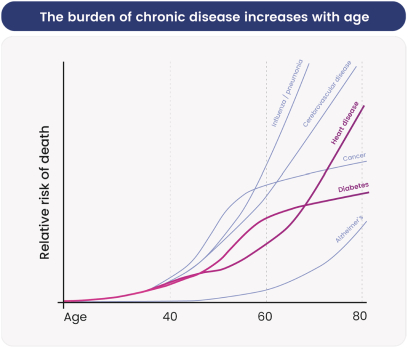
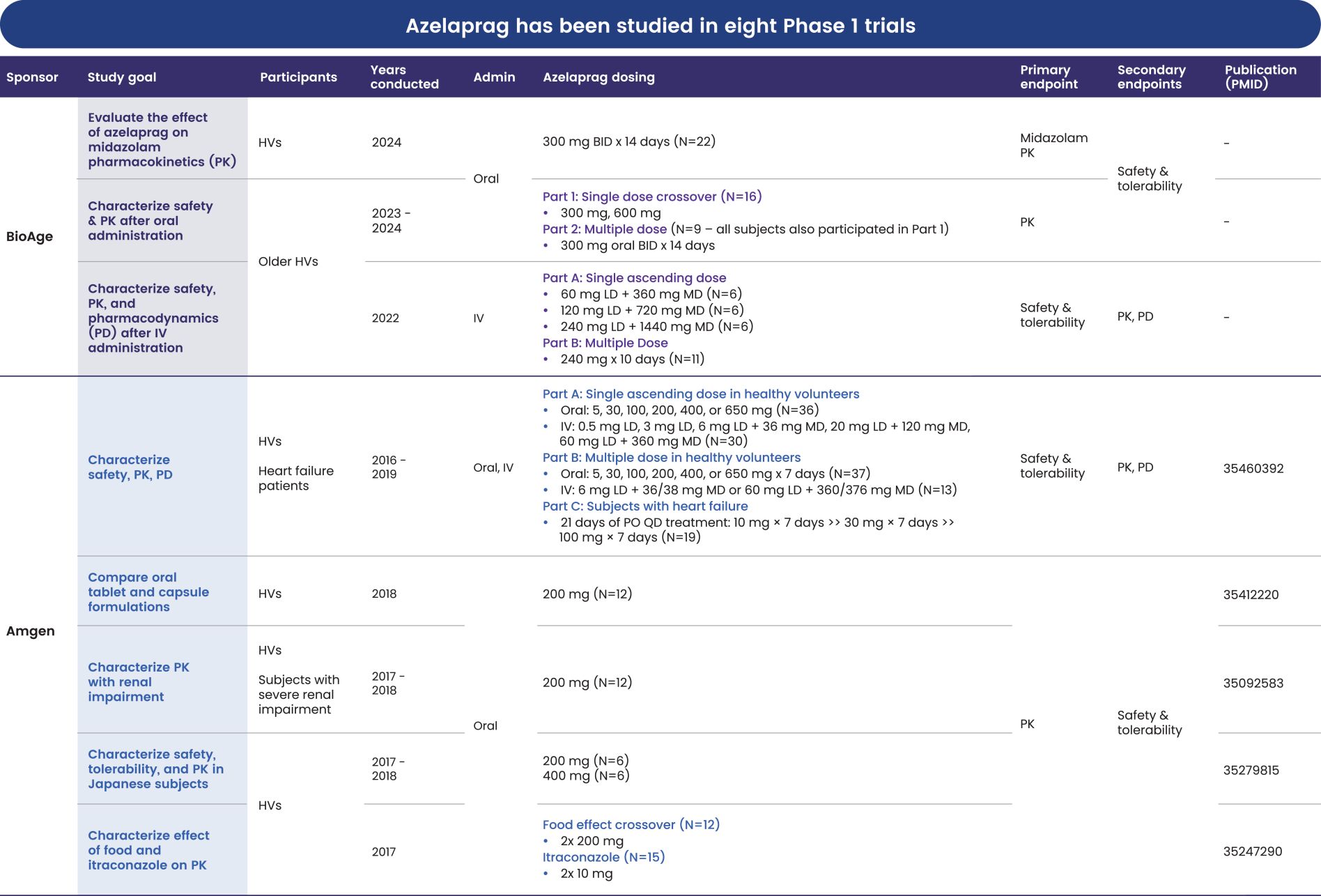
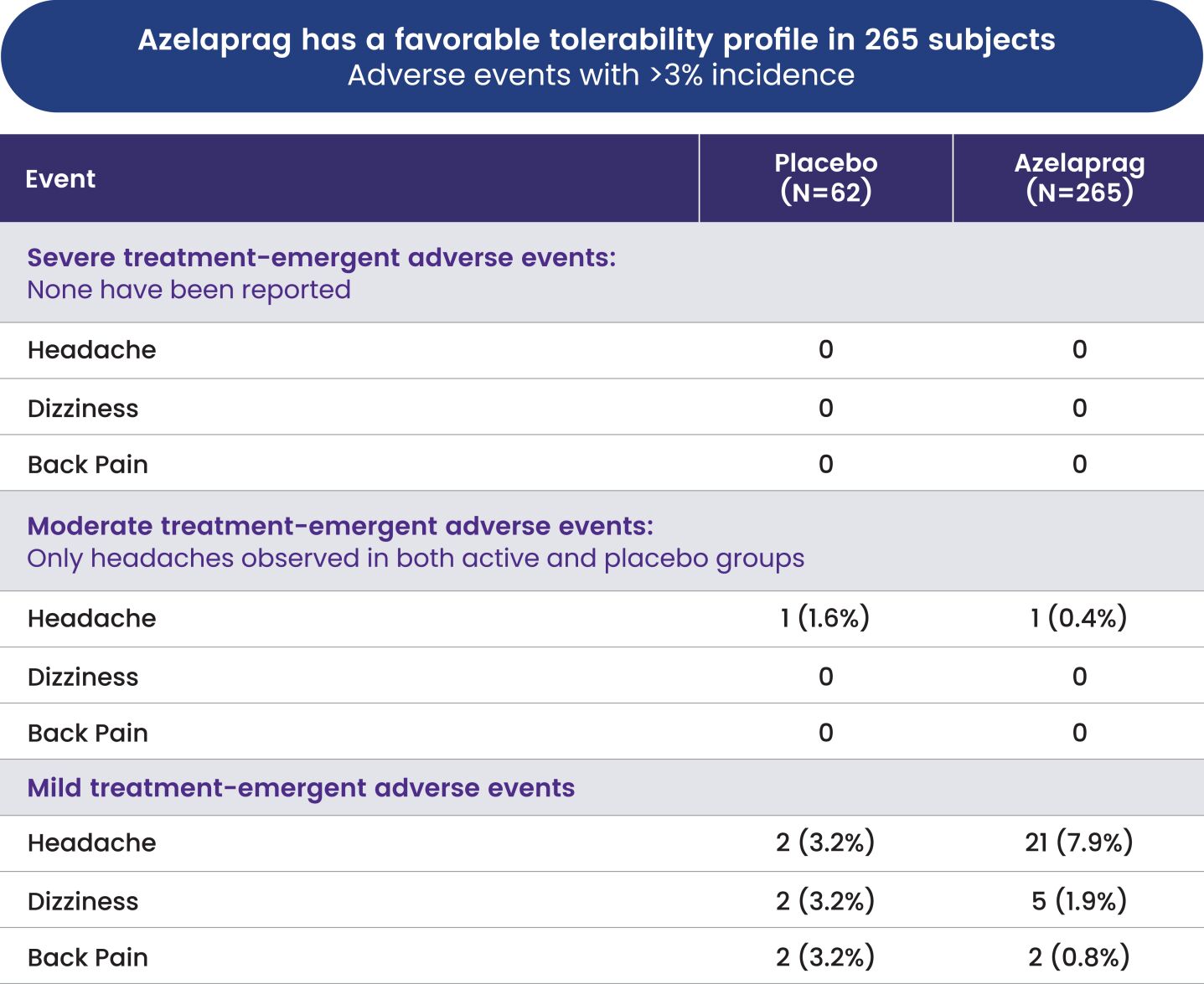
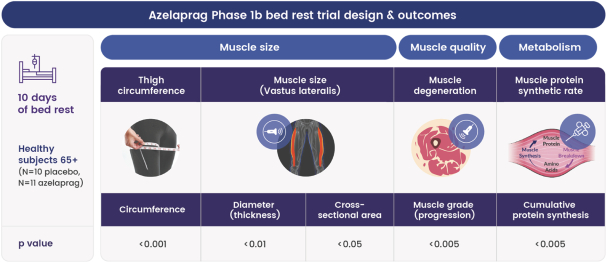
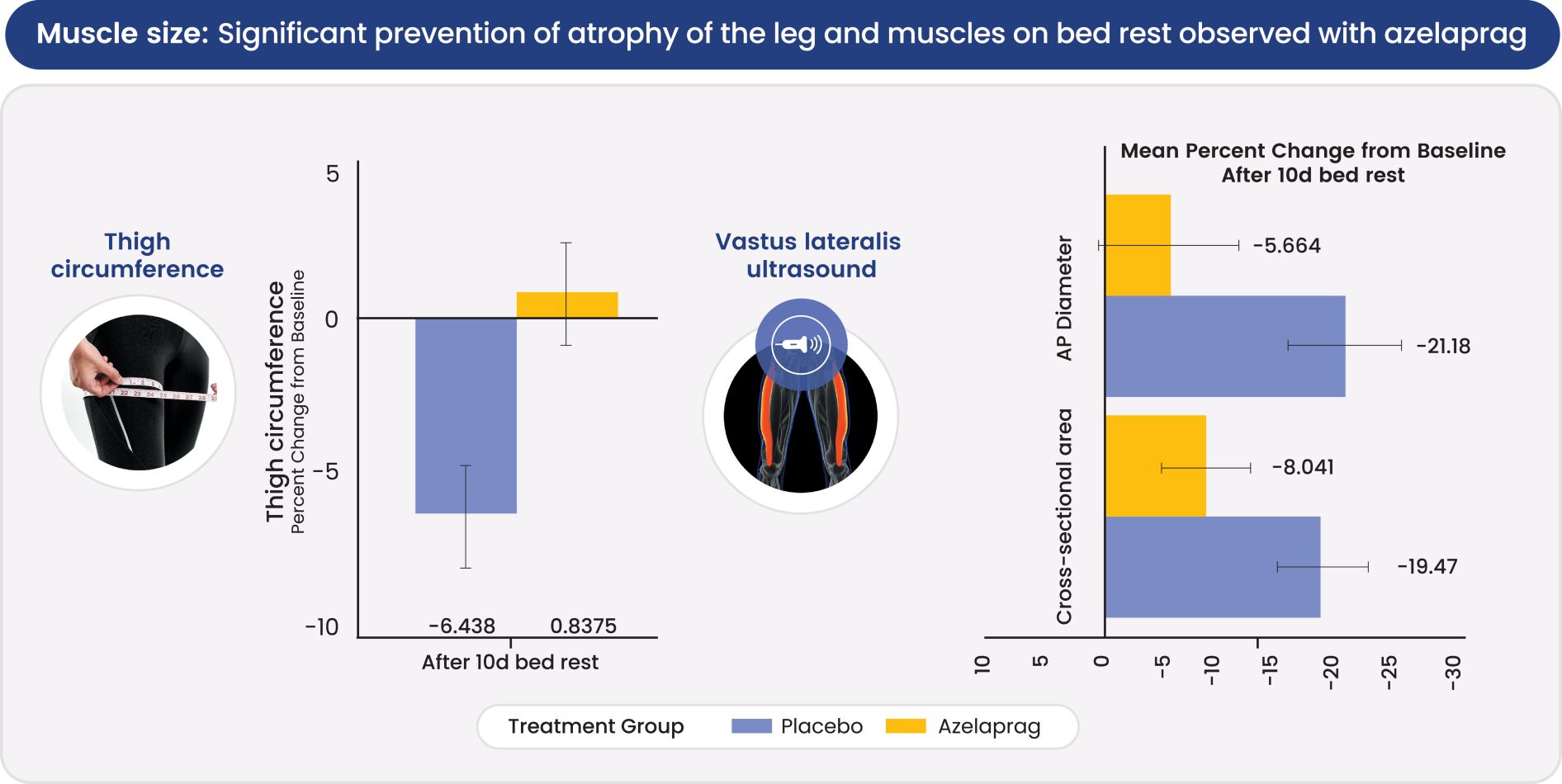
Australia, Brazil, Canada, China, Europe (with validation in 40 European states), India, Japan, Korea Mexico, Singapore, Taiwan and 23 other jurisdictions, and nine pending foreign national applications, including applications in Argentina, Egypt, Europe, Gulf Cooperation Council (GCC), Libya and Thailand. With respect to the 1 patent family that specifically and generically claims azelaprag, there are 10 issued U.S. patents, 77 issued foreign patents, and five pending foreign applications. U.S. Patent No. 9,573,936, U.S. Patent No. 9,868,721 and U.S. Patent No. 10,221,162 generically and specifically claim the drug substance azelaprag and each expires in 2036, without taking into account patent term adjustments, terminal disclaimers, or potential future extensions, and assuming payment of all appropriate maintenance fees. Foreign patents in this family expire and pending foreign applications are expected to expire in 2036, without taking into account potential future supplementary protection certificates and assuming payment of all appropriate annuity fees. The nine patent families that are directed to various structural analogs all expire between 2037 and 2039, without taking into account patent term adjustments, terminal disclaimers, or potential future extensions and assuming payment of all appropriate maintenance fees for the U.S. patents and without taking into account potential future supplementary protection certificates and assuming payment of all appropriate annuities for foreign patents in these families.
As of June 30, 2024, we had also in-licensed one patent family from INSERM relating to use of the class of apelin receptor agonists for treating sarcopenia. This patent family includes one U.S. Patent, and foreign national patents in Japan and Europe (with validation in 5 European states), which patents are expected to expire in 2032, without taking into account any patent term adjustments, or extensions, and assuming payment of all appropriate maintenance fees.
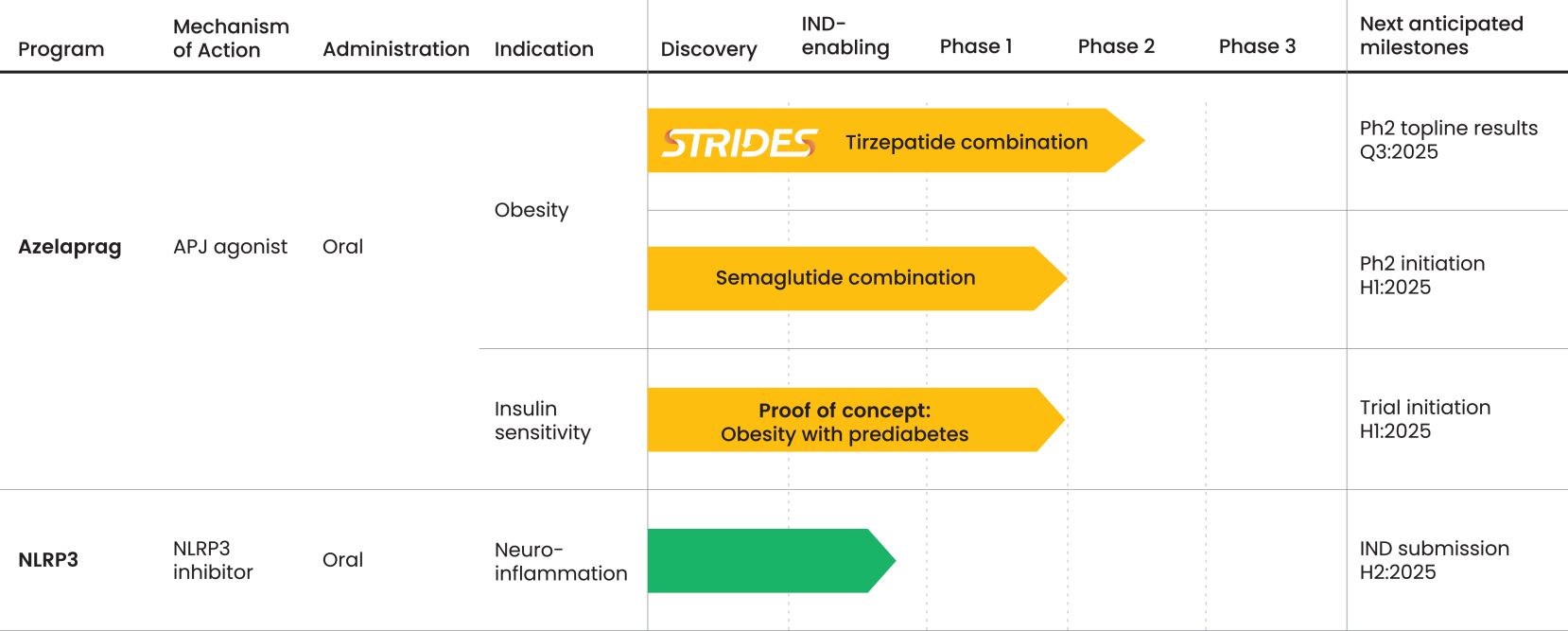
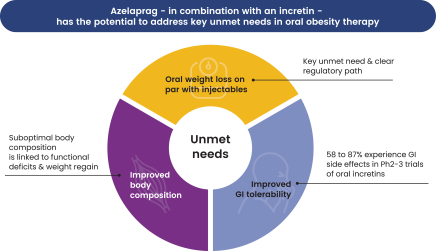
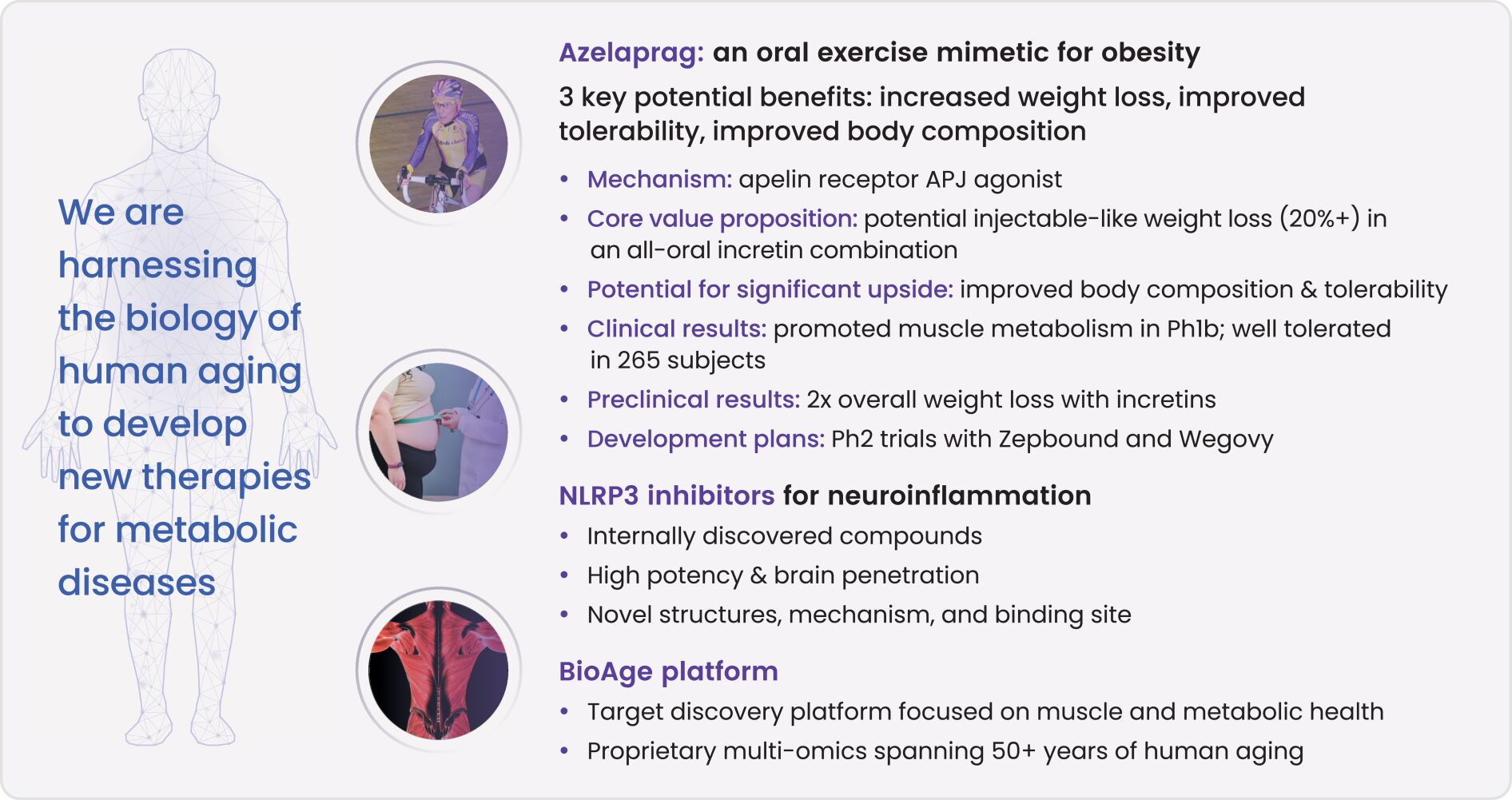
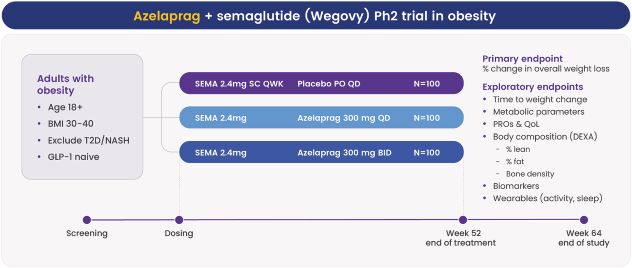
As of June 30, 2024, we owned seven patent families relating to methods of using azelaprag, including therapeutic uses for frailty, muscle atrophy, or obesity. These patent families include 14 pending U.S. provisional applications, seven pending U.S. and PCT non-provisional applications, and 14 pending foreign national applications, including applications in Australia, Brazil, Canada, China, Europe, Israel, Japan, Korea, Mexico, New Zealand, Singapore and Taiwan. Any patents that may issue from our pending patent applications or claim priority to pending provisional applications are expected to expire between 2042 and 2045, without taking into account any patent term adjustments, extensions or terminal disclaimers, and assuming payment of all appropriate maintenance fees.
NLRP3 Inhibitor Program
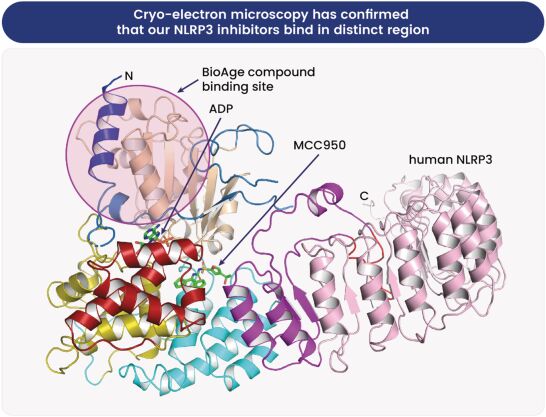
As of June 30, 2024, we owned six patent families relating to novel NLRP3 (nucleotide binding oligomerization domain-like receptor family pyrin domain-containing 3) inhibitors and related methods. One of these patent families is co-owned with HitGen, Inc. The six patent families include 3 issued U.S. patents (one co-owned with HitGen, that is under our exclusive control, and two solely-owned by BioAge), four pending U.S. provisional applications, eight pending U.S. and PCT non-provisional applications, and 28 pending foreign national applications, including applications in 24 jurisdictions, including Argentina, Australia, Canada, China, Europe, Eurasia, Japan, Korea and Taiwan. Patent term is based on the effective filing date of each family. Of the 3 issued patents, two will expire on March 23, 2042, and one will expire on January 27, 2043, without taking into account any patent term adjustments, extensions or terminal disclaimers, and assuming payment of all appropriate maintenance fees. Future patents that result from pending applications in these families are projected to expire on one of March 23, 2042; January 27, 2043; June 9, 2044; September 12, 2044; October 4, 2044; or March 26, 2045, without taking into account any patent term adjustments, extensions, or terminal disclaimers, and assuming payment of all appropriate maintenance fees.
Platform Technology and Discovery Program
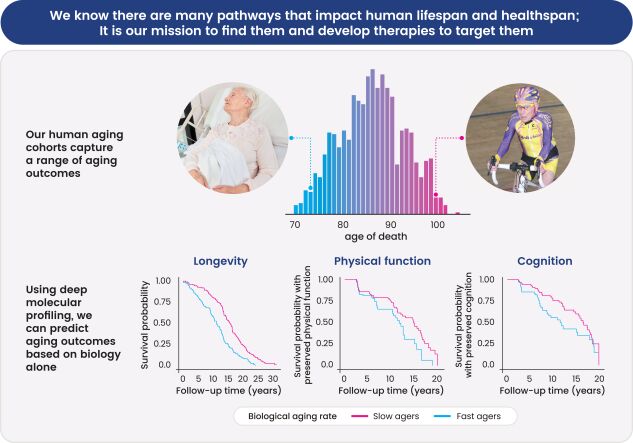
As of June 30, 2024, we owned 3 patent families relating to platform technology for identifying pathways for healthy aging and druggable targets, and 1 patent family relating to a class of therapeutic fusion proteins that bind endogenous RAGE ligands. These patent families include 4 issued U. S. patents, one issued Japanese patent, 3 pending U.S. applications, and 3 pending foreign national applications, including applications in Canada, and Europe. U.S. Patent No. 11,881,311 expires September 23, 2041, inclusive of patent term adjustment, and without taking into account any potential future extension. U.S. Patent No. 11,445,981 expires August 11, 2039, inclusive of patent term adjustment, and without taking into account any potential future extension. U.S. Patent
154