UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| |
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2024
OR
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-42279
BIOAGE LABS, INC.
(Exact Name of Registrant as Specified in its Charter)
| |
Delaware | 47-4721157 |
( State or other jurisdiction of incorporation or organization) | (I.R.S. Employer
Identification No.) |
1445A South 50th Street Richmond, CA | 94804 |
(Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (510) 806-1445
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Stock, par value $0.00001 per share | | BIOA | | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☐ No ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
Large accelerated filer | | ☐ |
| Accelerated filer |
| ☐ |
Non-accelerated filer | | ☒ |
| Smaller reporting company |
| ☒ |
Emerging growth company | | ☒ | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of October 31, 2024, the registrant had 35,848,738 shares of common stock, $0.00001 par value per share, outstanding.
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q (this Quarterly Report) contains forward-looking statements. All statements other than statements of historical facts contained in this Quarterly Report are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “aim,” “may,” “will,” “should,” “expect,” “forecast,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. All statements other than statements of historical fact contained in this Quarterly Report, including without limitation statements regarding our plans to develop and commercialize our product candidates, the timing and results of our ongoing or planned preclinical studies and clinical trials, risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, the timing of and our ability to obtain and maintain regulatory approvals, the clinical utility of our product candidates, our commercialization, marketing and manufacturing capabilities and strategy, our expectations about the willingness of healthcare professionals to use our product candidates, the sufficiency of our cash and cash equivalents, general economic, industry and market conditions, including fluctuating interest rates and inflation, a potential federal government shutdown, actual or perceived instability in the global banking system and changes in the U.S. presidential administration, and the plans and objectives of management for future operations and capital expenditures are forward-looking statements.
The forward-looking statements in this Quarterly Report are only predictions and are based largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this Quarterly Report and are subject to a number of known and unknown risks, uncertainties and assumptions, including those described under the sections in this Quarterly Report entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Quarterly Report.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. We intend the forward-looking statements contained in this Quarterly Report to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act.
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
BIOAGE LABS, INC.
Unaudited Condensed Consolidated Balance Sheets
(in thousands, except share and per share information)
| | | | | | | | |
| | September 30, | | | December 31, | |
| | 2024 | | | 2023 | |
Assets | | | | | | |
Current Assets: | | | | | | |
Cash and cash equivalents | | $ | 334,474 | | | $ | 21,644 | |
Restricted cash | | | — | | | | 3,313 | |
Prepaid expenses and other current assets | | | 1,993 | | | | 349 | |
Total current assets | | | 336,467 | | | | 25,306 | |
Investments | | | 100 | | | | 100 | |
Property and equipment, net | | | 543 | | | | 323 | |
Operating right-of-use assets, net | | | 271 | | | | 195 | |
Total assets | | $ | 337,381 | | | $ | 25,924 | |
Liabilities | | | | | | |
Current Liabilities: | | | | | | |
Accounts payable | | $ | 2,098 | | | $ | 1,866 | |
Accrued expenses and other current liabilities | | | 10,709 | | | | 7,938 | |
Current portion of term loan | | | 6,000 | | | | 6,000 | |
Operating lease liabilities, current | | | 273 | | | | 194 | |
Convertible promissory notes | | | — | | | | 20,674 | |
Convertible promissory notes embedded derivative liability | | | — | | | | 18,183 | |
Deferred grant income | | | — | | | | 3,313 | |
Total current liabilities | | | 19,080 | | | | 58,168 | |
Term loan | | | 3,940 | | | | 8,201 | |
Warrant liability | | | 613 | | | | 229 | |
Total liabilities | | | 23,633 | | | | 66,598 | |
Redeemable convertible preferred stock, par value of $0.00001, 31,634,362 shares authorized as of December 31, 2023, and 31,465,128 shares issued and outstanding as of December 31, 2023; aggregate liquidation preference of $131,864 as of December 31, 2023; no shares issued and outstanding as of September 30, 2024 | | | — | | | | 132,722 | |
Commitments and Contingencies (Note 8) | | | | | | |
Stockholders’ Equity (Deficit) | | | | | | |
Common stock, $0.00001 par value; 500,000,000 and 52,400,000 shares authorized as of September 30, 2024 and December 31, 2023, respectively; 34,196,821 and 1,673,314 shares issued and outstanding as of September 30, 2024 and December 31, 2023, respectively | | | — | | | | — | |
Preferred stock, $0.00001 par value; 10,000,000 shares authorized as of September 30, 2024; no shares issued and outstanding as of September 30, 2024; no shares authorized, issued, or outstanding as of December 31, 2023 | | | — | | | | — | |
Additional paid-in-capital | | | 545,321 | | | | 8,142 | |
Accumulated other comprehensive income | | | 109 | | | | 164 | |
Accumulated deficit | | | (231,682 | ) | | | (181,702 | ) |
Total stockholders’ equity (deficit) | | | 313,748 | | | | (173,396 | ) |
Total liabilities and stockholders’ equity (deficit) | | $ | 337,381 | | | $ | 25,924 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOAGE LABS, INC.
Unaudited Condensed Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share information)
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | Nine Months Ended | |
| | September 30, | | | September 30, | |
| | 2024 | | | 2023 | | | 2024 | | | 2023 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 20,019 | | | $ | 6,532 | | | $ | 39,811 | | | $ | 23,804 | |
General and administrative | | | 4,731 | | | | 3,355 | | | | 13,021 | | | | 11,000 | |
Total operating expenses | | | 24,750 | | | | 9,887 | | | | 52,832 | | | | 34,804 | |
Loss from operations | | | (24,750 | ) | | | (9,887 | ) | | | (52,832 | ) | | | (34,804 | ) |
Other income (expense), net: | | | | | | | | | | | | |
Interest expense | | | (388 | ) | | | (2,403 | ) | | | (2,048 | ) | | | (5,235 | ) |
Interest and other income | | | 2,037 | | | | 499 | | | | 5,534 | | | | 2,052 | |
Loss from changes in fair value of warrants and
derivative liabilities | | | (306 | ) | | | (2,834 | ) | | | (384 | ) | | | (4,909 | ) |
Loss on extinguishment of debt | | | — | | | | — | | | | (250 | ) | | | — | |
Total other income (expense), net | | | 1,343 | | | | (4,738 | ) | | | 2,852 | | | | (8,092 | ) |
Net loss | | $ | (23,407 | ) | | $ | (14,625 | ) | | $ | (49,980 | ) | | $ | (42,896 | ) |
Net loss per share attributable to common stockholders, basic
and diluted | | $ | (6.70 | ) | | $ | (8.74 | ) | | $ | (21.76 | ) | | $ | (25.64 | ) |
Weighted-average common shares outstanding, basic and dilutive | | | 3,494,580 | | | | 1,672,726 | | | | 2,297,397 | | | | 1,672,701 | |
Comprehensive loss: | | | | | | | | | | | | |
Net loss | | | (23,407 | ) | | | (14,625 | ) | | | (49,980 | ) | | | (42,896 | ) |
Foreign currency translation adjustment | | | 58 | | | | 35 | | | | 55 | | | | 67 | |
Total comprehensive loss | | $ | (23,349 | ) | | $ | (14,590 | ) | | $ | (49,925 | ) | | $ | (42,829 | ) |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOAGE LABS, INC.
Unaudited Condensed Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands, except share information)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Accumulated | | | | | | | |
| | Redeemable Convertible | | | | | | | | | | Additional | | | Other | | | | | | Total | |
| | Preferred Stock | | | | Common stock | | | Paid-In | | | Comprehensive | | | Accumulated | | | Stockholders' | |
| | Shares | | | Amount | | | | Shares | | | Amount | | | Capital | | | Income | | | Deficit | | | Equity (Deficit) | |
Balance, December 31, 2023 | | | 31,465,128 | | | $ | 132,722 | | | | | 1,673,314 | | | $ | — | | | $ | 8,142 | | | $ | 164 | | | $ | (181,702 | ) | | $ | (173,396 | ) |
Series D redeemable convertible preferred stock | | | 49,713,402 | | | | 169,458 | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Conversion of convertible promissory notes into Series D-1 redeemable convertible preferred stock | | | 11,887,535 | | | | 40,651 | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock upon exercise of options | | | — | | | | — | | | | | 280 | | | | — | | | | 3 | | | | — | | | | — | | | | 3 | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 852 | | | | — | | | | — | | | | 852 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | 21 | | | | — | | | | 21 | |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (12,992 | ) | | | (12,992 | ) |
Balance, March 31, 2024 | | | 93,066,065 | | | $ | 342,831 | | | | | 1,673,594 | | | $ | — | | | $ | 8,997 | | | $ | 185 | | | $ | (194,694 | ) | | $ | (185,512 | ) |
Issuance of common stock upon exercise of options | | | — | | | | — | | | | | 50,070 | | | | — | | | | 422 | | | | — | | | | — | | | | 422 | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 1,558 | | | | — | | | | — | | | | 1,558 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | (18 | ) | | | — | | | | (18 | ) |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (13,581 | ) | | | (13,581 | ) |
Balance, June 30, 2024 | | | 93,066,065 | | | $ | 342,831 | | | | | 1,723,664 | | | $ | — | | | $ | 10,977 | | | $ | 167 | | | $ | (208,275 | ) | | $ | (197,131 | ) |
Issuance of common shares through initial public offering, net of underwriting discounts, commissions, and issuance costs | | | — | | | | — | | | | | 11,000,000 | | | | — | | | | 179,623 | | | | — | | | | — | | | | 179,623 | |
Issuance of common shares through concurrent private placement, net of placement agent fee | | | — | | | | — | | | | | 588,888 | | | | — | | | | 9,858 | | | | — | | | | — | | | | 9,858 | |
Conversion of convertible preferred stock into common stock | | | (93,066,065 | ) | | | (342,831 | ) | | | | 20,854,632 | | | | — | | | | 342,831 | | | | — | | | | — | | | | 342,831 | |
Issuance of common stock upon exercise of options | | | — | | | | — | | | | | 29,637 | | | | — | | | | 184 | | | | — | | | | — | | | | 184 | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 1,848 | | | | — | | | | — | | | | 1,848 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | (58 | ) | | | — | | | | (58 | ) |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | $ | (23,407 | ) | | | (23,407 | ) |
Balance, September 30, 2024 | | | — | | | $ | — | | | | | 34,196,821 | | | $ | — | | | $ | 545,321 | | | $ | 109 | | | $ | (231,682 | ) | | $ | 313,748 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Accumulated | | | | | | | |
| | Redeemable Convertible | | | | | | | | | | Additional | | | Other | | | | | | Total | |
| | Preferred Stock | | | | Common stock | | | Paid-In | | | Comprehensive | | | Accumulated | | | Stockholders' | |
| | Shares | | | Amount | | | | Shares | | | Amount | | | Capital | | | Income | | | Deficit | | | Equity (Deficit) | |
Balance, December 31, 2022 | | | 31,465,128 | | | $ | 132,722 | | | | | 1,672,663 | | | $ | — | | | $ | 5,122 | | | $ | 167 | | | $ | (117,848 | ) | | $ | (112,559 | ) |
Issuance of common stock upon exercise of options | | | — | | | | — | | | | | 22 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 734 | | | | — | | | | — | | | | 734 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | (15 | ) | | | — | | | | (15 | ) |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (11,084 | ) | | | (11,084 | ) |
Balance, March 31, 2023 | | | 31,465,128 | | | $ | 132,722 | | | | | 1,672,685 | | | $ | — | | | $ | 5,856 | | | $ | 152 | | | $ | (128,932 | ) | | $ | (122,924 | ) |
Issuance of common stock upon
exercise of options | | | — | | | | — | | | | | 22 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 758 | | | | — | | | | — | | | | 758 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | 47 | | | | — | | | | 47 | |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (17,187 | ) | | | (17,187 | ) |
Balance, June 30, 2023 | | | 31,465,128 | | | $ | 132,722 | | | | | 1,672,707 | | | $ | — | | | $ | 6,614 | | | $ | 199 | | | $ | (146,119 | ) | | $ | (139,306 | ) |
Issuance of common stock upon
exercise of options | | | — | | | | — | | | | | 22 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | | — | | | | — | | | | 757 | | | | — | | | | — | | | | 757 | |
Foreign currency translation adjustment | | | — | | | | — | | | | | — | | | | — | | | | — | | | | 35 | | | | — | | | | 35 | |
Net loss | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | (14,625 | ) | | | (14,625 | ) |
Balance, September 30, 2023 | | | 31,465,128 | | | $ | 132,722 | | | | | 1,672,729 | | | $ | — | | | $ | 7,371 | | | $ | 234 | | | $ | (160,744 | ) | | $ | (153,139 | ) |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOAGE LABS, INC.
Unaudited Condensed Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | |
| | Nine Months Ended | |
| | September 30, | |
| | 2024 | | | 2023 | |
OPERATING ACTIVITIES | | | | | | |
Net loss | | $ | (49,980 | ) | | $ | (42,896 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Stock-based compensation expense | | | 4,258 | | | | 2,249 | |
Depreciation expense | | | 120 | | | | 121 | |
Loss on extinguishment of debt | | | 250 | | | | — | |
Non-cash interest expense | | | 987 | | | | 4,573 | |
Non-cash lease expense | | | 3 | | | | 2 | |
Loss from changes in fair value on derivative liability and warrants | | | 384 | | | | 4,910 | |
Changes in operating assets and liabilities: | | | | | | |
Prepaid expenses and other current assets | | | (1,644 | ) | | | (145 | ) |
Accounts payable | | | 132 | | | | (1,705 | ) |
Accrued expenses and other current liabilities | | | 2,419 | | | | 719 | |
Deferred grant income | | | (3,313 | ) | | | 3,313 | |
Net cash used in operating activities | | | (46,384 | ) | | | (28,859 | ) |
INVESTING ACTIVITIES | | | | | | |
Purchase of property and equipment | | | (340 | ) | | | (166 | ) |
Purchases of investments | | | — | | | | (100 | ) |
Net cash used in investing activities | | | (340 | ) | | | (266 | ) |
FINANCING ACTIVITIES | | | | | | |
Proceeds from issuance of convertible notes | | | — | | | | 23,500 | |
Issuance costs paid on convertible notes | | | — | | | | (46 | ) |
Proceeds from term loan | | | — | | | | 12,500 | |
Issuance costs paid on term loan | | | — | | | | (4 | ) |
Proceeds from Series D issuance | | | 170,000 | | | | — | |
Issuance costs paid on Series D issuance | | | (542 | ) | | | — | |
Proceeds from initial public offering, net of underwriting discounts and commissions | | | 184,140 | | | | — | |
Issuance costs paid on initial public offering and private placement | | | (3,268 | ) | | | |
Proceeds from issuance of common shares through private placement, net of placement agent fees | | | 9,858 | | | | — | |
Term loan principal payments | | | (4,500 | ) | | | — | |
Proceeds from issuance of common shares upon stock option exercises | | | 609 | | | | — | |
Net cash provided by financing activities | | | 356,297 | | | | 35,950 | |
Effect of changes in exchange rate on cash, cash equivalents, and restricted cash | | | (56 | ) | | | 65 | |
Net increase in cash, cash equivalents and restricted cash | | | 309,517 | | | | 6,890 | |
Cash, cash equivalents and restricted cash at beginning of period | | | 24,957 | | | | 27,644 | |
Cash, cash equivalents and restricted cash at end of period | | $ | 334,474 | | | $ | 34,534 | |
| | | | | | |
Supplemental non-cash disclosure: | | | | | | |
Unpaid equity issuance costs included in accounts payable and accrued expenses | | $ | 1,249 | | | $ | — | |
Conversion of convertible promissory notes into Series D-1 redeemable convertible preferred stock | | $ | 40,651 | | | $ | — | |
Conversion of redeemable convertible preferred stock into common stock upon initial public offering | | $ | 342,831 | | | $ | — | |
Cash paid for interest | | $ | 1,145 | | | $ | 687 | |
Right-of-use assets obtained in exchange for lease obligation | | $ | 282 | | | $ | 407 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOAGE LABS, INC.
Unaudited Notes to Condensed Consolidated Financial Statements
Note 1. Basis of Presentation
Nature of Business
BioAge Labs, Inc. (the “Company”), is a clinical-stage biotechnology company developing therapeutic product candidates for metabolic diseases, such as obesity. The Company’s lead product candidate, azelaprag, is an orally available small molecule that has been well-tolerated in over 265 individuals in seven Phase 1 clinical trials to date. The Company is also developing orally available, brain-penetrant inhibitors of NLRP3, a key driver of neuroinflammation, which is linked to many diseases including obesity.
The Company was incorporated in 2015 in the State of Delaware and is headquartered in Richmond, California.
On September 25, 2024, the Company completed its initial public offering (“IPO”) in which the Company issued and sold 11,000,000 shares of its common stock, at a public offering price of $18.00 per share and received approximately $179.6 million in net proceeds, after deducting underwriting discounts and commission of approximately $13.9 million and offering expenses of approximately $4.4 million.
On September 25, 2024, in a concurrent private placement with Sofinnova Venture Partners, XI, L.P., an existing stockholder, the Company issued and sold 588,888 shares of its common stock at a price of $18.00 per share and received approximately $9.9 million in net proceeds, after deducting placement agent fees of approximately $0.7 million.
Liquidity and Capital Resources
Since inception, the Company’s operations have consisted primarily of organizing and staffing the Company, business planning, raising capital, establishing its intellectual property portfolio, acquiring or discovering product candidates, research and development activities for its product candidates, establishing arrangements with third parties for the manufacture of its product candidates and component materials, and providing general and administrative support for these operations. The Company has not generated any product revenue to date.
The Company has incurred losses and negative cash flows from operations since inception and had an accumulated deficit of $231.7 million and $181.7 million as of September 30, 2024 and December 31, 2023, respectively. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant sales of its products currently in development. As of September 30, 2024, the Company had cash and cash equivalents of $334.5 million. On October 1, 2024, the underwriters of the Company's IPO elected to exercise in full their option to purchase 1,650,000 additional shares of the Company's common stock at the IPO price of $18.00 per share. The Company received approximately $27.6 million in net proceeds, after deducting underwriter fees of approximately $2.1 million.
Current cash and cash equivalents are sufficient to fund planned operations for at least one year after the date these condensed consolidated financial statements are issued. Accordingly, these condensed consolidated financial statements have been prepared on a going concern basis and do not include any adjustments to the amounts and classification of assets and liabilities that may be necessary in the event the Company can no longer continue as a going concern.
Until such time, if ever, the Company can generate substantial product revenues, it expects to finance its cash needs through equity offerings, debt financings or other capital sources, which could include collaborations, strategic alliances or licensing arrangements. To the extent that the Company raises additional capital through the sale of equity or convertible debt securities, the ownership interests of its existing stockholders may be diluted, and the terms of these securities may include liquidation or other preferences that could adversely affect the rights of such stockholders. Debt financing, if available, may involve agreements that include restrictive covenants that limit the Company’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, that could adversely impact the Company’s ability to conduct its business. If the Company raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, the Company may have to relinquish valuable rights to the Company’s technologies, future revenue streams, research program or product candidates, or grant licenses on terms that may not be favorable to the Company. If the Company is unable to raise additional funds through equity or debt financings when needed, the Company may be required to delay, limit, reduce or terminate its product development or future commercialization efforts or grant rights to develop and market product candidates that the Company would otherwise prefer to develop and market itself.
Note 2. Basis of Presentation and Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying condensed consolidated financial statements have been prepared in conformity with United States of America generally accepted accounting principles (“GAAP”) and applicable rules and regulations of the Securities and Exchange Commission (the “SEC”) regarding annual financial reporting. Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) promulgated by the Financial Accounting Standards Board (“FASB”). The condensed consolidated financial statements include the accounts of BioAge Labs, Inc. and its wholly owned subsidiary, BioAge Labs PTY LTD. BioAge Labs PTY LTD was incorporated in Australia in December 2020. All intercompany accounts and transactions have been eliminated in consolidation.
Reverse Stock Split
On September 17, 2024, the Company amended its amended and restated certificate of incorporation in order to effect a 1-for-4.4626 reverse stock split of its outstanding shares of common stock (the “Reverse Stock Split”). As a result of the Reverse Stock Split, every 4.4626 shares of the Company’s common stock issued or outstanding were automatically reclassified into one new share of common stock, subject to the treatment of fractional shares as described below, without any action on the part of the holders. All historical share and per-share amounts reflected throughout the accompanying condensed consolidated financial statements have been retroactively adjusted to reflect the Reverse Stock Split as if the split occurred as of the earliest period presented. The Reverse Stock Split did not affect the number of authorized shares of common stock or the par value of the common stock. No fractional shares were issued in connection with the Reverse Stock Split.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the condensed consolidated financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates.
Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the condensed consolidated financial statements in the period they are determined to be necessary. Areas that require management’s estimates include the fair values of common and redeemable convertible preferred stock (prior to the IPO), warrant liability, embedded derivative liability, stock-based compensation expense assumptions, valuation of deferred tax assets, and accruals for research and development expenses.
Foreign Currency
Results of foreign operations are translated from their functional currency into U.S. dollars (reporting currency) using average exchange rates in effect during the year while assets and liabilities are translated into U.S. dollars using exchange rates in effect at the balance sheet date. The resulting foreign currency translation adjustments are recorded in accumulated other comprehensive income (loss). Transaction gains and losses resulting from exchange rate changes on transactions denominated in currencies other than the U.S. dollar are included in operations in the period in which the transaction occurs.
Segments
The Company operates and manages its business as one reportable and operating segment, which is the business of extending healthy human life by targeting molecular causes of aging. The Company’s Chief Executive Officer, who is the chief operating decision maker, reviews financial information on an aggregate basis for allocating and evaluating financial performance. All long-lived assets are maintained in, and all losses are attributable to, the United States of America.
Cash, Cash Equivalents, and Restricted Cash
The Company considers all highly liquid investments that have original maturities of three months or less when acquired to be cash equivalents. Cash and cash equivalents as of September 30, 2024 and December 31, 2023 consisted of bank deposits and money market mutual funds invested in short-term U.S. government obligations. As of December 31, 2023 the Company had $3.3 million in restricted cash related to the Wellcome Leap Commercial Research Funding Agreement (Note 9). As of September 30, 2024, the Company did not have any restricted cash.
Concentrations of Credit Risk
Cash and cash equivalents are financial instruments that are potentially subject to concentrations of credit risk to the extent they exceed the federal depository insurance limits. The Company is exposed to credit risk in the event of default by the financial institutions holding its cash and cash equivalents to the extent recorded in the balance sheets. While the Company has not experienced any losses in such accounts, the failure of Silicon Valley Bank (“SVB”) in 2023, at which the Company held cash and cash equivalents in multiple accounts, potentially exposed the Company to significant credit risk. The Federal Deposit Insurance Corporation (“FDIC”) issued a statement on March 13, 2023 that they intended to take action to fully protect SVB depositors, which they did on March 27, 2023, by making SVB a division of First Citizens Bank. As of the date of the issuance of these condensed consolidated financial statements, the Company has full access to and control over all of its cash and cash equivalents. The Company has no financial instruments with off-balance sheet risk of loss.
Risks and Uncertainties
The Company faces risks and uncertainties associated with companies in the biotechnology industry, including but not limited to the uncertainty of success of its preclinical studies and clinical trials, regulatory approval of product candidates, uncertainty of market acceptance of products, competition from substitute products and larger companies, the need for additional financing, compliance with government regulations, dependence on third parties, recruiting and retaining skilled personnel, and dependence on key members of management.
The Company’s product candidates require approvals from the U.S. Food and Drug Administration (“FDA”) and comparable foreign regulatory agencies prior to commercial sales in their respective jurisdictions. There can be no assurance that any product candidates will receive the necessary approvals. If the Company was denied approval, approval was delayed or the Company was unable to maintain approval for any product candidate, it could have a materially adverse impact on the Company.
Property and Equipment, Net
Property and equipment, net is carried at cost less accumulated depreciation. Depreciation is computed over the estimated useful lives of the respective assets using the straight-line method. Useful lives of property and equipment range from three to five years. Operating lease leasehold improvements are amortized over the lesser of the useful lives of the leasehold improvements or the lease term. Upon retirement or sale, the costs of the assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is credited or charged to operations. Maintenance and repairs are expensed as incurred. Asset improvements are capitalized.
Impairment of Long-lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows which the assets are expected to generate. If such assets are considered to be impaired, the impairment is measured by the excess of the carrying amount of the assets over fair value less the costs to sell the assets, generally determined using the projected discounted future net cash flows arising from the asset. The Company did not recognize any impairment of long-lived assets during the nine months ended September 30, 2024 or the year ended December 31, 2023.
Redeemable Convertible Preferred Stock
The Company records redeemable convertible preferred stock net of issuance costs on the date of issuance, which represents the carrying value. Redeemable convertible preferred stock is classified outside of stockholders’ deficit as temporary equity on the accompanying condensed consolidated balance sheets as events triggering the liquidation preferences, including a deemed liquidation event, are not solely within the Company’s control. As of December 31, 2023, the Company had not remeasured redeemable convertible preferred stock.
Upon the closing of the IPO, all of the Company’s outstanding shares of redeemable convertible preferred stock automatically converted into 20,854,632 shares of common stock. As of September 30, 2024, the Company had no redeemable convertible preferred stock outstanding.
Convertible Promissory Notes and Embedded Derivative Liability
Convertible promissory notes are recorded at the issued value. Debt discount and issuance costs, consisting of legal and other fees directly related to the debt, are offset against gross proceeds from the issuance of the convertible promissory notes and are amortized to
interest expense over the life of the debt based on the effective interest method. Amortization expense is presented in interest expense in the condensed consolidated statement of operations and comprehensive loss.
The Company reviews the terms of its convertible promissory notes to determine whether there are conversion features or embedded derivative instruments including embedded conversion options that are required to be bifurcated and accounted for separately as a derivative financial instrument. In circumstances where the convertible promissory notes contain more than one embedded derivative instrument, including conversion options that are required to be bifurcated, the bifurcated derivative instruments are accounted for as a single compound instrument. When convertible debt contains embedded derivative instruments that are to be bifurcated and accounted for separately, the total proceeds allocated to the convertible host instruments are first allocated to the fair value of the bifurcated derivative instrument. The remaining proceeds, if any, are then allocated to the convertible instruments themselves, usually resulting in those instruments being recorded at a discount from their face amount.
As of December 31, 2023, the Company had bifurcated embedded derivatives related to its convertible promissory notes that are accounted for separately as derivative liabilities. Derivative liabilities are initially recorded at fair value and subsequently revalued at each reporting date with changes in fair value recognized separately on the condensed consolidated statement of operations and comprehensive loss. Derivative liabilities are presented separately in the condensed consolidated balance sheet. The Convertible Promissory Notes and related embedded derivative liability converted into Series D-1 Redeemable Convertible Preferred Stock on February 1, 2024.
Term Loan
Term loans are measured at net proceeds less debt discounts and issuance costs, which are accreted to the face value of the term loan over its expected term using the effective interest method. The Company considers whether there are any embedded features in its debt instruments that require bifurcation and separate accounting as derivative financial instruments pursuant to ASC Topic 815, Derivatives and Hedging (Note 5).
Warrant Liability
Freestanding warrants for the Company’s common stock are classified as liabilities and recorded at fair value, with any change in fair value recognized as a component of other income (loss). Such warrant liabilities are subject to re-measurement at each balance sheet date until the earlier of the exercise of the warrants, expiration, or the completion of a change in control event. Upon exercise, the warrant liability would be reclassified to additional paid-in capital, at its then fair value.
Research and Development Expenses
Research and development costs are expensed as incurred and include all direct and indirect costs associated with the development of the Company’s product candidates and other research programs. These expenses consist primarily of personnel costs, stock-based compensation charges, consulting fees, and payments to third parties for research, development, and manufacturing services as well as other allocated facility-related costs and overhead expenses. Nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities are capitalized and expensed as the goods are delivered or the related services are performed.
Accrued Research and Development Expenses
The Company records accruals for estimated costs of research, preclinical studies, clinical trials, and manufacturing, which are significant components of research and development expenses. A substantial portion of the Company’s ongoing research and development activities is conducted by third-party service providers, clinical research organizations (“CROs”), and clinical manufacturing organizations (“CMOs”). The Company’s contracts with CROs generally include pass-through fees such as laboratory supplies and services, regulatory expenses, investigator fees, travel costs and other miscellaneous costs, including shipping and printing fees. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided to the Company under such contracts. The Company accrues the costs incurred under agreements with these third parties based on estimates of actual work completed in accordance with the respective agreements. The Company determines the estimated costs through discussions with internal personnel and external service providers as to the progress, or stage of completion or actual timeline (start-date and end-date) of the services and the agreed-upon fees to be paid for such services. In the event the Company makes advance payments, the payments are recorded as a prepaid expense and recognized as the services are performed.
As actual costs become known, including subsequent to the reporting date, the Company adjusts its accruals. Although the Company does not expect its estimates to be materially different from amounts actually incurred, such estimates for the status and timing
of services performed relative to the actual status and timing of services performed may vary and could result in the Company reporting amounts that are too high or too low in any particular period. The Company’s accrual is dependent, in part, upon the receipt of timely and accurate reporting from CROs and other third-party vendors. Variations in the assumptions used to estimate accruals including, but not limited to, the number of patients enrolled, the rate of patient enrollment and the actual services performed, may vary from the Company’s estimates, resulting in adjustments to clinical trial expenses in future periods. Changes in these estimates that result in material changes to the Company’s accruals could materially affect its financial condition and results of operations.
Acquired In-Process Research and Development Expenses
Acquired in-process research and development expense consists of payments incurred in connection with the acquisition or licensing of products or technologies that do not meet the definition of a business under FASB ASC Topic 805, Business Combinations. Costs incurred in obtaining technology licenses including upfront and milestone payments incurred under licensing agreements are recorded as expense in the period in which they are incurred, provided that the licensed technology, method or process has no alternative future uses other than for the specific research and development activities. Such payments are classified as cash flows from operating activities in the Company’s condensed consolidated statements of cash flows. Milestone payments within the Company’s licensing arrangements are recognized when achievement of the milestone payment is legally due and payable. To the extent products are commercialized and future economic benefit has been established, commercial milestones that become probable are capitalized and amortized over the estimated remaining useful life of the intellectual property. In addition, the Company accrues royalty expense and sublicense nonroyalty payments, as applicable, for the amount it is obligated to pay, with adjustments as sales are made.
Stock-Based Compensation
The Company’s stock-based compensation program allows for grants of stock options and restricted stock awards. Grants are awarded to employees and non-employees, including directors.
Compensation cost for the Company’s stock-based payments to employees, non-employees and directors, are based on estimated fair value of the awards on the date of grant. The Company estimates the fair value of options granted using the Black-Scholes option pricing model for stock option grants to both employees and non-employees.
The Company’s stock-based compensation awards are subject to service-based vesting conditions. Compensation expense related to awards to employees, directors and non-employees with service-based vesting conditions is recognized on a straight-line basis based on the vesting date fair value over the associated service period of the award, which is generally the vesting term.
Income Taxes
Income taxes are accounted for under the asset and liability method in accordance with ASC Topic 740, Income Taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and the operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured at the balance sheet date using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Deferred tax assets are reduced by a valuation allowance if it is more likely than not that these assets may not be realized. The Company determines whether it is more-likely-than-not that a tax position will be sustained upon examination. If it is not more-likely-than-not that a position will be sustained, none of the benefit attributable to the position is recognized. The tax benefit to be recognized for any tax position that meets the more-likely-than-not recognition threshold is calculated as the largest amount that is more than 50% likely of being realized upon resolution of the contingency. The Company accounts for interest and penalties related to uncertain tax positions as part of its provision for income taxes. No interest or penalties were charged to the Company related to uncertain tax positions for the nine months ended September 30, 2024 or year ended December 31, 2023.
Leases
The Company determines if an arrangement is a lease at the inception of the arrangement. Operating leases are included in right-of-use assets, current portion of operating lease liability, and operating lease liability, net of current portion in the accompanying condensed consolidated balance sheets. Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease right-of-use assets and liabilities are recognized at the lease commencement date based on the present value of lease payments over the lease term. In determining the present value of lease payments, the Company uses its incremental borrowing rate based on the information available at the lease commencement date. The operating lease right-of-use assets also include any lease payments made and exclude lease incentives. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise any such options. Lease expense is recognized on a straight-line basis over the expected lease term. The Company
has elected not to separate lease and non-lease components, such as common area maintenance charges, and instead it accounts for these as a single lease component. Leases with an initial term of 12 months or less are not recorded on the balance sheet, unless they include an option to purchase the underlying asset or to extend the lease that the Company is reasonably certain to exercise.
Comprehensive Loss
Comprehensive loss is defined as a change in equity of a business enterprise during a period, resulting from transactions from non-owner sources. Comprehensive loss is comprised of net loss and other comprehensive income (loss). The Company’s other comprehensive loss consists of foreign currency translation adjustments. Total comprehensive loss for all periods presented has been disclosed in the condensed consolidated statements of operations and comprehensive loss.
Net Loss Per Share Attributable to Common Stockholders
Basic net loss per share attributable to common stockholders is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period, without consideration for potentially dilutive securities.
Diluted net loss per share attributable to common stockholders is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common stock and potentially dilutive securities outstanding for the period. For purposes of the diluted net loss per share attributable to common stockholders’ calculation, redeemable convertible preferred stock, stock options, and warrants are considered to be potentially dilutive securities.
The Company applies the two-class method to calculate its basic and diluted net loss per share attributable to common stockholders as the Company has issued shares that meet the definition of participating securities. The two-class method is an earnings allocation formula that treats a participating security as having rights to earnings that otherwise would have been available to common stockholders. Participating securities consist of common stock and redeemable convertible preferred stock. The Company’s participating securities contractually entitle the holders of such shares to participate in dividends, but do not contractually require the holders of such shares to participate in losses of the Company. Accordingly, in periods in which the Company reports a net loss, such losses are not allocated to such participating securities.
Accordingly, in periods in which the Company reports a net loss, diluted net loss per share attributable to common stockholders is the same as basic net loss per share attributable to common stockholders, since dilutive common shares are not assumed to have been issued if their effect is anti-dilutive.
Fair Value of Financial Instruments
GAAP establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company.
Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances.
Fair value is established as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, an established three-tier fair value hierarchy distinguishes between the following:
•Level 1 inputs are quoted prices in active markets that are accessible at the market date for identical assets or liabilities.
•Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly.
•Level 3 inputs are unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the assets or liability. Financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value instrument.
The carrying amounts of the Company’s other current assets, accounts payable, accrued expenses and other current liabilities reported in the condensed consolidated financial statements approximate their fair values due to their short-term nature.
Recent Accounting Pronouncements Not Yet Adopted
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, to improve its income tax disclosure requirements. Under the ASU, entities must annually (i) disclose specific categories in the rate reconciliation, (ii) provide additional information for reconciling items that meet a quantitative threshold, and (iii) disclose more detailed information about income taxes paid, including by jurisdiction; pretax income (or loss) from continuing operations; and income tax expense (or benefit). The ASU is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not expect this update to have a material impact on its condensed consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure of incremental segment information on an annual and interim basis. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024 on a retrospective basis. The Company is currently evaluating the potential impact that this standard may have on its condensed consolidated financial statements and related disclosures.
Note 3. Fair Value Measurements
The following fair value hierarchy table presents information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | |
| | September 30, 2024 | |
| | (Level 1) | | | (Level 2) | | | (Level 3) | | | Total | |
Assets: | | | | | | | | | | | | |
Cash equivalents | | $ | 334,181 | | | $ | — | | | $ | — | | | $ | 334,181 | |
Liabilities: | | | | | | | | | | | | |
Warrant liability | | $ | — | | | $ | — | | | $ | 613 | | | $ | 613 | |
| | | | | | | | | | | | | | | | |
| | December 31, 2023 | |
| | (Level 1) | | | (Level 2) | | | (Level 3) | | | Total | |
Assets: | | | | | | | | | | | | |
Cash equivalents | | $ | 21,061 | | | $ | — | | | $ | — | | | $ | 21,061 | |
Liabilities: | | | | | | | | | | | | |
Convertible promissory notes embedded derivative liability | | $ | — | | | $ | — | | | $ | 18,183 | | | $ | 18,183 | |
Warrant liability | | | — | | | | — | | | | 229 | | | | 229 | |
Total liabilities | | $ | — | | | $ | — | | | $ | 18,412 | | | $ | 18,412 | |
As of September 30, 2024, the Company revalued the warrant liability using its publicly traded stock price under the Black-Scholes valuation method. The valuation of warrant liabilities as of September 30, 2024 resulted in an increase in warrant liability of $0.4 million from $0.2 million as of December 31, 2023 to $0.6 million as of September 30, 2024.
Cash Equivalents
Cash equivalents include U.S. government obligation money market mutual funds that have a maturity of three months or less from the original acquisition date. The Company’s cash equivalents are classified using Level 1 inputs within the fair value hierarchy because they are valued using quoted market prices.
Convertible Promissory Notes Embedded Derivative Liability
The Company’s Convertible Promissory Notes (as defined in Note 5) contained equity conversion options, and certain repayment features, that were identified as a single compound embedded derivative requiring bifurcation from the Convertible Promissory Notes. The Company estimated the fair value of the convertible promissory note embedded derivative liabilities on issuance using a with-and-without scenario analysis. The estimated probability and timing of underlying events triggering the conversion and liquidity repayment features as well as discount rates, volatility and share prices are inputs used to determine the estimated fair value of the embedded derivative. The Convertible Promissory Notes and related embedded derivative liability converted into Series D-1 Redeemable Convertible Preferred Stock on February 1, 2024.
Warrant Liability
As of September 30, 2024, warrants representing 31,690 shares of common stock were outstanding. These warrants are classified as a liability since the warrants meet the classification requirements for liability accounting pursuant to ASC 815. This liability is subject to remeasurement at each balance sheet date until the warrants are exercised or expire, and any change in fair value is recognized in the Company’s condensed statements of operations. The Company classifies the warrant liability within Level 3 of the fair value hierarchy as the assessed fair value is based on both observable and unobservable market inputs including the Company's stock price, risk-free rate, and volatility.
Note 4. Balance Sheet Components
Property and Equipment
Property and equipment consisted of the following (in thousands):
| | | | | | | | |
| | September 30, | | | December 31, | |
| | 2024 | | | 2023 | |
Lab equipment | | $ | 652 | | | $ | 366 | |
Computer equipment and software | | | 323 | | | | 323 | |
Furniture and fixtures | | | 53 | | | | 53 | |
Construction-in-progress | | | 55 | | | | — | |
Property and equipment, gross | | $ | 1,083 | | | $ | 742 | |
Accumulated depreciation | | | (540 | ) | | | (419 | ) |
Property and equipment, net | | $ | 543 | | | $ | 323 | |
Depreciation expense was less than $0.1 million for each of the three months ended September 30, 2024 and 2023 and $0.1 million for each of the nine months ended September 30, 2024 and 2023.
Accrued Expenses and Other Current Liabilities
Accrued expenses consisted of the following (in thousands):
| | | | | | | | |
| | September 30, | | | December 31, | |
| | 2024 | | | 2023 | |
Research and development expenses | | $ | 6,006 | | | $ | 2,516 | |
Payroll and related costs | | | 2,967 | | | | 4,033 | |
Other | | | 1,736 | | | | 1,389 | |
Total accrued expenses and other current liabilities | | $ | 10,709 | | | $ | 7,938 | |
Note 5. Debt
Convertible Promissory Notes
In February 2023, the Company issued four convertible promissory notes with an aggregate principal amount of $23.5 million. Each note has an interest rate of 4% per annum and a maturity date of May 10, 2024 (the “Convertible Promissory Notes”). The notes and any accrued but unpaid interest were convertible at either the date of a qualified financing of at least $20.0 million (a “Qualified Financing”), or on the maturity date, at the option of the respective holder, and are convertible into the same securities issued in the Qualified Financing, or if no qualified financing occurs prior to maturity, then shall be convertible into the Company’s Series C Preferred Stock.
Upon a Qualified Financing, the Convertible Promissory Notes automatically convert into shares of the Company’s redeemable convertible preferred stock on the same conditions applicable for the Qualified Financing at a conversion price equal to the lowest price per share paid in the Qualified Financing multiplied by a discount factor ranging from 0.6 to 1.0 depending on the timing of the Qualified Financing.
On February 1, 2024, in connection with the closing of the Series D redeemable convertible preferred stock financing, the Convertible Promissory Notes (including accrued interest) and related embedded derivative liability converted into 11,887,535 shares of Series D-1 redeemable convertible preferred stock at a discount factor of 0.6 relative to the price paid by the Series D investors. The conversion resulted in a $0.3 million loss on extinguishment of the Convertible Promissory Notes.
Term Loan
In May 2022, the Company entered into a loan and security agreement (the “Loan Agreement”) with SVB Innovative Credit Growth Fund IX, LP and Innovative Credit Growth Fund VIII-A, LP, (collectively, the “Lenders”) pursuant to which the Company was eligible to borrow, and the Lenders are obligated to fund up to $25.0 million in borrowing capacity across two potential tranches (the “Term Loan”). At the closing of the Loan Agreement in May 2022, the Company drew $2.5 million from the first tranche (the “Initial Term Loan”) and in May 2023 the Company drew $12.5 million from the second tranche (the “Additional Term Loan”).
In connection with the Initial Term Loan of $2.5 million, the Company issued to the Lenders warrants to purchase 19,420 shares of the Company’s common stock. The warrants expire on May 20, 2032 and had a fair value of $125,602 at issuance. Similarly, in connection with the Additional Term Loan draw, the Company issued an additional warrant to purchase 5,548 shares of the Company’s common stock. The warrants expire on May 20, 2032 and had a fair value of $37,050 at issuance. As a result, proceeds from the debt equal to the fair value were allocated to these warrants and are amortized as part of the debt discount over the life of the Term Loan.
Interest for the Term Loan accrues at a floating per annum rate equal to the greater of (i) the Prime rate plus 4.00% or (ii) 7.50%. Interest is due monthly on the first business day of each month, commencing in June 2022. The Term Loan is scheduled to mature on April 1, 2026 and commencing on November 1, 2023 the Company is required to make monthly principal payments. The Company may prepay all of the outstanding principal balance of the Term Loan, at its option, prior to the maturity date subject to a prepayment premium of 1.0%. The prepayment premium will apply to any mandatory or voluntary prepayment. In addition, the Company will also be required to pay a final payment fee equal to 4.4% of the total amount borrowed.
The Company’s obligations under the Loan Agreement are subject to acceleration upon the occurrence of customary events of default, including payment default, insolvency and the occurrence of certain events having a material adverse effect on the Company, including (but not limited to) material adverse effects upon the business, operations, properties, assets or financial condition of the Company and its subsidiaries, taken as a whole.
The Loan Agreement includes positive and negative covenants that the Company must comply with and is secured by the assets of the Company that are pledged as collateral.
Debt issuance costs, including the fair value of the warrants, have been treated as debt discounts in the condensed consolidated balance sheet and together with the final payment are being amortized to interest expense throughout the life of the Term Loan using the effective interest rate method. As of September 30, 2024 and December 31, 2023, there were unamortized issuance costs and debt discounts of less than $0.1 million, which are recorded as a direct deduction from the Term Loan in the condensed consolidated balance sheet. Interest expense related to the Loan Agreement was $1.4 million and $0.9 million for the nine months ended September 30, 2024 and 2023, respectively. As of September 30, 2024 and December 31, 2023, the stated rate on the Term Loan was 12.0% and 12.5%, respectively. As of September 30, 2024, the effective interest rate on the Term Loan, including the amortization of the debt discount and accretion of the final payment, was 16.4% for the Initial Term Loan and 14.8% Additional Term Loan. The carrying amount of the Term Loan is subject to variable interest rates, which are based on current market rates, and as such, approximate fair value.
The components of the Term Loan balance were (in thousands):
| | | | |
| | September 30, 2024 | |
Principal loan balance | | $ | 9,500 | |
Final fee | | | 487 | |
Unamortized debt discount | | | (47 | ) |
Total Term Loan | | $ | 9,940 | |
Less current portion of Term loan | | | (6,000 | ) |
Term loan | | $ | 3,940 | |
As of September 30, 2024, the estimated future principal payments under the Term Loan are as follows (in thousands):
| | | | |
Year ending December 31, 2024 | | Total Principal
Payments | |
2024 (excluding the nine months ended September 30, 2024) | | $ | 1,500 | |
2025 | | | 6,000 | |
2026 | | | 2,000 | |
Principal amount of Term Loan | | $ | 9,500 | |
Note 6. Capital Structure
Upon the closing of the IPO, all of the Company’s outstanding shares of redeemable convertible preferred stock automatically converted into 20,854,632 shares of common stock.
There are 500,000,000 and 52,400,000 shares of common stock authorized as of September 30, 2024 and December 31, 2023, respectively. In connection with the IPO, the Company's board of directors authorized 10,000,000 shares of preferred stock. As of September 30, 2024, there were no preferred shares issued and outstanding. The Company had 34,196,821 and 1,673,314 shares of common stock issued and outstanding as of September 30, 2024 and December 31, 2023. Common stock reserved for future issuance, on an as-if-converted basis, as of September 30, 2024 and December 31, 2023, consisted of the following:
| | | | | | | | |
| | September 30,
2024 | | | December 31,
2023 | |
Redeemable convertible preferred stock, issued and outstanding | | | — | | | | 7,050,825 | |
Stock options, issued and outstanding | | | 5,248,778 | | | | 2,364,083 | |
Stock options, authorized for future issuance | | | 3,139,534 | | | | 614,041 | |
Warrants, issued and outstanding | | | 31,690 | | | | 31,690 | |
Total common stock reserved for future issuance | | | 8,420,002 | | | | 10,060,639 | |
Note 7. Stock-Based Compensation
Stock Option Plans
Under the terms of its stock option plans, the Company’s board of directors may grant stock options to employees, directors and consultants. The Company issued stock options under the 2015 Equity Incentive Plan, as amended (the “2015 plan”) until September 2024, when the 2024 Equity Incentive Plan (“2024 Plan”) was adopted. There are no remaining shares available to be granted under the 2015 Plan. There were 4,738,311 stock options outstanding under the 2015 Plan as of September 30, 2024.
The 2024 Plan authorizes the award of incentive stock options ("ISOs"), which are intended to qualify for tax treatment under Section 422 of the U.S. Internal Revenue Code of 1986, as amended, and nonqualified stock options, Restricted Stock Awards, Stock Appreciation Rights, Restricted Stock Units, (each as defined in the 2024 Plan), performance awards and stock bonus awards. The 2024 Plan initially reserved 3,650,000 shares of the Company's common stock, which includes any reserved shares not issued or subject to outstanding grants under the 2015 Plan on the effective date of the 2024 Plan, for issuance pursuant to awards granted under our 2024 Plan. The number of shares reserved for issuance under the 2024 Plan will increase automatically on January 1 of each of the first ten calendar years during the term of the 2024 Plan by the number of shares equal to the lesser of 5% of the aggregate number of all classes of the Company's common stock and the total number of shares of the Company's common stock subject to any pre-funded warrants, in each case, as issued and outstanding as of the immediately preceding December 31, or a number as may be determined by the Company's
board of directors. Pursuant to the 2024 Plan, ISOs may be granted only to employees of the Company. The Company may grant all other types of awards to its employees, directors and consultants.
As of September 30, 2024, 3,139,534 shares were available for future grants under the 2024 Plan. The Plan permits the granting of incentive stock options, non-statutory stock options, stock awards, and stock purchase rights. The terms of the agreements are determined by the board of directors. The Company’s stock options have a maximum term of 10 years and vest based on the terms in the agreements, generally over 4 years.
Employee Stock Purchase Plan
In September 2024, the Company adopted the 2024 Employee Stock Purchase Plan (the "2024 ESPP"). The 2024 ESPP enables eligible employees to purchase shares of the Company's common stock with accumulated payroll deductions. The Company has initially reserved 330,000 shares of its common stock for sale under the 2024 ESPP. The aggregate number of shares issued over the term of the 2024 ESPP, subject to stock-splits, recapitalizations or similar events, may not exceed 3,300,000 shares of the Company's common stock. As of September 30, 2024, there were a total of 330,000 shares available for future purchase under the 2024 ESPP.
The following table summarizes the stock option activity for the nine months ended September 30, 2024:
| | | | | | | | | | | | | | | | | | | | |
| | Shares
Available to
Grant | | | Number of
Options | | | Weighted-
Average
Exercise
Price | | | Weighted-
Average
Remaining
Contractual
Life (Years) | | | Aggregate
Intrinsic
Value (in
thousands) | |
Balance-December 31, 2023 | | | 614,041 | | | | 2,364,083 | | | $ | 8.38 | | | | 7.4 | | | $ | 2,864 | |
Change in authorized shares | | | 5,490,175 | | | | — | | | | — | | | | | | | |
Granted | | | (3,016,584 | ) | | | 3,016,584 | | | | 10.23 | | | | | | | |
Exercised | | | — | | | | (79,987 | ) | | | 7.62 | | | | | | | |
Forfeited/expired | | | 51,902 | | | | (51,902 | ) | | | 7.31 | | | | | | | |
Balance-September 30, 2024 | | | 3,139,534 | | | | 5,248,778 | | | $ | 9.47 | | | | 8.4 | | | $ | 59,491 | |
Vested and Exercisable-September 30, 2024 | | | | | | 1,951,828 | | | $ | 8.08 | | | | 6.7 | | | $ | 24,833 | |
The fair value of the stock options that were exercised during the nine months ended September 30, 2024 and 2023 were $1.0 million and less than $0.1 million, respectively.
The grant date fair value of stock options granted during the nine months ended September 30, 2024 and 2023 was $10.23 and $10.85, respectively, and were estimated on the date of grant using a Black-Scholes option pricing model with the following weighted-average assumptions:
| | | | | | | | |
| | Nine months ended
September 30, | |
| | 2024 | | | 2023 | |
Weighted-average risk-free interest rate | | | 4.4 | % | | | 3.8 | % |
Expected term of stock options (in years) | | 6.0 years | | | 6.0 years | |
Weighted-average expected stock price volatility | | | 109.9 | % | | | 91.8 | % |
Estimated dividend yield | | | — | | | | — | |
Stock-based compensation expense recorded as research and development and general and administrative expenses in the statements of operations and comprehensive loss is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended
September 30, | | | Nine Months Ended
September 30, | |
| | | 2024 | | | 2023 | | | 2024 | | | 2023 | |
Research and development | | | $ | 555 | | | $ | 270 | | | $ | 1,331 | | | $ | 819 | |
General and administrative | | | | 1,293 | | | | 487 | | | | 2,927 | | | | 1,430 | |
Total stock-based compensation expense | | | $ | 1,848 | | | $ | 757 | | | $ | 4,258 | | | $ | 2,249 | |
As of September 30, 2024, there was $27.2 million of unrecognized compensation cost that is expected to be recognized over a weighted average period of 3.3 years.
Note 8. Commitments and Contingencies
Indemnification
The Company entered into indemnification agreements with directors and certain officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. No demands have been made upon the Company to provide indemnification under such agreements, and thus, there are no claims that the Company is aware of that could have a material effect on the condensed consolidated financial statements. The Company also maintains director and officer insurance, which may cover certain liabilities arising from the Company’s obligation to indemnify its directors and officers. To date, the Company has not incurred any costs and have not accrued any liabilities in the condensed consolidated financial statements as a result of these provisions.
Legal Proceedings
The Company is not a party to any litigation and does not have contingency reserves established for any litigation liabilities.
Employee Benefit Plan
The Company maintains a defined contribution 401(k) plan, under which employee contributions are voluntary and are determined on an individual basis, limited by the maximum amounts allowable under federal tax regulations. The Company provides an automatic matching contribution of employee contributions into the plan up to a maximum of 4% of employee deferral. The Company’s matching contributions to employees were $0.1 million for each of the three months ended September 30, 2024 and 2023 and $0.4 million for each of the nine months ended September 30, 2024 and 2023.
Leases
In August 2017, the Company entered into an agreement to lease approximately 6,436 square feet of office and lab space in Richmond California, which the Company uses for its corporate offices and research facility (the “Richmond Lease”). The Richmond lease had an initial term of three years but was amended in October 2017 and August 2019 to add additional space for a total of 18,829 square feet and to extend the term of the lease through February 2023. In January 2023, the Company entered into an amendment which extended the term of the lease through August 2024. In March 2024, the Company entered into an amendment which extended the term of the lease through August 2025. The Richmond Lease includes escalating rent payments but does not provide for any renewal options. The Company recognizes rent expense on a straight-line basis over the lease term. The Richmond lease does not provide a bargain purchase option nor does it transfer ownership at any point during the lease to the Company and is classified as an operating lease.
As of September 30, 2024, the remaining lease term was 0.9 years and the discount rate used to determine the operating leases liability was 12.5%.
Cash paid for amounts included in the measurement of operating lease liabilities was $0.2 million for the nine months ended September 30, 2024 and 2023, respectively, and was included in net cash used in operating activities in the Company’s condensed consolidated statement of cash flows.
Future Minimum rental payments of $0.1 million and $0.2 million will be made in 2024 and 2025, respectively. Rent expense was $0.1 million for each of the three months ended September 30, 2024 and 2023 and $0.3 million for each of the nine months ended September 30, 2024 and 2023. Variable lease payments related to operating leases for the nine months ended September 30, 2024 and 2023 were not material.
On September 4, 2024, the Company executed a new lease agreement (“Emeryville Lease”) with a projected commencement in January 2025 and an initial term of 6 years. The annual base rent is $0.7 million per year, subject to an annual upward adjustment of 3.0%. The lease commencement date, for accounting purposes, was not reached as of September 30, 2024 and therefore the lease is not included in the Company’s operating lease right-of-use asset or operating lease liabilities as of September 30, 2024.
Note 9. Wellcome Leap Commercial Research Funding Agreement
In September 2023, the Company entered into a Commercial Research Funding Agreement with Wellcome Leap, Inc. (the “Wellcome Leap Agreement”) in which Wellcome Leap was to fund certain research and development work performed by the Company. In connection with the Wellcome Leap Agreement, the Company entered into a statement of work in which the Company was to evaluate Azelaprag’s efficacy at preventing muscle atrophy and frailty during hospitalization in chronic obstructive pulmonary disease (“COPD”) patients through a Phase 2 clinical trial (the “COPD Trial”).
Also, in September 2023, Wellcome Leap made a payment of $3.3 million to the Company to cover costs to be incurred related to the COPD Trial (the “Grant Funds”). As the Grant Funds are maintained in a separate bank account from the Company’s other funds and are only to be expended on the COPD Trial, it was determined that the Grant Funds represented restricted cash and are classified as such in the condensed consolidated balance sheet as of December 31, 2023.
In March 2024, the Company informed Wellcome Leap that it planned to terminate the COPD Trial due to concerns regarding commercial feasibility and on May 31, 2024, the Company and Wellcome Leap terminated the Wellcome Leap Agreement (the “Wellcome Leap Termination”). In connection with the Wellcome Leap Termination, the Company returned $2.4 million of unused Grant Funds received to Wellcome Leap in June 2024.
Note 10. Income Taxes
The Company estimates an annual effective tax rate of 0% for the year ending December 31, 2024 as the Company incurred losses for the nine months ended September 30, 2024 and expects to continue to incur losses through the remainder of fiscal year ending December 31, 2024, resulting in an estimated net loss for both financial statement and tax purposes for the year ending December 31, 2024. Therefore, no federal, state or foreign income taxes are expected and none have been recorded at this time.
Due to the Company’s history of losses since inception, there is not enough evidence at this time to support that the Company will generate future income of a sufficient amount and nature to utilize the benefits of its net deferred tax assets. Accordingly, the deferred tax assets have been reduced by a full valuation allowance, since the Company does not currently believe that realization of its deferred tax assets is more likely than not.
On September 30, 2024, the Company had no unrecognized tax benefits that would reduce the Company’s effective tax rate if recognized.
Note 11. Net Loss Per Share Attributable to Common Stockholders
The following table sets forth the computation of the basic and diluted net loss per share attributable to common stockholders (in thousands except for share and per share data):
| | | | | | | | | | | | | | | | |
| | Three months ended
September 30, | | | Nine months ended
September 30, | |
| | 2024 | | | 2023 | | | 2024 | | | 2023 | |
Numerator: | | | | | | | | | | | | |
Net loss | | $ | (23,407 | ) | | $ | (14,625 | ) | | $ | (49,980 | ) | | $ | (42,896 | ) |
Denominator: | | | | | | | | | | | | |
Weighted-average shares of common stock outstanding used to compute net loss per share attributable to common stockholders,
basic and diluted | | | 3,494,580 | | | | 1,672,726 | | | | 2,297,397 | | | | 1,672,701 | |
Net loss per share attributable to common stockholders, basic and diluted: | | $ | (6.70 | ) | | $ | (8.74 | ) | | $ | (21.76 | ) | | $ | (25.64 | ) |
The Company’s potentially dilutive securities have been excluded from the computation of diluted net loss per share attributable to common stockholders as the effect would be antidilutive. Therefore, the weighted-average number of shares of common stock outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same. Potentially dilutive securities that were not included in the diluted per share calculations because they would be anti-dilutive were as follows:
| | | | | | | | |
| | September 30,
2024 | | | September 30,
2023 | |
Series A-1 redeemable convertible preferred stock on an as if converted basis | | | — | | | | 1,065,172 | |
Series A-2 redeemable convertible preferred stock on an as if converted basis | | | — | | | | 660,615 | |
Series A-3 redeemable convertible preferred stock on an as if converted basis | | | — | | | | 45,673 | |
Series A-4 redeemable convertible preferred stock on an as if converted basis | | | — | | | | 6,194 | |
Series B redeemable convertible preferred stock on an as if converted basis | | | — | | | | 1,670,599 | |
Series C redeemable convertible preferred stock on an as if converted basis | | | — | | | | 3,602,572 | |
Stock options, issued and outstanding | | | 5,248,778 | | | | 2,382,765 | |
Warrants to purchase common stock | | | 31,690 | | | | 31,690 | |
Total | | | 5,280,468 | | | | 9,465,280 | |
Note 12. Subsequent Events
On October 1, 2024, the underwriters of the Company's IPO elected to exercise in full their option to purchase 1,650,000 additional shares of the Company's common stock at the IPO price of $18.00 per share. The Company received approximately $27.6 million in net proceeds, after deducting placement agent fees of approximately $2.1 million.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our condensed consolidated financial statements and the related notes and other financial information included elsewhere in this Quarterly Report and with our audited financial statements and the notes thereto for the year ended December 31, 2023 included in a final prospectus dated September 25, 2024 filed with the Securities and Exchange Commission pursuant to Rule 424(b) under the Securities Act of 1933, as amended. This discussion and analysis and other parts of this Quarterly Report contain forward-looking statements based upon our current plans and expectations that involve risks, uncertainties and assumptions, such as statements regarding our plans, objectives, expectations, intentions and beliefs. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the section titled “Risk Factors” and elsewhere in this Quarterly Report. You should carefully read the section titled “Risk Factors” to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Special Note Regarding Forward-Looking Statements.”
Overview
We are a clinical-stage biopharmaceutical company developing therapeutic product candidates for metabolic diseases, such as obesity, by targeting the biology of human aging. Our technology platform and differentiated human datasets enable us to identify promising targets based on insights into molecular changes that drive aging. Our primary focus is metabolic disease, one of the greatest global healthcare challenges. Azelaprag, our lead product candidate, is an orally available small molecule that has been well-tolerated in 265 individuals across eight Phase 1 clinical trials. In preclinical obesity models, azelaprag demonstrated the ability to more than double the weight loss induced by a glucagon-like-peptide-1 receptor (GLP-1R) agonist while also restoring normal body composition and muscle function. These preclinical results are supported by our Phase 1b clinical trial in older adults on bed rest where we observed decreased muscle atrophy, preservation of muscle quality and improved metabolism in subjects treated with azelaprag over a 10-day period. We plan to assess azelaprag’s potential to drive significant improvements in weight loss when combined with a GLP-1R agonist in two Phase 2 clinical trials. While the results of these preclinical studies and early clinical trials have demonstrated the potential use of azelaprag for the treatment of metabolic disease, they may not be predictive of the results of later-stage clinical trials. The ongoing STRIDES clinical trial will assess azelaprag in combination with tirzepatide, marketed as Zepbound® by Eli Lilly (Lilly), with topline results anticipated in the third quarter of 2025. The STRIDES 2 clinical trial will assess azelaprag in combination with semaglutide, marketed as Wegovy® by Novo Nordisk, with initiation expected in the first half of 2025 and topline results expected in the second half of 2026. We believe these trials will directly support our ultimate therapeutic goal of developing an all-oral combination product for obesity. We also intend to initiate the STRIDES T2D Phase 2 clinical trial of azelaprag monotherapy in type 2 diabetes in the first half of 2025 with topline results anticipated in the second half of 2025. We are also developing orally-available small molecule brain-penetrant NLRP3 inhibitors for the treatment of diseases driven by neuroinflammation. We anticipate submitting an Investigational New Drug application (IND) for an NLRP3 inhibitor in the second half of 2025 and, if cleared, initiating a Phase 1 clinical trial in the first half of 2026.
Our portfolio of product candidates is summarized in the figure below:
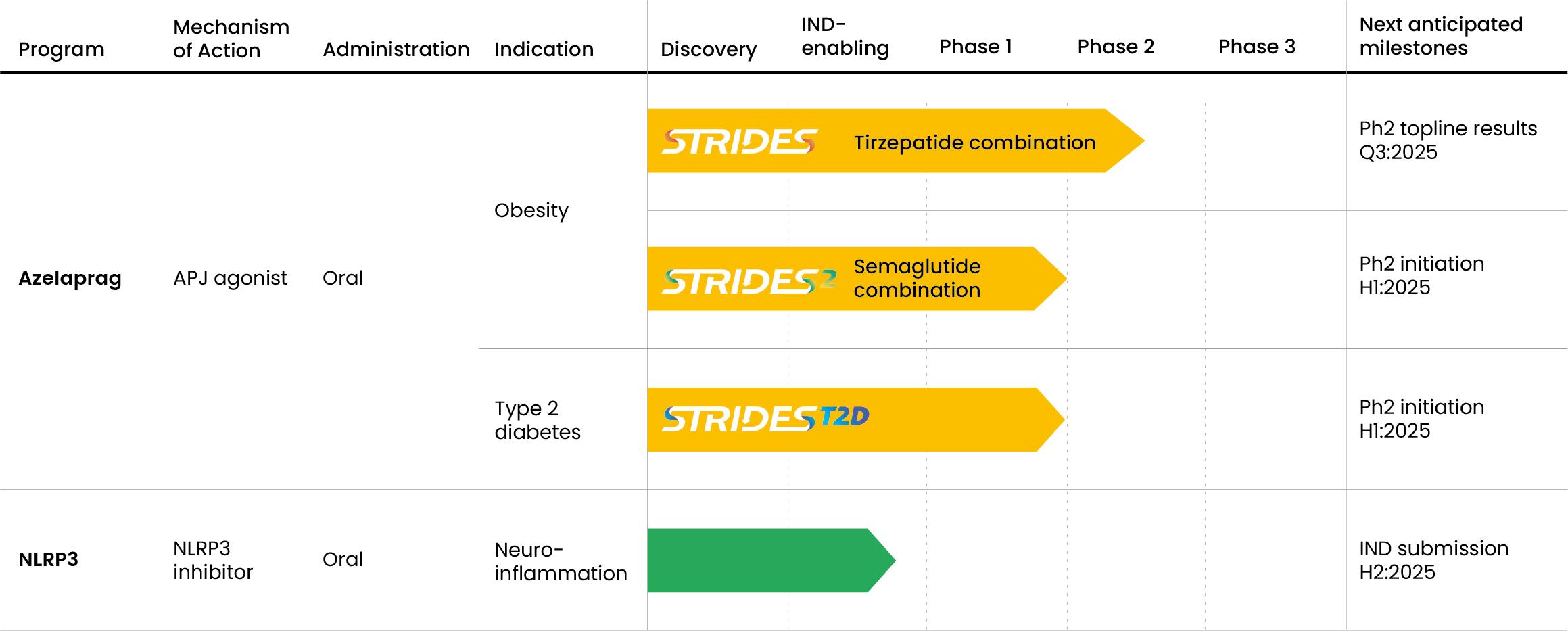
Since our inception in 2015, we have devoted substantially all of our efforts to organizing and staffing our company, business planning, raising capital, establishing our intellectual property portfolio, acquiring or discovering product candidates, research and development activities for our product candidates, establishing arrangements with third parties for the manufacture of our product candidates and component materials, and providing general and administrative support for these operations. We do not have any products approved for sale and have not generated any revenue from product sales. To date, we have financed our operations primarily with proceeds from sales of shares of our redeemable convertible preferred stock. From inception, through September 30, 2024, we have raised aggregate gross proceeds of approximately $529.5 million through the sale and issuance of our common stock, redeemable convertible preferred stock and convertible promissory notes. Our primary uses of capital are, and we expect will continue to be, research and development services, compensation and related expenses, and general overhead costs.
We have incurred significant operating losses and negative cash flows since inception. Our ability to generate product revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of azelaprag and any future product candidates. Our net losses were $50.0 million and $42.9 million for the nine months ended September 30, 2024 and 2023, respectively. As of September 30, 2024, we had an accumulated deficit of $231.7 million. We expect to continue to incur net operating losses for the foreseeable future, and we expect our research and development expenses, general and administrative expenses, and capital expenditures will increase substantially in connection with our ongoing activities, particularly if, and as, we:
•continue to progress the development of our lead product candidate, azelaprag;
•explore additional indications for our existing product candidates;
•discover and develop any future product candidates;
•obtain, expand, maintain, defend and enforce our intellectual property portfolio;
•manufacture, or have manufactured, preclinical, clinical and potentially commercial supplies of azelaprag and any future product candidates;
•seek regulatory approvals for azelaprag or any future product candidates that successfully complete clinical trials, if any;
•establish a sales, marketing and distribution infrastructure to commercialize azelaprag or any future product candidates, if approved;
•seek to identify, evaluate and establish licenses, collaborations or other strategic partnerships;
•hire additional clinical, scientific and management personnel, as well as administrative staff to support the growth of our business;
•add operational, financial and management information systems and personnel; and
•incur additional legal, accounting and other costs associated with operating as a public company.
Our net losses may fluctuate significantly from period to period, depending on the timing of factors above.
We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for a product candidate. In addition, if we obtain regulatory approval for a product candidate and do not enter into a third-party commercialization partnership, we expect to incur significant expenses related to developing our commercialization capability to support product sales, marketing, manufacturing and distribution activities.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, which could include licenses, collaborations, or other strategic partnerships. Adequate additional funds may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our existing stockholders may be diluted, and the terms of these securities may include liquidation or other preferences that could adversely affect the rights of such stockholders. Debt financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, which could adversely impact our ability to conduct our business. If we raise additional funds through licenses, collaborations, or other strategic partnerships with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research program or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. There is no assurance that we will ever be profitable or
generate positive cash flow from operating activities. Our ability to raise additional funds may also be adversely impacted by potential worsening global macroeconomic, industry and market conditions in either domestic or international markets, as well as economic conditions specifically affecting industries in which we operate, including but not limited to, actual or perceived instability in the banking industry, potential uncertainty with respect to the U.S. federal debt ceiling and budget and potential government shutdowns related thereto, labor shortages, supply chain disruptions, potential recession, inflation and changing interest rates and political instability and military hostilities in multiple geographies, such as the conflicts in Ukraine, the Middle East and tensions between China and Taiwan.
Because of the numerous risks and uncertainties associated with development of product candidates, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
We oversee and manage third party Contract Development and Manufacturing Organizations (CDMOs) to support development and manufacture of azelaprag for our preclinical and clinical trials. We expect to enter into commercial supply agreements with commercial manufacturers prior to any potential regulatory approval of azelaprag or any future product candidates. We continue to develop a commercial route for azelaprag manufacture in alignment with our program timeline. We believe our current manufacturers are able to supply the upcoming clinical trials and additional CDMOs may be on-boarded at later stages of clinical and commercial development.
As of September 30, 2024, we had $334.5 million in cash and cash equivalents. Based on our current operating plan, we estimate that our existing cash and cash equivalents as of the date of this Quarterly Report, together with the net proceeds received in October 2024 from the exercise in full of the option granted to the underwriters of our IPO to purchase additional shares of our common stock, will be sufficient to fund our operations and capital expenses into 2029. However, we have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. See the section titled "Liquidity and Capital Resources" included elsewhere in this Quarterly Report.
Exclusive License Agreement with Amgen, Inc.
On April 5, 2021, we entered into an exclusive license agreement (the Amgen Agreement) with Amgen Inc. (Amgen) pursuant to which Amgen granted us an exclusive, worldwide license, with the right to sublicense (subject to certain conditions), under Amgen’s rights in specified patents relating to Amgen’s proprietary compound, AMG 986 (azelaprag), a novel apelin J receptor agonist, to research, develop, and commercialize azelaprag in all diagnostic, preventative or therapeutic uses. Amgen also granted us a non-exclusive, worldwide license, with the right to sublicense (subject to certain conditions), under Amgen’s rights in specified know-how relating to azelaprag, including research reports, clinical data, manufacturing processes, regulatory documents, and other information pertaining to azelaprag, to research, develop, and commercialize azelaprag in all diagnostic, preventative or therapeutic uses. Although we maintain the exclusive rights described above with respect to the specified patents, Amgen retains research-only rights solely for Amgen’s internal research. All right, title and interest to inventions conceived or created by a party under the Amgen Agreement that are exclusively related to azelaprag will be owned exclusively by us, regardless of inventorship.
Under the Amgen Agreement, we are obligated to use commercially reasonable efforts to develop and commercialize at least one licensed product in each of the United States, European Union, Japan and the rest of the world (ROW). If we fail to materially develop or commercialize such products for twelve months in the United States, European Union, Japan, or ROW, and such failure is not due to reasons out of our control, in addition to other available remedies, Amgen may terminate our agreement with respect to the failing region, subject to a cure period.
In consideration for the rights granted under the Amgen Agreement, we paid an upfront fee of $1.0 million and issued Amgen 846,152 shares of our Series C redeemable convertible preferred stock which converted into 189,609 shares of our common stock in connection with the completion of our IPO. Additionally, we paid Amgen a $1.0 million development milestone in the nine months ended September 30, 2024 related to initiation of the first Phase 2 Clinical trial of azelaprag. We may also be required to pay up to an additional $119.0 million in the aggregate for future development, regulatory and commercial milestone payments, as well as tiered royalties at percentages ranging in the low- to upper-single digits on future net sales by us and our sublicensees of licensed products, if any. Royalties are paid on a product-by-product basis and commence with respect to a particular country upon the first commercial sale in such country and terminate in such country on the latest to occur of the date on which such product is no longer covered by a valid claim in such country, the loss of regulatory exclusivity for such product in such country, and for a specified time period after the first commercial sale of such product in such country. Such royalties may be decreased if, among other reasons, we are required to pay a third party for rights to intellectual property for the exploitation of a licensed product in a given country, but in no event be reduced in aggregate by a specified percentage.
The term of the Amgen Agreement will end on a licensed product-by-licensed product basis and country-by-country basis upon the expiration of our obligation to pay royalties to Amgen with respect to such licensed products in such countries. We may terminate the Amgen Agreement in its entirety for convenience upon a specified written notice period. Amgen has the right to terminate the agreement if we, or one of our affiliates or sublicensees, challenges the patentability, enforceability, or validity of a licensed patent, subject to a cure period. Additionally, either party will be able to terminate the Amgen Agreement for the other party’s uncured material breach or bankruptcy.
Components of Our Results of Operations
Revenue
We have not generated any product revenue since our inception and do not expect to generate any revenue from the sale of products or from other sources in the near future, if at all. If our development efforts for our lead product candidate, azelaprag or additional product candidates that we may develop in the future are successful and result in marketing approval or if we enter into collaboration or license agreements with third parties, we may generate revenue from a combination of product sales or payments from such collaboration or license agreements.
Operating Expenses
Our operating expenses consist of (i) research and development expenses and (ii) general and administrative expenses.
Research and Development Expense
Research and development expenses account for a significant portion of our operating expenses and consist primarily of costs incurred in connection with the discovery, preclinical development, clinical development and manufacturing of azelaprag and potential future product candidates, and include:
Direct Costs:
•expenses incurred under agreements with contract research organizations (CROs) that are primarily engaged in the oversight and conduct of our clinical trials; CDMOs that are primarily engaged to provide drug substance and product for our clinical trials, research and development programs, as well as investigative sites and consultants that conduct our clinical trials, preclinical studies and other scientific development services;
•the cost of acquiring and manufacturing preclinical and clinical trial materials, including manufacturing registration and validation batches;
•costs of outside consultants, including their fees and related travel expenses;
•costs related to compliance with quality and regulatory requirements; and
•payments made under third-party licensing agreements.
Indirect Costs:
•personnel-related expenses including, salaries, bonuses, benefits, stock-based compensation expenses and other related costs for individuals involved in research and development activities; and
•allocated facilities and other expenses not directly tied to a program.
We expense research and development costs as incurred. We recognize direct development costs based on an evaluation of the progress to completion of specific tasks using information provided to us by our vendors or our estimate of the level of service that has been performed at each reporting date. Payments for these development activities are based on the terms of the individual agreements, which may differ from the pattern of costs incurred, and are reflected in our financial statements as prepaid expenses or accrued expenses.
A significant portion of our research and development costs to date have been third-party direct costs, which we track on an individual product candidate basis after a product candidate progresses to the clinic. However, our indirect costs are not directly tied to any one program and are deployed across our programs. As such, we do not track these costs on a specific program basis. We utilize third party contractors for our research and development activities and CDMOs for our manufacturing activities and we do not have our own manufacturing facilities.
Research and development activities are central to our business model. We expect that our research and development expenses will continue to increase substantially for the foreseeable future as we advance azelaprag into multiple Phase 2 clinical trials, progress the NLRP3 inhibitors that we are developing for the treatment of neuroinflammation toward the submission of an IND application and into a Phase 1 clinical trial, continue to discover and develop additional product candidates, expand our headcount and costs related to our existing and potential future intellectual property licenses. Later stages of clinical development generally have higher development costs than those in earlier stages, primarily due to the increased size and duration of later-stage clinical trials. There are numerous factors associated with the successful development and commercialization of any product candidates we may develop in the future, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development. Additionally, future commercial and regulatory factors beyond our control will impact our clinical development program and plans.
Our research and development expenses may vary significantly in the future based on factors, such as:
•the number and scope of preclinical and IND-enabling studies;
•per patient trial costs;
•the number of trials required for approval;
•the number of sites included in the trials;
•the countries in which the trials are conducted;
•the length of time required to enroll eligible patients;
•the number of patients that participate in the trials;
•the drop-out or discontinuation rates of patients;
•potential additional safety monitoring requested by regulatory agencies;
•the duration of patient participation in the trials and follow-up;
•the cost and timing of manufacturing our product candidates;
•the phase of development of our product candidates;
•the efficacy and safety profile of our product candidates;
•the extent to which we establish additional collaboration or license agreements; and
•whether we choose to partner any of our product candidates and the terms of such partnership.
Changes in the outcome of any of these variables with respect to the development of azelaprag or any future product candidates in preclinical and clinical development could mean a significant change in the costs and timing associated with the development of these product candidates. For example, if the U.S. Food and Drug Administration, European Medicines Agency or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect, or if we experience significant delays in enrollment in any clinical trials following the applicable regulatory authority’s acceptance and clearance, we could be required to expend significant additional financial resources and time to complete clinical development than we currently expect. We may never obtain regulatory approval for any product candidates that we develop.
The successful development of azelaprag or any product candidates we may develop in the future is highly uncertain. Therefore, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the development and commercialization of azelaprag and any other product candidates we may develop. We are also unable to predict when,
if ever, material net cash inflows will commence from the sale of azelaprag or any future product candidate, if approved. This is due to the numerous risks and uncertainties associated with product development.
General and Administrative Expense
General and administrative expenses consist primarily of personnel-related expenses, including salaries, bonuses, benefits, and stock-based compensation expenses for individuals in executive, finance, corporate, business development, and administrative functions. Other significant general and administrative expenses include legal fees relating to patent, intellectual property and corporate matters, and fees paid for accounting, consulting and other professional services, and allocated expenses for rent, insurance and other operating costs.
We expect that our general and administrative expenses will continue to increase in the foreseeable future as our business expands to support our continued research and development activities, including any future clinical trials. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, among other expenses. We also anticipate increased expenses associated with being a public company, including costs for audit, legal, regulatory and tax-related services related to compliance with the rules and regulations of the SEC, listing standards applicable to companies listed on a national securities exchange, director and officer insurance premiums and investor relations costs. In addition, if we obtain regulatory approval for our current product candidate or any product candidates we may develop in the future and do not enter into a third-party commercialization collaboration, we expect to incur significant expenses related to building a sales and marketing team to support product sales, marketing and distribution activities.
Other (Income) Expense, Net
Interest Expense
Interest expense consists of interest incurred on both our convertible promissory notes and term loan.
Interest and Other Income
Interest and other income primarily consist of interest income generated from interest bearing cash accounts.
Gain (Loss) from Changes in Fair Value of Warrants and Derivative Liabilities
Gain (loss) on changes in fair value consists of assessed changes in fair value of liabilities carried at fair value, including warrants to purchase our common stock and the embedded derivative liability associated with our convertible promissory notes.
Loss on Extinguishment of Convertible Promissory Notes
Loss on extinguishment of convertible promissory notes consists of the difference between the carrying value of our convertible promissory notes (including accrued interest) and related embedded derivative liability and the fair value of shares issued upon conversion of our convertible promissory notes into our Series D-1 Redeemable Convertible Preferred Stock in February 2024.
Income Taxes
Since our inception, we have not recorded any income tax benefits for the net losses we have incurred in each period or for our research and development tax credits, as we believe, based upon the weight of available evidence, that it is more likely than not that all of our net operating loss carryforwards and tax credits will not be realized. As of December 31, 2023, we had U.S. federal and state net operating loss carryforwards of $83.8 million and $13.4 million, respectively, which expire at various dates beginning in 2035. These attributes may be subject to Section 382 limitation and we have not performed a formal assessment. As of September 30, 2024 and December 31, 2023, we have recorded a full valuation allowance against our deferred tax assets.
Results of Operations
Comparison of the Three Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations for each of the periods presented (in thousands, except percentages):
| | | | | | | | | | | | | | | | |
| | Three Months Ended
September 30, | | | | | | | |
| | 2024 | | | 2023 | | | $ Change | | | % Change | |
| | (unaudited) | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 20,019 | | | $ | 6,532 | | | $ | 13,487 | | | | 206 | % |
General and administrative | | | 4,731 | | | | 3,355 | | | | 1,376 | | | | 41 | % |
Total operating expenses | | | 24,750 | | | | 9,887 | | | | 14,863 | | | | 150 | % |
Loss from operations | | $ | (24,750 | ) | | $ | (9,887 | ) | | $ | (14,863 | ) | | | 150 | % |
Other income (expense), net: | | | | | | | | | | | | |
Interest expense | | | (388 | ) | | | (2,403 | ) | | | 2,015 | | | | -84 | % |
Interest and other income | | | 2,037 | | | | 499 | | | | 1,538 | | | | 308 | % |
Loss from changes in fair value on derivative liability and
warrants | | | (306 | ) | | | (2,834 | ) | | | 2,528 | | | | -89 | % |
Total other income (expense), net | | | 1,343 | | | | (4,738 | ) | | | 6,081 | | | | -128 | % |
Net loss | | $ | (23,407 | ) | | $ | (14,625 | ) | | $ | (8,782 | ) | | | 60 | % |
Research and Development Expenses
The following table summarizes our research and development expenses for each of the periods presented (in thousands, except percentages):
| | | | | | | | | | | | | | | | |
| | Three Months Ended
September 30, | | | | | | | |
| | 2024 | | | 2023 | | | $ Change | | | % Change | |
| | (unaudited) | | | | | | | |
Direct costs: | | | | | | | | | | | | |
azelaprag | | $ | 12,977 | | | $ | 1,021 | | | $ | 11,956 | | | | 1171 | % |
NLRP3 | | $ | 1,501 | | | $ | 1,439 | | | $ | 62 | | | | 4 | % |
Other programs | | $ | 1 | | | $ | 94 | | | $ | (93 | ) | | | -99 | % |
Indirect costs: | | | | | | | | | | | | |
Personnel-related expenses (including stock-based compensation
expense) | | | 4,153 | | | | 3,486 | | | | 667 | | | | 19 | % |
Allocated facility and other expenses | | | 1,387 | | | | 492 | | | | 895 | | | | 182 | % |
Total research and development expenses | | $ | 20,019 | | | $ | 6,532 | | | $ | 13,487 | | | | 206 | % |
Research and development expenses increased by $13.5 million from $6.5 million for the three months ended September 30, 2023 to $20.0 million for the three months ended September 30, 2024. The increase in research and development expenses was primarily attributable to a $12.0 million increase in costs related to the development of azelaprag driven by the ongoing Phase 2 STRIDES trial and costs related to the manufacture of azelaprag. Further contributing to the increase in research and development expenses was a $0.7 million increase in personnel-related expenses associated with an increase in stock-based compensation expense from option grants issued in 2024 to employees as well as an increase in recruiting fees associated with research and development personnel; and a $0.9 million increase in allocated facility and other expenses.
General and Administrative Expenses
General and administrative expenses increased by $1.3 million from $3.4 million for the three months ended September 30, 2023 to $4.7 million for the three months ended September 30, 2024. The increase was primarily attributable to an increase in stock-based compensation expense associated with option grants issued in 2024 to employees, executives, board members and advisors.
Other Income (Expense), Net
Other income (expense), net increased by approximately $6.0 million from $4.7 million of other expense for the three months ended September 30, 2023 to $1.3 million of other income for the three months ended September 30, 2024. This increase in other income was primarily attributable to a $1.5 million increase in interest income driven by our higher cash and cash equivalents balance, a $2.5 million decrease in losses from changes in fair value primarily related to the embedded derivative liability associated with our convertible promissory notes as these notes converted into Series D-1 redeemable convertible preferred stock in February 2024, and a $2.0 million decrease in interest expense as our convertible promissory notes converted into Series D-1 redeemable convertible preferred stock in February 2024.
Comparison of the Nine Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations for each of the periods presented (in thousands, except percentages):
| | | | | | | | | | | | | | | | |
| | Nine Months Ended
September 30, | | | | | | | |
| | 2024 | | | 2023 | | | $ Change | | | % Change | |
| | (unaudited) | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 39,811 | | | $ | 23,804 | | | $ | 16,007 | | | | 67 | % |
General and administrative | | | 13,021 | | | | 11,000 | | | | 2,021 | | | | 18 | % |
Total operating expenses | | | 52,832 | | | | 34,804 | | | | 18,028 | | | | 52 | % |
Loss from operations | | $ | (52,832 | ) | | $ | (34,804 | ) | | $ | (18,028 | ) | | | 52 | % |
Other income (expense), net: | | | | | | | | | | | | |
Interest expense | | | (2,048 | ) | | | (5,235 | ) | | | 3,187 | | | | -61 | % |
Interest and other income | | | 5,534 | | | | 2,052 | | | | 3,482 | | | | 170 | % |
Loss from changes in fair value on derivative liability and
warrants | | | (384 | ) | | | (4,909 | ) | | | 4,525 | | | | -92 | % |
Loss on extinguishment of convertible promissory notes | | | (250 | ) | | | - | | | | (250 | ) | | | -100 | % |
Total other income (expense), net | | | 2,852 | | | | (8,092 | ) | | | 10,944 | | | | -135 | % |
Net loss | | $ | (49,980 | ) | | $ | (42,896 | ) | | $ | (7,084 | ) | | | 17 | % |
Research and Development Expenses
The following table summarizes our research and development expenses for each of the periods presented (in thousands, except percentages):
| | | | | | | | | | | | | | | | |
| | Nine Months Ended
September 30, | | | | | | | |
| | 2024 | | | 2023 | | | $ Change | | | % Change | |
| | (unaudited) | | | | | | | |
Direct costs: | | | | | | | | | | | | |
azelaprag | | $ | 20,192 | | | $ | 3,653 | | | $ | 16,539 | | | | 453 | % |
NLRP3 | | $ | 3,573 | | | $ | 5,958 | | | | (2,385 | ) | | | -40 | % |
Other programs | | | 7 | | | | 514 | | | | (507 | ) | | | -99 | % |
Indirect costs: | | | | | | | | | | | | |
Personnel-related expenses (including stock-based compensation
expense) | | | 11,843 | | | | 10,151 | | | | 1,692 | | | | 17 | % |
Allocated facility and other expenses | | | 4,196 | | | | 3,528 | | | | 668 | | | | 19 | % |
Total research and development expenses | | $ | 39,811 | | | $ | 23,804 | | | $ | 16,007 | | | | 67 | % |
Research and development expenses increased by $16.0 million from $23.8 million for the nine months ended September 30, 2023 to $39.8 million for the nine months ended September 30, 2024. The increase was primarily attributable to a $16.5 million increase in costs related to the clinical development of azelaprag as it progressed toward Phase 2 trials in combination with a GLP-1R agonist and a $1.7 million increase in personnel-related expenses associated with an increase in stock-based compensation expense from option grants issued in 2024 to employees as well as an increase in recruiting fees associated with research and development personnel; partially offset by $2.4 million and $0.5 million decreases in direct costs related to the NLRP3 program and other programs, respectively, as we
have focused our development spend primarily on azelaprag and a $0.7 million decrease in allocated facility and other expenses primarily related to lab services.
General and Administrative Expenses
General and administrative expenses increased by $2.0 million from $11.0 million for the nine months ended September 30, 2023 to $13.0 million for the nine months ended September 30, 2024. The increase was primarily attributable to an increase in stock-based compensation expense associated with option grants issued in April 2024 to employees and executives.
Other Income (Expense), Net
Other income (expense), net increased by approximately $11.0 million from $8.1 million of other expense for the nine months ended September 30, 2023 to $2.9 million of other income for the nine months ended September 30, 2024. This increase in other income was primarily attributable to a $3.5 million increase in interest income driven by our higher cash and cash equivalents balance, a $4.5 million decrease in losses from changes in fair value primarily related to the embedded derivative liability associated with our convertible promissory notes as these notes converted into Series D-1 redeemable convertible preferred stock in February 2024, and a $3.2 million decrease in interest expense as our convertible promissory notes converted into Series D-1 redeemable convertible preferred stock in February 2024. These increases were partially offset by a $0.3 million loss on extinguishment of convertible promissory notes associated with conversion of the convertible promissory notes into Series D-1 redeemable convertible preferred stock in February 2024.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have incurred significant losses in each period and on an aggregate basis. We have not yet commercialized any product candidates, and we do not expect to generate revenue from sales of any product candidates or from other sources for the foreseeable future, if at all. As of September 30, 2024, we had $334.5 million in cash and cash equivalents, and we had an accumulated deficit of $231.7 million. From inception through September 30, 2024, we have raised aggregate gross proceeds of approximately $529.5 million through the sale and issuance of our common stock, redeemable convertible preferred stock and convertible promissory notes.
In May 2022, we entered into a loan and security agreement (the Loan Agreement) with SVB Innovative Credit Growth Fund IX, LP and Innovative Credit Growth Fund VIII-A, LP pursuant to which we were able to borrow up to an aggregate of $25.0 million across two potential tranches until December 31, 2023 (the Term Loan). The Loan Agreement has a floating interest rate of the higher of the Wall Street Journal Prime rate plus 4.00% or 7.5%. The amounts borrowed under the Loan Agreement are scheduled to mature on April 1, 2026 and commencing on November 1, 2023 we are required to make monthly principal payments. In addition, we will also be required to pay a final payment fee equal to 4.4% of the total amount borrowed. As of September 30, 2024, we had $9.5 million outstanding under the Loan Agreement. See Note 5 to our unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report for further discussion of the Loan Agreement.
Cash Flows
The following table provides information regarding our cash flows for each of the periods presented (in thousands):
| | | | | | | | |
| | Nine Months Ended
September 30, | |
| | 2024 | | | 2023 | |
| | (unaudited) | |
Net cash used in operating activities | | $ | (46,384 | ) | | $ | (28,859 | ) |
Net cash used in investing activities | | | (340 | ) | | | (266 | ) |
Net cash provided by financing activities | | | 356,297 | | | | 35,950 | |
Effects of exchange rate changes on cash, cash equivalents, and restricted cash | | | (56 | ) | | | 65 | |
Net increase in cash, cash equivalents, and restricted cash | | $ | 309,517 | | | $ | 6,890 | |
Net Cash Used in Operating Activities
Net cash used in operating activities for the nine months ended September 30, 2024 was $46.4 million, and was primarily due to our net loss of $50.0 million, which included non-cash charges of $4.3 million related to stock-based compensation expense, $1.0 million related to non-cash interest expense, $0.4 million related to losses from changes in fair value on warrants to purchase our current stock, and a $0.3 million loss on extinguishment of convertible promissory notes.
Net cash used in operating activities for the nine months ended September 30, 2023 was $28.9 million, and was primarily due to our net loss of $42.9 million, which included non-cash charges of $4.9 million related to losses from changes in fair value on warrants and derivative liabilities, $4.6 million of non-cash interest expense, and $2.2 million related to stock-based compensation expense.
Net Cash Used in Investing Activities
Net cash used in investing activities was $0.3 million for each of the nine months ended September 30, 2024 and 2023.
Net Cash Provided by Financing Activities
Net cash provided by financing activities during the nine months ended September 30, 2024 was $356.3 million, resulting from $180.9 million in net proceeds from our IPO, $9.8 million in net proceeds from the sale of our common stock through private a private placement transaction, $169.5 million in net proceeds from the issuance and sale of our Series D redeemable convertible preferred stock and $0.6 million in proceeds from stock option exercises partially offset by $4.5 million in principal payments on our Term Loan.
Net cash provided by financing activities during the nine months ended September 30, 2023 was $36.0 million, resulting from proceeds of $23.5 million received from the issuance and sale of convertible promissory notes and $12.5 million in proceeds from the Term Loan.
Funding Requirements
Our primary uses of capital are, and we expect will continue to be, research and development services, compensation and related expenses and general overhead costs. We expect to continue to incur significant expenses and operating losses for the foreseeable future. In addition, we expect to incur additional costs associated with operating as a public company. We anticipate that our expenses will increase significantly in connection with our ongoing activities.
Based on our current operating plan, we estimate that our existing cash and cash equivalents as of the date of this Quarterly Report, together with the net proceeds received in October 2024 from the exercise in full of the option granted to the underwriters of our IPO to purchase additional shares of our common stock, will be sufficient to fund our operations and capital expenses into 2029. However, we have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect.
Because of the numerous risks and uncertainties associated with research, development and commercialization of product candidates, we are unable to estimate the exact amount of our working capital requirements. Our future funding requirements will depend on, and could increase significantly as a result of, many factors, including:
•the timing, cost and progress of preclinical and clinical development activities;
•the cost of regulatory submissions and timing of regulatory approvals;
•the number and scope of preclinical and clinical programs we decide to pursue;
•the progress of the development efforts of parties with whom we may in the future enter into licenses, collaborations or other strategic partnerships;
•the timing and amount of milestone and other payments we are obligated to make under our Amgen Agreement or any future license agreements;
•the cash requirements of any future acquisitions or discovery of product candidates;
•our ability to establish and maintain licenses, collaborations or other strategic partnerships with third parties on favorable terms, if at all;
•the costs involved in prosecuting and enforcing patent and other intellectual property claims;
•the costs of manufacturing our product candidates by third parties;
•the cost of commercialization activities if azelaprag or any future product candidates are approved for sale, including marketing, sales and distribution costs;
•our efforts to enhance operational systems and hire additional personnel, including personnel to support development of our product candidates; and
•our need to implement additional internal systems and infrastructure, including financial and reporting systems to satisfy our obligations as a public company.
A change in the outcome of any of these or other variables with respect to the development of azelaprag or any product or development candidate we may develop in the future could significantly change the costs and timing associated with our development plans. Further, our operating plans may change in the future, and we may need additional funds to meet operational needs and capital requirements associated with such operating plans.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, which could include licenses, collaborations, or other strategic partnerships. We currently have no credit facility or committed sources of capital. Adequate additional funds may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our existing stockholders may be diluted, and the terms of these securities may include liquidation or other preferences that could adversely affect the rights of such stockholders. Debt financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, that could adversely impact our ability to conduct our business. If we raise additional funds through licenses, collaborations, or other strategic partnerships with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research program or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. There is no assurance that we will ever be profitable or generate positive cash flow from operating activities.
Contractual Obligations and Other Commitments
Lease Obligations
We lease office and lab space at our corporate headquarters in Richmond, California (the Headquarters Lease). The Headquarters lease is accounted for as an operating lease and expires on August 31, 2025. As of September 30, 2024, our non-cancellable base rent lease obligations related to the Headquarters Lease were $0.3 million all of which is due within the next 12 months.
The Company executed a lease for office and lab space in Emeryville, California on September 4, 2024 (the Emeryville lease). The lease commencement date for the Emeryville Lease was not reached as of September 30, 2024, for accounting purposes, and therefore is not capitalized in the condensed consolidated balance sheet as of September 30, 2024. Non-cancellable base rent lease obligations as of September 30, 2024 were $4.4 million of which $0.4 million is due within the next 12 months.
Purchase and Other Obligations
We enter into contracts in the normal course of business with CROs, CDMOs and other third-party vendors for preclinical research studies and testing, clinical trials and testing and manufacturing services. Most contracts do not contain minimum purchase commitments and are cancellable by us upon written notice. Payments due upon cancellation consist of payments for services provided or expenses incurred, including non-cancelable obligations of our service provided up to one year after the date of cancellation.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with United States Generally Accepted Accounting Principles (GAAP). The preparation of these condensed consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the reported amounts of income and expenses during the reporting period. We continually evaluate our estimates and judgments used in preparing our condensed consolidated financial statements and related disclosures. All estimates affect reported amounts of assets, liabilities, income and expenses. These estimates and judgments are also
based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known.
Our critical accounting policies and estimates are described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Critical Accounting Policies and Estimates” in our audited financial statements and the notes thereto for the year ended December 31, 2023 included in a final prospectus dated September 25, 2024 filed with the Securities and Exchange Commission pursuant to Rule 424(b) under the Securities Act of 1933, as amended. There were no material changes to these accounting policies during the three months ended September 30, 2024.
Internal Controls Over Financial Reporting
A company’s internal control over financial reporting is a process designed by, or under the supervision of, a company’s principal executive and principal financial officers, or persons performing similar functions, and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that it is reasonably possible that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
In connection with our preparation and the audit of our consolidated financial statements as of and for the years ended December 31, 2023 and 2022, management identified material weaknesses, as defined under the Exchange Act and by the Public Company Accounting Oversight Board (United States), in our internal control over financial reporting. The material weaknesses we identified related to the overall control environment as we had insufficient internal resources with appropriate accounting and finance knowledge and expertise to design, implement, document and operate effective internal controls around our financial reporting process and the lack of effective information technology general controls.
We have begun to implement measures designed to improve our internal control over financial reporting to remediate these material weaknesses, including (1) formalizing our processes and internal control documentation and strengthening supervisory reviews by our financial management; (2) hiring additional qualified accounting and finance personnel with technical accounting and financial reporting experience in the application of complex areas of GAAP, (3) engaging financial consultants and collaborating with our internal audit consultants to enable the implementation of internal control over financial reporting, and (4) improving segregation of duties among accounting and finance personnel in the preparation and review of account reconciliations and journal entries. We are also reviewing and improving the design of our general information technology controls including managing user access and privileged access, managing changes in the information system and segregation of duties with the systems supporting our accounting and reporting processes.
While we are implementing these measures, we cannot assure you that these efforts will remediate our material weaknesses in a timely manner, or at all, or prevent misstatements of our financial statements in the future. If we are unable to successfully remediate our material weaknesses, or identify any future material weaknesses, the accuracy and timing of our financial reporting may be adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports, and the market price of our common stock may decline as a result.
Emerging Growth Company and Smaller Reporting Company Status
Under Section 107(b) of the JOBS Act an “emerging growth company” can delay the adoption of new or revised accounting standards until such time as those standards would apply to private companies. We have elected this exemption to delay adopting new or revised accounting standards until such time as those standards apply to private companies. Where allowable we have early adopted certain standards as described in Note 2 of our unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report As a result, our condensed consolidated financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates. We will continue to remain an “emerging growth company” until the earliest of the following: (i) the last day of the fiscal year following the fifth anniversary of the date of the completion of our IPO; (ii) the last day of the fiscal year in which our total annual gross revenue is equal to or more than $1.235 billion; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We are also a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We will continue to be a smaller reporting company if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million.
If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our cash equivalents are held in money market funds that are invested in U.S. Treasury securities and our Term Loan has a variable interest rate that fluctuates with the U.S. prime rate.
Interest income is sensitive to changes in the general level of interest rates. However, due to the short-term maturities of our cash equivalents, we do not believe a hypothetical 100 basis point increase or decrease in interest rates during any of the periods presented would have had a material impact on our condensed consolidated financial statements included elsewhere in this Quarterly Report.
Interest expense is sensitive to changes in the general level of interest rates as our Term Loan incurs interest at a floating per annum rate equal to the U.S. prime rate plus 4.00% with an interest rate floor of 7.5%. However, we do not believe a hypothetical 100 basis point increase or decrease in interest rates during any of the periods presented would have had a material impact on our condensed consolidated financial statements included elsewhere in this Quarterly Report.
Credit Risk
Our primary exposure to credit risk is through financial instruments and consist primarily of cash and cash equivalents. We regularly maintain deposits in accredited financial institutions in excess of federally insured limits. As of September 30, 2024, we held cash deposits at Silicon Valley Bank in excess of FDIC insured limits.
Foreign Currency Exchange Risk
All of our employees and our operations are currently located in the United States and our expenses are generally denominated in U.S. dollars. We therefore are not currently exposed to significant market risk related to changes in foreign currency exchange rates. However, we have contracted with and may continue to contract with non-U.S. vendors who we may pay in local currency. Our operations may be subject to fluctuations in foreign currency exchange rates in the future. To date, foreign currency transaction gains and losses have not been material to our financial statements, and we have not had a formal hedging program with respect to foreign currency. We do not believe a hypothetical 100 basis point increase or decrease in exchange rates during any of the periods presented would have had a material effect on our condensed consolidated financial statements included elsewhere in this Quarterly Report.
Effects of Inflation
Inflation generally affects us by increasing our cost of labor and research and development costs. We do not believe that inflation had a material effect on our business, results of operations, or financial condition, or on our condensed consolidated financial statements included elsewhere in this Quarterly Report.
Item 4. Controls and Procedures.
Management’s Evaluation of our Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer (our principal executive officer and principal financial officer, respectively), we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under Exchange Act as of September 30, 2024. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as
appropriate, to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on our management’s evaluation (with the participation of our Chief Executive Officer and our Chief Financial Officer), as of the end of the period covered by this report, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures were ineffective due to the material weaknesses described below.
Material Weaknesses in Internal Control Over Financial Reporting
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
In connection with our preparation and the audit of our consolidated financial statements as of and for the years ended December 31, 2023 and 2022, we identified material weaknesses in our internal control over financial reporting that continued to exist as of September 30, 2024. Specifically, the control deficiencies related to (i) insufficient identification and assessment of risks impacting the design, implementation and operating effectiveness of internal controls over financial reporting and (ii) insufficient evaluation and determination as to whether components of internal control were present and functioning based upon evidence maintained for activity level controls, including management review controls, across substantially all of our financial statement areas. Management also determined that it did not maintain effective information technology controls in the areas of user access, change management and segregation of duties, within the systems supporting our accounting and reporting processes.
To our knowledge, these material weaknesses did not result in any material misstatements to the condensed consolidated financial statements.
Remediation of the Previously Reported Material Weaknesses
The material weaknesses previously reported are currently undergoing remediation. We are implementing measures designed to improve our internal control over financial reporting to remediate the material weaknesses, including (1) formalizing our processes and internal control documentation and strengthening supervisory reviews by our financial management; (2) hiring additional qualified accounting and finance personnel with technical accounting and financial reporting experience in the application of complex areas of GAAP, (3) engaging financial consultants and collaborating with our internal audit consultants to enable the implementation of internal control over financial reporting, and (4) improving segregation of duties among accounting and finance personnel in the preparation and review of account reconciliations and journal entries. We are also reviewing and improving the design of our general information technology controls including managing user access and privileged access, managing changes in the information system and segregation of duties with the systems supporting our accounting and reporting processes.
These additional resources and procedures are designed to enable us to broaden the scope and quality of our internal review of underlying information related to financial reporting and to formalize and enhance our internal control procedures. With the oversight of senior management and our audit committee, we have begun taking steps to remediate the underlying causes of the material weaknesses.
Changes in Internal Control over Financial Reporting
Except for the changes in internal control as referenced above for the remediation of previously reported material weaknesses, there have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the quarter ended September 30, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 1A. Risk Factors.
Investing in our common stock involves a high degree of risk. Before making your decision to invest in shares of our common stock, you should carefully consider the risks described below, together with the other information contained in this Quarterly Report, including in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in our condensed consolidated financial statements and the related notes included elsewhere in this Quarterly Report. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of or that we deem immaterial may also become important factors that adversely affect our business. We cannot assure you that any of the events discussed below will not occur. These events could have a material and adverse impact on our business, financial condition, results of operations and prospects. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Factors Summary
Our business is subject to a number of risks and uncertainties, including, among others, the following:
•We are a clinical-stage biopharmaceutical company with a limited operating history, have not completed any clinical trials beyond Phase 1b and have no products approved for commercial sale, which may make it difficult for investors to evaluate our business, likelihood of success and viability. We will need substantial additional funds to pursue our business objectives, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development programs, commercialization efforts or other operations.
•We have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We are not currently profitable, and may never achieve or sustain profitability. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline.
•We will require substantial additional capital to finance our operations and achieve our goals. If we are unable to raise capital when needed or on terms acceptable to us, we may be forced to delay, reduce or eliminate our research or development programs, any future commercialization efforts or other operations.
•We are substantially dependent on the success of our lead product candidate, azelaprag, which is currently in clinical development, and for which we have not completed a Phase 2 efficacy trial and any future product candidates we may develop. If we are unable to advance the development of, receive regulatory approval for and ultimately successfully commercialize azelaprag or any future product candidates we may develop, or experience significant delays in doing so, our business will be materially harmed.
•Drug development is a lengthy and expensive process, the outcome of clinical testing is inherently uncertain, and results of earlier studies and trials may not be predictive of future trial results. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of azelaprag and any future product candidates for many reasons, including a failure to replicate positive results from earlier preclinical studies or clinical trials in ongoing or future preclinical studies or clinical trials.
•We are developing our lead product candidate, azelaprag, and may develop future product candidates, in combination with other therapies, which would expose us to additional risks.
•We expect to expand our development, clinical and regulatory capabilities and operations as we grow, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
•Our quarterly and annual operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
•Negative results or publicity for one obesity drug could have a substantial impact on all drugs and product candidates for the treatment of obesity, including ours.
•We have identified material weaknesses in our internal control over financial reporting. If our remediation of the material weaknesses is not effective, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock
•We rely, and intend to continue to rely, on third parties to conduct our clinical trials and perform some of our research and preclinical studies. If these third parties do not satisfactorily carry out their contractual duties, fail to comply with applicable regulatory requirements or do not meet expected deadlines, our development programs may be delayed or subject to increased costs or we may be unable to obtain regulatory approval, each of which may have an adverse effect on our business, financial condition, results of operations and prospects.
•The manufacture of pharmaceutical products, including our product candidates, such as azelaprag, is complex. Our third-party manufacturers may encounter difficulties in production, which could delay or entirely halt their ability to supply our product candidates for clinical trials or, if approved, for commercial sale.
Risks Related to Our Financial Position, Limited Operating History and Need for Additional Capital
We are a clinical-stage biopharmaceutical company with a limited operating history, have not completed any clinical trials beyond Phase 1b and have no products approved for commercial sale, which may make it difficult for investors to evaluate our business, likelihood of success and viability.
We are a clinical-stage biopharmaceutical company with a limited operating history on which to base your investment decision. Drug development is a highly speculative undertaking and involves a substantial degree of risk. It entails substantial upfront capital expenditures and significant risk that any product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval or become commercially viable. We commenced operations in 2015, have no products approved for commercial sale and have never generated any revenue. To date, we have devoted substantially all of our resources to identifying, acquiring and developing our product candidates and licensed technologies, building our pipeline, performing research, conducting preclinical studies and early-stage clinical trials, organizing and staffing our company, business planning, establishing and maintaining our intellectual property portfolio, establishing arrangements with third parties for the manufacture of our product candidates, raising capital and providing general and administrative support for these operations.
To date, we have funded our operations with proceeds from sales of our redeemable convertible preferred stock, convertible notes, proceeds from the sale of our common stock, and stock option exercises. From inception through September 30, 2024, we received an aggregate of $293.8 million in gross proceeds from sales of our redeemable convertible preferred stock, an aggregate of $26.4 million in gross proceeds from sales of our convertible notes, $208.6 in gross proceeds from sales of our common stock, and $0.7 million in proceeds from stock option exercises.
We have not yet demonstrated an ability to successfully complete any clinical trials beyond our Phase 1 and Phase 1b clinical trials for azelaprag, obtain regulatory approvals, manufacture a commercial-scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. As a result, it may be more difficult for you to accurately predict our likelihood of success and viability than it could be if we had a longer operating history.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors and risks frequently experienced by clinical-stage biopharmaceutical companies. We also may need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We have not yet demonstrated an ability to successfully overcome such risks and difficulties, or to make such a transition. If we do not adequately address these risks and difficulties or successfully make such a transition, our business will suffer.
We have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We are not currently profitable, and may never achieve or sustain profitability. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline.
We have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We do not have any products approved for sale and have not generated any product revenue since our inception. If our lead product candidate, azelaprag, nor any future product candidates are successfully developed, approved and commercialized, we may never generate significant revenue, if we generate any revenue at all. Our net losses were $50.0 million and $42.9 million for the nine months ended September 30, 2024 and 2023, respectively. As of September 30, 2024, we had an accumulated deficit of $231.7 million. Substantially all of our losses have resulted from expenses incurred in connection with the development of, and in-licensing of
intellectual property related to, azelaprag, the research and development of our NLRP3 programs, our longitudinal human aging platform and from general and administrative costs associated with our operations. Azelaprag and any future product candidates will require substantial additional development time and resources before we would be able to apply for or receive regulatory approvals and begin generating revenue from product sales. We expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase substantially in connection with our additional ongoing and planned clinical trials for azelaprag including our ongoing STRIDES clinical trial for azelaprag in combination with tirzepatide and our planned Phase 2 clinical trial in combination with semaglutide, our planned initiation of an insulin sensitivity proof-of-concept trial of azelaprag monotherapy to support potential indication expansion; and our planned IND submission and Phase 1 clinical trial for an NLRP3 inhibitor for the treatment of neuroinflammation; and as we continue our development of, seek regulatory approval for and potentially commercialize azelaprag and any future product candidates we may develop and become a public company.
In addition, in May 2022, we entered into a loan and security agreement (the Loan Agreement) with SVB Innovative Credit Growth Fund IX, LP and Innovative Credit Growth Fund VIII-A, LP (collectively, the Lenders) pursuant to which we were able to borrow up to an aggregate of $25.0 million across two potential tranches until December 31, 2023 (the Term Loan). The Term Loan is secured by a lien covering substantially all of our assets, but not including our intellectual property or non-assignable licenses. In connection with the Term Loan, the Lenders were concurrently issued warrants to purchase 24,968 shares of our common stock at an exercise price of $10.26 per share, with a term of 10 years. The Loan Agreement required us to pay monthly interest payments until November 1, 2023, after which we commenced monthly principal payments. As of September 30, 2024 we had $9.5 million outstanding principal under the Term Loan. The Term Loan matures by April 1, 2026. For additional information about the Loan Agreement, see Note 5 to our audited consolidated financial statements and unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report.
To become and remain profitable, we must succeed in developing, obtaining regulatory approvals for, and eventually commercializing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing clinical trials of azelaprag and identifying, discovering, developing, in-licensing or acquiring any future product candidates, obtaining regulatory approval for azelaprag and any future product candidates, and manufacturing, marketing, and selling any products for which we may obtain regulatory approval. We are only in the preliminary stages of these activities. We may never succeed in these activities and, even if we do, may never generate revenue that is significant enough to achieve profitability. Because of the numerous risks and uncertainties associated with biopharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable may have an adverse effect on the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product candidates, achieve our strategic objectives or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will require substantial additional capital to finance our operations and achieve our goals. If we are unable to raise capital when needed or on terms acceptable to us, we may be forced to delay, reduce or eliminate our research or development programs, any future commercialization efforts or other operations.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. Our operations have consumed substantial amounts of cash since inception, and we expect our expenses to increase substantially in connection with our ongoing activities, particularly as we advance azelaprag and any future product candidates through clinical development. We expect increased expenses as we continue our research and development, continue our clinical trials, initiate additional clinical trials, seek to expand our product pipeline and clinical applications, seek regulatory approval for our current and future product candidates and invest in our organization. In addition, if we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. Because the outcome of any preclinical study or clinical trial is highly uncertain, we cannot reasonably estimate the actual amount of capital necessary to successfully complete the development and commercialization of our product candidates. Furthermore, we expect to incur additional costs associated with operating as a public company that we did not incur as a private company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations.
We had $334.5 million in cash and cash equivalents as of September 30, 2024. Based on our current operating plan, we estimate that our existing cash and cash equivalents as of the date of this Quarterly Report, together with the net proceeds received in October 2024 from the exercise in full of the option granted to the underwriters of our IPO to purchase additional shares of our common stock, will be sufficient to fund our operations and capital expenses into 2029. Changes beyond our control may occur that would cause us to use our available capital before that time, including changes in and progress of our drug development activities and changes in regulation. Our future capital requirements will be dependent on many factors, including:
•the progress, timing and results of preclinical studies and clinical trials for azelaprag or any future product candidates;
•the extent to which we develop, in-license or acquire any future product candidates or technologies;
•the number of future product candidates and additional indications for our current product candidates we may pursue, and the preclinical studies and clinical trials necessary to develop them;
•the costs, timing and outcome of seeking regulatory approvals of our current or future product candidates;
•the scope and costs of making arrangements with third-party manufacturers, or establishing manufacturing capabilities, for both clinical and commercial supplies of our current or future product candidates;
•the costs involved in growing our organization to the size needed to allow for the research, development and potential commercialization of our current or future product candidates;
•the costs associated with commercializing any approved product candidates, including establishing sales, marketing, market access and distribution capabilities;
•to the extent we pursue strategic collaborations, including collaborations to commercialize azelaprag or any of our future product candidates, our ability to establish and maintain collaborations on favorable terms, if at all, as well as the timing and amount of any milestone or royalty payments we are required to make or are eligible to receive under such collaborations or our current licenses;
•the costs associated with completing any post-marketing studies or trials required by the U.S. Food and Drug Administration (FDA) or other regulatory authorities;
•the revenue, if any, received from commercial sales of azelaprag or any of our future product candidates, if any are approved;
•the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims that we may become subject to, including any litigation costs and the outcome of such litigation; and
•the costs associated with potential product liability claims, including the costs associated with obtaining insurance against such claims and with defending against such claims.
We will require additional capital to complete our planned clinical development programs for our current product candidates in order to seek regulatory approval, and we anticipate needing to raise additional capital to complete the development of, and eventually commercialize, our product candidates, if approved. Adequate additional financing may not be available to us on favorable terms, or at all. Our ability to raise additional funds will be dependent on financial, economic and market conditions, geopolitical issues and other factors, over which we may have limited or no control. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. If adequate funds are not available on commercially acceptable terms when needed, we may be forced to delay, reduce or terminate the development or commercialization, if approved, of all or part of our research programs or product candidates or we may be unable to take advantage of future business opportunities. Furthermore, any additional capital-raising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our current and any future product candidates, if approved. Changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we currently anticipate, and we may need to seek additional funds sooner than planned.
We will be required to obtain further funding through public or private equity financings, debt financings, collaborative agreements, licensing arrangements or other sources of financing, which may dilute our stockholders or restrict our operating activities. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, each investor’s ownership interests will be diluted, and the terms may include liquidation or other preferences that adversely affect each investor’s rights as a stockholder. Debt financing or preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Such restrictions could adversely impact our ability to conduct our operations and execute our business plan. If we raise additional funds through upfront payments or milestone payments pursuant to strategic collaborations with third parties, we may have to relinquish valuable rights to our product candidates or grant licenses on terms that are not favorable to us.
Our failure to raise capital as and when needed or on acceptable terms could significantly harm our business, financial condition, results of operations and prospects and cause the price of our common stock to decline, and we may have to delay, reduce the scope of, suspend or eliminate one or more of our research or drug development programs, preclinical studies, clinical trials or future commercialization efforts.
Risk Related to Research, Discovery, Development, Regulatory Approval and Commercialization of our
Product Candidates
We are substantially dependent on the success of our lead product candidate, azelaprag, which is currently in clinical development, and for which we have not completed a Phase 2 efficacy trial and any future product candidates we may develop. If we are unable to advance the development of, receive regulatory approval for and ultimately successfully commercialize azelaprag or any future product candidates we may develop, or experience significant delays in doing so, our business will be materially harmed.
Our future success is highly dependent on our ability to timely complete successful clinical trials, obtain regulatory approval for, and then successfully commercialize our lead product candidate azelaprag and any future product candidates, which may never occur. We are early in our development efforts with respect to azelaprag, for which we recently completed our Phase 1 and Phase 1b clinical trials. We are developing brain-penetrant structurally novel small molecule inhibitors of NLRP3 that have a novel binding site, which are in earlier stages of development. We currently have no products that are approved for sale in any jurisdiction. There can be no assurance that azelaprag or any future product candidates we develop will achieve success in its respective clinical trials or obtain regulatory approval. We may also become dependent on other product candidates that we may develop or acquire in the future. Given our early stage of development, it may be several years, if at all, before we have demonstrated the safety and efficacy of a product candidate sufficient to warrant approval for commercialization.
Our ability to generate product revenue, which we do not expect will occur for many years, if ever, will be heavily dependent on the successful development and eventual commercialization of azelaprag and any future product candidates. The success of azelaprag and any future product candidates will be dependent on several factors, including the following:
•successful and timely completion of preclinical studies and clinical trials demonstrating attractive, competitive target product profiles for our product candidates;
•clearance of INDs by the FDA or other similar clinical trial applications from other regulatory authorities for our future clinical trials for our pipeline product candidates;
•timely and successful enrollment of patients in, and completion of, clinical trials with favorable results;
•demonstration of safety, efficacy and acceptable risk-benefit profiles of our product candidates to the satisfaction of the FDA and other comparable foreign regulatory agencies;
•receipt of regulatory approvals from applicable regulatory authorities, if granted, including the completion of any required post-marketing studies or trials and available funding to perform any post- marketing commitments;
•raising additional funds necessary to complete clinical development of and commercialize our current or future product candidates;
•obtaining, protecting and enforcing our patent, trade secret and other intellectual property and regulatory exclusivity for our current and future product candidates;
•making arrangements with third-party manufacturers, or establishing manufacturing capabilities, for both clinical and commercial supplies of our current and future product candidates and ensuring a resilient, effective supply chain that produces supply that outpaces demand;
•developing and implementing marketing and reimbursement strategies, as well as adequate demand forecasts for supply and sales planning;
•establishing sales, marketing and distribution capabilities and launching commercial sales of our products, if and when approved, whether alone or in collaboration with others in a market where promotional sales approaches are rapidly moving to digital platforms;
•demonstration of product characteristics attractive to physicians, patients, advocates, payors and caregivers;
•acceptance of our products, if and when approved, by patients, the medical community and third-party payors underpinned by adequate health economic data and a meaningful value proposition;
•effectively competing with other therapies, including those that have not yet entered the market;
•obtaining and maintaining third-party payor coverage and adequate reimbursement in both public and private payor spaces, given the significant number of obese patients in the United States who my benefit from our product candidates;
•obtaining appropriate support from patient advocacy organizations;
•addressing any delays in our clinical trials resulting from any major natural disasters, health pandemics or significant political events; and
•maintaining a continued acceptable safety profile of the products following approval.
Many of these factors are beyond our control, and it is possible that none of our product candidates will ever obtain regulatory approval even if we expend substantial time and resources seeking such approval. If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business. For example, our business could be harmed if results of our ongoing or planned clinical trials of azelaprag show unexpected adverse events or a lack of efficacy in the indications we intend to treat or do not meet the clinical endpoints, or if we experience other regulatory or developmental issues.
Due to our limited resources and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates over other potential product candidates. These decisions may prove to have been wrong and may adversely affect our ability to develop our own programs or our attractiveness as a commercial partner, and may ultimately have an impact on our commercial success.
Because we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount of resources to allocate to each. Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources away from better opportunities. Similarly, any decisions to delay, terminate or collaborate with third parties in respect of certain product development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the biopharmaceutical industry, in particular for azelaprag, our business, financial condition and results of operations would be materially adversely affected.
Drug development is a lengthy and expensive process, the outcome of clinical testing is inherently uncertain, and results of earlier studies and trials may not be predictive of future trial results. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of azelaprag and any future product candidates for many reasons, including a failure to replicate positive results from earlier preclinical studies or clinical trials in ongoing or future preclinical studies or clinical trials.
Our lead product candidate, azelaprag, is in clinical development, and the risk of failure is high. It is impossible to predict when or if azelaprag or any future product candidates will prove effective and safe in humans or will receive regulatory approval. To obtain the requisite regulatory approvals to commercialize any product candidate, we must demonstrate through extensive preclinical studies and lengthy, complex and expensive clinical trials that our product candidates are safe and effective in humans. Clinical testing can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of azelaprag or any future product candidates, or a competitor’s product candidate in the same class, may not be predictive of the results of later-stage clinical trials. For example, as is common in early-stage clinical trials, our Phase 1b bed rest atrophy clinical trial of azelaprag, conducted in a small number of healthy older individuals, evaluated a number of pharmacodynamic endpoints and biomarkers without correction for multiplicity. These results on measures of muscle size, quality and metabolism may not be replicated in later-stage clinical trials with different trial designs and patient populations. Interim, topline or preliminary results of a clinical trial are not necessarily indicative of final results. We may be unable to establish benefit on clinical endpoints that applicable regulatory authorities would consider clinically meaningful, and a clinical trial can fail at any stage of testing. Differences in trial design between early-stage clinical trials and later-stage clinical trials make it difficult to extrapolate the results of earlier clinical trials to later clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in clinical trials have nonetheless failed to obtain regulatory approval of their products. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or to unfavorable safety profiles, notwithstanding promising results in earlier trials. There is typically a high rate of failure of product candidates proceeding through clinical trials, particularly in the earlier stages of development. Most product candidates that commence clinical trials are never approved as products, and there can be no assurance that any of our future clinical trials will ultimately be successful or support clinical development of azelaprag or any future product candidates.
We may experience delays in initiating or completing clinical trials. We also may experience numerous unforeseen events during, or as a result of, any future clinical trials that we could conduct that could delay or prevent our ability to receive regulatory approval or commercialize azelaprag or any future product candidates, including:
•regulators, institutional review boards (IRBs) or ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site, or may halt or suspend an ongoing clinical trial;
•we may experience delays in reaching or fail to reach agreement on acceptable terms with prospective trial sites and prospective contract research organizations (CROs) the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
•clinical trial sites deviating from the trial protocol or dropping out of a trial;
•clinical trials of any product candidates may fail to show safety or efficacy, produce negative or inconclusive results and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials or we may decide to abandon product development programs;
•failure of our current or future product candidates in clinical trials to demonstrate important functional or patient-reported outcomes;
•the number of subjects required for clinical trials of any product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or subjects may drop out of these clinical trials or fail to return for post-treatment follow-up at a higher rate than we anticipate;
•our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the clinical trial protocol or drop out of the trial, which may require that we add new clinical trial sites or investigators;
•we may elect, or regulators, IRBs, or ethics committees may require, that we or our investigators, suspend or terminate clinical research or trials for various reasons, including noncompliance with regulatory requirements or a finding that the participants in our trials are being exposed to unacceptable health risks;
•the cost of clinical trials of azelaprag or any future product candidates may be greater than we anticipate, and we may not have sufficient funds to complete such trials;
•the quality of azelaprag or any future product candidates or other materials necessary to conduct clinical trials of azelaprag or any future product candidates may be inadequate to initiate or complete a given clinical trial;
•our inability to manufacture sufficient quantities of azelaprag or any future product candidates for use in clinical trials;
•our inability to meet drug specifications suitable for use in clinical trials and commercial applications;
•reports from clinical testing of other therapies may raise safety or efficacy concerns about azelaprag or any future product candidates;
•the receipt of feedback from regulatory authorities that requires us to modify the design of our clinical trials;
•our failure to establish an appropriate safety profile for a product candidate based on clinical or preclinical data for such product candidate as well as data emerging from other molecules in the same class as azelaprag or any future product candidates; and
•the FDA or other regulatory authorities may require us to submit additional data such as long-term toxicology studies or impose other requirements before permitting us to initiate a clinical trial.
We could also encounter delays if a clinical trial is suspended or terminated by us, the IRBs of the institutions in which such trials are being conducted, or the FDA or other regulatory authorities, or if a clinical trial is recommended for suspension or termination by the Data Safety Monitoring Board for such trial. A suspension or termination may be imposed due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements, including the FDA’s Good Clinical Practice (GCP) regulations, or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product or treatment, failure to establish or achieve clinically meaningful trial endpoints, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Clinical studies may also be delayed or terminated as a result of ambiguous or negative interim results. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of azelaprag or any future product candidates. Further, the FDA or other regulatory authorities may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after they have reviewed and commented on the design for our clinical trials. For example,
while the FDA has issued a draft guidance on developing products for weight management that describes appropriate efficacy endpoints for pivotal trials for product candidates for weight management, the guidance does not address endpoints related to change in body composition. While the FDA agreed with our primary endpoint of percent change in body weight for STRIDES, for change in body composition or muscle-related parameters we are currently examining or may examine in the future, we expect that we will have to demonstrate how any change in such parameters translates to clinical benefit.
We cannot predict with any certainty the schedule for commencement and completion of future clinical trials. Further, conducting clinical trials in foreign countries, as we have done and may do in the future for our product candidates, presents additional risks that may delay completion of our clinical trials. These risks include the failure of enrolled patients in foreign countries to adhere to clinical protocol as a result of differences in healthcare services or cultural customs, managing additional administrative burdens associated with foreign regulatory schemes, as well as political and economic risks relevant to such foreign countries.
If we are required to conduct additional clinical trials or other testing of our current or future product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our current or future product candidates or other testing in a timely manner, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may incur unplanned costs, be delayed in seeking and obtaining regulatory approval, if we receive such approval at all, receive more limited or restrictive regulatory approval, be subject to additional post-marketing testing requirements or have the drug removed from the market after obtaining regulatory approval.
Additionally, if the results of our clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with our product candidates, we may:
•be delayed in obtaining regulatory approval, if at all;
•obtain approval for indications or patient populations that are not as broad as intended or desired or may have restricted duration expectations or guidance;
•obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
•be subject to additional post-marketing testing requirements;
•be required to perform additional clinical trials to support approval or be subject to additional post- marketing testing requirements;
•have regulatory authorities withdraw, or suspend, their approval of the drug or impose restrictions on its distribution in the form of a Risk Evaluation and Mitigation Strategy (REMS);
•be subject to the addition of labeling statements, such as warnings or contraindications;
•experience damage to our reputation.
Our drug development costs will also increase if we experience delays in testing or obtaining regulatory approvals. Also, delays in obtaining regulatory approval may increase commercialization costs if the competitive environment becomes more intense prior to market entry. We do not know whether any of our preclinical studies or clinical trials will begin as planned, need to be restructured or be completed on schedule, if at all.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or other regulatory authorities. The FDA or other regulatory authorities may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable foreign regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or other regulatory authority, as the case may be, and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate. We may make formulation or manufacturing changes to our product candidates, in which case we may need to conduct additional preclinical studies to bridge our modified product candidates to earlier versions. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our current or any future product candidates could be negatively impacted, and our ability to generate revenues from our current or future product candidates may be delayed or eliminated entirely.
We are developing our lead product candidate, azelaprag, and may develop future product candidates, in combination with other therapies, which would expose us to additional risks.
We are currently developing our lead product candidate, azelaprag, for use in combination with certain incretins for the treatment of obesity, and we may develop other product candidates for use in combination with other therapies in the future. For example, our ongoing and planned Phase 2 trials of azelaprag are in combination with tirzepatide and semaglutide. The development of product candidates for use in combination with another product may present challenges that are not faced for single agent product candidates. Each of our Phase 2 trials of azelaprag in combination with tirzepatide and semaglutide, respectively, are designed to evaluate efficacy and it is possible that the results of these trials or future trials of azelaprag in combination with tirzepatide or semaglutide could show that azelaprag does not sufficiently contribute to the observed effects of individuals who participate in these trials. Even if any of our current or future product candidates were to receive regulatory approval or be commercialized for use in combination with other existing therapies, we would continue to be subject to the risks that the FDA or other comparable foreign regulatory authorities could revoke approval of the therapy used in combination with any of our product candidates, or safety, efficacy, manufacturing or supply issues could arise with these existing therapies. In addition, it is possible that existing therapies with which our product candidates are approved for use could themselves fall out of favor or be relegated to later lines of treatment. This could result in the FDA or similar foreign regulatory authorities requiring us to conduct additional clinical trials, the need to identify other combination therapies for our product candidates or our own products being removed from the market or being less successful commercially.
If the FDA or other comparable foreign regulatory authorities do not approve or withdraw their approval of these other therapies, or if safety, efficacy, commercial adoption, manufacturing or supply issues arise with the therapies we choose to evaluate in combination with any of our current or future product candidates, we may be unable to obtain approval of or successfully market any one or all of the current or future product candidates we develop. Additionally, if the third-party providers of therapies or therapies in development used in combination with our current or future product candidates are unable to produce sufficient quantities for clinical trials or for commercialization of our current or future product candidates, such as in connection with our material transfer agreement with Eli Lilly (Lilly) for certain amounts of tirzepatide to be used in connection with our planned clinical trials of azelaprag, or if the cost of combination therapies are prohibitive, our development and commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations and prospects.
Preliminary, topline or interim data from our clinical trials that we announce or publish from time to time may change as more patient data become available and/or are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary, topline or interim data from our clinical trials, such as preliminary, topline or interim or data analysis from our ongoing and planned Phase 2 clinical trials of azelaprag. These data and related findings and conclusions may only reflect certain endpoints rather than all endpoints and are subject to change. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the preliminary or topline results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated.
Preliminary or topline data also remain subject to review and verification procedures that may result in the final data being materially different from the preliminary or topline data we previously published. As a result, preliminary and topline data should be viewed with caution until the final data are available. In addition, we may report preliminary data or interim analyses of the clinical trials we may conduct and complete, which are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Adverse changes between preliminary or interim data and final data could significantly harm our business and prospects. Further, additional disclosure of preliminary or interim data by us, including, for example, preliminary or interim data that becomes available to us from our ongoing and planned Phase 2 clinical trials of azelaprag or by our competitors in the future could result in volatility in the price of our common stock.
Further, the information we choose to publicly disclose regarding a particular study or clinical trial is typically selected from a more extensive amount of available information. You or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or our business. If the preliminary, topline or interim data that we report differ from later, final or actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, financial condition, results of operations and prospects.
We may not be successful in applying our longitudinal human aging platform to identify additional targets with therapeutic and commercial potential or in the discovery and development of commercially viable product candidates for us or our collaborators.
We use our longitudinal human aging platform to identify and prioritize potential drug targets, to assess the likelihood that we can develop a product candidate that interacts with the target to elicit the desired therapeutic effect, and to transition these insights efficiently into well supported therapeutic candidates. While we believe our platform will increase the likelihood of producing additional product candidates that provide meaningful clinical benefit, past success in identifying potential product candidates does not assure future success for our internal drug discovery programs. Our longitudinal human aging platform is novel, and we may not succeed in applying our platform to identify additional drug targets or transition these targets into promising future product candidates. We similarly cannot provide any assurance that, even if we do successfully identify additional targets, we will be able to successfully develop future product candidates and advance any such future product candidates into and through clinical development. Therefore, we are unable to predict the time and cost associated with the identification and development of any future product candidate or whether the application of our platform will result in the identification, development and ultimately regulatory approval of any future product candidates.
Efforts through our platform to identify, discover, acquire or in-license, and ultimately develop, product candidates require substantial technical, financial and human resources, whether or not any such future product candidates are ultimately identified. Our efforts may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development or regulatory approval for many reasons, including the following:
•the methodology used may not be successful in identifying any future potential product candidates;
•competitors may develop alternatives that render any product candidates we develop obsolete;
•any product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
•a product candidate may be shown, in subsequent preclinical or clinical investigations, to have harmful side effects or characteristics that indicate it is unlikely to be effective, or otherwise would not meet applicable regulatory criteria;
•a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
•a product candidate may not be accepted as safe and effective by physicians, patients, the medical community or third-party payors.
Our future growth may be dependent, in part, on our ability to operate in foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future growth may be dependent, in part, on our ability to develop and commercialize azelaprag, if approved, and any future product candidates in foreign markets for which we may rely on collaboration with third parties. We are not permitted to market or promote azelaprag or any future product candidates before we receive regulatory approval from the applicable regulatory authority in that foreign market and may never receive such regulatory approval for azelaprag or any future product candidates. To obtain separate regulatory approval in many other countries, we must comply with numerous and varying regulatory requirements of such countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales, pricing and distribution of azelaprag or any future product candidates, and we cannot predict success in these jurisdictions. If we fail to comply with the regulatory requirements in international markets and receive applicable regulatory approvals, our target market will be reduced and our ability to realize the full market potential of azelaprag or any future product candidates will be harmed, and our business will be adversely affected. We may not obtain foreign regulatory approvals on a timely basis, if at all. Our failure to obtain approval of any of azelaprag or any future product candidates by regulatory authorities in another country may significantly diminish the commercial prospects of that product candidate and our business, financial condition, results of operations and prospects could be materially and adversely affected. Moreover, even if we obtain approval of azelaprag or any future product candidates and ultimately commercialize azelaprag or any future product candidates in foreign markets, we would be subject to the risks and uncertainties, including the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements and reduced protection of intellectual property rights in some foreign countries.
We may experience difficulty enrolling or keeping patients in our clinical trials, which could delay or prevent us from proceeding with, or otherwise adversely affect, clinical trials of our product candidates.
Our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition could reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, it is possible that we will be required to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which could negatively impact the number of patients who are available for our clinical trials in such clinical trial site.
Delays related to patient enrollment and difficulties related to patient retention may result in increased costs or may affect the timing or outcome of our future clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates. Further, if patients drop out of our clinical trials, miss scheduled doses or follow-up visits, or otherwise fail to follow clinical trial protocols, the integrity of data from our clinical trials may be compromised or not accepted by the FDA or other regulatory authorities, which would represent a significant setback for the applicable program.
Our current or future product candidates may not achieve adequate market acceptance among physicians, patients or their families, healthcare payors and others in the medical community necessary for commercial success.
Even if our current or future product candidates receive regulatory approval, they may not gain adequate market acceptance among physicians, patients or their families, third-party payors and others in the medical community. The degree of market acceptance of any of our approved product candidates will be dependent on a number of factors, including:
•the efficacy, durability and safety profile as demonstrated in clinical trials compared to alternative treatments, in addition to patient-reported outcomes;
•the timing of market introduction of the product candidate, as well as competitive products;
•the clinical indications for which a product candidate is approved;
•restrictions on the use of product candidates in the labeling approved by regulatory authorities, such as boxed warnings or contraindications in labeling, or a REMS, if any, which may not be required of alternative treatments and competitor products;
•the potential and perceived advantages of our current or future product candidates over alternative treatments;
•the cost of treatment in relation to alternative treatments and the cost/benefit ratios of each;
•the availability of coverage and adequate reimbursement by third-party payors, including government authorities, given the significant number of obese patients in the United States, and timing of relevant formulary decision-making resulting in this coverage and reimbursement;
•the availability of an approved product for use as a combination therapy;
•relative convenience and ease of administration in relation to competition;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the effectiveness of sales, marketing efforts and market access;
•publicity relating to our product candidates or those of our competitors; and
•the approval of new therapies for the same indications.
If any of our current or future product candidates are approved but do not achieve an adequate level of acceptance by physicians, hospitals, healthcare payors and patients, we may not generate or derive sufficient revenue from that product candidate and our financial results would be negatively impacted.
We have never commercialized a product candidate as a company before and currently lack the comprehensive, fully staffed expertise, personnel and resources to successfully commercialize any products on our own or together with suitable collaborators. If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any product we may develop, we may not be successful in commercializing those products if they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sales, marketing or distribution of any current or future product candidates. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future and if any of our product candidates are approved, we may choose to build a focused sales, marketing and commercial support infrastructure to sell, or participate in sales activities with collaborators for some of our current or future product candidates.
There are risks involved with both establishing our own commercial capabilities and entering into arrangements with third parties to perform these services. For example, factors that may inhibit our efforts to commercialize any approved product candidates include:
•the inability to recruit and retain adequate numbers of effective sales, marketing, coverage or reimbursement, customer service, medical affairs and other support personnel;
•the inability of sales personnel to obtain access to or persuade adequate numbers of decision makers to utilize any future approved product candidates;
•the inability of reimbursement professionals to negotiate arrangements for formulary access, reimbursement and other acceptance by payors;
•the inability to price any of our current or future product candidates at a sufficient price point to ensure an adequate and attractive level of profitability;
•restricted or closed distribution channels that make it difficult to distribute our current or future product candidates to segments of the patient population;
•the lack of complementary product candidates to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product candidate lines; and
•unforeseen costs and expenses associated with creating an independent commercialization organization.
If the commercial launch of a product candidate, if approved, for which we recruit a sales force and establish marketing and other commercialization capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our commercialization personnel.
If we enter into arrangements with third parties to perform sales, marketing, commercial support and distribution services, our sales revenue or the profitability of sales revenue may be lower than if we were to do so ourselves. In addition, we may not be successful in entering into arrangements with third parties to commercialize our product candidates or may be unable to do so on terms that are favorable to us. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our product candidates effectively. If we do not establish commercialization capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates, if approved.
Risks Related to Our Business and Operations
Our future performance is dependent on our ability to retain key employees and to attract, retain and motivate qualified personnel and manage our human capital.
Our ability to compete in the highly competitive biotechnology and biopharmaceutical industries is largely dependent on our ability to attract, motivate and retain highly qualified managerial, clinical, scientific and medical personnel. We are highly dependent on the scientific and management expertise of Dr. Fortney, our Chief Executive Officer, the other members of our management team and other key employees and advisors. We currently do not maintain “key person” life insurance on these individuals or any of our employees. This lack of insurance means that we may not have adequate compensation for the loss of the services of any such individuals. The loss of one or more members of our management team or other key employees or advisors could delay our research and development programs and have a material and adverse effect on our business, financial condition, results of operations and prospects. We are dependent on the continued service of our technical personnel, because of the highly technical nature of drug development and the specific knowledge related to azelaprag or any future product candidates and technologies, and the specialized nature of the regulatory approval process. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty.
In addition, job candidates and existing employees often consider the value of the stock awards they receive in connection with their employment. If the perceived benefits of our stock awards decline, either because we are a public company or for other reasons, it may harm our ability to recruit and retain highly skilled employees. Our employees may be more likely to leave us if the shares they own have significantly appreciated in value relative to the original purchase prices of the shares, or if the exercise prices of the options that they hold are significantly below the market price of our common stock, particularly after the expiration of the lock-up agreements described herein.
We are currently a remote-based company, with a majority of our employees working remotely, and we primarily conduct our in-person operations at our research facility in Richmond, California. This region is headquarters to many other biopharmaceutical companies and academic and research institutions. Competition for skilled personnel in our market, and nationally, is intense and may limit our ability to hire and retain highly qualified personnel on acceptable terms or at all. We also face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. Our industry has experienced a high rate of turnover of management personnel in recent years. Our future performance will be dependent in large part on our continued ability to attract and retain highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can discover and develop product candidates will be limited, which could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Our quarterly and annual operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
•variations in the level of expense related to the ongoing development of azelaprag or any future development programs;
•results of preclinical studies and clinical trials, or the addition or termination of future clinical trials or funding support by us, or existing or future collaborators or licensing partners;
•our ability to enroll patients in clinical trials and the timing of enrollment;
•the need to conduct unanticipated clinical trials or trials that are larger or more complex than anticipated;
•our execution of any additional collaboration, licensing or similar arrangements, and the timing of payments we may make or receive under existing or future arrangements or the termination or modification of any such existing or future arrangements;
•any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved;
•additions and departures of key personnel;
•strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
•regulatory developments affecting azelaprag or any future product candidates or those of our competitors;
•potential unforeseen business disruptions that increase our costs or expenses;
•effects of global macroeconomic events, such as inflation, geopolitical conflicts, pandemics, natural disasters and supply chain issues, on our business and operations; and
•changes in general market and economic conditions.
If our quarterly or annual operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly or annual fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially. We believe that quarterly or annual comparisons of our financial results are not necessarily meaningful and should not be relied on as an indication of our future performance.
We expect to expand our development, clinical and regulatory capabilities and operations as we grow, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to increase the number of our employees and the scope of our operations, particularly in the areas of clinical development, clinical operations, manufacturing, late-stage regulatory affairs, finance, accounting, business operations, public company compliance, communications and other corporate development functions, and, if azelaprag or any future product candidates receive regulatory approval, sales, marketing and distribution capabilities. If we acquire additional product candidates or enter into future collaborations, we may have to further expand our employee base beyond our current projections, which may include further preclinical research and development or later-stage regulatory operations. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth and with developing sales, marketing and distribution infrastructure, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources.
If we are not able to effectively manage growth and expand our operations, we may not be able to successfully implement the tasks necessary to further develop and commercialize, if approved, azelaprag or any future product candidates and, accordingly, we may not achieve our research, development and commercialization goals.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do, if at all.
The development and commercialization of new drug products is highly competitive, and specifically the development and commercialization of therapeutics for the treatment of obesity is particularly competitive. Our current and any future product candidates, if approved, will face significant competition, including from well- established, currently marketed therapies or recommended standards of care, and our failure to demonstrate a meaningful improvement to the existing standards of care may prevent us from achieving significant market penetration. Many of our competitors have significantly greater resources and experience than we do, and we may not be able to successfully compete. We face substantial competition from multiple sources, including large and specialty biopharmaceutical and biotechnology companies, academic research institutions and governmental agencies and public and private research institutions.
Our lead product candidate, azelaprag, initially under development as a combination therapy for the treatment of obesity, if approved, would face competition from other approved treatments, some of which have already achieved commercial success. To compete successfully, we will need to differentiate our combination therapy, if approved, from currently marketed drugs as well as those that may be approved in the future, meaning that we will have to demonstrate that the relative cost, method of administration, safety, tolerability or efficacy of our combination therapy provides a better alternative or complement to existing and new therapies. Our commercial opportunity and likelihood of success will be reduced or eliminated if our azelaprag combination therapy is not ultimately demonstrated to be safer, more effective, more conveniently administered, or less expensive than the current standards of care. Furthermore, even if an azelaprag combination therapy is able to achieve these attributes, acceptance of such combination therapy may be inhibited by the reluctance of physicians to switch from existing therapies, or if physicians choose to reserve our azelaprag combination therapy for use in limited circumstances.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than we have. If we obtain regulatory approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our current or any future product candidates, the ease with which our current or any future product candidates can be administered and the extent to which participants accept relatively new routes of administration, the timing and scope of regulatory approvals for these product candidates, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing products could present superior treatment alternatives,
including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our current or any future product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan. Mergers and acquisitions in the biopharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified management and other personnel and establishing clinical trial sites and participants registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
The estimates of market opportunity and forecasts of market growth included in this Quarterly Report may prove to be smaller than we believe, and even if the markets in which we compete achieve the forecasted growth, our business may not grow at similar rates, or at all.
We intend to initially focus our product candidate development on treatments for metabolic diseases, such as obesity. Our projections of addressable patient populations within any particular disease state that may benefit from treatment with our product candidates are based on our estimates. Market opportunity estimates and growth forecasts included in this Quarterly Report are subject to significant uncertainty and are based on assumptions and estimates. These estimates, which have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations and market research, may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. Similarly, the percent of the population with obesity and metabolic diseases could be lower than we anticipate. In both instances, the pool of potential patients that azelaprag could address could be substantially smaller than we anticipate. Additionally, the potentially addressable patient population for our product candidates may not ultimately be amenable to treatment with our product candidates. Our market opportunity may also be limited by future competitor treatments that enter the market. If any of our estimates prove to be inaccurate, the market opportunity for any product candidate that we or our strategic partners develop could be significantly diminished and have an adverse material impact on our business.
Negative results or publicity for one obesity drug could have a substantial impact on all drugs and product candidates for the treatment of obesity, including ours.
Our business can be affected by adverse publicity or negative public perception about us, our competitors, our product candidates or products, if approved, or our industry or competitors generally. Adverse publicity may include publicity about metabolic disease treatments or GLP-1R agonists generally, the efficacy, safety and quality of azelaprag, as well as of the broader category of obesity products, including any products that azelaprag are intended to be used in combination with, and regulatory investigations, regardless of whether these investigations involve us or the business practices or products of our competitors or our customers. Any adverse publicity or negative public perception could have a material adverse effect on our business, financial condition and results of operations. Further, any adverse effects in our clinical trials, even if not ultimately attributable to our product candidates, and the resulting publicity could result in withdrawal of clinical trial participants, and a decrease in demand for any such product candidates. Our business, financial condition and results of operations could be adversely affected if any of our product candidates or products, if approved, or any similar products distributed by other companies are alleged to be or are proved to be harmful to consumers or to have unanticipated and unwanted health consequences.
Our business entails a significant risk of product liability, and our ability to obtain sufficient insurance coverage could have a material and adverse effect on our business, financial condition, results of operations and prospects. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit, delay or cease commercialization of our products.
When we conduct clinical trials of our current and any future product candidates, we may be exposed to significant product liability risks inherent in the development, testing, manufacturing and marketing of therapeutic treatments. Product liability claims could delay or prevent completion of our development programs. If we succeed in marketing products, if approved, such claims could result in an FDA investigation of the safety and effectiveness of our products, our manufacturing processes and facilities or our marketing programs and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used or suspension or withdrawal of approvals. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit, delay or cease the commercialization of our products. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, termination of clinical trial sites or entire trial programs, withdrawal of clinical trial participants, injury to our reputation and significant negative media attention, significant costs to defend the related litigation, a diversion of management’s time and our resources from our business operations, substantial monetary awards to trial participants or patients, loss of revenue, the inability to commercialize any products that we may develop and a decline in our stock price.
We currently maintain approximately $19.0 million in general liability insurance and product liability insurance in the aggregate. We may, however, need to obtain higher levels of insurance coverage for later stages of clinical development or marketing any of our product candidates. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material and adverse effect on our business, financial condition, results of operations and prospects. Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of our product candidates. Although we will maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies will also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our employees, independent contractors, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading, and we may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to comply with FDA regulations, provide true, complete and accurate information to the FDA or other regulatory authorities, comply with manufacturing standards we may establish, comply with healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. If we obtain FDA approval of any of our current or future product candidates and begin commercializing those products in the United States, our potential exposure under these laws will increase significantly, and our costs associated with compliance with these laws will likely increase. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material and adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA or other regulatory authorities exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, integrity oversight and reporting obligations, or reputational harm.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies. Although we try to ensure that individuals working for or collaborating with us do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information proprietary to these third parties or our employees’ former employers, or that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. We may be subject to claims that patents and applications we have filed to protect inventions of our employees, consultants, advisors or other third parties, even those related to one or more of our product candidates, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees. If our defenses to these claims fail, in addition to requiring us to pay monetary damages, a court could prohibit us from using technologies or features that are essential to our product candidates, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. Moreover, any such litigation or the threat thereof may adversely affect our reputation, our ability to form strategic alliances or sublicense our rights to collaborators, engage with scientific advisors or hire employees or consultants, each of which would have an adverse effect on our business, results of operations and financial condition. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
We may engage in strategic transactions that could increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, subject us to other risks, adversely affect our liquidity, increase our expenses and present significant distractions to our management.
From time to time, we may consider strategic transactions, such as acquisitions of companies, asset purchases and out-licensing or in-licensing of intellectual property, products or technologies. Additional potential transactions that we may consider include a variety of business arrangements, including spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and investments. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. Any future transactions could increase our near and long-term expenditures, result in potentially dilutive issuances of our equity securities, including our common stock, or the incurrence of debt, contingent liabilities, amortization expenses or acquired in-process research and development expenses, any of which could affect our financial condition, liquidity and results of operations. Future acquisitions may also require us to obtain additional financing, which may not be available on favorable terms or at all. These transactions may never be successful and may require significant time and attention of our management. In addition, the integration of any business that we may acquire in the future may disrupt our existing business and may be a complex, risky and costly endeavor for which we may never realize the full benefits. Furthermore, we may experience losses related to investments in other companies, including as a result of failure to realize expected benefits or the materialization of unexpected liabilities or risks, which could have a material negative effect on our results of operations and financial condition. Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any additional transactions that we do complete could have a material adverse effect on our business, results of operations, financial condition and prospects.
In May 2022, we entered into the Loan Agreement and the Term Loan we entered into in connection with the Loan Agreement restricts our ability to pursue certain mergers, acquisitions or consolidations that we may believe to be in our best interest.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses during our history and do not expect to become profitable in the near future, and we may never achieve profitability. Under current law unused U.S. federal net operating losses generated in tax years beginning after December 31, 2017, will not expire and may be carried forward indefinitely but the deductibility of such federal net operating losses for any year is limited to no more than 80% of current year taxable income (without regard to certain deductions). In addition, both our current and our future net operating losses and other tax attributes may be subject to limitation under Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended (the Code), if we undergo, or have undergone, an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in our equity ownership by certain stockholders or groups of stockholders over a three-year period. It is possible that we have undergone one or more “ownership changes” in the past. We may also undergo ownership changes in the future as a result of the IPO and the concurrent private placement and/or other shifts in the ownership of our capital stock, some of which may be outside of our control, which may further limit our ability to use our pre-change net operating loss carryforwards and other pre-change tax attributes (such as research tax credits) to offset our post-change income or taxes. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. In addition, at the state level, there may be periods during which the use of net operating losses is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. As a result, even if we attain profitability, we may be unable to use all or a material portion of our net operating losses and other tax attributes, which could adversely affect our future cash flows.
Changes in tax laws or regulations that are applied adversely to us may have a material adverse effect on our business, cash flows, financial condition or results of operations.
New income, sales, use or other tax laws, statutes, rules or regulations could be enacted at any time, which could adversely affect our business operations and financial performance. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to us. For example, future changes in corporate tax rates, the realization of net deferred tax assets relating to our operations, the taxation of foreign earnings, and the deductibility of expenses could have a material impact on the value of our deferred tax assets, could result in significant one-time charges, and could increase our future U.S. tax expense. In addition, for tax years beginning after December 31, 2021, current law requires taxpayers to capitalize and amortize certain research and development expenditures over five years if incurred in the United States and fifteen years if incurred in foreign jurisdictions, rather than deducting them concurrently. Although there have been legislative proposals to repeal or defer the capitalization requirement to later years, there can be no assurance that the provision will be repealed or otherwise modified.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. We designed our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and
communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. For example, our directors or executive officers could inadvertently fail to disclose a new relationship or arrangement causing us to fail to make any related party transaction disclosures. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected. In addition, we do not have a formal risk management program for identifying and addressing risks to our business in other areas.
We have identified material weaknesses in our internal control over financial reporting. If our remediation of the material weaknesses is not effective, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock
We have identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. In preparing the financial statements as of and for the years ended December 31, 2023 and 2022, management determined it had not maintained appropriately designed entity-level controls impacting the control environment, risk assessment procedures and monitoring activities to prevent or detect material misstatements to our condensed consolidated financial statements, which constituted material weaknesses. Specifically, the control deficiencies related to (i) insufficient identification and assessment of risks impacting the design, implementation and operating effectiveness of internal controls over financial reporting and (ii) insufficient evaluation and determination as to whether components of internal control were present and functioning based upon evidence maintained for activity level controls, including management review controls, across substantially all of our financial statement areas. Management also determined that it did not maintain effective information technology controls in the areas of user access, change management and segregation of duties, within the systems supporting our accounting and reporting processes.
To remediate these material weaknesses, we are in the process of implementing measures designed to improve our internal control over financial reporting. We have hired additional accounting personnel with technical accounting and financial reporting experience and have implemented improved process level and management review controls. We are currently collaborating with our internal audit consultants to enable the implementation of appropriate internal controls over financial reporting. We will also review and improve the design of our general information technology controls including managing user access, change management, and segregation of duties within the systems supporting our accounting and reporting processes.
We cannot assure you that the measures we have taken to date, and are continuing to implement, will be sufficient to remediate the material weaknesses we have identified or avoid potential future material weaknesses. If the steps we take do not correct the material weaknesses in a timely manner, we will be unable to conclude that we maintain effective internal control over financial reporting. Accordingly, there could continue to be a reasonable possibility that a material misstatement of our financial statements would not be prevented or detected on a timely basis.
If we fail to remediate our existing material weaknesses or identify new material weaknesses in our internal controls over financial reporting, if we are unable to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, if we are unable to conclude that our internal controls over financial reporting are effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal controls over financial reporting when we are no longer an emerging growth company, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could be negatively affected. As a result of such failures, we could also become subject to investigations by the stock exchange on which our securities are listed, the SEC or other regulatory authorities, and become subject to litigation from investors and stockholders, which could harm our reputation and financial condition or divert financial and management resources from our regular business activities.
We and the third parties with whom we work are, or may in the future be, subject to stringent and changing data privacy and security obligations.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, “process”) certain personal information and other sensitive information, including our proprietary and confidential business data, trade secrets, employee data, intellectual property, data we collect about trial participants in connection with clinical trials, and other sensitive data. The global data protection landscape is rapidly evolving and we are or may
become subject to numerous data privacy and security obligations, such as various state, federal and foreign laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements and other obligations that govern the processing of personal, sensitive or confidential information by us and on our behalf, and we may be subject to new or additional data protection laws and regulations and face increased scrutiny from regulators as our business grows. The legislative and regulatory landscape for data privacy and security continues to evolve in jurisdictions worldwide, and there has been an increasing focus on these issues with the potential to affect our business.
Various federal, state, local and foreign legislative and regulatory bodies, or self-regulatory organizations, may expand current laws, rules or regulations, enact new laws, rules or regulations or issue revised rules or guidance regarding data privacy and security that could result in fines or injunctions. Implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future, and we cannot yet determine the impact future laws, regulations, standards, or perception of their requirements may have on our business. This evolution may create uncertainty in our business, affect our ability to operate in certain jurisdictions or to process personal information, necessitate the acceptance of more onerous obligations in our contracts, result in liability or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase in the future. Any failure or perceived failure by us to comply with federal, state or foreign laws or regulations, our internal policies and procedures or our contracts governing our processing of personal, sensitive or confidential information could result in negative publicity, government investigations and enforcement actions, claims by third parties and damage to our reputation, any of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including comprehensive consumer privacy laws, sector-specific privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), data breach notification laws, laws regarding on-line marketing, and other similar laws (e.g., wiretapping laws). For example, the Health Insurance Portability and Accountability Act of 1996, as amended by as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH) (collectively HIPAA), include a privacy rule and security rule that impose among other things, certain requirements relating to the privacy, security, transmission, and breach of individually identifiable health information. We may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA. Depending on the facts and circumstances, we could be subject to significant penalties if we violate HIPAA.
Certain states have also adopted comparable privacy and security laws and regulations, which govern the privacy, processing and protection of health-related and other personal information. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners.
Over a dozen states have also passed comprehensive consumer privacy laws, and similar laws are being considered in several other states, as well as at the federal and local levels, some of which we may become subject to. For example, the California Consumer Privacy Act of 2018 (as amended by the California Privacy Rights Act of 2020) (CCPA) imposes obligations on businesses that meet certain thresholds that process the personal information of California residents (including employees based in California). These obligations include, but are not limited to, providing specific disclosures in privacy notices and affording California residents certain rights related to their personal information. The CCPA also provides for fines of up to $7,500 per intentional violation and allows private litigants affected by certain data breaches to recover significant statutory damages. The 2020 amendments to the CCPA also created the California Privacy Protection Agency, a new enforcement agency whose sole responsibility is to enforce the CCPA and is empowered to create new CCPA regulations. In addition to government activity, privacy advocacy groups and technology and other industries are considering various new, additional or different self-regulatory standards that may place additional burdens on us. In addition to government activity, privacy advocacy groups and technology and other industries continue to consider new or revised self-regulatory standards that may place additional burdens on us.
Outside the United States, the European Union’s General Data Protection Regulation (EU GDPR) and the United Kingdom’s GDPR (UK GDPR) impose strict requirements for processing the personal data of individuals. Among other requirements, the GDPR and UK GDPR (and certain other foreign jurisdictions) regulate the cross- border transfer of personal data, which could make it more difficult for us to transfer information across jurisdictions (such as transferring or receiving personal data that originates in the European Union (EU), or the United Kingdom to countries such as the United States which are not considered by the EU or United Kingdom to provide adequate protection of personal data). In October 2022, the EU-U.S. Data Privacy Framework was implemented, and the European Commission adopted an adequacy decision on July 10, 2023 that set conditions for personal data transfers from the EU to certified companies in the United States without additional safeguards in place. While we strive to adhere to all requirements to transfer information across jurisdictions using safeguards endorsed by government guidance (such as using the Standard Contractual Clauses approved by the European Commission), we must still adapt to changing guidance and will follow any anticipated litigation closely. As the regulatory guidance and enforcement landscape in relation to data transfers continue to develop, we could suffer additional costs,
complaints and/or regulatory investigations or fines; we may have to stop using certain tools and vendors and make other operational changes; and/or it could adversely affect our business, financial condition, results of operations and prospects.
Any such changes in the law related to the use of personal information or data could compromise our ability to pursue our growth strategy effectively or even prevent us from providing certain products in jurisdictions in which we currently operate or may operate in the future. Complying with these numerous, complex and often changing regulations is expensive and difficult, and failure to comply with any data privacy or security laws, whether by us, one of our third-party Contract Development and Manufacturing Organizations (CDMOs), partners or another third party, could adversely affect our business, financial condition, results of operations and prospects and result in expenses which include, but are not limited to: investigation costs, material fines and penalties, compensatory, special, punitive and statutory damages, litigation, consent orders regarding our privacy and security practices, requirements that we provide notices, credit monitoring services and/or credit restoration services or other relevant services to impacted individuals, adverse actions against our licenses to do business, reputational damage and injunctive relief.
In addition to data privacy and security laws, we are also bound by contractual obligations related to data privacy and security. We may be contractually required to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, rules and regulations or other legal obligations relating to privacy or any inadvertent or unauthorized use or disclosure of data that we store or handle as part of operating our business. Any of these events could adversely affect our reputation, business, or financial condition, including but not limited to: loss of customers; interruptions or stoppages in our business operations (including clinical trials); inability to process personal information or to operate in certain jurisdictions; limited ability to develop or commercialize our products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to our business model or operations.
We cannot assure you that our CROs, CDMOs or other third-party service providers with access to our or our suppliers’, manufacturers’, clinical trial participants’ and employees’ sensitive information for which we are responsible will not breach contractual obligations imposed by us, or that they will not experience data security incidents, which could have a corresponding effect on our business, including putting us in breach of our obligations under privacy laws and regulations and/or which could in turn adversely affect our business, financial condition, results of operations and prospects. Our contractual measures and our own privacy and security-related safeguards cannot completely protect us from the risks associated with the third-party processing of such information. Any of the foregoing could adversely affect our business, financial condition, results of operations and prospects.
We also publicly post our privacy policies and practices concerning our collection, use, disclosure and other processing of the personal information provided to us. Although we endeavor to comply with our public statements and documentation, we may at times fail to do so or be perceived to have failed to do so. Our publication of our privacy policies and other statements we publish that provide promises and assurances about privacy and security can subject us to potential state and federal action if they are found to be deceptive, unfair or misrepresentative of our actual practices. Any actual or perceived failure by us to comply with federal, state or foreign laws, rules or regulations, industry standards, contractual or other legal obligations, or any actual, perceived or suspected cybersecurity incident, whether or not resulting in unauthorized access to, or acquisition, release or transfer of personal information or other data, may result in enforcement actions and prosecutions, private litigation, significant fines, penalties and censure, claims for damages by customers and other affected individuals, regulatory inquiries and investigations or adverse publicity and could cause our customers to lose trust in us, any of which could adversely affect our business, financial condition, results of operations and prospects.
We are dependent on the efficient and uninterrupted operation of our information technology systems, and those systems, or those of our third-party service providers, may be impacted by security incidents, cyberattacks, loss of data and other disruptions, which could adversely impact our business.
We are increasingly dependent on information technology systems and infrastructure to operate our business. In the ordinary course of business, we collect, store, generate, transfer, and transmit (collectively “process”) confidential information (such as intellectual property, proprietary business data and patient data). It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such information. We also outsource elements of our information technology systems and operations to third parties (such as vendors, contractors and consultants), and as a result we rely on and take steps designed to manage a number of third- parties who have access to and process our confidential information.
While we take steps designed to detect, mitigate, and remediate vulnerabilities in our information systems, we may not detect or be able to remediate all such vulnerabilities. Further, we may experience delays in developing and deploying remedial measures and patches designed to address identified vulnerabilities, if at all. Despite the implementation of these security measures, our information technology systems and those of our third-party vendors and other contractors and consultants have been in the past and may be in the future potentially vulnerable to service interruptions, system malfunction, accidents by our employees or third-party service providers, natural disasters, terrorism, war, global pandemics, and telecommunication and electrical failures. We may also experience security incidents from inadvertent or intentional actions by our employees, third-party vendors, contractors, consultants, business partners and/or
other third parties, including theft, fraud or unauthorized access to or use of our information technology systems, or attack or damage from hacking, cyberattacks or supply chain attacks by malicious third parties and sophisticated nation-state and nation-state- supported actors, which may compromise our system infrastructure, or that of our third-party vendors and other contractors and consultants, impede our ability to conduct business, delay our financial reporting or lead to data leakage. Any of the above concerns could apply to our third-party suppliers and vendors as well.
The risk of a security incident or disruption, particularly through cyberattacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity, and sophistication of attempted attacks and intrusions from around the world have increased. We may not be able to anticipate all types of security threats, nor implement preventive measures effective against all such security threats. Any breach, loss or compromise of confidential proprietary, or personal information may also subject us to liability, government enforcement actions (for example, investigations, fines, penalties, audits, and inspections), additional reporting requirements and/or oversight, restrictions on processing sensitive information (including personal data), litigation (including class claims), indemnification obligations, negative publicity, reputational harm, monetary fund diversions, diversion of management attention, interruptions in our operations (including availability of data), financial loss and other similar harms. If the information technology systems of our third-party vendors and other contractors and consultants become subject to disruptions or security incidents, we may have insufficient recourse against such third parties and we may have to expend significant resources to mitigate the impact of such an event, and to develop and implement protections to prevent future events of this nature from occurring.
Further, remote work may increase the risks to our information technology systems and data, as remotely working employees utilize network connections, computers and devices outside our premises or network, including working at home, while in transit or in public locations.
Disruptions of our information technology systems or those of our third-party vendors and other contractors and consultants, or security breaches could result in the loss, misappropriation and/or unauthorized access, use, or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property or proprietary business information) and claims by our counterparties that we have failed to comply with legal or contractual obligations, which could result in financial, legal, business, and reputational harm to us.
There can be no assurance that the limitations of liability in our contracts would be enforceable or adequate to protect us from liabilities and damage and we may not have adequate insurance coverage to cover losses, or all types of costs, expenses and losses, we could incur with respect to security breaches or disruptions. The successful assertion of one or more large claims against us that exceeds our available insurance coverage, or results in changes to our insurance policies (including premium increases or the imposition of large deductible or co-insurance requirements), could have an adverse effect on our business. In addition, we cannot be sure that our existing insurance coverage and coverage for errors and omissions will continue to be available on acceptable terms or that our insurers will not deny coverage as to any future claim.
We are an “emerging growth company” and a “smaller reporting company” and the reduced reporting requirements applicable to emerging growth companies or smaller reporting companies could make our common stock less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (i) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (ii) reduced disclosure obligations regarding executive compensation in this Quarterly Report and our periodic reports and proxy statements and (iii) exemptions from the requirements of holding nonbinding advisory stockholder votes on executive compensation and stockholder approval of any golden parachute payments not approved previously. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data in this Quarterly Report.
We could be an emerging growth company until December 31, 2029, although circumstances could cause us to lose that status earlier, including if we are deemed to be a “large accelerated filer,” which occurs when the market value of our common stock that is held by non-affiliates equals or exceeds $700.0 million as of the prior September 30, or if we have total annual gross revenue of $1.235 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31, or if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, in which case we would no longer be an emerging growth company immediately.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards. Until the date that we are no longer an “emerging growth company” or affirmatively and irrevocably opt out of the exemption provided by Section 7(a)(2)(B) of the Securities Act, upon issuance of a new or revised accounting standard that applies to our financial statements and that has a different effective date for public and private companies, we will disclose the date on which adoption is required for non-emerging growth companies and the date on which we will adopt the recently issued accounting standard.
We are also a “smaller reporting company” as defined in the Exchange Act. We will continue to be a smaller reporting company if either (i) the market value of our common stock held by non-affiliates is less than $250.0 million, measured as of the last business day of our most recently completed second quarter or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our common stock held by non-affiliates is less than $700.0 million. We may continue to be a smaller reporting company even after we cease to be an emerging growth company, so we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements, we are not required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Risks Related to Our Reliance on Third Parties
We may, in the future, seek to enter into collaborations or other agreements with third parties for the discovery, development and commercialization of product candidates, if approved, and we may not be successful in doing so. If those collaborations are not successful, we may not be able to capitalize on the market potential of azelaprag and any other current or future product candidates.
We may in the future seek third-party collaborators for research, development and commercialization of azelaprag or future product candidates. Biopharmaceutical companies are our prior and likely future collaborators for any marketing, distribution, development, licensing or broader collaboration arrangements. With respect to our existing collaboration agreements, and what we expect will be the case with any future collaboration agreements, we have and would expect to have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Moreover, our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our technology currently pose, and will continue to pose, the following risks to us:
•collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations and may not perform their obligations as expected;
•collaborators may de-emphasize or not pursue development and commercialization of any product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborators’ strategic focus, including as a result of a sale or disposition of a business unit or development function, or available funding or external factors such as an acquisition that diverts resources or creates competing priorities;
•collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with any product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
•a collaborator with marketing and distribution rights to multiple products may not commit sufficient resources to the marketing and distribution of our product, if approved, relative to other products;
•collaborators may not properly obtain, maintain, defend or enforce our intellectual property rights or may use our proprietary information and intellectual property in such a way as to invite litigation or other intellectual property related proceedings that could jeopardize or invalidate our proprietary information and intellectual property or expose us to potential litigation or other intellectual property related proceedings;
•disputes may arise between the collaborators and us that result in the delay or termination of the research, development or, if approved, commercialization of any product candidates or that result in costly litigation or arbitration that diverts management attention and resources;
•collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or, if approved, commercialization of the applicable product candidates;
•collaboration agreements may not lead to development or, if approved, commercialization of product candidates in the most efficient manner or at all; and
•if a future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or, if approved, commercialization program could be delayed, diminished or terminated.
If our collaborations do not result in the successful development and commercialization of product candidates, or if one of our collaborators terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. Furthermore, even if we receive such payments, they will likely result in payment obligations under license agreements with our licensors, which could be substantial. If we do not receive the funding we expect under these collaboration agreements, or if the funding is substantially offset by payment obligations to our licensors, our development of product candidates could be delayed, and we may need additional resources to develop product candidates. In addition, if one of our collaborators terminates its agreement with us, we may find it more difficult to find a suitable replacement collaborator or attract new collaborators, and our development programs may be delayed or the perception of us in the business and financial communities could be adversely affected.
As a result of the foregoing, our current and any future collaboration agreements may not lead to development or commercialization of our product candidates in the most efficient manner or at all. Moreover, if a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated. Any failure to successfully develop or commercialize our product candidates pursuant to our current or any future collaboration agreements could have a material and adverse effect on our business, financial condition, results of operations and prospects.
We rely, and intend to continue to rely, on third parties to conduct our clinical trials and perform some of our research and preclinical studies. If these third parties do not satisfactorily carry out their contractual duties, fail to comply with applicable regulatory requirements or do not meet expected deadlines, our development programs may be delayed or subject to increased costs or we may be unable to obtain regulatory approval, each of which may have an adverse effect on our business, financial condition, results of operations and prospects.
We do not have the ability to independently conduct all aspects of our clinical trials ourselves. As a result, we are dependent on third parties to conduct our ongoing and planned clinical trials of azelaprag and any future product candidates, as well as potentially preclinical studies of certain future product candidates. The timing of the initiation and completion of these trials will therefore be partially controlled by such third parties and may result in delays to our development programs. Since such third parties partially control the progress of these trials, they may also publish the data related to these trials prior to obtaining or without our approval for doing so. For example, we expect CROs, independent clinical investigators and consultants to play a significant role in the conduct of these trials and the subsequent collection and analysis of data. However, these investigators, CROs and other third parties are not our employees, and we will not be able to control all aspects of their activities. Nevertheless, we are responsible for ensuring that each clinical trial is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the investigators, CROs and other third parties does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with GCP requirements, which are regulations and guidelines enforced by the FDA for product candidates in clinical development. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, clinical trial investigators and clinical trial sites. If we or any of our CROs or clinical trial sites fail to comply with applicable GCP requirements, the data generated in our clinical trials may be deemed unreliable, and the FDA may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that our clinical trials comply with GCPs. In addition, our clinical trials must be conducted with product produced under current Good Manufacturing Practices (cGMP) regulations. Our failure or the failure of third parties on whom we rely to comply with these regulations may require us to stop and/or repeat clinical trials, which would delay the regulatory approval process.
There is no guarantee that any such CROs, clinical trial investigators or other third parties on which we rely will devote adequate time and resources to our development activities or perform as contractually required. In addition, these third parties may be subject to supply chain or inflationary pressures that limit their ability to achieve anticipated timelines or result in a greater cost to us. For example, we are aware of recurrent shortages of non-human primates available for preclinical studies and although that is not expected to impact our current business, if we begin new product development programs we could be subject to longer development times or difficulty completing necessary research. If any of these third parties fail to meet expected deadlines, adhere to our clinical protocols or meet regulatory requirements, otherwise perform in a substandard manner, or terminate their engagements with us, the timelines for our development programs may be extended or delayed or our development activities may be suspended or terminated. If our clinical trial site terminates for any reason, we may experience the loss of follow-up information on subjects enrolled in such clinical trial unless we are able to transfer those subjects to another qualified clinical trial site, which may be difficult or impossible.
For example, we entered into a material transfer agreement with Lilly, under which Lilly has agreed to manufacture and supply us with a certain quantity (which may be increased by mutual consent) of tirzepatide so we can sponsor a clinical trial in which azelaprag and tirzepatide are co-administered concomitantly or sequentially. If we experience difficulties procuring such products, we could be delayed or even prevented from proceeding with the clinical trials of our product candidates.
In addition, with respect to investigator-sponsored trials that may be conducted, we would not control the design or conduct of these trials, and it is possible that the FDA will not view these investigator-sponsored trials as providing adequate support for future clinical trials or market approval, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results. We expect that such arrangements will provide us certain information rights with respect to the investigator-sponsored trials, including access to and the ability to use and reference the data, including for our own regulatory submissions, resulting from the investigator-sponsored trials. However, we would not have control over the timing and reporting of the data from investigator-sponsored trials, nor would we own the data from the investigator-sponsored trials. If we are unable to confirm or replicate the results from the investigator- sponsored trials or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development. Further, if investigators or institutions breach their obligations with respect to the clinical development of our product candidates, or if the data proves to be inadequate compared to the firsthand knowledge we might have gained had the investigator-sponsored trials been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected. The investigators may design clinical trials with clinical endpoints that are more difficult to achieve, or in other ways that increase the risk of negative clinical trial results compared to clinical trials that we may design on our own. Negative results in investigator-sponsored clinical trials could have a material adverse effect on our efforts to obtain regulatory approval for our product candidates and the public perception of our product candidates. Additionally, the FDA may disagree with the sufficiency of our right of reference to the preclinical, manufacturing or clinical data generated by these investigator-sponsored trials, or our interpretation of preclinical, manufacturing or clinical data from these investigator-sponsored trials. If so, the FDA may require us to obtain and submit additional preclinical, manufacturing, or clinical data.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors for whom they may also be conducting clinical trials or other pharmaceutical product development activities that could harm our competitive position. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approval for azelaprag and any future product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our products.
The manufacture of pharmaceutical products, including our product candidates, such as azelaprag, is complex. Our third-party manufacturers may encounter difficulties in production, which could delay or entirely halt their ability to supply our product candidates for clinical trials or, if approved, for commercial sale.
We do not have any manufacturing facilities, and we currently contract with certain third-party manufacturers, which are located in China. We rely, and expect to continue to rely, on third parties for the manufacture of our product candidates and related raw materials for preclinical and clinical testing, product development purposes, to support regulatory application submissions, as well as for commercial manufacture if any of our product candidates obtain regulatory approval. In addition, we expect to contract with analytical laboratories for release and stability testing of our product candidates. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts and cause the FDA to withdraw certain designations, including orphan drug designation. For example, we cannot be sure to what extent the supply chain issues caused by geopolitical uncertainty and public health epidemics, may impact our ability to procure sufficient supplies for the development of our product candidates and what, if any, impact that may have on our facilities and operations in the region, including but not limited to a decrease or disruption of production, increased costs of production or other interruptions in our supply chain. In addition, any disruption in production or inability of our manufacturers, specifically in China, to produce adequate quantities to meet our needs, whether as a result of a natural disaster or other causes, could impair our ability to operate our business on a day-to-day basis and to continue our development of our product candidates.
Furthermore, since some of our third-party manufacturers are located in China, we are exposed to the possibility of product supply disruption and increased costs in the event of changes in the policies of the United States or Chinese governments, political unrest or unstable economic conditions in China. In addition, certain Chinese biotechnology companies may become subject to trade restrictions, sanctions, other regulatory requirements, or proposed legislation by the U.S. government, which could restrict or even prohibit our ability to work with such entities, thereby potentially disrupting the supply of material to us. For example, the recently proposed BIOSECURE Act introduced in the U.S. House of Representatives, as well as a substantially similar bill in the U.S. Senate, target U.S. government contracts, grants and loans for entities that use equipment and services from certain named Chinese biotechnology companies. If enacted as presently proposed, the BIOSECURE Act would, among other things, prohibit U.S. federal agencies from entering into or renewing any contract with any entity that uses biotechnology equipment or services produced or provided by a “biotechnology company of concern” to perform that contract as well as authorize the U.S. government to name additional Chinese “biotechnology companies of concern.” The BIOSECURE Act defines a “biotechnology company of concern” to include WuXi Apptec and its affiliates (WuXi). We are presently party to agreements with WuXi, pursuant to which WuXi provides development and manufacturing services to us. If these bills become law, or similar laws are passed, they would have the potential to severely restrict our
ability to work with Chinese biotechnology manufacturing companies without losing the ability to contract with, or otherwise receive funding from, the U.S. government. We cannot predict what actions may ultimately be taken with respect to trade relations between the United States and China or other countries, what products and services may be subject to such actions or what actions may be taken by China or the other countries in retaliation.
Any of these matters could materially adversely affect our business, financial condition and results of operations. In addition, disruptions in logistics routes and transportation capabilities could disrupt our supply chain. And, if we experience unexpected spikes in demand over time, we risk running out of our necessary supplies.
We may be unable to enter into additional agreements with third-party manufacturers or suppliers on favorable terms. Our anticipated reliance on a limited number of third party-manufacturers or suppliers exposes us to the following risks:
•reliance on the third party for regulatory, compliance and quality assurance;
•reliance on the third party for product development, analytical testing and data generation to support regulatory applications;
•operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier, the issuance of an FDA Form 483 notice or warning letter or other enforcement action by the FDA or other regulatory authority;
•the possible breach of the manufacturing agreement by the third party;
•the possible misappropriation of our proprietary information, including our trade secrets and know-how;
•the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us;
•carrier disruptions or increased costs that are beyond our control; and
•failure to deliver our drugs under specified storage conditions and in a timely manner.
Third-party manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside of the United States. If the FDA determines that our CDMOs are not in compliance with FDA laws and regulations, including those governing cGMPs, the FDA may not approve a new drug application (NDA) until the deficiencies are corrected or we replace the manufacturer in our application with a manufacturer that is in compliance. Moreover, our failure, or the failure of our third-party manufacturers and suppliers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products. In addition, approved products and the facilities at which they are manufactured are required to maintain ongoing compliance with extensive FDA requirements and the requirements of other similar agencies, including ensuring that quality control and manufacturing procedures conform to cGMP requirements. As such, our CDMOs are subject to continual review and periodic inspections to assess compliance with cGMPs. Furthermore, although we do not have day-to-day control over the operations of our CDMOs, we are responsible for ensuring compliance with applicable laws and regulations, including cGMPs.
Our product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. As a result, we may not obtain access to these facilities on a priority basis or at all. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
As we prepare for later-stage clinical trials and potential commercialization, we will need to take steps to increase the scale of production of our product candidates. We have not yet scaled up the manufacturing process for any of our product candidates apart from azelaprag and may need to scale further to support future supply needs for any of our product candidates. Third-party manufacturers may be unable to successfully increase the manufacturing capacity for any of our product candidates in a timely or cost-effective manner, or at all. In addition, quality issues may arise during scale-up or commercial activities. For example, if microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or regulatory approval. We expect to have an arrangement in place for a redundant supply or a second source for the active pharmaceutical ingredients of API in 2024. If our current CDMOs cannot perform as agreed, we may be required to replace such CDMOs. Although we believe that there are several potential alternative manufacturers who could manufacture our product candidates, we may incur added costs and delays in identifying and qualifying any such replacement manufacturer or be able to reach agreement with any alternative manufacturer. In this case, our clinical trials supply could be delayed significantly as we establish alternative supply sources. In addition, if we are required to change CDMOs for any reason, we will be required to verify that the new CDMO maintains facilities and procedures that comply with quality standards and with all applicable regulations. We will also need to verify, such as through a manufacturing comparability study, that any new manufacturing process will produce our product candidate according to the specifications previously submitted to the FDA or another regulatory authority. The delays associated with the verification of a new CDMO could negatively affect our ability to develop product candidates or commercialize our products in a timely manner or within budget. In addition, changes in manufacturers often involve changes in manufacturing procedures and processes, which could require that we conduct bridging studies between our prior clinical supply used in our clinical trials and that of any new manufacturer. We may be unsuccessful in demonstrating the comparability of clinical supplies, which could require the conduct of additional clinical trials. Further, our third- party manufacturers may experience manufacturing or shipping difficulties due to resource constraints or as a result of natural disasters, labor disputes, unstable political environments or public health epidemics.
Our current and anticipated future dependence upon others for the manufacture of our product candidates or products may adversely affect our future profit margins and our ability to commercialize any products that obtain regulatory approval on a timely and competitive basis.
If we, or any contract manufacturers or suppliers we engage, fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We and our third-party contractors are subject to numerous federal, state, local and foreign environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources, including any available insurance. We could also be held liable for unexpected safety events that could happen in our business offices.
In addition, our leasing and operation of real property may subject us to liability pursuant to certain of these laws or regulations. Under existing United States environmental laws and regulations, current or previous owners or operators of real property and entities that disposed or arranged for the disposal of hazardous substances may be held strictly, jointly and severally liable for the cost of investigating or remediating contamination caused by hazardous substance releases, even if they did not know of and were not responsible for the releases.
We could incur significant costs and liabilities which may adversely affect our financial condition and operating results for failure to comply with such laws and regulations, including, among other things, civil or criminal fines and penalties, property damage and personal injury claims, costs associated with upgrades to our facilities or changes to our operating procedures, or injunctions limiting or altering our operations.
Although we maintain liability insurance to cover us for costs and expenses we may incur due to injuries to our employees, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations, which are becoming increasingly more stringent, may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Risks Related to Intellectual Property
If we do not obtain patent term extension for any product candidates we may develop, our business may be harmed.
Depending upon the timing, duration and specifics of any FDA regulatory approval of azelaprag and any other product candidates we may develop and our technology, our U.S. patents or one or more U.S. patents that may issue in the future based on a patent application that we license or own may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved product, a method for using it or a method for manufacturing it may be extended. The application for the extension must be submitted prior to the expiration of the patent for which extension is sought and within 60 days of FDA approval. A patent that covers multiple products for which approval is sought can only be extended in connection with one of the approvals.
However, we may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. In addition, to the extent we wish to pursue patent term extension based on a patent that we in-license from a third party, we would need the cooperation of that third party. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Patent terms may be insufficient to protect our competitive position on azelaprag and any future product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various patent term adjustments or extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering azelaprag or any future product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products identical or similar to ours.
Obtaining and maintaining our patent protection is dependent on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the U.S. Patent and Trademark Office (USPTO) and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and/or rely on our outside counsel to pay these fees due to the USPTO and non-U.S. governmental patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
Changes in U.S. patent and ex-U.S. patent laws could diminish the value of patents in general, thereby impairing our ability to protect our current or future product candidates.
Changes in either the patent laws or interpretation of the patent laws in the United States or in other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. In the United States, numerous recent changes to the patent laws and proposed changes to the rules of the USPTO may have a significant impact on our ability to protect our technology and enforce our intellectual property rights.
For example, the Leahy-Smith Act includes a number of significant changes to United States patent law. These changes include provisions that affect the way patent applications are prosecuted, redefine prior art, provide more efficient and cost-effective avenues for competitors to challenge the validity of patents, and enable third-party submission of prior art to the USPTO during patent prosecution
and additional procedures to attack the validity of a patent at USPTO-administered post- grant proceedings, including post-grant review, inter partes review, and derivation proceedings.
Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith Act, the United States transitioned to a first-to-file system in which, assuming that the other statutory requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. As such, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, the patent positions of companies in the development and commercialization of pharmaceuticals and biologics are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future. For example, in the case, Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that claims to certain DNA molecules are not patentable. In Amgen Inc. v. Sanofi, the Federal Circuit held that claims with functional language may face high hurdles in fulfilling the enablement requirement. Recent decisions raise questions regarding the award of patent term adjustment (PTA) for patents where related patents have been issued without a PTA. Thus, it cannot be said with certainty how PTA will or will not be viewed in future and whether patent expiration dates may be impacted. We cannot predict how this and future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents. Any similar adverse changes in the patent laws of other jurisdictions could also have a material adverse effect on our business, financial condition, results of operations and prospects.
Furthermore, in Europe, a new unitary patent system took effect June 1, 2023, which will significantly impact European patents, including those granted before the introduction of such a system. Under the unitary patent system, European applications have the option, upon grant of a patent, of becoming a Unitary Patent which will be subject to the jurisdiction of the Unitary Patent Court (UPC). As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty of any litigation. Patents granted before the implementation of the UPC will have the option of opting out of the jurisdiction of the UPC and remaining as national patents in the UPC countries. Patents that remain under the jurisdiction of the UPC will be potentially vulnerable to a single UPC-based revocation challenge that, if successful, could invalidate the patent in all countries who are signatories to the UPC. We cannot predict with certainty the long-term effects of any potential changes.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our current or future trademarks or trade names may be challenged, infringed, circumvented or declared generic or descriptive or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest.
During trademark registration proceedings, we may receive rejections of our applications by the USPTO or in other foreign jurisdictions. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected. We may license our trademarks and trade names to third parties, such as distributors. Although these license agreements may provide guidelines for how our trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names.
Moreover, any name we have proposed to use with our therapeutic candidate in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. Similar requirements exist in Europe. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA (or an equivalent administrative body in a foreign jurisdiction) objects to any of our proposed proprietary product names, it may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. Furthermore, in many countries, owning and maintaining a trademark registration may not provide an adequate defense against a subsequent infringement claim asserted by the owner of a senior trademark. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. If we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to develop products that are similar to our product candidates but that are not covered by the claims of the patents that we own or license;
•we or our licensors or collaborators might not have been the first to make the inventions covered by the issued patents or patent application that we own or license;
•we or our licensors or collaborators might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•it is possible that the pending patent applications we own or license will not lead to issued patents;
•issued patents that we own or license may be held invalid or unenforceable, as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may have an adverse effect on our business;
•we may fail to adequately protect and police our trademarks and trade secrets; and
•we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, it could significantly harm our business, financial condition, results of operations and prospects.
Our rights to develop and commercialize our lead product candidate, azelaprag, as well as certain future products, are or may be subject to the terms and conditions of license agreements.
We have in the past licensed, and may in future license, certain patent rights and proprietary technology from third parties that are important or necessary to the development of our product candidates. For example, On April 5, 2021, we entered into an exclusive license agreement (the Amgen Agreement) with Amgen Inc. (Amgen), pursuant to which we have an exclusive, worldwide license, with the right to sublicense (subject to certain conditions), under Amgen’s rights in specified patents relating to Amgen’s clinical-stage apelin receptor APJ agonist azelaprag (named AMG 986 by Amgen) as well as their other APJ agonists. The Amgen Agreement imposes various diligence, milestone payment, royalty, insurance, indemnification and other obligations on us. If we breach any material obligation, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and Amgen
may have the right to terminate the license. If the license is terminated, we may be unable to develop, manufacture, sell, or use azelaprag and Amgen may allow a competitor to license the covered technology instead.
Out-license agreements we may enter into in the future may include exclusivity terms limiting our ability to develop product candidates that may compete with the relevant licensed target or product. If such exclusivity restrictions prevent us from developing or commercializing our technologies in a way that we deem necessary to gain or maintain our competitive advantage, it may have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
We may not have complete control in the preparation, filing, prosecution, maintenance, enforcement and defense of patents and patent applications covering the technology that we license from third parties. For example, under the Amgen Agreement, we have the first right to file, prosecute, maintain and enforce the licensed patents, and Amgen has the option to take over prosecution, maintenance and enforcement activities should we decline to take such actions. Amgen also has the right to comment on prosecution and maintenance activities, and cooperate on enforcement activities. It is possible that our licensors’ enforcement of patents against infringers or defense of such patents against challenges of validity or claims of enforceability may be less vigorous than if we had conducted them ourselves, or may not be conducted in accordance with our best interests. We cannot be certain that these patents and patent applications will be prepared, filed, prosecuted, maintained, enforced and defended in a manner consistent with the best interests of our business. If our licensors fail to prosecute, maintain, enforce and defend such patents, or lose rights to those patents or patent applications, the rights we have licensed may be reduced or eliminated, our right to develop and commercialize any of our product candidates we may develop that are the subject of such licensed rights could be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
Our licensors may have relied on third-party consultants or collaborators or on funds from third parties such that our licensors are not the sole and exclusive owners of the patents we in-licensed. If other third parties have ownership rights to our in-licensed patents, the license granted to us in jurisdictions where the consent of a co-owner is necessary to grant such a license may not be valid and such co-owners may be able to license such patents to our competitors, and our competitors could market competing products and technology. In addition, our rights to our in-licensed patents and patent applications are dependent, in part, on inter-institutional or other operating agreements between the joint owners of such in-licensed patents and patent applications. If one or more of such joint owners breaches such inter-institutional or operating agreements, our rights to such in-licensed patents and patent applications may be adversely affected. Any of these events could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
If we breach our license agreements it could have a material adverse effect on our commercialization efforts for azelaprag and any future product candidates.
We are party to the Amgen Agreement that enables us to utilize certain of Amgen’s intellectual property in the development and commercialization of azelaprag, and we may in the future enter into more such license agreements with third parties under which we license the use, development and commercialization rights to current or future product candidates or technology from third parties.
These intellectual property license agreements may require us to comply with various obligations, including diligence obligations such as development and commercialization obligations, as well as potential royalty and milestone payments and other obligations. If we fail to comply with our obligations under any of these license agreements, use the licensed intellectual property in an unauthorized manner, we are subject to bankruptcy- related proceedings or otherwise materially breach any of these license agreements, the terms of the license granted may be materially modified, such as by rendering currently exclusive licenses non-exclusive, or it may give our licensors the right to terminate the applicable license agreement, in whole or in part. Generally, the loss of or termination of our rights under the Amgen Agreement, or any other licenses we may acquire in the future, could harm our business, financial condition, results of operations and prospects.
We may also, in the future, enter into license agreements with third parties under which we are a sublicensee. If our sublicense or fails to comply with its obligations under its upstream license agreement with its licensor, the licensor may have the right to terminate the upstream license, which may result in termination of our sublicense. If this were to occur, we would no longer have rights to the applicable intellectual property unless we are able to secure our own direct license with the owner of the relevant rights, which we may not be able to do on reasonable terms, or at all, which may impact our ability to continue to develop and commercialize product candidates incorporating the relevant intellectual property.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including:
•the scope of rights granted under the Amgen Agreement and other interpretation-related issues;
•whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•our right to sublicense patent and other intellectual property rights to third parties under collaborative development relationships;
•our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization product candidates, and what activities satisfy those diligence obligations;
•the calculation of total payment amount due if we develop multiple products under the license agreement(s);
•our right to transfer or assign the license;
•the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
•whether and the extent to which inventors are able to contest the assignment of their rights to our licensors.
If disputes over intellectual property that we have licensed or license in the future prevent or impair our ability to maintain our current licensing arrangements on acceptable terms or at all, we may be unable to successfully develop and commercialize the affected product candidates, which could have material adverse effect on our business. In addition, if disputes arise as to ownership of licensed intellectual property, our ability to pursue or enforce the licensed patent rights may be jeopardized. If we or our licensors fail to adequately protect this intellectual property, our ability to commercialize our products could suffer. Further, certain of our future license agreements with third parties may limit or delay our ability to consummate certain transactions, may impact the value of those transactions or may limit our ability to pursue certain activities (e.g., we may in the future enter into license agreements that are not assignable or transferable, or that require the licensor’s express consent in order for an assignment or transfer to take place).
Third-party claims of intellectual property infringement may prevent or delay our product discovery and development efforts.
Our commercial success depends in part on our ability to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries, as well as administrative proceedings for challenging patents, including interference, derivation, inter partes review, post grant review, and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our product candidates and/or proprietary technologies infringe their intellectual property rights. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to our product candidates and programs. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others. Moreover, it is not always clear to industry participants, including us, which patents cover various types of drugs, products or their methods of use or manufacture that may be relevant to our product candidates. Thus, because of the large number of patents issued and patent applications filed in our fields, there may be a risk that third parties may allege they have patent rights encompassing our product candidates, technologies or methods. If any such patent were to be asserted against us, we may have defenses against any such action, including that these patents would not be infringed by our product candidates and/or that these patents are not valid. However, if these patents were asserted against us and our defenses to such an action were unsuccessful, unless we obtain a license to these patents, which may not be available on commercially reasonable terms, or at all, we could be liable for damages and precluded from commercializing azelaprag in certain indications, which could have a material adverse effect on our business, financial condition, cash flows or results of operations.
If a third-party claims that we infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
•infringement and other intellectual property claims which, regardless of merit, may be expensive and time-consuming to litigate and may divert our management’s attention from our core business;
•substantial damages for infringement, which we may have to pay if a court decides that the product candidate or technology at issue infringes on or violates the third party’s rights, and, if the court finds that the infringement was willful, we could be ordered to pay treble damages and the patent owner’s attorneys’ fees;
•a court prohibiting us from developing, manufacturing, marketing or selling our product candidates, or from using our proprietary technologies, unless the third party licenses its product rights to us, which it is not required to do;
•if a license is available from a third party, we may have to pay substantial royalties, upfront fees and other amounts, and/or grant cross-licenses to intellectual property rights for our products, if any; and
•redesigning our product candidates or processes so they do not infringe, which may not be possible or may require substantial monetary expenditures and time.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations or could otherwise have a material adverse effect on our business, results of operations, financial condition and prospects.
Third parties may assert that we are employing their proprietary technology without authorization. Generally, conducting clinical trials and other development activities in the United States is protected under the Safe Harbor exemption as set forth in 35 U.S.C. § 271. If and when azelaprag or any future product candidate is approved by the FDA, a certain third party may then seek to enforce its patent by filing a patent infringement lawsuit against us. While we do not believe that any claims of such patent that could otherwise materially adversely affect commercialization of our product candidates, if approved, are valid and enforceable, we may be incorrect in this belief, or we may not be able to prove it in a litigation. In this regard, patents issued in the United States by law enjoy a presumption of validity that can be rebutted only with evidence that is “clear and convincing,” a heightened standard of proof. There may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our product candidates, molecules used in or formed during the manufacturing process, or the product candidate itself, the holders of any such patents may be able to block our ability to commercialize the product candidate unless we obtained a license under the applicable patents, or until such patents expire or they are finally determined to be held invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, manufacturing process or methods of use, the holders of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtained a license or until such patent expires or is finally determined to be held invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business. Even if we obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Even if such a license is available, it may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
Lastly, we may need to indemnify our customers and distributors against claims relating to the infringement of intellectual property rights of third parties related to our product candidates, including azelaprag. Third parties may assert infringement claims against our customers or distributors. These claims may require us to initiate or defend protracted and costly litigation on behalf of our customers or distributors, regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of our customers, suppliers or distributors, or may be required to obtain licenses for the product candidates or services they use. If we cannot obtain all necessary licenses on commercially reasonable terms, our customers may be forced to stop using our products, if approved, or services.
We may not be able to protect our intellectual property rights throughout the world.
Although we have pending patent applications in the United States and other countries, filing, prosecuting, maintaining, enforcing and defending patents in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates, and our patents, the patents of our licensors, or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many foreign countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or our licensors’ patents or marketing of competing products in violation of our proprietary rights. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents or the patents of our licensors at risk of being invalidated or interpreted narrowly and our patent applications or the patent applications of our licensors at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, we rely on the protection of our trade secrets, including unpatented know-how, technology and other proprietary information to maintain our competitive position. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants and advisors, we cannot provide any assurances that all such agreements have been duly executed, and any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
Moreover, third parties may still obtain this information or may come upon this or similar information independently, and we would have no right to prevent them from using that technology or information to compete with us. If any of these events occurs or if we otherwise lose protection for our trade secrets, the value of this information may be greatly reduced, and our competitive position would be harmed. If we do not apply for patent protection prior to such publication or if we cannot otherwise maintain the confidentiality of our proprietary technology and other confidential information, then our ability to obtain patent protection or to protect our trade secret information may be jeopardized.
Third-party claims of intellectual property infringement, misappropriation or other violations against us or our collaborators could be expensive and time consuming and may prevent or delay the development and commercialization of our product candidates.
Our commercial success depends in part on our ability to avoid infringing, misappropriating and otherwise violating the patents and other intellectual property rights of third parties. There is a substantial amount of complex litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries, as well as administrative proceedings for challenging patents, including interference, derivation and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. Such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical products and techniques without payment, or limit the duration of the patent protection of our technology. As discussed above, recently,
due to changes in U.S. law referred to as patent reform, new procedures including inter partes review and post-grant review have also been implemented. As stated above, this reform adds uncertainty to the possibility of challenge to our patent rights in the future.
Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we are commercializing or plan to commercialize azelaprag. As the biotechnology and pharmaceutical industries expand and more patents are issued, and as we gain greater visibility and market exposure as a public company, the risk increases that azelaprag or any future product candidates, and commercializing activities may give rise to claims of infringement of the patent rights of others. We cannot assure you that azelaprag or any future product candidates will not infringe existing or future patents owned by third parties. We may not be aware of patents that have already been issued for which a third party, such as a competitor in the fields in which we are developing azelaprag or our future product candidates, might accuse us of infringing. It is also possible that patents owned by third parties of which we are aware, but which we do not believe we infringe or that we believe we have valid defenses to any claims of patent infringement, could be found to be infringed by us. It is not unusual that corresponding patents issued in different countries have different scopes of coverage, such that in one country a third-party patent does not pose a material risk, but in another country, the corresponding third-party patent may pose a material risk to azelaprag and any future product candidates. As such, we monitor third-party patents in the relevant pharmaceutical markets. In addition, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that we may infringe.
In the event that any third-party claims that we infringe their patents or that we are otherwise employing their proprietary technology without authorization and initiates litigation against us, even if we believe such claims are without merit, a court of competent jurisdiction could hold that such patents are valid, enforceable and infringed by us. Defense of infringement claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and other employee resources from our business, and may impact our reputation. In the event of a successful claim of infringement against us, we may be enjoined from further developing or commercializing the infringing products or technologies. In addition, we may be required to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties and/or redesign our infringing products or technologies, which may be impossible or require substantial time and monetary expenditure. Such licenses may not be available on commercially reasonable terms or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms or at all, we may be unable to commercialize the infringing products or technologies or such commercialization efforts may be significantly delayed, which could in turn significantly harm our business. In addition, we may in the future pursue patent challenges with respect to third-party patents, including as a defense against the foregoing infringement claims. The outcome of such challenges is unpredictable.
Even if resolved in our favor, the foregoing proceedings could be very expensive, particularly for a company of our size, and time-consuming. Such proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such proceedings adequately. Some of our competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than we can because of greater financial resources. Such proceedings may also absorb significant time of our technical and management personnel and distract them from their normal responsibilities. Uncertainties resulting from such proceedings could impair our ability to compete in the marketplace. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition or results of operations.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, consultants, collaborators or other third parties have an interest in our patent rights, any potential trade secrets, or other intellectual property as an inventor, co-inventor or owner of any potential trade secrets. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates and other proprietary technologies we may develop. Litigation may be necessary to defend against these and other claims challenging inventorship or our patent rights, any potential trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our product candidates and other proprietary technologies we may develop. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to our management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Third party claims or litigation alleging infringement of patents or other proprietary rights, or seeking to invalidate our patents or other proprietary rights, may delay or prevent the development and commercialization of our current or future product candidates or technologies.
Our commercial success depends in part on our avoiding infringement and other violations of the patents and proprietary rights of third parties. The intellectual property landscape around obesity and metabolic diseases drug development is highly dynamic and there is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biopharmaceutical industry. Potential litigation could include patent infringement lawsuits, derivation and administrative law proceedings, inter partes review and post-grant review before the USPTO, as well as oppositions and similar processes in foreign jurisdictions. As the fields of treating obesity and metabolic diseases continue to expand and more patents are issued, and as we gain greater visibility and market exposure as a public company, the risk increases that our product candidates or other business activities may be subject to claims of infringement of the patent and other proprietary rights of third parties. Third parties may assert that we are infringing their patents or employing their proprietary technology without authorization. Also, there may be third party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates or technologies may infringe.
Defense of third-party claims of patent infringement or violation of intellectual property rights involves substantial litigation expense and would be a substantial diversion of management and employee time and resources from our business. Some third parties may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise funds necessary to continue our operations or could otherwise have a material adverse effect on our business, financial condition, results of operations and prospects. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, third parties may obtain patent rights in the future and claim that use of our product candidates or other technologies infringe upon these rights. If any third-party patents were held by a court of competent jurisdiction to cover our product candidates, or any aspect of their manufacture or use, the holders of any such patents may be able to block our ability to commercialize such product candidate or technology unless we obtain a license under the applicable patents, or until such patents expire. Such a license may not be available on commercially reasonable terms, or at all. In addition, we may be subject to claims that we are infringing other intellectual property rights, such as trademarks or copyrights, or misappropriating the trade secrets of others, and to the extent that our employees, consultants or contractors use intellectual property or proprietary information owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful infringement or other intellectual property claim against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our affected products or technologies, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms.
The scope of a patent claim is a legal determination made by the courts. It is informed by the written disclosure of a patent, the patent’s prosecution history, and other intrinsic and extrinsic factors. Our interpretation of a patent claim may not be adopted during a patent litigation alleging infringement by our products. If a court does not adopt our claim interpretation and determines that our product candidates are covered by a third-party patent, we may be held liable for damages. Similarly, we may incorrectly predict whether a third-party patent application will issue with claims that cover one or more of our product candidates. If our claim interpretations are not adopted by the USPTO during prosecution of a third-party patent application, or by a court in a patent infringement dispute, our ability to develop and market our product candidates may be harmed.
Moreover, we, or one of our licensors, may have to participate in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge priority of invention or other features of patentability. If we or our licensors are unsuccessful in any validity (including any patent oppositions) or inventorship disputes to which we or they are subject, we may lose valuable intellectual property rights through the loss of one or more of our owned, licensed or optioned patents, or such patent claims may be narrowed, invalidated or held unenforceable, or through loss of exclusive ownership of or the exclusive right to use our owned or in-licensed patents. In the event of loss of patent rights as a result of any of these disputes, we may be required to obtain licenses from third parties, including parties involved in any such proceedings. If we are unable to obtain such licenses, we may need to cease the development, manufacture and commercialization of one or more of the product candidates or technologies we may develop. The loss of exclusivity or the
narrowing of our patent claims could limit our ability to stop others from using or commercializing similar or identical technology and product candidates. Even if we or our licensors are successful in such a proceeding, it could result in substantial costs and be a distraction to management and other employees.
Furthermore, the patent landscape is crowded and highly competitive. Numerous third-party United States and foreign issued patents and pending patent applications exist in the fields in which we are developing product candidates, and they may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. Ongoing research and development is taking place by several companies, universities, and other institutions. There can be no assurance that our operations do not, or will not in the future, infringe, misappropriate or otherwise violate existing or future third-party patents or other intellectual property rights. Identification of third-party patent rights that may be relevant to our operations is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases, and publication timelines. We cannot guarantee that any patent searches we may conduct are complete or thorough enough to identify every third-party patent and pending application in the United States and/or abroad that is relevant to or necessary for the development and commercialization of our product candidates in any country.
We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be unable to further develop and commercialize one or more of our product candidates, which could harm our business significantly. We cannot provide any assurances that third party patents do not exist which might be enforced against our product candidates resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties or other forms of compensation to third parties.
If we are unable to obtain and maintain patent protection or other necessary rights for any of our current or future product candidates and technology, or if the scope of the patent protection obtained is not sufficiently broad or our rights under our patents (owned, co-owned or licensed) is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our products and technology may be adversely affected.
Our success is dependent in part on our ability to obtain and maintain proprietary or intellectual property protection in the United States and other countries for our current product candidates or any future product candidates, as well as our core technologies, including our manufacturing know-how. We strive to protect and enhance the proprietary technology, inventions and improvements that are commercially important to the development of our business by seeking, maintaining and defending our intellectual property, whether developed internally or licensed from third parties. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen and maintain our proprietary position in obesity and metabolic disease drug development. Additionally, we intend to utilize regulatory protection afforded through rare drug designations, data exclusivity and market exclusivity as well as patent term extensions, where available.
The patent position of biotechnology and biopharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has in recent years been the subject of much litigation. The degree of patent protection we require to successfully compete in the marketplace may be unavailable or severely limited in some cases and may not adequately protect our rights or permit us to gain or keep any competitive advantage. We cannot provide any assurances that any of our own or licensed patent applications will mature into issued patents, and cannot provide any assurances that any such patents, if issued, will include claims with a scope sufficient to protect our current and future product candidates or otherwise provide any competitive advantage. Additionally, patents can be enforced only in those jurisdictions in which the patent has issued. Furthermore, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally twenty years after its first nonprovisional U.S. filing. The natural expiration of a patent outside of the United States varies in accordance with provisions of applicable local law, but is generally 20 years from the earliest local filing date. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
Moreover, our exclusive license to azelaprag may be subject to certain retained rights, which may adversely impact our competitive position. Our licensed patent portfolio may not provide us with adequate and continuing patent protection sufficient to exclude others from commercializing products similar to our product candidates, including generic versions of such products. In addition, the patent portfolio licensed to us is, or may be, licensed to third parties outside our licensed field, and such third parties may have certain enforcement rights. Thus, patents licensed to us could be put at risk of being invalidated or interpreted narrowly in litigation filed by or against another licensee or in administrative proceedings brought by or against another licensee in response to such litigation or for other reasons.
Other parties have developed technologies that may be related or competitive to our own and such parties may have filed or may file patent applications, or may have received or may receive patents, claiming inventions that may overlap or conflict with those claimed in our own patent applications or issued patents. Publication of discoveries in the scientific literature lags behind the actual discoveries,
and patent applications in the United States and in other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether the inventors of our patents and applications were the first to make the inventions claimed in those patents or pending patent applications, or that they were the first to file for patent protection of such inventions. Further, we cannot assure you that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. As a result, the issuance, scope, validity and commercial value of our patent rights cannot be predicted with any certainty. Further, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
In addition, the patent prosecution process is expensive and time-consuming, and we or our licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, the scope of the claims initially submitted for examination may be significantly narrowed by the time they issue, if at all. It is also possible that we or our licensors will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We cannot provide any assurances that we will be able to pursue or obtain additional patent protection based on our research and development efforts, or that any such patents or other intellectual property we generate will provide any competitive advantage.
Even if we acquire patent protection that we expect should enable us to maintain competitive advantage, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability. Third parties, including former employees, consultants, collaborators and competitors, may challenge the inventorship, scope, validity, or enforceability thereof, which may result in such patents being narrowed, invalidated or held unenforceable. If issued, our patents may be challenged in patent offices in the United States and abroad, or in court. For example, we may be subject to a third party submission of prior art to the USPTO challenging the validity of one or more claims of our patents, once issued. Such submissions may also be made prior to a patent’s issuance, precluding the granting of a patent based on one of our patent applications. We may become involved in opposition, reexamination, inter partes review, post-grant review, derivation, interference, or similar proceedings in the United States or abroad challenging the claims of our patents, once issued. Furthermore, patents may be challenged in court, once issued. Competitors may claim that they invented the inventions claimed in such patents or patent applications, or may have filed patent applications before the inventors of our patents did. A competitor may also claim that we are infringing its patents and that we therefore cannot practice our technology as claimed under our patent applications and patents, if issued. As a result, one or more claims of our patents may be narrowed or invalidated. In litigation, a competitor could claim that our patents, if issued, are not valid for a number of reasons. If a court agrees, we would lose our rights to those challenged patents.
Even if they are unchallenged, our patents and pending patent applications, if issued, may not provide us with any meaningful protection or prevent competitors from designing around our patent claims to circumvent our patents by developing similar or alternative technologies or therapeutics in a non-infringing manner. For example, even if we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention if the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. If the patent protection provided by the patents and patent applications we hold or pursue with respect to our product candidates is not sufficiently broad to impede such competition, our ability to successfully commercialize our product candidates could be negatively affected, which would harm our business.
Certain regulatory exclusivities may be available, however, the scope of such regulatory exclusivities is subject to change, and may not provide us with adequate and continuing protection sufficient to exclude others from commercializing products similar to our product candidates.
Risks Related to Government Regulation
Disruptions at the FDA, the SEC and other government agencies or comparable regulatory authorities caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, otherwise prevent new products and services from being developed, approved or commercialized in a timely manner or at all, or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA or other regulatory authorities to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, statutory, regulatory and policy changes, and other events that may otherwise affect the FDA’s or comparable foreign regulatory authorities’ ability to perform routine functions. In addition, government funding of the SEC, and other government agencies or comparable foreign regulatory authorities on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA or other regulatory authorities may also slow the time necessary for new drugs to be reviewed and/or approved, which would adversely affect our business. For example, in 2024, the U.S. government was on the verge of a shutdown and has previously shut down several times, and certain regulatory agencies, such as the FDA and the SEC, had to furlough critical employees and stop critical activities. If a prolonged government shutdown occurs, or if geopolitical or global health concerns prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns or delays could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
In addition, three decisions from the U.S. Supreme Court in July 2024 may lead to an increase in litigation against regulatory agencies that could create uncertainty and thus negatively impact our business. The first decision overturned established precedent that required courts to defer to regulatory agencies’ interpretations of ambiguous statutory language. The second decision overturned regulatory agencies’ ability to impose civil penalties in administrative proceedings. The third decision extended the statute of limitations within which entities may challenge agency actions. These cases may result in increased litigation by industry against regulatory agencies and impact how such agencies choose to pursue enforcement and compliance actions. However, the specific, lasting effects of these decisions, which may vary within different judicial districts and circuits, is unknown. We also cannot predict the extent to which FDA and SEC regulations, policies, and decisions may become subject to increasing legal challenges, delays, and changes.
Existing, recently enacted and future legislation may increase the difficulty and cost for us to obtain regulatory approval of and commercialize our product candidates and decrease the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any products for which we obtain regulatory approval.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the regulatory approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent regulatory approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs, including costs of pharmaceuticals. There has been heightened governmental scrutiny over the manner in which manufacturers set prices for their products, which has resulted in several presidential executive orders, Congressional inquiries, and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare and Medicaid, and reform government program reimbursement methodologies for drug products. For example, on August 2, 2011, the Budget Control Act of 2011 imposed, subject to certain temporary suspension periods, 2% reductions in Medicare payments to providers per fiscal year starting April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2032, unless additional Congressional action is taken. In December 2020, CMS issued a final rule implementing significant manufacturer price reporting changes under the Medicaid Drug Rebate Program, including an alternative rebate calculation for line extensions that is tied to the price increases of the original drug, and Best Price reporting related to certain value-based purchasing arrangements. Under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs is eliminated. Elimination of this cap may, in some cases, require pharmaceutical manufacturers to pay more in rebates than they receive on the sale of products.
Recently, several healthcare reform initiatives culminated in the enactment of the Inflation Reduction Act (the IRA) in August 2022, which, among other things, allows United States Health and Human Services (HHS) to directly negotiate the selling price of a statutorily specified number of drugs and biologics each year that CMS reimburses under Medicare Part B and Part D. Only high-expenditure single-source drugs that have been approved for at least 7 years (11 years for single-source biologics) are eligible to be selected for negotiation by CMS, with the negotiated price taking effect two years after the selection year. Negotiations for Medicare Part D products begin in 2024 with the negotiated price taking effect in 2026, and negotiations for Medicare Part B products begin in 2026 with the negotiated price taking effect in 2028. In August 2023, HHS announced the ten Medicare Part D drugs and biologics that it selected for negotiations. HHS will announce the negotiated maximum fair prices by September 1, 2024. This price cap, which cannot exceed a statutory ceiling price, will come into effect on January 1, 2026, and will represent a significant discount from average prices to wholesalers and direct purchasers. The IRA also imposes rebates on Medicare Part D and Part B drugs whose prices have increased at a rate greater than the rate of inflation. In addition, the law eliminates the “donut hole” under Medicare Part D beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and requiring manufacturers to subsidize, through a newly
established manufacturer discount program, 10% of Part D enrollees’ prescription costs for brand drugs below the out-of-pocket maximum, and 20% once the out-of-pocket maximum has been reached. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in Patient Protection and Affordable Care Act (ACA) marketplaces through plan year 2025. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including significant civil monetary penalties. These provisions may be subject to legal challenges. For example, the provisions related to the negotiation of selling prices of high-expenditure single-source drugs and biologics have been challenged in multiple lawsuits brought by pharmaceutical manufacturers. The outcome of these lawsuits is uncertain, and some IRA drug discount provisions have not been challenged in litigation. Thus, while it is unclear how the IRA will be implemented, it will likely have a significant impact on the pharmaceutical industry and the pricing of azelaprag or any future product candidates.
At the state level, legislatures are increasingly enacting laws and implementing regulations designed to control pharmaceutical and biological product pricing, including restrictions or prohibitions on certain marketing practices, reporting of specified categories of remuneration provided to health care practitioners, and reporting and justification of price increases greater than a specified level. In some cases, states have designed programs to encourage importation from other countries and bulk purchasing, though the federal government has not yet approved any such plans. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for pharmaceuticals and other healthcare products and services, which could result in reduced demand for azelaprag or any future product candidates or companion diagnostics or additional pricing pressures.
We expect that other healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
The insurance coverage and reimbursement status of newly approved products are uncertain. Failure to obtain or maintain coverage and adequate reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
Sales of our product candidates, if approved, will depend, in part, on the extent to which such products will be covered by third-party payors, such as government health care programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly limiting coverage and/or reducing reimbursements for medical products and services. A third-party payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. Further, one payor’s determination to provide coverage for a drug product does not ensure that other payors will also provide coverage for the drug product. Coverage policies and third-party payor reimbursement rates may change at any time. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (CMS) as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors often, but not always, follow CMS’s decisions regarding coverage and reimbursement. Decreases in third-party payor reimbursement or a decision by a third-party payor to not cover any of our product candidates, if approved, could reduce physician usage of our product candidates, and have a material adverse effect on our sales, results of operations and financial condition. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain FDA approvals. Nonetheless, our product candidates may not be considered medically necessary or cost-effective.
Our operations and relationships with healthcare providers, healthcare organizations, customers and third- party payors will be subject to applicable anti-bribery, anti-kickback, fraud and abuse, transparency and other healthcare laws and regulations, which could expose us to, among other things, enforcement actions, criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
Our current and future arrangements with healthcare providers, healthcare organizations, third-party payors and customers expose us to broadly applicable anti-bribery, fraud and abuse and other healthcare laws and regulations that may constrain the business or
financial arrangements and relationships through which we research, market, sell and distribute any of our product candidates, if approved. Restrictions under applicable federal and state anti-bribery and healthcare laws and regulations, include the following:
•the federal Anti-Kickback Statute, which prohibits, among other things, individuals and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under a federal and state healthcare program such as Medicare and Medicaid. The term remuneration has been broadly interpreted to include anything of value. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•the federal criminal and civil false claims and civil monetary penalties laws, including the federal False Claims Act, which can be enforced through civil whistleblower or qui tam actions against individuals or entities, and the Federal Civil Monetary Penalties Law, which prohibit, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. Moreover, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act;
•HIPAA and its implementing regulations, which imposes criminal and civil liability, prohibits, among other things, knowingly and willfully executing, or attempting to execute a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•HIPAA, as amended by HITECH, and their respective implementing regulations, which impose obligations on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services involving the storage, use or disclosure of individually identifiable health information for or on behalf of a covered entity and their covered subcontractors, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information;
•the federal Physician Payments Sunshine Act, which requires certain manufacturers of covered drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program, with certain exceptions, to report annually to CMS information related to certain payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other health care professionals (such as physician assistants and certain advance practices nurses), and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members, with the information made publicly available on a searchable website;
•the Foreign Corrupt Practices Act which prohibits U.S. businesses and their representatives from offering to pay, paying, promising to pay or authorizing the payment of money or anything of value to a foreign official in order to influence any act or decision of the foreign official in his or her official capacity or to secure any other improper advantage in order to obtain or retain business;
•analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, that may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; and
•certain state laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and drug pricing information, and state and local laws that require the registration of biopharmaceutical sales representatives.
Efforts to ensure that our current and future business arrangements with third parties comply with applicable healthcare laws and regulations could involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any such requirements, we may be subject to significant penalties,
including civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, integrity oversight and reporting obligations, or reputational harm, any of which could adversely affect our financial results. These risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction. For example, even if the FDA grants regulatory approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we or any partner we work with fail to comply with the regulatory requirements in international markets or fail to receive applicable regulatory approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Adverse side effects or other safety risks associated with azelaprag or any future product candidates we may develop could delay or preclude approval, cause us to suspend or discontinue clinical trials or abandon further development, change the design of our clinical trials, limit the commercial profile of an approved product, or result in significant negative consequences following regulatory approval, if any.
As is the case with small molecules generally, it is likely that there may be adverse side effects associated with the use of azelaprag or any future product candidates. For example, we have observed certain adverse events such as mild headaches and back pain and dizziness, which were higher in our placebo patients than in our active patients, in our clinical trials of azelaprag. Our clinical trials may reveal significant adverse events not seen in our preclinical studies or prior clinical trials and may result in a safety or tolerability profile that could delay or prevent regulatory approval or market acceptance of azelaprag or any future product candidates. Undesirable or clinically unmanageable side effects observed in our clinical trials for our product candidates could occur and cause us or regulatory authorities to interrupt, delay or halt our clinical trials and could result in more restrictive labeling than anticipated or the delay or denial of regulatory approval by the FDA or other regulatory authorities. If additional adverse events, serious adverse events (SAEs) or other side effects are observed in any of our clinical trials that are atypical of, or more severe than, the known side effects of the respective class of agents that each of our product candidates are a part of, we may have difficulty recruiting participants to our clinical trials, participants may drop out of our trials, or we may be required to abandon those trials or our development efforts of one or more product candidates altogether. Furthermore, clinical trials by their nature utilize a sample of the potential patient population. With a limited number of subjects and limited duration of exposure, rare and severe side effects of our product candidates or those of our competitors may only be uncovered with a significantly larger number of patients exposed to the drug. Undesirable or clinically unmanageable side effects observed in our clinical trials for our product candidates could also occur following discontinuation of azelaprag or any future product candidates with sufficient recovery periods, and we will need to monitor the severity and duration of side effects in our clinical trials. If such effects are more severe, less reversible than we expect or not reversible at all, we may decide or be required to perform additional studies or to halt or delay further clinical development of azelaprag, which could result in the delay or denial of regulatory approval by the FDA or other regulatory authorities. Adverse events and SAEs that emerge during clinical investigation of or treatment with azelaprag or any future product candidates may be deemed to be related to our product candidates. Moreover, if our product candidates are associated with undesirable side effects in clinical trials or have characteristics that are unexpected, we may elect to abandon or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk- benefit perspective, which may limit the commercial expectations for our product candidates, if approved. This may require longer and more extensive clinical development, or regulatory authorities may increase the amount of data and information required to approve, market or maintain approval for azelaprag or any future product candidates and could result in warnings and precautions in our product labeling or a restrictive REMS. This may also result in an inability to obtain approval of azelaprag or any future product candidates. We, the FDA or other regulatory authorities or an IRB or ethics committee may suspend clinical trials of a product candidate at any time for various reasons, including a belief that participants in such trials are being exposed to unacceptable health risks or adverse side effects. Even if the side effects do not preclude the product candidate from obtaining
or maintaining regulatory approval, undesirable side effects may inhibit market acceptance of the approved product due to its tolerability versus other therapies. Further, it is possible that, as we test our product candidates in larger, longer and more extensive clinical trials, including with different dosing regimens, or as the use of our drug candidates becomes more widespread following any regulatory approval, illnesses, injuries, discomforts and other adverse events that were observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by patients. Any of these developments could materially harm our business, financial condition, results of operations and prospects.
We plan to conduct future clinical trials at sites outside the United States. The FDA may not accept data from trials conducted in such locations, and the conduct of trials outside the United States could subject us to additional delays and expense.
We have conducted one Phase 1 trial of azelaprag in a study of older patients in New Zealand. The acceptance by the FDA or other regulatory authorities of trial data from clinical trials conducted outside their jurisdiction may be subject to certain conditions or may not be accepted at all.
Where foreign clinical trial data are not intended to serve as the sole basis for approval, the FDA will not accept the data as support for an application for marketing approval unless the trial is well-designed and well- conducted in accordance with GCP requirements and the FDA is able to validate the data from the trial through an onsite inspection if deemed necessary. Many foreign regulatory authorities have similar approval requirements. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA or any comparable foreign regulatory authority will accept data from trials conducted outside of the U.S. or the applicable jurisdiction. If the FDA or any comparable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which could be costly and time-consuming, and which may result in current or future product candidates that we may develop not receiving approval for commercialization in the applicable jurisdiction.
Conducting clinical trials outside the U.S. also exposes us to additional risks, including risks associated with:
•additional foreign regulatory requirements;
•foreign exchange fluctuations;
•compliance with foreign manufacturing, customs, shipment and storage requirements;
•cultural differences in medical practice and clinical research;
•diminished protection of intellectual property in some countries; and
•interruptions or delays in our trials resulting from geopolitical events, such as war or terrorism.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
U.S. and foreign anti-corruption, anti-money laundering, export control, sanctions and other trade laws and regulations prohibit, among other things, companies and their employees, agents, CROs, CDMOs, legal counsel, accountants, consultants, contractors and other partners from authorizing, promising, offering, providing, soliciting, or receiving directly or indirectly, corrupt or improper payments or anything else of value to or from recipients in the public or private sector. Export control and sanctions laws may also prohibit or limit our ability to sell or provide our drug candidates to embargoed countries, regions, governments, persons and entities. Violations of these laws can result in substantial criminal fines and civil penalties, imprisonment, the loss of trade privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We also expect our non-U.S. activities to increase over time. We expect to rely on third parties for research, preclinical studies and clinical trials and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. We can be held liable for the corrupt or other illegal activities of our personnel, agents, or partners, even if we do not explicitly authorize or have prior knowledge of such activities.
Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
Risks Related to Our Common Stock
Anti-takeover provisions in our charter documents and under Delaware law could prevent or delay an acquisition of us, which may be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and our restated bylaws contain provisions that could delay or prevent a change in control of our company. These provisions could also make it difficult for stockholders to elect directors who are not nominated by current members of our board of directors or take other corporate actions, including effecting changes in our management. These provisions:
•establish a classified board of directors so that not all members of our board of directors are elected at one time;
•permit only the board of directors to establish the number of directors and fill vacancies on the board of directors;
•provide that directors may only be removed “for cause” and only with the approval of two-thirds of our stockholders;
•require super-majority voting to amend some provisions in our restated certificate of incorporation and restated bylaws;
•authorize the issuance of “blank check” preferred stock that our board of directors could use to implement a stockholder rights plan;
•eliminate the ability of our stockholders to call special meetings of stockholders;
•prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders;
•prohibit cumulative voting; and
•establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon by stockholders at annual stockholder meetings.
In addition, Section 203 of the Delaware General Corporation Law (DGCL), may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions on mergers, business combinations and other transactions between us and holders of 15% or more of our common stock.
The exclusive forum provisions in our organizational documents may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or employees, or the underwriters of any offering giving rise to such claim, which may discourage lawsuits with respect to such claims.
Our restated certificate of incorporation, to the fullest extent permitted by law, provides that the Court of Chancery of the State of Delaware is the exclusive forum for: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the DGCL, our restated certificate of incorporation, or our restated bylaws; or any action asserting a claim that is governed by the internal affairs doctrine. This exclusive forum provision does not apply to suits brought to enforce a duty or liability created by the Exchange Act. It could apply, however, to a suit that falls within one or more of the categories enumerated in the exclusive forum provision. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or other employees, or the underwriters of any offering giving rise to such claims, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provisions contained in our restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, financial condition, results of operations and prospects.
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all claims brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Our restated bylaws provide that the federal district courts of the United States will, to the fullest extent permitted by law, be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act, or the Federal Forum Provision, including for all causes of action asserted against any defendant named in such complaint. For the avoidance of doubt, this provision is intended to benefit and may be enforced by us, our directors, officers, other employees, agents, and the underwriters to any offering giving rise to such complaint, and any other professional person or entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering. Our decision to adopt a Federal Forum Provision followed a decision by the Supreme Court of the State of Delaware holding that such provisions are facially valid under Delaware law. While federal or other state courts may not follow the holding of the Delaware Supreme Court or may determine that the Federal Forum Provision should be enforced in a particular case, application of the Federal Forum Provision means that suits brought by our stockholders to enforce any duty or liability created by the Securities Act must be brought in federal court and cannot be brought in state court, and our stockholders cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 27 of the Exchange Act creates exclusive
federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. In addition, neither the exclusive forum provision nor the Federal Forum Provision applies to suits brought to enforce any duty or liability created by the Exchange Act. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder must be brought in federal court, and our stockholders cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
Any person or entity purchasing or otherwise acquiring or holding any interest in any of our securities shall be deemed to have notice of and consented to our exclusive forum provisions, including the Federal Forum Provision. These provisions may limit a stockholders’ ability to bring a claim, and may result in increased costs for a stockholder to bring such a claim, in a judicial forum of their choosing for disputes with us or our directors, officers, other employees or agents, which may discourage lawsuits against us and our directors, officers, other employees or agents.
The market price of our common stock is likely to be highly volatile, and you could lose all or part of your investment.
The trading price of our common stock is likely to continue to be highly volatile and subject to wide fluctuations in response to various factors, some of which we cannot control. As a result of this volatility, investors may not be able to sell their common stock at or above the price initially paid for the stock. The market price for our common stock may be influenced by many factors, including the other risks described in this “Risk Factors” section and the following:
•results of preclinical studies and clinical trials of any product candidates, or those of our competitors or our existing or future collaborators or licensing partners;
•the timing and enrollment status of our clinical trials;
•regulatory or legal developments in the United States or other countries, especially changes in laws or regulations applicable to any product candidates;
•the success or failure of competitive products or technologies;
•introductions and announcements of new product candidates by us, any future commercialization partners, or our competitors, and the timing of these introductions or announcements;
•actions taken by regulatory agencies with respect to any product candidates, clinical studies, and, if approved, manufacturing process or sales and marketing terms;
•actual or anticipated variations in our financial results or those of companies that are perceived to be similar to us;
•the success of our efforts to acquire or in-license additional technologies or product candidates;
•developments concerning any future collaborations, including but not limited to those with development and commercialization partners if any product candidates are approved;
•market conditions in the pharmaceutical and biotechnology sectors;
•announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures or capital commitments;
•developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for any product candidates;
•our ability or inability to raise additional capital and the terms on which we are able to raise it, if at all;
•the recruitment or departure of key personnel;
•changes in the structure of healthcare payment systems;
•actual or anticipated changes in earnings estimates, development timelines or changes in stock market analyst recommendations regarding our common stock, other comparable companies or our industry generally;
•our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•announcement and expectation of additional financing efforts;
•speculation in the press or investment community;
•fluctuations of trading volume of our common stock;
•sales of shares of our common stock by us, insiders or our stockholders;
•the concentrated ownership of our common stock;
•expiration of market stand-off or lock-up agreements;
•changes in accounting principles;
•actions instituted by activist shareholders or others;
•terrorist acts, acts of war or periods of widespread civil unrest;
•natural disasters and other calamities, including global pandemics such as the COVID-19 pandemic; and
•general economic, industry and market conditions, including rising interest rates and inflation.
In addition, the stock market in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular, have experienced extreme price and volume fluctuations that have been often unrelated or disproportionate to the operating performance of the issuer. Furthermore, the trading price of our common stock may be adversely affected by third parties trying to drive down the market price. Short sellers and others, some of whom post anonymously on social media, may be positioned to profit if our stock declines and their activities can negatively affect our stock price. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in this “Risk Factors” section, could have a dramatic and adverse impact on the market price of our common stock.
We do not currently intend to pay dividends on our common stock and, consequently, our stockholders’ ability to achieve a return on their investment will be dependent on appreciation of the value of our common stock.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. We do not intend to declare or pay any cash dividends on our capital stock in the foreseeable future. As a result, any investment return on our common stock will be dependent on increases in the value for our common stock, which is not certain. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have any control over the industry or securities analysts, or the content and opinions included in their reports. If no or few securities or industry analysts continue or commence coverage of us, the trading price for our common stock could be impacted negatively. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our preclinical studies and clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of such analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause a decline in our stock price or trading volume.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that our stockholders intend to sell in the public market before or after the lock-up and other legal restrictions on resale lapse in connection with our IPO, the market price of our stock could decline significantly. Each of our officers, directors and substantially all of our stockholders have entered into lock-up agreements that, among other things and subject to certain exceptions, restrict their ability to sell or transfer their shares. The lock-up agreements will expire on March 24, 2025. However, Goldman Sachs & Co. LLC, Morgan Stanley & Co. LLC and Jefferies LLC may, in their sole discretion, permit our officers, directors and other stockholders who are subject to the lock-up agreements to sell shares prior to March 25, 2025.
The holders of an aggregate of 20,854,632 shares of our outstanding common stock as of September 30, 2024 will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration
statements that we may file for ourselves or our stockholders. We also have registered shares of common stock that we may issue under our equity incentive plans. These shares, are freely tradeable in the public market upon issuance, subject to the 180-day lock-up period under the lock-up agreements described above.
We cannot predict what effect, if any, sales of our shares in the public market or the availability of shares for sale will have on the market price of our common stock. However, future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of our outstanding options, or the perception that such sales may occur, could adversely affect the market price of our common stock.
We also expect that significant additional capital may be needed in the future to continue our planned operations. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. To the extent that additional capital is raised through the sale and issuance of shares of our common stock or other securities convertible into shares of our common stock, our stockholders will be diluted. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares of our common stock, could reduce the market price of our common stock.
General Risk Factors
Our current in-person operations are located in Richmond, California, and we or the third parties on whom we depend may be adversely affected by natural disasters, terrorist activity, pandemics, geo-political actions in the United States and in foreign countries, and other events beyond our control, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster. Geo-political actions could increase the uncertainties and costs surrounding the prosecution or maintenance of our patent applications or those of any current or future licensors and the maintenance, enforcement or defense of our issued patents or those of any current or future licensors.
While we are currently a remote-based company with a majority of our employees working remotely, our current in-person operations are located in our research facility in Richmond, California. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, pandemic, medical epidemic, power shortage, telecommunication failure or other natural or manmade accidents or incidents that result in us being unable to fully utilize our facilities, or the manufacturing facilities of our CDMOs may have a material and adverse effect on our ability to operate our business and have significant negative consequences on our financial and operating conditions. If our facilities, or the manufacturing facilities of our CDMOs, are unable to operate because of an accident or incident or for any other reason, including an inability to use all or a significant portion of our headquarters, damages to critical infrastructure, such as our research facilities or the manufacturing facilities of our CDMOs, or other disruptions to operations, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Our employees often conduct business outside of any facilities leased by us. These locations may be subject to additional security and other risk factors due to the limited control of our employees. The disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at these facilities, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses.
Unstable market and economic conditions and adverse developments affecting the financial services industry, such as actual events or concerns involving inflation, liquidity, defaults or nonperformance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations, and its financial condition and results of operations.
From time to time, the global credit and financial markets have experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. The financial markets and the global economy may also be adversely affected by the current or anticipated impact of military conflict, terrorism or other geopolitical events. Sanctions imposed by the United States and other countries in response to such conflicts, including the one in Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. Russia’s ongoing incursion of Ukraine has created extreme volatility in the global capital markets and is expected to have further global economic consequences, including disruptions of the global supply chain and energy markets; it is possible that the ensuing Israel-Hamas conflict may have similar effects. In addition, adverse developments that affect financial institutions, such as events involving liquidity that are rumored or actual, have in the past and may in the future lead to market-wide liquidity problems. For example, in March 2023, Silicon Valley Bank (SVB), one of our banking partners, was closed by the California Department of Financial Protection
and Innovation, which appointed the Federal Deposit Insurance Corporation (FDIC) as receiver. We previously kept substantially all of our cash and investments with SVB, the substantial majority of which was held in a custodial account with another institution, for which SVB Asset Management was the advisor. While we were afforded full access to our cash and investments with SVB, we may be impacted by other disruptions to the U.S. banking system, including potential delays in our ability to transfer funds whether held with SVB or otherwise. The closure of any additional national or regional commercial banks could lead to further economic instability. Although the Department of the Treasury, the Federal Reserve and the FDIC have taken steps to mitigate these risks, widespread demands for customer withdrawals or other liquidity needs of financial institutions for immediately liquidity may still occur in the future. We regularly maintain cash balances at third-party financial institutions in excess of the FDIC insurance limit and there is no guarantee that the federal government would provide access to uninsured funds in the future in the event of the closure of other banks or financial institutions, or that they would do so in a timely fashion.
Although we have not experienced any adverse impact to our liquidity or to our current and projected business operations, financial condition or results of operations, uncertainty remains over liquidity concerns in the broader financial services industry, and our business, our business partners, or industry as a whole may be adversely impacted in ways that we cannot predict at this time. Inflation and rapid increases in interest rates have led to a decline in the trading value of previously issued government securities with interest rates below current market interest rates.
In addition, if any of our suppliers or other parties with whom we conduct business are unable to access funds pursuant to such instruments or lending arrangements with any financial institution, such parties’ ability to pay their obligations to us or to enter into new commercial arrangements requiring additional payments to us could be adversely affected. In this regard, counterparties to SVB credit agreements and arrangements, and third parties such as beneficiaries of letters of credit (among others), may experience direct impacts from the closure of SVB, and uncertainty remains over liquidity concerns in the broader financial services industry. Similar impacts have occurred in the past, such as during the 2008-2010 financial crisis.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an emerging growth company or smaller reporting company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of Nasdaq and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain sufficient coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. The increased costs will decrease our net income or increase our net loss, and the increased costs may require us to reduce costs in other areas of our business.
Moreover, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock is likely to be volatile. The stock market in general, and Nasdaq and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs, divert our management’s attention and resources from other business concerns and damage our reputation, which could seriously harm our business, financial condition, results of operations and prospects.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
(a) Recent Sales of Unregistered Equity Securities
None.
(b) Use of Proceeds from Initial Public Offering and Concurrent Private Placement
On September 25, 2024, our Registration Statement on Form S-1 (No. 333-281901) was declared effective by the SEC, pursuant to which we issued and sold an aggregate of 12,650,000 shares of common stock (inclusive of 1,650,000 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares) at a public offering price of $18.00 per share for aggregate gross proceeds of $227.7 million and aggregate net cash proceeds of $211.8 million, after deducting approximately $15.9 million in underwriting discounts and commissions. Concurrently with the initial public offer, we also completed a private placement, in which we issued and sold an aggregate of 588,888 shares of our common stock at a price of $18.00 per share to Sofinnova Venture Partners, IX, L.P. The aggregate cash purchase price of the private placement shares was $10.6 million, resulting in aggregate net cash proceeds of $9.9 million, after deducting approximately $0.7 in placement agent fees. Our IPO and concurrent private placement closed on September 27, 2024. Goldman Sachs & Co, LLC, Morgan Stanley, Jefferies LLC and Citigroup acted as joint book-running managers for the offering and placement agents for the concurrent private placement. In connection with our IPO and concurrent private placement, no payments for such expenses were made directly or indirectly to (i) any of our officers or directors or their associates, (ii) any persons owning 10% or more of any class of our equity securities or (iii) any of our affiliates.
There has been no material change in the planned use of proceeds from our initial public offering and concurrent private placement as described in our final prospectus filed with the SEC pursuant to Rule 242(b)(4) under the Securities Act on September 26, 2024.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable
Item 5. Other Information.
None.
Item 6. Exhibits.
| | | | | | | | | | | | |
Exhibit Number |
| Description |
Form |
File No. |
Exhibit |
Filing Date |
Filed Herewith |
|
|
|
|
|
|
3.1 |
| Restated Certificate of Incorporation. |
|
|
|
| X |
3.2 |
| Restated Bylaws. |
|
|
|
| X |
4.1 |
| Form of Common Stock Certificate. | S-1 | 333-281901 | 4.1 | 09/03/2024 |
|
10.1 |
| Form of Indemnity Agreement. | S-1/A | 333-281901 | 10.1 | 09/18/2024 |
|
10.2 |
| 2024 Equity Incentive Plan and forms of award agreements. | S-1/A | 333-281901 | 10.3 | 09/18/2024 |
|
10.3 |
| 2024 Employee Stock Purchase Plan and forms of award agreements. | S-1/A | 333-281901 | 10.4 | 09/18/2024 |
|
10.4 |
| Change in Control and Severance Plan. | S-1/A | 333-281901 | 10.5 | 09/18/2024 |
|
10.5 |
| Offer Letter by and between the Registrant and Kristen Fortney, dated September 17, 2024. | S-1/A | 333-281901 | 10.10 | 09/18/2024 |
|
10.6 |
| Offer Letter by and between the Registrant and Eric Morgen, dated September 17, 2024. | S-1/A | 333-281901 | 10.11 | 09/18/2024 |
|
10.7 |
| Offer Letter by and between the Registrant and Paul Rubin, dated September 17, 2024. | S-1/A | 333-281901 | 10.12 | 09/18/2024 |
|
10.8 |
| Share Purchase Agreement, dated as of September 25, 2024, by and among Company and the Purchaser. | 8-K | 001-42279 | 10.1 | 09/27/2024 |
|
10.9 | | Office and Laboratory Lease by and between the Company and ES East, LLC, dated August 23, 2024. | | | | | X |
31.1 |
| Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
| X |
31.2 |
| Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
| X |
32.1* |
| Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
| X |
32.2* |
| Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
| X |
101.INS |
| Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
|
|
| X |
101.SCH |
| Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
|
|
|
| X |
104 |
| Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
| X |
* This certification is deemed not filed for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | |
|
| BioAge Labs, Inc. |
|
|
|
|
Date: November 7, 2024 |
| By: | /s/ Kristen Fortney |
|
|
| Kristen Fortney, Ph.D. |
|
|
| Chief Executive Officer, President and Director |
|
|
| (Principal Executive Officer) |
|
|
|
|
Date: November 7, 2024 |
| By: | /s/ Dov Goldstein |
|
|
| Dov Goldstein, M.D. |
|
|
| Chief Financial Officer |
|
|
| (Principal Financial Officer) |
