise, and disposition of post-merger warrants, and (2) the ownership, exercise and disposition of incentive warrants that are issued pursuant to the exercise of post-merger warrants, in each case, that are generally expected to be applicable to AgeX stockholders who are U.S. holders (as defined in the section titled “Matters Being Submitted to a Vote of AgeX’s Stockholders—Proposal No. 2: Approval of an Amendment to the Certificate of Incorporation of AgeX Effecting the Reverse Stock Split—Material U.S. Federal Income Tax Consequences of the Reverse Stock Split” beginning on page 187 of this proxy statement/prospectus/information statement) assuming the Merger is consummated as contemplated herein. This summary is based on current law, including the provisions of the Code, Treasury Regulations promulgated thereunder, judicial authority, and administrative rulings and practice, each as in effect as of the date of this proxy statement/prospectus/information statement and all of which are subject to change, possibly with retroactive effect.
This summary is not a complete description of all tax consequences of the Warrant Distribution and the ownership, exercise and disposition of post-merger warrants or incentive warrants and does not address U.S. federal income tax consequences that may be relevant to particular AgeX stockholder in light of their personal circumstances or to AgeX stockholders who are subject to special treatment under U.S. federal income tax laws, such as AgeX stockholder who: do not hold their AgeX common stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment); are banks, insurance companies, tax-exempt entities, mutual funds, financial institutions, real estate investment trusts, regulated investment companies, government entities or broker-dealers; hold their AgeX common stock as “qualified small business stock” under Section 1202 of the Code or as “Section 1244 stock” under Section 1244 of the Code; hold their AgeX common stock as part of a hedging, “straddle,” conversion or other integrated transaction or are treated as having sold their AgeX common stock pursuant to the constructive sale provisions of the Code; are not U.S. holders; acquired their AgeX common stock pursuant to the exercise of compensatory options, or in other compensatory transactions; acquired their AgeX common stock pursuant to the exercise of warrants or conversion rights under convertible instruments; are subject to special tax accounting rules under Section 451(b) of the Code; hold their AgeX common stock through individual retirement or other tax-deferred accounts; acquired their AgeX common stock in a transaction subject to the gain rollover provisions of Section 1045 of the Code; have a functional currency other than the U.S. dollar; or are partnerships or entities or arrangements classified as partnerships or disregarded entities for U.S. federal income tax purposes, S corporations, or other pass-through entities (including hybrid entities) and investors therein.
In addition, this summary does not address (i) the tax consequences of the Warrant Distribution and the ownership, exercise, and disposition of post-merger warrants under U.S. federal non-income tax law (including estate, gift, or other non-income taxes), (ii) the tax consequences of the Warrant Distribution and the ownership, exercise, and disposition of post-merger warrants under state, local or non-U.S. tax laws, (iii) the impact of the alternative minimum tax provisions of the Code (including the 15% minimum tax applicable to the adjusted financial statement income of certain corporations) or the Medicare contribution tax on net investment income, or (iv) the tax consequences of transactions effectuated before, subsequent to or concurrently with the Merger (whether or not any such transactions are consummated in connection with the Warrant Distribution).
If an entity that is treated as a partnership for U.S. federal income tax purposes holds AgeX common stock prior to the Warrant Distribution, the tax treatment of a partner in such partnership will generally depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partner should consult its tax advisors as to the tax consequences of the Warrant Distribution.
No opinions of counsel or rulings from the IRS have been requested or obtained in connection with the Warrant Distribution and the ownership, exercise, and disposition of post-merger warrants or the incentive warrants. Accordingly, all AgeX stockholders should consult their own tax advisors as to the specific tax consequences to them of the Warrant Distribution and the ownership, exercise, and disposition of post-merger warrants and incentive warrants, including applicable tax reporting requirements.
IN VIEW OF THE FOREGOING AND BECAUSE THE FOLLOWING DISCUSSION IS INTENDED AS A GENERAL SUMMARY ONLY, EACH AGEX STOCKHOLDER SHOULD CONSULT SUCH STOCKHOLDER’S TAX ADVISOR REGARDING THE TAX CONSEQUENCES, INCLUDING THE APPLICABLE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES, AND ANY TAX REPORTING REQUIREMENTS OF THE WARRANT DISTRIBUTION AND THE OWNERSHIP, EXERCISE, AND DISPOSITION OF POST-MERGER WARRANTS AND INCENTIVE WARRANTS AND RELATED TRANSACTIONS IN LIGHT OF SUCH STOCKHOLDER’S OWN TAX SITUATION.
Tax Consequences of the Warrant Distribution and the Ownership, Exercise and Disposition of Post-Merger Warrants
Tax Consequences of the Warrant Distribution
The Warrant Distribution is intended to be treated as a non-taxable distribution under Section 305(a) of the Code. If, however, the Warrant Distribution were treated as a distribution subject to Section 305(b) of the Code, a U.S. holder would be treated for U.S. federal income tax purposes as receiving a distribution equal to the fair market value of the post-merger warrants received in the Warrant Distribution. In such case, the Warrant Distribution would generally be taxable as a dividend to the extent paid out of AgeX’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles). The remainder of this discussion assumes that the Warrant Distribution will be treated as a non-taxable distribution under Section 305(a) of the Code.
Tax Basis and Holding Period in the Post-Merger Warrants
Except as provided in the following sentence, a U.S. holder’s tax basis in the post-merger warrants received in the Warrant Distribution will be zero. However, a U.S. holder’s aggregate tax basis in its AgeX common stock will be allocated between such AgeX common stock and the post-merger warrants received in the Warrant Distribution in proportion to their respective fair market values on the date of the Warrant Distribution if either (i) the fair market value of the Warrants received by a U.S. holder is equal to at least 15% of the fair market value of the AgeX common stock with respect to which such warrants are received, or (ii) the U.S. holder irrevocably elects, on its U.S. federal income tax return for the taxable year in which the Warrant Distribution occurs, to allocate a portion of its tax basis in its AgeX common stock to the post-merger warrants received in the Warrant Distribution.
The holding period of the post-merger warrants received by a U.S. holder in the Warrant Distribution will generally include the holding period of the AgeX common stock with respect to which such post-merger warrants are received.
Possible Constructive Distributions with respect to the Post-Merger Warrants
The number of shares of AgeX common stock that a U.S. holder is entitled to receive upon the exercise of a post-merger warrant and the exercise price of the post-merger warrant are subject to certain anti-dilution adjustments. Certain of these adjustments (including adjustments as a result of a taxable distribution to holders of AgeX common stock) could cause a U.S. holder to be deemed to receive a “constructive distribution” that is includible in income for U.S. federal income tax purposes. AgeX stockholders should consult their tax advisors regarding the possibility of constructive distributions with respect to the post-merger warrants.
Lapse of a Post-Merger Warrant
A U.S. holder will not recognize gain or loss with respect to a post-merger warrant received in the Warrant Distribution upon the failure to exercise such warrant prior to expiration. If a post-merger warrant expires unexercised, any portion of the U.S. holder’s tax basis in its AgeX common stock that was previously allocated to such warrant, as described above, will generally be allocated back to such AgeX common stock. AgeX stockholders should consult their tax advisors regarding the ability to recognize a loss (if any) with respect to post-merger warrants that expire unexercised after a disposition of the AgeX common stock with respect to which the post-merger warrants were received.
Exercise of a Post-Merger Warrant
The exercise of a post-merger warrant received in the Warrant Distribution will generally not be a taxable event to a U.S. holder. A U.S. holder’s aggregate tax basis in the AgeX common stock and incentive warrants acquired upon the exercise of a post-merger warrant will generally equal the exercise price of the post-merger warrants plus the U.S. holder’s tax basis in the post-merger warrant (if any), and such aggregated basis will be allocated between such AgeX common stock and the incentive warrants in proportion to their respective fair market values on the date of the exercise. The holding period of the AgeX common stock and incentive warrants acquired upon the exercise of a post-merger warrants will generally begin on the date of exercise of such post-merger warrants.
Sale or Other Taxable Disposition of a Post-Merger Warrant
A U.S. holder generally will recognize gain or loss on the sale or other taxable disposition of a post-merger warrant equal to the difference between the amount realized on the disposition and the U.S. holder’s tax basis in the post-merger warrants (if any). Any such gain or loss will generally be capital gain or loss, and will be long-term capital gain or loss if the U.S. holder’s holding period for the warrant disposed of (determined as described above) exceeds one year. For non-corporate taxpayers, long-term capital gains are generally eligible for preferential U.S. federal income tax rates. The deductibility of capital losses is subject to limitations.
Tax Consequences the Ownership, Exercise and Disposition of Incentive Warrants
Tax Basis and Holding Period in the Incentive Warrants
As discussed above in “— Exercise of a Post-Merger Warrant”, a U.S. holder’s aggregate tax basis in the AgeX common stock and incentive warrants acquired upon the exercise of a post-merger warrant will generally equal the exercise price of the post-merger warrant plus the U.S. holder’s tax basis in the post-merger warrant (if any), and such aggregated basis will be allocated between such AgeX common stock and the incentive warrants in proportion to their respective fair market values on the date of the exercise. The holding period of the incentive warrants acquired upon the exercise of a post-merger warrants will generally begin on the date of exercise of such post-merger warrants.
Possible Constructive Distributions with respect to the Incentive Warrants
The number of shares of AgeX common stock that a U.S. holder is entitled to receive upon the exercise of an incentive warrant and the exercise price of the incentive warrant are subject to certain anti-dilution adjustments. Certain of these adjustments (including adjustments as a result of a taxable distribution to holders of AgeX common stock) could a U.S. holder to be deemed to receive a “constructive distribution” that is includible in income for U.S. federal income tax purposes. U.S. holders should consult their tax advisors regarding the possibility of constructive distributions with respect to the incentive warrants.
Lapse of an Incentive Warrant
If an incentive warrant is allowed to lapse unexercised, a U.S. holder generally will recognize a capital loss equal to such holder’s tax basis in the incentive warrants.
Exercise of an Incentive Warrant
The exercise of an incentive warrant generally will not be a taxable event to a U.S. holder. A U.S. holder’s aggregate tax basis in the AgeX common stock acquired upon the exercise of an incentive warrant will generally equal the exercise price of the incentive warrant plus the U.S. holder’s tax basis in the incentive warrant. The holding period of the AgeX common acquired upon the exercise of an incentive warrants will generally begin on the date of exercise of such incentive warrant.
Sale or Other Taxable Disposition of an Incentive Warrant
A U.S. holder will generally recognize gain or loss on the sale or other taxable disposition of an incentive warrant equal to the difference between the amount realized on the disposition and the U.S. holder’s tax basis in the incentive warrant. Any such gain or loss will generally be capital gain or loss, and will be long-term capital gain or loss if the U.S. holder’s holding period for the warrant disposed of (determined as described above) exceeds one year. For non-corporate taxpayers, long-term capital gains are generally eligible for preferential U.S. federal income tax rates. The deductibility of capital losses is subject to limitations.
Required Vote; Recommendation of the AgeX Board
The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to is required to approve Proposal No. 3. Abstentions and broker non-votes, if any, will have no effect on the outcome of this proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX’S COMMON STOCKHOLDERS VOTE “FOR” THE WARRANT ISSUANCE PROPOSAL. THE APPROVAL OF EACH OF PROPOSAL NOS. 1, 2, 3, 4 AND 5 IS REQUIRED TO CONSUMMATE THE MERGER.
PROPOSAL NO. 4 (THE a&R charter PROPOSAL):
APPROVAL OF the adoption of the combined company charter
At the AgeX special meeting, AgeX’s common stockholders will be asked to approve the adoption of the Combined Company Charter in the form attached to this proxy statement/prospectus/information statement as Annex B, which, in the judgment of the AgeX Board, is necessary to adequately address the needs of the combined company following the consummation of the Merger. If the Merger is consummated, the certificate of incorporation of the combined company will be the Combined Company Charter.
Required Vote; Recommendation of the AgeX Board
The affirmative vote of the holders of a majority of AgeX common stock, the holders of a majority of AgeX Series A preferred stock and the holders of a majority of AgeX Series B preferred stock entitled to vote at the AgeX special meeting, each voting as a separate class, is required for the approval of Proposal No. 4. Abstentions and broker non-votes, if any, will be counted as “shares entitled to vote” for Proposal No. 4 and therefore will have the same effect of a vote “AGAINST” this proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX’S COMMON STOCKHOLDERS VOTE “FOR” THE A&R CHARTER PROPOSAL. THE APPROVAL OF EACH OF PROPOSAL NOS. 1, 2, 3, 4 AND 5 IS REQUIRED TO CONSUMMATE THE MERGER.
PROPOSAL NO. 5 (THE 2024 equity incentive plan PROPOSAL):
APPROVAL OF THE Serina Therapeutics, INc. 2024 Equity INcentive Plan
At the AgeX special meeting, AgeX’s common stockholders will be asked to approve the 2024 Equity Incentive Plan. The AgeX Board has approved the 2024 Equity Incentive Plan, subject to stockholder approval at the special meeting.
The purpose of the 2024 Equity Incentive Plan is to promote and closely align the interests of the combined company’s employees, officers, non-employee directors, and other service providers and the combined company’s stockholders by providing stock-based compensation. The objectives of the 2024 Equity Incentive Plan are to attract and retain the best available personnel for positions of substantial responsibility and to motivate Participants to optimize the profitability and growth of the combined company through incentives that are consistent with the combined company’s goals and that link the personal interests of Participants to those of the combined company’s stockholders.
Summary of the 2024 Equity Incentive Plan
The following description of the 2024 Equity Incentive Plan is not intended to be complete and is qualified in its entirety by the complete text of the 2024 Equity Incentive Plan, a copy of which is attached as Annex C to this proxy statement/prospectus/information statement. AgeX stockholders are urged to read the 2024 Equity Incentive Plan in its entirety. Any capitalized terms that are used in this summary description but not defined here or elsewhere in this proxy statement have the meanings assigned to them in the 2024 Equity Incentive Plan.
Administration
The 2024 Equity Incentive Plan will be administered by the board of directors of the combined company, or such other committee designated by the combined company’s board of directors to administer the 2024 Equity Incentive Plan, which is referred to herein as the “2024 EIP Administrator.” The 2024 EIP Administrator has broad authority, subject to the provisions of the 2024 Equity Incentive Plan, to administer and interpret the 2024 Equity Incentive Plan and Awards granted thereunder. All decisions and actions of the 2024 EIP Administrator are final and binding. To the maximum extent permissible under Applicable Law, the combined company board of directors may also delegate any or all of its authority to one or more committees composed of two or more non-employee directors of the combined company. Such committee will act pursuant to a vote of the majority of its members or, in the case of a committee comprised of only two members, the unanimous consent of its members.
Stock Subject to the 2024 Equity Incentive Plan
A total of 1,725,000 shares of the combined company’s common stock will be available for the grant of Awards under the 2024 Equity Incentive Plan. The shares of combined company common stock issuable under the 2024 Equity Incentive Plan will be shares of authorized but unissued or reacquired common stock, including shares repurchased by the combined company on the open market or otherwise. The maximum number of shares of the combined company common stock that may be issued pursuant to the exercise of Incentive Stock Options granted under the 2024 Equity Incentive Plan will not exceed 1,725,000.
On cancellation, forfeiture, or expiration of an Award under the 2024 Equity Incentive Plan, in whole or in part, the number of shares of combined company common stock subject to such Award will again become available for grant under the 2024 Equity Incentive Plan. Shares subject to an Award under the 2024 Equity Incentive Plan will not again be made available for issuance or delivery under the 2024 Equity Incentive Plan if such shares are (a) shares tendered in payment of an Option, (b) shares delivered or withheld by the combined company to satisfy any tax withholding obligation, (c) shares covered by a stock-settled Stock Appreciation Right or other Awards that were not issued upon the settlement of the Award, or (d) shares repurchased by the combined company using Option proceeds.
Eligibility
Prospective or current members of the board, employees (including executive officers), and other service providers of the combined company and its affiliates are eligible to participate in the 2024 Equity Incentive Plan. Only employees of the combined company may be granted Incentive Stock Options under the 2024 Equity Incentive Plan.
Types of Awards
Stock Options
All Options granted under the 2024 Equity Incentive Plan will be evidenced by a written agreement with the Participant, which provides, among other things, the term of the Option, which may not generally exceed 10 years (or 5 years for grants of Incentive Stock Options to Ten Percent Shareholders), and other terms and conditions. The Option Exercise Price for any Option granted may not be less than the Fair Market Value per share of combined company common stock on the grant date (or 110% of such Fair Market Value for grants of Incentive Stock Options to Ten Percent Shareholders); provided that an Option may be granted with a lower Option Exercise Price if such Option is granted pursuant to an assumption or substitution of another Option that satisfied the provisions of section 409A or 424(a) of the Code, as applicable.
Stock Appreciation Rights
Stock Appreciation Rights may be granted alone or in conjunction with all or part of an Option. Upon exercising a Stock Appreciation Right, the Participant is entitled to receive the amount equal to the number of shares of combined company common stock subject to the Stock Appreciation Right that is being exercised multiplied by the excess by which the Fair Market Value of combined company common stock at the time of exercise exceeds the exercise price of the Stock Appreciation Right. All freestanding Stock Appreciation Rights shall be granted subject to the same terms and conditions as applicable to Options, including exercise price and term, as set forth above, and all tandem Stock Appreciation Rights shall have the same exercise price as the Option to which they relate. Stock Appreciation Rights may be settled in combined company common stock, cash, Restricted Stock, or a combination thereof, at the 2024 EIP Administrator’s discretion.
Restricted Stock and RSUs
The 2024 Equity Incentive Plan provides for Awards of actual shares of combined company common stock or hypothetical combined company common stock units having a value equal to the Fair Market Value of an identical number of shares of combined company common stock. The 2024 EIP Administrator will determine the restrictions and conditions applicable to each Award of Restricted Stock or Restricted Stock Units, which may include performance vesting conditions. Participants are entitled to receive all dividends and other distributions paid with respect to shares of combined company common stock subject to Restricted Stock Awards. Participants are entitled to receive Dividend Equivalents with respect to shares of combined company Common Stock subject to Restricted Stock Units only to the extent provided by the 2024 EIP Administrator.
Notwithstanding the above, dividends or Dividend Equivalents will be withheld with respect to Restricted Stock or Restricted Stock Units that are subject to restrictions until the date the restrictions are released.
Transferability
Awards generally may not be sold, transferred for value, pledged, assigned or otherwise alienated or hypothecated by a Participant other than by will or the laws of descent and distribution or upon approval by the 2024 EIP Administrator, to the extent provided in the Award Agreement or by subsequent consent granted by the 2024 EIP Administrator. Each Option may be exercisable only by the Participant during his or her lifetime.
Adjustments
In the event any change is made to the combined company’s outstanding common stock as a result of any reorganization, reclassification, combination of shares, stock split, reverse stock split, spin-off, dividend or distribution of securities, property or cash (other than regular, quarterly cash dividends), or any other event or transaction that affects the number or kind of shares of combined company common stock outstanding, then equitable and proportional adjustments will be made to the maximum number and class(es) of securities issuable under the 2024 Equity Incentive Plan (including pursuant to Incentive Stock Options). The terms of any outstanding Award shall also be equitably adjusted by the 2024 EIP Administrator as to price, number, or kind of shares of combined company common stock subject to such Award, vesting, and other terms to reflect the foregoing events.
Change in Control
In the discretion of the 2024 EIP Administrator, any Award Agreement may provide, or the 2024 EIP Administrator may provide by amendment of any Award Agreement or otherwise, notwithstanding any provision of the 2024 Equity Incentive Plan to the contrary, that in the event of a Change in Control, Options and/or Stock Appreciation Rights will become immediately exercisable and/or the restrictions will be released immediately with respect to all or a specified portion of the shares of Restricted Stock or Restricted Stock Units. In addition, in the event of a Change in Control, the 2024 EIP Administrator may, in its discretion and upon at least 10 days’ advance notice to the affected persons, cancel any outstanding Awards and pay to the holders thereof, in cash or stock, or any combination thereof, the value of such Awards based upon the price per share of combined company common stock received or to be received by other stockholders of the combined company in the event. In the case of any Option or Stock Appreciation Right with an exercise price that equals or exceeds the price paid for a share of combined company common stock in connection with the Change in Control, the 2024 EIP Administrator may cancel the Option or Stock Appreciation Right without the payment of consideration therefor.
Clawback/Recoupment
Awards granted under the 2024 Equity Incentive Plan will be subject to recoupment in accordance with any law, government regulation, or stock exchange listing requirement (or any policy adopted by the combined company pursuant to any such law, government regulation or stock exchange listing requirement).
Amendment and Termination
The combined company’s board of directors has the right to amend or terminate the 2024 Equity Incentive Plan at any time, provided certain amendments may not be made without the combined company stockholder approval in compliance with Applicable Law. No amendment to the 2024 Equity Incentive Plan or an Award or Award Agreement may be made that would materially impair the rights of the Participant without such Participant’s consent.
Federal Income Tax Considerations
The following is a summary of the U.S. federal income tax treatment applicable to the combined company and the Participants who receive Awards under the 2024 Equity Incentive Plan based on the federal income tax laws in effect on the date of this proxy statement. This summary is not intended to be exhaustive and does not address all matters relevant to a particular Participant based on their specific circumstances. The summary expressly does not discuss the income tax laws of any state, municipality, or non-U.S. taxing jurisdiction, or the gift, estate, excise (including the rules applicable to deferred compensation under Section 409A of the Code), or tax laws other than U.S. federal income tax law. Because individual circumstances may vary, AgeX recommends that all Participants consult their own tax advisor concerning the tax implications of Awards granted under the 2024 Equity Incentive Plan.
Incentive Stock Options
Options granted under the 2024 Equity Incentive Plan may be either Incentive Stock Options, which satisfy the requirements of Section 422 of the Code, or Non-qualified Stock Options, which are not intended to meet such requirements.
No taxable income is recognized by the Optionholder at the time of the Option grant, and no taxable income is recognized for ordinary income tax purposes at the time the Option is exercised, although taxable income may arise at that time for alternative minimum tax purposes. Unless there is a “disqualifying disposition,” as described below, the Optionholder will recognize long-term capital gain in an amount equal to the excess, if any, of (i) the amount realized upon the sale or other disposition of the purchased shares over (ii) the Option Exercise Price paid for the shares. A disqualifying disposition occurs if the disposition is less than two years after the date of grant or less than one year after the exercise date. If there is a disqualifying disposition of the shares, then the excess of (i) the Fair Market Value of those shares on the exercise date or (if less) the amount realized upon such sale or disposition over (ii) the Option Exercise Price paid for the shares will be taxable as ordinary income to the Optionholder. Any additional gain or loss recognized upon the disposition will be a capital gain or loss.
If the Optionholder makes a disqualifying disposition of the purchased shares, then the combined company will be entitled to an income tax deduction for the taxable year in which such disposition occurs equal to the amount of ordinary income recognized by the Optionholder as a result of the disposition. The combined company will not be entitled to any income tax deduction if the Optionholder makes a qualifying disposition of the shares.
Nonqualified Stock Options
No taxable income is recognized by an Optionholder upon the grant of a Non-qualified Stock Option. The Optionholder in general will recognize ordinary income, in the year in which the Option is exercised, equal to the excess of the Fair Market Value of the purchased shares on the exercise date over the Option Exercise Price paid for the shares, and the Optionholder will be required to satisfy the tax withholding requirements applicable to such income. The combined company will be entitled to an income tax deduction equal to the amount of ordinary income recognized by the Optionholder with respect to the exercised Non-qualified Stock Option.
Stock Appreciation Rights
No taxable income is recognized upon receipt of a Stock Appreciation Right. The Participant will recognize ordinary income in the year in which the Stock Appreciation Right is exercised, in an amount equal to the excess of the Fair Market Value of the underlying shares of combined company common stock on the exercise date over the base price in effect for the exercised right, and the Participant will be required to satisfy the tax withholding requirements applicable to such income. The combined company will be entitled to an income tax deduction equal to the amount of ordinary income recognized by the Participant in connection with the exercise of the Stock Appreciation Right.
Restricted Stock Awards
A Participant who receives unvested shares of combined company common stock will not recognize any taxable income at the time those shares are granted but will have to report as ordinary income, as and when those shares subsequently vest, an amount equal to the excess of (i) the Fair Market Value of the shares on the vesting date over (ii) the cash consideration (if any) paid for the shares. The Participant may, however, elect under Section 83(b) of the Code to include as ordinary income in the year the unvested shares are issued an amount equal to the excess of (a) the Fair Market Value of those shares on the issue date over (b) the cash consideration (if any) paid for such shares. If the Section 83(b) election is made, the Participant will not recognize any additional income as and when the shares subsequently vest. The combined company will be entitled to an income tax deduction equal to the amount of ordinary income recognized by the Participant at the time such ordinary income is recognized by the Participant.
Restricted Stock Units
Generally, no taxable income is recognized upon the grant of Restricted Stock Units. The Participant will recognize ordinary income in the year in which the Award is settled in shares or cash. The amount of that income will be equal to the Fair Market Value of the shares on the date of issuance or the amount of the cash paid in settlement of the Award, and the Participant will be required to satisfy the tax withholding requirements applicable to the income. The combined company will be entitled to an income tax deduction equal to the amount of ordinary income recognized by the Participant at the time the shares are issued or the cash amount is paid.
Deductibility of Executive Compensation
Section 162(m) of the Code limits the deductibility for federal income tax purposes of certain compensation paid to any “covered employee” in excess of $1 million. For purposes of Section 162(m) of the Code, the term “covered employee” includes any individual who serves as chief executive officer, chief financial officer, or one of the other three most highly compensated executive officers for 2017 or any subsequent calendar year. It is expected that compensation deductions for any covered employee with respect to awards granted under the 2024 Equity Incentive Plan will be subject to the $1 million annual deduction limitation. The 2024 Equity Incentive Plan Administrator may grant Awards under the 2024 Equity Incentive Plan or otherwise that are or may become non-deductible when it believes doing so is in the best interests of the combined company and the combined company’s stockholders.
New Plan Benefits
AgeX cannot currently determine the benefits or number of shares subject to Awards that may be granted in the future to eligible Participants under the 2024 Equity Incentive Plan because the grant of Awards and terms of such Awards are to be determined in the sole discretion of the 2024 EIP Administrator. As of , 20 , the closing price of a share of AgeX’s common stock was $ .
Registration with the SEC
The combined company intends to file with the SEC a registration statement on Form S-8 covering the shares reserved for issuance under the 2024 Equity Incentive Plan in the first quarter of 2024.
Required Vote; Recommendation of the AgeX Board
The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote is required to approve Proposal No. 5. Abstentions and broker non-votes, if any, will have no effect on the outcome of this proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX’S COMMON STOCKHOLDERS VOTE “FOR” THE 2024 EQUITY INCENTIVE PLAN PROPOSAL. THE APPROVAL OF EACH OF PROPOSAL NOS. 1, 2, 3, 4 AND 5 IS REQUIRED TO CONSUMMATE THE MERGER.
PROPOSAL NO. 6 (THE ADJOURNMENT PROPOSAL):
APPROVAL OF POSSIBLE ADJOURNMENT OF THE AGEX SPECIAL MEETING
If AgeX fails to receive a sufficient number of votes to approve the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal, AgeX may propose to adjourn the AgeX special meeting for the purpose of soliciting additional proxies to approve the Stock Issuance Proposal, the Reverse Stock Split Proposal, the Warrant Issuance Proposal, the A&R Charter Proposal or the 2024 Equity Incentive Plan Proposal.
If on the date of the AgeX special meeting, or a date preceding the date on which the AgeX special meeting is scheduled, AgeX reasonably believes that (i) it will not receive proxies sufficient to obtain the required vote to approve the AgeX Proposals, whether or not a quorum would be present, or (ii) it will not have sufficient shares of AgeX common stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the AgeX special meeting, AgeX may postpone or adjourn, or make one or more successive postponements or adjournments of, the AgeX special meeting as long as the date of the AgeX special meeting is not postponed or adjourned more than an aggregate of 30 calendar days in connection with any postponements or adjournments.
Required Vote; Recommendation of the AgeX Board
The affirmative vote of a majority of the voting power of the shares of AgeX common stock present or represented by proxy at the AgeX special meeting and entitled to vote is required to approve Proposal No. 6. Abstentions and broker non-votes, if any, will have no effect on the outcome of this proposal.
The Merger is not conditioned upon the approval of the Adjournment Proposal.
THE AGEX BOARD RECOMMENDS THAT AGEX STOCKHOLDERS VOTE “FOR” THE ADJOURNMENT PROPOSAL, IF NECESSARY.
DESCRIPTION OF AGEX’S BUSINESS
References to “we,” “us” and “our” in this section titled “Description of AgeX’s Business” refer to AgeX.
Overview of Business
We are a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases. Our mission is to apply our comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions. We believe that demand for therapeutics addressing such conditions is on the rise, commensurate with the demographic shift of aging in the United States and many other industrialized countries.
Our proprietary technology, based on telomerase-mediated cellular immortality and regenerative biology, allows us to utilize telomerase-expressing regenerative PSCs for the manufacture of cell-based therapies to regenerate tissues afflicted with age-related chronic degenerative disease. We own or have licenses to a number of patents and patent applications used in the generation of these product candidates, including intellectual property related to PureStem® technology. Our technology platform also includes UniverCyte™ which uses the HLA-G gene to potentially confer low immune observability to cells, so as to suppress rejection of transplanted cells and tissues. AgeX plans to use or license the use of this patented technology to produce genetically-modified master cell banks of pluripotent stem cells that can then be differentiated into any young cell type of the human body that now express the immune tolerogenic molecule.
Our product candidates in the discovery stage include two cell-based therapies derived from telomerase-positive PSCs and two product candidates derived from our proprietary iTRTM technology. We have also sponsored a research program to derive neural stem cells from PSCs to treat degenerative diseases such as Huntington’s Disease. We will need to conduct or sponsor research and development work, or license our technology to other biotechnology or pharma companies interested in furthering research and development in order to develop these cell- and drug-based therapies, each targeting large unmet needs in age-related medicine.
Recent Developments
Termination of License and Sublicence
On November 9, 2023, AgeX sent a notice to the licensee and the sublicensees of patent rights for certain uses of HyStem® hydrogel products, informing them that AgeX was exercising its rights to terminate, respectively: (i) a License Agreement, dated August 17, 2017, as amended, by and between AgeX and Lineage Cell Therapeutics, Inc (formerly BioTime, Inc.) and (ii) a Sublicense Agreement, dated August 17, 2017, as amended, by and among AgeX, Lineage Cell Therapeutics, Inc., and OrthoCyte Corporation. The terminations will become effective sixty days after the termination notices were given.
The license agreement and sublicense agreement granted AgeX rights to use certain HyStem® hydrogel patents on a non-exclusive, world-wide, royalty bearing basis, but only for therapeutic uses with (a) cells covered by specified patents related to human embryonic progenitor cell technology, brown adipose fat progenitors, vascular cell progenitors, and human pluripotent stem cell lines and technology, and (b) products covered by specified AgeX iTR patents, and in either case only for use outside the fields of (i) orthopedic indications, (ii) ophthalmological indications, and (iii) medical aesthetics, as defined in the license and sublicense agreements.
AgeX’s decision to terminate the license and sublicence was based on certain considerations, including the scope of the rights licensed or sublicensed to AgeX, the extent to which HyStem® was used in AgeX research and development programs, the expected availability of alternative products for use as a cell transplant matrix or for therapeutic molecular iTR drug delivery that could be used in AgeX product development programs, and the expected cost of maintaining the license and sublicense rights to use HyStem®, including annual payment obligations and fees that would become payable upon a change of control of AgeX such as the expected Merger with Serina.
Lineage Cell Therapeutics, Inc. (Lineage) is the original parent corporation of AgeX. OrthoCyte Corporation is a subsidiary of Lineage. HyStem® is a registered trademark of Lineage.
Amendment to Preferred Stock and Remediation of Stock Exchange Listing Deficiency
On July 24, 2023, AgeX issued shares of AgeX Series A preferred stock and AgeX Series B preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence with the intent of adding $36 million to stockholders equity to eliminate a stockholders equity deficiency that caused AgeX to be out of compliance with the NYSE American continued listing standards. However, upon subsequent consideration in consultation with AgeX’s independent registered public accountants, AgeX determined that, in accordance with applicable guidance to GAAP, the deemed liquidation preference provisions of the Preferred Stock could be considered contingent redemption provisions that are not solely within AgeX’s control, requiring that the Preferred Stock be presented outside of permanent equity in the mezzanine section of the condensed consolidated balance sheet as of September 30, 2023. To comply with the NYSE American listing requirement by permitting the Preferred Stock to qualify now as permanent equity, on November 7, 2023, Section 3(b) of the terms of the Series A Preferred Stock and Series B Preferred Stock was amended (i) to clarify that certain change of control or disposition of asset transactions would be treated as a deemed liquidation if the applicable transaction is approved by the Board of Directors or stockholders of AgeX, and (ii) to provide that in case of such a deemed liquidation transaction holders of Preferred Stock would receive the same type of consideration as that distributed or paid to holders of AgeX common stock. AgeX has informed the NYSE American of the accounting issue and the remedy that has been implemented and AgeX believes it is in compliance with NYSE American’s continued listing standards.
Additional Loans Under the 2022 Secured Note
On October 3, 2023, AgeX drew $500,000 of its credit available under the 2022 Secured Note. On October 31, 2023 AgeX drew the final $500,000 of the credit line available under the 2022 Secured Note.
Increase in Secured Note Line of Credit
On November 9, 2023, AgeX and Juvenescence entered into the Allonge and Fifth Amendment to Amended and Restated Convertible Promissory Note (the 2022 Secured Note Fifth Amendment) that increases the amount of the line of credit available to AgeX by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. Concurrently with the execution of the 2022 Secured Note Fifth Amendment, AgeX also entered into an additional Pledge Agreement to add shares of a subsidiary to the collateral under the Security Agreement, and AgeX’s subsidiaries ReCyte, Reverse Bio, and UniverXome each entered into a Guaranty Agreement and Joinder Agreement pursuant to which each of them agreed to guaranty AgeX’s obligations to Juvenescence pursuant to the Secured Note, as amended by the 2022 Secured Note Fifth Amendment, and to grant Juvenescence a security interest in their respective assets pursuant to the Security Agreement to secure their obligations to Juvenescence.
Overview of Our Product Candidates
Our product pipeline includes two cell-based and two iTR-based product candidates in development.
Our lead cell-based therapeutic candidates in development are AGEX-BAT1 and AGEX-VASC1:
| ● | AGEX-BAT1 is our lead cell therapy product candidate in the discovery stage of development utilizing PSC-derived brown adipocytes for the treatment of certain age-related metabolic disorders such as Type II (adult-onset) diabetes and obesity. |
| ● | AGEX-VASC1 is a cell-based therapy in the discovery stage of development comprised of young regenerative vascular-forming cells. AGEX-VASC1 may restore vascular support in aged ischemic tissues such as in peripheral vascular disease and ischemic heart disease. |
Our lead small molecule drug-based therapeutic candidate for iTRTM in discovery is AGEX-iTR1547 and our lead biologic candidate for iTR is AGEX-iTR1550:
| ● | AGEX-iTR1547 is a drug-based formulation and AGEX-iTR1550 (also known as Renelon™) is a gene delivery technology, both of which are in the discovery stage of development. Initial indications for use may include scarless wound repair. |
Our research related to the reprogramming of aging has also led to novel insights into cancer. We have filed patent applications on inventions that relate to these discoveries. These technologies may provide novel targets for cancer therapy and diagnosis. One such cancer therapeutic in the early stages of development is designated “EPROTM” (embryonic promoter-regulated oncolysis). EPRO is an oncolytic gene therapy strategy that may provide a novel means of selectively destroying an array of different types of cancer cells. Successful development of EPRO will be dependent, in part, on the availability of financing and licensing or joint development opportunities.
Our currently marketed research products include cGMP ES Cells (human embryonic stem or hES) cells produced under cGMP and PSC-derived cells for research:
| ● | cGMP PSC lines and PSC-derived cells for research: Through our ESI BIO division, we market cGMP PSC lines as well as PSC-derived cells. |
Overview of Our Technology Platforms
The technology underlying our product development programs is based on telomerase-mediated cellular immortality and regenerative biology. By “telomerase-mediated cellular immortality” we refer to the fact that cells that express sufficient levels of a protein called telomerase are capable of replicating without limit. By “regenerative biology,” we refer to novel methods to regenerate tissues afflicted with age-related chronic degenerative disease such as peripheral vascular disease and ischemic heart disease as well as age-related metabolic disorders such as those associated with Type II diabetes and obesity, as well as others. We utilize telomerase-expressing regenerative Pluripotent Stem Cells, or PSCs, for the manufacture of cell-based therapies. We own or have licensed numerous patents and patent applications covering methods and compositions relating to this technology platform.
We believe our core technology platforms provide us with a strong foundation for successfully addressing many of the diseases of ageing by focusing on broad therapeutic applicability and commercially scalable technologies:
1. PureStem®: AgeX’s allogeneic cell derivation and manufacturing platform, based on human embryonic progenitors, which are cells in state of development between embryonic stem cells and adult cells. We believe PureStem has the potential to solve several major challenges faced by the cell therapy industry by generating cellular therapeutics which would:
| ● | be commercialized as “off-the-shelf” products; |
| ● | be pure and industrially scalable; |
| ● | have lower cost of goods per unit; |
| ● | be amenable to traditional pharmaceutical supply chain logistics’ |
| ● | have the potential for acceptable reimbursement prices, unlike the relatively expensive autologous products; and |
| ● | have higher clinical adoption from expected cost savings and more simplified processes. |
In addition, we believe PureStem cells may have advantages over mesenchymal stem cells (MSCs), which may only survive transiently in the body and exert any short-term benefit by releasing paracrine factors, which may limit their potential of MSCx.
MSCs neither engraft nor become specialized cells. On the other hand, cells derived from PureStem progenitors will be engineered to be young, not prone to the disadvantages associated with older cells, and are expected to become permanently engrafted in the body to deliver a true regenerative outcome. To date, AgeX has isolated more than 200 cell types from PureStem.
2. UniverCyte™: AgeX’s pioneering technology designed to genetically modify allogeneic donor cells to potentially become hypoimmunogenic/universal, so they can potentially be transplanted into all patients in an off-the- shelf manner, without the normal need for human leukocyte antigen (HLA) matching between donor and receipt or immunosuppression. UniverCyte utilizes a potent molecule called HLA-G. HLA is a group of related proteins that helps the immune system distinguish the body’s own proteins from proteins made by foreign invaders such as viruses and bacteria. HLA-G’s only known physiological role in nature is to prevent destruction of a semi-allogeneic fetus by the maternal immune system. We believe that UniverCyte could potentially avoid immune rejection of transplanted cells, solving a major challenge facing the allogeneic cell therapy industry. In addition to utilizing UniverCyte™ for its own future cell therapy products, AgeX may make UniverCyte™ available to other cell therapy companies through licensing arrangements.
3. Induced Tissue Regeneration (iTRTM): The aim of iTR is to return aged cells back to a youthful state, thereby inducing a capacity for scarless regeneration characteristic of early developing tissues, without reverting cells to pluripotency. This technology is sometimes referred to as “partial reprogramming” or “epigenetic reprogramming of aging.” We believe this novel approach may trigger complete regeneration of cells, and potentially even complex tissues, damaged as a result of age-related degenerative processes or trauma. The premise behind iTR is that aging, and in turn degenerative diseases of old age, are a result of the loss of two characteristics of cells; namely, replicative immortality and regenerative capacity. These two characteristics are present in embryonic cells but are lost at the embryonic to fetal transition (EFT). With this loss, humans can no longer generate new cells or repair damaged cells scarlessly and in sufficient numbers to maintain health. We discovered that cells begin expressing the gene COX7A1 at the EFT when regeneration is commonly lost. Therefore, we believe the gene may be a key inhibitor of cellular regeneration. For example, we have discovered that restoring a regenerative pattern of COX7A1 gene expression may facilitate hair regeneration in mouse models. In addition, we have invented multiple platforms for delivering iTR using small molecules as well as biologic strategies such as those using gene therapy to transiently express reprogramming factors. We have filed patent applications on the use of iTR in a wide array of degenerative conditions including cancer.
4. ESI Cell Lines: AgeX has six clinical-grade human embryonic stem cell lines, they are distinguished as the first clinical-grade human pluripotent stem cell lines created under current Good Manufacturing Practice as described in Cell Stem Cell (2007;1:490-4). They are listed on the NIH Stem Cell Registry in the USA and are among the best characterized and documented stem cell lines in the world. ESI-053 is among only a few pluripotent stem cell lines from which a derived cell therapy product candidate has been granted FDA IND clearance for human studies. The FDA cleared an IND application from ImStem Biotechnology, one of our sublicensees, for a MSC product derived from ESI-053 for multiple sclerosis. This was believed to be the first MSC product derived from a pluripotent stem line to be accepted for a human trial by the FDA. The ESI cell lines are available as research or clinical grade product, and have been offered since 2006.
The Merger
On August 29, 2023, we entered into the Merger Agreement with Serina and Merger Sub, pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into Serina, with Serina surviving as our wholly owned subsidiary. For additional information, see the sections titled “The Merger” and “The Merger Agreement” beginning on pages 134 and 153 of this proxy statement/prospectus/information statement, respectively.
Prior to the closing of the Merger, any assets of AgeX other than the Legacy Assets will be transferred into Newco. In consideration of the transfer of such assets, Newco will assume (i) any indebtedness of AgeX issued to Juvenescence that has not been previously converted into AgeX Series A preferred stock or AgeX Series B preferred stock, which will be secured by the Legacy Assets and (ii) all other liabilities of AgeX in existence as of the Effective Time (other than Transaction Expenses (as defined below)). If the potential spinoff of Reverse Bio is not completed, it is currently expected that the Legacy Assets would include the assets related to Reverse Bio.
If the Merger is completed, the combined company will primarily focus on developing Serina’s product candidates, which are described on page 231 under the section titled “Description of Serina’s Business.” If the Merger is not completed, we expect to continue to execute on our current business strategies as more fully described under the section titled “—Business Strategy” below while seeking out and evaluating potential strategic alternatives with respect to our assets and development programs.
Additional Information
AgeX was incorporated in the State of Delaware on January 6, 2017. Our common stock began trading on the NYSE American on November 29, 2018 and trade under the symbol “AGE.” Our principal executive offices are located at 1101 Marina Village Parkway, Suite 201, Alameda, CA 94501, and our phone number at that address is (510) 671-8370. Our website address is www.agexinc.com. The information on, or that can be accessed through our website is not part of this proxy statement/prospectus/information statement. We make available, free of charge through our website, our most recent annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after the reports are electronically filed with or furnished to the SEC.
iTRTM, UniverCyteTM, Renelon™, and EPRO™ are trademarks of AgeX Therapeutics, Inc. HyStem® and PureStem® are registered trademarks of Lineage Cell Therapeutics, Inc.
Emerging Growth Company
We are an “emerging growth company” under the Jumpstart our Business Startups Act of 2012 or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
| ● | reduced disclosure about our executive compensation arrangements; |
| ● | no non-binding stockholder advisory votes on executive compensation or golden parachute arrangements; and |
| ● | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We will remain an “emerging growth company” until the earliest of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt securities during the previous three years; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended (the Exchange Act).
The JOBS Act permits an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we have elected to comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements were previously consolidated with those of our former parent company Lineage which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Business Strategy
We believe our four proprietary platform technologies, PureStem® for cell derivation and manufacturing, UniverCyte™ for generation of hypoimmunogenic cells and iTRTM for reversing the age of cells already in the body present AgeX with a multiplicity of attractive opportunities which we may pursue. Given these platform technologies may be highly desirable to multiple academic and biopharma companies due to their broad applicability and potentially important clinical and commercial benefits, AgeX plans to pursue different business models for these platforms:
| ● | Co-Development and Licensing: Our PureStem® and UniverCyte™ technologies as well as our ESI cell lines may have applications in the development of a broad range of cell therapy products. We will seek opportunities to license these AgeX technologies to other cell therapy or biopharma companies to bring in early revenue streams, especially for therapies that AgeX does not presently intend to develop. |
| ● | Cellular Therapy: AgeX presently does not have the laboratory and research staff required to conduct in-house research and development for its product candidates, including AGEX-BAT1 and AGEX-VASC1. Instead, AgeX may conduct research and development of those product candidates through a variety of alternative strategies, including but not limited to, sponsoring research and development work at research laboratories at universities or other educational institutions, entering into co-development and marketing arrangements with researchers or other companies in the cell therapy or biopharma industry, and engaging contract service providers to conduct research and development and manufacturing for AgeX for particular product candidates. Our sponsored Huntington’s Disease research program at the University of California, Irvine (UCI) has led to the organization of an AgeX subsidiary, of which we are presently the majority shareholder, to pursue clinical studies of the use of derived neural stem cell to treat that disease. The new subsidiary is still in the organizational stage and commencement of clinical study work will depend on its ability to obtain financing through grants or third-party investment. |
| ● | Reverse Bioengineering, Inc. (Reverse Bio): Partial cellular reprogramming using our iTRTM technology may one day allow us to revert aged or diseased cells inside the body back to a more youthful, healthy and functional state. We incorporated Reverse Bio as an AgeX subsidiary to develop our iTRTM platform. Reverse Bio is intended to allow for a dedicated focus on iTRTM in terms of equity financing and advancing our iTRTM technology to proof-of-concept in an animal model. We have assigned to Reverse Bio our patent portfolio for iTR development, but the future operations of Reverse Bio will depend in large measure on its ability to raise its own capital. |
Each of these models may provide particular benefits to AgeX in terms of financing and efficiency of operations. However, each alternative has potential disadvantages as well. If AgeX out-licenses its technology it will avoid the costs and risks of research and development, clinical trials, regulatory approval, manufacturing, and commercialization of product candidates, but the revenues AgeX would receive from commercialization of products developed under those arrangements would likely be limited to royalties on product sales and potentially licensing fees and milestone payments representing a relatively small portion of total product revenues. Similarly, co-development and marketing or similar arrangements would permit AgeX to share costs and risks but would also require AgeX to share revenues from the product candidates that may be successfully developed and commercialized. See elsewhere in this proxy statement prospectus for information about certain risks associated with reliance on arrangements with third parties for research, product development, clinical trials, manufacturing, and commercializing product candidates.
We plan to finance our iTRTM and AGEX-BAT1 research and development through Reverse Bio. To the extent that such financing is obtained through the sale of capital stock or other equity securities to investors or other biopharma companies by Reverse Bio, or the sale of Reverse Bio shares held by AgeX, our equity interest in Reverse Bio and our iTRTM and AGEX-BAT1 business would be diluted.
However, if the Merger is completed, the combined company will primarily focus on developing Serina’s product candidates, which are described on page 231 under the section titled “Description of Serina’s Business.” See the section titled “—The Merger” above.
Our Technology Platforms
PureStem® Technology
Regulatory approval of cell- and tissue-based products require high standards of quality control. In the case of stem cell-derived products, there is a high standard for ensuring the known identity, purity, and reproducibility of the cells to be administered. PSCs provide certain advantages over adult stem cell products when used in the manufacture of cell-based therapeutics for the treatment of age-related disease. These advantages include:
| ● | The replicative immortality of the PSCs which facilitates the indefinite scale-up of PSC master cell banks for the manufacture of uniform product, as well as an immortal substrate for targeted genetic modifications. |
| ● | Since most PSCs maintain long and stable telomere lengths, the replicative capacity of derived differentiated cell types is typically longer (younger) than adult or even fetal-derived cells. |
| ● | Using PureStem® technology, it is possible to clonally expand hundreds of purified, identified, and reproducibly scalable cell types that retain regenerative potential (have not passed the regeneration limit). |
PureStem® technology is based on the observation that embryonic anlagen of many tissues in the human body are naturally comprised of highly proliferative cells with relatively long telomere length. Therefore, it is possible to generate clonal lineages of these cells in vitro. Cells derived from adult tissues commonly permanently cease to divide after a certain number of doublings, a condition known as senescence. In addition, adult and even fetal tissues largely contain differentiated cells often with limited or no capacity of replication in vitro. As a result, the clonal expansion of human embryonic progenitor cell types allows not only a novel and more facile point of scalability but also generates populations of cells that are multipotent instead of pluripotent, and therefore markedly easier to define identity, purity, and potency.
We have studied the fate of over 200 diverse PureStem cell lines in thousands of differentiation conditions. This was accomplished by thawing individual cryopreserved PureStem cell lines, culturing them in the laboratory, and then exposing the cells to factors that differentiate cells such as protein growth and differentiation factors, hormones, and small molecules implicated in causing cells to change from one type of cell into another (differentiation). Using individual cells from the over 200 diverse PureStem cell lines previously isolated and cryopreserved, we treated the diverse cells with thousands of differentiation conditions, prepared RNA, and determined the gene expression pattern of the cells using gene expression microarrays. These experiments have shown that the PureStem cell lines display site-specific markers that identify not only the type of cells, but also where in the body the cells would normally reside. Therefore, in the example of cartilage cells, it was possible to produce diverse types of cartilage in this manner. We have licensed from our former parent company Lineage PureStem applications outside of orthopedics, medical aesthetics, and certain ophthalmological applications.
We have chosen two PureStem applications for our initial product development based on unmet medical need along with other factors. The first product candidates are AGEX-BAT1, brown adipose tissue or BAT cells for the treatment of metabolic disorders such as obesity or Type II diabetes, and AGEX-VASC1, vascular endothelial progenitors for the treatment of age-related ischemic disease such as that leading to peripheral vascular disease and ischemic heart disease.
UniverCyte™
Our UniverCyte™ technology uses a proprietary, novel, modified form of HLA-G and is intended to permit donor cells to be transplanted into patients without donor-patient tissue matching and without administering immunosuppressant medication. Immunosuppressive drugs can reduce patient resistance to infectious diseases and cancers as well as cause organ and other toxicities. Reducing or eliminating the need for immunosuppressants after cell transplantation by use of hypoimmunogenic cells may make therapies universally available. We plan to use or license the use of this patented technology to produce genetically-modified master cell banks of pluripotent stem cells that can then be differentiated into any young cell type of the human body that now express the immune tolerogenic molecule.
Products and Product Candidates
Our Therapeutic Product Candidates
AGEX-BAT1 - Brown Adipose Tissue (BAT) Progenitors
Brown adipose tissue (BAT) is abundant early in life but lost precipitously with age. This tissue is believed to generate heat through expression of a gene called UCP1. In addition, the high levels of glucose and lipid uptake by the tissue is believed to balance metabolism in young people. In contrast, central obesity and Type II diabetes has been correlated with low levels of BAT.

Figure 6. Human tissue-derived BAT cells (left) stained red for the presence of UCP1 show a minority of cells being true BAT cells. PureStem-derived AGEX-BAT1 cells are uniformly UCP1 positive.
The demonstration in published literature in the public domain that the transplantation of BAT from young mice to obese diabetic mice resulted in weight loss and increased insulin sensitivity has led to a search for a source of industrially-scalable clinical grade BAT cells as well as an appropriate matrix for lipotransfer. There currently is no FDA-approved matrix for cell transplantation. As shown in Figure 6, the AGEX-BAT1 progenitors strongly express the BAT marker UCP1 when induced to differentiate and show a relatively high degree of purity compared to human tissue-derived BAT.
We entered into a Sponsored Research Agreement with Ohio State University using AGEX-BAT1 in mice to determine whether transplantation of AgeX-BAT1 cells may lead to improvements in diet-induced obesity, metabolic health including glucose metabolism, and cardiac function. For purposes of this proof of concept work, two different cell transplant matrices were tested, HyStem® and a 3-D silk scaffold. We consider this work to be an early stage study and expect to conduct or sponsor additional research on the potential therapeutic benefits of AGEX-BAT1.
AGEX-VASC1 - Vascular Progenitors
PureStem® technology can also yield highly purified embryonic vascular components. As shown below, select clonal lines express markers such as VE-Cadherin (CDH5) and PECAM1, as well as VWF and other markers of venous, arterial, and lymphatic endothelium. Flow cytometry shows purity indistinguishable from 100%.
In addition to vascular endothelial cells, we have characterized vascular smooth muscle cell progenitors. This makes it possible for us to construct two of the key cellular components of arterial vessels, such as those compromised in coronary artery disease.
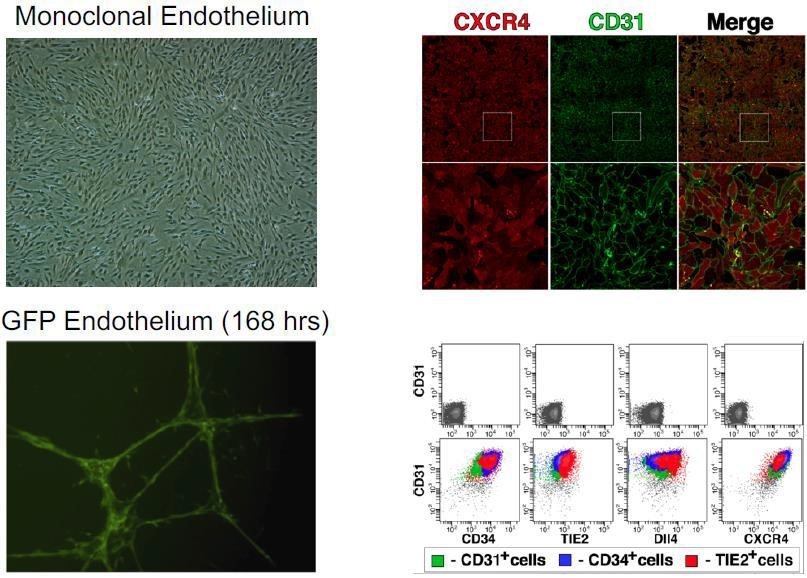
Figure 7. PureStem-derived vascular endothelial cell lines are capable of regenerating young vasculature (bottom left) and appear to have essentially 100% purity by fluorescence activated cell sorting analysis.
HyStem® hydrogels have been successfully used in cell transplantation research in animal models and in a CE marked product developed by Lineage for autologous adipose tissue preparations to restore and/or augment facial volume as a treatment of facial lipoatrophy after subcutaneous fat volume loss. AGEX-iTR1547 - Induced Tissue Regeneration (iTRTM)
Leveraging our assets in pluripotency and bioinformatics, we have performed research manipulating cellular immortality and regenerative biology in human cells. In 2010, our scientists while at Lineage demonstrated the reversal of the developmental aging of human cells using transcriptional reprogramming technology. In 2017, we published certain markers of the Weismann barrier, and the high prevalence of a reversion back before the Weismann barrier in diverse cancer cell types cultured in vitro.
We extended this research to determine whether reprogramming can be modified to only reverse the aging of cells back before the Weismann Barrier, not back to pluripotency. We have utilized for example the gene COX7A1 as a marker of cells that have lost regenerative potential (crossed the Weismann Barrier). As shown in Figure 8, our proprietary formulation AGEX-iTR1547 has demonstrated initial capability of reducing the expression of the marker gene COX7A1 back to before the Weismann Barrier without reverting the cells to pluripotency. When implemented in vivo, this partial reprogramming, or iTR, would be expected to induce tissue regeneration, and when combined with telomerase, may be able to modulate both cellular immortality and regenerative biology for therapeutic effect. In addition to the small molecule product candidate designated iTR1547, we have invented biological interventions based, for example, on gene therapy. Our inventions relating to iTR biologics disclose both DNA and RNA-based strategies. Our gene delivery iTR product candidate is designated iTR1550. We are performing research to optimize AGEX-iTR1547 and in parallel a gene delivery formulation designated AGEX-iTR1550 in order to initiate preclinical studies of one or both of the agents on the scarless regeneration of the skin.

Figure 8. PSCs such as ES Cells and PureStem EP Cells display a regenerative capacity like cells that have not crossed the Weismann Barrier. During pre- and post-natal development, skin cells become increasingly incapable of scarless regeneration as reflected in increasing COX7A1 expression. iPS cell reprograming reverts cells back to pluripotency, while AgeX-iTR1547 reverts cells back only to a point prior to the Weismann Barrier (regenerative state).
Status and Development Plan
The product candidates we have chosen are in the discovery stage of development. Prior to filing an investigational new drug application (IND) for the initiation of clinical trials of our initial product candidates, AGEX-BAT1, AGEX-VASC1, and AGEX-ITR1547/AGEX-iTR1550, a number of important research and development goals will need to be achieved, including discovery-level research for the qualification of reagents used in the manufacture of the product, completion of the standard operating procedures (SOPs) to be used, completion of the methods and documentation for characterization of the product; and producing and testing the genetic modifications in the master cell banks of the pluripotent stem cells under cGMP in order to produce product that will not illicit immune rejection following transplantation. In addition, we will be required to expand the numbers of the pluripotent stem cell master cell banks for future use; produce working cell banks from which the product will be manufactured for clinical trials; produce the relevant product under cGMP conditions; and expand the number of relevant cells and cryopreserve them under cGMP conditions. In addition, we will be required to design the pre-clinical studies including the study endpoints, perform biosafety testing and release the first clinical batch based on preliminary characterization results, and complete full product characterization. Biosafety testing will necessarily include pilot testing in animals such as (NOD/SCID) mice, dosing spiking studies at early and later endpoints, tumorgenicity and biodistribution studies to determine whether the cells form undesired tumors or migrate to inappropriate sites respectively in the animal. Lastly, we will need to define the clinical trial and regulatory strategy and hold various meetings with the U.S. Food and Drug Administration (FDA), as well as successfully submit an IND to the FDA and receive clearance to begin trials. Thereafter, we will need to demonstrate safety and efficacy of the product in human clinical trials in Phase I and II trials, and continued safety and efficacy for achieving the desired endpoint in Phase III trials, potentially then leading to product registration. See “Risk Factors—Risks Related to Our Business Operations” for discussion of risks relating to product development and clinical trials. These include, but are not limited to, failure to successfully complete the aforementioned studies due to the failure of the product, processes, or skills of our employees, unforeseen delays in the development process, failure to raise requisite financing, or failure to receive permission from the FDA to advance product development. To the extent we license development of one or more product candidates to third parties or enter into collaboration arrangements for product development, our licensees or collaborators would need to undertake and achieve the foregoing goals.
Because our product candidates are still in the discovery stage, our choice of product candidates and development plans are subject to change based on a variety of factors. We may determine to abandon the development of one or more of our product candidates, or we may prioritize the development of one or more product candidates, or we may select or acquire and prioritize the development of new product candidates. Our choice and prioritization of product candidates for development will be influenced by a variety of factors, including but not limited to:
| ● | Results of our laboratory research and any animal and clinical trials that we or any licensees or collaborators may conduct; |
| ● | Our ability to enter into licensing or collaborative arrangements with other biotechnology or biopharma companies or universities with their own laboratory facilities and research staffs to conduct research and development of one or more product candidates; |
| ● | Our analysis of third-party competitive and alternative technology that may lead us to conclude that our product candidates or technologies may be non-competitive or obsolete; |
| ● | Our analysis of market demand and market prices for the products we plan to develop could lead us to conclude that market conditions are not favorable for receiving an adequate return on our investment in product development and commercialization; |
| ● | The amount of capital that we will have for our development programs and our projected costs for those programs; |
| ● | The issuance of patents to third parties that might block our use of the same or similar technology to develop a product candidate; and |
| ● | The views of the FDA and comparable foreign regulatory agencies on the pre-clinical product characterization studies required to file an IND in order to initiate human clinical testing of a therapeutic product candidate or to attain marketing approval for that product candidate, or to obtain an investigational device exemption for clinical trials, or clearance for a 510(k) application to market a medical device. |
Other Products and Product Candidates
Neural Stem Cells
During January 2020, we began a research collaboration under a Sponsored Research Agreement with the University of California at Irvine using our PureStem® technology to derive neural stem cells, with the goal of developing cellular therapies to treat neurological disorders and diseases. The primary goal of the research will be to develop a robust method of deriving neural stem cells from PSCs in sufficient quantity and with sufficient purity and identity for use in cell based therapy. The initial focus will be on Huntington’s disease, while other potential targets may include Parkinson’s, Alzheimer’s, and stroke. UCI has already accumulated safety and efficacy animal data that may support an IND submission to the FDA for the commencement of clinical trials to treat Huntington’s disease. This collaboration led to the organization of an AgeX subsidiary, NeuroAirmid, jointly owned with certain researchers from UCI to pursue clinical development and commercialization of cell therapies derived using licensed inventions arising from the research program, as well as certain patent pending technology for neural stem cell derivation, and certain technical data to support IND submissions. This subsidiary is still in the organizational stage and commencement of clinical study work will depend on its ability to obtain financing through grants or third-party investment.
ESI BIO Research Products
We, through our ESI BIO research product division, market a number of products related to pluripotent stem cells including research-grade as well as cGMP-grade human PSC lines. We plan to contract with third parties where the third parties to allow them to utilize cGMP PSC lines in defined fields of application in exchange for certain compensation including the payment of royalties to us if they are successful in developing and commercializing a product.
Subsidiaries
AgeX has four subsidiaries, Reverse Bio, ReCyte Therapeutics, Inc. (ReCyte), NeuroAirmid Therapeutics, Inc. (NeuroAirmid) and Merger Sub. Reverse Bio, ReCyte and NeuroAirmid are early stage pre-clinical research and development companies.
AgeX intends to develop its iTRTM platform and AGEX-BAT1 through Reverse Bio. Reverse Bio will allow for a dedicated focus on iTRTM in terms of equity financing and advancing the iTRTM technology to proof-of-concept in an animal model. AgeX’s patent portfolio for iTR and AGEX-BAT1 development have been assigned to Reverse Bio, but the future operations of Reverse Bio will depend in large measure on its ability to raise its own capital. ReCyte is involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX owns 100%, 94.8%, and 50% of the outstanding capital stock of Reverse Bio, ReCyte, and NeuroAirmid, respectively. We expect that our ownership interests in Reverse Bio and NeuroAirmid will be diluted if those subsidiaries are successful in obtaining financing from investors or product development collaborators. All material intercompany accounts and transactions have been eliminated in consolidation.
Manufacturing
We presently do not have any manufacturing facilities and we will need to rely on third party contract manufacturers for the production of our cell lines and product candidates and to comply with quality manufacturing processes and controls.
Facilities
We presently occupy 101 square feet of leased office space in a building in an office and research park at 1101 Marina Village Parkway, Suite 201, Alameda, California pursuant to a lease that is set to expire on December 31, 2023. We do not have our own research laboratory facilities.
Commercialization Plan
With the exception of our research product sales which generate a trivial amount of revenues, we currently have no commercialized or marketed products such as FDA-approved drugs in our portfolio. As a result, we have not yet assembled an infrastructure for sales and marketing. At the point in time, if ever, that our product candidates approach clearance or approval, we plan to develop a commercial plan that may initially include strategic marketing partnerships.
Intellectual Property
Patents and Trade Secrets
We rely primarily on patents and contractual obligations with employees and third parties to protect our proprietary rights. We have sought, and intend to continue to seek, appropriate patent protection for important and strategic components of our proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There are no assurances that any of our intellectual property rights will guarantee protection or market exclusivity for our products and product candidates. We also use license agreements both to access technologies developed by other companies and universities and to convey certain intellectual property rights to others. Our financial success will be dependent, in part, on our ability to obtain commercially valuable patent claims, to protect and enforce our intellectual property rights, and to operate without infringing upon the proprietary rights of others if we are unable to obtain enabling licenses.
The patents for our core programs are summarized below.
AGEX-BAT1
Brown Adipose Tissue (BAT) Progenitor Cells: The pending patent applications related to BAT progenitor cells, which are owned by AgeX, include U.S. and international patent applications. The applications are directed to the differentiation of pluripotent stem cells (including hES cells) into progenitor cell types capable of making the cellular components of brown fat. The patents also describe culture and purification methods. The approximate expiration dates of the BAT patents, if issued, will range from 2034 to 2036.
AGEX-VASC1
Vascular Progenitors: The pending patent application pertaining to purified vascular progenitor cells and embryonic vascular components are owned by AgeX or an AgeX subsidiary or licensed from Lineage. The patents include U.S. patent applications and are directed to methods to enhance vascular tube networks, compositions of pericyte progenitor cells, compositions of exosomes containing angiogenic molecules, compositions of vascular and lymphatic cells, and methods to culture and purify the cells or components thereof. The approximate expiration dates of the vascular progenitor patents, if issued, range from 2032 to 2038. We plan to file an international patent application claiming priority from a pending US provisional application by the filing deadline, which could lead to a patent that if issued would expire in 2039. AGEX-iTR1547 and AGEX-iTR1550
Induced Tissue Regeneration (iTRTM): The pending patent applications related to the iTR programs, which are owned by AgeX, include applications pending, for example, in the United States, Australia, Canada, China, Europe, Japan and a pending international patent application. These patents and patent applications are directed to compositions and methods for healing damaged tissue using the iTR treatment methods. The patent applications are also directed to treatment methods by regenerating aging tissue by modulating genes involved in tissue regeneration, including reprogramming cells and tissues back to a regenerative state. The approximate expiration dates of the iTR patents, if issued, will range from 2034 to 2041.
Other AGEX Licensed and Sublicensed Patents
PureStem® Progenitor Cells: The patents and pending applications related to our PureStem® technology include patents and applications in the United States, Canada, Europe and Australia. These patents are directed to methods for generating diverse isolated progenitor cell lines which generally do not express COX7A1 and combinations of other methods for employing pluripotent stem cell lines suitable for clinical use. The pending applications are directed to clonally purified human embryonic progenitor cell lines and methods for reproducible, large scale production of clonally purified human embryonic progenitor cells, compositions and methods for generating diverse cell types, and assays useful in identifying hES cell lines and pluripotent cells resulting from the transcriptional reprogramming of somatic cells that have embryonic telomere length. The approximate expiration date of the PureStem® issued patents is 2031 and the approximate date of expiration of the pending patents, if issued, will range from 2029 to 2032.
The PureStem® patent portfolio includes patents and pending applications licensed from Advanced Cell Technology, Inc., which later became Ocata Therapeutics, Inc. (Ocata). The Ocata issued patents cover methods for reprogramming animal differentiated somatic cells to undifferentiated cells and methods for producing differentiated progenitor cells using morula-derived or inner cell mass cells from a blastocyst and expire from approximately 2023 to 2026. The Ocata pending applications relate to methods for the derivation of cells that have a reduced differentiation potential using PSCs, methods for reprogramming animal differentiated somatic cells to undifferentiated cells and methods for producing differentiated progenitor cells using morula-derived or inner cell mass cells from a blastocyst. The Ocata pending patents, if issued, will expire between 2023 and 2026.
ESI Human Embryonic Stem Cell (hES) Cell Lines: AgeX licenses rights to the ES Cell International Pte. Ltd. patent portfolio with patents issued in the United States, Australia, Israel, UK, Singapore, Japan, and applications pending in the US and Europe. The patents are directed to methods for the differentiation of or enhancing the differentiation of stem cells into cardiomyocytes, neural cells, and pancreatic endoderm cells, compositions of pancreatic progenitor cells, methods of promoting the attachment, survival and/or proliferation of substantially undifferentiated stem cells in culture, methods for identifying and selecting cardiomyocytes, methods of freezing stem cells or progenitor cells, methods for identifying cardiogenic factors, compositions and methods for modulating spontaneous differentiation of a stem cell, methods of modulating the differentiation of undifferentiated, pluripotent human embryonic stem cells in culture, isolated endodermal progenitor cells, methods for transducing human embryonic stem cells, cell culture systems. The pending applications are directed to methods for the differentiation of hES cells into the three cell lineages, including for example cardiomyocytes, skeletal muscle cells, vascular endothelial cells, and pancreatic endoderm cells, as well as various culture and purification methods and compositions and methods of treatment. The ESI issued patents will expire from 2023 to 2027, and the approximate date of expiration of the pending patents, if issued, will range from 2023 to 2027.
UniverCyteTM Human Leukocyte Antigen-G (HLA-G) Technology: In August 2018, we acquired from Escape Therapeutics patents and patent applications related to HLA-G-modified cells and methods of generating allogeneic cells with reduced risk of being rejected by patients regardless of the HLA class I haplotype. The patents and pending application related to our HLA-G modified cells technology include patents issued in the United States, Australia and Japan and applications are pending in the United States, Australia, Canada, China, Europe, Japan, Korea, and Singapore. The patents are directed to cells which are genetically modified to express a HLA-G and have reduced immunogenicity, and nucleic acid compositions useful for generating the genetically modified cells. The pending applications are directed to compositions and methods for generating cells which are genetically modified to express HLA-G having reduced immunogenicity, nucleic acid compositions useful for generating the genetically modified cells, and methods of producing artificial tissues using the genetically modified cells. The approximate expiration date of the UniverCyte™ HLA-G issued patents is 2033 and the approximate date of expiration of the pending patents, if issued, will also be 2033. We intend to use the UniverCyte™ technology in the development of our two lead product candidates, AGEX-BAT1 and AGEX-VASC1 for the treatment of Type II diabetes and peripheral vascular disease and ischemic heart disease, respectively. In addition, we may seek to license out or form collaborations for the use of our UniverCyte™ technology.
General Risks Related to Obtaining and Enforcing Patent Protection
There is a risk that any patent applications that we file and any patents that we hold or later obtain could be challenged by third parties and be declared invalid or infringing on third-party claims. Litigation, interferences, oppositions, inter partes reviews or other proceedings are, have been and may in the future be necessary in some instances to determine the validity and scope of certain of our proprietary rights, and in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. We may also face challenges to our patent and regulatory protections covering our products by third parties, including manufacturers of generics and biosimilars that may choose to launch or attempt to launch their products before the expiration of our patent or regulatory exclusivity. Litigation, interference, oppositions, inter partes reviews, administrative challenges or other similar types of proceedings are unpredictable and may be protracted, expensive and distracting to management. The outcome of such proceedings could adversely affect the validity and scope of our patent or other proprietary rights, hinder our ability to manufacture and market our products, require us to seek a license for the infringed product or technology or result in the assessment of significant monetary damages against us that may exceed any amounts that we may accrue on our consolidated financial statements as a reserve for contingent liabilities. An adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing or selling our products. Furthermore, payments under any licenses that we are able to obtain would reduce our profits derived from the covered products and services.
The enforcement of patent rights often requires litigation against third-party infringers, and such litigation can be costly to pursue. Even if we succeed in having new patents issued or in defending any challenge to issued patents, there is no assurance that our patents will be comprehensive enough to provide us with meaningful patent protection against our competitors.
In addition to relying on patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We have entered into intellectual property, invention, and non-disclosure agreements with our employees, and it is our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Our Licensing Arrangements
License Agreement with Lineage: iTRTM, PureStem® and Telomere Length
Concurrently with the contribution of assets to us by Lineage under an Asset Contribution and Separation Agreement, we entered into a License Agreement with Lineage pursuant to which Lineage has licensed to us, with rights to sublicense, certain intellectual property, including patents and patent applications and know-how for use in the development, manufacture and commercialization of products or services for the prevention, treatment, amelioration, diagnosis or monitoring of all human and non-human animal diseases and conditions except for the field of medical products, devices and services for the reserved Lineage Exclusive Field of orthopedic, ophthalmic, and medical aesthetic uses. In addition, Lineage retains an option right, on terms to be negotiated, to license iTR patents in research, development, manufacturing and commercialization of treatments based on iTR in the Lineage Exclusive Field. The licensed patents and know-how relate generally to (a) Lineage’s PureStem® human embryonic progenitor cell lines, and (b) telomere length and DNA quality control analysis in pluripotent stem cells.
The Lineage patent rights licensed to us are exclusive and worldwide except for existing third-party licenses, and for medical products, devices, and services related to tendon. We additionally received an option to license certain Lineage retained rights outside of orthopedic indications unless a license grant would compete with a Lineage program or products in the Lineage Exclusive Field.
The License Agreement contains customary provisions pertaining to patent maintenance, enforcement, and defense and related cost allocations, insurance, indemnification, and termination of the license in the event of a breach or default by a party, or the bankruptcy or other insolvency event with respect to a party.
Additional License and Sublicense Agreements
Lineage and certain Lineage subsidiaries also entered into agreements pursuant to which they have licensed or sublicense to us, on a non-exclusive, world-wide, royalty bearing basis, certain additional patents and patent rights and know-how relating to human embryonic progenitor cell technology and human pluripotent stem cell lines and technology for use outside the Lineage Exclusive Fields, or in the case of certain sublicense rights, fields previously licensed to third parties.
Sublicense of Certain Progenitor Patents
Lineage has granted to us a sublicense of certain patents licensed to Lineage that pertain to the derivation of human embryonic progenitor cell lines. The sublicense will permit us to use the sublicensed patents for the treatment, palliation, diagnosis, or prevention of any disease, disorder or health condition outside of the Lineage Exclusive Field. The sublicense expires the later of July 10, 2028 or the latest expiration date of a sublicensed patent, unless terminated earlier pursuant to the terms of the sublicense.
We will pay Lineage a royalty on “net sales,” as defined in the sublicense agreement, until the royalty payments to Lineage’s licensor by Lineage total $1.2 million and thereafter will pay to Lineage a low single digit royalty on its own net sales and a low double digit royalty on sublicensing consideration.
If we grant a sublicense to use the patents, we will pay Lineage a portion of any consideration received for a sublicense, including but not limited to, upfront payments and milestones, and non-cash exchanges or considerations, but not payments for developing a product, service or process. If we become obligated to pay royalties to one or more affiliates of Lineage for the use of patent rights related to this sublicense and as a result, the royalties payable to Lineage with respect to royalties under the sublicense plus the royalties payable to the affiliates would exceed a designated amount of net sales, the royalties due to Lineage may be reduced but not less than the designated amount. In addition, we will pay to Lineage a royalty on “net sales,” as defined in the sublicense agreement, by the sublicensee. If we become obligated to pay royalties to one or more affiliates of Lineage for the use of patent rights related to this sublicense and as a result, the royalties payable to Lineage with respect to sales by a sublicensee plus the royalties payable to the affiliates would exceed a designated amount of net sales, the royalty due on net sales by the sublicensee may be reduced but not less than the designated amount.
The sublicense agreement includes reciprocal cross-licenses between Lineage and us with respect to any new patents that may be issued based on the use of the sublicensed patents. Any such license to Lineage will be exclusive in the Lineage Exclusive Field and nonexclusive in all other licensed fields. Any such license from Lineage to us will be for use outside the Lineage Exclusive Field and for medical products or services involving tendon. Each license will be for a term of 10 years.
The foregoing description of the sublicense agreement is qualified in its entirety by reference to the sublicense agreement, a copy of which is filed as an exhibit to our Registration Statement on Form 10 and is incorporated herein by reference.
ESI License
Lineage’s subsidiary ES Cell International Pte (ESI) has granted to us non-exclusive rights to certain ESI patents and human pluripotent stem cell lines, or ESI Cell Lines, for use outside of the ESI Exclusive Field of orthopedic, ophthalmic, medical aesthetic, and spinal cord injury uses, and outside certain other fields for which ESI has previously granted licenses. We will pay ESI a 2% royalty on “net sales,” as defined in the license agreement. If we become obligated to pay royalties to one or more third party or to Lineage for the use of patent rights related to this license and as a result the royalties payable to ESI with respect to this license agreement plus the royalties payable to such third party or Lineage would exceed a designated amount of net sales, the royalty due on net sales by the sublicensee may be reduced. The patent license expires upon the latest expiration date of a licensed patent, unless terminated earlier pursuant to the terms of the license. All other rights under the license are terminable by either party under the conditions specified in the license.
If we grant rights to any third party to use ESI Cell Lines derived under cGMP, we will pay ESI a share of all consideration that we receive as consideration for the grant of those rights, including all cash and non-cash consideration but not royalties. We are not permitted to grant sublicenses to the licensed ESI patents but may sublicense the use of ESI Cell Lines.
AgeX also will pay ESI 5% of any fees that AgeX may receive for providing third parties with a “drug master file” for submission to the FDA or similar regulatory agencies in other jurisdictions that may be used to provide confidential detailed information about facilities, processes or articles used in the manufacturing, processing, packaging and storing of one or more human drugs, including but not limited to biologics, cell lines and cell products.
The foregoing description of the ESI License Agreement is qualified in its entirety by reference to the ESI License Agreement, a copy of which is filed as an exhibit to our Registration Statement on Form 10 and is incorporated herein by reference.
Competition
The biotechnology industry is highly competitive and characterized by rapid change (even disruptive advances) that challenge the ability of any one company to maintain leadership. Therefore, we face competition on multiple fronts, including from other biotechnology companies, large pharmaceutical companies, academic institutions and government research entities. We believe the competitive advantages of our technology platform and resulting product candidates arise from the large market opportunities addressed by our product candidates, their anticipated safety profile, the expected cost of manufacture of off-the-shelf products, our intellectual property, as well the fundamental and widespread role of cell aging and regeneration in human age-related degenerative disease.
There are numerous biotechnology companies developing therapeutics for human aging, with each company often focusing on a specific molecular pathway within cells. For example, ResTORbio, Inc. is developing modulators of the mechanistic target of rapamycin (mTOR) pathway to treat immunological and cardiovascular disorders. Calico Life Sciences LLC is a Google-founded research and development company aimed at identifying molecular pathways that control animal lifespan and translating these insights into novel therapeutics designed to increase human healthspan. Calico has not disclosed its lead product development plans. Unity Biotechnology, Inc. focuses on cellular senescence, in particular, the use of agents that can target senescent cells for selective ablation (senolysis). Unity’s stated targeted age-related diseases include osteoarthritis as well as other ophthalmological and pulmonary diseases. In addition, Altos Labs, Inc. (Altos) has reportedly received funding commitments in excess of $3 billion for research and development of products relating to age-reprogramming. The initial technology focus disclosed by Altos may compete with the iTR program within AgeX and its subsidiary Reverse Bio.
Our therapeutic product candidates in development are likely to face competition from a large number of companies and technological strategies including therapeutics intended to address our lead indications, including:
| ● | Type II diabetes: current standard of care treatments (though not necessarily focused on the root cause of the disease) include dieting and exercise programs to reduce weight, or pharmacological interventions with a wide array of medications, including: Metformin (Glucophage, Glumetza, or others); (DiaBeta, Glynase), glipizide (Glucotrol) and glimepiride (Amaryl); Meglitinides (repaglinide (Prandin) and nateglinide (Starlix)); Thiazolidinediones (rosiglitazone (Avandia) and pioglitazone (Actos)); DPP-4 (sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta)); GLP-1 receptor agonists (exenatide (Byetta) and liraglutide (Victoza)); SGLT2 inhibitors (canagliflozin (Invokana) and dapagliflozin (Farxiga)); and insulin therapy (Insulin glulisine (Apidra), Insulin lispro (Humalog), Insulin asgpart (Novolog), Insulin glargine (Lantus), Insulin detemir (Levemir), Insulin isophane (Humulin N, Novolin N)). |
| ● | Vascular ischemia, including myocardial ischemia: current standard of care treatments including dieting, lowered intake of cholesterol, daily aspirin as a blood thinner; pharmacological agents including but not limited to nitrates as vasodilators (nitroglycerin sublingual tablet (Nitrostat), nitroglycerin transdermal ointment (Nitro-Bid), and isosorbide mononitrate and dinitrate (Isordil, Isordil Titradose, Dilatrate-SR)); beta blockers (atenolol (Tenormin), metoprolol (Lopressor, Toprol XL), and nadolol (Corgard)); calcium channel blockers (amlodipine (Norvasc), amlodipine and atorvastatin (Caduet), amlodipine and benazepril (Lotrel), diltiazem (Cardizem), felodipine (Cardene, Cardene SR), and verapamil (Calan); cholesterol-lowering medications such as statins atorvastatin (Lipitor), rosuvastatin (Crestor), and simvastatin (Zocor); Angiotensin-converting enzyme (ACE) inhibitors (Ranolazine (Ranexa), benazepril (Lotensin), and lisinopril (Prinivil, Zestril, Qbrelis); and surgical procedures to increase circulation including but not limited to angioplasty and stenting, coronary artery bypass surgery, and enhanced external counterpulsation. |
Many of our competitors have greater financial, collaborative, technical, regulatory, and human resources as well as products more advanced in development than our product pipeline, including products already marketed for our target indications. As a result, these competitors may have great success in obtaining regulatory approvals, reimbursement, or market acceptance. Our competitors, may have greater success in attracting qualified personnel, recruiting clinical trial sites, or in establishing strategic partnerships with larger pharmaceutical companies to fund large late-stage clinical trials or product marketing. In addition, our future business could be limited should our competitors commercialize products demonstrated to be more effective, safer, or less expensive than our comparable products.
Government Regulation and Product Approval
Government authorities at the federal, state, and local level, and in other countries, extensively regulate among other things, the development, testing, manufacture, quality, approval, safety, efficacy, distribution, labeling, packaging, storage, record keeping, marketing, import/export, and promotion of drugs, biologics, and medical devices. Authorities also heavily regulate many of these activities for human cells, tissues, and cellular and tissue-based products (HCT/Ps).
FDA and Foreign Regulation of Therapeutic Products
The FDA and foreign regulatory authorities will regulate our proposed products as drugs, biologicals, or medical devices, depending upon such factors as: the use to which the product will be put, the chemical composition of the product, and the interaction of the product with the human body. In the United States, the FDA regulates drugs and biologicals under the Federal Food, Drug and Cosmetic Act (FDCA), the Public Health Service Act (PHSA), and implementing regulations. In addition, establishments that manufacture human cells, tissues, and cellular and tissue-based products are subject to additional registration and listing requirements, including current good tissue practice regulations. To the extent AgeX develops cellular and tissue-based products or therapies, its products will be subject to review by the FDA staff in its Center for Biologics Evaluation and Research (CBER) Office of Cellular, Tissue, and Gene Therapies. In some instances, AgeX’s clinical study protocol for a cell therapy product must be reviewed by the National Institute of Health through its Recombinant DNA Advisory Committee.
Any human drug and biological products that we may develop for testing, marketing, or use in the United States will be subject to rigorous FDA review and approval procedures. After testing in animals to evaluate the potential efficacy and safety of the product candidate, an IND submission must be made to the FDA to obtain authorization for human testing. Extensive clinical testing, which is generally done in three phases, must then be undertaken at a hospital or medical center to demonstrate optimal use, safety, and efficacy of each product in humans. Each clinical study is conducted under the auspices of an independent institutional review board (IRB). The IRB will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical trials are generally conducted in three “phases.” Phase I clinical trials are conducted in a small number of healthy volunteers or volunteers with the target disease or condition to assess safety. Phase II clinical trials are conducted with groups of patients afflicted with the target disease or condition in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In some cases, an initial trial is conducted in diseased patients to assess both preliminary efficacy and preliminary safety, in which case it is referred to as a Phase I/II trial. Phase III trials are large-scale, multi-center, comparative trials and are conducted with patients afflicted with the target disease or condition in order to provide enough data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the clinical trial based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the intended patient population. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuing process. The time and expense required to perform this clinical testing can far exceed the time and expense of the research and development initially required to create the product.
No action can be taken to market any therapeutic product in the U.S. until an appropriate New Drug Application (NDA) or BLA has been approved by the FDA. Submission of the application is no guarantee that the FDA will find it complete and accept it for filing. If an application is accepted for filing, following the FDA’s review, the FDA may grant marketing approval, request additional information, or deny the application if it determines that the application does not provide an adequate basis for approval. FDA regulations also restrict the export of therapeutic products for clinical use prior to FDA approval. To date, the FDA has not granted marketing approval to any pluripotent stem-based therapeutic products and it is possible that the FDA or foreign regulatory agencies may subject our product candidates to additional or more stringent review than drugs or biologicals derived from other technologies.
The FDA offers several programs to expedite development of products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing treatments. A product may be eligible for breakthrough therapy designation if it treats a serious or life-threatening disease or condition and preliminary clinical evidence indicates it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. In 2017, FDA established a new regenerative medicine advanced therapy (RMAT) designation as part of its implementation of the 21st Century Cures Act. An RMAT is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions that is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and preliminary clinical evidence indicates that it has the potential to address unmet medical needs for such a disease or condition. RMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. Once approved, when appropriate, the FDA can permit fulfillment of post-approval requirements under accelerated approval through the submission of clinical evidence, clinical studies, patient registries, or other sources of real world evidence such as electronic health records; through the collection of larger confirmatory datasets; or through post-approval monitoring of all patients treated with the therapy prior to approval.
Some of our future products may be eligible for RMAT designation. There is no assurance that the FDA will grant breakthrough therapy, accelerated approval or RMAT status to any of our product candidates.
In addition to regulations in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a drug candidate, we must obtain approval by the comparable regulatory authorities of foreign countries or economic areas, such as the European Union, before we can commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Combination Products
If we develop any products that are used with medical devices, they may be considered combination products, which are defined by the FDA to include products comprised of two or more regulated components or parts such as a biologic and a device. When regulated independently, biologics and devices each have their own regulatory requirements. However, regulatory requirements for a combination product comprised of a biologic administered with a delivery device can be more complex, because in addition to the individual regulatory requirements for each component, additional combination product regulatory requirements may apply.
510(k) Medical Devices & Notification
Product marketing in the U.S. for most Class II and limited Class I devices typically follows a 510(k) pathway. To obtain 510(k) clearance, a manufacturer must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a legally marketed device, referred to as the predicate device. A predicate device may be a previously 510(k) cleared device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for submission of PMA applications, or a product classification created by FDA when it granted de novo authorization. The manufacturer must show that the proposed device has the same intended use as the predicate device, and it either has the same technological characteristics, or it is shown to be equally safe and effective and does not raise different questions of safety and effectiveness as compared to the predicate device.
There are three types of 510(k)s: traditional; special, for devices that are modified and the modification needs a new 510(k) but the modification does not affect the intended use or alter the fundamental scientific technology of the device; and abbreviated, for devices that conform to a recognized standard. The special and abbreviated 510(k)s are intended to streamline review. The FDA intends to process special 510(k)s within 30 days of receipt and abbreviated 510(k)s within 90 days of receipt. Though statutorily required to clear a traditional 510(k) within 90 days of receipt, the clearance pathway for traditional 510(k)s can take substantially longer.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained.
Post-Approval Matters
Even after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval for the use of a product as a treatment for clinical indications other than those initially targeted. Data resulting from these clinical trials may result in expansions or restrictions to the labeled indications for which a product has already been approved.
FDA Regulation of Manufacturing
The FDA regulates the manufacturing process of pharmaceutical products, human tissue and cell products, and medical devices, requiring that they be produced in compliance with cGMP. The FDA regulates and inspects equipment, facilities, laboratories, and processes used in the manufacturing and testing of products prior to providing approval to market products. If after receiving approval from the FDA, a material change is made to manufacturing equipment or to the location or manufacturing process, additional regulatory review may be required. The FDA also conducts regular, periodic visits to re-inspect the equipment, facilities, laboratories and processes of manufacturers following an initial approval. If, as a result of those inspections, the FDA determines that that equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against the manufacturer, including suspension of manufacturing operations. Issues pertaining to manufacturing equipment, facilities or processes may also delay the approval of new products undergoing FDA review.
Federal Funding of Research
The NIH has adopted guidelines on the use of hES cells in federally funded research, consistent with President Obama’s Executive Order which rescinded President Bush’s Executive Orders that permitted federal funding of research on hES cells using only the limited number of hES cell lines. The central focus of the guidelines is to assure that hES cells used in federally funded research are derived from human embryos that were created for reproductive purposes, are no longer needed for this purpose, and are voluntarily donated for research purposes with the informed written consent of the donors. Those hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research.
California State Regulations
The state of California has adopted legislation and regulations that require institutions that conduct stem cell research to notify, and in certain cases obtain approval from, a Stem Cell Research Oversight Committee (SCRO Committee) before conducting the research. Under certain California regulations, all hES cell lines that will be used in our research must be acceptably derived. California regulations further require certain records to be maintained with respect to stem cell research and the materials used. AgeX programs that involve the use of stem cells will be reviewed by a SCRO Committee to confirm compliance with federal and state guidelines. The hES cell lines that we use are all on the NIH registry of lines that have been reviewed and meet standards for federal funding grants.
Health Insurance Portability and Accountability Act
Under HIPAA, HHS has issued regulations to protect the privacy and security of protected health information used or disclosed by health care providers. HIPAA also regulates standardization of data content, codes, and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties.
The requirements under these regulations may periodically change and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements. New laws governing privacy may also be adopted in the future. We can provide no assurance that we will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Federal and State Fraud and Abuse Laws
We are also subject to various laws pertaining to healthcare “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal to solicit, offer, receive or pay any remuneration in exchange for or to induce the referral of business, including the purchase or prescription of a particular drug that is reimbursed by a state or federal program. The term “remuneration” has been broadly interpreted to include anything of value. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act (ACA), among other things, amended the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate, in order to commit a violation. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as by the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid). Liability under the false claims laws may also arise when a violation of certain laws or regulations related to the underlying products (e.g., violations regarding improper promotional activity or unlawful payments) contributes to the submission of a false claim.
Additionally, the FCPA prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Healthcare Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. There have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs.
In particular, the ACA has had, and is expected to continue to have, a significant impact on the healthcare industry. The ACA was designed to expand coverage for the uninsured while at the same time containing overall healthcare costs. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare providers and entities, and a significant number of provisions are not yet, or have only recently become, effective.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, and there may be additional challenges and amendments to the ACA in the future, including efforts to implement changes to the law that may impact reimbursement for drugs and biologics.
Further, there has been heightened government scrutiny over the manner in which manufacturers set prices for their marketed pharmaceutical products. Such scrutiny has resulted in several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to pharmaceutical product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. Such proposals have included, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B and to allow some states to negotiate drug prices under Medicaid. The Department of Health and Human Services has already started the process of soliciting feedback on some of these measures and, at the same, is immediately implementing others under its existing authority. Although some of these and other proposals will require authorization through additional legislation to become effective, Congress and the President are likely to continue to seek new legislative and/or administrative measures to control drug costs. At the state level, legislatures have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
It is uncertain whether and how future legislation, whether domestic or foreign, could affect prospects for our product candidates or what actions foreign, federal, state, or private payors for health care treatment and services may take in response to any such health care reform proposals or legislation. Adoption of price controls and other cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures reforms may prevent or limit our ability to generate revenue, attain profitability or commercialize our product candidates.
Moreover, the DSCSA imposes obligations on manufacturers of pharmaceutical products, among others, related to product tracking and tracing. While some requirements of the DSCSA began in November 2014, many key requirements, development of standards, and the system for product tracing will continue to be phased in until 2023. Among the requirements of the DSCSA, manufacturers will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by manufacturers will eventually be required to be done electronically. Manufacturers will also be required to verify that purchasers of the manufacturers’ products are appropriately licensed. Further, under this new legislation, manufacturers will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or that are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Reimbursement
Medicare, Medicaid, and Third-Party Reimbursement Programs
Sales of the therapeutic products and medical devices that we and our subsidiaries may develop will depend, in part, on the extent to which the costs of those products will be covered by third-party payors, such as government health programs, commercial insurance, and managed healthcare organizations.
The containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. In the United States, the federal and many state governments have adopted or proposed initiatives relating to Medicaid and other health programs that may limit reimbursement or increase rebates that providers are required to pay to the state. The ACA increased many of the mandatory discounts and rebates and imposed a new Branded Prescription Pharmaceutical Manufacturers and Importers fee payable by manufacturers. Provisions of the Inflation Reduction Act of 2022 may impact the prices of drug products that are sold in the United States, particularly through Medicare programs. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease.
In addition to government regulation, managed care organizations in the United States, which include medical insurance companies, medical plan administrators, health-maintenance organizations, hospital and physician alliances and pharmacy benefit managers, continue to put pressure on the price and usage of healthcare products. Managed care organizations and third-party payers seek to contain healthcare expenditures, and their purchasing strength has been increasing due to their consolidation into fewer, larger organizations and a growing number of enrolled patients. Adoption of price controls, cost-containment measures, and more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If third-party payors do not consider the products we develop to be cost-effective compared to other therapies, they may not cover our products as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Other legislative and regulatory actions that would have a significant impact include: changes to coverage and payment for biosimilars, including the current Medicare biosimilar coverage and payment policies intended to encourage biosimilar adoption, or other policies that provide easier substitution or reimbursement advantages. The adoption of new healthcare legislation remains uncertain, but impending changes will likely impact the number of patient lives covered, the quality of the insurance, Medicaid eligibility and the level of patient protections provided.
We face similar issues outside of the United States. In some non-U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the EU provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for aa medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability of placing a medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the EU do not follow price structures of the United States and generally tend to be significantly lower.
Employees
As of September 30, 2023, we employed five persons on a full-time basis and four persons on a part-time basis. Three of these employees hold a Ph.D.
Legal Proceedings
AgeX may from time to time be a party to litigation and subject to claims incident to the ordinary course of business. In the future, AgeX may become a party to an increasing number of litigation matters and claims, including in connection with Merger Agreement and the transactions contemplated thereby. The outcome of litigation and claims cannot be predicted with certainty, and the resolution of these matters could materially affect AgeX’s future results of operations, cash flows or financial position. AgeX not presently involved in any material litigation or proceedings, and to its knowledge no material litigation or proceedings are contemplated.
DESCRIPTION OF SERINA’S BUSINESS
References to “we,” “us” and “our” in this section titled “Description of Serina’s Business” refer to Serina.
Overview
Serina is a clinical-stage biotechnology company developing a pipeline of wholly-owned drug product candidates to treat neurological diseases and pain. Serina’s POZ drug delivery technology is designed to enable certain existing drugs and novel drug candidates to be modified in a way that may provide an increase in efficacy and safety of the resulting polymeric drug conjugate. Serina’s proprietary POZ technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection.
The therapeutic agents in Serina’s product candidates are typically well-understood and marketed drugs that are effective but are limited by pharmacokinetic (PK) profiles that can include toxicity, side effects and short half-life. We believe that by using POZ technology, drugs with narrow therapeutic windows can be designed to maintain more desirable and stable levels in the blood. We believe that POZ technology can be applied to small molecules, proteins, antibody drug conjugates, and other classes of molecules.
The chemical attachment of water-soluble polymers, in particular polyethylene glycol (PEG), to drugs has become a valuable technique for improving the properties of pharmaceuticals. This technique has been successfully employed in producing many FDA-approved drugs. Serina believes that there is an unmet need for polymer delivery technology that addresses the limitations of PEG and other biocompatible polymers and that the POZ technology has the following advantages:
| ● | Synthesis and stability – POZ is produced using inexpensive starting materials in essentially a “one pot” synthesis and is stable at room temperature; |
| ● | Non-immunogenic or reduced immunogenicity – in studies to date, POZ has not elicited an immune response or stimulated significant development of antibodies to POZ; |
| ● | Drug loading and release – POZ enables greater drug loading than PEG, with highly programmable drug release kinetics to enable continuous drug delivery; |
| ● | No accumulation –studies to date indicate that POZ does not accumulate in tissues when given at dose levels anticipated to be given to humans, is not metabolized, and is cleared almost entirely through renal filtration; and |
| ● | Targeting – POZ offers multiple substitution sites, which allows for simultaneous attachment of several copies of a drug along with a targeting molecule. |
Serina’s business is largely focused on the development of a wholly-owned pipeline of POZ-enabled drug candidates for central nervous system (CNS) indications, including Parkinson’s disease, epilepsy, and pain. Serina’s lead product candidate, SER 252 (POZ-apomorphine), is a POZ conjugate of the potent dopamine agonist apomorphine being developed for the treatment of Parkinson’s disease and is in preclinical development. SER 252 is designed to provide CDS via a subcutaneous injection delivered one to two times per week. CDS is a long-sought clinical strategy for Parkinson’s disease that currently approved therapies fall short of delivering. Other POZ-conjugate candidates, including SER 227 (POZ-buprenorphine) for long-acting pain relief and SER 228 (POZ-cannabidiol) for epilepsy, are in preclinical development. We intend to develop other potential applications of the POZ technology, such as therapeutics delivered through ADCs and lipid-nanoparticle (LNP), through partnerships. Serina’s SER 214, a POZ conjugate of rotigotine for the treatment of early Parkinson’s and Restless Leg Syndrome, was Serina’s first product candidate advanced into human study and has completed a Phase Ia study in 19 subjects. Serina intends to out-license this product candidate.
Serina is led by a management team with extensive experience in drug discovery, platform technologies, clinical development, general management, financing, and business development transactions. Serina’s team also has specific experience in developing polymer therapeutics, including at Shearwater Polymers, Inc. (Shearwater), a company founded by Serina Executive Chairman J. Milton Harris and Serina’s former Chief Scientific Officer Michael Bentley, where they developed the first-generation polymer technology based on PEG conjugation, a technology platform that has been licensed to develop a wide array of “PEGylated” drugs. Shearwater was acquired by Inhale Therapeutics in 2001. Inhale subsequently changed its name to Nektar Therapeutics, Inc. The PEG technology platform has resulted in thirty-two (32) FDA approved drugs.
The members of Serina’s senior management team are:
| ● | Serina’s Chief Executive Officer and director, Randall Moreadith, M.D, Ph.D., has more than 13 years of experience as a life sciences CEO and over 25 years in executive roles. Prior to joining Serina, Dr. Moreadith served as the Chief Development Officer at Nektar Therapeutics where he led a clinical and drug development program that successfully moved several drugs into clinical trials, including the launch of NKTR-102 into four clinical cancer indications and the out-licensing efforts for the approved drug Movantik®. He has served in executive roles at Cardium Therapeutics, Inc., Renovis, Inc. and was a co-founder of ThromboGenics, Ltd. (now Oxurion). |
| ● | Serina’s Executive Chairman of the Serina Board and Co-Founder, J. Milton Harris, Ph.D., has more than 30 years of experience as a senior life sciences executive. Prior to founding Serina, he founded and was CEO of Shearwater. Shearwater successfully patented, manufactured, and partnered PEG technology that enabled multiple drug products including Neulasta® (Amgen) and Pegasys® (Roche). Dr. Harris has also served on the board of directors of HudsonAlpha Institute for Biotechnology since its founding in 2004. |
| ● | Serina’s Chief Operating Officer, Tacey Viegas, Ph.D., has more than 30 years of pharmaceutical industry experience in drug discovery, delivery, and development. Prior to joining Serina, he previously worked at R.P. Scherer Corporation (now Catalent, Inc.), MDV Technologies, Inc., BioCryst Pharmaceuticals, Inc. and Shearwater. During his tenures at Shearwater and BioCryst, he was a co-inventor of nalexogol (Movantik®) and was involved in the early development of peramivir (Rapivab™). |
| ● | Serina’s Chief Financial Officer and Board Director, Steve Ledger, has more than 30 years of experience in private capital and public company equity investment management as an investor and strategic advisor. He is a Managing Partner at Form & Fiction Ventures, a seed stage venture capital investment firm and a co-founder and director at Entourage Genomics, Inc., a bioinformatics software company. |
In addition to its management team, Serina has engaged a board of scientific advisors to advise Serina on its drug development efforts and offer technical guidance to help shape Serina’s scientific and strategic direction. While there are no formal rules or procedures for Serina’s scientific advisory board, Serina’s scientific advisors hold meetings throughout the year where they interact with the appropriate members of the Serina management team in real-time, lending expertise and guidance as needed. The members of the scientific advisory board may receive equity and cash compensation pursuant to their respective consulting agreements with Serina.
Serina’s scientific advisory board members are:
| ● | C. Warren Olanow, MD., is Professor Emeritus in and former Chairman of the Department of Neurology and Department of Neurosciences at the Mount Sinai School of Medicine in New York. He has served on the board of directors of the National Space Biomedical Research Institute and the executive committee of the Michael J. Fox Foundation Scientific Advisory Board, and he is the former Chairman of the Scientific Advisory Board of the Bachmann-Strauss Dystonia & Parkinson Foundation. Dr. Olanow is the former Co-Editor-in-Chief of the journal, Movement Disorders. He has been principal investigator of numerous studies leading to approval of drugs and devices for treating neurodegenerative diseases. |
| ● | William Schmidt, Ph.D., is President of NorthStar Consulting, LLC, which specializes in providing advice on preclinical and clinical studies of novel analgesic drugs. Dr. Schmidt has more than 30 years of experience in pain medicine pharmaceutical development. He is President and CEO of Catalina Pharma, part-time Senior Vice President of Global Clinical Development for Helixmith Inc., part-time Vice President of Clinical Development for EicOsis, LLC and Chief Medical Officer for Ensysce Biosciences. |
| ● | Gaurav Sahay., Ph.D., is a professor in the Department of Pharmaceutical Sciences and co-Director for the Center of Innovative Drug Delivery and Imaging (CIDDI), at the College of Pharmacy at Oregon State University. Dr. Sahay’s lab is developing novel nanotechnology-based platforms including lipid-based nanoparticles for effective delivery of messenger RNA therapeutics for treatment of cystic fibrosis, retinal degeneration and against SARS-CoV2. He has more than 60-peer-reviewed publications in top tier journals including Science Advances, Nature, Nature Communications, Nature Biotechnology, Nature Nanotechnology, Journal of Controlled Release, and Nano Letters. |
Serina’s Development Pipeline
We believe that Serina’s POZ platform delivery technology has potential for use across a broad range of payloads and indications. Among our development candidates, Serina intends to internally advance SER 252 for advanced Parkison’s disease and SER 227 for long-acting pain relief. Serina will not advance SER 214 internally and will seek to partner on any further development of this candidate. Serina is evaluating whether to internally advance SER 228 or to partner this candidate. Serina is advancing its research and development efforts for POZ technology in lipid nanoparticle delivered ribonucleic acid (RNA) vaccines for infectious diseases. Serina intends to advance additional applications of the POZ platform via out-licensing, co-development, or other partnership arrangements. These applications may include other CNS disorders and POZ LNP disease fields.

1. SER 214 will not be advanced internally. Serina will seek to partner on any further clinical development.
2. SER 252 IND-enabling studies were initiated August 2023 and are anticipated to be completed in the third quarter of 2024. Serina intends to advance SER 252 into Phase I clinical trials in 2025 for patients with advanced Parkinson’s disease.
3. SER 227 has completed IND-enabling studies and is currently evaluating the design of a Phase I clinical trial to present to the FDA in the first quarter of 2024. Prospective timing on the start of a Phase I study is late 2024 to early 2025 time period, subject to raising additional capital (in addition to any proceeds expected to be received from Juvenescence through the exercise of post-merger warrants).
4. SER 228 has not yet initiated IND-enabling studies. Serina anticipates advancing this candidate into IND-enabling studies in 2024, subject to raising additional capital (in addition to any proceeds expected to be received from Juvenescence through the exercise of post-merger warrants).
Serina’s Strategy
Serina’s strategy is to develop and commercialize polymer therapeutics based on conjugation of suitable small molecules to our proprietary POZ. While prior polymer technologies such as PEG focused primarily on protein conjugation, Serina envisioned the need to develop a polymer therapeutics platform that could address the vast universe of small molecules and program their release. Serina has demonstrated in multiple animal studies that POZ can program the release of small molecules, particularly those that have solubility challenges and PK limitations. Serina believes that specific POZ conjugated small molecules can be delivered continuously following a single injection. As a “platform technology,” Serina anticipates this technology has the potential for development of drug candidates across a broad range of payloads and indications. Serina intends to focus on its current pipeline of candidates and selectively explore new molecules for potential internal development. In parallel, Serina intends to expand its collaboration activity with prospective partners that have compounds that could potentially benefit from the Serina POZ polymer platform technology. To achieve this, Serina intends to:
| ● | Advance its lead program, SER 252, which addresses a large unmet need for continuous dopaminergic stimulation for late stage Parkinson’s patients; |
| ● | Develop its other CNS product candidates, SER 227 for long-acting pain relief, and SER 228 for treatment refractory epilepsy; |
| ● | Seek to create economic and strategic value through POZ LNP licensing and partnerships across multiple RNA-based therapeutic indications; |
| ● | Seek to create economic and strategic value for other applications of the POZ platform, including ADCs; |
| ● | Continue to expand upon applications of the POZ platform with a view to capitalize on the potential of the technology to enable additional payloads and product opportunities; and |
| ● | Continue to expand a network of academic and biopharmaceutical collaborations and commercial partnerships to develop and potentially validate other applications of the POZ platform. |
Company and POZ Development Background
Over the past 25 years, chemical attachment of water-soluble polymers, in particular, polyethylene glycol, to drugs has become a valuable technique for improving the properties of pharmaceuticals. This technique has been successfully employed in producing second-generation forms of several FDA-approved drugs.
A polymer drug delivery approach involves using polymers, which are large molecules composed of repeating subunits, to design drug delivery systems that can control the release of medications in a controlled and targeted manner. Polymer-based drug delivery systems can enhance the therapeutic outcomes of drugs by optimizing their PKs, improving patient compliance, and reducing side effects. Some key aspects of polymer drug delivery are as follows:
| ● | Controlled Release: Polymers can be engineered to release drugs at a controlled and predetermined rate. This enables sustained drug levels in the body, reducing the need for frequent dosing and maintaining therapeutic concentrations over an extended period. |
| ● | Targeted Delivery: Polymer drug delivery systems can be designed to target specific tissues, cells, or organs with a high concentration of the drug while minimizing systemic exposure. This precision targeting reduces the exposure of healthy tissues to the drug, minimizes side effects, and increases the drug’s effectiveness. |
| ● | Improved Bioavailability: Some drugs have low solubility or stability in the body, limiting their absorption and therapeutic efficacy. Polymers can be used to improve the solubility and stability of such drugs, allowing for better bioavailability. |
| ● | Protection of Labile Drugs: Polymers can protect labile or sensitive drugs from degradation in the harsh conditions of the gastrointestinal tract, allowing for oral delivery of drugs that would otherwise be ineffective when taken orally. |
| ● | Reduced Toxicity: Polymer encapsulation can help reduce the toxicity of certain drugs by controlling their release and preventing peak concentrations that can lead to adverse effects. |
| ● | Long-Acting Formulations: Polymer-based formulations can extend the duration of drug action, reducing the frequency of administration. This is particularly useful for chronic conditions where adherence to treatment is crucial. |
| ● | Biodegradable Polymers: Some polymer drug delivery systems are made from biodegradable materials that gradually break down in the body, eliminating the need for removal or extraction of delivery devices. |
Common polymer-based drug delivery systems include:
| ● | Microspheres and nanoparticles: Tiny particles made from biocompatible polymers that encapsulate and release drugs. |
| ● | Hydrogels: Cross-linked polymer networks that can hold a large amount of water and release drugs in response to environmental cues. |
| ● | Implants: Solid polymer devices that can be surgically implanted for long-term drug delivery. |
| ● | Liposomes: Lipid-based vesicles that can encapsulate drugs and deliver them to specific sites. |
Polymer drug delivery approaches offer versatility and customization, allowing for the design of drug delivery systems tailored to the specific needs of a drug and its intended therapeutic application. These approaches have the potential to enhance the safety and efficacy of medications while improving patient compliance and convenience.
Serina was formed with the goal of inventing a polymer that was distinct from PEG that could be used for modification of drugs. Research at Serina over the past fifteen years has led to the development of a new polymer technology based on poly(2-ethyl-2-oxazoline). Serina’s POZ technology is designed to be a “platform technology” in that Serina anticipates that other products can be developed using the same basic polymer.
Serina was first to develop and patent methods to produce polymers of POZ suitable for pharmaceutical applications. For the purposes of illustration, a simple form of POZ with ethyl side chains (pendent groups) and a carboxylic acid end group is shown below (note that in this structure the number of repeating units can be several hundred, with the individual units coming one after another):

Figure 1: Illustration of POZ with ethyl pendent groups and carboxylic acid end group
Figure 1: SER 214 Schematic representation of POZ-rotigotine. Rotigotine is attached to a 20 kDa pendent POZ polymer employing copper-catalyzed “click chemistry.” Each molecule of SER 214 contains approximately ten pendent rotigotine molecules.
During the synthesis of polymers of POZ, the expanding polymer incorporates a pendent 2-ethy-2-oxazoline pentynyl group. This pendent alkyne is capable of undergoing quantitative “click chemistry” with an azide to form a stable triazole ring structure. Click chemistry is an industry standard quantitative method for the efficient coupling of two molecules. The drug(s) of interest, in this case rotigotine, undergoes attachment of an azide-linker to a suitable chemical handle on the molecule. Rotigotine is a dopamine agonist and is the active drug ingredient in SER 214. All of the product candidates in our pipeline have a phenolic hydroxyl to which a variety of linkers may be attached depending on the nature of chemical release we desire. Cleavable linkers are designed to connect two molecular entities, and when exposed to the appropriate trigger (such as changes in pH, enzymatic activity), they undergo a chemical reaction that leads to the release of the linked components. Enzymatic activity requires water at biological pH. For example, SER 214 contains a 3-propionate linker that attaches rotigotine to the polymer backbone. The attachment of the linker to the hydroxyl group in these product candidates forms a stable ester linkage. Stable linkers are commonly used in various chemical and biological applications where the goal is to maintain the structural integrity and stability of the linked molecules. Serina believes that there is a single esterase activity in humans (and monkeys) in the vascular compartment that cleaves the ester thereby continuously releasing the attached drug. This enzyme is known as butyrylcholinesterase. When the drug is injected subcutaneously, the lymphatics clear the injection and eventually the drug finds its way to the vascular compartment. If the polymer has an extended circulation half-life in the body, the enzyme will continuously cleave the attached drug based on the PKs of the polymer conjugate in the circulation. Serina has empirically determined that a 20 kD polymer of POZ provides a one-week circulation time in both monkeys and humans.
Serina scientists have been able to engineer the foundational chemistry in the simple POZ structure in Figure 1 shown above to replace some of the pendent ethyl groups with reactive pendent acetylene groups. These reactive groups are used to attach drugs via linkages that can be programmed to provide controlled release of the drug. Serina scientists use “click chemistry,” which is a facile and quantitative method for attachment of azide-containing linker/drug conjugates to pendent acetylenes within POZ polymers. Serina’s first clinical drug candidate, SER 214, contains a POZ polymer backbone with multiple pendent groups to which are attached the potent dopamine agonist rotigotine.
Serina developed the idea of attaching rotigotine to POZ to develop a drug that would provide continuous dopaminergic stimulation in Parkinson’s disease patients who are responsive to dopamine agonists. Experimental studies in animals and humans suggest that CDS may prevent some of the adverse side effects of taking other dopamine agonists such as levodopa, which include “wearing off” and levodopa induced dyskinesias (LIDs). Based on studies using the industry standard monkey model of Parkinson’s disease, Serina progressed the development of SER 214, including completing the necessary basic science to design and create SER 214, obtaining the issuance of two key patents in 2013 that cover SER 214 composition of matter and use, advancing SER 214 through initial proof-of-concept in animal models (rat and monkey) of Parkinsonism, and receiving IND approval from the FDA in 2015.
Serina completed a successful SER 214 Phase Ia clinical study in 19 subjects in July 2017. Through the development of SER 214, Serina demonstrated the applicability of the POZ platform to an indication with an unmet need and significant commercial potential, advancing into humans a drug designed to provide continuous dopaminergic stimulation to treat Parkinson’s disease and restless legs syndrome. SER 214 set the stage for Serina to pursue other molecules having the appropriate chemical handle for attachment and release from the POZ polymer.
In September 2015, Serina entered into an agreement with AbbVie Inc. for research and development efforts to create high drug to antibody ratio (DAR) ADCs. Serina’s POZ polymer technology allows for the attachment of multiple copies of a toxin to a single polymer, and then that POZ toxin is attached to multiple sites on the antibody. Serina believes that this approach has the potential to create POZ polymer ADCs with DARs well above the standard technology approach of approximately 2-4 toxins per antibody. Serina believes that this approach may be particularly useful in targeting antigens on the surface of cancers that are in low abundance (less than 10,000 copies per cell) and this may produce a greater chance of killing the targeted cancer. In January 2018, the collaboration was terminated when the companies could not reach agreeable terms to continue the studies. With limited resources to support POZ ADC development internally, this application of the POZ platform was not actively advanced. Serina is currently not pursuing the preclinical study of the POZ ADC application internally and is seeking to advance the program with partners. Serina believes that POZ ADC technology continues to have promising potential and that ADCs represent one of the most promising areas of cancer research today. There are 12 FDA-approved ADCs, with over 170 novel ADCs in the clinical stage of development.
In June 2020, Serina and Jazz Pharmaceuticals, Ltd. (Jazz) entered into an exclusive license agreement for polymer conjugates of cannabidiol, specifically Serina product candidates SER 228 and SER 229. The agreement granted Jazz exclusive rights to certain intellectual property, know-how, and proprietary technologies. The licensing agreement included a one-time upfront payment, milestone payments payable to Serina upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology. In July 2021, the collaboration and license agreement was terminated after Jazz acquired GW Pharmaceuticals, Inc. (GW) and its FDA approved drug Epidiolex®, a cannabidiol based drug, and the GW pipeline of cannabinoid product candidates.
In October 2023, Serina entered into a non-exclusive license agreement with Pfizer, Inc. to use Serina’s POZ polymer technology for use in lipid nanoparticle drug delivery formulations. The agreement grants Pfizer non-exclusive rights to certain intellectual property, know-how, and proprietary technologies. Under the terms of the agreement, Pfizer is authorized to develop, manufacture, market, and commercialize products incorporating the licensed technology with respect to a specific POZ polymer structure in one field. The agreement outlines the protection and enforcement of intellectual property rights related to the licensed technology. Pfizer is obligated to use commercially reasonable diligent efforts to develop and commercialize licensed products, and to use such efforts to accomplish specified development and commercial objectives. The agreement includes a one-time upfront payment of less than $5 million, milestone payments payable to Serina upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology in accordance with the terms outlined in the license agreement.
Serina’s Product Candidates
Serina intends to focus on its current pipeline of wholly-owned candidates and selectively explore new molecules for potential internal development, co-development and partnering. Current candidates include:
SER 214 (POZ-rotigotine) is the first product from Serina’s pipeline to be advanced into humans. Serina initiated a Phase Ia trial July 2015 in 19 stably treated Parkinson’s subjects. The trial was completed in January 2017 with data published in a June 2020 article in Movement Disorders. This was a single and multi-dose, dose-escalation study in patients who were not experiencing significant motor fluctuations. Patients in the study were allowed to be on existing therapy for Parkinson’s disease, or be on no therapy, but have a definitive diagnosis of Parkinson’s disease. Serina has not internally advanced SER 214 beyond Phase Ia and will seek to partner on any further development. Serina believes the SER 214 program, while not being advanced internally, provided data important to the development of a POZ dopamine agonist (such as rotigotine and apomorphine) conjugate to enable CDS in Parkinson’s patients. This research led to the development of SER 252.
SER 252 (POZ-apomorphine) has entered IND-enabling studies. Studies were initiated in August 2023 and are anticipated to be completed in the third quarter of 2024. Serina intends to advance SER 252 into Phase I clinical trials in 2025 for patients with advanced Parkinson’s disease. The treatment of advanced Parkinson’s disease relies on multiple therapies, including levodopa (L-DOPA), compounds that inhibit the breakdown of L-DOPA in the brain (catechol-O-methyl transferase, or COMT; for example, opicopone), dopamine agonists (transdermal rotigotine; for example, NeuproTM) and others. L-DOPA in escalating doses is the mainstay of therapy for advanced Parkinson’s Disease but is also the proximate cause of levodopa-induced dyskinesias (LIDS), one of the most troubling complications of prolonged high dose L-DOPA therapy. Approximately 90% of Parkinson’s disease patients who use L-DOPA for ten years will develop irreversible LIDS. An infusion therapy known as Apo-go (apomorphine) is available in the European Union, or EU, but is not yet available in the United States. Apo-go must be administered as a 12–16-hour continuous infusion through an electronic pump and a standard insulin infusion set. While effective in reducing daily “OFF” time, and simultaneously increasing daily “ON” time without troublesome dyskinesia, its use frequently requires a healthcare provider to hook up the device and infusion each day and remove it at night. “OFF” time refers to the time period the patient is unable to perform routine daily activities. “ON” time refers to those periods where the patient is able to perform routine daily activities. Apo-go is confounded by significant skin reactions in approximately 40% of patients, often leading to permanent scarring (nodules) on the abdomen. Serina’s preclinical studies in monkeys suggest SER 252 may be administered as a single subcutaneous injection twice a week, provides continuous delivery of apomorphine and has no skin liabilities. Its use is designed to be administered in the convenience of the patient’s home without the need for a healthcare provider. Serina believes that SER 252 may allow some patients to titrate completely off L-DOPA, thus simultaneously addressing the LIDS that is associated with its prolonged use.
SER 227 (POZ-buprenorphine) has completed IND-enabling studies. Subject to raising additional capital (in addition to any proceeds expected to be received from Juvenescence through the exercise of post-merger warrants), Serina intends to advance SER 227 into Phase Ia SAD trial in normal volunteers in 2024 to support its eventual use in the treatment of post-operative pain, a strategy that will simultaneously provide analgesia and target the initial gateway to use of potential addictive opioids in the immediate post-operative period. The opioid crisis in the United States claimed approximately 80,000 lives in 2022. Patients who are administered a prescription for an opioid following either major or minor surgery are fifteen times more likely to transition to chronic use of an opioid within 90-180 days than those who do not fill a prescription. SER 227 is designed to provide immediate and sustained release of buprenorphine following a single subcutaneous injection at the time of surgery, with the intended effect of providing immediate analgesia by the time the patient is in the recovery room. The sustained release kinetics for approximately eight days may prevent abuse of an opioid in the event patients take them.
SER 228 (POZ-cannabidiol) has completed early dose-ranging studies in rats and monkeys and expects to enter IND-enabling studies in 2024, subject to raising additional capital (in addition to any proceeds expected to be received from Juvenescence through the exercise of post-merger warrants). If the IND-enabling studies are successfully completed in 2024, and if Serina has sufficient funding, Serina intends to advance SER 228 into a Phase Ia trial in children with refractory epilepsy in 2025. The treatment of childhood epilepsy remains a pressing challenge. CBD, a compound found in Cannabis, has been shown to be an effective treatment for refractory epilepsy (approved product Epidiolex®, developed by GW Pharmaceuticals and acquired by Jazz Pharmaceuticals). Epidiolex® is a sesame seed oil preparation that must be taken twice daily at high doses (20 mg/kg) and is associated with high first-pass metabolism, low oral bioavailability, and diarrhea. Serina is developing SER 228 as a single weekly subcutaneous injection that is predicted to cover the PK profile of twice daily Epidiolex®.
Serina’s POZ platform is designed as an enabling technology for the development of multiple cannabinoids, all of which have limited PK profiles and solubility issues. Cannabis contains over 100 cannabinoid compounds and some of these compounds have already shown promise in preclinical studies in animals. The cannabinoid compounds appear to be constrained by limited PK, low oral bioavailability and high first pass metabolism. Serina has attached cannabidivarin (CBDV), tetrahydrocannabinol (THC), cannabigerol (CBG) and cannabichromene (CBC) to POZ polymers and early studies suggest they can be delivered as single weekly subcutaneous injections to provide continuous drug delivery. The spectrum of potential clinical applications of POZ-cannabinoids include some of the most challenging disorders in neuroscience such as autism spectrum disorder (CBDV), Huntington’s disease and neuroprotection (CBG), chemotherapy-induced nausea, vomiting and spasticity in multiple sclerosis (THC) and traumatic brain injury, and inflammatory diseases (CBC). Serina believes that POZ technology is an enabling technology platform for many of the cannabinoids that may be found to have therapeutic potential in humans.
POZ-lipids. Serina is advancing pre-clinical research focused on POZ-lipids as a non-immunogenic (or markedly reduced immunogenic) alternative to the PEG-lipids in the lipid nanoparticles (LNP) currently approved for the Covid-19 vaccines developed by Pfizer/BioNTech and Moderna. Both of the currently approved vaccines contain 1-2 mol% PEG-lipid, which is required to stabilize the LNP and prevent fusion of nascent particles. Serina has prepared LNPs from POZ-lipids and demonstrated these can stably incorporate oligonucleotides and transfect cell lines. Serina believes that it is likely that the global population is being progressively immunized against PEG due to the presence of PEG-lipids in the currently approved vaccines, combined with upwards of 70% of the population already having some level of anti-PEG antibodies. PEG is used at low molecular weights in a wide range of consumer products including cosmetics, toothpaste, deodorants, and laxatives. Anti-PEG antibodies are implicated in some of the serious adverse reactions such as anaphylactic reactions seen following the Covid-19 vaccine administration. Most infectious disease experts believe that Covid-19 will become an endemic challenge and that booster immunizations will be required. Serina believes that the anti-PEG antibody issue potentially has the unintended consequence of compromising the efficacy of the next generation of vaccines due to accelerated blood clearance. Serina has entered into feasibility studies with two major pharmaceutical companies toward the goal of developing “PEG-alternative” LNP vaccines for infectious diseases. The LNP delivered RNA therapeutics field represents one of the most promising areas of drug development, with over 1,200 LNP delivered RNA-based therapeutics in development. Serina’s intent is to license the POZ technology to companies developing LNP approaches in infectious diseases, cancer immunology, and gene therapy.
Parkinson’s Disease
Parkinson’s disease is a chronic, disabling disorder that results from a deficiency of dopamine in the brain. Dopamine deficiency results from a degeneration of dopaminergic neurons in a portion of the brain known as the substantia nigra. Treatment for Parkinson’s disease has focused on mechanisms for delivery of dopamine precursors (levodopa, which can be delivered by daily pills or intestinal infusion), or on mechanisms that delay or prevent the breakdown of dopamine in the brain. The majority of patients who are diagnosed with Parkinson’s disease are treated with oral formulations of levodopa that include carbidopa, a compound that inhibits the breakdown of levodopa. Other approaches include selective inhibitors of monoamine oxidase enzymes in the brain (MAO-B), or dopamine agonists, such as rotigotine (the active ingredient in the transdermal patch NeuproTM), ropinirole or pramepexole. In almost all instances of oral treatment with any of these compounds, patients may experience “wearing off” where the drug fails to deliver an adequate dopaminergic stimulus after being used for months to years or the drugs may promote a disabling side effect known as dyskinesia (involuntary movements of the extremities). Serina believes that there is a major need for new therapies that can treat patients with Parkinson’s disease, extend their productive years, and ameliorate these unwanted side effects.
In recent years, the clinical strategy of continuous dopaminergic stimulation has been advanced in both animal and human studies. Most oral drugs, including the transdermal patch Neupro, deliver dopaminergic stimulation in a phasic peak and trough fashion. Animal studies show that such phasic alterations in dopaminergic tone may result in accelerated degeneration of dopaminergic neurons in the brain, and lead to motor complications known as dyskinesia. An animal study where one group of naïve MPTP monkeys received an apomorphine rod implant capable of providing continuous deliver of apomorphine and another group received an intermittent injection of apomorphine every 8 hours indicated that continuous delivery of apomorphine via the rod implant prevented any motor complications in MPTP monkeys, whereas intermittent apomorphine injections led to dyskinesis development within 2 days. These results underscore the challenge of treating Parkinson’s disease patients with intermittent stimulation. Animal studies also suggest that continuous delivery of apomorphine appears to delay the degeneration of dopaminergic neurons in the brains of affected animals, thus delaying some of the side effects of dopaminergic therapy as well as the onset of late-stage Parkinsonism. In a double-blind, double-dummy study in humans employing an intestinal infusion of levodopa-carbidopa gel (LCIG) in advanced Parkinson’s patients, continuous intestinal infusion of levodopa gel was shown to be superior in outcomes to oral, sustained release levodopa-carbidopa capsules. The results showed that an intestinal infusion of levodopa-carbidopa gel in the proximal small intestine (delivered by a percutaneous catheter) increased patients’ “ON” time without troublesome dyskinesia by approximately two hours per day, and decreased “OFF” time by the same amount. The approach has been approved by the FDA (AbbVie, Duodopa). This represented a substantial new treatment option for advanced Parkinson’s disease patients. Notwithstanding the significant increase in patient clinical outcomes in advanced Parkinson’s patients, the approach is invasive, requires surgery to place a percutaneous catheter (with risk of infection, perforation of the intestine or inadvertent removal), frequent loading of levodopa-carbidopa gel packs, and frequent flushing to keep the catheter flowing.
Serina’s POZ technology platform has attributes that Serina believes has the potential to allow POZ-conjugates to deliver on the approach of prolonging the PKs of attached drugs, thus enabling continuous drug delivery. The potent dopamine agonist rotigotine is attached to pendent POZ with programmable linkers that allow the rotigotine to be released slowly, following a single subcutaneous injection of SER 214 in a standard insulin syringe. Animal studies in rats and monkeys have shown that SER 214 appears to provide a continuous state of dopaminergic tone for approximately one week following a single subcutaneous injection. Repeat-dose studies in rats (12 weeks), dogs (single dose maximum tolerated dose study) and monkeys (results of a 4-week study, plus the 90-day study in cynomolgus macaques that was part of the IND toxicology program) show the week-to-week variation in plasma release of rotigotine (plasma half-life) and drug exposure do not change dramatically following a single subcutaneous injection each week.
SER 214 – Provides Continuous Release of Rotigotine Following a Single Weekly Subcutaneous Injection in Parkinson’s Disease Patients with Early Stable Disease
SER 214 is the first product from Serina’s pipeline to be advanced into humans and has completed the Phase Ia study in 19 subjects. The Phase Ia study demonstrated SER 214 was well-tolerated when delivered in a standard insulin syringe. The data for the final two weeks of Cohort 3 (high dose cohort) are shown in Figure 3. Subjects received an initial dose in week one of 50 mg SER 214, followed by a single dose of 100 mg SER 214 in week two, and then followed by a single 200 mg dose of SER 214 in weeks three and four. This dosing was followed by a one-week wash-out period. SER 214 demonstrated predictable PK that paralleled the PK results observed in pre-clinical studies in monkeys. The results of the PK for released drug (rotigotine) are shown below, along with the published data for the 3 mg transdermal rotigotine patch.
Figure 2: Pharmacokinetic profile of SER 214 in Parkinson’s disease subjects in Phase Ia

Figure 2: PK profile of released drug in Parkinson’s disease subjects who received an initial dose of 50 mg SER 214, followed by a single dose of 100 mg SER 214, and then two weekly doses of 200 mg SER 214. Levels of released drug (rotigotine) are indicated in ng/ml. The yellow shaded area is the therapeutic window for rotigotine. Also shown in green is a 24-hour period for plasma levels of rotigotine from the 3 mg NeuproÔ patch (UCB, published data). From the data, it would appear a weekly injection of SER 214 of 200 mg (in 1 mL) approximates the levels of rotigotine from the daily 3 mg transdermal patch.
The results in Figure 2 show that a single weekly injection of SER 214 provided continuous drug delivery in the predicted therapeutic window where plasma levels of rotigotine would be expected to provide control of symptoms of early Parkinson’s (yellow shaded area). The 200 mg dose of SER 214 provided plasma levels of rotigotine that are approximately the same as the 3 mg Neupro™ patch but did so for an entire week. In data not shown, plasma levels of released rotigotine fell below detectable levels approximately nine days after the final injection, indicating that released rotigotine is released at a near steady-state level for an entire week following a single weekly subcutaneous injection.
The Phase Ia study also measured the potential for efficacy, which relies on a change from baseline in the Unified Parkinson’s Disease Rating Score (UPDRS). One component of the UPDRS measures the change in motor scores; this is known as UPDRS (Part III) and is measured by the physician who evaluates if there is a change in scores when the patient returns to the clinic for follow-up. A change from baseline of negative scores indicates improvement and the FDA has used UPDRS (Part III) as part of an approvable endpoint. In Cohort 3, subjects had a mean change from baseline of approximately - 6 at day 28 – indicating that SER 214 may be improving their signs and/or symptoms of Parkinson’s disease. There appeared to be a dose-dependent change from baseline as the doses were increased. This is shown in Figure 3 below.
Figure 3:
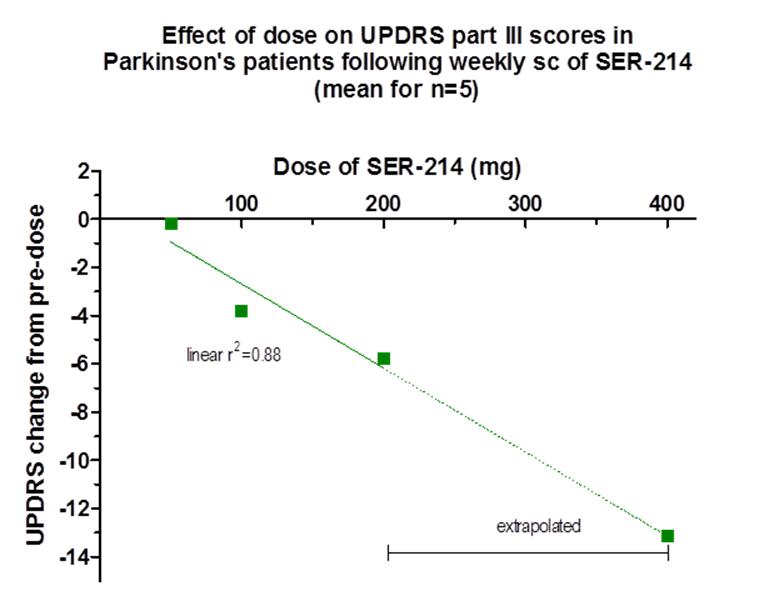
Figure 3: Effect of dose of SER 214 on change from baseline in UPDRS (Part III)
Subjects who completed the final two weeks of dosing were shown to be at steady-state release. The plot above shows the relationship between dose and change from baseline in UPDRS (Part III). At the highest dose evaluated (200 mg) there was an approximate -6 point change in UPDRS. When extrapolated to a 400 mg dose, which would approximate the 6 mg Neupro™ patch in terms of released rotigotine, there would be an estimated -13 point change in UPDRS (Part III). Serina believes such a degree of change would achieve an approvable endpoint. The extrapolated data was not an actual result of the study and there can be no assurance that this result is able to be achieved. The method of extrapolation is industry standard based on a near linear relationship (shown in figure as linear r2 = 0.88) with the data from the actual study.
The relationship between dose and steady-state PK levels of released rotigotine revealed a linear relationship as shown in Figure 4. Extrapolation of the dose of SER 214 to higher doses of 400-600 mg is predicted to result in steady-state levels of rotigotine equivalent to the 6 mg and 8 mg Neupro™ patch. Such higher doses would require a device to deliver the higher volumes (2-3 mL).
Figure 4:

Figure 4: Relationship between dose of SER 214 administered in the last two weeks of dosing in the 50 mg, 100 mg and 200 mg cohorts and mean (+/- SD) steady-state levels of released rotigotine.
In summary, the Phase Ia study demonstrated SER 214 is well-tolerated injection when administered subcutaneously in stable patients with early signs of Parkinson’s disease. Steady-state levels of released rotigotine were linear with dose, and physician assessment of UPDRS (Part III) suggested evidence of a dose-dependent decline from baseline - even in these stably treated patients. Serina believes that SER 214 is a promising product candidate that may be used to treat patients with early Parkinson’s disease, and market research suggests patients and physicians would readily use this approach at home without the need for an office visit or home health care professional. In June 2020, the results of the Phase Ia trial were published in Movement Disorders. Serina has not internally advanced SER 214 beyond Phase Ia and will seek to partner on any further development.
SER 252 (POZ-apomorphine) – Addressing the Need for a Potent Dopamine Agonist to Treat Advanced Parkinson’s disease
Serina believes that the same polymer chemistry used in SER 214 to provide continuous delivery of rotigotine might be used to provide continuous release of other drugs that would be effective in advanced PD. One such drug is apomorphine, one of the most potent dopamine agonists known and the one most closely aligned chemically with the natural substance in the brain (dopamine). Apomorphine is approved as a “rescue” medication that is delivered as an injection (Apokyn injection) analogous to an epinephrine injection for acute anaphylaxis. In Europe and some other parts of the world, there is an approved liquid formulation called Apo-go (Britannia Pharmaceuticals, not yet approved in the United States). In PD patients who develop severe “OFF” periods as a result of advancing disease associated with the “wearing off” of their medications, an Apokyn injection can promote “ON” periods within a few minutes. Many patients carry an Apokyn injection pen as a precaution for abrupt “OFF” periods. In contrast, Apo-go can be administered via an electronic pump as a daily subcutaneous infusion 12-16 hours a day. In July 2018, the first randomized, placebo-controlled study of Apo-go versus placebo was published (known as the TOLEDO study). The results showed that daily subcutaneous delivery of apomorphine for 12-16 hours during the waking day resulted in a significant reduction of daily “OFF” time of approximately 2 hours.
While Apo-go resulted in a substantial improvement in “OFF” time, the subcutaneous route of administration is confounded by severe skin reactions. An example of these types of skin reactions is shown in Figure 5.
Figure 5: Skin reactions from subcutaneous Apo-go administration

Figure 5: A patient with advanced Parkinson’s disease who uses daily subcutaneous infusions of Apo-Go to reduce “OFF” time. Subcutaneous delivery of apomorphine resulted in ulceration of the skin with draining abscesses, and in rare instances, skin necrosis.
In the TOLEDO study, approximately 60% of subjects dose-adjusted their daily infusions of Apo-go downward based on skin reactions, and about 40% of patients experienced nodule formation. Nodules that result from subcutaneous apomorphine generally do not resolve.
While Apo-go represents a non-surgical approach to treatment of advanced disease in PD patients and provides the same degree of improvement in “OFF” time as the LCIG catheter and deep brain stimulation (DBS), many patients do not tolerate the daily subcutaneous infusions. The daily set-up often requires a healthcare provider to come in each day to help the patient administer the drug. Serina believes that development of a more convenient method of delivering apomorphine, without having to use a complicated infusion device or confounded by serious skin reactions, would be a major contribution to patient care.
SER 252 (POZ-apomorphine)
In early 2018, Serina initiated work on developing a polymer conjugate of apomorphine that could be delivered as a subcutaneous injection that is devoid of any skin reactions. The first step was attachment of apomorphine to the polymer. The chemistry of attachment and controlled release required attachment of ester-linked groups to both of the hydroxyl groups in apomorphine; one ester linkage attaches the apomorphine to the polymer backbone (linking group) and the other ester linkage caps the second hydroxyl (capping group). In the course of these studies, Serina discovered that the rate-limiting step in the release of apomorphine from the polymer was the release of the “capping linker.” After three and a half years of dedicated efforts to control the release kinetics of apomorphine, Serina identified SER 252 as the IND candidate. Importantly, SER 252 provides linear dose kinetics when administered in multi-dose studies in monkeys as shown in Figure 6 below.
Figure 6: Dose response of SER 252 in monkeys
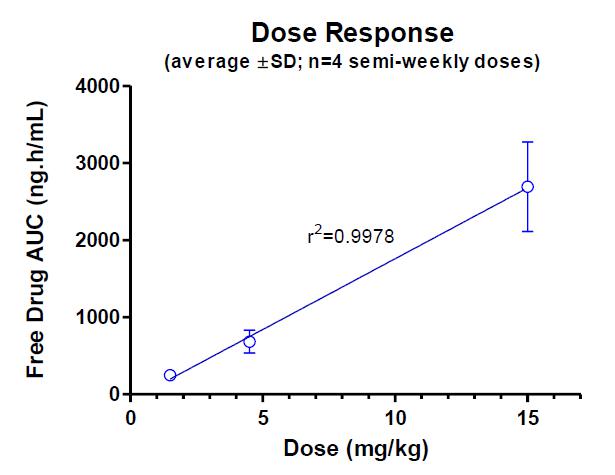
Figure 6: Young adult monkeys were dosed subcutaneous with SER 252 semi-weekly (n=4) at three different dose levels (1.5 mg equivalents Apo/kg, 4.5 mg equivalents Apo/kg and 15 mg equivalents Apo/kg) and daily levels of released apomorphine were determined. The AUC (area under the curve) for each set of doses was plotted versus dose administered.
PK Simulations for SER 252 in Patients with Advanced Disease
Although studies in humans are required for confirmation, Serina conducted a simulation of human PK based on the results from its studies in monkeys. The PK parameters of SER 252 in monkeys were modeled with a standard one-compartment fit of the data with V/F (volume of distribution) derived from imputed data from NeuroDerm, Ltd. published human PK on ND0701, an apomorphine product being developed by NeuroDerm; the V/F was 13 L/kg. The following results, which represent industry standard methodology for simulation, were obtained:
Figure 7:

Figure 7: Simulations for human PK. The simulation demonstrated that doses from 0.25 mg eq Apo/kg to 1 mg eq Apo/kg would cover the PK profile of Apo-go and provide a range of doses that Serina intends to evaluate in early studies in humans.
There can be no assurance that the simulated result is able to be achieved. Serina believes these simulations suggest it may be possible to dose patients with advanced PD with a subcutaneous injection that would provide levels of released apomorphine similar to the Apo-go infusion pump (a) without a trough level that would lead to an “OFF” period, (b) no need to have a healthcare provider hook up the device each day, and (c) no onerous skin reactions. Serina believes that the requisite trough level is likely in the range of about 3-5 ng/mL free apomorphine; plasma levels of apomorphine that “rescue” an acute “OFF” period about 4-5 ng/mL and persist for approximately 50 minutes (data from Cynapsus/Sunovion publications on development of a buccal apomorphine). The range of doses employed in an individual patient will likely vary, but the doses envisioned may potentially treat advanced disease and provide consistent “ON” time without troublesome dyskinesia and prevent the patient going into an “OFF” state. In multiple experiments in monkeys, SER 252 has not led to any skin reactions as a single or multi-dose regimen to date. Serina believes that it may be possible that this product candidate can be delivered in the patient’s home without the need for a healthcare provider.
Serina has initiated IND-enabling studies with SER 252. We anticipate SER 252 could enter the Phase I SAD study in advanced PD in late 2024. If the results show SER 252 is well-tolerated with predictable PK, we expect to proceed to a Phase I MAD study in advanced patients in 2025. The results of the combined SAD/MAD studies will measure not only safety, PK and tolerability in these advanced patients but also daily “OFF” and “ON” time and will inform the design of the Phase II study. If Phase I is successful, Serina plans to have a meeting with the FDA to aid in design of a Phase II study.
Summary of Serina’s Parkinson’s Disease Programs
Serina believes that SER 214 could be used primarily in patients with early Parkinson’s disease and that SER 252 could be used in patients with advanced disease. A critically important observation was made in the TOLEDO study in that many patients down-titrated their oral L-DOPA doses by greater than 50% from their baseline dose. L-DOPA therapy is considered the mainstay of treatment of both early and advanced Parkinson’s disease, despite evidence that dopamine agonists are equally effective for early disease and do not promote the same degree of dyskinesia as levodopa. Approximately 50% of PD patients will develop levodopa induced dyskinesia (LIDS) within five years, and 90% within 10 years, of initiation of levodopa therapy. In one specialized center in Scotland, the Lees group has shown that fastidious titration of apomorphine (Apo-go) during the waking day can lead to complete off-titration of levodopa with a dramatic reduction in motor complications (45 of 64 patients achieved apomorphine monotherapy and titrated completely off oral levodopa). Patients who achieved monotherapy had an increase in “ON” time to 85% of the waking day. If SER 252 effectively prevents “OFF” time and delays or prevents dyskinesia in advanced disease, it might be possible to use SER 252 in patients with mid-stage disease to achieve significant control of symptoms without initiating levodopa therapy. At present, we are not aware of an effective alternative to oral levodopa. For patients who experience advancing disease that no longer responds to oral agonists, the default approach is to continue to escalate doses of levodopa with the consequence of accelerated onset of motor complications. Serina believes that there is significant potential for SER 252 to become such an alternative, potentially leading to a paradigm shift in how patients with Parkinson’s disease might be treated. Serina believes that SER 214 and SER 252 may be administered in the home setting with the convenience and compliance of a single or twice-weekly subcutaneous injection that does not require a healthcare provider to administer, and patients may not need to continue therapy with levodopa (levodopa sparing strategy).
SER 227 (POZ-buprenorphine) – An Injectable Drug for Management of Post-Operative Pain That Simultaneously Obviates the Need to Discharge a Patient on an Opioid
Buprenorphine is a mixed mu-agonist/antagonist that is 30-50 times more potent than morphine at binding to the mu-opioid receptor. The mu-opioid receptor (MOR) is the target of drugs in the opioid class, of which morphine is the prototype opioid. Other synthetic opioids include oxycodone and fentanyl. When molecules bind the MOR, they “blunt” the perception of pain at the level of the brain and are often associated with euphoria. Buprenorphine is an approved drug available in a transdermal formulation (Butrans) as well as an injectable formulation (Buprenex), but it does not promote euphoria and is thus considered to have low abuse liability. Buprenorphine has several mechanisms of action, one of which is a potent sodium channel inhibitor activity similar to lidocaine-like drugs, and thus has mild anesthetic effects locally when injected. Finally, buprenorphine is believed to have anti-hyperanalgesic effects at both local and central nociceptors that may contribute to its activity as a pain drug.
Buprenex has a relatively short half-life, which limits its usefulness as a post-operative pain drug following local injection in humans. However, Serina believes that buprenorphine’s multiple mechanisms of action make it a particularly attractive candidate for an injectable pain drug if the PK can be enhanced.
SER 226/227 – Synthetic Scheme
Buprenorphine was attached to POZ with the aim to increase its PK profile, in much the same way as SER 214. The synthetic scheme is shown below:
Figure 8: Synthetic scheme for linker attachment to buprenorphine

Source: Serina
Buprenorphine (compound 1) is converted to an azide-derivative by coupling the linker to the single phenolic hydroxyl to generate compound 2.
Compound 2 is then purified, and it is attached to the 20 kDa pendent POZ polymer employing “click chemistry”, as shown in Figure 9 below.
Figure 9: Synthetic scheme for POZ-buprenorphine – SER 226/227

Source: Serina
Figure 9: Compound 2 is attached to a pendent POZ polymer employing “click chemistry” to generate the desired SER 226 (alpha-methyl linker) or SER 227 (propyl linker, shown above).
The PK profiles of SER 226 (2-propionate linker) and SER 227 (3-propionate linker) have shown that a single injection has a prolonged profile with detectable buprenorphine levels out to seven days in rat studies. SER 227 releases buprenorphine at an essentially “steady-state,” similar to the PK results we observed for SER 214 in animals and subsequently in humans. These results suggested that the PK of buprenorphine has the potential to be markedly extended employing a POZ polymer approach, and that the levels of buprenorphine may be titrated to a level where buprenorphine has been shown to have analgesic effects in humans.
SER 226/227 – Evaluation in the Brennan Model
Serina advanced both conjugates into the Brennan model of post-operative pain. In brief, the Brennan model is an industry standard pain model that employs rats that have received a unilateral 1 cm incision in the fat pad of the paw in the hind limb. This wound has enhanced sensitivity to painful stimuli for up to seven days (tactile allodynia), and analgesics may be evaluated via injection into the wound site or systemically to determine if there is an analgesic effect. Both SER 226 and SER 227 produced prompt and sustained analgesia in the Brennan model that exceeded the known activity of both morphine and Exparel (a liposomal formulation of bupivacaine, Pacira Pharmaceuticals, Inc.). This is shown below in Figure 10.
Figure 10:

Figure 10: Analgesic activity of SER 226 and SER 227 versus morphine in the Brennan Model in the rat.
Rats were administered a single subcutaneous injection of either SER 226, SER 227, saline or morphine at the time of wound closure. Animals were then evaluated for withdrawal threshold employing a von Frey monofilament. Withdrawal thresholds were measured as a function of time for up to 144 hours. A low withdrawal threshold indicates little or no analgesia when the wound area is challenged with the von Frey monofilament. In this model, morphine provides immediate analgesia that returns to baseline (no analgesia) within 3-4 hours. Exparel (a liposomal preparation of bupivacaine that is also an approved pain drug) would provide immediate analgesia that would return to baseline (no analgesia) within 4-6 hours. Both SER 226 and SER 227 provide immediate and prolonged analgesia that remained statistically significant out to 48 hours.
The release kinetics for both SER 226 and SER 227 were also evaluated in monkeys. The results of that study are shown below in Figure 11.
Figure 11:

Figure 11: Pharmacokinetic Profile of Released Buprenorphine from SER 226 and SER 227.
A single subcutaneous injection of SER 226 or SER 227 was administered to adult male monkeys at a dose of 1.5 mg/kg. At 3, 6, 12 and 24 hours, and then daily for 10 days, plasma samples were taken for determination of released buprenorphine. SER 226 releases buprenorphine quickly and generates very high levels of released buprenorphine approaching 100 ng/mL within 24 hours. SER 227 reaches Cmax at approximately 1-3 hours and remains at near steady-state release for > 3 days without fluctuations in plasma levels.
The data in Figure 11 suggests that SER 227 has the desired PK profile for a long-acting analgesic that could be administered in the immediate post-operative period and potentially achieve therapeutic levels of released buprenorphine in the therapeutic window for analgesia (1-5 ng/ml).
Serina’s target product profile for POZ-buprenorphine in humans is as follows:
| | ● | A single injectable product that can be administered in the immediate post-operative period; |
| | ● | Release of buprenorphine that provides prompt (within approximately two hours) plasma levels within the therapeutic window for analgesia; |
| | ● | Sustained release of buprenorphine systemically (in the blood) that provides greater than three days of steady-state levels, such that patients would not need to transition to an intravenous or oral opioid; |
| | ● | Release of buprenorphine at plasma levels that would also prevent the euphoria associated with other mu-agonists, such as morphine or oxycodone, should these be taken in the immediate three to four days following injection of SER 227; and |
| | ● | Prolonged analgesia for three to four days, whereupon patients would be able to transition to over-the-counter analgesics that are non-addictive such as acetaminophen or non-steroidal anti-inflammatory drugs. |
We are not aware of the target product profile described above being achievable by any injectable drug currently available. Exparel is an approved drug that is a liposomal formulation of bupivacaine that has a profile of about 12-24 hours of analgesia following injection into the wound bed (Exparel, Pacira Therapeutics). Research indicates that approximately 35-40% of patients who have undergone surgery (other than minor surgical procedures) will transition to an oral drug on day two to three, and in some cases an opioid depending on the type of surgical procedure. We believe that this is one of the contributing reasons for the “opioid epidemic” in the United States today. There are estimated to be more than two million people in the United States that are addicted to prescription pain drugs, and approximately 150 people die per day in the United States from opioid overdose. This is a major burden on the health care system in addition to the emotional burden on the addicted individual and the family. Serina believes that a product like SER 227 would represent a significant advance in the treatment of post-operative pain by providing up to three to four days of analgesia without transition to opioids due to its extended half-life, high activity at the mu-receptor, local lidocaine-like effect, and anti-hyperalgesia.
Finally, as buprenorphine is itself now an approved drug for the treatment of addiction as an implant (Probuphine, Titan Pharmaceuticals), a once-monthly injection (Sublocade, Indivior Pharmaceuticals), and as a sublingual formulation (Suboxone), it is possible that anyone who would take SER 227 post-operatively would not respond to that opioid with a “high,” as SER 227 may blunt the euphoria often experienced with opioids. Serina believes that SER 227 has the potential to be an excellent post-operative pain drug for many types of surgeries, while targeting the initial gateway of exposure to opioids in the hospital that may lead to subsequent opioid use disorder.
Buprenorphine is as Effective as Morphine Delivered by PCA
The current standard of treatment for post-operative pain, in particular for in-hospital procedures (those that require a hospital stay), is the administration of opioid drugs. This is frequently accomplished by an intravenous administration of morphine (or similar opioid) that can be controlled by the patient (patient-controlled analgesia, or PCA). In general, patients receive PCA for one to two days and are transitioned to an oral opioid on day two to three at which time they undergo progressive ambulation in preparation for discharge. Patients are often discharged with a prescription for an opioid with instructions to use as needed for control of pain.
There is a concern among some pain specialists that buprenorphine may not be as effective as morphine in the acute post-operative period. In a recent meta-analysis of studies which randomized patients to either IV morphine (via PCA) or buprenorphine in its current approved formulation (Buprenex), it was found that:
| | ● | Buprenorphine was equal to morphine in analgesic efficacy; |
| | ● | Buprenorphine had a ceiling effect on respiratory depression but not analgesia; |
| | ● | Respiratory depression increased with increase in morphine dose; |
| | ● | There was no difference for major side effects such as nausea and sedation; |
| | ● | There was no significant difference in the need for rescue analgesia or the time to rescue analgesia; and |
| | ● | Buprenorphine was superior to morphine for some troublesome adverse events. |
Serina believes that the view that buprenorphine might not be as effective as morphine is not supported by randomized, well-controlled studies.
Impact of Discharging a Patient with a Prescription for an Opioid
In one of the largest published studies of its kind (Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017), researchers made important observations regarding the impact of discharging a patient with a prescription for an opioid. In the event opioid-naïve patients fill a prescription for an opioid following discharge, independent of whether they have had minor or major surgery, the study found:
| | ● | The patient became 15 times (6-7% of patients) more likely to transition to chronic use of an opioid within the next 90-180 days; |
| | ● | If patients are never exposed to an addictive opioid (such as morphine via PCA, or oral oxycodone) the incidence of transitioning to opioid use disorder is less than 0.4%; and |
| | ● | Predictors of new and persistent opioid use were a history of substance abuse (tobacco, alcohol), mood disorders (primarily anxiety disorders) and pre-operative pain disorders where there was prior use of an opioid. |
Serina believes that the development of SER 227 for control of post-operative pain may not only provide adequate analgesia, but simultaneously target the initial gateway to exposure of potentially addictive opioids. The market potential and competitive landscape for this program were assessed in formal market research conducted by inVentiv Health in 2018. In September 2019, the NIH, through the National Institute of Neurological Disorders and Stroke (NINDS), awarded Serina $1.7 million over two years to support the pre-clinical development of SER 227. The award is part of the NIH HEAL Initiative (Helping to End Addiction Long-term). The NIH HEAL Initiative award has funded the IND-enabling studies to prepare SER 227 for Phase Ia clinical trials.
POZ Cannabinoid Conjugates – Potential Product Candidates in Early Pre-clinical Studies
Cannabis extracts have been used to treat glaucoma, pain, nausea and vomiting, muscle spasms, insomnia, anxiety, and epilepsy. Evidence for efficacy varies substantially for different indications, with the best data in painful HIV-associated sensory neuropathy, chronic pain, chemotherapy-induced nausea and vomiting, and spasms in patients with multiple sclerosis. More recently, an enriched extract from cannabis has shown great promise in the treatment of refractory epilepsy in infantile epileptic syndromes such as Dravet syndrome and Lennox-Gastaux syndrome. In June 2018, Epidiolex® was approved by the FDA for use in children with those syndromes. In 2020, Epidiolex® was approved for tuberous sclerosis complex (TSC), a rare genetic disorder associated with benign tumors throughout the body but also a high incidence of refractory epilepsy. Other medicinal uses for cannabis have been proposed, but for many of these additional indications the use of cannabis extracts (or pure compounds from cannabis) will have to be examined in well-controlled clinical trials.
The approval of Epidiolex® was a milestone in the development of a new drug class for refractory epilepsy as there had not been a new anti-epileptic drug (AED) class in almost 20 years. However, its use is confounded by significant side effects. In the treatment group, the combined incidence of diarrhea and vomiting was 46% compared to 15% in the placebo group – suggesting that the adverse events are the combination of sesame seed oil + CBD, not just sesame seed oil alone. Other side effects are also significant and include fatigue, anorexia, and somnolence. It is possible that this side effect profile may be due to the phasic peak and trough PK profile of the mixture. Cannabidiol is insoluble in aqueous solutions and is dissolved in sesame seed oil. At the recommended dose of 20 mg/kg/day in divided doses, an average 40 kg child will need to consume almost 250 mL of sesame seed oil each month in divided doses.
Serina initiated work with one of the authorities in cannabinoid therapy for seizures at the University of Alabama at Birmingham, Dr. Jerzy Szaflarski, in 2016. Data provided by Dr. Szaflarski showed that among patients with refractory epilepsy that have been using Epidiolex® since 2014, approximately 30% of adults and 50% of children use maximal doses of anti-diarrheal therapy and diapers to deal with the diarrhea side effects of treatment.
SER 228 (POZ-cannabidiol) for the Treatment of Intractable Epilepsy
Two initial cannabinoid compounds were identified as attractive candidates for a POZ polymer approach based upon an accessible chemical handle, potential limitations in terms of solubility and undesirable PK properties (for example, a short half-life or need to deliver orally in a solution to solubilize the drug). These compounds are cannabidiol (CBD) and tetrahydrocannabinol (THC). CBD and THC are among the approximately 120 known terpenophenolic compounds (collectively known as cannabinoids) in the plant cannabis. Their chemical structures are shown below.
Figure 12: Chemical structures of cannabidiol (CBD, left) and tetrahydrocannabinol (THC, right)
Figure 12: Chemical structures of the major non-psychoactive cannabidiol and psychoactive delta-9- tetrahydrocannabinol compounds in Cannabis. THC is the component in the plant that is associated with a “high”; CBD has no similar psychoactive activity. The chemical point of attachment to POZ is indicated by the circle on each chemical structure.
Source: Serina
SER 228/229 – Synthetic Scheme
Serina believes that CBD is an ideal candidate for a POZ polymer approach. The synthetic scheme for POZ-cannabidiol takes advantage of the chemical handle(s) indicated in the structure above. This is illustrated below.
Figure 13: Synthetic scheme for POZ-cannabidiol – SER 228/229
Source: Serina
Figure 13: Cannabidiol is first derivatized with the linker of interest (acetyl, SER 228; alpha-methyl, SER 229) and attached to a 20 kDa POZ polymer employing “click chemistry” as seen previously for other POZ-conjugates. The final product is then purified over ion-exchange chromatography.
Pharmacokinetic Study of SER 228/229 in Normal Rats
Serina obtained a Schedule I license from the DEA in the second quarter of 2016. The research study protocol was approved by both the DEA and FDA and studies commenced in the Serina laboratories in May 2016. CBD has been attached by two different linkers to a 20 kDa POZ polymer and the initial results in normal rats are shown below.
Figure 14:

Figure 14: PK profile of SER 228/229 in normal rats. CBD was attached to POZ via two different linkers to produce two potential candidate drugs; SER 228 employs an acetyl linker at one of the two phenolic hydroxyls, and SER 229 employs an alpha-methyl linker. These were dosed in normal rats via a single subcutaneous injection (at doses of 1.5 and 4.5 mg/kg) and plasma levels of CBD were obtained each day for seven days.
The PK profiles shown in Figure 14 demonstrated that POZ-cannabidiol has a prolonged PK profile in the rat. The PK profile of either SER 228 or SER 229 at a dose of 1.5 mg/kg in the rat was shown to be very similar to the PK profile of SER 214 in the rat (data not shown).
SER 214 has been shown to produce continuous drug delivery in humans following a single weekly subcutaneous injection. As the polymer backbone in SER 214 is a 20 kD polymer, Serina would expect that similar profiles might be obtained with other POZ-conjugates that employ a 20 kDa polymer. The POZ-cannabidiol conjugates have subsequently been advanced in single-dose PK studies in monkeys, with variable drug loaded 20 kDa POZ polymers. These data are shown in Figure 15.
Figure 15:

Figure 15: Plasma levels of released CBD following a single injection of the indicated POZ conjugates.
Adult male monkeys (N=3) were administered a single subcutaneous injection of the indicated POZ-conjugates at 4.5 mg/kg. SER 228 has CBD linked to the polymer via an acetate linker, whereas SER 229 employs an alpha-methyl linker. The respective POZ-conjugates were variably loaded with CBD as well. SER 229 PA is comprised of an additional “clicked” moiety – propionic acid – that is predicted to increase solubility. In prior studies, we have observed that decreasing the drug load in some instances leads to higher degrees of drug release both in vitro and in vivo.
In the approval trials of Epidiolex®, patients were dosed at variable doses of the sesame seed oil preparation, from 5 mg/kg to 50 mg/kg. It is unclear what the dose correlation is to seizure control and published data suggest that patients may respond across a wide range of doses. Serina sponsored a study with epilepsy experts at the University of Alabama at Birmingham who have treated approximately 200 patients (adult and pediatric) who are participants in the Expanded Access Program sponsored by GW Pharmaceuticals, Inc. (GW). Published data and results from our single-dose PK studies in monkeys suggest that in children with refractory epilepsy there is a response at lower plasma levels than those required in adults. Plasma PK determinations of CBD showed that at the approved dose of 20 mg/kg/day of Epidiolex® the levels were approximately 150 ng/mL (with a wide standard deviation, as would be predicted for a drug with limited PK). Serina believes that PK levels between 50-150 ng/mL is the target level for a POZ-cannabidiol conjugate that would achieve efficacy in Dravet syndrome and some other pediatric epilepsies based on published data. The multi-dose monkey PK results suggest that POZ-cannabidiol may have the potential to dramatically change the treatment paradigm for this disabling disorder by providing a single weekly injection that delivers CBD continuously. As shown below, we have identified SER 228 as the POZ-cannabidiol candidate for IND-enabling studies.
Figure 16

Figure 16: Multi-dose pharmacokinetics of release of CBD following a single weekly subcutaneous injection of either SER 228 or SER 229 at 7-8% drug load. SER 228 or SER 229 were dosed at 1.5 mg/kg in young adult monkeys for four successive weeks followed by a two-week washout period. Both conjugates provided virtually identical PK profiles. Serina has chosen SER 228 for IND-enabling studies.
The multi-dose PK data above were simulated to predict human PK based upon a standard one-compartment fit of the data. There can be no assurance that the simulated result is able to be achieved. The results are shown below.
Figure 17: SER 228 simulation of predicted human PK modeling compared to twice daily Epidiolex®
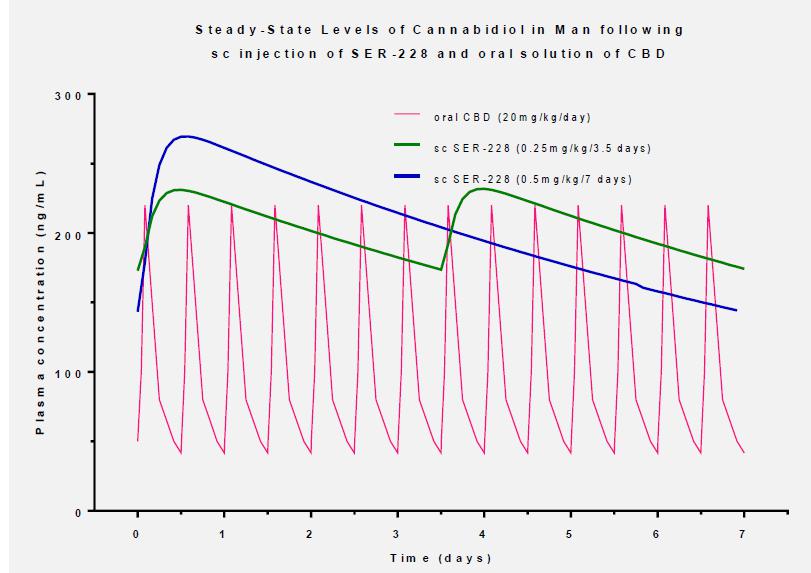
Figure 17: The multi-dose monkey PK was used to simulate human PK for SER 228 as a weekly or twice-weekly subcutaneous injection at doses of 0.5 mg/kg or 0.25 mg/kg, respectively. Epidiolex® data are derived from published information.
The results of the simulation illustrated in Figure 17 shows a single or twice-weekly subcutaneous injection of SER 228 may provide continuous release of CBD at levels that exceed the known PK of the 20 mg/kg/day dose of Epidiolex®. The simulation used the one-compartment model to predict the PK levels of CBD release from SER 228. In this modeling, Serina relied on the published volume of distribution for CBD in humans, and modeled the data for a two-fold accumulation of CBD that occurs in humans (data from the FDA Advisory Committee 2018). The steady-state levels from the simulation of SER 228 for human PK lie well above the target level of ~ 150 ng/mL CBD and trough levels of CBD release may not approach the trough levels of Epidiolex® (40 ng/mL). Serina has chosen SER 228 for further development and believes that a single or twice-weekly injection may represent a major contribution to patient care compared to CBD in a twice daily, orally delivered sesame seed oil preparation. There can be no assurance that the simulated result is able to be achieved.
SER 232 (POZ-tetrahydrocannabinol) - A Once Weekly Injectable for the Treatment of Chemotherapy-induced Nausea and Vomiting (CINV)
Serina obtained a Schedule I license from the DEA in early 2016, and the FDA has approved the research protocol for the delta-9-tetrahydrocannabinol (THC) program in early 2017. The chemical synthesis of SER 232 conjugates has been completed.
SER 232 – Synthetic Scheme
The synthetic scheme for SER 232 is virtually the same as in SER 228/229 except that the molecule is THC. This is shown below.
Figure 18: Synthetic scheme for POZ-tetrahydrocannabinol – SER 232

Source: Serina
THC is derivatized with the appropriate linker and coupled to a 20 kDa POZ polymer to generate SER 232 by employing “click chemistry.”
Serina will likely employ the same linkers that have been used in the SER 228/229 program to attach THC to the 20 kDa POZ polymer and evaluate release rates. As there is a single chemical handle in THC, and its chemical structure is very similar to CBD, we anticipate that programming THC release rates should be readily achievable. POZ-tetrahydrocannabinol is expected to be programmed to achieve relatively steady-state PK release, much like SER 214.
There is no animal model that has proven to be a reliable model for therapeutic potential of THC to treat CINV. The development of POZ-tetrahydrocannabinol is expected to rely on historical information regarding the PK levels that may be safe and efficacious in multiple potential clinical disorders, including CINV, spasticity in multiple sclerosis, neuropathic pain, dyskinesia in Parkinson’s disease, weight loss in AIDS and cancer patients and aggression / agitation in autism spectrum disorders and Alzheimer’s disease.
POZ-cannabinoids – Potential Enabling Technology for Other Cannabinoids Found in Cannabis (CBDV, CBG, CBC)
We believe that polymers of POZ could provide similar PK profiles for other cannabinoids. Serina has identified three additional cannabinoids for early preclinical studies – cannabidivarin (CBDV), cannabigerol (CBG) and cannabichromeme (CBC). Serina believes that cannabinoids as a class of compounds may hold potential for a myriad of CNS disorders.
Figure 19: Cannabinoids as a class of compounds may target some of the most challenging disorders in CNS and inflammatory disease. Many of the cannabinoids shown have multiple targets
Source: Serina

POZ-lipids – Development of a non-immunogenic lipid nanoparticle (LNP) by replacing the PEG-lipid in current infectious disease vaccines with a POZ-lipid
In early 2020, the formulations of the RNA-based Covid-19 vaccines from Pfizer/BioNTech and Moderna were revealed to use a PEG lipid nanoparticle delivery technology. In July 2021 and April 2022, Serina entered into feasibility study agreements (FSAs) with two major pharmaceutical companies to explore the potential of Serina’s POZ-lipid technology as a possible non-immunogenic replacement for the PEG polymer lipid nanoparticle formulation (PEG-lipid LNP).
Serina has demonstrated in animal experiments that rabbits do not produce detectable antibodies to the POZ polymer and Serina’s repeat-dose studies in animals and humans suggest the absence of an accelerated blood clearance phenomenon for POZ-conjugates. Accelerated blood clearance (ABC) has been demonstrated to occur in animals and in humans and Serina believes that it is likely due to the development of anti-PEG antibodies. In a study in mice, scientists at Moderna demonstrated that anti-PEG antibodies begin to clear LNPs by the second dose, and subsequent dosing of an mRNA LNP resulted in complete clearance of the LNP by the third, fourth and fifth dosing.
Both of the currently approved Covid-19 RNA-based vaccines contain 1-2 mol% PEG-lipid, which is required to stabilize the LNP and prevent fusion of nascent particles. In addition to the possibility that PEG may be the culprit in acute serious adverse events such as anaphylactic reactions, it is possible the efficacy of subsequent vaccines may be compromised. Many experts believe that Covid-19 will become an endemic challenge and that booster immunizations will be required. Formulation of a LNP with a POZ-lipid may provide a “PEG-free” alternative to development of future LNP delivered RNA vaccines for infectious diseases, cancer immunotherapy and rare diseases.
Serina has patents which cover the use of POZ-lipids for the formation of therapeutic LNPs, and we are continuing to advance the chemistry of POZ-lipids in the Serina laboratories. Serina intends to license the POZ-lipid technology to companies developing LNP approaches, potentially providing a vaccine delivery system with reduced immunogenicity compared to the current PEG-based standard approaches.
Licensing, Collaboration and Partnership Agreements
In early 2021, Serina entered into FSAs with several large pharmaceutical and biotechnology companies to advance POZ-lipids as a replacement strategy for PEG-lipids in the current mRNA vaccines. After two years of work with these partners, Serina entered into licensing discussions to advance POZ-lipids as a replacement for PEG-lipids.
In October 2023, Serina entered into a non-exclusive license agreement with Pfizer, Inc. to use Serina’s POZ polymer technology for use in lipid nanoparticle drug delivery formulations. The agreement grants Pfizer non-exclusive rights to certain intellectual property, know-how, and proprietary technologies. Under the terms of the agreement, Pfizer is authorized to develop, manufacture, market, and commercialize products incorporating the licensed technology with respect to a specific POZ polymer structure in one field. The agreement outlines the protection and enforcement of intellectual property rights related to the licensed technology. Pfizer is obligated to use commercially reasonable diligent efforts to develop and commercialize licensed products, and to use such efforts to accomplish specified development and commercial objectives. The agreement includes a one-time upfront payment of less than $5 million, milestone payments payable to Serina upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology in accordance with the terms outlined in the license agreement.
Clinical Manufacturing and Supply Agreement
In May 2023, Serina entered into a manufacturing agreement to manufacture SER 252 for IND enabling preclinical studies and clinical supply. Under the terms of the agreement, the manufacturer will manufacture and test SER 252 under cGMP. Serina retains all rights to SER 252 which includes data, methods, and process modifications.
Intellectual Property
Intellectual property is of vital importance in Serina’s field and in biotechnology generally. Serina seeks to protect and enhance proprietary technology, inventions, and improvements that are commercially important to the development of its business by seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. Serina will also seek to rely on regulatory protection afforded through inclusion in expedited development and review, data exclusivity, market exclusivity and patent term extensions where available.
Serina has sought patent protection in the United States and internationally related to the POZ technology. Serina owns an extensive issued patent estate covering POZ technology and certain product candidates enabled by the POZ technology. Serina has applied for additional patents seeking to further protect and extend the POZ patent portfolio owned by Serina. Such applications may not result in issued patents and, even if patents do issue, such patents may not be in a form or scope that will provide Serina with meaningful protection for its product candidates. Serina also relies on trade secrets that are important to the development of its business. Trade secrets are difficult to protect and provide Serina with only limited protection, as trade secrets do not protect against independent development of technology by third parties.
Serina expects to file additional patent applications in support of current and new clinical candidates as well as in support of new applications of POZ platform technology. Serina’s commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of current and future product candidates and the methods used to develop and manufacture them, as well as successfully defending any such patents against third party challenges and operating without infringing on the proprietary rights of others. Serina’s ability to stop third parties from making, using, selling, offering to sell, or importing its product candidates will depend on the extent to which Serina has rights under valid and enforceable patents or trade secrets that cover these activities. Serina cannot be sure that patents will be granted with respect to any of its pending patent applications or with respect to any patent applications filed by Serina in the future, nor can Serina be sure that any patents that may be granted in the future will be commercially useful in protecting its product candidates, discovery programs and processes.
The terms of individual patents depend upon the legal term of the patents in the countries in which they are obtained. In most countries in which Serina files, including the United States, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent or may be shortened if a patent is terminally disclaimed over an earlier filed patent. In the United States, the term of a patent that covers an FDA approved drug may also be eligible for extension, which permits patent term restoration to account for the patent term lost during the FDA regulatory review process. The Hatch Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the subject drug candidate is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent applicable to an approved drug may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar provisions to extend the term of a patent that covers an approved drug are available in Europe and other foreign jurisdictions. In the future, if and when Serina products receive FDA approval, Serina expects to apply for patent term extensions on patents covering those products. Serina plans to seek patent term extensions to any issued patents it may obtain in any jurisdiction where such patent term extensions are available, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with Serina’s assessment that such extensions should be granted, and if granted, the length of such extensions.
In some instances, Serina has submitted and expects to submit patent applications directly to the USPTO as provisional patent applications. Corresponding non provisional patent applications must be filed not later than 12 months after the provisional application filing date. While Serina intends to timely file non provisional patent applications relating to its provisional patent applications, Serina cannot predict whether any such patent applications will result in the issuance of patents that provide it with any competitive advantage.
Serina files U.S. non provisional applications and Patent Cooperation Treaty (PCT) applications that claim the benefit of the priority date of earlier filed provisional applications, when applicable. The PCT system allows a single application to be filed within 12 months of the original priority date of the patent application, and to designate all of the PCT member states in which national patent applications can later be pursued based on the international patent application filed under the PCT. The PCT searching authority performs a patentability search and issues a non-binding patentability opinion which can be used to evaluate the chances of success for the national applications in foreign countries prior to having to incur the filing fees. Although a PCT application does not issue as a patent, it allows the applicant to seek protection in any of the member states through national phase applications. At the end of the period of two and a half years from the first priority date of the patent application, separate patent applications can be pursued in any of the PCT member states either by direct national filing or, in some cases by filing through a regional patent organization, such as the European Patent Office. The PCT system delays expenses, allows a limited evaluation of the chances of success for national/regional patent applications and enables substantial savings where applications are abandoned within the first two and a half years of filing.
For all patent applications, Serina determines claiming strategy on a case by case basis. Advice of counsel and Serina business model and needs are always considered. Serina seeks to file patents containing claims for protection of all useful applications of its proprietary technologies and any products, as well as all new applications and/or uses that Serina discovers for existing technologies and products, assuming these are strategically valuable. Serina continuously reassesses the number and type of patent applications, as well as the pending and issued patent claims to pursue maximum coverage and value for its processes, and compositions, given existing patent office rules and regulations. Further, claims may be modified during patent prosecution to meet Serina’s intellectual property and business needs.
Serina recognizes that the ability to obtain patent protection and the degree of such protection depends on a number of factors, including the extent of the prior art, the novelty and nonobviousness of the invention, and the ability to satisfy the enablement requirement of the patent laws. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted or further altered even after patent issuance. Consequently, Serina may not obtain or maintain adequate patent protection for any of its future product candidates or for its technology platform. Serina cannot predict whether the patent applications it is currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that Serina holds may be challenged, circumvented, or invalidated by third parties.
In addition to patent protection, Serina also relies on trade secrets, know how, other proprietary information and continuing technological innovation to develop and maintain its competitive position. Serina seeks to protect and maintain the confidentiality of proprietary information to protect aspects of its business that are not amenable to, or that it does not consider appropriate for, patent protection. Although Serina takes steps to protect its proprietary information and trade secrets, including through contractual means with its employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to its trade secrets or disclose its technology. Thus, Serina may not be able to meaningfully protect its trade secrets. It is Serina’s policy to require its employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with Serina. These agreements provide that all confidential information concerning Serina’s business or financial affairs developed or made known to the individual during the course of the individual’s relationship with Serina is to be kept confidential and not disclosed to third parties except in specific circumstances. Serina’s agreements with employees also provide that all inventions conceived by the employee in the course of employment with Serina or from the employee’s use of its confidential information are Serina’s exclusive property. However, such confidentiality agreements and invention assignment agreements can be breached, and Serina may not have adequate remedies for any such breach. In addition, Serina’s trade secrets may otherwise become known or be independently discovered by competitors. To the extent that Serina’s consultants, contractors, or collaborators use intellectual property owned by others in their work for Serina, disputes may arise as to the rights in related or resulting trade secrets, know how and inventions.
The patent positions of biotechnology companies are generally uncertain and involve complex legal, scientific, and factual questions. Serina’s commercial success will also depend in part on not infringing upon the proprietary rights of third parties. Third party patents could require Serina to alter its development or commercial strategies, or its products or processes, obtain licenses or cease certain activities. Serina’s breach of any license agreements or its failure to obtain a license to proprietary rights required to develop or commercialize its future products may have a material adverse impact on it. If third parties prepare and file patent applications in the United States that also claim technology to which Serina has rights, Serina may have to participate in interference or derivation proceedings in the USPTO to determine priority of invention. For more information, see “Risk Factors—Risks Related to Serina—Risks Related to Intellectual Property.”
When available to expand market exclusivity, Serina’s strategy is to obtain, or license additional intellectual property related to current or contemplated development platforms, core elements of technology and/or clinical candidates.
Serina-Owned Intellectual Property
Serina has been issued a series of patents on various forms of POZ. The following is a list of patents that have been issued and published pending applications in the United States and worldwide. The list is presented by the individual family dockets.
SER 01 Family: “Activated Polyoxazolines and Compositions Comprising the Same” (J. M. Harris et al.).
This family of patents provides methods of synthesis and compositions of terminally activated linear poly(oxazolines) having various terminal functional groups suitable for attaching to other molecules such as enzymes, proteins, lipids, and antibodies by stable linkages.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| Japan | | Granted | | Feb-28-2008 | | 2009-551999 | | Sep-19-2014 | | 5,615,558 |
| Korea (South) | | Granted | | Feb-28-2008 | | 10-2009-7020124 | | Mar-30-2015 | | 10-1508617 |
| Korea (South) | | Granted | | Oct-25-2013 | | 10-2013-7028233 | | Mar-30-2015 | | 10-1508621 |
| US | | Granted | | Feb-28-2008 | | 12/529,001 | | May-17-2011 | | 7,943,141 |
| US | | Granted | | Mar-08-2017 | | 15/453,686 | | Oct-02-2018 | | 10,086,084 |
| US | | Granted | | Sep-27-2018 | | 16/144,358 | | Dec-15-2020 | | 10,864,276 |
SER 02 Family: “Multi-armed Forms of Activated Polyoxazoline and Methods of Synthesis Thereof” (J. M. Harris et al.)
This patent provides branched poly(oxazolines) having a terminal activating group that can be linked to a second moiety such as a drug molecule. The branched or multi-arm composition generally comprises two or more linear poly(oxazolines) linked to a single branching point.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| US | | Granted | | Sep-29-2008 | | 12/680,448 | | Jan-03-2012 | | 8,088,884 |
SER 03/07 Family: “Multifunctional Forms of Polyoxazoline Copolymers and Drug Compositions Comprising the Same” (K. Yoon et al.)
This family of patents provides for poly(oxazolines) having a set of pendent functional groups on the polymer backbone and a terminal functional group, wherein the pendent functional groups and the terminal functional groups are chemically orthogonal to one another. This family also covers mixtures of oxazoline monomers on the same polymer backbone e.g. ethyl oxazoline and pentynyl oxazoline. This family covers every class of molecule attached to POZ, including but not limited to small molecules, proteins, oligonucleotides, and lipids where the attached molecule can be a therapeutic, diagnostic, or targeting molecule. The family also covers such poly(oxazoline) polymers linked to various target molecules, such as therapeutic agents and targeting agents and of using such conjugates in the treatment or diagnosis of cancer:
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| US | | Granted | | Jan-12-2009 | | 12/744,472 | | Feb-07-2012 | | 8,110,651 |
| US | | Granted (CIP) | | May-25-2010 | | 12/787,241 | | Jan-24-2012 | | 8,101,706 |
| US | | Granted (CIP) | | Jan-23-2012 | | 13/356,552 | | Aug-06-2013 | | 8,501,899 |
| US | | Granted (CIP) | | Feb-06-2012 | | 13/367,128 | | Oct-27-2015 | | 9,169,354 |
| US | | Granted (CIP) | | Jul-08-2016 | | 15/205,671 | | Jan-01-2019 | | 10,166,294 |
| US | | Granted (CIP) | | Dec-28-2018 | | 16/235,936 | | Jan-04-2022 | | 11,213,588 |
| US | | Pending | | Jan-04-2022 | | 17/568,042 | | | | |
| China | | Granted | | Jan-12-2009 | | 200980106276.5 | | Dec-12-2012 | | 1098857 |
| Japan | | Granted | | Jan-12-2009 | | 2010-542410 | | Apr-04-2014 | | 5,514,736 |
| Korea (South) | | Granted | | Jan-12-2009 | | 10-2010-7017208 | | Jan-20-2015 | | 10-1486449 |
| Belgium | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| Belgium | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| Belgium | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| Switzerland | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| Switzerland | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| Switzerland | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| Germany | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| Germany | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| Germany | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| Denmark | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| France | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| France | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| France | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| United Kingdom | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| United Kingdom | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| United Kingdom | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| Netherlands | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| Netherlands | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| Netherlands | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
| Sweden | | Granted | | Jan-12-2009 | | 09701187.8 | | Sep-04-2013 | | 2,235,090 |
| Sweden | | Granted | | Jan-12-2009 | | 13181892.4 | | Mar-23-2016 | | 2,669,313 |
| Sweden | | Granted | | Jan-12-2009 | | 16154587.6 | | Jul-19-2017 | | 3,042,922 |
SER 08 family: “Poly(oxazoline) with Inert Terminating Groups, Polyoxazolines Prepared from Protected Initiating Groups and Related Compounds” (M. D. Bentley et al.)
This family of patents relates to linear, branched, multi-arm and pendent POZ with initiating functional groups. This patent family also covers compositions of POZ conjugates of phospholipids and glycolipids for use in making lipid nanoparticles (LNP) :
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| China | | Granted | | Jul-10-2009 | | 200980135512.6 | | Jun-25-2014 | | 102149749B |
| Japan | | Granted | | Jul-10-2009 | | 2011-517658 | | Nov-07-2014 | | 5,642,673 |
| Korea (South) | | Granted | | Jul-10-2009 | | 10-2011-7003132 | | Aug-04-2016 | | 10-167334 |
| US | | Granted | | Jul-10-2009 | | 13/003,306 | | Nov-11-2014 | | 8,883,211 |
| US | | Granted | | Nov-10-2014 | | 14/537,516 | | Mar-15-2016 | | 9,284,411 |
SER 16 Family: “Subcutaneous Delivery of Poly(oxazoline) Conjugates” (R. W. Moreadith et al.)
This family of patents provides for poly(oxazoline) conjugates of dopamine agonists and subcutaneous delivery of these conjugates for treatment of conditions related to dopamine insufficiency, such as Parkinson’s disease. In particular, this family includes claims to conjugates containing rotigotine, i.e., SER 214. The ʼ633 patent (US) has broader claims and would cover other molecules administered as subcutaneous injections, including but not limited to SER 226/227, SER 228/229 SER 232 and SER 252.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| US | | Granted | | Jun-15-2012 | | 13/524,994 | | Feb-26-2013 | | 8,383,093 |
| US | | Granted | | Feb-22-2013 | | 13/774,304 | | Dec-03-2013 | | 8,597,633 |
| US | | Granted | | Jun-29-2016 | | 15/197,336 | | Jun-11-2019 | | 10,314,837 |
| US | | Granted | | Jun-10-2019 | | 16/436,590 | | Jan-18-2022 | | 11,224,595 |
| US | | Granted | | Apr-05-2017 | | 15/480,122 | | Oct-01-2019 | | 10,426,768 |
| US | | Allowed | | Sep-30-2019 | | 16/588,761 | | Apr-12-2022 | | 11,298,350 |
| Canada | | Granted | | Nov-01-2012 | | 2,854,361 | | Aug-11-2020 | | 2,854,361 |
| Japan | | Granted | | Nov-01-2012 | | 2014-540093 | | Jul-21-2017 | | 6,177,787 |
| Japan | | Granted | | Jul-11-2017 | | 2017-135578 | | Apr-26-2019 | | 6,517,281 |
| Japan | | Granted | | Apr-16-2019 | | 2019-077583 | | May-12-2021 | | 6,883,605 |
| Korea (South) | | Granted | | Nov-01-2012 | | 10-2014-7014846 | | May-21-2020 | | 10-2115862 |
| Belgium | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| Switzerland | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| Germany | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| Denmark | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| United Kingdom | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| France | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| Netherlands | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
| Sweden | | Granted | | Nov-01-2012 | | 12846647.1 | | Oct-09-2019 | | 2,773,379 |
SER 18 Family: “Polyoxazoline Antibody Drug Conjugates” (R. W. Moreadith et al.)
This patent family describes and claims polymer-ADCs to treat human disease. They provide a large drug-antibody-ratio (DAR) which is beyond current technology.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| China | | Granted | | Jul-31-2015 | | 201580052259.3 | | Mar-13-2020 | | 3716373 |
| Japan | | Granted | | Jul-31-2015 | | 2017-505548 | | May-15-2020 | | 6,704,900 |
| US | | Granted | | Jul-31-2015 | | 14/815,718 | | Sep-11-2018 | | 10,071,168 |
| US | | Granted | | Sep-10-2018 | | 16/126,798 | | Jul-21-2021 | | 11,065,340 |
SER 22 Family: “Cleavable Conjugates of Catechol Compounds and Water-Soluble Polymers and Methods of Treatment Using the Same” (M. D. Bentley et al.)
This family covers conjugates including a water-soluble polymer linked to a compound including a catechol moiety via a cleavable linkage, wherein the cleavable linkage is formed between the water-soluble polymer and a first phenolic hydroxyl group of the catechol moiety and a second phenolic hydroxyl group of the catechol moiety is linked to a blocking group. The rate of hydrolytic release of the compound including the catechol moiety is controlled, at least in part, through structure or design of the blocking group on the second phenolic hydroxyl group of the catechol moiety such that the rate of hydrolytic release of the compound including the catechol moiety can be tuned through structural design of the group on the second phenolic hydroxyl group of the catechol moiety.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| Australia | | Pending | | Jul-27-2019 | | 2019309523 | | | | |
| Canada | | Pending | | Jul-27-2019 | | 3,107,317 | | | | |
| China | | Pending | | Jul-27-2019 | | CN201980063964 | | | | |
| Hong Kong | | Published | | Jan-26-2022 | | 62022045767.0 | | | | |
| Europe | | Pending | | Jul-27-2019 | | 19841823.8 | | | | |
| Israel | | Pending | | Jul-27-2019 | | 280364 | | | | |
| Japan | | Pending | | Jul-27-2019 | | 2021-504354 | | | | |
| Korea (South) | | Pending | | Jul-27-2019 | | 10-2021-7006020 | | | | |
| New Zealand | | Pending | | Jul-27-2019 | | 772903 | | | | |
| Singapore | | Pending | | Jul-27-2019 | | 11202100593P | | | | |
| US | | Granted | | Jan-27-2021 | | 17/263,723 | | Sept-26-2023 | | 11,766,132 |
SER 23 Family: “Polyoxazoline-Drug Conjugates with Novel Pharmacokinetic Properties” (J. M. Harris et al.)
Serina has cultivated a patent family related to all polymer conjugates of cannabinoids, including and not limited to SER 228, SER 229, SER 232 and the upcoming pipeline of POZ-cannabinoids of cannabidivarin, cannabigerol and cannabichromene.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| World Intellectual Property Org. (WIPO) | | Pending | | Jun-29-2020 | | PCT/US2020/040140 | | | | |
| Australia | | Pending | | Dec-23-2021 | | 2020301324 | | | | |
| Canada | | Pending | | Dec-21-2021 | | 3,144,774 | | | | |
| China | | Pending | | Jun-29-2020 | | CN202080060438 | | | | |
| Europe | | Pending | | Dec-20-2021 | | 20830744.7 | | | | |
| Israel | | Pending | | Dec-21-2021 | | 28921721 | | | | |
| Japan | | Pending | | Dec-24-2021 | | 2021576959 | | | | |
| Korea | | Pending | | Jan-27-2022 | | 10-2022-7003221 | | | | |
| New Zealand | | Pending | | Jun-29-2020 | | 783931 | | | | |
| Singapore | | Pending | | Jun-29-2020 | | 11202114046V | | | | |
| US | | Pending | | Dec-21-2021 | | 17/621,613 | | | | |
SER 24 family: “Polyoxazoline-lipid Conjugates and Lipid Nanoparticles and Pharmaceutical Compositions Including Same” (J. M. Harris et al.)
Serina has begun to cultivate a patent family related to the chemical synthesis of polymer conjugates of lipids that may be used to make lipid nanoparticles. The patent application discloses multiple different architectures of POZ, including but not limited to releasable linkages, stable linkages, therapeutic oligonucleotides (DNA, mRNA) and use in manufacture of vaccines for infectious diseases and cancer immunotherapy.
| Country | | Status | | Application Date | | Application Number | | Grant Date | | Grant Number |
| US | | Pending | | Feb-09-2021 | | 63/147,470 | | | | |
| US | | Pending | | Feb-04-2022 | | 17/665,190 | | | | |
| PCT | | Pending | | Feb-04-2022 | | PCT/US22/15314 | | | | |
| Canada | | Pending | | Jul-24-2023 | | 3206128 | | | | |
| China | | To be filed-entering by Oct-09-2023) (with 2-mo. extension) | | TBD | | TBD | | | | |
| Japan | | Pending | | Aug-08-2023 | | Awaiting filing receipt with app. no. | | | | |
| Israel | | Pending | | Jul-26-2023 | | 304773 | | | | |
| Europe | | Pending | | Aug-24-2023 | | 22753169.6 | | | | |
| Australia | | Pending | | Jul-24-2023 | | 2022219902 | | | | |
| New Zealand | | Pending | | Jul-25-2023 | | 802213 | | | | |
| South Korea | | Pending | | Sept-6-2023 | | 10-2023-7030341 | | | | |
Competition
Serina faces substantial competition from multiple sources, including large and specialty pharmaceutical, biopharmaceutical, and biotechnology companies, academic research institutions and governmental agencies, and public and private research institutions. Serina’s competitors compete with it on the level of the technologies employed, or on the level of development of product candidates. In addition, many small biotechnology companies have formed collaborations with large, established companies to (i) obtain support for their research, development, and commercialization of products or (ii) combine several treatment approaches to develop longer lasting or more efficacious treatments that may potentially directly compete with Serina’s current or future product candidates. Serina anticipates it will continue to face increasing competition as new therapies and combinations thereof, technologies, and data emerge within the field of drug delivery generally and, furthermore, within the treatment of diseases in which Serina expects to compete in the future.
In addition to the current treatments for patients, numerous commercial and academic preclinical studies and clinical trials are being undertaken by many parties to assess novel technologies and product candidates in the field of CNS disorders. Results from these studies and trials have fueled increasing levels of interest in the CNS field.
Several companies are in the business of developing and marketing polymer-modified therapeutics, with the majority using PEG polymers. The covalent attachment of PEG to therapeutic agents, termed PEGylation, is a well-established and clinically proven drug delivery approach to improve the PK and pharmacodynamics of drugs. Specifically, PEGylation can improve the parent drug’s solubility, extend its circulation time, and reduce its immunogenicity, with minimal undesirable properties. PEGylation technology has been applied to various therapeutic modalities or payloads including small molecules, aptamers, peptides, and proteins, leading to over 30 FDA approved PEGylated drugs currently in use and many investigational PEGylated agents under clinical trials.
Serina’s drug delivery technology competitors include:
| ● | Nektar Therapeutics, Inc. (Nektar) acquired Shearwater in 2001. Serina’s founders previously founded Shearwater. Shearwater developed the first generations of PEG, which became Nektar’s core technology platform after the acquisition. Nektar remains at the forefront of development of PEGylated compounds for use in humans. Nektar focuses primarily on developing products into early or mid-stage clinical trials before seeking a licensing partner to complete development (as in the example of NKTR-118, which was licensed to AstraZeneca in 2009 and is an approved product in both the US (under the name Movantik®) and the EU (under the name Moventig™). Despite the proven success of attaching PEG to proteins to enhance their pharmacokinetic properties, Nektar has had a series of recent setbacks with their approaches. In 2022 the Phase 3 trial for PEGylated IL-2 (bempegaldesleuken) failed in combination with Opdivo, resulting in Bristol-Myers Squibb returning the asset to Nektar. Nektar laid off 70% of its workforce as a result of the trial failure. In April 2023 Eli Lilly returned the IL-2 asset known as rezpeg to Nektar after it failed to meet clinical endpoints in a Phase 2 trial in atopic dermatitis. This prompted another reduction in workforce of 60%. The failures of these programs underscore the challenges of advancing some PEGylated product candidates beyond Phase 1 and into later stages of development. |
| ● | Mersana Therapeutics Inc. (Mersana) develops ADCs by attaching toxins to a delivery system comprised of 60 kDa modified biodegradable dextran (doloflexin, dolosynthen), and then attaching this to multiple sites on an antibody. This polymer delivery system is complex and despite its success in preclinical animal models of cancer Mersana’s pipeline of dolosynthen ADCs has failed to advance through a randomized Phase 2 clinical trial to date. In 2018 the company began work on a newer version of its ADC technology referred to as immunosynthen which creates a more precise drug antibody ratio (DAR) aimed at immunostimulation. Mersana has ongoing partnerships with Merck KGaA and Asana to advance their newer platform programs into additional preclinical models and Phase 1. |
| ● | Amunix, Inc. (Amunix) invented and patented a technology based upon E. coli synthesis of a tri-peptide (XTEN). These polypeptides have been shown to improve the pharmacokinetics of proteins in animals and in humans. Preliminary results suggest this peptide is broken down in the body and is non-immunogenic. Amunix’s technology has only been applied to proteins to date. Amunix advanced several of its proprietary XTEN conjugates into late-stage development with Roche and Biogen. In 2021 the company advanced its XTEN technology and began focusing on immune modulation of T-cells via a protease cleavable “mask” polypeptide (PRO-XTEN). The strategy is aimed at cleaving the mask polypeptide only in the tumor microenvironment to “unmask” the T-cell engagers and cytokines locally in the tumor. In 2021 Amunix was acquired by Sanofi. |
Serina believes that its POZ Platform™ has the advantages of each of the major technology competitors without any known added risk to the development profile.
Product Competition
SER 214 – POZ-rotigotine (Parkinson’s disease)
Parkinson’s disease continues to be a progressive disorder and available treatments are symptomatic rather than disease-modifying. Treatment differs in early and late stage disease. However, levodopa and dopamine agonists are central to treatment in both stages. Levodopa has been used to treat Parkinson’s disease for many years and is considered to be one of the most effective treatments available in the Parkinson’s disease drug market. However, as the condition progresses, patients may begin to experience side effects from levodopa treatment, such as involuntary movements (known as dyskinesia). In recent years, several new drugs have been developed that offer promising results in clinical trials. Other Parkinson’s disease drugs include MAO-B inhibitors and COMT inhibitors. MAO-B inhibitors work by breaking down dopamine, while COMT inhibitors prevent dopamine from being broken down. These drugs can be used alone or in combination with each other or with levodopa.
Rotigotine is a non-ergolinic dopamine receptor agonist (not a chemical member of the ergot class of drugs). In vitro binding studies and functional assays indicate a high affinity for the dopaminergic system (Dl, D2, D3, D4, D5 receptors), particularly D3 receptors. Rotigotine is thought to elicit its beneficial effect by activation of the D3, D2 and Dl receptors of the caudate-putamen region of the brain.
Rotigotine is marketed by UCB, S.A. as Neupro®. Neupro® delivers rotigotine via a daily application of a transdermal patch and is indicated for the treatment of Parkinson’s disease and for the treatment of moderate-to-severe RLS. In early-stage Parkinson’s disease, the recommended starting dose of Neupro® is 2 mg / 24 hours. Dose may be increased on a weekly basis if additional therapeutic effect is needed, by 2 mg / 24 hours. The maximum recommended dose for early stage Parkinson’s disease is 6 mg / 24 hours. In patients with late stage disease the recommended starting dose is 4 mg / 24 hours up to a maximum of 8 mg / 24 hours (20).
SER 227 – POZ-buprenorphine (long-acting analgesic for post-operative pain)
The post-surgical pain marketplace is crowded with older generic drugs as well as drugs with novel mechanisms of action. The numerous reports of patient dissatisfaction with current approaches for many opioid and non-opioid drugs currently in development illustrate that there is still an unmet medical need in the area. Not surprisingly, the opioid drugs in development are mainly centered on lowering abuse potential and reducing adverse events. The non-opioid drugs under development consist of novel combinations of established drugs or drugs with novel mechanisms of action that make them compatible with the multimodal approach to pain management.
Among the non-opioid drugs currently being developed, Heron Therapeutics, Inc.’s pipeline of approved products includes Sustol (granisetron injection), Cinvanti (apepritant injection, emulsion), Zynrelef (bupivacaine + meloxicam, injection) and Aponvie (apepritant emulsion, injection). Durect Corporation’s POSIMIR®, Innocoll AG’s Xaracoll® and Heron Therapeutics’ Zynrelef employ the local anesthetic, bupivacaine, as the central element of their pain control strategy. The efficacy of bupivacaine has been demonstrated by Pacira Pharmaceuticals, Inc. (Pacira) in its development and commercialization of the drug Exparel®, the first extended-release version of bupivacaine approved in the United States for administration into the surgical site for post-surgical pain control. Zynrelef is now approved as an extended-release formulation.
Exparel® was approved by the FDA in October 2011 for administration into the surgical site for post-surgical pain control. Exparel® is a long-acting sustained release formulation of bupivacaine (brand name: Marcaine) formulated using Pacira’s proprietary DepoFoam® technology (a multilayer liposomal vesicle that encapsulates a drug and releases it slowly over time without altering the released drug). The half-life of Exparel® is approximately ten times longer than bupivacaine – approximately 24 hours versus approximately 2.7 hours, respectively (data based on a pharmacokinetic study and half-life does not appear to be proportional to duration of treatment). Reports have indicated that surgeons use Exparel® with bupivacaine (for more immediate pain relief) as well as potentially with NSAIDS and steroids. The Exparel® label prohibits or discourages other combinations and specifically prohibits combination with lidocaine.
SER 227 is a POZ-polymer conjugate of buprenorphine, a potent mixed mu-agonist/antagonist that binds with high affinity to the mu-receptor. When bound to the mu-receptor, it triggers the mu-agonist pathway leading to potent analgesia (pain relief), but it is neither addictive nor does it produce a euphoria. Buprenorphine is fifty times more potent than morphine in animals and humans, and as such could be used in the setting of post-operative pain relief. Indeed, buprenorphine injection (Buprenex®) was approved over 35 years ago for control of post-operative pain, but its pharmacokinetic profile was suboptimal for continued dosing, and it was not a commercial success for human use. It is widely used in the care of veterinary animals for post-operative pain control. Buprenorphine is a well-characterized and approved drug for opioid use disorder (OUD), but the formulations that are approved for that indication would not be suitable for pain relief in the immediate post-operative period. The implant (Probuphine, Titan Pharmaceuticals) and the monthly injection (Sublocade, Indivior) are approved for chronic therapy in subjects who are not naïve to opioids, and those formulations provide weeks to months of release of buprenorphine. While those formulations are approved for OUD, they are not used for post-operative pain control as they do not have a desirable PK profile. In the rat and monkey studies conducted by Serina in 2017, SER 227 was found to promote prompt and sustained analgesia in the Brennan model in the rat with a > 3 day profile of steady-state release in the monkey. This profile would likely provide sustained analgesia in humans as well. The Company believes that such a product would not only provide adequate analgesia in the immediate post-operative period, but would prevent the need to use other opioids such as morphine or oxycodone to control pain. Thus, SER 227 would simultaneously target the initial gateway in the hospital that results in patients being exposed to addictive opioids.
In 2017, Serina commissioned inVentiv Health to conduct a market research report on the potential use of SER 227 in the treatment of post-operative pain. The report involved surveys among health care providers, surgeons, anesthesiologists, and health care payers. If approved by the FDA, and if effective, SER 227 would be indicated for use in the in-hospital setting for major surgical procedures (thoracotomy, abdominoplasty, major abdominal surgery, knee, and hip replacements).
SER 228 (POZ-cannabidiol) and SER 232 (POZ-tetrahydrocannabinol)
There has been anecdotal evidence of the medical benefits of cannabis for centuries. However, it is only in the last few decades that the pharmaceutical industry has been able to investigate the plant’s clinical benefit through the development of pharmaceutical agents based on the major two components (cannabidiol (CBD) and tetrahydrocannabinol (THC)). The advancement of these as pure pharmaceutical preparations into controlled clinical trials that are acceptable to regulatory authorities is an area of active clinical research.
GW has clinically validated the use of CBD in its development of Epidiolex®, a proprietary sesame seed formulation of relatively pure plant-derived CBD (98% oil-based CBD extract of known and constant composition). This oily mix has been approved for the treatment of severe, rare childhood-onset, treatment-resistant epilepsy disorders including Dravet syndrome (DS), Lennox-Gastaut syndrome (LGS), and Tuberous Sclerosis Complex (TSC). Epidiolex® was approved by the FDA in June 2018, and became available in November 2018, for the treatment of seizures associated with LGS and DS in patients two years of age or older. It is the first prescription pharmaceutical formulation of highly purified, plant-derived CBD, and the first in a new category of anti-epileptic drugs. Epidiolex® when approved was a Schedule 1 drug. However, the Drug Enforcement Agency (DEA) put Epidiolex® in Schedule V, the least restrictive schedule of the Controlled Substances Act, in October 2018. GW was acquired by Jazz Pharmaceuticals (Jazz) in May 2021
Zynerba Pharmaceuticals (Zynerba) is developing ZYN002 (Zygel), a synthetic CBD formulation with a permeation enhancer gel for transdermal delivery. In late 2017, Zynerba announced the results of the Phase II study in adults with non-convulsive and convulsive seizures; the trial failed to meet the pre-specified endpoint. A post-hoc analysis of the data suggested there was a high response rate in the placebo group that may have confounded the study results. Zynerba continued the development of ZYN002 for both refractory epilepsy, osteoarthritis, and Fragile X Syndrome (FXS), with a focus on rare forms of epilepsy. Zygel failed to meet the primary endpoint in the osteoarthritis trial but in the INSPIRE trial of patients with the 22q11.2 deletion syndrome the open label Phase 2 trial met the primary endpoint. In August 2023 Zynerba was acquired by Harmony Biosciences (Harmony). Harmony will continue the development of Zygel for 22q11.2 deletion syndrome and will advance Zygel into Phase 3 for FXS.
GW developed a buccal spray known as nabiximols or Sativex®, which comprises delta-9-tetrahydrocannabinol (27 mg/mL) and cannabidiol (25 mg/mL), as an analgesic agent for the treatment of neuropathic pain, spasticity, cancer pain and for neuroprotection in Huntington’s disease. The product, which contains soft extracts from Cannabis sativa, is primarily indicated for the treatment of multiple sclerosis (MS) related spasticity. It has been launched in the UK, Canada, Denmark, Germany, Ireland, Italy, Finland, Austria, Poland, Portugal, Israel, Norway, Spain, Czech Republic, the Netherlands, New Zealand, Sweden, and Switzerland. Nabiximols has also been registered in Australia, Belgium, Iceland, Kuwait, Luxembourg, Slovakia, and France. Nabiximols is also awaiting approval for this indication in several countries in the Middle East. In Canada, the product is also launched for the adjunctive treatment of cancer pain and neuropathic pain in adults with MS. It is marketed in Israel for neuropathic pain in adults with MS. Clinical trials are underway in cancer pain in the US and Europe. Phase I/II development is underway in UK and Germany. Despite approval in the territories above, Sativex is not approved for any indication in the US. Phase III development for MS-related neuropathic pain has been discontinued in the UK, France, Spain and Czech Republic.
The drug Marinol® is one of the oldest pharmaceutical compositions of THC and was initially commercialized by AbbVie as dronabinol capsules. Dronabinol is a man-made compound containing THC in standard concentrations. It was approved by the FDA in 1985 for the management of loss of appetite associated with weight loss in AIDS and prevention of chemotherapy induced nausea and vomiting (CINV) in patients who have failed to respond to standard of care. Insys Therapeutics developed Syndros™ (dronabinol in an oral solution) for the same indications with approval in July 2016.
Meda Pharmaceuticals, Inc. markets Cesamet®. Cesamet® is nabilone, a synthetic THC, in capsules and is indicated for the treatment of CINV. Both Marinol™ and Cesamet® contain low amounts of THC, and absorption from the gut demonstrates low bioavailability and high first pass metabolism.
Vaccines that Employ Lipid Nanoparticle (LNP) Delivery of RNA
Serina’s patent estate includes POZ conjugates of lipids that may prove to be useful in development of LNP technologies. The exploitation of LNP technology for the delivery of infectious disease vaccines, gene therapies and cancer immunotherapies has exploded in the last three years. Following the approvals of the Pfizer/BioNTech and Moderna mRNA vaccines for SARS-CoV-2 (COVID-19 disease) in December 2020 hundreds of companies including both large and small pharma, and small biotechnology companies such as Serina, have advanced their pipelines with novel approaches for development of LNP approaches to disease.
Industry reports show over 1,200 LNP delivered therapeutics currently in development. This rapid growth in development activity, while presenting prospective partnering opportunities for Serina, highlights the level of competition in the field. Many of Serina’s competitors, either alone or in combination with their respective strategic partners, have significantly greater financial resources and expertise in research and development, manufacturing, the regulatory approval process, and marketing than Serina does. Mergers and acquisition activity in the pharmaceutical, biopharmaceutical and biotechnology sector is likely to result in greater resource concentration among a smaller number of Serina’s competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through sizeable collaborative arrangements with established companies. These competitors also compete with Serina in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, Serina’s programs.
Serina’s commercial opportunity could be reduced or eliminated if one or more of its competitors develop and commercialize products that are safer, more effective, better tolerated, or of greater convenience or economic benefit than Serina’s proposed product offering. Serina’s competitors also may be in a position to obtain FDA or other regulatory approval for their products more rapidly, resulting in a stronger or dominant market position before Serina is able to enter the market. The key competitive factors affecting the success of all of Serina’s programs are likely to be product safety, efficacy, convenience, and treatment cost.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state, and local level, and in other countries, extensively regulate, among other things, the research, development, testing, approval, manufacturing, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import, and export of biopharmaceutical products. In addition, sponsors of biopharmaceutical products participating in Medicaid, Medicare, and other government health care programs are required to comply with mandatory price reporting, discount, and rebate requirements. Serina, along with its third party contractors, will be required to navigate the various preclinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which Serina wishes to conduct studies or seek approval or licensure of its product candidates. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with compliance with applicable statutes and regulations, requires the expenditure of substantial time and financial resources.
FDA Regulation
In the United States, the FDA regulates biologics under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Services Act, or PHSA, and their implementing regulations. The FDA further has issued a growing body of guidance documents, which, while not binding, provide the agency’s current interpretation of its statutes and regulations. Failure to comply with the applicable U.S. requirements may subject an applicant to administrative or judicial sanctions, such as FDA refusal to approve pending biologics license applications, or BLAs, or the agency’s issuance of warning letters, or the imposition of fines, civil penalties, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution brought by the FDA and the U.S. Department of Justice or other governmental entities.
The process required by the FDA before product candidates may be marketed in the United States generally involves the following:
completion of preclinical (or nonclinical) laboratory tests and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations;
submission to the FDA of an IND, which must become effective before human clinical trials may begin at United States clinical trial sites;
approval by an institutional review board, or IRB, for each clinical site, or centrally, before each trial may be initiated;
performance of adequate and well controlled human clinical trials to establish the product candidate’s safety, purity, potency, and efficacy for its intended use, performed in accordance with good clinical practice, or GCP, as well as IND regulations and other clinical trial related regulations;
development of manufacturing processes to ensure the product candidate’s identity, strength, quality, purity, and potency in compliance with current good manufacturing practice, cGMP, regulations;
submission to the FDA of a BLA;
satisfactory completion of an FDA advisory committee review, if applicable;
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product candidate is produced to assess compliance with cGMPs, and to assure that the facilities, methods, and controls are adequate to preserve the therapeutics’ identity, strength, quality, purity, and potency, as well as satisfactory completion of potential FDA inspection of selected clinical sites and selected clinical investigators to determine GCP compliance; and
FDA review and approval of the BLA to permit commercial marketing for particular indications for use.
Preclinical Studies and IND Submission
The testing and approval process of product candidates requires substantial time, effort, and financial resources. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease. Preclinical studies include laboratory evaluation of chemistry, pharmacology, toxicity, and product formulation, and may involve in vitro testing or in vivo animal studies to assess the potential for toxicity, adverse events, and other safety characteristics of the product candidate, and in some cases to establish a rationale for therapeutic use. Such studies must generally be conducted in accordance with FDA GLP regulations. The Consolidated Appropriations Act for 2023, signed into law on December 29, 2022, (P.L. 117 328) amended the FDCA and the Public Health Service Act to specify that nonclinical testing for drugs and biologics may, but is not required to, include in vivo animal studies. According to the amended language, a sponsor may fulfill nonclinical testing requirements by completing various in vitro assays (e.g., cell based assays, organ chips, or microphysiological systems), in silico studies (i.e., computer modeling), other human or nonhuman biology based tests (e.g., bioprinting), or in vivo animal studies.
Prior to commencing the first clinical trial at a U.S. investigational site with a product candidate, an IND sponsor must submit the results of the nonclinical tests and literature, together with manufacturing information, analytical data, any available clinical data, or literature (including data from clinical trials conducted outside of the United States), and proposed clinical study protocols among other things, to the FDA as part of an IND. An IND is a request from a clinical study sponsor to obtain authorization from the FDA to administer an investigational drug or biologic product to humans, as well as authorization to administer the product candidate to humans in accordance with a specific clinical trial protocol. Some long term nonclinical testing to further establish the safety profile of the product candidate, as well as manufacturing process development and product quality evaluation, continues after the IND is submitted.
An IND goes into effect upon notification by FDA or automatically 30 days after receipt by the FDA, unless the FDA, within the 30 day time period, notifies the applicant of safety concerns or questions related to one or more proposed clinical trials and places the trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve all outstanding concerns or questions posed by the FDA before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns or non-compliance with applicable regulations. As a result, submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development.
Clinical Trials
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with federal regulations and GCP requirements, which include the requirements that all research subjects provide their informed consent in writing for their participation in any clinical trial, as well as review and approval of the trial by an IRB. Investigators must also provide certain information to the clinical trial sponsors to allow the sponsors to make certain financial disclosures to the FDA. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the trial procedures, the parameters to be used in monitoring safety, the effectiveness criteria to be evaluated, and a statistical analysis plan. A protocol for each clinical trial, and any subsequent protocol amendments, must be submitted to the FDA as part of the IND. In addition, an IRB at each site participating in the clinical trial, or a central IRB, must review and approve the plan for any clinical trial, informed consent forms, and communications to trial subjects before a trial commences at that site. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits, and whether the planned human subject protections are adequate. The IRB must continue to oversee the clinical trial while it is being conducted. If a product candidate is being investigated for multiple intended indications, separate INDs may also be required. Status reports summarizing the progress of the clinical trials must be submitted at least annually to the FDA and the IRB and more frequently if suspected unexpected serious adverse reactions occur, findings from other studies suggest a significant risk to humans exposed to the biologic, findings from animal or in vitro testing suggest a significant risk for human subjects, or other significant safety information is found.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions on various grounds, including if the agency believes that the clinical trial either is not being conducted in accordance with regulatory requirements or presents an unacceptable risk to the clinical trial patients. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements or if the trial poses an unexpected serious harm to subjects. The FDA or an IRB may also impose conditions on the conduct of a clinical trial. Clinical trial sponsors may also choose to discontinue clinical trials as a result of risks to subjects, a lack of favorable results, or changing business priorities. Some clinical trials also include oversight by an independent group of qualified experts organized by the trial sponsor, known as an independent data monitoring committee, or IDMC, which provides authorization for whether a trial may move forward at designated check points based on review of certain data from the trial, to which only the IDMC has access, and may recommend halting the trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy.
Sponsors of clinical trials of certain FDA regulated products generally must register and disclose certain clinical trial information to a public registry maintained by the National Institutes of Health, or NIH. In particular, information related to the investigational product, patient population, phase of investigation, trial sites and investigators and other aspects of the clinical trial is made public as part of the registration of the clinical trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs. Although sponsors are also obligated to disclose the results of their clinical trials after completion, disclosure of the results may be delayed in some cases for up to two years after the date of completion of the trial. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. The NIH’s Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and the government has brought enforcement actions against non-compliant clinical trial sponsors. Sponsors or distributors of investigational products for the diagnosis, monitoring, or treatment of one or more serious diseases or conditions must also have a publicly available policy on evaluating and responding to requests for expanded access requests.
The manufacture of investigational biologics for the conduct of human clinical trials is subject to cGMP requirements. Investigational biologics and their therapeutic substances that are imported into the United States are also subject to regulation by the FDA. Further, the export of investigational products outside of the United States is subject to regulatory requirements of the receiving country as well as U.S. export requirements under the FDCA.
In general, for purposes of BLA approval, human clinical trials are typically conducted in three sequential phases, which may overlap or be combined.
Phase 1 The product candidate is initially administered to healthy human volunteers and tested for safety, dosage tolerance, structure activity relationships, mechanism of action, absorption, metabolism, distribution, and excretion. In the case of some products for severe or life threatening diseases, such as cancer, especially when the product may be too inherently toxic to administer ethically to healthy volunteers, the initial human testing is often conducted in patients with the target disease or condition. If possible, Phase 1 trials may also be used to gain an initial indication of product effectiveness.
Phase 2 Studies are conducted in limited subject populations with a specified disease or condition to evaluate preliminary efficacy, identify optimal dosages, dosage tolerance and schedule, possible adverse effects and safety risks, and expanded evidence of safety. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive clinical trials.
Phase 3 Clinical trials are undertaken with expanded subject populations, generally at geographically dispersed clinical trial sites, to generate sufficient data to provide statistically significant evidence of clinical efficacy and safety of the product candidate, to establish the overall risk benefit profile of the product candidate, and to provide adequate information for the labeling of the product candidate. Typically, two adequate, well controlled trials are required by the FDA for biological product approval. Under some limited circumstances, however, the FDA may approve a BLA based upon a single clinical trial plus confirmatory evidence from a post market trial or, alternatively, a single large, robust, well controlled multicenter trial without confirmatory evidence.
Additional kinds of data may also help to support a BLA, such as patient experience and real world data. For appropriate indications sought through supplemental BLAs, data summaries may provide marketing application support. For genetically targeted products and variant protein targeted products intended to address an unmet medical need in one or more patient subgroups with a serious or life threatening rare disease or condition, the FDA may allow a sponsor to rely upon data and information previously developed by the sponsor or for which the sponsor has a right of reference, that was submitted previously to support an approved application for a product that incorporates or utilizes the same or similar genetically targeted technology or a product that is the same or utilizes the same variant protein targeted drug as the product that is the subject of the application.
The FDA may also require, or companies may voluntarily conduct, additional clinical trials for the same indication after a product is approved. These post approval trials, referred to as Phase 4 clinical trials, are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 trials as a condition of approval of a BLA. The results of Phase 4 studies can confirm or refute the effectiveness of a product candidate and can provide important safety information.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product candidate as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, manufacturers must develop methods for testing the identity, strength, quality, potency, and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
In the Consolidated Appropriations Act for 2023, Congress amended the FDCA to require sponsors of a Phase 3 clinical trial, or other “pivotal study” of a new drug to support marketing authorization, to submit a diversity action plan for such clinical trial. The action plan must include the sponsor’s diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. A sponsor must submit a diversity action plan to FDA by the time the sponsor submits the trial protocol to the agency for review. The FDA may grant a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect Phase 3 trial planning and timing or what specific information FDA will expect in such plans, but if FDA objects to a sponsor’s diversity action plan and requires the sponsor to amend the plan or take other actions, it may delay trial initiation.
Marketing Application Submission, Review by the FDA, and Marketing Approval
Assuming successful completion of the required clinical and preclinical testing in accordance with all applicable regulatory requirements, the results of product development, including chemistry, manufacture, and controls information, nonclinical studies, and clinical trial results, including negative or ambiguous results as well as positive findings, are all submitted to the FDA, along with the proposed labeling, as part of a BLA requesting approval to market the product for one or more indications. A BLA must contain sufficient evidence of the biological product candidate’s safety, purity, potency and efficacy for its proposed indication or indications. Data may come from company sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act, as amended, or PDUFA, each BLA submission is subject to a substantial application user fee, and the sponsor of an approved BLA is also subject to an annual program fee. The FDA adjusts the PDUFA user fees on an annual basis. The application user fee must be paid at the time of the first submission of the application, even if the application is being submitted on a rolling basis. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Product candidates that are designated as orphan products are also not subject to application user fees unless the application also includes a non orphan indication.
In addition, under the Pediatric Research Equity Act, or PREA, a BLA or supplement to a BLA for a new active ingredient, indication, dosage form, dosage regimen, or route of administration, must contain data that are adequate to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. A sponsor who is planning to submit a marketing application for a product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration must submit an initial Pediatric Study Plan, or PSP, within sixty days of an end of Phase 2 meeting or, if there is no such meeting, as early as practicable before the initiation of the Phase 3 or Phase 2/3 clinical trial. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including trial objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach an agreement on the PSP. A sponsor can submit amendments to an agreed upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from pre clinical studies, early phase clinical trials or other clinical development programs. Orphan products are exempt from the PREA requirements.
The FDA Reauthorization Act of 2017 introduced a provision regarding required pediatric studies. Under this statute, for product candidates intended for the treatment of adult cancer which are directed at molecular targets that the FDA determines to be substantially relevant to the growth or progression of pediatric cancer, original application sponsors must submit, with the marketing application, reports from molecularly targeted pediatric cancer investigations designed to yield clinically meaningful pediatric study data, gathered using appropriate formulations for each applicable age group, to inform potential pediatric labeling. The FDA may, on its own initiative or at the request of the applicant, grant deferrals or waivers of some or all of this data, as above. Unlike PREA, orphan products are not exempt from this requirement.
The FDA also may require submission of a risk evaluation and mitigation strategy, or REMS, if it determines that a REMS is necessary to ensure that the benefits of the product candidate outweigh the risks and to assure safe use of the biological product. The REMS plan could include medication guides, physician communication plans, assessment plans and/or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools. The FDA determines the requirement for a REMS, as well as the specific REMS provisions, on a case by case basis. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS. The FDA will not approve a BLA without a REMS, if required. An assessment of the REMS must also be conducted at set intervals. Following product approval, a REMS may also be required by the FDA if new safety information is discovered and the FDA determines that a REMS is necessary to ensure that the benefits of the product outweigh the risks.
Once the FDA receives an application, it has 60 days to review the BLA to determine if it is substantially complete to permit a substantive review, before it accepts the application for filing. The FDA may request additional information rather than accept an application for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in depth substantive review.
Under the goals and policies agreed to by the FDA under PDUFA, the FDA has set the review goal of completing its review of 90% of BLAs within ten months of the filing date for a standard application and within six months of the filing date for an application with priority review. For all original BLAs, the ten and six month time periods run from the filing date; for all other submissions, including resubmissions, efficacy supplements and other supplements, the FDA’s stated review time periods, ranging from two to ten months, run from the submission date. This review goal is referred to as the PDUFA date. The PDUFA date is only a goal, and it is not uncommon for FDA review of a BLA to extend beyond the PDUFA date. The review process and the PDUFA date may also be extended if the FDA requests, or the sponsor otherwise provides, substantial additional information or clarification regarding the submission.
The FDA may also refer certain applications to an advisory committee. Before approving a product candidate for which no active ingredient has previously been approved by the FDA, the FDA must either refer that product candidate to an external advisory committee or provide in an action letter a summary of the reasons why the FDA did not refer the product candidate to an advisory committee. The FDA may also refer other product candidates to an advisory committee if FDA believes that the advisory committee’s expertise would be beneficial. An advisory committee is typically a panel that includes clinicians and other experts, which review, evaluate, and make a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making product approval decisions.
The FDA reviews a BLA to determine, among other things, whether a product candidate meets the agency’s approval standards, such as whether the application includes sufficient evidence that the product candidate is safe and effective for the proposed indications, and whether the manufacturing methods and controls are adequate to assure and preserve the product’s identity, strength, quality, potency, and purity. As part of its review, the FDA likely will re analyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process. Before approving a marketing application, the FDA typically will inspect the facility or facilities where the product is manufactured, referred to as a pre-approval inspection. The FDA will not approve an application unless it determines that the manufacturing processes and facilities, including contract manufacturers and subcontractors, are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a marketing application the FDA will inspect one or more clinical trial sites to assure compliance with applicable IND trial requirements and GCP. To assure cGMP and GCP compliance, an applicant must incur significant expenditure of time, money, and effort in the areas of training, record keeping, production, and quality control.
After evaluating the marketing application and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a Complete Response Letter, or CRL. A CRL indicates that the review cycle of the application is complete, and the application will not be approved in its present form, and it describes all of the specific deficiencies that the FDA identified. A CRL generally contains a statement of specific conditions that must be met in order to secure final approval of the marketing application and may require additional clinical or preclinical testing for the FDA to reconsider the application. The deficiencies identified may be minor, for example, requiring labeling changes; or major, for example, requiring additional Phase 3 clinical trials. If a CRL is issued, the applicant may either: resubmit the marketing application, addressing all of the deficiencies identified in the letter; withdraw the application; or request an opportunity for a hearing. The FDA has the goal of reviewing 90% of application resubmissions in either two or six months of the resubmission date, depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA may issue an approval letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications.
Even if the FDA approves a product, it may limit the approved indications or populations for use of the product, require that contraindications, warnings, or precautions be included in the product labeling, including a boxed warning, require that post approval studies, including Phase 4 clinical trials, be conducted to further assess a product’s safety and efficacy after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms under a REMS which can materially affect the potential market and profitability of the product. The FDA also may not approve label statements that are necessary for successful commercialization and marketing or may prevent or limit further marketing of a product based on the results of post marketing trials or surveillance programs.
After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval. The FDA may also withdraw the product approval if compliance with regulatory standards are not maintained or if problems occur after the product reaches the marketplace. Further, should new safety information arise, additional testing, product labeling, or FDA notification may be required.
Patent Term Restoration
Depending upon the timing, duration, and specifics of FDA approval of Serina’s biological product candidates, some of Serina’s U.S. patents may be eligible for limited patent term extension. These patent term extensions permit a patent restoration term of up to five years as compensation for any patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one half the time between the effective date of an IND, and the submission date of a BLA, plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension, and the extension must be applied for prior to expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Biosimilars and Exclusivity
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated approval pathway for biological products shown to be biosimilar to or interchangeable with an FDA licensed reference biological product. To date, a number of biosimilars have been licensed under the BPCIA, and numerous biosimilars have been approved in Europe. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be demonstrated through analytical studies, animal studies, and a clinical trial or trials. There must be no difference between the reference product and a biosimilar in mechanism of action, conditions of use, route of administration, dosage form, and strength. A biosimilar product may be deemed interchangeable with the reference product if it meets the higher hurdle of demonstrating that it can be expected to produce the same clinical results as the reference product in any given patient and, for products administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic without such alternation or switching. Upon licensure by the FDA, an interchangeable biosimilar may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product, although to date no such products have been approved for marketing in the United States. Complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being worked out by the FDA. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
A reference biologic is granted 12 years of data exclusivity from the time of first licensure of the product, and the first approved interchangeable biological product will be granted an exclusivity period of up to one year after it is first commercially marketed. However, certain changes and supplements to an approved BLA, and subsequent applications filed by the same sponsor, manufacturer, licensor, predecessor in interest, or other related entity do not qualify for the 12 year exclusivity period. As part of the Consolidated Appropriations Act for 2023, Congress amended the PHSA in order to permit multiple interchangeable products approved on the same day to receive and benefit from this one year exclusivity period. If pediatric studies are performed and accepted by the FDA as responsive to a written request, the 12 year exclusivity period will be extended for an additional six months. In addition, the FDA will not accept an application for a biosimilar or interchangeable product based on the reference biological product until four years after the date of first licensure of the reference product. “First licensure” typically means the initial date the particular product at issue was licensed in the United States. Date of first licensure does not include the date of licensure of (and a new period of exclusivity is not available for) a supplement for the reference product for a subsequent application filed by the same sponsor or manufacturer of the reference product (or licensor, predecessor in interest or other related entity) for a change (not including a modification to the structure of the biological product) that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device or strength or for a modification to the structure of the biological product that does not result in a change in safety, purity or potency. Therefore, one must determine whether a new product includes a modification to the structure of a previously licensed product that results in a change in safety, purity, or potency to assess whether the licensure of the new product is a first licensure that triggers its own period of exclusivity. Whether a subsequent application, if approved, warrants exclusivity as the “first licensure” of a biological product is determined on a case by case basis with data submitted by the sponsor.
The BPCIA is complex and continues to be interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the 12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate impact, implementation, and impact of the BPCIA is subject to significant uncertainty.
Pediatric Exclusivity
Pediatric exclusivity is a type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity or listed patents. This six month exclusivity may be granted if a sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application. The issuance of a written request does not require the sponsor to undertake the described studies.
Orphan Product Designation and Exclusivity
The Orphan Drug Act provides incentives for the development of products for rare diseases or conditions. Specifically, sponsors may apply for and receive Orphan Drug Designation, or ODD, if a product candidate is intended to treat a rare disease or condition, which is generally a disease or condition affecting less than 200,000 individuals in the United States, or affecting more than 200,000 in the United States and for which there is no reasonable expectation that the cost of developing and making the product available in the United States will be recovered from United States sales. Additionally, sponsors must present a plausible hypothesis for clinical superiority to obtain ODD if there is a product already approved by the FDA that that is considered by the FDA to be the same as the already approved product and is intended for the same indication. This hypothesis for clinical superiority must be demonstrated to obtain orphan exclusivity. Orphan drug designation must be requested before submitting a marketing application for the product candidate and does not convey any advantage in or shorten the duration of the regulatory review and approval process. If granted, ODD entitles the applicant to financial incentives such as opportunities for grant funding towards clinical study costs, tax advantages, and certain user fee waivers. After the FDA grants ODD, the identity of the therapeutic agent and its potential orphan use will be disclosed publicly by the FDA; the posting will also indicate whether the drug or biologic is no longer designated as an orphan product. More than one product candidate may receive an orphan designation for the same indication.
In addition, if a product candidate receives FDA approval for the indication for which it has ODD, the product is generally entitled to orphan exclusivity, which means the FDA may not approve any other application to market a product containing the same active moiety for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity. A product is clinically superior if it is safer, more effective or makes a major contribution to patient care. Thus, orphan drug exclusivity could block the approval of one of Serina’s potential products for seven years if a competitor obtains approval of the same product as defined by the FDA and Serina is not able to show the clinical superiority of its product candidate or if its product candidate’s indication is determined to be contained within the competitor’s product orphan indication. In addition, the FDA will not recognize orphan drug exclusivity if a sponsor fails to demonstrate upon approval that the product is clinically superior to a previously approved product containing the same active moiety for the same orphan condition, regardless of whether or not the previously approved product was designated an orphan drug or had orphan drug exclusivity. A product that has received ODD may not receive orphan exclusivity if it is approved for a use that is broader than the indication for which it received the designation. Orphan exclusivity does not prevent the FDA from approving a different drug or biological product for the same disease or condition, or the same product for a different disease or condition.
Recent court cases have challenged FDA’s approach to determining the scope of orphan drug exclusivity; however, at this time the agency continues to apply its long standing interpretation of the governing regulations and has stated that it does not plan to change any orphan drug implementing regulations.
Fast Track, Breakthrough Therapy and Priority Review Designations
The FDA is authorized to designate certain products for expedited development or review if they are intended for the treatment of serious or life threatening diseases or conditions and demonstrate the potential to address unmet medical needs or present a significant improvement over existing therapy. These programs include fast track designation, breakthrough therapy designation and priority review designation.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product candidate is intended to treat a serious or life threatening disease or condition and demonstrates the potential to address an unmet medical need by providing a therapy where none exists or a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. Fast track designation provides opportunities for more frequent interactions with the FDA review team to expedite development and review of the product. In addition, the FDA may initiate review of sections of a marketing application before the application is complete. This “rolling review” is available if the applicant provides and the FDA approves a schedule for the submission of the application sections and the sponsor pays any required user fees upon submission of the first section of the application. In some cases, a product with fast track designation may be eligible for accelerated approval or priority review if the relevant criteria are met. The FDA may rescind, or the sponsor may forfeit, fast track designation if the designation is no longer supported by data emerging from the clinical trial process.
Under the provisions of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, a sponsor may request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biologic that is intended, alone or in combination with one or more other drug or biologic, to treat a serious or life threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Products designated as breakthrough therapies are eligible for the same benefits described above for fast-track designation, as well as intensive guidance on an efficient development program beginning as early as Phase 1 trials, and a commitment from the FDA to involve senior managers and experienced review staff in a proactive collaborative and cross disciplinary review. Drugs or biologics designated as breakthrough therapies are also eligible for accelerated approval of their respective marketing applications.
Finally, the FDA may grant priority review designation to product candidates that are intended to treat serious conditions and, if approved, would provide significant improvements in the safety or effectiveness over existing therapies. The FDA determines at the time that the marketing application is submitted, on a case by case basis, whether the proposed drug or biologic represents a significant improvement in treatment, prevention or diagnosis of disease when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment limiting reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority review designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months for an original application from the date of filing.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. Furthermore, fast track designation, breakthrough therapy designation and priority review do not change the standards for approval and may not ultimately expedite the development or approval process.
Accelerated Approval
In addition, products studied for their safety and effectiveness in treating serious or life threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval from the FDA and may be approved on the basis of adequate and well controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a drug or biologic when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA will require that a sponsor of a drug receiving accelerated approval perform post marketing clinical trials to verify and describe the predicted effect on IMM or other clinical endpoint, and the product may be subject to expedited withdrawal procedures. Drugs and biologics granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a drug or biologic, such as an effect on IMM. The FDA has limited experience with accelerated approvals based on intermediate clinical endpoints but has indicated that such endpoints generally may support accelerated approval when the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate long term clinical benefit of a drug or biologic.
The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a drug or biologic, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. For example, accelerated approval has been used extensively in the development and approval of drugs and biologics for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large clinical trials to demonstrate a clinical or survival benefit.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post approval confirmatory studies to verify and describe the drug’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post marketing compliance requirements, including the completion of Phase 4 or post approval clinical trials to establish the effect on the clinical endpoint. Failure to conduct required post approval studies, or to confirm the predicted clinical benefit of the product during post marketing studies, would allow the FDA to withdraw approval of the drug. As part of the Consolidated Appropriations Act for 2023, Congress provided FDA additional statutory authority to mitigate potential risks to patients from continued marketing of ineffective drugs previously granted accelerated approval. Under the act’s amendments to the FDCA, FDA may require the sponsor of a product granted accelerated approval to have a confirmatory trial underway prior to approval. The sponsor must also submit progress reports on a confirmatory trial every six months until the trial is complete, and such reports are published on FDA’s website. The amendments also give FDA the option of using expedited procedures to withdraw product approval if the sponsor’s confirmatory trial fails to verify the claimed clinical benefits of the product.
All promotional materials for product candidates being considered and approved under the accelerated approval program are subject to prior review by the FDA.
Post-Approval Requirements
Any products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, monitoring and record keeping requirements, reporting of adverse experiences with the product, periodic reporting requirements, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, as well as advertising and promotion requirements, which include, among others, standards for direct to consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved uses (known as off label use), limitations on industry sponsored scientific and educational activities, and requirements for promotional activities involving the Internet.
After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval of a new BLA or a supplement, which may require the applicant to develop additional data or conduct additional pre clinical studies and clinical trials. The FDA may also place other conditions on approvals, including the requirement for a REMS, to assure the safe use of the product. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products.
In addition, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the quality and long term stability of the product. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports and returned or salvaged products. The manufacturing facilities for Serina’s product candidates must meet cGMP requirements and satisfy the FDA or comparable foreign regulatory authorities before any product is approved and Serina’s commercial products can be manufactured. Serina relies, and expects to continue to rely, on third parties for the production of clinical and commercial quantities of its products in accordance with cGMP regulations. These third-party manufacturers must comply with cGMP regulations that require, among other things, quality control and quality assurance, the maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Manufacturers, including third party manufacturers, and other entities involved in the manufacture and distribution of approved biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Future inspections by the FDA and other regulatory agencies may identify compliance issues at the facilities of Serina’s CMOs that may disrupt production or distribution or require substantial resources to correct. In addition, the discovery of conditions that violate these rules, including failure to conform to cGMP regulations, could result in enforcement actions, and the discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including, among other things, voluntary recall and regulatory sanctions as described below.
The FDA also strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. A company can make only those claims relating to a product that are approved by the FDA. Physicians, in their independent professional medical judgment, may prescribe legally available products for unapproved indications that are not described in the product’s labeling and that differ from those tested and approved by the FDA. Biopharmaceutical companies, however, are required to promote their products only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off label uses may be subject to significant liability, including, but not limited to, criminal and civil penalties under the FDCA and False Claims Act, exclusion from participation in federal health care programs, mandatory compliance programs under corporate integrity agreements, suspension and debarment from government contracts, and refusal of orders under existing government contracts.
Moreover, the Drug Supply Chain Security Act, or DSCSA, was enacted with the aim of building an electronic system to identify and trace certain prescription drugs distributed in the United States, including most biological products. The DSCSA imposes phased in and resource intensive obligations on biopharmaceutical manufacturers, wholesale distributors, and dispensers related to product tracking and tracing over a 10 year period that is expected to culminate in November 2023. Among the requirements of this legislation, manufacturers are required to provide certain information regarding the products to wholesale distributors and dispensers to which product ownership is transferred, label products with a product identifier, and keep certain records regarding the product. A manufacturer must also verify that purchasers of the manufacturer’s products are appropriately licensed. Further, under this legislation, manufacturers have product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products that would result in serious adverse health consequences of death to humans, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
FDA’s post market requirements are continuously evolving, and additional requirements may apply. For instance, in March 2020, the U.S. Congress passed the Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, which includes various provisions regarding FDA drug shortage reporting requirements, as well as provisions regarding supply chain security, such as risk management plan requirements, and the promotion of supply chain redundancy and domestic manufacturing. Any changes of law may require that Serina modify how it conducts its business and may require additional expenditure to ensure that Serina is in compliance.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in significant regulatory actions. Such actions may include refusal to approve pending applications, license or approval suspension or revocation, imposition of a clinical hold or termination of clinical trials, warning letters, untitled letters, cyber letters, modification of promotional materials or labeling, provision of corrective information, imposition of post market requirements including the need for additional testing, imposition of distribution or other restrictions under a REMS, product recalls, product seizures or detentions, refusal to allow imports or exports, total or partial suspension of production or distribution, FDA debarment, injunctions, fines, consent decrees, corporate integrity agreements, suspension and debarment from government contracts, and refusal of orders under existing government contracts, exclusion from participation in federal and state health care programs, restitution, disgorgement, or civil or criminal penalties, including fines and imprisonment, and adverse publicity, among other adverse consequences.
Additional Controls for Biologics
To help reduce the increased risk of the introduction of adventitious agents, the PHSA emphasizes the importance of manufacturing controls for products whose attributes cannot be precisely defined. The PHSA also provides authority to the FDA to immediately suspend licenses in situations where there exists a danger to public health, to prepare or procure products in the event of shortages and critical public health needs, and to authorize the creation and enforcement of regulations to prevent the introduction or spread of communicable diseases in the United States and between states.
After a BLA is approved, the product may also be subject to official lot release as a condition of approval. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing the results of all of the manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products before releasing the lots for distribution by the manufacturer.
In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of biological products.
Fraud and Abuse, Data Privacy and Security, and Transparency Laws and Regulations
Although Serina currently does not have any products on the market, its business activities and current and future arrangements with investigators, health care professionals, consultants, third party payors and customers may be subject to regulation and enforcement by numerous federal and state regulatory and law enforcement authorities in the United States in addition to the FDA, including potentially the Department of Justice, the Department of Health and Human Services and its various divisions, including the Centers for Medicare and Medicaid Services, or CMS, and the Health Resources and Services Administration, the Department of Veterans Affairs, the Department of Defense, and state and local governments. Serina’s business activities must comply with numerous health care laws, including but not limited to, Anti-Kickback and false claims laws and regulations as well as data privacy and security laws and regulations, which are described below, as well as state and federal consumer protection and unfair competition laws.
The federal Anti-Kickback Statute, which regulates, among other things, marketing practices, educational programs, pricing policies, and relationships with health care providers or other entities, prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting, or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order, or the referral to another for the furnishing or arranging for the furnishing of any item or service reimbursable under Medicare, Medicaid, or other federal health care programs, in whole or in part. The term “remuneration” has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between biopharmaceutical industry members on one hand and prescribers, purchasers, formulary managers, and beneficiaries on the other. There are certain statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly, and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case by case basis based on a cumulative review of all of its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal health care covered business, including purchases of products paid by federal health care programs, the statute has been violated. The Patient Protection and Affordable Care Act, or ACA, of 2010, as amended, also modified the intent requirement under the Anti-Kickback Statute to a stricter standard, such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA also provided that a violation of the federal Anti-Kickback Statute is grounds for the government or a whistleblower to assert that a claim for payment of items or services resulting from such violation constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act, or FCA, prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to, or approval by, the federal government, knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or avoiding, decreasing, or concealing an obligation to pay money to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. The civil False Claims Act has been used to assert liability on the basis of kickbacks and other improper referrals, improperly reported government pricing metrics such as Best Price or Average Manufacturer Price, improper use of Medicare provider or supplier numbers when detailing a provider of services, improper promotion of off label uses, and allegations as to misrepresentations with respect to products, contract requirements, and services rendered. Intent to deceive is not required to establish liability under the civil False Claims Act. Civil False Claims Act actions may be brought by the government or may be brought by private individuals on behalf of the government, called “qui tam” actions. If the government decides to intervene in a qui tam action and prevails in the lawsuit, the individual will share in the proceeds from any fines or settlement funds. If the government declines to intervene, the individual may pursue the case alone. The civil FCA provides for treble damages and a civil penalty for each false claim, such as an invoice or pharmacy claim for reimbursement, which can aggregate into millions of dollars. For these reasons, since 2004, False Claims Act lawsuits against biopharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements regarding certain sales practices and promoting off label uses. Civil False Claims act liability may further be imposed for known Medicare or Medicaid overpayments, for example, overpayments caused by understated rebate amounts that are not refunded within 60 days of discovering the overpayment, even if the overpayment was not caused by a false or fraudulent act. In addition, conviction, or civil judgment for violating the FCA may result in exclusion from federal health care programs, suspension and debarment from government contracts, and refusal of orders under existing government contracts.
The government may further prosecute conduct constituting a false claim under the criminal False Claims Act.
The criminal False Claims Act prohibits the making or presenting of a claim to the government knowing such claim to be false, fictitious, or fraudulent and, unlike the civil False Claims Act, requires proof of intent to submit a false claim.
The civil monetary penalties statute is another potential statute under which biopharmaceutical companies may be subject to enforcement. Among other things, the civil monetary penalties statue imposes fines against any person who is determined to have knowingly presented, or caused to be presented, claims to a federal health care program that the person knows, or should know, is for an item or service that was not provided as claimed or is false or fraudulent.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, also created federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, a health care benefit program, regardless of whether the payor is public or private, in connection with the delivery or payment for health care benefits, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing, or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, health care benefits, items, or services relating to health care matters. Additionally, the ACA amended the intent requirement of certain of these criminal statutes under HIPAA so that a person or entity no longer needs to have actual knowledge of the statute, or the specific intent to violate it, to have committed a violation.
Under the federal Physician Payments Sunshine Act and its implementing regulations, manufacturers of biologics for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program (with certain exceptions) must make annual reports to CMS regarding payments and other transfers of value made to or at the request of covered recipients, such as, but not limited to, physicians, physician assistants, nurse practitioners, clinical nurse specialists, and certified registered nurse anesthetists and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family. Certain payments for clinical trials are included within the ambit of this law. CMS makes the reported information publicly available.
Further, Serina may be subject to data privacy and security regulation by both the federal government and the states in which it conducts its business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, and its respective implementing regulations impose requirements on covered entities relating to the privacy, security, and transmission of individually identifiable health information, known as protected health information. Among other things, the HITECH Act, through its implementing regulations, makes HIPAA’s security standards and certain privacy standards directly applicable to business associates, defined as a person or organization, other than a member of a covered entity’s workforce, that creates, receives, maintains, or transmits protected health information on behalf of a covered entity for a function or activity regulated by HIPAA. The HITECH Act also strengthened the civil and criminal penalties that may be imposed against covered entities, business associates, and individuals, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, other federal and state laws, such as the California Consumer Privacy Act, may govern the privacy and security of health and other information in certain circumstances, many of which differ from each other in significant ways and may not be preempted by HIPAA, thus complicating compliance efforts.
Many states have also adopted laws similar to each of the above federal laws, which may be broader in scope and apply to items or services reimbursed by any third party payor, including commercial insurers. Certain state laws also regulate sponsors’ use of prescriber identifiable data. Certain states also require implementation of commercial compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments or the provision of other items of value that may be made to health care providers and other potential referral sources; impose restrictions on marketing practices; or require sponsors to track and report information related to payments, gifts, and other items of value to physicians and other health care providers. Furthermore, to distribute products commercially, Serina must comply with state laws requiring the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Recently, states have enacted or are considering legislation intended to make drug prices more transparent and deter significant price increases, typically as consumer protection laws. These laws may affect our future sales, marketing, and other promotional activities by imposing administrative and compliance burdens.
If Serina’s operations are found to be in violation of any of the laws or regulations described above or any other laws that apply to it, Serina may be subject to penalties or other enforcement actions, including criminal and significant civil monetary penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government health care programs, corporate integrity agreements, suspension and debarment from government contracts and non procurement transactions such as grants, and refusal of orders under existing government contracts, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of Serina’s operations, any of which could adversely affect Serina’s ability to operate its business and its results operations. Any action against us for violation of these laws, even if Serina successfully defends against it, could cause Serina to incur significant legal expenses and divert its management’s attention from the operation of its business.
To the extent that any of Serina’s products are sold in a foreign country, Serina may be subject to similar foreign laws and regulations, which may include, for instance, applicable post marketing requirements, including safety surveillance, anti fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to health care professionals.
Coverage and Reimbursement Generally
The commercial success of Serina’s product candidates and its ability to commercialize any approved product candidates successfully will depend in part on the extent to which governmental payor programs at the federal and state levels, including Medicare and Medicaid, private health insurers, and other third party payors provide coverage for and establish adequate reimbursement levels for Serina’s product candidates. Government authorities, private health insurers, and other organizations generally decide which therapeutics they will pay for and establish reimbursement levels for health care. A growing trend in recent years is the containment of health care costs.
Accordingly, governmental payors are increasingly trying control therapeutic prices through reimbursement restrictions, rebates, mandatory discounts, and formulary restrictions, among other strategies.
Medicare is a federal health care program administered by the federal government that covers individuals aged 65 and over as well as individuals with certain disabilities. Drugs and biologics may be covered under one or more sections of Medicare depending on the nature of the product and the conditions associated with and site of administration. For example, under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which provide coverage for outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Parts A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level.
Medicare Part B covers most injectable drugs and biologics given in an in-patient setting and some products administered by a licensed medical provider in hospital outpatient departments and doctors’ offices. Medicare Part B is administered by Medicare Administrative Contractors, which generally have the responsibility of making coverage decisions. Subject to certain payment adjustments and limits, Medicare generally pays for a Part B covered drug or biologic based on a percentage of manufacturer reported average sales price, which is regularly updated. Serina believes that its product candidates, which are intended to be administered by a health care professional in a clinical environment, will be subject to the Medicare Part B rules.
In the United States, the European Union, and other markets for Serina’s product candidates, government authorities and third party payors are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which often has resulted in average selling prices lower than they would otherwise be and sometimes at or below the provider’s acquisition cost. In the United States, it is also common for government and private health plans to use coverage determinations to leverage rebates from sponsors in order to reduce the plans’ net costs. These restrictions and limitations influence the purchase of health care services and products and lower the realization on sponsors’ sales of prescription therapeutics. Third party payors are developing increasingly sophisticated methods of controlling health care costs. Third party payors may limit coverage to specific therapeutic products on an approved list, or formulary, which might not include all of the FDA approved products for a particular indication or might impose high copayment amounts to influence patient choice. Third party payors also control costs by requiring prior authorization or imposing other dispensing restrictions before covering certain products and by broadening therapeutic classes to increase competition. Third party payors are increasingly challenging the price and examining the medical necessity and cost effectiveness of medical products and services, in addition to their safety and efficacy. Absent clinical differentiators, third party payors may treat products as therapeutically equivalent and base formulary decisions on net cost. To lower the prescription cost, sponsors frequently rebate a portion of the prescription price to the third party payors. Recently, purchasers and third party payors have begun to focus on the value of new therapeutics and have sought agreements in which price is based on achievement of performance metrics.
Federal programs also impose price controls through mandatory ceiling prices on purchases by federal agencies and federally funded hospitals and clinics and mandatory rebates on retail pharmacy prescriptions paid by Medicaid and Tricare. By example, payment or reimbursement of prescription therapeutics by Medicaid or Medicare requires sponsors to submit certified pricing information to CMS. The Medicaid Drug Rebate statute and state statutes requires sponsors to calculate and report price points, which are used to determine mandatory rebate payments or negotiate supplemental rebate payments on both the state and federal level and Medicaid payment rates for certain therapeutics. For therapeutics paid under Medicare Part B, sponsors must also calculate and report their Average Sales Price, which is used to determine the Medicare Part B payment rate. Furthermore, as a condition of receiving Medicare Part B reimbursement for eligible drugs or biologicals, the manufacturer is required to participate in other government health care programs, including the Medicaid Rebate Program and the 340B Drug Pricing Program. The Medicaid Drug Rebate Program requires biopharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of HHS as a condition for states to receive federal matching funds for the manufacturer’s outpatient therapeutic products furnished to Medicaid patients. Under the 340B Drug Pricing Program, the manufacturer must extend discounts to entities that participate in the program. In addition, therapeutics covered by certain government payor programs are subject to an additional inflation penalty which can substantially increase rebate payments. Certain states have also enacted laws that require that manufacturers report certain pricing information, including drug price increases. States laws may also limit the amount that prices may be increased or require negotiation of supplemental rebates for new drugs entering the market at price points determined to be high. Refusal to negotiate supplemental rebates can negatively affect market access and provider reimbursement.
Private payors often rely on the lead of the governmental payors in rendering coverage and reimbursement determinations. Therefore, achieving favorable CMS coverage and reimbursement is usually a significant gating issue for successful introduction of a new product. In addition, government programs as a condition of participation mandate fixed discounts or rebates from sponsors regardless of formulary position or utilization and may utilize mechanisms such as formulary placement to attain further price reductions, which can greatly reduce realization on the sale.
Further, the increased emphasis on managed health care in the United States and on country and regional pricing and reimbursement controls in the European Union will put additional pressure on product pricing, reimbursement, and utilization, which may adversely affect Serina’s future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, competition within therapeutic classes, judicial decisions and governmental laws and regulations related to Medicare, Medicaid, and health care reform, biopharmaceutical coverage and reimbursement policies, and pricing in general. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third party payors to reimburse all or part of the associated health care costs. Sales of Serina’s product candidates will therefore depend substantially, both domestically and abroad, on the extent to which the costs of Serina’s products will be paid by health maintenance, managed care, pharmacy benefit and similar health care management organizations, or reimbursed by government health administration authorities, such as Medicare and Medicaid, private health insurers, and other third party payors.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost effectiveness of Serina’s product candidate to currently available therapies (so called health technology assessment, or HTA) in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. Other member states allow companies to fix their own prices for drug products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of Serina’s products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to by significantly lower.
As a result of the above, Serina may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost effectiveness of Serina’s products, in addition to the costs required to obtain marketing approvals in the United States and in other jurisdictions. Serina’s product candidates may not be considered medically necessary or cost effective, or the rebate percentages required to secure coverage may not yield an adequate margin over cost. Additionally, companies are increasingly finding it necessary to establish bridge programs to assist patients access new therapies during protracted initial coverage determination periods.
Moreover, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved or that significant price concessions will not be required to avoid restrictive conditions. High health plan co payment requirements may result in patients refusing prescriptions or seeking alternative therapies. Adequate third party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on Serina’s investment in therapeutic development. Coverage policies and third party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which Serina receives regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future. Legislative proposals to reform health care or reduce costs under government insurance programs may result in lower reimbursement for Serina’s products and product candidates or exclusion of Serina’s products and product candidates from coverage. The cost containment measures that health care payors and providers are instituting and any health care reform could significantly reduce Serina’s revenues from the sale of any approved product candidates. Serina cannot provide any assurances that it will be able to obtain and maintain third party coverage or adequate reimbursement for Serina’s product candidates in whole or in part.
Health Care Reform Measures
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the health care system that could prevent or delay marketing approval of product and therapeutic candidates, restrict or regulate post approval activities, and affect the ability to profitably sell product and therapeutic candidates that obtain marketing approval. The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of Serina’s product and therapeutic candidates. If Serina is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if Serina is not able to maintain regulatory compliance, Serina may lose any marketing approval that it otherwise may have obtained and it may not achieve or sustain profitability, which would adversely affect Serina’s business, prospects, financial condition, and results of operations. Moreover, among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in health care systems with the stated goals of containing health care costs, improving quality and/or expanding access.
For example, the Patient Protection and the ACA, was enacted in March 2010, and has had a significant impact on the health care industry in the United States. The ACA expanded coverage for the uninsured while at the same time containing overall health care costs. With regard to biopharmaceutical products, the ACA, among other things, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees on manufacturers of certain branded prescription drugs, and created a new Medicare Part D coverage gap discount program. Additionally, the CREATES Act, which became law on December 20, 2019, aims to address the concern articulated by both the FDA and others in the industry that some brand manufacturers have improperly restricted the distribution of their products, including by invoking the existence of a REMS for certain products, to deny generic product developers access to samples of brand products. Because generic product developers need samples to conduct certain comparative testing required by the FDA, some have attributed the inability to timely obtain samples as a cause of delay in the entry of generic products. To remedy this concern, the CREATES Act establishes a private cause of action that permits a generic product developer to sue the brand manufacturer to compel it to furnish the necessary samples on “commercially reasonable, market based terms.” Whether and how generic product developments will use this new pathway, as well as the likely outcome of any legal challenges to provisions of the CREATES Act, remain highly uncertain and its potential effects on any of Serina’s future commercial products are unknown.
Following several years of litigation in the federal courts, in June 2021, the U.S. Supreme Court upheld the ACA when it dismissed a legal challenge to the ACA’s constitutionality. Further legislative and regulatory changes under the ACA remain possible, but it is unknown what form any such changes or any law would take and how or whether it may affect the biopharmaceutical industry as a whole or Serina’s business in the future. Serina expects that changes or additions to the ACA, the Medicare and Medicaid programs, and changes stemming from other health care reform measures, especially with regard to health care access, financing, or other legislation in individual states, could have a material adverse effect on the health care industry in the United States.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA that affect health care expenditures. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and was extended by the Consolidated Appropriations Act for 2023 and will remain in effect through 2032 unless additional Congressional action is taken.
Moreover, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. In May 2019, HHS issued a final rule to allow Medicare Advantage plans the option to use step therapy for Part B drugs beginning January 1, 2020. This final rule codified a HHS policy change that was effective January 1, 2019.
More recently, in August 2022, President Biden signed into the law the Inflation Reduction Act of 2022, or the IRA. Among other things, the IRA has multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the drug product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease.
Individual states in the United States have also increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In December 2020, the U.S. Supreme Court held unanimously that federal law does not preempt the states’ ability to regulate PBMs and other members of the health care and pharmaceutical supply chain, an important decision that may lead to further and more aggressive efforts by states in this area.
Serina cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. Serina expects that additional state and federal health care reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for health care products and services, including any future drug products for which Serina secures marketing approval.
The Foreign Corrupt Practices Act
The FCPA prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party, or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. Activities that violate the FCPA, even if they occur wholly outside the United States, can result in criminal and civil fines, imprisonment, disgorgement, oversight, and suspension and debarment from government contracts, and refusal of orders under existing government contracts.
EU Drug Development
In the EU, Serina’s product candidates and products, should they receive marketing authorization in the EU, will be subject to extensive regulatory requirements. As in the United States, medicinal products can be marketed only if a marketing authorization from the competent regulatory agencies has been obtained. Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU member states have transposed and applied the provisions of the Directive differently. This has led to some variations in the member state regimes.
In April 2014, the Clinical Trials Regulation, (EU) No 536/2014, was adopted and it became effective on January 31, 2022. The Clinical Trials Regulation will be directly applicable in all of the EU Member States, repealing the current Clinical Trials Directive 2001/20/EC. The extent to which ongoing clinical trials will be governed by the Clinical Trials Regulation will depend on when the clinical trial is initiated or on the duration of an ongoing trial. As of January 2023, all new clinical trials must comply with the Clinical Trials Regulation. In addition, any clinical trial that was already under way as of January 1, 2023, and continues for more than three years from the day on which the Clinical Trials Regulation becomes applicable (i.e., January 31, 2025), the Clinical Trials Regulation will at that time begin to apply to the clinical trial.
The Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the regulation include: a streamlined application procedure via a single entry point, the “EU portal” or Clinical Trial Information System, or CTIS; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed by the competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted (Member States concerned). Part II is assessed separately by each Member State concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the concerned EU Member State.
European Data Collection
The collection and use of personal health data in the EU is governed by the General Data Protection Regulation, or GDPR. The GDPR applies to any company established in the European Economic Area, or EEA, (which includes the EU Member States plus Iceland, Liechtenstein, and Norway) and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EEA or the monitoring of the behavior of data subjects in the EEA. The GDPR establishes stringent requirements applicable to the processing of personal data, including strict requirements relating to the validity of consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct data protection impact assessments for “high risk” processing, limitations on retention of personal data, special provisions affording greater protection to and requiring additional compliance measures for “special categories of personal data” including health and genetic information of data subjects, mandatory data breach notification (in certain circumstances), “privacy by design” requirements, and direct obligations on service providers acting as processors. The GDPR also prohibits the international transfer of personal data from the EEA to countries outside of the EEA unless made to a country deemed to have adequate data privacy laws by the European Commission or a data transfer mechanism has been put in place. Failure to comply with the GDPR requirements may subject an entity to litigation, regulatory investigations, enforcement notices and/or fines of up to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
The GDPR may also impose additional compliance obligations relating to the transfer of data between companies and their subsidiaries or other business partners. For example, on July 16, 2020, the Court of Justice of the European Union (CJEU), issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C 311/18), called Schrems II. This decision (a) calls into question commonly relied upon data transfer mechanisms as between the EU Member States and the United States (such as the Standard Contractual Clauses) and (b) invalidates the EU U.S. Privacy Shield on which many companies had relied as an acceptable mechanism for transferring such data from the EU to the United States. The CJEU is the highest court in Europe and the Schrems II decision heightens the burden on data importers to assess U.S. national security laws on their business and future actions of EU data protection authorities are difficult to predict.
United Kingdom Regulation
As of January 1, 2021, EU law no longer directly applies in the United Kingdom. The United Kingdom has adopted existing EU medicines regulation as standalone UK legislation with some amendments to reflect procedural and other requirements with respect to marketing authorizations and other regulatory provisions.
In order to market medicines in the United Kingdom, manufacturers must hold a UK authorization. On January 1, 2021, all EU marketing authorizations were converted to UK marketing authorizations subject to a manufacturer opt out. UK medicines legislation is subject to future regulatory change under the Medicines and Medical Devices Act 2021, which sets out a framework for the adoption of medicines regulation. Guidance issued by the Medicines and Healthcare products Regulatory Agency, or MHRA, states that the United Kingdom will have the power to take into account marketing authorizations made under the EU decentralized and mutual recognition procedures. In addition, the MHRA’s guidance has been updated to refer to new national licensing procedures including new routes of evaluation for novel and biotechnological products.
Different rules will apply in Northern Ireland following implementation of the Northern Ireland Protocol. In Northern Ireland, EU central marketing applications will continue to apply.
The Trade and Cooperation Agreement between the EU and the United Kingdom contains an Annex in relation to medicinal products with the objective of facilitating availability of medicines, promotion of public health and consumer protection in respect of medicinal products. The Annex provides for mutual recognition of good manufacturing practice (GMP) inspections and certificates, meaning that manufacturing facilities do not need to undergo duplicate inspections for the two markets. The Annex establishes a Working Group on Medicinal Products to deal with matters under the Trade and Cooperation Agreement, facilitate co-operation and for the carrying out of technical discussions. It is expected that further bilateral discussions will continue with respect to regulatory areas not the subject of the Trade and Cooperation Agreement, including pharmacovigilance. The Trade and Cooperation Agreement also does not include reciprocal arrangements for the recognition of batch testing certification. However, the United Kingdom has listed approved countries, including the EEA which will enable UK importers and wholesales to recognize certain certification and regulatory standards. The European Commission has not adopted such recognition procedures.
Relatedly, following the United Kingdom’s withdrawal from the EU, the GDPR has been implemented in the United Kingdom (as the UK GDPR). The UK GDPR sits alongside the UK Data Protection Act 2018 which implements certain derogations in the EU GDPR into United Kingdom law. Under the UK GDPR, companies not established in the United Kingdom but who process personal data in relation to the offering of goods or services to individuals in the United Kingdom, or to monitor their behavior will be subject to the UK GDPR the requirements of which are (at this time) largely aligned with those under the EU GDPR and as such, may lead to similar compliance and operational costs with potential fines of up to £17.5 million or 4% of global turnover. In 2022, the UK government proposed and debated the Data Protection and Digital Information Bill to harmonize the 2018 Data Protection Act, UK GDPR, and the Privacy and Electronic Communications Regulations under one legislative framework. However, progress on the bill stalled as the government continues to assess the most optimal approach to data protection reform.
Rest of the World Regulation
For other countries outside of the EU and the United States, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. Additionally, clinical trials to support applications for marketing authorization in such jurisdictions must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. If Serina fails to comply with applicable foreign regulatory requirements, Serina may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution.
Human Capital
As of November 6, 2023, Serina had eleven employees, nine of whom were full time, four of whom have Ph.D. or M.D. degrees, and eight are engaged in research and development and manufacturing activities. Serina does not have any employees represented by a labor union or covered under a collective bargaining agreement.
Talent Acquisition and Retention
Serina recognizes its employees largely contribute to its success. To this end, Serina supports business growth by seeking to attract and retain best in class talent. Serina uses internal and external resources to recruit highly skilled candidates for open positions. Serina believes that it is able to attract and retain superior talent as measured by its minimal turnover rate and high employee service tenure.
Total Rewards
Serina’s total rewards philosophy has been to create investment in its workforce by offering a competitive compensation and benefits package. Serina provides employees with compensation packages including base salary, annual incentive bonuses, and long-term equity incentive awards. Serina also offers comprehensive employee benefits, such as life, disability, and health insurance, health savings and flexible spending accounts, paid time off, and a 401(k) plan. It is Serina’s express intent to be an employer of choice in its industry by providing a market competitive compensation and benefits package.
Diversity, Equity, and Inclusion
Serina believes that a diverse workforce is critical to its success. Serina’s mission is to value differences in races, ethnicities, religions, nationalities, genders, ages, and sexual orientations, as well as education, skill sets, and experience. Serina is focused on inclusive hiring practices, fair and equitable treatment, organizational flexibility, and training and resources.
Training and Development
Serina believes in encouraging employees in becoming lifelong learners by providing ongoing learning and leadership training opportunities. While Serina strives to provide real time recognition of employee performance, it has a formal annual review process not only to determine pay and equity adjustments tied to individual contributions, but to identify areas where training and development may be needed.
Facilities
Serina’s current headquarters is comprised of 7,641 square feet of office and laboratory space. The lease term is two years for the office space and five years for the laboratory space. The current annual rent is $224,802 with a no annual increase. The lease termination date is October 31, 2025 for the office space and January 31, 2028 for the laboratory space. Serina believes that the space is adequate to meet its near term needs.
Legal Proceedings
From time to time, Serina may become involved in litigation or legal proceedings relating to claims arising from the ordinary course of its business. Serina is not currently a party to any material legal proceedings.
AGEX MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of AgeX’s financial condition and results of operations should be read in conjunction with AgeX’s consolidated financial statements and notes thereto appearing elsewhere in this proxy statement/prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this proxy statement/prospectus, including information with respect to AgeX’s plans and strategy for AgeX’s business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, AgeX’s actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. References to “we,” “us” and “our” in this section titled “AgeX Management’s Discussion and Analysis of Financial Condition and Results of Operations” refer to AgeX.
Emerging Growth Company Status
The Jumpstart our Business Startups Act of 2012 (JOBS Act) permits an “emerging growth company” such as AgeX to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we elected to comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements were consolidated with those of Lineage Cell Therapeutics, Inc., which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Recent Developments
Amendment to Preferred Stock and Remediation of Stock Exchange Listing Deficiency
On July 24, 2023, AgeX issued shares of AgeX Series A preferred stock and AgeX Series B preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence with the intent of adding $36 million to stockholders equity to eliminate a stockholders equity deficiency that caused AgeX to be out of compliance with the NYSE American continued listing standards. However, upon subsequent consideration in consultation with AgeX’s independent registered public accountants, AgeX determined that, in accordance with applicable guidance to GAAP, the deemed liquidation preference provisions of the Preferred Stock could be considered contingent redemption provisions that are not solely within AgeX’s control, requiring that the Preferred Stock be presented outside of permanent equity in the mezzanine section of the condensed consolidated balance sheet as of September 30, 2023. To comply with the NYSE American listing requirement by permitting the Preferred Stock to qualify now as permanent equity, on November 7, 2023, Section 3(b) of the terms of the Series A Preferred Stock and Series B Preferred Stock was amended (i) to clarify that certain change of control or disposition of asset transactions would be treated as a deemed liquidation if the applicable transaction is approved by the Board of Directors or stockholders of AgeX, and (ii) to provide that in case of such a deemed liquidation transaction holders of Preferred Stock would receive the same type of consideration as that distributed or paid to holders of AgeX common stock. AgeX has informed the NYSE American of the accounting issue and the remedy that has been implemented and AgeX believes it is in compliance with NYSE American’s continued listing standards.
Merger Agreement
On August 29, 2023, we entered into the Merger Agreement with Serina and Merger Sub, pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into Serina, with Serina surviving as our wholly owned subsidiary. For additional information, see the sections titled “The Merger” and “The Merger Agreement” beginning on pages 134 and 153 of this proxy statement/prospectus/information statement, respectively.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant. For a more complete description of the exchange ratio, please see the section titled “The Merger Agreement—Merger Consideration and Exchange Ratio.”
Concurrently with the execution of the Merger Agreement, AgeX, Serina and Juvenescence entered into the Side Letter, which will become effective immediately prior to the closing of the Merger. The Side Letter provides, among other things, that (i) immediately prior to the Effective Time, Juvenescence will cancel all out of the money AgeX warrants held by Juvenescence; (ii) Juvenescence will exercise all post-merger warrants it holds to provide the combined company an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; (iii) Juvenescence will not sell any shares of AgeX Series A preferred stock or AgeX Series B preferred stock and will take all actions necessary to convert all of such preferred stock into AgeX common stock before the reverse stock split; (iv) Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of certain loans to AgeX; and (v) Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loan agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. For additional information, see the sections titled “Agreements Related to the Merger—Side Letter” beginning on page 179 of this proxy statement/prospectus.
The following discussion and analysis of AgeX’s financial condition and results of operations does not reflect material changes to AgeX’s business, assets, liabilities, financial condition, operations, management, and prospects that will occur if the Merger is consummated.
Additional Loans Under the 2022 Secured Note
On October 3, 2023, AgeX drew $500,000 of its credit available under the 2022 Secured Note. On October 31, 2023 AgeX drew the final $500,000 of the credit line available under the 2022 Secured Note.
Increase in Secured Note Line of Credit
On November 9, 2023, AgeX and Juvenescence entered into the 2022 Secured Note Fifth Amendment that increases the amount of the line of credit available to AgeX by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. Concurrently with the execution of the 2022 Secured Note Fifth Amendment, AgeX also entered into an additional Pledge Agreement to add shares of a subsidiary to the collateral under the Security Agreement, and AgeX’s subsidiaries ReCyte, Reverse Bio, and UniverXome each entered into a Guaranty Agreement and Joinder Agreement pursuant to which each of them agreed to guaranty AgeX’s obligations to Juvenescence pursuant to the 2022 Secured Note, as amended by the 2022 Secured Note Fifth Amendment, and to grant Juvenescence a security interest in their respective assets pursuant to the Security Agreement to secure their obligations to Juvenescence.
Overview
We are a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases. Our initial discovery and preclinical programs focus on utilizing brown adipose tissue in targeting diabetes, obesity, and heart disease; and induced tissue regeneration in utilizing the human body’s own abilities to scarlessly regenerate tissues damaged from age or trauma. We may also pursue other early-stage pre-clinical programs.
Since inception, our operations have focused on building our technology platform, identifying potential product candidates, establishing and protecting our intellectual property and raising capital.
Since inception, we have incurred significant operating losses and we will need to obtain additional financing in order to continue our operations, including our research and development programs. See “—Liquidity and Capital Resources” for a discussion of our available capital resources and our need for financing. Our net loss from continuing operations before interest and other expenses were $7.0 million and $8.0 million for the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, we had an accumulated deficit of $116.2 million. Our net loss from operations before interest and other expenses were $4.0 million and $3.6 million for the six months ended June 30, 2023 and 2022, respectively. As of June 30, 2023, we had an accumulated deficit of $122.2 million. We expect to continue to incur operating losses and negative cash flows for the foreseeable future.
Critical Accounting Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts in our consolidated financial statements and related notes. Our significant accounting policies are described in Note 2, Summary of Significant Accounting Policies, to our consolidated financial statements included elsewhere in this proxy statement/prospectus. We have identified below our critical accounting policies and estimates that we believe require the greatest amount of judgment. On an ongoing basis, we evaluate our estimates that are subject to significant judgment, including those related to going concern assessment of consolidated financial statements, allocations and adjustments necessary for carve-out basis of presentation, including the separate return method for income taxes, useful lives associated with long-lived assets, including evaluation of asset impairment, allowances for uncollectible accounts receivables, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards or other equity instruments and liability classified warrants. Actual results could differ materially from those estimates. On an ongoing basis, we evaluate our estimates compared to historical experience and trends, which form the basis for making judgments about the carrying value of assets and liabilities. To the extent that there are material differences between our estimates and our actual results, our future consolidated financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe the assumptions and estimates associated with the following have the greatest potential impact on our consolidated financial statements. Management believes that there have been no significant changes during the six months ended June 30, 2023 to the items that we disclosed as our critical accounting policies and estimates in Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2022, except as disclosed in Note 2, Basis of Presentation and Summary of Significant Accounting Policies, of our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement.
Going Concern Assessment
We assess going concern uncertainty for our consolidated financial statements to determine if we have sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date our consolidated financial statements are issued, which is referred to as the “look-forward period” as defined by FASB’s ASU No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to us, we consider various scenarios, forecasts, projections, and estimates, and we make certain key assumptions, including the timing and nature of projected cash expenditures or programs, among other factors, and our ability to delay or curtail those expenditures or programs within the look-forward period in accordance with ASU No. 2014-15, if necessary.
Principles of Consolidation
AgeX’s consolidated financial statements include the accounts of its subsidiaries and certain research and development departments. AgeX consolidated its direct and indirect wholly owned or majority owned subsidiaries because AgeX has the ability to control their operating and financial decisions and policies through its ownership, and the noncontrolling interest is reflected as a separate element of stockholders’ deficit on AgeX’s consolidated balance sheets.
AgeX’s consolidated balance sheets at December 31, 2022 and 2021 do not include consolidated assets and liabilities of LifeMap Sciences, Inc. (LifeMap Sciences) due to the deconsolidation of LifeMap Sciences on March 15, 2021.
AgeX’s consolidated statements of operations for the year ended December 31, 2021 include LifeMap Sciences’ consolidated results for the period through March 15, 2021 rather than the day immediately preceding the deconsolidation due to the conversion of $1,761,296 of LifeMap Sciences’ indebtedness to AgeX into shares of LifeMap Sciences common stock on March 15, 2021, followed by the completion of the cash-out merger on the same day.
Long-Lived Intangible Assets
Long-lived intangible assets, consisting primarily of acquired patents, acquired in-process research and development with alternative future uses, patent applications, and licenses to use certain patents, are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets, generally over 10 years.
Impairment of Long-Lived Assets
Long-lived assets, including long-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, we evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment recognized is measured by the amount by which the carrying amount exceeds the estimated fair value of the assets. Through December 31, 2022, there have been no such impairment losses.
Accounting for Warrants
We determine the accounting classification of warrants we issue, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate us to settle the warrants or the underlying shares by paying cash or other assets, and warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet the liability classification under ASC 480-10, we assess the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, in order to conclude equity classification, we also assess whether the warrants are indexed to our common stock and whether the warrants are classified as equity under ASC 815-40 or other U.S. GAAP. After all such assessments, we conclude whether the warrants are classified as liability or equity. Liability classified warrants require fair value accounting at issuance and subsequent to initial issuance with all changes in fair value after the issuance date recorded in the statements of operations. Equity classified warrants only require fair value accounting at issuance with no changes recognized subsequent to the issuance date. We do not have any liability classified warrants as of any period presented. See Note 5, Related Party Transactions, to our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information regarding warrants.
Stock-Based Compensation
We recognize compensation expense related to employee stock option grants and other equity based awards, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation (ASC 718).
We use the Black-Scholes option pricing model for estimating the fair value of options granted under our the Incentive Plan. The fair value of each restricted stock or restricted stock unit grant, if any, is determined based on the value of the common stock granted or sold. We have elected to treat stock-based awards with time-based service conditions as a single award and recognize stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options can more reliably be measured than the fair value of services received. We record compensation expense based on the then-current fair values of the stock options at the grant date in accordance with ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which simplifies the accounting for non-employee share-based payment transactions. We adopted ASU 2018-07 on January 1, 2019. As we had one stock option grant issued to a nonemployee as of the adoption date and one additional stock option grant during 2019 to the same nonemployee, the application of the new standard did not have a material impact on our consolidated financial statements. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
The Black-Scholes option pricing model requires us to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield.
The fair value of the shares of common stock underlying the stock options has historically been determined by the AgeX Board. Because there was no public market for our common stock prior to November 29, 2018, the AgeX Board determined the fair value of the common stock at the time of the grant of options prior to that date by considering a number of objective and subjective factors including contemporaneous sales of our common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, amongst other factors. The fair value was determined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled Valuation of Privately Held Company Equity Securities Issued as Compensation. Since our common stock began publicly trading on the NYSE American, the fair value of our common stock underlying stock options has been valued based on prevailing market prices.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. We estimate the expected term of options granted based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because our common stock had no publicly traded history prior to November 29, 2018, for the years ended December 31, 2022 and 2021, we estimated the expected volatility using our own stock price volatility to the extent applicable or a combination of our stock price volatility and the stock price volatility of peer companies, for a period equal to the expected term of the options. The peer companies used include selected public companies within the biotechnology industry with comparable characteristics to AgeX, including similarity in size, lines of business, market capitalization, revenue and financial leverage.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of our stock options.
The dividend yield assumption is based on our history and expectation of dividend payouts. We have never declared or paid any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the consolidated statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes, and a tax deficiency arises when the compensation cost exceeds the tax deduction.
Stock-based compensation expense for the years ended December 31, 2022 and 2021 consists of stock-based compensation under the Incentive Plan, and stock-based compensation of AgeX’s subsidiaries that have their own stock option plans.
Our consolidated subsidiary ReCyte and our former subsidiary LifeMap Sciences had their own share-based compensation plans. For share-based compensation awards granted by those privately-held consolidated subsidiaries under their respective equity plans, we determined the fair value of the options granted under those plans using similar methodologies and assumptions we used for our stock options discussed above. None of our consolidated subsidiaries have granted stock options or other equity awards for the years ended December 31, 2022 and 2021.
Although the fair value of stock options is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and therefore the amount of compensation expense recognized in the consolidated financial statements.
Income Taxes
As of December 31, 2022, the deferred tax assets and liabilities presented in Note 9, Income Taxes, included elsewhere in this proxy statement/prospectus, including net operating loss carryforwards and research and development credits, represent the tax attributes of AgeX and its subsidiaries.
We account for income taxes in accordance with ASC 740, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and enacted rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. Our judgments, estimates and projections regarding future taxable income may change over time due to changes, among other factors, in market conditions, changes in tax laws, and tax planning strategies. If our assumptions and consequently our estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on our consolidated financial statements.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. We provided a reserve against our federal and California research and development credits generated. The carryforward amounts for these credits have been reported net of these reserves. Accordingly, no accrued interest and penalties related to unrecognized tax benefits have been recorded as of December 31, 2022 and 2021. We do not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months. We are currently unaware of any tax issues under review.
Revenue Recognition
We recognize revenue in a manner that depicts the transfer of control of a product or a service to a customer and reflects the amount of the consideration it expects to receive in exchange for such product or service. In doing so, we follow a five-step approach: (i) identify the contract with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) the customer obtains control of the product or service. We consider the terms of a contract and all relevant facts and circumstances when applying the revenue recognition standard. We apply the revenue recognition standard, including the use of any practical expedients, consistently to contracts with similar characteristics and in similar circumstances.
Subscription and advertisement revenues – LifeMap Sciences sold subscription-based products, including research databases and software tools, for biomedical, gene, and disease research. LifeMap Sciences sold these subscriptions primarily through the internet to biotech and pharmaceutical companies worldwide. LifeMap Sciences’ principal subscription product was the GeneCards® Suite, which includes the GeneCards® human gene database, and the MalaCards™ human disease database.
LifeMap Sciences’ performance obligations for subscriptions included a license of intellectual property related to its genetic information packages and premium genetic information tools. These licenses were deemed functional licenses that provide customers with a “right to access” to LifeMap Sciences’ intellectual property during the subscription period and, accordingly, revenue was recognized over a period of time, which was generally the subscription period. Payments were typically received at the beginning of a subscription period and revenue was recognized according to the type of subscription sold. For subscription contracts in which the subscription term commenced before a payment was due, LifeMap Sciences recorded an account receivable because the subscription was earned over time and billed the customer according to the contract terms. LifeMap Sciences deferred subscription revenues primarily represented subscriptions for which cash payment was received for the subscription term, but the subscription term was not completed as of the balance sheet date reported.
LifeMap Sciences licensed from third parties the databases and software it commercializes and had a contractual obligation to pay royalties to the licensor on subscriptions sold. These costs were included in operating loss from discontinued operations on the consolidated statements of operations when the cash was received and the royalty obligation was incurred, as the royalty payments do not qualify for capitalization of costs to fulfill a contract under ASC 340-40, Other Assets and Deferred Costs – Contracts with Customers.
LifeMap Sciences did not generate sufficient revenues to meet its operating expenses. On March 15, 2021, LifeMap Sciences was acquired by a third party in a cash-out merger. See Note 3, Disposition and Deconsolidation of LifeMap Sciences, to our consolidated financial statements included elsewhere in this proxy statement/prospectus.
Grant revenues – AgeX accounts for grants received to perform research and development services in accordance with ASC 730-20, Research and Development Arrangements. At the inception of the grant, we perform an assessment as to whether the grant is a liability or a contract to perform research and development services for others. If AgeX or a subsidiary receiving the grant is obligated to repay the grant funds to the grantor regardless of the outcome of the research and development activities, then AgeX is required to estimate and recognize that liability. Alternatively, if AgeX or a subsidiary receiving the grant is not required to repay, or if it is required to repay the grant funds only if the research and development activities are successful, then the grant agreement is accounted for as a contract to perform research and development services for others, in which case, grant revenue is recognized when the related research and development expenses are incurred.
In applying the provisions of Topic 606, we have determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. In the absence of applicable guidance under U.S. GAAP, our policy is to recognize grant revenue when the related costs are incurred, provided that the applicable conditions under the government contracts have been met. Only costs that are allowable under the grant award, certain government regulations and the National Institutes of Health’s supplemental policy and procedure manual may be claimed for reimbursement, and the reimbursements are subject to routine audits from governmental agencies from time to time. Costs incurred are recorded in research and development expenses on the accompanying consolidated statements of operations.
On April 8, 2020, we were awarded a grant of up to approximately $386,000 from the NIH. The NIH grant provides funding for continued development of our technologies for treating stroke. The grant funds were made available by the NIH as allowable expenses were incurred. We recognized no grant revenues for the year ended December 31, 2022 as we had expended the full amount available under this grant as of December 31, 2021.
ESI BIO research products – Through ESI BIO, our research product division, we market a number of products related to human pluripotent stem cells, including research-grade PSC lines and PSC lines produced under current good manufacturing practices or cGMP. We offer cells from PSC lines to customers under contracts that permit the customers to utilize PSC lines for the research, development, and commercialization of cell-based therapies or other products in defined fields of application. The compensation to us for providing the PSC line cells under such contracts may include up-front payments, milestone payments related to product development, regulatory matters, and commercialization, and the payment of royalties on sales of products developed from our PSC lines. Revenues from the sale of research products are included in other revenues.
Arrangements with multiple performance obligations – Future contracts with customers may include multiple performance obligations. For such arrangements, we will allocate revenue to each performance obligation based on its relative standalone selling price. We generally determine or estimate standalone selling prices based on the prices charged, or that would be charged, to customers for that product or service. As of and for the years ended December 31, 2022 and 2021, we did not have significant arrangements with multiple performance obligations.
Research and Development
Research and development expenses consist primarily of personnel costs and related benefits, stock-based compensation, amortization of intangible assets, and collaborative and contracted research and development fees. Research and development costs which have an alternative future use will be capitalized as intangible assets, and costs with no future benefit or alternative use will be expensed as incurred. Research and development expenses incurred and reimbursed by grants from third parties approximate the grant revenues recognized in the consolidated statements of operations.
General and Administrative
General and administrative expenses include employee and director compensation allocated to general and administrative expenses, consulting fees other than those paid for science-related consulting, license fees paid to third parties to acquire patents or licenses to use patents and other technology, professional fees to maintain patents and trademarks, minimum annual royalties stipulated under license and sublicense agreements with third parties, insurance costs allocated to general and administrative expenses, stock exchange-related costs, depreciation expense, marketing costs, legal and accounting costs, office rent, and other miscellaneous expenses which are allocated to general and administrative expense.
Impact of COVID-19 pandemic
The global outbreak of the coronavirus COVID-19, and the various attempts throughout the world to contain it, have created significant volatility, uncertainty and disruption. In response to government directives and guidelines, health care advisories and employee and other concerns, we have altered certain aspects of our operations. A number of our employees have had to work remotely from home and those on site have had to follow our social distance guidelines, which could impact their productivity. COVID-19 could also disrupt our operations due to absenteeism by infected or ill members of management or other employees, or absenteeism by members of management and other employees who cannot effectively work remotely but who elect not to come to work due to the illness affecting others in our office or in the laboratory facilities of third parties undertaking research work for us, or due to quarantines. COVID-19 illness could also impact members of the AgeX Board resulting in absenteeism from meetings of the directors or committees of directors and making it more difficult to convene the quorums of the full the AgeX Board or its committees needed to conduct meetings for the management of our affairs.
The completion of work at UCI under our Sponsored Research Agreement for the derivation of neural stem cells was slowed by COVID-19 safety procedures. We could experience similar delays under future out-sourced research arrangements.
The full extent to which the COVID-19 pandemic and the various responses might impact our business, operations and financial results in the future will depend on numerous evolving factors that we will not be able to accurately predict, including: the duration and scope of the pandemic; governmental, business and individuals’ actions that have been and continue to be taken in response to the pandemic; and the availability, effectiveness, and cost to access to current or future COVID-19 tests, vaccines and therapies. Due to the uncertain scope and duration of the COVID-19 pandemic and uncertain timing of any recovery or normalization, we are currently unable to estimate the resulting impacts on our operations and financial results. We will continue to actively monitor the issues raised by the COVID-19 pandemic and may take further actions that alter our operations, as may be required by federal, state, local or foreign authorities, or that we determine are in the best interests of our employees, any customers and stockholders. It is not clear what the potential effects any such alterations or modifications may have on our business, including the effects on our financial results.
Financial Operations Overview
Up until our disposition of LifeMap Sciences, our revenues were principally derived from subscription and advertising revenues from LifeMap Sciences’ online databases based upon applicable subscription or advertising periods. LifeMap Sciences was acquired by a third party through a cash-out merger during March 2021 and, as a result of that merger transaction, we no longer own interest in LifeMap Sciences and will no longer recognize any post-merger revenues attributable to LifeMap Sciences’ business. We do not have any therapeutic products approved for sale and have generated insignificant revenues from commercialized product sales, and we do not expect to generate any significant revenues from product sales for the foreseeable future.
Our operating expenses consist of research and development expenses primarily from our preclinical programs and general and administrative expenses. Since the layoffs of mostly research personnel in April 2020, research and development work has been scaled back and contracted out to third party service providers within the imposed budgetary constraints under our Secured Convertible Facility Agreement, dated March 30, 2020, between AgeX and Juvenescence (the 2020 Loan Agreement), our 2022 Secured Note and our 2023 Secured Note. As of June 30, 2023, AgeX had approximately $8,000,000, $17,400,000 and $10,400,000 outstanding under the 2020 Loan Agreement, the 2022 Secured Note and the 2023 Secured Note, respectively. Accordingly, the historical amounts of expense presented and discussed in this proxy statement/prospectus/information statement are likely not going to be indicative of expenses during future periods. See “—Liquidity and Capital Resources” for a discussion of our available capital resources and our need for financing.
Results of Operations
Comparison of Three and Six Months Ended June 30, 2023 and 2022
Revenues and Cost of Sales
During the three and six months ended June 30, 2023, we recognized revenues of $9,000 and $19,000, respectively, from the sale of research products. During the three and six months ended June 30, 2022, we recognized revenues of $12,000 and $17,000, respectively from the sale of research products.
During the three and six months ended June 30, 2023, we recognized cost of sales of $5,000 and $6,000, respectively, from the sale of research products. During the three and six months ended June 30, 2022, we recognized cost of sales of $6,000 and $7,000, respectively from the sale of research products.
Operating Expenses
We continue to maintain a minimal workforce since our May 1, 2020 reduction in force which resulted in the layoff of most of our research and development personnel and certain administrative personnel. The following table shows our consolidated operating expenses for the periods presented (in thousands).
| | | Three Months Ended June 30, | | | $ Increase/ | | | % Increase/ | |
| | | 2023 | | | 2022 | | | (Decrease) | | | (Decrease) | |
| Research and development expenses | | $ | 160 | | | $ | 259 | | | $ | (99 | ) | | | (38.2 | )% |
| General and administrative expenses | | | 1,730 | | | | 1,338 | | | | 392 | | | | 29.3 | % |
| | | Six Months Ended June 30, | | | $ Increase/ | | | % Increase/ | |
| | | 2023 | | | 2022 | | | (Decrease) | | | (Decrease) | |
| Research and development expenses | | $ | 334 | | | $ | 655 | | | $ | (321 | ) | | | (49.0 | )% |
| General and administrative expenses | | | 3,723 | | | | 2,998 | | | | 725 | | | | 24.2 | % |
Research and development expenses
Research and development expenses for the three months ended June 30, 2023 decreased by approximately $0.1 million to $0.16 million from $0.26 million during the same period in 2022. The net decrease was primarily attributable to a reduction of $0.1 million in outside research and services allocable to research and development expenses.
Research and development expenses for the six months ended June 30, 2023 decreased by approximately $0.32 million to $0.33 million from $0.66 million during the same period in 2022. The net decrease was primarily attributable to reductions of $0.2 million in outside research and services allocable to research and development expenses and $0.1 million in salaries to employees and related payroll expenses, including noncash stock-based compensation expense, and general laboratory supplies and expenses.
General and administrative expenses
General and administrative expenses for the three months ended June 30, 2023 increased by $0.4 million to $1.7 million as compared to $1.3 million during the same period in 2022. The net increase is attributable to increases of $0.5 million in professional fees for legal services, consulting expenses incurred in connection with due diligence, and other expenses related to the possible merger between AgeX and Serina, $0.1 million in professional fees for accounting and tax services, and reversal of certain withholding taxes that were reversed during the period in prior year, and $0.1 million in investor relations related expenses and insurance expense. These increases were offset to some extent by a $0.2 million decrease in noncash stock-based compensation to employees, consultants and directors, and $0.1 million in patent and license maintenance related fees.
General and administrative expenses for the six months ended June 30, 2023 increased by $0.7 million to $3.7 million as compared to $3 million during the same period in 2022. The net increase is attributable to increases of $0.9 million in professional fees for legal services, consulting expenses incurred in connection with due diligence and other expenses related to the possible merger between AgeX and Serina, $0.1 million in professional fees for other non-recurring legal services, and $0.1 million in investor relations related expenses and insurance expense. These increases were offset to some extent by a $0.3 million decrease in noncash stock-based compensation to employees, consultants and directors, and $0.1 million in patent and license maintenance related fees.
See Notes 4, Convertible Note Receivable and 5, Related Party Transactions, to our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement for further discussion about the Merger.
General and administrative expenses include employee and director compensation allocated to general and administrative expenses, consulting fees other than those paid for science-related consulting, facilities and equipment rent and maintenance related expenses, insurance costs allocated to general and administrative expenses, stock exchange-related costs, depreciation expense, marketing costs, legal and accounting costs, and other miscellaneous expenses which are allocated to general and administrative expense.
Other expense, net
Total other expense, net for the three months ended June 30, 2023 consists primarily of $1 million amortization of deferred debt issuance costs to interest expense and other debt related expenses included in interest expense offset by $0.2 million net interest income primarily earned from a $10,000,000 loan under the terms of our Convertible Promissory Note to Serina (the AgeX-Serina Note). Total other expense, net for the three months ended June 30, 2022 consists primarily of $0.9 million amortization of deferred debt issuance costs to interest expense and other debt related expenses included in interest expense and $0.2 million unrealized loss on change in fair value of warrants issued to Juvenescence in connection with borrowings under the 2022 Secured Note.
Total other expense, net for the six months ended June 30, 2023 consists primarily of $2.1 million amortization of deferred debt issuance costs to interest expense and other debt related expenses included in interest expense offset by $0.2 million net interest income primarily earned from the AgeX-Serina Note. Total other expense, net for the six months ended June 30, 2022 consists primarily of $1.4 million amortization of deferred debt issuance costs to interest expense and other debt related expenses included in interest expense and $0.3 million unrealized loss on change in fair value of warrants issued to Juvenescence in connection with borrowings under the 2022 Secured Note.
See Notes 4, Convertible Note Receivable, 5, Related Party Transactions, and 6, Warrant Liability, to our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreement with Serina, loan agreements with Juvenescence, and liability classified warrants.
Income taxes
For the three and six months ended June, 30, 2023 and 2022, AgeX experienced a loss; therefore, no income tax provision was recorded for the three and six months ended June, 30, 2023 and 2022.
Due to losses incurred for all periods presented, we did not record a provision or benefit for income taxes. A valuation allowance will be provided when it is more likely than not that some portion of the deferred tax assets will not be realized. We established a full valuation allowance for all deferred tax assets for the periods presented due to the uncertainty of realizing future tax benefits from our net operating loss carryforwards and other deferred tax assets.
For years beginning after December 31, 2021, the Tax Cuts and Jobs Act of 2017 (the 2017 Tax Act) requires companies to capitalize their research and experimentation expenditures as defined under Section 174 and amortize those expenditures on a straight-line bases over a period of 5 years. Previously AgeX was able to immediately expense such costs. It is possible that Congress will defer or eliminate the ultimate implementation of this provision. AgeX has sufficient federal net operation loss carryforwards to offset the impact of this provision.
Comparison of Years Ended December 31, 2022 and 2021
The following comparisons exclude the impact of the operations of LifeMap Sciences which have been presented in our consolidated financial results as discontinued operations. See Note 3, Disposition and Deconsolidation of LifeMap Sciences, to our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for a discussion of discontinued operations.
Revenues and Cost of Sales
The amounts in the table below show our consolidated revenues by source and cost of sales for the years ended December 31, 2022 and 2021 (in thousands).
| | | Year Ended December 31, | | | $ +Increase/ | | | % +Increase/ | |
| | | 2022 | | | 2021 | | | -Decrease | | | -Decrease | |
| Grant revenues | | $ | - | | | $ | 104 | | | $ | -104 | | | $ | -100.0 | % |
| Other revenues | | | 34 | | | | 40 | | | | -6 | | | | -15.0 | % |
| Total revenues | | | 34 | | | | 144 | | | | -110 | | | | -76.4 | % |
| Cost of sales | | | (13 | ) | | | (19 | ) | | | -6 | | | | -31.6 | % |
| Gross profit | | $ | 21 | | | $ | 125 | | | $ | -104 | | | | -83.2 | % |
During the year ended December 31, 2021, we recognized income of approximately $104,000 from a grant awarded by the NIH. During the year ended December 31, 2022, we did not recognize any grant revenues as we had expended the full amount available under the NIH grant as of December 31, 2021.
During the years ended December 31, 2022 and 2021, we recognized $34,000 and $40,000, respectively, from the sale of research products. Revenues from the sale of research products are included in other revenues.
Operating Expenses
We have maintained a minimal work force since the May 1, 2020 reduction in force which resulted in the layoff of most of our research and development personnel and certain administrative personnel. The following table shows our consolidated operating expenses for the years ended December 31, 2022 and 2021 (in thousands).
| | | Year Ended December 31, | | | $ +Increase/ | | | % +Increase/ | |
| | | 2022 | | | 2021 | | | -Decrease | | | -Decrease | |
| Research and development expenses | | $ | 1,025 | | | $ | 1,456 | | | $ | -431 | | | | -29.6 | % |
| General and administrative expenses | | | 5,971 | | | | 6,708 | | | | -737 | | | | -11.0 | % |
Research and Development Expenses
Research and development expenses for the year ended December 31, 2022 decreased by more than $0.4 million to $1.0 million as compared to approximately $1.5 million in 2021. The net decrease was primarily attributable to decreases of: $0.2 million in scientific consulting; $0.1 million in outside research and services expenses; $0.1 million in fees incurred related to sponsored research agreement with a university; and $0.1 million in cash and noncash stock-based compensation expense to employees.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2022 decreased by $0.7 million to $6.0 million as compared to $6.7 million in 2021. The net decrease is attributable to decreases of $0.4 million in consulting expenses, $0.2 million in professional fees for legal services, $0.2 million in annual minimum royalties due under license agreements and patent and license maintenance related fees and $0.1 million in noncash stock-based compensation expense to directors. These decreases were offset to some extent by increases of $0.1 million in insurance expense and $0.1 million in professional fees for tax and accounting services.
Other Expense, Net
Other expense, net in 2022 consists primarily of $3.3 million of amortization of deferred debt issuance costs on loans from Juvenescence to interest expense, and $0.2 million change in fair value of warrants issued to Juvenescence in connection with borrowings under the 2022 Secured Note. Other expense, net in 2021 consists primarily of $1.1 million of amortization of deferred debt issuance costs on loans from Juvenescence to interest expense offset by approximately $437,000 of gain recognized upon forgiveness of our loan in the amount of $432,952 from a bank under the Paycheck Protection Program (PPP Loan) on February 19, 2021. See Notes 5, Related Party Transactions, and 6, Warrant Liability, to our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreements with Juvenescence and liability classified warrants.
Income Taxes
Beginning in 2018, the 2017 Tax Act subjects a U.S. stockholder to tax on Global Intangible Low Tax Income “GILTI” earned by certain foreign subsidiaries. In general, GILTI is the excess of a U.S. shareholder’s total net foreign income over a deemed return on tangible assets. The provision further allows a deduction of 50% of GILTI, however this deduction is limited to the company’s pre-GILTI U.S. income. For the year ended December 31, 2021, AgeX’s foreign entity operated at a loss; therefore, no GILTI was included in income. For the year ended December 31, 2022, there was no income or loss related to foreign activities as the entity deconsolidated from AgeX on March 15, 2021. Current interpretations under ASC 740 state that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense. We have elected to account for GILTI as a current period expense when incurred.
For the year ended December 31, 2022, we experienced a domestic loss from continuing operations therefore, no income tax provision was recorded for the year ended December 31, 2022.
As of December 31, 2022, we had net operating loss carryforwards of approximately $52.8 million for U.S. federal income tax purposes. In general, NOLs and other tax credit carryforwards generated by legal entities in a consolidated federal tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the consolidated federal tax group. However, under the Tax Matters Agreement, dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX (the Tax Matters Agreement), any use of a member’s NOLs and other tax credit carryforwards by the other member is subject to reimbursement by the benefiting member for the actual tax benefit realized.
As of December 31, 2022, we had net operating losses of approximately $14.9 million for California purposes.
Federal net operating losses generated on or prior to December 31, 2017, expire in varying amounts between 2028 and 2037, while federal net operating losses generated after December 31, 2017, carryforward indefinitely. The state net operating losses expire in varying amounts between 2028 and 2042.
As of December 31, 2022, we had research and development tax credit carryforwards for federal and state tax purposes of $0.9 million and $0.6 million, respectively. The federal tax credits expire between 2028 and 2042, while the state tax credits have no expiration date.
As of December 31, 2022, we had capital loss carryforwards for federal and state tax purposes of $12.4 million and $5.9 million, respectively. The federal and California capital loss carryforwards will expire in 2026.
A valuation allowance is provided when it is more likely than not that all or some portion of the deferred tax assets will not be realized. We established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
Liquidity and Capital Resources
Operating Losses and Going Concern Considerations
We have incurred operating losses and negative cash flows since inception and had an accumulated deficit of $122.2 million as of June 30, 2023 and $116.2 million as of December 31, 2022. We expect to continue to incur operating losses and negative cash flows. We have been funding our operations with loans from Juvenescence. As of October 31, 2023, we had drawn down in full the remaining credit available to us from Juvenescence pursuant to the 2022 Secured Note, and we estimate that our cash on hand as of that date will be sufficient to fund our operations through the end of November 2023. However, on November 9, 2023, we entered into the 2022 Secured Note Fifth Amendment that increases the amount of the line of credit available to us by $4,400,000 under the 2022 Secured Note, subject to the terms of the 2022 Secured Note as so amended and Juvenescence’s discretion to approve and fund each of our future draws of that additional amount of credit.
We have made certain adjustments to our operating plans and budgets to reduce our projected cash expenditures in order to extend the period over which we can continue our operations with our available cash resources. These adjustments entailed down-sizing of our leased office space effective January 1, 2021, a staff force reduction during 2020, primarily impacting research and development personnel, and the elimination of our leased laboratory facility. These down-sizing adjustments to our operations will require the deferral of certain work on the development of our product candidates and technologies. However, notwithstanding those adjustments, based on our most recent projected cash flows, our cash and cash equivalents and the amount of the 2022 Secured Note line of credit available to us from Juvenescence, including the $4,400,000 increase in the line of credit, and the proceeds we may receive from the sale of additional shares of our common stock in “at-the-market” transactions through our sales agreement (the Sales Agreement) with Chardan as a sales agent, would not be sufficient to satisfy our anticipated operating and other funding requirements for the next twelve months from the date of filing of our Quarterly Report on Form 10-Q for the six months ended June 30, 2023. These factors raise substantial doubt regarding our ability to continue as a going concern. See Note 5, Related Party Transactions, to our condensed consolidated interim financial statements and our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreements with Juvenescence. We will need to raise additional capital in the near term to be able to meet our operating expenses.
As of June 30, 2023, we had borrowed a total of approximately $35,800,000 (including accrued origination fees) from Juvenescence pursuant to the 2020 Loan Agreement, the 2022 Secured Note and the 2023 Secured Note. The loans from Juvenescence that remain outstanding prohibit us and our subsidiaries ReCyte Therapeutics and Reverse Bio from borrowing funds from other lenders or engaging in certain other transactions without the consent of Juvenescence unless we repay all amounts owed to Juvenescence. AgeX and three of its subsidiaries have granted Juvenescence a security interest and lien on substantially all of their assets to secure AgeX’s obligations under the 2022 Secured Note, and AgeX has granted Juvenescence a security interest and lien on substantially all of AgeX’s assets to secure AgeX’s obligations for loans under the 2023 Secured Note. On July 21, 2023, AgeX and Juvenescence entered into an Exchange Agreement (the 2023 Exchange Agreement) pursuant to which AgeX issued to Juvenescence 211,600 shares of a newly authorized AgeX Series A preferred stock and 148,400 shares of a newly authorized AgeX Series B preferred stock in exchange for the cancellation of a total of $36,000,000 of indebtedness consisting of the outstanding principal amount of certain loans made by Juvenescence to AgeX and loan origination fees accrued with respect to those loans.
The outstanding amounts under the 2022 Secured Note and the 2023 Secured Note will become due and payable upon maturity on February 14, 2024 and March 13, 2026, respectively. These factors and the impact of potential dilution through the issuance of shares of our common stock upon the conversion of the Juvenescence loans or shares of AgeX Series A preferred stock and Series B preferred stock into AgeX common stock and the exercise of warrants issued to Juvenescence in connection with the Juvenescence loans could make AgeX less attractive to new equity investors and could impair our ability to finance our operations or the operations of our subsidiaries unless Juvenescence agrees, in its discretion, to lend us additional funds.
Through the Sales Agreement with Chardan, we may sell during any 12 month period an amount of common stock in “at-the-market” transactions not to exceed one-third of the aggregate market value of our common stock held by stockholders who would not be considered “affiliates” of AgeX, determined in accordance with applicable SEC rules. We do not have any other committed sources of funds for additional financing.
Although we have been able to reduce our operating expenses by eliminating internal research and development activities and focusing instead on out-sourcing research and development and seeking licensing arrangements for our technologies, this approach has also made it more difficult for us to make progress in developing our target product candidates and technologies, which in turn may make it more difficult for us to raise capital.
The unavailability or inadequacy of financing to meet future capital needs could force us to modify, curtail, delay, or suspend some or all aspects of planned operations.
To the extent that we are able to raise additional capital through the sale of AgeX equity or convertible debt securities or the sale of equity or convertible debt securities of any of our subsidiaries, the ownership interest of our present stockholders will be diluted, and the terms of any securities we or our subsidiaries issue may include liquidation or other preferences that adversely affect the rights of our common stockholders. Additional debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, and may involve the issuance of convertible debt or stock purchase warrants that would dilute the equity interests of our stockholders. If we raise funds through additional strategic partnerships or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
Summary of Cash Flows
The following table summarizes the major sources and uses of cash for the periods set forth below (in thousands):
| | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | |
| Net cash provided by (used in): | | | | | | | | |
| Operating activities | | $ | (3,884 | ) | | $ | (3,382 | ) |
| Investing activities | | | (10,000 | ) | | | - | |
| Financing activities | | | 13,500 | | | | 3,500 | |
| Net change in cash, cash equivalents, and restricted cash | | $ | (384 | ) | | $ | 118 | |
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| Net cash provided by (used in): | | | | | | | | |
| Operating activities from continuing operations | | $ | (5,939 | ) | | $ | (7,765 | ) |
| Operating activities from discontinued operations | | | - | | | | (90 | ) |
| Investing activities from continuing operations | | | - | | | | 716 | |
| Investing activities from discontinued operations | | | - | | | | (50 | ) |
| Financing activities from continuing operations | | | 6,000 | | | | 7,496 | |
| Financing activities from discontinued operations | | | - | | | | (250 | ) |
| Net change in cash, cash equivalents, and restricted cash | | $ | 61 | | | $ | 57 | |
Operating Activities
Net loss attributable to us for the six months ended June 30, 2023 amounted to $6 million. Net cash used in operating activities during this period amounted to $3.9 million. The $2.1 million difference between the net loss attributable to us and net cash used in operating activities during the six months ended June 30, 2023 was primarily attributable to $2.1 million in amortization of intangible assets and deferred debt issuance costs, $0.7 million in prepaid expenses and other current assets, $0.2 million in related party payables, and $0.1 million in stock-based compensation expense. The impact of these non-cash items was offset to some extent by a $0.7 million payment of a financed insurance premium liability, $0.2 million in accrued interest on convertible note receivable, and $0.1 million net change in working capital from operating activities. See Notes 4, Convertible Note Receivable, and 5, Related Party Transactions, to our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreement with Serina and our loan agreements with Juvenescence.
Net loss attributable to us for the year ended December 31, 2022 amounted to $10.5 million. Net cash used in operating activities from continuing operations during this period amounted to $5.9 million. The difference between the net loss attributable to us and net cash used in operating activities from continuing operations during the year ended December 31, 2022 was primarily attributable to $3.3 million in amortization of intangible assets and deferred debt issuance costs, $1.1 million net change in working capital from operating activities, $0.8 million in stock-based compensation expense, $0.2 million loss on change in fair value of warrants, and more than $0.2 million in related party payables. These amounts were offset to some extent by $1.0 million of financed insurance premiums and $0.1 million in net loss attributable to noncontrolling interest. See Notes 5, Related Party Transactions, and 6, Warrant Liability, to our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreements with Juvenescence and liability classified warrants.
Investing Activities
During the six months ended June 30, 2023, net cash used by investing activities is entirely comprised of the $10 million loan made to Serina. See Note 4, Convertible Note Receivable, to our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about the AgeX-Serina Note.
During the six months ended June 30, 2023 and the year ended December 31, 2022, AgeX had no investing activities.
Financing Activities
During the six months ended June 30, 2023, net cash provided by financing activities amounted to $13.5 million which was entirely attributable to amounts drawn under the credit facilities from Juvenescence. See Notes 5, Related Party Transactions, and 12, Subsequent Events, to our condensed consolidated interim financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreements with Juvenescence.
During the year ended December 31, 2022, net cash provided by financing activities from continuing operations amounted to $6.0 million, which was attributable to the $0.5 million drawn against the final remaining funds available under the 2020 Loan Agreement and the $12.7 million drawn against the $13.2 million made available under the 2022 Secured Note entered into with Juvenescence in February 2022 of which $7.2 million was used to refinance the $7.0 million outstanding principal amount of the loans and a $160,000 origination fee due under the Loan Facility Agreement, dated August 13, 2019, between AgeX and Juvenescence (as amended, the 2019 Loan Agreement). See Note 5, Related Party Transactions, to our consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information about our loan agreements with Juvenescence.
Off-Balance Sheet Arrangements
As of December 31, 2022, we did not have any off-balance sheet arrangements, as defined in Item 303(a) (4) (ii) of SEC Regulation S-K.
SERINA MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of Serina’s financial condition and results of operations together with the section titled “Unaudited Pro Forma Condensed Combined Financial Information” and Serina’s audited consolidated financial statements and related notes for the period ended December 31, 2022, along with our unaudited consolidated financial statements and related notes for the period ended June 30, 2023 included elsewhere in this proxy statement/prospectus/information statement. Some of the information contained in this discussion and analysis or set forth elsewhere in this proxy statement/prospectus/information statement, including information with respect to our plans and strategy for our business and related financing, includes forward looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the section titled “Risk Factors” our actual results could differ materially from the results described in or implied by the forward looking statements contained in the following discussion and analysis. You should carefully read the section titled “Risk Factors” to gain an understanding of the important factors that could cause actual results to differ materially from our forward looking statements. Please also see the section titled “Cautionary Note Regarding Forward Looking Statements.” References to “the Company,” “we,” “us” and “our” in this section titled “Serina Management’s Discussion and Analysis of Financial Condition and Results of Operations” refer to Serina.
Overview
Serina is a clinical-stage biotechnology company developing a pipeline of wholly owned drug product candidates to treat neurological diseases and pain. Serina’s POZ drug delivery technology is designed to enable certain existing drugs and novel drug candidates to be modified in a way that may provide an increase in efficacy and safety of the resulting polymeric drug conjugate. Serina’s proprietary POZ technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection. The therapeutic agents in Serina’s product candidates are typically well-understood and marketed drugs that are effective but are limited by pharmacokinetic profiles that can include toxicity, side effects and short half-life. Serina believes that by using POZ technology, drugs with narrow therapeutic windows can be designed to maintain more desirable and stable levels in the blood.
Our operations through June 30, 2023, have been financed primarily by aggregate net proceeds of $46.91 million from the issuance of convertible preferred stock and convertible notes. Since our inception in 2006, we have had significant annual operating losses. Our operating loss was $1.91 million, $0.92 million, $2.27 million and $1.63 million for the six months ended June 30, 2023 and 2022 and the years ended December 31, 2022 and 2021, respectively. As of June 30, 2023, we had an accumulated deficit of $36.0 million and $8.7 million in cash and cash equivalents.
Our losses from operations, negative operating cash flows and accumulated deficit, as well as the additional capital needed to fund operations within one year of the unaudited consolidated financial statement issuance date, raise substantial doubt about our ability to continue as a going concern. We expect to incur substantial expenditures in the foreseeable future for the development of our product candidates and will require additional financing to continue this development. Our unaudited consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement have been prepared on a basis that assumes that we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. Our unaudited consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our accounts payable and accrued expenses. We expect to continue to incur net losses for the foreseeable future, and we expect our research and development expenses, general and administrative expenses, and capital expenditures will continue to increase. In particular, we expect our expenses to increase as we continue our development of, and seek regulatory approvals for, our product candidates, as well as hire additional personnel, pay fees to outside consultants, attorneys, and accountants, and, if the Merger is consummated, incur other increased costs associated with being a public company. In addition, if and when we seek and obtain regulatory approval to commercialize any product candidate, we will also incur increased expenses in connection with commercialization and marketing of any such product. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the timing of our clinical trials and our expenditures on other research and development activities. We anticipate that our expenses will increase significantly in connection with our ongoing activities as we:
| | ● | advance our lead product candidate, SER 252 into Phase I clinical trials; |
| | ● | advance our other product candidates; |
| | ● | advance our preclinical programs to clinical trials; |
| | ● | further invest in our pipeline; |
| | ● | seek regulatory approval for our investigational medicines; |
| | ● | maintain, expand, protect, and defend our intellectual property portfolio; |
| | ● | secure facilities to support continued growth in our research, development, and commercialization efforts; |
| | ● | increase our headcount to support our development efforts and to expand our clinical development team. |
We believe that our cash on hand, along with the approximately $15 million of cash proceeds expected to be received from Juvenescence through the exercise of all the post-merger warrants it holds pursuant to the terms of the Side Letter if the Merger is consummated, will enable us to fund our operations through calendar year 2025 based on our current plan. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. If the Merger is not consummated, there is substantial doubt about the Company’s ability to continue as a going concern. The financial statements are prepared using GAAP as applicable to a going concern. Assuming the Merger is consummated, to finance our operations beyond calendar 2025, we will need to raise additional capital, which cannot be assured. We have not had any products approved for sale. We do not expect to generate any product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution. As a result, until such time, if ever, that we can generate substantial product revenue, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, including collaborations, licenses, or similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed or on favorable terms, if at all. Any failure to raise capital as and when needed could have a negative impact on our financial condition and on our ability to pursue our business plans and strategies, including our research and development activities. If we are unable to raise capital, we will need to delay, reduce, or terminate planned activities to reduce costs.
The COVID-19 pandemic continues to evolve. While it appears its most severe effects have subsided, COVID-19 could reemerge or new public health threats could appear. The future impact of the COVID-19 pandemic or a similar health disruption is highly uncertain and subject to change. We cannot predict the full extent of potential delays or impacts on our business, our clinical trials, health care systems, third parties with whom we engage or the global economy as a whole, but if we or any of the third parties with whom we engage, including personnel at contract manufacturing operations, or CMOs, and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timeline presently planned could be materially and adversely impacted. While we have been able to continue to execute our overall business plan, some of our business activities have taken longer to complete than anticipated. Overall, we recognize the challenges of product development during a pandemic, and we will continue to closely monitor events as they develop and plan for alternative and mitigating measures if needed.
On August 29, 2023, Serina entered into the Merger Agreement with AgeX. The transaction is expected to close in the first quarter of 2024.
Prior to the execution of the Merger Agreement, Serina issued a convertible promissory note (or the AgeX-Serina Note) to AgeX in the principal amount of $10 million as described elsewhere in this proxy statement/prospectus/information statement. Immediately prior to completion of the merger, the AgeX-Serina Note will be converted into Serina capital stock as a capital contribution. It is expected that the funds provided by the AgeX-Serina Note, together with the approximately additional $15 million of proceeds from the Juvenescence through the post-merger warrant cash exercises, are expected to provide working capital for the combined company to help fund operations through calendar year 2025.
Recent Developments
On August 29, 2023, Serina entered into the Merger Agreement with AgeX and Merger Sub, pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Serina will merge with and into Merger Sub, with Serina surviving as a wholly owned subsidiary of AgeX. The combined company will be renamed “Serina Therapeutics, Inc.”. For additional information, see the sections titled “The Merger” and “The Merger Agreement” beginning on pages 134 and 153 of this proxy statement/prospectus/information statement, respectively.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant.
Concurrently with the execution of the Merger Agreement, Serina, AgeX and Juvenescence entered into the Side Letter, which will become effective immediately prior to the closing of the Merger. The Side Letter provides, among other things, that (i) immediately prior to the Effective Time, Juvenescence will cancel all out of the money AgeX warrants held by Juvenescence; (ii) Juvenescence will exercise all of the post-merger warrants it holds to provide the combined company approximately an additional $15 million in capital according to the following schedule: (x) at least one-third on or before May 31, 2024, (y) at least one-third on or before November 30, 2024, and (z) at least one-third on or before June 30, 2025; (iii) Juvenescence will not sell any shares of AgeX Series A preferred stock or AgeX Series B preferred stock and will take all actions necessary to effect the AgeX preferred stock conversion, whereby all of such preferred stock will be converted into AgeX common stock prior to the reverse stock split; (iv) Juvenescence will release all security interests, guarantees, pledges, assignments and other forms of collateral that it may have in AgeX’s assets pursuant to the terms of certain loans to AgeX; and (v) Juvenescence will consent to a newly formed subsidiary of AgeX assuming AgeX’s obligations with respect to loans agreements and promissory notes governing loans payable to Juvenescence, including obligations for amounts currently owed and future advances of loan funds, and Juvenescence shall release AgeX from those loan obligations. For additional information, see the sections titled “Agreements Related to the Merger—Side Letter” beginning on page 179 of this proxy statement/prospectus/information statement.
In October 2023, we entered into a non-exclusive license agreement with Pfizer, Inc. to use our POZ polymer technology for use in lipid nanoparticle drug delivery formulations. The agreement grants Pfizer non-exclusive rights to certain intellectual property, know-how, and proprietary technologies. Under the terms of the agreement, Pfizer is authorized to develop, manufacture, market, and commercialize products incorporating the licensed technology with respect to a specific POZ polymer structure in one field. The agreement outlines the protection and enforcement of intellectual property rights related to the licensed technology. Pfizer is obligated to use commercially reasonable diligent efforts to develop and commercialize licensed products, and to use such efforts to accomplish specified development and commercial objectives. The agreement includes a one-time upfront payment in the single-digit millions, milestone payments payable to Serina upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology in accordance with the terms outlined in the license agreement.
Components of Operating Results
Operating Expenses
Our operating expenses since inception have consisted primarily of research and development expenses and general and administrative costs.
Research and Development
Our research and development expenses consist primarily of costs incurred for the development of our product candidates and our drug discovery efforts, which include:
● personnel costs, which include salaries, benefits and equity-based compensation expense;
● expenses incurred under agreements with consultants and contract organizations that conduct research and development activities on our behalf;
● costs related to production of preclinical and clinical materials, including fees paid to contract manufacturers;
● laboratory and vendor expenses related to the execution of preclinical studies and planned clinical trials; and
● laboratory supplies and equipment used for internal research and development activities.
We expense all research and development costs in the periods in which they are incurred. Costs for certain research and development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and service providers.
Our research and development expenses are not currently tracked on a program-by-program basis. We use our personnel and infrastructure resources across multiple research and development programs directed toward identifying and developing product candidates. Substantially all our research and development costs in the six months ended June 30, 2023, and the years ended December 31, 2022, and 2021, were incurred on the development of our preclinical candidates and advancing research on our POZ lipid technology.
We expect our research and development expenses to increase substantially for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates, including investments in conducting clinical trials, manufacturing and otherwise advancing our programs. The process of conducting the clinical research necessary to obtain regulatory approval is costly and time consuming, and the successful development of our product candidates is highly uncertain.
Because of the numerous risks and uncertainties associated with product development and the current stage of development of our product candidates and programs, we cannot reasonably estimate or know the nature, timing, and estimated costs necessary to complete the remainder of the development of our product candidates or programs. We are also unable to predict if, when, or to what extent we will obtain approval and generate revenues from the commercialization and sale of any of our product candidates. The duration, costs and timing of preclinical studies and clinical trials and development of our product candidates will depend on a variety of factors, including:
| ● | successful completion of preclinical studies and initiation of clinical trials for future product candidates; |
| ● | successful enrollment and completion of clinical trials for our current product candidates; |
| ● | data from our clinical programs that support an acceptable risk benefit profile of our product candidates in the intended patient populations; acceptance by the U.S. Food and Drug Administration, or FDA, or other applicable regulatory agencies of the Investigational New Drug, or IND, applications, clinical trial applications and/or other regulatory filings for SER 252 and other product candidates. |
| ● | expansion and maintenance of a workforce of experienced scientists and others to continue to develop our product candidates; |
| ● | successful application for and receipt of marketing approvals from applicable regulatory authorities; |
| ● | obtainment and maintenance of intellectual property protection and regulatory exclusivity for our product candidates; |
| ● | making of arrangements with contract manufacturing organizations for, or establishment of, commercial manufacturing capabilities; |
| ● | establishment of sales, marketing and distribution capabilities and successful launch of commercial sales of our product candidates, if and when approved, whether alone or in collaboration with others; |
| ● | acceptance of our product candidates, if and when approved, by patients, the medical community and third party payors; |
| ● | effective competition with other therapies; |
| ● | obtainment and maintenance of coverage, adequate pricing, and adequate reimbursement from third party payors, including government payors; |
| ● | maintenance, enforcement, defense, and protection of our rights in our intellectual property portfolio; |
| ● | avoidance of infringement, misappropriation, or other violations with respect to others’ intellectual property or proprietary rights; and |
| ● | maintenance of a continued acceptable safety profile of our products following receipt of any marketing approvals. |
We may never succeed in achieving regulatory approval for any of our product candidates. We may obtain unexpected results from our preclinical studies and clinical trials. We may elect to discontinue, delay, or modify clinical trials of some product candidates or focus on others. A change in the outcome of any of these factors could mean a significant change in the costs and timing associated with the development of our current and future preclinical and clinical product candidates. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of clinical development, or if we experience significant delays in execution of or enrollment in any of our preclinical studies or clinical trials, we could be required to expend significant additional financial resources and time on the completion of preclinical and clinical development.
Research and development activities account for a significant portion of our operating expenses. We expect our research and development expenses to increase for the foreseeable future as we continue to implement our business strategy, which includes advancing SER 252 and our other product candidates through clinical development, expanding our research and development efforts, including hiring additional personnel to support our research and development efforts, and seeking regulatory approvals for our product candidates that successfully complete clinical trials. In addition, product candidates in later stages of clinical development generally incur higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later stage clinical trials. As a result, we expect our research and development expenses to increase as our product candidates advance into later stages of clinical development. However, we do not believe that it is possible at this time to accurately project total program specific expenses through commercialization. There are numerous factors associated with the successful commercialization of any of our product candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development.
General and Administrative Expenses
Our general and administrative expenses consist primarily of personnel costs, including equity-based compensation, and other expenses for outside professional services, including legal, recruiting, audit and accounting and facility related costs not otherwise included in research and development expenses. Personnel costs consist of salaries, benefits and equity-based compensation expense for our personnel in executive and other administrative functions. We expect our general and administrative expenses to increase over the next several years to support our continued research and development activities, manufacturing activities, increased costs of expanding our operations and operating as a public company. These increases will likely include increases related to the hiring of additional personnel and legal, regulatory, and other fees and services associated with maintaining compliance with the NYSE American, the NYSE American Company Guide and Securities and Exchange Commission, or SEC, requirements, director and officer insurance costs and investor relations costs associated with being a public company.
Other Income/(Expense)
Our other income is comprised of interest from cash equivalents.
Our other expense is comprised of expenses related to the change in fair value of the embedded derivative and interest accrued from the convertible notes.
Results of Operations
Comparison of the Six Months Ended June 30, 2023 and 2022
The following sets forth our results of operations for the six months ended June 30, 2023 as compared to the six months ended June 30, 2022:
| | | | | | | | | Change | |
| | | 2023 | | | 2022 | | | Amount | | | Percent | |
| Revenues: | | | | | | | | | | | | | | | | |
| Contract Revenue | | $ | - | | | $ | 500,000 | | | $ | (500,000 | ) | | | -100 | % |
| Grant Revenue | | | 37,004 | | | | - | | | | 37,004 | | | | 100 | % |
| Total Revenue | | | 37,004 | | | | 500,000 | | | | (462,996 | ) | | | -93 | % |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 878,188 | | | | 884,588 | | | | (6,400 | ) | | | -1 | % |
| General and administrative | | | 1,066,360 | | | | 534,434 | | | | 531,926 | | | | 100 | % |
| Total operating expenses | | | 1,944,548 | | | | 1,419,022 | | | | 525,526 | | | | 37 | % |
| Loss from operations | | | (1,907,544 | ) | | | (919,022 | ) | | | (988,522 | ) | | | 108 | % |
| Other income (expense): | | | | | | | | | | | | | | | | |
| Loss on equity securities, net | | | - | | | | (7,586 | ) | | | 7,586 | | | | -100 | % |
| Interest expense | | | (281,703 | ) | | | - | | | | (281,703 | ) | | | 100 | % |
| Interest and dividend income | | | 88,075 | | | | 1,791 | | | | 86,284 | | | | 4818 | % |
| Fair value inception adjustment on convertible promissory notes | | | 2,240,000 | | | | - | | | | 2,240,000 | | | | 100 | % |
| Change in fair value of convertible promissory notes | | | 1,864,362 | | | | - | | | | 1,864,362 | | | | 100 | % |
| Change in fair value of warrant liability | | | 463,404 | | | | (485,833 | ) | | | 949,237 | | | | -195 | % |
| Other income | | | - | | | | 1,084 | | | | (1,084 | ) | | | -100 | % |
| Net income (loss) | | $ | 2,466,594 | | | $ | (1,409,566 | ) | | $ | 3,876,160 | | | | -275 | % |
Revenues
Contract revenue for the six months ended June 30, 2023, was $0 compared to $500,000 for the six months ended June 30, 2022. The decrease of $500,000 was due primarily to the absence of any contract revenue for the six months ended June 30, 2023, compared to the six months ended June 30, 2022. Grant revenue for the six months ended June 30, 2023, was $37,004 compared to $0 for the six months ended June 30, 2022. The increase of $37,004 was due to the Innovate Alabama Supplemental Grant awarded by the Alabama Innovation Corporation on August 24, 2022 recognized as revenue as eligible expenses under the grant were incurred.
Research and development expenses
Research and development expenses included costs associated with research performed pursuant to government grant agreements, including internal research, direct and indirect internal costs related to specific projects, employee compensation and benefits, included stock-based compensation expense, for employees in engineering and design, as well as fees paid to other that conduct certain research activities on Serina’s behalf.
Research and development expenses decreased by $6,400 and were $878,188 for the six months ended June 30, 2023, compared to $884,588 for the six months ended June 30, 2022. The approximately flat level of expenditures recognized in the periods was due to the Company’s strategy to control operating costs until additional working capital could be raised.
General and administrative expenses
General and administrative expenses were $1,066,360 for the six months ended June 30, 2023, compared to $534,434 for the six months ended June 30, 2022. The increase of $531,926 was due primarily to increases of approximately $139,000 in professional fees, approximately $252,000 in legal expenses, approximately $34,600 in general and administrative salaries, and approximately $91,000 in dues and subscriptions due to an annual fee for a new online platform for pharmaceutical companies.
Other income (expense)
Interest and dividend income for the six months ended June 30, 2023 was $88,075 compared to $1,791 for the six months ended June 30, 2022. The increase of $86,284 was primarily due to higher interest earning cash balances.
Interest expense for the six months ended June 30, 2023 was $281,703 compared to $0 for the six months ended June 30, 2022. The increase of $281,703 was due to the interest recognized on the outstanding $1.45 million in Convertible Notes (or the Serina Convertible Notes) issued between June 2022 through February 2023 and the $10.0 million AgeX-Serina Note issued to AgeX in March 2023.
The aggregate change in the fair value of the Serina Convertible Notes, the AgeX-Serina Note and Series A-5 preferred share warrants for the six months ended June 30, 2023, was $4,567,766. The change in the fair value of the Serina Convertible Notes and Series A-5 preferred share warrants for the six months ended June 30, 2022, was $(485,833). The increase of $5,053,599 million was due primarily to the at inception fair value adjustment of $2,240,000, the change in the fair market value of the AgeX-Serina Note issued to AgeX in March 2023 of $1,864,362 during the period, and the change in the fair market value of the Series A-5 preferred share warrants.
Results of Operations
Comparison of the Years Ended December 31, 2022 and 2021
The following sets forth our results of operations for the year ended December 31, 2022 as compared to the year ended December 31, 2021:
| | | | | | | | | Change | |
| | | 2022 | | | 2021 | | | Amount | | | Percent | |
| Revenues: | | | | | | | | | | | | | | | | |
| Contract Revenue | | $ | 500,000 | | | $ | 3,039,184 | | | $ | (2,539,184 | ) | | | -84 | % |
| Grant Revenue | | | 91,500 | | | | 425,569 | | | | (334,069 | ) | | | -78 | % |
| Total Revenue | | | 591,500 | | | | 3,464,753 | | | | (2,873,253 | ) | | | -83 | % |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 1,573,085 | | | | 3,798,593 | | | | (2,225,508 | ) | | | -59 | % |
| General and administrative | | | 1,288,783 | | | | 1,297,668 | | | | (8,885 | ) | | | -1 | % |
| Total operating expenses | | | 2,861,868 | | | | 5,096,261 | | | | (2,234,393 | ) | | | -44 | % |
| Loss from operations | | | (2,270,368 | ) | | | (1,631,508 | ) | | | (638,860 | ) | | | -39 | % |
| Other income (expense): | | | | | | | | | | | | | | | | |
| Loss on equity securities, net | | | (7,585 | ) | | | (6,452 | ) | | | (1,133 | ) | | | 18 | % |
| Interest expense | | | (15,878 | ) | | | (3,094 | ) | | | (12,784 | ) | | | 413 | % |
| Interest and dividend income | | | 1,757 | | | | 10,859 | | | | (9,102 | ) | | | -84 | % |
| Fair value inception adjustment on convertible promissory notes | | | (179,000 | ) | | | - | | | | (179,000 | ) | | | 100 | % |
| Change in fair value of convertible promissory notes | | | (88,000 | ) | | | - | | | | (88,000 | ) | | | 100 | % |
| Change in fair value of warrant liability | | | (124,118 | ) | | | 205,287 | | | | (329,405 | ) | | | -160 | % |
| Other income | | | 1,084 | | | | - | | | | 1,084 | | | | 100 | % |
| Forgiveness of SBA PPP loan | | | - | | | | 187,133 | | | | (187,133 | ) | | | -100 | % |
| Loss on dissolution of subsidiary | | | - | | | | (26,928 | ) | | | 26,928 | | | | -100 | % |
| Net loss | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) | | $ | (1,417,405 | ) | | | 112 | % |
Revenues
Contract revenue for the year ended December 31, 2022, was $500,000 compared to $3,039,184 for the year ended December 31, 2021. The decrease of $2,539,184 was due primarily to completion of revenue from a licensing agreement in 2021. Grant revenue for the year ended December 31, 2022, was $91,500 compared to $425,569 for the year ended December 31, 2021. The decrease of $334,069 was due to the timing of the grant program related to product candidate SER 227 in each year in which grant revenue is recognized as eligible expenses under the grants are incurred.
Research and development expenses
Research and development expenses were $1,573,085 for the year ended December 31, 2022, compared to $3,798,593 for the year ended December 31, 2021. The decrease of $2,225,508 was due to decreases of approximately $980,300 in the cost of outside lab analysis, approximately $1,119,200 of expenses related to contract manufacturing, approximately $119,700 in labor and related costs and approximately $68,000 in chemical costs, partially offset by an increase of approximately $67,300 in legal related expenses.
General and administrative expenses
General and administrative expenses were $1,288,783 for the year ended December 31, 2022, compared to $1,297,668 for the year ended December 31, 2021. The approximately flat level of expenditures recognized between the years was due to the Company’s strategy to control operating costs until additional working capital could be raised.
Other income (expense)
Interest and dividend income for the year ended December 31, 2022 was $1,757 compared to $10,859 for the year ended December 31, 2021. The decrease of $9,102 was mainly due to lower interest earning cash balances.
Interest expense for the year ended December 31, 2022 was $15,878 compared to $3,094 for the year ended December 31, 2021. The increase of $12,784 was due to the interest on the outstanding Serina Convertible Notes issued to certain Serina preferred stockholders between June 2022 and November 2022.
The aggregate change in the fair value of Serina Convertible Notes issued in 2022 was $267,000. Of this amount, $179,000 related to the fair value adjustment at the inception of the notes and $88,000 related to the change in fair value of the Convertible Notes during the year. No similar notes were issued and outstanding during the year ended December 31, 2021.
The change in the fair value of Series A-5 preferred stock warrants was $329,405 during the year ended December 31, 2022, as compared to the year ended December 31, 2021. The decrease was the result of the warrants mark to market adjustments.
The gain on forgiveness of the SBA PPP Loan recognized in the year ended December 31, 2021 was $187,133. No similar SBA PPA Loans remained outstanding during the year ended December 31, 2022.
Liquidity and Capital Resources
Sources of Liquidity
Our operations from inception through June 30, 2023 have been financed primarily by aggregate net proceeds of $46.91 million from the issuance of convertible preferred stock and convertible notes. Since inception, we have had significant operating losses. Our operating loss was $1,907,544, $919,022, $2,270,368, and $1,631,508 for the six months ended June 30, 2023 and 2022 and years ended December 31, 2022 and 2021, respectively. As of June 30, 2023, we had an accumulated deficit of $35,979,888 and $8,722,084 in cash and cash equivalents. Our primary use of cash is to fund operating expenses, which consist primarily of research and development expenditures, and to a lesser extent, general and administrative expenditures. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
Our losses from operations, negative operating cash flows and accumulated deficit, as well as the additional capital needed to fund operations within one year of the unaudited consolidated financial statement issuance date, raise substantial doubt about our ability to continue as a going concern. We expect to incur substantial expenditures in the foreseeable future for the development of our product candidates and will require additional financing to continue this development. The unaudited consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement have been prepared on a basis that assumes that we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The unaudited consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Funding Requirements
Any product candidates we may develop may never achieve commercialization, and we anticipate that we will continue to incur losses for the foreseeable future. We expect that our research and development expenses, general and administrative expenses, and capital expenditures will continue to increase. As a result, until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings or other capital sources, including potential collaborations, licenses, and other similar arrangements. Our primary uses of capital are, and we expect will continue to be, costs related to pre-clinical clinical research, clinical studies, manufacturing, and development services; compensation and related expenses; costs relating to the build out of our laboratories at our headquarters; license payments or milestone obligations that may arise; laboratory expenses and costs for related supplies; manufacturing costs; legal and other regulatory expenses and general overhead costs.
We believe that our cash on hand, along with the approximately $15 million of cash proceeds expected to be received from Juvenescence through the exercise of all the post-merger warrants it holds pursuant to the terms of the Side Letter if the Merger is consummated, will enable us to fund our operations through calendar year 2025 based on our current plan. To finance our operations beyond that point, we will need to raise additional capital, which cannot be assured. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. We will continue to require additional financing to advance our current product candidates through clinical development, to develop, acquire or in license other potential product candidates and to fund operations for the foreseeable future. We will continue to seek funds through equity offerings, debt financings or other capital sources, including potential collaborations, licenses, and other similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. If we do raise additional capital through public or private equity offerings, the ownership interest of our existing stockholders, including investors in this offering, will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. Any failure to raise capital as and when needed could have a negative impact on our financial condition and on our ability to pursue our business plans and strategies. If we are unable to raise capital, we will need to delay, reduce, or terminate planned activities to reduce costs.
Because of the numerous risks and uncertainties associated with research, development, and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| ● | the impacts of the COVID 19 pandemic; |
| ● | the progress, costs and results of IND enabling studies for our lead product candidate SER 252 and our potential future clinical trials for SER 252; |
| ● | the scope, progress, results and costs of discovery research, preclinical development, laboratory testing and clinical trials for our other product candidates; |
| ● | the costs, timing, and outcome of regulatory review of our product candidates; |
| ● | our ability to enter into contract manufacturing arrangements for supply of active pharmaceutical |
| ● | ingredient, or API, and manufacture of our product candidates and the terms of such arrangements; |
| ● | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such arrangements; |
| ● | the payment or receipt of milestones and receipt of other collaboration based revenues, if any; the costs and timing of any future commercialization activities, including product manufacturing, sales, marketing, and distribution, for any of our product candidates for which we may receive marketing approval; |
| ● | the amount and timing of revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval; |
| ● | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property and proprietary rights and defending any intellectual property related claims; |
| ● | the extent to which we acquire or in license other products, product candidates, technologies, or data referencing rights; |
| ● | the ability to receive additional non dilutive funding, including grants from organizations and foundations; and |
| ● | the costs of operating as a public company |
Further, our operating plans may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated product development programs.
Cash Flows
The following table summarizes our cash flows for the six months ended June 30, 2023 and 2022 and the years ended December 31, 2022 and 2021:
Comparison of the Six Months Ended June 30, 2023 and 2022
| | | Six Months Ended June 30, | | | | | | | |
| | | 2023 | | | 2022 | | | $ Change | | | % Change | |
| Net cash used in operating activities | | $ | (1,572,596 | ) | | $ | (840,275 | ) | | $ | (732,321 | ) | | | 87 | % |
| Net cash (used in) provided by investing activities | | | (315,341 | ) | | | 990,652 | | | | (1,305,993 | ) | | | -132 | % |
| Net cash provided by financing activities | | | 10,077,792 | | | | 229,453 | | | | 9,848,339 | | | | 4292 | % |
| Net increase in cash | | $ | 8,189,855 | | | $ | 379,830 | | | $ | 7,810,025 | | | | 2056 | % |
Comparison of the Years Ended December 31, 2022 and 2021
| | | Year Ended December 31, | | | | | | | |
| | | 2022 | | | 2021 | | | $ Change | | | % Change | |
| Net cash used in operating activities | | $ | (2,075,624 | ) | | $ | (2,250,775 | ) | | $ | 175,151 | | | | -8 | % |
| Net cash provided by investing activities | | | 980,937 | | | | 823,321 | | | | 157,616 | | | | 19 | % |
| Net cash provided by financing activities | | | 1,312,070 | | | | 1,077,245 | | | | 234,825 | | | | 22 | % |
| Net increase (decrease) in cash | | $ | 217,383 | | | $ | (350,209 | ) | | $ | 567,592 | | | | -162 | % |
Operating Activities
Net cash used in operating activities increased by $732,321 to $1,572,596 during the six months ended June 30, 2023, from $840,275 during the six months ended June 30, 2022. Net cash used in operating activities is impacted by our net income (loss) adjusted for certain non-cash items, including changes in the fair value of convertible notes and warrants, among other non-cash items as well as the effect of changes in operating assets and liabilities. The increase was primarily due to the non-cash change in the fair value of the Serina Convertible Notes and the AgeX-Serina Note outstanding of $4,104,362 and the non-cash change in fair value of the Series A-5 preferred share warrants outstanding of $463,404. These increases were offset by changes related to various asset and liability accounts, notably an increase in accounts payable of approximately $217,000 and accrued interest of approximately $282,000.
Net cash used in operating activities decreased by $175,151 to $2,075,624 during the year ended December 31, 2022, from $2,250,775 during the year ended December 31, 2021. The decrease was primarily due to the decrease of contract liabilities of approximately $154,000. This decrease was partially offset by changes in asset and liability accounts such as accounts payable and operating lease liabilities.
Investing Activities
Net cash (used in) provided by investing activities decreased by $1,305,993 to $(315,341) during the six months ended June 30, 2023 from $990,652 during the six months ended June 30, 2022. The net cash used during the six months ended June 30, 2023 related entirely to purchases of capital equipment of approximately $315,000. The net cash provided by in the six months ended June 30, 2022 related entirely to proceeds of $991,000 from the sale of equity investments.
Net cash provided by investing activities increased by $157,616 to $980,937 during the year ended December 31, 2022 from $823,321 during the year ended December 31, 2021. The net cash provided by in the year ended December 31, 2022 related substantially to proceeds of $991,000 from the sale of equity investments. The net cash provided by in the year ended December 31, 2021 related to proceeds of $932,000 from the sale of equity investments offset by net cash used of approximately $85,000 to purchase of capital equipment as well as net cash used of approximately $24,000 to acquire a financed lease asset.
Financing Activities
Net cash provided by financing activities for the six months ended June 30, 2023 was $10,077,792 comprised of $10,100,000 in net proceeds from the issuance of $100,000 of Serina Convertible Notes, $10,000,000 from the issuance of the AgeX-Serina Note, $936 in proceeds from common stock issued, partially offset by $23,144 in principal repayments on finance lease liabilities.
Net cash provided by financing activities for the six months ended June 30, 2022 was $229,453 comprised of $250,000 from the issuance of Serina Convertible Notes, partially offset by $20,547 in principal repayments on finance lease liabilities.
Net cash provided by financing activities for the year ended December 31, 2022 was $1,312,070, comprised of $5,000,000 in net proceeds from the issuance of Serina Convertible Notes to certain Serina preferred stockholders, $4,422 in proceeds from common stock issued, partially offset by $3,650,000 in repayments of Serina Convertible Notes and $42,352 in principal repayments on finance lease liabilities.
Net cash provided by financing activities for the year ended December 31, 2021 was $1,077,245, comprised of $90,152 in net proceeds from preferred stock issued and $1,000,000 from the collection of a stock subscription receivable, partially offset by $12,907 in principal repayments on finance lease liabilities.
Off-Balance Sheet Arrangements
As of June 30, 2023 and December 31, 2022, we did not have any relationships with special purpose or variable interest entities or other which would have been established for the purpose of facilitating off-balance sheet arrangements or other off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Critical Accounting Policies and Use of Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Going Concern
Our evaluation of our ability to continue as a going concern requires us to evaluate our future sources and uses of cash sufficient to fund our currently expected operations in conducting research and development activities one year from the date our audited consolidated financial statements are issued. We evaluate the probability associated with each source and use of cash resources in making our going concern determination. The research and development of pharmaceutical products is inherently subject to uncertainty.
Research and Development Costs
We will incur substantial expenses associated with manufacturing, pre-clinical research, and clinical studies. Accounting for pre-clinical research and clinical studies relating to activities performed by contract research organizations, or CROs, and other external vendors requires management to exercise significant estimates in regard to the timing and accounting for these expenses. We estimate costs of research and development activities conducted by service providers, which include the conduct of sponsored research, pre-clinical research, clinical studies, and contract manufacturing activities. The diverse nature of services being provided under CROs and other arrangements, the different compensation arrangements that exist for each type of service and the lack of timely information related to certain pre-clinical and clinical activities complicates the estimation of accruals for services rendered by CROs and other vendors in connection with pre-clinical research and clinical studies. We record the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and include these costs in the accrued expenses or prepaid expenses on the balance sheets and within research and development expense on the consolidated statements of operations. In estimating the duration of a clinical study, we evaluate the start up, treatment and wrap up periods, compensation arrangements and services rendered attributable to each clinical trial and fluctuations are regularly tested against payment plans and trial completion assumptions.
We estimate these costs based on factors such as estimates of the work completed and budget provided and in accordance with agreements established with our collaboration partners and third-party service providers. We make significant judgments and estimates in determining the accrued liabilities and prepaid expense balances in each reporting period. As actual costs become known, we adjust our accrued liabilities or prepaid expenses. We have not experienced any material differences between accrued costs and actual costs incurred since our inception.
Our expenses related to clinical trials will be based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that may be used to conduct and manage clinical trials on our behalf. We will accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we will modify our estimates of accrued expenses accordingly on a prospective basis.
Fair market value of common stock
As there has been no public market for our common stock, the estimated fair value of our common stock has been determined by the Serina Board as of the date of each option grant, with input from management, considering Serina’s most recently available third-party valuations of common stock and the Serina Board’s assessment of additional objective and subjective factors it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant. These third-party valuations were performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation. The common stock valuations were prepared using the option pricing method. These third-party valuations were performed at various dates, which resulted in valuations of our common stock of $0.99 per share as of December 31, 2022 and $0.06 per share as of March 31, 2021.
Leases
ASU No. 2016 02, Leases (ASC 842), establishes a right of use model (ROU) that requires a lessee to recognize a ROU asset and corresponding lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the unaudited statement of operations as well as the reduction of the right of use asset.
At the inception of an arrangement, Serina determines whether the arrangement is or contains a lease based on specific facts and circumstances, the existence of an identified asset(s), if any, and Serina’s control over the use of the identified asset(s), if applicable. Operating lease liabilities and their corresponding ROU assets are recorded based on the present value of future lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, Serina will utilize the incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
Serina has elected to combine lease and non lease components as a single component. Operating leases are recognized on the consolidated balance sheet as ROU lease assets, current lease liabilities and non-current lease liabilities. Fixed rents are included in the calculation of the lease balances, while variable costs paid for certain operating and pass through costs are excluded. Lease expense is recognized over the expected term on a straight-line basis.
Stock based Compensation
Prior to the proposed Merger, we issued equity-based compensation awards through the granting of options, which generally vest over four years. We account for equity-based compensation in accordance with Accounting Standards Codification, or ASC, 718, Compensation Stock Compensation, or ASC 718. In accordance with ASC 718, compensation cost is measured at estimated fair value at grant date and is included as compensation expense over the vesting period during which service is provided in exchange for the award. Compensation cost of awards that contain a performance condition are recognized when success is considered probable during the performance period.
We use the Black Scholes option pricing model, or Black Scholes, to determine fair value of our options. Black Scholes includes various assumptions, including the fair value of common shares, expected life of incentive shares, the expected volatility and the expected risk-free interest rate. These assumptions reflect our best estimates, but they involve inherent uncertainties based on market conditions generally outside our control. As a result, if other assumptions had been used, equity-based compensation cost could have been materially impacted. Furthermore, if we use different assumptions for future grants, equity-based compensation cost could be materially impacted in future periods.
The risk-free interest rate is estimated using the weighted average rate of return on U.S. Treasury notes with a life that approximates the expected life of the option. The expected term of options granted to employees was calculated using the simplified method, which represents the average of the contractual term of the option and the weighted average vesting period of the option. Serina uses the simplified method because it does not have sufficient historical option exercise data to provide a reasonable basis upon which to estimate expected term. The contractual life of the option was used for the expected life of options granted to nonemployees. Expected volatility is based on the weighted average of the historical volatility of a peer group of publicly traded companies. The assumed dividend yield is based upon Serina’s expectation of not paying dividends in the foreseeable future.
No stock options to purchase common stock were granted during the six months ended June 30, 2023.
We granted stock options to purchase 50,000 shares of common stock in the year ended December 31, 2022. The fair value of our awards in the year ended December 31, 2022 has been estimated using Black Scholes based on the following assumptions: expected term of 10 years; volatility of 88.57%; risk free rate of 3.96%; and no expectation of dividends.
We granted stock options to purchase 2,036,450 shares of common stock during the year ended December 31, 2021. The fair value of our awards in the year ended December 31, 2021 has been estimated using Black Scholes based on the following assumptions: expected term range of 5.15 – 10 years; volatility of 68.70%; risk free rate range of 0.73% - 0.81%; and no expectation of dividends.
We will continue to use judgment in evaluating the assumptions utilized for our equity-based compensation expense calculations on a prospective basis. In addition to the assumptions used in the Black Scholes model, the amount of equity-based compensation expense we recognize in our consolidated financial statements includes incentive share forfeitures as they occurred.
As there has been no public market for our common shares to date, the Serina Board, with input from management, has determined the estimated fair value of our common shares as of the date of each option grant considering our then most recently available third-party valuation of common shares. Valuations are updated when facts and circumstances indicate that the most recent valuation is no longer valid, such as changes in the stage of our development efforts, various exit strategies and their timing, and other scientific developments that could be related to the valuation of our company, or, at a minimum, annually. Third-party valuations were performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation.
The estimates of fair value of our common stock are highly complex and subjective. There are significant judgments and estimates inherent in the determination of the fair value of our common shares. These judgments and estimates include assumptions regarding our future operating performance, the time to completing a liquidity event, the related valuations associated with these events, and the determinations of the appropriate valuation methods at each valuation date. The assumptions underlying these valuations represent our best estimates, which involve inherent uncertainties and the application of management judgment. If we had made different assumptions, our equity-based compensation expense, net loss, and net loss per share applicable to common stockholders could have been materially different. Following the completion of this Merger, we intend to determine the fair value of our common stock based on the closing price of our common stock as reported by New York American Stock Exchange on the date of grant.
Refer to Note 6, Redeemable Convertible Preferred Stock and Note 11, Stock Warrants within the June 30, 2023 financial statements for details on the equity-based awards outstanding.



Derivative Financial Instruments
On March 15, 2023, the Company issued a Convertible Promissory Note (or the AgeX-Serina Note) in the amount of $10,000,000 to AgeX. The AgeX-Serina Note bears interest at 7% per annum and matures on March 15, 2026. The Company issued the AgeX-Serina Note to provide for general working capital needs. In connection with the issuance of the AgeX-Serina Note, AgeX is entitled to elect one member to the board of directors of the Company and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
The AgeX-Serina Note has various conversion options. The principal balance of the AgeX-Serina Note with accrued interest will automatically convert into the Company’s preferred stock if the Company raises at least $25,000,000 through the sale of shares of the Company’s preferred stock. The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by the Company’s then fully diluted capitalization. AgeX has the option to convert the AgeX-Serina Note into the Company’s preferred stock after a sale of the Company’s preferred stock regardless of the amount sold by the Company. AgeX may (i) at its election, upon a change of control (as defined in the AgeX-Serina Note), convert the AgeX-Serina Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the AgeX-Serina Note, plus interest, or (b) into the highest ranking shares of the Company then issued at a conversion price equal to the lowest price per share at which the most senior series of the Company’s shares has been sold in a single transaction or a series of related transactions through which the Company raised at least $5,000,000 or (ii) if the AgeX-Serina Note remains outstanding as of the maturity date, AgeX may convert the AgeX-Serina Note into the most senior shares of the Company issued at the time of conversion at a conversion price equal to the capped price. Upon the consummation of a merger between the Company and AgeX, the AgeX-Serina Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of the Company.
The Company evaluated the AgeX-Serina Note in accordance with ASC Topic 815, Derivatives and Hedging, and determined it contains certain variable share settlement features tied to the price of a future financing which were not considered clearly and closely related to the host instruments. These provisions included automatic conversion upon the event of a Qualified Financing, the Holder’s option to convert the AgeX-Serina Note upon a Non-Qualified Financing, and the Holder’s option to convert or request repayment upon sale of the Company. The Notes also contained a Change in Control Put and a Default Put which were not clearly and closely related to the host instrument. The Company elected to initially and subsequently measure the Notes in their entirety at fair value, with changes in fair value recognized in earnings. The fair value inception date adjustment on the instrument is recorded as a component of other income in the Company’s statements of operations.
FASB ASC 825-10-25 allows the Company to elect the fair value option for recording financial instruments when they are initially recognized or if there is an event that requires re-measurement of the instruments at fair value, such as a significant modification of the debt. The Company elected the fair value option because they believed it to be the most appropriate method of encompassing the credit risk and exercise behavior that a market participant would consider when valuing the hybrid financial instrument.
As of March 15, 2023 (inception), the outstanding principal and interest on the AgeX-Serina Note was approximately $10,000,000 and the fair value recorded on the balance sheet was $7,760,000. From March 15, 2023 to June 30, 2023, the change in fair value was approximately $1,684,000 and was recorded as a component of other income in the Company’s statements of operations.
From June 2022 through February 2023, the Company issued interest-bearing Convertible Promissory Notes (the Serina Convertible Notes) to various investors in the principal amount of $1,450,000. The Notes incur interest at 6% per annum and are payable by the Company two years from their issuance date. The Company may not be voluntarily prepaid. Upon a Qualified Equity Financing event in which the Company sells shares of Preferred Stock for aggregate proceeds of at least $15 million, the principal and outstanding interest on the Notes will automatically convert into shares of the Company’s Preferred Stock issued in the Qualified Financing at a conversion price of the lesser of i) a 20% discount to the price paid by purchasers in the Qualified Financing and ii) the quotient resulted from dividing $100 million by the fully diluted capitalization of the Company immediately prior to the Qualified Financing. If the Company enters into a Non-Qualified Equity Financing (less than $15 million in proceeds), the Holder has the option to convert the Notes into shares of the Company’s Preferred Stock issued in the Non-Qualified Financing at the price paid per share. The Company may also choose to optionally convert the Notes into Series A-5 Preferred Stock at a price of $13 per share, and a warrant to purchase shares of Series A-5 Preferred Stock with an exercise price of $20.00, and an expiration date of December 31, 2024.If a Change in Control or an IPO occurs prior to a Qualified Financing, then the Holder has the option to convert outstanding principal and interest into common stock at a price per share equal to an amount obtained by dividing i) the Post-Money Valuation Cap ($100,000,000) by ii) the Fully Diluted Capitalization immediately prior to the conversion. Upon a change in control, the Holder may also elect to require the Company to repay the outstanding principal and accrued but unpaid interest in cash.
The Company evaluated the Notes in accordance with ASC Topic 815, Derivatives and Hedging, and determined they contained certain variable share settlement features tied to the price of a future financing which were not considered clearly and closely related to the host instruments. These provisions included mandatory conversion upon the event of a Qualified Financing and the Holder’s option to convert the Notes upon a Non-Qualified Financing. The Notes also contained a Change in Control Put and a Default Put which were not clearly and closely related to the host instrument. The Company elected to initially and subsequently measure the Notes in their entirety at fair value, with changes in fair value recognized in earnings. The fair value inception date adjustment on the instrument is recorded as a component of other income in the Company’s statements of operations. The change in fair value of the instrument since inception date is recorded on a separate line item as a component of other income in the Company’s statements of operations.
Refer to Note 14, Convertible Promissory Notes to the June 30, 2023 financial statements for additional details on the Notes.
Recently Adopted Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in the notes to our consolidated financial statements for the years December 31, 2022 and 2021 appearing elsewhere in this prospectus.
Contractual Obligations and Commitments
The following table summarizes our contractual lease obligations as of June 30, 2023:
| | | Operating Leases | | | Finance Leases | |
| Six Months Ended December 31, 2023 | | $ | 93,152 | | | $ | 27,598 | |
| 2024 | | | 143,466 | | | | 38,055 | |
| 2025 | | | 116,592 | | | | 629 | |
| 2026 | | | 116,592 | | | | - | |
| 2027 | | | 116,592 | | | | - | |
| Thereafter | | | 9,716 | | | | - | |
| Total undiscounted lease payments | | | 596,110 | | | | 66,282 | |
| Less: imputed interest | | | (77,025 | ) | | | (4,750 | ) |
| Total lease obligations | | | 519,085 | | | | 61,532 | |
We enter into contracts in the normal course of business with third-party service providers for pre-clinical research, clinical studies, testing, manufacturing, supplier, and other services and products for operating purposes. We have not included our payment obligations under these contracts in the table as these contracts generally provide for termination upon notice, and therefore, we believe that our non-cancelable obligations under these agreements are not material, and we cannot reasonably estimate the timing of if and when they will occur. We could also enter into additional pre-clinical research, clinical studies, testing, manufacturing, supplier, and other agreements in the future, which may require up-front payments and even long-term commitments of cash.
MANAGEMENT FOLLOWING THE MERGER
The following table provides information regarding the expected directors and executive officers of the combined company following the closing of the Merger:
Name | | Age | | Position |
| Steve Ledger | | 64 | | Interim Chief Executive Officer and Class I Director |
| Andrea Park | | 51 | | Interim Chief Financial Officer and Chief Accounting Officer |
| Randall Moreadith, M.D., Ph.D. | | 69 | | Chief Scientific Officer |
| Tacey Viegas, Ph.D. | | 66 | | Chief Operating Officer; Secretary |
| Gregory M. Bailey, M.D. | | 67 | | Chairman of the Board and Class III Director |
| J. Milton Harris, Ph.D. | | 83 | | Class I Director |
| Remy Gross | | 53 | | Class II Director |
| Richard Marshall, M.D., Ph.D. | | 56 | | Class III Director |
Executive Officers
Steve Ledger has served as Serina’s Chief Financial Officer since June 2021 and as a member of Serina’s Board of Directors since December 2022. Mr. Ledger will serve as the Interim Chief Executive Officer of the combined company. Serina has engaged an executive search firm to recruit a permanent Chief Executive Officer for the combined company. Mr. Ledger will cease to be the Interim Chief Executive Officer of the combined company but will remain as a Class I Director upon the hiring of the Chief Executive Officer. Mr. Ledger has more than 35 years of experience as an investor, board member, advisor, and in operational roles with early-stage companies. From 2018 to the present, Mr. Ledger serves as Managing Partner of Form & Fiction Ventures, Inc. (FFV), a venture studio that launches and invests in startup and seed stage companies focused on socially responsible initiatives. Mr. Ledger is a co-founder and board director of Entourage Genomics, Inc., a bioinformatics software company formed by FFV in June 2023. From 2018 to February 2022, Mr. Ledger served as an advisor at Caldwell Sutter Capital, Inc., an SEC registered broker dealer and investment management firm focused on value-based equity and debt securities. From 2002 to 2012, Mr. Ledger was the founder and managing member of Tamalpais Partners, LLC, the general partner to funds focused on special situations in the small capitalization public equity markets. Mr. Ledger received a B.A. in Economics from the University of Connecticut.
Andrea E. Park has served as Chief Financial Officer of AgeX since May 2020. Ms. Park served as AgeX’s Vice President of Finance and Controller from October 2019. Ms. Park will serve as the Interim Chief Financial Officer and Chief Accounting Officer of the combined company. Upon the hiring of the Chief Financial Officer, Ms. Park will cease to be the Interim Chief Financial Officer of the combined company but will remain the Chief Accounting Officer of the combined company. Ms. Park’s career spans over 24 years of public accounting and finance experience. Before joining AgeX, Ms. Park served as Vice President of Finance and Controller from June 2016 to September 2019 and as Corporate Controller from August 2009 to June 2016 for Lineage Cell Therapeutics, Inc. (Lineage, formerly BioTime, Inc.). While at Lineage, Ms. Park was directly involved in the accounting and financial reporting of the public spin off and eventually the deconsolidation of three of its then subsidiaries including Asterias Biotherapeutics, Inc., Oncocyte Corporation and AgeX. Earlier in her career from 1996 to 2003, she worked in the audit and assurance practice at Deloitte. Ms. Park has a B.A. in Business Economics with Concentration in Accounting from the University of California, Santa Barbara.
Randall Moreadith, M.D., Ph.D. has served as Serina’s President and Chief Executive Officer and as a member of the Serina Board since September 2010. Dr. Moreadith will serve as Chief Scientific Officer of the combined company. From July 2009 to December 2009, Dr. Moreadith was Chief Development Officer at Nektar Therapeutics (Nektar) where he led clinical and drug development programs that successfully moved several of the Nektar’s PEGylated small molecule drugs into clinical trials for four clinical indications (ovarian, breast, cervical and colorectal cancer) and the out-licensing efforts for the approved product now known as Movantik®. Prior to Nektar, Dr. Moreadith served as the Executive Vice President and Chief Medical Officer of Cardium Therapeutics, Inc. where he led the advancement of novel DNA-based adenoviral therapeutics into Phase IIb and Phase III late-stage development, from 2006 to 2008. Prior to Cardium, Dr. Moreadith served as Chief Medical Officer of Renovis, Inc. where he led the Clinical, Regulatory and Quality Assurance Groups in 2004 to 2005. From 1996 to 2003, Dr. Moreadith was co-Founder, President, and Chief Operating Officer of ThromboGenics, Ltd. (now Oxurion), a leader in the field of thrombosis drug development. During his tenure at ThromboGenics, the company advanced four biologics into mid-stage development, with one product later approved (Ocriplasmin™). Dr. Moreadith received his M.D. from Duke University and is trained clinically in Internal Medicine and Cardiovascular Diseases. Dr. Moreadith received his Ph.D. from Johns Hopkins University and following his Fellowship in Cardiology at Duke University, he joined the laboratory of Professor Philip Leder where he was a Howard Hughes Medical Institute Fellow in Genetics at Harvard Medical School. Dr. Moreadith was a member of the faculty of the University of Texas Southwestern Medical Center.
Tacey Viegas, Ph.D. has served as Serina’s Chief Operating Officer since 2006. Dr. Viegas will serve as Chief Operating Officer and Secretary of the combined company. Dr. Viegas has managed the discovery and early development activities for both synthetically- and biologically-derived therapeutic agents in the areas of oncology, neurology, influenza, psoriasis, and wound care. He has numerous patents and publications in the area of polymer therapeutics and pharmaceutics. Prior to joining Serina, Dr. Viegas served as Senior Director of Chemistry Manufacturing and Controls at Nektar. Prior to Nektar, Dr. Viegas was Executive Director in product development at Biocryst Pharmaceuticals, Inc. (BioCryst). During his combined tenures at Nektar and BioCryst, he was a co-inventor of nalexogol (Movantik®), etirinotecan pegol and was involved in the early development of peramivir (Rapivab™). Prior to Biocryst, Dr. Viegas was a Director, Product Development at MDV Technologies, Inc. from 1989 to 1994. Dr. Viegas received his B.S. in Chemistry and Pharmacy from Bangalore University, and his M.S. and Ph.D. in Pharmaceutical Sciences from the University of Mississippi.
Non-Employee Directors
Gregory H. Bailey, M.D. has served on the AgeX Board since August 2018 and served as the Chair of the AgeX Board from October 2018 until May 2022. Dr. Bailey has served as a member of the Serina Board since March 2023. Dr. Bailey is currently Executive Chairman of Juvenescence. From October 2017 until January 2023, Dr. Bailey served as the Chief Executive Officer of Juvenescence. Dr. Bailey is also a board director of Manx Financial Group, plc, BioHaven Pharmaceuticals, Inc., SalvaRx Group, plc., and Portage Biotech, Inc. Dr. Bailey founded and served as a director of a number of private and public companies and previously served as a managing partner of Palantir Group, Inc., a merchant bank involved in a number of biotech company startups and financings. Dr. Bailey practiced emergency medicine for ten years before entering finance. Dr. Bailey received his M.D. from the University of Western Ontario.
J. Milton Harris, Ph.D. has served as Chair of the Serina Board of Directors since he co-founded Serina in 2006. Dr. Harris has more than 30 years of experience as a senior life sciences executive. Prior to founding Serina, he was the Founder and Chief Executive Officer of Shearwater Polymers, Inc. (Shearwater). Shearwater was founded by Dr. Harris in 1992 and sold in 2001 to Inhale Therapeutics, Inc. (Inhale Therapeutics, Inc., subsequently changed its name to Nektar Therapeutics, Inc.). Shearwater successfully patented, manufactured, and partnered PEG technology that enabled multiple drug products including Neulasta® (Amgen) and Pegasys® (Roche). Dr. Harris has also served on the board of directors of HudsonAlpha Institute for Biotechnology since its founding in 2004. Dr. Harris earned a B.S. from McGill University, where he also was awarded an honorary Sc.D., and a Ph.D. from the Massachusetts Institute of Technology. Dr. Harris has co-authored more than 200 publications and is a co-inventor on more than 75 patents.
Remy Gross has served as Vice President, Business Development & Technology Advancement at the Buck Institute for Research (Buck) on Aging from 2006. Mr. Gross has advised and helped create multiple new biopharmaceutical startups at Buck, including Unity Biotechnology, Inc., Aeovian Therapeutics, Inc., and BhB Therapeutics, Inc. Prior to Buck, Mr. Gross held increasingly senior roles at Shearwater from 1994 to 2001, and at Nektar after its acquisition of Shearwater from 2001 to 2005, including Vice President of Operations. In 2013, Mr. Gross co-founded RCP Companies, Inc., a boutique real estate company providing acquisition, development, and asset management. Mr. Gross serves as a board director of BhB Therapeutics, Inc., Napa Therapeutics, Inc., and Selah Therapeutics, Inc. Mr. Gross serves on several non-profit boards including MidCity Accelerator Foundation, Hatch HSV and k3innovation. Mr. Gross received a B.S. in Chemistry from Loyola University New Orleans.
Dr. Richard Marshall, CBE, M.D., Ph.D. has served as the Chief Executive Officer of Juvenescence from January 2023. Dr. Marshall is a physician scientist and highly experienced executive with a 20-year track record of leadership in pharmaceutical Research & Development. From September 2019 to January 2023, Dr. Marshall was Senior Vice President and Global Head of Respiratory & Immunology Development at AstraZeneca plc, overseeing the development and approval of five new medicines. This included the SARS CoV-2 vaccine, Vaxzevria®, and combination antibody, EvushieldTM. In 2021 he was recognized in the Queen’s Honours List with a CBE for his contribution to UK science and the Covid response. From 2002 to 2018, Dr. Marshall held increasingly senior roles at GlaxoSmithKline plc, including Vice President of Fibrosis R&D. Dr. Marshall received a Bachelor of Science in Neuroscience, a Bachelor of Medicine, a Bachelor of Surgery, and a Doctor of Philosophy in Medical Sciences from University College London and has held visiting professor and honorary consultant roles in thoracic medicine at Newcastle University and the Royal Brompton Hospital. Dr. Marshall has co-authored more than 60 original publications in journals including The Lancet and The New England Journal of Medicine.
Family Relationships
There are no family relationships among any of the combined company’s proposed directors and executive officers.
Composition of the Board of Directors Following the Merger
At the Effective Time, the combined company is expected to initially have a seven-member Combined Company Board, comprised of (a) Remy Gross, designated by both AgeX and Serina, (b) J. Milton Harris and Steven Ledger, each as a Serina designee, (c) Gregory Bailey and Richard Marshall, each as an AgeX designee, and (d) and , each as an independent director, until their respective successors are duly elected or appointed and qualified or their earlier death, resignation or removal. The Combined Company Board will be divided into three classes. Each class has a three-year term. Vacancies on the Combined Company Board may be filled only by a majority of the directors then in office. A director elected by the Combined Company Board to fill a vacancy in a class, including vacancies created by an increase in the number of directors, shall serve for the remainder of the full term of that class and until the director’s successor is duly elected and qualified.
It is anticipated that these directors will be appointed to the three staggered director classes of the Combined Board as follows:
| ● | Class I directors (expiring in 2024): Mr. Ledger and Mr. Harris; |
| ● | Class II directors (expiring in 2025): Mr. Bailey and Mr. Marshall; and |
| ● | Class III directors (expiring in 2026): Mr. Gross, and . |
Rule 803 of the rules set forth in the NYSE American Company Guide (the NYSE American Listing Rules) requires a majority of a listed company’s board of directors to be comprised of independent directors. In addition, the NYSE American Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act. Under Rule 803 of the NYSE American Listing Rules, a director will only qualify as an “independent director” if, in the opinion of the Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries.
It is anticipated that each individual expected to serve on the Combined Company Board upon the completion of the Merger, other than and , will qualify as an independent director under NYSE American listing standards.
Board Leadership Structure
The Combined Company Board is expected to have separate positions of Chair of the Combined Company Board and Chief Executive Officer in order to reinforce the independence of the board of directors from management, create an environment that encourages objective oversight of management’s performance and enhance the effectiveness of the board of directors as a whole. As such, the Combined Company Board shall be chaired by Dr. Bailey, and Mr. Ledger will serve as the combined company’s Interim Chief Executive Officer.
Role of Board in Risk Oversight
One of the key functions of the Combined Company Board will be to oversee the combined company’s risk management process. The Combined Company Board is not anticipated to have a standing risk management committee, but rather expects to administer this oversight function directly through the Combined Company Board as a whole, as well as through various standing committees of the Combined Company Board that address risks inherent in their respective areas of oversight. In particular, the Combined Company Board is responsible for monitoring and assessing strategic risk exposure and the combined company’s Audit Committee will have the responsibility to consider and discuss the major financial risk exposures and the steps its management should take to monitor and control such exposures, including guidelines and policies to govern the process by which risk assessment and management will be undertaken. The Audit Committee is also expected to monitor compliance with legal and regulatory requirements. The combined company’s Compensation Committee will also assess and monitor whether the combined company’s compensation plans, policies and programs comply with applicable legal and regulatory requirements.
Committees of the Combined Company Board
The Combined Company Board will have an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee, each of which will have the composition and the responsibilities described below.
Audit Committee
The purpose of the Audit Committee of the Combined Company Board will be to oversee the corporate accounting and financial reporting processes and audits of financial statements. For this purpose, the Audit Committee will perform several functions, including among other things:
| ● | evaluate the independent registered public accounting firm’s qualifications, independence and performance; |
| ● | determine whether to retain or terminate the engagement of the existing independent registered public accounting firm or to appoint and engage a different independent registered public accounting firm; |
| ● | review all relationships between any prospective independent registered accounting firm and the combined company that may reasonably be thought to bear on independence prior to engagement of any prospective independent registered accounting firm; |
| ● | review and approve the scope of the annual audit and pre-approves the audit and non-audit fees and services; |
| ● | obtain and review a report by the independent outside auditor describing that firm’s internal quality-control procedures on an annual basis; |
| ● | annually review all relationships between the independent outside auditor and the combined company that may reasonably be thought to bear on independence; |
| ● | review and monitor compliance with programs and policies designed to ensure compliance with laws and regulations and the Code of Business Conduct and Ethics, including review and approval of all related party transactions on an ongoing basis; |
| ● | establish procedures for the receipt, retention and treatment of complaints received regarding accounting, internal accounting controls or auditing matters; |
| ● | discuss with management and the independent registered public accounting firm the results of the annual audit and the review of the combined company’s quarterly financial statements; |
| ● | approve the retention of the independent outside auditor to perform any proposed permissible non-audit services; |
| ● | monitor the rotation of partners of the independent outside auditors on the combined company’s engagement team in accordance with requirements established by the SEC; |
| ● | discuss on a periodic basis, or as appropriate, with management the policies and procedures with respect to risk assessment and risk management; |
| ● | review the combined company’s financial statements and its management’s discussion and analysis of financial condition and results of operations to be included in the combined company’s annual and quarterly reports to be filed with the SEC; |
| ● | consider and adopt policies regarding Audit Committee preapproval of employment by the combined company of individuals employed or formerly employed by the combined company’s independent outside auditor; |
| ● | investigate any reports received through the ethics helpline and report to the Combined Company Board periodically with respect to the information received through the ethics helpline and any related investigations; |
| ● | review the combined company’s critical accounting policies and estimates; and |
| ● | review the audit committee charter and the committee’s performance at least annually. |
Following the completion of the Merger, the members of the combined company’s Audit Committee are expected to be , who is expected to be the Chair, and . All members of the Audit Committee will meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the NYSE American and at least one member of the Audit Committee will qualify as an audit committee financial expert as defined under the applicable rules of the SEC and have the requisite financial sophistication as defined under the applicable rules and regulations of the NYSE American. Under the rules of the SEC, members of the Audit Committee must also meet heightened independence standards. AgeX and Serina believe that, following the completion of the Merger, each member of the Audit Committee will be independent under the applicable rules of the SEC and the NYSE American. The Audit Committee will operate under a written charter that will satisfy the applicable standards of the SEC and the NYSE American.
Compensation Committee
The Compensation Committee of the Combined Company Board will oversee policies relating to compensation and benefits of officers and employees. The Compensation Committee will review and approve corporate goals and objectives relevant to compensation of the executive officers, evaluates the performance of these officers in light of those goals and objectives and approves the compensation of these officers based on such evaluations. The Compensation Committee will also review and approve or make recommendations to the Combined Company Board regarding the issuance of stock options and other awards under the combined company’s stock plans to the executive officers. The Compensation Committee will review and evaluate, at least annually, the performance of the Compensation Committee and its members, including compliance by the Compensation Committee with its charter.
The Chief Executive Officer may not participate in, or be present during, any deliberations or determinations of the Compensation Committee regarding his compensation or individual performance objectives. The proposed charter of the Compensation Committee will grant the Compensation Committee full access to all books, records, facilities and personnel of the company. In addition, under its proposed charter, the Compensation Committee will have the authority to obtain, at the expense of the Combined Company, advice and assistance from compensation consultants and internal and external legal, accounting or other advisors and other external resources the Compensation Committee considers necessary or appropriate in the performance of its duties. The Compensation Committee will have direct responsibility for the oversight of the work of any consultants or advisers engaged for the purpose of advising the Compensation Committee. In particular, the Compensation Committee will have the sole authority to retain, in its sole discretion, compensation consultants to assist in its evaluation of executive and director compensation, including the authority to approve the consultant’s reasonable fees and other retention terms. Under its proposed charter, the Compensation Committee may select, or receive advice from, a compensation consultant, legal counsel or other adviser to the Compensation Committee, other than in-house legal counsel and certain other types of advisers, only after taking into consideration six factors, prescribed by the SEC and the NYSE American, that bear upon the adviser’s independence; however, there is no requirement that any adviser be independent.
Following the completion of the Merger, the members of the Compensation Committee are expected to be , who is expected to be the Chair, and . To qualify as independent to serve on the combined company’s Compensation Committee, the NYSE American Listing Rules require a director not to accept any consulting, advisory, or other compensatory fee from the combined company, other than for service on the Combined Company Board, and that the Combined Company Board consider whether a director is affiliated with the combined company and, if so, whether such affiliation would impair the director’s judgment as a member of the combined company’s Compensation Committee. AgeX and Serina believe that, after the completion of the Merger, the composition of the Compensation Committee will meet the requirements for independence under, and the functioning of such Compensation Committee will comply with any applicable requirements of the rules and regulations of the SEC and the NYSE American.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee of the Combined Company Board will be responsible for making recommendations to the Combined Company Board regarding candidates for directorships and the size and composition of the Combined Company Board. In addition, the Nominating and Corporate Governance Committee will be responsible for overseeing corporate governance policies and reporting and making recommendations to the Combined Company Board concerning governance matters.
The Combined Company Board and the Nominating and Corporate Governance Committee do not expect to set any specific minimum qualifications that a prospective nominee would need in order to be nominated to serve on the Combined Company Board. Rather, in evaluating any new nominee or incumbent director, the Nominating and Corporate Governance Committee will consider whether the particular person has the knowledge, skills, experience, and expertise needed to manage business affairs in light of the skills, experience, and expertise of the other members of the Combined Company Board as a whole. The Nominating and Corporate Governance Committee will also consider whether a nominee or incumbent director has any conflicts of interest with the combined company that might conflict with the Code of Business Conduct and Ethics or that might otherwise interfere with their ability to perform their duties in a manner that is in the best interest of the combined company and its stockholders. The Nominating and Corporate Governance Committee will also consider whether including a prospective director on the Combined Company Board will result in a board composition that complies with (a) applicable state corporate laws, (b) applicable federal and state securities laws, and (c) the rules of the SEC and each stock exchange on which the combined company’s shares are listed.
The Combined Company Board and the Nominating and Corporate Governance Committee do not expect to adopt specific policies with respect to a particular mix or diversity of skills, experience, expertise, perspectives, and background that nominees should have. However, the Combined Company Board is expected to be assembled with a focus on attaining a board comprised of people with substantial experience in bioscience, the pharmaceutical industry, corporate management, and finance.
Following the completion of the Merger, the members of the Nominating and Corporate Governance Committee are expected to be , who will serve as Chair, and . Each of the members of the combined company’s Nominating and Corporate Governance Committee will be independent under the applicable rules and regulations of the NYSE American relating to nominating and corporate governance committee independence.
Compensation Committee Interlocks and Inside Participation
In connection with the completion of the Merger, the Combined Company Board is expected to select members of the Compensation Committee. Each member of the Compensation Committee is expected to be a “non-employee” director within the meaning of Rule 16b-3 of the rules promulgated under the Exchange Act and independent within the meaning of the independent director guidelines of the NYSE American. None of the proposed combined company’s executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers who is proposed to serve on the Combined Company Board or Compensation Committee following the completion of the Merger.
Code of Business Conduct and Ethics
The Combined Company Board will maintain a Code of Business Conduct and Ethics that applies to all directors, officers, and employees. The Code of Business Conduct and Ethics, and any applicable waivers or amendments, will be made available on the Combined Company’s website.
AGEX EXECUTIVE AND DIRECTOR COMPENSATION
Executive Officer Compensation
AgeX is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, and a “smaller reporting company” as defined in the rules and regulations of the SEC. As an emerging growth company and as a smaller reporting company, AgeX may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies or smaller reporting companies. Accordingly, this proxy statement/prospectus/information statement includes reduced disclosure about AgeX’s executive compensation arrangements.
Summary Compensation Table
The following table sets forth the compensation awarded to, earned by, or paid to AgeX’s Chief Executive Officer during fiscal year 2022 and the two highest paid individuals who were serving as executive officers as of December 31, 2022 (AgeX’s Named Executive Officers) in respect of their service to AgeX for the fiscal years ended December 31, 2022 and 2021.
| Name and principal position | | Year | | Salary | | | Option Awards(1) | | | All Other Compensation(2) | | | Total | |
| Michael D. West(3) | | 2022 | | $ | 546,782 | | | $ | - | | | $ | 15,250 | | | $ | 562,032 | |
| Chief Executive Officer | | 2021 | | | 546,782 | | | | 137,501 | (4) | | | 14,500 | | | | 698,783 | |
| | | | | | | | | | | | | | | | | | | |
| Andrea E. Park | | 2022 | | | 281,228 | | | | - | | | | 14,061 | | | | 295,289 | |
| Chief Financial Officer | | 2021 | | | 266,019 | | | | 85,938 | (5) | | | 13,301 | | | | 365,258 | |
| | | | | | | | | | | | | | | | | | | |
| Nafees N. Malik(6) | | 2022 | | | 282,272 | (7) | | | - | | | | - | | | | 282,272 | |
| Chief Operating Officer | | 2021 | | | 282,272 | (7) | | | 85,938 | (5) | | | - | | | | 368,210 | |
| (1) | Amounts shown in this column do not reflect dollar amounts actually received by AgeX’s Named Executive Officers. Instead, these amounts reflect the aggregate grant date fair value of each stock option granted, computed in accordance with the provisions of FASB ASC Topic 718, Compensation-Stock Compensation. AgeX used the Black-Scholes Pricing Model to compute option fair values based on applicable exercise and stock prices, an expected option term, volatility assumptions, and risk-free interest rates. AgeX’s Named Executive Officers will only realize compensation upon exercise of the stock options and to the extent the trading price of AgeX’s common stock is greater than the exercise price of such stock options at the time of exercise. One fourth of the options will vest upon completion of 12 full months of continuous employment measured from the date of grant, and the balance of the options vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based on the completion of each month of continuous service as an employee or director of AgeX or its subsidiaries. |
| (2) | Amounts represent 401(k) matching contributions by AgeX for the periods presented unless described otherwise. |
| (3) | On August 9, 2023, Dr. West stepped down as Chief Executive Officer and Dr. Joanne Hackett was appointed as Interim Chief Executive Officer. As described elsewhere in this proxy statement/prospectus/information statement, immediately following the Merger, Steve Ledger, currently Chief Financial Officer of Serina, is expected to serve as Interim Chief Executive Officer of the combined company. |
| (4) | Dr. West’s equity awards in 2021 reflect the fair value of 120,000 stock options awarded in June 2021. |
| (5) | Equity awards in 2021 to Ms. Park and Dr. Malik reflect the fair value of 75,000 stock options awarded in June 2021. |
| (6) | Dr. Malik serves as AgeX’s Chief Operating Officer as a consultant, with his services provided by Juvenescence. Dr. Malik devotes a majority of his time to AgeX’s operations and AgeX reimburses Juvenescence for his services. |
| (7) | Amounts represent consulting fees paid to Juvenescence for Dr. Malik’s services to AgeX. |
Employment Agreements and Change of Control Provisions
Michael D. West: During 2022, AgeX and Dr. West were party to an employment agreement, effective October 18, 2018 (the West Employment Agreement). Pursuant to the West Employment Agreement, Dr. West’s annual base salary was initially set at $525,000. Under the West Employment Agreement, Dr. West was eligible to earn an annual incentive cash bonus with a target of no less than 50% of annual base salary. Actual bonus amounts were based on Dr. West’s attainment of individual performance goals at target levels set by the AgeX Board for the applicable calendar year. If such performance goals for the applicable year were fully achieved, the AgeX Board could approve a bonus amount exceeding the target bonus level.
Under the West Employment Agreement, Dr. West was granted options to purchase 500,000 shares of AgeX’s common stock with an exercise price of $3.00 per share, with one fourth of the options vesting following 12 full months of continuous service as an employee of AgeX, measured from the date of grant, and the balance vesting in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous service as an employee of AgeX.
On August 9, 2023, AgeX and Dr. West entered into a Transition Services and Separation Agreement (the Transition Agreement) pursuant to which Dr. West stepped down as Chief Executive Officer of AgeX but agreed to continue to serve as Chief Executive Officer and as a director of AgeX’s subsidiary Reverse Bio during a “Transition Period.” The Transition Period ended on October 31, 2023. If Dr. West complies with and does not revoke the Transition Agreement and the supplemental release set forth therein, (i) AgeX will transfer to Dr. West title to certain laboratory and other equipment that AgeX has fully amortized for financial reporting purposes, and (ii) Dr. West’s outstanding vested AgeX stock options as of August 9, 2023 will remain exercisable until October 9, 2027.
Under the West Employment Agreement, and as affirmed in the Transition Agreement, Dr. West has agreed to certain covenants regarding confidential information and assignment of inventions, as well as a covenant not to solicit AgeX’s employees during Dr. West’s employment with AgeX and for one year thereafter. The West Employment Agreement also includes a covenant not to compete with AgeX during his employment.
Andrea E. Park: AgeX has entered into an employment agreement with AgeX’s Chief Financial Officer, Andrea E. Park, effective May 15, 2020 (the Park Employment Agreement). Pursuant to the Park Employment Agreement, Ms. Park’s annual base salary was initially set at $265,000. Under the Park Employment Agreement, Ms. Park is eligible to earn an annual incentive cash bonus with a target of no less than 40% of annual base salary. Actual bonus amounts will be based on Ms. Park’s attainment of individual performance goals at target levels set by the AgeX Board for the applicable calendar year. If such performance goals for the applicable year are fully achieved, the AgeX Board may approve a bonus amount exceeding the target bonus level.
Under the Park Employment Agreement, Ms. Park has been granted options to purchase 300,000 shares of AgeX’s common stock with an exercise price of $0.738 per share, with one fourth of the options vesting following 12 full months of continuous service as an employee of AgeX, measured from the date of grant, and the balance vesting in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous service as an employee of AgeX. Such options expire on the earliest of (1) 10 years from the date of grant, (2) three months after Ms. Park’s ceases to provide continuous service to AgeX (other than due to death or disability) or (3) one year after Ms. Park ceases to provide continuous service to AgeX due to death or disability.
Pursuant to the Park Employment Agreement, Ms. Park is entitled to severance benefits under certain circumstances.
If AgeX terminates Ms. Park’s employment without “cause” or she resigns for “good reason” at any time (each as defined in the Park Employment Agreement), she will be entitled to (1) 9 months base salary, (2) all accrued but unpaid salary earned prior to or as of the date of termination or resignation, (3) full payment of Ms. Park’s pro-rated target bonus due for such year and (4) for a period of six months, all benefits under any health insurance plan of AgeX. In addition, if AgeX terminates Ms. Park’s employment without “cause” or she resigns for “good reason,” (1) all of Ms. Park’s outstanding equity awards that would otherwise have vested during the 12 months following termination or resignation will become fully vested and exercisable immediately and (2) with respect to any outstanding vested but unexercised options, the exercise period following termination or resignation will be extended to the earlier of (A) 12 months after termination or (B) the natural expiration date of the applicable option. If AgeX terminates Ms. Park’s employment without “cause,” or she resigns for “good reason,” following a “change of control,” (as defined in the Park Employment Agreement) (1) Ms. Park will be entitled to all of the benefits and payments that she would have been entitled to if her employment had been otherwise terminated without “cause” or if she resigned for “good reason,” as set forth above, and (2) all of Ms. Park’s unvested options and restricted stock units, if any, will become fully vested and exercisable immediately. The severance compensation may be paid in a lump sum or, at AgeX’s election, in installments consistent with the payment of Ms. Park’s salary while employed by AgeX. In order to receive the severance benefits, Ms. Park must execute a general release of all claims against AgeX.
Nafees N. Malik Mr. Malik does not have an employment agreement with AgeX, but has been provided certain acceleration rights with respect to his equity awards by the Compensation Committee of the AgeX Board. On June 4, 2021, the Compensation Committee of the AgeX Board approved by way of a meeting certain acceleration rights of the equity awards held by Mr. Malik. If Mr. Malik is terminated by AgeX without “cause” or resigns for “good reason” (each as defined in the June 4, 2021 Compensation Committee Meeting) within 12 months following a change of control of AgeX, then Mr. Malik would be entitled to, subject to signing and not revoking a release, the full vesting of all of Mr. Malik’s unvested options and restricted stock units (if any), which will become exercisable immediately. Further, with respect to any outstanding vested but unexercised options held by Mr. Malik, the exercise period following termination or resignation will be extended to the earlier of (A) 12 months after termination or (B) the natural expiration date of the applicable option. Mr. Malik is not entitled to receive any severance benefits from AgeX under any circumstances.
Outstanding Equity Awards at 2022 Fiscal Year End
The following table summarizes certain information concerning outstanding stock options and restricted stock units (RSUs) granted by AgeX under the Incentive Plan and held by AgeX’s Named Executive Officers as of December 31, 2022.
| | | | | Option Awards | | | Stock Awards | |
| | | | | Number of Securities Underlying Unexercised Options | | | Option Exercise | | | Option Expiration | | Number of Shares or Units of Stock That Have Not | | | Market Value of Shares or Units of Stock That Have Not | |
| Name | | Grant Date | | Exercisable(1) | | | Unexercisable | | | Price | | | Date | | Vested | | | Vested(2) | |
| Michael D. West | | 6/4/2021 | | | 45,000 | | | | 75,000 | | | $ | 1.45 | | | 6/3/2031 | | | - | | | | - | |
| | | 3/11/2019 | | | 93,752 | | | | 6,248 | | | $ | 4.28 | | | 3/10/2029 | | | 3,125 | (3) | | $ | 1,725 | |
| | | 10/18/2018 | | | 500,000 | | | | - | | | $ | 3.00 | | | 10/17/2028 | | | - | | | | - | |
| | | 10/10/2017 | | | 660,000 | | | | - | | | $ | 2.00 | | | 10/9/2027 | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Andrea E. Park | | 6/4/2021 | | | 28,125 | | | | 46,875 | | | $ | 1.45 | | | 6/3/2031 | | | - | | | | - | |
| | | 5/21/2020 | | | 193,750 | | | | 106,250 | | | $ | 0.738 | | | 5/20/2030 | | | - | | | | - | |
| | | 10/1/2019 | | | 15,833 | | | | 4,167 | | | $ | 1.77 | | | 9/30/2029 | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Nafees N. Malik | | 6/4/2021 | | | 28,125 | | | | 46,875 | | | $ | 1.45 | | | 6/3/2031 | | | - | | | | - | |
| | | 3/11/2019 | | | 65,626 | | | | 4,374 | | | $ | 4.28 | | | 3/10/2029 | | | - | | | | - | |
| | | 10/18/2018 | | | 350,000 | | | | - | | | $ | 3.00 | | | 10/17/2028 | | | - | | | | - | |
| | (1) | Vesting of all options is subject to continued service as an employee, director and/or consultant of AgeX or a subsidiary on the applicable vesting date. One fourth of the options vested or will vest on the first anniversary of the date of grant, and the remaining balance of the options vested or will vest in 36 equal monthly installments thereafter. As described above, effective August 9, 2023, Dr. West forfeited all of his unvested stock options and, subject to his continued compliance with the terms and conditions set forth in the Transition Agreement, his vested stock options will remain exercisable until October 9, 2027. |
| | (2) | Value calculated based on $0.5519 closing price of AgeX common stock on the NYSE American on December 30, 2022. |
| | (3) | Represents RSUs which have vested or will vest according to the following schedule: 12,500 of the shares vested on March 11, 2020, and the remaining 37,500 of the shares vested or will vest in equal quarterly installments over a period of 3 years through March 11, 2023. Each RSU represents a contingent right to receive one share of AgeX common stock. |
Risk Considerations and Recoupment Policies
The Compensation Committee of the AgeX Board considers, in establishing and reviewing the executive compensation program, whether the program encourages unnecessary or excessive risk taking. Most of AgeX’s executive compensation arrangements include a fixed salary that provides a steady income so that executives do not feel pressured to focus exclusively on stock price performance or short term financial targets to the detriment of AgeX’s long-term operational and strategic objectives. AgeX supplements fixed salaries with discretionary bonus awards based on the executive’s performance as well as the performance of AgeX. The stock options and RSUs that AgeX has granted to its executive officers under the Incentive Plan vest over four years, assuring that the executives take a long-term perspective in viewing their equity ownership. Although AgeX has not adopted compensation plans, or made incentive awards, based on quantified financial performance measures, it has adopted a clawback policy intended to comply with Section 811 of the NYSE American Company Guide. In the event of certain restatements of AgeX financial statements, the Clawback Policy will require AgeX to recoup from its executive officers compensation that is granted, earned or vested based wholly or in part upon the attainment of a financial reporting measure, to the extent such compensation (a) was granted during the three fiscal years preceding a determination that AgeX financial statements must be restated, and (b) exceeds the amount of compensation that would have been granted had the grant been based on the restated financial statement amounts. A copy of the Clawback Policy has been posted on our internet website and can be found at www.agexinc.com.
Other Compensation Plans
AgeX does not have any pension plans, defined benefit plans, or non-qualified deferred compensation plans. AgeX may make contributions to 401(k) plan accounts for participating executive officers and other employees.
Non-Employee Director Compensation
Directors and members of committees of the AgeX Board who are AgeX’s employees are entitled to receive compensation as employees but are not compensated for serving as directors or attending meetings of the AgeX Board or committees of the AgeX Board. All directors are entitled to reimbursements for their out-of-pocket expenses incurred in attending meetings of the AgeX Board or committees of the AgeX Board.
For the year ended December 31, 2022, non-employee directors were entitled to receive the following annual cash fees for serving as a director or chair of a designated committee, pro-rated as applicable for length of time served in any particular position.
| ● | Service as a director: $35,000 |
| ● | Chair of the AgeX Board: $60,000 |
| ● | Chair of Audit Committee: $10,000 |
| ● | Chair of Compensation Committee: $5,000 |
| ● | Chair of Nominating & Corporate Governance Committee: $5,000 |
The following table summarizes certain information concerning the compensation paid during the past fiscal year to each of the persons who served as directors during the year ended December 31, 2022 and who were not AgeX’s employees on the date the compensation was earned.
Name | | Fees Earned or Paid in Cash | | | Option Awards (1) | | | Total | |
| Gregory H. Bailey | | $ | 49,010 | | | $ | - | | | $ | 49,010 | |
| Joanne M. Hackett (2) | | $ | 55,740 | | | $ | 47,325 | | | $ | 103,065 | |
| Michael H. May | | $ | 44,875 | | | $ | - | | | $ | 44,875 | |
| (1) | In accordance with SEC rules, the amounts shown reflect the aggregate grant date fair value of stock awards granted to Non-Employee Directors during 2022, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718 (FASB ASC 718). The grant date fair value for the stock options is measured based on the closing price of AgeX’s common stock on the date of grant. See Note 8, Stock-Based Awards to AgeX’s consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for details as to the assumptions used to determine the fair value of the awards. As of December 31, 2022, Dr. Bailey held 165,000 stock options, Dr. Hackett held 65,000 stock options and Dr. May held 126,534 stock options. The foregoing options held by each of the directors are fully vested as of December 31, 2022. |
| (2) | Dr. Hackett was elected as a director on December 29, 2021. On February 2, 2022, Dr. Hackett was awarded 65,000 stock options which had a fair value of $47,325 on the grant date. These options became exercisable in four equal calendar quarters and were fully vested as of December 31, 2022. |
SERINA EXECUTIVE AND DIRECTOR COMPENSATION
Executive Officer Compensation
This section discusses the material components of the executive compensation program for Serina’s executive officers who are named in the 2022 Summary Compensation Table below. In 2022, Serina’s “named executive officers” and their positions were:
| ● | Randall Moreadith, M.D., Ph.D., President and Chief Executive Officer; and |
| ● | Tacey Viegas, Ph.D., Chief Operating Officer. |
None of Serina’s other executive officers received total compensation in excess of $100,000 in 2022.
Summary Compensation Table
The following table sets forth information concerning the compensation of Serina’s named executive officers for the years indicated.
| Name and Principal Position | | Year | | | Salary | | | Bonus | | | Stock Awards | | | Option Awards (1) | | | All Other Compensation (2) | | | Total | |
| Randall Moreadith, M.D., Ph.D. | | 2022 | | | $ | 323,772 | | | $ | 13,490 | | | | — | | | | — | | | $ | 12,146 | | | $ | 349,408 | |
| President and Chief Executive Officer | | 2021 | | | $ | 305,445 | | | $ | 25,455 | | | | — | | | $ | 22,075 | | | $ | 11,373 | | | $ | 364,348 | |
| Tacey Viegas, Ph.D. | | 2022 | | | $ | 256,807 | | | $ | 10,700 | | | | — | | | | — | | | $ | 21,273 | | | $ | 288,780 | |
| Chief Operating Officer | | 2021 | | | $ | 242,271 | | | $ | 20,189 | | | | — | | | $ | 12,656 | | | $ | 23,752 | | | $ | 298,868 | |
| (1) | Amounts represent the aggregate grant date fair value of stock options issued during the years indicated, computed in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification Topic 718 (ASC Topic 718), rather than the amounts paid to or realized by the named individual. We provide information regarding the assumptions used to calculate the value of these awards in Note 9 to the consolidated financial statements for the years ended December 31, 2022 and 2021 included in this proxy statement/prospectus/information statement. |
| (2) | Serina’s named executive officers received perquisites or other personal benefits in 2021 and 2022 towards payments of premiums for health, vision, short-term disability, long-term disability and life insurance. |
Narrative Disclosure to Summary Compensation Table
2022 Salaries and Bonuses
Each of Dr. Moreadith and Dr. Viegas received a base salary in 2022 to provide a fixed component of compensation reflecting the executive’s skill set, experience, role, and responsibilities. Annual base salaries of executives are established at the date of hire and then reviewed periodically by the Serina Board.
For 2022, Serina’s executive bonus program was not based on pre-determined performance metrics. Serina’s executive officers were eligible to receive cash incentive compensation awarded at the discretion of Serina’s Board of up to 25% of such executive officer’s base salary.
2022 Equity Compensation
Serina did not grant stock awards or stock options to any of its named executive officers in 2022. In 2021 Serina granted stock options to certain of its employees and service providers, including its named executive officers. Serina’s stock options generally allow employees to purchase shares of Serina common stock at a price per share equal to the fair market value of Serina common stock on the date of grant, as determined by Serina’s Board.
Other Elements of Compensation
During their employment, Serina’s named executive officers are eligible to participate in employee benefit plans and programs to the same extent and on the same terms as Serina’s other full-time employees generally. We do not maintain any defined benefit pension plans or any nonqualified deferred compensation plans. Serina offers eligible full-time employees, including the NEOs, the opportunity to participate in a tax-qualified 401(k) plan. Employees can contribute eligible earnings up to the Internal Revenue Service’s annual limits on a before-tax basis, which is generally $22,500 for 2023.
Outstanding Equity Awards at 2022 Fiscal Year-End
The following table summarizes the outstanding equity incentive plan awards for each named executive officer as of December 31, 2022.
| | | Option Awards | | Stock Awards | |
| Name | | Grant Date | | Number of securities underlying unexercised options (#) exercisable | | | Number of securities underlying unexercised options (#) unexercisable | | | Option exercise price ($) | | | Option expiration date | | Number of shares or units of stock that have not vested (#) | | | Market value of shares of units of stock that have not vested ($) | |
| Randall Moreadith | | 5/6/2021(1) | | | 533,550 | | | | — | | | $ | 0.06 | | | 5/6/2031 | | | — | | | | — | |
| | | 7/29/2021(2) | | | 96,000 | | | | — | | | $ | 0.06 | | | 7/29/2031 | | | — | | | | — | |
| Tacey Viegas | | 5/6/2021(3) | | | 208,392 | | | | — | | | $ | 0.06 | | | 5/6/2031 | | | — | | | | — | |
| | | 7/29/2021(4) | | | 141,608 | | | | — | | | $ | 0.06 | | | 7/29/2031 | | | — | | | | — | |
| (1) | This option fully vested as of the grant date. |
| (2) | This option fully vested as of February 15, 2023 pursuant to an amendment to the named executive officer’s nonqualified stock option agreement. |
| (3) | This option fully vested as of the grant date. |
| (4) | This option fully vested as of February 15, 2023 pursuant to an amendment to the named executive officer’s nonqualified stock option agreement. |
Executive Compensation Arrangements
Below are descriptions of Serina’s employment arrangements with Serina’s named executive officers, each of whom is employed “at will.”
Employment of Dr. Moreadith
Dr. Moreadith’s employment with Serina is not subject to any written agreement between Dr. Moreadith and Serina. Dr. Moreadith’s base salary is reviewed periodically by the Serina Board and the Serina Board may, but is not required to, increase his base salary at its discretion. For the year ended December 31, 2022, Dr. Moreadith’s annual base salary was $323,772.
Employment of Dr. Viegas
Dr. Viegas’ employment with Serina is not subject to any written agreement between Dr. Viegas and Serina. Dr. Viegas’ base salary is reviewed periodically by the Serina Board and the Serina Board may, but is not required to, increase his base salary at its discretion. For the year ended December 31, 2022, Dr. Viegas’ annual base salary was $256,807.
Post-Employment Compensation and Change in Control Payments and Benefits
Neither Dr. Moreadith nor Dr. Viegas is party to any agreement, plan or policy providing post-employment severance or change in control payments or benefits.
Compensation Changes in Connection with Merger
In connection with the Merger, we may enter into new or additional compensation arrangements with Serina’s named executive officers. The terms of any such arrangements are not yet known.
Director Compensation
During Serina’s year ended December 31, 2022, none of the non-employee directors who served on the Serina Board received compensation from Serina for their services as a director. In connection with the Merger, the Serina Board may approve new compensation arrangements for its board of directors. The terms of any such arrangements are not yet known.
The table below shows the aggregate number of outstanding option awards held as of December 31, 2022 by each non-employee director who was serving as of December 31, 2022. None of Serina’s non-employee directors held any other equity awards as of December 31, 2022.
| Name | | Number of Options Outstanding | |
| Barbara Fisk | | | — | |
| James R. Hudson, Jr. | | | — | |
| Gregory Bailey | | | — | |
| J. Milton Harris, Ph.D. | | | 72,000 | |
Incentive Compensation Plans
Serina’s Board and stockholders previously adopted the Serina 2017 Stock Option Plan.
The following descriptions of the Serina 2017 Stock Option Plan is qualified by reference to the full text of the plan, which is included as an exhibit to the registration statement of which this prospectus is a part.
Serina’s 2017 Stock Option Plan
Serina’s 2017 Stock Option Plan was approved by Serina’s Board and stockholders in January 2017.
Authorized Shares. The Serina 2017 Plan will continue to govern outstanding awards granted thereunder. As of September 30, 2023, options to purchase an aggregate of 1,756,816 shares of Serina’s common stock were outstanding under the Serina 2017 Stock Option Plan, and an additional aggregate of 20,550 shares of Serina’s common stock were reserved for future grants under Serina’s 2017 Stock Option Plan. Upon consummation of the Merger, the Serina 2017 Stock Option Plan will be frozen and no new awards will be granted thereunder.
Plan Administration. Subject to the terms of Serina’s 2017 Stock Option Plan, the Serina Board has the full authority to construe and interpret Serina’s 2017 Stock Option Plan. Serina’s 2017 Stock Option Plan provides that the interpretation and construction by the Serina Board of any provision of Serina’s 2017 Stock Option Plan or of any option granted thereunder is final and binding. The Serina Board is authorized, in its discretion, to select the eligible employees, officers, directors, consultants, and advisors of Serina to whom options are to be granted, the number of shares to be subject to such options, whether such options will be incentive stock options or nonstatutory stock options, any exceptions to non-transferability, any provisions relating to vesting, and the periods during which options may be exercised.
Stock Options. Each option is evidenced by a stock option agreement between Serina and the employee or other eligible person. Options granted under Serina’s 2017 Stock Option Plan are exercisable during a period fixed by the Serina Board, provided that no option will be exercisable after the expiration of 10 years from the date of its grant, and no incentive stock option granted to a person who at the time of the grant owns stock possessing more than 10% of the total combined voting power of all classes of stock of Serina will be exercisable after the expiration of five years from the date of its grant.
Serina’s 2017 Stock Option Plan provides for appropriate adjustments in the aggregate numbers of shares issuable, and in the number of shares subject to Serina’s 2017 Stock Option Plan and/or outstanding options and/or the option price of outstanding options granted, in the event of certain changes in Serina’s capitalization.
Options granted under Serina’s 2017 Stock Option Plan may be exercised, to the extent the right to exercise has accrued, in whole or in any lesser amount authorized by the option agreement at any time prior to the expiration or termination of such options. Exercise is accomplished by written notice to Serina accompanied by payment in full of the option price of the shares to be purchased.
An option may not be assigned or transferred by the employee or other eligible person except by will or by applicable laws of descent and distribution, and, during the lifetime of an employee or other such person may be exercised or accepted only by the employee or other such person. No transfer of an option by the participant by will or by the laws of descent will be effective unless Serina shall have been furnished with written notice thereof and such evidence as the Serina Board may deem necessary to establish the validity of the transfer and the acceptance by the transferee or transferees of the terms and conditions of Serina’s 2017 Stock Option Plan and applicable stock option agreement.
RELATED PERSON TRANSACTIONS OF THE COMBINED COMPANY
AgeX Related Party Transactions
Related Person Transactions Policy and Procedures
The AgeX Board has adopted a written Related Person Transaction Policy, which applies to transactions exceeding $120,000 in which any of AgeX’s officers, directors, beneficial owners of more than 5% of the outstanding shares of AgeX common stock, or any member of their immediate family, has a direct or indirect material interest, determined in accordance with the policy. Those transactions are referred to as Related Person Transactions. A Related Person Transaction will be subject to review and approval by the Audit Committee of the AgeX Board prior to effectiveness or consummation, to the extent practical. The Audit Committee will review the relevant information available to it about the Related Person Transaction. The Audit Committee may approve or ratify the Related Person Transaction only if the Audit Committee determines that, under the circumstances, the transaction is in, or is not in conflict with, AgeX’s best interests.
Certain Related Person Transactions
Compensation of AgeX’s Interim Chief Executive Officer
On August 9, 2023, AgeX entered into a Consulting Agreement with Dr. Hackett pursuant to which she will receive a fee in the amount of $160,000 per year for services rendered as Interim Chief Executive Officer of AgeX. Dr. Hackett will not be eligible to participate in any AgeX retirement, pension, life, health, accident and disability insurance, or other similar employee benefit plans for AgeX executive officers or employees other than the Incentive Plan.
Compensation of AgeX’s Chief Operating Officer
Since October 2018, AgeX’s Chief Operating Officer, Dr. Malik, who is an employee of Juvenescence, has been devoting a majority of his time to AgeX’s operations for which AgeX reimburses Juvenescence for his services on an agreed upon fixed annual rate of approximately $272,000 from October 18, 2018 through March 10, 2019 and approximately $283,000 from March 11, 2019 through December 31, 2022. Additionally, Dr. Malik received a $50,000 bonus in March 2019. As of December 31, 2022 AgeX had accrued approximately $141,000 payable to Juvenescence for Dr. Malik’s services rendered.
2019 Loan Agreement and Warrant Agreement
On August 13, 2019, AgeX and Juvenescence entered into the 2019 Loan Agreement pursuant to which Juvenescence has provided to AgeX a $2.0 million line of credit for a period of 18 months. On February 10, 2021, AgeX entered into an amendment (the First Amendment) to the 2019 Loan Agreement. The First Amendment extended the maturity date of loans under the 2019 Loan Agreement to February 14, 2022 and increased the amount of the loan facility by $4.0 million. On November 8, 2021, AgeX entered into Amendment No. 2 (the Second Amendment) to the 2019 Loan Agreement. The Second Amendment increased the amount of the loan facility by another $1.0 million. As of December 31, 2021, AgeX had borrowed all of the $7.0 million total line of credit under the 2019 Loan Agreement. Concurrent with the first draw down of funds under the 2019 Loan Agreement, AgeX issued to Juvenescence 19,000 shares of AgeX common stock, with an approximate value of $56,000. On February 14, 2022, AgeX refinanced the $7.0 million outstanding principal amount of the loans and a $160,000 origination fee due under the 2019 Loan Agreement. See the discussion below regarding the 2022 Secured Note and repayment of the amounts borrowed under the 2019 Loan Agreement.
As consideration for the line of credit under the 2019 Loan Agreement, AgeX issued to Juvenescence warrants to purchase 150,000 shares of AgeX common stock, with an exercise price of $2.60 per share, which was the volume weighted average price on the NYSE American (VWAP) of AgeX common stock over the twenty trading days prior to the date the warrants were issued. The warrants expired on August 12, 2022.
2020 Loan Agreement and New Warrant Agreement
On March 30, 2020, AgeX and Juvenescence entered into the 2020 Loan Agreement, which was amended on March 13, 2023 to extend the maturity date by one year, pursuant to which AgeX borrowed $8.0 million from Juvenescence. During July 2023, the full $8 million of 2020 Loan Agreement indebtedness was extinguished in exchange for shares of AgeX Series A preferred stock pursuant to the 2023 Exchange Agreement between AgeX and Juvenescence described below.
Common Stock and 2020 Warrants – Under the terms of the 2020 Loan Agreement, AgeX issued to Juvenescence 28,500 shares of AgeX common stock as an arrangement fee for the loan facility when AgeX borrowed an aggregate of $3.0 million, and AgeX issued to Juvenescence warrants to purchase a total of 3,670,663 shares of AgeX common stock (the 2020 Warrants). The number of 2020 Warrants issued was determined as follows: each time AgeX received an advance of funds under the 2020 Loan Agreement, AgeX issued to Juvenescence a number of 2020 Warrants equal to 50% of the number determined by dividing the amount of the advance by the applicable market price of AgeX common stock. The market price for each 2020 Warrant when issued was the closing price per share of AgeX common stock on the NYSE American on the date of the applicable notice from AgeX requesting a draw of funds that triggered the obligation to issue the 2020 Warrant. The exercise price of the 2020 Warrants is the applicable market price of AgeX common stock. Each of the 2020 Warrants will expire at 5:00 p.m. New York time three years after the date of its issuance. AgeX had issued to Juvenescence 2020 Warrants to purchase 3,670,663 shares of AgeX common stock with exercise prices ranging from $0.70 per share to $1.895 per share representing the market closing price on the NYSE American of AgeX common stock on the one day prior to delivery of the drawdown notices. As of September 12, 2023, 1,900,166 2020 Warrants had expired and 1,770,497 remained outstanding with exercise prices ranging from $0.81 per share to $1.895 per share. The number of shares issuable upon exercise of the 2020 Warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
2022 Secured Convertible Promissory Note and Security Agreement
On February 14, 2022, AgeX and Juvenescence entered into the 2022 Secured Note pursuant to which Juvenescence agreed to provide to AgeX a $13,160,000 line of credit for a period of 12 months. AgeX drew an initial $8,160,000 of the line of credit and used $7,160,000 to refinance the outstanding principal and the loan origination fees under the 2019 Loan Agreement with Juvenescence. On February 9, 2023, AgeX and Juvenescence entered into an Amended and Restated Secured Convertible Promissory Note which amends and restates the 2022 Secured Note and added $2 million to the line of credit available to be borrowed by AgeX under the 2022 Secured Note subject to Juvenescence’s discretion to approve each loan draw. On May 9, 2023, AgeX and Juvenescence entered into an Allonge and Second Amendment to Amended and Restated Convertible Promissory Note (the 2022 Secured Note Second Amendment) that increased the amount of the line of credit available to AgeX by $4,000,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. On June 2, 2023, AgeX and Juvenescence entered into a Third Amendment to Amended and Restated Convertible Promissory Note, to provide that (i) AgeX may draw on the available portion of the line of credit under the 2022 Secured Note until the earlier of the date a Qualified Offering (as defined in the 2022 Secured Note) is consummated by AgeX or October 31, 2023 (subject to Juvenescence’s discretion to approve each loan draw as provided in the 2022 Secured Note), (ii) AgeX will not be obligated to issue additional common stock purchase warrants to Juvenescence in connection with the receipt of loan funds made available pursuant to the 2022 Secured Note Second Amendment, and (iii) the definition of the Reverse Financing Condition as defined in the 2022 Secured Note was amended to extend to June 20, 2023, the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by Reverse Bio. On July 31, 2023, AgeX and Juvenescence entered into a Fourth Amendment to the 2022 Secured Note to provide that (i) the definition of Reverse Financing Condition is amended to extend to October 31, 2023, the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by AgeX’s subsidiary Reverse Bio, and (ii) Juvenescence may convert the outstanding amount of the 2022 Secured Note loans or any portion of such loans into AgeX common stock without restriction by the “19.9% Cap” if Juvenescence elects to convert those amounts at a conversion price or prices equal to the “Drawdown Market Prices” applicable to such loan amounts in lieu of a lower conversion price set with reference to the current market price of AgeX common stock at the time of conversion. The 19.9% Cap is a provision of the 2022 Secured Note that limits the amount of common stock that Juvenescence may acquire through the conversion of 2022 Secured Note loans in order to comply with NYSE American requirements pertaining to the amount of shares that a listed company, such as AgeX, may sell at a price less than the market prices prevailing at the time the loans were made (the Drawdown Market Prices) without shareholder approval.
The date on which the outstanding principal balance of the 2022 Secured Note will become due and payable shall be February 14, 2024. As an arrangement fee for the 2022 Secured Note, AgeX will pay Juvenescence an origination fee in an amount equal to 4% of the amount each draw of loan funds, which will accrue as each draw is funded, and an additional 4% of all the total amount of funds drawn that will accrue following the end of the 12 month period during which funds may be drawn from the line of credit. The origination fee will become due and payable on the maturity date of the 2022 Secured Note or in a pro rata amount with any prepayment of in whole or in part of the outstanding principal balance of the 2022 Secured Note.
During July 2023, $17,992,800 of indebtedness and accrued loan origination fees under the 2022 Secured Note was extinguished in exchange for shares of AgeX Series A preferred stock and Series B preferred stock pursuant to the 2023 Exchange Agreement described below.
On October 3, 2023, AgeX drew $500,000 of its credit available under the 2022 Secured Note. On October 31, 2023 AgeX drew the final $500,000 of the credit line available under the 2022 Secured Note.
On November 9, 2023, AgeX and Juvenescence entered into the 2022 Secured Note Fifth Amendment that increases the amount of the line of credit available to AgeX by $4,400,000, subject to the terms of the 2022 Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit.
Conversion of Loan Amounts into Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance and any accrued but unpaid origination fees into AgeX common stock or “units” if AgeX raises at least $10,000,000 through sale of AgeX common stock (or AgeX common stock paired with warrants or other convertible securities in “units”). The conversion price per share or units shall be the lowest price at which such shares or units are sold. Juvenescence may convert the principal balance and accrued origination fee in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice of Juvenescence’s election to convert the 2022 Secured Note, in whole or in part, into AgeX common stock.
2022 Warrants – Upon each draw down of funds under the 2022 Secured Note prior to June 2, 2023, AgeX issued to Juvenescence warrants to purchase shares of AgeX common stock (the 2022 Warrants). The 2022 Warrants are governed by the terms of a Warrant Agreement, as amended by a Reaffirmation and Amendment Agreement, between AgeX and Juvenescence. The number of 2022 Warrants issued is equal to 50% of the number determined by dividing the amount of the applicable loan draw by the applicable market price. The market price was the last closing price per share of AgeX common stock on the NYSE American preceding the delivery of the notice from AgeX requesting a draw of funds that triggered the obligation to issue 2022 Warrants. The exercise price of the 2022 Warrants is the applicable market price. Each of the 2022 Warrants will expire at 5:00 p.m. New York time three years after the date of its issuance.
As of September 12, 2023, AgeX had issued to Juvenescence 2022 Warrants to purchase a total of 10,357,086 shares of AgeX common stock. The exercise prices of the 2022 Warrants range from $0.59 per share to $0.88 per share representing the market closing price of AgeX common stock on the NYSE American on the one day prior to delivery of the applicable drawdown notices. The number of shares issuable upon exercise of the 2022 Warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
Default Provisions –The loan balance and origination fees may become immediately due and payable prior to the mandatory repayment date if an Event of Default as defined in the 2022 Secured Note occurs. Events of Default under the 2022 Secured Note include the following: (a) AgeX fails to pay any principal amount payable by it in the manner and at the time provided under and in accordance with the 2022 Secured Note; (b) AgeX fails to pay any other amount payable by it in the manner and at the time provided under and in accordance with the 2022 Secured Note or the Security Agreement described below or any other agreement executed in connection with the 2022 Secured Note (the Loan Documents) and the failure is not remedied within three business days; (c) AgeX fails to perform any of its covenants or obligations or fails to satisfy any of the conditions under the 2022 Secured Note or any other Loan Document and, and such failure (if capable of remedy) remains unremedied to the satisfaction of Juvenescence (in its sole discretion) for 10 business days after the earlier of (i) notice requiring its remedy has been given by Juvenescence to AgeX and (ii) actual knowledge of the failure by senior officers of AgeX; (d) if any indebtedness of AgeX in excess of $100,000 becomes due and payable, or a breach or other circumstance arises thereunder such that Juvenescence is entitled to declare such indebtedness due and payable, prior to its due date, or any indebtedness of AgeX in excess of $25,000 is not paid on its due date; (e) AgeX stops payment of its debts generally or ceases or threatens to cease to carry on its business or is unable to pay its debts as they fall due or is deemed by a court of competent jurisdiction to be unable to pay its debts as they fall due, or enters into any arrangements with its creditors generally; (f) if (i) an involuntary proceeding (other than a proceeding instituted by Juvenescence or an affiliate of Juvenescence) shall be commenced or an involuntary petition shall be filed seeking liquidation, reorganization or other relief in respect of AgeX and any subsidiary, or of all or a substantial part of its assets, under any federal, state or foreign bankruptcy, insolvency, receivership or similar law now or hereafter in effect or (ii) an involuntary appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for AgeX or a subsidiary or for a substantial part of its assets occurs (other than in a proceeding instituted by Juvenescence or an affiliate of Juvenescence), and, in any such case, such proceeding shall continue undismissed and unstayed for sixty (60) consecutive days without having been dismissed, bonded or discharged or an order of relief is entered in any such proceeding; (g) it becomes unlawful for AgeX to perform all or any of its obligations under the 2022 Secured Note or any authorization, approval, consent, license, exemption, filing, registration or other requirement of any governmental, judicial or public body or authority necessary to enable AgeX to comply with its obligations under the 2022 Secured Note or to carry on its business is not obtained or, having been obtained, is modified in a manner that precludes AgeX or its subsidiaries from conducting their business in any material respect, or is revoked, suspended, withdrawn or withheld or fails to remain in full force and effect; (h) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX or a subsidiary if such process is not released, vacated or fully bonded within 60 calendar days after its issue or levy; (i) any injunction, order, judgment or decision of any court is entered or issued which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX or a subsidiary to carry on its business or to pay amounts owed to Juvenescence under the 2022 Secured Note; (j) AgeX, whether in a single transaction or a series of related transactions, sells, leases, licenses, consigns, transfers or otherwise disposes of any material portion of its assets (with any such disposition with respect to any asset or assets with a fair value of at least $250,000 being deemed material), other than (i) certain permitted investments (ii) sales, transfers and dispositions of inventory in the ordinary course of business, (iii) any termination of a lease of real or personal property that is not necessary in the ordinary course of the AgeX’s business, could not reasonably be expected to have a material adverse effect and does not result from AgeX’s default, and (iv) any sale, lease, license, consignment, transfer or other disposition of assets that are no longer necessary in the ordinary course of business or which has been approved in writing by Juvenescence; (k) any of the following shall occur: (i) the security and/or liens created by the Security Agreement or any other Loan Document shall at any time cease to constitute valid and perfected security and/or liens on any material portion of the collateral intended to be covered thereby; (ii) except for expiration in accordance with its terms, the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall for whatever reason be terminated or shall cease to be in full force and effect; (iii) the enforceability of the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall be contested by AgeX or a subsidiary; (iv) AgeX shall assert that its obligations under the 2022 Secured Note or any other Loan Document shall be invalid or unenforceable; or (v) a loss, theft, damage or destruction occurs with respect to a material portion of the collateral; (l) there is any change in the financial condition of AgeX and its subsidiaries which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX to perform any of its obligations under the 2022 Secured Note; and (m) any representation, warranty or statement made, repeated or deemed made or repeated by AgeX in the 2022 Secured Note, or pursuant to the Loan Documents, is incomplete, untrue, incorrect or misleading in any material respect when made, repeated or deemed made.
Security Agreement – AgeX has entered into a Security Agreement granting Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs under the 2022 Secured Note, Juvenescence will have the right to foreclose on the assets pledged as collateral. Concurrently with the execution of the 2022 Secured Note Fifth Amendment, AgeX also entered into an additional Pledge Agreement to add shares of a subsidiary to the collateral under the Security Agreement, and AgeX’s subsidiaries ReCyte, Reverse Bio, and UniverXome each entered into a Guaranty Agreement and Joinder Agreement pursuant to which each of them agreed to guaranty AgeX’s obligations to Juvenescence pursuant to the Secured Note, as amended by the 2022 Secured Note Fifth Amendment, and to grant Juvenescence a security interest in their respective assets pursuant to the Security Agreement to secure their obligations to Juvenescence.
The 2023 Secured Convertible Promissory Note and Security Agreement
On March 13, 2023, AgeX and Juvenescence entered into a $10 Million Secured Convertible Promissory Note (the 2023 Secured Note) pursuant to which Juvenescence has loaned to AgeX $10,000,000. AgeX used the loan proceeds to finance a $10,000,000 loan to Serina which is evidenced by a promissory note payable by Serina to AgeX. In lieu of accrued interest, AgeX will pay Juvenescence an origination fee in an amount equal to 7% of the loan funds disbursed to AgeX, which will accrue in two installments. During July 2023, the $10,000,000 principal balance of the 2022 Secured Note indebtedness and a portion of the loan origination fee was extinguished in exchange for shares of AgeX Series B preferred stock pursuant to the 2023 Exchange Agreement described below.
Conversion of Loan Amounts into Common Stock – AgeX may convert the any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. If less than $25,000,000 is raised through the sale of AgeX common stock or units, the conversion price per share or units shall be the lowest price at which shares or units are sold. If at least $25,000,000 is raised, the conversion price per share shall be 85% of the “Market Price” of AgeX common stock determined as provided in the 2023 Secured Note. Juvenescence may convert accrued origination fees into AgeX common stock at the market price per share of AgeX common stock. stock is listed.
Amended Security Agreement – AgeX has entered into an Amended and Restated Security Agreement that amended the February 14, 2022 Security Agreement between AgeX and Juvenescence and added the 2023 Secured Note to the obligations secured by the Security Agreement.
Debt Exchanged for Preferred Stock and Remediation of Stock Exchange Listing Deficiency
In order to eliminate a stockholders equity deficiency and to regain compliance with the continued listing requirements of the NYSE American, on July 24, 2023, AgeX issued to Juvenescence 211,600 shares of a newly authorized Series A preferred stock and 148,400 shares of a newly authorized Series B preferred stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of loans then outstanding under the 2020 Loan Agreement, the 2022 Secured Note, and the 2023 Secured Note, plus the loan origination fees accrued with respect to the 2022 Secured Note and a portion of the loan origination fees accrued pursuant to the 2023 Secured Note. The cancellation of indebtedness in exchange for the Preferred Stock was conducted pursuant to the 2023 Exchange Agreement between AgeX and Juvenescence.
Dividends and Liquidation Preference
Shares of Series A preferred stock and Series B preferred stock are not entitled to receive any payment or distribution of cash or other dividends. In the event of any voluntary or involuntary liquidation, dissolution or other winding up of the affairs of AgeX, subject to the preferences and other rights of any senior stock, before any assets of AgeX shall be distributed to holders of common stock or other junior stock, all of the assets of AgeX available for distribution to stockholders shall be distributed among the holders of the Preferred Stock and any other “parity stock” that may be issued ranking parri passu with the Preferred Stock with respect to liquidation rights, in proportion to the number of shares of Series B preferred Stock and parity stock held by each such holder as of the record date for the determination of holders of Series A preferred stock, Series B preferred stock, and parity stock entitled to receive such distribution, until AgeX shall have distributed to the holders of those shares an amount of assets having a value equal to the subscription price per share. If the assets of AgeX shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the holders of Series A preferred stock, Series B preferred stock and parity stock shall be ratably distributed among such holders. The (i) acquisition of AgeX by another entity by means of any transaction or series of transactions (including, without limitation, any reorganization, merger or consolidation) in which the stockholders of AgeX immediately before such transaction or series of transactions do not own a majority of the outstanding stock of the surviving or acquiring corporation upon completion of such transaction or series of transactions or (ii) a sale of all or substantially all of the assets of AgeX in a single transaction or series of related transactions, shall be deemed a liquidation.
Conversion of Preferred Stock into Common Stock
Each share of Preferred Stock shall be convertible into a number of shares of AgeX common stock determined by dividing (x) a number equal to the number of dollars and cents comprising the subscription price, by (y) a number equal to the number of dollars and cents comprising the conversion price. The subscription price per share of Preferred Stock is $100 which was paid through the exchange of indebtedness for shares of Preferred Stock. The conversion price per share of Series A preferred stock or Series B preferred stock is $0.72 which was the closing price of AgeX common stock on the NYSE American on the last trading day immediately preceding the execution of the Exchange Agreement.
Preferred Stock shall be convertible into AgeX common stock at the election of the holder of shares of Preferred Stock at any time and from time to time. The outstanding shares of Series A preferred stock shall automatically be converted into AgeX common stock without any further act of AgeX or its stockholders (the Automatic Conversion) upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina, or a subsidiary thereof; and (y) February 1, 2024. Further, if the holders of at least a majority of the outstanding shares of Series A preferred stock approve or consent to the Automatic Conversion of the shares of that series, then the outstanding shares of Series A preferred stock shall be converted into common stock upon such approval or consent. The outstanding shares of Series B preferred stock shall automatically be converted into common stock without any further act of AgeX or its stockholders upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina or a subsidiary thereof; and (y) February 1, 2024, provided that such conversion is not limited by the 19.9% Cap or the 50% Cap as described below; and if Automatic Conversion would then be limited by the 19.9% Cap or the 50% Cap, the Automatic Conversion shall take place on the tenth day after such stockholder approvals have been obtained as may be required to permit such Automatic Conversion without the limitations of the 19.9% Cap and the 50% Cap. Further, if the holders of at least a majority of the outstanding shares of Series B preferred stock approve or consent to the Automatic Conversion of the shares of that series, and the conversion is not then limited by the 19.9% Cap or the 50% Cap, then the outstanding shares of Series B preferred stock shall be converted into common stock upon such approval or consent.
If under the rules of the NYSE American or any other national securities exchange on which AgeX common stock may be listed, approval by AgeX stockholders would be required in connection with the issuance of common stock in excess of the “19.9% Cap” upon any conversion of Series B preferred stock, then unless and until such stockholder approval has been obtained, the maximum number of shares of common stock that may be issued upon conversion of all shares of Series B preferred stock shall be an amount equal to the 19.9% Cap. The 19.9% Cap means 7,550,302 shares of common stock, which is 19.9% of the shares of common stock outstanding on February 14, 2022 when the 2022 Secured Note, a portion of which has not been approved by AgeX stockholders for conversion into common stock without regard to the 19.9% Cap and 50% Cap, was issued.
If under the rules of the NYSE American or any other national securities exchange on which AgeX common stock may be listed, approval by AgeX stockholders would be required in connection with the issuance of common stock in excess of the 50% Cap upon any conversion of Series B preferred stock, then unless and until such stockholder approval has been obtained, the maximum number of shares of common stock that may be issued to a holder of Series B preferred stock upon conversion of such shares shall be an amount that, when added to other shares of common stock owned by such holder immediately prior to such conversion would equal one share less than 50% of the outstanding shares of AgeX common stock.
Adjustment of conversion price and subscription price
If AgeX shall (a) declare a dividend or make a distribution on its common stock in shares of common stock, (b) subdivide or reclassify the outstanding common stock into a greater number of shares, or (c) combine or reclassify the outstanding common stock into a smaller number of shares, the conversion price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. If AgeX shall (i) declare a dividend or make a distribution on a series of Preferred Stock in shares of Preferred Stock, (ii) subdivide or reclassify the outstanding shares of a series of Preferred Stock into a greater number of shares, or (iii) combine or reclassify the outstanding shares of a series of Preferred Stock into a smaller number of shares, the subscription price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. Successive adjustments in the conversion price or subscription price, as applicable, shall be made whenever any event specified above shall occur.
Voting Rights
The following matters shall require the approval of the holders of a majority of the shares of a series of Preferred Stock then outstanding, voting as a separate class: (i) creation of any Preferred Stock ranking as senior stock to the series with respect to liquidation preferences; (ii) repurchase of any shares of common stock or other junior stock except shares issued pursuant to or in connection with a compensation or incentive plan or agreement approved by the AgeX Board for any officers, directors, employees or consultants of AgeX; (iii) any sale, conveyance, or other disposition of all or substantially all AgeX’s property or business, or any liquidation or dissolution of AgeX, or a merger into or consolidation with any other corporation (other than a wholly-owned subsidiary corporation) but only to the extent that the DGCL requires that such transaction be approved by each class or series of Preferred Stock; (iv) any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock; or (v) any amendment of AgeX’s Certificate of Incorporation or Bylaws that results in any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock. However, the terms of the Preferred Stock do not restrict or limit the rights and powers of the AgeX Board to fix by resolution the rights, preferences, and privileges of, and restrictions and limitations on, stock ranking as parity stock or junior stock to a series of Preferred Stock. Except as may otherwise be required by the DGCL, as the same may be amended from time to time, the Preferred Stock will have no other voting rights.
Registration Rights Agreements
AgeX entered into a Registration Rights Agreement and certain amendments to the original agreement, pursuant to which it has agreed to register for sale under the Securities Act all shares of AgeX common stock presently held by Juvenescence or that may be acquired by Juvenescence through the exercise of common stock purchase warrants that they hold or that they may acquire pursuant to the 2020 Loan Agreement and the 2022 Secured Note, and shares that they may acquire through the conversion of loans under the 2020 Loan Agreement and the 2022 Secured Note, including principal and accrued interest, and the amount of the loan origination fee under the 2022 Secured Note. AgeX has filed a registration statement on Form S-3, which has become effective under the Securities Act, for offerings on a delayed or continuous basis covering 16,447,500 shares of AgeX common stock held by Juvenescence and 3,248,246 shares of AgeX common stock that may be issued upon the exercise of a portion of the warrants held by Juvenescence. Juvenescence retains the right to require AgeX to register additional shares of AgeX common stock that Juvenescence may acquire through the exercise of warrants or the conversion of 2020 Loan Agreement loans, Secured Note loans, and the origination fee under the 2022 Secured Note. AgeX is obligated to pay the fees and expenses of each registered offering under such registration rights agreement except for underwriting discounts and commissions. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
During July 2023, AgeX and Juvenescence entered into a Registration Rights Agreement pursuant to which AgeX has agreed to use commercially reasonable efforts to register the for sale under the Securities Act the shares of common stock issuable upon conversion of Preferred Stock. A registration statement must be filed upon request of Juvenescence if Form S-3 is available to AgeX. Juvenescence will also have “piggy-back” registration rights if AgeX files a registration statement for the sale of shares for itself or other stockholders, subject to certain customary exceptions based on the nature of the registration statement. AgeX will bear the expenses of the registration statement but not underwriting or broker’s commissions related to the sale of the common stock. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
Stockholder Approval of Certain Matters
2020 Loan Agreement, Secured Note, 2020 Warrants, and 2022 Warrants
In order to comply with applicable NYSE American listing requirements, the 2020 Loan Agreement and the 2022 Secured Note and the related Warrant Agreements governing the 2020 Warrants and 2022 Warrants placed certain limits on the number of shares of AgeX common stock that may be issued to Juvenescence upon conversion of outstanding loan amounts or exercise of the 2020 Warrants or 2022 Warrants prior to stockholder approval of the issuance of shares to Juvenescence that would result in (a) Juvenescence receiving additional shares in excess of a 19.9% Cap based on the number of shares of AgeX common stock outstanding as of March 30, 2020 in the case of a conversion of the outstanding loan amounts under the 2020 Loan Agreement into AgeX common stock, or outstanding as of February 14, 2022 in the case of a conversion of the outstanding loan amounts under Secured Note into AgeX common stock, for less than the greater of book value or the applicable tranche market values of AgeX common stock as of March 20, 2020 or February 14, 2022, or (b) Juvenescence owning 50% or more of the outstanding AgeX common stock (the 50% Cap) in the case of a conversion of the outstanding loan amounts under the 2020 Loan Agreement or the 2022 Secured Note or the exercise of the 2020 Warrants or 2022 Warrants. As required by the terms of the 2020 Loan Agreement and the 2022 Secured Note, AgeX sought and obtained the vote of AgeX stockholders approving (i) the ability of AgeX and Juvenescence to convert the loans under the 2020 Loan Agreement and the 2022 Secured Note into shares of AgeX common stock under the applicable loan conversion provisions even if the conversion would result in (a) Juvenescence receiving additional shares in excess of the 19.9% Cap or the 50% Cap limits, and (ii) the ability of Juvenescence to exercise its 2020 Warrants and 2022 Warrants even if the exercise would cause Juvenescence’s ownership of AgeX common stock to equal or exceed the 50% Cap limit.
Indemnification Agreements
On March 13, 2023, AgeX executed a Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (the Letter of Indemnification) pursuant to which AgeX agreed to indemnify Equiniti Trust Company, LLC (the Equiniti Indemnity) from and against any and all claims, damages, liabilities or losses arising out of the transfer of all of the AgeX common stock held by Juvenescence to its wholly-owned subsidiary, Juvenescence US Corp. (the Share Transfer). In connection with the execution of the Letter of Indemnification, AgeX and Juvenescence entered into a Transfer of Shares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and all claims, damages, liabilities or losses arising out of the Share Transfer or the Equiniti Indemnity.
Serina Related Party Transactions
Related Person Transactions Policy and Procedures
While Serina does not have a formal written policy or procedure for the review, approval or ratification of related party transactions, the Serina Board reviews and considers the interests of its directors, executive officers and principal stockholders in its review and consideration of transactions and obtains the approval of non-interested directors when it determines that such approval is appropriate under the circumstances.
Certain Related Person Transactions
Convertible Note
On March 15, 2023, Serina entered into a Convertible Note Purchase Agreement (the AgeX-Serina Note Purchase Agreement) with AgeX, pursuant to which AgeX lent to Serina an aggregate principal amount of $10,000,000 as evidenced by the AgeX-Serina Note on that date. Interest on the principal amount under the AgeX-Serina Note accrues on the unpaid principal amount at a simple interest rate equal to 7% per annum, computed on the basis of the 360-day year of twelve 30-day months. The outstanding principal balance of the AgeX-Serina Note will become due and payable on March 15, 2026.
Currently, the AgeX-Serina Note provides that upon the consummation of a merger between AgeX and Serina, the AgeX-Serina Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of Serina. Pursuant to the Merger Agreement, the AgeX-Serina Note will be amended prior to the closing of the Merger so that it automatically converts immediately prior to the Merger into shares of Serina capital stock, which shares will be canceled for no consideration in connection with the Merger, and will be treated as a capital contribution.
The outstanding principal balance of the AgeX-Serina Note with accrued interest may become immediately due and payable prior to the stated maturity date if an Event of Default as defined in the AgeX-Serina Note occurs. In addition to this and any other remedy, both in equity and in law, upon the occurrence of an Event of Default, an interest rate of 10% per annum and computed on the basis of the 360-day year of twelve 30-day months, shall apply to the Convertible Amount until fully paid. Events of Default under the AgeX-Serina Note include: (i) the commission of any act of bankruptcy by Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (ii) the execution by Serina of a general assignment for the benefit of creditors, (iii) the filing by or against Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) of a petition in bankruptcy or any petition for relief under the federal bankruptcy act (or, in each case, under any similar insolvency law) or the continuation of such petition without dismissal for a period of 60 calendar days or more, (iv) the appointment of a receiver or trustee to take possession of the property or assets of Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (v) failure of Serina to pay any amount due under the AgeX-Serina Note when due, which failure to pay is not cured by Serina within 5 business days of written notice thereof, (vi) unless waived by AgeX, Serina’s material breach of any representation, warranty or covenant of Serina under the AgeX-Serina Note Purchase Agreement, AgeX-Serina Note or other agreements entered in connection therewith, which breach, if curable, is not cured by Serina within 10 business days of written notice by AgeX thereof, (vii) Serina or any subsidiary shall default on any of its obligations under any indebtedness which default causes the indebtedness thereunder to (x) become prematurely due and payable, (y) be placed on demand or (z) become capable of being declared by or on behalf of a creditor thereunder to be prematurely due and payable or being placed on demand, in each case, as a result of such default or any provision having a similar effect (howsoever prescribed), (viii) any monetary judgment, writ or similar final process shall be entered or filed against Serina, any subsidiary or any of their respective property or other assets for more than $250,000, and such judgment, writ or similar final process shall remain unvacated, unbonded or unstayed for a period of 45 calendar days, and (ix) Serina experiences a Material Adverse Effect (as defined in the AgeX-Serina Note Purchase Agreement).
The AgeX-Serina Note Purchase Agreement and AgeX-Serina Note each includes certain covenants that among other matters require financial reporting and impose certain restrictions, including (i) restrictions on the incurrence of additional indebtedness by Serina and its subsidiaries; (ii) requiring that Serina use note proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, and for general working capital; and (iii) prohibiting Serina from entering into any material sale or transfer transactions outside of the ordinary course of business, other than in a merger between AgeX and Serina, without the consent of AgeX.
In connection with the issuance of the AgeX-Serina Note, AgeX was entitled to elect one member to the Serina Board and receive certain information and inspection rights as well as participation rights for subsequent equity issuances. AgeX designated Gregory Bailey, Executive Chairman of Juvenescence and a member of the AgeX Board, to serve on the Serina Board.
DESCRIPTION OF COMBINED COMPANY CAPITAL STOCK
The following information is a summary of the material terms of the capital stock of the combined company (upon effectiveness of the Merger). You are encouraged to read the Combined Company Charter and the Combined Company Bylaws, which, following completion of the Merger, will be substantially in the forms attached hereto as Annex A and Annex F of this proxy statement/prospectus/information statement, respectively. For more information, see the comparison of relative rights described in the section titled “Comparison of Rights of Stockholders of AgeX, Serina and the Combined Company” of this proxy statement/prospectus/information statement.
General
The combined company’s authorized capital stock will consist of 40,000,000 shares of common stock, par value $0.0001 per share, and 5,000,000 shares of preferred stock, par value $0.0001 per share.
Common Stock
Voting. Each holder of record of common stock will have one vote for each share of common stock which is outstanding on all matters on which stockholders are entitled to vote generally. The holders of shares of common stock will not have cumulative voting rights. Except as otherwise required by law, holders of common stock will not be entitled to vote on any amendment to the Combined Company Charter that relates solely to the terms, number of shares, powers, designations, preferences or relative, participating, optional or other special rights, or to qualifications, limitations or restrictions thereof, of one or more outstanding series of preferred stock if the holders of such affected series are entitled to vote thereon pursuant to the Combined Company Charter or pursuant to the DGCL.
Other Rights. Holders of the combined company shares will not have any dividend, liquidation, preemptive, subscription or conversion rights, and there will not be any redemption or sinking fund provisions applicable to the combined company common stock.
Preferred Stock
The Combined Company Board is authorized to issue from time to time, without further vote or action by the stockholders, up to an aggregate of 5,000,000 shares of preferred stock in one or more series. The Combined Company Board is authorized, within the limitations and restrictions stated in the Combined Company Charter, to determine or alter the rights, preferences, privileges and restrictions granted to or imposed on any wholly unissued series of preferred shares, and the number of shares constituting such series and the designation thereof.
The combined company currently has no plans to issue any shares of preferred stock, but the combined company believes that the ability to issue shares of preferred stock without the expense and delay of a special stockholders’ meeting will provide the combined company with increased flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that might arise. The Combined Company Board could issue shares of preferred stock having conversion privileges or having voting, dividend and liquidation rights superior to those of the shares of common stock, which could adversely affect the voting power of the common stockholders, including the loss of voting control to others, and delay, defer or prevent a change in control of the combined company without further action by the stockholders. This could discourage an acquisition attempt or other transaction that stockholders might believe to be in their best interests or in which they might receive a premium for their stock over the then-market price of the stock.
If the Combined Company Board were to issue a new series of preferred stock, the issuance of such shares could:
| ● | decrease the amount of earnings and assets available for distribution to any common stockholders; |
| ● | make removal of the combined company management more difficult; |
| ● | result in restrictions upon the payment of dividends and other distributions to any common stockholders; |
| ● | delay or prevent a change in control of the combined company; and |
| ● | limit the price that investors are willing to pay in the future for the shares of common stock. |
Anti-takeover Effects of Delaware Law and Provisions of the Combined Company Charter and the Combined Company Bylaws
The combined company is subject to the provisions of Section 203 of the DGCL. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| ● | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| ● | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (i) shares owned by persons who are directors and also officers and (ii) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| ● | on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| ● | any merger or consolidation involving the corporation and the interested stockholder; |
| ● | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| ● | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; or |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with, or controlling, or controlled by, the entity or person. The term “owner” is broadly defined to include any person that, individually, with or through that person’s affiliates or associates, among other things, beneficially owns the stock, or has the right to acquire the stock, whether or not the right is immediately exercisable, under any agreement or understanding or upon the exercise of warrants or options or otherwise or has the right to vote the stock under any agreement or understanding, or has an agreement or understanding with the beneficial owner of the stock for the purpose of acquiring, holding, voting or disposing of the stock.
The restrictions in Section 203 do not apply to corporations that have elected, in the manner provided in Section 203, not to be subject to Section 203 of the DGCL or, with certain exceptions, which do not have a class of voting stock that is listed on a national securities exchange or held of record by more than 2,000 stockholders. The Combined Company Charter and the Combined Company Bylaws do not opt out of Section 203.
Section 203 could delay or prohibit mergers or other takeover or change in control attempts with respect to the combined company and, accordingly, may discourage attempts to acquire the combined company even though such a transaction may offer its stockholders the opportunity to sell their stock at a price above the prevailing market price.
Classified Board of Directors; Removal of Directors; Filling Vacancies. The Combined Company Charter provides that the directors shall be divided into three classes, with each class having a three-year term expiring on a staggered basis. In addition, the Combined Company Charter and Combined Company Bylaws provide that any or all of the directors (other than the directors elected by the holders of any series of preferred stock, voting separately as a series or together with one or more other such series) may be removed only for cause and only by the affirmative vote of the holders of at least a majority in voting power of all the then outstanding shares of stock of the combined company entitled to vote thereon, voting together as a single class. Subject to the rights granted to the holders of any one or more series of Preferred Stock then outstanding, any newly-created directorship on the Combined Company Board that results from an increase in the number of directors and any vacancy occurring on the Combined Company Board (whether by death, resignation, retirement, disqualification, removal or other cause) shall be filled only by a majority of the directors then in office, even if less than a quorum, or by a sole remaining director (and not by the stockholders). Each director so elected shall hold office until the next annual meeting of the stockholders and until a successor has been elected and qualified.
Special Meetings and Notice Procedures. The Combined Company Bylaws provide that, except as otherwise required by law and subject to the rights of the holders of any series of preferred stock, special meetings of stockholders may only be called by or at the direction of the board of directors or the chair of the board of directors. Stockholders shall not be permitted to propose business to be brought before a special meeting of stockholders and the only matters that may be brought before a special meeting are the matters specified in the notice of meeting given by or at the direction of the person calling the meeting. In addition, the Combined Company Bylaws establish an advance written notice procedure for stockholders seeking to nominate candidates for election to the Combined Company Board or to propose matters to be acted upon at stockholders’ meetings. As a result, these provisions of the Combined Company Bylaws may delay stockholder actions with respect to business combinations or a change in management and may make it more difficult for third parties to acquire control of the combined company.
Advanced Notice Bylaws. For director nominations or other business to be properly brought before an annual meeting by a stockholder, such stockholder must generally provide notice to the Secretary of the combined company no later than 90 days and no more than 120 days prior to the first anniversary of the date of the prior year’s annual meeting; provided, that if the date of the annual meeting is advanced by more than 30 days prior to or delayed by more than 70 days after the anniversary of the preceding year’s annual meeting or if no annual meeting was held in the preceding year, such notice must be delivered no more than 120 days prior to such annual meeting nor less than the later of (i) 90 days prior to such annual meeting and (ii) ten days after the day on which public disclosure of the date of such meeting is first made.
Exclusive Jurisdiction. The Combined Company Charter provide that unless the combined company selects or consents in writing to the selection of an alternative forum, (i) any derivative action or proceeding brought on behalf of the combined company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer or other employee or stockholder of the combined company to the combined company or the combined company’s stockholders, creditors or other constituents, (iii) any action asserting a claim against the combined company or any current or former director or officer of the combined company arising pursuant to any provision of the DGCL or the Combined Company Charter or the Combined Company Bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) any action asserting a claim governed by the internal affairs doctrine.
No Action by Consent. Any action required or permitted to be taken by the stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by a consent or consents (written, electronic, or otherwise) of such stockholders.
Amendment to the Combined Company Charter and the Combined Company Bylaws. Pursuant to the DGCL, an amendment to the Combined Company Charter generally requires: (a) recommendation of the Combined Company Board; (b) the affirmative vote of a majority of the voting power of the outstanding stock entitled to vote; and (c) the affirmative vote of a majority of the voting power of the outstanding stock of each class entitled to vote; provided that any provision of Articles 5, 6, 7, 8, and 9 of the Combined Company Charter can only be altered, amended or repealed upon the affirmative vote of the holders of at least two-thirds of the combined company’s capital stock entitled to vote generally in an election of directors, voting together as a single class. Furthermore, the Combined Company Charter and Combined Company Bylaws provide that the Combined Company Board can make, alter, amend and repeal the Combined Company Bylaws subject to the power of the stockholders of the combined company to alter, amend or repeal the bylaws; provided, however, that with respect to the powers of stockholders to make, alter, amend or repeal the bylaws, the affirmative vote of the holders of at least two-thirds of the combined company’s capital stock entitled to vote generally in an election of directors, voting together as a single class, shall be required to make, alter amend or repeal the Combined Company Bylaws or to adopt any provision inconsistent therewith.
Authorized but Unissued Shares of Capital Stock. The authorized but unissued shares of the combined company’s common stock and the combined company’s preferred stock will be available for future issuance following the closing of the Merger without stockholder approval, subject to any limitations imposed by the listing requirements of the NYSE American. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved shares of the combined company’s common stock and the combined company’s preferred stock could make it more difficult or discourage an attempt to obtain control of the combined company by means of a proxy contest, tender offer, merger or otherwise.
Post-Merger Warrants
Prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock three post-merger warrants for each five shares of AgeX common stock held by such holder. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one incentive warrant and will expire on July 31, 2025. Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock and will expire on the four-year anniversary of the Closing Date.
For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 179 of this proxy statement/prospectus/information statement.
Incentive Warrants
Each incentive warrant will be exercisable for one share of AgeX common stock at a price equal to $18.00, subject to certain anti-dilution adjustments pursuant to the terms of the warrant agreement, and will expire on the four-year anniversary of the Closing Date.
For additional information, see the section titled “Agreements Related to the Merger—Warrant Agreement” beginning on page 179 of this proxy statement/prospectus/information statement.
COMPARISON OF RIGHTS OF STOCKHOLDERS OF AGEX, SERINA and the combined company
General
AgeX is incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of AgeX currently, and will continue to be, governed by the DGCL. Serina is incorporated under the laws of the State of Alabama and, accordingly, the rights of the stockholders of Serina are currently governed by the ABCL. If the Merger is completed, the AgeX stockholders and Serina stockholders will become the stockholders of the combined company. After giving effect to the Merger, the rights of stockholders of the combined company and the relative powers of the Combined Company Board will be governed by the Combined Company Charter, the Combined Company Bylaws and by the DGCL. Each share of the common stock of the combined company will be issued pursuant to, and will carry with it the rights and obligations set forth in, the Combined Company Charter. This section summarizes material differences between the rights of AgeX stockholders and Serina stockholders before consummation of the Merger and the rights of the combined company stockholders after consummation of the Merger. These differences in stockholder rights result from the differences between the respective organizational documents of AgeX, Serina and the combined company and the applicable governing law.
The following summary does not include a description of rights or obligations under the U.S. federal securities laws or relevant NYSE American listing requirements or standards.
The following summary is not a complete statement of the rights of the stockholders of AgeX, Serina or the combined company or a complete description of the specific provisions referred to below. The identification of specific differences is not intended to indicate that other equally significant or more significant differences do not exist. This summary is qualified in its entirety by reference to AgeX’s, Serina’s and the combined company’s organizational documents, which you are urged to read carefully.
The form of the Combined Company Charter, the form of the Combined Company Bylaws, the AgeX Charter, the AgeX Bylaws, Serina’s amended and restated certificate of incorporation (the Serina Charter) and Serina’s second amended and restated bylaws (the Serina Bylaws) are attached hereto as Annex B, Annex F, Exhibit 3.1, Exhibit 3.2, Exhibit 3.3 and Exhibit 3.4, respectively, of this proxy statement/prospectus/information statement and are incorporated herein by reference.
| AgeX | | Serina | | Combined Company |
| Authorized Capital Stock |
| |
AgeX’s authorized capital stock consists of 200,000,000 shares of common stock, $0.0001 par value per share, and 5,000,000 shares of preferred stock, $0.0001 par value per share. Holders of AgeX common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to AgeX common stock. The rights, preferences and privileges of the holders of AgeX common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of AgeX preferred stock that it may designate in the future. | | The Serina Charter authorizes the issuance of up to 15,000,000 shares of common stock, $0.01 par value per share, and 10,000,000 shares of preferred stock, $0.01 par value per share (the Serina Preferred Stock), of which 400,000 shares are designated Series A Preferred Stock, 300,000 shares are designated Series A-1 Preferred Stock, 1,122,077 shares are designated Series A-2 Preferred Stock, 499,200 shares are designated Series A-3 Preferred Stock, 718,997 shares are designated Series A-4 Preferred Stock and 2,000,000 shares are designated Series A-5 Preferred Stock. | | The combined company’s authorized capital stock will consist of 40,000,000 shares of common stock, $0.0001 par value per share, and 5,000,000 shares of preferred stock, $0.0001 par value per share. |
| | | | | |
| Common Stock |
| |
| AgeX’s authorized capital stock consists of 200,000,000 shares of common stock, $0.0001 par value per share. | | Serina is authorized to issue up to 15,000,000 shares of common stock, $0.01 par value per share. The Serina Charter provides that every stockholder shall be entitled to one vote for each share of common stock of Serina held by them (including all Serina Preferred Stock on an as-converted basis) as of the record date for such meeting. | | The combined company will be authorized to issue capital stock up to 40,000,000 shares of common stock, par value $0.0001 per share. Each holder of record of the combined company common stock, as such, shall have one vote for each share of common stock which is outstanding in his, her or its name on the stock records of the combined company on the record date, as provided by the Combined Company Bylaws, on all matters on which stockholders are entitled to vote generally. |
| | | | | |
| Preferred Stock |
| |
The AgeX Board is authorized, subject to limitations prescribed by Delaware law, to issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of its qualifications, limitations or restrictions. AgeX has 211,600 shares of Series A preferred stock and 148,400 shares of Series B preferred stock issued and outstanding (collectively, the AgeX preferred stock). | | The Serina Charter provides that each holder of shares of Serina Preferred Stock shall, subject to certain conditions, have the right to convert such shares into shares of Serina common stock at any time in accordance with the Serina Charter. | | The Combined Company Board will be authorized to issue from time to time, without further vote or action by the stockholders, up to an aggregate of 5,000,000 shares of preferred stock in one or more series. The Combined Company Board will be authorized, within the limitations and restrictions stated in the Combined Company Charter, to determine or alter the rights, preferences, privileges and restrictions granted to or imposed on any wholly unissued series of preferred shares, and the number of shares constituting such series and the designation thereof. |
Subject to certain limitations and restrictions, the outstanding shares of AgeX preferred stock shall automatically be converted into AgeX common stock without any further act of AgeX or its stockholders upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina, or a subsidiary thereof; and (y) February 1, 2024. Further, if the holders of at least a majority of the outstanding shares of AgeX preferred stock approve or consent to such automatic conversion of the shares of that series, then the outstanding shares of AgeX preferred stock of that series shall be converted into AgeX common stock upon such approval or consent. AgeX preferred stock shall be convertible into common stock at the election of the holder of shares of preferred stock at any time and from time to time. Certain matters require the approval of the holders of a majority of the shares of AgeX preferred stock, including but not limiting to (i) creation of any preferred stock ranking as senior stock to the AgeX preferred stock with respect to liquidation preferences; (ii) any sale, conveyance, or other disposition of all or substantially all AgeX’s property or business, or any liquidation or dissolution of AgeX, or a merger into or consolidation with any other corporation, but only to the extent that the DGCL requires that such approval; and (iii) any amendment of the AgeX Charter or AgeX Bylaws that results in any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on AgeX preferred stock. | | Each share of Serina Preferred Stock is convertible into a number of Serina common stock determined by dividing the applicable original issue price by the applicable conversion price in effect at the time of conversion. The applicable conversion price of each series of Serina Preferred Stock are subject to further adjustment if the outstanding shares of Serina common stock are subdivided by stock dividend split or otherwise or if the outstanding shares of Serina common stock are combined or consolidated by reclassification or otherwise. The current conversion prices of the Serina Preferred Stock are equal to the applicable original issue prices of each series of Serina Preferred Stock and each share of Serina Preferred Stock is convertible into 1.00 share of Serina common stock. The Serina Charter provides that for so long as any shares of its Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock or Series A-5 Preferred Stock is outstanding, Serina shall first obtaining the affirmative vote or written consent of the holders of at least 60% of the outstanding shares of such series of preferred stock, voting or consenting together as a separate class, before altering or changing the rights, preferences or privileges of the such preferred stock set forth in the Serina Charter. | | The combined company currently has no plans to issue any shares of preferred stock, but the combined company believes that the ability to issue shares of preferred stock without the expense and delay of a special stockholders’ meeting will provide the combined company with increased flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that might arise. The Combined Company Board could issue shares of preferred stock having conversion privileges or having voting, dividend and liquidation rights superior to those of the shares of common stock, which could adversely affect the voting power of the common stockholders, including the loss of voting control to others, and delay, defer or prevent a change in control of the combined company without further action by the stockholders. This could discourage an acquisition attempt or other transaction that stockholders might believe to be in their best interests or in which they might receive a premium for their stock over the then-market price of the stock. |
| Number of Directors |
| |
| The AgeX Charter and AgeX Bylaws provide the number of directors shall be set from time to time by the AgeX Board and shall be no less than one and not more than three directors. The AgeX Board currently has three members. | | The Serina Bylaws provide that the number of directors shall be set from time to time by the Serina Board and shall be no less than one and not more than nine directors. The Serina Charter provides for the election of one or more directors as specified in the Serina Bylaws. | | The Combined Company Charter and Combined Company Bylaws provide the number of directors shall be set from time to time by the Combined Company Board. At the Effective Time, the combined company is expected to initially have a seven-member board. |
| | | | | |
| Stockholder Nominations and Proposals |
| |
| The AgeX Bylaws provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder’s notice. | | None. | | The Combined Company Bylaws provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder’s notice. |
| | | | | |
| Classification of Board of Directors |
| |
| The AgeX Charter and AgeX Bylaws do not provide for the division of the AgeX Board into staggered classes. | | The Serina Charter and the Serina Bylaws do not provide for the division of the Serina Board into staggered classes. | | The Combined Company Charter provides that the directors shall be divided into three classes, with each class having a three-year term expiring on a staggered basis. |
| Removal of Directors |
| |
| The AgeX Bylaws provide that entire AgeX Board or any individual director may be removed as provided by the DGCL. | | The Serina Bylaws provide that, any director may be removed from the Serina Board at any time, with or without cause, by the holders of a majority of the shares then entitled to vote in an election of directors. If a director is elected by a voting group of stockholders, only the stockholders of that voting group may participate in the vote to remove that director. | | The Combined Company Charter and Combined Company Bylaws provide that any or all of the directors (other than the directors elected by the holders of any series of preferred stock, voting separately as a series or together with one or more other such series) may be removed only for cause and only by the affirmative vote of the holders of at least a majority in voting power of all the then outstanding shares of stock of the combined company entitled to vote thereon, voting together as a single class. |
| | | | | |
| Vacancies on the Board of Directors |
| |
| Any vacancies on the AgeX Board resulting from death, resignation, or removal of any director, the increase in the number of directors authorized, or if the Court of Chancery has removed a director for conviction of a felony, or if the stockholders fail, at any meeting of stockholders at which any director or directors are elected, to elect the number of directors to be voted for at that meeting, shall, except as otherwise provided by law, be filled by a majority of the remaining directors, though less than a quorum, or by the sole remaining director. Each director so elected shall hold office until the next annual meeting of the stockholders and until a successor has been elected and qualified. The stockholders may elect a director or directors at any time to fill any vacancy or vacancies not filled by the directors, but any such election by written consent shall require the consent of a majority of the outstanding shares entitled to vote. | | The Serina Bylaws provide that vacancies occurring on its board of directors may be filled by affirmative vote of the majority of directors then in office, even if less than a quorum, or the holders of a majority of the shares then entitled to vote in an election of directors. If the vacant office was held by a director elected by a voting group of stockholders, only the holders of shares of that voting group are entitled to vote to fill the vacancy if it is filled by the stockholders, and only the remaining directors elected by that voting group, even if less than a quorum, are entitled to fill the vacancy if it is filled by the directors. | | Subject to the rights granted to the holders of any one or more series of Preferred Stock then outstanding, any newly-created directorship on the Combined Company Board that results from an increase in the number of directors and any vacancy occurring on the Combined Company Board (whether by death, resignation, retirement, disqualification, removal or other cause) shall be filled only by a majority of the directors then in office, even if less than a quorum, or by a sole remaining director (and not by the stockholders). Each director so elected shall hold office until the next annual meeting of the stockholders and until a successor has been elected and qualified. |
| Cumulative Voting |
| |
| AgeX stockholders do not have cumulative voting rights. | | The Serina Charter states that stockholders shall not be entitled to cumulative voting in the elections of directors. | | The Combined Company Charter provides that the holders of shares of common stock shall not have cumulative voting rights. |
| | | | | |
| Stockholder Action by Written Consent |
| |
| The AgeX Bylaws provide that any action that may be taken at any annual or special meeting of stockholders may be taken without a meeting and without prior notice, if a consent in writing, setting forth the action so taken, is signed by the holders of outstanding shares having not less than the minimum number of votes that would be necessary to authorize or take that action at a meeting at which all shares entitled to vote on that action were present and voted. | | The Serina Bylaws provide that any action required by the ABCL or permitted to be taken at any annual or special meeting of stockholders, may be taken without a meeting, without prior notice and without a vote, if a consent or consents in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. | | Any action required or permitted to be taken by the combined company’s stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by a consent or consents (written, electronic, or otherwise) of such stockholders. |
| | | | | |
| Notice of Stockholder Meeting |
| |
The AgeX Bylaws provide that written notice of a meeting of the stockholders shall be given not less than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote at such meeting. The notice shall specify the place, if any, date and time of the meeting, the means of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, the record date for determining the stockholders entitled to vote at the meeting, if such date is different from the record date for determining stockholders entitled to notice of the meeting, and, in the case of a special meeting, the purpose or purposes for which the meeting is called. Notice need not be given to any stockholder who submits a written waiver of notice signed by him or her before or after the time stated therein. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends a meeting for the express purpose of objecting at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. | | The Serina Bylaws provide that notice of each annual or special meeting of the stockholders shall be given to each stockholder of record entitled to vote at such meeting not less than ten and nor more than 60 days before the day of the meeting to each stockholder entitled to vote at such meeting. Every such notice shall state the date, time, place, if any, the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting must include the record date for determining the stockholders entitled to vote at the meeting. Notice of a special meeting shall also include a description of the purpose or purposes for which the meeting is called. A stockholder’s attendance at a meeting shall constitute a waiver of objections to lack of or defective notice of a stockholders’ meeting or to consideration of particular matters at the meeting, in each case to the extent provided in the ABCL. | | The Combined Company Bylaws provide that written notice of a meeting of the stockholders shall be given not less than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote at such meeting. The notice shall specify the place, if any, date and hour of the meeting, the means of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, and, in the case of a special meeting, the purpose or purposes for which the meeting is called. Notice may be waived in writing, signed by the person entitled to notice thereof, or by electronic transmission by such person, either before or after such meeting, and will be waived by any stockholder by the stockholder’s attendance thereat in person, by remote communication, if applicable, or by proxy, except when the stockholder attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. |
| Special Stockholder Meetings |
| |
| The AgeX Bylaws provide that a special meeting of stockholders may be called at any time by the chairman of the AgeX Board, a majority of the directors then in office even if less than a quorum of the authorized number of directors, or by one or more stockholders entitled to cast not less than 10% of the votes eligible to the cast at that meeting. | | The Serina Bylaws provide that a special meeting of the stockholders may be called at any time by the Serina Board. | | The Combined Company Bylaws provide that, except as otherwise required by law and subject to the rights of the holders of any series of preferred stock, special meetings of stockholders may only be called by or at the direction of the board of directors or the chair of the board of directors. |
| | | | | |
| Indemnification |
| |
The DGCL permits a corporation to indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed legal action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe that the person’s conduct was unlawful. The AgeX Charter provides that the liability of the AgeX directors to AgeX or its stockholders for monetary damages for breach of fiduciary duty as a director is eliminated to the fullest extent permissible under DGCL; provided that this limitation of the liability does not apply: (i) for any breach of the director’s duty of loyalty to AgeX or its stockholders; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; (iii) under § 174 of the DGCL; or (iv) for any transaction from which the director derived an improper personal benefit. The corporation is authorized to indemnify directors, officers, and agents to the fullest extent permissible under Delaware law. AgeX is authorized to indemnify directors, officers, and agents to the fullest extent permissible under DGCL. | |
The ABCL provides that a corporation may indemnify an individual made a party to a proceeding because such individual is or was a director or officer of the corporation against liability incurred in a proceeding if: (i) such individual’s conduct was in good faith; (ii) the individual reasonably believed (a) the conduct was in its best interest of the corporation in the case of conduct performed in an official capacity; and (b) in all other cases, the conduct was at least not opposed to the corporation’s best interest; and (iii) in the case of any criminal proceeding the individual had no reasonable cause to believe the conduct was unlawful. Under the ABCL a corporation may not indemnify a director or officer in connection with a proceeding by or in the right of the corporation in which the director or officer has not met the relevant standard of conduct; or in connection with any other proceeding with respect to conduct for which the director or officer received an improper financial benefit. Under the ABCL, a corporation may advance funds to pay for or reimburse expenses incurred in connection with a proceeding by a director or officer. Serina is authorized to indemnify directors, officers, and agents to the fullest extent permissible under law, including the ABCL. Serina has entered into separate indemnification agreements with certain of its directors in addition to the indemnification provided for in the Serina Charter and the Serina Bylaws. Pursuant to Serina’s Charter, the Serina directors shall not be personally liable to Serina or its stockholders for monetary damages for any actions or failures to act, except liability for (i) the amount of improper financial benefit received, (ii) an intentional infliction of harm on Serina or its stockholders, (iii) a violation of Section 10A-2A-8.32 of the ABCL, (iv) an intentional violation of law, or (v) a breach of the director’s duty of loyalty to Serina or its stockholders. | | The DGCL permits a corporation to indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed legal action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe that the person’s conduct was unlawful. The Combined Company Bylaws provide that, to the fullest extent not prohibited by the DGCL or any other applicable law, the combined company shall indemnify its directors and executive officers; provided, however, that the combined company may modify the extent of such indemnification by individual contracts with its directors and executive officers. The Combined Company Charter provides that no director of the combined company, and no officer of the combined company serving in such capacity, shall be personally liable to the combined company or its stockholders for monetary damages for breach of fiduciary duty as a director or officer to the fullest extent permissible under the DGCL or other Delaware law. |
| | | | | |
| Amendment of Certificate of Incorporation |
| |
| Pursuant to the DGCL, an amendment to the AgeX Charter generally requires: (a) recommendation of the AgeX Board; (b) the affirmative vote of a majority of the voting power of the outstanding stock entitled to vote; and (c) the affirmative vote of a majority of the voting power of the outstanding stock of each class entitled to vote. The AgeX Charter does not include any additional conditions on its amendment. | | Serina may amend, alter, change or repeal any provision of the Serina Charter, in a manner prescribed by statute; provided that any such amendment may be subject to the protective provisions described above. | | Pursuant to the DGCL, an amendment to the Combined Company Charter generally requires: (a) recommendation of the Combined Company Board; (b) the affirmative vote of a majority of the voting power of the outstanding stock entitled to vote; and (c) the affirmative vote of a majority of the voting power of the outstanding stock of each class entitled to vote; provided that any provision of Articles 5, 6, 7, 8, and 9 of the Combined Company Charter can only be altered, amended or repealed upon the affirmative vote of the holders of at least two-thirds of the combined company’s capital stock entitled to vote generally in an election of directors, voting together as a single class. |
| Amendment of Bylaws |
| |
| The AgeX Charter and AgeX Bylaws provide that the AgeX Bylaws can be altered, amended or repealed (i) by AgeX’s stockholders by a majority of the voting power of the shares represented and entitled to vote or (ii) by the AgeX Board by a majority vote of the board members present at any board meeting where a quorum is present. | | Under the Serina Charter, the Serina Board is expressly authorized to make, repeal or alter any or all of its bylaws. The Serina Bylaws provide that the bylaws may be amended or repealed by the Serina Board; provided that the stockholders may adopt or amend a bylaw that fixes a greater quorum or voting requirement for stockholders and such bylaw may not be adopted, amended or repealed by the Serina Board and any bylaw that fixes a greater quorum or voting requirements for the Serina Board may be amended or repealed only by the stockholders if originally adopted by the stockholders or by either the stockholders or the Serina Board if originally adopted by the Serina Board. | | The Combined Company Charter and Combined Company Bylaws provide that the Combined Company Board can make, alter, amend and repeal the Combined Company Bylaws subject to the power of the stockholders of the corporation to alter, amend or repeal the bylaws; provided, however, that with respect to the powers of stockholders to make, alter, amend or repeal the bylaws, the affirmative vote of the holders of at least two-thirds of the combined company’s capital stock entitled to vote generally in an election of directors, voting together as a single class, shall be required to make, alter amend or repeal the Combined Company Bylaws or to adopt any provision inconsistent therewith. |
| | | | | |
| Forum Selection |
| |
The AgeX Charter and the AgeX Bylaws do not provide for the forum selection provisions. | | The Serina Charter provides that unless Serina consents in writing to the selection of an alternative forum, the Circuit Court of Madison County Alabama shall be the sole and exclusive forum for (i) any claim that is based upon a violation of a duty under the laws of the State of Alabama by a current or former director, officer or stockholder in their capacities as such, (ii) any derivative action or proceeding brought on behalf of Serina, (iii) any action asserting a claim arising pursuant to any provision of the ABCL, the Serina Charter or the Serina Bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine. If the Circuit Court of Madison County does not have jurisdiction, then another circuit court of the State of Alabama shall be the sole and exclusive forum for such claims and actions and if no circuit court of the State of Alabama has jurisdiction, then the federal district court for the Northern District of Alabama sitting in Madison County, Alabama shall be the sole and exclusive forum for such claims and actions. | | The Combined Company Charter provides that unless the combined company selects or consents in writing to the selection of an alternative forum, (i) any derivative action or proceeding brought on behalf of the combined company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer or other employee or stockholder of the combined company to the combined company or the combined company’s stockholders, creditors or other constituents, (iii) any action asserting a claim against the combined company or any current or former director or officer of the combined company arising pursuant to any provision of the DGCL or the Combined Company Charter or the Combined Company Bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) any action asserting a claim governed by the internal affairs doctrine. |
PRINCIPAL STOCKHOLDERS OF AGEX
References to “we,” “us” and “our” in this section titled “Principal Stockholders of AgeX” refer to AgeX.
The following table sets forth information regarding the beneficial ownership of AgeX common stock as of November 3, 2023, by (i) each of our Named Executive Officers, (ii) each of our directors, (iii) all of our directors and executive officers as a group; and (iv) each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of AgeX common stock. Our calculation of the percentage of beneficial ownership is based on 37,951,261 shares of AgeX common stock outstanding as of November 3, 2023.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are currently exercisable or exercisable within 60 days of November 3, 2023, and restricted stock units that will vest within 60 days of November 3, 2023. Shares of AgeX common stock issuable pursuant to stock options and restricted stock units currently exercisable or exercisable within 60 days of November 3, 2023, and restricted stock units that will vest within 60 days of November 3, 2023, are deemed outstanding for computing the percentage of the person holding such equity awards and the percentage of any group of which the person is a member but are not deemed outstanding for computing the percentage of any other person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all shares they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 16 of the Exchange Act.
| Name of Beneficial Owner | | Number of Shares Beneficially Owned | | | Percentage of Shares Beneficially Owned | |
| 5% Stockholders | | | | | | | | |
| Juvenescence Limited and certain affiliates (1) | | | 81,995,827 | | | | 79.22 | % |
| Broadwood Partners, L.P. and certain affiliates (2) | | | 3,003,446 | | | | 7.9 | % |
| | | | | | | | | |
| Named Executive Officers and Directors | | | | | | | | |
| Michael D. West (3) | | | 1,371,982 | | | | 3.5 | % |
| Andrea E. Park (4) | | | 335,976 | | | | * | |
| Nafees N. Malik (5) | | | 466,875 | | | | 1.2 | % |
| Gregory H. Bailey (6) | | | 165,000 | | | | * | |
| Joanne M. Hackett (6) | | | 65,000 | | | | * | |
| Michael H. May (6) | | | 126,534 | | | | * | |
| Jean-Christophe Renondin(7) | | | 25,821 | | | | * | |
| All executive officers and directors as a group (8 persons) (8) | | | 2,625,446 | | | | 6.5 | % |
| | * | Less than 1% |
| | | |
| | (1) | Includes 16,447,500 shares of AgeX common stock held by Juvenescence US Corp, a wholly-owned US subsidiary of Juvenescence, Limited, 11,539,348 shares of AgeX common stock that may be acquired upon the exercise of common stock purchase warrants, 4,008,980 shares of AgeX common stock that may be acquired through the conversion of $2,500,000 of certain outstanding loans into shares of AgeX common stock at an assumed conversion price of $0.06236 per share based on the closing price of AgeX common stock on the NYSE American on October 30, 2023, and 29,388,888 shares of AgeX common stock that may be acquired through the conversion of 211,600 shares of Series A preferred stock, and 20,611,111 shares of AgeX common stock that may be acquired through the conversion of 148,400 shares of Series B preferred stock. The address of Juvenescence is 18 Athol Street, Douglas, Isle of Man IM1 1JA. The foregoing information is based solely on a Schedule 13D/A filed with the SEC on November 1, 2023, which provides information only as of October 31, 2023 and consequently, Juvenescence’s beneficial ownership may have changed since that date. |
| | (2) | Includes 2,997,156 shares of AgeX common stock owned by Broadwood Partners, L.P. and 6,290 shares of AgeX common stock owned by Neal Bradsher. Broadwood Capital, Inc. is the general partner of Broadwood Partners, L.P. Neal Bradsher is the President of Broadwood Capital, Inc. Mr. Bradsher and Broadwood Capital, Inc. have disclaimed beneficial ownership of the shares owned by Broadwood Partners, L.P. except to the extent of their respective pecuniary interests in such shares. The address of these entities is 142 West 57th Street, 11th Floor, New York, NY 10019. The foregoing information is based solely on a Schedule 13G filed with the SEC on December 10, 2018, which provides information only as of November 28, 2018, and, consequently, the beneficial ownership of these reporting entities or persons may have changed since that date. |
| | (3) | Includes 1,330,000 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable. |
| | | |
| | (4) | Includes 335,625 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days. Excludes 59,375 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| | | |
| | (5) | Consists entirely shares of common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days. Excludes 28,125 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| | | |
| | (6) | Consists entirely shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable. |
| | | |
| | (7) | Consists entirely shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days. |
| | | |
| | (8) | Includes 2,582,980 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are presently exercisable or that will become exercisable within 60 days. Excludes 89,375 shares of AgeX common stock that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
PRINCIPAL STOCKHOLDERS OF SERINA
The following table sets forth certain information with respect to the beneficial ownership of Serina common stock, on an as-converted to common stock basis assuming a 1 to 1 conversion of Serina preferred stock to Serina common stock, as of November 5, 2023, for:
| ● | each person, or group of affiliated persons, known by Serina to beneficially own more than 5% of Serina’s outstanding shares of Serina capital stock; |
| ● | each of Serina’s directors; |
| ● | each of Serina’s named executive officers; and |
| ● | all directors and executive officers of Serina as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of November 5, 2023 through the exercise of stock options or other rights. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of capital stock held by that person.
The percentage of shares beneficially owned is computed on the basis of 5,987,562 shares of Serina common stock outstanding as of November 5, 2023, after giving effect to the automatic conversion of all 3,520,128 shares of Serina preferred stock outstanding as of November 5, 2023, into 3,520,128 shares of Serina common stock, assuming a 1 to 1 conversion of Serina preferred stock to Serina common stock, and includes 2,467,434 shares of Serina common stock outstanding as of November 5, 2023. Shares of Serina common stock that a person has the right to acquire within 60 days of November 5, 2023, are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless noted otherwise, the address of all listed stockholders is 601 Genome Way, Suite 2001, Huntsville, Alabama 35806.
| | | Beneficial Ownership as of November, 2023 | |
| Beneficial Owner | | Number of Shares(1) | | | Percent of Total | |
| 5% and Greater Stockholders: | | | | | | | | |
| Puffinus L.P.(2) | | | 1,004,482 | | | | 16.56 | % |
| Helen W. McMillan(3) | | | 857,354 | | | | 14.00 | % |
| Randall Moreadith(4) | | | 629,550 | | | | 9.51 | % |
| Michael D. Bentley(5) | | | 572,000 | | | | 9.44 | % |
| Ruth C. Bentley(6) | | | 572,000 | | | | 9.44 | % |
| Barbara M. Fisk(7) | | | 544,609 | | | | 9.04 | % |
| Miguel A. Loya(8) | | | 509,039 | | | | 8.39 | % |
| Tacey Viegas(9) | | | 410,000 | | | | 6.47 | % |
| John Benton Harris(10) | | | 311,638 | | | | 5.20 | % |
| Directors and Named Executive Officers: | | | | | | | | |
| Randall Moreadith(4) | | | 629,550 | | | | 9.51 | % |
| Michael D. Bentley(5) | | | 572,000 | | | | 9.44 | % |
| Barbara M. Fisk(7) | | | 544,609 | | | | 9.04 | % |
| Tacey Viegas(9) | | | 410,000 | | | | 6.47 | % |
| James R. Hudson, Jr.(11) | | | 212,000 | | | | 3.54 | % |
| Steve Ledger(12) | | | 116,666 | | | | 1.90 | % |
| J. Milton Harris(13) | | | 72,000 | | | | 1.19 | % |
| Gregory Bailey | | | — | | | | — | |
| All executive officers and directors as a group (8 persons)(14) | | | 2,556,825 | | | | 35.21 | % |
| ● | Represents beneficial ownership of less than 1%. |
| (1) | The number of shares assumes a 1 to 1 conversion of Serina preferred stock to Serina common stock. The actual number of shares of Serina common stock that a holder of Serina preferred stock may receive upon conversion of a share of Serina preferred stock may be different. |
| (2) | Consists of (i) 750,000 shares of Serina common stock, (ii) 100,634 shares of Serina common stock issuable upon the conversion of 100,634 shares of Serina Series A-2 preferred stock, (iii) 76,924 shares of Serina common stock issuable upon the conversion of 76,924 shares of Serina Series A-5 preferred stock and (iv) 76,924 shares of Serina common stock subject to warrants that may be exercised within 60 days of November 5, 2023. |
| (3) | Consists of (i) 98,000 shares of Serina common stock issuable upon the conversion of 98,000 shares of Serina Series A preferred stock, (ii) 251,571 shares of Serina common stock issuable upon the conversion of 251,571 shares of Serina Series A-2 preferred stock, (iii) 80,000 shares of Serina common stock issuable upon the conversion of 80,000 shares of Serina Series A-3 preferred stock, (iv) 153,847 shares of Serina common stock issuable upon the conversion of 153,847 shares of Serina Series A-4 preferred stock, (v) 136,968 shares of Serina common stock issuable upon the conversion of 136,968 shares of Serina Series A-5 preferred stock and (iv) 136,968 shares of Serina common stock subject to warrants that may be exercised within 60 days of November 5, 2023. |
| (4) | Consists of 629,550 shares of Serina common stock subject to options that are exercisable within 60 days of November 5, 2023. |
| (5) | Consists of (i) 250,000 shares of Serina common stock, (ii) 72,000 shares of Serina common stock subject to options that are exercisable within 60 days of November 5, 2023 and (iii) 250,000 shares of Serina common stock held by Ruth C. Bentley. Ruth C. Bentley is the spouse and member of Michael D. Bentley’s household. |
| (6) | Consists of (i) 250,000 shares of Serina common stock, (ii) 250,000 shares of Serina common stock held by Michael D. Bentley and (iii) 72,000 shares of Serina common stock subject to options that are exercisable within 60 days of November 5, 2023 by Michael D. Bentley. Michael D. Bentley is the spouse and member of Ruth C. Bentley’s household. |
| (7) | Consists of (i) 75,000 shares of Serina common stock issuable upon the conversion of 75,000 shares of Serina Series A-1 preferred stock, (ii) 75,476 shares of Serina common stock issuable upon the conversion of 75,476 shares of Serina Series A-2 preferred stock, (iii) 40,000 shares of Serina common stock issuable upon the conversion of 40,000 shares of Serina Series A-3 preferred stock, (iii) 11,539 shares of Serina common stock issuable upon the conversion of 11,539 shares of Serina Series A-5 preferred stock held by the Timothy Fisk 2012 GST-Exempt Family Trust, (iv) 11,539 shares of Serina common stock subject to warrants held by the Timothy Fisk 2012 GST-Exempt Family Trust that may be exercised within 60 days of November 5, 2023, (v) 4,000 shares of Serina common stock issuable upon the conversion of 4,000 shares of Serina Series A-3 preferred stock held by the Emily M. Robertson GST Trust, (vi) 50,317 shares of Serina common stock issuable upon the conversion of 50,317 shares of Serina Series A-2 preferred stock held by Orion Strategic Investments II, L.P., (vii) 40,000 shares of Serina common stock issuable upon the conversion of 40,000 shares of Serina Series A-3 preferred stock held by Orion Strategic Investments II, L.P., (viii) 100,000 shares of Serina common stock issuable upon the conversion of 100,000 shares of Serina Series A preferred stock held by Stoneway Enterprises, LLC, (ix) 90,584 shares of Serina common stock issuable upon the conversion of 90,584 shares of Serina Series A-2 preferred stock held by Stoneway Enterprises, LLC, (x) 23,077 shares of Serina common stock issuable upon the conversion of 23,077 shares of Serina Series A-5 preferred stock held by Stoneway Enterprises, LLC and (xi) 23,077 shares of Serina common stock subject to warrants held by Stoneway Enterprises, LLC that may be exercised within 60 days of November 5, 2023. Barbara M. Fisk is (a) the trustee of the Timothy Fisk 2012 GST-Exempt Family Trust and the Emily M. Robertson GST Trust and may be deemed to beneficially own the shares held by the Timothy Fisk 2012 GST-Exempt Family Trust and the Emily M. Robertson GST Trust, (b) a manager of Stoneway Enterprises, LLC and may be deemed to beneficially own the shares held by Stoneway Enterprises, LLC and (c) an authorized representative of Orion Strategic Investments II, L.P. and may be deemed to beneficially own the shares held by Orion Strategic Investments II, L.P. |
| (8) | Consists of (i) 355,191 shares of Serina common stock issuable upon the conversion of 355,191 shares of Serina Series A-4 preferred stock, (ii) 76,924 shares of Serina common stock issuable upon the conversion of 76,924 shares of Serina Series A-5 preferred stock and (iii) 76,924 shares of Serina common stock subject to warrants that may be exercised within 60 days of November 5, 2023. |
| (9) | Consists of (i) 60,000 shares of Serina common stock and (ii) 350,000 shares of Serina common stock subject to options that are exercisable within 60 days of November, 2023. |
| (10) | Consists of (i) 250,000 shares of Serina common stock held by the J. Milton Harris Family Trust dated 12/19/12 and (ii) 61,638 shares of Serina common stock issuable upon the conversion of 61,638 shares of Serina Series A-2 preferred stock held by the J. Milton Harris Family Trust dated 12/19/12. John Berton Harris is the trustee of the Harris Family Trust dated 12/19/12 and may be deemed to beneficially own the shares held by the Harris Family Trust dated 12/19/12. |
| (11) | Consists of (i) 200,000 shares of Serina common stock issuable upon the conversion of 200,000 shares of Serina Series A preferred stock and (ii) 12,000 shares of Serina common stock issuable upon the conversion of 12,000 shares of Serina Series A-3 preferred stock. |
| (12) | Consists of 116,666 shares of Serina common stock subject to options that are exercisable within 60 days of November 5, 2023. |
| (13) | Consists of 72,000 shares of Serina common stock subject to options that are exercisable within 60 days of November, 2023. |
| (14) | Consists of (i) 1,282,659.00 shares of Serina common stock held by, or issuable upon conversion to, Serina’s executive officers and directors and (ii) 1,274,166.00 shares of Serina common stock subject to options or warrants that are exercisable within 60 days of November 5, 2023. |
PRINCIPAL STOCKHOLDERS OF THE COMBINED COMPANY
The following table and the related notes present certain information with respect to the beneficial ownership of the common stock of the combined company upon consummation of the Merger, assuming the closing of the Merger occurred on , , by: (i) each person or group of affiliated persons known by Serina or AgeX to become the beneficial owner of more than 5% of the common stock of the combined company upon the closing of the Merger; (ii) each of the directors of the combined company; (iii) each of the executive officers of the combined company; and (iv) all executive officers and directors of the combined company as a group.
Unless otherwise indicated in the footnotes to this table, Serina and AgeX believe that each of the persons named in this table have sole voting and investment power with respect to the shares indicated as beneficially owned.
The following table assumes (i) no exercise of outstanding options to purchase shares of AgeX common stock or Serina common stock prior to the closing of the Merger, (ii) an exchange ratio of , (iii) that the closing of the Merger occurred on , (iv) that immediately prior to the Merger, AgeX will have shares of its common stock outstanding and Serina will have shares of its common stock outstanding, (v) a reverse stock split of -for- , to be implemented immediately prior to the closing of the Merger, and (vi) the shares of AgeX common stock being reduced to shares as a result of the reverse stock split and the shares of Serina common stock being reduced to a total of shares as a result of the exchange ratio and the reverse stock split. Based on these assumptions, there will be a total of shares of combined company common stock outstanding upon the closing of the Merger, after giving effect to the reverse stock split.
Shares of the combined company’s common stock that may be acquired by an individual or group within 60 days of , pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of the combined company’s common stock of any other person shown in the table. Unless otherwise indicated, the address for the following stockholders is: c/o AgeX Therapeutics, Inc. 1101 Marina Village Parkway, Suite 201, Alameda, California 945013.
| | | Beneficial Ownership
as of , 2023 | |
| Beneficial Owner | | Number of
Shares | | | Percent of
Total | |
| 5% and Greater Stockholders: | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Directors and Named Executive Officers(1): | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| ● | Represents beneficial ownership of less than 1%. |
| (1) | The directors of the combined company upon consummation of the Merger have not been determined. Such individuals will be included in this table in a subsequent filing prior to the time this Registration Statement is declared effective under the Securities Act. |
USE OF PROCEEDS
The combined company will not receive any proceeds from the sale by the Selling Stockholder of the Resale Shares covered hereby. However, upon any cash exercise of the post-merger warrants or the incentive warrants by any holders thereof, the combined company will receive cash proceeds per warrant equal to the exercise price of the post-merger warrants or the incentive warrants. Each post-merger warrant will have an exercise price equal to 13.20 per warrant (on a post-reverse stock split basis). Each incentive warrant will have an exercise price equal to $18.00 per warrant (on a post-reverse stock split basis). The combined company intends to use such proceeds to help fund its operations.
DETERMINATION OF OFFERING PRICE
The prices at which the shares of common stock of the combined company covered by the registration statement on Form S-1 of which the accompanying proxy statement/prospectus/information statement forms a part may actually be sold will be determined by the prevailing public market price for shares of the combined company’s common stock, by negotiations between any of the Selling Stockholder and buyers of the combined company’s common stock in private transactions or as otherwise described in “Plan of Distribution.”
SELLING STOCKHOLDER
The Resale Shares offered by this proxy statement/prospectus/information statement may be offered from time to time by the Selling Stockholder. The Selling Stockholder may sell some, all or none of the Resale Shares. AgeX does not know how long the Selling Stockholder will hold the post-merger warrants or incentive warrants before exercising them or the Resale Shares offered hereunder before selling them. The Selling Stockholder may have sold or transferred, in transactions exempt from the registration requirements of the Securities Act, some or all of the Resale Shares since the date of this proxy statement/prospectus/information statement. Information about the Selling Stockholder may change over time. As used in this proxy statement/prospectus/information statement, the term “Selling Stockholder” includes the Selling Stockholder listed below, and any donee, pledgee, transferee or other successor in interest selling shares received after the date of this proxy statement/prospectus/information statement from a Selling Stockholder as a gift, pledge, or other non-sale related transfer.
The following table sets forth the name of the Selling Stockholder, the number of shares of AgeX common stock (including shares underlying post-merger warrants and incentive warrants held by such Selling Stockholder) and the percentage of AgeX common stock beneficially owned by the Selling Stockholder prior to this offering, the number of shares that may be offered under this proxy statement/prospectus/information statement by the Selling Stockholder and the number of shares of AgeX common stock and the percentage of AgeX common stock to be beneficially owned by the Selling Stockholder after completion of this offering, assuming that all shares offered hereunder are sold as contemplated herein. The number of shares in the column “Shares of common stock beneficially owned to be sold in the offering” represents all of the shares that the Selling Stockholder may offer under this proxy statement/prospectus/information statement.
There will be shares of AgeX common stock outstanding upon the consummation of the Merger.
Beneficial ownership is determined under the rules of the SEC and generally includes voting or investment power over securities. Except in cases where community property laws apply or as indicated in the footnotes to this table, we believe that each stockholder identified in the table possesses sole voting and investment power over all shares of common stock shown as beneficially owned by the stockholder. Shares of common stock subject to options and warrants that are exercisable within 60 days of the date of this proxy statement/prospectus/information statement are considered outstanding and beneficially owned by the person holding the options and warrants for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
| | | Shares of AgeX common
stock beneficially
owned prior to
this offering | | | Shares of AgeX
common stock
offered
pursuant to
this proxy statement/prospectus/information statement | | | Shares of AgeX common stock
beneficially owned after
this offering (assuming the sale of all shares that may be sold hereunder) | |
Name of Selling Stockholder | | Shares
of
common
stock(2) | | | Percentage
of total
outstanding
common
stock (%)(1) | | | Shares of
common
stock | | | Shares of
common
stock | | | Percentage
of total
outstanding
common
stock (%) | |
| Juvenescence Limited and certain affiliates | | | | | | | | % | | | | | | | - | | | | - | |
| (1) | Based upon shares of AgeX common stock outstanding as of , 2023. |
| (2) | Shares listed consist of (i) shares of AgeX common stock, (ii) shares of AgeX common stock issuable upon exercise of the post-merger warrants and (iii) shares of AgeX common stock issuable upon exercise of the incentive warrants that will be held by Juvenescence US Corp, a wholly owned subsidiary of Juvenescence, upon the consummation of the Merger. The address of Juvenescence is 18 Athol Street, Douglas, Isle of Man IM1 1JA. |
PLAN OF DISTRIBUTION
The Selling Stockholder and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any or all of its securities covered hereby on the principal trading market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholder may use any one or more of the following methods when selling securities:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and |
| ● | resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately negotiated transactions; |
| ● | in underwritten offerings; |
| ● | settlement of short sales; |
| ● | in transactions through broker-dealers that agree with the Selling Stockholder to sell a specified number of such securities at a stipulated price per security; |
| ● | through the writing or settlement of options or other hedging transactions, whether through an |
| ● | options exchange or otherwise; |
| ● | a combination of any such methods of sale; or |
| ● | any other method permitted pursuant to applicable law. |
The Selling Stockholder or any donee, pledgee, transferee, etc. (hereinafter, a Selling Stockholder) may also sell the securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this proxy statement/prospectus/information statement. Broker-dealers engaged by a Selling Stockholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from a Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this proxy statement/prospectus/information statement, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, a Selling Stockholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. A Selling Stockholder may also sell securities short and deliver the securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell the securities. A Selling Stockholder may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities covered by the registration statement on Form S-1 of which the accompanying proxy statement/prospectus/information statement is a part, which securities such broker-dealer or other financial institution may resell pursuant to this proxy statement/prospectus/information statement (as supplemented or amended to reflect such transaction).
The Selling Stockholder and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. The Selling Stockholder has informed AgeX and the combined company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the Securities.
AgeX and the combined company are required to pay certain fees and expenses incurred by the AgeX and the combined company incident to the registration of the securities. AgeX and the combined company has agreed to indemnify the Selling Stockholder against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
AgeX and the combined company agree to keep this proxy statement/prospectus/information statement effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholder without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for AgeX or the combined company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this proxy statement/prospectus/information statement or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholder will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of common stock by the Selling Stockholder or any other person. AgeX and the combined company will make copies of this proxy statement/prospectus/information statement available to the Selling Stockholder and have informed the Selling Stockholder of the need to deliver a copy of this proxy statement/prospectus/information statement to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL MATTERS
Certain legal matters, including the legality of the securities offered, will be passed upon for us by Gibson, Dunn & Crutcher LLP, San Francisco, California. Additional legal matters may be passed upon for AgeX or the combined company or any underwriters, dealers or agents, by counsel that will be named in the applicable prospectus supplement.
EXPERTS
AgeX
The consolidated financial statements of AgeX Therapeutics, Inc. (AgeX) as of December 31, 2022 and 2021 and for the years then ended included in this proxy statement/prospectus/information statement have been audited by WithumSmith+Brown, PC, an independent registered public accounting firm, as stated in their report. Such consolidated financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing. This report on the consolidated financial statements contains an explanatory paragraph regarding AgeX’s ability to continue as a going concern.
Serina
The consolidated financial statements of Serina Therapeutics, Inc. (Serina) as of and for the years ended December 31, 2022 and 2021 included in this proxy statement/prospectus/information statement have been audited by Frazier & Deeter, LLC, an independent registered public accounting firm, as stated in their report. Such consolidated financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing. This report on the consolidated financial statements contains an explanatory paragraph regarding Serina’s ability to continue as a going concern and a restatement of Serina’s December 31, 2022 and 2021 consolidated financial statements.
WHERE YOU CAN FIND MORE INFORMATION
AgeX has filed with the SEC a registration statement on Form S-4/S-1 (including exhibits, schedules, and amendments) under the Securities Act with respect to the securities offered by this proxy statement/prospectus/information statement. This proxy statement/prospectus/information statement is a part of the registration statement and constitutes a prospectus of AgeX, as well as a proxy statement of AgeX for the AgeX special meeting and an information statement for the purpose of Serina for its written consent.
This proxy statement/prospectus/information statement does not contain all the information set forth in the registration statement. For further information about AgeX and the securities to be registered in connection with the Merger, you should refer to the registration statement. Statements contained in this proxy statement/prospectus/information statement relating to the contents of any contract, agreement or other document are not necessarily complete and are qualified in all respects by the complete text of the applicable contract, agreement or other document, a copy of which has been filed as an exhibit to the registration statement.
AgeX is subject to the reporting and information requirements of the Exchange Act and, as a result, files, or will file, periodic reports, proxy statements and other information with the SEC. The SEC maintains an website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The SEC’s website can be found at http://www.sec.gov. AgeX also maintains a website at https://www.agexinc.com/ and makes available free of charge through this website AgeX’s annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Exchange Act. AgeX makes these reports available through AgeX’s website as soon as reasonably practicable after AgeX electronically files such reports with, or furnishes such reports to, the SEC. The information contained on, or that can be accessed through, AgeX’s website is not a part of this proxy statement/prospectus/information statement.
This proxy statement/prospectus/information statement does not constitute an offer to sell, or a solicitation of an offer to purchase, the securities offered by this proxy statement/prospectus/information statement, or the solicitation of a proxy, in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer, solicitation of an offer or proxy solicitation in such jurisdiction. Neither the delivery of this proxy statement/prospectus/information statement nor any distribution of securities pursuant to this proxy statement/prospectus/information statement shall, under any circumstances, create any implication that there has been no change in the information set forth or incorporated into this proxy statement/prospectus/information statement by reference or in AgeX’s affairs since the date of this proxy statement/prospectus/information statement.
AgeX has supplied all information contained in this proxy statement/prospectus/information statement relating to AgeX and its business, and Serina has supplied all information contained in this proxy statement/prospectus/information statement relating to Serina and its business.
If you would like to request documents from AgeX or Serina, please send a request in writing or by telephone to either AgeX or Serina at the following addresses:
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 945013
Attention: Andrea Park
Phone Number: (510) 671-8370
Email: information@agexinc.com
Subject Line: Request for Proxy Materials | Serina Therapeutics, Inc.
601 Genome Way, Suite 2001
Huntsville, Alabama 35806
(256) 327-9630
investor.relations@serinatherapeutics.com |
Householding of Proxy Materials
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports with respect to two or more stockholders sharing the same address by delivering a single proxy statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
A number of brokers with account holders who are AgeX stockholders will be householding AgeX’s proxy materials. A single copy of this proxy statement/prospectus/information statement will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once a stockholder has received notice from its broker that they will be householding communications to such stockholder’s address, householding will continue until such stockholder is notified otherwise or until the stockholder revokes his or her consent. If, at any time, a stockholder no longer wishes to participate in householding and would prefer to receive a separate copy of this proxy statement/prospectus/information statement and any annual disclosure documents, it may notify its broker, or direct its written request to AgeX Therapeutics, Inc. at AgeX’s principal executive offices at 1101 Marina Village Parkway, Suite 201, Alameda, California 94501, Attention: Investor Relations. Stockholders who currently receive multiple copies of the proxy statement/prospectus/information statement and annual disclosure documents at their address and would like to request householding of their communications should contact their broker.
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
On August 29, 2023, AgeX Therapeutics, Inc. (AgeX) entered into the Agreement and Plan of Merger and Reorganization (as may be amended from time to time, the Merger Agreement) with Serina Therapeutics Inc. (Serina) and Canaria Transaction Corporation (Merger Sub), pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the Merger). AgeX following the Merger is referred to herein as the “combined company.”
At the effective time of the Merger (the Effective Time): (i) each outstanding share of common stock, $0.01 par value per share, of Serina (Serina common stock) (after giving effect to the conversion of each share of preferred stock of Serina into Serina common stock (the Serina preferred stock conversion) and including all such shares that are so converted other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of common stock of AgeX, $0.0001 par value per share (AgeX common stock) equal to the exchange ratio (the exchange ratio) determined in accordance with the Merger Agreement; (ii) each outstanding and unexercised option to purchase shares of Serina common stock (Serina option) immediately prior to the Effective Time under the Serina Therapeutics, Inc. 2017 Stock Option Plan, as amended (the Serina Plan) will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option; and (iii) each outstanding and unexercised warrant to purchase shares of Serina common stock (Serina warrant) immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Through the application of the exchange ratio, immediately following closing of the Merger, the stockholders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the aggregate number of outstanding shares of AgeX common stock immediately after the Effective Time, and the stockholders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the aggregate number of outstanding shares of AgeX common stock immediately after the Effective Time, in each case on a pro forma fully-diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion (as defined elsewhere in the proxy statement/prospectus/information statement) and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant.
AgeX will ask its stockholders to approve an amendment to its certificate of incorporation to effect a reverse stock split, which approval is also necessary to complete the transactions contemplated by the Merger Agreement. Upon the effectiveness of the amendment to its certificate of incorporation effecting the reverse stock split, the outstanding shares of AgeX common stock will be combined into a lesser number of shares resulting in approximately 2,500,000 shares of AgeX common stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed upon by the respective AgeX and Serina boards of directors. As the proposed reverse stock split ratio of AgeX common stock is not definitive and is expected to occur following receipt of stockholder approval and prior to the consummation of the Merger, the unaudited pro forma condensed combined financial information does not reflect the reverse stock split, unless otherwise stated.
Following the reverse stock split and the AgeX preferred stock conversion and prior to the consummation of the Merger (the Closing), AgeX will issue to each holder of AgeX common stock as of the close of business on a business day following the reverse stock split and the AgeX preferred stock conversion and prior to the closing of the Merger (the Warrant Dividend Record Date) three warrants (each, a post-merger warrant) for each five shares of AgeX common stock issued and outstanding held by such holder as of the Warrant Dividend Record Date. Each post-merger warrant will be exercisable at an exercise price equal to $13.20 per warrant (such exercise price reflecting the planned reverse stock split) for (i) one share of AgeX common stock and (ii) one warrant (each, an incentive warrant). Each incentive warrant will be exercisable at an exercise price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split) for one share of AgeX common stock. Each post-merger warrant will expire on July 31, 2025, and each incentive warrant will expire on the four-year anniversary of the Closing. Each post-merger warrant and incentive warrant will be issued pursuant to the terms of a warrant agreement to be entered into by AgeX and a warrant agent in connection with the closing of the Merger.
The unaudited pro forma condensed combined financial information presents the combination of the financial information of AgeX and Serina to give effect to the Merger, which has been accounted for as a reverse recapitalization under accounting principles generally accepted in the United States of America (GAAP). Serina is considered the accounting acquirer for financial reporting purposes. This determination is primarily based on the expectation that, immediately following the Merger:
| | ● | The pre-Merger stockholders of Serina are expected to own approximately 75% of the combined company and to hold the majority of voting rights in the combined company; |
| | ● | The pre-Merger stockholders of Serina will have the right to appoint or approve a majority of the directors on the combined company’s board of directors; |
| | ● | Certain current members of the Serina executive management team are expected to assume key leadership roles of the combined company; and |
| | ● | The combined company intends to focus primarily on developing Serina’s product candidates. |
The transaction is expected to be accounted for as a reverse recapitalization of AgeX by Serina similar to as if Serina had issued equity for the net assets of AgeX. As a result of Serina being treated as the accounting acquirer, Serina’s assets and liabilities will be recorded at their pre-Merger carrying amounts with no goodwill or other intangible assets recorded. AgeX’s assets and liabilities will be measured and recognized at their fair values as of the Effective Time of the Merger, which are expected to approximate the carrying value of the acquired cash and other non-operating net assets. Any difference between the consideration transferred and the fair value of the net assets of AgeX following determination of the actual purchase consideration for AgeX will be reflected as an adjustment to additional paid-in capital. Upon consummation of the Merger, the historical financial statements of Serina will become the historical consolidated financial statements of the combined company.
The unaudited pro forma condensed combined balance sheet assumes that the Merger took place on June 30, 2023, and combines the historical balance sheets of AgeX and Serina as of such date. The unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2023 and the year ended December 31, 2022 assumes that the Merger took place as of January 1, 2022 and combines the historical results of AgeX and Serina for those periods then ended. The unaudited pro forma condensed combined financial information was prepared pursuant to the rules and regulations of Article 11 of SEC Regulation S-X, as amended.
The unaudited pro forma condensed combined financial information is provided for illustrative purposes only, does not necessarily reflect what the actual consolidated results of operations and financial position would have been had the acquisition occurred on the dates assumed and may not be useful in predicting the future consolidated results of operations or financial position.
The unaudited pro forma condensed combined financial information is based on the assumptions and adjustments that are described in the accompanying notes. Accordingly, the pro forma adjustments are preliminary, subject to further revision as additional information becomes available and additional analyses are performed and have been made solely for the purpose of providing unaudited pro forma condensed combined financial information. Differences between these preliminary accounting and estimates and the final accounting conclusions and amounts may occur as a result of changes in initial assumptions in the determination of the accounting acquirer and related accounting, and the amount of cash used in AgeX’s operations, and other changes in AgeX’s assets and liabilities, which are expected to be completed after the closing of the Merger, may occur and these differences could have a material impact on the unaudited pro forma condensed combined financial information and the combined company’s future results of operations and financial position.
The unaudited pro forma condensed combined financial information does not give effect to the potential impact of current financial conditions, regulatory matters, operating efficiencies or other savings or expenses that may be associated with the integration of the two companies. The actual results reported in periods following the Merger may differ significantly from those reflected in the unaudited pro forma condensed combined financial information presented herein for a number of reasons, including, but not limited to, differences in the assumptions used to prepare this unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information is derived from:
| | ● | the historical audited consolidated financial statements of AgeX for the years ended December 31, 2022, and 2021 included elsewhere in this proxy statement/prospectus/information statement; |
| | ● | the historical unaudited condensed consolidated financial information of AgeX as of and for the six months ended June 30, 2023 included elsewhere in this proxy statement/prospectus/information statement; |
| | ● | the historical audited financial statements of Serina for the years ended December 31, 2022, and 2021 included elsewhere in this this proxy statement/prospectus/information statement; and |
| | ● | the historical unaudited condensed financial statements of Serina as of and for the six months ended June 30, 2023, included elsewhere in this this proxy statement/prospectus/information statement. |
Such unaudited pro forma condensed financial information has been prepared on a basis consistent with the financial statements of AgeX. This information should be read together with the financial statements of AgeX and Serina and related notes thereto, the discussion of the financial condition and results of operations of AgeX and Serina in the section entitled “AgeX Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Serina Management’s Discussion and Analysis of Financial Condition and Results of Operations,” respectively; and other information included elsewhere in this this proxy statement/prospectus/information statement.
The unaudited pro forma condensed combined financial information has been prepared in a manner consistent with the accounting policies adopted by Serina. Accounting rules require evaluation of certain assumptions, estimates, or determination of financial statement classifications. The accounting policies of AgeX may materially vary from those of Serina. During preparation of the unaudited pro forma condensed combined financial information, management has performed a preliminary analysis and is not aware of any material differences, and accordingly, this unaudited pro forma condensed combined financial information assumes no material differences in accounting policies. Following the completion of the Merger, management will perform a more detailed review of the accounting policies of AgeX and Serina in order to determine if differences in accounting policies require adjustment or reclassification of AgeX’s results of operations or reclassification of assets or liabilities to conform to Serina’s accounting policies and classifications. As a result of that review, differences could be identified between the accounting policies of the two companies that, when conformed, could have a material impact on the future combined financial statements if the transaction is consummated.
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF JUNE 30, 2023
(in thousands, except share and per share data)
| | | As Reported | | | Pre-Transaction
Accounting | | | | | Adjusted | | | Transaction
Accounting | | | | | Pro Forma
Combined | |
| | | AgeX | | | Serina | | | Adjustments | | | Notes | | AgeX | | | Serina | | | Adjustments | | | Notes | | Total | |
| ASSETS | | | | | | | | | | | | | | | | | | | | | | | | | |
| Current Assets: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 261 | | | $ | 8,722 | | | $ | 3,886 | | | D | | $ | 4,147 | | | $ | 8,722 | | | $ | (3,174 | ) | | H | | $ | 8,810 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | (885 | ) | | I | | | | |
| Accounts and grants receivable, net | | | 6 | | | | - | | | | - | | | | | | 6 | | | | - | | | | - | | | | | | 6 | |
| Prepaid expenses and other current assets | | | 1,083 | | | | 15 | | | | - | | | | | | 1,083 | | | | 15 | | | | - | | | | | | 1,098 | |
| Total current assets | | | 1,350 | | | | 8,737 | | | | | | | | | | 5,236 | | | | 8,737 | | | | (4,059 | ) | | | | | 9,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Restricted cash | | | 50 | | | | - | | | | - | | | | | | 50 | | | | - | | | | - | | | | | | 50 | |
| Property and equipment, net | | | - | | | | 376 | | | | - | | | | | | - | | | | 376 | | | | - | | | | | | 376 | |
| Right of use assets - operating leases | | | - | | | | 504 | | | | - | | | | | | - | | | | 504 | | | | - | | | | | | 504 | |
| Right of use assets - finance leases | | | - | | | | 121 | | | | - | | | | | | - | | | | 121 | | | | - | | | | | | 121 | |
| Intangible assets, net | | | 673 | | | | - | | | | - | | | | | | 673 | | | | - | | | | - | | | | | | 673 | |
| Convertible note receivable | | | 10,204 | | | | - | | | | - | | | | | | 10,204 | | | | - | | | | (10,204 | ) | | J | | | - | |
| TOTAL ASSETS | | $ | 12,277 | | | $ | 9,738 | | | | | | | | | $ | 16,163 | | | $ | 9,738 | | | $ | (14,263 | ) | | | | $ | 11,638 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Current Liabilities: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 961 | | | $ | 660 | | | | (78 | ) | | E | | $ | 961 | | | $ | 582 | | | $ | (204 | ) | | J | | $ | 1,339 | |
| Loans due to Juvenescence, net of debt issuance costs, current portion | | | 22,943 | | | | - | | | | (25,616 | ) | | A | | | 3,688 | | | | - | | | | - | | | | | | 3,688 | |
| | | | | | | | | | | | 2,475 | | | B | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | 3,886 | | | D | | | | | | | | | | | | | | | | | | |
| Related party payables, net | | | 230 | | | | - | | | | - | | | | | | 230 | | | | - | | | | - | | | | | | 230 | |
| Contract liability | | | - | | | | 116 | | | | - | | | | | | - | | | | 116 | | | | - | | | | | | 116 | |
| Insurance premium liability and other current liabilities | | | 371 | | | | - | | | | - | | | | | | 371 | | | | - | | | | - | | | | | | 371 | |
| Current portion of operating lease liabilities | | | - | | | | 142 | | | | - | | | | | | - | | | | 142 | | | | - | | | | | | 142 | |
| Current portion of finance lease liabilities | | | - | | | | 51 | | | | - | | | | | | - | | | | 51 | | | | - | | | | | | 51 | |
| Convertible promissory notes | | | - | | | | 7,613 | | | | (1,537 | ) | | E | | | - | | | | 10,000 | | | | (10,000 | ) | | J | | | - | |
| | | | | | | | | | | | 3,924 | | | F | | | | | | | | | | | | | | | | | | |
| Total current liabilities | | | 24,505 | | | | 8,582 | | | | | | | | | | 5,250 | | | | 10,891 | | | | (10,204 | ) | | | | | 5,937 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Warrant liability | | | - | | | | 613 | | | | 845 | | | E | | | - | | | | 1,458 | | | | 32,055 | | | K | | | 33,513 | |
| Operating lease liabilities, net of current portion | | | - | | | | 377 | | | | - | | | | | | - | | | | 377 | | | | - | | | | | | 377 | |
| Finance lease liabilities, net of current portion | | | - | | | | 11 | | | | - | | | | | | - | | | | 11 | | | | - | | | | | | 11 | |
| Loans due to Juvenescence, net of debt issuance costs, net of current portion | | | 10,068 | | | | - | | | | (10,384 | ) | | A | | | - | | | | - | | | | - | | | | | | - | |
| | | | | | | | | | | | 316 | | | B | | | | | | | | | | | | | | | | | | |
| Total liabilities | | | 34,573 | | | | 9,583 | | | | | | | | | | 5,250 | | | | 12,737 | | | | 21,851 | | | | | | 39,838 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Redeemable convertible preferred stock, Serina | | | - | | | | 35,442 | | | | 770 | | | E | | | - | | | | 46,488 | | | | (46,488 | ) | | N | | | - | |
| | | | | | | | | | | | 10,276 | | | G | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stockholders’ Equity (Deficit): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Preferred stock, Series A, AgeX | | | - | | | | - | | | | 21,160 | | | A | | | - | | | | - | | | | - | | | | | | - | |
| | | | | | | | | | | | (21,160 | ) | | C | | | | | | | | | | | | | | | | | | |
| Preferred stock, Series B, AgeX | | | - | | | | - | | | | 14,840 | | | A | | | - | | | | - | | | | - | | | | | | - | |
| | | | | | | | | | | | (14,840 | ) | | C | | | | | | | | | | | | | | | | | | |
| Common stock, AgeX, $0.0001 par value, 200,000,000 shares authorized; and 37,951,261 shares issued and outstanding | | | 4 | | | | - | | | | 5 | | | C | | | 9 | | | | - | | | | (9 | ) | | L | | | - | |
| Common stock, Serina, $0.01 par value, 15,000,000 shares authorized; and 2,144,800 shares issued and outstanding | | | - | | | | 22 | | | | - | | | | | | - | | | | 22 | | | | - | | | | | | 22 | |
| Additional paid-in capital | | | 99,977 | | | | 671 | | | | 35,995 | | | C | | | 135,972 | | | | 671 | | | | (135,972 | ) | | L | | | 26,017 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | 10,913 | | | M | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | (32,055 | ) | | K | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | 46,488 | | | N | | | | |
| Accumulated deficit | | | (122,156 | ) | | | (35,980 | ) | | | (2,791 | ) | | B | | | (124,947 | ) | | | (50,180 | ) | | | 124,947 | | | L | | | (54,239 | ) |
| | | | | | | | | | | | (3,924 | ) | | F | | | | | | | | | | | (3,174 | ) | | H | | | | |
| | | | | | | | | | | | (10,276 | ) | | G | | | | | | | | | | | (885 | ) | | I | | | | |
| Total pro forma combined stockholders’ equity (deficit) | | | (22,175 | ) | | | (35,287 | ) | | | | | | | | | 11,034 | | | | (49,487 | ) | | | 10,253 | | | | | | (28,200 | ) |
| Noncontrolling interest | | | (121 | ) | | | - | | | | - | | | | | | (121 | ) | | | - | | | | 121 | | | L | | | - | |
| Total stockholders’ equity (deficit) | | | (22,296 | ) | | | (35,287 | ) | | | | | | | | | 10,913 | | | | (49,487 | ) | | | 10,374 | | | | | | (28,200 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | $ | 12,277 | | | $ | 9,738 | | | | | | | | | $ | 16,163 | | | $ | 9,738 | | | $ | (14,263 | ) | | | | $ | 11,638 | |
The accompanying notes are an integral part of this unaudited pro forma condensed combined financial information.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
SIX MONTHS ENDED JUNE 30, 2023
(in thousands, except share and per share data)
| | | As Reported | | | Pre-Transaction
Accounting | | | | | Adjusted | | | Transaction
Accounting | | | | | Pro Forma
Combined | |
| | | AgeX | | | Serina | | | Adjustments | | | Notes | | AgeX | | | Serina | | | Adjustments | | | Notes | | Total | |
| REVENUES | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contract revenues | | $- | | | $- | | | $ | - | | | | | $ | - | | | $ | - | | | $ | - | | | | | $ | - | |
| Grant revenues | | | - | | | | 37 | | | | - | | | | | | - | | | | 37 | | | | - | | | | | | 37 | |
| Other revenues | | | 19 | | | | - | | | | - | | | | | | 19 | | | | - | | | | - | | | | | | 19 | |
| Total revenues | | | 19 | | | | 37 | | | | | | | | | | 19 | | | | 37 | | | | - | | | | | | 56 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of sales | | | 6 | | | | - | | | | - | | | | | | 6 | | | | - | | | | - | | | | | | 6 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 13 | | | | 37 | | | | | | | | | | 13 | | | | 37 | | | | - | | | | | | 50 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development expenses | | | 334 | | | | 878 | | | | - | | | | | | 334 | | | | 878 | | | | - | | | | | | 1,212 | |
| General and administrative expenses | | | 3,723 | | | | 1,066 | | | | - | | | | | | 3,723 | | | | 1,066 | | | | - | | | | | | 4,789 | |
| Total operating expenses | | | 4,057 | | | | 1,944 | | | | | | | | | | 4,057 | | | | 1,944 | | | | - | | | | | | 6,001 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (4,044 | ) | | | (1,907 | ) | | | | | | | | | (4,044 | ) | | | (1,907 | ) | | | - | | | | | | (5,951 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| OTHER EXPENSE, NET | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest income (expense), net | | | (1,892 | ) | | | (194 | ) | | | - | | | | | | (1,892 | ) | | | (194 | ) | | | 2,096 | | | EE | | | 88 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | 204 | | | FF | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | (204 | ) | | GG | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | 78 | | | HH | | | | |
| Change in fair value of warrants | | | (35 | ) | | | 463 | | | | - | | | | | | (35 | ) | | | 463 | | | | - | | | | | | 428 | |
| Fair value inception adjustment on convertible promissory notes | | | - | | | | 2,240 | | | | (2,240 | ) | | BB | | | - | | | | - | | | | - | | | | | | - | |
| Change in fair value of convertible promissory notes | | | - | | | | 1,864 | | | | (180 | ) | | AA | | | - | | | | - | | | | - | | | | | | - | |
| | | | | | | | | | | | (1,684 | ) | | BB | | | | | | | | | | | | | | | | | | |
| Other income, net | | | 7 | | | | - | | | | - | | | | | | 7 | | | | - | | | | - | | | | | | 7 | |
| Total other expense, net | | | (1,920 | ) | | | 4,373 | | | | | | | | | | (1,920 | ) | | | 269 | | | | 2,174 | | | | | | 523 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET INCOME (LOSS) | | | (5,964 | ) | | | 2,466 | | | | | | | | | | (5,964 | ) | | | (1,638 | ) | | | 2,174 | | | | | | (5,428 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss attributable to noncontrolling interest | | | 18 | | | | - | | | | - | | | | | | 18 | | | | - | | | | - | | | | | | 18 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET INCOME (LOSS) ATTRIBUTABLE TO COMBINED COMPANY | | $ | (5,946 | ) | | $ | 2,466 | | | | | | | | | $ | (5,946 | ) | | $ | (1,638 | ) | | $ | 2,174 | | | | | $ | (5,410 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET INCOME (LOSS) PER COMMON SHARE | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.16 | ) | | $ | 1.11 | | | | | | | | | | | | | | | | | | | | | | | $ | (0.02 | ) |
| Diluted | | $ | (0.16 | ) | | $ | 0.33 | | | | | | | | | | | | | | | | | | | | | | | $ | (0.02 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 37,951,261 | | | | 2,221,100 | | | | | | | | | | | | | | | | | | | | | II | | | 351,788,608 | |
| Diluted | | | 37,951,261 | | | | 7,602,825 | | | | | | | | | | | | | | | | | | | | | II | | | 351,788,608 | |
The accompanying notes are an integral part of this unaudited pro forma condensed combined financial information.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
YEAR ENDED DECEMBER 31, 2022
(in thousands, except share and per share data)
| | | As Reported | | | Pre-Transaction
Accounting | | | | | Adjusted | | | Transaction
Accounting | | | | | Pro Forma
Combined | |
| | | AgeX | | | Serina | | | Adjustments | | | Notes | | AgeX | | | Serina | | | Adjustments | | | Notes | | Total | |
| REVENUES | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contract revenues | | $ | - | | | $ | 500 | | | $ | - | | | | | $ | - | | | $ | 500 | | | $ | - | | | | | $ | 500 | |
| Grant revenues | | | - | | | | 92 | | | | - | | | | | | - | | | | 92 | | | | - | | | | | | 92 | |
| Other revenues | | | 34 | | | | - | | | | - | | | | | | 34 | | | | - | | | | - | | | | | | 34 | |
| Total revenues | | | 34 | | | | 592 | | | | | | | | | | 34 | | | | 592 | | | | - | | | | | | 626 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of sales | | | 13 | | | | - | | | | - | | | | | | 13 | | | | - | | | | - | | | | | | 13 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 21 | | | | 592 | | | | | | | | | | 21 | | | | 592 | | | | - | | | | | | 613 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development expenses | | | 1,025 | | | | 1,573 | | | | - | | | | | | 1,025 | | | | 1,573 | | | | - | | | | | | 2,598 | |
| General and administrative expenses | | | 5,971 | | | | 1,289 | | | | - | | | | | | 5,971 | | | | 1,289 | | | | 3,174 | | | CC | | | 11,319 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | 885 | | | DD | | | | |
| Total operating expenses | | | 6,996 | | | | 2,862 | | | | | | | | | | 6,996 | | | | 2,862 | | | | 4,059 | | | | | | 13,917 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,975 | ) | | | (2,270 | ) | | | | | | | | | (6,975 | ) | | | (2,270 | ) | | | (4,059 | ) | | | | | (13,304 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| OTHER EXPENSE, NET | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest income (expense), net | | | (3,335 | ) | | | (14 | ) | | | - | | | | | | (3,335 | ) | | | (14 | ) | | | 3,335 | | | EE | | | (14 | ) |
| Change in fair value of warrants | | | (225 | ) | | | (124 | ) | | | - | | | | | | (225 | ) | | | (124 | ) | | | - | | | | | | (349 | ) |
| Fair value inception adjustment on convertible promissory notes | | | - | | | | (179 | ) | | | 179 | | | AA | | | - | | | | - | | | | - | | | | | | - | |
| Change in fair value of convertible promissory notes | | | - | | | | (88 | ) | | | 88 | | | AA | | | - | | | | - | | | | - | | | | | | - | |
| Loss on equity securities, net | | | - | | | | (8 | ) | | | - | | | | | | - | | | | (8 | ) | | | - | | | | | | (8 | ) |
| Other income, net | | | 13 | | | | 1 | | | | - | | | | | | 13 | | | | 1 | | | | - | | | | | | 14 | |
| Total other expense, net | | | (3,547 | ) | | | (412 | ) | | | | | | | | | (3,547 | ) | | | (145 | ) | | | 3,335 | | | | | | (357 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET LOSS | | | (10,522 | ) | | | (2,682 | ) | | | | | | | | | (10,522 | ) | | | (2,415 | ) | | | (724 | ) | | | | | (13,661 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss attributable to noncontrolling interest | | | 60 | | | | - | | | | - | | | | | | 60 | | | | - | | | | - | | | | | | 60 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET LOSS ATTRIBUTABLE TO COMBINED COMPANY | | $ | (10,462 | ) | | $ | (2,682 | ) | | | | | | | | $ | (10,462 | ) | | $ | (2,415 | ) | | $ | (724 | ) | | | | $ | (13,601 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| NET LOSS PER COMMON SHARE | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.28 | ) | | $ | (1.25 | ) | | | | | | | | | | | | | | | | | | | | | | $ | (0.04 | ) |
| Diluted | | $ | (0.28 | ) | | $ | (1.25 | ) | | | | | | | | | | | | | | | | | | | | | | $ | (0.04 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 37,949,196 | | | | 2,145,002 | | | | | | | | | | | | | | | | | | | | | II | | | 350,235,142 | |
| Diluted | | | 37,949,196 | | | | 2,145,002 | | | | | | | | | | | | | | | | | | | | | II | | | 350,235,142 | |
The accompanying notes are an integral part of this unaudited pro forma condensed combined financial information.
NOTES TO THE UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
(in thousands, except share and per share data)
Note 1: Description of the Transaction
AgeX, Serina, and Canaria have entered into the Merger Agreement, pursuant to which, among other, matters and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX. At the Effective Time, each outstanding share of Serina common stock (other than shares held by Serina, AgeX, Merger Sub or any of their respective subsidiaries and appraisal shares) will be automatically converted solely into the right to receive a number of shares of AgeX common stock equal to the exchange ratio. The exchange ratio is currently estimated to be equal to approximately 0.83217216 shares of AgeX common stock for each share of Serina common stock, which estimated exchange ratio assumes (i) the Actual Closing Price (as defined elsewhere in this proxy statement/prospectus/information statement) of a share of AgeX common stock is equal to $12.00 per share (on a post-reverse stock split basis), (ii) the number of Company Outstanding Shares (as defined elsewhere in this proxy statement/prospectus/information statement) is equal to 9,012,558, (iii) the number of Company Merger Shares (as defined elsewhere in this proxy statement/prospectus/information statement) is equal to 7,500,000 and (iv) the implementation of the reverse stock split and the AgeX preferred stock conversion prior to the consummation of the Merger, as described in the accompanying proxy statement/prospectus/information statement. There can be no assurance that any of these assumptions will be accurate when the final exchange ratio is determined.
At the Effective Time, (i) each outstanding and unexercised Serina option immediately prior to the Effective Time under the Serina Plan will be converted into and become an option to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the option and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume the Serina Plan and each Serina option in accordance with the terms of the Serina Plan and the terms of the Serina option, and (ii) each outstanding and unexercised Serina warrant immediately prior to the Effective Time, if any, will be converted into and become a warrant to purchase AgeX common stock, with the number of shares of AgeX common stock subject to the warrant and exercise price being appropriately adjusted to reflect the exchange ratio, and AgeX will assume each Serina warrant in accordance with its terms.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of common stock of the combined company, and equity holders of AgeX immediately prior to the closing of the Merger are expected to own approximately 25% of the outstanding shares of common stock of the combined company, in each case, on a pro forma fully diluted basis, subject to certain assumptions and exclusions, including the Actual Closing Price of AgeX common stock being equal to or greater than $12.00 per share (on a post-reverse stock split basis), giving effect to the reverse stock split and the AgeX preferred stock conversion and excluding the impact of any post-merger warrant, incentive warrant or the issuance of any share of AgeX common stock upon exercise of any post-merger warrant or incentive warrant.
Consummation of the Merger is subject to certain closing conditions, including, among other things, approval by the AgeX and Serina stockholders.
Note 2: Basis of Presentation
The unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended by SEC Final Rule Release No. 33-10786, Amendments to Financial Disclosures About Acquired and Disposed Businesses. In accordance with Release No. 33-10786, the unaudited condensed combined pro forma statements of operations reflect transaction accounting adjustments. The historical financial information of AgeX and Serina has been adjusted in the unaudited pro forma condensed combined financial information to reflect transaction accounting adjustments related to the Merger in accordance with GAAP.
The unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2023, and for the year ended December 31, 2022, combines the historical results of AgeX and Serina for the respective periods presented to give pro forma effect to the Merger was if such had occurred on January 1, 2022. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 combines the historical balance sheets of AgeX and Serina to give pro forma effect to the Merger as if such transactions were completed on June 30, 2023. The pro forma information does not purport to represent what the actual consolidated results of operations of the combined company would have been if the Merger had occurred on January 1, 2022, nor is it necessarily indicative of the future consolidated results of operations of the combined company. The actual results of operations of the combined company will likely differ, perhaps materially, from the pro forma amounts reflected herein due to a variety of factors, including access to additional information, changes in value not currently identified, and changes in operating results following the closing date of the Merger and the date of the pro forma financial information.
The pro forma financial information does not give effect to any anticipated synergies, operating efficiencies, tax savings, or cost savings that may be associated with the Merger and the related transactions. The unaudited pro forma condensed combined financial information does not reflect the income tax effects of the pro forma adjustments, as management believes income tax adjustments to not be meaningful given the combined entity incurred significant losses during the historical periods presented.
AgeX and Serina have incurred certain non-recurring charges and AgeX and Serina anticipate that additional non-recurring charges will be incurred in connection with the Merger, the substantial majority of which consist of transaction costs related to financial advisors, legal services and professional accounting services. Such non-recurring charges could affect the future results of the combined company in the period in which such charges are incurred; however, these costs are not expected to be incurred in any period beyond 12 months from the closing date of the Merger. Accordingly, the unaudited pro forma condensed combined statement of operations for the year ended December 31, 2022, reflect the effects of these non-recurring charges, which are not accrued for in the historical balance sheets of AgeX and Serina as of June 30, 2023.
Note 3: Accounting Treatment for the Merger
For accounting purposes, Serina is considered to be the acquiring company and the Merger is expected to be accounted for as a reverse recapitalization of AgeX by Serina. This determination is primarily based on the expectation that, immediately following the Merger:
| | ● | The pre-Merger stockholders of Serina are expected to own approximately 75% of the combined company and to hold the majority of voting rights in the combined company; |
| | ● | The pre-Merger stockholders of Serina will have the right to appoint or approve a majority of the directors on the combined company’s board of directors; |
| | ● | Certain current members of the Serina executive management team are expected to continue in key leadership roles of the combined company; and |
| | ● | The combined company intends to primarily focus on developing Serina’s product candidates, and it is anticipated that the combined company will not continue to develop AgeX’s product candidates. |
Under reverse recapitalization accounting, Serina’s assets and liabilities will be recorded at their pre-Merger carrying amounts with no goodwill or other intangible assets recorded. The assets and liabilities of AgeX will be recorded, as of the completion of the Merger, at their fair value, which are expected to approximate the carrying value of the acquired cash and other non-operating net assets. Fair value was determined in accordance with the fair value concepts defined in ASC 820, Fair Value Measurements and Disclosures (ASC 820). Fair value is defined in ASC 820 as “the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.” Fair value measurements can be highly subjective and can involve a high degree of estimation. Any difference between the final fair value of the consideration transferred and the fair value of the net assets of AgeX following determination of the actual purchase price consideration for AgeX will be reflected as an adjustment to additional paid-in capital. As a result, any change in fair value of the consideration transferred is not expected to materially affect the unaudited pro forma condensed combined financial information. The subsequent financial statements of Serina will reflect the combined operations of Serina, as the acquirer for accounting purposes together with a deemed issuance of shares, equivalent to the shares held by the stockholders of the legal acquirer, AgeX, immediately prior to the Effective Time, and a recapitalization of the equity of the accounting acquirer, Serina.
For purposes of these unaudited pro forma condensed combined financial information, the estimated purchase price consideration consists of the following:
| (in thousands, except share and per share data) | | | |
| Estimated number of shares of the combined company to be owned by AgeX’s stockholders (i) | | | 87,947,152 | |
| Multiplied by the estimated fair value per share of AgeX’s common stock (ii) | | $ | 0.89 | |
| Total | | $ | 78,273 | |
| Post-merger warrants issued to AgeX stockholders (iii) | | $ | 32,055 | |
| Total estimated purchase price consideration | | $ | 110,328 | |
(i) Reflects the number of shares of common stock of the combined company that AgeX stockholders are expected to own as of the Effective Time pursuant to the Merger Agreement. This amount is calculated, for purposes of this unaudited pro forma condensed combined financial information, based on the shares of AgeX common stock outstanding as of June 30, 2023 and giving effect to the AgeX preferred stock conversion. On July 21, 2023, AgeX entered into the certain Exchange Agreement (the 2023 Exchange Agreement) with Juvenescence Limited (Juvenescence) pursuant to which AgeX issued to Juvenescence 211,600 shares of a newly authorized Series A preferred stock and 148,400 shares of a newly authorized Series B preferred stock in exchange for the cancellation of a total of $36,000 of indebtedness consisting of the outstanding principal amount of certain loans made by Juvenescence to AgeX and loan origination fees accrued with respect to those loans. The cancellation of indebtedness in exchange for the preferred stock was conducted pursuant to the 2023 Exchange Agreement. The transaction closed on July 24, 2023. By completing the exchange of indebtedness for shares of Series A preferred stock and Series B preferred stock, AgeX satisfied stockholders equity requirements to under the NYSE American continued listing requirements. Accordingly, the NYSE American staff withdrew its delisting determination and a scheduled hearing of AgeX’s appeal of that determination was cancelled. The NYSE American has approved the listing of the 36,939,190 shares of AgeX common stock into which the Series A and Series B preferred stock is presently convertible. In order to comply with Section 713 of the NYSE American Company Guide, the issuance of an additional 13,060,809 shares of AgeX common stock upon conversion of shares of Series B preferred stock is currently restricted by a “cap” prohibiting issuance of those additional shares without the prior approval of AgeX stockholders.
(ii) Reflects the price per share of AgeX common stock, which is the closing bid price of AgeX common stock as reported by NYSE American on June 30, 2023. The fair value of common stock included in the estimated purchase price consideration will change based on fluctuations in the share price of AgeX common stock and the number of equity instruments held by preexisting stockholders of AgeX at the Effective Time.
(iii) Reflects the fair value of post-merger warrants to be issued to the holders of AgeX common stock (including Juvenescence) prior to the consummation of the Merger. The fair value of the post-merger warrants was determined using level 3 inputs utilizing the Black-Scholes-Merton option pricing model.
The actual purchase price consideration transferred for the net assets of AgeX will vary based on, among other things, the exchange ratio and the Actual Closing Price of AgeX common stock, and those differences could be material. As such, the estimated purchase price consideration reflected in these unaudited pro forma condensed combined financial statements does not purport to represent what the actual purchase price consideration will be when the Merger is completed. The actual purchase price will fluctuate until the Effective Time, and the final valuation of the purchase price consideration could differ significantly from the current estimate.
Note 4: Transaction Accounting Adjustments
Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet as of June 30, 2023
Pre-Transaction Accounting Adjustments
The pre-transaction accounting adjustments included in the unaudited pro forma condensed combined balance sheet as of June 30, 2023, are as follows:
| A | Represents a reduction of $25,616 to the current portion of loans due to Juvenescence and $10,384 to the non-current portion of loans due to Juvenescence upon the conversion of the historical loans due to Juvenescence that were converted into 211,600 shares of AgeX Series A preferred stock and 148,400 shares of AgeX Series B preferred stock on July 24, 2023 pursuant to the 2023 Exchange Agreement. The subscription price per share of AgeX Series A preferred stock and AgeX Series B preferred stock was $100 per share. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects the AgeX Series A preferred stock of $21,160 and AgeX Series B preferred stock of $14,840. |
| B | Represents an adjustment of $2,475 to the current portion of loans due to Juvenescence and $316 to the non-current portion of loans due to Juvenescence to derecognize the debt issuance costs expensed upon the conversion of the historical loans due to Juvenescence that were converted into 211,600 shares of AgeX Series A preferred stock and 148,400 shares of AgeX Series B preferred stock on July 24, 2023 with a corresponding increase in accumulated deficit pursuant to the 2023 Exchange Agreement. |
| C | As a condition to the closing of the Merger, represents the conversion of 211,600 shares of AgeX Series A preferred stock and 148,400 shares of AgeX Series B preferred stock into 50,000,000 shares of AgeX common stock. Each share of AgeX preferred stock is convertible into a number of shares of AgeX common stock determined by dividing (x) a number equal to the number of dollars and cents comprising the subscription price, by (y) a number equal to the number of dollars and cents comprising the conversion price. The conversion price per share of AgeX Series A preferred stock and AgeX Series B preferred stock is $0.72, which was the closing price of AgeX common stock on the NYSE American on the last trading day immediately preceding the execution of the 2023 Exchange Agreement. |
| D | As a condition to the closing of the Merger, AgeX must have on hand at least $500 of immediately spendable non-restricted cash net of all payables and other liabilities (including Transaction Expenses (as defined elsewhere in this proxy statement/prospectus/information statement)). The adjustment represents additional loans due to Juvenescence necessary for AgeX to obtain to satisfy the closing condition to the Merger. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects an increase to cash with a corresponding increase in loans due to Juvenescence, net of debt issuance costs, current portion of $3,886. The additional loans due to Juvenescence would not be considered as additional payables in accordance with this closing condition. |
| E | As a condition to the closing of the Merger, represents the holders of Serina’s convertible promissory notes (other than the AgeX-Serina Note held by AgeX) comprising a principal amount of $1,450 and accrued interest of $78 election to convert such notes into shares of Serina Series A-5 Preferred Stock prior to the closing of the Merger. As of June 30, 2023, the fair value of Serina’s convertible promissory notes (other than the AgeX-Serina Note held by AgeX) was $1,537. On July 26, 2023, all of the convertible promissory notes (excluding the AgeX-Serina Note held by AgeX) were converted into 117,903 shares of Serina Series A-5 Preferred Stock. The holders of the convertible promissory notes (other than the AgeX-Serina Note held by AgeX) also received warrants to purchase an additional 117,903 shares of Serina Series A-5 Preferred Stock. The fair value of the warrants was $845 as of June 30, 2023. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects a reduction to convertible promissory notes of $1,537, a reduction of accrued interest recorded in accounts payable and accrued liabilities of $78, an increase to warrant liability of $845, and an increase to redeemable convertible preferred stock of $770. |
| F | Upon the consummation of the Merger, the $10,000 convertible promissory note between AgeX and Serina remains outstanding and becomes an intercompany asset of AgeX and an intercompany liability of Serina. The adjustment reverses any previous fair value adjustments recorded by Serina to bring the balance of the convertible promissory note liability back to its principal amount of $10,000. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects an increase to the convertible promissory note liability of $3,924 with a corresponding adjustment to accumulated deficit. |
| G | Represents an adjustment of $10,276 to Serina’s redeemable convertible preferred stock to reflect the preferred stock at its max redemption value as of June 30, 2023. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects an increase to redeemable convertible preferred stock with a corresponding increase in accumulated deficit. |
Transaction Accounting Adjustments
The transaction accounting adjustments included in the unaudited pro forma condensed combined balance sheet as of June 30, 2023 are as follows:
| H | Represents payment of AgeX’s estimated transaction costs subsequent to June 30, 2023. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects payment of these estimated costs with a corresponding increase in accumulated deficit, which excludes amounts which were previously paid and expensed of $934 during the six months ended June 30, 2023. Refer to the table below for a summary of estimated transaction costs associated with the Merger: |
| (in thousands) | | | |
| Advisory, legal, and other professional fees | | $ | 930 | |
| D&O insurance | | | 2,000 | |
| Other transaction costs and fees | | | 244 | |
| Total | | $ | 3,174 | |
| I | Represents payment of Serina’s estimated transaction costs subsequent to June 30, 2023. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects payment of these estimated costs with a corresponding increase in accumulated deficit, which excludes amounts which were previously paid and expensed of $220 during the six months ended June 30, 2023. Refer to the table below for a summary of estimated transaction costs associated with the Merger: |
| (in thousands) | | | |
| Advisory, legal, and other professional fees | | $ | 885 | |
| Total | | $ | 885 | |
| J | Upon the consummation of the Merger, the aggregate principal amount of $10,000 pursuant to the AgeX-Serina Note between AgeX and Serina remains outstanding and becomes an intercompany asset of AgeX and an intercompany liability of Serina that is eliminated in the combined company’s consolidation. As of June 30, 2023, accrued interest on the AgeX-Serina Note was $204. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 reflects a reduction to the AgeX-Serina Note asset recorded by AgeX of $10,204 comprising the principal and accrued interest, a reduction of accrued interest recorded in accounts payable and accrued liabilities of $204 recorded by Serina, and a reduction to the AgeX-Serina Note liability of $10,000 recorded by Serina. |
| K | Represents the estimated fair value of post-merger warrants to be issued to the holders of AgeX common stock (including Juvenescence) prior to the consummation of the Merger. The Black-Scholes-Merton option pricing model was utilized to determine the fair value of the warrants. A total of 39,868,484 post-merger warrants (on a pre-reverse stock split basis) are expected to be issued to Juvenescence and a total of 12,899,772 post-merger warrants (on a pre-reverse stock split basis) are expected to be issued to other AgeX stockholders. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 include an estimated adjustment of $32,055 to the post-merger warrant liability representing the fair value of the post-merger warrants issued with a corresponding change to additional paid-in capital. |
| L | Represents the derecognition of the AgeX adjusted stockholders’ equity (deficit) balances. The unaudited pro forma condensed combined balance sheet as of June 30, 2023 include an adjustment of $9 to AgeX common stock, $135,972 to additional paid-in capital, $124,947 to accumulated deficit, and $121 to noncontrolling interest. |
| M | Represents an adjustment of $10,913 to account for the adjusted AgeX net assets acquired in the reverse recapitalization as of June 30, 2023. |
| N | Represents an adjustment of $46,488 to account for the conversion of Serina’s adjusted redeemable convertible preferred stock into shares of common stock in the combined company. |
Adjustments to the Unaudited Pro Forma Condensed Combined Statements of Operations for the Six Months Ended June 30, 2023, and Year Ended December 31, 2022
Pre-Transaction Accounting Adjustments
The pre-transaction accounting adjustments included in the unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2023, and year ended December 31, 2022, are as follows:
| AA | Represents the derecognition of Serina’s as reported changes in the fair value at inception and through the end of the period of the convertible promissory notes (other than the AgeX-Serina Note held by AgeX) of $(180) and $267 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023 and the year ended December 31, 2022, respectively. |
| BB | Represents the derecognition of Serina’s as reported change in fair value of the AgeX-Serina Note held by AgeX at its inception on March 15, 2023 of $2,240 and through June 30, 2023 of $1,684 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023 and the year ended December 31, 2022, respectively. Upon the consummation of the Merger, the aggregate principal amount of $10,000 pursuant to the AgeX-Serina Note between AgeX and Serina remains outstanding and becomes an intercompany asset of AgeX and an intercompany liability of Serina that is eliminated in the combined company’s consolidation. |
Transaction Accounting Adjustments
The transaction accounting adjustments included in the unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2023, and year ended December 31, 2022, are as follows:
| CC | Recognition of estimated transaction costs related to the Merger expected to be incurred by AgeX in the amount of $3,174 that would be expensed. The unaudited pro forma condensed combined statement of operations reflects payment of these costs as an increase in general and administrative expense for the year ended December 31, 2022. The unaudited pro forma condensed combined statement of operations already includes amounts that were previously paid and expensed of $934 during the six months ended June 30, 2023. |
| DD | Recognition of estimated transaction costs related to the Merger expected to be incurred by Serina in the amount of $885 that would be expensed. The unaudited pro forma condensed combined statement of operations reflects payment of these costs as an increase in general and administrative expense for the year ended December 31, 2022. The unaudited pro forma condensed combined statement of operations already includes amounts that were previously paid and expensed of $220 during the six months ended June 30, 2023. |
| EE | Represents the derecognition of AgeX’s as reported interest expense of $2,096 and $3,335 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023 and the year ended December 31, 2022 respectively resulting from the conversion of the historical loans due to Juvenescence that through the 2023 Exchange Agreement entered into by AgeX and Juvenescence on July 21, 2023, and closed on July 24, 2023, whereby the loan obligations totaling $36,000 owed to Juvenescence by AgeX were converted into 211,600 shares of AgeX Series A preferred stock and 148,400 shares of AgeX Series B preferred stock on July 24, 2023 pursuant to the 2023 Exchange Agreement. |
| FF | Represents the derecognition of Serina’s as reported interest expense from the convertible promissory note with AgeX of $204 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023. Upon the consummation of the Merger, the $10,000 convertible promissory note between AgeX and Serina remains outstanding and becomes an intercompany asset of AgeX and an intercompany liability of Serina with the balances eliminated in consolidation. |
| GG | Represents the derecognition of AgeX’s as reported interest income from the AgeX-Serina Note of $204 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023. Upon the consummation of the Merger, the $10,000 AgeX-Serina Note between AgeX and Serina remains outstanding and becomes an intercompany asset of AgeX and an intercompany liability of Serina with the balances eliminated in consolidation. |
| HH | Represents the derecognition of Serina’s as reported interest expense from the convertible promissory notes (other than AgeX-Serina Note held by AgeX) of $78 in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2023. |
| II | The pro forma basic and diluted earnings per share of capital stock of AgeX and Serina have been adjusted to reflect the pro forma net loss for the six months ended June 30, 2023 and the year ended December 31, 2022. In addition, the number of shares of capital stock of AgeX and Serina used in calculating the pro forma combined basic and diluted net loss per share has been adjusted to reflect the estimated total number of shares of common stock of the combined company for the respective periods. |
For the six months ended June 30, 2023, the pro forma weighted average shares of capital stock of AgeX and Serina outstanding has been calculated as follows:
| (in thousands, except share and per share data) | | | |
| Historical Serina weighted average shares of Serina common stock outstanding | | | 2,221,100 | |
| Impact of historical Serina options to purchase shares converted into options to purchase AgeX common stock as of January 1, 2022 on an as exercised basis | | | 1,990,150 | |
| Impact of historical Serina Series A Preferred Stock outstanding on an as converted basis (giving effect to the Serina preferred stock conversion as of January 1, 2022) (iv) | | | 4,886,830 | |
| Total | | | 9,098,080 | |
| Application of the exchange ratio to historical Serina weighted average shares of Serina capital stock outstanding on an as converted basis | | | 28.9992 | |
| Adjusted Serina weighted average shares outstanding on an as converted basis | | | 263,837,348 | |
| Historical AgeX weighted average shares of AgeX common stock outstanding | | | 37,951,261 | |
| Impact of historical AgeX weighted average shares of AgeX Series A preferred stock and Series B preferred stock outstanding on an as converted basis (giving effect to the AgeX preferred stock conversion as of January 1, 2022) | | | 49,999,999 | |
| Total pro forma weighted average shares outstanding | | | 351,788,608 | |
For the year ended December 31, 2022, the pro forma weighted average shares of capital stock of AgeX and Serina outstanding has been calculated as follows:
| (in thousands, except share and per share data) | | | |
| Historical Serina weighted average shares of Serina common stock outstanding | | | 2,145,002 | |
| Impact of historical Serina options to purchase shares converted into options to purchase AgeX common stock as of January 1, 2022 on an as exercised basis | | | 2,012,750 | |
| Impact of historical Serina Series A Preferred outstanding on an as converted basis (giving effect to the Serina preferred stock conversion as of January 1, 2022) (iv) | | | 4,886,830 | |
| Total | | | 9,044,582 | |
| Application of the exchange ratio to historical Serina weighted average shares of Serina capital stock outstanding on an as converted basis | | | 28.9992 | |
| Adjusted Serina weighted average shares outstanding on an as converted basis | | | 262,285,947 | |
| Historical AgeX weighted average shares of AgeX common stock outstanding | | | 37,949,196 | |
| Impact of historical AgeX weighted average shares of AgeX Series A preferred stock and Series B preferred stock outstanding on an as converted basis (giving effect to the AgeX preferred stock conversion as of January 1, 2022) | | | 49,999,999 | |
| Total pro forma weighted average shares outstanding | | | 350,235,142 | |
(iv) The AgeX common stock allocated to the Serina Series A Preferred Stock is based on the “Minimum Share Price” of $12.00 (on a post-reverse split basis) as defined in the Merger Agreement.
As the proposed reverse stock split ratio of AgeX common stock is not definitive and is expected to occur following receipt of stockholder approval and prior to the consummation of the Merger, the exchange ratio and the estimated weighted average shares of AgeX common stock issued to Serina stockholders have not been adjusted to give retrospective effect to the reverse stock split.
INDEX TO FINANCIAL STATEMENTS
AgeX Therapeutics, Inc.
Index to Consolidated Financial Statements
Serina Therapeutics, Inc. Financial Statements
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
AgeX Therapeutics, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of AgeX Therapeutics, Inc. and Subsidiaries (collectively, the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1, the Company has had recurring losses and negative operating cash flows since inception, an accumulated deficit at December 31, 2022, and insufficient cash and cash equivalents and loan proceeds at December 31, 2022 to fund operations for twelve months from the date of issuance. All of these matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the Audit Committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements; and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Intangible Assets Impairment Assessment – Long-Lived Intangible Assets
Description of the Matter
As described in Note 2 to the consolidated financial statements, the Company’s long-lived net intangible assets, which consisted primarily of patents and acquired in-process research and development, had a balance of $0.7 million as of December 31, 2022.
Long-lived intangible assets are assessed for impairment whenever events or changes in circumstances indicate the carrying amounts of the assets may not be recoverable. Recoverability of a long-lived intangible asset that will continue to be used in the Company’s operations is measured by comparing the carrying amount of the asset to the forecasted undiscounted future cash flows related to the asset.
Auditing the Company’s impairment analysis of its long-lived intangible assets is complex because of the significant judgment used by management in the identification of events that suggest an asset group may not be recoverable, and the highly subjective assumptions used by management in the impairment testing process.
How We Addressed the Matter in Our Audit
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, obtaining an understanding of and evaluating management’s process for identifying potential impairment events; evaluating the appropriateness of the cash flow model used in the impairment testing process; testing the completeness, accuracy, and relevance of underlying data used in the model; and evaluating the reasonableness of the significant assumptions used by management, including the future cash flow projections. We evaluated the reasonableness of management’s assumptions for future cash flow projections in consideration of (i) the current and past performance of the asset group, (ii) the consistency with external market and industry data, (iii) peer companies and (iv) whether these assumptions were consistent with evidence obtained in other areas of the audit.
| /s/ WithumSmith+Brown, PC | |
| | |
| We have served as the Company’s auditor since 2017. | |
| San Francisco, California | |
| March 31, 2023 | |
| | |
| PCAOB ID Number 100 | |
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value amounts)
| | | | | 2021 | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 645 | | | $ | 584 | |
| Accounts and grants receivable, net | | | 4 | | | | 25 | |
| Prepaid expenses and other current assets | | | 1,804 | | | | 1,625 | |
| Total current assets | | | 2,453 | | | | 2,234 | |
| | | | | | | | | |
| Restricted cash | | | 50 | | | | 50 | |
| Intangible assets, net | | | 738 | | | | 870 | |
| Convertible note receivable | | | | | | | | |
| TOTAL ASSETS | | $ | 3,241 | | | $ | 3,154 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 1,034 | | | $ | 771 | |
| Loan due to Juvenescence, net of debt issuance costs, current portion | | | 7,646 | | | | 7,140 | |
| Related party payables, net | | | 141 | | | | 70 | |
| Warrant liability | | | 180 | | | | - | |
| Insurance premium liability and other current liabilities | | | 1,077 | | | | 986 | |
| Total current liabilities | | | 10,078 | | | | 8,967 | |
| | | | | | | | | |
| Loan due to Juvenescence, net of debt issuance costs, net of current portion | | | 10,478 | | | | 6,062 | |
| TOTAL LIABILITIES | | | 20,556 | | | | 15,029 | |
| | | | | | | | | |
| Commitments and contingencies (Note 10) | | | - | | | | - | |
| | | | | | | | | |
| Stockholders’ deficit: | | | | | | | | |
| Preferred stock, $0.0001 par value, 5,000 shares authorized; none issued and outstanding | | | - | | | | - | |
| Common stock, $0.0001 par value, 200,000 and 100,000 shares authorized, respectively; 37,949 and 37,941 shares issued and outstanding, respectively | | | 4 | | | | 4 | |
| Additional paid-in capital | | | 98,994 | | | | 93,912 | |
| Accumulated deficit | | | (116,210) | | | | (105,748) | |
| Total AgeX Therapeutics, Inc. stockholders’ deficit | | | (17,212) | | | | (11,832) | |
| Noncontrolling interest | | | (103) | | | | (43) | |
| Total stockholders’ deficit | | | (17,315) | | | | (11,875) | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 3,241 | | | $ | 3,154 | |
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| | | 2022 | | | 2021 | |
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| REVENUES | | | | | | |
| Grant revenues | | $ | - | | | $ | 104 | |
| Other revenues | | | 34 | | | | 40 | |
| Total revenues | | | 34 | | | | 144 | |
| | | | | | | | | |
| Cost of sales | | | (13) | | | | (19) | |
| | | | | | | | | |
| Gross profit | | | 21 | | | | 125 | |
| | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | |
| Research and development | | | 1,025 | | | | 1,456 | |
| General and administrative | | | 5,971 | | | | 6,708 | |
| Total operating expenses | | | 6,996 | | | | 8,164 | |
| | | | | | | | | |
| Gain on deconsolidation of LifeMap Sciences (Note 3) | | | - | | | | 106 | |
| | | | | | | | | |
| Loss from operations | | | (6,975) | | | | (7,933) | |
| | | | | | | | | |
| OTHER EXPENSE, NET | | | | | | | | |
| Interest expense, net | | | (3,335) | | | | (1,097) | |
| Change in fair value of warrants (Note 6) | | | (225) | | | | - | |
| Other income, net | | | 13 | | | | 448 | |
| Total other expense, net | | | (3,547) | | | | (649) | |
| | | | | | | | | |
| NET LOSS FROM CONTINUING OPERATIONS | | | (10,522) | | | | (8,582) | |
| | | | | | | | | |
| NET LOSS FROM DISCONTINUED OPERATIONS (Note 3) | | | - | | | | (103) | |
| | | | | | | | | |
| NET LOSS | | | (10,522) | | | | (8,685) | |
| Net loss attributable to noncontrolling interest from continuing operations | | | 60 | | | | 3 | |
| Net loss attributable to noncontrolling interest from discontinued operations | | | - | | | | 7 | |
| Net loss attributable to noncontrolling interest | | | | | | | | |
| | | | | | | | | |
| NET LOSS ATTRIBUTABLE TO AGEX | | $ | (10,462) | | | $ | (8,675) | |
| | | | | | | | | |
| NET LOSS PER COMMON SHARE: | | | | | | | | |
| BASIC AND DILUTED | | | | | | | | |
| NET LOSS PER COMMON SHARE: BASIC AND DILUTED | | | | | | | | |
| Continuing operations | | $ | (0.28) | | | $ | (0.23) | |
| Discontinued operations | | | - | | | | - | |
| | $ | (0.28) | | | $ | (0.23) | |
| WEIGHTED-AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | | | | | | | | |
| WEIGHTED-AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: BASIC AND DILUTED | | | | | | | | |
| BASIC AND DILUTED | | | 37,945 | | | | 37,886 | |
| | | | | | | | | |
| AMOUNTS ATTRIBUTABLE TO AGEX: | | | | | | | | |
| Loss from continuing operations | | $ | (10,462) | | | $ | (8,579) | |
| Loss from discontinued operations | | | - | | | | (96) | |
| NET LOSS ATTRIBUTABLE TO AGEX | | $ | (10,462) | | | $ | (8,675) | |
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| | | 2022 | | | 2021 | |
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| NET LOSS | | $ | (10,522) | | | $ | (8,685) | |
| Other comprehensive expense, net of tax: | | | | | | | | |
| Foreign currency translation adjustments from discontinued operations | | | - | | | | (143) | |
| COMPREHENSIVE LOSS | | | (10,522) | | | | (8,828) | |
| Less: Comprehensive loss attributable to noncontrolling interest from continuing operations | | | 60 | | | | 3 | |
| Less: Comprehensive loss attributable to noncontrolling interest from discontinued operations | | | - | | | | 7 | |
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AGEX COMMON STOCKHOLDERS | | $ | (10,462) | | | $ | (8,818) | |
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(In thousands)
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Comprehensive Income | | | Stockholders’ Deficit | |
| | | Common Stock | | | Additional | | | | | | | | | Accumulated Other | | | Total | |
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Comprehensive Income | | | Stockholders’ Deficit | |
| BALANCE AT DECEMBER 31, 2020 | | | 37,691 | | | $ | 4 | | | $ | 91,810 | | | $ | (97,073) | | | $ | (280) | | | $ | 143 | | | $ | (5,396) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock | | | 242 | | | | - | | | | 475 | | | | - | | | | - | | | | - | | | | 475 | |
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | | | 8 | | | | - | | | | (7) | | | | - | | | | - | | | | - | | | | (7) | |
| Issuance of warrants | | | - | | | | - | | | | 757 | | | | - | | | | - | | | | - | | | | 757 | |
| Stock-based compensation | | | - | | | | - | | | | 1,003 | | | | - | | | | - | | | | - | | | | 1,003 | |
| Transactions with noncontrolling interests – LifeMap Sciences | | | - | | | | - | | | | (269) | | | | - | | | | 269 | | | | - | | | | - | |
| Deconsolidation of LifeMap Sciences | | | - | | | | - | | | | 143 | | | | - | | | | (22) | | | | (143) | | | | (22) | |
| Fair value of liability classified warrants issued | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | - | | | | - | | | | - | | | | (8,675) | | | | (10) | | | | - | | | | (8,685) | |
| BALANCE AT DECEMBER 31, 2021 | | | 37,941 | | | | 4 | | | | 93,912 | | | | (105,748) | | | | (43) | | | | - | | | | (11,875) | |
| Balance | | | 37,941 | | | | 4 | | | | 93,912 | | | | (105,748 | ) | | | (43 | ) | | | - | | | | (11,875 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | | | 8 | | | | - | | | | (4) | | | | - | | | | - | | | | - | | | | (4) | |
| Issuance of warrants | | | - | | | | - | | | | 178 | | | | - | | | | - | | | | - | | | | 178 | |
| Fair value of liability classified warrants issued | | | - | | | | - | | | | 4,148 | | | | - | | | | - | | | | - | | | | 4,148 | |
| Stock-based compensation | | | - | | | | - | | | | 760 | | | | - | | | | - | | | | - | | | | 760 | |
| Net loss | | | - | | | | - | | | | - | | | | (10,462) | | | | (60) | | | | - | | | | (10,522) | |
| BALANCE AT DECEMBER 31, 2022 | | | 37,949 | | | $ | 4 | | | $ | 98,994 | | | $ | (116,210) | | | $ | (103) | | | $ | - | | | $ | (17,315) | |
| Balance | | | 37,949 | | | $ | 4 | | | $ | 98,994 | | | $ | (116,210 | ) | | $ | (103 | ) | | $ | - | | | $ | (17,315 | ) |
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | 2022 | | | 2021 | |
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| OPERATING ACTIVITIES: | | | | | | | | |
| Net loss attributable to AgeX from continuing operations | | $ | (10,462) | | | $ | (8,579) | |
| Net loss attributable to noncontrolling interest | | | (60) | | | | (3) | |
| Adjustments to reconcile net loss attributable to AgeX to net cash used in operating activities: | | | | | | | | |
| Gain on deconsolidation of LifeMap Sciences (Note 3) | | | - | | | | (106) | |
| Gain on extinguishment of debt (Paycheck Protection Program loan) | | | - | | | | (437) | |
| Change in fair value of warrants (Note 6) | | | 225 | | | | - | |
| Amortization of intangible assets | | | 132 | | | | 131 | |
| Amortization of debt issuance costs | | | 3,137 | | | | 1,114 | |
| Stock-based compensation | | | 760 | | | | 999 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts and grants receivable | | | 21 | | | | 128 | |
| Prepaid expenses and other current assets | | | 896 | | | | 760 | |
| Interest on convertible note receivable | | | | | | | | |
| Accounts payable and accrued liabilities | | | 144 | | | | (772) | |
| Related party payables | | | 255 | | | | - | |
| Insurance premium liability | | | (983) | | | | (921) | |
| Other current liabilities | | | (4) | | | | (79) | |
| Net cash used in operating activities from continuing operations | | | (5,939) | | | | (7,765) | |
| Net cash used in operating activities from discontinued operations (Note 3) | | | - | | | | (90) | |
| Net cash used in operating activities | | | (5,939) | | | | (7,855) | |
| | | | | | | | | |
| INVESTING ACTIVITIES: | | | | | | | | |
| Proceeds from the sale of LifeMap Sciences (Note 3) | | | - | | | | 466 | |
| Partial collection on loan due from LifeMap Sciences | | | - | | | | 250 | |
| Net cash provided by investing activities from continuing operations | | | - | | | | 716 | |
| Deconsolidation of cash and cash equivalents from discontinued operations (Note 3) | | | - | | | | (50) | |
| Cash advanced on convertible note receivable | | | | | | | | |
| Net cash provided by investing activities | | | - | | | | 666 | |
| | | | | | | | | |
| FINANCING ACTIVITIES: | | | | | | | | |
| Draw down on loan facilities from Juvenescence | | | 6,000 | | | | 7,000 | |
| Proceeds from the issuance of common stock | | | - | | | | 496 | |
| Net cash provided by financing activities from continuing operations | | | 6,000 | | | | 7,496 | |
| Partial payment on loan due to AgeX from discontinued operations (Note 3) | | | - | | | | (250) | |
| Net cash provided by financing activities | | | 6,000 | | | | 7,246 | |
| | | | | | | | | |
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | | | 61 | | | | 57 | |
| | | | | | | | | |
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | | | | | | | | |
| At beginning of the year | | | 634 | | | | 577 | |
| At end of the year | | $ | 695 | | | $ | 634 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | |
| Cash paid during the year for interest | | $ | 14 | | | $ | 13 | |
| | | | | | | | | |
| SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES: | | | | | | | | |
| Issuance of common stock upon vesting of restricted stock units (Note 8) | | $ | 8 | | | $ | 16 | |
| Issuance of warrants for debt issuance under the 2020 Loan Agreement | | $ | 178 | | | $ | 757 | |
| Issuance of warrants for debt issuance under the Secured Note (Note 6) | | $ | 4,148 | | | $ | - | |
| Debt refinanced with new debt (Note 5) | | $ | 7,160 | | | $ | - | |
See accompanying notes to the consolidated financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization, Basis of Presentation and Liquidity
Organization, Business Overview and Liquidity
AgeX Therapeutics, Inc. (“AgeX,” “we,” “our,” or “us”) was incorporated in January 2017 in the state of Delaware as a subsidiary of Lineage Cell Therapeutics, Inc. (“Lineage,” formerly known as BioTime, Inc.), a publicly traded, clinical-stage biotechnology company.
AgeX is a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases. AgeX’s mission is to apply its comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions.
AgeX’s main technology platforms and product candidates are:
| ● | PureStem® PSC-derived clonal embryonic progenitor cell lines that may be capable of generating a broad range of cell types for use in cell-based therapies; |
| ● | UniverCyte™ which uses the HLA-G gene to suppress rejection of transplanted cells and tissues to confer low immune observability to cells; |
| ● | AGEX-BAT1 using adipose brown fat cells for metabolic diseases such as Type II diabetes; |
| ● | AGEX-VASC1 using vascular progenitor cells to treat tissue ischemia; and |
| ● | Induced tissue regeneration or iTR technology to regenerate or rejuvenate cells to treat a variety of degenerative diseases including those associated with aging, as well as other potential tissue regeneration applications such as scarless wound repair. |
AgeX is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012.
Lineage’s Sale of Significant Ownership Interest in AgeX to Juvenescence
On August 30, 2018, Lineage consummated the sale of 14,400,000 shares of common stock of AgeX owned by Lineage to Juvenescence Limited (“Juvenescence”). Prior to the transaction, Juvenescence owned 5.6% of AgeX’s issued and outstanding common stock. Upon completion of the transaction, Lineage’s ownership in AgeX was reduced from 80.4% to 40.2% of AgeX’s issued and outstanding shares of common stock, and Juvenescence’s ownership in AgeX was increased from 5.6% to 45.8% of AgeX’s issued and outstanding shares of common stock. AgeX did not receive any proceeds from the transaction. As a result of that transaction, AgeX ceased to be a subsidiary of Lineage because Lineage experienced a “loss of control” of a subsidiary, as defined by U.S. GAAP. Loss of control is deemed to have occurred when, among other things, a parent company owns less than a majority of the outstanding common stock in the subsidiary, lacks a controlling financial interest in the subsidiary and, is unable to unilaterally control the subsidiary through other means such as having, or being able to obtain, the power to elect a majority of the subsidiary’s Board of Directors based solely on contractual rights or ownership of shares holding a majority of the voting power of the subsidiary’s voting securities. All of these loss-of-control factors were present with respect to Lineage’s ownership interest in AgeX as of August 30, 2018. Accordingly, Lineage deconsolidated AgeX’s consolidated financial statements and results from its consolidated financial statements and results beginning on August 30, 2018.
On November 28, 2018 (the “Distribution Date”), Lineage distributed to its shareholders, on a pro rata basis, 12,697,028 shares of the AgeX common stock it then held (the “Distribution”). Immediately after the Distribution, Lineage retained 1,718,972 shares of AgeX common stock, representing approximately 4.8% of the common stock then issued and outstanding. Following the Distribution, AgeX common stock began publicly trading on the NYSE American under the symbol “AGE.”
Disposition and Deconsolidation of LifeMap Sciences
On March 6, 2021, AgeX and its then majority-owned subsidiary LifeMap Sciences, Inc. (“LifeMap Sciences”) entered into an Agreement and Plan of Merger (the “LifeMap Merger Agreement”) with Atlas Capital Partners Limited, a British Virgin Islands company limited by shares (“Atlas”), and GCLMS Acquisition Corporation (“GCLMS”), a Delaware corporation that was a wholly-owned subsidiary of Atlas. On March 15, 2021, the merger was completed pursuant to the terms of the LifeMap Merger Agreement. As a result of the merger, GCLMS merged into LifeMap Sciences and (a) the shares of LifeMap Sciences common stock outstanding at the time of the merger entitled the holders of those shares to receive a pro rata portion of a $500,000 cash payment for all shares of LifeMap Sciences common stock in the aggregate (the “LifeMap Merger Consideration”), with each LifeMap Sciences shareholder’s pro rata portion of the LifeMap Merger Consideration to be determined in accordance with the number of shares of LifeMap Sciences common stock owned by such shareholder as a percentage of shares of LifeMap Sciences common stock outstanding immediately before the effective date of the merger, and (b) the outstanding shares of GCLMS common stock were converted into shares of LifeMap Sciences common stock so that Atlas is now the sole shareholder of LifeMap Sciences.
AgeX received approximately $466,400 in cash as its pro rata share of the LifeMap Merger Consideration in the merger. Prior to and as a condition to the merger under the terms of the LifeMap Merger Agreement, $1,761,296 of LifeMap Sciences’ indebtedness to AgeX was converted into shares of LifeMap Sciences common stock. LifeMap Sciences also paid AgeX $250,000 in cash to pay off a portion of LifeMap Sciences’ indebtedness to AgeX that was not converted into shares of LifeMap Sciences common stock.
As a result of the completion of the cash-out merger on March 15, 2021, LifeMap Sciences is no longer a subsidiary of AgeX. Accordingly, AgeX has deconsolidated LifeMap Sciences’ consolidated financial statements and consolidated results of operations from AgeX, effective March 15, 2021 (the “LifeMap Deconsolidation”), in accordance with Accounting Standards Codification (“ASC”) 810-10-40, Consolidation.
See Note 3, Disposition and Deconsolidation of LifeMap Sciences for additional information regarding the disposition and deconsolidation of LifeMap Sciences.
Going Concern
AgeX primarily finances its operations through loans from its largest stockholder Juvenescence. AgeX has incurred operating losses and negative cash flows since inception and had an accumulated deficit of $116.2 million as of December 31, 2022. AgeX expects to continue to incur operating losses and negative cash flows.
Based on a strategic review of its operations, giving consideration to the status of its product development programs, human resources, capital needs and resources, and current conditions in the capital markets, AgeX’s board of directors and management have adopted operating plans and budgets to extend the period over which AgeX can continue its operations with its available cash resources. Notwithstanding those operating plans and budgets, based on AgeX’s most recent projected cash flows AgeX believes that its cash and cash equivalents of $0.6 million as of December 31, 2022 plus the loan facilities provided by Juvenescence to advance up to an additional $1.0 million for operating capital discussed in Note 11, Subsequent Events and the proceeds we may receive from the sale of additional shares of our common stock in “at-the-market” transactions through a Sales Agreement with Chardan Capital Markets, LLC (“Chardan”) as a sales agent, would not be sufficient to satisfy AgeX’s anticipated operating and other funding requirements for the next twelve months from the issuance of these consolidated financial statements. These conditions raise substantial doubt about AgeX’s ability to continue as a going concern. AgeX will need to obtain substantial additional funding in connection with its continuing operations. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Principles of Consolidation
The consolidated financial statements of AgeX are presented in accordance with U.S. GAAP. AgeX’s consolidated financial statements include the accounts of its subsidiaries and certain research and development departments. AgeX consolidated its direct and indirect wholly-owned or majority-owned subsidiaries because AgeX has the ability to control their operating and financial decisions and policies through its ownership, and the noncontrolling interest is reflected as a separate element of stockholders’ deficit on AgeX’s consolidated balance sheets.
AgeX’s consolidated balance sheets at December 31, 2022 and 2021 do not include LifeMap Sciences’ consolidated assets and liabilities due to the deconsolidation of LifeMap Sciences on March 15, 2021. LifeMap Sciences’ consolidated financial statements and consolidated results of operations include its wholly-owned and consolidated subsidiary, LifeMap Sciences, Ltd.
AgeX’s consolidated statements of operations for the year ended December 31, 2021 include LifeMap Sciences’ consolidated results for the period through March 15, 2021 rather than the day immediately preceding the deconsolidation due to the conversion of $1,761,296 of LifeMap Sciences’ indebtedness to AgeX into shares of LifeMap Sciences common stock on March 15, 2021 followed by the completion of the cash-out merger on the same day.
AgeX has three subsidiaries, Reverse Bioengineering, Inc. (“Reverse Bio”), ReCyte Therapeutics, Inc. (“ReCyte”), and NeuroAirmid Therapeutics, Inc. (“NeuroAirmid”). Reverse Bio is a wholly owned subsidiary of AgeX through which AgeX plans to finance its iTRTM research and development efforts. To the extent that such financing is obtained through the sale of capital stock or other equity securities to investors or other biopharma companies by Reverse Bio, AgeX’s equity interest in Reverse Bio and its iTRTM business would be diluted. ReCyte is an early stage pre-clinical research and development company involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX owns 94.8% of the outstanding capital stock of ReCyte. NeuroAirmid is jointly owned by AgeX with the University of California – Irvine and certain researchers and was recently organized to pursue clinical development and commercialization of cell therapies, focusing initially on Huntington’s Disease. AgeX owns 50% of the outstanding capital stock of NeuroAirmid. AgeX consolidates NeuroAirmid despite not having majority ownership interest as it has the ability to influence decision making and financial results through contractual rights and obligations as per ASC 810, Consolidation.
All material intercompany accounts and transactions between AgeX and its subsidiaries have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period with consideration given to materiality. Significant estimates and assumptions which are subject to significant judgment include those related to going concern assessment of consolidated financial statements, allocations and adjustments necessary for carve-out basis of presentation, including the separate return method for income taxes, useful lives associated with long-lived assets, including evaluation of asset impairment, allowances for uncollectible accounts receivables, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards or other equity instruments and liability classified warrants. Actual results could differ materially from those estimates. To the extent there are material differences between the estimates and actual results, AgeX’s future results of operations will be affected. See Note 6, Warrant Liability for discussion on estimated change in fair value of warrant liability.
Transactions with Noncontrolling Interests of Subsidiaries
AgeX accounts for a change in ownership interests in its subsidiaries that does not result in a change of control of the subsidiary under the provisions of ASC 810-10-45-23, Consolidation – Other Presentation Matters, which prescribes the accounting for changes in ownership interest that do not result in a change in control of the subsidiary, as defined by U.S. GAAP, before and after the transaction. Under this guidance, changes in a controlling stockholder’s ownership interest that do not result in a change of control, as defined by U.S. GAAP, in the subsidiary are accounted for as equity transactions. Accordingly, if the controlling stockholder retains control, no gain or loss is recognized in the statements of operations of the controlling stockholder. Similarly, the controlling stockholder will not record any additional acquisition adjustments to reflect its subsequent purchases of additional shares in the subsidiary if there is no change of control. Only a proportional and immediate transfer of carrying value between the controlling and the noncontrolling stockholders occurs based on the respective ownership percentages.
Liquidity and Impact of COVID-19
In addition to general economic and capital market trends and conditions, AgeX’s ability to raise sufficient additional capital to finance its operations from time to time will depend on a number of factors specific to AgeX’s operations such as operating expenses and progress in out-licensing its technologies and development of its product candidates. Although AgeX has been able to reduce its operating expenses by eliminating internal research and development activities and focusing instead on out-sourcing research and development and seeking licensing arrangements for AgeX technologies, this approach has also made it more difficult for AgeX to make progress in developing its target product candidates and technologies, which in turn may make it more difficult for AgeX to raise capital. The availability of financing also may be adversely impacted by the COVID-19 pandemic which disrupt aspects of AgeX’s operations. The extent to which the ongoing COVID-19 pandemic will ultimately impact AgeX’s business, results of operations, financial condition, or cash flows is highly uncertain and difficult to predict because it will depend on many factors that are outside AgeX’s control. The unavailability or inadequacy of financing to meet future capital needs could force AgeX to modify, curtail, delay, or suspend some or all aspects of planned operations. Sales of additional equity securities could result in the dilution of the interests of its stockholders. AgeX cannot assure that adequate financing will be available on favorable terms, if at all.
2. Summary of Significant Accounting Policies
Basis of Presentation and Summary of Significant Accounting Policies
Going Concern Assessment
AgeX assesses going concern uncertainty for its consolidated financial statements to determine if AgeX has sufficient cash and cash equivalents on hand and working capital to operate for a period of at least one year from the date the consolidated financial statements are issued or are available to be issued, which is referred to as the “look-forward period” as defined by Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2014-15. As part of this assessment, based on conditions that are known and reasonably knowable to AgeX, AgeX will consider various scenarios, forecasts, projections, and estimates, and AgeX will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, and its ability to delay or curtail those expenditures or programs, if necessary, among other factors. Based on this assessment, as necessary or applicable, AgeX makes certain assumptions concerning its ability to curtail or delay research and development programs and expenditures within the look-forward period in accordance with ASU No. 2014-15 (see Note 1, Organization, Basis of Presentation and Liquidity).
Fair Value Measurements of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of the financial statement presentation date.
The carrying values of cash equivalents, accounts receivable and accounts payable, are carried at, or approximate, fair value as of the reporting date because of their short-term nature. The credit facility is reported at fair value as it bears market rates of interest. Fair values for the AgeX’s warrant liabilities are estimated by utilizing valuation models that consider current and expected stock prices, volatility, dividends, market interest rates, forward yield curves and discount rates. Such amounts and the recognition of such amounts are subject to significant estimates that may change in the future.
To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value (ASC 820-10-50, Fair Value Measurements and Disclosures):
| ● | Level 1 – Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 – Inputs to the valuation methodology include quoted prices for similar assets or liabilities in active markets, and inputs that are observable for the assets or liabilities, either directly or indirectly, for substantially the full term of the financial instruments. |
| ● | Level 3 – Inputs to the valuation methodology are unobservable; that reflect management’s own assumptions about the assumptions market participants would make and significant to the fair value. |
The following table summarizes fair value measurements by level as of December 31, 2022 for liabilities measured at fair value on a recurring basis (in thousands):
Schedule of Fair Value of Assets and Liabilities Measured on Recurring Basis
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| Warrant liability | | $ | - | | | $ | - | | | $ | 180 | | | $ | 180 | |
There were no warrant liabilities as of December 31, 2021. See Note 6, Warrant Liability for additional information on accounting for liability classified warrants and level 3 rollforward table.
In determining fair value, AgeX utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, AgeX has no financial assets recorded at fair value on a recurring basis, except for cash and cash equivalents primarily consisting of money market funds. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input.
The carrying amounts of accounts receivable, net, prepaid expenses and other current assets, related party amounts due to affiliates, accounts payable, accrued liabilities and other current liabilities approximate fair values because of the short-term nature of these items.
The accounting guidance establishes a hierarchy which requires an entity to maximize the use of quoted market prices and minimize the use of unobservable inputs. An asset or liability’s level is based on the lowest level of input that is significant to the fair value measurement. Fair value estimates are reviewed at the origination date and again at each applicable measurement date and interim or annual financial reporting dates, as applicable for the financial instrument, and are based upon certain market assumptions and pertinent information available to management at those times.
The methods and significant inputs and assumptions utilized in estimating the fair value of the warrant liabilities, as well as the respective hierarchy designations, are discussed further in Note 6, Warrant Liability. The warrant liability measurement is considered a Level 3 measurement based on the availability of market data and inputs and the significance of any unobservable inputs as of the measurement date.
Cash and Cash Equivalents
AgeX considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. As of December 31, 2022 and 2021, AgeX’s cash balances totaled $0.6 million and $0.6 million, respectively, and consist entirely of bank account deposits and amounts held in money market funds.
Concentrations of Credit Risk
Concentration of credit risk and other risks and uncertainties
Financial instruments that potentially subject AgeX to significant concentrations of credit risk consist primarily of cash and cash equivalents. AgeX limits the amount of credit exposure of cash balances by maintaining its accounts in high credit quality financial institutions. Cash equivalent deposits with financial institutions may occasionally exceed the limits of insurance on bank deposits; however, AgeX has not experienced any losses on such accounts.
Restricted Cash
Cash, cash equivalents, and restricted cash
In accordance with ASU 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash, a reconciliation of AgeX’s cash and cash equivalents in the consolidated balance sheets to cash, cash equivalents and restricted cash in the consolidated statements of cash flows for all periods presented is as follows (in thousands):
Schedule of Cash, Cash Equivalents and Restricted Cash
| | | | | | | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| Cash and cash equivalents | | $ | 645 | | | $ | 584 | |
| Restricted cash (1) | | | 50 | | | | 50 | |
| Cash, cash equivalents, and restricted cash as shown in the consolidated statements of cash flows | | $ | 695 | | | $ | 634 | |
| (1) | Restricted cash entirely represents the deposit required to maintain AgeX’s corporate credit card program. All restricted cash was included in deposits in the consolidated balance sheets. |
Accounts Receivable, Net
AgeX establishes an allowance for doubtful accounts based on the evaluation of the collectability of its receivables after considering a variety of factors, including the length of time receivables are past due, significant events that may impair the customer’s ability to pay, such as a bankruptcy filing or deterioration in the customer’s operating results or financial position, and historical experience. If circumstances related to customers change, estimates of the recoverability of receivables would be further adjusted. There were no amounts reserved for doubtful accounts as of December 31, 2022 and 2021.
Long-Lived Intangible Assets, Net
Long-lived intangible assets, consisting primarily of acquired in-process research and development (“IPR&D”) and patents is stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful life of 10 years (see Note 4, Selected Balance Sheet Components).
Impairment of Long-Lived Assets
AgeX assesses the impairment of long-lived assets whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. AgeX’s long-lived assets consist entirely of intangible assets. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss, equal to the excess of the carrying value of the asset over its fair value, is recorded. As of December 31, 2022, there has been no impairment of long-lived assets.
Leases
AgeX accounts for leases in accordance with ASU 2016-02, Leases (Topic 842) (“ASC 842”), and its subsequent amendments affecting AgeX, which are: (i) ASU 2018-10, Codification Improvements to Topic 842, Leases, and (ii) ASU 2018-11, Leases (Topic 842): Targeted improvements, using the modified retrospective method. AgeX management determines if an arrangement is a lease at inception. Leases are classified as either financing or operating, with classification affecting the pattern of expense recognition in the consolidated statements of operations. When determining whether a lease is a financing lease or an operating lease, ASC 842 does not specifically define criteria to determine “major part of remaining economic life of the underlying asset” and “substantially all of the fair value of the underlying asset.” For lease classification determination, AgeX continues to use (i) 75% or greater to determine whether the lease term is a major part of the remaining economic life of the underlying asset and (ii) 90% or greater to determine whether the present value of the sum of lease payments is substantially all of the fair value of the underlying asset. Under the available practical expedients, and as applicable, AgeX accounts for the lease and non-lease components as a single lease component. AgeX recognizes right-of-use (“ROU”) assets and lease liabilities for leases with terms greater than twelve months in the consolidated balance sheets.
ROU assets represent an entity’s right to use an underlying asset during the lease term and lease liabilities represent an entity’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. If the lease agreement does not provide an implicit rate in the contract, an entity uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The lease terms may include options to extend or terminate the lease when it is reasonably certain that the entity will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term. AgeX does not capitalize leases that have terms of twelve months or less.
On November 3, 2020, AgeX entered into a one-year lease effective January 1, 2021 for 135 square feet of office space in a building in an office and research park in Alameda, California. Base monthly rent was $947 over the lease term. AgeX renewed this lease for 12 months effective January 1, 2022 for base monthly rent of $1,074. In October 2022, AgeX entered into a new 12 month lease for a slightly less space in the same building effective January 1, 2023 for base monthly rent of $844. AgeX elected to not apply the recognition requirements under ASC 842 and instead recognized the lease payments as lease costs on a straight-line basis over the lease term, as the amount of the lease payments were not deemed material.
Accounting for Warrants
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480-10, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable provisions of U.S. GAAP. After all relevant assessments, AgeX concludes whether the warrants are classified as liability or equity. Liability classified warrants require fair value accounting at issuance and subsequent to initial issuance with all changes in fair value after the issuance date recorded in the statements of operations. Equity classified warrants only require fair value accounting at issuance with no changes recognized subsequent to the issuance date. AgeX has liability classified warrants as of December 31, 2022. See Notes 5, Related Party Transactions and 6, Warrant Liability for additional information regarding AgeX warrants.
Stock-Based Compensation
AgeX recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation.
AgeX estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures for grants prior to 2017, over the requisite service period. Upon adoption of ASU 2016-09 on January 1, 2017 as further discussed below, forfeitures are accounted for as they occur instead of based on the number of awards that were expected to vest prior to adoption of ASU 2016-09.
AgeX uses the Black-Scholes option pricing model for estimating the fair value of options granted under the Incentive Plan. The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. AgeX has elected to treat stock-based payment awards with time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options and restricted stock units can more reliably be measured than the fair value of services received. AgeX records compensation expense based on the then-current fair values of the stock options and restricted stock units at the grant date. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
The Black-Scholes option pricing model requires AgeX to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield (see Note 8, Stock-Based Awards).
The fair value of the shares of common stock underlying the stock options is determined in accordance with the Incentive Plan and is based on prevailing market prices on the NYSE American where AgeX common stock is traded.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. AgeX estimates the expected term of options granted using the “simplified method” provided under Staff Accounting
Bulletin, Topic 14, or SAB Topic 14.
Because AgeX’s common stock has public trading history of fewer than five years, AgeX has estimated the expected volatility using its own stock price volatility to the extent applicable or a combination of its stock price volatility and the stock price volatility of peer companies, for a period equal to the expected term of the options, which may exceed five years. The peer companies used include selected public companies within the biotechnology industry with comparable characteristics to AgeX, including similarity in size, lines of business, market capitalization, revenue and financial leverage.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of AgeX’s stock options.
The dividend yield assumption is based on AgeX’s history and expectation of dividend payouts. AgeX has never declared or paid any cash dividends on its common stock, and AgeX does not anticipate paying any cash dividends in the foreseeable future.
All excess tax benefits and tax deficiencies from stock-based compensation awards accounted for under ASC 718 are recognized as an income tax benefit or expense, respectively, in the consolidated statements of operations. An excess income tax benefit arises when the tax deduction of a share-based award for income tax purposes exceeds the compensation cost recognized for financial reporting purposes and, a tax deficiency arises when the compensation cost exceeds the tax deduction.
Stock-based compensation expense for the years ended December 31, 2022 and 2021 consists of stock-based compensation under the AgeX 2017 Equity Incentive Plan (see Note 8, Stock-Based Awards).
Certain of AgeX’s consolidated subsidiaries have had their own share-based compensation plans; however, there are no awards granted and outstanding under those plans as of December 31, 2022 and 2021. For share-based compensation awards granted by privately held consolidated subsidiaries under their respective equity plans, AgeX determines the fair value of the options granted under those plans using similar methodologies and assumptions AgeX used for its stock options discussed above.
Although the fair value of stock options and restricted stock units is determined in accordance with FASB guidance, changes in the assumptions and allocations can materially affect the estimated value and therefore the amount of compensation expense recognized in the consolidated financial statements.
Income Taxes
AgeX accounts for income taxes in accordance with ASC 740, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and enacted rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. AgeX’s judgments, estimates and projections regarding future taxable income may change over time due to changes, among other factors, in market conditions, changes in tax laws, and tax planning strategies. If AgeX’s assumptions and consequently its estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on AgeX’s consolidated financial statements.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. AgeX recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. No unrecognized tax benefits have been recorded and no amounts were accrued for the payment of interest and penalties as of December 31, 2022 and 2021. AgeX does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months. AgeX is currently unaware of any tax issues under review.
Beginning in 2018, the 2017 Tax Act subjects a U.S. stockholder to tax on Global Intangible Low Tax Income “GILTI” earned by certain foreign subsidiaries. In general, GILTI is the excess of a U.S. shareholder’s total net foreign income over a deemed return on tangible assets. The provision further allows a deduction of 50% of GILTI, however this deduction is limited to the company’s pre-GILTI U.S. income. For the year ended December 31, 2021, AgeX’s foreign entity operated at a loss; therefore, no GILTI was included in income. For the year ended December 31, 2022, there was no income or loss related to foreign activity as the entity deconsolidated from AgeX on March 15, 2021. Current interpretations under ASC 740 state that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense. We have elected to account for GILTI as a current period expense when incurred.
Revenue Recognition
AgeX recognizes revenue in a manner that depicts the transfer of control of a product or a service to a customer and reflects the amount of the consideration it expects to receive in exchange for such product or service. In doing so, AgeX follows a five-step approach: (i) identify the contract with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) the customer obtains control of the product or service. AgeX considers the terms of a contract and all relevant facts and circumstances when applying the revenue recognition standard. AgeX applies the revenue recognition standard, including the use of any practical expedients, consistently to contracts with similar characteristics and in similar circumstances.
In the applicable paragraphs below, AgeX has summarized its revenue recognition policies for its various revenue sources in accordance with Topic 606.
Revenue recognition by source and geography – Revenues are recognized when control of the promised goods or services is transferred to customers, or in the case of governmental entities funding a grant, when allowable expenses are incurred, in an amount that reflects the consideration AgeX or a subsidiary, depending on which company has the customer or the grant, expects to be entitled to in exchange for those goods or services.
The following table presents AgeX’s consolidated revenues disaggregated by source for continuing operations (in thousands):
Schedule of Disaggregated of Revenues
| | | | | | | |
| | | Year Ended December 31, | |
| REVENUES: | | 2022 | | | 2021 | |
| Grant revenues | | $ | - | | | $ | 104 | |
| Other revenues | | | 34 | | | | 40 | |
| Total revenues | | $ | 34 | | | $ | 144 | |
The following table presents consolidated revenues for continuing operations (in thousands), disaggregated by geography, based on the billing addresses of customers:
Schedule of Disaggregated Geographical Revenue
| | | | | | | |
| | | Year Ended December 31, | |
| REVENUES: | | 2022 | | | 2021 | |
| United States | | $ | 10 | | | $ | 107 | |
| Foreign | | | 24 | | | | 37 | |
| Total revenues | | $ | 34 | | | $ | 144 | |
Subscription and advertisement revenues – LifeMap Sciences sold subscription-based products, including research databases and software tools, for biomedical, gene, and disease research. LifeMap Sciences sold these subscriptions primarily through the internet to biotech and pharmaceutical companies worldwide. LifeMap Sciences’ principal subscription product was the GeneCards® Suite, which includes the GeneCards® human gene database, and the MalaCards™ human disease database. LifeMap Sciences’ performance obligations for subscriptions included a license of intellectual property related to its genetic information packages and premium genetic information tools. These licenses were deemed functional licenses that provide customers with a “right to access” to LifeMap Sciences’ intellectual property during the subscription period and, accordingly, revenue was recognized over a period of time, which was generally the subscription period. Payments were typically received at the beginning of a subscription period and revenue was recognized according to the type of subscription sold. For subscription contracts in which the subscription term commenced before a payment was due, LifeMap Sciences recorded an account receivable because the subscription was earned over time and billed the customer according to the contract terms. LifeMap Sciences deferred subscription revenues primarily represented subscriptions for which cash payment was received for the subscription term, but the subscription term was not completed as of the balance sheet date reported.
LifeMap Sciences licensed from third parties the databases and software it commercialized and had a contractual obligation to pay royalties to the licensor on subscriptions sold. These costs were included in operating loss from discontinued operations on the consolidated statements of operations when the cash was received and the royalty obligation was incurred as the royalty payments did not qualify for capitalization of costs to fulfill a contract under ASC 340-40, Other Assets and Deferred Costs - Contracts with Customers.
LifeMap Sciences recognized $0.3 million in subscription and advertisement revenues for the year ended December 31, 2021, which is included in operating loss from discontinued operations, and is for revenues earned through March 15, 2021. As a result of the LifeMap Deconsolidation, AgeX does not expect to earn subscription and advertising revenues in subsequent accounting periods. See Note 3, Disposition and Deconsolidation of LifeMap Sciences for further information on the disposition and deconsolidation of LifeMap Sciences.
Grant revenues – AgeX accounts for grants received to perform research and development services in accordance with ASC 730-20, Research and Development Arrangements. At the inception of the grant, we perform an assessment as to whether the grant is a liability or a contract to perform research and development services for others. If AgeX or a subsidiary receiving the grant is obligated to repay the grant funds to the grantor regardless of the outcome of the research and development activities, then AgeX is required to estimate and recognize that liability. Alternatively, if AgeX or a subsidiary receiving the grant is not required to repay, or if it is required to repay the grant funds only if the research and development activities are successful, then the grant agreement is accounted for as a contract to perform research and development services for others, in which case, grant revenue is recognized when the related research and development expenses are incurred.
In applying the provisions of Topic 606, AgeX has determined that government grants are out of the scope of Topic 606 because the government entities do not meet the definition of a “customer”, as defined by Topic 606, as there is not considered to be a transfer of control of good or services to the government entities funding the grant. In the absence of applicable guidance under U.S. GAAP, our policy is to recognize grant revenue when the related costs are incurred, provided that the applicable conditions under the government contracts have been met. Only costs that are allowable under the grant award, certain government regulations and the National Institutes of Health’s supplemental policy and procedure manual may be claimed for reimbursement, and the reimbursements are subject to routine audits from governmental agencies from time to time. Costs incurred are recorded in research and development expenses on the accompanying consolidated statements of operations.
On April 8, 2020, AgeX was awarded a grant of up to approximately $386,000 from the National Institutes of Health (“NIH”). The NIH grant provided funding for continued development of AgeX’s technologies for treating stroke. The grant funds were made available by the NIH to AgeX as allowable expenses were incurred. For the year ended December 31, 2021, AgeX incurred approximately $104,000 of allowable expenses under the NIH grant and recognized a corresponding amount of grant revenues. AgeX recognized no grant revenues for the year ended December 31, 2022 as AgeX had expended the full amount available under this grant as of December 31, 2021.
ESI BIO research products – AgeX, through its ESI BIO research product division, markets a number of products related to human pluripotent stem cells (“PSC lines”), including research-grade PSC lines and PSC lines produced under current good manufacturing practices or “cGMP.” AgeX offers cells from PSC lines to customers under contracts that permit the customers to utilize PSC lines for the research, development, and commercialization of cell-based therapies or other products in defined fields of application. The compensation to AgeX for providing the PSC line cells under such contracts may include up-front payments, milestone payments related to product development, regulatory matters, and commercialization, and the payment of royalties on sales of products developed from AgeX PSC lines. Revenues from the sale of research products are included in other revenues.
Arrangements with multiple performance obligations – AgeX may enter into contracts with customers that include multiple performance obligations. For such arrangements, AgeX will allocate revenue to each performance obligation based on its relative standalone selling price. AgeX will determine or estimate standalone selling prices based on the prices charged, or that would be charged, to customers for that product or service. As of and for the years ended December 31, 2022 and 2021, AgeX did not have significant arrangements with multiple performance obligations.
Research and Development
Research and development expenses consist primarily of personnel costs and related benefits, stock-based compensation, amortization of intangible assets, and collaborative and contracted research and development fees. Research and development costs which have an alternative future use will be capitalized as intangible assets, and costs with no future benefit or alternative use will be expensed as incurred. Research and development expenses incurred and reimbursed by grants from third parties approximate the grant revenues recognized in the consolidated statements of operations.
General and Administrative
General and administrative expenses include employee and director compensation allocated to general and administrative expenses, consulting fees other than those paid for science-related consulting, license fees paid to third parties to acquire patents or licenses to use patents and other technology, professional fees to maintain patents and trademarks, minimum annual royalties stipulated under license and sublicense agreements with third parties, insurance costs allocated to general and administrative expenses, stock exchange-related costs, depreciation expense, marketing costs, legal and accounting costs, office rent, and other miscellaneous expenses which are allocated to general and administrative expense.
Foreign Currency Transaction Gains and Losses
For transactions denominated in other than the functional currency of AgeX or its subsidiaries, AgeX recognizes transaction gains and losses in the consolidated statements of operations and classifies the gain or loss based on the nature of the item that generated it.
Segments
AgeX’s executive management team, as a group, represents the entity’s chief operating decision makers. To date, AgeX’s executive management team has viewed AgeX’s operations as one segment that includes the research and development of regenerative medicine technologies targeting the diseases of aging and metabolic disorders, oncology, and neurological diseases and disorders, blood and vascular system diseases and disorders, and pluripotent cell technologies. As a result, the financial information disclosed materially represents all of the financial information related to AgeX’s sole operating segment.
Basic and Diluted Net Loss per Share Attributable to Common Stockholders
Basic loss per share is calculated by dividing net loss attributable to AgeX common stockholders by the weighted-average number of shares of common stock outstanding, net of unvested restricted stock or restricted stock units, subject to repurchase by AgeX, if any, during the period. Diluted loss per share is calculated by dividing the net income attributable to AgeX common stockholders, if any, by the weighted-average number of shares of common stock outstanding, adjusted for the effects of potentially dilutive common stock issuable under outstanding stock options, warrants, and restricted stock units, using the treasury-stock method, and convertible preferred stock, if any, using the if-converted method, and treasury stock held by subsidiaries, if any.
For the years ended December 31, 2022 and 2021, because AgeX reported a net loss attributable to common stockholders, all potentially dilutive common stock, comprised of stock options, restricted stock units and warrants, is antidilutive.
The following weighted-average common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| Stock options | | | 3,297 | | | | 3,145 | |
| Warrants (1) | | | 9,558 | | | | 3,492 | |
| Restricted stock units | | | 10 | | | | 23 | |
| (1) | As of December 31, 2022 and 2021, AgeX issued Juvenescence warrants to purchase 12,129,260 and 3,512,098 shares, respectively, of AgeX common stock as consideration for the loan agreements discussed in Note 5, Related Party Transactions. |
Recently Adopted Accounting Pronouncements
In May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). The amendment in this update addresses how an issuer should account for modifications made to equity-classified written call options. The guidance in the standard requires the issuer to treat a modification of an equity-classified call option that does not cause the instrument to become liability-classified as an exchange of the original call option for a new call option. This guidance applies whether the modification is structured as an amendment to the terms and conditions of the call option or as termination of the original call option and issuance of a new call option. The Emerging Issues Task Force (EITF) concluded that the recognition of the modification depends on the nature of the transaction in which a warrant is modified. If there is more than one element in a transaction (for example, if the modification involves both a debt modification and an equity issuance), then the guidance requires the issuer to allocate the effect of the option modification to each element. Amendments in the new standard are effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. An entity should apply the amendments prospectively to modifications or exchanges occurring on or after the effective date of the amendments. Early adoption is permitted for all entities, including adoption in an interim period. If an entity elects to early adopt the amendments in this ASU in an interim period, the guidance should be applied as of the beginning of the fiscal year that includes that interim period. AgeX adopted this standard as of January 1, 2022, and it did not have a material impact on the consolidated financial statements.
In November 2021, the FASB issued ASU 2021-10, Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance. The amendments in this update require disclosures about transactions with a government that have been accounted for by analogizing to a grant or contribution accounting model to increase transparency about (1) the types of transactions, (2) the accounting for the transactions, and (3) the effect of the transactions on an entity’s financial statements. The amendments are effective for all entities within their scope, which excludes not-for-profit entities and employee benefit plans, for financial statements issued for annual periods beginning after December 15, 2021. Early application of the amendment is permitted. AgeX adopted this standard as of January 1, 2022, and it did not have a material impact on the consolidated financial statements.
Recently Issued Accounting Pronouncements Not Yet Adopted
In June 2016, the FASB issued ASU 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, and subsequent amendments to the initial guidance under ASU 2018-19, ASU 2019-04, ASU 2019-05 and ASU 2019-10, which amends the current approach to estimate credit losses on certain financial assets. This ASU requires immediate recognition of management’s estimates of current expected credit losses. Under the prior model, losses were recognized only as they were incurred, which FASB has noted delayed recognition of expected losses that might not yet have met the threshold of being probable. The standard is applicable to all financial assets (and net investment in leases) that are not accounted for at fair value through net income, such as trade receivables, loans, debt securities, and net investment in leases, thereby bringing consistency in accounting treatment across different types of financial instruments and requiring consideration of a broader range of variables when forming loss estimates. Subsequent changes in the valuation allowance are recorded in current earnings and reversal of previous losses are permitted. ASU 2016-13 is effective for AgeX beginning January 1, 2023 and is not expected to have a material impact on the consolidated financial statements.
In March 2022, the FASB issued ASU 2022-02, Financial Instruments – Credit Losses (Topic 326): Troubled Debt Restructurings and Vintage Disclosures, which amends the accounting for credit losses on financial instruments. This amendment eliminates the recognition and measurement guidance on troubled debt restructurings for creditors that have adopted the new credit losses guidance in ASC 326 and requires enhanced disclosures about loan modifications for borrowers experiencing financial difficulty. The new guidance also requires public business entities to present gross write-offs by year of origination in their vintage disclosures. The guidance is effective for AgeX on January 1, 2023, including interim periods. Early adoption is permitted, and the amendment applied prospectively, except for the recognition and remeasurement of troubled debt restructurings. Entities can elect to adopt the guidance on troubled debt restructurings using either a prospective or modified retrospective transition. If an entity elects to apply a modified retrospective transition, it will record a cumulative effect adjustment to retained earnings in the period of adoption. AgeX does not expect the adoption of this guidance to have a material impact on the consolidated financial statements.
CARES Act
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security (“CARES”) Act was enacted and signed into law. The CARES Act, among other things, includes provisions relating to refundable payroll tax credits, deferment of employer side social security payments, net operating loss carryback periods, alternative minimum tax credit refunds, modifications to the net interest deduction limitations, and technical corrections to tax depreciation methods for qualified improvement property. AgeX reviewed the provisions of the CARES Act but does not expect it to have a material impact to its tax provision or its consolidated financial statements. AgeX obtained a loan under the Paycheck Protection Program under the CARES Act, the repayment of which was forgiven in February 2021.
3. Disposition and Deconsolidation of LifeMap Sciences
Discontinued Operations
On March 6, 2021, AgeX and LifeMap Sciences entered into the LifeMap Merger Agreement with Atlas and GCLMS. On March 15, 2021, the merger was completed pursuant to the terms of the LifeMap Merger Agreement. As a result of the merger, GCLMS merged into LifeMap Sciences and (a) the shares of LifeMap Sciences common stock outstanding at the time of the merger entitled the holders of those shares to receive a pro rata portion of the $500,000 total LifeMap Merger Consideration, with each LifeMap Sciences shareholder’s pro rata portion of the LifeMap Merger Consideration determined in accordance with the number of shares of LifeMap Sciences common stock owned by such shareholder as a percentage of shares of LifeMap Sciences common stock outstanding immediately before the effective date of the merger, and (b) the outstanding shares of GCLMS common stock were converted into shares of LifeMap Sciences common stock so that Atlas is now the sole shareholder of LifeMap Sciences.
AgeX received approximately $466,400 in cash as its pro rata share of the LifeMap Merger Consideration in the merger. Prior to and as a condition to the merger under the terms of the LifeMap Merger Agreement, $1,761,296 of LifeMap Sciences’ indebtedness to AgeX was converted into shares of LifeMap Sciences common stock. LifeMap Sciences also paid AgeX $250,000 in cash to pay off a portion of LifeMap Sciences’ indebtedness to AgeX that was not converted into shares of LifeMap Sciences common stock.
The results of operations and cash flows for LifeMap Sciences are reported as discontinued operations under U.S. GAAP in accordance with ASC 205-20, Discontinued Operations, for all periods presented in our consolidated financial statements. AgeX will not have any continuing involvement in LifeMap Sciences subsequent to the consummation of the merger on March 15, 2021. The following table presents the operating results of LifeMap Sciences that have been treated as discontinued operations for the periods presented:
Schedule of Discontinued Operations
| | | Year Ended December 31, 2021 | |
| Net revenues | | $ | 277 | |
| Costs, operating and other expenses | | | (380) | |
| Loss from discontinued operations | | | (103) | |
| Net loss from discontinued operations attributable to noncontrolling interest | | | 7 | |
| Loss from discontinued operations (1) | | $ | (96) | |
| (1) | Does not include $106,000 gain on the deconsolidation of LifeMap Sciences recognized by AgeX. When dispositions occur in the normal course of business, gains or losses on the sale of such businesses or assets are recognized in the income statement. The gain on the sale of LifeMap Sciences is presented in the line item Gain on deconsolidation of LifeMap Sciences. There were no gains or losses resulting from the sale of businesses or assets that did not meet the criteria for a discontinued operation during the year ended December 31, 2021. |
Deconsolidation
As a result of the completion of the cash-out merger on March 15, 2021, LifeMap Sciences is no longer a subsidiary of AgeX. Effective March 15, 2021, AgeX deconsolidated LifeMap Sciences’ consolidated financial statements and consolidated results of operations from those of AgeX under U.S. GAAP ASC 810-10-40-4, Deconsolidation of a Subsidiary or Derecognition of a Group of Assets, due to the disposition of LifeMap Sciences on that date.
AgeX’s consolidated balance sheets at December 31, 2022 and 2021 do not include LifeMap Sciences’ consolidated assets and liabilities due to the deconsolidation of LifeMap Sciences on March 15, 2021.
AgeX’s consolidated statement of operations for the year ended December 31, 2021 includes LifeMap Sciences’ consolidated results for the period through March 15, 2021 rather than the day immediately preceding the LifeMap Deconsolidation due to the conversion of $1,761,296 of LifeMap Sciences’ indebtedness to AgeX into shares of LifeMap Sciences common stock on March 15, 2021 followed by the completion of the cash-out merger on the same day.
AgeX recognized a gain of $106,000 from the LifeMap Deconsolidation. The sale of LifeMap Sciences was a taxable transaction to AgeX; however, no income tax is due as the transaction resulted in a taxable loss primarily due to AgeX’s tax basis in the subsidiary.
4. Selected Balance Sheet Components
Intangible Assets, Net
On August 13, 2018, AgeX entered into an Asset Purchase Agreement (the “Purchase Agreement”) with Escape Therapeutics, Inc. (“Escape”) pursuant to which AgeX acquired certain patents and patent applications related primarily to methods of modifying cells and tissues and certain pluripotent stem cell lines so as to reduce their risk of being rejected when transplanted. This technology is called “UniverCyte™.” AgeX paid Escape $1,072,436 in cash and issued 80,000 shares of AgeX common stock, with an approximate value of $240,000, for aggregate acquisition cost of $1.3 million for the UniverCyte™ assets. The Purchase Agreement was considered an asset acquisition rather than a business combination in accordance with ASC 805-50, Business Combinations.
ASC 730-10-25(c), Research and Development – Intangible Assets Purchased from Others, provides guidance for acquisition and capitalization of the cost of intangible assets purchased from others in an asset acquisition that have alternative future uses in other research and development projects. These intangible assets are referred to as acquired IPR&D with alternative future uses and are accounted for as intangible assets and amortized to research and development over their useful life. Acquired IPR&D in an asset acquisition that does not have any alternative future uses is expensed under the same guidance. As an initial focus, AgeX intends to use the UniverCyte™ technology in the development of its two lead products, AGEX-BAT1 and AGEX-VASC1 for the treatment of Type II diabetes and cardiovascular aging, respectively. Accordingly, AgeX recorded the UniverCyte™ technology acquired from Escape as IPR&D intangible assets with alternative future uses in accordance with ASC 730-10-25(c) and is amortizing those assets to research and development expense over their estimated 10-year useful life.
In addition to the purchase price, AgeX will pay Escape a royalty of less than 1% on net sales of products, processes and services under the acquired patents, if the assets are commercialized. Additional shares of AgeX common stock totaling up to $4.3 million of market value will also be issued to Escape upon the attainment of development and regulatory approval milestones by AgeX for each product covered by the acquired patents. Contingent consideration in an asset acquisition is generally recorded when probable and estimable in accordance with ASC 450, Contingencies. Accordingly, none of the milestone payments have been accrued since the attainment of any milestone in the Purchase Agreement was not probable as of December 31, 2022.
AgeX has also agreed to engage Escape’s chief executive officer as a consultant for a period of up to three years to assist AgeX in utilizing the acquired patents. AgeX paid $200,000 per year in consulting fees as services were performed, included in research and development expenses up until the agreement expired in August 2021.
AgeX estimated the future undiscounted cash flows expected to be received from the assets developed through the use of the UniverCyte™ technology when commercialized. The estimate of the future undiscounted cash flows considered AgeX’s financial condition and the royalties that may become payable to Escape.
At December 31, 2022 and 2021, intangible assets, primarily consisting of acquired IPR&D and patents, and accumulated amortization were as follows (in thousands):
Schedule of Intangible Assets, Net
| | | | | | | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| Intangible assets | | $ | 1,312 | | | $ | 1,312 | |
| Accumulated amortization | | | (574) | | | | (442) | |
| Total intangible assets, net | | $ | 738 | | | $ | 870 | |
AgeX recognized $132,000 and $131,000 in amortization expense of intangible assets for continuing operations, included in research and development expenses, for the years ended December 31, 2022 and 2021, respectively.
Amortization expense of intangible assets for discontinued operations for the year ended December 31, 2021 amounted to $89,000. See Note 3, Disposition and Deconsolidation of LifeMap Sciences for discussion of discontinued operations.
Amortization of intangible assets for periods subsequent to December 31, 2022 is as follows (in thousands):
Schedule of Amortization Assets
| Year Ending December 31, | | Amortization Expense | |
| 2023 | | $ | 131 | |
| 2024 | | | 132 | |
| 2025 | | | 131 | |
| 2026 | | | 131 | |
| Thereafter | | | 213 | |
| Total | | $ | 738 | |
Accounts Payable and Accrued Liabilities
At December 31, 2022 and 2021, accounts payable and accrued liabilities were comprised of the following (in thousands):
Schedule of Accounts Payable and Accrued Liabilities
| | | | | | | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| Accounts payable | | $ | 568 | | | $ | 193 | |
| Accrued compensation | | | 193 | | | | 212 | |
| Accrued vendors and other expenses | | | 273 | | | | 366 | |
| Total accounts payable and accrued liabilities | | $ | 1,034 | | | $ | 771 | |
5. Related Party Transactions
2019 Loan Agreement
On August 13, 2019, AgeX and Juvenescence entered into a Loan Facility Agreement (the “2019 Loan Agreement”) pursuant to which Juvenescence provided to AgeX a $2.0 million line of credit for a period of 18 months. On February 10, 2021, AgeX entered into an amendment (the “First Amendment”) to the 2019 Loan Agreement which extended the maturity date of loans under the 2019 Loan Agreement to February 14, 2022 and increased the amount of the loan facility by $4.0 million. On November 8, 2021, AgeX entered into Amendment No. 2 to the 2019 Loan Agreement which increased the amount of the loan facility by another $1.0 million. As of December 31, 2021, AgeX had borrowed all of the $7.0 million total line of credit under the 2019 Loan Agreement, as amended. On February 14, 2022, AgeX refinanced the $7.0 million outstanding principal amount of the loans and a $160,000 origination fee due under the 2019 Loan Agreement, as amended. See discussion regarding 2022 Secured Convertible Promissory Note within this Note 5.
2020 Loan Agreement
On March 30, 2020, AgeX and Juvenescence entered into a new Secured Convertible Facility Agreement (the “2020 Loan Agreement”) pursuant to which Juvenescence provided to AgeX an $8.0 million line of credit for a period of 18 months. Through December 31, 2022, AgeX had drawn the full $8.0 million line of credit. AgeX issued to Juvenescence 28,500 shares of AgeX common stock as an arrangement fee for the loan facility when AgeX borrowed an aggregate of $3.0 million under the 2020 Loan Agreement, and AgeX issued to Juvenescence warrants to purchase a total of 3,670,663 shares of AgeX common stock (“2020 Warrants”). The number of 2020 Warrants issued was determined by the warrant formula described below. The repayment date for the outstanding principal balance of the loan under the 2020 Loan Agreement will be March 30, 2024 (see Note 11, Subsequent Events).
Default Provisions – The loan balance may become immediately due and payable prior to the mandatory repayment date if an Event of Default occurs. Events of Default under the 2020 Loan Agreement include the following: (i) AgeX fails to pay any amount in the manner and at the time provided in the 2020 Loan Agreement and the failure to pay is not remedied within 10 business days; (ii) AgeX fails to perform any of its obligations under the 2020 Loan Agreement and if the failure can be remedied it is not remedied to the satisfaction of Juvenescence within 10 business days after notice to AgeX; (iii) other indebtedness for money borrowed in excess of $100,000 becomes due and payable or can be declared due and payable prior to its due date or if indebtedness for money borrowed in excess of $25,000 is not paid when due; (iv) AgeX stops payment of its debts generally or discontinues its business or becomes unable to pay its debts as they become due or enters into any arrangement with creditors generally; (v) AgeX becoming insolvent or in liquidation or administration or other insolvency procedures, or a receiver, trustee or similar officer is appointed in respect of all or any part of its assets and such appointment continues undischarged or unstayed for sixty days; (vi) it becomes illegal for AgeX to perform its obligations under the 2020 Loan Agreement or any governmental permit, license, consent, exemption or similar requirement for AgeX to perform its obligations under the 2020 Loan Agreement or to carry out its business is not obtained or ceases to remain in effect; (vii) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX if such process is not released, vacated or fully bonded within sixty calendar days after its issue or levy; (viii) any injunction, order or judgement of any court is entered or issued which in the opinion of Juvenescence materially and adversely affects the ability of AgeX to carry out its business or to pay amounts owed to Juvenescence under the 2020 Loan Agreement; (ix) there is a change in AgeX’s financial condition that in the opinion of Juvenescence materially and adversely affects, or is likely to so affect, its ability to perform any of its obligations under the 2020 Loan Agreement; (x) AgeX or a designated subsidiary sells, leases, licenses, consigns, transfers, or otherwise disposes of a material part of their assets other than inventory in the ordinary course of business or certain intercompany transactions, or certain other limited permitted transactions, unless Juvenescence approves; (xi) AgeX or a designated subsidiary contests the validity of its obligations under the 2020 Loan Agreement or other related agreement with Juvenescence; (xii) any representation, warranty, or other statement made by AgeX or a designated subsidiary under the 2020 Loan Agreement is incomplete, untrue, incorrect, or misleading: and (xiii) AgeX or a designated subsidiary suspends or ceases to carry on all or a material part of its business or threatens to do so.
2020 Warrants — Under the terms of the 2020 Loan Agreement, each time AgeX received an advance of funds under the 2020 Loan Agreement, AgeX issued to Juvenescence a number of 2020 Warrants equal to 50% of the number determined by dividing the amount of the advance by the applicable Market Price. The Market Price set each 2020 Warrant when issued was the closing price per share of AgeX common stock on the NYSE American on the date of the applicable notice from AgeX requesting a draw of funds that triggered the obligation to issue the 2020 Warrant. The exercise price of the 2020 Warrants is the applicable Market Price. The 2020 Warrants will expire at 5:00 p.m. New York time three years after the date of issue. As of December 31, 2022, AgeX had issued to Juvenescence 2020 Warrants to purchase 3,670,663 shares of AgeX common stock. The exercise prices of the 2020 Warrants issued through December 31, 2022 range from $0.70 per share to $1.895 per share representing the market closing price on the NYSE American of AgeX common stock on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
Conversion of Loan Amounts to Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. The conversion price per share or units shall be the lowest price at which shares or units are sold. Juvenescence may convert the loan balance in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice Juvenescence’s election to convert the loan or a portion thereof into common stock.
2022 Secured Convertible Promissory Note and Security Agreement
On February 14, 2022, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (the “Secured Note”) pursuant to which Juvenescence agreed to provide to AgeX a $13,160,000 line of credit for a period of 12 months. AgeX drew an initial $8,160,000 of the line of credit and used $7,160,000 to refinance the outstanding principal and the loan origination fees under the 2019 Loan Agreement with Juvenescence. During the year ended December 31, 2022, AgeX borrowed an additional $4.5 million under the Secured Note. During January 2023, AgeX borrowed an additional $500,000 under the Secured Note and during February 2023 the Secured Note was amended and restated to increase the total line of credit available by $2,000,000 and the date by which additional funds can be drawn was extended to May 9, 2023. The outstanding principal balance of the Secured Note, as amended will become due and payable on February 14, 2024. See Note 11, Subsequent Events for additional information on the amended and restated Secured Note.
As an arrangement fee for the Secured Note, AgeX will pay Juvenescence an origination fee in an amount equal to 4% of the amount each draw of loan funds, which will accrue as each draw is funded, and an additional 4% of all the total amount of funds drawn that will accrue following the end of the period during which funds may be drawn from the line of credit. The origination fee will become due and payable on the repayment date or in a pro rata amount with any prepayment of in whole or in part of the outstanding principal balance of the Secured Note.
2022 Warrants – Upon each drawdown of funds under the Secured Note, AgeX will issue to Juvenescence warrants to purchase shares of AgeX common stock (“2022 Warrants”). The 2022 Warrants will be governed by the terms of a Warrant Agreement between AgeX and Juvenescence. The number of 2022 Warrants to be issued will be equal to 50% of the number determined by dividing the amount of the applicable loan draw by the applicable Market Price. The Market Price will be the last closing price per share of AgeX common stock on the NYSE American or other national securities exchange preceding the delivery of the notice from AgeX requesting a draw of funds that triggers the obligation to issue 2022 Warrants; provided, however that if AgeX common stock is not traded on a national securities exchange the Market Price shall be determined with reference to closing prices quoted or bid and asked prices on an interdealer quotation system averaged over twenty consecutive trading days. The exercise price of the 2022 Warrants will be the applicable Market Price. The 2022 Warrants will expire at 5:00 p.m. New York time three years after the date of issue.
As of December 31, 2022, AgeX had issued to Juvenescence 2022 Warrants to purchase 8,458,597 shares of AgeX common stock. The exercise prices of the 2022 Warrants issued through December 31, 2022 range from $0.59 per share to $0.88 per share representing the market closing price of AgeX common stock on the NYSE American on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
Conversion of Loan Amounts to Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. The conversion price per share or units shall be the lowest price at which shares or units are sold. Juvenescence may convert the loan balance in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice Juvenescence’s election to convert the loan or a portion thereof into common stock.
Default Provisions – The loan balance and origination fees may become immediately due and payable prior to the mandatory repayment date if an Event of Default occurs. Events of Default under the Secured Note include the following: (a) AgeX fails to pay any principal amount payable by it in the manner and at the time provided under and in accordance with the Secured Note; (b) AgeX fails to pay any other amount payable by it in the manner and at the time provided under and in accordance with the Secured Note or the Security Agreement described below or any other agreement executed in connection with the Secured Note (the “Loan Documents”) and the failure is not remedied within three business days; (c) AgeX fails to perform any of its covenants or obligations or fail to satisfy any of the conditions under the Secured Note or any other Loan Document and, such failure (if capable of remedy) remains unremedied to the satisfaction of Juvenescence (in its sole discretion) for 10 business days after the earlier of (i) notice requiring its remedy has been given by Juvenescence to AgeX and (ii) actual knowledge of the failure by senior officers of AgeX; (d) if any indebtedness of AgeX in excess of $100,000 becomes due and payable, or a breach or other circumstance arises thereunder such that Juvenescence is entitled to declare such indebtedness due and payable, prior to its due date, or any indebtedness of AgeX in excess of $25,000 is not paid on its due date; (e) AgeX stops payment of its debts generally or ceases or threatens to cease to carry on its business or is unable to pay its debts as they fall due or is deemed by a court of competent jurisdiction to be unable to pay its debts as they fall due, or enters into any arrangements with its creditors generally; (f) if (i) an involuntary proceeding (other than a proceeding instituted by Juvenescence or an affiliate of Juvenescence) shall be commenced or an involuntary petition shall be filed seeking liquidation, reorganization or other relief in respect of AgeX and any subsidiary, or of all or a substantial part of its assets, under any federal, state or foreign bankruptcy, insolvency, receivership or similar law now or hereafter in effect or (ii) an involuntary appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for AgeX or a subsidiary or for a substantial part of its assets occurs (other than in a proceeding instituted by Juvenescence or an affiliate of Juvenescence), and, in any such case, such proceeding shall continue undismissed and unstayed for sixty (60) consecutive days without having been dismissed, bonded or discharged or an order of relief is entered in any such proceeding; (g) it becomes unlawful for AgeX to perform all or any of its obligations under the Secured Note or any authorization, approval, consent, license, exemption, filing, registration or other requirement of any governmental, judicial or public body or authority necessary to enable AgeX to comply with its obligations under the Secured Note or to carry on its business is not obtained or, having been obtained, is modified in a manner that precludes AgeX or its subsidiaries from conducting their business in any material respect, or is revoked, suspended, withdrawn or withheld or fails to remain in full force and effect; (h) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX or a subsidiary if such process is not released, vacated or fully bonded within 60 calendar days after its issue or levy; (i) any injunction, order, judgment or decision of any court is entered or issued which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX or a subsidiary to carry on its business or to pay amounts owed to Juvenescence under the Secured Note; (j) AgeX, whether in a single transaction or a series of related transactions, sells, leases, licenses, consigns, transfers or otherwise disposes of any material portion of its assets (with any such disposition with respect to any asset or assets with a fair value of at least $250,000 being deemed material), other than (i) certain permitted investments (ii) sales, transfers and dispositions of inventory in the ordinary course of business, (iii) any termination of a lease of real or personal property that is not necessary in the ordinary course of the AgeX’s business, could not reasonably be expected to have a material adverse effect and does not result from AgeX’s default, and (iv) any sale, lease, license, consignment, transfer or other disposition of assets that are no longer necessary in the ordinary course of business or which has been approved in writing by Juvenescence; (k) any of the following shall occur: (i) the security and/or liens created by the Security Agreement or any other Loan Document shall at any time cease to constitute valid and perfected security and/or liens on any material portion of the collateral intended to be covered thereby; (ii) except for expiration in accordance with its terms, the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall for whatever reason be terminated or shall cease to be in full force and effect; (iii) the enforceability of the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall be contested by AgeX or a subsidiary; (iv) AgeX shall assert that its obligations under the Secured Note or any other Loan Document shall be invalid or unenforceable; or (v) a loss, theft, damage or destruction occurs with respect to a material portion of the collateral; (l) there is any change in the financial condition of AgeX and its subsidiaries which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX to perform any of its obligations under the Secured Note; and (m) any representation, warranty or statement made, repeated or deemed made or repeated by AgeX in the Secured Note, or pursuant to the Loan Documents, is incomplete, untrue, incorrect or misleading in any material respect when made, repeated or deemed made.
Restrictive Covenants – The Secured Note includes certain covenants that among other matters such as financial reporting: (i) impose financial restrictions on AgeX while the Secured Note remains unpaid, including restrictions on the incurrence of additional indebtedness by AgeX and its subsidiaries, except that AgeX’s subsidiary Reverse Bio will be permitted to incur debt convertible into equity not guaranteed or secured by the assets of AgeX or any other AgeX subsidiary, and the restrictions on the incurrence of indebtedness applicable to Reverse Bio will end if it raises more than $15.0 million in debt or equity financing by May 15, 2023 (ii) require that AgeX use loan proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, for general working capital, and for repayment of all or a portion of AgeX’s indebtedness to Juvenescence; and (iii) prohibit AgeX from making additional investments in subsidiaries, unless AgeX obtains the written consent of Juvenescence to a transaction that otherwise would be prohibited or restricted.
Security Agreement – AgeX has entered into a Security Agreement granting Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs, Juvenescence will have the right to foreclose on the assets pledged as collateral.
Registration Rights
AgeX entered into certain Registration Rights Agreements pursuant to which it has agreed to register for sale under the Securities Act all shares of AgeX common stock presently held by Juvenescence or that may be acquired by Juvenescence through the exercise of common stock purchase warrants that they hold or that they may acquire pursuant to the 2020 Loan Agreement, and shares that they may acquire through the conversion of the loans into AgeX common stock. AgeX has filed a registration statement on Form S-3, which has become effective under the Securities Act, for offerings on a delayed or continuous basis covering 16,447,500 shares of our common stock held by Juvenescence and 3,248,246 shares of AgeX common stock that may be issued upon the exercise of warrants held by Juvenescence. Juvenescence retains the right to require AgeX to register additional shares of common stock that Juvenescence may acquire through the exercise of warrants or the conversion of loans. AgeX is obligated to pay the fees and expenses of each registered offering under such registration rights agreement except for underwriting discounts and commissions. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
AgeX entered into an amendment to its Registration Rights Agreement with Juvenescence to include the 2022 Warrants and underlying shares and any shares issuable upon the conversion of the Secured Note into common stock as registrable securities under the Registration Rights Agreement.
Debt Issuance Costs
In accordance with ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs, all debt issuance costs are recorded as a discount on the debt and amortized to interest expense over the term of the loan agreement using the effective interest method. Direct debt issuance costs include but are not limited to legal fees, debt origination fees, estimated fair market value of common stock and warrants issued in connection with the loan agreements, and NYSE American additional listing fees for the underlying shares of warrants issued with each drawdown of funds.
The following table summarizes the debt issuance costs and the debt balances net of debt issuance costs by loan agreement as of December 31, 2022 (in thousands):
Schedule of Debt Issuance Costs and Debt Balances
| | | Drawdown of Funds | | | Origination Fee | | | Amount Refinanced | | | Total Debt | | | Debt Issuance Costs | | | Amortization of Debt Issuance Costs | | | Total Debt, Net | |
| 2019 Loan Agreement | | $ | 7,000 | | | $ | 160 | | | $ | (7,160) | | | $ | - | | | $ | (494) | | | $ | 494 | | | $ | - | |
| 2020 Loan Agreement | | | 8,000 | | | | - | | | | - | | | | 8,000 | | | | (2,805) | | | | 2,451 | | | | 7,646 | |
| 2022 Secured Note | | | 12,660 | | | | 691 | | | | - | | | | 13,351 | | | | (4,720) | | | | 1,847 | | | | 10,478 | |
| | | $ | 27,660 | | | $ | 851 | | | $ | (7,160) | | | $ | 21,351 | | | $ | (8,019) | | | $ | 4,792 | | | $ | 18,124 | |
The following table summarizes the debt issuance costs and the debt balances net of debt issuance costs by loan agreement as of December 31, 2021 (in thousands):
| | | Drawdown of Funds | | | Origination Fee | | | Amount Refinanced | | | Total Debt | | | Debt Issuance Costs | | | Amortization of Debt Issuance Costs | | | Total Debt, Net | |
| 2019 Loan Agreement | | $ | 7,000 | | | $ | 160 | | | $ | - | | | $ | 7,160 | | | $ | (494) | | | $ | 474 | | | $ | 7,140 | |
| 2020 Loan Agreement | | | 7,500 | | | | - | | | | - | | | | 7,500 | | | | (2,620) | | | | 1,182 | | | | 6,062 | |
| | | $ | 14,500 | | | $ | 160 | | | $ | - | | | $ | 14,660 | | | $ | (3,114) | | | $ | 1,656 | | | $ | 13,202 | |
Related Party Payables
Since October 2018, AgeX’s Chief Operating Officer (“COO”), who is also an employee of Juvenescence, has been devoting a majority of his time to AgeX’s operations. AgeX reimburses Juvenescence for his services on an agreed-upon fixed annual amount of approximately $280,000. As of December 31, 2022 and 2021, AgeX had approximately $141,000 and $70,000, respectively, payable to Juvenescence for COO services rendered, included in related party payables, net of certain expenses owed by Juvenescence to AgeX, on the consolidated balance sheets.
6. Warrant Liability
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, Distinguishing Liabilities from Equity, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or the warrants must or may require AgeX to issue a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable provisions of U.S. GAAP.
As a condition of each amount drawn from the Secured Note, on receipt of each amount drawn AgeX shall grant to Juvenescence a number of warrants equal to 50% of the gross value of the relevant advance made. The gross value is the quotient of the drawdown amount and the exercise price. The exercise price is based on the market closing price of AgeX’s common stock on the NYSE American on the one day preceding the delivery of the relevant drawdown notice (see Note 5, Related Party Transactions).
Given AgeX’s history, it is AgeX’s judgement that it is more likely than not that AgeX will utilize the full credit available under the Secured Note and accordingly warrants will be issued for each of the advances made within the credit availability period consisting of the 12 month period starting February 14, 2022. After all relevant assessments, AgeX determined that the warrants issued under the Secured Note require classification as a liability pursuant to ASC 480, Distinguishing Liabilities from Equity. In accordance with the accounting guidance, the number of warrants that would have been issued if the amount of credit under the Secured Note was drawn in full is measured at the inception date at fair value and recognized as a warrant liability on the balance sheet, adjusted for the fair value of warrants actually issued upon each advance, and subsequently the number of warrants that may be issued for the remaining credit available is re-measured at each reporting period with changes being recorded as a component of net other expense in the consolidated statements of operations.
The fair value of the warrant liabilities was measured using a Black-Scholes option pricing model. Significant inputs into the model at the inception date when warrants were issued upon receipt of amounts drawn during the period, and as of the reporting period end remeasurement dates are as follows:
Schedule of Warrants Liabilities And Stock Option Awards using a Black-Scholes Option-Pricing Model
| Black-Scholes Assumptions | | Exercise Price (1) | | | Warrant Expiration Date (2) | | Stock Price (3) | | | Interest Rate (annual) (4) | | | Volatility (annual) (5) | | | Time to Maturity (Years) | | | Calculated Fair Value per Share | |
| Inception Date: 2/14/2022 | | $ | 0.780 | | | 13-Feb-25 | | $ | 0.691 | | | | 1.80% | | | | 122.99% | | | | 3 | | | $ | 0.486 | |
| Issuance Date: 2/14/2022 | | $ | 0.780 | | | 13-Feb-25 | | $ | 0.691 | | | | 1.80% | | | | 122.99% | | | | 3 | | | $ | 0.486 | |
| Issuance Date: 2/15/2022 | | $ | 0.780 | | | 14-Feb-25 | | $ | 0.747 | | | | 1.80% | | | | 123.28% | | | | 3 | | | $ | 0.535 | |
| Period Ended 3/31/2022 | | $ | 0.940 | | | 30-Mar-25 | | $ | 0.854 | | | | 2.45% | | | | 123.28% | | | | 3 | | | $ | 0.607 | |
| Issuance Date: 4/4/2022 | | $ | 0.880 | | | 3-Apr-25 | | $ | 0.819 | | | | 2.61% | | | | 123.31% | | | | 3 | | | $ | 0.585 | |
| Issuance Date: 6/6/2022 | | $ | 0.711 | | | 5-Jun-25 | | $ | 0.800 | | | | 2.94% | | | | 122.62% | | | | 3 | | | $ | 0.592 | |
| Period Ended 6/30/2022 | | $ | 0.600 | | | 29-Jun-25 | | $ | 0.576 | | | | 2.99% | | | | 122.21% | | | | 3 | | | $ | 0.413 | |
| Issuance Date: 8/16/2022 | | $ | 0.670 | | | 15-Aug-25 | | $ | 0.640 | | | | 3.19% | | | | 121.37% | | | | 3 | | | $ | 0.457 | |
| Period Ended 9/30/2022 | | $ | 0.610 | | | 29-Sep-25 | | $ | 0.562 | | | | 4.25% | | | | 121.49% | | | | 3 | | | $ | 0.401 | |
| Issuance Date: 10/21/2022 | | $ | 0.690 | | | 20-Oct-25 | | $ | 0.620 | | | | 4.52% | | | | 120.51% | | | | 3 | | | $ | 0.439 | |
| Issuance Date: 12/14/2022 | | $ | 0.590 | | | 13-Dec-25 | | $ | 0.540 | | | | 3.94% | | | | 120.01% | | | | 3 | | | $ | 0.381 | |
| Period Ended 12/31/2022 | | $ | 0.550 | | | 30-Dec-25 | | $ | 0.552 | | | | 4.22% | | | | 119.31% | | | | 3 | | | $ | 0.396 | |
| (1) | Based on the market closing price of AgeX’s common stock on the NYSE American on the one day prior to the debt inception date and each presented period ending date and also the market closing price of AgeX’s common stock on the NYSE American on the one trading day preceding the delivery of the relevant drawdown notice in accordance with terms per the Secured Note. |
| (2) | Warrants are exercisable over a three-year period from each issuance date. |
| (3) | Based on the market price of AgeX’s common stock on the NYSE American as of each date presented. |
| (4) | Interest rate for U.S. Treasury Bonds, as of each date presented, as published by the U.S. Federal Reserve. |
| (5) | Based on the historical daily volatility of AgeX common stock as of each date presented. |
The warrants outstanding and fair values at each of the respective valuation dates are summarized below:
Schedule of Warrant Outstanding and Fair Values
| Warrant Liability | | Credit Line and Draw Amounts (in thousands) | | | Warrants | | | Fair Value per Share | | | Fair Value (in thousands) | |
| Fair value as of January 1, 2022 | | $ | - | | | | - | | | $ | - | | | $ | - | |
| Fair value at initial measurement date of 2/14/2022 | | | 13,160(1) | | | | 8,435,897(2) | | | | 0.4864 | | | | 4,103 | |
| Fair value of warrants issued on 2/14/2022 | | | (7,160)(3) | | | | (4,589,743)(4) | | | | 0.4864 | | | | (2,232) | |
| Fair value of warrants issued on 2/15/2022 | | | (1,000)(3) | | | | (641,025)(4) | | | | 0.5349 | | | | (343) | |
| Fair value of warrants issued on 4/4/2022 | | | (1,000)(3) | | | | (568,440)(4) | | | | 0.5854 | | | | (333) | |
| Fair value of warrants issued on 6/6/2022 | | | (1,000)(3) | | | | (703,234)(4) | | | | 0.5924 | | | | (417) | |
| Fair value of warrants issued on 8/16/2022 | | | (1,000)(3) | | | | (746,380)(4) | | | | 0.4569 | | | | (341) | |
| Fair value of warrants issued on 10/21/2022 | | | (500)(3) | | | | (362,318)(4) | | | | 0.4386 | | | | (159) | |
| Fair value of warrants issued on 12/14/2022 | | | (1,000)(3) | | | | (847,457)(4) | | | | 0.3810 | | | | (323) | |
| Change in fair value of warrant liability | | | - | | | | - | | | | - | | | | 225 | |
| Fair value as of December 31, 2022 | | $ | 500(1) | | | | 454,545(2) | | | $ | 0.3960 | | | $ | 180 | |
| (1) | Amount of credit available under the Secured Note on date of inception and as of each period end date. |
| (2) | Number of warrants issuable if the amount of credit available were drawn for measurement as of inception date and subsequently for remeasurement as of each period end date. |
| (3) | Amount of drawdown as of each date presented. |
| (4) | Number of warrants issued upon receipt of amounts drawn against the Secured Note as of each date presented. |
During the year ended December 31, 2022, AgeX recorded a loss of $225,000 on changes in the fair value of warrant liability. There were no warrant liabilities or corresponding changes in valuation during the year ended December 31, 2021.
The warrant liabilities are considered Level 3 liabilities on the fair value hierarchy as the determination of fair value includes various assumptions about future activities and AgeX’s stock prices and historical volatility as inputs. During the year ended December 31, 2022, none of the warrants issued were exercised.
7. Stockholders’ Deficit
Preferred Stock
AgeX is authorized to issue up to 5,000,000 shares of preferred stock, $0.0001 par value per share. At December 31, 2022 and 2021, there were no preferred shares issued and outstanding.
Common Stock
AgeX has 200,000,000 shares of common stock, $0.0001 par value per share, authorized. The holders of AgeX’s common stock are entitled to receive ratably dividends when, as, and if declared by the Board of Directors out of funds legally available. Upon liquidation, dissolution, or winding up, the holders of AgeX common stock are entitled to receive ratably the net assets available after the payment of all debts and other liabilities and subject to the prior rights of AgeX outstanding preferred shares, if any.
The holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of AgeX stockholders. The holders of common stock have no preemptive, subscription, or redemption rights. The outstanding shares of common stock are fully paid and non-assessable.
As of December 31, 2022 and 2021, there were 37,949,196 and 37,941,220 shares of AgeX common stock issued and outstanding, respectively.
Issuance of Warrants by AgeX
In connection with the $12,660,000 of drawdowns of loan funds from Juvenescence under the Secured Note during the year ended December 31, 2022, AgeX has issued to Juvenescence 2022 Warrants to purchase 8,458,597 shares of AgeX common stock.
As consideration for $8.0 million in loans made to AgeX under the 2020 Loan Agreement, AgeX issued to Juvenescence warrants to purchase 3,670,663 shares of AgeX common stock.
See Notes 5, Related Party Transactions and 6, Warrant Liability for additional information on warrants issued to Juvenescence.
At-the-Market Offering Facility
On January 8, 2021, AgeX entered into a Sales Agreement with Chardan, relating to the sale of shares of AgeX common stock, in at-the-market transactions. In accordance with the terms of the Sales Agreement, AgeX may offer and sell shares of AgeX common stock having an aggregate offering price of up to $12.6 million from time to time through Chardan, acting as the sales agent. The actual market value of common shares that AgeX may sell through its Sales Agreement with Chardan during any 12 month period will be limited to one-third of aggregate market value of our common stock held by stockholders that would not be considered “affiliates” of AgeX, determined in accordance with applicable SEC rules. During the years ended December 31, 2022 and 2021, AgeX raised nil and $496,000, respectively, in gross proceeds through the sale of shares of common stock through the Sales Agreement with Chardan.
8. Stock-Based Awards
Equity Incentive Plan
Under the 2017 Equity Incentive Plan, as amended (the “Incentive Plan”), AgeX has reserved 8,500,000 shares of common stock for the grant of stock options or the sale of restricted stock (“Restricted Stock”) or for the settlement of restricted stock units which are hypothetical units issued with reference to common stock (“Restricted Stock Units” or “RSUs”). AgeX may also grant stock appreciation rights (“SARs”) under the Incentive Plan. The Plan also permits AgeX to issue such other securities as its Board of Directors (the “Board”) or the Compensation Committee (the “Committee”) administering the Incentive Plan may determine. Awards of stock options, Restricted Stock, SARs, and RSUs (“Awards”) may be granted under the Incentive Plan to AgeX employees, directors, and consultants.
Awards may vest and thereby become exercisable or have restrictions on forfeiture lapse on the date of grant or in periodic installments or upon the attainment of performance goals, or upon the occurrence of specified events.
No person shall be granted, during any one year period, options to purchase, or SARs with respect to, more than 1,000,000 shares in the aggregate, or any Awards of Restricted Stock or RSUs with respect to more than 500,000 shares in the aggregate. If an Award is to be settled in cash, the number of shares on which the Award is based shall not count toward the individual share limit.
No Awards may be granted under the Incentive Plan more than ten years after the date upon which the Incentive Plan was adopted by the Board, and no options or SARS granted under the Incentive Plan may be exercised after the expiration of ten years from the date of grant.
Stock Options
Options granted under the Incentive Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended (the “Code”), or “non-qualified” stock options that do not qualify incentive stock options. Incentive stock options may be granted only to AgeX employees and employees of subsidiaries. The exercise price of stock options granted under the Incentive Plan must be equal to the fair market of AgeX common stock on the date the option is granted. In the case of an optionee who, at the time of grant, owns more than 10% of the combined voting power of all classes of AgeX stock, the exercise price of any incentive stock option must be at least 110% of the fair market value of the common stock on the grant date, and the term of the option may be no longer than five years. The aggregate fair market value of common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.
The exercise price of an option may be payable in cash or in common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock, or other legal consideration for the issuance of stock as the Board or Committee may approve.
Generally, options will be exercisable only while the optionee remains an employee, director or consultant, or during a specific period thereafter, but in the case of the termination of an employee, director, or consultant’s services due to death or disability, the period for exercising a vested option shall be extended to the earlier of 12 months after termination or the expiration date of the option.
Restricted Stock and RSUs
In lieu of granting options, AgeX may enter into purchase agreements with employees under which they may purchase or otherwise acquire Restricted Stock or RSUs subject to such vesting, transfer, and repurchase terms, and other restrictions. The price at which Restricted Stock may be issued or sold will be not less than 100% of fair market value. Employees or consultants, but not executive officers or directors, who purchase Restricted Stock may be permitted to pay for their shares by delivering a promissory note or an installment payment agreement that may be secured by a pledge of their Restricted Stock. Restricted Stock may also be issued for services actually performed by the recipient prior to the issuance of the Restricted Stock. Unvested Restricted Stock for which AgeX has not received payment may be forfeited, or AgeX may have the right to repurchase unvested shares upon the occurrence of specified events, such as termination of employment.
Subject to the restrictions set with respect to the particular Award, a recipient of Restricted Stock generally shall have the rights and privileges of a stockholder, including the right to vote the Restricted Stock and the right to receive dividends; provided that, any cash dividends and stock dividends with respect to the Restricted Stock shall be withheld for the recipient’s account, and interest may be credited on the amount of the cash dividends withheld. The cash dividends or stock dividends so withheld and attributable to any particular share of Restricted Stock (and earnings thereon, if applicable) shall be distributed to the recipient in cash or, at the discretion of the Board or Committee, in shares of common stock having a fair market value equal to the amount of such dividends, if applicable, upon the release of restrictions on the Restricted Stock and, if the Restricted Stock is forfeited, the recipient shall have no right to the dividends.
The terms and conditions of a grant of RSUs shall be determined by the Board or Committee. No shares of common stock shall be issued at the time a RSU is granted. A recipient of RSUs shall have no voting rights with respect to the RSUs. Upon the expiration of the restrictions applicable to a RSU, AgeX will either issue to the recipient, without charge, one share of common stock per RSU or cash in an amount equal to the fair market value of one share of common stock.
At the discretion of the Board or Committee, each RSU (representing one share of common stock) may be credited with cash and stock dividends paid in respect of one share (“Dividend Equivalents”). Dividend Equivalents shall be withheld for the recipient’s account, and interest may be credited on the amount of cash Dividend Equivalents withheld. Dividend Equivalents credited to a recipient’s account and attributable to any particular RSU (and earnings thereon, if applicable) shall be distributed in cash or in shares of common stock having a fair market value equal to the amount of the Dividend Equivalents and earnings, if applicable, upon settlement of the RSU. If a RSU is forfeited, the recipient shall have no right to the related Dividend Equivalents.
SARs
A SAR is the right to receive, upon exercise, an amount payable in cash or shares, or a combination of shares and cash, equal to the number of shares subject to the SAR that is being exercised, multiplied by the excess of (a) the fair market value of a common stock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement. SARs may be granted either as free standing SARs or in tandem with options. No SAR may be exercised later than 10 years after the date of grant.
The exercise price of a SAR shall not be less than 100% of the fair market value of one share of common stock on the date of grant. A SAR granted in conjunction with an option shall have the same exercise price as the related option, shall be transferable only upon the same terms and conditions as the related option, and shall be exercisable only to the same extent as the related option; provided, however, that the SAR by its terms shall be exercisable only when the fair market value per share exceeds the exercise price per share of the SAR or related option. Upon any exercise of a SAR granted in tandem with an option, the number of shares for which the related option shall be exercisable shall be reduced by the number of shares for which the SAR has been exercised. The number of shares for which a SAR issued in tandem with an option shall be exercisable shall be reduced by the number of shares for which the related option has been exercised.
Equity Incentive Plan Awards
A summary of the Incentive Plan activity and related information follows (in thousands except weighted-average exercise price):
Summary of Stock Option Activity
| | | Shares Available for Grant | | | Number of Options Outstanding | | | Number of RSUs Outstanding | | | Weighted- Average Exercise Price | |
| Balance at January 1, 2021 | | | 1,046 | | | | 2,854 | | | | 28 | | | $ | 2.51 | |
| Increase option pool | | | 500 | | | | - | | | | - | | | | - | |
| Options granted | | | (568) | | | | 568 | | | | - | | | | 1.46 | |
| Options forfeited, cancelled or expired | | | 57 | | | | (57) | | | | - | | | | 2.56 | |
| Restricted stock units vested | | | - | | | | - | | | | (12) | | | | - | |
| Balance at December 31, 2021 | | | 1,035 | | | | 3,365 | | | | 16 | | | $ | 2.32 | |
| Increase option pool | | | 4,000 | | | | - | | | | - | | | | - | |
| Options granted | | | (105) | | | | 105 | | | | - | | | | 0.79 | |
| Options forfeited, cancelled or expired | | | 209 | | | | (209) | | | | - | | | | 2.82 | |
| Restricted stock units vested | | | - | | | | - | | | | (13) | | | | - | |
| Balance at December 31, 2022 | | | 5,139 | | | | 3,261 | | | | 3 | | | $ | 2.25 | |
| Options exercisable at December 31, 2022 | | | | | | | 2,913 | | | | | | | $ | 2.37 | |
There were no exercises of stock options during the years ended December 31, 2022 and 2021. Total proceeds if all options granted and outstanding as of December 31, 2022 were exercised would be approximately $7.3 million.
At December 31, 2022, AgeX had approximately $0.3 million of total unrecognized compensation costs related to the stock options and unvested RSUs under the Incentive Plan that will be recognized as expense over a weighted-average period of approximately 2.02 years.
The aggregate intrinsic value of options outstanding was $1,700 and options exercisable was nil as of December 31, 2022.
Stock-Based Compensation Expense
AgeX recorded stock-based compensation expense in the following categories on the accompanying consolidated statements of operations for the years ended December 31, 2022 and 2021 (in thousands):
Schedule of Stock Based Compensation Expense
| | | 2022 | | | 2021 | |
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| Research and development – continuing operations | | $ | 32 | | | $ | 62 | |
| General and administrative – continuing operations | | | 728 | | | | 937 | |
| Total stock-based compensation expense – continuing operations | | | 760 | | | | 999 | |
| General and administrative – discontinued operations | | | - | | | | 4 | |
| Total stock-based compensation expense | | $ | 760 | | | $ | 1,003 | |
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2022 and 2021 was $0.70 per share and $1.15 per share, respectively, using the Black-Scholes option pricing model with the following weighted-average assumptions:
Schedule of Weighted Average Assumptions to Calculate Fair Value of Stock Options
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| Grant Price | | $ | 0.79 | | | $ | 1.46 | |
| Expected life (in years) | | | 5.58 | | | | 5.71 | |
| Risk-free interest rates | | | 1.74% | | | | 0.99% | |
| Volatility | | | 130.71% | | | | 102.34% | |
| Dividend yield | | | -% | | | | -% | |
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If AgeX had made different assumptions, its stock-based compensation expense and net loss for the years ended December 31, 2022 and 2021 may have been significantly different. See Note 2, Summary of Significant Accounting Policies for a discussion of the factors used in determining these assumptions.
AgeX does not recognize deferred income taxes for incentive stock option compensation expense and records a tax deduction only when a disqualified disposition has occurred.
9. Income Taxes
Net loss from operations before income taxes as of December 31, 2022 and 2021 are approximately $ million and $ million, respectively.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
The primary components of the net deferred tax assets and liabilities as of December 31, 2022 and 2021 were as follows (in thousands):
Schedule of Components of Deferred Tax Assets and Liabilities
| | | | | | | |
| | | December 31, | |
| Deferred tax assets/(liabilities): | | 2022 | | | 2021 | |
| Net operating loss carryforwards | | $ | 12,408 | | | $ | 12,000 | |
| Capital loss carryforwards | | | 3,120 | | | | 3,120 | |
| Research and development credit carryforwards | | | 1,138 | | | | 1,426 | |
| Patents and fixed assets | | | 879 | | | | 901 | |
| Stock-based compensation | | | 643 | | | | 690 | |
| Capitalized Research Expenses | | | 211 | | | | - | |
| Other, net | | | 62 | | | | 80 | |
| Valuation allowance | | | (18,461) | | | | (18,217) | |
| Total net deferred tax assets | | $ | - | | | $ | - | |
A valuation allowance is provided when it is more likely than not that all or some portion of the deferred tax assets will not be realized. AgeX established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
Income taxes differed from the amounts computed by applying the U.S. federal income tax rate indicated to pretax losses from operations as a result of the following:
Schedule of Income Tax Rate Reconciliation
| | | December 31, | |
| | | 2022 | | | 2021 | |
| Computed tax benefit at federal statutory rate | | | 21% | | | | 21% | |
| Research and development and other credits | | | 1% | | | | 1% | |
| State tax benefit, net of effect on federal income taxes | | | (7)% | | | | 14% | |
| Permanent differences | | | -% | | | | (1)% | |
| Stock Based Compensation | | | (2)% | | | | -% | |
| Loss and deconsolidation of LifeMap Sciences | | | -% | | | | (31)% | |
| Debt finance equity costs | | | (6)% | | | | -% | |
| Return to provision & other adjustments | | | (5)% | | | | -% | |
| Change in valuation allowance | | | (2)% | | | | (4)% | |
| | | -% | | | | -% | |
AgeX has established an accrual for uncertain tax positions related to its U.S. research and development credits. As of December 31, 2022 and 2021, there was no accrued interest related to uncertain tax positions. AgeX does not believe it is reasonably possible that its unrecognized tax benefits will significantly change in the next twelve months. A reconciliation of beginning and ending balances for unrecognized tax benefits is as follows (in thousands):
Schedule of Unrecognized Tax Benefits
| | | 2022 | | | 2021 | |
| | | December 31, | |
| | | 2022 | | | 2021 | |
| Balance at January 1 | | $ | - | | | $ | - | |
| Additions for tax positions related to the current year | | | 23 | | | | - | |
| Additions for tax positions related to prior years | | | 356 | | | | - | |
| Reductions for tax positions related to prior years | | | - | | | | - | |
| Reductions related to settlements | | | - | | | | - | |
| Reductions related to a lapse of statute | | | - | | | | - | |
| Balance at December 31 | | $ | 379 | | | $ | - | |
AgeX monitors proposed and issued tax law, regulations, and cases to determine the potential impact of uncertain income tax positions. At December 31, 2022, AgeX had not identified any potential subsequent events that would have a material impact on unrecognized income tax benefits within the next twelve months.
As of December 31, 2022, AgeX has net operating loss carryforwards of approximately $52.8 million for U.S. federal income tax purposes. In general, NOLs and other tax credit carryforwards generated by legal entities in a consolidated federal tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the consolidated federal tax group.
As of December 31, 2022, AgeX has net operating losses of approximately $14.9 million for California purposes. As AgeX and its subsidiaries have been included in the combined California tax return with Lineage, up to the date of deconsolidation on August 30, 2018, those state net operating losses will remain with AgeX. In general, NOLs and other tax credit carryforwards generated by legal entities in a combined state tax group are available to other members of the tax group depending on the nature of the transaction that a member may enter into while still in the combined state tax group. Federal net operating losses generated on or prior to December 31, 2017, expire in varying amounts between 2028 and 2037, while federal net operating losses generated after December 31, 2017, carryforward indefinitely. The state net operating losses expire in varying amounts between 2028 and 2042.
As of December 31, 2022, AgeX has research and development tax credit carryforwards for federal and state tax purposes of $0.9 million and $0.6 million, respectively. The federal tax credits expire between 2028 and 2042, while the state tax credits have no expiration date.
As of December 31, 2022, AgeX has capital loss carryforwards for federal and state tax purposes of $12.4 million and $5.9 million, respectively. The federal and California capital loss carryforwards will expire in 2026.
Effective for tax years beginning after December 31, 2021, taxpayers are required to capitalize any expenses incurred that are considered incidental to research and experimentation (“R&E”) activities under IRC Section 174. While taxpayers historically had the option of deducting these expenses under IRC Section 174, the December 2017 Tax Cuts and Jobs Act mandates capitalization and amortization of R&E expenses for tax years beginning after December 31, 2021. Expenses incurred in connection with R&E activities in the US must be amortized over a 5-year period if incurred, and R&E expenses incurred outside the US must be amortized over a 15-year period. R&E activities are broader in scope than qualified research activities considered under IRC Section 41 (relating to the research tax credit). For the year ended December 31, 2022, the Company performed an analysis based on available guidance and determined that it will continue to be in a loss position even after the required capitalization and amortization of its R&E expenses. We will continue to monitor this issue for future developments, but we does not expect R&E capitalization and amortization to require us to pay cash taxes now or in the near future.
Beginning in 2018, the 2017 Tax Act subjects a U.S. stockholder to tax on Global Intangible Low Tax Income “GILTI” earned by certain foreign subsidiaries. In general, GILTI is the excess of a U.S. stockholder’s total net foreign income over a deemed return on tangible assets. The provision further allows a deduction of 50% of GILTI, however this deduction is limited to the company’s pre-GILTI U.S. income. For the year ended December 31, 2021, AgeX’s foreign entity operated at an immaterial loss; therefore, no GILTI was included in income. For the year ended December 31, 2022, there was no income or loss related to foreign activity as the entity deconsolidated from AgeX on March 15, 2021. Current interpretations under ASC 740 state that an entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense. We have elected to account for GILTI as a current period expense when incurred.
For the year ended December 31, 2022, we experienced a domestic loss from continuing operations; therefore, no income tax provision was recorded for the year ended December 31, 2022.
The sale of LifeMap Sciences resulted in a taxable loss primarily due to AgeX’s tax basis in the subsidiary.
Other Income Tax Matters
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by NOL carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
AgeX and its subsidiaries may be subject to potential income tax examination by U.S. federal or states authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. AgeX filed its first consolidated federal tax return in 2018. AgeX and its current subsidiaries are not subject to tax examination by federal tax authorities for tax years beginning before 2019 and for state tax authorities beginning before 2018. However, the tax authorities may still make adjustments to the net operating loss and credit carryforwards used in open years by AgeX or any of its subsidiaries. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
10. Commitments and Contingencies
Office Lease Agreement
Effective January 1, 2021, AgeX relocated its principal offices to a 135 square foot leased space in a building located in an office and research park in Alameda, California. Base monthly rent was $947 for the first year of the lease term. In September 2021, AgeX extended its office lease for another year, effective January 1, 2022, at a monthly rent of $1,074. In October 2022, AgeX entered into a new one-year lease that became effective January 1, 2023 for a different space, comprising of 101 square feet, in the same building, at a monthly rent of $844. The lease also includes office furniture rental, janitorial services, utilities, and internet service.
ASC 842
For the office lease, AgeX has elected to not apply the recognition requirements under ASC 842 and instead recognized the lease payments as lease costs on a straight-line basis over the lease term, as the amount of the lease payments is not deemed material.
There were no future minimum lease commitments as of December 31, 2022.
Litigation – General
AgeX is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When AgeX is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, AgeX will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, AgeX discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. AgeX is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Tax Filings
AgeX tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes AgeX has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the consolidated financial statements.
Employment Contracts
AgeX has entered into employment contracts with certain executive officers. Under the provisions of the contracts, AgeX may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations.
Indemnification
In the normal course of business, AgeX may provide indemnifications of varying scope under AgeX’s agreements with other companies or consultants, typically for AgeX’s research and development programs. Pursuant to these agreements, AgeX will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with AgeX’s research and development. Indemnification provisions could also cover third-party infringement claims with respect to patent rights, copyrights, or other intellectual property licensed from AgeX to third parties. Office and laboratory leases will also generally indemnify the lessor with respect to certain matters that may arise during the term of the lease. The sales agreement between AgeX and Chardan also includes indemnification provisions pursuant to which the parties have agreed to indemnify each other from certain liabilities that could arise from the offer and sale of AgeX common stock through the ATM facility, including liabilities under the Securities Act. Similarly, the Registration Rights Agreement between Juvenescence and AgeX includes indemnification provisions pursuant to which the parties will indemnify each other from certain liabilities in connection with the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act. The term of these indemnification obligations will generally continue in effect after the termination or expiration of the particular license, lease, or agreement to which they relate. The potential future payments AgeX could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, AgeX has not been subject to any claims or demands for indemnification. AgeX also maintains various liability insurance policies that limit AgeX’s financial exposure. As a result, AgeX believes the fair value of these indemnification agreements is minimal. Accordingly, AgeX has not recorded any liabilities for these agreements as of December 31, 2022 and 2021.
Paycheck Protection Program Loan
On April 13, 2020, AgeX obtained a loan in the amount of $432,952 from a bank under the Paycheck Protection Program (the “PPP Loan”). The PPP Loan bore interest at a rate of 1% per annum. No payments were due on the PPP Loan during a six month deferral period commencing on the date of the promissory note. Commencing one month after the expiration of the deferral period, and continuing on the same day of each month thereafter until the maturity date of the PPP Loan, monthly payments of principal and interest became due, in an amount required to fully amortize the principal amount outstanding on the PPP Loan by the maturity date. The maturity date was April 13, 2022. The principal amount of the PPP Loan was subject to forgiveness under the Paycheck Protection Program (the “Program”) to the extent of PPP Loan proceeds that were used to pay expense permitted by the Program, including payroll, rent, and utilities during the time frame permitted by the Program. On February 19, 2021, the PPP Loan was forgiven in full.
On December 27, 2020, the Consolidated Appropriations Act of 2021 was signed into law, retroactively allowing a federal deduction of the expenses that gave rise to the PPP Loan forgiveness. California does not allow a deduction for these expenses for publicly traded companies.
Notice of Delisting
On June 1, 2020, AgeX received a letter (the “Deficiency Letter”) from the staff of the NYSE American (the “Exchange”) indicating that AgeX does not meet certain of the Exchange’s continued listing standards as set forth in Section 1003(a)(i) of the Exchange Company Guide in that AgeX has stockholders’ equity of less than $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years. Pursuant to Section 1009 of the Exchange Company Guide and as provided in the Deficiency Letter AgeX provided the Exchange staff with a plan (the “Compliance Plan”) advising the Exchange staff of action AgeX has taken and will take that would bring AgeX into compliance with the Exchange’s continued listing standards by December 1, 2021. The Exchange staff has accepted the Compliance Plan.
On April 15, 2021, AgeX regained compliance with all of the Exchange’s continued listing standards set forth in Part 10 of the Exchange Company Guide. Specially, the Exchange has resolved the continued listing deficiency with respect to Section 1003(a)(i) of the Exchange Company Guide.
On November 17, 2021, AgeX received a second deficiency letter (“2021 Deficiency Letter”) from the staff of the Exchange indicating that AgeX does not meet certain of the Exchange’s continued listing standards as set forth in Section 1003(a)(i) and (ii) of the Exchange Company Guide in that AgeX had stockholders equity of less than $2,000,000 and had incurred losses from continuing operations and/or net losses during the two most recent fiscal years, and that AgeX had stockholders equity of less than $4,000,000 and had incurred losses from continuing operations and/or net losses during three out of four of the most recent fiscal years. Pursuant to Section 1009 of the Exchange Company Guide and as provided in the 2021 Deficiency Letter, AgeX provided the Exchange staff with an updated plan (the “2021 Plan”) during December 2021 advising the Exchange staff of action that AgeX had taken and will take that would bring AgeX into compliance with the Exchange’s continued listing standards. The Exchange staff accepted the 2021 Plan which was subsequently revised by AgeX in the course of the Exchange’s continued monitoring of AgeX’s progress to attain compliance with the continued listing standards. During November 2022, the Exchange sent AgeX a notification indicating that the Exchange has accepted the revised 2021 Plan and has granted AgeX an extension of time to regain compliance with the Exchange’s continued listing standards as set forth in Section 1003(a)(i) and (ii) of the Exchange Company Guide by increasing stockholders equity to not less than $4,000,000. The Exchange staff will periodically review AgeX’s adherence to the revised 2021 Plan milestones. If we are not in compliance with the continued listing standards by May 17, 2023, or if AgeX does not make progress consistent with the revised 2021 Plan during the plan period, the Exchange will initiate delisting proceedings as appropriate.
AgeX intends to make arrangements to have its common stock quoted on an interdealer quotation system if its common stock is delisted from the Exchange.
11. Subsequent Events
2022 Secured Convertible Promissory Note
On January 25, 2023, AgeX borrowed an additional $500,000 under the Secured Note. In connection with that draw of loan funds under the Secured Note, AgeX issued to Juvenescence warrants to purchase 340,136 shares of AgeX common stock at an exercise price of $0.735 per share.
Amended and Restated Secured Convertible Promissory Note
On February 9, 2023, AgeX and Juvenescence entered into an Amended and Restated Secured Convertible Promissory Note which amends and restates the February 14, 2022 Secured Note and provides that AgeX may borrow up to an additional $2,000,000 from Juvenescence until May 9, 2023 subject to Juvenescence’s discretion to approve each loan draw. References to the Secured Note include the Amended and Restated Secured Note. AgeX may not draw more than $1 million in any single draw. The date on which the outstanding principal balance of the Secured Note will become due and payable shall be February 14, 2024, consistent with the original terms of the Secured Note.
The other material provisions of the Secured Note, including but not limited to those pertaining to loan origination fees, default provisions, provisions governing the rights of AgeX and Juvenescence to convert the loan balance and any accrued but unpaid origination fees into shares of AgeX common stock, and provisions requiring AgeX to issue common stock purchase warrants to Juvenescence in connection with borrowings remain in effect and will apply to any draw down of the additional $2,000,000 credit available under the Secured Note as amended.
AgeX’s obligations under the Secured Note, including with respect to any additional borrowings, will continue to be secured by the security interests in AgeX assets granted to Juvenescence pursuant to the Security Agreement.
On February 15, 2023, AgeX drew $1,000,000 of its available credit under the Secured Note. In connection with that draw of loan funds, AgeX issued to Juvenescence warrants to purchase 801,924 shares of AgeX common stock at an exercise price of $0.6235 per share, the closing price of AgeX common stock on the NYSE American on February 10, 2023, the trading day immediately preceding delivery of AgeX’s drawdown notice of loan funds.
2020 Loan Agreement
On March 13, 2023, the 2020 Loan Agreement was amended to extend the maturity date to March 30, 2024.
$10 Million Secured Convertible Promissory Note
On March 13, 2023, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (the “$10 Million Secured Note”) pursuant to which Juvenescence has loaned to AgeX $10,000,000. AgeX used the loan proceeds to finance the purchase of a Convertible Promissory Note (the “Serina Note”) issued by Serina Therapeutics Inc., an Alabama corporation (“Serina”) to AgeX on March 15, 2023, pursuant to the terms of a Convertible Note Purchase Agreement (the “Serina Note Purchase Agreement”)
The outstanding principal balance of the $10 Million Secured Note will become due and payable on March 13, 2026. In lieu of accrued interest, AgeX will pay Juvenescence an origination fee in an amount equal to 7% of the loan funds disbursed to AgeX, which will accrue in two installments. The origination fee will become due and payable on the earliest to occur of (i) conversion of the $10 Million Secured Note into shares of AgeX common stock, (ii) repayment of the $10 Million Secured Note in whole or in part (provided that the origination fee shall be prorated for the amount of any partial repayment), and (iii) the acceleration of the maturity date of the $10 Million Secured Note following an Event of Default as defined in the $10 Million Secured Note.
If (a) AgeX and Serina have not entered into a definitive merger agreement by June 13, 2023; (b) a merger between AgeX and Serina is terminated or either party gives notice to terminate the merger agreement; or (c) the merger is not consummated by March 13, 2024, then AgeX may, after written notice to Juvenescence, pay and satisfy in full the principal balance and accrued origination fees under the $10 Million Secured Note by tendering to Juvenescence the Serina Note and shares of capital stock of Serina, if any, that may have been issued to AgeX upon conversion of the Serina Note in whole or in part.
AgeX may convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. If less than $25,000,000 is raised through the sale of common stock or units, the conversion price per share or units shall be the lowest price at which shares or units are sold. If at least $25,000,000 is raised, the conversion price per share shall be 85% of the “Market Price” of AgeX common stock determined as provided in the $10 Million Secured Note.
Juvenescence may convert the outstanding principal amount of the $10 Million Secured Note plus the accrued origination fee into AgeX common stock at the market price per share of AgeX common stock. Juvenescence may not convert the $10 Million Secured Note to AgeX common stock before the earlier of (i) a merger between AgeX and Serina, and (ii) March 13, 2024. Any conversion of the $10 Million Secured Note into AgeX common stock is subject to certain restrictions to comply with applicable requirements of the NYSE American where AgeX common stock is listed.
The outstanding principal amount of the $10 Million Secured Note and the accrued origination fee may become immediately due and payable prior to the maturity date if an Event of Default as defined in the $10 Million Secured Note occurs. Events of Default under the $10 Million Secured Note are substantially the same events as those constituting an Event of Default under the Secured Note except that such events pertain to the $10 Million Secured Note and related loan documents.
The $10 Million Secured Note includes certain covenants that among other matters require financial reporting and impose certain restrictions on AgeX that are substantially the same as those under the Secured Note.
AgeX has entered into an Amended and Restated Security Agreement that amends the February 14, 2022 Security Agreement between AgeX and Juvenescence and adds the $10 Million Secured Note to the obligations secured by the Security Agreement. The Security Agreement grants Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs, Juvenescence will have the right to foreclose on the assets pledged as collateral.
Serina Note
On March 15, 2023, AgeX and Serina entered into the Serina Note Purchase Agreement, pursuant to which AgeX has agreed to lend to Serina an aggregate principal amount of $10,000,000 as evidenced by the Serina Note. Interest on the principal amount under the Serina Note accrues on the unpaid principal amount at a simple interest rate equal to 7% per annum, computed on the basis of the 360-day year of twelve 30-day months. The outstanding principal balance of the Serina Note will become due and payable on March 15, 2026.
In connection with the issuance of the Serina Note, AgeX is entitled to elect one member to the board of directors of Serina and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
The principal balance of the Serina Note with accrued interest will automatically convert into Serina preferred stock if Serina raises at least $25,000,000 through the sale of shares of Serina preferred stock. The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by Serina’s then fully diluted capitalization. AgeX has the option to convert the Serina Note into Serina preferred stock after a sale of Serina preferred stock regardless of the amount sold by Serina.
AgeX may (i) at its election, upon a change of control (as defined in the Serina Note), convert the Serina Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the Serina Note, plus interest, or (b) into the highest ranking shares of Serina then issued at a conversion price equal to the lowest price per share at which the most senior series of Serina shares has been sold in a single transaction or a series of related transactions through which Serina raised at least $5,000,000 or (ii) if the Serina Note remains outstanding as of the maturity date, AgeX may convert the Serina Note into the most senior shares of Serina issued at the time of conversion at a conversion price equal to the capped price.
Upon the consummation of a merger between AgeX and Serina, the Serina Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of Serina.
The outstanding principal balance of the Serina Note with accrued interest may become immediately due and payable prior to the stated maturity date if an Event of Default as defined in the Serina Note occurs. In addition to this and any other remedy, both in equity and in law, upon the occurrence of an Event of Default, an interest rate of 10% per annum and computed on the basis of the 360-day year of twelve 30-day months, shall apply to the Convertible Amount until fully paid. Events of Default under the Serina Note include: (i) the commission of any act of bankruptcy by Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (ii) the execution by Serina of a general assignment for the benefit of creditors, (iii) the filing by or against Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) of a petition in bankruptcy or any petition for relief under the federal bankruptcy act (or, in each case, under any similar insolvency law) or the continuation of such petition without dismissal for a period of 60 calendar days or more, (iv) the appointment of a receiver or trustee to take possession of the property or assets of Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (v) failure of Serina to pay any amount due under the Serina Note when due, which failure to pay is not cured by Serina within 5 business days of written notice thereof, (vi) unless waived by AgeX, Serina’s material breach of any representation, warranty or covenant of Serina under the Serina Note Purchase Agreement, Serina Note or other agreements entered in connection therewith, which breach, if curable, is not cured by Serina within 10 business days of written notice by AgeX thereof, (vii) Serina or any subsidiary shall default on any of its obligations under any indebtedness which default causes the indebtedness thereunder to (x) become prematurely due and payable, (y) be placed on demand or (z) become capable of being declared by or on behalf of a creditor thereunder to be prematurely due and payable or being placed on demand, in each case, as a result of such default or any provision having a similar effect (howsoever prescribed), (viii) any monetary judgment, writ or similar final process shall be entered or filed against Serina, any subsidiary or any of their respective property or other assets for more than $250,000, and such judgment, writ or similar final process shall remain unvacated, unbonded or unstayed for a period of 45 calendar days, and (ix) Serina experiences a Material Adverse Effect (as defined in the Serina Note Purchase Agreement).
The Serina Note Purchase Agreement and Serina Note each includes certain covenants that among other matters require financial reporting and impose certain restrictions, including (i) restrictions on the incurrence of additional indebtedness by Serina and its subsidiaries; (ii) requiring that Serina use note proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, and for general working capital; and (iii) prohibiting Serina from entering into any material sale or transfer transactions outside of the ordinary course of business, other than in a merger between AgeX and Serina, without the consent of AgeX.
Subordination Agreement
In connection with the issuance of the Serina Note, Serina, each other holder of Serina indebtedness (each a “Serina Lender”), and AgeX entered into a Subordination Agreement, dated March 15, 2023, pursuant to which each Serina Lender agreed to subordinate to AgeX’s rights of repayment with respect to the obligations owed under the Serina Note Purchase Agreement and the Serina Note (i) all Serina indebtedness owed to such Serina Lender under certain convertible notes between each Serina Lender and Serina, which aggregate principal amount of all of such convertible notes equals $1,450,000, and (ii) any related security interests.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except par value amounts)
(unaudited)
| | | | | | | | | |
| | | June 30, 2023 | | | December 31, 2022 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 261 | | | $ | 645 | |
| Accounts and grants receivable, net | | | 6 | | | | 4 | |
| Prepaid expenses and other current assets | | | 1,083 | | | | 1,804 | |
| Total current assets | | | 1,350 | | | | 2,453 | |
| | | | | | | | | |
| Restricted cash | | | 50 | | | | 50 | |
| Intangible assets, net | | | 673 | | | | 738 | |
| Convertible note receivable | | | 10,204 | | | | - | |
| TOTAL ASSETS | | $ | 12,277 | | | $ | 3,241 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 961 | | | $ | 1,034 | |
| Loans due to Juvenescence, net of debt issuance costs, current portion | | | 22,943 | | | | 7,646 | |
| Related party payables, net | | | 230 | | | | 141 | |
| Warrant liability | | | - | | | | 180 | |
| Insurance premium liability and other current liabilities | | | 371 | | | | 1,077 | |
| Total current liabilities | | | 24,505 | | | | 10,078 | |
| | | | | | | | | |
| Loans due to Juvenescence, net of debt issuance costs, net of current portion | | | 10,068 | | | | 10,478 | |
| TOTAL LIABILITIES | | | 34,573 | | | | 20,556 | |
| | | | | | | | | |
| Commitments and contingencies (Note 11) | | | - | | | | - | |
| | | | | | | | | |
| Stockholders’ deficit: | | | | | | | | |
| Preferred stock, $0.0001 par value, 5,000 shares authorized; none issued and outstanding | | | - | | | | - | |
| Common stock, $0.0001 par value, 200,000 shares authorized; and 37,951 and 37,949 shares issued and outstanding | | | 4 | | | | 4 | |
| Additional paid-in capital | | | 99,977 | | | | 98,994 | |
| Accumulated deficit | | | (122,156 | ) | | | (116,210 | ) |
| Total AgeX Therapeutics, Inc. stockholders’ deficit | | | (22,175 | ) | | | (17,212 | ) |
| Noncontrolling interest | | | (121 | ) | | | (103 | ) |
| Total stockholders’ deficit | | | (22,296 | ) | | | (17,315 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 12,277 | | | $ | 3,241 | |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
(unaudited)
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| REVENUES | | | | | | | | | | | | | | | | |
| Revenues | | $ | 9 | | | $ | 12 | | | $ | 19 | | | $ | 17 | |
| Cost of sales | | | 5 | | | | 6 | | | | 6 | | | | 7 | |
| | | | | | | | | | | | | | | | | |
| Gross profit | | | 4 | | | | 6 | | | | 13 | | | | 10 | |
| | | | | | | | | | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | | | | | | | | | |
| Research and development | | | 160 | | | | 259 | | | | 334 | | | | 655 | |
| General and administrative | | | 1,730 | | | | 1,338 | | | | 3,723 | | | | 2,998 | |
| Total operating expenses | | | 1,890 | | | | 1,597 | | | | 4,057 | | | | 3,653 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (1,886 | ) | | | (1,591 | ) | | | (4,044 | ) | | | (3,643 | ) |
| | | | | | | | | | | | | | | | | |
| OTHER EXPENSE, NET: | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (792 | ) | | | (863 | ) | | | (1,892 | ) | | | (1,434 | ) |
| Change in fair value of warrants | | | (5 | ) | | | (168 | ) | | | (35 | ) | | | (255 | ) |
| Other income, net | | | 4 | | | | 4 | | | | 7 | | | | 7 | |
| Total other expense, net | | | (793 | ) | | | (1,027 | ) | | | (1,920 | ) | | | (1,682 | ) |
| | | | | | | | | | | | | | | | | |
| NET LOSS | | | (2,679 | ) | | | (2,618 | ) | | | (5,964 | ) | | | (5,325 | ) |
| Net loss attributable to noncontrolling interest | | | 10 | | | | - | | | | 18 | | | | 1 | |
| | | | | | | | | | | | | | | | | |
| NET LOSS ATTRIBUTABLE TO AGEX | | $ | (2,669 | ) | | $ | (2,618 | ) | | $ | (5,946 | ) | | $ | (5,324 | ) |
| | | | | | | | | | | | | | | | | |
| NET LOSS PER COMMON SHARE: | | | | | | | | | | | | | | | | |
| NET LOSS PER COMMON SHARE: BASIC AND DILUTED | | | | | | | | | | | | | | | | |
| BASIC AND DILUTED | | $ | (0.07 | ) | | $ | (0.07 | ) | | $ | (0.16 | ) | | $ | (0.14 | ) |
| | | | | | | | | | | | | | | | |
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | | | | | | | | | | | | | | | | |
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: BASIC AND DILUTED | | | | | | | | | | | | | | | | |
| BASIC AND DILUTED | | | 37,951 | | | | 37,943 | | | | 37,950 | | | | 37,943 | |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands)
(unaudited)
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| NET LOSS | | $ | (2,679 | ) | | $ | (2,618 | ) | | $ | (5,964 | ) | | $ | (5,325 | ) |
| Less: Comprehensive loss attributable to noncontrolling interest | | | 10 | | | | - | | | | 18 | | | | 1 | |
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AGEX COMMON STOCKHOLDERS | | $ | (2,669 | ) | | $ | (2,618 | ) | | $ | (5,946 | ) | | $ | (5,324 | ) |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(in thousands)
(unaudited)
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Stockholders’ Deficit | |
| | | Three Months Ended June 30, 2023 | |
| | | AgeX’s Stockholders’ Deficit | | | | |
| | | Common Stock | | | Additional | | | | | | | | | Total | |
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Stockholders’ Deficit | |
| BALANCE AT MARCH 31, 2023 | | | 37,951 | | | $ | 4 | | | $ | 99,589 | | | $ | (119,487 | ) | | $ | (111 | ) | | $ | (20,005 | ) |
| Fair value of liability classified warrants issued | | | - | | | | - | | | | 353 | | | | - | | | | - | | | | 353 | |
| Stock-based compensation | | | - | | | | - | | | | 35 | | | | - | | | | - | | | | 35 | |
| Net loss | | | - | | | | - | | | | - | | | | (2,669 | ) | | | (10 | ) | | | (2,679 | ) |
| BALANCE AT JUNE 30, 2023 | | | 37,951 | | | $ | 4 | | | $ | 99,977 | | | $ | (122,156 | ) | | $ | (121 | ) | | $ | (22,296 | ) |
| Balance | | | 37,951 | | | $ | 4 | | | $ | 99,977 | | | $ | (122,156 | ) | | $ | (121 | ) | | $ | (22,296 | ) |
| | | Three Months Ended June 30, 2022 | |
| | | AgeX’s Stockholders’ Deficit | | | | |
| | | Common Stock | | | Additional | | | | | | | | | Total | |
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Stockholders’ Deficit | |
| BALANCE AT MARCH 31, 2022 | | | 37,943 | | | $ | 4 | | | $ | 96,903 | | | $ | (108,454 | ) | | $ | (44 | ) | | $ | (11,591 | ) |
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | | | 2 | | | | - | | | | (1 | ) | | | - | | | | - | | | | (1 | ) |
| Fair value of liability classified warrants issued | | | - | | | | - | | | | 750 | | | | - | | | | - | | | | 750 | |
| Stock-based compensation | | | - | | | | - | | | | 198 | | | | - | | | | - | | | | 198 | |
| Net loss | | | - | | | | - | | | | - | | | | (2,618 | ) | | | - | | | | (2,618 | ) |
| BALANCE AT JUNE 30, 2022 | | | 37,945 | | | $ | 4 | | | $ | 97,850 | | | $ | (111,072 | ) | | $ | (44 | ) | | $ | (13,262 | ) |
| Balance | | | 37,945 | | | $ | 4 | | | $ | 97,850 | | | $ | (111,072 | ) | | $ | (44 | ) | | $ | (13,262 | ) |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(in thousands)
(unaudited)
| | | Six Months Ended June 30, 2023 | |
| | | AgeX’s Stockholders’ Deficit | | | | |
| | | Common Stock | | | Additional | | | | | | | | | Total | |
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Stockholders’ Deficit | |
| BALANCE AT DECEMBER 31, 2022 | | | 37,949 | | | $ | 4 | | | $ | 98,994 | | | $ | (116,210 | ) | | $ | (103 | ) | | $ | (17,315 | ) |
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | | | 2 | | | | - | | | | (1 | ) | | | - | | | | - | | | | (1 | ) |
| Fair value of liability classified warrants issued | | | - | | | | - | | | | 879 | | | | - | | | | - | | | | 879 | |
| Stock-based compensation | | | - | | | | - | | | | 105 | | | | - | | | | - | | | | 105 | |
| Net loss | | | - | | | | - | | | | - | | | | (5,946 | ) | | | (18 | ) | | | (5,964 | ) |
| BALANCE AT JUNE 30, 2023 | | | 37,951 | | | $ | 4 | | | $ | 99,977 | | | $ | (122,156 | ) | | $ | (121 | ) | | $ | (22,296 | ) |
| Balance | | | 37,951 | | | $ | 4 | | | $ | 99,977 | | | $ | (122,156 | ) | | $ | (121 | ) | | $ | (22,296 | ) |
| | | Six Months Ended June 30, 2022 | |
| | | AgeX’s Stockholders’ Deficit | | | | |
| | | Common Stock | | | Additional | | | | | | | | | Total | |
| | | Number of Shares | | | Par Value | | | Paid-In Capital | | | Accumulated Deficit | | | Noncontrolling Interest | | | Stockholders’ Deficit | |
| BALANCE AT DECEMBER 31, 2021 | | | 37,941 | | | $ | 4 | | | $ | 93,912 | | | $ | (105,748 | ) | | $ | (43 | ) | | $ | (11,875 | ) |
| Balance | | | 37,941 | | | $ | 4 | | | $ | 93,912 | | | $ | (105,748 | ) | | $ | (43 | ) | | $ | (11,875 | ) |
| Issuance of common stock upon vesting of restricted stock units, net of shares retired to pay employee’s taxes | | | 4 | | | | - | | | | (2 | ) | | | - | | | | - | | | | (2 | ) |
| Issuance of warrants | | | - | | | | - | | | | 178 | | | | - | | | | - | | | | 178 | |
| Fair value of liability classified warrants issued | | | - | | | | - | | | | 3,325 | | | | - | | | | - | | | | 3,325 | |
| Stock-based compensation | | | - | | | | - | | | | 437 | | | | - | | | | - | | | | 437 | |
| Net loss | | | - | | | | - | | | | - | | | | (5,324 | ) | | | (1 | ) | | | (5,325 | ) |
| BALANCE AT JUNE 30, 2022 | | | 37,945 | | | $ | 4 | | | $ | 97,850 | | | $ | (111,072 | ) | | $ | (44 | ) | | $ | (13,262 | ) |
| Balance | | | 37,945 | | | $ | 4 | | | $ | 97,850 | | | $ | (111,072 | ) | | $ | (44 | ) | | $ | (13,262 | ) |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| | | 2023 | | | 2022 | |
| | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | |
| OPERATING ACTIVITIES: | | | | | | | | |
| Net loss attributable to AgeX | | $ | (5,946 | ) | | $ | (5,324 | ) |
| Net loss attributable to noncontrolling interest | | | (18 | ) | | | (1 | ) |
| Adjustments to reconcile net loss attributable to AgeX to net cash used in operating activities: | | | | | | | | |
| Change in fair value of warrants | | | 35 | | | | 255 | |
| Amortization of intangible assets | | | 65 | | | | 66 | |
| Amortization of debt issuance costs | | | 1,976 | | | | 1,355 | |
| Stock-based compensation | | | 105 | | | | 437 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts and grants receivable | | | (2 | ) | | | 13 | |
| Prepaid expenses and other current assets | | | 721 | | | | 614 | |
| Interest on convertible note receivable | | | (204 | ) | | | - | |
| Accounts payable and accrued liabilities | | | (96 | ) | | | (207 | ) |
| Related party payables | | | 186 | | | | 65 | |
| Insurance premium liability | | | (711 | ) | | | (653 | ) |
| Other current liabilities | | | 5 | | | | (2 | ) |
| Net cash used in operating activities | | | (3,884 | ) | | | (3,382 | ) |
| | | | | | | | | |
| INVESTING ACTIVITIES: | | | | | | | | |
| Cash advanced on convertible note receivable | | | (10,000 | ) | | | - | |
| Net cash used in investing activities | | | (10,000 | ) | | | - | |
| | | | | | | | | |
| FINANCING ACTIVITIES: | | | | | | | | |
| Drawdown on loan facilities from Juvenescence | | | 13,500 | | | | 3,500 | |
| Net cash provided by financing activities | | | 13,500 | | | | 3,500 | |
| | | | | | | | | |
| NET CHANGE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH | | | (384 | ) | | | 118 | |
| | | | | | | | | |
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH: | | | | | | | | |
| At beginning of the period | | | 695 | | | | 634 | |
| At end of the period | | $ | 311 | | | $ | 752 | |
See accompanying notes to these condensed consolidated interim financial statements.
AGEX THERAPEUTICS, INC. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS
(unaudited)
1. Organization, Business Overview and Liquidity
AgeX Therapeutics, Inc. (“AgeX”) was incorporated in January 2017 in the state of Delaware. AgeX is a biotechnology company focused on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases. AgeX’s mission is to apply its comprehensive experience in fundamental biological processes of human aging to a broad range of age-associated medical conditions.
AgeX’s proprietary technology, based on telomerase-mediated cellular immortality and regenerative biology, allows AgeX to utilize telomerase-expressing regenerative pluripotent stem cells (“PSCs”) for the manufacture of cell-based therapies to regenerate tissues afflicted with age-related chronic degenerative disease. AgeX’s main technology platforms and product candidates are:
| ● | PureStem® PSC-derived clonal embryonic progenitor cell lines that may be capable of generating a broad range of cell types for use in cell-based therapies; |
| ● | UniverCyte™ which uses the HLA-G gene to suppress rejection of transplanted cells and tissues to confer low immune observability to cells; |
| ● | AGEX-BAT1 using adipose brown fat cells for metabolic diseases such as Type II diabetes; |
| ● | AGEX-VASC1 using vascular progenitor cells to treat tissue ischemia; and |
| ● | Induced tissue regeneration or iTR technology to regenerate or rejuvenate cells to treat a variety of degenerative diseases including those associated with aging, as well as other potential tissue regeneration applications such as scarless wound repair. |
Restructuring Plans
During March 2023, AgeX borrowed $10,000,000 from Juvenescence Limited (“Juvenescence”) under the terms of a Secured Convertible Promissory Note (the “$10 Million Secured Note”) and used the loan proceeds to make a $10,000,000 loan under the terms of a Convertible Promissory Note to Serina (the “Serina Note”), in order to provide financing to Serina Therapeutics, Inc. (“Serina”) in contemplation of corporate restructuring plans that include a potential merger between AgeX and Serina in which AgeX would be the surviving company. Serina has developed a proprietary drug delivery polymer technology. AgeX’s restructuring plans also include a potential spinoff of AgeX’s subsidiary Reverse Bioengineering, Inc. (“Reverse Bio”) through a distribution of some or all of the shares of capital stock of Reverse Bio held by AgeX to AgeX stockholders following a financing of Reverse Bio through the sale of shares of Reverse Bio common stock to private investors (the “Reverse Bio Financing”). If the Reverse Bio spinoff is completed, Reverse Bio would become a separate publicly traded company.
No definitive agreement regarding a merger between AgeX and Serina has been negotiated or executed nor has the merger been approved by the respective boards of directors of AgeX and Serina. Further, a merger cannot be consummated unless approved by the stockholders of AgeX and Serina. Accordingly, there is no assurance that AgeX and Serina will reach agreement on the terms of a merger or that, if such an agreement is reached, the stockholders of AgeX and Serina will approve the merger.
Definitive agreements regarding the Reverse Bio Financing and a Reverse Bio spinoff have not yet been executed, nor has AgeX’s board of directors approved the Reverse Bio spinoff. Accordingly, there is a risk that the Reverse Bio Financing and the Reverse Bio spinoff may never be consummated.
Emerging Growth Company
AgeX is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012.
Going Concern
AgeX primarily finances its operations through loans from its largest stockholder Juvenescence. AgeX has incurred operating losses and negative cash flows since inception and had an accumulated deficit of $122.2 million as of June 30, 2023. AgeX expects to continue to incur operating losses and negative cash flows.
Based on a strategic review of its operations, giving consideration to the status of its product development programs, human resources, capital needs and resources, and current conditions in the capital markets, AgeX’s board of directors and management have adopted operating plans and budgets to extend the period over which AgeX can continue its operations with its available cash resources. Notwithstanding those operating plans and budgets, based on AgeX’s most recent projected cash flows AgeX believes that its cash and cash equivalents of $0.3 million as of June 30, 2023 plus the loan facilities provided by Juvenescence to advance up to an additional $4 million to AgeX, and the proceeds AgeX may receive from the sale of additional shares of its common stock in “at-the-market” transactions through a Sales Agreement with Chardan Capital, LLC (“Chardan”) as a sales agent, would not be sufficient to satisfy AgeX’s anticipated operating and other funding requirements for the next twelve months from the issuance of these condensed consolidated interim financial statements. These conditions raise substantial doubt about AgeX’s ability to continue as a going concern. AgeX will need to obtain substantial additional funding in connection with its continuing operations. The financial statements do not include any adjustments to the amount and classification of assets and liabilities that may be necessary should AgeX not continue as a going concern.
Liquidity and Impact of COVID-19
In addition to general economic and capital market trends and conditions, AgeX’s ability to raise sufficient additional capital to finance its operations from time to time will depend on a number of factors specific to AgeX’s operations such as operating expenses and progress in out-licensing its technologies and development of its product candidates. Although AgeX has been able to reduce its operating expenses, with the exception of certain non-recurring expenses incurred related to the possible merger between AgeX and Serina, by eliminating internal research and development activities and focusing instead on outsourcing research and development and seeking licensing arrangements for AgeX technologies, this approach has also made it more difficult for AgeX to make progress in developing its target product candidates and technologies, which in turn may make it more difficult for AgeX to raise capital. The availability of financing also may be adversely impacted by the COVID-19 pandemic to the extent it disrupts aspects of AgeX’s operations. The extent to which the ongoing COVID-19 pandemic will ultimately impact AgeX’s business, results of operations, financial condition, or cash flows is highly uncertain and difficult to predict because it will depend on many factors that are outside AgeX’s control. The unavailability or inadequacy of financing to meet future capital needs could force AgeX to modify, curtail, delay, or suspend some or all aspects of planned operations. Sales of additional equity securities could result in the dilution of the interests of its stockholders. AgeX cannot assure that adequate financing will be available on favorable terms, if at all.
2. Basis of Presentation and Summary of Significant Accounting Policies
The unaudited condensed consolidated interim financial statements presented herein, and discussed below, have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. In accordance with those rules and regulations certain information and footnote disclosures normally included in comprehensive consolidated financial statements have been condensed or omitted. The condensed consolidated balance sheet as of December 31, 2022 was derived from the audited consolidated financial statements at that date but does not include all the information and footnotes required by U.S. GAAP. These condensed consolidated interim financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in AgeX’s Annual Report on Form 10-K for the year ended December 31, 2022.
The accompanying condensed consolidated interim financial statements, in the opinion of management, include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of AgeX’s financial condition and results of operations. The condensed consolidated results of operations are not necessarily indicative of the results to be expected for any other interim period or for the entire year.
Principles of consolidation
The consolidated financial statements include the accounts of AgeX and its subsidiaries in which AgeX has a controlling financial interest. The consolidated financial statements also include certain variable interest entities in which AgeX is the primary beneficiary (as described in more detail below). For consolidated entities where AgeX has less than 100% of ownership, AgeX records net loss attributable to noncontrolling interest on the consolidated statement of operations equal to the percentage of the ownership interest retained in such entities by the respective noncontrolling parties. The noncontrolling interest is reflected as a separate element of stockholders’ deficit on AgeX’s consolidated balance sheets. Any material intercompany transactions and balances have been eliminated upon consolidation.
AgeX assesses whether it is the primary beneficiary of a variable interest entity (“VIE”) at the inception of the arrangement and at each reporting date. This assessment is based on its power to direct the activities of the VIE that most significantly impact the VIE’s economic performance and AgeX’s obligation to absorb losses or the right to receive benefits from the VIE that could potentially be significant to the VIE. If the entity is within the scope of the variable interest model and meets the definition of a VIE, AgeX considers whether it must consolidate the VIE or provide additional disclosures regarding its involvement with the VIE. If AgeX determines that it is the primary beneficiary of the VIE, AgeX will consolidate the VIE. This analysis is performed at the initial investment in the entity or upon any reconsideration event. For entities AgeX holds as an equity investment that are not consolidated under the VIE model, AgeX will consider whether its investment constitutes a controlling financial interest in the entity and therefore should be considered for consolidation under the voting interest model.
AgeX has three subsidiaries, Reverse Bio, ReCyte Therapeutics, Inc. (“ReCyte”), and NeuroAirmid Therapeutics, Inc. (“NeuroAirmid”). Reverse Bio is a wholly owned subsidiary of AgeX through which AgeX plans to finance its iTRTM research and development efforts. AgeX is actively seeking equity financing for Reverse Bio and to the extent that such Reverse Bio Financing is obtained through the sale of capital stock or other equity securities by Reverse Bio, AgeX’s equity interest in Reverse Bio and its iTRTM business would be diluted. AgeX’s restructuring plans also include a potential spinoff of Reverse Bio through a distribution of some or all of the shares of capital stock of Reverse Bio held by AgeX to AgeX stockholders following the Reverse Bio Financing. ReCyte is an early stage pre-clinical research and development company involved in stem cell-derived endothelial and cardiovascular related progenitor cells for the treatment of vascular disorders and ischemic conditions. AgeX owns 94.8% of the outstanding capital stock of ReCyte. NeuroAirmid is jointly owned by AgeX with the University of California – Irvine and certain researchers and was recently organized to pursue clinical development and commercialization of cell therapies, focusing initially on Huntington’s Disease. AgeX owns 50% of the outstanding capital stock of NeuroAirmid. AgeX consolidates NeuroAirmid despite not having majority ownership interest as it has the ability to influence decision making and financial results through contractual rights and obligations as per Accounting Standards Codification (“ASC”) 810, Consolidation.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect (i) the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and (ii) the reported amounts of revenues and expenses during the reporting period, in each case with consideration given to materiality. Significant estimates and assumptions which are subject to significant judgment include those related to going concern assessment of consolidated financial statements, useful lives associated with long-lived assets, including evaluation of asset impairment, allowances for uncollectible accounts receivables, loss contingencies, deferred income taxes and tax reserves, including valuation allowances related to deferred income taxes, determining the fair value of AgeX’s embedded derivatives in the convertible notes payable and receivable, and assumptions used to value stock-based awards or other equity instruments and liability classified warrants. Actual results could differ materially from those estimates. The financial information for private companies may not be available and, even if available, that information may be limited and/or unreliable. To the extent there are material differences between the estimates and actual results, AgeX’s future results of operations will be affected.
See Note 6, Warrant Liability, for discussion on estimated change in fair value of warrant liability.
Concentration of credit risk and other risks and uncertainties
Financial instruments that potentially subject AgeX to concentrations of risk consist principally of cash equivalents and a convertible note receivable. AgeX maintains its cash deposits in Federal Deposit Insurance Corporation insured financial institutions within the federally insured limits. Even if balances were to exceed the federally insured limits, AgeX does not believe that it would be exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
AgeX also monitors the creditworthiness of the borrower of the convertible promissory note. AgeX believes that any concentration of credit risk in a convertible note receivable was mitigated in part by (i) AgeX’s right to convert loan amounts owed to AgeX into shares of equity securities of the borrower in the event the borrower completes a financing in at least a designated amount, and (ii) AgeX’s right to tender the convertible note receivable to a lender to settle a convertible note payable. See Notes 4, Convertible Note Receivable and 5, Related Party Transactions.
Product candidates developed by AgeX and its subsidiaries will require approvals or clearances from the United States Food and Drug Administration or foreign regulatory agencies prior to commercial sales. There can be no assurance that any of the product candidates being developed or planned to be developed by AgeX or its subsidiaries will receive any of the required approvals or clearances. If regulatory approval or clearance were to be denied or any such approval or clearance was to be delayed, it would have a material adverse impact on AgeX.
Fair value measurements of financial instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of the financial statement presentation date.
The carrying values of cash equivalents, accounts receivable and accounts payable, are carried at, or approximate, fair value as of the reporting date because of their short-term nature. The convertible note receivable is reported at fair value as it bears market rates of interest. Fair values for the AgeX’s warrant liabilities are estimated by utilizing valuation models that consider current and expected stock prices, volatility, dividends, market interest rates, forward yield curves and discount rates. Such amounts and the recognition of such amounts are subject to significant estimates that may change in the future.
To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value (ASC 820-10-50, Fair Value Measurements and Disclosures):
| ● | Level 1 – Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 – Inputs to the valuation methodology include observable quoted prices (other than quoted market prices included within Level 1) for similar assets or liabilities in active markets, and inputs that are observable for the assets or liabilities, either directly or indirectly, for substantially the full term of the financial instruments. |
| ● | Level 3 – Inputs to the valuation methodology are unobservable; that reflect management’s own assumptions about the assumptions market participants would make and significant to the fair value. |
In determining fair value, AgeX utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, AgeX has no financial assets recorded at fair value on a recurring basis, except for cash and cash equivalents primarily consisting of money market funds. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input. The carrying amounts of accounts receivable, net, prepaid expenses and other current assets, related party amounts due to affiliates, accounts payable, accrued liabilities and other current liabilities approximate fair values because of the short-term nature of these items. The discounted conversion prices triggered by certain qualified events in the Serina Note and the $10 Million Secured Note are Level 3 on the fair value hierarchy and subject to fair valuation at inception and remeasurement at each reporting period. The fair value of the discounted conversion prices under both notes were determined to have an immaterial value at inception and life to date of the notes, as the probability of a future qualifying event is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period. For additional information regarding the convertible notes and derivatives, see Notes 4, Convertible Note Receivable, 5, Related Party Transactions, and 12, Subsequent Events.
The accounting guidance establishes a hierarchy which requires an entity to maximize the use of quoted market prices and minimize the use of unobservable inputs. An asset or liability’s level is based on the lowest level of input that is significant to the fair value measurement. Fair value estimates are reviewed at the origination date and again at each applicable measurement date and interim or annual financial reporting dates, as applicable for the financial instrument, and are based upon certain market assumptions and pertinent information available to management at those times.
The methods and significant inputs and assumptions utilized in estimating the fair value of the warrant liabilities, as well as the respective hierarchy designations are discussed further in Note 6, Warrant Liability. The warrant liability measurement is considered a Level 3 measurement based on the availability of market data and inputs and the significance of any unobservable inputs as of the measurement date. As of June 30, 2023, AgeX has utilized the full credit subject to warrants, and accordingly, the warrants were fully issued for each of the advances of loan funds under the Secured Note.
See Note 6, Warrant Liability, for additional information on accounting for liability classified warrants and certain Level 3 warrant valuation tables.
Cash, cash equivalents, and restricted cash
In accordance with Accounting Standards Update (“ASU”) 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash, a reconciliation of AgeX’s cash and cash equivalents in the condensed consolidated balance sheets to cash, cash equivalents and restricted cash in the condensed consolidated statements of cash flows for all periods presented is as follows (in thousands):
Schedule of Cash, Cash Equivalents and Restricted Cash
| | | June 30, 2023 (unaudited) | | | December 31, 2022 | |
| Cash and cash equivalents | | $ | 261 | | | $ | 645 | |
| Restricted cash (1) | | | 50 | | | | 50 | |
| Cash, cash equivalents, and restricted cash as shown in the condensed consolidated statements of cash flows | | $ | 311 | | | $ | 695 | |
| (1) | Restricted cash entirely represents the deposit required to maintain AgeX’s corporate credit card program. |
Long-lived intangible assets, net
Long-lived intangible assets, consisting primarily of acquired in-process research and development (“IPR&D”) and patents is stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful life of 10 years. See Note 3, Selected Balance Sheet Components.
Impairment of long-lived assets
AgeX assesses the impairment of long-lived assets whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. AgeX’s long-lived assets consists entirely of intangible assets. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss, equal to the excess of the carrying value of the asset over its fair value, is recorded. As of June 30, 2023, there has been no impairment of long-lived assets.
Leases
AgeX accounts for leases in accordance with ASU 2016-02, Leases (Topic 842) (“ASC 842”), and its subsequent amendments affecting AgeX: (i) ASU 2018-10, Codification Improvements to Topic 842, Leases, and (ii) ASU 2018-11, Leases (Topic 842): Targeted Improvements, using the modified retrospective method. AgeX management determines if an arrangement is a lease at inception. Leases are classified as either financing or operating, with classification affecting the pattern of expense recognition in the consolidated statements of operations. When determining whether a lease is a financing lease or an operating lease, ASC 842 does not specifically define criteria to determine “major part of remaining economic life of the underlying asset” and “substantially all of the fair value of the underlying asset.” For lease classification determination, AgeX continues to use (i) 75% or greater to determine whether the lease term is a major part of the remaining economic life of the underlying asset and (ii) 90% or greater to determine whether the present value of the sum of lease payments is substantially all of the fair value of the underlying asset. Under the available practical expedients, and as applicable, AgeX accounts for the lease and non-lease components as a single lease component. AgeX recognizes right-of-use (“ROU”) assets and lease liabilities for leases with terms greater than twelve months in the condensed consolidated balance sheets.
ROU assets represent an entity’s right to use an underlying asset during the lease term and lease liabilities represent an entity’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. If the lease agreement does not provide an implicit rate in the contract, an entity uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. The lease terms may include options to extend or terminate the lease when it is reasonably certain that the entity will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term. AgeX does not capitalize leases that have terms of twelve months or less.
AgeX leases office space in Alameda, California. For 2022 base monthly rent was $1,074 and for 2023 base monthly rent is $844 for slightly less space at the same building. AgeX has elected to not apply the recognition requirements under ASC 842 for the lease agreements and instead recognizes the lease payments as lease cost on a straight-line basis over the lease term as lease payments are not deemed material.
Accounting for warrants
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable U.S. GAAP. After all relevant assessments, AgeX concludes whether the warrants are classified as liability or equity. Liability classified warrants require fair value accounting at issuance and subsequent to initial issuance with all changes in fair value after the issuance date recorded in the statements of operations. Equity classified warrants only require fair value accounting at issuance with no changes recognized subsequent to the issuance date. AgeX has liability classified warrants as of June 30, 2023. See Notes 5, Related Party Transactions and 6, Warrant Liability, for additional information regarding warrants.
Revenue recognition
AgeX recognizes revenue in a manner that depicts the transfer of control of a product or a service to a customer and reflects the amount of the consideration it expects to receive in exchange for such product or service. In doing so, AgeX follows a five-step approach: (i) identify the contract with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) the customer obtains control of the product or service. AgeX considers the terms of a contract and all relevant facts and circumstances when applying the revenue recognition standard. AgeX applies the revenue recognition standard, including the use of any practical expedients, consistently to contracts with similar characteristics and in similar circumstances.
ESI BIO Research Products – AgeX, through its ESI BIO research product division, markets a number of products related to human pluripotent stem cells (“PSC lines”), including research-grade PSC lines and PSC lines produced under current good manufacturing practices or “cGMP”. AgeX offers cells from PSC lines to customers under contracts that permit the customers to utilize PSC lines for the research, development, and commercialization of cell-based therapies or other products in defined fields of application. The compensation to AgeX for providing the PSC line cells under such contracts may include up-front payments, milestone payments related to product development, regulatory matters, and commercialization, and the payment of royalties on sales of products developed from AgeX PSC lines. Revenues from the sale of research products have not been significant during the periods presented in the condensed consolidated interim financial statements included in this Report.
Arrangements with multiple performance obligations – AgeX may enter into contracts with customers that include multiple performance obligations. For such arrangements, AgeX will allocate revenue to each performance obligation based on its relative standalone selling price. AgeX will determine or estimate standalone selling prices based on the prices charged, or that would be charged, to customers for that product or service. As of June 30, 2023 and December 31, 2022, AgeX did not have significant arrangements with multiple performance obligations.
Research and development
Research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, amortization of intangible assets, outside consultants and contractors, sponsored research agreements with certain universities, and suppliers, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Research and development expenses incurred and reimbursed by grants from third parties or governmental agencies if any and as applicable, approximate the respective revenues recognized in the condensed consolidated statements of operations.
General and administrative
General and administrative expenses consist primarily of compensation and related benefits, including stock-based compensation, for executive and corporate personnel, and professional and consulting fees.
Basic and diluted net loss per share attributable to common stockholders
Basic loss per share is calculated by dividing net loss attributable to AgeX common stockholders by the weighted average number of shares of common stock outstanding, net of unvested restricted stock or restricted stock units, subject to repurchase by AgeX, if any, during the period. Diluted loss per share is calculated by dividing the net income attributable to AgeX common stockholders, if any, by the weighted average number of shares of common stock outstanding, adjusted for the effects of potentially dilutive common stock issuable under outstanding stock options, warrants, and restricted stock units, using the treasury-stock method, and convertible preferred stock, if any, using the if-converted method, and treasury stock held by subsidiaries, if any.
For the three and six months ended June 30, 2023 and 2022, because AgeX reported a net loss attributable to common stockholders, all potentially dilutive common stock, comprised of stock options, restricted stock units and warrants, is antidilutive.
The following weighted average common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Stock options | | | 3,261 | | | | 3,274 | | | | 3,261 | | | | 3,333 | |
| Warrants (1) | | | 13,246 | | | | 9,794 | | | | 13,013 | | | | 8,099 | |
| Restricted stock units | | | - | | | | 12 | | | | 1 | | | | 13 | |
| (1) | As of June 30, 2023 and 2022, AgeX had issued Juvenescence warrants to purchase 12,503,522 and 10,323,105 shares, respectively, of AgeX common stock as consideration for certain loan agreements discussed in Note 5, Related Party Transactions. |
Reclassifications
Certain reclassifications have been made to the prior period’s condensed consolidated interim financial statements to conform to current year presentation. Additionally, certain financial information is presented on a rounded basis, which may cause minor differences.
Recently adopted accounting pronouncements
In June 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, and subsequent amendments to the initial guidance under ASU 2018-19, ASU 2019-04, ASU 2019-05 and ASU 2019-10, which amends the current approach to estimate credit losses on certain financial assets. This ASU requires immediate recognition of management’s estimates of current expected credit losses. Under the prior model, losses were recognized only as they were incurred, which FASB has noted delayed recognition of expected losses that might not yet have met the threshold of being probable. The standard is applicable to all financial assets (and net investment in leases) that are not accounted for at fair value through net income, such as trade receivables, loans, debt securities, and net investment in leases, thereby bringing consistency in accounting treatment across different types of financial instruments and requiring consideration of a broader range of variables when forming loss estimates. Subsequent changes in the valuation allowance are recorded in current earnings and reversal of previous losses are permitted. AgeX adopted this standard as of January 1, 2023, and it did not have a material impact on the condensed consolidated interim financial statements.
In March 2022, the FASB issued ASU No. 2022-01, Derivatives and Hedging (Topic 815): Fair Value Hedging – Portfolio Layer Method, which clarifies the guidance in ASC 815 on fair value hedge accounting of interest rate risk for portfolios of financial assets. The ASU amends the guidance in ASU 2017-12 (released on August 28, 2017) that, among other things, established the “last-of-layer” method for making the fair value hedge accounting for these portfolios more accessible. ASU 2022-01 renames that method the “portfolio layer” method and addresses feedback from stakeholders regarding its application. AgeX adopted this standard as of January 1, 2023, and it did not have a material impact on the condensed consolidated interim financial statements.
In March 2022, the FASB issued ASU No. 2022-02, Financial Instruments – Credit Losses (Topic 326): Troubled Debt Restructurings and Vintage Disclosures, which amends the accounting for credit losses on financial instruments. This amendment eliminates the recognition and measurement guidance on troubled debt restructurings for creditors that have adopted the new credit losses guidance in ASC 326 and requires enhanced disclosures about loan modifications for borrowers experiencing financial difficulty. The new guidance also requires public business entities to present gross write-offs by year of origination in their vintage disclosures. The guidance became effective for AgeX on January 1, 2023 and includes interim periods. Entities can elect to adopt the guidance on troubled debt restructurings using either a prospective or modified retrospective transition. If an entity elects to apply a modified retrospective transition, it will record a cumulative effect adjustment to retained earnings in the period of adoption. This ASU did not have a material impact on the condensed consolidated interim financial statements.
On July 14, 2023, the FASB issued ASU No. 2023-02, Presentation of Financial Statements (Topic 205), Income Statement – Reporting Comprehensive Income (Topic 220), Distinguishing Liabilities from Equity (Topic 480), Equity (Topic 505), and Compensation – Stock Compensation, which amends or supersedes various SEC paragraphs within the codification to conform to past announcements and guidance issued by the SEC. Specifically, the ASU responds to (1) the issuance of SEC Staff Accounting Bulletin (SAB) 120; (2) the SEC staff announcement at the March 24, 2022, EITF meeting; and (3) SAB Topic 6.B, “Accounting Series Release No. 280 — General Revision of Regulation S-X: Income or Loss Applicable to Common Stock.” This ASU is effective immediately and did not have a material impact on AgeX’s condensed consolidated interim financial statements.
3. Selected Balance Sheet Components
Intangible assets, net
At June 30, 2023 and December 31, 2022, intangible assets, primarily consisting of acquired IPR&D and patents, and accumulated amortization were as follows (in thousands):
Schedule of Intangible Assets, Net
| | | June 30, 2023 (unaudited) | | | December 31, 2022 | |
| Intangible assets | | $ | 1,312 | | | $ | 1,312 | |
| Accumulated amortization | | | (639 | ) | | | (574 | ) |
| Total intangible assets, net | | $ | 673 | | | $ | 738 | |
AgeX recognized $32,000 and $65,000 in amortization expense of intangible assets, included in research and development expenses, for the three and six months ended June 30, 2023, respectively and $33,000 and $66,000 for the same periods in 2022, respectively.
Amortization of intangible assets for periods subsequent to June 30, 2023 is as follows (in thousands):
Schedule of Amortization Assets
| Year Ending December 31, | | Amortization Expense | |
| 2023 | | $ | 66 | |
| 2024 | | | 131 | |
| 2025 | | | 131 | |
| 2026 | | | 132 | |
| Thereafter | | | 213 | |
| Total | | $ | 673 | |
Accounts payable and accrued liabilities
At June 30, 2023 and December 31, 2022, accounts payable and accrued liabilities were comprised of the following (in thousands):
Schedule of Accounts Payable and Accrued Liabilities
| | | June 30, 2023 (unaudited) | | | December 31, 2022 | |
| Accounts payable | | $ | 506 | | | $ | 568 | |
| Accrued compensation | | | 199 | | | | 193 | |
| Accrued vendors and other expenses | | | 256 | | | | 273 | |
| Total accounts payable and accrued liabilities | | $ | 961 | | | $ | 1,034 | |
4. Convertible Note Receivable
On March 15, 2023, AgeX and Serina entered into a Convertible Note Purchase Agreement (the “Serina Note Purchase Agreement”), pursuant to which AgeX lent to Serina an aggregate principal amount of $10,000,000 as evidenced by the Serina Note on that date. Interest on the principal amount under the Serina Note accrues on the unpaid principal amount at a simple interest rate equal to 7% per annum, computed on the basis of the 360-day year of twelve 30-day months. The outstanding principal balance of the Serina Note will become due and payable on March 15, 2026.
In connection with the issuance of the Serina Note, AgeX is entitled to elect one member to the board of directors of Serina and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
The principal balance of the Serina Note with accrued interest will automatically convert into Serina preferred stock if Serina raises at least $25,000,000 through the sale of shares of Serina preferred stock (“qualifying event”). The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by Serina’s then fully diluted capitalization. AgeX has the option to convert the Serina Note into Serina preferred stock after a sale of Serina preferred stock regardless of the amount sold by Serina. AgeX evaluated the 20% discounted conversion feature of the Serina Note under ASC 815-15, Derivatives and Hedging—Embedded Derivatives, and concluded that it was an embedded derivative which should be bifurcated from the note and accounted for separately. The 20% discount was determined to have an immaterial value at inception and life to date of the Serina Note, as the probability of a future qualifying event is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period and any adjustments will be included in Interest (income) expense, net in the Other (income) expense, net section of the condensed consolidated statements of operations.
AgeX may (i) at its election, upon a change of control (as defined in the Serina Note), convert the Serina Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the Serina Note, plus interest, or (b) into the highest ranking shares of Serina then issued at a conversion price equal to the lowest price per share at which the most senior series of Serina shares has been sold in a single transaction or a series of related transactions through which Serina raised at least $5,000,000 or (ii) if the Serina Note remains outstanding as of the maturity date, AgeX may convert the Serina Note into the most senior shares of Serina issued at the time of conversion at a conversion price equal to the capped price.
Upon the consummation of a merger between AgeX and Serina, the Serina Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of Serina.
The outstanding principal balance of the Serina Note with accrued interest may become immediately due and payable prior to the stated maturity date if an Event of Default as defined in the Serina Note occurs. In addition to this and any other remedy, both in equity and in law, upon the occurrence of an Event of Default, an interest rate of 10% per annum and computed on the basis of the 360-day year of twelve 30-day months, shall apply to the Convertible Amount until fully paid. Events of Default under the Serina Note include: (i) the commission of any act of bankruptcy by Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (ii) the execution by Serina of a general assignment for the benefit of creditors, (iii) the filing by or against Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X) of a petition in bankruptcy or any petition for relief under the federal bankruptcy act (or, in each case, under any similar insolvency law) or the continuation of such petition without dismissal for a period of 60 calendar days or more, (iv) the appointment of a receiver or trustee to take possession of the property or assets of Serina or any Significant Subsidiary (as such term is defined in Rule 1-02(w) of Regulation S-X), (v) failure of Serina to pay any amount due under the Serina Note when due, which failure to pay is not cured by Serina within 5 business days of written notice thereof, (vi) unless waived by AgeX, Serina’s material breach of any representation, warranty or covenant of Serina under the Serina Note Purchase Agreement, Serina Note or other agreements entered in connection therewith, which breach, if curable, is not cured by Serina within 10 business days of written notice by AgeX thereof, (vii) Serina or any subsidiary shall default on any of its obligations under any indebtedness which default causes the indebtedness thereunder to (x) become prematurely due and payable, (y) be placed on demand or (z) become capable of being declared by or on behalf of a creditor thereunder to be prematurely due and payable or being placed on demand, in each case, as a result of such default or any provision having a similar effect (howsoever prescribed), (viii) any monetary judgment, writ or similar final process shall be entered or filed against Serina, any subsidiary or any of their respective property or other assets for more than $250,000, and such judgment, writ or similar final process shall remain unvacated, unbonded or unstayed for a period of 45 calendar days, and (ix) Serina experiences a Material Adverse Effect (as defined in the Serina Note Purchase Agreement).
The Serina Note Purchase Agreement and Serina Note each includes certain covenants that among other matters require financial reporting and impose certain restrictions, including (i) restrictions on the incurrence of additional indebtedness by Serina and its subsidiaries; (ii) requiring that Serina use note proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, and for general working capital; and (iii) prohibiting Serina from entering into any material sale or transfer transactions outside of the ordinary course of business, other than in a merger between AgeX and Serina, without the consent of AgeX.
Subordination Agreement
In connection with the issuance of the Serina Note, Serina, each other holder of Serina indebtedness (each a “Serina Lender”), and AgeX entered into a Subordination Agreement, dated March 15, 2023, pursuant to which each Serina Lender agreed to subordinate to AgeX’s rights of repayment with respect to the obligations owed under the Serina Note Purchase Agreement and the Serina Note (i) all Serina indebtedness owed to such Serina Lender under certain convertible notes between each Serina Lender and Serina, which aggregate principal amount of all of such convertible notes equals $1,450,000, and (ii) any related security interests.
5. Related Party Transactions
As disclosed in Note 12, Subsequent Events, during July 2023 AgeX and Juvenescence entered into an Exchange Agreement pursuant to which AgeX issued shares of Series A Preferred Stock and Series B Preferred Stock to Juvenescence in exchange for the extinguishment of a total of $36 million of indebtedness under the 2020 Loan Agreement, the Secured Note, and the $10 Million Secured Note discussed below. The unused portion of the line of credit under the Secured Note remains available to AgeX subject to the terms and conditions of the Secured Note.
2019 Loan Agreement
On August 13, 2019, AgeX and Juvenescence entered into a Loan Facility Agreement (the “2019 Loan Agreement”) pursuant to which Juvenescence provided to AgeX a $2 million line of credit for a period of 18 months. On February 10, 2021, AgeX entered into an amendment (the “First Amendment”) to the 2019 Loan Agreement which extended the maturity date of loans under the 2019 Loan Agreement to February 14, 2022, and increased the amount of the loan facility by $4 million. On November 8, 2021, AgeX entered into Amendment No. 2 to the 2019 Loan Agreement which increased the amount of the loan facility by another $1 million. As of December 31, 2021, AgeX had borrowed all of the $7 million total line of credit under the 2019 Loan Agreement, as amended. On February 14, 2022, AgeX refinanced the $7 million outstanding principal amount of the loans and a $160,000 origination fee due under the 2019 Loan Agreement, as amended. See discussion regarding the 2022 Secured Convertible Promissory Note within this Note 5.
2020 Loan Agreement
On March 30, 2020, AgeX and Juvenescence entered into a new Secured Convertible Facility Agreement (the “2020 Loan Agreement”) pursuant to which Juvenescence provided to AgeX an $8 million line of credit for a period of 18 months. Through June 30, 2023, AgeX had drawn the full $8 million line of credit. AgeX issued to Juvenescence 28,500 shares of AgeX common stock as an arrangement fee for the loan facility when AgeX borrowed an aggregate of $3 million under the 2020 Loan Agreement, and AgeX issued to Juvenescence warrants to purchase a total of 3,670,663 shares of AgeX common stock (“2020 Warrants”) as determined by the warrant formula described below of which 2,146,436 are outstanding as of June 30, 2023. On March 13, 2023, the 2020 Loan Agreement was amended to extend the maturity date to March 30, 2024. During July 2023 the full $8 million of 2020 Loan Agreement indebtedness was extinguished in exchange for shares of Series A Preferred Stock and Series B Preferred Stock pursuant to the Exchange Agreement. See Note 12, Subsequent Events.
2020 Warrants — Under the terms of the 2020 Loan Agreement, each time AgeX received an advance of funds under the 2020 Loan Agreement, AgeX issued to Juvenescence a number of 2020 Warrants equal to 50% of the number determined by dividing the amount of the advance by the applicable Market Price. The Market Price set each 2020 Warrant when issued was the closing price per share of AgeX common stock on the NYSE American on the date of the applicable notice from AgeX requesting a draw of funds that triggered the obligation to issue the 2020 Warrant. The 2020 Warrants will expire at 5:00 p.m. New York time three years after the date of issue. The exercise prices of the 2020 Warrants issued through June 30, 2023 range from $0.70 per share to $1.895 per share representing the market closing price on the NYSE American of AgeX common stock on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
2022 Secured Convertible Promissory Note and Security Agreement
On February 14, 2022, AgeX and Juvenescence entered into a Secured Convertible Promissory Note (the “Secured Note”) pursuant to which Juvenescence agreed to provide to AgeX a $13,160,000 line of credit for a period of 12 months. AgeX drew an initial $8,160,000 of the line of credit and used $7,160,000 to refinance the outstanding principal and the loan origination fees under the 2019 Loan Agreement with Juvenescence. On February 9, 2023, AgeX and Juvenescence entered into an Amended and Restated Secured Convertible Promissory Note which amends and restates the Secured Note and added $2 million to the line of credit available to be borrowed by AgeX under the Secured Note subject to Juvenescence’s discretion to approve each loan draw. On May 9, 2023, AgeX and Juvenescence entered into an Allonge and Second Amendment to Amended and Restated Convertible Promissory Note (the “Second Amendment”) that increased the amount of the line of credit available to AgeX by $4,000,000, subject to the terms of the Secured Note and Juvenescence’s discretion to approve and fund each of AgeX’s future draws of that additional amount of credit. On June 2, 2023, AgeX and Juvenescence entered into a Third Amendment to Amended and Restated Convertible Promissory Note (the “Third Amendment’), to provide that (i) AgeX may draw on the available portion of the line of credit under the Secured Note until the earlier of the date a Qualified Offering as defined in the Secured Note is consummated by AgeX or October 31, 2023 (subject to Juvenescence’s discretion to approve each loan draw as provided in the Secured Note), (ii) AgeX will not be obligated to issue additional common stock purchase warrants to Juvenescence in connection with the receipt of loan funds made available pursuant to the Second Amendment, and (iii) the definition of Reverse Financing Condition was amended to extend to June 20, 2023 the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by Reverse Bioengineering, Inc. The date on which the outstanding principal balance of the Secured Note will become due and payable shall be February 14, 2024. See Note 12, Subsequent Events for information regarding a Fourth Amendment to the Secured Note.
During the six months ended June 30, 2023, AgeX borrowed $3,500,000 of the available credit under the Secured Note. As of June 30, 2023, AgeX had borrowed a total of $16,160,000 under the Secured Note. During July 2023 the $17,992,800 of Secured Note indebtedness, including $16,160,000 borrowed as of June 30, 2023 plus $500,000 borrowed during July 2023 and accrued loan origination fees, was extinguished in exchange for shares of Series A Preferred Stock and Series B Preferred Stock pursuant to the Exchange Agreement. See Note 12, Subsequent Events.
As an arrangement fee for the Secured Note, AgeX will pay Juvenescence an origination fee in an amount equal to 4% of the amount each draw of loan funds, which will accrue as each draw is funded, and an additional 4% of all the total amount of funds drawn that will accrue following the end of the period during which funds may be drawn from the line of credit. The origination fee will become due and payable on the repayment date or in a pro rata amount with any prepayment of in whole or in part of the outstanding principal balance of the Secured Note. See Note 12, Subsequent Events, regarding the exchange of indebtedness, including accrued origination fees, by Juvenescence for AgeX preferred stock.
2022 Warrants – Upon each drawdown of funds under the Secured Note prior to June 2, 2023 when the Third Amendment went into effect, AgeX issued to Juvenescence warrants to purchase shares of AgeX common stock (“2022 Warrants”). The 2022 Warrants are governed by the terms of a Warrant Agreement between AgeX and Juvenescence. The number of 2022 Warrants issued with respect to each draw of loan funds was equal to 50% of the number determined by dividing the amount of the applicable loan draw by the applicable Market Price. The Market Price was the last closing price per share of AgeX common stock on the NYSE American o preceding the delivery of the notice from AgeX requesting the draw of funds that triggered the obligation to issue 2022 Warrants. The exercise price of the 2022 Warrants is the applicable Market Price used to determine the number of Warrants issued. The 2022 Warrants will expire at 5:00 p.m. New York time three years after the date of issue.
During the six months ended June 30, 2023, AgeX issued to Juvenescence 2022 Warrants to purchase 1,898,489 shares of AgeX Common Stock. As of June 30, 2023, AgeX had issued to Juvenescence 2022 Warrants to purchase a total of 10,357,086 shares of AgeX common stock. The exercise prices of the 2022 Warrants issued through June 30, 2023 range from $0.59 per share to $0.88 per share representing the market closing price of AgeX common stock on the NYSE American on the one day prior to delivery of the drawdown notices. The number of shares issuable upon exercise of the warrants and the exercise price per share are subject to adjustment upon the occurrence of certain events such as a stock split or reverse split or combination of the common stock, stock dividend, recapitalization or reclassification of the common stock, and similar events.
Conversion of Loan Amounts to Common Stock – In lieu of repayment of funds borrowed, AgeX may convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. The conversion price per share or units shall be the lowest price at which shares or units are sold. Juvenescence may convert the loan balance in whole or in part into AgeX common stock at any time at Juvenescence’s election at the closing price per share of AgeX common stock on the NYSE American or other national securities exchange on the date prior to the date Juvenescence gives AgeX notice Juvenescence’s election to convert the loan or a portion thereof into common stock.
Default Provisions – The loan balance and origination fees may become immediately due and payable prior to the mandatory repayment date if an Event of Default occurs. Events of Default under the Secured Note include the following: (a) AgeX fails to pay any principal amount payable by it in the manner and at the time provided under and in accordance with the Secured Note; (b) AgeX fails to pay any other amount payable by it in the manner and at the time provided under and in accordance with the Secured Note or the Security Agreement described below or any other agreement executed in connection with the Secured Note (the “Loan Documents”) and the failure is not remedied within three business days; (c) AgeX fails to perform any of its covenants or obligations or fail to satisfy any of the conditions under the Secured Note or any other Loan Document and, such failure (if capable of remedy) remains unremedied to the satisfaction of Juvenescence (in its sole discretion) for 10 business days after the earlier of (i) notice requiring its remedy has been given by Juvenescence to AgeX and (ii) actual knowledge of the failure by senior officers of AgeX; (d) if any indebtedness of AgeX in excess of $100,000 becomes due and payable, or a breach or other circumstance arises thereunder such that Juvenescence is entitled to declare such indebtedness due and payable, prior to its due date, or any indebtedness of AgeX in excess of $25,000 is not paid on its due date; (e) AgeX stops payment of its debts generally or ceases or threatens to cease to carry on its business or is unable to pay its debts as they fall due or is deemed by a court of competent jurisdiction to be unable to pay its debts as they fall due, or enters into any arrangements with its creditors generally; (f) if (i) an involuntary proceeding (other than a proceeding instituted by Juvenescence or an affiliate of Juvenescence) shall be commenced or an involuntary petition shall be filed seeking liquidation, reorganization or other relief in respect of AgeX and any subsidiary, or of all or a substantial part of its assets, under any federal, state or foreign bankruptcy, insolvency, receivership or similar law now or hereafter in effect or (ii) an involuntary appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for AgeX or a subsidiary or for a substantial part of its assets occurs (other than in a proceeding instituted by Juvenescence or an affiliate of Juvenescence), and, in any such case, such proceeding shall continue undismissed and unstayed for sixty (60) consecutive days without having been dismissed, bonded or discharged or an order of relief is entered in any such proceeding; (g) it becomes unlawful for AgeX to perform all or any of its obligations under the Secured Note or any authorization, approval, consent, license, exemption, filing, registration or other requirement of any governmental, judicial or public body or authority necessary to enable AgeX to comply with its obligations under the Secured Note or to carry on its business is not obtained or, having been obtained, is modified in a manner that precludes AgeX or its subsidiaries from conducting their business in any material respect, or is revoked, suspended, withdrawn or withheld or fails to remain in full force and effect; (h) the issuance or levy of any judgment, writ, warrant of attachment or execution or similar process against all or any material part of the property or assets of AgeX or a subsidiary if such process is not released, vacated or fully bonded within 60 calendar days after its issue or levy; (i) any injunction, order, judgment or decision of any court is entered or issued which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX or a subsidiary to carry on its business or to pay amounts owed to Juvenescence under the Secured Note; (j) AgeX, whether in a single transaction or a series of related transactions, sells, leases, licenses, consigns, transfers or otherwise disposes of any material portion of its assets (with any such disposition with respect to any asset or assets with a fair value of at least $250,000 being deemed material), other than (i) certain permitted investments (ii) sales, transfers and dispositions of inventory in the ordinary course of business, (iii) any termination of a lease of real or personal property that is not necessary in the ordinary course of the AgeX’s business, could not reasonably be expected to have a material adverse effect and does not result from AgeX’s default, and (iv) any sale, lease, license, consignment, transfer or other disposition of assets that are no longer necessary in the ordinary course of business or which has been approved in writing by Juvenescence; (k) any of the following shall occur: (i) the security and/or liens created by the Security Agreement or any other Loan Document shall at any time cease to constitute valid and perfected security and/or liens on any material portion of the collateral intended to be covered thereby; (ii) except for expiration in accordance with its terms, the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall for whatever reason be terminated or shall cease to be in full force and effect; (iii) the enforceability of the Security Agreement or any other Loan Document pursuant to which a lien is granted by AgeX in favor of Juvenescence shall be contested by AgeX or a subsidiary; (iv) AgeX shall assert that its obligations under the Secured Note or any other Loan Document shall be invalid or unenforceable; or (v) a loss, theft, damage or destruction occurs with respect to a material portion of the collateral; (l) there is any change in the financial condition of AgeX and its subsidiaries which, in the opinion of Juvenescence, materially and adversely affects, or is reasonably likely so to affect, the ability of AgeX to perform any of its obligations under the Secured Note; and (m) any representation, warranty or statement made, repeated or deemed made or repeated by AgeX in the Secured Note, or pursuant to the Loan Documents, is incomplete, untrue, incorrect or misleading in any material respect when made, repeated or deemed made.
Restrictive Covenants – The Secured Note includes certain covenants that among other matters such as financial reporting: (i) impose financial restrictions on AgeX while the Secured Note remains unpaid, including restrictions on the incurrence of additional indebtedness by AgeX and its subsidiaries, except that AgeX’s subsidiary Reverse Bio will be permitted to incur debt convertible into equity not guaranteed or secured by the assets of AgeX or any other AgeX subsidiary, and the restrictions on the incurrence of indebtedness applicable to Reverse Bio will end if it raises more than $15 million in debt or equity financing by October 31, 2023 (ii) require that AgeX use loan proceeds and funds that may be raised through certain equity offerings only for research and development work, professional and administrative expenses, for general working capital, and for repayment of all or a portion of AgeX’s indebtedness to Juvenescence; and (iii) prohibit AgeX from making additional investments in subsidiaries, unless AgeX obtains the written consent of Juvenescence to a transaction that otherwise would be prohibited or restricted.
Security Agreement – AgeX has entered into a Security Agreement granting Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default occurs, Juvenescence will have the right to foreclose on the assets pledged as collateral.
$10 Million Secured Convertible Promissory Note
On March 13, 2023, AgeX and Juvenescence entered into a $10 Million Secured Convertible Promissory Note (the “$10 Million Secured Note”) pursuant to which Juvenescence has loaned to AgeX $10,000,000. AgeX used the loan proceeds to finance the $10,000,000 loan to Serina under the Serina Note. See Note 4, Convertible Note Receivable, for further information on the Serina Note and the related Serina Note Purchase Agreement. See Note 12, Subsequent Events, for debt exchanged for preferred stock on July 24, 2023.
The outstanding principal balance of the $10 Million Secured Note was scheduled to become due and payable on March 13, 2026. In lieu of accrued interest, AgeX will pay Juvenescence an origination fee in an amount equal to 7% of the loan funds disbursed to AgeX, which will accrue in two installments. The origination fee will become due and payable on the earliest to occur of (i) conversion of the $10 Million Secured Note into shares of AgeX common stock, (ii) repayment of the $10 Million Secured Note in whole or in part (provided that the origination fee shall be prorated for the amount of any partial repayment), and (iii) the acceleration of the maturity date of the $10 Million Secured Note following an Event of Default as defined in the $10 Million Secured Note.
If (a) AgeX and Serina have not entered into a definitive merger agreement by August 31, 2023; (b) a merger between AgeX and Serina is terminated or either party gives notice to terminate the merger agreement; or (c) the merger is not consummated by March 13, 2024, then AgeX may, after written notice to Juvenescence, pay and satisfy in full any unpaid portion of the principal balance and accrued origination fees under the $10 Million Secured Note by tendering to Juvenescence the Serina Note and shares of capital stock of Serina, if any, that may have been issued to AgeX upon conversion of the Serina Note in whole or in part.
AgeX may convert the loan balance and any accrued but unpaid origination fee into AgeX common stock or “units” if AgeX consummates a sale of common stock (or common stock paired with warrants or other convertible securities in “units”) in which the gross sale proceeds are at least $10,000,000. If less than $25,000,000 is raised through the sale of common stock or units, the conversion price per share or units shall be the lowest price at which shares or units are sold. If at least $25,000,000 is raised, the conversion price per share shall be 85% of the “Market Price” of AgeX common stock determined as provided in the $10 Million Secured Note. AgeX evaluated the 15% discounted conversion feature of the $10 Million Secured Note under ASC 815-15, Derivatives and Hedging—Embedded Derivatives, and concluded that it was an embedded derivative which should be bifurcated from the $10 Million Secured Note and accounted for separately. The 15% discount was determined to have an immaterial value at inception and life to date of the $10 Million Secured Note, as the probability of a future financing event described above is remote. The likelihood of the future qualifying event will be evaluated at the end of each reporting period and any adjustments will be included in Interest (income) expense, net in the Other (income) expense, net section of the condensed consolidated statements of operations.
Juvenescence may convert the outstanding principal amount of the $10 Million Secured Note plus the accrued origination fee into AgeX common stock at the market price per share of AgeX common stock. Juvenescence may not convert the $10 Million Secured Note to AgeX common stock before the earlier of (i) a merger between AgeX and Serina, and (ii) March 13, 2024. Any conversion of the $10 Million Secured Note into AgeX common stock is subject to certain restrictions to comply with applicable requirements of the NYSE American where AgeX common stock is listed. See Note 12, Subsequent Events concerning an amendment to the $10 Million Secured Note.
The $10 Million Secured Note includes certain covenants that among other matters require financial reporting and impose certain restrictions on AgeX that are substantially the same as those under the Secured Note.
During July 2023 the $10 Million Secured Note indebtedness, plus a portion of the accrued loan origination fees, was extinguished pursuant to the Exchange Agreement. See Note 12, Subsequent Events.
AgeX has entered into an Amended and Restated Security Agreement that amends the February 14, 2022 Security Agreement between AgeX and Juvenescence and adds the $10 Million Secured Note to the obligations secured by the Security Agreement. The Security Agreement grants Juvenescence a security interest in substantially all of the assets of AgeX, including a security interest in shares of AgeX subsidiaries that hold certain assets, as collateral for AgeX’s loan obligations. If an Event of Default as defined in the $10 Million Secured Note occurs, Juvenescence will have the right to foreclose on the assets pledged as collateral.
Registration Rights
AgeX entered into certain Registration Rights Agreements, as amended, pursuant to which AgeX has agreed to register for sale under the Securities Act of 1933, as amended (the “Securities Act”) all shares of AgeX common stock presently held by Juvenescence or that may be acquired by Juvenescence through the exercise of common stock purchase warrants that they hold or that they may acquire pursuant to the 2020 Loan Agreement and pursuant to the Secured Note, and shares that they may acquire through the conversion of those loans into AgeX common stock. AgeX has filed a registration statement on Form S-3, which has become effective under the Securities Act, for offerings on a delayed or continuous basis covering 16,447,500 shares of AgeX common stock held by Juvenescence and 3,248,246 shares of AgeX common stock that may be issued upon the exercise of warrants held by Juvenescence. Juvenescence retains the right to require AgeX to register additional shares of common stock that Juvenescence may acquire through the exercise of warrants or the conversion of loans. AgeX is obligated to pay the fees and expenses of each registered offering under such registration rights agreement except for underwriting discounts and commissions. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
Debt Issuance Costs
In accordance with ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs, all debt issuance costs are recorded as a discount on the debt and amortized to interest expense over the term of the applicable loan agreement using the effective interest method. Direct debt issuance costs include but are not limited to legal fees, debt origination fees, estimated fair market value of common stock and warrants issued in connection with the loan agreement, and NYSE American additional listing fees for the underlying shares of warrants issued with each drawdown of funds.
The following table summarizes the debt issuance costs and the debt balances net of debt issuance costs by loan agreement as of June 30, 2023 (in thousands):
Schedule of Debt Issuance Costs and Debt Balances
| | | Drawdown of Funds | | | Origination Fee | | | Total Debt | | | Debt Issuance Costs | | | Amortization of Debt Issuance Costs | | | Total Debt, Net | |
| Current | | | | | | | | | | | | | | | | | | | | | | | | |
| 2020 Loan Agreement | | $ | 8,000 | | | $ | - | | | $ | 8,000 | | | $ | (2,806 | ) | | $ | 2,806 | | | $ | 8,000 | |
| Secured Note | | | 16,160 | | | | 1,258 | | | | 17,418 | | | | (5,909 | ) | | | 3,434 | | | | 14,943 | |
| Total current, net | | | 24,160 | | | | 1,258 | | | | 25,418 | | | | (8,715 | ) | | | 6,240 | | | | 22,943 | |
| Non-current | | | | | | | | | | | | | | | | | | | | | | | | |
| $10 Million Secured Note | | | 10,000 | | | | 384 | | | | 10,384 | | | | (350 | ) | | | 34 | | | | 10,068 | |
| Total debt, net | | $ | 34,160 | | | $ | 1,642 | | | $ | 35,802 | | | $ | (9,065 | ) | | $ | 6,274 | | | $ | 33,011 | |
Related Party Payables
Since October 2018, AgeX’s Chief Operating Officer (“COO”), who is also an employee of Juvenescence, is devoting a majority of his time to AgeX’s operations. AgeX reimburses Juvenescence for his services on an agreed-upon fixed annual amount of approximately $280,000. AgeX reimburses Juvenescence for services provided by other Juvenescence employees on a work order basis under a shared services agreement effective January 1, 2023. As of June 30, 2023 and December 31, 2022, AgeX had approximately $230,000 and $141,000, respectively, payable to Juvenescence included in related party payables, net, on the condensed consolidated balance sheets.
Indemnification Agreements
On March 13, 2023, AgeX executed that certain Letter of Indemnification in Lieu of or Supplemental to a Medallion Signature Guarantee (“Letter of Indemnification”), pursuant to which AgeX agreed to indemnify American Stock Transfer & Trust Company, LLC and its affiliates, successors and assigns (the “AST Indemnity”) from and against any and all claims, damages, liabilities or losses arising out of the transfer of all of the AgeX common stock held by Juvenescence to its wholly-owned subsidiary, Juvenescence US Corp. (the “Share Transfer”). In connection with AgeX’s execution of the Letter of Indemnification, AgeX and Juvenescence entered into that certain Transfer of Shares of AgeX Therapeutics, Inc. Common Stock – Indemnification Agreement, pursuant to which Juvenescence agreed to indemnify AgeX against any and all claims, damages, liabilities or losses arising out of the Share Transfer or AST Indemnity.
6. Warrant Liability
AgeX determines the accounting classification of warrants it issues, as either liability or equity, by first assessing whether the warrants meet liability classification in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity, then in accordance with ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock. Under ASC 480, Distinguishing Liabilities from Equity, warrants are considered liability classified if the warrants are mandatorily redeemable, obligate AgeX to settle the warrants or the underlying shares by paying cash or other assets, or warrants that must or may require settlement by issuing a variable number of shares. If warrants do not meet liability classification under ASC 480-10, AgeX assesses the requirements under ASC 815-40, which states that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability classification under ASC 815-40, and in order to conclude equity classification, AgeX also assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable U.S. GAAP.
As a condition of each amount drawn up to $15,160,000 from the Secured Note, on receipt of each amount drawn AgeX granted to Juvenescence a number of warrants equal to 50% of the gross value of the relevant advance made. The gross value is the quotient of the drawdown amount and the exercise price. The exercise price was based on the market closing price of AgeX’s common stock on the NYSE American on the one day preceding the delivery of the relevant drawdown notice. See Note 5, Related Party Transactions.
AgeX has utilized the full credit available under the Secured Note that is subject to warrants and accordingly the warrants were issued for each of the advances of loan funds under the Secured Note. After all relevant assessments, AgeX determined that the warrants issued under the Secured Note require classification as a liability pursuant to ASC 480, Distinguishing Liabilities from Equity. In accordance with the accounting guidance, for each reporting period prior to the full drawdown of the entire $15,160,000 of the Secured Note line of credit subject to warrants, the amount of warrant liability was determined and recognized on the balance sheet for the applicable reporting period based on the number of warrants that would have been issued if $15,160,000 of the Secured Note line of credit was drawn. The amount of warrant liability attributed to the expected future issuance of warrants upon subsequent loan draws was subsequently adjusted for the fair value of warrants actually issued upon each loan draw, and the number of warrants that could be issued for the remaining credit available was re-measured for the applicable reporting period with changes being recorded as a component of net other expense in the condensed consolidated statements of operations.
Under the Third Amendment, AgeX is not obligated to issue additional warrants to Juvenescence in connection with the receipt of loan funds up to $4 million made available pursuant to the Second Amendment. See Note 5, Related Party Transactions, for further details of the Second Amendment and the Third Amendment.
The fair value of the warrant liabilities was measured using a Black-Scholes option pricing model. Significant inputs into the model at the inception date, the date when warrants were issued upon receipt of amounts drawn during the period, and as of the reporting period end remeasurement dates are as follows:
Schedule of Warrants Liabilities And Stock Option Awards using a Black-Scholes Option-Pricing Model
| Black-Scholes Assumptions | | Exercise Price (1) | | | Warrant Expiration Date (2) | | Stock Price (3) | | | Interest Rate (annual)(4) | | | Volatility (annual) (5) | | | Time to Maturity (Years) | | | Calculated Fair Value per Share | |
| Inception Date: 2/14/2022 | | $ | 0.780 | | | 2/13/2025 | | $ | 0.691 | | | | 1.80 | % | | | 122.99 | % | | | 3 | | | $ | 0.486 | |
| Issuance Date: 2/14/2022 | | $ | 0.780 | | | 2/13/2025 | | $ | 0.691 | | | | 1.80 | % | | | 122.99 | % | | | 3 | | | $ | 0.486 | |
| Issuance Date: 2/15/2022 | | $ | 0.780 | | | 2/14/2025 | | $ | 0.747 | | | | 1.80 | % | | | 123.28 | % | | | 3 | | | $ | 0.535 | |
| Period Ended 3/31/2022 | | $ | 0.940 | | | 3/30/2025 | | $ | 0.854 | | | | 2.45 | % | | | 123.28 | % | | | 3 | | | $ | 0.607 | |
| Issuance Date: 4/4/2022 | | $ | 0.880 | | | 4/3/2025 | | $ | 0.819 | | | | 2.61 | % | | | 123.31 | % | | | 3 | | | $ | 0.585 | |
| Issuance Date: 6/6/2022 | | $ | 0.711 | | | 6/5/2025 | | $ | 0.800 | | | | 2.94 | % | | | 122.62 | % | | | 3 | | | $ | 0.592 | |
| Period Ended 6/30/2022 | | $ | 0.600 | | | 6/29/2025 | | $ | 0.576 | | | | 2.99 | % | | | 122.21 | % | | | 3 | | | $ | 0.413 | |
| Issuance Date: 8/16/2022 | | $ | 0.670 | | | 8/15/2025 | | $ | 0.640 | | | | 3.19 | % | | | 121.37 | % | | | 3 | | | $ | 0.457 | |
| Period Ended 9/30/2022 | | $ | 0.610 | | | 9/29/2025 | | $ | 0.562 | | | | 4.25 | % | | | 121.49 | % | | | 3 | | | $ | 0.401 | |
| Issuance Date: 10/21/2022 | | $ | 0.690 | | | 10/20/2025 | | $ | 0.620 | | | | 4.52 | % | | | 120.51 | % | | | 3 | | | $ | 0.439 | |
| Issuance Date: 12/14/2022 | | $ | 0.590 | | | 12/13/2025 | | $ | 0.540 | | | | 3.94 | % | | | 120.01 | % | | | 3 | | | $ | 0.381 | |
| Period Ended 12/31/2022 | | $ | 0.550 | | | 12/30/2025 | | $ | 0.552 | | | | 4.22 | % | | | 119.31 | % | | | 3 | | | $ | 0.396 | |
| Issuance Date: 1/25/2023 | | $ | 0.735 | | | 1/24/2026 | | $ | 0.751 | | | | 3.84 | % | | | 119.17 | % | | | 3 | | | $ | 0.540 | |
| Inception Date: 2/9/2023 | | $ | 0.703 | | | 2/8/2026 | | $ | 0.660 | | | | 4.15 | % | | | 118.94 | % | | | 3 | | | $ | 0.466 | |
| Issuance Date: 2/15/2023 | | $ | 0.624 | | | 2/14/2026 | | $ | 0.600 | | | | 4.35 | % | | | 118.93 | % | | | 3 | | | $ | 0.426 | |
| Period Ended 3/31/2023 | | $ | 0.661 | | | 3/30/2026 | | $ | 0.663 | | | | 3.81 | % | | | 113.43 | % | | | 3 | | | $ | 0.459 | |
| Issuance Date: 4/4/2023 | | $ | 0.661 | | | 4/3/2026 | | $ | 0.673 | | | | 3.60 | % | | | 113.01 | % | | | 3 | | | $ | 0.466 | |
| (1) | Based on the market closing price of AgeX’s common stock on the NYSE American on the day prior to each debt Inception Date, on each presented period ending date, and one day prior to the delivery of the relevant drawdown notice in accordance with terms of the Secured Note (with such drawdown notice delivery date being shown as the Issuance Date in the table). For this purpose, the date on which the Secured Note was amended and restated to increase the line of credit by $2,000,000 was treated as a new Inception Date for that portion of the line of credit. |
| (2) | Warrants are exercisable over a three-year period from each Issuance Date. |
| (3) | Based on the market price of AgeX’s common stock on the NYSE American as of each date presented. |
| (4) | Interest rate for U.S. Treasury Bonds, as of each date presented, as published by the U.S. Federal Reserve. |
| (5) | Based on the historical daily volatility of AgeX common stock as of each date presented. |
The warrants outstanding and fair values at each of the respective valuation dates are summarized below:
Schedule of Warrant Outstanding and Fair Values
| Warrant Liability | | Credit Line and Draw Amounts (in thousands) | | | Warrants | | | Fair Value per Share | | | Fair Value (in thousands) | |
| Fair value as of January 1, 2022 | | $ | - | | | | - | | | $ | - | | | $ | - | |
| Fair value at initial measurement date of 2/14/2022 | | | 13,160 | (1) | | | 8,435,897 | (2) | | | 0.4864 | | | | 4,103 | |
| Fair value of warrants issued on 2/14/2022 | | | (7,160 | )(3) | | | (4,589,743 | )(4) | | | 0.4864 | | | | (2,232 | ) |
| Fair value of warrants issued on 2/15/2022 | | | (1,000 | )(3) | | | (641,025 | )(4) | | | 0.5349 | | | | (343 | ) |
| Fair value of warrants issued on 4/4/2022 | | | (1,000 | )(3) | | | (568,440 | )(4) | | | 0.5854 | | | | (333 | ) |
| Fair value of warrants issued on 6/6/2022 | | | (1,000 | )(3) | | | (703,234 | )(4) | | | 0.5924 | | | | (417 | ) |
| Fair value of warrants issued on 8/16/2022 | | | (1,000 | )(3) | | | (746,380 | )(4) | | | 0.4569 | | | | (341 | ) |
| Fair value of warrants issued on 10/21/2022 | | | (500 | )(3) | | | (362,318 | )(4) | | | 0.4386 | | | | (159 | ) |
| Fair value of warrants issued on 12/14/2022 | | | (1,000 | )(3) | | | (847,457 | )(4) | | | 0.3810 | | | | (323 | ) |
| Change in fair value of warrants | | | - | | | | - | | | | - | | | | 225 | |
| Fair value as of December 31, 2022 | | $ | 500 | (1) | | | 454,545 | (2) | | $ | 0.3960 | | | $ | 180 | |
| Fair value of warrants issued on 1/25/2023 | | | (500 | )(3) | | | (340,136 | )(4) | | | 0.5395 | | | | (184 | ) |
| Fair value at initial measurement date of 2/9/2023 | | | 2,000 | (1) | | | 1,422,879 | (2) | | | 0.4657 | | | | 663 | |
| Fair value of warrants issued on 2/15/2023 | | | (1,000 | )(3) | | | (801,924 | )(4) | | | 0.4263 | | | | (342 | ) |
| Fair value of warrants issued on 4/4/2023 | | | (1,000 | )(3) | | | (756,429 | )(4) | | | 0.4660 | | | | (352 | ) |
| Change in fair value of warrants | | | - | | | | - | | | | - | | | | 35 | |
| Fair value as of June 30, 2023 | | $ | - | (1) | | | - | (2) | | $ | - | | | $ | - | |
| (1) | Amount of credit available under the Secured Note on date of inception and as of each period end date. For this purpose, the date on which the Secured Note was amended and restated to increase the line of credit by $2,000,000 was treated as a new Inception Date for that portion of the line of credit. |
| (2) | Number of warrants issuable, as applicable, (a) if the amount of credit available was drawn for measurement as of the applicable inception date, or (b) subsequently for remeasurement as of each period end date. |
| (3) | Amount of drawdown as of the date presented. |
| (4) | Number of warrants issued upon receipt of amounts drawn against the Secured Note as of the date presented. |
During the three and six months ended June 30, 2023, AgeX recorded a loss on change in fair value of warrants of $5,000 and $35,000, respectively. During the three and six months ended June 30, 2022, AgeX recorded a loss on change in fair value of warrants of $168,000 and $255,000, respectively.
The warrant liabilities are considered Level 3 liabilities on the fair value hierarchy as the determination of fair value includes various assumptions about future activities and AgeX’s stock prices and historical volatility as inputs. None of the warrants issued have been exercised.
7. Stockholders’ Deficit
Preferred Stock
AgeX is authorized to issue up to 5,000,000 shares of $0.0001 par value preferred stock. At June 30, 2023 and December 31, 2022, there were no preferred shares issued and outstanding. See Note 12, Subsequent Events, for information concerning the issuance of shares of Series A Preferred Stock and Series B Preferred Stock in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence.
Common Stock
AgeX has 200,000,000 shares of $0.0001 par value common stock authorized. At June 30, 2023 and December 31, 2022, there were 37,951,261 and 37,949,196 shares of AgeX common stock issued and outstanding, respectively.
Issuance and Sale of Warrants by AgeX
In connection with the $2,500,000 of drawdowns of loan funds from Juvenescence under the Secured Note during the six months ended June 30, 2023, AgeX issued to Juvenescence 2022 Warrants to purchase 1,898,489 shares of AgeX common stock. See Note 6, Warrant Liability.
Pursuant to the Third Amendment, no warrants were issued in connection with the $1,000,000 of additional drawdowns of loan funds after June 1, 2023. See Note 5, Related Party Transactions, for further details of the Second Amendment and the Third Amendment.
At-the-Market Offering Facility
On January 8, 2021, AgeX entered into a sales agreement with Chardan relating to the sale of shares of AgeX common stock, par value $0.0001 per share, through an at-the-market (“ATM”) offering as described in the prospectus supplement filed with the Form S-3 which was declared effective by the SEC on January 29, 2021. In accordance with the terms of the sales agreement, AgeX may offer and sell shares of AgeX common stock having an aggregate offering price of up to $12.6 million from time to time through Chardan, acting as the sales agent. The actual market value of shares of common stock that AgeX may sell through the ATM offering during any 12 month period will be limited to one-third of the aggregate market value of AgeX common stock held by stockholders that would not be considered “affiliates” of AgeX, determined in accordance with applicable SEC rules. During the six months ended June 30, 2023 and 2022, no proceeds were raised through the sale of shares of common stock under the ATM.
8. Stock-Based Awards
Equity Incentive Plan Awards
AgeX has an Equity Incentive Plan (the “Plan”) under which a maximum of 8,500,000 shares of common stock are available for the grant of stock options, the sale of restricted stock, the settlement of restricted stock units, and the grant of stock appreciation rights. The Plan also permits AgeX to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine.
A summary of AgeX stock option activity under the Plan and related information follows (in thousands, except weighted average exercise price):
Summary of Stock Option Activity
| | | Shares Available for Grant | | | Number of Options Outstanding | | | Number of RSUs Outstanding | | | Weighted- Average Exercise Price | |
| Balance at December 31, 2022 | | | 5,139 | | | | 3,261 | | | | 3 | | | $ | 2.25 | |
| Restricted stock units vested | | | - | | | | - | | | | (3 | ) | | | - | |
| Balance at June 30, 2023 | | | 5,139 | | | | 3,261 | | | | - | | | $ | 2.25 | |
| Options exercisable at June 30, 2023 | | | | | | | 3,016 | | | | | | | $ | 2.34 | |
There have been no exercises of stock options to date.
Stock-based Compensation Expense
AgeX recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with ASC 718, Compensation – Stock Compensation (“ASC 718”). AgeX estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures for grants prior to 2017, over the requisite service period. Upon adoption of ASU 2016-09 on January 1, 2017 as further discussed below, forfeitures are accounted for as they occur instead of based on the number of awards that were expected to vest prior to adoption of ASU 2016-09.
AgeX uses the Black-Scholes option pricing model for estimating the fair value of options granted under AgeX’s 2017 Equity Incentive Plan (the “Incentive Plan”). The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. AgeX has elected to treat stock-based payment awards with time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718. Stock option awards issued to non-employees, principally consultants or outside contractors, as applicable, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options and restricted stock units can more reliably be measured than the fair value of services received. AgeX records compensation expense based on the then-current fair values of the stock options and restricted stock units at the grant date. Compensation expense for non-employee grants is recorded on a straight-line basis in the consolidated statements of operations.
Operating expenses include stock-based compensation expense as follows (in thousands):
Schedule of Stock Based Compensation Expense
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| Research and development | | $ | 2 | | | $ | 8 | | | $ | 7 | | | $ | 17 | |
| General and administrative | | | 33 | | | | 190 | | | | 98 | | | | 420 | |
| Total stock-based compensation expense | | $ | 35 | | | $ | 198 | | | $ | 105 | | | $ | 437 | |
The fair value of each option award is estimated on the date of grant using a Black-Scholes option pricing model applying the weighted-average assumptions including expected life, risk-free interest rates, volatility, and dividend yield. The assumptions that were used to calculate the grant date fair value of employee and non-employee stock option grants for the three and six months ended June 30, 2022 were as follows:
Schedule of Weighted Average Assumptions to Calculate Fair Value of Stock Options
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2023(1) | | | 2022 | | | 2023(1) | | | 2022 | |
| Grant price | | $ | - | | | $ | 0.71 | | | $ | - | | | $ | 0.79 | |
| Market price | | $ | - | | | $ | 0.71 | | | $ | - | | | $ | 0.79 | |
| Expected life (in years) | | | - | | | | 6.08 | | | | - | | | | 5.58 | |
| Volatility | | | - | | | | 128.35 | % | | | - | | | | 130.71 | % |
| Risk-free interest rates | | | - | | | | 2.90 | % | | | - | | | | 1.74 | % |
| Dividend yield | | | - | | | | - | % | | | - | | | | - | % |
| (1) | There were no stock options granted under the Plan during the three and six months ended June 30, 2023. |
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If AgeX had made different assumptions, its stock-based compensation expense and net loss for the six months ended June 30, 2023 and 2022 may have been significantly different.
AgeX does not recognize deferred income taxes for incentive stock option compensation expense and records a tax deduction only when a disqualified disposition has occurred.
9. Income Taxes
The provision for income taxes for interim periods is determined using an estimated annual effective tax rate in accordance with ASC 740-270, Income Taxes, Interim Reporting. The effective tax rate may be subject to fluctuations during the year as new information is obtained, which may affect the assumptions used to estimate the annual effective tax rate, including factors such as valuation allowances against deferred tax assets, the recognition or de-recognition of tax benefits related to uncertain tax positions, if any, and changes in or the interpretation of tax laws in jurisdictions where AgeX conducts business.
For the three and six months ended June 30, 2023 and 2022, AgeX experienced a loss; therefore, no income tax provision was recorded for the three and six months ended June 30, 2023 and 2022.
Due to losses incurred for all periods presented, AgeX did not record a provision or benefit for income taxes. A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. AgeX established a full valuation allowance for all of its deferred tax assets for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
10. Supplemental Cash Flow Information
Non-cash investing and financing transactions presented separately from the condensed consolidated statements of cash flows for the six months ended June 30, 2023 and 2022 are as follows (in thousands):
Schedule of Non-cash Investing and Financing Transactions
| | | 2023 | | | 2022 | |
| | | Six Months Ended June 30, | |
| | | 2023 | | | 2022 | |
| Cash paid during the period for interest | | $ | 23 | | | $ | 12 | |
| Issuance of common stock upon vesting of restricted stock units (Note 8) | | $ | 2 | | | $ | 5 | |
| Issuance of warrants for debt issuance under the 2020 Loan Agreement | | $ | - | | | $ | 178 | |
| Fair value of liability classified warrants at debt inception date (Note 6) | | $ | 663 | | | $ | 3,325 | |
| Debt refinanced with new debt (Note 5) | | $ | - | | | $ | 7,160 | |
11. Commitments and Contingencies
Office Lease Agreement
AgeX leases office space in Alameda, California. For 2022 base monthly rent was $1,074 and for 2023 base monthly rent is $844 for slightly less space at the same building. The lease also includes office furniture rental, janitorial services, utilities, and internet service.
ASC 842
For the office lease, AgeX has elected to not apply the recognition requirements under ASC 842 as lease cost on a straight-line basis over the lease term because the amount of the lease payments is not deemed material.
There were no future minimum lease commitments as of June 30, 2023.
Litigation – General
AgeX is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When AgeX is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, AgeX will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, AgeX discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. AgeX is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Tax Filings
AgeX tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes AgeX has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the unaudited condensed consolidated interim financial statements.
Employment Contracts
AgeX has entered into employment contracts with certain executive officers. Under the provisions of the contracts, AgeX may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations.
Indemnification
In the normal course of business, AgeX may provide indemnifications of varying scope under AgeX’s agreements with other companies or consultants, typically for AgeX’s research and development programs. Pursuant to these agreements, AgeX will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with AgeX’s research and development. Indemnification provisions could also cover third-party infringement claims with respect to patent rights, copyrights, or other intellectual property licensed from AgeX to third parties. Office and laboratory leases will also generally indemnify the lessor with respect to certain matters that may arise during the term of the lease. The sales agreement between AgeX and Chardan also includes indemnification provisions pursuant to which the parties have agreed to indemnify each other from certain liabilities that could arise from the offer and sale of AgeX common stock through the ATM facility, including liabilities under the Securities Act. Similarly, the Registration Rights Agreement between Juvenescence and AgeX includes indemnification provisions pursuant to which the parties will indemnify each other from certain liabilities in connection with the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act. AgeX has also agreed to provide the AST Indemnity pursuant to the Letter of Indemnification described in Note 5, Related Party Transactions. The term of these indemnification obligations will generally continue in effect after the termination or expiration of the particular license, lease, or agreement to which they relate. The potential future payments AgeX could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, AgeX has not been subject to any claims or demands for indemnification. AgeX also maintains various liability insurance policies that limit AgeX’s financial exposure and in the case of the AST Indemnity AgeX has received a cross-indemnity from Juvenescence against all claims, damages, liabilities or losses arising out of the AST Indemnity. As a result, AgeX believes the fair value of these indemnification agreements is minimal. Accordingly, AgeX has not recorded any liabilities for these agreements to date.
Notice of Delisting
On April 20, 2023, AgeX received a letter (the “2023 Deficiency Letter”) from the staff of the Exchange indicating that AgeX does not meet certain of the Exchange’s continued listing standards as set forth in Sections 1003(a)(i), (ii), and (iii) of the Exchange Company Guide in that AgeX has stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and has incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and has reported losses from continuing operations and/or net losses in its five most recent fiscal years. The 2023 Deficiency Letter states that as AgeX remains subject to the conditions set forth in prior letters from the Exchange with respect to AgeX’s deficiencies in stockholders equity, and if AgeX is not in compliance with all of the Exchange’s stockholders equity standards, or does not make progress consistent with AgeX’s Exchange approved plan to come into compliance with the Exchange’s continued listing standards, by May 17, 2023, the Exchange will initiate delisting proceedings as appropriate.
On May 17, 2023 AgeX received a notice from the staff of the Exchange indicating that they intend to commence proceedings to delist AgeX common stock from the Exchange based upon AgeX’s non-compliance with the stockholders’ equity requirements set forth in Sections 1003(a)(i), (ii) and (iii) of the Exchange’s Company Guide by the end of a compliance plan period that expired on May 17, 2023. Specifically, AgeX does not meet the continued listing standards because it has stockholders equity of less than (A) $2,000,000 and has incurred losses from continuing operations and/or net losses during its two most recent fiscal years, (B) $4,000,000 and has incurred losses from continuing operations and/or net losses during three out of four of its most recent fiscal years, and (C) $6,000,000 or more and has reported losses from continuing operations and/or net losses in its five most recent fiscal years.
On May 24, 2023, AgeX filed a request for a review of the delisting determination by a Committee of the Board of Directors of the Exchange. On May 31, 2023, AgeX received a notice from the staff of the Exchange which scheduled a hearing for July 25, 2023.
On July 24, 2023, AgeX increased its stockholders equity and remediated the deficiency by issuing shares of preferred stock to Juvenescence in exchange for the extinguishment of $36 million of indebtedness owed to Juvenescence. See Note 12, Subsequent Events, for further details.
12. Subsequent Events
Additional Loans Under Secured Note
On July 5, 2023 and on August 1, 2023, AgeX drew $500,000 of its credit available under the Secured Note on each date.
Amendment of Secured Note
On July 31, 2023, AgeX and Juvenescence entered into a Fourth Amendment (the “Fourth Amendment”) to the Secured Note to provide that (i) the definition of Reverse Financing Condition is amended to extend to October 31, 2023 the referenced deadline for fulfillment of the condition to permit borrowing or other incurrence of indebtedness by AgeX’s subsidiary Reverse Bio, and (ii) Juvenescence may convert the outstanding amount of the Secured Note loans or any portion of such loans into AgeX common stock without restriction by the “19.9% Cap” if Juvenescence elects to convert those amounts at a conversion price or prices equal to the “Drawdown Market Prices” applicable to such loan amounts in lieu of a lower conversion price set with reference to the current market price of AgeX common stock at the time of conversion. The 19.9% Cap is a provision of the Secured Note that limits the amount of common stock that Juvenescence may acquire through the conversion of Secured Note loans in order to comply with NYSE American requirements pertaining to the amount of shares that a listed company, such as AgeX, may sell at a price less than the market prices prevailing at the time the loans were made (the “Drawdown Market Prices”) without shareholder approval.
Amendment of $10 Million Secured Note
On July 31, 2023, AgeX and Juvenescence also entered into an amendment to the $10 Million Secured Note that mirrors the amendments of the Secured Note described above, and also creates an earlier time window, ending October 31, 2023, during which Juvenescence may elect to convert any amount outstanding under the $10 Million Secured Note into shares of AgeX common stock. After October 31, 2023, Juvenescence may convert outstanding amounts under the $10 Million Secured Note into AgeX common stock on any date more than ninety (90) days after the earlier of (a) the occurrence of a Qualified Merger as defined, and (b) March 13, 2024.
Debt Exchanged for Preferred Stock and Remediation of Stock Exchange Listing Deficiency
On July 24, 2023, AgeX issued to Juvenescence 211,600 shares of a newly authorized Series A Preferred Stock and 148,400 shares of a newly authorized Series B Preferred Stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of loans then outstanding under the 2020 Loan Agreement, the Secured Note, and the $10 Million Secured Note, plus the loan origination fees accrued with respect to the 2022 Secured Note and a portion of the loan origination fees accrued pursuant to the $10 Million Secured Note. The cancellation of indebtedness in exchange for the Preferred Stock was conducted pursuant to the Exchange Agreement between AgeX and Juvenescence. By completing the exchange of indebtedness for shares of Series A Preferred Stock and Series B Preferred Stock (collectively referred to as the “Preferred Stock”), AgeX now has sufficient stockholders equity to meet the NYSE American continued listing requirements to have at least $6 million of stockholders’ equity. Accordingly, the NYSE American staff withdrew its May 17, 2023 delisting determination and the scheduled hearing of AgeX’s appeal of that determination was cancelled.
The NYSE American approved the listing of the 36,939,190 shares of AgeX common stock into which the Preferred Stock is presently convertible. In order to comply with Section 713 of the NYSE American Company Guide, the issuance of an additional 13,060,809 shares of AgeX common stock upon conversion of shares of Series B Preferred Stock is currently restricted by a “cap” prohibiting issuance of those additional shares without the prior approval of AgeX stockholders.
Summary of Preferred Stock
The following is a summary of the principal terms of the Series A Preferred Stock and Series B Preferred Stock (collectively “Preferred Stock”). This discussion of the Preferred Stock is a summary only, does not purport to be complete, and is qualified in all respects by the full terms of the Series A Preferred Stock and Series B Preferred Stock, including the respective powers, designations, preferences, rights qualifications, limitations, and restrictions of each such series of Preferred Stock, are set forth in the respective forms of Certificate of Designation of each such series of Preferred Stock.
Dividends
The Preferred Stock is not entitled to receive any payment or distribution of cash or other dividends.
Liquidation Preference
In the event of any voluntary or involuntary liquidation, dissolution or other winding up of the affairs of AgeX, subject to the preferences and other rights of any senior stock, before any assets of AgeX shall be distributed to holders of common stock or other junior stock, all of the assets of AgeX available for distribution to stockholders shall be distributed among the holders of Series A Preferred Stock and Series B Preferred Stock and any other “parity stock” that may be issued ranking parri passu with those series of Preferred Stock with respect to liquidation rights, in proportion to the number of shares of Series B Preferred Stock and parity stock held by each such holder as of the record date for the determination of holders of Series A Preferred Stock, Series B Preferred Stock, and parity stock entitled to receive such distribution, until AgeX shall have distributed to the holders of those shares an amount of assets having a value equal to the subscription price per share. If the assets of AgeX shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the holders of Series A Preferred Stock, Series B Preferred Stock and parity stock shall be ratably distributed among such holders. The (i) acquisition of AgeX by another entity by means of any transaction or series of transactions (including, without limitation, any reorganization, merger or consolidation) in which the stockholders of AgeX immediately before such transaction or series of transactions do not own a majority of the outstanding stock of the surviving or acquiring corporation upon completion of such transaction or series of transactions or (ii) a sale of all or substantially all of the assets of AgeX in a single transaction or series of related transactions, shall be deemed a liquidation.
Conversion of Preferred Stock into Common Stock
Each share of Preferred Stock shall be convertible into a number of shares of AgeX common stock determined by dividing (x) a number equal to the number of dollars and cents comprising the subscription price, by (y) a number equal to the number of dollars and cents comprising the conversion price. The subscription price per share of Preferred Stock is $100 which was paid through the exchange of indebtedness for shares of Preferred Stock. The conversion price per share of Series A Preferred Stock or Series B Preferred Stock is $0.72 which was the closing price of AgeX common stock on the NYSE American on the last trading day immediately preceding the execution of the Exchange Agreement.
Optional Conversion.
Preferred Stock shall be convertible into common stock at the election of the holder of shares of Preferred Stock at any time and from time to time.
Automatic Conversion.
The outstanding shares of Series A Preferred Stock shall automatically be converted into common stock without any further act of AgeX or its stockholders (“Automatic Conversion”) upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina, or a subsidiary thereof; and (y) February 1, 2024. Further, if the holders of at least a majority of the outstanding shares of Series A Preferred Stock approve or consent to the Automatic Conversion of the shares of that series, then the outstanding shares of Series A Preferred Stock shall be converted into common stock upon such approval or consent.
The outstanding shares of Series B Preferred Stock shall automatically be converted into common stock without any further act of AgeX or its stockholders upon the earliest of: (x) the date on which AgeX or a subsidiary shall have consummated a merger with Serina or a subsidiary thereof; and (y) February 1, 2024, provided that such conversion is not limited by the 19.9% Cap or the 50% Cap as described below; and if Automatic Conversion would then be limited by the 19.9% Cap or the 50% Cap, the Automatic Conversion shall take place on the tenth day after such stockholder approvals have been obtained as may be required to permit such Automatic Conversion without the limitations of the 19.9% Cap and the 50% Cap. Further, if the holders of at least a majority of the outstanding shares of Series B Preferred Stock approve or consent to the Automatic Conversion of the shares of that series, and the conversion is not then limited by the 19.9% Cap or the 50% Cap, then the outstanding shares of Series B Preferred Stock shall be converted into common stock upon such approval or consent.
Certain Limitations on Conversion of Series B Preferred Stock
If under the rules of the NYSE American or any other national securities exchange on which AgeX common stock may be listed, approval by AgeX stockholders would be required in connection with the issuance of common stock in excess of the “19.9% Cap” upon any conversion of Series B Preferred Stock, then unless and until such stockholder approval has been obtained, the maximum number of shares of common stock that may be issued upon conversion of all shares of Series B Preferred Stock shall be an amount equal to the 19.9% Cap. The 19.9% Cap means 7,550,302 shares of common stock, which is 19.9% of the shares of common stock outstanding on February 14, 2022 when the Secured Note, a portion of which has not been approved by AgeX stockholders for conversion into common stock without regard to the 19.9% Cap and 50% Cap, was issued.
If under the rules of the NYSE American or any other national securities exchange on which AgeX common stock may be listed, approval by AgeX stockholders would be required in connection with the issuance of common stock in excess of the 50% Cap upon any conversion of Series B Preferred Stock, then unless and until such stockholder approval has been obtained, the maximum number of shares of common stock that may be issued to a holder of Series B Preferred Stock upon conversion of such shares shall be an amount that, when added to other shares of common stock owned by such holder immediately prior to such conversion would equal one share less than the 50% Cap.
Adjustment of conversion price and subscription price
If AgeX shall (a) declare a dividend or make a distribution on its common stock in shares of common stock, (b) subdivide or reclassify the outstanding common stock into a greater number of shares, or (c) combine or reclassify the outstanding common stock into a smaller number of shares, the conversion price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. If AgeX shall (i) declare a dividend or make a distribution on a series of Preferred Stock in shares of Preferred Stock, (ii) subdivide or reclassify the outstanding shares of a series of Preferred Stock into a greater number of shares, or (iii) combine or reclassify the outstanding shares of a series of Preferred Stock into a smaller number of shares, the subscription price in effect at the time of the record date for such dividend or distribution or the effective date of such subdivision, combination or reclassification shall be proportionately adjusted. Successive adjustments in the conversion price or subscription price, as applicable, shall be made whenever any event specified above shall occur.
No Fractional Shares
No fractional share of common stock or scrip shall be issued upon conversion of Preferred Stock. Instead of any fractional share of common stock which would otherwise be issuable upon conversion of any Preferred Stock, AgeX will pay a cash adjustment in respect of such fractional interest in an amount equal to that fractional interest at the then fair value determined in accordance with the terms of the Preferred Stock.
Voting Rights
The following matters shall require the approval of the holders of a majority of the shares of a series of Preferred Stock then outstanding, voting as a separate class: (i) creation of any Preferred Stock ranking as senior stock to the series with respect to liquidation preferences; (ii) repurchase of any shares of common stock or other junior stock except shares issued pursuant to or in connection with a compensation or incentive plan or agreement approved by the Board of Directors for any officers, directors, employees or consultants of AgeX; (iii) any sale, conveyance, or other disposition of all or substantially all AgeX’s property or business, or any liquidation or dissolution of AgeX, or a merger into or consolidation with any other corporation (other than a wholly-owned subsidiary corporation) but only to the extent that the Delaware General Corporation Law requires that such transaction be approved by each class or series of Preferred Stock; (iv) any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock; or (v) any amendment of AgeX’s Certificate of Incorporation or Bylaws that results in any adverse change in the powers, preferences and rights of, and the qualifications, limitations or restrictions on, the series of Preferred Stock. However, the terms of the Preferred Stock do not restrict or limit the rights and powers of the Board of Directors to fix by resolution the rights, preferences, and privileges of, and restrictions and limitations on, stock ranking as parity stock or junior stock to a series of Preferred Stock. Except as may otherwise be required by the Delaware General Corporation Law, as the same may be amended from time to time, the Preferred Stock will have no other voting rights.
Governing Law
The powers, designations, preferences, rights, qualifications, limitations, and restrictions of either series of Preferred Stock, the validity, authorization and issuance of such Preferred Stock, and the conversion of such Preferred Stock into common stock shall be governed by and construed and enforced in accordance with the internal laws of the State of Delaware, without regard to the principles of conflict of laws thereof, and all legal proceedings pursuant or with respect to or concerning such matters (a “Proceeding”), whether brought by or against a holder of Preferred Stock or AgeX or any of their respective directors, officers, stockholders, employees or agents, shall be commenced in the state and federal courts sitting in the State of Delaware (the “Delaware Courts”). The Preferred Stock provides that (a) AgeX and each holder of Preferred Stock irrevocably submits to the exclusive jurisdiction of the Delaware Courts for the adjudication of any Proceeding, and irrevocably waives, and agrees not to assert in any Proceeding any claim that they are not personally subject to the jurisdiction of such Delaware Courts, or such Delaware Courts are an improper or inconvenient venue for such Proceeding, and (b) AgeX and each holder of Preferred Stock irrevocably waives personal service of process and consents to process being served in any such Proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to such party and agrees that such service shall constitute good and sufficient service of process and notice
Registration Rights Agreement
AgeX and Juvenescence have entered into a Registration Rights Agreement pursuant to which AgeX has agreed to use commercially reasonable efforts to register the for sale under the Securities Act the shares of common stock issuable upon conversion of Preferred Stock. A registration statement must be filed upon request of Juvenescence if Form S-3 is available to AgeX. Juvenescence will also have “piggy-back” registration rights if AgeX files a registration statement for the sale of shares for itself or other stockholders, subject to certain customary exceptions based on the nature of the registration statement. AgeX will bear the expenses of the registration statement but not underwriting or broker’s commissions related to the sale of the common stock. AgeX and Juvenescence will indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
Research Grant Award
On August 11, 2023, AgeX received a Notice of Award from the National Institutes of Health (NIH, National Heart, Lung, and Blood Institute) for a grant of approximately $341,000 for a research project entitled Novel Highly Regenerative and Scalable Progenitor Cell Exosomes for Treating Peripheral Artery Disease. The grant provides one year of funding for continued development of AgeX’s technologies toward treating cardiovascular disease.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022 AND 2021
TABLE OF CONTENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
of Serina Therapeutics, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Serina Therapeutics, Inc. (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations, redeemable convertible preferred stock and stockholders’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of their operations and cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt About the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has losses from operations, negative operating cash flows, accumulated deficit, and additional capital needs. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and auditing standards generally accepted in the United States of America (GAAS). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ Frazier & Deeter, LLC |
We have served as the Company’s auditor since 2021.
Tampa, Florida
November 10, 2023
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
AS OF December 31, 2022 AND 2021
| | | 2022 | | | 2021 | |
| ASSETS | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash | | $ | 532,229 | | | $ | 314,846 | |
| Prepaid expenses | | | 16,346 | | | | 17,853 | |
| Accounts receivable | | | - | | | | 1,109 | |
| Investments in equity securities | | | - | | | | 996,447 | |
| | | | | | | | | |
| Total Current Assets | | | 548,575 | | | | 1,330,255 | |
| Property and equipment, net | | | 91,569 | | | | 122,893 | |
| Right of use assets - operating leases | | | 84,752 | | | | 234,764 | |
| Right of use assets - finance leases | | | 133,146 | | | | 156,495 | |
| | | | | | | | | |
| Total Assets | | $ | 858,042 | | | $ | 1,844,407 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS - CONTINUED
AS OF December 31, 2022 AND 2021
LIABILITIES, REDEEMABLE CONVERTIBLE PREFFERED STOCK,
AND STOCKHOLDERS’ DEFICIT
| | | 2022 | | | 2021 | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 143,337 | | | $ | 145,468 | |
| Credit card payable | | | 5,520 | | | | 4,307 | |
| Payroll liabilities | | | 6,925 | | | | 9,898 | |
| Contract liabilities | | | 153,500 | | | | - | |
| Current portion of operating lease liabilities | | | 80,696 | | | | 169,442 | |
| Current portion of finance lease liabilities | | | 47,708 | | | | 42,352 | |
| | | | | | | | | |
| Total Current Liabilities | | | 437,686 | | | | 371,467 | |
| | | | | | | | | |
| Warrant liability | | | 1,076,766 | | | | 952,648 | |
| Convertible promissory notes | | | 1,617,000 | | | | - | |
| Operating lease liabilities, net of current portion | | | 26,283 | | | | 101,701 | |
| Finance lease liabilities, net of current portion | | | 36,968 | | | | 84,676 | |
| | | | | | | | | |
| Total Liabilities | | | 3,194,703 | | | | 1,510,492 | |
| | | | | | | | | |
| Redeemable Convertible Preferred Stock, Par Value $0.01 (Note 5) | | | 35,441,500 | | | | 35,441,500 | |
| | | | | | | | | |
| Stockholders’ Deficit | | | | | | | | |
| Common stock , $0.01 par value, 15,000,000 shares authorized; 2,218,500 and 2,144,800 shares issued and outstanding at December 31, 2022 and 2021, respectively | | | 22,185 | | | | 21,448 | |
| Additional paid-in capital | | | 646,136 | | | | 635,341 | |
| Accumulated deficit | | | (38,446,482 | ) | | | (35,764,374 | ) |
| | | | | | | | | |
| Total Stockholders’ Deficit | | | (37,778,161 | ) | | | (35,107,585 | ) |
| | | | | | | | |
| Total Liabilities, Redeemable Convertible Preferred Stock, and Stockholders’ Deficit | | $ | 858,042 | | | $ | 1,844,407 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED December 31, 2022 AND 2021
| | | 2022 | | | 2021 | |
| Revenue | | | | | | | | |
| Contract revenue | | $ | 500,000 | | | $ | 3,039,184 | |
| Grant revenue | | | 91,500 | | | | 425,569 | |
| | | | | | | | | |
| Total Revenue | | | 591,500 | | | | 3,464,753 | |
| | | | | | | | | |
| Operating Expenses | | | | | | | | |
| Research and development expenses | | | 1,573,085 | | | | 3,798,593 | |
| General and administrative expenses | | | 1,288,783 | | | | 1,297,668 | |
| | | | | | | | | |
| Total Operating Expenses | | | 2,861,868 | | | | 5,096,261 | |
| | | | | | | | | |
| Operating Loss | | | (2,270,368 | ) | | | (1,631,508 | ) |
| | | | | | | | | |
| Other Income (Expense) | | | | | | | | |
| Loss on equity securities, net | | | (7,585 | ) | | | (6,452 | ) |
| Interest expense | | | (15,878 | ) | | | (3,094 | ) |
| Interest and dividend income | | | 1,757 | | | | 10,859 | |
| Fair value inception adjustment on convertible | | | | | | | | |
| promissory notes | | | (179,000 | ) | | | - | |
| Change in fair value of convertible promissory notes | | | (88,000 | ) | | | - | |
| Change in fair value of warrant liability | | | (124,118 | ) | | | 205,287 | |
| Other income | | | 1,084 | | | | - | |
| Forgiveness of SBA PPP loan | | | - | | | | 187,133 | |
| Loss on dissolution of subsidiary | | | - | | | | (26,928 | ) |
| | | | | | | | | |
| Total Other (Expense) Income | | | (411,740 | ) | | | 366,805 | |
| | | | | | | | | |
| Net Loss | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| | | | | | | | | |
| Net Loss Per Common Share | | | | | | | | |
| Basic | | $ | (1.25 | ) | | $ | (0.59 | ) |
| Diluted | | $ | (1.25 | ) | | $ | (0.59 | ) |
| | | | | | | | | |
| Weighted-Average Shares Used in Computation of Net Loss Per Common Share | | | | | | | | |
| Basic | | | 2,145,002 | | | | 2,144,800 | |
| Diluted | | | 2,145,002 | | | | 2,144,800 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED December 31, 2022 AND 2021
| | | Redeemable Convertible Preferred Stock | |
| | | | | | Preferred | | | | | | Preferred | | | | | | Preferred | | | | | | Preferred | |
| | | | | | Stock -Series | | | | | | Stock -Series | | | | | | Stock -Series | | | | | | Stock -Series | |
| | | Shares | | | A Amount | | | Shares | | | A-1 Amount | | | Shares | | | A-2 Amount | | | Shares | | | A-3 Amount | |
| As of December 31, 2020 | | | 400,000 | | | $ | 2,000,000 | | | | 300,000 | | | $ | 1,998,000 | | | | 1,117,013 | | | $ | 11,085,291 | | | | 499,200 | | | $ | 6,240,000 | |
| Issuance of preferred stock | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| As of December 31, 2021 | | | 400,000 | | | | 2,000,000 | | | | 300,000 | | | | 1,998,000 | | | | 1,117,013 | | | | 11,085,291 | | | | 499,200 | | | | 6,240,000 | |
| As of December 31, 2022 | | | 400,000 | | | $ | 2,000,000 | | | | 300,000 | | | $ | 1,998,000 | | | | 1,117,013 | | | $ | 11,085,291 | | | | 499,200 | | | $ | 6,240,000 | |
| | Redeemable Convertible Preferred Stock | |
| | | | | | Preferred | | | | | | Preferred | | | | | | | |
| | | | | | Stock -Series | | | | | | Stock -Series | | | | | | | |
| | | Shares | | | A-4 Amount | | | Shares | | | A-5 Amount | | | Total Shares | | | Total Amount | |
| As of December 31, 2020 | | | 718,997 | | | $ | 9,346,961 | | | | 360,081 | | | $ | 4,681,096 | | | | 3,395,291 | | | $ | 35,351,348 | |
| Issuance of preferred stock | | | - | | | | - | | | | 6,934 | | | | 90,152 | | | | 6,934 | | | | 90,152 | |
| As of December 31, 2021 | | | 718,997 | | | | 9,346,961 | | | | 367,015 | | | | 4,771,248 | | | | 3,402,225 | | | | 35,441,500 | |
| As of December 31, 2022 | | | 718,997 | | | $ | 9,346,961 | | | | 367,015 | | | $ | 4,771,248 | | | | 3,402,225 | | | $ | 35,441,500 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT - CONTINUED
FOR THE YEARS ENDED December 31, 2022 AND 2021
| | | | | | | | | | | | Stock | | | | | | Non- | | | Total | |
| | | Common Stock | | | Common Stock | | | Additional Paid | | | Subscriptions | | | Accumulated | | | Controlling | | | Stockholders’ | |
| | | Shares | | | Amount | | | in Capital | | | Receivable | | | Deficit | | | Interest | | | Equity (Deficit) | |
| As of December 31, 2020 - continued | | | 2,144,800 | | | | 21,448 | | | | 597,761 | | | | (1,000,769 | ) | | | (34,520,876 | ) | | | 3,537 | | | | (34,898,899 | ) |
| Dissolution of Canaria Therapeutics, Inc. | | | - | | | | - | | | | (45,365 | ) | | | 769 | | | | 21,205 | | | | (3,537 | ) | | | (26,928 | ) |
| Collection of stock subscription receivable | | | - | | | | - | | | | - | | | | 1,000,000 | | | | - | | | | - | | | | 1,000,000 | |
| Stock based compensation | | | - | | | | - | | | | 82,945 | | | | - | | | | - | | | | - | | | | 82,945 | |
| Consolidated net loss | | | - | | | | - | | | | - | | | | - | | | | (1,264,703 | ) | | | - | | | | (1,264,703 | ) |
| As of December 31, 2021 - continued | | | 2,144,800 | | | | 21,448 | | | | 635,341 | | | | - | | | | (35,764,374 | ) | | | - | | | | (35,107,585 | ) |
| Exercise of stock options | | | 73,700 | | | | 737 | | | | 3,685 | | | | - | | | | - | | | | - | | | | 4,422 | |
| Stock based compensation | | | - | | | | - | | | | 7,110 | | | | - | | | | - | | | | - | | | | 7,110 | |
| Consolidated net loss | | | - | | | | - | | | | - | | | | - | | | | (2,682,108 | ) | | | - | | | | (2,682,108 | ) |
| As of December 31, 2022 - continued | | | 2,218,500 | | | $ | 22,185 | | | $ | 646,136 | | | $ | - | | | $ | (38,446,482 | ) | | $ | - | | | $ | (37,778,161 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED December 31, 2022 AND 2021
| | | 2022 | | | 2021 | |
| Cash Flows from Operating Activities | | | | | | | | |
| Net loss | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| | | | | | | | | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 61,272 | | | | 33,368 | |
| Loss on disposal of property and equipment | | | 3,083 | | | | 380 | |
| Stock based compensation | | | 7,110 | | | | 82,945 | |
| Loss on equity securities, net | | | 7,585 | | | | 6,452 | |
| Reinvested interest and dividend income | | | (1,757 | ) | | | (10,624 | ) |
| Change in fair value of warrant liability | | | 124,118 | | | | (205,287 | ) |
| Non-cash lease expense | | | 150,012 | | | | 75,546 | |
| Fair value inception adjustment on convertible promissory notes | | | 179,000 | | | | - | |
| Change in fair value of convertible promissory notes | | | 88,000 | | | | - | |
| Forgiveness of SBA PPP loan | | | - | | | | (187,133 | ) |
| Loss on dissolution of subsidiary | | | - | | | | (26,928 | ) |
| Changes in asset and liability accounts: | | | | | | | | |
| Accounts receivable | | | 1,109 | | | | 1,001,284 | |
| Contract assets | | | - | | | | 273,044 | |
| Prepaid expenses | | | 1,507 | | | | 284 | |
| Accounts payable | | | (2,131 | ) | | | (367,289 | ) |
| Credit card payable | | | 1,213 | | | | 1,481 | |
| Accrued rent | | | - | | | | (13,437 | ) |
| Payroll liabilities | | | (2,973 | ) | | | (2,940 | ) |
| Operating lease liabilities | | | (164,164 | ) | | | (40,075 | ) |
| Contract liabilities | | | 153,500 | | | | (1,607,143 | ) |
| | | | | | | | | |
| Net Cash Used in Operating Activities | | | (2,075,624 | ) | | | (2,250,775 | ) |
| | | | | | | | | |
| Cash Flows from Investing Activities | | | | | | | | |
| Proceeds from sale of investments in equity securities | | | 990,619 | | | | 931,755 | |
| Purchases of property and equipment | | | (9,682 | ) | | | (84,929 | ) |
| Acquisition of right of use asset - finance lease | | | - | | | | (23,505 | ) |
| | | | | | | | | |
| Net Cash Provided by Investing Activities | | | 980,937 | | | | 823,321 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS - CONTINUED
FOR THE YEARS ENDED December 31, 2022 AND 2021
| | | 2022 | | | 2021 | |
| Cash Flows from Financing Activities | | | | | | |
| Issuance of convertible promissory notes | | | 5,000,000 | | | | - | |
| Repayment of convertible promissory note | | | (3,650,000 | ) | | | - | |
| Proceeds from common stock issued | | | 4,422 | | | | - | |
| Principal repayments on finance lease liabilities | | | (42,352 | ) | | | (12,907 | ) |
| Proceeds from preferred stock issued | | | - | | | | 90,152 | |
| Collection of stock subscription receivable | | | - | | | | 1,000,000 | |
| Net Cash Provided by Financing Activities | | | 1,312,070 | | | | 1,077,245 | |
| Net Increase (Decrease) in Cash | | | 217,383 | | | | (350,209 | ) |
| Cash, Beginning of Year | | | 314,846 | | | | 665,055 | |
| Cash, End of Year | | $ | 532,229 | | | $ | 314,846 | |
| Supplemental Schedule of Noncash Investing and Financing Activities | | | | | | | | |
| Cash paid for interest | | $ | 15,878 | | | $ | 3,094 | |
| Fixed asset purchases in accounts payable as of December 31 | | $ | - | | | $ | 39,060 | |
| Acquisition of right of use assets in exchange for finance lease liabilities | | $ | - | | | $ | 139,935 | |
The accompanying notes are an integral part of these consolidated financial statements.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies
Nature of Business
Serina Therapeutics, Inc. (the “Company” or “Serina”) is a privately-held, clinical-stage biotechnology company developing a pipeline of wholly owned drug product candidates to treat neurological diseases and pain. Serina’s POZ drug delivery technology is designed to enable certain existing drugs and novel drug candidates to be modified in a way that may provide an increase in efficacy and safety of the resulting polymeric drug conjugate. Serina’s proprietary POZ technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection. Serina was the majority shareholder of Canaria Therapeutics, Inc. (“Canaria”) from its inception through its dissolution in 2021. Canaria was founded in 2018 and had minimal activity during the year ended December 31,2021. Serina was founded in 2006 and is headquartered in Huntsville, Alabama.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Serina and its subsidiary, Canaria (collectively, the Company). In July 2021, Canaria was dissolved.
Basis of Accounting
The accompanying consolidated financial statements reflect the operations of the Company and have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
Liquidity
The Company incurred net losses of approximately $2,700,000 for the year ended December 31, 2022. The Company used approximately $2,100,000 in net cash from operating activities for the year ended December 31, 2022 and has historically incurred losses from operations and expects to continue to generate negative cash flows as the Company implements its business plan. There is substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements are prepared using GAAP as applicable to a going concern.
The Company had cash on hand of approximately $7,600,000 as of September 30, 2023. As discussed in Note 17, subsequent to year-end the Company raised $10,100,000 in additional debt. The debt is expected to fund operations through approximately March 2024.
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, technical risks associated with the successful research, development and manufacturing of therapeutic candidates, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations, and the ability to secure additional capital to fund operations. Therapeutic candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel and infrastructure.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies - Continued
Liquidity - Continued
Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will realize revenue from product sales. The Company largely relies on raising capital from equity investors for funding its operations. Some funding is obtained through licensing agreements or other arrangements with commercial entities.
As a result of recurring losses from operations and recurring negative cash flows from operations, there is uncertainty regarding the Company’s ability to maintain liquidity sufficient to operate its business effectively. If sufficient capital is not available, the Company would be required to delay, limit, reduce, or terminate its product development or future commercialization efforts or grant rights to develop and market therapeutic candidates to other entities. There can be no assurance that the Company will be able to raise additional funds or that the terms and conditions of any future financings will be workable or acceptable to the Company or its shareholders. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Cash
Cash includes cash held in readily available checking and money market accounts.
Accounts Receivable
Accounts receivable consists primarily of payments due to the Company on contracts with customers. Bad debts arising from contracts are estimated using the allowance method. Management determines the allowance for doubtful accounts based on historical losses and current economic conditions. On a continuing basis, management analyzes delinquent receivables; and once receivables are determined to be uncollectible, they are written off through a charge against an existing allowance account or against earnings. No allowance for doubtful accounts has been provided at December 31, 2021, and there were no accounts receivable at December 31, 2022.
Contract Assets
Contract assets include the unbilled amount typically resulting from sales under contracts where the revenue recognized exceeds the amounts billed to the customer. The amounts may not exceed their estimated net realizable value. Contract assets are classified as current based on the Company’s contract operating cycle.
Contract Liabilities
Contract liabilities include billings in excess of revenue recognized. Contract liabilities are classified as current based on the Company’s contract operating cycle and reported on a contract-by-contract basis, net of revenue recognized, at the end of each reporting period.
Investments in Equity Securities
Investments in equity securities consist of mutual funds at fair value which are highly liquid. These investments are therefore classified as current assets and as trading securities. Net realized and unrealized gains and losses on trading securities are included in net earnings. For the purpose of determining realized gains and losses, the cost of securities sold is based on specific identification.
Property and Equipment
Property and equipment are carried at cost less accumulated depreciation. The costs of additions and betterments are capitalized and expenditures for repairs and maintenance are expensed as incurred. When items of property and equipment are sold or retired, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is included in the statements of operations. Depreciation of property and equipment is provided utilizing the straight-line method over the range of lives used of the respective assets, which is 3 - 10 years.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies - Continued
Property and Equipment - Continued
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if its carrying amount exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposal of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. No impairment loss was recognized during the years ended December 31, 2022 and 2021, respectively.
Revenue Recognition
Contract revenue - The Company recognizes contract revenue under licensing agreements with commercial entities. Under revenue sharing licensing agreements, the Company receives reimbursement for eligible costs as well as payments upon the achievement of certain milestones as defined by the contract. These licensing agreements provide for the Company to receive a certain percentage of revenue from sales of their product; however, no sales have occurred to date. Under other licensing agreements, the Company receives payment for the production and use of their polymer technology.
The Company accounts for a contract after it has been approved by all parties to the arrangement, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and it is probable that substantially all of the consideration entitled in exchange for the goods or services that will be transferred to the customer will be collected.
Each contract is assessed at inception to determine whether it should be combined with other contracts. When making this determination, factors such as whether two or more contracts were negotiated or executed at or near the same time or were negotiated with an overall profit objective. If combined, the Company treats the combined contracts as a single contract for revenue recognition purposes.
The Company evaluates the services promised in each contract at inception to determine whether the contract should be accounted for as having one or more performance obligations. The services in the contracts are typically not distinct from one another due to the requirements to perform under the contract. Accordingly, the contracts are typically accounted for as one performance obligation.
The Company determines the transaction price for each contract based on the consideration expected to be received for the services being provided under the contract. For contracts where a portion of the price may vary, the Company estimates variable consideration at the most likely amount, which is included in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur. The Company analyzes the risk of a significant reversal and if necessary, constrains the amount of variable consideration recognized in order to mitigate the risk. At inception of a contract, the transaction price is estimated based on current rights and do not contemplate future modifications (including unexercised options) or follow-on contracts until they become legally enforceable. Depending on the nature of the modification, the Company considers whether to account for the modification as an adjustment to the existing contract or as a separate contract.
For contracts with multiple performance obligations, the transaction price is allocated to each performance obligation based on the estimated standalone selling price of the service underlying each performance obligation. Revenue is recognized as performance obligations are satisfied and the customer obtains control of the service. Substantially all of the revenue is recognized over time using the input method as the Company performs under the contract because control of the work in process transfers continuously to the customer.
For performance obligations in which control does not continuously transfer to the customer, revenue is recognized at the point in time that each performance obligation is fully satisfied.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies - Continued
Revenue Recognition - Continued
Grant revenue - The Company recognizes revenue in accordance with the grant accounting under a cost-reimbursement grant model with a grant from a federal agency and a grant from a commercial entity under federal funding. Under the cost-reimbursement grant, the Company receives reimbursement for eligible costs as defined by the contract. Under the commercial grant, the Company received substantially all funding upon award of the grant, and recognizes revenue as eligible expenses under the grant are incurred. The Company has concluded that it is a principal in these transactions and, accordingly, recognizes revenue in the gross amount of consideration to which it is entitled from the agency in exchange for the services provided. The periods of performance for the cost-reimbursable grant was September 2019 - June 2021. The period of performance for the commercial grant is August 2022 - December 2023.
At December 31, 2022, the Company is obligated under a grant to continue its research and development related to the treatment of neurological disorders and stroke. The Company expects to complete the remaining performance obligation and recognize the remainder of contract liabilities during the year ending December 31, 2023.
Milestone payments are recognized as licensing revenue upon the achievement of specified milestones if (i) the milestone is substantive in nature and the achievement of the milestone was not probable at the inception of the agreement; and (ii) the Company has a right to payment. Any milestone payments received prior to satisfying these revenue recognition criteria are recorded as deferred revenue.
Various economic factors affect revenues and cash flows. Services are primarily provided under grants from U.S. Government agencies and a licensing agreement with a commercial entity, with amounts invoiced quarterly or upon the achievement of a milestone and typically being collected within 1 month.
Research and Development
Research and development costs are expensed as they are incurred and include compensation for scientists, support personnel, outside contracted services, and material costs associated with product development. The Company continually evaluates new product opportunities and engages in intensive research and product development efforts. Research and development expenses include both direct costs tied to a specific contract or grant, and indirect costs.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains cash in accounts with high quality, federally insured financial institutions. The balances in these accounts may be in excess of federally insured limits and were in excess by approximately $282,000 and $61,000 at December 31, 2022 and 2021, respectively.
For the year ended December 31, 2022, 85% of the Company’s revenue was related to a single customer. As of December 31, 2021, 100% of the Company’s accounts receivable was related to a licensing agreement, and for the year then ended, 69% of its revenue was related to the same agreement.
Fair Value of Financial Instruments
The Company has adopted ASC Topic 820 for certain financial instruments measured as fair value on a recurring basis. ASC Topic 820 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States, and expands disclosures about fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC Topic 820 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies - Continued
Fair Value of Financial Instruments - Continued
The Company has adopted ASC Topic 820 for certain financial instruments measured as fair value on a recurring basis. ASC Topic 820 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States, and expands disclosures about fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC Topic 820 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements).
The three levels of inputs that may be used to measure fair value are as follows:
| | ● | Level 1: Quoted prices in active markets for identical assets or liabilities. |
| | | |
| | ● | Level 2: Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets with insufficient volume or infrequent transactions (less active markets), or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated with observable market data for substantially the full term of the assets or liabilities. Level 2 inputs also include non-binding market consensus prices that can be corroborated with observable market data, as well as quoted prices that were adjusted for security-specific restrictions. |
| | | |
| | ● | Level 3: Unobservable inputs to the valuation methodology are significant to the measurement of the fair value of assets or liabilities. Level 3 inputs also include non-binding market consensus prices or non-binding broker quotes that we were unable to corroborate with observable market data. |
Redeemable Convertible Preferred Stock
The Company recorded redeemable convertible preferred stock at fair value upon issuance, net of any issuance costs. The Company classified stock that was redeemable in circumstances outside of the Company’s control outside of permanent equity.
Earnings (Loss) per Share
Basic earnings (loss) per share (“EPS”) of common stock is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period.
Diluted EPS give effect to all dilutive potential common shares outstanding during the period using the treasury stock method for stock options and warrants and the if-converted method for redeemable, convertible preferred stock and convertible promissory notes. In computing diluted EPS, the average stock price for the period is used to determine the number of shares assumed to be purchased from the exercise of stock options and/or warrants. Diluted EPS excluded all dilutive potential shares if their effect is anti-dilutive.
Basic loss per common share is computed based on the weighted average number of shares outstanding during the period. Diluted loss per share is computed in a manner similar to the basic loss per share, except the weighted-average number of shares outstanding is increased to include all common shares, including those with the potential to be issued by virtue of convertible debt and other such convertible instruments. Diluted loss per share contemplates a complete conversion to common shares of all convertible instruments only if they are dilutive in nature with regards to earnings per share.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
December 31, 2022 AND 2021
Note 1 - Summary of Significant Accounting Policies - Continued
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the consolidated financial statements and consist of taxes currently due plus deferred taxes related primarily to differences between the basis of loss carryovers and depreciation differences for financial and income tax reporting. Deferred taxes represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which the differences are expected to be recovered or settled.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, the Chief Executive Officer, in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business as one operating segment in the United States.
Reclassification of Prior Year Presentation
Certain prior year amounts have been reclassified for consistency with the current year presentation. This change in classification does not affect previously reported net loss.
Note 2 - Accounts Receivable and Contract Balances
The beginning and ending accounts receivable and contract balances were as follows as of December 31:
| | | 2022 | | | 2021 | | | 2020 | |
| Accounts receivable | | $ | - | | | $ | 1,109 | | | $ | 1,002,393 | |
| Unbilled revenue | | $ | - | | | $ | - | | | $ | 273,044 | |
| Deferred revenue | | $ | 153,500 | | | $ | - | | | $ | 1,607,143 | |
There were no contracts in progress at December 31, 2021; therefore there were no reportable contract assets or liabilities.
Receipts of $153,500 were included in contract liabilities at December 31, 2022 related to a contract in progress.
Note 3 - Profit Sharing Plan
Serina has established a 401(k) profit sharing plan (the PSP) for all eligible employees of Serina. The PSP provides for eligible employee contributions subject to certain annual Internal Revenue Code limits. For participants who are age 50 or older during any calendar year, additional employee contributions are allowed under the PSP, subject to Internal Revenue Code limits.
Employer contributions, if any, may include matching contributions and profit sharing contributions, both of which are made on a discretionary basis and are subject to service and employment requirements. Employer matching contributions and employer profit sharing contributions vest based on a graded vesting schedule. Serina made no discretionary employer matching or employer profit sharing contributions for the years ended December 31, 2022 and 2021.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 4 - Common Stock
The holders of the common stock are entitled to one vote for each share of common stock on all matters that may be submitted to the holders of common stock of Serina. The holders of common stock shall vote together with the holders of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock as a single class. Serina shall not plan a merger or conversion, sale or dispose of property, or dissolve the Company without the written consent or affirmative vote of the entire single class.
Note 5 - Redeemable Convertible Preferred Stock
Serina has authority to issue 10,000,000 shares of preferred stock with a par value of $0.01 per share. All outstanding preferred stock is redeemable upon a Deemed Liquidation Event and convertible and has been classified as temporary stockholders’ equity at December 31, 2022 and 2021, as the shares are redeemable for cash or other assets upon the occurrence of an event that is not solely within the control of the Company.
As of December 31, 2022 and 2021, all outstanding preferred stock was not initially redeemable and was not considered probable of being redeemed upon consideration of the events that would constitute a Deemed Liquidation Event.
Redeemable convertible preferred stock was as follows as of December 31, 2022 and 2021:
Preference Order | | Designation
| | Shares Designated | | | Shares Issued | | | Shares Outstanding | | | Issue Price per Share | | | Liquidation Preference | |
| #1 | | Series A Preferred Stock | | | 400,000 | | | | 400,000 | | | | 400,000 | | | $ | 5.00 | | | $ | 2,000,000 | |
| #2 | | Series A-1 Preferred Stock | | | 300,000 | | | | 300,000 | | | | 300,000 | | | $ | 6.66 | | | $ | 1,998,000 | |
| #3 | | Series A-2 Preferred Stock | | | 1,122,077 | | | | 1,117,013 | | | | 1,117,013 | | | $ | 9.93793 | | | $ | 11,085,291 | |
| #4 | | Series A-3 Preferred Stock | | | 499,200 | | | | 499,200 | | | | 499,200 | | | $ | 12.50 | | | $ | 6,240,000 | |
| #5 | | Series A-4 Preferred Stock | | | 718,997 | | | | 718,997 | | | | 718,997 | | | $ | 13.00 | | | $ | 9,346,961 | |
| #6 | | Series A-5 Preferred Stock | | | 2,000,000 | | | | 367,015 | | | | 367,015 | | | $ | 13.00 | | | $ | 4,771,248 | |
| | | | | | 5,040,274 | | | | 3,402,225 | | | | 3,402,225 | | | | | | | $ | 35,441,500 | |
Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock all have the same rights, preferences, powers, privileges, restrictions, qualifications, and limitations.
Dividends
From and after the date of the issuance of any shares of Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock, they accrued dividends at the rate per annum of 6% of the original issue price until June 30, 2019. The dividends accrued from day to day, whether or not declared, compounded annually, were calculated on the basis of a 365 day year, and were cumulative. Effective June 30, 2019, Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock no longer accrued dividends. The holders of the Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock were entitled to receive dividends when and if declared by the Board of Directors. The Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall not accrue dividends. Dividends on Preferred Stock are in preference to and prior to any payment of any dividends on common stock. As of December 31, 2022 and 2021, $10,276,653 in Preferred Stock dividends have been accrued, but not declared. As such, these dividends are not currently an obligation of the Company.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 5 - Redeemable Convertible Preferred Stock - Continued
Convertibility
Each share of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall be convertible, at the option of the holder, at any time and from time to time, and without the payment of additional consideration by the holder into common stock. The number of shares will be determined by dividing the original issue price by the conversion price of the Preferred Stock. The conversion price initially is the same as the issue price of the Preferred Stock, but may be adjusted based on certain events as provided in the amended Articles of Incorporation.
Liquidation Preference
In the event of Deemed Liquidation Event for Serina (described below), the holders of the various series of Preferred Stock are entitled to receive prior to, and in preference to, any distribution to the holders of common stock or any other class or series of stock ranking on liquidation junior to the series of Preferred Stock they hold, an amount equal to the original issue price plus any accrued dividends (excepting Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock) together with any other dividends declared that have not yet been paid. The preference order is noted above.
The following events will constitute a Deemed Liquidation Event:
| | a) | The consummation of a plan of merger or conversion in accordance with various sections of Alabama Business Corporation Law (“ABCL”), with some exceptions as provided in the amended Articles of Incorporation; |
| | b) | The sale, lease, exchange, license, or other disposal, in a single transaction or series of related transactions, of all, or substantially all, of the corporation’s property (with or without goodwill), other than in the usual course of business in accordance with Section 10A-2A-12.02 of the ABCL; |
| | c) | Dissolution of the corporation in accordance with Section 10A-2A-14.02 of the ABCLE, or liquidation or winding-up of the business and affairs of the corporation; or |
| | d) | The sale, lease, exchange, license, or other disposal, other than in the usual and regular course of business, of all, or substantially all, of the intellectual property of the corporation in a single transaction or a series of related transactions. |
The holders of at least 60% of the Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock, acting as a single class of stock, may elect that any such events described above shall not be a Deemed Liquidation Event as provided in the amended Articles of Incorporation.
The Series A Preferred Stock liquidation preference, the Series A-1 Preferred Stock liquidation preference, the Series A-2 liquidation preference, the Series A-3 liquidation preference, the Series A-4 liquidation preference, and the Series A-5 liquidation preference shall be on parity with one another. In the event that upon liquidation or dissolution, the assets and funds of the Company are insufficient to permit the payment to holders of shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock the full amount to which they shall be entitled, the holders of shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock then outstanding and any class or series of stock ranking on liquidation on a parity with the Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall share in any distribution of the remaining assets available for distribution in proportion to the respective amounts which would otherwise be payable in respect of the shares held by them upon such distribution if all amounts payable on or with respect to such shares were paid in full.
After the distributions described above have been paid in full, the remaining assets of the Company available for distribution shall be distributed pro-rata to the holders of the shares of common stock.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 6 - Income Taxes
Deferred tax assets and liabilities reflect the effects of tax losses, credits, and the future income tax effects of temporary differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases and are measured using enacted tax rates that apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
At December 31, 2022, the Company had the following carryovers subject to expiration:
| Carryover | | Amount | | | Begin to Expire In | |
| 1231 loss carryover | | $ | 798 | | | | 2024 | |
| Net capital loss carryover | | $ | 17,472 | | | | 2026 | |
| NOL carryover - Federal - indefinite life | | $ | 12,027,696 | | | | N/A | |
| NOL carryover - Federal - subject to expiration | | $ | 23,207,997 | | | | 2027 | |
| NOL carryover - State | | $ | 35,509,627 | | | | 2023 | |
| Charitable contributions carryover | | $ | 6,875 | | | | 2027 | |
| General business credit carryover ESB* | | $ | 129,310 | | | | 2030 | |
| General business credit carryover NonESB* | | $ | 1,824,263 | | | | 2026 | |
The Company’s effective tax rate is 0% for the periods ended December 31, 2022 and December 31, 2021. The Company’s effective tax rate is different than what would be expected if the federal statutory rate was applied to income before income taxes primarily because certain expenses are deductible for financial reporting purposes that are not deductible for tax purposes and valuation allowances applied to the Company’s net deferred tax assets. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The major temporary differences that give rise to the deferred tax assets and liabilities are as follows: NOL carryovers, general business credit carryovers, charitable contribution carryovers, operating and finance lease assets and liabilities, and depreciation and amortization.
The Company evaluates the recoverability of these future tax deductions and credits by assessing the adequacy of future expected taxable income from all sources, including reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. To the extent that management does not consider it more likely than not that a deferred tax asset will be recovered, a valuation allowance is established. The Company has provided a valuation allowance against the deferred income taxes at December 31, 2022 and 2021. The valuation allowance increased by $1,004,315 during the year ended December 31, 2022.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 6 - Income Taxes - Continued
The components of the deferred income tax asset and liability as of December 31, 2022 and 2021 are as follows:
| | | 2022 | | | 2021 | |
| Deferred Tax Assets (Liabilities): | | | | | | | | |
| General business credit carryover ESB | | $ | 129,310 | | | $ | 129,310 | |
| General business credit carryover NonESB | | | 1,824,263 | | | | 1,824,263 | |
| 1231 loss carryover | | | 219 | | | | 168 | |
| Net capital loss carryover | | | 4,805 | | | | 4,106 | |
| NOL carryover - Federal | | | 7,399,496 | | | | 6,909,074 | |
| NOL carryover - State | | | 2,308,126 | | | | 2,183,730 | |
| Research and development | | | 389,339 | | | | - | |
| Unrealized loss on investments | | | - | | | | 1,195 | |
| Charitable contributions carryover | | | 6,875 | | | | - | |
| Right of use assets - operating leases | | | (23,307 | ) | | | (64,560 | ) |
| Right of use assets - finance leases | | | - | | | | (43,036 | ) |
| Operating lease liabilities | | | 29,419 | | | | 74,564 | |
| Finance lease liabilities | | | - | | | | 34,933 | |
| Depreciation of property and equipment | | | 20,776 | | | | 31,259 | |
| Total deferred tax asset | | | 12,089,321 | | | | 11,085,006 | |
| Valuation allowance | | | (12,089,321 | ) | | | (11,085,006 | ) |
| Net Deferred Tax Asset | | $ | - | | | $ | - | |
The Company only recognizes tax benefits from an uncertain tax position if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statement from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. To date, the Company has not recognized such tax benefits in its financial statements.
On January 1, 2022, a provision of the Tax Cuts and Jobs Act of 2017 went into effect which eliminates the option to deduct research and development expenditures currently and requires tax payers to amortize such costs incurred in the U.S. over five years and 15 years for costs incurred overseas. While it is possible that Congress may modify or repeal this provision retroactively, the Company implemented such provision into its accounting for income taxes for the year ended December 31, 2022.
Note 7 - Leases
The Company leases many of its operating and office facilities for various terms under long-term, non-cancelable operating lease agreements. The leases expire at various dates through 2024 and provide for renewal periods of two years. In the normal course of business, it is expected that these leases will be renewed or replaced by leases on other properties. The Company also leases equipment for various terms under long-term, non-cancelable finance lease agreements. These leases expire at various dates through 2025.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 7 - Leases - Continued
The following represents information on leases as of and for the years ended December 31:
| | | 2022 | | | 2021 | |
| Finance lease cost (including amortization of right-of use assets of $23,349 and $6,945 and interest on lease liabilities of $12,843 and $3,094 in 2022 and 2021, respectively) | | $ | 36,192 | | | $ | 10,039 | |
| Operating lease expense | | | 101,399 | | | | 140,846 | |
| Total lease cost | | $ | 137,591 | | | $ | 150,885 | |
| Other information | | | | | | | | |
| Weighted average remaining lease term (yrs) - finance leases | | | 1.71 | | | | 2.71 | |
| Weighted average remaining lease term (yrs) - operating leases | | | 1.30 | | | | 1.42 | |
| Weighted average discount rate - finance leases | | | 11.90 | % | | | 11.90 | % |
| Weighted average discount rate - operating leases | | | 6.67 | % | | | 6.67 | % |
The following is a maturity analysis of the annual undiscounted cash flows of the lease liabilities as of December 31, 2022:
| | | Operating | | | Finance | |
| | | Leases | | | Leases | |
| 2023 | | $ | 84,781 | | | $ | 55,195 | |
| 2024 | | | 26,869 | | | | 38,055 | |
| 2025 | | | - | | | | 625 | |
| Total future minimum lease payments | | | 111,650 | | | | 93,875 | |
| Less: imputed interest | | | (4,671 | ) | | | (9,199 | ) |
| Total lease liabilities | | | 106,979 | | | | 84,676 | |
| Less: current portion | | | (80,696 | ) | | | (47,708 | ) |
| Lease liabilities, non-current | | $ | 26,283 | | | $ | 36,968 | |
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 8 - Property and Equipment, net
Property and equipment at December 31, 2022 and 2021 consists of:
| | | 2022 | | | 2021 | |
| Computer equipment | | $ | 24,061 | | | $ | 24,061 | |
| Equipment | | | 353,343 | | | | 348,017 | |
| Software | | | 81,676 | | | | 81,676 | |
| Total property and equipment | | | 459,080 | | | | 453,754 | |
| Less accumulated depreciation | | | (367,511 | ) | | | (330,861 | ) |
| Total property and equipment, net | | $ | 91,569 | | | $ | 122,893 | |
Note 9 - Stock Option Plan
Stock Option Plan Description
In 2007, the Company’s Board of Directors (“the Board”) adopted the Serina Therapeutics, Inc. 2007 Stock Option Plan (the 2007 Plan) that provides for the granting of stock options to employees. The 2007 Plan as amended in March 2014 reserved 2,000,000 shares of common stock for grant. Options are granted at the discretion of the Board. Options granted under the 2007 Plan shall be either incentive stock options (ISOs) or nonstatutory stock options (NSOs), as designated by the Board. The options will be granted at an exercise price set by the Board at the time of grant, but in no event will the price for ISOs be less than 100% of the fair market value of the common stock on the date of the grant, or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, not less than 110%; and not less than the par value of the common stock at grant, in the case of NSOs.
In 2017, the Serina Therapeutics, Inc. 2007 Plan by its terms expired, except with respect to unexercised stock options outstanding under the 2007 Plan. No future stock options shall be awarded under the 2007 Plan.
In 2017, the Company’s Board adopted the Serina Therapeutics, Inc. 2017 Stock Option Plan (the 2017 Plan) that provides for the granting of stock options to employees. The 2017 Plan reserved 1,000,000 shares of common stock for grant. In 2021, the 2017 Plan was amended to increase the reserved shares to 2,000,000, and then later in 2021 further amended to increase the reserved shares to 2,100,000. Options are granted at the discretion of the Board. Options granted under the 2017 Plan shall be either incentive stock options (ISOs) or nonstatutory stock options (NSOs), as designated by the Board. The options will be granted at an exercise price set by the Board at the time of grant, but in no event will the price for ISOs be less than 100% of the fair market value of the common stock on the date of the grant or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, not less than 110%; and not less than the par value of the common stock at grant, in the case of NSOs.
Vesting and Exercisability
Stock options are exercisable at such time or times and subject to such terms and conditions as determined by the Board at or after grant. If the Board provides, in its sole discretion, that any stock option is exercisable only in installments, the Board may waive such installment exercise provisions at any time at or after the grant, in whole or in part, based on such factors as the Board shall determine, in its sole discretion.
The terms of the grants are generally for ten years (or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, the term shall be five years) and vest over a period of time as determined on the date of the grant.
Stock-Based Compensation
The Company follows the provisions of the FASB ASC 718, Compensation - Stock Compensation (“FASB ASC 718”). FASB ASC 718 requires all share-based payments to employees, including grants of employee stock options, to be recognized as compensation expense in the consolidated financial statements based on their fair value at the grant date.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 9 - Stock Option Plan - Continued
Valuation and Expense
The Company recognizes compensation expense related to stock options on a straight-line basis over the requisite service period of the awards, which is generally three to four years. The Company estimates the fair value of options on the date of grant using the Black-Scholes-Merton option pricing model with the following assumptions:
| | | 2022 | | | 2021 | |
| Expected volatility | | | 88.57 | % | | | 68.70 | % |
| Expected term (in years) | | | 10.00 | | | | 5.15 - 10.00 | |
| Risk-free interest rate | | | 3.96 | % | | | 0.73% - 0.81 | % |
| Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected volatility is estimated using the historical volatility of an industry sector index for the years ended December 31, 2022 and 2021. The Company estimates the expected term using historical option exercise data to determine the expected employee exercise behavior. The risk-free interest rate is the yield on a U.S. Treasury zero-coupon issue with a remaining term equal to or approximating the expected term of the option at the grant date.
Using the Black-Scholes-Merton option pricing model, management has determined that the options issued in 2022 and 2021 have a grant date fair value of $0.05 and $0.03 - $0.04 per share, respectively.
The Company recorded stock-based compensation expense related to the stock options of $7,110 and $82,945 during the years ended December 31, 2022 and 2021, respectively. In 2021, all options outstanding as of December 31, 2020 were canceled and replaced, with the exception of 3,300 options, which were not replaced due to termination of the grantee. Total replacement options issued were 1,294,042. Incremental compensation cost of $878 and $35,533 was recognized during the years ended December 31, 2022 and 2021, respectively, as a result of the accounting for the modification required by FASB ASC 718. As of December 31, 2022, the Company had $22,728 of unrecognized compensation cost related to nonvested stock options.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Details of stock option activity for the years ended December 31, 2022 and 2021 are as follows:
| | | | | | | Weighted | |
| | | | Number of | | | Average | |
| | | | Options | | | Exercise Price | |
| Outstanding at December 31, 2020 | | | | 1,297,342 | | | $ | 1.35 | |
| Canceled | | | | (1,297,342 | ) | | | 1.35 | |
| Granted | | | | 2,036,450 | | | | 0.06 | |
| Outstanding at December 31, 2021 | | | | 2,036,450 | | | | 0.06 | |
| Exercised | | | | (73,700 | ) | | | 0.06 | |
| Granted | | | | 50,000 | | | | 0.06 | |
| Outstanding at December 31, 2022 | | | | 2,012,750 | | | $ | 0.06 | |
The following table summarizes information about stock options exercisable at December 31, 2022:
| | | | | | | Weighted Average | | | | |
| | | | Number | | | Remaining | | | Weighted | |
| Exercise | | | of Shares | | | Contractual | | | Average | |
| Price | | | Exercisable | | | Life in Years | | | Exercise Price | |
| $ | 0.06 | | | | 1,463,943 | | | | 8.74 | | | $ | 0.06 | |
The following table summarizes information about nonvested stock options at December 31, 2022:
| | | | Nonvested Options | | | Weighted Average
Grant Date Fair Value | |
| Nonvested options at December 31, 2021 | | | | 656,742 | | | $ | 0.04 | |
| Granted | | | | 50,000 | | | | 0.05 | |
| Vested | | | | (84,235 | ) | | | 0.04 | |
| Exercised | | | | (73,700 | ) | | | 0.03 | |
| Forfeited/cancelled | | | | - | | | | - | |
| Nonvested options at December 31, 2022 | | | | 548,807 | | | $ | 0.05 | |
Note 10 - Stock Warrants
Grants
During the year ended December 31, 2021, the Company granted warrants to purchase 6,934 shares of Series A-5 Preferred Stock at an exercise price of $20.00 per share. No warrants were granted during the year ended December 31, 2022. The warrants are accounted for as a liability and remeasured to fair value at each reporting period.
Vesting and Exercisability
The warrants vest immediately upon grant. All outstanding warrants at December 31, 2022 are exercisable at any time prior to close of business on December 31, 2024.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Valuation and Expense
The Company estimates the fair value of warrants using the Black-Scholes-Merton option pricing model with the following assumptions at December 31, 2022 and 2021:
| | | To Calculate Fair Value of
Warrant Liability at December 31, | |
| | | 2022 | | | 2021 | |
| Expected volatility | | | 88.57 | % | | | 68.70 | % |
| Expected term (in years) | | | 1.00 - 1.50 | | | | 1.50 - 2.00 | |
| Risk-free interest rate | | | 4.41 - 4.73 | % | | | 1.26 | % |
| Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
| | | | | | | | | | | | | Change in Fair Value | | | Change in Fair Value | |
| | | | | | | | | | | | | of Warrant Liability | | | of Warrant Liability | |
| | | | 2022 | | | 2021 | | | 2020 | | | in 2022 | | | in 2021 | |
| Fair value of warrant liability | | | $ | 1,076,766 | | | $ | 952,648 | | | $ | 1,157,935 | | | $ | 124,118 | | | $ | (205,287 | ) |
Expected volatility is estimated using the historical volatility of comparable public entities. The Company estimates the expected term using historical option exercise data to determine the expected employee exercise behavior. The risk-free interest rate is the yield on a U.S. Treasury zero-coupon issue with a remaining term equal to or approximating the expected term of the option at the grant date.
The Company recorded an expense related to the warrants due to an increase in the liability of $124,118 during the year ended December 31, 2022, and a benefit related to the warrants due to a decrease in the liability of $205,287 during the year ended December 31, 2021, which are recorded in other expenses and income on the Company’s consolidated statements of operations.
Details of stock warrant activity for the year ended December 31, 2022, are as follows:
| | | | | | Weighted | |
| | | Number of | | | Average | |
| | | Warrants | | | Exercise Price | |
| Outstanding at December 31, 2020 | | | 360,081 | | | $ | 20.00 | |
| Issued | | | 6,934 | | | | 20.00 | |
| Outstanding at December 31, 2021 | | | 367,015 | | | | 20.00 | |
| Outstanding at December 31, 2022 | | | 367,015 | | | $ | 20.00 | |
Note 11 - Long Term Debt
In 2020, the Company obtained an unsecured note payable in the amount of $187,133 under the Paycheck Protection Program (PPP) as part of the Coronavirus Aid, Relief, and Economic Security Act. Loans obtained under the PPP are eligible for debt forgiveness through guarantees provided to the lender by the U.S. Small Business Administration (“SBA”). The Company received notification from the SBA in 2021 that the loan had been fully forgiven. The Company recognized $187,133 in forgiveness of debt income in 2021.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 12 - Investments in Marketable Equity Securities
Realized and unrealized gains and losses for marketable equity securities for the years ended December 31, 2022 and 2021, are summarized below:
| | | 2022 | | | 2021 | |
| Realized (loss) gain for equity securities sold | | $ | (7,585 | ) | | $ | 5,609 | |
| Unrealized loss on equity securities held | | | - | | | | (12,061 | ) |
| | | | | | | | | |
| Total loss on equity securities | | $ | (7,585 | ) | | $ | (6,452 | ) |
Note 13 - Research and Development
At December 31, 2022, the Company is performing under a grant provided by a corporation via funding provided by a federal government agency to continue its research and development related to treatment of neurological disorders and strokes. The grant is structured such that the Company received $245,000 upon execution of the award, and recognizes eligible costs as they are incurred. Grant revenue and related research and development costs under the grant was $91,500 for the year ended December 31, 2022.
The Company performed under a grant provided by a federal governmental agency for research and development related to opioids during the year ended December 31, 2021. The grant was structured as a cost-reimbursement grant and was completed in 2021. Grant revenue and related research and development costs under the grant was $425,569 for the year ended December 31, 2021.
The Company performed under a contract for licensing with a research and development component with a commercial entity during the year ended December 31, 2021. The research and development component was structured as cost-reimbursable. This contract was terminated in 2021. Research and development revenue and related costs under this contract were $782,041 for the year ended December 31, 2021.
Note 14 - Commitments and Contingencies
The Company is subject to legal proceedings, claims, and litigation arising in the ordinary course of business. In the opinion of management, all such matters would be adequately covered by insurance or accruals or would be such that disposition would not have a material adverse effect on the consolidated financial position, results of operations, or cash flows of the Company.
Note 15 - Convertible Promissory Notes
From June 2022 through December 31, 2022, the Company issued interest-bearing Convertible Promissory Notes (the “Notes”) to various third-party investors in the principal amount of $1,350,000. In addition, on November 10, 2022, the Company issued a Convertible Promissory Note to a related party investor in the principal amount of $3,650,000 which was repaid in full on December 14, 2022. The Notes incur interest at 6% per annum and are payable by the Company two years from their issuance date. The Company may not voluntarily prepay. Upon a Qualified Equity Financing event in which the Company sells shares of Preferred Stock for aggregate proceeds of at least $15 million, the principal and outstanding interest on the Notes will automatically convert into shares of the Company’s Preferred Stock issued in the Qualified Financing at a conversion price of the lesser of i) a 20% discount to the price paid by purchasers in the Qualified Financing and ii) the quotient resulted from dividing $100 million by the fully diluted capitalization of the Company immediately prior to the Qualified Financing. If the Company enters into a Non-Qualified Equity Financing (less than $15 million in proceeds), the Holder has the option to convert the Notes into shares of the Company’s Preferred Stock issued in the Non-Qualified Financing at the price paid per share. The Company may also choose to optionally convert the Notes into Series A-5 Preferred Stock at a price of $13 per share, and a warrant to purchase shares of Series A-5 Preferred Stock with an exercise price of $20.00, and an expiration date of December 31, 2024.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
If a Change in Control or an IPO occurs prior to a Qualified Financing, then the Holder has the option to convert outstanding principal and interest into common stock at a price per share equal to an amount obtained by dividing i) the Post-Money Valuation Cap ($100,000,000) by ii) the Fully Diluted Capitalization immediately prior to the conversion. Upon a change in control, the Holder may also elect to require the Company to repay the outstanding principal and accrued but unpaid interest in cash.
The Company evaluated the Notes in accordance with ASC Topic 815, Derivatives and Hedging, and determined they contained certain variable share settlement features tied to the price of a future financing which were not considered clearly and closely related to the host instruments. These provisions included mandatory conversion upon the event of a Qualified Financing and the Holder’s option to convert the Notes upon a Non-Qualified Financing. The Notes also contained a Change in Control Put and a Default Put which were not clearly and closely related to the host instrument. The Company elected to initially and subsequently measure the Notes in their entirety at fair value, with changes in fair value recognized in earnings. The fair value inception date adjustment on the instrument is recorded as a component of other income in the Company’s consolidated statements of operations. The change in fair value of the instrument since inception date is recorded on a separate line item as a component of other income in the Company’s consolidated statements of operations.
FASB ASC 825-10-25 allows the Company to elect the fair value option for recording financial instruments when they are initially recognized or if there is an event that requires re-measurement of the instruments at fair value, such as a significant modification of the debt. The Company elected the fair value option because they believed it to be the most appropriate method of encompassing the credit risk and exercise behavior that a market participant would consider when valuing the hybrid financial instrument.
Because the Notes are carried in their entirety at fair value, the value of the compound embedded conversion features and the redemption features are embodied in that fair value. The Company estimated the fair value of the hybrid instrument based on a probability weighted cash flow analysis. A Monte Carlo model, which considered the present value of the cash flows under the different scenarios using a credit risk adjusted rate, was considered by management to be the most appropriate method of encompassing credit risk and exercise behavior. Inputs used to value the hybrid instrument at inception (July 19, 2022) included, (i) present value of future cash flows using a credit risk adjusted rate of 16%, (ii) remaining term of approximately 2 years, (iii) volatility of 90% based on the volatility of comparable guideline public companies, (iv) closing stock price on the valuation date, and (v) 70% probability the Notes will be converted upon a Non-Qualified Financing, at the expected price of the Non-Qualified Financing, and 30% probability the Notes will be optionally converted at their stated conversion price ($13 per share plus one warrant). Management expects the probability of a change in control, default, or Qualified Financing event occurring over the remaining term of the Notes to be minimal. However, this assumption will be considered each reporting period. Material changes due to instrument-specific credit risk are recorded in Other Comprehensive Income with all other changes in value being recorded in net income.
As of July 19, 2022, the outstanding principal amount of the Notes was $1,350,000 and the fair value of the hybrid instrument, recorded on the consolidated balance sheet, was $1,529,000. As of December 31, 2022, the outstanding principal and interest on the Notes was approximately $1,384,000 and the fair value recorded on the consolidated balance sheet was $1,617,000. From July 19, 2022 to December 31, 2022, the change in value was approximately $88,000 and was recorded as a component of other income in the Company’s consolidated statements of operations.
Company had liabilities that they elected to be measured at fair value on a recurring basis as follows at December 31, 2022. There were no convertible promissory notes at December 31, 2021.
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
| Assets | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Liabilities: | | | | | | | | | | | | |
| Convertible Notes at fair value | | $ | 1,617,000 | | | $ | - | | | $ | - | | | $ | 1,617,000 | |
The following is a reconciliation of the beginning and ending balances for the liability measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the year December 31, 2022:
| Balance as of December 31, 2021 | | $ | - | |
| Convertible debt issuance | | | 1,529,000 | |
| Loss on change in fair value | | | 88,000 | |
| Balance as of December 31, 2022 | | $ | 1,617,000 | |
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 16 - Net Loss per Common Share
Net loss per common share is calculated in accordance with ASC Topic 260, Earnings Per Share. Basic and diluted net loss per common share attributable to common shareholders is calculated as follows for the years ended December 31, 2022 and 2021:
| | 2022 | | | 2021 | |
| Basic net loss per common share allocable to common shareholders | | | | | | |
| NUMERATOR | | | | | | | | |
| Net loss | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| Less: net earnings allocable to participating securities | | | - | | | | - | |
| Net loss allocable to common shareholders | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| DENOMINATOR | | | | | | | | |
| Weighted average shares of common stock outstanding used to calculate basic net loss per common share | | | 2,145,002 | | | | 2,144,800 | |
| Basic net loss per common share allocable to common shareholders | | $ | (1.25 | ) | | $ | (0.59 | ) |
| | | | | | | | | |
| Diluted net loss per common share allocable to shareholders | | | | | | | | |
| NUMERATOR | | | | | | | | |
| Net loss allocable to common shareholders | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| Add back: interest on convertible promissory notes | | | - | | | | - | |
| Net loss allocable to common shareholders | | $ | (2,682,108 | ) | | $ | (1,264,703 | ) |
| DENOMINATOR | | | | | | | | |
| Weighted average shares of common stock outstanding used to calculate basic net loss per common share | | | 2,145,002 | | | | 2,144,800 | |
| Add: dilutive effect of stock options | | | - | | | | - | |
| Add: dilutive effect of warrants | | | - | | | | - | |
| Add: dilutive effect of common stock issued for convertible promissory notes | | | - | | | | - | |
| Add: dilutive effect of redeemable convertible preferred stock | | | - | | | | - | |
| Weighted average shares of common stock outstanding used to calculate diluted net loss per common share | | | 2,145,002 | | | | 2,144,800 | |
| Diluted net loss per common share allocable to common shareholders | | $ | (1.25 | ) | | $ | (0.59 | ) |
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 16 - Net Loss per Common Share - Continued
The following table sets forth the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per common share for the years ended December 31, 2022 and 2021 because to do so would be anti-dilutive:
| | | 2022 | | | 2021 | |
| Redeemable convertible preferred stock | | | 3,402,225 | | | | 3,402,225 | |
| Convertible promissory notes | | | 220,008 | | | | - | |
| Stock options | | | 2,012,750 | | | | 2,036,450 | |
| Warrants | | | 367,015 | | | | 367,015 | |
| Total anti-dilutive securities | | | 6,001,998 | | | | 5,805,690 | |
Note 17 - Subsequent Events
Management’s Evaluation
Management has evaluated subsequent events through November 10, 2023, the date the consolidated financial statements were issued.
Conversion of Promissory Notes
On July 26, 2023, all of the Convertible Promissory Notes described in Note 15 (excluding the AgeX Note) previously issued for a total principal amount of $1,450,000 and accrued interest of $82,695 were converted to 117,903 shares of Series A-5 Preferred Stock. As provided for in the note agreements, the holders of the notes also received warrants to purchase an additional 117,903 shares of Series A-5 Preferred Stock.
Convertible Note
On March 15, 2023, The Company issued a convertible promissory note (the “AgeX Note”) in the amount of $10,000,000 to AgeX Therapeutics (“AgeX”). The AgeX Note bears interest at 7% per annum and matures on March 15, 2026. The Company issued the AgeX Note to provide for general working capital needs. As described further in this note, subsequent to the date of these financial statements, the Company and AgeX have entered into a merger agreement. In connection with the issuance of the AgeX Note, AgeX is entitled to elect one member to the board of directors of the Company and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
The AgeX Note has various conversion options. The principal balance of the AgeX Note with accrued interest will automatically convert into the Company’s preferred stock if the Company raises at least $25,000,000 through the sale of shares of the Company’s preferred stock. The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by the Company’s then fully diluted capitalization. AgeX has the option to convert the AgeX Note into the Company’s preferred stock after a sale of the Company’s preferred stock regardless of the amount sold by the Company. AgeX may (i) at its election, upon a change of control (as defined in the AgeX Note), convert the AgeX Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the AgeX Note, plus interest, or (b) into the highest ranking shares of the Company then issued at a conversion price equal to the lowest price per share at which the most senior series of the Company’s shares has been sold in a single transaction or a series of related transactions through which the Company raised at least $5,000,000 or (ii) if the AgeX Note remains outstanding as of the maturity date, AgeX may convert the AgeX Note into the most senior shares of the Company issued at the time of conversion at a conversion price equal to the capped price. Upon the consummation of a merger between the Company and AgeX, the AgeX Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of the Company.
SERINA THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
DECEMBER 31, 2022 AND 2021
Note 17 - Subsequent Events - Continued
License Agreement
In October 2023, the Company entered into a non-exclusive license agreement with Pfizer, Inc. to use the Company’s POZ polymer technology for use in lipid nanoparticle drug delivery formulations. The agreement grants Pfizer non-exclusive rights to certain intellectual property, know-how, and proprietary technologies. Under the terms of the agreement, Pfizer is authorized to develop, manufacture, market, and commercialize products incorporating the licensed technology with respect to a specific POZ polymer structure in one field. The agreement outlines the protection and enforcement of intellectual property rights related to the licensed technology. Pfizer is obligated to use commercially reasonable diligent efforts to develop and commercialize licensed products, and to use such efforts to accomplish specified development and commercial objectives. The agreement includes a one-time upfront payment in the single-digit millions, milestone payments payable to the Company upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology in accordance with the terms outlined in the license agreement.
Merger Agreement
On August 29, 2023, the Company entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with AgeX and Canaria Transaction Corporation, an Alabama corporation and wholly owned subsidiary of AgeX (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including that the Merger is approved by the stockholders of AgeX and the stockholders of Serina, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the “Merger”). There is no assurance the necessary approvals by AgeX stockholders and Serina stockholders will be obtained or that the other conditions to the Merger as provided in the Merger Agreement will be met.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of AgeX common stock, and stockholders of AgeX immediately prior to the closing of the Merger are expected to own, excluding the impact of the exercise of any of the Post-Merger Warrants or Incentive Warrants described below, approximately 25% of the outstanding shares of AgeX common stock, in each case, on a fully diluted basis, subject to certain assumptions, including giving effect to a planned reverse stock split and the conversion of AgeX Preferred Stock into shares of AgeX common stock. Accordingly, the Merger is anticipated to be accounted for as a reverse acquisition, with the Company treated as the acquiror for financial accounting purposes.
Prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock three warrants (each, a “Post-Merger Warrant”) for each five shares of AgeX common stock held by such stockholder. Each Post-Merger Warrant will be exercisable for (i) one share of AgeX common stock and (ii) one warrant (each, an “Incentive Warrant”) at an exercise price equal to $13.20 per warrant (such exercise price reflecting a planned reverse stock split of AgeX common stock) and will expire on July 31, 2025. Each Incentive Warrant will be exercisable for one share of AgeX common stock at a price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split of AgeX common stock) and will expire on the four-year anniversary of the closing of the Merger. Each Post-Merger Warrant and Incentive Warrant will be issued pursuant to the terms of a warrant agreement to be entered into by AgeX and a warrant agent in connection with the closing of the Merger.
SERINA THERAPEUTICS, INC.
FINANCIAL STATEMENTS
JUNE 30, 2023 and 2022
TABLE OF CONTENTS
FINANCIAL STATEMENTS
SERINA THERAPEUTICS, INC.
BALANCE SHEETS
AS OF JUNE 30, 2023 AND DECEMBER 31, 2022
| | | (Unaudited) | | | | |
| | | June 30, | | | December 31, | |
| | | 2023 | | | 2022 | |
| ASSETS | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash | | $ | 8,722,084 | | | $ | 532,229 | |
| Prepaid expenses | | | 15,197 | | | | 16,346 | |
| Total Current Assets | | | 8,737,281 | | | | 548,575 | |
| Property and equipment, net | | | 376,008 | | | | 91,569 | |
| Right of use assets - operating leases | | | 503,935 | | | | 84,752 | |
| Right of use assets - finance leases | | | 121,472 | | | | 133,146 | |
| Total Assets | | $ | 9,738,696 | | | $ | 858,042 | |
| | | | | | | | | |
| LIABILITIES, REDEEMABLE CONVERTIBLE PREFERRED STOCK, AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 360,255 | | | $ | 143,337 | |
| Credit card payable | | | 14,063 | | | | 5,520 | |
| Accrued interest | | | 281,703 | | | | - | |
| Payroll liabilities | | | 4,368 | | | | 6,925 | |
| Contract liabilities | | | 116,496 | | | | 153,500 | |
| Current portion of operating lease liabilities | | | 141,709 | | | | 80,696 | |
| Current portion of finance lease liabilities | | | 50,638 | | | | 47,708 | |
| Convertible promissory notes | | | 7,612,638 | | | | - | |
| Total Current Liabilities | | | 8,581,870 | | | | 437,686 | |
| Warrant liability | | | 613,362 | | | | 1,076,766 | |
| Convertible promissory notes | | | - | | | | 1,617,000 | |
| Operating lease liabilities, net of current portion | | | 377,376 | | | | 26,283 | |
| Finance lease liabilities, net of current portion | | | 10,894 | | | | 36,968 | |
| Total Liabilities | | | 9,583,502 | | | | 3,194,703 | |
| | | | | | | | | |
| Redeemable Convertible Preferred Stock | | | | | | | | |
| Redeemable convertible preferred stock, $0.01 par value; 10,000,000 authorized; 3,402,225 issued and outstanding | | | 35,441,500 | | | | 35,441,500 | |
| | | | | | | | | |
| Stockholders’ Deficit | | | | | | | | |
| Common stock, $0.01 par value, 15,000,000 shares authorized, 2,234,100 and 2,218,500 shares issued and outstanding at June 30, 2023 and December 31, 2022, respectively | | | 22,341 | | | | 22,185 | |
| Additional paid-in capital | | | 671,241 | | | | 646,136 | |
| Accumulated deficit | | | (35,979,888 | ) | | | (38,446,482 | ) |
| Total Stockholders’ Deficit | | | (35,286,306 | ) | | | (37,778,161 | ) |
| | | | | | | | | |
| Total Liabilities, Redeemable Convertible Preferred Stock, and Stockholders’ Deficit | | $ | 9,738,696 | | | $ | 858,042 | |
The accompanying notes are an integral part of these financial statements.
SERINA THERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
FOR THE SIX MONTHS ENDED
| | | (Unaudited) | | | (Unaudited) | |
| | | June 30, | | | June 30, | |
| | | 2023 | | | 2022 | |
| Revenue | | | | | | | | |
| Contract revenue | | $ | - | | | $ | 500,000 | |
| Grant revenue | | | 37,004 | | | | - | |
| Total Revenue | | | 37,004 | | | | 500,000 | |
| Operating Expenses | | | | | | | | |
| Research and development expenses | | | 878,188 | | | | 884,588 | |
| General and administrative expenses | | | 1,066,360 | | | | 534,434 | |
| Total Operating Expenses | | | 1,944,548 | | | | 1,419,022 | |
| Operating Loss | | | (1,907,544 | ) | | | (919,022 | ) |
| Other Income (Expense) | | | | | | | | |
| Loss on equity securities, net | | | - | | | | (7,586 | ) |
| Interest expense | | | (281,703 | ) | | | - | |
| Interest and dividend income | | | 88,075 | | | | 1,791 | |
| Fair value inception adjustment on convertible promissory notes | | | 2,240,000 | | | | - | |
| Change in fair value of convertible promissory notes | | | 1,864,362 | | | | - | |
| Change in fair value of warrant liability | | | 463,404 | | | | (485,833 | ) |
| Other income | | | - | | | | 1,084 | |
| Total Other Income (Expense) | | | 4,374,138 | | | | (490,544 | ) |
| | | | | | | | | |
| Net Income (Loss) | | $ | 2,466,594 | | | $ | (1,409,566 | ) |
| | | | | | | | | |
| Net Earnings (Loss) Per Common Share | | | | | | | | |
| Basic | | $ | 1.11 | | | $ | (0.66 | ) |
| Diluted | | $ | 0.33 | | | $ | (0.66 | ) |
| | | | | | | | | |
Weighted-Average Shares Used in Computation of Net
Earnings (Loss) Per Common Share | | | | | | | | |
| Basic | | | 2,221,100 | | | | 2,144,800 | |
| Diluted | | | 7,602,825 | | | | 2,144,800 | |
The accompanying notes are an integral part of these financial statements.
SERINA THERAPEUTICS, INC.
STATEMENT OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
FOR THE SIX MONTHS ENDED JUNE 30, 2023 AND 2022
| (Unaudited) | | Redeemable Convertible Preferred Stock | |
| | | Preferred Stock – Series A | | | Preferred Stock – Series A-1 | | | Preferred Stock – Series A-2 | | | Preferred Stock – Series A-3 | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| As of December 31, 2021 | | | 400,000 | | | $ | 2,000,000 | | | | 300,000 | | | $ | 1,998,000 | | | | 1,117,013 | | | $ | 11,085,291 | | | | 499,200 | | | $ | 6,240,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2022 | | | 400,000 | | | | 2,000,000 | | | | 300,000 | | | | 1,998,000 | | | | 1,117,013 | | | | 11,085,291 | | | | 499,200 | | | | 6,240,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2022 | | | 400,000 | | | | 2,000,000 | | | | 300,000 | | | | 1,998,000 | | | | 1,117,013 | | | | 11,085,291 | | | | 499,200 | | | | 6,240,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2023 | | | 400,000 | | | $ | 2,000,000 | | | | 300,000 | | | $ | 1,998,000 | | | | 1,117,013 | | | $ | 11,085,291 | | | | 499,200 | | | $ | 6,240,000 | |
| (Unaudited) | | Redeemable Convertible Preferred Stock | |
| | | Preferred Stock – Series A-4 | | | Preferred Stock – Series A-5 | | | Preferred Stock – Totals | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | |
| As of December 31, 2021 | | | 718,997 | | | $ | 9,346,961 | | | | 367,015 | | | $ | 4,771,248 | | | | 3,402,225 | | | $ | 35,441,500 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2022 | | | 718,997 | | | | 9,346,961 | | | | 367,015 | | | | 4,771,248 | | | | 3,402,225 | | | | 35,441,500 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2022 | | | 718,997 | | | | 9,346,961 | | | | 367,015 | | | | 4,771,248 | | | | 3,402,225 | | | | 35,441,500 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2023 | | | 718,997 | | | $ | 9,346,961 | | | | 367,015 | | | $ | 4,771,248 | | | | 3,402,225 | | | $ | 35,441,500 | |
The accompanying notes are an integral part of these financial statements.
SERINA THERAPEUTICS, INC.
STATEMENT OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
FOR THE SIX MONTHS ENDED JUNE 30, 2023 AND 2022
| (Unaudited) | | Common Stock Shares | | | Common Stock Amount | | | Additional Paid in Capital | | | Accumulated Deficit | | | Total Stockholders’ Equity (Deficit) | |
| As of December 31, 2021 | | | 2,144,800 | | | $ | 21,448 | | | $ | 635,341 | | | $ | (35,764,374 | ) | | $ | (35,107,585 | ) |
| Stock based compensation | | | - | | | | - | | | | 1,852 | | | | - | | | | 1,852 | |
| Net loss | | | - | | | | - | | | | - | | | | (1,409,566 | ) | | | (1,409,566 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2022 | | | 2,144,800 | | | $ | 21,448 | | | $ | 637,193 | | | $ | (37,173,940 | ) | | $ | (36,515,299 | ) |
| (Unaudited) | | Common Stock Shares | | | Common Stock Amount | | | Additional Paid in Capital | | | Accumulated Deficit | | | Total Stockholders’ Equity (Deficit) | |
| As of December 31, 2022 | | | 2,218,500 | | | $ | 22,185 | | | $ | 646,136 | | | $ | (38,446,482 | ) | | $ | (37,778,161 | ) |
| Exercise of stock options | | | 15,600 | | | | 156 | | | | 780 | | | | - | | | | 936 | |
| Stock based compensation | | | - | | | | - | | | | 24,325 | | | | - | | | | 24,325 | |
| Net income | | | - | | | | - | | | | - | | | | 2,466,594 | | | | 2,466,594 | |
| | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2023 | | | 2,234,100 | | | $ | 22,341 | | | $ | 671,241 | | | $ | (35,979,888 | ) | | $ | (35,286,306 | ) |
The accompanying notes are an integral part of these financial statements.
SERINA THERAPEUTICS, INC.
STATEMENT OF CASH FLOWS
FOR THE SIX MONTHS ENDED
| | | (Unaudited) | | | (Unaudited) | |
| | | June 30, | | | June 30, | |
| | | 2023 | | | 2022 | |
| Cash Flows from Operating Activities | | | | | | | | |
| Net income (loss) | | $ | 2,466,594 | | | $ | (1,409,566 | ) |
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 42,576 | | | | 27,359 | |
| Loss on disposal of property & equipment | | | - | | | | 3,083 | |
| Stock based compensation | | | 24,325 | | | | 1,852 | |
| Loss on equity securities, net | | | - | | | | 7,586 | |
| Reinvested interest and dividend income | | | - | | | | (1,791 | ) |
| Change in fair value of warrant liability | | | (463,404 | ) | | | 485,833 | |
| Non-cash lease expense | | | 78,122 | | | | 73,672 | |
| Fair value inception adjustment on convertible promissory notes | | | (2,240,000 | ) | | | - | |
| Change in fair value of convertible promissory notes | | | (1,864,362 | ) | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | - | | | | 1,109 | |
| Prepaid expenses | | | 1,149 | | | | - | |
| Accounts payable | | | 216,918 | | | | 56,155 | |
| Credit card payable | | | 8,543 | | | | (575 | ) |
| Accrued interest | | | 281,703 | | | | - | |
| Payroll liabilities | | | (2,557 | ) | | | (4,244 | ) |
| Operating lease liabilities | | | (85,199 | ) | | | (80,748 | ) |
| Contract liabilities | | | (37,004 | ) | | | - | |
| Net Cash Used in Operating Activities | | | (1,572,596 | ) | | | (840,275 | ) |
| | | | | | | | | |
| Cash Flows from Investing Activities | | | | | | | | |
| Proceeds from sale of investments in equity securities | | | - | | | | 990,652 | |
| Purchases of property and equipment | | | (315,341 | ) | | | - | |
| Net Cash (Used in) Provided by Investing Activities | | | (315,341 | ) | | | 990,652 | |
| | | | | | | | | |
| Cash Flows from Financing Activities | | | | | | | | |
| Proceeds from issuance of convertible promissory notes | | | 10,100,000 | | | | 250,000 | |
| Proceeds from exercise of stock options | | | 936 | | | | - | |
| Principal repayments on finance lease liabilities | | | (23,144 | ) | | | (20,547 | ) |
| Net Cash Provided by Financing Activities | | | 10,077,792 | | | | 229,453 | |
| | | | | | | | | |
| Net Increase in Cash | | | 8,189,855 | | | | 379,830 | |
| Cash, Beginning of Period | | | 532,229 | | | | 314,846 | |
| Cash, End of Period | | $ | 8,722,084 | | | $ | 694,676 | |
The accompanying notes are an integral part of these financial statements.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Note 1 - Summary of Significant Accounting Policies
Nature of Business
Serina Therapeutics, Inc. (the “Company” or “Serina”) is a privately-held clinical-stage biotechnology company developing a pipeline of wholly owned drug product candidates to treat neurological diseases and pain. Serina’s POZ drug delivery technology is designed to enable certain existing drugs and novel drug candidates to be modified in a way that may provide an increase in efficacy and safety of the resulting polymeric drug conjugate. Serina’s proprietary POZ technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection. Serina is headquartered in Huntsville, Alabama.
Basis of Accounting
The accompanying financial statements reflect the operations of the Company and have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
Liquidity
The Company recognized net income of approximately $2,467,000 for the six months ended June 30, 2023. The Company used approximately $1,600,000 in net cash from operating activities for the six months ended June 30, 2023 and has historically incurred losses from operations and expects to continue to generate negative cash flows as the Company implements its business plan. There is substantial doubt about the Company’s ability to continue as a going concern. The financial statements are prepared using GAAP as applicable to a going concern.
The Company had cash on hand of approximately $8,700,000 as of June 30, 2023. As discussed in Note 14, during the six months ended June 30, 2023, the Company raised $10,100,000 in additional debt. The debt is expected to fund operations through approximately March 2024.
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, technical risks associated with the successful research, development and manufacturing of therapeutic candidates, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations, and the ability to secure additional capital to fund operations. Therapeutic candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel, and infrastructure. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will realize revenue from product sales. The Company largely relies on raising capital from equity investors for funding its operations. Some funding is obtained through licensing agreements or other arrangements with commercial entities.
As a result of recurring losses from operations and recurring negative cash flows from operations, there is uncertainty regarding the Company’s ability to maintain liquidity sufficient to operate its business effectively. If sufficient capital is not available, the Company would be required to delay, limit, reduce, or terminate its product development or future commercialization efforts or grant rights to develop and market therapeutic candidates to other entities. There can be no assurance that the Company will be able to raise additional funds or that the terms and conditions of any future financings will be workable or acceptable to the Company or its shareholders. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Cash
Cash includes cash held in readily available checking and money market accounts.
Contract Liabilities
Contract liabilities include billings in excess of revenue recognized. Contract liabilities are classified as current based on the Company’s contract operating cycle and reported on a contract-by-contract basis, net of revenue recognized, at the end of each reporting period.
Property and Equipment
Property and equipment are carried at cost less accumulated depreciation. The costs of additions and betterments are capitalized and expenditures for repairs and maintenance are expensed as incurred. When items of property and equipment are sold or retired, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is included in the statements of operations. Depreciation of property and equipment is provided utilizing the straight-line method over the range of lives used of the respective assets, which is 3 - 10 years.
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if its carrying amount exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposal of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. No impairment loss was recognized during the six months ended June 30, 2023 and 2022, respectively.
Revenue Recognition
The Company recognizes revenue under a grant contract with a commercial entity under federal funding and under licensing agreements with commercial entities in accordance with ASC 606, Revenue from Contracts with Customers. Under the commercial grant, the Company received substantially all funding upon award of the grant, and recognizes revenue as eligible expenses under the grant are incurred. The Company has concluded that it is a principal in these transactions and, accordingly, recognizes revenue in the gross amount of consideration to which it is entitled from the agency in exchange for the services provided. The period of performance for the commercial grant is August 2022 - December 2023.
Under revenue sharing licensing agreements, the Company receives reimbursement for eligible costs as well as payments upon the achievement of certain milestones as defined by the contract. These licensing agreements provide for the Company to receive a certain percentage of revenue from sales of their product; however, no sales have occurred to date.
Under other licensing agreements, the Company receives payment for the production and use of their polymer technology.
The Company accounts for a contract after is has been approved by all parties to the arrangement, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and collection is probable.
Each contract is assessed at inception to determine whether it should be combined with other contracts. When making this determination, factors such as whether two or more contracts were negotiated or executed at or near the same time or were negotiated with an overall profit objective. If combined, the Company treats the combined contracts as a single contract for revenue recognition purposes.
The Company evaluates the services promised in each contract at inception to determine whether the contract should be accounted for as having one or more performance obligations. The services in the contracts are typically not distinct from one another due to the requirements to perform under the contract. Accordingly, the contracts are typically accounted for as one performance obligation.
The Company determines the transaction price for each contract based on the consideration expected to be received for the services being provided under the contract. For contracts where a portion of the price may vary, the Company estimates variable consideration at the most likely amount, which is included in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur. The Company analyzes the risk of a significant reversal and if necessary, constrains the amount of variable consideration recognized in order to mitigate the risk. At inception of a contract, the transaction price is estimated based on current rights and do not contemplate future modifications (including unexercised options) or follow-on contracts until they become legally enforceable. Depending on the nature of the modification, the Company considers whether to account for the modification as an adjustment to the existing contract or as a separate contract.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
For contracts with multiple performance obligations, the transaction price is allocated to each performance obligation based on the estimated standalone selling price of the service underlying each performance obligation. Revenue is recognized as performance obligations are satisfied and the customer obtains control of the service. Substantially all of the revenue is recognized over time using the input method as the Company performs under the contract because control of the work in process transfers continuously to the customer.
For performance obligations in which control does not continuously transfer to the customer, revenue is recognized at the point in time that each performance obligation is fully satisfied.
At June 30, 2023, the Company is obligated under a grant to continue its research and development related to the treatment of neurological disorders and stroke. The Company expects to complete the remaining performance obligation and recognize the remainder of deferred revenue during the year ended December 31, 2023.
Milestone payments are recognized as licensing revenue upon the achievement of specified milestones if (i) the milestone is substantive in nature and the achievement of the milestone was not probable at the inception of the agreement; and (ii) the Company has a right to payment. Any milestone payments received prior to satisfying these revenue recognition criteria are recorded as deferred revenue.
Various economic factors affect revenues and cash flows. Services are primarily provided under grants from U.S. Government agencies and a licensing agreement with a commercial entity, with amounts invoiced quarterly or upon the achievement of a milestone and typically being collected within one month.
Research and Development
Research and development costs are expensed as they are incurred and include compensation for scientists, support personnel, outside contracted services, and material costs associated with product development. The Company continually evaluates new product opportunities and engages in intensive research and product development efforts. Research and development expenses include both direct costs tied to a specific contract or grant, and indirect costs.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. The Company maintains cash in accounts with high quality, federally insured financial institutions. At times, the balances in these accounts may be in excess of federally insured limits and were in excess by approximately $8,500,000 and $280,000 at June 30, 2023 and December 31, 2022, respectively.
For the six months ended June 30, 2023, 100% of the Company’s revenue was related to a single grantor. For the six months ended June 30, 2022, 100% of the Company’s revenue was related to a single contract with a customer.
Fair Value of Financial Instruments
The Company has adopted ASC Topic 820 for certain financial instruments measured as fair value on a recurring basis. ASC Topic 820 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States, and expands disclosures about fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC Topic 820 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements).
The three levels of inputs that may be used to measure fair value are as follows:
| | ● | Level 1: Quoted prices in active markets for identical assets or liabilities. |
| | | |
| | ● | Level 2: Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets with insufficient volume or infrequent transactions (less active markets), or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated with observable market data for substantially the full term of the assets or liabilities. Level 2 inputs also include non-binding market consensus prices that can be corroborated with observable market data, as well as quoted prices that were adjusted for security-specific restrictions. |
| | | |
| | ● | Level 3: Unobservable inputs to the valuation methodology are significant to the measurement of the fair value of assets or liabilities. Level 3 inputs also include non-binding market consensus prices or non-binding broker quotes that we were unable to corroborate with observable market data. |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The Company has elected to measure the convertible promissory notes at fair value on a recurring basis.
Redeemable Convertible Preferred Stock
The Company recorded redeemable convertible preferred stock at fair value upon issuance, net of any issuance costs. The Company classified stock that was redeemable in circumstances outside of the Company’s control outside of permanent equity.
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due plus deferred taxes related primarily to differences between the basis of loss carryovers and depreciation differences for financial and income tax reporting. Deferred taxes represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which the differences are expected to be recovered or settled.
Earnings (Loss) Per Share
Basic earnings (loss) per share (“EPS”) of common stock is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period.
Diluted EPS give effect to all dilutive potential common shares outstanding during the period using the treasury stock method for stock options and warrants and the if-converted method for redeemable, convertible preferred stock and convertible promissory notes. In computing diluted EPS, the average stock price for the period is used to determine the number of shares assumed to be purchased from the exercise of stock options and/or warrants. Diluted EPS excluded all dilutive potential shares if their effect is anti-dilutive.
Basic loss per common share is computed based on the weighted average number of shares outstanding during the period. Diluted loss per share is computed in a manner similar to the basic loss per share, except the weighted-average number of shares outstanding is increased to include all common shares, including those with the potential to be issued by virtue of convertible debt and other such convertible instruments. Diluted loss per share contemplates a complete conversion to common shares of all convertible instruments only if they are dilutive in nature with regards to earnings per share.
Segments
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, the Chief Executive Officer, in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business as one operating segment in the United States of America.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Note 2 – Fair Value Measurements
The Company had the following assets and liabilities measured at fair value on a recurring basis at June 30, 2023.
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
| Assets | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | | | | | |
| Convertible promissory notes | | | 7,612,638 | | | | - | | | | - | | | | 7,612,638 | |
| Warrant liability | | | 613,362 | | | | - | | | | - | | | | 613,362 | |
| Total | | $ | 8,226,000 | | | $ | - | | | $ | - | | | $ | 8,226,000 | |
The Company had the following assets and liabilities measured at fair value on a recurring basis at December 31, 2022.
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
| Assets | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | | | | | |
| Convertible promissory notes | | | 1,617,000 | | | | - | | | | - | | | | 1,617,000 | |
| Warrant liability | | | 1,076,766 | | | | - | | | | - | | | | 1,076,766 | |
| Total | | $ | 2,693,766 | | | $ | - | | | $ | - | | | $ | 2,693,766 | |
The following is a reconciliation of the beginning and ending balances for the liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the six months ended June 30, 2023 and the year ended December 31, 2022:
| | | Convertible Promissory Notes | | | Warrant Liability | |
| Balance as of December 31, 2021 | | $ | - | | | $ | 952,648 | |
| Convertible debt issuance | | | 1,529,000 | | | | - | |
| Change in fair value | | | 88,000 | | | | 124,118 | |
| Balance as of December 31, 2022 | | | 1,617,000 | | | | 1,076,766 | |
| | | Convertible Promissory Notes | | | Warrant Liability | |
| Balance as of December 31, 2022 | | $ | 1,617,000 | | | $ | 1,076,766 | |
| Convertible debt issuance | | | 10,100,000 | | | | - | |
| Inception adjustment | | | (2,240,000 | ) | | | - | |
| Change in fair value | | | (1,864,362 | ) | | | (463,404 | ) |
| Balance as of June 30, 2023 | | $ | 7,612,638 | | | $ | 613,362 | |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Note 3 - Contract Balances
The beginning and ending contract balances were as follows:
| | | June 30, 2023 | | | December 31, 2022 | | | December 31, 2021 | |
| Deferred revenue | | $ | 116,496 | | | $ | 153,500 | | | $ | - | |
Receipts of $116,496 were included in contract liabilities at June 30, 2023 related to a contract in progress.
Note 4 - Profit Sharing Plan
Serina has established a 401(k) profit sharing plan (the PSP) for all eligible employees of Serina. The PSP provides for eligible employee contributions subject to certain annual Internal Revenue Code limits. For participants who are age 50 or older during any calendar year, additional employee contributions are allowed under the PSP, subject to Internal Revenue Code limits.
Employer contributions, if any, may include matching contributions and profit sharing contributions, both of which are made on a discretionary basis and are subject to service and employment requirements. Employer matching contributions and employer profit sharing contributions vest based on a graded vesting schedule. Serina made no discretionary employer matching or employer profit sharing contributions for the six months ended June 30, 2023 and 2022.
Note 5 - Common Stock
The holders of the common stock are entitled to one vote for each share of common stock on all matters that may be submitted to the holders of common stock of Serina. The holders of common stock shall vote together with the holders of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock as a single class. Serina shall not plan a merger or conversion, sale or dispose of property, or dissolve the Company without the written consent or affirmative vote of the entire single class.
Note 6 - Redeemable Convertible Preferred Stock
Serina has authority to issue 10,000,000 shares of preferred stock with a par value of $0.01 per share. All outstanding preferred stock is redeemable upon a Deemed Liquidation Event and convertible and has been classified as temporary stockholders’ equity at June 30, 2023 and December 31, 2022, as the shares are redeemable for cash or other assets upon the occurrence of an event that is not solely within the control of the Company. As of June 30, 2023 and December 31, 2022, all outstanding preferred stock was not initially redeemable and was not considered probable of being redeemed upon consideration of the events that would constitute a Deemed Liquidation Event.
Redeemable convertible preferred stock was as follows as of June 30, 2023 and December 31, 2022:
| Preference Order | | Designation | | Shares Designated | | | Shares Issued | | | Shares Outstanding | | | Issue Price per Share | | | Liquidation Preference | |
| #1 | | Series A Preferred Stock | | | 400,000 | | | | 400,000 | | | | 400,000 | | | $ | 5.00 | | | $ | 2,000,000 | |
| #2 | | Series A-1 Preferred Stock | | | 300,000 | | | | 300,000 | | | | 300,000 | | | $ | 6.66 | | | $ | 1,998,000 | |
| #3 | | Series A-2 Preferred Stock | | | 1,117,013 | | | | 1,117,013 | | | | 1,117,013 | | | $ | 9.93793 | | | $ | 11,085,291 | |
| #4 | | Series A-3 Preferred Stock | | | 499,200 | | | | 499,200 | | | | 499,200 | | | $ | 12.50 | | | $ | 6,240,000 | |
| #5 | | Series A-4 Preferred Stock | | | 718,997 | | | | 718,997 | | | | 718,997 | | | $ | 13.00 | | | $ | 9,346,961 | |
| #6 | | Series A-5 Preferred Stock | | | 2,000,000 | | | | 367,015 | | | | 367,015 | | | $ | 13.00 | | | $ | 4,771,248 | |
| | | | | | 5,035,210 | | | | 3,402,225 | | | | 3,402,225 | | | | | | | $ | 35,441,500 | |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock all have the same rights, preferences, powers, privileges, restrictions, qualifications, and limitations.
Dividends
From and after the date of the issuance of any shares of Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock, they accrued dividends at the rate per annum of 6% of the original issue price until June 30, 2019. The dividends accrued from day to day, whether or not declared, compounded annually, were calculated on the basis of a 365-day year, and were cumulative. Effective June 30, 2019, Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock no longer accrued dividends. The holders of the Series A Preferred Stock, Series A-1 Preferred Stock, and Series A-2 Preferred Stock were entitled to receive dividends when and if declared by the Board of Directors. The Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall not accrue dividends. Dividends on Preferred Stock are in preference to and prior to any payment of any dividends on common stock. As of June 30, 2023 and December 31, 2022, $10,276,653 in Preferred Stock dividends have been accrued, but not declared. As such, these dividends are not currently an obligation of the Company.
Convertibility
Each share of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall be convertible, at the option of the holder, at any time and from time to time, and without the payment of additional consideration by the holder into common stock. The number of shares will be determined by dividing the original issue price by the conversion price of the Preferred Stock. The conversion price initially is the same as the issue price of the Preferred Stock, but may be adjusted based on certain events as provided in the amended Articles of Incorporation.
Liquidation Preference
In the event of Deemed Liquidation Event for Serina (described below), the holders of the various series of Preferred Stock are entitled to receive prior to, and in preference to, any distribution to the holders of common stock or any other class or series of stock ranking on liquidation junior to the series of Preferred Stock they hold, an amount equal to the original issue price plus any accrued dividends (excepting Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock) together with any other dividends declared that have not yet been paid. The preference order is noted above.
The following events will constitute a Deemed Liquidation Event:
| | a) | The consummation of a plan of merger or conversion in accordance with various sections of Alabama Business Corporation Law (“ABCL”), with some exceptions as provided in the amended Articles of Incorporation; |
| | | |
| | b) | The sale, lease, exchange, license, or other disposal, in a single transaction or series of related transactions, of all, or substantially all, of the corporation’s property (with or without goodwill), other than in the usual course of business in accordance with Section 10A-2A-12.02 of the ABCL; |
| | | |
| | c) | Dissolution of the corporation in accordance with Section 10A-2A-14.02 of the ABCLE, or liquidation or winding-up of the business and affairs of the corporation; or |
| | | |
| | d) | The sale, lease, exchange, license, or other disposal, other than in the usual and regular course of business, of all, or substantially all, of the intellectual property of the corporation in a single transaction or a series of related transactions. |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The holders of at least 60% of the Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock, acting as a single class of stock, may elect that any such events described above shall not be a Deemed Liquidation Event as provided in the amended Articles of Incorporation.
The Series A Preferred Stock liquidation preference, the Series A-1 Preferred Stock liquidation preference, the Series A-2 liquidation preference, the Series A-3 liquidation preference, the Series A-4 liquidation preference, and the Series A-5 liquidation preference shall be on parity with one another. In the event that upon liquidation or dissolution, the assets and funds of the Company are insufficient to permit the payment to holders of shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock the full amount to which they shall be entitled, the holders of shares of Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock then outstanding and any class or series of stock ranking on liquidation on a parity with the Series A Preferred Stock, Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series A-3 Preferred Stock, Series A-4 Preferred Stock, and Series A-5 Preferred Stock shall share in any distribution of the remaining assets available for distribution in proportion to the respective amounts which would otherwise be payable in respect of the shares held by them upon such distribution if all amounts payable on or with respect to such shares were paid in full.
After the distributions described above have been paid in full, the remaining assets of the Company available for distribution shall be distributed pro-rata to the holders of the shares of common stock.
Note 7 - Income Taxes
Deferred tax assets and liabilities reflect the effects of tax losses, credits, and the future income tax effects of temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and are measured using enacted tax rates that apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The Company’s effective tax rate is 0% for the six months ended June 30, 2023 and 2022. The Company’s effective tax rate is different than what would be expected if the federal statutory rate was applied to income before income taxes primarily because certain expenses are deductible for financial reporting purposes that are not deductible for tax purposes and valuation allowances applied to the Company’s net deferred tax assets. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The major temporary differences that give rise to the deferred tax assets and liabilities are as follows: NOL carryovers, general business credit carryovers, charitable contribution carryovers, operating and finance lease assets and liabilities, and depreciation and amortization.
The Company evaluates the recoverability of these future tax deductions and credits by assessing the adequacy of future expected taxable income from all sources, including reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. To the extent that management does not consider it more likely than not that a deferred tax asset will be recovered, a valuation allowance is established. The Company has provided a valuation allowance against the deferred income taxes at June 30, 2023 and December 31, 2022. The valuation allowance increased by $147,382 during the six months ended June 30, 2023.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The components of the deferred income tax asset and liability as of June 30, 2023 and December 31, 2022 are as follows:
| | | June 30, 2023 | | | December 31, 2022 | |
| Deferred Tax Assets (Liabilities): | | | | | | | | |
| General business credit carryover ESB | | $ | 129,310 | | | $ | 129,310 | |
| General business credit carryover NonESB | | | 1,824,263 | | | | 1,824,263 | |
| 1231 loss carryover | | | 219 | | | | 219 | |
| Net capital loss carryover | | | 4,805 | | | | 4,805 | |
| NOL carryover - Federal | | | 7,771,458 | | | | 7,399,496 | |
| NOL carryover - State | | | 2,395,855 | | | | 2,308,126 | |
| Research and development | | | - | | | | 389,339 | |
| Charitable contributions carryover | | | 6,875 | | | | 6,875 | |
| Right of use assets - operating leases | | | (12,266 | ) | | | (23,307 | ) |
| Operating lease liabilities | | | 16,432 | | | | 29,419 | |
| Depreciation of property and equipment | | | 99,752 | | | | 20,776 | |
| Total deferred tax asset | | | 12,236,703 | | | | 12,089,321 | |
| Valuation allowance | | | (12,236,703 | ) | | | (12,089,321 | ) |
| Net Deferred Tax Asset | | $ | - | | | $ | - | |
The Company only recognizes tax benefits from an uncertain tax position if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statement from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. To date, the Company has not recognized such tax benefits in its financial statements.
Note 8 - Leases
The Company leases many of its operating and office facilities for various terms under long-term, non-cancelable operating lease agreements. The leases expire at various dates through 2024 and provide for renewal periods of 2 years. In the normal course of business, it is expected that these leases will be renewed or replaced by leases on other properties. The Company also leases equipment for various terms under long-term, non-cancelable finance lease agreements. These leases expire at various dates through 2025.
The following represents information on leases as of and for the six months ended June 30:
| | | 2023 | | | 2022 | |
| Finance lease cost (including amortization of right-of use assets of $11,675 and $11,675 and interest on lease liabilities of $4,454 and $7,051 in 2023 and 2022, respectively) | | $ | 16,129 | | | $ | 18,726 | |
| Operating lease expense | | | 95,059 | | | | 83,531 | |
| | | | | | | | | |
| Total lease cost | | $ | 111,188 | | | $ | 102,257 | |
| | | | | | | | | |
| Other information | | | | | | | | |
| Weighted average remaining lease term (years) - finance | | | 1.14 | | | | 1.71 | |
| Weighted average remaining lease term (years) - operating | | | 4.14 | | | | 1.30 | |
| Weighted average discount rate - finance leases | | | 11.90 | % | | | 11.90 | % |
| Weighted average discount rate - operating leases | | | 6.67 | % | | | 6.67 | % |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The following is a maturity analysis of the annual undiscounted cash flows of the lease liabilities as of June 30, 2023:
| | | Operating Leases | | | Finance Leases | |
| Six months ended December 31, 2023 | | $ | 93,152 | | | $ | 27,598 | |
| Year ending December 31, 2024 | | | 143,466 | | | | 38,055 | |
| Year ending December 31, 2025 | | | 116,592 | | | | 629 | |
| Year ending December 31, 2026 | | | 116,592 | | | | - | |
| Year ending December 31, 2027 | | | 116,592 | | | | - | |
| Thereafter | | | 9,716 | | | | - | |
| Total undiscounted lease payments | | | 596,110 | | | | 66,282 | |
| Less: imputed interest | | | (77,025 | ) | | | (4,750 | ) |
| Total lease obligations | | | 519,085 | | | | 61,532 | |
| Less: current portion | | | (141,709 | ) | | | (50,638 | ) |
| Long-term lease obligations | | $ | 377,376 | | | $ | 10,894 | |
Right of use assets - operating leases obtained in exchange for new operating lease liabilities was $497,306 for the six months ended June 30, 2023.
Note 9 - Property and Equipment
Property and equipment at June 30, 2023 and December 31, 2022 consists of:
| | | June 30, 2023 | | | December 31, 2022 | |
| Computer | | $ | 28,021 | | | $ | 24,061 | |
| Equipment | | | 649,881 | | | | 353,343 | |
| Software | | | 96,517 | | | | 81,676 | |
| Total property and equipment | | | 774,419 | | | | 459,080 | |
| Less: accumulated depreciation | | | (398,411 | ) | | | (367,511 | ) |
| Property and equipment, net | | $ | 376,008 | | | $ | 91,569 | |
Note 10 - Stock Option Plan
Stock Option Plan Description
In 2007, the Company’s Board of Directors (“the Board”) adopted the Serina Therapeutics, Inc. 2007 Stock Option Plan (the 2007 Plan) that provides for the granting of stock options to employees. The 2007 Plan as amended in March 2014 reserved 2,000,000 shares of common stock for grant. Options are granted at the discretion of the Board. Options granted under the 2007 Plan shall be either incentive stock options (ISOs) or nonstatutory stock options (NSOs), as designated by the Board. The options will be granted at an exercise price set by the Board at the time of grant, but in no event will the price for ISOs be less than 100% of the fair market value of the common stock on the date of the grant, or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, not less than 110%; and not less than the par value of the common stock at grant, in the case of NSOs.
In 2017, the Serina Therapeutics, Inc. 2007 Plan by its terms expired, except with respect to unexercised stock options outstanding under the 2007 Plan. No future stock options shall be awarded under the 2007 Plan.
In 2017, the Company’s Board adopted the Serina Therapeutics, Inc. 2017 Stock Option Plan (the 2017 Plan) that provides for the granting of stock options to employees. The 2017 Plan reserved 1,000,000 shares of common stock for grant. In 2021, the 2017 Plan was amended to increase the reserved shares to 2,000,000, and then later in 2021 further amended to increase the reserved shares to 2,100,000. Options are granted at the discretion of the Board. Options granted under the 2017 Plan shall be either incentive stock options (ISOs) or nonstatutory stock options (NSOs), as designated by the Board. The options will be granted at an exercise price set by the Board at the time of grant, but in no event will the price for ISOs be less than 100% of the fair market value of the common stock on the date of the grant or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, not less than 110%; and not less than the par value of the common stock at grant, in the case of NSOs.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Vesting and Exercisability
Stock options are exercisable at such time or times and subject to such terms and conditions as determined by the Board at or after grant. If the Board provides, in its sole discretion, that any stock option is exercisable only in installments, the Board may waive such installment exercise provisions at any time at or after the grant, in whole or in part, based on such factors as the Board shall determine, in its sole discretion.
The terms of the grants are generally for ten years (or in the case of an option holder who owns more than 10% of the total combined voting power of all classes of stock of Serina, the term shall be five years) and vest over a period of time as determined on the date of the grant.
Stock-Based Compensation
The Company follows the provisions of the FASB ASC 718, Compensation - Stock Compensation (“FASB ASC 718”). FASB ASC 718 requires all share-based payments to employees, including grants of employee stock options, to be recognized as compensation expense in the financial statements based on their fair value at the grant date.
Valuation and Expense
The Company recognizes compensation expense related to stock options on a straight-line basis over the requisite service period of the awards, which is generally three to four years. The Company estimates the fair value of options on the date of grant using the Black-Scholes-Merton option pricing model. There were no options issued during the six months ended June 30, 2023 or 2022.
The Company recorded stock-based compensation expense related to the stock options of $24,359 and $1,852 during the six months ended June 30, 2023 and 2022, respectively.
In 2021, all options outstanding as of December 31, 2020 were canceled and replaced, with the exception of 3,300 options, which were not replaced due to termination of the grantee. Total replacement options issued were 1,294,042. Incremental compensation cost of $113 and $219 was recognized during the six months ended June 30, 2023 and 2022, respectively, as a result of the accounting for the modification required by FASB ASC 718.
In February 2023, by vote of the Board all remaining unvested options became fully vested. Therefore as of June 30, 2023, the Company had no unrecognized compensation cost related to nonvested stock options.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Details of stock option activity for the six months ended June 30, 2023 and 2022 are as follows:
| | | Number of Options | | | Weighted Average Exercise Price | |
| Outstanding at December 31, 2021 | | | 2,036,450 | | | $ | 0.06 | |
| Outstanding at June 30, 2022 | | | 2,036,450 | | | $ | 0.06 | |
| | | Number of Options | | | Weighted Average Exercise Price | |
| Outstanding at December 31, 2022 | | | 2,012,750 | | | $ | 0.06 | |
| Forfeited | | | (7,000 | ) | | | 0.06 | |
| Exercised | | | (15,600 | ) | | | 0.06 | |
| Outstanding at June 30, 2023 | | | 1,990,150 | | | $ | 0.06 | |
The following table summarizes information about stock options exercisable at June 30, 2023:
| Exercise Price | | | Number of Shares Exercisable | | | Weighted Average Remaining Contractual Life in Years | | | Weighted Average Exercise Price | |
| $ | 0.06 | | | | 1,990,150 | | | | 7.98 | | | $ | 0.06 | |
The following table summarizes information about nonvested stock options for the six months ended June 30, 2023 and 2022:
| | | Nonvested Options | | | Weighted Average
Grant Date Fair Value | |
| Nonvested options at December 31, 2021 | | | 656,742 | | | $ | 0.04 | |
| Vested | | | (12,000 | ) | | | 0.03 | |
| Nonvested options at June 30, 2022 | | | 644,742 | | | | 0.04 | |
| | | Nonvested Options | | | Weighted Average Grant Date Fair Value | |
| Nonvested options at December 31, 2022 | | | 548,807 | | | $ | 0.05 | |
| Forfeited/cancelled | | | (1,000 | ) | | | 0.03 | |
| Vested | | | (547,807 | ) | | | 0.04 | |
| Nonvested options at June 30, 2023 | | | - | | | $ | - | |
Note 11 - Stock Warrants
Grants
No warrants were granted during the six months ended June 30, 2023 and 2022.
The warrants contain both put and call options. The Company may call the warrants at any time following the date the warrants become exercisable. The warrants are accounted for as a liability and remeasured to fair value at each reporting period. The liability as of June 30, 2023 and December 31, 2022 was $613,362 and $1,076,766, respectively.
Vesting and Exercisability
The warrants vest immediately upon grant. All outstanding warrants at June 30, 2023 are exercisable at any time prior to close of business on December 31, 2024.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Valuation and Expense
The Company estimates the fair value of warrants using the Black-Scholes-Merton option pricing model with the following assumptions at the reporting date:
| | | June 30, 2023 | | | June 30, 2022 | |
| Expected volatility | | | 92 | % | | | 88 | % |
| Expected term (in years) | | | 0.5 - 1.0 | | | | 1.5 - 2.0 | |
| Risk-free interest rate | | | 5.40% - 5.47 | % | | | 2.80% - 2.92 | % |
| Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
Expected volatility is estimated using the historical volatility of comparable public entities. The Company estimates the expected term using historical option exercise data to determine the expected employee exercise behavior. The risk-free interest rate is the yield on a U.S. Treasury zero-coupon issue with a remaining term equal to or approximating the expected term of the option at the grant date.
Related to the warrants, the Company recorded an expense of $463,404 due to the increase in the liability and a benefit of $485,833 due to the decrease in the liability during the quarters ended June 30, 2023 and 2022, respectively.
Details of stock warrant activity for the six months ended June 30, 2023 and 2022, are as follows:
| | | Number of Warrants | | | Weighted Average Exercise Price | |
| Outstanding at December 31, 2021 | | | 367,015 | | | $ | 20.00 | |
| Outstanding at June 30, 2022 | | | 367,015 | | | | 20.00 | |
| Outstanding at December 31, 2022 | | | 367,015 | | | | 20.00 | |
| Outstanding at June 30, 2023 | | | 367,015 | | | $ | 20.00 | |
Note 12 - Research and Development
At June 30, 2023, the Company is performing under a grant provided by a corporation via funding provided by a federal government agency to continue its research and development related to treatment of neurological disorders and strokes. The grant is structured such that the Company received $245,000 upon execution of the award in August 2022 and recognizes eligible costs as they are incurred. Grant revenue and related research and development costs under the grant was $37,002 for the six months ended June 30, 2023.
Note 13 - Commitments and Contingencies
The Company is subject to legal proceedings, claims, and litigation arising in the ordinary course of business. In the opinion of management, all such matters would be adequately covered by insurance or accruals or would be such that disposition would not have a material adverse effect on the financial position, results of operations, or cash flows of the Company.
Note 14 - Convertible Promissory Notes
On March 15, 2023, The Company issued a convertible promissory note (the “AgeX-Serina Note”) in the amount of $10,000,000 to AgeX Therapeutics (“AgeX”). The AgeX-Serina Note bears interest at 7% per annum and matures on March 15, 2026. The Company issued the AgeX-Serina Note to provide for general working capital needs. As described in Note 16, subsequent to the date of these financial statements, the Company and AgeX have entered into a merger agreement. In connection with the issuance of the AgeX-Serina Note, AgeX is entitled to elect one member to the board of directors of the Company and receive certain information and inspection rights as well as participation rights for subsequent equity issuances.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The AgeX-Serina Note has various conversion options. The principal balance of the AgeX-Serina Note with accrued interest will automatically convert into the Company’s preferred stock if the Company raises at least $25,000,000 through the sale of shares of the Company’s preferred stock. The conversion price per share shall be the lower of (a) 80% of the lowest price at which the shares of preferred stock were sold, and (b) a “capped price” equal to $105,000,000 divided by the Company’s then fully diluted capitalization. AgeX has the option to convert the AgeX-Serina Note into the Company’s preferred stock after a sale of the Company’s preferred stock regardless of the amount sold by the Company. AgeX may (i) at its election, upon a change of control (as defined in the AgeX-Serina Note), convert the AgeX-Serina Note in whole or in part into either (a) cash in an amount equal to 100% of the outstanding principal amount of the AgeX-Serina Note, plus interest, or (b) into the highest ranking shares of the Company then issued at a conversion price equal to the lowest price per share at which the most senior series of the Company’s shares has been sold in a single transaction or a series of related transactions through which the Company raised at least $5,000,000 or (ii) if the AgeX-Serina Note remains outstanding as of the maturity date, AgeX may convert the AgeX-Serina Note into the most senior shares of the Company issued at the time of conversion at a conversion price equal to the capped price. Upon the consummation of a merger between the Company and AgeX, the AgeX-Serina Note would remain outstanding and become an intercompany asset of AgeX and an intercompany liability of the Company.
The Company evaluated the AgeX-Serina Note in accordance with ASC Topic 815, Derivatives and Hedging, and determined it contains certain variable share settlement features tied to the price of a future financing which were not considered clearly and closely related to the host instruments. These provisions included automatic conversion upon the event of a Qualified Financing, the Holder’s option to convert the AgeX-Serina Note upon a Non-Qualified Financing, and the Holder’s option to convert or request repayment upon sale of the Company. The AgeX-Serina Note also contains a Change in Control Put and a Default Put which were not clearly and closely related to the host instrument. The Company elected to initially and subsequently measure the AgeX-Serina Note in its entirety at fair value, with changes in fair value recognized in earnings. The fair value inception date adjustment on the instrument is recorded as a component of other income in the Company’s statements of operations.
The change in fair value of the instrument since inception date is recorded on a separate line item as a component of other income in the Company’s statements of operations.
FASB ASC 825-10-25 allows the Company to elect the fair value option for recording financial instruments when they are initially recognized or if there is an event that requires re-measurement of the instruments at fair value, such as a significant modification of the debt. The Company elected the fair value option because they believed it to be the most appropriate method of encompassing the credit risk and exercise behavior that a market participant would consider when valuing the hybrid financial instrument.
As of March 15, 2023 (inception), the outstanding principal on the AgeX-Serina Note was approximately $10,000,000 and the fair value recorded on the balance sheet was $7,760,000. From March 15, 2023 to June 30, 2023, the change in value was approximately $1,684,000 and was recorded as a component of other income in the Company’s statements of operations.
From June 2022 through February 2023, the Company issued interest-bearing Convertible Promissory Notes (the “Serina Convertible Notes”) to various investors in the principal amount of $1,450,000. The Serina Convertible Notes incur interest at 6% per annum and are payable by the Company two years from their issuance date. The Company may not voluntarily prepay the Serina Convertible Notes. Upon a Qualified Equity Financing event in which the Company sells shares of Preferred Stock for aggregate proceeds of at least $15 million, the principal and outstanding interest on the Serina Convertible Notes will automatically convert into shares of the Company’s Preferred Stock issued in the Qualified Financing at a conversion price of the lesser of i) a 20% discount to the price paid by purchasers in the Qualified Financing and ii) the quotient resulted from dividing $100 million by the fully diluted capitalization of the Company immediately prior to the Qualified Financing. If the Company enters into a Non-Qualified Equity Financing (less than $15 million in proceeds), the Holder has the option to convert the Serina Convertible Notes into shares of the Company’s Preferred Stock issued in the Non-Qualified Financing at the price paid per share. The Company may also choose to optionally convert the Serina Convertible Notes into Series A-5 Preferred Stock at a price of $13 per share, and a warrant to purchase shares of Series A-5 Preferred Stock with an exercise price of $20.00, and an expiration date of December 31, 2024. If a Change in Control or an IPO occurs prior to a Qualified Financing, then the Holder has the option to convert outstanding principal and interest into common stock at a price per share equal to an amount obtained by dividing i) the Post-Money Valuation Cap ($100,000,000) by ii) the Fully Diluted Capitalization immediately prior to the conversion. Upon a change in control, the Holder may also elect to require the Company to repay the outstanding principal and accrued but unpaid interest in cash.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The Company evaluated the Serina Convertible Notes in accordance with ASC Topic 815, Derivatives and Hedging, and determined they contained certain variable share settlement features tied to the price of a future financing which were not considered clearly and closely related to the host instruments. These provisions included mandatory conversion upon the event of a Qualified Financing and the Holder’s option to convert the Serina Convertible Notes upon a Non-Qualified Financing. The Serina Convertible Notes also contained a Change in Control Put and a Default Put which were not clearly and closely related to the host instrument. The Company elected to initially and subsequently measure the Serina Convertible Notes in their entirety at fair value, with changes in fair value recognized in earnings. The fair value inception date adjustment on the instrument is recorded as a component of other income in the Company’s statements of operations. The change in fair value of the instrument since inception date is recorded on a separate line item as a component of other income in the Company’s statements of operations.
Because the Serina Convertible Notes are carried in their entirety at fair value, the value of the compound embedded conversion features and the redemption features are embodied in that fair value. The Company estimated the fair value of the hybrid instrument based on a probability weighted cash flow analysis. A Monte Carlo model, which considered the present value of the cash flows under the different scenarios using a credit risk adjusted rate, was considered by management to be the most appropriate method of encompassing credit risk and exercise behavior. Inputs used to value the hybrid instrument at inception (July 19, 2022) included, (i) present value of future cash flows using a credit risk adjusted rate of 16%, (ii) remaining term of approximately 2 years, (iii) volatility of 90% based on the volatility of comparable guideline public companies, (iv) closing stock price on the valuation date, and (v) 70% probability the Serina Convertible Notes will be converted upon a Non-Qualified Financing, at the expected price of the Non-Qualified Financing, and 30% probability the Serina Convertible Notes will be optionally converted at their stated conversion price ($13 per share plus one warrant).
Management expects the probability of a change in control, default, or Qualified Financing event occurring over the remaining term of the Serina Convertible Notes to be minimal. However, this assumption will be considered each reporting period. Material changes due to instrument-specific credit risk are recorded in Other Comprehensive Income with all other changes in value being recorded in net income.
As of June 30, 2023, the outstanding principal on the Serina Convertible Notes was approximately $1,450,000 and the fair value recorded on the balance sheet was $1,536,638. From January 1, 2023 to June 30, 2023, the change in value was approximately $180,362 and was recorded as a component of other income in the Company’s statements of operations. For the six months ended June 30, 2023, interest expense associated with the Serina Convertible Notes issued between June 2022 and February 2023 was approximately $78,000. For the six months ended June 30, 2023, interest expense associated with the AgeX-Serina Note was approximately $204,000.
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
Note 15 - Net Earnings (Loss) Per Common Share
Net earnings (loss) per common share is calculated in accordance with ASC 260, Earnings Per Share. Basic and diluted net earnings (loss) per common share attributable to common shareholders is calculated as follows for the six months ended June 30, 2023 and 2022:
| | | 2023 | | | 2022 | |
| Basic net earnings (loss) per common share allocable to common shareholders | | | | | | | | |
| | | | | | | | | |
| NUMERATOR | | | | | | | | |
| Net income (loss) | | $ | 2,466,594 | | | $ | (1,409,566 | ) |
| Less: net earnings allocable to participating securities | | | - | | | | - | |
| Net earnings (loss) allocable to common shareholders | | | 2,466,594 | | | | (1,409,566 | ) |
| DENOMINATOR | | | | | | | | |
| Weighted-average shares of common stock outstanding used to calculate basic net earnings (loss) per common share | | | 2,221,100 | | | | 2,144,800 | |
| | | | | | | | | |
| Basic net earnings (loss) per common share allocable to common | | | | | | | | |
| shareholders | | $ | 1.11 | | | $ | (0.66 | ) |
| | | | | | | | | |
| Diluted net earnings (loss) per common share allocable to common shareholders | | | | | | | | |
| | | | | | | | |
| NUMERATOR | | | | | | | | |
| Net earnings (loss) allocable to common shareholders | | $ | 2,466,594 | | | $ | (1,409,566 | ) |
| Add back: interest on convertible promissory notes | | | 76,498 | | | | - | |
| Net earnings (loss) allocable to common shareholders | | | 2,543,092 | | | | (1,409,566 | ) |
| DENOMINATOR | | | | | | | | |
Weighted-average shares of common stock outstanding used to calculate basic net earnings (loss) per common share
| | | 2,221,100 | | | | 2,144,800 | |
| Add: dilutive effect of stock options | | | 1,863,484 | | | | - | |
| Add: dilutive effect of warrants | | | - | | | | - | |
| Add: dilutive effect of common stock issued for convertible promissory notes | | | 116,016 | | | | - | |
| Add: dilutive effect of redeemable convertible preferred stock | | | 3,402,225 | | | | - | |
| Weighted-average shares of common stock outstanding used to calculate diluted net earnings (loss) per common share | | | 7,602,825 | | | | 2,144,800 | |
| | | | | | | | | |
| Diluted net earnings (loss) per common share attributable to common shareholders | | $ | 0.33 | | | $ | (0.66 | ) |
SERINA THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(UNAUDITED)
The following table sets forth the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net earnings (loss) per common share for the six months ended June 30, 2023 and 2022 because to do so would be anti-dilutive:
| | | 2023 | | | 2022 | |
| Redeemable convertible preferred stock | | | - | | | | 3,402,225 | |
| Convertible promissory notes | | | - | | | | 40,992 | |
| Stock options | | | - | | | | 2,036,450 | |
| Warrants | | | - | | | | 367,015 | |
| Total anti-dilutive securities | | | - | | | | 5,846,682 | |
Note 16 - Subsequent Events
Convertible Notes
On July 26, 2023, all of the Convertible Promissory Notes described in Note 14 (excluding the AgeX-Serina Note) previously issued for a total principal amount of $1,450,000 and accrued interest of $82,695 were converted to 117,903 shares of Series A-5 Preferred Stock. As provided for in the note agreements, the holders of the notes also received warrants to purchase an additional 117,903 shares of Series A-5 Preferred Stock.
Merger Agreement
On August 29, 2023, the Company entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) with AgeX Therapeutics, Inc. and Canaria Transaction Corporation, an Alabama corporation and wholly owned subsidiary of AgeX (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including that the Merger is approved by the stockholders of AgeX and the stockholders of Serina, Merger Sub will be merged with and into Serina, with Serina surviving as a wholly owned subsidiary of AgeX (the “Merger”). There is no assurance the necessary approvals by AgeX stockholders and Serina stockholders will be obtained or that the other conditions to the Merger as provided in the Merger Agreement will be met.
Immediately following the Merger, equity holders of Serina immediately prior to the closing of the Merger are expected to own approximately 75% of the outstanding shares of AgeX common stock, and stockholders of AgeX immediately prior to the closing of the Merger are expected to own, excluding the impact of the exercise of any of the Post-Merger Warrants or Incentive Warrants described below, approximately 25% of the outstanding shares of AgeX common stock, in each case, on a fully diluted basis, subject to certain assumptions, including giving effect to a planned reverse stock split and the conversion of AgeX Preferred Stock into shares of AgeX common stock. Accordingly, the Merger is anticipated to be accounted for as a reverse acquisition, with the Company treated as the acquiror for financial accounting purposes.
Prior to the closing of the Merger, AgeX will issue to each holder of AgeX common stock three warrants (each, a“Post-Merger Warrant”) for each five shares of AgeX common stock held by such stockholder. Each Post-Merger Warrant will be exercisable for (i) one share of AgeX common stock and (ii) one warrant (each, an “Incentive Warrant”) at an exercise price equal to $13.20 per warrant (such exercise price reflecting a planned reverse stock split of AgeX common stock) and will expire on July 31, 2025. Each Incentive Warrant will be exercisable for one share of AgeX common stock at a price equal to $18.00 per warrant (such exercise price reflecting the planned reverse stock split of AgeX common stock) and will expire on the four-year anniversary of the closing of the Merger. Each Post-Merger Warrant and Incentive Warrant will be issued pursuant to the terms of a warrant agreement to be entered into by AgeX and a warrant agent in connection with the closing of the Merger.
License Agreement
In October 2023, the Company entered into a non-exclusive license agreement with Pfizer, Inc. to use the Company’s POZ polymer technology for use in lipid nanoparticle drug delivery formulations. The agreement grants Pfizer non-exclusive rights to certain intellectual property, know-how, and proprietary technologies. Under the terms of the agreement, Pfizer is authorized to develop, manufacture, market, and commercialize products incorporating the licensed technology with respect to a specific POZ polymer structure in one field. The agreement outlines the protection and enforcement of intellectual property rights related to the licensed technology. Pfizer is obligated to use commercially reasonable diligent efforts to develop and commercialize licensed products, and to use such efforts to accomplish specified development and commercial objectives. The agreement includes a one-time upfront payment of less than $5 million, milestone payments payable to the Company upon the achievement of specific development, regulatory, and commercial milestones, and a royalty on net sales of products incorporating the licensed technology in accordance with the terms outlined in the license agreement.
Annex A
AGREEMENT AND PLAN OF MERGER
AND REORGANIZATION
among:
AgeX Therapeutics, Inc.,
a Delaware corporation;
CANARIA TRANSACTION CORPORATION,
an Alabama corporation; and
Serina THERAPEUTICS, INC.,
an Alabama corporation
Dated as of August 29, 2023
TABLE OF CONTENTS
| | | Page |
| | | |
| SECTION 1. | DESCRIPTION OF TRANSACTION | 2 |
| | | |
| 1.1 | The Merger | 2 |
| 1.2 | Effects of the Merger | 2 |
| 1.3 | Closing; Effective Time | 2 |
| 1.4 | Certificate of Incorporation and Bylaws; Directors and Officers | 3 |
| 1.5 | Conversion of Shares | 4 |
| 1.6 | Closing of the Company’s Transfer Books | 5 |
| 1.7 | Delivery of Merger Consideration | 6 |
| 1.8 | Appraisal Shares | 7 |
| 1.9 | Further Action | 8 |
| 1.10 | Withholding | 8 |
| | | |
| SECTION 2. | REPRESENTATIONS AND WARRANTIES OF THE COMPANY | 8 |
| | | |
| 2.1 | Due Organization; Subsidiaries | 8 |
| 2.2 | Organizational Documents | 9 |
| 2.3 | Authority; Binding Nature of Agreement | 9 |
| 2.4 | Vote Required | 10 |
| 2.5 | Non-Contravention; Consents | 10 |
| 2.6 | Capitalization | 11 |
| 2.7 | Financial Statements | 13 |
| 2.8 | Absence of Changes | 14 |
| 2.9 | Absence of Undisclosed Liabilities | 15 |
| 2.10 | Title to Assets | 15 |
| 2.11 | Real Property; Leasehold | 15 |
| 2.12 | Intellectual Property | 16 |
| 2.13 | Agreements, Contracts and Commitments | 18 |
| 2.14 | Compliance; Permits; Restrictions | 20 |
| 2.15 | Legal Proceedings; Orders | 22 |
| 2.16 | Tax Matters | 22 |
| 2.17 | Employee and Labor Matters; Benefit Plans | 25 |
| 2.18 | Environmental Matters | 28 |
| 2.19 | Insurance | 29 |
| 2.20 | No Financial Advisors | 29 |
| 2.21 | Disclosure | 29 |
| 2.22 | Transactions with Affiliates | 30 |
| 2.23 | Anti-Bribery | 30 |
| 2.24 | Disclaimer of Other Representations or Warranties | 31 |
| | | |
| SECTION 3. | REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB | 31 |
| | | |
| 3.1 | Due Organization; Subsidiaries | 31 |
| 3.2 | Organizational Documents | 32 |
| 3.3 | Authority; Binding Nature of Agreement | 32 |
| 3.4 | Vote Required | 33 |
| 3.5 | Non-Contravention; Consents | 33 |
| 3.6 | Capitalization | 34 |
TABLE OF CONTENTS
(continued)
| | | Page |
| 3.7 | SEC Filings; Financial Statements | 36 |
| 3.8 | Absence of Changes | 38 |
| 3.9 | Absence of Undisclosed Liabilities | 38 |
| 3.10 | Title to Assets | 38 |
| 3.11 | Real Property; Leasehold | 39 |
| 3.12 | Intellectual Property | 39 |
| 3.13 | Agreements, Contracts and Commitments | 42 |
| 3.14 | Compliance; Permits; Restrictions | 44 |
| 3.15 | Legal Proceedings; Orders | 45 |
| 3.16 | Tax Matters | 45 |
| 3.17 | Employee and Labor Matters; Benefit Plans | 48 |
| 3.18 | Environmental Matters | 51 |
| 3.19 | Transactions with Affiliates | 52 |
| 3.20 | Insurance | 52 |
| 3.21 | No Financial Advisors | 52 |
| 3.22 | Disclosure | 52 |
| 3.23 | Anti-Bribery | 53 |
| 3.24 | Valid Issuance | 53 |
| 3.25 | Disclaimer of Other Representations or Warranties | 53 |
| | | |
| SECTION 4. | CERTAIN COVENANTS OF THE PARTIES | 53 |
| | | |
| 4.1 | Operation of Parent’s Business | 53 |
| 4.2 | Operation of the Company’s Business | 56 |
| 4.3 | Access and Investigation | 59 |
| 4.4 | Parent Non-Solicitation | 60 |
| 4.5 | Company Non-Solicitation | 61 |
| 4.6 | Notification of Certain Matters | 61 |
| 4.7 | Potentially Transferable Assets | 62 |
| 4.8 | Parent Legacy Note | 62 |
| 4.9 | Post-Merger Warrant | 63 |
| 4.10 | Sarbanes-Oxley Certification. | 63 |
| 4.11 | Parent Preferred Stock. | 63 |
| 4.12 | Company Notes | 63 |
| 4.13 | Company Incentive Plan and Parent Incentive Plan. | 63 |
| | | |
| SECTION 5. | ADDITIONAL AGREEMENTS OF THE PARTIES | 64 |
| | | |
| 5.1 | Registration Statement; Proxy Statement | 64 |
| 5.2 | Company Information Statement; Stockholder Written Consent | 65 |
| 5.3 | Parent Stockholders’ Meeting | 67 |
| 5.4 | Regulatory Approvals | 71 |
| 5.5 | Options and Warrants. | 71 |
| 5.6 | Indemnification of Officers and Directors | 73 |
| 5.7 | Additional Agreements | 75 |
| 5.8 | Public Announcement | 75 |
| 5.9 | Listing | 76 |
TABLE OF CONTENTS
(continued)
| | | Page |
| 5.10 | Tax Matters | 76 |
| 5.11 | Directors and Officers | 77 |
| 5.12 | Termination of Certain Agreements and Rights | 77 |
| 5.13 | Section 16 Matters | 77 |
| 5.14 | Cooperation | 78 |
| 5.15 | Allocation Certificates | 78 |
| 5.16 | Company Financial Statements | 78 |
| 5.17 | Takeover Statutes | 79 |
| 5.18 | Stockholder Litigation | 79 |
| 5.19 | Parent Benefit Plans | 79 |
| 5.20 | Company 280G Actions. | 80 |
| | | |
| SECTION 6. | CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY | 80 |
| | | |
| 6.1 | No Restraints | 80 |
| 6.2 | Stockholder Approval | 80 |
| 6.3 | Listing | 80 |
| 6.4 | Effectiveness of Registration Statement | 81 |
| 6.5 | Side Letter | 81 |
| 6.6 | NYSE Reverse Split. | 81 |
| | | |
| SECTION 7. | ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB | 81 |
| | | |
| 7.1 | Accuracy of Representations | 81 |
| 7.2 | Performance of Covenants | 81 |
| 7.3 | Documents | 82 |
| 7.4 | No Company Material Adverse Effect | 82 |
| 7.5 | Termination of Investor Agreements | 82 |
| 7.6 | Company Lock-Up Agreements | 82 |
| 7.7 | Company Stockholder Written Consent | 82 |
| 7.8 | Appraisal Shares | 82 |
| 7.9 | FIRPTA Certificate | 82 |
| 7.10 | Company Notes. | 82 |
| | | |
| SECTION 8. | ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY | 83 |
| | | |
| 8.1 | Accuracy of Representations | 83 |
| 8.2 | Performance of Covenants | 83 |
| 8.3 | Documents | 83 |
| 8.4 | No Parent Material Adverse Effect | 83 |
| 8.5 | Termination of Contracts | 84 |
| 8.6 | Parent Lock-Up Agreements | 84 |
| 8.7 | Actual Closing Price and Post-Merger Warrants. | 84 |
| 8.8 | Cash at Closing. | 84 |
| | | |
| SECTION 9. | TERMINATION | 84 |
| | | |
| 9.1 | Termination | 84 |
TABLE OF CONTENTS
(continued)
| | | Page |
| 9.2 | Effect of Termination | 87 |
| 9.3 | Expenses; Termination Fees | 87 |
| | | |
| SECTION 10. | MISCELLANEOUS PROVISIONS | 90 |
| | | |
| 10.1 | Non-Survival of Representations and Warranties | 90 |
| 10.2 | Amendment | 90 |
| 10.3 | Waiver | 91 |
| 10.4 | Entire Agreement; Counterparts; Exchanges by Electronic Transmission | 91 |
| 10.5 | Applicable Law; Jurisdiction | 91 |
| 10.6 | Attorneys’ Fees | 92 |
| 10.7 | Assignability | 92 |
| 10.8 | Notices | 92 |
| 10.9 | Cooperation | 93 |
| 10.10 | Severability | 93 |
| 10.11 | Other Remedies; Specific Performance | 93 |
| 10.12 | No Third Party Beneficiaries | 94 |
| 10.13 | Construction | 94 |
Exhibits:
| | Exhibit A | Certain Definitions |
| | Exhibit B-1 | Form of Company Stockholder Support Agreement |
| | Exhibit B-2 | Form of Parent Stockholder Support Agreement |
| | Exhibit C-1 | Form of Company Lock-Up Agreement |
| | Exhibit C-2 | Form of Parent Lock-Up Agreement |
| | Exhibit D | Form of Company Stockholder Written Consent |
| | Exhibit E | Surviving Corporation Amended and Restated Certificate of Incorporation |
| | Exhibit F | Parent Amended and Restated Certificate of Incorporation |
| | Exhibit G | Surviving Corporation Amended and Restated Bylaws |
| | Exhibit H | Parent Amended and Restated Bylaws |
| | Exhibit I | Amended and Restated Company Incentive Plan |
| | Exhibit J | Amended and Restated Parent Incentive Plan |
| | Exhibit K | New Incentive Plan |
| | Exhibit L | Post-Closing Officers and Directors |
| | Exhibit M | Side Letter |
Schedules:
Company Disclosure Schedule
Parent Disclosure Schedule
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
THIS AGREEMENT AND PLAN OF MERGER AND REORGANIZATION (this “Agreement”) is made and entered into as of August 29, 2023, by and among AgeX Therapeutics, Inc., a Delaware corporation (“Parent”), CANARIA TRANSACTION CORPORATION, an Alabama corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Serina Therapeutics, Inc., an Alabama corporation (the “Company”). Certain capitalized terms used in this Agreement are defined in Exhibit A.
RECITALS
A. Parent and the Company intend to effect a merger of Merger Sub with and into the Company (the “Merger”) in accordance with this Agreement and the ABCL. Upon consummation of the Merger, Merger Sub will cease to exist and the Company will become a wholly owned subsidiary of Parent.
B. The Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code, and by executing this Agreement, the Parties hereby adopt a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a).
C. The Parent Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders, (ii) authorized, approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of shares of Parent Common Stock to the stockholders of the Company pursuant to the terms of this Agreement, the change of control of Parent and other actions contemplated by this Agreement, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters and such other actions as contemplated by this Agreement.
D. The Merger Sub Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger Sub and its sole stockholder, (ii) authorized, approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the sole stockholder of Merger Sub votes to adopt this Agreement and thereby approve the Contemplated Transactions.
E. The Company Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) authorized, approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote to approve the Company Stockholder Matters and such other actions as contemplated by this Agreement.
F. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to Parent’s willingness to enter into this Agreement, (a) the officers, directors and stockholders of the Company listed in Section A-1 of the Company Disclosure Schedule (the “Company Signatories”) (solely in their capacity as stockholders of the Company), are executing support agreements in favor of Parent in substantially the form attached hereto as Exhibit B-1 (the “Company Stockholder Support Agreement”) and (b) the officers, directors and stockholders of the Company listed in Section A-2 of the Company Disclosure Schedule (the “Company Lock-Up Signatories”) (solely in their capacity as stockholders of the Company) are executing lock-up agreements in substantially the form attached hereto as Exhibit C-1 (the “Company Lock-Up Agreement”).
G. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to the Company’s willingness to enter into this Agreement, (a) the officers, directors and stockholders of Parent listed in Section A-1 of the Parent Disclosure Schedule (solely in their capacity as stockholders of Parent) are executing support agreements in favor of the Company in substantially the form attached hereto as Exhibit B-2 (the “Parent Stockholder Support Agreement”) and (b) the officers, directors and stockholders of Parent listed in Section A-2 of the Parent Disclosure Schedule (solely in their capacity as stockholders of Parent) are executing lock-up agreements in substantially the form attached hereto as Exhibit C-2 (the “Parent Lock-Up Agreement”).
H. It is expected that, within three (3) Business Days after the Registration Statement is declared effective under the Securities Act, the holders of shares of Company Capital Stock sufficient to approve the Company Stockholder Matters as required under the ABCL and the Company’s certificate of incorporation and the Company’s bylaws (hereinafter collectively referred to as the “Company’s Organizational Documents”) will execute and deliver an action by written consent in substantially the form attached hereto as Exhibit D (each, a “Company Stockholder Written Consent” and collectively, the “Company Stockholder Written Consents”).
AGREEMENT
The Parties, intending to be legally bound, agree as follows:
SECTION 1. DESCRIPTION OF TRANSACTION
1.1 The Merger. Upon the terms and subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub shall be merged with and into the Company, and the separate existence of Merger Sub shall cease. The Company will continue as the surviving corporation in the Merger (the “Surviving Corporation”).
1.2 Effects of the Merger. The Merger shall have the effects set forth in this Agreement, the Statement of Merger and in the applicable provisions of the ABCL. As a result of the Merger, the Company will become a wholly owned subsidiary of Parent.
1.3 Closing; Effective Time. Unless this Agreement is earlier terminated pursuant to the provisions of Section 9.1, and subject to the satisfaction or waiver of the conditions set forth in Sections 6, 7 and 8, the consummation of the Merger (the “Closing”) shall take place remotely as promptly as practicable (but in no event later than the second (2nd) Business Day following the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth in Sections 6, 7 and 8, other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of each of such conditions), or at such other time, date and place as Parent and the Company may mutually agree in writing. The date on which the Closing actually takes place is referred to as the “Closing Date.” At the Closing, the Parties shall cause the Merger to be consummated by executing and filing with the Secretary of State of the State of Alabama a Statement of Merger with respect to the Merger, satisfying the applicable requirements of the ABCL and in a form reasonably acceptable to Parent and the Company (the “Statement of Merger”). The Merger shall become effective at the time of the filing of such Statement of Merger with the Secretary of State of the State of Alabama or at such later time as may be specified in such Statement of Merger with the consent of Parent and the Company (the time as of which the Merger becomes effective being referred to as the “Effective Time”).
1.4 Certificate of Incorporation and Bylaws; Directors and Officers. At the Effective Time:
(a) the certificate of incorporation of the Surviving Corporation shall be amended and restated in its entirety at or immediately prior to the Effective Time as set forth in Exhibit E, to, among other things, change the name of the Surviving Corporation to “Serina Operating Company, Inc.” or such other name as shall be mutually agreed upon by Parent and the Company prior to filing such amendment and restatement, and shall be the certificate of incorporation of the Surviving Corporation until thereafter amended as provided by the ABCL and such amended and restated certificate of incorporation;
(b) the certificate of incorporation of Parent shall be amended and restated in its entirety, as contemplated in Section 5.3(a)(v) (the “First Amended and Restated Certificate of Incorporation”) and as set forth in Exhibit F, at or immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and the First Amended and Restated Certificate of Incorporation; provided, however, that at or immediately prior to the filing of the First Amended and Restated Certificate of Incorporation, Parent shall file an amendment to its current certificate of incorporation to (i) as contemplated by Section 5.3(a)(i), effect the NYSE Reverse Split and (ii) make such other changes as shall be mutually agreed upon by Parent and the Company prior to filing such amendment;
(c) the bylaws of the Surviving Corporation shall be amended and restated in their entirety at or immediately prior to the Effective Time as set forth in Exhibit G, and shall be the bylaws of the Surviving Corporation until thereafter amended as provided by the ABCL and such amended and restated bylaws;
(d) the bylaws of the Parent shall be amended and restated in their entirety at or immediately prior to the Effective Time as set forth in Exhibit H, and shall be the bylaws of the Parent until thereafter amended as provided by the DGCL and such amended and restated bylaws;
(e) the directors and officers of Parent, each to hold office in accordance with the First Amended and Restated Certificate of Incorporation and bylaws of Parent, shall be as set forth in Section 5.11 after giving effect to the provisions of Section 5.11, or such other persons as shall be mutually agreed upon by Parent and the Company; and
(f) the directors and officers of the Surviving Corporation, each to hold office in accordance with the certificate of incorporation and bylaws of the Surviving Corporation, shall be the directors and officers of Parent, or such other persons as shall be mutually agreed upon by Parent and the Company.
1.5 Conversion of Shares.
(a) At the Effective Time, by virtue of the Merger and without any further action on the part of Parent, Merger Sub, the Company or any stockholder of the Company or Parent:
(i) any shares of Company Capital Stock held as treasury stock by the Company or held or owned by Parent, Merger Sub or any Subsidiary of Parent or the Company immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; and
(ii) subject to Section 1.5(c), each share of Company Capital Stock outstanding immediately prior to the Effective Time (excluding shares to be canceled pursuant to Section 1.5(a)(i) and excluding Appraisal Shares), after giving effect to the Preferred Stock Conversion, shall be automatically converted solely into the right to receive a number of shares of Parent Common Stock equal to the Exchange Ratio (the “Merger Consideration”).
(b) If any shares of Company Capital Stock outstanding immediately prior to the Effective Time are unvested or are subject to a repurchase option or a risk of forfeiture under any applicable restricted stock purchase agreement or other similar agreement with the Company, then the shares of Parent Common Stock issued in exchange for such shares of Company Capital Stock at the Effective Time will to the same extent be unvested and subject to the same repurchase option or risk of forfeiture, and such shares of Parent Common Stock shall accordingly be marked with appropriate legends. The Company shall take all actions that may be necessary to ensure that, from and after the Effective Time, Parent is entitled to exercise any such repurchase option or other right set forth in any such restricted stock purchase agreement or other agreement in accordance with its terms.
(c) No fractional shares of Parent Common Stock shall be issued in connection with the Merger, and no certificates or scrip for any such fractional shares shall be issued. Any holder of Company Capital Stock who would otherwise be entitled to receive a fraction of a share of Parent Common Stock (after aggregating all fractional shares of Parent Common Stock issuable to such holder) shall receive from Parent, in lieu of such fractional share and upon surrender by such holder of a letter of transmittal in accordance with Section 1.7 and any accompanying documents as required therein: (i) one share of Parent Common Stock, if the aggregate amount of fractional shares of Parent Common Stock such holder of Company Capital Stock would otherwise be entitled to is equal to or exceeds 0.50; or (ii) no shares of Parent Common Stock, if the aggregate amount of fractional shares of Parent Common Stock such holder of Company Capital Stock would otherwise be entitled to is less than 0.50.
(d) All Company Options outstanding immediately prior to the Effective Time under the Company Incentive Plan shall be treated in accordance with Section 5.5(a).
(e) All Company Warrants outstanding immediately prior to the Effective Time shall be treated in accordance with Section 5.5(c)
(f) Each share of common stock, $0.0001 par value per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be converted into and exchanged for one validly issued, fully paid and nonassessable share of common stock, $0.0001 par value per share, of the Surviving Corporation. Each stock certificate or book-entry share of Merger Sub evidencing ownership of any such shares shall, as of the Effective Time, evidence ownership of such shares of common stock of the Surviving Corporation.
(g) If, between the time of calculating the Exchange Ratio and the Effective Time, the outstanding shares of Company Capital Stock or Parent Common Stock shall have been changed into, or exchanged for, a different number of shares or a different class, by reason of any stock dividend, subdivision, reclassification, recapitalization, split (including the NYSE Reverse Split to the extent such split has not been previously taken into account in calculating the Exchange Ratio), combination or exchange of shares or other like change, the Exchange Ratio shall, to the extent necessary, be equitably adjusted to reflect such change to the extent necessary to provide the holders of Company Capital Stock, Parent Common Stock, Company Options and Company Warrants with the same economic effect as contemplated by this Agreement prior to such stock dividend, subdivision, reclassification, recapitalization, split (including the NYSE Reverse Split), combination or exchange of shares or other like change; provided, however, that nothing herein will be construed to permit the Company or Parent to take any action with respect to Company Capital Stock or Parent Common Stock, respectively, that is prohibited or not expressly permitted by the terms of this Agreement.
1.6 Closing of the Company’s Transfer Books. At the Effective Time: (a) all shares of Company Capital Stock outstanding immediately prior to the Effective Time shall be treated in accordance with Section 1.5(a), and all holders of certificates or book-entry shares representing shares of Company Capital Stock that were outstanding immediately prior to the Effective Time shall cease to have any rights as stockholders of the Company; and (b) the stock transfer books of the Company shall be closed with respect to all shares of Company Capital Stock outstanding immediately prior to the Effective Time. No further transfer of any such shares of Company Capital Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a valid certificate previously representing any shares of Company Capital Stock outstanding immediately prior to the Effective Time (including any certificates representing the Company Preferred Stock or Company Warrants that were converted or exercised in connection with the conversion of Company Preferred Stock or exercise of Company Warrants, as applicable) (a “Company Stock Certificate”) is presented to the Exchange Agent or to the Surviving Corporation, such Company Stock Certificate shall be canceled and shall be exchanged as provided in Sections 1.5 and 1.7.
1.7 Delivery of Merger Consideration.
(a) On or prior to the Closing Date, Parent and the Company shall agree upon and select a reputable bank, transfer agent or trust company to act as exchange agent in the Merger (the “Exchange Agent”). At the Effective Time, Parent shall deposit with the Exchange Agent evidence of book-entry shares representing the Parent Common Stock issuable pursuant to Section 1.5(a). The Parent Common Stock so deposited with the Exchange Agent, together with any dividends or distributions received by the Exchange Agent with respect to such shares, are referred to collectively as the “Exchange Fund.”
(b) Promptly after the Effective Time, the Parties shall cause the Exchange Agent to mail to the Persons who were record holders of shares of Company Capital Stock that were converted into the right to receive the Merger Consideration: (i) a letter of transmittal in customary form and containing such provisions as Parent may reasonably specify (including a provision confirming that delivery of any Company Stock Certificates shall be effected, and risk of loss and title to such Company Stock Certificates shall pass, only upon proper delivery of such Company Stock Certificates to the Exchange Agent); and (ii) instructions for effecting the surrender of any Company Stock Certificates, or uncertificated shares of Company Capital Stock, in exchange for shares of Parent Common Stock. Upon surrender of a Company Stock Certificate or other reasonable evidence of the ownership of uncertificated Company Capital Stock to the Exchange Agent for exchange, together with a duly executed letter of transmittal and such other documents as may be reasonably required by the Exchange Agent or Parent (including a properly completed IRS Form W-9 or the appropriate version of IRS Form W-8, as applicable): (A) the holder of such Company Capital Stock shall be entitled to receive in exchange therefor shares representing the Merger Consideration (in a number of whole shares of Parent Common Stock) that such holder has the right to receive pursuant to the provisions of Section 1.5(a) (B) such Company Stock Certificate so surrendered shall be canceled. Until surrendered as contemplated by this Section 1.7(b), each Company Stock Certificate shall be deemed, from and after the Effective Time, to represent only the right to receive shares of Parent Common Stock representing the Merger Consideration. If any Company Stock Certificate shall have been lost, stolen or destroyed, Parent may, in its discretion and as a condition precedent to the delivery of any shares of Parent Common Stock, require the owner of such lost, stolen or destroyed Company Stock Certificate to provide an applicable affidavit with respect to such Company Stock Certificate and post a bond indemnifying Parent against any claim suffered by Parent related to the lost, stolen or destroyed Company Stock Certificate as Parent may reasonably request. In the event of a transfer of ownership of a Company Stock Certificate that is not registered in the transfer records of the Company, payment of the Merger Consideration may be made to a Person other than the Person in whose name such Company Stock Certificate so surrendered is registered if such Company Stock Certificate shall be properly endorsed or otherwise be in proper form for transfer and the Person requesting such payment shall pay any transfer or other Taxes required by reason of the transfer or establish to the satisfaction of Parent that such Taxes have been paid or are not applicable. The Merger Consideration and any dividends or other distributions as are payable pursuant to Section 1.7(c) shall be deemed to have been in full satisfaction of all rights pertaining to Company Capital Stock formerly represented by such Company Stock Certificates.
(c) No dividends or other distributions declared or made with respect to Parent Common Stock with a record date on or after the Effective Time shall be paid to the holder of any unsurrendered Company Stock Certificate with respect to the shares of Parent Common Stock that such holder has the right to receive in the Merger until such holder surrenders such Company Stock Certificate, together with a duly executed letter of transmittal and such other documents as may be reasonably required by the Exchange Agent or Parent, or provides an affidavit of loss, theft or destruction in lieu thereof in accordance with this Section 1.7 (at which time (or, if later, on the applicable payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws, to receive all such dividends and distributions, without interest).
(d) Any portion of the Exchange Fund that remains undistributed to holders of Company Capital Stock as of the date that is one (1) year after the Closing Date shall be delivered to Parent upon demand, and any holders of Company Stock Certificates who have not theretofore surrendered their Company Stock Certificates in accordance with this Section 1.7 shall thereafter look only to Parent for satisfaction of their claims for Parent Common Stock and any dividends or distributions with respect to shares of Parent Common Stock.
(e) No Party to this Agreement shall be liable to any holder of any Company Capital Stock or to any other Person with respect to any shares of Parent Common Stock (or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable abandoned property Law, escheat Law or similar Law.
1.8 Appraisal Shares.
(a) Notwithstanding any provision of this Agreement to the contrary, shares of Company Capital Stock that are outstanding immediately prior to the Effective Time and which are held by any Person who is entitled to exercise that Person’s appraisal rights and properly perfects that Person’s appraisal rights with respect to such shares of Company Capital Stock in accordance with the ABCL (collectively, the “Appraisal Shares”) shall not be converted into or represent the right to receive the Merger Consideration described in Section 1.5 attributable to such Appraisal Shares. Such stockholders shall be entitled to receive payment of the “fair value” (as defined in Section 10A-2A-13.01 et seq. of the ABCL (the “ABCL Article 13”)) of such shares of Company Capital Stock held by them in accordance with the ABCL, unless and until such stockholders fail to perfect or waive, withdraw or lose the right to appraisal under ABCL Article 13. All Appraisal Shares held by stockholders who have failed to perfect or who have waived, withdrawn, or lost the right to appraisal rights under ABCL Article 13 of such shares of Company Capital Stock under the ABCL (whether occurring before, at or after the Effective Time) shall thereupon be deemed to be converted into and to have become exchangeable for, as of the Effective Time, the right to receive the Merger Consideration, without interest, attributable to such Appraisal Shares upon their surrender in the manner provided in Sections 1.5 and 1.7.
(b) The Company shall give prompt written notice to Parent of any demand received by the Company from a holder of shares of Company Common Stock pursuant to Section 13.21 of ABCL Article 13, and Parent shall have the right to participate in all negotiations and proceedings with respect to such demands. Prior to the Effective Time, the Company shall not, without the prior written consent of Parent (not to be unreasonably withheld, delayed or conditioned), make any payment with respect to, or settle or offer to settle, any such demands, or waive any failure by any holder of Company Common Stock to timely deliver a written demand for appraisal or the taking of any other action by any such holder as may be necessary to perfect appraisal rights under the ABCL, or agree to do any of the foregoing.
1.9 Further Action. If, at any time after the Effective Time, any further action is determined by the Surviving Corporation to be necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Corporation with full right, title and possession of and to all rights and property of the Company, then the officers and directors of the Surviving Corporation shall be fully authorized, and shall use their and its commercially reasonable efforts (in the name of the Company, in the name of Merger Sub, in the name of the Surviving Corporation and otherwise) to take such action.
1.10 Withholding. The Parties and the Exchange Agent shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of Company Capital Stock or any other Person such amounts as such Party or the Exchange Agent reasonably determines it is required to deduct and withhold under the Code or any other Law with respect to the making of such payment. To the extent that amounts are so deducted and withheld and paid to the appropriate Governmental Body, such deducted and withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of whom such deduction and withholding was made. To the extent it is determined that any such deduction or withholding is required in respect of payment to a holder of Company Capital Stock (other than by reason of failure of the Company to provide the FIRPTA Certificate in accordance with Section 7.9 or such holder to provide an IRS Form W-9 or appropriate IRS Form W-8 to the Exchange Agent or in respect of any payments in the nature of compensation), the Parties shall use commercially reasonable efforts (including using commercially reasonable efforts to cause the Exchange Agent) (x) to notify the Person in respect of which such deduction or withholding is being made and (y) to the extent permitted by applicable Law, cooperate with such Person to the extent reasonably requested to establish an exemption or reduction of, or otherwise minimize, such deduction and withholding.
SECTION 2. REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Subject to Section 10.13(h), except as set forth in the written disclosure schedule delivered by the Company to Parent (the “Company Disclosure Schedule”), the Company represents and warrants to Parent and Merger Sub as follows:
2.1 Due Organization; Subsidiaries.
(a) The Company is a corporation duly incorporated, validly existing and in good standing under the Laws of the State of Alabama and has all necessary corporate power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used; and (iii) to perform its obligations under all Contracts by which it is bound, except where the failure to have such power or authority would not reasonably be expected to prevent or materially delay the ability of the Company to consummate the Contemplated Transactions.
(b) The Company is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Company Material Adverse Effect.
(c) The Company has no Subsidiaries, except for the Entities identified in Section 2.1(c) of the Company Disclosure Schedule; and neither the Company nor any of the Entities identified in Section 2.1(c) of the Company Disclosure Schedule own any capital stock of, or any equity, ownership or profit sharing interest of any nature in, or controls directly or indirectly, any other Entity other than the Entities identified in Section 2.1(c) of the Company Disclosure Schedule. Each of the Company’s Subsidiaries is a corporation or other legal entity duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its organization and has all necessary corporate or other power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not be reasonably expected to have a Company Material Adverse Effect.
(d) Neither the Company nor any of its Subsidiaries are or have otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Neither the Company nor any of its Subsidiaries have agreed or is obligated to make, or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Neither the Company nor any of its Subsidiaries have, at any time, been a general partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
2.2 Organizational Documents. The Company has made available to Parent accurate and complete copies of the Organizational Documents of the Company and each of its Subsidiaries in effect as of the date of this Agreement. Neither the Company nor any of its Subsidiaries are in breach or violation of its respective Organizational Documents in any material respect.
2.3 Authority; Binding Nature of Agreement. The Company has all necessary corporate power and authority to enter into this Agreement and, subject, with respect to the Company, to receipt of the Required Company Stockholder Vote, to perform its obligations under this Agreement and to consummate the Contemplated Transactions. The Company Board (at meetings duly called and held or by written consent in lieu of a meeting) has: (a) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders; (b) authorized, approved and declared advisable this Agreement and the Contemplated Transactions; (c) recommended, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote to approve the Company Stockholder Matters; and (d) approved the Company Stockholder Support Agreements and the transactions contemplated thereby.
This Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution and delivery by Parent and Merger Sub, constitutes the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to the Enforceability Exceptions.
2.4 Vote Required. The affirmative vote (or written consent) of the holders of at least (a) 60% of the then outstanding shares of Series A Preferred Stock voting as a separate class, (b) 60% of the then outstanding shares of Series A-1 Preferred Stock voting as a separate class, (c) 60% of the then outstanding shares of Series A-2 Preferred Stock voting as a separate class, (d) 60% of the then outstanding shares of Series A-3 Preferred Stock voting as a separate class, (e) 60% of the then outstanding shares of Series A-4 Preferred Stock voting as a separate class, (f) 60% of the then outstanding shares of Series A-5 Preferred Stock voting as a separate class, and (g) a majority of the then outstanding shares of the capital stock of the Company on an as-converted to Company Common Stock basis and as a single class (collectively, the “Required Company Stockholder Vote”), is the only vote (or written consent) of the holders of any class or series of Company Capital Stock necessary to adopt and approve this Agreement and approve the Contemplated Transactions.
2.5 Non-Contravention; Consents. Subject to obtaining the Required Company Stockholder Vote and the filing of the Statement of Merger required by the ABCL, neither (a) the execution, delivery, or performance of this Agreement by the Company, nor (b) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(i) contravene, conflict with or result in a violation of any of the provisions of the Organizational Documents of the Company or any of its Subsidiaries;
(ii) contravene, conflict with or result in a violation of, or give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any order, writ, injunction, judgment or decree to which the Company or any of its Subsidiaries, or any of the assets owned or used by the Company or any of its Subsidiaries, is subject, except as would not reasonably be expected to constitute, individually or in the aggregate, a Company Material Adverse Effect;
(iii) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by the Company or its Subsidiaries, except as would not reasonably be expected to constitute, individually or in the aggregate, a Company Material Adverse Effect;
(iv) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Company Material Contract, or give any Person the right to: (A) declare a default or exercise any remedy under any Company Material Contract; (B) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Company Material Contract; (C) accelerate the maturity or performance of any Company Material Contract; or (D) cancel, terminate or modify any term of any Company Material Contract, except in the case of any non-material breach, default, penalty or modification; or
(v) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by the Company or any of its Subsidiaries (except for Permitted Encumbrances).
Except for (A) any Consent set forth in Section 2.5 of the Company Disclosure Schedule under any Company Material Contract, (B) the Required Company Stockholder Vote, (C) the filing of the Statement of Merger with the Secretary of State of the State of Alabama pursuant to the ABCL, and (D) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings, as may be required under applicable federal and state securities Laws, neither the Company nor any of its Subsidiaries was, is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (x) the execution, delivery or performance of this Agreement, or (y) the consummation of the Contemplated Transactions, which if individually or in the aggregate were not given or obtained, would reasonably be expected to prevent or materially delay the ability of the Company to consummate the Contemplated Transactions. The Company Board has taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in the ABCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Company Stockholder Support Agreements, the Company Lock-Up Agreements and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement, the Company Stockholder Support Agreements, the Company Lock-Up Agreements or any of the Contemplated Transactions.
2.6 Capitalization.
(a) The authorized Company Capital Stock as of the date of this Agreement consists of (i) 15,000,000 shares of Company Common Stock, par value $0.01 per share, of which 2,234,100 shares are issued and outstanding as of the date of this Agreement, and (ii) 10,000,000 shares of preferred stock, par value $0.01 per share (the “Company Preferred Stock”), 400,000 shares of which have been designated Series A Preferred Stock (the “Series A Preferred Stock”), all of which have been issued and are outstanding as of the date of this Agreement, 300,000 shares of which have been designated Series A-1 Preferred Stock (the “Series A-1 Preferred Stock”), all of which have been issued and are outstanding as of the date of this Agreement, 1,122,077 shares of which have been designated Series A-2 Preferred Stock (the “Series A-2 Preferred Stock”), 1,117,013 shares of which have been issued and are outstanding on the date of this Agreement, 499,200 shares of which have been designated Series A-3 Preferred Stock (the “Series A-3 Preferred Stock”), all of which have been issued and are outstanding on the date of this Agreement, 718,997 shares of which have been designated Series A-4 Preferred Stock (the “Series A-4 Preferred Stock”), all of which have been issued and are outstanding on the date of this Agreement, 4,620,000 shares of which have been designated Series A-5 Preferred Stock (the “Series A-5 Preferred Stock”), 484,918 shares of which have been issued and are outstanding on the date of this Agreement, and the balance is available for designation by the Company Board. Section 2.6(a) of the Company Disclosure Schedule lists, as of the date of this Agreement (A) each record holder of issued and outstanding Company Capital Stock and the number and type of shares of Company Capital Stock held by such holder; (B)(1) each holder of issued and outstanding Company Warrants, (2) the number and type of shares subject to each Company Warrant, (3) the exercise price of each Company Warrant and (4) the termination date of each Company Warrant; and (C)(1) each holder of issued and outstanding Company Notes, (2) the number and type of shares subject to each Company Note, (3) the conversion triggers for each Company Note and (4) the maturity date of each Company Note.
(b) All of the outstanding shares of Company Common Stock and Company Preferred Stock have been duly authorized and validly issued, and are fully paid and nonassessable. Except as set forth in the Investor Agreements or Section 2.6(b) of the Company Disclosure Schedule, none of the outstanding shares of Company Capital Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Company Capital Stock is subject to any right of first refusal in favor of the Company. Except as contemplated herein and in the Investor Agreements, there is no Company Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Company Capital Stock. The Company is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Company Capital Stock or other securities. Section 2.6(b) of the Company Disclosure Schedule accurately and completely lists all repurchase rights held by the Company with respect to shares of Company Capital Stock (including shares issued pursuant to the exercise of stock options) and specifies which of those repurchase rights are currently exercisable.
(c) Except for (i) the Serina Therapeutics, Inc. 2017 Stock Option Plan, as amended (the “Company Incentive Plan”), (ii) the Company Warrants as set forth in Section 2.6(a) of the Company Disclosure Schedule, and (iii) the Company Notes as set forth in Section 2.6(a) of the Company Disclosure Schedule, the Company does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the date of this Agreement, the Company has reserved 2,100,000 shares of Company Common Stock for issuance under the Company Incentive Plan, the Company has granted Company Options for 2,086,450 shares of Company Common Stock, of which 1,990,150 Company Options are fully vested but have not yet been exercised, and 20,550 shares of Company Common Stock remain available for future issuance of Company Options pursuant to the Company Incentive Plan. Section 2.6(c) of the Company Disclosure Schedule sets forth the following information with respect to each Company Option outstanding as of the date of this Agreement: (i) the name of the optionee; (ii) the number of shares of Company Common Stock subject to the Company Option at the time of grant; (iii) the number of shares of Company Common Stock subject to the Company Option as of the date of this Agreement; (iv) the exercise price of the Company Option; (v) the date on which the Company Option was granted; (vi) the applicable vesting schedule, including the number of vested and unvested Company Options as of the date of this Agreement; (vii) the date on which the Company Option expires; and (viii) whether the Company Option is intended to constitute an “incentive stock option” (as defined in the Code) or a non-qualified stock option. The Company has made available to Parent an accurate and complete copy of the Company Incentive Plan and all stock option agreements evidencing outstanding options granted thereunder. Section 2.6(c) of the Company Disclosure Schedule sets forth a list of Company Options that have accelerated vesting in connection with the closing of the Contemplated Transactions.
(d) Except for Company Warrants as set forth in Section 2.6(a) of the Company Disclosure Schedule, Company Notes as set forth in Section 2.6(a) of the Company Disclosure Schedule, and the Company Options set forth in Section 2.6(c) of the Company Disclosure Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of the Company or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of the Company or any of its Subsidiaries; or (iii) condition or circumstance that could be reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of the Company or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to the Company or any of its Subsidiaries.
(e) All outstanding shares of Company Common Stock, Company Preferred Stock, Company Options, Company Warrants, and Company Notes and other securities of the Company have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Laws, and (ii) all requirements set forth in applicable Contracts.
2.7 Financial Statements.
(a) Section 2.7(a) of the Company Disclosure Schedules includes true and complete copies of (i) the Company’s audited consolidated balance sheets at December 31, 2022, 2021, and 2020, together with related audited consolidated statements of operations, preferred stock and stockholders’ deficit and cash flows, and notes thereto, of the Company for the fiscal years then ended and (ii) the Company Unaudited Interim Balance Sheet, together with the unaudited consolidated statements of operations, preferred stock and stockholders’ deficit and cash flows of the Company for the period reflected in the Company Unaudited Interim Balance Sheet (collectively, the “Company Financials”). The Company Financials were prepared in accordance with GAAP applied on a consistent basis unless otherwise noted therein throughout the periods covered thereby (except as may be indicated in the notes to such Company Financials and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments, none of which are reasonably expected to be material in amount) and fairly present, in all material respects, the financial position and operating results of the Company and its consolidated Subsidiaries as of the dates and for the periods indicated therein.
(b) The Company and each of its Subsidiaries maintains accurate books and records reflecting their assets and liabilities and maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of the financial statements in conformity with GAAP and to maintain accountability of the Company’s and its Subsidiaries’ assets; (iii) access to the Company’s and its Subsidiaries’ assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for the Company’s and its Subsidiaries’ assets is compared with the existing assets at regular intervals and appropriate action is taken with respect to any differences; and (v) accounts, notes and other payables are recorded accurately, and proper and adequate procedures are implemented to effect the collection or payment thereof on a current and timely basis. The Company and each of its Subsidiaries maintain internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
(c) Section 2.7(c) of the Company Disclosure Schedule lists, and the Company has delivered to Parent accurate and complete copies of the documentation creating or governing, all securitization transactions and “off-balance sheet arrangements” (as described in Instruction 8 to Item 303(b) of Regulation S-K as promulgated under the Securities Act) effected by the Company or any of its Subsidiaries since January 1, 2020.
(d) Since January 1, 2020, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of the Company, the Company Board or any committee thereof. Except as set forth in Section 2.7(d) of the Company Disclosure Schedule, since January 1, 2020, neither the Company nor its independent auditors have identified (i) any significant deficiency or material weakness in the design or operation of the system of internal accounting controls utilized by the Company and its Subsidiaries, (ii) any fraud, whether or not material, that involves the Company, any of its Subsidiaries, the Company’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by the Company and its Subsidiaries or (iii) any claim or allegation regarding any of the foregoing.
2.8 Absence of Changes. Except as set forth in Section 2.8 of the Company Disclosure Schedule and except for reasonable and good faith actions or omissions taken to comply with applicable Law or guidance by a Governmental Body in connection with the COVID-19 pandemic, between the date of the Company Unaudited Interim Balance Sheet and the date of this Agreement, the Company has conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto, including the Contemplated Transactions) and there has not been any (a) Company Material Adverse Effect or (b) action, event or occurrence that would have required the consent of Parent pursuant to Section 4.2(b) of this Agreement had such action, event or occurrence taken place after the execution and delivery of this Agreement.
2.9 Absence of Undisclosed Liabilities. As of the date of this Agreement, neither the Company nor any of its Subsidiaries have any liability, indebtedness, obligation, expense, claim, deficiency, guaranty or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or otherwise (whether or not required to be reflected in the financial statements in accordance with GAAP) (each a “Liability”), individually or in the aggregate, of a type required to be recorded or reflected on a balance sheet or disclosed in the footnotes thereto under GAAP except for: (a) Liabilities disclosed, reflected or reserved against in the Company Unaudited Interim Balance Sheet; (b) normal and recurring current Liabilities that have been incurred by the Company or its Subsidiaries since the date of the Company Unaudited Interim Balance Sheet in the Ordinary Course of Business and which are not in excess of $100,000 in the aggregate; (c) Liabilities for performance of obligations of the Company or any of its Subsidiaries under Company Material Contracts which have not resulted from a breach of such Company Material Contracts, breach of warranty, tort, infringement or violation of Law; (d) Liabilities incurred in connection with the Contemplated Transactions; and (e) Liabilities described in Section 2.9 of the Company Disclosure Schedule.
2.10 Title to Assets. The Company and each of its Subsidiaries owns, and have good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it, including: (a) all tangible assets reflected on the Company Unaudited Interim Balance Sheet; and (b) all other tangible assets reflected in the books and records of the Company or any of its Subsidiaries as being owned by the Company or such Subsidiary. All of such assets are owned or, in the case of leased assets, leased by the Company or its applicable Subsidiary free and clear of any Encumbrances, other than Permitted Encumbrances.
2.11 Real Property; Leasehold. Neither the Company nor any of its Subsidiaries owns or has ever owned any real property. The Company has made available to Parent an accurate and complete list of all real properties with respect to which the Company directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by the Company or any of its Subsidiaries, and the Company has made available to Parent copies of all leases under which any such real property is possessed (the “Company Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. The Company’s or its applicable Subsidiary’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and the Company or its applicable Subsidiary has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances.
2.12 Intellectual Property.
(a) Section 2.12(a) of the Company Disclosure Schedule accurately identifies (i) the name of the applicant/registrant, (ii) the jurisdiction of application/registration, (iii) the application or registration number and (iv) any other co-owners, for each item of Registered IP owned in whole or in part by the Company or its Subsidiaries (the “Company Owned Registered IP”). Each of the patents and patent applications included in the Company Owned Registered IP properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. To the Company’s Knowledge, (A) the Company Owned Registered IP is valid, enforceable and subsisting, (B) none of the Company Owned Registered IP has been misused, withdrawn, canceled or abandoned, and (C) all application, registration, issuance, renewal and maintenance fees for the Company Owned Registered IP having a final due date on or before the date of this Agreement have been timely paid in full. To the Company’s Knowledge, with respect to each item of Company Owned Registered IP and each patent application from which such Company Owned Registered IP claims priority, all statements made and information presented to the applicable patent office by or on behalf of the Company or its Subsidiaries or any inventor thereof, or their respective patent counsel, during the prosecution thereof are accurate and complete and comply with 37 CFR 1.56. As of the date of this Agreement, no interference, opposition, reissue, reexamination or other proceeding of any nature (other than initial examination proceedings) is pending or, to the Company’s Knowledge, threatened in writing, in which the scope, validity, enforceability or ownership of any Company Owned Registered IP is being or has been contested or challenged.
(b) The Company or its Subsidiaries solely owns all right, title and interest in and to all Company IP (other than as disclosed in Section 2.12(a) of the Company Disclosure Schedule and except for any failure to own or have such right to use, or have the right to bring actions that would not reasonably be expected to have a Company Material Adverse Effect), free and clear of all Encumbrances other than Permitted Encumbrances and, to the Company’s Knowledge, has the right, pursuant to a Company In-bound License to use all other material Intellectual Property Rights used by the Company or its Subsidiaries in their respective businesses as currently conducted or as proposed to be conducted as of the date of this Agreement. The Company IP and the Intellectual Property Rights licensed to the Company or its Subsidiaries pursuant to a Company In-bound License (the “Company In-Licensed IP”) are all the Intellectual Property Rights necessary to operate the business of the Company and its Subsidiaries as currently conducted and to the Company’s Knowledge, as proposed to be conducted, as of the date of this Agreement. No Company Associate owns or has any claim, right (whether or not currently exercisable) or interest to or in any Company IP, and each Company Associate involved in the creation or development of any material Company IP, pursuant to such Company Associate’s activities on behalf of the Company or its Subsidiaries, has signed a valid, enforceable written agreement containing a present assignment of all of such Company Associate’s rights in such Company IP to the Company or its Subsidiaries (without further payment being owed to any such Company Associate and without any restrictions or obligations on the Company’s or its Subsidiaries’ ownership or use thereof) and confidentiality provisions protecting the Company IP, which, to the Company’s Knowledge, has not been breached by such Company Associate. Without limiting the foregoing, the Company and its Subsidiaries have taken commercially reasonable steps to protect, maintain and enforce all Company IP, and as required, Company In-Licensed IP, including the secrecy, confidentiality and value of trade secrets and other confidential information therein (as applicable), and to the Company’s Knowledge there have been no unauthorized disclosures by Company or its Subsidiaries of any confidential information or trade secrets associated with Company IP or Company In-Licensed IP. Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated hereby will conflict with, alter or impair any of the Company’s or its Subsidiaries’ or, following the Closing, Parent’s, rights in or to any Company IP or Company In-Licensed IP or cause any payments of any kind to be due or payable to any Person.
(c) Other than the Company IP identified as co-owned in Section 2.12(a) of the Company Disclosure Schedule, to the Company’s Knowledge, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational or academic institution has been used, in whole or in part, to create any Company IP or any Company In-Licensed IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership or other rights (including any “march in” rights or a right to direct the location of manufacturing of products) to such Company IP or the right to receive royalties or other consideration for the practice of such Company IP.
(d) Section 2.12(d) of the Company Disclosure Schedule sets forth each license agreement pursuant to which the Company or any of its Subsidiaries (i) is granted a license under any Intellectual Property Right owned by any third party that is used by the Company or its Subsidiaries in its business as currently conducted or, to the Company’s Knowledge, as proposed to be conducted as of the date of this Agreement (each a “Company In-bound License”) or (ii) grants to any third party a license, option, covenant not to sue or other right under any Company IP or any Company In-Licensed IP (each a “Company Out-bound License”) (provided, that Company In-bound Licenses shall not include material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, commercially available Software-as-a-Service offerings, off-the-shelf software licenses or generally available patent license agreements, in each case entered into in the Ordinary Course of Business on a non-exclusive basis and that do not grant any commercial rights to any products or services of the Company or its Subsidiaries; and Company Out-bound Licenses shall not include material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, or non-exclusive outbound licenses, in each case entered into in the Ordinary Course of Business on a non-exclusive basis and that do not grant any commercial rights to any products or services of the Company or its Subsidiaries). Neither the Company nor its Subsidiaries nor, to the Company’s Knowledge, any other party to any Company In-bound License or Company Out-bound License has breached or is in breach of any of its obligations under any Company In-bound License or Company Out-bound License.
(e) To the Company’s Knowledge: (i) the operation of the businesses of the Company and its Subsidiaries as currently conducted or as proposed to be conducted as of the date of this Agreement does not infringe or misappropriate or otherwise violate any Intellectual Property Right owned by any other Person; and (ii) no other Person is infringing, misappropriating or otherwise violating any Company IP or any Intellectual Property rights exclusively licensed to the Company or its Subsidiaries. As of the date of this Agreement, no Legal Proceeding is pending (or, to the Company’s Knowledge, is threatened in writing) (A) against the Company or its Subsidiaries alleging that the operation of the businesses of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or (B) by the Company or its Subsidiaries alleging that another Person has infringed, misappropriated or otherwise violated any of the Company IP or any Company In-Licensed IP. Since January 1, 2020, neither the Company nor any of its Subsidiaries have received any written notice or other written communication alleging that the operation of the business of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of the Company IP or, to the Company’s Knowledge, any Company In-Licensed IP (i) is subject to any pending or outstanding injunction, directive, order, decree, settlement, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by the Company or its Subsidiaries or (ii) would reasonably be expected to adversely affect the validity, scope, use, registrability, or enforceability thereof.
(g) To the Company’s Knowledge, the Company, its Subsidiaries and the operation of the Company’s and its Subsidiaries’ business are in substantial compliance with all applicable Laws pertaining to data privacy and data security of any personally identifiable information and sensitive business information (collectively, “Sensitive Data”) except to the extent that such noncompliance has not and would not reasonably be expected to have a Company Material Adverse Effect. To the Company’s Knowledge, since January 1, 2020, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of the Company or its Subsidiaries, (ii) no violations of any security policy of the Company or its Subsidiaries regarding any such Sensitive Data, (iii) no unauthorized access or unauthorized use of any Sensitive Data used in the business of the Company or its Subsidiaries and (iv) no unintended or improper disclosure of any personally identifiable information in the possession, custody or control of the Company or its Subsidiaries, or a contractor or agent acting on behalf of the Company or its Subsidiaries, in each case of (i) through (iv), except as would not reasonably be expected to, individually or in the aggregate, have a Company Material Adverse Effect.
(h) None of the Company or its Subsidiaries are now nor has ever been a member or promoter of, or a contributor to, any industry standards body or any similar organization that would reasonably be expected to require or obligate any of the Company or its Subsidiaries to grant or offer to any other Person any license or right to any Company IP or Company In-Licensed IP.
2.13 Agreements, Contracts and Commitments.
(a) Section 2.13(a) of the Company Disclosure Schedule lists the following Company Contracts in effect as of the date of this Agreement (other than any Company Benefit Plans) (each, a “Company Material Contract” and collectively, the “Company Material Contracts”):
(i) each Company Contract that would be a material contract as defined in Item 601(b)(10) of Regulation S-K as promulgated under the Securities Act (assuming the Company was subject to the public reporting requirements of the Exchange Act);
(ii) each Company Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(iii) each Company Contract containing (A) any covenant limiting the freedom of the Company, its Subsidiaries or the Surviving Corporation to engage in any line of business or compete with any Person, (B) any “most-favored nations” pricing provisions or marketing or distribution rights related to any products or territory, (C) any exclusivity provision, (D) any agreement to purchase a minimum quantity of goods or services, or (E) any material non-solicitation provisions applicable to the Company or any of its Subsidiaries;
(iv) each Company Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $50,000 pursuant to its express terms and not cancelable without penalty;
(v) each Company Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(vi) each Company Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit in excess of $50,000 or creating any material Encumbrances with respect to any assets of the Company or any of its Subsidiaries or any loans or debt obligations with officers or directors of the Company or any of its Subsidiaries;
(vii) each Company Contract requiring payment by or to the Company or any of its Subsidiaries after the date of this Agreement in excess of $300,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to any pre-clinical or clinical development activities of the Company or any of its Subsidiaries; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, collaboration, development or other agreement currently in force under which the Company or any of its Subsidiaries have continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which the Company or any of its Subsidiaries have continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by the Company or any of its Subsidiaries; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of the Company or any of its Subsidiaries or any Contract to sell, distribute or commercialize any products or service of the Company or any of its Subsidiaries, in each case, except for Contracts entered into in the Ordinary Course of Business;
(viii) each Company Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing advisory services to the Company in connection with the Contemplated Transactions;
(ix) each Company Real Estate Lease;
(x) each Company Contract with any Governmental Body;
(xi) each Company Out-bound License and Company In-bound License;
(xii) each Company Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of the Company or any of its Subsidiaries; or
(xiii) any other Company Contract that is not terminable at will (with no penalty or payment) by the Company or its Subsidiaries, as applicable, and (A) which involves payment or receipt by the Company or its Subsidiaries after the date of this Agreement under any such agreement, contract or commitment of more than $300,000 in the aggregate, or (B) that is material to the business or operations of the Company and its Subsidiaries, taken as a whole.
(b) The Company has delivered or made available to Parent accurate and complete copies of all Company Material Contracts, including all amendments thereto. Except as set forth in Section 2.13(b) of the Company Disclosure Schedule, there are no Company Material Contracts that are not in written form. As of the date of this Agreement, none of the Company, any of its Subsidiaries, nor, to the Company’s Knowledge, any other party to a Company Material Contract, has breached, violated or defaulted under, or received notice that it breached, violated or defaulted under, any of the terms or conditions of, or Laws applicable to, any Company Material Contract in such manner as would permit any other party to cancel or terminate any such Company Material Contract, or would permit any other party to seek damages or pursue other legal remedies which would reasonably be expected to be material to the Company or its business or operations. As to the Company and its Subsidiaries, as of the date of this Agreement, each Company Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Company Material Contract to change, any material amount paid or payable to the Company or any of its Subsidiaries under any Company Material Contract or any other material term or provision of any Company Material Contract.
2.14 Compliance; Permits; Restrictions.
(a) The Company and each of its Subsidiaries are, and since January 1, 2020, have been, in compliance in all material respects with all applicable Laws, including the Federal Food, Drug, and Cosmetic Act (“FDCA”), the U.S. Food and Drug Administration (“FDA”) regulations adopted thereunder, the Public Health Service Act and any other similar Law administered or promulgated by the FDA or other comparable Governmental Body responsible for the regulation of the development, clinical testing, manufacturing, sale, marketing, distribution and importation or exportation of drug and biopharmaceutical products (each, a “Drug Regulatory Agency”), except for any noncompliance, either individually or in the aggregate, which would not be material to the Company. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to the Company’s Knowledge, threatened against the Company or any of its Subsidiaries. There is no agreement, judgment, injunction, order or decree by or with a Drug Regulatory Agency binding upon the Company or any of its Subsidiaries which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of material property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted, (ii) is reasonably likely to have an adverse effect on the Company’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
(b) The Company and its Subsidiaries hold all required Governmental Authorizations which are material to the operation of the business of the Company and its Subsidiaries as currently conducted (the “Company Permits”). Section 2.14(b) of the Company Disclosure Schedule identifies each Company Permit. The Company and its Subsidiaries hold all right, title and interest in and to all Company Permits free and clear of any Encumbrance. The Company and each of its Subsidiaries are in material compliance with the terms of the Company Permits. No Legal Proceeding is pending or, to the Company’s Knowledge, threatened, which seeks to revoke, limit, suspend, or materially modify any Company Permit. The rights and benefits of each Company Permit will be available to the Surviving Corporation or its Subsidiaries, as applicable, immediately after the Effective Time on terms substantially identical to those enjoyed by the Company and its Subsidiaries as of the date of this Agreement and immediately prior to the Effective Time.
(c) There are no proceedings pending or, to the Company’s Knowledge, threatened with respect to an alleged material violation by the Company or any of its Subsidiaries of the FDCA, FDA regulations adopted thereunder, the Controlled Substances Act, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) The Company is not currently conducting or addressing, and to the Company’s Knowledge there is no basis to expect that it will be required to conduct or address, any corrective actions, including, without limitation, product recalls or clinical holds.
(e) All clinical, pre-clinical and other studies and tests conducted by or, to the Company’s Knowledge, on behalf of, or sponsored by, the Company or any of its Subsidiaries, or in which the Company or any of its Subsidiaries or their respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. Since January 1, 2020, neither the Company nor any of its Subsidiaries have received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or, to the Company’s Knowledge, threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, the Company or any of its Subsidiaries or in which the Company or any of its Subsidiaries or their respective current products or product candidates have participated.
(f) Neither the Company nor any of its Subsidiaries are the subject of any pending or, to the Company’s Knowledge, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Company’s Knowledge, neither the Company nor any of its Subsidiaries have committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. None of the Company, any of its Subsidiaries or any of their respective officers, employees or agents has been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. To the Company’s Knowledge, no debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or threatened against the Company, any of its Subsidiaries or any of their respective officers, employees or agents.
2.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no pending Legal Proceeding and, to the Company’s Knowledge, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) the Company, (B) any of its Subsidiaries, (C) any director or officer of the Company (in his or her capacity as such) or (D) any of the material assets owned or used by the Company or its Subsidiaries; or (ii) that challenges, or that would reasonably be expected to have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b) Except as set forth in Section 2.15(b) of the Company Disclosure Schedule, since January 1, 2020, no Legal Proceeding has been pending against the Company or any of its Subsidiaries that resulted in material liability to the Company or any of its Subsidiaries.
(c) There is no order, writ, injunction, judgment or decree to which the Company or any of its Subsidiaries, or any of the material assets owned or used by the Company or any of its Subsidiaries, is subject. To the Company’s Knowledge, no officer or other employee of the Company or any of its Subsidiaries are subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of the Company or any of its Subsidiaries or to any material assets owned or used by the Company or any of its Subsidiaries.
2.16 Tax Matters.
(a) The Company and each of its Subsidiaries have timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No written claim has ever been made by any Governmental Body in any jurisdiction where the Company or any of its Subsidiaries does not file a particular Tax Return or pay a particular Tax that the Company or such Subsidiary is subject to taxation by that jurisdiction.
(b) All income and other material Taxes due and owing by the Company or any of its Subsidiaries (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of the Company and its Subsidiaries did not, as of the date of the Company Unaudited Interim Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Company Unaudited Interim Balance Sheet. Since the date of the Company Unaudited Interim Balance Sheet, neither the Company nor any of its Subsidiaries have incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that the Company or any of its Subsidiaries are or were required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose. The Company and its Subsidiaries have complied in all material respects with applicable Laws relating to information reporting and record retention (including to the extent necessary to claim any exemption from sales Tax collection and maintaining adequate and current resale certificates to support any such claimed exemptions).
(d) There are no Encumbrances for material Taxes (other than Taxes not yet due and payable) upon any of the assets of the Company or any of its Subsidiaries.
(e) No deficiencies for income or other material Taxes with respect to the Company or any of its Subsidiaries have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and, to the Company’s Knowledge, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries (or any of their predecessors) have waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) Neither the Company nor any of its Subsidiaries have been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(g) Neither the Company nor any of its Subsidiaries are a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
(h) Neither the Company nor any of its Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes made on or prior to the Closing Date; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law) entered into on or prior to the Closing Date; (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (vii) application of Section 367(d) of the Code to any transfer of intangible property on or prior to the Closing Date; (viii) application of Sections 951 or 951A of the Code (or any similar provision of state, local or foreign Law) to any income received or accrued on or prior to the Closing Date; or (ix) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law) made on or prior to the Closing Date. The Company has not made any election under Section 965(h) of the Code.
(i) Neither the Company nor any of its Subsidiaries have ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is the Company) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Neither the Company nor any of its Subsidiaries have any Liability for any material amount of Taxes of any Person (other than the Company and any of its Subsidiaries) under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign Law), by contract, as a transferee or successor, or otherwise.
(j) Neither the Company nor any of its Subsidiaries (i) are a “controlled foreign corporation” as defined in Section 957 of the Code, (ii) is a “passive foreign investment company” within the meaning of Section 1297 of the Code, or (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized.
(k) Neither the Company nor any of its Subsidiaries have participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “reportable transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(l) Neither the Company nor any of its Subsidiaries have taken any action, nor to the Company’s Knowledge is there any fact, that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
(m) Neither the Company nor any of its Subsidiaries have availed itself of any Tax relief pursuant to any Pandemic Response Laws that could reasonably be expected to materially impact the Tax payment and/or Tax reporting obligations of Parent and its Affiliates (including the Company and its Subsidiaries) after the Closing Date.
For purposes of this Section 2.16, each reference to the Company or any of its Subsidiaries shall be deemed to include any Person that was liquidated into, merged with, or otherwise a predecessor to, the Company or any of its Subsidiaries.
2.17 Employee and Labor Matters; Benefit Plans.
(a) Section 2.17(a) of the Company Disclosure Schedule lists, as of the date of this Agreement, all material Company Benefit Plans. “Company Benefit Plan” means each (i) “employee benefit plan,” as defined in Section 3(3) of ERISA, whether or not subject to ERISA, and (ii) any other pension, retirement, deferred compensation, excess benefit, profit-sharing, bonus, incentive, equity or equity-based, phantom equity, employment (other than at-will employment offer letters on Company’s standard form that may be terminated without notice and with no penalty to Company or any of its Subsidiaries and other than individual Company Options, or other compensatory equity award grants made pursuant to Company’s standard forms, in which case only representative standard forms of such agreements shall be scheduled), consulting, severance, change-of-control, stay or retention, medical, dental, vision, health, prescription drug, life, disability, cafeteria plan, flexible spending account, employee assistance program, travel assistance, accidental death & dismemberment, group insurance, retiree medical or life insurance, paid-time-off, holiday, welfare or fringe benefit plan, program, agreement, contract, or arrangement (other than regular salary or wages) (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in each case, maintained, contributed to, or required to be contributed to, by the Company or any of its Subsidiaries for the benefit of any current or former employee, director, officer, or independent contractor of the Company or any of its Subsidiaries or under which the Company or any of its Subsidiaries have or could have any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Section 414 of the Code with any other person).
(b) As applicable with respect to each material Company Benefit Plan, the Company has made available to Parent, true and complete copies of (i) each material Company Benefit Plan, including all amendments thereto, and in the case of an unwritten material Company Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements, insurance, and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the three most recently filed annual reports on Form 5500 and all schedules thereto, (v) the most recent IRS determination or opinion letter, (vi) the three most recent nondiscrimination testing reports, actuarial reports and financial statements, (vii) all notices and filings concerning IRS, United States Department of Labor or other Governmental Body audits or investigations since January 1, 2020, (viii) each written report constituting a valuation of Company Capital Stock for purposes of Sections 409A or 422 of the Code, whether prepared internally by the Company or by an outside, third-party valuation firm, and (ix) all material written materials provided to employees or participants relating to the amendment, termination, establishment, or increase or decrease in benefits under any Company Benefit Plan.
(c) Each Company Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other applicable Laws.
(d) The Company Benefit Plans that are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and that are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code, and, to the Company’s Knowledge, nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Company Benefit Plan.
(e) None of the Company or any Company ERISA Affiliate sponsors, maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, or has within the past six (6) years sponsored, maintained, contributed to, or been required to contribute to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code), or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA), and none of the Company or any Company ERISA Affiliate has, within the preceding six (6) years, incurred a complete or partial withdrawal from any “multiemployer plan” or otherwise incurred any liability under Section 4202 of ERISA.
(f) There are no pending audits or investigations by any Governmental Body involving any Company Benefit Plan, and no pending or, to the Company’s Knowledge, threatened claims (except for individual claims for benefits payable in the normal operation of the Company Benefit Plans), suits, or proceedings involving any Company Benefit Plan. All contributions and premium payments required to have been made under any of the Company Benefit Plans or by applicable Law have been timely made, and neither the Company nor any of its Subsidiaries have any liability for any unpaid contributions with respect to any Company Benefit Plan (other than contributions that are accrued in the Ordinary Course of Business).
(g) Neither the Company nor any of its Subsidiaries, nor, to the Company’s Knowledge, any fiduciary, trustee, or administrator of any Company Benefit Plan has engaged in, or in connection with the Contemplated Transactions will engage in, any transaction with respect to any Company Benefit Plan that would subject any such Company Benefit Plan, the Company or any of its Subsidiaries or Parent to a Tax, penalty, or liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) No Company Benefit Plan provides (i) death, medical, dental, vision, life insurance, or other welfare benefits beyond termination of service or retirement, other than coverage mandated by Law (including COBRA, for which the covered individual pays the full cost of coverage), or (ii) death or retirement benefits under a Company Benefit Plan qualified under Section 401(a) of the Code, and neither the Company nor any of its Subsidiaries have made a written or oral representation promising the same.
(i) Except as set forth in Section 2.17(i) of the Company Disclosure Schedules, neither the execution of this Agreement, nor the consummation of the Contemplated Transactions will either alone or in connection with any other event(s) (i) result in any payment becoming due to any current or former employee, director, officer, independent contractor or other service provider of the Company or any of its Subsidiaries, (ii) increase any amount of compensation or benefits otherwise payable to any current or former employee, director, officer, independent contractor or other service provider of the Company or any of its Subsidiaries, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Company Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Company Benefit Plan or (v) limit the right to merge, amend or terminate any Company Benefit Plan.
(j) Neither the execution of this Agreement, nor the consummation of the Contemplated Transactions (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Section 280G of the Code) with respect to the Company and its Subsidiaries of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Section 280G of the Code), determined without regard to the application of Section 280G(b)(5) of the Code.
(k) Each Company Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in all material respects in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder.
(l) No Person has any “gross-up” agreements with the Company or any of its Subsidiaries or other assurance of reimbursement or compensation by the Company or any of its Subsidiaries for any Taxes imposed under Section 409A or Section 4999 of the Code.
(m) The Company does not have any Company Benefit Plan that is maintained for service providers located outside of the United States.
(n) Neither the Company nor any of its Subsidiaries are a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union or labor organization representing any of its employees, and there is no labor union or labor organization representing or, to the Company’s Knowledge, purporting to represent or seeking to represent any employees of the Company or its Subsidiaries, including through the filing of a petition for representation election.
(o) The Company and each of its Subsidiaries is, and since January 1, 2020, has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including, without limitation, worker classification, discrimination, wrongful termination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to employees of the Company or any of its Subsidiaries, each of the Company and its Subsidiaries, since January 1, 2020: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries, and other payments, benefits or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or penalties for failure to comply with any of the foregoing in any material respect, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body with respect to unemployment compensation benefits, disability, social security or other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business or as required by Law). There are no actions, suits, claims, charges, demands, lawsuits, investigations, audits or administrative matters pending or, to the Company’s Knowledge, threatened or reasonably anticipated against the Company or any of its Subsidiaries relating to any current or former employee, applicant for employment, consultant, employment agreement or Company Benefit Plan (other than routine claims for benefits). All employees of the Company and its Subsidiaries are employed “at-will” and their employment can be terminated without advance notice or payment of severance.
(p) Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to each individual who currently renders services to the Company or any of its Subsidiaries, the Company and each of its Subsidiaries have accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, the Company and each of its Subsidiaries have accurately classified him or her as overtime eligible or overtime ineligible under all applicable Laws. Neither the Company nor any of its Subsidiaries have any material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee, (b) any employee leased from another employer, or (c) any employee currently or formerly classified as exempt from overtime wages.
(q) There is not and has not been since January 1, 2020, nor is there or has there been since January 1, 2020, any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to the Company’s Knowledge, any union organizing activity, against the Company or any of its Subsidiaries. No event has occurred, and, to the Company’s Knowledge, no condition or circumstance exists, that might directly or indirectly give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute.
2.18 Environmental Matters. The Company and each of its Subsidiaries are in compliance, and since January 1, 2020, have complied, with all applicable Environmental Laws, which compliance includes the possession by the Company and its Subsidiaries of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to result in a Company Material Adverse Effect. Neither the Company nor any of its Subsidiaries have received since January 1, 2020, any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that the Company or any of its Subsidiaries are not in compliance with or has liability pursuant to any Environmental Law and, to the Company’s Knowledge, there are no circumstances that would reasonably be expected to prevent or interfere with the Company’s or any of its Subsidiaries’ compliance with any Environmental Law in the future, except where such failure to comply would not reasonably be expected to have a Company Material Adverse Effect. To the Company’s Knowledge, no current or (during the time a prior property was leased or controlled by the Company or any of its Subsidiaries) prior property leased or controlled by the Company or any of its Subsidiaries have had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of the Company or any of its Subsidiaries pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the consummation of the Contemplated Transactions. Prior to the date of this Agreement, the Company has provided or otherwise made available to Parent true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of the Company or any of its Subsidiaries with respect to any property leased or controlled by the Company or any of its Subsidiaries or any business operated by them.
2.19 Insurance. The Company has delivered or made available to Parent accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of the Company and each of its Subsidiaries, as of the date of this Agreement. Each of such insurance policies is in full force and effect and the Company and each of its Subsidiaries are in compliance in all material respects with the terms thereof. Other than customary end-of-policy notifications from insurance carriers, since January 1, 2020, neither the Company nor any of its Subsidiaries have received any notice or other communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. The Company and each of its Subsidiaries have provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against the Company or any of its Subsidiaries for which the Company or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed the Company or any of its Subsidiaries of its intent to do so.
2.20 No Financial Advisors. Except as set forth in Section 2.20 of the Company Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of the Company or any of its Subsidiaries.
2.21 Disclosure. The information supplied by or on behalf of the Company and each of its Subsidiaries for inclusion or incorporation by reference in the Registration Statement, or to be included or supplied by or on behalf of the Company and each of its Subsidiaries for inclusion in any filing pursuant to Rule 165 and Rule 425 under the Securities Act or Rule 14a-12 under the Exchange Act (each a “Regulation M-A Filing”), shall not at the time the Registration Statement or any such Regulation M-A Filing is filed with the SEC, at any time it is amended or supplemented or at the time the Registration Statement is declared effective by the SEC, as applicable, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements therein not false or misleading, or omit to state any material fact necessary to correct any statement therein that has become false or misleading. The information supplied by or on behalf of the Company and each of its Subsidiaries for inclusion or incorporation by reference in the Proxy Statement, which information shall be deemed to include all information about or related to the Company or any of its Subsidiaries and/or the Company Stockholder Matters, shall not, on the date the Proxy Statement is first mailed to Parent’s stockholders, or at the time of the Parent Stockholders’ Meeting or as of the Effective Time, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements made in the Proxy Statement not false or misleading, or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies for the Parent Stockholders’ Meeting that has become false or misleading.
2.22 Transactions with Affiliates.
(a) Except as set forth in Section 2.22(a) of the Company Disclosure Schedule, there are no material transactions or relationships, since January 1, 2020, between, on one hand, the Company or any of its Subsidiaries and, on the other hand, any (i) executive officer or director of the Company or, to the Company’s Knowledge, any of its Subsidiaries or any of such executive officer’s or director’s immediate family members, (ii) owner of more than 5% of the voting power of the outstanding Company Capital Stock or (iii) to the Company’s Knowledge, any “related person” (within the meaning of Item 404 of Regulation S-K as promulgated under the Securities Act) of any such executive officer, director or stockholder (other than the Company or its Subsidiaries) in the case of each of (i), (ii) or (iii) that is of the type that would be required to be disclosed under Item 404 of Regulation S-K as promulgated under the Securities Act (assuming the Company was subject to the public reporting requirements of the Exchange Act).
(b) Section 2.22(b) of the Company Disclosure Schedule lists each stockholders’ agreement, voting agreement, registration rights agreement, co-sale agreement or other similar Contract between the Company and any holders of Company Capital Stock, including any such Contract granting any Person investor rights, rights of first refusal, rights of first offer, registration rights, director designation rights or similar rights (collectively, the “Investor Agreements”).
2.23 Anti-Bribery. None of the Company or any of its Subsidiaries or any of their respective directors, officers, employees or, to the Company’s Knowledge, agents or any other Person acting on their behalf has, directly or indirectly, made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of the Foreign Corrupt Practices Act of 1977, the UK Bribery Act of 2010 or any other anti-bribery or anti-corruption Law (collectively, the “Anti-Bribery Laws”). Neither the Company nor any of its Subsidiaries are or has been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
2.24 Disclaimer of Other Representations or Warranties. Except as previously set forth in this Section 2 or in any certificate delivered by the Company to Parent and/or Merger Sub pursuant to this Agreement, the Company makes no representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
SECTION 3. REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB
Subject to Section 10.13(h), except (a) as set forth in the written disclosure schedule delivered by Parent to the Company (the “Parent Disclosure Schedule”) or (b) as disclosed in the Parent SEC Documents filed with, or furnished to, the SEC at least two Business Days prior to the date of this Agreement and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system that is reasonably apparent on the face of such disclosure to be applicable to the representations and warranties herein (but (i) without giving effect to any amendment thereof filed with, or furnished to, the SEC on or after the date of this Agreement and (ii) excluding any disclosures contained under the heading “Risk Factors” and any disclosure of risks included in any “forward-looking statements” disclaimer or in any other section to the extent they are forward-looking statements or cautionary, predictive or forward-looking in nature), Parent and Merger Sub represent and warrant to the Company as follows:
3.1 Due Organization; Subsidiaries.
(a) Each of Parent and Merger Sub is a corporation duly incorporated, validly existing and in good standing under the Laws of its jurisdiction of incorporation, and has all necessary corporate power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used; and (iii) to perform its obligations under all Contracts by which it is bound, except where the failure to have such power or authority would not reasonably be expected to prevent or materially delay the ability of Parent and Merger Sub to consummate the Contemplated Transactions. Since the date of its incorporation, Merger Sub has not engaged in any activities other than activities incident to its formation or in connection with or as contemplated by this Agreement.
(b) Parent is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Parent Material Adverse Effect.
(c) Parent has no Subsidiaries, except for the Entities identified in Section 3.1(c) of the Parent Disclosure Schedule; and neither Parent nor any of the Entities identified in Section 3.1(c) of the Parent Disclosure Schedule owns any capital stock of, or any equity, ownership or profit sharing interest of any nature in, or controls directly or indirectly, any other Entity other than the Entities identified in Section 3.1(c) of the Parent Disclosure Schedule. Each of Parent’s Subsidiaries is a corporation or other legal entity duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its organization and has all necessary corporate or other power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not be reasonably expected to have a Parent Material Adverse Effect.
(d) Neither Parent nor any of its Subsidiaries are or has otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Neither Parent nor any of its Subsidiaries have agreed or is obligated to make, or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Neither Parent nor any of its Subsidiaries have, at any time, been a general partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership, or other Entity.
3.2 Organizational Documents. Parent has made available to the Company accurate and complete copies of the Organizational Documents of Parent and each of its Subsidiaries in effect as of the date of this Agreement. Neither Parent nor any of its Subsidiaries are in breach or violation of its respective Organizational Documents in any material respect.
3.3 Authority; Binding Nature of Agreement. Each of Parent and Merger Sub has all necessary corporate power and authority to enter into this Agreement and, subject, with respect to Parent, to receipt of the Required Parent Stockholder Vote and, with respect to Merger Sub, the adoption of this Agreement by Parent in its capacity as sole stockholder of Merger Sub, to perform its obligations under this Agreement and to consummate the Contemplated Transactions. The Parent Board (at meetings duly called and held or by written consent in lieu of a meeting) has: (a) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders; (b) authorized, approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of shares of Parent Common Stock to the stockholders of the Company pursuant to the terms of this Agreement, the change of control of Parent and other actions contemplated by this Agreement; (c) recommended, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters; and (d) approved the Parent Stockholder Support Agreements and the transactions contemplated thereby. The Merger Sub Board (by written consent) has: (x) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Merger Sub and its sole stockholder; (y) authorized, approved and declared advisable this Agreement and the Contemplated Transactions; and (z) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the sole stockholder of Merger Sub vote to approve this Agreement and the Contemplated Transactions. This Agreement has been duly executed and delivered by Parent and Merger Sub and, assuming the due authorization, execution and delivery by the Company, constitutes the legal, valid and binding obligation of Parent and Merger Sub, enforceable against each of Parent and Merger Sub in accordance with its terms, subject to the Enforceability Exceptions.
3.4 Vote Required. The affirmative vote (i) of the holders of a majority of the outstanding Parent Common Stock, Parent Series A Preferred Stock and Parent Series B Preferred Stock, each as a separate class, is the only vote of the holders of any class or series of Parent Capital Stock necessary to approve the proposal in Sections 5.3(a)(v), (ii) of a majority of the votes cast by the Parent Common Stock is the only vote of the holders of any class or series of Parent Capital Stock necessary to approve the proposals in Section 5.3(a)(i), Section 5.3(a)(iii), Sections 5.3(a)(iv), Sections 5.3(a)(vi), Section 5.3(a)(vii) and Section 5.3(a)(viii) and (iii) if determined to be required by Parent and subject to the structure and timing of the Asset Dispositions, if any, of the holders of a majority of the votes cast by the Parent Common Stock and of the holders of a majority of the outstanding Parent Series A Preferred Stock and Parent Series B Preferred Stock, each as a separate class, is the only vote of the holders of any class or series of Parent Capital Stock necessary to approve the proposals in Section 5.3(a)(ii) (the “Required Parent Stockholder Vote”).
3.5 Non-Contravention; Consents. Subject to obtaining the Required Parent Stockholder Vote, the adoption of this Agreement (effective immediately following the execution of this Agreement) by Parent as the sole stockholder of Merger Sub and the filing of the Statement of Merger required by the ABCL, neither (a) the execution, delivery or performance of this Agreement by Parent or Merger Sub, nor (b) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(i) contravene, conflict with or result in a violation of any of the provisions of the Organizational Documents of Parent or any of its Subsidiaries;
(ii) contravene, conflict with or result in a violation of, or give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any remedy or obtain any relief under, any Law or any order, writ, injunction, judgment or decree to which Parent or any of its Subsidiaries, or any of the assets owned or used by Parent or any of its Subsidiaries, is subject, except as would not reasonably be expected to constitute, individually or in the aggregate, a Parent Material Adverse Effect;
(iii) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Parent or its Subsidiaries, except as would not reasonably be expected to constitute, individually or in the aggregate, a Parent Material Adverse Effect;
(iv) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Parent Material Contract, or give any Person the right to: (A) declare a default or exercise any remedy under any Parent Material Contract; (B) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Parent Material Contract; (C) accelerate the maturity or performance of any Parent Material Contract; or (D) cancel, terminate or modify any term of any Parent Material Contract, except in the case of any non-material breach, default, penalty or modification; or
(v) result in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by Parent or any of its Subsidiaries (except for Permitted Encumbrances).
Except for (A) any Consent set forth in Section 3.5 of the Parent Disclosure Schedule under any Parent Material Contract, (B) the Required Parent Stockholder Vote, (C) the filing of the Statement of Merger with the Secretary of State of the State of Alabama pursuant to the ABCL, and (D) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities Laws, neither Parent nor any of its Subsidiaries were, are or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (x) the execution, delivery or performance of this Agreement, or (y) the consummation of the Contemplated Transactions, which if individually or in the aggregate were not given or obtained, would reasonably be expected to prevent or materially delay the ability of Parent and Merger Sub to consummate the Contemplated Transactions. The Parent Board and the Merger Sub Board have taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in the ABCL and Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Parent Stockholder Support Agreements, the Parent Lock-Up Agreements, and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement, the Parent Stockholder Support Agreements, the Parent Lock-Up Agreements or any of the Contemplated Transactions.
3.6 Capitalization.
(a) The authorized capital stock of Parent consists of (i) 100,000,000 shares of Parent Common Stock, par value $0.0001 per share, of which 37,951,261 shares have been issued and are outstanding as of the close of business on the Reference Date, and (ii) 5,000,000 shares of preferred stock of Parent, par value $0.0001 per share (the “Parent Preferred Stock”), 211,600 shares of which have been designated as Series A Preferred Stock (the “Parent Series A Preferred Stock”), all of which have been issued and are outstanding as of the date of this Agreement, and 148,400 shares of which have been designated as Series B Preferred Stock (the “Parent Series B Preferred Stock”), all of which have been issued and are outstanding as of the date of this Agreement, and the balance is available for designation by the Parent Board. Parent does not hold any shares of its capital stock in its treasury. Section 3.6(a) of the Parent Disclosure Schedule lists, on the date of the Reference Date, (A) each holder of issued and outstanding Parent Preferred Stock, (B) each holder of issued and outstanding Parent Warrants, (C) the number and type of shares subject to each Parent Warrant, (D) the exercise price of each Parent Warrant and (E) the termination date of each Parent Warrant.
(b) All of the outstanding shares of Parent Common Stock and Parent Preferred Stock have been duly authorized and validly issued, and are fully paid and nonassessable. None of the outstanding shares of Parent Capital Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Parent Common Stock is subject to any right of first refusal in favor of Parent. Except as contemplated herein and as set forth in Section 3.6(b)(i) of the Parent Disclosure Schedule, there is no Parent Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Parent Capital Stock. Except as set forth in Section 3.6(b)(ii) of the Parent Disclosure Schedule, Parent is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Parent Capital Stock or other securities. Section 3.6(b)(iii) of the Parent Disclosure Schedule accurately and completely describes all repurchase rights held by Parent with respect to shares of Parent Capital Stock (including shares issued pursuant to the exercise of stock options) and specifies which of those repurchase rights are currently exercisable.
(c) Except for the Parent Incentive Plan, Parent does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the date of the Reference Date, Parent has reserved 4,000,000 shares of Parent Common Stock for issuance under the Parent Incentive Plan, of which no shares have been issued and are currently outstanding, of which no shares are subject to Parent’s right of repurchase, 3,286,800 shares have been reserved for issuance upon exercise of Parent Options previously granted and currently outstanding under the Parent Incentive Plan, no shares have been reserved for issuance upon the settlement of Parent RSUs granted under the Parent Incentive Plan that are outstanding as of the close of business on the Reference Date and 713,200 shares remain available for future issuance pursuant to the Parent Incentive Plan. Section 3.6(c) of the Parent Disclosure Schedule sets forth the following information with respect to each Parent Option outstanding as of the Reference Date: (i) the name of the optionee; (ii) the number of shares of Parent Common Stock subject to such Parent Option at the time of grant; (iii) the number of shares of Parent Common Stock subject to such Parent Option as of the Reference Date; (iv) the exercise price of such Parent Option; (v) the date on which such Parent Option was granted; (vi) the applicable vesting schedule, including the number of vested and unvested shares as of the Reference Date; (vii) the date on which such Parent Option expires; and (viii) whether such Parent Option is an “incentive stock option” (as defined in the Code) or a non-qualified stock option. Parent has made available to the Company accurate and complete copies of equity incentive plans pursuant to which Parent has equity-based awards, the forms of all award agreements evidencing such equity-based awards and evidence of board and stockholder approval of the Parent Incentive Plan and any amendments thereto.
(d) Except for the Parent Warrants, the Parent Incentive Plan, including the Parent Options, the Parent RSUs, and as otherwise set forth in Section 3.6(d) of the Parent Disclosure Schedule, as of the Reference Date there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of Parent or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of Parent or any of its Subsidiaries; (iii) stockholder rights plan (or similar plan commonly referred to as a “poison pill”) or Contract under which Parent or any of its Subsidiaries are or may become obligated to sell or otherwise issue any shares of its capital stock or any other securities; or (iv) condition or circumstance that could be reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of Parent or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to Parent or any of its Subsidiaries.
(e) All outstanding shares of Parent Common Stock, Parent Preferred Stock, Parent Options, Parent RSUs, Parent Warrants and other securities of Parent have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Laws, and (ii) all requirements set forth in applicable Contracts.
3.7 SEC Filings; Financial Statements.
(a) Other than such documents that can be obtained on the SEC’s website at www.sec.gov, Parent has delivered or made available to the Company accurate and complete copies of all registration statements, proxy statements, Certifications (as defined below) and other statements, reports, schedules, forms and other documents filed by Parent with the SEC since March 29, 2022 (the “Parent SEC Documents”). All material statements, reports, schedules, forms and other documents required to have been filed by Parent or its officers with the SEC have been so filed on a timely basis. As of the time it was filed with the SEC (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the Parent SEC Documents complied in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and, as of the time they were filed, none of the Parent SEC Documents contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The certifications and statements required by (i) Rule 13a-14 under the Exchange Act and (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the Parent SEC Documents (collectively, the “Certifications”) are accurate and complete and comply as to form and content with all applicable Laws. As used in this Section 3.7, the term “file” and variations thereof shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The financial statements (including any related notes) contained or incorporated by reference in the Parent SEC Documents: (i) complied as to form in all material respects with the published rules and regulations of the SEC applicable thereto; (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements or, in the case of unaudited financial statements, except as permitted by the SEC on Form 10-Q under the Exchange Act, and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments that are not reasonably expected to be material in amount) applied on a consistent basis unless otherwise noted therein throughout the periods indicated; and (iii) fairly present, in all material respects, the financial position of Parent and its consolidated Subsidiaries as of the respective dates thereof and the results of operations and cash flows of Parent and its consolidated Subsidiaries for the periods covered thereby. Other than as expressly disclosed in the Parent SEC Documents filed prior to the date of this Agreement, there has been no material change in Parent’s accounting methods or principles that would be required to be disclosed in Parent’s financial statements in accordance with GAAP. The books of account and other financial records of Parent and each of its Subsidiaries are true and complete in all material respects.
(c) As of the date of this Agreement, Parent is in compliance in all material respects with the applicable provisions of the Sarbanes-Oxley Act and the applicable current listing and governance rules and regulations of NYSE.
(d) Parent maintains a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including policies and procedures designed to provide reasonable assurance (i) that Parent maintains records that in reasonable detail accurately and fairly reflect Parent’s transactions and dispositions of assets, (ii) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, (iii) that receipts and expenditures are made only in accordance with authorizations of management and the Parent Board and (iv) regarding prevention or timely detection of the unauthorized acquisition, use or disposition of Parent’s assets that could have a material effect on Parent’s financial statements. Parent has evaluated the effectiveness of Parent’s internal control over financial reporting as of December 31, 2022, and, to the extent required by applicable Law, presented in any applicable Parent SEC Document that is a report on Form 10-K or Form 10-Q (or any amendment thereto) its conclusions about the effectiveness of the internal control over financial reporting as of the end of the period covered by such report or amendment based on such evaluation. Parent has disclosed, based on its most recent evaluation of internal control over financial reporting, to Parent’s auditors and the audit committee of the Parent Board (and made available to the Company a summary of the significant aspects of such disclosure) (A) all significant deficiencies and material weaknesses, if any, in the design or operation of internal control over financial reporting that are reasonably likely to adversely affect Parent’s ability to record, process, summarize and report financial information and (B) any known fraud, whether or not material, that involves management or other employees who have a significant role in Parent’s or its Subsidiaries’ internal control over financial reporting. Parent has not identified, based on its most recent evaluation of internal control over financial reporting, any material weaknesses in the design or operation of Parent’s internal control over financial reporting.
(e) Parent maintains “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) that are reasonably designed to ensure that information required to be disclosed by Parent in the periodic reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the required time periods, and that all such information is accumulated and communicated to Parent’s management as appropriate to allow timely decisions regarding required disclosure and to make the Certifications.
(f) To the Knowledge of Parent, Parent’s auditor has at all times since the date of enactment of the Sarbanes-Oxley Act been: (i) a registered public accounting firm (as defined in Section 2(a)(12) of the Sarbanes-Oxley Act); (ii) with respect to Parent, “independent” with respect to Parent within the meaning of Regulation S-X under the Exchange Act; and (iii) to the Knowledge of Parent, in compliance with subsections (g) through (l) of Section 10A of the Exchange Act and the rules and regulations promulgated by the SEC and the Public Company Accounting Oversight Board thereunder.
(g) Except as set forth in Section 3.7(g) of the Parent Disclosure Schedule, since March 29, 2022, Parent has not received any comment letter from the SEC or the staff thereof or any correspondence from NYSE or the staff thereof relating to the delisting or maintenance of listing of the Parent Common Stock on NYSE that has not been disclosed in the Parent SEC Documents. Parent has not disclosed any unresolved comments.
(h) Since March 29, 2022, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, or general counsel of Parent, the Parent Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls required by the Sarbanes-Oxley Act.
3.8 Absence of Changes. Except as set forth in Section 3.8 of the Parent Disclosure Schedule and except for reasonable and good faith actions or omissions taken to comply with applicable Law or guidance by a Governmental Body in connection with the COVID-19 pandemic, between the date of the Parent Balance Sheet and the date of this Agreement, Parent has conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto, including the Contemplated Transactions) and there has not been any (a) Parent Material Adverse Effect or (b) action, event or occurrence that would have required the consent of the Company pursuant to Section 4.1(b) had such action, event or occurrence taken place after the execution and delivery of this Agreement.
3.9 Absence of Undisclosed Liabilities. As of the date of this Agreement, neither Parent nor any of its Subsidiaries have any Liability, individually or in the aggregate, of a type required to be recorded or reflected on Parent’s balance sheet or disclosed in the footnotes thereto under GAAP except for: (a) Liabilities disclosed, reflected or reserved against in the Parent Balance Sheet; (b) normal and recurring current Liabilities that have been incurred by Parent or any of its Subsidiaries since the date of the Parent Balance Sheet in the Ordinary Course of Business and which are not in excess of $100,000 in the aggregate; (c) Liabilities for performance of obligations of Parent or any of its Subsidiaries under Parent Material Contracts which have not resulted from a breach of such Parent Material Contracts, breach of warranty, tort, infringement or violation of Law; (d) Liabilities incurred in connection with the Contemplated Transactions; and (e) Liabilities described in Section 3.9 of the Parent Disclosure Schedule.
3.10 Title to Assets. Parent and each of its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it, including: (a) all tangible assets reflected on the Parent Balance Sheet; and (b) all other tangible assets reflected in the books and records of Parent or any of its Subsidiaries as being owned by Parent or such Subsidiary. All of such assets are owned or, in the case of leased assets, leased by Parent or its applicable Subsidiary free and clear of any Encumbrances, other than Permitted Encumbrances.
3.11 Real Property; Leasehold. Neither Parent nor any of its Subsidiaries owns or has ever owned any real property. Parent has made available to the Company (a) an accurate and complete list of all real properties with respect to which Parent directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by Parent or any of its Subsidiaries, and (b) copies of all leases under which any such real property is possessed (the “Parent Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. Parent’s or its applicable Subsidiary’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and Parent or its applicable Subsidiary has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances.
3.12 Intellectual Property.
(a) Section 3.12(a) of the Parent Disclosure Schedule identifies each item of Registered IP owned in whole or in part by Parent or its Subsidiaries (“Parent Owned Registered IP”). To Parent’s Knowledge, each of the patents and patent applications included in the Parent Owned Registered IP properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. To Parent’s Knowledge, (A) Parent Owned Registered IP is valid, enforceable and subsisting, (B) none of Parent Owned Registered IP has been misused, withdrawn, canceled or abandoned, and (C) all application, registration, issuance, renewal and maintenance fees for Parent Owned Registered IP having a final due date on or before the date of this Agreement have been timely paid in full. To Parent’s Knowledge, with respect to each item of Parent Owned Registered IP and each patent application from which such Parent Owned Registered IP claims priority, all statements made and information presented to the applicable patent office by or on behalf of Parent or its Subsidiaries or any inventor thereof, or their respective patent counsel, during the prosecution thereof are accurate and complete and comply with 37 CFR 1.56. As of the date of this Agreement, no interference, opposition, reissue, reexamination or other proceeding of any nature (other than initial examination proceedings) is pending or, to Parent’s Knowledge, threatened in writing, in which the scope, validity, enforceability or ownership of any Parent Owned Registered IP is being or has been contested or challenged.
(b) Parent or its Subsidiaries solely owns all right, title and interest in and to all Parent IP (other than as disclosed in Section 3.12(a) of the Parent Disclosure Schedule and except for any failure to own or have such right to use, or have the right to bring actions that would not reasonably be expected to have a Parent Material Adverse Effect), free and clear of all Encumbrances other than Permitted Encumbrances and, to Parent’s Knowledge, has the right, pursuant to a Parent In-bound License to use all other material Intellectual Property Rights used by Parent or its Subsidiaries in their respective businesses as currently conducted. The Parent IP and the Intellectual Property Rights licensed to Parent or its Subsidiaries pursuant to a Parent In-bound License (the “Parent In-Licensed IP”) are all the Intellectual Property Rights necessary to operate the business of Parent and its Subsidiaries as currently conducted and to the Parent’s Knowledge, as proposed to be conducted, as of the date of this Agreement. No Parent Associate owns or has any claim, right (whether or not currently exercisable) or interest to or in any Parent IP, and each Parent Associate involved in the creation or development of any material Parent IP, pursuant to such Parent Associate’s activities on behalf of Parent or its Subsidiaries, has signed a valid, enforceable written agreement containing a present assignment of all of such Parent Associate’s each Parent Associate involved in the creation or development of any material Parent IP, pursuant to such Parent Associate’s activities on behalf of Parent or its Subsidiaries, has signed a valid, enforceable written agreement containing a present assignment of all such Parent Associate’s rights in such material Parent IP to Parent or its Subsidiaries (without further payment being owed to any such Parent Associate and without any restrictions or obligations on Parent’s or its Subsidiaries’ ownership or use thereof) and confidentiality provisions protecting the Parent IP, which, to Parent’s Knowledge, has not been materially breached by such Parent Associate. Without limiting the foregoing, Parent and its Subsidiaries have taken commercially reasonable steps to protect, maintain and enforce all Parent IP, and, as required, Parent In-Licensed IP, including the secrecy, confidentiality and value of trade secrets and other confidential information therein (as applicable), and to Parent’s Knowledge there have been no unauthorized disclosures by Parent or its Subsidiaries of any confidential information or trade secrets associated with Parent IP or Parent In-Licensed IP.
(c) Except as disclosed in Section 3.12(c) of the Parent Disclosure Schedule, to Parent’s Knowledge, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational or academic institution has been used, in whole or in part, to create any Parent IP or any Parent In-Licensed IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership or other rights (including any “march in” rights or a right to direct the location of manufacturing of products) to such Parent IP or the right to receive royalties or other consideration for the practice of such Parent IP.
(d) Section 3.12(d) of Parent Disclosure Schedule sets forth each license agreement pursuant to which Parent or any of its Subsidiaries (i) is granted a license under any Intellectual Property Right owned by any third party that is used by Parent or any of its Subsidiaries in its business as currently conducted or, to Parent’s Knowledge, as proposed to be conducted as of the date of this Agreement (each a “Parent In-bound License”) or (ii) grants to any third party a license under any material Parent IP or any material Intellectual Property Right licensed to Parent or any of its Subsidiaries under a Parent In-bound License (each a “Parent Out-bound License”) (provided, that Parent In-bound Licenses shall not include material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, commercially available Software-as-a-Service offerings, off-the-shelf software licenses or generally available patent license agreements, in each case, entered into in the Ordinary Course of Business on a non-exclusive basis and that do not grant any commercial rights to any products or services of Parent or any of its Subsidiaries; and Parent Out-bound Licenses shall not include material transfer agreements, clinical trial agreements, services agreements, non-disclosure agreements, or non-exclusive outbound licenses entered into in the Ordinary Course of Business on a non-exclusive basis and that do not grant any commercial rights to any products or services of Parent or any of its Subsidiaries). Neither Parent nor its Subsidiaries nor, to Parent’s Knowledge, any other party to any Parent In-bound License or Parent Out-bound License has breached or is in breach of any of its obligations under any Parent In-bound License or Parent Out-bound License.
(e) To Parent’s Knowledge, (i) the operation of the businesses of Parent and its Subsidiaries as currently conducted or as proposed to be conducted as of the date of this Agreement does not infringe or misappropriate or otherwise violate any other Intellectual Property Right owned by any other Person; and (ii) no other Person is infringing, misappropriating or otherwise violating any Parent IP or any Intellectual Property Rights exclusively licensed to Parent or any of its Subsidiaries. As of the date of this Agreement, no Legal Proceeding is pending (or, to Parent’s Knowledge, is threatened in writing) (A) against Parent or any of its Subsidiaries alleging that the operation of the businesses of Parent or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or (B) by Parent or any of its Subsidiaries alleging that another Person has infringed, misappropriated or otherwise violated any of Parent IP or any Parent In- Licensed IP. Since January 1, 2020, neither Parent nor any of its Subsidiaries have received any written notice or other written communication alleging that the operation of the businesses of Parent or any of its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of the Parent IP or, to the Parent’s Knowledge, any Parent In-Licensed IP (i) is subject to any pending or outstanding injunction, directive, order, decree, settlement, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by the Parent or its Subsidiaries or (ii) would reasonably be expected to adversely affect the validity, scope, use, registrability, or enforceability thereof.
(g) To Parent’s Knowledge, the Parent, its Subsidiaries, and the operation of Parent’s and its Subsidiaries’ business are in substantial compliance with all applicable Laws pertaining to data privacy and data security of Sensitive Data, except to the extent that such noncompliance has not and would not reasonably be expected to have a Parent Material Adverse Effect. To Parent’s Knowledge, since January 1, 2020, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of Parent or its Subsidiaries, (ii) no violations of any security policy of Parent or its Subsidiaries regarding any such Sensitive Data, (iii) no unauthorized access or unauthorized use of any Sensitive Data used in the business of Parent or its Subsidiaries and (iv) no unintended or improper disclosure of any personally identifiable information in the possession, custody or control of Parent or its Subsidiaries or a contractor or agent acting on behalf of Parent or its Subsidiaries, in each case of (i) through (iv), except as would not reasonably be expected to, individually or in the aggregate, have a Parent Material Adverse Effect.
(h) None of Parent or its Subsidiaries are now or has ever been a member or promoter of, or a contributor to, any industry standards body or any similar organization that would reasonably be expected to require or obligate Parent or any of its Subsidiaries to grant or offer to any other Person any license or right to any Parent IP or Parent In-Licensed IP.
3.13 Agreements, Contracts and Commitments.
(a) Section 3.13 of the Parent Disclosure Schedule lists the following Parent Contracts in effect as of the date of this Agreement (other than any Parent Benefit Plan) (each, a “Parent Material Contract” and collectively, the “Parent Material Contracts”):
(i) a material contract as defined in Item 601(b)(10) of Regulation S-K as promulgated under the Securities Act;
(ii) each Parent Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(iii) each Parent Contract containing (A) any covenant limiting the freedom of Parent or any of its Subsidiaries to engage in any line of business or compete with any Person, (B) any “most-favored nations” pricing provisions or marketing or distribution rights related to any products or territory, (C) any exclusivity provision, (D) any agreement to purchase minimum quantity of goods or services, or (E) any material non-solicitation provisions applicable to Parent or any of its Subsidiaries;
(iv) each Parent Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $50,000 pursuant to its express terms and not cancelable without penalty;
(v) each Parent Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(vi) each Parent Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit in excess of $50,000 or creating any material Encumbrances with respect to any assets of Parent or any of its Subsidiaries or any loans or debt obligations with officers or directors of Parent or any of its Subsidiaries;
(vii) each Parent Contract requiring payment by or to Parent or any of its Subsidiaries after the date of this Agreement in excess of $300,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to any pre-clinical or clinical development activities of Parent or any of its Subsidiaries; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, collaboration, development or other agreement currently in force under which Parent or any of its Subsidiaries have continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which Parent or any of its Subsidiaries have continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by Parent or any of its Subsidiaries; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of Parent or any of its Subsidiaries or any Contract to sell, distribute or commercialize any products or service of Parent or any of its Subsidiaries, in each case, except for Contracts entered into in the Ordinary Course of Business;
(viii) each Parent Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing advisory services to Parent in connection with the Contemplated Transactions;
(ix) each Parent Real Estate Lease;
(x) each Parent Contract with any Governmental Body;
(xi) each Parent Out-bound License and Parent In-bound License;
(xii) each Parent Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of Parent or any of its Subsidiaries; or
(xiii) any other Parent Contract that is not terminable at will (with no penalty or payment) by Parent or its Subsidiaries, as applicable, and (A) which involves payment or receipt by Parent or its Subsidiaries after the date of this Agreement under any such agreement, contract or commitment of more than $300,000 in the aggregate, or (B) that is material to the business or operations of Parent and its Subsidiaries, taken as a whole.
(b) Parent has delivered or made available to the Company accurate and complete copies of all Parent Material Contracts, including all amendments thereto. There are no Parent Material Contracts that are not in written form. As of the date of this Agreement, none of Parent, any of its Subsidiaries or, to Parent’s Knowledge, any other party to a Parent Material Contract, has breached, violated or defaulted under, or received notice that it breached, violated or defaulted under, any of the terms or conditions of, or Laws applicable to, any Parent Material Contract in such manner as would permit any other party to cancel or terminate any such Parent Material Contract, or would permit any other party to seek damages or pursue other legal remedies which would reasonably be expected to be material to Parent or its business or operations. As to Parent and its Subsidiaries, as of the date of this Agreement, each Parent Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Parent Material Contract to change, any material amount paid or payable to Parent or any of its Subsidiaries under any Parent Material Contract or any other material term or provision of any Parent Material Contract.
3.14 Compliance; Permits; Restrictions.
(a) Parent and each of its Subsidiaries are, and since January 1, 2020, have been, in compliance in all material respects with all applicable Laws, including the FDCA, the FDA regulations adopted thereunder, the Public Health Service Act and any other similar Law administered or promulgated by the FDA or other Drug Regulatory Agency, except for any noncompliance, either individually or in the aggregate, which would not be material to Parent. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to Parent’s Knowledge, threatened against Parent or any of its Subsidiaries. There is no agreement, judgment, injunction, order or decree by or with a Drug Regulatory Agency binding upon Parent or any of its Subsidiaries which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of Parent or any of its Subsidiaries, any acquisition of material property by Parent or any of its Subsidiaries or the conduct of business by Parent or any of its Subsidiaries as currently conducted, (ii) is reasonably likely to have an adverse effect on Parent’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
(b) Parent and its Subsidiaries hold all required Governmental Authorizations which are material to the operation of the business of Parent and its Subsidiaries as currently conducted (the “Parent Permits”). Section 3.14(b) of the Parent Disclosure Schedule identifies each Parent Permit. Parent and its Subsidiaries hold all right, title and interest in and to all Parent Permits free and clear of any Encumbrance. Parent and each of its Subsidiaries are in material compliance with the terms of the Parent Permits. No Legal Proceeding is pending or, to Parent’s Knowledge, threatened, which seeks to revoke, limit, suspend, or materially modify any Parent Permit.
(c) There are no proceedings pending or, to the Parent’s Knowledge, threatened with respect to an alleged material violation by Parent or any of its Subsidiaries of the FDCA, FDA regulations adopted thereunder, the Controlled Substances Act, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) Parent is not currently conducting or addressing, and to Parent’s Knowledge there is no basis to expect that it will be required to conduct or address, any corrective actions, including, without limitation, product recalls or clinical holds.
(e) All clinical, pre-clinical and other studies and tests conducted by or, to Parent’s Knowledge, on behalf of, or sponsored by, Parent or any of its Subsidiaries, or in which Parent or any of its Subsidiaries or their respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. Since January 1, 2020, neither Parent nor any of its Subsidiaries have received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or, to Parent’s Knowledge, threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, Parent or any of its Subsidiaries or in which Parent or any of its Subsidiaries or their respective current products or product candidates have participated.
(f) Neither Parent nor any of its Subsidiaries are the subject of any pending or, to Parent’s Knowledge, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To Parent’s Knowledge, neither Parent nor any of its Subsidiaries have committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. None of Parent, any of its Subsidiaries or any of their respective officers, employees or agents have been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. To Parent’s Knowledge, no debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or threatened against Parent, any of its Subsidiaries or any of their respective officers, employees or agents.
3.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no pending Legal Proceeding and, to Parent’s Knowledge, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) Parent, (B) any of its Subsidiaries, (C) any Parent Associate (in his or her capacity as such) or (D) any of the material assets owned or used by Parent or its Subsidiaries; or (ii) that challenges, or that would reasonably be expected to have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b) Except as set forth in Section 3.15(b) of the Parent Disclosure Schedule, since January 1, 2020, no Legal Proceeding has been pending against Parent or any of its Subsidiaries that resulted in material liability to Parent or any of its Subsidiaries.
(c) There is no order, writ, injunction, judgment or decree to which Parent or any of its Subsidiaries, or any of the material assets owned or used by Parent or any of its Subsidiaries, is subject. To Parent’s Knowledge, no officer or other employee of Parent or any of its Subsidiaries is subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of Parent or any of its Subsidiaries or to any material assets owned or used by Parent or any of its Subsidiaries.
3.16 Tax Matters.
(a) Parent and each of its Subsidiaries have timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No written claim has ever been made by any Governmental Body in any jurisdiction where Parent or any of its Subsidiaries does not file a particular Tax Return or pay a particular Tax that Parent or such Subsidiary is subject to taxation by that jurisdiction.
(b) All income and other material Taxes due and owing by Parent or any of its Subsidiaries (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of Parent and its Subsidiaries did not, as of the date of the Parent Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Parent Balance Sheet. Since the date of the Parent Balance Sheet, neither Parent nor any of its Subsidiaries have incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that Parent or any of its Subsidiaries are or were required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose. The Parent and its Subsidiaries have complied in all material respects with applicable Laws relating to information reporting and record retention (including to the extent necessary to claim any exemption from sales Tax collection and maintaining adequate and current resale certificates to support any such claimed exemptions).
(d) There are no Encumbrances for material Taxes (other than Taxes not yet due and payable) upon any of the assets of Parent or any of its Subsidiaries.
(e) No deficiencies for income or other material Taxes with respect to Parent or any of its Subsidiaries have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and, to Parent’s Knowledge, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of Parent or any of its Subsidiaries. Neither Parent nor any of its Subsidiaries (or any of their predecessors) has waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) Neither Parent nor any of its Subsidiaries have been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(g) Neither Parent nor any of its Subsidiaries are a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
(h) Neither Parent nor any of its Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes made on or prior to the Closing Date; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law) entered into on or prior to the Closing Date; (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received or deferred revenue accrued on or prior to the Closing Date; (vii) application of Section 367(d) of the Code to any transfer of intangible property on or prior to the Closing Date; (viii) application of Sections 951 or 951A of the Code (or any similar provision of state, local or foreign Law) to any income received or accrued on or prior to the Closing Date; or (ix) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law) made on or prior to the Closing Date. Parent has not made any election under Section 965(h) of the Code.
(i) Neither Parent nor any of its Subsidiaries have ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is Parent) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Neither Parent nor any of its Subsidiaries have any Liability for any material amount of Taxes of any Person (other than Parent and any of its Subsidiaries) under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign Law), by contract, as a transferee or successor, or otherwise.
(j) Neither Parent nor any of its Subsidiaries (i) are a “controlled foreign corporation” as defined in Section 957 of the Code; (ii) is a “passive foreign investment company” within the meaning of Section 1297 of the Code; or (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized.
(k) Neither Parent nor any of its Subsidiaries have participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “reportable transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(l) Neither Parent nor any of its Subsidiaries have taken any action, nor to Parent’s Knowledge is there any fact, that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
(m) Neither Parent nor any of its Subsidiaries have availed itself of any Tax relief pursuant to any Pandemic Response Laws that could reasonably be expected to materially impact the Tax payment and/or Tax reporting obligations of Parent and its Affiliates (including the Company and its Subsidiaries) after the Closing Date.
For purposes of this Section 3.16, each reference to Parent or any of its Subsidiaries shall be deemed to include any Person that was liquidated into, merged with, or is otherwise a predecessor to, Parent of any of its Subsidiaries.
3.17 Employee and Labor Matters; Benefit Plans.
(a) Section 3.17(a) of the Parent Disclosure Schedule lists, as of the date of this Agreement, all material Parent Benefit Plans. “Parent Benefit Plan” means each (i) “employee benefit plan,” as defined in Section 3(3) of ERISA, whether or not subject to ERISA, and (ii) any other pension, retirement, deferred compensation, excess benefit, profit-sharing, bonus, incentive, equity or equity-based, phantom equity, employment (other than at-will employment offer letters on Parent’s standard form that may be terminated without notice and with no penalty to Parent or any of its Subsidiaries and other than individual Parent Options, Parent RSUs, or other compensatory equity award grants made pursuant to Parent’s standard forms, in which case only representative standard forms of such agreements shall be scheduled), consulting, severance, change-of-control, stay or retention, medical, dental, vision, health, prescription drug, life, disability, cafeteria plan, flexible spending account, employee assistance program, travel assistance, accidental death & dismemberment, group insurance, retiree medical or life insurance, paid-time-off, holiday, welfare or fringe benefit plan, program, agreement, contract, or arrangement (other than regular salary or wages) (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in each case, maintained, contributed to, or required to be contributed to, by Parent or any of its Subsidiaries for the benefit of any current or former employee, director, officer, or independent contractor of Parent or any of its Subsidiaries or under which Parent or any of its Subsidiaries have or could have any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Section 414 of the Code with any other person).
(b) As applicable with respect to each material Parent Benefit Plan, Parent has made available to the Company, true and complete copies of (i) each material Parent Benefit Plan, including all amendments thereto, and in the case of an unwritten material Parent Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements, insurance, and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the three most recently filed annual reports on Form 5500 and all schedules thereto, (v) the most recent IRS determination or opinion letter, (vi) the three most recent nondiscrimination testing reports, actuarial reports, and financial statements, (vii) all notices and filings concerning IRS, United States Department of Labor, or other Governmental Body audits or investigations since January 1, 2020, (viii) each written report constituting a valuation of Parent’s capital stock for purposes of Sections 409A or 422 of the Code, whether prepared internally by Parent or by an outside, third-party valuation firm, and (ix) all material written materials provided to employees or participants relating to the amendment, termination, establishment, or increase or decrease in benefits under any Parent Benefit Plan.
(c) Each Parent Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other applicable Laws.
(d) The Parent Benefit Plans that are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and that are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and, to Parent’s Knowledge, nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Parent Benefit Plan.
(e) None of Parent or any Parent ERISA Affiliate sponsors, maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, or has within the past six (6) years sponsored, maintained, contributed to, or been required to contribute to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code), or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA), and none of Parent or any Parent ERISA Affiliate has, within the preceding six (6) years, incurred a complete or partial withdrawal from any “multiemployer plan” or otherwise incurred any liability under Section 4202 of ERISA.
(f) There are no pending audits or investigations by any Governmental Body involving any Parent Benefit Plan, and no pending or, to Parent’s Knowledge, threatened claims (except for individual claims for benefits payable in the normal operation of the Parent Benefit Plans), suits, or proceedings involving any Parent Benefit Plan. All contributions and premium payments required to have been made under any of the Parent Benefit Plans or by applicable Law have been timely made, and neither Parent nor any of its Subsidiaries have any liability for any unpaid contributions with respect to any Parent Benefit Plan (other than contributions that are accrued in the Ordinary Course of Business).
(g) Neither Parent nor any of its Subsidiaries, nor, to Parent’s Knowledge, any fiduciary, trustee, or administrator of any Parent Benefit Plan, has engaged in, or in connection with the Contemplated Transactions will engage in, any transaction with respect to any Parent Benefit Plan that would subject any such Parent Benefit Plan, Parent or any of its Subsidiaries or the Company to a Tax, penalty, or liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) No Parent Benefit Plan provides (i) death, medical, dental, vision, life insurance or other welfare benefits beyond the termination of service or retirement, other than coverage mandated by Law (including COBRA, for which the covered individual pays the full cost of coverage) or (ii) death or retirement benefits under a Parent Benefit Plan qualified under Section 401(a) of the Code, and neither the Parent nor any of its Subsidiaries have made a written or oral representation promising the same.
(i) Except as set forth in Section 3.17(i) of the Parent Disclosure Schedules, neither the execution of this Agreement, nor the consummation of the Contemplated Transactions will either alone or in connection with any other event(s) (i) result in any payment becoming due to any current or former employee, director, officer, independent contractor or other service provider of Parent or any of its Subsidiaries, (ii) increase any amount of compensation or benefits otherwise payable to any current or former employee, director, officer, independent contractor or other service provider of Parent or any of its Subsidiaries, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Parent Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Parent Benefit Plan or (v) limit the right to merge, amend or terminate any Parent Benefit Plan.
(j) Neither the execution of this Agreement, nor the consummation of the Contemplated Transactions (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Section 280G of the Code) with respect to Parent and its Subsidiaries of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Section 280G of the Code), determined without regard to the application of Section 280G(b)(5) of the Code.
(k) Each Parent Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in all material respects in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder.
(l) No Person has any “gross-up” agreements with Parent or any of its Subsidiaries or other assurance of reimbursement or compensation by Parent or any of its Subsidiaries for any Taxes imposed under Section 409A or Section 4999 of the Code.
(m) Parent does not have any Parent Benefit Plan that is maintained for service providers located outside of the United States.
(n) Neither Parent nor any of its Subsidiaries are a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union or labor organization representing any of its employees, and there is no labor union or labor organization representing or, to Parent’s Knowledge, purporting to represent or seeking to represent any employees of Parent or its Subsidiaries, including through the filing of a petition for representation election.
(o) Parent and each of its Subsidiaries is, and since January 1, 2020, has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including, without limitation, worker classification, discrimination, wrongful termination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to Parent or any of its Subsidiaries, with respect to employees of Parent or any of its Subsidiaries, each of Parent and its Subsidiaries, since January 1, 2020: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries and other payments, benefits, or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or penalties for failure to comply with any of the foregoing in any material respect, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body, with respect to unemployment compensation benefits, disability, social security or other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business or as required by Law). There are no actions, suits, claims, charges, demands, lawsuits, investigations, audits or administrative matters pending or, to Parent’s Knowledge, threatened or reasonably anticipated against Parent or any of its Subsidiaries relating to any current or former employee, applicant for employment, consultant, employment agreement or Parent Benefit Plan (other than routine claims for benefits). All employees of Parent and its Subsidiaries are employed “at-will” and their employment can be terminated without advance notice or payment of severance.
(p) Except as would not be reasonably likely to result in a material liability to Parent or any of its Subsidiaries, with respect to each individual who currently renders services to Parent or any of its Subsidiaries, Parent and each of its Subsidiaries have accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, Parent and each of its Subsidiaries have accurately classified him or her as overtime eligible or overtime ineligible under all applicable Laws. Neither Parent nor any of its Subsidiaries have any material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee, (b) any employee leased from another employer, or (c) any employee currently or formerly classified as exempt from overtime wages.
(q) There is not and has not been since January 1, 2020, nor is there or has there been since January 1, 2020, any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to Parent’s Knowledge, any union organizing activity, against Parent or any of its Subsidiaries. No event has occurred, and, to Parent’s Knowledge, no condition or circumstance exists, that might directly or indirectly give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute.
3.18 Environmental Matters. Parent and each of its Subsidiaries are in compliance, and since January 1, 2020, have complied, with all applicable Environmental Laws, which compliance includes the possession by Parent and its Subsidiaries of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to result in a Parent Material Adverse Effect. Neither Parent nor any of its Subsidiaries have received since January 1, 2020, any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that Parent or any of its Subsidiaries are not in compliance with or has liability pursuant to any Environmental Law and, to Parent’s Knowledge, there are no circumstances that would reasonably be expected to prevent or interfere with Parent’s or any of its Subsidiaries’ compliance with any Environmental Law in the future, except where such failure to comply would not reasonably be expected to have a Parent Material Adverse Effect. To the Parent’s Knowledge, no current or (during the time a prior property was leased or controlled by Parent or any of its Subsidiaries) prior property leased or controlled by Parent or any of its Subsidiaries have had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of Parent or any of its Subsidiaries pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the consummation of the Contemplated Transactions. Prior to the date of this Agreement, Parent has provided or otherwise made available to the Company true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of Parent or any of its Subsidiaries with respect to any property leased or controlled by Parent or any of its Subsidiaries or any business operated by them.
3.19 Transactions with Affiliates. Except as set forth in the Parent SEC Documents filed prior to the date of this Agreement, since the date of Parent’s proxy statement filed in 2022 with the SEC, no event has occurred that would be required to be reported by Parent pursuant to Item 404 of Regulation S-K as promulgated under the Securities Act.
3.20 Insurance. Parent has delivered or made available to the Company accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of Parent and each of its Subsidiaries, as of the date of this Agreement. Each of such insurance policies is in full force and effect and Parent and each of its Subsidiaries are in compliance in all material respects with the terms thereof. Other than customary end-of-policy notifications from insurance carriers, since January 1, 2020, neither Parent nor any of its Subsidiaries have received any notice or other communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. Parent and each of its Subsidiaries have provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against Parent or any of its Subsidiaries for which Parent or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed Parent or any of its Subsidiaries of its intent to do so.
3.21 No Financial Advisors. Except as set forth in Section 3.21 of the Parent Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of Parent or any of its Subsidiaries.
3.22 Disclosure. The information supplied by or on behalf of Parent and each of its Subsidiaries for inclusion or incorporation by reference in the Registration Statement, or to be included or supplied by or on behalf of Parent and each of its Subsidiaries for inclusion in any Regulation M-A Filing, shall not at the time the Registration Statement or any such Regulation M-A Filing is filed with the SEC, at any time it is amended or supplemented or at the time the Registration Statement is declared effective by the SEC, as applicable, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements therein not false or misleading, or omit to state any material fact necessary to correct any statement therein that has become false or misleading. The information supplied by or on behalf of Parent and each of its Subsidiaries for inclusion or incorporation by reference in the Proxy Statement, which information shall be deemed to include all information about or related to Parent and each of its Subsidiaries, the Parent Stockholder Matters and the Parent Stockholder Meeting, shall not, on the date the Proxy Statement is first mailed to Parent’s stockholders, or at the time of the Parent Stockholders’ Meeting or as of the Effective Time, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements made in the Proxy Statement not false or misleading, or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies for the Parent Stockholders’ Meeting that has become false or misleading.
3.23 Anti-Bribery. None of Parent, any of its Subsidiaries or any of their respective directors, officers, employees or, to Parent’s Knowledge, agents or any other Person acting on their behalf has directly or indirectly made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of Anti-Bribery Laws. Neither Parent nor any of its Subsidiaries are or has been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
3.24 Valid Issuance. The Parent Common Stock to be issued in the Merger will, when issued in accordance with the provisions of this Agreement, be validly issued, fully paid and nonassessable.
3.25 Disclaimer of Other Representations or Warranties. Except as previously set forth in this Section 3 or in any certificate delivered by Parent or Merger Sub to the Company pursuant to this Agreement, neither Parent nor Merger Sub makes any representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities, or operations, and any such other representations or warranties are hereby expressly disclaimed.
SECTION 4. CERTAIN COVENANTS OF THE PARTIES
4.1 Operation of Parent’s Business.
(a) Except (i) as set forth in Section 4.1(a) of the Parent Disclosure Schedule, (ii) as expressly permitted by or required in accordance with this Agreement, including in connection with the Asset Dispositions pursuant to Section 4.7, (iii) as required by applicable Law or (iv) as may be consented to in writing by the Company (which consent shall not be unreasonably withheld, delayed or conditioned), during the period commencing on the date of this Agreement and continuing until the earlier to occur of the termination of this Agreement pursuant to Section 9 and the Effective Time (the “Pre-Closing Period”): each of Parent and its Subsidiaries shall (A) conduct its business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Parent Material Contracts and (B) continue to pay material outstanding accounts payable and other material current Liabilities (including payroll) when due and payable.
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth in Section 4.1(b) of the Parent Disclosure Schedule, (iii) as required by applicable Law, (iv) in connection with the Asset Dispositions pursuant to Section 4.7 or the winding down of Parent’s prior research and development activities (including the termination of ongoing contractual obligations related to Parent’s current products or product candidates), or (v) with the prior written consent of the Company (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, Parent shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except repurchases of shares of Parent Common Stock from terminated employees, directors or consultants of Parent or in connection with the payment of the exercise price and/or withholding Taxes incurred upon the exercise, settlement or vesting of any award or purchase rights granted under the Parent Incentive Plan in accordance with the terms of such award in effect on the date of this Agreement);
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of Parent or any of its Subsidiaries (except for shares of Parent Common Stock issued upon the valid exercise of outstanding Parent Options or Parent Warrants or upon settlement of Parent RSUs); (B) any option, warrant or right to acquire any capital stock or any other security, other than Parent Options or Parent RSUs granted to directors, employees and service providers in the Ordinary Course of Business which are included in the calculation of the Parent Outstanding Shares; or (C) any instrument convertible into or exchangeable for any capital stock or other security of Parent or any of its Subsidiaries;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its or its Subsidiaries’ Organizational Documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(v) (A) lend money to any Person (except for the advancement of reasonable and customary expenses to employees, directors and consultants in the Ordinary Course of Business), (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, (D) other than the incurrence or payment of Transaction Expenses, make any capital expenditure in excess of $100,000 of the budgeted capital expenditure amounts set forth in Parent’s operating budget delivered to the Company concurrently with the execution of this Agreement (the “Parent Budget”) or (E) forgive any loans to any Persons, including Parent’s employees, officers, directors or Affiliates;
(vi) other than as required by applicable Law or the terms of any Parent Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Parent Benefit Plan; (B) cause or permit any Parent Benefit Plan to be amended; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or bonus or other compensation or remuneration payable to, any of its directors, officers, consultants or employees, other than increases in base salary and annual cash bonus opportunities and payments made, in each case, in the Ordinary Course of Business; (D) hire any officer or employee; or (E) increase the severance or change of control benefits offered to any current or new employees, directors or consultants;
(vii) recognize any labor union or labor organization, except as otherwise required by applicable Law and after prior written consent of the Company (which consent shall not be unreasonably withheld, delayed or conditioned);
(viii) enter into any material transaction other than in the Ordinary Course of Business;
(ix) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any Encumbrance with respect to such assets or properties, except in the Ordinary Course of Business;
(x) sell, assign, transfer, license, sublicense or otherwise dispose of any material Parent IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
(xi) make, change or revoke any material Tax election, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability or submit any voluntary disclosure application, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than seven (7) months), or adopt or change any material accounting method in respect of Taxes;
(xii) enter into, materially amend or terminate any Parent Material Contract;
(xiii) except as otherwise set forth in the Parent Budget and the incurrence or payment of any Transaction Expenses, make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed the aggregate amount of the Parent Budget by $100,000;
(xiv) other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xv) initiate or settle any Legal Proceeding or other claim or dispute involving or against Parent or any Subsidiary of Parent;
(xvi) enter into or amend a Parent Material Contract that would reasonably be expected to prevent or materially impede, interfere with, hinder or delay the consummation of the Contemplated Transactions; or
(xvii) agree, resolve or commit to do any of the foregoing.
(c) Nothing contained in this Agreement shall give the Company, directly or indirectly, the right to control or direct the operations of Parent prior to the Effective Time. Prior to the Effective Time, Parent shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations.
4.2 Operation of the Company’s Business.
(a) Except (i) as set forth in Section 4.2(a) of the Company Disclosure Schedule, (ii) as expressly permitted by or required in accordance with this Agreement, (iii) as required by applicable Law, or (iv) as may be consented to in writing by Parent (which consent shall not be unreasonably withheld, delayed or conditioned), during the Pre-Closing Period: each of the Company and its Subsidiaries shall (A) conduct its business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Company Material Contracts and (B) continue to pay material outstanding accounts payable and other material current Liabilities (including payroll) when due and payable.
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth in Section 4.2(b) of the Company Disclosure Schedule, (iii) as required by applicable Law or (iv) with the prior written consent of Parent (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, the Company shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except repurchases of shares of Company Common Stock from terminated employees, directors or consultants of Company or in connection with the payment of the exercise price and/or withholding Taxes incurred upon the exercise, settlement or vesting of any award or purchase rights granted under the Company Incentive Plan in accordance with the terms of such award in effect on the date of this Agreement);
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of the Company or any of its Subsidiaries (except for shares of Company Common Stock issued upon the valid exercise of outstanding Company Options or Company Warrants); (B) any option, warrant or right to acquire any capital stock or any other security, other than Company options or restricted stock unit awards granted to directors, employees and service providers in the Ordinary Course of Business which are included in the calculation of the Company Outstanding Shares; or (C) any instrument convertible into or exchangeable for any capital stock or other security of the Company or any of its Subsidiaries;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its or its Subsidiaries’ Organizational Documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(v) (A) lend money to any Person (except for the advancement of reasonable and customary expenses to employees, directors and consultants in the Ordinary Course of Business), (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, (D) other than the incurrence or payment of Transaction Expenses, make any capital expenditure in excess of $100,000 of the budgeted capital expenditure amounts set forth in the Company operating budget delivered to Parent concurrently with the execution of this Agreement (the “Company Budget”) or (E) forgive any loans to any Persons, including the Company’s employees, officers, directors or Affiliates;
(vi) other than as required by applicable Law or the terms of any Company Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Company Benefit Plan; (B) cause or permit any Company Benefit Plan to be amended; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or bonus or other compensation or remuneration payable to, any of its directors, officers, consultants or employees, other than increases in base salary and annual cash bonus opportunities and payments made, in each case, in the Ordinary Course of Business; (D) increase the severance or change of control benefits offered to any current or new employees, directors or consultants; or (E) terminate or give notice to any officer other than for cause;
(vii) recognize any labor union or labor organization, except as otherwise required by applicable Law and after prior written consent of Parent (which consent shall not be unreasonably withheld, delayed or conditioned);
(viii) enter into any material transaction other than in the Ordinary Course of Business;
(ix) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any Encumbrance with respect to such assets or properties, except in the Ordinary Course of Business;
(x) sell, assign, transfer, license, sublicense or otherwise dispose of any Company IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
(xi) make, change or revoke any material Tax election, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability or submit any voluntary disclosure application, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than seven (7) months), or adopt or change any material accounting method in respect of Taxes;
(xii) enter into, materially amend or terminate any Company Material Contract (other than statements of work entered into or amended in the Ordinary Course of Business);
(xiii) except as otherwise set forth in the Company Budget and the incurrence or payment of any Transaction Expenses, make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed the aggregate amount of the Company Budget by $150,000;
(xiv) other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xv) initiate or settle any Legal Proceeding or other claim or dispute involving or against the Company or any Subsidiary of the Company; or
(xvi) enter into or amend a Contract that would reasonably be expected to prevent or materially impede, interfere with, hinder or delay the consummation of the Contemplated Transactions; or
(xvii) agree, resolve or commit to do any of the foregoing.
(c) Nothing contained in this Agreement shall give Parent, directly or indirectly, the right to control or direct the operations of the Company prior to the Effective Time. Prior to the Effective Time, the Company shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations.
4.3 Access and Investigation. Subject to the terms of the Confidentiality Agreement, which the Parties agree will continue in full force following the date of this Agreement, during the Pre-Closing Period, upon reasonable notice, Parent, on the one hand, and the Company, on the other hand, shall and shall use commercially reasonable efforts to cause such Party’s Representatives to: (a) provide the other Party and such other Party’s Representatives with reasonable access during normal business hours to such Party’s Representatives, personnel, property and assets and to all existing books, records, Tax Returns, work papers and other documents and information relating to such Party and its Subsidiaries; (b) provide the other Party and such other Party’s Representatives with such copies of the existing books, records, Tax Returns, work papers, product data, and other documents and information relating to such Party and its Subsidiaries, and with such additional financial, operating and other data and information regarding such Party and its Subsidiaries as the other Party may reasonably request; (c) permit the other Party’s officers and other employees to meet, upon reasonable notice and during normal business hours, with the chief financial officer and other officers and managers of such Party responsible for such Party’s financial statements and the internal controls of such Party to discuss such matters as the other Party may reasonably deem necessary or appropriate; and (d) make available to the other Party copies of unaudited financial statements, material operating and financial reports prepared for senior management or the board of directors of such Party, and any material notice, report or other document filed with or sent to or received from any Governmental Body in connection with the Contemplated Transactions. Any investigation conducted by either Parent or the Company pursuant to this Section 4.3 shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the other Party.
Notwithstanding the foregoing, any Party may restrict the foregoing access to the extent that any Law, and, with respect to the Company, any contract listed in Section 4.3 of the Company Disclosure Schedule, applicable to such Party requires such Party to restrict or prohibit access to any such properties or information or may redact any of the foregoing documents or reports to the extent necessary to preserve the attorney-client privilege under any circumstances in which such privilege may be jeopardized by the disclosure of such document or report.
4.4 Parent Non-Solicitation.
(a) Parent agrees that, during the Pre-Closing Period, neither it nor any of its Subsidiaries shall, nor shall it or any of its Subsidiaries authorize any of their respective Representatives to, directly or indirectly, other than relating to communicating, discussing, negotiating or consummating the Asset Dispositions: (i) solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnish any non-public information regarding Parent or any of its Subsidiaries to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engage in discussions (other than to inform any Person of the existence of the provisions in this Section 4.4) or negotiations with any Person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approve, endorse or recommend any Acquisition Proposal (subject to Section 5.3); (v) execute or enter into any letter of intent or any Contract contemplating or otherwise relating to any Acquisition Transaction (other than a confidentiality agreement permitted under this Section 4.4(a)); or (vi) publicly propose to do any of the foregoing; provided, however, that, notwithstanding anything contained in this Section 4.4 and subject to compliance with this Section 4.4, prior to obtaining the Required Parent Stockholder Vote, Parent and its Subsidiaries may furnish non-public information regarding Parent or any of its Subsidiaries to, and enter into discussions or negotiations with, any Person in response to a bona fide Acquisition Proposal by such Person, which the Parent Board determines in good faith, after consultation with Parent’s outside financial advisors and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (and is not withdrawn) if: (A) none of Parent, any of its Subsidiaries or any of their respective Representatives shall have breached this Section 4.4 in any material respect; (B) the Parent Board concludes in good faith based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of the Parent Board under applicable Law; (C) substantially contemporaneously with furnishing any such non-public information to such Person, Parent gives the Company notice of Parent’s intention to furnish nonpublic information to, or enter into discussions with, such Person and furnishes such non-public information to the Company (to the extent such information has not been previously furnished by Parent to the Company); and (D) Parent receives from such Person an executed confidentiality agreement containing provisions (including nondisclosure provisions, use restrictions, non-solicitation provisions, no hire and “standstill” provisions), in the aggregate, at least as favorable to Parent as those contained in the Confidentiality Agreement. Without limiting the generality of the foregoing, Parent acknowledges and agrees that, in the event any Representative of Parent or any of its Subsidiaries (whether or not such Representative is purporting to act on behalf of Parent or any of its Subsidiaries) takes any action that, if taken by Parent or any of its Subsidiaries, would constitute a breach of this Section 4.4, the taking of such action by such Representative shall be deemed to constitute a breach of this Section 4.4 by Parent for purposes of this Agreement.
(b) If Parent, any of its Subsidiaries or any of their respective Representatives receives an Acquisition Proposal or Acquisition Inquiry at any time during the Pre-Closing Period, then Parent shall promptly (and in no event later than one (1) Business Day after Parent becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise the Company orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the material terms thereof). Parent shall keep the Company reasonably informed with respect to the status and material terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or proposed material modification thereto.
(c) Parent shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any Person that relate to any Acquisition Proposal or Acquisition Inquiry (other than any Asset Disposition) that has not already been terminated as of the date of this Agreement and request the destruction or return of any non-public information of Parent or any of its Subsidiaries provided to such Person as soon as practicable after the date of this Agreement.
4.5 Company Non-Solicitation.
(a) The Company agrees that, during the Pre-Closing Period, neither it nor any of its Subsidiaries shall, nor shall it or any of its Subsidiaries authorize any of their respective Representatives to, directly or indirectly: (i) solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnish any non-public information regarding the Company or any of its Subsidiaries to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engage in discussions (other than to inform any Person of the existence of the provisions in this Section 4.5) or negotiations with any Person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approve, endorse or recommend any Acquisition Proposal; (v) execute or enter into any letter of intent or any Contract contemplating or otherwise relating to any Acquisition Transaction; or (vi) publicly propose to do any of the foregoing. Without limiting the generality of the foregoing, the Company acknowledges and agrees that, in the event any Representative of the Company or any of its Subsidiaries (whether or not such Representative is purporting to act on behalf of the Company or any of its Subsidiaries) takes any action that, if taken by the Company, would constitute a breach of this Section 4.5, the taking of such action by such Representative shall be deemed to constitute a breach of this Section 4.5 by the Company for purposes of this Agreement.
(b) If the Company, any of its Subsidiaries or any of their respective Representatives receives an Acquisition Proposal or Acquisition Inquiry at any time during the Pre-Closing Period, then the Company shall promptly (and in no event later than one (1) Business Day after the Company becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise Parent orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the material terms thereof). The Company shall keep Parent reasonably informed with respect to the status and material terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or proposed material modification thereto.
(c) The Company shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any Person that relate to any Acquisition Proposal or Acquisition Inquiry that has not already been terminated as of the date of this Agreement and request the destruction or return of any non-public information of the Company or any of its Subsidiaries provided to such Person as soon as practicable after the date of this Agreement.
4.6 Notification of Certain Matters.
(a) During the Pre-Closing Period, the Company shall promptly (and in no event later than one (1) Business Day after the Company becomes aware of the same) notify Parent (and, if in writing, furnish copies of any relevant documents) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting the Company or its Subsidiaries are commenced, or, to the Company’s Knowledge, threatened against the Company or its Subsidiaries or, to the Company’s Knowledge, any director or officer of the Company or its Subsidiaries; (iii) the Company becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of the Company to comply with any covenant or obligation of the Company; in each case that could reasonably be expected to make the timely satisfaction of any of the conditions set forth in Sections 6 or 7, as applicable, impossible or materially less likely. No notification given to Parent pursuant to this Section 4.6(a) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of the Company or any of its Subsidiaries contained in this Agreement or the Company Disclosure Schedule for purposes of Sections 6 and 7, as applicable.
(b) During the Pre-Closing Period, Parent shall promptly (and in no event later than one (1) Business Day after Parent becomes aware of the same) notify the Company (and, if in writing, furnish copies of any relevant documents) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting Parent or any of its Subsidiaries are commenced, or, to Parent’s Knowledge, threatened against Parent or any of its Subsidiaries or, to Parent’s Knowledge, any director or officer of Parent or any of its Subsidiaries; (iii) Parent becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of Parent to comply with any covenant or obligation of Parent or Merger Sub; in each case that could reasonably be expected to make the timely satisfaction of any of the conditions set forth in Sections 6 or 8, as applicable, impossible or materially less likely. No notification given to the Company pursuant to this Section 4.6(b) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of Parent or any of its Subsidiaries contained in this Agreement or the Parent Disclosure Schedule for purposes of Sections 6 and 8, as applicable.
4.7 Potentially Transferable Assets. Parent shall be entitled, but under no obligation, to sell, transfer, license, assign, dividend, spin-off, reclassification, corporate rearrangement, scheme of arrangement or otherwise divest the Potentially Transferable Assets to one or more third parties in one or a series of transactions prior to or substantially concurrently with the Closing (each an “Asset Disposition” and collectively, the “Asset Dispositions”); provided, that any such Asset Disposition shall require, to the extent consistent with applicable Laws, the written consent of the Company, not to be unreasonably withheld, conditioned or delayed, if such Asset Disposition would create any post-disposition material Liabilities for Parent following the Closing. Each Party acknowledges that Parent may not be successful in completing, or may determine not to proceed with, any Asset Dispositions. For clarity, if the Asset Dispositions are not completed prior to, concurrently with, or immediately following the Closing, the Potentially Transferable Assets shall be retained by Parent.
4.8 Parent Legacy Note. Prior to the Closing, any Parent IP and other assets and technology of Parent that remain after the Asset Disposition other than the Parent Excluded Assets (“Parent Legacy Assets”) shall be sold, transferred, licensed, assigned, or conveyed into a newly formed Subsidiary of Parent (“Newco”). In consideration of the transfer of such assets, Newco shall assume (i) the Parent Legacy Note, which will be secured by the Parent Legacy Assets and (ii) all other Liabilities of Parent in existence as of the Effective Time (for the avoidance of doubt, such Liabilities shall not include Transaction Expenses).
4.9 Post-Merger Warrant. Prior to the Closing, Parent shall issue to the holders of shares of Parent Common Stock three Post-Merger Warrants for each five shares of Parent Common Stock held by such holder. Each Post-Merger Warrant shall have the terms set forth in a warrant agreement to be entered into by Parent and a warrant agent (the “Warrant Agreement”). The Warrant Agreement will be on terms reasonably satisfactory to the Company. The parties agree that the Warrant Agreements shall not be assignable or transferable (except in the case of the death of a natural person) and shall not be listed on any securities exchange.
4.10 Sarbanes-Oxley Certification. The principal executive officer and the principal financial officer of Parent shall have provided, with regard to any SEC Document file (or to be filed) with the SEC on or after the date of this Agreement, any required certification in the form required under Rule 13a-14 under the Exchange Act and 18 U.S.C. 1350.
4.11 Parent Preferred Stock. Subject to requisite Parent stockholder approval and in accordance with the Side Letter, Juvenescence Limited, a company incorporated under the Isle of Man (“Juvenescence”) will convert all of the Parent Preferred Stock owned by Juvenescence, or an Affiliate thereof, into Parent Common Stock prior to the NYSE Reverse Split.
4.12 Company Notes. Any Company Note issued to Parent shall be amended prior to Closing so they automatically convert immediately prior to the Merger into shares of Company Capital Stock (which for the avoidance of doubt, such shares of Company Capital Stock will be treated as set forth in Section 1.5(a)(i)).
4.13 Company Incentive Plan and Parent Incentive Plan. The Company agrees to cause the Amended and Restated Company Incentive Plan, attached hereto as Exhibit I, to become effective prior to the Effective Time. Parent agrees to cause the Amended and Restated Parent Incentive Plan, attached hereto as Exhibit J, to become effective prior to the Effective Time. The Parties agree that such amendment and restatement shall not require stockholder approval of either the Company or the Parent.
SECTION 5. ADDITIONAL AGREEMENTS OF THE PARTIES
5.1 Registration Statement; Proxy Statement.
(a) As promptly as practicable after the date of this Agreement, and in no event more than sixty (60) days following the date of this Agreement unless agreed to in writing by the Parties, the Parties shall prepare, and Parent shall cause to be filed with the SEC, the Registration Statement, in which the Proxy Statement will be included as a prospectus. Parent covenants and agrees that the Registration Statement (and the letter to Parent’s stockholders, a notice of meeting and form of proxy included therewith) will not, at the time that the Proxy Statement or any amendments or supplements thereto are filed with the SEC or are first mailed to Parent’s stockholders, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The Company covenants and agrees that the information provided by or on behalf of the Company or its Subsidiaries or their respective Representatives to Parent for inclusion in the Registration Statement (including the Company’s audited consolidated financial statements for the fiscal years ended 2022 and 2021, or the Company Interim Financial Statements, as the case may be) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make such information not misleading. Notwithstanding the foregoing, Parent makes no covenant, representation or warranty with respect to statements made in the Registration Statement (and the letter to Parent’s stockholders, notice of meeting and form of proxy included therewith), if any, based on information provided by or on behalf of the Company or any of its Representatives for inclusion therein, and the Company makes no covenant, representation or warranty with respect to statements made in the Registration Statement (and the letter to Parent’s stockholders, notice of meeting and form of proxy included therewith), if any, other than with respect to the information provided by or on behalf of the Company, any of its Subsidiaries or any of their respective Representatives for inclusion therein. The Company and its legal counsel shall be given a reasonable opportunity to review and comment on the Registration Statement, including all amendments and supplements thereto, prior to the filing thereof with the SEC, and on the response to any comments of the SEC on the Registration Statement, prior to the filing thereof with the SEC. Parent shall use commercially reasonable efforts to cause the Registration Statement and the Proxy Statement to comply with the applicable rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to have the Registration Statement declared effective under the Securities Act as promptly as practicable after it is filed with the SEC. Each of the Parties shall use commercially reasonable efforts to cause the Proxy Statement to be mailed to Parent’s stockholders as promptly as practicable after the Registration Statement is declared effective under the Securities Act. Each Party shall promptly furnish to the other Party all information concerning such Party and such Party’s Affiliates and such Party’s stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.1. If Parent, Merger Sub or the Company becomes aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Registration Statement or Proxy Statement, as the case may be, then such Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate with such other Parties in filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to Parent’s stockholders. The Company and Parent shall each use commercially reasonable efforts to cause the Registration Statement and the Proxy Statement to comply with applicable federal and state securities Laws requirements.
(b) The Parties shall reasonably cooperate with each other and provide, and require their respective Representatives to provide, the other Party and its Representatives, with all true, correct and complete information regarding such Party or its Subsidiaries that is required by Law to be included in the Registration Statement and the Proxy Statement or reasonably requested by the other Party to be included in the Registration Statement and the Proxy Statement.
(c) Parent and the Company shall mutually agree on the form and substance of a Current Report on Form 8-K setting forth the anticipated Exchange Ratio as of the anticipated Closing Date, which the Parties shall cause to be filed with the SEC as early as practicable prior to the Parent Stockholders’ Meeting.
(d) If, in connection with the preparation and filing of the Registration Statement or any other filing required by applicable Law or the SEC’s review thereof, the SEC requests or requires that a tax opinion with respect to the U.S. federal income tax consequences of the Merger be prepared and submitted (a “Tax Opinion”), (i) the Company, Parent and Merger Sub shall each use their reasonable best efforts to deliver to Gibson, Dunn & Crutcher LLP, Bradley Arant Boult Cummings LLP or such other counsel as is reasonably acceptable to Parent and the Company, respectively, in connection with any Tax Opinion to be rendered by such counsel, customary Tax representation letters satisfactory to such counsel, dated and executed as of the date such relevant filing shall have been declared effective by the SEC and such other date(s) as determined to be reasonably necessary by such counsel in connection with the preparation and filing of such Registration Statement or any other filing required by applicable Law, and (ii) the Company and Parent shall each use its reasonable best efforts to cause Gibson, Dunn & Crutcher LLP, Bradley Arant Boult Cummings LLP or such other counsel as is reasonably acceptable to Parent and the Company, respectively, to furnish a Tax Opinion, subject to customary assumptions and limitations, to the effect that the Intended Tax Treatment should apply to the Merger. Gibson, Dunn & Crutcher LLP, Bradley Arant Boult Cummings LLP or such other counsel as is reasonably acceptable to Parent and the Company shall be entitled to rely on the Tax representation letters in rendering the Tax Opinions. For the avoidance of doubt, in no event shall any such Tax Opinion be a condition to Closing.
5.2 Company Information Statement; Stockholder Written Consent.
(a) Promptly after the Registration Statement shall have been declared effective under the Securities Act, and in any event no later than three (3) Business Days thereafter, the Company shall prepare, with the cooperation of Parent, and cause to be mailed to Company’s stockholders an information statement, which shall include a copy of the Proxy Statement (the “Information Statement”), to solicit the approval by written consent from the Company stockholders sufficient for the Required Company Stockholder Vote in lieu of a meeting pursuant to the ABCL, for purposes of (i) adopting and approving this Agreement and the Contemplated Transactions, (ii) acknowledging that the approval given thereby is irrevocable and that such stockholder is aware of its appraisal rights for its shares of Company Capital Stock pursuant to ABCL Article 13, a true and correct copy of which will be attached thereto, and that such stockholder has received and read a copy of ABCL Article 13, (iii) acknowledging that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares of Company Capital Stock in connection with the Merger and thereby waives any rights to receive payment of the fair value of its shares of Company Capital Stock under the ABCL, (iv) electing that the consummation of the Merger (and any plan of merger related thereto) is not a Deemed Liquidation Event, as such term is defined in the Company’s Organizational Documents, and (v) electing an automatic conversion of each share of Company Preferred Stock into shares of Company Common Stock immediately prior to the Effective Time in accordance with the relevant provisions of the Company’s Organizational Documents (the “Preferred Stock Conversion”) (collectively, the “Company Stockholder Matters”). Under no circumstances shall the Company assert that any other approval or consent is necessary by its stockholders to approve this Agreement and the Contemplated Transactions. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with this Section 5.2(a) shall be subject to Parent’s advance review and reasonable approval. The Parties shall reasonably cooperate with each other and provide, and require their respective Representatives to provide, the other Party and its Representatives with all true, correct and complete information regarding such Party or its Subsidiaries that is required by Law to be included in the Information Statement or reasonably requested by the other Party to be included in the Information Statement.
(b) The Company covenants and agrees that the Information Statement, including any pro forma financial statements included therein (and the letter to stockholders and form of Company Stockholder Written Consent included therewith), will not, at the time that the Information Statement or any amendment or supplement thereto is first mailed, distributed or otherwise made available to the stockholders of the Company, at the time of receipt of the Required Company Stockholder Vote and at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. Notwithstanding the foregoing, the Company makes no covenant, representation or warranty with respect to statements made in the Information Statement (and the letter to the stockholders and form of Company Stockholder Written Consent included therewith), if any, based on information furnished in writing by Parent, any of its Subsidiaries or any of their respective Representatives for inclusion therein. Each of the Parties shall use commercially reasonable efforts to cause the Information Statement to comply with the applicable rules and regulations promulgated by the SEC and applicable federal and state securities Laws requirements in all material respects.
(c) Promptly following receipt of the Required Company Stockholder Vote, the Company shall prepare and mail a notice (the “Stockholder Notice”) to every stockholder of the Company that did not execute the Company Stockholder Written Consent. The Stockholder Notice shall (i) be a statement to the effect that the Company Board determined that the Merger is advisable in accordance with the ABCL and in the best interests of the stockholders of the Company and authorized, approved and adopted this Agreement, the Merger and the other Contemplated Transactions, (ii) provide the stockholders of the Company to whom it is sent with notice of the actions taken in the Company Stockholder Written Consent, including the adoption and approval of this Agreement, the Merger and the other Contemplated Transactions in accordance with the ABCL and the Organizational Documents of the Company and (iii) include a description of the appraisal rights of the Company’s stockholders available under the ABCL, along with such other information as is required thereunder and pursuant to applicable Law. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with this Section 5.2(c) shall be subject to Parent’s advance review and reasonable approval.
(d) The Company agrees that: (i) the Company Board shall recommend that the Company’s stockholders vote to approve the Company Stockholder Matters and shall use commercially reasonable efforts to solicit such approval from each of the Company Signatories within the time set forth in Section 5.2(a) (the recommendation of the Company Board that the Company’s stockholders vote to adopt and approve the Company Stockholder Matters being referred to as the “Company Board Recommendation”); and (ii) the Company Board Recommendation shall not be withdrawn or modified (and the Company Board shall not publicly propose to withdraw or modify the Company Board Recommendation) in a manner adverse to Parent, and no resolution by the Company Board or any committee thereof to withdraw or modify the Company Board Recommendation in a manner adverse to Parent or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal shall be adopted or proposed (the actions set forth in the foregoing clause (ii), collectively, a “Company Board Adverse Recommendation Change”).
(e) The Company’s obligation to solicit the consent of its stockholders to sign the Company Stockholder Written Consent in accordance with Section 5.2(a) and Section 5.2(d) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or other Acquisition Proposal.
5.3 Parent Stockholders’ Meeting.
(a) Promptly after the Registration Statement has been declared effective by the SEC under the Securities Act, Parent shall take all action necessary under applicable Law to call, give notice of and hold a meeting of the holders of Parent Common Stock for the purpose of seeking approval of this Agreement and the Contemplated Transactions, including:
(i) the amendment of Parent’s certificate of incorporation to effect the NYSE Reverse Split;
(ii) if applicable, the consummation of the Asset Dispositions pursuant to Section 4.7;
(iii) the issuance of Parent Common Stock or other securities of Parent that represent (or are convertible into) more than twenty percent (20%) of the shares of Parent Common Stock outstanding immediately prior to the Merger to the holders of Company Capital Stock, Company Options and Company Warrants in connection with the Contemplated Transactions and the change of control of Parent resulting from the Contemplated Transactions, in each case pursuant to the NYSE rules;
(iv) the issuance of the Post-Merger Warrants to the holders of Parent Common Stock in connection with the Contemplated Transactions;
(v) the amendment and restatement of the Parent’s certificate of incorporation, attached hereto as Exhibit F, upon the Effective Time;
(vi) the adoption of the new Parent Incentive Plan, attached hereto as Exhibit K, along with the reservation of 1,725,000 shares of Parent Common Stock for use under the new Parent Incentive Plan;
(vii) if not previously approved by Parent’s stockholders, the issuance of shares of Parent Common Stock that represent more than twenty percent (20%) of the shares of Parent Common Stock outstanding immediately prior to conversion of the Parent Series B Preferred Stock in connection with the conversion of the Parent Preferred Stock required in Section 4.11 and the change of control of Parent resulting from the conversion of the Parent Preferred Stock, in each case pursuant to the NYSE rules; and
(viii) any other proposals the Parties deem necessary or desirable to consummate the Contemplated Transactions.
(the matters contemplated by Section 5.3(a)(i) through Section 5.3(a)(viii) are referred to as the “Parent Stockholder Matters,” and such meeting, the “Parent Stockholders’ Meeting”).
(b) The Parent Stockholders’ Meeting shall be held as promptly as practicable after the Registration Statement is declared effective under the Securities Act and, in any event, no later than forty-five (45) calendar days after the effective date of the Registration Statement. Parent shall take reasonable measures to ensure that all proxies solicited in connection with the Parent Stockholders’ Meeting are solicited in compliance with all applicable Laws. Notwithstanding anything to the contrary contained herein, if on the date of the Parent Stockholders’ Meeting, or a date preceding the date on which the Parent Stockholders’ Meeting is scheduled, Parent reasonably believes that (i) it will not receive proxies sufficient to obtain the Required Parent Stockholder Vote, whether or not a quorum would be present, or (ii) it will not have sufficient shares of Parent Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the Parent Stockholders’ Meeting, Parent may postpone or adjourn, or make one or more successive postponements or adjournments of, the Parent Stockholders’ Meeting as long as the date of the Parent Stockholders’ Meeting is not postponed or adjourned more than an aggregate of sixty (60) calendar days in connection with any postponements or adjournments.
(c) Parent agrees that, subject to Section 5.3(d): (i) the Parent Board shall recommend that the holders of Parent Common Stock vote to approve the Parent Stockholder Matters and shall use commercially reasonable efforts to solicit such approval within the timeframe set forth in Section 5.3(b) above, (ii) the Proxy Statement shall include a statement to the effect that the Parent Board recommends that Parent’s stockholders vote to approve the Parent Stockholder Matters (the recommendation of the Parent Board with respect to the Parent Stockholder Matters being referred to as the “Parent Board Recommendation”); and (iii) the Parent Board Recommendation shall not be withheld, amended, withdrawn or modified (and the Parent Board shall not publicly propose to withhold, amend, withdraw or modify the Parent Board Recommendation) in a manner adverse to the Company, and no resolution by the Parent Board or any committee thereof to withdraw or modify the Parent Board Recommendation in a manner adverse to the Company or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal shall be adopted or proposed (the actions set forth in the foregoing clause (iii), collectively, a “Parent Board Adverse Recommendation Change”).
(d) Notwithstanding anything to the contrary contained in this Agreement, and subject to compliance with Section 4.4 and this Section 5.3(d), if at any time prior to the approval of the Parent Stockholder Matters at the Parent Stockholders’ Meeting by the Required Parent Stockholder Vote:
(i) if Parent has received a bona fide Acquisition Proposal (which Acquisition Proposal did not arise out of a material breach of Section 4.4) from any Person that has not been withdrawn and after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer, (x) the Parent Board may make a Parent Board Adverse Recommendation Change or (y) Parent may terminate this Agreement pursuant to Section 9.1(j) to enter into a Permitted Alternative Agreement with respect to such Superior Offer, if and only if: (A) the Parent Board determines in good faith, after consultation with Parent’s outside legal counsel, that the failure to do so would be reasonably likely to be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law; (B) Parent shall have given the Company prior written notice of its intention to consider making a Parent Board Adverse Recommendation Change or terminate this Agreement pursuant to Section 9.1(j) at least four (4) Business Days prior to making any such Parent Board Adverse Recommendation Change or termination (a “Determination Notice”; and such period, the “Parent Notice Period”) (which notice shall not constitute a Parent Board Adverse Recommendation Change); and (C)(1) Parent shall have provided to the Company a summary of the material terms and conditions of the Acquisition Proposal in accordance with Section 4.4(b), (2) Parent shall, and shall have caused its Representatives to, during the Parent Notice Period, negotiate in good faith with the Company (to the extent the Company desires to negotiate) to enable the Company to propose in writing an offer binding on the Company to effect such adjustments to the terms and conditions of this Agreement so that such Acquisition Proposal ceases to constitute a Superior Offer, and (3) after considering the results of such negotiations and giving effect to the proposals made by the Company, if any, after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer and that the failure to make the Parent Board Adverse Recommendation Change or terminate this Agreement pursuant to Section 9.1(j) would be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law; provided, that (x) the Company receives written notice from Parent confirming that the Parent Board has determined to change its recommendation during the Parent Notice Period, which notice shall include a description in reasonable detail of the reasons for such Parent Board Adverse Recommendation Change and written copies of any relevant proposed transaction agreements with any party making a potential Superior Offer or to terminate this Agreement pursuant to Section 9.1(j) during the Parent Notice Period; (y) during any Parent Notice Period, the Company shall be entitled to deliver to Parent one or more counterproposals to such Acquisition Proposal and Parent will, and cause its Representatives to, negotiate with the Company in good faith (to the extent the Company desires to negotiate) to enable the Company to propose in writing an offer binding on the Company to effect such adjustments to the terms and conditions of this Agreement so that the applicable Acquisition Proposal ceases to constitute a Superior Offer; and (z) in the event of any material amendment to any Superior Offer (including any revision in price or percentage of the combined company that Parent’s stockholders would receive as a result of such potential Superior Offer), Parent shall be required to provide the Company with notice of such material amendment and the Parent Notice Period shall be extended, if applicable, to ensure that at least two (2) Business Days remain in the Parent Notice Period following such notification during which the parties shall comply again with the requirements of this Section 5.3(d) and the Parent Board shall not make a Parent Board Adverse Recommendation Change or terminate this Agreement pursuant to Section 9.1(j) prior to the end of such Parent Notice Period as so extended (it being understood that there may be multiple extensions); and
(ii) other than in connection with an Acquisition Proposal, the Parent Board may make a Parent Board Adverse Recommendation Change in response to a Parent Change in Circumstance, if and only if: (A) the Parent Board determines in good faith, after consultation with Parent’s outside legal counsel, that the failure to do so would be reasonably likely to be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law; (B) Parent shall have given the Company a Determination Notice at least four (4) Business Days prior to making any such Parent Board Adverse Recommendation Change; and (C)(1) Parent shall have specified the Parent Change in Circumstance in reasonable detail, including the material facts and circumstances related to the applicable Parent Change in Circumstance, (2) Parent shall have given the Company the four (4) Business Days after the Determination Notice to propose revisions to the terms of this Agreement or make another proposal, and shall have made its Representatives reasonably available to negotiate in good faith with the Company (to the extent the Company desires to do so) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by the Company, if any, after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that the failure to make the Parent Board Adverse Recommendation Change in response to such Parent Change in Circumstance or terminate this Agreement pursuant to Section 9.1(j) would be inconsistent with its fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law. For the avoidance of doubt, the provisions of this Section 5.3(d)(ii) shall also apply to any material change to the facts and circumstances relating to such Parent Change in Circumstance and require a new Determination Notice, except that the references to four (4) Business Days shall be deemed to be two (2) Business Days.
(e) Nothing contained in this Agreement shall prohibit Parent or the Parent Board from (i) complying with Rules 14d-9 and 14e-2(a) promulgated under the Exchange Act, (ii) issuing a “stop, look and listen” communication or similar communication of the type contemplated by Section 14d-9(f) under the Exchange Act or (iii) otherwise making any disclosure to Parent’s stockholders; provided, however, that any disclosure made by Parent or the Parent Board pursuant to the foregoing shall be limited to a statement that Parent is unable to take a position with respect to the bidder’s tender offer unless the Parent Board determines in good faith, after consultation with its outside legal counsel, that failure to make additional disclosure would be inconsistent with its fiduciary duties under applicable Law. Parent shall not withdraw or modify in a manner adverse to the Company the Parent Board Recommendation unless specifically permitted pursuant to the terms of Section 5.3(d). Unless the Parent Board has effected a Parent Board Adverse Recommendation Change in accordance with this Section 5.3 and this Agreement is otherwise terminated pursuant to Section 9.1, Parent’s obligation to call, give notice of and hold the Parent Stockholders’ Meeting in accordance with Section 5.3(b) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Acquisition Proposal, or by any Parent Board Adverse Recommendation Change.
5.4 Regulatory Approvals. Each Party shall use commercially reasonable efforts to file or otherwise submit, as soon as practicable after the date of this Agreement, all applications, notices, reports, filings and other documents required to be filed by such Party with or otherwise submitted by such Party to any Governmental Body with respect to the Contemplated Transactions, to submit promptly any additional information requested by any such Governmental Body, and to keep the other Party promptly informed of any communication from or to any Governmental Body.
5.5 Options and Warrants.
(a) At the Effective Time, each Company Option that is outstanding and unexercised immediately prior to the Effective Time under the Company Incentive Plan, whether or not vested, shall be converted into and become an option to purchase Parent Common Stock, and Parent shall assume the Company Incentive Plan (if necessary) and each such Company Option in accordance with the terms (as in effect as of the date of this Agreement) of the Company Incentive Plan and the terms of the stock option agreement by which such Company Option is evidenced (but with changes to such documents as Parent in good faith determines are appropriate to reflect the substitution of the Company Options by Parent to purchase shares of Parent Common Stock). All rights, terms, and restrictions with respect to Company Common Stock under Company Options assumed by Parent shall thereupon be converted into rights with respect to Parent Common Stock. Accordingly, from and after the Effective Time: (i) each Company Option assumed by Parent may be exercised solely for shares of Parent Common Stock; (ii) the number of shares of Parent Common Stock subject to each Company Option assumed by Parent shall be determined in accordance with the Allocation Certificate as provided in Section 5.15; (iii) the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Option assumed by Parent shall be determined in accordance with the Allocation Certificate as provided in Section 5.15; and (iv) any restriction on the exercise of any Company Option assumed by Parent shall continue in full force and effect and the term, exercisability, vesting schedule, and other provisions of such Company Option shall otherwise remain unchanged; provided, however, that: (A) the determination in the Allocation Certificate of the number of shares of Parent Common Stock subject to each Company Option assumed by Parent shall be based on the same ratio as that applied to the Company Common Stock to determine the number of shares of Parent Common Stock each holder of Company Common Stock shall receive; (B) the determination in the Allocation Certificate of the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Option assumed by Parent shall be adjusted equitably to reflect the ratio described in clause (A); (C) to the extent provided under the terms of the respective stock option agreements governing the Company Options and the Company Incentive Plan, Parent may amend the terms of the Company Options and the Company Incentive Plan, in accordance with the terms thereof, to reflect Parent’s substitution of the Company Options with options to purchase Parent Common Stock (such as by making any change in control or similar definition relate to Parent and having any provision that provides for the adjustment of Company Options upon the occurrence of certain corporate events relate to corporate events that relate to Parent and/or Parent Common Stock), and such Company Options shall be subject to further adjustment as appropriate and necessary to reflect any stock split, division or subdivision of shares, stock dividend, reverse stock split, consolidation of shares, reclassification, recapitalization, or other similar transaction with respect to Parent Common Stock subsequent to the Effective Time; and (D) the Parent Board or a committee thereof shall succeed to the authority and responsibility of the Company Board or any committee thereof with respect to each Company Option assumed by Parent. Each Company Option so assumed by Parent is a nonqualified stock option (that is, an option that is not intended to qualify as an incentive stock option as defined in Section 422 of the Code), and, further, the assumption of such Company Option pursuant to this Section 5.5(a) shall be effected in a manner that satisfies the requirements of Section 409A of the Code and the Treasury Regulations promulgated thereunder (including the applicable portions of Treasury Regulation Section 1.424-1 as applied under Treasury Regulation Section 1.409A-1(b)(5)(v)(D)), and this Section 5.5(a) will be construed consistent with this intent.
(b) Parent shall file with the SEC, promptly, but no later than thirty (30) calendar days, after the Effective Time, a registration statement on Form S-8 (or any successor form), if available for use by Parent, relating to the shares of Parent Common Stock that are issuable with respect to Company Options assumed by Parent in accordance with Section 5.5(a).
(c) At the Effective Time, each Company Warrant that is outstanding and unexercised as of immediately prior to the Effective Time, if any, shall be converted into and become a warrant to purchase Parent Common Stock and Parent shall assume each such Company Warrant in accordance with its terms. All rights with respect to Company Capital Stock under Company Warrants assumed by Parent shall thereupon be converted into rights with respect to Parent Common Stock. Accordingly, from and after the Effective Time: (i) each Company Warrant assumed by Parent may be exercised solely for shares of Parent Common Stock; (ii) the number of shares of Parent Common Stock subject to each Company Warrant assumed by Parent shall be determined in accordance with the Allocation Certificate as provided in Section 5.15; (iii) the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Warrant assumed by Parent shall be determined in accordance with the Allocation Certificate as provided in Section 5.15; and (iv) any restriction on any Company Warrant assumed by Parent shall continue in full force and effect and the term and other provisions of such Company Warrant shall otherwise remain unchanged; provided, however, that: (A) the determination in the Allocation Certificate of the number of shares of Parent Common Stock subject to each Company Warrant assumed by Parent shall be based on the same ratio as that applied to the Company Common Stock to determine the number of shares of Parent Common Stock each holder of Company Common Stock shall receive; and (B) the determination in the Allocation Certificate of the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Warrant assumed by Parent shall be adjusted equitably to reflect the ratio described in clause (A).
(d) Prior to the Effective Time, the Company shall take all actions that may be necessary (under the Company Incentive Plan, the Company Warrants and otherwise) to effectuate the provisions of this Section 5.5 and to ensure that, from and after the Effective Time, holders of Company Options and Company Warrants have no rights with respect thereto other than those specifically provided in this Section 5.5.
(e) Immediately prior to the NYSE Reverse Split, each Out of the Money Parent Option that is outstanding and unexercised as of immediately prior to the NYSE Reverse Split, if any, shall be canceled without the payment of consideration therefor. In accordance with the terms and conditions set forth in the Parent Incentive Plan, Parent shall notify each holder of an Out of the Money Parent Option that each such Out of the Money Parent Option shall become exercisable at least ten days prior to the NYSE Reverse Split and shall thereafter be canceled.
5.6 Indemnification of Officers and Directors.
(a) From the Effective Time through the sixth (6th) anniversary of the date on which the Effective Time occurs, each of Parent and the Surviving Corporation, jointly and severally, shall indemnify and hold harmless each person who is now, or has been at any time prior to the date of this Agreement, or who becomes prior to the Effective Time, a director, officer, fiduciary or agent of Parent or the Company and their respective Subsidiaries, respectively (the “D&O Indemnified Parties”), against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements, incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was a director, officer, fiduciary or agent of Parent or of the Company or their respective Subsidiaries, whether asserted or claimed prior to, at or after the Effective Time, in each case, to the fullest extent permitted under applicable Law for directors or officers of corporations in the relevant jurisdiction. Each D&O Indemnified Party will be entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Parent and the Surviving Corporation, jointly and severally, upon receipt by Parent or the Surviving Corporation from the D&O Indemnified Party of a request therefor; provided, that any such person to whom expenses are advanced provides an undertaking to Parent, to the extent then required by applicable Law, to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
(b) The provisions of the Organizational Documents of Parent or any of its Subsidiaries with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of Parent or any of its Subsidiaries that are set forth in the Organizational Documents of Parent or any of its Subsidiaries as of the date of this Agreement shall not be amended, modified or repealed for a period of six (6) years from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of Parent or any of its Subsidiaries, unless such modification is required by applicable Law. The Organizational Documents of the Surviving Corporation shall contain, and Parent shall cause the Organizational Documents of the Surviving Corporation to so contain, provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers as those set forth in the Organizational Documents of Parent as of the date of this Agreement.
(c) From and after the Effective Time, (i) the Surviving Corporation shall fulfill and honor in all respects the obligations of the Company or any of its Subsidiaries to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Organizational Documents of the Company or any of its Subsidiaries and pursuant to any indemnification agreements between the Company or any of its Subsidiaries and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time and (ii) Parent shall fulfill and honor in all respects the obligations of Parent or any of its Subsidiaries to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Organizational Documents of Parent or any of its Subsidiaries and pursuant to any indemnification agreements between Parent or any of its Subsidiaries and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time.
(d) From and after the Effective Time, Parent shall maintain directors’ and officers’ liability insurance policies, with an effective date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies similarly situated to Parent. In addition, Parent shall purchase, prior to the Effective Time, a six (6)-year prepaid “tail policy” for the non-cancellable extension of the directors’ and officers’ liability coverage of Parent’s and any of its Subsidiaries’ existing directors’ and officers’ insurance policies and Parent’s existing fiduciary liability insurance policies (if any), in each case, for a claims reporting or discovery period of at least six (6) years from and after the Effective Time with respect to any claim related to any period of time at or prior to the Effective Time. During the term of the “tail” policy, Parent shall not take any action following the Effective Time to cause such “tail” policy to be canceled or any provision therein to be amended or waived in any manner that would adversely affect in any material respect the rights of its or any of its Subsidiaries’ former and current officers and directors.
(e) From and after the Effective Time, Parent shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the persons referred to in this Section 5.6 in connection with their successful enforcement of the rights provided to such persons in this Section 5.6.
(f) All rights to exculpation, indemnification and advancement of expenses for acts or omissions occurring at or prior to the Effective Time, whether asserted or claimed prior to, at or after the Closing, now existing in favor of the current or former directors, officers or employees, as the case may be, of Parent or the Company or any of their respective Subsidiaries as provided in their respective Organizational Documents or in any agreement shall survive the Merger and shall continue in full force and effect. The provisions of this Section 5.6 are intended to be in addition to the rights otherwise available to the current and former officers and directors of Parent and the Company and any of their respective Subsidiaries by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their representatives.
(g) From and after the Effective Time, in the event Parent or the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that the successors and assigns of Parent or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 5.6. Parent shall cause the Surviving Corporation to perform all of the obligations of the Surviving Corporation under this Section 5.6. The obligations set forth in this Section 5.6 shall not be terminated, amended or otherwise modified in any manner that adversely affects any D&O Indemnified Party, or any person who is a beneficiary under the policies referred to in this Section 5.6 and their heirs and representatives, without the prior written consent of such affected D&O Indemnified Party or other person
5.7 Additional Agreements. The Parties shall (a) use commercially reasonable efforts to cause to be taken all actions necessary to consummate the Contemplated Transactions and (b) reasonably cooperate with the other Parties and provide the other Parties with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to enable the Surviving Corporation to continue to meet its obligations under this Agreement following the Closing. Without limiting the generality of the foregoing, each Party to this Agreement: (i) shall make all filings and other submissions (if any) and give all notices (if any) required to be made and given by such Party in connection with the Contemplated Transactions; (ii) shall use reasonable best efforts to obtain each Consent (if any) reasonably required to be obtained (pursuant to any applicable Law or Contract, or otherwise) by such Party in connection with the Contemplated Transactions or for such Contract (with respect to Contracts set forth in Section 5.7 of the Company Disclosure Schedule or Section 5.7 of the Parent Disclosure Schedule, as applicable) to remain in full force and effect; (iii) shall use commercially reasonable efforts to lift any injunction prohibiting, or any other legal bar to, the Contemplated Transactions; and (iv) shall use commercially reasonable efforts to satisfy the conditions precedent to the consummation of this Agreement.
5.8 Public Announcement. The initial press release relating to this Agreement shall be a joint press release issued by the Company and Parent and thereafter Parent and the Company shall consult with each other before issuing any further press release(s) or otherwise making any public statement or making any announcement to Parent Associates or Company Associates (to the extent not previously issued or made in accordance with this Agreement) with respect to the Contemplated Transactions and shall not issue any such press release, public statement or announcement to Parent Associates or Company Associates without the other Party’s written consent (which shall not be unreasonably withheld, conditioned or delayed). Notwithstanding the foregoing: (a) each Party may, without such consultation or consent, make any public statement in response to specific questions from the press, analysts, investors or those attending industry conferences, make internal announcements to employees and make disclosures in Parent SEC Documents, so long as any such statements, announcements or disclosures are consistent with and do not disclose material information not previously disclosed in previous press releases, public disclosures or public statements made jointly by the Parties (or individually, if approved by the other Party) in compliance with this Section 5.8; (b) a Party may, without the prior consent of the other Party hereto but subject to giving advance notice to the other Party of, and consulting with the other Party regarding, the text of such press release, announcement or statement, issue any such press release or make any such public announcement or statement which Parent shall have determined in good faith, upon the advice of outside legal counsel, is required by any applicable Law; and (c) Parent need not consult with the Company in connection with such portion of any press release, public statement or filing to be issued or made pursuant to Section 5.3(e) or with respect to any Acquisition Proposal or Parent Board Adverse Recommendation Change.
5.9 Listing. Parent shall use its commercially reasonable efforts, (a) to maintain its existing listing on NYSE until the Closing Date and to obtain approval of the listing of the combined company on NYSE; (b) without derogating from the generality of the requirements of the foregoing clause (a) and to the extent required by the rules and regulations of NYSE, (i) to prepare and submit to NYSE a notification form for the listing of the shares of Parent Common Stock to be issued in connection with the Contemplated Transactions, and (ii) to cause such shares to be approved for listing (subject to official notice of issuance); (c) to effect the NYSE Reverse Split and (d) to the extent required by to Section 101 of the NYSE American Company Guide, to file an initial listing application for the Parent Common Stock on NYSE (the “NYSE Listing Application”) and to cause such NYSE Listing Application to be conditionally approved prior to the Effective Time. The Parties will use commercially reasonable efforts to coordinate with respect to compliance with NYSE rules and regulations. The Company will cooperate with Parent as reasonably requested by Parent with respect to the NYSE Listing Application and promptly furnish to Parent all information concerning the Company and its stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.9.
5.10 Tax Matters.
(a) For United States federal income Tax purposes, (i) the Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “Intended Tax Treatment”), and (ii) this Agreement is intended to be, and is hereby adopted as, a “plan of reorganization” for purposes of Section 354 and 361 of the Code and Treasury Regulations Sections 1.368-2(g) and 1.368-3(a), to which Parent, Merger Sub and the Company are parties under Section 368(b) of the Code.
(b) The Parties shall use their respective commercially reasonable efforts to cause the Merger to qualify, and will not take any action or cause any action to be taken which action would reasonably be expected to prevent the Merger from qualifying, for the Intended Tax Treatment. The Parties shall use commercially reasonable efforts to operate the Surviving Corporation so as to meet the “continuity of business enterprise” requirement. The Parties shall not file any U.S. federal, state or local Tax Return in a manner that is inconsistent with the Intended Tax Treatment, unless otherwise required by applicable Law.
5.11 Directors and Officers. The Parties shall use reasonable best efforts and take all necessary action so that (a) effective as of the Effective Time, the Parent Board is comprised of seven (7) members, with two (2) of such members designated by Parent, two (2) of such members designated by the Company, two (2) of such member whom shall meet NYSE director independence requirements and be designated by mutual agreement of Parent and the Company, and one (1) member which shall be the Company’s Chief Executive Officer as of immediately following the Closing, (b) the Persons listed in Exhibit L under the heading “Officers” are elected or appointed, as applicable, to the positions of officers of Parent, as set forth therein, to serve in such positions effective as of the Effective Time until successors are duly appointed and qualified in accordance with applicable Law and (c) the Persons listed in Exhibit L under the heading “Directors” are elected or appointed, as applicable, to the positions of directors of Parent, as set forth therein, to serve in such positions effective as of the Effective Time until successors are duly appointed and qualified in accordance with applicable Law. If any Person listed in Exhibit L is unable or unwilling to serve as an officer of Parent, as set forth therein, as of the Effective Time, the Parties shall mutually agree upon a successor. If any Person listed in Exhibit L is unable or unwilling to serve as a director of Parent, as set forth therein, as of the Effective Time, the Party appointing such Person (as set forth on Exhibit L) shall designate a successor. The Persons listed in Exhibit L under the heading “Board Designees - Parent” shall be Parent’s designees pursuant to clause (a) of this Section 5.11 (which list may be changed by Parent at any time prior to the Closing by written notice to the Company to include different board designees who are reasonably acceptable to the Company). The Persons listed in Exhibit L under the heading “Board Designees - Company” shall be the Company’s designees pursuant to clause (a) of this Section 5.11 (which list may be changed by the Company at any time prior to the Closing by written notice to Parent to include different board designees who are reasonably acceptable to Parent). The Persons listed in Exhibit L under the heading “Board Designees - Independent” shall be the independent members mutually agreed upon between the Company and Parent pursuant to clause (a) of this Section 5.11 (which list may be changed by mutual written agreement of the Company and Parent at any time prior to the Closing).
5.12 Termination of Certain Agreements and Rights.
(a) The Company shall cause any Investor Agreements (excluding the Company Stockholder Support Agreements) to be terminated immediately prior to the Effective Time, without any liability being imposed on the part of Parent or the Surviving Corporation.
(b) Parent agrees to use commercially reasonable efforts to (a) terminate, assign or fully perform all Parent Contracts (except (i) Parent Contracts with obligations that do not exceed $25,000 for Parent over a 12-month period, (ii) Parent Contracts set forth on Schedule 5.12 of the Parent Disclosure Schedule and (iii) any other Parent Contract agreed to by Parent and Company) (the “Specified Parent Contracts”) and (b) fully satisfy, waive or otherwise discharge all obligations of Parent under all Specified Parent Contracts, in each case prior to the Closing.
5.13 Section 16 Matters. Prior to the Effective Time, Parent and the Company shall take all such steps as may be required (to the extent permitted under applicable Laws) to cause any acquisitions of Parent Common Stock, restricted stock awards to acquire Parent Common Stock and any options to purchase Parent Common Stock in connection with the Contemplated Transactions, by each individual who is reasonably expected to become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Parent, to be exempt under Rule 16b-3 promulgated under the Exchange Act. Promptly following the date of this Agreement and at least ten (10) calendar days prior to the Closing Date, the Company shall furnish the following information to Parent for each individual who, immediately after the Effective Time, will become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Parent: (a) the number of shares of Company Capital Stock owned by such individual and expected to be exchanged for shares of Parent Common Stock pursuant to the Merger, and (b) the number of other derivative securities (if any) with respect to Company Capital Stock owned by such individual and expected to be converted into shares of Parent Common Stock, restricted stock awards to acquire Parent Common Stock or derivative securities with respect to Parent Common Stock in connection with the Merger.
5.14 Cooperation. Each Party shall cooperate reasonably with the other Party and shall provide the other Party with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to enable the combined entity to continue to meet its obligations following the Effective Time.
5.15 Allocation Certificates.
(a) The Company will prepare and deliver to Parent no later than three (3) Business Days prior to the Closing Date a certificate signed by the Chief Financial Officer of the Company in a form reasonably acceptable to Parent setting forth (as of immediately prior to the Effective Time) (i) each holder of Company Capital Stock, Company Options and Company Warrants, (ii) such holder’s name, address and email address; (iii) the number and type of Company Capital Stock held and/or underlying the Company Options and Company Warrants as of the immediately prior to the Effective Time for each such holder; (iv) the number of shares of Parent Common Stock to be issued to such holder, or to underlie any Parent Option, Parent Warrant or Parent RSU to be issued to such holder, pursuant to this Agreement in respect of the Company Capital Stock, Company Options or Company Warrants held by such holder as of immediately prior to the Effective Time; and (v) the exercise price of any Parent Option, Parent Warrant or Parent RSU to be issued to such holder, pursuant to this Agreement in respect of the Company Options or Company Warrants held by such holder as of immediately prior to the Effective Time (the “Allocation Certificate”).
(b) Parent will prepare and deliver to the Company at least three (3) Business Days prior to the Closing Date a certificate signed by the Chief Financial Officer of Parent in a form reasonably acceptable to the Company, setting forth, as of immediately prior to the Effective Time (i) each record holder of Parent Common Stock, Parent Warrants, Parent Options or Parent RSUs, (ii) such record holder’s name and address and (iii) the number of shares of Parent Common Stock held and/or underlying the Parent Warrants, Parent Options or Parent RSUs as of the Effective Time for such holder (the “Parent Outstanding Shares Certificate”).
5.16 Company Financial Statements. As promptly as reasonably practicable following the date of this Agreement: (i) and no later than fifteen (15) calendar days after the date hereof, the Company will furnish to Parent audited consolidated financial statements for the fiscal year ended 2022 (the “Company Audited Financial Statements”); and (ii) the Company will furnish to Parent unaudited interim consolidated financial statements for each interim period completed prior to Closing that would be required to be included in the Registration Statement or any periodic report due prior to the Closing if the Company were subject to the periodic reporting requirements under the Securities Act or the Exchange Act (the “Company Interim Financial Statements”). Each of the Company Audited Financial Statements and the Company Interim Financial Statements will be suitable for inclusion in the Proxy Statement and the Registration Statement and prepared in accordance with GAAP as applied on a consistent basis during the periods involved (except in each case as described in the notes thereto) and on that basis will present fairly, in all material respects, the consolidated financial position and the results of operations, changes in stockholders’ equity, and cash flows of the Company and its consolidated Subsidiaries as of the dates of and for the periods referred to in the Company Financial Statements, the Company Audited Financial Statements and Company Interim Financial Statements, as the case may be.
5.17 Takeover Statutes. If any Takeover Statute is or may become applicable to the Contemplated Transactions, each of the Company, the Company Board, Parent and the Parent Board, as applicable, shall grant such approvals and take such actions as are necessary so that the Contemplated Transactions may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise act to eliminate or minimize the effects of such Takeover Statute on the Contemplated Transactions.
5.18 Stockholder Litigation. Until the earlier of the termination of this Agreement in accordance with its terms or the Effective Time, Parent shall (a) promptly advise the Company in writing of any stockholder litigation or investigation against it or its directors relating to this Agreement, the Merger or the Contemplated Transactions and keep the Company fully informed regarding such stockholder litigation and (b) give the Company the opportunity to participate in the defense or settlement of any stockholder litigation or investigation relating to this Agreement or any of the Contemplated Transactions, and not settle any such litigation or investigation without the Company’s written consent, which will not be unreasonably withheld, conditioned or delayed.
5.19 Parent Benefit Plans. No later than five (5) Business Days before the Closing Date, the Parent shall terminate, effective as of the day immediately preceding the date the Company becomes a member of the same controlled group of corporations (as defined in Section 414(b) of the Code) as Parent, any and all 401(k) plans maintained by the Parent or any of its Subsidiaries. The Parent shall promptly provide Company with evidence that the 401(k) plan(s) of the Parent and/or its Subsidiaries have been terminated pursuant to resolutions of the Parent’s Board of Directors or the board of directors of its Subsidiaries, as applicable. The form and substance of such resolutions shall be subject to review and approval of the Company, which shall not be unreasonably withheld, conditioned or delayed. The Parent shall also take such other actions in furtherance of terminating any such 401(k) plan(s) as Company may reasonably request. The intent of the Parties is to have the Company’s 401(k) plan and its other employee benefit plans become the employee benefit plans of the Parent and its Subsidiaries in order to reduce the disruption of the employees of the Company and avoid the duplication of plans. Following the Effective Time, the parties agree to cooperate to transition all Parent Benefit Plans (other than the Parent Incentive Plan, employment agreements, and severance arrangements) to Company Benefit Plans as soon after the Effective Time as practicable. The Parent shall promptly provide Company with evidence that such Parent Benefit Plans are being transitioned and terminated pursuant to resolutions of the Parent’s Board of Directors or the board of directors of its Subsidiaries, as applicable. The form and substance of such resolutions shall be subject to review and approval of the Company, which shall not be unreasonably withheld. The Parent shall also take such other actions in furtherance of transitioning and terminating such Parent Benefit Plans as the Company may reasonably request.
5.20 Company 280G Actions. To the extent that any “disqualified individual” (within the meaning of Section 280G(c) of the Code and the regulations thereunder) has the right to receive any payments or benefits that could be deemed to constitute “parachute payments” (within the meaning of Section 280G(b)(2) of the Code and the regulations thereunder), then, the Company shall (i) no later than five Business Days prior to the Effective Time, solicit and use reasonable efforts to obtain from each such “disqualified individual” a waiver of such disqualified individual’s rights to some or all of such payments or benefits (to the extent such waiver is obtained, these amounts are referred to as the “Waived 280G Benefits”) so that any remaining payments and/or benefits shall not be deemed to be “excess parachute payments” (within the meaning of Section 280G(a) of the Code and the regulations thereunder), and (ii) no later than three Business Days prior to the Effective Time, with respect to each individual who agrees to the waiver described in clause (i), submit to a vote or consent of the holders of the Company Capital Stock entitled to vote on such matters, in the manner required under Section 280G(b)(5) of the Code and the regulations promulgated thereunder, along with disclosure intended to satisfy such requirements (including Q&A 7 of Section 1.280G-1 of such regulations), the right of any such “disqualified individual” to receive the Waived 280G Benefits. Prior to soliciting such waivers and approval, the Company shall provide drafts of such waivers and disclosure and approval materials to Parent for its review and comment no later than two Business Days prior to soliciting such waivers and soliciting such approvals. Prior to the Effective Time, the Company shall deliver to Parent evidence reasonably acceptable to Parent that a vote or consent of the holders of the Company Capital Stock was solicited in accordance with the foregoing provisions of this Section 5.20 and that either (i) the requisite number of votes or consents of the holders of the Company Capital Stock was obtained with respect to the Waived 280G Benefits (the “280G Approval”) or (ii) the 280G Approval was not obtained.
SECTION 6. CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY
The obligations of each Party to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction or, to the extent permitted by applicable Law, the written waiver by each of the Parties, at or prior to the Closing, of each of the following conditions:
6.1 No Restraints. No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the Contemplated Transactions shall have been issued by any court of competent jurisdiction or other Governmental Body of competent jurisdiction and remain in effect and there shall not be any Law which has the effect of making the consummation of the Contemplated Transactions illegal.
6.2 Stockholder Approval. (a) Parent shall have obtained the Required Parent Stockholder Vote and (b) the Company shall have obtained the Required Company Stockholder Vote.
6.3 Listing. (a) The existing shares of Parent Common Stock shall have been continually listed on NYSE as of and from the date of this Agreement through the Closing Date and (b) the shares of Parent Common Stock to be issued in the Contemplated Transactions pursuant to this Agreement shall have been approved for listing (subject to official notice of issuance) on NYSE as of the Closing.
6.4 Effectiveness of Registration Statement. The Registration Statement shall have become effective in accordance with the provisions of the Securities Act, and shall not be subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the Registration Statement that has not been withdrawn.
6.5 Side Letter. Parent, the Company and Juvenescence shall have entered into a letter agreement in substantially the form attached hereto as Exhibit M (the “Side Letter”). Juvenescence shall have converted all of the Parent Preferred Stock into Parent Common Stock prior to the NYSE Reverse Split.
6.6 NYSE Reverse Split. The NYSE Reverse Split shall have occurred and the shares of Parent Common Stock outstanding immediately prior to the Effective Time shall be approximately 2,500,000.
SECTION 7. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB
The obligations of Parent and Merger Sub to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by Parent, at or prior to the Closing, of each of the following conditions:
7.1 Accuracy of Representations. The Company Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The representations and warranties of the Company contained in this Agreement (other than the Company Fundamental Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on and as of the Closing Date except (a) in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Company Material Adverse Effect (without giving effect to any references therein to any Company Material Adverse Effect or other materiality qualifications), or (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Company Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
7.2 Performance of Covenants. The Company shall have performed or complied with in all material respects all agreements and covenants required to be performed or complied with by it under this Agreement at or prior to the Effective Time.
7.3 Documents. Parent shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of the Company certifying (i) that the conditions set forth in Sections 7.1, 7.2, 7.5 and 7.6 have been duly satisfied and (ii) that the information set forth in the Allocation Certificate delivered by the Company in accordance with Section 5.15 is true and accurate in all respects as of the Closing Date; and
(b) the Allocation Certificate.
7.4 No Company Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Company Material Adverse Effect that is continuing.
7.5 Termination of Investor Agreements. The Investor Agreements shall have been terminated (or will be terminated as of the Closing).
7.6 Company Lock-Up Agreements. Parent shall have received the Company Lock-Up Agreements duly executed by each of the Company Lock-Up Signatories and each executive officer and director of the Company who is elected or appointed, as applicable, as an executive officer and director of Parent as of immediately following the Closing, each of which shall be in full force and effect as of immediately following the Effective Time.
7.7 Company Stockholder Written Consent. The Company Stockholder Written Consent executed by each Company Signatory shall be in full force and effect.
7.8 Appraisal Shares. The holders of no more than 5% of shares of Company Capital Stock shall have exercised statutory appraisal rights pursuant to ABCL Article 13 with respect to such shares of Company Capital Stock.
7.9 FIRPTA Certificate. Parent shall have received (i) an original signed statement from the Company that the Company is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury Regulations Section 1.1445-2(c)(3) and 1.897-2(h), and (ii) an original signed notice from the Company to be delivered to the IRS in accordance with the provisions of Treasury Regulations Section 1.897-2(h)(2), together with written authorization for Parent to deliver such notice to the IRS on behalf of the Company following the Closing, each dated as of the Closing Date, duly executed by an authorized officer of the Company, and in form and substance reasonably acceptable to Parent.
7.10 Company Notes. The holders of the Company Notes, other than any Company Note held by the Parent, shall have elected to convert the Company Notes into Company Common Stock prior to the Effective Time.
SECTION 8. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY
The obligations of the Company to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by the Company, at or prior to the Closing, of each of the following conditions:
8.1 Accuracy of Representations. The Parent Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The representations and warranties of Parent and Merger Sub contained in this Agreement (other than the Parent Fundamental Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on and as of the Closing Date except (a) in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Parent Material Adverse Effect (without giving effect to any references therein to any Parent Material Adverse Effect or other materiality qualifications), or (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Parent Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
8.2 Performance of Covenants. Parent and Merger Sub shall have performed or complied with in all material respects all of their agreements and covenants required to be performed or complied with by each of them under this Agreement at or prior to the Effective Time.
8.3 Documents. The Company shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of Parent certifying that the conditions set forth in Sections 8.1, 8.2, and 8.4 have been duly satisfied;
(b) the Parent Outstanding Shares Certificate; and
(c) a written resignation, in a form reasonably satisfactory to the Company, dated as of the Closing Date and effective as of the Closing, executed by each of the directors of Parent who are not to continue as directors of Parent after the Closing pursuant to Section 5.11 hereof.
8.4 No Parent Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Parent Material Adverse Effect that is continuing.
8.5 Termination of Contracts. The Company shall have received evidence, in form and substance reasonably satisfactory to it, that the Specified Parent Contracts have been (a) terminated, assigned, or fully performed by Parent and (b) all obligations of Parent thereunder have been fully satisfied, waived or otherwise discharged.
8.6 Parent Lock-Up Agreements. The Parent Lock-Up Agreements will continue to be in full force and effect immediately following the Effective Time.
8.7 Actual Closing Price and Post-Merger Warrants. (a) The Actual Closing Price of a share of the Parent Common Stock must not be less than the Minimum Share Price and (b) Juvenescence must hold at least 1,133,308 Post-Merger Warrants upon the Parent’s issuance of the Post-Merger Warrants.
8.8 Cash at Closing. Immediately prior to the Closing, Parent must have on hand at least $500,000 (USD) of immediately spendable nonrestricted cash, net of all payables and other Liabilities (for the avoidance of doubt, such payables and Liabilities shall include Transaction Expenses).
SECTION 9. TERMINATION
9.1 Termination. This Agreement may be terminated prior to the Effective Time (whether before or after approval of the Company Stockholder Matters by the Company’s stockholders and whether before or after approval of the Parent Stockholder Matters by Parent’s stockholders, unless otherwise specified below):
(a) by mutual written consent of Parent and the Company;
(b) by either Parent or the Company if the Contemplated Transactions shall not have been consummated by February 29, 2024 (subject to possible extension as provided in this Section 9.1(b), the “End Date”); provided, however, that the right to terminate this Agreement under this Section 9.1(b) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party’s (or, in the case of Parent, Merger Sub’s) action or failure to act has been a principal cause of the failure of the Contemplated Transactions to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement, provided, further, however, that, in the event that a request for additional information has been made by any Governmental Body, or in the event that the SEC has not declared the Registration Statement effective under the Securities Act by the date which is thirty (30) calendar days prior to the End Date, then Parent shall be entitled to extend the End Date for an additional sixty (60) calendar days by written notice to the Company;
(c) by either Parent or the Company if a court of competent jurisdiction or other Governmental Body shall have issued a final and nonappealable order, decree or ruling, or shall have taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the Contemplated Transactions;
(d) by Parent if the Company Stockholder Written Consent shall not have been obtained within three (3) Business Days of the Registration Statement becoming effective in accordance with the provisions of the Securities Act; provided, however, that once the Company Stockholder Written Consent has been obtained, Parent may not terminate this Agreement pursuant to this Section 9.1(d);
(e) by either Parent or the Company if (i) the Parent Stockholders’ Meeting (including any adjournments and postponements thereof) shall have been held and completed and Parent’s stockholders shall have taken a final vote on the Parent Stockholder Matters and (ii) the Parent Stockholder Matters shall not have been approved at the Parent Stockholders’ Meeting (or at any adjournment or postponement thereof) by the Required Parent Stockholder Vote; provided, however, that the right to terminate this Agreement under this Section 9.1(e) shall not be available to Parent where the failure to obtain the Required Parent Stockholder Vote shall have been caused by the action or failure to act of Parent and such action or failure to act constitutes a material breach by Parent of this Agreement;
(f) by the Company (at any time prior to the approval of the Parent Stockholder Matters by the Required Parent Stockholder Vote) if a Parent Triggering Event shall have occurred;
(g) by Parent (at any time prior to the Required Company Stockholder Vote being obtained) if a Company Triggering Event shall have occurred;
(h) by the Company, upon a breach of any representation, warranty, covenant, or agreement set forth in this Agreement by Parent or Merger Sub or if any representation or warranty of Parent or Merger Sub shall have become inaccurate, in either case, such that the conditions set forth in Section 8.1 or Section 8.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided, that the Company is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided, further, that if such inaccuracy in Parent’s or Merger Sub’s representations and warranties or breach by Parent or Merger Sub is curable by the End Date by Parent or Merger Sub, then this Agreement shall not terminate pursuant to this Section 9.1(h) as a result of such particular breach or inaccuracy until the earlier of (i) the End Date and (ii) the expiration of a thirty (30) calendar day period commencing upon delivery of written notice from the Company to Parent or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to this Section 9.1(h) (it being understood that this Agreement shall not terminate pursuant to this Section 9.1(h) as a result of such particular breach or inaccuracy if such breach by Parent or Merger Sub is cured prior to such termination becoming effective);
(i) by Parent, upon a breach of any representation, warranty, covenant, or agreement set forth in this Agreement by the Company or if any representation or warranty of the Company shall have become inaccurate, in either case, such that the conditions set forth in Section 7.1 or Section 7.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided, that Parent is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided, further, that if such inaccuracy in the Company’s representations and warranties or breach by the Company is curable by the End Date by the Company then this Agreement shall not terminate pursuant to this Section 9.1(i) as a result of such particular breach or inaccuracy until the earlier of (i) the End Date and (ii) the expiration of a thirty (30) calendar day period commencing upon delivery of written notice from Parent to the Company of such breach or inaccuracy and its intention to terminate pursuant to this Section 9.1(i) (it being understood that this Agreement shall not terminate pursuant to this Section 9.1(i) as a result of such particular breach or inaccuracy if such breach by the Company is cured prior to such termination becoming effective);
(j) by Parent, at any time prior to the approval of the Parent Stockholder Matters by the Required Parent Stockholder Vote, if (i) Parent has received a Superior Offer, (ii) Parent has complied with its obligations under Section 4.4 and Section 5.3(d), (iii) the Parent Board shall have determined in good faith, after consultation with its outside legal counsel, that the failure to terminate this Agreement would be reasonably likely to be inconsistent with its fiduciary duties under applicable Law, (iv) Parent concurrently terminates this Agreement and enters into a Permitted Alternative Agreement with respect to such Superior Offer and (v) Parent concurrently pays to the Company the amount contemplated by Section 9.3(b)(ii);
(k) by Parent, if the Company Audited Financial Statements have not been provided by the Company to Parent in accordance with Section 5.16(i); or
(l) by Company, if the condition set forth in Section 8.7(a) is not satisfied and is not, to the extent permitted by applicable Law, waived by the Company at or prior to the Closing; provided, however, that if the Company exercises its termination rights under this Section 9.1(l) then the Company shall provide the Parent with written notice five (5) days prior to such termination and the Parent shall have the option, but not the obligation, to, within five (5) business days of receipt by the Parent of that written notice, provide the Company with a written offer to adjust the Exchange Ratio such that additional Parent Common Stock shall be issued to the stockholders of the Company in the amount necessary to equal the Target Merger Consideration Minimum Value, and if the Parent provides that written offer, the Company may not exercise its termination rights under this Section 9.1(l) and the condition set forth in Section 8.7(a) shall be deemed to have been satisfied.
(m) by Company, if the condition set forth in Section 8.7(b) is not satisfied and is not, to the extent permitted by applicable Law, waived by the Company at or prior to the Closing; provided, however, that if the Company exercises its termination rights under this Section 9.1(m) then the Company shall provide the Parent with written notice five (5) days prior to such termination and the Parent shall have the option, but not the obligation, to, within five (5) business days of receipt by the Parent of that written notice, to provide a sufficient number of additional holders of Post-Merger Warrants who have agreed to, and are bound by, the Side Letter such that the aggregate number of Post-Merger Warrants subject to the Side letter is at least 1,133,308, and if the Parent provides the said additional Post-Merger Warrant holders to be bound by the Side Letter, then the Company may not exercise its termination rights under this Section 9.1(m) and the condition set forth in Section 8.7(b) shall be deemed to have been satisfied.
(n) by Company, if the condition set forth in Section 8.8 is not satisfied and is not, to the extent permitted by applicable Law, waived by the Company at or prior to the Closing; provided, however, that if the Company exercises its termination rights under this Section 9.1(n), then the Company shall provide the Parent with written notice prior to such termination and the Parent shall have the option, but not the obligation, to, within five (5) business days of receipt by the Parent of that written notice, obtain sufficient net funds to meet the condition set forth in Section 8.8, and if the Parent obtains such sufficient net funds to meet the condition set forth in Section 8.8, then the Company may not exercise its termination rights under this Section 9.1(n) and the condition set forth in Section 8.8 shall be deemed to have been satisfied.
The Party desiring to terminate this Agreement pursuant to this Section 9.1, shall give the other Party written notice of such termination, specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
9.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 9.1, this Agreement shall be of no further force or effect; provided, however, that (a) Section 5.8, this Section 9.2, Section 9.3, Section 10 and the definitions of the defined terms in such Sections (including the definitions of such defined terms set forth in Exhibit A) shall survive the termination of this Agreement and shall remain in full force and effect, and (b) the termination of this Agreement and the provisions of Section 9.3 shall not relieve any Party of any liability for intentional fraud or for any willful and material breach of any representation, warranty, covenant, obligation or other provision contained in this Agreement.
9.3 Expenses; Termination Fees.
(a) Except as set forth in this Section 9.3, the Transaction Expenses shall be paid by the Party incurring such expenses, whether or not the Merger is consummated.
(b) If:
(i) (A) this Agreement is terminated by the Company pursuant to Section 9.1(b), Section 9.1(e) or Section 9.1(h), (B) an Acquisition Proposal with respect to Parent shall have been publicly announced, disclosed or otherwise communicated to Parent or the Parent Board at any time after the date of this Agreement but prior to the termination of this Agreement (which shall not have been withdrawn) and (C) within twelve (12) months after the date of such termination, Parent enters into a definitive agreement with respect to a Subsequent Transaction or consummates a Subsequent Transaction in respect of the Acquisition Proposal referred to in clause (B); or
(ii) (A) this Agreement is terminated by the Company pursuant to Section 9.1(f) (or, at the time this Agreement is terminated, the Company had the right to terminate this Agreement pursuant to Section 9.1(f)) or (B) this Agreement is terminated by Parent pursuant to Section 9.1(j),
then the Parent shall pay to the Company a nonrefundable fee in an amount equal to $1,000,000 (the “Company Termination Fee”) upon the consummation of such Subsequent Transaction or termination of this Agreement, as applicable, plus any amount payable to the Company pursuant to Section 9.3(f).
(c) If:
(i) (A) this Agreement is terminated by Parent pursuant to Section 9.1(b), Section 9.1(d), or Section 9.1(i), (B) an Acquisition Proposal with respect to the Company shall have been publicly announced, disclosed or otherwise communicated to the Company or the Company Board at any time after the date of this Agreement but prior to the termination of this Agreement (which shall not have been withdrawn) and (C) within twelve (12) months after the date of such termination, the Company enters into a definitive agreement with respect to a Subsequent Transaction or consummates a Subsequent Transaction in respect of the Acquisition Proposal referred to in clause (B); or
(ii) this Agreement is terminated by Parent pursuant to Section 9.1(g) (or, at the time this Agreement is terminated, Parent had the right to terminate this Agreement pursuant to Section 9.1(g)),
then the Company shall pay to Parent a nonrefundable fee in an amount equal to $1,000,000 (the “Parent Termination Fee”) upon the consummation of such Subsequent Transaction or termination of this Agreement, as applicable, plus any amount payable to Parent pursuant to Section 9.3(f).
(d) (i) If this Agreement is terminated by the Company pursuant to Section 9.1(h) or (ii) in the event of the failure of the Company to consummate the transactions to be contemplated at the Closing solely as a result of a Parent Material Adverse Effect as set forth in Section 8.4 (provided, that at such time all of the other conditions precedent to Parent’s obligation to close set forth in Section 6 and Section 7 have been satisfied by the Company, are capable of being satisfied by the Company or have been waived by Parent), then Parent shall reimburse the Company for all reasonable out-of-pocket fees and expenses incurred by the Company in connection with this Agreement and the Contemplated Transactions (such expenses, collectively, the “Third Party Expenses”), up to a maximum of $1,000,000, by wire transfer of same-day funds within ten (10) Business Days following the date on which the Company submits to Parent true and correct copies of reasonable documentation supporting such Third Party Expenses; provided, however, that such Third Party Expenses shall not include (i) any amounts paid to Juvenescence or any Affiliate thereof, or (ii) any amounts for financial advisors to the Company except for reasonably documented out-of-pocket expenses otherwise reimbursable by the Company to such financial advisors pursuant to the terms of the Company’s engagement letter or similar arrangement with such financial advisors.
(e) (i) If this Agreement is terminated by Parent pursuant to Section 9.1(i) or (ii) in the event of the failure of Parent to consummate the transactions to be consummated to the Closing solely as a result of a Company Material Adverse Effect as set forth in Section 7.5 (provided, that at such time all of the other conditions precedent to the Company’s obligation to close set forth in Section 6 and Section 8 have been satisfied by Parent are capable of being satisfied by Parent or have been waived by the Company), the Company shall reimburse Parent for all Third Party Expenses incurred by Parent up to a maximum of $1,000,000, by wire transfer of same-day funds within ten (10) Business Days following the date on which Parent submits to the Company true and correct copies of reasonable documentation supporting such Third Party Expenses; provided, however, that such Third Party Expenses shall not include (i) any amounts paid to Juvenescence or any Affiliate thereof, or (ii) any amounts for financial advisors to Parent except for reasonably documented out-of-pocket expenses otherwise reimbursable by Parent to such financial advisors pursuant to the terms of Parent’s engagement letter or similar arrangement with such financial advisors.
(f) The Company agrees it shall be entitled only to a Company Termination Fee or the reimbursement of Third Party Expenses, but not both, and the Parent agrees that it shall be entitled only to a Parent Termination Fee or the reimbursement of Third Party Expenses, but not both. Any Company Termination Fee, Parent Termination Fee, or Third Party Expenses due under this Section 9.3 shall be paid by wire transfer of same day funds. If a Party fails to pay when due any amount payable by it under this Section 9.3, then (i) such Party shall reimburse the other Party for reasonable costs and expenses (including reasonable fees and disbursements of counsel) incurred in connection with the collection of such overdue amount and the enforcement by the other Party of its rights under this Section 9.3, and (ii) such Party shall pay to the other Party interest on such overdue amount (for the period commencing as of the date such overdue amount was originally required to be paid and ending on the date such overdue amount is actually paid to other Party in full) at a rate per annum equal to the “prime rate” (as published in The Wall Street Journal or any successor thereto) in effect on the date such overdue amount was originally required to be paid plus three percent (3%).
(g) The Parties agree that, subject to Section 9.2, (i) payment of the Company Termination Fee or Third Party Expenses, as the case may be, shall, in the circumstances in which it is owed in accordance with the terms of this Agreement, constitute the sole and exclusive remedy of the Company following the termination of this Agreement under the circumstances described in Section 9.3(b), it being understood that in no event shall Parent be required to pay the amounts payable pursuant to this Section 9.3 on more than one occasion and (ii) following payment of the Company Termination Fee or Third Party Expenses, as the case may be, (x) Parent shall have no further liability to the Company in connection with or arising out of this Agreement or the termination thereof, any breach of this Agreement by Parent giving rise to such termination, or the failure of the Contemplated Transactions to be consummated, (y) neither the Company nor any of its Affiliates shall be entitled to bring or maintain any other claim, action or proceeding against Parent or Merger Sub or seek to obtain any recovery, judgment or damages of any kind against such Parties (or any partner, member, stockholder, director, officer, employee, Subsidiary, Affiliate, agent or other Representative of such Parties) in connection with or arising out of this Agreement or the termination thereof, any breach by any such Parties giving rise to such termination or the failure of the Contemplated Transactions to be consummated and (z) the Company and its Affiliates shall be precluded from any other remedy against Parent, Merger Sub and their respective Affiliates, at law or in equity or otherwise, in connection with or arising out of this Agreement or the termination thereof, any breach by such Party giving rise to such termination or the failure of the Contemplated Transactions to be consummated; provided, however, that nothing in this Section 9.3(g) shall limit the rights of Parent and Merger Sub under Section 10.11.
(h) The Parties agree that, subject to Section 9.2, (i) payment of the Parent Termination Fee or Third Party Expenses, as the case may be, shall, in the circumstances in which it is owed in accordance with the terms of this Agreement, constitute the sole and exclusive remedy of Parent following the termination of this Agreement under the circumstances described in Section 9.3(c), it being understood that in no event shall the Company be required to pay the amounts payable pursuant to this Section 9.3 on more than one occasion and (ii) following payment of the Parent Termination Fee or Third Party Expenses, as the case may be, (x) the Company shall have no further liability to Parent in connection with or arising out of this Agreement or the termination thereof, any breach of this Agreement by the Company giving rise to such termination, or the failure of the Contemplated Transactions to be consummated, (y) neither Parent nor any of its Affiliates shall be entitled to bring or maintain any other claim, action or proceeding against the Company or seek to obtain any recovery, judgment or damages of any kind against the Company (or any partner, member, stockholder, director, officer, employee, Subsidiary, Affiliate, agent or other Representative of the Company) in connection with or arising out of this Agreement or the termination thereof, any breach by the Company giving rise to such termination or the failure of the Contemplated Transactions to be consummated and (z) Parent and its Affiliates shall be precluded from any other remedy against the Company and its Affiliates, at law or in equity or otherwise, in connection with or arising out of this Agreement or the termination thereof, any breach by such Party giving rise to such termination or the failure of the Contemplated Transactions to be consummated; provided, however, that nothing in this Section 9.3(h) shall limit the rights of the Company under Section 10.11.
(i) Each of the Parties acknowledges that (i) the agreements contained in this Section 9.3 are an integral part of the Contemplated Transactions, (ii) without these agreements, the Parties would not enter into this Agreement and (iii) any amount payable pursuant to this Section 9.3 is not a penalty, but rather is liquidated damages in a reasonable amount that will compensate the applicable Party in the circumstances in which such amount is payable.
SECTION 10. MISCELLANEOUS PROVISIONS
10.1 Non-Survival of Representations and Warranties. The representations and warranties of the Company, Parent and Merger Sub contained in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the Effective Time, and only the covenants that by their terms survive the Effective Time and this Section 10 shall survive the Effective Time.
10.2 Amendment. This Agreement may be amended with the approval of the respective boards of directors of the Company, Merger Sub and Parent at any time (whether before or after obtaining the Required Company Stockholder Vote or before or after obtaining the Required Parent Stockholder Vote); provided, however, that after any such approval of this Agreement by a Party’s stockholders, no amendment shall be made which by Law requires further approval of such stockholders without the further approval of such stockholders. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Company, Merger Sub and Parent.
10.3 Waiver.
(a) No failure on the part of any Party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any Party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
(b) No Party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such Party and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given.
10.4 Entire Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement, the Company Disclosure Schedule, the Parent Disclosure Schedule and the other agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the Parties with respect to the subject matter hereof and thereof; provided, however, that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect in accordance with its terms. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all Parties by electronic transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of this Agreement.
10.5 Applicable Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions, each of the Parties: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the United States District Court for the District of Delaware or, to the extent that neither of the foregoing courts has jurisdiction, the Superior Court of the State of Delaware; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 10.5; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 10.8 of this Agreement; and (f) irrevocably and unconditionally waives the right to trial by jury. Notwithstanding the foregoing, all matters relating to the fiduciary obligations of the Company Board and the internal affairs of the Company or the Company Board (including, without limitation, the interpretation of the Company’s Organizational Documents and the ABCL) shall be governed by and construed in accordance with the Laws of Alabama without regard to the conflicts of law principles thereof to the extent that such principles would direct a matter to another jurisdiction.
10.6 Attorneys’ Fees. In any action at law or suit in equity to enforce this Agreement or the rights of any of the Parties, the prevailing Party in such action or suit (as determined by a court of competent jurisdiction) shall be entitled to recover its reasonable out-of-pocket attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
10.7 Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective successors and permitted assigns; provided, however, that neither this Agreement nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no effect.
10.8 Notices. All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder (a) one (1) Business Day after being sent for the next Business Day delivery, fees prepaid, via a reputable international overnight courier service, (b) upon delivery in the case of delivery by hand, or (c) on the date delivered in the place of delivery if sent by email (with a written or electronic confirmation of delivery) prior to 5:00 p.m. New York time, otherwise on the next succeeding Business Day, in each case to the intended recipient as set forth below:
if to Parent or Merger Sub:
AgeX Therapeutics, Inc.
1101 Marina Village Parkway, Suite 201
Alameda, California 94501
Attention: Chief Financial Officer
with a copy to (which shall not constitute notice):
Gibson, Dunn & Crutcher LLP
555 Mission Street
San Francisco, California 94105-0921
| | Attention: | Robert Phillips |
| | | Chris Trester |
Email:
if to the Company:
Serina Therapeutics, Inc.
601 Genome Way, Suite 2001
Huntsville, Alabama 35806
Attention: Steve Ledger
Email:
with a copy to (which shall not constitute notice):
Bradley Arant Boult Cummings LLP
200 Clinton Avenue, Suite 900
Huntsville, Alabama 35801
Attention: Scott E. Ludwig
Email:
10.9 Cooperation. Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by the other Party to evidence or reflect the Contemplated Transactions and to carry out the intent and purposes of this Agreement.
10.10 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or provision of this Agreement is invalid or unenforceable, the Parties agree that the court making such determination shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the Parties agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term or provision.
10.11 Other Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that any Party does not perform the provisions of this Agreement (including failing to take such actions as are required of it hereunder to consummate this Agreement) in accordance with their specified terms or otherwise breaches such provisions. Accordingly, the Parties acknowledge and agree that the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof in any court of the United States or any state thereof having jurisdiction, in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity. Any Party seeking an injunction or injunctions to prevent breaches of this Agreement shall not be required to provide any bond, surety or other security in connection with any such order or injunction.
10.12 No Third Party Beneficiaries. Other than (a) the Parties and the D&O Indemnified Parties to the extent of their respective rights pursuant to Section 5.6 and (b) Juvenescence to the extent of its rights pursuant to Sections 5.11 and 6.5, nothing in this Agreement, express or implied, is intended to or shall confer upon any Person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
10.13 Construction.
(a) References to “cash,” “dollars” or “$” are to U.S. dollars.
(b) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(c) The Parties have participated jointly in the negotiating and drafting of this Agreement and agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting Party shall not be applied in the construction or interpretation of this Agreement, and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provision of this Agreement.
(d) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(e) Except as otherwise indicated, all references in this Agreement to “Sections,” “Exhibits” and “Schedules” are intended to refer to Sections of this Agreement and Exhibits and Schedules to this Agreement, respectively.
(f) Any reference to legislation or to any provision of any legislation shall include any modification, amendment, re- enactment thereof, any legislative provision substituted therefore and all rules, regulations, and statutory instruments issued or related to such legislations.
(g) The bold-faced headings and table of contents contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
(h) The Parties agree that each of the Company Disclosure Schedule and the Parent Disclosure Schedule shall be arranged in sections and subsections corresponding to the numbered and lettered sections and subsections contained in this Agreement. The disclosures in any section or subsection of the Company Disclosure Schedule or the Parent Disclosure Schedule shall qualify other sections and subsections in this Agreement to the extent it is readily apparent on its face from a reading of the disclosure that such disclosure is applicable to such other sections and subsections.
(i) Each of “delivered” or “made available” means, with respect to any documentation, that prior to 11:59 p.m. (New York time) on the date that is two (2) calendar days prior to the date of this Agreement (i) a copy of such material has been posted to and made available by a Party to the other Party and its Representatives in the electronic data room maintained by such disclosing Party or (ii) such material is disclosed in the Parent SEC Documents filed with the SEC prior to the date hereof and publicly made available on the SEC’s Electronic Data Gathering Analysis and Retrieval system.
(j) Whenever the last day for the exercise of any privilege or the discharge of any duty hereunder shall fall upon a Saturday, Sunday, or any date on which banks in New York, New York are authorized or obligated by Law to be closed, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day which is a regular Business Day.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
(Remainder of page intentionally left blank)
AGREEMENT AND PLAN OF MERGER
AND REORGANIZATION
(Signature Page for AgeX Therapeutics, Inc. and Merger Sub)
| | AgeX Therapeutics, Inc. |
| | | |
| | By: | /s/ Joanne Hackett, Ph.D. |
| | | |
| | Name: | Joanne Hackett, Ph.D. |
| | Title: | Interim Chief Executive Officer |
| | | |
| | CANARIA TRANSACTION CORPORATION |
| | | |
| | By: | /s/ Joanne Hackett, Ph.D. |
| | | |
| | Name: | Joanne Hackett, Ph.D. |
| | Title: | Director and Secretary |
[Signature Page to Agreement and Plan of Merger and Reorganization]
AGREEMENT AND PLAN OF MERGER
AND REORGANIZATION
(Signature Page for Serina Therapeutics, Inc.)
| | SERINA THERAPEUTICS, INC. |
| | | |
| | By: | /s/ Randall Moreadith |
| | | Randall Moreadith, its President and Chief Executive Officer |
[Signature Page to Agreement and Plan of Merger and Reorganization]
EXHIBIT A
CERTAIN DEFINITIONS
For purposes of this Agreement (including this Exhibit A):
“ABCL” means the Alabama Business Corporation Law.
“Acquisition Inquiry” means, with respect to a Party, an inquiry, indication of interest or request for information (other than an inquiry, an indication of interest or request for information made or submitted by the Company, on the one hand, or Parent, on the other hand, to the other Party) that could reasonably be expected to lead to an Acquisition Proposal; provided, however, that the term “Acquisition Inquiry” shall not include the Merger or the other Contemplated Transactions or any transactions related to the Asset Dispositions.
“Acquisition Proposal” means, with respect to a Party, any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of the Company or any of its Affiliates, on the one hand, or by or on behalf of Parent or any of its Affiliates, on the other hand, to the other Party) contemplating or otherwise relating to any Acquisition Transaction with such Party, other than the Asset Dispositions.
“Acquisition Transaction” means any transaction or series of related transactions (other than the Asset Dispositions) involving:
(i) any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which a Party is a constituent entity; (ii) in which a Person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of Persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of a Party or any of its Subsidiaries; or (iii) in which a Party or any of its Subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such Party or any of its Subsidiaries; or
(ii) any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of a Party and its Subsidiaries, taken as a whole.
“Actual Closing Price” means the volume weighted average closing trading price of a share of the Parent Common Stock as reported on the NYSE for the ten (10) Trading Days preceding the date that is the fifth (5th) business day preceding the Effective Time.
“Affiliate” of a Person means any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Agreement” shall have the meaning set forth in the Preamble.
“Allocation Certificate” shall have the meaning set forth in Section 5.15(a).
“Business Day” means any day other than a Saturday, Sunday or other day on which banks in New York, New York are authorized or obligated by Law to be closed.
“Code” means the Internal Revenue Code of 1986, as amended.
“Company Associate” means any current or former employee, independent contractor, officer or director of the Company or any of its Subsidiaries.
“Company Board” means the board of directors of the Company.
“Company Capital Stock” means the Company Common Stock and the Company Preferred Stock.
“Company Common Stock” means the Common Stock, $0.01 par value per share, of the Company.
“Company Contract” means any Contract: (a) to which the Company or any of its Subsidiaries is a party; (b) by which the Company or any of its Subsidiaries or any Company IP or any other asset of the Company or its Subsidiaries are or may become bound or under which the Company or any of its Subsidiaries have, or may become subject to, any obligation; or (c) under which the Company or any of its Subsidiaries have or may acquire any right or interest.
“Company ERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with the Company or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Company Fundamental Representations” means the representations and warranties of the Company set forth in Sections 2.1 (Due Organization; Subsidiaries.), 2.3 (Authority; Binding Nature of Agreement), 2.4 (Vote Required), 2.6(a) and (c) (Capitalization) and 2.20 (No Financial Advisors).
“Company IP” means all Intellectual Property Rights that are owned or co-owned or purported to be owned or co-owned by the Company or its Subsidiaries.
“Company Material Adverse Effect” means any Effect that, considered together with all other Effects, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of the Company or its Subsidiaries, taken as a whole; provided, however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Company Material Adverse Effect: (a) general business, political or economic conditions generally affecting the industry in which the Company and its Subsidiaries operate, (b) acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions, (c) changes in financial, banking or securities markets, (d) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP), (e) the announcement of this Agreement or the pendency of the Contemplated Transactions, (f) the taking of any action required to be taken by this Agreement, or (g) continued losses from operations or decreases in cash balances of the Company or any of its Subsidiaries or on a consolidated basis among the Company and its Subsidiaries; except, in each case, with respect to clauses (a) through (c), to the extent disproportionately affecting the Company and its Subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which the Company and its Subsidiaries operate.
“Company Notes” means convertible notes or similar instruments that convert into shares of Company Capital Stock issued by the Company.
“Company Options” means options or other rights to purchase shares of Company Capital Stock issued by the Company pursuant to the Company Incentive Plan.
“Company Triggering Event” shall be deemed to have occurred if: (a) the Company Board shall have made a Company Board Adverse Recommendation Change; (b) the Company Board or any committee thereof shall have publicly approved, endorsed or recommended any Acquisition Proposal; or (c) the Company shall have entered into any letter of intent or similar document or any Contract relating to any Acquisition Proposal.
“Company Unaudited Interim Balance Sheet” means the unaudited consolidated balance sheet of the Company and its consolidated Subsidiaries for the period ended March 31, 2023, provided to Parent prior to the date of this Agreement.
“Company Warrant” means the warrants to purchase capital stock of the Company listed in Section 2.6(a) of the Company Disclosure Schedule.
“Company’s Knowledge” or “Knowledge of the Company” means the actual knowledge of Randall Moreadith, Steve Ledger, and Tacey Viegas and such knowledge as such Persons would reasonably be expected to have obtained in the ordinary course of their performance of their employment or consulting duties to the Company or any of its Subsidiaries.
“Confidentiality Agreement” means the Confidentiality Agreement, dated as of October 20, 2022, between the Company and Parent.
“Consent” means any approval, consent, ratification, permission, waiver or authorization (including any Governmental Authorization).
“Contemplated Transactions” means the Merger and the other transactions and actions contemplated by this Agreement, including the NYSE Reverse Split, the Asset Dispositions, and the Post-Merger Warrants.
“Contract” means, with respect to any Person, any written agreement, contract, subcontract, lease (whether for real or personal property), mortgage, license, sublicense or other legally binding commitment or undertaking of any nature to which such Person is a party or by which such Person or any of its assets are bound or affected under applicable Law.
“COVID-19” means the novel coronavirus (SARS-CoV-2) and related variants thereof.
“DGCL” means the General Corporation Law of the State of Delaware.
“Effect” means any effect, change, event, circumstance or development.
“Encumbrance” means any lien, pledge, hypothecation, charge, mortgage, security interest, lease, license, option, easement, reservation, servitude, adverse title, claim, infringement, interference, right of first refusal, preemptive right, community property interest or restriction or encumbrance of any nature (including any restriction on the voting of any security, any restriction on the transfer of any security or other asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any asset and any restriction on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Enforceability Exceptions” means the (a) Laws of general application relating to bankruptcy, insolvency and the relief of debtors; and (b) rules of law governing specific performance, injunctive relief and other equitable remedies.
“Entity” means any corporation (including any non-profit corporation), partnership (including any general partnership, limited partnership or limited liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint stock company), firm, society or other enterprise, association, organization or entity, and each of its successors.
“Environmental Law” means any federal, state, local or foreign Law relating to pollution or protection of human health or the environment (including ambient air, surface water, groundwater, land surface or subsurface strata), including any Law or regulation relating to emissions, discharges, releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Exchange Ratio” means, subject to Section 1.5(g), the following ratio: the quotient obtained by dividing (a) the Company Merger Shares by (b) the Company Outstanding Shares, in which:
| | ● | “Company Allocation Percentage” means 75.00%. |
| | | |
| | ● | “Company Merger Shares” means the product (rounded to the nearest whole share) obtained by multiplying (a) Post-Closing Parent Shares by (b) the Company Allocation Percentage. |
| | ● | “Company Outstanding Shares” means the total number of shares of Company Capital Stock outstanding immediately prior to the Effective Time after giving effect to the Preferred Stock Conversion, expressed on a fully-diluted and as-converted to Company Common Stock basis assuming, without limitation or duplication, (i) the exercise of all Company Options outstanding as of immediately prior to the Effective Time, (ii) the issuance of shares of Company Capital Stock upon the conversion of all Company Notes (but excluding any Company Notes issued to Parent) and (iii) the issuance of shares of Company Capital Stock in respect of all other outstanding options, restricted stock awards, restricted stock units, or rights to receive such shares, whether conditional or unconditional and including any outstanding options, restricted stock awards, restricted stock units or rights triggered by or associated with the consummation of the Merger (but excluding any shares of Company Capital Stock reserved for issuance other than with respect to outstanding Company Options under the Company Incentive Plan as of immediately prior to the Effective Time). No Company Warrants shall be included in the total number of shares of Company Common Stock outstanding for purposes of determining the Company Outstanding Shares. |
| | | |
| | ● | “Parent Outstanding Shares” means, subject to Section 1.5(g) (that addresses, among other things, the NYSE Reverse Split) and the immediately following sentence, the total number of shares of Parent Common Stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as-converted to Parent Common Stock basis and using the treasury stock method (and shall include, for the avoidance of doubt, all Parent Note Shares, Parent Common Stock to be received upon the conversion of the Parent Preferred Stock, In the Money Parent Options and Parent Warrants), but assuming, without limitation or duplication, the issuance of shares of Parent Common Stock in respect of all Parent Options, Parent RSUs, Parent Warrants and other outstanding options, warrants or rights to receive such shares, in each case, outstanding as of immediately prior to the Effective Time (assuming cashless exercise using the Parent Closing Price), whether conditional or unconditional and including any outstanding options, warrants or rights triggered by or associated with the consummation of the Merger (but excluding any shares of Parent Common Stock reserved for issuance other than with respect to outstanding Parent Options, Parent RSUs and Parent Warrants as of immediately prior to the Effective Time and as set forth above). No (i) Post-Merger Warrants, or (ii) Incentive Warrants, in each case, shall be included in the total number of shares of Parent Common Stock outstanding for purposes of determining the Parent Outstanding Shares. |
| | | |
| | ● | “Post-Closing Parent Shares” means the quotient obtained by dividing (a) the Parent Outstanding Shares by (b)(i) 1.00 minus (ii) the Company Allocation Percentage. |
“GAAP” means generally accepted accounting principles and practices in effect from time to time within the United States applied consistently throughout the period involved.
“Governmental Authorization” means any: (a) permit, license, certificate, franchise, permission, variance, exception, order, clearance, registration, qualification or authorization issued, granted, given or otherwise made available by or under the authority of any Governmental Body or pursuant to any Law; or (b) right under any Contract with any Governmental Body.
“Governmental Body” means any: (a) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction of any nature; (b) federal, state, local, municipal, foreign or other government; (c) governmental or quasi-governmental authority of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any taxing authority); or (d) self-regulatory organization (including NYSE).
“Hazardous Materials” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products or by-products.
“Incentive Warrants” means the warrants of Parent, each whole warrant exercisable for one share of Parent Common Stock at a price equal to 1.5 multiplied by the Minimum Share Price, and expiring on the four year anniversary of the Closing Date, upon the terms and conditions set forth in the Warrant Agreement.
“Intellectual Property Rights” means and includes all past, present, and future rights of the following types, which may exist or be created under the laws of any jurisdiction in the world: (a) rights associated with works of authorship, including exclusive exploitation rights, copyrights, moral rights, software, databases, and mask works; (b) trademarks, service marks, trade dress, logos, trade names and other source identifiers, domain names and URLs and similar rights and any goodwill associated therewith; (c) rights associated with trade secrets, know how, inventions, invention disclosures, methods, processes, protocols, specifications, techniques and other forms of technology; (d) patents and industrial property rights; and (e) other similar proprietary rights in intellectual property of every kind and nature; (f) rights of privacy and publicity; and (g) all registrations, renewals, extensions, statutory invention registrations, provisionals, continuations, continuations-in-part, divisions, or reissues of, and applications for, any of the rights referred to in clauses “(a)” through “(f)” above (whether or not in tangible form and including all tangible embodiments of any of the foregoing, such as samples, studies and summaries), along with all rights to prosecute and perfect the same through administrative prosecution, registration, recordation or other administrative proceeding, and all causes of action and rights to sue or seek other remedies arising from or relating to the foregoing.
“In the Money Parent Options and Parent Warrants” shall mean any Parent Options and Parent Warrants with an exercise price less than the Parent Closing Price.
“IRS” means the United States Internal Revenue Service.
“Juvenescence Note Conversion” means that certain Exchange Agreement entered into by and between Parent and Juvenescence on July 21, 2023, and closed on July 24, 2023, whereby certain notes totaling $36,000,000 owed to Juvenescence by Parent were converted into 211,600 shares of Parent Series A Preferred Stock and 148,400 shares Parent Series B Preferred Stock.
“Law” means any federal, state, national, foreign, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body (including under the authority of NYSE or the Financial Industry Regulatory Authority).
“Legal Proceeding” means any action, suit, litigation, arbitration, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Body or any arbitrator or arbitration panel.
“Merger Sub Board” means the board of directors of Merger Sub.
“Minimum Share Price” means $12.00.
“NYSE” means the New York Stock Exchange, including the NYSE American or such other NYSE market on which shares of Parent Common Stock are then listed.
“NYSE Reverse Split” means a reverse stock split of all outstanding shares of Parent Common Stock that is effected by Parent for the purpose of resulting in approximately 2,500,000 shares of Parent Common Stock being outstanding immediately prior to the Effective Time, unless otherwise mutually agreed to by Parent and the Company.
“Ordinary Course of Business” means, in the case of each of the Company and Parent, such actions taken in the ordinary course of its and its Subsidiaries’ normal operations and consistent with its and its Subsidiaries’ past practices; provided, however, that the Ordinary Course of Business of (a) Parent shall also include actions required to effect the Asset Dispositions or effect the winding down of Parent’s prior research and development activities (including the termination of ongoing contractual obligations relating to Parent’s current products or product candidates), and (b) each Party shall also include any actions expressly required or permitted by this Agreement, including the Contemplated Transactions.
“Organizational Documents” means, with respect to any Person (other than an individual), (a) the certificate or articles of association or incorporation or organization or limited partnership or limited liability company, and any joint venture, limited liability company, operating or partnership agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such Person and (b) all bylaws, regulations and similar documents or agreements relating to the organization or governance of such Person, in each case, as amended or supplemented.
“Out of the Money Parent Options or Parent Warrants” shall mean Parent Options and Parent Warrants with an exercise price equal to or greater than the Parent Closing Price.
“Pandemic Response Laws” means the Coronavirus Aid, Relief, and Economic Security Act, the Families First Coronavirus Response Act, the COVID-related Tax Relief Act of 2020, the Presidential Memorandum on Deferring Payroll Tax Obligations in Light of the Ongoing COVID-19 Disaster (as issued on August 8, 2020, and including any administrative or other guidance published with respect thereto by any Tax authority (including IRS Notice 2020-65)), and any other similar or additional U.S. federal, state, or local or non-U.S. Law, or administrative guidance intended to benefit taxpayers in response to the COVID-19 pandemic and associated economic downturn.
“Parent Associate” means any current or former employee, independent contractor, officer or director of Parent or any of its Subsidiaries.
“Parent Balance Sheet” means the audited balance sheet of Parent as of December 31, 2022, included in Parent’s Report on Form 10-K for the annual period ended December 31, 2022, as filed with the SEC.
“Parent Board” means the board of directors of Parent.
“Parent Change in Circumstance” means a material development or change in circumstances (other than any such event, development or change to the extent related to (A) any Acquisition Proposal, Acquisition Inquiry or the consequences thereof or (B) the fact, in and of itself, that Parent meets or exceeds internal budgets, plans or forecasts of its revenues, earnings or other financial performance or results of operations) that affects the business, assets or operations of Parent that occurs or arises after the date of this Agreement.
“Parent Closing Price” means the volume weighted average closing trading price of a share of Parent Common Stock on NYSE for the five (5) consecutive trading days ending three (3) trading days immediately prior to the date of the public announcement of this Agreement.
“Parent Capital Stock” means the Parent Common Stock and the Parent Preferred Stock.
“Parent Common Stock” means the Common Stock, $0.0001 par value per share, of Parent.
“Parent Contract” means any Contract: (a) to which Parent or any of its Subsidiaries is a party; (b) by which Parent or any of its Subsidiaries or any Parent IP or any other asset of Parent or its Subsidiaries are or may become bound or under which Parent or any of its Subsidiaries have, or may become subject to, any obligation; or (c) under which Parent or any of its Subsidiaries have or may acquire any right or interest.
“Parent ERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with Parent or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Parent Excluded Assets” means the right, title and interest in and to the enumerated assets in Section C of the Parent Disclosure Schedule.
“Parent Fundamental Representations” means the representations and warranties of Parent and Merger Sub set forth in Sections 3.1 (Due Organization; Subsidiaries), 3.3 (Authority; Binding Nature of Agreement), 3.4 (Vote Required), 3.6(a) and (c) (Capitalization) and 3.21 (No Financial Advisors).
“Parent Incentive Plan” means Parent’s 2017 Equity Incentive Plan.
“Parent IP” means all Intellectual Property Rights that are owned or co-owned or purported to be owned or co-owned by Parent or its Subsidiaries.
“Parent Legacy Note” means any Parent Notes not converted in the Juvenescence Note Conversion.
“Parent Material Adverse Effect” means any Effect that, considered together with all other Effects, has or would reasonably be expected to have a material adverse effect on the business, condition (financial or otherwise), assets, liabilities or results of operations of Parent or its Subsidiaries, taken as a whole; provided, however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Parent Material Adverse Effect: (a) general business, political or economic conditions generally affecting the industry in which Parent and its Subsidiaries operate, (b) acts of war, the outbreak or escalation of armed hostilities, acts of terrorism, earthquakes, wildfires, hurricanes or other natural disasters, health emergencies, including pandemics (including COVID-19 and any evolutions or mutations thereof) and related or associated epidemics, disease outbreaks or quarantine restrictions, (c) changes in financial, banking or securities markets, (d) any change in the stock price or trading volume of Parent Common Stock (it being understood, however, that any Effect causing or contributing to any change in stock price or trading volume of Parent Common Stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such Effects are otherwise excepted from this definition), (e) the failure of Parent to meet internal or analysts’ expectations or projections or the results of operations of Parent, (f) any changes in or affecting clinical trial programs or studies conducted by or on behalf of Parent or its Subsidiaries, including any adverse data, event or outcome arising out of or related to any such programs or studies, (g) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP), (h) the announcement of this Agreement or the pendency of the Contemplated Transactions, (i) the Asset Dispositions, (j) any reduction in the amount of Parent’s cash and cash equivalents as a result of expenditures made by Parent related to wind down activities of Parent associated with the termination of its research and development activities (including the termination of ongoing contractual obligations relating to Parent current products or product candidates), (k) resulting from the taking of any action required to be taken by this Agreement or (l) as set forth in Section B of the Parent Disclosure Schedule, except in each case, with respect to clauses (a) through (c), to the extent disproportionately affecting Parent and its Subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which Parent and its Subsidiaries operate.
“Parent Notes” means (i) that certain Secured Convertible Promissory Note, dated March 13, 2023, by and between Parent and Juvenescence; (ii) that certain Secured Convertible Promissory Note, dated February 14, 2022, by and between Parent and Juvenescence; (iii) that certain Convertible Facility Agreement by and among Parent, Juvenescence, ReCyte Therapeutics, Inc., and Reverse Bioengineering, Inc, dated March 30, 2020; (iv) that certain Amended and Restated Secured Convertible Promissory Note, dated February 9, 2023, by and between Parent and Juvenescence, as amended on March 13, 2023, and May 9, 2023; (v) any other indebtedness entered into by and between Parent and Juvenescence after the date hereof.
“Parent Note Shares” means any shares of Parent Common Stock that are issued or issuable upon conversion of the Parent Notes (excluding the Parent Legacy Note).
“Parent Options” means options or other rights to purchase shares of Parent Common Stock issued by Parent.
“Parent RSUs” means any restricted stock unit award granted pursuant to the Parent Incentive Plan or otherwise.
“Parent Triggering Event” shall be deemed to have occurred if: (a) Parent shall have failed to include in the Proxy Statement the Parent Board Recommendation or shall have made a Parent Board Adverse Recommendation Change; (b) the Parent Board or any committee thereof shall have approved, endorsed or recommended any Acquisition Proposal; (c) Parent shall have entered into any letter of intent or similar document or any Contract relating to any Acquisition Proposal (other than a confidentiality agreement permitted pursuant to Section 4.4); or (d) Parent or any director or officer of Parent shall have willfully and intentionally breached the provisions set forth in Section 4.4 or Section 5.3 of this Agreement.
“Parent Unit” means the units of Parent, each unit consisting of one share of Parent Common Stock and one Incentive Warrant.
“Parent Warrants” means the warrants to purchase capital stock of Parent listed in Section 3.6(a) of the Parent Disclosure Schedule.
“Parent’s Knowledge” or “Knowledge of Parent” means the actual knowledge of the members of the Parent Board and Parent’s Chief Executive Officer, Chief Financial Officer and Chief Operating Officer and such knowledge as such Persons would reasonably be expected to have obtained in the ordinary course of their performance of their director or employment duties, as applicable, to Parent or any of its Subsidiaries.
“Party” or “Parties” means the Company, Merger Sub and Parent.
“Permitted Alternative Agreement” means a definitive agreement that contemplates or otherwise relates to an Acquisition Transaction that constitutes a Superior Offer.
“Permitted Encumbrance” means: (a) any liens for current Taxes not yet due and payable or for Taxes that are being contested in good faith and for which adequate reserves have been made on the Company Unaudited Interim Balance Sheet or the Parent Balance Sheet, as applicable; (b) minor liens that have arisen in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract from the value of the assets or properties subject thereto or materially impair the operations of the Company or any of its Subsidiaries or Parent or any of its Subsidiaries, as applicable; (c) statutory liens to secure obligations to landlords, lessors or renters under leases or rental agreements; (d) deposits or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance or similar programs mandated by Law; (e) non-exclusive licenses of Intellectual Property Rights or options to such non-exclusive licenses granted by the Company or any of its Subsidiaries or Parent or any of its Subsidiaries, as applicable, in the Ordinary Course of Business; and (f) statutory liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims for labor, materials or supplies.
“Person” means any individual, Entity or Governmental Body.
“Post-Merger Warrant” means the warrants of Parent, each whole warrant exercisable for one Parent Unit at a price equal to 1.1 multiplied by the Minimum Share Price, and expiring on July 31, 2025, upon the terms and conditions set forth in the Warrant Agreement.
“Potentially Transferable Assets” means the Reverse Bio Business.
“Proxy Statement” means the definitive proxy statement/prospectus to be sent to Parent’s stockholders in connection with the Parent Stockholders’ Meeting.
“Reference Date” means August 14, 2023.
“Registered IP” means all Intellectual Property Rights that are registered or issued under the authority of any Governmental Body, including all patents, registered copyrights, registered mask works, and registered trademarks, service marks and trade dress, registered domain names, and all applications for any of the foregoing.
“Registration Statement” means the registration statement on Form S-4 (or any other applicable form under the Securities Act to register Parent Common Stock) to be filed with the SEC by Parent registering the public offering and sale of Parent Common Stock to some or all holders of Company Capital Stock in the Merger, including all shares of Parent Common Stock to be issued in exchange for all shares of Company Capital Stock in the Merger, as said registration statement may be amended prior to the time it is declared effective by the SEC.
“Representatives” means directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors and representatives.
“Reverse Bio Business” means the right, title and interest in and to the enumerated assets in Section D of the Parent Disclosure Schedule to the extent relating exclusively to the business of Reverse Bioengineering, Inc., a wholly-owned subsidiary of Parent, and the liabilities and obligations arising out of, relating to or otherwise in respect thereof.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002.
“SEC” means the United States Securities and Exchange Commission.
“SEC Document” means any registration statement, proxy statement, form, document, report, notice or other filing filed by a Person or any Subsidiary of a Person with the SEC pursuant to the securities Laws.
“Securities Act” means the Securities Act of 1933, as amended.
“Subsequent Transaction” means any Acquisition Transaction (with all references to 20% in the definition of Acquisition Transaction being treated as references to 50% for these purposes).
“Subsidiary” means, an Entity of a Person that such Person directly or indirectly owns or purports to own, beneficially or of record, (a) a number of voting securities or other interests in such Entity that is sufficient to enable such Person to elect at least a majority of the members of such Entity’s board of directors or other governing body, or (b) at least 50% of the outstanding equity, voting, beneficial or financial interests in such Entity.
“Superior Offer” means an unsolicited bona fide written Acquisition Proposal (with all references to 20% in the definition of Acquisition Transaction being treated as references to greater than 50% for these purposes) that: (a) was not obtained or made as a direct or indirect result of a breach of (or in violation of) this Agreement; and (b) is on terms and conditions that the Parent Board determines in good faith, based on such matters that it deems relevant (including the likelihood of consummation thereof and the financing terms thereof), as well as any written offer by the other Party to this Agreement to amend the terms of this Agreement, and following consultation with its outside legal counsel and outside financial advisors, if any, are more favorable, from a financial point of view, to Parent’s stockholders than the terms of the Contemplated Transactions and is not subject to any financing condition (and if financing is required, such financing is then fully committed to the third party).
“Target Merger Consideration Minimum Value” means an amount equal to the Minimum Share Price multiplied by the anticipated aggregate number (approximately 7,500,000) of shares of Parent Common Stock to be issued (prior to any adjustment that may be made under Section 9.1(l)) to the Company stockholders.
“Takeover Statute” means any “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover Law.
“Tax” means any federal, state, local, foreign or other tax, including any income, capital gain, gross receipts, capital stock, profits, transfer, estimated, registration, stamp, premium, escheat, unclaimed property, customs duty, ad valorem, occupancy, occupation, alternative, add- on, windfall profits, value added, severance, property, business, production, sales, use, license, excise, franchise, employment, payroll, social security, disability, unemployment, workers’ compensation, national health insurance, withholding and any escheat or unclaimed property obligations, or other taxes, duties, fees, assessments or governmental charges, surtaxes or deficiencies thereof in the nature of a tax, whether or not disputed and however denominated, and including any fine, penalty, addition to tax or interest imposed by a Governmental Body with respect thereto.
“Tax Return” means any return (including any information return), report, statement, declaration, estimate, schedule, notice, notification, form, election, certificate or other document or information, and any amendment or supplement to any of the foregoing, filed with or submitted to, or required to be filed with or submitted to, any Governmental Body in connection with the determination, assessment, collection or payment of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Law relating to any Tax.
“Trading Day” means any day on which the NYSE is open for trading; provided, that a “Trading Day” includes only those days that have a scheduled closing time of 4:00 p.m. (Eastern Time).
“Transaction Expenses” means, with respect to each Party, all fees and expenses incurred by such Party at or prior to the Effective Time in connection with the Contemplated Transactions and this Agreement, including (a) any fees and expenses of legal counsel and accountants and the maximum amount of fees and expenses payable to financial advisors, investment bankers, brokers, consultants, and other advisors of such Party in connection with the negotiation, preparation and execution of this Agreement and the consummation of the Contemplated Transactions (including in connection with any stockholder litigation relating to this Agreement or any of the Contemplated Transactions), including finders’ fees; (b) only with respect to Parent, fees paid to the SEC in connection with filing the Registration Statement, the Proxy Statement, and any amendments and supplements thereto, with the SEC; (c) only with respect to Parent, any fees and expenses in connection with the printing, mailing and distribution of the Registration Statement and any amendments and supplements thereto; (d) 50% of the fees and expenses payable to NYSE in connection with the NYSE Listing Application; (e) any bonus, severance, change-in-control or retention payments or similar payment obligations (including payments with “single-trigger” provisions triggered at and as of the Closing) that become due or payable to any director, officer, employee or consultant of such Party or any of its Subsidiaries in connection with the consummation of the Contemplated Transactions; (f) only with respect to Parent, any fees and expenses in connection with the D&O tail policy described in Section 5.6(d); (g) only with respect to Parent, any notice payments, change-of-control payments, fines or other payments to be made by Parent in connection with terminating any existing Contract to which Parent is a party or winding down any of Parent’s clinical trial obligations; and (i) only with respect to Parent, any wind-down costs of Parent associated with discontinued lab, research and development and related operations.
“Treasury Regulations” means the United States Treasury regulations promulgated under the Code.
Annex B
FIRST AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
AGEX Therapeutics, INC.
The undersigned, AgeX Therapeutics, Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware, for the purpose of amending and restating its Certificate of Incorporation in its entirety, DOES HEREBY CERTIFY:
FIRST: That the current name of the corporation is AgeX Therapeutics, Inc., and the original Certificate of Incorporation of this corporation was filed with the Secretary of State of Delaware on January 6, 2017, as amended by that Certificate of Amendment filed on December 8, 2022, and as further amended by that Certificate of Amendment filed on ______________, 2023.
SECOND: That at a meeting of the board of directors (the “Board”) of AgeX Therapeutics, Inc., the Board approved, subject to approval by the stockholders, The First Amended and Restated Certificate of Incorporation of this corporation attached hereto as Exhibit A.
THIRD: That thereafter, pursuant to a resolution of the Board, a meeting of the stockholders of said corporation was dully called and held upon notice in accordance with Section 222 of the General Corporation Law of the State of Delaware at which meeting the necessary number of shares as required by statue were voted in favor of The First Amended and Restated Certificate of Incorporation, attached hereto as Exhibit A.
FOURTH: The First Amended and Restated Certificate of Incorporation of this corporation as set forth in Exhibit A has been duly adopted in accordance with the provisions of Sections 242 and 245 of the General Corporation Law of the State of Delaware by the directors and stockholders of the corporation.
FIFTH: The First Amended and Restated Certificate of Incorporation so adopted reads in full as set forth in Exhibit A and is incorporated herein by reference.
IN WITNESS WHEREOF, this corporation has caused this First Amended and Restated Certificate of Incorporation to be signed by its President and duly authorized on the ____ day of __________________, 2024.
| | AgeX Therapeutics, Inc. |
| | | |
| | By: | |
| | | As its President |
EXHIBIT A
FIRST AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
SERINA Therapeutics, Inc.
Article 1
Name
The name of the corporation is Serina Therapeutics, Inc.
Article 2
Address
The address of the registered office of the corporation in the State of Delaware is 1675 South State Street, Suite B, Dover, Delaware 19901 in Kent County. The name of its registered agent at such address is Capitol Services, Inc.
Article 3
Purpose
The purpose of the corporation is to engage in any lawful act or activity for which a corporation may be organized under the DGCL.
Article 4
Capital Stock
4.1 Authorized Shares. The corporation is authorized to issue two classes of stock, which shall be designated “Common Stock” and “Preferred Stock.” The number of shares of Common Stock which the corporation is authorized to issue is forty million (40,000,000), with a par value of $0.0001 per share. The number of shares of Preferred Stock which the corporation is authorized to issue is five million (5,000,000), with a par value of $0.0001 per share.
4.2 Preferred Stock. Shares of Preferred Stock may be issued in one or more series, from time to time, with each such series to consist of such number of shares and to have such voting powers, full or limited, or no voting powers, and such designations, preferences and relative, participating, optional or other special rights, and the qualifications, limitations or restrictions thereof, as shall be stated in the resolution or resolutions providing for the issuance of such series adopted by the board of directors (the “Board of Directors”) of the corporation, and the Board of Directors is hereby expressly vested with the authority, to the full extent now or hereafter provided by law, to adopt any such resolution or resolutions. The authority of the Board of Directors with respect to each series of Preferred Stock shall include, but not be limited to, determination of the following:
(i) The number of shares constituting that series and the distinctive designation of that series;
(ii) The dividend rate or rates on the shares of that series, the terms and conditions upon which and the periods in respect of which dividends shall be payable, whether dividends shall be cumulative, and, if so, from which date or dates, and the relative rights of priority, if any, of payment of dividends on shares of that series;
(iii) Whether that series shall have voting rights, in addition to the voting rights provided by law, and, if so, the terms of such voting rights;
(iv) Whether that series shall have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the Board of Directors shall determine;
(v) Whether the shares of that series shall be redeemable, and, if so, the terms and conditions of such redemption, including the date or dates upon or after which they shall be redeemable, and the amount per share payable in the event of redemption, which amount may vary under different conditions and at different redemption dates;
(vi) Whether that series shall have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund;
(vii) The rights of the shares of that series in the event of voluntary or involuntary liquidation, distribution of assets, dissolution or winding up of the corporation, and the relative rights of priority, if any, of payment of shares of that series; and
(viii) Any other relative rights, powers, and preferences, and the qualifications, limitations and restrictions thereof, of that series.
4.3 Voting Rights. Each holder of record of Common Stock, as such, shall have one vote for each share of Common Stock which is outstanding in his, her or its name on the books of the corporation on all matters on which stockholders are entitled to vote generally. The holders of shares of Common Stock shall not have cumulative voting rights. Except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to this Certificate of Incorporation (including any certificate of designation relating to any series of Preferred Stock) that relates solely to the terms, number of shares, powers, designations, preferences or relative, participating, optional or other special rights (including, without limitation, voting rights), or to qualifications, limitations or restrictions thereof, of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together with the holders of one or more other such series, to vote thereon pursuant to this Certificate of Incorporation (including any certificate of designation relating to any series of Preferred Stock) or pursuant to the DGCL. Except as otherwise required by law, holders of any series of Preferred Stock shall be entitled to only such voting rights, if any, as shall expressly be granted thereto by this Certificate of Incorporation (including any certificate of designation relating to such series of Preferred Stock).
4.4 No Class Vote on Changes in Authorized Number of Shares. The number of authorized shares of Preferred Stock or Common Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority in voting power of the stock of the corporation entitled to vote thereon irrespective of the provisions of Section 242(b)(2) of the DGCL (or any successor provision thereto), and no vote of the holders of any of the Common Stock or the Preferred Stock voting separately as a class shall be required therefor, unless a vote of any such holder is required pursuant to this Certificate of Incorporation (including any certificate of designation relating to any series of Preferred Stock).
Article 5
Board of Directors
5.1 Authority/Number of Directors. Except as otherwise provided in this Certificate of Incorporation or the DGCL, the business and affairs of the corporation shall be managed by or under the direction of the Board of Directors. Except as otherwise provided for or fixed pursuant to the provisions of Article 4 (including any certificate of designation with respect to any series of Preferred Stock) and this Article 5 relating to the rights of the holders of any series of Preferred Stock to elect additional directors, the precise number of directors shall be determined from time to time exclusively by resolution adopted by the Board of Directors.
5.2 Classified Board of Directors. Subject to the special right of the holders of one or more series of Preferred Stock to elect directors, the Board of Directors, shall be classified with respect to the time for which they severally hold office into three classes, as nearly equal in number as possible. The initial Class I Directors shall serve for a term expiring at the first annual meeting of stockholders of the corporation following the effective date of this Certificate of Incorporation; the initial Class II Directors shall serve for a term expiring at the second annual meeting of stockholders following the effective date of this Certificate of Incorporation; and the initial Class III Directors shall serve for a term expiring at the third annual meeting of stockholders following the effective date of this Certificate of Incorporation. Each director in each class shall hold office until his or her successor is duly elected and qualified. At each annual meeting of stockholders beginning with the first annual meeting of stockholders following the effective date of this Certificate of Incorporation, the successors of the class of directors whose term expires at that annual meeting shall be elected to hold office for a term expiring at the annual meeting of stockholders to be held in the third year following the year of their election, with each director in each such class to hold office until his or her successor is duly elected and qualified. If the number of such directors is changed, any increase or decrease shall be apportioned by the Board of Directors among the classes so as to maintain the number of directors in each class as nearly equal as possible, and any such additional director of any class elected to fill a newly created directorship resulting from an increase in such class shall hold office for a term that shall coincide with the remaining term of that class, but in no case shall a decrease in the number of directors remove or shorten the term of any incumbent director. The Board of Directors is authorized to assign members of the Board of Directors already in office to their respective class.
5.3 Vacancies. Subject to the rights granted to the holders of any one or more series of Preferred Stock then outstanding, any newly-created directorship on the Board of Directors that results from an increase in the number of directors and any vacancy occurring on the Board of Directors (whether by death, resignation, retirement, disqualification, removal or other cause) shall be filled only by a majority of the directors then in office, even if less than a quorum, or by a sole remaining director (and not by the stockholders). Any director elected to fill a vacancy or newly created directorship shall hold office until the next election of the class for which such director shall have been chosen and until his or her successor shall be elected and qualified, or until his or her earlier death, resignation, retirement, disqualification or removal.
5.4 Removal. Any or all of the directors (other than the directors elected by the holders of any series of Preferred Stock of the corporation, voting separately as a series or together with one or more other such series, as the case may be) may be removed only for cause and only by the affirmative vote of the holders of at least a majority in voting power of all the then outstanding shares of stock of the corporation entitled to vote thereon, voting together as a single class.
5.5 Election. Elections of directors need not be by written ballot unless the bylaws shall so provide.
5.6 Election by Holders of Preferred Stock. During any period when the holders of any series of Preferred Stock, voting separately as a series or together with one or more series, have the right to elect additional directors, then upon commencement and for the duration of the period during which such right continues: (i) the then otherwise total authorized number of directors of the corporation shall automatically be increased by such specified number of directors, and the holders of such Preferred Stock shall be entitled to elect the additional directors so provided for or fixed pursuant to said provisions, and (ii) each such additional director shall serve until such director’s successor shall have been duly elected and qualified, or until such director’s right to hold such office terminates pursuant to said provisions, whichever occurs earlier, subject to his or her earlier death, resignation, retirement, disqualification or removal. Notwithstanding any other provision of this Certificate of Incorporation, except as otherwise provided by the Board of Directors in the resolution or resolutions establishing such series, whenever the holders of any series of Preferred Stock having such right to elect additional directors are divested of such right pursuant to the provisions of such stock, the terms of office of all such additional directors elected by the holders of such stock, or elected to fill any vacancies resulting from the death, resignation, disqualification or removal of such additional directors, shall forthwith terminate (in which case each such director shall thereupon cease to be qualified as, and shall cease to be, a director) and the total authorized number of directors of the corporation shall automatically be reduced accordingly.
Article 6
Limitation of Director and Officer Liability
6.1 Limitation of Liability. To the fullest extent that the General Corporation Law of the State of Delaware or any other law of the State of Delaware as it exists on the date hereof or as it may hereafter be amended permits the limitation or elimination of the liability of directors and officers, no director of the corporation, and with respect to officers serving as such on or after the effective date of this Certificate of Incorporation, no officer of the corporation, shall be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director or officer.
6.2 Effect of Amendment. Neither the amendment nor repeal of this Article 6, or any portion thereof, nor the adoption of any provision of this Certificate of Incorporation, nor, to the fullest extent permitted by the DGCL, any modification of law shall eliminate, reduce or otherwise adversely affect any right or protection of a current or former director or officer of the corporation existing hereunder with respect to any act or omission occurring prior to such amendment, repeal. adoption, or modification.
Article 7
Meetings of Stockholders
7.1 No Action by Consent. Any action required or permitted to be taken by the stockholders of the corporation must be effected at a duly called annual or special meeting of stockholders and may not be effected by a consent or consents (written, electronic, or otherwise) of such stockholders; provided, however, that any action required or permitted to be taken by the holders of Preferred Stock, voting separately as a series or separately as a class with one or more other such series, may be taken by consent, without a meeting, without prior notice and without a vote, but only if that right is expressly provided by this Certificate of Incorporation (including any certificate of designation relating to that series of Preferred Stock).
7.2 Annual Meetings. An annual meeting of stockholders for the election of directors to succeed those whose terms expire and for the transaction of such other business as may properly come before the meeting, shall be held at such place, if any, on such date, and at such time as shall be fixed exclusively by resolution of the Board of Directors or a duly authorized committee thereof.
7.3 Special Meetings. Except as otherwise required by law and subject to the rights of the holders of any series of Preferred Stock, special meetings of the stockholders of the corporation for any purpose or purposes may be called at any time only by or at the direction of the Board of Directors or the chair of the Board of Directors. Stockholders shall not be permitted to propose business to be brought before a special meeting of stockholders and the only matters that may be brought before a special meeting are the matters specified in the notice of meeting given by or at the direction of the person calling the meeting. The Board of Directors may postpone, reschedule or cancel any special meeting of stockholders.
7.4 Advance Notice. Advance notice of stockholder nominations for the election of directors and of business to be brought by stockholders before any meeting of the stockholders of the corporation shall be given in the manner and to the extent provided in the bylaws of the corporation.
Article 8
Amendment of the Certificate of Incorporation and Bylaws
8.1 Certificate of Incorporation. Notwithstanding any other provisions of this Certificate of Incorporation (including any certificate of designation relating to any series of Preferred Stock), and notwithstanding that a lesser percentage may be permitted from time to time by applicable law, no provision of Articles 5, 6, 7, 8, and 9 may be altered, amended or repealed in any respect (including by merger, division, conversion, transfer, domestication, consolidation or otherwise), nor may any provision inconsistent therewith be adopted, unless such alteration, amendment, repeal or adoption is approved by the affirmative vote of the holders of at least two-thirds (66 2/3%) of the capital stock of the corporation entitled to vote generally in an election of directors, voting together as a single class.
8.2 Bylaws. In furtherance and not in limitation of the powers conferred by law, the board of directors is expressly authorized to make, alter, amend and repeal the bylaws of the corporation subject to the power of the stockholders of the corporation to alter, amend or repeal the bylaws; provided, however, that with respect to the powers of stockholders to make, alter, amend or repeal the bylaws, the affirmative vote of the holders of at least two-thirds (66 2/3%) of the capital stock of the corporation entitled to vote generally in an election of directors, voting together as a single class, shall be required to make, alter amend or repeal the bylaws of the corporation or to adopt any provision inconsistent therewith.
Article 9
Corporate Governance Matters
9.1 Validity and Construction. If any provision or provisions of this Certificate of Incorporation shall be held to be invalid, illegal or unenforceable as applied to any circumstance for any reason whatsoever: (i) the validity, legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Certificate of Incorporation (including, without limitation, each portion of any paragraph of this Certificate of Incorporation containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) shall not, to the fullest extent permitted by law, in any way be affected or impaired thereby and (ii) to the fullest extent permitted by law, the provisions of this Certificate of Incorporation (including, without limitation, each such portion of any paragraph of this Certificate of Incorporation containing any such provision held to be invalid, illegal or unenforceable) shall be construed so as to permit the corporation to protect its directors, officers, employees and agents from personal liability in respect of their good faith service or for the benefit of the corporation to the fullest extent permitted by law.
9.2 Forum Selection. Unless the corporation consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or if such court does not have subject matter jurisdiction another state or federal court (as appropriate) located within the State of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of the corporation, (ii) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer or other employee or stockholder of the corporation to the corporation or the corporation’s stockholders, creditors or other constituents, (iii) any action asserting a claim against the corporation or any current or former director or officer of the corporation arising pursuant to any provision of the DGCL or this Certificate of Incorporation or the bylaws (as either may be amended and/or restated from time to time) or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) any action asserting a claim governed by the internal affairs doctrine. Unless the corporation consents in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the federal securities laws of the United States of America.
In addition to the above, if any action the subject matter of which is within the scope of this Section 9.2 is filed in a court other than a court located within the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to: (a) the personal jurisdiction of the state and federal courts located within the State of Delaware in connection with any action brought in any such court to enforce this Section 9.2 (an “Enforcement Action”), and (b) having service of process made upon such stockholder in any such Enforcement Action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
To the fullest extent permitted by law, any person or entity purchasing or otherwise acquiring or holding any interest in shares of capital stock of the corporation shall be deemed to have notice of and consented to the provisions of this Section 9.2 and this Certificate of Incorporation. Failure to enforce the foregoing provisions would cause the corporation irreparable harm and the corporation shall be entitled to equitable relief, including injunction and specific performance, to enforce the forgoing provisions.
Annex C
SERINA THERAPEUTICS, INC.
2024 EQUITY INCENTIVE PLAN
1. Purpose; Eligibility.
1.1 General Purpose. The name of this plan is the Serina Therapeutics, Inc. 2024 Equity Incentive Plan. The purposes of the Plan are to (a) enable the Company to attract and retain the types of Employees, Consultants, and Directors who will contribute to the Company’s long-range success; (b) provide incentives that align the interests of Employees, Consultants, and Directors with those of the stockholders of the Company; and (c) promote the success of the Company’s business.
1.2 Eligible Award Recipients. The persons eligible to receive Awards are the Employees, Consultants, and Directors of the Company.
1.3 Available Awards. Awards that may be granted under the Plan include: (a) Incentive Stock Options, (b) Non-qualified Stock Options, (c) Stock Appreciation Rights, and (d) Stock Awards.
2. Definitions.
“Applicable Laws” means the requirements related to or implicated by the administration of the Plan under applicable state corporate law, United States federal and state securities laws, the Code, any stock exchange or quotation system on which the shares of Common Stock are listed or quoted, and the applicable laws of any foreign country or jurisdiction where Awards are granted under the Plan.
“Award” means any right granted under the Plan, including an Incentive Stock Option, a Non-qualified Stock Option, a Stock Appreciation Right, or a Stock Award.
“Award Agreement” means a written agreement, contract, certificate, or other instrument or document evidencing the terms and conditions of an individual Award granted under the Plan, which may, in the discretion of the Company, be transmitted electronically to any Participant. Each Award Agreement will be subject to the terms and conditions of the Plan.
“AgeX” means AgeX Therapeutics, Inc., a Delaware corporation, and any successor company or any parent company.
“Board” means the Board of Directors of Serina, as constituted at any time.
“Cause” means:
With respect to any Employee or Consultant: (a) if the Employee or Consultant is a party to an employment or service agreement with the Company and such agreement provides for a definition of Cause, the definition contained therein; or (b) if no such agreement exists, or if such agreement does not define Cause: (i) the commission of, or plea of guilty or no contest to, a felony or a crime involving moral turpitude or the commission of any other act involving willful malfeasance or material fiduciary breach with respect to the Company; (ii) conduct that results in or is reasonably likely to result in harm to the reputation or business of the Company; (iii) willful conversion or misappropriation of corporate funds; (iv) gross negligence or willful misconduct with respect to the Company; or (v) material violation of any state or federal securities law.
With respect to any Director, a determination by a majority of the disinterested Board members that the Director has engaged in any of the following: (a) malfeasance in office; (b) gross misconduct or neglect; (c) false or fraudulent misrepresentation inducing the Director’s appointment;(d) willful conversion or misappropriation of corporate funds; or (e) repeated failure to participate in Board meetings on a regular basis despite having received proper notice of the meetings in advance.
The Committee, in its absolute discretion, shall determine the effect of all matters and questions relating to whether a Participant has been discharged for Cause.
“Change in Control” (a) the direct or indirect sale, transfer, conveyance, or other disposition (other than by way of merger or consolidation), in one or a series of related transactions, of all or substantially all of the properties or assets of the Company, taken as a whole, to any Person that is not a subsidiary of the Company; (b) the date that is 10 business days prior to the consummation of a complete liquidation or dissolution of the Company; (c) the acquisition by any Person of Beneficial Ownership of 50% or more (on a fully diluted basis) of either (i) the then-outstanding shares of Common Stock of the Company, taking into account as outstanding for this purpose such Common Stock issuable upon the exercise of options or warrants, the conversion of convertible stock or debt, and the exercise of any similar right to acquire such Common Stock (the “Outstanding Company Common Stock”) or (ii) the combined voting power of the then-outstanding voting securities of the Company entitled to vote generally in the election of Directors (the “Outstanding Company Voting Securities”); provided, however, that for purposes of this Plan, the following acquisitions will not constitute a Change in Control: (A) any acquisition by the Company or any Subsidiary, (B) any acquisition by any employee benefit plan sponsored or maintained by the Company or any Subsidiary, (C) any acquisition that complies with clauses, (i), (ii), and (iii) of subsection (d) of this definition, or (D) in respect of an Award held by a particular Participant, any acquisition by the Participant or any group of persons including the Participant (or any entity controlled by the Participant or any group of persons including the Participant); or (d) the consummation of a reorganization, merger, consolidation, statutory share exchange, or similar form of corporate transaction involving the Company that requires the approval of the Company’s stockholders, whether for such transaction or the issuance of securities in the transaction (a “Business Combination”), unless immediately following such Business Combination: (i) more than 50% of the total voting power of (A) the entity resulting from such Business Combination (the “Surviving Company”), or (B) if applicable, the ultimate parent entity that directly or indirectly has beneficial ownership of sufficient voting securities eligible to elect a majority of the members of the board of directors (or the analogous governing body) of the Surviving Company (the “Parent Company”), is represented by the Outstanding Company Voting Securities that were outstanding immediately prior to such Business Combination (or, if applicable, is represented by shares into which the Outstanding Company Voting Securities were converted pursuant to such Business Combination), and such voting power among the holders thereof is in substantially the same proportion as the voting power of the Outstanding Company Voting Securities among the holders thereof immediately prior to the Business Combination; (ii) no Person (other than any employee benefit plan sponsored or maintained by the Surviving Company or the Parent Company) is or becomes the Beneficial Owner, directly or indirectly, of 50% or more of the total voting power of the outstanding voting securities eligible to elect members of the board of directors of the Parent Company (or the analogous governing body) (or, if there is no Parent Company, the Surviving Company); and (iii) at least a majority of the members of the board of directors (or the analogous governing body) of the Parent Company (or, if there is no Parent Company, the Surviving Company) following the consummation of the Business Combination were Board members at the time of the Board’s approval of the execution of the initial agreement providing for such Business Combination.
“Code” means the Internal Revenue Code of 1986, as it may be amended from time to time. Any reference to a section of the Code will be deemed to include a reference to any regulations promulgated thereunder.
“Committee” means a committee of the Board appointed by the Board to administer the Plan in accordance with Section 3.3 and Section 3.4.
“Common Stock” means the common stock, par value $0.0001 per share, of Serina, or such other securities of Serina as may be designated by the Board or Committee from time to time in substitution thereof.
“Company” means Serina and any or all of its Subsidiaries.
“Consultant” means any individual who is engaged by the Company to render consulting or advisory services.
“Continuous Service” means that the Participant’s service with the Company, whether as an Employee, Consultant, or Director, is not interrupted or terminated. The Participant’s Continuous Service will not be deemed to have terminated merely because of a change in the capacity in which the Participant renders service to the Company as an Employee, Consultant, or Director or a change in the entity for which the Participant renders such service (such as a change of employment from one Subsidiary to another Subsidiary), provided that there is no interruption or termination of the Participant’s Continuous Service; provided further that if any Award is subject to Section 409A of the Code, this sentence will only be given effect to the extent consistent with Section 409A of the Code. For example, a change in status from an Employee to a Director will not constitute an interruption of Continuous Service. The Board or Committee, in its sole discretion, may determine whether Continuous Service will be considered interrupted in the case of any leave of absence approved by the Board or Committee, such as sick leave, military leave, or any other personal or family leave of absence. The Board or Committee, in its sole discretion, may determine whether a Company transaction, such as a sale or spin-off of a division or Subsidiary that employs a Participant, will be deemed to result in a termination of Continuous Service for purposes of affected Awards, and such decision will be final, conclusive, and binding.
“Director” means a member of the Board.
“Disability” means that the Participant is unable to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment; provided, however, for purposes of determining the term of an Incentive Stock Option pursuant to Section 6.10 hereof, the term Disability will have the meaning ascribed to it under Section 22(e)(3) of the Code. The determination of whether an individual has a Disability will be determined by the Board or Committee or under procedures adopted by the Board or Committee. Except for a determination of Disability within the meaning of Section 22(e)(3) of the Code for purposes of an Incentive Stock Option, the Board or Committee may rely on any determination that a Participant is disabled for purposes of benefits under any long-term disability plan maintained by the Company in which a Participant participates.
“Effective Date” means the date as of which this Plan was adopted by the board of directors of AgeX.
“Employee” means any person employed by the Company; provided, that, for purposes of determining eligibility to receive Incentive Stock Options, an Employee means an employee of the Company or a parent corporation within the meaning of Code Section 424. Mere service as a Director or payment of a director’s fee by the Company will not be sufficient to constitute “employment” by the Company.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Fair Market Value” means, as of any date, the value of the Common Stock as determined below. If the Common Stock is listed on any national stock exchange, inter-dealer quotation system, or over-the-counter market that reports closing prices, including without limitation, the New York Stock Exchange, NYSE MKT, Nasdaq, or the OTC Bulletin Board, the Fair Market Value will be the closing price of a share of Common Stock (or if no sales were reported the closing price on the date immediately preceding such date) as quoted on such exchange or system on the day of determination, as reported in the Wall Street Journal or such other source as the Board or Committee deems reliable. In the absence of an established market for the Common Stock, the Fair Market Value will be determined in good faith by the Board or Committee, using such methods as the Board or Committee determines to be reasonable under the circumstances, and such determination will be conclusive and binding on all persons.
“Free Standing Rights” has the meaning set forth in Section 7.1(a).
“Good Reason” means: (a) if an Employee or Consultant is a party to an employment or service agreement with the Company and such agreement provides for a definition of Good Reason, the definition contained therein; or (b) if no such agreement exists or if such agreement does not define Good Reason, the occurrence of one or more of the following without the Participant’s express written consent, which circumstances are not remedied by the Company within thirty (30) days of its receipt of a written notice from the Participant describing the applicable circumstances (which notice must be provided by the Participant within ninety (90) days of the Participant’s knowledge of the applicable circumstances): (i) any material increase in the Participant’s duties (other than by way of promotion attendant with additional responsibilities, authority, or title and an increase in salary commensurate therewith), (ii) any material diminution of responsibilities, authority, title, status, or reporting structure; (iii) a material reduction in the Participant’s base salary or bonus opportunity; or (iv) a geographical relocation of the Participant’s principal office location by more than fifty (50) miles.
“Grant Date” means the date on which the Board or Committee adopts a resolution, or takes other appropriate action, expressly granting an Award to a Participant that specifies the key terms and conditions of the Award or, if a later date is set forth in such resolution, then such date as is set forth in such resolution.
“Incentive Stock Option” means an Option intended to qualify as an incentive stock option within the meaning of Section 422 of the Code.
“Non-Employee Director” means a Director who is a “non-employee director” within the meaning of Rule 16b-3.
“Non-qualified Stock Option” means an Option that by its terms does not qualify or is not intended to qualify as an Incentive Stock Option.
“Officer” means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act and the rules and regulations promulgated thereunder.
“Option” means an Incentive Stock Option or a Non-qualified Stock Option granted pursuant to the Plan.
“Optionholder” means a person to whom an Option is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Option.
“Option Exercise Price” means the price at which a share of Common Stock may be purchased upon the exercise of an Option.
“Participant” means an eligible person to whom an Award is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Award.
“Performance Goals” means one or more goals established by the Board or Committee that must be attained by Serina or a Subsidiary, or a division, business unit or operational unit of Serina or a Subsidiary in order for an Award to vest or for the determination of the amount of an Award. A Performance Goal may be based on financial results or performance or upon the attainment of any other goal or milestone designated by the Board or Committee such as, by way of example only and not by way of limitation, the attainment of a specified amount of sales, revenues, or net income, an increase in the Fair Market Value of the Common Stock, or the commencement or successful completion of a clinical trial of a new drug, biological product, or medical device.
“Permitted Transferee” means: (a) a member of the Optionholder’s immediate family (child, stepchild, grandchild, parent, stepparent, grandparent, spouse, former spouse, sibling, niece, nephew, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, including adoptive relationships), any person sharing the Optionholder’s household (other than a tenant or employee), a trust in which these persons have more than 50% of the beneficial interest, a foundation in which these persons (or the Optionholder) control the management of assets, and any other entity in which these persons (or the Optionholder) own more than 50% of the voting interests; and (b) in conjunction with the exercise of an Option, and for the purpose of obtaining financing for such exercise, the Optionholder may arrange for a securities broker/dealer to exercise an Option on the Optionholder’s behalf, to the extent necessary to obtain funds required to pay the exercise price of the Option, provided that the Fair Market Value of the Common Stock determined as of the date immediately before the date of such transfer exceeded the exercise price of the Option.
“Plan” means this Serina Therapeutics, Inc. 2024 Equity Incentive Plan, as amended and/or amended and restated from time to time.
“Related Rights” has the meaning set forth in Section 7.1(a).
“Restricted Period” has the meaning set forth in Section 7.2(a).
“Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act or any successor to Rule 16b-3, as in effect from time to time.
“Securities Act” means the Securities Act of 1933, as amended.
“Serina” means Serina Therapeutics, Inc., a Delaware corporation, and any successor company or any parent company.
“Stock Appreciation Right” means the right pursuant to an Award granted under Section 7.1 to receive, upon exercise, an amount payable in cash or shares equal to the number of shares subject to the Stock Appreciation Right that is being exercised multiplied by the excess of (a) the Fair Market Value of a share of Common Stock on the date the Award is exercised, over (b) the exercise price specified in the Stock Appreciation Right Award Agreement.
“Stock Award” means any Award granted pursuant to Section 7.2(a).
“Subsidiary” means (i) any corporation or other entity in which the Company possesses directly or indirectly equity interests representing at least 50% of the total ordinary voting power or at least 50% of the total value of all classes of equity interests of such corporation or other entity and (ii) any other entity in which the Company has a direct or indirect economic interest that is designated as a Subsidiary by the Board or Committee.
“Ten Percent Shareholder” means a person who owns (or is deemed to own pursuant to Section 424(d) of the Code) stock possessing more than 10% of the total combined voting power of all classes of stock of the Company or of any of its Subsidiaries.
“Voting Securities” means any class or series of stock or other securities entitling the holder vote for the election of Directors generally but will exclude any such security that entitles the holder to designate, appoint, or vote for the election of a minority of the Directors.
3. Administration.
3.1 Authority of Committee. The Plan will be administered by the Board or, in the Board’s sole discretion, by a Committee. Subject to the terms of the Plan, the Board or Committee will have the authority:
(a) to construe and interpret the Plan and apply its provisions;
(b) to promulgate, amend, and rescind rules and regulations relating to the administration of the Plan;
(c) to authorize any person to execute, on behalf of the Company, any instrument required to carry out the purposes of the Plan;
(d) to determine when Awards are to be granted under the Plan and the applicable Grant Date;
(e) from time to time to select those Participants to whom Awards will be granted;
(f) to determine the number of shares of Common Stock to be made subject to each Award;
(g) to determine whether each Option is to be an Incentive Stock Option or a Non-qualified Stock Option;
(h) to prescribe the terms and conditions of each Award, including, without limitation, the exercise price and medium of payment and vesting provisions, and to specify the provisions of the Award Agreement relating to such grant;
(i) to amend any outstanding Awards, including for the purpose of modifying the time or manner of vesting, or the term of any outstanding Award; provided, however, that if any such amendment impairs a Participant’s rights or increases a Participant’s obligations under his or her Award or creates or increases a Participant’s federal income tax liability with respect to an Award, such amendment will also be subject to the Participant’s consent;
(j) to determine the duration and purpose of leaves of absences which may be granted to a Participant without constituting termination of their employment for purposes of the Plan, which periods will be no shorter than the periods generally applicable to Employees under the Company’s employment policies;
(k) to make decisions with respect to outstanding Awards that may become necessary upon a change in corporate control or an event that triggers anti-dilution adjustments;
(l) to interpret, administer, reconcile any inconsistency in, correct any defect in and/or supply any omission in the Plan and any instrument or agreement relating to, or Award granted under, the Plan; and
(m) to exercise discretion to make any and all other determinations which it determines to be necessary or advisable for the administration of the Plan.
The Board or Committee also may modify the purchase price or the exercise price of any outstanding Award, consistent with requirements under the Code applicable to the Award, provided that if the modification effects a repricing, stockholder approval will be required before the repricing is effective. As used in this paragraph, repricing means (i) reduction in the exercise price of an outstanding Option or SAR, and (ii) cancellation of an “underwater” or “out-of-the money” Award in exchange for other Awards or cash. An “underwater” or “out-of-the money” Award is one for which the exercise price is greater than the Fair Market Value of the underlying Common Stock.
3.2 Decisions Final. All decisions made by the Board or Committee pursuant to the provisions of the Plan will be final and binding on the Company and the Participants.
3.3 Delegation. The Board may delegate administration of the Plan to a committee or committees of the Board, and the term “Committee” will apply to any such committee. The Board may abolish the Committee at any time and revest in the Board the administration of the Plan. The members of the Committee will be appointed by and serve at the pleasure of the Board. From time to time, the Board may increase or decrease the size of the Committee, add additional members to, remove members (with or without cause) from, appoint new members in substitution therefor, and fill vacancies, however caused, in the Committee. The Committee will act pursuant to a vote of the majority of its members or, in the case of a Committee comprised of only two members, the unanimous consent of its members, whether present or not, or by the written consent of the majority of its members and minutes will be kept of all of its meetings and copies thereof will be provided to the Board. Subject to the limitations prescribed by the Plan and the Board, the Committee may establish and follow such rules and regulations for the conduct of its business as it may determine to be advisable.
3.4 Committee Composition. Except as otherwise determined by the Board, the Committee will consist solely of two or more Non-Employee Directors. The Board will have discretion to determine whether or not it intends to comply with the exemption requirements of Rule 16b-3. However, if the Board intends to satisfy such exemption requirements, with respect to any insider subject to Section 16 of the Exchange Act, the Committee will be a compensation committee of the Board that at all times consists solely of two or more Non-Employee Directors. Nothing herein will create an inference that an Award is not validly granted under the Plan in the event Awards are granted under the Plan by a compensation committee of the Board that does not at all times consist solely of two or more Non-Employee Directors.
4. Shares Subject to the Plan.
4.1 Subject to adjustment in accordance with Section 11, a total of 1,750,000 shares of Common Stock will be available for the grant of Awards under the Plan. The shares of Common Stock issuable under the Plan will be shares of authorized but unissued or reacquired Common Stock, including shares repurchased by the Company on the open market or otherwise. During the terms of the Awards, the Company shall keep available at all times the number of shares of Common Stock required to satisfy such Awards.
4.2 Any shares of Common Stock subject to an Award that is cancelled, forfeited, or expires prior to exercise or realization, either in full or in part, will again become available for issuance under the Plan. Notwithstanding anything to the contrary contained herein, shares subject to an Award under the Plan will not again be made available for issuance or delivery under the Plan if such shares are (a) shares tendered in payment of an Option, (b) shares delivered or withheld by the Company to satisfy any tax withholding obligation, (c) shares covered by a stock-settled Stock Appreciation Right or other Awards that were not issued upon the settlement of the Award, or (d) shares repurchased by the Company using Option proceeds.
4.3 Subject to adjustment in accordance with Section 11, the aggregate maximum number of shares of Common Stock that may be issued on the exercise of Incentive Stock Options will be 1,750,000 shares of Common Stock.
5. Eligibility.
5.1 Eligibility for Specific Awards. Incentive Stock Options may be granted only to Employees. Awards other than Incentive Stock Options may be granted to Employees, Consultants and Directors. Awards may be granted to individuals whom the Committee determines are reasonably expected to become Employees, Consultants and Directors; provided that such grant and the Grant Date will become effective only the individual becoming an Employee, Consultant, or Director.
5.2 Ten Percent Shareholders. A Ten Percent Shareholder will not be granted an Incentive Stock Option unless the Option Exercise Price is at least 110% of the Fair Market Value of the Common Stock at the Grant Date and the Option is not exercisable after the expiration of five years from the Grant Date.
6. Option Provisions.
Each Option granted under the Plan will be evidenced by an Award Agreement. Each Option so granted will be subject to the conditions set forth in this Section 6, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award Agreement. All Options will be separately designated Incentive Stock Options or Non-qualified Stock Options at the time of grant, and, if certificates are issued, a separate certificate or certificates will be issued for shares of Common Stock purchased on exercise of each type of Option. Notwithstanding the foregoing, the Company will have no liability to any Participant or any other person if an Option designated as an Incentive Stock Option fails to qualify as such at any time or if an Option is determined to constitute “nonqualified deferred compensation” within the meaning of Section 409A of the Code and the terms of such Option do not satisfy the requirements of Section 409A of the Code. The provisions of separate Options need not be identical, but each Option will include (through incorporation of provisions hereof by reference in the Option or otherwise) the substance of each of the following provisions:
6.1 Term. An Option will expire, and thereafter no longer be exercisable, on such date as the Board or Committee may designate; provided, however, no Option will be exercisable after the expiration of 10 years from the Grant Date, and no Incentive Stock Option granted to a Ten Percent Shareholder will be exercisable after the expiration of 5 years from the Grant Date. The expiration date of each Option will be stated in the Award Agreement pertaining to the Option.
6.2 Exercise Price of An Incentive Stock Option. Subject to the provisions of Section 5.2 pertaining to Incentive Stock Options granted to Ten Percent Shareholders, the Option Exercise Price of each Incentive Stock Option will be not less than 100% of the Fair Market Value of the Common Stock subject to the Option on the Grant Date. Notwithstanding the foregoing, an Incentive Stock Option may be granted with an Option Exercise Price lower than that set forth in the preceding sentence if such Option is granted pursuant to an assumption or substitution for another option in a manner satisfying the provisions of Section 424(a) of the Code.
6.3 Exercise Price of a Non-qualified Stock Option. The Option Exercise Price of each Non-qualified Stock Option will be not less than 100% of the Fair Market Value of the Common Stock subject to the Option on the Grant Date. Notwithstanding the foregoing, a Non-qualified Stock Option may be granted with an Option Exercise Price lower than that set forth in the preceding sentence if such Option is granted pursuant to an assumption or substitution for another option in a manner satisfying the provisions of Section 409A of the Code.
6.4 Consideration. The Option Exercise Price of Common Stock acquired pursuant to an Option will be paid, to the extent permitted by applicable statutes and regulations, either (a) in cash or by certified or bank check at the time the Option is exercised or (b) to the extent approved by the Board or Committee, the Option Exercise Price may be paid: (i) by delivery to the Company of other Common Stock, duly endorsed for transfer to the Company, with a Fair Market Value on the date of delivery equal to the Option Exercise Price (or portion thereof) due for the number of shares being acquired; (ii) a “cashless” exercise program established with a broker pursuant to which the broker exercises or arranges for the coordination of the exercise of the Option with the sale of some or all of the underlying Common Stock; (iii) any combination of the foregoing methods; or (iv) in any other form of consideration that is legal consideration for the issuance of Common Stock and that may be acceptable to the Board or Committee. Unless otherwise specifically provided in the Option, the exercise price of Common Stock acquired pursuant to an Option that is paid by delivery to the Company of other Common Stock acquired, directly or indirectly from the Company, will be paid only by shares of the Common Stock of the Company that have been held for more than six months (or such longer or shorter period of time required to avoid a charge to earnings for financial accounting purposes). Notwithstanding the foregoing, during any period for which the Common Stock is publicly traded (i.e., the Common Stock is listed on any national securities exchange or an interdealer quotation system, or is traded in an over-the-counter market that reports closing prices) an exercise by a Director or Officer that involves or may involve a direct or indirect extension of credit or arrangement of an extension of credit by the Company, directly or indirectly, in violation of Section 402(a) of the Sarbanes-Oxley Act of 2002 is prohibited with respect to any Award under this Plan.
6.5 Transferability of An Incentive Stock Option. An Incentive Stock Option will not be transferable except by will or by the laws of descent and distribution and will be exercisable during the lifetime of the Optionholder only by the Optionholder.
6.6 Transferability of a Non-qualified Stock Option. A Non-qualified Stock Option may, in the sole discretion of the Board or Committee, be transferable to a Permitted Transferee, upon approval by the Board or Committee, to the extent provided in the Award Agreement or by subsequent consent granted by the Board or Committee. If the Non-qualified Stock Option does not provide for transferability or consent to transfer to a Permitted Transferee is not granted by the Board or Committee, then the Non-qualified Stock Option will not be transferable except by will or by the laws of descent and distribution and will be exercisable during the lifetime of the Optionholder only by the Optionholder.
6.7 Vesting of Options. Each Option may, but need not, vest and therefore become exercisable in periodic instalments as determined by the Board or Committee or based upon the attainment of a Performance Goal or the occurrence of a specified event. The vesting provisions of individual Options may vary. No Option may be exercised for a fraction of a share of Common Stock.
6.8 Termination of Continuous Service. Unless otherwise provided in an Award Agreement or in an employment agreement the terms of which have been approved by the Board or Committee, in the event an Optionholder’s Continuous Service terminates (other than upon the Optionholder’s death or Disability), the Optionholder may exercise his or her Option (to the extent that the Optionholder was entitled to exercise such Option as of the date of termination) but only within such period of time ending on the earlier of (a) the date three months following the termination of the Optionholder’s Continuous Service or (b) the expiration of the term of the Option as set forth in the Award Agreement The Board or Committee or its respective delegate, in its sole discretion, may determine whether a Company transaction, such as a sale or spin-off of a division or subsidiary that employs a Participant, shall be deemed to result in a termination of Continuous Service for purposes of affected Awards, and such decision shall be final, conclusive and binding. If, after termination, the Optionholder does not exercise his or her Option within the time specified in the Award Agreement, the Option will terminate.
6.9 Extension of Termination Date. An Optionholder’s Award Agreement may also provide that if the exercise of the Option following the termination of the Optionholder’s Continuous Service for any reason would be prohibited at any time because the issuance of shares of Common Stock would violate the registration requirements under the Securities Act or any other state or federal securities law or the rules of any securities exchange or interdealer quotation system, then the Option will terminate on the earlier of (a) the expiration of the term of the Option in accordance with Section 6.1 or (b) the expiration of a period after termination of the Participant’s Continuous Service that is three months after the end of the period during which the exercise of the Option would be in violation of such registration or other securities law requirements.
6.10 Disability of Optionholder. Unless otherwise provided in an Award Agreement, in the event that an Optionholder’s Continuous Service terminates as a result of the Optionholder’s Disability, the Optionholder may exercise his or her Option (to the extent that the Optionholder was entitled to exercise such Option as of the date of termination), but only within such period of time ending on the earlier of (a) the date 12 months following such termination or (b) the expiration of the term of the Option as set forth in the Award Agreement. If, after termination, the Optionholder does not exercise his or her Option within the time specified herein or in the Award Agreement, the Option will terminate.
6.11 Death of Optionholder. Unless otherwise provided in an Award Agreement, in the event an Optionholder’s Continuous Service terminates as a result of the Optionholder’s death, then the Option may be exercised (to the extent the Optionholder was entitled to exercise such Option as of the date of death) by the Optionholder’s estate, executor, or personal representative, by a person who acquired the right to exercise the Option by bequest, but only within the period ending on the earlier of (a) the date 12 months following the date of death or (b) the expiration of the term of such Option as set forth in the Award Agreement. If, after the Optionholder’s death, the Option is not exercised within the time specified herein or in the Award Agreement, the Option will terminate.
6.12 Incentive Stock Option $100,000 Limitation. To the extent that the aggregate Fair Market Value (determined at the time of grant) of Common Stock with respect to which Incentive Stock Options are exercisable for the first time by any Optionholder during any calendar year (under all plans of the Company) exceeds $100,000, the Options or portions thereof which exceed such limit (according to the order in which they were granted) will be treated as Non-qualified Stock Options.
7. Provisions of Awards Other Than Options.
7.1 Stock Appreciation Rights.
(a) General
Each Stock Appreciation Right granted under the Plan will be evidenced by an Award Agreement. Each Stock Appreciation Right so granted will be subject to the conditions set forth in this Section 7.1, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award Agreement. Stock Appreciation Rights may be granted alone (“Free Standing Rights”) or in tandem with an Option granted under the Plan (“Related Rights”).
(b) Grant Requirements
Any Related Right that relates to a Non-qualified Stock Option may be granted at the same time the Option is granted or at any time thereafter but before the exercise or expiration of the Option. Any Related Right that relates to an Incentive Stock Option must be granted at the same time the Incentive Stock Option is granted.
(c) Term of Stock Appreciation Rights
The term of a Stock Appreciation Right granted under the Plan will be determined by the Board or Committee; provided, however, no Stock Appreciation Right will be exercisable later than the tenth anniversary of the Grant Date.
(d) Vesting of Stock Appreciation Rights
Each Stock Appreciation Right may, but need not, vest and therefore become exercisable in periodic instalments as determined by the Board or Committee or based upon the attainment of a Performance Goal or the occurrence of a specified event. The vesting provisions of individual Stock Appreciation Rights may vary. No Stock Appreciation Right may be exercised for a fraction of a share of Common Stock.
(e) Exercise and Payment
Upon exercise of a Stock Appreciation Right, the holder will be entitled to receive from the Company an amount equal to the number of shares of Common Stock subject to the Stock Appreciation Right that is being exercised multiplied by the excess of (i) the Fair Market Value of a share of Common Stock on the date the Award is exercised, over (ii) the exercise price specified in the Stock Appreciation Right or related Option. Payment with respect to the exercise of a Stock Appreciation Right will be made on the date of exercise. Payment will be made in the form of shares of Common Stock (with or without restrictions as to substantial risk of forfeiture and transferability, as determined by the Board or Committee in its sole discretion), cash or a combination thereof, as determined by the Board or Committee.
(f) Exercise Price
The exercise price of a Free Standing Stock Appreciation Right will be determined by the Board or Committee, but will not be less than 100% of the Fair Market Value of one share of Common Stock on the Grant Date of the Stock Appreciation Right. A Related Right granted simultaneously with or subsequent to the grant of an Option and in conjunction therewith or in the alternative thereto will have the same exercise price as the related Option, will be transferable only upon the same terms and conditions as the related Option, and will be exercisable only to the same extent as the related Option; provided, however, that a Stock Appreciation Right, by its terms, will be exercisable only when the Fair Market Value per share of Common Stock subject to the Stock Appreciation Right and related Option exceeds the exercise price per share thereof. No Stock Appreciation Rights may be granted in tandem with an Option unless the Board or Committee determines that the requirements of Section 7.1(b) are satisfied.
(g) Reduction in the Underlying Option Shares
Upon any exercise of a Related Right, the number of shares of Common Stock for which any related Option will be exercisable will be reduced by the number of shares for which the Stock Appreciation Right has been exercised. The number of shares of Common Stock for which a Related Right will be exercisable will be reduced upon any exercise of any related Option by the number of shares of Common Stock for which such Option has been exercised.
7.2 Stock Awards.
(a) General
A Stock Award is an Award of actual shares of Common Stock (“Restricted Stock”) or hypothetical Common Stock units (“Restricted Stock Units”) having a value equal to the Fair Market Value of an identical number of shares of Common Stock. A Stock Award may, but need not, provide that such Stock Award may not be sold, assigned, transferred or otherwise disposed of, or pledged or hypothecated as collateral for a loan or as security for the performance of any obligation or for any other purpose for such period as the Board or Committee shall determine (the “Restricted Period”). Each Stock Award granted under the Plan will be evidenced by an Award Agreement. Each Stock Award so granted will be subject to the conditions set forth in this Section 7.2, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award Agreement.
(b) Restricted Stock and Restricted Stock Units
(i) Each Participant granted Restricted Stock shall execute and deliver to the Company an Award Agreement with respect to the Restricted Stock setting forth the applicable payment terms, if any, for the Restricted Stock, and restrictions and other terms and conditions applicable to such Restricted Stock.
(ii) Restricted Stock may be issued to a Participant without payment or without the delivery of a promissory note or instalment payment agreement only for services actually performed by the Participant prior to the issuance of the Restricted Stock.
(iii) In the case of Restricted Stock sold to a Participant on an instalment payment basis, the Company may require, as a condition of the grant, that the Participant execute and deliver to the Company a promissory note or instalment payment agreement and a stock pledge or security agreement, and a blank stock power with respect to the Restricted Stock, in such form and containing such terms as the Board or Committee may require. No Restricted Stock will be sold to an Officer or Director on instalment payment terms that would constitute an extension of credit in violation of Section 402(a) of the Sarbanes-Oxley Act of 2002.
(iv) If the Committee determines that the Restricted Stock shall be held by the Company or in escrow rather than delivered to the Participant pending the release of the applicable restrictions, the Committee may require the Participant to additionally execute and deliver to the Company (A) an escrow agreement satisfactory to the Committee, if applicable and (B) the appropriate blank stock power with respect to the Restricted Stock covered by such agreement.
(v) If a Participant fails to execute an agreement evidencing an Award of Restricted Stock and, if applicable, a promissory note or instalment payment agreement, stock pledge or security agreement, escrow agreement, and stock power, the Award will be null and void. Subject to the restrictions set forth in the Award, the Participant generally will have the rights and privileges of a stockholder as to such Restricted Stock, including the right to vote such Restricted Stock and the right to receive dividends; provided that, any cash dividends and stock dividends with respect to the Restricted Stock will be withheld by the Company for the Participant’s account, and interest may be credited on the amount of the cash dividends withheld at a rate and subject to such terms as determined by the Board or Committee. The cash dividends or stock dividends so withheld and attributable to any particular share of Restricted Stock (and earnings thereon, if applicable) will be distributed to the Participant in cash or, at the discretion of the Board or Committee, in shares of Common Stock having a Fair Market Value equal to the amount of such dividends, if applicable, upon the release of restrictions on such share and, if such share is forfeited, the Participant will have no right to such dividends.
(vi) The terms and conditions of a grant of Restricted Stock Units will be reflected in an Award Agreement. No shares of Common Stock will be issued at the time a Restricted Stock Unit is granted, and the Company will not be required to set aside a fund for the payment of any such Award. A Participant will have no voting rights with respect to any Restricted Stock Units granted hereunder. At the discretion of the Committee, each Restricted Stock Unit (representing one share of Common Stock) may be credited with cash and stock dividends paid by the Company in respect of one share of Common Stock (“Dividend Equivalents”). Dividend Equivalents will be withheld by the Company for the Participant’s account, and interest may be credited on the amount of cash Dividend Equivalents withheld at a rate and subject to such terms as determined by the Board or Committee. Dividend Equivalents credited to a Participant’s account and attributable to any particular Restricted Stock Unit (and earnings thereon, if applicable) will be distributed in cash or, at the discretion of the Board or Committee, in shares of Common Stock having a Fair Market Value equal to the amount of such Dividend Equivalents and earnings, if applicable, to the Participant upon settlement of such Restricted Stock Unit and, if such Restricted Stock Unit is forfeited, the Participant will have no right to such Dividend Equivalents.
(c) Restrictions
(i) Restricted Stock awarded to a Participant will be subject to the following restrictions until the expiration of the Restricted Period, and to such other terms and conditions as may be set forth in the applicable Award Agreement: (A) if an escrow arrangement is used, the Participant will not be entitled to delivery of the stock certificate; (B) the shares will be subject to the restrictions on transferability set forth in the Award Agreement; (C) the shares will be subject to forfeiture to the extent provided in the applicable Award Agreement; and (D) to the extent such shares are forfeited, the stock certificates will be returned to the Company, and all rights of the Participant to such shares and as a stockholder with respect to such shares will terminate without further obligation on the part of the Company.
(ii) Restricted Stock Units awarded to any Participant will be subject to (A) forfeiture until the expiration of the Restricted Period, and satisfaction of any applicable Performance Goals during such period, to the extent provided in the applicable Award Agreement, and to the extent such Restricted Stock Units are forfeited, all rights of the Participant to such Restricted Stock Units will terminate without further obligation on the part of the Company and (B) such other terms and conditions as may be set forth in the applicable Award Agreement.
(iii) The Board or Committee will have the authority to remove any or all of the restrictions on the Restricted Stock and Restricted Stock Units whenever it may determine that, by reason of changes in Applicable Laws or other changes in circumstances arising after the date the Restricted Stock or Restricted Stock Units are granted, such action is appropriate.
(d) Restricted Period
With respect to Stock Awards, the Restricted Period will commence on the Grant Date and end at the time or times set forth on a schedule established by the Board or Committee in the applicable Award Agreement. The Board or Committee may, but will not be required to, provide for an acceleration of the expiration of a Restricted Period upon the occurrence of a specified event.
(e) Delivery of Restricted Stock and Settlement of Restricted Stock Units
Upon the expiration of the Restricted Period with respect to any shares of Restricted Stock, the restrictions set forth in Section 7.2(c) and the applicable Award Agreement will be of no further force or effect with respect to such shares, except as set forth in the applicable Award Agreement. If an escrow arrangement is used, upon such expiration, the Company shall deliver to the Participant, or his or her beneficiary, without charge, the stock certificate evidencing the shares of Restricted Stock which have not then been forfeited and with respect to which the Restricted Period has expired (provided, that no fractional shares will be issued) and any cash dividends or stock dividends credited to the Participant’s account with respect to such Restricted Stock and the interest thereon, if any. Upon the expiration of the Restricted Period with respect to any outstanding Restricted Stock Units, the Company shall deliver to the Participant, or his or her beneficiary, without charge, one share of Common Stock for each such outstanding Restricted Stock Unit (“Vested Unit”); provided, however, that, if explicitly provided in the applicable Award Agreement, the Company may, in its sole discretion, elect to pay cash or part cash and part Common Stock in lieu of delivering only shares of Common Stock for Vested Units. If a cash payment is made in lieu of delivering shares of Common Stock, the amount of such payment will be equal to the Fair Market Value of the Common Stock as of the date on which the Restricted Period lapsed with respect to each Vested Unit.
(f) Stock Restrictions
Each certificate representing Restricted Stock awarded under the Plan will, in addition to any other legends as may be required by law or by the Board or Committee, bear a legend to the following effect:
THESE SHARES MAY BE TRANSFERRED ONLY IN ACCORDANCE WITH THE TERMS OF AN AGREEMENT BETWEEN THE COMPANY AND THE STOCKHOLDER, A COPY OF WHICH IS ON FILE WITH THE SECRETARY OF THE COMPANY.
8. Securities Law Compliance.
All Awards, including all Options, Stock Appreciation Rights, and Stock Awards granted under the Plan will be subject to the requirement that, if at any time the Board or the Committee shall determine, in its discretion, that the listing upon any securities exchange, or the registration under the Securities Act, or registration or qualification under any state law is required for the grant, exercise, issue, or sale of any Options, Stock Appreciation Rights, Common Stock, or Restricted Stock Units under the Plan, or the consent or approval of any government regulatory body, is necessary or desirable as a condition of, or in connection therewith, such Option, Stock Appreciation Rights, or Stock Award may not be exercised in whole or in part unless such listing, registration, qualification, consent or approval will have been effected or obtained free of any conditions not acceptable to the Board or the Committee. Furthermore, if the Board or the Committee determines that any amendment to any Award (including, but not limited to, an increase in the exercise price of any Option or Stock Award) is necessary or desirable in connection with the registration or qualification of any of its shares under any state securities or “blue sky” law, then the Board or the Committee will have the unilateral right to make such changes without the consent of the Participant to whom the Award was granted.
8.1 Each Award Agreement will provide that no shares of Common Stock will be purchased or sold thereunder unless and until (i) any then applicable requirements of state or federal laws and regulatory agencies have been fully complied with to the satisfaction of the Company and its counsel and (ii) if required to do so by the Company, the Participant has executed and delivered to the Company a letter of investment intent in such form and containing such provisions as the Committee may require.
8.2 Except as may otherwise be required by the Securities Act, the Company will not be required to register under the Securities Act the Plan, any Award or any Option, Stock Appreciation Right, Restricted Stock, Restricted Stock Unit, or any Common Stock issued or issuable pursuant to any such Award, and the Company will have no liability for any delay in issuing or failure to issue or sell any Option, Stock Appreciation Right, Common Stock, or Restricted Stock Unit prior to the date on which a registration statement under the Securities Act becomes effective with respect to the offer, sale, and issuance of such Award, Option, Stock Appreciation Right, Restricted Stock, Restricted Stock Unit, or Common Stock.
9. Use of Proceeds from Stock.
Proceeds from the sale of Common Stock pursuant to Awards, or upon exercise thereof, will constitute general funds of the Company.
10. Miscellaneous.
10.1 Acceleration of Exercisability and Vesting. The Board or Committee will have the power to accelerate the time at which an Award may first be exercised or the time during which an Award or any part thereof will vest in accordance with the Plan, notwithstanding the provisions in the Award stating the time at which it may first be exercised or the time during which it will vest.
10.2 Stockholder Rights. Except as provided in the Plan or an Award Agreement, no Participant will be deemed to be the holder of, or to have any of the rights of a holder with respect to, any shares of Common Stock subject to such Award unless and until such Participant has satisfied all requirements for exercise of the Award pursuant to its terms and no adjustment will be made for dividends (ordinary or extraordinary, whether in cash, securities or other property) or distributions of other rights for which the record date is prior to the date such Common Stock certificate is issued, except as provided in Section 11 hereof.
10.3 No Employment or Other Service Rights. Nothing in the Plan or any instrument executed or Award granted pursuant thereto will confer upon any Participant any right to continue to serve the Company in the capacity in effect at the time the Award was granted or will affect the right of the Company to terminate (a) the employment of an Employee with or without notice and with or without Cause, except as may otherwise be provided in a written employment agreement between the Company and the Participant, or (b) the service of a Director pursuant to the By-laws of Serina or a Subsidiary, and any applicable provisions of the corporate law of the state in which Serina or the Subsidiary is incorporated, as the case may be.
10.4 Withholding Obligations. To the extent provided by the terms of an Award Agreement or as may be approved by the Board or Committee, a Participant may satisfy any federal, state or local tax withholding obligation relating to the exercise or acquisition of Common Stock under an Award by any of the following means (in addition to the Company’s right to withhold from any compensation paid to the Participant by the Company) or by a combination of such means: (a) tendering a cash payment; (b) authorizing the Company to withhold shares of Common Stock from the shares of Common Stock otherwise issuable to the Participant as a result of the exercise or acquisition of Common Stock under the Award, provided, however, that no shares of Common Stock are withheld with a value exceeding the minimum amount of tax required to be withheld by law; or (c) delivering to the Company previously owned and unencumbered shares of Common Stock.
11. Adjustments Upon Changes in Stock.
In the event of changes in the outstanding Common Stock or in the capital structure of the Company by reason of any stock or extraordinary cash dividend, stock split, reverse stock split, an extraordinary corporate transaction such as any recapitalization, reorganization, merger, consolidation, combination, exchange, or other relevant change in capitalization occurring after the Grant Date of any Award, Awards granted under the Plan and any Award Agreements, including the exercise price of Options and Stock Appreciation Rights and the number of shares of Common Stock subject to such Options, Stock Appreciation Rights, or Stock Awards, the maximum number of shares of Common Stock subject to all Awards stated in Section 4, and the maximum number of shares of Common Stock with respect to which any one person may be granted Awards during any period stated in Section 4 will be equitably adjusted or substituted, as to the number, price or kind of a share of Common Stock or other consideration subject to such Awards to the extent necessary to preserve the economic intent of such Award. In the case of adjustments made pursuant to this Section 11, unless the Board or Committee specifically determines that such adjustment is in the best interests of the Company, Board or the Committee shall, in the case of Incentive Stock Options, ensure that any adjustments under this Section 11 will not constitute a modification, extension or renewal of the Incentive Stock Options within the meaning of Section 424(h)(3) of the Code and in the case of Non-qualified Stock Options, ensure that any adjustments under this Section 11 will not constitute a modification of such Non-qualified Stock Options within the meaning of Section 409A of the Code. Any adjustments made under this Section 11 will be made in a manner which does not adversely affect the exemption provided pursuant to Rule 16b-3 under the Exchange Act. The Company shall give each Participant notice of an adjustment hereunder and, upon notice, such adjustment will be conclusive and binding for all purposes.
12. Effect of Change in Control.
12.1 In the discretion of the Board and the Committee, any Award Agreement may provide, or the Board or the Committee may provide by amendment of any Award Agreement or otherwise, notwithstanding any provision of the Plan to the contrary, that in the event of a Change in Control, Options and/or Stock Appreciation Rights will become immediately exercisable with respect to all or a specified portion of the shares subject to such Options or Stock Appreciation Rights, and/or the Restricted Period will expire immediately with respect to all or a specified portion of the shares of Restricted Stock or Restricted Stock Units.
12.2 In addition, in the event of a Change in Control, the Committee may, in its discretion and upon at least 10 days’ advance notice to the affected persons, cancel any outstanding Awards and pay to the holders thereof, in cash or stock, or any combination thereof, the value of such Awards based upon the price per share of Common Stock received or to be received by other stockholders of the Company in the event. In the case of any Option or Stock Appreciation Right with an exercise price (or SAR Exercise Price in the case of a Stock Appreciation Right) that equals or exceeds the price paid for a share of Common Stock in connection with the Change in Control, the Committee may cancel the Option or Stock Appreciation Right without the payment of consideration therefor.
12.3 The obligations of the Company under the Plan will be binding upon any successor corporation or organization resulting from the merger, consolidation or other reorganization of the Company, or upon any successor corporation or organization succeeding to all or substantially all of the assets and business of the Company and its Subsidiaries, taken as a whole.
13. Amendment of the Plan and Awards.
13.1 Amendment of Plan. The Board at any time, and from time to time, may amend or terminate the Plan. However, except as provided in Section 11 relating to adjustments upon changes in Common Stock, and Section 13.3 and Section 14.14, no amendment will be effective unless approved by the stockholders of the Company to the extent stockholder approval is necessary to satisfy any Applicable Laws. At the time of such amendment, the Board shall determine, upon advice from counsel, whether such amendment will be contingent on stockholder approval.
13.2 Stockholder Approval. The Board may, in its sole discretion, submit any amendment to the Plan or any Award for stockholder approval. Further, the Board or Committee may make the payment of any Award contingent upon stockholder approval.
13.3 No Impairment of Rights. Rights under any Award granted before amendment of the Plan will not be impaired by any amendment of the Plan unless (a) the Company requests the consent of the Participant and the Participant consents in writing, or (b) the Award was granted subject to the terms of the amendment.
14. General Provisions.
14.1 Forfeiture Events. The Committee may specify in an Award Agreement that the Participant’s rights, payments and benefits with respect to an Award will be subject to reduction, cancellation, forfeiture or recoupment upon the occurrence of certain events, in addition to applicable vesting conditions of an Award. Such events may include, without limitation, breach of non-competition, non-solicitation, confidentiality, or other restrictive covenants that are contained in the Award Agreement or otherwise applicable to the Participant, a termination of the Participant’s Continuous Service for Cause, or other conduct by the Participant that is detrimental to the business or reputation of the Company and/or its Subsidiaries.
14.2 Clawback. Notwithstanding any other provisions in this Plan, any Award which is subject to recovery under any law, government regulation or stock exchange listing requirement, will be subject to such deductions and clawback as may be required to be made pursuant to such law, government regulation or stock exchange listing requirement (or any policy adopted by the Company pursuant to any such law, government regulation or stock exchange listing requirement).
14.3 Other Compensation Arrangements. Nothing contained in this Plan will prevent the Board or Committee from adopting other or additional compensation arrangements, subject to stockholder approval if such approval is required; and such arrangements may be either generally applicable or applicable only in specific cases.
14.4 Sub-plans. The Committee may from time to time establish sub-plans under the Plan for purposes of satisfying blue sky, securities, tax or other laws of various jurisdictions in which the Company intends to grant Awards. Any sub-plans will contain such limitations and other terms and conditions as the Committee determines are necessary or desirable. All sub-plans will be deemed a part of the Plan, but each sub-plan will apply only to the Participants in the jurisdiction for which the sub-plan was designed.
14.5 Deferral of Awards. The Committee may establish one or more programs under the Plan to permit selected Participants the opportunity to elect to defer receipt of consideration upon exercise of an Award, satisfaction of performance criteria, or other event that absent the election would entitle the Participant to payment or receipt of shares of Common Stock or other consideration under an Award. The Committee may establish the election procedures, the timing of such elections, the mechanisms for payments of, and accrual of interest or other earnings, if any, on amounts, shares or other consideration so deferred, and such other terms, conditions, rules and procedures that the Committee deems advisable for the administration of any such deferral program.
14.6 Unfunded Plan. The Plan will be unfunded. Neither the Company, the Board nor the Committee will be required to establish any special or separate fund or to segregate any assets to assure the performance of its obligations under the Plan.
14.7 Recapitalizations. Each Award Agreement will contain provisions required to reflect the provisions of Section 11.
14.8 Delivery. Subject to Section 8 and Section 7.2(c), upon exercise of an Option or Stock Appreciation Right or Restricted Stock Unit granted under this Plan, the Company shall issue Common Stock or pay any amounts due within a reasonable period of time thereafter. A period of 30 days will be considered a reasonable period of time.
14.9 No Fractional Shares. No fractional shares of Common Stock will be issued or delivered pursuant to the Plan. The Board or Committee shall determine whether cash, additional Awards or other securities or property will be issued or paid in lieu of fractional shares of Common Stock or whether any fractional shares should be rounded down, forfeited, or otherwise eliminated.
14.10 Other Provisions. The Award Agreements authorized under the Plan may contain such other provisions not inconsistent with this Plan, including, without limitation, restrictions upon the exercise of the Awards, as the Committee may deem advisable.
14.11 Section 409A. The Plan is intended to comply with Section 409A of the Code to the extent subject thereto, and, accordingly, to the maximum extent permitted, the Plan will be interpreted and administered to be in compliance therewith. Any payments described in the Plan that are due within the short-term deferral period described in Treasury Regulation Section 1.409A-1(b)(4) will not be treated as deferred compensation unless Applicable Laws require otherwise. Notwithstanding anything to the contrary in the Plan, to the extent required to avoid accelerated taxation and tax penalties under Section 409A of the Code, amounts that would otherwise be payable and benefits that would otherwise be provided pursuant to the Plan during the six (6) month period immediately following the Participant’s termination of Continuous Service will instead be paid on the first payroll date after the six-month anniversary of the Participant’s separation from service (or the Participant’s death, if earlier). Notwithstanding the foregoing, neither the Company nor the Committee will have any obligation to take any action to prevent the assessment of any excise tax or penalty on any Participant under Section 409A of the Code and neither the Company nor the Committee will have any liability to any Participant for such tax or penalty.
14.12 Disqualifying Dispositions. Any Participant who makes a “disposition” (as defined in Section 424 of the Code) of all or any portion of shares of Common Stock acquired upon exercise of an Incentive Stock Option within two years from the Grant Date of such Incentive Stock Option or within one year after the issuance of the shares of Common Stock acquired upon exercise of such Incentive Stock Option will be required to immediately advise the Company in writing as to the occurrence of the sale and the price realized upon the sale of such shares of Common Stock.
14.13 Section 16. It is the intent of the Company that the Plan satisfy, and be interpreted in a manner that satisfies, the applicable requirements of Rule 16b-3 as promulgated under Section 16 of the Exchange Act so that Participants will be entitled to the benefit of Rule 16b-3, or any other rule promulgated under Section 16 of the Exchange Act, and will not be subject to short-swing liability under Section 16 of the Exchange Act. Accordingly, if the operation of any provision of the Plan would conflict with the intent expressed in this Section 14.13, such provision to the extent possible will be interpreted and/or deemed amended so as to avoid such conflict.
14.14 Expenses. The costs of administering the Plan will be paid by the Company.
14.15 Severability. If any of the provisions of the Plan or any Award Agreement is held to be invalid, illegal or unenforceable, whether in whole or in part, such provision will be deemed modified to the extent, but only to the extent, of such invalidity, illegality or unenforceability and the remaining provisions will not be affected thereby.
14.16 Plan Headings. The headings in the Plan are for purposes of convenience only and are not intended to define or limit the construction of the provisions hereof.
14.17 Non-Uniform Treatment. The determinations of the Board or Committee under the Plan need not be uniform and may be made selectively among persons who are eligible to receive, or actually receive, Awards. Without limiting the generality of the foregoing, the Board and Committee will be entitled to make non-uniform and selective determinations, amendments and adjustments, and to enter into non-uniform and selective Award Agreements.
15. Effective Date of Plan.
The Plan will become effective as of the Effective Date, but no Award will be exercised (or, in the case of a stock Award, will be granted) unless and until the Plan has been approved by the stockholders of the Company, which approval will be within twelve (12) months before or after the date the Plan is adopted by the Board.
16. Termination or Suspension of the Plan.
The Plan will terminate automatically on [__________, 20__], which is ten years from the date the Plan was approved by AgeX’s board of directors. No Award will be granted pursuant to the Plan after such date, but Awards theretofore granted may extend beyond that date.
17. Effect of Dissolution, Merger or Other Reorganization.
Upon the dissolution or liquidation of Serina, or upon a reorganization, merger or consolidation of Serina as a result of which the outstanding Common Stock or other securities of the class then subject to Awards are changed into or exchanged for cash or property or securities not of Serina’s issue, or upon a sale of substantially all the property of Serina to, or the acquisition of more than eighty percent (80%) of the Voting Securities of Serina then outstanding by, another corporation or person, this Plan will terminate, and all unexercised Awards theretofore granted hereunder will terminate, unless provision can be made in writing in connection with such transaction for the continuance of the Plan and/or for the assumption of Awards theretofore granted, or the substitution for Awards options or other rights covering the shares of a successor corporation, or a parent or a subsidiary thereof, with appropriate adjustments as to the number and kind of shares and prices, in which event the Plan and Awards theretofore granted will continue in the manner and under the terms so provided, subject to such adjustments. The grant of an Award pursuant to the Plan will not affect in any way the right or power of Serina or any Subsidiary or parent corporation to make adjustments, reclassifications, reorganizations or changes or its capital or business structure or to merge or to consolidate or to dissolve, liquidate or sell, or transfer all or any part of its business or assets.
18. Choice of Law.
The law of the State of Delaware will govern all questions concerning the construction, validity and interpretation of this Plan, without regard to such state’s conflict of law rules.
Adopted by the board of directors on [__________, ____].
Approved by stockholders on [__________, ____].
Annex D









ANNEX e*
CERTIFICATE OF AMENDMENT
TO THE
CERTIFICATE OF INCORPORATION, AS AMENDED,
OF
AGEX THERAPEUTICS, INC.
*To be included by amendment.
Annex F
AMENDED AND RESTATED
BYLAWS
OF
SERINA THERAPEUTICS, INC.
(A DELAWARE CORPORATION)
ARTICLE 1
OFFICES
Section 1.1 Registered Office. The registered office of the corporation in the State of Delaware shall be in the City of Dover, County of Kent.
Section 1.2 Other Offices. The corporation shall also have and maintain an office or principal place of business at such place as may be fixed by the Board of Directors, and may also have offices at such other places, both within and without the State of Delaware, as the Board of Directors may from time to time determine or the business of the corporation may require.
ARTICLE 2
CORPORATE SEAL
The Board of Directors may adopt a corporate seal. The corporate seal shall consist of a die bearing the name of the corporation and the inscription, “Corporate Seal-Delaware.” Said seal may be used by causing it or a facsimile thereof to be impressed or affixed or reproduced or otherwise.
ARTICLE 3
STOCKHOLDERS’ MEETINGS
Section 3.1 Place of Meetings. Meetings of the stockholders of the corporation may be held at such place, either within or without the State of Delaware, as may be determined from time to time by the Board of Directors, or, if not so designated, then at the principal offices of the corporation required to be maintained pursuant to Section 1.2 of these bylaws (“Bylaws”). The Board of Directors may, in its sole discretion, determine that the meeting shall not be held at any place, but may instead be held solely by means of remote communication as provided under the General Corporation Law of the State of Delaware (the “DGCL”).
Section 3.2 Annual Meetings.
(a) The annual meeting of the stockholders of the corporation, for the purpose of the election of directors and for such other business as may properly come before it, shall be held on such date and at such time as may be designated from time to time by the Board of Directors or a duly authorized committee thereof. At an annual meeting, only such business shall be conducted as is a proper matter for stockholder action under the DGCL and as shall have been properly brought before the meeting. Matters shall be properly brought before the annual meeting only as follows: (i) brought before the meeting and specified pursuant to the corporation’s notice of meeting (or any supplement thereto) of stockholders; (ii) otherwise brought specifically by or at the direction of the Board of Directors; and (iii) by any stockholder of the corporation who was a stockholder of record as of the time of the giving of the stockholder’s notice provided for in Section 3.2(b) below and as of the record date for the meeting who is entitled to vote at the meeting and who complied with the notice procedures set forth in Section 3.2(b) below; provided, that if such matter is proposed on behalf of a beneficial owner, it may only be properly brought before the meeting if such beneficial owner was the beneficial owner of shares of the corporation at the time of the giving of the stockholder’s notice provided for in Section 3.2(b) below. Clause (iii) above shall be the exclusive means for a stockholder to make nominations and submit other business (other than matters properly included in the corporation’s notice of meeting of stockholders and proxy statement under Rule 14a-8 under the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder (the “1934 Act”)) before an annual meeting of stockholders. The Board of Directors may postpone, reschedule or cancel any annual meeting of stockholders previously called by the Board of Directors.
(b) At an annual meeting of the stockholders, the following procedures shall apply in order for a matter to be properly brought before the meeting by a stockholder.
(i) For nominations for the election to the Board of Directors to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 3.2(a) of these Bylaws, the stockholder must deliver written notice to the Secretary at the principal executive offices of the corporation on a timely basis as set forth in Section 3.2(b)(iii) and must update and supplement the information contained in such written notice on a timely basis as set forth in Section 3.2(c). Such stockholder’s notice shall set forth (or include, as appropriate): (A) as to each nominee such stockholder proposes to nominate at the meeting: (1) the name, age, business address and residence address of such nominee, (2) the principal occupation or employment of such nominee, (3) the class and number of shares of each class of capital stock of the corporation which are owned of record and beneficially by such nominee and list of any pledge of or encumbrances on such shares, (4) the date or dates on which such shares were acquired and the investment intent of such acquisition, (5) a statement whether such nominee, if elected, (x) intends to tender, promptly following such person’s failure to receive the required vote for election or re-election at the next meeting at which such person would face election or re-election, a resignation effective upon acceptance of such resignation by the Board of Directors, in accordance with the corporation’s corporate governance policies and guidelines related to conflicts of interest and confidentiality, stock ownership and trading policies and guidelines and any other policies and guidelines applicable to directors, and (y) currently intends to serve as a director for the full term for which such person is standing for election, (6) with respect to each nominee for election or re-election to the Board of Directors, a completed and signed questionnaire, representation and agreement required by Section 3.2(e) of these Bylaws, and (7) such other information concerning such nominee as would be required to be disclosed in a proxy statement soliciting proxies for the election of such nominee as a director in an election contest (even if an election contest is not involved), or that is otherwise required to be disclosed or provided to the corporation pursuant to Section 14 of the 1934 Act and the rules and regulations promulgated thereunder (including such person’s written consent to being named in a proxy statement and associated proxy card as a nominee of the stockholder and to serving as a director if elected); and (B) the information required by Section 3.2(b)(iv). The corporation may require any proposed nominee to furnish such other information as it may reasonably require to determine the eligibility of such proposed nominee to serve as a director of the corporation and to determine the independence of such proposed nominee (as such term is used in any applicable stock exchange listing requirements or applicable law) or to determine the eligibility of such proposed nominee to serve on any committee or sub-committee of the Board of Directors under any applicable stock exchange listing requirements or applicable law, or that the Board of Directors determines, in its sole discretion, could be material to a reasonable stockholder’s understanding of the background, qualifications, experience, independence, or lack thereof, of such proposed nominee. The number of nominees a stockholder may nominate for election at the annual meeting on its own behalf (or in the case of a stockholder giving the notice on behalf of a beneficial owner, the number of nominees a stockholder may nominate for election at the annual meeting on behalf of the beneficial owner) shall not exceed the number of directors to be elected at such annual meeting.
(ii) For business other than (i) proposals sought to be included in the corporation’s proxy statement pursuant to Rule 14a-8 under the 1934 Act, or (ii) nominations for the election to the Board of Directors to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 3.2(a) of these Bylaws, the stockholder must deliver written notice to the Secretary at the principal executive offices of the corporation on a timely basis as set forth in Section 3.2(b)(iii), and must update and supplement such written notice on a timely basis as set forth in Section 3.2(c). Such stockholder’s notice shall set forth: (A) as to each matter such stockholder proposes to bring before the meeting, a brief description of the business desired to be brought before the meeting, the text of the proposal or business (including the text of any resolutions proposed for consideration and in the event that such business includes a proposal to amend these Bylaws, the language of the proposed amendment), the reasons for conducting such business at the meeting, and any material interest (including any anticipated benefit of such business to any Proponent (as defined below) other than solely as a result of its ownership of the corporation’s capital stock, that is material to any Proponent individually, or to the Proponents in the aggregate) in such business of any Proponent; and (B) all of the information required by Section 3.2(b)(iv).
(iii) To be timely, the written notice required by Section 3.2(b)(i) or 3.2(b)(ii) must be received by the Secretary at the principal executive offices of the corporation not later than the close of business on the ninetieth (90th) day nor earlier than the close of business on the one hundred twentieth (120th) day prior to the first anniversary of the prior year’s annual meeting of stockholders; provided, however, that in the event that the date of the annual meeting is advanced more than thirty (30) days prior to, or delayed by more than seventy (70) days after, the anniversary of the preceding year’s annual meeting or if no annual meeting was held in the preceding year, for notice by the stockholder to be timely, such stockholder’s written notice must be delivered to the Secretary not earlier than the close of business on the one hundred twentieth (120th) day prior to such annual meeting and not later than the close of business on the ninetieth (90th) day prior to such annual meeting or the tenth (10th) day following the day on which public announcement of the date of such meeting is first made by the corporation, whichever is later. Notwithstanding the foregoing, in no event shall an adjournment or postponement (or the public announcement thereof) of an annual meeting for which notice has been given or with respect to which there has been a public announcement of the date of the meeting commence a new time period for the giving of a stockholder’s notice as described above.
(iv) The written notice required by Section 3.2(b)(i) or 3.2(b)(ii) shall also set forth, as of the date of the notice and as to the stockholder, including all control persons, as defined below, of a stockholder that is an entity, giving the notice and the beneficial owner, if any, on whose behalf the nomination or proposal is made, including all control persons of a beneficial owner that is an entity (each, a “Proponent” and collectively, the “Proponents,” including any person who is a member of a “group” (as such term is used in Rule 13d-5 promulgated under the 1934 Act) with any other Proponent with respect to the stock of the corporation, including any proposed nominee, and any person Acting in Concert, as defined below, with any Proponent): (A) the name and address of the stockholder giving notice, as they appear on the corporation’s books, and of each other Proponent; (B) the class, series and number of shares of the corporation that are owned beneficially (within the meaning of Rule 13d-3 under the 1934 Act) and of record by each Proponent (provided, that for purposes of this Section 3.2(b)(iv), such Proponent shall in all events be deemed to beneficially own all shares of any class or series of capital stock of the corporation as to which such Proponent has a right to acquire beneficial ownership at any time in the future); (C) a description of any agreement, arrangement or understanding (whether oral or in writing, and regardless to whether it relates specifically to the corporation) with respect to such nomination or proposal (and/or the voting of shares of any class or series of capital stock of the corporation) between or among any Proponent and any of its affiliates or associates and/or any other persons (including their names), including, in the case of a nominee, including any agreement, arrangement or understanding (whether oral or in writing) relating to any compensation or payments to be paid to any such proposed nominee(s); (D) a representation that the stockholder giving the notice is a holder of record of shares of the corporation entitled to vote at the meeting and that such stockholder (or its qualified representative, as defined below) intends to appear in person or by proxy at the meeting to nominate the person or persons specified in the notice (with respect to a notice under Section 3.2(b)(i)) or to propose the business that is specified in the notice (with respect to a notice under Section 3.2(b)(ii)); (E) a representation that the beneficial owner, if any, on whose behalf the nomination or proposal is made is the beneficial owner of shares of the corporation; (F) a representation as to whether the Proponents or any other participant (as defined in Item 4 of Schedule 14A under the Exchange Act) will engage in a solicitation with respect to such nomination or proposal and, if so, the name of each participant in such solicitation and the amount of the cost of solicitation that has been and will be borne, directly or indirectly, by each participant in such solicitation and a representation whether the stockholder intends or is part of a group that intends (x) to solicit proxies from the required number of the corporation’s shares of capital stock entitled to vote in the election of directors in support of any proposed nominee in accordance with and as required by Rule 14a-19 promulgated under the 1934 Act, (y) to deliver, or make available, a proxy statement and form of proxy to holders of at least the percentage of the corporation’s voting shares required to approve or adopt the proposal or elect the nominee and/or (z) to otherwise solicit proxies or votes from stockholders in support of any nominee or any such proposal; (G) a description of all Derivative Transactions (as defined below) by each Proponent during the previous twelve (12) month period, including the date of the transactions and the class, series and number of securities involved in, and the material economic or voting terms of, such Derivative Transactions; (H) a certification that each Proponent has complied with all applicable federal, state and other legal requirements in connection with such Proponent’s acquisition of shares of capital stock or other securities of the corporation and/or such Proponent’s acts or omissions as a stockholder or beneficial owner of the corporation; and (I) any other information relating to the Proponents required to be disclosed in a proxy statement or other filings made in connection with solicitations of proxies for, as applicable, the proposal and/or for the election of directors in a contested election pursuant to and in accordance with Section 14 of the 1934 Act and the rules and regulations promulgated thereunder (whether or not such Proponent intends to deliver a proxy statement or conduct a proxy solicitation).
For purposes of Sections 3.2 and 3.3, a “Derivative Transaction” means any agreement, arrangement, interest or understanding entered into by, or on behalf or for the benefit of, any Proponent or any of its affiliates or associates, whether record or beneficial:
(i) the value of which is derived in whole or in part from the value of any class or series of shares or other securities of the corporation,
(ii) which otherwise provides any direct or indirect opportunity to gain or share in any gain derived from a change in the value of securities of the corporation,
(iii) the effect or intent of which is to mitigate loss, manage risk or benefit from changes in value or price with respect to any securities of the corporation, or
(iv) which provides the right to vote or increase or decrease the voting power of, such Proponent, or any of its affiliates or associates, directly or indirectly, with respect to any securities of the corporation, which agreement, arrangement, interest or understanding may include, without limitation, any option, warrant, debt position, note, bond, convertible security, swap, stock appreciation right or similar right, short position, profit interest, hedge, right to dividends, voting agreement, performance-related fee or arrangement to borrow or lend shares (whether or not subject to payment, settlement, exercise or conversion in any such class or series), and any proportionate interest of such Proponent in the securities of the corporation held by any general or limited partnership, or any limited liability company, of which such Proponent is, directly or indirectly, a general partner or managing member.
A person shall be deemed to be “Acting in Concert” with another person for purposes of these Bylaws if such person knowingly acts (whether or not pursuant to an express agreement, arrangement or understanding) in concert with, or towards a common goal relating to the management, governance or control of the corporation in parallel with, such other person where (A) each person is conscious of the other person’s conduct or intent and this awareness is an element in their decision-making processes and (B) at least one additional factor suggests that such persons intend to act in concert or in parallel, which such additional factors may include, without limitation, exchanging information (whether publicly or privately), attending meetings, conducting discussions, or making or soliciting invitations to act in concert or in parallel; provided, that a person shall not be deemed to be Acting in Concert with any other person solely as a result of the solicitation or receipt of revocable proxies or consents from such other person in response to a solicitation made pursuant to, and in accordance with, Section 14(a) of the Exchange Act by way of a proxy or consent solicitation statement filed on Schedule 14A. A person Acting in Concert with another person shall be deemed to be Acting in Concert with any third party who is also Acting in Concert with such other person.
For purposes of this Section 3.2, a “control person” of a stockholder that is an entity or a beneficial owner that is an entity includes each individual who is a director, executive officer, general partner, or managing member of such entity or of any other entity that has or shares control of that entity.
(c) A stockholder providing written notice required by Section 3.2(b)(i) or (ii) shall update and supplement such notice in writing, if necessary, so that the information (other than the representations required by Section 3.2(b)(iv)(F)) provided or required to be provided in such notice is true and correct in all material respects as of (i) the record date for the determination of stockholders entitled to notice of the meeting and (ii) the date that is five (5) business days prior to the meeting and, in the event of any adjournment or postponement thereof, five (5) business days prior to any adjournment or postponement thereof; provided, that no such update or supplement shall cure or affect the accuracy (or inaccuracy) of any representations made by any Proponent, any of its affiliates or associates, or a nominee or the validity (or invalidity) of any nomination or proposal that failed to comply with this Section 3.2 or is rendered invalid as a result of any inaccuracy therein. In the case of an update and supplement pursuant to clause (i) of this Section 3.2(c), such update and supplement shall be delivered to the Secretary at the principal executive offices of the corporation not later than five (5) business days after the later of the record date for the determination of stockholders entitled to notice of the meeting or the public announcement of such record date. In the case of an update and supplement pursuant to clause (ii) of this Section 3.2(c), such update and supplement shall be delivered to the Secretary at the principal executive offices of the corporation not later than two (2) business days prior to the date for the meeting, and, in the event of any adjournment or postponement thereof, two (2) business days prior to any adjournment or postponement thereof.
(d) Notwithstanding anything in Section 3.2(b)(iii) to the contrary, in the event that the number of directors is increased and there is no public announcement naming all of the nominees for director or specifying the size of the increased Board of Directors made by the corporation at least ten (10) days before the last day a stockholder may deliver a notice of nomination in accordance with Section 3.2(b)(iii), a stockholder’s notice required by this Section 3.2 and which complies with the requirements in Section 3.2(b)(i), other than the timing requirements in Section 3.2(b)(iii), shall also be considered timely, but only with respect to nominees for any new positions created by such increase, if it shall be received by the Secretary at the principal executive offices of the corporation not later than the close of business on the tenth (10th) day following the day on which such public announcement is first made by the corporation.
(e) To be eligible to be a nominee for election or re-election as a director of the corporation pursuant to a nomination under clause (iii) of Section 3.2(a), such nominee or a person on his or her behalf must deliver (in accordance with the time periods prescribed for delivery of notice under Section 3.2(b)(iii) or 3.2(d), as applicable) to the Secretary at the principal executive offices of the corporation a completed and signed written questionnaire with respect to the background, qualifications, stock ownership and independence of such proposed nominee and the background of any other person or entity on whose behalf the nomination is being made (which questionnaire shall be provided by the Secretary upon written request) and a written representation and agreement (in the form provided by the Secretary upon written request) that such person (i) is not and will not become a party to (A) any agreement, arrangement or understanding with, and has not given any commitment or assurance to, any person or entity as to how such person, if elected as a director of the corporation, will act or vote on any issue or question (a “Voting Commitment”) that has not been disclosed to the corporation in the questionnaire or (B) any Voting Commitment that could limit or interfere with such person’s ability to comply, if elected as a director of the corporation, with such person’s fiduciary duties under applicable law; (ii) is not and will not become a party to any agreement, arrangement or understanding with any person or entity other than the corporation with respect to any direct or indirect compensation, reimbursement or indemnification in connection with service or action as a director of the corporation or as a nominee that has not been disclosed to the corporation in the questionnaire; and (iii) in such person’s individual capacity and on behalf of any person or entity on whose behalf the nomination is being made, would be in compliance, if elected as a director of the corporation, and will comply with, all applicable publicly disclosed corporate governance, conflict of interest, confidentiality and stock ownership and trading policies and guidelines of the corporation, and (iv) if elected as a director of the corporation, intends to serve the entire term until the next meeting at which such candidate would face re-election.
(f) A person shall not be eligible for election or re-election as a director unless the person is nominated, in the case of an annual meeting, in accordance with clause (i), (ii), or (iii) of Section 3.2(a), or, in the case of a special meeting, in accordance with clause (c)(ii) of Section 3.3 of these Bylaws. Except as otherwise required by law, the chair of the meeting shall have the power and duty to determine whether a nomination or any business proposed to be brought before an annual or special meeting was made, or proposed, as the case may be, in accordance with the procedures and requirements set forth in these Bylaws and, if any proposed nomination or business is not in compliance with these Bylaws (including, without limitation, compliance with Rule 14a-19), or the Proponent does not act in accordance with the representations required in this Section 3.2, to declare that such proposal or nomination shall not be presented for stockholder action at the meeting and shall be disregarded, or that such business shall not be transacted, notwithstanding that such proposal or nomination is set forth in the corporation’s proxy statement, notice of meeting or other proxy materials and notwithstanding that proxies or votes in respect of such nomination or such business may have been solicited or received. Notwithstanding anything in these Bylaws to the contrary, unless otherwise required by law, if (i) a stockholder intending to make a nomination at a meeting pursuant to Section 3.2(b)(i) (or Section 3.3(c) with respect to a special meeting) or to propose business at a meeting pursuant to Section 3.2(b)(ii) does not provide the information in the stockholder’s notice required under Section 3.2(b)(i) or 3.2(b)(ii), as applicable, and Section 3.2(b)(iv) within the applicable time periods specified in this Section 3.2, or Section 3.3 as applicable, (including any update and supplement required under Section 3.2(c)), (ii) the stockholder (or a qualified representative of the stockholder) does not appear at the meeting to make such nomination or to propose such business (whether pursuant to the requirements of these Bylaws or in accordance with Rule 14a-8 under the 1934 Act), or (iii) the Proponents shall not have acted in accordance with the representations required under Section 3.2(b)(iv)(F), such nomination or proposal shall not be presented for stockholder action at the meeting and shall be disregarded (and any such nominee shall be disqualified), as determined by the chair of the meeting as described above, notwithstanding that proxies in respect of such nominations or such business may have been solicited or received. To be considered a qualified representative of a stockholder for purposes of these Bylaws, a person must be a duly authorized officer, manager or partner of such stockholder or authorized by a writing executed by such stockholder (or a reliable reproduction of the writing) delivered to the corporation prior to the making of such nomination or proposal at such meeting (and in any event not fewer than five (5) business days before the meeting) stating that such person is authorized to act for such stockholder as proxy at the meeting of stockholders. Notwithstanding anything to the contrary in these Bylaws, unless otherwise required by law, if any Proponent (i) provides notice pursuant to Rule 14a-19(b) under the 1934 Act with respect to any proposed nominee and either subsequently (x) fails to comply with the requirements of Rule 14a-19 under the 1934 Act (or fails to timely provide reasonable evidence sufficient to satisfy the corporation that such Proponent has met the requirements of Rule 14a-19(a)(3) under the 1934 Act in accordance with the following sentence) or (y) fails to inform the corporation that they no longer plan to solicit proxies in accordance with the requirements of Rule 14a-19 under the 1934 Act by delivering a written notice to the Secretary at the principal executive offices of the corporation within two (2) business days after the occurrence of such change, then the nomination of each such proposed nominee shall be disregarded (and such nominees shall be disqualified), notwithstanding that the nominee is included as a nominee in the corporation’s proxy statement, notice of meeting or other proxy materials for any annual meeting (or any supplement thereto) and notwithstanding that proxies or votes in respect of the election of such proposed nominees may have been received by the corporation (which proxies and votes shall be disregarded). If any Proponent provides notice pursuant to Rule 14a-19(b) under the 1934 Act, such Proponent shall deliver to the corporation, no later than five (5) business days prior to the applicable meeting, reasonable evidence that it has met the requirements of Rule 14a-19(a)(3) under the 1934 Act. Notwithstanding anything to the contrary set forth herein, and for the avoidance of doubt, the nomination of any person whose name is included as a nominee in the corporation’s proxy statement, notice of meeting or other proxy materials for any annual meeting (or any supplement thereto) as a result of any notice provided by any Proponent and any of its affiliates of associates pursuant to Rule 14a-19(b) promulgated under the 1934 Act with respect to such proposed nominee and whose nomination is not made by or at the direction of the Board of Directors or any authorized committee thereof shall not be deemed (for purposes of clause (i) of Section 3.2(a) or otherwise) to have been made pursuant to the corporation’s notice of meeting (or any supplement thereto) and any such nominee may only be nominated by a Proponent pursuant to clause (iii) of Section 3.2(a) (and, in the case of a special meeting of stockholders pursuant to and to the extent permitted under Section 3.3(c)).
(g) Notwithstanding the foregoing provisions of this Section 3.2, in order to include information with respect to a stockholder proposal in the proxy statement and form of proxy for a stockholders’ meeting, a stockholder must also comply with all applicable requirements of the 1934 Act and the rules and regulations thereunder. Nothing in these Bylaws shall be deemed to affect any rights of stockholders to request inclusion of proposals in the corporation’s proxy statement pursuant to Rule 14a-8 under the 1934 Act; provided, however, that any references in these Bylaws to the 1934 Act or the rules and regulations thereunder are not intended to and shall not limit the requirements applicable to proposals and/or nominations to be considered pursuant to Section 3.2(a) of these Bylaws.
(h) For purposes of Sections 3.2 and 3.3,
(i) “public announcement” shall mean disclosure in a press release reported by the Dow Jones News Service, Associated Press or comparable national news service or in a document publicly filed by the corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the 1934 Act;
(ii) “affiliates” and “associates” shall have the meanings set forth in Rule 405 under the 1933 Act; and
(iii) “close of business” shall mean 5:00 p.m. local time at the principal place of business of the corporation on any calendar day, whether or not the day is a business day.
Section 3.3 Special Meetings.
(a) Except as otherwise required by law and subject to the rights of the holders of any series of Preferred Stock, special meetings of the stockholders of the corporation for any purpose or purposes may be called at any time only by or at the direction of the Board of Directors or the chair of the Board of Directors. The Board of Directors may postpone, reschedule or cancel any special meeting of stockholders.
(b) Stockholders shall not be permitted to propose business to be brought before a special meeting of stockholders and the only matters that may be brought before a special meeting are the matters specified in the notice of meeting given by or at the direction of the person calling the meeting. The Board of Directors shall determine the time and place of such special meeting. Upon determination of the time and place of the meeting, the officer receiving the request shall cause notice to be given to the stockholders entitled to vote, in accordance with the provisions of Section 3.4 of these Bylaws. Nothing contained in this paragraph (b) shall be construed as limiting, fixing, or affecting the time when a meeting of stockholders called by action of the Board of Directors may be held.
(c) Nominations of persons for election to the Board of Directors may be made at a special meeting of stockholders at which directors are to be elected pursuant to the corporation’s notice of meeting (i) by or at the direction of the Board of Directors or (ii) by any stockholder of the corporation who is a stockholder of record at the time of giving notice provided for in these Bylaws who shall be entitled to vote at the meeting and who delivers written notice to the Secretary of the corporation setting forth the information required by Section 3.2(b)(i) and (iv); provided, that if such nominee(s) are proposed on behalf of a beneficial owner, such nominations may only be properly brought before the meeting if such beneficial owner was the beneficial owner of shares of the corporation at the time of the giving of the stockholder’s notice set forth in this paragraph. In the event the corporation calls a special meeting of stockholders for the purpose of electing of one or more directors to the Board of Directors, any such stockholder of record may nominate a person or persons (as the case may be), for election to such position(s) as specified in the corporation’s notice of meeting, if the stockholder’s notice required by this Section 3.3(c) that includes the information required by Section 3.2(b)(i) and (iv) of these Bylaws shall be delivered to the Secretary at the principal executive offices of the corporation not earlier than the close of business on the one hundred twentieth (120th) day prior to such special meeting and not later than the close of business on the later of the ninetieth (90th) day prior to such meeting or the tenth (10th) day following the day on which public announcement is first made of the date of the special meeting and of the nominees proposed by the Board of Directors to be elected at such meeting. The stockholder shall also update and supplement such information as required under Section 3.2(c). In no event shall an adjournment or postponement (or the public announcement thereof) of a special meeting commence a new time period for the giving of a stockholder’s notice as described above. The number of nominees a stockholder may nominate for election at the special meeting (or in the case of a stockholder giving the notice on behalf of a beneficial owner, the number of nominees a stockholder may nominate for election at the special meeting on behalf of such beneficial owner) shall not exceed the number of directors to be elected at such special meeting.
(d) Notwithstanding the foregoing provisions of this Section 3.3, a stockholder must also comply with all applicable requirements of the 1934 Act and the rules and regulations thereunder with respect to matters set forth in this Section 3.3. Nothing in these Bylaws shall be deemed to affect any rights of stockholders to request inclusion of proposals in the corporation’s proxy statement pursuant to Rule 14a--8 under the 1934 Act; provided, however, that any references in these Bylaws to the 1934 Act or the rules and regulations thereunder are not intended to and shall not limit the requirements applicable to nominations for the election to the Board of Directors to be considered pursuant to Section 3.3(c) of these Bylaws.
Section 3.4 Notice of Meetings. Except as otherwise provided by law, notice, given in writing or by electronic transmission in the manner provided by Section 232 of the DGCL, of each meeting of stockholders shall be given not less than ten (10) nor more than sixty (60) days before the date of the meeting to each stockholder entitled to vote at such meeting, such notice to specify the place, if any, date and hour, in the case of special meetings, the purpose or purposes of the meeting, and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at any such meeting. If mailed, notice is given when deposited in the United States mail, postage prepaid, directed to the stockholder at such stockholder’s address as it appears on the records of the corporation. Notice of the time, place, if any, and purpose of any meeting of stockholders may be waived in writing, signed by the person entitled to notice thereof, or by electronic transmission by such person, either before or after such meeting, and will be waived by any stockholder by the stockholder’s attendance thereat in person, by remote communication, if applicable, or by proxy, except when the stockholder attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Any stockholder so waiving notice of such meeting shall be bound by the proceedings of any such meeting in all respects as if due notice thereof had been given.
Section 3.5 Quorum. At all meetings of stockholders, except where otherwise provided by statute or by the Certificate of Incorporation, or by these Bylaws, the presence, in person, by remote communication, if applicable, or by proxy duly authorized, of the holders of a majority of the voting power of all the then-outstanding shares of stock entitled to vote shall constitute a quorum for the transaction of business. In the absence of a quorum, any meeting of stockholders may be adjourned, from time to time, either by the chair of the meeting or by vote of the holders of a majority of the voting power of the shares represented thereat, but no other business shall be transacted at such meeting. The stockholders present at a duly called or convened meeting, at which a quorum is present, may continue to transact business until adjournment, notwithstanding the withdrawal of enough stockholders to leave less than a quorum. Except as otherwise provided by statute or by applicable stock exchange rules or the rules of The New York Stock Exchange, or by the Certificate of Incorporation or these Bylaws, the affirmative vote of the majority of shares present in person, by remote communication, if applicable, or represented by proxy at the meeting and entitled to vote generally on the subject matter shall be the act of the stockholders. Where a separate vote by a class or classes or series is required, except where otherwise provided by the statute or by the Certificate of Incorporation or these Bylaws, a majority of the voting power of the then-outstanding shares of such class or classes or series, present in person, by remote communication, if applicable, or represented by proxy duly authorized, shall constitute a quorum entitled to take action with respect to that vote on that matter. Except where otherwise provided by statute or by the Certificate of Incorporation or these Bylaws, the affirmative vote of the majority of the voting power of the shares of such class or classes or series present in person, by remote communication, if applicable, or represented by proxy at the meeting shall be the act of such class or classes or series.
Section 3.6 Adjournment and Notice of Adjourned Meetings. Any meeting of stockholders, whether annual or special, may be adjourned from time to time either by the chair of the meeting or by the vote of a majority of the voting power of the shares casting votes present in person, by remote communication, if applicable, or represented by proxy at the meeting. When a meeting is adjourned to another time or place, if any, (including an adjournment taken to address a technical failure to convene or continue a meeting using remote communication), notice need not be given of the adjourned meeting if the time and place, if any, thereof, and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such adjourned meeting are (i) announced at the meeting at which the adjournment is taken, (ii) displayed, during the time scheduled for the meeting, on the same electronic network used to enable stockholders and proxy holders to participate in the meeting by means of remote communication, or (iii) set forth in the notice of meeting given in accordance with Section 3.4. At the adjourned meeting, the corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than thirty (30) days or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
Section 3.7 Voting Rights. For the purpose of determining those stockholders entitled to vote at any meeting of the stockholders, except as otherwise provided by law, only persons in whose names shares stand on the stock records of the corporation on the record date, as provided in Section 3.9 of these Bylaws, shall be entitled to vote at any meeting of stockholders. Unless otherwise provided in the corporation’s Certificate of Incorporation each stockholder, shall at every meeting of the stockholders, be entitled to one vote for each share of capital stock having voting power held by such stockholder. Every person entitled to vote shall have the right to do so in person, by remote communication, if applicable, or by an agent or agents authorized by a proxy granted in accordance with the DGCL. An agent so appointed need not be a stockholder. No proxy shall be voted or acted upon after three (3) years from its date of creation unless the proxy provides for a longer period. Any stockholder directly or indirectly soliciting proxies from other stockholders must use a proxy card color other than white, which shall be reserved for the exclusive use by the Board of Directors.
Section 3.8 Joint Owners of Stock. If shares or other securities having voting power stand of record in the names of two (2) or more persons, whether fiduciaries, members of a partnership, joint tenants, tenants in common, tenants by the entirety, or otherwise, or if two (2) or more persons have the same fiduciary relationship respecting the same shares, unless the Secretary is given written notice to the contrary and is furnished with a copy of the instrument or order appointing them or creating the relationship wherein it is so provided, their acts with respect to voting shall have the following effect: (a) if only one (1) votes, his act binds all; (b) if more than one (1) votes, the act of the majority so voting binds all; (c) if more than one (1) votes, but the vote is evenly split on any particular matter, each faction may vote the securities in question proportionally, or may apply to the Delaware Court of Chancery for relief as provided in the DGCL, Section 217(b). If the instrument filed with the Secretary shows that any such tenancy is held in unequal interests, a majority or even-split for the purpose of subsection (c) shall be a majority or even-split in interest.
Section 3.9 List of Stockholders. The officer in charge of the stock ledger of the corporation or the transfer agent shall prepare, no later than the tenth day before each meeting of stockholders, a complete list of the stockholders entitled to vote at the meeting; provided, however, if the record date for determining the stockholders entitled to vote is less than 10 days before the meeting date, the list shall reflect the stockholders entitled to vote as of the tenth day before the meeting date, arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. Nothing contained in this section shall require the corporation to include electronic mail addresses or other electronic contact information on such list. Such list shall be open to the examination of any stockholder for any purpose germane to the meeting for a period of 10 days ending on the day before the meeting date: (i) on a reasonably accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the meeting, or (ii) during ordinary business hours, at the principal place of business of the corporation. In the event that the corporation determines to make the list available on an electronic network, the corporation may take reasonable steps to ensure that such information is available only to stockholders of the corporation. For purposes of this Section 3.9, “stock ledger” means 1 or more records administered by or on behalf of the corporation in which the names of all of the corporation’s stockholders of record, the address and number of shares registered in the name of each such stockholder, and all issuances and transfers of stock of the corporation are recorded in accordance with § 224 of the DGCL. The stock ledger shall be the only evidence as to who are the stockholders entitled by this Section 3.9 to examine the list required by this Section 3.9 or to vote in person or by proxy at any meeting of stockholders.
Section 3.10 No Action Without Meeting. Any action required or permitted to be taken by the stockholders of the corporation must be effected at a duly called annual or special meeting of stockholders and may not be effected by a consent or consents (written, electronic, or otherwise) of such stockholders.
Section 3.11 Organization.
(a) At every meeting of stockholders, the Chair of the Board of Directors, or, if a Chair has not been appointed or is absent, the Chief Executive Officer and President, or, if the Chief Executive Officer and President has not been appointed or is absent, a chair of the meeting chosen by the a majority of the Board of Directors shall act as chair of the meeting. The Secretary, or in his or her absence any person appointed by the chair of the meeting, shall act as secretary of the meeting.
(b) The corporation shall be entitled to make such rules or regulations for the conduct of meetings of stockholders as it shall deem necessary, appropriate or convenient. Subject to such rules and regulations of the Board of Directors, if any, the chair of the meeting shall have the right and authority to prescribe such rules, regulations and procedures and to do all such acts as, in the judgment of such chair, are necessary, appropriate or convenient for the proper conduct of the meeting, including, without limitation, establishing an agenda or order of business for the meeting, making a determination concerning whether business is properly brought before the meeting, rules and procedures for maintaining order at the meeting and the safety of those present, limitations on participation in such meeting to stockholders of record of the corporation and their duly authorized and constituted proxies and such other persons as the chair shall permit, restrictions on entry to the meeting after the time fixed for the commencement thereof, limitations on the time allotted to questions or comments by participants and regulation of the opening and closing of the polls for balloting on matters which are to be voted on by ballot. The date and time of the opening and closing of the polls for each matter upon which the stockholders will vote at the meeting shall be announced at the meeting. Unless and to the extent determined by the chair of the meeting, meetings of stockholders shall not be required to be held in accordance with rules of parliamentary procedure.
ARTICLE 4
DIRECTORS
Section 4.1 Number and Term of Office.
(a) Except as otherwise provided for or fixed pursuant to the provisions of the Certificate of Incorporation relating to the rights of the holders of any series of Preferred Stock to elect additional directors, the precise number of directors shall be determined from time to time exclusively by resolution adopted by the Board of Directors. Directors need not be stockholders unless so required by the Certificate of Incorporation.
(b) At any meeting of stockholders for the election of one or more directors at which a quorum is present, each such director shall be elected by the affirmative vote of the majority of the votes cast with respect to that director, provided that if the number of nominees at such meeting, determined at any time, including as of the record date for such meeting, exceeds the number of directors to be elected (a “Contested Election”), the directors shall be elected by the vote of a plurality of the shares represented in person or by proxy at any such meeting and entitled to vote on the election of directors. For purposes of this Section, a majority of the votes cast means that the number of shares voted “for” a director must exceed the number of votes cast as “against” for that director. “Abstentions” and “broker non-votes” with respect to that director’s election shall not be counted as votes cast. In an election other than a Contested Election, stockholders will be given the choice to cast votes “for” or “against” the election of directors or to “abstain” from such vote and shall not have the ability to cast any other vote with respect to such election of directors. In a Contested Election, stockholders will be given the choice to cast “for” or “withhold” votes for the election of directors and shall not have the ability to cast any other vote with respect to such election of directors. If a director then serving on the Board of Directors does not receive the necessary votes, the director shall offer to tender his or her resignation to the Board. The Nominating and Corporate Governance Committee or other committee that may be designated by the Board will make a recommendation to the Board on whether to accept or reject the resignation, or whether other action should be taken. The Board will act on such committee’s recommendation and publicly disclose its decision and the rationale within 90 days from the date of the certification of the election results. In making their decision, the Committee and the Board will evaluate the best interests of the Company and its stockholders and shall consider all factors and information deemed relevant. The director who tenders his or her resignation will not participate in the Committee’s recommendation or the Board’s decision.
Section 4.2 Nomination of Director Candidates. Nominations for the election of Directors at the annual meeting, by or at the direction of the Board of Directors, may be made by any nominating committee or person appointed by the Board of Directors. Nominations may also be made by any stockholder of record of the corporation entitled to vote for the election of directors at the annual meeting who complies with the notice procedures set forth in Section 3.2 hereof. Nominations for the election of directors at a special meeting of stockholders shall be made pursuant to the procedures of Section 3.3 hereof.
Section 4.3 Powers. The powers of the corporation shall be exercised, its business conducted and its property controlled by or under the direction of the Board of Directors, except as may be otherwise provided by statute or by the Certificate of Incorporation.
Section 4.4 Classes of Directors. Subject to the next sentence hereof and to the rights of the holders of any series of Preferred Stock to elect additional directors under specified circumstances, the Board of Directors is and shall remain divided into three classes (Class I, Class II, and Class III), with the directors in each class serving for a term expiring at the third annual meeting of stockholders held after their election. The initial terms of the members of the Board of Directors shall be as follows: the initial Class I Directors shall serve for a term expiring at the second annual meeting of stockholders of the corporation following the effective date of the First Amended and Restated Certificate of Incorporation of the corporation; the initial Class II Directors shall serve for a term expiring at the third annual meeting of stockholders following the effective date of the First Amended and Restated Certificate of Incorporation of the corporation; and the initial Class III Directors shall serve for a term expiring at the fourth annual meeting of stockholders following the effective date of the First Amended and Restated Certificate of Incorporation of the corporation. Each director in each class shall hold office until his or her successor is duly elected and qualified. At each annual meeting of stockholders beginning with the first annual meeting of stockholders following the effective date of the Fist Amended and Restated Certificate of Incorporation, the successors of the class of directors whose term expires at that annual meeting shall be elected to hold office for a term expiring at the annual meeting of stockholders to be held in the third year following the year of their election, with each director in each such class to hold office until his or her successor is duly elected and qualified. Notwithstanding the foregoing provisions of this Section 4.4, each director shall serve until his successor is duly elected and qualified or until his death, resignation or removal. No decrease in the number of directors constituting the Board of Directors shall shorten the term of any incumbent director.
Section 4.5 Vacancies. Subject to the rights granted to the holders of any one or more series of Preferred Stock then outstanding, any newly-created directorship on the Board of Directors that results from an increase in the number of directors and any vacancy occurring on the Board of Directors (whether by death, resignation, retirement, disqualification, removal or other cause) shall be filled only by a majority of the directors then in office, even if less than a quorum, or by a sole remaining director (and not by the stockholders). Any director elected to fill a vacancy or newly created directorship shall hold office until the next election of the class for which such director shall have been chosen and until his or her successor shall be elected and qualified, or until his or her earlier death, resignation, retirement, disqualification or removal.
Section 4.6 Resignation. Any director may resign at any time by delivering his or her notice in writing or by electronic transmission to the Secretary, such resignation to specify whether it will be effective at a particular time, upon receipt by the Secretary or at the pleasure of the Board of Directors. If no such specification is made, it shall be deemed effective at the pleasure of the Board of Directors. When one or more directors shall resign from the Board of Directors, effective at a future date, a majority of the directors then in office, including those who have so resigned, shall have power to fill such vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each Director so chosen shall hold office for the unexpired portion of the term of the Director whose place shall be vacated and until his successor shall have been duly elected and qualified.
Section 4.7 Removal. Any or all of the directors (other than the directors elected by the holders of any series of Preferred Stock of the corporation, voting separately as a series or together with one or more other such series, as the case may be) may be removed only for cause and only by the affirmative vote of the holders of at least a majority in voting power of all the then outstanding shares of stock of the corporation entitled to vote thereon, voting together as a single class.
Section 4.8 Meetings.
(a) Regular Meetings. Unless otherwise restricted by the Certificate of Incorporation, regular meetings of the Board of Directors may be held at any time or date and at any place within or without the State of Delaware which has been designated by the Board of Directors. No notice shall be required for regular meetings of the Board of Directors.
(b) Special Meetings. Unless otherwise restricted by the Certificate of Incorporation, special meetings of the Board of Directors may be held at any time and place within or without the State of Delaware whenever called in writing, including electronic communication, by the Chair of the Board, the Chief Executive Officer and President, any two directors or any one director in the event there is only one director in office.
(c) Meetings by Electronic Communications Equipment. Any member of the Board of Directors, or of any committee thereof, may participate in a meeting by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and participation in a meeting by such means shall constitute presence in person at such meeting.
(d) Notice of Special Meetings. Notice of the time and place of all special meetings of the Board of Directors shall be delivered orally or in writing, by telephone, including a voice messaging system or other system or technology designed to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other electronic means, during normal business hours, at least twenty-four (24) hours before the date and time of the meeting. If notice is sent by US mail, it shall be sent by first class mail, charges prepaid, at least three (3) days before the date of the meeting.
(e) Waiver of Notice. Notice of any meeting may be waived in writing, or by electronic transmission, at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends the meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. The transaction of all business at any meeting of the Board of Directors, or any committee thereof, however called or noticed, or wherever held, shall be as valid as though the business was transacted at a meeting duly held after regular call and notice, if a quorum be present and if, either before or after the meeting, each of the directors not present who did not receive notice shall sign a written waiver of notice or shall waive notice by electronic transmission. All such waivers shall be filed with the corporate records or made a part of the minutes of the meeting.
Section 4.9 Quorum and Voting.
(a) Unless the Certificate of Incorporation requires a greater number, and except with respect to indemnification questions arising under Section 12.1 hereof, for which a quorum shall be one-third of the exact number of directors fixed from time to time, and except with respect to certain transactions questions arising under Section 9.1, for which a quorum is set by Section 9.2 hereof, a quorum of the Board of Directors shall consist of a majority of the exact number of directors fixed from time to time by the Board of Directors in accordance with the Certificate of Incorporation; provided, however, at any meeting whether a quorum be present or otherwise, a majority of the directors present may adjourn from time to time until the time fixed for the next regular meeting of the Board of Directors, without notice other than by announcement at the meeting.
(b) At each meeting of the Board of Directors at which a quorum is present, all questions and business shall be determined by the affirmative vote of a majority of the directors present, unless a different vote be required by law, the Certificate of Incorporation or these Bylaws.
Section 4.10. Action without Meeting. Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, any action required or permitted to be taken at any meeting of the Board of Directors or of any committee thereof may be taken without a meeting, if all members of the Board of Directors or committee, as the case may be, consent thereto in writing or by electronic transmission, and such writing or writings or transmission or transmissions are filed with the minutes of proceedings of the Board of Directors or committee. Such filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained in electronic form.
Section 4.11 Fees and Compensation. Directors shall be entitled to such compensation for their services as may be approved by the Board of Directors, including, if so approved, by resolution of the Board of Directors, a fixed sum and expenses of attendance, if any, for attendance at each regular or special meeting of the Board of Directors and at any meeting of a committee of the Board of Directors. Nothing herein contained shall be construed to preclude any director from serving the corporation in any other capacity as an officer, agent, employee, or otherwise and receiving compensation therefor.
Section 4.12 Committees.
(a) Committees. The Board of Directors may, from time to time, appoint such committees as may be permitted by law. Such other committees appointed by the Board of Directors shall consist of one (1) or more members of the Board of Directors and shall have such powers and perform such duties as may be prescribed by the resolution or resolutions creating such committees.
(b) Term. The Board of Directors, subject to any requirements of any outstanding series of Preferred Stock, and the provisions of subsection (a) of this Section 4.12 may at any time increase or decrease the number of members of a committee or terminate the existence of a committee. The membership of a committee member shall terminate on the date of his death or voluntary resignation from the committee or from the Board of Directors. The Board of Directors may at any time for any reason remove any individual committee member and the Board of Directors may fill any committee vacancy created by death, resignation, removal or increase in the number of members of the committee. The Board of Directors may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee, and, in addition, in the absence or disqualification of any member of a committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member.
(c) Meetings. Unless the Board of Directors shall otherwise provide, regular meetings of any committee appointed pursuant to this Section 4.12 shall be held at such times and places as are determined by the Board of Directors, or by any such committee, and when notice thereof has been given to each member of such committee, no further notice of such regular meetings need be given thereafter. Special meetings of any such committee may be held at any place which has been determined from time to time by such committee, and may be called by the chair of such committee or a member of such committee, upon notice to the members of such committee of the time and place of such special meeting given in the manner provided for the giving of notice to members of the Board of Directors of the time and place of special meetings of the Board of Directors. Notice of any special meeting of any committee may be waived in writing or by electronic transmission at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends such special meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Unless otherwise provided by the Board of Directors in the resolutions authorizing the creation of the committee, a majority of the authorized number of members of any such committee shall constitute a quorum for the transaction of business, and the act of a majority of those present at any meeting at which a quorum is present shall be the act of such committee.
Section 4.13 Organization. At every meeting of the directors, the Chair of the Board of Directors, or, if a Chair has not been appointed or is absent, the Chief Executive Officer and President (if a director), or, if the Chief Executive Officer and President has not been appointed or is absent the most senior Vice President (if a director), or, in the absence of any such person, a chair of the meeting chosen by a majority of the directors present, shall preside over the meeting. The Secretary, or in his or her absence, any person directed to do so by the chair of the meeting, shall act as secretary of the meeting.
ARTICLE 5
OFFICERS
Section 5.1 Officers Designated. The officers of the corporation shall include, if and when designated by the Board of Directors, the Chair of the Board of Directors (provided that notwithstanding anything to the contrary contained in these Bylaws, the Chair of the Board of Directors shall not be deemed an officer of the corporation unless so designated by the Board of Directors), the Chief Executive Officer and President, one or more Vice Presidents, the Secretary, the Chief Financial Officer and the Treasurer. The Board of Directors may also appoint one or more Assistant Secretaries, Assistant Treasurers and such other officers and agents with such powers and duties as it shall deem necessary. The Board of Directors may assign such additional titles to one or more of the officers as it shall deem appropriate. Any one person may hold any number of offices of the corporation at any one time unless specifically prohibited therefrom by law. The salaries and other compensation of the officers of the corporation shall be fixed by or in the manner designated by the Board of Directors.
Section 5.2 Tenure and Duties of Officers.
(a) General. All officers shall hold office at the pleasure of the Board of Directors and until their successors shall have been duly elected and qualified, unless sooner removed. Any officer elected or appointed by the Board of Directors may be removed at any time by the Board of Directors. If the office of any officer becomes vacant for any reason, the vacancy may be filled by the Board of Directors.
(b) Duties of Chair of the Board of Directors. The Chair of the Board of Directors, when present, shall preside at all meetings of the stockholders and the Board of Directors. The Chair of the Board of Directors shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time.
(c) Duties of Chief Executive Officer and President. The Chief Executive Officer and President shall preside at all meetings of the stockholders and at all meetings of the Board of Directors, unless the Chair of the Board of Directors has been appointed and is present. The Chief Executive Officer and President shall, subject to the control of the Board of Directors, have general supervision, direction and control of the business and officers of the corporation, perform other duties commonly incident to the office, and shall also perform such other duties and have such other powers, as the Board of Directors shall designate from time to time.
(d) Duties of Vice Presidents. The Vice Presidents may assume and perform the duties of the President in the absence or disability of the President or whenever the office of President is vacant. The Vice Presidents shall perform other duties commonly incident to their office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
(e) Duties of Secretary. The Secretary shall attend all meetings of the stockholders and of the Board of Directors and shall record all acts and proceedings thereof in the minute book of the corporation. The Secretary shall give notice in conformity with these Bylaws of all meetings of the stockholders and of all meetings of the Board of Directors and any committee thereof requiring notice. The Secretary shall perform all other duties provided for in these Bylaws and other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time. The Chief Executive Officer and President may direct any Assistant Secretary or other office or director to assume and perform the duties of the Secretary in the absence or disability of the Secretary, and each Assistant Secretary shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the Chief Executive Officer and President shall designate from time to time.
(f) Duties of Chief Financial Officer. The Chief Financial Officer shall keep or cause to be kept the books of account of the corporation in a thorough and proper manner and shall render statements of the financial affairs of the corporation in such form and as often as required by the Board of Directors or the Chief Executive Officer and President. The Chief Financial Officer, subject to the order of the Board of Directors, shall have the custody of all funds and securities of the corporation. The Chief Financial Officer shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the Chief Executive Officer and President shall designate from time to time. The Chief Executive Officer and President may direct the Vice President of Finance, Treasurer or any Assistant Treasurer, or the Controller or Assistant Controller, to assume and perform the duties of the Chief Financial Officer in the absence or disability of the Chief Financial Officer and, in the absence or disability of the Chief Financial Officer, each Vice President of Finance, Treasurer or any Assistant Treasurer, or the Controller or Assistance Controller shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the Chief Executive Officer and President shall designate from time to time.
(g) Treasurer. The Treasurer shall have such duties as may be specified by the Chief Financial Officer to assist the Chief Financial Officer in the performance of his or her duties to perform such other duties and have other powers as may from time to time be prescribed by the Board of Directors or the Chief Executive Officer and President.
(h) Assistant Treasurer. The Chief Executive Officer and President may direct any Assistant Treasurer to assume and perform the duties of the Treasurer in the absence or disability of the Treasurer, and each Assistant Treasurer shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the Chief Executive Officer and President shall designate from time to time.
Section 5.3 Delegation of Authority. The Board of Directors may from time to time delegate the powers or duties of any officer to any other officer or agent, notwithstanding any provision hereof.
Section 5.4 Resignations. Any officer may resign at any time by giving notice in writing or by electronic transmission to the Board of Directors or to the President or to the Secretary. Any such resignation shall be effective when received by the person or persons to whom such notice is given, unless a later time is specified therein, in which event the resignation shall become effective at such later time. Unless otherwise specified in such notice, the acceptance of any such resignation shall not be necessary to make it effective. Any resignation shall be without prejudice to the rights, if any, of the corporation under any contract with the resigning officer.
Section 5.5 Removal. Any officer may be removed from office at any time, either with or without cause, by the affirmative vote of a majority of the directors in office at the time, or by the unanimous written or electronic consent of the directors in office at the time, or by any committee of the Board of Directors or by superior officers upon whom such power of removal may have been conferred by the Board of Directors.
ARTICLE 6
EXECUTION OF CORPORATE INSTRUMENTS AND VOTING
OF SECURITIES OWNED BY THE CORPORATION
Section 6.1 Execution of Corporate Instruments.
(a) The Board of Directors may, in its discretion, determine the method and designate the signatory officer or officers, or other person or persons, to execute on behalf of the corporation any corporate instrument or document, or to sign on behalf of the corporation the corporate name without limitation, or to enter into contracts on behalf of the corporation, except where otherwise provided by law or these Bylaws, and such execution or signature shall be binding upon the corporation.
(b) Unless otherwise specifically determined by the Board of Directors or otherwise required by law, promissory notes, deeds of trust, mortgages and other evidences of indebtedness of the corporation, and other corporate instruments or documents requiring the corporate seal, if any, and certificates of shares of stock owned by the corporation, shall be executed, signed or endorsed by the Chair of the Board of Directors, or the Chief Executive Officer and President or any Vice President, and by the Secretary or Treasurer or any Assistant Secretary or Assistant Treasurer. All other instruments and documents requiring the corporate signature, but not requiring the corporate seal, may be executed as aforesaid or in such other manner as may be directed by the Board of Directors.
(c) Unless authorized or ratified by the Board of Directors or within the agency power of an officer, no officer, agent or employee shall have any power or authority to bind the corporation by any contract or engagement or to pledge the corporation’s credit or to render it liable for any purpose or for any amount.
(d) All checks and drafts drawn on banks or other depositaries on funds to the credit of the corporation or in special accounts of the corporation shall be signed by such person or persons as the Board of Directors shall authorize so to do.
Section 6.2 Voting of Securities Owned by the Corporation. All stock and other securities of other corporations owned or held by the corporation for itself, or for other parties in any capacity, shall be voted, and all proxies with respect thereto shall be executed, by the person authorized so to do by resolution of the Board of Directors, or, in the absence of such authorization, by the Chair of the Board of Directors, the Chief Executive Officer and President, or any Vice President.
ARTICLE 7
SHARES OF STOCK
Section 7.1 Form and Execution of Certificates. The shares of the corporation shall be represented by certificates, or shall be uncertificated. Certificates for the shares of stock of the corporation shall be in such form as is consistent with the Certificate of Incorporation and applicable law. Every holder of stock in the corporation represented by certificates shall be entitled to have a certificate signed by or in the name of the corporation by the Chair of the Board of Directors, or the Chief Executive Officer and President, or any Vice President and by the Treasurer or Assistant Treasurer or the Secretary or Assistant Secretary, certifying the number of shares owned by the holder in the corporation. Any or all of the signatures on the certificate may be facsimiles. In case any officer, transfer agent, or registrar who has signed or whose facsimile signature has been placed upon a certificate shall have ceased to be such officer, transfer agent, or registrar before such certificate is issued, it may be issued with the same effect as if he were such officer, transfer agent, or registrar at the date of issue. Each certificate shall state upon the face or back thereof, in full or in summary, all of the powers, designations, preferences, and rights, and the limitations or restrictions of the shares authorized to be issued or shall, except as otherwise required by law, set forth on the face or back a statement that the corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative, participating, optional, or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights. Within a reasonable time after the issuance or transfer of uncertificated stock, the corporation shall send to the registered owner thereof a written notice containing the information required by the DGCL or a statement that the corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
Section 7.2 Lost Certificates. A new certificate or certificates shall be issued in place of any certificate or certificates theretofore issued by the corporation alleged to have been lost, stolen, or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen, or destroyed and on such terms and conditions as the corporation may require. The corporation may require, as a condition precedent to the issuance of a new certificate or certificates, the owner of such lost, stolen, or destroyed certificate or certificates, or the owner’s legal representative, to agree to indemnify the corporation in such manner as it shall require or to give the corporation a surety bond in such form and amount as it may direct as indemnity against any claim that may be made against the corporation with respect to the certificate alleged to have been lost, stolen, or destroyed.
Section 7.3 Transfers.
(a) Transfers of record of shares of stock of the corporation shall be made only upon the corporation’s books by the holders thereof, in person or by attorney duly authorized, and upon the surrender of a properly endorsed certificate or certificates for a like number of shares and proper evidence of compliance with other conditions of applicable law, by contract or otherwise to rightful transfer.
(b) Upon receipt of proper transfer instructions and proper evidence of compliance of other conditions of applicable law, by contract or otherwise to rightful transfer from the registered owner of the uncertificated or certificated shares, such uncertificated or certificated shares, as applicable, shall be cancelled and issuance of new equivalent uncertificated shares or certificated shares shall be made to the person entitled thereto and the transaction shall be recorded upon the books of the corporation.
(c) The corporation shall have power to enter into and perform any agreement with any number of stockholders of any one or more classes of stock of the corporation to restrict the transfer of shares of stock of the corporation of any one or more classes owned by such stockholders in any manner not prohibited by the DGCL.
Section 7.4 Fixing Record Dates.
(a) In order that the corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the Board of Directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which record date shall, subject to applicable law, not be more than sixty (60) nor less than ten (10) days before the date of such meeting. If no record date is fixed by the Board of Directors, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board of Directors may fix a new record date for the adjourned meeting.
(b) In order that the corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of stock, or for the purpose of any other lawful action, the Board of Directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than sixty (60) days prior to such action. If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto.
Section 7.5 Registered Stockholders. The corporation shall be entitled to recognize the exclusive right of a person registered on its books as the owner of shares to receive dividends, and to vote as such owner, and shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of any other person whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of Delaware.
ARTICLE 8
OTHER SECURITIES OF THE CORPORATION
All bonds, debentures and other corporate securities of the corporation, other than stock certificates (covered in Section 7.1), may be signed by the Chair of the Board of Directors, the Chief Executive Officer and President, or any Vice President, or such other person as may be authorized by the Board of Directors, and the corporate seal impressed thereon or a facsimile of such seal imprinted thereon and attested by the signature of the Secretary or an Assistant Secretary, or the Chief Financial Officer or Treasurer or an Assistant Treasurer; provided, however, that where any such bond, debenture or other corporate security shall be authenticated by the manual signature, or where permissible facsimile signature, of a trustee under an indenture pursuant to which such bond, debenture or other corporate security shall be issued, the signatures of the persons signing and attesting the corporate seal on such bond, debenture or other corporate security may be the imprinted facsimile of the signatures of such persons. Interest coupons appertaining to any such bond, debenture or other corporate security, authenticated by a trustee as aforesaid, shall be signed by the Treasurer or an Assistant Treasurer of the corporation or such other person as may be authorized by the Board of Directors or bear imprinted thereon the facsimile signature of such person. In case any officer who shall have signed or attested any bond, debenture or other corporate security, or whose facsimile signature shall appear thereon or on any such interest coupon, shall have ceased to be such officer before the bond, debenture or other corporate security so signed or attested shall have been delivered, such bond, debenture or other corporate security nevertheless may be adopted by the corporation and issued and delivered as though the person who signed the same or whose facsimile signature shall have been used thereon had not ceased to be such officer of the corporation.
ARTICLE 9
CERTAIN TRANSACTIONS
Section 9.1 Transactions with Interested Parties. No contract or transaction between the corporation and one or more of its directors or officers, or between the corporation and any other corporation, partnership, association or other organization in which one or more of its directors or officers, are directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the Board of Directors or committee thereof which authorized the contract or transaction or solely because any such director’s or officer’s votes are counted for such purposes, if:
(a) the material facts as to the director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the Board of Directors or committee, and the Board of Directors or committee in good faith authorizes the contract or transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; or
(b) the material facts as to the director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the stockholders entitled to vote thereon, and the contract or transaction is specifically approved in good faith by vote of the stockholders; or
(c) the contract or transaction is fair as to the corporation as of the time it is authorized, approved or ratified, by the Board of Directors, a committee thereof, or the stockholders.
Section 9.2 Quorum. Interested directors may be counted in determining the presence of a quorum at a meeting of the Board of Directors or of a committee thereof, which authorizes the contract or transaction.
ARTICLE 10
DIVIDENDS
Section 10.1 Declaration of Dividends. Dividends upon the capital stock of the corporation, subject to the provisions of the Certificate of Incorporation and applicable law, if any, may be declared by the Board of Directors pursuant to law at any regular or special meeting. Dividends may be paid in cash, in property, or in shares of the capital stock, subject to the provisions of the Certificate of Incorporation and applicable law.
Section 10.2 Dividend Reserve. Before payment of any dividend, there may be set aside out of any funds of the corporation available for dividends such sum or sums as the Board of Directors from time to time, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the corporation, or for such other purpose as the Board of Directors shall think conducive to the interests of the corporation, and the Board of Directors may modify or abolish any such reserve in the manner in which it was created.
ARTICLE 11
FISCAL YEAR
The fiscal year of the corporation shall be fixed by resolution of the Board of Directors.
ARTICLE 12
INDEMNIFICATION
Section 12.1. Indemnification of Directors, Executive Officers, Other Officers, Employees and Other Agents.
(a) Directors and Executive Officers. The corporation shall indemnify its directors and executive officers (for the purposes of this Article 12, “executive officers” shall have the meaning defined in Rule 3b-7 promulgated under the 1934 Act) to the fullest extent not prohibited by the DGCL or any other applicable law; provided, however, that the corporation may modify the extent of such indemnification by individual contracts with its directors and executive officers.
(b) Other Officers, Employees and Other Agents. The corporation shall have power to indemnify its other officers, employees and other agents as set forth in the DGCL or any other applicable law. The Board of Directors shall have the power to delegate the determination of whether indemnification shall be given to any such person (other than as specified in clause (a) above) to such officers or other persons as the Board of Directors shall determine.
(c) Expenses. The corporation shall advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director or executive officer, of the corporation, or is or was serving at the request of the corporation as a director or executive officer of another corporation, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefor, all expenses incurred by any director or executive officer in connection with such proceeding; provided, however, that if the DGCL requires, an advancement of expenses incurred by a director or an executive officer in his or her capacity as a director or an executive officer (and not in any other capacity in which service was or is rendered by such indemnitee, including, without limitation, service to an employee benefit plan) shall be made only upon delivery to the corporation of an undertaking (hereinafter an “undertaking”), by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal (hereinafter a “final adjudication”) that such indemnitee is not entitled to be indemnified for such expenses under this Section 12.1 or otherwise.
Notwithstanding the foregoing, unless otherwise determined pursuant to paragraph (e) of this Section 12.1, no advance shall be made by the corporation to an executive officer of the corporation (except by reason of the fact that such executive officer is or was a director of the corporation, in which event this paragraph shall not apply) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made (i) by a majority vote of directors who were not parties to the proceeding, even if not a quorum, or (ii) by a committee of such directors designated by a majority vote of such directors, even though less than a quorum, or (iii) if there are no such directors, or such directors so direct, by independent legal counsel in a written opinion, that the facts known to the decision-making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the corporation.
(d) Enforcement. Without the necessity of entering into an express contract, all rights to indemnification and advances to directors and executive officers under this Section 12.1 shall be deemed to be contractual rights and be effective to the same extent and as if provided for in a contract between the corporation and the director or executive officer. Any right to indemnification or advances granted by this Section 12.1 to a director or executive officer shall be enforceable by or on behalf of the person holding such right in any court of competent jurisdiction if (i) the claim for indemnification or advances is denied, in whole or in part, or (ii) no disposition of such claim is made within ninety (90) days of request therefor. The claimant in such enforcement action, if successful in whole or in part, shall be entitled to be paid also the expense of prosecuting the claim. In connection with any claim for indemnification, the corporation shall be entitled to raise as a defense to any such action that the claimant has not met the standards of conduct that make it permissible under the DGCL or any other applicable law for the corporation to indemnify the claimant for the amount claimed. In connection with any claim by an executive officer of the corporation (except in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such executive officer is or was a director of the corporation) for advances, the corporation shall be entitled to raise a defense as to any such action clear and convincing evidence that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the corporation, or with respect to any criminal action or proceeding that such person acted without reasonable cause to believe that his conduct was lawful. Neither the failure of the corporation (including its directors who are not parties to such action, a committee of such directors, independent legal counsel or its stockholders) to have made a determination prior to the commencement of such action that indemnification of the claimant is proper in the circumstances because he has met the applicable standard of conduct set forth in the DGCL or any other applicable law, nor an actual determination by the corporation (including its directors who are not parties to such action, a committee of such directors, independent legal counsel or its stockholders) that the claimant has not met such applicable standard of conduct, shall be a defense to the action or create a presumption that claimant has not met the applicable standard of conduct. In any suit brought by a director or executive officer to enforce a right to indemnification or to an advancement of expenses hereunder or brought by the corporation to recover an advancement of expenses pursuant to the terms of any undertaking, the burden of proving that the director or executive officer is not entitled to be indemnified, or to such advancement of expenses under this Section 12.1 or otherwise shall be on the corporation.
(e) Non-Exclusivity of Rights. The rights conferred on any person by this Section 12.1 shall not be exclusive of any other right which such person may have or hereafter acquire under any applicable statute, provision of the Certificate of Incorporation, Bylaws, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding office. The corporation is specifically authorized to enter into individual contracts with any or all of its directors, officers, employees or agents respecting indemnification and advances, to the fullest extent not prohibited by the DGCL, or by any other applicable law.
(f) Survival of Rights. The rights conferred on any person by this Bylaw shall continue as to a person who has ceased to be a director or executive officer and shall inure to the benefit of the heirs, executors and administrators of such a person.
(g) Insurance. To the fullest extent permitted by the DGCL, or any other applicable law, the corporation, upon approval by the Board of Directors, may purchase insurance on behalf of any person required or permitted to be indemnified pursuant to this Section 12.1.
(h) Amendments. Any amendment, alteration or repeal of this Section 12.1 that adversely affects any right of an indemnitee or its successors shall be prospective only and shall not limit or eliminate any such right with respect to any proceeding involving any occurrence or alleged occurrence of any action or omission to act that took place prior to such amendment or repeal.
(i) Saving Clause. If this Section 12.1 or any portion hereof shall be invalidated on any ground by any court of competent jurisdiction, then the corporation shall nevertheless indemnify each director and executive officer to the full extent not prohibited by any applicable portion of this Section 12.1 that shall not have been invalidated, or by any other applicable law. If this Section 12.1 shall be invalid due to the application of the indemnification provisions of another jurisdiction, then the corporation shall indemnify each director and executive officer to the full extent under any other applicable law.
(j) Certain Definitions. For the purposes of this Bylaw, the following definitions shall apply:
(1) The term “proceeding” shall be broadly construed and shall include, without limitation, the investigation, preparation, prosecution, defense, settlement, arbitration and appeal of, and the giving of testimony in, any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative.
(2) The term “expenses” shall be broadly construed and shall include, without limitation, court costs, attorneys’ fees, witness fees, fines, amounts paid in settlement or judgment, interest assessments and any other costs and expenses of any nature or kind incurred in connection with any proceeding.
(3) The term the “corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, shall stand in the same position under the provisions of this Section 12.1 with respect to the resulting or surviving corporation as he would have with respect to such constituent corporation if its separate existence had continued.
(4) References to a “director,” “executive officer,” “officer,” “employee,” or “agent” of the corporation shall include, without limitation, situations where such person is serving at the request of the corporation as, respectively, a director, executive officer, officer, employee, trustee or agent of another corporation, partnership, joint venture, trust or other enterprise.
(5) References to “other enterprises” shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to an employee benefit plan; and references to “serving at the request of the corporation” shall include any service as a director, officer, employee or agent of the corporation which imposes duties on, or involves services by, such director, officer, employee, or agent with respect to an employee benefit plan, its participants, or beneficiaries; and a person who acted in good faith and in a manner he reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this Section 12.1.
ARTICLE 13
NOTICES
(a) Notice to Stockholders. Notice to stockholders of stockholder meetings shall be given as provided in Section 3.4 herein. Without limiting the manner by which notice may otherwise be given effectively to stockholders under any agreement or contract with such stockholder, and except as otherwise required by law, notice to stockholders for purposes other than stockholder meetings may be sent by US mail or nationally recognized overnight courier, or by facsimile, telegraph or telex or by electronic mail or other electronic transmission in the manner provided in Section 232 of the DGCL.
(b) Notice to Directors. Any notice required to be given to any director may be given by the method stated in subsection (a), or by overnight delivery service, facsimile, telex or telegram, except that such notice other than one which is delivered personally shall be sent to such address as such director shall have filed in writing with the Secretary, or, in the absence of such filing, to the last known post office address of such director.
(c) Affidavit of Mailing. An affidavit of mailing, executed by a duly authorized and competent employee of the corporation or its transfer agent appointed with respect to the class of stock affected, or other agent, specifying the name and address or the names and addresses of the stockholder or stockholders, or director or directors, to whom any such notice or notices was or were given, and the time and method of giving the same, shall in the absence of fraud, be prima facie evidence of the facts therein contained.
(d) Time Notices Deemed Given. All notices given by mail or by overnight delivery service, as above provided, shall be deemed to have been given as at the time of mailing, and all notices given by facsimile, telex or telegram or by electronic mail or other electronic means shall be deemed to have been given as of the sending time recorded at time of transmission.
(e) Failure to Receive Notice. The period or limitation of time within which any stockholder may exercise any option or right, or enjoy any privilege or benefit, or be required to act, or within which any director may exercise any power or right, or enjoy any privilege, pursuant to any notice sent in the manner above provided, shall not be affected or extended in any manner by the failure of such stockholder or such director to receive such notice.
(f) Methods of Notice. It shall not be necessary that the same method of giving notice be employed in respect of all recipients of notice, but one permissible method may be employed in respect of any one or more, and any other permissible method or methods may be employed in respect of any other or others.
(g) Notice to Person with Whom Communication Is Unlawful. Whenever notice is required to be given, under any provision of law or of the Certificate of Incorporation or Bylaws of the corporation, to any person with whom communication is unlawful, the giving of such notice to such person shall not be required and there shall be no duty to apply to any governmental authority or agency for a license or permit to give such notice to such person. Any action or meeting which shall be taken or held without notice to any such person with whom communication is unlawful shall have the same force and effect as if such notice had been duly given. In the event that the action taken by the corporation is such as to require the filing of a certificate under any provision of the DGCL, the certificate shall state, if such is the fact and if notice is required, that notice was given to all persons entitled to receive notice except such persons with whom communication is unlawful.
(h) Notice to Stockholders Sharing an Address. Except as otherwise prohibited under DGCL, any notice given under the provisions of DGCL, the Certificate of Incorporation or the Bylaws shall be effective if given by a single written notice to stockholders who share an address if consented to by the stockholders at that address to whom such notice is given. Such consent shall have been deemed to have been given if such stockholder fails to object in writing to the corporation within sixty (60) days of having been given notice by the corporation of its intention to send the single notice. Any consent shall be revocable by the stockholder by written notice to the corporation.
ARTICLE 14
AMENDMENTS
Subject to the limitation set forth in Section 12.1(h) of these Bylaws or the provisions of the Certificate of Incorporation, the Board of Directors is expressly empowered to adopt, amend or repeal the Bylaws of the corporation. The stockholders shall also have power to adopt, amend or repeal the Bylaws of the corporation; provided, however, that, in addition to any vote of the holders of any class or series of stock of the corporation required by law or by the Certificate of Incorporation, such action by the stockholders shall require the affirmative vote of the holders of at least two-thirds (66-2/3%) of the voting power of all of the then-outstanding shares of the capital stock of the corporation entitled to vote generally in the election of directors, voting together as a single class.
[Certificate of Adoption attached on next page]
CERTIFICATE OF ADOPTION OF
AMENDED AND RESTATED BYLAWS
OF
SERINA THERAPEUTICS, INC.
The undersigned hereby certifies that they are the duly elected, qualified, and acting Secretary of Serina Therapeutics, Inc., a Delaware corporation (the “Corporation”), and that the foregoing amended and restated bylaws were adopted as the Corporation’s bylaws as of _________________, by the Corporation’s Board of Directors.
The undersigned has executed this Certificate as of ______________.
____________________________
___________________
As its Secretary
PART II
INFORMATION NOT REQUIRED IN PROXY STATEMENT/PROSPECTUS/INFORMATION
STATEMENT
Item 13. Other Expenses of Issuance and Distribution*
The following table sets forth the costs and expenses in connection with the distribution of the securities being registered hereunder. All amounts are estimates except the SEC registration fee.
| SEC registration fee | | $ | 7,036.83 | |
| Legal fees and expenses | | $ | * | |
| Accounting fees and expenses | | $ | * | |
| Printing and miscellaneous expenses | | $ | * | |
| Total | | $ | * | |
* The amount of securities and the expenses cannot be estimated at this time. An estimate of the costs and expenses in connection with the distribution of the securities being registered hereunder will be included by amendment.
Item 15. Recent Sales of Unregistered Securities
During the three years preceding the filing of this registration statement on Form S-4/S-1, of which this proxy statement/prospectus/information statement is a part, the registrant has granted or issued the following securities of the registrant which were not registered under the Securities Act.
On July 24, 2023, AgeX issued to Juvenescence 211,600 shares of AgeX Series A preferred stock and 148,400 shares of AgeX Series B preferred stock in exchange for the cancellation of a total of $36 million of indebtedness consisting of the outstanding principal amount of loans then outstanding under the 2020 Loan Agreement, the 2022 Secured Note, and the 2023 Secured Note, plus the loan origination fees accrued with respect to the 2022 Secured Note and a portion of the loan origination fees accrued pursuant to the 2023 Secured Note. The cancellation of indebtedness in exchange for Series A preferred stock and AgeX Series B preferred stock was conducted pursuant to the 2023 Exchange Agreement between AgeX and Juvenescence.
The NYSE American approved the listing of the 36,939,190 shares of AgeX common stock into which AgeX preferred stock is presently convertible. In order to comply with Section 713 of the NYSE American Company Guide, the issuance of an additional 13,060,809 shares of AgeX common stock upon conversion of shares of AgeX Series B preferred stock is currently restricted by a “cap” prohibiting issuance of those additional shares without the prior approval of AgeX stockholders.
The sales of the above securities were exempt from the registration requirements of the Securities Act in reliance on the exemptions afforded by Section 4(a)(2) of the Securities Act. No sales mentioned above involved underwriters, underwriting discounts or commissions or public offerings of securities of the registrant.
Item 20. Indemnification of Directors and Officers
The registrant’s Certificate of Incorporation provides that the liability of the directors to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director is eliminated to the fullest extent permissible under Delaware law, except for the liability of a director: (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; (iii) under §174 of the Delaware General Corporation Law; or (iv) for any transaction from which the director derived an improper personal benefit. The corporation is authorized to indemnify directors, officers, and agents to the fullest extent permissible under Delaware law.
Section 145 of the Delaware General Corporation Law permits a corporation to indemnify any director or officer of the corporation against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. In a derivative action, (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that the defendant is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
The foregoing summary is subject to the complete text of the applicable statutes and the Certificate of Incorporation and Bylaws of the registrant and is qualified in its entirety by reference to such documents.
The registrant also maintains directors’ and officers’ liability insurance coverage and may enter into indemnification agreements with its officers and directors.
Item 21. Exhibits
| (a) | The following exhibits are filed herewith or incorporated herein by reference: |
| Exhibit | | | | Incorporated by
Reference | | Filed | | To be Filed by | | Previously |
| Number | | Exhibit Description | | Form | | Date | | Number | | Herewith | | Amendment | | Filed |
| 2.1† | | Agreement and Plan of Merger and Reorganization, dated as of August 29, 2023, by and among AgeX Therapeutics, Inc., Canaria Transaction Corporation and Serina Therapeutics, Inc. | | 8-K | | 8/30/2023 | | 2.1 | | | | | | X |
| 2.2 | | Agreement and Plan of Merger, dated March 6, 2021, by Atlas Capital Partners Limited, GCLMS Acquisition Corporation, LifeMap Sciences, Inc. and AgeX Therapeutics, Inc. | | | | | | | | | | | | X |
| 3.1 | | Certificate of Incorporation, as amended, of AgeX Therapeutics, Inc. | | 8-K | | 12/12/2022 | | 3.1 | | | | | | X |
| 3.2 | | Bylaws of AgeX Therapeutics, Inc. | | 10-12(b) | | 6/8/2018 | | | | | | | | X |
| 3.3 | | Amended and Restated Certificate of Incorporation of Serina Therapeutics, Inc. | | | | | | | | X | | | | |
| 3.4 | | Second Amended and Restated Bylaws of Serina Therapeutics, Inc. | | | | | | | | X | | | | |
| 4.1 | | Specimen of Common Stock Certificate AgeX Therapeutics, Inc. | | 10-12(b) A-2 | | 8/30/2018 | | 4.1 | | | | | | X |
| 4.2 | | Form of Warrant included in Warrant Agreement dated March 30, 2020. | | 10-K | | 3/30/2020 | | 10.25 | | | | | | X |
| Exhibit | | | | Incorporated by
Reference | | Filed | | To be Filed by | | Previously |
| Number | | Exhibit Description | | Form | | Date | | Number | | Herewith | | Amendment | | Filed |
| 4.3 | | Form of Warrant included in Warrant Agreement dated February 14, 2022. | | 8-K | | 2/15/2022 | | 4.1 | | | | | | X |
| 4.4 | | Description of Securities of AgeX Therapeutics, Inc. | | | | | | | | | | | | X |
| 4.5 | | Certificate of Designation of Series A Preferred Stock | | 8-K | | 7/21/2023 | | 4.1 | | | | | | X |
| 4.6 | | Certificate of Designation of Series B Preferred Stock | | 8-K | | 7/21/2023 | | 4.2 | | | | | | X |
| 4.7 | | Amended and Restated Certificate of Designation of Series A Preferred Stock | | | | | | | | | | X | | |
| 4.8 | | Amended and Restated Certificate of Designation of Series B Preferred Stock | | | | | | | | | | X | | |
| 5.1 | | Opinion of Gibson, Dunn & Crutcher LLP | | | | | | | | | | X | | |
| 10.1# | | Asset Contribution and Separation Agreement dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-Q | | 11/9/2017 | | 10.1 | | | | | | X |
| 10.2# | | License Agreement, dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-Q | | 11/9/2017 | | 10.2 | | | | | | X |
| 10.3+ | | AgeX Therapeutics, Inc. 2017 Equity Incentive Plan. | | S-8 | | 1/30/2019 | | 99.1 | | | | | | X |
| 10.4+ | | Form of AgeX Therapeutics, Inc. Employee Stock Option Agreement. | | S-8 | | 1/30/2019 | | 99.2 | | | | | | X |
| 10.5+ | | Form of AgeX Therapeutics, Inc. Non-Employee Director Stock Option Agreement. | | S-8 | | 1/30/2019 | | 99.3 | | | | | | X |
| 10.6+ | | Form of AgeX Therapeutics, Inc. Restricted Stock Agreement. | | S-8 | | 1/30/2019 | | 99.4 | | | | | | X |
| 10.7+ | | Form of AgeX Therapeutics, Inc. Restricted Stock Unit Agreement. | | S-8 | | 1/30/2019 | | 99.5 | | | | | | X |
| 10.8# | | Sublicense Agreement, dated September 26, 2017, between Lineage Cell Technology, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.7 | | | | | | X |
| 10.9 | | First Amendment, dated November 8, 2017, to License Agreement, dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.8 | | | | | | X |
| 10.10# | | Sublicense Agreement, dated August 17, 2017, by and among OrthoCyte Corporation, Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.9 | | | | | | X |
| 10.11 | | First Amendment, dated November 8, 2017, to Sublicense Agreement, dated August 17, 2017, between OrthoCyte Corporation, Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.10 | | | | | | X |
| 10.12# | | License Agreement, dated August 17, 2017, by and between ES Cell International Ptd Ltd., Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.11 | | | | | | X |
| 10.13+ | | Employment Agreement, by and between AgeX Therapeutics, Inc. and Hal Sternberg, dated August 21, 2017. | | 10-12(b) | | 6/8/2018 | | 10.17 | | | | | | X |
| 10.14 | | Tax Matters Agreement, dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.15 | | | | | | X |
| Exhibit | | | | Incorporated by
Reference | | Filed | | To be Filed by | | Previously |
| Number | | Exhibit Description | | Form | | Date | | Number | | Herewith | | Amendment | | Filed |
| 10.15 | | Form of Registration Rights Agreement. | | 10-12(b) A-1 | | 7/19/2018 | | 10.16 | | | | | | X |
| 10.16# | | License Agreement, dated August 17, 2017, between Lineage Cell Therapeutics, Inc. and AgeX Therapeutics, Inc. | | 10-12(b) A-1 | | 7/19/2018 | | 10.17 | | | | | | X |
| 10.17+ | | Employment Agreement, by and between AgeX Therapeutics, Inc. and Michael D. West, dated October 18, 2018. | | 10-12(b) A-3 | | 10/22/2018 | | 10.19 | | | | | | X |
| 10.18 | | Registration Rights Agreement, dated August 13, 2019, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 10-Q | | 8/14/2019 | | 10.3 | | | | | | X |
| 10.19 | | Secured Convertible Facility Agreement, dated March 30, 2020, by and among AgeX Therapeutics, Inc., ReCyte Therapeutics, Inc., Reverse Bioengineering, Inc., and Juvenescence Limited. | | 10-K | | 3/30/2020 | | 10.24 | | | | | | X |
| 10.20 | | Warrant Agreement, dated March 30, 2020, between AgeX Therapeutics, Inc. and Juvenescence Limited, including form of warrant. | | 10-K | | 3/30/2020 | | 10.25 | | | | | | X |
| 10.21 | | Amendment No. 1 to Registration Rights Agreement, dated March 30, 2020, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 10-K | | 3/30/2020 | | 10.26 | | | | | | X |
| 10.22+ | | Employment Agreement, by and between AgeX Therapeutics, Inc. and Andrea E. Park, dated May 15, 2020. | | 10-Q | | 8/14/2020 | | 10.3 | | | | | | X |
| 10.23 | | First Amendment to Secured Convertible Facility Agreement, dated July 21, 2020, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 10-Q | | 8/14/2020 | | 10.1 | | | | | | X |
| 10.24 | | First Amendment to Warrant Agreement, dated July 21, 2020, between AgeX Therapeutics, Inc. and Juvenescence Limited | | 10-Q | | 8/14/2020 | | 10.2 | | | | | | X |
| 10.25 | | Second Amendment to Secured Convertible Facility Agreement, dated November 12, 2020, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 10-Q | | 11/16/2020 | | 10.1 | | | | | | X |
| 10.26 | | Amendment No. 2 to Registration Rights Agreement, dated February 10, 2021, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 2/11/2021 | | 10.2 | | | | | | X |
| 10.27 | | Amendment No. 2 to Loan Facility Agreement, dated November 8, 2021, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 11/9/2021 | | 10.1 | | | | | | X |
| 10.28+ | | Amendment to AgeX Therapeutics, Inc. 2017 Equity Incentive Plan. | | S-8 | | 1/4/2022 | | 99.1 | | | | | | X |
| 10.29† | | Secured Note dated February 14, 2022, executed by AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 2/15/2022 | | 10.1 | | | | | | X |
| Exhibit | | | | Incorporated by
Reference | | Filed | | To be Filed by | | Previously |
| Number | | Exhibit Description | | Form | | Date | | Number | | Herewith | | Amendment | | Filed |
| 10.30† | | Security Agreement, dated February 14, 2022, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 2/15/2022 | | 10.2 | | | | | | X |
| 10.31 | | Warrant Agreement, dated February 14, 2022, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 2/15/2022 | | 10.3 | | | | | | X |
| 10.32 | | Amendment No. 3 to Registration Rights Agreement, dated February 14, 2022, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 2/15/2022 | | 10.4 | | | | | | X |
| 10.33+ | | Amendment to AgeX Therapeutics, Inc. 2017 Equity Incentive Plan | | 8-K | | 12/12/2022 | | 10.1 | | | | | | X |
| 10.34† | | Amended and Restated Secured Convertible Promissory Note, dated February 9, 2023, executed by AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 2/10/2023 | | 10.1 | | | | | | X |
| 10.35 | | Reaffirmation Agreement, dated February 9, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 2/10/2023 | | 10.2 | | | | | | X |
| 10.36† | | Secured Convertible Promissory Note dated March 13, 2023, executed by AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 3/15/2023 | | 10.1 | | | | | | X |
| 10.37† | | Amended and Restated Security Agreement, dated March 13, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 3/15/2023 | | 10.2 | | | | | | X |
| 10.38† | | Convertible Note Purchase Agreement, dated March 15, 2023, between AgeX Therapeutics, Inc. and Serina Therapeutics, Inc. | | 8-K | | 3/15/2023 | | 10.3 | | | | | | X |
| 10.39 | | Convertible Promissory Note, dated March 15, 2023, between AgeX Therapeutics, Inc. and Serina Therapeutics, Inc. | | 8-K | | 3/15/2023 | | 10.4 | | | | | | X |
| 10.40† | | Subordination Agreement, dated March 15, 2023, between AgeX Therapeutics, Inc., Serina Therapeutics, Inc. and the other investors signatory thereto | | 8-K | | 3/15/2023 | | 10.5 | | | | | | X |
| 10.41† | | Third Amendment to that certain Secured Convertible Facility Agreement, dated March 30, 2020, by and between AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 3/15/2023 | | 10.6 | | | | | | X |
| 10.42 | | Allonge and Second Amendment to Amended and Restated Convertible Promissory Note dated May 9, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | 10-Q | | 5/12/2023 | | 10.9 | | | | | | X |
| 10.43 | | Third Amendment to Amended and Restated Secured Convertible Promissory Note, dated June 2, 2023, executed by AgeX Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 6/8/2023 | | 10.1 | | | | | | X |
| Exhibit | | | | Incorporated by
Reference | | Filed | | To be Filed by | | Previously |
| Number | | Exhibit Description | | Form | | Date | | Number | | Herewith | | Amendment | | Filed |
| 10.44 | | Exchange Agreement, dated July 21, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 7/21/2023 | | 10.1 | | | | | | X |
| 10.45 | | Registration Rights Agreement, dated July 21, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited | | 8-K | | 7/21/2023 | | 10.2 | | | | | | X |
| 10.46 | | Fourth Amendment to Amended and Restated Secured Convertible Promissory Note, executed by AgeX Therapeutics, Inc. and Juvenescence Limited on July 31, 2023. | | 8-K | | 8/4/2023 | | 10.1 | | | | | | X |
| 10.47 | | Amendment to Secured Convertible Promissory Note, executed by AgeX Therapeutics, Inc. and Juvenescence Limited on July 31, 2023. | | 8-K | | 8/4/2023 | | 10.2 | | | | | | X |
| 10.48+† | | Transition Services and Separation Agreement, dated August 9, 2023, between AgeX Therapeutics, Inc. and Michael D. West. | | 10-Q | | 8/14/2023 | | 10.7 | | | | | | X |
| 10.49+ | | Consulting Agreement, dated August 9, 2023, between AgeX Therapeutics, Inc. and Joanne Hackett | | 10-Q | | 8/14/2023 | | 10.8 | | | | | | X |
| 10.50 | | Form of AgeX Therapeutics, Inc. Stockholder Support Agreement. | | 8-K | | 8/30/2023 | | 10.1 | | | | | | X |
| 10.51 | | Form of Serina Therapeutics, Inc. Stockholder Support Agreement. | | 8-K | | 8/30/2023 | | 10.2 | | | | | | X |
| 10.52 | | Form of AgeX Therapeutics, Inc. Lock-Up Agreement. | | 8-K | | 8/30/2023 | | 10.3 | | | | | | X |
| 10.53 | | Form of Serina Therapeutics, Inc. Lock-Up Agreement. | | 8-K | | 8/30/2023 | | 10.4 | | | | | | X |
| 10.54 | | Letter Agreement, dated August 29, 2023, by and among AgeX Therapeutics, Inc., Serina Therapeutics, Inc. and Juvenescence Limited. | | 8-K | | 8/30/2023 | | 10.5 | | | | | | X |
| 10.55 | | Allonge and Fifth Amendment to Amended and Restated Convertible Promissory Note, dated November 9, 2023, between AgeX Therapeutics, Inc. and Juvenescence Limited. | | | | | | | | X | | | | |
| 10.56† | | Form of Pledge Agreement by AgeX Therapeutics, Inc. | | | | | | | | X | | | | |
| 10.57 | | Guaranty Agreement, dated November 9, 2023, between Reverse Bioengineering, Inc., ReCyte Therapeutics, Inc., UniverXome Bioengineering, Inc. and Juvenescence Limited. | | | | | | | | X | | | | |
| 10.58† | | Joinder Agreement, dated November 9, 2023, between Reverse Bioengineering, Inc., ReCyte Therapeutics, Inc., UniverXome Bioengineering, Inc., AgeX Therapeutics, Inc. and Juvenescence Limited. | | | | | | | | X | | | | |
| 21.1 | | List of Subsidiaries of AgeX Therapeutics, Inc. | | | | | | | | | | | | X |
| 23.1 | | Consent of WithumSmith+Brown, PC, independent registered public accounting firm of AgeX Therapeutics, Inc. | | | | | | | | X | | | | |
| 23.2 | | Consent of Frazier & Deeter, LLC, independent registered public accounting firm of Serina Therapeutics, Inc. | | | | | | | | X | | | | |
| 23.3 | | Consent of Gibson, Dunn & Crutcher LLP (included in Exhibit 5.1) | | | | | | | | | | X | | |
| 24.1 | | Power of Attorney (included on the signature page of this Registration Statement on Form S-4/S-1) | | | | | | | | X | | | | |
| 99.1 | | Form of AgeX Therapeutics, Inc. proxy card | | | | | | | | | | X | | |
| 99.2 | | Form of Certificate of Amendment to Certificate of Incorporation, as amended, of AgeX Therapeutics, Inc. — Reverse Stock Split (included as Annex E to the proxy statement/prospectus/information statement and incorporated herein by reference). | | | | | | | | | | X | | |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| † | Portions of this exhibit have been omitted in accordance with Item 601(b)(10) of Regulation S-K. |
| # | Confidential treatment has been granted with respect to portions of this exhibit (indicated by asterisks) and those portions have been separately filed by Lineage Cell Therapeutics, Inc. with the Securities and Exchange Commission. |
(b) Financial Statements
The financial statements filed with this registration statement on Form S-4/S-1 are set forth on the Index to Financial Statements and is incorporated herein by reference.
(d) Filing Fee
See Exhibit 107
Item 22. Undertakings
| (b) | The undersigned registrant hereby undertakes as follows: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | to include any prospectus required by Section 10(a)(3) of the Securities Act; |
| (ii) | to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement; notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of securities, in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (6) | That prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this registration statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form. |
| (7) | That every prospectus (i) that is filed pursuant to paragraph (7) immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (8) | To respond to requests for information that is incorporated by reference into this proxy statement/prospectus/information statement pursuant to Items 4, 10(b), 11, or 13 of this form, within one (1) business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means; this includes information contained in documents filed subsequent to the effective date of this registration statement through the date of responding to the request. |
| | |
| (9) | To supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in this registration statement when it became effective. |
| (10) | Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event a claim of indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in a successful defense of any action, suit or proceeding) is asserted by such director, officer, or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant to the requirements of the Securities Act, as amended, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on each of Form S-4 and Form S-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned thereunto duly authorized, in the City of Alameda, State of California, on this 14th day of November, 2023.
| | AGEX THERAPEUTICS, INC. |
| | |
| | By: | /s/ Andrea E. Park |
| | | Andrea E. Park |
| | | Chief Financial Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Joanne M. Hackett and Andrea E. Park as his or her true and lawful attorney-in-fact and agent, with full powers of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments and any related registration statements filed pursuant to Rule 462 and otherwise), and to file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent and full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that said attorney-in-fact and agent, or any substitute or resubstitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Joanne M. Hackett | | Interim Chief Executive Officer and Chair of the Board | | November 14, 2023 |
| JOANNE M. HACKETT, PH.D. | | (Principal Executive Officer) | | |
| | | | | |
| /s/ Andrea E. Park | | Chief Financial Officer | | November 14, 2023 |
| ANDREA E. PARK | | (Principal Financial and Accounting Officer) | | |
| | | | | |
| /s/ Gregory Bailey | | Director | | November 14, 2023 |
| GREGORY BAILEY, M.D. | | | | |
| | | | | |
| /s/ Michael H. May | | Director | | November 14, 2023 |
| MICHAEL H. MAY, PH.D. | | | | |
| | | | | |
| /s/ Jean-Christophe Renondin | | Director | | November 14, 2023 |
| JEAN-CHRISTOPHE RENONDIN, M.D. | | | | |




































